User login
Chemo avoidance pays off for some women with HER2+ early BC
The secondary primary endpoint results from the PHERgain study, presented at the annual meeting of the American Society of Clinical Oncology, provide more evidence to support a strategy that avoids chemotherapy as long as patients show signs of response to hormone therapy via PET scans. The results revealed that 98.8% (95% confidence interval, 96.3-100.0) of 86 patients who received treatment with trastuzumab and pertuzumab – but no chemotherapy – remained cancer free and alive 3 years after surgery (invasive disease–free survival).
“Only 1 out of 86 patients experience disease recurrence ... in those patients who never received chemotherapy,” said study lead author Javier Cortés, MD, PhD, an oncologist with Ramón y Cajal University Hospital, Madrid, during his presentation at the meeting.
As Dr. Cortés noted, HER2-targeted therapies such as trastuzumab have improved lifespans in women with HER2-positive early breast cancer, sparking interest in whether chemotherapy can be de-escalated. The PHERgain study examines whether it can be avoided entirely.
The primary endpoint results of the multicenter, open-label, noncomparative study were released in The Lancet Oncology in 2021.
Study methods and results
At 45 hospitals in Europe, patients with HER2-positive, stage I-IIIA, invasive, operable breast cancer were randomly assigned between 2017 and 2019 to receive chemotherapy prior to surgery (n = 71, group A) or to only receive hormone therapy with trastuzumab and pertuzumab, unless PET scans suggested they needed chemotherapy because they weren’t properly responding (n = 285, group B).
At a median follow-up of 5.7 months, 86 patients in the latter group had a pathological complete response and therefore met the first primary endpoint.
The new analysis tracked patients for 3 years after they underwent surgery (n = 63 and 267 for patients in groups A and B, respectively). As previously noted, at a median follow-up of 43.3 months (range, 2.4-63.0 months), only 1 of 86 patients in group B who didn’t receive chemotherapy had a recurrence of cancer (a locoregional ipsilateral recurrence). The 98.8% invasive disease–free survival rate was higher that what was seen for patients in group B as a whole (95.4% invasive disease–free survival, 95% CI, 92.8%-98.0%, P < .001). The 95.4% met the study’s second primary endpoint.
Treatment-related adverse events were higher in the group that received chemotherapy only (group A) versus group B (experiencing an adverse event grade of at least 3, 61.8% vs. 32.9%, respectively, P < .001; serious adverse events, 27.9% vs. 13.8%, respectively; P = .01). Those in group B who didn’t receive any chemotherapy had very few treatment-related adverse events that were considered being greater than a grade 3 (1.2%) and no treatment-related serious adverse events. The researchers reported that there were no treatment-related deaths.
In an interview, Kevin Kalinsky, MD, MS, an oncologist at Emory University Hospital, Atlanta, and cochair of the session where the study data was presented, said the “intriguing and meaningful [findings] highlight the fact that not everyone may need chemotherapy.” In the big picture, the results reflect a movement toward “individualized, personalized medicine, and moving away from one size fits all.”
Should clinicians embrace the study’s strategy, and what are the costs?
“There may be a need for additional evaluation in a large phase 3 trial,” Dr. Kalinsky said.
There was no discussion about cost during the ASCO presentation. However, Dr. Kalinsky noted that there will be cost savings if patients don’t need chemotherapy. But he added that insurers in the United States don’t always cover the PET scans that are needed to evaluate whether patients are responding to hormone therapy.
The study is funded by Roche and sponsored by MedSIR. Dr. Cortes has multiple disclosures, including stock/other ownership in Leuko, MedSIR, and Nektar and honoraria from AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung. Dr. Kalinsky has no disclosures.
The secondary primary endpoint results from the PHERgain study, presented at the annual meeting of the American Society of Clinical Oncology, provide more evidence to support a strategy that avoids chemotherapy as long as patients show signs of response to hormone therapy via PET scans. The results revealed that 98.8% (95% confidence interval, 96.3-100.0) of 86 patients who received treatment with trastuzumab and pertuzumab – but no chemotherapy – remained cancer free and alive 3 years after surgery (invasive disease–free survival).
“Only 1 out of 86 patients experience disease recurrence ... in those patients who never received chemotherapy,” said study lead author Javier Cortés, MD, PhD, an oncologist with Ramón y Cajal University Hospital, Madrid, during his presentation at the meeting.
As Dr. Cortés noted, HER2-targeted therapies such as trastuzumab have improved lifespans in women with HER2-positive early breast cancer, sparking interest in whether chemotherapy can be de-escalated. The PHERgain study examines whether it can be avoided entirely.
The primary endpoint results of the multicenter, open-label, noncomparative study were released in The Lancet Oncology in 2021.
Study methods and results
At 45 hospitals in Europe, patients with HER2-positive, stage I-IIIA, invasive, operable breast cancer were randomly assigned between 2017 and 2019 to receive chemotherapy prior to surgery (n = 71, group A) or to only receive hormone therapy with trastuzumab and pertuzumab, unless PET scans suggested they needed chemotherapy because they weren’t properly responding (n = 285, group B).
At a median follow-up of 5.7 months, 86 patients in the latter group had a pathological complete response and therefore met the first primary endpoint.
The new analysis tracked patients for 3 years after they underwent surgery (n = 63 and 267 for patients in groups A and B, respectively). As previously noted, at a median follow-up of 43.3 months (range, 2.4-63.0 months), only 1 of 86 patients in group B who didn’t receive chemotherapy had a recurrence of cancer (a locoregional ipsilateral recurrence). The 98.8% invasive disease–free survival rate was higher that what was seen for patients in group B as a whole (95.4% invasive disease–free survival, 95% CI, 92.8%-98.0%, P < .001). The 95.4% met the study’s second primary endpoint.
Treatment-related adverse events were higher in the group that received chemotherapy only (group A) versus group B (experiencing an adverse event grade of at least 3, 61.8% vs. 32.9%, respectively, P < .001; serious adverse events, 27.9% vs. 13.8%, respectively; P = .01). Those in group B who didn’t receive any chemotherapy had very few treatment-related adverse events that were considered being greater than a grade 3 (1.2%) and no treatment-related serious adverse events. The researchers reported that there were no treatment-related deaths.
In an interview, Kevin Kalinsky, MD, MS, an oncologist at Emory University Hospital, Atlanta, and cochair of the session where the study data was presented, said the “intriguing and meaningful [findings] highlight the fact that not everyone may need chemotherapy.” In the big picture, the results reflect a movement toward “individualized, personalized medicine, and moving away from one size fits all.”
Should clinicians embrace the study’s strategy, and what are the costs?
“There may be a need for additional evaluation in a large phase 3 trial,” Dr. Kalinsky said.
There was no discussion about cost during the ASCO presentation. However, Dr. Kalinsky noted that there will be cost savings if patients don’t need chemotherapy. But he added that insurers in the United States don’t always cover the PET scans that are needed to evaluate whether patients are responding to hormone therapy.
The study is funded by Roche and sponsored by MedSIR. Dr. Cortes has multiple disclosures, including stock/other ownership in Leuko, MedSIR, and Nektar and honoraria from AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung. Dr. Kalinsky has no disclosures.
The secondary primary endpoint results from the PHERgain study, presented at the annual meeting of the American Society of Clinical Oncology, provide more evidence to support a strategy that avoids chemotherapy as long as patients show signs of response to hormone therapy via PET scans. The results revealed that 98.8% (95% confidence interval, 96.3-100.0) of 86 patients who received treatment with trastuzumab and pertuzumab – but no chemotherapy – remained cancer free and alive 3 years after surgery (invasive disease–free survival).
“Only 1 out of 86 patients experience disease recurrence ... in those patients who never received chemotherapy,” said study lead author Javier Cortés, MD, PhD, an oncologist with Ramón y Cajal University Hospital, Madrid, during his presentation at the meeting.
As Dr. Cortés noted, HER2-targeted therapies such as trastuzumab have improved lifespans in women with HER2-positive early breast cancer, sparking interest in whether chemotherapy can be de-escalated. The PHERgain study examines whether it can be avoided entirely.
The primary endpoint results of the multicenter, open-label, noncomparative study were released in The Lancet Oncology in 2021.
Study methods and results
At 45 hospitals in Europe, patients with HER2-positive, stage I-IIIA, invasive, operable breast cancer were randomly assigned between 2017 and 2019 to receive chemotherapy prior to surgery (n = 71, group A) or to only receive hormone therapy with trastuzumab and pertuzumab, unless PET scans suggested they needed chemotherapy because they weren’t properly responding (n = 285, group B).
At a median follow-up of 5.7 months, 86 patients in the latter group had a pathological complete response and therefore met the first primary endpoint.
The new analysis tracked patients for 3 years after they underwent surgery (n = 63 and 267 for patients in groups A and B, respectively). As previously noted, at a median follow-up of 43.3 months (range, 2.4-63.0 months), only 1 of 86 patients in group B who didn’t receive chemotherapy had a recurrence of cancer (a locoregional ipsilateral recurrence). The 98.8% invasive disease–free survival rate was higher that what was seen for patients in group B as a whole (95.4% invasive disease–free survival, 95% CI, 92.8%-98.0%, P < .001). The 95.4% met the study’s second primary endpoint.
Treatment-related adverse events were higher in the group that received chemotherapy only (group A) versus group B (experiencing an adverse event grade of at least 3, 61.8% vs. 32.9%, respectively, P < .001; serious adverse events, 27.9% vs. 13.8%, respectively; P = .01). Those in group B who didn’t receive any chemotherapy had very few treatment-related adverse events that were considered being greater than a grade 3 (1.2%) and no treatment-related serious adverse events. The researchers reported that there were no treatment-related deaths.
In an interview, Kevin Kalinsky, MD, MS, an oncologist at Emory University Hospital, Atlanta, and cochair of the session where the study data was presented, said the “intriguing and meaningful [findings] highlight the fact that not everyone may need chemotherapy.” In the big picture, the results reflect a movement toward “individualized, personalized medicine, and moving away from one size fits all.”
Should clinicians embrace the study’s strategy, and what are the costs?
“There may be a need for additional evaluation in a large phase 3 trial,” Dr. Kalinsky said.
There was no discussion about cost during the ASCO presentation. However, Dr. Kalinsky noted that there will be cost savings if patients don’t need chemotherapy. But he added that insurers in the United States don’t always cover the PET scans that are needed to evaluate whether patients are responding to hormone therapy.
The study is funded by Roche and sponsored by MedSIR. Dr. Cortes has multiple disclosures, including stock/other ownership in Leuko, MedSIR, and Nektar and honoraria from AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung. Dr. Kalinsky has no disclosures.
AT ASCO 2023
Breast cancer: Meta-analysis supports ovarian suppression/ablation
Those who didn’t take tamoxifen – a standard treatment today – seemed to gain an especially large benefit.
The randomized studies, which included 14,999 subjects, suggest that ovarian suppression/ablation can provide a “substantial and persistent benefit for premenopausal women,” said study lead author and medical statistician Richard G. Gray, MA, MSc, of the University of Oxford (England), in a presentation at the annual meeting of the American Society of Clinical Oncology.
The study authors sought to better understand the value of ovarian suppression/ablation, which may prevent estrogen from stimulating residual cancer after treatment. According to the study abstract, premenopausal women with estrogen receptor–positive tumors may be at special risk of cancer recurrence because of this phenomenon.
Recently published research has supported hormone therapy targeting the ovaries in this population.
“Ovarian suppression with an aromatase inhibitor should become the preferred initial hormone therapy recommendation for all premenopausal women with high-risk (i.e., grade 3, T2, and age less than 35 years) estrogen receptor–positive breast cancer,” declared a 2022 editorial in the Journal of Clinical Oncology that noted the positive findings of a 13-year follow-up analysis of 2 studies.
Study methods and results
For the meta-analysis released at ASCO, researchers examined 25 trials that randomized women with breast cancer who were premenopausal. In some cases, the women went through menopause during the trials, and in some other cases, ovarian suppression/ablation brought on early menopause.
Among women who had received no chemotherapy or remained premenopausal after chemotherapy (n = 7,213), cancer recurred within 15 years in 41% of the controls and 28.9% of the ovarian suppression/ablation group, (relative risk, 0.70; 95% confidence interval, 0.63-0.78; P < .00001).
Among these same women, breast cancer mortality at 20 years was 34.7% in the controls and 23.8% in the ovarian suppression/ablation group (RR, 0.71; 95% CI, 0.62-0.81; P < .00001).
The researchers also looked at the same group of women and divided it into those who didn’t take tamoxifen (2,362) and those who did take tamoxifen (4,851). The drug is now the preferred option “for treatment of breast cancer.”
Among those who did not take tamoxifen, the recurrence rate at 15 years was 56.5% among controls versus 39.0% among those in the ovarian suppression/ablation group (RR, 0.61; 95% CI, 0.52-0.72; P < .00001). The gap shrunk in those who did take tamoxifen: recurrence occurred in 30.3% of the control group and 25.8% of the ovarian suppression/ablation group (RR, 0.80; 95% CI, 0.70-0.93; P = .002).
Tamoxifen on its own seems to have powerful positive effect
The findings suggest that tamoxifen on its own has a powerful positive effect, leaving less extra benefit for ovarian suppression/ablation to provide, said Mr. Gray.
The meta-analysis didn’t examine cost or cost-effectiveness.
Kevin Kalinsky, MD, MS, an oncologist at Emory University Hospital, Atlanta, cochair of the session where the meta-analysis data was presented, said in an interview that the new research shows that “patients can really benefit from ovarian function suppression.” Even so, recent trials suggested that the strategy is uncommon, used by less than 20% of high-risk patients.
Dr. Kalinsky noted that suppressing the ovaries with medication or removing the ovaries entirely can cause early menopause and eliminate fertility.
“There can be definitely be side effects like hot flashes and tolerability issues,” he said, “along with an impact on quality of life.”
According to the U.K. organization Breast Cancer Now,“ovarian suppression achieved by hormone therapy or surgery is more likely to cause menopausal symptoms than a natural menopause.” In addition, “research has shown that younger women are more likely to stop taking hormone therapy early if they don’t get help with possible side effects.”
It’s important for patients and providers to have full discussions about possible strategies, Dr. Kalinsky said.
No information about study funding was provided. Dr. Kalinsky and Mr. Gray had no financial conflicts.
Those who didn’t take tamoxifen – a standard treatment today – seemed to gain an especially large benefit.
The randomized studies, which included 14,999 subjects, suggest that ovarian suppression/ablation can provide a “substantial and persistent benefit for premenopausal women,” said study lead author and medical statistician Richard G. Gray, MA, MSc, of the University of Oxford (England), in a presentation at the annual meeting of the American Society of Clinical Oncology.
The study authors sought to better understand the value of ovarian suppression/ablation, which may prevent estrogen from stimulating residual cancer after treatment. According to the study abstract, premenopausal women with estrogen receptor–positive tumors may be at special risk of cancer recurrence because of this phenomenon.
Recently published research has supported hormone therapy targeting the ovaries in this population.
“Ovarian suppression with an aromatase inhibitor should become the preferred initial hormone therapy recommendation for all premenopausal women with high-risk (i.e., grade 3, T2, and age less than 35 years) estrogen receptor–positive breast cancer,” declared a 2022 editorial in the Journal of Clinical Oncology that noted the positive findings of a 13-year follow-up analysis of 2 studies.
Study methods and results
For the meta-analysis released at ASCO, researchers examined 25 trials that randomized women with breast cancer who were premenopausal. In some cases, the women went through menopause during the trials, and in some other cases, ovarian suppression/ablation brought on early menopause.
Among women who had received no chemotherapy or remained premenopausal after chemotherapy (n = 7,213), cancer recurred within 15 years in 41% of the controls and 28.9% of the ovarian suppression/ablation group, (relative risk, 0.70; 95% confidence interval, 0.63-0.78; P < .00001).
Among these same women, breast cancer mortality at 20 years was 34.7% in the controls and 23.8% in the ovarian suppression/ablation group (RR, 0.71; 95% CI, 0.62-0.81; P < .00001).
The researchers also looked at the same group of women and divided it into those who didn’t take tamoxifen (2,362) and those who did take tamoxifen (4,851). The drug is now the preferred option “for treatment of breast cancer.”
Among those who did not take tamoxifen, the recurrence rate at 15 years was 56.5% among controls versus 39.0% among those in the ovarian suppression/ablation group (RR, 0.61; 95% CI, 0.52-0.72; P < .00001). The gap shrunk in those who did take tamoxifen: recurrence occurred in 30.3% of the control group and 25.8% of the ovarian suppression/ablation group (RR, 0.80; 95% CI, 0.70-0.93; P = .002).
Tamoxifen on its own seems to have powerful positive effect
The findings suggest that tamoxifen on its own has a powerful positive effect, leaving less extra benefit for ovarian suppression/ablation to provide, said Mr. Gray.
The meta-analysis didn’t examine cost or cost-effectiveness.
Kevin Kalinsky, MD, MS, an oncologist at Emory University Hospital, Atlanta, cochair of the session where the meta-analysis data was presented, said in an interview that the new research shows that “patients can really benefit from ovarian function suppression.” Even so, recent trials suggested that the strategy is uncommon, used by less than 20% of high-risk patients.
Dr. Kalinsky noted that suppressing the ovaries with medication or removing the ovaries entirely can cause early menopause and eliminate fertility.
“There can be definitely be side effects like hot flashes and tolerability issues,” he said, “along with an impact on quality of life.”
According to the U.K. organization Breast Cancer Now,“ovarian suppression achieved by hormone therapy or surgery is more likely to cause menopausal symptoms than a natural menopause.” In addition, “research has shown that younger women are more likely to stop taking hormone therapy early if they don’t get help with possible side effects.”
It’s important for patients and providers to have full discussions about possible strategies, Dr. Kalinsky said.
No information about study funding was provided. Dr. Kalinsky and Mr. Gray had no financial conflicts.
Those who didn’t take tamoxifen – a standard treatment today – seemed to gain an especially large benefit.
The randomized studies, which included 14,999 subjects, suggest that ovarian suppression/ablation can provide a “substantial and persistent benefit for premenopausal women,” said study lead author and medical statistician Richard G. Gray, MA, MSc, of the University of Oxford (England), in a presentation at the annual meeting of the American Society of Clinical Oncology.
The study authors sought to better understand the value of ovarian suppression/ablation, which may prevent estrogen from stimulating residual cancer after treatment. According to the study abstract, premenopausal women with estrogen receptor–positive tumors may be at special risk of cancer recurrence because of this phenomenon.
Recently published research has supported hormone therapy targeting the ovaries in this population.
“Ovarian suppression with an aromatase inhibitor should become the preferred initial hormone therapy recommendation for all premenopausal women with high-risk (i.e., grade 3, T2, and age less than 35 years) estrogen receptor–positive breast cancer,” declared a 2022 editorial in the Journal of Clinical Oncology that noted the positive findings of a 13-year follow-up analysis of 2 studies.
Study methods and results
For the meta-analysis released at ASCO, researchers examined 25 trials that randomized women with breast cancer who were premenopausal. In some cases, the women went through menopause during the trials, and in some other cases, ovarian suppression/ablation brought on early menopause.
Among women who had received no chemotherapy or remained premenopausal after chemotherapy (n = 7,213), cancer recurred within 15 years in 41% of the controls and 28.9% of the ovarian suppression/ablation group, (relative risk, 0.70; 95% confidence interval, 0.63-0.78; P < .00001).
Among these same women, breast cancer mortality at 20 years was 34.7% in the controls and 23.8% in the ovarian suppression/ablation group (RR, 0.71; 95% CI, 0.62-0.81; P < .00001).
The researchers also looked at the same group of women and divided it into those who didn’t take tamoxifen (2,362) and those who did take tamoxifen (4,851). The drug is now the preferred option “for treatment of breast cancer.”
Among those who did not take tamoxifen, the recurrence rate at 15 years was 56.5% among controls versus 39.0% among those in the ovarian suppression/ablation group (RR, 0.61; 95% CI, 0.52-0.72; P < .00001). The gap shrunk in those who did take tamoxifen: recurrence occurred in 30.3% of the control group and 25.8% of the ovarian suppression/ablation group (RR, 0.80; 95% CI, 0.70-0.93; P = .002).
Tamoxifen on its own seems to have powerful positive effect
The findings suggest that tamoxifen on its own has a powerful positive effect, leaving less extra benefit for ovarian suppression/ablation to provide, said Mr. Gray.
The meta-analysis didn’t examine cost or cost-effectiveness.
Kevin Kalinsky, MD, MS, an oncologist at Emory University Hospital, Atlanta, cochair of the session where the meta-analysis data was presented, said in an interview that the new research shows that “patients can really benefit from ovarian function suppression.” Even so, recent trials suggested that the strategy is uncommon, used by less than 20% of high-risk patients.
Dr. Kalinsky noted that suppressing the ovaries with medication or removing the ovaries entirely can cause early menopause and eliminate fertility.
“There can be definitely be side effects like hot flashes and tolerability issues,” he said, “along with an impact on quality of life.”
According to the U.K. organization Breast Cancer Now,“ovarian suppression achieved by hormone therapy or surgery is more likely to cause menopausal symptoms than a natural menopause.” In addition, “research has shown that younger women are more likely to stop taking hormone therapy early if they don’t get help with possible side effects.”
It’s important for patients and providers to have full discussions about possible strategies, Dr. Kalinsky said.
No information about study funding was provided. Dr. Kalinsky and Mr. Gray had no financial conflicts.
AT ASCO 2023
Buprenorphine update: Looser rules and a helpful injectable
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
AT APA 2023
Blood cancer patient takes on bias and ‘gaslighting’
Diagnosed with Hodgkin lymphoma in 2021, Ms. Ngon underwent port surgery to allow chemotherapy to be administered. Her right arm lost circulation and went numb, so she sought guidance from her blood cancer specialist. He dismissed her worries, saying that her tumors were pinching a nerve. She’d get better, he predicted, after more chemo.
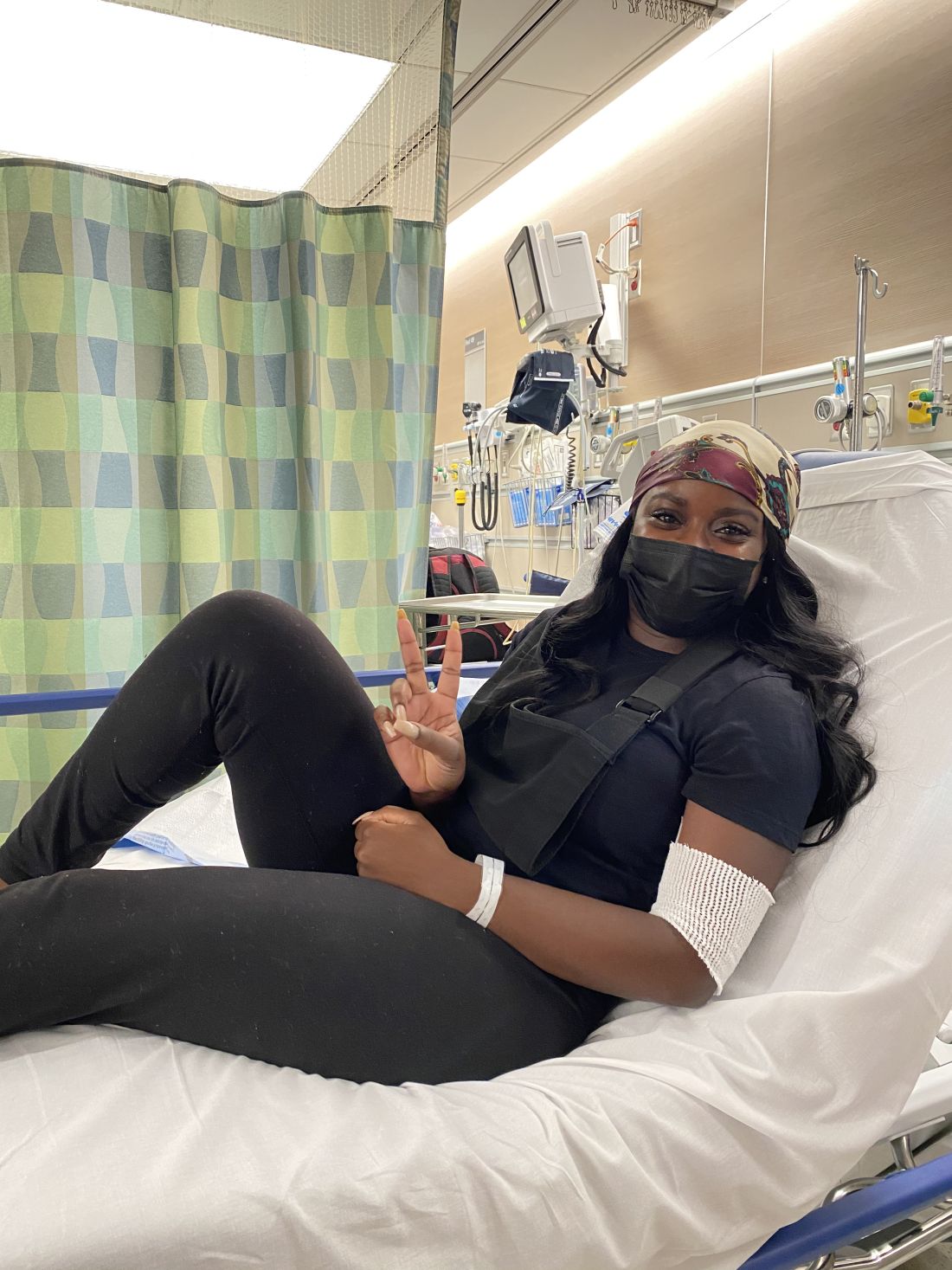
“I knew in my body that something was wrong,” Ms. Ngon recalled. When the oncologist continued to downplay her concerns, she and a fellow communications specialist sat down together in the hospital lobby to draft an email to her physician. “We were trying to articulate the urgency in an email that expresses that I’m not being dramatic. We had to do it in a way that didn’t insult his intelligence: ‘Respectfully, you’re the doctor, but I know something is wrong.’ ”
In essence, Ms. Ngon was trying to be diplomatic and not trigger her oncologist’s defenses, while still convincing him to take action. Her approach to getting her doctor’s attention worked. He referred Ms. Ngon to a radiologist, who discovered that she had blood clots in her arm. Ms. Ngon then landed in the ICU for a week, as clinicians tried to break up the clots.
“I was the perfect person for this to happen to, because of my job and education. But it makes me sad because I understand I was in a fortunate position, with a background in communication. Most people don’t have that,” Ms. Ngon said.
This and other negative experiences during her medical saga inspired Ms. Ngon to partner with the Lymphoma Research Foundation in order to spread the word about unique challenges facing patients like her: people of color.
Ms. Ngon, who is Black, said her goal as a patient advocate is to “empower communities of color to speak up for themselves and hold oncologists responsible for listening and understanding differences across cultures.” And she wants to take a stand against the “gaslighting” of patients.
African Americans with hematologic disease like Ms. Ngon face a higher risk of poor outcomes than Whites, even as they are less likely than Whites to develop certain blood cancers. The reasons for this disparity aren’t clear, but researchers suspect they’re related to factors such as poverty, lack of insurance, genetics, and limited access to high-quality care.
Some researchers have blamed another factor: racism. A 2022 study sought to explain why Black and Hispanic patients with acute myeloid leukemia in urban areas have higher mortality rates than Whites, “despite more favorable genetics and younger age” (hazard ratio, 1.59, 95% confidence interval, 1.15-2.22 and HR, 1.25; 95% CI, 0.88-1.79). The study authors determined that “structural racism” – which they measured by examining segregation and “disadvantage” in neighborhoods where patients lived – accounted for nearly all of the disparities.
Ms. Ngon said her experiences and her awareness about poorer outcomes in medicine for African Americans – such as higher death rates for Black women during pregnancy – affect how she interacts with clinicians. “I automatically assume a barrier between me and my doctors, and it’s their responsibility to dismantle it.”
Making an connection with a physician can make a huge difference, she said. “I walked into my primary care doctor’s office and saw that she was a Latino woman. My guard went down, and I could feel her care for me as a human being. Whether that was because she was also a woman of color or not, I don’t know. But I did feel more cared for.”
However, Ms. Ngon could not find a Black oncologist to care for her in New York City, and that’s no surprise.
Ethnic and gender diversity remains an immense challenge in the hematology/oncology field. According to the American Society of Clinical Oncology, only about a third of oncologists are women, and the percentages identifying themselves as Black/African American and Hispanic are just 2.3% and 5.8%, respectively.
These numbers don’t seem likely to budge much any time soon. An analysis of medical students in U.S. oncology training programs from 2015-2020 found that just 3.8% identified themselves as Black/African American and 5.1% as Hispanic/Latino versus 52.15% as White and 31% as Asian/Pacific Islander/Native Hawaiian.
Ms. Ngon encountered challenges on other fronts during her cancer care. When she needed a wig during chemotherapy, a list of insurer-approved shops didn’t include any that catered to African Americans. Essentially, she said, she was being told that she couldn’t “purchase a wig from a place that makes you feel comfortable and from a woman who understand your needs as a Black woman. It needs to be from these specific shops that really don’t cater to my community.”
She also found it difficult to find fellow patients who shared her unique challenges. “I remember when I was diagnosed, I was looking through the support groups on Facebook, trying to find someone Black to ask about whether braiding my hair might stop it from falling out.”
Now, Ms. Ngon is in remission. And she’s happy with her oncologist, who’s White. “He listened to me, and he promised me that I would have the most boring recovery process ever, after everything I’d experienced. That explains a lot of why I felt so comfortable with him.”
She hopes to use her partnership with the Lymphoma Research Foundation to be a resource for people of color and alert them to the support that’s available for them. “I would love to let them know how to advocate for themselves as patients, how to trust their bodies, how to push back if they feel like they’re not getting the care that they deserve.”
Ms. Ngon would also like to see more support for medical students of color. “I hope to exist in a world one day where it wouldn’t be so hard to find an oncologist who looks like me in a city as large as this one,” she said.
As for oncologists, she urged them to “go the extra mile and really, really listen to what patients are saying. It’s easier said than done because there are natural biases in this world, and it’s hard to overcome those obstacles. But to not be heard and have to push every time. It was just exhausting to do that on top of trying to beat cancer.”
Diagnosed with Hodgkin lymphoma in 2021, Ms. Ngon underwent port surgery to allow chemotherapy to be administered. Her right arm lost circulation and went numb, so she sought guidance from her blood cancer specialist. He dismissed her worries, saying that her tumors were pinching a nerve. She’d get better, he predicted, after more chemo.

“I knew in my body that something was wrong,” Ms. Ngon recalled. When the oncologist continued to downplay her concerns, she and a fellow communications specialist sat down together in the hospital lobby to draft an email to her physician. “We were trying to articulate the urgency in an email that expresses that I’m not being dramatic. We had to do it in a way that didn’t insult his intelligence: ‘Respectfully, you’re the doctor, but I know something is wrong.’ ”
In essence, Ms. Ngon was trying to be diplomatic and not trigger her oncologist’s defenses, while still convincing him to take action. Her approach to getting her doctor’s attention worked. He referred Ms. Ngon to a radiologist, who discovered that she had blood clots in her arm. Ms. Ngon then landed in the ICU for a week, as clinicians tried to break up the clots.
“I was the perfect person for this to happen to, because of my job and education. But it makes me sad because I understand I was in a fortunate position, with a background in communication. Most people don’t have that,” Ms. Ngon said.
This and other negative experiences during her medical saga inspired Ms. Ngon to partner with the Lymphoma Research Foundation in order to spread the word about unique challenges facing patients like her: people of color.
Ms. Ngon, who is Black, said her goal as a patient advocate is to “empower communities of color to speak up for themselves and hold oncologists responsible for listening and understanding differences across cultures.” And she wants to take a stand against the “gaslighting” of patients.
African Americans with hematologic disease like Ms. Ngon face a higher risk of poor outcomes than Whites, even as they are less likely than Whites to develop certain blood cancers. The reasons for this disparity aren’t clear, but researchers suspect they’re related to factors such as poverty, lack of insurance, genetics, and limited access to high-quality care.
Some researchers have blamed another factor: racism. A 2022 study sought to explain why Black and Hispanic patients with acute myeloid leukemia in urban areas have higher mortality rates than Whites, “despite more favorable genetics and younger age” (hazard ratio, 1.59, 95% confidence interval, 1.15-2.22 and HR, 1.25; 95% CI, 0.88-1.79). The study authors determined that “structural racism” – which they measured by examining segregation and “disadvantage” in neighborhoods where patients lived – accounted for nearly all of the disparities.
Ms. Ngon said her experiences and her awareness about poorer outcomes in medicine for African Americans – such as higher death rates for Black women during pregnancy – affect how she interacts with clinicians. “I automatically assume a barrier between me and my doctors, and it’s their responsibility to dismantle it.”
Making an connection with a physician can make a huge difference, she said. “I walked into my primary care doctor’s office and saw that she was a Latino woman. My guard went down, and I could feel her care for me as a human being. Whether that was because she was also a woman of color or not, I don’t know. But I did feel more cared for.”
However, Ms. Ngon could not find a Black oncologist to care for her in New York City, and that’s no surprise.
Ethnic and gender diversity remains an immense challenge in the hematology/oncology field. According to the American Society of Clinical Oncology, only about a third of oncologists are women, and the percentages identifying themselves as Black/African American and Hispanic are just 2.3% and 5.8%, respectively.
These numbers don’t seem likely to budge much any time soon. An analysis of medical students in U.S. oncology training programs from 2015-2020 found that just 3.8% identified themselves as Black/African American and 5.1% as Hispanic/Latino versus 52.15% as White and 31% as Asian/Pacific Islander/Native Hawaiian.
Ms. Ngon encountered challenges on other fronts during her cancer care. When she needed a wig during chemotherapy, a list of insurer-approved shops didn’t include any that catered to African Americans. Essentially, she said, she was being told that she couldn’t “purchase a wig from a place that makes you feel comfortable and from a woman who understand your needs as a Black woman. It needs to be from these specific shops that really don’t cater to my community.”
She also found it difficult to find fellow patients who shared her unique challenges. “I remember when I was diagnosed, I was looking through the support groups on Facebook, trying to find someone Black to ask about whether braiding my hair might stop it from falling out.”
Now, Ms. Ngon is in remission. And she’s happy with her oncologist, who’s White. “He listened to me, and he promised me that I would have the most boring recovery process ever, after everything I’d experienced. That explains a lot of why I felt so comfortable with him.”
She hopes to use her partnership with the Lymphoma Research Foundation to be a resource for people of color and alert them to the support that’s available for them. “I would love to let them know how to advocate for themselves as patients, how to trust their bodies, how to push back if they feel like they’re not getting the care that they deserve.”
Ms. Ngon would also like to see more support for medical students of color. “I hope to exist in a world one day where it wouldn’t be so hard to find an oncologist who looks like me in a city as large as this one,” she said.
As for oncologists, she urged them to “go the extra mile and really, really listen to what patients are saying. It’s easier said than done because there are natural biases in this world, and it’s hard to overcome those obstacles. But to not be heard and have to push every time. It was just exhausting to do that on top of trying to beat cancer.”
Diagnosed with Hodgkin lymphoma in 2021, Ms. Ngon underwent port surgery to allow chemotherapy to be administered. Her right arm lost circulation and went numb, so she sought guidance from her blood cancer specialist. He dismissed her worries, saying that her tumors were pinching a nerve. She’d get better, he predicted, after more chemo.

“I knew in my body that something was wrong,” Ms. Ngon recalled. When the oncologist continued to downplay her concerns, she and a fellow communications specialist sat down together in the hospital lobby to draft an email to her physician. “We were trying to articulate the urgency in an email that expresses that I’m not being dramatic. We had to do it in a way that didn’t insult his intelligence: ‘Respectfully, you’re the doctor, but I know something is wrong.’ ”
In essence, Ms. Ngon was trying to be diplomatic and not trigger her oncologist’s defenses, while still convincing him to take action. Her approach to getting her doctor’s attention worked. He referred Ms. Ngon to a radiologist, who discovered that she had blood clots in her arm. Ms. Ngon then landed in the ICU for a week, as clinicians tried to break up the clots.
“I was the perfect person for this to happen to, because of my job and education. But it makes me sad because I understand I was in a fortunate position, with a background in communication. Most people don’t have that,” Ms. Ngon said.
This and other negative experiences during her medical saga inspired Ms. Ngon to partner with the Lymphoma Research Foundation in order to spread the word about unique challenges facing patients like her: people of color.
Ms. Ngon, who is Black, said her goal as a patient advocate is to “empower communities of color to speak up for themselves and hold oncologists responsible for listening and understanding differences across cultures.” And she wants to take a stand against the “gaslighting” of patients.
African Americans with hematologic disease like Ms. Ngon face a higher risk of poor outcomes than Whites, even as they are less likely than Whites to develop certain blood cancers. The reasons for this disparity aren’t clear, but researchers suspect they’re related to factors such as poverty, lack of insurance, genetics, and limited access to high-quality care.
Some researchers have blamed another factor: racism. A 2022 study sought to explain why Black and Hispanic patients with acute myeloid leukemia in urban areas have higher mortality rates than Whites, “despite more favorable genetics and younger age” (hazard ratio, 1.59, 95% confidence interval, 1.15-2.22 and HR, 1.25; 95% CI, 0.88-1.79). The study authors determined that “structural racism” – which they measured by examining segregation and “disadvantage” in neighborhoods where patients lived – accounted for nearly all of the disparities.
Ms. Ngon said her experiences and her awareness about poorer outcomes in medicine for African Americans – such as higher death rates for Black women during pregnancy – affect how she interacts with clinicians. “I automatically assume a barrier between me and my doctors, and it’s their responsibility to dismantle it.”
Making an connection with a physician can make a huge difference, she said. “I walked into my primary care doctor’s office and saw that she was a Latino woman. My guard went down, and I could feel her care for me as a human being. Whether that was because she was also a woman of color or not, I don’t know. But I did feel more cared for.”
However, Ms. Ngon could not find a Black oncologist to care for her in New York City, and that’s no surprise.
Ethnic and gender diversity remains an immense challenge in the hematology/oncology field. According to the American Society of Clinical Oncology, only about a third of oncologists are women, and the percentages identifying themselves as Black/African American and Hispanic are just 2.3% and 5.8%, respectively.
These numbers don’t seem likely to budge much any time soon. An analysis of medical students in U.S. oncology training programs from 2015-2020 found that just 3.8% identified themselves as Black/African American and 5.1% as Hispanic/Latino versus 52.15% as White and 31% as Asian/Pacific Islander/Native Hawaiian.
Ms. Ngon encountered challenges on other fronts during her cancer care. When she needed a wig during chemotherapy, a list of insurer-approved shops didn’t include any that catered to African Americans. Essentially, she said, she was being told that she couldn’t “purchase a wig from a place that makes you feel comfortable and from a woman who understand your needs as a Black woman. It needs to be from these specific shops that really don’t cater to my community.”
She also found it difficult to find fellow patients who shared her unique challenges. “I remember when I was diagnosed, I was looking through the support groups on Facebook, trying to find someone Black to ask about whether braiding my hair might stop it from falling out.”
Now, Ms. Ngon is in remission. And she’s happy with her oncologist, who’s White. “He listened to me, and he promised me that I would have the most boring recovery process ever, after everything I’d experienced. That explains a lot of why I felt so comfortable with him.”
She hopes to use her partnership with the Lymphoma Research Foundation to be a resource for people of color and alert them to the support that’s available for them. “I would love to let them know how to advocate for themselves as patients, how to trust their bodies, how to push back if they feel like they’re not getting the care that they deserve.”
Ms. Ngon would also like to see more support for medical students of color. “I hope to exist in a world one day where it wouldn’t be so hard to find an oncologist who looks like me in a city as large as this one,” she said.
As for oncologists, she urged them to “go the extra mile and really, really listen to what patients are saying. It’s easier said than done because there are natural biases in this world, and it’s hard to overcome those obstacles. But to not be heard and have to push every time. It was just exhausting to do that on top of trying to beat cancer.”
Quick medication, better communication linked to less violence at inpatient psych unit
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
AT APA 2023
Adult tonsillectomies work and they’re cost effective
A new randomized trial offers rare insight into outcomes in adult tonsillectomy, a surgical procedure that’s commonly performed in the United States yet falling out of favor. Tonsillectomies are both clinically effective and cost-effective in adult patients with recurrent acute tonsillitis, a British team reports.
The researchers declined to weigh in on whether the procedure is actually better than nonsurgical management. Still, “here at last, we have a substantial piece of scientific evidence which shows that, compared with nonsurgical management, removal of tonsils has a significant impact on the number of sore throat days and on the cost of managing sore throat disease in adults,” said study lead author Janet A. Wilson, MBChB, MD, an emerita professor of otolaryngology at Newcastle University (England), in an interview.
The study was published in The Lancet.
Tonsillectomies have become much less common over the past several decades as questions have arisen about their value. In the United States, the number of procedures performed each year plunged from a high of 1.4 million in 1959 to an estimated 286,000 tonsillectomies performed in children under 15 and 120,000 in people aged 15 in 2010.
It’s harder for adults to tolerate tonsillectomies than children, Dr. Wilson said. In children, surgeons can easily remove tonsils by scraping them off the throat’s side walls. But, she said, “an adult tonsillectomy is more akin to taking off the skin of an unripe orange, so it’s harder work for the surgeon and more traumatizing for the wall of the adult patient’s pharynx. We can only assume that this greater amount of fibrous tissue reflects the cumulative effect of infections over a period of years.”
While tonsillectomies are still performed hundreds of times a day in adults in the United States, a 2014 Cochrane Library review found there’s “insufficient information “to support them versus nonsurgical care as treatments to reduce sore throats.”
For the new multicenter, open-label, randomized study, researchers randomly assigned patients aged 16 and older with recurrent acute tonsillitis to immediate tonsillectomy or nonsurgical management, which Dr. Wilson said can include cold fluids, honey, analgesics/anti-inflammatories. and anesthetic throat lozenges. The study was conducted between 2015 and 2018.
Ultimately, there were 224 and 204 patients, respectively, in the two groups (average age = 23, [19-30], 78% female, 90% White).
Patients who underwent tonsillectomies versus nonsurgical treatment had fewer sore throats over 2 years (median 23 days [IQR 11-46 days] vs. 30 days [14-65 days]) with an incident rate ratio of 0.53 (95% confidence interval, 0.43-0.65, P < 0.0001) after adjustment for clinic site and baseline severity.
The study also shows that “adults who have severe recurrent throat infections with a frequency of seven episodes within 1 year, five or more for 2 consecutive years, or three or more in 3 consecutive years will suffer fewer days of sore throat in the 2 years following tonsillectomy than if they had kept their tonsils,” Dr. Wilson said.
The study doesn’t examine longer-term consequences. A 2018 study of children linked tonsillectomies to “significantly increased relative risk of later respiratory, allergic, and infectious diseases.”
In the new study, nearly 4 in 10 (39%) of the tonsillectomy patients had adverse events linked to the surgeries, and bleeding (19%) was the most common adverse effect. The researchers also estimated that “tonsillectomy has a high probability of being considered cost-effective.”
“Whichever way the results were analyzed and confounding variables allowed for, the result always seems to be the same: Tonsillectomy applied using current qualifying criteria was a worthwhile procedure,” Dr. Wilson said.
Dr. Wilson noted that tonsillectomy patients will suffer a persistent sore throat after surgery, “about the same as a bad episode of tonsillitis.” And she said patients will need to adjust their diet for a few days and take 1-2 weeks off work.
In an interview, internal medicine physician Noel Deep, MD, of Antigo, Wisc., said antibiotics are a common treatment for tonsillitis in primary care clinics. According to him, the United States doesn’t have guidelines for tonsillectomies in adults. He believes they can be considered if tonsillitis keeps recurring three to five times a year and disrupts quality of life.
Dr. Deep said the new study “reinforces the benefit of tonsillectomies. Several studies from Germany, Sweden, Finland, and the United Kingdom have demonstrated benefits of tonsillectomies, but they were only for short periods of less than a year and lacked long-term data.”
He noted that “there is no clear evidence as to when to recommend tonsillectomies.” Clinicians should talk to patients about the potential that tonsillectomies will reduce sore throat episodes and cost the patient less in the long run, he said. It’s also important, he said, to make sure tonsillitis is bacterial before prescribing antibiotics.
The United Kingdom’s National Institute for Health Research funded the study. Dr. Wilson disclosed support for meetings/travel from ENT Scotland, and the other authors report various disclosures, including grants and contracts. Dr. Deep serves on the editorial advisory board of Internal Medicine News and is chair of the American Medical Association Council on Science and Public Health.
A new randomized trial offers rare insight into outcomes in adult tonsillectomy, a surgical procedure that’s commonly performed in the United States yet falling out of favor. Tonsillectomies are both clinically effective and cost-effective in adult patients with recurrent acute tonsillitis, a British team reports.
The researchers declined to weigh in on whether the procedure is actually better than nonsurgical management. Still, “here at last, we have a substantial piece of scientific evidence which shows that, compared with nonsurgical management, removal of tonsils has a significant impact on the number of sore throat days and on the cost of managing sore throat disease in adults,” said study lead author Janet A. Wilson, MBChB, MD, an emerita professor of otolaryngology at Newcastle University (England), in an interview.
The study was published in The Lancet.
Tonsillectomies have become much less common over the past several decades as questions have arisen about their value. In the United States, the number of procedures performed each year plunged from a high of 1.4 million in 1959 to an estimated 286,000 tonsillectomies performed in children under 15 and 120,000 in people aged 15 in 2010.
It’s harder for adults to tolerate tonsillectomies than children, Dr. Wilson said. In children, surgeons can easily remove tonsils by scraping them off the throat’s side walls. But, she said, “an adult tonsillectomy is more akin to taking off the skin of an unripe orange, so it’s harder work for the surgeon and more traumatizing for the wall of the adult patient’s pharynx. We can only assume that this greater amount of fibrous tissue reflects the cumulative effect of infections over a period of years.”
While tonsillectomies are still performed hundreds of times a day in adults in the United States, a 2014 Cochrane Library review found there’s “insufficient information “to support them versus nonsurgical care as treatments to reduce sore throats.”
For the new multicenter, open-label, randomized study, researchers randomly assigned patients aged 16 and older with recurrent acute tonsillitis to immediate tonsillectomy or nonsurgical management, which Dr. Wilson said can include cold fluids, honey, analgesics/anti-inflammatories. and anesthetic throat lozenges. The study was conducted between 2015 and 2018.
Ultimately, there were 224 and 204 patients, respectively, in the two groups (average age = 23, [19-30], 78% female, 90% White).
Patients who underwent tonsillectomies versus nonsurgical treatment had fewer sore throats over 2 years (median 23 days [IQR 11-46 days] vs. 30 days [14-65 days]) with an incident rate ratio of 0.53 (95% confidence interval, 0.43-0.65, P < 0.0001) after adjustment for clinic site and baseline severity.
The study also shows that “adults who have severe recurrent throat infections with a frequency of seven episodes within 1 year, five or more for 2 consecutive years, or three or more in 3 consecutive years will suffer fewer days of sore throat in the 2 years following tonsillectomy than if they had kept their tonsils,” Dr. Wilson said.
The study doesn’t examine longer-term consequences. A 2018 study of children linked tonsillectomies to “significantly increased relative risk of later respiratory, allergic, and infectious diseases.”
In the new study, nearly 4 in 10 (39%) of the tonsillectomy patients had adverse events linked to the surgeries, and bleeding (19%) was the most common adverse effect. The researchers also estimated that “tonsillectomy has a high probability of being considered cost-effective.”
“Whichever way the results were analyzed and confounding variables allowed for, the result always seems to be the same: Tonsillectomy applied using current qualifying criteria was a worthwhile procedure,” Dr. Wilson said.
Dr. Wilson noted that tonsillectomy patients will suffer a persistent sore throat after surgery, “about the same as a bad episode of tonsillitis.” And she said patients will need to adjust their diet for a few days and take 1-2 weeks off work.
In an interview, internal medicine physician Noel Deep, MD, of Antigo, Wisc., said antibiotics are a common treatment for tonsillitis in primary care clinics. According to him, the United States doesn’t have guidelines for tonsillectomies in adults. He believes they can be considered if tonsillitis keeps recurring three to five times a year and disrupts quality of life.
Dr. Deep said the new study “reinforces the benefit of tonsillectomies. Several studies from Germany, Sweden, Finland, and the United Kingdom have demonstrated benefits of tonsillectomies, but they were only for short periods of less than a year and lacked long-term data.”
He noted that “there is no clear evidence as to when to recommend tonsillectomies.” Clinicians should talk to patients about the potential that tonsillectomies will reduce sore throat episodes and cost the patient less in the long run, he said. It’s also important, he said, to make sure tonsillitis is bacterial before prescribing antibiotics.
The United Kingdom’s National Institute for Health Research funded the study. Dr. Wilson disclosed support for meetings/travel from ENT Scotland, and the other authors report various disclosures, including grants and contracts. Dr. Deep serves on the editorial advisory board of Internal Medicine News and is chair of the American Medical Association Council on Science and Public Health.
A new randomized trial offers rare insight into outcomes in adult tonsillectomy, a surgical procedure that’s commonly performed in the United States yet falling out of favor. Tonsillectomies are both clinically effective and cost-effective in adult patients with recurrent acute tonsillitis, a British team reports.
The researchers declined to weigh in on whether the procedure is actually better than nonsurgical management. Still, “here at last, we have a substantial piece of scientific evidence which shows that, compared with nonsurgical management, removal of tonsils has a significant impact on the number of sore throat days and on the cost of managing sore throat disease in adults,” said study lead author Janet A. Wilson, MBChB, MD, an emerita professor of otolaryngology at Newcastle University (England), in an interview.
The study was published in The Lancet.
Tonsillectomies have become much less common over the past several decades as questions have arisen about their value. In the United States, the number of procedures performed each year plunged from a high of 1.4 million in 1959 to an estimated 286,000 tonsillectomies performed in children under 15 and 120,000 in people aged 15 in 2010.
It’s harder for adults to tolerate tonsillectomies than children, Dr. Wilson said. In children, surgeons can easily remove tonsils by scraping them off the throat’s side walls. But, she said, “an adult tonsillectomy is more akin to taking off the skin of an unripe orange, so it’s harder work for the surgeon and more traumatizing for the wall of the adult patient’s pharynx. We can only assume that this greater amount of fibrous tissue reflects the cumulative effect of infections over a period of years.”
While tonsillectomies are still performed hundreds of times a day in adults in the United States, a 2014 Cochrane Library review found there’s “insufficient information “to support them versus nonsurgical care as treatments to reduce sore throats.”
For the new multicenter, open-label, randomized study, researchers randomly assigned patients aged 16 and older with recurrent acute tonsillitis to immediate tonsillectomy or nonsurgical management, which Dr. Wilson said can include cold fluids, honey, analgesics/anti-inflammatories. and anesthetic throat lozenges. The study was conducted between 2015 and 2018.
Ultimately, there were 224 and 204 patients, respectively, in the two groups (average age = 23, [19-30], 78% female, 90% White).
Patients who underwent tonsillectomies versus nonsurgical treatment had fewer sore throats over 2 years (median 23 days [IQR 11-46 days] vs. 30 days [14-65 days]) with an incident rate ratio of 0.53 (95% confidence interval, 0.43-0.65, P < 0.0001) after adjustment for clinic site and baseline severity.
The study also shows that “adults who have severe recurrent throat infections with a frequency of seven episodes within 1 year, five or more for 2 consecutive years, or three or more in 3 consecutive years will suffer fewer days of sore throat in the 2 years following tonsillectomy than if they had kept their tonsils,” Dr. Wilson said.
The study doesn’t examine longer-term consequences. A 2018 study of children linked tonsillectomies to “significantly increased relative risk of later respiratory, allergic, and infectious diseases.”
In the new study, nearly 4 in 10 (39%) of the tonsillectomy patients had adverse events linked to the surgeries, and bleeding (19%) was the most common adverse effect. The researchers also estimated that “tonsillectomy has a high probability of being considered cost-effective.”
“Whichever way the results were analyzed and confounding variables allowed for, the result always seems to be the same: Tonsillectomy applied using current qualifying criteria was a worthwhile procedure,” Dr. Wilson said.
Dr. Wilson noted that tonsillectomy patients will suffer a persistent sore throat after surgery, “about the same as a bad episode of tonsillitis.” And she said patients will need to adjust their diet for a few days and take 1-2 weeks off work.
In an interview, internal medicine physician Noel Deep, MD, of Antigo, Wisc., said antibiotics are a common treatment for tonsillitis in primary care clinics. According to him, the United States doesn’t have guidelines for tonsillectomies in adults. He believes they can be considered if tonsillitis keeps recurring three to five times a year and disrupts quality of life.
Dr. Deep said the new study “reinforces the benefit of tonsillectomies. Several studies from Germany, Sweden, Finland, and the United Kingdom have demonstrated benefits of tonsillectomies, but they were only for short periods of less than a year and lacked long-term data.”
He noted that “there is no clear evidence as to when to recommend tonsillectomies.” Clinicians should talk to patients about the potential that tonsillectomies will reduce sore throat episodes and cost the patient less in the long run, he said. It’s also important, he said, to make sure tonsillitis is bacterial before prescribing antibiotics.
The United Kingdom’s National Institute for Health Research funded the study. Dr. Wilson disclosed support for meetings/travel from ENT Scotland, and the other authors report various disclosures, including grants and contracts. Dr. Deep serves on the editorial advisory board of Internal Medicine News and is chair of the American Medical Association Council on Science and Public Health.
FROM THE LANCET
Breast cancer outcomes are worse for Black men
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
FROM ESMO BREAST CANCER 2023
Black patients most likely to be restrained in EDs, Latino patients least likely
SAN FRANCISCO – .
In contrast, Hispanic/Latino patients were less likely to be restrained than both Black and White patients, researchers reported in a poster presented at the annual meeting of the American Psychiatric Association. The study authors also found that clinicians rarely turned to restraints, using them in just 2,712 of 882,390 ED visits (0.3%) over a 7-year period.
The study doesn’t examine why the disparities exist. But lead author Erika Chang-Sing, a medical student at Yale University, New Haven, Conn., said in an interview that it’s clear that racial bias is the cause of the differences in restraint rates among White, Black, and Hispanics/Latino patients. “We think that there are multiple contributing factors to the higher rates of restraint for Black patients brought to the hospital by police, and all of them are rooted in systemic racism,” she said, adding that “the lower odds of restraint in the Hispanic or Latino group are also rooted in systemic racism and inequity.”
According to Ms. Chang-Sing, researchers launched the study to gain insight into the use of the restraints in the Southeast and to see what’s happening in light of the recent publicizing of killings of Black people by police. Being taken to the hospital by police “might contribute both to the individual patient’s behavior and the health care provider’s assessment of risk in determining whether or not to apply restraints,” she said.
Other research has linked ethnicity to higher rates of restraint use. For example, a 2021 study of 32,054 cases of patients under mandatory psychiatric hold in 11 Massachusetts emergency rooms found that Black (adjusted odds ratio, 1.22) and Hispanic (aOR, 1.45) patients were more likely to be restrained than White patients.
For the new study, researchers retrospectively tracked 885,102 emergency room visits at three North Carolina emergency departments from 2015 to 2022, including 9,130 who were brought in by police and 2,712 who were physically restrained because of the perceived risk of violence. “Providers use restraints, or straps, to secure the patient’s wrists and ankles to the bed,” Ms. Chang-Sing said.
Among all patients, 52.5% were Black, but 66% of those who were restrained were Black. The numbers for White patients were 35.7% and 23.9%, respectively, and 5.7% and 3.2% for Hispanics/Latino patients. Black patients were less likely than White patients to get a psychiatric primary emergency department diagnosis (aOR, 0.67), but those in that category were more likely than their White counterparts to be restrained (aOR, 1.36).
The higher risk of restraint use in Black patients overall disappeared when researchers adjusted their statistics to account for the effects of sex, age, and type of insurance (aOR, 0.86). Ms. Chang-Sing said the study team is reanalyzing the data since they think insurance may not be a confounder.
Why might Hispanic/Latino ethnicity be protective against restraint use? “This may be due to language barriers, fear of law enforcement, and avoidance of the hospital in the first place,” Ms. Chang-Sing said.
Emergency physician Wendy Macias-Konstantopoulos, MD, MPH, MBA, of Harvard Medical School and Massachusetts General Hospital, both in Boston, coauthored the 2021 study on police restraints. In an interview, she said the new findings add to previous research by providing data about the role played by the police who bring patients to the ED. She added that there is no evidence that certain populations simply need more restraints.
What can be done to reduce disparities in restraint use? Mental health teams can make a difference by responding to mental health emergencies, Ms. Chang-Sing said. “These providers can be instrumental in communicating to patients that the intention is to care for them, not to punish them.”
Another strategy is to increase the number of clinics and crisis response centers, she said. Hospital-based crisis response teams can also be helpful, she said. “Because these teams are focused only on behavioral emergencies, they can be more thoughtful in avoiding the use of restraints.”
No study funding was reported. The study authors and Dr. Macias-Konstantopoulos have no disclosures.
SAN FRANCISCO – .
In contrast, Hispanic/Latino patients were less likely to be restrained than both Black and White patients, researchers reported in a poster presented at the annual meeting of the American Psychiatric Association. The study authors also found that clinicians rarely turned to restraints, using them in just 2,712 of 882,390 ED visits (0.3%) over a 7-year period.
The study doesn’t examine why the disparities exist. But lead author Erika Chang-Sing, a medical student at Yale University, New Haven, Conn., said in an interview that it’s clear that racial bias is the cause of the differences in restraint rates among White, Black, and Hispanics/Latino patients. “We think that there are multiple contributing factors to the higher rates of restraint for Black patients brought to the hospital by police, and all of them are rooted in systemic racism,” she said, adding that “the lower odds of restraint in the Hispanic or Latino group are also rooted in systemic racism and inequity.”
According to Ms. Chang-Sing, researchers launched the study to gain insight into the use of the restraints in the Southeast and to see what’s happening in light of the recent publicizing of killings of Black people by police. Being taken to the hospital by police “might contribute both to the individual patient’s behavior and the health care provider’s assessment of risk in determining whether or not to apply restraints,” she said.
Other research has linked ethnicity to higher rates of restraint use. For example, a 2021 study of 32,054 cases of patients under mandatory psychiatric hold in 11 Massachusetts emergency rooms found that Black (adjusted odds ratio, 1.22) and Hispanic (aOR, 1.45) patients were more likely to be restrained than White patients.
For the new study, researchers retrospectively tracked 885,102 emergency room visits at three North Carolina emergency departments from 2015 to 2022, including 9,130 who were brought in by police and 2,712 who were physically restrained because of the perceived risk of violence. “Providers use restraints, or straps, to secure the patient’s wrists and ankles to the bed,” Ms. Chang-Sing said.
Among all patients, 52.5% were Black, but 66% of those who were restrained were Black. The numbers for White patients were 35.7% and 23.9%, respectively, and 5.7% and 3.2% for Hispanics/Latino patients. Black patients were less likely than White patients to get a psychiatric primary emergency department diagnosis (aOR, 0.67), but those in that category were more likely than their White counterparts to be restrained (aOR, 1.36).
The higher risk of restraint use in Black patients overall disappeared when researchers adjusted their statistics to account for the effects of sex, age, and type of insurance (aOR, 0.86). Ms. Chang-Sing said the study team is reanalyzing the data since they think insurance may not be a confounder.
Why might Hispanic/Latino ethnicity be protective against restraint use? “This may be due to language barriers, fear of law enforcement, and avoidance of the hospital in the first place,” Ms. Chang-Sing said.
Emergency physician Wendy Macias-Konstantopoulos, MD, MPH, MBA, of Harvard Medical School and Massachusetts General Hospital, both in Boston, coauthored the 2021 study on police restraints. In an interview, she said the new findings add to previous research by providing data about the role played by the police who bring patients to the ED. She added that there is no evidence that certain populations simply need more restraints.
What can be done to reduce disparities in restraint use? Mental health teams can make a difference by responding to mental health emergencies, Ms. Chang-Sing said. “These providers can be instrumental in communicating to patients that the intention is to care for them, not to punish them.”
Another strategy is to increase the number of clinics and crisis response centers, she said. Hospital-based crisis response teams can also be helpful, she said. “Because these teams are focused only on behavioral emergencies, they can be more thoughtful in avoiding the use of restraints.”
No study funding was reported. The study authors and Dr. Macias-Konstantopoulos have no disclosures.
SAN FRANCISCO – .
In contrast, Hispanic/Latino patients were less likely to be restrained than both Black and White patients, researchers reported in a poster presented at the annual meeting of the American Psychiatric Association. The study authors also found that clinicians rarely turned to restraints, using them in just 2,712 of 882,390 ED visits (0.3%) over a 7-year period.
The study doesn’t examine why the disparities exist. But lead author Erika Chang-Sing, a medical student at Yale University, New Haven, Conn., said in an interview that it’s clear that racial bias is the cause of the differences in restraint rates among White, Black, and Hispanics/Latino patients. “We think that there are multiple contributing factors to the higher rates of restraint for Black patients brought to the hospital by police, and all of them are rooted in systemic racism,” she said, adding that “the lower odds of restraint in the Hispanic or Latino group are also rooted in systemic racism and inequity.”
According to Ms. Chang-Sing, researchers launched the study to gain insight into the use of the restraints in the Southeast and to see what’s happening in light of the recent publicizing of killings of Black people by police. Being taken to the hospital by police “might contribute both to the individual patient’s behavior and the health care provider’s assessment of risk in determining whether or not to apply restraints,” she said.
Other research has linked ethnicity to higher rates of restraint use. For example, a 2021 study of 32,054 cases of patients under mandatory psychiatric hold in 11 Massachusetts emergency rooms found that Black (adjusted odds ratio, 1.22) and Hispanic (aOR, 1.45) patients were more likely to be restrained than White patients.
For the new study, researchers retrospectively tracked 885,102 emergency room visits at three North Carolina emergency departments from 2015 to 2022, including 9,130 who were brought in by police and 2,712 who were physically restrained because of the perceived risk of violence. “Providers use restraints, or straps, to secure the patient’s wrists and ankles to the bed,” Ms. Chang-Sing said.
Among all patients, 52.5% were Black, but 66% of those who were restrained were Black. The numbers for White patients were 35.7% and 23.9%, respectively, and 5.7% and 3.2% for Hispanics/Latino patients. Black patients were less likely than White patients to get a psychiatric primary emergency department diagnosis (aOR, 0.67), but those in that category were more likely than their White counterparts to be restrained (aOR, 1.36).
The higher risk of restraint use in Black patients overall disappeared when researchers adjusted their statistics to account for the effects of sex, age, and type of insurance (aOR, 0.86). Ms. Chang-Sing said the study team is reanalyzing the data since they think insurance may not be a confounder.
Why might Hispanic/Latino ethnicity be protective against restraint use? “This may be due to language barriers, fear of law enforcement, and avoidance of the hospital in the first place,” Ms. Chang-Sing said.
Emergency physician Wendy Macias-Konstantopoulos, MD, MPH, MBA, of Harvard Medical School and Massachusetts General Hospital, both in Boston, coauthored the 2021 study on police restraints. In an interview, she said the new findings add to previous research by providing data about the role played by the police who bring patients to the ED. She added that there is no evidence that certain populations simply need more restraints.
What can be done to reduce disparities in restraint use? Mental health teams can make a difference by responding to mental health emergencies, Ms. Chang-Sing said. “These providers can be instrumental in communicating to patients that the intention is to care for them, not to punish them.”
Another strategy is to increase the number of clinics and crisis response centers, she said. Hospital-based crisis response teams can also be helpful, she said. “Because these teams are focused only on behavioral emergencies, they can be more thoughtful in avoiding the use of restraints.”
No study funding was reported. The study authors and Dr. Macias-Konstantopoulos have no disclosures.
AT APA 2023
Venetoclax boosts ibrutinib in high-risk CLL
Of 45 patients, 57% reached U-MRD at 12 months, and 55% reached complete remission, according to the study, published in Leukemia.
By adding venetoclax, “you can get very deep remissions in high-risk patients with ibrutinib,” lead author Philip A. Thompson, MBBS, a hematologist-oncologist with the University of Melbourne and Peter MacCallum Cancer Center, also in Melbourne, said in an interview. “This is a significant advance for really high-risk patients.”
According to Dr. Thompson, Bruton’s tyrosine kinase inhibitors like ibrutinib have revolutionized the treatment of high-risk CLL by forcing the disease into remission for several years and allowing patients to avoid stem cell transplants. “But the drug doesn’t eradicate the disease,” he said, “so eventually these patients develop progression.”
The current hope, he said, is to use a combination therapy like ibrutinib and venetoclax to send CLL into remission with lower chance of drug resistance and then allow patients to stop taking the medications.
Previous research has supported the combination of ibrutinib and venetoclax in CLL in the frontline setting, and the European Commission approved it in 2022 for that use. But “ours is the first [study] that looked at patients who’ve been on ibrutinib for a long time and added venetoclax,” Dr. Thompson said. In some cases, he said, patients in the study had been on ibrutinib for several years.
For the new study, researchers at the University of Texas MD Anderson Cancer Center in Houston – where Dr. Thompson used to work – tracked 45 patients (average age, 68.5 years; 51-80 years) with CLL or small lymphocytic lymphoma who had MRD but no clinical disease progression. They all had at least 1 high-risk feature such as a mutated TP53. They’d been on ibrutinib for a median of 32 months (12-73 months), and two were in complete remission but with MRD.
An intention-to-treat analysis found that 71% reached U-MRD when they finished taking venetoclax. “We were actually pleasantly surprised by the high rate of undetectable MRD,” Dr. Thompson said.
At a median 41-month follow-up, 11% of patients had progressed, but none had died of CLL or Richter transformation, a deadly complication of CLL. “The main side effects were neutropenia and diarrhea, which we were manageable,” Dr. Thompson said.
It’s not clear why the drug combination is especially effective, he said, but it may be because the medications are synergistic. According to the National Cancer Institute, synergy in medicine refers to “the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
The findings suggest that “you can get deep remissions in high-risk patients with ibrutinib and venetoclax with very with good tolerability and very low risk of on-treatment progression,” Dr. Thompson said. “We don’t yet have enough progression events to talk about retreatment data, but we do feel that retreatment with Bruton’s tyrosine kinase inhibitors – plus or minus venetoclax – will be successful in the vast majority of patients.”
The combination can be given off label in the United States, Dr. Thompson added. As for expense, adding venetoclax will double the cost of ibrutinib. The two drugs are some of the most expensive medications in the world. But patients will save money if they can stop therapy when they reach remission.
In an interview, hematologist-oncologist Kerry A. Rogers, MD, of Ohio State University, Columbus, who is not involved with the study, praised the research: “While small, this study does say quite a bit about this as a strategy to help people discontinue ibrutinib prior to resistance developing.”
She noted that Bruton’s tyrosine kinase inhibitors “are generally given as a continuous monotherapy, and venetoclax is usually given for a fixed duration in combination with an anti-CD20 antibody.”
Going forward, she said, “the fact that the study was in high-risk patients who have the most to gain from such a combination suggests that similar or better rates of undetectable minimal residual disease might be seen in non–high-risk groups. Additional follow-up should be reported as well as use of this strategy in a larger group of patients before this should be considered a standard approach.”
AbbVie funding the study and provided study drugs. MD Anderson Cancer Center conducted the study and discloses funding from the National Cancer Institute. Dr. Thompson reported ties with AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, BeiGene, and Genentech. The other study authors reported multiple disclosures. Dr. Rogers disclosed relationships with Genentech, AbbVie, Novartis, Janssen, Pharmacyclics, BeiGene, Lilly, and AstraZeneca.
Of 45 patients, 57% reached U-MRD at 12 months, and 55% reached complete remission, according to the study, published in Leukemia.
By adding venetoclax, “you can get very deep remissions in high-risk patients with ibrutinib,” lead author Philip A. Thompson, MBBS, a hematologist-oncologist with the University of Melbourne and Peter MacCallum Cancer Center, also in Melbourne, said in an interview. “This is a significant advance for really high-risk patients.”
According to Dr. Thompson, Bruton’s tyrosine kinase inhibitors like ibrutinib have revolutionized the treatment of high-risk CLL by forcing the disease into remission for several years and allowing patients to avoid stem cell transplants. “But the drug doesn’t eradicate the disease,” he said, “so eventually these patients develop progression.”
The current hope, he said, is to use a combination therapy like ibrutinib and venetoclax to send CLL into remission with lower chance of drug resistance and then allow patients to stop taking the medications.
Previous research has supported the combination of ibrutinib and venetoclax in CLL in the frontline setting, and the European Commission approved it in 2022 for that use. But “ours is the first [study] that looked at patients who’ve been on ibrutinib for a long time and added venetoclax,” Dr. Thompson said. In some cases, he said, patients in the study had been on ibrutinib for several years.
For the new study, researchers at the University of Texas MD Anderson Cancer Center in Houston – where Dr. Thompson used to work – tracked 45 patients (average age, 68.5 years; 51-80 years) with CLL or small lymphocytic lymphoma who had MRD but no clinical disease progression. They all had at least 1 high-risk feature such as a mutated TP53. They’d been on ibrutinib for a median of 32 months (12-73 months), and two were in complete remission but with MRD.
An intention-to-treat analysis found that 71% reached U-MRD when they finished taking venetoclax. “We were actually pleasantly surprised by the high rate of undetectable MRD,” Dr. Thompson said.
At a median 41-month follow-up, 11% of patients had progressed, but none had died of CLL or Richter transformation, a deadly complication of CLL. “The main side effects were neutropenia and diarrhea, which we were manageable,” Dr. Thompson said.
It’s not clear why the drug combination is especially effective, he said, but it may be because the medications are synergistic. According to the National Cancer Institute, synergy in medicine refers to “the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
The findings suggest that “you can get deep remissions in high-risk patients with ibrutinib and venetoclax with very with good tolerability and very low risk of on-treatment progression,” Dr. Thompson said. “We don’t yet have enough progression events to talk about retreatment data, but we do feel that retreatment with Bruton’s tyrosine kinase inhibitors – plus or minus venetoclax – will be successful in the vast majority of patients.”
The combination can be given off label in the United States, Dr. Thompson added. As for expense, adding venetoclax will double the cost of ibrutinib. The two drugs are some of the most expensive medications in the world. But patients will save money if they can stop therapy when they reach remission.
In an interview, hematologist-oncologist Kerry A. Rogers, MD, of Ohio State University, Columbus, who is not involved with the study, praised the research: “While small, this study does say quite a bit about this as a strategy to help people discontinue ibrutinib prior to resistance developing.”
She noted that Bruton’s tyrosine kinase inhibitors “are generally given as a continuous monotherapy, and venetoclax is usually given for a fixed duration in combination with an anti-CD20 antibody.”
Going forward, she said, “the fact that the study was in high-risk patients who have the most to gain from such a combination suggests that similar or better rates of undetectable minimal residual disease might be seen in non–high-risk groups. Additional follow-up should be reported as well as use of this strategy in a larger group of patients before this should be considered a standard approach.”
AbbVie funding the study and provided study drugs. MD Anderson Cancer Center conducted the study and discloses funding from the National Cancer Institute. Dr. Thompson reported ties with AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, BeiGene, and Genentech. The other study authors reported multiple disclosures. Dr. Rogers disclosed relationships with Genentech, AbbVie, Novartis, Janssen, Pharmacyclics, BeiGene, Lilly, and AstraZeneca.
Of 45 patients, 57% reached U-MRD at 12 months, and 55% reached complete remission, according to the study, published in Leukemia.
By adding venetoclax, “you can get very deep remissions in high-risk patients with ibrutinib,” lead author Philip A. Thompson, MBBS, a hematologist-oncologist with the University of Melbourne and Peter MacCallum Cancer Center, also in Melbourne, said in an interview. “This is a significant advance for really high-risk patients.”
According to Dr. Thompson, Bruton’s tyrosine kinase inhibitors like ibrutinib have revolutionized the treatment of high-risk CLL by forcing the disease into remission for several years and allowing patients to avoid stem cell transplants. “But the drug doesn’t eradicate the disease,” he said, “so eventually these patients develop progression.”
The current hope, he said, is to use a combination therapy like ibrutinib and venetoclax to send CLL into remission with lower chance of drug resistance and then allow patients to stop taking the medications.
Previous research has supported the combination of ibrutinib and venetoclax in CLL in the frontline setting, and the European Commission approved it in 2022 for that use. But “ours is the first [study] that looked at patients who’ve been on ibrutinib for a long time and added venetoclax,” Dr. Thompson said. In some cases, he said, patients in the study had been on ibrutinib for several years.
For the new study, researchers at the University of Texas MD Anderson Cancer Center in Houston – where Dr. Thompson used to work – tracked 45 patients (average age, 68.5 years; 51-80 years) with CLL or small lymphocytic lymphoma who had MRD but no clinical disease progression. They all had at least 1 high-risk feature such as a mutated TP53. They’d been on ibrutinib for a median of 32 months (12-73 months), and two were in complete remission but with MRD.
An intention-to-treat analysis found that 71% reached U-MRD when they finished taking venetoclax. “We were actually pleasantly surprised by the high rate of undetectable MRD,” Dr. Thompson said.
At a median 41-month follow-up, 11% of patients had progressed, but none had died of CLL or Richter transformation, a deadly complication of CLL. “The main side effects were neutropenia and diarrhea, which we were manageable,” Dr. Thompson said.
It’s not clear why the drug combination is especially effective, he said, but it may be because the medications are synergistic. According to the National Cancer Institute, synergy in medicine refers to “the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
The findings suggest that “you can get deep remissions in high-risk patients with ibrutinib and venetoclax with very with good tolerability and very low risk of on-treatment progression,” Dr. Thompson said. “We don’t yet have enough progression events to talk about retreatment data, but we do feel that retreatment with Bruton’s tyrosine kinase inhibitors – plus or minus venetoclax – will be successful in the vast majority of patients.”
The combination can be given off label in the United States, Dr. Thompson added. As for expense, adding venetoclax will double the cost of ibrutinib. The two drugs are some of the most expensive medications in the world. But patients will save money if they can stop therapy when they reach remission.
In an interview, hematologist-oncologist Kerry A. Rogers, MD, of Ohio State University, Columbus, who is not involved with the study, praised the research: “While small, this study does say quite a bit about this as a strategy to help people discontinue ibrutinib prior to resistance developing.”
She noted that Bruton’s tyrosine kinase inhibitors “are generally given as a continuous monotherapy, and venetoclax is usually given for a fixed duration in combination with an anti-CD20 antibody.”
Going forward, she said, “the fact that the study was in high-risk patients who have the most to gain from such a combination suggests that similar or better rates of undetectable minimal residual disease might be seen in non–high-risk groups. Additional follow-up should be reported as well as use of this strategy in a larger group of patients before this should be considered a standard approach.”
AbbVie funding the study and provided study drugs. MD Anderson Cancer Center conducted the study and discloses funding from the National Cancer Institute. Dr. Thompson reported ties with AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, BeiGene, and Genentech. The other study authors reported multiple disclosures. Dr. Rogers disclosed relationships with Genentech, AbbVie, Novartis, Janssen, Pharmacyclics, BeiGene, Lilly, and AstraZeneca.
FROM LEUKEMIA
PARP inhibitors and breast cancer: Questions remain about wider use
oncologists explained at the European Society for Medical Oncology Breast Cancer annual congress.
For now, the drugs are only approved in high-risk germline BRCA mutation (gBRCAmut) early breast cancer, oncologist Kevin Punie, MD, of Saint Augustine Hospital in Wilrijk, Belgium, said during a session at the meeting. Combining the drugs with chemotherapy “has not yet demonstrated significant benefits, and this is irrespective whether platinum was part of the chemotherapy backbone.”
PARP is a kind of enzyme that repairs damaged DNA in cells, especially cancerous ones. PARP inhibitors block the enzyme, potentially leading more cancer cells to die, the Dana-Farber Cancer Institute states.
In a separate presentation during the same session, oncologist Andrew Tutt, MBChB, PhD, noted that a study he led – a phase 3, double-blinded, randomized 2021 trial – found that patients with BRCA1- or BRCA2-mutated breast cancer who took the PARP inhibitor olarapib (Lynparza) versus placebo had improved outcomes on several measures, including 3-year invasive disease-free survival (85.9% vs. 77.1%, P < .001). However, the study noted that “olaparib had limited effects on global patient-reported quality of life.”
Dr. Tutt, of the Institute of Cancer Research, London, and Kings College London, said 57% of patients who took olarapib suffered nausea versus 24% of those who took placebo, and fatigue and anemia were also more common in the olarapib group. Anemia can be severe and lead to transfusions in some cases.
As Dr. Punie explained, there are many reasons to consider combining PARP inhibitors with other treatments such as chemotherapy, immunotherapy, and radiotherapy. The combinations may have synergetic effects, and they could have potential in both the neoadjuvant and adjuvant settings.
The combination of the PARP inhibitor olaparib and endocrine therapy is now approved by the European Medicines Agency for the adjuvant treatment of certain patients with germline BRCA1/2 mutations who have HER2-negative, high-risk early breast cancer, Dr. Punie noted.
The 2021 study led by Dr. Tutt reported that treatment or safety differences were found in those who received both olaparib and endocrine therapy versus those who only received olarapib.
So far, Dr. Punie said, “we not yet have enough clinical evidence to say that there’s really synergy between PNP inhibitors and other anticancer therapies.” According to the National Institutes of Health, medical synergy “describes the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
In regard to chemotherapy, it makes sense that PARP inhibitors would be helpful in combination, Dr. Punie said. DNA damage to cancer cells accumulates during chemotherapy, he said, and they’re more depending on PARP for repair.
Study results so far have been mixed. A 2022 study, for example, found that adding the experimental PARP inhibitor veliparib to the chemotherapy regimen carboplatin-paclitaxel didn’t improve outcomes, he said. A similar study examining the addition of olaparib to carboplatin-paclitaxel is ongoing.
As for combining radiotherapy and PARP inhibitors, Dr. Punie said that preclinical findings are promising, and research is underway. There’s also ongoing research into combining PARP inhibitors with immunotherapy.
Off-label use of olaparib with immunotherapy or sequential treatment may be appropriate in the setting of adjuvant gBRCAmut triple-negative breast cancer with residual disease, he said.
During his presentation, Dr. Tutt called for researchers to investigate the use of PARP inhibitors in the de-escalation of treatment in lower-risk gBRCAmut disease.
“Clearly, some patients require chemotherapy, and we know patients respond very well to neoadjuvant chemotherapy if they have a BRCA mutation, but we don’t yet know who we can de-escalate in,” he said.
He also highlighted the need to reduce anemia in patients on PARP inhibitors, “particularly if we’re moving into lower-risk populations or possibly considering prevention trials.
“The study of PARP inhibitor resistance ... is now urgent, so that we can address it,” he said.
Dr. Punie disclosed financial relationships with AstraZeneca, Eli Lilly, Exact Sciences, Focus Patient, Medscape, MSD, Mundi Pharma, Need, Novartis, Pierre Fabre, Pfizer, F. Hoffmann–La Roche, Sanofi, Seagen, and PharmaMar. Dr. Tutt disclosed financial relationships with Artios, Gilead, MD Anderson, Merck KGaA, Pfizer, Vertex, AstraZeneca, EM Partners, Medscape Education, CRUK, Inbiomotion, Myriad Genetics, and Breast Cancer Now.
oncologists explained at the European Society for Medical Oncology Breast Cancer annual congress.
For now, the drugs are only approved in high-risk germline BRCA mutation (gBRCAmut) early breast cancer, oncologist Kevin Punie, MD, of Saint Augustine Hospital in Wilrijk, Belgium, said during a session at the meeting. Combining the drugs with chemotherapy “has not yet demonstrated significant benefits, and this is irrespective whether platinum was part of the chemotherapy backbone.”
PARP is a kind of enzyme that repairs damaged DNA in cells, especially cancerous ones. PARP inhibitors block the enzyme, potentially leading more cancer cells to die, the Dana-Farber Cancer Institute states.
In a separate presentation during the same session, oncologist Andrew Tutt, MBChB, PhD, noted that a study he led – a phase 3, double-blinded, randomized 2021 trial – found that patients with BRCA1- or BRCA2-mutated breast cancer who took the PARP inhibitor olarapib (Lynparza) versus placebo had improved outcomes on several measures, including 3-year invasive disease-free survival (85.9% vs. 77.1%, P < .001). However, the study noted that “olaparib had limited effects on global patient-reported quality of life.”
Dr. Tutt, of the Institute of Cancer Research, London, and Kings College London, said 57% of patients who took olarapib suffered nausea versus 24% of those who took placebo, and fatigue and anemia were also more common in the olarapib group. Anemia can be severe and lead to transfusions in some cases.
As Dr. Punie explained, there are many reasons to consider combining PARP inhibitors with other treatments such as chemotherapy, immunotherapy, and radiotherapy. The combinations may have synergetic effects, and they could have potential in both the neoadjuvant and adjuvant settings.
The combination of the PARP inhibitor olaparib and endocrine therapy is now approved by the European Medicines Agency for the adjuvant treatment of certain patients with germline BRCA1/2 mutations who have HER2-negative, high-risk early breast cancer, Dr. Punie noted.
The 2021 study led by Dr. Tutt reported that treatment or safety differences were found in those who received both olaparib and endocrine therapy versus those who only received olarapib.
So far, Dr. Punie said, “we not yet have enough clinical evidence to say that there’s really synergy between PNP inhibitors and other anticancer therapies.” According to the National Institutes of Health, medical synergy “describes the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
In regard to chemotherapy, it makes sense that PARP inhibitors would be helpful in combination, Dr. Punie said. DNA damage to cancer cells accumulates during chemotherapy, he said, and they’re more depending on PARP for repair.
Study results so far have been mixed. A 2022 study, for example, found that adding the experimental PARP inhibitor veliparib to the chemotherapy regimen carboplatin-paclitaxel didn’t improve outcomes, he said. A similar study examining the addition of olaparib to carboplatin-paclitaxel is ongoing.
As for combining radiotherapy and PARP inhibitors, Dr. Punie said that preclinical findings are promising, and research is underway. There’s also ongoing research into combining PARP inhibitors with immunotherapy.
Off-label use of olaparib with immunotherapy or sequential treatment may be appropriate in the setting of adjuvant gBRCAmut triple-negative breast cancer with residual disease, he said.
During his presentation, Dr. Tutt called for researchers to investigate the use of PARP inhibitors in the de-escalation of treatment in lower-risk gBRCAmut disease.
“Clearly, some patients require chemotherapy, and we know patients respond very well to neoadjuvant chemotherapy if they have a BRCA mutation, but we don’t yet know who we can de-escalate in,” he said.
He also highlighted the need to reduce anemia in patients on PARP inhibitors, “particularly if we’re moving into lower-risk populations or possibly considering prevention trials.
“The study of PARP inhibitor resistance ... is now urgent, so that we can address it,” he said.
Dr. Punie disclosed financial relationships with AstraZeneca, Eli Lilly, Exact Sciences, Focus Patient, Medscape, MSD, Mundi Pharma, Need, Novartis, Pierre Fabre, Pfizer, F. Hoffmann–La Roche, Sanofi, Seagen, and PharmaMar. Dr. Tutt disclosed financial relationships with Artios, Gilead, MD Anderson, Merck KGaA, Pfizer, Vertex, AstraZeneca, EM Partners, Medscape Education, CRUK, Inbiomotion, Myriad Genetics, and Breast Cancer Now.
oncologists explained at the European Society for Medical Oncology Breast Cancer annual congress.
For now, the drugs are only approved in high-risk germline BRCA mutation (gBRCAmut) early breast cancer, oncologist Kevin Punie, MD, of Saint Augustine Hospital in Wilrijk, Belgium, said during a session at the meeting. Combining the drugs with chemotherapy “has not yet demonstrated significant benefits, and this is irrespective whether platinum was part of the chemotherapy backbone.”
PARP is a kind of enzyme that repairs damaged DNA in cells, especially cancerous ones. PARP inhibitors block the enzyme, potentially leading more cancer cells to die, the Dana-Farber Cancer Institute states.
In a separate presentation during the same session, oncologist Andrew Tutt, MBChB, PhD, noted that a study he led – a phase 3, double-blinded, randomized 2021 trial – found that patients with BRCA1- or BRCA2-mutated breast cancer who took the PARP inhibitor olarapib (Lynparza) versus placebo had improved outcomes on several measures, including 3-year invasive disease-free survival (85.9% vs. 77.1%, P < .001). However, the study noted that “olaparib had limited effects on global patient-reported quality of life.”
Dr. Tutt, of the Institute of Cancer Research, London, and Kings College London, said 57% of patients who took olarapib suffered nausea versus 24% of those who took placebo, and fatigue and anemia were also more common in the olarapib group. Anemia can be severe and lead to transfusions in some cases.
As Dr. Punie explained, there are many reasons to consider combining PARP inhibitors with other treatments such as chemotherapy, immunotherapy, and radiotherapy. The combinations may have synergetic effects, and they could have potential in both the neoadjuvant and adjuvant settings.
The combination of the PARP inhibitor olaparib and endocrine therapy is now approved by the European Medicines Agency for the adjuvant treatment of certain patients with germline BRCA1/2 mutations who have HER2-negative, high-risk early breast cancer, Dr. Punie noted.
The 2021 study led by Dr. Tutt reported that treatment or safety differences were found in those who received both olaparib and endocrine therapy versus those who only received olarapib.
So far, Dr. Punie said, “we not yet have enough clinical evidence to say that there’s really synergy between PNP inhibitors and other anticancer therapies.” According to the National Institutes of Health, medical synergy “describes the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
In regard to chemotherapy, it makes sense that PARP inhibitors would be helpful in combination, Dr. Punie said. DNA damage to cancer cells accumulates during chemotherapy, he said, and they’re more depending on PARP for repair.
Study results so far have been mixed. A 2022 study, for example, found that adding the experimental PARP inhibitor veliparib to the chemotherapy regimen carboplatin-paclitaxel didn’t improve outcomes, he said. A similar study examining the addition of olaparib to carboplatin-paclitaxel is ongoing.
As for combining radiotherapy and PARP inhibitors, Dr. Punie said that preclinical findings are promising, and research is underway. There’s also ongoing research into combining PARP inhibitors with immunotherapy.
Off-label use of olaparib with immunotherapy or sequential treatment may be appropriate in the setting of adjuvant gBRCAmut triple-negative breast cancer with residual disease, he said.
During his presentation, Dr. Tutt called for researchers to investigate the use of PARP inhibitors in the de-escalation of treatment in lower-risk gBRCAmut disease.
“Clearly, some patients require chemotherapy, and we know patients respond very well to neoadjuvant chemotherapy if they have a BRCA mutation, but we don’t yet know who we can de-escalate in,” he said.
He also highlighted the need to reduce anemia in patients on PARP inhibitors, “particularly if we’re moving into lower-risk populations or possibly considering prevention trials.
“The study of PARP inhibitor resistance ... is now urgent, so that we can address it,” he said.
Dr. Punie disclosed financial relationships with AstraZeneca, Eli Lilly, Exact Sciences, Focus Patient, Medscape, MSD, Mundi Pharma, Need, Novartis, Pierre Fabre, Pfizer, F. Hoffmann–La Roche, Sanofi, Seagen, and PharmaMar. Dr. Tutt disclosed financial relationships with Artios, Gilead, MD Anderson, Merck KGaA, Pfizer, Vertex, AstraZeneca, EM Partners, Medscape Education, CRUK, Inbiomotion, Myriad Genetics, and Breast Cancer Now.
FROM ESMO BREAST CANCER 2023