User login
Hire Hard, Manage Easy
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
© 2019 Society of Hospital Medicine
Focused Ethnography of Diagnosis in Academic Medical Centers
Diagnostic error—defined as a failure to establish an accurate and timely explanation of the patient’s health problem—is an important source of patient harm.1 Data suggest that all patients will experience at least 1 diagnostic error in their lifetime.2-4 Not surprisingly, diagnostic errors are among the leading categories of paid malpractice claims in the United States.5
Despite diagnostic errors being morbid and sometimes deadly in the hospital,6,7 little is known about how residents and learners approach diagnostic decision making. Errors in diagnosis are believed to stem from cognitive or system failures,8 with errors in cognition believed to occur due to rapid, reflexive thinking operating in the absence of a more analytical, deliberate process. System-based problems (eg, lack of expert availability, technology barriers, and access to data) have also been cited as contributors.9 However, whether and how these apply to trainees is not known.
Therefore, we conducted a focused ethnography of inpatient medicine teams (ie, attendings, residents, interns, and medical students) in 2 affiliated teaching hospitals, aiming to (a) observe the process of diagnosis by trainees and (b) identify methods to improve the diagnostic process and prevent errors.
METHODS
We designed a multimethod, focused ethnographic study to examine diagnostic decision making in hospital settings.10,11 In contrast to anthropologic ethnographies that study entire fields using open-ended questions, our study was designed to examine the process of diagnosis from the perspective of clinicians engaged in this activity.11 This approach allowed us to capture diagnostic decisions and cognitive and system-based factors in a manner currently lacking in the literature.12
Setting and Participants
Between January 2016 and May 2016, we observed the members of four inpatient internal medicine teaching teams at 2 affiliated teaching hospitals. We purposefully selected teaching teams for observation because they are the primary model of care in academic settings and we have expertise in carrying out similar studies.13,14 Teaching teams typically consisted of a medical attending (senior-level physician), 1 senior resident (a second- or third-year postgraduate trainee), two interns (a trainee in their first postgraduate year), and two to four medical students. Teams were selected at random using existing schedules and followed Monday to Friday so as to permit observation of work on call and noncall days. Owing to manpower limitations, weekend and night shifts were not observed. However, overnight events were captured during morning rounds.
Most of the teams began rounds at 8:30 AM. Typically, rounds lasted for 90–120 min and concluded with a recap (ie, “running the list”) with a review of explicit plans for patients after they had been evaluated by the attending. This discussion often occurred in the team rooms, with the attending leading the discussion with the trainees.
Data Collection
A multidisciplinary team, including clinicians (eg, physicians, nurses), nonclinicians (eg, qualitative researchers, social scientists), and healthcare engineers, conducted the observations. We observed preround activities of interns and residents before arrival of the attending (7:00 AM - 8:30 AM), followed by morning rounds with the entire team, and afternoon work that included senior residents, interns, and students.
To capture multiple aspects of the diagnostic process, we collected data using field notes modeled on components of the National Academy of Science model for diagnosis (Appendix).1,15 This model encompasses phases of the diagnostic process (eg, data gathering, integration, formulation of a working diagnosis, treatment delivery, and outcomes) and the work system (team members, organization, technology and tools, physical environment, tasks).
Focus Groups and Interviews
At the end of weekly observations, we conducted focus groups with the residents and one-on- one interviews with the attendings. Focus groups with the residents were conducted to encourage a group discussion about the diagnostic process. Separate interviews with the attendings were performed to ensure that power differentials did not influence discussions. During focus groups, we specifically asked about challenges and possible solutions to improve diagnosis. Experienced qualitative methodologists (J.F., M.H., M.Q.) used semistructured interview guides for discussions (Appendix).
Data Analysis
After aggregating and reading the data, three reviewers (V.C., S.K., S.S.) began inductive analysis by handwriting notes and initial reflective thoughts to create preliminary codes. Multiple team members then reread the original field notes and the focus group/interview data to refine the preliminary codes and develop additional codes. Next, relationships between codes were identified and used to develop key themes. Triangulation of data collected from observations and interview/focus group sessions was carried out to compare data that we surmised with data that were verbalized by the team. The developed themes were discussed as a group to ensure consistency of major findings.
Ethical and Regulatory Oversight
This study was reviewed and approved by the Institutional Review Boards at the University of Michigan Health System (HUM-00106657) and the VA Ann Arbor Healthcare System (1-2016-010040).
RESULTS
Four teaching teams (4 attendings, 4 senior residents, 9 interns, and 14 medical students) were observed over 33 distinct shifts and 168 hours. Observations included morning rounds (96 h), postround call days (52 h), and postround non-call days (20 h). Morning rounds lasted an average of 127 min (range: 48-232 min) and included an average of 9 patients (range: 4-16 patients).
Themes Regarding the Diagnostic Process
We identified the following 4 primary themes related to the diagnostic process in teaching hospitals: (1) diagnosis is a social phenomenon; (2) data necessary to make diagnoses are fragmented; (3) distractions undermine the diagnostic process; and (4) time pressures interfere with diagnostic decision making (Appendix Table 1).
(1) Diagnosis is a Social Phenomenon.
Team members viewed the process of diagnosis as a social exchange of facts, findings, and strategies within a defined structure. The opportunity to discuss impressions with others was valued as a means to share, test, and process assumptions.
“Rounds are the most important part of the process. That is where we make most decisions in a collective, collaborative way with the attending present. We bounce ideas off each other.” (Intern)
Typical of social processes, variations based on time of day and schedule were observed. For instance, during call days, learners gathered data and formed working diagnosis and treatment plans with minimal attending interaction. This separation of roles and responsibilities introduced a hierarchy within diagnosis as follows:
“The interns would not call me first; they would talk to the senior resident and then if the senior thought he should chat with me, then they would call. But for the most part, they gather information and come up with the plan.” (Attending).
The work system was suited to facilitate social interactions. For instance, designated rooms (with team members informally assigned to a computer) provided physical proximity of the resident to interns and medical students. In this space, numerous informal discussions between team members (eg, “What do you think about this test?” “I’m not sure what to do about this finding.” “Should I call a [consult] on this patient?”) were observed. Although proximity to each other was viewed as beneficial, dangers to the social nature of diagnosis in the form of anchoring (ie, a cognitive bias where emphasis is placed on the first piece of data)16 were also mentioned. Similarly, the paradox associated with social proof (ie, the pressure to assume conformity within a group) was also observed as disagreement between team members and attendings rarely occurred during observations.
“I mean, they’re the attending, right? It’s hard to argue with them when they want a test or something done. When I do push back, it’s rare that others will support me–so it’s usually me and the attending.” (Resident)
“I would push back if I think it’s really bad for the patient or could cause harm–but the truth is, it doesn’t happen much.” (Intern)
(2) Data Necessary to Make Diagnoses are Fragmented
Team members universally cited fragmentation in data delivery, retrieval, and processing as a barrier to diagnosis. Team members indicated that test results might not be looked at or acted upon in a timely manner, and participants pointed to the electronic medical record as a source of this challenge.
“Before I knew about [the app for Epic], I would literally sit on the computer to get all the information we would need on rounds. Its key to making decisions. We often say we will do something, only to find the test result doesn’t support it–and then we’re back to square 1.” (Intern)
Information used by teams came from myriad sources (eg, patients, family members, electronic records) and from various settings (eg, emergency department, patient rooms, discussions with consultants). Additionally, test results often appeared without warning. Thus, availability of information was poorly aligned with clinical duties.
“They (the lab) will call us when a blood culture is positive or something is off. That is very helpful but it often comes later in the day, when we’re done with rounds.” (Resident)
The work system was highlighted as a key contributor to data fragmentation. Peculiarities of our electronic medical record (EMR) and how data were collected, stored, or presented were described as “frustrating,” and “unsafe,” by team members. Correspondingly, we frequently observed interns asking for assistance for tasks such as ordering tests or finding information despite being “trained” to use the EMR.
“People have to learn how to filter, how to recognize the most important points and link data streams together in terms of causality. But we assume they know where to find that information. It’s actually a very hard thing to do, for both the house staff and me.” (Attending)
(3) Distractions Undermine the Diagnostic Process
Distractions often created cognitive difficulties. For example, ambient noise and interruptions from neighbors working on other teams were cited as barriers to diagnosis. In addition, we observed several team members using headphones to drown out ambient noise while working on the computer.
“I know I shouldn’t do it (wear headphones), but I have no other way of turning down the noise so I can concentrate.” (Intern)
Similarly, the unpredictable nature and the volume of pages often interrupted thinking about diagnosis.
“Sometimes the pager just goes off all the time and (after making sure its not an urgent issue), I will just ignore it for a bit, especially if I am in the middle of something. It would be great if I could finish my thought process knowing I would not be interrupted.” (Resident)
To mitigate this problem, 1 attending described how he would proactively seek out nurses caring for his patients to “head off” questions (eg, “I will renew the restraints and medications this morning,” and “Is there anything you need in terms of orders for this patient that I can take care of now?”) that might lead to pages. Another resident described his approach as follows:
“I make it a point to tell the nurses where I will be hanging out and where they can find me if they have any questions. I tell them to come talk to me rather than page me since that will be less distracting.” (Resident).
Most of the interns described documentation work such as writing admission and progress notes in negative terms (“an academic exercise,” “part of the billing activity”). However, in the context of interruptions, some described this as helpful.
“The most valuable part of the thinking process was writing the assessment and plan because that’s actually my schema for all problems. It literally is the only time where I can sit and collect my thoughts to formulate a diagnosis and plan.” (Intern)
(4) Time Pressures Interfere With Diagnostic Decision Making
All team members spoke about the challenge of finding time for diagnosis during the workday. Often, they had to skip learning sessions for this purpose.
“They tell us we should go to morning report or noon conference but when I’m running around trying to get things done. I hate having to choose between my education and doing what’s best for the patient–but that’s often what it comes down to.” (Intern)
When specifically asked whether setting aside dedicated time to specifically review and formulate diagnoses would be valuable, respondents were uniformly enthusiastic. Team members described attentional conflicts as being the worst when “cross covering” other teams on call days, as their patient load effectively doubled during this time. Of note, cross-covering occurred when teams were also on call—and thus took them away from important diagnostic activities such as data gathering or synthesis for patients they were admitting.
“If you were to ever design a system where errors were likely–this is how you would design it: take a team with little supervision, double their patient load, keep them busy with new challenging cases and then ask questions about patients they know little about.” (Resident)
DISCUSSION
Although diagnostic errors have been called “the next frontier for patient safety,”17 little is known about the process, barriers, and facilitators to diagnosis in teaching hospitals. In this focused ethnography conducted at 2 academic medical centers, we identified multiple cognitive and system-level challenges and potential strategies to improve diagnosis from trainees engaged in this activity. Key themes identified by those we observed included the social nature of diagnosis, fragmented information delivery, constant distractions and interruptions, and time pressures. In turn, these insights allow us to generate strategies that can be applied to improve the diagnostic process in teaching hospitals.
Our study underscores the importance of social interactions in diagnosis. In contrast, most of the interventions to prevent diagnostic errors target individual providers through practices such as metacognition and “thinking about thinking.”18-20 These interventions are based on Daniel Kahnemann’s work on dual thought process. Type 1 thought processes are fast, subconscious, reflexive, largely intuitive, and more vulnerable to error. In contrast, Type 2 processes are slower, deliberate, analytic, and less prone to error.21 Although an individual’s Type 2 thought capacity is limited, a major goal of cognitive interventions is to encourage Type 2 over Type 1 thinking, an approach termed “de-biasing.”22-24 Unfortunately, cognitive interventions testing such approaches have suffered mixed results–perhaps because of lack of focus on collective wisdom or group thinking, which may be key to diagnosis from our findings.9,25 In this sense, morning rounds were a social gathering used to strategize and develop care plans, but with limited time to think about diagnosis.26 Introduction of defined periods for individuals to engage in diagnostic activities such as de-biasing (ie, asking “what else could this be)27 before or after rounds may provide an opportunity for reflection and improving diagnosis. In addition, embedding tools such as diagnosis expanders and checklists within these defined time slots28,29 may prove to be useful in reflecting on diagnosis and preventing diagnostic errors.
An unexpected yet important finding from this study were the challenges posed by distractions and the physical environment. Potentially maladaptive workarounds to these interruptions included use of headphones; more productive strategies included updating nurses with plans to avert pages and creating a list of activities to ensure that key tasks were not forgotten.30,31 Applying lessons from aviation, a focused effort to limit distractions during key portions of the day, might be worth considering for diagnostic safety.32 Similarly, improving the environment in which diagnosis occurs—including creating spaces that are quiet, orderly, and optimized for thinking—may be valuable.33Our study has limitations. First, our findings are limited to direct observations; we are thus unable to comment on how unobserved aspects of care (eg, cognitive processes) might have influenced our findings. Our observations of clinical care might also have introduced a Hawthorne effect. However, because we were closely integrated with teams and conducted focus groups to corroborate our assessments, we believe that this was not the case. Second, we did not identify diagnostic errors or link processes we observed to errors. Third, our approach is limited to 2 teaching centers, thereby limiting the generalizability of findings. Relatedly, we were only able to conduct observations during weekdays; differences in weekend and night resources might affect our insights.
The cognitive and system-based barriers faced by clinicians in teaching hospitals suggest that new methods to improve diagnosis are needed. Future interventions such as defined “time-outs” for diagnosis, strategies focused on limiting distractions, and methods to improve communication between team members are novel and have parallels in other industries. As challenges to quantify diagnostic errors abound,34 improving cognitive- and system-based factors via reflection through communication, concentration, and organization is necessary to improve medical decision making in academic medical centers.
Disclosures
None declared for all coauthors.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data. Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). Dr. Singh is partially supported by Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the Department of Veterans Affairs.
1. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. http://www.nap.edu/21794. Accessed November 1; 2016:2015. https://doi.org/10.17226/21794.
2. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. http://dx.doi.org/10.1001/archinternmed.2009.333. PubMed
3. Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: A necropsy study. Lancet. 2000;355(9220):2027-2031. http://dx.doi.org/10.1016/S0140-6736(00)02349-7. PubMed
4. Winters B, Custer J, Galvagno SM Jr, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. http://dx.doi.org/10.1136/bmjqs-2012-000803. PubMed
5. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. http://dx.doi.org/10.1136/bmjqs-2012-001550. PubMed
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77(10):981-992. http://dx.doi.org/10.1097/00001888-200210000-00009. PubMed
7. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2018;27(1):53-60. 10.1136/bmjqs-2017-006774. PubMed
8. van Noord I, Eikens MP, Hamersma AM, de Bruijne MC. Application of root cause analysis on malpractice claim files related to diagnostic failures. Qual Saf Health Care. 2010;19(6):e21. http://dx.doi.org/10.1136/qshc.2008.029801. PubMed
9. Croskerry P, Petrie DA, Reilly JB, Tait G. Deciding about fast and slow decisions. Acad Med. 2014;89(2):197-200. 10.1097/ACM.0000000000000121. PubMed
10. Higginbottom GM, Pillay JJ, Boadu NY. Guidance on performing focused ethnographies with an emphasis on healthcare research. Qual Rep. 2013;18(9):1-6. https://doi.org/10.7939/R35M6287P.
11. Savage J. Participative observation: standing in the shoes of others? Qual Health Res. 2000;10(3):324-339. http://dx.doi.org/10.1177/104973200129118471. PubMed
12. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2002.
13. Harrod M, Weston LE, Robinson C, Tremblay A, Greenstone CL, Forman J. “It goes beyond good camaraderie”: A qualitative study of the process of becoming an interprofessional healthcare “teamlet.” J Interprof Care. 2016;30(3):295-300. http://dx.doi.org/10.3109/13561820.2015.1130028. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. http://dx.doi.org/10.12788/jhm.2763. PubMed
15. Mulhall A. In the field: notes on observation in qualitative research. J Adv Nurs. 2003;41(3):306-313. http://dx.doi.org/10.1046/j.1365-2648.2003.02514.x. PubMed
16. Zwaan L, Monteiro S, Sherbino J, Ilgen J, Howey B, Norman G. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual Saf. 2017;26(2):104-110. http://dx.doi.org/10.1136/bmjqs-2015-005014. PubMed
17. Singh H, Graber ML. Improving diagnosis in health care--the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. http://dx.doi.org/10.1056/NEJMp1512241. PubMed
18. Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448. http://dx.doi.org/10.1056/NEJMp1303712. PubMed
19. van den Berge K, Mamede S. Cognitive diagnostic error in internal medicine. Eur J Intern Med. 2013;24(6):525-529. http://dx.doi.org/10.1016/j.ejim.2013.03.006. PubMed
20. Norman G, Sherbino J, Dore K, et al. The etiology of diagnostic errors: A controlled trial of system 1 versus system 2 reasoning. Acad Med. 2014;89(2):277-284. 10.1097/ACM.0000000000000105 PubMed
21. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. http://dx.doi.org/10.1136/bmjqs-2016-005267. PubMed
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: Origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-iiii64. http://dx.doi.org/10.1136/bmjqs-2012-001712. PubMed
23. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: Impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-iiii72. http://dx.doi.org/10.1136/bmjqs-2012-001713. PubMed
24. Reilly JB, Ogdie AR, Von Feldt JM, Myers JS. Teaching about how doctors think: a longitudinal curriculum in cognitive bias and diagnostic error for residents. BMJ Qual Saf. 2013;22(12):1044-1050. http://dx.doi.org/10.1136/bmjqs-2013-001987. PubMed
25. Schmidt HG, Mamede S, van den Berge K, van Gog T, van Saase JL, Rikers RM. Exposure to media information about a disease can cause doctors to misdiagnose similar-looking clinical cases. Acad Med. 2014;89(2):285-291. http://dx.doi.org/10.1097/ACM.0000000000000107. PubMed
26. Hess BJ, Lipner RS, Thompson V, Holmboe ES, Graber ML. Blink or think: can further reflection improve initial diagnostic impressions? Acad Med. 2015;90(1):112-118. http://dx.doi.org/10.1097/ACM.0000000000000550. PubMed
27. Lambe KA, O’Reilly G, Kelly BD, Curristan S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual Saf. 2016;25(10):808-820. http://dx.doi.org/10.1136/bmjqs-2015-004417. PubMed
28. Graber ML, Kissam S, Payne VL, et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535-557. http://dx.doi.org/10.1136/bmjqs-2011-000149. PubMed
29. McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):381-389. http://dx.doi.org/10.7326/0003-4819-158-5-201303051-00004. PubMed
30. Wray CM, Chaudhry S, Pincavage A, et al. Resident shift handoff strategies in US internal medicine residency programs. JAMA. 2016;316(21):2273-2275. http://dx.doi.org/10.1001/jama.2016.17786. PubMed
31. Choo KJ, Arora VM, Barach P, Johnson JK, Farnan JM. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014;9(3):169-175. http://dx.doi.org/10.1002/jhm.2150. PubMed
32. Carayon P, Wood KE. Patient safety - the role of human factors and systems engineering. Stud Health Technol Inform. 2010;153:23-46.
.http://dx.doi.org/10.1001/jama.2015.13453 PubMed
34. McGlynn EA, McDonald KM, Cassel CK. Measurement is essential for improving diagnosis and reducing diagnostic error: A report from the Institute of Medicine. JAMA. 2015;314(23):2501-2502.
.http://dx.doi.org/10.1136/bmjqs-2013-001812 PubMed
33. Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. BMJ Qual Saf. 2014;23(3):196-205. PubMed
Diagnostic error—defined as a failure to establish an accurate and timely explanation of the patient’s health problem—is an important source of patient harm.1 Data suggest that all patients will experience at least 1 diagnostic error in their lifetime.2-4 Not surprisingly, diagnostic errors are among the leading categories of paid malpractice claims in the United States.5
Despite diagnostic errors being morbid and sometimes deadly in the hospital,6,7 little is known about how residents and learners approach diagnostic decision making. Errors in diagnosis are believed to stem from cognitive or system failures,8 with errors in cognition believed to occur due to rapid, reflexive thinking operating in the absence of a more analytical, deliberate process. System-based problems (eg, lack of expert availability, technology barriers, and access to data) have also been cited as contributors.9 However, whether and how these apply to trainees is not known.
Therefore, we conducted a focused ethnography of inpatient medicine teams (ie, attendings, residents, interns, and medical students) in 2 affiliated teaching hospitals, aiming to (a) observe the process of diagnosis by trainees and (b) identify methods to improve the diagnostic process and prevent errors.
METHODS
We designed a multimethod, focused ethnographic study to examine diagnostic decision making in hospital settings.10,11 In contrast to anthropologic ethnographies that study entire fields using open-ended questions, our study was designed to examine the process of diagnosis from the perspective of clinicians engaged in this activity.11 This approach allowed us to capture diagnostic decisions and cognitive and system-based factors in a manner currently lacking in the literature.12
Setting and Participants
Between January 2016 and May 2016, we observed the members of four inpatient internal medicine teaching teams at 2 affiliated teaching hospitals. We purposefully selected teaching teams for observation because they are the primary model of care in academic settings and we have expertise in carrying out similar studies.13,14 Teaching teams typically consisted of a medical attending (senior-level physician), 1 senior resident (a second- or third-year postgraduate trainee), two interns (a trainee in their first postgraduate year), and two to four medical students. Teams were selected at random using existing schedules and followed Monday to Friday so as to permit observation of work on call and noncall days. Owing to manpower limitations, weekend and night shifts were not observed. However, overnight events were captured during morning rounds.
Most of the teams began rounds at 8:30 AM. Typically, rounds lasted for 90–120 min and concluded with a recap (ie, “running the list”) with a review of explicit plans for patients after they had been evaluated by the attending. This discussion often occurred in the team rooms, with the attending leading the discussion with the trainees.
Data Collection
A multidisciplinary team, including clinicians (eg, physicians, nurses), nonclinicians (eg, qualitative researchers, social scientists), and healthcare engineers, conducted the observations. We observed preround activities of interns and residents before arrival of the attending (7:00 AM - 8:30 AM), followed by morning rounds with the entire team, and afternoon work that included senior residents, interns, and students.
To capture multiple aspects of the diagnostic process, we collected data using field notes modeled on components of the National Academy of Science model for diagnosis (Appendix).1,15 This model encompasses phases of the diagnostic process (eg, data gathering, integration, formulation of a working diagnosis, treatment delivery, and outcomes) and the work system (team members, organization, technology and tools, physical environment, tasks).
Focus Groups and Interviews
At the end of weekly observations, we conducted focus groups with the residents and one-on- one interviews with the attendings. Focus groups with the residents were conducted to encourage a group discussion about the diagnostic process. Separate interviews with the attendings were performed to ensure that power differentials did not influence discussions. During focus groups, we specifically asked about challenges and possible solutions to improve diagnosis. Experienced qualitative methodologists (J.F., M.H., M.Q.) used semistructured interview guides for discussions (Appendix).
Data Analysis
After aggregating and reading the data, three reviewers (V.C., S.K., S.S.) began inductive analysis by handwriting notes and initial reflective thoughts to create preliminary codes. Multiple team members then reread the original field notes and the focus group/interview data to refine the preliminary codes and develop additional codes. Next, relationships between codes were identified and used to develop key themes. Triangulation of data collected from observations and interview/focus group sessions was carried out to compare data that we surmised with data that were verbalized by the team. The developed themes were discussed as a group to ensure consistency of major findings.
Ethical and Regulatory Oversight
This study was reviewed and approved by the Institutional Review Boards at the University of Michigan Health System (HUM-00106657) and the VA Ann Arbor Healthcare System (1-2016-010040).
RESULTS
Four teaching teams (4 attendings, 4 senior residents, 9 interns, and 14 medical students) were observed over 33 distinct shifts and 168 hours. Observations included morning rounds (96 h), postround call days (52 h), and postround non-call days (20 h). Morning rounds lasted an average of 127 min (range: 48-232 min) and included an average of 9 patients (range: 4-16 patients).
Themes Regarding the Diagnostic Process
We identified the following 4 primary themes related to the diagnostic process in teaching hospitals: (1) diagnosis is a social phenomenon; (2) data necessary to make diagnoses are fragmented; (3) distractions undermine the diagnostic process; and (4) time pressures interfere with diagnostic decision making (Appendix Table 1).
(1) Diagnosis is a Social Phenomenon.
Team members viewed the process of diagnosis as a social exchange of facts, findings, and strategies within a defined structure. The opportunity to discuss impressions with others was valued as a means to share, test, and process assumptions.
“Rounds are the most important part of the process. That is where we make most decisions in a collective, collaborative way with the attending present. We bounce ideas off each other.” (Intern)
Typical of social processes, variations based on time of day and schedule were observed. For instance, during call days, learners gathered data and formed working diagnosis and treatment plans with minimal attending interaction. This separation of roles and responsibilities introduced a hierarchy within diagnosis as follows:
“The interns would not call me first; they would talk to the senior resident and then if the senior thought he should chat with me, then they would call. But for the most part, they gather information and come up with the plan.” (Attending).
The work system was suited to facilitate social interactions. For instance, designated rooms (with team members informally assigned to a computer) provided physical proximity of the resident to interns and medical students. In this space, numerous informal discussions between team members (eg, “What do you think about this test?” “I’m not sure what to do about this finding.” “Should I call a [consult] on this patient?”) were observed. Although proximity to each other was viewed as beneficial, dangers to the social nature of diagnosis in the form of anchoring (ie, a cognitive bias where emphasis is placed on the first piece of data)16 were also mentioned. Similarly, the paradox associated with social proof (ie, the pressure to assume conformity within a group) was also observed as disagreement between team members and attendings rarely occurred during observations.
“I mean, they’re the attending, right? It’s hard to argue with them when they want a test or something done. When I do push back, it’s rare that others will support me–so it’s usually me and the attending.” (Resident)
“I would push back if I think it’s really bad for the patient or could cause harm–but the truth is, it doesn’t happen much.” (Intern)
(2) Data Necessary to Make Diagnoses are Fragmented
Team members universally cited fragmentation in data delivery, retrieval, and processing as a barrier to diagnosis. Team members indicated that test results might not be looked at or acted upon in a timely manner, and participants pointed to the electronic medical record as a source of this challenge.
“Before I knew about [the app for Epic], I would literally sit on the computer to get all the information we would need on rounds. Its key to making decisions. We often say we will do something, only to find the test result doesn’t support it–and then we’re back to square 1.” (Intern)
Information used by teams came from myriad sources (eg, patients, family members, electronic records) and from various settings (eg, emergency department, patient rooms, discussions with consultants). Additionally, test results often appeared without warning. Thus, availability of information was poorly aligned with clinical duties.
“They (the lab) will call us when a blood culture is positive or something is off. That is very helpful but it often comes later in the day, when we’re done with rounds.” (Resident)
The work system was highlighted as a key contributor to data fragmentation. Peculiarities of our electronic medical record (EMR) and how data were collected, stored, or presented were described as “frustrating,” and “unsafe,” by team members. Correspondingly, we frequently observed interns asking for assistance for tasks such as ordering tests or finding information despite being “trained” to use the EMR.
“People have to learn how to filter, how to recognize the most important points and link data streams together in terms of causality. But we assume they know where to find that information. It’s actually a very hard thing to do, for both the house staff and me.” (Attending)
(3) Distractions Undermine the Diagnostic Process
Distractions often created cognitive difficulties. For example, ambient noise and interruptions from neighbors working on other teams were cited as barriers to diagnosis. In addition, we observed several team members using headphones to drown out ambient noise while working on the computer.
“I know I shouldn’t do it (wear headphones), but I have no other way of turning down the noise so I can concentrate.” (Intern)
Similarly, the unpredictable nature and the volume of pages often interrupted thinking about diagnosis.
“Sometimes the pager just goes off all the time and (after making sure its not an urgent issue), I will just ignore it for a bit, especially if I am in the middle of something. It would be great if I could finish my thought process knowing I would not be interrupted.” (Resident)
To mitigate this problem, 1 attending described how he would proactively seek out nurses caring for his patients to “head off” questions (eg, “I will renew the restraints and medications this morning,” and “Is there anything you need in terms of orders for this patient that I can take care of now?”) that might lead to pages. Another resident described his approach as follows:
“I make it a point to tell the nurses where I will be hanging out and where they can find me if they have any questions. I tell them to come talk to me rather than page me since that will be less distracting.” (Resident).
Most of the interns described documentation work such as writing admission and progress notes in negative terms (“an academic exercise,” “part of the billing activity”). However, in the context of interruptions, some described this as helpful.
“The most valuable part of the thinking process was writing the assessment and plan because that’s actually my schema for all problems. It literally is the only time where I can sit and collect my thoughts to formulate a diagnosis and plan.” (Intern)
(4) Time Pressures Interfere With Diagnostic Decision Making
All team members spoke about the challenge of finding time for diagnosis during the workday. Often, they had to skip learning sessions for this purpose.
“They tell us we should go to morning report or noon conference but when I’m running around trying to get things done. I hate having to choose between my education and doing what’s best for the patient–but that’s often what it comes down to.” (Intern)
When specifically asked whether setting aside dedicated time to specifically review and formulate diagnoses would be valuable, respondents were uniformly enthusiastic. Team members described attentional conflicts as being the worst when “cross covering” other teams on call days, as their patient load effectively doubled during this time. Of note, cross-covering occurred when teams were also on call—and thus took them away from important diagnostic activities such as data gathering or synthesis for patients they were admitting.
“If you were to ever design a system where errors were likely–this is how you would design it: take a team with little supervision, double their patient load, keep them busy with new challenging cases and then ask questions about patients they know little about.” (Resident)
DISCUSSION
Although diagnostic errors have been called “the next frontier for patient safety,”17 little is known about the process, barriers, and facilitators to diagnosis in teaching hospitals. In this focused ethnography conducted at 2 academic medical centers, we identified multiple cognitive and system-level challenges and potential strategies to improve diagnosis from trainees engaged in this activity. Key themes identified by those we observed included the social nature of diagnosis, fragmented information delivery, constant distractions and interruptions, and time pressures. In turn, these insights allow us to generate strategies that can be applied to improve the diagnostic process in teaching hospitals.
Our study underscores the importance of social interactions in diagnosis. In contrast, most of the interventions to prevent diagnostic errors target individual providers through practices such as metacognition and “thinking about thinking.”18-20 These interventions are based on Daniel Kahnemann’s work on dual thought process. Type 1 thought processes are fast, subconscious, reflexive, largely intuitive, and more vulnerable to error. In contrast, Type 2 processes are slower, deliberate, analytic, and less prone to error.21 Although an individual’s Type 2 thought capacity is limited, a major goal of cognitive interventions is to encourage Type 2 over Type 1 thinking, an approach termed “de-biasing.”22-24 Unfortunately, cognitive interventions testing such approaches have suffered mixed results–perhaps because of lack of focus on collective wisdom or group thinking, which may be key to diagnosis from our findings.9,25 In this sense, morning rounds were a social gathering used to strategize and develop care plans, but with limited time to think about diagnosis.26 Introduction of defined periods for individuals to engage in diagnostic activities such as de-biasing (ie, asking “what else could this be)27 before or after rounds may provide an opportunity for reflection and improving diagnosis. In addition, embedding tools such as diagnosis expanders and checklists within these defined time slots28,29 may prove to be useful in reflecting on diagnosis and preventing diagnostic errors.
An unexpected yet important finding from this study were the challenges posed by distractions and the physical environment. Potentially maladaptive workarounds to these interruptions included use of headphones; more productive strategies included updating nurses with plans to avert pages and creating a list of activities to ensure that key tasks were not forgotten.30,31 Applying lessons from aviation, a focused effort to limit distractions during key portions of the day, might be worth considering for diagnostic safety.32 Similarly, improving the environment in which diagnosis occurs—including creating spaces that are quiet, orderly, and optimized for thinking—may be valuable.33Our study has limitations. First, our findings are limited to direct observations; we are thus unable to comment on how unobserved aspects of care (eg, cognitive processes) might have influenced our findings. Our observations of clinical care might also have introduced a Hawthorne effect. However, because we were closely integrated with teams and conducted focus groups to corroborate our assessments, we believe that this was not the case. Second, we did not identify diagnostic errors or link processes we observed to errors. Third, our approach is limited to 2 teaching centers, thereby limiting the generalizability of findings. Relatedly, we were only able to conduct observations during weekdays; differences in weekend and night resources might affect our insights.
The cognitive and system-based barriers faced by clinicians in teaching hospitals suggest that new methods to improve diagnosis are needed. Future interventions such as defined “time-outs” for diagnosis, strategies focused on limiting distractions, and methods to improve communication between team members are novel and have parallels in other industries. As challenges to quantify diagnostic errors abound,34 improving cognitive- and system-based factors via reflection through communication, concentration, and organization is necessary to improve medical decision making in academic medical centers.
Disclosures
None declared for all coauthors.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data. Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). Dr. Singh is partially supported by Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the Department of Veterans Affairs.
Diagnostic error—defined as a failure to establish an accurate and timely explanation of the patient’s health problem—is an important source of patient harm.1 Data suggest that all patients will experience at least 1 diagnostic error in their lifetime.2-4 Not surprisingly, diagnostic errors are among the leading categories of paid malpractice claims in the United States.5
Despite diagnostic errors being morbid and sometimes deadly in the hospital,6,7 little is known about how residents and learners approach diagnostic decision making. Errors in diagnosis are believed to stem from cognitive or system failures,8 with errors in cognition believed to occur due to rapid, reflexive thinking operating in the absence of a more analytical, deliberate process. System-based problems (eg, lack of expert availability, technology barriers, and access to data) have also been cited as contributors.9 However, whether and how these apply to trainees is not known.
Therefore, we conducted a focused ethnography of inpatient medicine teams (ie, attendings, residents, interns, and medical students) in 2 affiliated teaching hospitals, aiming to (a) observe the process of diagnosis by trainees and (b) identify methods to improve the diagnostic process and prevent errors.
METHODS
We designed a multimethod, focused ethnographic study to examine diagnostic decision making in hospital settings.10,11 In contrast to anthropologic ethnographies that study entire fields using open-ended questions, our study was designed to examine the process of diagnosis from the perspective of clinicians engaged in this activity.11 This approach allowed us to capture diagnostic decisions and cognitive and system-based factors in a manner currently lacking in the literature.12
Setting and Participants
Between January 2016 and May 2016, we observed the members of four inpatient internal medicine teaching teams at 2 affiliated teaching hospitals. We purposefully selected teaching teams for observation because they are the primary model of care in academic settings and we have expertise in carrying out similar studies.13,14 Teaching teams typically consisted of a medical attending (senior-level physician), 1 senior resident (a second- or third-year postgraduate trainee), two interns (a trainee in their first postgraduate year), and two to four medical students. Teams were selected at random using existing schedules and followed Monday to Friday so as to permit observation of work on call and noncall days. Owing to manpower limitations, weekend and night shifts were not observed. However, overnight events were captured during morning rounds.
Most of the teams began rounds at 8:30 AM. Typically, rounds lasted for 90–120 min and concluded with a recap (ie, “running the list”) with a review of explicit plans for patients after they had been evaluated by the attending. This discussion often occurred in the team rooms, with the attending leading the discussion with the trainees.
Data Collection
A multidisciplinary team, including clinicians (eg, physicians, nurses), nonclinicians (eg, qualitative researchers, social scientists), and healthcare engineers, conducted the observations. We observed preround activities of interns and residents before arrival of the attending (7:00 AM - 8:30 AM), followed by morning rounds with the entire team, and afternoon work that included senior residents, interns, and students.
To capture multiple aspects of the diagnostic process, we collected data using field notes modeled on components of the National Academy of Science model for diagnosis (Appendix).1,15 This model encompasses phases of the diagnostic process (eg, data gathering, integration, formulation of a working diagnosis, treatment delivery, and outcomes) and the work system (team members, organization, technology and tools, physical environment, tasks).
Focus Groups and Interviews
At the end of weekly observations, we conducted focus groups with the residents and one-on- one interviews with the attendings. Focus groups with the residents were conducted to encourage a group discussion about the diagnostic process. Separate interviews with the attendings were performed to ensure that power differentials did not influence discussions. During focus groups, we specifically asked about challenges and possible solutions to improve diagnosis. Experienced qualitative methodologists (J.F., M.H., M.Q.) used semistructured interview guides for discussions (Appendix).
Data Analysis
After aggregating and reading the data, three reviewers (V.C., S.K., S.S.) began inductive analysis by handwriting notes and initial reflective thoughts to create preliminary codes. Multiple team members then reread the original field notes and the focus group/interview data to refine the preliminary codes and develop additional codes. Next, relationships between codes were identified and used to develop key themes. Triangulation of data collected from observations and interview/focus group sessions was carried out to compare data that we surmised with data that were verbalized by the team. The developed themes were discussed as a group to ensure consistency of major findings.
Ethical and Regulatory Oversight
This study was reviewed and approved by the Institutional Review Boards at the University of Michigan Health System (HUM-00106657) and the VA Ann Arbor Healthcare System (1-2016-010040).
RESULTS
Four teaching teams (4 attendings, 4 senior residents, 9 interns, and 14 medical students) were observed over 33 distinct shifts and 168 hours. Observations included morning rounds (96 h), postround call days (52 h), and postround non-call days (20 h). Morning rounds lasted an average of 127 min (range: 48-232 min) and included an average of 9 patients (range: 4-16 patients).
Themes Regarding the Diagnostic Process
We identified the following 4 primary themes related to the diagnostic process in teaching hospitals: (1) diagnosis is a social phenomenon; (2) data necessary to make diagnoses are fragmented; (3) distractions undermine the diagnostic process; and (4) time pressures interfere with diagnostic decision making (Appendix Table 1).
(1) Diagnosis is a Social Phenomenon.
Team members viewed the process of diagnosis as a social exchange of facts, findings, and strategies within a defined structure. The opportunity to discuss impressions with others was valued as a means to share, test, and process assumptions.
“Rounds are the most important part of the process. That is where we make most decisions in a collective, collaborative way with the attending present. We bounce ideas off each other.” (Intern)
Typical of social processes, variations based on time of day and schedule were observed. For instance, during call days, learners gathered data and formed working diagnosis and treatment plans with minimal attending interaction. This separation of roles and responsibilities introduced a hierarchy within diagnosis as follows:
“The interns would not call me first; they would talk to the senior resident and then if the senior thought he should chat with me, then they would call. But for the most part, they gather information and come up with the plan.” (Attending).
The work system was suited to facilitate social interactions. For instance, designated rooms (with team members informally assigned to a computer) provided physical proximity of the resident to interns and medical students. In this space, numerous informal discussions between team members (eg, “What do you think about this test?” “I’m not sure what to do about this finding.” “Should I call a [consult] on this patient?”) were observed. Although proximity to each other was viewed as beneficial, dangers to the social nature of diagnosis in the form of anchoring (ie, a cognitive bias where emphasis is placed on the first piece of data)16 were also mentioned. Similarly, the paradox associated with social proof (ie, the pressure to assume conformity within a group) was also observed as disagreement between team members and attendings rarely occurred during observations.
“I mean, they’re the attending, right? It’s hard to argue with them when they want a test or something done. When I do push back, it’s rare that others will support me–so it’s usually me and the attending.” (Resident)
“I would push back if I think it’s really bad for the patient or could cause harm–but the truth is, it doesn’t happen much.” (Intern)
(2) Data Necessary to Make Diagnoses are Fragmented
Team members universally cited fragmentation in data delivery, retrieval, and processing as a barrier to diagnosis. Team members indicated that test results might not be looked at or acted upon in a timely manner, and participants pointed to the electronic medical record as a source of this challenge.
“Before I knew about [the app for Epic], I would literally sit on the computer to get all the information we would need on rounds. Its key to making decisions. We often say we will do something, only to find the test result doesn’t support it–and then we’re back to square 1.” (Intern)
Information used by teams came from myriad sources (eg, patients, family members, electronic records) and from various settings (eg, emergency department, patient rooms, discussions with consultants). Additionally, test results often appeared without warning. Thus, availability of information was poorly aligned with clinical duties.
“They (the lab) will call us when a blood culture is positive or something is off. That is very helpful but it often comes later in the day, when we’re done with rounds.” (Resident)
The work system was highlighted as a key contributor to data fragmentation. Peculiarities of our electronic medical record (EMR) and how data were collected, stored, or presented were described as “frustrating,” and “unsafe,” by team members. Correspondingly, we frequently observed interns asking for assistance for tasks such as ordering tests or finding information despite being “trained” to use the EMR.
“People have to learn how to filter, how to recognize the most important points and link data streams together in terms of causality. But we assume they know where to find that information. It’s actually a very hard thing to do, for both the house staff and me.” (Attending)
(3) Distractions Undermine the Diagnostic Process
Distractions often created cognitive difficulties. For example, ambient noise and interruptions from neighbors working on other teams were cited as barriers to diagnosis. In addition, we observed several team members using headphones to drown out ambient noise while working on the computer.
“I know I shouldn’t do it (wear headphones), but I have no other way of turning down the noise so I can concentrate.” (Intern)
Similarly, the unpredictable nature and the volume of pages often interrupted thinking about diagnosis.
“Sometimes the pager just goes off all the time and (after making sure its not an urgent issue), I will just ignore it for a bit, especially if I am in the middle of something. It would be great if I could finish my thought process knowing I would not be interrupted.” (Resident)
To mitigate this problem, 1 attending described how he would proactively seek out nurses caring for his patients to “head off” questions (eg, “I will renew the restraints and medications this morning,” and “Is there anything you need in terms of orders for this patient that I can take care of now?”) that might lead to pages. Another resident described his approach as follows:
“I make it a point to tell the nurses where I will be hanging out and where they can find me if they have any questions. I tell them to come talk to me rather than page me since that will be less distracting.” (Resident).
Most of the interns described documentation work such as writing admission and progress notes in negative terms (“an academic exercise,” “part of the billing activity”). However, in the context of interruptions, some described this as helpful.
“The most valuable part of the thinking process was writing the assessment and plan because that’s actually my schema for all problems. It literally is the only time where I can sit and collect my thoughts to formulate a diagnosis and plan.” (Intern)
(4) Time Pressures Interfere With Diagnostic Decision Making
All team members spoke about the challenge of finding time for diagnosis during the workday. Often, they had to skip learning sessions for this purpose.
“They tell us we should go to morning report or noon conference but when I’m running around trying to get things done. I hate having to choose between my education and doing what’s best for the patient–but that’s often what it comes down to.” (Intern)
When specifically asked whether setting aside dedicated time to specifically review and formulate diagnoses would be valuable, respondents were uniformly enthusiastic. Team members described attentional conflicts as being the worst when “cross covering” other teams on call days, as their patient load effectively doubled during this time. Of note, cross-covering occurred when teams were also on call—and thus took them away from important diagnostic activities such as data gathering or synthesis for patients they were admitting.
“If you were to ever design a system where errors were likely–this is how you would design it: take a team with little supervision, double their patient load, keep them busy with new challenging cases and then ask questions about patients they know little about.” (Resident)
DISCUSSION
Although diagnostic errors have been called “the next frontier for patient safety,”17 little is known about the process, barriers, and facilitators to diagnosis in teaching hospitals. In this focused ethnography conducted at 2 academic medical centers, we identified multiple cognitive and system-level challenges and potential strategies to improve diagnosis from trainees engaged in this activity. Key themes identified by those we observed included the social nature of diagnosis, fragmented information delivery, constant distractions and interruptions, and time pressures. In turn, these insights allow us to generate strategies that can be applied to improve the diagnostic process in teaching hospitals.
Our study underscores the importance of social interactions in diagnosis. In contrast, most of the interventions to prevent diagnostic errors target individual providers through practices such as metacognition and “thinking about thinking.”18-20 These interventions are based on Daniel Kahnemann’s work on dual thought process. Type 1 thought processes are fast, subconscious, reflexive, largely intuitive, and more vulnerable to error. In contrast, Type 2 processes are slower, deliberate, analytic, and less prone to error.21 Although an individual’s Type 2 thought capacity is limited, a major goal of cognitive interventions is to encourage Type 2 over Type 1 thinking, an approach termed “de-biasing.”22-24 Unfortunately, cognitive interventions testing such approaches have suffered mixed results–perhaps because of lack of focus on collective wisdom or group thinking, which may be key to diagnosis from our findings.9,25 In this sense, morning rounds were a social gathering used to strategize and develop care plans, but with limited time to think about diagnosis.26 Introduction of defined periods for individuals to engage in diagnostic activities such as de-biasing (ie, asking “what else could this be)27 before or after rounds may provide an opportunity for reflection and improving diagnosis. In addition, embedding tools such as diagnosis expanders and checklists within these defined time slots28,29 may prove to be useful in reflecting on diagnosis and preventing diagnostic errors.
An unexpected yet important finding from this study were the challenges posed by distractions and the physical environment. Potentially maladaptive workarounds to these interruptions included use of headphones; more productive strategies included updating nurses with plans to avert pages and creating a list of activities to ensure that key tasks were not forgotten.30,31 Applying lessons from aviation, a focused effort to limit distractions during key portions of the day, might be worth considering for diagnostic safety.32 Similarly, improving the environment in which diagnosis occurs—including creating spaces that are quiet, orderly, and optimized for thinking—may be valuable.33Our study has limitations. First, our findings are limited to direct observations; we are thus unable to comment on how unobserved aspects of care (eg, cognitive processes) might have influenced our findings. Our observations of clinical care might also have introduced a Hawthorne effect. However, because we were closely integrated with teams and conducted focus groups to corroborate our assessments, we believe that this was not the case. Second, we did not identify diagnostic errors or link processes we observed to errors. Third, our approach is limited to 2 teaching centers, thereby limiting the generalizability of findings. Relatedly, we were only able to conduct observations during weekdays; differences in weekend and night resources might affect our insights.
The cognitive and system-based barriers faced by clinicians in teaching hospitals suggest that new methods to improve diagnosis are needed. Future interventions such as defined “time-outs” for diagnosis, strategies focused on limiting distractions, and methods to improve communication between team members are novel and have parallels in other industries. As challenges to quantify diagnostic errors abound,34 improving cognitive- and system-based factors via reflection through communication, concentration, and organization is necessary to improve medical decision making in academic medical centers.
Disclosures
None declared for all coauthors.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data. Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). Dr. Singh is partially supported by Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the Department of Veterans Affairs.
1. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. http://www.nap.edu/21794. Accessed November 1; 2016:2015. https://doi.org/10.17226/21794.
2. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. http://dx.doi.org/10.1001/archinternmed.2009.333. PubMed
3. Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: A necropsy study. Lancet. 2000;355(9220):2027-2031. http://dx.doi.org/10.1016/S0140-6736(00)02349-7. PubMed
4. Winters B, Custer J, Galvagno SM Jr, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. http://dx.doi.org/10.1136/bmjqs-2012-000803. PubMed
5. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. http://dx.doi.org/10.1136/bmjqs-2012-001550. PubMed
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77(10):981-992. http://dx.doi.org/10.1097/00001888-200210000-00009. PubMed
7. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2018;27(1):53-60. 10.1136/bmjqs-2017-006774. PubMed
8. van Noord I, Eikens MP, Hamersma AM, de Bruijne MC. Application of root cause analysis on malpractice claim files related to diagnostic failures. Qual Saf Health Care. 2010;19(6):e21. http://dx.doi.org/10.1136/qshc.2008.029801. PubMed
9. Croskerry P, Petrie DA, Reilly JB, Tait G. Deciding about fast and slow decisions. Acad Med. 2014;89(2):197-200. 10.1097/ACM.0000000000000121. PubMed
10. Higginbottom GM, Pillay JJ, Boadu NY. Guidance on performing focused ethnographies with an emphasis on healthcare research. Qual Rep. 2013;18(9):1-6. https://doi.org/10.7939/R35M6287P.
11. Savage J. Participative observation: standing in the shoes of others? Qual Health Res. 2000;10(3):324-339. http://dx.doi.org/10.1177/104973200129118471. PubMed
12. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2002.
13. Harrod M, Weston LE, Robinson C, Tremblay A, Greenstone CL, Forman J. “It goes beyond good camaraderie”: A qualitative study of the process of becoming an interprofessional healthcare “teamlet.” J Interprof Care. 2016;30(3):295-300. http://dx.doi.org/10.3109/13561820.2015.1130028. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. http://dx.doi.org/10.12788/jhm.2763. PubMed
15. Mulhall A. In the field: notes on observation in qualitative research. J Adv Nurs. 2003;41(3):306-313. http://dx.doi.org/10.1046/j.1365-2648.2003.02514.x. PubMed
16. Zwaan L, Monteiro S, Sherbino J, Ilgen J, Howey B, Norman G. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual Saf. 2017;26(2):104-110. http://dx.doi.org/10.1136/bmjqs-2015-005014. PubMed
17. Singh H, Graber ML. Improving diagnosis in health care--the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. http://dx.doi.org/10.1056/NEJMp1512241. PubMed
18. Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448. http://dx.doi.org/10.1056/NEJMp1303712. PubMed
19. van den Berge K, Mamede S. Cognitive diagnostic error in internal medicine. Eur J Intern Med. 2013;24(6):525-529. http://dx.doi.org/10.1016/j.ejim.2013.03.006. PubMed
20. Norman G, Sherbino J, Dore K, et al. The etiology of diagnostic errors: A controlled trial of system 1 versus system 2 reasoning. Acad Med. 2014;89(2):277-284. 10.1097/ACM.0000000000000105 PubMed
21. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. http://dx.doi.org/10.1136/bmjqs-2016-005267. PubMed
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: Origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-iiii64. http://dx.doi.org/10.1136/bmjqs-2012-001712. PubMed
23. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: Impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-iiii72. http://dx.doi.org/10.1136/bmjqs-2012-001713. PubMed
24. Reilly JB, Ogdie AR, Von Feldt JM, Myers JS. Teaching about how doctors think: a longitudinal curriculum in cognitive bias and diagnostic error for residents. BMJ Qual Saf. 2013;22(12):1044-1050. http://dx.doi.org/10.1136/bmjqs-2013-001987. PubMed
25. Schmidt HG, Mamede S, van den Berge K, van Gog T, van Saase JL, Rikers RM. Exposure to media information about a disease can cause doctors to misdiagnose similar-looking clinical cases. Acad Med. 2014;89(2):285-291. http://dx.doi.org/10.1097/ACM.0000000000000107. PubMed
26. Hess BJ, Lipner RS, Thompson V, Holmboe ES, Graber ML. Blink or think: can further reflection improve initial diagnostic impressions? Acad Med. 2015;90(1):112-118. http://dx.doi.org/10.1097/ACM.0000000000000550. PubMed
27. Lambe KA, O’Reilly G, Kelly BD, Curristan S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual Saf. 2016;25(10):808-820. http://dx.doi.org/10.1136/bmjqs-2015-004417. PubMed
28. Graber ML, Kissam S, Payne VL, et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535-557. http://dx.doi.org/10.1136/bmjqs-2011-000149. PubMed
29. McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):381-389. http://dx.doi.org/10.7326/0003-4819-158-5-201303051-00004. PubMed
30. Wray CM, Chaudhry S, Pincavage A, et al. Resident shift handoff strategies in US internal medicine residency programs. JAMA. 2016;316(21):2273-2275. http://dx.doi.org/10.1001/jama.2016.17786. PubMed
31. Choo KJ, Arora VM, Barach P, Johnson JK, Farnan JM. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014;9(3):169-175. http://dx.doi.org/10.1002/jhm.2150. PubMed
32. Carayon P, Wood KE. Patient safety - the role of human factors and systems engineering. Stud Health Technol Inform. 2010;153:23-46.
.http://dx.doi.org/10.1001/jama.2015.13453 PubMed
34. McGlynn EA, McDonald KM, Cassel CK. Measurement is essential for improving diagnosis and reducing diagnostic error: A report from the Institute of Medicine. JAMA. 2015;314(23):2501-2502.
.http://dx.doi.org/10.1136/bmjqs-2013-001812 PubMed
33. Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. BMJ Qual Saf. 2014;23(3):196-205. PubMed
1. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. http://www.nap.edu/21794. Accessed November 1; 2016:2015. https://doi.org/10.17226/21794.
2. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. http://dx.doi.org/10.1001/archinternmed.2009.333. PubMed
3. Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: A necropsy study. Lancet. 2000;355(9220):2027-2031. http://dx.doi.org/10.1016/S0140-6736(00)02349-7. PubMed
4. Winters B, Custer J, Galvagno SM Jr, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. http://dx.doi.org/10.1136/bmjqs-2012-000803. PubMed
5. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. http://dx.doi.org/10.1136/bmjqs-2012-001550. PubMed
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77(10):981-992. http://dx.doi.org/10.1097/00001888-200210000-00009. PubMed
7. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2018;27(1):53-60. 10.1136/bmjqs-2017-006774. PubMed
8. van Noord I, Eikens MP, Hamersma AM, de Bruijne MC. Application of root cause analysis on malpractice claim files related to diagnostic failures. Qual Saf Health Care. 2010;19(6):e21. http://dx.doi.org/10.1136/qshc.2008.029801. PubMed
9. Croskerry P, Petrie DA, Reilly JB, Tait G. Deciding about fast and slow decisions. Acad Med. 2014;89(2):197-200. 10.1097/ACM.0000000000000121. PubMed
10. Higginbottom GM, Pillay JJ, Boadu NY. Guidance on performing focused ethnographies with an emphasis on healthcare research. Qual Rep. 2013;18(9):1-6. https://doi.org/10.7939/R35M6287P.
11. Savage J. Participative observation: standing in the shoes of others? Qual Health Res. 2000;10(3):324-339. http://dx.doi.org/10.1177/104973200129118471. PubMed
12. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2002.
13. Harrod M, Weston LE, Robinson C, Tremblay A, Greenstone CL, Forman J. “It goes beyond good camaraderie”: A qualitative study of the process of becoming an interprofessional healthcare “teamlet.” J Interprof Care. 2016;30(3):295-300. http://dx.doi.org/10.3109/13561820.2015.1130028. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. http://dx.doi.org/10.12788/jhm.2763. PubMed
15. Mulhall A. In the field: notes on observation in qualitative research. J Adv Nurs. 2003;41(3):306-313. http://dx.doi.org/10.1046/j.1365-2648.2003.02514.x. PubMed
16. Zwaan L, Monteiro S, Sherbino J, Ilgen J, Howey B, Norman G. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual Saf. 2017;26(2):104-110. http://dx.doi.org/10.1136/bmjqs-2015-005014. PubMed
17. Singh H, Graber ML. Improving diagnosis in health care--the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. http://dx.doi.org/10.1056/NEJMp1512241. PubMed
18. Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448. http://dx.doi.org/10.1056/NEJMp1303712. PubMed
19. van den Berge K, Mamede S. Cognitive diagnostic error in internal medicine. Eur J Intern Med. 2013;24(6):525-529. http://dx.doi.org/10.1016/j.ejim.2013.03.006. PubMed
20. Norman G, Sherbino J, Dore K, et al. The etiology of diagnostic errors: A controlled trial of system 1 versus system 2 reasoning. Acad Med. 2014;89(2):277-284. 10.1097/ACM.0000000000000105 PubMed
21. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. http://dx.doi.org/10.1136/bmjqs-2016-005267. PubMed
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: Origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-iiii64. http://dx.doi.org/10.1136/bmjqs-2012-001712. PubMed
23. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: Impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-iiii72. http://dx.doi.org/10.1136/bmjqs-2012-001713. PubMed
24. Reilly JB, Ogdie AR, Von Feldt JM, Myers JS. Teaching about how doctors think: a longitudinal curriculum in cognitive bias and diagnostic error for residents. BMJ Qual Saf. 2013;22(12):1044-1050. http://dx.doi.org/10.1136/bmjqs-2013-001987. PubMed
25. Schmidt HG, Mamede S, van den Berge K, van Gog T, van Saase JL, Rikers RM. Exposure to media information about a disease can cause doctors to misdiagnose similar-looking clinical cases. Acad Med. 2014;89(2):285-291. http://dx.doi.org/10.1097/ACM.0000000000000107. PubMed
26. Hess BJ, Lipner RS, Thompson V, Holmboe ES, Graber ML. Blink or think: can further reflection improve initial diagnostic impressions? Acad Med. 2015;90(1):112-118. http://dx.doi.org/10.1097/ACM.0000000000000550. PubMed
27. Lambe KA, O’Reilly G, Kelly BD, Curristan S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual Saf. 2016;25(10):808-820. http://dx.doi.org/10.1136/bmjqs-2015-004417. PubMed
28. Graber ML, Kissam S, Payne VL, et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535-557. http://dx.doi.org/10.1136/bmjqs-2011-000149. PubMed
29. McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):381-389. http://dx.doi.org/10.7326/0003-4819-158-5-201303051-00004. PubMed
30. Wray CM, Chaudhry S, Pincavage A, et al. Resident shift handoff strategies in US internal medicine residency programs. JAMA. 2016;316(21):2273-2275. http://dx.doi.org/10.1001/jama.2016.17786. PubMed
31. Choo KJ, Arora VM, Barach P, Johnson JK, Farnan JM. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014;9(3):169-175. http://dx.doi.org/10.1002/jhm.2150. PubMed
32. Carayon P, Wood KE. Patient safety - the role of human factors and systems engineering. Stud Health Technol Inform. 2010;153:23-46.
.http://dx.doi.org/10.1001/jama.2015.13453 PubMed
34. McGlynn EA, McDonald KM, Cassel CK. Measurement is essential for improving diagnosis and reducing diagnostic error: A report from the Institute of Medicine. JAMA. 2015;314(23):2501-2502.
.http://dx.doi.org/10.1136/bmjqs-2013-001812 PubMed
33. Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. BMJ Qual Saf. 2014;23(3):196-205. PubMed
© 2018 Society of Hospital Medicine
Perception of Resources Spent on Defensive Medicine and History of Being Sued Among Hospitalists: Results from a National Survey
Annual healthcare costs in the United States are over $3 trillion and are garnering significant national attention.1 The United States spends approximately 2.5 times more per capita on healthcare when compared to other developed nations.2 One source of unnecessary cost in healthcare is defensive medicine. Defensive medicine has been defined by Congress as occurring “when doctors order tests, procedures, or visits, or avoid certain high-risk patients or procedures, primarily (but not necessarily) because of concern about malpractice liability.”3
Though difficult to assess, in 1 study, defensive medicine was estimated to cost $45 billion annually.4 While general agreement exists that physicians practice defensive medicine, the extent of defensive practices and the subsequent impact on healthcare costs remain unclear. This is especially true for a group of clinicians that is rapidly increasing in number: hospitalists. Currently, there are more than 50,000 hospitalists in the United States,5 yet the prevalence of defensive medicine in this relatively new specialty is unknown. Inpatient care is complex and time constraints can impede establishing an optimal therapeutic relationship with the patient, potentially raising liability fears. We therefore sought to quantify hospitalist physician estimates of the cost of defensive medicine and assess correlates of their estimates. As being sued might spur defensive behaviors, we also assessed how many hospitalists reported being sued and whether this was associated with their estimates of defensive medicine.
METHODS
Survey Questionnaire
In a previously published survey-based analysis, we reported on physician practice and overuse for 2 common scenarios in hospital medicine: preoperative evaluation and management of uncomplicated syncope.6 After responding to the vignettes, each physician was asked to provide demographic and employment information and malpractice history. In addition, they were asked the following: In your best estimation, what percentage of healthcare-related resources (eg, hospital admissions, diagnostic testing, treatment) are spent purely because of defensive medicine concerns? __________% resources
Survey Sample & Administration
The survey was sent to a sample of 1753 hospitalists, randomly identified through the Society of Hospital Medicine’s (SHM) database of members and annual meeting attendees. It is estimated that almost 30% of practicing hospitalists in the United States are members of the SHM.5 A full description of the sampling methodology was previously published.6 Selected hospitalists were mailed surveys, a $20 financial incentive, and subsequent reminders between June and October 2011.
The study was exempted from institutional review board review by the University of Michigan and the VA Ann Arbor Healthcare System.
Variables
The primary outcome of interest was the response to the “% resources” estimated to be spent on defensive medicine. This was analyzed as a continuous variable. Independent variables included the following: VA employment, malpractice insurance payer, employer, history of malpractice lawsuit, sex, race, and years practicing as a physician.
Statistical Analysis
Analyses were conducted using SAS, version 9.4 (SAS Institute). Descriptive statistics were first calculated for all variables. Next, bivariable comparisons between the outcome variables and other variables of interest were performed. Multivariable comparisons were made using linear regression for the outcome of estimated resources spent on defensive medicine. A P value of < 0.05 was considered statistically significant.
RESULTS
Of the 1753 surveys mailed, 253 were excluded due to incorrect addresses or because the recipients were not practicing hospitalists. A total of 1020 were completed and returned, yielding a 68% response rate (1020 out of 1500 eligible). The hospitalist respondents were in practice for an average of 11 years (range 1-40 years). Respondents represented all 50 states and had a diverse background of experience and demographic characteristics, which has been previously described.6
Resources Estimated Spent on Defensive Medicine
Hospitalists reported, on average, that they believed defensive medicine accounted for 37.5% (standard deviation, 20.2%) of all healthcare spending. Results from the multivariable regression are presented in the Table. Hospitalists affiliated with a VA hospital reported 5.5% less in resources spent on defensive medicine than those not affiliated with a VA hospital (32.2% VA vs 37.7% non-VA, P = 0.025). For every 10 years in practice, the estimate of resources spent on defensive medicine decreased by 3% (P = 0.003). Those who were male (36.4% male vs 39.4% female, P = 0.023) and non-Hispanic white (32.5% non-Hispanic white vs 44.7% other, P ≤ 0.001) also estimated less resources spent on defensive medicine. We did not find an association between a hospitalist reporting being sued and their perception of resources spent on defensive medicine.
Risk of Being Sued
Over a quarter of our sample (25.6%) reported having been sued at least once for medical malpractice. The proportion of hospitalists that reported a history of being sued generally increased with more years of practice (Figure). For those who had been in practice for at least 20 years, more than half (55%) had been sued at least once during their career.
DISCUSSION
In a national survey, hospitalists estimated that almost 40% of all healthcare-related resources are spent purely because of defensive medicine concerns. This estimate was affected by personal demographic and employment factors. Our second major finding is that over one-quarter of a large random sample of hospitalist physicians reported being sued for malpractice.
Hospitalist perceptions of defensive medicine varied significantly based on employment at a VA hospital, with VA-affiliated hospitalists reporting less estimated spending on defensive medicine. This effect may reflect a less litigious environment within the VA, even though physicians practicing within the VA can be reported to the National Practitioner Data Bank.7 The different environment may be due to the VA’s patient mix (VA patients tend to be poorer, older, sicker, and have more mental illness)8; however, it could also be due to its de facto practice of a form of enterprise liability, in which, by law, the VA assumes responsibility for negligence, sheltering its physicians from direct liability.
We also found that the higher the number of years a hospitalist reported practicing, the lower the perception of resources being spent on defensive medicine. The reason for this finding is unclear. There has been a recent focus on high-value care and overspending, and perhaps younger hospitalists are more aware of these initiatives and thus have higher estimates. Additionally, non-Hispanic white male respondents estimated a lower amount spent on defensive medicine compared with other respondents. This is consistent with previous studies of risk perception which have noted a “white male effect” in which white males generally perceive a wide range of risks to be lower than female and non-white individuals, likely due to sociopolitical factors.9 Here, the white male effect is particularly interesting, considering that male physicians are almost 2.5 times as likely as female physicians to report being sued.10
Similar to prior studies,11 there was no association with personal liability claim experience and perceived resources spent on defensive medicine. It is unclear why personal experience of being sued does not appear to be associated with perceptions of defensive medicine practice. It is possible that the fear of being sued is worse than the actual experience or that physicians believe that lawsuits are either random events or inevitable and, as a result, do not change their practice patterns.
The lifetime risk of being named in a malpractice suit is substantial for hospitalists: in our study, over half of hospitalists in practice for 20 years or more reported they had been sued. This corresponds with the projection made by Jena and colleagues,12 which estimated that 55% of internal medicine physicians will be sued by the age of 45, a number just slightly higher than the average for all physicians.
Our study has important limitations. Our sample was of hospitalists and therefore may not be reflective of other medical specialties. Second, due to the nature of the study design, the responses to spending on defensive medicine may not represent actual practice. Third, we did not confirm details such as place of employment or history of lawsuit, and this may be subject to recall bias. However, physicians are unlikely to forget having been sued. Finally, this survey is observational and cross-sectional. Our data imply association rather than causation. Without longitudinal data, it is impossible to know if years of practice correlate with perceived defensive medicine spending due to a generational effect or a longitudinal effect (such as more confidence in diagnostic skills with more years of practice).
Despite these limitations, our survey has important policy implications. First, we found that defensive medicine is perceived by hospitalists to be costly. Although physicians likely overestimated the cost (37.5%, or an estimated $1 trillion is far higher than previous estimates of approximately 2% of all healthcare spending),4 it also demonstrates the extent to which physicians feel as though the medical care that is provided may be unnecessary. Second, at least a quarter of hospitalist physicians have been sued, and the risk of being named as a defendant in a lawsuit increases the longer they have been in clinical practice.
Given these findings, policies aimed to reduce the practice of defensive medicine may help the rising costs of healthcare. Reducing defensive medicine requires decreasing physician fears of liability and related reporting. Traditional tort reforms (with the exception of damage caps) have not been proven to do this. And damage caps can be inequitable, hard to pass, and even found to be unconstitutional in some states.13 However, other reform options hold promise in reducing liability fears, including enterprise liability, safe harbor legislation, and health courts.13 Finally, shared decision-making models may also provide a method to reduce defensive fears as well.6
Acknowledgments
The authors thank the Society of Hospital Medicine, Dr. Scott Flanders, Andrew Hickner, and David Ratz for their assistance with this project.
Disclosure
The authors received financial support from the Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs Health Services Research and Development Center for Clinical Management Research, the University of Michigan Specialist-Hospitalist Allied Research Program, and the Ann Arbor University of Michigan VA Patient Safety Enhancement Program.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs, or the Society of Hospital Medicine.
1. Centers for Medicare & Medicaid Services. National Health Expenditures 2014 Highlights. 2015; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed on July 28, 2016.
2. OECD. Health expenditure per capita. Health at a Glance 2015. Paris: OECD Publishing; 2015.
3. U.S. Congress, Office of Technology Assessment. Defensive Medicine and Medical Malpractice. Washington, DC: U.S. Government Printing Office; July 1994. OTA-H-602.
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. PubMed
5. Society of Hospital Medicine. Society of Hospital Medicine: Membership. 2017; http://www.hospitalmedicine.org/Web/Membership/Web/Membership/Membership_Landing_Page.aspx?hkey=97f40c85-fdcd-411f-b3f6-e617bc38a2c5. Accessed on January 5, 2017.
6. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. PubMed
7. Pugatch MB. Federal tort claims and military medical malpractice. J Legal Nurse Consulting. 2008;19(2):3-6.
8. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Santa Monica, CA: RAND Corporation; 2015. PubMed
9. Finucane ML, Slovic P, Mertz CK, Flynn J, Satterfield TA. Gender, race, and perceived risk: the ‘white male’ effect. Health, Risk & Society. 2000;2(2):159-172.
10. Unwin E, Woolf K, Wadlow C, Potts HW, Dacre J. Sex differences in medico-legal action against doctors: a systematic review and meta-analysis. BMC Med. 2015;13:172. PubMed
11. Glassman PA, Rolph JE, Petersen LP, Bradley MA, Kravitz RL. Physicians’ personal malpractice experiences are not related to defensive clinical practices. J Health Polit Policy Law. 1996;21(2):219-241. PubMed
12. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636. PubMed
13. Mello MM, Studdert DM, Kachalia A. The medical liability climate and prospects for reform. JAMA. 2014;312(20):2146-2155. PubMed
Annual healthcare costs in the United States are over $3 trillion and are garnering significant national attention.1 The United States spends approximately 2.5 times more per capita on healthcare when compared to other developed nations.2 One source of unnecessary cost in healthcare is defensive medicine. Defensive medicine has been defined by Congress as occurring “when doctors order tests, procedures, or visits, or avoid certain high-risk patients or procedures, primarily (but not necessarily) because of concern about malpractice liability.”3
Though difficult to assess, in 1 study, defensive medicine was estimated to cost $45 billion annually.4 While general agreement exists that physicians practice defensive medicine, the extent of defensive practices and the subsequent impact on healthcare costs remain unclear. This is especially true for a group of clinicians that is rapidly increasing in number: hospitalists. Currently, there are more than 50,000 hospitalists in the United States,5 yet the prevalence of defensive medicine in this relatively new specialty is unknown. Inpatient care is complex and time constraints can impede establishing an optimal therapeutic relationship with the patient, potentially raising liability fears. We therefore sought to quantify hospitalist physician estimates of the cost of defensive medicine and assess correlates of their estimates. As being sued might spur defensive behaviors, we also assessed how many hospitalists reported being sued and whether this was associated with their estimates of defensive medicine.
METHODS
Survey Questionnaire
In a previously published survey-based analysis, we reported on physician practice and overuse for 2 common scenarios in hospital medicine: preoperative evaluation and management of uncomplicated syncope.6 After responding to the vignettes, each physician was asked to provide demographic and employment information and malpractice history. In addition, they were asked the following: In your best estimation, what percentage of healthcare-related resources (eg, hospital admissions, diagnostic testing, treatment) are spent purely because of defensive medicine concerns? __________% resources
Survey Sample & Administration
The survey was sent to a sample of 1753 hospitalists, randomly identified through the Society of Hospital Medicine’s (SHM) database of members and annual meeting attendees. It is estimated that almost 30% of practicing hospitalists in the United States are members of the SHM.5 A full description of the sampling methodology was previously published.6 Selected hospitalists were mailed surveys, a $20 financial incentive, and subsequent reminders between June and October 2011.
The study was exempted from institutional review board review by the University of Michigan and the VA Ann Arbor Healthcare System.
Variables
The primary outcome of interest was the response to the “% resources” estimated to be spent on defensive medicine. This was analyzed as a continuous variable. Independent variables included the following: VA employment, malpractice insurance payer, employer, history of malpractice lawsuit, sex, race, and years practicing as a physician.
Statistical Analysis
Analyses were conducted using SAS, version 9.4 (SAS Institute). Descriptive statistics were first calculated for all variables. Next, bivariable comparisons between the outcome variables and other variables of interest were performed. Multivariable comparisons were made using linear regression for the outcome of estimated resources spent on defensive medicine. A P value of < 0.05 was considered statistically significant.
RESULTS
Of the 1753 surveys mailed, 253 were excluded due to incorrect addresses or because the recipients were not practicing hospitalists. A total of 1020 were completed and returned, yielding a 68% response rate (1020 out of 1500 eligible). The hospitalist respondents were in practice for an average of 11 years (range 1-40 years). Respondents represented all 50 states and had a diverse background of experience and demographic characteristics, which has been previously described.6
Resources Estimated Spent on Defensive Medicine
Hospitalists reported, on average, that they believed defensive medicine accounted for 37.5% (standard deviation, 20.2%) of all healthcare spending. Results from the multivariable regression are presented in the Table. Hospitalists affiliated with a VA hospital reported 5.5% less in resources spent on defensive medicine than those not affiliated with a VA hospital (32.2% VA vs 37.7% non-VA, P = 0.025). For every 10 years in practice, the estimate of resources spent on defensive medicine decreased by 3% (P = 0.003). Those who were male (36.4% male vs 39.4% female, P = 0.023) and non-Hispanic white (32.5% non-Hispanic white vs 44.7% other, P ≤ 0.001) also estimated less resources spent on defensive medicine. We did not find an association between a hospitalist reporting being sued and their perception of resources spent on defensive medicine.
Risk of Being Sued
Over a quarter of our sample (25.6%) reported having been sued at least once for medical malpractice. The proportion of hospitalists that reported a history of being sued generally increased with more years of practice (Figure). For those who had been in practice for at least 20 years, more than half (55%) had been sued at least once during their career.
DISCUSSION
In a national survey, hospitalists estimated that almost 40% of all healthcare-related resources are spent purely because of defensive medicine concerns. This estimate was affected by personal demographic and employment factors. Our second major finding is that over one-quarter of a large random sample of hospitalist physicians reported being sued for malpractice.
Hospitalist perceptions of defensive medicine varied significantly based on employment at a VA hospital, with VA-affiliated hospitalists reporting less estimated spending on defensive medicine. This effect may reflect a less litigious environment within the VA, even though physicians practicing within the VA can be reported to the National Practitioner Data Bank.7 The different environment may be due to the VA’s patient mix (VA patients tend to be poorer, older, sicker, and have more mental illness)8; however, it could also be due to its de facto practice of a form of enterprise liability, in which, by law, the VA assumes responsibility for negligence, sheltering its physicians from direct liability.
We also found that the higher the number of years a hospitalist reported practicing, the lower the perception of resources being spent on defensive medicine. The reason for this finding is unclear. There has been a recent focus on high-value care and overspending, and perhaps younger hospitalists are more aware of these initiatives and thus have higher estimates. Additionally, non-Hispanic white male respondents estimated a lower amount spent on defensive medicine compared with other respondents. This is consistent with previous studies of risk perception which have noted a “white male effect” in which white males generally perceive a wide range of risks to be lower than female and non-white individuals, likely due to sociopolitical factors.9 Here, the white male effect is particularly interesting, considering that male physicians are almost 2.5 times as likely as female physicians to report being sued.10
Similar to prior studies,11 there was no association with personal liability claim experience and perceived resources spent on defensive medicine. It is unclear why personal experience of being sued does not appear to be associated with perceptions of defensive medicine practice. It is possible that the fear of being sued is worse than the actual experience or that physicians believe that lawsuits are either random events or inevitable and, as a result, do not change their practice patterns.
The lifetime risk of being named in a malpractice suit is substantial for hospitalists: in our study, over half of hospitalists in practice for 20 years or more reported they had been sued. This corresponds with the projection made by Jena and colleagues,12 which estimated that 55% of internal medicine physicians will be sued by the age of 45, a number just slightly higher than the average for all physicians.
Our study has important limitations. Our sample was of hospitalists and therefore may not be reflective of other medical specialties. Second, due to the nature of the study design, the responses to spending on defensive medicine may not represent actual practice. Third, we did not confirm details such as place of employment or history of lawsuit, and this may be subject to recall bias. However, physicians are unlikely to forget having been sued. Finally, this survey is observational and cross-sectional. Our data imply association rather than causation. Without longitudinal data, it is impossible to know if years of practice correlate with perceived defensive medicine spending due to a generational effect or a longitudinal effect (such as more confidence in diagnostic skills with more years of practice).
Despite these limitations, our survey has important policy implications. First, we found that defensive medicine is perceived by hospitalists to be costly. Although physicians likely overestimated the cost (37.5%, or an estimated $1 trillion is far higher than previous estimates of approximately 2% of all healthcare spending),4 it also demonstrates the extent to which physicians feel as though the medical care that is provided may be unnecessary. Second, at least a quarter of hospitalist physicians have been sued, and the risk of being named as a defendant in a lawsuit increases the longer they have been in clinical practice.
Given these findings, policies aimed to reduce the practice of defensive medicine may help the rising costs of healthcare. Reducing defensive medicine requires decreasing physician fears of liability and related reporting. Traditional tort reforms (with the exception of damage caps) have not been proven to do this. And damage caps can be inequitable, hard to pass, and even found to be unconstitutional in some states.13 However, other reform options hold promise in reducing liability fears, including enterprise liability, safe harbor legislation, and health courts.13 Finally, shared decision-making models may also provide a method to reduce defensive fears as well.6
Acknowledgments
The authors thank the Society of Hospital Medicine, Dr. Scott Flanders, Andrew Hickner, and David Ratz for their assistance with this project.
Disclosure
The authors received financial support from the Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs Health Services Research and Development Center for Clinical Management Research, the University of Michigan Specialist-Hospitalist Allied Research Program, and the Ann Arbor University of Michigan VA Patient Safety Enhancement Program.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs, or the Society of Hospital Medicine.
Annual healthcare costs in the United States are over $3 trillion and are garnering significant national attention.1 The United States spends approximately 2.5 times more per capita on healthcare when compared to other developed nations.2 One source of unnecessary cost in healthcare is defensive medicine. Defensive medicine has been defined by Congress as occurring “when doctors order tests, procedures, or visits, or avoid certain high-risk patients or procedures, primarily (but not necessarily) because of concern about malpractice liability.”3
Though difficult to assess, in 1 study, defensive medicine was estimated to cost $45 billion annually.4 While general agreement exists that physicians practice defensive medicine, the extent of defensive practices and the subsequent impact on healthcare costs remain unclear. This is especially true for a group of clinicians that is rapidly increasing in number: hospitalists. Currently, there are more than 50,000 hospitalists in the United States,5 yet the prevalence of defensive medicine in this relatively new specialty is unknown. Inpatient care is complex and time constraints can impede establishing an optimal therapeutic relationship with the patient, potentially raising liability fears. We therefore sought to quantify hospitalist physician estimates of the cost of defensive medicine and assess correlates of their estimates. As being sued might spur defensive behaviors, we also assessed how many hospitalists reported being sued and whether this was associated with their estimates of defensive medicine.
METHODS
Survey Questionnaire
In a previously published survey-based analysis, we reported on physician practice and overuse for 2 common scenarios in hospital medicine: preoperative evaluation and management of uncomplicated syncope.6 After responding to the vignettes, each physician was asked to provide demographic and employment information and malpractice history. In addition, they were asked the following: In your best estimation, what percentage of healthcare-related resources (eg, hospital admissions, diagnostic testing, treatment) are spent purely because of defensive medicine concerns? __________% resources
Survey Sample & Administration
The survey was sent to a sample of 1753 hospitalists, randomly identified through the Society of Hospital Medicine’s (SHM) database of members and annual meeting attendees. It is estimated that almost 30% of practicing hospitalists in the United States are members of the SHM.5 A full description of the sampling methodology was previously published.6 Selected hospitalists were mailed surveys, a $20 financial incentive, and subsequent reminders between June and October 2011.
The study was exempted from institutional review board review by the University of Michigan and the VA Ann Arbor Healthcare System.
Variables
The primary outcome of interest was the response to the “% resources” estimated to be spent on defensive medicine. This was analyzed as a continuous variable. Independent variables included the following: VA employment, malpractice insurance payer, employer, history of malpractice lawsuit, sex, race, and years practicing as a physician.
Statistical Analysis
Analyses were conducted using SAS, version 9.4 (SAS Institute). Descriptive statistics were first calculated for all variables. Next, bivariable comparisons between the outcome variables and other variables of interest were performed. Multivariable comparisons were made using linear regression for the outcome of estimated resources spent on defensive medicine. A P value of < 0.05 was considered statistically significant.
RESULTS
Of the 1753 surveys mailed, 253 were excluded due to incorrect addresses or because the recipients were not practicing hospitalists. A total of 1020 were completed and returned, yielding a 68% response rate (1020 out of 1500 eligible). The hospitalist respondents were in practice for an average of 11 years (range 1-40 years). Respondents represented all 50 states and had a diverse background of experience and demographic characteristics, which has been previously described.6
Resources Estimated Spent on Defensive Medicine
Hospitalists reported, on average, that they believed defensive medicine accounted for 37.5% (standard deviation, 20.2%) of all healthcare spending. Results from the multivariable regression are presented in the Table. Hospitalists affiliated with a VA hospital reported 5.5% less in resources spent on defensive medicine than those not affiliated with a VA hospital (32.2% VA vs 37.7% non-VA, P = 0.025). For every 10 years in practice, the estimate of resources spent on defensive medicine decreased by 3% (P = 0.003). Those who were male (36.4% male vs 39.4% female, P = 0.023) and non-Hispanic white (32.5% non-Hispanic white vs 44.7% other, P ≤ 0.001) also estimated less resources spent on defensive medicine. We did not find an association between a hospitalist reporting being sued and their perception of resources spent on defensive medicine.
Risk of Being Sued
Over a quarter of our sample (25.6%) reported having been sued at least once for medical malpractice. The proportion of hospitalists that reported a history of being sued generally increased with more years of practice (Figure). For those who had been in practice for at least 20 years, more than half (55%) had been sued at least once during their career.
DISCUSSION
In a national survey, hospitalists estimated that almost 40% of all healthcare-related resources are spent purely because of defensive medicine concerns. This estimate was affected by personal demographic and employment factors. Our second major finding is that over one-quarter of a large random sample of hospitalist physicians reported being sued for malpractice.
Hospitalist perceptions of defensive medicine varied significantly based on employment at a VA hospital, with VA-affiliated hospitalists reporting less estimated spending on defensive medicine. This effect may reflect a less litigious environment within the VA, even though physicians practicing within the VA can be reported to the National Practitioner Data Bank.7 The different environment may be due to the VA’s patient mix (VA patients tend to be poorer, older, sicker, and have more mental illness)8; however, it could also be due to its de facto practice of a form of enterprise liability, in which, by law, the VA assumes responsibility for negligence, sheltering its physicians from direct liability.
We also found that the higher the number of years a hospitalist reported practicing, the lower the perception of resources being spent on defensive medicine. The reason for this finding is unclear. There has been a recent focus on high-value care and overspending, and perhaps younger hospitalists are more aware of these initiatives and thus have higher estimates. Additionally, non-Hispanic white male respondents estimated a lower amount spent on defensive medicine compared with other respondents. This is consistent with previous studies of risk perception which have noted a “white male effect” in which white males generally perceive a wide range of risks to be lower than female and non-white individuals, likely due to sociopolitical factors.9 Here, the white male effect is particularly interesting, considering that male physicians are almost 2.5 times as likely as female physicians to report being sued.10
Similar to prior studies,11 there was no association with personal liability claim experience and perceived resources spent on defensive medicine. It is unclear why personal experience of being sued does not appear to be associated with perceptions of defensive medicine practice. It is possible that the fear of being sued is worse than the actual experience or that physicians believe that lawsuits are either random events or inevitable and, as a result, do not change their practice patterns.
The lifetime risk of being named in a malpractice suit is substantial for hospitalists: in our study, over half of hospitalists in practice for 20 years or more reported they had been sued. This corresponds with the projection made by Jena and colleagues,12 which estimated that 55% of internal medicine physicians will be sued by the age of 45, a number just slightly higher than the average for all physicians.
Our study has important limitations. Our sample was of hospitalists and therefore may not be reflective of other medical specialties. Second, due to the nature of the study design, the responses to spending on defensive medicine may not represent actual practice. Third, we did not confirm details such as place of employment or history of lawsuit, and this may be subject to recall bias. However, physicians are unlikely to forget having been sued. Finally, this survey is observational and cross-sectional. Our data imply association rather than causation. Without longitudinal data, it is impossible to know if years of practice correlate with perceived defensive medicine spending due to a generational effect or a longitudinal effect (such as more confidence in diagnostic skills with more years of practice).
Despite these limitations, our survey has important policy implications. First, we found that defensive medicine is perceived by hospitalists to be costly. Although physicians likely overestimated the cost (37.5%, or an estimated $1 trillion is far higher than previous estimates of approximately 2% of all healthcare spending),4 it also demonstrates the extent to which physicians feel as though the medical care that is provided may be unnecessary. Second, at least a quarter of hospitalist physicians have been sued, and the risk of being named as a defendant in a lawsuit increases the longer they have been in clinical practice.
Given these findings, policies aimed to reduce the practice of defensive medicine may help the rising costs of healthcare. Reducing defensive medicine requires decreasing physician fears of liability and related reporting. Traditional tort reforms (with the exception of damage caps) have not been proven to do this. And damage caps can be inequitable, hard to pass, and even found to be unconstitutional in some states.13 However, other reform options hold promise in reducing liability fears, including enterprise liability, safe harbor legislation, and health courts.13 Finally, shared decision-making models may also provide a method to reduce defensive fears as well.6
Acknowledgments
The authors thank the Society of Hospital Medicine, Dr. Scott Flanders, Andrew Hickner, and David Ratz for their assistance with this project.
Disclosure
The authors received financial support from the Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs Health Services Research and Development Center for Clinical Management Research, the University of Michigan Specialist-Hospitalist Allied Research Program, and the Ann Arbor University of Michigan VA Patient Safety Enhancement Program.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs, or the Society of Hospital Medicine.
1. Centers for Medicare & Medicaid Services. National Health Expenditures 2014 Highlights. 2015; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed on July 28, 2016.
2. OECD. Health expenditure per capita. Health at a Glance 2015. Paris: OECD Publishing; 2015.
3. U.S. Congress, Office of Technology Assessment. Defensive Medicine and Medical Malpractice. Washington, DC: U.S. Government Printing Office; July 1994. OTA-H-602.
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. PubMed
5. Society of Hospital Medicine. Society of Hospital Medicine: Membership. 2017; http://www.hospitalmedicine.org/Web/Membership/Web/Membership/Membership_Landing_Page.aspx?hkey=97f40c85-fdcd-411f-b3f6-e617bc38a2c5. Accessed on January 5, 2017.
6. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. PubMed
7. Pugatch MB. Federal tort claims and military medical malpractice. J Legal Nurse Consulting. 2008;19(2):3-6.
8. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Santa Monica, CA: RAND Corporation; 2015. PubMed
9. Finucane ML, Slovic P, Mertz CK, Flynn J, Satterfield TA. Gender, race, and perceived risk: the ‘white male’ effect. Health, Risk & Society. 2000;2(2):159-172.
10. Unwin E, Woolf K, Wadlow C, Potts HW, Dacre J. Sex differences in medico-legal action against doctors: a systematic review and meta-analysis. BMC Med. 2015;13:172. PubMed
11. Glassman PA, Rolph JE, Petersen LP, Bradley MA, Kravitz RL. Physicians’ personal malpractice experiences are not related to defensive clinical practices. J Health Polit Policy Law. 1996;21(2):219-241. PubMed
12. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636. PubMed
13. Mello MM, Studdert DM, Kachalia A. The medical liability climate and prospects for reform. JAMA. 2014;312(20):2146-2155. PubMed
1. Centers for Medicare & Medicaid Services. National Health Expenditures 2014 Highlights. 2015; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed on July 28, 2016.
2. OECD. Health expenditure per capita. Health at a Glance 2015. Paris: OECD Publishing; 2015.
3. U.S. Congress, Office of Technology Assessment. Defensive Medicine and Medical Malpractice. Washington, DC: U.S. Government Printing Office; July 1994. OTA-H-602.
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. PubMed
5. Society of Hospital Medicine. Society of Hospital Medicine: Membership. 2017; http://www.hospitalmedicine.org/Web/Membership/Web/Membership/Membership_Landing_Page.aspx?hkey=97f40c85-fdcd-411f-b3f6-e617bc38a2c5. Accessed on January 5, 2017.
6. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. PubMed
7. Pugatch MB. Federal tort claims and military medical malpractice. J Legal Nurse Consulting. 2008;19(2):3-6.
8. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Santa Monica, CA: RAND Corporation; 2015. PubMed
9. Finucane ML, Slovic P, Mertz CK, Flynn J, Satterfield TA. Gender, race, and perceived risk: the ‘white male’ effect. Health, Risk & Society. 2000;2(2):159-172.
10. Unwin E, Woolf K, Wadlow C, Potts HW, Dacre J. Sex differences in medico-legal action against doctors: a systematic review and meta-analysis. BMC Med. 2015;13:172. PubMed
11. Glassman PA, Rolph JE, Petersen LP, Bradley MA, Kravitz RL. Physicians’ personal malpractice experiences are not related to defensive clinical practices. J Health Polit Policy Law. 1996;21(2):219-241. PubMed
12. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636. PubMed
13. Mello MM, Studdert DM, Kachalia A. The medical liability climate and prospects for reform. JAMA. 2014;312(20):2146-2155. PubMed
© 2018 Society of Hospital Medicine
How Exemplary Teaching Physicians Interact with Hospitalized Patients
Approximately a century ago, Francis Peabody taught that “the secret of the care of the patient is in caring for the patient.”1 His advice remains true today. Despite the advent of novel diagnostic tests, technologically sophisticated interventional procedures, and life-saving medications, perhaps the most important skill a bedside clinician can use is the ability to connect with patients.
The literature on patient-physician interaction is vast2-11 and generally indicates that exemplary bedside clinicians are able to interact well with patients by being competent, trustworthy, personable, empathetic, and effective communicators. “Etiquette-based medicine,” first proposed by Kahn,12 emphasizes the importance of certain behaviors from physicians, such as introducing yourself and
Yet, improving patient-physician interactions remains necessary. A recent systematic review reported that almost half of the reviewed studies on the patient-physician relationship published between 2000 and 2014 conveyed the idea that the patient-physician relationship is deteriorating.13
As part of a broader study to understand the behaviors and approaches of exemplary inpatient attending physicians,14-16 we examined how 12 carefully selected physicians interacted with their patients during inpatient teaching rounds.
METHODS
Overview
We conducted a multisite study using an exploratory, qualitative approach to inquiry, which has been described previously.14-16 Our primary purpose was to study the attributes and behaviors of outstanding general medicine attendings in the setting of inpatient rounds. The focus of this article is on the attendings’ interactions with patients.
We used a modified snowball sampling approach17 to identify 12 exemplary physicians. First, we contacted individuals throughout the United States who were known to the principal investigator (S.S.) and asked for suggestions of excellent clinician educators (also referred to as attendings) for potential inclusion in the study. In addition to these personal contacts, other individuals unknown to the investigative team were contacted and asked to provide suggestions for attendings to include in the study. Specifically, the US News & World Report 2015 Top Medical Schools: Research Rankings,18 which are widely used to represent the best U.S. hospitals, were reviewed in an effort to identify attendings from a broad range of medical schools. Using this list, we identified other medical schools that were in the top 25 and were not already represented. We contacted the division chiefs of general internal (or hospital) medicine, chairs and chiefs of departments of internal medicine, and internal medicine residency program directors from these medical schools and asked for recommendations of attendings from both within and outside their institutions whom they considered to be great inpatient teachers.
This sampling method resulted in 59 potential participants. An internet search was conducted on each potential participant to obtain further information about the individuals and their institutions. Both personal characteristics (medical education, training, and educational awards) and organizational characteristics (geographic location, hospital size and affiliation, and patient population) were considered so that a variety of organizations and backgrounds were represented. Through this process, the list was narrowed to 16 attendings who were contacted to participate in the study, of which 12 agreed. The number of attendings examined was appropriate because saturation of metathemes can occur in as little as 6 interviews, and data saturation occurs at 12 interviews.19 The participants were asked to provide a list of their current learners (ie, residents and medical students) and 6 to 10 former learners to contact for interviews and focus groups.
Data Collection
Observations
Two researchers conducted the one-day site visits. One was a physician (S.S.) and the other a medical anthropologist (M.H.), and both have extensive experience in qualitative methods. The only exception was the site visit at the principal investigator’s own institution, which was conducted by the medical anthropologist and a nonpracticing physician who was unknown to the participants. The team structure varied slightly among different institutions but in general was composed of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Each site visit began with observing the attendings (n = 12) and current learners (n = 57) on morning rounds, which included their interactions with patients. These observations lasted approximately 2 to 3 hours. The observers took handwritten field notes, paying particular attention to group interactions, teaching approaches, and patient interactions. The observers stood outside the medical team circle and remained silent during rounds so as to be unobtrusive to the teams’ discussions. The observers discussed and compared their notes after each site visit.
Interviews and Focus Groups
The research team also conducted individual, semistructured interviews with the attendings (n = 12), focus groups with their current teams (n = 46), and interviews or focus groups with their former learners (n = 26). Current learners were asked open-ended questions about their roles on the teams, their opinions of the attendings, and the care the attendings provide to their patients. Because they were observed during rounds, the researchers asked for clarification about specific interactions observed during the teaching rounds. Depending on availability and location, former learners either participated in in-person focus groups or interviews on the day of the site visit, or in a later telephone interview. All interviews and focus groups were audio recorded and transcribed.
This study was deemed to be exempt from regulation by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could refuse to answer any question.
Data Analysis
Data were analyzed using a thematic analysis approach,20 which involves reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. The patterns are then grouped into themes to help further explain the findings.21 The research team members (S.S. and M.H.) met after the first site visit and developed initial ideas about meanings and possible patterns. One team member (M.H.) read all the transcripts from the site visit and, based on the data, developed a codebook to be used for this study. This process was repeated after every site visit, and the coding definitions were refined as necessary. All transcripts were reviewed to apply any new codes when they developed. NVivo® 10 software (QSR International, Melbourne, Australia) was used to assist with the qualitative data analysis.
To ensure consistency and identify relationships between codes, code reports listing all the data linked to a specific code were generated after all the field notes and transcripts were coded. Once verified, codes were grouped based on similarities and relationships into prominent themes related to physician-patient interactions by 2 team members (S.S. and M.H.), though all members reviewed them and concurred.
RESULTS
C are for the Patient’s Well-Being
The attendings we observed appeared to openly care for their patients’ well-being and were focused on the patients’ wants and needs. We noted that attendings were generally very attentive to the patients’ comfort. For example, we observed one attending sending the senior resident to find the patient’s nurse in order to obtain additional pain medications. The attending said to the patient several times, “I’m sorry you’re in so much pain.” When the team was leaving, she asked the intern to stay with the patient until the medications had been administered.
The attendings we observed could also be considered patient advocates, ensuring that patients received superb care. As one learner said about an attending who was attempting to have his patient listed for a liver transplant, “He is the biggest advocate for the patient that I have ever seen.” Regarding the balance between learning biomedical concepts and advocacy, another learner noted the following: “… there is always a teaching aspect, but he always makes sure that everything is taken care of for the patient…”
Building rapport creates and sustains bonds between people. Even though most of the attendings we observed primarily cared for hospitalized patients and had little long-term continuity with them, the attendings tended to take special care to talk with their patients about topics other than medicine to form a bond. This bonding between attending and patient was appreciated by learners. “Probably the most important thing I learned about patient care would be taking the time and really developing that relationship with patients,” said one of the former learners we interviewed. “There’s a question that he asks to a lot of our patients,” one learner told us, “especially our elderly patients, that [is], ‘What’s the most memorable moment in your life?’ So, he asks that question, and patient[s] open up and will share.”
The attendings often used touch to further solidify their relationships with their patients. We observed one attending who would touch her patients’ arms or knees when she was talking with them. Another attending would always shake the patient’s hand when leaving. Another attending would often lay his hand on the patient’s shoulder and help the patient sit up during the physical examination. Such humanistic behavior was noticed by learners. “She does a lot of comforting touch, particularly at the end of an exam,” said a current learner.
C onsideration of the “Big Picture”
Our exemplary attendings kept the “big picture” (that is, the patient’s overall medical and social needs) in clear focus. They behaved in a way to ensure that the patients understood the key points of their care and explained so the patients and families could understand. A current learner said, “[The attending] really makes sure that the patient understands what’s going on. And she always asks them, ‘What do you understand, what do you know, how can we fill in any blanks?’ And that makes the patient really involved in their own care, which I think is important.” This reflection was supported by direct observations. Attendings posed the following questions at the conclusion of patient interactions: “Tell me what you know.” “Tell me what our plan is.” “What did the lung doctors tell you yesterday?” These questions, which have been termed “teach-back” and are crucial for health literacy, were not meant to quiz the patient but rather to ensure the patient and family understood the plan.
We noticed that the attendings effectively explained clinical details and the plan of care to the patient while avoiding medical jargon. The following is an example of one interaction with a patient: “You threw up and created a tear in the food tube. Air got from that into the middle of the chest, not into the lungs. Air isn’t normally there. If it is just air, the body will reabsorb [it]... But we worry about bacteria getting in with the air. We need to figure out if it is an infection. We’re still trying to figure it out. Hang in there with us.” One learner commented, “… since we do bedside presentations, he has a great way of translating our gibberish, basically, to real language the patient understands.”
Finally, the attendings anticipated what patients would need in the outpatient setting. We observed that attendings stressed what the next steps would be during transitions of care. As one learner put it, “But he also thinks ahead; what do they need as an outpatient?” Another current learner commented on how another attending always asked about the social situations of his patients stating, “And then there is the social part of it. So, he is very much interested [in] where do they live? What is their support system? So, I think it has been a very holistic approach to patient care.”
R espect for the Patient
The attendings we observed were steadfastly respectful toward patients. As one attending told us, “The patient’s room is sacred space, and it’s a privilege for us to be there. And if we don’t earn that privilege, then we don’t get to go there.” We observed that the attendings generally referred to the patient as Mr. or Ms. (last name) rather than the patient’s first name unless the patient insisted. We also noticed that many of the attendings would introduce the team members to the patients or ask each member to introduce himself or herself. They also tended to leave the room and patient the way they were found, for example, by pushing the patient’s bedside table so that it was back within his or her reach or placing socks back onto the patient’s feet.
We noted that many of our attendings used appropriate humor with patients and families. As one learner explained, “I think Dr. [attending] makes most of our patients laugh during rounds. I don’t know if you noticed, but he really puts a smile on their face[s] whenever he walks in. … Maybe it would catch them off guard the first day, but after that, they are so happy to see him.”
Finally, we noticed that several of our attendings made sure to meet the patient at eye level during discussions by either kneeling or sitting on a chair. One of the attendings put it this way: “That’s a horrible power dynamic when you’re an inpatient and you’re sick and someone’s standing over you telling you things, and I like to be able to make eye contact with people, and often times that requires me to kneel down or to sit on a stool or to sit on the bed. … I feel like you’re able to connect with the people in a much better way…” Learners viewed this behavior favorably. As one told us, “[The attending] gets down to their level and makes sure that all of their questions are answered. So that is one thing that other attendings don’t necessarily do.”
DISCUSSION
In our national, qualitative study of 12 exemplary attending physicians, we found that these clinicians generally exhibited the following behaviors with patients. First, they were personable and caring and made significant attempts to connect with their patients. This occasionally took the form of using touch to comfort patients. Second, they tended to seek the “big picture” and tried to understand what patients would need upon hospital discharge. They communicated plans clearly to patients and families and inquired if those plans were understood. Finally, they showed respect toward their patients without fail. Such respect took many forms but included leaving the patient and room exactly as they were found and speaking with patients at eye level.
Our findings are largely consistent with other key studies in this field. Not surprisingly, the attendings we observed adhered to the major suggestions that Branch and colleagues2 put forth more than 15 years ago to improve the teaching of the humanistic dimension of the patient-physician relationship. Examples include greeting the patient, introducing team members and explaining each person’s role, asking open-ended questions, providing patient education, placing oneself at the same level as the patient, using appropriate touch, and being respectful. Weissmann et al.22 also found similar themes in their study of teaching physicians at 4 universities from 2003 to 2004. In that study, role-modeling was the primary method used by physician educators to teach the humanistic aspects of medical care, including nonverbal communication (eg, touch and eye contact), demonstration of respect, and building a personal connection with the patients.22In a focus group-based study performed at a teaching hospital in Boston, Ramani and Orlander23 concluded that both participating teachers and learners considered the patient’s bedside as a valuable venue to learn humanistic skills. Unfortunately, they also noted that there has been a decline in bedside teaching related to various factors, including documentation requirements and electronic medical records.23 Our attendings all demonstrated the value of teaching at a patient’s bedside. Not only could physical examination skills be demonstrated but role-modeling of interpersonal skills could be observed by learners.
Block and colleagues24 observed 29 interns in 732 patient encounters in 2 Baltimore training programs using Kahn’s “etiquette-based medicine” behaviors as a guide.12 They found that interns introduced themselves 40% of the time, explained their role 37% of the time, touched patients on 65% of visits (including as part of the physical examination), asked open-ended questions 75% of the time, and sat down with patients during only 9% of visits.24 Tackett et al.7 observed 24 hospitalists who collectively cared for 226 unique patients in 3 Baltimore-area hospitals. They found that each of the following behaviors was performed less than 30% of the time: explains role in care, shakes hand, and sits down.7 However, our attendings appeared to adhere to these behaviors to a much higher extent, though we did not quantify the interactions. This lends support to the notion that effective patient-physician interactions are the foundation of great teaching.
The attendings we observed (most of whom are inpatient based) tended to the contextual issues of the patients, such as their home environments and social support. Our exemplary physicians did what they could to ensure that patients received the appropriate follow-up care upon discharge.
Our study has important limitations. First, it was conducted in a limited number of US hospitals. The institutions represented were generally large, research-intensive, academic medical centers. Therefore, our findings may not apply to settings that are different from the hospitals studied. Second, our study included only 12 attendings and their learners, which may also limit the study’s generalizability. Third, we focused exclusively on teaching within general medicine rounds. Thus, our findings may not be generalizable to other subspecialties. Fourth, attendings were selected through a nonexhaustive method, increasing the potential for selection bias. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Former-learner responses were subject to recall bias. Finally, the study design is susceptible to observer bias. Attempts to reduce this included the diversity of the observers (ie, both a clinician and a nonclinician, the latter of whom was unfamiliar with medical education) and review of the data and coding by multiple research team members to ensure validity. Although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team attempted to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Limitations notwithstanding, we believe that our multisite study is important given the longstanding imperative to improve patient-physician interactions. We found empirical support for behaviors proposed by Branch and colleagues2 and Kahn12 in order to enhance these relationships. While others have studied attendings and their current learners,22 we add to the literature by also examining former learners’ perspectives on how the attendings’ teaching and role-modeling have created and sustained a lasting impact. The key findings of our national, qualitative study (care for the patient’s well-being, consideration of the “big picture,” and respect for the patient) can be readily adopted and honed by physicians to improve their interactions with hospitalized patients.
A cknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Veterans Affairs.
F unding
Dr. Saint provided funding for this study using a University of Michigan endowment.
Disclosure
The authors declare no conflicts of interest.
1. Peabody FW. The care of the patient. JAMA. 1927;88(12):877-882. PubMed
2. Branch WT, Jr., Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
3. Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19(11):1163-1165. PubMed
4. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
5. Osmun WE, Brown JB, Stewart M, Graham S. Patients’ attitudes to comforting touch in family practice. Can Fam Physician. 2000;46:2411-2416. PubMed
6. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
7. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
8. Gallagher TH, Levinson W. A prescription for protecting the doctor-patient relationship. Am J Manag Care. 2004;10(2, pt 1):61-68. PubMed
9. Braddock CH, 3rd, Snyder L. The doctor will see you shortly. The ethical significance of time for the patient-physician relationship. J Gen Intern Med. 2005;20(11):1057-1062. PubMed
10. Ong LM, de Haes JC, Hoos AM, Lammes FB. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903-918. PubMed
11. Lee SJ, Back AL, Block SD, Stewart SK. Enhancing physician-patient communication. Hematology Am Soc Hematol Educ Program. 2002:464-483. PubMed
12. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
13. Hoff T, Collinson GE. How Do We Talk About the Physician-Patient Relationship? What the Nonempirical Literature Tells Us. Med Care Res Rev. 2016. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. PubMed
15. Houchens N, Harrod M, Fowler KE, Moody S., Saint S. Teaching “how” to think instead of “what” to think: how great inpatient physicians foster clinical reasoning. Am J Med. In Press.
16. Harrod M, Saint S, Stock RW. Teaching Inpatient Medicine: What Every Physician Needs to Know. New York, NY: Oxford University Press; 2017.
17. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications Inc; 2013.
18. US News and World Report. Best Medical Schools: Research. 2014; http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed on September 16, 2016.
19. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
20. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. PubMed
21. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
22. Weissmann PF, Branch WT, Gracey CF, Haidet P, Frankel RM. Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661-667. PubMed
23. Ramani S, Orlander JD. Human dimensions in bedside teaching: focus group discussions of teachers and learners. Teach Learn Med. 2013;25(4):312-318. PubMed
24. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette-based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
Approximately a century ago, Francis Peabody taught that “the secret of the care of the patient is in caring for the patient.”1 His advice remains true today. Despite the advent of novel diagnostic tests, technologically sophisticated interventional procedures, and life-saving medications, perhaps the most important skill a bedside clinician can use is the ability to connect with patients.
The literature on patient-physician interaction is vast2-11 and generally indicates that exemplary bedside clinicians are able to interact well with patients by being competent, trustworthy, personable, empathetic, and effective communicators. “Etiquette-based medicine,” first proposed by Kahn,12 emphasizes the importance of certain behaviors from physicians, such as introducing yourself and
Yet, improving patient-physician interactions remains necessary. A recent systematic review reported that almost half of the reviewed studies on the patient-physician relationship published between 2000 and 2014 conveyed the idea that the patient-physician relationship is deteriorating.13
As part of a broader study to understand the behaviors and approaches of exemplary inpatient attending physicians,14-16 we examined how 12 carefully selected physicians interacted with their patients during inpatient teaching rounds.
METHODS
Overview
We conducted a multisite study using an exploratory, qualitative approach to inquiry, which has been described previously.14-16 Our primary purpose was to study the attributes and behaviors of outstanding general medicine attendings in the setting of inpatient rounds. The focus of this article is on the attendings’ interactions with patients.
We used a modified snowball sampling approach17 to identify 12 exemplary physicians. First, we contacted individuals throughout the United States who were known to the principal investigator (S.S.) and asked for suggestions of excellent clinician educators (also referred to as attendings) for potential inclusion in the study. In addition to these personal contacts, other individuals unknown to the investigative team were contacted and asked to provide suggestions for attendings to include in the study. Specifically, the US News & World Report 2015 Top Medical Schools: Research Rankings,18 which are widely used to represent the best U.S. hospitals, were reviewed in an effort to identify attendings from a broad range of medical schools. Using this list, we identified other medical schools that were in the top 25 and were not already represented. We contacted the division chiefs of general internal (or hospital) medicine, chairs and chiefs of departments of internal medicine, and internal medicine residency program directors from these medical schools and asked for recommendations of attendings from both within and outside their institutions whom they considered to be great inpatient teachers.
This sampling method resulted in 59 potential participants. An internet search was conducted on each potential participant to obtain further information about the individuals and their institutions. Both personal characteristics (medical education, training, and educational awards) and organizational characteristics (geographic location, hospital size and affiliation, and patient population) were considered so that a variety of organizations and backgrounds were represented. Through this process, the list was narrowed to 16 attendings who were contacted to participate in the study, of which 12 agreed. The number of attendings examined was appropriate because saturation of metathemes can occur in as little as 6 interviews, and data saturation occurs at 12 interviews.19 The participants were asked to provide a list of their current learners (ie, residents and medical students) and 6 to 10 former learners to contact for interviews and focus groups.
Data Collection
Observations
Two researchers conducted the one-day site visits. One was a physician (S.S.) and the other a medical anthropologist (M.H.), and both have extensive experience in qualitative methods. The only exception was the site visit at the principal investigator’s own institution, which was conducted by the medical anthropologist and a nonpracticing physician who was unknown to the participants. The team structure varied slightly among different institutions but in general was composed of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Each site visit began with observing the attendings (n = 12) and current learners (n = 57) on morning rounds, which included their interactions with patients. These observations lasted approximately 2 to 3 hours. The observers took handwritten field notes, paying particular attention to group interactions, teaching approaches, and patient interactions. The observers stood outside the medical team circle and remained silent during rounds so as to be unobtrusive to the teams’ discussions. The observers discussed and compared their notes after each site visit.
Interviews and Focus Groups
The research team also conducted individual, semistructured interviews with the attendings (n = 12), focus groups with their current teams (n = 46), and interviews or focus groups with their former learners (n = 26). Current learners were asked open-ended questions about their roles on the teams, their opinions of the attendings, and the care the attendings provide to their patients. Because they were observed during rounds, the researchers asked for clarification about specific interactions observed during the teaching rounds. Depending on availability and location, former learners either participated in in-person focus groups or interviews on the day of the site visit, or in a later telephone interview. All interviews and focus groups were audio recorded and transcribed.
This study was deemed to be exempt from regulation by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could refuse to answer any question.
Data Analysis
Data were analyzed using a thematic analysis approach,20 which involves reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. The patterns are then grouped into themes to help further explain the findings.21 The research team members (S.S. and M.H.) met after the first site visit and developed initial ideas about meanings and possible patterns. One team member (M.H.) read all the transcripts from the site visit and, based on the data, developed a codebook to be used for this study. This process was repeated after every site visit, and the coding definitions were refined as necessary. All transcripts were reviewed to apply any new codes when they developed. NVivo® 10 software (QSR International, Melbourne, Australia) was used to assist with the qualitative data analysis.
To ensure consistency and identify relationships between codes, code reports listing all the data linked to a specific code were generated after all the field notes and transcripts were coded. Once verified, codes were grouped based on similarities and relationships into prominent themes related to physician-patient interactions by 2 team members (S.S. and M.H.), though all members reviewed them and concurred.
RESULTS
C are for the Patient’s Well-Being
The attendings we observed appeared to openly care for their patients’ well-being and were focused on the patients’ wants and needs. We noted that attendings were generally very attentive to the patients’ comfort. For example, we observed one attending sending the senior resident to find the patient’s nurse in order to obtain additional pain medications. The attending said to the patient several times, “I’m sorry you’re in so much pain.” When the team was leaving, she asked the intern to stay with the patient until the medications had been administered.
The attendings we observed could also be considered patient advocates, ensuring that patients received superb care. As one learner said about an attending who was attempting to have his patient listed for a liver transplant, “He is the biggest advocate for the patient that I have ever seen.” Regarding the balance between learning biomedical concepts and advocacy, another learner noted the following: “… there is always a teaching aspect, but he always makes sure that everything is taken care of for the patient…”
Building rapport creates and sustains bonds between people. Even though most of the attendings we observed primarily cared for hospitalized patients and had little long-term continuity with them, the attendings tended to take special care to talk with their patients about topics other than medicine to form a bond. This bonding between attending and patient was appreciated by learners. “Probably the most important thing I learned about patient care would be taking the time and really developing that relationship with patients,” said one of the former learners we interviewed. “There’s a question that he asks to a lot of our patients,” one learner told us, “especially our elderly patients, that [is], ‘What’s the most memorable moment in your life?’ So, he asks that question, and patient[s] open up and will share.”
The attendings often used touch to further solidify their relationships with their patients. We observed one attending who would touch her patients’ arms or knees when she was talking with them. Another attending would always shake the patient’s hand when leaving. Another attending would often lay his hand on the patient’s shoulder and help the patient sit up during the physical examination. Such humanistic behavior was noticed by learners. “She does a lot of comforting touch, particularly at the end of an exam,” said a current learner.
C onsideration of the “Big Picture”
Our exemplary attendings kept the “big picture” (that is, the patient’s overall medical and social needs) in clear focus. They behaved in a way to ensure that the patients understood the key points of their care and explained so the patients and families could understand. A current learner said, “[The attending] really makes sure that the patient understands what’s going on. And she always asks them, ‘What do you understand, what do you know, how can we fill in any blanks?’ And that makes the patient really involved in their own care, which I think is important.” This reflection was supported by direct observations. Attendings posed the following questions at the conclusion of patient interactions: “Tell me what you know.” “Tell me what our plan is.” “What did the lung doctors tell you yesterday?” These questions, which have been termed “teach-back” and are crucial for health literacy, were not meant to quiz the patient but rather to ensure the patient and family understood the plan.
We noticed that the attendings effectively explained clinical details and the plan of care to the patient while avoiding medical jargon. The following is an example of one interaction with a patient: “You threw up and created a tear in the food tube. Air got from that into the middle of the chest, not into the lungs. Air isn’t normally there. If it is just air, the body will reabsorb [it]... But we worry about bacteria getting in with the air. We need to figure out if it is an infection. We’re still trying to figure it out. Hang in there with us.” One learner commented, “… since we do bedside presentations, he has a great way of translating our gibberish, basically, to real language the patient understands.”
Finally, the attendings anticipated what patients would need in the outpatient setting. We observed that attendings stressed what the next steps would be during transitions of care. As one learner put it, “But he also thinks ahead; what do they need as an outpatient?” Another current learner commented on how another attending always asked about the social situations of his patients stating, “And then there is the social part of it. So, he is very much interested [in] where do they live? What is their support system? So, I think it has been a very holistic approach to patient care.”
R espect for the Patient
The attendings we observed were steadfastly respectful toward patients. As one attending told us, “The patient’s room is sacred space, and it’s a privilege for us to be there. And if we don’t earn that privilege, then we don’t get to go there.” We observed that the attendings generally referred to the patient as Mr. or Ms. (last name) rather than the patient’s first name unless the patient insisted. We also noticed that many of the attendings would introduce the team members to the patients or ask each member to introduce himself or herself. They also tended to leave the room and patient the way they were found, for example, by pushing the patient’s bedside table so that it was back within his or her reach or placing socks back onto the patient’s feet.
We noted that many of our attendings used appropriate humor with patients and families. As one learner explained, “I think Dr. [attending] makes most of our patients laugh during rounds. I don’t know if you noticed, but he really puts a smile on their face[s] whenever he walks in. … Maybe it would catch them off guard the first day, but after that, they are so happy to see him.”
Finally, we noticed that several of our attendings made sure to meet the patient at eye level during discussions by either kneeling or sitting on a chair. One of the attendings put it this way: “That’s a horrible power dynamic when you’re an inpatient and you’re sick and someone’s standing over you telling you things, and I like to be able to make eye contact with people, and often times that requires me to kneel down or to sit on a stool or to sit on the bed. … I feel like you’re able to connect with the people in a much better way…” Learners viewed this behavior favorably. As one told us, “[The attending] gets down to their level and makes sure that all of their questions are answered. So that is one thing that other attendings don’t necessarily do.”
DISCUSSION
In our national, qualitative study of 12 exemplary attending physicians, we found that these clinicians generally exhibited the following behaviors with patients. First, they were personable and caring and made significant attempts to connect with their patients. This occasionally took the form of using touch to comfort patients. Second, they tended to seek the “big picture” and tried to understand what patients would need upon hospital discharge. They communicated plans clearly to patients and families and inquired if those plans were understood. Finally, they showed respect toward their patients without fail. Such respect took many forms but included leaving the patient and room exactly as they were found and speaking with patients at eye level.
Our findings are largely consistent with other key studies in this field. Not surprisingly, the attendings we observed adhered to the major suggestions that Branch and colleagues2 put forth more than 15 years ago to improve the teaching of the humanistic dimension of the patient-physician relationship. Examples include greeting the patient, introducing team members and explaining each person’s role, asking open-ended questions, providing patient education, placing oneself at the same level as the patient, using appropriate touch, and being respectful. Weissmann et al.22 also found similar themes in their study of teaching physicians at 4 universities from 2003 to 2004. In that study, role-modeling was the primary method used by physician educators to teach the humanistic aspects of medical care, including nonverbal communication (eg, touch and eye contact), demonstration of respect, and building a personal connection with the patients.22In a focus group-based study performed at a teaching hospital in Boston, Ramani and Orlander23 concluded that both participating teachers and learners considered the patient’s bedside as a valuable venue to learn humanistic skills. Unfortunately, they also noted that there has been a decline in bedside teaching related to various factors, including documentation requirements and electronic medical records.23 Our attendings all demonstrated the value of teaching at a patient’s bedside. Not only could physical examination skills be demonstrated but role-modeling of interpersonal skills could be observed by learners.
Block and colleagues24 observed 29 interns in 732 patient encounters in 2 Baltimore training programs using Kahn’s “etiquette-based medicine” behaviors as a guide.12 They found that interns introduced themselves 40% of the time, explained their role 37% of the time, touched patients on 65% of visits (including as part of the physical examination), asked open-ended questions 75% of the time, and sat down with patients during only 9% of visits.24 Tackett et al.7 observed 24 hospitalists who collectively cared for 226 unique patients in 3 Baltimore-area hospitals. They found that each of the following behaviors was performed less than 30% of the time: explains role in care, shakes hand, and sits down.7 However, our attendings appeared to adhere to these behaviors to a much higher extent, though we did not quantify the interactions. This lends support to the notion that effective patient-physician interactions are the foundation of great teaching.
The attendings we observed (most of whom are inpatient based) tended to the contextual issues of the patients, such as their home environments and social support. Our exemplary physicians did what they could to ensure that patients received the appropriate follow-up care upon discharge.
Our study has important limitations. First, it was conducted in a limited number of US hospitals. The institutions represented were generally large, research-intensive, academic medical centers. Therefore, our findings may not apply to settings that are different from the hospitals studied. Second, our study included only 12 attendings and their learners, which may also limit the study’s generalizability. Third, we focused exclusively on teaching within general medicine rounds. Thus, our findings may not be generalizable to other subspecialties. Fourth, attendings were selected through a nonexhaustive method, increasing the potential for selection bias. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Former-learner responses were subject to recall bias. Finally, the study design is susceptible to observer bias. Attempts to reduce this included the diversity of the observers (ie, both a clinician and a nonclinician, the latter of whom was unfamiliar with medical education) and review of the data and coding by multiple research team members to ensure validity. Although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team attempted to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Limitations notwithstanding, we believe that our multisite study is important given the longstanding imperative to improve patient-physician interactions. We found empirical support for behaviors proposed by Branch and colleagues2 and Kahn12 in order to enhance these relationships. While others have studied attendings and their current learners,22 we add to the literature by also examining former learners’ perspectives on how the attendings’ teaching and role-modeling have created and sustained a lasting impact. The key findings of our national, qualitative study (care for the patient’s well-being, consideration of the “big picture,” and respect for the patient) can be readily adopted and honed by physicians to improve their interactions with hospitalized patients.
A cknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Veterans Affairs.
F unding
Dr. Saint provided funding for this study using a University of Michigan endowment.
Disclosure
The authors declare no conflicts of interest.
Approximately a century ago, Francis Peabody taught that “the secret of the care of the patient is in caring for the patient.”1 His advice remains true today. Despite the advent of novel diagnostic tests, technologically sophisticated interventional procedures, and life-saving medications, perhaps the most important skill a bedside clinician can use is the ability to connect with patients.
The literature on patient-physician interaction is vast2-11 and generally indicates that exemplary bedside clinicians are able to interact well with patients by being competent, trustworthy, personable, empathetic, and effective communicators. “Etiquette-based medicine,” first proposed by Kahn,12 emphasizes the importance of certain behaviors from physicians, such as introducing yourself and
Yet, improving patient-physician interactions remains necessary. A recent systematic review reported that almost half of the reviewed studies on the patient-physician relationship published between 2000 and 2014 conveyed the idea that the patient-physician relationship is deteriorating.13
As part of a broader study to understand the behaviors and approaches of exemplary inpatient attending physicians,14-16 we examined how 12 carefully selected physicians interacted with their patients during inpatient teaching rounds.
METHODS
Overview
We conducted a multisite study using an exploratory, qualitative approach to inquiry, which has been described previously.14-16 Our primary purpose was to study the attributes and behaviors of outstanding general medicine attendings in the setting of inpatient rounds. The focus of this article is on the attendings’ interactions with patients.
We used a modified snowball sampling approach17 to identify 12 exemplary physicians. First, we contacted individuals throughout the United States who were known to the principal investigator (S.S.) and asked for suggestions of excellent clinician educators (also referred to as attendings) for potential inclusion in the study. In addition to these personal contacts, other individuals unknown to the investigative team were contacted and asked to provide suggestions for attendings to include in the study. Specifically, the US News & World Report 2015 Top Medical Schools: Research Rankings,18 which are widely used to represent the best U.S. hospitals, were reviewed in an effort to identify attendings from a broad range of medical schools. Using this list, we identified other medical schools that were in the top 25 and were not already represented. We contacted the division chiefs of general internal (or hospital) medicine, chairs and chiefs of departments of internal medicine, and internal medicine residency program directors from these medical schools and asked for recommendations of attendings from both within and outside their institutions whom they considered to be great inpatient teachers.
This sampling method resulted in 59 potential participants. An internet search was conducted on each potential participant to obtain further information about the individuals and their institutions. Both personal characteristics (medical education, training, and educational awards) and organizational characteristics (geographic location, hospital size and affiliation, and patient population) were considered so that a variety of organizations and backgrounds were represented. Through this process, the list was narrowed to 16 attendings who were contacted to participate in the study, of which 12 agreed. The number of attendings examined was appropriate because saturation of metathemes can occur in as little as 6 interviews, and data saturation occurs at 12 interviews.19 The participants were asked to provide a list of their current learners (ie, residents and medical students) and 6 to 10 former learners to contact for interviews and focus groups.
Data Collection
Observations
Two researchers conducted the one-day site visits. One was a physician (S.S.) and the other a medical anthropologist (M.H.), and both have extensive experience in qualitative methods. The only exception was the site visit at the principal investigator’s own institution, which was conducted by the medical anthropologist and a nonpracticing physician who was unknown to the participants. The team structure varied slightly among different institutions but in general was composed of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Each site visit began with observing the attendings (n = 12) and current learners (n = 57) on morning rounds, which included their interactions with patients. These observations lasted approximately 2 to 3 hours. The observers took handwritten field notes, paying particular attention to group interactions, teaching approaches, and patient interactions. The observers stood outside the medical team circle and remained silent during rounds so as to be unobtrusive to the teams’ discussions. The observers discussed and compared their notes after each site visit.
Interviews and Focus Groups
The research team also conducted individual, semistructured interviews with the attendings (n = 12), focus groups with their current teams (n = 46), and interviews or focus groups with their former learners (n = 26). Current learners were asked open-ended questions about their roles on the teams, their opinions of the attendings, and the care the attendings provide to their patients. Because they were observed during rounds, the researchers asked for clarification about specific interactions observed during the teaching rounds. Depending on availability and location, former learners either participated in in-person focus groups or interviews on the day of the site visit, or in a later telephone interview. All interviews and focus groups were audio recorded and transcribed.
This study was deemed to be exempt from regulation by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could refuse to answer any question.
Data Analysis
Data were analyzed using a thematic analysis approach,20 which involves reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. The patterns are then grouped into themes to help further explain the findings.21 The research team members (S.S. and M.H.) met after the first site visit and developed initial ideas about meanings and possible patterns. One team member (M.H.) read all the transcripts from the site visit and, based on the data, developed a codebook to be used for this study. This process was repeated after every site visit, and the coding definitions were refined as necessary. All transcripts were reviewed to apply any new codes when they developed. NVivo® 10 software (QSR International, Melbourne, Australia) was used to assist with the qualitative data analysis.
To ensure consistency and identify relationships between codes, code reports listing all the data linked to a specific code were generated after all the field notes and transcripts were coded. Once verified, codes were grouped based on similarities and relationships into prominent themes related to physician-patient interactions by 2 team members (S.S. and M.H.), though all members reviewed them and concurred.
RESULTS
C are for the Patient’s Well-Being
The attendings we observed appeared to openly care for their patients’ well-being and were focused on the patients’ wants and needs. We noted that attendings were generally very attentive to the patients’ comfort. For example, we observed one attending sending the senior resident to find the patient’s nurse in order to obtain additional pain medications. The attending said to the patient several times, “I’m sorry you’re in so much pain.” When the team was leaving, she asked the intern to stay with the patient until the medications had been administered.
The attendings we observed could also be considered patient advocates, ensuring that patients received superb care. As one learner said about an attending who was attempting to have his patient listed for a liver transplant, “He is the biggest advocate for the patient that I have ever seen.” Regarding the balance between learning biomedical concepts and advocacy, another learner noted the following: “… there is always a teaching aspect, but he always makes sure that everything is taken care of for the patient…”
Building rapport creates and sustains bonds between people. Even though most of the attendings we observed primarily cared for hospitalized patients and had little long-term continuity with them, the attendings tended to take special care to talk with their patients about topics other than medicine to form a bond. This bonding between attending and patient was appreciated by learners. “Probably the most important thing I learned about patient care would be taking the time and really developing that relationship with patients,” said one of the former learners we interviewed. “There’s a question that he asks to a lot of our patients,” one learner told us, “especially our elderly patients, that [is], ‘What’s the most memorable moment in your life?’ So, he asks that question, and patient[s] open up and will share.”
The attendings often used touch to further solidify their relationships with their patients. We observed one attending who would touch her patients’ arms or knees when she was talking with them. Another attending would always shake the patient’s hand when leaving. Another attending would often lay his hand on the patient’s shoulder and help the patient sit up during the physical examination. Such humanistic behavior was noticed by learners. “She does a lot of comforting touch, particularly at the end of an exam,” said a current learner.
C onsideration of the “Big Picture”
Our exemplary attendings kept the “big picture” (that is, the patient’s overall medical and social needs) in clear focus. They behaved in a way to ensure that the patients understood the key points of their care and explained so the patients and families could understand. A current learner said, “[The attending] really makes sure that the patient understands what’s going on. And she always asks them, ‘What do you understand, what do you know, how can we fill in any blanks?’ And that makes the patient really involved in their own care, which I think is important.” This reflection was supported by direct observations. Attendings posed the following questions at the conclusion of patient interactions: “Tell me what you know.” “Tell me what our plan is.” “What did the lung doctors tell you yesterday?” These questions, which have been termed “teach-back” and are crucial for health literacy, were not meant to quiz the patient but rather to ensure the patient and family understood the plan.
We noticed that the attendings effectively explained clinical details and the plan of care to the patient while avoiding medical jargon. The following is an example of one interaction with a patient: “You threw up and created a tear in the food tube. Air got from that into the middle of the chest, not into the lungs. Air isn’t normally there. If it is just air, the body will reabsorb [it]... But we worry about bacteria getting in with the air. We need to figure out if it is an infection. We’re still trying to figure it out. Hang in there with us.” One learner commented, “… since we do bedside presentations, he has a great way of translating our gibberish, basically, to real language the patient understands.”
Finally, the attendings anticipated what patients would need in the outpatient setting. We observed that attendings stressed what the next steps would be during transitions of care. As one learner put it, “But he also thinks ahead; what do they need as an outpatient?” Another current learner commented on how another attending always asked about the social situations of his patients stating, “And then there is the social part of it. So, he is very much interested [in] where do they live? What is their support system? So, I think it has been a very holistic approach to patient care.”
R espect for the Patient
The attendings we observed were steadfastly respectful toward patients. As one attending told us, “The patient’s room is sacred space, and it’s a privilege for us to be there. And if we don’t earn that privilege, then we don’t get to go there.” We observed that the attendings generally referred to the patient as Mr. or Ms. (last name) rather than the patient’s first name unless the patient insisted. We also noticed that many of the attendings would introduce the team members to the patients or ask each member to introduce himself or herself. They also tended to leave the room and patient the way they were found, for example, by pushing the patient’s bedside table so that it was back within his or her reach or placing socks back onto the patient’s feet.
We noted that many of our attendings used appropriate humor with patients and families. As one learner explained, “I think Dr. [attending] makes most of our patients laugh during rounds. I don’t know if you noticed, but he really puts a smile on their face[s] whenever he walks in. … Maybe it would catch them off guard the first day, but after that, they are so happy to see him.”
Finally, we noticed that several of our attendings made sure to meet the patient at eye level during discussions by either kneeling or sitting on a chair. One of the attendings put it this way: “That’s a horrible power dynamic when you’re an inpatient and you’re sick and someone’s standing over you telling you things, and I like to be able to make eye contact with people, and often times that requires me to kneel down or to sit on a stool or to sit on the bed. … I feel like you’re able to connect with the people in a much better way…” Learners viewed this behavior favorably. As one told us, “[The attending] gets down to their level and makes sure that all of their questions are answered. So that is one thing that other attendings don’t necessarily do.”
DISCUSSION
In our national, qualitative study of 12 exemplary attending physicians, we found that these clinicians generally exhibited the following behaviors with patients. First, they were personable and caring and made significant attempts to connect with their patients. This occasionally took the form of using touch to comfort patients. Second, they tended to seek the “big picture” and tried to understand what patients would need upon hospital discharge. They communicated plans clearly to patients and families and inquired if those plans were understood. Finally, they showed respect toward their patients without fail. Such respect took many forms but included leaving the patient and room exactly as they were found and speaking with patients at eye level.
Our findings are largely consistent with other key studies in this field. Not surprisingly, the attendings we observed adhered to the major suggestions that Branch and colleagues2 put forth more than 15 years ago to improve the teaching of the humanistic dimension of the patient-physician relationship. Examples include greeting the patient, introducing team members and explaining each person’s role, asking open-ended questions, providing patient education, placing oneself at the same level as the patient, using appropriate touch, and being respectful. Weissmann et al.22 also found similar themes in their study of teaching physicians at 4 universities from 2003 to 2004. In that study, role-modeling was the primary method used by physician educators to teach the humanistic aspects of medical care, including nonverbal communication (eg, touch and eye contact), demonstration of respect, and building a personal connection with the patients.22In a focus group-based study performed at a teaching hospital in Boston, Ramani and Orlander23 concluded that both participating teachers and learners considered the patient’s bedside as a valuable venue to learn humanistic skills. Unfortunately, they also noted that there has been a decline in bedside teaching related to various factors, including documentation requirements and electronic medical records.23 Our attendings all demonstrated the value of teaching at a patient’s bedside. Not only could physical examination skills be demonstrated but role-modeling of interpersonal skills could be observed by learners.
Block and colleagues24 observed 29 interns in 732 patient encounters in 2 Baltimore training programs using Kahn’s “etiquette-based medicine” behaviors as a guide.12 They found that interns introduced themselves 40% of the time, explained their role 37% of the time, touched patients on 65% of visits (including as part of the physical examination), asked open-ended questions 75% of the time, and sat down with patients during only 9% of visits.24 Tackett et al.7 observed 24 hospitalists who collectively cared for 226 unique patients in 3 Baltimore-area hospitals. They found that each of the following behaviors was performed less than 30% of the time: explains role in care, shakes hand, and sits down.7 However, our attendings appeared to adhere to these behaviors to a much higher extent, though we did not quantify the interactions. This lends support to the notion that effective patient-physician interactions are the foundation of great teaching.
The attendings we observed (most of whom are inpatient based) tended to the contextual issues of the patients, such as their home environments and social support. Our exemplary physicians did what they could to ensure that patients received the appropriate follow-up care upon discharge.
Our study has important limitations. First, it was conducted in a limited number of US hospitals. The institutions represented were generally large, research-intensive, academic medical centers. Therefore, our findings may not apply to settings that are different from the hospitals studied. Second, our study included only 12 attendings and their learners, which may also limit the study’s generalizability. Third, we focused exclusively on teaching within general medicine rounds. Thus, our findings may not be generalizable to other subspecialties. Fourth, attendings were selected through a nonexhaustive method, increasing the potential for selection bias. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Former-learner responses were subject to recall bias. Finally, the study design is susceptible to observer bias. Attempts to reduce this included the diversity of the observers (ie, both a clinician and a nonclinician, the latter of whom was unfamiliar with medical education) and review of the data and coding by multiple research team members to ensure validity. Although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team attempted to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Limitations notwithstanding, we believe that our multisite study is important given the longstanding imperative to improve patient-physician interactions. We found empirical support for behaviors proposed by Branch and colleagues2 and Kahn12 in order to enhance these relationships. While others have studied attendings and their current learners,22 we add to the literature by also examining former learners’ perspectives on how the attendings’ teaching and role-modeling have created and sustained a lasting impact. The key findings of our national, qualitative study (care for the patient’s well-being, consideration of the “big picture,” and respect for the patient) can be readily adopted and honed by physicians to improve their interactions with hospitalized patients.
A cknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Veterans Affairs.
F unding
Dr. Saint provided funding for this study using a University of Michigan endowment.
Disclosure
The authors declare no conflicts of interest.
1. Peabody FW. The care of the patient. JAMA. 1927;88(12):877-882. PubMed
2. Branch WT, Jr., Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
3. Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19(11):1163-1165. PubMed
4. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
5. Osmun WE, Brown JB, Stewart M, Graham S. Patients’ attitudes to comforting touch in family practice. Can Fam Physician. 2000;46:2411-2416. PubMed
6. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
7. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
8. Gallagher TH, Levinson W. A prescription for protecting the doctor-patient relationship. Am J Manag Care. 2004;10(2, pt 1):61-68. PubMed
9. Braddock CH, 3rd, Snyder L. The doctor will see you shortly. The ethical significance of time for the patient-physician relationship. J Gen Intern Med. 2005;20(11):1057-1062. PubMed
10. Ong LM, de Haes JC, Hoos AM, Lammes FB. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903-918. PubMed
11. Lee SJ, Back AL, Block SD, Stewart SK. Enhancing physician-patient communication. Hematology Am Soc Hematol Educ Program. 2002:464-483. PubMed
12. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
13. Hoff T, Collinson GE. How Do We Talk About the Physician-Patient Relationship? What the Nonempirical Literature Tells Us. Med Care Res Rev. 2016. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. PubMed
15. Houchens N, Harrod M, Fowler KE, Moody S., Saint S. Teaching “how” to think instead of “what” to think: how great inpatient physicians foster clinical reasoning. Am J Med. In Press.
16. Harrod M, Saint S, Stock RW. Teaching Inpatient Medicine: What Every Physician Needs to Know. New York, NY: Oxford University Press; 2017.
17. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications Inc; 2013.
18. US News and World Report. Best Medical Schools: Research. 2014; http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed on September 16, 2016.
19. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
20. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. PubMed
21. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
22. Weissmann PF, Branch WT, Gracey CF, Haidet P, Frankel RM. Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661-667. PubMed
23. Ramani S, Orlander JD. Human dimensions in bedside teaching: focus group discussions of teachers and learners. Teach Learn Med. 2013;25(4):312-318. PubMed
24. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette-based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
1. Peabody FW. The care of the patient. JAMA. 1927;88(12):877-882. PubMed
2. Branch WT, Jr., Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
3. Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19(11):1163-1165. PubMed
4. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
5. Osmun WE, Brown JB, Stewart M, Graham S. Patients’ attitudes to comforting touch in family practice. Can Fam Physician. 2000;46:2411-2416. PubMed
6. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
7. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
8. Gallagher TH, Levinson W. A prescription for protecting the doctor-patient relationship. Am J Manag Care. 2004;10(2, pt 1):61-68. PubMed
9. Braddock CH, 3rd, Snyder L. The doctor will see you shortly. The ethical significance of time for the patient-physician relationship. J Gen Intern Med. 2005;20(11):1057-1062. PubMed
10. Ong LM, de Haes JC, Hoos AM, Lammes FB. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903-918. PubMed
11. Lee SJ, Back AL, Block SD, Stewart SK. Enhancing physician-patient communication. Hematology Am Soc Hematol Educ Program. 2002:464-483. PubMed
12. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
13. Hoff T, Collinson GE. How Do We Talk About the Physician-Patient Relationship? What the Nonempirical Literature Tells Us. Med Care Res Rev. 2016. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. PubMed
15. Houchens N, Harrod M, Fowler KE, Moody S., Saint S. Teaching “how” to think instead of “what” to think: how great inpatient physicians foster clinical reasoning. Am J Med. In Press.
16. Harrod M, Saint S, Stock RW. Teaching Inpatient Medicine: What Every Physician Needs to Know. New York, NY: Oxford University Press; 2017.
17. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications Inc; 2013.
18. US News and World Report. Best Medical Schools: Research. 2014; http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed on September 16, 2016.
19. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
20. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. PubMed
21. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
22. Weissmann PF, Branch WT, Gracey CF, Haidet P, Frankel RM. Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661-667. PubMed
23. Ramani S, Orlander JD. Human dimensions in bedside teaching: focus group discussions of teachers and learners. Teach Learn Med. 2013;25(4):312-318. PubMed
24. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette-based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
© 2017 Society of Hospital Medicine
A Longitudinal Study of Transfusion Utilization in Hospitalized Veterans
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, [email protected].
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, [email protected].
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, [email protected].
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Mass Confusion
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
© 2017 Society of Hospital Medicine
Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study
Clinician educators face numerous obstacles to their joint mission of facilitating learning while also ensuring high-quality and patient-centered care. Time constraints, including the institution of house officer duty hour limitations,1 shorter lengths of stay for hospitalized patients,2 and competing career responsibilities, combine to create a dynamic learning environment. Additionally, clinician educators must balance the autonomy of their learners with the safety of their patients. They must teach to multiple learning levels and work collaboratively with multiple disciplines to foster an effective team-based approach to patient care. Yet, many clinician educators have no formal training in pedagogical methods.3 Such challenges necessitate increased attention to the work of excellent clinician educators and their respective teaching approaches.
Many studies of clinical teaching rely primarily on survey data of attributes of good clinical teachers.3-7 While some studies have incorporated direct observations of teaching8,9 or interviews with clinician educators or learners,10,11 few have incorporated multiple perspectives from the current team and from former learners in order to provide a comprehensive picture of team-based learning.12
The goal of this study was to gain a thorough understanding, through multiple perspectives, of the techniques and behaviors used by exemplary educators within actual clinical environments. We studied attitudes, behaviors, and approaches of 12 such inpatient clinician educators.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. This approach was used to study the techniques and behaviors of excellent attendings during inpatient general medicine rounds. A modified snowball sampling approach13 was used, meaning individuals known to one member of the research team (SS) were initially contacted and asked to identify clinician educators (also referred to as attendings) for potential inclusion in the study. In an effort to identify attendings from a broad range of medical schools, the “2015 U.S. News and World Report Top Medical Schools: Research” rankings14 were also reviewed, with priority given to the top 25, as these are widely used to represent the best US hospitals. In an attempt to invite attendings from diverse institutions, additional medical schools not in the top 25 as well as historically black medical schools were also included. Division chiefs and chairs of internal medicine and/or directors of internal medicine residency programs at these schools were contacted and asked for recommendations of attendings, both within and outside their institutions, who they considered to be great inpatient teachers. In addition, key experts who have won teaching awards or were known to be specialists in the field of medical education were asked to nominate one or two other outstanding attendings.
By using this sampling method, 59 potential participants were identified. An internet search was conducted to obtain information about the potential participants and their institutions. Organizational characteristics such as geographic location, hospital size and affiliation, and patient population, as well as individual characteristics such as gender, medical education and training, and educational awards received were considered so that a diversity of organizations and backgrounds was represented. The list was narrowed down to 16 attendings who were contacted via e-mail and asked to participate. Interested participants were asked for a list of their current team members and 6 to 10 former learners to contact for interviews and focus groups. Former learners were included in an effort to better understand lasting effects on learners from their exemplary teaching attendings. A total of 12 attending physicians agreed to participate (Table 1). Literature on field methods has shown that 12 interviews are found to be adequate in accomplishing data saturation.15 Although 2 attendings were located at the same institution, we decided to include them given that both are recognized as master clinician educators and were each recommended by several individuals from various institutions. Hospitals were located throughout the US and included both university-affiliated hospitals and Veterans Affairs medical centers. Despite efforts to include physicians from historically black colleges and universities, only one attending was identified, and they declined the request to participate.
Data Collection
Observations. The one-day site visits were mainly conducted by two research team members, a physician (SS) and a medical anthropologist (MH), both of whom have extensive experience in qualitative methods. Teams were not uniform but were generally comprised of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Occasionally, a pharmacist, clinical assistant, or other health professional accompanied the team on rounds. Not infrequently, the bedside nurse would explicitly be included in the discussion regarding his or her specific patient. Each site visit began with observing attendings (N = 12) and current learners (N = 57) during rounds. Each research team member recorded their own observations via handwritten field notes, paying particular attention to group interactions, teaching approach, conversations occurring within and peripheral to the team, patient-team interactions, and the physical environment. By standing outside of the medical team circle and remaining silent during rounds, research team members remained unobtrusive to the discussion and process of rounds. Materials the attendings used during their teaching rounds were also documented and collected. Rounds generally lasted 2 to 3 hours. After each site visit, the research team met to compare and combine field notes.
Interviews and Focus Groups. The research team then conducted individual, semi-structured interviews with the attendings, focus groups with their current team (N = 46), and interviews or focus groups with their former learners (N = 26; Supplement 1). Eleven of the current team members observed during rounds were unable to participate in the focus groups due to clinical duties. Because the current learners who participated in the focus groups were also observed during rounds, the research team was able to ask them open-ended questions regarding teaching rounds and their roles as learners within this environment. Former learners who were still at the hospital participated in separate focus groups or interviews. Former learners who were no longer present at the hospital were contacted by telephone and individually interviewed by one research team member (MH). All interviews and focus groups were audio-recorded and transcribed.
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a thematic analysis approach.16 Thematic analysis entails reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. Once patterns have been identified, they are grouped according to similarity into themes, which help to further explain the findings.17
After the first site visit was completed, the research team members that participated (SS and MH) met to develop initial ideas about meanings and possible patterns. All transcripts were read by one team member (MH) and, based on review of the data, codes were developed, defined, and documented in a codebook. This process was repeated after every site visit using the codebook to expand or combine codes and refine definitions as necessary. If a new code was added, the previously coded data were reviewed to apply the new code. NVivo® 10 software (QSR International; Melbourne, Australia) was used to manage the data.
Once all field notes and transcripts were coded (MH), the code reports, which list all data described within a specific code, were run to ensure consistency and identify relationships between codes. Once coding was verified, codes were grouped based on similarities and relationships into salient themes by 3 members of the research team (NH, MH, and SM). Themes, along with their supporting codes, were then further defined to understand how these attendings worked to facilitate excellent teaching in clinical settings.
RESULTS
The coded interview data and field notes were categorized into broad, overlapping themes. Three of these major themes include (1) fostering positive relationships, (2) patient-centered teaching, and (3) collaboration and coaching. Table 2 lists each theme, salient behaviors, examples, and selected quotes that further elucidate its meaning.
Fostering Positive Relationships
Attending physicians took observable steps to develop positive relationships with their team members, which in turn created a safe learning environment. For instance, attendings used learners’ first names, demonstrated interest in their well-being, deployed humor, and generally displayed informal actions—uncrossed arms, “fist bump” when recognizing learners’ success, standing outside the circle of team members and leaning in to listen—during learner interactions. Attendings also made it a priority to get to know individuals on a personal level. As one current learner put it, “He asks about where we are from. He will try to find some kind of connection that he can establish with not only each of the team members but also with each of the patients.”
Additionally, attendings built positive relationships with their learners by responding thoughtfully to their input, even when learners’ evaluations of patients required modification. In turn, learners reported feeling safe to ask questions, admit uncertainty, and respectfully disagree with their attendings. As one attending reflected, “If I can get them into a place where they feel like the learning environment is someplace where they can make a mistake and know that that mistake does not necessarily mean that it’s going to cost them in their evaluation part, then I feel like that’s why it’s important.”
To build rapport and create a safe learning environment, attendings used a number of strategies to position themselves as learners alongside their team members. For instance, attendings indicated that they wanted their ideas questioned because they saw it as an opportunity to learn. Moreover, in conversations with learners, attendings demonstrated humility, admitting when they did not know something. One former learner noted, “There have been times when he has asked [a] question…nobody knows and then he admits that he doesn’t know either. So everybody goes and looks it up…The whole thing turns out to be a fun learning experience.”
Attendings demonstrated respect for their team members’ time by reading about patients before rounds, identifying learning opportunities during rounds, and integrating teaching points into the daily work of patient care. Teaching was not relegated exclusively to the conference room or confined to the traditional “chalk talk” before or after rounds but rather was assimilated into daily workflow. They appeared to be responsive to the needs of individual patients and the team, which allowed attendings to both directly oversee their patients’ care and overcome the challenges of multiple competing demands for time. The importance of this approach was made clear by one current learner who stated “…she does prepare before, especially you know on call days, she does prepare for the new patients before coming in to staff, which is really appreciated… it saves a lot of time on rounds.”
Attendings also included other health professionals in team discussions. Attendings used many of the same relationship-building techniques with these professionals as they did with learners and patients. They consistently asked these professionals to provide insight and direction in patients’ plans of care. A former learner commented, “He always asks the [nurse] what is her impression of the patient...he truly values the [nurse’s] opinion of the patient.” One attending reiterated this approach, stating “I don’t want them to think that anything I have to say is more valuable than our pharmacist or the [nurse].”
Patient-Centered Teaching
Attending physicians modeled numerous teaching techniques that focused learning around the patient. Attendings knew their patients well through review of the medical records, discussion with the patient, and personal examination. This preparation allowed attendings to focus on key teaching points in the context of the patient. One former learner noted, “He tended to bring up a variety of things that really fit well into the clinical scenario. So whether that is talking about what is the differential for a new symptom that just came up for this patient or kind of here is a new paper talking about this condition or maybe some other pearl of physical exam for a patient that has a certain physical condition.”
Attendings served as effective role models by being directly involved in examining and talking with patients as well as demonstrating excellent physical examination and communication techniques. One current learner articulated the importance of learning these skills by observing them done well: “I think he teaches by example and by doing, again, those little things: being attentive to the patients and being very careful during exams…I think those are things that you teach people by doing them, not by saying you need to do this better during the patient encounter.”
Collaboration and Coaching
Attending physicians used varied collaboration and coaching techniques to facilitate learning across the entire care team. During rounds, attendings utilized visual aids to reinforce key concepts and simplify complex topics. They also collaborated by using discussion rather than lecture to engage with team members. For instance, attendings used Socratic questioning, asking questions that lead learners through critical thinking and allow them to solve problems themselves, to guide learners’ decision-making. One former learner reported, “He never gives you the answer, and he always asks your opinion; ‘So what are your thoughts on this?’”
Coaching for success, rather than directing the various team members, was emphasized. Attendings did not wish to be seen as the “leaders” of the team. During rounds, one attending was noted to explain his role in ensuring that the team was building connections with others: “When we have a bad outcome, if it feels like your soul has been ripped out, then you’ve done something right. You’ve made that connection with the patient. My job, as your coach, was to build communication between all of us so we feel vested in each other and our patients.”
Attendings also fostered clinical reasoning skills in their learners by encouraging them to verbalize their thought processes aloud in order to clarify and check for understanding. Attendings also placed emphasis not simply on memorizing content but rather prioritization of the patient’s problems and thinking step by step through individual medical problems. One current learner applauded an attending who could “come up with schematics of how to approach problems rather than feeding us factual information of this paper or this trial.”
Additionally, attendings facilitated learning across the entire care team by differentiating their teaching to meet the needs of multiple learning levels. While the entire team was explicitly included in the learning process, attendings encouraged learners to play various roles, execute tasks, and answer questions depending on their educational level. Attendings positioned learners as leaders of the team by allowing them to talk without interruption and by encouraging them to take ownership of their patients’ care. One former learner stated, “She set expectations…we would be the ones who would be running the team, that you know it would very much be our team and that she is there to advise us and provide supervision but also safety for the patients as well.”
CONCLUSION
This study reveals the complex ways effective attendings build rapport, create a safe learning environment, utilize patient-centered teaching strategies, and engage in collaboration and coaching with all members of the team. These findings provide a framework of shared themes and their salient behaviors that may influence the success of inpatient general medicine clinician educators (Table 3).
There is a broad and voluminous literature on the subject of outstanding clinical teaching characteristics, much of which has shaped various faculty development curricula for decades. This study sought not to identify novel approaches of inpatient teaching necessarily but rather to closely examine the techniques and behaviors of clinician educators identified as exemplary. The findings affirm and reinforce the numerous, well-documented lists of personal attributes, techniques, and behaviors that resonate with learners, including creating a positive environment, demonstrating enthusiasm and interest in the learner, reading facial expressions, being student-centered, maintaining a high level of clinical knowledge, and utilizing effective communication skills.18-24 The strengths of this study lie within the nuanced and rich observations and discussions that move beyond learners’ Likert scale evaluations and responses.3-7,12 Input was sought from multiple perspectives on the care team, which provided detail from key stakeholders. Out of these comprehensive data arose several conclusions that extend the research literature on medical education.
In their seminal review, Sutkin et al.18 demonstrate that two thirds of characteristics of outstanding clinical teachers are “noncognitive” and that, “Perhaps what makes a clinical educator truly great depends less on the acquisition of cognitive skills such as medical knowledge and formulating learning objectives, and more on inherent, relationship-based, noncognitive attributes. Whereas cognitive abilities generally involve skills that may be taught and learned, albeit with difficulty, noncognitive abilities represent personal attributes, such as relationship skills, personality types, and emotional states, which are more difficult to develop and teach.”18 Our study, thus, adds to the literature by (1) highlighting examples of techniques and behaviors that encompass the crucial “noncognitive” arena and (2) informing best practices in teaching clinical medicine, especially those that resonate with learners, for future faculty development.
The findings highlight the role that relationships play in the teaching and learning of team-based medicine. Building rapport and sustaining successful relationships are cornerstones of effective teaching.18 For the attendings in this study, this manifested in observable, tangible behaviors such as greeting others by name, joking, using physical touch, and actively involving all team members, regardless of role or level of education. Previous literature has highlighted the importance of showing interest in learners.7,19,25-27 This study provides multiple and varied examples of ways in which interest might be displayed.
For patients, the critical role of relationships was evidenced through rapport building and attention to patients as people outside their acute hospitalization. For instance, attendings regularly put patients’ medical issues into context and anticipated future outpatient challenges. To the authors’ knowledge, previous scholarship has not significantly emphasized this form of contextualized medicine, which involves the mindful consideration of the ongoing needs patients may experience upon transitions of care.
Several participants highlighted humility as an important characteristic of effective clinician educators. Attendings recognized that the field produces more new knowledge than can possibly be assimilated and that uncertainty is a mainstay of modern medical care. Attendings frequently utilized self-deprecation to acknowledge doubt, a technique that created a collaborative environment in which learners also felt safe to ask questions. These findings support the viewpoints by Reilly and Beckman that humility and an appreciation for questions and push-back from learners encourage lifelong learning through role modeling.19,23 In responding to the interviewer’s question “And what happens when [the attending] is wrong?” one learner simply stated, “He makes fun of himself.”
This study has several limitations. First, it was conducted in a limited number of US based healthcare systems. The majority of institutions represented were larger, research intensive hospitals. While these hospitals were purposefully selected to provide a range in geography, size, type, and access to resources, the findings may differ in other settings. Second, it was conducted with a limited number of attendings and learners, which may limit the study’s generalizability. However, enough interviews were conducted to reach data saturation.15 Because evidence for a causal relationship between quality teaching and student and patient outcomes is lacking,18 we must rely on imperfect proxies for teaching excellence, including awards and recognition. This study attempted to identify exemplary educators through various means, but it is recognized that bias is likely. Third, because attendings provided lists of former learners, selection and recall biases may have been introduced, as attendings may have more readily identified former learners with whom they formed strong relationships. Fourth, focus was placed exclusively on teaching and learning within general medicine rounds. This was because there would be ample opportunity for teaching on this service, the structure of the teams and the types of patients would be comparable across sites, and the principal investigator was also a general medicine attending and would have a frame of reference for these types of rounds. Due to this narrow focus, the findings may not be generalizable to other subspecialties. Fifth, attendings were selected through a nonexhaustive method. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Finally, although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team did attempt to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Using a combination of interviews, focus group discussions, and direct observation, we identified consistent techniques and behaviors of excellent teaching attendings during inpatient general medicine rounds. We hope that all levels of clinician educators may use them to elevate their own teaching.
Disclosure
Dr. Saint is on a medical advisory board of Doximity, a new social networking site for physicians, and receives an honorarium. He is also on the scientific advisory board of Jvion, a healthcare technology company. Drs. Houchens, Harrod, Moody, and Ms. Fowler have no conflicts of interest.
1. Accreditation Council for Graduate Medical Education. Common program requirements. 2011. http://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed September 16, 2016.
2. Healthcare Cost and Utilization Project. Overview statistics for inpatient hospital stays. HCUP Facts and Figures: Statistics on Hospital-Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
3. Busari JO, W eggelaar NM, Knottnerus AC, Greidanus PM, Scherpbier AJ. How medical residents perceive the quality of supervision provided by attending doctors in the clinical setting. Med Educ. 2005;39(7):696-703. PubMed
4. Smith CA, Varkey AB, Evans AT, Reilly BM. Evaluating the performance of inpatient attending physicians: a new instrument for today’s teaching hospitals. J Gen Intern Med. 2004;19(7):766-771. PubMed
5. Elnicki DM, Cooper A. Medical students’ perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635-639. PubMed
6. Buchel TL, Edwards FD. Characteristics of effective clinical teachers. Fam Med. 2005;37(1):30-35. PubMed
7. Guarino CM, Ko CY, Baker LC, Klein DJ, Quiter ES, Escarce JJ. Impact of instructional practices on student satisfaction with attendings’ teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7-12. PubMed
8. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. PubMed
9. Beckman TJ. Lessons learned from a peer review of bedside teaching. Acad Med. 2004;79(4):343-346. PubMed
10. Wright SM, Carrese JA. Excellence in role modelling: insight and perspectives from the pros. CMAJ. 2002;167(6):638-643. PubMed
11. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
12. Bergman K, Gaitskill T. Faculty and student perceptions of effective clinical teachers: an extension study. J Prof Nurs. 1990;6(1):33-44. PubMed
13. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications, Inc.; 2013.
14. U.S. News and World Report. Best Medical Schools: Research. 2014. http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed September 16, 2016.
15. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
16. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101.
17. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
18. Sutkin G, Wagner E, Harris I, Schiffer R. What makes a good clinical teacher in medicine? A review of the literature. Acad Med. 2008;83(5):452-466. PubMed
19. Beckman TJ, Lee MC. Proposal for a collaborative approach to clinical teaching. Mayo Clin Proc. 2009;84(4):339-344. PubMed
20. Ramani S. Twelve tips to improve bedside teaching. Med Teach. 2003;25(2):112-115. PubMed
21. Irby DM. What clinical teachers in medicine need to know. Acad Med. 1994;69(5):333-342. PubMed
22. Wiese J, ed. Teaching in the Hospital. Philadelphia, PA: American College of Physicians; 2010.
23. Reilly BM. Inconvenient truths about effective clinical teaching. Lancet. 2007;370(9588):705-711. PubMed
24. Branch WT Jr, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
25. McLeod PJ, Harden RM. Clinical teaching strategies for physicians. Med Teach. 1985;7(2):173-189. PubMed
26. Pinsky LE, Monson D, Irby DM. How excellent teachers are made: reflecting on success to improve teaching. Adv Health Sci Educ Theory Pract. 1998;3(3):207-215. PubMed
27. Ullian JA, Bland CJ, Simpson DE. An alternative approach to defining the role of the clinical teacher. Acad Med. 1994;69(10):832-838. PubMed
Clinician educators face numerous obstacles to their joint mission of facilitating learning while also ensuring high-quality and patient-centered care. Time constraints, including the institution of house officer duty hour limitations,1 shorter lengths of stay for hospitalized patients,2 and competing career responsibilities, combine to create a dynamic learning environment. Additionally, clinician educators must balance the autonomy of their learners with the safety of their patients. They must teach to multiple learning levels and work collaboratively with multiple disciplines to foster an effective team-based approach to patient care. Yet, many clinician educators have no formal training in pedagogical methods.3 Such challenges necessitate increased attention to the work of excellent clinician educators and their respective teaching approaches.
Many studies of clinical teaching rely primarily on survey data of attributes of good clinical teachers.3-7 While some studies have incorporated direct observations of teaching8,9 or interviews with clinician educators or learners,10,11 few have incorporated multiple perspectives from the current team and from former learners in order to provide a comprehensive picture of team-based learning.12
The goal of this study was to gain a thorough understanding, through multiple perspectives, of the techniques and behaviors used by exemplary educators within actual clinical environments. We studied attitudes, behaviors, and approaches of 12 such inpatient clinician educators.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. This approach was used to study the techniques and behaviors of excellent attendings during inpatient general medicine rounds. A modified snowball sampling approach13 was used, meaning individuals known to one member of the research team (SS) were initially contacted and asked to identify clinician educators (also referred to as attendings) for potential inclusion in the study. In an effort to identify attendings from a broad range of medical schools, the “2015 U.S. News and World Report Top Medical Schools: Research” rankings14 were also reviewed, with priority given to the top 25, as these are widely used to represent the best US hospitals. In an attempt to invite attendings from diverse institutions, additional medical schools not in the top 25 as well as historically black medical schools were also included. Division chiefs and chairs of internal medicine and/or directors of internal medicine residency programs at these schools were contacted and asked for recommendations of attendings, both within and outside their institutions, who they considered to be great inpatient teachers. In addition, key experts who have won teaching awards or were known to be specialists in the field of medical education were asked to nominate one or two other outstanding attendings.
By using this sampling method, 59 potential participants were identified. An internet search was conducted to obtain information about the potential participants and their institutions. Organizational characteristics such as geographic location, hospital size and affiliation, and patient population, as well as individual characteristics such as gender, medical education and training, and educational awards received were considered so that a diversity of organizations and backgrounds was represented. The list was narrowed down to 16 attendings who were contacted via e-mail and asked to participate. Interested participants were asked for a list of their current team members and 6 to 10 former learners to contact for interviews and focus groups. Former learners were included in an effort to better understand lasting effects on learners from their exemplary teaching attendings. A total of 12 attending physicians agreed to participate (Table 1). Literature on field methods has shown that 12 interviews are found to be adequate in accomplishing data saturation.15 Although 2 attendings were located at the same institution, we decided to include them given that both are recognized as master clinician educators and were each recommended by several individuals from various institutions. Hospitals were located throughout the US and included both university-affiliated hospitals and Veterans Affairs medical centers. Despite efforts to include physicians from historically black colleges and universities, only one attending was identified, and they declined the request to participate.
Data Collection
Observations. The one-day site visits were mainly conducted by two research team members, a physician (SS) and a medical anthropologist (MH), both of whom have extensive experience in qualitative methods. Teams were not uniform but were generally comprised of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Occasionally, a pharmacist, clinical assistant, or other health professional accompanied the team on rounds. Not infrequently, the bedside nurse would explicitly be included in the discussion regarding his or her specific patient. Each site visit began with observing attendings (N = 12) and current learners (N = 57) during rounds. Each research team member recorded their own observations via handwritten field notes, paying particular attention to group interactions, teaching approach, conversations occurring within and peripheral to the team, patient-team interactions, and the physical environment. By standing outside of the medical team circle and remaining silent during rounds, research team members remained unobtrusive to the discussion and process of rounds. Materials the attendings used during their teaching rounds were also documented and collected. Rounds generally lasted 2 to 3 hours. After each site visit, the research team met to compare and combine field notes.
Interviews and Focus Groups. The research team then conducted individual, semi-structured interviews with the attendings, focus groups with their current team (N = 46), and interviews or focus groups with their former learners (N = 26; Supplement 1). Eleven of the current team members observed during rounds were unable to participate in the focus groups due to clinical duties. Because the current learners who participated in the focus groups were also observed during rounds, the research team was able to ask them open-ended questions regarding teaching rounds and their roles as learners within this environment. Former learners who were still at the hospital participated in separate focus groups or interviews. Former learners who were no longer present at the hospital were contacted by telephone and individually interviewed by one research team member (MH). All interviews and focus groups were audio-recorded and transcribed.
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a thematic analysis approach.16 Thematic analysis entails reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. Once patterns have been identified, they are grouped according to similarity into themes, which help to further explain the findings.17
After the first site visit was completed, the research team members that participated (SS and MH) met to develop initial ideas about meanings and possible patterns. All transcripts were read by one team member (MH) and, based on review of the data, codes were developed, defined, and documented in a codebook. This process was repeated after every site visit using the codebook to expand or combine codes and refine definitions as necessary. If a new code was added, the previously coded data were reviewed to apply the new code. NVivo® 10 software (QSR International; Melbourne, Australia) was used to manage the data.
Once all field notes and transcripts were coded (MH), the code reports, which list all data described within a specific code, were run to ensure consistency and identify relationships between codes. Once coding was verified, codes were grouped based on similarities and relationships into salient themes by 3 members of the research team (NH, MH, and SM). Themes, along with their supporting codes, were then further defined to understand how these attendings worked to facilitate excellent teaching in clinical settings.
RESULTS
The coded interview data and field notes were categorized into broad, overlapping themes. Three of these major themes include (1) fostering positive relationships, (2) patient-centered teaching, and (3) collaboration and coaching. Table 2 lists each theme, salient behaviors, examples, and selected quotes that further elucidate its meaning.
Fostering Positive Relationships
Attending physicians took observable steps to develop positive relationships with their team members, which in turn created a safe learning environment. For instance, attendings used learners’ first names, demonstrated interest in their well-being, deployed humor, and generally displayed informal actions—uncrossed arms, “fist bump” when recognizing learners’ success, standing outside the circle of team members and leaning in to listen—during learner interactions. Attendings also made it a priority to get to know individuals on a personal level. As one current learner put it, “He asks about where we are from. He will try to find some kind of connection that he can establish with not only each of the team members but also with each of the patients.”
Additionally, attendings built positive relationships with their learners by responding thoughtfully to their input, even when learners’ evaluations of patients required modification. In turn, learners reported feeling safe to ask questions, admit uncertainty, and respectfully disagree with their attendings. As one attending reflected, “If I can get them into a place where they feel like the learning environment is someplace where they can make a mistake and know that that mistake does not necessarily mean that it’s going to cost them in their evaluation part, then I feel like that’s why it’s important.”
To build rapport and create a safe learning environment, attendings used a number of strategies to position themselves as learners alongside their team members. For instance, attendings indicated that they wanted their ideas questioned because they saw it as an opportunity to learn. Moreover, in conversations with learners, attendings demonstrated humility, admitting when they did not know something. One former learner noted, “There have been times when he has asked [a] question…nobody knows and then he admits that he doesn’t know either. So everybody goes and looks it up…The whole thing turns out to be a fun learning experience.”
Attendings demonstrated respect for their team members’ time by reading about patients before rounds, identifying learning opportunities during rounds, and integrating teaching points into the daily work of patient care. Teaching was not relegated exclusively to the conference room or confined to the traditional “chalk talk” before or after rounds but rather was assimilated into daily workflow. They appeared to be responsive to the needs of individual patients and the team, which allowed attendings to both directly oversee their patients’ care and overcome the challenges of multiple competing demands for time. The importance of this approach was made clear by one current learner who stated “…she does prepare before, especially you know on call days, she does prepare for the new patients before coming in to staff, which is really appreciated… it saves a lot of time on rounds.”
Attendings also included other health professionals in team discussions. Attendings used many of the same relationship-building techniques with these professionals as they did with learners and patients. They consistently asked these professionals to provide insight and direction in patients’ plans of care. A former learner commented, “He always asks the [nurse] what is her impression of the patient...he truly values the [nurse’s] opinion of the patient.” One attending reiterated this approach, stating “I don’t want them to think that anything I have to say is more valuable than our pharmacist or the [nurse].”
Patient-Centered Teaching
Attending physicians modeled numerous teaching techniques that focused learning around the patient. Attendings knew their patients well through review of the medical records, discussion with the patient, and personal examination. This preparation allowed attendings to focus on key teaching points in the context of the patient. One former learner noted, “He tended to bring up a variety of things that really fit well into the clinical scenario. So whether that is talking about what is the differential for a new symptom that just came up for this patient or kind of here is a new paper talking about this condition or maybe some other pearl of physical exam for a patient that has a certain physical condition.”
Attendings served as effective role models by being directly involved in examining and talking with patients as well as demonstrating excellent physical examination and communication techniques. One current learner articulated the importance of learning these skills by observing them done well: “I think he teaches by example and by doing, again, those little things: being attentive to the patients and being very careful during exams…I think those are things that you teach people by doing them, not by saying you need to do this better during the patient encounter.”
Collaboration and Coaching
Attending physicians used varied collaboration and coaching techniques to facilitate learning across the entire care team. During rounds, attendings utilized visual aids to reinforce key concepts and simplify complex topics. They also collaborated by using discussion rather than lecture to engage with team members. For instance, attendings used Socratic questioning, asking questions that lead learners through critical thinking and allow them to solve problems themselves, to guide learners’ decision-making. One former learner reported, “He never gives you the answer, and he always asks your opinion; ‘So what are your thoughts on this?’”
Coaching for success, rather than directing the various team members, was emphasized. Attendings did not wish to be seen as the “leaders” of the team. During rounds, one attending was noted to explain his role in ensuring that the team was building connections with others: “When we have a bad outcome, if it feels like your soul has been ripped out, then you’ve done something right. You’ve made that connection with the patient. My job, as your coach, was to build communication between all of us so we feel vested in each other and our patients.”
Attendings also fostered clinical reasoning skills in their learners by encouraging them to verbalize their thought processes aloud in order to clarify and check for understanding. Attendings also placed emphasis not simply on memorizing content but rather prioritization of the patient’s problems and thinking step by step through individual medical problems. One current learner applauded an attending who could “come up with schematics of how to approach problems rather than feeding us factual information of this paper or this trial.”
Additionally, attendings facilitated learning across the entire care team by differentiating their teaching to meet the needs of multiple learning levels. While the entire team was explicitly included in the learning process, attendings encouraged learners to play various roles, execute tasks, and answer questions depending on their educational level. Attendings positioned learners as leaders of the team by allowing them to talk without interruption and by encouraging them to take ownership of their patients’ care. One former learner stated, “She set expectations…we would be the ones who would be running the team, that you know it would very much be our team and that she is there to advise us and provide supervision but also safety for the patients as well.”
CONCLUSION
This study reveals the complex ways effective attendings build rapport, create a safe learning environment, utilize patient-centered teaching strategies, and engage in collaboration and coaching with all members of the team. These findings provide a framework of shared themes and their salient behaviors that may influence the success of inpatient general medicine clinician educators (Table 3).
There is a broad and voluminous literature on the subject of outstanding clinical teaching characteristics, much of which has shaped various faculty development curricula for decades. This study sought not to identify novel approaches of inpatient teaching necessarily but rather to closely examine the techniques and behaviors of clinician educators identified as exemplary. The findings affirm and reinforce the numerous, well-documented lists of personal attributes, techniques, and behaviors that resonate with learners, including creating a positive environment, demonstrating enthusiasm and interest in the learner, reading facial expressions, being student-centered, maintaining a high level of clinical knowledge, and utilizing effective communication skills.18-24 The strengths of this study lie within the nuanced and rich observations and discussions that move beyond learners’ Likert scale evaluations and responses.3-7,12 Input was sought from multiple perspectives on the care team, which provided detail from key stakeholders. Out of these comprehensive data arose several conclusions that extend the research literature on medical education.
In their seminal review, Sutkin et al.18 demonstrate that two thirds of characteristics of outstanding clinical teachers are “noncognitive” and that, “Perhaps what makes a clinical educator truly great depends less on the acquisition of cognitive skills such as medical knowledge and formulating learning objectives, and more on inherent, relationship-based, noncognitive attributes. Whereas cognitive abilities generally involve skills that may be taught and learned, albeit with difficulty, noncognitive abilities represent personal attributes, such as relationship skills, personality types, and emotional states, which are more difficult to develop and teach.”18 Our study, thus, adds to the literature by (1) highlighting examples of techniques and behaviors that encompass the crucial “noncognitive” arena and (2) informing best practices in teaching clinical medicine, especially those that resonate with learners, for future faculty development.
The findings highlight the role that relationships play in the teaching and learning of team-based medicine. Building rapport and sustaining successful relationships are cornerstones of effective teaching.18 For the attendings in this study, this manifested in observable, tangible behaviors such as greeting others by name, joking, using physical touch, and actively involving all team members, regardless of role or level of education. Previous literature has highlighted the importance of showing interest in learners.7,19,25-27 This study provides multiple and varied examples of ways in which interest might be displayed.
For patients, the critical role of relationships was evidenced through rapport building and attention to patients as people outside their acute hospitalization. For instance, attendings regularly put patients’ medical issues into context and anticipated future outpatient challenges. To the authors’ knowledge, previous scholarship has not significantly emphasized this form of contextualized medicine, which involves the mindful consideration of the ongoing needs patients may experience upon transitions of care.
Several participants highlighted humility as an important characteristic of effective clinician educators. Attendings recognized that the field produces more new knowledge than can possibly be assimilated and that uncertainty is a mainstay of modern medical care. Attendings frequently utilized self-deprecation to acknowledge doubt, a technique that created a collaborative environment in which learners also felt safe to ask questions. These findings support the viewpoints by Reilly and Beckman that humility and an appreciation for questions and push-back from learners encourage lifelong learning through role modeling.19,23 In responding to the interviewer’s question “And what happens when [the attending] is wrong?” one learner simply stated, “He makes fun of himself.”
This study has several limitations. First, it was conducted in a limited number of US based healthcare systems. The majority of institutions represented were larger, research intensive hospitals. While these hospitals were purposefully selected to provide a range in geography, size, type, and access to resources, the findings may differ in other settings. Second, it was conducted with a limited number of attendings and learners, which may limit the study’s generalizability. However, enough interviews were conducted to reach data saturation.15 Because evidence for a causal relationship between quality teaching and student and patient outcomes is lacking,18 we must rely on imperfect proxies for teaching excellence, including awards and recognition. This study attempted to identify exemplary educators through various means, but it is recognized that bias is likely. Third, because attendings provided lists of former learners, selection and recall biases may have been introduced, as attendings may have more readily identified former learners with whom they formed strong relationships. Fourth, focus was placed exclusively on teaching and learning within general medicine rounds. This was because there would be ample opportunity for teaching on this service, the structure of the teams and the types of patients would be comparable across sites, and the principal investigator was also a general medicine attending and would have a frame of reference for these types of rounds. Due to this narrow focus, the findings may not be generalizable to other subspecialties. Fifth, attendings were selected through a nonexhaustive method. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Finally, although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team did attempt to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Using a combination of interviews, focus group discussions, and direct observation, we identified consistent techniques and behaviors of excellent teaching attendings during inpatient general medicine rounds. We hope that all levels of clinician educators may use them to elevate their own teaching.
Disclosure
Dr. Saint is on a medical advisory board of Doximity, a new social networking site for physicians, and receives an honorarium. He is also on the scientific advisory board of Jvion, a healthcare technology company. Drs. Houchens, Harrod, Moody, and Ms. Fowler have no conflicts of interest.
Clinician educators face numerous obstacles to their joint mission of facilitating learning while also ensuring high-quality and patient-centered care. Time constraints, including the institution of house officer duty hour limitations,1 shorter lengths of stay for hospitalized patients,2 and competing career responsibilities, combine to create a dynamic learning environment. Additionally, clinician educators must balance the autonomy of their learners with the safety of their patients. They must teach to multiple learning levels and work collaboratively with multiple disciplines to foster an effective team-based approach to patient care. Yet, many clinician educators have no formal training in pedagogical methods.3 Such challenges necessitate increased attention to the work of excellent clinician educators and their respective teaching approaches.
Many studies of clinical teaching rely primarily on survey data of attributes of good clinical teachers.3-7 While some studies have incorporated direct observations of teaching8,9 or interviews with clinician educators or learners,10,11 few have incorporated multiple perspectives from the current team and from former learners in order to provide a comprehensive picture of team-based learning.12
The goal of this study was to gain a thorough understanding, through multiple perspectives, of the techniques and behaviors used by exemplary educators within actual clinical environments. We studied attitudes, behaviors, and approaches of 12 such inpatient clinician educators.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. This approach was used to study the techniques and behaviors of excellent attendings during inpatient general medicine rounds. A modified snowball sampling approach13 was used, meaning individuals known to one member of the research team (SS) were initially contacted and asked to identify clinician educators (also referred to as attendings) for potential inclusion in the study. In an effort to identify attendings from a broad range of medical schools, the “2015 U.S. News and World Report Top Medical Schools: Research” rankings14 were also reviewed, with priority given to the top 25, as these are widely used to represent the best US hospitals. In an attempt to invite attendings from diverse institutions, additional medical schools not in the top 25 as well as historically black medical schools were also included. Division chiefs and chairs of internal medicine and/or directors of internal medicine residency programs at these schools were contacted and asked for recommendations of attendings, both within and outside their institutions, who they considered to be great inpatient teachers. In addition, key experts who have won teaching awards or were known to be specialists in the field of medical education were asked to nominate one or two other outstanding attendings.
By using this sampling method, 59 potential participants were identified. An internet search was conducted to obtain information about the potential participants and their institutions. Organizational characteristics such as geographic location, hospital size and affiliation, and patient population, as well as individual characteristics such as gender, medical education and training, and educational awards received were considered so that a diversity of organizations and backgrounds was represented. The list was narrowed down to 16 attendings who were contacted via e-mail and asked to participate. Interested participants were asked for a list of their current team members and 6 to 10 former learners to contact for interviews and focus groups. Former learners were included in an effort to better understand lasting effects on learners from their exemplary teaching attendings. A total of 12 attending physicians agreed to participate (Table 1). Literature on field methods has shown that 12 interviews are found to be adequate in accomplishing data saturation.15 Although 2 attendings were located at the same institution, we decided to include them given that both are recognized as master clinician educators and were each recommended by several individuals from various institutions. Hospitals were located throughout the US and included both university-affiliated hospitals and Veterans Affairs medical centers. Despite efforts to include physicians from historically black colleges and universities, only one attending was identified, and they declined the request to participate.
Data Collection
Observations. The one-day site visits were mainly conducted by two research team members, a physician (SS) and a medical anthropologist (MH), both of whom have extensive experience in qualitative methods. Teams were not uniform but were generally comprised of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Occasionally, a pharmacist, clinical assistant, or other health professional accompanied the team on rounds. Not infrequently, the bedside nurse would explicitly be included in the discussion regarding his or her specific patient. Each site visit began with observing attendings (N = 12) and current learners (N = 57) during rounds. Each research team member recorded their own observations via handwritten field notes, paying particular attention to group interactions, teaching approach, conversations occurring within and peripheral to the team, patient-team interactions, and the physical environment. By standing outside of the medical team circle and remaining silent during rounds, research team members remained unobtrusive to the discussion and process of rounds. Materials the attendings used during their teaching rounds were also documented and collected. Rounds generally lasted 2 to 3 hours. After each site visit, the research team met to compare and combine field notes.
Interviews and Focus Groups. The research team then conducted individual, semi-structured interviews with the attendings, focus groups with their current team (N = 46), and interviews or focus groups with their former learners (N = 26; Supplement 1). Eleven of the current team members observed during rounds were unable to participate in the focus groups due to clinical duties. Because the current learners who participated in the focus groups were also observed during rounds, the research team was able to ask them open-ended questions regarding teaching rounds and their roles as learners within this environment. Former learners who were still at the hospital participated in separate focus groups or interviews. Former learners who were no longer present at the hospital were contacted by telephone and individually interviewed by one research team member (MH). All interviews and focus groups were audio-recorded and transcribed.
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a thematic analysis approach.16 Thematic analysis entails reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. Once patterns have been identified, they are grouped according to similarity into themes, which help to further explain the findings.17
After the first site visit was completed, the research team members that participated (SS and MH) met to develop initial ideas about meanings and possible patterns. All transcripts were read by one team member (MH) and, based on review of the data, codes were developed, defined, and documented in a codebook. This process was repeated after every site visit using the codebook to expand or combine codes and refine definitions as necessary. If a new code was added, the previously coded data were reviewed to apply the new code. NVivo® 10 software (QSR International; Melbourne, Australia) was used to manage the data.
Once all field notes and transcripts were coded (MH), the code reports, which list all data described within a specific code, were run to ensure consistency and identify relationships between codes. Once coding was verified, codes were grouped based on similarities and relationships into salient themes by 3 members of the research team (NH, MH, and SM). Themes, along with their supporting codes, were then further defined to understand how these attendings worked to facilitate excellent teaching in clinical settings.
RESULTS
The coded interview data and field notes were categorized into broad, overlapping themes. Three of these major themes include (1) fostering positive relationships, (2) patient-centered teaching, and (3) collaboration and coaching. Table 2 lists each theme, salient behaviors, examples, and selected quotes that further elucidate its meaning.
Fostering Positive Relationships
Attending physicians took observable steps to develop positive relationships with their team members, which in turn created a safe learning environment. For instance, attendings used learners’ first names, demonstrated interest in their well-being, deployed humor, and generally displayed informal actions—uncrossed arms, “fist bump” when recognizing learners’ success, standing outside the circle of team members and leaning in to listen—during learner interactions. Attendings also made it a priority to get to know individuals on a personal level. As one current learner put it, “He asks about where we are from. He will try to find some kind of connection that he can establish with not only each of the team members but also with each of the patients.”
Additionally, attendings built positive relationships with their learners by responding thoughtfully to their input, even when learners’ evaluations of patients required modification. In turn, learners reported feeling safe to ask questions, admit uncertainty, and respectfully disagree with their attendings. As one attending reflected, “If I can get them into a place where they feel like the learning environment is someplace where they can make a mistake and know that that mistake does not necessarily mean that it’s going to cost them in their evaluation part, then I feel like that’s why it’s important.”
To build rapport and create a safe learning environment, attendings used a number of strategies to position themselves as learners alongside their team members. For instance, attendings indicated that they wanted their ideas questioned because they saw it as an opportunity to learn. Moreover, in conversations with learners, attendings demonstrated humility, admitting when they did not know something. One former learner noted, “There have been times when he has asked [a] question…nobody knows and then he admits that he doesn’t know either. So everybody goes and looks it up…The whole thing turns out to be a fun learning experience.”
Attendings demonstrated respect for their team members’ time by reading about patients before rounds, identifying learning opportunities during rounds, and integrating teaching points into the daily work of patient care. Teaching was not relegated exclusively to the conference room or confined to the traditional “chalk talk” before or after rounds but rather was assimilated into daily workflow. They appeared to be responsive to the needs of individual patients and the team, which allowed attendings to both directly oversee their patients’ care and overcome the challenges of multiple competing demands for time. The importance of this approach was made clear by one current learner who stated “…she does prepare before, especially you know on call days, she does prepare for the new patients before coming in to staff, which is really appreciated… it saves a lot of time on rounds.”
Attendings also included other health professionals in team discussions. Attendings used many of the same relationship-building techniques with these professionals as they did with learners and patients. They consistently asked these professionals to provide insight and direction in patients’ plans of care. A former learner commented, “He always asks the [nurse] what is her impression of the patient...he truly values the [nurse’s] opinion of the patient.” One attending reiterated this approach, stating “I don’t want them to think that anything I have to say is more valuable than our pharmacist or the [nurse].”
Patient-Centered Teaching
Attending physicians modeled numerous teaching techniques that focused learning around the patient. Attendings knew their patients well through review of the medical records, discussion with the patient, and personal examination. This preparation allowed attendings to focus on key teaching points in the context of the patient. One former learner noted, “He tended to bring up a variety of things that really fit well into the clinical scenario. So whether that is talking about what is the differential for a new symptom that just came up for this patient or kind of here is a new paper talking about this condition or maybe some other pearl of physical exam for a patient that has a certain physical condition.”
Attendings served as effective role models by being directly involved in examining and talking with patients as well as demonstrating excellent physical examination and communication techniques. One current learner articulated the importance of learning these skills by observing them done well: “I think he teaches by example and by doing, again, those little things: being attentive to the patients and being very careful during exams…I think those are things that you teach people by doing them, not by saying you need to do this better during the patient encounter.”
Collaboration and Coaching
Attending physicians used varied collaboration and coaching techniques to facilitate learning across the entire care team. During rounds, attendings utilized visual aids to reinforce key concepts and simplify complex topics. They also collaborated by using discussion rather than lecture to engage with team members. For instance, attendings used Socratic questioning, asking questions that lead learners through critical thinking and allow them to solve problems themselves, to guide learners’ decision-making. One former learner reported, “He never gives you the answer, and he always asks your opinion; ‘So what are your thoughts on this?’”
Coaching for success, rather than directing the various team members, was emphasized. Attendings did not wish to be seen as the “leaders” of the team. During rounds, one attending was noted to explain his role in ensuring that the team was building connections with others: “When we have a bad outcome, if it feels like your soul has been ripped out, then you’ve done something right. You’ve made that connection with the patient. My job, as your coach, was to build communication between all of us so we feel vested in each other and our patients.”
Attendings also fostered clinical reasoning skills in their learners by encouraging them to verbalize their thought processes aloud in order to clarify and check for understanding. Attendings also placed emphasis not simply on memorizing content but rather prioritization of the patient’s problems and thinking step by step through individual medical problems. One current learner applauded an attending who could “come up with schematics of how to approach problems rather than feeding us factual information of this paper or this trial.”
Additionally, attendings facilitated learning across the entire care team by differentiating their teaching to meet the needs of multiple learning levels. While the entire team was explicitly included in the learning process, attendings encouraged learners to play various roles, execute tasks, and answer questions depending on their educational level. Attendings positioned learners as leaders of the team by allowing them to talk without interruption and by encouraging them to take ownership of their patients’ care. One former learner stated, “She set expectations…we would be the ones who would be running the team, that you know it would very much be our team and that she is there to advise us and provide supervision but also safety for the patients as well.”
CONCLUSION
This study reveals the complex ways effective attendings build rapport, create a safe learning environment, utilize patient-centered teaching strategies, and engage in collaboration and coaching with all members of the team. These findings provide a framework of shared themes and their salient behaviors that may influence the success of inpatient general medicine clinician educators (Table 3).
There is a broad and voluminous literature on the subject of outstanding clinical teaching characteristics, much of which has shaped various faculty development curricula for decades. This study sought not to identify novel approaches of inpatient teaching necessarily but rather to closely examine the techniques and behaviors of clinician educators identified as exemplary. The findings affirm and reinforce the numerous, well-documented lists of personal attributes, techniques, and behaviors that resonate with learners, including creating a positive environment, demonstrating enthusiasm and interest in the learner, reading facial expressions, being student-centered, maintaining a high level of clinical knowledge, and utilizing effective communication skills.18-24 The strengths of this study lie within the nuanced and rich observations and discussions that move beyond learners’ Likert scale evaluations and responses.3-7,12 Input was sought from multiple perspectives on the care team, which provided detail from key stakeholders. Out of these comprehensive data arose several conclusions that extend the research literature on medical education.
In their seminal review, Sutkin et al.18 demonstrate that two thirds of characteristics of outstanding clinical teachers are “noncognitive” and that, “Perhaps what makes a clinical educator truly great depends less on the acquisition of cognitive skills such as medical knowledge and formulating learning objectives, and more on inherent, relationship-based, noncognitive attributes. Whereas cognitive abilities generally involve skills that may be taught and learned, albeit with difficulty, noncognitive abilities represent personal attributes, such as relationship skills, personality types, and emotional states, which are more difficult to develop and teach.”18 Our study, thus, adds to the literature by (1) highlighting examples of techniques and behaviors that encompass the crucial “noncognitive” arena and (2) informing best practices in teaching clinical medicine, especially those that resonate with learners, for future faculty development.
The findings highlight the role that relationships play in the teaching and learning of team-based medicine. Building rapport and sustaining successful relationships are cornerstones of effective teaching.18 For the attendings in this study, this manifested in observable, tangible behaviors such as greeting others by name, joking, using physical touch, and actively involving all team members, regardless of role or level of education. Previous literature has highlighted the importance of showing interest in learners.7,19,25-27 This study provides multiple and varied examples of ways in which interest might be displayed.
For patients, the critical role of relationships was evidenced through rapport building and attention to patients as people outside their acute hospitalization. For instance, attendings regularly put patients’ medical issues into context and anticipated future outpatient challenges. To the authors’ knowledge, previous scholarship has not significantly emphasized this form of contextualized medicine, which involves the mindful consideration of the ongoing needs patients may experience upon transitions of care.
Several participants highlighted humility as an important characteristic of effective clinician educators. Attendings recognized that the field produces more new knowledge than can possibly be assimilated and that uncertainty is a mainstay of modern medical care. Attendings frequently utilized self-deprecation to acknowledge doubt, a technique that created a collaborative environment in which learners also felt safe to ask questions. These findings support the viewpoints by Reilly and Beckman that humility and an appreciation for questions and push-back from learners encourage lifelong learning through role modeling.19,23 In responding to the interviewer’s question “And what happens when [the attending] is wrong?” one learner simply stated, “He makes fun of himself.”
This study has several limitations. First, it was conducted in a limited number of US based healthcare systems. The majority of institutions represented were larger, research intensive hospitals. While these hospitals were purposefully selected to provide a range in geography, size, type, and access to resources, the findings may differ in other settings. Second, it was conducted with a limited number of attendings and learners, which may limit the study’s generalizability. However, enough interviews were conducted to reach data saturation.15 Because evidence for a causal relationship between quality teaching and student and patient outcomes is lacking,18 we must rely on imperfect proxies for teaching excellence, including awards and recognition. This study attempted to identify exemplary educators through various means, but it is recognized that bias is likely. Third, because attendings provided lists of former learners, selection and recall biases may have been introduced, as attendings may have more readily identified former learners with whom they formed strong relationships. Fourth, focus was placed exclusively on teaching and learning within general medicine rounds. This was because there would be ample opportunity for teaching on this service, the structure of the teams and the types of patients would be comparable across sites, and the principal investigator was also a general medicine attending and would have a frame of reference for these types of rounds. Due to this narrow focus, the findings may not be generalizable to other subspecialties. Fifth, attendings were selected through a nonexhaustive method. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Finally, although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team did attempt to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Using a combination of interviews, focus group discussions, and direct observation, we identified consistent techniques and behaviors of excellent teaching attendings during inpatient general medicine rounds. We hope that all levels of clinician educators may use them to elevate their own teaching.
Disclosure
Dr. Saint is on a medical advisory board of Doximity, a new social networking site for physicians, and receives an honorarium. He is also on the scientific advisory board of Jvion, a healthcare technology company. Drs. Houchens, Harrod, Moody, and Ms. Fowler have no conflicts of interest.
1. Accreditation Council for Graduate Medical Education. Common program requirements. 2011. http://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed September 16, 2016.
2. Healthcare Cost and Utilization Project. Overview statistics for inpatient hospital stays. HCUP Facts and Figures: Statistics on Hospital-Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
3. Busari JO, W eggelaar NM, Knottnerus AC, Greidanus PM, Scherpbier AJ. How medical residents perceive the quality of supervision provided by attending doctors in the clinical setting. Med Educ. 2005;39(7):696-703. PubMed
4. Smith CA, Varkey AB, Evans AT, Reilly BM. Evaluating the performance of inpatient attending physicians: a new instrument for today’s teaching hospitals. J Gen Intern Med. 2004;19(7):766-771. PubMed
5. Elnicki DM, Cooper A. Medical students’ perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635-639. PubMed
6. Buchel TL, Edwards FD. Characteristics of effective clinical teachers. Fam Med. 2005;37(1):30-35. PubMed
7. Guarino CM, Ko CY, Baker LC, Klein DJ, Quiter ES, Escarce JJ. Impact of instructional practices on student satisfaction with attendings’ teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7-12. PubMed
8. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. PubMed
9. Beckman TJ. Lessons learned from a peer review of bedside teaching. Acad Med. 2004;79(4):343-346. PubMed
10. Wright SM, Carrese JA. Excellence in role modelling: insight and perspectives from the pros. CMAJ. 2002;167(6):638-643. PubMed
11. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
12. Bergman K, Gaitskill T. Faculty and student perceptions of effective clinical teachers: an extension study. J Prof Nurs. 1990;6(1):33-44. PubMed
13. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications, Inc.; 2013.
14. U.S. News and World Report. Best Medical Schools: Research. 2014. http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed September 16, 2016.
15. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
16. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101.
17. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
18. Sutkin G, Wagner E, Harris I, Schiffer R. What makes a good clinical teacher in medicine? A review of the literature. Acad Med. 2008;83(5):452-466. PubMed
19. Beckman TJ, Lee MC. Proposal for a collaborative approach to clinical teaching. Mayo Clin Proc. 2009;84(4):339-344. PubMed
20. Ramani S. Twelve tips to improve bedside teaching. Med Teach. 2003;25(2):112-115. PubMed
21. Irby DM. What clinical teachers in medicine need to know. Acad Med. 1994;69(5):333-342. PubMed
22. Wiese J, ed. Teaching in the Hospital. Philadelphia, PA: American College of Physicians; 2010.
23. Reilly BM. Inconvenient truths about effective clinical teaching. Lancet. 2007;370(9588):705-711. PubMed
24. Branch WT Jr, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
25. McLeod PJ, Harden RM. Clinical teaching strategies for physicians. Med Teach. 1985;7(2):173-189. PubMed
26. Pinsky LE, Monson D, Irby DM. How excellent teachers are made: reflecting on success to improve teaching. Adv Health Sci Educ Theory Pract. 1998;3(3):207-215. PubMed
27. Ullian JA, Bland CJ, Simpson DE. An alternative approach to defining the role of the clinical teacher. Acad Med. 1994;69(10):832-838. PubMed
1. Accreditation Council for Graduate Medical Education. Common program requirements. 2011. http://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed September 16, 2016.
2. Healthcare Cost and Utilization Project. Overview statistics for inpatient hospital stays. HCUP Facts and Figures: Statistics on Hospital-Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
3. Busari JO, W eggelaar NM, Knottnerus AC, Greidanus PM, Scherpbier AJ. How medical residents perceive the quality of supervision provided by attending doctors in the clinical setting. Med Educ. 2005;39(7):696-703. PubMed
4. Smith CA, Varkey AB, Evans AT, Reilly BM. Evaluating the performance of inpatient attending physicians: a new instrument for today’s teaching hospitals. J Gen Intern Med. 2004;19(7):766-771. PubMed
5. Elnicki DM, Cooper A. Medical students’ perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635-639. PubMed
6. Buchel TL, Edwards FD. Characteristics of effective clinical teachers. Fam Med. 2005;37(1):30-35. PubMed
7. Guarino CM, Ko CY, Baker LC, Klein DJ, Quiter ES, Escarce JJ. Impact of instructional practices on student satisfaction with attendings’ teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7-12. PubMed
8. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. PubMed
9. Beckman TJ. Lessons learned from a peer review of bedside teaching. Acad Med. 2004;79(4):343-346. PubMed
10. Wright SM, Carrese JA. Excellence in role modelling: insight and perspectives from the pros. CMAJ. 2002;167(6):638-643. PubMed
11. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
12. Bergman K, Gaitskill T. Faculty and student perceptions of effective clinical teachers: an extension study. J Prof Nurs. 1990;6(1):33-44. PubMed
13. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications, Inc.; 2013.
14. U.S. News and World Report. Best Medical Schools: Research. 2014. http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed September 16, 2016.
15. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
16. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101.
17. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
18. Sutkin G, Wagner E, Harris I, Schiffer R. What makes a good clinical teacher in medicine? A review of the literature. Acad Med. 2008;83(5):452-466. PubMed
19. Beckman TJ, Lee MC. Proposal for a collaborative approach to clinical teaching. Mayo Clin Proc. 2009;84(4):339-344. PubMed
20. Ramani S. Twelve tips to improve bedside teaching. Med Teach. 2003;25(2):112-115. PubMed
21. Irby DM. What clinical teachers in medicine need to know. Acad Med. 1994;69(5):333-342. PubMed
22. Wiese J, ed. Teaching in the Hospital. Philadelphia, PA: American College of Physicians; 2010.
23. Reilly BM. Inconvenient truths about effective clinical teaching. Lancet. 2007;370(9588):705-711. PubMed
24. Branch WT Jr, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
25. McLeod PJ, Harden RM. Clinical teaching strategies for physicians. Med Teach. 1985;7(2):173-189. PubMed
26. Pinsky LE, Monson D, Irby DM. How excellent teachers are made: reflecting on success to improve teaching. Adv Health Sci Educ Theory Pract. 1998;3(3):207-215. PubMed
27. Ullian JA, Bland CJ, Simpson DE. An alternative approach to defining the role of the clinical teacher. Acad Med. 1994;69(10):832-838. PubMed
© 2017 Society of Hospital Medicine
Taking the Detour
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal <12 mm).
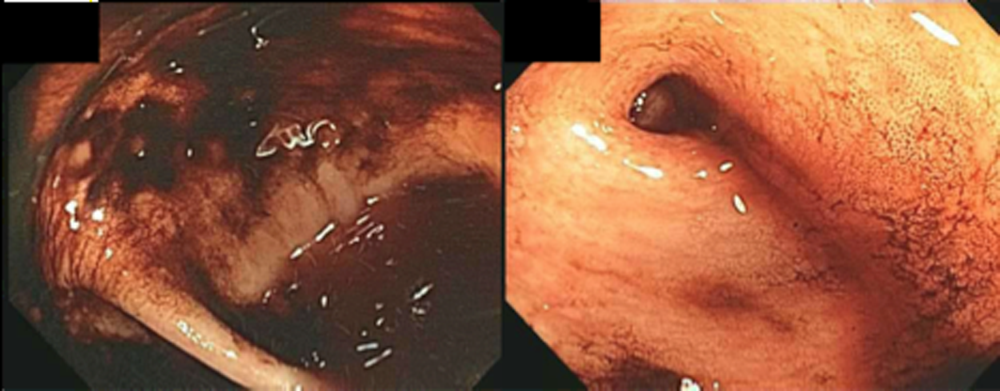
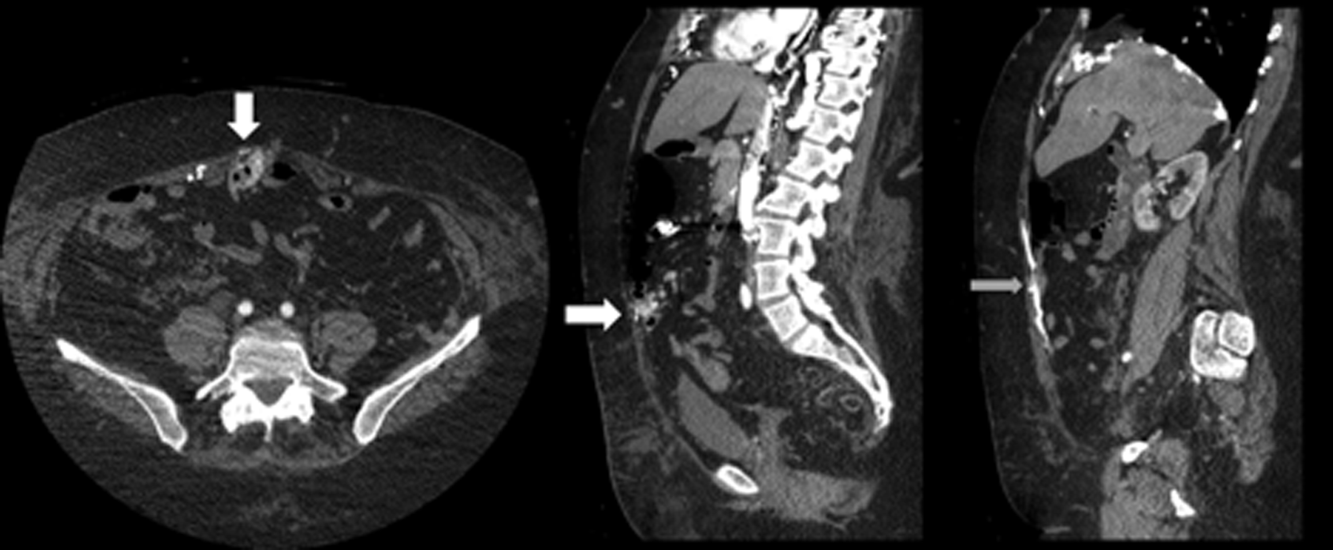
The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal <12 mm).


The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal <12 mm).


The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
Bigger Than His Bite
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.
Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.


Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.


Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
Evaluating an Academic Hospitalist Service
Improving quality while reducing costs remains important for hospitals across the United States, including the approximately 150 hospitals that are part of the Veterans Affairs (VA) healthcare system.[1, 2] The field of hospital medicine has grown rapidly, leading to predictions that the majority of inpatient care in the United States eventually will be delivered by hospitalists.[3, 4] In 2010, 57% of US hospitals had hospitalists on staff, including 87% of hospitals with 200 beds,[5] and nearly 80% of VA hospitals.[6]
The demand for hospitalists within teaching hospitals has grown in part as a response to the mandate to reduce residency work hours.[7] Furthermore, previous research has found that hospitalist care is associated with modest reductions in length of stay (LOS) and weak but inconsistent differences in quality.[8] The educational effect of hospitalists has been far less examined. The limited number of studies published to date suggests that hospitalists may improve resident learning and house‐officer satisfaction in academic medical centers and community teaching hospitals[9, 10, 11] and provide positive experiences for medical students12,13; however, Wachter et al reported no significant changes in clinical outcomes or patient, faculty, and house‐staff satisfaction in a newly designed hospital medicine service in San Francisco.[14] Additionally, whether using hospitalists influences nurse‐physician communication[15] is unknown.
Recognizing the limited and sometimes conflicting evidence about the hospitalist model, we report the results of a 3‐year quasi‐experimental evaluation of the experience at our medical center with academic hospitalists. As part of a VA Systems Redesign Improvement Capability Grantknown as the Hospital Outcomes Program of Excellence (HOPE) Initiativewe created a hospitalist‐based medicine team focused on quality improvement, medical education, and patient outcomes.
METHODS
Setting and Design
The main hospital of the VA Ann Arbor Healthcare System, located in Ann Arbor, Michigan, operates 105 acute‐care beds and 40 extended‐care beds. At the time of this evaluation, the medicine service consisted of 4 internal medicine teamsGold, Silver, Burgundy, and Yelloweach of which was responsible for admitting patients on a rotating basis every fourth day, with limited numbers of admissions occurring between each team's primary admitting day. Each team is led by an attending physician, a board‐certified (or board‐eligible) general internist or subspecialist who is also a faculty member at the University of Michigan Medical School. Each team has a senior medical resident, 2 to 3 interns, and 3 to 5 medical students (mostly third‐year students). In total, there are approximately 50 senior medical residents, 60 interns, and 170 medical students who rotate through the medicine service each year. Traditional rounding involves the medical students and interns receiving sign‐out from the overnight team in the morning, then pre‐rounding on each patient by obtaining an interval history, performing an exam, and checking any test results. A tentative plan of care is formed with the senior medical resident, usually by discussing each patient very quickly in the team room. Attending rounds are then conducted, with the physician team visiting each patient one by one to review and plan all aspects of care in detail. When time allows, small segments of teaching may occur during these attending work rounds. This system had been in place for >20 years.
Resulting in part from a grant received from the VA Systems Redesign Central Office (ie, the HOPE Initiative), the Gold team was modified in July 2009 and an academic hospitalist (S.S.) was assigned to head this team. Specific hospitalists were selected by the Associate Chief of Medicine (S.S.) and the Chief of Medicine (R.H.M.) to serve as Gold team attendings on a regular basis. The other teams continued to be overseen by the Chief of Medicine, and the Gold team remained within the medicine service. Characteristics of the Gold and nonGold team attendings can be found in Table 1. The 3 other teams initially were noninterventional concurrent control groups. However, during the second year of the evaluation, the Silver team adopted some of the initiatives as a result of the preliminary findings observed on Gold. Specifically, in the second year of the evaluation, approximately 42% of attendings on the Silver team were from the Gold team. This increased in the third year to 67% of coverage by Gold team attendings on the Silver team. The evaluation of the Gold team ended in June 2012.
| Characteristic | Gold Team | Non‐Gold Teams |
|---|---|---|
| Total number of attendings | 14 | 57 |
| Sex, % | ||
| Male | 79 | 58 |
| Female | 21 | 42 |
| Median years postresidency (range) | 10 (130) | 7 (141) |
| Subspecialists, % | 14 | 40 |
| Median days on service per year (range) | 53 (574) | 30 (592) |
The clinical interventions implemented on the Gold team were quality‐improvement work and were therefore exempt from institutional review board review. Human subjects' approval was, however, received to conduct interviews as part of a qualitative assessment.
Clinical Interventions
Several interventions involving the clinical care delivered were introduced on the Gold team, with a focus on improving communication among healthcare workers (Table 2).
| Clinical Interventions | Educational Interventions |
|---|---|
| Modified structure of attending rounds | Modified structure of attending rounds |
| Circle of Concern rounds | Attending reading list |
| Clinical Care Coordinator | Nifty Fifty reading list for learners |
| Regular attending team meetings | Website to provide expectations to learners |
| Two‐month per year commitment by attendings |
Structure of Attending Rounds
The structure of morning rounds was modified on the Gold team. Similar to the traditional structure, medical students and interns on the Gold team receive sign‐out from the overnight team in the morning. However, interns and students may or may not conduct pre‐rounds on each patient. The majority of time between sign‐out and the arrival of the attending physician is spent on work rounds. The senior resident leads rounds with the interns and students, discussing each patient while focusing on overnight events and current symptoms, new physical‐examination findings, and laboratory and test data. The plan of care to be presented to the attending is then formulated with the senior resident. The attending physician then leads Circle of Concern rounds with an expanded team, including a charge nurse, a clinical pharmacist, and a nurse Clinical Care Coordinator. Attending rounds tend to use an E‐AP format: significant Events overnight are discussed, followed by an Assessment & Plan by problem for the top active problems. Using this model, the attendings are able to focus more on teaching and discussing the patient plan than in the traditional model (in which the learner presents the details of the subjective, objective, laboratory, and radiographic data, with limited time left for the assessment and plan for each problem).
Circle of Concern Rounds
Suzanne Gordon described the Circle of Concern in her book Nursing Against the Odds.[16] From her observations, she noted that physicians typically form a circle to discuss patient care during rounds. The circle expands when another physician joins the group; however, the circle does not similarly expand to include nurses when they approach the group. Instead, nurses typically remain on the periphery, listening silently or trying to communicate to physicians' backs.[16] Thus, to promote nurse‐physician communication, Circle of Concern rounds were formally introduced on the Gold team. Each morning, the charge nurse rounds with the team and is encouraged to bring up nursing concerns. The inpatient clinical pharmacist is also included 2 to 3 times per week to help provide education to residents and students and perform medication reconciliation.
Clinical Care Coordinator
The role of the nurse Clinical Care Coordinatoralso introduced on the Gold teamis to provide continuity of patient care, facilitate interdisciplinary communication, facilitate patient discharge, ensure appropriate appointments are scheduled, communicate with the ambulatory care service to ensure proper transition between inpatient and outpatient care, and help educate residents and students on VA procedures and resources.
Regular Gold Team Meetings
All Gold team attendings are expected to dedicate 2 months per year to inpatient service (divided into half‐month blocks), instead of the average 1 month per year for attendings on the other teams. The Gold team attendings, unlike the other teams, also attend bimonthly meetings to discuss strategies for running the team.
Educational Interventions
Given the high number of learners on the medicine service, we wanted to enhance the educational experience for our learners. We thus implemented various interventions, in addition to the change in the structure of rounds, as described below.
Reading List for Learners: The Nifty Fifty
Because reading about clinical medicine is an integral part of medical education, we make explicit our expectation that residents and students read something clinically relevant every day. To promote this, we have provided a Nifty Fifty reading list of key articles. The PDF of each article is provided, along with a brief summary highlighting key points.
Reading List for Gold Attendings and Support Staff
To promote a common understanding of leadership techniques, management books are provided to Gold attending physicians and other members of the team (eg, Care Coordinator, nurse researcher, systems redesign engineer). One book is discussed at each Gold team meeting (Table 3), with participants taking turns leading the discussion.
| Book Title | Author(s) |
|---|---|
| The One Minute Manager | Ken Blanchard and Spencer Johnson |
| Good to Great | Jim Collins |
| Good to Great and the Social Sectors | Jim Collins |
| The Checklist Manifesto: How to Get Things Right | Atul Gawande |
| The Five Dysfunctions of a Team: A Leadership Fable | Patrick Lencioni |
| Getting to Yes: Negotiating Agreement Without Giving In | Roger Fisher, William Ury, and Bruce Patton |
| The Effective Executive: The Definitive Guide to Getting the Right Things Done | Peter Drucker |
| A Sense of Urgency | John Kotter |
| The Power of Positive Deviance: How Unlikely Innovators Solve the World's Toughest Problems | Richard Pascale, Jerry Sternin, and Monique Sternin |
| On the Mend: Revolutionizing Healthcare to Save Lives and Transform the Industry | John Toussaint and Roger Gerard |
| Outliers: The Story of Success | Malcolm Gladwell |
| Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care | Suzanne Gordon |
| How the Mighty Fall and Why Some Companies Never Give In | Jim Collins |
| What the Best College Teachers Do | Ken Bain |
| The Creative Destruction of Medicine | Eric Topol |
| What Got You Here Won't Get You There: How Successful People Become Even More Successful! | Marshall Goldsmith |
Website
A HOPE Initiative website was created (
Qualitative Assessment
To evaluate our efforts, we conducted a thorough qualitative assessment during the third year of the program. A total of 35 semistructured qualitative interviews were conducted with patients and staff from all levels of the organization, including senior leadership. The qualitative assessment was led by research staff from the Center for Clinical Management Research, who were minimally involved in the redesign effort and could provide an unbiased view of the initiative. Field notes from the semistructured interviews were analyzed, with themes developed using a descriptive approach and through discussion by a multidisciplinary team, which included building team consensus on findings that were supported by clear evidence in the data.[17]
Quantitative Outcome Measures
Clinical Outcomes
To determine if our communication and educational interventions had an impact on patient care, we used hospital administrative data to evaluate admission rates, LOS, and readmission rates for all 4 of the medicine teams. Additional clinical measures were assessed as needed. For example, we monitored the impact of the clinical pharmacist during a 4‐week pilot study by asking the Clinical Care Coordinator to track the proportion of patient encounters (n=170) in which the clinical pharmacist changed management or provided education to team members. Additionally, 2 staff surveys were conducted. The first survey focused on healthcare‐worker communication and was given to inpatient nurses and physicians (including attendings, residents, and medical students) who were recently on an inpatient medical service rotation. The survey included questions from previously validated communication measures,[18, 19, 20] as well as study‐specific questions. The second survey evaluated the new role of the Clinical Care Coordinator (Appendix). Both physicians and nurses who interacted with the Gold team's Clinical Care Coordinator were asked to complete this survey.
Educational Outcomes
To assess the educational interventions, we used learner evaluations of attendings, by both residents and medical students, and standardized internal medicine National Board of Medical Examiners Subject Examination (or shelf) scores for third‐year medical students. A separate evaluation of medical student perceptions of the rounding structure introduced on the Gold team using survey design has already been published.[21]
Statistical Analyses
Data from all sources were analyzed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Outliers for the LOS variable were removed from the analysis. Means and frequency distributions were examined for all variables. Student t tests and [2] tests of independence were used to compare data between groups. Multivariable linear regression models controlling for time (preintervention vs postintervention) were used to assess the effect of the HOPE Initiative on patient LOS and readmission rates. In all cases, 2‐tailed P values of 0.05 or less were considered statistically significant.
Role of the Funding Source
The VA Office of Systems Redesign provided funding but was not involved in the design or conduct of the study, data analysis, or preparation of the manuscript.
RESULTS
Clinical Outcomes
Patient Outcomes
Our multivariable linear regression analysis, controlling for time, showed a significant reduction in LOS of approximately 0.3 days on all teams after the HOPE Initiative began (P=0.004). There were no significant differences between the Gold and non‐Gold teams in the multivariate models when controlling for time for any of the patient‐outcome measures. The number of admissions increased for all 4 medical teams (Figure 1), but, as shown in Figures 2 and 3, the readmission rates for all teams remained relatively stable over this same period of time.

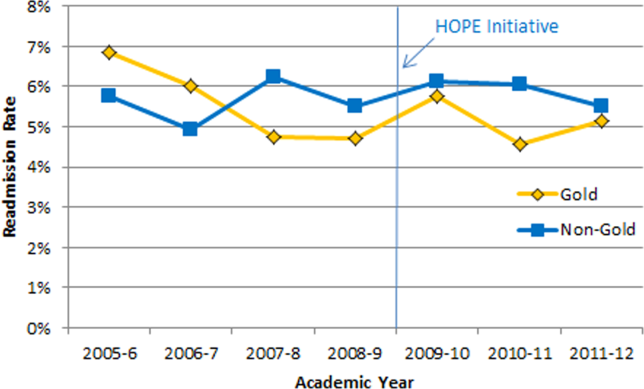

Clinical Pharmacist on Gold Team Rounds
The inpatient clinical pharmacist changed the management plan for 22% of the patients seen on rounds. Contributions from the clinical pharmacist included adjusting the dosing of ordered medication and correcting medication reconciliation. Education and pharmaceutical information was provided to the team in another 6% of the 170 consecutive patient encounters evaluated.
Perception of Circle of Concern Rounds
Circle of Concern rounds were generally well‐received by both nurses and physicians. In a healthcare‐worker communication survey, completed by 38 physicians (62% response rate) and 48 nurses (54% response rate), the majority of both physicians (83%) and nurses (68%) felt Circle of Concern rounds improved communication.
Nurse Perception of Communication
The healthcare‐worker communication survey asked inpatient nurses to rate communication between nurses and physicians on each of the 4 medicine teams. Significantly more nurses were satisfied with communication with the Gold team (71%) compared with the other 3 medicine teams (53%; P=0.02) (Figure 4).
Perception of the Clinical Care Coordinator
In total, 20 physicians (87% response rate) and 10 nurses (56% response rate) completed the Clinical Care Coordinator survey. The physician results were overwhelmingly positive: 100% were satisfied or very satisfied with the role; 100% felt each team should have a Clinical Care Coordinator; and 100% agreed or strongly agreed that the Clinical Care Coordinator ensures that appropriate follow‐up is arranged, provides continuity of care, assists with interdisciplinary communication, and helps facilitate discharge. The majority of nurses was also satisfied or very satisfied with the Clinical Care Coordinator role and felt each team should have one.
Educational Outcomes
House Officer Evaluation of Attendings
Monthly evaluations of attending physicians by house officers (Figure 5) revealed that prior to the HOPE Initiative, little differences were observed between teams, as would be expected because attending assignment was largely random. After the intervention date of July 2009, however, significant differences were noted, with Gold team attendings receiving significantly higher teaching evaluations immediately after the introduction of the HOPE Initiative. Although ratings for Gold attendings remained more favorable, the difference was no longer statistically significant in the second and third year of the initiative, likely due to Gold attendings serving on other medicine teams, which contributed to an improvement in ratings of all attendings.
Medical Student Evaluation of Attendings
Monthly evaluations of attending physicians by third‐year medical students (Figure 6) revealed differences between the Gold attendings and all others, with the attendings that joined the Gold team in 2009 receiving higher teaching evaluations even before the HOPE Initiative started. However, this difference remained statistically significant in years 2 and 3 postinitiative, despite the addition of 4 new junior attendings.
Medical Student Medicine Shelf Scores
The national average on the shelf exam, which reflects learning after the internal medicine third‐year clerkship, has ranged from 75 to 78 for the past several years, with University of Michigan students averaging significantly higher scores prior to and after the HOPE Initiative. However, following the HOPE Initiative, third‐year medical students on the Gold team scored significantly higher on the shelf exam compared with their colleagues on the non‐Gold teams (84 vs 82; P=0.006). This difference in the shelf exam scores, although small, is statistically significant. It represents a measurable improvement in shelf scores in our system and demonstrates the potential educational benefit for the students. Over this same time period, scores on the United States Medical Licensing Exam, given to medical students at the beginning of their third year, remained stable (233 preHOPE Initiative; 234 postHOPE Initiative).
Qualitative Assessment
Qualitative data collected as part of our evaluation of the HOPE Initiative also suggested that nurse‐physician communication had improved since the start of the project. In particular, they reported positively on the Gold team in general, the Circle of Concern rounds, and the Clinical Care Coordinator (Table 4).
| Staff Type | Statement1 |
|---|---|
| |
| Nurse | [Gold is] above and beyond other [teams]. Other teams don't run as smoothly. |
| Nurse | There has been a difference in communication [on Gold]. You can tell the difference in how they communicate with staff. We know the Clinical Care Coordinator or charge nurse is rounding with that team, so there is more communication. |
| Nurse | The most important thing that has improved communication is the Circle of Concern rounds. |
| Physician | [The Gold Clinical Care Coordinator] expedites care, not only what to do but who to call. She can convey the urgency. On rounds she is able to break off, put in an order, place a call, talk to a patient. Things that we would do at 11 AM she gets to at 9 AM. A couple of hours may not seem like much, but sometimes it can make the difference between things happening that day instead of the next. |
| Physician | The Clinical Care Coordinator is completely indispensable. Major benefit to providing care to Veterans. |
| Physician | I like to think Gold has lifted all of the teams to a higher level. |
| Medical student | It may be due to personalities vs the Gold [team] itself, but there is more emphasis on best practices. Are we following guidelines even if it is not related to the primary reason for admission? |
| Medical student | Gold is very collegial and nurses/physicians know one another by name. Physicians request rather than order; this sets a good example to me on how to approach the nurses. |
| Chief resident | [Gold attendings] encourage senior residents to take charge and run the team, although the attending is there for back‐up and support. This provides great learning for the residents. Interns and medical students also are affected because they have to step up their game as well. |
DISCUSSION
Within academic medical centers, hospitalists are expected to care for patients, teach, and help improve the quality and efficiency of hospital‐based care.[7] The Department of Veterans Affairs runs the largest integrated healthcare system in the United States, with approximately 80% of VA hospitals having hospital medicine programs. Overall, one‐third of US residents perform part of their residency training at a VA hospital.[22, 23] Thus, the effects of a system‐wide change at a VA hospital may have implications throughout the country. We studied one such intervention. Our primary findings are that we were able to improve communication and learner education with minimal effects on patient outcomes. While overall LOS decreased slightly postintervention, after taking into account secular trends, readmission rates did not.
We are not the first to evaluate a hospital medicine team using a quasi‐experimental design. For example, Meltzer and colleagues evaluated a hospitalist program at the University of Chicago Medical Center and found that, by the second year of operation, hospitalist care was associated with significantly shorter LOS (0.49 days), reduced costs, and decreased mortality.[24] Auerbach also evaluated a newly created hospital medicine service, finding decreased LOS (0.61 days), lower costs, and lower risk of mortality by the second year of the program.[25]
Improving nurse‐physician communication is considered important for avoiding medical error,[26] yet there has been limited empirical study of methods to improve communication within the medical profession.[27] Based both on our surveys and qualitative interviews, healthcare‐worker communication appeared to improve on the Gold team during the study. A key component of this improvement is likely related to instituting Circle of Concern rounds, in which nurses joined the medical team during attending rounds. Such an intervention likely helped to address organizational silence[28] and enhance the psychological safety of the nursing staff, because the attending physician was proactive about soliciting the input of nurses during rounds.[29] Such leader inclusivenesswords and deeds exhibited by leaders that invite and appreciate others' contributionscan aid interdisciplinary teams in overcoming the negative effects of status differences, thereby promoting collaboration.[29] The inclusion of nurses on rounds is also relationship‐building, which Gotlib Conn and colleagues found was important to improved interprofessional communication and collaboration.[30] In the future, using a tool such as the Teamwork Effectiveness Assessment Module (TEAM) developed by the American Board of Internal Medicine[31] could provide further evaluation of the impact on interprofessional teamwork and communication.
The focus on learner education, though evaluated in prior studies, is also novel. One previous survey of medical students showed that engaging students in substantive discussions is associated with greater student satisfaction.[32] Another survey of medical students found that attendings who were enthusiastic about teaching, inspired confidence in knowledge and skills, provided useful feedback, and encouraged increased student responsibility were viewed as more effective teachers.[33] No previous study that we are aware of, however, has looked at actual educational outcomes, such as shelf scores. The National Board of Medical Examiners reports that the Medicine subject exam is scaled to have a mean of 70 and a standard deviation of 8.[34] Thus, a mean increase in score of 2 points is small, but not trivial. This shows improvement in a hard educational outcome. Additionally, 2 points, although small in the context of total score and standard deviation, may make a substantial difference to an individual student in terms of overall grade, and, thus, residency applications. Our finding that third‐year medical students on the Gold team performed significantly better than University of Michigan third‐year medical students on other teams is an intriguing finding that warrants confirmation. On the other hand, this finding is consistent with a previous report evaluating learner satisfaction in which Bodnar et al found improved ratings of quantity and quality of teaching on teams with a nontraditional structure (Gold team).[21] Moreover, despite relatively few studies, the reason underlying the educational benefit of hospitalists should surprise few. The hospitalist model ensures that learners are supervised by physicians who are experts in the care of hospitalized patients.[35] Hospitalists hired at teaching hospitals to work on services with learners are generally chosen because they possess superior educational skills.[7]
Our findings should be interpreted in the context of the following limitations. First, our study focused on a single academically affiliated VA hospital. As other VA hospitals are pursuing a similar approach (eg, the Houston and Detroit VA medical centers), replicating our results will be important. Second, the VA system, although the largest integrated healthcare system in the United States, has unique characteristicssuch as an integrated electronic health record and predominantly male patient populationthat may make generalizations to the larger US healthcare system challenging. Third, there was a slightly lower response rate among nurses on a few of the surveys to evaluate our efforts; however, this rate of response is standard at our facility. Finally, our evaluation lacks an empirical measure of healthcare‐worker communication, such as incident reports.
Despite these limitations, our results have important implications. Using both quantitative and qualitative assessment, we found that academic hospitalists have the ability to improve healthcare‐worker communication and enhance learner education without increasing LOS. These findings are directly applicable to VA medical centers and potentially applicable to other academic medical centers.
Acknowledgments
The authors thank Milisa Manojlovich, PhD, RN, Edward Kennedy, MS, and Andrew Hickner, MSI, for help with preparation of this manuscript.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability grant. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs. Dr. Saint reports receiving travel reimbursement for giving invited talks at the Society of Hospital Medicine's National Meeting, as well as serving on the advisory boards of Doximity and Jvion.
APPENDIX
Survey to Evaluate the Care Coordinator Position
| Yes | No | Not Sure | |
| Q1. Are you familiar with the role of the Care Coordinator on the Gold Service (Susan Lee)? | 1 | 2 | 3 |
Please indicate how much you agree or disagree with the statements below.
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | Don't Know | |
| Q2. The Care Coordinator ensures that appropriate primary care follow‐up and any other appropriate services are arranged. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q3. The Care Coordinator provides continuity of patient care on the Gold Service. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q4. The Care Coordinator helps educate House Officers and Medical Students on VA processes (e.g., CPRS). | 1 | 2 | 3 | 4 | 5 | 9 |
| Q5. The Care Coordinator assists with interdisciplinary communication between the medical team and other services (e.g., nursing, ambulatory care, pharmacy, social work) | 1 | 2 | 3 | 4 | 5 | 9 |
| Q6. The Care Coordinator helps facilitate patient discharge. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q7. The Care Coordinator initiates communication with the ambulatory care teams to coordinate care. | 1 | 2 | 3 | 4 | 5 | 9 |
| Yes | No | |
| Q8. Are you a physician (attending or resident), or medical student who has been on more than one medical team at the VA (Gold, Silver, Burgundy, or Yellow)? | 1 | 2 |
If no, please skip to Q13
If yes, comparing your experience on the Gold Service (with the Care Coordinator) to your experience on any of the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q9. To what extent does the presence of a Care Coordinator affect patient care? | 1 | 2 | 3 | 4 |
| Q10. To what extent does the presence of a Care Coordinator improve patient flow? | 1 | 2 | 3 | 4 |
| Q11. To what extent does the presence of a Care Coordinator assist with education? | 1 | 2 | 3 | 4 |
| Q12. To what extent does the presence of a Care Coordinator contribute to attending rounds? | 1 | 2 | 3 | 4 |
| Yes | No | |
| Q13. Do you work [as a nurse] in ambulatory care? | 1 | 2 |
If no, please skip to Q17.
If yes, comparing your experience with the Gold Service (with the Care Coordinator) to the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q14. To what extent does the presence of a Care Coordinator improve coordination of care between inpatient and outpatient services? | 1 | 2 | 3 | 4 |
| Q15. To what extent does the presence of a Care Coordinator help identify high risk patients who require follow‐up? | 1 | 2 | 3 | 4 |
| Q16. To what extent does the presence of a Care Coordinator ensure follow‐up appointments are scheduled? | 1 | 2 | 3 | 4 |
| Yes | No | Not Sure | |
| Q17. Do you think each medical team should have a Care Coordinator? | 1 | 2 | 3 |
| Q18. Are there any additional tasks or duties you think would improve the effectiveness of the Care Coordinator? |
| Very Satisfied | Satisfied | Neutral | Dissatisfied | Very Dissatisfied | |
| Q19. Overall how satisfied are you with the role of the Care Coordinator on the Gold Service? | 1 | 2 | 3 | 4 | 5 |
| Q20. Do you have any other comments about the role of the Care Coordinator? |
| Q21. What is your position? |
| 1. Physician (attending or resident) or medical student |
| 2. Nurse (inpatient or ambulatory care) |
- Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine of the National Academies. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- . Growth in care provided by hospitalists. N Engl J Med. 2009;360(26):2789–2791.
- American Hospital Association. AHA Annual Survey of Hospitals, 2010. Chicago, IL: Health Forum, LLC; 2010.
- , , , . Preventing hospital‐acquired infections: a national survey of practices reported by U.S. hospitals in 2005 and 2009. J Gen Intern Med. 2012;27(7):773–779.
- , . Hospitalists in teaching hospitals: opportunities but not without danger. J Gen Intern Med. 2004;19(4):392–393.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , . Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490–498.
- , , , , , . Resident satisfaction on an academic hospitalist service: time to teach. Am J Med. 2002;112(7):597–601.
- , , , et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19(4):293–301.
- , . Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships. J Hosp Med. 2007;2(1):17–22.
- , , , . Medical student evaluation of the quality of hospitalist and nonhospitalist teaching faculty on inpatient medicine rotations. Acad Med. 2004;79(1):78–82.
- , , , , . Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education. JAMA. 1998;279(19):1560–1565.
- . Nurse/physician communication through a sensemaking lens: shifting the paradigm to improve patient safety. Med Care. 2010;48(11):941–946.
- . Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care. Ithaca, NY: Cornell University Press; 2005.
- . Focus on research methods: whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Development of an instrument to measure collaboration and satisfaction about care decisions. J Adv Nurs. 1994;20(1):176–182.
- . Development of the practice environment scale of the Nursing Work Index. Res Nurs Health. 2002;25(3):176–188.
- , , . Does the structure of inpatient rounds affect medical student education? Int J Med Educ. 2013;4:96–100.
- U.S. Department of Veterans Affairs, Office of Academic Affiliations. Medical and Dental Education Program. Available at: http://www.va. gov/oaa/GME_default.asp. Published 2012. Accessed May 08, 2013.
- , . Graduate medical education, 2011–2012. JAMA. 2012;308(21):2264–2279.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859–865.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . ‘It depends': medical residents' perspectives on working with nurses. Am J Nurs. 2009;109(7):34–44.
- , . Organizational silence: a barrier to change and development in a pluralistic world. Acad Manage Rev. 2000;25(4):706–725.
- , . Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav. 2006;27:941–966.
- , , , , . Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012;12:437.
- , , , , , . A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485–2492.
- , , , , , . Impact of instructional practices on student satisfaction with attendings' teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7–12.
- , . Medical students' perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635–639.
- National Board of Medical Examiners Subject Examination Program. Internal Medicine Advanced Clinical Examination, score interpretation guide. Available at: http://www.nbme.org/PDF/SampleScoreReports/Internal_Medicine_ACE_Score_Report.pdf. Published 2011. Accessed September 13, 2013.
- . The impact of hospitalists on medical education and the academic health system. Ann Intern Med. 1999;130(4 part 2):364–367.
Improving quality while reducing costs remains important for hospitals across the United States, including the approximately 150 hospitals that are part of the Veterans Affairs (VA) healthcare system.[1, 2] The field of hospital medicine has grown rapidly, leading to predictions that the majority of inpatient care in the United States eventually will be delivered by hospitalists.[3, 4] In 2010, 57% of US hospitals had hospitalists on staff, including 87% of hospitals with 200 beds,[5] and nearly 80% of VA hospitals.[6]
The demand for hospitalists within teaching hospitals has grown in part as a response to the mandate to reduce residency work hours.[7] Furthermore, previous research has found that hospitalist care is associated with modest reductions in length of stay (LOS) and weak but inconsistent differences in quality.[8] The educational effect of hospitalists has been far less examined. The limited number of studies published to date suggests that hospitalists may improve resident learning and house‐officer satisfaction in academic medical centers and community teaching hospitals[9, 10, 11] and provide positive experiences for medical students12,13; however, Wachter et al reported no significant changes in clinical outcomes or patient, faculty, and house‐staff satisfaction in a newly designed hospital medicine service in San Francisco.[14] Additionally, whether using hospitalists influences nurse‐physician communication[15] is unknown.
Recognizing the limited and sometimes conflicting evidence about the hospitalist model, we report the results of a 3‐year quasi‐experimental evaluation of the experience at our medical center with academic hospitalists. As part of a VA Systems Redesign Improvement Capability Grantknown as the Hospital Outcomes Program of Excellence (HOPE) Initiativewe created a hospitalist‐based medicine team focused on quality improvement, medical education, and patient outcomes.
METHODS
Setting and Design
The main hospital of the VA Ann Arbor Healthcare System, located in Ann Arbor, Michigan, operates 105 acute‐care beds and 40 extended‐care beds. At the time of this evaluation, the medicine service consisted of 4 internal medicine teamsGold, Silver, Burgundy, and Yelloweach of which was responsible for admitting patients on a rotating basis every fourth day, with limited numbers of admissions occurring between each team's primary admitting day. Each team is led by an attending physician, a board‐certified (or board‐eligible) general internist or subspecialist who is also a faculty member at the University of Michigan Medical School. Each team has a senior medical resident, 2 to 3 interns, and 3 to 5 medical students (mostly third‐year students). In total, there are approximately 50 senior medical residents, 60 interns, and 170 medical students who rotate through the medicine service each year. Traditional rounding involves the medical students and interns receiving sign‐out from the overnight team in the morning, then pre‐rounding on each patient by obtaining an interval history, performing an exam, and checking any test results. A tentative plan of care is formed with the senior medical resident, usually by discussing each patient very quickly in the team room. Attending rounds are then conducted, with the physician team visiting each patient one by one to review and plan all aspects of care in detail. When time allows, small segments of teaching may occur during these attending work rounds. This system had been in place for >20 years.
Resulting in part from a grant received from the VA Systems Redesign Central Office (ie, the HOPE Initiative), the Gold team was modified in July 2009 and an academic hospitalist (S.S.) was assigned to head this team. Specific hospitalists were selected by the Associate Chief of Medicine (S.S.) and the Chief of Medicine (R.H.M.) to serve as Gold team attendings on a regular basis. The other teams continued to be overseen by the Chief of Medicine, and the Gold team remained within the medicine service. Characteristics of the Gold and nonGold team attendings can be found in Table 1. The 3 other teams initially were noninterventional concurrent control groups. However, during the second year of the evaluation, the Silver team adopted some of the initiatives as a result of the preliminary findings observed on Gold. Specifically, in the second year of the evaluation, approximately 42% of attendings on the Silver team were from the Gold team. This increased in the third year to 67% of coverage by Gold team attendings on the Silver team. The evaluation of the Gold team ended in June 2012.
| Characteristic | Gold Team | Non‐Gold Teams |
|---|---|---|
| Total number of attendings | 14 | 57 |
| Sex, % | ||
| Male | 79 | 58 |
| Female | 21 | 42 |
| Median years postresidency (range) | 10 (130) | 7 (141) |
| Subspecialists, % | 14 | 40 |
| Median days on service per year (range) | 53 (574) | 30 (592) |
The clinical interventions implemented on the Gold team were quality‐improvement work and were therefore exempt from institutional review board review. Human subjects' approval was, however, received to conduct interviews as part of a qualitative assessment.
Clinical Interventions
Several interventions involving the clinical care delivered were introduced on the Gold team, with a focus on improving communication among healthcare workers (Table 2).
| Clinical Interventions | Educational Interventions |
|---|---|
| Modified structure of attending rounds | Modified structure of attending rounds |
| Circle of Concern rounds | Attending reading list |
| Clinical Care Coordinator | Nifty Fifty reading list for learners |
| Regular attending team meetings | Website to provide expectations to learners |
| Two‐month per year commitment by attendings |
Structure of Attending Rounds
The structure of morning rounds was modified on the Gold team. Similar to the traditional structure, medical students and interns on the Gold team receive sign‐out from the overnight team in the morning. However, interns and students may or may not conduct pre‐rounds on each patient. The majority of time between sign‐out and the arrival of the attending physician is spent on work rounds. The senior resident leads rounds with the interns and students, discussing each patient while focusing on overnight events and current symptoms, new physical‐examination findings, and laboratory and test data. The plan of care to be presented to the attending is then formulated with the senior resident. The attending physician then leads Circle of Concern rounds with an expanded team, including a charge nurse, a clinical pharmacist, and a nurse Clinical Care Coordinator. Attending rounds tend to use an E‐AP format: significant Events overnight are discussed, followed by an Assessment & Plan by problem for the top active problems. Using this model, the attendings are able to focus more on teaching and discussing the patient plan than in the traditional model (in which the learner presents the details of the subjective, objective, laboratory, and radiographic data, with limited time left for the assessment and plan for each problem).
Circle of Concern Rounds
Suzanne Gordon described the Circle of Concern in her book Nursing Against the Odds.[16] From her observations, she noted that physicians typically form a circle to discuss patient care during rounds. The circle expands when another physician joins the group; however, the circle does not similarly expand to include nurses when they approach the group. Instead, nurses typically remain on the periphery, listening silently or trying to communicate to physicians' backs.[16] Thus, to promote nurse‐physician communication, Circle of Concern rounds were formally introduced on the Gold team. Each morning, the charge nurse rounds with the team and is encouraged to bring up nursing concerns. The inpatient clinical pharmacist is also included 2 to 3 times per week to help provide education to residents and students and perform medication reconciliation.
Clinical Care Coordinator
The role of the nurse Clinical Care Coordinatoralso introduced on the Gold teamis to provide continuity of patient care, facilitate interdisciplinary communication, facilitate patient discharge, ensure appropriate appointments are scheduled, communicate with the ambulatory care service to ensure proper transition between inpatient and outpatient care, and help educate residents and students on VA procedures and resources.
Regular Gold Team Meetings
All Gold team attendings are expected to dedicate 2 months per year to inpatient service (divided into half‐month blocks), instead of the average 1 month per year for attendings on the other teams. The Gold team attendings, unlike the other teams, also attend bimonthly meetings to discuss strategies for running the team.
Educational Interventions
Given the high number of learners on the medicine service, we wanted to enhance the educational experience for our learners. We thus implemented various interventions, in addition to the change in the structure of rounds, as described below.
Reading List for Learners: The Nifty Fifty
Because reading about clinical medicine is an integral part of medical education, we make explicit our expectation that residents and students read something clinically relevant every day. To promote this, we have provided a Nifty Fifty reading list of key articles. The PDF of each article is provided, along with a brief summary highlighting key points.
Reading List for Gold Attendings and Support Staff
To promote a common understanding of leadership techniques, management books are provided to Gold attending physicians and other members of the team (eg, Care Coordinator, nurse researcher, systems redesign engineer). One book is discussed at each Gold team meeting (Table 3), with participants taking turns leading the discussion.
| Book Title | Author(s) |
|---|---|
| The One Minute Manager | Ken Blanchard and Spencer Johnson |
| Good to Great | Jim Collins |
| Good to Great and the Social Sectors | Jim Collins |
| The Checklist Manifesto: How to Get Things Right | Atul Gawande |
| The Five Dysfunctions of a Team: A Leadership Fable | Patrick Lencioni |
| Getting to Yes: Negotiating Agreement Without Giving In | Roger Fisher, William Ury, and Bruce Patton |
| The Effective Executive: The Definitive Guide to Getting the Right Things Done | Peter Drucker |
| A Sense of Urgency | John Kotter |
| The Power of Positive Deviance: How Unlikely Innovators Solve the World's Toughest Problems | Richard Pascale, Jerry Sternin, and Monique Sternin |
| On the Mend: Revolutionizing Healthcare to Save Lives and Transform the Industry | John Toussaint and Roger Gerard |
| Outliers: The Story of Success | Malcolm Gladwell |
| Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care | Suzanne Gordon |
| How the Mighty Fall and Why Some Companies Never Give In | Jim Collins |
| What the Best College Teachers Do | Ken Bain |
| The Creative Destruction of Medicine | Eric Topol |
| What Got You Here Won't Get You There: How Successful People Become Even More Successful! | Marshall Goldsmith |
Website
A HOPE Initiative website was created (
Qualitative Assessment
To evaluate our efforts, we conducted a thorough qualitative assessment during the third year of the program. A total of 35 semistructured qualitative interviews were conducted with patients and staff from all levels of the organization, including senior leadership. The qualitative assessment was led by research staff from the Center for Clinical Management Research, who were minimally involved in the redesign effort and could provide an unbiased view of the initiative. Field notes from the semistructured interviews were analyzed, with themes developed using a descriptive approach and through discussion by a multidisciplinary team, which included building team consensus on findings that were supported by clear evidence in the data.[17]
Quantitative Outcome Measures
Clinical Outcomes
To determine if our communication and educational interventions had an impact on patient care, we used hospital administrative data to evaluate admission rates, LOS, and readmission rates for all 4 of the medicine teams. Additional clinical measures were assessed as needed. For example, we monitored the impact of the clinical pharmacist during a 4‐week pilot study by asking the Clinical Care Coordinator to track the proportion of patient encounters (n=170) in which the clinical pharmacist changed management or provided education to team members. Additionally, 2 staff surveys were conducted. The first survey focused on healthcare‐worker communication and was given to inpatient nurses and physicians (including attendings, residents, and medical students) who were recently on an inpatient medical service rotation. The survey included questions from previously validated communication measures,[18, 19, 20] as well as study‐specific questions. The second survey evaluated the new role of the Clinical Care Coordinator (Appendix). Both physicians and nurses who interacted with the Gold team's Clinical Care Coordinator were asked to complete this survey.
Educational Outcomes
To assess the educational interventions, we used learner evaluations of attendings, by both residents and medical students, and standardized internal medicine National Board of Medical Examiners Subject Examination (or shelf) scores for third‐year medical students. A separate evaluation of medical student perceptions of the rounding structure introduced on the Gold team using survey design has already been published.[21]
Statistical Analyses
Data from all sources were analyzed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Outliers for the LOS variable were removed from the analysis. Means and frequency distributions were examined for all variables. Student t tests and [2] tests of independence were used to compare data between groups. Multivariable linear regression models controlling for time (preintervention vs postintervention) were used to assess the effect of the HOPE Initiative on patient LOS and readmission rates. In all cases, 2‐tailed P values of 0.05 or less were considered statistically significant.
Role of the Funding Source
The VA Office of Systems Redesign provided funding but was not involved in the design or conduct of the study, data analysis, or preparation of the manuscript.
RESULTS
Clinical Outcomes
Patient Outcomes
Our multivariable linear regression analysis, controlling for time, showed a significant reduction in LOS of approximately 0.3 days on all teams after the HOPE Initiative began (P=0.004). There were no significant differences between the Gold and non‐Gold teams in the multivariate models when controlling for time for any of the patient‐outcome measures. The number of admissions increased for all 4 medical teams (Figure 1), but, as shown in Figures 2 and 3, the readmission rates for all teams remained relatively stable over this same period of time.



Clinical Pharmacist on Gold Team Rounds
The inpatient clinical pharmacist changed the management plan for 22% of the patients seen on rounds. Contributions from the clinical pharmacist included adjusting the dosing of ordered medication and correcting medication reconciliation. Education and pharmaceutical information was provided to the team in another 6% of the 170 consecutive patient encounters evaluated.
Perception of Circle of Concern Rounds
Circle of Concern rounds were generally well‐received by both nurses and physicians. In a healthcare‐worker communication survey, completed by 38 physicians (62% response rate) and 48 nurses (54% response rate), the majority of both physicians (83%) and nurses (68%) felt Circle of Concern rounds improved communication.
Nurse Perception of Communication
The healthcare‐worker communication survey asked inpatient nurses to rate communication between nurses and physicians on each of the 4 medicine teams. Significantly more nurses were satisfied with communication with the Gold team (71%) compared with the other 3 medicine teams (53%; P=0.02) (Figure 4).

Perception of the Clinical Care Coordinator
In total, 20 physicians (87% response rate) and 10 nurses (56% response rate) completed the Clinical Care Coordinator survey. The physician results were overwhelmingly positive: 100% were satisfied or very satisfied with the role; 100% felt each team should have a Clinical Care Coordinator; and 100% agreed or strongly agreed that the Clinical Care Coordinator ensures that appropriate follow‐up is arranged, provides continuity of care, assists with interdisciplinary communication, and helps facilitate discharge. The majority of nurses was also satisfied or very satisfied with the Clinical Care Coordinator role and felt each team should have one.
Educational Outcomes
House Officer Evaluation of Attendings
Monthly evaluations of attending physicians by house officers (Figure 5) revealed that prior to the HOPE Initiative, little differences were observed between teams, as would be expected because attending assignment was largely random. After the intervention date of July 2009, however, significant differences were noted, with Gold team attendings receiving significantly higher teaching evaluations immediately after the introduction of the HOPE Initiative. Although ratings for Gold attendings remained more favorable, the difference was no longer statistically significant in the second and third year of the initiative, likely due to Gold attendings serving on other medicine teams, which contributed to an improvement in ratings of all attendings.

Medical Student Evaluation of Attendings
Monthly evaluations of attending physicians by third‐year medical students (Figure 6) revealed differences between the Gold attendings and all others, with the attendings that joined the Gold team in 2009 receiving higher teaching evaluations even before the HOPE Initiative started. However, this difference remained statistically significant in years 2 and 3 postinitiative, despite the addition of 4 new junior attendings.

Medical Student Medicine Shelf Scores
The national average on the shelf exam, which reflects learning after the internal medicine third‐year clerkship, has ranged from 75 to 78 for the past several years, with University of Michigan students averaging significantly higher scores prior to and after the HOPE Initiative. However, following the HOPE Initiative, third‐year medical students on the Gold team scored significantly higher on the shelf exam compared with their colleagues on the non‐Gold teams (84 vs 82; P=0.006). This difference in the shelf exam scores, although small, is statistically significant. It represents a measurable improvement in shelf scores in our system and demonstrates the potential educational benefit for the students. Over this same time period, scores on the United States Medical Licensing Exam, given to medical students at the beginning of their third year, remained stable (233 preHOPE Initiative; 234 postHOPE Initiative).
Qualitative Assessment
Qualitative data collected as part of our evaluation of the HOPE Initiative also suggested that nurse‐physician communication had improved since the start of the project. In particular, they reported positively on the Gold team in general, the Circle of Concern rounds, and the Clinical Care Coordinator (Table 4).
| Staff Type | Statement1 |
|---|---|
| |
| Nurse | [Gold is] above and beyond other [teams]. Other teams don't run as smoothly. |
| Nurse | There has been a difference in communication [on Gold]. You can tell the difference in how they communicate with staff. We know the Clinical Care Coordinator or charge nurse is rounding with that team, so there is more communication. |
| Nurse | The most important thing that has improved communication is the Circle of Concern rounds. |
| Physician | [The Gold Clinical Care Coordinator] expedites care, not only what to do but who to call. She can convey the urgency. On rounds she is able to break off, put in an order, place a call, talk to a patient. Things that we would do at 11 AM she gets to at 9 AM. A couple of hours may not seem like much, but sometimes it can make the difference between things happening that day instead of the next. |
| Physician | The Clinical Care Coordinator is completely indispensable. Major benefit to providing care to Veterans. |
| Physician | I like to think Gold has lifted all of the teams to a higher level. |
| Medical student | It may be due to personalities vs the Gold [team] itself, but there is more emphasis on best practices. Are we following guidelines even if it is not related to the primary reason for admission? |
| Medical student | Gold is very collegial and nurses/physicians know one another by name. Physicians request rather than order; this sets a good example to me on how to approach the nurses. |
| Chief resident | [Gold attendings] encourage senior residents to take charge and run the team, although the attending is there for back‐up and support. This provides great learning for the residents. Interns and medical students also are affected because they have to step up their game as well. |
DISCUSSION
Within academic medical centers, hospitalists are expected to care for patients, teach, and help improve the quality and efficiency of hospital‐based care.[7] The Department of Veterans Affairs runs the largest integrated healthcare system in the United States, with approximately 80% of VA hospitals having hospital medicine programs. Overall, one‐third of US residents perform part of their residency training at a VA hospital.[22, 23] Thus, the effects of a system‐wide change at a VA hospital may have implications throughout the country. We studied one such intervention. Our primary findings are that we were able to improve communication and learner education with minimal effects on patient outcomes. While overall LOS decreased slightly postintervention, after taking into account secular trends, readmission rates did not.
We are not the first to evaluate a hospital medicine team using a quasi‐experimental design. For example, Meltzer and colleagues evaluated a hospitalist program at the University of Chicago Medical Center and found that, by the second year of operation, hospitalist care was associated with significantly shorter LOS (0.49 days), reduced costs, and decreased mortality.[24] Auerbach also evaluated a newly created hospital medicine service, finding decreased LOS (0.61 days), lower costs, and lower risk of mortality by the second year of the program.[25]
Improving nurse‐physician communication is considered important for avoiding medical error,[26] yet there has been limited empirical study of methods to improve communication within the medical profession.[27] Based both on our surveys and qualitative interviews, healthcare‐worker communication appeared to improve on the Gold team during the study. A key component of this improvement is likely related to instituting Circle of Concern rounds, in which nurses joined the medical team during attending rounds. Such an intervention likely helped to address organizational silence[28] and enhance the psychological safety of the nursing staff, because the attending physician was proactive about soliciting the input of nurses during rounds.[29] Such leader inclusivenesswords and deeds exhibited by leaders that invite and appreciate others' contributionscan aid interdisciplinary teams in overcoming the negative effects of status differences, thereby promoting collaboration.[29] The inclusion of nurses on rounds is also relationship‐building, which Gotlib Conn and colleagues found was important to improved interprofessional communication and collaboration.[30] In the future, using a tool such as the Teamwork Effectiveness Assessment Module (TEAM) developed by the American Board of Internal Medicine[31] could provide further evaluation of the impact on interprofessional teamwork and communication.
The focus on learner education, though evaluated in prior studies, is also novel. One previous survey of medical students showed that engaging students in substantive discussions is associated with greater student satisfaction.[32] Another survey of medical students found that attendings who were enthusiastic about teaching, inspired confidence in knowledge and skills, provided useful feedback, and encouraged increased student responsibility were viewed as more effective teachers.[33] No previous study that we are aware of, however, has looked at actual educational outcomes, such as shelf scores. The National Board of Medical Examiners reports that the Medicine subject exam is scaled to have a mean of 70 and a standard deviation of 8.[34] Thus, a mean increase in score of 2 points is small, but not trivial. This shows improvement in a hard educational outcome. Additionally, 2 points, although small in the context of total score and standard deviation, may make a substantial difference to an individual student in terms of overall grade, and, thus, residency applications. Our finding that third‐year medical students on the Gold team performed significantly better than University of Michigan third‐year medical students on other teams is an intriguing finding that warrants confirmation. On the other hand, this finding is consistent with a previous report evaluating learner satisfaction in which Bodnar et al found improved ratings of quantity and quality of teaching on teams with a nontraditional structure (Gold team).[21] Moreover, despite relatively few studies, the reason underlying the educational benefit of hospitalists should surprise few. The hospitalist model ensures that learners are supervised by physicians who are experts in the care of hospitalized patients.[35] Hospitalists hired at teaching hospitals to work on services with learners are generally chosen because they possess superior educational skills.[7]
Our findings should be interpreted in the context of the following limitations. First, our study focused on a single academically affiliated VA hospital. As other VA hospitals are pursuing a similar approach (eg, the Houston and Detroit VA medical centers), replicating our results will be important. Second, the VA system, although the largest integrated healthcare system in the United States, has unique characteristicssuch as an integrated electronic health record and predominantly male patient populationthat may make generalizations to the larger US healthcare system challenging. Third, there was a slightly lower response rate among nurses on a few of the surveys to evaluate our efforts; however, this rate of response is standard at our facility. Finally, our evaluation lacks an empirical measure of healthcare‐worker communication, such as incident reports.
Despite these limitations, our results have important implications. Using both quantitative and qualitative assessment, we found that academic hospitalists have the ability to improve healthcare‐worker communication and enhance learner education without increasing LOS. These findings are directly applicable to VA medical centers and potentially applicable to other academic medical centers.
Acknowledgments
The authors thank Milisa Manojlovich, PhD, RN, Edward Kennedy, MS, and Andrew Hickner, MSI, for help with preparation of this manuscript.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability grant. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs. Dr. Saint reports receiving travel reimbursement for giving invited talks at the Society of Hospital Medicine's National Meeting, as well as serving on the advisory boards of Doximity and Jvion.
APPENDIX
Survey to Evaluate the Care Coordinator Position
| Yes | No | Not Sure | |
| Q1. Are you familiar with the role of the Care Coordinator on the Gold Service (Susan Lee)? | 1 | 2 | 3 |
Please indicate how much you agree or disagree with the statements below.
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | Don't Know | |
| Q2. The Care Coordinator ensures that appropriate primary care follow‐up and any other appropriate services are arranged. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q3. The Care Coordinator provides continuity of patient care on the Gold Service. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q4. The Care Coordinator helps educate House Officers and Medical Students on VA processes (e.g., CPRS). | 1 | 2 | 3 | 4 | 5 | 9 |
| Q5. The Care Coordinator assists with interdisciplinary communication between the medical team and other services (e.g., nursing, ambulatory care, pharmacy, social work) | 1 | 2 | 3 | 4 | 5 | 9 |
| Q6. The Care Coordinator helps facilitate patient discharge. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q7. The Care Coordinator initiates communication with the ambulatory care teams to coordinate care. | 1 | 2 | 3 | 4 | 5 | 9 |
| Yes | No | |
| Q8. Are you a physician (attending or resident), or medical student who has been on more than one medical team at the VA (Gold, Silver, Burgundy, or Yellow)? | 1 | 2 |
If no, please skip to Q13
If yes, comparing your experience on the Gold Service (with the Care Coordinator) to your experience on any of the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q9. To what extent does the presence of a Care Coordinator affect patient care? | 1 | 2 | 3 | 4 |
| Q10. To what extent does the presence of a Care Coordinator improve patient flow? | 1 | 2 | 3 | 4 |
| Q11. To what extent does the presence of a Care Coordinator assist with education? | 1 | 2 | 3 | 4 |
| Q12. To what extent does the presence of a Care Coordinator contribute to attending rounds? | 1 | 2 | 3 | 4 |
| Yes | No | |
| Q13. Do you work [as a nurse] in ambulatory care? | 1 | 2 |
If no, please skip to Q17.
If yes, comparing your experience with the Gold Service (with the Care Coordinator) to the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q14. To what extent does the presence of a Care Coordinator improve coordination of care between inpatient and outpatient services? | 1 | 2 | 3 | 4 |
| Q15. To what extent does the presence of a Care Coordinator help identify high risk patients who require follow‐up? | 1 | 2 | 3 | 4 |
| Q16. To what extent does the presence of a Care Coordinator ensure follow‐up appointments are scheduled? | 1 | 2 | 3 | 4 |
| Yes | No | Not Sure | |
| Q17. Do you think each medical team should have a Care Coordinator? | 1 | 2 | 3 |
| Q18. Are there any additional tasks or duties you think would improve the effectiveness of the Care Coordinator? |
| Very Satisfied | Satisfied | Neutral | Dissatisfied | Very Dissatisfied | |
| Q19. Overall how satisfied are you with the role of the Care Coordinator on the Gold Service? | 1 | 2 | 3 | 4 | 5 |
| Q20. Do you have any other comments about the role of the Care Coordinator? |
| Q21. What is your position? |
| 1. Physician (attending or resident) or medical student |
| 2. Nurse (inpatient or ambulatory care) |
Improving quality while reducing costs remains important for hospitals across the United States, including the approximately 150 hospitals that are part of the Veterans Affairs (VA) healthcare system.[1, 2] The field of hospital medicine has grown rapidly, leading to predictions that the majority of inpatient care in the United States eventually will be delivered by hospitalists.[3, 4] In 2010, 57% of US hospitals had hospitalists on staff, including 87% of hospitals with 200 beds,[5] and nearly 80% of VA hospitals.[6]
The demand for hospitalists within teaching hospitals has grown in part as a response to the mandate to reduce residency work hours.[7] Furthermore, previous research has found that hospitalist care is associated with modest reductions in length of stay (LOS) and weak but inconsistent differences in quality.[8] The educational effect of hospitalists has been far less examined. The limited number of studies published to date suggests that hospitalists may improve resident learning and house‐officer satisfaction in academic medical centers and community teaching hospitals[9, 10, 11] and provide positive experiences for medical students12,13; however, Wachter et al reported no significant changes in clinical outcomes or patient, faculty, and house‐staff satisfaction in a newly designed hospital medicine service in San Francisco.[14] Additionally, whether using hospitalists influences nurse‐physician communication[15] is unknown.
Recognizing the limited and sometimes conflicting evidence about the hospitalist model, we report the results of a 3‐year quasi‐experimental evaluation of the experience at our medical center with academic hospitalists. As part of a VA Systems Redesign Improvement Capability Grantknown as the Hospital Outcomes Program of Excellence (HOPE) Initiativewe created a hospitalist‐based medicine team focused on quality improvement, medical education, and patient outcomes.
METHODS
Setting and Design
The main hospital of the VA Ann Arbor Healthcare System, located in Ann Arbor, Michigan, operates 105 acute‐care beds and 40 extended‐care beds. At the time of this evaluation, the medicine service consisted of 4 internal medicine teamsGold, Silver, Burgundy, and Yelloweach of which was responsible for admitting patients on a rotating basis every fourth day, with limited numbers of admissions occurring between each team's primary admitting day. Each team is led by an attending physician, a board‐certified (or board‐eligible) general internist or subspecialist who is also a faculty member at the University of Michigan Medical School. Each team has a senior medical resident, 2 to 3 interns, and 3 to 5 medical students (mostly third‐year students). In total, there are approximately 50 senior medical residents, 60 interns, and 170 medical students who rotate through the medicine service each year. Traditional rounding involves the medical students and interns receiving sign‐out from the overnight team in the morning, then pre‐rounding on each patient by obtaining an interval history, performing an exam, and checking any test results. A tentative plan of care is formed with the senior medical resident, usually by discussing each patient very quickly in the team room. Attending rounds are then conducted, with the physician team visiting each patient one by one to review and plan all aspects of care in detail. When time allows, small segments of teaching may occur during these attending work rounds. This system had been in place for >20 years.
Resulting in part from a grant received from the VA Systems Redesign Central Office (ie, the HOPE Initiative), the Gold team was modified in July 2009 and an academic hospitalist (S.S.) was assigned to head this team. Specific hospitalists were selected by the Associate Chief of Medicine (S.S.) and the Chief of Medicine (R.H.M.) to serve as Gold team attendings on a regular basis. The other teams continued to be overseen by the Chief of Medicine, and the Gold team remained within the medicine service. Characteristics of the Gold and nonGold team attendings can be found in Table 1. The 3 other teams initially were noninterventional concurrent control groups. However, during the second year of the evaluation, the Silver team adopted some of the initiatives as a result of the preliminary findings observed on Gold. Specifically, in the second year of the evaluation, approximately 42% of attendings on the Silver team were from the Gold team. This increased in the third year to 67% of coverage by Gold team attendings on the Silver team. The evaluation of the Gold team ended in June 2012.
| Characteristic | Gold Team | Non‐Gold Teams |
|---|---|---|
| Total number of attendings | 14 | 57 |
| Sex, % | ||
| Male | 79 | 58 |
| Female | 21 | 42 |
| Median years postresidency (range) | 10 (130) | 7 (141) |
| Subspecialists, % | 14 | 40 |
| Median days on service per year (range) | 53 (574) | 30 (592) |
The clinical interventions implemented on the Gold team were quality‐improvement work and were therefore exempt from institutional review board review. Human subjects' approval was, however, received to conduct interviews as part of a qualitative assessment.
Clinical Interventions
Several interventions involving the clinical care delivered were introduced on the Gold team, with a focus on improving communication among healthcare workers (Table 2).
| Clinical Interventions | Educational Interventions |
|---|---|
| Modified structure of attending rounds | Modified structure of attending rounds |
| Circle of Concern rounds | Attending reading list |
| Clinical Care Coordinator | Nifty Fifty reading list for learners |
| Regular attending team meetings | Website to provide expectations to learners |
| Two‐month per year commitment by attendings |
Structure of Attending Rounds
The structure of morning rounds was modified on the Gold team. Similar to the traditional structure, medical students and interns on the Gold team receive sign‐out from the overnight team in the morning. However, interns and students may or may not conduct pre‐rounds on each patient. The majority of time between sign‐out and the arrival of the attending physician is spent on work rounds. The senior resident leads rounds with the interns and students, discussing each patient while focusing on overnight events and current symptoms, new physical‐examination findings, and laboratory and test data. The plan of care to be presented to the attending is then formulated with the senior resident. The attending physician then leads Circle of Concern rounds with an expanded team, including a charge nurse, a clinical pharmacist, and a nurse Clinical Care Coordinator. Attending rounds tend to use an E‐AP format: significant Events overnight are discussed, followed by an Assessment & Plan by problem for the top active problems. Using this model, the attendings are able to focus more on teaching and discussing the patient plan than in the traditional model (in which the learner presents the details of the subjective, objective, laboratory, and radiographic data, with limited time left for the assessment and plan for each problem).
Circle of Concern Rounds
Suzanne Gordon described the Circle of Concern in her book Nursing Against the Odds.[16] From her observations, she noted that physicians typically form a circle to discuss patient care during rounds. The circle expands when another physician joins the group; however, the circle does not similarly expand to include nurses when they approach the group. Instead, nurses typically remain on the periphery, listening silently or trying to communicate to physicians' backs.[16] Thus, to promote nurse‐physician communication, Circle of Concern rounds were formally introduced on the Gold team. Each morning, the charge nurse rounds with the team and is encouraged to bring up nursing concerns. The inpatient clinical pharmacist is also included 2 to 3 times per week to help provide education to residents and students and perform medication reconciliation.
Clinical Care Coordinator
The role of the nurse Clinical Care Coordinatoralso introduced on the Gold teamis to provide continuity of patient care, facilitate interdisciplinary communication, facilitate patient discharge, ensure appropriate appointments are scheduled, communicate with the ambulatory care service to ensure proper transition between inpatient and outpatient care, and help educate residents and students on VA procedures and resources.
Regular Gold Team Meetings
All Gold team attendings are expected to dedicate 2 months per year to inpatient service (divided into half‐month blocks), instead of the average 1 month per year for attendings on the other teams. The Gold team attendings, unlike the other teams, also attend bimonthly meetings to discuss strategies for running the team.
Educational Interventions
Given the high number of learners on the medicine service, we wanted to enhance the educational experience for our learners. We thus implemented various interventions, in addition to the change in the structure of rounds, as described below.
Reading List for Learners: The Nifty Fifty
Because reading about clinical medicine is an integral part of medical education, we make explicit our expectation that residents and students read something clinically relevant every day. To promote this, we have provided a Nifty Fifty reading list of key articles. The PDF of each article is provided, along with a brief summary highlighting key points.
Reading List for Gold Attendings and Support Staff
To promote a common understanding of leadership techniques, management books are provided to Gold attending physicians and other members of the team (eg, Care Coordinator, nurse researcher, systems redesign engineer). One book is discussed at each Gold team meeting (Table 3), with participants taking turns leading the discussion.
| Book Title | Author(s) |
|---|---|
| The One Minute Manager | Ken Blanchard and Spencer Johnson |
| Good to Great | Jim Collins |
| Good to Great and the Social Sectors | Jim Collins |
| The Checklist Manifesto: How to Get Things Right | Atul Gawande |
| The Five Dysfunctions of a Team: A Leadership Fable | Patrick Lencioni |
| Getting to Yes: Negotiating Agreement Without Giving In | Roger Fisher, William Ury, and Bruce Patton |
| The Effective Executive: The Definitive Guide to Getting the Right Things Done | Peter Drucker |
| A Sense of Urgency | John Kotter |
| The Power of Positive Deviance: How Unlikely Innovators Solve the World's Toughest Problems | Richard Pascale, Jerry Sternin, and Monique Sternin |
| On the Mend: Revolutionizing Healthcare to Save Lives and Transform the Industry | John Toussaint and Roger Gerard |
| Outliers: The Story of Success | Malcolm Gladwell |
| Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care | Suzanne Gordon |
| How the Mighty Fall and Why Some Companies Never Give In | Jim Collins |
| What the Best College Teachers Do | Ken Bain |
| The Creative Destruction of Medicine | Eric Topol |
| What Got You Here Won't Get You There: How Successful People Become Even More Successful! | Marshall Goldsmith |
Website
A HOPE Initiative website was created (
Qualitative Assessment
To evaluate our efforts, we conducted a thorough qualitative assessment during the third year of the program. A total of 35 semistructured qualitative interviews were conducted with patients and staff from all levels of the organization, including senior leadership. The qualitative assessment was led by research staff from the Center for Clinical Management Research, who were minimally involved in the redesign effort and could provide an unbiased view of the initiative. Field notes from the semistructured interviews were analyzed, with themes developed using a descriptive approach and through discussion by a multidisciplinary team, which included building team consensus on findings that were supported by clear evidence in the data.[17]
Quantitative Outcome Measures
Clinical Outcomes
To determine if our communication and educational interventions had an impact on patient care, we used hospital administrative data to evaluate admission rates, LOS, and readmission rates for all 4 of the medicine teams. Additional clinical measures were assessed as needed. For example, we monitored the impact of the clinical pharmacist during a 4‐week pilot study by asking the Clinical Care Coordinator to track the proportion of patient encounters (n=170) in which the clinical pharmacist changed management or provided education to team members. Additionally, 2 staff surveys were conducted. The first survey focused on healthcare‐worker communication and was given to inpatient nurses and physicians (including attendings, residents, and medical students) who were recently on an inpatient medical service rotation. The survey included questions from previously validated communication measures,[18, 19, 20] as well as study‐specific questions. The second survey evaluated the new role of the Clinical Care Coordinator (Appendix). Both physicians and nurses who interacted with the Gold team's Clinical Care Coordinator were asked to complete this survey.
Educational Outcomes
To assess the educational interventions, we used learner evaluations of attendings, by both residents and medical students, and standardized internal medicine National Board of Medical Examiners Subject Examination (or shelf) scores for third‐year medical students. A separate evaluation of medical student perceptions of the rounding structure introduced on the Gold team using survey design has already been published.[21]
Statistical Analyses
Data from all sources were analyzed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Outliers for the LOS variable were removed from the analysis. Means and frequency distributions were examined for all variables. Student t tests and [2] tests of independence were used to compare data between groups. Multivariable linear regression models controlling for time (preintervention vs postintervention) were used to assess the effect of the HOPE Initiative on patient LOS and readmission rates. In all cases, 2‐tailed P values of 0.05 or less were considered statistically significant.
Role of the Funding Source
The VA Office of Systems Redesign provided funding but was not involved in the design or conduct of the study, data analysis, or preparation of the manuscript.
RESULTS
Clinical Outcomes
Patient Outcomes
Our multivariable linear regression analysis, controlling for time, showed a significant reduction in LOS of approximately 0.3 days on all teams after the HOPE Initiative began (P=0.004). There were no significant differences between the Gold and non‐Gold teams in the multivariate models when controlling for time for any of the patient‐outcome measures. The number of admissions increased for all 4 medical teams (Figure 1), but, as shown in Figures 2 and 3, the readmission rates for all teams remained relatively stable over this same period of time.



Clinical Pharmacist on Gold Team Rounds
The inpatient clinical pharmacist changed the management plan for 22% of the patients seen on rounds. Contributions from the clinical pharmacist included adjusting the dosing of ordered medication and correcting medication reconciliation. Education and pharmaceutical information was provided to the team in another 6% of the 170 consecutive patient encounters evaluated.
Perception of Circle of Concern Rounds
Circle of Concern rounds were generally well‐received by both nurses and physicians. In a healthcare‐worker communication survey, completed by 38 physicians (62% response rate) and 48 nurses (54% response rate), the majority of both physicians (83%) and nurses (68%) felt Circle of Concern rounds improved communication.
Nurse Perception of Communication
The healthcare‐worker communication survey asked inpatient nurses to rate communication between nurses and physicians on each of the 4 medicine teams. Significantly more nurses were satisfied with communication with the Gold team (71%) compared with the other 3 medicine teams (53%; P=0.02) (Figure 4).

Perception of the Clinical Care Coordinator
In total, 20 physicians (87% response rate) and 10 nurses (56% response rate) completed the Clinical Care Coordinator survey. The physician results were overwhelmingly positive: 100% were satisfied or very satisfied with the role; 100% felt each team should have a Clinical Care Coordinator; and 100% agreed or strongly agreed that the Clinical Care Coordinator ensures that appropriate follow‐up is arranged, provides continuity of care, assists with interdisciplinary communication, and helps facilitate discharge. The majority of nurses was also satisfied or very satisfied with the Clinical Care Coordinator role and felt each team should have one.
Educational Outcomes
House Officer Evaluation of Attendings
Monthly evaluations of attending physicians by house officers (Figure 5) revealed that prior to the HOPE Initiative, little differences were observed between teams, as would be expected because attending assignment was largely random. After the intervention date of July 2009, however, significant differences were noted, with Gold team attendings receiving significantly higher teaching evaluations immediately after the introduction of the HOPE Initiative. Although ratings for Gold attendings remained more favorable, the difference was no longer statistically significant in the second and third year of the initiative, likely due to Gold attendings serving on other medicine teams, which contributed to an improvement in ratings of all attendings.

Medical Student Evaluation of Attendings
Monthly evaluations of attending physicians by third‐year medical students (Figure 6) revealed differences between the Gold attendings and all others, with the attendings that joined the Gold team in 2009 receiving higher teaching evaluations even before the HOPE Initiative started. However, this difference remained statistically significant in years 2 and 3 postinitiative, despite the addition of 4 new junior attendings.

Medical Student Medicine Shelf Scores
The national average on the shelf exam, which reflects learning after the internal medicine third‐year clerkship, has ranged from 75 to 78 for the past several years, with University of Michigan students averaging significantly higher scores prior to and after the HOPE Initiative. However, following the HOPE Initiative, third‐year medical students on the Gold team scored significantly higher on the shelf exam compared with their colleagues on the non‐Gold teams (84 vs 82; P=0.006). This difference in the shelf exam scores, although small, is statistically significant. It represents a measurable improvement in shelf scores in our system and demonstrates the potential educational benefit for the students. Over this same time period, scores on the United States Medical Licensing Exam, given to medical students at the beginning of their third year, remained stable (233 preHOPE Initiative; 234 postHOPE Initiative).
Qualitative Assessment
Qualitative data collected as part of our evaluation of the HOPE Initiative also suggested that nurse‐physician communication had improved since the start of the project. In particular, they reported positively on the Gold team in general, the Circle of Concern rounds, and the Clinical Care Coordinator (Table 4).
| Staff Type | Statement1 |
|---|---|
| |
| Nurse | [Gold is] above and beyond other [teams]. Other teams don't run as smoothly. |
| Nurse | There has been a difference in communication [on Gold]. You can tell the difference in how they communicate with staff. We know the Clinical Care Coordinator or charge nurse is rounding with that team, so there is more communication. |
| Nurse | The most important thing that has improved communication is the Circle of Concern rounds. |
| Physician | [The Gold Clinical Care Coordinator] expedites care, not only what to do but who to call. She can convey the urgency. On rounds she is able to break off, put in an order, place a call, talk to a patient. Things that we would do at 11 AM she gets to at 9 AM. A couple of hours may not seem like much, but sometimes it can make the difference between things happening that day instead of the next. |
| Physician | The Clinical Care Coordinator is completely indispensable. Major benefit to providing care to Veterans. |
| Physician | I like to think Gold has lifted all of the teams to a higher level. |
| Medical student | It may be due to personalities vs the Gold [team] itself, but there is more emphasis on best practices. Are we following guidelines even if it is not related to the primary reason for admission? |
| Medical student | Gold is very collegial and nurses/physicians know one another by name. Physicians request rather than order; this sets a good example to me on how to approach the nurses. |
| Chief resident | [Gold attendings] encourage senior residents to take charge and run the team, although the attending is there for back‐up and support. This provides great learning for the residents. Interns and medical students also are affected because they have to step up their game as well. |
DISCUSSION
Within academic medical centers, hospitalists are expected to care for patients, teach, and help improve the quality and efficiency of hospital‐based care.[7] The Department of Veterans Affairs runs the largest integrated healthcare system in the United States, with approximately 80% of VA hospitals having hospital medicine programs. Overall, one‐third of US residents perform part of their residency training at a VA hospital.[22, 23] Thus, the effects of a system‐wide change at a VA hospital may have implications throughout the country. We studied one such intervention. Our primary findings are that we were able to improve communication and learner education with minimal effects on patient outcomes. While overall LOS decreased slightly postintervention, after taking into account secular trends, readmission rates did not.
We are not the first to evaluate a hospital medicine team using a quasi‐experimental design. For example, Meltzer and colleagues evaluated a hospitalist program at the University of Chicago Medical Center and found that, by the second year of operation, hospitalist care was associated with significantly shorter LOS (0.49 days), reduced costs, and decreased mortality.[24] Auerbach also evaluated a newly created hospital medicine service, finding decreased LOS (0.61 days), lower costs, and lower risk of mortality by the second year of the program.[25]
Improving nurse‐physician communication is considered important for avoiding medical error,[26] yet there has been limited empirical study of methods to improve communication within the medical profession.[27] Based both on our surveys and qualitative interviews, healthcare‐worker communication appeared to improve on the Gold team during the study. A key component of this improvement is likely related to instituting Circle of Concern rounds, in which nurses joined the medical team during attending rounds. Such an intervention likely helped to address organizational silence[28] and enhance the psychological safety of the nursing staff, because the attending physician was proactive about soliciting the input of nurses during rounds.[29] Such leader inclusivenesswords and deeds exhibited by leaders that invite and appreciate others' contributionscan aid interdisciplinary teams in overcoming the negative effects of status differences, thereby promoting collaboration.[29] The inclusion of nurses on rounds is also relationship‐building, which Gotlib Conn and colleagues found was important to improved interprofessional communication and collaboration.[30] In the future, using a tool such as the Teamwork Effectiveness Assessment Module (TEAM) developed by the American Board of Internal Medicine[31] could provide further evaluation of the impact on interprofessional teamwork and communication.
The focus on learner education, though evaluated in prior studies, is also novel. One previous survey of medical students showed that engaging students in substantive discussions is associated with greater student satisfaction.[32] Another survey of medical students found that attendings who were enthusiastic about teaching, inspired confidence in knowledge and skills, provided useful feedback, and encouraged increased student responsibility were viewed as more effective teachers.[33] No previous study that we are aware of, however, has looked at actual educational outcomes, such as shelf scores. The National Board of Medical Examiners reports that the Medicine subject exam is scaled to have a mean of 70 and a standard deviation of 8.[34] Thus, a mean increase in score of 2 points is small, but not trivial. This shows improvement in a hard educational outcome. Additionally, 2 points, although small in the context of total score and standard deviation, may make a substantial difference to an individual student in terms of overall grade, and, thus, residency applications. Our finding that third‐year medical students on the Gold team performed significantly better than University of Michigan third‐year medical students on other teams is an intriguing finding that warrants confirmation. On the other hand, this finding is consistent with a previous report evaluating learner satisfaction in which Bodnar et al found improved ratings of quantity and quality of teaching on teams with a nontraditional structure (Gold team).[21] Moreover, despite relatively few studies, the reason underlying the educational benefit of hospitalists should surprise few. The hospitalist model ensures that learners are supervised by physicians who are experts in the care of hospitalized patients.[35] Hospitalists hired at teaching hospitals to work on services with learners are generally chosen because they possess superior educational skills.[7]
Our findings should be interpreted in the context of the following limitations. First, our study focused on a single academically affiliated VA hospital. As other VA hospitals are pursuing a similar approach (eg, the Houston and Detroit VA medical centers), replicating our results will be important. Second, the VA system, although the largest integrated healthcare system in the United States, has unique characteristicssuch as an integrated electronic health record and predominantly male patient populationthat may make generalizations to the larger US healthcare system challenging. Third, there was a slightly lower response rate among nurses on a few of the surveys to evaluate our efforts; however, this rate of response is standard at our facility. Finally, our evaluation lacks an empirical measure of healthcare‐worker communication, such as incident reports.
Despite these limitations, our results have important implications. Using both quantitative and qualitative assessment, we found that academic hospitalists have the ability to improve healthcare‐worker communication and enhance learner education without increasing LOS. These findings are directly applicable to VA medical centers and potentially applicable to other academic medical centers.
Acknowledgments
The authors thank Milisa Manojlovich, PhD, RN, Edward Kennedy, MS, and Andrew Hickner, MSI, for help with preparation of this manuscript.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability grant. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs. Dr. Saint reports receiving travel reimbursement for giving invited talks at the Society of Hospital Medicine's National Meeting, as well as serving on the advisory boards of Doximity and Jvion.
APPENDIX
Survey to Evaluate the Care Coordinator Position
| Yes | No | Not Sure | |
| Q1. Are you familiar with the role of the Care Coordinator on the Gold Service (Susan Lee)? | 1 | 2 | 3 |
Please indicate how much you agree or disagree with the statements below.
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | Don't Know | |
| Q2. The Care Coordinator ensures that appropriate primary care follow‐up and any other appropriate services are arranged. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q3. The Care Coordinator provides continuity of patient care on the Gold Service. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q4. The Care Coordinator helps educate House Officers and Medical Students on VA processes (e.g., CPRS). | 1 | 2 | 3 | 4 | 5 | 9 |
| Q5. The Care Coordinator assists with interdisciplinary communication between the medical team and other services (e.g., nursing, ambulatory care, pharmacy, social work) | 1 | 2 | 3 | 4 | 5 | 9 |
| Q6. The Care Coordinator helps facilitate patient discharge. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q7. The Care Coordinator initiates communication with the ambulatory care teams to coordinate care. | 1 | 2 | 3 | 4 | 5 | 9 |
| Yes | No | |
| Q8. Are you a physician (attending or resident), or medical student who has been on more than one medical team at the VA (Gold, Silver, Burgundy, or Yellow)? | 1 | 2 |
If no, please skip to Q13
If yes, comparing your experience on the Gold Service (with the Care Coordinator) to your experience on any of the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q9. To what extent does the presence of a Care Coordinator affect patient care? | 1 | 2 | 3 | 4 |
| Q10. To what extent does the presence of a Care Coordinator improve patient flow? | 1 | 2 | 3 | 4 |
| Q11. To what extent does the presence of a Care Coordinator assist with education? | 1 | 2 | 3 | 4 |
| Q12. To what extent does the presence of a Care Coordinator contribute to attending rounds? | 1 | 2 | 3 | 4 |
| Yes | No | |
| Q13. Do you work [as a nurse] in ambulatory care? | 1 | 2 |
If no, please skip to Q17.
If yes, comparing your experience with the Gold Service (with the Care Coordinator) to the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q14. To what extent does the presence of a Care Coordinator improve coordination of care between inpatient and outpatient services? | 1 | 2 | 3 | 4 |
| Q15. To what extent does the presence of a Care Coordinator help identify high risk patients who require follow‐up? | 1 | 2 | 3 | 4 |
| Q16. To what extent does the presence of a Care Coordinator ensure follow‐up appointments are scheduled? | 1 | 2 | 3 | 4 |
| Yes | No | Not Sure | |
| Q17. Do you think each medical team should have a Care Coordinator? | 1 | 2 | 3 |
| Q18. Are there any additional tasks or duties you think would improve the effectiveness of the Care Coordinator? |
| Very Satisfied | Satisfied | Neutral | Dissatisfied | Very Dissatisfied | |
| Q19. Overall how satisfied are you with the role of the Care Coordinator on the Gold Service? | 1 | 2 | 3 | 4 | 5 |
| Q20. Do you have any other comments about the role of the Care Coordinator? |
| Q21. What is your position? |
| 1. Physician (attending or resident) or medical student |
| 2. Nurse (inpatient or ambulatory care) |
- Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine of the National Academies. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- . Growth in care provided by hospitalists. N Engl J Med. 2009;360(26):2789–2791.
- American Hospital Association. AHA Annual Survey of Hospitals, 2010. Chicago, IL: Health Forum, LLC; 2010.
- , , , . Preventing hospital‐acquired infections: a national survey of practices reported by U.S. hospitals in 2005 and 2009. J Gen Intern Med. 2012;27(7):773–779.
- , . Hospitalists in teaching hospitals: opportunities but not without danger. J Gen Intern Med. 2004;19(4):392–393.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , . Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490–498.
- , , , , , . Resident satisfaction on an academic hospitalist service: time to teach. Am J Med. 2002;112(7):597–601.
- , , , et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19(4):293–301.
- , . Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships. J Hosp Med. 2007;2(1):17–22.
- , , , . Medical student evaluation of the quality of hospitalist and nonhospitalist teaching faculty on inpatient medicine rotations. Acad Med. 2004;79(1):78–82.
- , , , , . Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education. JAMA. 1998;279(19):1560–1565.
- . Nurse/physician communication through a sensemaking lens: shifting the paradigm to improve patient safety. Med Care. 2010;48(11):941–946.
- . Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care. Ithaca, NY: Cornell University Press; 2005.
- . Focus on research methods: whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Development of an instrument to measure collaboration and satisfaction about care decisions. J Adv Nurs. 1994;20(1):176–182.
- . Development of the practice environment scale of the Nursing Work Index. Res Nurs Health. 2002;25(3):176–188.
- , , . Does the structure of inpatient rounds affect medical student education? Int J Med Educ. 2013;4:96–100.
- U.S. Department of Veterans Affairs, Office of Academic Affiliations. Medical and Dental Education Program. Available at: http://www.va. gov/oaa/GME_default.asp. Published 2012. Accessed May 08, 2013.
- , . Graduate medical education, 2011–2012. JAMA. 2012;308(21):2264–2279.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859–865.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . ‘It depends': medical residents' perspectives on working with nurses. Am J Nurs. 2009;109(7):34–44.
- , . Organizational silence: a barrier to change and development in a pluralistic world. Acad Manage Rev. 2000;25(4):706–725.
- , . Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav. 2006;27:941–966.
- , , , , . Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012;12:437.
- , , , , , . A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485–2492.
- , , , , , . Impact of instructional practices on student satisfaction with attendings' teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7–12.
- , . Medical students' perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635–639.
- National Board of Medical Examiners Subject Examination Program. Internal Medicine Advanced Clinical Examination, score interpretation guide. Available at: http://www.nbme.org/PDF/SampleScoreReports/Internal_Medicine_ACE_Score_Report.pdf. Published 2011. Accessed September 13, 2013.
- . The impact of hospitalists on medical education and the academic health system. Ann Intern Med. 1999;130(4 part 2):364–367.
- Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine of the National Academies. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- . Growth in care provided by hospitalists. N Engl J Med. 2009;360(26):2789–2791.
- American Hospital Association. AHA Annual Survey of Hospitals, 2010. Chicago, IL: Health Forum, LLC; 2010.
- , , , . Preventing hospital‐acquired infections: a national survey of practices reported by U.S. hospitals in 2005 and 2009. J Gen Intern Med. 2012;27(7):773–779.
- , . Hospitalists in teaching hospitals: opportunities but not without danger. J Gen Intern Med. 2004;19(4):392–393.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , . Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490–498.
- , , , , , . Resident satisfaction on an academic hospitalist service: time to teach. Am J Med. 2002;112(7):597–601.
- , , , et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19(4):293–301.
- , . Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships. J Hosp Med. 2007;2(1):17–22.
- , , , . Medical student evaluation of the quality of hospitalist and nonhospitalist teaching faculty on inpatient medicine rotations. Acad Med. 2004;79(1):78–82.
- , , , , . Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education. JAMA. 1998;279(19):1560–1565.
- . Nurse/physician communication through a sensemaking lens: shifting the paradigm to improve patient safety. Med Care. 2010;48(11):941–946.
- . Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care. Ithaca, NY: Cornell University Press; 2005.
- . Focus on research methods: whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Development of an instrument to measure collaboration and satisfaction about care decisions. J Adv Nurs. 1994;20(1):176–182.
- . Development of the practice environment scale of the Nursing Work Index. Res Nurs Health. 2002;25(3):176–188.
- , , . Does the structure of inpatient rounds affect medical student education? Int J Med Educ. 2013;4:96–100.
- U.S. Department of Veterans Affairs, Office of Academic Affiliations. Medical and Dental Education Program. Available at: http://www.va. gov/oaa/GME_default.asp. Published 2012. Accessed May 08, 2013.
- , . Graduate medical education, 2011–2012. JAMA. 2012;308(21):2264–2279.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859–865.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . ‘It depends': medical residents' perspectives on working with nurses. Am J Nurs. 2009;109(7):34–44.
- , . Organizational silence: a barrier to change and development in a pluralistic world. Acad Manage Rev. 2000;25(4):706–725.
- , . Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav. 2006;27:941–966.
- , , , , . Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012;12:437.
- , , , , , . A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485–2492.
- , , , , , . Impact of instructional practices on student satisfaction with attendings' teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7–12.
- , . Medical students' perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635–639.
- National Board of Medical Examiners Subject Examination Program. Internal Medicine Advanced Clinical Examination, score interpretation guide. Available at: http://www.nbme.org/PDF/SampleScoreReports/Internal_Medicine_ACE_Score_Report.pdf. Published 2011. Accessed September 13, 2013.
- . The impact of hospitalists on medical education and the academic health system. Ann Intern Med. 1999;130(4 part 2):364–367.
© 2013 Society of The Authors. Journal of Hospital Medicine published by Wiley Periodicals, Inc. on behalf of Society of Hospital Medicine.