User login
DIAMOND: Adding patiromer helps optimize HF meds, foils hyperkalemia
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.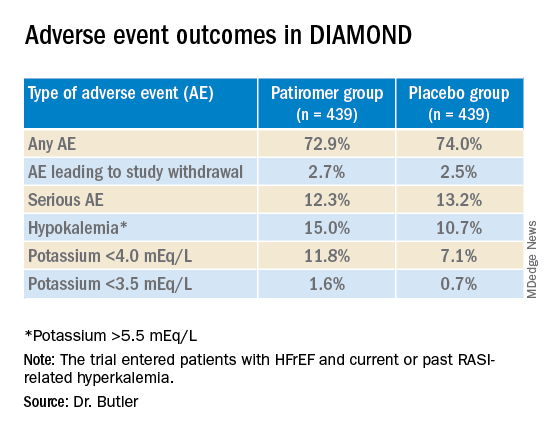
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
New HF guidelines feature ‘quad’ therapy, tweaked terminology
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
VALOR-HCM: Novel drug may delay, avert invasive therapy in OHCM
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.
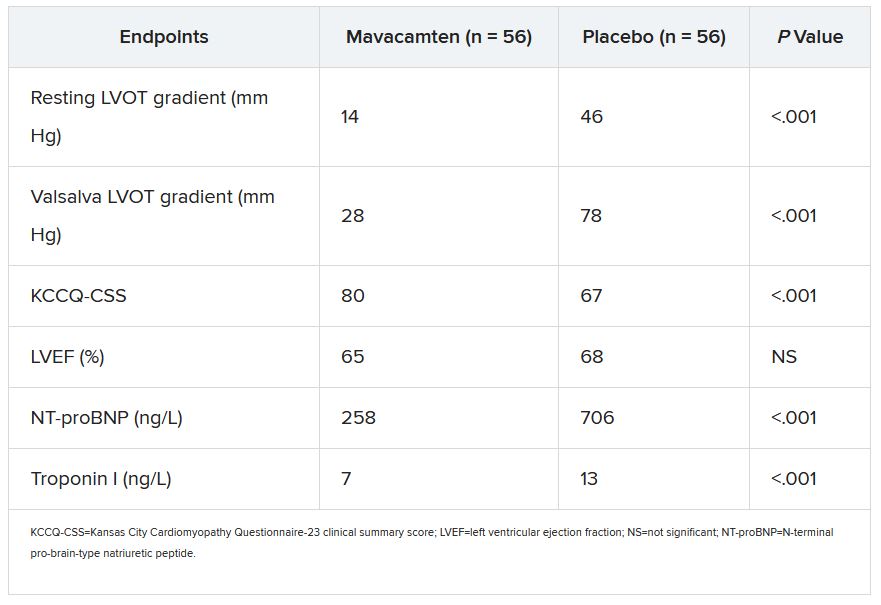
The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.

The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.

The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Calcium scores predict sudden-death risk in preclinical CAD
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
Hybrid ACC 2022 resurrects the live scientific session
Regardless of the pandemic’s sometimes mercurial behavior, the cardiology community appears set to reclaim valued traditions perhaps taken for granted in the pre-COVID era.
They include the bustling scientific congress and its myriad educational and networking prospects, along with pleiotropic effects like unplanned reunions with colleagues and catching up face-to-face with old friends.
That seems evident in the growing number of registrants for live attendance at at the annual scientific sessions of the American College of Cardiology, set for this Saturday through Monday in Washington as well as virtually, for a global reach that was unattainable in the pre-COVID era.
Registrations had hit the 11,000 mark and were picking up speed in recent weeks, ACC 2022 cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said at a mid-March presentation to the media.
They had reached about 12,880 and were still climbing a week before the conference, the ACC confirmed to this news organization. By then the professional registration had surpassed 9,900, of whom more than two-thirds reported plans to attend in person.
Dr. Morris said there had been 117 international submissions for what turned out to be 39 coveted spots on the meeting’s Late-Breaking Clinical Trial (LBCT) and Featured Clinical Research agenda spread across eight separate sessions.
On-site participants at the Walter E. Washington Convention Center should head for the Main Tent in Hall D for all LBCT presentations; venues for the Featured Clinical Research sessions are as noted below. Their real-time virtual equivalents will reside on the online platform’s Hot Topics channel. All noted session times are Eastern Daylight Time.
Saturday, April 2, 9:30 a.m.–10:30 a.m. Joint American College of Cardiology/Journal of the American College of Cardiology LBCT (I)
Leading off the conference’s first LBCT session, the randomized VALOR-HCM trial explored whether 16 weeks of mavacamten (MyoKardia) could help patients with severe obstructive hypertrophic cardiomyopathy (HCM) avoid septal reduction therapy, either surgical or by alcohol ablation.
The 22-center VALOR-HCM trial with an estimated enrollment of 100 follows EXPLORER-HCM, which in 2020 suggested the novel myosin-inhibiting agent could improve symptoms, exercise capacity, cardiac remodeling, and quality of life in such patients.
Simply advising people with heart failure (HF) to consume less salt is one thing, but it’s another to show them clinical trial evidence that it might help keep them out of the hospital. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure) study, conducted at 27 sites in six countries, sought to provide that evidence.
The trial randomly assigned 1,000 patients with NYHA class 2-3 HF to consume no more than 1,500 mg/day in sodium or to receive standard advice to limit sodium intake, and followed them for a year for the endpoint of death from any cause, cardiovascular (CV) hospitalization, or CV emergency department visit.
SODIUM-HF “may provide a rigorous evidence base for sodium restriction in patients with heart failure and may truly change our practice and how we recommend dietary modification,” ACC 2022 vice chair Douglas E. Drachman, MD, Massachusetts General Hospital, Boston, said at the media presentation.
In the same session, the CHAP (Chronic Hypertension and Pregnancy) study explored whether blood pressure (BP) control in pregnant women with new or untreated chronic hypertension could help avert preeclampsia, poor fetal outcomes, and other adverse events.
CHAP assigned about 2,400 women to receive either stepwise antihypertensive therapy to a BP goal of 140/90 mm Hg or lower or no such meds unless their BP reached or exceeded 160/105 mm Hg. Stepwise therapy featured either labetalol or extended-release nifedipine to start, the other agent added as necessary.
The LBCT block also includes the POISE-3 (Perioperative Ischemic Evaluation-3) comparison of the hemostatic agent tranexamic acid vs. placebo in nearly 10,000 patients undergoing noncardiac surgery. A separate randomization of the same cohort, to be reported at a Monday LBCT session, compared pre- and perioperative BP-control strategies.
Saturday, April 2, 12:00 p.m.–1:15 p.m. Featured Clinical Research I. Room 143A
This session features a subgroup analysis by age from the REVERSE-IT trial, which had previously showcased the monoclonal antibody bentracimab (PhaseBio Pharmaceuticals) for its ability to reverse the antiplatelet effects of ticagrelor.
REVERSE-IT is accompanied on the schedule by several secondary-endpoint presentations from trials whose primary outcomes have already been presented at meetings or in the journals.
They include the SCORED trial of sotagliflozin in patients with diabetes and chronic kidney disease (CKD); COMPLETE, which explored complete revascularization of multivessel coronary disease at primary stenting; and the FAME-3 comparison of coronary bypass surgery (CABG) vs. percutaneous coronary intervention (PCI) guided by fractional flow reserve (FFR) readings.
The session is to conclude with EDIT-CMD, which was a small, randomized assessment of diltiazem for improving microvascular dysfunction in patients with chronic angina despite nonobstructive coronary disease.
Sunday, April 3, 8:00 a.m.–9:15 a.m. Joint American College of Cardiology/Journal of the American Medical Association LBCT (II)
The SuperWIN (Supermarket Web Intervention) study tested an innovative strategy for community-based promotion of healthy lifestyle choices: point-of-purchase dietary education for grocery shoppers with an online instructional component, and follow-up to determine whether it influenced future food choices.
“Dietary interventions are notoriously difficult for us to implement, let alone to study scientifically,” Dr. Drachman observed. “So we think that there may be opportunity for dietary interventions to be best implemented at grocery stores where people are doing their shopping for food.”
SuperWIN compared supermarket shoppers with at least one CV risk factor who participated in the education intervention to a nonintervention control group for any changes in their DASH scores. The scores reflected consistency with the venerable DASH diet based on participants’ food purchases over 3 months.
In the same session, the MITIGATE trial explored whether daily administration of icosapent ethyl (Vascepa) might cut the risk of upper respiratory infection (especially from SARS-CoV-2 or seasonal influenza virus) in persons 50 or older with a history of clinical coronary, neurovascular, or peripheral vascular disease or revascularization. The trial has an estimated enrollment of 39,600.
Accompanying SuperWIN and MITIGATE are studies of several dyslipidemia drugs, including the discontinued antisense agent vupanorsen (Pfizer), as tested in TRANSLATE-TIMI 70; the PCSK9 inhibitor alirocumab (Praluent), explored for its effects on coronary plaque volume and composition in the PACMAN-AMI trial; and the APOLLO trial, a phase 1 evaluation of SLN360 (Silence Therapeutics), a short interfering ribonucleic acid (siRNA) that suppresses the molecular machinery in the liver that produces lipoprotein(a), or Lp(a).
The 32-patient APOLLO trial’s recently released top-line results suggested that SLN360 at varying dosages reduced Lp(a) levels by about one-half to more than 90%. Although elevated Lp(a) is known to track with CV risk, it remains to be shown whether dropping Lp(a) levels pharmacologically is protective.
Sunday, April 3, 9:45 a.m.–11:00 a.m. Joint American College of Cardiology/New England Journal of Medicine LBCT (III)
The meeting’s all-HF late-breaker session includes the METEORIC-HF trial, which compared the myotropic agent omecamtiv mecarbil (Cytokinetics) against placebo for effects on exercise performance over 20 weeks. The trial entered 276 patients with HF with reduced ejection fraction (HFrEF) and reduced peak VO2.
The GALACTIC-HF trial had previously suggested that the drug improved the risk of HF-related events or CV death in more than 8000 patients with HFrEF, those with the lowest ejection fractions benefiting the most.
This block of trials also features DIAMOND, the latest trial with a gemologic name to look at the potassium sequestrant patiromer (Veltassa) for any protection against hyperkalemia, a familiar side effect of renin-angiotensin-aldosterone inhibitors. DIAMOND tested patiromer in 878 patients with HFrEF who were on beta-blockers and other HF-appropriate medications and had a history of drug-associated hyperkalemia.
Previously, the AMBER trial of patients with CKD or refractory hypertension on spironolactone had suggested the drug might be protective enough against hyperkalemia to allow higher and more consistent dosing of BP-lowering agents.
Also in the session: the randomized IVVE (Influenza Vaccine to Prevent Adverse Vascular Events) trial, with an estimated 5,000 patients with HF in Africa, Asia, and the Middle East; PROMPT-HF, with a projected 1,310 HF patients and billed as a cluster-randomized pragmatic trial of a strategy for improving guideline-directed outpatient medical therapy; and MAVA-LTE, the long-term extension study of an estimated 310 patients who were in the MAVERICK-HCM and EXPLORER-HCM mavacamten trials.
Sunday, April 3, 12:15–1:30 p.m. Featured Clinical Research II. Main Tent, Hall D
The arrhythmia-centric session includes PARTITA, with its estimated 590 patients with primary- or secondary-prevention implantable cardioverter-defibrillators (ICDs). The trial followed them initially for burden of untreated nonsustained ventricular tachycardia (VT) or events treated with anti-tachycardia pacing. Then it randomly assigned those who experienced a first appropriate ICD shock to either immediate VT ablation or standard care. The latter included ablation on next occurrence of arrhythmic storm.
Investigational oral factor XIa inhibitors, viewed by many as potentially safer as anticoagulants than contemporary oral inhibitors of factor Xa, are now on the scene and include milvexian (Bristol-Myers Squibb/Janssen) and, lately, asundexian (BAY 2433334; Bayer). The latter agent was compared to the factor Xa inhibitor apixaban (Eliquis) in 753 patients with AF in the phase 2 PACIFIC-AF trial, which looked at the newer drug’s safety and optimal dosing.
Also on the bill: a long-term follow-up of the mAFA-2 (Mobile AF Application 2) extension study, which explored the value of a smartphone-based atrial fibrillation (AF) screening app for improving risk of AF-related events; a presentation billed as “Residual Leaks Post Left Atrial Appendage Occlusion”; and one that declares “low rates of guideline-directed care” to be “associated with higher mortality” in patients with pacemakers or ICDs.
Monday, April 4, 8:30 a.m.–9:45 a.m. LBCT IV
This session is to open with the PROTECT trial, which sought to determine whether perioperative “aggressive warming” may be cardioprotective in patients with CV risk factors undergoing noncardiac surgery. Its estimated 5,100 patients were randomly assigned to a procedure that achieves normothermia, that is 37° C (98.6° F), vs. standard care in which patients’ core temperature may decline to no further than 35.5° C (95.9° F).
Next on the list are a second POISE-3 comparison of BP-control strategies comparing hypotension avoidance vs. hypertension avoidance in patients undergoing noncardiac surgery; the pivotal CLASP 2 TR trial of patients with symptomatic tricuspid regurgitation on optimal medical therapy with vs. without treatment with the Edwards PASCAL Transcatheter Repair System; and one said to provide “insights from the Corevalve US Pivotal and SURTAVI trials” on 5-year incidence, timing, and predictors of hemodynamic valve deterioration transcatheter and surgical aortic bioprostheses.”
Rounding out the block of presentations: the ADAPT-TAVR comparison of the factor Xa inhibitor edoxaban (Lixiana) to dual-antiplatelet therapy for prevention of leaflet thrombosis after successful transcatheter aortic valve replacement (TAVR). The 235-patient trial was conducted at five centers in South Korea, Hong Kong, and Taiwan.
Monday, April 4, 11:00–12:15 p.m. LBCT V
This session includes the FLAVOUR randomized comparison of PCI guided by either FFR or intravascular ultrasound (IVUS) in 1,700 patients with 40%-70% stenoses. The patients from centers in China and South Korea were followed for death from any cause, MI, or any repeat revascularization at 24 months.
Also scheduled: the 2-year report on 4,000 patients with ST-segment elevation MI (STEMI) in the ACC-sponsored quality improvement program GHATI (Global Heart Attack Treatment Initiative); the GIPS-4 myocardial protection study of an estimated 380 patients with STEMI assigned to receive pre- and post-PCI infusions of sodium thiosulfate or placebo, with infarct size at 4 months as the primary endpoint; and a randomized test of an arrhythmia-monitoring implant for influence on clinical outcomes in 802 patients with a history of MI but no pacemaker or ICD indication, called BIO-GUARD-MI,
Last in the session: the Chocolate Touch Study of peripheral-artery angioplasty using a drug-coated balloon (DCB) with a confectionery name that treats lesions not with theobromine, but the antiproliferative mainstay paclitaxel.
The randomized comparison of the Chocolate Touch DCB (TriReme Medical) and the more established Lutonix DCB (Bard) assigned a projected 585 patients with symptomatic peripheral vascular disease to treatment of superficial femoral or popliteal artery lesions with one of the two paclitaxel-coated balloon catheters.
Monday, April 4, 12:45–2 p.m. Featured Clinical Research III. Room 143A
The final session features five subgroup analyses or other updates from trials that have already reported their primary outcomes. Among them is the SPYRAL HTN-ON MED trial, which helped to revitalize hopes for renal denervation therapy as a catheter-based treatment for drug-resistant hypertension by showing significant effects on both systolic and diastolic blood pressure. The new data follow the trial’s more than 400 patients out to 3 years.
There is also a symptom and quality-of-life analysis from the 530-patient EMPULSE trial of 530 patients with stabilized acute HF assigned in-hospital to start on empagliflozin (Jardiance) or placebo. The trial made a splash last year when it reported a significant improvement in risk for death or HF rehospitalization for its patients put on the SGLT2 inhibitor.
A secondary analysis from CANTOS is also featured; the trial had randomly assigned more than 10,000 patients with recent acute MI and elevated C-reactive protein (CRP) levels to receive or not receive the anti-inflammatory canakinumab (Ilaris). Those assigned to active therapy showed benefits for a range of outcomes, including CV mortality and stroke, but no decreases in cholesterol levels. Billing for the new CANTOS analysis promises insights on the “differential impact of residual inflammatory risk and residual cholesterol risk among atherosclerosis patients with and without chronic kidney disease.”
The session also features “trends and final results” from the NACMI (North American COVID-19 Myocardial Infarction) registry, which had shown excellent primary-PCI results without compromise of door-to-balloon times in patients with confirmed SARS-CoV-2 infection; and a FIDELITY analysis of cardiorenal endpoints by history of CV disease in the study’s more than 13,000 patients with diabetes and CKD assigned to placebo or finerenone (Kerendia), a mineralocorticoid receptor antagonist.
A version of this article first appeared on Medscape.com.
Regardless of the pandemic’s sometimes mercurial behavior, the cardiology community appears set to reclaim valued traditions perhaps taken for granted in the pre-COVID era.
They include the bustling scientific congress and its myriad educational and networking prospects, along with pleiotropic effects like unplanned reunions with colleagues and catching up face-to-face with old friends.
That seems evident in the growing number of registrants for live attendance at at the annual scientific sessions of the American College of Cardiology, set for this Saturday through Monday in Washington as well as virtually, for a global reach that was unattainable in the pre-COVID era.
Registrations had hit the 11,000 mark and were picking up speed in recent weeks, ACC 2022 cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said at a mid-March presentation to the media.
They had reached about 12,880 and were still climbing a week before the conference, the ACC confirmed to this news organization. By then the professional registration had surpassed 9,900, of whom more than two-thirds reported plans to attend in person.
Dr. Morris said there had been 117 international submissions for what turned out to be 39 coveted spots on the meeting’s Late-Breaking Clinical Trial (LBCT) and Featured Clinical Research agenda spread across eight separate sessions.
On-site participants at the Walter E. Washington Convention Center should head for the Main Tent in Hall D for all LBCT presentations; venues for the Featured Clinical Research sessions are as noted below. Their real-time virtual equivalents will reside on the online platform’s Hot Topics channel. All noted session times are Eastern Daylight Time.
Saturday, April 2, 9:30 a.m.–10:30 a.m. Joint American College of Cardiology/Journal of the American College of Cardiology LBCT (I)
Leading off the conference’s first LBCT session, the randomized VALOR-HCM trial explored whether 16 weeks of mavacamten (MyoKardia) could help patients with severe obstructive hypertrophic cardiomyopathy (HCM) avoid septal reduction therapy, either surgical or by alcohol ablation.
The 22-center VALOR-HCM trial with an estimated enrollment of 100 follows EXPLORER-HCM, which in 2020 suggested the novel myosin-inhibiting agent could improve symptoms, exercise capacity, cardiac remodeling, and quality of life in such patients.
Simply advising people with heart failure (HF) to consume less salt is one thing, but it’s another to show them clinical trial evidence that it might help keep them out of the hospital. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure) study, conducted at 27 sites in six countries, sought to provide that evidence.
The trial randomly assigned 1,000 patients with NYHA class 2-3 HF to consume no more than 1,500 mg/day in sodium or to receive standard advice to limit sodium intake, and followed them for a year for the endpoint of death from any cause, cardiovascular (CV) hospitalization, or CV emergency department visit.
SODIUM-HF “may provide a rigorous evidence base for sodium restriction in patients with heart failure and may truly change our practice and how we recommend dietary modification,” ACC 2022 vice chair Douglas E. Drachman, MD, Massachusetts General Hospital, Boston, said at the media presentation.
In the same session, the CHAP (Chronic Hypertension and Pregnancy) study explored whether blood pressure (BP) control in pregnant women with new or untreated chronic hypertension could help avert preeclampsia, poor fetal outcomes, and other adverse events.
CHAP assigned about 2,400 women to receive either stepwise antihypertensive therapy to a BP goal of 140/90 mm Hg or lower or no such meds unless their BP reached or exceeded 160/105 mm Hg. Stepwise therapy featured either labetalol or extended-release nifedipine to start, the other agent added as necessary.
The LBCT block also includes the POISE-3 (Perioperative Ischemic Evaluation-3) comparison of the hemostatic agent tranexamic acid vs. placebo in nearly 10,000 patients undergoing noncardiac surgery. A separate randomization of the same cohort, to be reported at a Monday LBCT session, compared pre- and perioperative BP-control strategies.
Saturday, April 2, 12:00 p.m.–1:15 p.m. Featured Clinical Research I. Room 143A
This session features a subgroup analysis by age from the REVERSE-IT trial, which had previously showcased the monoclonal antibody bentracimab (PhaseBio Pharmaceuticals) for its ability to reverse the antiplatelet effects of ticagrelor.
REVERSE-IT is accompanied on the schedule by several secondary-endpoint presentations from trials whose primary outcomes have already been presented at meetings or in the journals.
They include the SCORED trial of sotagliflozin in patients with diabetes and chronic kidney disease (CKD); COMPLETE, which explored complete revascularization of multivessel coronary disease at primary stenting; and the FAME-3 comparison of coronary bypass surgery (CABG) vs. percutaneous coronary intervention (PCI) guided by fractional flow reserve (FFR) readings.
The session is to conclude with EDIT-CMD, which was a small, randomized assessment of diltiazem for improving microvascular dysfunction in patients with chronic angina despite nonobstructive coronary disease.
Sunday, April 3, 8:00 a.m.–9:15 a.m. Joint American College of Cardiology/Journal of the American Medical Association LBCT (II)
The SuperWIN (Supermarket Web Intervention) study tested an innovative strategy for community-based promotion of healthy lifestyle choices: point-of-purchase dietary education for grocery shoppers with an online instructional component, and follow-up to determine whether it influenced future food choices.
“Dietary interventions are notoriously difficult for us to implement, let alone to study scientifically,” Dr. Drachman observed. “So we think that there may be opportunity for dietary interventions to be best implemented at grocery stores where people are doing their shopping for food.”
SuperWIN compared supermarket shoppers with at least one CV risk factor who participated in the education intervention to a nonintervention control group for any changes in their DASH scores. The scores reflected consistency with the venerable DASH diet based on participants’ food purchases over 3 months.
In the same session, the MITIGATE trial explored whether daily administration of icosapent ethyl (Vascepa) might cut the risk of upper respiratory infection (especially from SARS-CoV-2 or seasonal influenza virus) in persons 50 or older with a history of clinical coronary, neurovascular, or peripheral vascular disease or revascularization. The trial has an estimated enrollment of 39,600.
Accompanying SuperWIN and MITIGATE are studies of several dyslipidemia drugs, including the discontinued antisense agent vupanorsen (Pfizer), as tested in TRANSLATE-TIMI 70; the PCSK9 inhibitor alirocumab (Praluent), explored for its effects on coronary plaque volume and composition in the PACMAN-AMI trial; and the APOLLO trial, a phase 1 evaluation of SLN360 (Silence Therapeutics), a short interfering ribonucleic acid (siRNA) that suppresses the molecular machinery in the liver that produces lipoprotein(a), or Lp(a).
The 32-patient APOLLO trial’s recently released top-line results suggested that SLN360 at varying dosages reduced Lp(a) levels by about one-half to more than 90%. Although elevated Lp(a) is known to track with CV risk, it remains to be shown whether dropping Lp(a) levels pharmacologically is protective.
Sunday, April 3, 9:45 a.m.–11:00 a.m. Joint American College of Cardiology/New England Journal of Medicine LBCT (III)
The meeting’s all-HF late-breaker session includes the METEORIC-HF trial, which compared the myotropic agent omecamtiv mecarbil (Cytokinetics) against placebo for effects on exercise performance over 20 weeks. The trial entered 276 patients with HF with reduced ejection fraction (HFrEF) and reduced peak VO2.
The GALACTIC-HF trial had previously suggested that the drug improved the risk of HF-related events or CV death in more than 8000 patients with HFrEF, those with the lowest ejection fractions benefiting the most.
This block of trials also features DIAMOND, the latest trial with a gemologic name to look at the potassium sequestrant patiromer (Veltassa) for any protection against hyperkalemia, a familiar side effect of renin-angiotensin-aldosterone inhibitors. DIAMOND tested patiromer in 878 patients with HFrEF who were on beta-blockers and other HF-appropriate medications and had a history of drug-associated hyperkalemia.
Previously, the AMBER trial of patients with CKD or refractory hypertension on spironolactone had suggested the drug might be protective enough against hyperkalemia to allow higher and more consistent dosing of BP-lowering agents.
Also in the session: the randomized IVVE (Influenza Vaccine to Prevent Adverse Vascular Events) trial, with an estimated 5,000 patients with HF in Africa, Asia, and the Middle East; PROMPT-HF, with a projected 1,310 HF patients and billed as a cluster-randomized pragmatic trial of a strategy for improving guideline-directed outpatient medical therapy; and MAVA-LTE, the long-term extension study of an estimated 310 patients who were in the MAVERICK-HCM and EXPLORER-HCM mavacamten trials.
Sunday, April 3, 12:15–1:30 p.m. Featured Clinical Research II. Main Tent, Hall D
The arrhythmia-centric session includes PARTITA, with its estimated 590 patients with primary- or secondary-prevention implantable cardioverter-defibrillators (ICDs). The trial followed them initially for burden of untreated nonsustained ventricular tachycardia (VT) or events treated with anti-tachycardia pacing. Then it randomly assigned those who experienced a first appropriate ICD shock to either immediate VT ablation or standard care. The latter included ablation on next occurrence of arrhythmic storm.
Investigational oral factor XIa inhibitors, viewed by many as potentially safer as anticoagulants than contemporary oral inhibitors of factor Xa, are now on the scene and include milvexian (Bristol-Myers Squibb/Janssen) and, lately, asundexian (BAY 2433334; Bayer). The latter agent was compared to the factor Xa inhibitor apixaban (Eliquis) in 753 patients with AF in the phase 2 PACIFIC-AF trial, which looked at the newer drug’s safety and optimal dosing.
Also on the bill: a long-term follow-up of the mAFA-2 (Mobile AF Application 2) extension study, which explored the value of a smartphone-based atrial fibrillation (AF) screening app for improving risk of AF-related events; a presentation billed as “Residual Leaks Post Left Atrial Appendage Occlusion”; and one that declares “low rates of guideline-directed care” to be “associated with higher mortality” in patients with pacemakers or ICDs.
Monday, April 4, 8:30 a.m.–9:45 a.m. LBCT IV
This session is to open with the PROTECT trial, which sought to determine whether perioperative “aggressive warming” may be cardioprotective in patients with CV risk factors undergoing noncardiac surgery. Its estimated 5,100 patients were randomly assigned to a procedure that achieves normothermia, that is 37° C (98.6° F), vs. standard care in which patients’ core temperature may decline to no further than 35.5° C (95.9° F).
Next on the list are a second POISE-3 comparison of BP-control strategies comparing hypotension avoidance vs. hypertension avoidance in patients undergoing noncardiac surgery; the pivotal CLASP 2 TR trial of patients with symptomatic tricuspid regurgitation on optimal medical therapy with vs. without treatment with the Edwards PASCAL Transcatheter Repair System; and one said to provide “insights from the Corevalve US Pivotal and SURTAVI trials” on 5-year incidence, timing, and predictors of hemodynamic valve deterioration transcatheter and surgical aortic bioprostheses.”
Rounding out the block of presentations: the ADAPT-TAVR comparison of the factor Xa inhibitor edoxaban (Lixiana) to dual-antiplatelet therapy for prevention of leaflet thrombosis after successful transcatheter aortic valve replacement (TAVR). The 235-patient trial was conducted at five centers in South Korea, Hong Kong, and Taiwan.
Monday, April 4, 11:00–12:15 p.m. LBCT V
This session includes the FLAVOUR randomized comparison of PCI guided by either FFR or intravascular ultrasound (IVUS) in 1,700 patients with 40%-70% stenoses. The patients from centers in China and South Korea were followed for death from any cause, MI, or any repeat revascularization at 24 months.
Also scheduled: the 2-year report on 4,000 patients with ST-segment elevation MI (STEMI) in the ACC-sponsored quality improvement program GHATI (Global Heart Attack Treatment Initiative); the GIPS-4 myocardial protection study of an estimated 380 patients with STEMI assigned to receive pre- and post-PCI infusions of sodium thiosulfate or placebo, with infarct size at 4 months as the primary endpoint; and a randomized test of an arrhythmia-monitoring implant for influence on clinical outcomes in 802 patients with a history of MI but no pacemaker or ICD indication, called BIO-GUARD-MI,
Last in the session: the Chocolate Touch Study of peripheral-artery angioplasty using a drug-coated balloon (DCB) with a confectionery name that treats lesions not with theobromine, but the antiproliferative mainstay paclitaxel.
The randomized comparison of the Chocolate Touch DCB (TriReme Medical) and the more established Lutonix DCB (Bard) assigned a projected 585 patients with symptomatic peripheral vascular disease to treatment of superficial femoral or popliteal artery lesions with one of the two paclitaxel-coated balloon catheters.
Monday, April 4, 12:45–2 p.m. Featured Clinical Research III. Room 143A
The final session features five subgroup analyses or other updates from trials that have already reported their primary outcomes. Among them is the SPYRAL HTN-ON MED trial, which helped to revitalize hopes for renal denervation therapy as a catheter-based treatment for drug-resistant hypertension by showing significant effects on both systolic and diastolic blood pressure. The new data follow the trial’s more than 400 patients out to 3 years.
There is also a symptom and quality-of-life analysis from the 530-patient EMPULSE trial of 530 patients with stabilized acute HF assigned in-hospital to start on empagliflozin (Jardiance) or placebo. The trial made a splash last year when it reported a significant improvement in risk for death or HF rehospitalization for its patients put on the SGLT2 inhibitor.
A secondary analysis from CANTOS is also featured; the trial had randomly assigned more than 10,000 patients with recent acute MI and elevated C-reactive protein (CRP) levels to receive or not receive the anti-inflammatory canakinumab (Ilaris). Those assigned to active therapy showed benefits for a range of outcomes, including CV mortality and stroke, but no decreases in cholesterol levels. Billing for the new CANTOS analysis promises insights on the “differential impact of residual inflammatory risk and residual cholesterol risk among atherosclerosis patients with and without chronic kidney disease.”
The session also features “trends and final results” from the NACMI (North American COVID-19 Myocardial Infarction) registry, which had shown excellent primary-PCI results without compromise of door-to-balloon times in patients with confirmed SARS-CoV-2 infection; and a FIDELITY analysis of cardiorenal endpoints by history of CV disease in the study’s more than 13,000 patients with diabetes and CKD assigned to placebo or finerenone (Kerendia), a mineralocorticoid receptor antagonist.
A version of this article first appeared on Medscape.com.
Regardless of the pandemic’s sometimes mercurial behavior, the cardiology community appears set to reclaim valued traditions perhaps taken for granted in the pre-COVID era.
They include the bustling scientific congress and its myriad educational and networking prospects, along with pleiotropic effects like unplanned reunions with colleagues and catching up face-to-face with old friends.
That seems evident in the growing number of registrants for live attendance at at the annual scientific sessions of the American College of Cardiology, set for this Saturday through Monday in Washington as well as virtually, for a global reach that was unattainable in the pre-COVID era.
Registrations had hit the 11,000 mark and were picking up speed in recent weeks, ACC 2022 cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said at a mid-March presentation to the media.
They had reached about 12,880 and were still climbing a week before the conference, the ACC confirmed to this news organization. By then the professional registration had surpassed 9,900, of whom more than two-thirds reported plans to attend in person.
Dr. Morris said there had been 117 international submissions for what turned out to be 39 coveted spots on the meeting’s Late-Breaking Clinical Trial (LBCT) and Featured Clinical Research agenda spread across eight separate sessions.
On-site participants at the Walter E. Washington Convention Center should head for the Main Tent in Hall D for all LBCT presentations; venues for the Featured Clinical Research sessions are as noted below. Their real-time virtual equivalents will reside on the online platform’s Hot Topics channel. All noted session times are Eastern Daylight Time.
Saturday, April 2, 9:30 a.m.–10:30 a.m. Joint American College of Cardiology/Journal of the American College of Cardiology LBCT (I)
Leading off the conference’s first LBCT session, the randomized VALOR-HCM trial explored whether 16 weeks of mavacamten (MyoKardia) could help patients with severe obstructive hypertrophic cardiomyopathy (HCM) avoid septal reduction therapy, either surgical or by alcohol ablation.
The 22-center VALOR-HCM trial with an estimated enrollment of 100 follows EXPLORER-HCM, which in 2020 suggested the novel myosin-inhibiting agent could improve symptoms, exercise capacity, cardiac remodeling, and quality of life in such patients.
Simply advising people with heart failure (HF) to consume less salt is one thing, but it’s another to show them clinical trial evidence that it might help keep them out of the hospital. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure) study, conducted at 27 sites in six countries, sought to provide that evidence.
The trial randomly assigned 1,000 patients with NYHA class 2-3 HF to consume no more than 1,500 mg/day in sodium or to receive standard advice to limit sodium intake, and followed them for a year for the endpoint of death from any cause, cardiovascular (CV) hospitalization, or CV emergency department visit.
SODIUM-HF “may provide a rigorous evidence base for sodium restriction in patients with heart failure and may truly change our practice and how we recommend dietary modification,” ACC 2022 vice chair Douglas E. Drachman, MD, Massachusetts General Hospital, Boston, said at the media presentation.
In the same session, the CHAP (Chronic Hypertension and Pregnancy) study explored whether blood pressure (BP) control in pregnant women with new or untreated chronic hypertension could help avert preeclampsia, poor fetal outcomes, and other adverse events.
CHAP assigned about 2,400 women to receive either stepwise antihypertensive therapy to a BP goal of 140/90 mm Hg or lower or no such meds unless their BP reached or exceeded 160/105 mm Hg. Stepwise therapy featured either labetalol or extended-release nifedipine to start, the other agent added as necessary.
The LBCT block also includes the POISE-3 (Perioperative Ischemic Evaluation-3) comparison of the hemostatic agent tranexamic acid vs. placebo in nearly 10,000 patients undergoing noncardiac surgery. A separate randomization of the same cohort, to be reported at a Monday LBCT session, compared pre- and perioperative BP-control strategies.
Saturday, April 2, 12:00 p.m.–1:15 p.m. Featured Clinical Research I. Room 143A
This session features a subgroup analysis by age from the REVERSE-IT trial, which had previously showcased the monoclonal antibody bentracimab (PhaseBio Pharmaceuticals) for its ability to reverse the antiplatelet effects of ticagrelor.
REVERSE-IT is accompanied on the schedule by several secondary-endpoint presentations from trials whose primary outcomes have already been presented at meetings or in the journals.
They include the SCORED trial of sotagliflozin in patients with diabetes and chronic kidney disease (CKD); COMPLETE, which explored complete revascularization of multivessel coronary disease at primary stenting; and the FAME-3 comparison of coronary bypass surgery (CABG) vs. percutaneous coronary intervention (PCI) guided by fractional flow reserve (FFR) readings.
The session is to conclude with EDIT-CMD, which was a small, randomized assessment of diltiazem for improving microvascular dysfunction in patients with chronic angina despite nonobstructive coronary disease.
Sunday, April 3, 8:00 a.m.–9:15 a.m. Joint American College of Cardiology/Journal of the American Medical Association LBCT (II)
The SuperWIN (Supermarket Web Intervention) study tested an innovative strategy for community-based promotion of healthy lifestyle choices: point-of-purchase dietary education for grocery shoppers with an online instructional component, and follow-up to determine whether it influenced future food choices.
“Dietary interventions are notoriously difficult for us to implement, let alone to study scientifically,” Dr. Drachman observed. “So we think that there may be opportunity for dietary interventions to be best implemented at grocery stores where people are doing their shopping for food.”
SuperWIN compared supermarket shoppers with at least one CV risk factor who participated in the education intervention to a nonintervention control group for any changes in their DASH scores. The scores reflected consistency with the venerable DASH diet based on participants’ food purchases over 3 months.
In the same session, the MITIGATE trial explored whether daily administration of icosapent ethyl (Vascepa) might cut the risk of upper respiratory infection (especially from SARS-CoV-2 or seasonal influenza virus) in persons 50 or older with a history of clinical coronary, neurovascular, or peripheral vascular disease or revascularization. The trial has an estimated enrollment of 39,600.
Accompanying SuperWIN and MITIGATE are studies of several dyslipidemia drugs, including the discontinued antisense agent vupanorsen (Pfizer), as tested in TRANSLATE-TIMI 70; the PCSK9 inhibitor alirocumab (Praluent), explored for its effects on coronary plaque volume and composition in the PACMAN-AMI trial; and the APOLLO trial, a phase 1 evaluation of SLN360 (Silence Therapeutics), a short interfering ribonucleic acid (siRNA) that suppresses the molecular machinery in the liver that produces lipoprotein(a), or Lp(a).
The 32-patient APOLLO trial’s recently released top-line results suggested that SLN360 at varying dosages reduced Lp(a) levels by about one-half to more than 90%. Although elevated Lp(a) is known to track with CV risk, it remains to be shown whether dropping Lp(a) levels pharmacologically is protective.
Sunday, April 3, 9:45 a.m.–11:00 a.m. Joint American College of Cardiology/New England Journal of Medicine LBCT (III)
The meeting’s all-HF late-breaker session includes the METEORIC-HF trial, which compared the myotropic agent omecamtiv mecarbil (Cytokinetics) against placebo for effects on exercise performance over 20 weeks. The trial entered 276 patients with HF with reduced ejection fraction (HFrEF) and reduced peak VO2.
The GALACTIC-HF trial had previously suggested that the drug improved the risk of HF-related events or CV death in more than 8000 patients with HFrEF, those with the lowest ejection fractions benefiting the most.
This block of trials also features DIAMOND, the latest trial with a gemologic name to look at the potassium sequestrant patiromer (Veltassa) for any protection against hyperkalemia, a familiar side effect of renin-angiotensin-aldosterone inhibitors. DIAMOND tested patiromer in 878 patients with HFrEF who were on beta-blockers and other HF-appropriate medications and had a history of drug-associated hyperkalemia.
Previously, the AMBER trial of patients with CKD or refractory hypertension on spironolactone had suggested the drug might be protective enough against hyperkalemia to allow higher and more consistent dosing of BP-lowering agents.
Also in the session: the randomized IVVE (Influenza Vaccine to Prevent Adverse Vascular Events) trial, with an estimated 5,000 patients with HF in Africa, Asia, and the Middle East; PROMPT-HF, with a projected 1,310 HF patients and billed as a cluster-randomized pragmatic trial of a strategy for improving guideline-directed outpatient medical therapy; and MAVA-LTE, the long-term extension study of an estimated 310 patients who were in the MAVERICK-HCM and EXPLORER-HCM mavacamten trials.
Sunday, April 3, 12:15–1:30 p.m. Featured Clinical Research II. Main Tent, Hall D
The arrhythmia-centric session includes PARTITA, with its estimated 590 patients with primary- or secondary-prevention implantable cardioverter-defibrillators (ICDs). The trial followed them initially for burden of untreated nonsustained ventricular tachycardia (VT) or events treated with anti-tachycardia pacing. Then it randomly assigned those who experienced a first appropriate ICD shock to either immediate VT ablation or standard care. The latter included ablation on next occurrence of arrhythmic storm.
Investigational oral factor XIa inhibitors, viewed by many as potentially safer as anticoagulants than contemporary oral inhibitors of factor Xa, are now on the scene and include milvexian (Bristol-Myers Squibb/Janssen) and, lately, asundexian (BAY 2433334; Bayer). The latter agent was compared to the factor Xa inhibitor apixaban (Eliquis) in 753 patients with AF in the phase 2 PACIFIC-AF trial, which looked at the newer drug’s safety and optimal dosing.
Also on the bill: a long-term follow-up of the mAFA-2 (Mobile AF Application 2) extension study, which explored the value of a smartphone-based atrial fibrillation (AF) screening app for improving risk of AF-related events; a presentation billed as “Residual Leaks Post Left Atrial Appendage Occlusion”; and one that declares “low rates of guideline-directed care” to be “associated with higher mortality” in patients with pacemakers or ICDs.
Monday, April 4, 8:30 a.m.–9:45 a.m. LBCT IV
This session is to open with the PROTECT trial, which sought to determine whether perioperative “aggressive warming” may be cardioprotective in patients with CV risk factors undergoing noncardiac surgery. Its estimated 5,100 patients were randomly assigned to a procedure that achieves normothermia, that is 37° C (98.6° F), vs. standard care in which patients’ core temperature may decline to no further than 35.5° C (95.9° F).
Next on the list are a second POISE-3 comparison of BP-control strategies comparing hypotension avoidance vs. hypertension avoidance in patients undergoing noncardiac surgery; the pivotal CLASP 2 TR trial of patients with symptomatic tricuspid regurgitation on optimal medical therapy with vs. without treatment with the Edwards PASCAL Transcatheter Repair System; and one said to provide “insights from the Corevalve US Pivotal and SURTAVI trials” on 5-year incidence, timing, and predictors of hemodynamic valve deterioration transcatheter and surgical aortic bioprostheses.”
Rounding out the block of presentations: the ADAPT-TAVR comparison of the factor Xa inhibitor edoxaban (Lixiana) to dual-antiplatelet therapy for prevention of leaflet thrombosis after successful transcatheter aortic valve replacement (TAVR). The 235-patient trial was conducted at five centers in South Korea, Hong Kong, and Taiwan.
Monday, April 4, 11:00–12:15 p.m. LBCT V
This session includes the FLAVOUR randomized comparison of PCI guided by either FFR or intravascular ultrasound (IVUS) in 1,700 patients with 40%-70% stenoses. The patients from centers in China and South Korea were followed for death from any cause, MI, or any repeat revascularization at 24 months.
Also scheduled: the 2-year report on 4,000 patients with ST-segment elevation MI (STEMI) in the ACC-sponsored quality improvement program GHATI (Global Heart Attack Treatment Initiative); the GIPS-4 myocardial protection study of an estimated 380 patients with STEMI assigned to receive pre- and post-PCI infusions of sodium thiosulfate or placebo, with infarct size at 4 months as the primary endpoint; and a randomized test of an arrhythmia-monitoring implant for influence on clinical outcomes in 802 patients with a history of MI but no pacemaker or ICD indication, called BIO-GUARD-MI,
Last in the session: the Chocolate Touch Study of peripheral-artery angioplasty using a drug-coated balloon (DCB) with a confectionery name that treats lesions not with theobromine, but the antiproliferative mainstay paclitaxel.
The randomized comparison of the Chocolate Touch DCB (TriReme Medical) and the more established Lutonix DCB (Bard) assigned a projected 585 patients with symptomatic peripheral vascular disease to treatment of superficial femoral or popliteal artery lesions with one of the two paclitaxel-coated balloon catheters.
Monday, April 4, 12:45–2 p.m. Featured Clinical Research III. Room 143A
The final session features five subgroup analyses or other updates from trials that have already reported their primary outcomes. Among them is the SPYRAL HTN-ON MED trial, which helped to revitalize hopes for renal denervation therapy as a catheter-based treatment for drug-resistant hypertension by showing significant effects on both systolic and diastolic blood pressure. The new data follow the trial’s more than 400 patients out to 3 years.
There is also a symptom and quality-of-life analysis from the 530-patient EMPULSE trial of 530 patients with stabilized acute HF assigned in-hospital to start on empagliflozin (Jardiance) or placebo. The trial made a splash last year when it reported a significant improvement in risk for death or HF rehospitalization for its patients put on the SGLT2 inhibitor.
A secondary analysis from CANTOS is also featured; the trial had randomly assigned more than 10,000 patients with recent acute MI and elevated C-reactive protein (CRP) levels to receive or not receive the anti-inflammatory canakinumab (Ilaris). Those assigned to active therapy showed benefits for a range of outcomes, including CV mortality and stroke, but no decreases in cholesterol levels. Billing for the new CANTOS analysis promises insights on the “differential impact of residual inflammatory risk and residual cholesterol risk among atherosclerosis patients with and without chronic kidney disease.”
The session also features “trends and final results” from the NACMI (North American COVID-19 Myocardial Infarction) registry, which had shown excellent primary-PCI results without compromise of door-to-balloon times in patients with confirmed SARS-CoV-2 infection; and a FIDELITY analysis of cardiorenal endpoints by history of CV disease in the study’s more than 13,000 patients with diabetes and CKD assigned to placebo or finerenone (Kerendia), a mineralocorticoid receptor antagonist.
A version of this article first appeared on Medscape.com.
Death of pig heart transplant patient is more a beginning than an end
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
DISCHARGE: CTA shows safety edge versus cath in intermediate-risk stable chest pain
Clinical outcomes over several years in the randomized trial – called DISCHARGE, with an enrollment of more than 3,500 – were statistically similar whether the patients were assigned to CTA or invasive coronary angiography (ICA) as their initial evaluation. Symptoms and quality-of-life measures were also similar.
But the patients assigned to the initial-CTA strategy, of whom fewer than a fourth went on to cardiac cath, showed far fewer procedure-related complications and less often went to coronary revascularization during the median follow-up of 3.5 years, the group reported March 4 in the New England Journal of Medicine.
Based on the findings, CTA “is a safe alternative to cardiac catheterization for patients with suspected CAD [coronary artery disease] that will likely change clinical practice worldwide by replacing invasive testing in patients with stable chest pain who can be expected to benefit” those with an intermediate pretest probability for obstructive disease, principal investigator Marc Dewey, MD, Charité – Universitätsmedizin Berlin, told this news organization.
None of the patient subgroups explored in the trial showed a significant clinical benefit from one strategy over the other, Dr. Dewey commented in an email.
The trial’s results don’t apply to patients unlike those entered, and in particular, he said, “ICA should remain the first test option in patients with high clinical pretest probability of obstructive CAD.”
Dr. Dewey is senior author on the study’s publication, which was timed to coincide with his presentation of the results at ECR 2022 Overture, an all-virtual scientific session of the European Congress of Radiology.
“This is the definitive study,” Matthew Budoff, MD, Lundquist Institute at Harbor-UCLA, Torrance, California, said in an interview. It suggests in a large population that the initial CTA strategy “is as good and maybe safer” in stable patients at intermediate risk compared with initial ICA. “I would say close to 75% or 80% of the patients that we see would fall into that range of risk” and be suitable for the testing algorithm used in the study, said Dr. Budoff, who was not part of the trial.
Invasive angiography would generally still be the initial approach for patients at greater than intermediate risk, such as those with breakthrough angina or electrocardiographic changes, he said. “I still think there’s a huge role for invasive angiography. It’s just a bit smaller now than it used to be for the lower-risk patient.”
The DISCHARGE trial, agreed cardiothoracic radiology specialist Rozemarijn Vliegenthart, MD, PhD, University of Groningen, the Netherlands, “shows that in patients with intermediate pretest probability, CTA should be used as a gatekeeper before invasive coronary angiography, instead of directly referring for invasive coronary angiography.”
It shows that “a CT-first approach” is both safe and clinically effective and even a trend suggesting better clinical outcomes, compared with ICA. And it demonstrates that “still, many diagnostic invasive coronary angiographies are performed unnecessarily,” Dr. Vliegenthart said as the invited discussant following Dr. Dewey’s presentation.
DISCOVER is only the latest in a series of major studies to explore how CTA best fits in with ICA, stress imaging, and other tests for evaluating patients with chest pain. For example, “the PROMISE trial and the SCOT-HEART trial found that CT was as good as or even better than functional testing. DISCHARGE, I think, confirms the safety of the CT strategy” and reaffirms that it is “at least as good” as an ICA-first approach, cardiologist Klaus F. Kofoed, MD, PhD, DMSc, Rigshospitalet University of Copenhagen, said when co-presenting the trial’s results with Dr. Dewey.
“We can now say CT may be suitable in intermediate-risk patients referred for ICA, particularly those with a clinical constellation suggesting a higher event risk, with abnormal or inconclusive functional test results, or with persistent symptoms despite medical treatment,” said Dr. Kofoed, who is on the DISCOVER steering committee.
The trial’s 3,561 patients with stable chest pain – at 26 experienced centers in 16 countries – were randomly assigned to undergo CTA or ICA as their initial diagnostic imaging approach. Entry required them to be at intermediate risk, defined as an estimated 10% to 60% probability of having obstructive CAD. Of note, women made up about 56% of both groups.
Imaging was positive for obstructive disease in 26% of the 1,808 patients in the CTA group and in the same proportion of the 1,753 who were assigned to ICA. Nonobstructive CAD was identified in 36% and 22%, respectively.
Importantly, 404 (22.3%) patients in the CTA group then underwent ICA, which identified obstructive CAD in 293 (72.5%).
With a complete follow-up in about 99% of patients, the report notes, the rate of the primary endpoint of major adverse cardiac events, or MACE (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) was 2.1% in the CTA group and in 3.0% in the ICA group. The adjusted hazard ratio of 0.70 (95% confidence interval, 0.46-1.07; P = .10) fell short of significance.
The corresponding HR for an “expanded primary outcome” that also included transient ischemic attack or major procedure-related complications was 0.60 (95% CI, 0.42-0.85) in favor of the CTA group.
As a “pragmatic trial,” DISCHARGE relied on clinically identified events for the endpoint assessments and did not require, for example, laboratory biomarker or neurologic imaging for confirmation, the report notes.
Major procedure-related complications during the initial management phase occurred in 0.5% of the CTA group, and 1.9% of those assigned to initial ICA (HR, 0.26; 95% CI, 0.13-0.55).
Coronary revascularization was less common in the CTA group during the trial’s follow-up, 14.2% versus 18.0% for those assigned to ICA (HR, 0.76; 95% CI, 0.65-0.90).
But the prevalences of angina during the final 4 weeks of follow-up, the group reported, were statistically similar at 8.8% and 7.5% for patients assigned to CTA and ICA, respectively.
The trial showed “no material difference” between the initial CTA versus ICA strategies for its MACE primary endpoint, observed Joseph Loscalzo, MD, PhD, Brigham and Women’s Hospital and Harvard Medical School, Boston, Mass., in an accompanying editorial.
“This result is probably a consequence of the lack of effect of revascularization on cardiovascular events among most patients with stable angina and the limited number of those with high-risk anatomy who would benefit from revascularization in the trial,” he writes.
That CTA was performed “significantly earlier than angiography, 3 days versus 12 days after enrollment,” may have led to earlier coronary revascularization in that group, and therefore is “a better outcome in patients whose anatomy would benefit from it.”
Dr. Loscalzo questioned several aspects of the trial design, which, for example, led to a more than 35% prevalence of patients with nonanginal chest pain among those randomized. Different criteria for classifying patients as “intermediate risk” might also have contributed to the fairly low prevalence of patients in either group ultimately identified with obstructive CAD, he proposes. That low prevalence “suggests that the overall trial population had a low risk of obstructive CAD rather than an intermediate risk.”
DISCHARGE was supported by grants from the European Union Seventh Framework Program, the Berlin Institute of Health, Rigshospitalet of the University of Copenhagen, the British Heart Foundation, and the German Research Foundation. Disclosures for the authors and editorialist are available at NEJM.org. Dr. Budoff has disclosed receiving grant support from General Electric. Dr. Vliegenthart discloses receiving grants from Siemens Healthineers and honorarium for speaking from Siemens Healthineers and Bayer.
A version of this article first appeared on Medscape.com.
Clinical outcomes over several years in the randomized trial – called DISCHARGE, with an enrollment of more than 3,500 – were statistically similar whether the patients were assigned to CTA or invasive coronary angiography (ICA) as their initial evaluation. Symptoms and quality-of-life measures were also similar.
But the patients assigned to the initial-CTA strategy, of whom fewer than a fourth went on to cardiac cath, showed far fewer procedure-related complications and less often went to coronary revascularization during the median follow-up of 3.5 years, the group reported March 4 in the New England Journal of Medicine.
Based on the findings, CTA “is a safe alternative to cardiac catheterization for patients with suspected CAD [coronary artery disease] that will likely change clinical practice worldwide by replacing invasive testing in patients with stable chest pain who can be expected to benefit” those with an intermediate pretest probability for obstructive disease, principal investigator Marc Dewey, MD, Charité – Universitätsmedizin Berlin, told this news organization.
None of the patient subgroups explored in the trial showed a significant clinical benefit from one strategy over the other, Dr. Dewey commented in an email.
The trial’s results don’t apply to patients unlike those entered, and in particular, he said, “ICA should remain the first test option in patients with high clinical pretest probability of obstructive CAD.”
Dr. Dewey is senior author on the study’s publication, which was timed to coincide with his presentation of the results at ECR 2022 Overture, an all-virtual scientific session of the European Congress of Radiology.
“This is the definitive study,” Matthew Budoff, MD, Lundquist Institute at Harbor-UCLA, Torrance, California, said in an interview. It suggests in a large population that the initial CTA strategy “is as good and maybe safer” in stable patients at intermediate risk compared with initial ICA. “I would say close to 75% or 80% of the patients that we see would fall into that range of risk” and be suitable for the testing algorithm used in the study, said Dr. Budoff, who was not part of the trial.
Invasive angiography would generally still be the initial approach for patients at greater than intermediate risk, such as those with breakthrough angina or electrocardiographic changes, he said. “I still think there’s a huge role for invasive angiography. It’s just a bit smaller now than it used to be for the lower-risk patient.”
The DISCHARGE trial, agreed cardiothoracic radiology specialist Rozemarijn Vliegenthart, MD, PhD, University of Groningen, the Netherlands, “shows that in patients with intermediate pretest probability, CTA should be used as a gatekeeper before invasive coronary angiography, instead of directly referring for invasive coronary angiography.”
It shows that “a CT-first approach” is both safe and clinically effective and even a trend suggesting better clinical outcomes, compared with ICA. And it demonstrates that “still, many diagnostic invasive coronary angiographies are performed unnecessarily,” Dr. Vliegenthart said as the invited discussant following Dr. Dewey’s presentation.
DISCOVER is only the latest in a series of major studies to explore how CTA best fits in with ICA, stress imaging, and other tests for evaluating patients with chest pain. For example, “the PROMISE trial and the SCOT-HEART trial found that CT was as good as or even better than functional testing. DISCHARGE, I think, confirms the safety of the CT strategy” and reaffirms that it is “at least as good” as an ICA-first approach, cardiologist Klaus F. Kofoed, MD, PhD, DMSc, Rigshospitalet University of Copenhagen, said when co-presenting the trial’s results with Dr. Dewey.
“We can now say CT may be suitable in intermediate-risk patients referred for ICA, particularly those with a clinical constellation suggesting a higher event risk, with abnormal or inconclusive functional test results, or with persistent symptoms despite medical treatment,” said Dr. Kofoed, who is on the DISCOVER steering committee.
The trial’s 3,561 patients with stable chest pain – at 26 experienced centers in 16 countries – were randomly assigned to undergo CTA or ICA as their initial diagnostic imaging approach. Entry required them to be at intermediate risk, defined as an estimated 10% to 60% probability of having obstructive CAD. Of note, women made up about 56% of both groups.
Imaging was positive for obstructive disease in 26% of the 1,808 patients in the CTA group and in the same proportion of the 1,753 who were assigned to ICA. Nonobstructive CAD was identified in 36% and 22%, respectively.
Importantly, 404 (22.3%) patients in the CTA group then underwent ICA, which identified obstructive CAD in 293 (72.5%).
With a complete follow-up in about 99% of patients, the report notes, the rate of the primary endpoint of major adverse cardiac events, or MACE (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) was 2.1% in the CTA group and in 3.0% in the ICA group. The adjusted hazard ratio of 0.70 (95% confidence interval, 0.46-1.07; P = .10) fell short of significance.
The corresponding HR for an “expanded primary outcome” that also included transient ischemic attack or major procedure-related complications was 0.60 (95% CI, 0.42-0.85) in favor of the CTA group.
As a “pragmatic trial,” DISCHARGE relied on clinically identified events for the endpoint assessments and did not require, for example, laboratory biomarker or neurologic imaging for confirmation, the report notes.
Major procedure-related complications during the initial management phase occurred in 0.5% of the CTA group, and 1.9% of those assigned to initial ICA (HR, 0.26; 95% CI, 0.13-0.55).
Coronary revascularization was less common in the CTA group during the trial’s follow-up, 14.2% versus 18.0% for those assigned to ICA (HR, 0.76; 95% CI, 0.65-0.90).
But the prevalences of angina during the final 4 weeks of follow-up, the group reported, were statistically similar at 8.8% and 7.5% for patients assigned to CTA and ICA, respectively.
The trial showed “no material difference” between the initial CTA versus ICA strategies for its MACE primary endpoint, observed Joseph Loscalzo, MD, PhD, Brigham and Women’s Hospital and Harvard Medical School, Boston, Mass., in an accompanying editorial.
“This result is probably a consequence of the lack of effect of revascularization on cardiovascular events among most patients with stable angina and the limited number of those with high-risk anatomy who would benefit from revascularization in the trial,” he writes.
That CTA was performed “significantly earlier than angiography, 3 days versus 12 days after enrollment,” may have led to earlier coronary revascularization in that group, and therefore is “a better outcome in patients whose anatomy would benefit from it.”
Dr. Loscalzo questioned several aspects of the trial design, which, for example, led to a more than 35% prevalence of patients with nonanginal chest pain among those randomized. Different criteria for classifying patients as “intermediate risk” might also have contributed to the fairly low prevalence of patients in either group ultimately identified with obstructive CAD, he proposes. That low prevalence “suggests that the overall trial population had a low risk of obstructive CAD rather than an intermediate risk.”
DISCHARGE was supported by grants from the European Union Seventh Framework Program, the Berlin Institute of Health, Rigshospitalet of the University of Copenhagen, the British Heart Foundation, and the German Research Foundation. Disclosures for the authors and editorialist are available at NEJM.org. Dr. Budoff has disclosed receiving grant support from General Electric. Dr. Vliegenthart discloses receiving grants from Siemens Healthineers and honorarium for speaking from Siemens Healthineers and Bayer.
A version of this article first appeared on Medscape.com.
Clinical outcomes over several years in the randomized trial – called DISCHARGE, with an enrollment of more than 3,500 – were statistically similar whether the patients were assigned to CTA or invasive coronary angiography (ICA) as their initial evaluation. Symptoms and quality-of-life measures were also similar.
But the patients assigned to the initial-CTA strategy, of whom fewer than a fourth went on to cardiac cath, showed far fewer procedure-related complications and less often went to coronary revascularization during the median follow-up of 3.5 years, the group reported March 4 in the New England Journal of Medicine.
Based on the findings, CTA “is a safe alternative to cardiac catheterization for patients with suspected CAD [coronary artery disease] that will likely change clinical practice worldwide by replacing invasive testing in patients with stable chest pain who can be expected to benefit” those with an intermediate pretest probability for obstructive disease, principal investigator Marc Dewey, MD, Charité – Universitätsmedizin Berlin, told this news organization.
None of the patient subgroups explored in the trial showed a significant clinical benefit from one strategy over the other, Dr. Dewey commented in an email.
The trial’s results don’t apply to patients unlike those entered, and in particular, he said, “ICA should remain the first test option in patients with high clinical pretest probability of obstructive CAD.”
Dr. Dewey is senior author on the study’s publication, which was timed to coincide with his presentation of the results at ECR 2022 Overture, an all-virtual scientific session of the European Congress of Radiology.
“This is the definitive study,” Matthew Budoff, MD, Lundquist Institute at Harbor-UCLA, Torrance, California, said in an interview. It suggests in a large population that the initial CTA strategy “is as good and maybe safer” in stable patients at intermediate risk compared with initial ICA. “I would say close to 75% or 80% of the patients that we see would fall into that range of risk” and be suitable for the testing algorithm used in the study, said Dr. Budoff, who was not part of the trial.
Invasive angiography would generally still be the initial approach for patients at greater than intermediate risk, such as those with breakthrough angina or electrocardiographic changes, he said. “I still think there’s a huge role for invasive angiography. It’s just a bit smaller now than it used to be for the lower-risk patient.”
The DISCHARGE trial, agreed cardiothoracic radiology specialist Rozemarijn Vliegenthart, MD, PhD, University of Groningen, the Netherlands, “shows that in patients with intermediate pretest probability, CTA should be used as a gatekeeper before invasive coronary angiography, instead of directly referring for invasive coronary angiography.”
It shows that “a CT-first approach” is both safe and clinically effective and even a trend suggesting better clinical outcomes, compared with ICA. And it demonstrates that “still, many diagnostic invasive coronary angiographies are performed unnecessarily,” Dr. Vliegenthart said as the invited discussant following Dr. Dewey’s presentation.
DISCOVER is only the latest in a series of major studies to explore how CTA best fits in with ICA, stress imaging, and other tests for evaluating patients with chest pain. For example, “the PROMISE trial and the SCOT-HEART trial found that CT was as good as or even better than functional testing. DISCHARGE, I think, confirms the safety of the CT strategy” and reaffirms that it is “at least as good” as an ICA-first approach, cardiologist Klaus F. Kofoed, MD, PhD, DMSc, Rigshospitalet University of Copenhagen, said when co-presenting the trial’s results with Dr. Dewey.
“We can now say CT may be suitable in intermediate-risk patients referred for ICA, particularly those with a clinical constellation suggesting a higher event risk, with abnormal or inconclusive functional test results, or with persistent symptoms despite medical treatment,” said Dr. Kofoed, who is on the DISCOVER steering committee.
The trial’s 3,561 patients with stable chest pain – at 26 experienced centers in 16 countries – were randomly assigned to undergo CTA or ICA as their initial diagnostic imaging approach. Entry required them to be at intermediate risk, defined as an estimated 10% to 60% probability of having obstructive CAD. Of note, women made up about 56% of both groups.
Imaging was positive for obstructive disease in 26% of the 1,808 patients in the CTA group and in the same proportion of the 1,753 who were assigned to ICA. Nonobstructive CAD was identified in 36% and 22%, respectively.
Importantly, 404 (22.3%) patients in the CTA group then underwent ICA, which identified obstructive CAD in 293 (72.5%).
With a complete follow-up in about 99% of patients, the report notes, the rate of the primary endpoint of major adverse cardiac events, or MACE (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) was 2.1% in the CTA group and in 3.0% in the ICA group. The adjusted hazard ratio of 0.70 (95% confidence interval, 0.46-1.07; P = .10) fell short of significance.
The corresponding HR for an “expanded primary outcome” that also included transient ischemic attack or major procedure-related complications was 0.60 (95% CI, 0.42-0.85) in favor of the CTA group.
As a “pragmatic trial,” DISCHARGE relied on clinically identified events for the endpoint assessments and did not require, for example, laboratory biomarker or neurologic imaging for confirmation, the report notes.
Major procedure-related complications during the initial management phase occurred in 0.5% of the CTA group, and 1.9% of those assigned to initial ICA (HR, 0.26; 95% CI, 0.13-0.55).
Coronary revascularization was less common in the CTA group during the trial’s follow-up, 14.2% versus 18.0% for those assigned to ICA (HR, 0.76; 95% CI, 0.65-0.90).
But the prevalences of angina during the final 4 weeks of follow-up, the group reported, were statistically similar at 8.8% and 7.5% for patients assigned to CTA and ICA, respectively.
The trial showed “no material difference” between the initial CTA versus ICA strategies for its MACE primary endpoint, observed Joseph Loscalzo, MD, PhD, Brigham and Women’s Hospital and Harvard Medical School, Boston, Mass., in an accompanying editorial.
“This result is probably a consequence of the lack of effect of revascularization on cardiovascular events among most patients with stable angina and the limited number of those with high-risk anatomy who would benefit from revascularization in the trial,” he writes.
That CTA was performed “significantly earlier than angiography, 3 days versus 12 days after enrollment,” may have led to earlier coronary revascularization in that group, and therefore is “a better outcome in patients whose anatomy would benefit from it.”
Dr. Loscalzo questioned several aspects of the trial design, which, for example, led to a more than 35% prevalence of patients with nonanginal chest pain among those randomized. Different criteria for classifying patients as “intermediate risk” might also have contributed to the fairly low prevalence of patients in either group ultimately identified with obstructive CAD, he proposes. That low prevalence “suggests that the overall trial population had a low risk of obstructive CAD rather than an intermediate risk.”
DISCHARGE was supported by grants from the European Union Seventh Framework Program, the Berlin Institute of Health, Rigshospitalet of the University of Copenhagen, the British Heart Foundation, and the German Research Foundation. Disclosures for the authors and editorialist are available at NEJM.org. Dr. Budoff has disclosed receiving grant support from General Electric. Dr. Vliegenthart discloses receiving grants from Siemens Healthineers and honorarium for speaking from Siemens Healthineers and Bayer.
A version of this article first appeared on Medscape.com.
How Lp(a) can help improve ASCVD risk assessment
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).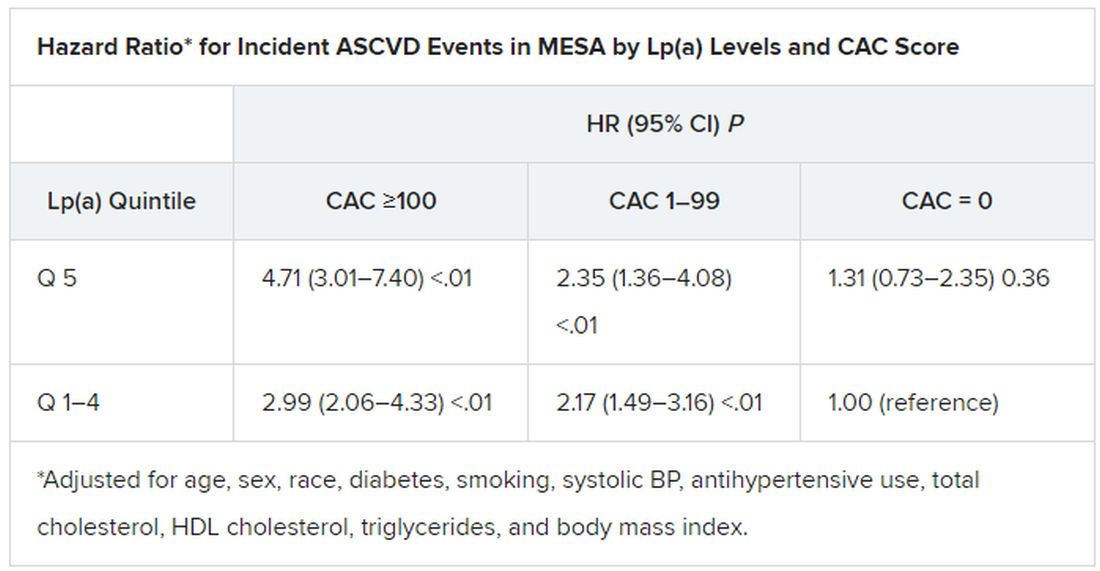
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).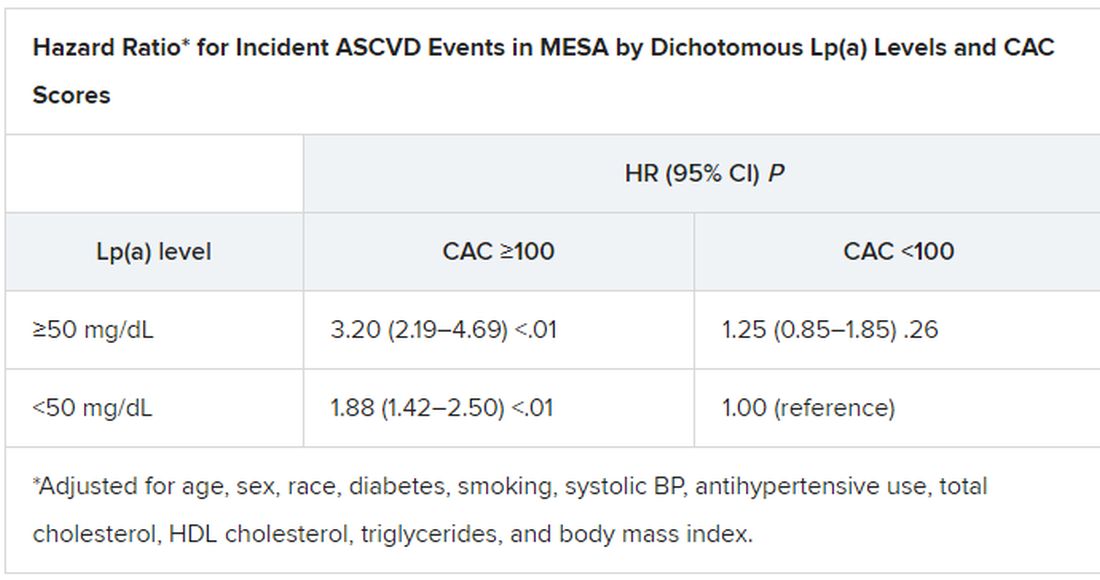
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FDA okays empagliflozin for HF regardless of ejection fraction
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
USPSTF tweaks primary prevention statin recommendations in new draft guidance
Given the expansive contemporary role of statins for primary cardiovascular disease (CVD) prevention, the language in the new U.S. Preventive Services Task Force draft guidance on their use in that setting may seem conservative. Even so, the proposed recommendations, open to public comment until March 21, take more recent data into account but don’t substantially vary from the 2016 USPSTF document they are intended to replace.
The task force concluded “with moderate certainty” that a statin prescription will clinically benefit adults aged 40-75 years without CVD but with at least one of several risk factors, such as dyslipidemia or diabetes, who have a 10-year CVD risk of at least 7.5%.
the new report states. That, says an accompanying USPSTF press release, means such people “may benefit from statin use and should decide with their clinician if taking a statin is right for them.”
Also, notes the report, the net benefit of statin therapy is “at least moderate” for individuals with a 10% or greater CVD risk over the next decade who, the press release states, “should take a statin to prevent a first heart attack or stroke.”
The evidence review on which the task force based the guidance, the report says, lacked sufficient basis for determining statin benefit versus risk in adults older than 75 years without a history of CVD. “In the absence of this evidence, clinicians should use their judgment as to whether to offer a statin to a patient in this age group,” according to the press release.
The review focused on 22 clinical trials for data on the statin benefits and saw significantly decreased associated risks for death from any cause, fatal or nonfatal stroke, and fatal or nonfatal myocardial infarction with treatment. The combined trial populations exceeded 85,000 for assessing all-cause mortality and 76,000 for each of the other two endpoints.
To assess any potential statin therapy harms, the evidence review covered 19 clinical trials with a combined enrollment of about 75,000 – two more trials than considered in the 2016 document – plus three observational studies with more than 400,000 participants. Statins were found not to be associated with an increased risk for study withdrawal because of adverse events, nor were there signs of greater risk for myalgia or new-onset diabetes, compared with placebo.
“A majority of the trials reviewed by the USPSTF used moderate-intensity statin therapy,” the report states. “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CVD in most persons.”
A version of this article first appeared on Medscape.com.
Given the expansive contemporary role of statins for primary cardiovascular disease (CVD) prevention, the language in the new U.S. Preventive Services Task Force draft guidance on their use in that setting may seem conservative. Even so, the proposed recommendations, open to public comment until March 21, take more recent data into account but don’t substantially vary from the 2016 USPSTF document they are intended to replace.
The task force concluded “with moderate certainty” that a statin prescription will clinically benefit adults aged 40-75 years without CVD but with at least one of several risk factors, such as dyslipidemia or diabetes, who have a 10-year CVD risk of at least 7.5%.
the new report states. That, says an accompanying USPSTF press release, means such people “may benefit from statin use and should decide with their clinician if taking a statin is right for them.”
Also, notes the report, the net benefit of statin therapy is “at least moderate” for individuals with a 10% or greater CVD risk over the next decade who, the press release states, “should take a statin to prevent a first heart attack or stroke.”
The evidence review on which the task force based the guidance, the report says, lacked sufficient basis for determining statin benefit versus risk in adults older than 75 years without a history of CVD. “In the absence of this evidence, clinicians should use their judgment as to whether to offer a statin to a patient in this age group,” according to the press release.
The review focused on 22 clinical trials for data on the statin benefits and saw significantly decreased associated risks for death from any cause, fatal or nonfatal stroke, and fatal or nonfatal myocardial infarction with treatment. The combined trial populations exceeded 85,000 for assessing all-cause mortality and 76,000 for each of the other two endpoints.
To assess any potential statin therapy harms, the evidence review covered 19 clinical trials with a combined enrollment of about 75,000 – two more trials than considered in the 2016 document – plus three observational studies with more than 400,000 participants. Statins were found not to be associated with an increased risk for study withdrawal because of adverse events, nor were there signs of greater risk for myalgia or new-onset diabetes, compared with placebo.
“A majority of the trials reviewed by the USPSTF used moderate-intensity statin therapy,” the report states. “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CVD in most persons.”
A version of this article first appeared on Medscape.com.
Given the expansive contemporary role of statins for primary cardiovascular disease (CVD) prevention, the language in the new U.S. Preventive Services Task Force draft guidance on their use in that setting may seem conservative. Even so, the proposed recommendations, open to public comment until March 21, take more recent data into account but don’t substantially vary from the 2016 USPSTF document they are intended to replace.
The task force concluded “with moderate certainty” that a statin prescription will clinically benefit adults aged 40-75 years without CVD but with at least one of several risk factors, such as dyslipidemia or diabetes, who have a 10-year CVD risk of at least 7.5%.
the new report states. That, says an accompanying USPSTF press release, means such people “may benefit from statin use and should decide with their clinician if taking a statin is right for them.”
Also, notes the report, the net benefit of statin therapy is “at least moderate” for individuals with a 10% or greater CVD risk over the next decade who, the press release states, “should take a statin to prevent a first heart attack or stroke.”
The evidence review on which the task force based the guidance, the report says, lacked sufficient basis for determining statin benefit versus risk in adults older than 75 years without a history of CVD. “In the absence of this evidence, clinicians should use their judgment as to whether to offer a statin to a patient in this age group,” according to the press release.
The review focused on 22 clinical trials for data on the statin benefits and saw significantly decreased associated risks for death from any cause, fatal or nonfatal stroke, and fatal or nonfatal myocardial infarction with treatment. The combined trial populations exceeded 85,000 for assessing all-cause mortality and 76,000 for each of the other two endpoints.
To assess any potential statin therapy harms, the evidence review covered 19 clinical trials with a combined enrollment of about 75,000 – two more trials than considered in the 2016 document – plus three observational studies with more than 400,000 participants. Statins were found not to be associated with an increased risk for study withdrawal because of adverse events, nor were there signs of greater risk for myalgia or new-onset diabetes, compared with placebo.
“A majority of the trials reviewed by the USPSTF used moderate-intensity statin therapy,” the report states. “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CVD in most persons.”
A version of this article first appeared on Medscape.com.