User login
Infectious disease pop quiz: Clinical challenges for the ObGyn
In this question-and-answer article (the first in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
1. What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
2. Which major organisms cause urinary tract infections (UTIs) in women?
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
3. What are the major complications of pyelonephritis in pregnancy?
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
4. What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
5. What are the major manifestations of congenital rubella syndrome?
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
6. Which vaccines are contraindicated in pregnancy?
Live virus vaccines should not be used in pregnancy because of the possibility of teratogenic effects. Live agents include the measles, mumps, and rubella (MMR) vaccine; live influenza vaccine (FluMist); oral polio vaccine; BCG (bacille Calmette-Guerin) vaccine; yellow fever vaccine; and smallpox vaccine.
7. What is the most appropriate treatment for trichomonas infection in pregnancy?
Trichomonas infection should be treated with oral metronidazole 500 mg twice daily for 7 days. Metronidazole also can be given as a single oral 2-g dose. This treatment is not quite as effective as the multidose regimen, but it may be appropriate for patients who are not likely to be adherent with the longer course of treatment.
Resistance to metronidazole is rare; in such instances, oral tinidazole 2 g in a single dose may be effective.
8. For uncomplicated gonorrhea in a pregnant woman, what is the most appropriate treatment?
The current recommendation from the Centers for Disease Control and Prevention for treatment of uncomplicated gonorrhea is a single 500-mg intramuscular dose of ceftriaxone. For the patient who is opposed to an intramuscular injection, an alternative treatment is cefixime 800 mg orally. With either of these regimens, if chlamydia infection cannot be excluded, the pregnant patient also should receive azithromycin 1,000 mg orally in a single dose. In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days should be used to cover for concurrent chlamydia infection.
In a patient with an allergy to β-lactam antibiotics, an alternative regimen for treatment of uncomplicated gonorrhea is intramuscular gentamicin 240 mg plus a single 2,000-mg dose of oral azithromycin. (St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.) ●
1. Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
2. Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In this question-and-answer article (the first in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
1. What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
2. Which major organisms cause urinary tract infections (UTIs) in women?
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
3. What are the major complications of pyelonephritis in pregnancy?
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
4. What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
5. What are the major manifestations of congenital rubella syndrome?
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
6. Which vaccines are contraindicated in pregnancy?
Live virus vaccines should not be used in pregnancy because of the possibility of teratogenic effects. Live agents include the measles, mumps, and rubella (MMR) vaccine; live influenza vaccine (FluMist); oral polio vaccine; BCG (bacille Calmette-Guerin) vaccine; yellow fever vaccine; and smallpox vaccine.
7. What is the most appropriate treatment for trichomonas infection in pregnancy?
Trichomonas infection should be treated with oral metronidazole 500 mg twice daily for 7 days. Metronidazole also can be given as a single oral 2-g dose. This treatment is not quite as effective as the multidose regimen, but it may be appropriate for patients who are not likely to be adherent with the longer course of treatment.
Resistance to metronidazole is rare; in such instances, oral tinidazole 2 g in a single dose may be effective.
8. For uncomplicated gonorrhea in a pregnant woman, what is the most appropriate treatment?
The current recommendation from the Centers for Disease Control and Prevention for treatment of uncomplicated gonorrhea is a single 500-mg intramuscular dose of ceftriaxone. For the patient who is opposed to an intramuscular injection, an alternative treatment is cefixime 800 mg orally. With either of these regimens, if chlamydia infection cannot be excluded, the pregnant patient also should receive azithromycin 1,000 mg orally in a single dose. In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days should be used to cover for concurrent chlamydia infection.
In a patient with an allergy to β-lactam antibiotics, an alternative regimen for treatment of uncomplicated gonorrhea is intramuscular gentamicin 240 mg plus a single 2,000-mg dose of oral azithromycin. (St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.) ●
In this question-and-answer article (the first in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
1. What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
2. Which major organisms cause urinary tract infections (UTIs) in women?
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
3. What are the major complications of pyelonephritis in pregnancy?
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
4. What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
5. What are the major manifestations of congenital rubella syndrome?
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
6. Which vaccines are contraindicated in pregnancy?
Live virus vaccines should not be used in pregnancy because of the possibility of teratogenic effects. Live agents include the measles, mumps, and rubella (MMR) vaccine; live influenza vaccine (FluMist); oral polio vaccine; BCG (bacille Calmette-Guerin) vaccine; yellow fever vaccine; and smallpox vaccine.
7. What is the most appropriate treatment for trichomonas infection in pregnancy?
Trichomonas infection should be treated with oral metronidazole 500 mg twice daily for 7 days. Metronidazole also can be given as a single oral 2-g dose. This treatment is not quite as effective as the multidose regimen, but it may be appropriate for patients who are not likely to be adherent with the longer course of treatment.
Resistance to metronidazole is rare; in such instances, oral tinidazole 2 g in a single dose may be effective.
8. For uncomplicated gonorrhea in a pregnant woman, what is the most appropriate treatment?
The current recommendation from the Centers for Disease Control and Prevention for treatment of uncomplicated gonorrhea is a single 500-mg intramuscular dose of ceftriaxone. For the patient who is opposed to an intramuscular injection, an alternative treatment is cefixime 800 mg orally. With either of these regimens, if chlamydia infection cannot be excluded, the pregnant patient also should receive azithromycin 1,000 mg orally in a single dose. In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days should be used to cover for concurrent chlamydia infection.
In a patient with an allergy to β-lactam antibiotics, an alternative regimen for treatment of uncomplicated gonorrhea is intramuscular gentamicin 240 mg plus a single 2,000-mg dose of oral azithromycin. (St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.) ●
1. Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
2. Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
1. Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
2. Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Vaccinations for the ObGyn’s toolbox
CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.
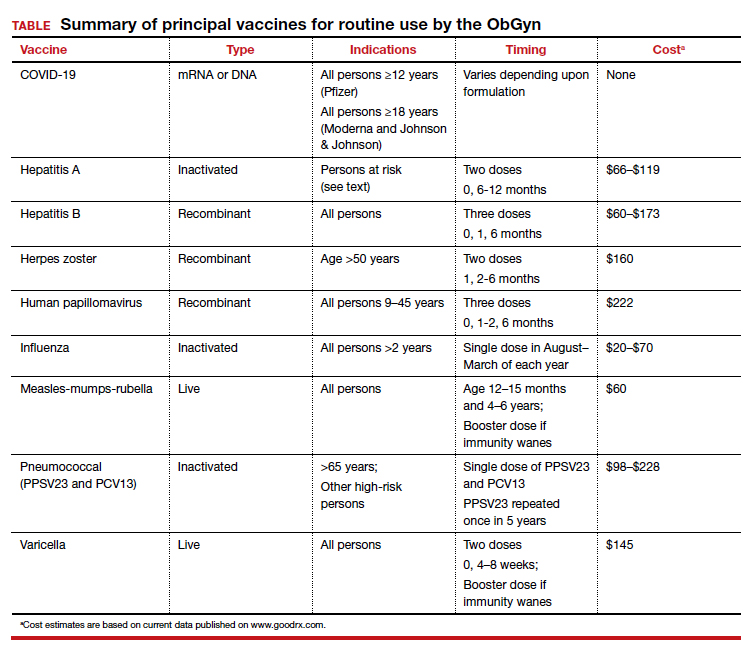
COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
Hepatitis in pregnancy: Sorting through the alphabet
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).
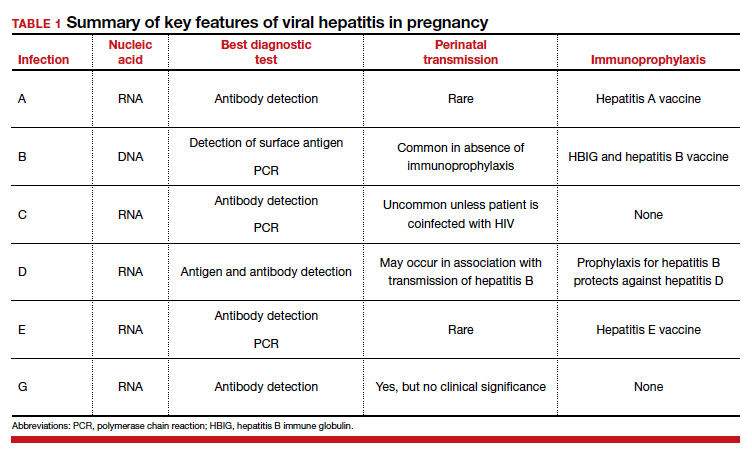
Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).
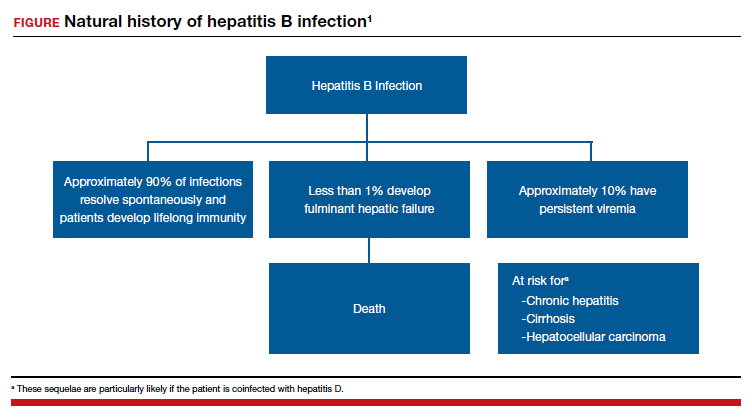

All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
Managing herpes simplex virus genital infection in pregnancy
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.
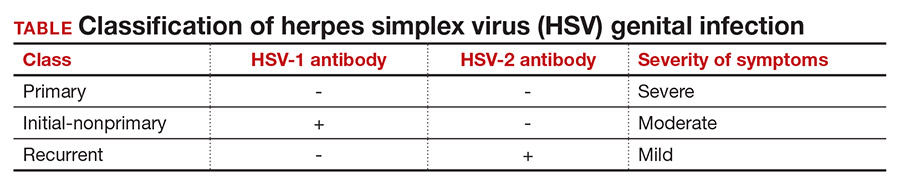
Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1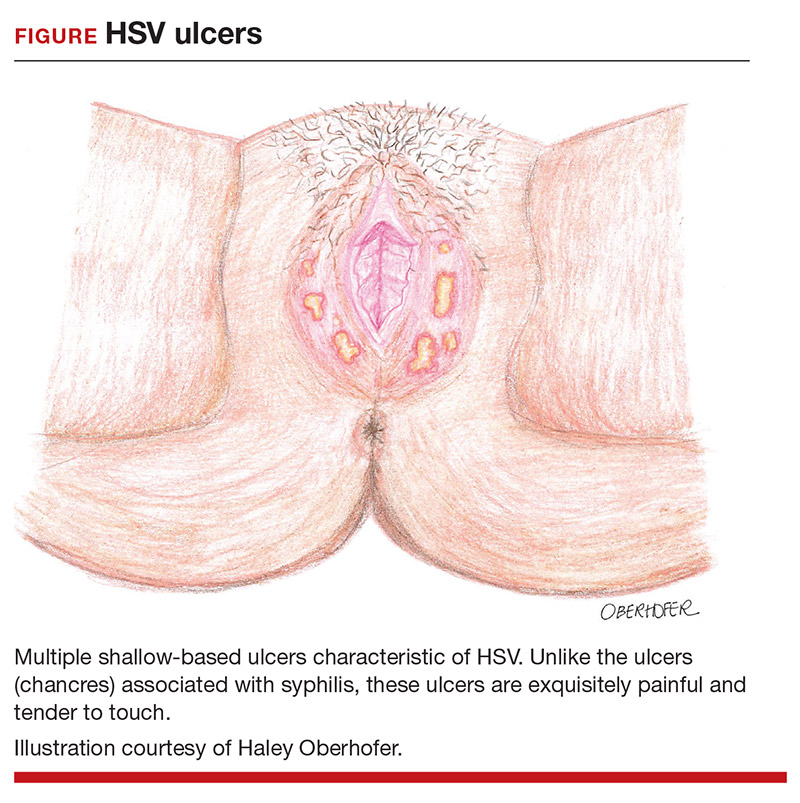
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
A case of BV during pregnancy: Best management approach
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.
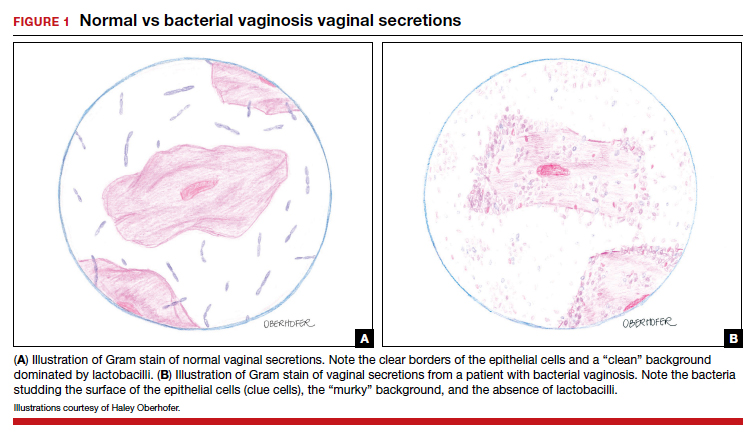
BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.
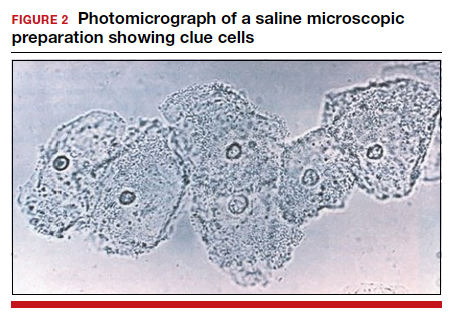
For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.
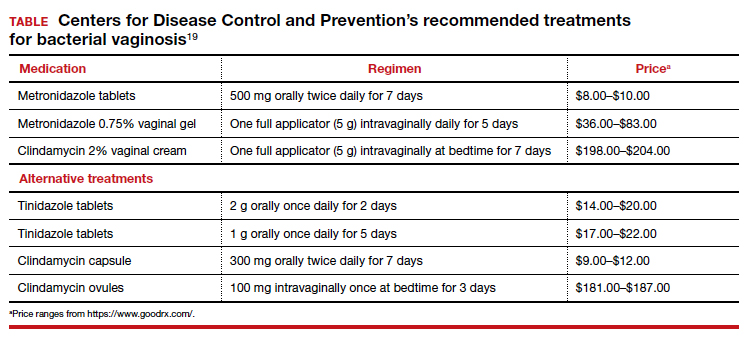
Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
Syphilis: Cutting risk through primary prevention and prenatal screening
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
Candidiasis: The essentials of diagnosis and treatment
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8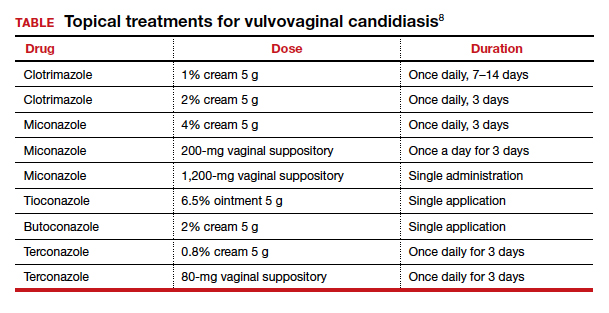
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
Managing Trichomonas vaginalis infections
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18
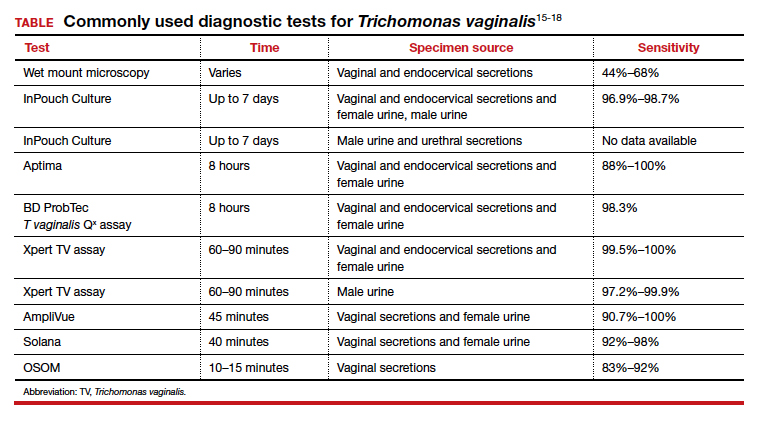
Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
Chlamydia trachomatis infections
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
COVID-19 during pregnancy: How would you proceed in this case of a novel and ominous emerging pathogen?
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).
Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.