User login
FDA warns of serious liver injury from HCV drugs Viekira Pak and Technivie
The Food and Drug Administration has issued a safety alert warning of possible serious liver injury as a result of the hepatitis C drugs Viekira Pak and Technivie, the agency announced Oct. 22.
A review of adverse events reported to manufacturer AbbVie and to the FDA MedWatch program identified serious side effects in patients with underlying liver cirrhosis, including hepatic decompensation and liver failure, the FDA said in a statement. Some of these cases resulted in liver transplantation or death, they said.
“These serious outcomes were reported mostly in patients taking Viekira Pak who had evidence of advanced cirrhosis even before starting treatment with it,” the statement said.
At least 26 cases submitted to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program are believed to be linked to Viekira Pak or Technivie, with most liver injuries occurring within 1-4 weeks of starting treatment, the FDA said.
Additionally, some cases “occurred in patients for whom these medicines were contraindicated or not recommended,” they said.
Viekira Pak, approved in December 2014, is a fixed-dose combination of dasabuvir, ombitasvir, paritaprevir, and ritonavir taken with or without ribavirin. Technivie, approved in July 2015, is a fixed-dose combination of ombitasvir, paritaprevir, and ritonavir, used in combination with ribavirin.
The FDA recommends that clinicians closely monitor patients for signs of serious liver disease such as ascites, hepatic encephalopathy, variceal hemorrhage, and increases in direct bilirubin in the blood. Additionally, it recommends that patients contact their provider immediately if they develop symptoms of liver injury such as fatigue, weakness, loss of appetite, nausea, vomiting, yellow eyes or skin, and light-colored stools.
Patients and providers can report adverse events to the FDA’s MedWatch program.
The Food and Drug Administration has issued a safety alert warning of possible serious liver injury as a result of the hepatitis C drugs Viekira Pak and Technivie, the agency announced Oct. 22.
A review of adverse events reported to manufacturer AbbVie and to the FDA MedWatch program identified serious side effects in patients with underlying liver cirrhosis, including hepatic decompensation and liver failure, the FDA said in a statement. Some of these cases resulted in liver transplantation or death, they said.
“These serious outcomes were reported mostly in patients taking Viekira Pak who had evidence of advanced cirrhosis even before starting treatment with it,” the statement said.
At least 26 cases submitted to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program are believed to be linked to Viekira Pak or Technivie, with most liver injuries occurring within 1-4 weeks of starting treatment, the FDA said.
Additionally, some cases “occurred in patients for whom these medicines were contraindicated or not recommended,” they said.
Viekira Pak, approved in December 2014, is a fixed-dose combination of dasabuvir, ombitasvir, paritaprevir, and ritonavir taken with or without ribavirin. Technivie, approved in July 2015, is a fixed-dose combination of ombitasvir, paritaprevir, and ritonavir, used in combination with ribavirin.
The FDA recommends that clinicians closely monitor patients for signs of serious liver disease such as ascites, hepatic encephalopathy, variceal hemorrhage, and increases in direct bilirubin in the blood. Additionally, it recommends that patients contact their provider immediately if they develop symptoms of liver injury such as fatigue, weakness, loss of appetite, nausea, vomiting, yellow eyes or skin, and light-colored stools.
Patients and providers can report adverse events to the FDA’s MedWatch program.
The Food and Drug Administration has issued a safety alert warning of possible serious liver injury as a result of the hepatitis C drugs Viekira Pak and Technivie, the agency announced Oct. 22.
A review of adverse events reported to manufacturer AbbVie and to the FDA MedWatch program identified serious side effects in patients with underlying liver cirrhosis, including hepatic decompensation and liver failure, the FDA said in a statement. Some of these cases resulted in liver transplantation or death, they said.
“These serious outcomes were reported mostly in patients taking Viekira Pak who had evidence of advanced cirrhosis even before starting treatment with it,” the statement said.
At least 26 cases submitted to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program are believed to be linked to Viekira Pak or Technivie, with most liver injuries occurring within 1-4 weeks of starting treatment, the FDA said.
Additionally, some cases “occurred in patients for whom these medicines were contraindicated or not recommended,” they said.
Viekira Pak, approved in December 2014, is a fixed-dose combination of dasabuvir, ombitasvir, paritaprevir, and ritonavir taken with or without ribavirin. Technivie, approved in July 2015, is a fixed-dose combination of ombitasvir, paritaprevir, and ritonavir, used in combination with ribavirin.
The FDA recommends that clinicians closely monitor patients for signs of serious liver disease such as ascites, hepatic encephalopathy, variceal hemorrhage, and increases in direct bilirubin in the blood. Additionally, it recommends that patients contact their provider immediately if they develop symptoms of liver injury such as fatigue, weakness, loss of appetite, nausea, vomiting, yellow eyes or skin, and light-colored stools.
Patients and providers can report adverse events to the FDA’s MedWatch program.
High cholesterol predicts RA in women
High serum cholesterol levels predicted rheumatoid arthritis in women, but not in men, report Dr. Carl Turesson of the department of clinical sciences at Lund (Sweden) University and his associates.
A study of 290 patients (151 men, 139 women), who participated in the Malmö Preventive Medicine Program health survey and were subsequently diagnosed with rheumatoid arthritis, found that the women had higher total cholesterol levels at baseline, compared with controls (odds ratio, 1.54; 95% confidence interval, 1.22-1.94). The association remained statistically significant when adjusted for smoking and a history of early menopause, Dr. Turesson and his colleagues reported.
Cholesterol did not significantly affect risk of RA in men (OR, 1.03; 95 % CI, 0.83-1.26). Triglycerides were not a risk factor in men or women, the authors wrote.
The findings “suggest hormone-related metabolic pathways in the early pathogenesis of RA and may have implications for disease prevention and CVD risk management,” the investigators said in the report.
Read the full study in Arthritis Research and Therapy.
High serum cholesterol levels predicted rheumatoid arthritis in women, but not in men, report Dr. Carl Turesson of the department of clinical sciences at Lund (Sweden) University and his associates.
A study of 290 patients (151 men, 139 women), who participated in the Malmö Preventive Medicine Program health survey and were subsequently diagnosed with rheumatoid arthritis, found that the women had higher total cholesterol levels at baseline, compared with controls (odds ratio, 1.54; 95% confidence interval, 1.22-1.94). The association remained statistically significant when adjusted for smoking and a history of early menopause, Dr. Turesson and his colleagues reported.
Cholesterol did not significantly affect risk of RA in men (OR, 1.03; 95 % CI, 0.83-1.26). Triglycerides were not a risk factor in men or women, the authors wrote.
The findings “suggest hormone-related metabolic pathways in the early pathogenesis of RA and may have implications for disease prevention and CVD risk management,” the investigators said in the report.
Read the full study in Arthritis Research and Therapy.
High serum cholesterol levels predicted rheumatoid arthritis in women, but not in men, report Dr. Carl Turesson of the department of clinical sciences at Lund (Sweden) University and his associates.
A study of 290 patients (151 men, 139 women), who participated in the Malmö Preventive Medicine Program health survey and were subsequently diagnosed with rheumatoid arthritis, found that the women had higher total cholesterol levels at baseline, compared with controls (odds ratio, 1.54; 95% confidence interval, 1.22-1.94). The association remained statistically significant when adjusted for smoking and a history of early menopause, Dr. Turesson and his colleagues reported.
Cholesterol did not significantly affect risk of RA in men (OR, 1.03; 95 % CI, 0.83-1.26). Triglycerides were not a risk factor in men or women, the authors wrote.
The findings “suggest hormone-related metabolic pathways in the early pathogenesis of RA and may have implications for disease prevention and CVD risk management,” the investigators said in the report.
Read the full study in Arthritis Research and Therapy.
BMI Negatively Associated With Acne Lesion Counts in Postadolescent Women
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
BMI negatively associated with acne lesion counts in postadolescent women
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Study identifies possible bipolar I subtype with externalizing features
Bipolar disorder might include a distinct subphenotype characterized by externalizing symptoms and specific genetic variations, report Shanker Swaminathan, Ph.D., and his colleagues.
A study of 2,505 bipolar I disorder patients categorized participants into two groups: externalizing (those with comorbid externalizing disorders, plus a subgroup of patients with externalizing symptoms before 15 years of age) and nonexternalizing, wrote Dr. Swaminathan, formerly with the department of medical and molecular genetics at Indiana University, Indianapolis, and now with Baylor College of Medicine, Houston.
Patients in the externalizing group showed significant differences in clinical features from the nonexternalizing group in multiple areas, including earlier age of onset of bipolar disorder, depression, and mania (P less than .001).
Additionally, those in the externalizing group had a higher frequency of self-harm incidents (P less than .001) and suicide attempts (P less than .008). These patients also were more likely to report a history of rapid switching and cycling (P less than .001), the authors reported.
Genetic analysis found numerous “nominally associated” SNPs, though none reached genome-wide significance, Dr. Swaminathan and his colleagues reported.
The findings “suggest the presence of an externalizing disorder subphenotype within [bipolar I disorder] warranting further investigation,” they concluded.
Read the full study here: J Affect Disord. 2015 Jun;178:206-14.
Bipolar disorder might include a distinct subphenotype characterized by externalizing symptoms and specific genetic variations, report Shanker Swaminathan, Ph.D., and his colleagues.
A study of 2,505 bipolar I disorder patients categorized participants into two groups: externalizing (those with comorbid externalizing disorders, plus a subgroup of patients with externalizing symptoms before 15 years of age) and nonexternalizing, wrote Dr. Swaminathan, formerly with the department of medical and molecular genetics at Indiana University, Indianapolis, and now with Baylor College of Medicine, Houston.
Patients in the externalizing group showed significant differences in clinical features from the nonexternalizing group in multiple areas, including earlier age of onset of bipolar disorder, depression, and mania (P less than .001).
Additionally, those in the externalizing group had a higher frequency of self-harm incidents (P less than .001) and suicide attempts (P less than .008). These patients also were more likely to report a history of rapid switching and cycling (P less than .001), the authors reported.
Genetic analysis found numerous “nominally associated” SNPs, though none reached genome-wide significance, Dr. Swaminathan and his colleagues reported.
The findings “suggest the presence of an externalizing disorder subphenotype within [bipolar I disorder] warranting further investigation,” they concluded.
Read the full study here: J Affect Disord. 2015 Jun;178:206-14.
Bipolar disorder might include a distinct subphenotype characterized by externalizing symptoms and specific genetic variations, report Shanker Swaminathan, Ph.D., and his colleagues.
A study of 2,505 bipolar I disorder patients categorized participants into two groups: externalizing (those with comorbid externalizing disorders, plus a subgroup of patients with externalizing symptoms before 15 years of age) and nonexternalizing, wrote Dr. Swaminathan, formerly with the department of medical and molecular genetics at Indiana University, Indianapolis, and now with Baylor College of Medicine, Houston.
Patients in the externalizing group showed significant differences in clinical features from the nonexternalizing group in multiple areas, including earlier age of onset of bipolar disorder, depression, and mania (P less than .001).
Additionally, those in the externalizing group had a higher frequency of self-harm incidents (P less than .001) and suicide attempts (P less than .008). These patients also were more likely to report a history of rapid switching and cycling (P less than .001), the authors reported.
Genetic analysis found numerous “nominally associated” SNPs, though none reached genome-wide significance, Dr. Swaminathan and his colleagues reported.
The findings “suggest the presence of an externalizing disorder subphenotype within [bipolar I disorder] warranting further investigation,” they concluded.
Read the full study here: J Affect Disord. 2015 Jun;178:206-14.
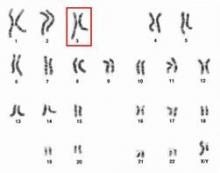
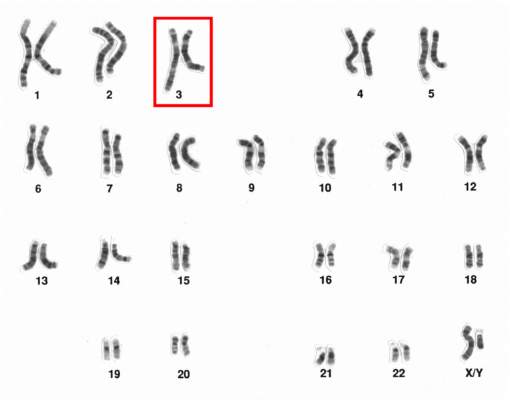
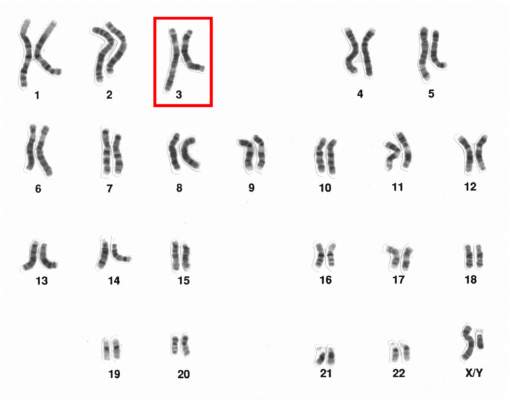
Chromosome 3 abnormalities linked to poor CML outcomes
Chromosome 3 abnormalities, specifically 3q26.2 rearrangements, were associated with treatment resistance and poor prognosis in patients with chronic myelogenous leukemia (CML), report Dr. Wei Wang and coauthors of the department of hematopathology at the University of Texas MD Anderson Cancer Center in Houston.
A study of 2,013 CML patients found that just 6% of those with 3q26.2 abnormalities achieved complete cytogenetic response during the course of tyrosine kinase inhibitor (TKI) treatment. Patients with other chromosome 3 abnormalities had a significantly better response rate of 42% the investigators found.
Additionally, patients with 3q26.2 chromosome rearrangements had significantly worse survival rates than those with abnormalities involving other chromosomes, with 2-year overall survival rates of 22% and 60%, respectively.
The lack of response to TKI treatment “raises the issue of how to manage these patients,” Dr. Wang and associates said in the report.
“TKIs themselves are not sufficient to control the disease with 3q26.2 abnormalities,” they added. “Intensive therapy, stem cell transplantation, or investigational therapy targeted to EVI1 should be considered,” concluded the authors, who declared that they had no competing financial interests.
Read the full article in Blood.
Chromosome 3 abnormalities, specifically 3q26.2 rearrangements, were associated with treatment resistance and poor prognosis in patients with chronic myelogenous leukemia (CML), report Dr. Wei Wang and coauthors of the department of hematopathology at the University of Texas MD Anderson Cancer Center in Houston.
A study of 2,013 CML patients found that just 6% of those with 3q26.2 abnormalities achieved complete cytogenetic response during the course of tyrosine kinase inhibitor (TKI) treatment. Patients with other chromosome 3 abnormalities had a significantly better response rate of 42% the investigators found.
Additionally, patients with 3q26.2 chromosome rearrangements had significantly worse survival rates than those with abnormalities involving other chromosomes, with 2-year overall survival rates of 22% and 60%, respectively.
The lack of response to TKI treatment “raises the issue of how to manage these patients,” Dr. Wang and associates said in the report.
“TKIs themselves are not sufficient to control the disease with 3q26.2 abnormalities,” they added. “Intensive therapy, stem cell transplantation, or investigational therapy targeted to EVI1 should be considered,” concluded the authors, who declared that they had no competing financial interests.
Read the full article in Blood.
Chromosome 3 abnormalities, specifically 3q26.2 rearrangements, were associated with treatment resistance and poor prognosis in patients with chronic myelogenous leukemia (CML), report Dr. Wei Wang and coauthors of the department of hematopathology at the University of Texas MD Anderson Cancer Center in Houston.
A study of 2,013 CML patients found that just 6% of those with 3q26.2 abnormalities achieved complete cytogenetic response during the course of tyrosine kinase inhibitor (TKI) treatment. Patients with other chromosome 3 abnormalities had a significantly better response rate of 42% the investigators found.
Additionally, patients with 3q26.2 chromosome rearrangements had significantly worse survival rates than those with abnormalities involving other chromosomes, with 2-year overall survival rates of 22% and 60%, respectively.
The lack of response to TKI treatment “raises the issue of how to manage these patients,” Dr. Wang and associates said in the report.
“TKIs themselves are not sufficient to control the disease with 3q26.2 abnormalities,” they added. “Intensive therapy, stem cell transplantation, or investigational therapy targeted to EVI1 should be considered,” concluded the authors, who declared that they had no competing financial interests.
Read the full article in Blood.
Chromosome 3 abnormalities linked to poor CML outcomes
Chromosome 3 abnormalities, specifically 3q26.2 rearrangements, were associated with treatment resistance and poor prognosis in patients with chronic myelogenous leukemia (CML), report Dr. Wei Wang and coauthors of the department of hematopathology at the University of Texas MD Anderson Cancer Center in Houston.
A study of 2,013 CML patients found that just 6% of those with 3q26.2 abnormalities achieved complete cytogenetic response during the course of tyrosine kinase inhibitor (TKI) treatment. Patients with other chromosome 3 abnormalities had a significantly better response rate of 42% the investigators found.
Additionally, patients with 3q26.2 chromosome rearrangements had significantly worse survival rates than those with abnormalities involving other chromosomes, with 2-year overall survival rates of 22% and 60%, respectively.
The lack of response to TKI treatment “raises the issue of how to manage these patients,” Dr. Wang and associates said in the report.
“TKIs themselves are not sufficient to control the disease with 3q26.2 abnormalities,” they added. “Intensive therapy, stem cell transplantation, or investigational therapy targeted to EVI1 should be considered,” concluded the authors, who declared that they had no competing financial interests.
Read the full article in Blood.
Chromosome 3 abnormalities, specifically 3q26.2 rearrangements, were associated with treatment resistance and poor prognosis in patients with chronic myelogenous leukemia (CML), report Dr. Wei Wang and coauthors of the department of hematopathology at the University of Texas MD Anderson Cancer Center in Houston.
A study of 2,013 CML patients found that just 6% of those with 3q26.2 abnormalities achieved complete cytogenetic response during the course of tyrosine kinase inhibitor (TKI) treatment. Patients with other chromosome 3 abnormalities had a significantly better response rate of 42% the investigators found.
Additionally, patients with 3q26.2 chromosome rearrangements had significantly worse survival rates than those with abnormalities involving other chromosomes, with 2-year overall survival rates of 22% and 60%, respectively.
The lack of response to TKI treatment “raises the issue of how to manage these patients,” Dr. Wang and associates said in the report.
“TKIs themselves are not sufficient to control the disease with 3q26.2 abnormalities,” they added. “Intensive therapy, stem cell transplantation, or investigational therapy targeted to EVI1 should be considered,” concluded the authors, who declared that they had no competing financial interests.
Read the full article in Blood.
Chromosome 3 abnormalities, specifically 3q26.2 rearrangements, were associated with treatment resistance and poor prognosis in patients with chronic myelogenous leukemia (CML), report Dr. Wei Wang and coauthors of the department of hematopathology at the University of Texas MD Anderson Cancer Center in Houston.
A study of 2,013 CML patients found that just 6% of those with 3q26.2 abnormalities achieved complete cytogenetic response during the course of tyrosine kinase inhibitor (TKI) treatment. Patients with other chromosome 3 abnormalities had a significantly better response rate of 42% the investigators found.
Additionally, patients with 3q26.2 chromosome rearrangements had significantly worse survival rates than those with abnormalities involving other chromosomes, with 2-year overall survival rates of 22% and 60%, respectively.
The lack of response to TKI treatment “raises the issue of how to manage these patients,” Dr. Wang and associates said in the report.
“TKIs themselves are not sufficient to control the disease with 3q26.2 abnormalities,” they added. “Intensive therapy, stem cell transplantation, or investigational therapy targeted to EVI1 should be considered,” concluded the authors, who declared that they had no competing financial interests.
Read the full article in Blood.
Clindamycin–Benzoyl Peroxide Combo Effective in Moderate and Severe Acne
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
Clindamycin–benzoyl peroxide combo effective in moderate and severe acne
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
PCORI approves $83 million for HCV, rare disease research
The Patient-Centered Outcomes Research Institute (PCORI) board has approved $83 million in funding for research on hepatitis C virus and other diseases, the group announced Sept. 28.
Two of the 26 awards, totaling $29.5 million, will be used towards studies on caring for HCV patients, PCORI said in a statement.
The HCV awards were the result of a “targeted funding opportunity PCORI issued in response to input from the health care community, which identified HCV infection as a top health concern,” the statement said. PCORI is an independent, nonprofit organization based in Washington.
Studies will focus on comparing different antiviral medication regimens and improving treatment adherence in injection drug users.
“New oral medications for HCV offer significant improvements over previous therapies, but they were tested in specialized settings with carefully selected groups of patients,” PCORI said. The group hopes the grants will help close these research gaps and provide evidence of long-term efficacy, so patients and providers can make informed treatment decisions.
The HCV trials “will involve national advocacy organizations, major professional associations, payers, or other key patient and stakeholder groups in their research design and implementation,” PCORI said.
Read more at the PCORI website.
The Patient-Centered Outcomes Research Institute (PCORI) board has approved $83 million in funding for research on hepatitis C virus and other diseases, the group announced Sept. 28.
Two of the 26 awards, totaling $29.5 million, will be used towards studies on caring for HCV patients, PCORI said in a statement.
The HCV awards were the result of a “targeted funding opportunity PCORI issued in response to input from the health care community, which identified HCV infection as a top health concern,” the statement said. PCORI is an independent, nonprofit organization based in Washington.
Studies will focus on comparing different antiviral medication regimens and improving treatment adherence in injection drug users.
“New oral medications for HCV offer significant improvements over previous therapies, but they were tested in specialized settings with carefully selected groups of patients,” PCORI said. The group hopes the grants will help close these research gaps and provide evidence of long-term efficacy, so patients and providers can make informed treatment decisions.
The HCV trials “will involve national advocacy organizations, major professional associations, payers, or other key patient and stakeholder groups in their research design and implementation,” PCORI said.
Read more at the PCORI website.
The Patient-Centered Outcomes Research Institute (PCORI) board has approved $83 million in funding for research on hepatitis C virus and other diseases, the group announced Sept. 28.
Two of the 26 awards, totaling $29.5 million, will be used towards studies on caring for HCV patients, PCORI said in a statement.
The HCV awards were the result of a “targeted funding opportunity PCORI issued in response to input from the health care community, which identified HCV infection as a top health concern,” the statement said. PCORI is an independent, nonprofit organization based in Washington.
Studies will focus on comparing different antiviral medication regimens and improving treatment adherence in injection drug users.
“New oral medications for HCV offer significant improvements over previous therapies, but they were tested in specialized settings with carefully selected groups of patients,” PCORI said. The group hopes the grants will help close these research gaps and provide evidence of long-term efficacy, so patients and providers can make informed treatment decisions.
The HCV trials “will involve national advocacy organizations, major professional associations, payers, or other key patient and stakeholder groups in their research design and implementation,” PCORI said.
Read more at the PCORI website.