User login
New vascular 0 + 5 training programs yield fewer PAD cases
The new vascular surgery training protocol may not reflect what is currently occurring in practice for peripheral artery occlusive disease, and may warrant further investigation, according to a study performed by John Phair, MD, and his colleagues.
“Peripheral arterial occlusive disease constitutes a substantial portion of clinical practice in vascular surgery and, as such, trainees must graduate with proficiency in endovascular and open procedures to become capable vascular surgeons,” they stated, but the new paradigm does not appear to be reflecting that reality.
The researchers compared case volume for 0 + 5 integrated vascular surgery residents (IVSR) in the chief and junior years with their 5 + 2 fellowship counterparts (VSF) for the treatment of peripheral arterial occlusive disease. An aggregate of 887 residents and fellows from 137 programs were identified. From 2012 to 2018, VSFs performed significantly more total peripheral procedures than IVSR surgeon chiefs in the treatment of peripheral arterial occlusive disease, with VSFs consistently performing 1.7-fold (P less than .001) and 1.6-fold (P less than .001) more total peripheral cases than their integrated vascular surgery residents chief and junior counterparts, respectively. When stratified by anatomic location, VSFs were found to have performed more peripheral arterial procedures in the aortoiliac, femoropopliteal, and infrapopliteal arteries, compared with IVSR chiefs, and they also performed more total peripheral procedures than IVSR surgeon juniors, the authors reported online in the journal Surgery (2019 E-pub; doi/10.1016/j.surg.2019.02.021).
“Although both training paradigms offer operative experiences that meet criteria for graduation, it seems that VSFs currently perform more endovascular and open cases than their IVSR counterparts for peripheral arterial occlusive disease during the final phase of training. These findings may be contradictory to current thinking regarding the vascular surgery training paradigms,” the researchers concluded.
There were no disclosures reported.
SOURCE: Phari J et al. Surgery 2019 E-pub; doi/10.1016/j.surg.2019.02.021).
The new vascular surgery training protocol may not reflect what is currently occurring in practice for peripheral artery occlusive disease, and may warrant further investigation, according to a study performed by John Phair, MD, and his colleagues.
“Peripheral arterial occlusive disease constitutes a substantial portion of clinical practice in vascular surgery and, as such, trainees must graduate with proficiency in endovascular and open procedures to become capable vascular surgeons,” they stated, but the new paradigm does not appear to be reflecting that reality.
The researchers compared case volume for 0 + 5 integrated vascular surgery residents (IVSR) in the chief and junior years with their 5 + 2 fellowship counterparts (VSF) for the treatment of peripheral arterial occlusive disease. An aggregate of 887 residents and fellows from 137 programs were identified. From 2012 to 2018, VSFs performed significantly more total peripheral procedures than IVSR surgeon chiefs in the treatment of peripheral arterial occlusive disease, with VSFs consistently performing 1.7-fold (P less than .001) and 1.6-fold (P less than .001) more total peripheral cases than their integrated vascular surgery residents chief and junior counterparts, respectively. When stratified by anatomic location, VSFs were found to have performed more peripheral arterial procedures in the aortoiliac, femoropopliteal, and infrapopliteal arteries, compared with IVSR chiefs, and they also performed more total peripheral procedures than IVSR surgeon juniors, the authors reported online in the journal Surgery (2019 E-pub; doi/10.1016/j.surg.2019.02.021).
“Although both training paradigms offer operative experiences that meet criteria for graduation, it seems that VSFs currently perform more endovascular and open cases than their IVSR counterparts for peripheral arterial occlusive disease during the final phase of training. These findings may be contradictory to current thinking regarding the vascular surgery training paradigms,” the researchers concluded.
There were no disclosures reported.
SOURCE: Phari J et al. Surgery 2019 E-pub; doi/10.1016/j.surg.2019.02.021).
The new vascular surgery training protocol may not reflect what is currently occurring in practice for peripheral artery occlusive disease, and may warrant further investigation, according to a study performed by John Phair, MD, and his colleagues.
“Peripheral arterial occlusive disease constitutes a substantial portion of clinical practice in vascular surgery and, as such, trainees must graduate with proficiency in endovascular and open procedures to become capable vascular surgeons,” they stated, but the new paradigm does not appear to be reflecting that reality.
The researchers compared case volume for 0 + 5 integrated vascular surgery residents (IVSR) in the chief and junior years with their 5 + 2 fellowship counterparts (VSF) for the treatment of peripheral arterial occlusive disease. An aggregate of 887 residents and fellows from 137 programs were identified. From 2012 to 2018, VSFs performed significantly more total peripheral procedures than IVSR surgeon chiefs in the treatment of peripheral arterial occlusive disease, with VSFs consistently performing 1.7-fold (P less than .001) and 1.6-fold (P less than .001) more total peripheral cases than their integrated vascular surgery residents chief and junior counterparts, respectively. When stratified by anatomic location, VSFs were found to have performed more peripheral arterial procedures in the aortoiliac, femoropopliteal, and infrapopliteal arteries, compared with IVSR chiefs, and they also performed more total peripheral procedures than IVSR surgeon juniors, the authors reported online in the journal Surgery (2019 E-pub; doi/10.1016/j.surg.2019.02.021).
“Although both training paradigms offer operative experiences that meet criteria for graduation, it seems that VSFs currently perform more endovascular and open cases than their IVSR counterparts for peripheral arterial occlusive disease during the final phase of training. These findings may be contradictory to current thinking regarding the vascular surgery training paradigms,” the researchers concluded.
There were no disclosures reported.
SOURCE: Phari J et al. Surgery 2019 E-pub; doi/10.1016/j.surg.2019.02.021).
FROM SURGERY
Ask patients, not devices, about impact of PAD
The ankle-brachial index (ABI) is a poor indicator of patient-centered and clinician-based evaluations of functional status in patients with intermittent claudication, according to the results of PORTRAIT, a prospective observational study of patients with newly diagnosed or an exacerbation of nonlimb-threatening peripheral arterial disease (PAD).
PORTRAIT studied 1,251 patients with intermittent claudication enrolled at 16 sites. Researchers studied the correlation of ABI values and Rutherford symptom classification with PAD-specific health status as measured by the Peripheral Artery Questionnaire (PAQ).
ABI values were categorized as mild (greater than 0.80), moderate (0.40-0.79), and severe (less than 0.40). Spearman rank correlation coefficients were calculated between raw ABI values and PAQ scores and between the Rutherford classification and PAQ scores.
ABI explained only 0.1%-2.1% of the variation in PAQ scores and the Rutherford classification had stronger but still modest associations with PAQ scores, according to the researchers.
“This large study of IC patients found that the PAQ offers a unique and complementary
measure of disease burden that is not captured by physiologic or clinician-observed classifications. The findings from this study highlight the clinical complexity of PAD
and the difficulty in using common hemodynamic and symptom measures to classify the impact of this disease on patients’ health status,” the researchers concluded.
Several authors reported serving as consultant for and/or receiving grants from various device and pharmaceutical companies involved with PAD. The senior author. owns the copyright to the Peripheral Artery Questionnaire that formed the basis for the study.
SOURCE: Johnston A et al. J Vasc Surg. 2018;69(3):906-12.
The ankle-brachial index (ABI) is a poor indicator of patient-centered and clinician-based evaluations of functional status in patients with intermittent claudication, according to the results of PORTRAIT, a prospective observational study of patients with newly diagnosed or an exacerbation of nonlimb-threatening peripheral arterial disease (PAD).
PORTRAIT studied 1,251 patients with intermittent claudication enrolled at 16 sites. Researchers studied the correlation of ABI values and Rutherford symptom classification with PAD-specific health status as measured by the Peripheral Artery Questionnaire (PAQ).
ABI values were categorized as mild (greater than 0.80), moderate (0.40-0.79), and severe (less than 0.40). Spearman rank correlation coefficients were calculated between raw ABI values and PAQ scores and between the Rutherford classification and PAQ scores.
ABI explained only 0.1%-2.1% of the variation in PAQ scores and the Rutherford classification had stronger but still modest associations with PAQ scores, according to the researchers.
“This large study of IC patients found that the PAQ offers a unique and complementary
measure of disease burden that is not captured by physiologic or clinician-observed classifications. The findings from this study highlight the clinical complexity of PAD
and the difficulty in using common hemodynamic and symptom measures to classify the impact of this disease on patients’ health status,” the researchers concluded.
Several authors reported serving as consultant for and/or receiving grants from various device and pharmaceutical companies involved with PAD. The senior author. owns the copyright to the Peripheral Artery Questionnaire that formed the basis for the study.
SOURCE: Johnston A et al. J Vasc Surg. 2018;69(3):906-12.
The ankle-brachial index (ABI) is a poor indicator of patient-centered and clinician-based evaluations of functional status in patients with intermittent claudication, according to the results of PORTRAIT, a prospective observational study of patients with newly diagnosed or an exacerbation of nonlimb-threatening peripheral arterial disease (PAD).
PORTRAIT studied 1,251 patients with intermittent claudication enrolled at 16 sites. Researchers studied the correlation of ABI values and Rutherford symptom classification with PAD-specific health status as measured by the Peripheral Artery Questionnaire (PAQ).
ABI values were categorized as mild (greater than 0.80), moderate (0.40-0.79), and severe (less than 0.40). Spearman rank correlation coefficients were calculated between raw ABI values and PAQ scores and between the Rutherford classification and PAQ scores.
ABI explained only 0.1%-2.1% of the variation in PAQ scores and the Rutherford classification had stronger but still modest associations with PAQ scores, according to the researchers.
“This large study of IC patients found that the PAQ offers a unique and complementary
measure of disease burden that is not captured by physiologic or clinician-observed classifications. The findings from this study highlight the clinical complexity of PAD
and the difficulty in using common hemodynamic and symptom measures to classify the impact of this disease on patients’ health status,” the researchers concluded.
Several authors reported serving as consultant for and/or receiving grants from various device and pharmaceutical companies involved with PAD. The senior author. owns the copyright to the Peripheral Artery Questionnaire that formed the basis for the study.
SOURCE: Johnston A et al. J Vasc Surg. 2018;69(3):906-12.
FROM THE JOURNAL OF VASCULAR SURGERY
HCC linked to mitochondrial damage, iron accumulation from HCV
according to an extensive literature review.
Although the mechanisms underlying the hepatocellular carcinoma development are not fully understood, it is known that oxidative stress exists to a greater degree in hepatitis C virus (HCV) infection, compared with other inflammatory liver diseases. Such stress has been proposed as a major mechanism of liver injury in patients with chronic HCV, the authors reported in Free Radical Biology and Medicine.
Patients with HCV have significant hepatocellular mitochondrial alterations, and iron accumulation is also a well-known characteristic in patients with chronic HCV. Such alterations in mitochondria and iron accumulation are closely related to oxidative stress, since the mitochondria are the main site of reactive oxygen species generation, and iron produces hydroxy radicals via the Fenton reaction, according to the review.
“The greatest concern is whether mitochondrial damage and iron metabolic dysregulation persist even after HCV eradication and to what extent such pathological conditions affect the development of HCC. Determining the molecular signaling that underlies the mitophagy induced by iron depletion is another topic of interest and is expected to lead to potential therapeutic approaches for multiple diseases,” the researchers concluded.
Support was from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development. The authors reported no disclosures.
SOURCE: Keisuke H et al. Free Radic Biol Med. 2019;133:193-9.
according to an extensive literature review.
Although the mechanisms underlying the hepatocellular carcinoma development are not fully understood, it is known that oxidative stress exists to a greater degree in hepatitis C virus (HCV) infection, compared with other inflammatory liver diseases. Such stress has been proposed as a major mechanism of liver injury in patients with chronic HCV, the authors reported in Free Radical Biology and Medicine.
Patients with HCV have significant hepatocellular mitochondrial alterations, and iron accumulation is also a well-known characteristic in patients with chronic HCV. Such alterations in mitochondria and iron accumulation are closely related to oxidative stress, since the mitochondria are the main site of reactive oxygen species generation, and iron produces hydroxy radicals via the Fenton reaction, according to the review.
“The greatest concern is whether mitochondrial damage and iron metabolic dysregulation persist even after HCV eradication and to what extent such pathological conditions affect the development of HCC. Determining the molecular signaling that underlies the mitophagy induced by iron depletion is another topic of interest and is expected to lead to potential therapeutic approaches for multiple diseases,” the researchers concluded.
Support was from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development. The authors reported no disclosures.
SOURCE: Keisuke H et al. Free Radic Biol Med. 2019;133:193-9.
according to an extensive literature review.
Although the mechanisms underlying the hepatocellular carcinoma development are not fully understood, it is known that oxidative stress exists to a greater degree in hepatitis C virus (HCV) infection, compared with other inflammatory liver diseases. Such stress has been proposed as a major mechanism of liver injury in patients with chronic HCV, the authors reported in Free Radical Biology and Medicine.
Patients with HCV have significant hepatocellular mitochondrial alterations, and iron accumulation is also a well-known characteristic in patients with chronic HCV. Such alterations in mitochondria and iron accumulation are closely related to oxidative stress, since the mitochondria are the main site of reactive oxygen species generation, and iron produces hydroxy radicals via the Fenton reaction, according to the review.
“The greatest concern is whether mitochondrial damage and iron metabolic dysregulation persist even after HCV eradication and to what extent such pathological conditions affect the development of HCC. Determining the molecular signaling that underlies the mitophagy induced by iron depletion is another topic of interest and is expected to lead to potential therapeutic approaches for multiple diseases,” the researchers concluded.
Support was from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development. The authors reported no disclosures.
SOURCE: Keisuke H et al. Free Radic Biol Med. 2019;133:193-9.
FROM FREE RADICAL BIOLOGY AND MEDICINE
Patient complications affect surgeons adversely
Psychological consequences of patient complications seem to be an important occupational health issue for surgeons, according to the results of an extensive literature review published in JAMA Surgery.
Sanket Srinivasa, PhD, of North Shore Hospital, Auckland, New Zealand, and colleagues assessed studies from MEDLINE, Embase, PubMed, Web of Science, and Google Scholar that examined the consequences of complications, adverse events, or error for surgeons published up to the search date of May 1, 2018. Studies pertaining to burnout alone, studies not conducted on surgeons or surgical trainees, and review articles with no original data were excluded. This final review of consisted of nine studies (10,702 unique participants) that explored the occurrence of patient complications and their affect on surgeons’ psychological well-being and their professional and personal lives.
All of the studies indicated that surgeons were affected emotionally after patient complications, which led to adverse consequences in their professional and personal lives. The study authors identified four themes from the literature.
- The adverse emotional influence of complications (including anxiety, guilt, sadness, shame, and interference with professional and leisure activities) after intraoperative adverse events; one study diagnosed acute traumatic stress (using valid diagnostic criteria) in one-third of their participants 1 month after a major surgical complication.
- Coping mechanisms used by surgeons and trainees (including limited discussion with colleagues, exercise, artistic or creative outlets, alcohol and substance abuse); emotion-focused coping strategies reported included rationalization, seeking reassurance, blaming oneself or others, and dissociation with self-distraction. Other adaptive strategies used included engaging in artistic endeavors and exercise, although maladaptive strategies were also adopted by some, including alcohol and substance use disorder.
- Institutional support mechanisms and barriers to support (including clinical conferences, discussion with mentors, and a perception that emotional distress would be perceived as a constitutional weakness). For example, surgical trainees in one study did not believe that morbidity and mortality meetings addressed the emotional needs of trainees, and respondents in another study pointed to poor institutional support with a competitive, unsympathetic surgical culture, with the morbidity and mortality meeting being regarded as accusatory and hostile without providing support.
- The consequences of complications in future clinical practice (including changes in practice, introduction of protocols, education of staff members, and participating in root-cause analysis). Participants in several studies believed that dealing with errors and complications improved their subsequent performance, For example, 92 of 123 respondents (74.8%) in one study believed that their professional ability was not impaired after a complication, and in another study half of the surgeons did not believe they should stop operating for a brief period after an intraoperative death. “However, respondents in other studies described a combination of anxiety and shock affecting their ability to rectify the operative problem in a practical sense immediately after an intraoperative complication. Some respondents reported impairment for weeks after the incident, describing ongoing rumination, difficulties in concentration, adversely affected clinical judgment, and loss of confidence,” according to the researchers. Surgeons in another study described an initial denial and minimization of the severity of the consequence potentially delaying the necessary treatment, while some surgeons reported avoiding or stopping certain operations as well as contemplating early retirement.
“Surgeons across the studies indicated that they deal with these problems in isolation with significant personal and clinical consequences. With primum non nocere remaining a cornerstone of medical practice as applied to patients, a similar philosophy needs to be embraced by the surgical community for the betterment of health of the profession,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Srinivasa S et al. JAMA Surg. 2019 Mar 27. doi: 10.1001/jamasurg.2018.5640.
Psychological consequences of patient complications seem to be an important occupational health issue for surgeons, according to the results of an extensive literature review published in JAMA Surgery.
Sanket Srinivasa, PhD, of North Shore Hospital, Auckland, New Zealand, and colleagues assessed studies from MEDLINE, Embase, PubMed, Web of Science, and Google Scholar that examined the consequences of complications, adverse events, or error for surgeons published up to the search date of May 1, 2018. Studies pertaining to burnout alone, studies not conducted on surgeons or surgical trainees, and review articles with no original data were excluded. This final review of consisted of nine studies (10,702 unique participants) that explored the occurrence of patient complications and their affect on surgeons’ psychological well-being and their professional and personal lives.
All of the studies indicated that surgeons were affected emotionally after patient complications, which led to adverse consequences in their professional and personal lives. The study authors identified four themes from the literature.
- The adverse emotional influence of complications (including anxiety, guilt, sadness, shame, and interference with professional and leisure activities) after intraoperative adverse events; one study diagnosed acute traumatic stress (using valid diagnostic criteria) in one-third of their participants 1 month after a major surgical complication.
- Coping mechanisms used by surgeons and trainees (including limited discussion with colleagues, exercise, artistic or creative outlets, alcohol and substance abuse); emotion-focused coping strategies reported included rationalization, seeking reassurance, blaming oneself or others, and dissociation with self-distraction. Other adaptive strategies used included engaging in artistic endeavors and exercise, although maladaptive strategies were also adopted by some, including alcohol and substance use disorder.
- Institutional support mechanisms and barriers to support (including clinical conferences, discussion with mentors, and a perception that emotional distress would be perceived as a constitutional weakness). For example, surgical trainees in one study did not believe that morbidity and mortality meetings addressed the emotional needs of trainees, and respondents in another study pointed to poor institutional support with a competitive, unsympathetic surgical culture, with the morbidity and mortality meeting being regarded as accusatory and hostile without providing support.
- The consequences of complications in future clinical practice (including changes in practice, introduction of protocols, education of staff members, and participating in root-cause analysis). Participants in several studies believed that dealing with errors and complications improved their subsequent performance, For example, 92 of 123 respondents (74.8%) in one study believed that their professional ability was not impaired after a complication, and in another study half of the surgeons did not believe they should stop operating for a brief period after an intraoperative death. “However, respondents in other studies described a combination of anxiety and shock affecting their ability to rectify the operative problem in a practical sense immediately after an intraoperative complication. Some respondents reported impairment for weeks after the incident, describing ongoing rumination, difficulties in concentration, adversely affected clinical judgment, and loss of confidence,” according to the researchers. Surgeons in another study described an initial denial and minimization of the severity of the consequence potentially delaying the necessary treatment, while some surgeons reported avoiding or stopping certain operations as well as contemplating early retirement.
“Surgeons across the studies indicated that they deal with these problems in isolation with significant personal and clinical consequences. With primum non nocere remaining a cornerstone of medical practice as applied to patients, a similar philosophy needs to be embraced by the surgical community for the betterment of health of the profession,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Srinivasa S et al. JAMA Surg. 2019 Mar 27. doi: 10.1001/jamasurg.2018.5640.
Psychological consequences of patient complications seem to be an important occupational health issue for surgeons, according to the results of an extensive literature review published in JAMA Surgery.
Sanket Srinivasa, PhD, of North Shore Hospital, Auckland, New Zealand, and colleagues assessed studies from MEDLINE, Embase, PubMed, Web of Science, and Google Scholar that examined the consequences of complications, adverse events, or error for surgeons published up to the search date of May 1, 2018. Studies pertaining to burnout alone, studies not conducted on surgeons or surgical trainees, and review articles with no original data were excluded. This final review of consisted of nine studies (10,702 unique participants) that explored the occurrence of patient complications and their affect on surgeons’ psychological well-being and their professional and personal lives.
All of the studies indicated that surgeons were affected emotionally after patient complications, which led to adverse consequences in their professional and personal lives. The study authors identified four themes from the literature.
- The adverse emotional influence of complications (including anxiety, guilt, sadness, shame, and interference with professional and leisure activities) after intraoperative adverse events; one study diagnosed acute traumatic stress (using valid diagnostic criteria) in one-third of their participants 1 month after a major surgical complication.
- Coping mechanisms used by surgeons and trainees (including limited discussion with colleagues, exercise, artistic or creative outlets, alcohol and substance abuse); emotion-focused coping strategies reported included rationalization, seeking reassurance, blaming oneself or others, and dissociation with self-distraction. Other adaptive strategies used included engaging in artistic endeavors and exercise, although maladaptive strategies were also adopted by some, including alcohol and substance use disorder.
- Institutional support mechanisms and barriers to support (including clinical conferences, discussion with mentors, and a perception that emotional distress would be perceived as a constitutional weakness). For example, surgical trainees in one study did not believe that morbidity and mortality meetings addressed the emotional needs of trainees, and respondents in another study pointed to poor institutional support with a competitive, unsympathetic surgical culture, with the morbidity and mortality meeting being regarded as accusatory and hostile without providing support.
- The consequences of complications in future clinical practice (including changes in practice, introduction of protocols, education of staff members, and participating in root-cause analysis). Participants in several studies believed that dealing with errors and complications improved their subsequent performance, For example, 92 of 123 respondents (74.8%) in one study believed that their professional ability was not impaired after a complication, and in another study half of the surgeons did not believe they should stop operating for a brief period after an intraoperative death. “However, respondents in other studies described a combination of anxiety and shock affecting their ability to rectify the operative problem in a practical sense immediately after an intraoperative complication. Some respondents reported impairment for weeks after the incident, describing ongoing rumination, difficulties in concentration, adversely affected clinical judgment, and loss of confidence,” according to the researchers. Surgeons in another study described an initial denial and minimization of the severity of the consequence potentially delaying the necessary treatment, while some surgeons reported avoiding or stopping certain operations as well as contemplating early retirement.
“Surgeons across the studies indicated that they deal with these problems in isolation with significant personal and clinical consequences. With primum non nocere remaining a cornerstone of medical practice as applied to patients, a similar philosophy needs to be embraced by the surgical community for the betterment of health of the profession,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Srinivasa S et al. JAMA Surg. 2019 Mar 27. doi: 10.1001/jamasurg.2018.5640.
FROM JAMA SURGERY
An HCV-infected population showed gaps in HBV testing, vaccination, and care
Assessment of a large cohort of hepatitis C virus (HCV)–infected patients revealed a high prevalence of current or past hepatitis B virus. However, within this cohort, there were notable gaps in HBV testing, directed care, and vaccination, according to Aaron M. Harris, MD, of the Centers for Disease Control and Prevention.
Dr. Harris and his colleagues abstracted patient-level data from the Grady Health System EHR in August 2016 to create an HCV patient registry. They found that, among 4,224 HCV-infected patients, 3,629 (86%) had test results for the hepatitis B surface antigen (HBsAg), with 43 (1.2%) being HBsAg positive.
“Our results identified a gap in care as a minority of HBsAg-positive patients with HCV coinfection received HBV DNA and/or e-antigen [HBeAg] testing,” the researchers stated.
Overall, only 2,342 (55.4%) patients had test results for all three HBV serologic markers. Among these, 789 (33.7%) were anti-HBc positive only, 678 (28.9%) were anti-HBc/anti-HBs positive, 190 (8.1%) were anti-HBs positive only, and 642 (27.4%) were HBV susceptible. In addition, only 50% of the HBV-susceptible patients received at least one dose of hepatitis B vaccine, according to the report published in Vaccine.
“Strategies are needed to increase hepatitis B testing, linkage to hepatitis B–directed care of HBV/HCV-coinfected patients, and to increase uptake in hepatitis B vaccination for HCV-infected patients within the Grady Health System,” the researchers concluded.
The study was funded by the CDC and the authors reported that they had no conflicts.
SOURCE: Harris AM et al. Vaccine. 2019;37:2188-93.
Assessment of a large cohort of hepatitis C virus (HCV)–infected patients revealed a high prevalence of current or past hepatitis B virus. However, within this cohort, there were notable gaps in HBV testing, directed care, and vaccination, according to Aaron M. Harris, MD, of the Centers for Disease Control and Prevention.
Dr. Harris and his colleagues abstracted patient-level data from the Grady Health System EHR in August 2016 to create an HCV patient registry. They found that, among 4,224 HCV-infected patients, 3,629 (86%) had test results for the hepatitis B surface antigen (HBsAg), with 43 (1.2%) being HBsAg positive.
“Our results identified a gap in care as a minority of HBsAg-positive patients with HCV coinfection received HBV DNA and/or e-antigen [HBeAg] testing,” the researchers stated.
Overall, only 2,342 (55.4%) patients had test results for all three HBV serologic markers. Among these, 789 (33.7%) were anti-HBc positive only, 678 (28.9%) were anti-HBc/anti-HBs positive, 190 (8.1%) were anti-HBs positive only, and 642 (27.4%) were HBV susceptible. In addition, only 50% of the HBV-susceptible patients received at least one dose of hepatitis B vaccine, according to the report published in Vaccine.
“Strategies are needed to increase hepatitis B testing, linkage to hepatitis B–directed care of HBV/HCV-coinfected patients, and to increase uptake in hepatitis B vaccination for HCV-infected patients within the Grady Health System,” the researchers concluded.
The study was funded by the CDC and the authors reported that they had no conflicts.
SOURCE: Harris AM et al. Vaccine. 2019;37:2188-93.
Assessment of a large cohort of hepatitis C virus (HCV)–infected patients revealed a high prevalence of current or past hepatitis B virus. However, within this cohort, there were notable gaps in HBV testing, directed care, and vaccination, according to Aaron M. Harris, MD, of the Centers for Disease Control and Prevention.
Dr. Harris and his colleagues abstracted patient-level data from the Grady Health System EHR in August 2016 to create an HCV patient registry. They found that, among 4,224 HCV-infected patients, 3,629 (86%) had test results for the hepatitis B surface antigen (HBsAg), with 43 (1.2%) being HBsAg positive.
“Our results identified a gap in care as a minority of HBsAg-positive patients with HCV coinfection received HBV DNA and/or e-antigen [HBeAg] testing,” the researchers stated.
Overall, only 2,342 (55.4%) patients had test results for all three HBV serologic markers. Among these, 789 (33.7%) were anti-HBc positive only, 678 (28.9%) were anti-HBc/anti-HBs positive, 190 (8.1%) were anti-HBs positive only, and 642 (27.4%) were HBV susceptible. In addition, only 50% of the HBV-susceptible patients received at least one dose of hepatitis B vaccine, according to the report published in Vaccine.
“Strategies are needed to increase hepatitis B testing, linkage to hepatitis B–directed care of HBV/HCV-coinfected patients, and to increase uptake in hepatitis B vaccination for HCV-infected patients within the Grady Health System,” the researchers concluded.
The study was funded by the CDC and the authors reported that they had no conflicts.
SOURCE: Harris AM et al. Vaccine. 2019;37:2188-93.
FROM VACCINE
FDA approves first two-drug tablet for HIV
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
SHM’s Research Shark Tank a resounding success
A few lucky hospitalists had the chance to compete for dedicated consultation time from experienced hospital medicine mentors during the SHM Annual Conference’s first Research Shark Tank.

During the Monday afternoon session, four hospitalist projects were each presented in a 5-minute “pitch” to three senior quality and research leaders in hospital medicine who served as the “sharks.” These pitches were followed by 7 minutes of moderated questions and feedback from the sharks and the audience. Sharks then “bid” on the projects, offering up to 2 hours of one-on-one consultation during the conference or as needed.
The four projects included a study of the use of off-site scribes listening in to patient/hospitalist interactions to eliminate the need for the doctor to be glued to the computer screen, which was presented by Thea Dalfino, MD, chief of hospital medicine at Albany (N.Y.) Memorial Hospital; a rethinking of medical education to emphasize the role of hospitalists as mentors to individual student “apprentices,” presented by Amulya Nagarur, MD, of the department of medicine at Massachusetts General Hospital, Boston, and Christiana Renner, MD, of University of Texas Southwestern Medical Center, Dallas; and a redesign of patient hospital gowns to optimize, comfort, morale, and functionality, presented by Cheryl Dellasega, PhD, professor of medicine and humanities at Penn State University, Hershey.
The winning project was presented by Meera Udayakumar, MD, medical director at the University of North Carolina REX Healthcare in Raleigh. She discussed “The Equalizer,” a computerized tool to optimize patient distribution among hospitalists in order to balance workflow in a practice.
In discussing the thinking behind this unique session, Luci Leykum, MD, SFHM, chief of the division of general and hospital medicine at the University of Texas, San Antonio, who served as one of the sharks, stated that: “We’ve always tried to do things to promote the pipeline of research in hospital medicine and to raise the visibility of research activities at the annual conference. In the past, we have done one-on-one ‘speed dating’ with mentors, but the research committee thought this format would be more interactive and that audience members could benefit from hearing the discussion.”
The other participating sharks were Andrew Auerbach, MD, MPH, MHM, professor of medicine at the University of California, San Francisco, and former editor of the Journal of Hospital Medicine, and Hardeep Singh, MD, MPH, chief of the health policy, quality, and informatics program at the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey Veterans Affairs Medical Center in Houston.
The selection process for those looking to pitch was rigorous. Projects submitted to the research committee had to focus on research, quality improvement, or medical education and be very specific to the practice of hospital medicine. In addition, the ideas needed to be relatively well developed, ideally with some pilot data. Applicants also needed to address a significant problem in hospital medicine, showcase an innovative approach, and make the case for how their solution would have short- and long-term effects.
Dr. Leykum said she was looking to see whether the pitched projects have clearly articulated questions that are important and interesting and whether the proposed methods would sufficiently answer those questions. She also considered what the implications were if the work was done.
Audience members had a chance to ask questions and, if they were interested, to potentially partner with presenters or adopt similar ideas at their own institutions. Attendees were exposed to innovative ways of solving problems that are common and ideas that have a big impact on the way problems are approached in hospital medicine.
“I think it was a fun, fast, interactive session, and it was interesting to see,” said Dr. Leykum. “Those of us who were the sharks know each other and each other’s work, so that was a fun dynamic.”
A few lucky hospitalists had the chance to compete for dedicated consultation time from experienced hospital medicine mentors during the SHM Annual Conference’s first Research Shark Tank.

During the Monday afternoon session, four hospitalist projects were each presented in a 5-minute “pitch” to three senior quality and research leaders in hospital medicine who served as the “sharks.” These pitches were followed by 7 minutes of moderated questions and feedback from the sharks and the audience. Sharks then “bid” on the projects, offering up to 2 hours of one-on-one consultation during the conference or as needed.
The four projects included a study of the use of off-site scribes listening in to patient/hospitalist interactions to eliminate the need for the doctor to be glued to the computer screen, which was presented by Thea Dalfino, MD, chief of hospital medicine at Albany (N.Y.) Memorial Hospital; a rethinking of medical education to emphasize the role of hospitalists as mentors to individual student “apprentices,” presented by Amulya Nagarur, MD, of the department of medicine at Massachusetts General Hospital, Boston, and Christiana Renner, MD, of University of Texas Southwestern Medical Center, Dallas; and a redesign of patient hospital gowns to optimize, comfort, morale, and functionality, presented by Cheryl Dellasega, PhD, professor of medicine and humanities at Penn State University, Hershey.
The winning project was presented by Meera Udayakumar, MD, medical director at the University of North Carolina REX Healthcare in Raleigh. She discussed “The Equalizer,” a computerized tool to optimize patient distribution among hospitalists in order to balance workflow in a practice.
In discussing the thinking behind this unique session, Luci Leykum, MD, SFHM, chief of the division of general and hospital medicine at the University of Texas, San Antonio, who served as one of the sharks, stated that: “We’ve always tried to do things to promote the pipeline of research in hospital medicine and to raise the visibility of research activities at the annual conference. In the past, we have done one-on-one ‘speed dating’ with mentors, but the research committee thought this format would be more interactive and that audience members could benefit from hearing the discussion.”
The other participating sharks were Andrew Auerbach, MD, MPH, MHM, professor of medicine at the University of California, San Francisco, and former editor of the Journal of Hospital Medicine, and Hardeep Singh, MD, MPH, chief of the health policy, quality, and informatics program at the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey Veterans Affairs Medical Center in Houston.
The selection process for those looking to pitch was rigorous. Projects submitted to the research committee had to focus on research, quality improvement, or medical education and be very specific to the practice of hospital medicine. In addition, the ideas needed to be relatively well developed, ideally with some pilot data. Applicants also needed to address a significant problem in hospital medicine, showcase an innovative approach, and make the case for how their solution would have short- and long-term effects.
Dr. Leykum said she was looking to see whether the pitched projects have clearly articulated questions that are important and interesting and whether the proposed methods would sufficiently answer those questions. She also considered what the implications were if the work was done.
Audience members had a chance to ask questions and, if they were interested, to potentially partner with presenters or adopt similar ideas at their own institutions. Attendees were exposed to innovative ways of solving problems that are common and ideas that have a big impact on the way problems are approached in hospital medicine.
“I think it was a fun, fast, interactive session, and it was interesting to see,” said Dr. Leykum. “Those of us who were the sharks know each other and each other’s work, so that was a fun dynamic.”
A few lucky hospitalists had the chance to compete for dedicated consultation time from experienced hospital medicine mentors during the SHM Annual Conference’s first Research Shark Tank.

During the Monday afternoon session, four hospitalist projects were each presented in a 5-minute “pitch” to three senior quality and research leaders in hospital medicine who served as the “sharks.” These pitches were followed by 7 minutes of moderated questions and feedback from the sharks and the audience. Sharks then “bid” on the projects, offering up to 2 hours of one-on-one consultation during the conference or as needed.
The four projects included a study of the use of off-site scribes listening in to patient/hospitalist interactions to eliminate the need for the doctor to be glued to the computer screen, which was presented by Thea Dalfino, MD, chief of hospital medicine at Albany (N.Y.) Memorial Hospital; a rethinking of medical education to emphasize the role of hospitalists as mentors to individual student “apprentices,” presented by Amulya Nagarur, MD, of the department of medicine at Massachusetts General Hospital, Boston, and Christiana Renner, MD, of University of Texas Southwestern Medical Center, Dallas; and a redesign of patient hospital gowns to optimize, comfort, morale, and functionality, presented by Cheryl Dellasega, PhD, professor of medicine and humanities at Penn State University, Hershey.
The winning project was presented by Meera Udayakumar, MD, medical director at the University of North Carolina REX Healthcare in Raleigh. She discussed “The Equalizer,” a computerized tool to optimize patient distribution among hospitalists in order to balance workflow in a practice.
In discussing the thinking behind this unique session, Luci Leykum, MD, SFHM, chief of the division of general and hospital medicine at the University of Texas, San Antonio, who served as one of the sharks, stated that: “We’ve always tried to do things to promote the pipeline of research in hospital medicine and to raise the visibility of research activities at the annual conference. In the past, we have done one-on-one ‘speed dating’ with mentors, but the research committee thought this format would be more interactive and that audience members could benefit from hearing the discussion.”
The other participating sharks were Andrew Auerbach, MD, MPH, MHM, professor of medicine at the University of California, San Francisco, and former editor of the Journal of Hospital Medicine, and Hardeep Singh, MD, MPH, chief of the health policy, quality, and informatics program at the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey Veterans Affairs Medical Center in Houston.
The selection process for those looking to pitch was rigorous. Projects submitted to the research committee had to focus on research, quality improvement, or medical education and be very specific to the practice of hospital medicine. In addition, the ideas needed to be relatively well developed, ideally with some pilot data. Applicants also needed to address a significant problem in hospital medicine, showcase an innovative approach, and make the case for how their solution would have short- and long-term effects.
Dr. Leykum said she was looking to see whether the pitched projects have clearly articulated questions that are important and interesting and whether the proposed methods would sufficiently answer those questions. She also considered what the implications were if the work was done.
Audience members had a chance to ask questions and, if they were interested, to potentially partner with presenters or adopt similar ideas at their own institutions. Attendees were exposed to innovative ways of solving problems that are common and ideas that have a big impact on the way problems are approached in hospital medicine.
“I think it was a fun, fast, interactive session, and it was interesting to see,” said Dr. Leykum. “Those of us who were the sharks know each other and each other’s work, so that was a fun dynamic.”
What does COMPASS mean for vascular surgeons?
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
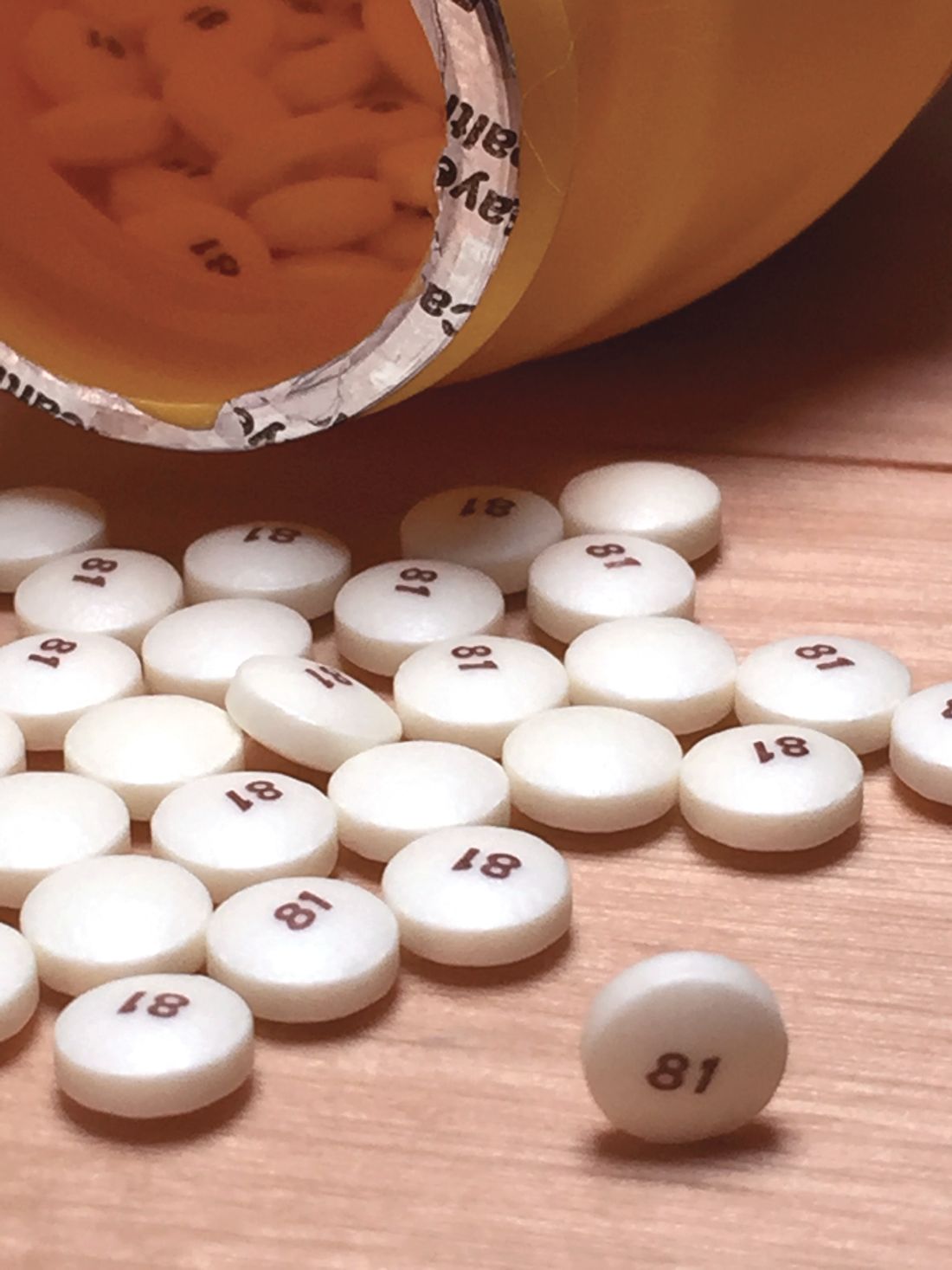
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
FROM CURRENT OPINION IN CARDIOLOGY
FDA advises alternatives to paclitaxel-coated devices for PAD, pending review
“Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending an ongoing safety review, according to the Food and Drug Administration.
The FDA conducted a preliminary analysis of long-term follow-up data (up to 5 years in some studies) of the pivotal premarket randomized trials for paclitaxel-coated products indicated for peripheral arterial disease (PAD). In a Letter to Healthcare providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
The three trials (totaling 975 patients) that had 5-year follow-up data demonstrated an approximately 50% increased risk of mortality in subjects treated with paclitaxel-coated devices vs. those treated with control devices (20.1% vs. 13.4% crude risk of death at 5 years), according to the agency.
The FDA indicated that these data “should be interpreted with caution for several reasons.” They cited a large variability in the risk estimate of mortality because of the limited amount of long-term data and pointed out that the studies were not designed to be pooled. In addition, the specific cause and mechanism of the increased mortality was unknown.
The FDA also announced that they are planning on convening an Advisory Committee meeting of the Circulatory System Devices Panel to address this issue, including plausible mechanisms for this mortality effect, a re-examination of the benefit-risk profile, modifications of current and future clinical trials regarding these devices, and guidance to any regulatory action, as needed. The timing of this meeting is to be announced within the upcoming weeks.
The FDA letter further stated that the agency intends to conduct additional analyses “to determine whether the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”
[email protected]
SOURCE: Food and Drug Administration Letter to Healthcare Providers. 2019 Mar 15.
“Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending an ongoing safety review, according to the Food and Drug Administration.
The FDA conducted a preliminary analysis of long-term follow-up data (up to 5 years in some studies) of the pivotal premarket randomized trials for paclitaxel-coated products indicated for peripheral arterial disease (PAD). In a Letter to Healthcare providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
The three trials (totaling 975 patients) that had 5-year follow-up data demonstrated an approximately 50% increased risk of mortality in subjects treated with paclitaxel-coated devices vs. those treated with control devices (20.1% vs. 13.4% crude risk of death at 5 years), according to the agency.
The FDA indicated that these data “should be interpreted with caution for several reasons.” They cited a large variability in the risk estimate of mortality because of the limited amount of long-term data and pointed out that the studies were not designed to be pooled. In addition, the specific cause and mechanism of the increased mortality was unknown.
The FDA also announced that they are planning on convening an Advisory Committee meeting of the Circulatory System Devices Panel to address this issue, including plausible mechanisms for this mortality effect, a re-examination of the benefit-risk profile, modifications of current and future clinical trials regarding these devices, and guidance to any regulatory action, as needed. The timing of this meeting is to be announced within the upcoming weeks.
The FDA letter further stated that the agency intends to conduct additional analyses “to determine whether the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”
[email protected]
SOURCE: Food and Drug Administration Letter to Healthcare Providers. 2019 Mar 15.
“Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending an ongoing safety review, according to the Food and Drug Administration.
The FDA conducted a preliminary analysis of long-term follow-up data (up to 5 years in some studies) of the pivotal premarket randomized trials for paclitaxel-coated products indicated for peripheral arterial disease (PAD). In a Letter to Healthcare providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
The three trials (totaling 975 patients) that had 5-year follow-up data demonstrated an approximately 50% increased risk of mortality in subjects treated with paclitaxel-coated devices vs. those treated with control devices (20.1% vs. 13.4% crude risk of death at 5 years), according to the agency.
The FDA indicated that these data “should be interpreted with caution for several reasons.” They cited a large variability in the risk estimate of mortality because of the limited amount of long-term data and pointed out that the studies were not designed to be pooled. In addition, the specific cause and mechanism of the increased mortality was unknown.
The FDA also announced that they are planning on convening an Advisory Committee meeting of the Circulatory System Devices Panel to address this issue, including plausible mechanisms for this mortality effect, a re-examination of the benefit-risk profile, modifications of current and future clinical trials regarding these devices, and guidance to any regulatory action, as needed. The timing of this meeting is to be announced within the upcoming weeks.
The FDA letter further stated that the agency intends to conduct additional analyses “to determine whether the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”
[email protected]
SOURCE: Food and Drug Administration Letter to Healthcare Providers. 2019 Mar 15.
Key clinical point: FDA advises that alternatives to paclitaxel-coated devices for PAD should be used for most patients.
Major finding: Five-year data demonstrated an approximately 50% increased risk of mortality in patients with paclitaxel-coated devices, compared with uncoated ones.
Study details: Preliminary FDA review of three trials with 975 patients.
Disclosures: Study is funded and performed by the FDA.
Source: Food and Drug Administration. Letter to Healthcare Providers. 2019 Mar 15.
Developing an HCV vaccine faces significant challenges
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
FROM GASTROENTEROLOGY