User login
Preventing early-onset group B streptococcal disease in newborns
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
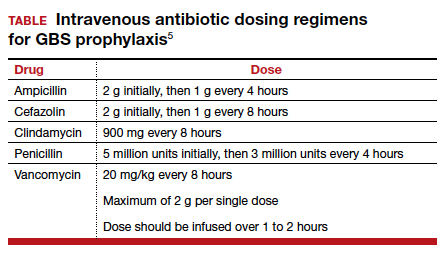
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.
5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
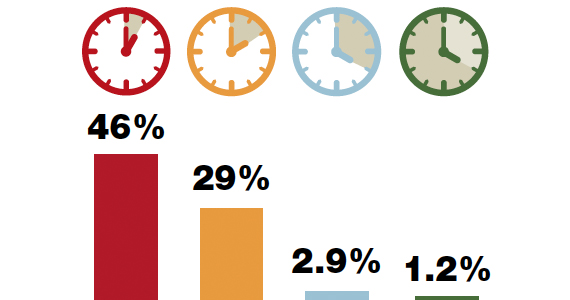
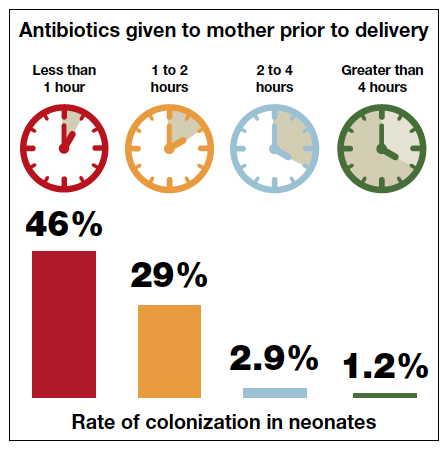
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.
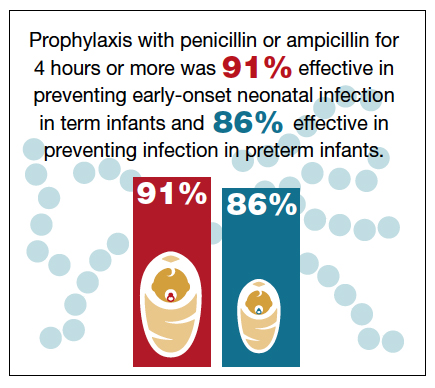
Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.
These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
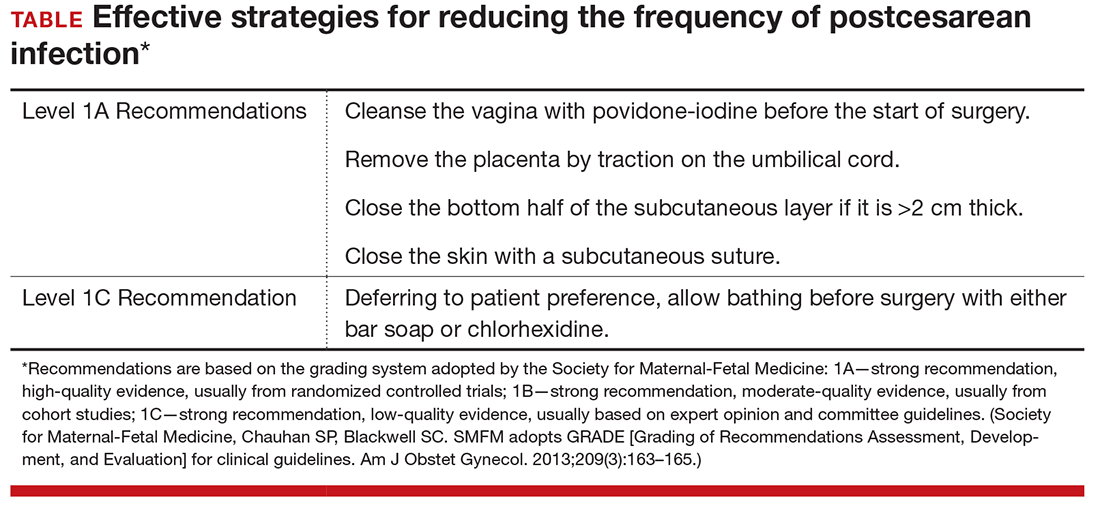
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.

5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.

Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.

These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.

5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.

Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.

These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
Considerations on the mode of delivery for pregnant women with hepatitis C infection
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
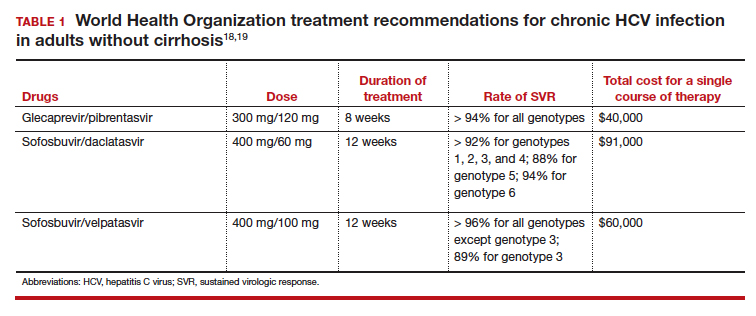
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11
Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
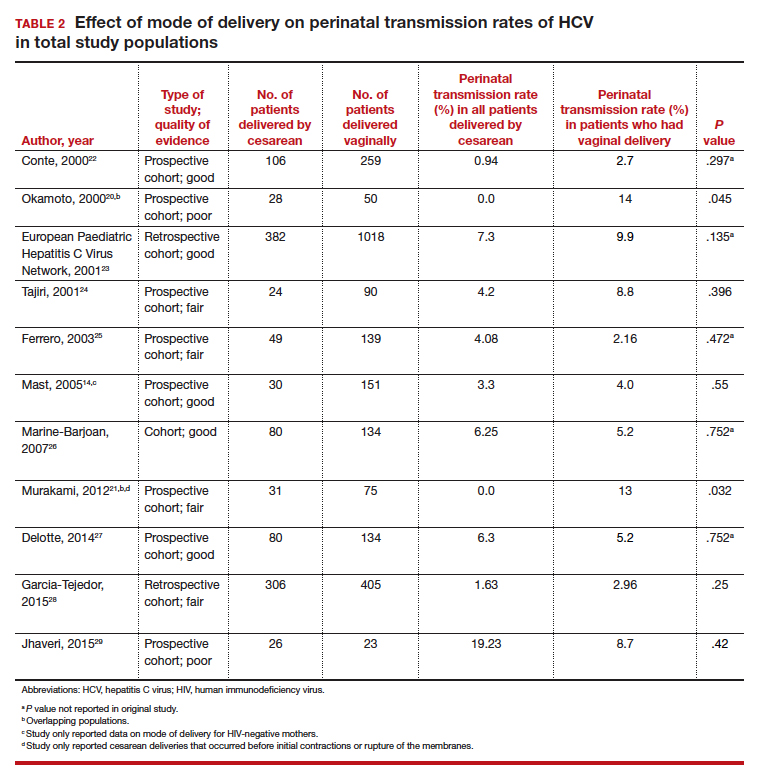
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
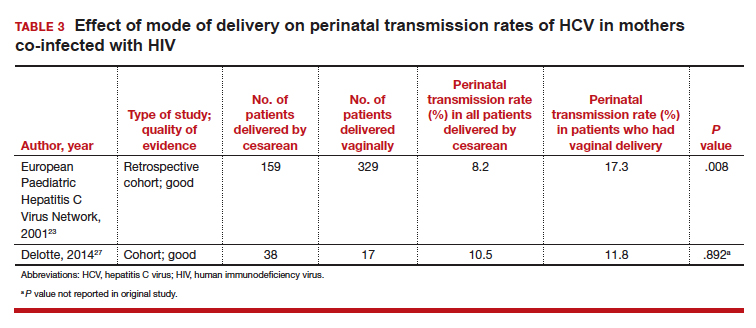
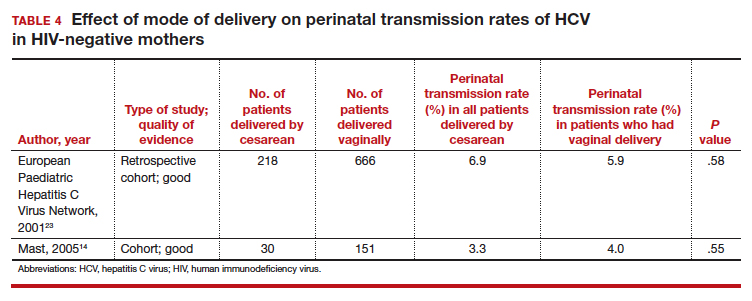
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
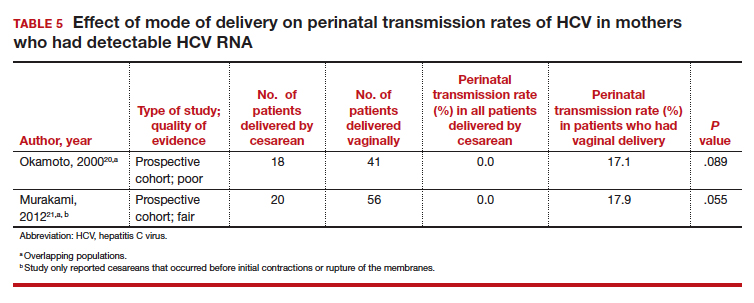
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
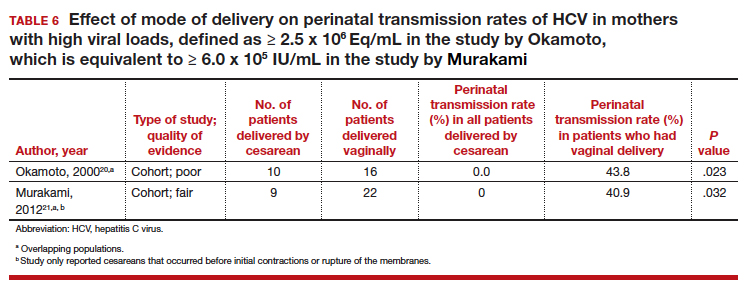
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21
Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
Pre-exposure prophylaxis for the prevention of HIV infection: Ready for prime time
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
2018 Update on infectious disease
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.
How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
IN THIS ARTICLE
- Vaginal cleansing before CD lowers risk of postop endometritis
- Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
- Antibiotic use, common in the obstetric population, raises risk for C difficile infection
- Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Which antibiotics should be used with caution in pregnant women with UTI?
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
Should we stop administering the influenza vaccine to pregnant women?
EXPERT COMMENTARY
Influenza can be a serious, even life-threatening infection, especially in pregnant women and their newborn infants.1 For that reason, the Centers for Disease Control and Prevention (CDC) and the American College of Obstetricians and Gynecologists (ACOG) strongly recommend that all pregnant women receive the inactivated influenza vaccine at the start of each flu season, regardless of trimester of exposure.
The most widely-used vaccine in the United States is the inactivated quadrivalent vaccine, which is intended for intramuscular administration in a single dose. The 2017-2018 version of this vaccine includes 2 influenza A antigens and 2 influenza B antigens. The first of the A antigens differs from last year's vaccine. The other 3 antigens are the same as in the 2016-2017 vaccine:
- A/Michigan/45/2015 (H1N1) pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus
- B/Phuket/3073/2013-like virus (B/Yamagota)
Several recent reports in large, diverse populations2-5 have demonstrated that the vaccine does not increase the risk of spontaneous abortion, stillbirth, preterm delivery, or congenital anomalies. Therefore, a recent report by Donahue and colleagues6 is surprising and is certainly worthy of our attention.
Related article:
Does influenza immunization during pregnancy confer flu protection to newborns?
Details of the study
Donahue and colleagues, experienced investigators, were tasked by the CDC with using information from the Vaccine Safety Datalink to specifically assess the safety of the influenza vaccine when administered early in pregnancy. They evaluated 485 women who had experienced a spontaneous abortion in 1 of 2 time periods: September 1, 2010 to April 28, 2011 and September 1, 2011 to April 28, 2012. These women were matched by last menstrual period with controls who subsequently had a liveborn infant or stillbirth at greater than 20 weeks of gestation. The exposure of interest was receipt of the monovalent H1N1 vaccine (H1N1pdm09), the inactivated trivalent vaccine (A/California/7/2009 H1N1 pdm09-like, A/Perth/16/2009 H3N2-like, and B/Brisbane/60/2008-like), or both in the 28 days immediately preceding the spontaneous abortion. The investigators also considered 2 other windows of exposure: 29 to 56 days and greater than 56 days. In addition, they controlled for the following potential confounding variables: maternal age, smoking history, presence of type 1 or 2 diabetes, prepregnancy BMI, and previous health care utilization.
Cases were significantly older than controls. They also were more likely to be African-American, to have had a history of greater than or equal to 2 spontaneous abortions, and to have smoked during pregnancy. The median gestational age at the time of spontaneous abortion was 7 weeks. Overall, the adjusted odds ratio (aOR) of spontaneous abortion within the 1- to 28-day window was 2.0 (95% confidence interval [CI], 1.1-3.6). There was not even a weak association in the other 2 windows of exposure. However, in women who received the pH1N1 vaccine in the previous flu season, the aOR was 7.7 (95% CI, 2.2-27.3). When women who had experienced 2 or more spontaneous abortions were excluded, the aOR remained significantly elevated at 6.5 (95% CI, 1.7-24.3). The aOR was 1.3 (95% CI, 0.7-2.7) in women who were not vaccinated with the pH1N1 vaccine in the previous flu season.
Donahue and colleagues6 offered several possible explanations for their observations. They noted that the pH1N1 vaccine seemed to cause at least mild increases in pro-inflammatory cytokines, particularly in pregnant compared with nonpregnant women. In addition, infection with the pH1N1 virus or vaccination with the pH1N1 vaccine induces an increase in T helper type-1 cells, which exert a pro-inflammatory effect. Excessive inflammation, in turn, may cause spontaneous abortion.
Related article:
5 ways to reduce infection risk during pregnancy
Study limitations
This study has several important limitations. First, the vaccine used in the investigation is not identical to the one used most commonly today. Second, although the number of women with spontaneous abortions is relatively large, the number who received the pH1N1-containing vaccine in consecutive years was relatively small. Third, this case-control study was able to estimate an odds ratio for the adverse outcome, but it could not prove causation, nor could it provide a precise estimate of absolute risk for a spontaneous miscarriage following the influenza vaccine. Finally, the authors, understandably,were unable to control for all the myriad factors that may increase the risk for spontaneous abortion.
With these limitations in mind, I certainly concur with the recommendations of the CDC and ACOG7 to continue our practice of routinely offering the influenza vaccine to virtually all pregnant women at the beginning of the flu season. For the vast majority of patients, the benefit of vaccination for both mother and baby outweighs the risk of an adverse effect. Traditionally, allergy to any of the vaccine components, principally egg protein and/or mercury, was considered a contraindication to vaccination. However, one trivalent vaccine preparation (Flublok, Protein Sciences Corporation) is now available that does not contain egg protein, and 3 trivalent preparations and at least 6 quadrivalent preparations are available that do not contain thimerosal (mercury). Therefore, allergy should rarely be a contraindication to vaccination.
In order to exercise an abundance of caution, I will not offer this vaccination in the first trimester to women who appear to be at increased risk for early pregnancy loss (women with spontaneous bleeding or a prior history of early loss). In these individuals, I will defer vaccination until the second trimester. I will also eagerly await the results of another CDC-sponsored investigation designed to evaluate the risks of spontaneous abortion in women who were vaccinated consecutively in the 2012–2013, 2013–2014, and 2014–2015 influenza seasons.6
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Louie JK, Acosta M, Jamieson DJ, Honein MA; California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35.
- Polyzos KA, Konstantelias AA, Pitsa CE, Falagas ME. Maternal influenza vaccination and risk for congenital malformations: a systematic review and meta-analysis. Obstet Gynecol. 2015;126(5):1075–1084.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122(3):659–667.
- Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120(3):532–537.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 468: influenza vaccination during pregnancy. Obstet Gynecol. 2010;116(4):1006–1007.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314–5322.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Updated September 13, 2017. Accessed October 2, 2017.
EXPERT COMMENTARY
Influenza can be a serious, even life-threatening infection, especially in pregnant women and their newborn infants.1 For that reason, the Centers for Disease Control and Prevention (CDC) and the American College of Obstetricians and Gynecologists (ACOG) strongly recommend that all pregnant women receive the inactivated influenza vaccine at the start of each flu season, regardless of trimester of exposure.
The most widely-used vaccine in the United States is the inactivated quadrivalent vaccine, which is intended for intramuscular administration in a single dose. The 2017-2018 version of this vaccine includes 2 influenza A antigens and 2 influenza B antigens. The first of the A antigens differs from last year's vaccine. The other 3 antigens are the same as in the 2016-2017 vaccine:
- A/Michigan/45/2015 (H1N1) pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus
- B/Phuket/3073/2013-like virus (B/Yamagota)
Several recent reports in large, diverse populations2-5 have demonstrated that the vaccine does not increase the risk of spontaneous abortion, stillbirth, preterm delivery, or congenital anomalies. Therefore, a recent report by Donahue and colleagues6 is surprising and is certainly worthy of our attention.
Related article:
Does influenza immunization during pregnancy confer flu protection to newborns?
Details of the study
Donahue and colleagues, experienced investigators, were tasked by the CDC with using information from the Vaccine Safety Datalink to specifically assess the safety of the influenza vaccine when administered early in pregnancy. They evaluated 485 women who had experienced a spontaneous abortion in 1 of 2 time periods: September 1, 2010 to April 28, 2011 and September 1, 2011 to April 28, 2012. These women were matched by last menstrual period with controls who subsequently had a liveborn infant or stillbirth at greater than 20 weeks of gestation. The exposure of interest was receipt of the monovalent H1N1 vaccine (H1N1pdm09), the inactivated trivalent vaccine (A/California/7/2009 H1N1 pdm09-like, A/Perth/16/2009 H3N2-like, and B/Brisbane/60/2008-like), or both in the 28 days immediately preceding the spontaneous abortion. The investigators also considered 2 other windows of exposure: 29 to 56 days and greater than 56 days. In addition, they controlled for the following potential confounding variables: maternal age, smoking history, presence of type 1 or 2 diabetes, prepregnancy BMI, and previous health care utilization.
Cases were significantly older than controls. They also were more likely to be African-American, to have had a history of greater than or equal to 2 spontaneous abortions, and to have smoked during pregnancy. The median gestational age at the time of spontaneous abortion was 7 weeks. Overall, the adjusted odds ratio (aOR) of spontaneous abortion within the 1- to 28-day window was 2.0 (95% confidence interval [CI], 1.1-3.6). There was not even a weak association in the other 2 windows of exposure. However, in women who received the pH1N1 vaccine in the previous flu season, the aOR was 7.7 (95% CI, 2.2-27.3). When women who had experienced 2 or more spontaneous abortions were excluded, the aOR remained significantly elevated at 6.5 (95% CI, 1.7-24.3). The aOR was 1.3 (95% CI, 0.7-2.7) in women who were not vaccinated with the pH1N1 vaccine in the previous flu season.
Donahue and colleagues6 offered several possible explanations for their observations. They noted that the pH1N1 vaccine seemed to cause at least mild increases in pro-inflammatory cytokines, particularly in pregnant compared with nonpregnant women. In addition, infection with the pH1N1 virus or vaccination with the pH1N1 vaccine induces an increase in T helper type-1 cells, which exert a pro-inflammatory effect. Excessive inflammation, in turn, may cause spontaneous abortion.
Related article:
5 ways to reduce infection risk during pregnancy
Study limitations
This study has several important limitations. First, the vaccine used in the investigation is not identical to the one used most commonly today. Second, although the number of women with spontaneous abortions is relatively large, the number who received the pH1N1-containing vaccine in consecutive years was relatively small. Third, this case-control study was able to estimate an odds ratio for the adverse outcome, but it could not prove causation, nor could it provide a precise estimate of absolute risk for a spontaneous miscarriage following the influenza vaccine. Finally, the authors, understandably,were unable to control for all the myriad factors that may increase the risk for spontaneous abortion.
With these limitations in mind, I certainly concur with the recommendations of the CDC and ACOG7 to continue our practice of routinely offering the influenza vaccine to virtually all pregnant women at the beginning of the flu season. For the vast majority of patients, the benefit of vaccination for both mother and baby outweighs the risk of an adverse effect. Traditionally, allergy to any of the vaccine components, principally egg protein and/or mercury, was considered a contraindication to vaccination. However, one trivalent vaccine preparation (Flublok, Protein Sciences Corporation) is now available that does not contain egg protein, and 3 trivalent preparations and at least 6 quadrivalent preparations are available that do not contain thimerosal (mercury). Therefore, allergy should rarely be a contraindication to vaccination.
In order to exercise an abundance of caution, I will not offer this vaccination in the first trimester to women who appear to be at increased risk for early pregnancy loss (women with spontaneous bleeding or a prior history of early loss). In these individuals, I will defer vaccination until the second trimester. I will also eagerly await the results of another CDC-sponsored investigation designed to evaluate the risks of spontaneous abortion in women who were vaccinated consecutively in the 2012–2013, 2013–2014, and 2014–2015 influenza seasons.6
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Influenza can be a serious, even life-threatening infection, especially in pregnant women and their newborn infants.1 For that reason, the Centers for Disease Control and Prevention (CDC) and the American College of Obstetricians and Gynecologists (ACOG) strongly recommend that all pregnant women receive the inactivated influenza vaccine at the start of each flu season, regardless of trimester of exposure.
The most widely-used vaccine in the United States is the inactivated quadrivalent vaccine, which is intended for intramuscular administration in a single dose. The 2017-2018 version of this vaccine includes 2 influenza A antigens and 2 influenza B antigens. The first of the A antigens differs from last year's vaccine. The other 3 antigens are the same as in the 2016-2017 vaccine:
- A/Michigan/45/2015 (H1N1) pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus
- B/Phuket/3073/2013-like virus (B/Yamagota)
Several recent reports in large, diverse populations2-5 have demonstrated that the vaccine does not increase the risk of spontaneous abortion, stillbirth, preterm delivery, or congenital anomalies. Therefore, a recent report by Donahue and colleagues6 is surprising and is certainly worthy of our attention.
Related article:
Does influenza immunization during pregnancy confer flu protection to newborns?
Details of the study
Donahue and colleagues, experienced investigators, were tasked by the CDC with using information from the Vaccine Safety Datalink to specifically assess the safety of the influenza vaccine when administered early in pregnancy. They evaluated 485 women who had experienced a spontaneous abortion in 1 of 2 time periods: September 1, 2010 to April 28, 2011 and September 1, 2011 to April 28, 2012. These women were matched by last menstrual period with controls who subsequently had a liveborn infant or stillbirth at greater than 20 weeks of gestation. The exposure of interest was receipt of the monovalent H1N1 vaccine (H1N1pdm09), the inactivated trivalent vaccine (A/California/7/2009 H1N1 pdm09-like, A/Perth/16/2009 H3N2-like, and B/Brisbane/60/2008-like), or both in the 28 days immediately preceding the spontaneous abortion. The investigators also considered 2 other windows of exposure: 29 to 56 days and greater than 56 days. In addition, they controlled for the following potential confounding variables: maternal age, smoking history, presence of type 1 or 2 diabetes, prepregnancy BMI, and previous health care utilization.
Cases were significantly older than controls. They also were more likely to be African-American, to have had a history of greater than or equal to 2 spontaneous abortions, and to have smoked during pregnancy. The median gestational age at the time of spontaneous abortion was 7 weeks. Overall, the adjusted odds ratio (aOR) of spontaneous abortion within the 1- to 28-day window was 2.0 (95% confidence interval [CI], 1.1-3.6). There was not even a weak association in the other 2 windows of exposure. However, in women who received the pH1N1 vaccine in the previous flu season, the aOR was 7.7 (95% CI, 2.2-27.3). When women who had experienced 2 or more spontaneous abortions were excluded, the aOR remained significantly elevated at 6.5 (95% CI, 1.7-24.3). The aOR was 1.3 (95% CI, 0.7-2.7) in women who were not vaccinated with the pH1N1 vaccine in the previous flu season.
Donahue and colleagues6 offered several possible explanations for their observations. They noted that the pH1N1 vaccine seemed to cause at least mild increases in pro-inflammatory cytokines, particularly in pregnant compared with nonpregnant women. In addition, infection with the pH1N1 virus or vaccination with the pH1N1 vaccine induces an increase in T helper type-1 cells, which exert a pro-inflammatory effect. Excessive inflammation, in turn, may cause spontaneous abortion.
Related article:
5 ways to reduce infection risk during pregnancy
Study limitations
This study has several important limitations. First, the vaccine used in the investigation is not identical to the one used most commonly today. Second, although the number of women with spontaneous abortions is relatively large, the number who received the pH1N1-containing vaccine in consecutive years was relatively small. Third, this case-control study was able to estimate an odds ratio for the adverse outcome, but it could not prove causation, nor could it provide a precise estimate of absolute risk for a spontaneous miscarriage following the influenza vaccine. Finally, the authors, understandably,were unable to control for all the myriad factors that may increase the risk for spontaneous abortion.
With these limitations in mind, I certainly concur with the recommendations of the CDC and ACOG7 to continue our practice of routinely offering the influenza vaccine to virtually all pregnant women at the beginning of the flu season. For the vast majority of patients, the benefit of vaccination for both mother and baby outweighs the risk of an adverse effect. Traditionally, allergy to any of the vaccine components, principally egg protein and/or mercury, was considered a contraindication to vaccination. However, one trivalent vaccine preparation (Flublok, Protein Sciences Corporation) is now available that does not contain egg protein, and 3 trivalent preparations and at least 6 quadrivalent preparations are available that do not contain thimerosal (mercury). Therefore, allergy should rarely be a contraindication to vaccination.
In order to exercise an abundance of caution, I will not offer this vaccination in the first trimester to women who appear to be at increased risk for early pregnancy loss (women with spontaneous bleeding or a prior history of early loss). In these individuals, I will defer vaccination until the second trimester. I will also eagerly await the results of another CDC-sponsored investigation designed to evaluate the risks of spontaneous abortion in women who were vaccinated consecutively in the 2012–2013, 2013–2014, and 2014–2015 influenza seasons.6
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Louie JK, Acosta M, Jamieson DJ, Honein MA; California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35.
- Polyzos KA, Konstantelias AA, Pitsa CE, Falagas ME. Maternal influenza vaccination and risk for congenital malformations: a systematic review and meta-analysis. Obstet Gynecol. 2015;126(5):1075–1084.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122(3):659–667.
- Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120(3):532–537.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 468: influenza vaccination during pregnancy. Obstet Gynecol. 2010;116(4):1006–1007.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314–5322.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Updated September 13, 2017. Accessed October 2, 2017.
- Louie JK, Acosta M, Jamieson DJ, Honein MA; California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35.
- Polyzos KA, Konstantelias AA, Pitsa CE, Falagas ME. Maternal influenza vaccination and risk for congenital malformations: a systematic review and meta-analysis. Obstet Gynecol. 2015;126(5):1075–1084.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122(3):659–667.
- Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120(3):532–537.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 468: influenza vaccination during pregnancy. Obstet Gynecol. 2010;116(4):1006–1007.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314–5322.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Updated September 13, 2017. Accessed October 2, 2017.
2017 Update on infectious disease
In this Update we review the results of 4 recent investigations that have important implications:
- the first analysis of the US Zika Virus Infection in Pregnancy Registry
- a study revealing an improved antibiotic regimen to prevent postcesarean infection
- an important new methodology for reducing the rate of perinatal transmission of hepatitis B virus (HBV) infection
- the risks and benefits of combination antiretroviral therapy (ART) in pregnancy.
Zika virus-associated birth defect rates similar regardless of symptom presence; first-trimester exposure has highest rate of anomalies
Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59-68.
Honein and colleagues provide a summary of the data from the US Zika Virus in Pregnancy Registry (a collaboration between the Centers for Disease Control and Prevention and state and local health departments), estimating the proportion of fetuses and infants with birth defects based on maternal symptoms of Zika virus infection and trimester of possible infection.
Related article:
Zika virus: Counseling considerations for this emerging perinatal threat
Details of the study
The authors evaluated the outcomes of 442 women who had laboratory evidence of a possible Zika virus infection during pregnancy. Overall, 26 infants (6%; 95% confidence interval (CI), 4%-8%) had evidence of birth defects related to the Zika virus. Of note, abnormalities were detected in 16 of the 271 children (6%; 95% CI, 4%-9%) born to women who were asymptomatic and 10 of 167 (6%; 95% CI, 3%-11%) children delivered to women with symptomatic infections.
The most common birth defect was microcephaly, although other serious central nervous system abnormalities were noted as well. Nine of 85 women (11%; 95% CI, 6%-19%) who had exposure only during the first trimester had infants with birth defects. There were no documented abnormalities in infants born to mothers who developed Zika virus infection only in the second or third trimester.
Related article:
Zika virus update: A rapidly moving target
Key study findings
This article is important for several reasons. First, the authors describe the largest series of pregnant women in the United States with Zika virus infection. All of these patients developed Zika virus infection as a result of foreign travel or exposure to sexual partners who had traveled to Zika virus endemic areas. Second, the authors confirmed findings that previously had been based only on mathematical models rather than on actual case series. Specifically, they demonstrated that the risk of a serious birth defect following first-trimester exposure to Zika virus infection was approximately 11%, with a 95% CI that extended from 6% to 19%. Finally, Honein and colleagues highlighted the key fact that the risk of a serious birth defect was comparable in mothers who had either an asymptomatic or a symptomatic infection, a finding that seems somewhat counterintuitive.
This study's critical observations are a "call to action" for clinicians who provide prenatal care.1,2 Proactive steps include:
- For patients considering pregnancy, strongly advise against travel to any area of the world where Zika virus is endemic until an effective vaccine is available to protect against this infection.
- For any woman with a newly diagnosed pregnancy, ask about travel to an endemic area.
- Inquire also about a pregnant woman's exposure to partners who live in, or who have traveled to, areas of the world where Zika virus infection is endemic.
- Be aware that both asymptomatic and symptomatic infection in the first trimester of pregnancy pose a grave risk to the fetus.
- Recognize that, although microcephaly is the principal abnormality associated with Zika virus infection, other central nervous system anomalies also may occur in these children. These include ventriculomegaly, subcortical calcifications, abnormalities of the corpus callosum, cerebral atrophy, and cerebellar abnormalities. In addition, infected infants may have arthrogryposis.
- Finally, as Honein and colleagues noted, laboratory testing for Zika virus infection is imperfect. In the early stages of infection or exposure, testing for Zika virus infection by polymerase chain reaction (PCR) in both serum and urine is the preferred test. After a period of 2 weeks, the preferred laboratory test is an immunoglobulin M (IgM) assay. Positive tests on the IgM assay must be confirmed by the plaque neutralization reduction test--a very important test for differentiating Zika virus infection from infection caused by other arboviruses, such as those that cause dengue fever and chikungunya.
Read about prophylaxis for postcesarean infection
Two antibiotics before cesarean delivery reduce infection rates further than one agent
Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231-1241.
Tita and colleagues reported the results of a multicenter trial that was designed to assess whether a combination of 2 antibiotics, including one that specifically targets ureaplasma species, provided more effective prophylaxis against postcesarean infection than single-agent prophylaxis.
Details of the study
The Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial was conducted at 14 centers in the United States and included 2,013 women who were at least at 24 weeks' gestation and who had a cesarean delivery during labor or after membrane rupture.
The authors randomly assigned 1,019 women to receive 500 mg of intravenous azithromycin plus conventional single-agent prophylaxis (usually cefazolin) and 994 women to receive a placebo plus conventional prophylaxis. The primary outcome was the composite of endometritis, wound infection, or other infection occurring within 6 weeks.
The authors observed that the primary outcome occurred in 62 women (6.1%) who received azithromycin plus conventional prophylaxis and in 119 women (12%) who received only single-agent prophylaxis. The relative risk of developing a postoperative infection was 0.51 in women who received the combined therapy. There were significant differences between the 2 groups in both the rates of endometritis (3.8% vs 6.1%, P = .02) and wound infection (2.4% vs 6.6%, P<.001). There were no differences between the groups in the frequency of the secondary neonatal composite outcome, which included neonatal death and serious neonatal complications.
Related article:
Preventing infection after cesarean delivery: 5 more evidence-based measures to consider
Efficacy of dual-agent prophylaxis
At present, the standard of care is to administer prophylactic antibiotics to all women having cesarean delivery, including women having a scheduled cesarean in the absence of labor or ruptured membranes. Multiple studies have shown clearly that prophylaxis reduces the frequency of endometritis and, in high-risk patient populations, wound infection, and that prophylaxis is most beneficial when administered prior to the time the surgical incision is made. The most commonly used drug for prophylaxis is cefazolin, a first-generation cephalosporin. The usual recommended dose is 2 g, administered immediately prior to surgery.3,4
Although most centers in the United States traditionally have used just a single antibiotic for prophylaxis, selected recent reports indicate that expanding the spectrum of activity of prophylactic antibiotics can result in additional beneficial effects. Specifically, Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection.5 They showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis. In a follow-up report from the same institution, Tita and colleagues demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.6 Of note, in both these investigations, the antibiotics were administered after cord clamping. In a subsequent report, Ward and Duff showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a combined rate of endometritis and wound infection that was less than 3%.7
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
C/SOAP trial confirmed lower infection rates with combined regimen
Results of the present study confirm the findings of these 3 investigations. The trial included a large sample size. The study was carefully designed, and the end points were clearly defined. It included only patients at increased risk for postoperative infection by virtue of being in labor or having ruptured membranes at the time of cesarean delivery. Patients who received standard prophylaxis, usually cefazolin, plus azithromycin had a significantly lower risk of postcesarean endometritis and wound infection compared with patients who received a single antibiotic. The overall risk of infection was reduced by an impressive 50%.
Based on the results of the C/SOAP trial, considered in conjunction with the 3 previously cited investigations,5-7 we believe that the standard approach to antibiotic prophylaxis should be to administer both cefazolin, in a dose of 2 g, plus azithromycin, in a dose of 500 mg, prior to surgery. Cefazolin can be administered as an intravenous bolus; azithromycin should be administered as a continuous infusion over a 60-minute period prior to surgery. Clinicians may anticipate very low rates of both endometritis and wound infection with this regimen.
Read about reducing HBV transmission
Tenofovir treatment in pregnant women with HBV reduces vertical transmission
Pan CQ, Duan Z, Dai E, et al; China Study Group for the Mother-to-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324-2334.
A multicenter, open-label, randomized, parallel-group investigation was conducted from March 2012 to June 2013 at academic tertiary care centers in 5 geographic regions of China. Two hundred mothers, who were positive for both hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) and who had HBV DNA concentrations of 200,000 IU/mL or greater, were randomly assigned in a 1:1 ratio to either tenofovir or to usual treatment. Exclusion criteria were coexistent viral infections or medical conditions, renal failure, laboratory abnormalities, fetal deformities, and use of many medications.
Related article:
5 ways to reduce infection risk during pregnancy
Details of the study
Women in the active treatment group received tenofovir 300 mg by mouth daily from 30 to 32 weeks' gestation until postpartum week 4. Patients were monitored every 4 weeks in the antepartum period for adverse events and laboratory abnormalities. In the postpartum period, mother-infant dyads were evaluated at weeks 4, 12, 24, and 28.
Primary outcomes were the rates of mother-to-child transmission and birth defects with, or without, tenofovir exposure. Secondary outcomes were the percentage of mothers who had an HBV DNA serum concentration of less than 200,000 IU/mL at delivery and the percentage of mothers with HBeAg or HBsAg loss or seroconversion at postpartum week 28. Safety outcomes included the adverse event profile of tenofovir in mothers and safety events in the mother-infant dyads. These outcomes encompassed all adverse events and drug discontinuations in patients who received at least one dose of tenofovir.
Sixty-eight percent of mothers in the tenofovir group, compared with 2% of mothers in the control group, had HBV levels less than 200,000 IU/mL at delivery (P<.001). The rate of mother-to-child HBV transmission at postpartum week 28 was lower in the tenofovir group. In the intention-to-treat analysis, the rate was 5% (95% CI, 1-10; 5 of 97 infants) in the tenofovir group versus 18% (95% CI, 10-26; 18 of 100 infants) in the control group (P = .007). In the per-protocol analysis, the rate was 0% (95% CI, 0-3; 0 of 92 infants) in the tenofovir group versus 7% (95% CI, 2-12; 6 of 88 infants) in the control group (P = .01). Maternal and infant safety profiles were similar between the 2 groups, with the exception of elevated creatinine kinase and alanine aminotransferase levels in mothers treated with tenofovir. Maternal HBV serologic titers did not differ significantly between the 2 groups.
Study strengths and limitations
This study's strengths include a multicenter, randomized controlled design, with strict inclusion and exclusion criteria. The results are clinically relevant and of global impact, with potential to decrease morbidity and mortality from HBV infection in children born to infected mothers.
A limitation, however, is that the study was probably underpowered to detect small differences in the rate of birth defects between the tenofovir and usual-care treatment groups. Additionally, some patients ceased taking tenofovir in the postpartum time period. Abrupt cessation may be associated with acute, severe HBV exacerbation.
HBV is a serious infection that can lead to liver failure and cirrhosis. HBV infection is most likely to have long-term sequelae if acquired in the perinatal period. If untreated, chronic HBV infection will develop in 80% to 90% of infants born to mothers positive for HBeAg. Current immunoprophylaxis for at-risk neonates is postnatal HBV vaccine in combination with hepatitis B immune globulin. Unfortunately, this immunoprophylaxis fails in 10% to 30% of infants born to mothers with an HBV DNA level of greater than 6 log 10 copies/mL. Thus, the observations of Pan and colleagues are welcome findings.
Based on the results of this study, we recommend the use of tenofovir to decrease HBV transmission during pregnancy for women with high viral loads.
Benefits of ART for reducing mother-to-baby HIV transmission outweigh higher risk of adverse outcomes
Fowler MG, Qin M, Fiscus SA, et al; IMPAACT 1077BF/1077FF PROMISE Study Team. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726-1737.
Part of the larger PROMISE (Promoting Maternal and Infant Survival Everywhere) trial, a study by Fowler and colleagues compared the relative efficacy and safety of various proven ART strategies for prevention of mother-to-child transmission of HIV infection in women with relatively high CD4 counts.
Details of the study
The trial was conducted at 14 sites in 7 countries. Patients were stratified according to HBV coinfection status and country of origin. The primary efficacy outcome was frequency of early infant HIV infection.
Women were randomly assigned to 1 of 3 treatment categories:
- zidovudine alone (zidovudine plus a single intrapartum dose of nevirapine, followed by 6 to 14 days of tenofovir plus emtricitabine postpartum)
- zidovudine-based ART (zidovudine in combination with lamivudine and lopinavir-ritonavir)
- tenofovir-based ART (tenofovir in combination with emtricitabine and lopinavir-ritonavir).
All regimens were continued through 6 to 14 days postpartum. All infants received nevirapine at birth and in the immediate postpartum period.
Two trial periods. During period 1 (April 2011-September 2012), safety data on tenofovir in pregnancy were limited. Women without HBV coinfection were assigned only to zidovudine alone or zidovudine-based ART. During period 2 (October 2012-October 2014), since more information about tenofovir use in pregnancy was available, the study protocol was modified to allow women to be assigned to any of the 3 regimens, regardless of their HBV status.
Inclusion criteria were as follows: CD4 count of at least 350 cells/mm3 (or country-specific threshold for initiating triple-drug ART, if that threshold was higher), gestation of at least 14 weeks and not in labor, no previous use of triple-drug ART, no clinical or immune-related indication for triple-drug ART, hemoglobin level of at least 6.5 g/dL, an absolute neutrophil count of at least 750 cells/mm3, an alanine aminotransferase level of less than 2.5 times the upper limit of normal range, an estimated creatinine clearance of greater than 60 mL/min, and no serious pregnancy complications. Patients were excluded if they had active tuberculosis, HBV infection requiring treatment, a structural or conduction heart defect, or a fetus with a serious congenital malformation.
Primary outcomes. The primary efficacy outcome was early infant HIV infection, defined as a positive infant HIV nucleic acid test result at birth or at 1 week postpartum. The primary safety outcome was a composite of adverse events.
Adverse events in mothers were defined as hematologic abnormalities, abnormal blood chemical values, or abnormal signs/symptoms during pregnancy through 1 week postpartum. Severe pregnancy composite outcomes were low birth weight (<2,500 g), preterm delivery before 37 weeks' gestation, spontaneous abortion (<20 weeks), stillbirth (≥20 weeks), or congenital anomaly. Adverse events in infants were defined as death from any cause, hematologic abnormalities or abnormal blood chemical values, and abnormal signs/symptoms through 1 week postpartum.
A total of 3,490 mother-infant sets were included in the analysis (2,261 during trial period 1 and 1,229 during trial period 2). Baseline maternal characteristics were well balanced between groups. Most women were African, young (median age, 26 years), and asymptomatic.
Related article:
2016 Update on infectious disease
Study results
The combined maternal ART-treated groups had significantly lower rates of early transmission of HIV infection compared with the zidovudine-alone group (0.5% vs 1.8%, -1.3 percentage points; CI, -2.1 to -0.4). The zidovudine-based ART-treated group had a significantly higher rate of infant HIV-free survival through postpartum week 1 than did the zidovudine-alone group (P = .001) or the tenofovir-based ART group (P = .002).
When examining trial periods 1 and 2 combined, the zidovudine-based ART group experienced significantly higher rates of any adverse event than those receiving zidovudine alone (21.1% vs 17.3%, P = .008) and higher rates of abnormal blood chemical values (5.8% vs 1.3%, P<.001). During period 2 alone, the tenofovir-based ART group had significantly higher rates of abnormal blood chemical values than did the zidovudine-alone group (2.9% vs 0.8%, P = .03). There were no significant differences between the 2 ART treatment groups. No maternal deaths occurred during the study, and the trial-drug discontinuation rate was low (2%-5%) and did not vary among the 3 groups.
During trial periods 1 and 2, the zidovudine-based ART group had significantly higher rates of adverse pregnancy outcomes than did the zidovudine-alone group (40% vs 27.5%, P<.001). These included low birth weight less than 2,500 g (23% vs 12%) and preterm delivery before 37 weeks (20.5% vs 13.1%). During trial period 2, the tenofovir-based ART group had significantly higher rates of adverse pregnancy outcomes than did the zidovudine-alone group (34.7% vs 27.2%, P = .04). There were no significant differences for any outcome between the 2 ART-treated groups, and there were no significant differences in stillbirth or spontaneous abortion and congenital anomalies among the 3 groups.
Regarding severe pregnancy outcomes, there were no significant differences (composite or individual) between the zidovudine-based ART group and the zidovudine-alone group. The tenofovir-based ART group experienced significantly higher rates of composite severe adverse pregnancy outcomes compared with the zidovudine-based ART group (9.2% vs 4.3%, P = .02), and very preterm birth before 34 weeks (6.0% vs 2.6%, P = .04).
Infant safety outcomes were also examined. There were no significant differences for composite or individual adverse neonatal outcomes other than death. The tenofovir-based ART group experienced a significantly higher rate of infant death than did the zidovudine-based ART group (4.4% vs 0.6%, P<.001). However, a post hoc analysis suggested that extreme prematurity contributed to the infant mortality.
Limitations of the study
This study had minor limitations. It divided patients into only 2 major categories with respect to gestational age--more than or less than 34 weeks. Some maternal medical conditions, such as malaria, were not controlled for. In addition, breastfeeding and formula feeding were combined for analysis, and we know that breastfeeding would inherently confer a higher risk of HIV transmission.
Nevertheless, this study was thoughtfully designed and carefully conducted, and the results are of significant global impact.
Although antenatal ART was associated with a higher risk of adverse maternal and neonatal outcomes when compared with zidovudine alone, these risks are outweighed by the benefit of significantly lower rates of early HIV transmission. Therefore, women who meet the World Health Organization's (WHO) eligibility criteria should be treated with combination ART during pregnancy. The WHO major eligibility criteria for ART during pregnancy are:
- CD4 count of ≤350 cells/mm3, irrespective of clinical staging
- clinical stage 3 or stage 4 disease, irrespective of CD4 cell count.
The WHO recommends starting ART at 14 weeks' gestation.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28-34.
- Chelliah A, Duff P. Zika virus update: a rapidly moving target. OBG Manag. 2016;28(8):17-26.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41-47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidenced-based methods to consider. OBG Manag. 2016;28(12):18-22.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51-56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1-e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1-e4.
- New guidance on prevention of mother-to-child transmission of HIV and infant feeding in the context of HIV. World Health Organization website. http://www.who.int/hiv/pub/mtct/PMTCTfactsheet/en/. Published July 20, 2010. Accessed June 16, 2017.
In this Update we review the results of 4 recent investigations that have important implications:
- the first analysis of the US Zika Virus Infection in Pregnancy Registry
- a study revealing an improved antibiotic regimen to prevent postcesarean infection
- an important new methodology for reducing the rate of perinatal transmission of hepatitis B virus (HBV) infection
- the risks and benefits of combination antiretroviral therapy (ART) in pregnancy.
Zika virus-associated birth defect rates similar regardless of symptom presence; first-trimester exposure has highest rate of anomalies
Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59-68.
Honein and colleagues provide a summary of the data from the US Zika Virus in Pregnancy Registry (a collaboration between the Centers for Disease Control and Prevention and state and local health departments), estimating the proportion of fetuses and infants with birth defects based on maternal symptoms of Zika virus infection and trimester of possible infection.
Related article:
Zika virus: Counseling considerations for this emerging perinatal threat
Details of the study
The authors evaluated the outcomes of 442 women who had laboratory evidence of a possible Zika virus infection during pregnancy. Overall, 26 infants (6%; 95% confidence interval (CI), 4%-8%) had evidence of birth defects related to the Zika virus. Of note, abnormalities were detected in 16 of the 271 children (6%; 95% CI, 4%-9%) born to women who were asymptomatic and 10 of 167 (6%; 95% CI, 3%-11%) children delivered to women with symptomatic infections.
The most common birth defect was microcephaly, although other serious central nervous system abnormalities were noted as well. Nine of 85 women (11%; 95% CI, 6%-19%) who had exposure only during the first trimester had infants with birth defects. There were no documented abnormalities in infants born to mothers who developed Zika virus infection only in the second or third trimester.
Related article:
Zika virus update: A rapidly moving target
Key study findings
This article is important for several reasons. First, the authors describe the largest series of pregnant women in the United States with Zika virus infection. All of these patients developed Zika virus infection as a result of foreign travel or exposure to sexual partners who had traveled to Zika virus endemic areas. Second, the authors confirmed findings that previously had been based only on mathematical models rather than on actual case series. Specifically, they demonstrated that the risk of a serious birth defect following first-trimester exposure to Zika virus infection was approximately 11%, with a 95% CI that extended from 6% to 19%. Finally, Honein and colleagues highlighted the key fact that the risk of a serious birth defect was comparable in mothers who had either an asymptomatic or a symptomatic infection, a finding that seems somewhat counterintuitive.
This study's critical observations are a "call to action" for clinicians who provide prenatal care.1,2 Proactive steps include:
- For patients considering pregnancy, strongly advise against travel to any area of the world where Zika virus is endemic until an effective vaccine is available to protect against this infection.
- For any woman with a newly diagnosed pregnancy, ask about travel to an endemic area.
- Inquire also about a pregnant woman's exposure to partners who live in, or who have traveled to, areas of the world where Zika virus infection is endemic.
- Be aware that both asymptomatic and symptomatic infection in the first trimester of pregnancy pose a grave risk to the fetus.
- Recognize that, although microcephaly is the principal abnormality associated with Zika virus infection, other central nervous system anomalies also may occur in these children. These include ventriculomegaly, subcortical calcifications, abnormalities of the corpus callosum, cerebral atrophy, and cerebellar abnormalities. In addition, infected infants may have arthrogryposis.
- Finally, as Honein and colleagues noted, laboratory testing for Zika virus infection is imperfect. In the early stages of infection or exposure, testing for Zika virus infection by polymerase chain reaction (PCR) in both serum and urine is the preferred test. After a period of 2 weeks, the preferred laboratory test is an immunoglobulin M (IgM) assay. Positive tests on the IgM assay must be confirmed by the plaque neutralization reduction test--a very important test for differentiating Zika virus infection from infection caused by other arboviruses, such as those that cause dengue fever and chikungunya.
Read about prophylaxis for postcesarean infection
Two antibiotics before cesarean delivery reduce infection rates further than one agent
Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231-1241.
Tita and colleagues reported the results of a multicenter trial that was designed to assess whether a combination of 2 antibiotics, including one that specifically targets ureaplasma species, provided more effective prophylaxis against postcesarean infection than single-agent prophylaxis.
Details of the study
The Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial was conducted at 14 centers in the United States and included 2,013 women who were at least at 24 weeks' gestation and who had a cesarean delivery during labor or after membrane rupture.
The authors randomly assigned 1,019 women to receive 500 mg of intravenous azithromycin plus conventional single-agent prophylaxis (usually cefazolin) and 994 women to receive a placebo plus conventional prophylaxis. The primary outcome was the composite of endometritis, wound infection, or other infection occurring within 6 weeks.
The authors observed that the primary outcome occurred in 62 women (6.1%) who received azithromycin plus conventional prophylaxis and in 119 women (12%) who received only single-agent prophylaxis. The relative risk of developing a postoperative infection was 0.51 in women who received the combined therapy. There were significant differences between the 2 groups in both the rates of endometritis (3.8% vs 6.1%, P = .02) and wound infection (2.4% vs 6.6%, P<.001). There were no differences between the groups in the frequency of the secondary neonatal composite outcome, which included neonatal death and serious neonatal complications.
Related article:
Preventing infection after cesarean delivery: 5 more evidence-based measures to consider
Efficacy of dual-agent prophylaxis
At present, the standard of care is to administer prophylactic antibiotics to all women having cesarean delivery, including women having a scheduled cesarean in the absence of labor or ruptured membranes. Multiple studies have shown clearly that prophylaxis reduces the frequency of endometritis and, in high-risk patient populations, wound infection, and that prophylaxis is most beneficial when administered prior to the time the surgical incision is made. The most commonly used drug for prophylaxis is cefazolin, a first-generation cephalosporin. The usual recommended dose is 2 g, administered immediately prior to surgery.3,4
Although most centers in the United States traditionally have used just a single antibiotic for prophylaxis, selected recent reports indicate that expanding the spectrum of activity of prophylactic antibiotics can result in additional beneficial effects. Specifically, Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection.5 They showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis. In a follow-up report from the same institution, Tita and colleagues demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.6 Of note, in both these investigations, the antibiotics were administered after cord clamping. In a subsequent report, Ward and Duff showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a combined rate of endometritis and wound infection that was less than 3%.7
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
C/SOAP trial confirmed lower infection rates with combined regimen
Results of the present study confirm the findings of these 3 investigations. The trial included a large sample size. The study was carefully designed, and the end points were clearly defined. It included only patients at increased risk for postoperative infection by virtue of being in labor or having ruptured membranes at the time of cesarean delivery. Patients who received standard prophylaxis, usually cefazolin, plus azithromycin had a significantly lower risk of postcesarean endometritis and wound infection compared with patients who received a single antibiotic. The overall risk of infection was reduced by an impressive 50%.
Based on the results of the C/SOAP trial, considered in conjunction with the 3 previously cited investigations,5-7 we believe that the standard approach to antibiotic prophylaxis should be to administer both cefazolin, in a dose of 2 g, plus azithromycin, in a dose of 500 mg, prior to surgery. Cefazolin can be administered as an intravenous bolus; azithromycin should be administered as a continuous infusion over a 60-minute period prior to surgery. Clinicians may anticipate very low rates of both endometritis and wound infection with this regimen.
Read about reducing HBV transmission
Tenofovir treatment in pregnant women with HBV reduces vertical transmission
Pan CQ, Duan Z, Dai E, et al; China Study Group for the Mother-to-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324-2334.
A multicenter, open-label, randomized, parallel-group investigation was conducted from March 2012 to June 2013 at academic tertiary care centers in 5 geographic regions of China. Two hundred mothers, who were positive for both hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) and who had HBV DNA concentrations of 200,000 IU/mL or greater, were randomly assigned in a 1:1 ratio to either tenofovir or to usual treatment. Exclusion criteria were coexistent viral infections or medical conditions, renal failure, laboratory abnormalities, fetal deformities, and use of many medications.
Related article:
5 ways to reduce infection risk during pregnancy
Details of the study
Women in the active treatment group received tenofovir 300 mg by mouth daily from 30 to 32 weeks' gestation until postpartum week 4. Patients were monitored every 4 weeks in the antepartum period for adverse events and laboratory abnormalities. In the postpartum period, mother-infant dyads were evaluated at weeks 4, 12, 24, and 28.
Primary outcomes were the rates of mother-to-child transmission and birth defects with, or without, tenofovir exposure. Secondary outcomes were the percentage of mothers who had an HBV DNA serum concentration of less than 200,000 IU/mL at delivery and the percentage of mothers with HBeAg or HBsAg loss or seroconversion at postpartum week 28. Safety outcomes included the adverse event profile of tenofovir in mothers and safety events in the mother-infant dyads. These outcomes encompassed all adverse events and drug discontinuations in patients who received at least one dose of tenofovir.
Sixty-eight percent of mothers in the tenofovir group, compared with 2% of mothers in the control group, had HBV levels less than 200,000 IU/mL at delivery (P<.001). The rate of mother-to-child HBV transmission at postpartum week 28 was lower in the tenofovir group. In the intention-to-treat analysis, the rate was 5% (95% CI, 1-10; 5 of 97 infants) in the tenofovir group versus 18% (95% CI, 10-26; 18 of 100 infants) in the control group (P = .007). In the per-protocol analysis, the rate was 0% (95% CI, 0-3; 0 of 92 infants) in the tenofovir group versus 7% (95% CI, 2-12; 6 of 88 infants) in the control group (P = .01). Maternal and infant safety profiles were similar between the 2 groups, with the exception of elevated creatinine kinase and alanine aminotransferase levels in mothers treated with tenofovir. Maternal HBV serologic titers did not differ significantly between the 2 groups.
Study strengths and limitations
This study's strengths include a multicenter, randomized controlled design, with strict inclusion and exclusion criteria. The results are clinically relevant and of global impact, with potential to decrease morbidity and mortality from HBV infection in children born to infected mothers.
A limitation, however, is that the study was probably underpowered to detect small differences in the rate of birth defects between the tenofovir and usual-care treatment groups. Additionally, some patients ceased taking tenofovir in the postpartum time period. Abrupt cessation may be associated with acute, severe HBV exacerbation.
HBV is a serious infection that can lead to liver failure and cirrhosis. HBV infection is most likely to have long-term sequelae if acquired in the perinatal period. If untreated, chronic HBV infection will develop in 80% to 90% of infants born to mothers positive for HBeAg. Current immunoprophylaxis for at-risk neonates is postnatal HBV vaccine in combination with hepatitis B immune globulin. Unfortunately, this immunoprophylaxis fails in 10% to 30% of infants born to mothers with an HBV DNA level of greater than 6 log 10 copies/mL. Thus, the observations of Pan and colleagues are welcome findings.
Based on the results of this study, we recommend the use of tenofovir to decrease HBV transmission during pregnancy for women with high viral loads.
Benefits of ART for reducing mother-to-baby HIV transmission outweigh higher risk of adverse outcomes
Fowler MG, Qin M, Fiscus SA, et al; IMPAACT 1077BF/1077FF PROMISE Study Team. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726-1737.
Part of the larger PROMISE (Promoting Maternal and Infant Survival Everywhere) trial, a study by Fowler and colleagues compared the relative efficacy and safety of various proven ART strategies for prevention of mother-to-child transmission of HIV infection in women with relatively high CD4 counts.
Details of the study
The trial was conducted at 14 sites in 7 countries. Patients were stratified according to HBV coinfection status and country of origin. The primary efficacy outcome was frequency of early infant HIV infection.
Women were randomly assigned to 1 of 3 treatment categories:
- zidovudine alone (zidovudine plus a single intrapartum dose of nevirapine, followed by 6 to 14 days of tenofovir plus emtricitabine postpartum)
- zidovudine-based ART (zidovudine in combination with lamivudine and lopinavir-ritonavir)
- tenofovir-based ART (tenofovir in combination with emtricitabine and lopinavir-ritonavir).
All regimens were continued through 6 to 14 days postpartum. All infants received nevirapine at birth and in the immediate postpartum period.
Two trial periods. During period 1 (April 2011-September 2012), safety data on tenofovir in pregnancy were limited. Women without HBV coinfection were assigned only to zidovudine alone or zidovudine-based ART. During period 2 (October 2012-October 2014), since more information about tenofovir use in pregnancy was available, the study protocol was modified to allow women to be assigned to any of the 3 regimens, regardless of their HBV status.
Inclusion criteria were as follows: CD4 count of at least 350 cells/mm3 (or country-specific threshold for initiating triple-drug ART, if that threshold was higher), gestation of at least 14 weeks and not in labor, no previous use of triple-drug ART, no clinical or immune-related indication for triple-drug ART, hemoglobin level of at least 6.5 g/dL, an absolute neutrophil count of at least 750 cells/mm3, an alanine aminotransferase level of less than 2.5 times the upper limit of normal range, an estimated creatinine clearance of greater than 60 mL/min, and no serious pregnancy complications. Patients were excluded if they had active tuberculosis, HBV infection requiring treatment, a structural or conduction heart defect, or a fetus with a serious congenital malformation.
Primary outcomes. The primary efficacy outcome was early infant HIV infection, defined as a positive infant HIV nucleic acid test result at birth or at 1 week postpartum. The primary safety outcome was a composite of adverse events.
Adverse events in mothers were defined as hematologic abnormalities, abnormal blood chemical values, or abnormal signs/symptoms during pregnancy through 1 week postpartum. Severe pregnancy composite outcomes were low birth weight (<2,500 g), preterm delivery before 37 weeks' gestation, spontaneous abortion (<20 weeks), stillbirth (≥20 weeks), or congenital anomaly. Adverse events in infants were defined as death from any cause, hematologic abnormalities or abnormal blood chemical values, and abnormal signs/symptoms through 1 week postpartum.
A total of 3,490 mother-infant sets were included in the analysis (2,261 during trial period 1 and 1,229 during trial period 2). Baseline maternal characteristics were well balanced between groups. Most women were African, young (median age, 26 years), and asymptomatic.
Related article:
2016 Update on infectious disease
Study results
The combined maternal ART-treated groups had significantly lower rates of early transmission of HIV infection compared with the zidovudine-alone group (0.5% vs 1.8%, -1.3 percentage points; CI, -2.1 to -0.4). The zidovudine-based ART-treated group had a significantly higher rate of infant HIV-free survival through postpartum week 1 than did the zidovudine-alone group (P = .001) or the tenofovir-based ART group (P = .002).
When examining trial periods 1 and 2 combined, the zidovudine-based ART group experienced significantly higher rates of any adverse event than those receiving zidovudine alone (21.1% vs 17.3%, P = .008) and higher rates of abnormal blood chemical values (5.8% vs 1.3%, P<.001). During period 2 alone, the tenofovir-based ART group had significantly higher rates of abnormal blood chemical values than did the zidovudine-alone group (2.9% vs 0.8%, P = .03). There were no significant differences between the 2 ART treatment groups. No maternal deaths occurred during the study, and the trial-drug discontinuation rate was low (2%-5%) and did not vary among the 3 groups.
During trial periods 1 and 2, the zidovudine-based ART group had significantly higher rates of adverse pregnancy outcomes than did the zidovudine-alone group (40% vs 27.5%, P<.001). These included low birth weight less than 2,500 g (23% vs 12%) and preterm delivery before 37 weeks (20.5% vs 13.1%). During trial period 2, the tenofovir-based ART group had significantly higher rates of adverse pregnancy outcomes than did the zidovudine-alone group (34.7% vs 27.2%, P = .04). There were no significant differences for any outcome between the 2 ART-treated groups, and there were no significant differences in stillbirth or spontaneous abortion and congenital anomalies among the 3 groups.
Regarding severe pregnancy outcomes, there were no significant differences (composite or individual) between the zidovudine-based ART group and the zidovudine-alone group. The tenofovir-based ART group experienced significantly higher rates of composite severe adverse pregnancy outcomes compared with the zidovudine-based ART group (9.2% vs 4.3%, P = .02), and very preterm birth before 34 weeks (6.0% vs 2.6%, P = .04).
Infant safety outcomes were also examined. There were no significant differences for composite or individual adverse neonatal outcomes other than death. The tenofovir-based ART group experienced a significantly higher rate of infant death than did the zidovudine-based ART group (4.4% vs 0.6%, P<.001). However, a post hoc analysis suggested that extreme prematurity contributed to the infant mortality.
Limitations of the study
This study had minor limitations. It divided patients into only 2 major categories with respect to gestational age--more than or less than 34 weeks. Some maternal medical conditions, such as malaria, were not controlled for. In addition, breastfeeding and formula feeding were combined for analysis, and we know that breastfeeding would inherently confer a higher risk of HIV transmission.
Nevertheless, this study was thoughtfully designed and carefully conducted, and the results are of significant global impact.
Although antenatal ART was associated with a higher risk of adverse maternal and neonatal outcomes when compared with zidovudine alone, these risks are outweighed by the benefit of significantly lower rates of early HIV transmission. Therefore, women who meet the World Health Organization's (WHO) eligibility criteria should be treated with combination ART during pregnancy. The WHO major eligibility criteria for ART during pregnancy are:
- CD4 count of ≤350 cells/mm3, irrespective of clinical staging
- clinical stage 3 or stage 4 disease, irrespective of CD4 cell count.
The WHO recommends starting ART at 14 weeks' gestation.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update we review the results of 4 recent investigations that have important implications:
- the first analysis of the US Zika Virus Infection in Pregnancy Registry
- a study revealing an improved antibiotic regimen to prevent postcesarean infection
- an important new methodology for reducing the rate of perinatal transmission of hepatitis B virus (HBV) infection
- the risks and benefits of combination antiretroviral therapy (ART) in pregnancy.
Zika virus-associated birth defect rates similar regardless of symptom presence; first-trimester exposure has highest rate of anomalies
Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59-68.
Honein and colleagues provide a summary of the data from the US Zika Virus in Pregnancy Registry (a collaboration between the Centers for Disease Control and Prevention and state and local health departments), estimating the proportion of fetuses and infants with birth defects based on maternal symptoms of Zika virus infection and trimester of possible infection.
Related article:
Zika virus: Counseling considerations for this emerging perinatal threat
Details of the study
The authors evaluated the outcomes of 442 women who had laboratory evidence of a possible Zika virus infection during pregnancy. Overall, 26 infants (6%; 95% confidence interval (CI), 4%-8%) had evidence of birth defects related to the Zika virus. Of note, abnormalities were detected in 16 of the 271 children (6%; 95% CI, 4%-9%) born to women who were asymptomatic and 10 of 167 (6%; 95% CI, 3%-11%) children delivered to women with symptomatic infections.
The most common birth defect was microcephaly, although other serious central nervous system abnormalities were noted as well. Nine of 85 women (11%; 95% CI, 6%-19%) who had exposure only during the first trimester had infants with birth defects. There were no documented abnormalities in infants born to mothers who developed Zika virus infection only in the second or third trimester.
Related article:
Zika virus update: A rapidly moving target
Key study findings
This article is important for several reasons. First, the authors describe the largest series of pregnant women in the United States with Zika virus infection. All of these patients developed Zika virus infection as a result of foreign travel or exposure to sexual partners who had traveled to Zika virus endemic areas. Second, the authors confirmed findings that previously had been based only on mathematical models rather than on actual case series. Specifically, they demonstrated that the risk of a serious birth defect following first-trimester exposure to Zika virus infection was approximately 11%, with a 95% CI that extended from 6% to 19%. Finally, Honein and colleagues highlighted the key fact that the risk of a serious birth defect was comparable in mothers who had either an asymptomatic or a symptomatic infection, a finding that seems somewhat counterintuitive.
This study's critical observations are a "call to action" for clinicians who provide prenatal care.1,2 Proactive steps include:
- For patients considering pregnancy, strongly advise against travel to any area of the world where Zika virus is endemic until an effective vaccine is available to protect against this infection.
- For any woman with a newly diagnosed pregnancy, ask about travel to an endemic area.
- Inquire also about a pregnant woman's exposure to partners who live in, or who have traveled to, areas of the world where Zika virus infection is endemic.
- Be aware that both asymptomatic and symptomatic infection in the first trimester of pregnancy pose a grave risk to the fetus.
- Recognize that, although microcephaly is the principal abnormality associated with Zika virus infection, other central nervous system anomalies also may occur in these children. These include ventriculomegaly, subcortical calcifications, abnormalities of the corpus callosum, cerebral atrophy, and cerebellar abnormalities. In addition, infected infants may have arthrogryposis.
- Finally, as Honein and colleagues noted, laboratory testing for Zika virus infection is imperfect. In the early stages of infection or exposure, testing for Zika virus infection by polymerase chain reaction (PCR) in both serum and urine is the preferred test. After a period of 2 weeks, the preferred laboratory test is an immunoglobulin M (IgM) assay. Positive tests on the IgM assay must be confirmed by the plaque neutralization reduction test--a very important test for differentiating Zika virus infection from infection caused by other arboviruses, such as those that cause dengue fever and chikungunya.
Read about prophylaxis for postcesarean infection
Two antibiotics before cesarean delivery reduce infection rates further than one agent
Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231-1241.
Tita and colleagues reported the results of a multicenter trial that was designed to assess whether a combination of 2 antibiotics, including one that specifically targets ureaplasma species, provided more effective prophylaxis against postcesarean infection than single-agent prophylaxis.
Details of the study
The Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) trial was conducted at 14 centers in the United States and included 2,013 women who were at least at 24 weeks' gestation and who had a cesarean delivery during labor or after membrane rupture.
The authors randomly assigned 1,019 women to receive 500 mg of intravenous azithromycin plus conventional single-agent prophylaxis (usually cefazolin) and 994 women to receive a placebo plus conventional prophylaxis. The primary outcome was the composite of endometritis, wound infection, or other infection occurring within 6 weeks.
The authors observed that the primary outcome occurred in 62 women (6.1%) who received azithromycin plus conventional prophylaxis and in 119 women (12%) who received only single-agent prophylaxis. The relative risk of developing a postoperative infection was 0.51 in women who received the combined therapy. There were significant differences between the 2 groups in both the rates of endometritis (3.8% vs 6.1%, P = .02) and wound infection (2.4% vs 6.6%, P<.001). There were no differences between the groups in the frequency of the secondary neonatal composite outcome, which included neonatal death and serious neonatal complications.
Related article:
Preventing infection after cesarean delivery: 5 more evidence-based measures to consider
Efficacy of dual-agent prophylaxis
At present, the standard of care is to administer prophylactic antibiotics to all women having cesarean delivery, including women having a scheduled cesarean in the absence of labor or ruptured membranes. Multiple studies have shown clearly that prophylaxis reduces the frequency of endometritis and, in high-risk patient populations, wound infection, and that prophylaxis is most beneficial when administered prior to the time the surgical incision is made. The most commonly used drug for prophylaxis is cefazolin, a first-generation cephalosporin. The usual recommended dose is 2 g, administered immediately prior to surgery.3,4
Although most centers in the United States traditionally have used just a single antibiotic for prophylaxis, selected recent reports indicate that expanding the spectrum of activity of prophylactic antibiotics can result in additional beneficial effects. Specifically, Tita and colleagues evaluated an indigent patient population with an inherently high rate of postoperative infection.5 They showed that adding azithromycin 500 mg to cefazolin significantly reduced the rate of postcesarean endometritis. In a follow-up report from the same institution, Tita and colleagues demonstrated that adding azithromycin also significantly reduced the frequency of wound infection.6 Of note, in both these investigations, the antibiotics were administered after cord clamping. In a subsequent report, Ward and Duff showed that the combination of azithromycin plus cefazolin administered preoperatively resulted in a combined rate of endometritis and wound infection that was less than 3%.7
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
C/SOAP trial confirmed lower infection rates with combined regimen
Results of the present study confirm the findings of these 3 investigations. The trial included a large sample size. The study was carefully designed, and the end points were clearly defined. It included only patients at increased risk for postoperative infection by virtue of being in labor or having ruptured membranes at the time of cesarean delivery. Patients who received standard prophylaxis, usually cefazolin, plus azithromycin had a significantly lower risk of postcesarean endometritis and wound infection compared with patients who received a single antibiotic. The overall risk of infection was reduced by an impressive 50%.
Based on the results of the C/SOAP trial, considered in conjunction with the 3 previously cited investigations,5-7 we believe that the standard approach to antibiotic prophylaxis should be to administer both cefazolin, in a dose of 2 g, plus azithromycin, in a dose of 500 mg, prior to surgery. Cefazolin can be administered as an intravenous bolus; azithromycin should be administered as a continuous infusion over a 60-minute period prior to surgery. Clinicians may anticipate very low rates of both endometritis and wound infection with this regimen.
Read about reducing HBV transmission
Tenofovir treatment in pregnant women with HBV reduces vertical transmission
Pan CQ, Duan Z, Dai E, et al; China Study Group for the Mother-to-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324-2334.
A multicenter, open-label, randomized, parallel-group investigation was conducted from March 2012 to June 2013 at academic tertiary care centers in 5 geographic regions of China. Two hundred mothers, who were positive for both hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) and who had HBV DNA concentrations of 200,000 IU/mL or greater, were randomly assigned in a 1:1 ratio to either tenofovir or to usual treatment. Exclusion criteria were coexistent viral infections or medical conditions, renal failure, laboratory abnormalities, fetal deformities, and use of many medications.
Related article:
5 ways to reduce infection risk during pregnancy
Details of the study
Women in the active treatment group received tenofovir 300 mg by mouth daily from 30 to 32 weeks' gestation until postpartum week 4. Patients were monitored every 4 weeks in the antepartum period for adverse events and laboratory abnormalities. In the postpartum period, mother-infant dyads were evaluated at weeks 4, 12, 24, and 28.
Primary outcomes were the rates of mother-to-child transmission and birth defects with, or without, tenofovir exposure. Secondary outcomes were the percentage of mothers who had an HBV DNA serum concentration of less than 200,000 IU/mL at delivery and the percentage of mothers with HBeAg or HBsAg loss or seroconversion at postpartum week 28. Safety outcomes included the adverse event profile of tenofovir in mothers and safety events in the mother-infant dyads. These outcomes encompassed all adverse events and drug discontinuations in patients who received at least one dose of tenofovir.
Sixty-eight percent of mothers in the tenofovir group, compared with 2% of mothers in the control group, had HBV levels less than 200,000 IU/mL at delivery (P<.001). The rate of mother-to-child HBV transmission at postpartum week 28 was lower in the tenofovir group. In the intention-to-treat analysis, the rate was 5% (95% CI, 1-10; 5 of 97 infants) in the tenofovir group versus 18% (95% CI, 10-26; 18 of 100 infants) in the control group (P = .007). In the per-protocol analysis, the rate was 0% (95% CI, 0-3; 0 of 92 infants) in the tenofovir group versus 7% (95% CI, 2-12; 6 of 88 infants) in the control group (P = .01). Maternal and infant safety profiles were similar between the 2 groups, with the exception of elevated creatinine kinase and alanine aminotransferase levels in mothers treated with tenofovir. Maternal HBV serologic titers did not differ significantly between the 2 groups.
Study strengths and limitations
This study's strengths include a multicenter, randomized controlled design, with strict inclusion and exclusion criteria. The results are clinically relevant and of global impact, with potential to decrease morbidity and mortality from HBV infection in children born to infected mothers.
A limitation, however, is that the study was probably underpowered to detect small differences in the rate of birth defects between the tenofovir and usual-care treatment groups. Additionally, some patients ceased taking tenofovir in the postpartum time period. Abrupt cessation may be associated with acute, severe HBV exacerbation.
HBV is a serious infection that can lead to liver failure and cirrhosis. HBV infection is most likely to have long-term sequelae if acquired in the perinatal period. If untreated, chronic HBV infection will develop in 80% to 90% of infants born to mothers positive for HBeAg. Current immunoprophylaxis for at-risk neonates is postnatal HBV vaccine in combination with hepatitis B immune globulin. Unfortunately, this immunoprophylaxis fails in 10% to 30% of infants born to mothers with an HBV DNA level of greater than 6 log 10 copies/mL. Thus, the observations of Pan and colleagues are welcome findings.
Based on the results of this study, we recommend the use of tenofovir to decrease HBV transmission during pregnancy for women with high viral loads.
Benefits of ART for reducing mother-to-baby HIV transmission outweigh higher risk of adverse outcomes
Fowler MG, Qin M, Fiscus SA, et al; IMPAACT 1077BF/1077FF PROMISE Study Team. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726-1737.
Part of the larger PROMISE (Promoting Maternal and Infant Survival Everywhere) trial, a study by Fowler and colleagues compared the relative efficacy and safety of various proven ART strategies for prevention of mother-to-child transmission of HIV infection in women with relatively high CD4 counts.
Details of the study
The trial was conducted at 14 sites in 7 countries. Patients were stratified according to HBV coinfection status and country of origin. The primary efficacy outcome was frequency of early infant HIV infection.
Women were randomly assigned to 1 of 3 treatment categories:
- zidovudine alone (zidovudine plus a single intrapartum dose of nevirapine, followed by 6 to 14 days of tenofovir plus emtricitabine postpartum)
- zidovudine-based ART (zidovudine in combination with lamivudine and lopinavir-ritonavir)
- tenofovir-based ART (tenofovir in combination with emtricitabine and lopinavir-ritonavir).
All regimens were continued through 6 to 14 days postpartum. All infants received nevirapine at birth and in the immediate postpartum period.
Two trial periods. During period 1 (April 2011-September 2012), safety data on tenofovir in pregnancy were limited. Women without HBV coinfection were assigned only to zidovudine alone or zidovudine-based ART. During period 2 (October 2012-October 2014), since more information about tenofovir use in pregnancy was available, the study protocol was modified to allow women to be assigned to any of the 3 regimens, regardless of their HBV status.
Inclusion criteria were as follows: CD4 count of at least 350 cells/mm3 (or country-specific threshold for initiating triple-drug ART, if that threshold was higher), gestation of at least 14 weeks and not in labor, no previous use of triple-drug ART, no clinical or immune-related indication for triple-drug ART, hemoglobin level of at least 6.5 g/dL, an absolute neutrophil count of at least 750 cells/mm3, an alanine aminotransferase level of less than 2.5 times the upper limit of normal range, an estimated creatinine clearance of greater than 60 mL/min, and no serious pregnancy complications. Patients were excluded if they had active tuberculosis, HBV infection requiring treatment, a structural or conduction heart defect, or a fetus with a serious congenital malformation.
Primary outcomes. The primary efficacy outcome was early infant HIV infection, defined as a positive infant HIV nucleic acid test result at birth or at 1 week postpartum. The primary safety outcome was a composite of adverse events.
Adverse events in mothers were defined as hematologic abnormalities, abnormal blood chemical values, or abnormal signs/symptoms during pregnancy through 1 week postpartum. Severe pregnancy composite outcomes were low birth weight (<2,500 g), preterm delivery before 37 weeks' gestation, spontaneous abortion (<20 weeks), stillbirth (≥20 weeks), or congenital anomaly. Adverse events in infants were defined as death from any cause, hematologic abnormalities or abnormal blood chemical values, and abnormal signs/symptoms through 1 week postpartum.
A total of 3,490 mother-infant sets were included in the analysis (2,261 during trial period 1 and 1,229 during trial period 2). Baseline maternal characteristics were well balanced between groups. Most women were African, young (median age, 26 years), and asymptomatic.
Related article:
2016 Update on infectious disease
Study results
The combined maternal ART-treated groups had significantly lower rates of early transmission of HIV infection compared with the zidovudine-alone group (0.5% vs 1.8%, -1.3 percentage points; CI, -2.1 to -0.4). The zidovudine-based ART-treated group had a significantly higher rate of infant HIV-free survival through postpartum week 1 than did the zidovudine-alone group (P = .001) or the tenofovir-based ART group (P = .002).
When examining trial periods 1 and 2 combined, the zidovudine-based ART group experienced significantly higher rates of any adverse event than those receiving zidovudine alone (21.1% vs 17.3%, P = .008) and higher rates of abnormal blood chemical values (5.8% vs 1.3%, P<.001). During period 2 alone, the tenofovir-based ART group had significantly higher rates of abnormal blood chemical values than did the zidovudine-alone group (2.9% vs 0.8%, P = .03). There were no significant differences between the 2 ART treatment groups. No maternal deaths occurred during the study, and the trial-drug discontinuation rate was low (2%-5%) and did not vary among the 3 groups.
During trial periods 1 and 2, the zidovudine-based ART group had significantly higher rates of adverse pregnancy outcomes than did the zidovudine-alone group (40% vs 27.5%, P<.001). These included low birth weight less than 2,500 g (23% vs 12%) and preterm delivery before 37 weeks (20.5% vs 13.1%). During trial period 2, the tenofovir-based ART group had significantly higher rates of adverse pregnancy outcomes than did the zidovudine-alone group (34.7% vs 27.2%, P = .04). There were no significant differences for any outcome between the 2 ART-treated groups, and there were no significant differences in stillbirth or spontaneous abortion and congenital anomalies among the 3 groups.
Regarding severe pregnancy outcomes, there were no significant differences (composite or individual) between the zidovudine-based ART group and the zidovudine-alone group. The tenofovir-based ART group experienced significantly higher rates of composite severe adverse pregnancy outcomes compared with the zidovudine-based ART group (9.2% vs 4.3%, P = .02), and very preterm birth before 34 weeks (6.0% vs 2.6%, P = .04).
Infant safety outcomes were also examined. There were no significant differences for composite or individual adverse neonatal outcomes other than death. The tenofovir-based ART group experienced a significantly higher rate of infant death than did the zidovudine-based ART group (4.4% vs 0.6%, P<.001). However, a post hoc analysis suggested that extreme prematurity contributed to the infant mortality.
Limitations of the study
This study had minor limitations. It divided patients into only 2 major categories with respect to gestational age--more than or less than 34 weeks. Some maternal medical conditions, such as malaria, were not controlled for. In addition, breastfeeding and formula feeding were combined for analysis, and we know that breastfeeding would inherently confer a higher risk of HIV transmission.
Nevertheless, this study was thoughtfully designed and carefully conducted, and the results are of significant global impact.
Although antenatal ART was associated with a higher risk of adverse maternal and neonatal outcomes when compared with zidovudine alone, these risks are outweighed by the benefit of significantly lower rates of early HIV transmission. Therefore, women who meet the World Health Organization's (WHO) eligibility criteria should be treated with combination ART during pregnancy. The WHO major eligibility criteria for ART during pregnancy are:
- CD4 count of ≤350 cells/mm3, irrespective of clinical staging
- clinical stage 3 or stage 4 disease, irrespective of CD4 cell count.
The WHO recommends starting ART at 14 weeks' gestation.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28-34.
- Chelliah A, Duff P. Zika virus update: a rapidly moving target. OBG Manag. 2016;28(8):17-26.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41-47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidenced-based methods to consider. OBG Manag. 2016;28(12):18-22.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51-56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1-e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1-e4.
- New guidance on prevention of mother-to-child transmission of HIV and infant feeding in the context of HIV. World Health Organization website. http://www.who.int/hiv/pub/mtct/PMTCTfactsheet/en/. Published July 20, 2010. Accessed June 16, 2017.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28-34.
- Chelliah A, Duff P. Zika virus update: a rapidly moving target. OBG Manag. 2016;28(8):17-26.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41-47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidenced-based methods to consider. OBG Manag. 2016;28(12):18-22.
- Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51-56.
- Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1-e3.
- Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1-e4.
- New guidance on prevention of mother-to-child transmission of HIV and infant feeding in the context of HIV. World Health Organization website. http://www.who.int/hiv/pub/mtct/PMTCTfactsheet/en/. Published July 20, 2010. Accessed June 16, 2017.
Should the length of treatment for trichomoniasis in women be reconsidered?
EXPERT COMMENTARY
Both the Centers for Disease Control and Prevention and the World Health Organization currently recommend that patients with trichomoniasis be treated with a single 2-g oral dose of metronidazole.1 Following treatment, the reported rates of repeat infection or persistent infection range from 5% to 31%. Repeat infection rates may be even higher in HIV-infected patients.
Repeat infections presumably result from a failure to treat the patient’s sexual partner(s) or from the patient’s exposure to a new partner. Persistent infections, however, may be the result of inadequate primary therapy, even though inherent resistance of the organism to metronidazole is quite rare. To date, no single study has shown that single-dose therapy is inferior to multidose therapy, but most of these studies lack sufficient power to completely exclude the possibility of a type-2 statistical error.2 To compare single-dose with multidose therapy for trichomoniasis in a more systematic manner, Howe and Kissinger conducted a meta-analysis, which was recently published in Sexually Transmitted Diseases.
Related article:
2016 Update on infectious disease
Details of the study
The investigators conducted a comprehensive literature search using Embase, Medline, and ClinicalTrials.gov; 6 articles were included in the final results, 4 of which were randomized controlled trials. Approximately 1,300 participants were included in the 6 trials. All of the patients in the single-dose treatment arms received a 2-g oral dose of metronidazole. In the multidose treatment arms for 2 studies the participants received metronidazole 250 mg orally 3 times daily for 7 days, and for 2 studies the dose was 200 mg 3 times daily for 7 days. The fifth study employed a 500-mg oral dose of metronidazole twice daily for 7 days. The final study used a 400-mg oral dose twice daily for 5 days. The key study end point was treatment failure.
Howe and Kissinger demonstrated that women who received the single 2-g dose were 1.87 times (95% CI, 1.23−2.82; P<.01) more likely to experience a treatment failure compared with women who received a multidose regimen. When the one study that focused only on HIV-infected women was excluded from analysis, the results were similar. The relative risk of treatment failure was 1.80 (95% CI, 1.07−3.02; P<.03).
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
Study limitations
The results of this meta-analysis are interesting and provocative. However, the analysis has several important limitations. Five of the 6 studies were published many years ago (1971, 1972, 1979, 1980, and 1982). The most recent study was published in 2010. The investigators used 4 different multidose regimens, with metronidazole doses ranging from 200 mg to 500 mg and duration of therapy ranging from 5 to 7 days. Four of the six investigations used saline microscopy as the definitive diagnostic test of treatment failure. Compared with culture or DNA testing, microscopy is not as accurate. Moreover, the timing of retesting varied in the studies, and some apparent treatment failures actually may have been due to reinfection. In addition, the studies did not consistently track the adequacy of treatment of the sexual partner.
To be sure, we would benefit from a new comparative study that included a large sample size, a consistent multidose regimen, rigorous treatment of the sexual partner(s), and more sophisticated diagnostic testing to define treatment failure. Pending the publication of such a study, however, I plan to alter my practice pattern and treat infected patients with a multidose regimen of metronidazole. I favor the regimen of 500 mg orally twice daily for 7 days because it is effective against both trichomoniasis and bacterial vaginosis, which is a common co-infection.
The twice-daily regimen is more convenient than the thrice-daily regimen and is not much more expensive than the single-dose regimen ($13 vs $4, http://www.goodrx.com). I will reserve the single 2-g dose of metronidazole for patients in whom treatment adherence is likely to be a problem or for patients in whom an immediate response to treatment is imperative (eg, a patient with preterm premature rupture of membranes or preterm labor).
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29−34.
EXPERT COMMENTARY
Both the Centers for Disease Control and Prevention and the World Health Organization currently recommend that patients with trichomoniasis be treated with a single 2-g oral dose of metronidazole.1 Following treatment, the reported rates of repeat infection or persistent infection range from 5% to 31%. Repeat infection rates may be even higher in HIV-infected patients.
Repeat infections presumably result from a failure to treat the patient’s sexual partner(s) or from the patient’s exposure to a new partner. Persistent infections, however, may be the result of inadequate primary therapy, even though inherent resistance of the organism to metronidazole is quite rare. To date, no single study has shown that single-dose therapy is inferior to multidose therapy, but most of these studies lack sufficient power to completely exclude the possibility of a type-2 statistical error.2 To compare single-dose with multidose therapy for trichomoniasis in a more systematic manner, Howe and Kissinger conducted a meta-analysis, which was recently published in Sexually Transmitted Diseases.
Related article:
2016 Update on infectious disease
Details of the study
The investigators conducted a comprehensive literature search using Embase, Medline, and ClinicalTrials.gov; 6 articles were included in the final results, 4 of which were randomized controlled trials. Approximately 1,300 participants were included in the 6 trials. All of the patients in the single-dose treatment arms received a 2-g oral dose of metronidazole. In the multidose treatment arms for 2 studies the participants received metronidazole 250 mg orally 3 times daily for 7 days, and for 2 studies the dose was 200 mg 3 times daily for 7 days. The fifth study employed a 500-mg oral dose of metronidazole twice daily for 7 days. The final study used a 400-mg oral dose twice daily for 5 days. The key study end point was treatment failure.
Howe and Kissinger demonstrated that women who received the single 2-g dose were 1.87 times (95% CI, 1.23−2.82; P<.01) more likely to experience a treatment failure compared with women who received a multidose regimen. When the one study that focused only on HIV-infected women was excluded from analysis, the results were similar. The relative risk of treatment failure was 1.80 (95% CI, 1.07−3.02; P<.03).
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
Study limitations
The results of this meta-analysis are interesting and provocative. However, the analysis has several important limitations. Five of the 6 studies were published many years ago (1971, 1972, 1979, 1980, and 1982). The most recent study was published in 2010. The investigators used 4 different multidose regimens, with metronidazole doses ranging from 200 mg to 500 mg and duration of therapy ranging from 5 to 7 days. Four of the six investigations used saline microscopy as the definitive diagnostic test of treatment failure. Compared with culture or DNA testing, microscopy is not as accurate. Moreover, the timing of retesting varied in the studies, and some apparent treatment failures actually may have been due to reinfection. In addition, the studies did not consistently track the adequacy of treatment of the sexual partner.
To be sure, we would benefit from a new comparative study that included a large sample size, a consistent multidose regimen, rigorous treatment of the sexual partner(s), and more sophisticated diagnostic testing to define treatment failure. Pending the publication of such a study, however, I plan to alter my practice pattern and treat infected patients with a multidose regimen of metronidazole. I favor the regimen of 500 mg orally twice daily for 7 days because it is effective against both trichomoniasis and bacterial vaginosis, which is a common co-infection.
The twice-daily regimen is more convenient than the thrice-daily regimen and is not much more expensive than the single-dose regimen ($13 vs $4, http://www.goodrx.com). I will reserve the single 2-g dose of metronidazole for patients in whom treatment adherence is likely to be a problem or for patients in whom an immediate response to treatment is imperative (eg, a patient with preterm premature rupture of membranes or preterm labor).
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Both the Centers for Disease Control and Prevention and the World Health Organization currently recommend that patients with trichomoniasis be treated with a single 2-g oral dose of metronidazole.1 Following treatment, the reported rates of repeat infection or persistent infection range from 5% to 31%. Repeat infection rates may be even higher in HIV-infected patients.
Repeat infections presumably result from a failure to treat the patient’s sexual partner(s) or from the patient’s exposure to a new partner. Persistent infections, however, may be the result of inadequate primary therapy, even though inherent resistance of the organism to metronidazole is quite rare. To date, no single study has shown that single-dose therapy is inferior to multidose therapy, but most of these studies lack sufficient power to completely exclude the possibility of a type-2 statistical error.2 To compare single-dose with multidose therapy for trichomoniasis in a more systematic manner, Howe and Kissinger conducted a meta-analysis, which was recently published in Sexually Transmitted Diseases.
Related article:
2016 Update on infectious disease
Details of the study
The investigators conducted a comprehensive literature search using Embase, Medline, and ClinicalTrials.gov; 6 articles were included in the final results, 4 of which were randomized controlled trials. Approximately 1,300 participants were included in the 6 trials. All of the patients in the single-dose treatment arms received a 2-g oral dose of metronidazole. In the multidose treatment arms for 2 studies the participants received metronidazole 250 mg orally 3 times daily for 7 days, and for 2 studies the dose was 200 mg 3 times daily for 7 days. The fifth study employed a 500-mg oral dose of metronidazole twice daily for 7 days. The final study used a 400-mg oral dose twice daily for 5 days. The key study end point was treatment failure.
Howe and Kissinger demonstrated that women who received the single 2-g dose were 1.87 times (95% CI, 1.23−2.82; P<.01) more likely to experience a treatment failure compared with women who received a multidose regimen. When the one study that focused only on HIV-infected women was excluded from analysis, the results were similar. The relative risk of treatment failure was 1.80 (95% CI, 1.07−3.02; P<.03).
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
Study limitations
The results of this meta-analysis are interesting and provocative. However, the analysis has several important limitations. Five of the 6 studies were published many years ago (1971, 1972, 1979, 1980, and 1982). The most recent study was published in 2010. The investigators used 4 different multidose regimens, with metronidazole doses ranging from 200 mg to 500 mg and duration of therapy ranging from 5 to 7 days. Four of the six investigations used saline microscopy as the definitive diagnostic test of treatment failure. Compared with culture or DNA testing, microscopy is not as accurate. Moreover, the timing of retesting varied in the studies, and some apparent treatment failures actually may have been due to reinfection. In addition, the studies did not consistently track the adequacy of treatment of the sexual partner.
To be sure, we would benefit from a new comparative study that included a large sample size, a consistent multidose regimen, rigorous treatment of the sexual partner(s), and more sophisticated diagnostic testing to define treatment failure. Pending the publication of such a study, however, I plan to alter my practice pattern and treat infected patients with a multidose regimen of metronidazole. I favor the regimen of 500 mg orally twice daily for 7 days because it is effective against both trichomoniasis and bacterial vaginosis, which is a common co-infection.
The twice-daily regimen is more convenient than the thrice-daily regimen and is not much more expensive than the single-dose regimen ($13 vs $4, http://www.goodrx.com). I will reserve the single 2-g dose of metronidazole for patients in whom treatment adherence is likely to be a problem or for patients in whom an immediate response to treatment is imperative (eg, a patient with preterm premature rupture of membranes or preterm labor).
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29−34.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29−34.
Preventing infection after cesarean delivery: 5 more evidence-based measures to consider
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.
Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.
Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.
Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
Zika virus update: A rapidly moving target
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.
Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.
How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.
How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.
How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.
How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
In this Article
- Confirming Zika virus infection
- Zika virus and Guillain-Barré syndrome
- Preventing sexual transmission