User login
Radiation and Medical Oncology Perspectives on Oligometastatic Disease Treatment
Radiation and Medical Oncology Perspectives on Oligometastatic Disease Treatment
The treatment of metastatic solid tumors has been based historically on systemic therapies, with the goal of delaying progression and extend life as long as possible, with tolerable treatment-related adverse events. Some exceptions were made for local treatment with surgery or radiotherapy (RT), often for patients with a single metastasis. A 1939 report describes a patient with renal adenocarcinoma and a solitary lung metastasis who underwent RT to the lung lesion after nephrectomy and subsequently partial lobectomy after the metastatic lesion progressed. The authors argued that if a metastasis appears solitary and accessible, it is plausible to remove it in addition to the primary growth.1
In 1995 Hellman and Weichselbaum proposed oligometastatic disease (OMD). They reasoned that malignancy exists along a spectrum from localized disease to widely disseminated disease, with OMD existing in between with a still-restricted tumor metastatic capacity. Appropriately selected patients with OMD may be candidates for prolonged disease-free survival or cure with the addition of local therapy to systemic therapy.2
The EORTC 4004 phase 2 randomized control trial (RCT) analyzed radiofrequency ablation (RFA) for colorectal liver metastases with systemic therapy vs systemic therapy alone for patients with ≤ 9 liver lesions.3 Systemic therapyconsisted of 5-FU/leucovorin/oxaliplatin, with bevacizumab added to the regimen 3.5 years into the study, per updated standard- of-care. This trial was the first to demonstrate the benefit of aggressive local treatment vs system treatment alone for OMD with a progression-free survival (PFS) benefit (16.8 vs 9.9 months; hazard ratio [HR], 0.63; P = .03) and overall survival (OS) benefit (45.3 vs 40.5 months; HR, 0.74; P = .02) with the addition of local treatment with RFA.
Since the presentations of the SABR-COMET phase 2 RCT and another study by Gomez et al at the American Society for Radiation Oncology (ASTRO) 2018 annual meeting, the paradigm for offering local RT for OMD has rapidly evolved. Both studies found PFS and OS benefits of RT for patients with OMD.4,5 Additional RCTs have since demonstrated that for properly selected patients with OMD, aggressive local RT improved PFS and OS.6-9 These small studies have led to larger RCTs to better understand who benefits from local consolidative treatment, particularly RT.10,11
There is a large degree of heterogeneity in how oncologists define and approach OMD treatment. The 2020 European Society for Radiotherapy and Oncology (ESTRO) and ASTRO consensus guidelines defined the OMD state as 1 to 5 metastatic lesions for which all metastatic sites are safely treatable.12 The purpose of this study was to evaluate perceptions and practice patterns among radiation oncologists and medical oncologists regarding the use of local RT for OMD across the Veterans Health Administration (VHA).
Methods
A 12-question survey was developed by the VHA Palliative Radiotherapy Task Force using the ESTRO-ASTRO consensus guidelines to define OMD. The survey was emailed to the VHA radiation oncology and medical oncology listservs on August 1, 2023. These listservs consist of physicians in these specialties either directly employed by the VHA or serve in its facilities as contractors. The original response closure date was August 11, 2023, but it was extended to August 18, 2023, to increase responses. No incentives were offered to respondents. Two email reminders were sent to the medical oncology listserv and 3 to the radiation oncology listserv. Descriptive statistics and X2 tests were used for data analysis. The impact of specialty and presence of an on-site department of radiation oncology were reviewed. This project was approved by the VHA National Oncology Program and National Radiation Oncology Program.
Results
The survey was sent to 125 radiation oncologists and 515 medical oncologists and 106 were completed for a 16.6% response rate. There were 59 (55.7%) radiation oncologist responses and 47 (44.3%) medical oncologist responses. Most (96.2%) respondents were board-certified, and 84 (79.2%) were affiliated with an academic center. Not every respondent answered every question (Table).
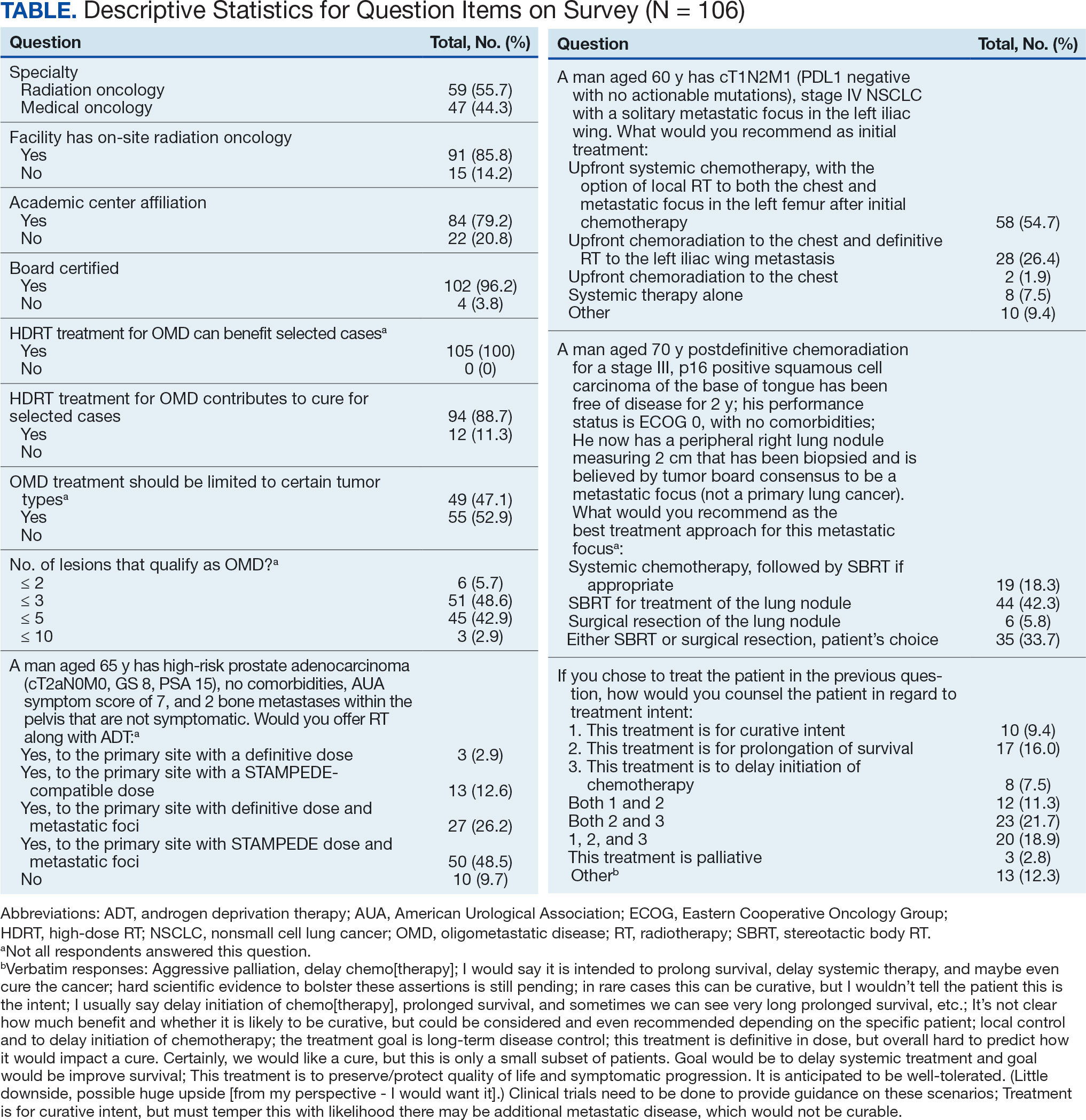
All respondents (n = 105) indicated there is a potential benefit of high-dose RT for appropriately selected cases. Ninety-four oncologists (88.7%) believed that RT for OMD contributes to cure (88.1% of radiation oncologists, 89.4% of medical oncologists; P = .84) for appropriately selected cases. Some respondents who did not believe RT for OMD contributes to cure added comments about other perceived benefits, such as local disease control for palliation, delaying systemic therapy with its associated toxicities, and prolongation of disease-free survival or OS. A higher percentage of respondents with academic affiliations believed high-dose RT contributes to cure, although this difference did not reach statistical significance (Figure 1).

Fifty-five respondents (51.9%; 55.2% radiation oncologists vs 50.0% medical oncologists; P = .60) responded that local RT for OMD treatment should not be limited by primary tumor type. Of respondents who responded that OMD treatment should be limited based on the type of primary tumor, many provided comments that argued there was a benefit for non-small cell lung cancer (NSCLC), prostate adenocarcinoma (PCa), and colorectal cancer.
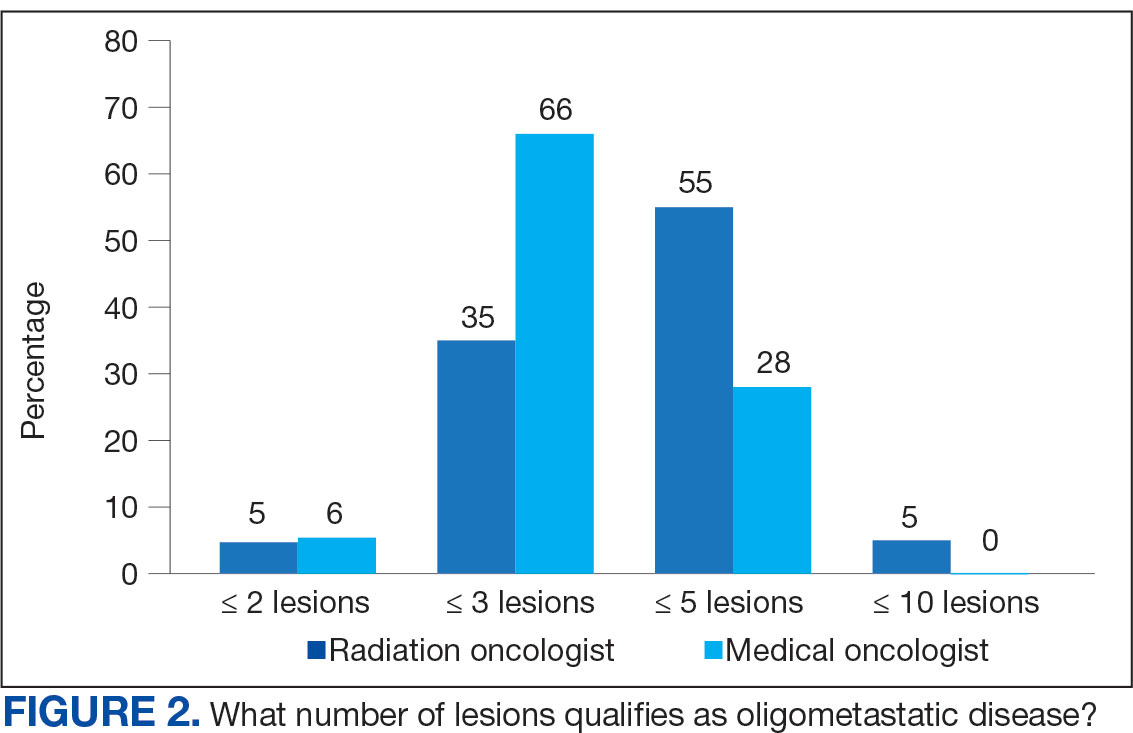
The definition of how many metastatic lesions qualify as OMD varied. A total of 48.6% of respondents defined OMD as ≤ 3 lesions and 42.9% answered ≤ 5 lesions. A majority of radiation oncologists (55.2%) classified ≤ 5 lesions as OMD, whereas a majority of medical oncologists (66.0%) considered ≤ 3 lesions as OMD (P = .006) (Figure 2).
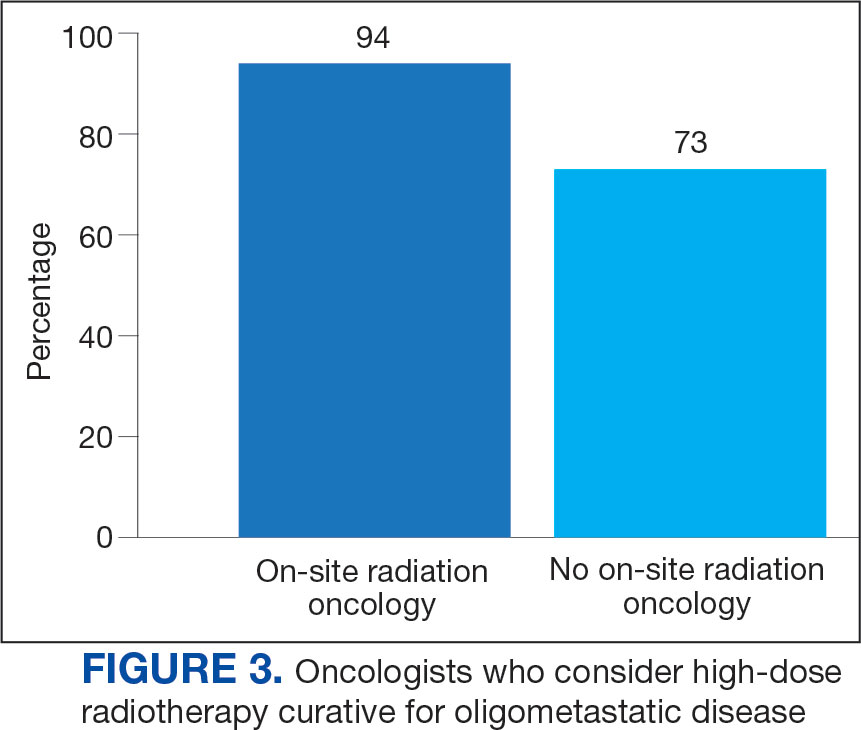
Thirty-six medical oncologists (76.6%) report having an on-site department of radiation oncology (Figure 3). This subgroup was more likely to consider local RT potentially curative compared with their medical oncology peers without on-site radiation oncology (94.4% vs 72.7%; P = .04).
Case Management
The 3 clinical cases demonstrated the heterogeneity of management approaches for OMD. The first described a man aged 65 years with PCa and 2 asymptomatic pelvic bone metastases. Ninety-three respondents (90.3%) recommended RT at the primary site and 74.8% recommended RT to both the primary site and metastatic foci. Sixty-three respondents (67.7%) recommended a STAMPEDE-compatible dose, and 30 (32.3%) recommended a definitive dose.
The second clinical case was a 60-year-old man with a cT1N2M1 NSCLC, with a solitary metastatic focus to the left iliac wing. Fifty-eight respondents (54.7%) recommended upfront systemic chemotherapy and the option of local therapy to the chest and metastatic focus after initial chemotherapy; 28 respondents (26.4%) recommended upfront chemoradiation to the chest and definitive radiation to the left iliac wing metastasis.
The third clinical case described a male aged 70 years with a history of a treated base of tongue squamous cell carcinoma, with a solitary metastatic focus within the right lung. Respondents could pick multiple treatment options and 85 (81.7%) favored upfront definitive local therapy with surgery or stereotactic body radiotherapy (SBRT), rather than upfront chemotherapy, with future consideration for local treatment. About half of respondents (51.8%) recommended SBRT and 41.2% would let the patient decide between surgery or SBRT. Additionally, 39.6% included in their patient counselling that the treatment may be for curative intent.
Discussion
The use of local treatment to increased PFS, OS, or even cure treatment for OMD has become more accepted since the 2018 ASTRO meeting.4,5 Palma et al analyzed a controlled primary malignancy of any histology and ≤ 5 metastatic lesions, with all lesions amenable to SBRT.4 With a median follow-up of 51 months when comparing the standard-of-care (SOC) arm and the SBRT arm, the 5-year PFS was not reached and the 5-year OS rates were 17.7% and 42.3% (P = .006), respectively. In the SBRT arm, about 1 in 5 patients survived > 5 years without a recurrence or disease progression, vs 0 patients in the control arm. There was a 29% rate of grade 2 or higher toxicity in the SBRT arm, including 3 deaths that were likely due to treatment. Subsequent trials, such as the phase 3 SABR-COMET-3 (1-3 metastases), phase 3 SABR-COMET-10 (4-10 metastases), and phase 1 ARREST (> 10 metastases) trials, have been specifically designed to minimize treatment-related toxicities.13-15
Gomez et al analyzed patients at 3 sites with a controlled NSCLC primary tumor and ≤ 3 metastases.5 At a follow-up of 38.8 months, the PFS was 4.4 months in the SOC arm vs 14.2 months in the RT and/or surgery local treatment arm (P = .02). There was also an OS benefit of 17.0 vs 41.2 months (P = .02), respectively.
Several RCTs soon followed that demonstrated improved PFS and OS with local radiotherapy for OMD; however, total metastatic ablation of the foci is necessary to attain these PFS and OS benefits.6-9 Still, an oncologic benefit has yet to be proven. The randomized NRGBR002 study phase 2/3 trial for oligometastatic breast cancer included patients with ≤ 4 extracranial metastases and controlled primary disease to metastasis-directed therapy (SBRT and/ or surgical resection) and systemic therapy vs systemic therapy alone.10 The study did not demonstrate improved PFS or OS at 3 years. However, for most breast cancers, especially with the rapid advancements in systemic therapy that have been achieved, longer follow-up may be necessary to detect a significant difference.
The prospective single-arm phase 2 SABR-5 trial retrospectively demonstrated important lessons about the timing of SBRT and systemic therapy.11 This study included patients with ≤ 5 metastases of any histology, and they received SBRT to all lesions. SABR-5 retrospectively compared patients who received upfront systemic therapy followed by SBRT vs another cohort that first received SBRT and did not receive systemic therapy until there was disease progression. Patients with oligo-progression were excluded, as it demonstrated systemic drug resistance. At a median follow-up time of 34 months, delayed systemic treatment was associated with shorter PFS (23 vs 34 months, respectively; P = .001), but not worse 3-year OS (80% vs 85%, respectively; P = .66). In addition, the delayed systemic treatment arm demonstrated a reduced risk of grade 2 or higher SBRT-related toxicity (odds ratio, 0.35; P < .001).
Similarly, the STOMP phase 2 trial analyzed the role of metastasis-directed therapy (MDT) in delaying initiation of androgen deprivation therapy (ADT) in a randomized phase 2 trial.16 This study included patients with asymptomatic PCa with a biochemical recurrence after primary treatment, 1 to 3 extracranial metastatic lesions, and serum testosterone levels > 50 ng/mL. Sixty-two patients were randomized 1:1 to either MDT (SBRT or surgery) of all lesions or surveillance. The 5-year ADT-free survival was 34% for MDT vs 8% for surveillance (P = .06).
VHA Radiation Oncology
The VHA has 138 departments of medical oncology, but only 41 departments of radiation oncology. Compared with medical oncologists without an on-site radiation oncology department, those with on-site departments were more likely to believe that local RT was potentially curative (94.4% vs 72.7%, respectively; P = .04). This finding suggests that a cancer center that includes both specialties has closer collaboration, which results in greater inclination to embrace local RT for OMD, as it has demonstrated PFS and OS benefits.
The radiation and medical oncologists surveyed had statistically significant differences in response by specialty regarding the maximal number of lesions still believed to constitute OMD. Most radiation oncologists classified ≤ 5 lesions as OMD, whereas most medical oncologists classified ≤ 3 lesions as OMD. This difference is not unexpected. There is no universally agreed-upon definition of OMD, and criteria differ across studies.
While the SABR-COMET trial did include ≤ 5 metastatic lesions, it was a phase 2 RCT, making subgroup analysis difficult. Ongoing phase 3 trials that are more specific in the number of metastases, comparing 1 to 3 vs 4 to 10 metastases (SABR-COMET-3 and SABR-COMET-10, respectively).13,14 There is even an ongoing phase 1 trial (ARREST) studying the potential benefits of treating (“restraining”) > 10 metastases, if dosimetrically feasible.15 Within the VHA, VA STARPORT is investigating MDT for recurrent or de novo hormone-sensitive metastatic PCa.17 The ongoing HALT phase 2/3 trial focuses on patients with actionable mutations to help determine management of oligo-progression in mutation-positive NSCLC.18
There was no significant difference by specialty in who responded that offering local RT for OMD treatment should not be limited by histology (55.2% of radiation oncologists and 50.0% of medical oncologists; P = .60). Oncologists could make the argument that some histologies (eg, pancreatic adenocarcinomas) have such poor prognoses that local RT would not meaningfully affect oncologic outcomes, while potentially adding toxicity, whereas others could point to improved systemic therapy regimens and the low toxicity rates with careful hypofractionation regimens. Of note, the 41-patient phase 2 EXTEND trial for pancreatic ductal adenocarcinoma suggested an oncologic benefit to MDT, with far better PFS and no grade ≥ 3 toxicities related to MDT.19 About half of respondents for each specialty believed the primary histology should affect the decision. Further clarification may emerge from phase 3 trials.
Of note, a 2023 study of 44 radiation and medical oncologists at 2 Harvard Medical School-affiliated hospitals found that for synchronous OMD, 50.0% of medical oncologists and 5.3% (P < .01) of radiation oncologists recommended systemic treatment, suggesting a greater divergence in approach than found in this study.20
Limitations
The response rate of 17.0% raised a potential for selection bias, but this rate is expected for a nonincentivized medical survey. A study by the American Board of Internal Medicine with 11 surveys and 6 weekly email contacts only generated a 23.7% response rate, while another study among physicians demonstrated a 4.5% response rate for email-based contact and 11.8% for mail-based contact.21,22 We could have asked participants questions regarding demographics and geography to ensure the survey represented a diverse sample of the medical community, although additional questions would likely suppress the response rate. Additional data collection about respondents may elucidate the rationale for differences in their responses, especially between the specialties. In a planned subsequent survey in several years, the question on the number of lesions that qualifies as OMD may be amended to reflect the context and dosimetry for the maximal number of metastases constituting OMD; the joint ESTRO-ASTRO consensus defined OMD as 1 to 5 metastatic lesions, but in which all metastatic sites must be safely treatable.12 Also, fewer example cases could be included to simplify the survey and boost response rates. A future survey may ask about the timing of SBRT and systemic therapy, and whether SBRT can safely delay systemic therapy.
Conclusions
Survey results demonstrated significant confidence among both radiation oncologists and medical oncologists that local RT for OMD improves outcomes, which is encouraging and a reflection of the recent evidence-based paradigm shift in viewing metastatic disease as a spectrum. However, there is a difference between radiation oncologists and medical oncologists in how they define OMD, and preferred treatment of the sample cases presented revealed nuanced differences by specialty. Close collaboration with radiation oncologists influences the belief of medical oncologists in the beneficial role of RT for OMD. As more phase 3 data for OMD local treatments emerge, additional investigation is needed on how beliefs and practice patterns evolve among radiation and medical oncologists.
- Barney JD, Churchill EJ. Adenocarcinoma of the kidney with metastasis to the lung. J Urology. 1939.
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8-10. doi:10.1200/JCO.1995.13.1.8
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 4004). Ann Oncol. 2012;23(10):2619-2626. doi:10.1093/annonc/mds053
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830- 2838. doi:10.1200/JCO.20.00818
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-1565. doi:10.1200/JCO.19.00201
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501. doi:10.1001/jamaoncol.2017.3501
- Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650-659. doi:10.1001/jamaoncol.2020.0147
- Wang XS, Bai YF, Verma V, et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated NSCLC. J Natl Cancer Inst 2023;115(6):742-748. doi:10.1093/jnci/djac015
- Tang C, Sherry AD, Haymaker C, et al. Addition of metastasis- directed therapy to intermittent hormone therapy for oligometastatic prostate cancer (EXTEND): a multicenter, randomized phase II trial. Am Soc Radiat Oncol Annu Meet. 2023;9(6):825-834. doi:10.1001/jamaoncol.2023.0161
- Chmura SJ, Winter KA, Woodward WA, et al. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol. 2022;40:1007. doi:10.1200/JCO.2022.40.16_suppl.1007
- Baker S, Lechner L, Liu M, et al. Upfront versus delayed systemic therapy in patients with oligometastatic cancer treated with SABR in the phase 2 SABR-5 trial. Int J Radiat Oncol Biol Phys. 2024;118(5):1497-1506. doi:10.1016/j.ijrobp.2023.11.007
- Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157-166. doi:10.1016/j.radonc.2020.04.003
- Olson R, Mathews L, Liu M, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1-3 oligometastatic tumors (SABR-COMET-3): study protocol for a randomized phase III trial. BMC Cancer. 2020;20(1):380. doi:10.1186/s12885-020-06876-4
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. 2019;19(1):816. doi:10.1186/s12885-019-5977-6
- Bauman GS, Corkum MT, Fakir H, et al. Ablative radiation therapy to restrain everything safely treatable (ARREST): study protocol for a phase I trial treating polymetastatic cancer with stereotactic radiotherapy. BMC Cancer. 2021;21(1):405. doi:10.1186/s12885-021-08136-5
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:suppl.
- Solanki AA, Campbell D, Carlson K, et al. Veterans Affairs seamless phase II/III randomized trial of standard systemic therapy with or without PET-directed local therapy for oligometastatic prostate cancer (VA STARPORT). J Clin Oncol. 2024;42:16.
- McDonald F, Guckenberger M, Popat S. EP08.03-005 HALT – Targeted therapy with or without dose-intensified radiotherapy in oligo-progressive disease in oncogene addicted lung tumours. J Thor Oncol. 2022;17:S492.
- Ludmir EB, Sherry AD, Fellman BM, et al. Addition of metastasis- directed therapy to systemic therapy for oligometastatic pancreatic ductal adenocarcinoma (EXTEND): a multicenter, randomized phase II trial. J Clin Oncol. 2024;42(32):3795-3805. doi:10.1200/JCO.24.00081
- Cho HL, Balboni T, Christ SB, et al. Is oligometastatic cancer curable? A survey of oncologist perspectives, decision making, and communication. Adv Radiat Oncol. 2023;8(5):101221. doi:10.1016/j.adro.2023.101221
- Barnhart BJ, Reddy SG, Arnold GK. Remind me again: physician response to web surveys: the effect of email reminders across 11 opinion survey efforts at the American Board of Internal Medicine from 2017 to 2019. Eval Health Prof. 2021;44(3):245-259. doi:10.1177/01632787211019445
- Murphy CC, Lee SJC, Geiger AM, et al. A randomized trial of mail and email recruitment strategies for a physician survey on clinical trial accrual. BMC Med Res Methodol. 2020;20(1):123. doi:10.1186/s12874-020-01014-x
The treatment of metastatic solid tumors has been based historically on systemic therapies, with the goal of delaying progression and extend life as long as possible, with tolerable treatment-related adverse events. Some exceptions were made for local treatment with surgery or radiotherapy (RT), often for patients with a single metastasis. A 1939 report describes a patient with renal adenocarcinoma and a solitary lung metastasis who underwent RT to the lung lesion after nephrectomy and subsequently partial lobectomy after the metastatic lesion progressed. The authors argued that if a metastasis appears solitary and accessible, it is plausible to remove it in addition to the primary growth.1
In 1995 Hellman and Weichselbaum proposed oligometastatic disease (OMD). They reasoned that malignancy exists along a spectrum from localized disease to widely disseminated disease, with OMD existing in between with a still-restricted tumor metastatic capacity. Appropriately selected patients with OMD may be candidates for prolonged disease-free survival or cure with the addition of local therapy to systemic therapy.2
The EORTC 4004 phase 2 randomized control trial (RCT) analyzed radiofrequency ablation (RFA) for colorectal liver metastases with systemic therapy vs systemic therapy alone for patients with ≤ 9 liver lesions.3 Systemic therapyconsisted of 5-FU/leucovorin/oxaliplatin, with bevacizumab added to the regimen 3.5 years into the study, per updated standard- of-care. This trial was the first to demonstrate the benefit of aggressive local treatment vs system treatment alone for OMD with a progression-free survival (PFS) benefit (16.8 vs 9.9 months; hazard ratio [HR], 0.63; P = .03) and overall survival (OS) benefit (45.3 vs 40.5 months; HR, 0.74; P = .02) with the addition of local treatment with RFA.
Since the presentations of the SABR-COMET phase 2 RCT and another study by Gomez et al at the American Society for Radiation Oncology (ASTRO) 2018 annual meeting, the paradigm for offering local RT for OMD has rapidly evolved. Both studies found PFS and OS benefits of RT for patients with OMD.4,5 Additional RCTs have since demonstrated that for properly selected patients with OMD, aggressive local RT improved PFS and OS.6-9 These small studies have led to larger RCTs to better understand who benefits from local consolidative treatment, particularly RT.10,11
There is a large degree of heterogeneity in how oncologists define and approach OMD treatment. The 2020 European Society for Radiotherapy and Oncology (ESTRO) and ASTRO consensus guidelines defined the OMD state as 1 to 5 metastatic lesions for which all metastatic sites are safely treatable.12 The purpose of this study was to evaluate perceptions and practice patterns among radiation oncologists and medical oncologists regarding the use of local RT for OMD across the Veterans Health Administration (VHA).
Methods
A 12-question survey was developed by the VHA Palliative Radiotherapy Task Force using the ESTRO-ASTRO consensus guidelines to define OMD. The survey was emailed to the VHA radiation oncology and medical oncology listservs on August 1, 2023. These listservs consist of physicians in these specialties either directly employed by the VHA or serve in its facilities as contractors. The original response closure date was August 11, 2023, but it was extended to August 18, 2023, to increase responses. No incentives were offered to respondents. Two email reminders were sent to the medical oncology listserv and 3 to the radiation oncology listserv. Descriptive statistics and X2 tests were used for data analysis. The impact of specialty and presence of an on-site department of radiation oncology were reviewed. This project was approved by the VHA National Oncology Program and National Radiation Oncology Program.
Results
The survey was sent to 125 radiation oncologists and 515 medical oncologists and 106 were completed for a 16.6% response rate. There were 59 (55.7%) radiation oncologist responses and 47 (44.3%) medical oncologist responses. Most (96.2%) respondents were board-certified, and 84 (79.2%) were affiliated with an academic center. Not every respondent answered every question (Table).

All respondents (n = 105) indicated there is a potential benefit of high-dose RT for appropriately selected cases. Ninety-four oncologists (88.7%) believed that RT for OMD contributes to cure (88.1% of radiation oncologists, 89.4% of medical oncologists; P = .84) for appropriately selected cases. Some respondents who did not believe RT for OMD contributes to cure added comments about other perceived benefits, such as local disease control for palliation, delaying systemic therapy with its associated toxicities, and prolongation of disease-free survival or OS. A higher percentage of respondents with academic affiliations believed high-dose RT contributes to cure, although this difference did not reach statistical significance (Figure 1).

Fifty-five respondents (51.9%; 55.2% radiation oncologists vs 50.0% medical oncologists; P = .60) responded that local RT for OMD treatment should not be limited by primary tumor type. Of respondents who responded that OMD treatment should be limited based on the type of primary tumor, many provided comments that argued there was a benefit for non-small cell lung cancer (NSCLC), prostate adenocarcinoma (PCa), and colorectal cancer.
The definition of how many metastatic lesions qualify as OMD varied. A total of 48.6% of respondents defined OMD as ≤ 3 lesions and 42.9% answered ≤ 5 lesions. A majority of radiation oncologists (55.2%) classified ≤ 5 lesions as OMD, whereas a majority of medical oncologists (66.0%) considered ≤ 3 lesions as OMD (P = .006) (Figure 2).

Thirty-six medical oncologists (76.6%) report having an on-site department of radiation oncology (Figure 3). This subgroup was more likely to consider local RT potentially curative compared with their medical oncology peers without on-site radiation oncology (94.4% vs 72.7%; P = .04).

Case Management
The 3 clinical cases demonstrated the heterogeneity of management approaches for OMD. The first described a man aged 65 years with PCa and 2 asymptomatic pelvic bone metastases. Ninety-three respondents (90.3%) recommended RT at the primary site and 74.8% recommended RT to both the primary site and metastatic foci. Sixty-three respondents (67.7%) recommended a STAMPEDE-compatible dose, and 30 (32.3%) recommended a definitive dose.
The second clinical case was a 60-year-old man with a cT1N2M1 NSCLC, with a solitary metastatic focus to the left iliac wing. Fifty-eight respondents (54.7%) recommended upfront systemic chemotherapy and the option of local therapy to the chest and metastatic focus after initial chemotherapy; 28 respondents (26.4%) recommended upfront chemoradiation to the chest and definitive radiation to the left iliac wing metastasis.
The third clinical case described a male aged 70 years with a history of a treated base of tongue squamous cell carcinoma, with a solitary metastatic focus within the right lung. Respondents could pick multiple treatment options and 85 (81.7%) favored upfront definitive local therapy with surgery or stereotactic body radiotherapy (SBRT), rather than upfront chemotherapy, with future consideration for local treatment. About half of respondents (51.8%) recommended SBRT and 41.2% would let the patient decide between surgery or SBRT. Additionally, 39.6% included in their patient counselling that the treatment may be for curative intent.
Discussion
The use of local treatment to increased PFS, OS, or even cure treatment for OMD has become more accepted since the 2018 ASTRO meeting.4,5 Palma et al analyzed a controlled primary malignancy of any histology and ≤ 5 metastatic lesions, with all lesions amenable to SBRT.4 With a median follow-up of 51 months when comparing the standard-of-care (SOC) arm and the SBRT arm, the 5-year PFS was not reached and the 5-year OS rates were 17.7% and 42.3% (P = .006), respectively. In the SBRT arm, about 1 in 5 patients survived > 5 years without a recurrence or disease progression, vs 0 patients in the control arm. There was a 29% rate of grade 2 or higher toxicity in the SBRT arm, including 3 deaths that were likely due to treatment. Subsequent trials, such as the phase 3 SABR-COMET-3 (1-3 metastases), phase 3 SABR-COMET-10 (4-10 metastases), and phase 1 ARREST (> 10 metastases) trials, have been specifically designed to minimize treatment-related toxicities.13-15
Gomez et al analyzed patients at 3 sites with a controlled NSCLC primary tumor and ≤ 3 metastases.5 At a follow-up of 38.8 months, the PFS was 4.4 months in the SOC arm vs 14.2 months in the RT and/or surgery local treatment arm (P = .02). There was also an OS benefit of 17.0 vs 41.2 months (P = .02), respectively.
Several RCTs soon followed that demonstrated improved PFS and OS with local radiotherapy for OMD; however, total metastatic ablation of the foci is necessary to attain these PFS and OS benefits.6-9 Still, an oncologic benefit has yet to be proven. The randomized NRGBR002 study phase 2/3 trial for oligometastatic breast cancer included patients with ≤ 4 extracranial metastases and controlled primary disease to metastasis-directed therapy (SBRT and/ or surgical resection) and systemic therapy vs systemic therapy alone.10 The study did not demonstrate improved PFS or OS at 3 years. However, for most breast cancers, especially with the rapid advancements in systemic therapy that have been achieved, longer follow-up may be necessary to detect a significant difference.
The prospective single-arm phase 2 SABR-5 trial retrospectively demonstrated important lessons about the timing of SBRT and systemic therapy.11 This study included patients with ≤ 5 metastases of any histology, and they received SBRT to all lesions. SABR-5 retrospectively compared patients who received upfront systemic therapy followed by SBRT vs another cohort that first received SBRT and did not receive systemic therapy until there was disease progression. Patients with oligo-progression were excluded, as it demonstrated systemic drug resistance. At a median follow-up time of 34 months, delayed systemic treatment was associated with shorter PFS (23 vs 34 months, respectively; P = .001), but not worse 3-year OS (80% vs 85%, respectively; P = .66). In addition, the delayed systemic treatment arm demonstrated a reduced risk of grade 2 or higher SBRT-related toxicity (odds ratio, 0.35; P < .001).
Similarly, the STOMP phase 2 trial analyzed the role of metastasis-directed therapy (MDT) in delaying initiation of androgen deprivation therapy (ADT) in a randomized phase 2 trial.16 This study included patients with asymptomatic PCa with a biochemical recurrence after primary treatment, 1 to 3 extracranial metastatic lesions, and serum testosterone levels > 50 ng/mL. Sixty-two patients were randomized 1:1 to either MDT (SBRT or surgery) of all lesions or surveillance. The 5-year ADT-free survival was 34% for MDT vs 8% for surveillance (P = .06).
VHA Radiation Oncology
The VHA has 138 departments of medical oncology, but only 41 departments of radiation oncology. Compared with medical oncologists without an on-site radiation oncology department, those with on-site departments were more likely to believe that local RT was potentially curative (94.4% vs 72.7%, respectively; P = .04). This finding suggests that a cancer center that includes both specialties has closer collaboration, which results in greater inclination to embrace local RT for OMD, as it has demonstrated PFS and OS benefits.
The radiation and medical oncologists surveyed had statistically significant differences in response by specialty regarding the maximal number of lesions still believed to constitute OMD. Most radiation oncologists classified ≤ 5 lesions as OMD, whereas most medical oncologists classified ≤ 3 lesions as OMD. This difference is not unexpected. There is no universally agreed-upon definition of OMD, and criteria differ across studies.
While the SABR-COMET trial did include ≤ 5 metastatic lesions, it was a phase 2 RCT, making subgroup analysis difficult. Ongoing phase 3 trials that are more specific in the number of metastases, comparing 1 to 3 vs 4 to 10 metastases (SABR-COMET-3 and SABR-COMET-10, respectively).13,14 There is even an ongoing phase 1 trial (ARREST) studying the potential benefits of treating (“restraining”) > 10 metastases, if dosimetrically feasible.15 Within the VHA, VA STARPORT is investigating MDT for recurrent or de novo hormone-sensitive metastatic PCa.17 The ongoing HALT phase 2/3 trial focuses on patients with actionable mutations to help determine management of oligo-progression in mutation-positive NSCLC.18
There was no significant difference by specialty in who responded that offering local RT for OMD treatment should not be limited by histology (55.2% of radiation oncologists and 50.0% of medical oncologists; P = .60). Oncologists could make the argument that some histologies (eg, pancreatic adenocarcinomas) have such poor prognoses that local RT would not meaningfully affect oncologic outcomes, while potentially adding toxicity, whereas others could point to improved systemic therapy regimens and the low toxicity rates with careful hypofractionation regimens. Of note, the 41-patient phase 2 EXTEND trial for pancreatic ductal adenocarcinoma suggested an oncologic benefit to MDT, with far better PFS and no grade ≥ 3 toxicities related to MDT.19 About half of respondents for each specialty believed the primary histology should affect the decision. Further clarification may emerge from phase 3 trials.
Of note, a 2023 study of 44 radiation and medical oncologists at 2 Harvard Medical School-affiliated hospitals found that for synchronous OMD, 50.0% of medical oncologists and 5.3% (P < .01) of radiation oncologists recommended systemic treatment, suggesting a greater divergence in approach than found in this study.20
Limitations
The response rate of 17.0% raised a potential for selection bias, but this rate is expected for a nonincentivized medical survey. A study by the American Board of Internal Medicine with 11 surveys and 6 weekly email contacts only generated a 23.7% response rate, while another study among physicians demonstrated a 4.5% response rate for email-based contact and 11.8% for mail-based contact.21,22 We could have asked participants questions regarding demographics and geography to ensure the survey represented a diverse sample of the medical community, although additional questions would likely suppress the response rate. Additional data collection about respondents may elucidate the rationale for differences in their responses, especially between the specialties. In a planned subsequent survey in several years, the question on the number of lesions that qualifies as OMD may be amended to reflect the context and dosimetry for the maximal number of metastases constituting OMD; the joint ESTRO-ASTRO consensus defined OMD as 1 to 5 metastatic lesions, but in which all metastatic sites must be safely treatable.12 Also, fewer example cases could be included to simplify the survey and boost response rates. A future survey may ask about the timing of SBRT and systemic therapy, and whether SBRT can safely delay systemic therapy.
Conclusions
Survey results demonstrated significant confidence among both radiation oncologists and medical oncologists that local RT for OMD improves outcomes, which is encouraging and a reflection of the recent evidence-based paradigm shift in viewing metastatic disease as a spectrum. However, there is a difference between radiation oncologists and medical oncologists in how they define OMD, and preferred treatment of the sample cases presented revealed nuanced differences by specialty. Close collaboration with radiation oncologists influences the belief of medical oncologists in the beneficial role of RT for OMD. As more phase 3 data for OMD local treatments emerge, additional investigation is needed on how beliefs and practice patterns evolve among radiation and medical oncologists.
The treatment of metastatic solid tumors has been based historically on systemic therapies, with the goal of delaying progression and extend life as long as possible, with tolerable treatment-related adverse events. Some exceptions were made for local treatment with surgery or radiotherapy (RT), often for patients with a single metastasis. A 1939 report describes a patient with renal adenocarcinoma and a solitary lung metastasis who underwent RT to the lung lesion after nephrectomy and subsequently partial lobectomy after the metastatic lesion progressed. The authors argued that if a metastasis appears solitary and accessible, it is plausible to remove it in addition to the primary growth.1
In 1995 Hellman and Weichselbaum proposed oligometastatic disease (OMD). They reasoned that malignancy exists along a spectrum from localized disease to widely disseminated disease, with OMD existing in between with a still-restricted tumor metastatic capacity. Appropriately selected patients with OMD may be candidates for prolonged disease-free survival or cure with the addition of local therapy to systemic therapy.2
The EORTC 4004 phase 2 randomized control trial (RCT) analyzed radiofrequency ablation (RFA) for colorectal liver metastases with systemic therapy vs systemic therapy alone for patients with ≤ 9 liver lesions.3 Systemic therapyconsisted of 5-FU/leucovorin/oxaliplatin, with bevacizumab added to the regimen 3.5 years into the study, per updated standard- of-care. This trial was the first to demonstrate the benefit of aggressive local treatment vs system treatment alone for OMD with a progression-free survival (PFS) benefit (16.8 vs 9.9 months; hazard ratio [HR], 0.63; P = .03) and overall survival (OS) benefit (45.3 vs 40.5 months; HR, 0.74; P = .02) with the addition of local treatment with RFA.
Since the presentations of the SABR-COMET phase 2 RCT and another study by Gomez et al at the American Society for Radiation Oncology (ASTRO) 2018 annual meeting, the paradigm for offering local RT for OMD has rapidly evolved. Both studies found PFS and OS benefits of RT for patients with OMD.4,5 Additional RCTs have since demonstrated that for properly selected patients with OMD, aggressive local RT improved PFS and OS.6-9 These small studies have led to larger RCTs to better understand who benefits from local consolidative treatment, particularly RT.10,11
There is a large degree of heterogeneity in how oncologists define and approach OMD treatment. The 2020 European Society for Radiotherapy and Oncology (ESTRO) and ASTRO consensus guidelines defined the OMD state as 1 to 5 metastatic lesions for which all metastatic sites are safely treatable.12 The purpose of this study was to evaluate perceptions and practice patterns among radiation oncologists and medical oncologists regarding the use of local RT for OMD across the Veterans Health Administration (VHA).
Methods
A 12-question survey was developed by the VHA Palliative Radiotherapy Task Force using the ESTRO-ASTRO consensus guidelines to define OMD. The survey was emailed to the VHA radiation oncology and medical oncology listservs on August 1, 2023. These listservs consist of physicians in these specialties either directly employed by the VHA or serve in its facilities as contractors. The original response closure date was August 11, 2023, but it was extended to August 18, 2023, to increase responses. No incentives were offered to respondents. Two email reminders were sent to the medical oncology listserv and 3 to the radiation oncology listserv. Descriptive statistics and X2 tests were used for data analysis. The impact of specialty and presence of an on-site department of radiation oncology were reviewed. This project was approved by the VHA National Oncology Program and National Radiation Oncology Program.
Results
The survey was sent to 125 radiation oncologists and 515 medical oncologists and 106 were completed for a 16.6% response rate. There were 59 (55.7%) radiation oncologist responses and 47 (44.3%) medical oncologist responses. Most (96.2%) respondents were board-certified, and 84 (79.2%) were affiliated with an academic center. Not every respondent answered every question (Table).

All respondents (n = 105) indicated there is a potential benefit of high-dose RT for appropriately selected cases. Ninety-four oncologists (88.7%) believed that RT for OMD contributes to cure (88.1% of radiation oncologists, 89.4% of medical oncologists; P = .84) for appropriately selected cases. Some respondents who did not believe RT for OMD contributes to cure added comments about other perceived benefits, such as local disease control for palliation, delaying systemic therapy with its associated toxicities, and prolongation of disease-free survival or OS. A higher percentage of respondents with academic affiliations believed high-dose RT contributes to cure, although this difference did not reach statistical significance (Figure 1).

Fifty-five respondents (51.9%; 55.2% radiation oncologists vs 50.0% medical oncologists; P = .60) responded that local RT for OMD treatment should not be limited by primary tumor type. Of respondents who responded that OMD treatment should be limited based on the type of primary tumor, many provided comments that argued there was a benefit for non-small cell lung cancer (NSCLC), prostate adenocarcinoma (PCa), and colorectal cancer.
The definition of how many metastatic lesions qualify as OMD varied. A total of 48.6% of respondents defined OMD as ≤ 3 lesions and 42.9% answered ≤ 5 lesions. A majority of radiation oncologists (55.2%) classified ≤ 5 lesions as OMD, whereas a majority of medical oncologists (66.0%) considered ≤ 3 lesions as OMD (P = .006) (Figure 2).

Thirty-six medical oncologists (76.6%) report having an on-site department of radiation oncology (Figure 3). This subgroup was more likely to consider local RT potentially curative compared with their medical oncology peers without on-site radiation oncology (94.4% vs 72.7%; P = .04).

Case Management
The 3 clinical cases demonstrated the heterogeneity of management approaches for OMD. The first described a man aged 65 years with PCa and 2 asymptomatic pelvic bone metastases. Ninety-three respondents (90.3%) recommended RT at the primary site and 74.8% recommended RT to both the primary site and metastatic foci. Sixty-three respondents (67.7%) recommended a STAMPEDE-compatible dose, and 30 (32.3%) recommended a definitive dose.
The second clinical case was a 60-year-old man with a cT1N2M1 NSCLC, with a solitary metastatic focus to the left iliac wing. Fifty-eight respondents (54.7%) recommended upfront systemic chemotherapy and the option of local therapy to the chest and metastatic focus after initial chemotherapy; 28 respondents (26.4%) recommended upfront chemoradiation to the chest and definitive radiation to the left iliac wing metastasis.
The third clinical case described a male aged 70 years with a history of a treated base of tongue squamous cell carcinoma, with a solitary metastatic focus within the right lung. Respondents could pick multiple treatment options and 85 (81.7%) favored upfront definitive local therapy with surgery or stereotactic body radiotherapy (SBRT), rather than upfront chemotherapy, with future consideration for local treatment. About half of respondents (51.8%) recommended SBRT and 41.2% would let the patient decide between surgery or SBRT. Additionally, 39.6% included in their patient counselling that the treatment may be for curative intent.
Discussion
The use of local treatment to increased PFS, OS, or even cure treatment for OMD has become more accepted since the 2018 ASTRO meeting.4,5 Palma et al analyzed a controlled primary malignancy of any histology and ≤ 5 metastatic lesions, with all lesions amenable to SBRT.4 With a median follow-up of 51 months when comparing the standard-of-care (SOC) arm and the SBRT arm, the 5-year PFS was not reached and the 5-year OS rates were 17.7% and 42.3% (P = .006), respectively. In the SBRT arm, about 1 in 5 patients survived > 5 years without a recurrence or disease progression, vs 0 patients in the control arm. There was a 29% rate of grade 2 or higher toxicity in the SBRT arm, including 3 deaths that were likely due to treatment. Subsequent trials, such as the phase 3 SABR-COMET-3 (1-3 metastases), phase 3 SABR-COMET-10 (4-10 metastases), and phase 1 ARREST (> 10 metastases) trials, have been specifically designed to minimize treatment-related toxicities.13-15
Gomez et al analyzed patients at 3 sites with a controlled NSCLC primary tumor and ≤ 3 metastases.5 At a follow-up of 38.8 months, the PFS was 4.4 months in the SOC arm vs 14.2 months in the RT and/or surgery local treatment arm (P = .02). There was also an OS benefit of 17.0 vs 41.2 months (P = .02), respectively.
Several RCTs soon followed that demonstrated improved PFS and OS with local radiotherapy for OMD; however, total metastatic ablation of the foci is necessary to attain these PFS and OS benefits.6-9 Still, an oncologic benefit has yet to be proven. The randomized NRGBR002 study phase 2/3 trial for oligometastatic breast cancer included patients with ≤ 4 extracranial metastases and controlled primary disease to metastasis-directed therapy (SBRT and/ or surgical resection) and systemic therapy vs systemic therapy alone.10 The study did not demonstrate improved PFS or OS at 3 years. However, for most breast cancers, especially with the rapid advancements in systemic therapy that have been achieved, longer follow-up may be necessary to detect a significant difference.
The prospective single-arm phase 2 SABR-5 trial retrospectively demonstrated important lessons about the timing of SBRT and systemic therapy.11 This study included patients with ≤ 5 metastases of any histology, and they received SBRT to all lesions. SABR-5 retrospectively compared patients who received upfront systemic therapy followed by SBRT vs another cohort that first received SBRT and did not receive systemic therapy until there was disease progression. Patients with oligo-progression were excluded, as it demonstrated systemic drug resistance. At a median follow-up time of 34 months, delayed systemic treatment was associated with shorter PFS (23 vs 34 months, respectively; P = .001), but not worse 3-year OS (80% vs 85%, respectively; P = .66). In addition, the delayed systemic treatment arm demonstrated a reduced risk of grade 2 or higher SBRT-related toxicity (odds ratio, 0.35; P < .001).
Similarly, the STOMP phase 2 trial analyzed the role of metastasis-directed therapy (MDT) in delaying initiation of androgen deprivation therapy (ADT) in a randomized phase 2 trial.16 This study included patients with asymptomatic PCa with a biochemical recurrence after primary treatment, 1 to 3 extracranial metastatic lesions, and serum testosterone levels > 50 ng/mL. Sixty-two patients were randomized 1:1 to either MDT (SBRT or surgery) of all lesions or surveillance. The 5-year ADT-free survival was 34% for MDT vs 8% for surveillance (P = .06).
VHA Radiation Oncology
The VHA has 138 departments of medical oncology, but only 41 departments of radiation oncology. Compared with medical oncologists without an on-site radiation oncology department, those with on-site departments were more likely to believe that local RT was potentially curative (94.4% vs 72.7%, respectively; P = .04). This finding suggests that a cancer center that includes both specialties has closer collaboration, which results in greater inclination to embrace local RT for OMD, as it has demonstrated PFS and OS benefits.
The radiation and medical oncologists surveyed had statistically significant differences in response by specialty regarding the maximal number of lesions still believed to constitute OMD. Most radiation oncologists classified ≤ 5 lesions as OMD, whereas most medical oncologists classified ≤ 3 lesions as OMD. This difference is not unexpected. There is no universally agreed-upon definition of OMD, and criteria differ across studies.
While the SABR-COMET trial did include ≤ 5 metastatic lesions, it was a phase 2 RCT, making subgroup analysis difficult. Ongoing phase 3 trials that are more specific in the number of metastases, comparing 1 to 3 vs 4 to 10 metastases (SABR-COMET-3 and SABR-COMET-10, respectively).13,14 There is even an ongoing phase 1 trial (ARREST) studying the potential benefits of treating (“restraining”) > 10 metastases, if dosimetrically feasible.15 Within the VHA, VA STARPORT is investigating MDT for recurrent or de novo hormone-sensitive metastatic PCa.17 The ongoing HALT phase 2/3 trial focuses on patients with actionable mutations to help determine management of oligo-progression in mutation-positive NSCLC.18
There was no significant difference by specialty in who responded that offering local RT for OMD treatment should not be limited by histology (55.2% of radiation oncologists and 50.0% of medical oncologists; P = .60). Oncologists could make the argument that some histologies (eg, pancreatic adenocarcinomas) have such poor prognoses that local RT would not meaningfully affect oncologic outcomes, while potentially adding toxicity, whereas others could point to improved systemic therapy regimens and the low toxicity rates with careful hypofractionation regimens. Of note, the 41-patient phase 2 EXTEND trial for pancreatic ductal adenocarcinoma suggested an oncologic benefit to MDT, with far better PFS and no grade ≥ 3 toxicities related to MDT.19 About half of respondents for each specialty believed the primary histology should affect the decision. Further clarification may emerge from phase 3 trials.
Of note, a 2023 study of 44 radiation and medical oncologists at 2 Harvard Medical School-affiliated hospitals found that for synchronous OMD, 50.0% of medical oncologists and 5.3% (P < .01) of radiation oncologists recommended systemic treatment, suggesting a greater divergence in approach than found in this study.20
Limitations
The response rate of 17.0% raised a potential for selection bias, but this rate is expected for a nonincentivized medical survey. A study by the American Board of Internal Medicine with 11 surveys and 6 weekly email contacts only generated a 23.7% response rate, while another study among physicians demonstrated a 4.5% response rate for email-based contact and 11.8% for mail-based contact.21,22 We could have asked participants questions regarding demographics and geography to ensure the survey represented a diverse sample of the medical community, although additional questions would likely suppress the response rate. Additional data collection about respondents may elucidate the rationale for differences in their responses, especially between the specialties. In a planned subsequent survey in several years, the question on the number of lesions that qualifies as OMD may be amended to reflect the context and dosimetry for the maximal number of metastases constituting OMD; the joint ESTRO-ASTRO consensus defined OMD as 1 to 5 metastatic lesions, but in which all metastatic sites must be safely treatable.12 Also, fewer example cases could be included to simplify the survey and boost response rates. A future survey may ask about the timing of SBRT and systemic therapy, and whether SBRT can safely delay systemic therapy.
Conclusions
Survey results demonstrated significant confidence among both radiation oncologists and medical oncologists that local RT for OMD improves outcomes, which is encouraging and a reflection of the recent evidence-based paradigm shift in viewing metastatic disease as a spectrum. However, there is a difference between radiation oncologists and medical oncologists in how they define OMD, and preferred treatment of the sample cases presented revealed nuanced differences by specialty. Close collaboration with radiation oncologists influences the belief of medical oncologists in the beneficial role of RT for OMD. As more phase 3 data for OMD local treatments emerge, additional investigation is needed on how beliefs and practice patterns evolve among radiation and medical oncologists.
- Barney JD, Churchill EJ. Adenocarcinoma of the kidney with metastasis to the lung. J Urology. 1939.
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8-10. doi:10.1200/JCO.1995.13.1.8
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 4004). Ann Oncol. 2012;23(10):2619-2626. doi:10.1093/annonc/mds053
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830- 2838. doi:10.1200/JCO.20.00818
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-1565. doi:10.1200/JCO.19.00201
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501. doi:10.1001/jamaoncol.2017.3501
- Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650-659. doi:10.1001/jamaoncol.2020.0147
- Wang XS, Bai YF, Verma V, et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated NSCLC. J Natl Cancer Inst 2023;115(6):742-748. doi:10.1093/jnci/djac015
- Tang C, Sherry AD, Haymaker C, et al. Addition of metastasis- directed therapy to intermittent hormone therapy for oligometastatic prostate cancer (EXTEND): a multicenter, randomized phase II trial. Am Soc Radiat Oncol Annu Meet. 2023;9(6):825-834. doi:10.1001/jamaoncol.2023.0161
- Chmura SJ, Winter KA, Woodward WA, et al. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol. 2022;40:1007. doi:10.1200/JCO.2022.40.16_suppl.1007
- Baker S, Lechner L, Liu M, et al. Upfront versus delayed systemic therapy in patients with oligometastatic cancer treated with SABR in the phase 2 SABR-5 trial. Int J Radiat Oncol Biol Phys. 2024;118(5):1497-1506. doi:10.1016/j.ijrobp.2023.11.007
- Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157-166. doi:10.1016/j.radonc.2020.04.003
- Olson R, Mathews L, Liu M, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1-3 oligometastatic tumors (SABR-COMET-3): study protocol for a randomized phase III trial. BMC Cancer. 2020;20(1):380. doi:10.1186/s12885-020-06876-4
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. 2019;19(1):816. doi:10.1186/s12885-019-5977-6
- Bauman GS, Corkum MT, Fakir H, et al. Ablative radiation therapy to restrain everything safely treatable (ARREST): study protocol for a phase I trial treating polymetastatic cancer with stereotactic radiotherapy. BMC Cancer. 2021;21(1):405. doi:10.1186/s12885-021-08136-5
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:suppl.
- Solanki AA, Campbell D, Carlson K, et al. Veterans Affairs seamless phase II/III randomized trial of standard systemic therapy with or without PET-directed local therapy for oligometastatic prostate cancer (VA STARPORT). J Clin Oncol. 2024;42:16.
- McDonald F, Guckenberger M, Popat S. EP08.03-005 HALT – Targeted therapy with or without dose-intensified radiotherapy in oligo-progressive disease in oncogene addicted lung tumours. J Thor Oncol. 2022;17:S492.
- Ludmir EB, Sherry AD, Fellman BM, et al. Addition of metastasis- directed therapy to systemic therapy for oligometastatic pancreatic ductal adenocarcinoma (EXTEND): a multicenter, randomized phase II trial. J Clin Oncol. 2024;42(32):3795-3805. doi:10.1200/JCO.24.00081
- Cho HL, Balboni T, Christ SB, et al. Is oligometastatic cancer curable? A survey of oncologist perspectives, decision making, and communication. Adv Radiat Oncol. 2023;8(5):101221. doi:10.1016/j.adro.2023.101221
- Barnhart BJ, Reddy SG, Arnold GK. Remind me again: physician response to web surveys: the effect of email reminders across 11 opinion survey efforts at the American Board of Internal Medicine from 2017 to 2019. Eval Health Prof. 2021;44(3):245-259. doi:10.1177/01632787211019445
- Murphy CC, Lee SJC, Geiger AM, et al. A randomized trial of mail and email recruitment strategies for a physician survey on clinical trial accrual. BMC Med Res Methodol. 2020;20(1):123. doi:10.1186/s12874-020-01014-x
- Barney JD, Churchill EJ. Adenocarcinoma of the kidney with metastasis to the lung. J Urology. 1939.
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8-10. doi:10.1200/JCO.1995.13.1.8
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 4004). Ann Oncol. 2012;23(10):2619-2626. doi:10.1093/annonc/mds053
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830- 2838. doi:10.1200/JCO.20.00818
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-1565. doi:10.1200/JCO.19.00201
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501. doi:10.1001/jamaoncol.2017.3501
- Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650-659. doi:10.1001/jamaoncol.2020.0147
- Wang XS, Bai YF, Verma V, et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated NSCLC. J Natl Cancer Inst 2023;115(6):742-748. doi:10.1093/jnci/djac015
- Tang C, Sherry AD, Haymaker C, et al. Addition of metastasis- directed therapy to intermittent hormone therapy for oligometastatic prostate cancer (EXTEND): a multicenter, randomized phase II trial. Am Soc Radiat Oncol Annu Meet. 2023;9(6):825-834. doi:10.1001/jamaoncol.2023.0161
- Chmura SJ, Winter KA, Woodward WA, et al. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol. 2022;40:1007. doi:10.1200/JCO.2022.40.16_suppl.1007
- Baker S, Lechner L, Liu M, et al. Upfront versus delayed systemic therapy in patients with oligometastatic cancer treated with SABR in the phase 2 SABR-5 trial. Int J Radiat Oncol Biol Phys. 2024;118(5):1497-1506. doi:10.1016/j.ijrobp.2023.11.007
- Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157-166. doi:10.1016/j.radonc.2020.04.003
- Olson R, Mathews L, Liu M, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1-3 oligometastatic tumors (SABR-COMET-3): study protocol for a randomized phase III trial. BMC Cancer. 2020;20(1):380. doi:10.1186/s12885-020-06876-4
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. 2019;19(1):816. doi:10.1186/s12885-019-5977-6
- Bauman GS, Corkum MT, Fakir H, et al. Ablative radiation therapy to restrain everything safely treatable (ARREST): study protocol for a phase I trial treating polymetastatic cancer with stereotactic radiotherapy. BMC Cancer. 2021;21(1):405. doi:10.1186/s12885-021-08136-5
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:suppl.
- Solanki AA, Campbell D, Carlson K, et al. Veterans Affairs seamless phase II/III randomized trial of standard systemic therapy with or without PET-directed local therapy for oligometastatic prostate cancer (VA STARPORT). J Clin Oncol. 2024;42:16.
- McDonald F, Guckenberger M, Popat S. EP08.03-005 HALT – Targeted therapy with or without dose-intensified radiotherapy in oligo-progressive disease in oncogene addicted lung tumours. J Thor Oncol. 2022;17:S492.
- Ludmir EB, Sherry AD, Fellman BM, et al. Addition of metastasis- directed therapy to systemic therapy for oligometastatic pancreatic ductal adenocarcinoma (EXTEND): a multicenter, randomized phase II trial. J Clin Oncol. 2024;42(32):3795-3805. doi:10.1200/JCO.24.00081
- Cho HL, Balboni T, Christ SB, et al. Is oligometastatic cancer curable? A survey of oncologist perspectives, decision making, and communication. Adv Radiat Oncol. 2023;8(5):101221. doi:10.1016/j.adro.2023.101221
- Barnhart BJ, Reddy SG, Arnold GK. Remind me again: physician response to web surveys: the effect of email reminders across 11 opinion survey efforts at the American Board of Internal Medicine from 2017 to 2019. Eval Health Prof. 2021;44(3):245-259. doi:10.1177/01632787211019445
- Murphy CC, Lee SJC, Geiger AM, et al. A randomized trial of mail and email recruitment strategies for a physician survey on clinical trial accrual. BMC Med Res Methodol. 2020;20(1):123. doi:10.1186/s12874-020-01014-x
Radiation and Medical Oncology Perspectives on Oligometastatic Disease Treatment
Radiation and Medical Oncology Perspectives on Oligometastatic Disease Treatment
The Availability of Advanced Radiation Oncology Technology Within VHA Radiation Oncology Centers
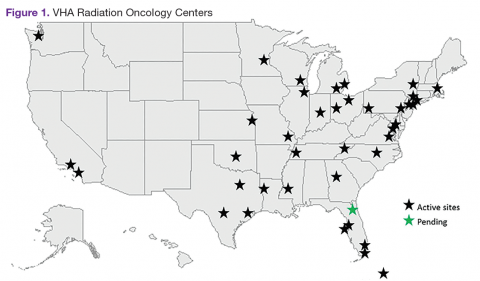
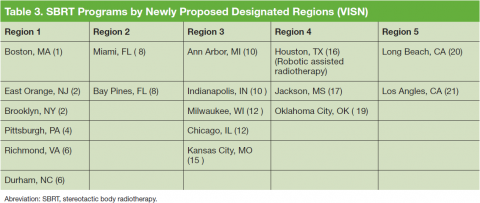
The VHA is the primary care provider for 20.4% of the more than 21.9 million military veterans.1 Surveys report that over a lifetime, an estimated 28.4% of U.S. veterans will receive some measure of their health care from the VHA.2 An estimated 40,000 new cancer cases are diagnosed each year from these veterans, resulting in a minimum of 175,000 veterans receiving cancer care in VHA facilities.3 The 39 VHA facilities currently with onsite radiation oncology practices annually provide radiation therapy to about 20,000 veterans (Figure 1).
Nationally, tumor control and toxicity outcomes have each improved over recent decades as advances have occurred in imaging, radiation treatment planning, and equipment for the delivery of radiotherapy.4 The VHA has kept pace with these technological advancements to the point where image-guided radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT), and stereotactic body radiotherapy (SBRT) are widely available at VHA centers. Additionally, all active VHA radiation oncology centers have earned accreditation from the American College of Radiology, while 3 new centers are in the process of gaining accreditation.
When technologies deemed to be medically necessary are not available onsite, these treatments are made available to veterans through referral to other VHA or non-VHA centers. Here, the authors present the results of a survey of VHA-based radiation oncologists to evaluate onsite availability of various radiation technologies.
Methods
The VHA Palliative Radiotherapy Task Force constructed an online survey and sent it to the 82 radiation oncologists practicing at the 38 VHA radiation oncology centers that were active at the time. After emailing the survey,follow-up phone calls were made to maximize response rates. The survey was conducted during the months of May and June of 2014.
In this survey, all 82 VHA radiation oncologists were queried on the availability of advanced radiation delivery technologies including IGRT, IMRT, and SBRT at their facilities. The authors also surveyed for presence of brachytherapy (BT) programs, stereotactic radiosurgery (SRS), and cone-beam computed tomography (CBCT). Information was collected regarding the extent to which physicians can treat cases requiring SRS and/or SBRT onsite vs through referral to another facility for treatment. These data were gathered from a survey conducted in conjunction with a larger survey on the practice and patterns of care in the treatment of patients with brain metastases within the VHA.5,6 The data presented here apply to radiation therapy in general and are not limited to the treatment of brain metastases.
Results
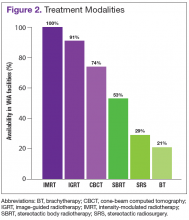
The overall response rate was 76% (62 of 82 radiation oncologists). At the time of the survey, 90% (34 of 38) of active VHA radiation oncology treatment facilities were represented. However as of May 2016, there are 40 active VHA radiation oncology centers. Figure 2 describes the availability of various treatment delivery systems. The data demonstrated 100% availability of IMRT. Respondents reported onsite availability of IGRT at 91%, CBCT at 74%, and SBRT at 53%. Treatment technologies that were not as widely available at VHA facilities with inherent radiation oncology practices included SRS at 29% and BT at 21%. For cases requiring SRS, 69% (40 of 58) of respondents who answered this question indicated that they refer patients to other VHA radiation oncology centers or VHA contracted private entities. This report is limited by the following factors:
- A narrow scope of practices was surveyed. The survey was solely sent to VHA physicians at 38 active VHA radiation oncology centers out of 144 VHA hospitals. Therefore the practices at VHA medical centers without active VHA radiation was not acquired with this survey.
- This survey only addresses availability of these newer treatment technologies, not their actual use, in treating cancers predominant within the VHA.
- Literature comparison in this report is based on current use of these technologies for some of the reports cited, rather than availability as this report reflects. As such, direct comparisons could be misleading.
Discussion
Although the total number of veterans has been decreasing in recent years, the number of veterans enrolling into VHA-related programs has been increasing and is expected to expand increase further in years to come.1,2 It is important for radiation oncologists to keep pace with new technologies to ensure their patients have access to the best possible treatments.
Advances in radiation oncology have allowed radiotherapy to evolve from the 2-dimensional treatments of the 1950s to the 1980s, to more targeted treatments that employ advanced imaging and complex planning. Modern techniques for delivery of radiotherapy are better at confining radiation dose to the tumor volume while minimizing the irradiation of normal structures. The use of cumbersome blocks, wedges, and tissue compensators has given way to treatment with internal collimation techniques such as IMRT, SBRT, and SRS. These techniques rely heavily on image guidance for tumor targeting. Four-dimensional planning and treatment allow radiation oncologists to track tumor and normal tissue motion, thereby increasing the accuracy and precision of radiation treatments.
As is true in the community, IMRT and IGRT are widely available within the VHA. According to a survey by Simpson and colleagues evaluating the use of IGRT in the U.S., 93% of radiation oncologists use IGRT.7 Similarly, the survey presented here demonstrates that 91% of VHA radiation oncologists report availability of IGRT at their centers. All VHA radiation oncologists surveyed report access to IMRT.
Shen’s recent report evaluating radiotherapy patterns of practice from 2002 to 2010 examined volume of payments for treatment delivery by codes for office-based IMRT.8 These authors noted an increase in the usage of IMRT as a percentage of external beam radiotherapy from 2002 to 2010 of 0% to 70%, respectively. They further noted during this period that IGRT use, based on total payments for treatment delivery, increased from 2.1% to 11.1%.
The reported use of onsite SBRT among VHA physicians is slightly less than that of community physicians. A survey study by Pan and colleagues demonstrated that 63.9% of U.S. radiation oncologists use SBRT, while in the survey study presented here, 53% of VHA radiation oncologists reported availability of onsite SBRT.9 Of note, the lack of availability of onsite SBRT at VHA centers does not preclude treatment with SBRT when medically necessary. These cases can be referred to other VHA or community centers with the requisite accreditation credentials. Because of the increasing use of SBRT and related technologies in the treatment of some cancers, an improved availability of SBRT in the future within the VHA will allow for some centers to participate in the Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR) trial, which was approved for open recruitment in 2015.
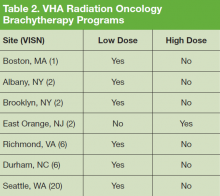
Although BT and SRS are not as widely available within the VHA as other evaluated technologies such as IGRT and IMRT, their availability mirrors a similar limited availability in the community.10-12 When necessary these services also can be provided for veterans through referral to other VHA or non-VHA centers.
The benefit of charged particle radiotherapy, such as proton beam radiotherapy, is limited to specific cancers.13 This technology is not widely available in the community or within the VHA. Because of a VHA policy currently in place permitting non-VHA care when needed, veterans who require treatment with charged particle radiotherapy are referred to accredited non-VHA radiation oncology centers when indicated.
Conclusion
In this survey, 92% of the VHA radiation oncology centers are accredited by the American College of Radiology. Further, VHA radiation oncologists respondents reported availability of treatment technologies in line with responses of physicians from community based surveys. The majority of VHA radiation oncologists report access to IMRT, IGRT, CBCT, and SBRT. While BT and SRS are not available onsite at the majority of the 40 VHA radiation oncology centers, this mirrors limited availability and use of these technologies in the community as well.
Acknowledgments
This article was based on a presentation at the ASCO Quality Care Symposium (October 17-18, 2014) in Boston, Massachusetts. Dawson GA, Cheuk AV, Jolly S, et al. Advanced radiation oncology technology within the Veterans Health Administration (VHA). J Clin Oncol. 214;32(suppl 30):52.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. National Center for Veterans Analysis and Statistics. 2010 National Survey of Veterans: reported plan to use VA health care in the future. U.S. Department of Veteran Affairs website. http://www.va.gov/vetdata/docs/QuickFacts/2010NSV_Quick_Fact_Final.pdf. Published December 2011. Accessed April 4, 2016.
2. National Center for Veterans Analysis and Statistics. 2010 National Survey Veterans: enrollment and usage of VA benefits and services. U.S. Department of Veteran Affairs website. http://www.va.gov/vedata/docs/quickfacts/Surveys-slideshow.pdf. Published August 15, 2011. Accessed April 4, 2016.
3. Zulling LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Mil Med. 2012;177(6):693-701.
4. International Atomic Energy Agency. Recent developments in the technology of radiation oncology. International Atomic Energy Agency website. https://www.iaea.org/About/Policy/GC/GC55/GC55InfDocuments/English/gc55inf-5-att1_en.pdf. Accessed April 4, 2016.
5. Dawson GA, Jolly S, Fosmire H, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P114) radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: supportive measures (Part 1 of 2 reports). Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain#sthash.fcB6idE7.dpuf. Published April 30, 2015. Accessed April 4, 2016.
6. Cheuk AV, Gutt R, Moghanaki D, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P118) Radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: part 2 clinical treatment patterns. Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain-part-2#sthash.fwW0g1RZ.dpuf. Published April 30, 2015. Accessed April 4, 2016.
7. Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey on the use of image-guided radiotherapy in the United States. Cancer. 2010;116(16):3953–3960.
8. Shen X, Showalter TN, Mishra MV, et al. Radiation oncology services in the modern era: evolving patterns of usage and payments in the office setting for medicare patients from 2000 to 2010. J Oncol Pract. 2014;10(4):e201-e207.
9. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117(19):4566-4572.
10. Mahmood U, Pugh T, Frank S, et al. Declining u se of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13(2):157-162.
11. Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109-e116.
12. Kong FM, Cuneo KC, Wang L, et al. Patterns of practice in radiation therapy for non-small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4(2):e133-e141.
13. Trikalinos TA, Terasawa T, Ip S, Raman G, Lau J. Particle Beam Radiation Therapies for Cancer. Technical Brief, No. 1. Rockville, MD: Agency for Healthcare Research and Quality; 2009.
The VHA is the primary care provider for 20.4% of the more than 21.9 million military veterans.1 Surveys report that over a lifetime, an estimated 28.4% of U.S. veterans will receive some measure of their health care from the VHA.2 An estimated 40,000 new cancer cases are diagnosed each year from these veterans, resulting in a minimum of 175,000 veterans receiving cancer care in VHA facilities.3 The 39 VHA facilities currently with onsite radiation oncology practices annually provide radiation therapy to about 20,000 veterans (Figure 1).
Nationally, tumor control and toxicity outcomes have each improved over recent decades as advances have occurred in imaging, radiation treatment planning, and equipment for the delivery of radiotherapy.4 The VHA has kept pace with these technological advancements to the point where image-guided radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT), and stereotactic body radiotherapy (SBRT) are widely available at VHA centers. Additionally, all active VHA radiation oncology centers have earned accreditation from the American College of Radiology, while 3 new centers are in the process of gaining accreditation.
When technologies deemed to be medically necessary are not available onsite, these treatments are made available to veterans through referral to other VHA or non-VHA centers. Here, the authors present the results of a survey of VHA-based radiation oncologists to evaluate onsite availability of various radiation technologies.
Methods
The VHA Palliative Radiotherapy Task Force constructed an online survey and sent it to the 82 radiation oncologists practicing at the 38 VHA radiation oncology centers that were active at the time. After emailing the survey,follow-up phone calls were made to maximize response rates. The survey was conducted during the months of May and June of 2014.
In this survey, all 82 VHA radiation oncologists were queried on the availability of advanced radiation delivery technologies including IGRT, IMRT, and SBRT at their facilities. The authors also surveyed for presence of brachytherapy (BT) programs, stereotactic radiosurgery (SRS), and cone-beam computed tomography (CBCT). Information was collected regarding the extent to which physicians can treat cases requiring SRS and/or SBRT onsite vs through referral to another facility for treatment. These data were gathered from a survey conducted in conjunction with a larger survey on the practice and patterns of care in the treatment of patients with brain metastases within the VHA.5,6 The data presented here apply to radiation therapy in general and are not limited to the treatment of brain metastases.
Results
The overall response rate was 76% (62 of 82 radiation oncologists). At the time of the survey, 90% (34 of 38) of active VHA radiation oncology treatment facilities were represented. However as of May 2016, there are 40 active VHA radiation oncology centers. Figure 2 describes the availability of various treatment delivery systems. The data demonstrated 100% availability of IMRT. Respondents reported onsite availability of IGRT at 91%, CBCT at 74%, and SBRT at 53%. Treatment technologies that were not as widely available at VHA facilities with inherent radiation oncology practices included SRS at 29% and BT at 21%. For cases requiring SRS, 69% (40 of 58) of respondents who answered this question indicated that they refer patients to other VHA radiation oncology centers or VHA contracted private entities. This report is limited by the following factors:
- A narrow scope of practices was surveyed. The survey was solely sent to VHA physicians at 38 active VHA radiation oncology centers out of 144 VHA hospitals. Therefore the practices at VHA medical centers without active VHA radiation was not acquired with this survey.
- This survey only addresses availability of these newer treatment technologies, not their actual use, in treating cancers predominant within the VHA.
- Literature comparison in this report is based on current use of these technologies for some of the reports cited, rather than availability as this report reflects. As such, direct comparisons could be misleading.
Discussion
Although the total number of veterans has been decreasing in recent years, the number of veterans enrolling into VHA-related programs has been increasing and is expected to expand increase further in years to come.1,2 It is important for radiation oncologists to keep pace with new technologies to ensure their patients have access to the best possible treatments.
Advances in radiation oncology have allowed radiotherapy to evolve from the 2-dimensional treatments of the 1950s to the 1980s, to more targeted treatments that employ advanced imaging and complex planning. Modern techniques for delivery of radiotherapy are better at confining radiation dose to the tumor volume while minimizing the irradiation of normal structures. The use of cumbersome blocks, wedges, and tissue compensators has given way to treatment with internal collimation techniques such as IMRT, SBRT, and SRS. These techniques rely heavily on image guidance for tumor targeting. Four-dimensional planning and treatment allow radiation oncologists to track tumor and normal tissue motion, thereby increasing the accuracy and precision of radiation treatments.
As is true in the community, IMRT and IGRT are widely available within the VHA. According to a survey by Simpson and colleagues evaluating the use of IGRT in the U.S., 93% of radiation oncologists use IGRT.7 Similarly, the survey presented here demonstrates that 91% of VHA radiation oncologists report availability of IGRT at their centers. All VHA radiation oncologists surveyed report access to IMRT.
Shen’s recent report evaluating radiotherapy patterns of practice from 2002 to 2010 examined volume of payments for treatment delivery by codes for office-based IMRT.8 These authors noted an increase in the usage of IMRT as a percentage of external beam radiotherapy from 2002 to 2010 of 0% to 70%, respectively. They further noted during this period that IGRT use, based on total payments for treatment delivery, increased from 2.1% to 11.1%.
The reported use of onsite SBRT among VHA physicians is slightly less than that of community physicians. A survey study by Pan and colleagues demonstrated that 63.9% of U.S. radiation oncologists use SBRT, while in the survey study presented here, 53% of VHA radiation oncologists reported availability of onsite SBRT.9 Of note, the lack of availability of onsite SBRT at VHA centers does not preclude treatment with SBRT when medically necessary. These cases can be referred to other VHA or community centers with the requisite accreditation credentials. Because of the increasing use of SBRT and related technologies in the treatment of some cancers, an improved availability of SBRT in the future within the VHA will allow for some centers to participate in the Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR) trial, which was approved for open recruitment in 2015.
Although BT and SRS are not as widely available within the VHA as other evaluated technologies such as IGRT and IMRT, their availability mirrors a similar limited availability in the community.10-12 When necessary these services also can be provided for veterans through referral to other VHA or non-VHA centers.
The benefit of charged particle radiotherapy, such as proton beam radiotherapy, is limited to specific cancers.13 This technology is not widely available in the community or within the VHA. Because of a VHA policy currently in place permitting non-VHA care when needed, veterans who require treatment with charged particle radiotherapy are referred to accredited non-VHA radiation oncology centers when indicated.
Conclusion
In this survey, 92% of the VHA radiation oncology centers are accredited by the American College of Radiology. Further, VHA radiation oncologists respondents reported availability of treatment technologies in line with responses of physicians from community based surveys. The majority of VHA radiation oncologists report access to IMRT, IGRT, CBCT, and SBRT. While BT and SRS are not available onsite at the majority of the 40 VHA radiation oncology centers, this mirrors limited availability and use of these technologies in the community as well.
Acknowledgments
This article was based on a presentation at the ASCO Quality Care Symposium (October 17-18, 2014) in Boston, Massachusetts. Dawson GA, Cheuk AV, Jolly S, et al. Advanced radiation oncology technology within the Veterans Health Administration (VHA). J Clin Oncol. 214;32(suppl 30):52.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
The VHA is the primary care provider for 20.4% of the more than 21.9 million military veterans.1 Surveys report that over a lifetime, an estimated 28.4% of U.S. veterans will receive some measure of their health care from the VHA.2 An estimated 40,000 new cancer cases are diagnosed each year from these veterans, resulting in a minimum of 175,000 veterans receiving cancer care in VHA facilities.3 The 39 VHA facilities currently with onsite radiation oncology practices annually provide radiation therapy to about 20,000 veterans (Figure 1).
Nationally, tumor control and toxicity outcomes have each improved over recent decades as advances have occurred in imaging, radiation treatment planning, and equipment for the delivery of radiotherapy.4 The VHA has kept pace with these technological advancements to the point where image-guided radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT), and stereotactic body radiotherapy (SBRT) are widely available at VHA centers. Additionally, all active VHA radiation oncology centers have earned accreditation from the American College of Radiology, while 3 new centers are in the process of gaining accreditation.
When technologies deemed to be medically necessary are not available onsite, these treatments are made available to veterans through referral to other VHA or non-VHA centers. Here, the authors present the results of a survey of VHA-based radiation oncologists to evaluate onsite availability of various radiation technologies.
Methods
The VHA Palliative Radiotherapy Task Force constructed an online survey and sent it to the 82 radiation oncologists practicing at the 38 VHA radiation oncology centers that were active at the time. After emailing the survey,follow-up phone calls were made to maximize response rates. The survey was conducted during the months of May and June of 2014.
In this survey, all 82 VHA radiation oncologists were queried on the availability of advanced radiation delivery technologies including IGRT, IMRT, and SBRT at their facilities. The authors also surveyed for presence of brachytherapy (BT) programs, stereotactic radiosurgery (SRS), and cone-beam computed tomography (CBCT). Information was collected regarding the extent to which physicians can treat cases requiring SRS and/or SBRT onsite vs through referral to another facility for treatment. These data were gathered from a survey conducted in conjunction with a larger survey on the practice and patterns of care in the treatment of patients with brain metastases within the VHA.5,6 The data presented here apply to radiation therapy in general and are not limited to the treatment of brain metastases.
Results
The overall response rate was 76% (62 of 82 radiation oncologists). At the time of the survey, 90% (34 of 38) of active VHA radiation oncology treatment facilities were represented. However as of May 2016, there are 40 active VHA radiation oncology centers. Figure 2 describes the availability of various treatment delivery systems. The data demonstrated 100% availability of IMRT. Respondents reported onsite availability of IGRT at 91%, CBCT at 74%, and SBRT at 53%. Treatment technologies that were not as widely available at VHA facilities with inherent radiation oncology practices included SRS at 29% and BT at 21%. For cases requiring SRS, 69% (40 of 58) of respondents who answered this question indicated that they refer patients to other VHA radiation oncology centers or VHA contracted private entities. This report is limited by the following factors:
- A narrow scope of practices was surveyed. The survey was solely sent to VHA physicians at 38 active VHA radiation oncology centers out of 144 VHA hospitals. Therefore the practices at VHA medical centers without active VHA radiation was not acquired with this survey.
- This survey only addresses availability of these newer treatment technologies, not their actual use, in treating cancers predominant within the VHA.
- Literature comparison in this report is based on current use of these technologies for some of the reports cited, rather than availability as this report reflects. As such, direct comparisons could be misleading.
Discussion
Although the total number of veterans has been decreasing in recent years, the number of veterans enrolling into VHA-related programs has been increasing and is expected to expand increase further in years to come.1,2 It is important for radiation oncologists to keep pace with new technologies to ensure their patients have access to the best possible treatments.
Advances in radiation oncology have allowed radiotherapy to evolve from the 2-dimensional treatments of the 1950s to the 1980s, to more targeted treatments that employ advanced imaging and complex planning. Modern techniques for delivery of radiotherapy are better at confining radiation dose to the tumor volume while minimizing the irradiation of normal structures. The use of cumbersome blocks, wedges, and tissue compensators has given way to treatment with internal collimation techniques such as IMRT, SBRT, and SRS. These techniques rely heavily on image guidance for tumor targeting. Four-dimensional planning and treatment allow radiation oncologists to track tumor and normal tissue motion, thereby increasing the accuracy and precision of radiation treatments.
As is true in the community, IMRT and IGRT are widely available within the VHA. According to a survey by Simpson and colleagues evaluating the use of IGRT in the U.S., 93% of radiation oncologists use IGRT.7 Similarly, the survey presented here demonstrates that 91% of VHA radiation oncologists report availability of IGRT at their centers. All VHA radiation oncologists surveyed report access to IMRT.
Shen’s recent report evaluating radiotherapy patterns of practice from 2002 to 2010 examined volume of payments for treatment delivery by codes for office-based IMRT.8 These authors noted an increase in the usage of IMRT as a percentage of external beam radiotherapy from 2002 to 2010 of 0% to 70%, respectively. They further noted during this period that IGRT use, based on total payments for treatment delivery, increased from 2.1% to 11.1%.
The reported use of onsite SBRT among VHA physicians is slightly less than that of community physicians. A survey study by Pan and colleagues demonstrated that 63.9% of U.S. radiation oncologists use SBRT, while in the survey study presented here, 53% of VHA radiation oncologists reported availability of onsite SBRT.9 Of note, the lack of availability of onsite SBRT at VHA centers does not preclude treatment with SBRT when medically necessary. These cases can be referred to other VHA or community centers with the requisite accreditation credentials. Because of the increasing use of SBRT and related technologies in the treatment of some cancers, an improved availability of SBRT in the future within the VHA will allow for some centers to participate in the Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR) trial, which was approved for open recruitment in 2015.
Although BT and SRS are not as widely available within the VHA as other evaluated technologies such as IGRT and IMRT, their availability mirrors a similar limited availability in the community.10-12 When necessary these services also can be provided for veterans through referral to other VHA or non-VHA centers.
The benefit of charged particle radiotherapy, such as proton beam radiotherapy, is limited to specific cancers.13 This technology is not widely available in the community or within the VHA. Because of a VHA policy currently in place permitting non-VHA care when needed, veterans who require treatment with charged particle radiotherapy are referred to accredited non-VHA radiation oncology centers when indicated.
Conclusion
In this survey, 92% of the VHA radiation oncology centers are accredited by the American College of Radiology. Further, VHA radiation oncologists respondents reported availability of treatment technologies in line with responses of physicians from community based surveys. The majority of VHA radiation oncologists report access to IMRT, IGRT, CBCT, and SBRT. While BT and SRS are not available onsite at the majority of the 40 VHA radiation oncology centers, this mirrors limited availability and use of these technologies in the community as well.
Acknowledgments
This article was based on a presentation at the ASCO Quality Care Symposium (October 17-18, 2014) in Boston, Massachusetts. Dawson GA, Cheuk AV, Jolly S, et al. Advanced radiation oncology technology within the Veterans Health Administration (VHA). J Clin Oncol. 214;32(suppl 30):52.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. National Center for Veterans Analysis and Statistics. 2010 National Survey of Veterans: reported plan to use VA health care in the future. U.S. Department of Veteran Affairs website. http://www.va.gov/vetdata/docs/QuickFacts/2010NSV_Quick_Fact_Final.pdf. Published December 2011. Accessed April 4, 2016.
2. National Center for Veterans Analysis and Statistics. 2010 National Survey Veterans: enrollment and usage of VA benefits and services. U.S. Department of Veteran Affairs website. http://www.va.gov/vedata/docs/quickfacts/Surveys-slideshow.pdf. Published August 15, 2011. Accessed April 4, 2016.
3. Zulling LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Mil Med. 2012;177(6):693-701.
4. International Atomic Energy Agency. Recent developments in the technology of radiation oncology. International Atomic Energy Agency website. https://www.iaea.org/About/Policy/GC/GC55/GC55InfDocuments/English/gc55inf-5-att1_en.pdf. Accessed April 4, 2016.
5. Dawson GA, Jolly S, Fosmire H, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P114) radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: supportive measures (Part 1 of 2 reports). Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain#sthash.fcB6idE7.dpuf. Published April 30, 2015. Accessed April 4, 2016.
6. Cheuk AV, Gutt R, Moghanaki D, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P118) Radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: part 2 clinical treatment patterns. Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain-part-2#sthash.fwW0g1RZ.dpuf. Published April 30, 2015. Accessed April 4, 2016.
7. Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey on the use of image-guided radiotherapy in the United States. Cancer. 2010;116(16):3953–3960.
8. Shen X, Showalter TN, Mishra MV, et al. Radiation oncology services in the modern era: evolving patterns of usage and payments in the office setting for medicare patients from 2000 to 2010. J Oncol Pract. 2014;10(4):e201-e207.
9. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117(19):4566-4572.
10. Mahmood U, Pugh T, Frank S, et al. Declining u se of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13(2):157-162.
11. Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109-e116.
12. Kong FM, Cuneo KC, Wang L, et al. Patterns of practice in radiation therapy for non-small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4(2):e133-e141.
13. Trikalinos TA, Terasawa T, Ip S, Raman G, Lau J. Particle Beam Radiation Therapies for Cancer. Technical Brief, No. 1. Rockville, MD: Agency for Healthcare Research and Quality; 2009.
1. National Center for Veterans Analysis and Statistics. 2010 National Survey of Veterans: reported plan to use VA health care in the future. U.S. Department of Veteran Affairs website. http://www.va.gov/vetdata/docs/QuickFacts/2010NSV_Quick_Fact_Final.pdf. Published December 2011. Accessed April 4, 2016.
2. National Center for Veterans Analysis and Statistics. 2010 National Survey Veterans: enrollment and usage of VA benefits and services. U.S. Department of Veteran Affairs website. http://www.va.gov/vedata/docs/quickfacts/Surveys-slideshow.pdf. Published August 15, 2011. Accessed April 4, 2016.
3. Zulling LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Mil Med. 2012;177(6):693-701.
4. International Atomic Energy Agency. Recent developments in the technology of radiation oncology. International Atomic Energy Agency website. https://www.iaea.org/About/Policy/GC/GC55/GC55InfDocuments/English/gc55inf-5-att1_en.pdf. Accessed April 4, 2016.
5. Dawson GA, Jolly S, Fosmire H, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P114) radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: supportive measures (Part 1 of 2 reports). Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain#sthash.fcB6idE7.dpuf. Published April 30, 2015. Accessed April 4, 2016.
6. Cheuk AV, Gutt R, Moghanaki D, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P118) Radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: part 2 clinical treatment patterns. Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain-part-2#sthash.fwW0g1RZ.dpuf. Published April 30, 2015. Accessed April 4, 2016.
7. Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey on the use of image-guided radiotherapy in the United States. Cancer. 2010;116(16):3953–3960.
8. Shen X, Showalter TN, Mishra MV, et al. Radiation oncology services in the modern era: evolving patterns of usage and payments in the office setting for medicare patients from 2000 to 2010. J Oncol Pract. 2014;10(4):e201-e207.
9. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117(19):4566-4572.
10. Mahmood U, Pugh T, Frank S, et al. Declining u se of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13(2):157-162.
11. Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109-e116.
12. Kong FM, Cuneo KC, Wang L, et al. Patterns of practice in radiation therapy for non-small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4(2):e133-e141.
13. Trikalinos TA, Terasawa T, Ip S, Raman G, Lau J. Particle Beam Radiation Therapies for Cancer. Technical Brief, No. 1. Rockville, MD: Agency for Healthcare Research and Quality; 2009.