User login
Most endometrial cancers treated with minimally invasive procedures
Of 3,730 women with endometrial cancer in the Society of Gynecologic Oncology Clinical Outcomes Registry (SGO-COR), 88.8% underwent minimally invasive procedures, reported Amanda Nickles Fader, MD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“When you have surgery with a gyn-oncologist who is specially trained in this type of surgery, we see that women have a very high likelihood of having the appropriate surgery, the minimally invasive surgery, and we thought that this benchmark of an 80% rate of minimally invasive surgery in these patients is very feasible and should be recognized as the standard of care,” Dr. Nickles Fader said in an interview.
Coinvestigator Summer B. Dewdney, MD, of Rush University Medical Center, Chicago, who was instrumental in creating and running the SGO-COR registry, said these findings are encouraging.
“We’re happy to see that rate. It’s the rate that it should be because minimally invasive surgery is the standard of care for endometrial cancer,” Dr. Dewdney said. She added, however, that data supplied to the registry come from gynecologic oncologists who are highly motivated to participate and follow best practice guidelines, which could skew the results slightly toward more favorable outcomes.
Results of the registry-based study are detailed in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Assessing adherence to guidelines
In 2015, the SGO Clinical Practice Committee and the American College of Obstetricians and Gynecologists issued a practice bulletin, which stated that “minimally invasive surgery should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”
Similarly, National Comprehensive Cancer Network guidelines for uterine cancer state that “minimally invasive surgery is the preferred approach when technically feasible” for treatment of endometrial cancer confined to the uterus.
Despite these recommendations, the overall rate of minimally invasive endometrial cancer surgery in the United States is reported be around to 60%, Dr. Nickles Fader and colleagues wrote.
With this in mind, the investigators set out to determine the rate of minimally invasive surgery in women with apparent stage I, II, or III endometrial cancer who underwent hysterectomy with or without staging from 2012 to 2017 at a center reporting to SGO-COR.
The team identified 3,730 women treated at 25 SGO-COR centers; 12 of which were university-affiliated centers and 13 of which were nonuniversity based. Most patients (83.2%) had stage I disease, 4.7% had stage II cancer, and 12.1% had stage III disease. The median patient age was 57 years. Most patients (88%) were white, and two-thirds (67.1%) were obese. In all, 80.4% of samples had endometrioid histology, and 77.7% were either grade 1 or 2.
Factors associated with minimally invasive surgery
The data showed that 88.8% of patients underwent a minimally invasive hysterectomy, composed of robotic-assisted procedures in 73.9% of cases, laparoscopy in 13.4%, and vaginal access in 1.6%.
The proportion of patients who underwent a minimally invasive procedure was significantly higher at nonuniversity centers, compared with academic centers (92.6% vs. 82.7%; P < .0001), but rates of minimally invasive procedures did not differ significantly across U.S. geographic regions.
Dr. Dewdney said that the higher proportion of open surgeries performed at university centers may be attributable to those centers treating patients with more advanced disease or rare aggressive cancers that may not be amenable to a minimally invasive approach.
In a multivariate analysis, factors associated with a failure to perform minimally invasive surgery were black race of the patient (adjusted odds ratio, 0.57), body mass index over 35 kg/m2 (aOR, 1.40), stage II disease (aOR, 0.49), stage III disease (aOR, 0.36), carcinosarcoma/leiomyosarcoma (aOR, 0.58), and university hospital (aOR, 3.46).
Looking at perioperative complications, the investigators found that laparotomy was associated with more in-hospital complications than minimally invasive procedures, including more unscheduled ICU stays (P < .001) and prolonged hospital stays (P = .0002).
Dr. Dewdney said that investigators are planning further registry-based studies focusing on ovarian cancer, uterine cancer, and cervical cancer.
Dr. Nickles Fader and Dr. Dewdney reported having no relevant conflicts of interest.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 63.
Of 3,730 women with endometrial cancer in the Society of Gynecologic Oncology Clinical Outcomes Registry (SGO-COR), 88.8% underwent minimally invasive procedures, reported Amanda Nickles Fader, MD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“When you have surgery with a gyn-oncologist who is specially trained in this type of surgery, we see that women have a very high likelihood of having the appropriate surgery, the minimally invasive surgery, and we thought that this benchmark of an 80% rate of minimally invasive surgery in these patients is very feasible and should be recognized as the standard of care,” Dr. Nickles Fader said in an interview.
Coinvestigator Summer B. Dewdney, MD, of Rush University Medical Center, Chicago, who was instrumental in creating and running the SGO-COR registry, said these findings are encouraging.
“We’re happy to see that rate. It’s the rate that it should be because minimally invasive surgery is the standard of care for endometrial cancer,” Dr. Dewdney said. She added, however, that data supplied to the registry come from gynecologic oncologists who are highly motivated to participate and follow best practice guidelines, which could skew the results slightly toward more favorable outcomes.
Results of the registry-based study are detailed in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Assessing adherence to guidelines
In 2015, the SGO Clinical Practice Committee and the American College of Obstetricians and Gynecologists issued a practice bulletin, which stated that “minimally invasive surgery should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”
Similarly, National Comprehensive Cancer Network guidelines for uterine cancer state that “minimally invasive surgery is the preferred approach when technically feasible” for treatment of endometrial cancer confined to the uterus.
Despite these recommendations, the overall rate of minimally invasive endometrial cancer surgery in the United States is reported be around to 60%, Dr. Nickles Fader and colleagues wrote.
With this in mind, the investigators set out to determine the rate of minimally invasive surgery in women with apparent stage I, II, or III endometrial cancer who underwent hysterectomy with or without staging from 2012 to 2017 at a center reporting to SGO-COR.
The team identified 3,730 women treated at 25 SGO-COR centers; 12 of which were university-affiliated centers and 13 of which were nonuniversity based. Most patients (83.2%) had stage I disease, 4.7% had stage II cancer, and 12.1% had stage III disease. The median patient age was 57 years. Most patients (88%) were white, and two-thirds (67.1%) were obese. In all, 80.4% of samples had endometrioid histology, and 77.7% were either grade 1 or 2.
Factors associated with minimally invasive surgery
The data showed that 88.8% of patients underwent a minimally invasive hysterectomy, composed of robotic-assisted procedures in 73.9% of cases, laparoscopy in 13.4%, and vaginal access in 1.6%.
The proportion of patients who underwent a minimally invasive procedure was significantly higher at nonuniversity centers, compared with academic centers (92.6% vs. 82.7%; P < .0001), but rates of minimally invasive procedures did not differ significantly across U.S. geographic regions.
Dr. Dewdney said that the higher proportion of open surgeries performed at university centers may be attributable to those centers treating patients with more advanced disease or rare aggressive cancers that may not be amenable to a minimally invasive approach.
In a multivariate analysis, factors associated with a failure to perform minimally invasive surgery were black race of the patient (adjusted odds ratio, 0.57), body mass index over 35 kg/m2 (aOR, 1.40), stage II disease (aOR, 0.49), stage III disease (aOR, 0.36), carcinosarcoma/leiomyosarcoma (aOR, 0.58), and university hospital (aOR, 3.46).
Looking at perioperative complications, the investigators found that laparotomy was associated with more in-hospital complications than minimally invasive procedures, including more unscheduled ICU stays (P < .001) and prolonged hospital stays (P = .0002).
Dr. Dewdney said that investigators are planning further registry-based studies focusing on ovarian cancer, uterine cancer, and cervical cancer.
Dr. Nickles Fader and Dr. Dewdney reported having no relevant conflicts of interest.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 63.
Of 3,730 women with endometrial cancer in the Society of Gynecologic Oncology Clinical Outcomes Registry (SGO-COR), 88.8% underwent minimally invasive procedures, reported Amanda Nickles Fader, MD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“When you have surgery with a gyn-oncologist who is specially trained in this type of surgery, we see that women have a very high likelihood of having the appropriate surgery, the minimally invasive surgery, and we thought that this benchmark of an 80% rate of minimally invasive surgery in these patients is very feasible and should be recognized as the standard of care,” Dr. Nickles Fader said in an interview.
Coinvestigator Summer B. Dewdney, MD, of Rush University Medical Center, Chicago, who was instrumental in creating and running the SGO-COR registry, said these findings are encouraging.
“We’re happy to see that rate. It’s the rate that it should be because minimally invasive surgery is the standard of care for endometrial cancer,” Dr. Dewdney said. She added, however, that data supplied to the registry come from gynecologic oncologists who are highly motivated to participate and follow best practice guidelines, which could skew the results slightly toward more favorable outcomes.
Results of the registry-based study are detailed in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Assessing adherence to guidelines
In 2015, the SGO Clinical Practice Committee and the American College of Obstetricians and Gynecologists issued a practice bulletin, which stated that “minimally invasive surgery should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”
Similarly, National Comprehensive Cancer Network guidelines for uterine cancer state that “minimally invasive surgery is the preferred approach when technically feasible” for treatment of endometrial cancer confined to the uterus.
Despite these recommendations, the overall rate of minimally invasive endometrial cancer surgery in the United States is reported be around to 60%, Dr. Nickles Fader and colleagues wrote.
With this in mind, the investigators set out to determine the rate of minimally invasive surgery in women with apparent stage I, II, or III endometrial cancer who underwent hysterectomy with or without staging from 2012 to 2017 at a center reporting to SGO-COR.
The team identified 3,730 women treated at 25 SGO-COR centers; 12 of which were university-affiliated centers and 13 of which were nonuniversity based. Most patients (83.2%) had stage I disease, 4.7% had stage II cancer, and 12.1% had stage III disease. The median patient age was 57 years. Most patients (88%) were white, and two-thirds (67.1%) were obese. In all, 80.4% of samples had endometrioid histology, and 77.7% were either grade 1 or 2.
Factors associated with minimally invasive surgery
The data showed that 88.8% of patients underwent a minimally invasive hysterectomy, composed of robotic-assisted procedures in 73.9% of cases, laparoscopy in 13.4%, and vaginal access in 1.6%.
The proportion of patients who underwent a minimally invasive procedure was significantly higher at nonuniversity centers, compared with academic centers (92.6% vs. 82.7%; P < .0001), but rates of minimally invasive procedures did not differ significantly across U.S. geographic regions.
Dr. Dewdney said that the higher proportion of open surgeries performed at university centers may be attributable to those centers treating patients with more advanced disease or rare aggressive cancers that may not be amenable to a minimally invasive approach.
In a multivariate analysis, factors associated with a failure to perform minimally invasive surgery were black race of the patient (adjusted odds ratio, 0.57), body mass index over 35 kg/m2 (aOR, 1.40), stage II disease (aOR, 0.49), stage III disease (aOR, 0.36), carcinosarcoma/leiomyosarcoma (aOR, 0.58), and university hospital (aOR, 3.46).
Looking at perioperative complications, the investigators found that laparotomy was associated with more in-hospital complications than minimally invasive procedures, including more unscheduled ICU stays (P < .001) and prolonged hospital stays (P = .0002).
Dr. Dewdney said that investigators are planning further registry-based studies focusing on ovarian cancer, uterine cancer, and cervical cancer.
Dr. Nickles Fader and Dr. Dewdney reported having no relevant conflicts of interest.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 63.
FROM SGO 2020
When to treat, delay, or omit breast cancer therapy in the face of COVID-19
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Investigators recommend expanding testing for Lynch syndrome
Strict adherence to pathology guidelines for interpreting immumohistochemical (IHC) staining in endometrial cancer samples may miss the opportunity to identify patients with Lynch syndrome, investigators cautioned.
Current College of American Pathologists recommendations say that any partial, indeterminate, or “heterogeneous” IHC staining for DNA mismatch repair proteins (MMRP) should be considered as intact expression staining.
However, a retrospective review of patients with endometrial cancer showed that 3 of 13 patients with Lynch syndrome had a small proportion of staining and would have been considered at low risk for Lynch syndrome if the reporting rules were followed to the letter, according to Courtney J. Riedinger, MD, and colleagues from the University of Tennessee Medical Center in Knoxville.
“IHC staining for mismatch repair proteins is a way to screen for who should get genetic testing [for Lynch syndrome],” Dr. Riedinger said in an interview. “The pathology guidelines say that any expression is intact staining, but we found some tumor specimens that have about only 20% staining, and we found that 3 of 27 patients with a range of 20%-60% expression had Lynch syndrome.”
The findings suggest that genetic testing for Lynch syndrome should be considered in patients with heterogeneous staining for MMRP, Dr. Riedinger and colleagues wrote in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled due to the COVID-19 pandemic.A recent systematic review estimated the prevalence of Lynch syndrome in women with endometrial cancer to be 3%. The authors of the review recommended universal screening for Lynch syndrome in women with endometrial cancer (Genet Med. 2019 Oct;21[10]:2167-2180).
As Dr. Riedinger and colleagues noted, screening for Lynch syndrome can be performed clinically with microsatellite instability testing or IHC staining for MMRP.
To determine the frequency of Lynch syndrome in women with endometrial cancer whose samples exhibited heterogeneous staining for MMRP, the investigators conducted a retrospective review.
They identified 455 women who underwent hysterectomy for endometrial cancer during 2012-2017. Of this group, samples from 315 patients had no MMRP loss, 92 had complete loss, and 48 had heterogeneous MMRP staining. Of the latter group, 21 samples were reported as intact, and 27 were reported as heterogeneous.
A total of 13 patients were identified as having Lynch syndrome, including 3 of the 27 patients with reported heterogeneous staining and 10 with reported complete loss of MMRP.
The investigators found the frequency of Lynch syndrome among patients with reported heterogeneous staining was not significantly different than that for patients with complete MMRP loss. In addition, there were no significant differences between samples with heterogeneous or complete loss of staining by type of MMRP.
“Our data suggest genetic testing for Lynch syndrome in patients with heterogeneous IHC staining for MMRP should be considered. Current reporting guidelines regarding MMRP expression in endometrial cancer patients need to be reevaluated,” Dr. Riedinger and colleagues concluded.
Dr. Riedinger reported no conflicts of interest. The study was internally funded.
SOURCE: Riedinger CJ et al. SGO 2020, Abstract 104.
Strict adherence to pathology guidelines for interpreting immumohistochemical (IHC) staining in endometrial cancer samples may miss the opportunity to identify patients with Lynch syndrome, investigators cautioned.
Current College of American Pathologists recommendations say that any partial, indeterminate, or “heterogeneous” IHC staining for DNA mismatch repair proteins (MMRP) should be considered as intact expression staining.
However, a retrospective review of patients with endometrial cancer showed that 3 of 13 patients with Lynch syndrome had a small proportion of staining and would have been considered at low risk for Lynch syndrome if the reporting rules were followed to the letter, according to Courtney J. Riedinger, MD, and colleagues from the University of Tennessee Medical Center in Knoxville.
“IHC staining for mismatch repair proteins is a way to screen for who should get genetic testing [for Lynch syndrome],” Dr. Riedinger said in an interview. “The pathology guidelines say that any expression is intact staining, but we found some tumor specimens that have about only 20% staining, and we found that 3 of 27 patients with a range of 20%-60% expression had Lynch syndrome.”
The findings suggest that genetic testing for Lynch syndrome should be considered in patients with heterogeneous staining for MMRP, Dr. Riedinger and colleagues wrote in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled due to the COVID-19 pandemic.A recent systematic review estimated the prevalence of Lynch syndrome in women with endometrial cancer to be 3%. The authors of the review recommended universal screening for Lynch syndrome in women with endometrial cancer (Genet Med. 2019 Oct;21[10]:2167-2180).
As Dr. Riedinger and colleagues noted, screening for Lynch syndrome can be performed clinically with microsatellite instability testing or IHC staining for MMRP.
To determine the frequency of Lynch syndrome in women with endometrial cancer whose samples exhibited heterogeneous staining for MMRP, the investigators conducted a retrospective review.
They identified 455 women who underwent hysterectomy for endometrial cancer during 2012-2017. Of this group, samples from 315 patients had no MMRP loss, 92 had complete loss, and 48 had heterogeneous MMRP staining. Of the latter group, 21 samples were reported as intact, and 27 were reported as heterogeneous.
A total of 13 patients were identified as having Lynch syndrome, including 3 of the 27 patients with reported heterogeneous staining and 10 with reported complete loss of MMRP.
The investigators found the frequency of Lynch syndrome among patients with reported heterogeneous staining was not significantly different than that for patients with complete MMRP loss. In addition, there were no significant differences between samples with heterogeneous or complete loss of staining by type of MMRP.
“Our data suggest genetic testing for Lynch syndrome in patients with heterogeneous IHC staining for MMRP should be considered. Current reporting guidelines regarding MMRP expression in endometrial cancer patients need to be reevaluated,” Dr. Riedinger and colleagues concluded.
Dr. Riedinger reported no conflicts of interest. The study was internally funded.
SOURCE: Riedinger CJ et al. SGO 2020, Abstract 104.
Strict adherence to pathology guidelines for interpreting immumohistochemical (IHC) staining in endometrial cancer samples may miss the opportunity to identify patients with Lynch syndrome, investigators cautioned.
Current College of American Pathologists recommendations say that any partial, indeterminate, or “heterogeneous” IHC staining for DNA mismatch repair proteins (MMRP) should be considered as intact expression staining.
However, a retrospective review of patients with endometrial cancer showed that 3 of 13 patients with Lynch syndrome had a small proportion of staining and would have been considered at low risk for Lynch syndrome if the reporting rules were followed to the letter, according to Courtney J. Riedinger, MD, and colleagues from the University of Tennessee Medical Center in Knoxville.
“IHC staining for mismatch repair proteins is a way to screen for who should get genetic testing [for Lynch syndrome],” Dr. Riedinger said in an interview. “The pathology guidelines say that any expression is intact staining, but we found some tumor specimens that have about only 20% staining, and we found that 3 of 27 patients with a range of 20%-60% expression had Lynch syndrome.”
The findings suggest that genetic testing for Lynch syndrome should be considered in patients with heterogeneous staining for MMRP, Dr. Riedinger and colleagues wrote in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled due to the COVID-19 pandemic.A recent systematic review estimated the prevalence of Lynch syndrome in women with endometrial cancer to be 3%. The authors of the review recommended universal screening for Lynch syndrome in women with endometrial cancer (Genet Med. 2019 Oct;21[10]:2167-2180).
As Dr. Riedinger and colleagues noted, screening for Lynch syndrome can be performed clinically with microsatellite instability testing or IHC staining for MMRP.
To determine the frequency of Lynch syndrome in women with endometrial cancer whose samples exhibited heterogeneous staining for MMRP, the investigators conducted a retrospective review.
They identified 455 women who underwent hysterectomy for endometrial cancer during 2012-2017. Of this group, samples from 315 patients had no MMRP loss, 92 had complete loss, and 48 had heterogeneous MMRP staining. Of the latter group, 21 samples were reported as intact, and 27 were reported as heterogeneous.
A total of 13 patients were identified as having Lynch syndrome, including 3 of the 27 patients with reported heterogeneous staining and 10 with reported complete loss of MMRP.
The investigators found the frequency of Lynch syndrome among patients with reported heterogeneous staining was not significantly different than that for patients with complete MMRP loss. In addition, there were no significant differences between samples with heterogeneous or complete loss of staining by type of MMRP.
“Our data suggest genetic testing for Lynch syndrome in patients with heterogeneous IHC staining for MMRP should be considered. Current reporting guidelines regarding MMRP expression in endometrial cancer patients need to be reevaluated,” Dr. Riedinger and colleagues concluded.
Dr. Riedinger reported no conflicts of interest. The study was internally funded.
SOURCE: Riedinger CJ et al. SGO 2020, Abstract 104.
FROM SGO 2020
Water-only fasting may reduce chemo modifications, hospital admissions
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
FROM SGO 2020
Promising efficacy with adavosertib in uterine serous carcinoma
The experimental agent adavosertib showed hints of efficacy against recurrent uterine serous carcinoma in early data from a phase 2 trial.
The overall response rate among 21 patients with advanced uterine serous carcinoma treated with adavosertib monotherapy was 30%, and an additional patient had an unconfirmed response at the time of data cutoff, reported Joyce F. Liu, MD, of the Dana-Farber Cancer Institute in Boston, and colleagues.
“These results were noteworthy for the preliminary response rate of 30% that was observed in the first cohort of patients on this study, especially as, on average, patients on this study had received three prior treatments for their cancer. For us, this was an exciting signal of activity, especially for a targeted therapy used by itself in this type of cancer,” Dr. Liu said in an interview.
Preliminary results of the phase 2 trial were published in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Adavosertib is a small molecule that inhibits the Wee1 kinase, a “gatekeeper” of the G2-M cell cycle checkpoint that is highly expressed and active in several types of cancer.
“Molecular characterization of these cancers has demonstrated that they have frequent p53 mutations as well as significant alterations in oncogenes,” Dr. Liu said. “These characteristics mean that these are cancers that may both have significant dysregulation of their cell cycle combined with high levels of replication stress. We hypothesized that these cancers could therefore be particularly vulnerable to further dysregulation of the cell cycle, which can be mediated by a drug such as adavosertib, which interrupts regulation of the G2-M cell cycle checkpoint by inhibiting the protein Wee1.”
Study details
The study enrolled women with recurrent uterine serous carcinoma. Patients were eligible if any disease component was considered to be serous, except for carcinosarcomas. The patients had a minimum of one prior platinum-based chemotherapy regimen (median 3, range 1-7), with no upper limit on prior lines of therapy required for eligibility.
Patients with microsatellite high/deficient mismatch repair disease had to have received prior therapy with programmed death-1/ligand-1 inhibitor, or to have been deemed ineligible for immunotherapy with a checkpoint inhibitor.
The patients received adavosertib at 300 mg daily on days 1 through 5 and days 8 through 12 of each 21-day cycle.
The trial would be considered successful if at least of 4 of 35 patients planned for accrual had a confirmed response or if 8 patients were progression free at 6 months. The coprimary endpoints are overall response rate of 20% or more, or a progression-free survival rate at 6 months of 30% or more.
Results and next steps
As of Aug. 20, 2019, the investigators had enrolled 27 patients, of whom 21 were evaluable for response.
The overall response rate was 30%, consisting of six confirmed partial responses. One additional patient had an unconfirmed response. Eleven of the 21 patients had stable disease, and 3 had disease progression.
Eleven patients remained on treatment with adavosertib at the time of data cutoff. Progression-free survival data were not mature.
The most frequent adverse events included anemia and diarrhea in 67% of patients each, nausea in 58%, and fatigue in 50%.
Frequent grade 3 or higher adverse effects included anemia, neutropenia, and syncope, all occurring in 21% of patients.
Dr. Liu said the investigators plan to present updated data from the study at a future meeting.
“We are planning additional cohorts in this study that will allow us to more deeply investigate why certain uterine serous cancer patients had very good responses to adavosertib and to identify potential biomarkers of response,” she said.
“Additionally, we plan to investigate whether adavosertib has similar activity in another type of uterine cancer, uterine carcinosarcoma, that shares many similar molecular characteristics with uterine serous carcinoma, including p53 mutations and oncogenic alterations, that might make it similarly vulnerable to targeting Wee1,” she said.
Dr. Liu disclosed ties with AstraZeneca, which supported the trial, as well as Merck and other companies.
SOURCE: Liu JF et al. SGO 2020, Abstract 7.
The experimental agent adavosertib showed hints of efficacy against recurrent uterine serous carcinoma in early data from a phase 2 trial.
The overall response rate among 21 patients with advanced uterine serous carcinoma treated with adavosertib monotherapy was 30%, and an additional patient had an unconfirmed response at the time of data cutoff, reported Joyce F. Liu, MD, of the Dana-Farber Cancer Institute in Boston, and colleagues.
“These results were noteworthy for the preliminary response rate of 30% that was observed in the first cohort of patients on this study, especially as, on average, patients on this study had received three prior treatments for their cancer. For us, this was an exciting signal of activity, especially for a targeted therapy used by itself in this type of cancer,” Dr. Liu said in an interview.
Preliminary results of the phase 2 trial were published in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Adavosertib is a small molecule that inhibits the Wee1 kinase, a “gatekeeper” of the G2-M cell cycle checkpoint that is highly expressed and active in several types of cancer.
“Molecular characterization of these cancers has demonstrated that they have frequent p53 mutations as well as significant alterations in oncogenes,” Dr. Liu said. “These characteristics mean that these are cancers that may both have significant dysregulation of their cell cycle combined with high levels of replication stress. We hypothesized that these cancers could therefore be particularly vulnerable to further dysregulation of the cell cycle, which can be mediated by a drug such as adavosertib, which interrupts regulation of the G2-M cell cycle checkpoint by inhibiting the protein Wee1.”
Study details
The study enrolled women with recurrent uterine serous carcinoma. Patients were eligible if any disease component was considered to be serous, except for carcinosarcomas. The patients had a minimum of one prior platinum-based chemotherapy regimen (median 3, range 1-7), with no upper limit on prior lines of therapy required for eligibility.
Patients with microsatellite high/deficient mismatch repair disease had to have received prior therapy with programmed death-1/ligand-1 inhibitor, or to have been deemed ineligible for immunotherapy with a checkpoint inhibitor.
The patients received adavosertib at 300 mg daily on days 1 through 5 and days 8 through 12 of each 21-day cycle.
The trial would be considered successful if at least of 4 of 35 patients planned for accrual had a confirmed response or if 8 patients were progression free at 6 months. The coprimary endpoints are overall response rate of 20% or more, or a progression-free survival rate at 6 months of 30% or more.
Results and next steps
As of Aug. 20, 2019, the investigators had enrolled 27 patients, of whom 21 were evaluable for response.
The overall response rate was 30%, consisting of six confirmed partial responses. One additional patient had an unconfirmed response. Eleven of the 21 patients had stable disease, and 3 had disease progression.
Eleven patients remained on treatment with adavosertib at the time of data cutoff. Progression-free survival data were not mature.
The most frequent adverse events included anemia and diarrhea in 67% of patients each, nausea in 58%, and fatigue in 50%.
Frequent grade 3 or higher adverse effects included anemia, neutropenia, and syncope, all occurring in 21% of patients.
Dr. Liu said the investigators plan to present updated data from the study at a future meeting.
“We are planning additional cohorts in this study that will allow us to more deeply investigate why certain uterine serous cancer patients had very good responses to adavosertib and to identify potential biomarkers of response,” she said.
“Additionally, we plan to investigate whether adavosertib has similar activity in another type of uterine cancer, uterine carcinosarcoma, that shares many similar molecular characteristics with uterine serous carcinoma, including p53 mutations and oncogenic alterations, that might make it similarly vulnerable to targeting Wee1,” she said.
Dr. Liu disclosed ties with AstraZeneca, which supported the trial, as well as Merck and other companies.
SOURCE: Liu JF et al. SGO 2020, Abstract 7.
The experimental agent adavosertib showed hints of efficacy against recurrent uterine serous carcinoma in early data from a phase 2 trial.
The overall response rate among 21 patients with advanced uterine serous carcinoma treated with adavosertib monotherapy was 30%, and an additional patient had an unconfirmed response at the time of data cutoff, reported Joyce F. Liu, MD, of the Dana-Farber Cancer Institute in Boston, and colleagues.
“These results were noteworthy for the preliminary response rate of 30% that was observed in the first cohort of patients on this study, especially as, on average, patients on this study had received three prior treatments for their cancer. For us, this was an exciting signal of activity, especially for a targeted therapy used by itself in this type of cancer,” Dr. Liu said in an interview.
Preliminary results of the phase 2 trial were published in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Adavosertib is a small molecule that inhibits the Wee1 kinase, a “gatekeeper” of the G2-M cell cycle checkpoint that is highly expressed and active in several types of cancer.
“Molecular characterization of these cancers has demonstrated that they have frequent p53 mutations as well as significant alterations in oncogenes,” Dr. Liu said. “These characteristics mean that these are cancers that may both have significant dysregulation of their cell cycle combined with high levels of replication stress. We hypothesized that these cancers could therefore be particularly vulnerable to further dysregulation of the cell cycle, which can be mediated by a drug such as adavosertib, which interrupts regulation of the G2-M cell cycle checkpoint by inhibiting the protein Wee1.”
Study details
The study enrolled women with recurrent uterine serous carcinoma. Patients were eligible if any disease component was considered to be serous, except for carcinosarcomas. The patients had a minimum of one prior platinum-based chemotherapy regimen (median 3, range 1-7), with no upper limit on prior lines of therapy required for eligibility.
Patients with microsatellite high/deficient mismatch repair disease had to have received prior therapy with programmed death-1/ligand-1 inhibitor, or to have been deemed ineligible for immunotherapy with a checkpoint inhibitor.
The patients received adavosertib at 300 mg daily on days 1 through 5 and days 8 through 12 of each 21-day cycle.
The trial would be considered successful if at least of 4 of 35 patients planned for accrual had a confirmed response or if 8 patients were progression free at 6 months. The coprimary endpoints are overall response rate of 20% or more, or a progression-free survival rate at 6 months of 30% or more.
Results and next steps
As of Aug. 20, 2019, the investigators had enrolled 27 patients, of whom 21 were evaluable for response.
The overall response rate was 30%, consisting of six confirmed partial responses. One additional patient had an unconfirmed response. Eleven of the 21 patients had stable disease, and 3 had disease progression.
Eleven patients remained on treatment with adavosertib at the time of data cutoff. Progression-free survival data were not mature.
The most frequent adverse events included anemia and diarrhea in 67% of patients each, nausea in 58%, and fatigue in 50%.
Frequent grade 3 or higher adverse effects included anemia, neutropenia, and syncope, all occurring in 21% of patients.
Dr. Liu said the investigators plan to present updated data from the study at a future meeting.
“We are planning additional cohorts in this study that will allow us to more deeply investigate why certain uterine serous cancer patients had very good responses to adavosertib and to identify potential biomarkers of response,” she said.
“Additionally, we plan to investigate whether adavosertib has similar activity in another type of uterine cancer, uterine carcinosarcoma, that shares many similar molecular characteristics with uterine serous carcinoma, including p53 mutations and oncogenic alterations, that might make it similarly vulnerable to targeting Wee1,” she said.
Dr. Liu disclosed ties with AstraZeneca, which supported the trial, as well as Merck and other companies.
SOURCE: Liu JF et al. SGO 2020, Abstract 7.
FROM SGO 2020
Younger gynecologic cancer patients at risk for early bone loss
Younger women treated for uterine or ovarian cancer are at increased risk for decreased bone mineral density and osteoporosis, especially in the first year after diagnosis, and they should be screened for bone changes, investigators advise.
This recommendation is based on results from a retrospective study of women, age 65 years and younger, all of whom underwent oophorectomy and most of whom received chemotherapy. Half of the women who had normal bone mineral density (BMD) at baseline were at risk for osteopenia or osteoporosis 5 years after diagnosis.
Rates of patients at risk for osteoporosis roughly doubled each year for the first 3 years of follow-up, reported study author Janelle Sobecki, MD, of the University of Wisconsin–Madison, and colleagues.
Their research is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“Clinicians should follow current National Comprehensive Cancer Network guidelines regarding bone mineral density screening in women under 65 years old with cancer who have undergone therapy affecting their ovarian function [ovarian removal and/or antiestrogen therapies],” Dr. Sobecki said in an interview.
“Our findings indicate women with gynecologic cancer undergoing ovarian removal and chemotherapy may warrant sooner bone density evaluation, as early as 1 year following treatment. Bone loss screening in this population is feasible using opportunistic CT imaging,” she added.
Current guidelines recommending routine BMD evaluation every 2 years in women who received treatments impairing ovarian function are based largely on data in breast cancer patients, but there is a paucity of data on women who undergo oophorectomy and cancer therapies, both of which are known risk factors for bone loss, Dr. Sobecki noted.
“Bone loss is an important issue for women’s cancer survivorship, particularly for women who we expect to have long survival,” she said. “Identifying bone loss early is important for long-term bone health and prevention of osteoporosis in cancer survivors.”
Patient analysis
To get a better picture of long-term BMD changes and osteoporosis risk in younger patients, Dr. Sobecki and colleagues conducted a retrospective cohort study of women with uterine or ovarian cancers who underwent oophorectomy from 2010 to 2015.
The investigators calculated CT-based L1 trabecular attenuation BMD measurements (Hounsfield units, HU) on CT scans performed at baseline and at 1 year, 3 years, 5 years, and beyond 5 years after cancer diagnosis.
Osteoporosis risk was defined based on HU. Less than 100 HU was deemed “concerning” for osteoporosis, 100-150 HU was suggestive of osteopenia, and more than 150 HU indicated normal BMD.
The investigators reviewed scans for 185 patients with a median age of 55 years and a mean body mass index of 32 kg/m2. Each patient had at least a baseline scan and one additional CT scan during follow-up.
The majority of patients (78.1%) had ovarian cancer, 78.1% underwent chemotherapy, and 17.1% were treated with external beam radiation. As of 2019, 118 patients (63.6%) were still alive.
Results and next steps
The investigators found that BMD decreased from a mean of 179.4 HU at baseline to 146.5 HU at 1 year, a significant decline (P < .001), and to 123.63 HU beyond 5 years (P < .001). As noted before, half of the patients with normal BMD at baseline were at risk for osteopenia or osteoporosis at 5 years.
The proportion of patients at risk for osteoporosis at baseline was 4.3%, compared with 7.4% at 1 year, 15.7% at 3 years, 18% at 5 years, and 23.3% beyond 5 years. BMD at baseline was a significant predictor for bone loss at all time points. In multivariate analysis, chemotherapy predicted bone loss at 1 year (P = .03), and current smoking predicted BMD decrease at 5 years (P < .01).
“We plan to further investigate the role of chemotherapy in bone loss in gynecologic cancer patients, including chemotherapy dose-related bone loss,” Dr. Sobecki said. “We also plan to investigate bone loss in older women [over the age of 65] undergoing treatment for gynecologic cancer as they may be at greater risk than their baseline age-related risk.”
This study was internally funded. Dr. Sobecki reported no conflicts of interest.
SOURCE: Sobecki J et al. SGO 2020, Abstract 130.
Younger women treated for uterine or ovarian cancer are at increased risk for decreased bone mineral density and osteoporosis, especially in the first year after diagnosis, and they should be screened for bone changes, investigators advise.
This recommendation is based on results from a retrospective study of women, age 65 years and younger, all of whom underwent oophorectomy and most of whom received chemotherapy. Half of the women who had normal bone mineral density (BMD) at baseline were at risk for osteopenia or osteoporosis 5 years after diagnosis.
Rates of patients at risk for osteoporosis roughly doubled each year for the first 3 years of follow-up, reported study author Janelle Sobecki, MD, of the University of Wisconsin–Madison, and colleagues.
Their research is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“Clinicians should follow current National Comprehensive Cancer Network guidelines regarding bone mineral density screening in women under 65 years old with cancer who have undergone therapy affecting their ovarian function [ovarian removal and/or antiestrogen therapies],” Dr. Sobecki said in an interview.
“Our findings indicate women with gynecologic cancer undergoing ovarian removal and chemotherapy may warrant sooner bone density evaluation, as early as 1 year following treatment. Bone loss screening in this population is feasible using opportunistic CT imaging,” she added.
Current guidelines recommending routine BMD evaluation every 2 years in women who received treatments impairing ovarian function are based largely on data in breast cancer patients, but there is a paucity of data on women who undergo oophorectomy and cancer therapies, both of which are known risk factors for bone loss, Dr. Sobecki noted.
“Bone loss is an important issue for women’s cancer survivorship, particularly for women who we expect to have long survival,” she said. “Identifying bone loss early is important for long-term bone health and prevention of osteoporosis in cancer survivors.”
Patient analysis
To get a better picture of long-term BMD changes and osteoporosis risk in younger patients, Dr. Sobecki and colleagues conducted a retrospective cohort study of women with uterine or ovarian cancers who underwent oophorectomy from 2010 to 2015.
The investigators calculated CT-based L1 trabecular attenuation BMD measurements (Hounsfield units, HU) on CT scans performed at baseline and at 1 year, 3 years, 5 years, and beyond 5 years after cancer diagnosis.
Osteoporosis risk was defined based on HU. Less than 100 HU was deemed “concerning” for osteoporosis, 100-150 HU was suggestive of osteopenia, and more than 150 HU indicated normal BMD.
The investigators reviewed scans for 185 patients with a median age of 55 years and a mean body mass index of 32 kg/m2. Each patient had at least a baseline scan and one additional CT scan during follow-up.
The majority of patients (78.1%) had ovarian cancer, 78.1% underwent chemotherapy, and 17.1% were treated with external beam radiation. As of 2019, 118 patients (63.6%) were still alive.
Results and next steps
The investigators found that BMD decreased from a mean of 179.4 HU at baseline to 146.5 HU at 1 year, a significant decline (P < .001), and to 123.63 HU beyond 5 years (P < .001). As noted before, half of the patients with normal BMD at baseline were at risk for osteopenia or osteoporosis at 5 years.
The proportion of patients at risk for osteoporosis at baseline was 4.3%, compared with 7.4% at 1 year, 15.7% at 3 years, 18% at 5 years, and 23.3% beyond 5 years. BMD at baseline was a significant predictor for bone loss at all time points. In multivariate analysis, chemotherapy predicted bone loss at 1 year (P = .03), and current smoking predicted BMD decrease at 5 years (P < .01).
“We plan to further investigate the role of chemotherapy in bone loss in gynecologic cancer patients, including chemotherapy dose-related bone loss,” Dr. Sobecki said. “We also plan to investigate bone loss in older women [over the age of 65] undergoing treatment for gynecologic cancer as they may be at greater risk than their baseline age-related risk.”
This study was internally funded. Dr. Sobecki reported no conflicts of interest.
SOURCE: Sobecki J et al. SGO 2020, Abstract 130.
Younger women treated for uterine or ovarian cancer are at increased risk for decreased bone mineral density and osteoporosis, especially in the first year after diagnosis, and they should be screened for bone changes, investigators advise.
This recommendation is based on results from a retrospective study of women, age 65 years and younger, all of whom underwent oophorectomy and most of whom received chemotherapy. Half of the women who had normal bone mineral density (BMD) at baseline were at risk for osteopenia or osteoporosis 5 years after diagnosis.
Rates of patients at risk for osteoporosis roughly doubled each year for the first 3 years of follow-up, reported study author Janelle Sobecki, MD, of the University of Wisconsin–Madison, and colleagues.
Their research is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“Clinicians should follow current National Comprehensive Cancer Network guidelines regarding bone mineral density screening in women under 65 years old with cancer who have undergone therapy affecting their ovarian function [ovarian removal and/or antiestrogen therapies],” Dr. Sobecki said in an interview.
“Our findings indicate women with gynecologic cancer undergoing ovarian removal and chemotherapy may warrant sooner bone density evaluation, as early as 1 year following treatment. Bone loss screening in this population is feasible using opportunistic CT imaging,” she added.
Current guidelines recommending routine BMD evaluation every 2 years in women who received treatments impairing ovarian function are based largely on data in breast cancer patients, but there is a paucity of data on women who undergo oophorectomy and cancer therapies, both of which are known risk factors for bone loss, Dr. Sobecki noted.
“Bone loss is an important issue for women’s cancer survivorship, particularly for women who we expect to have long survival,” she said. “Identifying bone loss early is important for long-term bone health and prevention of osteoporosis in cancer survivors.”
Patient analysis
To get a better picture of long-term BMD changes and osteoporosis risk in younger patients, Dr. Sobecki and colleagues conducted a retrospective cohort study of women with uterine or ovarian cancers who underwent oophorectomy from 2010 to 2015.
The investigators calculated CT-based L1 trabecular attenuation BMD measurements (Hounsfield units, HU) on CT scans performed at baseline and at 1 year, 3 years, 5 years, and beyond 5 years after cancer diagnosis.
Osteoporosis risk was defined based on HU. Less than 100 HU was deemed “concerning” for osteoporosis, 100-150 HU was suggestive of osteopenia, and more than 150 HU indicated normal BMD.
The investigators reviewed scans for 185 patients with a median age of 55 years and a mean body mass index of 32 kg/m2. Each patient had at least a baseline scan and one additional CT scan during follow-up.
The majority of patients (78.1%) had ovarian cancer, 78.1% underwent chemotherapy, and 17.1% were treated with external beam radiation. As of 2019, 118 patients (63.6%) were still alive.
Results and next steps
The investigators found that BMD decreased from a mean of 179.4 HU at baseline to 146.5 HU at 1 year, a significant decline (P < .001), and to 123.63 HU beyond 5 years (P < .001). As noted before, half of the patients with normal BMD at baseline were at risk for osteopenia or osteoporosis at 5 years.
The proportion of patients at risk for osteoporosis at baseline was 4.3%, compared with 7.4% at 1 year, 15.7% at 3 years, 18% at 5 years, and 23.3% beyond 5 years. BMD at baseline was a significant predictor for bone loss at all time points. In multivariate analysis, chemotherapy predicted bone loss at 1 year (P = .03), and current smoking predicted BMD decrease at 5 years (P < .01).
“We plan to further investigate the role of chemotherapy in bone loss in gynecologic cancer patients, including chemotherapy dose-related bone loss,” Dr. Sobecki said. “We also plan to investigate bone loss in older women [over the age of 65] undergoing treatment for gynecologic cancer as they may be at greater risk than their baseline age-related risk.”
This study was internally funded. Dr. Sobecki reported no conflicts of interest.
SOURCE: Sobecki J et al. SGO 2020, Abstract 130.
FROM SGO 2020
Case fatality rate for COVID-19 near 1.4%, increases with age
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.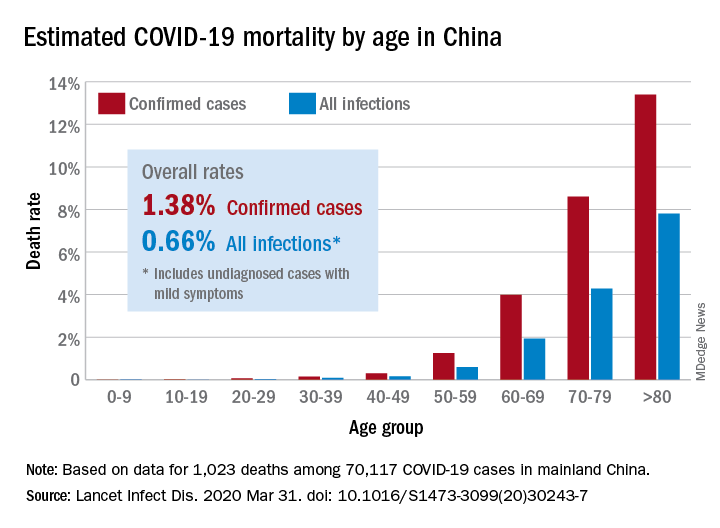
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.
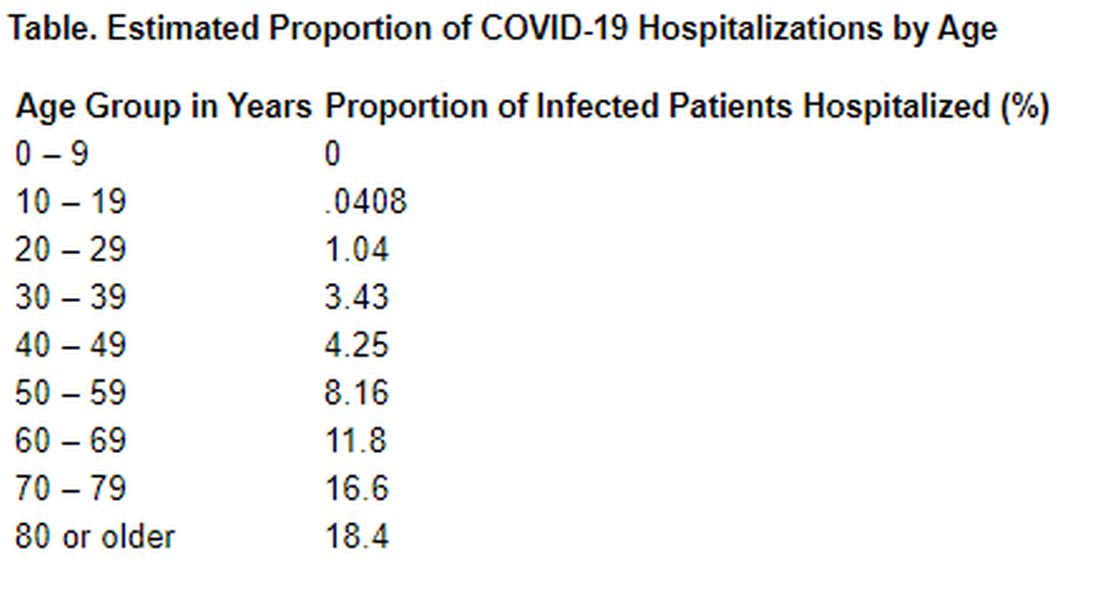
“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.

“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.

“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Financial toxicity is a common complication of gynecologic cancers
More than one-fifth of patients being treated for gynecologic malignancies experience financial toxicity, results of a single-center study suggest.
Among 5,188 patients treated for gynecologic cancers, 1,155 (22%) experienced financial toxicity, measured by bills sent to collection, financial assistance, bankruptcy, or similar measures, reported Emeline Aviki, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and colleagues.
“In any clinical study reporting that over 20% of patients develop a serious complication as a result of treatment, financial toxicity in this case, future efforts to address the complication are critically important,” Dr. Aviki said in an interview.
Her group’s study is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Study details
To address financial problems patients with gynecologic cancer face, MSKCC assembled a multidisciplinary team that included the strategy and innovation department, the patient financial services department, medical oncologists, radiation oncologists, and surgical oncologists.
The team’s first priority was to measure the prevalence of financial burden among the center’s patients using readily available institutional data. Financial toxicity was defined as one or more of the following:
- Two or more bills sent for collection.
- Application for and granting of a time-payment plan.
- Bill settlement.
- Bankruptcy.
- Enrollment in a financial assistance plan.
- Finance-related social work visit.
In a univariate analysis, factors significantly associated with financial toxicity, and the proportion of patients in each category affected, included cervical cancer (31%), stage 3 (29%) or 4 disease (27%), age younger than 30 years (32%), nonpartnered marital status (28%), black (38%) or Hispanic (33%) race/ethnicity, self-pay (42%) or commercial insurance (26%), clinical trial participation (27%), nine or more imaging studies (33%), one or more emergency department visits (31%), inpatient stays of 20 days or longer (35%), and 20 or more outpatient clinic visits (35%).
In a multivariate analysis controlling for disease and demographics, factors that remained significantly associated with financial toxicity (P < .05) included younger age, nonpartnered marital status, black and Hispanic race/ethnicity, commercial insurance, more imaging studies, and more outpatient physician visits.
Implications for patients and providers
“We were really surprised to see the significant increase in financial toxicity associated with patients undergoing more frequent imaging studies,” Dr. Aviki said. “There are randomized controlled studies showing that patients with ovarian cancer do not benefit from more frequent surveillance imaging. However, many providers across the country still order scans every 3 or 4 months. With this new data showing increased financial toxicity in patients who undergo more frequent scans, I think many will pause before ordering their next surveillance scan or at least have the conversation with patients to make sure no financial harm is being done.”
Dr. Aviki and colleagues used the data from this study to create a risk-stratification tool that can be employed to identify patients with gynecologic cancers who are at increased risk for financial toxicity, who can then be offered help through patient financial services.
In addition, the investigators are working to improve provider knowledge about the costs and financial implications surveillance imaging can have for patients.
“When considering interventions that might reduce patient financial burden, we questioned what role providers should play in patient affordability issues,” Dr. Aviki said. “Many providers may believe it is unethical to be informed of their patient’s risk of financial toxicity as it may affect their treatment recommendations. Others may believe it is important for them to be fully aware of any and all treatment-related risks their patients face.”
To get a better sense of how providers see their role in patient finances and care affordability, Dr. Aviki and colleagues surveyed more than 350 attending physicians at MSKCC. The investigators plan to use the results to develop provider-focused interventions.
The study was internally funded. Dr. Aviki reported no conflicts of interest.
SOURCE: Aviki EM et al. SGO 2020, Abstract 144.
More than one-fifth of patients being treated for gynecologic malignancies experience financial toxicity, results of a single-center study suggest.
Among 5,188 patients treated for gynecologic cancers, 1,155 (22%) experienced financial toxicity, measured by bills sent to collection, financial assistance, bankruptcy, or similar measures, reported Emeline Aviki, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and colleagues.
“In any clinical study reporting that over 20% of patients develop a serious complication as a result of treatment, financial toxicity in this case, future efforts to address the complication are critically important,” Dr. Aviki said in an interview.
Her group’s study is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Study details
To address financial problems patients with gynecologic cancer face, MSKCC assembled a multidisciplinary team that included the strategy and innovation department, the patient financial services department, medical oncologists, radiation oncologists, and surgical oncologists.
The team’s first priority was to measure the prevalence of financial burden among the center’s patients using readily available institutional data. Financial toxicity was defined as one or more of the following:
- Two or more bills sent for collection.
- Application for and granting of a time-payment plan.
- Bill settlement.
- Bankruptcy.
- Enrollment in a financial assistance plan.
- Finance-related social work visit.
In a univariate analysis, factors significantly associated with financial toxicity, and the proportion of patients in each category affected, included cervical cancer (31%), stage 3 (29%) or 4 disease (27%), age younger than 30 years (32%), nonpartnered marital status (28%), black (38%) or Hispanic (33%) race/ethnicity, self-pay (42%) or commercial insurance (26%), clinical trial participation (27%), nine or more imaging studies (33%), one or more emergency department visits (31%), inpatient stays of 20 days or longer (35%), and 20 or more outpatient clinic visits (35%).
In a multivariate analysis controlling for disease and demographics, factors that remained significantly associated with financial toxicity (P < .05) included younger age, nonpartnered marital status, black and Hispanic race/ethnicity, commercial insurance, more imaging studies, and more outpatient physician visits.
Implications for patients and providers
“We were really surprised to see the significant increase in financial toxicity associated with patients undergoing more frequent imaging studies,” Dr. Aviki said. “There are randomized controlled studies showing that patients with ovarian cancer do not benefit from more frequent surveillance imaging. However, many providers across the country still order scans every 3 or 4 months. With this new data showing increased financial toxicity in patients who undergo more frequent scans, I think many will pause before ordering their next surveillance scan or at least have the conversation with patients to make sure no financial harm is being done.”
Dr. Aviki and colleagues used the data from this study to create a risk-stratification tool that can be employed to identify patients with gynecologic cancers who are at increased risk for financial toxicity, who can then be offered help through patient financial services.
In addition, the investigators are working to improve provider knowledge about the costs and financial implications surveillance imaging can have for patients.
“When considering interventions that might reduce patient financial burden, we questioned what role providers should play in patient affordability issues,” Dr. Aviki said. “Many providers may believe it is unethical to be informed of their patient’s risk of financial toxicity as it may affect their treatment recommendations. Others may believe it is important for them to be fully aware of any and all treatment-related risks their patients face.”
To get a better sense of how providers see their role in patient finances and care affordability, Dr. Aviki and colleagues surveyed more than 350 attending physicians at MSKCC. The investigators plan to use the results to develop provider-focused interventions.
The study was internally funded. Dr. Aviki reported no conflicts of interest.
SOURCE: Aviki EM et al. SGO 2020, Abstract 144.
More than one-fifth of patients being treated for gynecologic malignancies experience financial toxicity, results of a single-center study suggest.
Among 5,188 patients treated for gynecologic cancers, 1,155 (22%) experienced financial toxicity, measured by bills sent to collection, financial assistance, bankruptcy, or similar measures, reported Emeline Aviki, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and colleagues.
“In any clinical study reporting that over 20% of patients develop a serious complication as a result of treatment, financial toxicity in this case, future efforts to address the complication are critically important,” Dr. Aviki said in an interview.
Her group’s study is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Study details
To address financial problems patients with gynecologic cancer face, MSKCC assembled a multidisciplinary team that included the strategy and innovation department, the patient financial services department, medical oncologists, radiation oncologists, and surgical oncologists.
The team’s first priority was to measure the prevalence of financial burden among the center’s patients using readily available institutional data. Financial toxicity was defined as one or more of the following:
- Two or more bills sent for collection.
- Application for and granting of a time-payment plan.
- Bill settlement.
- Bankruptcy.
- Enrollment in a financial assistance plan.
- Finance-related social work visit.
In a univariate analysis, factors significantly associated with financial toxicity, and the proportion of patients in each category affected, included cervical cancer (31%), stage 3 (29%) or 4 disease (27%), age younger than 30 years (32%), nonpartnered marital status (28%), black (38%) or Hispanic (33%) race/ethnicity, self-pay (42%) or commercial insurance (26%), clinical trial participation (27%), nine or more imaging studies (33%), one or more emergency department visits (31%), inpatient stays of 20 days or longer (35%), and 20 or more outpatient clinic visits (35%).
In a multivariate analysis controlling for disease and demographics, factors that remained significantly associated with financial toxicity (P < .05) included younger age, nonpartnered marital status, black and Hispanic race/ethnicity, commercial insurance, more imaging studies, and more outpatient physician visits.
Implications for patients and providers
“We were really surprised to see the significant increase in financial toxicity associated with patients undergoing more frequent imaging studies,” Dr. Aviki said. “There are randomized controlled studies showing that patients with ovarian cancer do not benefit from more frequent surveillance imaging. However, many providers across the country still order scans every 3 or 4 months. With this new data showing increased financial toxicity in patients who undergo more frequent scans, I think many will pause before ordering their next surveillance scan or at least have the conversation with patients to make sure no financial harm is being done.”
Dr. Aviki and colleagues used the data from this study to create a risk-stratification tool that can be employed to identify patients with gynecologic cancers who are at increased risk for financial toxicity, who can then be offered help through patient financial services.
In addition, the investigators are working to improve provider knowledge about the costs and financial implications surveillance imaging can have for patients.
“When considering interventions that might reduce patient financial burden, we questioned what role providers should play in patient affordability issues,” Dr. Aviki said. “Many providers may believe it is unethical to be informed of their patient’s risk of financial toxicity as it may affect their treatment recommendations. Others may believe it is important for them to be fully aware of any and all treatment-related risks their patients face.”
To get a better sense of how providers see their role in patient finances and care affordability, Dr. Aviki and colleagues surveyed more than 350 attending physicians at MSKCC. The investigators plan to use the results to develop provider-focused interventions.
The study was internally funded. Dr. Aviki reported no conflicts of interest.
SOURCE: Aviki EM et al. SGO 2020, Abstract 144.
FROM SGO 2020
Vaginal artesunate quells CIN 2/3 lesions, clears HPV
In a small study, a self-administered vaginal insert containing the antimalarial agent artesunate resolved cervical intraepithelial neoplasia (CIN) 2/3 lesions in two-thirds of patients and cleared human papillomavirus (HPV) genotypes in nearly half of women whose lesions disappeared.
Among 28 women with biopsy-confirmed CIN 2/3 who used the inserts prior to a planned standard-of-care resection, histologic regression of lesions occurred in 19 patients. In 9 of the 19 women, there was clearance of baseline HPV genotypes.
These results were reported in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. The study results were also published in Gynecologic Oncology.
An unexpected treatment
“The implications of having a safe, inexpensive, self-administered, shelf-stable, nonsurgical treatment for HPV intraepithelial disease, not only here in the U.S., but also extending to low-resource settings,” are self-evident, said study author Cornelia L. Trimble, MD, of Johns Hopkins University in Baltimore.
“This could change the entire landscape of care,” Dr. Trimble said in an interview. “Who’d have thunk that a freaking Chinese herbal medicine derived from the bark of a tree could have this effect?”
Artesunate is a derivative of artemisinin, an antimalarial isolated from the plant Artemisia annua, which is used in traditional Chinese medicine. According to the Centers for Disease Control and Prevention, intravenous artesunate is the first-line drug for treatment of severe malaria in the United States.
However, artesunate is neither approved by the Food and Drug Administration nor commercially available in the United States. The CDC provides artesunate to U.S. clinicians on an as-needed basis.
In addition to its antimalarial activity, artesunate has been shown to have a cytotoxic effect on squamous cells transformed by HPV in vitro. Dr. Trimble and colleagues are also testing a topical form of the drug for the treatment of vulvar intraepithelial neoplasia.
Patients, dosing, and efficacy
In the current study, Dr. Trimble and colleagues enrolled adult immunocompetent women with CIN 2/3, visible residual lesions, and detectable HPV. The patients were assigned sequentially to one of four treatment groups: one 5-day cycle of 50-mg inserts or one, two, or three 5-day cycles using 200-mg inserts.
The patients were instructed to place the inserts at bedtime using a vaginal applicator, followed by a tampon, and then remove the inserts in the morning.
In a modified intention-to-treat analysis including all women who received at least one dose of artesunate and who had endpoint data available, 19 of 28 (67.9%) had histologic regression of CIN lesions. Of the 19 patients, 9 (47.4%) had clearance of all HPV genotypes that had been present at baseline.
Asked how the investigators could distinguish between the treatment effect of the inserts and spontaneous clearance of lesions seen as part of the natural history of CIN in some patients, Dr. Trimble pointed to two observations suggesting an immunologic effect from treatment.
Specifically, although there was lesion regression to CIN 1 or less in all treatment groups, the patients who had only a single treatment cycle had a longer time to regression than those who received two or three cycles.
Additionally, among the nine patients who had viral clearance, three had clearance at the same study time point where histologic regression was observed. For the other six patients, the virus did not clear until several weeks following lesion regression.
These two observations suggest the therapeutic effect of artesunate is recognized by the immune system, which may stimulate a localized immune-mediated cytotoxic effect, Dr. Trimble said.
Safety and next steps
The safety analysis showed that side effects were generally mild and well tolerated. There were 161 adverse events among 29 women for whom safety data were available. The most frequently reported adverse events were vaginal itching (n = 13), vaginal pain (n = 12), vaginal discharge (n = 8), spotting (n = 6), uterine cramping (n = 6), vaginal dryness (n = 4), pelvic pain (n = 1), perineal pain (n = 1), and dyspareunia (n = 1).
Grade 2 adverse events included vaginal yeast infection (n = 6), bacterial vaginosis (n = 2), vaginal inflammation (n = 2), urinary tract infection (n = 2), and noninfective cystitis (n = 1). There were no grade 3 or 4 adverse events reported, and three women reported no noticeable side effects.
Dr. Trimble and colleagues are continuing to study immune responses in cervical tissues and are examining the composition and functions of the cervicovaginal metagenome, looking at bacterial, viral, and fungal components. The team has joined with collaborators at the University of Texas MD Anderson Cancer Center in Houston to look for immune markers in longitudinally collected, subject-matched cervical swabs.
Frantz Viral Therapeutics supplied the artesunate vaginal inserts and partial financial support for this study. Dr. Trimble disclosed relationships with a range of companies and organizations outside this work.
SOURCE: Trimble C L et al. SGO 2020, Abstract LBA 1.
In a small study, a self-administered vaginal insert containing the antimalarial agent artesunate resolved cervical intraepithelial neoplasia (CIN) 2/3 lesions in two-thirds of patients and cleared human papillomavirus (HPV) genotypes in nearly half of women whose lesions disappeared.
Among 28 women with biopsy-confirmed CIN 2/3 who used the inserts prior to a planned standard-of-care resection, histologic regression of lesions occurred in 19 patients. In 9 of the 19 women, there was clearance of baseline HPV genotypes.
These results were reported in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. The study results were also published in Gynecologic Oncology.
An unexpected treatment
“The implications of having a safe, inexpensive, self-administered, shelf-stable, nonsurgical treatment for HPV intraepithelial disease, not only here in the U.S., but also extending to low-resource settings,” are self-evident, said study author Cornelia L. Trimble, MD, of Johns Hopkins University in Baltimore.
“This could change the entire landscape of care,” Dr. Trimble said in an interview. “Who’d have thunk that a freaking Chinese herbal medicine derived from the bark of a tree could have this effect?”
Artesunate is a derivative of artemisinin, an antimalarial isolated from the plant Artemisia annua, which is used in traditional Chinese medicine. According to the Centers for Disease Control and Prevention, intravenous artesunate is the first-line drug for treatment of severe malaria in the United States.
However, artesunate is neither approved by the Food and Drug Administration nor commercially available in the United States. The CDC provides artesunate to U.S. clinicians on an as-needed basis.
In addition to its antimalarial activity, artesunate has been shown to have a cytotoxic effect on squamous cells transformed by HPV in vitro. Dr. Trimble and colleagues are also testing a topical form of the drug for the treatment of vulvar intraepithelial neoplasia.
Patients, dosing, and efficacy
In the current study, Dr. Trimble and colleagues enrolled adult immunocompetent women with CIN 2/3, visible residual lesions, and detectable HPV. The patients were assigned sequentially to one of four treatment groups: one 5-day cycle of 50-mg inserts or one, two, or three 5-day cycles using 200-mg inserts.
The patients were instructed to place the inserts at bedtime using a vaginal applicator, followed by a tampon, and then remove the inserts in the morning.
In a modified intention-to-treat analysis including all women who received at least one dose of artesunate and who had endpoint data available, 19 of 28 (67.9%) had histologic regression of CIN lesions. Of the 19 patients, 9 (47.4%) had clearance of all HPV genotypes that had been present at baseline.
Asked how the investigators could distinguish between the treatment effect of the inserts and spontaneous clearance of lesions seen as part of the natural history of CIN in some patients, Dr. Trimble pointed to two observations suggesting an immunologic effect from treatment.
Specifically, although there was lesion regression to CIN 1 or less in all treatment groups, the patients who had only a single treatment cycle had a longer time to regression than those who received two or three cycles.
Additionally, among the nine patients who had viral clearance, three had clearance at the same study time point where histologic regression was observed. For the other six patients, the virus did not clear until several weeks following lesion regression.
These two observations suggest the therapeutic effect of artesunate is recognized by the immune system, which may stimulate a localized immune-mediated cytotoxic effect, Dr. Trimble said.
Safety and next steps
The safety analysis showed that side effects were generally mild and well tolerated. There were 161 adverse events among 29 women for whom safety data were available. The most frequently reported adverse events were vaginal itching (n = 13), vaginal pain (n = 12), vaginal discharge (n = 8), spotting (n = 6), uterine cramping (n = 6), vaginal dryness (n = 4), pelvic pain (n = 1), perineal pain (n = 1), and dyspareunia (n = 1).
Grade 2 adverse events included vaginal yeast infection (n = 6), bacterial vaginosis (n = 2), vaginal inflammation (n = 2), urinary tract infection (n = 2), and noninfective cystitis (n = 1). There were no grade 3 or 4 adverse events reported, and three women reported no noticeable side effects.
Dr. Trimble and colleagues are continuing to study immune responses in cervical tissues and are examining the composition and functions of the cervicovaginal metagenome, looking at bacterial, viral, and fungal components. The team has joined with collaborators at the University of Texas MD Anderson Cancer Center in Houston to look for immune markers in longitudinally collected, subject-matched cervical swabs.
Frantz Viral Therapeutics supplied the artesunate vaginal inserts and partial financial support for this study. Dr. Trimble disclosed relationships with a range of companies and organizations outside this work.
SOURCE: Trimble C L et al. SGO 2020, Abstract LBA 1.
In a small study, a self-administered vaginal insert containing the antimalarial agent artesunate resolved cervical intraepithelial neoplasia (CIN) 2/3 lesions in two-thirds of patients and cleared human papillomavirus (HPV) genotypes in nearly half of women whose lesions disappeared.
Among 28 women with biopsy-confirmed CIN 2/3 who used the inserts prior to a planned standard-of-care resection, histologic regression of lesions occurred in 19 patients. In 9 of the 19 women, there was clearance of baseline HPV genotypes.
These results were reported in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. The study results were also published in Gynecologic Oncology.
An unexpected treatment
“The implications of having a safe, inexpensive, self-administered, shelf-stable, nonsurgical treatment for HPV intraepithelial disease, not only here in the U.S., but also extending to low-resource settings,” are self-evident, said study author Cornelia L. Trimble, MD, of Johns Hopkins University in Baltimore.
“This could change the entire landscape of care,” Dr. Trimble said in an interview. “Who’d have thunk that a freaking Chinese herbal medicine derived from the bark of a tree could have this effect?”
Artesunate is a derivative of artemisinin, an antimalarial isolated from the plant Artemisia annua, which is used in traditional Chinese medicine. According to the Centers for Disease Control and Prevention, intravenous artesunate is the first-line drug for treatment of severe malaria in the United States.
However, artesunate is neither approved by the Food and Drug Administration nor commercially available in the United States. The CDC provides artesunate to U.S. clinicians on an as-needed basis.
In addition to its antimalarial activity, artesunate has been shown to have a cytotoxic effect on squamous cells transformed by HPV in vitro. Dr. Trimble and colleagues are also testing a topical form of the drug for the treatment of vulvar intraepithelial neoplasia.
Patients, dosing, and efficacy
In the current study, Dr. Trimble and colleagues enrolled adult immunocompetent women with CIN 2/3, visible residual lesions, and detectable HPV. The patients were assigned sequentially to one of four treatment groups: one 5-day cycle of 50-mg inserts or one, two, or three 5-day cycles using 200-mg inserts.
The patients were instructed to place the inserts at bedtime using a vaginal applicator, followed by a tampon, and then remove the inserts in the morning.
In a modified intention-to-treat analysis including all women who received at least one dose of artesunate and who had endpoint data available, 19 of 28 (67.9%) had histologic regression of CIN lesions. Of the 19 patients, 9 (47.4%) had clearance of all HPV genotypes that had been present at baseline.
Asked how the investigators could distinguish between the treatment effect of the inserts and spontaneous clearance of lesions seen as part of the natural history of CIN in some patients, Dr. Trimble pointed to two observations suggesting an immunologic effect from treatment.
Specifically, although there was lesion regression to CIN 1 or less in all treatment groups, the patients who had only a single treatment cycle had a longer time to regression than those who received two or three cycles.
Additionally, among the nine patients who had viral clearance, three had clearance at the same study time point where histologic regression was observed. For the other six patients, the virus did not clear until several weeks following lesion regression.
These two observations suggest the therapeutic effect of artesunate is recognized by the immune system, which may stimulate a localized immune-mediated cytotoxic effect, Dr. Trimble said.
Safety and next steps
The safety analysis showed that side effects were generally mild and well tolerated. There were 161 adverse events among 29 women for whom safety data were available. The most frequently reported adverse events were vaginal itching (n = 13), vaginal pain (n = 12), vaginal discharge (n = 8), spotting (n = 6), uterine cramping (n = 6), vaginal dryness (n = 4), pelvic pain (n = 1), perineal pain (n = 1), and dyspareunia (n = 1).
Grade 2 adverse events included vaginal yeast infection (n = 6), bacterial vaginosis (n = 2), vaginal inflammation (n = 2), urinary tract infection (n = 2), and noninfective cystitis (n = 1). There were no grade 3 or 4 adverse events reported, and three women reported no noticeable side effects.
Dr. Trimble and colleagues are continuing to study immune responses in cervical tissues and are examining the composition and functions of the cervicovaginal metagenome, looking at bacterial, viral, and fungal components. The team has joined with collaborators at the University of Texas MD Anderson Cancer Center in Houston to look for immune markers in longitudinally collected, subject-matched cervical swabs.
Frantz Viral Therapeutics supplied the artesunate vaginal inserts and partial financial support for this study. Dr. Trimble disclosed relationships with a range of companies and organizations outside this work.
SOURCE: Trimble C L et al. SGO 2020, Abstract LBA 1.
FROM SGO 2020
SABR slashes progression of advanced prostate cancer
Patients with oligometastatic disease who received stereotactic ablative radiotherapy (SABR) had a significant threefold decrease in disease progression at 6 months compared with patients who were randomly assigned to observation alone.
“Local control for SABR-treated lesions was excellent, and the adverse effects associated with SABR were mild and did not appear to affect quality of life,” say the investigators, led by Ryan Phillips, MD, PhD, from the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore.
“Although the approach is controversial, many men are interested in avoiding the unpleasant adverse effects and potential health risks of androgen deprivation therapy (ADT) for as long as is reasonable,” they write.
These results come from outcomes of the Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer (ORIOLE) phase 2 trial and were published online March 26 in JAMA Oncology.
An intriguing finding from the study was evidence that SABR may evoke an immune response.
“We observed enhanced differential clonotype expansion, clusters of similar expanded T-cell receptors, and a clinical benefit to greater baseline clonality seen only in participants treated with SABR,” said senior author Phuoc T. Tran, MD, PhD, from the department of radiation oncology and molecular radiation sciences at Johns Hopkins.
“This the only data I’m aware of that really shows that radiation in isolation can cause a systemic immune response,” Tran told Medscape Medical News.
Previous studies suggesting an immune response with radiation have been confounded by coadministration of radiation and chemotherapy, he said.
Drive the Disease to ‘Near Extinction’
SABR appears to “alter the natural history of prostate oligometastatic disease by removing or greatly affecting signals that promote further development of micrometastatic disease,” write the authors of accompanying editorial.
This hypothesis is consistent with the oligometastatic paradigm, which postulates that this is a transient phase that offers “a window of opportunity for cancer cure if equilibrium-phase lesions are ablated before polymetastatic escape occurs,” write Carlo Greco, MD, and Zvi Fuks, MD, both from the Champalimaud Centre for the Unknown, Lisbon, Portugal; Fuks is also affiliated with Memorial Sloan Kettering Cancer Center, New York.
“Taken together, these observations support the hypothesis that all detectable oligometastatic lesions should be systematically ablated, if feasible, in an effort to maximize oligometastatic cancer cure,” Greco and Fuks comment.
However, there is no clear consensus on what constitutes oligometastatic disease, they point out, and they warn that “a numerical-based decision to withhold lesion ablation may represent a strategic error, potentially reducing the benefit of metastasis-directed therapy (MDT) in the treatment of oligometastatic cancer.”
“We speculate that, if clinically and technically feasible, there should be no restriction to MDT at first oligometastatic presentation and as sequential lesions appear, regardless of lesion numbers, because each lesion may potentially constitute a generator of evolving metastatogenic clonogens,” they add.
Multiple rounds of MDT theoretically could “drive the disease to near extinction” and prevent the development of polymetastatic disease, they contend.
Study Details
For their study, Phillips and colleagues enrolled 54 men with recurrent hormone-sensitive prostate cancer with one, two, or three metastases detectable on conventional imaging who had not received ADT within 6 months of enrollment, or for a total of 3 or more years.
The patients were randomly assigned in a 2:1 ratio to receive either SABR, with dose and fractionation based on the size and location of each lesion, or to observation. The median age in each group was 68 years.
Prostate-specific membrane antigen (PSMA)-targeted PET-CT, a relatively new technique that is more accurate than conventional imaging, was performed during treatment planning and at day 180 of follow-up for patients assigned to SABR, but the investigators were blinded to the results to prevent bias in target-lesion selection.
The primary endpoint of progression at 6 months was measured by prostate-specific antigen increase, radiographic evidence, symptomatic progression, ADT initiation for any reason, or death.
This primary endpoint occurred in 7 of 36 men (19%) who were treated with SABR, compared with 11 of 18 (61%) who underwent observation (P = .005).
Median progression-free survival (PFS) was not reached in the SABR group vs. 5.8 months in the observation group, translating into a hazard ratio of 0.30 (P = .002).
Of the 36 men treated with SABR, 16 had baseline PET-avid lesions that, because of investigator blinding, were not included in the treatment fields.
Among all SABR-treated patients, the rate of progression at 6 months among men for whom all lesions were treated was 5%, compared with 38% for men with any lesions outside the treatment field (P = .03). The median PFS for patients with no untreated lesions was not reached, vs. 11.8 months for men in whom PET-avid lesions were missed (HR, 0.26; P = .006).
Oligometastatic vs. Polymetastatic
In an interview with Medscape Medical News, Tran noted that using PSMA PET-CT for imaging appears to be very important for identifying those patients with numerous or disseminated (polymetastatic) lesions, compared with those patients with limited disease who are most likely to benefit from SABR. He emphasized, however, that the PSMA PET-CT findings were an exploratory endpoint that required further validation.
Another secondary, exploratory endpoint of the study was the use of a circulating tumor DNA (ctDNA) analysis by the CAPP-Seq (cancer personalized profiling by deep sequencing) method.
“We show, in a discovery fashion, that certain mutations in the circulation seem to distinguish patients who would benefit from SABR vs those who would not,” he said.
The study was supported by the Nesbitt-McMaster Foundation, Ronald Rose and Joan Lazar, the Movember Foundation and Prostate Cancer Foundation, and the National Cancer Institute. Phillips reported receiving consulting fees and honoraria from RefleXion Medical outside the submitted work. Tran holds a licensed patent related to ablative radiotherapy compounds and methods (Natsar Pharmaceuticals). Several other authors reported financial disclosures. The full list can be found with the original article. Editorialists Greco and Fuks reported serving as cofounders of and owning stock in Ceramedix Holding, LLC.
This article first appeared on Medscape.com.
Patients with oligometastatic disease who received stereotactic ablative radiotherapy (SABR) had a significant threefold decrease in disease progression at 6 months compared with patients who were randomly assigned to observation alone.
“Local control for SABR-treated lesions was excellent, and the adverse effects associated with SABR were mild and did not appear to affect quality of life,” say the investigators, led by Ryan Phillips, MD, PhD, from the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore.
“Although the approach is controversial, many men are interested in avoiding the unpleasant adverse effects and potential health risks of androgen deprivation therapy (ADT) for as long as is reasonable,” they write.
These results come from outcomes of the Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer (ORIOLE) phase 2 trial and were published online March 26 in JAMA Oncology.
An intriguing finding from the study was evidence that SABR may evoke an immune response.
“We observed enhanced differential clonotype expansion, clusters of similar expanded T-cell receptors, and a clinical benefit to greater baseline clonality seen only in participants treated with SABR,” said senior author Phuoc T. Tran, MD, PhD, from the department of radiation oncology and molecular radiation sciences at Johns Hopkins.
“This the only data I’m aware of that really shows that radiation in isolation can cause a systemic immune response,” Tran told Medscape Medical News.
Previous studies suggesting an immune response with radiation have been confounded by coadministration of radiation and chemotherapy, he said.
Drive the Disease to ‘Near Extinction’
SABR appears to “alter the natural history of prostate oligometastatic disease by removing or greatly affecting signals that promote further development of micrometastatic disease,” write the authors of accompanying editorial.
This hypothesis is consistent with the oligometastatic paradigm, which postulates that this is a transient phase that offers “a window of opportunity for cancer cure if equilibrium-phase lesions are ablated before polymetastatic escape occurs,” write Carlo Greco, MD, and Zvi Fuks, MD, both from the Champalimaud Centre for the Unknown, Lisbon, Portugal; Fuks is also affiliated with Memorial Sloan Kettering Cancer Center, New York.
“Taken together, these observations support the hypothesis that all detectable oligometastatic lesions should be systematically ablated, if feasible, in an effort to maximize oligometastatic cancer cure,” Greco and Fuks comment.
However, there is no clear consensus on what constitutes oligometastatic disease, they point out, and they warn that “a numerical-based decision to withhold lesion ablation may represent a strategic error, potentially reducing the benefit of metastasis-directed therapy (MDT) in the treatment of oligometastatic cancer.”
“We speculate that, if clinically and technically feasible, there should be no restriction to MDT at first oligometastatic presentation and as sequential lesions appear, regardless of lesion numbers, because each lesion may potentially constitute a generator of evolving metastatogenic clonogens,” they add.
Multiple rounds of MDT theoretically could “drive the disease to near extinction” and prevent the development of polymetastatic disease, they contend.
Study Details
For their study, Phillips and colleagues enrolled 54 men with recurrent hormone-sensitive prostate cancer with one, two, or three metastases detectable on conventional imaging who had not received ADT within 6 months of enrollment, or for a total of 3 or more years.
The patients were randomly assigned in a 2:1 ratio to receive either SABR, with dose and fractionation based on the size and location of each lesion, or to observation. The median age in each group was 68 years.
Prostate-specific membrane antigen (PSMA)-targeted PET-CT, a relatively new technique that is more accurate than conventional imaging, was performed during treatment planning and at day 180 of follow-up for patients assigned to SABR, but the investigators were blinded to the results to prevent bias in target-lesion selection.
The primary endpoint of progression at 6 months was measured by prostate-specific antigen increase, radiographic evidence, symptomatic progression, ADT initiation for any reason, or death.
This primary endpoint occurred in 7 of 36 men (19%) who were treated with SABR, compared with 11 of 18 (61%) who underwent observation (P = .005).
Median progression-free survival (PFS) was not reached in the SABR group vs. 5.8 months in the observation group, translating into a hazard ratio of 0.30 (P = .002).
Of the 36 men treated with SABR, 16 had baseline PET-avid lesions that, because of investigator blinding, were not included in the treatment fields.
Among all SABR-treated patients, the rate of progression at 6 months among men for whom all lesions were treated was 5%, compared with 38% for men with any lesions outside the treatment field (P = .03). The median PFS for patients with no untreated lesions was not reached, vs. 11.8 months for men in whom PET-avid lesions were missed (HR, 0.26; P = .006).
Oligometastatic vs. Polymetastatic
In an interview with Medscape Medical News, Tran noted that using PSMA PET-CT for imaging appears to be very important for identifying those patients with numerous or disseminated (polymetastatic) lesions, compared with those patients with limited disease who are most likely to benefit from SABR. He emphasized, however, that the PSMA PET-CT findings were an exploratory endpoint that required further validation.
Another secondary, exploratory endpoint of the study was the use of a circulating tumor DNA (ctDNA) analysis by the CAPP-Seq (cancer personalized profiling by deep sequencing) method.
“We show, in a discovery fashion, that certain mutations in the circulation seem to distinguish patients who would benefit from SABR vs those who would not,” he said.
The study was supported by the Nesbitt-McMaster Foundation, Ronald Rose and Joan Lazar, the Movember Foundation and Prostate Cancer Foundation, and the National Cancer Institute. Phillips reported receiving consulting fees and honoraria from RefleXion Medical outside the submitted work. Tran holds a licensed patent related to ablative radiotherapy compounds and methods (Natsar Pharmaceuticals). Several other authors reported financial disclosures. The full list can be found with the original article. Editorialists Greco and Fuks reported serving as cofounders of and owning stock in Ceramedix Holding, LLC.
This article first appeared on Medscape.com.
Patients with oligometastatic disease who received stereotactic ablative radiotherapy (SABR) had a significant threefold decrease in disease progression at 6 months compared with patients who were randomly assigned to observation alone.
“Local control for SABR-treated lesions was excellent, and the adverse effects associated with SABR were mild and did not appear to affect quality of life,” say the investigators, led by Ryan Phillips, MD, PhD, from the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore.
“Although the approach is controversial, many men are interested in avoiding the unpleasant adverse effects and potential health risks of androgen deprivation therapy (ADT) for as long as is reasonable,” they write.
These results come from outcomes of the Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer (ORIOLE) phase 2 trial and were published online March 26 in JAMA Oncology.
An intriguing finding from the study was evidence that SABR may evoke an immune response.
“We observed enhanced differential clonotype expansion, clusters of similar expanded T-cell receptors, and a clinical benefit to greater baseline clonality seen only in participants treated with SABR,” said senior author Phuoc T. Tran, MD, PhD, from the department of radiation oncology and molecular radiation sciences at Johns Hopkins.
“This the only data I’m aware of that really shows that radiation in isolation can cause a systemic immune response,” Tran told Medscape Medical News.
Previous studies suggesting an immune response with radiation have been confounded by coadministration of radiation and chemotherapy, he said.
Drive the Disease to ‘Near Extinction’
SABR appears to “alter the natural history of prostate oligometastatic disease by removing or greatly affecting signals that promote further development of micrometastatic disease,” write the authors of accompanying editorial.
This hypothesis is consistent with the oligometastatic paradigm, which postulates that this is a transient phase that offers “a window of opportunity for cancer cure if equilibrium-phase lesions are ablated before polymetastatic escape occurs,” write Carlo Greco, MD, and Zvi Fuks, MD, both from the Champalimaud Centre for the Unknown, Lisbon, Portugal; Fuks is also affiliated with Memorial Sloan Kettering Cancer Center, New York.
“Taken together, these observations support the hypothesis that all detectable oligometastatic lesions should be systematically ablated, if feasible, in an effort to maximize oligometastatic cancer cure,” Greco and Fuks comment.
However, there is no clear consensus on what constitutes oligometastatic disease, they point out, and they warn that “a numerical-based decision to withhold lesion ablation may represent a strategic error, potentially reducing the benefit of metastasis-directed therapy (MDT) in the treatment of oligometastatic cancer.”
“We speculate that, if clinically and technically feasible, there should be no restriction to MDT at first oligometastatic presentation and as sequential lesions appear, regardless of lesion numbers, because each lesion may potentially constitute a generator of evolving metastatogenic clonogens,” they add.
Multiple rounds of MDT theoretically could “drive the disease to near extinction” and prevent the development of polymetastatic disease, they contend.
Study Details
For their study, Phillips and colleagues enrolled 54 men with recurrent hormone-sensitive prostate cancer with one, two, or three metastases detectable on conventional imaging who had not received ADT within 6 months of enrollment, or for a total of 3 or more years.
The patients were randomly assigned in a 2:1 ratio to receive either SABR, with dose and fractionation based on the size and location of each lesion, or to observation. The median age in each group was 68 years.
Prostate-specific membrane antigen (PSMA)-targeted PET-CT, a relatively new technique that is more accurate than conventional imaging, was performed during treatment planning and at day 180 of follow-up for patients assigned to SABR, but the investigators were blinded to the results to prevent bias in target-lesion selection.
The primary endpoint of progression at 6 months was measured by prostate-specific antigen increase, radiographic evidence, symptomatic progression, ADT initiation for any reason, or death.
This primary endpoint occurred in 7 of 36 men (19%) who were treated with SABR, compared with 11 of 18 (61%) who underwent observation (P = .005).
Median progression-free survival (PFS) was not reached in the SABR group vs. 5.8 months in the observation group, translating into a hazard ratio of 0.30 (P = .002).
Of the 36 men treated with SABR, 16 had baseline PET-avid lesions that, because of investigator blinding, were not included in the treatment fields.
Among all SABR-treated patients, the rate of progression at 6 months among men for whom all lesions were treated was 5%, compared with 38% for men with any lesions outside the treatment field (P = .03). The median PFS for patients with no untreated lesions was not reached, vs. 11.8 months for men in whom PET-avid lesions were missed (HR, 0.26; P = .006).
Oligometastatic vs. Polymetastatic
In an interview with Medscape Medical News, Tran noted that using PSMA PET-CT for imaging appears to be very important for identifying those patients with numerous or disseminated (polymetastatic) lesions, compared with those patients with limited disease who are most likely to benefit from SABR. He emphasized, however, that the PSMA PET-CT findings were an exploratory endpoint that required further validation.
Another secondary, exploratory endpoint of the study was the use of a circulating tumor DNA (ctDNA) analysis by the CAPP-Seq (cancer personalized profiling by deep sequencing) method.
“We show, in a discovery fashion, that certain mutations in the circulation seem to distinguish patients who would benefit from SABR vs those who would not,” he said.
The study was supported by the Nesbitt-McMaster Foundation, Ronald Rose and Joan Lazar, the Movember Foundation and Prostate Cancer Foundation, and the National Cancer Institute. Phillips reported receiving consulting fees and honoraria from RefleXion Medical outside the submitted work. Tran holds a licensed patent related to ablative radiotherapy compounds and methods (Natsar Pharmaceuticals). Several other authors reported financial disclosures. The full list can be found with the original article. Editorialists Greco and Fuks reported serving as cofounders of and owning stock in Ceramedix Holding, LLC.
This article first appeared on Medscape.com.


