User login
Chest pain • shortness of breath • fever and nausea • Dx?
THE CASE
A 38-year-old Hispanic man was brought to the emergency department (ED) after losing consciousness and falling at home, striking his elbow, head, and neck. For the past week, he’d had palpitations, shortness of breath, mild swelling of the lower extremities, fever, nausea, and fatigue. He had also been experiencing squeezing chest pain that worsened with exertion and was only partially relieved by nitroglycerin.
The patient did not have any rashes and denied having contact with anyone who was sick. He said that he’d been bitten by mosquitos during recent outdoor activities. His medical history included hypertension, hemorrhagic basal ganglia stroke, hyperlipidemia, sleep apnea, metabolic syndrome, and gout. The patient denied smoking or using illicit drugs.
In the ED, his temperature was 101°F, heart rate was 112 beats/min, blood pressure was 175/100 mm Hg, and respiratory rate was 18 breaths/min. His head and neck exam was normal, with no neck stiffness. A lung exam revealed bilateral basal crackles, and a neurologic exam showed residual right-sided weakness due to the hemorrhagic stroke one year ago.
Lab test results revealed the following: white blood cell (WBC) count, 13,000/mm3 with relative monocytosis (14%); lymphocytosis (44%) with normal neutrophils and no bands; hemoglobin, 12 g/dL; hematocrit, 36/mm3; and platelets, 300,000/mm3. Liver function tests were within normal limits. Urinalysis was unremarkable. His troponin I level was elevated at 1.385 ng/dL. In addition to the tachycardia, his electrocardiogram (EKG) showed left axis deviation, left atrial enlargement, left anterior fascicular block, and diffuse nonspecific ST and T wave abnormalities. Chest x-ray was unremarkable except for cardiomegaly. A computed tomography (CT) scan of his head showed residual changes from the previous stroke.
The patient was admitted with a provisional diagnosis of systemic inflammatory response syndrome (SIRS), syncope, non–ST elevation myocardial infarction (NSTEMI), and acute heart failure. The patient had continuous EKG monitoring and serial assessments of his troponin levels. He was also given aspirin, metoprolol 25 mg BID, lisinopril 10 mg/d, furosemide 40 mg IV, isosorbide mononitrate 60 mg/d, and atorvastatin 40 mg/d.
The patient’s cardiac enzymes subsequently decreased. A left heart catheterization was performed, which showed minimum irregularities of the left anterior descending artery (< 20% narrowing) and an ejection fraction (EF) of 35%, without any evidence of obstructive coronary artery disease (CAD). An echocardiogram revealed systolic dysfunction, with an EF of 35% to 40% and global hypokinesis without any apical ballooning or pericardial effusion. (An echocardiogram performed 6 months earlier had shown normal systolic function, an EF of 60% to 65%, and no wall motion abnormalities.) Blood, urine, and fungal cultures were negative; stool studies for ova and parasites were also negative. A lower extremity venous Doppler was negative for deep vein thrombosis.
THE DIAGNOSIS
Because our patient had SIRS, troponinemia, acute systolic dysfunction, and global hypokinesis without any evidence of obstructive CAD, we considered a diagnosis of viral myocarditis. Serologic studies for echovirus, coxsackievirus B, parvovirus B19, adenovirus, and human herpesvirus 6 (HHV-6) all came back negative. However, an enzyme-linked immunosorbent assay (ELISA) for West Nile virus (WNV) was positive. WNV infection was confirmed with a positive plaque reduction neutralization test and a positive qualitative polymerase chain reaction (PCR) assay, which established a diagnosis of WNV myocarditis.
DISCUSSION
While most individuals infected with WNV are asymptomatic, 20% to 40% of patients will exhibit symptoms.1-4 Typical presentations of WNV infection include West Nile fever and neuroinvasive disease. West Nile fever is a self-limited illness characterized by a low-grade fever, headache, malaise, back pain, myalgia, and anorexia for 3 to 6 days.2 Neuroinvasive disease caused by WNV may present as encephalitis, meningitis, or flaccid paralysis.5 Atypical presentations of the virus include rhabdomyolysis,6 fatal hemorrhagic fever with multi-organ failure and palpable purpura,7 hepatitis,8 pancreatitis,9 central diabetes insipidus,10 and myocarditis.11
Although WNV has been linked to myocarditismin animals,12 few human cases of WNV myocarditis11,13 or cardiomyopathy14 have been reported. Viral myocarditis often leads to the development of dilated cardiomyopathy, and myocardial damage may result from direct virus-induced cytotoxicity, T cell-mediated immune response to the virus, or apoptosis.15 Some research suggests that immune-mediated mechanisms play a primary role in myocardial damage. Caforio et al16 found that anti-alpha myosin antibodies were present in 34% of myocarditis patients. In a follow-up study, these antibodies were shown to persist for up to 6 months, which far surpasses the viral cardiac replication timeline of 2 to 3 weeks,17 suggesting that damage occurring after that time is primarily an autoimmune process.
The differential diagnosis for WNV myocarditis includes myocardial stunning from demand ischemia related to SIRS, Takotsubo cardiomyopathy (stress cardiomyopathy), and Dressler’s syndrome. For our patient, myocardial stunning from demand ischemia was less likely because he had no obstructive coronary disease or focal hypokinesis. In addition, the persistence of left ventricular systolic dysfunction and global hypokinesis demonstrated in a repeat echocardiogram during follow-up 6 months later reinforced the likelihood of myocarditis.
The patient’s chest pain with syncope, elevated troponin level, and nonspecific EKG changes in the absence of obstructive CAD raised the possibility of Takotsubo cardiomyopathy. The characteristic echocardiogram finding in Takotsubo cardiomyopathy is transient apical ballooning with akinesis or hypokinesis in the apical and/or mid ventricular regions (typical variant) or isolated midventricular hypokinesis (apical sparing variant). Our patient’s echocardiogram did not show any of these focal wall motion abnormalities, but instead showed global hypokinesis. In addition, the persistence of systolic dysfunction during the repeat echocardiogram and the patient’s lack of psychological distress made the diagnosis of Takotsubo cardiomyopathy unlikely.
Dressler’s syndrome, which is also known as post-myocardial infarction (MI) syndrome, typically presents weeks to months after MI as pleuritic chest pain with a pericardial rub, elevated inflammatory markers, typical EKG changes (diffuse ST-segment elevation and PR-segment depression), and pericardial effusion. This did not fit our patient’s presentation.
Supportive care for heart failure is the mainstay of treatment
The standard treatment for WNV myocarditis is supportive care. Diuretics are used as needed for fluid overload, along with angiotensin-converting enzyme inhibitors and beta-blockers for cardiomyopathy with decreased EF.
Our patient’s dyspnea improved with treatment of furosemide 40 mg IV BID, and his blood pressure was controlled with metoprolol 25 mg BID and lisinopril 10 mg BID. His chest pain and fever resolved when his blood pressure improved. He was discharged home after 7 days on the furosemide, metoprolol, and lisinopril, in addition to isosorbide mononitrate 30 mg/d, atorvastatin 40 mg/d, and aspirin 325 mg/d. An echocardiogram performed 6 months later showed persistent systolic dysfunction, with an EF of 35% and global wall motion abnormalities.
THE TAKEAWAY
In addition to acute coronary syndrome, consider alternate etiologies in patients who present with chest pain and elevated cardiac biomarkers, particularly if diagnostic work-up is negative for obstructive coronary artery disease. WNV myocarditis should be considered as a diagnosis when a patient’s symptoms suggest acute coronary syndrome but are accompanied by fever, headache, and other constitutional symptoms, especially during mosquito season or a WNV outbreak.
1. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807-1814.
2. Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272-277.
3. Brown JA, Factor DL, Tkachenko N, et al. West Nile viremic blood donors and risk factors for subsequent West Nile fever. Vector Borne Zoonotic Dis. 2007;7:479-488.
4. Zou S, Foster GA, Dodd RY, et al. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354-1361.
5. Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286-300.
6. Montgomery SP, Chow CC, Smith SW, et al. Rhabdomyolysis in patients with west nile encephalitis and meningitis. Vector Borne Zoonotic Dis. 2005;5:252-257.
7. Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42:1527-1535.
8. Georges AJ, Lesbordes JL, Georges-Courbot MC, et al. Fatal hepatitis from West Nile virus. Ann Inst Pasteur Virol. 1988;138:237.
9. Perelman A, Stern J. Acute pancreatitis in West Nile Fever. Am J Trop Med Hyg. 1974;23:1150-1152.
10. Sherman-Weber S, Axelrod P. Central diabetes insipidus complicating West Nile encephalitis. Clin Infect Dis. 2004;38:1042-1043.
11. Pergam SA, DeLong CE, Echevarria L, et al. Myocarditis in West Nile virus infection. Am J Trop Med Hyg. 2006;75:1232-1233.
12. van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637-657.
13. Kushawaha A, Jadonath S, Mobarakai N. West nile virus myocarditis causing a fatal arrhythmia: a case report. Cases J. 2009;2:7147.
14. Khouzam RN. Significant cardiomyopathy secondary to West Nile virus infection. South Med J. 2009;102:527-528.
15. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091-1100.
16. Caforio AL, Goldman JH, Haven AJ, et al. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270-275.
17. Lauer B, Schannwell M, Kühl U, et al. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11-18.
THE CASE
A 38-year-old Hispanic man was brought to the emergency department (ED) after losing consciousness and falling at home, striking his elbow, head, and neck. For the past week, he’d had palpitations, shortness of breath, mild swelling of the lower extremities, fever, nausea, and fatigue. He had also been experiencing squeezing chest pain that worsened with exertion and was only partially relieved by nitroglycerin.
The patient did not have any rashes and denied having contact with anyone who was sick. He said that he’d been bitten by mosquitos during recent outdoor activities. His medical history included hypertension, hemorrhagic basal ganglia stroke, hyperlipidemia, sleep apnea, metabolic syndrome, and gout. The patient denied smoking or using illicit drugs.
In the ED, his temperature was 101°F, heart rate was 112 beats/min, blood pressure was 175/100 mm Hg, and respiratory rate was 18 breaths/min. His head and neck exam was normal, with no neck stiffness. A lung exam revealed bilateral basal crackles, and a neurologic exam showed residual right-sided weakness due to the hemorrhagic stroke one year ago.
Lab test results revealed the following: white blood cell (WBC) count, 13,000/mm3 with relative monocytosis (14%); lymphocytosis (44%) with normal neutrophils and no bands; hemoglobin, 12 g/dL; hematocrit, 36/mm3; and platelets, 300,000/mm3. Liver function tests were within normal limits. Urinalysis was unremarkable. His troponin I level was elevated at 1.385 ng/dL. In addition to the tachycardia, his electrocardiogram (EKG) showed left axis deviation, left atrial enlargement, left anterior fascicular block, and diffuse nonspecific ST and T wave abnormalities. Chest x-ray was unremarkable except for cardiomegaly. A computed tomography (CT) scan of his head showed residual changes from the previous stroke.
The patient was admitted with a provisional diagnosis of systemic inflammatory response syndrome (SIRS), syncope, non–ST elevation myocardial infarction (NSTEMI), and acute heart failure. The patient had continuous EKG monitoring and serial assessments of his troponin levels. He was also given aspirin, metoprolol 25 mg BID, lisinopril 10 mg/d, furosemide 40 mg IV, isosorbide mononitrate 60 mg/d, and atorvastatin 40 mg/d.
The patient’s cardiac enzymes subsequently decreased. A left heart catheterization was performed, which showed minimum irregularities of the left anterior descending artery (< 20% narrowing) and an ejection fraction (EF) of 35%, without any evidence of obstructive coronary artery disease (CAD). An echocardiogram revealed systolic dysfunction, with an EF of 35% to 40% and global hypokinesis without any apical ballooning or pericardial effusion. (An echocardiogram performed 6 months earlier had shown normal systolic function, an EF of 60% to 65%, and no wall motion abnormalities.) Blood, urine, and fungal cultures were negative; stool studies for ova and parasites were also negative. A lower extremity venous Doppler was negative for deep vein thrombosis.
THE DIAGNOSIS
Because our patient had SIRS, troponinemia, acute systolic dysfunction, and global hypokinesis without any evidence of obstructive CAD, we considered a diagnosis of viral myocarditis. Serologic studies for echovirus, coxsackievirus B, parvovirus B19, adenovirus, and human herpesvirus 6 (HHV-6) all came back negative. However, an enzyme-linked immunosorbent assay (ELISA) for West Nile virus (WNV) was positive. WNV infection was confirmed with a positive plaque reduction neutralization test and a positive qualitative polymerase chain reaction (PCR) assay, which established a diagnosis of WNV myocarditis.
DISCUSSION
While most individuals infected with WNV are asymptomatic, 20% to 40% of patients will exhibit symptoms.1-4 Typical presentations of WNV infection include West Nile fever and neuroinvasive disease. West Nile fever is a self-limited illness characterized by a low-grade fever, headache, malaise, back pain, myalgia, and anorexia for 3 to 6 days.2 Neuroinvasive disease caused by WNV may present as encephalitis, meningitis, or flaccid paralysis.5 Atypical presentations of the virus include rhabdomyolysis,6 fatal hemorrhagic fever with multi-organ failure and palpable purpura,7 hepatitis,8 pancreatitis,9 central diabetes insipidus,10 and myocarditis.11
Although WNV has been linked to myocarditismin animals,12 few human cases of WNV myocarditis11,13 or cardiomyopathy14 have been reported. Viral myocarditis often leads to the development of dilated cardiomyopathy, and myocardial damage may result from direct virus-induced cytotoxicity, T cell-mediated immune response to the virus, or apoptosis.15 Some research suggests that immune-mediated mechanisms play a primary role in myocardial damage. Caforio et al16 found that anti-alpha myosin antibodies were present in 34% of myocarditis patients. In a follow-up study, these antibodies were shown to persist for up to 6 months, which far surpasses the viral cardiac replication timeline of 2 to 3 weeks,17 suggesting that damage occurring after that time is primarily an autoimmune process.
The differential diagnosis for WNV myocarditis includes myocardial stunning from demand ischemia related to SIRS, Takotsubo cardiomyopathy (stress cardiomyopathy), and Dressler’s syndrome. For our patient, myocardial stunning from demand ischemia was less likely because he had no obstructive coronary disease or focal hypokinesis. In addition, the persistence of left ventricular systolic dysfunction and global hypokinesis demonstrated in a repeat echocardiogram during follow-up 6 months later reinforced the likelihood of myocarditis.
The patient’s chest pain with syncope, elevated troponin level, and nonspecific EKG changes in the absence of obstructive CAD raised the possibility of Takotsubo cardiomyopathy. The characteristic echocardiogram finding in Takotsubo cardiomyopathy is transient apical ballooning with akinesis or hypokinesis in the apical and/or mid ventricular regions (typical variant) or isolated midventricular hypokinesis (apical sparing variant). Our patient’s echocardiogram did not show any of these focal wall motion abnormalities, but instead showed global hypokinesis. In addition, the persistence of systolic dysfunction during the repeat echocardiogram and the patient’s lack of psychological distress made the diagnosis of Takotsubo cardiomyopathy unlikely.
Dressler’s syndrome, which is also known as post-myocardial infarction (MI) syndrome, typically presents weeks to months after MI as pleuritic chest pain with a pericardial rub, elevated inflammatory markers, typical EKG changes (diffuse ST-segment elevation and PR-segment depression), and pericardial effusion. This did not fit our patient’s presentation.
Supportive care for heart failure is the mainstay of treatment
The standard treatment for WNV myocarditis is supportive care. Diuretics are used as needed for fluid overload, along with angiotensin-converting enzyme inhibitors and beta-blockers for cardiomyopathy with decreased EF.
Our patient’s dyspnea improved with treatment of furosemide 40 mg IV BID, and his blood pressure was controlled with metoprolol 25 mg BID and lisinopril 10 mg BID. His chest pain and fever resolved when his blood pressure improved. He was discharged home after 7 days on the furosemide, metoprolol, and lisinopril, in addition to isosorbide mononitrate 30 mg/d, atorvastatin 40 mg/d, and aspirin 325 mg/d. An echocardiogram performed 6 months later showed persistent systolic dysfunction, with an EF of 35% and global wall motion abnormalities.
THE TAKEAWAY
In addition to acute coronary syndrome, consider alternate etiologies in patients who present with chest pain and elevated cardiac biomarkers, particularly if diagnostic work-up is negative for obstructive coronary artery disease. WNV myocarditis should be considered as a diagnosis when a patient’s symptoms suggest acute coronary syndrome but are accompanied by fever, headache, and other constitutional symptoms, especially during mosquito season or a WNV outbreak.
THE CASE
A 38-year-old Hispanic man was brought to the emergency department (ED) after losing consciousness and falling at home, striking his elbow, head, and neck. For the past week, he’d had palpitations, shortness of breath, mild swelling of the lower extremities, fever, nausea, and fatigue. He had also been experiencing squeezing chest pain that worsened with exertion and was only partially relieved by nitroglycerin.
The patient did not have any rashes and denied having contact with anyone who was sick. He said that he’d been bitten by mosquitos during recent outdoor activities. His medical history included hypertension, hemorrhagic basal ganglia stroke, hyperlipidemia, sleep apnea, metabolic syndrome, and gout. The patient denied smoking or using illicit drugs.
In the ED, his temperature was 101°F, heart rate was 112 beats/min, blood pressure was 175/100 mm Hg, and respiratory rate was 18 breaths/min. His head and neck exam was normal, with no neck stiffness. A lung exam revealed bilateral basal crackles, and a neurologic exam showed residual right-sided weakness due to the hemorrhagic stroke one year ago.
Lab test results revealed the following: white blood cell (WBC) count, 13,000/mm3 with relative monocytosis (14%); lymphocytosis (44%) with normal neutrophils and no bands; hemoglobin, 12 g/dL; hematocrit, 36/mm3; and platelets, 300,000/mm3. Liver function tests were within normal limits. Urinalysis was unremarkable. His troponin I level was elevated at 1.385 ng/dL. In addition to the tachycardia, his electrocardiogram (EKG) showed left axis deviation, left atrial enlargement, left anterior fascicular block, and diffuse nonspecific ST and T wave abnormalities. Chest x-ray was unremarkable except for cardiomegaly. A computed tomography (CT) scan of his head showed residual changes from the previous stroke.
The patient was admitted with a provisional diagnosis of systemic inflammatory response syndrome (SIRS), syncope, non–ST elevation myocardial infarction (NSTEMI), and acute heart failure. The patient had continuous EKG monitoring and serial assessments of his troponin levels. He was also given aspirin, metoprolol 25 mg BID, lisinopril 10 mg/d, furosemide 40 mg IV, isosorbide mononitrate 60 mg/d, and atorvastatin 40 mg/d.
The patient’s cardiac enzymes subsequently decreased. A left heart catheterization was performed, which showed minimum irregularities of the left anterior descending artery (< 20% narrowing) and an ejection fraction (EF) of 35%, without any evidence of obstructive coronary artery disease (CAD). An echocardiogram revealed systolic dysfunction, with an EF of 35% to 40% and global hypokinesis without any apical ballooning or pericardial effusion. (An echocardiogram performed 6 months earlier had shown normal systolic function, an EF of 60% to 65%, and no wall motion abnormalities.) Blood, urine, and fungal cultures were negative; stool studies for ova and parasites were also negative. A lower extremity venous Doppler was negative for deep vein thrombosis.
THE DIAGNOSIS
Because our patient had SIRS, troponinemia, acute systolic dysfunction, and global hypokinesis without any evidence of obstructive CAD, we considered a diagnosis of viral myocarditis. Serologic studies for echovirus, coxsackievirus B, parvovirus B19, adenovirus, and human herpesvirus 6 (HHV-6) all came back negative. However, an enzyme-linked immunosorbent assay (ELISA) for West Nile virus (WNV) was positive. WNV infection was confirmed with a positive plaque reduction neutralization test and a positive qualitative polymerase chain reaction (PCR) assay, which established a diagnosis of WNV myocarditis.
DISCUSSION
While most individuals infected with WNV are asymptomatic, 20% to 40% of patients will exhibit symptoms.1-4 Typical presentations of WNV infection include West Nile fever and neuroinvasive disease. West Nile fever is a self-limited illness characterized by a low-grade fever, headache, malaise, back pain, myalgia, and anorexia for 3 to 6 days.2 Neuroinvasive disease caused by WNV may present as encephalitis, meningitis, or flaccid paralysis.5 Atypical presentations of the virus include rhabdomyolysis,6 fatal hemorrhagic fever with multi-organ failure and palpable purpura,7 hepatitis,8 pancreatitis,9 central diabetes insipidus,10 and myocarditis.11
Although WNV has been linked to myocarditismin animals,12 few human cases of WNV myocarditis11,13 or cardiomyopathy14 have been reported. Viral myocarditis often leads to the development of dilated cardiomyopathy, and myocardial damage may result from direct virus-induced cytotoxicity, T cell-mediated immune response to the virus, or apoptosis.15 Some research suggests that immune-mediated mechanisms play a primary role in myocardial damage. Caforio et al16 found that anti-alpha myosin antibodies were present in 34% of myocarditis patients. In a follow-up study, these antibodies were shown to persist for up to 6 months, which far surpasses the viral cardiac replication timeline of 2 to 3 weeks,17 suggesting that damage occurring after that time is primarily an autoimmune process.
The differential diagnosis for WNV myocarditis includes myocardial stunning from demand ischemia related to SIRS, Takotsubo cardiomyopathy (stress cardiomyopathy), and Dressler’s syndrome. For our patient, myocardial stunning from demand ischemia was less likely because he had no obstructive coronary disease or focal hypokinesis. In addition, the persistence of left ventricular systolic dysfunction and global hypokinesis demonstrated in a repeat echocardiogram during follow-up 6 months later reinforced the likelihood of myocarditis.
The patient’s chest pain with syncope, elevated troponin level, and nonspecific EKG changes in the absence of obstructive CAD raised the possibility of Takotsubo cardiomyopathy. The characteristic echocardiogram finding in Takotsubo cardiomyopathy is transient apical ballooning with akinesis or hypokinesis in the apical and/or mid ventricular regions (typical variant) or isolated midventricular hypokinesis (apical sparing variant). Our patient’s echocardiogram did not show any of these focal wall motion abnormalities, but instead showed global hypokinesis. In addition, the persistence of systolic dysfunction during the repeat echocardiogram and the patient’s lack of psychological distress made the diagnosis of Takotsubo cardiomyopathy unlikely.
Dressler’s syndrome, which is also known as post-myocardial infarction (MI) syndrome, typically presents weeks to months after MI as pleuritic chest pain with a pericardial rub, elevated inflammatory markers, typical EKG changes (diffuse ST-segment elevation and PR-segment depression), and pericardial effusion. This did not fit our patient’s presentation.
Supportive care for heart failure is the mainstay of treatment
The standard treatment for WNV myocarditis is supportive care. Diuretics are used as needed for fluid overload, along with angiotensin-converting enzyme inhibitors and beta-blockers for cardiomyopathy with decreased EF.
Our patient’s dyspnea improved with treatment of furosemide 40 mg IV BID, and his blood pressure was controlled with metoprolol 25 mg BID and lisinopril 10 mg BID. His chest pain and fever resolved when his blood pressure improved. He was discharged home after 7 days on the furosemide, metoprolol, and lisinopril, in addition to isosorbide mononitrate 30 mg/d, atorvastatin 40 mg/d, and aspirin 325 mg/d. An echocardiogram performed 6 months later showed persistent systolic dysfunction, with an EF of 35% and global wall motion abnormalities.
THE TAKEAWAY
In addition to acute coronary syndrome, consider alternate etiologies in patients who present with chest pain and elevated cardiac biomarkers, particularly if diagnostic work-up is negative for obstructive coronary artery disease. WNV myocarditis should be considered as a diagnosis when a patient’s symptoms suggest acute coronary syndrome but are accompanied by fever, headache, and other constitutional symptoms, especially during mosquito season or a WNV outbreak.
1. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807-1814.
2. Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272-277.
3. Brown JA, Factor DL, Tkachenko N, et al. West Nile viremic blood donors and risk factors for subsequent West Nile fever. Vector Borne Zoonotic Dis. 2007;7:479-488.
4. Zou S, Foster GA, Dodd RY, et al. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354-1361.
5. Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286-300.
6. Montgomery SP, Chow CC, Smith SW, et al. Rhabdomyolysis in patients with west nile encephalitis and meningitis. Vector Borne Zoonotic Dis. 2005;5:252-257.
7. Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42:1527-1535.
8. Georges AJ, Lesbordes JL, Georges-Courbot MC, et al. Fatal hepatitis from West Nile virus. Ann Inst Pasteur Virol. 1988;138:237.
9. Perelman A, Stern J. Acute pancreatitis in West Nile Fever. Am J Trop Med Hyg. 1974;23:1150-1152.
10. Sherman-Weber S, Axelrod P. Central diabetes insipidus complicating West Nile encephalitis. Clin Infect Dis. 2004;38:1042-1043.
11. Pergam SA, DeLong CE, Echevarria L, et al. Myocarditis in West Nile virus infection. Am J Trop Med Hyg. 2006;75:1232-1233.
12. van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637-657.
13. Kushawaha A, Jadonath S, Mobarakai N. West nile virus myocarditis causing a fatal arrhythmia: a case report. Cases J. 2009;2:7147.
14. Khouzam RN. Significant cardiomyopathy secondary to West Nile virus infection. South Med J. 2009;102:527-528.
15. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091-1100.
16. Caforio AL, Goldman JH, Haven AJ, et al. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270-275.
17. Lauer B, Schannwell M, Kühl U, et al. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11-18.
1. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807-1814.
2. Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272-277.
3. Brown JA, Factor DL, Tkachenko N, et al. West Nile viremic blood donors and risk factors for subsequent West Nile fever. Vector Borne Zoonotic Dis. 2007;7:479-488.
4. Zou S, Foster GA, Dodd RY, et al. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354-1361.
5. Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286-300.
6. Montgomery SP, Chow CC, Smith SW, et al. Rhabdomyolysis in patients with west nile encephalitis and meningitis. Vector Borne Zoonotic Dis. 2005;5:252-257.
7. Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42:1527-1535.
8. Georges AJ, Lesbordes JL, Georges-Courbot MC, et al. Fatal hepatitis from West Nile virus. Ann Inst Pasteur Virol. 1988;138:237.
9. Perelman A, Stern J. Acute pancreatitis in West Nile Fever. Am J Trop Med Hyg. 1974;23:1150-1152.
10. Sherman-Weber S, Axelrod P. Central diabetes insipidus complicating West Nile encephalitis. Clin Infect Dis. 2004;38:1042-1043.
11. Pergam SA, DeLong CE, Echevarria L, et al. Myocarditis in West Nile virus infection. Am J Trop Med Hyg. 2006;75:1232-1233.
12. van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637-657.
13. Kushawaha A, Jadonath S, Mobarakai N. West nile virus myocarditis causing a fatal arrhythmia: a case report. Cases J. 2009;2:7147.
14. Khouzam RN. Significant cardiomyopathy secondary to West Nile virus infection. South Med J. 2009;102:527-528.
15. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091-1100.
16. Caforio AL, Goldman JH, Haven AJ, et al. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270-275.
17. Lauer B, Schannwell M, Kühl U, et al. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11-18.
Lack of energy, petechiae, elevated PSA level—Dx?
THE CASE
A 57-year-old Hispanic man sought treatment because he had been feeling tired for a few weeks. He had not seen a physician for 15 years. When he came in, his temperature was 98.8°F, blood pressure was 132/82 mm Hg, pulse was 82 beats/min, respiration rate was 16 breaths/min, and oxygen saturation was 93% on room air. Examination of the head, neck, and respiratory and cardiovascular systems was normal. Skin examination showed petechiae and bruising on his abdomen, left ankle, right thigh, and bilateral shin area. His abdomen was nontender with no organomegaly. There was no focal neurological finding or spinal tenderness. Our patient had no chills, chest pains, shortness of breath, headache, dizziness, or loss of consciousness. There was no hematemesis, melena, hematuria, edema, or weight loss. He had no medical or surgical history and denied substance abuse or taking any medications recently; he did use alcohol previously.
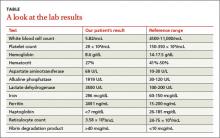
Results of some initial lab tests were abnormal, including a decreased white blood cell count (5.82/mcL), platelet count (29 x 103/mcL), hemoglobin (8.6 g/dL), and hematocrit (27%) (TABLE). A peripheral blood smear showed decreased normocytic red blood cells and scattered schistocytes. His prostate-specific antigen (PSA) level was elevated at 212 ng/mL.
The patient’s coagulation profile was normal, and his von Willebrand factor (vWF) protease (ADAMTS-13) level was within normal limits (13.83). Antineutrophil cytoplasmic antibody and antinuclear antibody tests were negative. Testing for pulmonary embolism was negative, as was testing for human immunodeficiency virus. An abdominal ultrasound was normal, as well.
THE DIAGNOSIS
Based on our patient’s abnormal blood test results and the presence of petechiae and bruising, we diagnosed thrombotic thrombocytopenic purpura (TTP). The patient’s elevated PSA prompted us to order computed tomography of the chest and abdomen, which showed an enlarged prostate gland and mixed lytic sclerotic lesions in T3 to T5 and T9 vertebrae and in his ribs. A bone marrow biopsy revealed metastatic prostatic adenocarcinoma and a bone scan confirmed multiple metastases in the spine, pelvis, and shoulders.
DISCUSSION
TTP is a rare disorder of increased clotting in small blood vessels throughout the body that can include thrombocytopenia, microangiopathic hemolytic anemia (MAHA), fever, renal dysfunction, and neurological deficits.1 It’s important to maintain a high index of suspicion for TTP because the condition is a hematologic medical emergency that can quickly cause multiorgan failure and death.2
Almost always an acquired condition, TTP can be idiopathic or secondary to another condition, such as collagen vascular diseases, transplants, certain drugs, infections, pregnancy, or cancer.3 In idiopathic TTP, the cause of the condition is believed to be reduced activity of ADAMTS-13, the protease that breaks vWF into smaller pieces—thus preventing the formation of unnecessary blood clots.
In cancer-associated TTP, which could be a complication resulting from chemotherapy or a manifestation of cancer itself,3 ADAMTS-13 level is normal and the condition is likely the result of an increased tumor cell load, which leads to endothelial damage and fragmentation of red blood cells (RBC) as they traverse the injured microvasculature.4 In an analysis of 154 cases of “solid” cancer-related MAHA, Lechner and Obermeier5 found 23 cases were related to prostate cancer, as was the case with our patient.
Treatment for TTP is plasma exchange. The mortality rate of untreated TTP can exceed 90%, but plasma exchange therapy has reduced that rate to <20%.6 It has been suggested that proteolysis of vWF may play a central role in the efficacy of plasma exchange for TTP.7
Our patient was hospitalized and received 2 units of packed RBCs. He also received plasma exchange for 9 days with minimal response. On Day 5, our patient was started on leuprorelin and parenteral steroids. Soon after, his platelet count rose to 33 × 103/mcL and lactate dehydrogenase decreased. He was discharged approximately one week after the steroids were started.
After several months of outpatient treatment with leuprorelin and bicalutamide, the patient’s platelet count normalized to 212 × 103/mcL (from 29 × 103/mcL), alkaline phosphatase decreased to 402 U/L (from 1919 U/L), and PSA levels trended downward to 8.63 ng/mL (from 212 ng/mL). He continued to receive care from our oncology clinic for the next several months and his PSA level continued to decline. However, at his last few visits, his PSA level had trended up, suggesting progression of his prostate cancer. The patient has not followed up with our clinic recently.
THE TAKEAWAY
Suspect TTP in patients who present with unexplained petechiae and bruising, and whose blood work reveals thrombocytopenia and MAHA.2 Patients with TTP who do not respond to plasma exchange should be evaluated for underlying cancer or other potential secondary causes.3 Patients with cancer-associated TTP may respond to steroid therapy.
1. Lichtin AE, Schreiber AD, Hurwitz S, et al. Efficacy of intensive plasmapheresis in thrombotic thrombocytopenic purpura. Archives Intern Med. 1987;147:2122-2126.
2. Blombery P, Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J Blood Med. 2014;5:15-23.
3. Chang JC, Naqvi T. Thrombotic thrombocytopenic purpura associated with bone marrow metastasis and secondary myelofibrosis in cancer. Oncologist. 2003;8:375-380.
4. Pirrotta MT, Bucalossi A, Forconi F, et al. Thrombotic thrombocytopenic purpura secondary to an occult adenocarcinoma. Oncologist. 2005;10:299-300.
5. Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore). 2012;91: 195-205.
6. Oberic L, Buffet, M, Scwarzinger M, et al; Reference Center for the Management of Thrombotic Microangiopathies. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009;14:769-779.
7. Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS 13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059-41063.
THE CASE
A 57-year-old Hispanic man sought treatment because he had been feeling tired for a few weeks. He had not seen a physician for 15 years. When he came in, his temperature was 98.8°F, blood pressure was 132/82 mm Hg, pulse was 82 beats/min, respiration rate was 16 breaths/min, and oxygen saturation was 93% on room air. Examination of the head, neck, and respiratory and cardiovascular systems was normal. Skin examination showed petechiae and bruising on his abdomen, left ankle, right thigh, and bilateral shin area. His abdomen was nontender with no organomegaly. There was no focal neurological finding or spinal tenderness. Our patient had no chills, chest pains, shortness of breath, headache, dizziness, or loss of consciousness. There was no hematemesis, melena, hematuria, edema, or weight loss. He had no medical or surgical history and denied substance abuse or taking any medications recently; he did use alcohol previously.
Results of some initial lab tests were abnormal, including a decreased white blood cell count (5.82/mcL), platelet count (29 x 103/mcL), hemoglobin (8.6 g/dL), and hematocrit (27%) (TABLE). A peripheral blood smear showed decreased normocytic red blood cells and scattered schistocytes. His prostate-specific antigen (PSA) level was elevated at 212 ng/mL.
The patient’s coagulation profile was normal, and his von Willebrand factor (vWF) protease (ADAMTS-13) level was within normal limits (13.83). Antineutrophil cytoplasmic antibody and antinuclear antibody tests were negative. Testing for pulmonary embolism was negative, as was testing for human immunodeficiency virus. An abdominal ultrasound was normal, as well.
THE DIAGNOSIS
Based on our patient’s abnormal blood test results and the presence of petechiae and bruising, we diagnosed thrombotic thrombocytopenic purpura (TTP). The patient’s elevated PSA prompted us to order computed tomography of the chest and abdomen, which showed an enlarged prostate gland and mixed lytic sclerotic lesions in T3 to T5 and T9 vertebrae and in his ribs. A bone marrow biopsy revealed metastatic prostatic adenocarcinoma and a bone scan confirmed multiple metastases in the spine, pelvis, and shoulders.
DISCUSSION
TTP is a rare disorder of increased clotting in small blood vessels throughout the body that can include thrombocytopenia, microangiopathic hemolytic anemia (MAHA), fever, renal dysfunction, and neurological deficits.1 It’s important to maintain a high index of suspicion for TTP because the condition is a hematologic medical emergency that can quickly cause multiorgan failure and death.2
Almost always an acquired condition, TTP can be idiopathic or secondary to another condition, such as collagen vascular diseases, transplants, certain drugs, infections, pregnancy, or cancer.3 In idiopathic TTP, the cause of the condition is believed to be reduced activity of ADAMTS-13, the protease that breaks vWF into smaller pieces—thus preventing the formation of unnecessary blood clots.
In cancer-associated TTP, which could be a complication resulting from chemotherapy or a manifestation of cancer itself,3 ADAMTS-13 level is normal and the condition is likely the result of an increased tumor cell load, which leads to endothelial damage and fragmentation of red blood cells (RBC) as they traverse the injured microvasculature.4 In an analysis of 154 cases of “solid” cancer-related MAHA, Lechner and Obermeier5 found 23 cases were related to prostate cancer, as was the case with our patient.
Treatment for TTP is plasma exchange. The mortality rate of untreated TTP can exceed 90%, but plasma exchange therapy has reduced that rate to <20%.6 It has been suggested that proteolysis of vWF may play a central role in the efficacy of plasma exchange for TTP.7
Our patient was hospitalized and received 2 units of packed RBCs. He also received plasma exchange for 9 days with minimal response. On Day 5, our patient was started on leuprorelin and parenteral steroids. Soon after, his platelet count rose to 33 × 103/mcL and lactate dehydrogenase decreased. He was discharged approximately one week after the steroids were started.
After several months of outpatient treatment with leuprorelin and bicalutamide, the patient’s platelet count normalized to 212 × 103/mcL (from 29 × 103/mcL), alkaline phosphatase decreased to 402 U/L (from 1919 U/L), and PSA levels trended downward to 8.63 ng/mL (from 212 ng/mL). He continued to receive care from our oncology clinic for the next several months and his PSA level continued to decline. However, at his last few visits, his PSA level had trended up, suggesting progression of his prostate cancer. The patient has not followed up with our clinic recently.
THE TAKEAWAY
Suspect TTP in patients who present with unexplained petechiae and bruising, and whose blood work reveals thrombocytopenia and MAHA.2 Patients with TTP who do not respond to plasma exchange should be evaluated for underlying cancer or other potential secondary causes.3 Patients with cancer-associated TTP may respond to steroid therapy.
THE CASE
A 57-year-old Hispanic man sought treatment because he had been feeling tired for a few weeks. He had not seen a physician for 15 years. When he came in, his temperature was 98.8°F, blood pressure was 132/82 mm Hg, pulse was 82 beats/min, respiration rate was 16 breaths/min, and oxygen saturation was 93% on room air. Examination of the head, neck, and respiratory and cardiovascular systems was normal. Skin examination showed petechiae and bruising on his abdomen, left ankle, right thigh, and bilateral shin area. His abdomen was nontender with no organomegaly. There was no focal neurological finding or spinal tenderness. Our patient had no chills, chest pains, shortness of breath, headache, dizziness, or loss of consciousness. There was no hematemesis, melena, hematuria, edema, or weight loss. He had no medical or surgical history and denied substance abuse or taking any medications recently; he did use alcohol previously.
Results of some initial lab tests were abnormal, including a decreased white blood cell count (5.82/mcL), platelet count (29 x 103/mcL), hemoglobin (8.6 g/dL), and hematocrit (27%) (TABLE). A peripheral blood smear showed decreased normocytic red blood cells and scattered schistocytes. His prostate-specific antigen (PSA) level was elevated at 212 ng/mL.
The patient’s coagulation profile was normal, and his von Willebrand factor (vWF) protease (ADAMTS-13) level was within normal limits (13.83). Antineutrophil cytoplasmic antibody and antinuclear antibody tests were negative. Testing for pulmonary embolism was negative, as was testing for human immunodeficiency virus. An abdominal ultrasound was normal, as well.
THE DIAGNOSIS
Based on our patient’s abnormal blood test results and the presence of petechiae and bruising, we diagnosed thrombotic thrombocytopenic purpura (TTP). The patient’s elevated PSA prompted us to order computed tomography of the chest and abdomen, which showed an enlarged prostate gland and mixed lytic sclerotic lesions in T3 to T5 and T9 vertebrae and in his ribs. A bone marrow biopsy revealed metastatic prostatic adenocarcinoma and a bone scan confirmed multiple metastases in the spine, pelvis, and shoulders.
DISCUSSION
TTP is a rare disorder of increased clotting in small blood vessels throughout the body that can include thrombocytopenia, microangiopathic hemolytic anemia (MAHA), fever, renal dysfunction, and neurological deficits.1 It’s important to maintain a high index of suspicion for TTP because the condition is a hematologic medical emergency that can quickly cause multiorgan failure and death.2
Almost always an acquired condition, TTP can be idiopathic or secondary to another condition, such as collagen vascular diseases, transplants, certain drugs, infections, pregnancy, or cancer.3 In idiopathic TTP, the cause of the condition is believed to be reduced activity of ADAMTS-13, the protease that breaks vWF into smaller pieces—thus preventing the formation of unnecessary blood clots.
In cancer-associated TTP, which could be a complication resulting from chemotherapy or a manifestation of cancer itself,3 ADAMTS-13 level is normal and the condition is likely the result of an increased tumor cell load, which leads to endothelial damage and fragmentation of red blood cells (RBC) as they traverse the injured microvasculature.4 In an analysis of 154 cases of “solid” cancer-related MAHA, Lechner and Obermeier5 found 23 cases were related to prostate cancer, as was the case with our patient.
Treatment for TTP is plasma exchange. The mortality rate of untreated TTP can exceed 90%, but plasma exchange therapy has reduced that rate to <20%.6 It has been suggested that proteolysis of vWF may play a central role in the efficacy of plasma exchange for TTP.7
Our patient was hospitalized and received 2 units of packed RBCs. He also received plasma exchange for 9 days with minimal response. On Day 5, our patient was started on leuprorelin and parenteral steroids. Soon after, his platelet count rose to 33 × 103/mcL and lactate dehydrogenase decreased. He was discharged approximately one week after the steroids were started.
After several months of outpatient treatment with leuprorelin and bicalutamide, the patient’s platelet count normalized to 212 × 103/mcL (from 29 × 103/mcL), alkaline phosphatase decreased to 402 U/L (from 1919 U/L), and PSA levels trended downward to 8.63 ng/mL (from 212 ng/mL). He continued to receive care from our oncology clinic for the next several months and his PSA level continued to decline. However, at his last few visits, his PSA level had trended up, suggesting progression of his prostate cancer. The patient has not followed up with our clinic recently.
THE TAKEAWAY
Suspect TTP in patients who present with unexplained petechiae and bruising, and whose blood work reveals thrombocytopenia and MAHA.2 Patients with TTP who do not respond to plasma exchange should be evaluated for underlying cancer or other potential secondary causes.3 Patients with cancer-associated TTP may respond to steroid therapy.
1. Lichtin AE, Schreiber AD, Hurwitz S, et al. Efficacy of intensive plasmapheresis in thrombotic thrombocytopenic purpura. Archives Intern Med. 1987;147:2122-2126.
2. Blombery P, Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J Blood Med. 2014;5:15-23.
3. Chang JC, Naqvi T. Thrombotic thrombocytopenic purpura associated with bone marrow metastasis and secondary myelofibrosis in cancer. Oncologist. 2003;8:375-380.
4. Pirrotta MT, Bucalossi A, Forconi F, et al. Thrombotic thrombocytopenic purpura secondary to an occult adenocarcinoma. Oncologist. 2005;10:299-300.
5. Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore). 2012;91: 195-205.
6. Oberic L, Buffet, M, Scwarzinger M, et al; Reference Center for the Management of Thrombotic Microangiopathies. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009;14:769-779.
7. Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS 13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059-41063.
1. Lichtin AE, Schreiber AD, Hurwitz S, et al. Efficacy of intensive plasmapheresis in thrombotic thrombocytopenic purpura. Archives Intern Med. 1987;147:2122-2126.
2. Blombery P, Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J Blood Med. 2014;5:15-23.
3. Chang JC, Naqvi T. Thrombotic thrombocytopenic purpura associated with bone marrow metastasis and secondary myelofibrosis in cancer. Oncologist. 2003;8:375-380.
4. Pirrotta MT, Bucalossi A, Forconi F, et al. Thrombotic thrombocytopenic purpura secondary to an occult adenocarcinoma. Oncologist. 2005;10:299-300.
5. Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore). 2012;91: 195-205.
6. Oberic L, Buffet, M, Scwarzinger M, et al; Reference Center for the Management of Thrombotic Microangiopathies. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009;14:769-779.
7. Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS 13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059-41063.