User login
Outcomes of ESBL‐EK UTI
Community‐onset urinary tract infection (UTI) is a common bacterial infection encountered in hospital medicine, accounting for >350,000 hospital admissions and $3.4 billion in healthcare costs annually.[1] High proportions of these infections are caused by Enterobacteriaceae, primarily Escherichia coli and Klebsiella species.[2] The prevalence of hospitalized patients with UTI caused by multidrug‐resistant E coli and Klebsiella species has increased.[3, 4, 5] Enterobacteriaceae can produce hydrolytic enzymes, specifically extended‐spectrum ‐lactamases (ESBL), which result in high rates of bacterial resistance to frequently used agents.[6] Global rates vary widely by region, and recent surveillance data showed increasing rates of clinical isolates in North America are ESBL producers.[7, 8, 9, 10] More alarming is the emergence of these resistant organisms in the community.[11]
In addition to ‐lactams, ESBL production is associated with resistance to other antibiotic classes (fluoroquinolones, aminoglycosides, and sulfonamides), and has become an important cause of failed therapy.[12, 13] Consequently, providing adequate and timely antibiotics can become convoluted due to fewer remaining treatment options. Therefore, carbapenems, which are less susceptible to hydrolysis by these enzymes, have become the preferred therapy for infection with ESBL‐producing pathogens.[3, 13, 14]
The consequences of ESBL production, mainly in bloodstream infection (BSI), are well characterized, including notable delays in receipt of appropriate antibiotic therapy, prolonged length of stay (LOS), and increased cost of care.[15, 16] Importantly, others have found higher rates of mortality.[17, 18, 19, 20, 21] However, the impact on outcomes specifically in UTI remains unclear. As a result of the prevalence of UTI admissions to the hospital, the increasing incidence of ESBLs, and the potential impact on the clinical course of care, additional study is required to support best practices for this common diagnosis.
PATIENTS AND METHODS
Study Subjects and Design
This was a retrospective, matched‐cohort analysis of patients admitted to the hospital with UTI (International Classification of Diseases, 9th Revision‐Clinical Modification code 599.0) caused by extended‐spectrum b‐lactamase‐producing Escherichia coli and Klebsiella species (ESBL‐EK). Patients admitted to Hartford Hospital from September 1, 2011 through August 31, 2012 with UTI present on admission (48 hours) were evaluated. Cases were patients 18 years of age, with a positive urine culture (104 CFU/mL) for an ESBL‐producing organism (48 hours of admission), who received antibiotic treatment directed at the positive culture for 48 hours, and beginning prior to availability of in vitro susceptibility results. Cases were identified by a detailed search of the microbiology department database of ESBL‐EK cultures. Only the first positive (index) culture for each patient was included. Bacteremia was defined as isolation of a blood culture organism identical to the one isolated from the urine culture. Patients were excluded if they were discharged, died, or placed on palliative care prior to or on the date of urine collection. Controls (patients admitted with UTI on admission caused by nonESBL‐EK) were matched to cases in a 1:1 fashion on the basis of isolated urinary pathogen, age (5 years), sex, and race. ESBL‐producing organisms were identified and classified according to the Clinical and Laboratory Standards Institute guidelines.[22]
Data Collection
Once patients were identified, the following information was collected from the patient's medical record by 2 investigators using a standardized case report form: demographic characteristics, comorbid conditions and severity of comorbidities using the Charlson comorbidity index, recent patient medical history, and clinical and economic attributes.[23]
The study was approved by the institutional review board of Hartford Hospital. An informed consent waiver was granted as all data were currently in existence and no patient‐specific interventions were conducted for the study. The collection of data was in compliance with the Health Insurance Portability and Accountability Act of 1996.
Outcomes and Definitions
Onset of UTI was defined as the date/time of the index culture collection. An initial antibiotic treatment was a course of therapy initiated empirically (prior to availability of in vitro susceptibility) and that continued for 48 hours. An appropriate empiric antibiotic was defined as an initial antibiotic that ultimately possessed in vitro activity against the isolated pathogen.
The primary clinical outcomes were initial antibiotic response and clinical response. Initial antibiotic response was defined as failure if there was lack of clinical improvement, as evident by a switch to an alternative antibiotic (excluding switches to similar/narrower‐spectrum agents and courses begun at discharge) or infection‐related mortality while receiving the initial antibiotic. Patients were deemed clinical success if they were clinically stable at discharge or end of therapy, whichever occurred first, with resolution of signs and symptoms of infection. Clinical failures were patients with (1) infection‐related mortality or (2) readmission to hospital with UTI within 30 days of discharge. Clinical response was chosen as a primary outcome because significant mortality attributed to UTI was not anticipated. Secondary clinical outcomes included: time to appropriate antibiotic therapy, mortality (all cause and infection related), and 30‐day readmission (all cause and UTI related). Patients were considered to have received appropriate antibiotic therapy when they had received their first dose of antibiotic with activity against the isolated pathogen based on the patient‐specific in vitro susceptibility results. Time to appropriate antibiotics was defined as the elapsed time (hours) between the index culture collection and the initial dose of appropriate antibiotic therapy. All‐cause mortality was defined as any cause of death at the end of hospitalization, whereas infection‐related mortality was defined as death occurring while receiving antibiotics for the index infection, without any other obvious cause of death.
The primary economic outcomes were hospital LOS, costs, and reimbursement. Antibiotic costs were calculated for each patient according to acquisition costs. Additional economic outcomes evaluated were net hospital reimbursement and primary payor. Net hospital reimbursement was calculated as the difference between hospital reimbursement and hospital costs for each patient. Hospital costs were calculated as the direct plus indirect hospitalization costs for each patient, as determined by our institutional accounting department. All economic values were reported in United States dollars.
Statistical Analysis
Statistical comparisons were performed between cases and controls using a paired t test or Wilcoxon signed rank test for continuous variables, where appropriate. Dichotomous variables were compared using the McNemar test. Multivariate logistic regression was performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to determine independent risk factors for ESBL‐EK, including all pertinent variables with a P value <0.1 in univariate analyses. All data were analyzed using SigmaStat version 2.03 (IBM/SPSS, Armonk, NY). A P value of 0.05 was considered statistically significant.
RESULTS
Patient Population
Between September 2011 and August 2012, there were 220 specimens of ESBL‐related infection and 2345 patients admitted with a UTI on admission. Eighty‐four were confirmed ESBL‐EK cases (3.6%), and 55 met criteria for inclusion. Twenty‐nine of these cases were excluded because the index culture was polymicrobial, for which the response to antibiotics for ESBL‐EK could not be elicited (n=22); they had incomplete medical records (n=4); or they did not have a matched control patient (n=3). Fifty‐five matched control patients were identified, resulting in 110 patients overall.
Patient demographics and baseline characteristics are shown in Table 1. Patients with ESBL‐EK UTI were more likely to have diabetes mellitus, chronic obstructive pulmonary disorder, and a history of recurrent UTIs. They were more likely to have recently received antibiotics, been hospitalized, or had isolation of an ESBL‐producing organism. No significant differences in Charlson comorbidity index, recent immunosuppressive therapy, or urinary catheterization were observed. Compared with controls, patients with ESBL‐EK UTI were more frequently transferred from another healthcare facility, although the difference was not statistically significant (P=0.06). In the multivariate regression model, diabetes mellitus (OR: 4.4, 95% CI: 1.711.5; P=0.002), history of recurrent UTIs (OR: 4.4, 95% CI: 1.810.9; P=0.001), and transfer from another healthcare facility (OR: 2.38, 95% CI: 1.05.7; P=0.05) were independently associated with ESBL‐EK UTI. Previous isolation of an ESBL‐producing organism (P<0.001) was unable to be included in the multivariate logistic regression model because only patients with UTI caused by ESBL‐EK were positive for this variable. However, as noted in Table 1, 27.2% of cases had isolation of an ESBL‐producing organism in the previous year.
| ESBL Positive, n=55 | ESBL Negative, n=55 | Pa | |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y, median (IQR) | 77 (6785) | 77 (6685) | 0.83b |
| Female | 36 (65.4) | 36 (65.4) | 1.00 |
| White | 42 (76.4) | 42 (76.4) | 1.00 |
| Black | 5 (9.1) | 5 (9.1) | 1.00 |
| Hispanic/Latino | 6 (10.9) | 6 (10.9) | 1.00 |
| Other | 2 (3.6) | 2 (3.6) | 1.00 |
| Comorbidities | |||
| Diabetes mellitus | 25 (45.5) | 10 (18.1) | 0.004 |
| COPD | 15 (27.2) | 6 (10.9) | 0.04 |
| Liver disease | 2 (3.6) | 1 (1.8) | 1.00 |
| Hemodialysis | 9 (16.4) | 6 (10.9) | 0.58 |
| Hematological malignancy | 3 (5.5) | 2 (3.6) | 1.00 |
| Solid malignancy | 13 (23.6) | 9 (16.4) | 0.45 |
| HIV/AIDS | 0 | 1 (1.8) | 1.00 |
| Age >65 years | 44 (80.0) | 43 (78.2) | 1.00 |
| Urinary abnormality | 15 (27.2) | 15 (27.2) | 1.00 |
| Charlson comorbidity index, median (IQR) | 3 (24) | 2 (13.8) | 0.19 |
| History | |||
| Previous hospitalizationc | 38 (69.1) | 24 (43.6) | 0.01 |
| Previous antibioticsd | 23 (41.8) | 12 (21.8) | 0.04 |
| Recent immunosuppressive therapye | 9 (16.4) | 3 (5.5) | 0.11 |
| History of recurrent UTIsf | 29 (52.7) | 12 (21.8) | 0.001 |
| History of urinary catheterizationg | 18 (32.7) | 14 (25.4) | 0.45 |
| Previous genitourinary procedure/surgery | 10 (18.2) | 6 (10.9) | 0.39 |
| Previous ESBL‐producing organismh | 15 (27.2) | 0 | <0.001 |
| Clinical features | |||
| Transfer from another healthcare facility | 27 (49.1) | 17 (21.0) | 0.06 |
| ICU admission | 12 (21.8) | 7 (12.7) | 0.33 |
| Bacteremia | 7 (12.7) | 5 (9.1) | 0.75 |
| Infectious Diseases consulted | 39 (70.1) | 16 (29.1) | <0.001 |
| Empiric ‐lactam (noncarbapenem) | 35 (63.6) | 50 (90.9) | 0.001 |
Isolate Characteristics
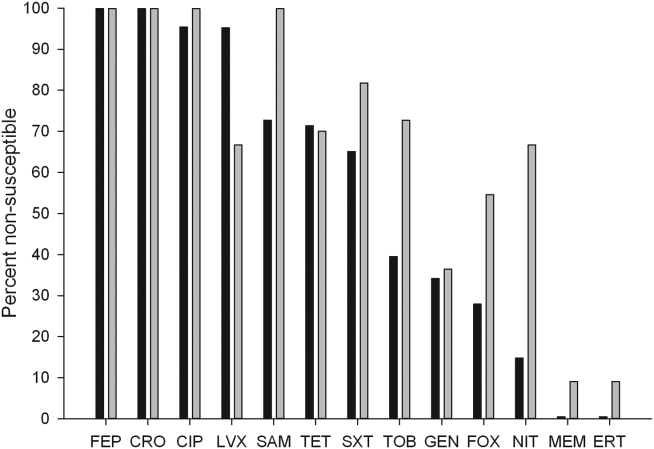
The distribution of causative pathogens in each cohort was: 44 (80%) Escherichia coli, 8 (14.5%) Klebsiella pneumoniae, and 3 (5.5%) Klebsiella oxytoca. In vitro nonsusceptibility profiles of the 55 ESBL‐EK cases are characterized in Figure 1. The most active agents were carbapenems, with 98.2% susceptibility to the entire isolate profile, whereas <10% of isolates were susceptible to the fluoroquinolones. All ESBL‐producing isolates were resistant to cefepime.
Failure on Initial Antibiotic Regimen
Initial antibiotic therapy is shown in Table 2. A majority of patients (87.2%) were initially treated with a ‐lactam. Empiric carbapenem use was greater in ESBL‐EK cases (18.1% vs 0%; P<0.001), and there were no other significant differences in the distribution of initial antibiotic therapy between cohorts. Less than one‐quarter of ESBL‐EK patients (23.6%) received appropriate initial therapy, whereas 98.2% of controls were initially treated appropriately (P<0.001).
| Initial Antibiotic | ESBL Positive, n=55 | ESBL Negative, n=55 | Pa |
|---|---|---|---|
| |||
| Ceftriaxone | 21 (38.1) | 32 (58.1) | 0.06 |
| Cefepime | 10 (18.2) | 12 (21.8) | 0.81 |
| Ertapenem | 7 (12.7) | 0 | 0.02 |
| Levofloxacin | 6 (10.9) | 4 (7.3) | 0.74 |
| Cefazolin | 0 | 4 (7.3) | 0.13 |
| Piperacillin‐tazobactam | 3 (5.5) | 0 | 0.24 |
| Ciprofloxacin | 2 (3.6) | 1 (1.8) | 1.00 |
| Doripenem | 2 (3.6) | 0 | 0.48 |
| Trimethoprim‐sulfamethoxazole | 2 (3.6) | 0 | 0.48 |
| Meropenem | 1 (1.8) | 0 | 1.00 |
| Cephalexin | 1 (1.8) | 0 | 1.00 |
| Aztreonam | 0 | 2 (3.6) | 0.48 |
Compared with controls, failure of initial antibiotic therapy was more common in patients with ESBL‐EK UTI, resulting in a significantly longer time to appropriate antibiotics (Table 3). Among ESBL‐EK UTI cases, failure of initial antibiotic therapy was greater for patients who received noncarbapenem ‐lactams (85.7%) as compared to those who empirically received a carbapenem (0%) (P<0.001). Antibiotic failure (>2 patients treated) in ESBL‐EK was highest with the following antibiotics: cefepime (100% [10/10]), piperacillin‐tazobactam (100% [3/3]), and ceftriaxone (76.2% [16/21]).
| ESBL Positive, n=55 | ESBL Negative, n=55 | Pa | |
|---|---|---|---|
| |||
| Clinical parameter | |||
| Initial antibiotic failure | 34 (61.8) | 3 (5.5) | <0.001 |
| Escalation to an alternative antibiotic | 33 (60.0) | 3 (5.5) | <0.001 |
| Time to appropriate antibiotics, h, median (IQR) | 51 (32.560.8) | 2.5 (1.07.2) | <0.001b |
| Appropriate empiric antibiotics | 13 (23.6) | 54 (98.2) | <0.001 |
| Clinical success | 47 (85.5) | 52 (94.5) | 0.23 |
| All‐cause mortality | 5 (9.1) | 1 (1.8) | 0.21 |
| Infection‐related mortality | 4 (7.2) | 1 (1.8) | 0.37 |
| All‐cause 30‐day readmission | 12 (21.8) | 15 (27.2) | 0.63 |
| UTI‐related 30‐day readmission | 4 (7.2) | 2 (3.6) | 0.68 |
| Economic parameterb | |||
| Length of stay, d, median (IQR) | 6 (48) | 4 (36) | 0.02 |
| Total hospital cost, median (IQR)c | 10,741 (684615,819) | 7,083 (566711,652) | 0.02 |
| Bed cost, % total cost, median (IQR) | 57.5 (51.666.0) | 63.8 (51.973.5) | 0.21 |
| Antibiotic cost, % total cost, median (IQR) | 0.5 (0.12.0) | 0.1(0.030.2) | <0.001 |
| Primary payor, n (%) | |||
| Medicare | 44 (80) | 44 (80) | 1.00 |
| Medicaid | 7 (12.7) | 3 (5.4) | 0.32 |
| Private insurance | 2 (3.6) | 2 (3.6) | 0.61 |
| Managed care | 2 (3.6) | 6 (10.9) | 0.27 |
Clinical Outcomes
There were no significant differences in clinical success, mortality, or 30‐day readmission between cohorts (Table 3). Among ESBL‐EK patients, those who received appropriate antibiotics within 48 hours were significantly more likely to achieve treatment success (100% vs 77.1%; P=0.04). All 8 ESBL‐EK treatment failures (4 infection‐related mortality and 4 UTI readmitted with the same ESBL pathogen) failed to receive appropriate antibiotics within 48 hours of culture collection.
More ESBL‐EK patients required a switch in their antibiotics. Within the subgroup of ESBL‐EK patients with an escalation in antibiotics, ertapenem was added to 19 of 33 (57.6%) cases.
Economic Outcomes
ESBL‐EK patients who received inappropriate initial therapy received longer antibiotic treatment courses than those empirically treated with a carbapenem (meanstandard deviation, 8.93.7 vs 6.23.2 days, respectively; P=0.04). When compared to non‐ESBL infection, ESBL‐EK patients required more days of antibiotic therapy (median 8 vs 5 days; P=0.03). The median LOS was significantly longer and total hospital costs were significantly greater for ESBL cases. Antibiotic costs contributed minimally to the overall cost, accounting for <1% (0.5% for cases vs 0.1% for controls), regardless of ESBL status. A comparison of economic outcomes is presented in Table 3. LOS among ESBL cases was not different between those discharged with and without continued antimicrobial therapy. Moreover, for those discharged on antimicrobial therapy, the utilization of either the oral or intravenous route did not delay discharge (data not shown).
The payor mix was similar between cases and controls. Given the predominately elderly population, Medicare was the primary payor for a majority of patients. Median differences in cost and reimbursement between cohorts (ESBL‐EK vs nonESBL‐EK) were $3658 (P=0.02) and $469 (P=0.56), respectively. As a result, median loss per patient with ESBL‐EK infection was $3189 when compared with controls.
Bacteremia
All cases of bacteremia were present on admission. Bacteremic UTI due to ESBL‐EK was associated with initial antibiotic failure (85.7% [6/7] vs 0% [0/5]; P=0.015), delayed appropriate therapy (median, 56 vs 2 hours; P=0.003), longer median hospital stays (11 vs 5 days; P=0.05), and higher median cost ($27,671 vs $5898; P=0.03) as compared with bacteremic UTI due to non‐ESBL‐EK. Infection‐related mortality occurred in 2 ESBL‐EK bacteremic patients, but no mortality was observed among the nonESBL‐EK bacteremic UTI cohort (28.6% [2/7] vs 0% [0/5]; P=0.47].
DISCUSSION
This matched cohort analysis revealed that ESBL‐EK has detrimental effects on the outcomes of patients admitted to the hospital with UTI. While matching for demographics and infecting pathogen, patients with ESBL‐EK UTI had diminished initial antibiotic response and considerably longer time to appropriate antibiotic therapy (48 hours longer) than their non‐ESBL comparator. Despite significant delays in appropriate therapy, we saw no attributable difference in clinical outcome and mortality; however, numerical trends toward increased risk were observed in ESBL‐EK patients. Although clinical response was largely unchanged, prolonged hospitals stays and increased cost of care were endured by ESBL‐EK patients.
Antimicrobial resistance, a primary factor in the postponement of appropriate antibiotic therapy, is a worrisome occurrence with meaningful clinical implications.16[21, 24] In BSI, delayed appropriate therapy due to ESBLs has been associated with prolonged LOS, increased costs, and increased mortality.[20, 25] However, the clinical significance of ESBL production on patient outcomes in UTI remains equivocal. To the best of our knowledge, this is the first matched control analysis evaluating the clinical and economic impact of ESBL specifically in UTI. We saw a 50% increase in the median LOS (from 4 to 6 days) and cost of care (additional $3658 per patient) in the ESBL‐EK UTI cohort. Albeit as small subpopulation, patients with bacteremic UTI due to ESBL‐EK had significantly longer hospital stays and increased cost of care as compared with nonESBL‐EK bacteremic UTI. These economic findings are consistent with data from other investigations. For example, a matched case‐control study of infection (51.5% UTI) due to ESBL‐producing E coli and K pneumoniae by Lautenbach and colleagues found an additional 60‐hour (72 vs 11.5 hours, P<0.001) delay in time to appropriate antibiotics for case patients, resulting in significantly longer LOS (1.8 times) and increased hospital charges (2.9 times).[17] With respect to costs, a matched case‐control analysis of ESBL‐EK in non‐UTI saw a 70% increase in cost ($41,353 vs $24,902).[26] Moreover, the increased cost in that study, like this one, was driven primarily by LOS (additional 9.7 days) and not drug utilization, with antibiotic costs representing <2% of the total hospitalization cost. Tumbarello and colleagues observed an approximate 50% increase in LOS and hospital costs in patients with BSI caused by ESBL‐producing E coli.[20]
Distinctive from those studies, we investigated the significance of ESBL production on hospital reimbursement. Despite the additional healthcare resource utilization (50% greater), we saw no appreciable increase (<5%) in median hospital reimbursement. Given that the primary payors between cohorts were comparable, infection with ESBL‐producing bacteria may result in a potential loss of income if optimal treatment is not initiated on admission.
The risk factors for development of UTI due to ESBL‐EK are well defined.[27, 28, 29, 30, 31, 32] Two scoring systems, an Italian and a Duke model, have identified patients at increased risk of harboring ESBL‐producing organisms on hospital admission.[33, 34] The features of each model center on established risk factors for ESBL‐EK UTI. In our study population, these scoring model features were more common in ESBL‐EK patients, supporting their potential application in UTI. However, because of our study design (infected controls) and matching criteria, only 2 features (recent antibiotics and previous hospitalization) achieved statistical significance as detectable risk factors in our population. Regardless, these data, coupled with increasing prevalence of UTI with ESBL‐producing bacteria, provide justification and advocacy for the empiric use of ESBL active antibiotics (ie, carbapenems) in certain high‐risk individuals, particularly those patients with a previous history of ESBL or those with multiple risk factors identified in our study as well as others (previous hospitalization, recent antibiotic exposure). Importantly, an aggressive de‐escalation strategy should be used to temper collateral damage for patients with non‐ESBL infections. Moreover, the utilization of oral therapies beyond the fluoroquinolones and trimethoprim‐sulfamethoxazole, such as fosfomycin and nitrofurantoin, coupled with coordinated transitions of care, may alleviate the demand for intravenous access in patients prepared for discharge.[35, 36]
This study is not without limitations. As only a distinct period in time was studied, we are unable to determine the implications of previous episodes of UTI on the current admission. Although the Charlson comorbidity score was not significantly different between the 2 patient cohorts, ESBL‐EK patients were more likely to have several demographic features (ie, diabetes mellitus, recurrent UTIs, previous hospitalization, and recent antibiotic exposure), which are to be expected in the ESBL population.[37, 38] Although these differences have been observed as expected, we believe the driver of prolonged LOS and increased costs of care stem from inappropriate initial antibiotic therapy as evident by the initial clinical failure observed in ESBL‐EK patients. It should also be noted that we defined antibiotics as appropriate based on the laboratory criteria for susceptibility, as such agents that have high concentration in the urinary tract (ie, fluoroquinolones) may be effective despite these laboratory definitions.[17] For instance, 10 of our ESBL‐EK patients never received appropriate antibiotics (3 fluoroquinolones, 7 noncarbapenem ‐lactams) as defined by laboratory‐based susceptibility testing, 8 of whom experienced clinical success. Previous studies have shown discordance between phenotypic ESBL‐related resistance profiles and outcomes.[17, 18, 19, 20, 21, 25, 39, 40]
In summary, recent increases in antimicrobial resistance present ongoing challenges in the treatment of hospitalized patients, as appropriate treatment options are extremely limited. Our findings strengthen the consortium of data stating that antimicrobial resistance unfavorably impacts patient outcomes.[41, 42] ESBL‐EK in UTI is associated with high rates of failure of initial antibiotic therapy, prolonged LOS, and increased cost of care. Furthermore, the added cost associated with UTI due to ESBL‐EK is not recognized in hospital reimbursement, as evident by a $3200 net loss relative to reimbursement. This loss appears to be solely due to the increased LOS, as antibiotic costs were <1% of cost of care. Moreover, these negative consequences could be even more pronounced at institutions with a higher prevalence of ESBL infection. A multidisciplinary approach (infection control, microbiology) using these data as a benchmark, may enhance the detection, treatment, and prevention of these ESBL‐producing organisms. With high levels of coresistance to noncarbapenem ‐lactams and fluoroquinolones, providers should be cognizant of organisms capable of producing ESBL when selecting initial antibiotic therapy in high‐risk populations. Early recognition and timely initiation of appropriate antibiotic therapy appear paramount to minimizing the burden of ESBL‐EK in patients admitted to the hospital with a UTI.
Acknowledgements
The authors thank Gilbert Fotunato for his assistance with administrative data retrieval.
Disclosures
This work is supported by a grant from Merck & Co., Inc. D.P.N. reports having received grant support and honorarium from Merck & Co., Inc. S.H.M and L.O.T report no conflicts of interest relevant to this article.
- , , , et al. Urologic diseases in America project: analytical methods and principal findings. J Urol. 2005;173(3):933–937.
- . Urinary tract pathogens in complicated infection and in elderly individuals. J Infect Dis. 2001;183(suppl 1):S5–S8.
- , , , , . Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009–2010. Diagn Microbiol Infect Dis. 2011;70(4):507–511.
- , . Extended‐spectrum β‐lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686.
- , , . Extended‐spectrum β‐lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis. 2010;23(4):320–326.
- . Defining an extended‐spectrum beta‐lactamase. Clin Microbiol Infect. 2008;14(suppl 5):3–10.
- , , , , , . Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J Antimicrob Chemother. 2007;60(5):1018–1029.
- , , , . Variations in the prevalence of strains expressing an extended‐spectrum β‐lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis. 2001;32(suppl 2):S94–S103.
- National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. Am J Infect Control. 2004;32(8):470–485.
- , . Prevalence and antimicrobial susceptibility data for extended‐spectrum β‐lactamase‐ and AmpC‐producing Enterobacteriaceae from the MYSTIC Program in Europe and the United States (1997–2004). Diagn Microbiol Infect Dis. 2005;53(4):257–264.
- , , , . Emergence of Enterobacteriaceae producing extended‐spectrum b‐lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005;56(1):52–59.
- , , , et al. Epidemiology of ciprofloxacin resistance and its relationship to extended‐spectrum B‐lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin Infect Dis. 2000;30(4):473–478.
- , , , , , Epidemiological investigation of fluoroquinolone resistance in infections due to extended‐spectrum‐β‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis. 2001;33(8):1288–1294.
- , , , . High levels of antimicrobial coresistance among extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae. Antimicrob Agents Chemother. 2005;49(5):2137–2139.
- . Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31(suppl 4):S131–S138.
- . The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82–S89.
- , , , , , Extended‐spectrum beta‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001:32(8):1162–1171.
- , , , et al. Predictors of mortality in patients with bloodstream infections caused by extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51(6):1987–1994.
- , . Mortality and delay in effective therapy associated with extended‐spectrum β lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta‐analysis. J Antimicrob Chemother. 2007;60(5):913–920.
- , , , et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended‐spectrum‐β‐lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. 2010;54(10):4085–4091.
- , , . Effects of confounders and intermediates on the association of bacteraemia caused by extended‐spectrum β‐lactamase‐producing Enterobacteriaceae and patient outcome: a meta‐analysis. J Antimicrob Chemother. 2012;67(6):1311–1320.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 21st informational supplement. M100‐S20. Wayne, PA: Clinical and Laboratory Standards Institute; 2010.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. Bloodstream infections caused by antibiotic‐resistant gram‐negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766.
- , , , , . Clinical implications of extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae bacteraemia. J Hosp Infect. 2002;52(2):99–106.
- , , , , . Impact of extended‐spectrum β‐lactamase‐producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol. 2006;27(11):1226–1232.
- , , , et al. Risk factors for community‐onset urinary tract infections due to Escherichia coli harbouring extended‐spectrum‐β‐lactamases. J Antimicrob Chemother. 2006:57(4):780–783.
- , , , et al. Community infections caused extended‐spectrum‐β‐lactamase‐producing Escherichia coli. Arch Intern Med. 2008;168(17):1897–1902.
- , , . Prevalence and risk factors for extended spectrum‐β‐lactamase‐producing uropathogens in urinary tract infection. Korean J Urol. 2010;51(7):492–497.
- , , , , . Extended‐spectrum β‐lactamase‐producing gram‐negative pathogens in community‐acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39(4):333–340.
- , , , et al. Epidemiology and genetic characteristics of extended‐spectrum‐β‐lactamase‐producing gram‐negative bacteria causing urinary tract infections in long‐term care facilities. J Antimicrob Chemother. 2012;67(12):2982–2987.
- , , , et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended‐spectrum beta‐lactamase (ESBL)‐producing enterobacteria. Int J Clin Pract. 2012;66(9):891–896.
- , , , et al. Identifying patients harboring extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011;55(7):3485–3490.
- , , , . Utility of a clinical risk factor scoring model in predicting infection with extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol. 2013;34(4):385–392.
- , , , , , . Fosfomycin in the treatment of extended spectrum beta‐lactamase‐producing Escherichia coli‐related lower urinary tract infections. Int J Amtimicrob Agents. 2007;29(1):62–65.
- , , , , . Nitrofurantoin in the treatment of extended‐spectrum beta‐lactamase‐producing Escherichia coli‐related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554–556.
- , , , et al. Epidemiology and clinical features of infections caused by extended‐spectrum beta‐lactamase‐producing Escherichia coli in nonhospitalized patients. J Clin Microb. 2004;42(3):1089–1094.
- , , , et al. Community infections caused by extended‐spectrum β‐lactamase‐producing Escherichia coli. Arch Intern Med. 2008:168(17):1897–1902.
- , , , et al. Extended‐spectrum beta‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med. 2002;28(12):1718–1723.
- , , , et al. Ceftazidime‐resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case‐control and molecular epidemiologic investigation. J Infect Dis. 1996;174(3):529–536.
- , . The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36(11):1433–1437.
- . Clinical implications of antimicrobial resistance for therapy. J Antimicrob Chemother. 2008;62(suppl 2):ii105–ii114.
Community‐onset urinary tract infection (UTI) is a common bacterial infection encountered in hospital medicine, accounting for >350,000 hospital admissions and $3.4 billion in healthcare costs annually.[1] High proportions of these infections are caused by Enterobacteriaceae, primarily Escherichia coli and Klebsiella species.[2] The prevalence of hospitalized patients with UTI caused by multidrug‐resistant E coli and Klebsiella species has increased.[3, 4, 5] Enterobacteriaceae can produce hydrolytic enzymes, specifically extended‐spectrum ‐lactamases (ESBL), which result in high rates of bacterial resistance to frequently used agents.[6] Global rates vary widely by region, and recent surveillance data showed increasing rates of clinical isolates in North America are ESBL producers.[7, 8, 9, 10] More alarming is the emergence of these resistant organisms in the community.[11]
In addition to ‐lactams, ESBL production is associated with resistance to other antibiotic classes (fluoroquinolones, aminoglycosides, and sulfonamides), and has become an important cause of failed therapy.[12, 13] Consequently, providing adequate and timely antibiotics can become convoluted due to fewer remaining treatment options. Therefore, carbapenems, which are less susceptible to hydrolysis by these enzymes, have become the preferred therapy for infection with ESBL‐producing pathogens.[3, 13, 14]
The consequences of ESBL production, mainly in bloodstream infection (BSI), are well characterized, including notable delays in receipt of appropriate antibiotic therapy, prolonged length of stay (LOS), and increased cost of care.[15, 16] Importantly, others have found higher rates of mortality.[17, 18, 19, 20, 21] However, the impact on outcomes specifically in UTI remains unclear. As a result of the prevalence of UTI admissions to the hospital, the increasing incidence of ESBLs, and the potential impact on the clinical course of care, additional study is required to support best practices for this common diagnosis.
PATIENTS AND METHODS
Study Subjects and Design
This was a retrospective, matched‐cohort analysis of patients admitted to the hospital with UTI (International Classification of Diseases, 9th Revision‐Clinical Modification code 599.0) caused by extended‐spectrum b‐lactamase‐producing Escherichia coli and Klebsiella species (ESBL‐EK). Patients admitted to Hartford Hospital from September 1, 2011 through August 31, 2012 with UTI present on admission (48 hours) were evaluated. Cases were patients 18 years of age, with a positive urine culture (104 CFU/mL) for an ESBL‐producing organism (48 hours of admission), who received antibiotic treatment directed at the positive culture for 48 hours, and beginning prior to availability of in vitro susceptibility results. Cases were identified by a detailed search of the microbiology department database of ESBL‐EK cultures. Only the first positive (index) culture for each patient was included. Bacteremia was defined as isolation of a blood culture organism identical to the one isolated from the urine culture. Patients were excluded if they were discharged, died, or placed on palliative care prior to or on the date of urine collection. Controls (patients admitted with UTI on admission caused by nonESBL‐EK) were matched to cases in a 1:1 fashion on the basis of isolated urinary pathogen, age (5 years), sex, and race. ESBL‐producing organisms were identified and classified according to the Clinical and Laboratory Standards Institute guidelines.[22]
Data Collection
Once patients were identified, the following information was collected from the patient's medical record by 2 investigators using a standardized case report form: demographic characteristics, comorbid conditions and severity of comorbidities using the Charlson comorbidity index, recent patient medical history, and clinical and economic attributes.[23]
The study was approved by the institutional review board of Hartford Hospital. An informed consent waiver was granted as all data were currently in existence and no patient‐specific interventions were conducted for the study. The collection of data was in compliance with the Health Insurance Portability and Accountability Act of 1996.
Outcomes and Definitions
Onset of UTI was defined as the date/time of the index culture collection. An initial antibiotic treatment was a course of therapy initiated empirically (prior to availability of in vitro susceptibility) and that continued for 48 hours. An appropriate empiric antibiotic was defined as an initial antibiotic that ultimately possessed in vitro activity against the isolated pathogen.
The primary clinical outcomes were initial antibiotic response and clinical response. Initial antibiotic response was defined as failure if there was lack of clinical improvement, as evident by a switch to an alternative antibiotic (excluding switches to similar/narrower‐spectrum agents and courses begun at discharge) or infection‐related mortality while receiving the initial antibiotic. Patients were deemed clinical success if they were clinically stable at discharge or end of therapy, whichever occurred first, with resolution of signs and symptoms of infection. Clinical failures were patients with (1) infection‐related mortality or (2) readmission to hospital with UTI within 30 days of discharge. Clinical response was chosen as a primary outcome because significant mortality attributed to UTI was not anticipated. Secondary clinical outcomes included: time to appropriate antibiotic therapy, mortality (all cause and infection related), and 30‐day readmission (all cause and UTI related). Patients were considered to have received appropriate antibiotic therapy when they had received their first dose of antibiotic with activity against the isolated pathogen based on the patient‐specific in vitro susceptibility results. Time to appropriate antibiotics was defined as the elapsed time (hours) between the index culture collection and the initial dose of appropriate antibiotic therapy. All‐cause mortality was defined as any cause of death at the end of hospitalization, whereas infection‐related mortality was defined as death occurring while receiving antibiotics for the index infection, without any other obvious cause of death.
The primary economic outcomes were hospital LOS, costs, and reimbursement. Antibiotic costs were calculated for each patient according to acquisition costs. Additional economic outcomes evaluated were net hospital reimbursement and primary payor. Net hospital reimbursement was calculated as the difference between hospital reimbursement and hospital costs for each patient. Hospital costs were calculated as the direct plus indirect hospitalization costs for each patient, as determined by our institutional accounting department. All economic values were reported in United States dollars.
Statistical Analysis
Statistical comparisons were performed between cases and controls using a paired t test or Wilcoxon signed rank test for continuous variables, where appropriate. Dichotomous variables were compared using the McNemar test. Multivariate logistic regression was performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to determine independent risk factors for ESBL‐EK, including all pertinent variables with a P value <0.1 in univariate analyses. All data were analyzed using SigmaStat version 2.03 (IBM/SPSS, Armonk, NY). A P value of 0.05 was considered statistically significant.
RESULTS
Patient Population
Between September 2011 and August 2012, there were 220 specimens of ESBL‐related infection and 2345 patients admitted with a UTI on admission. Eighty‐four were confirmed ESBL‐EK cases (3.6%), and 55 met criteria for inclusion. Twenty‐nine of these cases were excluded because the index culture was polymicrobial, for which the response to antibiotics for ESBL‐EK could not be elicited (n=22); they had incomplete medical records (n=4); or they did not have a matched control patient (n=3). Fifty‐five matched control patients were identified, resulting in 110 patients overall.
Patient demographics and baseline characteristics are shown in Table 1. Patients with ESBL‐EK UTI were more likely to have diabetes mellitus, chronic obstructive pulmonary disorder, and a history of recurrent UTIs. They were more likely to have recently received antibiotics, been hospitalized, or had isolation of an ESBL‐producing organism. No significant differences in Charlson comorbidity index, recent immunosuppressive therapy, or urinary catheterization were observed. Compared with controls, patients with ESBL‐EK UTI were more frequently transferred from another healthcare facility, although the difference was not statistically significant (P=0.06). In the multivariate regression model, diabetes mellitus (OR: 4.4, 95% CI: 1.711.5; P=0.002), history of recurrent UTIs (OR: 4.4, 95% CI: 1.810.9; P=0.001), and transfer from another healthcare facility (OR: 2.38, 95% CI: 1.05.7; P=0.05) were independently associated with ESBL‐EK UTI. Previous isolation of an ESBL‐producing organism (P<0.001) was unable to be included in the multivariate logistic regression model because only patients with UTI caused by ESBL‐EK were positive for this variable. However, as noted in Table 1, 27.2% of cases had isolation of an ESBL‐producing organism in the previous year.
| ESBL Positive, n=55 | ESBL Negative, n=55 | Pa | |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y, median (IQR) | 77 (6785) | 77 (6685) | 0.83b |
| Female | 36 (65.4) | 36 (65.4) | 1.00 |
| White | 42 (76.4) | 42 (76.4) | 1.00 |
| Black | 5 (9.1) | 5 (9.1) | 1.00 |
| Hispanic/Latino | 6 (10.9) | 6 (10.9) | 1.00 |
| Other | 2 (3.6) | 2 (3.6) | 1.00 |
| Comorbidities | |||
| Diabetes mellitus | 25 (45.5) | 10 (18.1) | 0.004 |
| COPD | 15 (27.2) | 6 (10.9) | 0.04 |
| Liver disease | 2 (3.6) | 1 (1.8) | 1.00 |
| Hemodialysis | 9 (16.4) | 6 (10.9) | 0.58 |
| Hematological malignancy | 3 (5.5) | 2 (3.6) | 1.00 |
| Solid malignancy | 13 (23.6) | 9 (16.4) | 0.45 |
| HIV/AIDS | 0 | 1 (1.8) | 1.00 |
| Age >65 years | 44 (80.0) | 43 (78.2) | 1.00 |
| Urinary abnormality | 15 (27.2) | 15 (27.2) | 1.00 |
| Charlson comorbidity index, median (IQR) | 3 (24) | 2 (13.8) | 0.19 |
| History | |||
| Previous hospitalizationc | 38 (69.1) | 24 (43.6) | 0.01 |
| Previous antibioticsd | 23 (41.8) | 12 (21.8) | 0.04 |
| Recent immunosuppressive therapye | 9 (16.4) | 3 (5.5) | 0.11 |
| History of recurrent UTIsf | 29 (52.7) | 12 (21.8) | 0.001 |
| History of urinary catheterizationg | 18 (32.7) | 14 (25.4) | 0.45 |
| Previous genitourinary procedure/surgery | 10 (18.2) | 6 (10.9) | 0.39 |
| Previous ESBL‐producing organismh | 15 (27.2) | 0 | <0.001 |
| Clinical features | |||
| Transfer from another healthcare facility | 27 (49.1) | 17 (21.0) | 0.06 |
| ICU admission | 12 (21.8) | 7 (12.7) | 0.33 |
| Bacteremia | 7 (12.7) | 5 (9.1) | 0.75 |
| Infectious Diseases consulted | 39 (70.1) | 16 (29.1) | <0.001 |
| Empiric ‐lactam (noncarbapenem) | 35 (63.6) | 50 (90.9) | 0.001 |
Isolate Characteristics
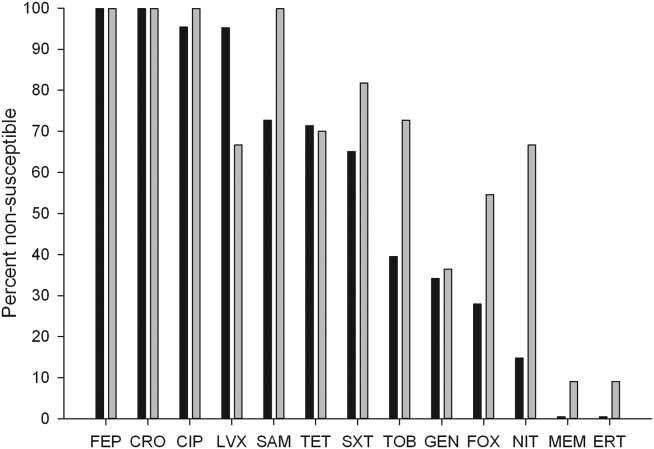
The distribution of causative pathogens in each cohort was: 44 (80%) Escherichia coli, 8 (14.5%) Klebsiella pneumoniae, and 3 (5.5%) Klebsiella oxytoca. In vitro nonsusceptibility profiles of the 55 ESBL‐EK cases are characterized in Figure 1. The most active agents were carbapenems, with 98.2% susceptibility to the entire isolate profile, whereas <10% of isolates were susceptible to the fluoroquinolones. All ESBL‐producing isolates were resistant to cefepime.

Failure on Initial Antibiotic Regimen
Initial antibiotic therapy is shown in Table 2. A majority of patients (87.2%) were initially treated with a ‐lactam. Empiric carbapenem use was greater in ESBL‐EK cases (18.1% vs 0%; P<0.001), and there were no other significant differences in the distribution of initial antibiotic therapy between cohorts. Less than one‐quarter of ESBL‐EK patients (23.6%) received appropriate initial therapy, whereas 98.2% of controls were initially treated appropriately (P<0.001).
| Initial Antibiotic | ESBL Positive, n=55 | ESBL Negative, n=55 | Pa |
|---|---|---|---|
| |||
| Ceftriaxone | 21 (38.1) | 32 (58.1) | 0.06 |
| Cefepime | 10 (18.2) | 12 (21.8) | 0.81 |
| Ertapenem | 7 (12.7) | 0 | 0.02 |
| Levofloxacin | 6 (10.9) | 4 (7.3) | 0.74 |
| Cefazolin | 0 | 4 (7.3) | 0.13 |
| Piperacillin‐tazobactam | 3 (5.5) | 0 | 0.24 |
| Ciprofloxacin | 2 (3.6) | 1 (1.8) | 1.00 |
| Doripenem | 2 (3.6) | 0 | 0.48 |
| Trimethoprim‐sulfamethoxazole | 2 (3.6) | 0 | 0.48 |
| Meropenem | 1 (1.8) | 0 | 1.00 |
| Cephalexin | 1 (1.8) | 0 | 1.00 |
| Aztreonam | 0 | 2 (3.6) | 0.48 |
Compared with controls, failure of initial antibiotic therapy was more common in patients with ESBL‐EK UTI, resulting in a significantly longer time to appropriate antibiotics (Table 3). Among ESBL‐EK UTI cases, failure of initial antibiotic therapy was greater for patients who received noncarbapenem ‐lactams (85.7%) as compared to those who empirically received a carbapenem (0%) (P<0.001). Antibiotic failure (>2 patients treated) in ESBL‐EK was highest with the following antibiotics: cefepime (100% [10/10]), piperacillin‐tazobactam (100% [3/3]), and ceftriaxone (76.2% [16/21]).
| ESBL Positive, n=55 | ESBL Negative, n=55 | Pa | |
|---|---|---|---|
| |||
| Clinical parameter | |||
| Initial antibiotic failure | 34 (61.8) | 3 (5.5) | <0.001 |
| Escalation to an alternative antibiotic | 33 (60.0) | 3 (5.5) | <0.001 |
| Time to appropriate antibiotics, h, median (IQR) | 51 (32.560.8) | 2.5 (1.07.2) | <0.001b |
| Appropriate empiric antibiotics | 13 (23.6) | 54 (98.2) | <0.001 |
| Clinical success | 47 (85.5) | 52 (94.5) | 0.23 |
| All‐cause mortality | 5 (9.1) | 1 (1.8) | 0.21 |
| Infection‐related mortality | 4 (7.2) | 1 (1.8) | 0.37 |
| All‐cause 30‐day readmission | 12 (21.8) | 15 (27.2) | 0.63 |
| UTI‐related 30‐day readmission | 4 (7.2) | 2 (3.6) | 0.68 |
| Economic parameterb | |||
| Length of stay, d, median (IQR) | 6 (48) | 4 (36) | 0.02 |
| Total hospital cost, median (IQR)c | 10,741 (684615,819) | 7,083 (566711,652) | 0.02 |
| Bed cost, % total cost, median (IQR) | 57.5 (51.666.0) | 63.8 (51.973.5) | 0.21 |
| Antibiotic cost, % total cost, median (IQR) | 0.5 (0.12.0) | 0.1(0.030.2) | <0.001 |
| Primary payor, n (%) | |||
| Medicare | 44 (80) | 44 (80) | 1.00 |
| Medicaid | 7 (12.7) | 3 (5.4) | 0.32 |
| Private insurance | 2 (3.6) | 2 (3.6) | 0.61 |
| Managed care | 2 (3.6) | 6 (10.9) | 0.27 |
Clinical Outcomes
There were no significant differences in clinical success, mortality, or 30‐day readmission between cohorts (Table 3). Among ESBL‐EK patients, those who received appropriate antibiotics within 48 hours were significantly more likely to achieve treatment success (100% vs 77.1%; P=0.04). All 8 ESBL‐EK treatment failures (4 infection‐related mortality and 4 UTI readmitted with the same ESBL pathogen) failed to receive appropriate antibiotics within 48 hours of culture collection.
More ESBL‐EK patients required a switch in their antibiotics. Within the subgroup of ESBL‐EK patients with an escalation in antibiotics, ertapenem was added to 19 of 33 (57.6%) cases.
Economic Outcomes
ESBL‐EK patients who received inappropriate initial therapy received longer antibiotic treatment courses than those empirically treated with a carbapenem (meanstandard deviation, 8.93.7 vs 6.23.2 days, respectively; P=0.04). When compared to non‐ESBL infection, ESBL‐EK patients required more days of antibiotic therapy (median 8 vs 5 days; P=0.03). The median LOS was significantly longer and total hospital costs were significantly greater for ESBL cases. Antibiotic costs contributed minimally to the overall cost, accounting for <1% (0.5% for cases vs 0.1% for controls), regardless of ESBL status. A comparison of economic outcomes is presented in Table 3. LOS among ESBL cases was not different between those discharged with and without continued antimicrobial therapy. Moreover, for those discharged on antimicrobial therapy, the utilization of either the oral or intravenous route did not delay discharge (data not shown).
The payor mix was similar between cases and controls. Given the predominately elderly population, Medicare was the primary payor for a majority of patients. Median differences in cost and reimbursement between cohorts (ESBL‐EK vs nonESBL‐EK) were $3658 (P=0.02) and $469 (P=0.56), respectively. As a result, median loss per patient with ESBL‐EK infection was $3189 when compared with controls.
Bacteremia
All cases of bacteremia were present on admission. Bacteremic UTI due to ESBL‐EK was associated with initial antibiotic failure (85.7% [6/7] vs 0% [0/5]; P=0.015), delayed appropriate therapy (median, 56 vs 2 hours; P=0.003), longer median hospital stays (11 vs 5 days; P=0.05), and higher median cost ($27,671 vs $5898; P=0.03) as compared with bacteremic UTI due to non‐ESBL‐EK. Infection‐related mortality occurred in 2 ESBL‐EK bacteremic patients, but no mortality was observed among the nonESBL‐EK bacteremic UTI cohort (28.6% [2/7] vs 0% [0/5]; P=0.47].
DISCUSSION
This matched cohort analysis revealed that ESBL‐EK has detrimental effects on the outcomes of patients admitted to the hospital with UTI. While matching for demographics and infecting pathogen, patients with ESBL‐EK UTI had diminished initial antibiotic response and considerably longer time to appropriate antibiotic therapy (48 hours longer) than their non‐ESBL comparator. Despite significant delays in appropriate therapy, we saw no attributable difference in clinical outcome and mortality; however, numerical trends toward increased risk were observed in ESBL‐EK patients. Although clinical response was largely unchanged, prolonged hospitals stays and increased cost of care were endured by ESBL‐EK patients.
Antimicrobial resistance, a primary factor in the postponement of appropriate antibiotic therapy, is a worrisome occurrence with meaningful clinical implications.16[21, 24] In BSI, delayed appropriate therapy due to ESBLs has been associated with prolonged LOS, increased costs, and increased mortality.[20, 25] However, the clinical significance of ESBL production on patient outcomes in UTI remains equivocal. To the best of our knowledge, this is the first matched control analysis evaluating the clinical and economic impact of ESBL specifically in UTI. We saw a 50% increase in the median LOS (from 4 to 6 days) and cost of care (additional $3658 per patient) in the ESBL‐EK UTI cohort. Albeit as small subpopulation, patients with bacteremic UTI due to ESBL‐EK had significantly longer hospital stays and increased cost of care as compared with nonESBL‐EK bacteremic UTI. These economic findings are consistent with data from other investigations. For example, a matched case‐control study of infection (51.5% UTI) due to ESBL‐producing E coli and K pneumoniae by Lautenbach and colleagues found an additional 60‐hour (72 vs 11.5 hours, P<0.001) delay in time to appropriate antibiotics for case patients, resulting in significantly longer LOS (1.8 times) and increased hospital charges (2.9 times).[17] With respect to costs, a matched case‐control analysis of ESBL‐EK in non‐UTI saw a 70% increase in cost ($41,353 vs $24,902).[26] Moreover, the increased cost in that study, like this one, was driven primarily by LOS (additional 9.7 days) and not drug utilization, with antibiotic costs representing <2% of the total hospitalization cost. Tumbarello and colleagues observed an approximate 50% increase in LOS and hospital costs in patients with BSI caused by ESBL‐producing E coli.[20]
Distinctive from those studies, we investigated the significance of ESBL production on hospital reimbursement. Despite the additional healthcare resource utilization (50% greater), we saw no appreciable increase (<5%) in median hospital reimbursement. Given that the primary payors between cohorts were comparable, infection with ESBL‐producing bacteria may result in a potential loss of income if optimal treatment is not initiated on admission.
The risk factors for development of UTI due to ESBL‐EK are well defined.[27, 28, 29, 30, 31, 32] Two scoring systems, an Italian and a Duke model, have identified patients at increased risk of harboring ESBL‐producing organisms on hospital admission.[33, 34] The features of each model center on established risk factors for ESBL‐EK UTI. In our study population, these scoring model features were more common in ESBL‐EK patients, supporting their potential application in UTI. However, because of our study design (infected controls) and matching criteria, only 2 features (recent antibiotics and previous hospitalization) achieved statistical significance as detectable risk factors in our population. Regardless, these data, coupled with increasing prevalence of UTI with ESBL‐producing bacteria, provide justification and advocacy for the empiric use of ESBL active antibiotics (ie, carbapenems) in certain high‐risk individuals, particularly those patients with a previous history of ESBL or those with multiple risk factors identified in our study as well as others (previous hospitalization, recent antibiotic exposure). Importantly, an aggressive de‐escalation strategy should be used to temper collateral damage for patients with non‐ESBL infections. Moreover, the utilization of oral therapies beyond the fluoroquinolones and trimethoprim‐sulfamethoxazole, such as fosfomycin and nitrofurantoin, coupled with coordinated transitions of care, may alleviate the demand for intravenous access in patients prepared for discharge.[35, 36]
This study is not without limitations. As only a distinct period in time was studied, we are unable to determine the implications of previous episodes of UTI on the current admission. Although the Charlson comorbidity score was not significantly different between the 2 patient cohorts, ESBL‐EK patients were more likely to have several demographic features (ie, diabetes mellitus, recurrent UTIs, previous hospitalization, and recent antibiotic exposure), which are to be expected in the ESBL population.[37, 38] Although these differences have been observed as expected, we believe the driver of prolonged LOS and increased costs of care stem from inappropriate initial antibiotic therapy as evident by the initial clinical failure observed in ESBL‐EK patients. It should also be noted that we defined antibiotics as appropriate based on the laboratory criteria for susceptibility, as such agents that have high concentration in the urinary tract (ie, fluoroquinolones) may be effective despite these laboratory definitions.[17] For instance, 10 of our ESBL‐EK patients never received appropriate antibiotics (3 fluoroquinolones, 7 noncarbapenem ‐lactams) as defined by laboratory‐based susceptibility testing, 8 of whom experienced clinical success. Previous studies have shown discordance between phenotypic ESBL‐related resistance profiles and outcomes.[17, 18, 19, 20, 21, 25, 39, 40]
In summary, recent increases in antimicrobial resistance present ongoing challenges in the treatment of hospitalized patients, as appropriate treatment options are extremely limited. Our findings strengthen the consortium of data stating that antimicrobial resistance unfavorably impacts patient outcomes.[41, 42] ESBL‐EK in UTI is associated with high rates of failure of initial antibiotic therapy, prolonged LOS, and increased cost of care. Furthermore, the added cost associated with UTI due to ESBL‐EK is not recognized in hospital reimbursement, as evident by a $3200 net loss relative to reimbursement. This loss appears to be solely due to the increased LOS, as antibiotic costs were <1% of cost of care. Moreover, these negative consequences could be even more pronounced at institutions with a higher prevalence of ESBL infection. A multidisciplinary approach (infection control, microbiology) using these data as a benchmark, may enhance the detection, treatment, and prevention of these ESBL‐producing organisms. With high levels of coresistance to noncarbapenem ‐lactams and fluoroquinolones, providers should be cognizant of organisms capable of producing ESBL when selecting initial antibiotic therapy in high‐risk populations. Early recognition and timely initiation of appropriate antibiotic therapy appear paramount to minimizing the burden of ESBL‐EK in patients admitted to the hospital with a UTI.
Acknowledgements
The authors thank Gilbert Fotunato for his assistance with administrative data retrieval.
Disclosures
This work is supported by a grant from Merck & Co., Inc. D.P.N. reports having received grant support and honorarium from Merck & Co., Inc. S.H.M and L.O.T report no conflicts of interest relevant to this article.
Community‐onset urinary tract infection (UTI) is a common bacterial infection encountered in hospital medicine, accounting for >350,000 hospital admissions and $3.4 billion in healthcare costs annually.[1] High proportions of these infections are caused by Enterobacteriaceae, primarily Escherichia coli and Klebsiella species.[2] The prevalence of hospitalized patients with UTI caused by multidrug‐resistant E coli and Klebsiella species has increased.[3, 4, 5] Enterobacteriaceae can produce hydrolytic enzymes, specifically extended‐spectrum ‐lactamases (ESBL), which result in high rates of bacterial resistance to frequently used agents.[6] Global rates vary widely by region, and recent surveillance data showed increasing rates of clinical isolates in North America are ESBL producers.[7, 8, 9, 10] More alarming is the emergence of these resistant organisms in the community.[11]
In addition to ‐lactams, ESBL production is associated with resistance to other antibiotic classes (fluoroquinolones, aminoglycosides, and sulfonamides), and has become an important cause of failed therapy.[12, 13] Consequently, providing adequate and timely antibiotics can become convoluted due to fewer remaining treatment options. Therefore, carbapenems, which are less susceptible to hydrolysis by these enzymes, have become the preferred therapy for infection with ESBL‐producing pathogens.[3, 13, 14]
The consequences of ESBL production, mainly in bloodstream infection (BSI), are well characterized, including notable delays in receipt of appropriate antibiotic therapy, prolonged length of stay (LOS), and increased cost of care.[15, 16] Importantly, others have found higher rates of mortality.[17, 18, 19, 20, 21] However, the impact on outcomes specifically in UTI remains unclear. As a result of the prevalence of UTI admissions to the hospital, the increasing incidence of ESBLs, and the potential impact on the clinical course of care, additional study is required to support best practices for this common diagnosis.
PATIENTS AND METHODS
Study Subjects and Design
This was a retrospective, matched‐cohort analysis of patients admitted to the hospital with UTI (International Classification of Diseases, 9th Revision‐Clinical Modification code 599.0) caused by extended‐spectrum b‐lactamase‐producing Escherichia coli and Klebsiella species (ESBL‐EK). Patients admitted to Hartford Hospital from September 1, 2011 through August 31, 2012 with UTI present on admission (48 hours) were evaluated. Cases were patients 18 years of age, with a positive urine culture (104 CFU/mL) for an ESBL‐producing organism (48 hours of admission), who received antibiotic treatment directed at the positive culture for 48 hours, and beginning prior to availability of in vitro susceptibility results. Cases were identified by a detailed search of the microbiology department database of ESBL‐EK cultures. Only the first positive (index) culture for each patient was included. Bacteremia was defined as isolation of a blood culture organism identical to the one isolated from the urine culture. Patients were excluded if they were discharged, died, or placed on palliative care prior to or on the date of urine collection. Controls (patients admitted with UTI on admission caused by nonESBL‐EK) were matched to cases in a 1:1 fashion on the basis of isolated urinary pathogen, age (5 years), sex, and race. ESBL‐producing organisms were identified and classified according to the Clinical and Laboratory Standards Institute guidelines.[22]
Data Collection
Once patients were identified, the following information was collected from the patient's medical record by 2 investigators using a standardized case report form: demographic characteristics, comorbid conditions and severity of comorbidities using the Charlson comorbidity index, recent patient medical history, and clinical and economic attributes.[23]
The study was approved by the institutional review board of Hartford Hospital. An informed consent waiver was granted as all data were currently in existence and no patient‐specific interventions were conducted for the study. The collection of data was in compliance with the Health Insurance Portability and Accountability Act of 1996.
Outcomes and Definitions
Onset of UTI was defined as the date/time of the index culture collection. An initial antibiotic treatment was a course of therapy initiated empirically (prior to availability of in vitro susceptibility) and that continued for 48 hours. An appropriate empiric antibiotic was defined as an initial antibiotic that ultimately possessed in vitro activity against the isolated pathogen.
The primary clinical outcomes were initial antibiotic response and clinical response. Initial antibiotic response was defined as failure if there was lack of clinical improvement, as evident by a switch to an alternative antibiotic (excluding switches to similar/narrower‐spectrum agents and courses begun at discharge) or infection‐related mortality while receiving the initial antibiotic. Patients were deemed clinical success if they were clinically stable at discharge or end of therapy, whichever occurred first, with resolution of signs and symptoms of infection. Clinical failures were patients with (1) infection‐related mortality or (2) readmission to hospital with UTI within 30 days of discharge. Clinical response was chosen as a primary outcome because significant mortality attributed to UTI was not anticipated. Secondary clinical outcomes included: time to appropriate antibiotic therapy, mortality (all cause and infection related), and 30‐day readmission (all cause and UTI related). Patients were considered to have received appropriate antibiotic therapy when they had received their first dose of antibiotic with activity against the isolated pathogen based on the patient‐specific in vitro susceptibility results. Time to appropriate antibiotics was defined as the elapsed time (hours) between the index culture collection and the initial dose of appropriate antibiotic therapy. All‐cause mortality was defined as any cause of death at the end of hospitalization, whereas infection‐related mortality was defined as death occurring while receiving antibiotics for the index infection, without any other obvious cause of death.
The primary economic outcomes were hospital LOS, costs, and reimbursement. Antibiotic costs were calculated for each patient according to acquisition costs. Additional economic outcomes evaluated were net hospital reimbursement and primary payor. Net hospital reimbursement was calculated as the difference between hospital reimbursement and hospital costs for each patient. Hospital costs were calculated as the direct plus indirect hospitalization costs for each patient, as determined by our institutional accounting department. All economic values were reported in United States dollars.
Statistical Analysis
Statistical comparisons were performed between cases and controls using a paired t test or Wilcoxon signed rank test for continuous variables, where appropriate. Dichotomous variables were compared using the McNemar test. Multivariate logistic regression was performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to determine independent risk factors for ESBL‐EK, including all pertinent variables with a P value <0.1 in univariate analyses. All data were analyzed using SigmaStat version 2.03 (IBM/SPSS, Armonk, NY). A P value of 0.05 was considered statistically significant.
RESULTS
Patient Population
Between September 2011 and August 2012, there were 220 specimens of ESBL‐related infection and 2345 patients admitted with a UTI on admission. Eighty‐four were confirmed ESBL‐EK cases (3.6%), and 55 met criteria for inclusion. Twenty‐nine of these cases were excluded because the index culture was polymicrobial, for which the response to antibiotics for ESBL‐EK could not be elicited (n=22); they had incomplete medical records (n=4); or they did not have a matched control patient (n=3). Fifty‐five matched control patients were identified, resulting in 110 patients overall.
Patient demographics and baseline characteristics are shown in Table 1. Patients with ESBL‐EK UTI were more likely to have diabetes mellitus, chronic obstructive pulmonary disorder, and a history of recurrent UTIs. They were more likely to have recently received antibiotics, been hospitalized, or had isolation of an ESBL‐producing organism. No significant differences in Charlson comorbidity index, recent immunosuppressive therapy, or urinary catheterization were observed. Compared with controls, patients with ESBL‐EK UTI were more frequently transferred from another healthcare facility, although the difference was not statistically significant (P=0.06). In the multivariate regression model, diabetes mellitus (OR: 4.4, 95% CI: 1.711.5; P=0.002), history of recurrent UTIs (OR: 4.4, 95% CI: 1.810.9; P=0.001), and transfer from another healthcare facility (OR: 2.38, 95% CI: 1.05.7; P=0.05) were independently associated with ESBL‐EK UTI. Previous isolation of an ESBL‐producing organism (P<0.001) was unable to be included in the multivariate logistic regression model because only patients with UTI caused by ESBL‐EK were positive for this variable. However, as noted in Table 1, 27.2% of cases had isolation of an ESBL‐producing organism in the previous year.
| ESBL Positive, n=55 | ESBL Negative, n=55 | Pa | |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y, median (IQR) | 77 (6785) | 77 (6685) | 0.83b |
| Female | 36 (65.4) | 36 (65.4) | 1.00 |
| White | 42 (76.4) | 42 (76.4) | 1.00 |
| Black | 5 (9.1) | 5 (9.1) | 1.00 |
| Hispanic/Latino | 6 (10.9) | 6 (10.9) | 1.00 |
| Other | 2 (3.6) | 2 (3.6) | 1.00 |
| Comorbidities | |||
| Diabetes mellitus | 25 (45.5) | 10 (18.1) | 0.004 |
| COPD | 15 (27.2) | 6 (10.9) | 0.04 |
| Liver disease | 2 (3.6) | 1 (1.8) | 1.00 |
| Hemodialysis | 9 (16.4) | 6 (10.9) | 0.58 |
| Hematological malignancy | 3 (5.5) | 2 (3.6) | 1.00 |
| Solid malignancy | 13 (23.6) | 9 (16.4) | 0.45 |
| HIV/AIDS | 0 | 1 (1.8) | 1.00 |
| Age >65 years | 44 (80.0) | 43 (78.2) | 1.00 |
| Urinary abnormality | 15 (27.2) | 15 (27.2) | 1.00 |
| Charlson comorbidity index, median (IQR) | 3 (24) | 2 (13.8) | 0.19 |
| History | |||
| Previous hospitalizationc | 38 (69.1) | 24 (43.6) | 0.01 |
| Previous antibioticsd | 23 (41.8) | 12 (21.8) | 0.04 |
| Recent immunosuppressive therapye | 9 (16.4) | 3 (5.5) | 0.11 |
| History of recurrent UTIsf | 29 (52.7) | 12 (21.8) | 0.001 |
| History of urinary catheterizationg | 18 (32.7) | 14 (25.4) | 0.45 |
| Previous genitourinary procedure/surgery | 10 (18.2) | 6 (10.9) | 0.39 |
| Previous ESBL‐producing organismh | 15 (27.2) | 0 | <0.001 |
| Clinical features | |||
| Transfer from another healthcare facility | 27 (49.1) | 17 (21.0) | 0.06 |
| ICU admission | 12 (21.8) | 7 (12.7) | 0.33 |
| Bacteremia | 7 (12.7) | 5 (9.1) | 0.75 |
| Infectious Diseases consulted | 39 (70.1) | 16 (29.1) | <0.001 |
| Empiric ‐lactam (noncarbapenem) | 35 (63.6) | 50 (90.9) | 0.001 |
Isolate Characteristics
The distribution of causative pathogens in each cohort was: 44 (80%) Escherichia coli, 8 (14.5%) Klebsiella pneumoniae, and 3 (5.5%) Klebsiella oxytoca. In vitro nonsusceptibility profiles of the 55 ESBL‐EK cases are characterized in Figure 1. The most active agents were carbapenems, with 98.2% susceptibility to the entire isolate profile, whereas <10% of isolates were susceptible to the fluoroquinolones. All ESBL‐producing isolates were resistant to cefepime.

Failure on Initial Antibiotic Regimen
Initial antibiotic therapy is shown in Table 2. A majority of patients (87.2%) were initially treated with a ‐lactam. Empiric carbapenem use was greater in ESBL‐EK cases (18.1% vs 0%; P<0.001), and there were no other significant differences in the distribution of initial antibiotic therapy between cohorts. Less than one‐quarter of ESBL‐EK patients (23.6%) received appropriate initial therapy, whereas 98.2% of controls were initially treated appropriately (P<0.001).
| Initial Antibiotic | ESBL Positive, n=55 | ESBL Negative, n=55 | Pa |
|---|---|---|---|
| |||
| Ceftriaxone | 21 (38.1) | 32 (58.1) | 0.06 |
| Cefepime | 10 (18.2) | 12 (21.8) | 0.81 |
| Ertapenem | 7 (12.7) | 0 | 0.02 |
| Levofloxacin | 6 (10.9) | 4 (7.3) | 0.74 |
| Cefazolin | 0 | 4 (7.3) | 0.13 |
| Piperacillin‐tazobactam | 3 (5.5) | 0 | 0.24 |
| Ciprofloxacin | 2 (3.6) | 1 (1.8) | 1.00 |
| Doripenem | 2 (3.6) | 0 | 0.48 |
| Trimethoprim‐sulfamethoxazole | 2 (3.6) | 0 | 0.48 |
| Meropenem | 1 (1.8) | 0 | 1.00 |
| Cephalexin | 1 (1.8) | 0 | 1.00 |
| Aztreonam | 0 | 2 (3.6) | 0.48 |
Compared with controls, failure of initial antibiotic therapy was more common in patients with ESBL‐EK UTI, resulting in a significantly longer time to appropriate antibiotics (Table 3). Among ESBL‐EK UTI cases, failure of initial antibiotic therapy was greater for patients who received noncarbapenem ‐lactams (85.7%) as compared to those who empirically received a carbapenem (0%) (P<0.001). Antibiotic failure (>2 patients treated) in ESBL‐EK was highest with the following antibiotics: cefepime (100% [10/10]), piperacillin‐tazobactam (100% [3/3]), and ceftriaxone (76.2% [16/21]).
| ESBL Positive, n=55 | ESBL Negative, n=55 | Pa | |
|---|---|---|---|
| |||
| Clinical parameter | |||
| Initial antibiotic failure | 34 (61.8) | 3 (5.5) | <0.001 |
| Escalation to an alternative antibiotic | 33 (60.0) | 3 (5.5) | <0.001 |
| Time to appropriate antibiotics, h, median (IQR) | 51 (32.560.8) | 2.5 (1.07.2) | <0.001b |
| Appropriate empiric antibiotics | 13 (23.6) | 54 (98.2) | <0.001 |
| Clinical success | 47 (85.5) | 52 (94.5) | 0.23 |
| All‐cause mortality | 5 (9.1) | 1 (1.8) | 0.21 |
| Infection‐related mortality | 4 (7.2) | 1 (1.8) | 0.37 |
| All‐cause 30‐day readmission | 12 (21.8) | 15 (27.2) | 0.63 |
| UTI‐related 30‐day readmission | 4 (7.2) | 2 (3.6) | 0.68 |
| Economic parameterb | |||
| Length of stay, d, median (IQR) | 6 (48) | 4 (36) | 0.02 |
| Total hospital cost, median (IQR)c | 10,741 (684615,819) | 7,083 (566711,652) | 0.02 |
| Bed cost, % total cost, median (IQR) | 57.5 (51.666.0) | 63.8 (51.973.5) | 0.21 |
| Antibiotic cost, % total cost, median (IQR) | 0.5 (0.12.0) | 0.1(0.030.2) | <0.001 |
| Primary payor, n (%) | |||
| Medicare | 44 (80) | 44 (80) | 1.00 |
| Medicaid | 7 (12.7) | 3 (5.4) | 0.32 |
| Private insurance | 2 (3.6) | 2 (3.6) | 0.61 |
| Managed care | 2 (3.6) | 6 (10.9) | 0.27 |
Clinical Outcomes
There were no significant differences in clinical success, mortality, or 30‐day readmission between cohorts (Table 3). Among ESBL‐EK patients, those who received appropriate antibiotics within 48 hours were significantly more likely to achieve treatment success (100% vs 77.1%; P=0.04). All 8 ESBL‐EK treatment failures (4 infection‐related mortality and 4 UTI readmitted with the same ESBL pathogen) failed to receive appropriate antibiotics within 48 hours of culture collection.
More ESBL‐EK patients required a switch in their antibiotics. Within the subgroup of ESBL‐EK patients with an escalation in antibiotics, ertapenem was added to 19 of 33 (57.6%) cases.
Economic Outcomes
ESBL‐EK patients who received inappropriate initial therapy received longer antibiotic treatment courses than those empirically treated with a carbapenem (meanstandard deviation, 8.93.7 vs 6.23.2 days, respectively; P=0.04). When compared to non‐ESBL infection, ESBL‐EK patients required more days of antibiotic therapy (median 8 vs 5 days; P=0.03). The median LOS was significantly longer and total hospital costs were significantly greater for ESBL cases. Antibiotic costs contributed minimally to the overall cost, accounting for <1% (0.5% for cases vs 0.1% for controls), regardless of ESBL status. A comparison of economic outcomes is presented in Table 3. LOS among ESBL cases was not different between those discharged with and without continued antimicrobial therapy. Moreover, for those discharged on antimicrobial therapy, the utilization of either the oral or intravenous route did not delay discharge (data not shown).
The payor mix was similar between cases and controls. Given the predominately elderly population, Medicare was the primary payor for a majority of patients. Median differences in cost and reimbursement between cohorts (ESBL‐EK vs nonESBL‐EK) were $3658 (P=0.02) and $469 (P=0.56), respectively. As a result, median loss per patient with ESBL‐EK infection was $3189 when compared with controls.
Bacteremia
All cases of bacteremia were present on admission. Bacteremic UTI due to ESBL‐EK was associated with initial antibiotic failure (85.7% [6/7] vs 0% [0/5]; P=0.015), delayed appropriate therapy (median, 56 vs 2 hours; P=0.003), longer median hospital stays (11 vs 5 days; P=0.05), and higher median cost ($27,671 vs $5898; P=0.03) as compared with bacteremic UTI due to non‐ESBL‐EK. Infection‐related mortality occurred in 2 ESBL‐EK bacteremic patients, but no mortality was observed among the nonESBL‐EK bacteremic UTI cohort (28.6% [2/7] vs 0% [0/5]; P=0.47].
DISCUSSION
This matched cohort analysis revealed that ESBL‐EK has detrimental effects on the outcomes of patients admitted to the hospital with UTI. While matching for demographics and infecting pathogen, patients with ESBL‐EK UTI had diminished initial antibiotic response and considerably longer time to appropriate antibiotic therapy (48 hours longer) than their non‐ESBL comparator. Despite significant delays in appropriate therapy, we saw no attributable difference in clinical outcome and mortality; however, numerical trends toward increased risk were observed in ESBL‐EK patients. Although clinical response was largely unchanged, prolonged hospitals stays and increased cost of care were endured by ESBL‐EK patients.
Antimicrobial resistance, a primary factor in the postponement of appropriate antibiotic therapy, is a worrisome occurrence with meaningful clinical implications.16[21, 24] In BSI, delayed appropriate therapy due to ESBLs has been associated with prolonged LOS, increased costs, and increased mortality.[20, 25] However, the clinical significance of ESBL production on patient outcomes in UTI remains equivocal. To the best of our knowledge, this is the first matched control analysis evaluating the clinical and economic impact of ESBL specifically in UTI. We saw a 50% increase in the median LOS (from 4 to 6 days) and cost of care (additional $3658 per patient) in the ESBL‐EK UTI cohort. Albeit as small subpopulation, patients with bacteremic UTI due to ESBL‐EK had significantly longer hospital stays and increased cost of care as compared with nonESBL‐EK bacteremic UTI. These economic findings are consistent with data from other investigations. For example, a matched case‐control study of infection (51.5% UTI) due to ESBL‐producing E coli and K pneumoniae by Lautenbach and colleagues found an additional 60‐hour (72 vs 11.5 hours, P<0.001) delay in time to appropriate antibiotics for case patients, resulting in significantly longer LOS (1.8 times) and increased hospital charges (2.9 times).[17] With respect to costs, a matched case‐control analysis of ESBL‐EK in non‐UTI saw a 70% increase in cost ($41,353 vs $24,902).[26] Moreover, the increased cost in that study, like this one, was driven primarily by LOS (additional 9.7 days) and not drug utilization, with antibiotic costs representing <2% of the total hospitalization cost. Tumbarello and colleagues observed an approximate 50% increase in LOS and hospital costs in patients with BSI caused by ESBL‐producing E coli.[20]
Distinctive from those studies, we investigated the significance of ESBL production on hospital reimbursement. Despite the additional healthcare resource utilization (50% greater), we saw no appreciable increase (<5%) in median hospital reimbursement. Given that the primary payors between cohorts were comparable, infection with ESBL‐producing bacteria may result in a potential loss of income if optimal treatment is not initiated on admission.
The risk factors for development of UTI due to ESBL‐EK are well defined.[27, 28, 29, 30, 31, 32] Two scoring systems, an Italian and a Duke model, have identified patients at increased risk of harboring ESBL‐producing organisms on hospital admission.[33, 34] The features of each model center on established risk factors for ESBL‐EK UTI. In our study population, these scoring model features were more common in ESBL‐EK patients, supporting their potential application in UTI. However, because of our study design (infected controls) and matching criteria, only 2 features (recent antibiotics and previous hospitalization) achieved statistical significance as detectable risk factors in our population. Regardless, these data, coupled with increasing prevalence of UTI with ESBL‐producing bacteria, provide justification and advocacy for the empiric use of ESBL active antibiotics (ie, carbapenems) in certain high‐risk individuals, particularly those patients with a previous history of ESBL or those with multiple risk factors identified in our study as well as others (previous hospitalization, recent antibiotic exposure). Importantly, an aggressive de‐escalation strategy should be used to temper collateral damage for patients with non‐ESBL infections. Moreover, the utilization of oral therapies beyond the fluoroquinolones and trimethoprim‐sulfamethoxazole, such as fosfomycin and nitrofurantoin, coupled with coordinated transitions of care, may alleviate the demand for intravenous access in patients prepared for discharge.[35, 36]
This study is not without limitations. As only a distinct period in time was studied, we are unable to determine the implications of previous episodes of UTI on the current admission. Although the Charlson comorbidity score was not significantly different between the 2 patient cohorts, ESBL‐EK patients were more likely to have several demographic features (ie, diabetes mellitus, recurrent UTIs, previous hospitalization, and recent antibiotic exposure), which are to be expected in the ESBL population.[37, 38] Although these differences have been observed as expected, we believe the driver of prolonged LOS and increased costs of care stem from inappropriate initial antibiotic therapy as evident by the initial clinical failure observed in ESBL‐EK patients. It should also be noted that we defined antibiotics as appropriate based on the laboratory criteria for susceptibility, as such agents that have high concentration in the urinary tract (ie, fluoroquinolones) may be effective despite these laboratory definitions.[17] For instance, 10 of our ESBL‐EK patients never received appropriate antibiotics (3 fluoroquinolones, 7 noncarbapenem ‐lactams) as defined by laboratory‐based susceptibility testing, 8 of whom experienced clinical success. Previous studies have shown discordance between phenotypic ESBL‐related resistance profiles and outcomes.[17, 18, 19, 20, 21, 25, 39, 40]
In summary, recent increases in antimicrobial resistance present ongoing challenges in the treatment of hospitalized patients, as appropriate treatment options are extremely limited. Our findings strengthen the consortium of data stating that antimicrobial resistance unfavorably impacts patient outcomes.[41, 42] ESBL‐EK in UTI is associated with high rates of failure of initial antibiotic therapy, prolonged LOS, and increased cost of care. Furthermore, the added cost associated with UTI due to ESBL‐EK is not recognized in hospital reimbursement, as evident by a $3200 net loss relative to reimbursement. This loss appears to be solely due to the increased LOS, as antibiotic costs were <1% of cost of care. Moreover, these negative consequences could be even more pronounced at institutions with a higher prevalence of ESBL infection. A multidisciplinary approach (infection control, microbiology) using these data as a benchmark, may enhance the detection, treatment, and prevention of these ESBL‐producing organisms. With high levels of coresistance to noncarbapenem ‐lactams and fluoroquinolones, providers should be cognizant of organisms capable of producing ESBL when selecting initial antibiotic therapy in high‐risk populations. Early recognition and timely initiation of appropriate antibiotic therapy appear paramount to minimizing the burden of ESBL‐EK in patients admitted to the hospital with a UTI.
Acknowledgements
The authors thank Gilbert Fotunato for his assistance with administrative data retrieval.
Disclosures
This work is supported by a grant from Merck & Co., Inc. D.P.N. reports having received grant support and honorarium from Merck & Co., Inc. S.H.M and L.O.T report no conflicts of interest relevant to this article.
- , , , et al. Urologic diseases in America project: analytical methods and principal findings. J Urol. 2005;173(3):933–937.
- . Urinary tract pathogens in complicated infection and in elderly individuals. J Infect Dis. 2001;183(suppl 1):S5–S8.
- , , , , . Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009–2010. Diagn Microbiol Infect Dis. 2011;70(4):507–511.
- , . Extended‐spectrum β‐lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686.
- , , . Extended‐spectrum β‐lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis. 2010;23(4):320–326.
- . Defining an extended‐spectrum beta‐lactamase. Clin Microbiol Infect. 2008;14(suppl 5):3–10.
- , , , , , . Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J Antimicrob Chemother. 2007;60(5):1018–1029.
- , , , . Variations in the prevalence of strains expressing an extended‐spectrum β‐lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis. 2001;32(suppl 2):S94–S103.
- National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. Am J Infect Control. 2004;32(8):470–485.
- , . Prevalence and antimicrobial susceptibility data for extended‐spectrum β‐lactamase‐ and AmpC‐producing Enterobacteriaceae from the MYSTIC Program in Europe and the United States (1997–2004). Diagn Microbiol Infect Dis. 2005;53(4):257–264.
- , , , . Emergence of Enterobacteriaceae producing extended‐spectrum b‐lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005;56(1):52–59.
- , , , et al. Epidemiology of ciprofloxacin resistance and its relationship to extended‐spectrum B‐lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin Infect Dis. 2000;30(4):473–478.
- , , , , , Epidemiological investigation of fluoroquinolone resistance in infections due to extended‐spectrum‐β‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis. 2001;33(8):1288–1294.
- , , , . High levels of antimicrobial coresistance among extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae. Antimicrob Agents Chemother. 2005;49(5):2137–2139.
- . Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31(suppl 4):S131–S138.
- . The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82–S89.
- , , , , , Extended‐spectrum beta‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001:32(8):1162–1171.
- , , , et al. Predictors of mortality in patients with bloodstream infections caused by extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51(6):1987–1994.
- , . Mortality and delay in effective therapy associated with extended‐spectrum β lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta‐analysis. J Antimicrob Chemother. 2007;60(5):913–920.
- , , , et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended‐spectrum‐β‐lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. 2010;54(10):4085–4091.
- , , . Effects of confounders and intermediates on the association of bacteraemia caused by extended‐spectrum β‐lactamase‐producing Enterobacteriaceae and patient outcome: a meta‐analysis. J Antimicrob Chemother. 2012;67(6):1311–1320.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 21st informational supplement. M100‐S20. Wayne, PA: Clinical and Laboratory Standards Institute; 2010.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. Bloodstream infections caused by antibiotic‐resistant gram‐negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766.
- , , , , . Clinical implications of extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae bacteraemia. J Hosp Infect. 2002;52(2):99–106.
- , , , , . Impact of extended‐spectrum β‐lactamase‐producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol. 2006;27(11):1226–1232.
- , , , et al. Risk factors for community‐onset urinary tract infections due to Escherichia coli harbouring extended‐spectrum‐β‐lactamases. J Antimicrob Chemother. 2006:57(4):780–783.
- , , , et al. Community infections caused extended‐spectrum‐β‐lactamase‐producing Escherichia coli. Arch Intern Med. 2008;168(17):1897–1902.
- , , . Prevalence and risk factors for extended spectrum‐β‐lactamase‐producing uropathogens in urinary tract infection. Korean J Urol. 2010;51(7):492–497.
- , , , , . Extended‐spectrum β‐lactamase‐producing gram‐negative pathogens in community‐acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39(4):333–340.
- , , , et al. Epidemiology and genetic characteristics of extended‐spectrum‐β‐lactamase‐producing gram‐negative bacteria causing urinary tract infections in long‐term care facilities. J Antimicrob Chemother. 2012;67(12):2982–2987.
- , , , et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended‐spectrum beta‐lactamase (ESBL)‐producing enterobacteria. Int J Clin Pract. 2012;66(9):891–896.
- , , , et al. Identifying patients harboring extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011;55(7):3485–3490.
- , , , . Utility of a clinical risk factor scoring model in predicting infection with extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol. 2013;34(4):385–392.
- , , , , , . Fosfomycin in the treatment of extended spectrum beta‐lactamase‐producing Escherichia coli‐related lower urinary tract infections. Int J Amtimicrob Agents. 2007;29(1):62–65.
- , , , , . Nitrofurantoin in the treatment of extended‐spectrum beta‐lactamase‐producing Escherichia coli‐related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554–556.
- , , , et al. Epidemiology and clinical features of infections caused by extended‐spectrum beta‐lactamase‐producing Escherichia coli in nonhospitalized patients. J Clin Microb. 2004;42(3):1089–1094.
- , , , et al. Community infections caused by extended‐spectrum β‐lactamase‐producing Escherichia coli. Arch Intern Med. 2008:168(17):1897–1902.
- , , , et al. Extended‐spectrum beta‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med. 2002;28(12):1718–1723.
- , , , et al. Ceftazidime‐resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case‐control and molecular epidemiologic investigation. J Infect Dis. 1996;174(3):529–536.
- , . The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36(11):1433–1437.
- . Clinical implications of antimicrobial resistance for therapy. J Antimicrob Chemother. 2008;62(suppl 2):ii105–ii114.
- , , , et al. Urologic diseases in America project: analytical methods and principal findings. J Urol. 2005;173(3):933–937.
- . Urinary tract pathogens in complicated infection and in elderly individuals. J Infect Dis. 2001;183(suppl 1):S5–S8.
- , , , , . Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009–2010. Diagn Microbiol Infect Dis. 2011;70(4):507–511.
- , . Extended‐spectrum β‐lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686.
- , , . Extended‐spectrum β‐lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis. 2010;23(4):320–326.
- . Defining an extended‐spectrum beta‐lactamase. Clin Microbiol Infect. 2008;14(suppl 5):3–10.
- , , , , , . Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J Antimicrob Chemother. 2007;60(5):1018–1029.
- , , , . Variations in the prevalence of strains expressing an extended‐spectrum β‐lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis. 2001;32(suppl 2):S94–S103.
- National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. Am J Infect Control. 2004;32(8):470–485.
- , . Prevalence and antimicrobial susceptibility data for extended‐spectrum β‐lactamase‐ and AmpC‐producing Enterobacteriaceae from the MYSTIC Program in Europe and the United States (1997–2004). Diagn Microbiol Infect Dis. 2005;53(4):257–264.
- , , , . Emergence of Enterobacteriaceae producing extended‐spectrum b‐lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005;56(1):52–59.
- , , , et al. Epidemiology of ciprofloxacin resistance and its relationship to extended‐spectrum B‐lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin Infect Dis. 2000;30(4):473–478.
- , , , , , Epidemiological investigation of fluoroquinolone resistance in infections due to extended‐spectrum‐β‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis. 2001;33(8):1288–1294.
- , , , . High levels of antimicrobial coresistance among extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae. Antimicrob Agents Chemother. 2005;49(5):2137–2139.
- . Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31(suppl 4):S131–S138.
- . The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82–S89.
- , , , , , Extended‐spectrum beta‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001:32(8):1162–1171.
- , , , et al. Predictors of mortality in patients with bloodstream infections caused by extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51(6):1987–1994.
- , . Mortality and delay in effective therapy associated with extended‐spectrum β lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta‐analysis. J Antimicrob Chemother. 2007;60(5):913–920.
- , , , et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended‐spectrum‐β‐lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. 2010;54(10):4085–4091.
- , , . Effects of confounders and intermediates on the association of bacteraemia caused by extended‐spectrum β‐lactamase‐producing Enterobacteriaceae and patient outcome: a meta‐analysis. J Antimicrob Chemother. 2012;67(6):1311–1320.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 21st informational supplement. M100‐S20. Wayne, PA: Clinical and Laboratory Standards Institute; 2010.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. Bloodstream infections caused by antibiotic‐resistant gram‐negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766.
- , , , , . Clinical implications of extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae bacteraemia. J Hosp Infect. 2002;52(2):99–106.
- , , , , . Impact of extended‐spectrum β‐lactamase‐producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol. 2006;27(11):1226–1232.
- , , , et al. Risk factors for community‐onset urinary tract infections due to Escherichia coli harbouring extended‐spectrum‐β‐lactamases. J Antimicrob Chemother. 2006:57(4):780–783.
- , , , et al. Community infections caused extended‐spectrum‐β‐lactamase‐producing Escherichia coli. Arch Intern Med. 2008;168(17):1897–1902.
- , , . Prevalence and risk factors for extended spectrum‐β‐lactamase‐producing uropathogens in urinary tract infection. Korean J Urol. 2010;51(7):492–497.
- , , , , . Extended‐spectrum β‐lactamase‐producing gram‐negative pathogens in community‐acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39(4):333–340.
- , , , et al. Epidemiology and genetic characteristics of extended‐spectrum‐β‐lactamase‐producing gram‐negative bacteria causing urinary tract infections in long‐term care facilities. J Antimicrob Chemother. 2012;67(12):2982–2987.
- , , , et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended‐spectrum beta‐lactamase (ESBL)‐producing enterobacteria. Int J Clin Pract. 2012;66(9):891–896.
- , , , et al. Identifying patients harboring extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011;55(7):3485–3490.
- , , , . Utility of a clinical risk factor scoring model in predicting infection with extended‐spectrum‐β‐lactamase‐producing Enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol. 2013;34(4):385–392.
- , , , , , . Fosfomycin in the treatment of extended spectrum beta‐lactamase‐producing Escherichia coli‐related lower urinary tract infections. Int J Amtimicrob Agents. 2007;29(1):62–65.
- , , , , . Nitrofurantoin in the treatment of extended‐spectrum beta‐lactamase‐producing Escherichia coli‐related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554–556.
- , , , et al. Epidemiology and clinical features of infections caused by extended‐spectrum beta‐lactamase‐producing Escherichia coli in nonhospitalized patients. J Clin Microb. 2004;42(3):1089–1094.
- , , , et al. Community infections caused by extended‐spectrum β‐lactamase‐producing Escherichia coli. Arch Intern Med. 2008:168(17):1897–1902.
- , , , et al. Extended‐spectrum beta‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med. 2002;28(12):1718–1723.
- , , , et al. Ceftazidime‐resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case‐control and molecular epidemiologic investigation. J Infect Dis. 1996;174(3):529–536.
- , . The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36(11):1433–1437.
- . Clinical implications of antimicrobial resistance for therapy. J Antimicrob Chemother. 2008;62(suppl 2):ii105–ii114.
© 2014 Society of Hospital Medicine