User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Early data support further study of ivosidenib in mIDH1 glioma
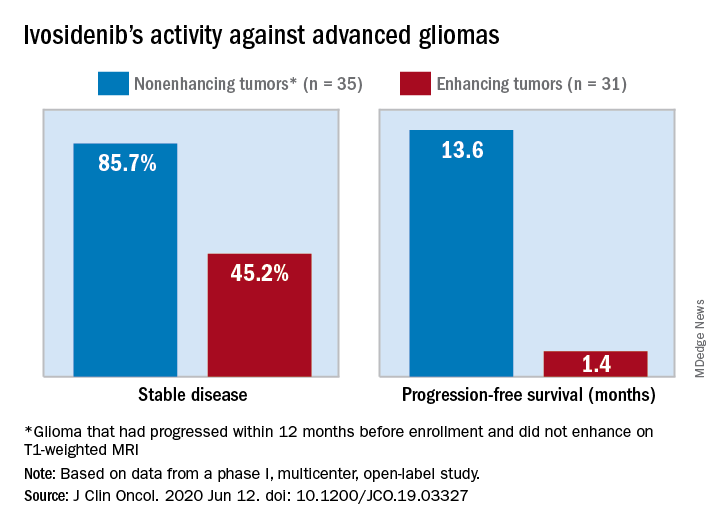
The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Breast density asymmetry might increase breast cancer risk
The 854 women in the study had been referred for biopsy after an abnormal mammogram.
Researchers used the mammograms to assess global bilateral asymmetry, which was the overall absolute difference in percent fibroglandular tissue volume (%FGV) between the ipsilateral (biopsied) breast and the contralateral (unaffected) breast.
The researchers also assessed local bilateral asymmetry, which was the perilesional %FGV difference in an area twice the size of, but excluding, the biopsy target, and the corresponding area in the unaffected breast.
The women were then divided into quartiles based on breast density asymmetry.
Most of the women had benign breast disease, including proliferative (43%) and nonproliferative (33%) disease, but 23% had carcinoma in situ or invasive breast cancer.
The trend for higher risk of in situ or invasive cancer with increasing breast density asymmetry was observed only in the local analysis. The odds ratio was 1.59 (95% confidence interval, 0.94-2.69) for women in the highest quartile of breast density asymmetry (absolute difference, > 8.23) versus those in the lowest quartile (absolute difference, ≤ –5.55; P = .067).
When compared with women who had proliferative benign disease, women with carcinoma in situ or invasive breast cancer “were more likely to be in the higher than lower quartiles,” said lead investigator Maeve Mullooly, PhD, a research fellow at the Royal College of Surgeons in Dublin.
There was no association between breast density asymmetry and traditional breast cancer risk factors such as age, body mass index, race, and hormone therapy. However, among women diagnosed with benign nonproliferative disease, women with a breast cancer family history were more likely to have higher overall breast density asymmetry.
Study rationale and details
Higher breast density is a known risk factor for breast cancer. Breast asymmetry also has been reported as a possible risk factor (Breast Cancer Res. 2006;8[2]:R14), and incorporation of breast density asymmetry into traditional risk factors in one study improved risk prediction (Breast Cancer Res. 2017 Mar 14;19[1]:29).
Building on that work, the goal of Dr. Mullooly’s study was to “learn how to better use breast density to inform breast cancer risk prediction,” she said.
To that end, her team turned to 854 women enrolled from 2007-2010 in the National Cancer Institute’s Breast Radiology Evaluation and Study of Tissues Project, a cross-sectional molecular epidemiologic study designed to understand how breast density measures are related to breast cancer etiology.
Most of the women were non-Hispanic white. The mean age was 51 years (range, 40-65), and the median body mass index was 25 kg/m2.
About three-quarters of the women (76%) had a breast density asymmetry of at least 2% on the global analysis, with 43% having higher %FGV in the biopsied breast and 33% having higher %FGV in the unaffected breast. In all, 89% of women had local breast density asymmetry, with higher density in the biopsied breast in 61% of women and higher density in the contralateral breast in 28%.
Next steps
This research is ongoing, and additional follow-up is planned, according to Dr. Mullooly. She said the researchers hope to apply more recent analytical techniques to the mammograms and to study the histologic differences in their breast biopsy specimens, among other steps, to see if stronger relationships with greater clinical utility emerge.
It was a “very well done study” with “very provocative data,” said presentation moderator Jennifer Wargo, MD, professor of genomic medicine and surgical oncology at the University of Texas MD Anderson Cancer Center in Houston.
She was interested in the planned next steps, particularly the histologic analysis of dense versus less dense breast tissue. There could be “differences in stroma or hormonal levels even at the microenvironmental level” that “represent a potential field defect, which later puts someone at risk,” she said, adding that it’s “great” that the work is continuing.
The National Cancer Institute funded the research. Dr. Mullooly reported no relevant disclosures. Dr. Wargo disclosed relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, GlaxoSmithKline, AstraZeneca, Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune, Merck, Biothera Pharmaceuticals, and Microbiome DX.
SOURCE: Mullooly M et al. AACR 2020, Abstract NG15.
The 854 women in the study had been referred for biopsy after an abnormal mammogram.
Researchers used the mammograms to assess global bilateral asymmetry, which was the overall absolute difference in percent fibroglandular tissue volume (%FGV) between the ipsilateral (biopsied) breast and the contralateral (unaffected) breast.
The researchers also assessed local bilateral asymmetry, which was the perilesional %FGV difference in an area twice the size of, but excluding, the biopsy target, and the corresponding area in the unaffected breast.
The women were then divided into quartiles based on breast density asymmetry.
Most of the women had benign breast disease, including proliferative (43%) and nonproliferative (33%) disease, but 23% had carcinoma in situ or invasive breast cancer.
The trend for higher risk of in situ or invasive cancer with increasing breast density asymmetry was observed only in the local analysis. The odds ratio was 1.59 (95% confidence interval, 0.94-2.69) for women in the highest quartile of breast density asymmetry (absolute difference, > 8.23) versus those in the lowest quartile (absolute difference, ≤ –5.55; P = .067).
When compared with women who had proliferative benign disease, women with carcinoma in situ or invasive breast cancer “were more likely to be in the higher than lower quartiles,” said lead investigator Maeve Mullooly, PhD, a research fellow at the Royal College of Surgeons in Dublin.
There was no association between breast density asymmetry and traditional breast cancer risk factors such as age, body mass index, race, and hormone therapy. However, among women diagnosed with benign nonproliferative disease, women with a breast cancer family history were more likely to have higher overall breast density asymmetry.
Study rationale and details
Higher breast density is a known risk factor for breast cancer. Breast asymmetry also has been reported as a possible risk factor (Breast Cancer Res. 2006;8[2]:R14), and incorporation of breast density asymmetry into traditional risk factors in one study improved risk prediction (Breast Cancer Res. 2017 Mar 14;19[1]:29).
Building on that work, the goal of Dr. Mullooly’s study was to “learn how to better use breast density to inform breast cancer risk prediction,” she said.
To that end, her team turned to 854 women enrolled from 2007-2010 in the National Cancer Institute’s Breast Radiology Evaluation and Study of Tissues Project, a cross-sectional molecular epidemiologic study designed to understand how breast density measures are related to breast cancer etiology.
Most of the women were non-Hispanic white. The mean age was 51 years (range, 40-65), and the median body mass index was 25 kg/m2.
About three-quarters of the women (76%) had a breast density asymmetry of at least 2% on the global analysis, with 43% having higher %FGV in the biopsied breast and 33% having higher %FGV in the unaffected breast. In all, 89% of women had local breast density asymmetry, with higher density in the biopsied breast in 61% of women and higher density in the contralateral breast in 28%.
Next steps
This research is ongoing, and additional follow-up is planned, according to Dr. Mullooly. She said the researchers hope to apply more recent analytical techniques to the mammograms and to study the histologic differences in their breast biopsy specimens, among other steps, to see if stronger relationships with greater clinical utility emerge.
It was a “very well done study” with “very provocative data,” said presentation moderator Jennifer Wargo, MD, professor of genomic medicine and surgical oncology at the University of Texas MD Anderson Cancer Center in Houston.
She was interested in the planned next steps, particularly the histologic analysis of dense versus less dense breast tissue. There could be “differences in stroma or hormonal levels even at the microenvironmental level” that “represent a potential field defect, which later puts someone at risk,” she said, adding that it’s “great” that the work is continuing.
The National Cancer Institute funded the research. Dr. Mullooly reported no relevant disclosures. Dr. Wargo disclosed relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, GlaxoSmithKline, AstraZeneca, Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune, Merck, Biothera Pharmaceuticals, and Microbiome DX.
SOURCE: Mullooly M et al. AACR 2020, Abstract NG15.
The 854 women in the study had been referred for biopsy after an abnormal mammogram.
Researchers used the mammograms to assess global bilateral asymmetry, which was the overall absolute difference in percent fibroglandular tissue volume (%FGV) between the ipsilateral (biopsied) breast and the contralateral (unaffected) breast.
The researchers also assessed local bilateral asymmetry, which was the perilesional %FGV difference in an area twice the size of, but excluding, the biopsy target, and the corresponding area in the unaffected breast.
The women were then divided into quartiles based on breast density asymmetry.
Most of the women had benign breast disease, including proliferative (43%) and nonproliferative (33%) disease, but 23% had carcinoma in situ or invasive breast cancer.
The trend for higher risk of in situ or invasive cancer with increasing breast density asymmetry was observed only in the local analysis. The odds ratio was 1.59 (95% confidence interval, 0.94-2.69) for women in the highest quartile of breast density asymmetry (absolute difference, > 8.23) versus those in the lowest quartile (absolute difference, ≤ –5.55; P = .067).
When compared with women who had proliferative benign disease, women with carcinoma in situ or invasive breast cancer “were more likely to be in the higher than lower quartiles,” said lead investigator Maeve Mullooly, PhD, a research fellow at the Royal College of Surgeons in Dublin.
There was no association between breast density asymmetry and traditional breast cancer risk factors such as age, body mass index, race, and hormone therapy. However, among women diagnosed with benign nonproliferative disease, women with a breast cancer family history were more likely to have higher overall breast density asymmetry.
Study rationale and details
Higher breast density is a known risk factor for breast cancer. Breast asymmetry also has been reported as a possible risk factor (Breast Cancer Res. 2006;8[2]:R14), and incorporation of breast density asymmetry into traditional risk factors in one study improved risk prediction (Breast Cancer Res. 2017 Mar 14;19[1]:29).
Building on that work, the goal of Dr. Mullooly’s study was to “learn how to better use breast density to inform breast cancer risk prediction,” she said.
To that end, her team turned to 854 women enrolled from 2007-2010 in the National Cancer Institute’s Breast Radiology Evaluation and Study of Tissues Project, a cross-sectional molecular epidemiologic study designed to understand how breast density measures are related to breast cancer etiology.
Most of the women were non-Hispanic white. The mean age was 51 years (range, 40-65), and the median body mass index was 25 kg/m2.
About three-quarters of the women (76%) had a breast density asymmetry of at least 2% on the global analysis, with 43% having higher %FGV in the biopsied breast and 33% having higher %FGV in the unaffected breast. In all, 89% of women had local breast density asymmetry, with higher density in the biopsied breast in 61% of women and higher density in the contralateral breast in 28%.
Next steps
This research is ongoing, and additional follow-up is planned, according to Dr. Mullooly. She said the researchers hope to apply more recent analytical techniques to the mammograms and to study the histologic differences in their breast biopsy specimens, among other steps, to see if stronger relationships with greater clinical utility emerge.
It was a “very well done study” with “very provocative data,” said presentation moderator Jennifer Wargo, MD, professor of genomic medicine and surgical oncology at the University of Texas MD Anderson Cancer Center in Houston.
She was interested in the planned next steps, particularly the histologic analysis of dense versus less dense breast tissue. There could be “differences in stroma or hormonal levels even at the microenvironmental level” that “represent a potential field defect, which later puts someone at risk,” she said, adding that it’s “great” that the work is continuing.
The National Cancer Institute funded the research. Dr. Mullooly reported no relevant disclosures. Dr. Wargo disclosed relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, GlaxoSmithKline, AstraZeneca, Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune, Merck, Biothera Pharmaceuticals, and Microbiome DX.
SOURCE: Mullooly M et al. AACR 2020, Abstract NG15.
FROM AACR 2020
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
FROM JAMA NETWORK OPEN
Real-world data support adjuvant immunotherapy for stage III melanoma
Among patients with stage IIIC disease, the 2-year survival rate was 70% in those who received immunotherapy and 59% in those who did not (P < .01). The median overall survival in this group was 32.8 months with immunotherapy and 28 months without it (P < .01).
Among patients with stage IIIA disease, the 2-year survival rate was 94% with immunotherapy and 91% without it (P = .03).
There was a trend toward a 2-year survival benefit with immunotherapy in patients with stage IIIB disease and in all 4,094 stage III patients, but the differences were not significant. The 2-year survival rate was 84% with immunotherapy and 81% without it among patients with stage IIIB disease (P = .35). The survival rates were 83% and 80%, respectively, in all stage III patients (P = .051).
This was an early analysis, noted investigator Justin Moyers, MD, of Loma Linda (Calif.) University. Ipilimumab was approved as adjuvant therapy for stage III melanoma patients in 2015, the year patients from this analysis were diagnosed.
“There’s really only 2 full years of survival data,” Dr. Moyers said. “I think given time, we will see a benefit amongst all the substages.”
In the meantime, “I would definitely not use this data to say whether or not [immunotherapy] should be given,” Dr. Moyers said.
The researchers were just using the database – which captures 52% of U.S. melanoma cases – to see if “real-world data mimics the clinical trial data,” Dr. Moyers said.
Overall, the findings support “adjuvant immunotherapy in the real-world setting,” he said.
The researchers also looked at treatment patterns in 2015-2016 across 8,160 patients with stage III melanoma, 4,094 of whom were included in the aforementioned survival analysis. There were 2,260 patients (27.7%) who received immunotherapy after surgery during that time period.
Uptake of adjuvant immunotherapy “was low to start, but those patients did better than ones who did not get” it, said AACR president Antoni Ribas, MD, PhD, of the University of California, Los Angeles, who was not involved in the study.
Immunotherapy recipients were younger, on average (54.8 years vs. 62.4 years). Patients with Charlson comorbidity scores above zero and those on Medicare were less likely to receive immunotherapy (18.4% Medicare vs. over 30% with other payers). There also were trends of decreased use with lower income and lower high school graduation rates.
The finding “highlights the negative impact of socioeconomic [factors] on access to proven therapy,” Dr. Ribas said.
As for low use among Medicare patients, uptake of new treatments, in general, “seems to be faster with private insurance,” he noted.
The study excluded patients who received systemic therapies other than immunotherapy, as well as those who received immunotherapy before surgery. Among study limitations, the specific immunotherapies patients received was unknown.
There was no external funding for this study. Dr. Moyers reported travel compensation from Astellas Pharmaceuticals in 2018. Dr. Ribas disclosed relationships with Amgen, Chugai, Merck, Sanofi, Tango, Arcus, Bioncotech, Compugen, CytomX, FLX Bio, ImaginAb, Isoplexis, Merus, Rgenix, and PACT.
SOURCE: Moyers J et al. AACR 2020, Abstract 4338.
Among patients with stage IIIC disease, the 2-year survival rate was 70% in those who received immunotherapy and 59% in those who did not (P < .01). The median overall survival in this group was 32.8 months with immunotherapy and 28 months without it (P < .01).
Among patients with stage IIIA disease, the 2-year survival rate was 94% with immunotherapy and 91% without it (P = .03).
There was a trend toward a 2-year survival benefit with immunotherapy in patients with stage IIIB disease and in all 4,094 stage III patients, but the differences were not significant. The 2-year survival rate was 84% with immunotherapy and 81% without it among patients with stage IIIB disease (P = .35). The survival rates were 83% and 80%, respectively, in all stage III patients (P = .051).
This was an early analysis, noted investigator Justin Moyers, MD, of Loma Linda (Calif.) University. Ipilimumab was approved as adjuvant therapy for stage III melanoma patients in 2015, the year patients from this analysis were diagnosed.
“There’s really only 2 full years of survival data,” Dr. Moyers said. “I think given time, we will see a benefit amongst all the substages.”
In the meantime, “I would definitely not use this data to say whether or not [immunotherapy] should be given,” Dr. Moyers said.
The researchers were just using the database – which captures 52% of U.S. melanoma cases – to see if “real-world data mimics the clinical trial data,” Dr. Moyers said.
Overall, the findings support “adjuvant immunotherapy in the real-world setting,” he said.
The researchers also looked at treatment patterns in 2015-2016 across 8,160 patients with stage III melanoma, 4,094 of whom were included in the aforementioned survival analysis. There were 2,260 patients (27.7%) who received immunotherapy after surgery during that time period.
Uptake of adjuvant immunotherapy “was low to start, but those patients did better than ones who did not get” it, said AACR president Antoni Ribas, MD, PhD, of the University of California, Los Angeles, who was not involved in the study.
Immunotherapy recipients were younger, on average (54.8 years vs. 62.4 years). Patients with Charlson comorbidity scores above zero and those on Medicare were less likely to receive immunotherapy (18.4% Medicare vs. over 30% with other payers). There also were trends of decreased use with lower income and lower high school graduation rates.
The finding “highlights the negative impact of socioeconomic [factors] on access to proven therapy,” Dr. Ribas said.
As for low use among Medicare patients, uptake of new treatments, in general, “seems to be faster with private insurance,” he noted.
The study excluded patients who received systemic therapies other than immunotherapy, as well as those who received immunotherapy before surgery. Among study limitations, the specific immunotherapies patients received was unknown.
There was no external funding for this study. Dr. Moyers reported travel compensation from Astellas Pharmaceuticals in 2018. Dr. Ribas disclosed relationships with Amgen, Chugai, Merck, Sanofi, Tango, Arcus, Bioncotech, Compugen, CytomX, FLX Bio, ImaginAb, Isoplexis, Merus, Rgenix, and PACT.
SOURCE: Moyers J et al. AACR 2020, Abstract 4338.
Among patients with stage IIIC disease, the 2-year survival rate was 70% in those who received immunotherapy and 59% in those who did not (P < .01). The median overall survival in this group was 32.8 months with immunotherapy and 28 months without it (P < .01).
Among patients with stage IIIA disease, the 2-year survival rate was 94% with immunotherapy and 91% without it (P = .03).
There was a trend toward a 2-year survival benefit with immunotherapy in patients with stage IIIB disease and in all 4,094 stage III patients, but the differences were not significant. The 2-year survival rate was 84% with immunotherapy and 81% without it among patients with stage IIIB disease (P = .35). The survival rates were 83% and 80%, respectively, in all stage III patients (P = .051).
This was an early analysis, noted investigator Justin Moyers, MD, of Loma Linda (Calif.) University. Ipilimumab was approved as adjuvant therapy for stage III melanoma patients in 2015, the year patients from this analysis were diagnosed.
“There’s really only 2 full years of survival data,” Dr. Moyers said. “I think given time, we will see a benefit amongst all the substages.”
In the meantime, “I would definitely not use this data to say whether or not [immunotherapy] should be given,” Dr. Moyers said.
The researchers were just using the database – which captures 52% of U.S. melanoma cases – to see if “real-world data mimics the clinical trial data,” Dr. Moyers said.
Overall, the findings support “adjuvant immunotherapy in the real-world setting,” he said.
The researchers also looked at treatment patterns in 2015-2016 across 8,160 patients with stage III melanoma, 4,094 of whom were included in the aforementioned survival analysis. There were 2,260 patients (27.7%) who received immunotherapy after surgery during that time period.
Uptake of adjuvant immunotherapy “was low to start, but those patients did better than ones who did not get” it, said AACR president Antoni Ribas, MD, PhD, of the University of California, Los Angeles, who was not involved in the study.
Immunotherapy recipients were younger, on average (54.8 years vs. 62.4 years). Patients with Charlson comorbidity scores above zero and those on Medicare were less likely to receive immunotherapy (18.4% Medicare vs. over 30% with other payers). There also were trends of decreased use with lower income and lower high school graduation rates.
The finding “highlights the negative impact of socioeconomic [factors] on access to proven therapy,” Dr. Ribas said.
As for low use among Medicare patients, uptake of new treatments, in general, “seems to be faster with private insurance,” he noted.
The study excluded patients who received systemic therapies other than immunotherapy, as well as those who received immunotherapy before surgery. Among study limitations, the specific immunotherapies patients received was unknown.
There was no external funding for this study. Dr. Moyers reported travel compensation from Astellas Pharmaceuticals in 2018. Dr. Ribas disclosed relationships with Amgen, Chugai, Merck, Sanofi, Tango, Arcus, Bioncotech, Compugen, CytomX, FLX Bio, ImaginAb, Isoplexis, Merus, Rgenix, and PACT.
SOURCE: Moyers J et al. AACR 2020, Abstract 4338.
FROM AACR 2020
Venetoclax plus LDAC tops LDAC alone in AML
At about 18 months’ follow-up in treatment naive acute myelogenous leukemia (AML) patients who were 75 years or older or otherwise unfit for intensive chemotherapy, median overall survival (OS) was 8.4 months when they were randomized to low-dose cytarabine (LDAC) plus the BCL-2 inhibitor venetoclax versus 4.1 months with LDAC plus placebo. The results from the phase 3 trial were reported at the virtual annual congress of the European Hematology Association.
The combination also improved rates of remission, event-free survival, and patient reported outcomes and lessened transfusion requirements. Adverse events were manageable.
The findings position venetoclax add-on with LDAC “as a potential new standard of care” for untreated patients ineligible for intensive chemotherapy, lead investigator Andrew Wei, MD, PhD, an AML researcher at Monash University, Melbourne, said at the meeting.
The study addresses a substantial unmet need. The median age at AML diagnosis is over 68 years old and comorbidities such as heart failure and reduced creatinine clearance are common, which make the risk of toxicity with standard chemotherapy too high. Single-agent alternatives are of limited benefit, so Dr. Wei’s group and others are looking for better options to plug the treatment gap when standard chemotherapy is contraindicated.
Several combinations are under investigation, including LDAC plus venetoclax, which appears to have a synergistic effect greater than either agent on its own, Dr. Wei and colleagues explained in their journal report, which was published online to coincide with his presentation (Blood. 2020 Jun 11;135(24):2137-45).
In a commentary, Bob Lowenberg, Ph, a hematologist with the Erasmus University Medical Center in Rotterdam, and Gerwin Huls, MD, PhD, of the University Medical Center Groningen, both in the Netherlands, said the study “represents a valuable although moderate step forward on the way to a better therapeutic future for the ‘unfit’ patient with AML” (Blood. 2020. Jun 11;135(24): 2114-5).
“A challenging AML population”
In the study, 143 patients were randomized to oral venetoclax 600 mg daily and 68 to placebo in 28-day cycles, on a background of LDAC 20 mg/m2 administered subcutaneously on days 1-10 of each cycle.
“This study enrolled a challenging AML population, with nearly 60% age ≥75 years and a high proportion of patients with secondary disease (38%), prior hypomethylating agent (HMA) treatment (20%), poor cytogenetic risk (32%), and TP53 mutations (15%), which are known factors associated with dismal prognosis in AML,” the investigators noted in their report.
There was a numerical benefit in OS at 12 months – the preplanned primary outcome – but it was not statistically significant. At 18 months, however, and after adjustment for a higher rate of secondary AML in the venetoclax arm and other confounders in a post hoc analysis, survival differences reached significance. The 4.3-month OS benefit with the combination translated into a 30% reduction in the risk of death (hazard ratio, 0.70; 95% confidence interval, 0.50-0.99; P = .04)
Survival outcomes “were particularly promising for patient subgroups with NPM1- (median OS, not reached) and IDH1/2-mutant AML (median OS, 19.4 months),” the team noted.
Complete remission (CR) were 48% in the venetoclax arm, compared with 13% in the placebo group, and 34% of venetoclax patients versus 3% of placebo patients went into remission after their first cycle. Venetoclax subjects also had longer median event free survival (4.7 months vs. 2 months); higher rates of red blood cell and platelet transfusion independence (37% vs. 16%); and higher rates of cytometric minimal residual disease levels below 0.1% (6% vs. 1%).
The findings correlated with “strong improvements” in patient-reported outcomes, including fatigue and quality of life, the investigators reported.
Risk mitigation
Grade 3 or higher adverse events (AEs) included febrile neutropenia (32% in the venetoclax arm versus 29% in the placebo group), neutropenia (47% venetoclax vs. 16% placebo), thrombocytopenia (45% vs. 37%), and anemia (25% vs. 22%). The eight cases of tumor lysis syndrome (TLS) were all in the venetoclax arm. Grade 3 or higher bleeding was higher in the venetoclax arm (11% versus 7%), but the incidence of fatal bleeding was similar between the groups (1.4% venetoclax versus 1.5%).
“Although the venetoclax arm showed modest increases in hematologic AEs, the rate of AEs leading to treatment discontinuation (24% vs. 25%) and the rate of serious AEs such as pneumonia” and sepsis “were nearly identical between” the arms, the team said.
The combination “is more myelosuppressive,” but the effects “were mostly mitigated by venetoclax dose interruptions and reductions.” To mitigate the TLS risk, patients were hospitalized for TLS evaluation and prophylaxis during the 4-day venetoclax ramp-up in the first treatment cycle and for 24 hours after the 600-mg target was reached. “I think this is an extremely important measure to avoid this small but important complication,” Dr. Wei said at the meeting.
A moderate step forward
Dr. Lowenberg and Dr. Huls noted in their commentary that, despite the favorable outcomes, “the results are still sobering with a rapid drop of the survival curves to values of [around] 25% or less within 18 months, and event-free survival rates even falling to considerably lower levels.”
Also, there was a “weak correlation between the relatively wide differences in comparative CR/CRi rates and the much smaller differences in survival,” perhaps “due to a limited depth of the complete responses following venetoclax-LDAC therapy or the early development of therapeutic resistance,” they said.
The commentary also noted another option, adding the hedgehog pathway inhibitor glasdegib, instead of venetoclax, to LDAC. It also improved survival in a similar randomized study in unfit AML and high-risk myelodysplastic syndrome patients, from a median survival of 4.9 months with LDAC alone to 8.8 months with the combination (Leukemia. 2019 Feb;33(2):379-389. doi: 10.1038/s41375-018-0312-9).
Dueling regimens
Another alternative approach – venetoclax plus the HMA agent azacitidine – garnered a lot of attention at the meeting when it was reported that the combination had a median overall survival of 14.7 months, versus 9.6 months with azacitidine alone, in patients ineligible for intensive chemotherapy. CR/CRi rates were 66% with the combination, versus 28%.
“It seems like the results were better with the combination of venetoclax and azacitidine” than venetoclax plus LDAC, said Gunnar Juliusson, MD, PhD, of Lund (Sweden) University, who moderated Dr. Wei’s presentation.
He wanted to know if there was a way to identify patients who would do better on one regimen versus the other and was curious about the fact that the azacitidine study used a dose of 400 mg venetoclax, instead of 600 mg.
Dr. Wei noted the high incidence of poor prognostic factors in his study, including prior HMA treatment in 20%, but also that “we don’t know for sure” if there’s a clinically meaningful benefit with the higher dose.
He also said the optimal number of venetoclax cycles for best response is unknown. For now, treatment is “recommend until either [disease] progression, dose intolerance, or patient or physician preference,” he noted. Venetoclax subjects in his study had a median of four treatment cycles versus two in the placebo group. Combination patients in the azacitidine study had a median of seven cycles versus 4.5 with placebo.
Venetoclax already carries an indication in the United States in combination with azacitidine, decitabine, or LDAC for newly-diagnosed AML in adults 75 years or older or who have comorbidities that preclude use of intensive induction chemotherapy, at a daily dosage of 400 mg with HMAs and 600 mg with LDAC.
Labeling notes that “continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
Both venetoclax trials were sponsored by the drug’s maker, AbbVie, which was involved with data interpretation and other matters. Dr. Wei is a consultant for and receives research funding from the company and also receives royalty payments in relation to venetoclax. The commentators did not have any competing financial interests. Disclosures, if any, were not reported for Dr. Juliusson.
SOURCE: Wei AH et al. EHA Congress, Abstract S136.
At about 18 months’ follow-up in treatment naive acute myelogenous leukemia (AML) patients who were 75 years or older or otherwise unfit for intensive chemotherapy, median overall survival (OS) was 8.4 months when they were randomized to low-dose cytarabine (LDAC) plus the BCL-2 inhibitor venetoclax versus 4.1 months with LDAC plus placebo. The results from the phase 3 trial were reported at the virtual annual congress of the European Hematology Association.
The combination also improved rates of remission, event-free survival, and patient reported outcomes and lessened transfusion requirements. Adverse events were manageable.
The findings position venetoclax add-on with LDAC “as a potential new standard of care” for untreated patients ineligible for intensive chemotherapy, lead investigator Andrew Wei, MD, PhD, an AML researcher at Monash University, Melbourne, said at the meeting.
The study addresses a substantial unmet need. The median age at AML diagnosis is over 68 years old and comorbidities such as heart failure and reduced creatinine clearance are common, which make the risk of toxicity with standard chemotherapy too high. Single-agent alternatives are of limited benefit, so Dr. Wei’s group and others are looking for better options to plug the treatment gap when standard chemotherapy is contraindicated.
Several combinations are under investigation, including LDAC plus venetoclax, which appears to have a synergistic effect greater than either agent on its own, Dr. Wei and colleagues explained in their journal report, which was published online to coincide with his presentation (Blood. 2020 Jun 11;135(24):2137-45).
In a commentary, Bob Lowenberg, Ph, a hematologist with the Erasmus University Medical Center in Rotterdam, and Gerwin Huls, MD, PhD, of the University Medical Center Groningen, both in the Netherlands, said the study “represents a valuable although moderate step forward on the way to a better therapeutic future for the ‘unfit’ patient with AML” (Blood. 2020. Jun 11;135(24): 2114-5).
“A challenging AML population”
In the study, 143 patients were randomized to oral venetoclax 600 mg daily and 68 to placebo in 28-day cycles, on a background of LDAC 20 mg/m2 administered subcutaneously on days 1-10 of each cycle.
“This study enrolled a challenging AML population, with nearly 60% age ≥75 years and a high proportion of patients with secondary disease (38%), prior hypomethylating agent (HMA) treatment (20%), poor cytogenetic risk (32%), and TP53 mutations (15%), which are known factors associated with dismal prognosis in AML,” the investigators noted in their report.
There was a numerical benefit in OS at 12 months – the preplanned primary outcome – but it was not statistically significant. At 18 months, however, and after adjustment for a higher rate of secondary AML in the venetoclax arm and other confounders in a post hoc analysis, survival differences reached significance. The 4.3-month OS benefit with the combination translated into a 30% reduction in the risk of death (hazard ratio, 0.70; 95% confidence interval, 0.50-0.99; P = .04)
Survival outcomes “were particularly promising for patient subgroups with NPM1- (median OS, not reached) and IDH1/2-mutant AML (median OS, 19.4 months),” the team noted.
Complete remission (CR) were 48% in the venetoclax arm, compared with 13% in the placebo group, and 34% of venetoclax patients versus 3% of placebo patients went into remission after their first cycle. Venetoclax subjects also had longer median event free survival (4.7 months vs. 2 months); higher rates of red blood cell and platelet transfusion independence (37% vs. 16%); and higher rates of cytometric minimal residual disease levels below 0.1% (6% vs. 1%).
The findings correlated with “strong improvements” in patient-reported outcomes, including fatigue and quality of life, the investigators reported.
Risk mitigation
Grade 3 or higher adverse events (AEs) included febrile neutropenia (32% in the venetoclax arm versus 29% in the placebo group), neutropenia (47% venetoclax vs. 16% placebo), thrombocytopenia (45% vs. 37%), and anemia (25% vs. 22%). The eight cases of tumor lysis syndrome (TLS) were all in the venetoclax arm. Grade 3 or higher bleeding was higher in the venetoclax arm (11% versus 7%), but the incidence of fatal bleeding was similar between the groups (1.4% venetoclax versus 1.5%).
“Although the venetoclax arm showed modest increases in hematologic AEs, the rate of AEs leading to treatment discontinuation (24% vs. 25%) and the rate of serious AEs such as pneumonia” and sepsis “were nearly identical between” the arms, the team said.
The combination “is more myelosuppressive,” but the effects “were mostly mitigated by venetoclax dose interruptions and reductions.” To mitigate the TLS risk, patients were hospitalized for TLS evaluation and prophylaxis during the 4-day venetoclax ramp-up in the first treatment cycle and for 24 hours after the 600-mg target was reached. “I think this is an extremely important measure to avoid this small but important complication,” Dr. Wei said at the meeting.
A moderate step forward
Dr. Lowenberg and Dr. Huls noted in their commentary that, despite the favorable outcomes, “the results are still sobering with a rapid drop of the survival curves to values of [around] 25% or less within 18 months, and event-free survival rates even falling to considerably lower levels.”
Also, there was a “weak correlation between the relatively wide differences in comparative CR/CRi rates and the much smaller differences in survival,” perhaps “due to a limited depth of the complete responses following venetoclax-LDAC therapy or the early development of therapeutic resistance,” they said.
The commentary also noted another option, adding the hedgehog pathway inhibitor glasdegib, instead of venetoclax, to LDAC. It also improved survival in a similar randomized study in unfit AML and high-risk myelodysplastic syndrome patients, from a median survival of 4.9 months with LDAC alone to 8.8 months with the combination (Leukemia. 2019 Feb;33(2):379-389. doi: 10.1038/s41375-018-0312-9).
Dueling regimens
Another alternative approach – venetoclax plus the HMA agent azacitidine – garnered a lot of attention at the meeting when it was reported that the combination had a median overall survival of 14.7 months, versus 9.6 months with azacitidine alone, in patients ineligible for intensive chemotherapy. CR/CRi rates were 66% with the combination, versus 28%.
“It seems like the results were better with the combination of venetoclax and azacitidine” than venetoclax plus LDAC, said Gunnar Juliusson, MD, PhD, of Lund (Sweden) University, who moderated Dr. Wei’s presentation.
He wanted to know if there was a way to identify patients who would do better on one regimen versus the other and was curious about the fact that the azacitidine study used a dose of 400 mg venetoclax, instead of 600 mg.
Dr. Wei noted the high incidence of poor prognostic factors in his study, including prior HMA treatment in 20%, but also that “we don’t know for sure” if there’s a clinically meaningful benefit with the higher dose.
He also said the optimal number of venetoclax cycles for best response is unknown. For now, treatment is “recommend until either [disease] progression, dose intolerance, or patient or physician preference,” he noted. Venetoclax subjects in his study had a median of four treatment cycles versus two in the placebo group. Combination patients in the azacitidine study had a median of seven cycles versus 4.5 with placebo.
Venetoclax already carries an indication in the United States in combination with azacitidine, decitabine, or LDAC for newly-diagnosed AML in adults 75 years or older or who have comorbidities that preclude use of intensive induction chemotherapy, at a daily dosage of 400 mg with HMAs and 600 mg with LDAC.
Labeling notes that “continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
Both venetoclax trials were sponsored by the drug’s maker, AbbVie, which was involved with data interpretation and other matters. Dr. Wei is a consultant for and receives research funding from the company and also receives royalty payments in relation to venetoclax. The commentators did not have any competing financial interests. Disclosures, if any, were not reported for Dr. Juliusson.
SOURCE: Wei AH et al. EHA Congress, Abstract S136.
At about 18 months’ follow-up in treatment naive acute myelogenous leukemia (AML) patients who were 75 years or older or otherwise unfit for intensive chemotherapy, median overall survival (OS) was 8.4 months when they were randomized to low-dose cytarabine (LDAC) plus the BCL-2 inhibitor venetoclax versus 4.1 months with LDAC plus placebo. The results from the phase 3 trial were reported at the virtual annual congress of the European Hematology Association.
The combination also improved rates of remission, event-free survival, and patient reported outcomes and lessened transfusion requirements. Adverse events were manageable.
The findings position venetoclax add-on with LDAC “as a potential new standard of care” for untreated patients ineligible for intensive chemotherapy, lead investigator Andrew Wei, MD, PhD, an AML researcher at Monash University, Melbourne, said at the meeting.
The study addresses a substantial unmet need. The median age at AML diagnosis is over 68 years old and comorbidities such as heart failure and reduced creatinine clearance are common, which make the risk of toxicity with standard chemotherapy too high. Single-agent alternatives are of limited benefit, so Dr. Wei’s group and others are looking for better options to plug the treatment gap when standard chemotherapy is contraindicated.
Several combinations are under investigation, including LDAC plus venetoclax, which appears to have a synergistic effect greater than either agent on its own, Dr. Wei and colleagues explained in their journal report, which was published online to coincide with his presentation (Blood. 2020 Jun 11;135(24):2137-45).
In a commentary, Bob Lowenberg, Ph, a hematologist with the Erasmus University Medical Center in Rotterdam, and Gerwin Huls, MD, PhD, of the University Medical Center Groningen, both in the Netherlands, said the study “represents a valuable although moderate step forward on the way to a better therapeutic future for the ‘unfit’ patient with AML” (Blood. 2020. Jun 11;135(24): 2114-5).
“A challenging AML population”
In the study, 143 patients were randomized to oral venetoclax 600 mg daily and 68 to placebo in 28-day cycles, on a background of LDAC 20 mg/m2 administered subcutaneously on days 1-10 of each cycle.
“This study enrolled a challenging AML population, with nearly 60% age ≥75 years and a high proportion of patients with secondary disease (38%), prior hypomethylating agent (HMA) treatment (20%), poor cytogenetic risk (32%), and TP53 mutations (15%), which are known factors associated with dismal prognosis in AML,” the investigators noted in their report.
There was a numerical benefit in OS at 12 months – the preplanned primary outcome – but it was not statistically significant. At 18 months, however, and after adjustment for a higher rate of secondary AML in the venetoclax arm and other confounders in a post hoc analysis, survival differences reached significance. The 4.3-month OS benefit with the combination translated into a 30% reduction in the risk of death (hazard ratio, 0.70; 95% confidence interval, 0.50-0.99; P = .04)
Survival outcomes “were particularly promising for patient subgroups with NPM1- (median OS, not reached) and IDH1/2-mutant AML (median OS, 19.4 months),” the team noted.
Complete remission (CR) were 48% in the venetoclax arm, compared with 13% in the placebo group, and 34% of venetoclax patients versus 3% of placebo patients went into remission after their first cycle. Venetoclax subjects also had longer median event free survival (4.7 months vs. 2 months); higher rates of red blood cell and platelet transfusion independence (37% vs. 16%); and higher rates of cytometric minimal residual disease levels below 0.1% (6% vs. 1%).
The findings correlated with “strong improvements” in patient-reported outcomes, including fatigue and quality of life, the investigators reported.
Risk mitigation
Grade 3 or higher adverse events (AEs) included febrile neutropenia (32% in the venetoclax arm versus 29% in the placebo group), neutropenia (47% venetoclax vs. 16% placebo), thrombocytopenia (45% vs. 37%), and anemia (25% vs. 22%). The eight cases of tumor lysis syndrome (TLS) were all in the venetoclax arm. Grade 3 or higher bleeding was higher in the venetoclax arm (11% versus 7%), but the incidence of fatal bleeding was similar between the groups (1.4% venetoclax versus 1.5%).
“Although the venetoclax arm showed modest increases in hematologic AEs, the rate of AEs leading to treatment discontinuation (24% vs. 25%) and the rate of serious AEs such as pneumonia” and sepsis “were nearly identical between” the arms, the team said.
The combination “is more myelosuppressive,” but the effects “were mostly mitigated by venetoclax dose interruptions and reductions.” To mitigate the TLS risk, patients were hospitalized for TLS evaluation and prophylaxis during the 4-day venetoclax ramp-up in the first treatment cycle and for 24 hours after the 600-mg target was reached. “I think this is an extremely important measure to avoid this small but important complication,” Dr. Wei said at the meeting.
A moderate step forward
Dr. Lowenberg and Dr. Huls noted in their commentary that, despite the favorable outcomes, “the results are still sobering with a rapid drop of the survival curves to values of [around] 25% or less within 18 months, and event-free survival rates even falling to considerably lower levels.”
Also, there was a “weak correlation between the relatively wide differences in comparative CR/CRi rates and the much smaller differences in survival,” perhaps “due to a limited depth of the complete responses following venetoclax-LDAC therapy or the early development of therapeutic resistance,” they said.
The commentary also noted another option, adding the hedgehog pathway inhibitor glasdegib, instead of venetoclax, to LDAC. It also improved survival in a similar randomized study in unfit AML and high-risk myelodysplastic syndrome patients, from a median survival of 4.9 months with LDAC alone to 8.8 months with the combination (Leukemia. 2019 Feb;33(2):379-389. doi: 10.1038/s41375-018-0312-9).
Dueling regimens
Another alternative approach – venetoclax plus the HMA agent azacitidine – garnered a lot of attention at the meeting when it was reported that the combination had a median overall survival of 14.7 months, versus 9.6 months with azacitidine alone, in patients ineligible for intensive chemotherapy. CR/CRi rates were 66% with the combination, versus 28%.
“It seems like the results were better with the combination of venetoclax and azacitidine” than venetoclax plus LDAC, said Gunnar Juliusson, MD, PhD, of Lund (Sweden) University, who moderated Dr. Wei’s presentation.
He wanted to know if there was a way to identify patients who would do better on one regimen versus the other and was curious about the fact that the azacitidine study used a dose of 400 mg venetoclax, instead of 600 mg.
Dr. Wei noted the high incidence of poor prognostic factors in his study, including prior HMA treatment in 20%, but also that “we don’t know for sure” if there’s a clinically meaningful benefit with the higher dose.
He also said the optimal number of venetoclax cycles for best response is unknown. For now, treatment is “recommend until either [disease] progression, dose intolerance, or patient or physician preference,” he noted. Venetoclax subjects in his study had a median of four treatment cycles versus two in the placebo group. Combination patients in the azacitidine study had a median of seven cycles versus 4.5 with placebo.
Venetoclax already carries an indication in the United States in combination with azacitidine, decitabine, or LDAC for newly-diagnosed AML in adults 75 years or older or who have comorbidities that preclude use of intensive induction chemotherapy, at a daily dosage of 400 mg with HMAs and 600 mg with LDAC.
Labeling notes that “continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
Both venetoclax trials were sponsored by the drug’s maker, AbbVie, which was involved with data interpretation and other matters. Dr. Wei is a consultant for and receives research funding from the company and also receives royalty payments in relation to venetoclax. The commentators did not have any competing financial interests. Disclosures, if any, were not reported for Dr. Juliusson.
SOURCE: Wei AH et al. EHA Congress, Abstract S136.
REPORTING FROM EHA CONGRESS
Azacitidine plus enasidenib improves response, but not survival, in mIDH2 AML
Azacitidine plus enasidenib improved complete and overall responses in newly diagnosed acute myelogenous leukemia with isocitrate dehydrogenase 2 gene mutations, compared with azacitidine alone, but it did not improve overall survival in an open-label, phase 2 trial reported at the virtual annual congress of the European Hematology Association.
“Given the very high cost of” enasidenib, and the lack of survival benefit, Gunnar Juliusson, MD, PhD, of Lund University, Sweden, who moderated the study presentation, wondered if it might make more sense to hold enasidenib in reserve until after progression on azacitidine.
“The challenge is going to be exactly” that, “trying to figure out [if] you use both things together” or in sequence. “You can look at it in both ways,” said lead investigator Courtney DiNardo, MD, associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
“We do know” that with enasidenib monotherapy, there’s “a decrement in the rates of remission and in the duration of response” and overall survival in the salvage setting, so there’s “a clear rationale to give it earlier rather than later,” but “I think this study in some ways provides a few more questions than it really answers,” she said at the meeting.
About 15% of AML patients have leukemogenic isocitrate dehydrogenase 2 (IDH2) mutations; enasidenib, an oral small molecule, inhibits the mutant enzyme. The older AML patients are, the more likely they are to have an IDH2 mutation, so the work “is relevant to our older chemotherapy ineligible population,” Dr. DiNardo said.
The trial was prompted by preclinical indications of synergy with azacitidine; alone, each agent has an overall response rate of about 30% in newly diagnosed AML, and a complete remission (CR) rate of about 20%, she explained.
Her team randomized 68 adults with newly diagnosed AML and an IDH2 mutation to enasidenib 100 mg daily on a 28-day cycle with subcutaneous azacitidine 75 mg/m2 for 7 days during the cycle, and 33 others to just the azacitidine alone.
Their subjects were ineligible for intensive chemotherapy and had intermediate to poor risk cytogenetics. The median age was 75 years, and Eastern Cooperative Oncology Group performance scores were 2 or less.
The overall response rate was 71% with the combination and 42% in the azacitidine alone arm (P = .0064). Fifty-three percent of combination patients, but 12% of azacitidine alone subjects, had complete remissions (P = .0001). The median duration of response with combination therapy was 24.1 months, versus 12.1 months.
Enasidenib plus azacitidine subjects also had greater drops in mutant IDH2 variant allele frequency (median 83.4% versus 17.7%, P < .01) and levels of the downstream oncometabolite 2-hydroxyglutarate (97.8% versus 54.3%; P < .01).
However, median OS was 22 months in both arms (HR 0.99, 95% CI 0.52, 1.87, P = .97). Although median event-free survival favored the combination – 17.2 months versus 10.8 – the results were not statistically significant (HR 0.59, 95% CI 0.30, 1.17, P = .13).
A possible reason for the lack of survival benefit, Dr. DiNardo said, was that seven azacitidine-alone patients (21%) went on to enasidenib after leaving the study, most commonly for disease progression, which occurred in 31% of combination patients versus 52% in the azacitidine-alone arm.
Combination subjects had a median of 10 treatment cycles, vs. 7 in the azacitidine-alone group. Grade 3-4 adverse events included thrombocytopenia (37% combination, 19% azacitidine-alone), neutropenia (35% vs. 22%), anemia (19% vs. 22%), and febrile neutropenia (15% vs. 16%). Grade 3-4 infections were more common with azacitidine monotherapy (31% vs. 18%).
Twelve enasidenib/azacitidine subjects (18%) developed isocitrate dehydrogenase differentiation syndrome, a complication that carries a black box warning in enasidenib’s label.
The work was funded by enasidenib marketer Celgene. Dr. DiNardo is an adviser to, and receives research funding from, the company. Dr. Juliusson’s disclosures, if any, were not reported.
SOURCE: DiNardo CD et al. EHA Congress, abstract S139.
Azacitidine plus enasidenib improved complete and overall responses in newly diagnosed acute myelogenous leukemia with isocitrate dehydrogenase 2 gene mutations, compared with azacitidine alone, but it did not improve overall survival in an open-label, phase 2 trial reported at the virtual annual congress of the European Hematology Association.
“Given the very high cost of” enasidenib, and the lack of survival benefit, Gunnar Juliusson, MD, PhD, of Lund University, Sweden, who moderated the study presentation, wondered if it might make more sense to hold enasidenib in reserve until after progression on azacitidine.
“The challenge is going to be exactly” that, “trying to figure out [if] you use both things together” or in sequence. “You can look at it in both ways,” said lead investigator Courtney DiNardo, MD, associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
“We do know” that with enasidenib monotherapy, there’s “a decrement in the rates of remission and in the duration of response” and overall survival in the salvage setting, so there’s “a clear rationale to give it earlier rather than later,” but “I think this study in some ways provides a few more questions than it really answers,” she said at the meeting.
About 15% of AML patients have leukemogenic isocitrate dehydrogenase 2 (IDH2) mutations; enasidenib, an oral small molecule, inhibits the mutant enzyme. The older AML patients are, the more likely they are to have an IDH2 mutation, so the work “is relevant to our older chemotherapy ineligible population,” Dr. DiNardo said.
The trial was prompted by preclinical indications of synergy with azacitidine; alone, each agent has an overall response rate of about 30% in newly diagnosed AML, and a complete remission (CR) rate of about 20%, she explained.
Her team randomized 68 adults with newly diagnosed AML and an IDH2 mutation to enasidenib 100 mg daily on a 28-day cycle with subcutaneous azacitidine 75 mg/m2 for 7 days during the cycle, and 33 others to just the azacitidine alone.
Their subjects were ineligible for intensive chemotherapy and had intermediate to poor risk cytogenetics. The median age was 75 years, and Eastern Cooperative Oncology Group performance scores were 2 or less.
The overall response rate was 71% with the combination and 42% in the azacitidine alone arm (P = .0064). Fifty-three percent of combination patients, but 12% of azacitidine alone subjects, had complete remissions (P = .0001). The median duration of response with combination therapy was 24.1 months, versus 12.1 months.
Enasidenib plus azacitidine subjects also had greater drops in mutant IDH2 variant allele frequency (median 83.4% versus 17.7%, P < .01) and levels of the downstream oncometabolite 2-hydroxyglutarate (97.8% versus 54.3%; P < .01).
However, median OS was 22 months in both arms (HR 0.99, 95% CI 0.52, 1.87, P = .97). Although median event-free survival favored the combination – 17.2 months versus 10.8 – the results were not statistically significant (HR 0.59, 95% CI 0.30, 1.17, P = .13).
A possible reason for the lack of survival benefit, Dr. DiNardo said, was that seven azacitidine-alone patients (21%) went on to enasidenib after leaving the study, most commonly for disease progression, which occurred in 31% of combination patients versus 52% in the azacitidine-alone arm.
Combination subjects had a median of 10 treatment cycles, vs. 7 in the azacitidine-alone group. Grade 3-4 adverse events included thrombocytopenia (37% combination, 19% azacitidine-alone), neutropenia (35% vs. 22%), anemia (19% vs. 22%), and febrile neutropenia (15% vs. 16%). Grade 3-4 infections were more common with azacitidine monotherapy (31% vs. 18%).
Twelve enasidenib/azacitidine subjects (18%) developed isocitrate dehydrogenase differentiation syndrome, a complication that carries a black box warning in enasidenib’s label.
The work was funded by enasidenib marketer Celgene. Dr. DiNardo is an adviser to, and receives research funding from, the company. Dr. Juliusson’s disclosures, if any, were not reported.
SOURCE: DiNardo CD et al. EHA Congress, abstract S139.
Azacitidine plus enasidenib improved complete and overall responses in newly diagnosed acute myelogenous leukemia with isocitrate dehydrogenase 2 gene mutations, compared with azacitidine alone, but it did not improve overall survival in an open-label, phase 2 trial reported at the virtual annual congress of the European Hematology Association.
“Given the very high cost of” enasidenib, and the lack of survival benefit, Gunnar Juliusson, MD, PhD, of Lund University, Sweden, who moderated the study presentation, wondered if it might make more sense to hold enasidenib in reserve until after progression on azacitidine.
“The challenge is going to be exactly” that, “trying to figure out [if] you use both things together” or in sequence. “You can look at it in both ways,” said lead investigator Courtney DiNardo, MD, associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
“We do know” that with enasidenib monotherapy, there’s “a decrement in the rates of remission and in the duration of response” and overall survival in the salvage setting, so there’s “a clear rationale to give it earlier rather than later,” but “I think this study in some ways provides a few more questions than it really answers,” she said at the meeting.
About 15% of AML patients have leukemogenic isocitrate dehydrogenase 2 (IDH2) mutations; enasidenib, an oral small molecule, inhibits the mutant enzyme. The older AML patients are, the more likely they are to have an IDH2 mutation, so the work “is relevant to our older chemotherapy ineligible population,” Dr. DiNardo said.
The trial was prompted by preclinical indications of synergy with azacitidine; alone, each agent has an overall response rate of about 30% in newly diagnosed AML, and a complete remission (CR) rate of about 20%, she explained.
Her team randomized 68 adults with newly diagnosed AML and an IDH2 mutation to enasidenib 100 mg daily on a 28-day cycle with subcutaneous azacitidine 75 mg/m2 for 7 days during the cycle, and 33 others to just the azacitidine alone.
Their subjects were ineligible for intensive chemotherapy and had intermediate to poor risk cytogenetics. The median age was 75 years, and Eastern Cooperative Oncology Group performance scores were 2 or less.
The overall response rate was 71% with the combination and 42% in the azacitidine alone arm (P = .0064). Fifty-three percent of combination patients, but 12% of azacitidine alone subjects, had complete remissions (P = .0001). The median duration of response with combination therapy was 24.1 months, versus 12.1 months.
Enasidenib plus azacitidine subjects also had greater drops in mutant IDH2 variant allele frequency (median 83.4% versus 17.7%, P < .01) and levels of the downstream oncometabolite 2-hydroxyglutarate (97.8% versus 54.3%; P < .01).
However, median OS was 22 months in both arms (HR 0.99, 95% CI 0.52, 1.87, P = .97). Although median event-free survival favored the combination – 17.2 months versus 10.8 – the results were not statistically significant (HR 0.59, 95% CI 0.30, 1.17, P = .13).
A possible reason for the lack of survival benefit, Dr. DiNardo said, was that seven azacitidine-alone patients (21%) went on to enasidenib after leaving the study, most commonly for disease progression, which occurred in 31% of combination patients versus 52% in the azacitidine-alone arm.
Combination subjects had a median of 10 treatment cycles, vs. 7 in the azacitidine-alone group. Grade 3-4 adverse events included thrombocytopenia (37% combination, 19% azacitidine-alone), neutropenia (35% vs. 22%), anemia (19% vs. 22%), and febrile neutropenia (15% vs. 16%). Grade 3-4 infections were more common with azacitidine monotherapy (31% vs. 18%).
Twelve enasidenib/azacitidine subjects (18%) developed isocitrate dehydrogenase differentiation syndrome, a complication that carries a black box warning in enasidenib’s label.
The work was funded by enasidenib marketer Celgene. Dr. DiNardo is an adviser to, and receives research funding from, the company. Dr. Juliusson’s disclosures, if any, were not reported.
SOURCE: DiNardo CD et al. EHA Congress, abstract S139.
FROM EHA CONGRESS
ID dermatology: Advancements, but new challenges, over 50 years
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
Combo exhibits activity in metastatic mucosal melanoma
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
FROM ASCO 2020
Compound CAR T – a double whammy with promise for AML
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
FROM EHA CONGRESS
Adding low-dose ipi to pembro seems safer, still effective for advanced melanoma
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
FROM ASCO 2020
Key clinical point: Low-dose ipilimumab (1 mg/kg) plus pembrolizumab given immediately after progression on a PD-1 antibody alone demonstrated antitumor activity and tolerability in patients with advanced melanoma, according to an investigator.
Major finding: There were 5 complete responses and 14 partial responses, for a response rate of 27%. The rate of grade 3/4 adverse events was 27%.
Study details: Phase 2 study of 70 patients, 61 of whom were evaluable for response.
Disclosures: The study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company.
Source: Olson D et al. ASCO 2020, Abstract 10004.