User login
2016 Update on infectious disease
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
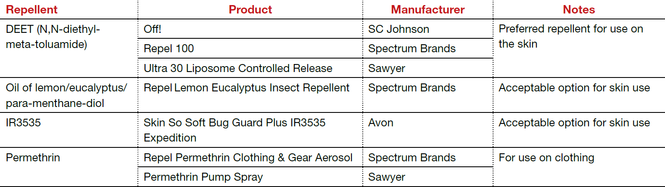
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.
Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
In this article
• Azithromycin vs doxycycline for chlamydia
• Insect repellents to prevent Zika virus
What insect repellents are safe during pregnancy?
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Zika virus: Counseling considerations for this emerging perinatal threat
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||
 |  | |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |
 |
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||
 |  | |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |
 |
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||
 |  | |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |
 |
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
In this Article
- Management strategies for pregnant patients with Zika virus exposure
- Fetal surveillance
- Perinatal counseling on exposure prevention
- Algorithm for evaluation and management
Which is the more effective treatment for uncomplicated skin infections—clindamycin or trimethoprim-sulfamethoxazole?
Miller and colleagues conducted their study in adults as well as children. Patients were included if they had either a discrete skin abscess or cellulitis, or both. They were excluded if they had one of the following:
- impetigo
- perirectal, genital, or hand infection
- a human or animal bite at the site of infection
- temperature of 38.5° C or higher
- immunocompromise
- morbid obesity
- prosthetic device at the site of infection.
The remaining patients then were stratified into one of 2 groups:
- those with a larger abscess (>5 cm in maximum diameter in adults, proportionally smaller in children) and/or cellulitis
- those who had a smaller abscess.
This study by Miller and colleagues focuses only on the patients in the former group.
Details of the trial
All discrete abscesses were incised and drained, and patients were randomly assigned to either:
- clindamycin, 300 mg 3 times daily for 10 days
- trimethoprim-sulfamethoxazole, 2 single-strength tablets orally twice daily for 10 days.
The primary endpoint was clinical cure at 7 to 10 days after completion of antibiotic therapy.
The study enrolled 524 patients—264 in the clindamycin group and 260 in the trimethoprim-sulfamethoxazole group. Approximately 30% of the patients were children. One hundred sixty patients (30.5%) had a discrete abscess, 280 (53.4%) had cellulitis, and 82 (15.6%) had both an abscess and cellulitis. An incision and drainage procedure was performed in 44.5% of patients. Slightly more than 50% of patients had a microbiological culture.
The most common organism isolated was S aureus (217 of 524 patients, or 41.4%), of which 167 (77%) were methicillin-resistant S aureus (MRSA). Of the 217 isolates identified as S aureus, 27 (12.4%) were resistant to clindamycin, and only one (0.5%) was resistant to trimethoprim-sulfamethoxazole.
Of the 466 patients who were fully evaluable, the rate of cure was 89.5% in the clindamycin group (95% confidence interval [CI], 85.2–93.7) and 88.2% in the trimethoprim-sulfamethoxazole group (95% CI, 83.7–92.7). The difference in the observed rate of clinical cure was not statistically significant.
Eleven of 15 patients in the clindamycin group who had clindamycin-resistant isolates were cured, compared with 77 of 84 patients with susceptible isolates (73.3% vs 91.7%; P = .06). At 1 month after treatment, cure rates remained similar. The overall rates of adverse effects in the 2 groups were similar, at 19%. No patient developed Clostridium difficile-associated diarrhea.
Skin infections can be life-threatening
Skin and skin-structure infections are common—and can influence the decision of when to perform a cesarean delivery, how to prepare the skin before surgery, and where to place the surgical incision. In some patients, these infections can be quite debilitating, even life-threatening. When a discrete abscess (furuncle, carbuncle) is present, the most likely organism is S aureus, and the majority of strains are MRSA. When cellulitis is present, S aureus is less likely, and the dominant organisms are usually streptococci, particularly Streptococcus pyogenes.
Abscesses of any size need incision and drainage and, in most cases, systemic antibiotic therapy. When cellulitis without a discrete abscess is present, the key to treatment is antibiotic therapy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For uncomplicated skin and skin-structure infections in immunocompetent women, oral clindamycin and oral trimethoprim-sulfamethoxazole are equally effective; both achieve cures in approximately 90% of patients.
Given that more strains of S aureus were resistant to clindamycin, trimethoprim-sulfamethoxazole may be the preferred agent. It also is less expensive and, in theory at least, less likely to cause drug-induced diarrhea.
Affected patients need to be followed closely because recurrences are common and, in isolated instances, serious complications such as sepsis can develop.
—Patrick Duff, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Miller and colleagues conducted their study in adults as well as children. Patients were included if they had either a discrete skin abscess or cellulitis, or both. They were excluded if they had one of the following:
- impetigo
- perirectal, genital, or hand infection
- a human or animal bite at the site of infection
- temperature of 38.5° C or higher
- immunocompromise
- morbid obesity
- prosthetic device at the site of infection.
The remaining patients then were stratified into one of 2 groups:
- those with a larger abscess (>5 cm in maximum diameter in adults, proportionally smaller in children) and/or cellulitis
- those who had a smaller abscess.
This study by Miller and colleagues focuses only on the patients in the former group.
Details of the trial
All discrete abscesses were incised and drained, and patients were randomly assigned to either:
- clindamycin, 300 mg 3 times daily for 10 days
- trimethoprim-sulfamethoxazole, 2 single-strength tablets orally twice daily for 10 days.
The primary endpoint was clinical cure at 7 to 10 days after completion of antibiotic therapy.
The study enrolled 524 patients—264 in the clindamycin group and 260 in the trimethoprim-sulfamethoxazole group. Approximately 30% of the patients were children. One hundred sixty patients (30.5%) had a discrete abscess, 280 (53.4%) had cellulitis, and 82 (15.6%) had both an abscess and cellulitis. An incision and drainage procedure was performed in 44.5% of patients. Slightly more than 50% of patients had a microbiological culture.
The most common organism isolated was S aureus (217 of 524 patients, or 41.4%), of which 167 (77%) were methicillin-resistant S aureus (MRSA). Of the 217 isolates identified as S aureus, 27 (12.4%) were resistant to clindamycin, and only one (0.5%) was resistant to trimethoprim-sulfamethoxazole.
Of the 466 patients who were fully evaluable, the rate of cure was 89.5% in the clindamycin group (95% confidence interval [CI], 85.2–93.7) and 88.2% in the trimethoprim-sulfamethoxazole group (95% CI, 83.7–92.7). The difference in the observed rate of clinical cure was not statistically significant.
Eleven of 15 patients in the clindamycin group who had clindamycin-resistant isolates were cured, compared with 77 of 84 patients with susceptible isolates (73.3% vs 91.7%; P = .06). At 1 month after treatment, cure rates remained similar. The overall rates of adverse effects in the 2 groups were similar, at 19%. No patient developed Clostridium difficile-associated diarrhea.
Skin infections can be life-threatening
Skin and skin-structure infections are common—and can influence the decision of when to perform a cesarean delivery, how to prepare the skin before surgery, and where to place the surgical incision. In some patients, these infections can be quite debilitating, even life-threatening. When a discrete abscess (furuncle, carbuncle) is present, the most likely organism is S aureus, and the majority of strains are MRSA. When cellulitis is present, S aureus is less likely, and the dominant organisms are usually streptococci, particularly Streptococcus pyogenes.
Abscesses of any size need incision and drainage and, in most cases, systemic antibiotic therapy. When cellulitis without a discrete abscess is present, the key to treatment is antibiotic therapy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For uncomplicated skin and skin-structure infections in immunocompetent women, oral clindamycin and oral trimethoprim-sulfamethoxazole are equally effective; both achieve cures in approximately 90% of patients.
Given that more strains of S aureus were resistant to clindamycin, trimethoprim-sulfamethoxazole may be the preferred agent. It also is less expensive and, in theory at least, less likely to cause drug-induced diarrhea.
Affected patients need to be followed closely because recurrences are common and, in isolated instances, serious complications such as sepsis can develop.
—Patrick Duff, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Miller and colleagues conducted their study in adults as well as children. Patients were included if they had either a discrete skin abscess or cellulitis, or both. They were excluded if they had one of the following:
- impetigo
- perirectal, genital, or hand infection
- a human or animal bite at the site of infection
- temperature of 38.5° C or higher
- immunocompromise
- morbid obesity
- prosthetic device at the site of infection.
The remaining patients then were stratified into one of 2 groups:
- those with a larger abscess (>5 cm in maximum diameter in adults, proportionally smaller in children) and/or cellulitis
- those who had a smaller abscess.
This study by Miller and colleagues focuses only on the patients in the former group.
Details of the trial
All discrete abscesses were incised and drained, and patients were randomly assigned to either:
- clindamycin, 300 mg 3 times daily for 10 days
- trimethoprim-sulfamethoxazole, 2 single-strength tablets orally twice daily for 10 days.
The primary endpoint was clinical cure at 7 to 10 days after completion of antibiotic therapy.
The study enrolled 524 patients—264 in the clindamycin group and 260 in the trimethoprim-sulfamethoxazole group. Approximately 30% of the patients were children. One hundred sixty patients (30.5%) had a discrete abscess, 280 (53.4%) had cellulitis, and 82 (15.6%) had both an abscess and cellulitis. An incision and drainage procedure was performed in 44.5% of patients. Slightly more than 50% of patients had a microbiological culture.
The most common organism isolated was S aureus (217 of 524 patients, or 41.4%), of which 167 (77%) were methicillin-resistant S aureus (MRSA). Of the 217 isolates identified as S aureus, 27 (12.4%) were resistant to clindamycin, and only one (0.5%) was resistant to trimethoprim-sulfamethoxazole.
Of the 466 patients who were fully evaluable, the rate of cure was 89.5% in the clindamycin group (95% confidence interval [CI], 85.2–93.7) and 88.2% in the trimethoprim-sulfamethoxazole group (95% CI, 83.7–92.7). The difference in the observed rate of clinical cure was not statistically significant.
Eleven of 15 patients in the clindamycin group who had clindamycin-resistant isolates were cured, compared with 77 of 84 patients with susceptible isolates (73.3% vs 91.7%; P = .06). At 1 month after treatment, cure rates remained similar. The overall rates of adverse effects in the 2 groups were similar, at 19%. No patient developed Clostridium difficile-associated diarrhea.
Skin infections can be life-threatening
Skin and skin-structure infections are common—and can influence the decision of when to perform a cesarean delivery, how to prepare the skin before surgery, and where to place the surgical incision. In some patients, these infections can be quite debilitating, even life-threatening. When a discrete abscess (furuncle, carbuncle) is present, the most likely organism is S aureus, and the majority of strains are MRSA. When cellulitis is present, S aureus is less likely, and the dominant organisms are usually streptococci, particularly Streptococcus pyogenes.
Abscesses of any size need incision and drainage and, in most cases, systemic antibiotic therapy. When cellulitis without a discrete abscess is present, the key to treatment is antibiotic therapy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For uncomplicated skin and skin-structure infections in immunocompetent women, oral clindamycin and oral trimethoprim-sulfamethoxazole are equally effective; both achieve cures in approximately 90% of patients.
Given that more strains of S aureus were resistant to clindamycin, trimethoprim-sulfamethoxazole may be the preferred agent. It also is less expensive and, in theory at least, less likely to cause drug-induced diarrhea.
Affected patients need to be followed closely because recurrences are common and, in isolated instances, serious complications such as sepsis can develop.
—Patrick Duff, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Does a high-dose influenza vaccine protect older adults to a greater extent than the standard-dose vaccine?
The objective of this investigation was to compare a standard-dose trivalent influenza vaccine with a high-dose vaccine in adults older than 65 years. The standard dose of the vaccine contained 15 µg of hemagglutinin per strain, and the high dose contained 60 µg hemagglutinin per strain. The study was conducted during the 2011–2012 and 2012–2013 flu seasons. Key outcome measures were efficacy, as assessed by the occurrence of laboratory-confirmed influenza at least 14 days after vaccination; immunogenicity of the vaccines; and frequency of adverse events.
Details of the study
The study involved 15,991 patients in the high-dose group and 15,998 patients in the standard-dose group. Two hundred twenty-eight participants (1.4%) in the high-dose group developed influenza, compared with 301 participants (1.9%) in the standard-dose group.
The overall efficacy of the high-dose vaccine was 24.2% (95% confidence interval [CI], 9.7–36.5), meaning that approximately 24% of influenza cases could have been prevented if the high-dose vaccine had been administered to all patients.
In the high-dose group, 8.3% of patients had at least 1 adverse event, compared with 9% in the standard-dose group (relative risk, 0.92; 95% CI, 0.85–0.99).
After vaccination, the hemagglutination inhibition titers were significantly higher in the high-dose group.
Fewer adverse events with the higher dose, but some events were graver
Influenza is a serious viral illness, and it can be associated with mortality in certain populations, such as very young children, pregnant women, and people older than 65 years.
As a general rule, older patients do not respond as well to the vaccine as younger patients do. The standard dose of vaccine provides about 50% protection against influenza in older patients, compared with approximately 60% to 65% in younger individuals. With the added protection of the high-dose vaccine (overall efficacy, 24.2%), approximately 62% of adults older than age 65 would be protected—a figure similar to that reported for younger patients.
The increase in effectiveness was achieved with no increase in the overall frequency of adverse effects. In fact, the frequency of adverse effects was actually slightly lower in the recipients of the higher dose. However, in 3 recipients of the high-dose vaccine the adverse effects were notable. One had a transient sixth cranial nerve palsy that started 1 day after vaccination. One had hypovolemic shock due to diarrhea that started 1 day after vaccination. One had acute disseminated encephalomyelitis that started 117 days after vaccination. All 3 patients recovered fully. No such serious events occurred in the standard-dose group.
Several barriers prevent widespread vaccination
The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices strongly recommends influenza vaccination for everyone over the age of 6 months. Barriers to widespread vaccination include reluctance on the part of the patient, failure on the part of the physician to advocate for vaccination, and cost of the vaccine for patients who have suboptimal insurance or no insurance.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
We should strongly advise older women in our practice to receive the high-dose influenza vaccine. We should caution them that the overall risk of adverse effects is actually lower than with the standard-dose vaccine but that serious effects can occur in rare instances.
—Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The objective of this investigation was to compare a standard-dose trivalent influenza vaccine with a high-dose vaccine in adults older than 65 years. The standard dose of the vaccine contained 15 µg of hemagglutinin per strain, and the high dose contained 60 µg hemagglutinin per strain. The study was conducted during the 2011–2012 and 2012–2013 flu seasons. Key outcome measures were efficacy, as assessed by the occurrence of laboratory-confirmed influenza at least 14 days after vaccination; immunogenicity of the vaccines; and frequency of adverse events.
Details of the study
The study involved 15,991 patients in the high-dose group and 15,998 patients in the standard-dose group. Two hundred twenty-eight participants (1.4%) in the high-dose group developed influenza, compared with 301 participants (1.9%) in the standard-dose group.
The overall efficacy of the high-dose vaccine was 24.2% (95% confidence interval [CI], 9.7–36.5), meaning that approximately 24% of influenza cases could have been prevented if the high-dose vaccine had been administered to all patients.
In the high-dose group, 8.3% of patients had at least 1 adverse event, compared with 9% in the standard-dose group (relative risk, 0.92; 95% CI, 0.85–0.99).
After vaccination, the hemagglutination inhibition titers were significantly higher in the high-dose group.
Fewer adverse events with the higher dose, but some events were graver
Influenza is a serious viral illness, and it can be associated with mortality in certain populations, such as very young children, pregnant women, and people older than 65 years.
As a general rule, older patients do not respond as well to the vaccine as younger patients do. The standard dose of vaccine provides about 50% protection against influenza in older patients, compared with approximately 60% to 65% in younger individuals. With the added protection of the high-dose vaccine (overall efficacy, 24.2%), approximately 62% of adults older than age 65 would be protected—a figure similar to that reported for younger patients.
The increase in effectiveness was achieved with no increase in the overall frequency of adverse effects. In fact, the frequency of adverse effects was actually slightly lower in the recipients of the higher dose. However, in 3 recipients of the high-dose vaccine the adverse effects were notable. One had a transient sixth cranial nerve palsy that started 1 day after vaccination. One had hypovolemic shock due to diarrhea that started 1 day after vaccination. One had acute disseminated encephalomyelitis that started 117 days after vaccination. All 3 patients recovered fully. No such serious events occurred in the standard-dose group.
Several barriers prevent widespread vaccination
The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices strongly recommends influenza vaccination for everyone over the age of 6 months. Barriers to widespread vaccination include reluctance on the part of the patient, failure on the part of the physician to advocate for vaccination, and cost of the vaccine for patients who have suboptimal insurance or no insurance.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
We should strongly advise older women in our practice to receive the high-dose influenza vaccine. We should caution them that the overall risk of adverse effects is actually lower than with the standard-dose vaccine but that serious effects can occur in rare instances.
—Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The objective of this investigation was to compare a standard-dose trivalent influenza vaccine with a high-dose vaccine in adults older than 65 years. The standard dose of the vaccine contained 15 µg of hemagglutinin per strain, and the high dose contained 60 µg hemagglutinin per strain. The study was conducted during the 2011–2012 and 2012–2013 flu seasons. Key outcome measures were efficacy, as assessed by the occurrence of laboratory-confirmed influenza at least 14 days after vaccination; immunogenicity of the vaccines; and frequency of adverse events.
Details of the study
The study involved 15,991 patients in the high-dose group and 15,998 patients in the standard-dose group. Two hundred twenty-eight participants (1.4%) in the high-dose group developed influenza, compared with 301 participants (1.9%) in the standard-dose group.
The overall efficacy of the high-dose vaccine was 24.2% (95% confidence interval [CI], 9.7–36.5), meaning that approximately 24% of influenza cases could have been prevented if the high-dose vaccine had been administered to all patients.
In the high-dose group, 8.3% of patients had at least 1 adverse event, compared with 9% in the standard-dose group (relative risk, 0.92; 95% CI, 0.85–0.99).
After vaccination, the hemagglutination inhibition titers were significantly higher in the high-dose group.
Fewer adverse events with the higher dose, but some events were graver
Influenza is a serious viral illness, and it can be associated with mortality in certain populations, such as very young children, pregnant women, and people older than 65 years.
As a general rule, older patients do not respond as well to the vaccine as younger patients do. The standard dose of vaccine provides about 50% protection against influenza in older patients, compared with approximately 60% to 65% in younger individuals. With the added protection of the high-dose vaccine (overall efficacy, 24.2%), approximately 62% of adults older than age 65 would be protected—a figure similar to that reported for younger patients.
The increase in effectiveness was achieved with no increase in the overall frequency of adverse effects. In fact, the frequency of adverse effects was actually slightly lower in the recipients of the higher dose. However, in 3 recipients of the high-dose vaccine the adverse effects were notable. One had a transient sixth cranial nerve palsy that started 1 day after vaccination. One had hypovolemic shock due to diarrhea that started 1 day after vaccination. One had acute disseminated encephalomyelitis that started 117 days after vaccination. All 3 patients recovered fully. No such serious events occurred in the standard-dose group.
Several barriers prevent widespread vaccination
The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices strongly recommends influenza vaccination for everyone over the age of 6 months. Barriers to widespread vaccination include reluctance on the part of the patient, failure on the part of the physician to advocate for vaccination, and cost of the vaccine for patients who have suboptimal insurance or no insurance.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
We should strongly advise older women in our practice to receive the high-dose influenza vaccine. We should caution them that the overall risk of adverse effects is actually lower than with the standard-dose vaccine but that serious effects can occur in rare instances.
—Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
5 ways to reduce infection risk during pregnancy
Pregnant women may be more severelyaffected by certain microorganisms than nonpregnant individuals.1 In a recent review, Kourtis and colleagues cited evidence for increased mortality risk for pregnant patients related to 5 specific infections.1 What are those infections and why does pregnancy put a woman at greater risk for adverse outcomes? I review these topics in this article and, based on this evidence, I suggest 5 specific ways to avoid infection during pregnancy.
Five infections that can lead to detrimental outcomes during pregnancy
Influenza
During the pandemic of 1918, maternal mortality was 27%. During the 1957 pandemic, 50% of influenza-related deaths among women of reproductive age occurred among pregnant women. In the 2009 H1N1 influenza A pandemic, pregnant women were clearly at increased risk for severe disease, reflected by an increased frequency of hospitalization and increased likelihood of admission to an intensive care unit.
Hepatitis E
Compared with nonpregnant women and men, pregnant women also are at markedly increased risk for mortality due to hepa-titis E infection, especially in Southeast Asia, the Middle East, and Africa. The pathophysiologic basis for this increase is not well understood. Interestingly, in a report from India, 33% to 43% of pregnant women infected with hepatitis E had such severe disease that they developed hepatic failure.
Herpes simplex virus
Pregnant women with primary herpes simplex virus (HSV) infection are at increased risk for hepatitis and for disseminated infection, compared with other nonpregnant adults. Only patients with obvious immunodeficiency disorders are at greater risk for disseminated HSV infection.
Malaria
Pregnant women are at significantly increased risk for acquiring Plasmodium falciparum malaria and developing severe, life-threatening disease. In multiple studies from the Asia-Pacific region, pregnant women have been threefold more likely to acquire malaria compared with nonpregnant individuals. In India, during the period 2004 to 2006, malaria was the most common cause of maternal death.
The most likely explanation for the deleterious effect of this particular form of malaria is the fact that the P falciparum parasites accumulate selectively in the placenta because they bind avidly to syncytiotrophoblastic chondroitin sulfate A. Intense inflammation in the placenta, in turn, can lead to early pregnancy loss, preterm delivery, and fetal infection.
Listeria
Another important infection to which pregnant women are particularly susceptible is listeriosis. Listeria monocytogenes may contaminate several types of food such as uncooked meats and vegetables, unpasteurized milk, and soft cheeses. The organism has a predilection to attack the placenta and fetus and can cause spontaneous abortion, stillbirth, preterm delivery, and neonatal infection. Hispanic women may be at unusually high risk for listeria.
Immune system changes during pregnancy
Certain subtle changes occur in the immune system during pregnancy, which may help explain the increased risk of acquired infection and subsequent adverse effects. These changes include1:
- Progesterone presence, which may suppress the maternal immune response and alter the balance between type-1 helper T-cell response and type-2 helper T-cell response. Type-2 cells stimulate B lymphocytes, increase antibody production, and suppress the cytotoxic T-lymphocyte response. The net effect of these changes is to decrease the robustness of cell-mediated immunity, which may impair the response of the pregnant patient to selected viral respiratory pathogens such as influenza virus.
- Increasing serum concentrations of estrogen and progesterone, which may lead to a reversible thymic involution.
- Serum concentrations of interferon-gamma, monocyte chemoattractant protein 1, and eotaxin are decreased in most pregnant women.
- Overall, serum concentrations of inflammatory cytokines are reduced and concentrations of cytokines that induce phagocytic-cell recruitment are increased.
5 ways to reduce infection risk during pregnancy
Pregnant patients clearly are not as immunosuppressed as patients receiving chemotherapy or high doses of systemic glucocorticoids. Nevertheless, the subtle alterations in their immune system just described make pregnant women increasingly susceptible to certain infections. Therefore, I suggest these take-home messages for reducing infection risk in your pregnant patients.
1. Vaccinate against the flu
All pregnant women should be vaccinated each year for influenza. If your patient becomes infected despite vaccination, treat her promptly with an antiviral medication such as oseltamivir and observe her carefully for evidence of superimposed bacterial pneumonia. If the latter complication develops, hospitalize the patient immediately and treat her with appropriate broad spectrum antibiotics. (See, “Don’t wait for rapid flu test results. Treat your pregnant patient with antiviral therapy!”)
Don’t wait for rapid flu test results. Treat your pregnant patient with antiviral therapy!
More than 17,000 deaths occurred worldwide in the 2009 H1N1 influenza A global pandemic.1 The dominant circulating virus in the US 2013–2014 influenza season was again H1N1. In California, H1N1 accounted for about 94% of subtyped specimens.2
In a recent case series,1 Louie and colleagues reviewed California Department of Public Health data on pregnant and postpartum women (6 weeks or less from delivery) with laboratory-confirmed influenza who died or required hospitalization in intensive care units in the 2013−2014 influenza season.
They found that, from September 29, 2013, through May 17, 2014, 17 pregnant women (median age, 29 years [range, 17−44 years]) with severe influenza were reported. Fifteen patients were hospitalized, 9 required mechanical ventilation, 5 required emergent cesarean delivery, and 4 died. Sixteen of the 17 patients were in the second or third trimester; one was in the first trimester. An additional patient was 36 days postpartum and required intensive care unit admission and mechanical ventilation for influenza-associated acute respiratory distress syndrome.
Only 2 patients, of the 14 with available information, received influenza vaccination during their pregnancy.
The 7 patients who tested positive for influenza by polymerase chain reaction also had rapid influenza diagnostic testing performed; only 1 patient had a positive rapid influenza diagnostic test result.
The authors point out that, although rapid influenza diagnostic tests produce very quick results, they can have poor sensitivity, depending on specimen type, patient age, and even virus type.3 Therefore, it is imperative to begin empiric antiviral therapy promptly in a pregnant or postpartum patient who has clinical manifestations of viral influenza regardless of rapid influenza diagnostic test results or vaccination status. Such manifestations include malaise, myalgias, arthalgias, fever, chills, cough, and pleuritic chest pain.
Treat patients with oseltamivir 75 mg orally twice daily for 5 days. If a patient is unable to take oral medications, she can be treated with zanamivir, 2 puffs inhaled twice daily for 5 days. To be most effective, treatment should be started within 48 hours of the onset of symptoms.
References
2. Ayscue P, Murray E, Uyeki T, et al. Influenza-associated intensive-care unit admissions and deaths—California, September 29, 2013-January 18, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(7):143−147.
3. Centers for Disease Control and Prevention. Evaluation of 11 commercially available rapid influenza diagnostic tests—United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2012;61(43):873−876.
2. Avoid hepatitis E−endemic areas
Ideally, our patients should avoid travel to areas of the world where hepatitis E is endemic. If travel cannot be avoided, the patient should receive the new hepatitis E vaccine. This vaccine is administered in a 3-dose series; in clinical trials, it has had an efficacy of 85% to 90%.2
If a patient acquires hepatitis E infection, she should receive aggressive supportive care, with hospitalization strongly considered because of the increased risk for hepatic failure.
The clinical manifestations of hepatitis Eare very similar to those of hepatitis A: fever, malaise, anorexia, nausea, pain and tenderness in the right upper quadrant, jaundice, darkened urine, and clay-colored stools. Laboratory abnormalities in affected patients include elevated transaminase enzymes, elevated bilirubin, positive immunoglobulin M antibody against hepatitis E virus, a fourfold increase in a prior immunoglobulin G antibody titer against hepatitis E virus, and a positive test for hepatitis E RNA.2
3. Treat patients with HSV infection to avoid an outbreak during delivery
Pregnant women who develop primary or recurrent HSV infection should be treated promptly with therapeutic doses of acyclovir or valacyclovir. Patients with frequent recurrences should receive daily anti-HSV prophylaxis throughout pregnancy. Other patients should be treated prophylactically from week 36 until delivery.
4. Recommend malaria prophylaxis when appropriate
If your pregnant patient is traveling to an area of the world where malaria is endemic, she should receive appropriate prophylaxis, especially against P falciparum.
5. Vaccinate during pregnancy and promptly treat developed infections
All infections in pregnant women should be treated in a timely manner with appropriate antibiotics. Moreover, we should make a firm effort to provide all pregnant women with the following vaccinations: influenza, Tdap, and hepatitis B (if susceptible). Select patients also should receive pneumococcal vaccine (those who are immunosuppressed; have chronic medical illnessess, particulary cardiopulmonary disease; or have had a splenectomy).
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211−2218.
2. Zhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914−922.
Pregnant women may be more severelyaffected by certain microorganisms than nonpregnant individuals.1 In a recent review, Kourtis and colleagues cited evidence for increased mortality risk for pregnant patients related to 5 specific infections.1 What are those infections and why does pregnancy put a woman at greater risk for adverse outcomes? I review these topics in this article and, based on this evidence, I suggest 5 specific ways to avoid infection during pregnancy.
Five infections that can lead to detrimental outcomes during pregnancy
Influenza
During the pandemic of 1918, maternal mortality was 27%. During the 1957 pandemic, 50% of influenza-related deaths among women of reproductive age occurred among pregnant women. In the 2009 H1N1 influenza A pandemic, pregnant women were clearly at increased risk for severe disease, reflected by an increased frequency of hospitalization and increased likelihood of admission to an intensive care unit.
Hepatitis E
Compared with nonpregnant women and men, pregnant women also are at markedly increased risk for mortality due to hepa-titis E infection, especially in Southeast Asia, the Middle East, and Africa. The pathophysiologic basis for this increase is not well understood. Interestingly, in a report from India, 33% to 43% of pregnant women infected with hepatitis E had such severe disease that they developed hepatic failure.
Herpes simplex virus
Pregnant women with primary herpes simplex virus (HSV) infection are at increased risk for hepatitis and for disseminated infection, compared with other nonpregnant adults. Only patients with obvious immunodeficiency disorders are at greater risk for disseminated HSV infection.
Malaria
Pregnant women are at significantly increased risk for acquiring Plasmodium falciparum malaria and developing severe, life-threatening disease. In multiple studies from the Asia-Pacific region, pregnant women have been threefold more likely to acquire malaria compared with nonpregnant individuals. In India, during the period 2004 to 2006, malaria was the most common cause of maternal death.
The most likely explanation for the deleterious effect of this particular form of malaria is the fact that the P falciparum parasites accumulate selectively in the placenta because they bind avidly to syncytiotrophoblastic chondroitin sulfate A. Intense inflammation in the placenta, in turn, can lead to early pregnancy loss, preterm delivery, and fetal infection.
Listeria
Another important infection to which pregnant women are particularly susceptible is listeriosis. Listeria monocytogenes may contaminate several types of food such as uncooked meats and vegetables, unpasteurized milk, and soft cheeses. The organism has a predilection to attack the placenta and fetus and can cause spontaneous abortion, stillbirth, preterm delivery, and neonatal infection. Hispanic women may be at unusually high risk for listeria.
Immune system changes during pregnancy
Certain subtle changes occur in the immune system during pregnancy, which may help explain the increased risk of acquired infection and subsequent adverse effects. These changes include1:
- Progesterone presence, which may suppress the maternal immune response and alter the balance between type-1 helper T-cell response and type-2 helper T-cell response. Type-2 cells stimulate B lymphocytes, increase antibody production, and suppress the cytotoxic T-lymphocyte response. The net effect of these changes is to decrease the robustness of cell-mediated immunity, which may impair the response of the pregnant patient to selected viral respiratory pathogens such as influenza virus.
- Increasing serum concentrations of estrogen and progesterone, which may lead to a reversible thymic involution.
- Serum concentrations of interferon-gamma, monocyte chemoattractant protein 1, and eotaxin are decreased in most pregnant women.
- Overall, serum concentrations of inflammatory cytokines are reduced and concentrations of cytokines that induce phagocytic-cell recruitment are increased.
5 ways to reduce infection risk during pregnancy
Pregnant patients clearly are not as immunosuppressed as patients receiving chemotherapy or high doses of systemic glucocorticoids. Nevertheless, the subtle alterations in their immune system just described make pregnant women increasingly susceptible to certain infections. Therefore, I suggest these take-home messages for reducing infection risk in your pregnant patients.
1. Vaccinate against the flu
All pregnant women should be vaccinated each year for influenza. If your patient becomes infected despite vaccination, treat her promptly with an antiviral medication such as oseltamivir and observe her carefully for evidence of superimposed bacterial pneumonia. If the latter complication develops, hospitalize the patient immediately and treat her with appropriate broad spectrum antibiotics. (See, “Don’t wait for rapid flu test results. Treat your pregnant patient with antiviral therapy!”)
Don’t wait for rapid flu test results. Treat your pregnant patient with antiviral therapy!
More than 17,000 deaths occurred worldwide in the 2009 H1N1 influenza A global pandemic.1 The dominant circulating virus in the US 2013–2014 influenza season was again H1N1. In California, H1N1 accounted for about 94% of subtyped specimens.2
In a recent case series,1 Louie and colleagues reviewed California Department of Public Health data on pregnant and postpartum women (6 weeks or less from delivery) with laboratory-confirmed influenza who died or required hospitalization in intensive care units in the 2013−2014 influenza season.
They found that, from September 29, 2013, through May 17, 2014, 17 pregnant women (median age, 29 years [range, 17−44 years]) with severe influenza were reported. Fifteen patients were hospitalized, 9 required mechanical ventilation, 5 required emergent cesarean delivery, and 4 died. Sixteen of the 17 patients were in the second or third trimester; one was in the first trimester. An additional patient was 36 days postpartum and required intensive care unit admission and mechanical ventilation for influenza-associated acute respiratory distress syndrome.
Only 2 patients, of the 14 with available information, received influenza vaccination during their pregnancy.
The 7 patients who tested positive for influenza by polymerase chain reaction also had rapid influenza diagnostic testing performed; only 1 patient had a positive rapid influenza diagnostic test result.
The authors point out that, although rapid influenza diagnostic tests produce very quick results, they can have poor sensitivity, depending on specimen type, patient age, and even virus type.3 Therefore, it is imperative to begin empiric antiviral therapy promptly in a pregnant or postpartum patient who has clinical manifestations of viral influenza regardless of rapid influenza diagnostic test results or vaccination status. Such manifestations include malaise, myalgias, arthalgias, fever, chills, cough, and pleuritic chest pain.
Treat patients with oseltamivir 75 mg orally twice daily for 5 days. If a patient is unable to take oral medications, she can be treated with zanamivir, 2 puffs inhaled twice daily for 5 days. To be most effective, treatment should be started within 48 hours of the onset of symptoms.
References
2. Ayscue P, Murray E, Uyeki T, et al. Influenza-associated intensive-care unit admissions and deaths—California, September 29, 2013-January 18, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(7):143−147.
3. Centers for Disease Control and Prevention. Evaluation of 11 commercially available rapid influenza diagnostic tests—United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2012;61(43):873−876.
2. Avoid hepatitis E−endemic areas
Ideally, our patients should avoid travel to areas of the world where hepatitis E is endemic. If travel cannot be avoided, the patient should receive the new hepatitis E vaccine. This vaccine is administered in a 3-dose series; in clinical trials, it has had an efficacy of 85% to 90%.2
If a patient acquires hepatitis E infection, she should receive aggressive supportive care, with hospitalization strongly considered because of the increased risk for hepatic failure.
The clinical manifestations of hepatitis Eare very similar to those of hepatitis A: fever, malaise, anorexia, nausea, pain and tenderness in the right upper quadrant, jaundice, darkened urine, and clay-colored stools. Laboratory abnormalities in affected patients include elevated transaminase enzymes, elevated bilirubin, positive immunoglobulin M antibody against hepatitis E virus, a fourfold increase in a prior immunoglobulin G antibody titer against hepatitis E virus, and a positive test for hepatitis E RNA.2
3. Treat patients with HSV infection to avoid an outbreak during delivery
Pregnant women who develop primary or recurrent HSV infection should be treated promptly with therapeutic doses of acyclovir or valacyclovir. Patients with frequent recurrences should receive daily anti-HSV prophylaxis throughout pregnancy. Other patients should be treated prophylactically from week 36 until delivery.
4. Recommend malaria prophylaxis when appropriate
If your pregnant patient is traveling to an area of the world where malaria is endemic, she should receive appropriate prophylaxis, especially against P falciparum.
5. Vaccinate during pregnancy and promptly treat developed infections
All infections in pregnant women should be treated in a timely manner with appropriate antibiotics. Moreover, we should make a firm effort to provide all pregnant women with the following vaccinations: influenza, Tdap, and hepatitis B (if susceptible). Select patients also should receive pneumococcal vaccine (those who are immunosuppressed; have chronic medical illnessess, particulary cardiopulmonary disease; or have had a splenectomy).
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Pregnant women may be more severelyaffected by certain microorganisms than nonpregnant individuals.1 In a recent review, Kourtis and colleagues cited evidence for increased mortality risk for pregnant patients related to 5 specific infections.1 What are those infections and why does pregnancy put a woman at greater risk for adverse outcomes? I review these topics in this article and, based on this evidence, I suggest 5 specific ways to avoid infection during pregnancy.
Five infections that can lead to detrimental outcomes during pregnancy
Influenza
During the pandemic of 1918, maternal mortality was 27%. During the 1957 pandemic, 50% of influenza-related deaths among women of reproductive age occurred among pregnant women. In the 2009 H1N1 influenza A pandemic, pregnant women were clearly at increased risk for severe disease, reflected by an increased frequency of hospitalization and increased likelihood of admission to an intensive care unit.
Hepatitis E
Compared with nonpregnant women and men, pregnant women also are at markedly increased risk for mortality due to hepa-titis E infection, especially in Southeast Asia, the Middle East, and Africa. The pathophysiologic basis for this increase is not well understood. Interestingly, in a report from India, 33% to 43% of pregnant women infected with hepatitis E had such severe disease that they developed hepatic failure.
Herpes simplex virus
Pregnant women with primary herpes simplex virus (HSV) infection are at increased risk for hepatitis and for disseminated infection, compared with other nonpregnant adults. Only patients with obvious immunodeficiency disorders are at greater risk for disseminated HSV infection.
Malaria
Pregnant women are at significantly increased risk for acquiring Plasmodium falciparum malaria and developing severe, life-threatening disease. In multiple studies from the Asia-Pacific region, pregnant women have been threefold more likely to acquire malaria compared with nonpregnant individuals. In India, during the period 2004 to 2006, malaria was the most common cause of maternal death.
The most likely explanation for the deleterious effect of this particular form of malaria is the fact that the P falciparum parasites accumulate selectively in the placenta because they bind avidly to syncytiotrophoblastic chondroitin sulfate A. Intense inflammation in the placenta, in turn, can lead to early pregnancy loss, preterm delivery, and fetal infection.
Listeria
Another important infection to which pregnant women are particularly susceptible is listeriosis. Listeria monocytogenes may contaminate several types of food such as uncooked meats and vegetables, unpasteurized milk, and soft cheeses. The organism has a predilection to attack the placenta and fetus and can cause spontaneous abortion, stillbirth, preterm delivery, and neonatal infection. Hispanic women may be at unusually high risk for listeria.
Immune system changes during pregnancy
Certain subtle changes occur in the immune system during pregnancy, which may help explain the increased risk of acquired infection and subsequent adverse effects. These changes include1:
- Progesterone presence, which may suppress the maternal immune response and alter the balance between type-1 helper T-cell response and type-2 helper T-cell response. Type-2 cells stimulate B lymphocytes, increase antibody production, and suppress the cytotoxic T-lymphocyte response. The net effect of these changes is to decrease the robustness of cell-mediated immunity, which may impair the response of the pregnant patient to selected viral respiratory pathogens such as influenza virus.
- Increasing serum concentrations of estrogen and progesterone, which may lead to a reversible thymic involution.
- Serum concentrations of interferon-gamma, monocyte chemoattractant protein 1, and eotaxin are decreased in most pregnant women.
- Overall, serum concentrations of inflammatory cytokines are reduced and concentrations of cytokines that induce phagocytic-cell recruitment are increased.
5 ways to reduce infection risk during pregnancy
Pregnant patients clearly are not as immunosuppressed as patients receiving chemotherapy or high doses of systemic glucocorticoids. Nevertheless, the subtle alterations in their immune system just described make pregnant women increasingly susceptible to certain infections. Therefore, I suggest these take-home messages for reducing infection risk in your pregnant patients.
1. Vaccinate against the flu
All pregnant women should be vaccinated each year for influenza. If your patient becomes infected despite vaccination, treat her promptly with an antiviral medication such as oseltamivir and observe her carefully for evidence of superimposed bacterial pneumonia. If the latter complication develops, hospitalize the patient immediately and treat her with appropriate broad spectrum antibiotics. (See, “Don’t wait for rapid flu test results. Treat your pregnant patient with antiviral therapy!”)
Don’t wait for rapid flu test results. Treat your pregnant patient with antiviral therapy!
More than 17,000 deaths occurred worldwide in the 2009 H1N1 influenza A global pandemic.1 The dominant circulating virus in the US 2013–2014 influenza season was again H1N1. In California, H1N1 accounted for about 94% of subtyped specimens.2
In a recent case series,1 Louie and colleagues reviewed California Department of Public Health data on pregnant and postpartum women (6 weeks or less from delivery) with laboratory-confirmed influenza who died or required hospitalization in intensive care units in the 2013−2014 influenza season.
They found that, from September 29, 2013, through May 17, 2014, 17 pregnant women (median age, 29 years [range, 17−44 years]) with severe influenza were reported. Fifteen patients were hospitalized, 9 required mechanical ventilation, 5 required emergent cesarean delivery, and 4 died. Sixteen of the 17 patients were in the second or third trimester; one was in the first trimester. An additional patient was 36 days postpartum and required intensive care unit admission and mechanical ventilation for influenza-associated acute respiratory distress syndrome.
Only 2 patients, of the 14 with available information, received influenza vaccination during their pregnancy.
The 7 patients who tested positive for influenza by polymerase chain reaction also had rapid influenza diagnostic testing performed; only 1 patient had a positive rapid influenza diagnostic test result.
The authors point out that, although rapid influenza diagnostic tests produce very quick results, they can have poor sensitivity, depending on specimen type, patient age, and even virus type.3 Therefore, it is imperative to begin empiric antiviral therapy promptly in a pregnant or postpartum patient who has clinical manifestations of viral influenza regardless of rapid influenza diagnostic test results or vaccination status. Such manifestations include malaise, myalgias, arthalgias, fever, chills, cough, and pleuritic chest pain.
Treat patients with oseltamivir 75 mg orally twice daily for 5 days. If a patient is unable to take oral medications, she can be treated with zanamivir, 2 puffs inhaled twice daily for 5 days. To be most effective, treatment should be started within 48 hours of the onset of symptoms.
References
2. Ayscue P, Murray E, Uyeki T, et al. Influenza-associated intensive-care unit admissions and deaths—California, September 29, 2013-January 18, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(7):143−147.
3. Centers for Disease Control and Prevention. Evaluation of 11 commercially available rapid influenza diagnostic tests—United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2012;61(43):873−876.
2. Avoid hepatitis E−endemic areas
Ideally, our patients should avoid travel to areas of the world where hepatitis E is endemic. If travel cannot be avoided, the patient should receive the new hepatitis E vaccine. This vaccine is administered in a 3-dose series; in clinical trials, it has had an efficacy of 85% to 90%.2
If a patient acquires hepatitis E infection, she should receive aggressive supportive care, with hospitalization strongly considered because of the increased risk for hepatic failure.
The clinical manifestations of hepatitis Eare very similar to those of hepatitis A: fever, malaise, anorexia, nausea, pain and tenderness in the right upper quadrant, jaundice, darkened urine, and clay-colored stools. Laboratory abnormalities in affected patients include elevated transaminase enzymes, elevated bilirubin, positive immunoglobulin M antibody against hepatitis E virus, a fourfold increase in a prior immunoglobulin G antibody titer against hepatitis E virus, and a positive test for hepatitis E RNA.2
3. Treat patients with HSV infection to avoid an outbreak during delivery
Pregnant women who develop primary or recurrent HSV infection should be treated promptly with therapeutic doses of acyclovir or valacyclovir. Patients with frequent recurrences should receive daily anti-HSV prophylaxis throughout pregnancy. Other patients should be treated prophylactically from week 36 until delivery.
4. Recommend malaria prophylaxis when appropriate
If your pregnant patient is traveling to an area of the world where malaria is endemic, she should receive appropriate prophylaxis, especially against P falciparum.
5. Vaccinate during pregnancy and promptly treat developed infections
All infections in pregnant women should be treated in a timely manner with appropriate antibiotics. Moreover, we should make a firm effort to provide all pregnant women with the following vaccinations: influenza, Tdap, and hepatitis B (if susceptible). Select patients also should receive pneumococcal vaccine (those who are immunosuppressed; have chronic medical illnessess, particulary cardiopulmonary disease; or have had a splenectomy).
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211−2218.
2. Zhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914−922.
1. Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211−2218.
2. Zhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914−922.
In this article
- Infections to avoid in pregnancy
- Don’t wait for rapid flu test results!
- 5 ways to reduce infection risk
Is azithromycin a good alternative to erythromycin for PPROM prophylaxis?
The objective of this investigation by Pierson and colleagues was to determine if there was any significant difference between erythyromycin and azithromycin, used in combination with ampicillin, for prophylaxis in women with PPROM.
Details of the study
The authors conducted a retrospective study of 168 women at 24 to 34 weeks’ gestation. At the discretion of the attending physician, patients received either ampicillin plus erythromycin or ampicillin plus azithromycin as their prophylactic antibiotic regimen. Patients were excluded from the study if they had a cerclage, a multiple gestation, a history of amniocentesis or fetal surgery, a history of abdominal trauma, or if they had a fetus with a lethal anomaly.
The primary study end point was the duration of the latency period between rupture of membranes and onset of labor. The secondary outcomes were gestational age at delivery, adverse drug effects, neonatal birth weight, Apgar scores, and rates of neonatal death, respiratory distress syndrome, and sepsis.
The mean (SD) duration of the latent period was 9.4 (10.4) days in the azithromycin group and 9.6 (13.2) days in the erythromycin group (P = .4). There also were no significant differences in any of the secondary outcome measures. Accordingly, the authors concluded that azithromycin was an acceptable alternative to erythromycin in the prophylactic antibiotic regimen for patients with PPROM.
Several factors make azithromycin the favored PPROM prophylactic option
In the original Maternal-Fetal Medicine Network trial of prophylactic antibiotics for PPROM, Mercer and colleagues1 used the combination regimen of ampicillin plus erythromycin. In this regimen, ampicillin primarily targets group B streptococci and Escherichia coli. Erythromycin specifically targets mycoplasma organisms, which can be part of the polymicrobial flora that causes chorioamnionitis. The drug also is effective against chlamydia.
However, erythromycin may cause troublesome gastrointestinal adverse effects, notably diarrhea, in some patients. Therefore, in recent years, several investigators have advocated use of azithromycin in lieu of erythromycin. Azithromycin has a similar spectrum of activity as erythromycin, but it has a more favorable pharmacokinetic profile. When given in a single oral dose of 1,000 mg, it has a half-life of 68 hours, compared with erythromycin’s half-life of 1.6 hours. Thus, it is much easier to administer. Moreover, it is usually much better tolerated than erythromycin and, now that generic versions of the drug are available, it is relatively inexpensive.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although this study is retrospective (Level II evidence), it is the first to demonstrate that, from the perspective of clinical effectiveness, azithromycin is comparable to erythromycin when used in combination with ampicillin for prophylaxis in patients with PPROM. For the reasons outlined above, I strongly favor azithromycin in lieu of erythromycin.
At our center we administer the drug in a single 1,000-mg oral dose. If the patient cannot tolerate oral medication at the time of admission, the drug can be administered intravenously.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278(12):989–995.
The objective of this investigation by Pierson and colleagues was to determine if there was any significant difference between erythyromycin and azithromycin, used in combination with ampicillin, for prophylaxis in women with PPROM.
Details of the study
The authors conducted a retrospective study of 168 women at 24 to 34 weeks’ gestation. At the discretion of the attending physician, patients received either ampicillin plus erythromycin or ampicillin plus azithromycin as their prophylactic antibiotic regimen. Patients were excluded from the study if they had a cerclage, a multiple gestation, a history of amniocentesis or fetal surgery, a history of abdominal trauma, or if they had a fetus with a lethal anomaly.
The primary study end point was the duration of the latency period between rupture of membranes and onset of labor. The secondary outcomes were gestational age at delivery, adverse drug effects, neonatal birth weight, Apgar scores, and rates of neonatal death, respiratory distress syndrome, and sepsis.
The mean (SD) duration of the latent period was 9.4 (10.4) days in the azithromycin group and 9.6 (13.2) days in the erythromycin group (P = .4). There also were no significant differences in any of the secondary outcome measures. Accordingly, the authors concluded that azithromycin was an acceptable alternative to erythromycin in the prophylactic antibiotic regimen for patients with PPROM.
Several factors make azithromycin the favored PPROM prophylactic option
In the original Maternal-Fetal Medicine Network trial of prophylactic antibiotics for PPROM, Mercer and colleagues1 used the combination regimen of ampicillin plus erythromycin. In this regimen, ampicillin primarily targets group B streptococci and Escherichia coli. Erythromycin specifically targets mycoplasma organisms, which can be part of the polymicrobial flora that causes chorioamnionitis. The drug also is effective against chlamydia.
However, erythromycin may cause troublesome gastrointestinal adverse effects, notably diarrhea, in some patients. Therefore, in recent years, several investigators have advocated use of azithromycin in lieu of erythromycin. Azithromycin has a similar spectrum of activity as erythromycin, but it has a more favorable pharmacokinetic profile. When given in a single oral dose of 1,000 mg, it has a half-life of 68 hours, compared with erythromycin’s half-life of 1.6 hours. Thus, it is much easier to administer. Moreover, it is usually much better tolerated than erythromycin and, now that generic versions of the drug are available, it is relatively inexpensive.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although this study is retrospective (Level II evidence), it is the first to demonstrate that, from the perspective of clinical effectiveness, azithromycin is comparable to erythromycin when used in combination with ampicillin for prophylaxis in patients with PPROM. For the reasons outlined above, I strongly favor azithromycin in lieu of erythromycin.
At our center we administer the drug in a single 1,000-mg oral dose. If the patient cannot tolerate oral medication at the time of admission, the drug can be administered intravenously.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The objective of this investigation by Pierson and colleagues was to determine if there was any significant difference between erythyromycin and azithromycin, used in combination with ampicillin, for prophylaxis in women with PPROM.
Details of the study
The authors conducted a retrospective study of 168 women at 24 to 34 weeks’ gestation. At the discretion of the attending physician, patients received either ampicillin plus erythromycin or ampicillin plus azithromycin as their prophylactic antibiotic regimen. Patients were excluded from the study if they had a cerclage, a multiple gestation, a history of amniocentesis or fetal surgery, a history of abdominal trauma, or if they had a fetus with a lethal anomaly.
The primary study end point was the duration of the latency period between rupture of membranes and onset of labor. The secondary outcomes were gestational age at delivery, adverse drug effects, neonatal birth weight, Apgar scores, and rates of neonatal death, respiratory distress syndrome, and sepsis.
The mean (SD) duration of the latent period was 9.4 (10.4) days in the azithromycin group and 9.6 (13.2) days in the erythromycin group (P = .4). There also were no significant differences in any of the secondary outcome measures. Accordingly, the authors concluded that azithromycin was an acceptable alternative to erythromycin in the prophylactic antibiotic regimen for patients with PPROM.
Several factors make azithromycin the favored PPROM prophylactic option
In the original Maternal-Fetal Medicine Network trial of prophylactic antibiotics for PPROM, Mercer and colleagues1 used the combination regimen of ampicillin plus erythromycin. In this regimen, ampicillin primarily targets group B streptococci and Escherichia coli. Erythromycin specifically targets mycoplasma organisms, which can be part of the polymicrobial flora that causes chorioamnionitis. The drug also is effective against chlamydia.
However, erythromycin may cause troublesome gastrointestinal adverse effects, notably diarrhea, in some patients. Therefore, in recent years, several investigators have advocated use of azithromycin in lieu of erythromycin. Azithromycin has a similar spectrum of activity as erythromycin, but it has a more favorable pharmacokinetic profile. When given in a single oral dose of 1,000 mg, it has a half-life of 68 hours, compared with erythromycin’s half-life of 1.6 hours. Thus, it is much easier to administer. Moreover, it is usually much better tolerated than erythromycin and, now that generic versions of the drug are available, it is relatively inexpensive.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although this study is retrospective (Level II evidence), it is the first to demonstrate that, from the perspective of clinical effectiveness, azithromycin is comparable to erythromycin when used in combination with ampicillin for prophylaxis in patients with PPROM. For the reasons outlined above, I strongly favor azithromycin in lieu of erythromycin.
At our center we administer the drug in a single 1,000-mg oral dose. If the patient cannot tolerate oral medication at the time of admission, the drug can be administered intravenously.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278(12):989–995.
Reference
- Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278(12):989–995.
2014 Update on infectious disease
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
UPDATE ON INFECTIOUS DISEASE
The past year has seen the publication of four studies with immediate relevance for clinicians:
- a retrospective, population-based cohort study that explores whether women who have chorioamnionitis in one pregnancy are at risk for the same type of infection in a subsequent pregnancy
- another retrospective cohort study that assesses the clinical utility of testing for gonorrhea and chlamydia before inserting an intrauterine device (IUD)
- an elegant primate experiment that highlights the value of azithromycin in subjects with chorioamnionitis
- a multicenter, randomized, nonblinded trial in seriously ill patients to determine whether daily bathing with chlorhexidine-impregnated washcloths can reduce the acquisition of multidrug-resistant organisms and the incidence of hospital-acquired bloodstream infection.
CHORIOAMNIONITIS IN ONE PREGNANCY IS LIKELY TO RECUR IN THE NEXT GENERATION
Cohen-Cline HN, Kahn TR, Hutter CM. A population-based study of the risk of repeat clinical chorioamnionitis in Washington State, 1989–2008. Am J Obstet Gynecol. 2012;207(6):473.e1–e7.
This retrospective, population-based cohort study (Level II evidence) is one of the few to examine the risk of recurrence for chorioamnionitis, and the findings are intriguing. Women who were infected during their first delivery were 3.43 times more likely to become infected in their second delivery than women who did not have chorioamnionitis in their first pregnancy (95% confidence interval [CI], 2.67–4.42; P <.001). This association persisted even after adjustment for potential confounders, such as age, ethnicity, presence of premature rupture of membranes (PROM), and internal fetal monitoring.
Chorioamnionitis is a common affliction
This infection complicates approximately 5% of term deliveries and a significantly higher percentage of preterm deliveries. The principal causative organisms are group B streptococci (GBS), Escherichia coli and other aerobic Gram-negative bacilli, both Gram-positive and Gram-negative anaerobes, and genital mycoplasmas.
The main risk factors for chorioamnionitis are:
- prematurity
- prolonged labor
- prolonged rupture of membranes
- multiple internal examinations
- internal fetal monitoring
- low socioeconomic status
- preexisting genital tract infection (eg, bacterial vaginosis, GBS colonization).
Infants delivered to infected mothers are at increased risk for sepsis, pneumonia, and meningitis. Severely infected infants, particularly those who are premature, are also at increased risk for cerebral palsy.
Details of the study
This investigation focused on women in Washington State who had a first pregnancy from 1989 through 2008 and then had at least one additional birth during the study period.
Participants included 6,219 women who had chorioamnionitis in their first pregnancy and 25,294 women who did not. Using logistic regression, Cohen-Cline and colleagues estimated the odds ratio for chorioamnionitis in the second delivery, taking into account the following potential confounders:
- maternal age
- ethnicity
- presence of PROM
- use of internal monitoring
- smoking.
As I stated above, women who had chorioamnionitis in their first pregnancy were 3.43 times as likely to have it again in their second pregnancy.
What this EVIDENCE means for practice
When a patient has a history of chorioamnionitis, we should do everything possible to reduce her risk for recurrent infection. For example, we should screen her for lower genital tract infections that predispose to chorioamnionitis:
-gonorrhea
-chlamydia
-bacterial vaginosis
-GBS.
If the patient has any of the first three infections, treat her immediately with the appropriate antibiotics. If she is colonized with GBS, administer one of the intrapartum antibiotic regimens recommended by the Centers for Disease Control and Prevention (CDC).
If the patient has a history of preterm PROM or spontaneous preterm delivery, initiate prophylaxis with progesterone and assess her cervical length periodically to determine whether cerclage is indicated.
During labor, make every effort to minimize the duration of ruptured membranes, the length of invasive monitoring, and the number of internal vaginal examinations.
At the earliest sign of intra-amniotic infection, treat the patient with broad-spectrum antibiotics, usually ampicillin plus gentamicin.
In low-risk populations, universal screening for sexually transmitted infections is probably unnecessary before IUD insertion
Sufrin CB, Postlethwaite D, Armstrong MA, et al. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol. 2012;120(6):1314–1321.
This retrospective cohort study (Level II evidence) focused on women who had an IUD inserted in a managed-care practice at Kaiser Permanente of Northern California during a 5-year period. Sufrin and colleagues compared the incidence of pelvic inflammatory disease (PID) within 90 days after insertion among women who were, and were not, screened for gonorrhea and chlamydia.
Among 57,728 IUD insertions, 47% involved women who were unscreened within 1 year of the procedure. Among women who were screened, 19% were tested on the day of IUD insertion.
The overall risk of PID in the study cohort was very low—0.54% (95% CI, 0.48–0.60). Investigators were unable to identify any significant difference in the risk of PID between women who had no screening versus those who were screened. Among women who were screened, same-day screening was equivalent to prescreening.
Investigators concluded that the most reasonable protocol is to screen on the basis of risk factors on the same day as IUD insertion. If the patient has obvious evidence of endocervicitis (ie, mucopurulent discharge), IUD insertion should be delayed. Otherwise, if the patient has risk factors for infection, screening should be followed by IUD insertion.
If the screen is positive, the patient should be treated in accordance with the latest CDC recommendations, and the IUD can be left in place.
Sufrin and colleagues concluded that adherence to this protocol would be associated with a very low, and clinically acceptable, risk of PID.
STI screening need not be an obstacle to IUD use
The IUD is an excellent method of contraception, and it is suitable for most patients. It is particularly useful for women who have difficulty remembering to take a pill each day or to use a barrier method of contraception at each episode of coitus.
Obstacles to more widespread use of the IUD include:
- high initial cost
- misconceptions on the part of the patient about the mechanism of action and adverse effects of the device
- cumbersome protocols that require multiple physician visits for counseling and sexually transmitted infection (STI) testing before the device is inserted.
What this EVIDENCE means for practice
This study provides reassurance that, at least in a relatively affluent managed-care population, universal testing for STIs is probably not necessary. When testing is indicated, it can be performed on the same day that the IUD is inserted, minimizing the number of office visits.
What is less clear is whether the same protocol can be applied to a population with a significantly higher prevalence of STIs. In such a population, universal screening for gonorrhea and chlamydia may be more prudent. However, screening still can be performed on the same day as IUD insertion.
In a primate model of intra-amniotic infection with Ureaplasma, maternal azithromycin prolonged gestation
Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207(6):475.e1–e14.
Grigsby and colleagues assessed the efficacy of azithromycin—with and without anti-inflammatory agents—in delaying preterm birth and minimizing fetal lung injury in a primate model. They found that azithromycin significantly prolonged gestation.
Details of the study
The study involved 16 chronically instrumented rhesus monkeys who received intra-amniotic inoculation with Ureaplasma parvum (107 colony-forming units/mL) and were then observed. When contractions began, as they invariably did, six monkeys received no treatment, five received intravenous (IV) azithromycin (12.5 mg/kg every 12 hours) for 10 days, and five received azithromycin plus dexamethasone and indomethacin.
Key outcome measures were the intra-amniotic concentration of proinflammatory mediators, the frequency of positive amniotic fluid cultures for U parvum, and the extent of histologic fetal lung injury.
In treated animals, the mean (SD) inoculation-to-delivery interval was 20.9 (1.4) days, compared with 13.7 (2.5) days in untreated monkeys (P <.05).
In addition, there was a twofold to threefold increase in the percentage of undelivered animals at 18 to 20 days after inoculation in the treatment group, compared with the no-treatment group. Treatment also significantly decreased the Ureaplasma colony count in the amniotic fluid, effectively eliminating the organism within 4 days.
In both treatment groups, the amniotic fluid concentration of proinflammatory mediators decreased significantly, compared with the untreated group. Treatment also significantly reduced the magnitude of deleterious histologic changes in the fetal lungs.
Somewhat surprisingly, dexamethasone and indomethacin did not enhance the treatment effect of azithromycin. Moreover, despite prolongation of pregnancy, all animals in the treatment group still delivered prematurely.
Why treatment should target genital mycoplasmas
Chorioamnionitis is an importance cause of preterm labor and preterm delivery. The principal pathogens are part of the normal vaginal flora: aerobic Gram-negative bacilli, aerobic Gram-positive cocci, anaerobes, and genital mycoplasmas.
Most treatment regimens for chorioamnionitis (eg, ampicillin plus gentamicin) do not specifically target the genital mycoplasmas. However, the most commonly recommended prophylactic antibiotic regimens for patients with preterm PROM include agents with specific action against mycoplasmas, namely erythromycin and azithromycin.
In this clinical setting, antibiotic prophylaxis prolongs the latency period and decreases the frequency of both maternal and fetal/neonatal infection.
This elegant basic science investigation sheds new light on the importance of the genital mycoplasmas in the pathogenesis of preterm labor and helps to explain why drugs like erythromycin and azithromycin may be so valuable in prolonging the latent period and reducing the frequency of infection and injury in the baby.
What this EVIDENCE means for practice
Because IV azithromycin rapidly achieved inhibitory concentrations in amniotic fluid and maintained these concentrations over 10 days of treatment, it significantly reduced the concentration of Ureaplasma in the amniotic fluid as well as the risk of histologic injury to the fetal lung.
Accordingly, I recommend that azithromycin remain a key component of the prophylactic regimen for patients with preterm PROM. It also may be advisable to add azithromycin to the usual combination of ampicillin plus gentamicin for empiric treatment of chorioamnionitis.
Daily bathing with chlorhexidine cloths can protect hospitalized patients from serious infection
Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–542.
This multicenter, randomized, nonblinded trial of 7,727 seriously ill patients sought to determine whether daily bathing with chlorhexidine-impregnated washcloths can decrease the acquisition of multidrug-resistant organisms and the incidence of hospital-acquired bloodstream infection.
Each day, patients in eight ICUs and one bone-marrow transplant unit bathed themselves, or were bathed by nursing staff, with 2% chlorhexidine-impregnated cloths or non–antimicrobial washcloths. All body surfaces except the face were cleansed. After 6 months, each unit changed to the other method of bathing.
Investigators focused on two outcomes:
- the prevalence of colonization of the nares with methicillin-resistant Staphylococcus aureus (MRSA) or colonization of the perirectal area with vancomycin-resistant enterococci (VRE)
- the frequency of hospital-acquired bloodstream infection (bacterial or fungal) detected more than 48 hours after admission to the unit.
The overall rate of MRSA or VRE acquisition was reduced by 23% when patients were bathed with chlorhexidine (5.10 versus 6.60 cases per 100 patient-days; P = .03). The overall rate of hospital-acquired bloodstream infection was reduced by 28% during the intervention period (4.78 vs 6.60 cases per 1,000 patient-days; P = .006).
In particular, the rate of central-catheter–associated bloodstream infection was 53% lower during the intervention (1.55 vs 3.30 cases per 1,000 catheter-days; P = .004).
The intervention had the greatest impact on infections caused by Gram-positive and fungal organisms.
The protective effect of chlorhexidine bathing was greatest among patients who had the longest length of stay in the unit.
Chlorhexidine did not cause an increased frequency of skin reactions. Moreover, use of the antiseptic washes did not cause the emergence of MRSA or VRA isolates with high-level resistance.
This study is of great interest in light of a recent report that demonstrated that preoperative preparation of the skin with chlorhexidine was more effective than preparation with povidone-iodine in reducing the risk of surgical-site infections after major operative procedures.1 Not only is chlorhexidine highly active against the usual bacteria that colonize the skin of hospitalized patients, it also has residual antibacterial activity that further decreases the colonization of the patient’s skin by microbes.
What this EVIDENCE means for practice
This study has two clear implications for ObGyns. First, chlorhexidine washes should be used by all patients who are scheduled for surgery, particularly those undergoing procedures that carry a relatively high risk of postoperative wound infection, such as total abdominal hysterectomy, radical hysterectomy, and cesarean delivery. In morbidly obese patients, particular attention should be directed to the skin beneath the abdominal panniculus.
Second, when we have seriously ill obstetric or gynecologic patients, especially those with long-term indwelling catheters who require prolonged hospitalization, we should order daily bathing (excluding the face) with chlorhexidine.
Reference
1. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
The past year has seen the publication of four studies with immediate relevance for clinicians:
- a retrospective, population-based cohort study that explores whether women who have chorioamnionitis in one pregnancy are at risk for the same type of infection in a subsequent pregnancy
- another retrospective cohort study that assesses the clinical utility of testing for gonorrhea and chlamydia before inserting an intrauterine device (IUD)
- an elegant primate experiment that highlights the value of azithromycin in subjects with chorioamnionitis
- a multicenter, randomized, nonblinded trial in seriously ill patients to determine whether daily bathing with chlorhexidine-impregnated washcloths can reduce the acquisition of multidrug-resistant organisms and the incidence of hospital-acquired bloodstream infection.
CHORIOAMNIONITIS IN ONE PREGNANCY IS LIKELY TO RECUR IN THE NEXT GENERATION
Cohen-Cline HN, Kahn TR, Hutter CM. A population-based study of the risk of repeat clinical chorioamnionitis in Washington State, 1989–2008. Am J Obstet Gynecol. 2012;207(6):473.e1–e7.
This retrospective, population-based cohort study (Level II evidence) is one of the few to examine the risk of recurrence for chorioamnionitis, and the findings are intriguing. Women who were infected during their first delivery were 3.43 times more likely to become infected in their second delivery than women who did not have chorioamnionitis in their first pregnancy (95% confidence interval [CI], 2.67–4.42; P <.001). This association persisted even after adjustment for potential confounders, such as age, ethnicity, presence of premature rupture of membranes (PROM), and internal fetal monitoring.
Chorioamnionitis is a common affliction
This infection complicates approximately 5% of term deliveries and a significantly higher percentage of preterm deliveries. The principal causative organisms are group B streptococci (GBS), Escherichia coli and other aerobic Gram-negative bacilli, both Gram-positive and Gram-negative anaerobes, and genital mycoplasmas.
The main risk factors for chorioamnionitis are:
- prematurity
- prolonged labor
- prolonged rupture of membranes
- multiple internal examinations
- internal fetal monitoring
- low socioeconomic status
- preexisting genital tract infection (eg, bacterial vaginosis, GBS colonization).
Infants delivered to infected mothers are at increased risk for sepsis, pneumonia, and meningitis. Severely infected infants, particularly those who are premature, are also at increased risk for cerebral palsy.
Details of the study
This investigation focused on women in Washington State who had a first pregnancy from 1989 through 2008 and then had at least one additional birth during the study period.
Participants included 6,219 women who had chorioamnionitis in their first pregnancy and 25,294 women who did not. Using logistic regression, Cohen-Cline and colleagues estimated the odds ratio for chorioamnionitis in the second delivery, taking into account the following potential confounders:
- maternal age
- ethnicity
- presence of PROM
- use of internal monitoring
- smoking.
As I stated above, women who had chorioamnionitis in their first pregnancy were 3.43 times as likely to have it again in their second pregnancy.
What this EVIDENCE means for practice
When a patient has a history of chorioamnionitis, we should do everything possible to reduce her risk for recurrent infection. For example, we should screen her for lower genital tract infections that predispose to chorioamnionitis:
-gonorrhea
-chlamydia
-bacterial vaginosis
-GBS.
If the patient has any of the first three infections, treat her immediately with the appropriate antibiotics. If she is colonized with GBS, administer one of the intrapartum antibiotic regimens recommended by the Centers for Disease Control and Prevention (CDC).
If the patient has a history of preterm PROM or spontaneous preterm delivery, initiate prophylaxis with progesterone and assess her cervical length periodically to determine whether cerclage is indicated.
During labor, make every effort to minimize the duration of ruptured membranes, the length of invasive monitoring, and the number of internal vaginal examinations.
At the earliest sign of intra-amniotic infection, treat the patient with broad-spectrum antibiotics, usually ampicillin plus gentamicin.
In low-risk populations, universal screening for sexually transmitted infections is probably unnecessary before IUD insertion
Sufrin CB, Postlethwaite D, Armstrong MA, et al. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol. 2012;120(6):1314–1321.
This retrospective cohort study (Level II evidence) focused on women who had an IUD inserted in a managed-care practice at Kaiser Permanente of Northern California during a 5-year period. Sufrin and colleagues compared the incidence of pelvic inflammatory disease (PID) within 90 days after insertion among women who were, and were not, screened for gonorrhea and chlamydia.
Among 57,728 IUD insertions, 47% involved women who were unscreened within 1 year of the procedure. Among women who were screened, 19% were tested on the day of IUD insertion.
The overall risk of PID in the study cohort was very low—0.54% (95% CI, 0.48–0.60). Investigators were unable to identify any significant difference in the risk of PID between women who had no screening versus those who were screened. Among women who were screened, same-day screening was equivalent to prescreening.
Investigators concluded that the most reasonable protocol is to screen on the basis of risk factors on the same day as IUD insertion. If the patient has obvious evidence of endocervicitis (ie, mucopurulent discharge), IUD insertion should be delayed. Otherwise, if the patient has risk factors for infection, screening should be followed by IUD insertion.
If the screen is positive, the patient should be treated in accordance with the latest CDC recommendations, and the IUD can be left in place.
Sufrin and colleagues concluded that adherence to this protocol would be associated with a very low, and clinically acceptable, risk of PID.
STI screening need not be an obstacle to IUD use
The IUD is an excellent method of contraception, and it is suitable for most patients. It is particularly useful for women who have difficulty remembering to take a pill each day or to use a barrier method of contraception at each episode of coitus.
Obstacles to more widespread use of the IUD include:
- high initial cost
- misconceptions on the part of the patient about the mechanism of action and adverse effects of the device
- cumbersome protocols that require multiple physician visits for counseling and sexually transmitted infection (STI) testing before the device is inserted.
What this EVIDENCE means for practice
This study provides reassurance that, at least in a relatively affluent managed-care population, universal testing for STIs is probably not necessary. When testing is indicated, it can be performed on the same day that the IUD is inserted, minimizing the number of office visits.
What is less clear is whether the same protocol can be applied to a population with a significantly higher prevalence of STIs. In such a population, universal screening for gonorrhea and chlamydia may be more prudent. However, screening still can be performed on the same day as IUD insertion.
In a primate model of intra-amniotic infection with Ureaplasma, maternal azithromycin prolonged gestation
Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207(6):475.e1–e14.
Grigsby and colleagues assessed the efficacy of azithromycin—with and without anti-inflammatory agents—in delaying preterm birth and minimizing fetal lung injury in a primate model. They found that azithromycin significantly prolonged gestation.
Details of the study
The study involved 16 chronically instrumented rhesus monkeys who received intra-amniotic inoculation with Ureaplasma parvum (107 colony-forming units/mL) and were then observed. When contractions began, as they invariably did, six monkeys received no treatment, five received intravenous (IV) azithromycin (12.5 mg/kg every 12 hours) for 10 days, and five received azithromycin plus dexamethasone and indomethacin.
Key outcome measures were the intra-amniotic concentration of proinflammatory mediators, the frequency of positive amniotic fluid cultures for U parvum, and the extent of histologic fetal lung injury.
In treated animals, the mean (SD) inoculation-to-delivery interval was 20.9 (1.4) days, compared with 13.7 (2.5) days in untreated monkeys (P <.05).
In addition, there was a twofold to threefold increase in the percentage of undelivered animals at 18 to 20 days after inoculation in the treatment group, compared with the no-treatment group. Treatment also significantly decreased the Ureaplasma colony count in the amniotic fluid, effectively eliminating the organism within 4 days.
In both treatment groups, the amniotic fluid concentration of proinflammatory mediators decreased significantly, compared with the untreated group. Treatment also significantly reduced the magnitude of deleterious histologic changes in the fetal lungs.
Somewhat surprisingly, dexamethasone and indomethacin did not enhance the treatment effect of azithromycin. Moreover, despite prolongation of pregnancy, all animals in the treatment group still delivered prematurely.
Why treatment should target genital mycoplasmas
Chorioamnionitis is an importance cause of preterm labor and preterm delivery. The principal pathogens are part of the normal vaginal flora: aerobic Gram-negative bacilli, aerobic Gram-positive cocci, anaerobes, and genital mycoplasmas.
Most treatment regimens for chorioamnionitis (eg, ampicillin plus gentamicin) do not specifically target the genital mycoplasmas. However, the most commonly recommended prophylactic antibiotic regimens for patients with preterm PROM include agents with specific action against mycoplasmas, namely erythromycin and azithromycin.
In this clinical setting, antibiotic prophylaxis prolongs the latency period and decreases the frequency of both maternal and fetal/neonatal infection.
This elegant basic science investigation sheds new light on the importance of the genital mycoplasmas in the pathogenesis of preterm labor and helps to explain why drugs like erythromycin and azithromycin may be so valuable in prolonging the latent period and reducing the frequency of infection and injury in the baby.
What this EVIDENCE means for practice
Because IV azithromycin rapidly achieved inhibitory concentrations in amniotic fluid and maintained these concentrations over 10 days of treatment, it significantly reduced the concentration of Ureaplasma in the amniotic fluid as well as the risk of histologic injury to the fetal lung.
Accordingly, I recommend that azithromycin remain a key component of the prophylactic regimen for patients with preterm PROM. It also may be advisable to add azithromycin to the usual combination of ampicillin plus gentamicin for empiric treatment of chorioamnionitis.
Daily bathing with chlorhexidine cloths can protect hospitalized patients from serious infection
Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–542.
This multicenter, randomized, nonblinded trial of 7,727 seriously ill patients sought to determine whether daily bathing with chlorhexidine-impregnated washcloths can decrease the acquisition of multidrug-resistant organisms and the incidence of hospital-acquired bloodstream infection.
Each day, patients in eight ICUs and one bone-marrow transplant unit bathed themselves, or were bathed by nursing staff, with 2% chlorhexidine-impregnated cloths or non–antimicrobial washcloths. All body surfaces except the face were cleansed. After 6 months, each unit changed to the other method of bathing.
Investigators focused on two outcomes:
- the prevalence of colonization of the nares with methicillin-resistant Staphylococcus aureus (MRSA) or colonization of the perirectal area with vancomycin-resistant enterococci (VRE)
- the frequency of hospital-acquired bloodstream infection (bacterial or fungal) detected more than 48 hours after admission to the unit.
The overall rate of MRSA or VRE acquisition was reduced by 23% when patients were bathed with chlorhexidine (5.10 versus 6.60 cases per 100 patient-days; P = .03). The overall rate of hospital-acquired bloodstream infection was reduced by 28% during the intervention period (4.78 vs 6.60 cases per 1,000 patient-days; P = .006).
In particular, the rate of central-catheter–associated bloodstream infection was 53% lower during the intervention (1.55 vs 3.30 cases per 1,000 catheter-days; P = .004).
The intervention had the greatest impact on infections caused by Gram-positive and fungal organisms.
The protective effect of chlorhexidine bathing was greatest among patients who had the longest length of stay in the unit.
Chlorhexidine did not cause an increased frequency of skin reactions. Moreover, use of the antiseptic washes did not cause the emergence of MRSA or VRA isolates with high-level resistance.
This study is of great interest in light of a recent report that demonstrated that preoperative preparation of the skin with chlorhexidine was more effective than preparation with povidone-iodine in reducing the risk of surgical-site infections after major operative procedures.1 Not only is chlorhexidine highly active against the usual bacteria that colonize the skin of hospitalized patients, it also has residual antibacterial activity that further decreases the colonization of the patient’s skin by microbes.
What this EVIDENCE means for practice
This study has two clear implications for ObGyns. First, chlorhexidine washes should be used by all patients who are scheduled for surgery, particularly those undergoing procedures that carry a relatively high risk of postoperative wound infection, such as total abdominal hysterectomy, radical hysterectomy, and cesarean delivery. In morbidly obese patients, particular attention should be directed to the skin beneath the abdominal panniculus.
Second, when we have seriously ill obstetric or gynecologic patients, especially those with long-term indwelling catheters who require prolonged hospitalization, we should order daily bathing (excluding the face) with chlorhexidine.
The past year has seen the publication of four studies with immediate relevance for clinicians:
- a retrospective, population-based cohort study that explores whether women who have chorioamnionitis in one pregnancy are at risk for the same type of infection in a subsequent pregnancy
- another retrospective cohort study that assesses the clinical utility of testing for gonorrhea and chlamydia before inserting an intrauterine device (IUD)
- an elegant primate experiment that highlights the value of azithromycin in subjects with chorioamnionitis
- a multicenter, randomized, nonblinded trial in seriously ill patients to determine whether daily bathing with chlorhexidine-impregnated washcloths can reduce the acquisition of multidrug-resistant organisms and the incidence of hospital-acquired bloodstream infection.
CHORIOAMNIONITIS IN ONE PREGNANCY IS LIKELY TO RECUR IN THE NEXT GENERATION
Cohen-Cline HN, Kahn TR, Hutter CM. A population-based study of the risk of repeat clinical chorioamnionitis in Washington State, 1989–2008. Am J Obstet Gynecol. 2012;207(6):473.e1–e7.
This retrospective, population-based cohort study (Level II evidence) is one of the few to examine the risk of recurrence for chorioamnionitis, and the findings are intriguing. Women who were infected during their first delivery were 3.43 times more likely to become infected in their second delivery than women who did not have chorioamnionitis in their first pregnancy (95% confidence interval [CI], 2.67–4.42; P <.001). This association persisted even after adjustment for potential confounders, such as age, ethnicity, presence of premature rupture of membranes (PROM), and internal fetal monitoring.
Chorioamnionitis is a common affliction
This infection complicates approximately 5% of term deliveries and a significantly higher percentage of preterm deliveries. The principal causative organisms are group B streptococci (GBS), Escherichia coli and other aerobic Gram-negative bacilli, both Gram-positive and Gram-negative anaerobes, and genital mycoplasmas.
The main risk factors for chorioamnionitis are:
- prematurity
- prolonged labor
- prolonged rupture of membranes
- multiple internal examinations
- internal fetal monitoring
- low socioeconomic status
- preexisting genital tract infection (eg, bacterial vaginosis, GBS colonization).
Infants delivered to infected mothers are at increased risk for sepsis, pneumonia, and meningitis. Severely infected infants, particularly those who are premature, are also at increased risk for cerebral palsy.
Details of the study
This investigation focused on women in Washington State who had a first pregnancy from 1989 through 2008 and then had at least one additional birth during the study period.
Participants included 6,219 women who had chorioamnionitis in their first pregnancy and 25,294 women who did not. Using logistic regression, Cohen-Cline and colleagues estimated the odds ratio for chorioamnionitis in the second delivery, taking into account the following potential confounders:
- maternal age
- ethnicity
- presence of PROM
- use of internal monitoring
- smoking.
As I stated above, women who had chorioamnionitis in their first pregnancy were 3.43 times as likely to have it again in their second pregnancy.
What this EVIDENCE means for practice
When a patient has a history of chorioamnionitis, we should do everything possible to reduce her risk for recurrent infection. For example, we should screen her for lower genital tract infections that predispose to chorioamnionitis:
-gonorrhea
-chlamydia
-bacterial vaginosis
-GBS.
If the patient has any of the first three infections, treat her immediately with the appropriate antibiotics. If she is colonized with GBS, administer one of the intrapartum antibiotic regimens recommended by the Centers for Disease Control and Prevention (CDC).
If the patient has a history of preterm PROM or spontaneous preterm delivery, initiate prophylaxis with progesterone and assess her cervical length periodically to determine whether cerclage is indicated.
During labor, make every effort to minimize the duration of ruptured membranes, the length of invasive monitoring, and the number of internal vaginal examinations.
At the earliest sign of intra-amniotic infection, treat the patient with broad-spectrum antibiotics, usually ampicillin plus gentamicin.
In low-risk populations, universal screening for sexually transmitted infections is probably unnecessary before IUD insertion
Sufrin CB, Postlethwaite D, Armstrong MA, et al. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol. 2012;120(6):1314–1321.
This retrospective cohort study (Level II evidence) focused on women who had an IUD inserted in a managed-care practice at Kaiser Permanente of Northern California during a 5-year period. Sufrin and colleagues compared the incidence of pelvic inflammatory disease (PID) within 90 days after insertion among women who were, and were not, screened for gonorrhea and chlamydia.
Among 57,728 IUD insertions, 47% involved women who were unscreened within 1 year of the procedure. Among women who were screened, 19% were tested on the day of IUD insertion.
The overall risk of PID in the study cohort was very low—0.54% (95% CI, 0.48–0.60). Investigators were unable to identify any significant difference in the risk of PID between women who had no screening versus those who were screened. Among women who were screened, same-day screening was equivalent to prescreening.
Investigators concluded that the most reasonable protocol is to screen on the basis of risk factors on the same day as IUD insertion. If the patient has obvious evidence of endocervicitis (ie, mucopurulent discharge), IUD insertion should be delayed. Otherwise, if the patient has risk factors for infection, screening should be followed by IUD insertion.
If the screen is positive, the patient should be treated in accordance with the latest CDC recommendations, and the IUD can be left in place.
Sufrin and colleagues concluded that adherence to this protocol would be associated with a very low, and clinically acceptable, risk of PID.
STI screening need not be an obstacle to IUD use
The IUD is an excellent method of contraception, and it is suitable for most patients. It is particularly useful for women who have difficulty remembering to take a pill each day or to use a barrier method of contraception at each episode of coitus.
Obstacles to more widespread use of the IUD include:
- high initial cost
- misconceptions on the part of the patient about the mechanism of action and adverse effects of the device
- cumbersome protocols that require multiple physician visits for counseling and sexually transmitted infection (STI) testing before the device is inserted.
What this EVIDENCE means for practice
This study provides reassurance that, at least in a relatively affluent managed-care population, universal testing for STIs is probably not necessary. When testing is indicated, it can be performed on the same day that the IUD is inserted, minimizing the number of office visits.
What is less clear is whether the same protocol can be applied to a population with a significantly higher prevalence of STIs. In such a population, universal screening for gonorrhea and chlamydia may be more prudent. However, screening still can be performed on the same day as IUD insertion.
In a primate model of intra-amniotic infection with Ureaplasma, maternal azithromycin prolonged gestation
Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207(6):475.e1–e14.
Grigsby and colleagues assessed the efficacy of azithromycin—with and without anti-inflammatory agents—in delaying preterm birth and minimizing fetal lung injury in a primate model. They found that azithromycin significantly prolonged gestation.
Details of the study
The study involved 16 chronically instrumented rhesus monkeys who received intra-amniotic inoculation with Ureaplasma parvum (107 colony-forming units/mL) and were then observed. When contractions began, as they invariably did, six monkeys received no treatment, five received intravenous (IV) azithromycin (12.5 mg/kg every 12 hours) for 10 days, and five received azithromycin plus dexamethasone and indomethacin.
Key outcome measures were the intra-amniotic concentration of proinflammatory mediators, the frequency of positive amniotic fluid cultures for U parvum, and the extent of histologic fetal lung injury.
In treated animals, the mean (SD) inoculation-to-delivery interval was 20.9 (1.4) days, compared with 13.7 (2.5) days in untreated monkeys (P <.05).
In addition, there was a twofold to threefold increase in the percentage of undelivered animals at 18 to 20 days after inoculation in the treatment group, compared with the no-treatment group. Treatment also significantly decreased the Ureaplasma colony count in the amniotic fluid, effectively eliminating the organism within 4 days.
In both treatment groups, the amniotic fluid concentration of proinflammatory mediators decreased significantly, compared with the untreated group. Treatment also significantly reduced the magnitude of deleterious histologic changes in the fetal lungs.
Somewhat surprisingly, dexamethasone and indomethacin did not enhance the treatment effect of azithromycin. Moreover, despite prolongation of pregnancy, all animals in the treatment group still delivered prematurely.
Why treatment should target genital mycoplasmas
Chorioamnionitis is an importance cause of preterm labor and preterm delivery. The principal pathogens are part of the normal vaginal flora: aerobic Gram-negative bacilli, aerobic Gram-positive cocci, anaerobes, and genital mycoplasmas.
Most treatment regimens for chorioamnionitis (eg, ampicillin plus gentamicin) do not specifically target the genital mycoplasmas. However, the most commonly recommended prophylactic antibiotic regimens for patients with preterm PROM include agents with specific action against mycoplasmas, namely erythromycin and azithromycin.
In this clinical setting, antibiotic prophylaxis prolongs the latency period and decreases the frequency of both maternal and fetal/neonatal infection.
This elegant basic science investigation sheds new light on the importance of the genital mycoplasmas in the pathogenesis of preterm labor and helps to explain why drugs like erythromycin and azithromycin may be so valuable in prolonging the latent period and reducing the frequency of infection and injury in the baby.
What this EVIDENCE means for practice
Because IV azithromycin rapidly achieved inhibitory concentrations in amniotic fluid and maintained these concentrations over 10 days of treatment, it significantly reduced the concentration of Ureaplasma in the amniotic fluid as well as the risk of histologic injury to the fetal lung.
Accordingly, I recommend that azithromycin remain a key component of the prophylactic regimen for patients with preterm PROM. It also may be advisable to add azithromycin to the usual combination of ampicillin plus gentamicin for empiric treatment of chorioamnionitis.
Daily bathing with chlorhexidine cloths can protect hospitalized patients from serious infection
Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–542.
This multicenter, randomized, nonblinded trial of 7,727 seriously ill patients sought to determine whether daily bathing with chlorhexidine-impregnated washcloths can decrease the acquisition of multidrug-resistant organisms and the incidence of hospital-acquired bloodstream infection.
Each day, patients in eight ICUs and one bone-marrow transplant unit bathed themselves, or were bathed by nursing staff, with 2% chlorhexidine-impregnated cloths or non–antimicrobial washcloths. All body surfaces except the face were cleansed. After 6 months, each unit changed to the other method of bathing.
Investigators focused on two outcomes:
- the prevalence of colonization of the nares with methicillin-resistant Staphylococcus aureus (MRSA) or colonization of the perirectal area with vancomycin-resistant enterococci (VRE)
- the frequency of hospital-acquired bloodstream infection (bacterial or fungal) detected more than 48 hours after admission to the unit.
The overall rate of MRSA or VRE acquisition was reduced by 23% when patients were bathed with chlorhexidine (5.10 versus 6.60 cases per 100 patient-days; P = .03). The overall rate of hospital-acquired bloodstream infection was reduced by 28% during the intervention period (4.78 vs 6.60 cases per 1,000 patient-days; P = .006).
In particular, the rate of central-catheter–associated bloodstream infection was 53% lower during the intervention (1.55 vs 3.30 cases per 1,000 catheter-days; P = .004).
The intervention had the greatest impact on infections caused by Gram-positive and fungal organisms.
The protective effect of chlorhexidine bathing was greatest among patients who had the longest length of stay in the unit.
Chlorhexidine did not cause an increased frequency of skin reactions. Moreover, use of the antiseptic washes did not cause the emergence of MRSA or VRA isolates with high-level resistance.
This study is of great interest in light of a recent report that demonstrated that preoperative preparation of the skin with chlorhexidine was more effective than preparation with povidone-iodine in reducing the risk of surgical-site infections after major operative procedures.1 Not only is chlorhexidine highly active against the usual bacteria that colonize the skin of hospitalized patients, it also has residual antibacterial activity that further decreases the colonization of the patient’s skin by microbes.
What this EVIDENCE means for practice
This study has two clear implications for ObGyns. First, chlorhexidine washes should be used by all patients who are scheduled for surgery, particularly those undergoing procedures that carry a relatively high risk of postoperative wound infection, such as total abdominal hysterectomy, radical hysterectomy, and cesarean delivery. In morbidly obese patients, particular attention should be directed to the skin beneath the abdominal panniculus.
Second, when we have seriously ill obstetric or gynecologic patients, especially those with long-term indwelling catheters who require prolonged hospitalization, we should order daily bathing (excluding the face) with chlorhexidine.
Reference
1. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
Reference
1. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
UPDATE: INFECTIOUS DISEASE
- 10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Meghan O. Schimpf (June 2012) - Gaps in Chlamydia testing threaten reproductive health, CDC warns
Janelle Yates, Senior Editor (Web exclusive, May 2012)
Dr. Duff reports no financial relationships relevant to this article.
In this Update, I’ve highlighted four interesting articles about infectious disease management in obstetric and gyn practice that appeared in the medical literature over the past 12 months:
- One describes a study that reminds physicians of the importance of an unusual manifestation of gonococcal infection
- A second article demonstrates the importance of making a change in the prophylactic antibiotic regimen provided to morbidly obese patients who are having a cesarean delivery
- A third describes an exciting development in the treatment of chronic hepatitis C virus infection
- The final article makes interesting observations about the proper duration of treatment for patients who have chorioamnionitis.
N gonorrhoeae causes illness beyond the urogenital tract
Bleich AT, Sheffield JS, Wendel GD, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119(3):597–602.
This article describes a retrospective review of 112 women who were admitted to Parkland Memorial Hospital in Dallas, Texas, from January 1975 through December 2008 and given a diagnosis of disseminated infection with Neisseria gonorrhoeae. Eighty (71%) of these women were not pregnant and were cared for on the internal medicine service; 32 (29%) were pregnant and were treated by faculty members and residents on the ObGyn service.
Over the course of the study, the frequency of disseminated gonococcal infection decreased significantly. Among pregnant women, the rate of infection was 11 for every 100,000 deliveries before 1980 and, after 1985, five for every 100,000 deliveries.
The most common clinical manifestation of disseminated gonococcal infection was arthritis. The most commonly affected joints were the knee, wrist, elbow, and ankle.
Other common clinical manifestations included dermatitis, fever, chills, and a purulent cervical discharge. Notably, the frequency of a purulent joint effusion was 50% in pregnant women and 70% in nonpregnant women—reflecting the fact that the duration of symptoms was approximately 3 days shorter in pregnant women than in nonpregnant women. Otherwise, the clinical presentation in pregnant women did not differ significantly from that of nonpregnant women.
In addition, the clinical course and the response to intravenous (IV) antibiotic therapy did not differ significantly between pregnant and nonpregnant women.
The authors were unable to document that disseminated gonococcal infection had any deleterious effect on the outcome of pregnancy among the patients studied. Although four of the 32 women delivered preterm, in only one instance was delivery related temporally to the disseminated gonococcal infection.
Commentary
Because of their experience treating women who have gonorrhea, I would say that most ObGyns think of N gonorrhoeae as causing localized infection in the lower genital tract (urethritis, endocervicitis, inflammatory proctitis) or upper genital tract (pelvic inflammatory disease). We should recognize, however, that gonorrhea also can cause prominent extra-pelvic findings, such as severe pharyngitis (in patients who practice orogenital intercourse) and perihepatitis (Fitz-Hugh-Curtis syndrome).
In addition, always bear in mind that, in rare instances, gonorrhea can become disseminated, causing quite serious illness. The most common extra-pelvic manifestation of disseminated gonococcal infection is arthritis. As noted in this study of a series of patients, the arthritis is usually polyarticular and affects medium or small joints.
The second most common manifestation of disseminated gonococcal infection is dermatitis. Characteristic lesions are raised, red or purple papules. These lesions are not a simple vasculitis; rather, they contain a high concentration of microorganisms.
Other possible manifestations of disseminated infection include pericarditis, endocarditis, and meningitis.
The diagnosis of disseminated gonococcal infection is usually made by clinical examination and culture of specimens from the genital tract, blood, or joint effusion.
Disseminated gonococcal infection usually responds promptly to intravenous antibiotic therapy.
Recommended therapy is ceftriaxone:
• 25 to 50 mg/kg/d IV for 7 days
or
• a single, daily, 25 to 50 mg/kg intramuscular dose, also for 7 days.
Continue therapy for 10 to 14 days if the patient has meningitis.
An alternative regimen is cefotaxime:
• 25 mg/kg/d IV for 7 days
or
• 25 mg/kg IM every 12 hours, also for 7 days.
Extend treatment for 10 to 14 days if meningitis is present.1
Obesity curtails effectiveness of antibiotic prophylaxis in cesarean delivery
Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
In this prospective study of the influence of an obese habitus on antibiotic prophylaxis during cesarean delivery, researchers divided 29 patients who were scheduled for cesarean into three groups, by body mass index (BMI):
- lean (BMI, <30; n = 10)
- obese (30–39.9; n = 10)
- extremely obese (>40; n = 9).
All patients were given a 2-g dose of IV cefazolin 30 to 60 minutes before surgery.
During delivery, the team took two specimens of adipose tissue: one immediately after the skin incision and one later, after fascia was closed. They also obtained a specimen of myometrial tissue after delivery and a blood specimen after surgery was completed.
The concentration of cefazolin was then measured in adipose and myometrial tissue and in serum.
Findings. The researchers demonstrated that the mean concentration of cefazolin in the initial specimen of adipose tissue was significantly higher in lean patients than in obese and extremely obese patients. All 10 women who had a BMI less than 30 had a serum cefazolin concentration greater than 4 μg/g—the theoretical break-point for defining resistance to cefazolin. The initial adipose tissue specimen from two of the 10 obese patients and three of the nine extremely obese patients showed cefazolin concentrations less than 4 μg/g.
Of particular interest, two women—both of whom had a BMI greater than 40—developed a wound infection that required antibiotic therapy. Their initial and subsequent adipose tissue concentrations of cefazolin were less than the 4 μg/g break-point for resistance.
The concentration of cefazolin in the patients’ myometrial and serum specimens demonstrated a pattern similar to what the researchers observed in adipose tissue, but these results were not statistically significant across BMI groups. In fact, the cefazolin concentration in all groups’ myometrial and serum specimens exceeded the minimum inhibitory concentration for most potential pathogens in the setting of cesarean delivery.
Commentary
Clearly, prophylactic antibiotics are indicated for all women who are having a cesarean delivery. Antibiotics have their greatest impact when administered before the surgical incision is made; to exert their full protective effect against endometritis and wound infection, however, antibiotics should reach a recognized therapeutic concentration—not only in serum and myometrium but in the subcutaneous tissue.
The customary dosage of cefazolin for cesarean delivery prophylaxis has been 1 g. This study demonstrated that, although a 2-g dose of cefazolin reached a therapeutic concentration in myometrial tissue and serum, it did not consistently do so in the adipose tissue of obese and extremely obese patients.
Pending further investigation, I strongly recommend that all women who have a BMI greater than 30 receive a 2-g dose of cefazolin 30 to 60 minutes before cesarean delivery. Future research is needed to determine whether an even higher dosage is necessary to achieve a therapeutic concentration in the subcutaneous tissue of morbidly obese patients.
New therapies promise a better outcome in hepatitis C
Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416.
The authors conducted an international Phase-3, randomized, double-blind, placebo-controlled trial of two different treatment modalities for chronic hepatitis C virus (HCV) infection. The authors assigned 1,088 patients who had HCV genotype-1 infection and who had not received prior therapy to one of three treatment groups:
- telaprevir (Incivek, Vertex Pharmaceuticals), an HCV genotype-1 protease inhibitor, combined with peginterferon alfa-2a (Pegasys, Genetech) plus ribavirin (Copegus, Genetech; Rebetol, Merck; etc.) for 12 weeks; patients then were given peginterferon alfa-2a plus ribavirin only for 12 additional weeks if HCV RNA was undetectable at weeks 4 and 12 or peginterferon alfa-2a plus ribavirin only for 36 weeks if HCV RNA was detectable at either time point (Group 1)
- telaprevir with peginterferon alfa-2a plus ribavirin for 8 weeks, then placebo with peginterferon alfa-2a plus ribavirin for 4 weeks, followed by 12 to 36 weeks of peginterferon alfa-2a plus ribavirin using the HCV RNA criteria applied to Group 1 (Group 2)
- placebo with peginterferon alfa-2a plus ribavirin for 12 weeks, followed by 36 weeks of peginterferon alfa-2a plus ribavirin (Group 3).
The primary endpoint of the trial was the percentage of patients who had undetectable plasma HCV RNA at 24 weeks after the last planned dose of the study drugs. The investigators considered that this endpoint represented a sustained virologic response.
Findings. Seventy-five percent of patients in Group 1 and 69% of those in Group 2 had a sustained virologic response. By comparison, only 44% of patients in Group 3 had a sustained response. The differences in outcome between Group 1 and Group 3, and between Group 2 and Group 3, were highly significant (P<.001). Virologic failure was more common among patients who had HCV genotype-1a infection than among those who had HCV genotype-1b infection.
The most common side effects noted by patients who received telaprevir were gastrointestinal irritation, rash, and anemia. Ten percent of patients in the telaprevir group discontinued therapy, compared with 7% in the peginterferon-ribavirin-alone group.
Commentary
Worldwide, approximately 170 million people have chronic hepatitis C, which is the most common indication for liver transplantation. Until recently, the principal treatments for hepatitis C were pegylated interferon alfa with ribavirin and without ribavirin; the response rate with these regimens was in the range of 55%. This study shows that adding telaprevir to regimens for HCV infection significantly improves prospects for long-term resolution of infection.
In some obstetric and gynecologic populations, HCV is more common than hepatitis B virus. Risk factors for hepatitis C include hepatitis B, intravenous drug abuse, and human immunodeficiency virus infection. HCV-infected women pose a risk to their sex partners; infected pregnant women can transmit the virus to their baby.
Unlike hepatitis A and hepatitis B, immunoprophylaxis is not available for hepatitis C. That reality is what makes the study by Jacobsen and colleagues so compelling: They have clearly demonstrated that multi-agent antiviral therapy might be able to truly cure this infection.
The lesson here for ObGyns? Screen at-risk patients and then refer the hepatitis C-seropositive ones to a specialist in gastroenterology, who can determine candidacy for one of the new treatment regimens.
Clearly, the prognosis for people who have hepatitis C is much better today than it was 20 years ago.
For how long should chorioamnionitis be treated?
Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119(6):1102-1105.
The authors conducted a retrospective review of 423 women who had been treated for chorioamnionitis at the University of Florida from 2005 to 2009.
Patients had been given IV ampicillin (2 g every 6 h) plus IV gentamicin (1.5 mg/kg every 8 h) as soon as the diagnosis of chorioamnionitis was established; postpartum, they were given only the one next scheduled dose of each antibiotic. Patients who had a cesarean received either metronidazole (500 mg) or clindamycin (900 mg) immediately after cord clamping to enhance coverage of anaerobic organisms.
The primary outcome was treatment failure, defined as persistent fever requiring continued antibiotics, surgical intervention, or administration of heparin for septic pelvic-vein thrombophlebitis.
Findings. Here is a breakdown of what the investigators found regarding the 282 women who delivered vaginally and the 141 who underwent cesarean delivery:
- Overall, 399 of the patients (94%; 95% confidence interval [CI], 92% and 96%) were treated successfully; 24 (6%; 95% CI, 3.7% and 8.3%) failed short-course treatment
- Of the 282 patients who delivered vaginally, 279 (99%; 95% CI, 98% and 100%) were cured with short-term therapy
- Of the 141 who delivered by cesarean, 120 (85%; 95% CI, 79% and 91%) were cured (P<.001).
- Seventeen of the total treatment failures had endometritis and responded quickly to continuation of antibiotics. Of the 17 patients with endometritis, 14 had a cesarean delivery.
- Seven patients had more serious complications: four, wound infection; three, septic pelvic-vein thrombophlebitis. All serious complications occurred after cesarean delivery.
- Of the four patients who had a wound infection, three had labor induced by misoprostol; their BMI was 44.8, 31.1, and 48.5, respectively. The fourth had a cesarean delivery at 29 weeks for preterm premature rupture of membranes (PPROM), chorioamnionitis, and malpresentation.
- Of the three patients who had septic pelvic-vein thrombophlebitis, two had labor induced by misoprostol. One had a BMI of 29.2; the other, 31.1. The third patient was delivered secondary to PPROM; her BMI was 40.3.
In addition, of the 21 treatment failures in the cesarean delivery group, 6 had prolonged rupture of membranes (ROM) and 10 had a BMI greater than 30. Six patients had both prolonged ROM and were obese or morbidly obese.
Of the 120 women who had a cesarean delivery and were treated successfully, 3 had prolonged ROM and 39 had a BMI greater than 30. None had both prolonged ROM and a BMI greater than 30.
Last, the difference between treatment failures and treatment successes in regard to the frequency of prolonged ROM or a BMI greater than 30 was highly significant (P<.01).
Commentary
In most published reports of patients who have chorioamnionitis, antibiotic treatment continues until the patient is afebrile and asymptomatic for 24 to 48 hours. This treatment approach has been based largely on expert opinion, however, not on Level-1 or Level-2 evidence.
In 2003, Edwards and Duff published a study of chorioamnionitis antibiotic regimens that compared single-dose postpartum treatment to extended treatment.2 This randomized controlled trial demonstrated that there was no statistically significant difference between patients who had only a single dose of postpartum antibiotics and those who received an extended course of medication (i.e., who were treated until they had been afebrile and asymptomatic for a minimum of 24 hours) in regard to adverse outcomes (2.9% and 4.3%, respectively). The study discussed here extends and refines the observations made in the 2003 Edwards and Duff randomized controlled trial.
The new study shows that a limited course of antibiotics was, overall, effective in treating 94% of patients with chorioamnionitis (95% CI, 92% and 96%). Only 1% of patients who delivered vaginally failed therapy, compared with 15% of patients who delivered by cesarean (P<.001). In the cesarean group, women who failed therapy were likely to 1) be obese or 2) have a relatively long duration of labor or ruptured membranes, or both. These patients may have benefitted from a more extended course of antibiotic therapy.
Based on this investigation, I strongly recommend a limited course of antibiotic therapy (ampicillin plus gentamicin) for women with chorioamnionitis who deliver vaginally. Patients who have had a cesarean delivery—particularly those who are obese or have had an extended duration of labor, or both—should be treated with antibiotics until they have been afebrile and asymptomatic for 24 hours.
We want to hear from you! Tell us what you think.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Edwards RK, Duff P. Single dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 Pt 1):957-961.
- 10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Meghan O. Schimpf (June 2012) - Gaps in Chlamydia testing threaten reproductive health, CDC warns
Janelle Yates, Senior Editor (Web exclusive, May 2012)
Dr. Duff reports no financial relationships relevant to this article.
In this Update, I’ve highlighted four interesting articles about infectious disease management in obstetric and gyn practice that appeared in the medical literature over the past 12 months:
- One describes a study that reminds physicians of the importance of an unusual manifestation of gonococcal infection
- A second article demonstrates the importance of making a change in the prophylactic antibiotic regimen provided to morbidly obese patients who are having a cesarean delivery
- A third describes an exciting development in the treatment of chronic hepatitis C virus infection
- The final article makes interesting observations about the proper duration of treatment for patients who have chorioamnionitis.
N gonorrhoeae causes illness beyond the urogenital tract
Bleich AT, Sheffield JS, Wendel GD, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119(3):597–602.
This article describes a retrospective review of 112 women who were admitted to Parkland Memorial Hospital in Dallas, Texas, from January 1975 through December 2008 and given a diagnosis of disseminated infection with Neisseria gonorrhoeae. Eighty (71%) of these women were not pregnant and were cared for on the internal medicine service; 32 (29%) were pregnant and were treated by faculty members and residents on the ObGyn service.
Over the course of the study, the frequency of disseminated gonococcal infection decreased significantly. Among pregnant women, the rate of infection was 11 for every 100,000 deliveries before 1980 and, after 1985, five for every 100,000 deliveries.
The most common clinical manifestation of disseminated gonococcal infection was arthritis. The most commonly affected joints were the knee, wrist, elbow, and ankle.
Other common clinical manifestations included dermatitis, fever, chills, and a purulent cervical discharge. Notably, the frequency of a purulent joint effusion was 50% in pregnant women and 70% in nonpregnant women—reflecting the fact that the duration of symptoms was approximately 3 days shorter in pregnant women than in nonpregnant women. Otherwise, the clinical presentation in pregnant women did not differ significantly from that of nonpregnant women.
In addition, the clinical course and the response to intravenous (IV) antibiotic therapy did not differ significantly between pregnant and nonpregnant women.
The authors were unable to document that disseminated gonococcal infection had any deleterious effect on the outcome of pregnancy among the patients studied. Although four of the 32 women delivered preterm, in only one instance was delivery related temporally to the disseminated gonococcal infection.
Commentary
Because of their experience treating women who have gonorrhea, I would say that most ObGyns think of N gonorrhoeae as causing localized infection in the lower genital tract (urethritis, endocervicitis, inflammatory proctitis) or upper genital tract (pelvic inflammatory disease). We should recognize, however, that gonorrhea also can cause prominent extra-pelvic findings, such as severe pharyngitis (in patients who practice orogenital intercourse) and perihepatitis (Fitz-Hugh-Curtis syndrome).
In addition, always bear in mind that, in rare instances, gonorrhea can become disseminated, causing quite serious illness. The most common extra-pelvic manifestation of disseminated gonococcal infection is arthritis. As noted in this study of a series of patients, the arthritis is usually polyarticular and affects medium or small joints.
The second most common manifestation of disseminated gonococcal infection is dermatitis. Characteristic lesions are raised, red or purple papules. These lesions are not a simple vasculitis; rather, they contain a high concentration of microorganisms.
Other possible manifestations of disseminated infection include pericarditis, endocarditis, and meningitis.
The diagnosis of disseminated gonococcal infection is usually made by clinical examination and culture of specimens from the genital tract, blood, or joint effusion.
Disseminated gonococcal infection usually responds promptly to intravenous antibiotic therapy.
Recommended therapy is ceftriaxone:
• 25 to 50 mg/kg/d IV for 7 days
or
• a single, daily, 25 to 50 mg/kg intramuscular dose, also for 7 days.
Continue therapy for 10 to 14 days if the patient has meningitis.
An alternative regimen is cefotaxime:
• 25 mg/kg/d IV for 7 days
or
• 25 mg/kg IM every 12 hours, also for 7 days.
Extend treatment for 10 to 14 days if meningitis is present.1
Obesity curtails effectiveness of antibiotic prophylaxis in cesarean delivery
Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
In this prospective study of the influence of an obese habitus on antibiotic prophylaxis during cesarean delivery, researchers divided 29 patients who were scheduled for cesarean into three groups, by body mass index (BMI):
- lean (BMI, <30; n = 10)
- obese (30–39.9; n = 10)
- extremely obese (>40; n = 9).
All patients were given a 2-g dose of IV cefazolin 30 to 60 minutes before surgery.
During delivery, the team took two specimens of adipose tissue: one immediately after the skin incision and one later, after fascia was closed. They also obtained a specimen of myometrial tissue after delivery and a blood specimen after surgery was completed.
The concentration of cefazolin was then measured in adipose and myometrial tissue and in serum.
Findings. The researchers demonstrated that the mean concentration of cefazolin in the initial specimen of adipose tissue was significantly higher in lean patients than in obese and extremely obese patients. All 10 women who had a BMI less than 30 had a serum cefazolin concentration greater than 4 μg/g—the theoretical break-point for defining resistance to cefazolin. The initial adipose tissue specimen from two of the 10 obese patients and three of the nine extremely obese patients showed cefazolin concentrations less than 4 μg/g.
Of particular interest, two women—both of whom had a BMI greater than 40—developed a wound infection that required antibiotic therapy. Their initial and subsequent adipose tissue concentrations of cefazolin were less than the 4 μg/g break-point for resistance.
The concentration of cefazolin in the patients’ myometrial and serum specimens demonstrated a pattern similar to what the researchers observed in adipose tissue, but these results were not statistically significant across BMI groups. In fact, the cefazolin concentration in all groups’ myometrial and serum specimens exceeded the minimum inhibitory concentration for most potential pathogens in the setting of cesarean delivery.
Commentary
Clearly, prophylactic antibiotics are indicated for all women who are having a cesarean delivery. Antibiotics have their greatest impact when administered before the surgical incision is made; to exert their full protective effect against endometritis and wound infection, however, antibiotics should reach a recognized therapeutic concentration—not only in serum and myometrium but in the subcutaneous tissue.
The customary dosage of cefazolin for cesarean delivery prophylaxis has been 1 g. This study demonstrated that, although a 2-g dose of cefazolin reached a therapeutic concentration in myometrial tissue and serum, it did not consistently do so in the adipose tissue of obese and extremely obese patients.
Pending further investigation, I strongly recommend that all women who have a BMI greater than 30 receive a 2-g dose of cefazolin 30 to 60 minutes before cesarean delivery. Future research is needed to determine whether an even higher dosage is necessary to achieve a therapeutic concentration in the subcutaneous tissue of morbidly obese patients.
New therapies promise a better outcome in hepatitis C
Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416.
The authors conducted an international Phase-3, randomized, double-blind, placebo-controlled trial of two different treatment modalities for chronic hepatitis C virus (HCV) infection. The authors assigned 1,088 patients who had HCV genotype-1 infection and who had not received prior therapy to one of three treatment groups:
- telaprevir (Incivek, Vertex Pharmaceuticals), an HCV genotype-1 protease inhibitor, combined with peginterferon alfa-2a (Pegasys, Genetech) plus ribavirin (Copegus, Genetech; Rebetol, Merck; etc.) for 12 weeks; patients then were given peginterferon alfa-2a plus ribavirin only for 12 additional weeks if HCV RNA was undetectable at weeks 4 and 12 or peginterferon alfa-2a plus ribavirin only for 36 weeks if HCV RNA was detectable at either time point (Group 1)
- telaprevir with peginterferon alfa-2a plus ribavirin for 8 weeks, then placebo with peginterferon alfa-2a plus ribavirin for 4 weeks, followed by 12 to 36 weeks of peginterferon alfa-2a plus ribavirin using the HCV RNA criteria applied to Group 1 (Group 2)
- placebo with peginterferon alfa-2a plus ribavirin for 12 weeks, followed by 36 weeks of peginterferon alfa-2a plus ribavirin (Group 3).
The primary endpoint of the trial was the percentage of patients who had undetectable plasma HCV RNA at 24 weeks after the last planned dose of the study drugs. The investigators considered that this endpoint represented a sustained virologic response.
Findings. Seventy-five percent of patients in Group 1 and 69% of those in Group 2 had a sustained virologic response. By comparison, only 44% of patients in Group 3 had a sustained response. The differences in outcome between Group 1 and Group 3, and between Group 2 and Group 3, were highly significant (P<.001). Virologic failure was more common among patients who had HCV genotype-1a infection than among those who had HCV genotype-1b infection.
The most common side effects noted by patients who received telaprevir were gastrointestinal irritation, rash, and anemia. Ten percent of patients in the telaprevir group discontinued therapy, compared with 7% in the peginterferon-ribavirin-alone group.
Commentary
Worldwide, approximately 170 million people have chronic hepatitis C, which is the most common indication for liver transplantation. Until recently, the principal treatments for hepatitis C were pegylated interferon alfa with ribavirin and without ribavirin; the response rate with these regimens was in the range of 55%. This study shows that adding telaprevir to regimens for HCV infection significantly improves prospects for long-term resolution of infection.
In some obstetric and gynecologic populations, HCV is more common than hepatitis B virus. Risk factors for hepatitis C include hepatitis B, intravenous drug abuse, and human immunodeficiency virus infection. HCV-infected women pose a risk to their sex partners; infected pregnant women can transmit the virus to their baby.
Unlike hepatitis A and hepatitis B, immunoprophylaxis is not available for hepatitis C. That reality is what makes the study by Jacobsen and colleagues so compelling: They have clearly demonstrated that multi-agent antiviral therapy might be able to truly cure this infection.
The lesson here for ObGyns? Screen at-risk patients and then refer the hepatitis C-seropositive ones to a specialist in gastroenterology, who can determine candidacy for one of the new treatment regimens.
Clearly, the prognosis for people who have hepatitis C is much better today than it was 20 years ago.
For how long should chorioamnionitis be treated?
Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119(6):1102-1105.
The authors conducted a retrospective review of 423 women who had been treated for chorioamnionitis at the University of Florida from 2005 to 2009.
Patients had been given IV ampicillin (2 g every 6 h) plus IV gentamicin (1.5 mg/kg every 8 h) as soon as the diagnosis of chorioamnionitis was established; postpartum, they were given only the one next scheduled dose of each antibiotic. Patients who had a cesarean received either metronidazole (500 mg) or clindamycin (900 mg) immediately after cord clamping to enhance coverage of anaerobic organisms.
The primary outcome was treatment failure, defined as persistent fever requiring continued antibiotics, surgical intervention, or administration of heparin for septic pelvic-vein thrombophlebitis.
Findings. Here is a breakdown of what the investigators found regarding the 282 women who delivered vaginally and the 141 who underwent cesarean delivery:
- Overall, 399 of the patients (94%; 95% confidence interval [CI], 92% and 96%) were treated successfully; 24 (6%; 95% CI, 3.7% and 8.3%) failed short-course treatment
- Of the 282 patients who delivered vaginally, 279 (99%; 95% CI, 98% and 100%) were cured with short-term therapy
- Of the 141 who delivered by cesarean, 120 (85%; 95% CI, 79% and 91%) were cured (P<.001).
- Seventeen of the total treatment failures had endometritis and responded quickly to continuation of antibiotics. Of the 17 patients with endometritis, 14 had a cesarean delivery.
- Seven patients had more serious complications: four, wound infection; three, septic pelvic-vein thrombophlebitis. All serious complications occurred after cesarean delivery.
- Of the four patients who had a wound infection, three had labor induced by misoprostol; their BMI was 44.8, 31.1, and 48.5, respectively. The fourth had a cesarean delivery at 29 weeks for preterm premature rupture of membranes (PPROM), chorioamnionitis, and malpresentation.
- Of the three patients who had septic pelvic-vein thrombophlebitis, two had labor induced by misoprostol. One had a BMI of 29.2; the other, 31.1. The third patient was delivered secondary to PPROM; her BMI was 40.3.
In addition, of the 21 treatment failures in the cesarean delivery group, 6 had prolonged rupture of membranes (ROM) and 10 had a BMI greater than 30. Six patients had both prolonged ROM and were obese or morbidly obese.
Of the 120 women who had a cesarean delivery and were treated successfully, 3 had prolonged ROM and 39 had a BMI greater than 30. None had both prolonged ROM and a BMI greater than 30.
Last, the difference between treatment failures and treatment successes in regard to the frequency of prolonged ROM or a BMI greater than 30 was highly significant (P<.01).
Commentary
In most published reports of patients who have chorioamnionitis, antibiotic treatment continues until the patient is afebrile and asymptomatic for 24 to 48 hours. This treatment approach has been based largely on expert opinion, however, not on Level-1 or Level-2 evidence.
In 2003, Edwards and Duff published a study of chorioamnionitis antibiotic regimens that compared single-dose postpartum treatment to extended treatment.2 This randomized controlled trial demonstrated that there was no statistically significant difference between patients who had only a single dose of postpartum antibiotics and those who received an extended course of medication (i.e., who were treated until they had been afebrile and asymptomatic for a minimum of 24 hours) in regard to adverse outcomes (2.9% and 4.3%, respectively). The study discussed here extends and refines the observations made in the 2003 Edwards and Duff randomized controlled trial.
The new study shows that a limited course of antibiotics was, overall, effective in treating 94% of patients with chorioamnionitis (95% CI, 92% and 96%). Only 1% of patients who delivered vaginally failed therapy, compared with 15% of patients who delivered by cesarean (P<.001). In the cesarean group, women who failed therapy were likely to 1) be obese or 2) have a relatively long duration of labor or ruptured membranes, or both. These patients may have benefitted from a more extended course of antibiotic therapy.
Based on this investigation, I strongly recommend a limited course of antibiotic therapy (ampicillin plus gentamicin) for women with chorioamnionitis who deliver vaginally. Patients who have had a cesarean delivery—particularly those who are obese or have had an extended duration of labor, or both—should be treated with antibiotics until they have been afebrile and asymptomatic for 24 hours.
We want to hear from you! Tell us what you think.
- 10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Meghan O. Schimpf (June 2012) - Gaps in Chlamydia testing threaten reproductive health, CDC warns
Janelle Yates, Senior Editor (Web exclusive, May 2012)
Dr. Duff reports no financial relationships relevant to this article.
In this Update, I’ve highlighted four interesting articles about infectious disease management in obstetric and gyn practice that appeared in the medical literature over the past 12 months:
- One describes a study that reminds physicians of the importance of an unusual manifestation of gonococcal infection
- A second article demonstrates the importance of making a change in the prophylactic antibiotic regimen provided to morbidly obese patients who are having a cesarean delivery
- A third describes an exciting development in the treatment of chronic hepatitis C virus infection
- The final article makes interesting observations about the proper duration of treatment for patients who have chorioamnionitis.
N gonorrhoeae causes illness beyond the urogenital tract
Bleich AT, Sheffield JS, Wendel GD, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119(3):597–602.
This article describes a retrospective review of 112 women who were admitted to Parkland Memorial Hospital in Dallas, Texas, from January 1975 through December 2008 and given a diagnosis of disseminated infection with Neisseria gonorrhoeae. Eighty (71%) of these women were not pregnant and were cared for on the internal medicine service; 32 (29%) were pregnant and were treated by faculty members and residents on the ObGyn service.
Over the course of the study, the frequency of disseminated gonococcal infection decreased significantly. Among pregnant women, the rate of infection was 11 for every 100,000 deliveries before 1980 and, after 1985, five for every 100,000 deliveries.
The most common clinical manifestation of disseminated gonococcal infection was arthritis. The most commonly affected joints were the knee, wrist, elbow, and ankle.
Other common clinical manifestations included dermatitis, fever, chills, and a purulent cervical discharge. Notably, the frequency of a purulent joint effusion was 50% in pregnant women and 70% in nonpregnant women—reflecting the fact that the duration of symptoms was approximately 3 days shorter in pregnant women than in nonpregnant women. Otherwise, the clinical presentation in pregnant women did not differ significantly from that of nonpregnant women.
In addition, the clinical course and the response to intravenous (IV) antibiotic therapy did not differ significantly between pregnant and nonpregnant women.
The authors were unable to document that disseminated gonococcal infection had any deleterious effect on the outcome of pregnancy among the patients studied. Although four of the 32 women delivered preterm, in only one instance was delivery related temporally to the disseminated gonococcal infection.
Commentary
Because of their experience treating women who have gonorrhea, I would say that most ObGyns think of N gonorrhoeae as causing localized infection in the lower genital tract (urethritis, endocervicitis, inflammatory proctitis) or upper genital tract (pelvic inflammatory disease). We should recognize, however, that gonorrhea also can cause prominent extra-pelvic findings, such as severe pharyngitis (in patients who practice orogenital intercourse) and perihepatitis (Fitz-Hugh-Curtis syndrome).
In addition, always bear in mind that, in rare instances, gonorrhea can become disseminated, causing quite serious illness. The most common extra-pelvic manifestation of disseminated gonococcal infection is arthritis. As noted in this study of a series of patients, the arthritis is usually polyarticular and affects medium or small joints.
The second most common manifestation of disseminated gonococcal infection is dermatitis. Characteristic lesions are raised, red or purple papules. These lesions are not a simple vasculitis; rather, they contain a high concentration of microorganisms.
Other possible manifestations of disseminated infection include pericarditis, endocarditis, and meningitis.
The diagnosis of disseminated gonococcal infection is usually made by clinical examination and culture of specimens from the genital tract, blood, or joint effusion.
Disseminated gonococcal infection usually responds promptly to intravenous antibiotic therapy.
Recommended therapy is ceftriaxone:
• 25 to 50 mg/kg/d IV for 7 days
or
• a single, daily, 25 to 50 mg/kg intramuscular dose, also for 7 days.
Continue therapy for 10 to 14 days if the patient has meningitis.
An alternative regimen is cefotaxime:
• 25 mg/kg/d IV for 7 days
or
• 25 mg/kg IM every 12 hours, also for 7 days.
Extend treatment for 10 to 14 days if meningitis is present.1
Obesity curtails effectiveness of antibiotic prophylaxis in cesarean delivery
Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
In this prospective study of the influence of an obese habitus on antibiotic prophylaxis during cesarean delivery, researchers divided 29 patients who were scheduled for cesarean into three groups, by body mass index (BMI):
- lean (BMI, <30; n = 10)
- obese (30–39.9; n = 10)
- extremely obese (>40; n = 9).
All patients were given a 2-g dose of IV cefazolin 30 to 60 minutes before surgery.
During delivery, the team took two specimens of adipose tissue: one immediately after the skin incision and one later, after fascia was closed. They also obtained a specimen of myometrial tissue after delivery and a blood specimen after surgery was completed.
The concentration of cefazolin was then measured in adipose and myometrial tissue and in serum.
Findings. The researchers demonstrated that the mean concentration of cefazolin in the initial specimen of adipose tissue was significantly higher in lean patients than in obese and extremely obese patients. All 10 women who had a BMI less than 30 had a serum cefazolin concentration greater than 4 μg/g—the theoretical break-point for defining resistance to cefazolin. The initial adipose tissue specimen from two of the 10 obese patients and three of the nine extremely obese patients showed cefazolin concentrations less than 4 μg/g.
Of particular interest, two women—both of whom had a BMI greater than 40—developed a wound infection that required antibiotic therapy. Their initial and subsequent adipose tissue concentrations of cefazolin were less than the 4 μg/g break-point for resistance.
The concentration of cefazolin in the patients’ myometrial and serum specimens demonstrated a pattern similar to what the researchers observed in adipose tissue, but these results were not statistically significant across BMI groups. In fact, the cefazolin concentration in all groups’ myometrial and serum specimens exceeded the minimum inhibitory concentration for most potential pathogens in the setting of cesarean delivery.
Commentary
Clearly, prophylactic antibiotics are indicated for all women who are having a cesarean delivery. Antibiotics have their greatest impact when administered before the surgical incision is made; to exert their full protective effect against endometritis and wound infection, however, antibiotics should reach a recognized therapeutic concentration—not only in serum and myometrium but in the subcutaneous tissue.
The customary dosage of cefazolin for cesarean delivery prophylaxis has been 1 g. This study demonstrated that, although a 2-g dose of cefazolin reached a therapeutic concentration in myometrial tissue and serum, it did not consistently do so in the adipose tissue of obese and extremely obese patients.
Pending further investigation, I strongly recommend that all women who have a BMI greater than 30 receive a 2-g dose of cefazolin 30 to 60 minutes before cesarean delivery. Future research is needed to determine whether an even higher dosage is necessary to achieve a therapeutic concentration in the subcutaneous tissue of morbidly obese patients.
New therapies promise a better outcome in hepatitis C
Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416.
The authors conducted an international Phase-3, randomized, double-blind, placebo-controlled trial of two different treatment modalities for chronic hepatitis C virus (HCV) infection. The authors assigned 1,088 patients who had HCV genotype-1 infection and who had not received prior therapy to one of three treatment groups:
- telaprevir (Incivek, Vertex Pharmaceuticals), an HCV genotype-1 protease inhibitor, combined with peginterferon alfa-2a (Pegasys, Genetech) plus ribavirin (Copegus, Genetech; Rebetol, Merck; etc.) for 12 weeks; patients then were given peginterferon alfa-2a plus ribavirin only for 12 additional weeks if HCV RNA was undetectable at weeks 4 and 12 or peginterferon alfa-2a plus ribavirin only for 36 weeks if HCV RNA was detectable at either time point (Group 1)
- telaprevir with peginterferon alfa-2a plus ribavirin for 8 weeks, then placebo with peginterferon alfa-2a plus ribavirin for 4 weeks, followed by 12 to 36 weeks of peginterferon alfa-2a plus ribavirin using the HCV RNA criteria applied to Group 1 (Group 2)
- placebo with peginterferon alfa-2a plus ribavirin for 12 weeks, followed by 36 weeks of peginterferon alfa-2a plus ribavirin (Group 3).
The primary endpoint of the trial was the percentage of patients who had undetectable plasma HCV RNA at 24 weeks after the last planned dose of the study drugs. The investigators considered that this endpoint represented a sustained virologic response.
Findings. Seventy-five percent of patients in Group 1 and 69% of those in Group 2 had a sustained virologic response. By comparison, only 44% of patients in Group 3 had a sustained response. The differences in outcome between Group 1 and Group 3, and between Group 2 and Group 3, were highly significant (P<.001). Virologic failure was more common among patients who had HCV genotype-1a infection than among those who had HCV genotype-1b infection.
The most common side effects noted by patients who received telaprevir were gastrointestinal irritation, rash, and anemia. Ten percent of patients in the telaprevir group discontinued therapy, compared with 7% in the peginterferon-ribavirin-alone group.
Commentary
Worldwide, approximately 170 million people have chronic hepatitis C, which is the most common indication for liver transplantation. Until recently, the principal treatments for hepatitis C were pegylated interferon alfa with ribavirin and without ribavirin; the response rate with these regimens was in the range of 55%. This study shows that adding telaprevir to regimens for HCV infection significantly improves prospects for long-term resolution of infection.
In some obstetric and gynecologic populations, HCV is more common than hepatitis B virus. Risk factors for hepatitis C include hepatitis B, intravenous drug abuse, and human immunodeficiency virus infection. HCV-infected women pose a risk to their sex partners; infected pregnant women can transmit the virus to their baby.
Unlike hepatitis A and hepatitis B, immunoprophylaxis is not available for hepatitis C. That reality is what makes the study by Jacobsen and colleagues so compelling: They have clearly demonstrated that multi-agent antiviral therapy might be able to truly cure this infection.
The lesson here for ObGyns? Screen at-risk patients and then refer the hepatitis C-seropositive ones to a specialist in gastroenterology, who can determine candidacy for one of the new treatment regimens.
Clearly, the prognosis for people who have hepatitis C is much better today than it was 20 years ago.
For how long should chorioamnionitis be treated?
Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119(6):1102-1105.
The authors conducted a retrospective review of 423 women who had been treated for chorioamnionitis at the University of Florida from 2005 to 2009.
Patients had been given IV ampicillin (2 g every 6 h) plus IV gentamicin (1.5 mg/kg every 8 h) as soon as the diagnosis of chorioamnionitis was established; postpartum, they were given only the one next scheduled dose of each antibiotic. Patients who had a cesarean received either metronidazole (500 mg) or clindamycin (900 mg) immediately after cord clamping to enhance coverage of anaerobic organisms.
The primary outcome was treatment failure, defined as persistent fever requiring continued antibiotics, surgical intervention, or administration of heparin for septic pelvic-vein thrombophlebitis.
Findings. Here is a breakdown of what the investigators found regarding the 282 women who delivered vaginally and the 141 who underwent cesarean delivery:
- Overall, 399 of the patients (94%; 95% confidence interval [CI], 92% and 96%) were treated successfully; 24 (6%; 95% CI, 3.7% and 8.3%) failed short-course treatment
- Of the 282 patients who delivered vaginally, 279 (99%; 95% CI, 98% and 100%) were cured with short-term therapy
- Of the 141 who delivered by cesarean, 120 (85%; 95% CI, 79% and 91%) were cured (P<.001).
- Seventeen of the total treatment failures had endometritis and responded quickly to continuation of antibiotics. Of the 17 patients with endometritis, 14 had a cesarean delivery.
- Seven patients had more serious complications: four, wound infection; three, septic pelvic-vein thrombophlebitis. All serious complications occurred after cesarean delivery.
- Of the four patients who had a wound infection, three had labor induced by misoprostol; their BMI was 44.8, 31.1, and 48.5, respectively. The fourth had a cesarean delivery at 29 weeks for preterm premature rupture of membranes (PPROM), chorioamnionitis, and malpresentation.
- Of the three patients who had septic pelvic-vein thrombophlebitis, two had labor induced by misoprostol. One had a BMI of 29.2; the other, 31.1. The third patient was delivered secondary to PPROM; her BMI was 40.3.
In addition, of the 21 treatment failures in the cesarean delivery group, 6 had prolonged rupture of membranes (ROM) and 10 had a BMI greater than 30. Six patients had both prolonged ROM and were obese or morbidly obese.
Of the 120 women who had a cesarean delivery and were treated successfully, 3 had prolonged ROM and 39 had a BMI greater than 30. None had both prolonged ROM and a BMI greater than 30.
Last, the difference between treatment failures and treatment successes in regard to the frequency of prolonged ROM or a BMI greater than 30 was highly significant (P<.01).
Commentary
In most published reports of patients who have chorioamnionitis, antibiotic treatment continues until the patient is afebrile and asymptomatic for 24 to 48 hours. This treatment approach has been based largely on expert opinion, however, not on Level-1 or Level-2 evidence.
In 2003, Edwards and Duff published a study of chorioamnionitis antibiotic regimens that compared single-dose postpartum treatment to extended treatment.2 This randomized controlled trial demonstrated that there was no statistically significant difference between patients who had only a single dose of postpartum antibiotics and those who received an extended course of medication (i.e., who were treated until they had been afebrile and asymptomatic for a minimum of 24 hours) in regard to adverse outcomes (2.9% and 4.3%, respectively). The study discussed here extends and refines the observations made in the 2003 Edwards and Duff randomized controlled trial.
The new study shows that a limited course of antibiotics was, overall, effective in treating 94% of patients with chorioamnionitis (95% CI, 92% and 96%). Only 1% of patients who delivered vaginally failed therapy, compared with 15% of patients who delivered by cesarean (P<.001). In the cesarean group, women who failed therapy were likely to 1) be obese or 2) have a relatively long duration of labor or ruptured membranes, or both. These patients may have benefitted from a more extended course of antibiotic therapy.
Based on this investigation, I strongly recommend a limited course of antibiotic therapy (ampicillin plus gentamicin) for women with chorioamnionitis who deliver vaginally. Patients who have had a cesarean delivery—particularly those who are obese or have had an extended duration of labor, or both—should be treated with antibiotics until they have been afebrile and asymptomatic for 24 hours.
We want to hear from you! Tell us what you think.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Edwards RK, Duff P. Single dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 Pt 1):957-961.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Edwards RK, Duff P. Single dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 Pt 1):957-961.