User login
Intermediate Care: Role for Hospitalists
Hospitalized patients are becoming increasingly complex. The care of such patients may be impacted by the limited resources of the general ward and might benefit from more intensive monitoring in an intensive care unit (ICU)‐like setting. In light of this problem, the intermediate care units (ImCU) may provide a cost‐effective alternative by providing higher levels of staffing tailored to patient needs, without incurring the cost of an ICU admission. The ImCU can reduce costs and improves ICU utilization for sicker patients, decrease ICU readmissions, promote greater flexibility in patient triage, and decrease mortality rates in hospital wards.18
The characteristics of ImCUs depend on resource availability, institutional infrastructure, and the organization and funding of the parent healthcare system. The ImCU may function as a step‐up or step‐down unit, or may provide specialty care for cardiac, neurologic, respiratory, or surgical conditions.811 These units can expand opportunities for co‐management and, at the same time, offer the occasion for training residents to follow up patients through different levels of care (from the general ward to ImCU). In the same way, the multidisciplinary approach of the ImCU can improve the center's teaching potential.
Characterizing the ImCU population requires the assessment of their severity of illness, which is crucial for the evaluation of risk‐adjusted outcomes. The present study evaluated the impact of a hospitalist‐led ImCU on observed‐to‐expected mortality ratios, as well as its role in co‐management and teaching.
PATIENTS AND METHODS
We performed a retrospective observational study, with data collected from April 2006 to April 2010 in a single academic medical center in Pamplona, Spain. The ImCU is a 9‐bed unit adjacent to, but independent from, the mixed ICU. Each bed is equipped with continuous telemetry, pulse oximetry, noninvasive arterial blood pressure, central venous pressure monitoring, and noninvasive pressure support ventilation. The signals are relayed to a central monitoring station and the nurse‐to‐patient ratio is 1:3.
The ImCU rounding team is multidisciplinary, and involves the hospital pharmacist, a nurse, the ImCU resident, the specialist or surgeon, and the attending hospitalist. After the triage process, ImCU patients were admitted to the attending hospitalist, who was responsible for admission and discharge of all ImCU patients. The hospitalist ordered diagnostic or therapeutic interventions as needed, with the exception of orders for procedures or consultations related with specialist/surgeon's specific needs.
Admission and discharge criteria for the ImCU were set according to guidelines defined by The American College of Critical Care Medicine,10 and also served as inclusion criteria for the present study. Exclusion criteria included: age less than 18 years old, severe respiratory failure, status epilepticus, and catastrophic brain illness. Patients admitted for drug administration and desensitization, and also ImCU readmissions, were excluded from data analysis. Patients came from medical and surgical wards, ICU, the operating room, and the emergency room.
A total of 756 patients were admitted to our ImCU during the study period. Patient demographics, past medical history, physiologic parameters at the time of admission, and survival to hospital discharge were recorded for all patients. Patient demographics include: age, gender, location before ImCU admission, length of stay before ImCU admission, reason for ImCU admission, anatomic site of surgery (if applicable), planned or unplanned admission, and infection status (nosocomial). Past medical history includes: the presence of arterial hypertension, diabetes, cirrhosis, chronic renal failure, chronic heart failure, cancer, hematological malignancy, chronic obstructive pulmonary disease (COPD), human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), immunosuppression, radiotherapy, chemotherapy, steroid treatment, and alcoholism. Physiologic parameters abstracted are described in Table 1. We used the Simplified Acute Physiology Score II (SAPS II),12 as a prognostic and severity score. SAPS II is the only previously validated score in intermediate care.13 In‐hospital mortality was the clinical outcome measured.
|
| Vital signs |
| Glasgow Coma Scale |
| Serum bilirubin |
| Serum creatinine |
| Urea nitrogen |
| Leucocyte count |
| Serum sodium |
| Serum potassium |
| Bicarbonate levels |
| Urinary output in the first 24 hr |
| Oxygenation and ventilatory support |
Data were entered into a computer database by the authors. Statistical analysis was not blinded, and was performed using SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean standard deviation or median (25%‐75% interquartile range). For nonparametric measure of statistical dependence of quantitative variables, we used Spearman's correlation coefficient. Discrimination was evaluated by calculating the area under receiver operating characteristic curve (AUROC).
The study protocol was approved by the institutional review board at the Clnica Universidad de Navarra in Pamplona, Spain.
RESULTS
Four hundred fifty‐six patients were included in data analysis. Three hundred patients were excluded: 61 low‐risk patients (drug administration and desensitization), 147 readmissions, and 92 patients for missing variables. Patient characteristics, including probability of death following ImCU admission and discharge location, are summarized in Table 2. The mean age was 65.6 years, and about 35% of patients had a SAPS II‐based risk of death higher than 25% at the time of ImCU admission. The median length of stay was 4 (3‐7) days.
| |
| Age (yr) | 65.6 14.3 |
| Gender | |
| Male | 283 (62.1%) |
| Female | 173 (37.9%) |
| Location prior to admission | |
| General ward | 252 (55.3%) |
| Emergency room | 96 (21.1%) |
| ICU | 63 (13.8%) |
| Operating room | 28 (6.1%) |
| Other hospital | 17 (3.7%) |
| Probability of in‐hospital mortality based on SAPS II | |
| <10% | 128 (28.1%) |
| 11%‐25% | 176 (38.6%) |
| 26%‐50% | 107 (23.4%) |
| >50% | 45 (9.9%) |
| Global expected mortality (in‐hospital) | 23.2% |
| Global observed mortality (in‐hospital) | 20.6% (94/456) |
| O/E mortality ratio | 0.89 |
| Discharge location | |
| General ward | 352/456 (77.2%) |
| ICU | 65/456 (14.3%) |
| Home | 1/456 (0.2%) |
| Other hospital | 11/456 (2.4%) |
| Death location | |
| ImCU | 27/456 (5.9%) |
| ICU (transferred patients) | 32/65* (49.2%) |
| General ward | 35/352* (9.9%) |
Outcomes
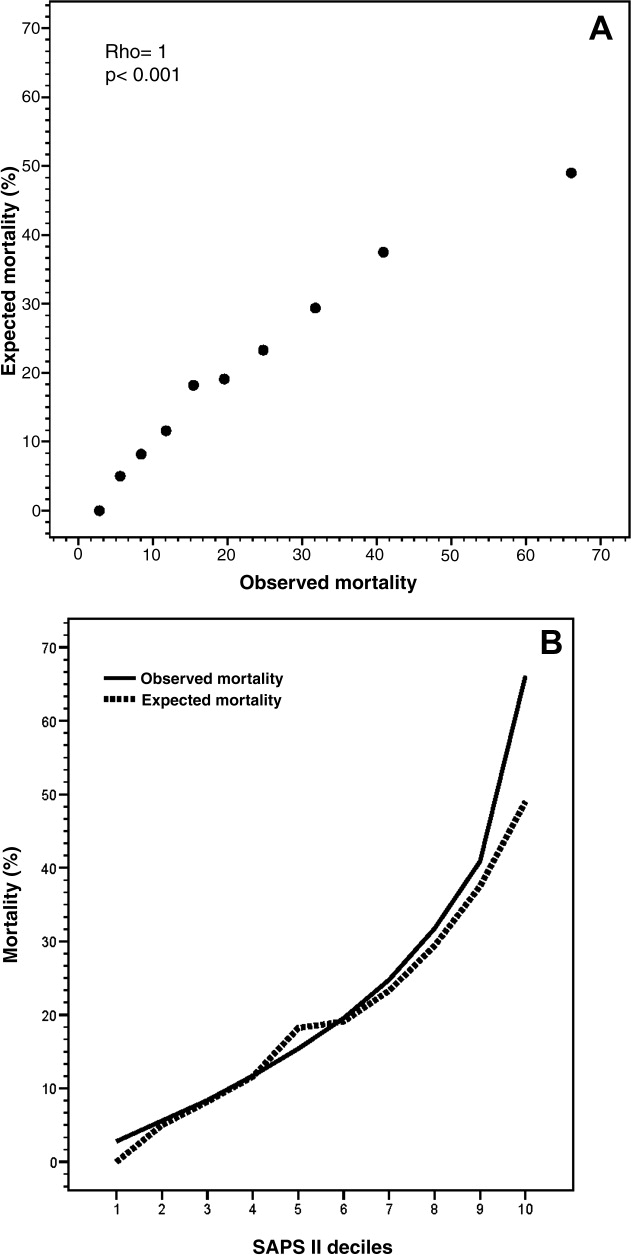
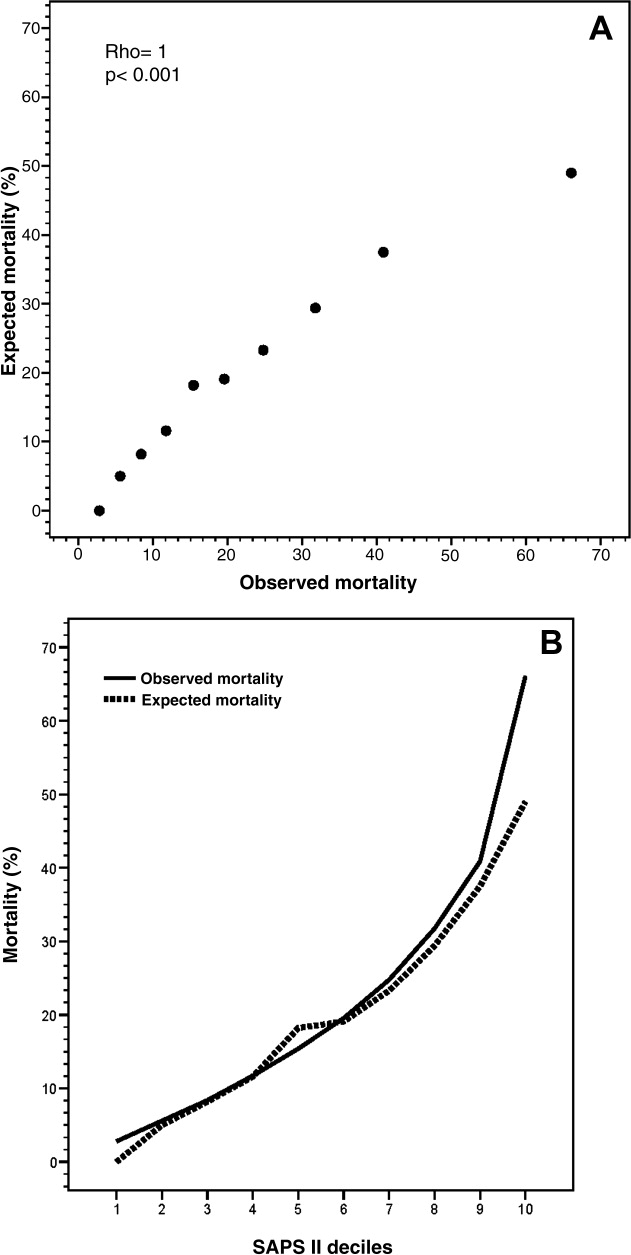
The mean SAPS II of the cohort was 37 12 points, and the expected mortality derived from this score was 23.2%. The observed in‐hospital mortality was 20.6% (94/456) resulting in an observed‐to‐expected mortality ratio of 0.89 (Table 2). Reasons for ImCU admission, as well as mortality ratios, are described in Table 3. The correlation between SAPS II predicted and observed death rates was accurate and statistically significant (Rho = 1.0, P < 0.001) (Figure 1). The AUROC for SAPS II predicting in‐hospital mortality was 0.75 (P < 0.001).
| Condition | Patients | SAPS II | Expected Mortality | Observed Mortality | O/E Ratio |
|---|---|---|---|---|---|
| |||||
| Respiratory failure | 153 (33.6%) | 36.1 9.7 | 21.5 15.3% | 25.5% (39) | 1.19 |
| Sepsis | 88 (19.3%) | 45.7 15.1 | 37.5 25.1% | 22.7% (20) | 0.61 |
| Cardiovascular | 72 (15.8%) | 35.7 11.0 | 21.3 16.6% | 23.6% (17) | 1.11 |
| Perioperative | 59 (12.9%) | 28.9 9.9 | 12.9 11.7% | 5.1% (3) | 0.40 |
| Complex monitoring | 34 (7.5%) | 33.2 12.1 | 19.1 16.3% | 14.7% (5) | 0.77 |
| GI complications | 33 (7.2%) | 32.1 8.3 | 15.6 10.7% | 12.1% (4) | 0.78 |
| Neurologic | 10 (2.2%) | 40.9 10.6 | 29.7 20.0% | 30.0% (3) | 1.01 |
| Liver failure | 7 (1.5%) | 42.1 17.2 | 30.9 29.4% | 42.9% (3) | 1.39 |
Co‐Management and Teaching
During the study period, 382/456 (83.8%) patients were co‐managed with 9 medical and 7 surgical teams (Table 4). From the period of 2006‐2008, a total of 37/106 (34.9%) patients were co‐managed with surgeons, and just 5/37 (13.5%) were co‐managed preoperatively before ImCU admission. In the next 2 years, the patient total increased to 69/106 (65.1%), and preoperative surgical co‐management significantly increased to 25/69 (36.2%) (P = 0.014).
| Medical | |||
|---|---|---|---|
| Surgical | |||
| |||
| Oncology | 100 (21.9%) | Neurology | 17 (3.7%) |
| Hepatology | 43 (9.4%) | Cardiology | 14 (3.1%) |
| Pulmonology | 36 (7.9%) | Nephrology | 14 (3.1%) |
| Hematology | 20 (4.4%) | Others | 13 (2.9%) |
| Gastroenterology | 19 (4.2%) | ||
| Total | 276 | ||
| General | 44 (9.6%) | Orthopedics | 6 (1.3%) |
| Vascular | 23 (5.0%) | Urology | 5 (1.1%) |
| Thoracic | 11 (2.4%) | Others | 10 (2.2%) |
| Neurosurgery | 7 (1.5%) | ||
| Total | 106 | ||
Our academic medical center enrolls 46 new residents every year. Since the creation of the ImCU in 2006, residents from different medical subspecialties and from general surgery received training in intermediate care and hospital medicine. All residents rotated into the ImCU for 1‐3 months working 8 hours a day. In 2006, when the unit was opened, 2 residents from internal medicine (4.3%) rotated in the ImCU. Thereafter, a significant increase in the number of training residents was observed, reaching 30.4% of the total resident pool (14/46) in 2010 (P = 0.002).
DISCUSSION
To the best of our knowledge, this is the first description of hospitalists in intermediate care. In Spain, where hospital medicine is early in development but expanding, critical and intermediate care units are usually staffed by intensivists or anesthesiologists. Staffing an ImCU with hospitalists, using a multidisciplinary co‐management model, is a novel staffing solution for acutely ill patients.
Approximately 35% of ICU patients are low risk, admitted mainly for monitoring purposes.9, 14 In contrast, some patients are treated on general wards when they should receive more intensive care and monitoring.15 Intermediate care units could improve cost containment and triage flexibility, while tailoring treatments according to patient needs. In general, ImCUs require lower nurse‐to‐patient ratios, and less expensive equipment and supplies than ICUs, while retaining the capability of responding appropriately to acute events.16 Moreover, patient and family satisfaction may be increased as a result of more liberal visitation policies and a less noisy environment.17
This study was not designed to measure the cost‐effectiveness of the ImCU. Surprisingly, there are few reports in the last 2 decades demonstrating the efficacy and cost containment of intermediate care. The majority of the studies were retrospective or uncontrolled observations.27 To our knowledge, only 1 randomized controlled trial1 and 1 multicenter prospective cost study exist.8 Further research is needed in this area, with larger, prospective randomized controlled trials, before the benefits and limitations of intermediate care can be fully determined.
Description of the ImCU patients depends on accurate severity scoring. The efficacy and reliability of these scores has been described only for ICU patients and their role for predicting mortality in the ImCU is uncertain. There is only 1 report using SAPS II in intermediate care, showing good discriminant power and calibration in a cohort of 433 patients.13 Auriant et al described, in that cohort, an observed mortality rate of 8.1% with an expected mortality rate of 8.7%.13 In contrast, our expected mortality rate was considerably higher (23.2%). Although ImCUs are generally created for low‐risk patients and monitoring purposes, our population was more similar to an ICU population, with very high risk for major complications and mortality.1823 The contribution of oncologic patients (22% of the total series; most of them with advanced disease, elevated SAPS II [42.2 13.6] and do‐not‐resuscitate orders), probably contributed to the higher acuity of our ImCU population. The correlation of our present data supports the value of SAPS II as a prognostic score in intermediate care, even for patients sicker than those reported by Auriant et al.13 Intermediate care is also a valuable setting to expand a co‐management model with different medical and surgical specialties.
Similarly, since the creation of the ImCU at our institution in 2006, there is a substantial increase in the number of residents rotating through our ImCU. Previous studies showed positive results of hospitalists as clinical educators in various settings.24, 25
In conclusion, intermediate care serves as an expansion of role for hospitalists at our institution; and clinicians, trainees, and patients may benefit from co‐management and teaching opportunities at this unique level of care. An ImCU led by hospitalists showed encouraging results in terms of observed‐to‐expected mortality ratios for acutely ill patients. SAPS II is a useful tool for prognostic evaluation of ImCU patients. However, results of this study should be confirmed with larger, prospective trials at multiple centers.
Acknowledgements
The authors thank Dr Efren Manjarrez for the final manuscript revision, and the ImCU Nursing Staff for their unconditional support in patient care.
- ,,,,,.The cost‐effectiveness of a special care unit to care for the chronically critically ill.J Nurs Adm.1995;25:47–53.
- ,.Noninvasive respiratory care unit. A cost‐effective solution for the future.Chest.1988;93:390–394.
- ,,,.The noninvasive respiratory care unit. Patterns of use and financial implications.Chest.1991;99:205–208.
- ,,,,,.Decreases in mortality on a large urban medical service by facilitating access to critical care. An alternative to rationing.Arch Intern Med.1988;148:1403–1405.
- ,,,.Impact of an intermediate care area on ICU utilization after cardiac surgery.Crit Care Med.1986;14:869–872.
- ,,.Closure of an intermediate care unit. Impact on critical care utilization.Chest.1993;104:876–881.
- ,.A case‐control study of patients readmitted to the intensive care unit.Crit Care Med.1993;21:1547–1553.
- ,,, et al.Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit.Respir Med.2005;99:894–900.
- ,,,,.A multicenter description of intermediate‐care patients. Comparison with ICU low‐risk monitor patients.Chest.2002;121:1253–1261.
- ,,, et al.Guidelines on admission and discharge for adult intermediate care units. American College of Critical Care Medicine of the Society of Critical Care Medicine.Crit Care Med.1998;26:607–610.
- ,.Do we need intermediate care units?Intensive Care Med.1999;25:1345–1349.
- ,,.A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study.JAMA.1993;270:2957–2963.
- ,,,,.Simplified acute physiology score II for measuring severity of illness in intermediate care units.Crit Care Med.1998;26:1368–1371.
- ,,,,,.The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost.Chest.1995;108:490–499.
- ,.Identifying patients with high risk of high cost.Chest1991;99:530–531.
- ,,.Structural models for intermediate care areas.Crit Care Med.1999;27:2266–2271.
- ,,,.Characteristics of pediatric intermediate care units in pediatric training programs.Crit Care Med.1991;19:1004–1007.
- ,,, et al.Prognostic performance and customization of the SAPS II: results of a multicenter Austrian study.Int Care Med.1999;25:192–197.
- ,,,,,.Comparison of Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II) scoring systems in a single Greek intensive care unit.Crit Care Med.2000;28:426–432.
- ,,,.External validation of the SAPS II, APACHE II and APACHE III prognostic models in South England: a multicentre study.Intensive Care Med.2003;29:249–256.
- ,,,,,.SAPS II revisited.Intensive Care Med.2005;31:416–423.
- ,,, et al.Mortality prediction using SAPS II: an update for French intensive care units.Crit Care.2005;9:R645–R652.
- ,,,.Predicting death and readmission after intensive care discharge.Br J Anaesth.2008;100:656–662.
- ,,, et al.Hospitalists as teachers.J Gen Intern Med.2004;19:8–15.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
Hospitalized patients are becoming increasingly complex. The care of such patients may be impacted by the limited resources of the general ward and might benefit from more intensive monitoring in an intensive care unit (ICU)‐like setting. In light of this problem, the intermediate care units (ImCU) may provide a cost‐effective alternative by providing higher levels of staffing tailored to patient needs, without incurring the cost of an ICU admission. The ImCU can reduce costs and improves ICU utilization for sicker patients, decrease ICU readmissions, promote greater flexibility in patient triage, and decrease mortality rates in hospital wards.18
The characteristics of ImCUs depend on resource availability, institutional infrastructure, and the organization and funding of the parent healthcare system. The ImCU may function as a step‐up or step‐down unit, or may provide specialty care for cardiac, neurologic, respiratory, or surgical conditions.811 These units can expand opportunities for co‐management and, at the same time, offer the occasion for training residents to follow up patients through different levels of care (from the general ward to ImCU). In the same way, the multidisciplinary approach of the ImCU can improve the center's teaching potential.
Characterizing the ImCU population requires the assessment of their severity of illness, which is crucial for the evaluation of risk‐adjusted outcomes. The present study evaluated the impact of a hospitalist‐led ImCU on observed‐to‐expected mortality ratios, as well as its role in co‐management and teaching.
PATIENTS AND METHODS
We performed a retrospective observational study, with data collected from April 2006 to April 2010 in a single academic medical center in Pamplona, Spain. The ImCU is a 9‐bed unit adjacent to, but independent from, the mixed ICU. Each bed is equipped with continuous telemetry, pulse oximetry, noninvasive arterial blood pressure, central venous pressure monitoring, and noninvasive pressure support ventilation. The signals are relayed to a central monitoring station and the nurse‐to‐patient ratio is 1:3.
The ImCU rounding team is multidisciplinary, and involves the hospital pharmacist, a nurse, the ImCU resident, the specialist or surgeon, and the attending hospitalist. After the triage process, ImCU patients were admitted to the attending hospitalist, who was responsible for admission and discharge of all ImCU patients. The hospitalist ordered diagnostic or therapeutic interventions as needed, with the exception of orders for procedures or consultations related with specialist/surgeon's specific needs.
Admission and discharge criteria for the ImCU were set according to guidelines defined by The American College of Critical Care Medicine,10 and also served as inclusion criteria for the present study. Exclusion criteria included: age less than 18 years old, severe respiratory failure, status epilepticus, and catastrophic brain illness. Patients admitted for drug administration and desensitization, and also ImCU readmissions, were excluded from data analysis. Patients came from medical and surgical wards, ICU, the operating room, and the emergency room.
A total of 756 patients were admitted to our ImCU during the study period. Patient demographics, past medical history, physiologic parameters at the time of admission, and survival to hospital discharge were recorded for all patients. Patient demographics include: age, gender, location before ImCU admission, length of stay before ImCU admission, reason for ImCU admission, anatomic site of surgery (if applicable), planned or unplanned admission, and infection status (nosocomial). Past medical history includes: the presence of arterial hypertension, diabetes, cirrhosis, chronic renal failure, chronic heart failure, cancer, hematological malignancy, chronic obstructive pulmonary disease (COPD), human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), immunosuppression, radiotherapy, chemotherapy, steroid treatment, and alcoholism. Physiologic parameters abstracted are described in Table 1. We used the Simplified Acute Physiology Score II (SAPS II),12 as a prognostic and severity score. SAPS II is the only previously validated score in intermediate care.13 In‐hospital mortality was the clinical outcome measured.
|
| Vital signs |
| Glasgow Coma Scale |
| Serum bilirubin |
| Serum creatinine |
| Urea nitrogen |
| Leucocyte count |
| Serum sodium |
| Serum potassium |
| Bicarbonate levels |
| Urinary output in the first 24 hr |
| Oxygenation and ventilatory support |
Data were entered into a computer database by the authors. Statistical analysis was not blinded, and was performed using SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean standard deviation or median (25%‐75% interquartile range). For nonparametric measure of statistical dependence of quantitative variables, we used Spearman's correlation coefficient. Discrimination was evaluated by calculating the area under receiver operating characteristic curve (AUROC).
The study protocol was approved by the institutional review board at the Clnica Universidad de Navarra in Pamplona, Spain.
RESULTS
Four hundred fifty‐six patients were included in data analysis. Three hundred patients were excluded: 61 low‐risk patients (drug administration and desensitization), 147 readmissions, and 92 patients for missing variables. Patient characteristics, including probability of death following ImCU admission and discharge location, are summarized in Table 2. The mean age was 65.6 years, and about 35% of patients had a SAPS II‐based risk of death higher than 25% at the time of ImCU admission. The median length of stay was 4 (3‐7) days.
| |
| Age (yr) | 65.6 14.3 |
| Gender | |
| Male | 283 (62.1%) |
| Female | 173 (37.9%) |
| Location prior to admission | |
| General ward | 252 (55.3%) |
| Emergency room | 96 (21.1%) |
| ICU | 63 (13.8%) |
| Operating room | 28 (6.1%) |
| Other hospital | 17 (3.7%) |
| Probability of in‐hospital mortality based on SAPS II | |
| <10% | 128 (28.1%) |
| 11%‐25% | 176 (38.6%) |
| 26%‐50% | 107 (23.4%) |
| >50% | 45 (9.9%) |
| Global expected mortality (in‐hospital) | 23.2% |
| Global observed mortality (in‐hospital) | 20.6% (94/456) |
| O/E mortality ratio | 0.89 |
| Discharge location | |
| General ward | 352/456 (77.2%) |
| ICU | 65/456 (14.3%) |
| Home | 1/456 (0.2%) |
| Other hospital | 11/456 (2.4%) |
| Death location | |
| ImCU | 27/456 (5.9%) |
| ICU (transferred patients) | 32/65* (49.2%) |
| General ward | 35/352* (9.9%) |
Outcomes
The mean SAPS II of the cohort was 37 12 points, and the expected mortality derived from this score was 23.2%. The observed in‐hospital mortality was 20.6% (94/456) resulting in an observed‐to‐expected mortality ratio of 0.89 (Table 2). Reasons for ImCU admission, as well as mortality ratios, are described in Table 3. The correlation between SAPS II predicted and observed death rates was accurate and statistically significant (Rho = 1.0, P < 0.001) (Figure 1). The AUROC for SAPS II predicting in‐hospital mortality was 0.75 (P < 0.001).

| Condition | Patients | SAPS II | Expected Mortality | Observed Mortality | O/E Ratio |
|---|---|---|---|---|---|
| |||||
| Respiratory failure | 153 (33.6%) | 36.1 9.7 | 21.5 15.3% | 25.5% (39) | 1.19 |
| Sepsis | 88 (19.3%) | 45.7 15.1 | 37.5 25.1% | 22.7% (20) | 0.61 |
| Cardiovascular | 72 (15.8%) | 35.7 11.0 | 21.3 16.6% | 23.6% (17) | 1.11 |
| Perioperative | 59 (12.9%) | 28.9 9.9 | 12.9 11.7% | 5.1% (3) | 0.40 |
| Complex monitoring | 34 (7.5%) | 33.2 12.1 | 19.1 16.3% | 14.7% (5) | 0.77 |
| GI complications | 33 (7.2%) | 32.1 8.3 | 15.6 10.7% | 12.1% (4) | 0.78 |
| Neurologic | 10 (2.2%) | 40.9 10.6 | 29.7 20.0% | 30.0% (3) | 1.01 |
| Liver failure | 7 (1.5%) | 42.1 17.2 | 30.9 29.4% | 42.9% (3) | 1.39 |
Co‐Management and Teaching
During the study period, 382/456 (83.8%) patients were co‐managed with 9 medical and 7 surgical teams (Table 4). From the period of 2006‐2008, a total of 37/106 (34.9%) patients were co‐managed with surgeons, and just 5/37 (13.5%) were co‐managed preoperatively before ImCU admission. In the next 2 years, the patient total increased to 69/106 (65.1%), and preoperative surgical co‐management significantly increased to 25/69 (36.2%) (P = 0.014).
| Medical | |||
|---|---|---|---|
| Surgical | |||
| |||
| Oncology | 100 (21.9%) | Neurology | 17 (3.7%) |
| Hepatology | 43 (9.4%) | Cardiology | 14 (3.1%) |
| Pulmonology | 36 (7.9%) | Nephrology | 14 (3.1%) |
| Hematology | 20 (4.4%) | Others | 13 (2.9%) |
| Gastroenterology | 19 (4.2%) | ||
| Total | 276 | ||
| General | 44 (9.6%) | Orthopedics | 6 (1.3%) |
| Vascular | 23 (5.0%) | Urology | 5 (1.1%) |
| Thoracic | 11 (2.4%) | Others | 10 (2.2%) |
| Neurosurgery | 7 (1.5%) | ||
| Total | 106 | ||
Our academic medical center enrolls 46 new residents every year. Since the creation of the ImCU in 2006, residents from different medical subspecialties and from general surgery received training in intermediate care and hospital medicine. All residents rotated into the ImCU for 1‐3 months working 8 hours a day. In 2006, when the unit was opened, 2 residents from internal medicine (4.3%) rotated in the ImCU. Thereafter, a significant increase in the number of training residents was observed, reaching 30.4% of the total resident pool (14/46) in 2010 (P = 0.002).
DISCUSSION
To the best of our knowledge, this is the first description of hospitalists in intermediate care. In Spain, where hospital medicine is early in development but expanding, critical and intermediate care units are usually staffed by intensivists or anesthesiologists. Staffing an ImCU with hospitalists, using a multidisciplinary co‐management model, is a novel staffing solution for acutely ill patients.
Approximately 35% of ICU patients are low risk, admitted mainly for monitoring purposes.9, 14 In contrast, some patients are treated on general wards when they should receive more intensive care and monitoring.15 Intermediate care units could improve cost containment and triage flexibility, while tailoring treatments according to patient needs. In general, ImCUs require lower nurse‐to‐patient ratios, and less expensive equipment and supplies than ICUs, while retaining the capability of responding appropriately to acute events.16 Moreover, patient and family satisfaction may be increased as a result of more liberal visitation policies and a less noisy environment.17
This study was not designed to measure the cost‐effectiveness of the ImCU. Surprisingly, there are few reports in the last 2 decades demonstrating the efficacy and cost containment of intermediate care. The majority of the studies were retrospective or uncontrolled observations.27 To our knowledge, only 1 randomized controlled trial1 and 1 multicenter prospective cost study exist.8 Further research is needed in this area, with larger, prospective randomized controlled trials, before the benefits and limitations of intermediate care can be fully determined.
Description of the ImCU patients depends on accurate severity scoring. The efficacy and reliability of these scores has been described only for ICU patients and their role for predicting mortality in the ImCU is uncertain. There is only 1 report using SAPS II in intermediate care, showing good discriminant power and calibration in a cohort of 433 patients.13 Auriant et al described, in that cohort, an observed mortality rate of 8.1% with an expected mortality rate of 8.7%.13 In contrast, our expected mortality rate was considerably higher (23.2%). Although ImCUs are generally created for low‐risk patients and monitoring purposes, our population was more similar to an ICU population, with very high risk for major complications and mortality.1823 The contribution of oncologic patients (22% of the total series; most of them with advanced disease, elevated SAPS II [42.2 13.6] and do‐not‐resuscitate orders), probably contributed to the higher acuity of our ImCU population. The correlation of our present data supports the value of SAPS II as a prognostic score in intermediate care, even for patients sicker than those reported by Auriant et al.13 Intermediate care is also a valuable setting to expand a co‐management model with different medical and surgical specialties.
Similarly, since the creation of the ImCU at our institution in 2006, there is a substantial increase in the number of residents rotating through our ImCU. Previous studies showed positive results of hospitalists as clinical educators in various settings.24, 25
In conclusion, intermediate care serves as an expansion of role for hospitalists at our institution; and clinicians, trainees, and patients may benefit from co‐management and teaching opportunities at this unique level of care. An ImCU led by hospitalists showed encouraging results in terms of observed‐to‐expected mortality ratios for acutely ill patients. SAPS II is a useful tool for prognostic evaluation of ImCU patients. However, results of this study should be confirmed with larger, prospective trials at multiple centers.
Acknowledgements
The authors thank Dr Efren Manjarrez for the final manuscript revision, and the ImCU Nursing Staff for their unconditional support in patient care.
Hospitalized patients are becoming increasingly complex. The care of such patients may be impacted by the limited resources of the general ward and might benefit from more intensive monitoring in an intensive care unit (ICU)‐like setting. In light of this problem, the intermediate care units (ImCU) may provide a cost‐effective alternative by providing higher levels of staffing tailored to patient needs, without incurring the cost of an ICU admission. The ImCU can reduce costs and improves ICU utilization for sicker patients, decrease ICU readmissions, promote greater flexibility in patient triage, and decrease mortality rates in hospital wards.18
The characteristics of ImCUs depend on resource availability, institutional infrastructure, and the organization and funding of the parent healthcare system. The ImCU may function as a step‐up or step‐down unit, or may provide specialty care for cardiac, neurologic, respiratory, or surgical conditions.811 These units can expand opportunities for co‐management and, at the same time, offer the occasion for training residents to follow up patients through different levels of care (from the general ward to ImCU). In the same way, the multidisciplinary approach of the ImCU can improve the center's teaching potential.
Characterizing the ImCU population requires the assessment of their severity of illness, which is crucial for the evaluation of risk‐adjusted outcomes. The present study evaluated the impact of a hospitalist‐led ImCU on observed‐to‐expected mortality ratios, as well as its role in co‐management and teaching.
PATIENTS AND METHODS
We performed a retrospective observational study, with data collected from April 2006 to April 2010 in a single academic medical center in Pamplona, Spain. The ImCU is a 9‐bed unit adjacent to, but independent from, the mixed ICU. Each bed is equipped with continuous telemetry, pulse oximetry, noninvasive arterial blood pressure, central venous pressure monitoring, and noninvasive pressure support ventilation. The signals are relayed to a central monitoring station and the nurse‐to‐patient ratio is 1:3.
The ImCU rounding team is multidisciplinary, and involves the hospital pharmacist, a nurse, the ImCU resident, the specialist or surgeon, and the attending hospitalist. After the triage process, ImCU patients were admitted to the attending hospitalist, who was responsible for admission and discharge of all ImCU patients. The hospitalist ordered diagnostic or therapeutic interventions as needed, with the exception of orders for procedures or consultations related with specialist/surgeon's specific needs.
Admission and discharge criteria for the ImCU were set according to guidelines defined by The American College of Critical Care Medicine,10 and also served as inclusion criteria for the present study. Exclusion criteria included: age less than 18 years old, severe respiratory failure, status epilepticus, and catastrophic brain illness. Patients admitted for drug administration and desensitization, and also ImCU readmissions, were excluded from data analysis. Patients came from medical and surgical wards, ICU, the operating room, and the emergency room.
A total of 756 patients were admitted to our ImCU during the study period. Patient demographics, past medical history, physiologic parameters at the time of admission, and survival to hospital discharge were recorded for all patients. Patient demographics include: age, gender, location before ImCU admission, length of stay before ImCU admission, reason for ImCU admission, anatomic site of surgery (if applicable), planned or unplanned admission, and infection status (nosocomial). Past medical history includes: the presence of arterial hypertension, diabetes, cirrhosis, chronic renal failure, chronic heart failure, cancer, hematological malignancy, chronic obstructive pulmonary disease (COPD), human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), immunosuppression, radiotherapy, chemotherapy, steroid treatment, and alcoholism. Physiologic parameters abstracted are described in Table 1. We used the Simplified Acute Physiology Score II (SAPS II),12 as a prognostic and severity score. SAPS II is the only previously validated score in intermediate care.13 In‐hospital mortality was the clinical outcome measured.
|
| Vital signs |
| Glasgow Coma Scale |
| Serum bilirubin |
| Serum creatinine |
| Urea nitrogen |
| Leucocyte count |
| Serum sodium |
| Serum potassium |
| Bicarbonate levels |
| Urinary output in the first 24 hr |
| Oxygenation and ventilatory support |
Data were entered into a computer database by the authors. Statistical analysis was not blinded, and was performed using SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean standard deviation or median (25%‐75% interquartile range). For nonparametric measure of statistical dependence of quantitative variables, we used Spearman's correlation coefficient. Discrimination was evaluated by calculating the area under receiver operating characteristic curve (AUROC).
The study protocol was approved by the institutional review board at the Clnica Universidad de Navarra in Pamplona, Spain.
RESULTS
Four hundred fifty‐six patients were included in data analysis. Three hundred patients were excluded: 61 low‐risk patients (drug administration and desensitization), 147 readmissions, and 92 patients for missing variables. Patient characteristics, including probability of death following ImCU admission and discharge location, are summarized in Table 2. The mean age was 65.6 years, and about 35% of patients had a SAPS II‐based risk of death higher than 25% at the time of ImCU admission. The median length of stay was 4 (3‐7) days.
| |
| Age (yr) | 65.6 14.3 |
| Gender | |
| Male | 283 (62.1%) |
| Female | 173 (37.9%) |
| Location prior to admission | |
| General ward | 252 (55.3%) |
| Emergency room | 96 (21.1%) |
| ICU | 63 (13.8%) |
| Operating room | 28 (6.1%) |
| Other hospital | 17 (3.7%) |
| Probability of in‐hospital mortality based on SAPS II | |
| <10% | 128 (28.1%) |
| 11%‐25% | 176 (38.6%) |
| 26%‐50% | 107 (23.4%) |
| >50% | 45 (9.9%) |
| Global expected mortality (in‐hospital) | 23.2% |
| Global observed mortality (in‐hospital) | 20.6% (94/456) |
| O/E mortality ratio | 0.89 |
| Discharge location | |
| General ward | 352/456 (77.2%) |
| ICU | 65/456 (14.3%) |
| Home | 1/456 (0.2%) |
| Other hospital | 11/456 (2.4%) |
| Death location | |
| ImCU | 27/456 (5.9%) |
| ICU (transferred patients) | 32/65* (49.2%) |
| General ward | 35/352* (9.9%) |
Outcomes
The mean SAPS II of the cohort was 37 12 points, and the expected mortality derived from this score was 23.2%. The observed in‐hospital mortality was 20.6% (94/456) resulting in an observed‐to‐expected mortality ratio of 0.89 (Table 2). Reasons for ImCU admission, as well as mortality ratios, are described in Table 3. The correlation between SAPS II predicted and observed death rates was accurate and statistically significant (Rho = 1.0, P < 0.001) (Figure 1). The AUROC for SAPS II predicting in‐hospital mortality was 0.75 (P < 0.001).

| Condition | Patients | SAPS II | Expected Mortality | Observed Mortality | O/E Ratio |
|---|---|---|---|---|---|
| |||||
| Respiratory failure | 153 (33.6%) | 36.1 9.7 | 21.5 15.3% | 25.5% (39) | 1.19 |
| Sepsis | 88 (19.3%) | 45.7 15.1 | 37.5 25.1% | 22.7% (20) | 0.61 |
| Cardiovascular | 72 (15.8%) | 35.7 11.0 | 21.3 16.6% | 23.6% (17) | 1.11 |
| Perioperative | 59 (12.9%) | 28.9 9.9 | 12.9 11.7% | 5.1% (3) | 0.40 |
| Complex monitoring | 34 (7.5%) | 33.2 12.1 | 19.1 16.3% | 14.7% (5) | 0.77 |
| GI complications | 33 (7.2%) | 32.1 8.3 | 15.6 10.7% | 12.1% (4) | 0.78 |
| Neurologic | 10 (2.2%) | 40.9 10.6 | 29.7 20.0% | 30.0% (3) | 1.01 |
| Liver failure | 7 (1.5%) | 42.1 17.2 | 30.9 29.4% | 42.9% (3) | 1.39 |
Co‐Management and Teaching
During the study period, 382/456 (83.8%) patients were co‐managed with 9 medical and 7 surgical teams (Table 4). From the period of 2006‐2008, a total of 37/106 (34.9%) patients were co‐managed with surgeons, and just 5/37 (13.5%) were co‐managed preoperatively before ImCU admission. In the next 2 years, the patient total increased to 69/106 (65.1%), and preoperative surgical co‐management significantly increased to 25/69 (36.2%) (P = 0.014).
| Medical | |||
|---|---|---|---|
| Surgical | |||
| |||
| Oncology | 100 (21.9%) | Neurology | 17 (3.7%) |
| Hepatology | 43 (9.4%) | Cardiology | 14 (3.1%) |
| Pulmonology | 36 (7.9%) | Nephrology | 14 (3.1%) |
| Hematology | 20 (4.4%) | Others | 13 (2.9%) |
| Gastroenterology | 19 (4.2%) | ||
| Total | 276 | ||
| General | 44 (9.6%) | Orthopedics | 6 (1.3%) |
| Vascular | 23 (5.0%) | Urology | 5 (1.1%) |
| Thoracic | 11 (2.4%) | Others | 10 (2.2%) |
| Neurosurgery | 7 (1.5%) | ||
| Total | 106 | ||
Our academic medical center enrolls 46 new residents every year. Since the creation of the ImCU in 2006, residents from different medical subspecialties and from general surgery received training in intermediate care and hospital medicine. All residents rotated into the ImCU for 1‐3 months working 8 hours a day. In 2006, when the unit was opened, 2 residents from internal medicine (4.3%) rotated in the ImCU. Thereafter, a significant increase in the number of training residents was observed, reaching 30.4% of the total resident pool (14/46) in 2010 (P = 0.002).
DISCUSSION
To the best of our knowledge, this is the first description of hospitalists in intermediate care. In Spain, where hospital medicine is early in development but expanding, critical and intermediate care units are usually staffed by intensivists or anesthesiologists. Staffing an ImCU with hospitalists, using a multidisciplinary co‐management model, is a novel staffing solution for acutely ill patients.
Approximately 35% of ICU patients are low risk, admitted mainly for monitoring purposes.9, 14 In contrast, some patients are treated on general wards when they should receive more intensive care and monitoring.15 Intermediate care units could improve cost containment and triage flexibility, while tailoring treatments according to patient needs. In general, ImCUs require lower nurse‐to‐patient ratios, and less expensive equipment and supplies than ICUs, while retaining the capability of responding appropriately to acute events.16 Moreover, patient and family satisfaction may be increased as a result of more liberal visitation policies and a less noisy environment.17
This study was not designed to measure the cost‐effectiveness of the ImCU. Surprisingly, there are few reports in the last 2 decades demonstrating the efficacy and cost containment of intermediate care. The majority of the studies were retrospective or uncontrolled observations.27 To our knowledge, only 1 randomized controlled trial1 and 1 multicenter prospective cost study exist.8 Further research is needed in this area, with larger, prospective randomized controlled trials, before the benefits and limitations of intermediate care can be fully determined.
Description of the ImCU patients depends on accurate severity scoring. The efficacy and reliability of these scores has been described only for ICU patients and their role for predicting mortality in the ImCU is uncertain. There is only 1 report using SAPS II in intermediate care, showing good discriminant power and calibration in a cohort of 433 patients.13 Auriant et al described, in that cohort, an observed mortality rate of 8.1% with an expected mortality rate of 8.7%.13 In contrast, our expected mortality rate was considerably higher (23.2%). Although ImCUs are generally created for low‐risk patients and monitoring purposes, our population was more similar to an ICU population, with very high risk for major complications and mortality.1823 The contribution of oncologic patients (22% of the total series; most of them with advanced disease, elevated SAPS II [42.2 13.6] and do‐not‐resuscitate orders), probably contributed to the higher acuity of our ImCU population. The correlation of our present data supports the value of SAPS II as a prognostic score in intermediate care, even for patients sicker than those reported by Auriant et al.13 Intermediate care is also a valuable setting to expand a co‐management model with different medical and surgical specialties.
Similarly, since the creation of the ImCU at our institution in 2006, there is a substantial increase in the number of residents rotating through our ImCU. Previous studies showed positive results of hospitalists as clinical educators in various settings.24, 25
In conclusion, intermediate care serves as an expansion of role for hospitalists at our institution; and clinicians, trainees, and patients may benefit from co‐management and teaching opportunities at this unique level of care. An ImCU led by hospitalists showed encouraging results in terms of observed‐to‐expected mortality ratios for acutely ill patients. SAPS II is a useful tool for prognostic evaluation of ImCU patients. However, results of this study should be confirmed with larger, prospective trials at multiple centers.
Acknowledgements
The authors thank Dr Efren Manjarrez for the final manuscript revision, and the ImCU Nursing Staff for their unconditional support in patient care.
- ,,,,,.The cost‐effectiveness of a special care unit to care for the chronically critically ill.J Nurs Adm.1995;25:47–53.
- ,.Noninvasive respiratory care unit. A cost‐effective solution for the future.Chest.1988;93:390–394.
- ,,,.The noninvasive respiratory care unit. Patterns of use and financial implications.Chest.1991;99:205–208.
- ,,,,,.Decreases in mortality on a large urban medical service by facilitating access to critical care. An alternative to rationing.Arch Intern Med.1988;148:1403–1405.
- ,,,.Impact of an intermediate care area on ICU utilization after cardiac surgery.Crit Care Med.1986;14:869–872.
- ,,.Closure of an intermediate care unit. Impact on critical care utilization.Chest.1993;104:876–881.
- ,.A case‐control study of patients readmitted to the intensive care unit.Crit Care Med.1993;21:1547–1553.
- ,,, et al.Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit.Respir Med.2005;99:894–900.
- ,,,,.A multicenter description of intermediate‐care patients. Comparison with ICU low‐risk monitor patients.Chest.2002;121:1253–1261.
- ,,, et al.Guidelines on admission and discharge for adult intermediate care units. American College of Critical Care Medicine of the Society of Critical Care Medicine.Crit Care Med.1998;26:607–610.
- ,.Do we need intermediate care units?Intensive Care Med.1999;25:1345–1349.
- ,,.A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study.JAMA.1993;270:2957–2963.
- ,,,,.Simplified acute physiology score II for measuring severity of illness in intermediate care units.Crit Care Med.1998;26:1368–1371.
- ,,,,,.The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost.Chest.1995;108:490–499.
- ,.Identifying patients with high risk of high cost.Chest1991;99:530–531.
- ,,.Structural models for intermediate care areas.Crit Care Med.1999;27:2266–2271.
- ,,,.Characteristics of pediatric intermediate care units in pediatric training programs.Crit Care Med.1991;19:1004–1007.
- ,,, et al.Prognostic performance and customization of the SAPS II: results of a multicenter Austrian study.Int Care Med.1999;25:192–197.
- ,,,,,.Comparison of Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II) scoring systems in a single Greek intensive care unit.Crit Care Med.2000;28:426–432.
- ,,,.External validation of the SAPS II, APACHE II and APACHE III prognostic models in South England: a multicentre study.Intensive Care Med.2003;29:249–256.
- ,,,,,.SAPS II revisited.Intensive Care Med.2005;31:416–423.
- ,,, et al.Mortality prediction using SAPS II: an update for French intensive care units.Crit Care.2005;9:R645–R652.
- ,,,.Predicting death and readmission after intensive care discharge.Br J Anaesth.2008;100:656–662.
- ,,, et al.Hospitalists as teachers.J Gen Intern Med.2004;19:8–15.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,,,,.The cost‐effectiveness of a special care unit to care for the chronically critically ill.J Nurs Adm.1995;25:47–53.
- ,.Noninvasive respiratory care unit. A cost‐effective solution for the future.Chest.1988;93:390–394.
- ,,,.The noninvasive respiratory care unit. Patterns of use and financial implications.Chest.1991;99:205–208.
- ,,,,,.Decreases in mortality on a large urban medical service by facilitating access to critical care. An alternative to rationing.Arch Intern Med.1988;148:1403–1405.
- ,,,.Impact of an intermediate care area on ICU utilization after cardiac surgery.Crit Care Med.1986;14:869–872.
- ,,.Closure of an intermediate care unit. Impact on critical care utilization.Chest.1993;104:876–881.
- ,.A case‐control study of patients readmitted to the intensive care unit.Crit Care Med.1993;21:1547–1553.
- ,,, et al.Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit.Respir Med.2005;99:894–900.
- ,,,,.A multicenter description of intermediate‐care patients. Comparison with ICU low‐risk monitor patients.Chest.2002;121:1253–1261.
- ,,, et al.Guidelines on admission and discharge for adult intermediate care units. American College of Critical Care Medicine of the Society of Critical Care Medicine.Crit Care Med.1998;26:607–610.
- ,.Do we need intermediate care units?Intensive Care Med.1999;25:1345–1349.
- ,,.A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study.JAMA.1993;270:2957–2963.
- ,,,,.Simplified acute physiology score II for measuring severity of illness in intermediate care units.Crit Care Med.1998;26:1368–1371.
- ,,,,,.The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost.Chest.1995;108:490–499.
- ,.Identifying patients with high risk of high cost.Chest1991;99:530–531.
- ,,.Structural models for intermediate care areas.Crit Care Med.1999;27:2266–2271.
- ,,,.Characteristics of pediatric intermediate care units in pediatric training programs.Crit Care Med.1991;19:1004–1007.
- ,,, et al.Prognostic performance and customization of the SAPS II: results of a multicenter Austrian study.Int Care Med.1999;25:192–197.
- ,,,,,.Comparison of Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II) scoring systems in a single Greek intensive care unit.Crit Care Med.2000;28:426–432.
- ,,,.External validation of the SAPS II, APACHE II and APACHE III prognostic models in South England: a multicentre study.Intensive Care Med.2003;29:249–256.
- ,,,,,.SAPS II revisited.Intensive Care Med.2005;31:416–423.
- ,,, et al.Mortality prediction using SAPS II: an update for French intensive care units.Crit Care.2005;9:R645–R652.
- ,,,.Predicting death and readmission after intensive care discharge.Br J Anaesth.2008;100:656–662.
- ,,, et al.Hospitalists as teachers.J Gen Intern Med.2004;19:8–15.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
Copyright © 2012 Society of Hospital Medicine