User login
Nitrite food additives may increase risk of type 2 diabetes
Consuming a large amount of nitrites from food additives versus none was associated with a greater risk of developing type 2 diabetes in the NutriNet-Santé study in France, researchers report.
However, a few experts who were not involved with this research question the strength of the findings because of study limitations.
The study involved more than 100,000 adults with a mean age of 43, and 79% were women.
Individuals with the highest intakes of nitrites from food additives (top third) had a 53% higher risk of developing type 2 diabetes during a median follow-up of 7 years compared with those with the lowest intake of this food additive after controlling for intake of sugars, red and processed meats, heme iron, salt, and saturated fatty acids. Consumption of nitrates from food additives was not associated with risk of type 2 diabetes.
“Our findings suggest a direct association between additives-originated nitrites and [type 2 diabetes] risk and corroborate previously suggested associations between total dietary nitrites and [type 2 diabetes],” the researchers report in an article published online in PLoS Medicine.
However, “as this is the first large-scale study finding these associations, these results need to be replicated in other large-scale cohorts,” senior author Mathilde Touvier, PhD, head of the Nutritional Epidemiology Research Team (EREN-CRESS), INSERM, INRAE, Sorbonne Paris Nord University, and lead author Bernard Srour, PhD, PharmD, a scientist at the same institution, said in a joint email to this news organization.
Short-term intervention studies to determine insulin resistance could also be tested, they add.
In the meantime, “this study adds further evidence to the existing strong link between nitrites and colorectal cancer risk, and supports the importance of further regulation of nitrites as food additives and nitrogen fertilizers,” they say.
According to Dr. Touvier and Dr. Srour, the takeaway message for clinicians is the finding that nitrites from food additives are associated with type 2 diabetes, “support existing guidelines recommending [limiting] the consumption of processed meats to prevent chronic diseases. However, the consumption of vegetables should be encouraged as they contain several beneficial compounds and contribute to chronic disease prevention.”
Some experts are skeptical
But three experts who were not involved with the research were skeptical about the conclusions, in comments made to the U.K. Science Media Centre.
“The fundamental weakness of this study is how the food additive intake was assessed,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London. “Estimates of intake were based on recalls of dietary intake on two separate occasions at the beginning of the study with no further estimates in the follow-up period of over 7 years,” he noted.
Other limitations include the relatively young age of the cohort and relatively low incidence of new cases of type 2 diabetes (about 1% of the study population over 7 years).
Moreover, the level of nitrite food additive ingestion is much lower than the acceptable daily intake. The findings would need to be replicated with appropriate adjustment for differences in body weight.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, said that “the study does not support the claim in the press release and paper that food additives are responsible for the increased risk.”
He pointed out that “nitrite from additives contributes only about 4%-6% of total nitrite intake in the population, and it is not clear why this should have a stronger impact on risk than nitrite from other sources,” such as nitrate found in food and water.
Duane Mellor, PhD, registered dietitian and senior lecturer, Aston University, Birmingham, England, said: “It could be questioned how accurate estimating intakes of individual additives like sodium nitrite, which was less than 1 mg per day from a record of just 2 days food intake per year, as it assumes people ate the same the other 363 days of the year.”
Moreover, “it is perhaps worth noting that the use of nitrites as an additive is often as sodium nitrite, which is used to cure meats like bacon, which if someone is seeking to reduce their risk of type 2 diabetes would be something people would be encouraged to eat less of [anyway].”
“The best way to reduce your risk of developing type 2 diabetes,” he said, “is to be physically active, maintain a healthy weight for you, and eat a varied diet based on vegetables, pulses, nuts, seeds, and fruit along with wholegrain and moderate intakes of dairy foods and meat (especially processed meats).”
Study details
Nitrites and nitrates are used as food additives to prevent bacterial growth, mainly in processed meats, and they are also found in foods (mainly green leafy vegetables) and water (nitrates from the use of nitrogen fertilizer can enter the water supply).
The researchers analyzed data from 104,168 participants in NutriNet-Santé who had no diabetes at baseline and who completed 24-hour dietary intake records. They investigated the association between exposure to nitrites and nitrates (in food and water or in additives) and incident type 2 diabetes.
Most nitrites came from food (95.3%), and less often from food additives (4.7%) and water (< 0.01%). The nitrites in foods were mainly from vegetables (60%) and seasonings (23%).
Most nitrates also came from food (93%), followed by water (6.9%) and food additives (0.1%). The nitrates in foods were mainly from vegetables (41%), processed meat (19%), and meat (17%).
During a median follow-up of 7.3 years, there were 969 incident cases of type 2 diabetes.
Compared with individuals in the lowest third of nitrites from food and water, those in the highest tertile had a 27% higher risk of incident type 2 diabetes, after adjusting for multiple variables (hazard ratio, 1.27; P = .009).
The risk of type 2 diabetes associated with the highest intake of nitrites from additives was as previously described, 53% higher, than that for those with the lowest intake.
There was no evidence of an association between nitrates and risk of type 2 diabetes.
The researchers acknowledge that study limitations include potential errors in assessment of nitrate and nitrate exposure, potential selection bias (participants in the web-based study may have had healthier behaviors than the general population), and potential unaccounted confounders (because it was an observational study).
A version of this article first appeared on Medscape.com.
Consuming a large amount of nitrites from food additives versus none was associated with a greater risk of developing type 2 diabetes in the NutriNet-Santé study in France, researchers report.
However, a few experts who were not involved with this research question the strength of the findings because of study limitations.
The study involved more than 100,000 adults with a mean age of 43, and 79% were women.
Individuals with the highest intakes of nitrites from food additives (top third) had a 53% higher risk of developing type 2 diabetes during a median follow-up of 7 years compared with those with the lowest intake of this food additive after controlling for intake of sugars, red and processed meats, heme iron, salt, and saturated fatty acids. Consumption of nitrates from food additives was not associated with risk of type 2 diabetes.
“Our findings suggest a direct association between additives-originated nitrites and [type 2 diabetes] risk and corroborate previously suggested associations between total dietary nitrites and [type 2 diabetes],” the researchers report in an article published online in PLoS Medicine.
However, “as this is the first large-scale study finding these associations, these results need to be replicated in other large-scale cohorts,” senior author Mathilde Touvier, PhD, head of the Nutritional Epidemiology Research Team (EREN-CRESS), INSERM, INRAE, Sorbonne Paris Nord University, and lead author Bernard Srour, PhD, PharmD, a scientist at the same institution, said in a joint email to this news organization.
Short-term intervention studies to determine insulin resistance could also be tested, they add.
In the meantime, “this study adds further evidence to the existing strong link between nitrites and colorectal cancer risk, and supports the importance of further regulation of nitrites as food additives and nitrogen fertilizers,” they say.
According to Dr. Touvier and Dr. Srour, the takeaway message for clinicians is the finding that nitrites from food additives are associated with type 2 diabetes, “support existing guidelines recommending [limiting] the consumption of processed meats to prevent chronic diseases. However, the consumption of vegetables should be encouraged as they contain several beneficial compounds and contribute to chronic disease prevention.”
Some experts are skeptical
But three experts who were not involved with the research were skeptical about the conclusions, in comments made to the U.K. Science Media Centre.
“The fundamental weakness of this study is how the food additive intake was assessed,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London. “Estimates of intake were based on recalls of dietary intake on two separate occasions at the beginning of the study with no further estimates in the follow-up period of over 7 years,” he noted.
Other limitations include the relatively young age of the cohort and relatively low incidence of new cases of type 2 diabetes (about 1% of the study population over 7 years).
Moreover, the level of nitrite food additive ingestion is much lower than the acceptable daily intake. The findings would need to be replicated with appropriate adjustment for differences in body weight.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, said that “the study does not support the claim in the press release and paper that food additives are responsible for the increased risk.”
He pointed out that “nitrite from additives contributes only about 4%-6% of total nitrite intake in the population, and it is not clear why this should have a stronger impact on risk than nitrite from other sources,” such as nitrate found in food and water.
Duane Mellor, PhD, registered dietitian and senior lecturer, Aston University, Birmingham, England, said: “It could be questioned how accurate estimating intakes of individual additives like sodium nitrite, which was less than 1 mg per day from a record of just 2 days food intake per year, as it assumes people ate the same the other 363 days of the year.”
Moreover, “it is perhaps worth noting that the use of nitrites as an additive is often as sodium nitrite, which is used to cure meats like bacon, which if someone is seeking to reduce their risk of type 2 diabetes would be something people would be encouraged to eat less of [anyway].”
“The best way to reduce your risk of developing type 2 diabetes,” he said, “is to be physically active, maintain a healthy weight for you, and eat a varied diet based on vegetables, pulses, nuts, seeds, and fruit along with wholegrain and moderate intakes of dairy foods and meat (especially processed meats).”
Study details
Nitrites and nitrates are used as food additives to prevent bacterial growth, mainly in processed meats, and they are also found in foods (mainly green leafy vegetables) and water (nitrates from the use of nitrogen fertilizer can enter the water supply).
The researchers analyzed data from 104,168 participants in NutriNet-Santé who had no diabetes at baseline and who completed 24-hour dietary intake records. They investigated the association between exposure to nitrites and nitrates (in food and water or in additives) and incident type 2 diabetes.
Most nitrites came from food (95.3%), and less often from food additives (4.7%) and water (< 0.01%). The nitrites in foods were mainly from vegetables (60%) and seasonings (23%).
Most nitrates also came from food (93%), followed by water (6.9%) and food additives (0.1%). The nitrates in foods were mainly from vegetables (41%), processed meat (19%), and meat (17%).
During a median follow-up of 7.3 years, there were 969 incident cases of type 2 diabetes.
Compared with individuals in the lowest third of nitrites from food and water, those in the highest tertile had a 27% higher risk of incident type 2 diabetes, after adjusting for multiple variables (hazard ratio, 1.27; P = .009).
The risk of type 2 diabetes associated with the highest intake of nitrites from additives was as previously described, 53% higher, than that for those with the lowest intake.
There was no evidence of an association between nitrates and risk of type 2 diabetes.
The researchers acknowledge that study limitations include potential errors in assessment of nitrate and nitrate exposure, potential selection bias (participants in the web-based study may have had healthier behaviors than the general population), and potential unaccounted confounders (because it was an observational study).
A version of this article first appeared on Medscape.com.
Consuming a large amount of nitrites from food additives versus none was associated with a greater risk of developing type 2 diabetes in the NutriNet-Santé study in France, researchers report.
However, a few experts who were not involved with this research question the strength of the findings because of study limitations.
The study involved more than 100,000 adults with a mean age of 43, and 79% were women.
Individuals with the highest intakes of nitrites from food additives (top third) had a 53% higher risk of developing type 2 diabetes during a median follow-up of 7 years compared with those with the lowest intake of this food additive after controlling for intake of sugars, red and processed meats, heme iron, salt, and saturated fatty acids. Consumption of nitrates from food additives was not associated with risk of type 2 diabetes.
“Our findings suggest a direct association between additives-originated nitrites and [type 2 diabetes] risk and corroborate previously suggested associations between total dietary nitrites and [type 2 diabetes],” the researchers report in an article published online in PLoS Medicine.
However, “as this is the first large-scale study finding these associations, these results need to be replicated in other large-scale cohorts,” senior author Mathilde Touvier, PhD, head of the Nutritional Epidemiology Research Team (EREN-CRESS), INSERM, INRAE, Sorbonne Paris Nord University, and lead author Bernard Srour, PhD, PharmD, a scientist at the same institution, said in a joint email to this news organization.
Short-term intervention studies to determine insulin resistance could also be tested, they add.
In the meantime, “this study adds further evidence to the existing strong link between nitrites and colorectal cancer risk, and supports the importance of further regulation of nitrites as food additives and nitrogen fertilizers,” they say.
According to Dr. Touvier and Dr. Srour, the takeaway message for clinicians is the finding that nitrites from food additives are associated with type 2 diabetes, “support existing guidelines recommending [limiting] the consumption of processed meats to prevent chronic diseases. However, the consumption of vegetables should be encouraged as they contain several beneficial compounds and contribute to chronic disease prevention.”
Some experts are skeptical
But three experts who were not involved with the research were skeptical about the conclusions, in comments made to the U.K. Science Media Centre.
“The fundamental weakness of this study is how the food additive intake was assessed,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London. “Estimates of intake were based on recalls of dietary intake on two separate occasions at the beginning of the study with no further estimates in the follow-up period of over 7 years,” he noted.
Other limitations include the relatively young age of the cohort and relatively low incidence of new cases of type 2 diabetes (about 1% of the study population over 7 years).
Moreover, the level of nitrite food additive ingestion is much lower than the acceptable daily intake. The findings would need to be replicated with appropriate adjustment for differences in body weight.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, said that “the study does not support the claim in the press release and paper that food additives are responsible for the increased risk.”
He pointed out that “nitrite from additives contributes only about 4%-6% of total nitrite intake in the population, and it is not clear why this should have a stronger impact on risk than nitrite from other sources,” such as nitrate found in food and water.
Duane Mellor, PhD, registered dietitian and senior lecturer, Aston University, Birmingham, England, said: “It could be questioned how accurate estimating intakes of individual additives like sodium nitrite, which was less than 1 mg per day from a record of just 2 days food intake per year, as it assumes people ate the same the other 363 days of the year.”
Moreover, “it is perhaps worth noting that the use of nitrites as an additive is often as sodium nitrite, which is used to cure meats like bacon, which if someone is seeking to reduce their risk of type 2 diabetes would be something people would be encouraged to eat less of [anyway].”
“The best way to reduce your risk of developing type 2 diabetes,” he said, “is to be physically active, maintain a healthy weight for you, and eat a varied diet based on vegetables, pulses, nuts, seeds, and fruit along with wholegrain and moderate intakes of dairy foods and meat (especially processed meats).”
Study details
Nitrites and nitrates are used as food additives to prevent bacterial growth, mainly in processed meats, and they are also found in foods (mainly green leafy vegetables) and water (nitrates from the use of nitrogen fertilizer can enter the water supply).
The researchers analyzed data from 104,168 participants in NutriNet-Santé who had no diabetes at baseline and who completed 24-hour dietary intake records. They investigated the association between exposure to nitrites and nitrates (in food and water or in additives) and incident type 2 diabetes.
Most nitrites came from food (95.3%), and less often from food additives (4.7%) and water (< 0.01%). The nitrites in foods were mainly from vegetables (60%) and seasonings (23%).
Most nitrates also came from food (93%), followed by water (6.9%) and food additives (0.1%). The nitrates in foods were mainly from vegetables (41%), processed meat (19%), and meat (17%).
During a median follow-up of 7.3 years, there were 969 incident cases of type 2 diabetes.
Compared with individuals in the lowest third of nitrites from food and water, those in the highest tertile had a 27% higher risk of incident type 2 diabetes, after adjusting for multiple variables (hazard ratio, 1.27; P = .009).
The risk of type 2 diabetes associated with the highest intake of nitrites from additives was as previously described, 53% higher, than that for those with the lowest intake.
There was no evidence of an association between nitrates and risk of type 2 diabetes.
The researchers acknowledge that study limitations include potential errors in assessment of nitrate and nitrate exposure, potential selection bias (participants in the web-based study may have had healthier behaviors than the general population), and potential unaccounted confounders (because it was an observational study).
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Fitbit figures: More steps per day cut type 2 diabetes risk
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
Intermittent fasting can lead to type 2 diabetes remission
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients trying to lose weight overestimate their diet quality
Only 28% of the participants had good agreement – defined as a difference of 6 points or less – between their perceived diet quality and its actual quality based on Healthy Eating Index–2015 (HEI) scores at the end of the 12-month intervention.
Even fewer – only 13% – had good agreement with their perceived and actual improvement in diet quality.
Jessica Cheng, PhD, Harvard School of Public Health, Boston, presented the findings in an oral session at the American Heart Association scientific sessions.
The study suggests that “patients can benefit from concrete advice on aspects of their diet that could most benefit by being changed,” Dr. Cheng said in an interview.
“But once they know what to change, they may need additional advice on how to make and sustain those changes. Providers may direct their patients to resources such as dietitians, medically tailored meals, MyPlate, healthy recipes, etc.,” she advised.
“The findings are not surprising given that dietary recalls are subject to recall bias and depend on the person’s baseline nutrition knowledge or literacy,” Deepika Laddu, PhD, who was not involved with this research, said in an interview.
Misperception of diet intake is common in individuals with overweight or obesity, and one 90-minute session with a dietitian is not enough, according to Dr. Laddu, assistant professor at the University of Illinois at Chicago.
“The Dietary Guidelines for Americans does a really nice job at presenting all of the options,” she said. However, “understanding what a healthy diet pattern is, or how to adopt it, is confusing, due to a lot of ‘noise’, that is, the mixed messaging and unproven health claims, which add to inadequacies in health or nutrition literacy.”
“It is important to recognize that changing dietary practices is behaviorally challenging and complex,” she emphasized.
People who are interested in making dietary changes need to have ongoing conversations with a qualified health care professional, which most often starts with their primary care clinician.
“Given the well-known time constraints during a typical clinical visit, beyond that initial conversation, it is absolutely critical that patients be referred to qualified healthcare professionals such as a registered dietitian, nurse practitioner, health coach/educator or diabetes educator, etc, for ongoing support.”
These providers can assess the patient’s initial diet, perceptions of a healthy diet, and diet goals, and address any gaps in health literacy, to enable the patient to develop long-lasting, realistic, and healthy eating behaviors.
Perceived vs. actual diet quality
Healthy eating is essential for heart and general health and longevity, but it is unclear if people who make lifestyle (diet and physical activity) changes to lose weight have an accurate perception of diet quality.
The researchers analyzed data from the SMARTER trial of 502 adults aged 35-58 living in the greater Pittsburgh area who were trying to lose weight.
Participants received a 90-minute weight loss counseling session addressing behavioral strategies and establishing dietary and physical activity goals. They all received instructions on how to monitor their diet, physical activity, and weight daily, using a smartphone app, a wristband tracker (Fitbit Charge 2), and a smart wireless scale. Half of the participants also received real-time personalized feedback on those behaviors, up to three times a day, via the study app.
The participants replied to two 24-hour dietary recall questionnaires at study entry and two questionnaires at 12 months.
Researchers analyzed data from the 116 participants who provided information about diet quality. At 1 year, they were asked to rate their diet quality, but also rate their diet quality 12 months earlier at baseline, on a scale of 0-100, where 100 is best.
The average weight loss at 12 months was similar in the groups with and without feedback from the app (roughly 3.2% of baseline weight), so the two study arms were combined. The participants had a mean age of 52 years; 80% were women and 87% were White. They had an average body mass index of 33 kg/m2.
Based on the information from the food recall questionnaires, the researchers calculated the patients’ HEI scores at the start and end of the study. The HEI score is a measure of how well a person’s diet adheres to the 2015-2020 Dietary Guidelines for Americans. It is based on an adequate consumption of nine types of foods – total fruits, whole fruits, total vegetables, greens and beans, total protein foods, seafood, and plant proteins (up to 5 points each), and whole grains, dairy, and fatty acids (up to 10 points each) – and reduced consumption of four dietary components – refined grains, sodium, added sugars, and saturated fats (up to 10 points each).
The healthiest diet has an HEI score of 100, and the Healthy People 2020 goal was an HEI score of 74, Dr. Cheng noted.
At 12 months, on average, the participants rated their diet quality at 70.5 points, whereas the researchers calculated that their average HEI score was only 56.
Participants thought they had improved their diet quality by about 20 points, Dr. Cheng reported. “However, the HEI would suggest they’ve improved it by 1.5 points, which is not a lot out of 100.”
“Future studies should examine the effects of helping people close the gap between their perceptions and objective diet quality measurements,” Dr. Cheng said in a press release from the AHA.
The study was funded by the National Heart, Lung, and Blood Institute, a division of the National Institutes of Health. Dr. Cheng and Dr. Laddu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Only 28% of the participants had good agreement – defined as a difference of 6 points or less – between their perceived diet quality and its actual quality based on Healthy Eating Index–2015 (HEI) scores at the end of the 12-month intervention.
Even fewer – only 13% – had good agreement with their perceived and actual improvement in diet quality.
Jessica Cheng, PhD, Harvard School of Public Health, Boston, presented the findings in an oral session at the American Heart Association scientific sessions.
The study suggests that “patients can benefit from concrete advice on aspects of their diet that could most benefit by being changed,” Dr. Cheng said in an interview.
“But once they know what to change, they may need additional advice on how to make and sustain those changes. Providers may direct their patients to resources such as dietitians, medically tailored meals, MyPlate, healthy recipes, etc.,” she advised.
“The findings are not surprising given that dietary recalls are subject to recall bias and depend on the person’s baseline nutrition knowledge or literacy,” Deepika Laddu, PhD, who was not involved with this research, said in an interview.
Misperception of diet intake is common in individuals with overweight or obesity, and one 90-minute session with a dietitian is not enough, according to Dr. Laddu, assistant professor at the University of Illinois at Chicago.
“The Dietary Guidelines for Americans does a really nice job at presenting all of the options,” she said. However, “understanding what a healthy diet pattern is, or how to adopt it, is confusing, due to a lot of ‘noise’, that is, the mixed messaging and unproven health claims, which add to inadequacies in health or nutrition literacy.”
“It is important to recognize that changing dietary practices is behaviorally challenging and complex,” she emphasized.
People who are interested in making dietary changes need to have ongoing conversations with a qualified health care professional, which most often starts with their primary care clinician.
“Given the well-known time constraints during a typical clinical visit, beyond that initial conversation, it is absolutely critical that patients be referred to qualified healthcare professionals such as a registered dietitian, nurse practitioner, health coach/educator or diabetes educator, etc, for ongoing support.”
These providers can assess the patient’s initial diet, perceptions of a healthy diet, and diet goals, and address any gaps in health literacy, to enable the patient to develop long-lasting, realistic, and healthy eating behaviors.
Perceived vs. actual diet quality
Healthy eating is essential for heart and general health and longevity, but it is unclear if people who make lifestyle (diet and physical activity) changes to lose weight have an accurate perception of diet quality.
The researchers analyzed data from the SMARTER trial of 502 adults aged 35-58 living in the greater Pittsburgh area who were trying to lose weight.
Participants received a 90-minute weight loss counseling session addressing behavioral strategies and establishing dietary and physical activity goals. They all received instructions on how to monitor their diet, physical activity, and weight daily, using a smartphone app, a wristband tracker (Fitbit Charge 2), and a smart wireless scale. Half of the participants also received real-time personalized feedback on those behaviors, up to three times a day, via the study app.
The participants replied to two 24-hour dietary recall questionnaires at study entry and two questionnaires at 12 months.
Researchers analyzed data from the 116 participants who provided information about diet quality. At 1 year, they were asked to rate their diet quality, but also rate their diet quality 12 months earlier at baseline, on a scale of 0-100, where 100 is best.
The average weight loss at 12 months was similar in the groups with and without feedback from the app (roughly 3.2% of baseline weight), so the two study arms were combined. The participants had a mean age of 52 years; 80% were women and 87% were White. They had an average body mass index of 33 kg/m2.
Based on the information from the food recall questionnaires, the researchers calculated the patients’ HEI scores at the start and end of the study. The HEI score is a measure of how well a person’s diet adheres to the 2015-2020 Dietary Guidelines for Americans. It is based on an adequate consumption of nine types of foods – total fruits, whole fruits, total vegetables, greens and beans, total protein foods, seafood, and plant proteins (up to 5 points each), and whole grains, dairy, and fatty acids (up to 10 points each) – and reduced consumption of four dietary components – refined grains, sodium, added sugars, and saturated fats (up to 10 points each).
The healthiest diet has an HEI score of 100, and the Healthy People 2020 goal was an HEI score of 74, Dr. Cheng noted.
At 12 months, on average, the participants rated their diet quality at 70.5 points, whereas the researchers calculated that their average HEI score was only 56.
Participants thought they had improved their diet quality by about 20 points, Dr. Cheng reported. “However, the HEI would suggest they’ve improved it by 1.5 points, which is not a lot out of 100.”
“Future studies should examine the effects of helping people close the gap between their perceptions and objective diet quality measurements,” Dr. Cheng said in a press release from the AHA.
The study was funded by the National Heart, Lung, and Blood Institute, a division of the National Institutes of Health. Dr. Cheng and Dr. Laddu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Only 28% of the participants had good agreement – defined as a difference of 6 points or less – between their perceived diet quality and its actual quality based on Healthy Eating Index–2015 (HEI) scores at the end of the 12-month intervention.
Even fewer – only 13% – had good agreement with their perceived and actual improvement in diet quality.
Jessica Cheng, PhD, Harvard School of Public Health, Boston, presented the findings in an oral session at the American Heart Association scientific sessions.
The study suggests that “patients can benefit from concrete advice on aspects of their diet that could most benefit by being changed,” Dr. Cheng said in an interview.
“But once they know what to change, they may need additional advice on how to make and sustain those changes. Providers may direct their patients to resources such as dietitians, medically tailored meals, MyPlate, healthy recipes, etc.,” she advised.
“The findings are not surprising given that dietary recalls are subject to recall bias and depend on the person’s baseline nutrition knowledge or literacy,” Deepika Laddu, PhD, who was not involved with this research, said in an interview.
Misperception of diet intake is common in individuals with overweight or obesity, and one 90-minute session with a dietitian is not enough, according to Dr. Laddu, assistant professor at the University of Illinois at Chicago.
“The Dietary Guidelines for Americans does a really nice job at presenting all of the options,” she said. However, “understanding what a healthy diet pattern is, or how to adopt it, is confusing, due to a lot of ‘noise’, that is, the mixed messaging and unproven health claims, which add to inadequacies in health or nutrition literacy.”
“It is important to recognize that changing dietary practices is behaviorally challenging and complex,” she emphasized.
People who are interested in making dietary changes need to have ongoing conversations with a qualified health care professional, which most often starts with their primary care clinician.
“Given the well-known time constraints during a typical clinical visit, beyond that initial conversation, it is absolutely critical that patients be referred to qualified healthcare professionals such as a registered dietitian, nurse practitioner, health coach/educator or diabetes educator, etc, for ongoing support.”
These providers can assess the patient’s initial diet, perceptions of a healthy diet, and diet goals, and address any gaps in health literacy, to enable the patient to develop long-lasting, realistic, and healthy eating behaviors.
Perceived vs. actual diet quality
Healthy eating is essential for heart and general health and longevity, but it is unclear if people who make lifestyle (diet and physical activity) changes to lose weight have an accurate perception of diet quality.
The researchers analyzed data from the SMARTER trial of 502 adults aged 35-58 living in the greater Pittsburgh area who were trying to lose weight.
Participants received a 90-minute weight loss counseling session addressing behavioral strategies and establishing dietary and physical activity goals. They all received instructions on how to monitor their diet, physical activity, and weight daily, using a smartphone app, a wristband tracker (Fitbit Charge 2), and a smart wireless scale. Half of the participants also received real-time personalized feedback on those behaviors, up to three times a day, via the study app.
The participants replied to two 24-hour dietary recall questionnaires at study entry and two questionnaires at 12 months.
Researchers analyzed data from the 116 participants who provided information about diet quality. At 1 year, they were asked to rate their diet quality, but also rate their diet quality 12 months earlier at baseline, on a scale of 0-100, where 100 is best.
The average weight loss at 12 months was similar in the groups with and without feedback from the app (roughly 3.2% of baseline weight), so the two study arms were combined. The participants had a mean age of 52 years; 80% were women and 87% were White. They had an average body mass index of 33 kg/m2.
Based on the information from the food recall questionnaires, the researchers calculated the patients’ HEI scores at the start and end of the study. The HEI score is a measure of how well a person’s diet adheres to the 2015-2020 Dietary Guidelines for Americans. It is based on an adequate consumption of nine types of foods – total fruits, whole fruits, total vegetables, greens and beans, total protein foods, seafood, and plant proteins (up to 5 points each), and whole grains, dairy, and fatty acids (up to 10 points each) – and reduced consumption of four dietary components – refined grains, sodium, added sugars, and saturated fats (up to 10 points each).
The healthiest diet has an HEI score of 100, and the Healthy People 2020 goal was an HEI score of 74, Dr. Cheng noted.
At 12 months, on average, the participants rated their diet quality at 70.5 points, whereas the researchers calculated that their average HEI score was only 56.
Participants thought they had improved their diet quality by about 20 points, Dr. Cheng reported. “However, the HEI would suggest they’ve improved it by 1.5 points, which is not a lot out of 100.”
“Future studies should examine the effects of helping people close the gap between their perceptions and objective diet quality measurements,” Dr. Cheng said in a press release from the AHA.
The study was funded by the National Heart, Lung, and Blood Institute, a division of the National Institutes of Health. Dr. Cheng and Dr. Laddu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
More weight loss with surgery than new obesity meds: meta-analysis
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.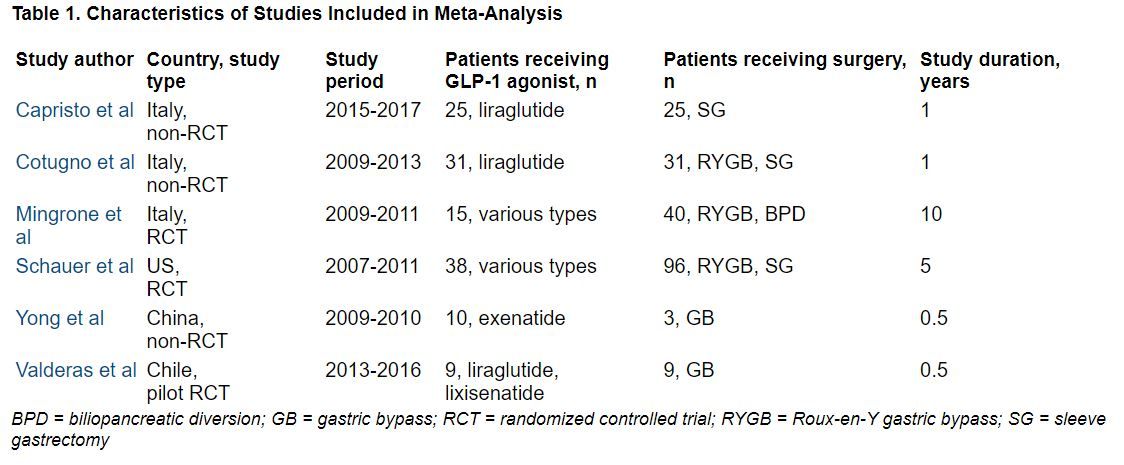
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK®
New dual-agonist weight-loss injection impressive, but early days
SAN DIEGO – A novel glucagonlike peptide-1 (GLP-1)/glucagon dual-receptor agonist, BI 456906, being developed by Boehringer Ingelheim and Zealand Pharma, led to “impressive” weight loss in a phase 2 dosing study of patients with overweight/obesity and type 2 diabetes – but this is early research.
Julio Rosenstock, MD, presented the study results, including weight loss and adverse events, at the annual meeting of the Obesity Society.
At the highest tested dose (1.8 mg twice weekly subcutaneous injections), 57% of patients lost at least 5% of their initial body weight and 35% lost at least 10% of their initial body weight at 16 weeks.
In contrast, among the patients who received a 1-mg semaglutide dose as a comparator, 38% lost at least 5% of their initial body weight and 16% lost at least 10% of their initial body weight at study end.
This is “very promising data as an anti-obesity compound,” said Dr. Rosenstock, professor of medicine, University of Texas Southwestern Medical Center in Dallas.
The researchers enrolled 411 adults and randomized them into eight groups of roughly 50 patients each.
They compared six doses of BI 456906 (from 0.3 mg/week to 1.8 mg twice weekly) versus 1 mg/week of the GLP-1 agonist semaglutide (Wegovy, Novo Nordisk) versus placebo.
Patients had a mean initial weight of 97 kg (214 pounds).
After 4 months, on average, patients who received the highest tested dose of BI 456906 lost 9% of their initial weight or roughly 8.7 kg (19 pounds).
Patients who received semaglutide lost 5.4% of their initial weight or roughly 5.2 kg (11.5 pounds), and patients who received placebo lost only 1.2% of their initial weight
The main adverse events were gastrointestinal.
‘Exciting data,’ but still early days
“This is very exciting data. It comes from another experienced company with a track record of successful products with a new compound in a class where other related compounds have shown efficacy and safety,” Dan Bessesen, MD, president of The Obesity Society, who was not involved with this research, told this news organization in an email.
“The degree of weight loss is impressive for a 16-week study,” Dr. Bessesen, professor of medicine in the division of endocrinology, metabolism and diabetes at the University of Colorado at Denver, Aurora, added. “The longer-term weight loss will likely be more.”
The side-effect profile is not particularly concerning and is like other drugs in this general class, he said.
However, he also noted a few caveats. This was only a phase 2 study, “so we should not make firm conclusions about efficacy from a study like this, as the number of subjects studied at each dose is relatively small and the follow-up not long.”
In addition, “the dose of semaglutide is the old ‘diabetes’ dose (1 mg) not the weight-loss dose of 2.4 mg or the new diabetes dose of 2 mg. It is not a real comparison with the maximal approved dose of semaglutide. So, we cannot say that it will be better than semaglutide.”
The next hurdle is the “need to see phase 3 studies in a larger group of patients studied for a longer time. Then [the company] will need FDA approval, so it may be a bit of time” before this drug potentially enters the marketplace.
The “bottom line” is that this potential new antiobesity/diabetes drug is “very promising, but [it is] still a little early to say where it ultimately will go.”
A1c results presented at EASD
To be included in this study, patients had to be 18-75 years old, have type 2 diabetes, a body mass index of 25-50 kg/m2, and hemoglobin A1c of 7%-10%, and be stable on metformin therapy.
The patients had a mean age of 57 years, and 57% were men. They had a mean A1c of 8.1%, a mean BMI of 34 kg/m2, and a mean waist circumference of 110 cm (43 inches).
“We just recently reported at the EASD conference last month, the effect of BI 456906 on A1c lowering,” Dr. Rosenstock said.
“It looks like the [drop in] A1c plateaus at 1.9%, which is pretty good when you consider the baseline A1c is around 8%. You get down to around 6%, which is what we regard as a very robust reduction in people with type 2 diabetes on metformin.”
The current analysis showed that patients who received doses of 0.3, 0.9, 1.8, and 2.7 mg/week of the novel drug lost 1.9%, 4.4%, 6.6%, and 6.7% of their initial body weight, respectively, after 16 weeks.
The patients who received 1.2 mg and 1.8 mg twice weekly lost even more weight, 7.2% and 9% of their initial weight, respectively.
At the highest dose, on average, patients lost 13 cm (5 inches) around their waist.
Adverse events were reported by 78% of the patients, most commonly nausea (34% of patients), vomiting (18%), and diarrhea (16%).
Only 1.3% of patients had a drug-related serious adverse event. A total of 16% of patients discontinued the therapy.
Most of the “gastrointestinal adverse events leading the treatment discontinuation were possibly dose and titration related,” Dr. Rosenstock said, “and it’s highly conceivable that for future studies a slower dose escalation may mitigate the occurrence of the gastrointestinal adverse events.”
BI 456906 was coinvented with Zealand Pharma. Under the licensing agreement, Boehringer Ingelheim funds all research, development, and commercialization.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A novel glucagonlike peptide-1 (GLP-1)/glucagon dual-receptor agonist, BI 456906, being developed by Boehringer Ingelheim and Zealand Pharma, led to “impressive” weight loss in a phase 2 dosing study of patients with overweight/obesity and type 2 diabetes – but this is early research.
Julio Rosenstock, MD, presented the study results, including weight loss and adverse events, at the annual meeting of the Obesity Society.
At the highest tested dose (1.8 mg twice weekly subcutaneous injections), 57% of patients lost at least 5% of their initial body weight and 35% lost at least 10% of their initial body weight at 16 weeks.
In contrast, among the patients who received a 1-mg semaglutide dose as a comparator, 38% lost at least 5% of their initial body weight and 16% lost at least 10% of their initial body weight at study end.
This is “very promising data as an anti-obesity compound,” said Dr. Rosenstock, professor of medicine, University of Texas Southwestern Medical Center in Dallas.
The researchers enrolled 411 adults and randomized them into eight groups of roughly 50 patients each.
They compared six doses of BI 456906 (from 0.3 mg/week to 1.8 mg twice weekly) versus 1 mg/week of the GLP-1 agonist semaglutide (Wegovy, Novo Nordisk) versus placebo.
Patients had a mean initial weight of 97 kg (214 pounds).
After 4 months, on average, patients who received the highest tested dose of BI 456906 lost 9% of their initial weight or roughly 8.7 kg (19 pounds).
Patients who received semaglutide lost 5.4% of their initial weight or roughly 5.2 kg (11.5 pounds), and patients who received placebo lost only 1.2% of their initial weight
The main adverse events were gastrointestinal.
‘Exciting data,’ but still early days
“This is very exciting data. It comes from another experienced company with a track record of successful products with a new compound in a class where other related compounds have shown efficacy and safety,” Dan Bessesen, MD, president of The Obesity Society, who was not involved with this research, told this news organization in an email.
“The degree of weight loss is impressive for a 16-week study,” Dr. Bessesen, professor of medicine in the division of endocrinology, metabolism and diabetes at the University of Colorado at Denver, Aurora, added. “The longer-term weight loss will likely be more.”
The side-effect profile is not particularly concerning and is like other drugs in this general class, he said.
However, he also noted a few caveats. This was only a phase 2 study, “so we should not make firm conclusions about efficacy from a study like this, as the number of subjects studied at each dose is relatively small and the follow-up not long.”
In addition, “the dose of semaglutide is the old ‘diabetes’ dose (1 mg) not the weight-loss dose of 2.4 mg or the new diabetes dose of 2 mg. It is not a real comparison with the maximal approved dose of semaglutide. So, we cannot say that it will be better than semaglutide.”
The next hurdle is the “need to see phase 3 studies in a larger group of patients studied for a longer time. Then [the company] will need FDA approval, so it may be a bit of time” before this drug potentially enters the marketplace.
The “bottom line” is that this potential new antiobesity/diabetes drug is “very promising, but [it is] still a little early to say where it ultimately will go.”
A1c results presented at EASD
To be included in this study, patients had to be 18-75 years old, have type 2 diabetes, a body mass index of 25-50 kg/m2, and hemoglobin A1c of 7%-10%, and be stable on metformin therapy.
The patients had a mean age of 57 years, and 57% were men. They had a mean A1c of 8.1%, a mean BMI of 34 kg/m2, and a mean waist circumference of 110 cm (43 inches).
“We just recently reported at the EASD conference last month, the effect of BI 456906 on A1c lowering,” Dr. Rosenstock said.
“It looks like the [drop in] A1c plateaus at 1.9%, which is pretty good when you consider the baseline A1c is around 8%. You get down to around 6%, which is what we regard as a very robust reduction in people with type 2 diabetes on metformin.”
The current analysis showed that patients who received doses of 0.3, 0.9, 1.8, and 2.7 mg/week of the novel drug lost 1.9%, 4.4%, 6.6%, and 6.7% of their initial body weight, respectively, after 16 weeks.
The patients who received 1.2 mg and 1.8 mg twice weekly lost even more weight, 7.2% and 9% of their initial weight, respectively.
At the highest dose, on average, patients lost 13 cm (5 inches) around their waist.
Adverse events were reported by 78% of the patients, most commonly nausea (34% of patients), vomiting (18%), and diarrhea (16%).
Only 1.3% of patients had a drug-related serious adverse event. A total of 16% of patients discontinued the therapy.
Most of the “gastrointestinal adverse events leading the treatment discontinuation were possibly dose and titration related,” Dr. Rosenstock said, “and it’s highly conceivable that for future studies a slower dose escalation may mitigate the occurrence of the gastrointestinal adverse events.”
BI 456906 was coinvented with Zealand Pharma. Under the licensing agreement, Boehringer Ingelheim funds all research, development, and commercialization.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A novel glucagonlike peptide-1 (GLP-1)/glucagon dual-receptor agonist, BI 456906, being developed by Boehringer Ingelheim and Zealand Pharma, led to “impressive” weight loss in a phase 2 dosing study of patients with overweight/obesity and type 2 diabetes – but this is early research.
Julio Rosenstock, MD, presented the study results, including weight loss and adverse events, at the annual meeting of the Obesity Society.
At the highest tested dose (1.8 mg twice weekly subcutaneous injections), 57% of patients lost at least 5% of their initial body weight and 35% lost at least 10% of their initial body weight at 16 weeks.
In contrast, among the patients who received a 1-mg semaglutide dose as a comparator, 38% lost at least 5% of their initial body weight and 16% lost at least 10% of their initial body weight at study end.
This is “very promising data as an anti-obesity compound,” said Dr. Rosenstock, professor of medicine, University of Texas Southwestern Medical Center in Dallas.
The researchers enrolled 411 adults and randomized them into eight groups of roughly 50 patients each.
They compared six doses of BI 456906 (from 0.3 mg/week to 1.8 mg twice weekly) versus 1 mg/week of the GLP-1 agonist semaglutide (Wegovy, Novo Nordisk) versus placebo.
Patients had a mean initial weight of 97 kg (214 pounds).
After 4 months, on average, patients who received the highest tested dose of BI 456906 lost 9% of their initial weight or roughly 8.7 kg (19 pounds).
Patients who received semaglutide lost 5.4% of their initial weight or roughly 5.2 kg (11.5 pounds), and patients who received placebo lost only 1.2% of their initial weight
The main adverse events were gastrointestinal.
‘Exciting data,’ but still early days
“This is very exciting data. It comes from another experienced company with a track record of successful products with a new compound in a class where other related compounds have shown efficacy and safety,” Dan Bessesen, MD, president of The Obesity Society, who was not involved with this research, told this news organization in an email.
“The degree of weight loss is impressive for a 16-week study,” Dr. Bessesen, professor of medicine in the division of endocrinology, metabolism and diabetes at the University of Colorado at Denver, Aurora, added. “The longer-term weight loss will likely be more.”
The side-effect profile is not particularly concerning and is like other drugs in this general class, he said.
However, he also noted a few caveats. This was only a phase 2 study, “so we should not make firm conclusions about efficacy from a study like this, as the number of subjects studied at each dose is relatively small and the follow-up not long.”
In addition, “the dose of semaglutide is the old ‘diabetes’ dose (1 mg) not the weight-loss dose of 2.4 mg or the new diabetes dose of 2 mg. It is not a real comparison with the maximal approved dose of semaglutide. So, we cannot say that it will be better than semaglutide.”
The next hurdle is the “need to see phase 3 studies in a larger group of patients studied for a longer time. Then [the company] will need FDA approval, so it may be a bit of time” before this drug potentially enters the marketplace.
The “bottom line” is that this potential new antiobesity/diabetes drug is “very promising, but [it is] still a little early to say where it ultimately will go.”
A1c results presented at EASD
To be included in this study, patients had to be 18-75 years old, have type 2 diabetes, a body mass index of 25-50 kg/m2, and hemoglobin A1c of 7%-10%, and be stable on metformin therapy.
The patients had a mean age of 57 years, and 57% were men. They had a mean A1c of 8.1%, a mean BMI of 34 kg/m2, and a mean waist circumference of 110 cm (43 inches).
“We just recently reported at the EASD conference last month, the effect of BI 456906 on A1c lowering,” Dr. Rosenstock said.
“It looks like the [drop in] A1c plateaus at 1.9%, which is pretty good when you consider the baseline A1c is around 8%. You get down to around 6%, which is what we regard as a very robust reduction in people with type 2 diabetes on metformin.”
The current analysis showed that patients who received doses of 0.3, 0.9, 1.8, and 2.7 mg/week of the novel drug lost 1.9%, 4.4%, 6.6%, and 6.7% of their initial body weight, respectively, after 16 weeks.
The patients who received 1.2 mg and 1.8 mg twice weekly lost even more weight, 7.2% and 9% of their initial weight, respectively.
At the highest dose, on average, patients lost 13 cm (5 inches) around their waist.
Adverse events were reported by 78% of the patients, most commonly nausea (34% of patients), vomiting (18%), and diarrhea (16%).
Only 1.3% of patients had a drug-related serious adverse event. A total of 16% of patients discontinued the therapy.
Most of the “gastrointestinal adverse events leading the treatment discontinuation were possibly dose and titration related,” Dr. Rosenstock said, “and it’s highly conceivable that for future studies a slower dose escalation may mitigate the occurrence of the gastrointestinal adverse events.”
BI 456906 was coinvented with Zealand Pharma. Under the licensing agreement, Boehringer Ingelheim funds all research, development, and commercialization.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK® 2022
Tirzepatide lowers weight across all groups with obesity
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK® 2022
Puzzling, unique ECG from pig-to-human transplanted heart
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
STEP TEENS: Semaglutide ‘gives hope’ to adolescents with obesity
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
FROM OBESITYWEEK® 2022
Marital stress tied to worse outcome in young MI patients
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2022