User login
How should you manage a depressed patient unresponsive to an SSRI?
The best approach among studied alternatives to manage a patient with treatment-resistant depression is not clear from the evidence. All of the options reviewed seem to have about a 25% to 30% success rate.
Switching to other antidepressants or augmenting with non-antidepressant drugs has the best supporting evidence (strength of recommendation [SOR]: B).1 Adding additional antidepressants (SOR: B), using psychotherapy (SOR: B), and initiating electroconvulsive therapy (ECT) (SOR: C) are options. Various antidepressants are used as add-on therapy. Psychotherapy is often recommended, though the evidence of benefit after a failed course of initial therapy is sparse. The evidence supporting use of ECT in treatment-resistant depression is weak.
Comparison among the options is based on expert opinion (SOR: C). Additional reports from the STAR*D trial may improve the quality of the evidence in the near future.
Optimize initial drug dose and duration, then change to a different medication if needed
David Schneider, MD
Sutter Health Center, Santa Rosa, Calif
Although “epidemics” of obesity and avian influenza steal headlines, depression remains an American scourge, with a lifetime prevalence of 13% and rising. Major depressive disorder is often a chronic or relapsing illness, with recurrence rates of more than 40% at 2 years. Treatment resistance may be associated with pain (which is a presenting symptom in two thirds of depressed patients), psychosocial factors, psychiatric comorbidities, or the presence of bipolar disorder rather than unipolar depression.
Various forms of counseling and psychotherapy, alone or in combination with medications, are effective in treating depression, and I recommend them liberally when resources permit. Like the authors of this review, I first optimize initial drug dose and duration, then change to a different medication if needed. Some evidence suggests benefit from a combined serotonin and norepinephrine agent, such as venlafaxine (Effexor) or imipramine (Tofranil), which may also alleviate pain. I often add a noradrenergic tricyclic antidepressant, such as nortriptyline or desipramine, or the newer agent mirtazapine (Remeron), to a selective serotonin reuptake inhibitor (SSRI) for augmentation. I encourage physicians not to fear tricyclics, although I am hesitant to use lithium, thyroxine, or atypical antipsychotics in depression because of their hazards.
Evidence summary
In general, strategies for addressing treatment-resistant depression have not been compared in head-to-head studies. Guidelines at this time are based mainly on expert opinion2,3 and gradually accumulating data from a few randomized controlled studies or low-quality cohort studies.
While it makes sense, as the question implies, to first optimize the dose and duration of SSRI treatment in treatment-resistant depression, it is not clear which strategy to employ next. Switch, augmentation, and combination strategies may each improve clinical outcomes, but which strategy is best is based on expert opinion at this time.
Optimize. The first step in treatment-resistant depression should be optimizing dose and duration of therapy.4 For fluoxetine (Prozac), based on a nonrandomized open trial, patients should receive 8 weeks of treatment before the SSRI course is deemed adequate. Only 23% of patients who have not responded to 8 weeks of fluoxetine respond to a still longer course of fluoxetine.
Switch. The strongest evidence is from the recent STAR*D trial, a randomized study that assigned patients in one arm of the study who had no relief from (or did not tolerate) therapy with citalopram (Celexa) to 1 of 3 drugs—sustained-release bupropion (Wellbutrin SR), sertraline (Zoloft), or extended-release venlafaxine (Effexor XR). The study concluded that approximately 1 in 4 patients have remission after switching to an antidepressant from another drug class.1 Further switches in antidepressant monotherapy have a low success rate (10%–20%).5
Add/combine. Mixed evidence supports combining different antidepressants. There is cohort study evidence that combining citalopram and bupropion is more effective than switching to the alternate antidepressant,6 but other cohort studies did not find a significant difference between switching and augmenting. An arm of the STAR*D trial added either sustained-release bupropion or buspirone (Buspar) to the failed citalopram therapy. Thirty percent of patients with depression unresponsive to citalopram had remission when bupropion-SR or buspirone was added.7 The STAR*D reports do not compare the 2 strategies of switching or combining drugs directly.
Augment. Evidence from a meta-analysis with aggregate data from 3 studies representing a total of 110 patients showed that augmentation of various antidepressants with lithium leads to improved outcomes (number needed to treat [NNT]=3.7).8 A cohort study of augmentation with an atypical antipsychotic agent such as aripiprazole (Abilify) suggest improved outcomes, but similar studies found no benefit.9 A small (23-patient) randomized trial of lamotrigine (Lamictal) suggests that it may augment the effect of fluoxetine.10
Psychotherapy. A systematic review of psychological therapies in treatment-resistant depression found 2 controlled studies (of cognitive therapy and cognitive behavioral therapy) out of 12 total studies meeting their inclusion criteria that demonstrated improved scores on the Hamilton Rating Scale for Depression. Further study of these therapies was recommended.11
ECT. The evidence supporting use of ECT for treatment-resistant depression comes from studies following failure of treatment with tricyclic antidepressants and monoamine oxidase (MAO) inhibitors. Methodological problems in these older studies do not permit an estimate of response rate.12
Recommendations by others
The American Psychiatric Association treatment guideline recommends changing antidepressant, adding or changing to psychotherapy, or ECT if no response to 4 to 8 weeks of the initial therapy in depression.13 A guideline from the University of Michigan recommends referral to a psychiatrist if patients have treatment refractory depression (defined in their guideline as failure of 2 successive trials of antidepressants).14 The Institute for Clinical Systems Improvement guideline recommends considering switch, augmentation, or other therapies (including adding or modifying psychotherapy).15
1. Rush AJ, Trivedi MH, Wisniewski SR, et al. STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006;354:1231-1242.
2. Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry 1999;60:142-156.
3. Nelson JC. Managing treatment-resistant major depression. J Clin Psychiatry 2003;64(suppl 1):5-12.
4. Quitkin FM, Petkova E, McGrath PJ, et al. When should a trial of fluoxetine for major depression be declared failed? Am J Psychiatry 2003;160:734-740.
5. Fava M, Rush AJ, Wisneiwski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: A STAR*D report. Am J Psychiatry 2006;163:1161-1172.
6. Lam RW, Hossie H, Solons K, Yatham LN. Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry 2004;65:337-340.
7. Trivedi MH and others for the STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006;354:1243-1252.
8. Bauer M, Döpfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999;19:427-434.
9. Worthington JJ, 3rd, Kinrys G, Wygant LE, Pollack MH. Aripriprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patients. Int Clin Psychopharm 2005;20:9-11.
10. Barbosa L, Berk M, Vorster M. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J Clin Psychiatry 2003;64:403-407.
11. McPherson S, Cairns P, Carlyle J, et al. The effectiveness of psychological treatments for treatment-resistant depression: a systematic review. Acta Psychiatr Scand 2005;111:331-340.
12. Devanand DP, Sackheim HA, Prudic J. Electroconvulsive therapy in the treatment-resistant patient. Psychiatr Clin North Am 1991;14:905-923.
13. American Psychiatric Association Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157(supplement):1-45.
14. Schwenk TL, et al. UMHS Depression Guideline. Updated: May 2004. Available at: www.med.umich.edu/depression/depressguidelines04.pdf. Accessed on November 13, 2006.
15. Institute for Clinical Systems Improvement (ICSI). Major depression in adults in primary care. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); updated May 2006. 81 p. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=180. Accessed on November 13, 2006.
The best approach among studied alternatives to manage a patient with treatment-resistant depression is not clear from the evidence. All of the options reviewed seem to have about a 25% to 30% success rate.
Switching to other antidepressants or augmenting with non-antidepressant drugs has the best supporting evidence (strength of recommendation [SOR]: B).1 Adding additional antidepressants (SOR: B), using psychotherapy (SOR: B), and initiating electroconvulsive therapy (ECT) (SOR: C) are options. Various antidepressants are used as add-on therapy. Psychotherapy is often recommended, though the evidence of benefit after a failed course of initial therapy is sparse. The evidence supporting use of ECT in treatment-resistant depression is weak.
Comparison among the options is based on expert opinion (SOR: C). Additional reports from the STAR*D trial may improve the quality of the evidence in the near future.
Optimize initial drug dose and duration, then change to a different medication if needed
David Schneider, MD
Sutter Health Center, Santa Rosa, Calif
Although “epidemics” of obesity and avian influenza steal headlines, depression remains an American scourge, with a lifetime prevalence of 13% and rising. Major depressive disorder is often a chronic or relapsing illness, with recurrence rates of more than 40% at 2 years. Treatment resistance may be associated with pain (which is a presenting symptom in two thirds of depressed patients), psychosocial factors, psychiatric comorbidities, or the presence of bipolar disorder rather than unipolar depression.
Various forms of counseling and psychotherapy, alone or in combination with medications, are effective in treating depression, and I recommend them liberally when resources permit. Like the authors of this review, I first optimize initial drug dose and duration, then change to a different medication if needed. Some evidence suggests benefit from a combined serotonin and norepinephrine agent, such as venlafaxine (Effexor) or imipramine (Tofranil), which may also alleviate pain. I often add a noradrenergic tricyclic antidepressant, such as nortriptyline or desipramine, or the newer agent mirtazapine (Remeron), to a selective serotonin reuptake inhibitor (SSRI) for augmentation. I encourage physicians not to fear tricyclics, although I am hesitant to use lithium, thyroxine, or atypical antipsychotics in depression because of their hazards.
Evidence summary
In general, strategies for addressing treatment-resistant depression have not been compared in head-to-head studies. Guidelines at this time are based mainly on expert opinion2,3 and gradually accumulating data from a few randomized controlled studies or low-quality cohort studies.
While it makes sense, as the question implies, to first optimize the dose and duration of SSRI treatment in treatment-resistant depression, it is not clear which strategy to employ next. Switch, augmentation, and combination strategies may each improve clinical outcomes, but which strategy is best is based on expert opinion at this time.
Optimize. The first step in treatment-resistant depression should be optimizing dose and duration of therapy.4 For fluoxetine (Prozac), based on a nonrandomized open trial, patients should receive 8 weeks of treatment before the SSRI course is deemed adequate. Only 23% of patients who have not responded to 8 weeks of fluoxetine respond to a still longer course of fluoxetine.
Switch. The strongest evidence is from the recent STAR*D trial, a randomized study that assigned patients in one arm of the study who had no relief from (or did not tolerate) therapy with citalopram (Celexa) to 1 of 3 drugs—sustained-release bupropion (Wellbutrin SR), sertraline (Zoloft), or extended-release venlafaxine (Effexor XR). The study concluded that approximately 1 in 4 patients have remission after switching to an antidepressant from another drug class.1 Further switches in antidepressant monotherapy have a low success rate (10%–20%).5
Add/combine. Mixed evidence supports combining different antidepressants. There is cohort study evidence that combining citalopram and bupropion is more effective than switching to the alternate antidepressant,6 but other cohort studies did not find a significant difference between switching and augmenting. An arm of the STAR*D trial added either sustained-release bupropion or buspirone (Buspar) to the failed citalopram therapy. Thirty percent of patients with depression unresponsive to citalopram had remission when bupropion-SR or buspirone was added.7 The STAR*D reports do not compare the 2 strategies of switching or combining drugs directly.
Augment. Evidence from a meta-analysis with aggregate data from 3 studies representing a total of 110 patients showed that augmentation of various antidepressants with lithium leads to improved outcomes (number needed to treat [NNT]=3.7).8 A cohort study of augmentation with an atypical antipsychotic agent such as aripiprazole (Abilify) suggest improved outcomes, but similar studies found no benefit.9 A small (23-patient) randomized trial of lamotrigine (Lamictal) suggests that it may augment the effect of fluoxetine.10
Psychotherapy. A systematic review of psychological therapies in treatment-resistant depression found 2 controlled studies (of cognitive therapy and cognitive behavioral therapy) out of 12 total studies meeting their inclusion criteria that demonstrated improved scores on the Hamilton Rating Scale for Depression. Further study of these therapies was recommended.11
ECT. The evidence supporting use of ECT for treatment-resistant depression comes from studies following failure of treatment with tricyclic antidepressants and monoamine oxidase (MAO) inhibitors. Methodological problems in these older studies do not permit an estimate of response rate.12
Recommendations by others
The American Psychiatric Association treatment guideline recommends changing antidepressant, adding or changing to psychotherapy, or ECT if no response to 4 to 8 weeks of the initial therapy in depression.13 A guideline from the University of Michigan recommends referral to a psychiatrist if patients have treatment refractory depression (defined in their guideline as failure of 2 successive trials of antidepressants).14 The Institute for Clinical Systems Improvement guideline recommends considering switch, augmentation, or other therapies (including adding or modifying psychotherapy).15
The best approach among studied alternatives to manage a patient with treatment-resistant depression is not clear from the evidence. All of the options reviewed seem to have about a 25% to 30% success rate.
Switching to other antidepressants or augmenting with non-antidepressant drugs has the best supporting evidence (strength of recommendation [SOR]: B).1 Adding additional antidepressants (SOR: B), using psychotherapy (SOR: B), and initiating electroconvulsive therapy (ECT) (SOR: C) are options. Various antidepressants are used as add-on therapy. Psychotherapy is often recommended, though the evidence of benefit after a failed course of initial therapy is sparse. The evidence supporting use of ECT in treatment-resistant depression is weak.
Comparison among the options is based on expert opinion (SOR: C). Additional reports from the STAR*D trial may improve the quality of the evidence in the near future.
Optimize initial drug dose and duration, then change to a different medication if needed
David Schneider, MD
Sutter Health Center, Santa Rosa, Calif
Although “epidemics” of obesity and avian influenza steal headlines, depression remains an American scourge, with a lifetime prevalence of 13% and rising. Major depressive disorder is often a chronic or relapsing illness, with recurrence rates of more than 40% at 2 years. Treatment resistance may be associated with pain (which is a presenting symptom in two thirds of depressed patients), psychosocial factors, psychiatric comorbidities, or the presence of bipolar disorder rather than unipolar depression.
Various forms of counseling and psychotherapy, alone or in combination with medications, are effective in treating depression, and I recommend them liberally when resources permit. Like the authors of this review, I first optimize initial drug dose and duration, then change to a different medication if needed. Some evidence suggests benefit from a combined serotonin and norepinephrine agent, such as venlafaxine (Effexor) or imipramine (Tofranil), which may also alleviate pain. I often add a noradrenergic tricyclic antidepressant, such as nortriptyline or desipramine, or the newer agent mirtazapine (Remeron), to a selective serotonin reuptake inhibitor (SSRI) for augmentation. I encourage physicians not to fear tricyclics, although I am hesitant to use lithium, thyroxine, or atypical antipsychotics in depression because of their hazards.
Evidence summary
In general, strategies for addressing treatment-resistant depression have not been compared in head-to-head studies. Guidelines at this time are based mainly on expert opinion2,3 and gradually accumulating data from a few randomized controlled studies or low-quality cohort studies.
While it makes sense, as the question implies, to first optimize the dose and duration of SSRI treatment in treatment-resistant depression, it is not clear which strategy to employ next. Switch, augmentation, and combination strategies may each improve clinical outcomes, but which strategy is best is based on expert opinion at this time.
Optimize. The first step in treatment-resistant depression should be optimizing dose and duration of therapy.4 For fluoxetine (Prozac), based on a nonrandomized open trial, patients should receive 8 weeks of treatment before the SSRI course is deemed adequate. Only 23% of patients who have not responded to 8 weeks of fluoxetine respond to a still longer course of fluoxetine.
Switch. The strongest evidence is from the recent STAR*D trial, a randomized study that assigned patients in one arm of the study who had no relief from (or did not tolerate) therapy with citalopram (Celexa) to 1 of 3 drugs—sustained-release bupropion (Wellbutrin SR), sertraline (Zoloft), or extended-release venlafaxine (Effexor XR). The study concluded that approximately 1 in 4 patients have remission after switching to an antidepressant from another drug class.1 Further switches in antidepressant monotherapy have a low success rate (10%–20%).5
Add/combine. Mixed evidence supports combining different antidepressants. There is cohort study evidence that combining citalopram and bupropion is more effective than switching to the alternate antidepressant,6 but other cohort studies did not find a significant difference between switching and augmenting. An arm of the STAR*D trial added either sustained-release bupropion or buspirone (Buspar) to the failed citalopram therapy. Thirty percent of patients with depression unresponsive to citalopram had remission when bupropion-SR or buspirone was added.7 The STAR*D reports do not compare the 2 strategies of switching or combining drugs directly.
Augment. Evidence from a meta-analysis with aggregate data from 3 studies representing a total of 110 patients showed that augmentation of various antidepressants with lithium leads to improved outcomes (number needed to treat [NNT]=3.7).8 A cohort study of augmentation with an atypical antipsychotic agent such as aripiprazole (Abilify) suggest improved outcomes, but similar studies found no benefit.9 A small (23-patient) randomized trial of lamotrigine (Lamictal) suggests that it may augment the effect of fluoxetine.10
Psychotherapy. A systematic review of psychological therapies in treatment-resistant depression found 2 controlled studies (of cognitive therapy and cognitive behavioral therapy) out of 12 total studies meeting their inclusion criteria that demonstrated improved scores on the Hamilton Rating Scale for Depression. Further study of these therapies was recommended.11
ECT. The evidence supporting use of ECT for treatment-resistant depression comes from studies following failure of treatment with tricyclic antidepressants and monoamine oxidase (MAO) inhibitors. Methodological problems in these older studies do not permit an estimate of response rate.12
Recommendations by others
The American Psychiatric Association treatment guideline recommends changing antidepressant, adding or changing to psychotherapy, or ECT if no response to 4 to 8 weeks of the initial therapy in depression.13 A guideline from the University of Michigan recommends referral to a psychiatrist if patients have treatment refractory depression (defined in their guideline as failure of 2 successive trials of antidepressants).14 The Institute for Clinical Systems Improvement guideline recommends considering switch, augmentation, or other therapies (including adding or modifying psychotherapy).15
1. Rush AJ, Trivedi MH, Wisniewski SR, et al. STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006;354:1231-1242.
2. Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry 1999;60:142-156.
3. Nelson JC. Managing treatment-resistant major depression. J Clin Psychiatry 2003;64(suppl 1):5-12.
4. Quitkin FM, Petkova E, McGrath PJ, et al. When should a trial of fluoxetine for major depression be declared failed? Am J Psychiatry 2003;160:734-740.
5. Fava M, Rush AJ, Wisneiwski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: A STAR*D report. Am J Psychiatry 2006;163:1161-1172.
6. Lam RW, Hossie H, Solons K, Yatham LN. Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry 2004;65:337-340.
7. Trivedi MH and others for the STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006;354:1243-1252.
8. Bauer M, Döpfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999;19:427-434.
9. Worthington JJ, 3rd, Kinrys G, Wygant LE, Pollack MH. Aripriprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patients. Int Clin Psychopharm 2005;20:9-11.
10. Barbosa L, Berk M, Vorster M. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J Clin Psychiatry 2003;64:403-407.
11. McPherson S, Cairns P, Carlyle J, et al. The effectiveness of psychological treatments for treatment-resistant depression: a systematic review. Acta Psychiatr Scand 2005;111:331-340.
12. Devanand DP, Sackheim HA, Prudic J. Electroconvulsive therapy in the treatment-resistant patient. Psychiatr Clin North Am 1991;14:905-923.
13. American Psychiatric Association Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157(supplement):1-45.
14. Schwenk TL, et al. UMHS Depression Guideline. Updated: May 2004. Available at: www.med.umich.edu/depression/depressguidelines04.pdf. Accessed on November 13, 2006.
15. Institute for Clinical Systems Improvement (ICSI). Major depression in adults in primary care. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); updated May 2006. 81 p. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=180. Accessed on November 13, 2006.
1. Rush AJ, Trivedi MH, Wisniewski SR, et al. STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006;354:1231-1242.
2. Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry 1999;60:142-156.
3. Nelson JC. Managing treatment-resistant major depression. J Clin Psychiatry 2003;64(suppl 1):5-12.
4. Quitkin FM, Petkova E, McGrath PJ, et al. When should a trial of fluoxetine for major depression be declared failed? Am J Psychiatry 2003;160:734-740.
5. Fava M, Rush AJ, Wisneiwski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: A STAR*D report. Am J Psychiatry 2006;163:1161-1172.
6. Lam RW, Hossie H, Solons K, Yatham LN. Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry 2004;65:337-340.
7. Trivedi MH and others for the STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006;354:1243-1252.
8. Bauer M, Döpfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999;19:427-434.
9. Worthington JJ, 3rd, Kinrys G, Wygant LE, Pollack MH. Aripriprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patients. Int Clin Psychopharm 2005;20:9-11.
10. Barbosa L, Berk M, Vorster M. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J Clin Psychiatry 2003;64:403-407.
11. McPherson S, Cairns P, Carlyle J, et al. The effectiveness of psychological treatments for treatment-resistant depression: a systematic review. Acta Psychiatr Scand 2005;111:331-340.
12. Devanand DP, Sackheim HA, Prudic J. Electroconvulsive therapy in the treatment-resistant patient. Psychiatr Clin North Am 1991;14:905-923.
13. American Psychiatric Association Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157(supplement):1-45.
14. Schwenk TL, et al. UMHS Depression Guideline. Updated: May 2004. Available at: www.med.umich.edu/depression/depressguidelines04.pdf. Accessed on November 13, 2006.
15. Institute for Clinical Systems Improvement (ICSI). Major depression in adults in primary care. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); updated May 2006. 81 p. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=180. Accessed on November 13, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
What is the diagnostic approach to a 1-year-old with chronic cough?
Very few studies examine the evaluation of chronic cough among young children. Based on expert opinion, investigation of chronic cough should begin with a detailed history, physical examination, and chest radiograph (strength of recommendation [SOR]: C, expert opinion).1
Before pursuing additional studies, remove potential irritants from the patient’s environment. Work-up for persisting cough should consider congenital anomalies and then be directed toward common causes of chronic cough like those seen in older children and adults, including postnasal drip syndrome, gastroesophageal reflux disease (GERD), and asthma (SOR: C).
Evidence summary
The data on which expert opinion is based comes from case series of chronic cough in adults and older children in the setting of a specialty clinic.2,3 A detailed history should attend to the neonatal course, feeding concerns, sleep issues, potential for foreign-body aspiration, medications, infectious exposures, family history of atopy or asthma, and exposures to environmental irritants such as tobacco smoke.1 A dry, barking, or brassy cough in infants suggests large airway obstruction; in older children, it is likely psychogenic. A wet, productive cough is associated with an infectious cause. A cough associated with throat clearing suggests GERD or postnasal drip syndrome.3
Chest radiography, although universally recommended, was only abnormal for 4% of older patients (age 6 years through adult) from one series.2 Chest radiography may be most helpful for infants at increased risk for foreign-body aspiration. Cough from passive smoke exposure should improve with removal of exposure. There is no information on how long to wait for improvement.1
If the initial evaluation is not revealing, further investigation should focus on congenital anomalies, asthma, postnasal drip, and GERD. Aberrant innominate artery and asthma were the most frequent diagnoses among children aged <18 months old referred for otolaryngology consultation.3 Because pulmonary function testing is not practical for infants, a trial of aggressive therapy in combination with a “cough diary” kept by the parents may be used to diagnose asthma.1 Sinus computed tomography films are not routinely recommended to evaluate for postnasal drip, as sinusitis among children does not correlate well with postnasal drip.1 GERD may present with chronic cough; however, there is insufficient evidence for a uniform approach to diagnosis of cough associated with reflux.4
After evaluating infants for common causes of chronic cough (or if suggested by the history or physical), less common causes should be explored (Table). Consider a sweat chloride test first, followed by tuberculin testing. More than one cause for chronic cough was found 23% of the time in adults.2 Multiple causes of cough may be less common among infants, though no data confirm this.
Though it includes a mixture of adults and older children, a case series from pulmonary specialists finds pulmonary function tests with methacholine challenge the most helpful test.2 Case series from otolaryngology find endoscopy to be the most helpful, but it also includes a mix of older patients.3 Therefore, it seems likely that primary care physicians already appropriately refer patients to the correct specialists for evaluation. The optimal time to refer patients is unknown. We identified no reports from primary care settings. The question is an appropriate topic for primary care research.
TABLE
Causes of chronic cough among children with normal chest radiograph
| Category | Diagnoses |
|---|---|
| Asthma | Cough-variant asthma, hyperactive airways after infection |
| Infectious | Chronic sinusitis, otitis media with effusion, chronic bronchitis, bronchiectasis, chronic Waldeyer’s ring infection, pertussis, parapertussis, adenovirus, tuberculosis |
Congenital
| Aberrant innominate artery, vascular rings, bronchogenic cyst, esophageal duplication, subglottic stenosis, tracheomalacia, tracheal and bronchial stenosis Gastroesophageal reflux, esophageal incoordination, tracheoesophageal fistula, cleft larynx, vocal cord paralysis, pharyngeal incoordination, achalasia, cricopharyngeal achalasia Tracheobronchial tree abnormalities, cystic fibrosis, immotile cilia syndrome, congenital heart disease, bronchopulmonary dysplasia |
| Psychogenic | Psychogenic cough |
| Traumatic | Foreign bodies of bronchus, trachea, larynx, nose, external auditory canal |
| Environmental | Tobacco exposure, low humidity, overheating, allergens, industrial pollutants |
| Otologic | Cerumen, foreign body, infection, neoplasm, hair |
| Neoplastic | Larynx: Subglottic hemangioma, papillomatosis Tracheobronchial tree: Papillomatosis, bronchial adenoma Mediastinal tumor causing tracheobronchial compression |
| Cardiovascular | Rheumatic fever, congestive heart failure, mitral stenosis |
| Adapted from: Holinger 1986.3 | |
Recommendations from others
A guideline from Finaldn suggests referral for investigations of asthma, allergy, and GERD. Infectious diseases, the presence of foreign bodies, and psychogenic causes should also be considered.5
Inquire about exposure to irritants, feeding habits, infection, family history of asthma
Phong Luu, MD
Baylor College of Medicine, Houston, Tex
A comprehensive detailed history is the first important diagnostic step in the approach to a chronic cough in a 1-year old. The primary care provider should inquire about the infant’s exposure to environmental irritants such as tobacco smoke, their feeding habits, possible foreign-body aspiration, infectious exposure, and the family history of asthma. Environmental pollution in areas such as where I practice (Houston, Texas) can be a significant factor in evaluating infant with chronic cough. A chest radiograph should be considered after a thorough physical examination.
After an initial evaluation, further investigation should focus on the common causes of chronic cough such as postnasal drip, asthma, and GERD. Because of the high frequency of postnasal drip, the patient can be empirically started on antihistamine/decongestion combination. The primary care provider may consider an empirical treatment for gastroesophageal reflux disease if suggested by the history and physical examination. If no improvement is seen after several weeks, the patient should be referred to the appropriate specialist for evaluation of asthma, congenital anomalies, or other less common cause of chronic cough.
1. Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998;114(2 Suppl):133S-181S.
2. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis 1990;141:640-647.
3. Holinger LD. Chronic cough in infants and children. Laryngoscope 1986;96:316-322.
4. Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 2001;32 Suppl 2:S1-31.
5. Finnish Medical Society Duodecim. Prolonged cough in children. Helsinki, Finland: Duodecim Medical Publications; 2001. Available at: www.guideline.gov/guidelines/FTNGC-2602.html. Accessed on November 11, 2004.
Very few studies examine the evaluation of chronic cough among young children. Based on expert opinion, investigation of chronic cough should begin with a detailed history, physical examination, and chest radiograph (strength of recommendation [SOR]: C, expert opinion).1
Before pursuing additional studies, remove potential irritants from the patient’s environment. Work-up for persisting cough should consider congenital anomalies and then be directed toward common causes of chronic cough like those seen in older children and adults, including postnasal drip syndrome, gastroesophageal reflux disease (GERD), and asthma (SOR: C).
Evidence summary
The data on which expert opinion is based comes from case series of chronic cough in adults and older children in the setting of a specialty clinic.2,3 A detailed history should attend to the neonatal course, feeding concerns, sleep issues, potential for foreign-body aspiration, medications, infectious exposures, family history of atopy or asthma, and exposures to environmental irritants such as tobacco smoke.1 A dry, barking, or brassy cough in infants suggests large airway obstruction; in older children, it is likely psychogenic. A wet, productive cough is associated with an infectious cause. A cough associated with throat clearing suggests GERD or postnasal drip syndrome.3
Chest radiography, although universally recommended, was only abnormal for 4% of older patients (age 6 years through adult) from one series.2 Chest radiography may be most helpful for infants at increased risk for foreign-body aspiration. Cough from passive smoke exposure should improve with removal of exposure. There is no information on how long to wait for improvement.1
If the initial evaluation is not revealing, further investigation should focus on congenital anomalies, asthma, postnasal drip, and GERD. Aberrant innominate artery and asthma were the most frequent diagnoses among children aged <18 months old referred for otolaryngology consultation.3 Because pulmonary function testing is not practical for infants, a trial of aggressive therapy in combination with a “cough diary” kept by the parents may be used to diagnose asthma.1 Sinus computed tomography films are not routinely recommended to evaluate for postnasal drip, as sinusitis among children does not correlate well with postnasal drip.1 GERD may present with chronic cough; however, there is insufficient evidence for a uniform approach to diagnosis of cough associated with reflux.4
After evaluating infants for common causes of chronic cough (or if suggested by the history or physical), less common causes should be explored (Table). Consider a sweat chloride test first, followed by tuberculin testing. More than one cause for chronic cough was found 23% of the time in adults.2 Multiple causes of cough may be less common among infants, though no data confirm this.
Though it includes a mixture of adults and older children, a case series from pulmonary specialists finds pulmonary function tests with methacholine challenge the most helpful test.2 Case series from otolaryngology find endoscopy to be the most helpful, but it also includes a mix of older patients.3 Therefore, it seems likely that primary care physicians already appropriately refer patients to the correct specialists for evaluation. The optimal time to refer patients is unknown. We identified no reports from primary care settings. The question is an appropriate topic for primary care research.
TABLE
Causes of chronic cough among children with normal chest radiograph
| Category | Diagnoses |
|---|---|
| Asthma | Cough-variant asthma, hyperactive airways after infection |
| Infectious | Chronic sinusitis, otitis media with effusion, chronic bronchitis, bronchiectasis, chronic Waldeyer’s ring infection, pertussis, parapertussis, adenovirus, tuberculosis |
Congenital
| Aberrant innominate artery, vascular rings, bronchogenic cyst, esophageal duplication, subglottic stenosis, tracheomalacia, tracheal and bronchial stenosis Gastroesophageal reflux, esophageal incoordination, tracheoesophageal fistula, cleft larynx, vocal cord paralysis, pharyngeal incoordination, achalasia, cricopharyngeal achalasia Tracheobronchial tree abnormalities, cystic fibrosis, immotile cilia syndrome, congenital heart disease, bronchopulmonary dysplasia |
| Psychogenic | Psychogenic cough |
| Traumatic | Foreign bodies of bronchus, trachea, larynx, nose, external auditory canal |
| Environmental | Tobacco exposure, low humidity, overheating, allergens, industrial pollutants |
| Otologic | Cerumen, foreign body, infection, neoplasm, hair |
| Neoplastic | Larynx: Subglottic hemangioma, papillomatosis Tracheobronchial tree: Papillomatosis, bronchial adenoma Mediastinal tumor causing tracheobronchial compression |
| Cardiovascular | Rheumatic fever, congestive heart failure, mitral stenosis |
| Adapted from: Holinger 1986.3 | |
Recommendations from others
A guideline from Finaldn suggests referral for investigations of asthma, allergy, and GERD. Infectious diseases, the presence of foreign bodies, and psychogenic causes should also be considered.5
Inquire about exposure to irritants, feeding habits, infection, family history of asthma
Phong Luu, MD
Baylor College of Medicine, Houston, Tex
A comprehensive detailed history is the first important diagnostic step in the approach to a chronic cough in a 1-year old. The primary care provider should inquire about the infant’s exposure to environmental irritants such as tobacco smoke, their feeding habits, possible foreign-body aspiration, infectious exposure, and the family history of asthma. Environmental pollution in areas such as where I practice (Houston, Texas) can be a significant factor in evaluating infant with chronic cough. A chest radiograph should be considered after a thorough physical examination.
After an initial evaluation, further investigation should focus on the common causes of chronic cough such as postnasal drip, asthma, and GERD. Because of the high frequency of postnasal drip, the patient can be empirically started on antihistamine/decongestion combination. The primary care provider may consider an empirical treatment for gastroesophageal reflux disease if suggested by the history and physical examination. If no improvement is seen after several weeks, the patient should be referred to the appropriate specialist for evaluation of asthma, congenital anomalies, or other less common cause of chronic cough.
Very few studies examine the evaluation of chronic cough among young children. Based on expert opinion, investigation of chronic cough should begin with a detailed history, physical examination, and chest radiograph (strength of recommendation [SOR]: C, expert opinion).1
Before pursuing additional studies, remove potential irritants from the patient’s environment. Work-up for persisting cough should consider congenital anomalies and then be directed toward common causes of chronic cough like those seen in older children and adults, including postnasal drip syndrome, gastroesophageal reflux disease (GERD), and asthma (SOR: C).
Evidence summary
The data on which expert opinion is based comes from case series of chronic cough in adults and older children in the setting of a specialty clinic.2,3 A detailed history should attend to the neonatal course, feeding concerns, sleep issues, potential for foreign-body aspiration, medications, infectious exposures, family history of atopy or asthma, and exposures to environmental irritants such as tobacco smoke.1 A dry, barking, or brassy cough in infants suggests large airway obstruction; in older children, it is likely psychogenic. A wet, productive cough is associated with an infectious cause. A cough associated with throat clearing suggests GERD or postnasal drip syndrome.3
Chest radiography, although universally recommended, was only abnormal for 4% of older patients (age 6 years through adult) from one series.2 Chest radiography may be most helpful for infants at increased risk for foreign-body aspiration. Cough from passive smoke exposure should improve with removal of exposure. There is no information on how long to wait for improvement.1
If the initial evaluation is not revealing, further investigation should focus on congenital anomalies, asthma, postnasal drip, and GERD. Aberrant innominate artery and asthma were the most frequent diagnoses among children aged <18 months old referred for otolaryngology consultation.3 Because pulmonary function testing is not practical for infants, a trial of aggressive therapy in combination with a “cough diary” kept by the parents may be used to diagnose asthma.1 Sinus computed tomography films are not routinely recommended to evaluate for postnasal drip, as sinusitis among children does not correlate well with postnasal drip.1 GERD may present with chronic cough; however, there is insufficient evidence for a uniform approach to diagnosis of cough associated with reflux.4
After evaluating infants for common causes of chronic cough (or if suggested by the history or physical), less common causes should be explored (Table). Consider a sweat chloride test first, followed by tuberculin testing. More than one cause for chronic cough was found 23% of the time in adults.2 Multiple causes of cough may be less common among infants, though no data confirm this.
Though it includes a mixture of adults and older children, a case series from pulmonary specialists finds pulmonary function tests with methacholine challenge the most helpful test.2 Case series from otolaryngology find endoscopy to be the most helpful, but it also includes a mix of older patients.3 Therefore, it seems likely that primary care physicians already appropriately refer patients to the correct specialists for evaluation. The optimal time to refer patients is unknown. We identified no reports from primary care settings. The question is an appropriate topic for primary care research.
TABLE
Causes of chronic cough among children with normal chest radiograph
| Category | Diagnoses |
|---|---|
| Asthma | Cough-variant asthma, hyperactive airways after infection |
| Infectious | Chronic sinusitis, otitis media with effusion, chronic bronchitis, bronchiectasis, chronic Waldeyer’s ring infection, pertussis, parapertussis, adenovirus, tuberculosis |
Congenital
| Aberrant innominate artery, vascular rings, bronchogenic cyst, esophageal duplication, subglottic stenosis, tracheomalacia, tracheal and bronchial stenosis Gastroesophageal reflux, esophageal incoordination, tracheoesophageal fistula, cleft larynx, vocal cord paralysis, pharyngeal incoordination, achalasia, cricopharyngeal achalasia Tracheobronchial tree abnormalities, cystic fibrosis, immotile cilia syndrome, congenital heart disease, bronchopulmonary dysplasia |
| Psychogenic | Psychogenic cough |
| Traumatic | Foreign bodies of bronchus, trachea, larynx, nose, external auditory canal |
| Environmental | Tobacco exposure, low humidity, overheating, allergens, industrial pollutants |
| Otologic | Cerumen, foreign body, infection, neoplasm, hair |
| Neoplastic | Larynx: Subglottic hemangioma, papillomatosis Tracheobronchial tree: Papillomatosis, bronchial adenoma Mediastinal tumor causing tracheobronchial compression |
| Cardiovascular | Rheumatic fever, congestive heart failure, mitral stenosis |
| Adapted from: Holinger 1986.3 | |
Recommendations from others
A guideline from Finaldn suggests referral for investigations of asthma, allergy, and GERD. Infectious diseases, the presence of foreign bodies, and psychogenic causes should also be considered.5
Inquire about exposure to irritants, feeding habits, infection, family history of asthma
Phong Luu, MD
Baylor College of Medicine, Houston, Tex
A comprehensive detailed history is the first important diagnostic step in the approach to a chronic cough in a 1-year old. The primary care provider should inquire about the infant’s exposure to environmental irritants such as tobacco smoke, their feeding habits, possible foreign-body aspiration, infectious exposure, and the family history of asthma. Environmental pollution in areas such as where I practice (Houston, Texas) can be a significant factor in evaluating infant with chronic cough. A chest radiograph should be considered after a thorough physical examination.
After an initial evaluation, further investigation should focus on the common causes of chronic cough such as postnasal drip, asthma, and GERD. Because of the high frequency of postnasal drip, the patient can be empirically started on antihistamine/decongestion combination. The primary care provider may consider an empirical treatment for gastroesophageal reflux disease if suggested by the history and physical examination. If no improvement is seen after several weeks, the patient should be referred to the appropriate specialist for evaluation of asthma, congenital anomalies, or other less common cause of chronic cough.
1. Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998;114(2 Suppl):133S-181S.
2. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis 1990;141:640-647.
3. Holinger LD. Chronic cough in infants and children. Laryngoscope 1986;96:316-322.
4. Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 2001;32 Suppl 2:S1-31.
5. Finnish Medical Society Duodecim. Prolonged cough in children. Helsinki, Finland: Duodecim Medical Publications; 2001. Available at: www.guideline.gov/guidelines/FTNGC-2602.html. Accessed on November 11, 2004.
1. Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998;114(2 Suppl):133S-181S.
2. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis 1990;141:640-647.
3. Holinger LD. Chronic cough in infants and children. Laryngoscope 1986;96:316-322.
4. Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 2001;32 Suppl 2:S1-31.
5. Finnish Medical Society Duodecim. Prolonged cough in children. Helsinki, Finland: Duodecim Medical Publications; 2001. Available at: www.guideline.gov/guidelines/FTNGC-2602.html. Accessed on November 11, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
When should patients with mitral valve prolapse get endocarditis prophylaxis?
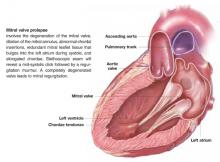
Patients with suspected mitral valve prolapse (MVP) ( Figure 1 ) should undergo echocardiography before any procedure that may place them at risk for bacteremia. Patients with MVP and documented absence of mitral regurgitation or valvular thickening likely do not need antibiotic prophylaxis against subacute bacterial endocarditis (SBE). Patients with MVP with documented mitral regurgitation, valvular thickening, or an unknown degree of valvular dysfunction may benefit from antibiotics during procedures that often lead to bacteremia (strength of recommendation: C).1
FIGURE 1 Mitral valve prolapse
Evidence summary
Only disease-oriented evidence and expert opinion address prevention for endocarditis. A randomized trial would require an estimated 6000 patients to demonstrate benefit.2
Endocarditis occurs in MVP at a rate of 0.1 cases/100 patient-years.3 However, MVP is the most common predisposing/precipitating cause of native valve endocarditis.4,5 In animal models, antibiotics prevent endocarditis following experimental bacteremia. The antibiotic can be administered either just before or up to 2 hours after the bacteremic event.2 It is worth noting that most bacteremia is not associated with medical procedures. Since endocarditis is often fatal, recommendations have been developed based on these animal models. Estimates of effectiveness of prophylaxis from case-control studies in humans (not limited to patients with MVP) estimate effectiveness from 49% to 91%.2
For patients with MVP who do not have evidence of mitral regurgitation on physical examination or echocardiography, the risk of morbidity may be greater from antibiotic therapy than the risk of endocarditis. Prophylaxis for these patients is not recommended. Patients with MVP associated with regurgitation are at moderate risk and may benefit from antibiotic prophylaxis.
Recommendations from others
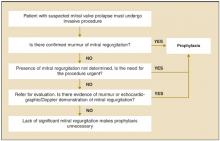
The American Heart Association has published recommendations in 1985,6 1990,7 and 1997.1 The 1997 recommendations are summarized in Figure 2 . The Swiss Working Group for Endocarditis Prophylaxis published similar recommendations in 2000.8 Recommended prophylactic regimens appear in Table 1. Table 2 shows a modified list of procedures for which prophylaxis is recommended.
FIGURE 2
Determining the need for antibiotic prophylaxis for patients with mitral valve prolapse
TABLE 1
Recommended prophylactic regimens for mitral valve prolaspe
| Situation | Medication | Dosage | |
|---|---|---|---|
| Dental, oral, respiratory, esophageal procedures | 1 hour before procedure | ||
| Standard prophylaxis | Amoxicillin | Adult:2 g | Child: 50 mg/kg |
| Allergy to penicillin | Clindamycin | Adult: 600 mg | Child: 20 mg/kg |
| Cephalexin | Adult: 2 g | Child: 50 mg/kg | |
| Azithromycin | Adult: 500 mg | Child: 15 mg/kg | |
| Genitourinary or non-esophageal gastrointestinal procedures | |||
| Moderate-risk patients | Amoxicillin | Adult: 2 g | Child: 50 mg/kg |
| 1 hour before procedure | |||
| Moderate-risk patients allergic to penicillin | Vancomycin | Adult: 1 g IV | Child: 20 mg/kg IV |
| Administer over 1-2 hrs; complete 30 minutes before procedure | |||
| High-risk patients | Add gentamicin to amoxicillin or vancomycin | 1.5 mg/kg (up to 120 mg) IV to be completed 30 minutes before procedure. If not allergic to penicillin, give penicillin give penicillin, give amoxicillin 1 g 6 hours after | |
| Modified from Dajani 1997.1 | |||
TABLE 2
Procedures for which endocarditis prophylaxis is, or is not, recommended
| Endocarditis prophylaxis recommended |
| Respiratory tract |
| Tonsillectomy or adenoidectomy |
| Surgical operations that involve respiratory mucosa |
| Bronchoscopy with a rigid bronchoscope |
| Gastrointestinal tract |
| Sclerotherapy for esophageal varices |
| Esophageal stricture dilation |
| Endoscopic retrograde cholangiography with biliary obstruction |
| Biliary tract surgery |
| Surgical operations that involve intestinal mucosa |
| Genitourinary tract |
| Prostatic surgery |
| Cystoscopy |
| Urethral dilation |
| Endocarditis prophylaxis not recommended |
| Respiratory tract |
| Endotracheal intubation |
| Flexible bronchoscopy, with or without biopsy |
| Tympanostomy tube insertion |
| Gastrointestinal tract |
| Endoscopy with or without gastrointestinal biopsy |
| Genitourinary tract |
| Circumcision |
| Vaginal hysterectomy |
| Vaginal delivery |
| Cesarean section |
| In uninfected tissue |
| Incision or biopsy of surgically scrubbed skin |
| Urethral catheterization |
| Uterine dilatation and curettage |
| Therapeutic abortion |
| Sterilization procedures |
| Insertion or removal of intrauterine devices |
| Cardiac |
| Transesophageal echocardiography |
| Cardiac catheterization, including balloon angioplasty and coronary stents |
| Implanted cardiac pacemakers, implanted defibrillators |
| Modified from Dajani et al, 1997.1 |
Guidelines assist decision-making regarding who needs SBE prophylaxis
David M. Bercaw, MD
Christiana Care Health Systems, Wilmington, Del
It is unfortunate, but not surprising, that the evidence for SBE prophylaxis for patients with MVP is disease-oriented evidence and expert opinion. Too often, the easy thing to do in a busy practice is not necessarily in the best interest of either the patient or the public. However—despite the low incidence of SBE—the high mortality of the disease and community standard of care often drive clinicians to write that prescription for antibiotics.
With the improved resolution and sensitivity of newer generations of echocardiograms, clinicians often face the dilemma of the patient with MVP and “trivial” or “mnimal” mitral regurgitation. Unfortunately, no guidelines assist us in our decision-making regarding these patients. Another consideration for the clinician is the American Heart Association’s recommendation for SBE prophylaxis for patients with MVP and thickened leaflets, regardless of whether there is associated mitral valve regurgitation.
One significant change that should lessen the frequency of unnecessary antibiotic prescribing was published recently. The echocardiographic criteria for diagnosing MVP were changed in the 2003 updated guidelines from the American College of Cardiology, American Heart Association, and American Society of Echocardiography. Valve prolapse of 2 mm or more above the mitral annulus is required for diagnosis.10 This change has effectively lowered the prevalence of MVP from 4% to 8% of the general population down to 2% to 3%.
1. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-1801.
2. Durack DT. Prevention of infective endocarditis. N Engl J Med 1995;332:38-44.
3. Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol 1995;75:1028-1032.
4. Awadallah SM, Kavey RE, Byrum CJ, Smith FC, Kveselis DA, Blackman MS. The changing pattern of infective endocarditis in childhood. Am J Cardiol 1991;68:90-94.
5. McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987;82:681-688.
6. Shulman ST, Amren DP, Bisno AL, et al. Prevention of bacterial endocarditis: A statement for health professionals by the Committee on Rheumatic Fever and Bacterial Endocarditis of the Council on Cardiovascular Diseases in the Young of the American Heart Association. Am J Dis Child 1985;139:232-235.
7. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990;264:2919-2922.
8. Moreillon P. Endocarditis prophylaxis revisited: experimental evidence of efficacy and new Swiss recommendations. Swiss Working Group for Endocarditis Prophylaxis. Schweiz Med Wochenschr 2000;130:1013-1026.
9. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Am Coll Cardiol. 2003;42:954-970.
Patients with suspected mitral valve prolapse (MVP) ( Figure 1 ) should undergo echocardiography before any procedure that may place them at risk for bacteremia. Patients with MVP and documented absence of mitral regurgitation or valvular thickening likely do not need antibiotic prophylaxis against subacute bacterial endocarditis (SBE). Patients with MVP with documented mitral regurgitation, valvular thickening, or an unknown degree of valvular dysfunction may benefit from antibiotics during procedures that often lead to bacteremia (strength of recommendation: C).1
FIGURE 1 Mitral valve prolapse
Evidence summary
Only disease-oriented evidence and expert opinion address prevention for endocarditis. A randomized trial would require an estimated 6000 patients to demonstrate benefit.2
Endocarditis occurs in MVP at a rate of 0.1 cases/100 patient-years.3 However, MVP is the most common predisposing/precipitating cause of native valve endocarditis.4,5 In animal models, antibiotics prevent endocarditis following experimental bacteremia. The antibiotic can be administered either just before or up to 2 hours after the bacteremic event.2 It is worth noting that most bacteremia is not associated with medical procedures. Since endocarditis is often fatal, recommendations have been developed based on these animal models. Estimates of effectiveness of prophylaxis from case-control studies in humans (not limited to patients with MVP) estimate effectiveness from 49% to 91%.2
For patients with MVP who do not have evidence of mitral regurgitation on physical examination or echocardiography, the risk of morbidity may be greater from antibiotic therapy than the risk of endocarditis. Prophylaxis for these patients is not recommended. Patients with MVP associated with regurgitation are at moderate risk and may benefit from antibiotic prophylaxis.
Recommendations from others
The American Heart Association has published recommendations in 1985,6 1990,7 and 1997.1 The 1997 recommendations are summarized in Figure 2 . The Swiss Working Group for Endocarditis Prophylaxis published similar recommendations in 2000.8 Recommended prophylactic regimens appear in Table 1. Table 2 shows a modified list of procedures for which prophylaxis is recommended.
FIGURE 2
Determining the need for antibiotic prophylaxis for patients with mitral valve prolapse
TABLE 1
Recommended prophylactic regimens for mitral valve prolaspe
| Situation | Medication | Dosage | |
|---|---|---|---|
| Dental, oral, respiratory, esophageal procedures | 1 hour before procedure | ||
| Standard prophylaxis | Amoxicillin | Adult:2 g | Child: 50 mg/kg |
| Allergy to penicillin | Clindamycin | Adult: 600 mg | Child: 20 mg/kg |
| Cephalexin | Adult: 2 g | Child: 50 mg/kg | |
| Azithromycin | Adult: 500 mg | Child: 15 mg/kg | |
| Genitourinary or non-esophageal gastrointestinal procedures | |||
| Moderate-risk patients | Amoxicillin | Adult: 2 g | Child: 50 mg/kg |
| 1 hour before procedure | |||
| Moderate-risk patients allergic to penicillin | Vancomycin | Adult: 1 g IV | Child: 20 mg/kg IV |
| Administer over 1-2 hrs; complete 30 minutes before procedure | |||
| High-risk patients | Add gentamicin to amoxicillin or vancomycin | 1.5 mg/kg (up to 120 mg) IV to be completed 30 minutes before procedure. If not allergic to penicillin, give penicillin give penicillin, give amoxicillin 1 g 6 hours after | |
| Modified from Dajani 1997.1 | |||
TABLE 2
Procedures for which endocarditis prophylaxis is, or is not, recommended
| Endocarditis prophylaxis recommended |
| Respiratory tract |
| Tonsillectomy or adenoidectomy |
| Surgical operations that involve respiratory mucosa |
| Bronchoscopy with a rigid bronchoscope |
| Gastrointestinal tract |
| Sclerotherapy for esophageal varices |
| Esophageal stricture dilation |
| Endoscopic retrograde cholangiography with biliary obstruction |
| Biliary tract surgery |
| Surgical operations that involve intestinal mucosa |
| Genitourinary tract |
| Prostatic surgery |
| Cystoscopy |
| Urethral dilation |
| Endocarditis prophylaxis not recommended |
| Respiratory tract |
| Endotracheal intubation |
| Flexible bronchoscopy, with or without biopsy |
| Tympanostomy tube insertion |
| Gastrointestinal tract |
| Endoscopy with or without gastrointestinal biopsy |
| Genitourinary tract |
| Circumcision |
| Vaginal hysterectomy |
| Vaginal delivery |
| Cesarean section |
| In uninfected tissue |
| Incision or biopsy of surgically scrubbed skin |
| Urethral catheterization |
| Uterine dilatation and curettage |
| Therapeutic abortion |
| Sterilization procedures |
| Insertion or removal of intrauterine devices |
| Cardiac |
| Transesophageal echocardiography |
| Cardiac catheterization, including balloon angioplasty and coronary stents |
| Implanted cardiac pacemakers, implanted defibrillators |
| Modified from Dajani et al, 1997.1 |
Guidelines assist decision-making regarding who needs SBE prophylaxis
David M. Bercaw, MD
Christiana Care Health Systems, Wilmington, Del
It is unfortunate, but not surprising, that the evidence for SBE prophylaxis for patients with MVP is disease-oriented evidence and expert opinion. Too often, the easy thing to do in a busy practice is not necessarily in the best interest of either the patient or the public. However—despite the low incidence of SBE—the high mortality of the disease and community standard of care often drive clinicians to write that prescription for antibiotics.
With the improved resolution and sensitivity of newer generations of echocardiograms, clinicians often face the dilemma of the patient with MVP and “trivial” or “mnimal” mitral regurgitation. Unfortunately, no guidelines assist us in our decision-making regarding these patients. Another consideration for the clinician is the American Heart Association’s recommendation for SBE prophylaxis for patients with MVP and thickened leaflets, regardless of whether there is associated mitral valve regurgitation.
One significant change that should lessen the frequency of unnecessary antibiotic prescribing was published recently. The echocardiographic criteria for diagnosing MVP were changed in the 2003 updated guidelines from the American College of Cardiology, American Heart Association, and American Society of Echocardiography. Valve prolapse of 2 mm or more above the mitral annulus is required for diagnosis.10 This change has effectively lowered the prevalence of MVP from 4% to 8% of the general population down to 2% to 3%.
Patients with suspected mitral valve prolapse (MVP) ( Figure 1 ) should undergo echocardiography before any procedure that may place them at risk for bacteremia. Patients with MVP and documented absence of mitral regurgitation or valvular thickening likely do not need antibiotic prophylaxis against subacute bacterial endocarditis (SBE). Patients with MVP with documented mitral regurgitation, valvular thickening, or an unknown degree of valvular dysfunction may benefit from antibiotics during procedures that often lead to bacteremia (strength of recommendation: C).1
FIGURE 1 Mitral valve prolapse
Evidence summary
Only disease-oriented evidence and expert opinion address prevention for endocarditis. A randomized trial would require an estimated 6000 patients to demonstrate benefit.2
Endocarditis occurs in MVP at a rate of 0.1 cases/100 patient-years.3 However, MVP is the most common predisposing/precipitating cause of native valve endocarditis.4,5 In animal models, antibiotics prevent endocarditis following experimental bacteremia. The antibiotic can be administered either just before or up to 2 hours after the bacteremic event.2 It is worth noting that most bacteremia is not associated with medical procedures. Since endocarditis is often fatal, recommendations have been developed based on these animal models. Estimates of effectiveness of prophylaxis from case-control studies in humans (not limited to patients with MVP) estimate effectiveness from 49% to 91%.2
For patients with MVP who do not have evidence of mitral regurgitation on physical examination or echocardiography, the risk of morbidity may be greater from antibiotic therapy than the risk of endocarditis. Prophylaxis for these patients is not recommended. Patients with MVP associated with regurgitation are at moderate risk and may benefit from antibiotic prophylaxis.
Recommendations from others
The American Heart Association has published recommendations in 1985,6 1990,7 and 1997.1 The 1997 recommendations are summarized in Figure 2 . The Swiss Working Group for Endocarditis Prophylaxis published similar recommendations in 2000.8 Recommended prophylactic regimens appear in Table 1. Table 2 shows a modified list of procedures for which prophylaxis is recommended.
FIGURE 2
Determining the need for antibiotic prophylaxis for patients with mitral valve prolapse
TABLE 1
Recommended prophylactic regimens for mitral valve prolaspe
| Situation | Medication | Dosage | |
|---|---|---|---|
| Dental, oral, respiratory, esophageal procedures | 1 hour before procedure | ||
| Standard prophylaxis | Amoxicillin | Adult:2 g | Child: 50 mg/kg |
| Allergy to penicillin | Clindamycin | Adult: 600 mg | Child: 20 mg/kg |
| Cephalexin | Adult: 2 g | Child: 50 mg/kg | |
| Azithromycin | Adult: 500 mg | Child: 15 mg/kg | |
| Genitourinary or non-esophageal gastrointestinal procedures | |||
| Moderate-risk patients | Amoxicillin | Adult: 2 g | Child: 50 mg/kg |
| 1 hour before procedure | |||
| Moderate-risk patients allergic to penicillin | Vancomycin | Adult: 1 g IV | Child: 20 mg/kg IV |
| Administer over 1-2 hrs; complete 30 minutes before procedure | |||
| High-risk patients | Add gentamicin to amoxicillin or vancomycin | 1.5 mg/kg (up to 120 mg) IV to be completed 30 minutes before procedure. If not allergic to penicillin, give penicillin give penicillin, give amoxicillin 1 g 6 hours after | |
| Modified from Dajani 1997.1 | |||
TABLE 2
Procedures for which endocarditis prophylaxis is, or is not, recommended
| Endocarditis prophylaxis recommended |
| Respiratory tract |
| Tonsillectomy or adenoidectomy |
| Surgical operations that involve respiratory mucosa |
| Bronchoscopy with a rigid bronchoscope |
| Gastrointestinal tract |
| Sclerotherapy for esophageal varices |
| Esophageal stricture dilation |
| Endoscopic retrograde cholangiography with biliary obstruction |
| Biliary tract surgery |
| Surgical operations that involve intestinal mucosa |
| Genitourinary tract |
| Prostatic surgery |
| Cystoscopy |
| Urethral dilation |
| Endocarditis prophylaxis not recommended |
| Respiratory tract |
| Endotracheal intubation |
| Flexible bronchoscopy, with or without biopsy |
| Tympanostomy tube insertion |
| Gastrointestinal tract |
| Endoscopy with or without gastrointestinal biopsy |
| Genitourinary tract |
| Circumcision |
| Vaginal hysterectomy |
| Vaginal delivery |
| Cesarean section |
| In uninfected tissue |
| Incision or biopsy of surgically scrubbed skin |
| Urethral catheterization |
| Uterine dilatation and curettage |
| Therapeutic abortion |
| Sterilization procedures |
| Insertion or removal of intrauterine devices |
| Cardiac |
| Transesophageal echocardiography |
| Cardiac catheterization, including balloon angioplasty and coronary stents |
| Implanted cardiac pacemakers, implanted defibrillators |
| Modified from Dajani et al, 1997.1 |
Guidelines assist decision-making regarding who needs SBE prophylaxis
David M. Bercaw, MD
Christiana Care Health Systems, Wilmington, Del
It is unfortunate, but not surprising, that the evidence for SBE prophylaxis for patients with MVP is disease-oriented evidence and expert opinion. Too often, the easy thing to do in a busy practice is not necessarily in the best interest of either the patient or the public. However—despite the low incidence of SBE—the high mortality of the disease and community standard of care often drive clinicians to write that prescription for antibiotics.
With the improved resolution and sensitivity of newer generations of echocardiograms, clinicians often face the dilemma of the patient with MVP and “trivial” or “mnimal” mitral regurgitation. Unfortunately, no guidelines assist us in our decision-making regarding these patients. Another consideration for the clinician is the American Heart Association’s recommendation for SBE prophylaxis for patients with MVP and thickened leaflets, regardless of whether there is associated mitral valve regurgitation.
One significant change that should lessen the frequency of unnecessary antibiotic prescribing was published recently. The echocardiographic criteria for diagnosing MVP were changed in the 2003 updated guidelines from the American College of Cardiology, American Heart Association, and American Society of Echocardiography. Valve prolapse of 2 mm or more above the mitral annulus is required for diagnosis.10 This change has effectively lowered the prevalence of MVP from 4% to 8% of the general population down to 2% to 3%.
1. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-1801.
2. Durack DT. Prevention of infective endocarditis. N Engl J Med 1995;332:38-44.
3. Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol 1995;75:1028-1032.
4. Awadallah SM, Kavey RE, Byrum CJ, Smith FC, Kveselis DA, Blackman MS. The changing pattern of infective endocarditis in childhood. Am J Cardiol 1991;68:90-94.
5. McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987;82:681-688.
6. Shulman ST, Amren DP, Bisno AL, et al. Prevention of bacterial endocarditis: A statement for health professionals by the Committee on Rheumatic Fever and Bacterial Endocarditis of the Council on Cardiovascular Diseases in the Young of the American Heart Association. Am J Dis Child 1985;139:232-235.
7. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990;264:2919-2922.
8. Moreillon P. Endocarditis prophylaxis revisited: experimental evidence of efficacy and new Swiss recommendations. Swiss Working Group for Endocarditis Prophylaxis. Schweiz Med Wochenschr 2000;130:1013-1026.
9. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Am Coll Cardiol. 2003;42:954-970.
1. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-1801.
2. Durack DT. Prevention of infective endocarditis. N Engl J Med 1995;332:38-44.
3. Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol 1995;75:1028-1032.
4. Awadallah SM, Kavey RE, Byrum CJ, Smith FC, Kveselis DA, Blackman MS. The changing pattern of infective endocarditis in childhood. Am J Cardiol 1991;68:90-94.
5. McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987;82:681-688.
6. Shulman ST, Amren DP, Bisno AL, et al. Prevention of bacterial endocarditis: A statement for health professionals by the Committee on Rheumatic Fever and Bacterial Endocarditis of the Council on Cardiovascular Diseases in the Young of the American Heart Association. Am J Dis Child 1985;139:232-235.
7. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990;264:2919-2922.
8. Moreillon P. Endocarditis prophylaxis revisited: experimental evidence of efficacy and new Swiss recommendations. Swiss Working Group for Endocarditis Prophylaxis. Schweiz Med Wochenschr 2000;130:1013-1026.
9. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Am Coll Cardiol. 2003;42:954-970.
Evidence-based answers from the Family Physicians Inquiries Network