User login
Telemedicine offers solution for late cancellations and no-show appointments
TOPLINE:
Converting late cancellations and no-show appointments to telemedicine visits increases access to care without the need for rescheduling, according to new research.
METHODOLOGY:
- Investigators identified adult rheumatology patients with late cancellations (within 24 hours of appointment) or impending no-show appointments from September 2020 to March 2023.
- These patients were contacted and were offered the option of converting their in-person appointment to a telemedicine visit, either by phone or video.
- The program was piloted at one clinic beginning Sept. 1, 2020, and was expanded to a second clinic on Sept. 1, 2021.
TAKEAWAY:
- Of 624 eligible visits, 516 (83%) were converted to telehealth visits. Phone visits were slightly more popular than video visits (54% vs. 46%, respectively).
- Patients who were older, who lived in a rural area, or who were on Medicare and Medicaid were more likely to opt for phone visits.
- The intervention resulted in an additional 258 hours of patient care.
- The reduction in lost revenue for phone versus video telemedicine visits was $7,298 ($39.19 per appointment).
IN PRACTICE:
“Our simple, targeted strategy of converting appointments to telehealth when an in-person appointment is identified as at-risk resulted in significant access gains and modest revenue loss reduction,” with net gains overall, the authors write.
SOURCE:
Sancia Ferguson MD, MPH, of the University of Wisconsin-Madison School of Medicine, presented the research at the annual meeting of the American College of Rheumatology, abstract 1007.
LIMITATIONS:
The study was conducted at two clinics in the UW Health system and may not be implementable in smaller practices.
DISCLOSURES:
Senior author Christie Bartels, MD, also of University of Washington-Madison School of Medicine, reports receiving a research grant from Pfizer unrelated to this study.
A version of this article appeared on Medscape.com.
TOPLINE:
Converting late cancellations and no-show appointments to telemedicine visits increases access to care without the need for rescheduling, according to new research.
METHODOLOGY:
- Investigators identified adult rheumatology patients with late cancellations (within 24 hours of appointment) or impending no-show appointments from September 2020 to March 2023.
- These patients were contacted and were offered the option of converting their in-person appointment to a telemedicine visit, either by phone or video.
- The program was piloted at one clinic beginning Sept. 1, 2020, and was expanded to a second clinic on Sept. 1, 2021.
TAKEAWAY:
- Of 624 eligible visits, 516 (83%) were converted to telehealth visits. Phone visits were slightly more popular than video visits (54% vs. 46%, respectively).
- Patients who were older, who lived in a rural area, or who were on Medicare and Medicaid were more likely to opt for phone visits.
- The intervention resulted in an additional 258 hours of patient care.
- The reduction in lost revenue for phone versus video telemedicine visits was $7,298 ($39.19 per appointment).
IN PRACTICE:
“Our simple, targeted strategy of converting appointments to telehealth when an in-person appointment is identified as at-risk resulted in significant access gains and modest revenue loss reduction,” with net gains overall, the authors write.
SOURCE:
Sancia Ferguson MD, MPH, of the University of Wisconsin-Madison School of Medicine, presented the research at the annual meeting of the American College of Rheumatology, abstract 1007.
LIMITATIONS:
The study was conducted at two clinics in the UW Health system and may not be implementable in smaller practices.
DISCLOSURES:
Senior author Christie Bartels, MD, also of University of Washington-Madison School of Medicine, reports receiving a research grant from Pfizer unrelated to this study.
A version of this article appeared on Medscape.com.
TOPLINE:
Converting late cancellations and no-show appointments to telemedicine visits increases access to care without the need for rescheduling, according to new research.
METHODOLOGY:
- Investigators identified adult rheumatology patients with late cancellations (within 24 hours of appointment) or impending no-show appointments from September 2020 to March 2023.
- These patients were contacted and were offered the option of converting their in-person appointment to a telemedicine visit, either by phone or video.
- The program was piloted at one clinic beginning Sept. 1, 2020, and was expanded to a second clinic on Sept. 1, 2021.
TAKEAWAY:
- Of 624 eligible visits, 516 (83%) were converted to telehealth visits. Phone visits were slightly more popular than video visits (54% vs. 46%, respectively).
- Patients who were older, who lived in a rural area, or who were on Medicare and Medicaid were more likely to opt for phone visits.
- The intervention resulted in an additional 258 hours of patient care.
- The reduction in lost revenue for phone versus video telemedicine visits was $7,298 ($39.19 per appointment).
IN PRACTICE:
“Our simple, targeted strategy of converting appointments to telehealth when an in-person appointment is identified as at-risk resulted in significant access gains and modest revenue loss reduction,” with net gains overall, the authors write.
SOURCE:
Sancia Ferguson MD, MPH, of the University of Wisconsin-Madison School of Medicine, presented the research at the annual meeting of the American College of Rheumatology, abstract 1007.
LIMITATIONS:
The study was conducted at two clinics in the UW Health system and may not be implementable in smaller practices.
DISCLOSURES:
Senior author Christie Bartels, MD, also of University of Washington-Madison School of Medicine, reports receiving a research grant from Pfizer unrelated to this study.
A version of this article appeared on Medscape.com.
Split-dose methotrexate speeds RA response over single dose
SAN DIEGO – A split dose of methotrexate (MTX) given orally once per week showed significantly higher efficacy in patients with rheumatoid arthritis at 16 weeks, compared with a single MTX dose weekly, according to new research. By 24 weeks, efficacy measures were similar for both groups.
However, fewer patients in the split-dose group needed additional disease-modifying antirheumatic drugs (DMARDs) to control disease activity.
MTX is a highly utilized, inexpensive drug for RA, but only about 30% of patients can achieve low disease activity or remission on MTX monotherapy, said Varun Dhir, MD, MBBS, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. He co-authored and presented the research at the annual meeting of the American College of Rheumatology.
Part of the problem is that “oral methotrexate absorption from the gut reduces as the doses go up,” Dr. Dhir noted, because the transport mechanism gets saturated. MTX delivered subcutaneously is one way to improve efficacy, but patients can be needle-averse, and in some countries, like India, pre-filled syringes are not available, he said.
There is pharmacokinetic data dating back 20 years that suggest split-dose MTX could be more efficacious. “However, there are no randomized controlled trials to date, and the guidelines therefore are silent on this approach,” Dr. Dhir said.
To address this question, Dr. Dhir and colleagues recruited patients with RA from six centers across India. Patients were aged 18-60 years, seropositive (rheumatoid factor or anti-citrullinated protein antibodies), and had a disease duration of 5 years or fewer. Patients had active disease, defined as at least four tender joints and at least two swollen joints, and were not taking any DMARDs except for hydroxychloroquine and/or low-dose prednisolone.
A total of 253 patients were randomly assigned to a single 25-mg dose or a split-dose of MTX once weekly (10 mg in the morning and 15 mg in the evening on the same day). The primary outcome was a European Alliance of Associations for Rheumatology (EULAR) good response at 24 weeks. At the 16-week mark, if patients had not achieved low disease activity based on a 28-joint Disease Activity Score (DAS28) greater than 3.2, a blinded assessor could add either leflunomide or sulfasalazine to the continued MTX therapy.
At baseline, there was no difference between the groups’ DAS28, but after 16 weeks, DAS28 was significantly lower in the split-dose group, compared with the single-dose group (4.4 vs. 5.1; P < .001), and a higher percentage of patients in the split-dose group had a EULAR good response.
About three-quarters (76.6%) of patients in the split-dose group experienced an improvement of at least 20% in ACR response criteria (ACR20), compared with 52% in the single-dose group. The split-dose group also had higher proportion of patients achieving ACR50 and ACR70.
About one-third of the split-dose group (35%) added an additional DMARD at 16 weeks, compared with 54.5% of the single-dose group (P = .005).
After 24 weeks, DAS28 scores remained lower in the split-dose group (4.1 vs. 4.5; P = .03), but there were no other differences in treatment responses. Health Assessment Questionnaire scores were the same between both groups at 16 and 24 weeks.
The primary outcome was not met, although Dr. Dhir noted a flaw in the study design that could have affected the results. By allowing patients to add additional DMARDs at 16 weeks, “there were two factors which were affecting the primary outcome” at 24 weeks, he told this news organization. “I feel there was a robust result at least at 16 weeks.”
While there were no major adverse events, the split-dose group had higher rates of transaminitis (elevated liver enzymes) during the study, and low white blood cell count was higher in the single-dose group at 24 weeks. There was no difference in MTX intolerance between the two groups.
“It looks like [the split-dose group] gets out of the block faster. It’s a faster effect,” although the other group did catch up, Janet Pope, MD, MPH, of Western University, London, Ont., said in an interview. She was not involved with the research. Two positive results were the earlier ACR responses in the split-dose group as well as fewer patients in that same group needing to add another DMARD to therapy.
“In my opinion, if it’s equal cost, why not try it and see?” she said.
In a separate presentation referring to the abstract, Joan Bathon, MD, director of rheumatology at Columbia University, New York City, noted that these results align with ACR 2021 recommendations. Dr. Bathon was not involved with this study but was on the writing committee establishing those 2021 guidelines.
“The recommendation – with low certainty of evidence – was that for patients who are intolerant to MTX, that split-dose of oral MTX is worth trying before you switch to a different DMARD,” she said. “I think these data support that concept.”
Dr. Dhir and Dr. Bathon had no relevant financial relationships. Dr. Pope disclosed financial relationships with AbbVie/Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, GlaxoSmithKline, Janssen, Mallinckrodt, Novartis, Organon, Pfizer, Sandoz, and Viatris.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A split dose of methotrexate (MTX) given orally once per week showed significantly higher efficacy in patients with rheumatoid arthritis at 16 weeks, compared with a single MTX dose weekly, according to new research. By 24 weeks, efficacy measures were similar for both groups.
However, fewer patients in the split-dose group needed additional disease-modifying antirheumatic drugs (DMARDs) to control disease activity.
MTX is a highly utilized, inexpensive drug for RA, but only about 30% of patients can achieve low disease activity or remission on MTX monotherapy, said Varun Dhir, MD, MBBS, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. He co-authored and presented the research at the annual meeting of the American College of Rheumatology.
Part of the problem is that “oral methotrexate absorption from the gut reduces as the doses go up,” Dr. Dhir noted, because the transport mechanism gets saturated. MTX delivered subcutaneously is one way to improve efficacy, but patients can be needle-averse, and in some countries, like India, pre-filled syringes are not available, he said.
There is pharmacokinetic data dating back 20 years that suggest split-dose MTX could be more efficacious. “However, there are no randomized controlled trials to date, and the guidelines therefore are silent on this approach,” Dr. Dhir said.
To address this question, Dr. Dhir and colleagues recruited patients with RA from six centers across India. Patients were aged 18-60 years, seropositive (rheumatoid factor or anti-citrullinated protein antibodies), and had a disease duration of 5 years or fewer. Patients had active disease, defined as at least four tender joints and at least two swollen joints, and were not taking any DMARDs except for hydroxychloroquine and/or low-dose prednisolone.
A total of 253 patients were randomly assigned to a single 25-mg dose or a split-dose of MTX once weekly (10 mg in the morning and 15 mg in the evening on the same day). The primary outcome was a European Alliance of Associations for Rheumatology (EULAR) good response at 24 weeks. At the 16-week mark, if patients had not achieved low disease activity based on a 28-joint Disease Activity Score (DAS28) greater than 3.2, a blinded assessor could add either leflunomide or sulfasalazine to the continued MTX therapy.
At baseline, there was no difference between the groups’ DAS28, but after 16 weeks, DAS28 was significantly lower in the split-dose group, compared with the single-dose group (4.4 vs. 5.1; P < .001), and a higher percentage of patients in the split-dose group had a EULAR good response.
About three-quarters (76.6%) of patients in the split-dose group experienced an improvement of at least 20% in ACR response criteria (ACR20), compared with 52% in the single-dose group. The split-dose group also had higher proportion of patients achieving ACR50 and ACR70.
About one-third of the split-dose group (35%) added an additional DMARD at 16 weeks, compared with 54.5% of the single-dose group (P = .005).
After 24 weeks, DAS28 scores remained lower in the split-dose group (4.1 vs. 4.5; P = .03), but there were no other differences in treatment responses. Health Assessment Questionnaire scores were the same between both groups at 16 and 24 weeks.
The primary outcome was not met, although Dr. Dhir noted a flaw in the study design that could have affected the results. By allowing patients to add additional DMARDs at 16 weeks, “there were two factors which were affecting the primary outcome” at 24 weeks, he told this news organization. “I feel there was a robust result at least at 16 weeks.”
While there were no major adverse events, the split-dose group had higher rates of transaminitis (elevated liver enzymes) during the study, and low white blood cell count was higher in the single-dose group at 24 weeks. There was no difference in MTX intolerance between the two groups.
“It looks like [the split-dose group] gets out of the block faster. It’s a faster effect,” although the other group did catch up, Janet Pope, MD, MPH, of Western University, London, Ont., said in an interview. She was not involved with the research. Two positive results were the earlier ACR responses in the split-dose group as well as fewer patients in that same group needing to add another DMARD to therapy.
“In my opinion, if it’s equal cost, why not try it and see?” she said.
In a separate presentation referring to the abstract, Joan Bathon, MD, director of rheumatology at Columbia University, New York City, noted that these results align with ACR 2021 recommendations. Dr. Bathon was not involved with this study but was on the writing committee establishing those 2021 guidelines.
“The recommendation – with low certainty of evidence – was that for patients who are intolerant to MTX, that split-dose of oral MTX is worth trying before you switch to a different DMARD,” she said. “I think these data support that concept.”
Dr. Dhir and Dr. Bathon had no relevant financial relationships. Dr. Pope disclosed financial relationships with AbbVie/Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, GlaxoSmithKline, Janssen, Mallinckrodt, Novartis, Organon, Pfizer, Sandoz, and Viatris.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A split dose of methotrexate (MTX) given orally once per week showed significantly higher efficacy in patients with rheumatoid arthritis at 16 weeks, compared with a single MTX dose weekly, according to new research. By 24 weeks, efficacy measures were similar for both groups.
However, fewer patients in the split-dose group needed additional disease-modifying antirheumatic drugs (DMARDs) to control disease activity.
MTX is a highly utilized, inexpensive drug for RA, but only about 30% of patients can achieve low disease activity or remission on MTX monotherapy, said Varun Dhir, MD, MBBS, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. He co-authored and presented the research at the annual meeting of the American College of Rheumatology.
Part of the problem is that “oral methotrexate absorption from the gut reduces as the doses go up,” Dr. Dhir noted, because the transport mechanism gets saturated. MTX delivered subcutaneously is one way to improve efficacy, but patients can be needle-averse, and in some countries, like India, pre-filled syringes are not available, he said.
There is pharmacokinetic data dating back 20 years that suggest split-dose MTX could be more efficacious. “However, there are no randomized controlled trials to date, and the guidelines therefore are silent on this approach,” Dr. Dhir said.
To address this question, Dr. Dhir and colleagues recruited patients with RA from six centers across India. Patients were aged 18-60 years, seropositive (rheumatoid factor or anti-citrullinated protein antibodies), and had a disease duration of 5 years or fewer. Patients had active disease, defined as at least four tender joints and at least two swollen joints, and were not taking any DMARDs except for hydroxychloroquine and/or low-dose prednisolone.
A total of 253 patients were randomly assigned to a single 25-mg dose or a split-dose of MTX once weekly (10 mg in the morning and 15 mg in the evening on the same day). The primary outcome was a European Alliance of Associations for Rheumatology (EULAR) good response at 24 weeks. At the 16-week mark, if patients had not achieved low disease activity based on a 28-joint Disease Activity Score (DAS28) greater than 3.2, a blinded assessor could add either leflunomide or sulfasalazine to the continued MTX therapy.
At baseline, there was no difference between the groups’ DAS28, but after 16 weeks, DAS28 was significantly lower in the split-dose group, compared with the single-dose group (4.4 vs. 5.1; P < .001), and a higher percentage of patients in the split-dose group had a EULAR good response.
About three-quarters (76.6%) of patients in the split-dose group experienced an improvement of at least 20% in ACR response criteria (ACR20), compared with 52% in the single-dose group. The split-dose group also had higher proportion of patients achieving ACR50 and ACR70.
About one-third of the split-dose group (35%) added an additional DMARD at 16 weeks, compared with 54.5% of the single-dose group (P = .005).
After 24 weeks, DAS28 scores remained lower in the split-dose group (4.1 vs. 4.5; P = .03), but there were no other differences in treatment responses. Health Assessment Questionnaire scores were the same between both groups at 16 and 24 weeks.
The primary outcome was not met, although Dr. Dhir noted a flaw in the study design that could have affected the results. By allowing patients to add additional DMARDs at 16 weeks, “there were two factors which were affecting the primary outcome” at 24 weeks, he told this news organization. “I feel there was a robust result at least at 16 weeks.”
While there were no major adverse events, the split-dose group had higher rates of transaminitis (elevated liver enzymes) during the study, and low white blood cell count was higher in the single-dose group at 24 weeks. There was no difference in MTX intolerance between the two groups.
“It looks like [the split-dose group] gets out of the block faster. It’s a faster effect,” although the other group did catch up, Janet Pope, MD, MPH, of Western University, London, Ont., said in an interview. She was not involved with the research. Two positive results were the earlier ACR responses in the split-dose group as well as fewer patients in that same group needing to add another DMARD to therapy.
“In my opinion, if it’s equal cost, why not try it and see?” she said.
In a separate presentation referring to the abstract, Joan Bathon, MD, director of rheumatology at Columbia University, New York City, noted that these results align with ACR 2021 recommendations. Dr. Bathon was not involved with this study but was on the writing committee establishing those 2021 guidelines.
“The recommendation – with low certainty of evidence – was that for patients who are intolerant to MTX, that split-dose of oral MTX is worth trying before you switch to a different DMARD,” she said. “I think these data support that concept.”
Dr. Dhir and Dr. Bathon had no relevant financial relationships. Dr. Pope disclosed financial relationships with AbbVie/Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, GlaxoSmithKline, Janssen, Mallinckrodt, Novartis, Organon, Pfizer, Sandoz, and Viatris.
A version of this article first appeared on Medscape.com.
AT ACR 2023
First referral guide issued for axial spondyloarthritis
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.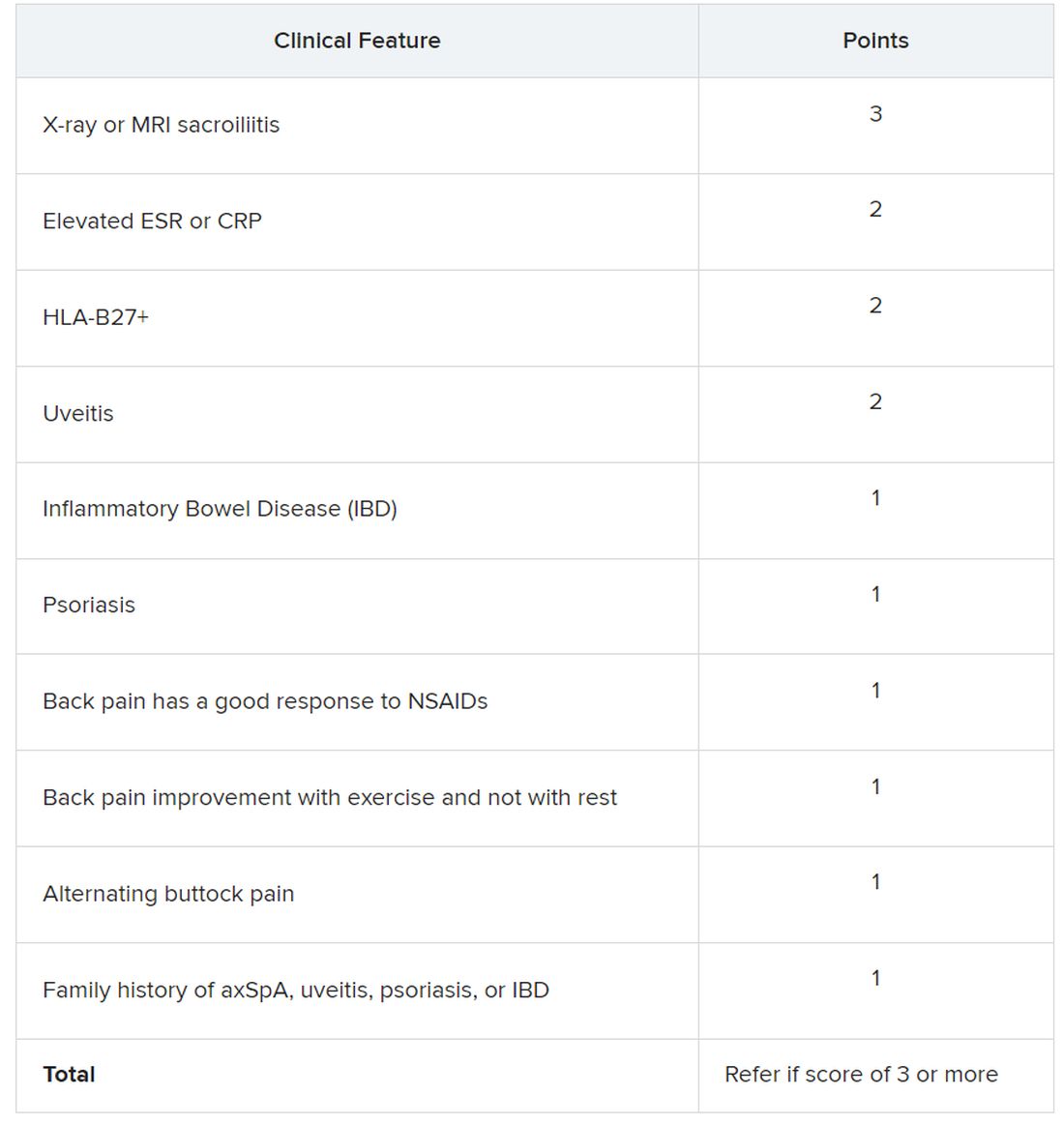
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
AT ACR 2023
TNF blockers not associated with poorer pregnancy outcomes
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
AT ACR 2023
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
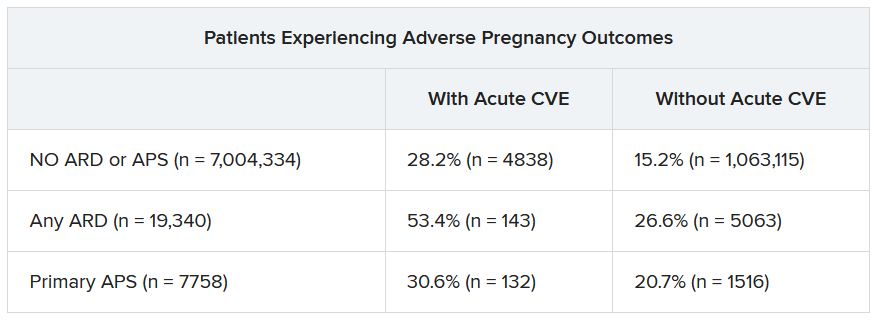
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
FDA OKs first ustekinumab biosimilar
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
FDA approves abatacept for pediatric patients with psoriatic arthritis
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
RA precision medicine using synovial biopsy ‘remains elusive’
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Tech encourages HIV prevention among women
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
FDA warns of hidden ingredients in arthritis, pain products
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.