User login
Multiple zoledronic acid doses may be needed after stopping denosumab
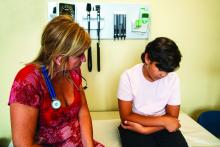
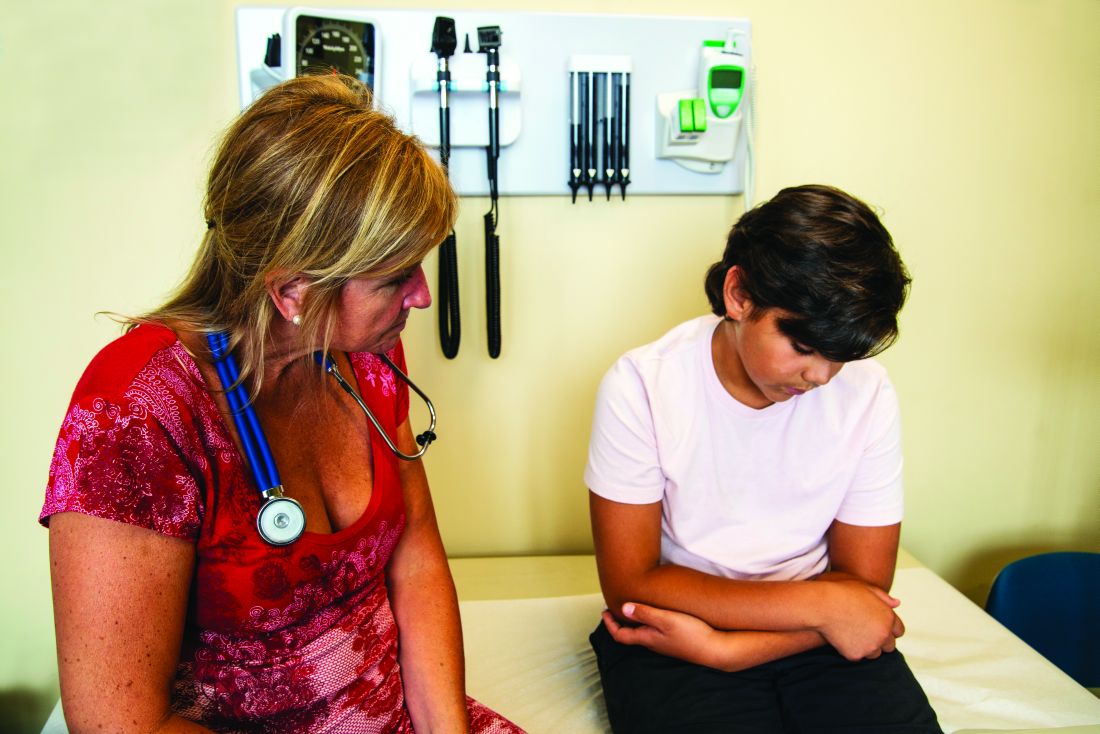
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
REPORTING FROM ASBMR 2019
Midurethral slings have low reoperation rates for stress urinary incontinence
according to a study published in Obstetrics & Gynecology.
Alexander A. Berger, MD, MPH, of the division of female pelvic medicine and reconstructive surgery at Kaiser Permanente in San Diego, and colleagues performed a retrospective cohort study of 17,030 patients with stress urinary incontinence (SUI) who underwent midurethral sling surgery between 2005 and 2016, examining the reoperation rate at 1 year, 5 years, and 9 years after the procedure, as well as secondary outcomes of mesh revision, mesh removal, and recurrence of SUI.
Overall, the rate of reoperation at 1 year was 2.1% (95% confidence interval, 1.9%-2.4%), was 4.5% at 5 years (95% CI, 4.1%-4.8%) and 6.0% at 9 years (95% CI, 5.5%-6.5%). Compared with white patients, there was a lower rate of reoperation among Asian or Pacific Islander patients.
The rate of reoperation involving mesh removal was 0.7% at 1 year (95% CI, 0.6%-0.8%), 1.0% at 5 years (95% CI, 0.8%-1.1%) and 1.1% at 9 years (95% CI, 0.9%-1.3%).
The rate of recurrent SUI leading to operation was 1.6% at 1 year (95% CI, 1.4%-1.8%), 3.9% at 5 years (95% CI, 3.5%-4.2%) and 5.2% at 9 years (95% CI, 4.7%-5.7%), with more reoperations occurring for patients who received a single-incision sling, rather than a retropubic sling (adjusted hazard ratio, 1.5; 95% CI, 1.06-2.11; P = .03), Dr. Berger and associates wrote.
Urogynecologists, ob.gyns., and urologists who use mesh for slings and reconstructive surgery have struggled to recommend synthetic mesh slings to their patients with SUI, said Patrick J. Woodman, DO, MS, program director of obstetrics and gynecology residency at Providence Health Ascension Macomb-Oakland, Warren (Mich.) Campus, said in an interview. In 2008, the Food and Drug Administration issued a public health notification for transvaginal placement of surgical mesh in patients with pelvic organ prolapse and SUI.
“Although some of the recommendations first made by the FDA were reasoned and reasonable, such as the need for direct, premarket, patient studies instead of the mostly administrative 510(k) ‘similar-to’ process that had been used previously, physicians and patients had been eagerly awaiting the outcomes of some of these clinical studies that would help answer some of the safety and efficacy questions that had been dogging the transvaginal use of mesh material for years,” he said.
“But, to everyone’s surprise, in April [2019] they called for a recall of all vaginal mesh products, even before the study data could be analyzed, written up and released,” added Dr. Woodman. “Companies were forced to halt production, pull stocks from the shelves, and halt and reverse shipments.”
One reason the results by Berger et al. show midurethral slings have had a good safety record is because of a small incision size and low amount of mesh, noted Dr. Woodman, who was not involved with the study. “This article seems to underline and highlight the fact that reoperation is rare for midurethral slings (for all reasons), but particularly for mesh erosion or exposure. This is well within the experience of most female pelvic medicine and reconstructive surgery and urologic surgeons, and incredibly less than the 8%-24% of mesh exposures reported in the variety of mesh exposure literature on vaginal mesh procedures.”
Despite this safety record, some women may still experience adverse events with midurethral slings, admitted Dr. Woodman. “The fact remains, if a surgeon drags a large piece of synthetic fabric through a ‘clean-contaminated’ vaginal environment, and buries this mesh under the skin of the vagina, and then rests this mesh against a long incision, some women’s immune systems will not be able to handle the resultant inflammation and bacterial load, despite antibiotics, vaginal prepping, and any number of coatings or soakings of the mesh.”
The researchers noted the study’s retrospective nature is one potential limitation, and the data has not been compiled by surgeon type or skill, or considered patients with complications that did not choose reoperations.
“But, the flip side is also true,” said Dr. Woodman, an Ob.Gyn News editorial advisor. “There may have been a number of individuals who had a surgical removal who did not need or warrant it due to the societal, family, or legal ‘suggestion’ that the mesh is now ‘dangerous’ and must be removed at all costs.”
Berger et al. “hit the nail on the head” with the study, including a large amount of patients that demonstrates the safety of midurethral slings, he said. “We need a solid body of unquestioned evidence of safety and effectiveness from which to base solid, evidence-based medical decisions. If there is a way to effectively use mesh to reinforce a vaginal repair in a high-risk woman (for example, with previous failed surgeries), then we have to take the stigma away from its use: because no one wants to use it now, even if it could help.
“The best we can hope for, as physicians, is a rehabilitation of reputation for vaginal mesh,” he concluded.
The study was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California. One coauthor reported receiving royalties from UptoDate and the American Urogynecologic Society Board Member for travel for board meetings. The other authors reported no relevant conflicts of interest. Dr. Woodman said he had no relevant financial disclosures.*
SOURCE: Berger AA et al. Obstet Gynecol. 2019 Oct 10. doi:10.1097/AOG.0000000000003526.
* Updated 10/14/2019
according to a study published in Obstetrics & Gynecology.
Alexander A. Berger, MD, MPH, of the division of female pelvic medicine and reconstructive surgery at Kaiser Permanente in San Diego, and colleagues performed a retrospective cohort study of 17,030 patients with stress urinary incontinence (SUI) who underwent midurethral sling surgery between 2005 and 2016, examining the reoperation rate at 1 year, 5 years, and 9 years after the procedure, as well as secondary outcomes of mesh revision, mesh removal, and recurrence of SUI.
Overall, the rate of reoperation at 1 year was 2.1% (95% confidence interval, 1.9%-2.4%), was 4.5% at 5 years (95% CI, 4.1%-4.8%) and 6.0% at 9 years (95% CI, 5.5%-6.5%). Compared with white patients, there was a lower rate of reoperation among Asian or Pacific Islander patients.
The rate of reoperation involving mesh removal was 0.7% at 1 year (95% CI, 0.6%-0.8%), 1.0% at 5 years (95% CI, 0.8%-1.1%) and 1.1% at 9 years (95% CI, 0.9%-1.3%).
The rate of recurrent SUI leading to operation was 1.6% at 1 year (95% CI, 1.4%-1.8%), 3.9% at 5 years (95% CI, 3.5%-4.2%) and 5.2% at 9 years (95% CI, 4.7%-5.7%), with more reoperations occurring for patients who received a single-incision sling, rather than a retropubic sling (adjusted hazard ratio, 1.5; 95% CI, 1.06-2.11; P = .03), Dr. Berger and associates wrote.
Urogynecologists, ob.gyns., and urologists who use mesh for slings and reconstructive surgery have struggled to recommend synthetic mesh slings to their patients with SUI, said Patrick J. Woodman, DO, MS, program director of obstetrics and gynecology residency at Providence Health Ascension Macomb-Oakland, Warren (Mich.) Campus, said in an interview. In 2008, the Food and Drug Administration issued a public health notification for transvaginal placement of surgical mesh in patients with pelvic organ prolapse and SUI.
“Although some of the recommendations first made by the FDA were reasoned and reasonable, such as the need for direct, premarket, patient studies instead of the mostly administrative 510(k) ‘similar-to’ process that had been used previously, physicians and patients had been eagerly awaiting the outcomes of some of these clinical studies that would help answer some of the safety and efficacy questions that had been dogging the transvaginal use of mesh material for years,” he said.
“But, to everyone’s surprise, in April [2019] they called for a recall of all vaginal mesh products, even before the study data could be analyzed, written up and released,” added Dr. Woodman. “Companies were forced to halt production, pull stocks from the shelves, and halt and reverse shipments.”
One reason the results by Berger et al. show midurethral slings have had a good safety record is because of a small incision size and low amount of mesh, noted Dr. Woodman, who was not involved with the study. “This article seems to underline and highlight the fact that reoperation is rare for midurethral slings (for all reasons), but particularly for mesh erosion or exposure. This is well within the experience of most female pelvic medicine and reconstructive surgery and urologic surgeons, and incredibly less than the 8%-24% of mesh exposures reported in the variety of mesh exposure literature on vaginal mesh procedures.”
Despite this safety record, some women may still experience adverse events with midurethral slings, admitted Dr. Woodman. “The fact remains, if a surgeon drags a large piece of synthetic fabric through a ‘clean-contaminated’ vaginal environment, and buries this mesh under the skin of the vagina, and then rests this mesh against a long incision, some women’s immune systems will not be able to handle the resultant inflammation and bacterial load, despite antibiotics, vaginal prepping, and any number of coatings or soakings of the mesh.”
The researchers noted the study’s retrospective nature is one potential limitation, and the data has not been compiled by surgeon type or skill, or considered patients with complications that did not choose reoperations.
“But, the flip side is also true,” said Dr. Woodman, an Ob.Gyn News editorial advisor. “There may have been a number of individuals who had a surgical removal who did not need or warrant it due to the societal, family, or legal ‘suggestion’ that the mesh is now ‘dangerous’ and must be removed at all costs.”
Berger et al. “hit the nail on the head” with the study, including a large amount of patients that demonstrates the safety of midurethral slings, he said. “We need a solid body of unquestioned evidence of safety and effectiveness from which to base solid, evidence-based medical decisions. If there is a way to effectively use mesh to reinforce a vaginal repair in a high-risk woman (for example, with previous failed surgeries), then we have to take the stigma away from its use: because no one wants to use it now, even if it could help.
“The best we can hope for, as physicians, is a rehabilitation of reputation for vaginal mesh,” he concluded.
The study was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California. One coauthor reported receiving royalties from UptoDate and the American Urogynecologic Society Board Member for travel for board meetings. The other authors reported no relevant conflicts of interest. Dr. Woodman said he had no relevant financial disclosures.*
SOURCE: Berger AA et al. Obstet Gynecol. 2019 Oct 10. doi:10.1097/AOG.0000000000003526.
* Updated 10/14/2019
according to a study published in Obstetrics & Gynecology.
Alexander A. Berger, MD, MPH, of the division of female pelvic medicine and reconstructive surgery at Kaiser Permanente in San Diego, and colleagues performed a retrospective cohort study of 17,030 patients with stress urinary incontinence (SUI) who underwent midurethral sling surgery between 2005 and 2016, examining the reoperation rate at 1 year, 5 years, and 9 years after the procedure, as well as secondary outcomes of mesh revision, mesh removal, and recurrence of SUI.
Overall, the rate of reoperation at 1 year was 2.1% (95% confidence interval, 1.9%-2.4%), was 4.5% at 5 years (95% CI, 4.1%-4.8%) and 6.0% at 9 years (95% CI, 5.5%-6.5%). Compared with white patients, there was a lower rate of reoperation among Asian or Pacific Islander patients.
The rate of reoperation involving mesh removal was 0.7% at 1 year (95% CI, 0.6%-0.8%), 1.0% at 5 years (95% CI, 0.8%-1.1%) and 1.1% at 9 years (95% CI, 0.9%-1.3%).
The rate of recurrent SUI leading to operation was 1.6% at 1 year (95% CI, 1.4%-1.8%), 3.9% at 5 years (95% CI, 3.5%-4.2%) and 5.2% at 9 years (95% CI, 4.7%-5.7%), with more reoperations occurring for patients who received a single-incision sling, rather than a retropubic sling (adjusted hazard ratio, 1.5; 95% CI, 1.06-2.11; P = .03), Dr. Berger and associates wrote.
Urogynecologists, ob.gyns., and urologists who use mesh for slings and reconstructive surgery have struggled to recommend synthetic mesh slings to their patients with SUI, said Patrick J. Woodman, DO, MS, program director of obstetrics and gynecology residency at Providence Health Ascension Macomb-Oakland, Warren (Mich.) Campus, said in an interview. In 2008, the Food and Drug Administration issued a public health notification for transvaginal placement of surgical mesh in patients with pelvic organ prolapse and SUI.
“Although some of the recommendations first made by the FDA were reasoned and reasonable, such as the need for direct, premarket, patient studies instead of the mostly administrative 510(k) ‘similar-to’ process that had been used previously, physicians and patients had been eagerly awaiting the outcomes of some of these clinical studies that would help answer some of the safety and efficacy questions that had been dogging the transvaginal use of mesh material for years,” he said.
“But, to everyone’s surprise, in April [2019] they called for a recall of all vaginal mesh products, even before the study data could be analyzed, written up and released,” added Dr. Woodman. “Companies were forced to halt production, pull stocks from the shelves, and halt and reverse shipments.”
One reason the results by Berger et al. show midurethral slings have had a good safety record is because of a small incision size and low amount of mesh, noted Dr. Woodman, who was not involved with the study. “This article seems to underline and highlight the fact that reoperation is rare for midurethral slings (for all reasons), but particularly for mesh erosion or exposure. This is well within the experience of most female pelvic medicine and reconstructive surgery and urologic surgeons, and incredibly less than the 8%-24% of mesh exposures reported in the variety of mesh exposure literature on vaginal mesh procedures.”
Despite this safety record, some women may still experience adverse events with midurethral slings, admitted Dr. Woodman. “The fact remains, if a surgeon drags a large piece of synthetic fabric through a ‘clean-contaminated’ vaginal environment, and buries this mesh under the skin of the vagina, and then rests this mesh against a long incision, some women’s immune systems will not be able to handle the resultant inflammation and bacterial load, despite antibiotics, vaginal prepping, and any number of coatings or soakings of the mesh.”
The researchers noted the study’s retrospective nature is one potential limitation, and the data has not been compiled by surgeon type or skill, or considered patients with complications that did not choose reoperations.
“But, the flip side is also true,” said Dr. Woodman, an Ob.Gyn News editorial advisor. “There may have been a number of individuals who had a surgical removal who did not need or warrant it due to the societal, family, or legal ‘suggestion’ that the mesh is now ‘dangerous’ and must be removed at all costs.”
Berger et al. “hit the nail on the head” with the study, including a large amount of patients that demonstrates the safety of midurethral slings, he said. “We need a solid body of unquestioned evidence of safety and effectiveness from which to base solid, evidence-based medical decisions. If there is a way to effectively use mesh to reinforce a vaginal repair in a high-risk woman (for example, with previous failed surgeries), then we have to take the stigma away from its use: because no one wants to use it now, even if it could help.
“The best we can hope for, as physicians, is a rehabilitation of reputation for vaginal mesh,” he concluded.
The study was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California. One coauthor reported receiving royalties from UptoDate and the American Urogynecologic Society Board Member for travel for board meetings. The other authors reported no relevant conflicts of interest. Dr. Woodman said he had no relevant financial disclosures.*
SOURCE: Berger AA et al. Obstet Gynecol. 2019 Oct 10. doi:10.1097/AOG.0000000000003526.
* Updated 10/14/2019
FROM OBSTETRICS & GYNECOLOGY
Much work to be done in optimizing treatment for transgender children
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASBMR 2019
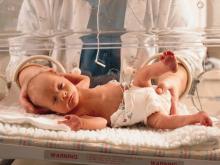
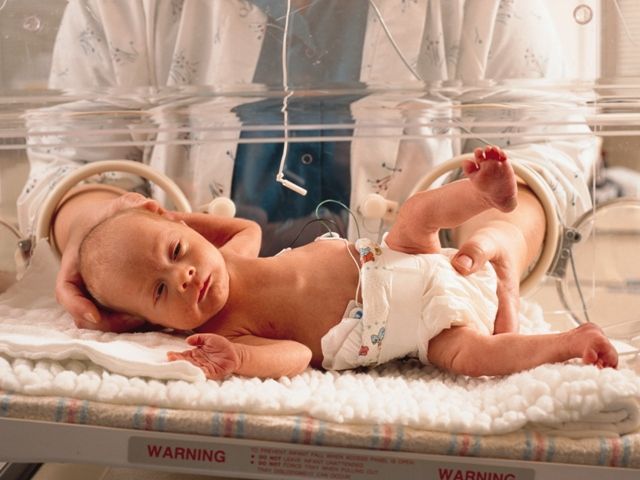
Faster enteral feeding does not up adverse outcomes risk in preterm infants
including moderate or severe neurodevelopmental disability and necrotizing enterocolitis, according to recent research published in the New England Journal of Medicine.
Although some data have shown rapidly increasing the speed of enteral-feeding volumes for preterm infants can raise the risk of necrotizing enterocolitis, these data are from observational case-control and uncontrolled studies, said Jon Dorling, MD, of the division of neonatal–perinatal medicine at Dalhousie University in Halifax, N.S., and colleagues in their study.
Dr. Doring and colleagues randomized 2,804 infants who were either very preterm or with a very low birth weight to receive daily milk increments at different volumes until the infants reached full feeding volume. Infants in the faster-increment group received daily milk at 30 mL per kg of body weight, while the slower-increment group received 18 mL per kg of body weight each day. The researchers analyzed infant survival without moderate or severe neurodevelopmental disability, with secondary outcomes of sepsis, necrotizing enterocolitis, and cerebral palsy at 24 months.
Overall, the researchers had information on the primary outcome for 87.4% of infants in the faster-increment group and 88.7% of infants in the slower-increment group. They found that 65.5% of infants in the faster-increment group and 68.1% of infants in the slower-increment group achieved an outcome of survival without moderate or severe neurodevelopmental disability at 24 months (adjusted risk ratio, 0.96; 95% confidence interval, 0.92-1.01; P equals .16). Secondary outcomes showed similar rates of adverse outcomes in the two groups, with 29.8% of infants in the faster-increment group and 31.1% of infants in the slower-increment group developing late-onset sepsis (aRR, 0.96; 95% CI, 0.86-1.07). Infants in the faster-increment group also had a similar rate of necrotizing enterocolitis (5.0%), compared with infants in the slower-increment group (5.6%) (aRR, 0.88; 95% CI, 0.68-1.16). Motor impairment was higher among infants in the faster-increment group (7.5%), compared with the slow-increment group (5.0%).
In the faster-increment group, the median number of days to reach full milk-feeding volumes was 7 vs. 10 in the slower-increment group.
“Although these feeding outcomes seem to favor faster increments, the risk of moderate or severe motor impairment was unexpectedly higher in the faster-increment group than in the slower-increment group,” the researchers said. “This observation is unexplained, and there were not more cases of late-onset sepsis or necrotizing enterocolitis in the faster-increment group.”
It is possible that it is a chance finding, since it was one of multiple secondary outcomes assessed, but biologically plausible explanations include increased cardiorespiratory events from pressure on the diaphragm or inability to absorb enteral nutrition,” they added.
The researchers said one potential limitation of the study was that it was unblinded.
This study was funded by the Health Technology Assessment Programme of the National Institute for Health Research. The authors reported various relationships with Baxter Bioscience, Chiesi Farmaceutici, Danone Early Life Nutrition, Fresenius Kabi USA LLC, National Institute for Health Research, Nestle Nutrition Institute, Nutrina, Medical Research Council, and Prolacta Biosciences in the form of consultancies, grants, travel reimbursement, board memberships, and editorial board appointments.
SOURCE: Doring J et al. N Eng J Med. 2019. doi: 10.1056/NEJMoa1816654.
including moderate or severe neurodevelopmental disability and necrotizing enterocolitis, according to recent research published in the New England Journal of Medicine.
Although some data have shown rapidly increasing the speed of enteral-feeding volumes for preterm infants can raise the risk of necrotizing enterocolitis, these data are from observational case-control and uncontrolled studies, said Jon Dorling, MD, of the division of neonatal–perinatal medicine at Dalhousie University in Halifax, N.S., and colleagues in their study.
Dr. Doring and colleagues randomized 2,804 infants who were either very preterm or with a very low birth weight to receive daily milk increments at different volumes until the infants reached full feeding volume. Infants in the faster-increment group received daily milk at 30 mL per kg of body weight, while the slower-increment group received 18 mL per kg of body weight each day. The researchers analyzed infant survival without moderate or severe neurodevelopmental disability, with secondary outcomes of sepsis, necrotizing enterocolitis, and cerebral palsy at 24 months.
Overall, the researchers had information on the primary outcome for 87.4% of infants in the faster-increment group and 88.7% of infants in the slower-increment group. They found that 65.5% of infants in the faster-increment group and 68.1% of infants in the slower-increment group achieved an outcome of survival without moderate or severe neurodevelopmental disability at 24 months (adjusted risk ratio, 0.96; 95% confidence interval, 0.92-1.01; P equals .16). Secondary outcomes showed similar rates of adverse outcomes in the two groups, with 29.8% of infants in the faster-increment group and 31.1% of infants in the slower-increment group developing late-onset sepsis (aRR, 0.96; 95% CI, 0.86-1.07). Infants in the faster-increment group also had a similar rate of necrotizing enterocolitis (5.0%), compared with infants in the slower-increment group (5.6%) (aRR, 0.88; 95% CI, 0.68-1.16). Motor impairment was higher among infants in the faster-increment group (7.5%), compared with the slow-increment group (5.0%).
In the faster-increment group, the median number of days to reach full milk-feeding volumes was 7 vs. 10 in the slower-increment group.
“Although these feeding outcomes seem to favor faster increments, the risk of moderate or severe motor impairment was unexpectedly higher in the faster-increment group than in the slower-increment group,” the researchers said. “This observation is unexplained, and there were not more cases of late-onset sepsis or necrotizing enterocolitis in the faster-increment group.”
It is possible that it is a chance finding, since it was one of multiple secondary outcomes assessed, but biologically plausible explanations include increased cardiorespiratory events from pressure on the diaphragm or inability to absorb enteral nutrition,” they added.
The researchers said one potential limitation of the study was that it was unblinded.
This study was funded by the Health Technology Assessment Programme of the National Institute for Health Research. The authors reported various relationships with Baxter Bioscience, Chiesi Farmaceutici, Danone Early Life Nutrition, Fresenius Kabi USA LLC, National Institute for Health Research, Nestle Nutrition Institute, Nutrina, Medical Research Council, and Prolacta Biosciences in the form of consultancies, grants, travel reimbursement, board memberships, and editorial board appointments.
SOURCE: Doring J et al. N Eng J Med. 2019. doi: 10.1056/NEJMoa1816654.
including moderate or severe neurodevelopmental disability and necrotizing enterocolitis, according to recent research published in the New England Journal of Medicine.
Although some data have shown rapidly increasing the speed of enteral-feeding volumes for preterm infants can raise the risk of necrotizing enterocolitis, these data are from observational case-control and uncontrolled studies, said Jon Dorling, MD, of the division of neonatal–perinatal medicine at Dalhousie University in Halifax, N.S., and colleagues in their study.
Dr. Doring and colleagues randomized 2,804 infants who were either very preterm or with a very low birth weight to receive daily milk increments at different volumes until the infants reached full feeding volume. Infants in the faster-increment group received daily milk at 30 mL per kg of body weight, while the slower-increment group received 18 mL per kg of body weight each day. The researchers analyzed infant survival without moderate or severe neurodevelopmental disability, with secondary outcomes of sepsis, necrotizing enterocolitis, and cerebral palsy at 24 months.
Overall, the researchers had information on the primary outcome for 87.4% of infants in the faster-increment group and 88.7% of infants in the slower-increment group. They found that 65.5% of infants in the faster-increment group and 68.1% of infants in the slower-increment group achieved an outcome of survival without moderate or severe neurodevelopmental disability at 24 months (adjusted risk ratio, 0.96; 95% confidence interval, 0.92-1.01; P equals .16). Secondary outcomes showed similar rates of adverse outcomes in the two groups, with 29.8% of infants in the faster-increment group and 31.1% of infants in the slower-increment group developing late-onset sepsis (aRR, 0.96; 95% CI, 0.86-1.07). Infants in the faster-increment group also had a similar rate of necrotizing enterocolitis (5.0%), compared with infants in the slower-increment group (5.6%) (aRR, 0.88; 95% CI, 0.68-1.16). Motor impairment was higher among infants in the faster-increment group (7.5%), compared with the slow-increment group (5.0%).
In the faster-increment group, the median number of days to reach full milk-feeding volumes was 7 vs. 10 in the slower-increment group.
“Although these feeding outcomes seem to favor faster increments, the risk of moderate or severe motor impairment was unexpectedly higher in the faster-increment group than in the slower-increment group,” the researchers said. “This observation is unexplained, and there were not more cases of late-onset sepsis or necrotizing enterocolitis in the faster-increment group.”
It is possible that it is a chance finding, since it was one of multiple secondary outcomes assessed, but biologically plausible explanations include increased cardiorespiratory events from pressure on the diaphragm or inability to absorb enteral nutrition,” they added.
The researchers said one potential limitation of the study was that it was unblinded.
This study was funded by the Health Technology Assessment Programme of the National Institute for Health Research. The authors reported various relationships with Baxter Bioscience, Chiesi Farmaceutici, Danone Early Life Nutrition, Fresenius Kabi USA LLC, National Institute for Health Research, Nestle Nutrition Institute, Nutrina, Medical Research Council, and Prolacta Biosciences in the form of consultancies, grants, travel reimbursement, board memberships, and editorial board appointments.
SOURCE: Doring J et al. N Eng J Med. 2019. doi: 10.1056/NEJMoa1816654.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Online assessment identifies excess steroid use in IBD patients
according to recent research in the journal Alimentary Pharmacology & Therapeutics.
Since measurement of excess corticosteroid use can be measured through an online assessment tool in clinical practice and long-term corticosteroid use is associated with adverse outcomes, it may be a quality marker for patients with IBD, wrote Christian P. Selinger, MD, from the Leeds (England) Gastroenterology Institute and colleagues. “Such key performance indicators have previously been lacking in IBD, unlike other disease areas such as diabetes and cardiovascular disease.”
Over a period of 3 months, Dr. Selinger and colleagues collected prospective data from 2,385 patients with IBD at 19 centers in England, Wales, and Scotland who had received steroids within the last year. The researchers divided the centers into groups based on whether they participated in the quality improvement program (7 centers), were new to the process of collecting data on steroid use (11 centers), or did not participate in the program (1 center). The seven centers that participated in the intervention were part of an audit that began in 2017, while the other centers were evaluated over a 3-month period between April and July 2017. Patients were asked questions about their steroid use, including whether the steroids were prescribed for their IBD, how long the course of steroids was, how many courses of steroids they received, and if they were able to stop using steroids without their symptoms returning.
The researchers found 14.8% of patients had an excess of steroids or were dependent on steroids, and patients at centers that participated in the quality improvement programs had a lower rate of exposure (23.8% vs. 31.0%; P less than .001) and a lower rate of steroid excess (11.5% vs. 17.1%; P less than .001) than did patients at sites that did not participate in the program. Centers with the improvement program also had steroid use decrease over time, from 30.0% in 2015 to 23.8% in 2017 (P = .003), and steroid excess also decreased from 13.8% at those centers to 11.5% during that time (P equals .17). The researchers noted that, in over half of cases (50.7%), the steroid excess was “avoidable.”
In patients with Crohn’s disease, those who had reduced steroid excess were more likely to be part of an intervention center (odds ratio, 0.72; 95% confidence interval, 0.46-0.97), at a center with a multidisciplinary team (OR, 0.54; 95% CI, 0.20-0.86), or receiving maintenance anti–tumor necrosis factor therapy (OR, 0.61; 95% CI, 0.24-0.95); in contrast, patients who received aminosalicylate were more likely to have steroid excess (OR, 1.72; 95% CI, 1.24-2.09). Steroid excess in ulcerative colitis (UC) patients was more likely among those receiving thiopurine monotherapy (OR, 1.97; 95% CI, 1.19‐3.01), while UC patients at an intervention center were less likely to have steroid excess (OR; 0.72; 95% CI, 0.45‐0.95).
The researchers said the online assessment is limited in assessing the reason for steroid excess and is unable to consider variables such as patient age, sex, IBD phenotype, and disease duration, but is a “simple, pragmatic tool” that can be used in real time in a clinical setting.
“This advances the case for steroid excess as a potential key performance indicator of quality in an IBD service, although in order for clinicians to benchmark their service and provide targets for improvements, any numerical goal attached to this key performance indicator would require consideration of case mix. Further data, including from national and international contexts, is needed,” concluded Dr. Selinger and colleagues.
The authors reported AbbVie provided the funding to develop the steroid assessment tool, as well as honoraria for invited attendees of the quality improvement plan, which the company also sponsored.
To help your patients better understand their treatment options, share AGA’s IBD patient education, which is online at www.gastro.org/practice-guidance/gi-patient-center/topic/inflammatory-bowel-disease.
SOURCE: Selinger CP et al. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15497.
according to recent research in the journal Alimentary Pharmacology & Therapeutics.
Since measurement of excess corticosteroid use can be measured through an online assessment tool in clinical practice and long-term corticosteroid use is associated with adverse outcomes, it may be a quality marker for patients with IBD, wrote Christian P. Selinger, MD, from the Leeds (England) Gastroenterology Institute and colleagues. “Such key performance indicators have previously been lacking in IBD, unlike other disease areas such as diabetes and cardiovascular disease.”
Over a period of 3 months, Dr. Selinger and colleagues collected prospective data from 2,385 patients with IBD at 19 centers in England, Wales, and Scotland who had received steroids within the last year. The researchers divided the centers into groups based on whether they participated in the quality improvement program (7 centers), were new to the process of collecting data on steroid use (11 centers), or did not participate in the program (1 center). The seven centers that participated in the intervention were part of an audit that began in 2017, while the other centers were evaluated over a 3-month period between April and July 2017. Patients were asked questions about their steroid use, including whether the steroids were prescribed for their IBD, how long the course of steroids was, how many courses of steroids they received, and if they were able to stop using steroids without their symptoms returning.
The researchers found 14.8% of patients had an excess of steroids or were dependent on steroids, and patients at centers that participated in the quality improvement programs had a lower rate of exposure (23.8% vs. 31.0%; P less than .001) and a lower rate of steroid excess (11.5% vs. 17.1%; P less than .001) than did patients at sites that did not participate in the program. Centers with the improvement program also had steroid use decrease over time, from 30.0% in 2015 to 23.8% in 2017 (P = .003), and steroid excess also decreased from 13.8% at those centers to 11.5% during that time (P equals .17). The researchers noted that, in over half of cases (50.7%), the steroid excess was “avoidable.”
In patients with Crohn’s disease, those who had reduced steroid excess were more likely to be part of an intervention center (odds ratio, 0.72; 95% confidence interval, 0.46-0.97), at a center with a multidisciplinary team (OR, 0.54; 95% CI, 0.20-0.86), or receiving maintenance anti–tumor necrosis factor therapy (OR, 0.61; 95% CI, 0.24-0.95); in contrast, patients who received aminosalicylate were more likely to have steroid excess (OR, 1.72; 95% CI, 1.24-2.09). Steroid excess in ulcerative colitis (UC) patients was more likely among those receiving thiopurine monotherapy (OR, 1.97; 95% CI, 1.19‐3.01), while UC patients at an intervention center were less likely to have steroid excess (OR; 0.72; 95% CI, 0.45‐0.95).
The researchers said the online assessment is limited in assessing the reason for steroid excess and is unable to consider variables such as patient age, sex, IBD phenotype, and disease duration, but is a “simple, pragmatic tool” that can be used in real time in a clinical setting.
“This advances the case for steroid excess as a potential key performance indicator of quality in an IBD service, although in order for clinicians to benchmark their service and provide targets for improvements, any numerical goal attached to this key performance indicator would require consideration of case mix. Further data, including from national and international contexts, is needed,” concluded Dr. Selinger and colleagues.
The authors reported AbbVie provided the funding to develop the steroid assessment tool, as well as honoraria for invited attendees of the quality improvement plan, which the company also sponsored.
To help your patients better understand their treatment options, share AGA’s IBD patient education, which is online at www.gastro.org/practice-guidance/gi-patient-center/topic/inflammatory-bowel-disease.
SOURCE: Selinger CP et al. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15497.
according to recent research in the journal Alimentary Pharmacology & Therapeutics.
Since measurement of excess corticosteroid use can be measured through an online assessment tool in clinical practice and long-term corticosteroid use is associated with adverse outcomes, it may be a quality marker for patients with IBD, wrote Christian P. Selinger, MD, from the Leeds (England) Gastroenterology Institute and colleagues. “Such key performance indicators have previously been lacking in IBD, unlike other disease areas such as diabetes and cardiovascular disease.”
Over a period of 3 months, Dr. Selinger and colleagues collected prospective data from 2,385 patients with IBD at 19 centers in England, Wales, and Scotland who had received steroids within the last year. The researchers divided the centers into groups based on whether they participated in the quality improvement program (7 centers), were new to the process of collecting data on steroid use (11 centers), or did not participate in the program (1 center). The seven centers that participated in the intervention were part of an audit that began in 2017, while the other centers were evaluated over a 3-month period between April and July 2017. Patients were asked questions about their steroid use, including whether the steroids were prescribed for their IBD, how long the course of steroids was, how many courses of steroids they received, and if they were able to stop using steroids without their symptoms returning.
The researchers found 14.8% of patients had an excess of steroids or were dependent on steroids, and patients at centers that participated in the quality improvement programs had a lower rate of exposure (23.8% vs. 31.0%; P less than .001) and a lower rate of steroid excess (11.5% vs. 17.1%; P less than .001) than did patients at sites that did not participate in the program. Centers with the improvement program also had steroid use decrease over time, from 30.0% in 2015 to 23.8% in 2017 (P = .003), and steroid excess also decreased from 13.8% at those centers to 11.5% during that time (P equals .17). The researchers noted that, in over half of cases (50.7%), the steroid excess was “avoidable.”
In patients with Crohn’s disease, those who had reduced steroid excess were more likely to be part of an intervention center (odds ratio, 0.72; 95% confidence interval, 0.46-0.97), at a center with a multidisciplinary team (OR, 0.54; 95% CI, 0.20-0.86), or receiving maintenance anti–tumor necrosis factor therapy (OR, 0.61; 95% CI, 0.24-0.95); in contrast, patients who received aminosalicylate were more likely to have steroid excess (OR, 1.72; 95% CI, 1.24-2.09). Steroid excess in ulcerative colitis (UC) patients was more likely among those receiving thiopurine monotherapy (OR, 1.97; 95% CI, 1.19‐3.01), while UC patients at an intervention center were less likely to have steroid excess (OR; 0.72; 95% CI, 0.45‐0.95).
The researchers said the online assessment is limited in assessing the reason for steroid excess and is unable to consider variables such as patient age, sex, IBD phenotype, and disease duration, but is a “simple, pragmatic tool” that can be used in real time in a clinical setting.
“This advances the case for steroid excess as a potential key performance indicator of quality in an IBD service, although in order for clinicians to benchmark their service and provide targets for improvements, any numerical goal attached to this key performance indicator would require consideration of case mix. Further data, including from national and international contexts, is needed,” concluded Dr. Selinger and colleagues.
The authors reported AbbVie provided the funding to develop the steroid assessment tool, as well as honoraria for invited attendees of the quality improvement plan, which the company also sponsored.
To help your patients better understand their treatment options, share AGA’s IBD patient education, which is online at www.gastro.org/practice-guidance/gi-patient-center/topic/inflammatory-bowel-disease.
SOURCE: Selinger CP et al. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15497.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS
Online assessment identifies excess steroid use in IBD patients
according to recent research in the journal Alimentary Pharmacology & Therapeutics.
Since measurement of excess corticosteroid use can be measured through an online assessment tool in clinical practice and long-term corticosteroid use is associated with adverse outcomes, it may be a quality marker for patients with IBD, wrote Christian P. Selinger, MD, from the Leeds (England) Gastroenterology Institute and colleagues. “Such key performance indicators have previously been lacking in IBD, unlike other disease areas such as diabetes and cardiovascular disease.”
Over a period of 3 months, Dr. Selinger and colleagues collected prospective data from 2,385 patients with IBD at 19 centers in England, Wales, and Scotland who had received steroids within the last year. The researchers divided the centers into groups based on whether they participated in the quality improvement program (7 centers), were new to the process of collecting data on steroid use (11 centers), or did not participate in the program (1 center). The seven centers that participated in the intervention were part of an audit that began in 2017, while the other centers were evaluated over a 3-month period between April and July 2017. Patients were asked questions about their steroid use, including whether the steroids were prescribed for their IBD, how long the course of steroids was, how many courses of steroids they received, and if they were able to stop using steroids without their symptoms returning.
The researchers found 14.8% of patients had an excess of steroids or were dependent on steroids, and patients at centers that participated in the quality improvement programs had a lower rate of exposure (23.8% vs. 31.0%; P less than .001) and a lower rate of steroid excess (11.5% vs. 17.1%; P less than .001) than did patients at sites that did not participate in the program. Centers with the improvement program also had steroid use decrease over time, from 30.0% in 2015 to 23.8% in 2017 (P = .003), and steroid excess also decreased from 13.8% at those centers to 11.5% during that time (P equals .17). The researchers noted that, in over half of cases (50.7%), the steroid excess was “avoidable.”
In patients with Crohn’s disease, those who had reduced steroid excess were more likely to be part of an intervention center (odds ratio, 0.72; 95% confidence interval, 0.46-0.97), at a center with a multidisciplinary team (OR, 0.54; 95% CI, 0.20-0.86), or receiving maintenance anti–tumor necrosis factor therapy (OR, 0.61; 95% CI, 0.24-0.95); in contrast, patients who received aminosalicylate were more likely to have steroid excess (OR, 1.72; 95% CI, 1.24-2.09). Steroid excess in ulcerative colitis (UC) patients was more likely among those receiving thiopurine monotherapy (OR, 1.97; 95% CI, 1.19‐3.01), while UC patients at an intervention center were less likely to have steroid excess (OR; 0.72; 95% CI, 0.45‐0.95).
The researchers said the online assessment is limited in assessing the reason for steroid excess and is unable to consider variables such as patient age, sex, IBD phenotype, and disease duration, but is a “simple, pragmatic tool” that can be used in real time in a clinical setting.
“This advances the case for steroid excess as a potential key performance indicator of quality in an IBD service, although in order for clinicians to benchmark their service and provide targets for improvements, any numerical goal attached to this key performance indicator would require consideration of case mix. Further data, including from national and international contexts, is needed,” concluded Dr. Selinger and colleagues.
The authors reported AbbVie provided the funding to develop the steroid assessment tool, as well as honoraria for invited attendees of the quality improvement plan, which the company also sponsored.
SOURCE: Selinger CP et al. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15497.
according to recent research in the journal Alimentary Pharmacology & Therapeutics.
Since measurement of excess corticosteroid use can be measured through an online assessment tool in clinical practice and long-term corticosteroid use is associated with adverse outcomes, it may be a quality marker for patients with IBD, wrote Christian P. Selinger, MD, from the Leeds (England) Gastroenterology Institute and colleagues. “Such key performance indicators have previously been lacking in IBD, unlike other disease areas such as diabetes and cardiovascular disease.”
Over a period of 3 months, Dr. Selinger and colleagues collected prospective data from 2,385 patients with IBD at 19 centers in England, Wales, and Scotland who had received steroids within the last year. The researchers divided the centers into groups based on whether they participated in the quality improvement program (7 centers), were new to the process of collecting data on steroid use (11 centers), or did not participate in the program (1 center). The seven centers that participated in the intervention were part of an audit that began in 2017, while the other centers were evaluated over a 3-month period between April and July 2017. Patients were asked questions about their steroid use, including whether the steroids were prescribed for their IBD, how long the course of steroids was, how many courses of steroids they received, and if they were able to stop using steroids without their symptoms returning.
The researchers found 14.8% of patients had an excess of steroids or were dependent on steroids, and patients at centers that participated in the quality improvement programs had a lower rate of exposure (23.8% vs. 31.0%; P less than .001) and a lower rate of steroid excess (11.5% vs. 17.1%; P less than .001) than did patients at sites that did not participate in the program. Centers with the improvement program also had steroid use decrease over time, from 30.0% in 2015 to 23.8% in 2017 (P = .003), and steroid excess also decreased from 13.8% at those centers to 11.5% during that time (P equals .17). The researchers noted that, in over half of cases (50.7%), the steroid excess was “avoidable.”
In patients with Crohn’s disease, those who had reduced steroid excess were more likely to be part of an intervention center (odds ratio, 0.72; 95% confidence interval, 0.46-0.97), at a center with a multidisciplinary team (OR, 0.54; 95% CI, 0.20-0.86), or receiving maintenance anti–tumor necrosis factor therapy (OR, 0.61; 95% CI, 0.24-0.95); in contrast, patients who received aminosalicylate were more likely to have steroid excess (OR, 1.72; 95% CI, 1.24-2.09). Steroid excess in ulcerative colitis (UC) patients was more likely among those receiving thiopurine monotherapy (OR, 1.97; 95% CI, 1.19‐3.01), while UC patients at an intervention center were less likely to have steroid excess (OR; 0.72; 95% CI, 0.45‐0.95).
The researchers said the online assessment is limited in assessing the reason for steroid excess and is unable to consider variables such as patient age, sex, IBD phenotype, and disease duration, but is a “simple, pragmatic tool” that can be used in real time in a clinical setting.
“This advances the case for steroid excess as a potential key performance indicator of quality in an IBD service, although in order for clinicians to benchmark their service and provide targets for improvements, any numerical goal attached to this key performance indicator would require consideration of case mix. Further data, including from national and international contexts, is needed,” concluded Dr. Selinger and colleagues.
The authors reported AbbVie provided the funding to develop the steroid assessment tool, as well as honoraria for invited attendees of the quality improvement plan, which the company also sponsored.
SOURCE: Selinger CP et al. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15497.
according to recent research in the journal Alimentary Pharmacology & Therapeutics.
Since measurement of excess corticosteroid use can be measured through an online assessment tool in clinical practice and long-term corticosteroid use is associated with adverse outcomes, it may be a quality marker for patients with IBD, wrote Christian P. Selinger, MD, from the Leeds (England) Gastroenterology Institute and colleagues. “Such key performance indicators have previously been lacking in IBD, unlike other disease areas such as diabetes and cardiovascular disease.”
Over a period of 3 months, Dr. Selinger and colleagues collected prospective data from 2,385 patients with IBD at 19 centers in England, Wales, and Scotland who had received steroids within the last year. The researchers divided the centers into groups based on whether they participated in the quality improvement program (7 centers), were new to the process of collecting data on steroid use (11 centers), or did not participate in the program (1 center). The seven centers that participated in the intervention were part of an audit that began in 2017, while the other centers were evaluated over a 3-month period between April and July 2017. Patients were asked questions about their steroid use, including whether the steroids were prescribed for their IBD, how long the course of steroids was, how many courses of steroids they received, and if they were able to stop using steroids without their symptoms returning.
The researchers found 14.8% of patients had an excess of steroids or were dependent on steroids, and patients at centers that participated in the quality improvement programs had a lower rate of exposure (23.8% vs. 31.0%; P less than .001) and a lower rate of steroid excess (11.5% vs. 17.1%; P less than .001) than did patients at sites that did not participate in the program. Centers with the improvement program also had steroid use decrease over time, from 30.0% in 2015 to 23.8% in 2017 (P = .003), and steroid excess also decreased from 13.8% at those centers to 11.5% during that time (P equals .17). The researchers noted that, in over half of cases (50.7%), the steroid excess was “avoidable.”
In patients with Crohn’s disease, those who had reduced steroid excess were more likely to be part of an intervention center (odds ratio, 0.72; 95% confidence interval, 0.46-0.97), at a center with a multidisciplinary team (OR, 0.54; 95% CI, 0.20-0.86), or receiving maintenance anti–tumor necrosis factor therapy (OR, 0.61; 95% CI, 0.24-0.95); in contrast, patients who received aminosalicylate were more likely to have steroid excess (OR, 1.72; 95% CI, 1.24-2.09). Steroid excess in ulcerative colitis (UC) patients was more likely among those receiving thiopurine monotherapy (OR, 1.97; 95% CI, 1.19‐3.01), while UC patients at an intervention center were less likely to have steroid excess (OR; 0.72; 95% CI, 0.45‐0.95).
The researchers said the online assessment is limited in assessing the reason for steroid excess and is unable to consider variables such as patient age, sex, IBD phenotype, and disease duration, but is a “simple, pragmatic tool” that can be used in real time in a clinical setting.
“This advances the case for steroid excess as a potential key performance indicator of quality in an IBD service, although in order for clinicians to benchmark their service and provide targets for improvements, any numerical goal attached to this key performance indicator would require consideration of case mix. Further data, including from national and international contexts, is needed,” concluded Dr. Selinger and colleagues.
The authors reported AbbVie provided the funding to develop the steroid assessment tool, as well as honoraria for invited attendees of the quality improvement plan, which the company also sponsored.
SOURCE: Selinger CP et al. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15497.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS
Key clinical point: An online assessment tool can be used to identify patients with inflammatory bowel disease (IBD) receiving an excess of steroids, and a quality improvement program lowered excess steroids at centers that implemented the program.
Major finding: Of patients in the study, 14.8% of patients were given excess steroids or were dependent on steroids, and patients at centers that participated in the quality improvement programs had a lower rate of exposure (23.8% vs. 31.0%; P less than .001) and a lower rate of steroid excess (11.5% vs. 17.1%; P less than .001) than did patients at sites that did not participate in the program.
Study details: A prospective study of 2,385 patients with IBD at 19 centers in England, Wales, and Scotland.
Disclosures: The authors reported AbbVie provided the funding to develop the steroid assessment tool, as well as honoraria for invited attendees of the quality improvement plan, which the company also sponsored.
Source: Selinger CP et al. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15497.
Romosozumab benefits prevail, despite renal insufficiency
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
REPORTING FROM ASBMR 2019
Consider cognitive reframing of osteoporosis to improve adherence
ORLANDO – Reframing the decision-making process for selecting osteoporosis treatment options may help increase adherence to medication, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“The language of this disease needs to be changed,” Deborah Gold, PhD, of Duke University, Durham, N.C., said in her presentation.
As medications for osteoporosis have changed over the decades – from estrogen in the 1940s and calcitonin in the 1970s to bisphosphonates in the 1990s – the delivery mechanisms of the drugs and the dosing intervals also have changed. However, long-term adherence to osteoporosis medication through various delivery mechanisms and doses have remained elusive, Dr. Gold said.
She cited the negative public and media response to osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) – two rare side effects of bisphosphonate treatment – as one reason why adherence to osteoporosis medications has changed.
While the more common side effects of osteoporosis medications are manageable, the emphasis in lay press has been on “rare and frightening side effects,” she said. A 2018 study found that reports in the media “strongly influence the level of awareness of osteoporosis and fracture risk” and that a gap exists between clinical recommendations and patient perceptions (J Endocrinol Invest. 2018;41[12]:1359-64. doi: 10.1007/s40618-018-0898-9). Over the same time period, another study found that 40.2% of high-risk patients hospitalized for a hip fracture were treated with osteoporosis medications in 2002, but that number declined to 20.5% in 2011 (J Bone Miner Res. 2014;29[9]:1929-37. doi: 10.1002/jbmr.2202).
Dr. Gold advised clinicians to counter negative patient perceptions about osteoporosis treatment by explaining the risks of treatment with medications as well as the risks of not undergoing treatment for osteoporosis. “A hip fracture is a lot more likely for nontreatment than ONJ or AFF are for treatment,” she said.
Dr. Gold also described the importance of listening to patients’ preferences for treatment as well as attempting to find an appropriate treatment they are likely to continue using. “This has been shown in the literature over and over again in other diseases,” she said. If “somebody says, ‘I can’t do needles,’ you can’t prescribe a medication that goes in through a needle.”
Assuring patients that they can visit again to address issues with treatment, change medications if needed, and discuss concerns about adverse outcomes such as ONJ and AFF is also relevant. “We need to promote osteoporosis understanding – not just osteoporosis – and we need to promote treatment to multiple sources,” she said.
When osteoporosis is characterized in terms of bone mineral density, T-scores, fragility fractures, appropriate exercises, and diet, there is plenty of opportunity for confusion or misunderstanding. Accurate, plain-language information, given both verbally and in handouts, works well , according to Dr. Gold.
“Health communication services – whether we’re talking about newspapers, magazines, radio shows, television shows, or even things that come out from organizations like the NOF [National Osteoporosis Foundation] and ASBMR – need to promote accurate information and not exaggerated negatives that we hear all the time,” she said.
“Cognitive reframing is not an easy thing; there’s no question,” Dr. Gold said. “I’m not standing up here telling you we can do it tomorrow. It will be difficult. But for those of us who know this disease and know how serious it is and know what the consequences are, we need to make a positive difference.”
Dr. Gold reported being a consultant for Amgen, Eli Lilly, and Radius Pharmaceuticals.
SOURCE: Gold D. ASBMR 2019. Adherence to Osteoporosis: A Conundrum of Significant Proportions.
ORLANDO – Reframing the decision-making process for selecting osteoporosis treatment options may help increase adherence to medication, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“The language of this disease needs to be changed,” Deborah Gold, PhD, of Duke University, Durham, N.C., said in her presentation.
As medications for osteoporosis have changed over the decades – from estrogen in the 1940s and calcitonin in the 1970s to bisphosphonates in the 1990s – the delivery mechanisms of the drugs and the dosing intervals also have changed. However, long-term adherence to osteoporosis medication through various delivery mechanisms and doses have remained elusive, Dr. Gold said.
She cited the negative public and media response to osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) – two rare side effects of bisphosphonate treatment – as one reason why adherence to osteoporosis medications has changed.
While the more common side effects of osteoporosis medications are manageable, the emphasis in lay press has been on “rare and frightening side effects,” she said. A 2018 study found that reports in the media “strongly influence the level of awareness of osteoporosis and fracture risk” and that a gap exists between clinical recommendations and patient perceptions (J Endocrinol Invest. 2018;41[12]:1359-64. doi: 10.1007/s40618-018-0898-9). Over the same time period, another study found that 40.2% of high-risk patients hospitalized for a hip fracture were treated with osteoporosis medications in 2002, but that number declined to 20.5% in 2011 (J Bone Miner Res. 2014;29[9]:1929-37. doi: 10.1002/jbmr.2202).
Dr. Gold advised clinicians to counter negative patient perceptions about osteoporosis treatment by explaining the risks of treatment with medications as well as the risks of not undergoing treatment for osteoporosis. “A hip fracture is a lot more likely for nontreatment than ONJ or AFF are for treatment,” she said.
Dr. Gold also described the importance of listening to patients’ preferences for treatment as well as attempting to find an appropriate treatment they are likely to continue using. “This has been shown in the literature over and over again in other diseases,” she said. If “somebody says, ‘I can’t do needles,’ you can’t prescribe a medication that goes in through a needle.”
Assuring patients that they can visit again to address issues with treatment, change medications if needed, and discuss concerns about adverse outcomes such as ONJ and AFF is also relevant. “We need to promote osteoporosis understanding – not just osteoporosis – and we need to promote treatment to multiple sources,” she said.
When osteoporosis is characterized in terms of bone mineral density, T-scores, fragility fractures, appropriate exercises, and diet, there is plenty of opportunity for confusion or misunderstanding. Accurate, plain-language information, given both verbally and in handouts, works well , according to Dr. Gold.
“Health communication services – whether we’re talking about newspapers, magazines, radio shows, television shows, or even things that come out from organizations like the NOF [National Osteoporosis Foundation] and ASBMR – need to promote accurate information and not exaggerated negatives that we hear all the time,” she said.
“Cognitive reframing is not an easy thing; there’s no question,” Dr. Gold said. “I’m not standing up here telling you we can do it tomorrow. It will be difficult. But for those of us who know this disease and know how serious it is and know what the consequences are, we need to make a positive difference.”
Dr. Gold reported being a consultant for Amgen, Eli Lilly, and Radius Pharmaceuticals.
SOURCE: Gold D. ASBMR 2019. Adherence to Osteoporosis: A Conundrum of Significant Proportions.
ORLANDO – Reframing the decision-making process for selecting osteoporosis treatment options may help increase adherence to medication, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“The language of this disease needs to be changed,” Deborah Gold, PhD, of Duke University, Durham, N.C., said in her presentation.
As medications for osteoporosis have changed over the decades – from estrogen in the 1940s and calcitonin in the 1970s to bisphosphonates in the 1990s – the delivery mechanisms of the drugs and the dosing intervals also have changed. However, long-term adherence to osteoporosis medication through various delivery mechanisms and doses have remained elusive, Dr. Gold said.
She cited the negative public and media response to osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) – two rare side effects of bisphosphonate treatment – as one reason why adherence to osteoporosis medications has changed.
While the more common side effects of osteoporosis medications are manageable, the emphasis in lay press has been on “rare and frightening side effects,” she said. A 2018 study found that reports in the media “strongly influence the level of awareness of osteoporosis and fracture risk” and that a gap exists between clinical recommendations and patient perceptions (J Endocrinol Invest. 2018;41[12]:1359-64. doi: 10.1007/s40618-018-0898-9). Over the same time period, another study found that 40.2% of high-risk patients hospitalized for a hip fracture were treated with osteoporosis medications in 2002, but that number declined to 20.5% in 2011 (J Bone Miner Res. 2014;29[9]:1929-37. doi: 10.1002/jbmr.2202).
Dr. Gold advised clinicians to counter negative patient perceptions about osteoporosis treatment by explaining the risks of treatment with medications as well as the risks of not undergoing treatment for osteoporosis. “A hip fracture is a lot more likely for nontreatment than ONJ or AFF are for treatment,” she said.
Dr. Gold also described the importance of listening to patients’ preferences for treatment as well as attempting to find an appropriate treatment they are likely to continue using. “This has been shown in the literature over and over again in other diseases,” she said. If “somebody says, ‘I can’t do needles,’ you can’t prescribe a medication that goes in through a needle.”
Assuring patients that they can visit again to address issues with treatment, change medications if needed, and discuss concerns about adverse outcomes such as ONJ and AFF is also relevant. “We need to promote osteoporosis understanding – not just osteoporosis – and we need to promote treatment to multiple sources,” she said.
When osteoporosis is characterized in terms of bone mineral density, T-scores, fragility fractures, appropriate exercises, and diet, there is plenty of opportunity for confusion or misunderstanding. Accurate, plain-language information, given both verbally and in handouts, works well , according to Dr. Gold.
“Health communication services – whether we’re talking about newspapers, magazines, radio shows, television shows, or even things that come out from organizations like the NOF [National Osteoporosis Foundation] and ASBMR – need to promote accurate information and not exaggerated negatives that we hear all the time,” she said.
“Cognitive reframing is not an easy thing; there’s no question,” Dr. Gold said. “I’m not standing up here telling you we can do it tomorrow. It will be difficult. But for those of us who know this disease and know how serious it is and know what the consequences are, we need to make a positive difference.”
Dr. Gold reported being a consultant for Amgen, Eli Lilly, and Radius Pharmaceuticals.
SOURCE: Gold D. ASBMR 2019. Adherence to Osteoporosis: A Conundrum of Significant Proportions.
REPORTING FROM ASBMR 2019
Osteoporosis remains a costly burden to older U.S. adults
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
REPORTING FROM ASBMR 2019
Systemic sclerosis raises risk of breast cancer, lung cancer, melanoma
in a population-linked cohort study published in Arthritis Care & Research.
Kathleen Morrisroe, MBBS, PhD, of St. Vincent’s Hospital Melbourne and colleagues matched deidentified patient data in the Australian Scleroderma Cohort Study (ASCS) with patients’ respective state cancer registry data between January 2008 and December 2015. The researchers also used the Australian Medical Benefit Schedule (MBS) to track health care costs for hospital admissions, presentations to the ED, other health visits, pathology, and imaging, as well as other associated costs for care, in each state. Based on this information, Dr. Morrisroe and colleagues calculated standardized incidence ratios (SIR) and standardized mortality ratios (SMR) for these patients by comparing them with the general population in Australia.
The results included 1,727 patients with systemic sclerosis (SSc) and cancer in the cohort, which consisted of mostly white (92.1%) women (85.9%) who had limited cutaneous SSc (73.9%). They were a mean of 46.6 years old when they were diagnosed with SSc and had a mean disease duration of 10.9 years. The incidence of cancer was 1.3% per year, and the overall prevalence for the cohort was 14.2%, which was higher than the general Australian population (SIR, 2.15; 95% confidence interval, 1.84-2.49). Breast cancer, melanoma, hematologic cancer, and lung cancer were the most common types of cancers found in the cohort, with early breast cancer (SIR, 3.07; 95% CI, 1.47-5.64), lung cancer (SIR, 3.07; 95% CI, 1.21-3.44), and early melanoma (SIR, 3.40; 95% CI, 1.10-7.93) having a higher incidence than the general population.
Patients with RNA polymerase III (RNAP) autoantibody had a higher incidence of early onset cancer (odds ratio, 2.9; P = .044), defined as a cancer diagnosis within 5 years of SSc diagnosis. Interstitial lung disease was also linked to an increased risk of lung cancer (OR, 2.83; P = .031), which persisted after the researchers performed a multivariate analysis.
Another factor that increased the overall risk of cancer was calcium channel blockers (OR, 1.47; P = .016), which also increased the risk of breast (OR, 1.61; P = .051) and melanoma-specific cancers (OR, 2.01; P = .042), a finding the researchers said was “unexpected, but has been reported in the literature with conflicting results.”
“This association is hypothesized to be related to the role of calcium in cell apoptosis, such as activation of the caspase pathway, induction of endonuclease activity and mitochondrial permeation,” Dr. Morrisroe and colleagues wrote.
SSc patients had more than a doubling of risk of mortality with incident cancer in comparison with SSc patients who did not have cancer (hazard ratio, 2.85; 95% CI, 1.51-5.37; P = .001). The average cost of health care annually for an SSc patient with cancer was AUD $1,496 (P less than .001), the researchers said.
This study was funded in part by Scleroderma Australia, Arthritis Australia, Actelion Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia, and Pfizer. Dr. Morrisroe reported receiving support from Arthritis Australia and Royal Australasian College of Physicians Research Establishment Fellowships. Another author reported receiving a fellowship from the National Health and Medical Research Council of Australia. The other authors reported no relevant conflicts of interest.
SOURCE: Morrisroe K et al. Arthritis Care Res. 2019 Sep 20. doi: 10.1002/acr.24076
in a population-linked cohort study published in Arthritis Care & Research.
Kathleen Morrisroe, MBBS, PhD, of St. Vincent’s Hospital Melbourne and colleagues matched deidentified patient data in the Australian Scleroderma Cohort Study (ASCS) with patients’ respective state cancer registry data between January 2008 and December 2015. The researchers also used the Australian Medical Benefit Schedule (MBS) to track health care costs for hospital admissions, presentations to the ED, other health visits, pathology, and imaging, as well as other associated costs for care, in each state. Based on this information, Dr. Morrisroe and colleagues calculated standardized incidence ratios (SIR) and standardized mortality ratios (SMR) for these patients by comparing them with the general population in Australia.
The results included 1,727 patients with systemic sclerosis (SSc) and cancer in the cohort, which consisted of mostly white (92.1%) women (85.9%) who had limited cutaneous SSc (73.9%). They were a mean of 46.6 years old when they were diagnosed with SSc and had a mean disease duration of 10.9 years. The incidence of cancer was 1.3% per year, and the overall prevalence for the cohort was 14.2%, which was higher than the general Australian population (SIR, 2.15; 95% confidence interval, 1.84-2.49). Breast cancer, melanoma, hematologic cancer, and lung cancer were the most common types of cancers found in the cohort, with early breast cancer (SIR, 3.07; 95% CI, 1.47-5.64), lung cancer (SIR, 3.07; 95% CI, 1.21-3.44), and early melanoma (SIR, 3.40; 95% CI, 1.10-7.93) having a higher incidence than the general population.
Patients with RNA polymerase III (RNAP) autoantibody had a higher incidence of early onset cancer (odds ratio, 2.9; P = .044), defined as a cancer diagnosis within 5 years of SSc diagnosis. Interstitial lung disease was also linked to an increased risk of lung cancer (OR, 2.83; P = .031), which persisted after the researchers performed a multivariate analysis.
Another factor that increased the overall risk of cancer was calcium channel blockers (OR, 1.47; P = .016), which also increased the risk of breast (OR, 1.61; P = .051) and melanoma-specific cancers (OR, 2.01; P = .042), a finding the researchers said was “unexpected, but has been reported in the literature with conflicting results.”
“This association is hypothesized to be related to the role of calcium in cell apoptosis, such as activation of the caspase pathway, induction of endonuclease activity and mitochondrial permeation,” Dr. Morrisroe and colleagues wrote.
SSc patients had more than a doubling of risk of mortality with incident cancer in comparison with SSc patients who did not have cancer (hazard ratio, 2.85; 95% CI, 1.51-5.37; P = .001). The average cost of health care annually for an SSc patient with cancer was AUD $1,496 (P less than .001), the researchers said.
This study was funded in part by Scleroderma Australia, Arthritis Australia, Actelion Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia, and Pfizer. Dr. Morrisroe reported receiving support from Arthritis Australia and Royal Australasian College of Physicians Research Establishment Fellowships. Another author reported receiving a fellowship from the National Health and Medical Research Council of Australia. The other authors reported no relevant conflicts of interest.
SOURCE: Morrisroe K et al. Arthritis Care Res. 2019 Sep 20. doi: 10.1002/acr.24076
in a population-linked cohort study published in Arthritis Care & Research.
Kathleen Morrisroe, MBBS, PhD, of St. Vincent’s Hospital Melbourne and colleagues matched deidentified patient data in the Australian Scleroderma Cohort Study (ASCS) with patients’ respective state cancer registry data between January 2008 and December 2015. The researchers also used the Australian Medical Benefit Schedule (MBS) to track health care costs for hospital admissions, presentations to the ED, other health visits, pathology, and imaging, as well as other associated costs for care, in each state. Based on this information, Dr. Morrisroe and colleagues calculated standardized incidence ratios (SIR) and standardized mortality ratios (SMR) for these patients by comparing them with the general population in Australia.
The results included 1,727 patients with systemic sclerosis (SSc) and cancer in the cohort, which consisted of mostly white (92.1%) women (85.9%) who had limited cutaneous SSc (73.9%). They were a mean of 46.6 years old when they were diagnosed with SSc and had a mean disease duration of 10.9 years. The incidence of cancer was 1.3% per year, and the overall prevalence for the cohort was 14.2%, which was higher than the general Australian population (SIR, 2.15; 95% confidence interval, 1.84-2.49). Breast cancer, melanoma, hematologic cancer, and lung cancer were the most common types of cancers found in the cohort, with early breast cancer (SIR, 3.07; 95% CI, 1.47-5.64), lung cancer (SIR, 3.07; 95% CI, 1.21-3.44), and early melanoma (SIR, 3.40; 95% CI, 1.10-7.93) having a higher incidence than the general population.
Patients with RNA polymerase III (RNAP) autoantibody had a higher incidence of early onset cancer (odds ratio, 2.9; P = .044), defined as a cancer diagnosis within 5 years of SSc diagnosis. Interstitial lung disease was also linked to an increased risk of lung cancer (OR, 2.83; P = .031), which persisted after the researchers performed a multivariate analysis.
Another factor that increased the overall risk of cancer was calcium channel blockers (OR, 1.47; P = .016), which also increased the risk of breast (OR, 1.61; P = .051) and melanoma-specific cancers (OR, 2.01; P = .042), a finding the researchers said was “unexpected, but has been reported in the literature with conflicting results.”
“This association is hypothesized to be related to the role of calcium in cell apoptosis, such as activation of the caspase pathway, induction of endonuclease activity and mitochondrial permeation,” Dr. Morrisroe and colleagues wrote.
SSc patients had more than a doubling of risk of mortality with incident cancer in comparison with SSc patients who did not have cancer (hazard ratio, 2.85; 95% CI, 1.51-5.37; P = .001). The average cost of health care annually for an SSc patient with cancer was AUD $1,496 (P less than .001), the researchers said.
This study was funded in part by Scleroderma Australia, Arthritis Australia, Actelion Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia, and Pfizer. Dr. Morrisroe reported receiving support from Arthritis Australia and Royal Australasian College of Physicians Research Establishment Fellowships. Another author reported receiving a fellowship from the National Health and Medical Research Council of Australia. The other authors reported no relevant conflicts of interest.
SOURCE: Morrisroe K et al. Arthritis Care Res. 2019 Sep 20. doi: 10.1002/acr.24076
FROM ARTHRITIS CARE & RESEARCH