User login
Stroke risk in new-onset atrial fib goes up with greater alcohol intake
There’s abundant evidence linking higher alcohol intake levels to greater stroke risk and, separately, increasing risk for new-onset atrial fibrillation (AFib). Less settled is whether moderate to heavy drinking worsens the risk for stroke in patients already in AFib and whether giving up alcohol can attenuate that risk. A new observational study suggests the answer to both questions is yes.
The risk for ischemic stroke was only around 1% over about 5 years in a Korean nationwide cohort of almost 98,000 patients with new-onset AFib. About half the patients followed were nondrinkers, as they had been before the study, 13% became abstinent soon after their AFib diagnosis, and 36% were currently drinkers.
But stroke risk went up about 30% with “moderate” current alcohol intake, compared with no intake, and by more than 40% for current drinkers reporting “heavy” alcohol intake, researchers found in an adjusted analysis.
However, abstainers who had mild to moderate alcohol-intake levels before their AFib diagnosis “had a similar risk of ischemic stroke as nondrinkers,” write the authors, led by So-Ryoung Lee, MD, PhD, and colleagues, Seoul National University Hospital, Republic of Korea, in their report published June 7 in the European Heart Journal. In a secondary analysis, binge drinking was also independently associated with risk for ischemic stroke.
The findings suggest that “alcohol abstinence after the diagnosis of AFib could reduce the risk of ischemic stroke,” they conclude. “Lifestyle interventions, including attention to alcohol consumption, should be encouraged as part of a comprehensive approach in the management of patients with a new diagnosis of AFib” for lowering the risk for stroke and other clinical outcomes.
“These results are pretty comparable to those obtained in the more general population,” David Conen, MD, MPH, not connected to the analysis, told this news organization.
In the study’s population with new-onset AFib, there is an alcohol-dependent risk for stroke “that goes up with increasing alcohol intake, which is more or less similar to that found without atrial fibrillation in previous studies,” said Dr. Conen, from the Population Health Research Institute, McMaster University, Hamilton, Ont.
The study, “which overall I think is very well done,” he said, is noteworthy for also suggesting that binge drinking, which was scrutinized in a secondary analysis, appeared independently to worsen the risk for stroke in its AFib population.
Dr. Conen said the observed 1% overall risk for stroke was very similar to the rate he and his colleagues saw in a recent combined analysis of two European cohorts with AFib that was usually longer standing; the median was 3 years. That analysis, in contrast, showed no significant association between increasing levels of alcohol intake and risk for stroke or systemic embolism.
However, “our confidence limits did not exclude the possibility of a small to moderate association,” he said. Given that, and the current study from Korea, there might indeed be “a weak association between alcohol consumption and stroke” in patients with AFib.
“Their results are just more precise because of the larger sample size. That’s why they were able to show those associations,” said Dr. Conen, who was senior author on the earlier report, which covered a pooled analysis of 3,852 patients with AFib in the BEAT-AF and SWISS-AF cohort studies. It was published January 25 in CMAJ, with lead author Philipp Reddiess, MD, Cardiovascular Research Institute Basel, Switzerland.
The two published studies contrast in other ways that are worth noting and together suggest the stroke rate might have been 1% in both by chance, Dr. Conen said. “The populations were pretty different.”
In the earlier study, for example, the overwhelmingly European patients had more comorbidities and had been in AFib for much longer; their mean age was 71 years; and 84% were on oral anticoagulation (OAC).
In contrast, the Korean cohort averaged 61 years in age and only about 24% were taking oral anticoagulants. Given their distribution of CHA2DS2-VASc scores and mean score of 2.3, more than twice as many should have been on OAC, Dr. Conen speculated. “Even if you take into account that some patients may have contraindications, this is clearly an underanticoagulated population.”
The European cohort might have been “a little bit more representative because atrial fibrillation is a disease of the elderly,” Dr. Conen said, but “the Korean paper has the advantage of being a population-based study.”
It involved 97,869 patients from a Korean national data base who were newly diagnosed with AFib from 2010 to 2016. Of the total, 49,781 (51%) were continuously nondrinkers before and after their diagnosis; 12,789 (13%) abstained from alcohol only after their AFib diagnosis; and 35,299 (36%) were drinkers during the follow-up, either because they continued to drink or newly started after their diagnosis.
Of the cohort, 3,120 were diagnosed with new ischemic stroke over a follow-up of 310,926 person-years, for a rate of 1 per 100 person-years.
The adjusted hazard ratio (HR) for ischemic stroke over a 5-year follow-up, compared with nondrinkers, was:
- 1.127 (95% confidence interval, 1.003-1.266) among abstainers
- 1.280 (95% CI, 1.166-1.405) for current drinkers
The corresponding HR, compared with current drinkers, was:
- 0.781 (95% CI, 0.712-0.858) for nondrinkers
- 0.880 (95% CI, 0.782-0.990) among abstainers
No significant interactions with ischemic stroke risk were observed in groups by sex, age, CHA2DS2-VASc score, or smoking status. The risk rose consistently with current drinking levels.
The overall stroke rate of 1% per year is “very low,” and “the absolute differences are small, even though there is a clear significant trend from nondrinking to drinking,” Dr. Conen said.
However, “the difference becomes more sizable when you compare heavy drinking to abstinence.”
Dr. Lee reports no conflicts of interest; disclosures for the other authors are in their report. Dr. Conen reports receiving speaker fees from Servier Canada; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
There’s abundant evidence linking higher alcohol intake levels to greater stroke risk and, separately, increasing risk for new-onset atrial fibrillation (AFib). Less settled is whether moderate to heavy drinking worsens the risk for stroke in patients already in AFib and whether giving up alcohol can attenuate that risk. A new observational study suggests the answer to both questions is yes.
The risk for ischemic stroke was only around 1% over about 5 years in a Korean nationwide cohort of almost 98,000 patients with new-onset AFib. About half the patients followed were nondrinkers, as they had been before the study, 13% became abstinent soon after their AFib diagnosis, and 36% were currently drinkers.
But stroke risk went up about 30% with “moderate” current alcohol intake, compared with no intake, and by more than 40% for current drinkers reporting “heavy” alcohol intake, researchers found in an adjusted analysis.
However, abstainers who had mild to moderate alcohol-intake levels before their AFib diagnosis “had a similar risk of ischemic stroke as nondrinkers,” write the authors, led by So-Ryoung Lee, MD, PhD, and colleagues, Seoul National University Hospital, Republic of Korea, in their report published June 7 in the European Heart Journal. In a secondary analysis, binge drinking was also independently associated with risk for ischemic stroke.
The findings suggest that “alcohol abstinence after the diagnosis of AFib could reduce the risk of ischemic stroke,” they conclude. “Lifestyle interventions, including attention to alcohol consumption, should be encouraged as part of a comprehensive approach in the management of patients with a new diagnosis of AFib” for lowering the risk for stroke and other clinical outcomes.
“These results are pretty comparable to those obtained in the more general population,” David Conen, MD, MPH, not connected to the analysis, told this news organization.
In the study’s population with new-onset AFib, there is an alcohol-dependent risk for stroke “that goes up with increasing alcohol intake, which is more or less similar to that found without atrial fibrillation in previous studies,” said Dr. Conen, from the Population Health Research Institute, McMaster University, Hamilton, Ont.
The study, “which overall I think is very well done,” he said, is noteworthy for also suggesting that binge drinking, which was scrutinized in a secondary analysis, appeared independently to worsen the risk for stroke in its AFib population.
Dr. Conen said the observed 1% overall risk for stroke was very similar to the rate he and his colleagues saw in a recent combined analysis of two European cohorts with AFib that was usually longer standing; the median was 3 years. That analysis, in contrast, showed no significant association between increasing levels of alcohol intake and risk for stroke or systemic embolism.
However, “our confidence limits did not exclude the possibility of a small to moderate association,” he said. Given that, and the current study from Korea, there might indeed be “a weak association between alcohol consumption and stroke” in patients with AFib.
“Their results are just more precise because of the larger sample size. That’s why they were able to show those associations,” said Dr. Conen, who was senior author on the earlier report, which covered a pooled analysis of 3,852 patients with AFib in the BEAT-AF and SWISS-AF cohort studies. It was published January 25 in CMAJ, with lead author Philipp Reddiess, MD, Cardiovascular Research Institute Basel, Switzerland.
The two published studies contrast in other ways that are worth noting and together suggest the stroke rate might have been 1% in both by chance, Dr. Conen said. “The populations were pretty different.”
In the earlier study, for example, the overwhelmingly European patients had more comorbidities and had been in AFib for much longer; their mean age was 71 years; and 84% were on oral anticoagulation (OAC).
In contrast, the Korean cohort averaged 61 years in age and only about 24% were taking oral anticoagulants. Given their distribution of CHA2DS2-VASc scores and mean score of 2.3, more than twice as many should have been on OAC, Dr. Conen speculated. “Even if you take into account that some patients may have contraindications, this is clearly an underanticoagulated population.”
The European cohort might have been “a little bit more representative because atrial fibrillation is a disease of the elderly,” Dr. Conen said, but “the Korean paper has the advantage of being a population-based study.”
It involved 97,869 patients from a Korean national data base who were newly diagnosed with AFib from 2010 to 2016. Of the total, 49,781 (51%) were continuously nondrinkers before and after their diagnosis; 12,789 (13%) abstained from alcohol only after their AFib diagnosis; and 35,299 (36%) were drinkers during the follow-up, either because they continued to drink or newly started after their diagnosis.
Of the cohort, 3,120 were diagnosed with new ischemic stroke over a follow-up of 310,926 person-years, for a rate of 1 per 100 person-years.
The adjusted hazard ratio (HR) for ischemic stroke over a 5-year follow-up, compared with nondrinkers, was:
- 1.127 (95% confidence interval, 1.003-1.266) among abstainers
- 1.280 (95% CI, 1.166-1.405) for current drinkers
The corresponding HR, compared with current drinkers, was:
- 0.781 (95% CI, 0.712-0.858) for nondrinkers
- 0.880 (95% CI, 0.782-0.990) among abstainers
No significant interactions with ischemic stroke risk were observed in groups by sex, age, CHA2DS2-VASc score, or smoking status. The risk rose consistently with current drinking levels.
The overall stroke rate of 1% per year is “very low,” and “the absolute differences are small, even though there is a clear significant trend from nondrinking to drinking,” Dr. Conen said.
However, “the difference becomes more sizable when you compare heavy drinking to abstinence.”
Dr. Lee reports no conflicts of interest; disclosures for the other authors are in their report. Dr. Conen reports receiving speaker fees from Servier Canada; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
There’s abundant evidence linking higher alcohol intake levels to greater stroke risk and, separately, increasing risk for new-onset atrial fibrillation (AFib). Less settled is whether moderate to heavy drinking worsens the risk for stroke in patients already in AFib and whether giving up alcohol can attenuate that risk. A new observational study suggests the answer to both questions is yes.
The risk for ischemic stroke was only around 1% over about 5 years in a Korean nationwide cohort of almost 98,000 patients with new-onset AFib. About half the patients followed were nondrinkers, as they had been before the study, 13% became abstinent soon after their AFib diagnosis, and 36% were currently drinkers.
But stroke risk went up about 30% with “moderate” current alcohol intake, compared with no intake, and by more than 40% for current drinkers reporting “heavy” alcohol intake, researchers found in an adjusted analysis.
However, abstainers who had mild to moderate alcohol-intake levels before their AFib diagnosis “had a similar risk of ischemic stroke as nondrinkers,” write the authors, led by So-Ryoung Lee, MD, PhD, and colleagues, Seoul National University Hospital, Republic of Korea, in their report published June 7 in the European Heart Journal. In a secondary analysis, binge drinking was also independently associated with risk for ischemic stroke.
The findings suggest that “alcohol abstinence after the diagnosis of AFib could reduce the risk of ischemic stroke,” they conclude. “Lifestyle interventions, including attention to alcohol consumption, should be encouraged as part of a comprehensive approach in the management of patients with a new diagnosis of AFib” for lowering the risk for stroke and other clinical outcomes.
“These results are pretty comparable to those obtained in the more general population,” David Conen, MD, MPH, not connected to the analysis, told this news organization.
In the study’s population with new-onset AFib, there is an alcohol-dependent risk for stroke “that goes up with increasing alcohol intake, which is more or less similar to that found without atrial fibrillation in previous studies,” said Dr. Conen, from the Population Health Research Institute, McMaster University, Hamilton, Ont.
The study, “which overall I think is very well done,” he said, is noteworthy for also suggesting that binge drinking, which was scrutinized in a secondary analysis, appeared independently to worsen the risk for stroke in its AFib population.
Dr. Conen said the observed 1% overall risk for stroke was very similar to the rate he and his colleagues saw in a recent combined analysis of two European cohorts with AFib that was usually longer standing; the median was 3 years. That analysis, in contrast, showed no significant association between increasing levels of alcohol intake and risk for stroke or systemic embolism.
However, “our confidence limits did not exclude the possibility of a small to moderate association,” he said. Given that, and the current study from Korea, there might indeed be “a weak association between alcohol consumption and stroke” in patients with AFib.
“Their results are just more precise because of the larger sample size. That’s why they were able to show those associations,” said Dr. Conen, who was senior author on the earlier report, which covered a pooled analysis of 3,852 patients with AFib in the BEAT-AF and SWISS-AF cohort studies. It was published January 25 in CMAJ, with lead author Philipp Reddiess, MD, Cardiovascular Research Institute Basel, Switzerland.
The two published studies contrast in other ways that are worth noting and together suggest the stroke rate might have been 1% in both by chance, Dr. Conen said. “The populations were pretty different.”
In the earlier study, for example, the overwhelmingly European patients had more comorbidities and had been in AFib for much longer; their mean age was 71 years; and 84% were on oral anticoagulation (OAC).
In contrast, the Korean cohort averaged 61 years in age and only about 24% were taking oral anticoagulants. Given their distribution of CHA2DS2-VASc scores and mean score of 2.3, more than twice as many should have been on OAC, Dr. Conen speculated. “Even if you take into account that some patients may have contraindications, this is clearly an underanticoagulated population.”
The European cohort might have been “a little bit more representative because atrial fibrillation is a disease of the elderly,” Dr. Conen said, but “the Korean paper has the advantage of being a population-based study.”
It involved 97,869 patients from a Korean national data base who were newly diagnosed with AFib from 2010 to 2016. Of the total, 49,781 (51%) were continuously nondrinkers before and after their diagnosis; 12,789 (13%) abstained from alcohol only after their AFib diagnosis; and 35,299 (36%) were drinkers during the follow-up, either because they continued to drink or newly started after their diagnosis.
Of the cohort, 3,120 were diagnosed with new ischemic stroke over a follow-up of 310,926 person-years, for a rate of 1 per 100 person-years.
The adjusted hazard ratio (HR) for ischemic stroke over a 5-year follow-up, compared with nondrinkers, was:
- 1.127 (95% confidence interval, 1.003-1.266) among abstainers
- 1.280 (95% CI, 1.166-1.405) for current drinkers
The corresponding HR, compared with current drinkers, was:
- 0.781 (95% CI, 0.712-0.858) for nondrinkers
- 0.880 (95% CI, 0.782-0.990) among abstainers
No significant interactions with ischemic stroke risk were observed in groups by sex, age, CHA2DS2-VASc score, or smoking status. The risk rose consistently with current drinking levels.
The overall stroke rate of 1% per year is “very low,” and “the absolute differences are small, even though there is a clear significant trend from nondrinking to drinking,” Dr. Conen said.
However, “the difference becomes more sizable when you compare heavy drinking to abstinence.”
Dr. Lee reports no conflicts of interest; disclosures for the other authors are in their report. Dr. Conen reports receiving speaker fees from Servier Canada; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
Are left atrial thrombi that defy preprocedure anticoagulation predictable?
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
Novel rehab program fights frailty, boosts capacity in advanced HF
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.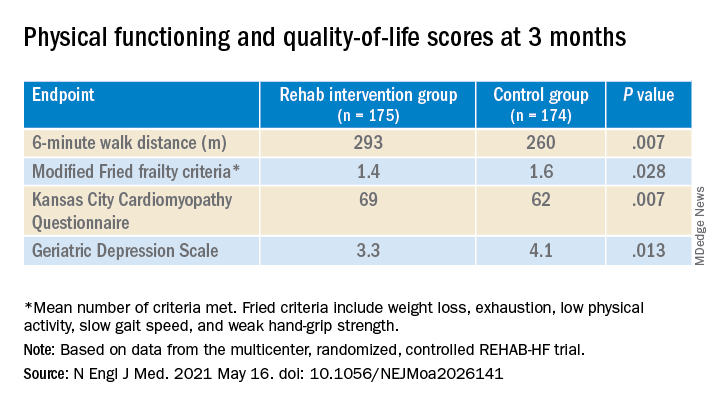
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
Fresh look at ISCHEMIA bolsters conservative message in stable CAD
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.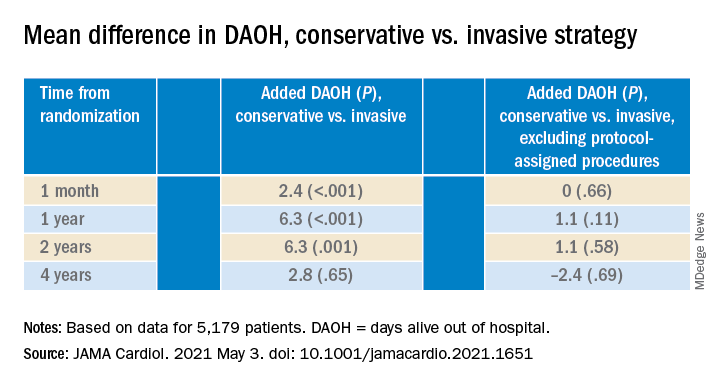
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
A ‘mess’ of a diagnosis: Is it type 2 MI or a nonischemic imposter?
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Modest clinical gain for AF screening of asymptomatic elderly: STROKESTOP
Some, perhaps many, previously unrecognized cases of atrial fibrillation (AF) will come to light in a screening program aimed at older asymptomatic adults. The key question is whether the challenges of such systematic but age-restricted AF screening in the community, with oral anticoagulation (OAC) offered to those found to have the arrhythmia, is worthwhile in preventing events such as death or stroke.
Now there is evidence supporting such a clinical benefit from a large, prospective, randomized trial. A screening program restricted to people 75 or 76 years of age in two Swedish communities, which called on them to use a handheld single-lead ECG system at home intermittently for 2 weeks, was followed by a slight drop in clinical events over about 7 years.
The 4% decline in risk (P = .045) in the STROKESTOP trial’s “intention-to-treat” (ITT) analysis yielded a number needed to treat of 91; that is, that many people had to be targeted by the screening program to prevent one primary-endpoint clinical event.
Those included ischemic stroke, systemic thromboembolism, hospitalization for severe bleeding, and death from any cause, investigators reported April 23 during the virtual European Heart Rhythm Association (EHRA) 2021 Congress.
If that benefit and its significance seem marginal, some secondary findings might be reassuring. Half the population of the target age in the two communities – 13,979 randomly selected people – were invited to join the trial and follow the screening protocol, comprising the ITT cohort. The other half, numbering 13,996, was not invited and served as control subjects.
However, only 51% of the ITT cohort accepted the invitation and participated in the trial; they represented the “as-treated” cohort, observed Emma Svennberg, MD, PhD, Karolinska Institute, Danderyd Hospital, Stockholm, who presented the analysis at the EHRA sessions.
The screening protocol identified untreated AF, whether previously known or unknown, in about 5% of the 7,165 as-treated screening participants; OAC was initiated in about three-fourths of those cases.
The as-treated group, on their own, benefited with a 24% drop in the prospectively defined secondary endpoint of ischemic stroke, compared with the entire control group.
The clinical benefit in the ITT population was “small but significant,” but over the same period in the as-treated cohort, there was a highly significant drop in risk for ischemic stroke, Dr. Svennberg said in an interview.
The trial’s lead message, she said, is that “screening for atrial fibrillation in an elderly population reduces the risk of death and ischemic stroke without increasing the risk of bleeding.”
Caveats: As-treated vs. ITT
But there are caveats that complicate interpretation of the trial and, Dr. Svennberg proposed, point to the importance of that interpretation of both the ITT and as-treated analyses.
“We detected significantly more atrial fibrillation in the group that was randomized to screening. A major strength of our study was that we referred all of those individuals for a structured follow-up within the study,” she said. “Although the focus of the follow-up was oral anticoagulant therapy, other risk factors were also assessed and managed, such as hypertension and diabetes.”
It’s possible that increased detection of AF followed by such structured management contributed to the observed benefit, Dr. Svennberg proposed.
However, the exclusion of those in the prespecified ITT population who declined to be screened or otherwise didn’t participate left an as-treated cohort that was healthier than the ITT population or the control group.
Indeed, the nonparticipating invitees were sicker, with significantly more diabetes, vascular disease, hypertension, and heart failure, and higher CHA2DS2VASc stroke risk scores than those who agreed to participate.
“We took a more difficult route in setting up this study, in that we identified all individuals aged 75 to 76 residing in our two regions and excluded no one,” Dr. Svennberg said in an interview. “That means even individuals with end-stage disease, severe dementia, bedridden in nursing homes, et cetera, were also randomized but perhaps not likely or eligible to participate.”
Therefore, some invitees were unable to join the study even as others might have declined “out of low interest” or other personal reasons, she said. “We believe that this mimics how a population-based screening program would be performed if done in our country.”
In the ITT analysis, screening successfully identified previously unknown or untreated cases of AF, which led to expanded OAC use and intensified risk-factor management, “which was key to a successful outcome.”
In the as-treated analysis, Dr. Svennberg said, “I think a combination of the intervention and the population being overall more healthy was driving the secondary endpoint.”
Systematic vs. opportunistic screening
Although “opportunistic screening in individuals aged 65 and older” is recommended by current European Society of Cardiology guidelines, systematic screening, such as that used in STROKESTOP, has a much weaker evidence base, observed Renate B. Schnabel, MD, PhD, University Heart & Vascular Center, Hamburg, Germany, as the invited discussant after the STROKESTOP presentation.
STROKESTOP “is one of the first studies, if not the first study,” to show a clinical benefit from screening for AF, Dr. Schnabel said.
Fewer-than-projected primary outcome events were seen during the trial, and event curves for screened and control participants didn’t start to separate until about 4 years into the study, she said. It therefore might take a long time for the screened elderly to realize the clinical benefits of screening.
Studies such as the recent SCREEN-AF and mSTOPS have amply shown that AF screening in the asymptomatic elderly can reveal previously unrecognized AF far more often than would be detected in routine practice, allowing them the opportunity to go on OAC. But the trials weren’t able to show whether the benefits of such management outweigh the risks or costs.
Indeed, on April 20, the U.S. Preventive Services Task Force (USPSTF) released a draft recommendation statement concluding that “the current evidence is insufficient to assess the balance of benefits and harms” associated with AF screening in asymptomatic people at least 50 years of age.
In STROKESTOP, however, benefit for the primary outcome reached significance in the prespecified ITT analysis and “appeared to be driven by the reduction in ischemic stroke incidence,” Dr. Schnabel said.
“The future guidelines have gained strong evidence to judge on systematic atrial fibrillation screening” as it was performed in the trial, she said. “How to implement atrial fibrillation screening, including systematic screening in health care systems across Europe and beyond, remains an open question.”
A randomized population
STROKESTOP considered all 75- and 76-year-olds living in Sweden’s Stockholm County (n = 23,888) and the Halland region (n = 4,880) and randomly assigned them to the ITT group or a control group, with stratification by sex, birth year, and geographic region. In both groups, 54.6% were female and the mean CHA2DS2VASc score was 3.5.
People assigned to the ITT cohort were invited to be screened and followed. Those who agreed to participate underwent a baseline ECG assessment to detect or rule out permanent AF. Guideline-based OAC and follow-up was offered to those found with the arrhythmia. Those in sinus rhythm with no history of AF used a handheld single-lead ECG recorder (Zenicor) for 30 seconds twice daily for 14 days.
Structured management, including OAC, was offered to anyone demonstrating sufficient AF, that is, at least one bout without p waves in one 30-second recording or at least two such episodes lasting 10-29 seconds during the 2-week screening period.
In the ITT analysis, the hazard ratio (HR) for the composite clinical primary endpoint was 0.96 (95% confidence interval, 0.920-0.999; P = .045), but in the as-treated analysis, the HR for ischemic stroke was 0.76 (95% CI, 0.68-0.87; P < .001).
“I believe that this will likely be generalizable to most countries’ elderly residents,” Dr. Svennberg said. “I think if we can find a significant difference in our elderly population in Sweden, most countries will be able to do so, or find even more significant results.”
That’s because “baseline detection of AF in Sweden is high,” she said, “so new detection is likely more difficult.” Also, in Sweden, “care can be sought without monetary concern, and prescriptions are provided at low costs to the patients.” Therefore, patients newly identified with AF, whether in studies or not, “would likely be started on therapy.”
It will be important to know whether the screening strategy is cost-effective, Dr. Schnabel said, because “the overall effect, with a hazard ratio of 0.96, is not too big, and costs incurred by systematic screening are comparatively high.”
STROKESTOP “now provides sound information for cost-effectiveness analyses, which to date have largely relied on assumptions.”
STROKESTOP was partially supported by Carl Bennet AB, Boehringer-Ingelheim, Bayer, Bristol-Meyers Squibb, and Pfizer. Dr. Svennberg disclosed receiving fees for lectures or consulting from Bayer, Bristol-Meyers Squibb, Pfizer, Boehringer-Ingelheim, Merck Sharp & Dohme, and Sanofi; and institutional grants from Roche Diagnostics and Carl Bennett Ltd.
A version of this article first appeared on Medscape.com.
Some, perhaps many, previously unrecognized cases of atrial fibrillation (AF) will come to light in a screening program aimed at older asymptomatic adults. The key question is whether the challenges of such systematic but age-restricted AF screening in the community, with oral anticoagulation (OAC) offered to those found to have the arrhythmia, is worthwhile in preventing events such as death or stroke.
Now there is evidence supporting such a clinical benefit from a large, prospective, randomized trial. A screening program restricted to people 75 or 76 years of age in two Swedish communities, which called on them to use a handheld single-lead ECG system at home intermittently for 2 weeks, was followed by a slight drop in clinical events over about 7 years.
The 4% decline in risk (P = .045) in the STROKESTOP trial’s “intention-to-treat” (ITT) analysis yielded a number needed to treat of 91; that is, that many people had to be targeted by the screening program to prevent one primary-endpoint clinical event.
Those included ischemic stroke, systemic thromboembolism, hospitalization for severe bleeding, and death from any cause, investigators reported April 23 during the virtual European Heart Rhythm Association (EHRA) 2021 Congress.
If that benefit and its significance seem marginal, some secondary findings might be reassuring. Half the population of the target age in the two communities – 13,979 randomly selected people – were invited to join the trial and follow the screening protocol, comprising the ITT cohort. The other half, numbering 13,996, was not invited and served as control subjects.
However, only 51% of the ITT cohort accepted the invitation and participated in the trial; they represented the “as-treated” cohort, observed Emma Svennberg, MD, PhD, Karolinska Institute, Danderyd Hospital, Stockholm, who presented the analysis at the EHRA sessions.
The screening protocol identified untreated AF, whether previously known or unknown, in about 5% of the 7,165 as-treated screening participants; OAC was initiated in about three-fourths of those cases.
The as-treated group, on their own, benefited with a 24% drop in the prospectively defined secondary endpoint of ischemic stroke, compared with the entire control group.
The clinical benefit in the ITT population was “small but significant,” but over the same period in the as-treated cohort, there was a highly significant drop in risk for ischemic stroke, Dr. Svennberg said in an interview.
The trial’s lead message, she said, is that “screening for atrial fibrillation in an elderly population reduces the risk of death and ischemic stroke without increasing the risk of bleeding.”
Caveats: As-treated vs. ITT
But there are caveats that complicate interpretation of the trial and, Dr. Svennberg proposed, point to the importance of that interpretation of both the ITT and as-treated analyses.
“We detected significantly more atrial fibrillation in the group that was randomized to screening. A major strength of our study was that we referred all of those individuals for a structured follow-up within the study,” she said. “Although the focus of the follow-up was oral anticoagulant therapy, other risk factors were also assessed and managed, such as hypertension and diabetes.”
It’s possible that increased detection of AF followed by such structured management contributed to the observed benefit, Dr. Svennberg proposed.
However, the exclusion of those in the prespecified ITT population who declined to be screened or otherwise didn’t participate left an as-treated cohort that was healthier than the ITT population or the control group.
Indeed, the nonparticipating invitees were sicker, with significantly more diabetes, vascular disease, hypertension, and heart failure, and higher CHA2DS2VASc stroke risk scores than those who agreed to participate.
“We took a more difficult route in setting up this study, in that we identified all individuals aged 75 to 76 residing in our two regions and excluded no one,” Dr. Svennberg said in an interview. “That means even individuals with end-stage disease, severe dementia, bedridden in nursing homes, et cetera, were also randomized but perhaps not likely or eligible to participate.”
Therefore, some invitees were unable to join the study even as others might have declined “out of low interest” or other personal reasons, she said. “We believe that this mimics how a population-based screening program would be performed if done in our country.”
In the ITT analysis, screening successfully identified previously unknown or untreated cases of AF, which led to expanded OAC use and intensified risk-factor management, “which was key to a successful outcome.”
In the as-treated analysis, Dr. Svennberg said, “I think a combination of the intervention and the population being overall more healthy was driving the secondary endpoint.”
Systematic vs. opportunistic screening
Although “opportunistic screening in individuals aged 65 and older” is recommended by current European Society of Cardiology guidelines, systematic screening, such as that used in STROKESTOP, has a much weaker evidence base, observed Renate B. Schnabel, MD, PhD, University Heart & Vascular Center, Hamburg, Germany, as the invited discussant after the STROKESTOP presentation.
STROKESTOP “is one of the first studies, if not the first study,” to show a clinical benefit from screening for AF, Dr. Schnabel said.
Fewer-than-projected primary outcome events were seen during the trial, and event curves for screened and control participants didn’t start to separate until about 4 years into the study, she said. It therefore might take a long time for the screened elderly to realize the clinical benefits of screening.
Studies such as the recent SCREEN-AF and mSTOPS have amply shown that AF screening in the asymptomatic elderly can reveal previously unrecognized AF far more often than would be detected in routine practice, allowing them the opportunity to go on OAC. But the trials weren’t able to show whether the benefits of such management outweigh the risks or costs.
Indeed, on April 20, the U.S. Preventive Services Task Force (USPSTF) released a draft recommendation statement concluding that “the current evidence is insufficient to assess the balance of benefits and harms” associated with AF screening in asymptomatic people at least 50 years of age.
In STROKESTOP, however, benefit for the primary outcome reached significance in the prespecified ITT analysis and “appeared to be driven by the reduction in ischemic stroke incidence,” Dr. Schnabel said.
“The future guidelines have gained strong evidence to judge on systematic atrial fibrillation screening” as it was performed in the trial, she said. “How to implement atrial fibrillation screening, including systematic screening in health care systems across Europe and beyond, remains an open question.”
A randomized population
STROKESTOP considered all 75- and 76-year-olds living in Sweden’s Stockholm County (n = 23,888) and the Halland region (n = 4,880) and randomly assigned them to the ITT group or a control group, with stratification by sex, birth year, and geographic region. In both groups, 54.6% were female and the mean CHA2DS2VASc score was 3.5.
People assigned to the ITT cohort were invited to be screened and followed. Those who agreed to participate underwent a baseline ECG assessment to detect or rule out permanent AF. Guideline-based OAC and follow-up was offered to those found with the arrhythmia. Those in sinus rhythm with no history of AF used a handheld single-lead ECG recorder (Zenicor) for 30 seconds twice daily for 14 days.
Structured management, including OAC, was offered to anyone demonstrating sufficient AF, that is, at least one bout without p waves in one 30-second recording or at least two such episodes lasting 10-29 seconds during the 2-week screening period.
In the ITT analysis, the hazard ratio (HR) for the composite clinical primary endpoint was 0.96 (95% confidence interval, 0.920-0.999; P = .045), but in the as-treated analysis, the HR for ischemic stroke was 0.76 (95% CI, 0.68-0.87; P < .001).
“I believe that this will likely be generalizable to most countries’ elderly residents,” Dr. Svennberg said. “I think if we can find a significant difference in our elderly population in Sweden, most countries will be able to do so, or find even more significant results.”
That’s because “baseline detection of AF in Sweden is high,” she said, “so new detection is likely more difficult.” Also, in Sweden, “care can be sought without monetary concern, and prescriptions are provided at low costs to the patients.” Therefore, patients newly identified with AF, whether in studies or not, “would likely be started on therapy.”
It will be important to know whether the screening strategy is cost-effective, Dr. Schnabel said, because “the overall effect, with a hazard ratio of 0.96, is not too big, and costs incurred by systematic screening are comparatively high.”
STROKESTOP “now provides sound information for cost-effectiveness analyses, which to date have largely relied on assumptions.”
STROKESTOP was partially supported by Carl Bennet AB, Boehringer-Ingelheim, Bayer, Bristol-Meyers Squibb, and Pfizer. Dr. Svennberg disclosed receiving fees for lectures or consulting from Bayer, Bristol-Meyers Squibb, Pfizer, Boehringer-Ingelheim, Merck Sharp & Dohme, and Sanofi; and institutional grants from Roche Diagnostics and Carl Bennett Ltd.
A version of this article first appeared on Medscape.com.
Some, perhaps many, previously unrecognized cases of atrial fibrillation (AF) will come to light in a screening program aimed at older asymptomatic adults. The key question is whether the challenges of such systematic but age-restricted AF screening in the community, with oral anticoagulation (OAC) offered to those found to have the arrhythmia, is worthwhile in preventing events such as death or stroke.
Now there is evidence supporting such a clinical benefit from a large, prospective, randomized trial. A screening program restricted to people 75 or 76 years of age in two Swedish communities, which called on them to use a handheld single-lead ECG system at home intermittently for 2 weeks, was followed by a slight drop in clinical events over about 7 years.
The 4% decline in risk (P = .045) in the STROKESTOP trial’s “intention-to-treat” (ITT) analysis yielded a number needed to treat of 91; that is, that many people had to be targeted by the screening program to prevent one primary-endpoint clinical event.
Those included ischemic stroke, systemic thromboembolism, hospitalization for severe bleeding, and death from any cause, investigators reported April 23 during the virtual European Heart Rhythm Association (EHRA) 2021 Congress.
If that benefit and its significance seem marginal, some secondary findings might be reassuring. Half the population of the target age in the two communities – 13,979 randomly selected people – were invited to join the trial and follow the screening protocol, comprising the ITT cohort. The other half, numbering 13,996, was not invited and served as control subjects.
However, only 51% of the ITT cohort accepted the invitation and participated in the trial; they represented the “as-treated” cohort, observed Emma Svennberg, MD, PhD, Karolinska Institute, Danderyd Hospital, Stockholm, who presented the analysis at the EHRA sessions.
The screening protocol identified untreated AF, whether previously known or unknown, in about 5% of the 7,165 as-treated screening participants; OAC was initiated in about three-fourths of those cases.
The as-treated group, on their own, benefited with a 24% drop in the prospectively defined secondary endpoint of ischemic stroke, compared with the entire control group.
The clinical benefit in the ITT population was “small but significant,” but over the same period in the as-treated cohort, there was a highly significant drop in risk for ischemic stroke, Dr. Svennberg said in an interview.
The trial’s lead message, she said, is that “screening for atrial fibrillation in an elderly population reduces the risk of death and ischemic stroke without increasing the risk of bleeding.”
Caveats: As-treated vs. ITT
But there are caveats that complicate interpretation of the trial and, Dr. Svennberg proposed, point to the importance of that interpretation of both the ITT and as-treated analyses.
“We detected significantly more atrial fibrillation in the group that was randomized to screening. A major strength of our study was that we referred all of those individuals for a structured follow-up within the study,” she said. “Although the focus of the follow-up was oral anticoagulant therapy, other risk factors were also assessed and managed, such as hypertension and diabetes.”
It’s possible that increased detection of AF followed by such structured management contributed to the observed benefit, Dr. Svennberg proposed.
However, the exclusion of those in the prespecified ITT population who declined to be screened or otherwise didn’t participate left an as-treated cohort that was healthier than the ITT population or the control group.
Indeed, the nonparticipating invitees were sicker, with significantly more diabetes, vascular disease, hypertension, and heart failure, and higher CHA2DS2VASc stroke risk scores than those who agreed to participate.
“We took a more difficult route in setting up this study, in that we identified all individuals aged 75 to 76 residing in our two regions and excluded no one,” Dr. Svennberg said in an interview. “That means even individuals with end-stage disease, severe dementia, bedridden in nursing homes, et cetera, were also randomized but perhaps not likely or eligible to participate.”
Therefore, some invitees were unable to join the study even as others might have declined “out of low interest” or other personal reasons, she said. “We believe that this mimics how a population-based screening program would be performed if done in our country.”
In the ITT analysis, screening successfully identified previously unknown or untreated cases of AF, which led to expanded OAC use and intensified risk-factor management, “which was key to a successful outcome.”
In the as-treated analysis, Dr. Svennberg said, “I think a combination of the intervention and the population being overall more healthy was driving the secondary endpoint.”
Systematic vs. opportunistic screening
Although “opportunistic screening in individuals aged 65 and older” is recommended by current European Society of Cardiology guidelines, systematic screening, such as that used in STROKESTOP, has a much weaker evidence base, observed Renate B. Schnabel, MD, PhD, University Heart & Vascular Center, Hamburg, Germany, as the invited discussant after the STROKESTOP presentation.
STROKESTOP “is one of the first studies, if not the first study,” to show a clinical benefit from screening for AF, Dr. Schnabel said.
Fewer-than-projected primary outcome events were seen during the trial, and event curves for screened and control participants didn’t start to separate until about 4 years into the study, she said. It therefore might take a long time for the screened elderly to realize the clinical benefits of screening.
Studies such as the recent SCREEN-AF and mSTOPS have amply shown that AF screening in the asymptomatic elderly can reveal previously unrecognized AF far more often than would be detected in routine practice, allowing them the opportunity to go on OAC. But the trials weren’t able to show whether the benefits of such management outweigh the risks or costs.
Indeed, on April 20, the U.S. Preventive Services Task Force (USPSTF) released a draft recommendation statement concluding that “the current evidence is insufficient to assess the balance of benefits and harms” associated with AF screening in asymptomatic people at least 50 years of age.
In STROKESTOP, however, benefit for the primary outcome reached significance in the prespecified ITT analysis and “appeared to be driven by the reduction in ischemic stroke incidence,” Dr. Schnabel said.
“The future guidelines have gained strong evidence to judge on systematic atrial fibrillation screening” as it was performed in the trial, she said. “How to implement atrial fibrillation screening, including systematic screening in health care systems across Europe and beyond, remains an open question.”
A randomized population
STROKESTOP considered all 75- and 76-year-olds living in Sweden’s Stockholm County (n = 23,888) and the Halland region (n = 4,880) and randomly assigned them to the ITT group or a control group, with stratification by sex, birth year, and geographic region. In both groups, 54.6% were female and the mean CHA2DS2VASc score was 3.5.
People assigned to the ITT cohort were invited to be screened and followed. Those who agreed to participate underwent a baseline ECG assessment to detect or rule out permanent AF. Guideline-based OAC and follow-up was offered to those found with the arrhythmia. Those in sinus rhythm with no history of AF used a handheld single-lead ECG recorder (Zenicor) for 30 seconds twice daily for 14 days.
Structured management, including OAC, was offered to anyone demonstrating sufficient AF, that is, at least one bout without p waves in one 30-second recording or at least two such episodes lasting 10-29 seconds during the 2-week screening period.
In the ITT analysis, the hazard ratio (HR) for the composite clinical primary endpoint was 0.96 (95% confidence interval, 0.920-0.999; P = .045), but in the as-treated analysis, the HR for ischemic stroke was 0.76 (95% CI, 0.68-0.87; P < .001).
“I believe that this will likely be generalizable to most countries’ elderly residents,” Dr. Svennberg said. “I think if we can find a significant difference in our elderly population in Sweden, most countries will be able to do so, or find even more significant results.”
That’s because “baseline detection of AF in Sweden is high,” she said, “so new detection is likely more difficult.” Also, in Sweden, “care can be sought without monetary concern, and prescriptions are provided at low costs to the patients.” Therefore, patients newly identified with AF, whether in studies or not, “would likely be started on therapy.”
It will be important to know whether the screening strategy is cost-effective, Dr. Schnabel said, because “the overall effect, with a hazard ratio of 0.96, is not too big, and costs incurred by systematic screening are comparatively high.”
STROKESTOP “now provides sound information for cost-effectiveness analyses, which to date have largely relied on assumptions.”
STROKESTOP was partially supported by Carl Bennet AB, Boehringer-Ingelheim, Bayer, Bristol-Meyers Squibb, and Pfizer. Dr. Svennberg disclosed receiving fees for lectures or consulting from Bayer, Bristol-Meyers Squibb, Pfizer, Boehringer-Ingelheim, Merck Sharp & Dohme, and Sanofi; and institutional grants from Roche Diagnostics and Carl Bennett Ltd.
A version of this article first appeared on Medscape.com.
HDL anti-inflammatory effects show prognostic potential
The high-density lipoprotein particle’s complexity as a mediator of cardiovascular risk was on display in a case-control study that, the researchers say, points to its anti-inflammatory capacity as potentially a worthy addition to standard CV risk assessments.
A measure of HDL anti-inflammatory capacity in a prospective community cohort was inversely related to future CV risk independent of HDL’s role in cholesterol transport, total cholesterol, and other established biomarkers, as well as any lipid-modifying therapy.
The current analysis “identified an impaired HDL anti-inflammatory capacity as a functional metric prospectively associated with increased cardiovascular risk in the general population,” observed the authors of the study, published April 12, 2021, in Circulation, led by Congzhuo Jia, MD, University of Groningen (the Netherlands).
“In contrast with the cholesterol efflux function of HDL that tracks moderately with HDL cholesterol levels,” they wrote, HDL anti-inflammatory capacity was not significantly correlated with actual levels of the lipoprotein or a major constituent, apolipoprotein A1 (apoA1). Nor was it correlated with levels of a more generalized inflammatory biomarker, C-reactive protein by high-sensitivity assay (hsCRP).
In a test of its independence as a prognosticator, HDL anti-inflammatory capacity significantly and meaningfully improved prediction of CV events in the study after it was added to the familiar Framingham risk equations.
Measurement of HDL anti-inflammatory properties, therefore, has the potential to improve current CV risk assessments in people without clinical heart disease, the authors proposed.
The study “adds to our understanding of the potential cardioprotective role of HDL,” Michael Miller, MD, University of Maryland, Baltimore, said in an interview.
“We’ve known for some time that HDL has anti-inflammatory properties in vitro, and my understanding is this is the first study to assess these anti-inflammatory properties in a clinical trial,” said Dr. Miller, who studies lipid metabolism and directs the Center for Preventive Cardiology at his center but isn’t an author of the report.
The study is part of a long line of research aiming to “untangle the complexities of HDL and try to get a better handle as to the properties that make it cardioprotective,” he said. For example, “high levels are not always associated with cardioprotection, and low levels don’t always imply increased risk.”
The current findings highlight a quality of HDL that might be prognostic but also independent of its concentrations, apoA1 content, or cholesterol efflux capacity, Dr. Miller noted. That makes HDL anti-inflammatory capacity a “promising feature” of HDL that, if confirmed in further studies, could potentially be brought into the mainstream for CV risk prediction. “But it’s too premature at this time.”
The study of participants in the population-based PREVEND cohort study compared 340 patients with a first CV event – CV death, ischemic heart disease, nonfatal MI, or coronary revascularization – over a median of about 10 years with the same number of participants without such events. The two cohorts of people from the same city in the Netherlands had been matched according to sex, smoking status, age, and HDL cholesterol levels at baseline.
No measured clinical or laboratory value, the group wrote, was significantly correlated with HDL anti-inflammatory capacity, defined here as ability to suppress vascular cell adhesion molecule-1 (VCAM-1) mRNA expression as induced by tumor necrosis factor–alpha in endothelial cells in vitro.
HDL anti-inflammatory capacity was significantly lower in the case cohort, compared with the control cohort (P < .001), and was inversely related to new CV events, at an odds ratio per 1 standard deviation of 0.74 (95% confidence interval, 0.61-0.90; P = .002). Covariate adjustments included body mass index; alcohol intake; diabetes and hypertension status; use of lipid-lowering medicine; levels of total cholesterol, apoA1, triglyceride, and hsCRP; and measures of renal function.
No significant association was seen between HDL anti-inflammatory capacity and cholesterol efflux capacity (coefficient of correlation, −0.02; P > .05). But both metrics were independently associated with CV disease events. The OR per 1 standard deviation was 0.74 (95% CI, 0.61-0.90; P = .002) for cholesterol efflux capacity and 0.66 (95% CI, 0.54-0.81; P < .001) for HDL anti-inflammatory capacity.
Adding HDL anti-inflammatory capacity to the Framingham risk score significantly improved its predictive power; its likelihood-ratio statistic rose from 10.50 to 20.40 (P = .002), the group wrote. The addition of cholesterol efflux capacity further elevated the risk score’s likelihood-ratio statistic to 32.84 (P = .0005).
The analysis has all the limitations of a case-control study, Dr. Miller said, but it does “show a potential reasonable association” between anti-inflammatory capacity and CV risk “that needs to be taken to the next level.”
For example, it could be explored in a controlled trial that tracks anti-inflammatory capacity in individuals who receive an intervention that is likely to improve the biomarker – such as weight loss, he proposed – and follows them for clinical outcomes.
“If you want to elevate the stature of the anti-inflammatory index,” Dr. Miller said, “you will need to show that it’s clinically meaningful.”
Dr. Jia reported no conflicts. Dr. Miller has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The high-density lipoprotein particle’s complexity as a mediator of cardiovascular risk was on display in a case-control study that, the researchers say, points to its anti-inflammatory capacity as potentially a worthy addition to standard CV risk assessments.
A measure of HDL anti-inflammatory capacity in a prospective community cohort was inversely related to future CV risk independent of HDL’s role in cholesterol transport, total cholesterol, and other established biomarkers, as well as any lipid-modifying therapy.
The current analysis “identified an impaired HDL anti-inflammatory capacity as a functional metric prospectively associated with increased cardiovascular risk in the general population,” observed the authors of the study, published April 12, 2021, in Circulation, led by Congzhuo Jia, MD, University of Groningen (the Netherlands).
“In contrast with the cholesterol efflux function of HDL that tracks moderately with HDL cholesterol levels,” they wrote, HDL anti-inflammatory capacity was not significantly correlated with actual levels of the lipoprotein or a major constituent, apolipoprotein A1 (apoA1). Nor was it correlated with levels of a more generalized inflammatory biomarker, C-reactive protein by high-sensitivity assay (hsCRP).
In a test of its independence as a prognosticator, HDL anti-inflammatory capacity significantly and meaningfully improved prediction of CV events in the study after it was added to the familiar Framingham risk equations.
Measurement of HDL anti-inflammatory properties, therefore, has the potential to improve current CV risk assessments in people without clinical heart disease, the authors proposed.
The study “adds to our understanding of the potential cardioprotective role of HDL,” Michael Miller, MD, University of Maryland, Baltimore, said in an interview.
“We’ve known for some time that HDL has anti-inflammatory properties in vitro, and my understanding is this is the first study to assess these anti-inflammatory properties in a clinical trial,” said Dr. Miller, who studies lipid metabolism and directs the Center for Preventive Cardiology at his center but isn’t an author of the report.
The study is part of a long line of research aiming to “untangle the complexities of HDL and try to get a better handle as to the properties that make it cardioprotective,” he said. For example, “high levels are not always associated with cardioprotection, and low levels don’t always imply increased risk.”
The current findings highlight a quality of HDL that might be prognostic but also independent of its concentrations, apoA1 content, or cholesterol efflux capacity, Dr. Miller noted. That makes HDL anti-inflammatory capacity a “promising feature” of HDL that, if confirmed in further studies, could potentially be brought into the mainstream for CV risk prediction. “But it’s too premature at this time.”
The study of participants in the population-based PREVEND cohort study compared 340 patients with a first CV event – CV death, ischemic heart disease, nonfatal MI, or coronary revascularization – over a median of about 10 years with the same number of participants without such events. The two cohorts of people from the same city in the Netherlands had been matched according to sex, smoking status, age, and HDL cholesterol levels at baseline.
No measured clinical or laboratory value, the group wrote, was significantly correlated with HDL anti-inflammatory capacity, defined here as ability to suppress vascular cell adhesion molecule-1 (VCAM-1) mRNA expression as induced by tumor necrosis factor–alpha in endothelial cells in vitro.
HDL anti-inflammatory capacity was significantly lower in the case cohort, compared with the control cohort (P < .001), and was inversely related to new CV events, at an odds ratio per 1 standard deviation of 0.74 (95% confidence interval, 0.61-0.90; P = .002). Covariate adjustments included body mass index; alcohol intake; diabetes and hypertension status; use of lipid-lowering medicine; levels of total cholesterol, apoA1, triglyceride, and hsCRP; and measures of renal function.
No significant association was seen between HDL anti-inflammatory capacity and cholesterol efflux capacity (coefficient of correlation, −0.02; P > .05). But both metrics were independently associated with CV disease events. The OR per 1 standard deviation was 0.74 (95% CI, 0.61-0.90; P = .002) for cholesterol efflux capacity and 0.66 (95% CI, 0.54-0.81; P < .001) for HDL anti-inflammatory capacity.
Adding HDL anti-inflammatory capacity to the Framingham risk score significantly improved its predictive power; its likelihood-ratio statistic rose from 10.50 to 20.40 (P = .002), the group wrote. The addition of cholesterol efflux capacity further elevated the risk score’s likelihood-ratio statistic to 32.84 (P = .0005).
The analysis has all the limitations of a case-control study, Dr. Miller said, but it does “show a potential reasonable association” between anti-inflammatory capacity and CV risk “that needs to be taken to the next level.”
For example, it could be explored in a controlled trial that tracks anti-inflammatory capacity in individuals who receive an intervention that is likely to improve the biomarker – such as weight loss, he proposed – and follows them for clinical outcomes.
“If you want to elevate the stature of the anti-inflammatory index,” Dr. Miller said, “you will need to show that it’s clinically meaningful.”
Dr. Jia reported no conflicts. Dr. Miller has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The high-density lipoprotein particle’s complexity as a mediator of cardiovascular risk was on display in a case-control study that, the researchers say, points to its anti-inflammatory capacity as potentially a worthy addition to standard CV risk assessments.
A measure of HDL anti-inflammatory capacity in a prospective community cohort was inversely related to future CV risk independent of HDL’s role in cholesterol transport, total cholesterol, and other established biomarkers, as well as any lipid-modifying therapy.
The current analysis “identified an impaired HDL anti-inflammatory capacity as a functional metric prospectively associated with increased cardiovascular risk in the general population,” observed the authors of the study, published April 12, 2021, in Circulation, led by Congzhuo Jia, MD, University of Groningen (the Netherlands).
“In contrast with the cholesterol efflux function of HDL that tracks moderately with HDL cholesterol levels,” they wrote, HDL anti-inflammatory capacity was not significantly correlated with actual levels of the lipoprotein or a major constituent, apolipoprotein A1 (apoA1). Nor was it correlated with levels of a more generalized inflammatory biomarker, C-reactive protein by high-sensitivity assay (hsCRP).
In a test of its independence as a prognosticator, HDL anti-inflammatory capacity significantly and meaningfully improved prediction of CV events in the study after it was added to the familiar Framingham risk equations.
Measurement of HDL anti-inflammatory properties, therefore, has the potential to improve current CV risk assessments in people without clinical heart disease, the authors proposed.
The study “adds to our understanding of the potential cardioprotective role of HDL,” Michael Miller, MD, University of Maryland, Baltimore, said in an interview.
“We’ve known for some time that HDL has anti-inflammatory properties in vitro, and my understanding is this is the first study to assess these anti-inflammatory properties in a clinical trial,” said Dr. Miller, who studies lipid metabolism and directs the Center for Preventive Cardiology at his center but isn’t an author of the report.
The study is part of a long line of research aiming to “untangle the complexities of HDL and try to get a better handle as to the properties that make it cardioprotective,” he said. For example, “high levels are not always associated with cardioprotection, and low levels don’t always imply increased risk.”
The current findings highlight a quality of HDL that might be prognostic but also independent of its concentrations, apoA1 content, or cholesterol efflux capacity, Dr. Miller noted. That makes HDL anti-inflammatory capacity a “promising feature” of HDL that, if confirmed in further studies, could potentially be brought into the mainstream for CV risk prediction. “But it’s too premature at this time.”
The study of participants in the population-based PREVEND cohort study compared 340 patients with a first CV event – CV death, ischemic heart disease, nonfatal MI, or coronary revascularization – over a median of about 10 years with the same number of participants without such events. The two cohorts of people from the same city in the Netherlands had been matched according to sex, smoking status, age, and HDL cholesterol levels at baseline.
No measured clinical or laboratory value, the group wrote, was significantly correlated with HDL anti-inflammatory capacity, defined here as ability to suppress vascular cell adhesion molecule-1 (VCAM-1) mRNA expression as induced by tumor necrosis factor–alpha in endothelial cells in vitro.
HDL anti-inflammatory capacity was significantly lower in the case cohort, compared with the control cohort (P < .001), and was inversely related to new CV events, at an odds ratio per 1 standard deviation of 0.74 (95% confidence interval, 0.61-0.90; P = .002). Covariate adjustments included body mass index; alcohol intake; diabetes and hypertension status; use of lipid-lowering medicine; levels of total cholesterol, apoA1, triglyceride, and hsCRP; and measures of renal function.
No significant association was seen between HDL anti-inflammatory capacity and cholesterol efflux capacity (coefficient of correlation, −0.02; P > .05). But both metrics were independently associated with CV disease events. The OR per 1 standard deviation was 0.74 (95% CI, 0.61-0.90; P = .002) for cholesterol efflux capacity and 0.66 (95% CI, 0.54-0.81; P < .001) for HDL anti-inflammatory capacity.
Adding HDL anti-inflammatory capacity to the Framingham risk score significantly improved its predictive power; its likelihood-ratio statistic rose from 10.50 to 20.40 (P = .002), the group wrote. The addition of cholesterol efflux capacity further elevated the risk score’s likelihood-ratio statistic to 32.84 (P = .0005).
The analysis has all the limitations of a case-control study, Dr. Miller said, but it does “show a potential reasonable association” between anti-inflammatory capacity and CV risk “that needs to be taken to the next level.”
For example, it could be explored in a controlled trial that tracks anti-inflammatory capacity in individuals who receive an intervention that is likely to improve the biomarker – such as weight loss, he proposed – and follows them for clinical outcomes.
“If you want to elevate the stature of the anti-inflammatory index,” Dr. Miller said, “you will need to show that it’s clinically meaningful.”
Dr. Jia reported no conflicts. Dr. Miller has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Medtronic recall of almost 240,000 ICDs is class I, FDA says
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
How to convince patients muscle pain isn’t a statin Achilles heel: StatinWISE
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
New light cast on type 2 MI aims to sharpen diagnosis, therapy
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.