User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Down syndrome arthritis: Distinct from JIA and missed in the clinic
ATLANTA – Pediatric Down syndrome arthritis is more aggressive and severe than juvenile idiopathic arthritis (JIA), but it’s underrecognized and undertreated, according to reports at the annual meeting of the American College of Rheumatology.
“The vast majority of parents don’t know their kids are at risk for arthritis,” and a lot of doctors don’t realize it, either. Meanwhile, children show up in the clinic a year or more into the process with irreversible joint damage, said pediatric rheumatologist Jordan Jones, DO, an assistant professor at the University of Missouri, Kansas City, and the lead investigator on a review of 36 children with Down syndrome (DS) in the national Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
One solution is to add routine musculoskeletal exams to American Academy of Pediatrics DS guidelines, something Dr. Jones said he and his colleagues are hoping to do.
Part of the problem is that children with DS have a hard time articulating and localizing pain, and it’s easy to attribute functional issues to DS itself. Charlene Foley, MD, PhD, from the National Centre for Paediatric Rheumatology in Dublin, said she’s seen “loads of cases” in which parents were told that their children were acting up, probably because of the DS, when they didn’t want to walk down stairs anymore or hold their parent’s hand.
She was the lead investigator on an Irish program that screened 503 DS children, about one-third of the country’s pediatric DS population, for arthritis; 33 cases were identified, including 18 new ones. Most of the children had polyarticular, rheumatoid factor–negative arthritis, and all of them were antinuclear antibody negative.
A key take-home from the work is that DS arthritis preferentially attacks the hands and wrists and was present exclusively in the hands and wrists of about one-third of the Irish cohort. “So, if you only have a second to examine a child or you can’t get them to sit still, just go straight for the hands, and have a low threshold for imaging,” Dr. Foley said.
DS arthritis is often considered a subtype of JIA, but findings from the studies call that into question and suggest the need for novel therapeutic targets, the investigators said.
The Irish team found that 42% of their subjects (14 of 33) had joint erosions, far more than the 14% of JIA children (3 of 21) who served as controls, and Dr. Foley and colleagues didn’t think that was solely because of delayed diagnosis. Also, at about 20 cases per 1,000, they estimated that arthritis was far more prevalent in DS than was JIA in the general pediatrics population.
Disease onset was at a mean of 7.1 years in Dr. Jones’ CARRA registry review, and mean delay to diagnosis was 11.5 months. The 36 children presented with an average of four affected joints. Only 22% (8 of 36) had elevated inflammatory markers; just one-third were positive for antinuclear antibody, and 17% for human leukocyte antigen B27. It means that “these kids can present with normal labs, even with very aggressive disease. The threshold of concern for arthritis has to be very high when you evaluate these children,” Dr. Jones said.
Treatment was initiated with disease-modifying antirheumatic drugs (DMARDs) in two-thirds of the registry children, often with a concomitant biologic, most commonly etanercept. Over half had at least one switch during a mean follow-up of 4.5 years; methotrexate was a leading culprit, frequently discontinued because of nausea and other problems, and biologics were changed for lack of effect. Active joint counts and physician assessments improved, but there were no significant changes in limited joint counts and health assessments.
In short, “the current therapies for JIA appear to be poorly tolerated, more toxic, and less effective in patients with Down syndrome. These kids don’t respond the same. They have a very high disease burden despite being treated aggressively,” Dr. Jones said.
That finding adds additional weight to the idea that DS arthritis is a distinct disease entity, with unique therapeutic targets. “Down syndrome has a lot of immunologic issues associated with it; maybe that’s it. I think in the next few years, we will be able to show that this is a different disease,” Dr. Jones said.
There was a boost in that direction from benchwork, also led and presented by Dr. Foley, that found significant immunologic, histologic, and genetic differences between JIA and DS arthritis, including lower CD19- and CD20-positive B-cell counts in DS arthritis and higher interferon-gamma and tumor necrosis factor–alpha production, greater synovial lining hyperplasia, and different minor allele frequencies.
There was no industry funding for the studies, and the investigators didn’t have any industry disclosures.
SOURCES: Jones J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2722; Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1817; and Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 781
ATLANTA – Pediatric Down syndrome arthritis is more aggressive and severe than juvenile idiopathic arthritis (JIA), but it’s underrecognized and undertreated, according to reports at the annual meeting of the American College of Rheumatology.
“The vast majority of parents don’t know their kids are at risk for arthritis,” and a lot of doctors don’t realize it, either. Meanwhile, children show up in the clinic a year or more into the process with irreversible joint damage, said pediatric rheumatologist Jordan Jones, DO, an assistant professor at the University of Missouri, Kansas City, and the lead investigator on a review of 36 children with Down syndrome (DS) in the national Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
One solution is to add routine musculoskeletal exams to American Academy of Pediatrics DS guidelines, something Dr. Jones said he and his colleagues are hoping to do.
Part of the problem is that children with DS have a hard time articulating and localizing pain, and it’s easy to attribute functional issues to DS itself. Charlene Foley, MD, PhD, from the National Centre for Paediatric Rheumatology in Dublin, said she’s seen “loads of cases” in which parents were told that their children were acting up, probably because of the DS, when they didn’t want to walk down stairs anymore or hold their parent’s hand.
She was the lead investigator on an Irish program that screened 503 DS children, about one-third of the country’s pediatric DS population, for arthritis; 33 cases were identified, including 18 new ones. Most of the children had polyarticular, rheumatoid factor–negative arthritis, and all of them were antinuclear antibody negative.
A key take-home from the work is that DS arthritis preferentially attacks the hands and wrists and was present exclusively in the hands and wrists of about one-third of the Irish cohort. “So, if you only have a second to examine a child or you can’t get them to sit still, just go straight for the hands, and have a low threshold for imaging,” Dr. Foley said.
DS arthritis is often considered a subtype of JIA, but findings from the studies call that into question and suggest the need for novel therapeutic targets, the investigators said.
The Irish team found that 42% of their subjects (14 of 33) had joint erosions, far more than the 14% of JIA children (3 of 21) who served as controls, and Dr. Foley and colleagues didn’t think that was solely because of delayed diagnosis. Also, at about 20 cases per 1,000, they estimated that arthritis was far more prevalent in DS than was JIA in the general pediatrics population.
Disease onset was at a mean of 7.1 years in Dr. Jones’ CARRA registry review, and mean delay to diagnosis was 11.5 months. The 36 children presented with an average of four affected joints. Only 22% (8 of 36) had elevated inflammatory markers; just one-third were positive for antinuclear antibody, and 17% for human leukocyte antigen B27. It means that “these kids can present with normal labs, even with very aggressive disease. The threshold of concern for arthritis has to be very high when you evaluate these children,” Dr. Jones said.
Treatment was initiated with disease-modifying antirheumatic drugs (DMARDs) in two-thirds of the registry children, often with a concomitant biologic, most commonly etanercept. Over half had at least one switch during a mean follow-up of 4.5 years; methotrexate was a leading culprit, frequently discontinued because of nausea and other problems, and biologics were changed for lack of effect. Active joint counts and physician assessments improved, but there were no significant changes in limited joint counts and health assessments.
In short, “the current therapies for JIA appear to be poorly tolerated, more toxic, and less effective in patients with Down syndrome. These kids don’t respond the same. They have a very high disease burden despite being treated aggressively,” Dr. Jones said.
That finding adds additional weight to the idea that DS arthritis is a distinct disease entity, with unique therapeutic targets. “Down syndrome has a lot of immunologic issues associated with it; maybe that’s it. I think in the next few years, we will be able to show that this is a different disease,” Dr. Jones said.
There was a boost in that direction from benchwork, also led and presented by Dr. Foley, that found significant immunologic, histologic, and genetic differences between JIA and DS arthritis, including lower CD19- and CD20-positive B-cell counts in DS arthritis and higher interferon-gamma and tumor necrosis factor–alpha production, greater synovial lining hyperplasia, and different minor allele frequencies.
There was no industry funding for the studies, and the investigators didn’t have any industry disclosures.
SOURCES: Jones J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2722; Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1817; and Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 781
ATLANTA – Pediatric Down syndrome arthritis is more aggressive and severe than juvenile idiopathic arthritis (JIA), but it’s underrecognized and undertreated, according to reports at the annual meeting of the American College of Rheumatology.
“The vast majority of parents don’t know their kids are at risk for arthritis,” and a lot of doctors don’t realize it, either. Meanwhile, children show up in the clinic a year or more into the process with irreversible joint damage, said pediatric rheumatologist Jordan Jones, DO, an assistant professor at the University of Missouri, Kansas City, and the lead investigator on a review of 36 children with Down syndrome (DS) in the national Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
One solution is to add routine musculoskeletal exams to American Academy of Pediatrics DS guidelines, something Dr. Jones said he and his colleagues are hoping to do.
Part of the problem is that children with DS have a hard time articulating and localizing pain, and it’s easy to attribute functional issues to DS itself. Charlene Foley, MD, PhD, from the National Centre for Paediatric Rheumatology in Dublin, said she’s seen “loads of cases” in which parents were told that their children were acting up, probably because of the DS, when they didn’t want to walk down stairs anymore or hold their parent’s hand.
She was the lead investigator on an Irish program that screened 503 DS children, about one-third of the country’s pediatric DS population, for arthritis; 33 cases were identified, including 18 new ones. Most of the children had polyarticular, rheumatoid factor–negative arthritis, and all of them were antinuclear antibody negative.
A key take-home from the work is that DS arthritis preferentially attacks the hands and wrists and was present exclusively in the hands and wrists of about one-third of the Irish cohort. “So, if you only have a second to examine a child or you can’t get them to sit still, just go straight for the hands, and have a low threshold for imaging,” Dr. Foley said.
DS arthritis is often considered a subtype of JIA, but findings from the studies call that into question and suggest the need for novel therapeutic targets, the investigators said.
The Irish team found that 42% of their subjects (14 of 33) had joint erosions, far more than the 14% of JIA children (3 of 21) who served as controls, and Dr. Foley and colleagues didn’t think that was solely because of delayed diagnosis. Also, at about 20 cases per 1,000, they estimated that arthritis was far more prevalent in DS than was JIA in the general pediatrics population.
Disease onset was at a mean of 7.1 years in Dr. Jones’ CARRA registry review, and mean delay to diagnosis was 11.5 months. The 36 children presented with an average of four affected joints. Only 22% (8 of 36) had elevated inflammatory markers; just one-third were positive for antinuclear antibody, and 17% for human leukocyte antigen B27. It means that “these kids can present with normal labs, even with very aggressive disease. The threshold of concern for arthritis has to be very high when you evaluate these children,” Dr. Jones said.
Treatment was initiated with disease-modifying antirheumatic drugs (DMARDs) in two-thirds of the registry children, often with a concomitant biologic, most commonly etanercept. Over half had at least one switch during a mean follow-up of 4.5 years; methotrexate was a leading culprit, frequently discontinued because of nausea and other problems, and biologics were changed for lack of effect. Active joint counts and physician assessments improved, but there were no significant changes in limited joint counts and health assessments.
In short, “the current therapies for JIA appear to be poorly tolerated, more toxic, and less effective in patients with Down syndrome. These kids don’t respond the same. They have a very high disease burden despite being treated aggressively,” Dr. Jones said.
That finding adds additional weight to the idea that DS arthritis is a distinct disease entity, with unique therapeutic targets. “Down syndrome has a lot of immunologic issues associated with it; maybe that’s it. I think in the next few years, we will be able to show that this is a different disease,” Dr. Jones said.
There was a boost in that direction from benchwork, also led and presented by Dr. Foley, that found significant immunologic, histologic, and genetic differences between JIA and DS arthritis, including lower CD19- and CD20-positive B-cell counts in DS arthritis and higher interferon-gamma and tumor necrosis factor–alpha production, greater synovial lining hyperplasia, and different minor allele frequencies.
There was no industry funding for the studies, and the investigators didn’t have any industry disclosures.
SOURCES: Jones J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2722; Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1817; and Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 781
REPORTING FROM ACR 2019
First autoimmune epilepsy RCT supports IVIG therapy
BALTIMORE –
Although the numbers of enrolled subjects was small, it was the first double-blind, placebo-controlled randomized trial in autoimmune epilepsy, the start of a level 1 evidence base. Until now, treatment has been based mostly on case reports and expert opinion. “We’ve clearly shown that immunotherapy works and that treating early makes a difference, much more so than antiseizure medications,” said lead author Divyanshu Dubey, MBBS, from the Mayo Clinic.
The lack of data has meant that “we couldn’t get insurance approval for IVIG, so people have generally leaned towards” high-dose intravenous steroids, which are problematic because LGI-1 antibody epilepsy is a disease of older people, in whom osteoporosis, underlying infections, and other problems complicate steroid use, Dr. Dubey said.
The trial also included three people with contactin-associated-protein-like-2 (CASPR2) antibody epilepsy, but they all wound up in the placebo arm, “so it’s hard to say anything about them,” Dr. Dubey said at the American Epilepsy Society annual meeting. The work was published shortly before the meeting (Ann Neurol. 2019 Nov 28. doi: 10.1002/ana.25655).
CASPR2 and LGI-1 are proteins found in brain cells; attack by antibodies triggers encephalitis and tens to hundreds of seizures per day. The seizures tend to diminish with time, but the cognitive damage caused by the encephalitis does not. “We’ve seen patients end up in nursing homes diagnosed with Alzheimer’s disease” because the conditions weren’t recognized and treated, Dr. Dubey said.
He and his team chose LGI-1 and CASPR2 epilepsy because of the potentially devastating consequences and because they are among the most common autoimmune epilepsies for which antibodies have been identified. There was also a hope that positive results might open up insurance coverage.
The trial randomized eight people to IVIG 0.5 g/kg on day 1; 1 g/kg on day 2; and 0.6 g/kg once at 3 and 5 weeks. Nine others were randomized to volume-matched IV saline placebo on the same schedule. After enrollment of 17 patients (LGI1-IgG, 14; CASPR2-IgG, 3) over 34 months, the study was terminated because of slow enrollment.
Although none of the LGI-1 subjects in the placebo group responded, two CASPR2 patients did, yielding an IVIG response rate of 75% versus 22% (2/9) in the placebo arm after week 5 (odds ratio, 10.5; 95% confidence interval, 1.1-98.9; P = .044).
Two of the LGI-1 subjects in the IVIG arm were completely seizure free after treatment. Results in both arms, meanwhile, did not correlate with concomitant antiseizure medications among those who were on them.
All eight IVIG patients showed stabilization or improvement in cognitive function, compared with two of five in the placebo arm, as gauged by Repeatable Battery for the Assessment of Neuropsychological Status scores. Patients in the IVIG arm gained a median of 3 points, while patients in the placebo arm lost a median of 1 point (P = .077).
At week 5, six patients with persistent seizures who were in the placebo group were switched to the IVIG regimen after unblinding; four (67%) reported more than a 50% reduction in seizures.
Responses did not correlate with LGI-1/CASPR2-IgG1-4 subclass, and there were no IVIG-associated adverse events. One IVIG patients fell because of a faciobrachial dystonic seizure, a classic sign of LGI-1 disease. Antibodies were not measured in the trial because they “do not correlate with severity of autoimmune epilepsy,” Dr. Dubey said.
The original plan was to enroll 30 subjects, but the investigators terminated the study after 18 because of slow enrollment. With knowledge of autoimmune epilepsy growing at Mayo, it was increasingly difficult to find immunotherapy-naive patients, he said.
All the subjects were between 60 and 70 years old, and the majority in both arms were men, which was not surprising because the conditions skew male, Dr. Dubey said. None of the patients had underlying tumors, which are known triggers of autoimmune epilepsy.
This work was funded by Grifols Shared Services, a maker of IVIG, and Option Care, a provider of home infusion equipment. Dr. Dubey said the company had no active role in the trial, but that the lack of insurance coverage for IVIG in autoimmune epilepsy was one of the drivers of the study. He disclosed research support from Grifols; another investigator is a consultant.
SOURCE: Dubey D et al. AES 2019, Abstract 1.292.
BALTIMORE –
Although the numbers of enrolled subjects was small, it was the first double-blind, placebo-controlled randomized trial in autoimmune epilepsy, the start of a level 1 evidence base. Until now, treatment has been based mostly on case reports and expert opinion. “We’ve clearly shown that immunotherapy works and that treating early makes a difference, much more so than antiseizure medications,” said lead author Divyanshu Dubey, MBBS, from the Mayo Clinic.
The lack of data has meant that “we couldn’t get insurance approval for IVIG, so people have generally leaned towards” high-dose intravenous steroids, which are problematic because LGI-1 antibody epilepsy is a disease of older people, in whom osteoporosis, underlying infections, and other problems complicate steroid use, Dr. Dubey said.
The trial also included three people with contactin-associated-protein-like-2 (CASPR2) antibody epilepsy, but they all wound up in the placebo arm, “so it’s hard to say anything about them,” Dr. Dubey said at the American Epilepsy Society annual meeting. The work was published shortly before the meeting (Ann Neurol. 2019 Nov 28. doi: 10.1002/ana.25655).
CASPR2 and LGI-1 are proteins found in brain cells; attack by antibodies triggers encephalitis and tens to hundreds of seizures per day. The seizures tend to diminish with time, but the cognitive damage caused by the encephalitis does not. “We’ve seen patients end up in nursing homes diagnosed with Alzheimer’s disease” because the conditions weren’t recognized and treated, Dr. Dubey said.
He and his team chose LGI-1 and CASPR2 epilepsy because of the potentially devastating consequences and because they are among the most common autoimmune epilepsies for which antibodies have been identified. There was also a hope that positive results might open up insurance coverage.
The trial randomized eight people to IVIG 0.5 g/kg on day 1; 1 g/kg on day 2; and 0.6 g/kg once at 3 and 5 weeks. Nine others were randomized to volume-matched IV saline placebo on the same schedule. After enrollment of 17 patients (LGI1-IgG, 14; CASPR2-IgG, 3) over 34 months, the study was terminated because of slow enrollment.
Although none of the LGI-1 subjects in the placebo group responded, two CASPR2 patients did, yielding an IVIG response rate of 75% versus 22% (2/9) in the placebo arm after week 5 (odds ratio, 10.5; 95% confidence interval, 1.1-98.9; P = .044).
Two of the LGI-1 subjects in the IVIG arm were completely seizure free after treatment. Results in both arms, meanwhile, did not correlate with concomitant antiseizure medications among those who were on them.
All eight IVIG patients showed stabilization or improvement in cognitive function, compared with two of five in the placebo arm, as gauged by Repeatable Battery for the Assessment of Neuropsychological Status scores. Patients in the IVIG arm gained a median of 3 points, while patients in the placebo arm lost a median of 1 point (P = .077).
At week 5, six patients with persistent seizures who were in the placebo group were switched to the IVIG regimen after unblinding; four (67%) reported more than a 50% reduction in seizures.
Responses did not correlate with LGI-1/CASPR2-IgG1-4 subclass, and there were no IVIG-associated adverse events. One IVIG patients fell because of a faciobrachial dystonic seizure, a classic sign of LGI-1 disease. Antibodies were not measured in the trial because they “do not correlate with severity of autoimmune epilepsy,” Dr. Dubey said.
The original plan was to enroll 30 subjects, but the investigators terminated the study after 18 because of slow enrollment. With knowledge of autoimmune epilepsy growing at Mayo, it was increasingly difficult to find immunotherapy-naive patients, he said.
All the subjects were between 60 and 70 years old, and the majority in both arms were men, which was not surprising because the conditions skew male, Dr. Dubey said. None of the patients had underlying tumors, which are known triggers of autoimmune epilepsy.
This work was funded by Grifols Shared Services, a maker of IVIG, and Option Care, a provider of home infusion equipment. Dr. Dubey said the company had no active role in the trial, but that the lack of insurance coverage for IVIG in autoimmune epilepsy was one of the drivers of the study. He disclosed research support from Grifols; another investigator is a consultant.
SOURCE: Dubey D et al. AES 2019, Abstract 1.292.
BALTIMORE –
Although the numbers of enrolled subjects was small, it was the first double-blind, placebo-controlled randomized trial in autoimmune epilepsy, the start of a level 1 evidence base. Until now, treatment has been based mostly on case reports and expert opinion. “We’ve clearly shown that immunotherapy works and that treating early makes a difference, much more so than antiseizure medications,” said lead author Divyanshu Dubey, MBBS, from the Mayo Clinic.
The lack of data has meant that “we couldn’t get insurance approval for IVIG, so people have generally leaned towards” high-dose intravenous steroids, which are problematic because LGI-1 antibody epilepsy is a disease of older people, in whom osteoporosis, underlying infections, and other problems complicate steroid use, Dr. Dubey said.
The trial also included three people with contactin-associated-protein-like-2 (CASPR2) antibody epilepsy, but they all wound up in the placebo arm, “so it’s hard to say anything about them,” Dr. Dubey said at the American Epilepsy Society annual meeting. The work was published shortly before the meeting (Ann Neurol. 2019 Nov 28. doi: 10.1002/ana.25655).
CASPR2 and LGI-1 are proteins found in brain cells; attack by antibodies triggers encephalitis and tens to hundreds of seizures per day. The seizures tend to diminish with time, but the cognitive damage caused by the encephalitis does not. “We’ve seen patients end up in nursing homes diagnosed with Alzheimer’s disease” because the conditions weren’t recognized and treated, Dr. Dubey said.
He and his team chose LGI-1 and CASPR2 epilepsy because of the potentially devastating consequences and because they are among the most common autoimmune epilepsies for which antibodies have been identified. There was also a hope that positive results might open up insurance coverage.
The trial randomized eight people to IVIG 0.5 g/kg on day 1; 1 g/kg on day 2; and 0.6 g/kg once at 3 and 5 weeks. Nine others were randomized to volume-matched IV saline placebo on the same schedule. After enrollment of 17 patients (LGI1-IgG, 14; CASPR2-IgG, 3) over 34 months, the study was terminated because of slow enrollment.
Although none of the LGI-1 subjects in the placebo group responded, two CASPR2 patients did, yielding an IVIG response rate of 75% versus 22% (2/9) in the placebo arm after week 5 (odds ratio, 10.5; 95% confidence interval, 1.1-98.9; P = .044).
Two of the LGI-1 subjects in the IVIG arm were completely seizure free after treatment. Results in both arms, meanwhile, did not correlate with concomitant antiseizure medications among those who were on them.
All eight IVIG patients showed stabilization or improvement in cognitive function, compared with two of five in the placebo arm, as gauged by Repeatable Battery for the Assessment of Neuropsychological Status scores. Patients in the IVIG arm gained a median of 3 points, while patients in the placebo arm lost a median of 1 point (P = .077).
At week 5, six patients with persistent seizures who were in the placebo group were switched to the IVIG regimen after unblinding; four (67%) reported more than a 50% reduction in seizures.
Responses did not correlate with LGI-1/CASPR2-IgG1-4 subclass, and there were no IVIG-associated adverse events. One IVIG patients fell because of a faciobrachial dystonic seizure, a classic sign of LGI-1 disease. Antibodies were not measured in the trial because they “do not correlate with severity of autoimmune epilepsy,” Dr. Dubey said.
The original plan was to enroll 30 subjects, but the investigators terminated the study after 18 because of slow enrollment. With knowledge of autoimmune epilepsy growing at Mayo, it was increasingly difficult to find immunotherapy-naive patients, he said.
All the subjects were between 60 and 70 years old, and the majority in both arms were men, which was not surprising because the conditions skew male, Dr. Dubey said. None of the patients had underlying tumors, which are known triggers of autoimmune epilepsy.
This work was funded by Grifols Shared Services, a maker of IVIG, and Option Care, a provider of home infusion equipment. Dr. Dubey said the company had no active role in the trial, but that the lack of insurance coverage for IVIG in autoimmune epilepsy was one of the drivers of the study. He disclosed research support from Grifols; another investigator is a consultant.
SOURCE: Dubey D et al. AES 2019, Abstract 1.292.
REPORTING FROM AES 2019
California researchers work to update EMS status epilepticus protocols
BALTIMORE – Investigators from the University of California, San Francisco, are working with medical directors across the state to update county emergency medical services protocols to ensure patients in status epilepticus get 10 mg IM midazolam in the field, per national treatment guidelines from the American Epilepsy Society.
The work comes in the wake of a recent research letter in JAMA where the UCSF team reported that, across 33 emergency medical services (EMS) in California, only 2 included 10 mg midazolam IM per the guidelines, advice based on randomized, controlled clinical trials that found it to be safe and effective for stopping prehospital seizures in adults.
“Making people aware of the problem [is having] an impact,” said investigator Elan Guterman, MD, a neurology hospitalist and assistant professor of neurology at the university.
In a follow-up review at the annual meeting of the American Epilepsy Society, the team took a deep dive into the situation in Alameda County, just east of San Francisco and including the city of Oakland, as an indicator of what’s been going on across the state.
Patients had to have an EMS record of active seizures, meaning more than two within 5 minutes or a single seizure lasting more than 5 minutes. Alameda ambulance crews, like most, carry intramuscular midazolam because it’s more shelf stable than the two other first-line options, lorazepam and diazepam, and doesn’t require an intravenous line.
Among the 2,494 adults treated for status epilepticus from 2013 to 2018, just 62% received intramuscular midazolam, and only 39% got a dose of 5 mg or more. Not a single patient received the recommended 10-mg IM injection.
In short, “at the time when it’s the most important to act quickly, patients were not receiving the care they needed,” and the problem isn’t likely limited to California, Dr. Guterman said.
When patients did get 5 mg or more, they were less likely to reseize and require additional doses (adjusted odds ratio, 0.59; 95% CI, 0.4-0.86). Also – and counterintuitively given the concern about benzodiazepines and respiratory depression – the team found that higher initial doses of 5 mg or more were actually associated with a lower need for respiratory support, including intubation (OR, 0.81; 95% CI, 0.67-0.99).
It’s possible ambulance crews were erring on the side of caution. People who got midazolam were more likely to have an established diagnosis of epilepsy (68% vs. 62%; P less than .01) and less likely to have been abusing drugs or alcohol (12.5% vs. 16.3%; P less than .01).
But an abundance of caution doesn’t fully explain it; even among people known to have epilepsy, many weren’t treated with midazolam and none at the appropriate dose.
Dr. Guterman thinks the bigger issue is what was reported in the research letter: Local EMS protocols simply haven’t been updated to include current best practices. EMS services might not even be aware of them, which is why she and her colleagues have been meeting with county medical directors.
“The first step is making sure the EMS world is aware of this gap in care, and motivating them to address it,” she said.
Patients in the study were a mean of 53 years old, and just over half were men.
There was no industry funding for the study, and Dr. Guterman didn’t report any relevant disclosures.
SOURCE: Guterman E et al. AES 2019, Abstract 1.394.
BALTIMORE – Investigators from the University of California, San Francisco, are working with medical directors across the state to update county emergency medical services protocols to ensure patients in status epilepticus get 10 mg IM midazolam in the field, per national treatment guidelines from the American Epilepsy Society.
The work comes in the wake of a recent research letter in JAMA where the UCSF team reported that, across 33 emergency medical services (EMS) in California, only 2 included 10 mg midazolam IM per the guidelines, advice based on randomized, controlled clinical trials that found it to be safe and effective for stopping prehospital seizures in adults.
“Making people aware of the problem [is having] an impact,” said investigator Elan Guterman, MD, a neurology hospitalist and assistant professor of neurology at the university.
In a follow-up review at the annual meeting of the American Epilepsy Society, the team took a deep dive into the situation in Alameda County, just east of San Francisco and including the city of Oakland, as an indicator of what’s been going on across the state.
Patients had to have an EMS record of active seizures, meaning more than two within 5 minutes or a single seizure lasting more than 5 minutes. Alameda ambulance crews, like most, carry intramuscular midazolam because it’s more shelf stable than the two other first-line options, lorazepam and diazepam, and doesn’t require an intravenous line.
Among the 2,494 adults treated for status epilepticus from 2013 to 2018, just 62% received intramuscular midazolam, and only 39% got a dose of 5 mg or more. Not a single patient received the recommended 10-mg IM injection.
In short, “at the time when it’s the most important to act quickly, patients were not receiving the care they needed,” and the problem isn’t likely limited to California, Dr. Guterman said.
When patients did get 5 mg or more, they were less likely to reseize and require additional doses (adjusted odds ratio, 0.59; 95% CI, 0.4-0.86). Also – and counterintuitively given the concern about benzodiazepines and respiratory depression – the team found that higher initial doses of 5 mg or more were actually associated with a lower need for respiratory support, including intubation (OR, 0.81; 95% CI, 0.67-0.99).
It’s possible ambulance crews were erring on the side of caution. People who got midazolam were more likely to have an established diagnosis of epilepsy (68% vs. 62%; P less than .01) and less likely to have been abusing drugs or alcohol (12.5% vs. 16.3%; P less than .01).
But an abundance of caution doesn’t fully explain it; even among people known to have epilepsy, many weren’t treated with midazolam and none at the appropriate dose.
Dr. Guterman thinks the bigger issue is what was reported in the research letter: Local EMS protocols simply haven’t been updated to include current best practices. EMS services might not even be aware of them, which is why she and her colleagues have been meeting with county medical directors.
“The first step is making sure the EMS world is aware of this gap in care, and motivating them to address it,” she said.
Patients in the study were a mean of 53 years old, and just over half were men.
There was no industry funding for the study, and Dr. Guterman didn’t report any relevant disclosures.
SOURCE: Guterman E et al. AES 2019, Abstract 1.394.
BALTIMORE – Investigators from the University of California, San Francisco, are working with medical directors across the state to update county emergency medical services protocols to ensure patients in status epilepticus get 10 mg IM midazolam in the field, per national treatment guidelines from the American Epilepsy Society.
The work comes in the wake of a recent research letter in JAMA where the UCSF team reported that, across 33 emergency medical services (EMS) in California, only 2 included 10 mg midazolam IM per the guidelines, advice based on randomized, controlled clinical trials that found it to be safe and effective for stopping prehospital seizures in adults.
“Making people aware of the problem [is having] an impact,” said investigator Elan Guterman, MD, a neurology hospitalist and assistant professor of neurology at the university.
In a follow-up review at the annual meeting of the American Epilepsy Society, the team took a deep dive into the situation in Alameda County, just east of San Francisco and including the city of Oakland, as an indicator of what’s been going on across the state.
Patients had to have an EMS record of active seizures, meaning more than two within 5 minutes or a single seizure lasting more than 5 minutes. Alameda ambulance crews, like most, carry intramuscular midazolam because it’s more shelf stable than the two other first-line options, lorazepam and diazepam, and doesn’t require an intravenous line.
Among the 2,494 adults treated for status epilepticus from 2013 to 2018, just 62% received intramuscular midazolam, and only 39% got a dose of 5 mg or more. Not a single patient received the recommended 10-mg IM injection.
In short, “at the time when it’s the most important to act quickly, patients were not receiving the care they needed,” and the problem isn’t likely limited to California, Dr. Guterman said.
When patients did get 5 mg or more, they were less likely to reseize and require additional doses (adjusted odds ratio, 0.59; 95% CI, 0.4-0.86). Also – and counterintuitively given the concern about benzodiazepines and respiratory depression – the team found that higher initial doses of 5 mg or more were actually associated with a lower need for respiratory support, including intubation (OR, 0.81; 95% CI, 0.67-0.99).
It’s possible ambulance crews were erring on the side of caution. People who got midazolam were more likely to have an established diagnosis of epilepsy (68% vs. 62%; P less than .01) and less likely to have been abusing drugs or alcohol (12.5% vs. 16.3%; P less than .01).
But an abundance of caution doesn’t fully explain it; even among people known to have epilepsy, many weren’t treated with midazolam and none at the appropriate dose.
Dr. Guterman thinks the bigger issue is what was reported in the research letter: Local EMS protocols simply haven’t been updated to include current best practices. EMS services might not even be aware of them, which is why she and her colleagues have been meeting with county medical directors.
“The first step is making sure the EMS world is aware of this gap in care, and motivating them to address it,” she said.
Patients in the study were a mean of 53 years old, and just over half were men.
There was no industry funding for the study, and Dr. Guterman didn’t report any relevant disclosures.
SOURCE: Guterman E et al. AES 2019, Abstract 1.394.
REPORTING FROM AES 2019
Hydroxychloroquine prevents congenital heart block recurrence in anti-Ro pregnancies
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
REPORTING FROM ACR 2019
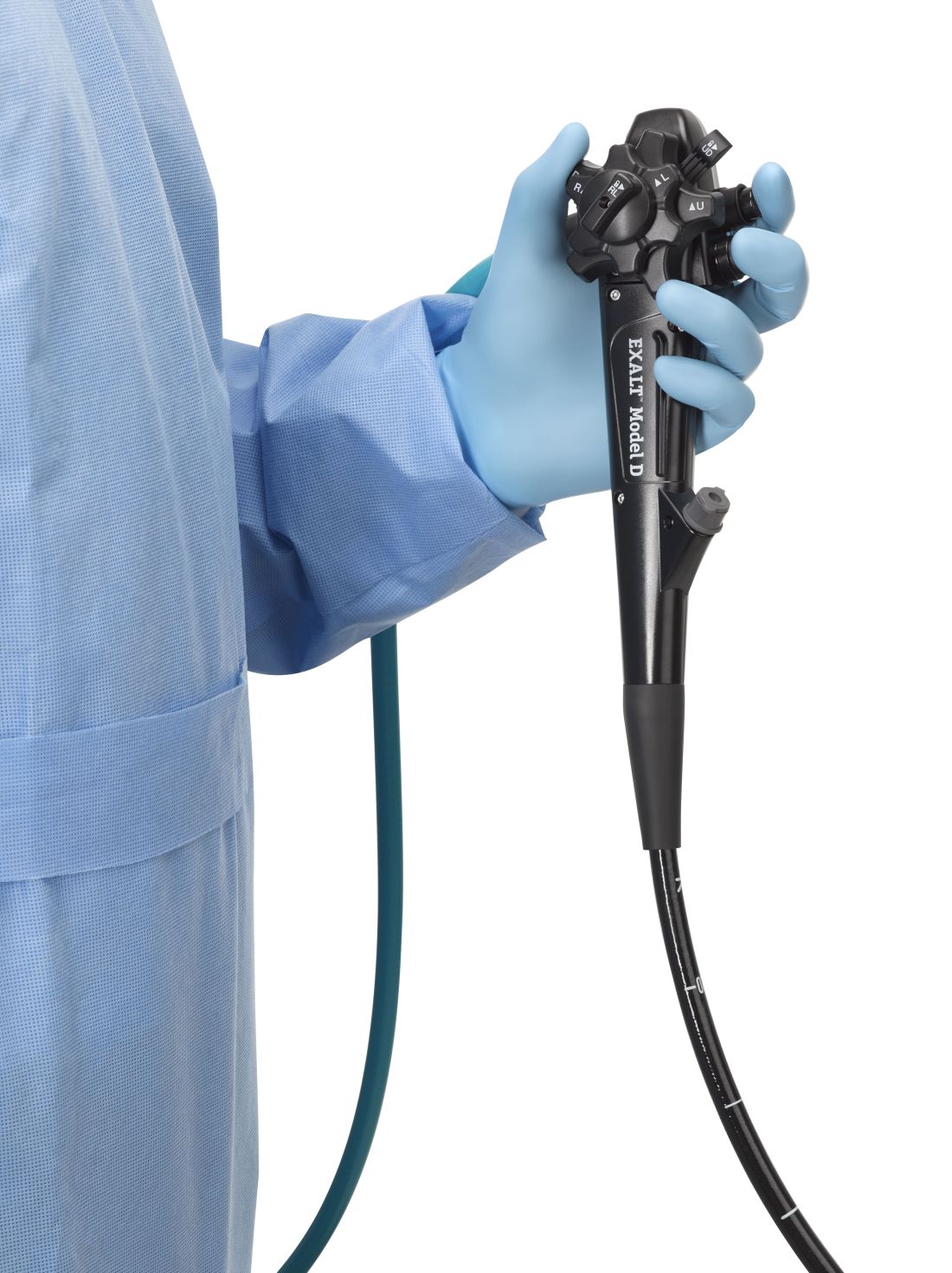
FDA clears first fully disposable duodenoscope
The Food and Drug Administration on Dec. 13 cleared Boston Scientific’s single-use duodenoscope, the Exalt Model D, for endoscopic retrograde cholangiopancreatography.
It’s the first disposable duodenoscope to hit the market in the wake of the agency’s August call for manufacturers and health care facilities to move to partially or fully disposable duodenoscopes. The goal is to eliminate the risk of spreading infections between patients from incomplete sterilization of traditional, multi-use scopes. The FDA also recently approved a Pentax duodenoscope with a disposable elevator, the most difficult part to clean.
The agency reported in April that 5.4% of samples from multi-use scopes test postive for Escherichia coli, Pseudomonas aeruginosa, or other “high-concern” organisms.
Boston Scientific spokesperson Kate Haranis said the Exalt Model D will be available in the first quarter of 2020, but the company is still working out how much it will charge.
Cost effectiveness will depend largely on the degree to which the price of the device is offset by the infections it prevents. It might prove particularly attractive to high-volume centers with higher than usual infection rates. It might also be of interest to smaller practices where the price of a multi-use scope doesn’t make sense for only a few procedures a year, said Gyanprakash Ketwaroo, MD, an interventional endoscopist and assistant professor of gastroenterology at Baylor University, Houston.
“The feel is a little different,” said Dr. Ketwaroo, who’s tried the new device, but “it’s pretty functional and probably okay to use in almost all endoscopic procedures that require ERCP.”
In a study funded by Boston Scientific, endoscopists reported a median overall satisfaction score of 9 out of 10 with the new scope (Clin Gastroenterol Hepatol. 2019 Nov 6. doi: 10.1016/j.cgh.2019.10.052).
As for using it at Baylor, Dr. Ketwaroo said, “we’re not sure yet; we are still evaluating it” and want to see if any problems emerge once it’s on the market. It’s also not clear if infection risks would be lower than with the disposable elevator model from Pentax, he added.
The Exalt Model D was granted breakthrough status by the FDA, and the agency worked closely with Boston Scientific to bring it to market.
“The availability of a fully disposable duodenoscope represents another major step forward for improving the safety of these devices, which are used in more than 500,000 procedures in the United States each year. The FDA continues to encourage innovative ways to improve the safety and effectiveness of these devices,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
Dr. Ketwaroo had no relevant financial disclosures.
The Food and Drug Administration on Dec. 13 cleared Boston Scientific’s single-use duodenoscope, the Exalt Model D, for endoscopic retrograde cholangiopancreatography.
It’s the first disposable duodenoscope to hit the market in the wake of the agency’s August call for manufacturers and health care facilities to move to partially or fully disposable duodenoscopes. The goal is to eliminate the risk of spreading infections between patients from incomplete sterilization of traditional, multi-use scopes. The FDA also recently approved a Pentax duodenoscope with a disposable elevator, the most difficult part to clean.
The agency reported in April that 5.4% of samples from multi-use scopes test postive for Escherichia coli, Pseudomonas aeruginosa, or other “high-concern” organisms.
Boston Scientific spokesperson Kate Haranis said the Exalt Model D will be available in the first quarter of 2020, but the company is still working out how much it will charge.
Cost effectiveness will depend largely on the degree to which the price of the device is offset by the infections it prevents. It might prove particularly attractive to high-volume centers with higher than usual infection rates. It might also be of interest to smaller practices where the price of a multi-use scope doesn’t make sense for only a few procedures a year, said Gyanprakash Ketwaroo, MD, an interventional endoscopist and assistant professor of gastroenterology at Baylor University, Houston.
“The feel is a little different,” said Dr. Ketwaroo, who’s tried the new device, but “it’s pretty functional and probably okay to use in almost all endoscopic procedures that require ERCP.”
In a study funded by Boston Scientific, endoscopists reported a median overall satisfaction score of 9 out of 10 with the new scope (Clin Gastroenterol Hepatol. 2019 Nov 6. doi: 10.1016/j.cgh.2019.10.052).
As for using it at Baylor, Dr. Ketwaroo said, “we’re not sure yet; we are still evaluating it” and want to see if any problems emerge once it’s on the market. It’s also not clear if infection risks would be lower than with the disposable elevator model from Pentax, he added.
The Exalt Model D was granted breakthrough status by the FDA, and the agency worked closely with Boston Scientific to bring it to market.
“The availability of a fully disposable duodenoscope represents another major step forward for improving the safety of these devices, which are used in more than 500,000 procedures in the United States each year. The FDA continues to encourage innovative ways to improve the safety and effectiveness of these devices,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
Dr. Ketwaroo had no relevant financial disclosures.
The Food and Drug Administration on Dec. 13 cleared Boston Scientific’s single-use duodenoscope, the Exalt Model D, for endoscopic retrograde cholangiopancreatography.
It’s the first disposable duodenoscope to hit the market in the wake of the agency’s August call for manufacturers and health care facilities to move to partially or fully disposable duodenoscopes. The goal is to eliminate the risk of spreading infections between patients from incomplete sterilization of traditional, multi-use scopes. The FDA also recently approved a Pentax duodenoscope with a disposable elevator, the most difficult part to clean.
The agency reported in April that 5.4% of samples from multi-use scopes test postive for Escherichia coli, Pseudomonas aeruginosa, or other “high-concern” organisms.
Boston Scientific spokesperson Kate Haranis said the Exalt Model D will be available in the first quarter of 2020, but the company is still working out how much it will charge.
Cost effectiveness will depend largely on the degree to which the price of the device is offset by the infections it prevents. It might prove particularly attractive to high-volume centers with higher than usual infection rates. It might also be of interest to smaller practices where the price of a multi-use scope doesn’t make sense for only a few procedures a year, said Gyanprakash Ketwaroo, MD, an interventional endoscopist and assistant professor of gastroenterology at Baylor University, Houston.
“The feel is a little different,” said Dr. Ketwaroo, who’s tried the new device, but “it’s pretty functional and probably okay to use in almost all endoscopic procedures that require ERCP.”
In a study funded by Boston Scientific, endoscopists reported a median overall satisfaction score of 9 out of 10 with the new scope (Clin Gastroenterol Hepatol. 2019 Nov 6. doi: 10.1016/j.cgh.2019.10.052).
As for using it at Baylor, Dr. Ketwaroo said, “we’re not sure yet; we are still evaluating it” and want to see if any problems emerge once it’s on the market. It’s also not clear if infection risks would be lower than with the disposable elevator model from Pentax, he added.
The Exalt Model D was granted breakthrough status by the FDA, and the agency worked closely with Boston Scientific to bring it to market.
“The availability of a fully disposable duodenoscope represents another major step forward for improving the safety of these devices, which are used in more than 500,000 procedures in the United States each year. The FDA continues to encourage innovative ways to improve the safety and effectiveness of these devices,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
Dr. Ketwaroo had no relevant financial disclosures.
FROM THE FDA
FDA clears first fully disposable duodenoscope
The Food and Drug Administration on Dec. 13 cleared Boston Scientific’s single-use duodenoscope, the Exalt Model D, for endoscopic retrograde cholangiopancreatography.
It’s the first disposable duodenoscope to hit the market in the wake of the agency’s August call for manufacturers and health care facilities to move to partially or fully disposable duodenoscopes. The goal is to eliminate the risk of spreading infections between patients from incomplete sterilization of traditional, multi-use scopes. The FDA also recently approved a Pentax duodenoscope with a disposable elevator, the most difficult part to clean.
The agency reported in April that 5.4% of samples from multi-use scopes test positive for Escherichia coli, Pseudomonas aeruginosa, or other “high-concern” organisms.
Boston Scientific spokesperson Kate Haranis said the Exalt Model D will be available in the first quarter of 2020, but the company is still working out how much it will charge.
Cost effectiveness will depend largely on the degree to which the price of the device is offset by the infections it prevents. It might prove particularly attractive to high-volume centers with higher than usual infection rates. It might also be of interest to smaller practices where the price of a multi-use scope doesn’t make sense for only a few procedures a year, said Gyanprakash Ketwaroo, MD, an interventional endoscopist and assistant professor of gastroenterology at Baylor University, Houston.
AGA is working with FDA to ensure physicians continue to have access to ERCP as new devices are introduced to the market and will continue to update members on the latest developments. The GI societies believe that device transitions can be incorporated over the life cycle of current instrumentation, to eliminate the potential for gaps in accessibility of care and to ensure that there is adequate efficacy and safety data to support the adoption of new technology. Review the GI societies’ guiding principles for continued scope evolution at https://www.gastro.org/news/gi-societies-advise-fda-on-duodenoscope-reprocessing.
Dr. Ketwaroo had no relevant financial disclosures.
The Food and Drug Administration on Dec. 13 cleared Boston Scientific’s single-use duodenoscope, the Exalt Model D, for endoscopic retrograde cholangiopancreatography.
It’s the first disposable duodenoscope to hit the market in the wake of the agency’s August call for manufacturers and health care facilities to move to partially or fully disposable duodenoscopes. The goal is to eliminate the risk of spreading infections between patients from incomplete sterilization of traditional, multi-use scopes. The FDA also recently approved a Pentax duodenoscope with a disposable elevator, the most difficult part to clean.
The agency reported in April that 5.4% of samples from multi-use scopes test positive for Escherichia coli, Pseudomonas aeruginosa, or other “high-concern” organisms.
Boston Scientific spokesperson Kate Haranis said the Exalt Model D will be available in the first quarter of 2020, but the company is still working out how much it will charge.
Cost effectiveness will depend largely on the degree to which the price of the device is offset by the infections it prevents. It might prove particularly attractive to high-volume centers with higher than usual infection rates. It might also be of interest to smaller practices where the price of a multi-use scope doesn’t make sense for only a few procedures a year, said Gyanprakash Ketwaroo, MD, an interventional endoscopist and assistant professor of gastroenterology at Baylor University, Houston.
AGA is working with FDA to ensure physicians continue to have access to ERCP as new devices are introduced to the market and will continue to update members on the latest developments. The GI societies believe that device transitions can be incorporated over the life cycle of current instrumentation, to eliminate the potential for gaps in accessibility of care and to ensure that there is adequate efficacy and safety data to support the adoption of new technology. Review the GI societies’ guiding principles for continued scope evolution at https://www.gastro.org/news/gi-societies-advise-fda-on-duodenoscope-reprocessing.
Dr. Ketwaroo had no relevant financial disclosures.
The Food and Drug Administration on Dec. 13 cleared Boston Scientific’s single-use duodenoscope, the Exalt Model D, for endoscopic retrograde cholangiopancreatography.
It’s the first disposable duodenoscope to hit the market in the wake of the agency’s August call for manufacturers and health care facilities to move to partially or fully disposable duodenoscopes. The goal is to eliminate the risk of spreading infections between patients from incomplete sterilization of traditional, multi-use scopes. The FDA also recently approved a Pentax duodenoscope with a disposable elevator, the most difficult part to clean.
The agency reported in April that 5.4% of samples from multi-use scopes test positive for Escherichia coli, Pseudomonas aeruginosa, or other “high-concern” organisms.
Boston Scientific spokesperson Kate Haranis said the Exalt Model D will be available in the first quarter of 2020, but the company is still working out how much it will charge.
Cost effectiveness will depend largely on the degree to which the price of the device is offset by the infections it prevents. It might prove particularly attractive to high-volume centers with higher than usual infection rates. It might also be of interest to smaller practices where the price of a multi-use scope doesn’t make sense for only a few procedures a year, said Gyanprakash Ketwaroo, MD, an interventional endoscopist and assistant professor of gastroenterology at Baylor University, Houston.
AGA is working with FDA to ensure physicians continue to have access to ERCP as new devices are introduced to the market and will continue to update members on the latest developments. The GI societies believe that device transitions can be incorporated over the life cycle of current instrumentation, to eliminate the potential for gaps in accessibility of care and to ensure that there is adequate efficacy and safety data to support the adoption of new technology. Review the GI societies’ guiding principles for continued scope evolution at https://www.gastro.org/news/gi-societies-advise-fda-on-duodenoscope-reprocessing.
Dr. Ketwaroo had no relevant financial disclosures.
ADA2 is a potent new biomarker for macrophage activation syndrome
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
REPORTING FROM ACR 2019
High infantile spasm risk should contraindicate sodium channel blocker antiepileptics
BALTIMORE – “This is scary and warrants caution,” said senior investigator and pediatric neurologist Shaun Hussain, MD, a pediatric neurologist at Mattel Children’s Hospital at UCLA. Because of the findings, “we are avoiding the use of voltage-gated sodium channel blockade in any child at risk for infantile spasms. More broadly, we are avoiding [them] in any infant if there is a good alternative medication, of which there are many in most cases.”
There have been a few previous case reports linking voltage-gated sodium channel blockers (SCBs) – which include oxcarbazepine, carbamazepine, lacosamide, and phenytoin – to infantile spasms, but they are still commonly used for infant seizures. There was some disagreement at UCLA whether there really was a link, so Dr. Hussain and his team took a look at the university’s experience. They matched 50 children with nonsyndromic epilepsy who subsequently developed video-EEG confirmed infantile spasms (cases) to 50 children who also had nonsyndromic epilepsy but did not develop spasms, based on follow-up duration and age and date of epilepsy onset.
The team then looked to see what drugs they had been on; it turned out that cases and controls were about equally as likely to have been treated with any specific antiepileptic, including SCBs. Infantile spasms were substantially more likely with SCB exposure in children with spasm risk factors, which also include focal cortical dysplasia, Aicardi syndrome, and other problems (HR 7.0; 95%; CI 2.5-19.8; P less than .001). Spasms were also more likely among even low-risk children treated with SCBs, although the trend was not statistically significant.
In the end, “we wonder how many cases of infantile spasms could [have been] prevented entirely if we had avoided sodium channel blockade,” Dr. Hussain said at the annual meeting of the American Epilepsy Society.
With so many other seizure options available – levetiracetam, topiramate, and phenobarbital, to name just a few – maybe it would be best “to stay away from” SCBs entirely in “infants with any form of epilepsy,” said lead investigator Jaeden Heesch, an undergraduate researcher who worked with Dr. Hussain.
It is unclear why SCBs increase infantile spasm risk; maybe nonselective voltage-gated sodium channel blockade interferes with proper neuron function in susceptible children, similar to the effects of sodium voltage-gated channel alpha subunit 1 mutations in Dravet syndrome, Dr. Hussain said. Perhaps the findings will inspire drug development. “If nonselective sodium channel blockade is bad, perhaps selective modulation of voltage-gated sodium currents [could be] beneficial or protective,” he said.
The age of epilepsy onset in the study was around 2 months. Children who went on to develop infantile spasms had an average of almost two seizures per day, versus fewer than one among controls, and were on an average of two, versus about 1.5 antiepileptics. The differences were not statistically significant.
The study looked at SCB exposure overall, but it’s possible that infantile spasm risk differs among the various class members.
The work was funded by the Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute. The investigators didn’t have any relevant disclosures.
SOURCE: Heesch J et al. AES 2019. Abstract 2.234.
BALTIMORE – “This is scary and warrants caution,” said senior investigator and pediatric neurologist Shaun Hussain, MD, a pediatric neurologist at Mattel Children’s Hospital at UCLA. Because of the findings, “we are avoiding the use of voltage-gated sodium channel blockade in any child at risk for infantile spasms. More broadly, we are avoiding [them] in any infant if there is a good alternative medication, of which there are many in most cases.”
There have been a few previous case reports linking voltage-gated sodium channel blockers (SCBs) – which include oxcarbazepine, carbamazepine, lacosamide, and phenytoin – to infantile spasms, but they are still commonly used for infant seizures. There was some disagreement at UCLA whether there really was a link, so Dr. Hussain and his team took a look at the university’s experience. They matched 50 children with nonsyndromic epilepsy who subsequently developed video-EEG confirmed infantile spasms (cases) to 50 children who also had nonsyndromic epilepsy but did not develop spasms, based on follow-up duration and age and date of epilepsy onset.
The team then looked to see what drugs they had been on; it turned out that cases and controls were about equally as likely to have been treated with any specific antiepileptic, including SCBs. Infantile spasms were substantially more likely with SCB exposure in children with spasm risk factors, which also include focal cortical dysplasia, Aicardi syndrome, and other problems (HR 7.0; 95%; CI 2.5-19.8; P less than .001). Spasms were also more likely among even low-risk children treated with SCBs, although the trend was not statistically significant.
In the end, “we wonder how many cases of infantile spasms could [have been] prevented entirely if we had avoided sodium channel blockade,” Dr. Hussain said at the annual meeting of the American Epilepsy Society.
With so many other seizure options available – levetiracetam, topiramate, and phenobarbital, to name just a few – maybe it would be best “to stay away from” SCBs entirely in “infants with any form of epilepsy,” said lead investigator Jaeden Heesch, an undergraduate researcher who worked with Dr. Hussain.
It is unclear why SCBs increase infantile spasm risk; maybe nonselective voltage-gated sodium channel blockade interferes with proper neuron function in susceptible children, similar to the effects of sodium voltage-gated channel alpha subunit 1 mutations in Dravet syndrome, Dr. Hussain said. Perhaps the findings will inspire drug development. “If nonselective sodium channel blockade is bad, perhaps selective modulation of voltage-gated sodium currents [could be] beneficial or protective,” he said.
The age of epilepsy onset in the study was around 2 months. Children who went on to develop infantile spasms had an average of almost two seizures per day, versus fewer than one among controls, and were on an average of two, versus about 1.5 antiepileptics. The differences were not statistically significant.
The study looked at SCB exposure overall, but it’s possible that infantile spasm risk differs among the various class members.
The work was funded by the Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute. The investigators didn’t have any relevant disclosures.
SOURCE: Heesch J et al. AES 2019. Abstract 2.234.
BALTIMORE – “This is scary and warrants caution,” said senior investigator and pediatric neurologist Shaun Hussain, MD, a pediatric neurologist at Mattel Children’s Hospital at UCLA. Because of the findings, “we are avoiding the use of voltage-gated sodium channel blockade in any child at risk for infantile spasms. More broadly, we are avoiding [them] in any infant if there is a good alternative medication, of which there are many in most cases.”
There have been a few previous case reports linking voltage-gated sodium channel blockers (SCBs) – which include oxcarbazepine, carbamazepine, lacosamide, and phenytoin – to infantile spasms, but they are still commonly used for infant seizures. There was some disagreement at UCLA whether there really was a link, so Dr. Hussain and his team took a look at the university’s experience. They matched 50 children with nonsyndromic epilepsy who subsequently developed video-EEG confirmed infantile spasms (cases) to 50 children who also had nonsyndromic epilepsy but did not develop spasms, based on follow-up duration and age and date of epilepsy onset.
The team then looked to see what drugs they had been on; it turned out that cases and controls were about equally as likely to have been treated with any specific antiepileptic, including SCBs. Infantile spasms were substantially more likely with SCB exposure in children with spasm risk factors, which also include focal cortical dysplasia, Aicardi syndrome, and other problems (HR 7.0; 95%; CI 2.5-19.8; P less than .001). Spasms were also more likely among even low-risk children treated with SCBs, although the trend was not statistically significant.
In the end, “we wonder how many cases of infantile spasms could [have been] prevented entirely if we had avoided sodium channel blockade,” Dr. Hussain said at the annual meeting of the American Epilepsy Society.
With so many other seizure options available – levetiracetam, topiramate, and phenobarbital, to name just a few – maybe it would be best “to stay away from” SCBs entirely in “infants with any form of epilepsy,” said lead investigator Jaeden Heesch, an undergraduate researcher who worked with Dr. Hussain.
It is unclear why SCBs increase infantile spasm risk; maybe nonselective voltage-gated sodium channel blockade interferes with proper neuron function in susceptible children, similar to the effects of sodium voltage-gated channel alpha subunit 1 mutations in Dravet syndrome, Dr. Hussain said. Perhaps the findings will inspire drug development. “If nonselective sodium channel blockade is bad, perhaps selective modulation of voltage-gated sodium currents [could be] beneficial or protective,” he said.
The age of epilepsy onset in the study was around 2 months. Children who went on to develop infantile spasms had an average of almost two seizures per day, versus fewer than one among controls, and were on an average of two, versus about 1.5 antiepileptics. The differences were not statistically significant.
The study looked at SCB exposure overall, but it’s possible that infantile spasm risk differs among the various class members.
The work was funded by the Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute. The investigators didn’t have any relevant disclosures.
SOURCE: Heesch J et al. AES 2019. Abstract 2.234.
REPORTING FROM AES 2019
Updated gout guidelines: Don’t let kidney function dictate allopurinol dosing
ATLANTA – Soon-to-be-published gout guidelines from the American College of Rheumatology will recommend dosing allopurinol above 300 mg/day to get serum urate below 6 mg/dL, even in people with renal impairment.
It’s the same strong treat-to-target recommendation the group made in its last outing in 2012, but “we now have more evidence to support it,” said co–lead author, rheumatologist, and epidemiologist Tuhina Neogi, MD, PhD, a professor of medicine at Boston University.
She gave a sneak preview of the new guidelines, which will be published in 2020, at the ACR annual meeting. They are under review, but she said the “major recommendations will remain the same.”
“There will still be controversy that we have not yet proven that a threshold of 6 mg/dL is better than a threshold of 7 mg/dL, but we know that” at physiologic pH and temperature, monosodium urate starts to crystallize out at 6.8 mg/dL. “Serum urate is not a perfect measure or total body urate, so we need to get urate to below at least 6 mg/dL,” she said, and perhaps lower in some.
A popular alternative in primary care – where most gout is managed – is to treat to avoid symptoms. It “has no evidence,” and people “end up getting tophaceous gout with joint destruction. Suppressive colchicine therapy does not manage underlying hyperuricemia,” Dr. Neogi said.
With the symptom approach, “patients are often [profoundly] dismayed” when they find out they have large tophi and joint damage because they weren’t managed properly. “Primary care physicians [don’t often] see that because those patients don’t go back to them,” she said.
Dr. Neogi suspects that, for rheumatologists, the biggest surprise in the new guidelines will be a deemphasis on lifestyle and dietary factors. They can be triggers, but “gout is increasingly recognized as largely genetically determined,” and the impact of other factors on serum urate is low. Plus, “patients are embarrassed” by gout, and even less comfortable being honest with physicians “if they think we are blaming them,” she said.
The new document will recommend allopurinol as the definitive first-line option for hyperuricemia. Febuxostat (Uloric) was put on pretty much equal footing in 2012, but now “we acknowledge” that allopurinol dosing in head-to-head trials – 300 mg/day or 200 mg/day with renal impairment – was too low for most people, “so to say febuxostat is equivalent or superior isn’t really fair.” The substantially higher cost of febuxostat was also taken into consideration, she said.
The ACR will broaden the indications for urate lowering beyond frequent flares, tophi, and radiologic joint damage to include conditional, shared decision-making recommendations for people who have less than two flares per year, those with kidney stones, and people with a first flare if they are particularly susceptible to a second – namely those with serum urate at or above 9 mg/dL and people with stage 3 or worse chronic kidney disease, who are less able to tolerate NSAIDs and colchicine for symptom treatment.
The group will also relax its advice against treating asymptomatic hyperuricemia. Febuxostat trials have shown a reduction in incident gout, but the number needed to treat was large, so the ACR will recommend shared decision making.
Inadequate allopurinol dosing, meanwhile, has been the bête noire of rheumatology for years, but there is still reluctance among many to go above 300 mg/day. Dr. Neogi said it’s because of a decades-old concern, “unsupported by any evidence, that higher doses may be detrimental in people with renal insufficiency.” It’s frustrating, she said, because “there is good data supporting the safety of increasing the dose above 300 mg/day even in those with renal impairment,” and not doing so opens the door to entirely preventable complications.
As for allopurinol hypersensitivity – another reason people shy away from higher dosing, especially in the renally impaired – the trick is to start low and slowly titrate allopurinol up to the target urate range. Asian and black people, especially, should be screened beforehand for the HLA-B*58:01 genetic variant that increases the risk of severe reactions. Both will be strong recommendations in the new guidelines.
Dr. Neogi didn’t have any relevant industry disclosures.
ATLANTA – Soon-to-be-published gout guidelines from the American College of Rheumatology will recommend dosing allopurinol above 300 mg/day to get serum urate below 6 mg/dL, even in people with renal impairment.
It’s the same strong treat-to-target recommendation the group made in its last outing in 2012, but “we now have more evidence to support it,” said co–lead author, rheumatologist, and epidemiologist Tuhina Neogi, MD, PhD, a professor of medicine at Boston University.
She gave a sneak preview of the new guidelines, which will be published in 2020, at the ACR annual meeting. They are under review, but she said the “major recommendations will remain the same.”
“There will still be controversy that we have not yet proven that a threshold of 6 mg/dL is better than a threshold of 7 mg/dL, but we know that” at physiologic pH and temperature, monosodium urate starts to crystallize out at 6.8 mg/dL. “Serum urate is not a perfect measure or total body urate, so we need to get urate to below at least 6 mg/dL,” she said, and perhaps lower in some.
A popular alternative in primary care – where most gout is managed – is to treat to avoid symptoms. It “has no evidence,” and people “end up getting tophaceous gout with joint destruction. Suppressive colchicine therapy does not manage underlying hyperuricemia,” Dr. Neogi said.
With the symptom approach, “patients are often [profoundly] dismayed” when they find out they have large tophi and joint damage because they weren’t managed properly. “Primary care physicians [don’t often] see that because those patients don’t go back to them,” she said.
Dr. Neogi suspects that, for rheumatologists, the biggest surprise in the new guidelines will be a deemphasis on lifestyle and dietary factors. They can be triggers, but “gout is increasingly recognized as largely genetically determined,” and the impact of other factors on serum urate is low. Plus, “patients are embarrassed” by gout, and even less comfortable being honest with physicians “if they think we are blaming them,” she said.
The new document will recommend allopurinol as the definitive first-line option for hyperuricemia. Febuxostat (Uloric) was put on pretty much equal footing in 2012, but now “we acknowledge” that allopurinol dosing in head-to-head trials – 300 mg/day or 200 mg/day with renal impairment – was too low for most people, “so to say febuxostat is equivalent or superior isn’t really fair.” The substantially higher cost of febuxostat was also taken into consideration, she said.
The ACR will broaden the indications for urate lowering beyond frequent flares, tophi, and radiologic joint damage to include conditional, shared decision-making recommendations for people who have less than two flares per year, those with kidney stones, and people with a first flare if they are particularly susceptible to a second – namely those with serum urate at or above 9 mg/dL and people with stage 3 or worse chronic kidney disease, who are less able to tolerate NSAIDs and colchicine for symptom treatment.
The group will also relax its advice against treating asymptomatic hyperuricemia. Febuxostat trials have shown a reduction in incident gout, but the number needed to treat was large, so the ACR will recommend shared decision making.
Inadequate allopurinol dosing, meanwhile, has been the bête noire of rheumatology for years, but there is still reluctance among many to go above 300 mg/day. Dr. Neogi said it’s because of a decades-old concern, “unsupported by any evidence, that higher doses may be detrimental in people with renal insufficiency.” It’s frustrating, she said, because “there is good data supporting the safety of increasing the dose above 300 mg/day even in those with renal impairment,” and not doing so opens the door to entirely preventable complications.
As for allopurinol hypersensitivity – another reason people shy away from higher dosing, especially in the renally impaired – the trick is to start low and slowly titrate allopurinol up to the target urate range. Asian and black people, especially, should be screened beforehand for the HLA-B*58:01 genetic variant that increases the risk of severe reactions. Both will be strong recommendations in the new guidelines.
Dr. Neogi didn’t have any relevant industry disclosures.
ATLANTA – Soon-to-be-published gout guidelines from the American College of Rheumatology will recommend dosing allopurinol above 300 mg/day to get serum urate below 6 mg/dL, even in people with renal impairment.
It’s the same strong treat-to-target recommendation the group made in its last outing in 2012, but “we now have more evidence to support it,” said co–lead author, rheumatologist, and epidemiologist Tuhina Neogi, MD, PhD, a professor of medicine at Boston University.
She gave a sneak preview of the new guidelines, which will be published in 2020, at the ACR annual meeting. They are under review, but she said the “major recommendations will remain the same.”
“There will still be controversy that we have not yet proven that a threshold of 6 mg/dL is better than a threshold of 7 mg/dL, but we know that” at physiologic pH and temperature, monosodium urate starts to crystallize out at 6.8 mg/dL. “Serum urate is not a perfect measure or total body urate, so we need to get urate to below at least 6 mg/dL,” she said, and perhaps lower in some.
A popular alternative in primary care – where most gout is managed – is to treat to avoid symptoms. It “has no evidence,” and people “end up getting tophaceous gout with joint destruction. Suppressive colchicine therapy does not manage underlying hyperuricemia,” Dr. Neogi said.
With the symptom approach, “patients are often [profoundly] dismayed” when they find out they have large tophi and joint damage because they weren’t managed properly. “Primary care physicians [don’t often] see that because those patients don’t go back to them,” she said.
Dr. Neogi suspects that, for rheumatologists, the biggest surprise in the new guidelines will be a deemphasis on lifestyle and dietary factors. They can be triggers, but “gout is increasingly recognized as largely genetically determined,” and the impact of other factors on serum urate is low. Plus, “patients are embarrassed” by gout, and even less comfortable being honest with physicians “if they think we are blaming them,” she said.
The new document will recommend allopurinol as the definitive first-line option for hyperuricemia. Febuxostat (Uloric) was put on pretty much equal footing in 2012, but now “we acknowledge” that allopurinol dosing in head-to-head trials – 300 mg/day or 200 mg/day with renal impairment – was too low for most people, “so to say febuxostat is equivalent or superior isn’t really fair.” The substantially higher cost of febuxostat was also taken into consideration, she said.
The ACR will broaden the indications for urate lowering beyond frequent flares, tophi, and radiologic joint damage to include conditional, shared decision-making recommendations for people who have less than two flares per year, those with kidney stones, and people with a first flare if they are particularly susceptible to a second – namely those with serum urate at or above 9 mg/dL and people with stage 3 or worse chronic kidney disease, who are less able to tolerate NSAIDs and colchicine for symptom treatment.
The group will also relax its advice against treating asymptomatic hyperuricemia. Febuxostat trials have shown a reduction in incident gout, but the number needed to treat was large, so the ACR will recommend shared decision making.
Inadequate allopurinol dosing, meanwhile, has been the bête noire of rheumatology for years, but there is still reluctance among many to go above 300 mg/day. Dr. Neogi said it’s because of a decades-old concern, “unsupported by any evidence, that higher doses may be detrimental in people with renal insufficiency.” It’s frustrating, she said, because “there is good data supporting the safety of increasing the dose above 300 mg/day even in those with renal impairment,” and not doing so opens the door to entirely preventable complications.
As for allopurinol hypersensitivity – another reason people shy away from higher dosing, especially in the renally impaired – the trick is to start low and slowly titrate allopurinol up to the target urate range. Asian and black people, especially, should be screened beforehand for the HLA-B*58:01 genetic variant that increases the risk of severe reactions. Both will be strong recommendations in the new guidelines.
Dr. Neogi didn’t have any relevant industry disclosures.
REPORTING FROM ACR 2019
Scalp EEG predicts temporal lobe resection success
BALTIMORE – In a review of 43 temporal lobe epilepsy patients at Yale University in New Haven, Conn., anteromedial temporal resection (AMTR) failed in every case in which initial ictal rhythm on scalp EEG spread beyond the medial temporal lobe to other brain regions within 10 seconds.
Among the 33 patients who had no spread on preoperative scalp EEG or who spread in 10 or more seconds, 31 (94%) had a good outcome, meaning they were seizure free or had only auras after AMTR. The findings could mean that scalp EEG can predict surgery outcome.
AMTR works in the majority of patients with refractory temporal lobe epilepsy, but about 10-20% continue to have seizures. Senior investigator Pue Farooque, DO, from Yale University wanted to find a way to identify patients likely to fail surgery beforehand to help counsel patients on what to expect and also to know when other treatment options might be a better bet.
“If you see seizures are spreading quickly to another area, like the frontal lobe or the temporal neocortex, you could implant RNS [responsive neurostimulation]” instead of doing an ATMR, “and that might improve your outcomes,” she said at the American Epilepsy Society’s annual meeting.
The findings are essentially the same as when the group used intracranial EEG to detect fast spread in a previous report, but scalp EEG is noninvasive and allows for easy preoperative assessment (JAMA Neurol. 2019 Apr 1;76[4]:462-9).
The team also found in their new study that diffuse hypometabolism in the entire temporal lobe on quantitative PET also predicted poor ATMR outcomes (P less than .001), but Dr. Farooque said more work is needed to quantify the finding. The investigators also plan to assess the predictive value of resting functional MRI.
The take home, she said, is that “we can do better” with epilepsy surgery, and “there are noninvasive markers we can use to help guide us.”
It’s unclear why more rapid seizure spread would predict AMTR failure. In the earlier study with intracranial EEG, the investigators said “the results are best explained by attributing epileptogenic potential to sites of early seizure spread that were not included in resection. This mechanism of failure implies that a distributed epileptogenic network rather than a single epileptogenic focus may underlie surgically refractory epilepsy.”
Patients in the new report had epilepsy for a mean of 24.4 years, and 25 (58%) were women; 30 cases (69%) were lesional, and follow-up was at least a year. The contralateral or lateralized seizure spread ranged from 1 to 63 seconds, with a mean of 18.5 seconds. Among patients who failed AMTR, seizure spread occurred at a mean of 7.1 seconds.
Electrographic pattern at onset and location of interictal epileptiform discharges did not predict outcome
There was no industry funding, and Dr. Farooque didn’t have any relevant disclosures.
SOURCE: Chiari J et al. AES 2019, Abstract 1.36.
BALTIMORE – In a review of 43 temporal lobe epilepsy patients at Yale University in New Haven, Conn., anteromedial temporal resection (AMTR) failed in every case in which initial ictal rhythm on scalp EEG spread beyond the medial temporal lobe to other brain regions within 10 seconds.
Among the 33 patients who had no spread on preoperative scalp EEG or who spread in 10 or more seconds, 31 (94%) had a good outcome, meaning they were seizure free or had only auras after AMTR. The findings could mean that scalp EEG can predict surgery outcome.
AMTR works in the majority of patients with refractory temporal lobe epilepsy, but about 10-20% continue to have seizures. Senior investigator Pue Farooque, DO, from Yale University wanted to find a way to identify patients likely to fail surgery beforehand to help counsel patients on what to expect and also to know when other treatment options might be a better bet.
“If you see seizures are spreading quickly to another area, like the frontal lobe or the temporal neocortex, you could implant RNS [responsive neurostimulation]” instead of doing an ATMR, “and that might improve your outcomes,” she said at the American Epilepsy Society’s annual meeting.
The findings are essentially the same as when the group used intracranial EEG to detect fast spread in a previous report, but scalp EEG is noninvasive and allows for easy preoperative assessment (JAMA Neurol. 2019 Apr 1;76[4]:462-9).
The team also found in their new study that diffuse hypometabolism in the entire temporal lobe on quantitative PET also predicted poor ATMR outcomes (P less than .001), but Dr. Farooque said more work is needed to quantify the finding. The investigators also plan to assess the predictive value of resting functional MRI.
The take home, she said, is that “we can do better” with epilepsy surgery, and “there are noninvasive markers we can use to help guide us.”
It’s unclear why more rapid seizure spread would predict AMTR failure. In the earlier study with intracranial EEG, the investigators said “the results are best explained by attributing epileptogenic potential to sites of early seizure spread that were not included in resection. This mechanism of failure implies that a distributed epileptogenic network rather than a single epileptogenic focus may underlie surgically refractory epilepsy.”
Patients in the new report had epilepsy for a mean of 24.4 years, and 25 (58%) were women; 30 cases (69%) were lesional, and follow-up was at least a year. The contralateral or lateralized seizure spread ranged from 1 to 63 seconds, with a mean of 18.5 seconds. Among patients who failed AMTR, seizure spread occurred at a mean of 7.1 seconds.
Electrographic pattern at onset and location of interictal epileptiform discharges did not predict outcome
There was no industry funding, and Dr. Farooque didn’t have any relevant disclosures.
SOURCE: Chiari J et al. AES 2019, Abstract 1.36.
BALTIMORE – In a review of 43 temporal lobe epilepsy patients at Yale University in New Haven, Conn., anteromedial temporal resection (AMTR) failed in every case in which initial ictal rhythm on scalp EEG spread beyond the medial temporal lobe to other brain regions within 10 seconds.
Among the 33 patients who had no spread on preoperative scalp EEG or who spread in 10 or more seconds, 31 (94%) had a good outcome, meaning they were seizure free or had only auras after AMTR. The findings could mean that scalp EEG can predict surgery outcome.
AMTR works in the majority of patients with refractory temporal lobe epilepsy, but about 10-20% continue to have seizures. Senior investigator Pue Farooque, DO, from Yale University wanted to find a way to identify patients likely to fail surgery beforehand to help counsel patients on what to expect and also to know when other treatment options might be a better bet.
“If you see seizures are spreading quickly to another area, like the frontal lobe or the temporal neocortex, you could implant RNS [responsive neurostimulation]” instead of doing an ATMR, “and that might improve your outcomes,” she said at the American Epilepsy Society’s annual meeting.
The findings are essentially the same as when the group used intracranial EEG to detect fast spread in a previous report, but scalp EEG is noninvasive and allows for easy preoperative assessment (JAMA Neurol. 2019 Apr 1;76[4]:462-9).
The team also found in their new study that diffuse hypometabolism in the entire temporal lobe on quantitative PET also predicted poor ATMR outcomes (P less than .001), but Dr. Farooque said more work is needed to quantify the finding. The investigators also plan to assess the predictive value of resting functional MRI.
The take home, she said, is that “we can do better” with epilepsy surgery, and “there are noninvasive markers we can use to help guide us.”
It’s unclear why more rapid seizure spread would predict AMTR failure. In the earlier study with intracranial EEG, the investigators said “the results are best explained by attributing epileptogenic potential to sites of early seizure spread that were not included in resection. This mechanism of failure implies that a distributed epileptogenic network rather than a single epileptogenic focus may underlie surgically refractory epilepsy.”
Patients in the new report had epilepsy for a mean of 24.4 years, and 25 (58%) were women; 30 cases (69%) were lesional, and follow-up was at least a year. The contralateral or lateralized seizure spread ranged from 1 to 63 seconds, with a mean of 18.5 seconds. Among patients who failed AMTR, seizure spread occurred at a mean of 7.1 seconds.
Electrographic pattern at onset and location of interictal epileptiform discharges did not predict outcome
There was no industry funding, and Dr. Farooque didn’t have any relevant disclosures.
SOURCE: Chiari J et al. AES 2019, Abstract 1.36.
REPORTING FROM AES 2019