User login
Suspect myopathy? Take this approach to the work-up
› Categorize patients with muscle complaints into suspected myositic, intrinsic, or toxic myopathy to help guide subsequent work-up. C
› Look for diffusely painful, swollen, or boggy-feeling muscles—as well as weakness and pain with exertion—in patients you suspect may have viral myopathy. C
› Consider electromyography and muscle biopsy for patients you suspect may have dermatomyositis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Marie C, a 75-year-old Asian woman, reports weakness in her legs and arms with unsteadiness when walking. She has a vague but persistent ache in her large muscles. Her symptoms have developed slowly over the past 3 months. She denies recent signs or symptoms of infection or other illness. Her medical history includes hypertension, hyperlipidemia, osteopenia, and obesity. Ms. C takes lisinopril 10 mg/d and atorvastatin, which was recently increased from 10 to 20 mg/d.
What would your next steps be in caring for this patient?
Patients who experience muscle-related symptoms such as pain, fatigue, or weakness often seek help from their family physician (FP). The list of possible causes of these complaints can be lengthy and vary greatly, from nonmyopathic conditions such as fibromyalgia to worrisome forms of myopathy such as inclusion body myositis or polymyositis. This article will help you to quickly identify which patients with muscle-related complaints should be evaluated for myopathy and what your work-up should include.
Myopathy or not?
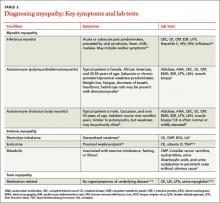
Distinguishing between myopathy and nonmyopathic muscle pain or weakness is the first step in evaluating patients with muscle-related complaints. Many conditions share muscle-related symptoms, but actual muscle damage is not always present (eg, fibromyalgia, chronic pain, and chronic fatigue syndromes).1 While there is some overlap in presentation between patients with myopathy and nonmyopathic conditions, there are important differences in symptoms, physical exam findings, and lab test results (TABLE 11-4). Notably, in myopathic disease, patients’ symptoms are usually progressive, vital signs are abnormal, and weakness is common, whereas patients with nonmyopathic disease typically have remitting and relapsing symptoms, normal vital signs, and no weakness.
Myopathy itself is divided into 3 categories—myositic, intrinsic, and toxic—which reflect the condition, or medication, that brought on the muscle damage (TABLE 22,4-15). Placing patients into one of these categories based on their risk factors, history, and physical exam findings can help to focus the diagnostic work-up on areas most likely to provide useful information.
Myositic myopathy can be caused by infection or autoimmunity
Myositic myopathies result in inflammatory destruction of muscle tissue. Patients with myositic myopathy often exhibit fever, malaise, weight loss, and general fatigue. Though weakness and pain are common, both can be variable or even absent in myositic myopathy.2,5 Myositic myopathy can be caused by infectious agents or can develop from an autoimmune disease.
Infectious myositic myopathy is one of the more common types of myopathy that FPs will encounter.2 Viruses such as influenza, parainfluenza, coxsackievirus, human immunodeficiency virus, cytomegalovirus, echovirus, adenovirus, Epstein-Barr, and hepatitis C are common causes.2,4,16 Bacterial and fungal myositides are relatively rare. Both most often occur as the result of penetrating trauma or immunocompromise, and are generally not subtle.2 Parasitic myopathy can occur from the invasion of skeletal muscle by trichinella after ingesting undercooked, infected meat.2 Although previously a more common problem, currently only 10 to 20 cases of trichinellosis are reported in the United States each year.17 Due to their rarity, bacterial, fungal, and parasitic myositides are not reviewed here.
Patients with a viral myositis often report prodromal symptoms such as fever, upper respiratory illness, or gastrointestinal distress one to 2 weeks before the onset of muscle complaints. Muscle pain is usually multifocal, involving larger, bilateral muscle groups, and may be associated with swelling.
Patients with viral myositis may exhibit diffusely painful, swollen, or boggy-feeling muscles as well as weakness and pain with exertion. Other signs of viral infection such as rash, fever, upper respiratory symptoms, or meningeal signs may be present. Severe signs include arrhythmia or respiratory failure due to cardiac muscle or diaphragm involvement, or signs of renal failure due to precipitation of myoglobin in the renal system (ie, rhabdomyolysis).2 If the infection affects the heart, patients may develop palpitations, pleuritic chest pain, or shortness of breath.2
Diagnosis of viral myositis relies heavily on clinical suspicion in patients with a fitting history and physical exam findings. Helpful lab tests include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine kinase (CK), and liver function tests (LFTs), all of which can be abnormal in viral myositis. Viral polymerase chain reaction, culture, or antigen testing may be helpful in severe or confusing cases, but in most cases such testing is unnecessary. Muscle biopsy is not recommended except in persistent cases, where definitive identification of the causative agent might alter treatment or when nonviral infection is suspected.2
Autoimmune myositic myopathy. Unlike infectious myopathies, autoimmune myopathies are usually chronic, subtle, and relatively rare. The 3 most common autoimmune myopathies—polymyositis, dermatomyositis, and inclusion body myositis—have a combined prevalence of approximately 10:100,000.6 Although these types of myopathies are uncommon, FPs will likely be the first to evaluate a patient with one of them.
Patients with an autoimmune myopathy typically complain of weakness and mild to moderate muscle pain, although pain may be absent. Compared to infectious myopathies, autoimmune myopathies usually exhibit a more indolent course. Patients with advanced disease may report fever, weight loss, shortness of breath from cardiomyopathy, heartburn from a weakened lower esophageal sphincter, and/or a rash.5
Physical examination may reveal symmetric, proximal muscle weakness. Atrophy is typically not seen until late in the disease. Skin exam usually is normal in patients with inclusion body myositis and polymyositis. The typical rash of dermatomyositis is a heliotrope (blue-purple) discoloration on the upper eyelids and a raised, violaceous, scaly eruption on the knuckles (Gottron’s papules).
Laboratory tests that can be helpful include CK, lactate dehydrogenase (LDH), aldolase, and LFTs (reflecting muscle injury, not liver involvement). For polymyositis and dermatomyositis, CK is the most sensitive lab test and often exhibits the highest elevation above normal.6 Conversely, CK is often normal or only mildly elevated in inclusion body myositis. Up to 80% of patients with autoimmune myopathy will have antinuclear antibodies.3,5 ESR and CRP levels are also often elevated.
Both electromyography (EMG) and muscle biopsy may be required to diagnose autoimmune myopathy, but these are typically done under the direction of a rheumatologist after an FP’s initial work-up is inconclusive.
Intrinsic myopathy: Suspect electrolyte problems, other causes
Intrinsic myopathy occurs in patients with electrolyte disorders, diseases of the endocrine system, or underlying metabolic dysfunction.
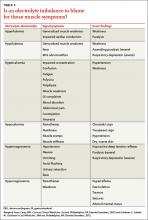
Electrolyte disorders. Muscle-related symptoms are unlikely to be the chief complaint of patients with severe electrolyte imbalance. However, a patient with mild to moderate electrolyte problems may develop muscle fatigue, weakness, or pain. TABLE 3 reviews other signs and symptoms of electrolyte abnormalities that may be helpful in establishing a diagnosis in a patient with muscle complaints.
Ordering a complete metabolic panel (CMP), CK, and urinalysis (UA) can help rule out electrolyte disorders. If electrolyte disorders are detected, an electrocardiogram is useful to evaluate for cardiac dysfunction. Once an electrolyte disorder is identified, investigate its underlying cause. Correcting the electrolyte disorder should help improve symptoms of myopathy.
Endocrine myopathy can be associated with hypothyroidism, hyperthyroidism, parathyroid disease, vitamin D deficiency, or Cushing syndrome.8-10,18,19 Although less common than some other causes, identifying endocrine myopathy is crucial because correcting the underlying disease will often improve multiple aspects of the patient’s health.
The presentation of endocrine myopathy may be subtle. Patients with hypothyroidism may experience muscle pain or weakness, fatigue, cold sensitivity, constipation, and dry skin.20 Muscle-related symptoms may be the only sign of endocrine myopathy in a patient who would otherwise be considered to have subclinical hypothyroidism.8,18 Hyperthyroidism can present with weight loss, heat intolerance, frequent bowel movements, tachycardia, and muscle weakness.21
Patients with parathyroid disease— especially patients with chronic renal failure—may report proximal muscle weakness, often in the lower extremities.19 Complaints of muscle weakness or pain can occur with severe vitamin D deficiency.10 Patients with Cushing syndrome often experience proximal weakness and weight gain.9
Patients with a personal or family history of endocrine disorders, previous thyroid surgery, or those taking medications that can impair thyroid function, such as lithium, amiodarone, or interferon, are at risk for endocrine myopathy.18-20 Suspect hyperparathyroidism in patients with chronic kidney disease who complain of weakness.
Vitamin D deficiency is relatively common, with at minimum 20% of elderly adults estimated to be deficient.10 Patients at risk for Cushing disease are most likely receiving pharmacologic doses of glucocorticoids, which can increase their risk of myopathy, or to have ectopic adrenocorticotropic hormone secretion.
Metabolic myopathy results from a lack of sufficient energy production in the muscle. The 3 main groups of metabolic myopathy are impaired muscle glycogenoses, disorders of fatty acid oxidation, and mitochondrial myopathies.7
Because metabolic myopathy can occur at any age, a thorough history and physical is crucial for diagnosis. Proximal weakness in metabolic myopathy is often associated with exercise intolerance, stressful illness, or fasting. Patients often present with dynamic abnormalities such as fatigue, muscle cramping, and even rhabdomyolysis during exertion.7
When evaluating patients you suspect may have metabolic myopathy, a physical exam may reveal muscle contractures, muscle swelling, or proximal muscle weakness. Patients with certain types of fatty acid oxidation disorders or mitochondrial disorders may also exhibit cardiomyopathy, neuropathy, retinopathy, ataxia, hearing loss, or other systemic manifestations.7
Basic labs for investigating suspected metabolic myopathy include serum electrolytes, glucose, LFTs, CK (which may or may not be elevated), lactate, ammonia, and UA for myoglobinuria. More advanced labs, such as serum total carnitine and acylcarnitine as well as urinary levels of dicarboxylic acids and acylglycines, may be needed if a metabolic disorder is strongly suspected.7 Muscle biopsy, EMG, and genetic testing can also prove helpful in diagnosis. Definitive diagnosis and treatment of metabolic myopathy usually requires a multidisciplinary team of providers, including subspecialty referral.
Toxic myopathy
Toxic myopathy refers to muscle damage caused by an exogenous chemical agent, most often a drug. The mechanism of toxicity is not always clear and may result from the activation of inflammatory responses similar to autoimmune myopathy.22 Toxic myopathies may result from several commonly used medications; cholesterol-lowering medications are a common culprit.13-15,23-25 Drug-induced myopathies vary in frequency and severity. For instance, in patients taking statins, the rate of myalgias is 6%, while the incidence of rhabdomyolysis is estimated to be 4 per 100,000, and is found most often in patients taking concomitant fibrates.23
Drug-induced toxic myopathy differs from previously discussed myopathies in that symptoms are usually more insidious, findings on exam are more often mixed muscular and neurologic, and lab abnormalities are usually more subtle.11,12 Symptoms of myopathy typically occur weeks or months after initiating a drug and usually improve or resolve within weeks after discontinuing the offending agent. Knowing the patient’s medication list and which medications cause certain patterns of myopathy symptoms can help guide the differential diagnosis (TABLE 411-15,22-25).
Risk factors for most medication-related myopathies are polypharmacy, renal or liver disease, and age over 50 years13-15,23-25 The physical exam for patients with drug- or toxin-related myopathy will most often reveal relatively minor abnormalities such as muscle tenderness and mild weakness, except for the most severe or advanced cases. Most patients will not have physical signs that suggest an underlying illness. CK levels and LFTs should be obtained. Basic chemistry and UA may also be helpful in patients with risk factors for renal disease.
CASE › Ms. C has been taking a statin for more than 10 years, and the dose was recently increased. You are aware that statin-related muscle injury can develop even after years of use, and suspect the statin may be causing her myopathy. You order a CK test, which is mildly elevated. You recommend discontinuing the statin. After 8 weeks off her statin, Ms. C’s Symptoms do not improve. Given her lack of systemic complaints, myositic myopathy from an infectious or rheumatologic cause seems unlikely. You begin to consider an intrinsic cause of myopathy, and order the following tests: a CMP, UA, thyroid-stimulating hormone, repeat CK, and vitamin D level. This testing reveals a vitamin D deficiency at 17 ng/ml (normal range: 30-74 ng/ml). You recommend vitamin D, 50,000 IU per week for 8 weeks. At follow-up, Ms. C's vitamin D level is 40. She says she feels better and her muscle complaints have resolved.
CORRESPONDENCE
Brent W. Smith, MD, Travis Air Force Base Family Medicine Residency, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
1. Huynh CN, Yanni LM, Morgan LA. Fibromyalgia: diagnosis and management for the primary healthcare provider. J Womens Health. 2008;8:1379-1387.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473-494.
3. Reichlin M, Arnett FC Jr. Multiplicity of antibodies in myositis sera. Arthritis Rheum. 1984;27:1150-1156.
4. Yoshino M, Suzuki S, Adachi K, et al. High incidence of acute myositis with type A influenza virus infection in the elderly. Intern Med. 2000;39:431-432.
5. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
6. Wilson FC, Ytterberg SR, St Sauver JL, et al. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35:445-447.
7. Smith EC, El-Gharbawy A, Koeberl DD. Metabolic myopathies: clinical features and diagnostic approach. Rheum Dis Clin N Am. 2011:37:201-217.
8. Reuters V, Teixeira Pde F, Vigário PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci. 2009;338:259-263.
9. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526-1540.
10. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
11. Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400-409.
12. Phillips PS, Haas RH, Bannykh S, et al; Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581-585.
13. Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41-44.
14. Posada C, García-Cruz A, García-Doval I, et al. Chloroquine-induced myopathy. Lupus. 2011;20:773-774.
15. Uri DS, Biavis M. Colchicine neuromyopathy. J Clin Rheumatol. 1996;2:163-166.
16. Mannix R, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118:2119-2125.
17. Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol. 2007;149:3-21.
18. Rodolico C, Toscano A, Benvenga S, et al. Myopathy as the persistently isolated symptomatology of primary autoimmune hypothyroidism. Thyroid.1998;8:1033-1038.
19. AACE/AAES Task Force on Primary Hyperparathyroidism. The American Association of Clinical Endocrinologists and The American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49-54.
20. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Pract. 2012;18:988-1028.
21. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
22. Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644-650.
23. Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23: 1182-1186.
24. Marot A, Morelle J, Chouinard VA, et al. Concomitant use of simvastatin and amiodarone resulting in severe rhabdomyolysis: a case report and review of the literature. Acta Clin Belg. 2011;66:134-136.
25. Peters BS, Winer J, Landon DN, et al. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q J Med. 1993;86:5-15.
› Categorize patients with muscle complaints into suspected myositic, intrinsic, or toxic myopathy to help guide subsequent work-up. C
› Look for diffusely painful, swollen, or boggy-feeling muscles—as well as weakness and pain with exertion—in patients you suspect may have viral myopathy. C
› Consider electromyography and muscle biopsy for patients you suspect may have dermatomyositis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Marie C, a 75-year-old Asian woman, reports weakness in her legs and arms with unsteadiness when walking. She has a vague but persistent ache in her large muscles. Her symptoms have developed slowly over the past 3 months. She denies recent signs or symptoms of infection or other illness. Her medical history includes hypertension, hyperlipidemia, osteopenia, and obesity. Ms. C takes lisinopril 10 mg/d and atorvastatin, which was recently increased from 10 to 20 mg/d.
What would your next steps be in caring for this patient?
Patients who experience muscle-related symptoms such as pain, fatigue, or weakness often seek help from their family physician (FP). The list of possible causes of these complaints can be lengthy and vary greatly, from nonmyopathic conditions such as fibromyalgia to worrisome forms of myopathy such as inclusion body myositis or polymyositis. This article will help you to quickly identify which patients with muscle-related complaints should be evaluated for myopathy and what your work-up should include.
Myopathy or not?
Distinguishing between myopathy and nonmyopathic muscle pain or weakness is the first step in evaluating patients with muscle-related complaints. Many conditions share muscle-related symptoms, but actual muscle damage is not always present (eg, fibromyalgia, chronic pain, and chronic fatigue syndromes).1 While there is some overlap in presentation between patients with myopathy and nonmyopathic conditions, there are important differences in symptoms, physical exam findings, and lab test results (TABLE 11-4). Notably, in myopathic disease, patients’ symptoms are usually progressive, vital signs are abnormal, and weakness is common, whereas patients with nonmyopathic disease typically have remitting and relapsing symptoms, normal vital signs, and no weakness.
Myopathy itself is divided into 3 categories—myositic, intrinsic, and toxic—which reflect the condition, or medication, that brought on the muscle damage (TABLE 22,4-15). Placing patients into one of these categories based on their risk factors, history, and physical exam findings can help to focus the diagnostic work-up on areas most likely to provide useful information.
Myositic myopathy can be caused by infection or autoimmunity
Myositic myopathies result in inflammatory destruction of muscle tissue. Patients with myositic myopathy often exhibit fever, malaise, weight loss, and general fatigue. Though weakness and pain are common, both can be variable or even absent in myositic myopathy.2,5 Myositic myopathy can be caused by infectious agents or can develop from an autoimmune disease.
Infectious myositic myopathy is one of the more common types of myopathy that FPs will encounter.2 Viruses such as influenza, parainfluenza, coxsackievirus, human immunodeficiency virus, cytomegalovirus, echovirus, adenovirus, Epstein-Barr, and hepatitis C are common causes.2,4,16 Bacterial and fungal myositides are relatively rare. Both most often occur as the result of penetrating trauma or immunocompromise, and are generally not subtle.2 Parasitic myopathy can occur from the invasion of skeletal muscle by trichinella after ingesting undercooked, infected meat.2 Although previously a more common problem, currently only 10 to 20 cases of trichinellosis are reported in the United States each year.17 Due to their rarity, bacterial, fungal, and parasitic myositides are not reviewed here.
Patients with a viral myositis often report prodromal symptoms such as fever, upper respiratory illness, or gastrointestinal distress one to 2 weeks before the onset of muscle complaints. Muscle pain is usually multifocal, involving larger, bilateral muscle groups, and may be associated with swelling.
Patients with viral myositis may exhibit diffusely painful, swollen, or boggy-feeling muscles as well as weakness and pain with exertion. Other signs of viral infection such as rash, fever, upper respiratory symptoms, or meningeal signs may be present. Severe signs include arrhythmia or respiratory failure due to cardiac muscle or diaphragm involvement, or signs of renal failure due to precipitation of myoglobin in the renal system (ie, rhabdomyolysis).2 If the infection affects the heart, patients may develop palpitations, pleuritic chest pain, or shortness of breath.2
Diagnosis of viral myositis relies heavily on clinical suspicion in patients with a fitting history and physical exam findings. Helpful lab tests include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine kinase (CK), and liver function tests (LFTs), all of which can be abnormal in viral myositis. Viral polymerase chain reaction, culture, or antigen testing may be helpful in severe or confusing cases, but in most cases such testing is unnecessary. Muscle biopsy is not recommended except in persistent cases, where definitive identification of the causative agent might alter treatment or when nonviral infection is suspected.2
Autoimmune myositic myopathy. Unlike infectious myopathies, autoimmune myopathies are usually chronic, subtle, and relatively rare. The 3 most common autoimmune myopathies—polymyositis, dermatomyositis, and inclusion body myositis—have a combined prevalence of approximately 10:100,000.6 Although these types of myopathies are uncommon, FPs will likely be the first to evaluate a patient with one of them.
Patients with an autoimmune myopathy typically complain of weakness and mild to moderate muscle pain, although pain may be absent. Compared to infectious myopathies, autoimmune myopathies usually exhibit a more indolent course. Patients with advanced disease may report fever, weight loss, shortness of breath from cardiomyopathy, heartburn from a weakened lower esophageal sphincter, and/or a rash.5
Physical examination may reveal symmetric, proximal muscle weakness. Atrophy is typically not seen until late in the disease. Skin exam usually is normal in patients with inclusion body myositis and polymyositis. The typical rash of dermatomyositis is a heliotrope (blue-purple) discoloration on the upper eyelids and a raised, violaceous, scaly eruption on the knuckles (Gottron’s papules).
Laboratory tests that can be helpful include CK, lactate dehydrogenase (LDH), aldolase, and LFTs (reflecting muscle injury, not liver involvement). For polymyositis and dermatomyositis, CK is the most sensitive lab test and often exhibits the highest elevation above normal.6 Conversely, CK is often normal or only mildly elevated in inclusion body myositis. Up to 80% of patients with autoimmune myopathy will have antinuclear antibodies.3,5 ESR and CRP levels are also often elevated.
Both electromyography (EMG) and muscle biopsy may be required to diagnose autoimmune myopathy, but these are typically done under the direction of a rheumatologist after an FP’s initial work-up is inconclusive.
Intrinsic myopathy: Suspect electrolyte problems, other causes
Intrinsic myopathy occurs in patients with electrolyte disorders, diseases of the endocrine system, or underlying metabolic dysfunction.
Electrolyte disorders. Muscle-related symptoms are unlikely to be the chief complaint of patients with severe electrolyte imbalance. However, a patient with mild to moderate electrolyte problems may develop muscle fatigue, weakness, or pain. TABLE 3 reviews other signs and symptoms of electrolyte abnormalities that may be helpful in establishing a diagnosis in a patient with muscle complaints.
Ordering a complete metabolic panel (CMP), CK, and urinalysis (UA) can help rule out electrolyte disorders. If electrolyte disorders are detected, an electrocardiogram is useful to evaluate for cardiac dysfunction. Once an electrolyte disorder is identified, investigate its underlying cause. Correcting the electrolyte disorder should help improve symptoms of myopathy.
Endocrine myopathy can be associated with hypothyroidism, hyperthyroidism, parathyroid disease, vitamin D deficiency, or Cushing syndrome.8-10,18,19 Although less common than some other causes, identifying endocrine myopathy is crucial because correcting the underlying disease will often improve multiple aspects of the patient’s health.
The presentation of endocrine myopathy may be subtle. Patients with hypothyroidism may experience muscle pain or weakness, fatigue, cold sensitivity, constipation, and dry skin.20 Muscle-related symptoms may be the only sign of endocrine myopathy in a patient who would otherwise be considered to have subclinical hypothyroidism.8,18 Hyperthyroidism can present with weight loss, heat intolerance, frequent bowel movements, tachycardia, and muscle weakness.21
Patients with parathyroid disease— especially patients with chronic renal failure—may report proximal muscle weakness, often in the lower extremities.19 Complaints of muscle weakness or pain can occur with severe vitamin D deficiency.10 Patients with Cushing syndrome often experience proximal weakness and weight gain.9
Patients with a personal or family history of endocrine disorders, previous thyroid surgery, or those taking medications that can impair thyroid function, such as lithium, amiodarone, or interferon, are at risk for endocrine myopathy.18-20 Suspect hyperparathyroidism in patients with chronic kidney disease who complain of weakness.
Vitamin D deficiency is relatively common, with at minimum 20% of elderly adults estimated to be deficient.10 Patients at risk for Cushing disease are most likely receiving pharmacologic doses of glucocorticoids, which can increase their risk of myopathy, or to have ectopic adrenocorticotropic hormone secretion.
Metabolic myopathy results from a lack of sufficient energy production in the muscle. The 3 main groups of metabolic myopathy are impaired muscle glycogenoses, disorders of fatty acid oxidation, and mitochondrial myopathies.7
Because metabolic myopathy can occur at any age, a thorough history and physical is crucial for diagnosis. Proximal weakness in metabolic myopathy is often associated with exercise intolerance, stressful illness, or fasting. Patients often present with dynamic abnormalities such as fatigue, muscle cramping, and even rhabdomyolysis during exertion.7
When evaluating patients you suspect may have metabolic myopathy, a physical exam may reveal muscle contractures, muscle swelling, or proximal muscle weakness. Patients with certain types of fatty acid oxidation disorders or mitochondrial disorders may also exhibit cardiomyopathy, neuropathy, retinopathy, ataxia, hearing loss, or other systemic manifestations.7
Basic labs for investigating suspected metabolic myopathy include serum electrolytes, glucose, LFTs, CK (which may or may not be elevated), lactate, ammonia, and UA for myoglobinuria. More advanced labs, such as serum total carnitine and acylcarnitine as well as urinary levels of dicarboxylic acids and acylglycines, may be needed if a metabolic disorder is strongly suspected.7 Muscle biopsy, EMG, and genetic testing can also prove helpful in diagnosis. Definitive diagnosis and treatment of metabolic myopathy usually requires a multidisciplinary team of providers, including subspecialty referral.
Toxic myopathy
Toxic myopathy refers to muscle damage caused by an exogenous chemical agent, most often a drug. The mechanism of toxicity is not always clear and may result from the activation of inflammatory responses similar to autoimmune myopathy.22 Toxic myopathies may result from several commonly used medications; cholesterol-lowering medications are a common culprit.13-15,23-25 Drug-induced myopathies vary in frequency and severity. For instance, in patients taking statins, the rate of myalgias is 6%, while the incidence of rhabdomyolysis is estimated to be 4 per 100,000, and is found most often in patients taking concomitant fibrates.23
Drug-induced toxic myopathy differs from previously discussed myopathies in that symptoms are usually more insidious, findings on exam are more often mixed muscular and neurologic, and lab abnormalities are usually more subtle.11,12 Symptoms of myopathy typically occur weeks or months after initiating a drug and usually improve or resolve within weeks after discontinuing the offending agent. Knowing the patient’s medication list and which medications cause certain patterns of myopathy symptoms can help guide the differential diagnosis (TABLE 411-15,22-25).
Risk factors for most medication-related myopathies are polypharmacy, renal or liver disease, and age over 50 years13-15,23-25 The physical exam for patients with drug- or toxin-related myopathy will most often reveal relatively minor abnormalities such as muscle tenderness and mild weakness, except for the most severe or advanced cases. Most patients will not have physical signs that suggest an underlying illness. CK levels and LFTs should be obtained. Basic chemistry and UA may also be helpful in patients with risk factors for renal disease.
CASE › Ms. C has been taking a statin for more than 10 years, and the dose was recently increased. You are aware that statin-related muscle injury can develop even after years of use, and suspect the statin may be causing her myopathy. You order a CK test, which is mildly elevated. You recommend discontinuing the statin. After 8 weeks off her statin, Ms. C’s Symptoms do not improve. Given her lack of systemic complaints, myositic myopathy from an infectious or rheumatologic cause seems unlikely. You begin to consider an intrinsic cause of myopathy, and order the following tests: a CMP, UA, thyroid-stimulating hormone, repeat CK, and vitamin D level. This testing reveals a vitamin D deficiency at 17 ng/ml (normal range: 30-74 ng/ml). You recommend vitamin D, 50,000 IU per week for 8 weeks. At follow-up, Ms. C's vitamin D level is 40. She says she feels better and her muscle complaints have resolved.
CORRESPONDENCE
Brent W. Smith, MD, Travis Air Force Base Family Medicine Residency, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
› Categorize patients with muscle complaints into suspected myositic, intrinsic, or toxic myopathy to help guide subsequent work-up. C
› Look for diffusely painful, swollen, or boggy-feeling muscles—as well as weakness and pain with exertion—in patients you suspect may have viral myopathy. C
› Consider electromyography and muscle biopsy for patients you suspect may have dermatomyositis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Marie C, a 75-year-old Asian woman, reports weakness in her legs and arms with unsteadiness when walking. She has a vague but persistent ache in her large muscles. Her symptoms have developed slowly over the past 3 months. She denies recent signs or symptoms of infection or other illness. Her medical history includes hypertension, hyperlipidemia, osteopenia, and obesity. Ms. C takes lisinopril 10 mg/d and atorvastatin, which was recently increased from 10 to 20 mg/d.
What would your next steps be in caring for this patient?
Patients who experience muscle-related symptoms such as pain, fatigue, or weakness often seek help from their family physician (FP). The list of possible causes of these complaints can be lengthy and vary greatly, from nonmyopathic conditions such as fibromyalgia to worrisome forms of myopathy such as inclusion body myositis or polymyositis. This article will help you to quickly identify which patients with muscle-related complaints should be evaluated for myopathy and what your work-up should include.
Myopathy or not?
Distinguishing between myopathy and nonmyopathic muscle pain or weakness is the first step in evaluating patients with muscle-related complaints. Many conditions share muscle-related symptoms, but actual muscle damage is not always present (eg, fibromyalgia, chronic pain, and chronic fatigue syndromes).1 While there is some overlap in presentation between patients with myopathy and nonmyopathic conditions, there are important differences in symptoms, physical exam findings, and lab test results (TABLE 11-4). Notably, in myopathic disease, patients’ symptoms are usually progressive, vital signs are abnormal, and weakness is common, whereas patients with nonmyopathic disease typically have remitting and relapsing symptoms, normal vital signs, and no weakness.
Myopathy itself is divided into 3 categories—myositic, intrinsic, and toxic—which reflect the condition, or medication, that brought on the muscle damage (TABLE 22,4-15). Placing patients into one of these categories based on their risk factors, history, and physical exam findings can help to focus the diagnostic work-up on areas most likely to provide useful information.
Myositic myopathy can be caused by infection or autoimmunity
Myositic myopathies result in inflammatory destruction of muscle tissue. Patients with myositic myopathy often exhibit fever, malaise, weight loss, and general fatigue. Though weakness and pain are common, both can be variable or even absent in myositic myopathy.2,5 Myositic myopathy can be caused by infectious agents or can develop from an autoimmune disease.
Infectious myositic myopathy is one of the more common types of myopathy that FPs will encounter.2 Viruses such as influenza, parainfluenza, coxsackievirus, human immunodeficiency virus, cytomegalovirus, echovirus, adenovirus, Epstein-Barr, and hepatitis C are common causes.2,4,16 Bacterial and fungal myositides are relatively rare. Both most often occur as the result of penetrating trauma or immunocompromise, and are generally not subtle.2 Parasitic myopathy can occur from the invasion of skeletal muscle by trichinella after ingesting undercooked, infected meat.2 Although previously a more common problem, currently only 10 to 20 cases of trichinellosis are reported in the United States each year.17 Due to their rarity, bacterial, fungal, and parasitic myositides are not reviewed here.
Patients with a viral myositis often report prodromal symptoms such as fever, upper respiratory illness, or gastrointestinal distress one to 2 weeks before the onset of muscle complaints. Muscle pain is usually multifocal, involving larger, bilateral muscle groups, and may be associated with swelling.
Patients with viral myositis may exhibit diffusely painful, swollen, or boggy-feeling muscles as well as weakness and pain with exertion. Other signs of viral infection such as rash, fever, upper respiratory symptoms, or meningeal signs may be present. Severe signs include arrhythmia or respiratory failure due to cardiac muscle or diaphragm involvement, or signs of renal failure due to precipitation of myoglobin in the renal system (ie, rhabdomyolysis).2 If the infection affects the heart, patients may develop palpitations, pleuritic chest pain, or shortness of breath.2
Diagnosis of viral myositis relies heavily on clinical suspicion in patients with a fitting history and physical exam findings. Helpful lab tests include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine kinase (CK), and liver function tests (LFTs), all of which can be abnormal in viral myositis. Viral polymerase chain reaction, culture, or antigen testing may be helpful in severe or confusing cases, but in most cases such testing is unnecessary. Muscle biopsy is not recommended except in persistent cases, where definitive identification of the causative agent might alter treatment or when nonviral infection is suspected.2
Autoimmune myositic myopathy. Unlike infectious myopathies, autoimmune myopathies are usually chronic, subtle, and relatively rare. The 3 most common autoimmune myopathies—polymyositis, dermatomyositis, and inclusion body myositis—have a combined prevalence of approximately 10:100,000.6 Although these types of myopathies are uncommon, FPs will likely be the first to evaluate a patient with one of them.
Patients with an autoimmune myopathy typically complain of weakness and mild to moderate muscle pain, although pain may be absent. Compared to infectious myopathies, autoimmune myopathies usually exhibit a more indolent course. Patients with advanced disease may report fever, weight loss, shortness of breath from cardiomyopathy, heartburn from a weakened lower esophageal sphincter, and/or a rash.5
Physical examination may reveal symmetric, proximal muscle weakness. Atrophy is typically not seen until late in the disease. Skin exam usually is normal in patients with inclusion body myositis and polymyositis. The typical rash of dermatomyositis is a heliotrope (blue-purple) discoloration on the upper eyelids and a raised, violaceous, scaly eruption on the knuckles (Gottron’s papules).
Laboratory tests that can be helpful include CK, lactate dehydrogenase (LDH), aldolase, and LFTs (reflecting muscle injury, not liver involvement). For polymyositis and dermatomyositis, CK is the most sensitive lab test and often exhibits the highest elevation above normal.6 Conversely, CK is often normal or only mildly elevated in inclusion body myositis. Up to 80% of patients with autoimmune myopathy will have antinuclear antibodies.3,5 ESR and CRP levels are also often elevated.
Both electromyography (EMG) and muscle biopsy may be required to diagnose autoimmune myopathy, but these are typically done under the direction of a rheumatologist after an FP’s initial work-up is inconclusive.
Intrinsic myopathy: Suspect electrolyte problems, other causes
Intrinsic myopathy occurs in patients with electrolyte disorders, diseases of the endocrine system, or underlying metabolic dysfunction.
Electrolyte disorders. Muscle-related symptoms are unlikely to be the chief complaint of patients with severe electrolyte imbalance. However, a patient with mild to moderate electrolyte problems may develop muscle fatigue, weakness, or pain. TABLE 3 reviews other signs and symptoms of electrolyte abnormalities that may be helpful in establishing a diagnosis in a patient with muscle complaints.
Ordering a complete metabolic panel (CMP), CK, and urinalysis (UA) can help rule out electrolyte disorders. If electrolyte disorders are detected, an electrocardiogram is useful to evaluate for cardiac dysfunction. Once an electrolyte disorder is identified, investigate its underlying cause. Correcting the electrolyte disorder should help improve symptoms of myopathy.
Endocrine myopathy can be associated with hypothyroidism, hyperthyroidism, parathyroid disease, vitamin D deficiency, or Cushing syndrome.8-10,18,19 Although less common than some other causes, identifying endocrine myopathy is crucial because correcting the underlying disease will often improve multiple aspects of the patient’s health.
The presentation of endocrine myopathy may be subtle. Patients with hypothyroidism may experience muscle pain or weakness, fatigue, cold sensitivity, constipation, and dry skin.20 Muscle-related symptoms may be the only sign of endocrine myopathy in a patient who would otherwise be considered to have subclinical hypothyroidism.8,18 Hyperthyroidism can present with weight loss, heat intolerance, frequent bowel movements, tachycardia, and muscle weakness.21
Patients with parathyroid disease— especially patients with chronic renal failure—may report proximal muscle weakness, often in the lower extremities.19 Complaints of muscle weakness or pain can occur with severe vitamin D deficiency.10 Patients with Cushing syndrome often experience proximal weakness and weight gain.9
Patients with a personal or family history of endocrine disorders, previous thyroid surgery, or those taking medications that can impair thyroid function, such as lithium, amiodarone, or interferon, are at risk for endocrine myopathy.18-20 Suspect hyperparathyroidism in patients with chronic kidney disease who complain of weakness.
Vitamin D deficiency is relatively common, with at minimum 20% of elderly adults estimated to be deficient.10 Patients at risk for Cushing disease are most likely receiving pharmacologic doses of glucocorticoids, which can increase their risk of myopathy, or to have ectopic adrenocorticotropic hormone secretion.
Metabolic myopathy results from a lack of sufficient energy production in the muscle. The 3 main groups of metabolic myopathy are impaired muscle glycogenoses, disorders of fatty acid oxidation, and mitochondrial myopathies.7
Because metabolic myopathy can occur at any age, a thorough history and physical is crucial for diagnosis. Proximal weakness in metabolic myopathy is often associated with exercise intolerance, stressful illness, or fasting. Patients often present with dynamic abnormalities such as fatigue, muscle cramping, and even rhabdomyolysis during exertion.7
When evaluating patients you suspect may have metabolic myopathy, a physical exam may reveal muscle contractures, muscle swelling, or proximal muscle weakness. Patients with certain types of fatty acid oxidation disorders or mitochondrial disorders may also exhibit cardiomyopathy, neuropathy, retinopathy, ataxia, hearing loss, or other systemic manifestations.7
Basic labs for investigating suspected metabolic myopathy include serum electrolytes, glucose, LFTs, CK (which may or may not be elevated), lactate, ammonia, and UA for myoglobinuria. More advanced labs, such as serum total carnitine and acylcarnitine as well as urinary levels of dicarboxylic acids and acylglycines, may be needed if a metabolic disorder is strongly suspected.7 Muscle biopsy, EMG, and genetic testing can also prove helpful in diagnosis. Definitive diagnosis and treatment of metabolic myopathy usually requires a multidisciplinary team of providers, including subspecialty referral.
Toxic myopathy
Toxic myopathy refers to muscle damage caused by an exogenous chemical agent, most often a drug. The mechanism of toxicity is not always clear and may result from the activation of inflammatory responses similar to autoimmune myopathy.22 Toxic myopathies may result from several commonly used medications; cholesterol-lowering medications are a common culprit.13-15,23-25 Drug-induced myopathies vary in frequency and severity. For instance, in patients taking statins, the rate of myalgias is 6%, while the incidence of rhabdomyolysis is estimated to be 4 per 100,000, and is found most often in patients taking concomitant fibrates.23
Drug-induced toxic myopathy differs from previously discussed myopathies in that symptoms are usually more insidious, findings on exam are more often mixed muscular and neurologic, and lab abnormalities are usually more subtle.11,12 Symptoms of myopathy typically occur weeks or months after initiating a drug and usually improve or resolve within weeks after discontinuing the offending agent. Knowing the patient’s medication list and which medications cause certain patterns of myopathy symptoms can help guide the differential diagnosis (TABLE 411-15,22-25).
Risk factors for most medication-related myopathies are polypharmacy, renal or liver disease, and age over 50 years13-15,23-25 The physical exam for patients with drug- or toxin-related myopathy will most often reveal relatively minor abnormalities such as muscle tenderness and mild weakness, except for the most severe or advanced cases. Most patients will not have physical signs that suggest an underlying illness. CK levels and LFTs should be obtained. Basic chemistry and UA may also be helpful in patients with risk factors for renal disease.
CASE › Ms. C has been taking a statin for more than 10 years, and the dose was recently increased. You are aware that statin-related muscle injury can develop even after years of use, and suspect the statin may be causing her myopathy. You order a CK test, which is mildly elevated. You recommend discontinuing the statin. After 8 weeks off her statin, Ms. C’s Symptoms do not improve. Given her lack of systemic complaints, myositic myopathy from an infectious or rheumatologic cause seems unlikely. You begin to consider an intrinsic cause of myopathy, and order the following tests: a CMP, UA, thyroid-stimulating hormone, repeat CK, and vitamin D level. This testing reveals a vitamin D deficiency at 17 ng/ml (normal range: 30-74 ng/ml). You recommend vitamin D, 50,000 IU per week for 8 weeks. At follow-up, Ms. C's vitamin D level is 40. She says she feels better and her muscle complaints have resolved.
CORRESPONDENCE
Brent W. Smith, MD, Travis Air Force Base Family Medicine Residency, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
1. Huynh CN, Yanni LM, Morgan LA. Fibromyalgia: diagnosis and management for the primary healthcare provider. J Womens Health. 2008;8:1379-1387.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473-494.
3. Reichlin M, Arnett FC Jr. Multiplicity of antibodies in myositis sera. Arthritis Rheum. 1984;27:1150-1156.
4. Yoshino M, Suzuki S, Adachi K, et al. High incidence of acute myositis with type A influenza virus infection in the elderly. Intern Med. 2000;39:431-432.
5. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
6. Wilson FC, Ytterberg SR, St Sauver JL, et al. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35:445-447.
7. Smith EC, El-Gharbawy A, Koeberl DD. Metabolic myopathies: clinical features and diagnostic approach. Rheum Dis Clin N Am. 2011:37:201-217.
8. Reuters V, Teixeira Pde F, Vigário PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci. 2009;338:259-263.
9. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526-1540.
10. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
11. Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400-409.
12. Phillips PS, Haas RH, Bannykh S, et al; Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581-585.
13. Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41-44.
14. Posada C, García-Cruz A, García-Doval I, et al. Chloroquine-induced myopathy. Lupus. 2011;20:773-774.
15. Uri DS, Biavis M. Colchicine neuromyopathy. J Clin Rheumatol. 1996;2:163-166.
16. Mannix R, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118:2119-2125.
17. Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol. 2007;149:3-21.
18. Rodolico C, Toscano A, Benvenga S, et al. Myopathy as the persistently isolated symptomatology of primary autoimmune hypothyroidism. Thyroid.1998;8:1033-1038.
19. AACE/AAES Task Force on Primary Hyperparathyroidism. The American Association of Clinical Endocrinologists and The American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49-54.
20. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Pract. 2012;18:988-1028.
21. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
22. Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644-650.
23. Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23: 1182-1186.
24. Marot A, Morelle J, Chouinard VA, et al. Concomitant use of simvastatin and amiodarone resulting in severe rhabdomyolysis: a case report and review of the literature. Acta Clin Belg. 2011;66:134-136.
25. Peters BS, Winer J, Landon DN, et al. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q J Med. 1993;86:5-15.
1. Huynh CN, Yanni LM, Morgan LA. Fibromyalgia: diagnosis and management for the primary healthcare provider. J Womens Health. 2008;8:1379-1387.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473-494.
3. Reichlin M, Arnett FC Jr. Multiplicity of antibodies in myositis sera. Arthritis Rheum. 1984;27:1150-1156.
4. Yoshino M, Suzuki S, Adachi K, et al. High incidence of acute myositis with type A influenza virus infection in the elderly. Intern Med. 2000;39:431-432.
5. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
6. Wilson FC, Ytterberg SR, St Sauver JL, et al. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35:445-447.
7. Smith EC, El-Gharbawy A, Koeberl DD. Metabolic myopathies: clinical features and diagnostic approach. Rheum Dis Clin N Am. 2011:37:201-217.
8. Reuters V, Teixeira Pde F, Vigário PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci. 2009;338:259-263.
9. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526-1540.
10. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
11. Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400-409.
12. Phillips PS, Haas RH, Bannykh S, et al; Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581-585.
13. Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41-44.
14. Posada C, García-Cruz A, García-Doval I, et al. Chloroquine-induced myopathy. Lupus. 2011;20:773-774.
15. Uri DS, Biavis M. Colchicine neuromyopathy. J Clin Rheumatol. 1996;2:163-166.
16. Mannix R, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118:2119-2125.
17. Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol. 2007;149:3-21.
18. Rodolico C, Toscano A, Benvenga S, et al. Myopathy as the persistently isolated symptomatology of primary autoimmune hypothyroidism. Thyroid.1998;8:1033-1038.
19. AACE/AAES Task Force on Primary Hyperparathyroidism. The American Association of Clinical Endocrinologists and The American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49-54.
20. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Pract. 2012;18:988-1028.
21. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
22. Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644-650.
23. Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23: 1182-1186.
24. Marot A, Morelle J, Chouinard VA, et al. Concomitant use of simvastatin and amiodarone resulting in severe rhabdomyolysis: a case report and review of the literature. Acta Clin Belg. 2011;66:134-136.
25. Peters BS, Winer J, Landon DN, et al. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q J Med. 1993;86:5-15.
What’s new in type 2 diabetes?
In April 2012, the American Diabetes Association (ADA) updated its guidelines for evaluating and treating type 2 diabetes mellitus (T2DM). In particular, the ADA acknowledges the value of an individualized, patient-centered approach that is less formulaic than its earlier guidelines. In this article, we highlight these and other recently published developments in the context of a case study. To help ensure follow-through on these newest recommendations, we also frame our review with the mnemonic, “ABCD IS diabetes.”
CASE JR is a 57-year-old man being seen for a regular follow-up appointment. His medical history includes T2DM, hypertension, and obesity. He is taking metformin 1000 mg twice daily, lisinopril 40 mg each morning, and amlodipine 10 mg each morning. He is current on his influenza and pneumococcal vaccinations. He does not smoke cigarettes. His physical exam and lab results reveal the following:
- blood pressure (BP), 132/70 mm Hg
- body mass index (BMI), 33 kg/m2
- glycosylated hemoglobin (A1C), 7.6%
- lipid profile: Total cholesterol, 185 mg/dL; high-density lipoprotein (HDL), 40 mg/dL; triglycerides (TG), 145 mg/dL; low-density lipoprotein (LDL), 90 mg/dL
Applying the “ABCD IS diabetes” mnemonic leads us through the following assessments.
Antiplatelets
In the past, guidelines have recommended that most patients with diabetes be placed on aspirin therapy. However, 2 trials published in 2008 failed to demonstrate significant reduction in cardiovascular disease (CVD) end points with aspirin use, raising questions about its effectiveness for primary CVD prevention in patients with diabetes.1,2 In 2010, the ADA, American Heart Association, and American College of Cardiology Foundation modified their recommendations for primary prevention,3 which remain unchanged in the 2012 ADA guidelines.4
Antiplatelet agents continue to play a role in primary prevention of CVD for patients with T2DM, but only after appropriate risk stratification.4 Consider low-dose aspirin therapy (75-162 mg/d) for patients with diabetes who have a 10-year Framingham risk >10%.4 (To calculate a patient’s 10-year risk, go to http://hp2010.nhlbihin.net/atpiii/calculator.asp.)
Many patients with T2DM seen in the primary care setting will reach this risk level and qualify for aspirin—in particular, men older than 50 years and women older than 60 with a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria.4 Aspirin therapy is not recommended for primary prevention in adults with diabetes at low risk for CVD (10-year Framingham risk <5%)—eg, men <50 and women <60 years without additional CVD risk factors.4 For patients with a 10-year Framingham risk between 5% and 10%, a decision to treat rests with the physician.
CASE Should JR be started on aspirin therapy for primary prevention of CVD? Initiating low-dose aspirin is recommended, assuming no contraindications, because his 10-year Framingham risk assessment is 11%.
A (antiplatelets): Consider low-dose aspirin therapy (75-162 mg/d) for diabetes patients with a 10-year Framingham risk >10%.
B (blood pressure): Individualize a patient’s goal for systolic blood pressure, aiming higher or lower than the customary systolic target of <130 mm Hg, as appropriate.
C (cholesterol): Recommend lifestyle changes and prescribe a statin, as needed, to achieve LDL goals in T2DM patients.
D (drug management): Use a patient-centered approach to achieve an individualized A1C goal. Metformin is the initial medication of choice. Select additional drug classes to balance adverse effects, cost, and effectiveness.
I (immunizations): Ensure that each T2DM patient receives influenza and pneumococcal vaccines, and the hepatitis B vaccine if <60 years.
S (surveillance): Confirm at each visit that annual surveillance testing for nephropathy, retinopathy, and peripheral neuropathy has been completed.
Blood pressure
The benefits of lowering BP in diabetes to <140 mm Hg systolic and <80 mm Hg diastolic have been established in randomized control trials.5-8 However, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that, in patients with T2DM, intensive BP lowering to <120 mm Hg systolic yielded no significant differences in fatal and nonfatal cardiovascular events compared with BP maintained between 130 and 140 mm Hg.9 Moreover, aggressive BP lowering may be associated with serious adverse events.10 The 2012 ADA guidelines state that a systolic BP goal of <130 mm Hg is appropriate for most patients; however, higher or lower BP targets may be individualized.4
Recommendations for adding a second antihypertensive agent and timing medication administration. For T2DM patients with hypertension, the 2012 guidelines recommend that you treat initially with either an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), if tolerated.4 When adding a second agent, the Avoiding Cardiovascular Events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial demonstrated reduced morbidity and mortality in patients receiving benazepril and amlodipine compared with those receiving benazepril and hydrochlorothiazide.11 As a result, amlodipine has joined diuretics as a preferred second oral antihypertensive agent after an ACEI or ARB.
If a patient is taking multiple BP medications, one or more should be taken at bedtime.4 Administering an antihypertensive at night results in better ambulatory BP control and reduces cardiovascular mortality.12
CASE JR is on maximal doses of 2 antihypertensive agents, and his BP is 132/70 mm Hg. His physician must individualize care and decide if adding a third agent is worth the risk of another medication when clear benefit has not been demonstrated. It is reasonable to continue his current regimen with the exception of changing his lisinopril dose to the evening and reassessing his BP control at his next visit.
Cholesterol
Controlling LDL remains the top priority of T2DM lipid management. In addition to lifestyle changes, statins are the primary means of achieving LDL goals. All patients with overt CVD should receive a statin.4 Also prescribe a statin for patients with diabetes who do not have CVD but who are older than 40 and have one or more cardiac risk factors, regardless of their baseline LDL cholesterol.4 The recommended LDL goal in T2DM patients continues to be <100 mg/dL. However, <70 mg/dL is a reasonable goal for those with known CVD.13
Using additional lipid-lowering agents besides a statin may improve cholesterol numbers, but not CVD outcomes. In the ACCORD study, adding fenofibrate to simvastatin did not decrease fatal cardiovascular events or nonfatal myocardial infarction and stroke compared with simvastatin given alone.14 The AIM-High study showed no difference in cardiovascular outcomes and a possible increase in ischemic stroke with combination niacin and statin compared with statin therapy alone.15 For now, lifestyle changes and statins remain the ideal modalities to achieve LDL goals.
CASE Should a statin be initiated for our patient? Since JR is over 40 without known CVD and has a cardiac risk factor of hypertension, he should be started on statin therapy regardless of his baseline LDL (90 mg/dL), which is already at goal (<100 mg/dL).
Drug management
Let the glycemic goal for each patient guide your medication management. The 2012 ADA recommendation for most adults is an A1C of <7%.4 More strict control (A1C <6.5%) may be appropriate for certain individuals with a long life expectancy, short duration of diabetes, and no significant micro- or macrovascular disease.4 Less strict control (A1C <8%) may be appropriate for individuals with significant comorbidities, shorter life expectancy, severe hypoglycemia, or long-standing T2DM that’s been difficult to control despite multiple medications, including insulin.4
Individualize treatment. In April 2012, the ADA released a position statement encouraging a patient-centered approach to managing hyperglycemia in T2DM.16 This statement contains a new treatment algorithm (available at: http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) that is less prescriptive than the previous 2009 algorithm and balances provider judgment, patient preference, and susceptibility to adverse effects in order to attain an individualized A1C target.16 Although a comprehensive review of T2DM pharmacotherapy is beyond the scope of this article, we will discuss the importance of metformin, familiarize prescribers with incretin-based therapy, and highlight recent safety concerns regarding thiazolidinediones (TZDs).
Metformin is first line. The 2012 ADA guidelines recommend prescribing metformin at the time of diagnosis of T2DM, in addition to advising lifestyle changes.4,16 The American College of Physicians (ACP) also recommends metformin as the first agent in diabetes management, citing the benefits of weight loss, improved lipid profiles, and decreased cardiovascular mortality.17 Adding a second medication to metformin at the time of diagnosis may be considered if the initial A1C value is >9%.16 Because robust comparative trials are lacking, the selection of additional medications beyond metformin depends on a patient-centered approach, with consideration of efficacy, adverse effect profile, and cost.16 The TABLE provides a succinct review of the key properties of diabetic medications that clinicians may discuss with their patients. All of the listed agents are valid second-line treatments, and you should select one based on the individual’s needs.
Incretin-based therapy. Among newer antihyperglycemic agents, incretins have drawn much attention and thus warrant special focus. The emphasis on these agents should not be interpreted as an implied endorsement for their second-line use. There are 2 main classes: dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Both act on the gut peptide GLP-1 to enhance glucose-stimulated insulin secretion and glucagon suppression.16
DPP-4 inhibitors promote the effects of endogenous GLP-1 by inhibiting its breakdown by the enzyme DPP-4. By increasing GLP-1, these agents achieve mild glucose lowering while remaining weight neutral.16 DPP-4 inhibitors can be combined with metformin and other oral agents and are not associated with hypoglycemia.16
Injectable GLP-1 receptor agonists provide supraphysiologic levels of GLP-1, resulting in increased insulin secretion, reduced glucagon secretion, delayed gastric emptying, increased satiety, and weight loss.16 Research has shown that exenatide can decrease mean weight by 7 kg over 2.4 years.18,19 Exenatide is dosed subcutaneously twice daily, while liraglutide is administered once daily. Once-weekly exenatide was approved by the US Food and Drug Administration (FDA) in February 2012. A recent study showed once-weekly exenatide lowered A1C levels, reduced weight, and caused fewer episodes of hypoglycemia compared with adding insulin glargine to the regimen when diabetes was uncontrolled on metformin (with or without a sulfonylurea).20 Patients may experience nausea, vomiting, and diarrhea at the onset of use of GLP-1 agonists.21 Slow titration and forewarning the patient of these adverse effects will help with compliance.
In October 2011, the FDA approved the use of exenatide with basal insulin. For patients already taking basal insulin with or without metformin or pioglitazone, adding exenatide resulted in improved A1C values and weight loss over a 30-week period.22 Reducing the dose of basal insulin at the initiation of exenatide helps decrease the incidence of hypoglycemia when considering this combination.22 Basal insulin lowers fasting glucose levels, while exenatide reduces postprandial glucose.
Although gaining in popularity, incretin therapy is being monitored for long-term safety. Cases of pancreatitis have been reported in both classes of medicines.4 Liraglutide has been associated with medullary thyroid cancer (MTC) in rodents.23,24 The FDA has recommended against using liraglutide and extended-release exenatide in patients with a personal or family history of MTC.16 Although the long-term safety of GLP-1 agonists and DPP-4 inhibitors is unknown, their novel mechanisms of action can prove useful for the right patient.
Concerns over TZDs. In addition to the FDA recommendation to avoid TZDs in patients with symptomatic heart failure, 2 studies have recently found that pioglitazone may be associated with an increased risk of bladder cancer.25,26 The FDA recommends avoiding use of pioglitazone in patients with active bladder cancer, and that it should be used with caution in patients with a history of cured bladder cancer. The European Medicines Agency also recommends against pioglitazone use in patients with uninvestigated macroscopic hematuria.27 The potential association between pioglitazone and bladder cancer requires further study. At this point, TZDs remain a valid second- or third-line treatment option in patients only after they are made aware of the potential risks and benefits.
CASE JR’s A1C of 7.6% is above his individualized goal of 7%. He feels he has maximized his efforts in the realm of lifestyle changes and is interested in another medication. Using the recommended patient-centered approach, we discuss with him the risks and benefits of each medication in the TABLE and we select the medication best suited to him based on adverse-effect profile.
TABLE
Matching diabetic medication attributes to patient needs
| Class | Medications | Actions | Benefits | Possible adverse effects and disadvantages | A1C-lowering (%) | Cost* |
|---|---|---|---|---|---|---|
| Biguanides | Metformin | ↓ Hepatic glucose production | Weight neutral or loss No hypoglycemia ↓ CV mortality | GI side effects Lactic acidosis Impaired B12 absorption Use caution or avoid in renal dysfunction | 1-2 | $ |
| Sulfonylureas | Gliclazide Glimepiride Glipizide Glyburide | ↑ Insulin secretion | Fast-onset glucose lowering | Hypoglycemia Lack of durable glycemic control Weight gain | 1-2 | $ |
| Meglitinides | Repaglinide Nateglinide | ↑ Insulin secretion | Improve meal-related insulin release and postprandial glucose | Hypoglycemia Weight gain | 0.1-2.1 | $$-$$$ |
| Thiazolidinediones | Pioglitazone | ↑ Insulin sensitivity | No hypoglycemia ↑ HDL ↓ Triglycerides | Bladder cancer concerns Edema Fracture risk Heart failure Weight gain | 0.5-1.4 | $$$ |
| GLP-1 receptor agonists | Exenatide Liraglutide | ↑ Insulin secretion ↓ Glucagon secretion Delayed gastric emptying Early satiety | Possible beta-cell preservation Weight loss | GI (nausea, vomiting, diarrhea) Injectable Medullary thyroid tumors in rodents Pancreatitis | 0.5-1.5 | $$$ |
| DPP-4 inhibitors | Linagliptin Saxagliptin Sitagliptin Vildagliptin | ↓ Glucagon secretion ↑ Insulin secretion | No hypoglycemia Weight neutral | Angioedema Pancreatitis | 0.5-0.8 | $$$ |
| Alpha-glucosidase inhibitors | Acarbose Miglitol | Delays carbohydrate absorption | Nonsystemic medication Reduces postprandial glucose | Frequent dosing GI side effects (abdominal cramping, flatulence) | 0.5-0.8 | $$ |
| Insulin | Aspart Detemir Glargine Lispro NPH Regular | Replaces endogenous insulin | Mimics physiology Rapidly effective | Hypoglycemia Weight gain | 1.5-3.5 | $-$$$ |
| CV, cardiovascular; DPP, dipeptidyl peptidase; GI, gastrointestinal; GLP, glucagon-like peptide, HDL, high-density lipoprotein. *Monthly cost of an average daily maintenance dose of available products: $, <$50; $$, $50.01-$100; $$$, >$100. Source: www.drugstore.com; accessed October 10, 2012. Adapted from: Reid TS. Options for intensifying diabetes treatment. J Fam Pract. 2011;9(suppl 1):S7-S10; American Diabetes Association Position Statement. Standards of Medical Care in Type 2 Diabetes-2012. Diabetes Care. 2012;35(suppl 1):S11-S63. | ||||||
Immunizations
An often overlooked but important part of the diabetes visit is reviewing the patient’s immunization history. Unless there are contraindications, all individuals with diabetes should receive the pneumococcal and annual influenza vaccines.4 In addition, the Advisory Committee on Immunization Practices now recommends hepatitis B virus (HBV) vaccine for unvaccinated adults with diabetes from ages 19 to 59.28 Unvaccinated adults with diabetes over age 60 should be vaccinated at the discretion of the provider after risk assessment.28 Patients may be at risk of contracting HBV in long-term care facilities where assisted blood sugar monitoring commonly occurs.28 Studies have shown that patients with diabetes may progress to chronic hepatitis B infection more often than patients without diabetes, and are at higher risk for nonalcoholic liver disease and hepatocellular carcinoma.29
CASE JR’s history shows that he is current on his influenza and pneumococcal vaccines. However, he doesn’t recall whether he’s been vaccinated against HBV. Serum testing reveals no previous immunization, and recommending HBV vaccine is appropriate.
Surveillance
The 2012 ADA recommendations do not include any new surveillance practices for microvascular disease. Providers should continue to offer the following screening to T2DM patients annually: urine albumin excretion testing and serum creatinine to assess for nephropathy, a comprehensive dilated eye exam to assess for retinopathy, and a foot exam to assess for distal symmetric polyneuropathy.4
CASE Each of these tests were performed (or ordered) for JR. We’ll see him again in 2 to 3 months for diabetes follow-up.
Acknowledgement
The authors thank Pamela Williams, MD, and Brent Smith, MD, for their guidance in the preparation of this article.
CORRESPONDENCE Jason C. McCarthy, MD, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
1. Ogawa H, Nakayama M, Morimoto T, et al. Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141.
2. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.-
3. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
4. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
6. Turner R, Holman R, Stratton I, et al. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
7. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
8. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
9. Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
10. Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799-2810.
11. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
12. Hermida R, Ayala D, Mojon A, et al. Influence of time of day of blood-pressure lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2001;34:1270-1276.
13. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
14. Cushman W, Evans G, Byington R, et al. The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
15. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
16. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
17. Qaseem A, Humphrey L, Sweet D, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218-231.
18. Varanasi A, Chaudhuri A, Dhindsa S, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192-200.
19. Vilsboll T, Christensen M, Junker A, et al. Effects of glucagon-like peptide-1 receptor agonist on weight loss: systemic review and meta-analyses of randomized controlled trials. BMJ. 2012;344:d7771.-
20. Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35:683-689.
21. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
22. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized controlled trial. Ann Intern Med. 2011;154:103-112.
23. Drucker DJ, Sherman SI, Bergenstal RM, et al. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027-2031.
24. Elashoff M, Matveyenko A, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156.
25. Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371.
26. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
27. European Medicines Agency. European Medicines Agency recommends new contra-indications and warnings for pioglitazone to reduce small increased risk of bladder cancer, updated July 7, 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07. Accessed March 22, 2012.
28. Sawyer M, Hoerger T. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
29. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.
In April 2012, the American Diabetes Association (ADA) updated its guidelines for evaluating and treating type 2 diabetes mellitus (T2DM). In particular, the ADA acknowledges the value of an individualized, patient-centered approach that is less formulaic than its earlier guidelines. In this article, we highlight these and other recently published developments in the context of a case study. To help ensure follow-through on these newest recommendations, we also frame our review with the mnemonic, “ABCD IS diabetes.”
CASE JR is a 57-year-old man being seen for a regular follow-up appointment. His medical history includes T2DM, hypertension, and obesity. He is taking metformin 1000 mg twice daily, lisinopril 40 mg each morning, and amlodipine 10 mg each morning. He is current on his influenza and pneumococcal vaccinations. He does not smoke cigarettes. His physical exam and lab results reveal the following:
- blood pressure (BP), 132/70 mm Hg
- body mass index (BMI), 33 kg/m2
- glycosylated hemoglobin (A1C), 7.6%
- lipid profile: Total cholesterol, 185 mg/dL; high-density lipoprotein (HDL), 40 mg/dL; triglycerides (TG), 145 mg/dL; low-density lipoprotein (LDL), 90 mg/dL
Applying the “ABCD IS diabetes” mnemonic leads us through the following assessments.
Antiplatelets
In the past, guidelines have recommended that most patients with diabetes be placed on aspirin therapy. However, 2 trials published in 2008 failed to demonstrate significant reduction in cardiovascular disease (CVD) end points with aspirin use, raising questions about its effectiveness for primary CVD prevention in patients with diabetes.1,2 In 2010, the ADA, American Heart Association, and American College of Cardiology Foundation modified their recommendations for primary prevention,3 which remain unchanged in the 2012 ADA guidelines.4
Antiplatelet agents continue to play a role in primary prevention of CVD for patients with T2DM, but only after appropriate risk stratification.4 Consider low-dose aspirin therapy (75-162 mg/d) for patients with diabetes who have a 10-year Framingham risk >10%.4 (To calculate a patient’s 10-year risk, go to http://hp2010.nhlbihin.net/atpiii/calculator.asp.)
Many patients with T2DM seen in the primary care setting will reach this risk level and qualify for aspirin—in particular, men older than 50 years and women older than 60 with a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria.4 Aspirin therapy is not recommended for primary prevention in adults with diabetes at low risk for CVD (10-year Framingham risk <5%)—eg, men <50 and women <60 years without additional CVD risk factors.4 For patients with a 10-year Framingham risk between 5% and 10%, a decision to treat rests with the physician.
CASE Should JR be started on aspirin therapy for primary prevention of CVD? Initiating low-dose aspirin is recommended, assuming no contraindications, because his 10-year Framingham risk assessment is 11%.
A (antiplatelets): Consider low-dose aspirin therapy (75-162 mg/d) for diabetes patients with a 10-year Framingham risk >10%.
B (blood pressure): Individualize a patient’s goal for systolic blood pressure, aiming higher or lower than the customary systolic target of <130 mm Hg, as appropriate.
C (cholesterol): Recommend lifestyle changes and prescribe a statin, as needed, to achieve LDL goals in T2DM patients.
D (drug management): Use a patient-centered approach to achieve an individualized A1C goal. Metformin is the initial medication of choice. Select additional drug classes to balance adverse effects, cost, and effectiveness.
I (immunizations): Ensure that each T2DM patient receives influenza and pneumococcal vaccines, and the hepatitis B vaccine if <60 years.
S (surveillance): Confirm at each visit that annual surveillance testing for nephropathy, retinopathy, and peripheral neuropathy has been completed.
Blood pressure
The benefits of lowering BP in diabetes to <140 mm Hg systolic and <80 mm Hg diastolic have been established in randomized control trials.5-8 However, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that, in patients with T2DM, intensive BP lowering to <120 mm Hg systolic yielded no significant differences in fatal and nonfatal cardiovascular events compared with BP maintained between 130 and 140 mm Hg.9 Moreover, aggressive BP lowering may be associated with serious adverse events.10 The 2012 ADA guidelines state that a systolic BP goal of <130 mm Hg is appropriate for most patients; however, higher or lower BP targets may be individualized.4
Recommendations for adding a second antihypertensive agent and timing medication administration. For T2DM patients with hypertension, the 2012 guidelines recommend that you treat initially with either an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), if tolerated.4 When adding a second agent, the Avoiding Cardiovascular Events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial demonstrated reduced morbidity and mortality in patients receiving benazepril and amlodipine compared with those receiving benazepril and hydrochlorothiazide.11 As a result, amlodipine has joined diuretics as a preferred second oral antihypertensive agent after an ACEI or ARB.
If a patient is taking multiple BP medications, one or more should be taken at bedtime.4 Administering an antihypertensive at night results in better ambulatory BP control and reduces cardiovascular mortality.12
CASE JR is on maximal doses of 2 antihypertensive agents, and his BP is 132/70 mm Hg. His physician must individualize care and decide if adding a third agent is worth the risk of another medication when clear benefit has not been demonstrated. It is reasonable to continue his current regimen with the exception of changing his lisinopril dose to the evening and reassessing his BP control at his next visit.
Cholesterol
Controlling LDL remains the top priority of T2DM lipid management. In addition to lifestyle changes, statins are the primary means of achieving LDL goals. All patients with overt CVD should receive a statin.4 Also prescribe a statin for patients with diabetes who do not have CVD but who are older than 40 and have one or more cardiac risk factors, regardless of their baseline LDL cholesterol.4 The recommended LDL goal in T2DM patients continues to be <100 mg/dL. However, <70 mg/dL is a reasonable goal for those with known CVD.13
Using additional lipid-lowering agents besides a statin may improve cholesterol numbers, but not CVD outcomes. In the ACCORD study, adding fenofibrate to simvastatin did not decrease fatal cardiovascular events or nonfatal myocardial infarction and stroke compared with simvastatin given alone.14 The AIM-High study showed no difference in cardiovascular outcomes and a possible increase in ischemic stroke with combination niacin and statin compared with statin therapy alone.15 For now, lifestyle changes and statins remain the ideal modalities to achieve LDL goals.
CASE Should a statin be initiated for our patient? Since JR is over 40 without known CVD and has a cardiac risk factor of hypertension, he should be started on statin therapy regardless of his baseline LDL (90 mg/dL), which is already at goal (<100 mg/dL).
Drug management
Let the glycemic goal for each patient guide your medication management. The 2012 ADA recommendation for most adults is an A1C of <7%.4 More strict control (A1C <6.5%) may be appropriate for certain individuals with a long life expectancy, short duration of diabetes, and no significant micro- or macrovascular disease.4 Less strict control (A1C <8%) may be appropriate for individuals with significant comorbidities, shorter life expectancy, severe hypoglycemia, or long-standing T2DM that’s been difficult to control despite multiple medications, including insulin.4
Individualize treatment. In April 2012, the ADA released a position statement encouraging a patient-centered approach to managing hyperglycemia in T2DM.16 This statement contains a new treatment algorithm (available at: http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) that is less prescriptive than the previous 2009 algorithm and balances provider judgment, patient preference, and susceptibility to adverse effects in order to attain an individualized A1C target.16 Although a comprehensive review of T2DM pharmacotherapy is beyond the scope of this article, we will discuss the importance of metformin, familiarize prescribers with incretin-based therapy, and highlight recent safety concerns regarding thiazolidinediones (TZDs).
Metformin is first line. The 2012 ADA guidelines recommend prescribing metformin at the time of diagnosis of T2DM, in addition to advising lifestyle changes.4,16 The American College of Physicians (ACP) also recommends metformin as the first agent in diabetes management, citing the benefits of weight loss, improved lipid profiles, and decreased cardiovascular mortality.17 Adding a second medication to metformin at the time of diagnosis may be considered if the initial A1C value is >9%.16 Because robust comparative trials are lacking, the selection of additional medications beyond metformin depends on a patient-centered approach, with consideration of efficacy, adverse effect profile, and cost.16 The TABLE provides a succinct review of the key properties of diabetic medications that clinicians may discuss with their patients. All of the listed agents are valid second-line treatments, and you should select one based on the individual’s needs.
Incretin-based therapy. Among newer antihyperglycemic agents, incretins have drawn much attention and thus warrant special focus. The emphasis on these agents should not be interpreted as an implied endorsement for their second-line use. There are 2 main classes: dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Both act on the gut peptide GLP-1 to enhance glucose-stimulated insulin secretion and glucagon suppression.16
DPP-4 inhibitors promote the effects of endogenous GLP-1 by inhibiting its breakdown by the enzyme DPP-4. By increasing GLP-1, these agents achieve mild glucose lowering while remaining weight neutral.16 DPP-4 inhibitors can be combined with metformin and other oral agents and are not associated with hypoglycemia.16
Injectable GLP-1 receptor agonists provide supraphysiologic levels of GLP-1, resulting in increased insulin secretion, reduced glucagon secretion, delayed gastric emptying, increased satiety, and weight loss.16 Research has shown that exenatide can decrease mean weight by 7 kg over 2.4 years.18,19 Exenatide is dosed subcutaneously twice daily, while liraglutide is administered once daily. Once-weekly exenatide was approved by the US Food and Drug Administration (FDA) in February 2012. A recent study showed once-weekly exenatide lowered A1C levels, reduced weight, and caused fewer episodes of hypoglycemia compared with adding insulin glargine to the regimen when diabetes was uncontrolled on metformin (with or without a sulfonylurea).20 Patients may experience nausea, vomiting, and diarrhea at the onset of use of GLP-1 agonists.21 Slow titration and forewarning the patient of these adverse effects will help with compliance.
In October 2011, the FDA approved the use of exenatide with basal insulin. For patients already taking basal insulin with or without metformin or pioglitazone, adding exenatide resulted in improved A1C values and weight loss over a 30-week period.22 Reducing the dose of basal insulin at the initiation of exenatide helps decrease the incidence of hypoglycemia when considering this combination.22 Basal insulin lowers fasting glucose levels, while exenatide reduces postprandial glucose.
Although gaining in popularity, incretin therapy is being monitored for long-term safety. Cases of pancreatitis have been reported in both classes of medicines.4 Liraglutide has been associated with medullary thyroid cancer (MTC) in rodents.23,24 The FDA has recommended against using liraglutide and extended-release exenatide in patients with a personal or family history of MTC.16 Although the long-term safety of GLP-1 agonists and DPP-4 inhibitors is unknown, their novel mechanisms of action can prove useful for the right patient.
Concerns over TZDs. In addition to the FDA recommendation to avoid TZDs in patients with symptomatic heart failure, 2 studies have recently found that pioglitazone may be associated with an increased risk of bladder cancer.25,26 The FDA recommends avoiding use of pioglitazone in patients with active bladder cancer, and that it should be used with caution in patients with a history of cured bladder cancer. The European Medicines Agency also recommends against pioglitazone use in patients with uninvestigated macroscopic hematuria.27 The potential association between pioglitazone and bladder cancer requires further study. At this point, TZDs remain a valid second- or third-line treatment option in patients only after they are made aware of the potential risks and benefits.
CASE JR’s A1C of 7.6% is above his individualized goal of 7%. He feels he has maximized his efforts in the realm of lifestyle changes and is interested in another medication. Using the recommended patient-centered approach, we discuss with him the risks and benefits of each medication in the TABLE and we select the medication best suited to him based on adverse-effect profile.
TABLE
Matching diabetic medication attributes to patient needs
| Class | Medications | Actions | Benefits | Possible adverse effects and disadvantages | A1C-lowering (%) | Cost* |
|---|---|---|---|---|---|---|
| Biguanides | Metformin | ↓ Hepatic glucose production | Weight neutral or loss No hypoglycemia ↓ CV mortality | GI side effects Lactic acidosis Impaired B12 absorption Use caution or avoid in renal dysfunction | 1-2 | $ |
| Sulfonylureas | Gliclazide Glimepiride Glipizide Glyburide | ↑ Insulin secretion | Fast-onset glucose lowering | Hypoglycemia Lack of durable glycemic control Weight gain | 1-2 | $ |
| Meglitinides | Repaglinide Nateglinide | ↑ Insulin secretion | Improve meal-related insulin release and postprandial glucose | Hypoglycemia Weight gain | 0.1-2.1 | $$-$$$ |
| Thiazolidinediones | Pioglitazone | ↑ Insulin sensitivity | No hypoglycemia ↑ HDL ↓ Triglycerides | Bladder cancer concerns Edema Fracture risk Heart failure Weight gain | 0.5-1.4 | $$$ |
| GLP-1 receptor agonists | Exenatide Liraglutide | ↑ Insulin secretion ↓ Glucagon secretion Delayed gastric emptying Early satiety | Possible beta-cell preservation Weight loss | GI (nausea, vomiting, diarrhea) Injectable Medullary thyroid tumors in rodents Pancreatitis | 0.5-1.5 | $$$ |
| DPP-4 inhibitors | Linagliptin Saxagliptin Sitagliptin Vildagliptin | ↓ Glucagon secretion ↑ Insulin secretion | No hypoglycemia Weight neutral | Angioedema Pancreatitis | 0.5-0.8 | $$$ |
| Alpha-glucosidase inhibitors | Acarbose Miglitol | Delays carbohydrate absorption | Nonsystemic medication Reduces postprandial glucose | Frequent dosing GI side effects (abdominal cramping, flatulence) | 0.5-0.8 | $$ |
| Insulin | Aspart Detemir Glargine Lispro NPH Regular | Replaces endogenous insulin | Mimics physiology Rapidly effective | Hypoglycemia Weight gain | 1.5-3.5 | $-$$$ |
| CV, cardiovascular; DPP, dipeptidyl peptidase; GI, gastrointestinal; GLP, glucagon-like peptide, HDL, high-density lipoprotein. *Monthly cost of an average daily maintenance dose of available products: $, <$50; $$, $50.01-$100; $$$, >$100. Source: www.drugstore.com; accessed October 10, 2012. Adapted from: Reid TS. Options for intensifying diabetes treatment. J Fam Pract. 2011;9(suppl 1):S7-S10; American Diabetes Association Position Statement. Standards of Medical Care in Type 2 Diabetes-2012. Diabetes Care. 2012;35(suppl 1):S11-S63. | ||||||
Immunizations
An often overlooked but important part of the diabetes visit is reviewing the patient’s immunization history. Unless there are contraindications, all individuals with diabetes should receive the pneumococcal and annual influenza vaccines.4 In addition, the Advisory Committee on Immunization Practices now recommends hepatitis B virus (HBV) vaccine for unvaccinated adults with diabetes from ages 19 to 59.28 Unvaccinated adults with diabetes over age 60 should be vaccinated at the discretion of the provider after risk assessment.28 Patients may be at risk of contracting HBV in long-term care facilities where assisted blood sugar monitoring commonly occurs.28 Studies have shown that patients with diabetes may progress to chronic hepatitis B infection more often than patients without diabetes, and are at higher risk for nonalcoholic liver disease and hepatocellular carcinoma.29
CASE JR’s history shows that he is current on his influenza and pneumococcal vaccines. However, he doesn’t recall whether he’s been vaccinated against HBV. Serum testing reveals no previous immunization, and recommending HBV vaccine is appropriate.
Surveillance
The 2012 ADA recommendations do not include any new surveillance practices for microvascular disease. Providers should continue to offer the following screening to T2DM patients annually: urine albumin excretion testing and serum creatinine to assess for nephropathy, a comprehensive dilated eye exam to assess for retinopathy, and a foot exam to assess for distal symmetric polyneuropathy.4
CASE Each of these tests were performed (or ordered) for JR. We’ll see him again in 2 to 3 months for diabetes follow-up.
Acknowledgement
The authors thank Pamela Williams, MD, and Brent Smith, MD, for their guidance in the preparation of this article.
CORRESPONDENCE Jason C. McCarthy, MD, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
In April 2012, the American Diabetes Association (ADA) updated its guidelines for evaluating and treating type 2 diabetes mellitus (T2DM). In particular, the ADA acknowledges the value of an individualized, patient-centered approach that is less formulaic than its earlier guidelines. In this article, we highlight these and other recently published developments in the context of a case study. To help ensure follow-through on these newest recommendations, we also frame our review with the mnemonic, “ABCD IS diabetes.”
CASE JR is a 57-year-old man being seen for a regular follow-up appointment. His medical history includes T2DM, hypertension, and obesity. He is taking metformin 1000 mg twice daily, lisinopril 40 mg each morning, and amlodipine 10 mg each morning. He is current on his influenza and pneumococcal vaccinations. He does not smoke cigarettes. His physical exam and lab results reveal the following:
- blood pressure (BP), 132/70 mm Hg
- body mass index (BMI), 33 kg/m2
- glycosylated hemoglobin (A1C), 7.6%
- lipid profile: Total cholesterol, 185 mg/dL; high-density lipoprotein (HDL), 40 mg/dL; triglycerides (TG), 145 mg/dL; low-density lipoprotein (LDL), 90 mg/dL
Applying the “ABCD IS diabetes” mnemonic leads us through the following assessments.
Antiplatelets
In the past, guidelines have recommended that most patients with diabetes be placed on aspirin therapy. However, 2 trials published in 2008 failed to demonstrate significant reduction in cardiovascular disease (CVD) end points with aspirin use, raising questions about its effectiveness for primary CVD prevention in patients with diabetes.1,2 In 2010, the ADA, American Heart Association, and American College of Cardiology Foundation modified their recommendations for primary prevention,3 which remain unchanged in the 2012 ADA guidelines.4
Antiplatelet agents continue to play a role in primary prevention of CVD for patients with T2DM, but only after appropriate risk stratification.4 Consider low-dose aspirin therapy (75-162 mg/d) for patients with diabetes who have a 10-year Framingham risk >10%.4 (To calculate a patient’s 10-year risk, go to http://hp2010.nhlbihin.net/atpiii/calculator.asp.)
Many patients with T2DM seen in the primary care setting will reach this risk level and qualify for aspirin—in particular, men older than 50 years and women older than 60 with a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria.4 Aspirin therapy is not recommended for primary prevention in adults with diabetes at low risk for CVD (10-year Framingham risk <5%)—eg, men <50 and women <60 years without additional CVD risk factors.4 For patients with a 10-year Framingham risk between 5% and 10%, a decision to treat rests with the physician.
CASE Should JR be started on aspirin therapy for primary prevention of CVD? Initiating low-dose aspirin is recommended, assuming no contraindications, because his 10-year Framingham risk assessment is 11%.
A (antiplatelets): Consider low-dose aspirin therapy (75-162 mg/d) for diabetes patients with a 10-year Framingham risk >10%.
B (blood pressure): Individualize a patient’s goal for systolic blood pressure, aiming higher or lower than the customary systolic target of <130 mm Hg, as appropriate.
C (cholesterol): Recommend lifestyle changes and prescribe a statin, as needed, to achieve LDL goals in T2DM patients.
D (drug management): Use a patient-centered approach to achieve an individualized A1C goal. Metformin is the initial medication of choice. Select additional drug classes to balance adverse effects, cost, and effectiveness.
I (immunizations): Ensure that each T2DM patient receives influenza and pneumococcal vaccines, and the hepatitis B vaccine if <60 years.
S (surveillance): Confirm at each visit that annual surveillance testing for nephropathy, retinopathy, and peripheral neuropathy has been completed.
Blood pressure
The benefits of lowering BP in diabetes to <140 mm Hg systolic and <80 mm Hg diastolic have been established in randomized control trials.5-8 However, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that, in patients with T2DM, intensive BP lowering to <120 mm Hg systolic yielded no significant differences in fatal and nonfatal cardiovascular events compared with BP maintained between 130 and 140 mm Hg.9 Moreover, aggressive BP lowering may be associated with serious adverse events.10 The 2012 ADA guidelines state that a systolic BP goal of <130 mm Hg is appropriate for most patients; however, higher or lower BP targets may be individualized.4
Recommendations for adding a second antihypertensive agent and timing medication administration. For T2DM patients with hypertension, the 2012 guidelines recommend that you treat initially with either an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), if tolerated.4 When adding a second agent, the Avoiding Cardiovascular Events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial demonstrated reduced morbidity and mortality in patients receiving benazepril and amlodipine compared with those receiving benazepril and hydrochlorothiazide.11 As a result, amlodipine has joined diuretics as a preferred second oral antihypertensive agent after an ACEI or ARB.
If a patient is taking multiple BP medications, one or more should be taken at bedtime.4 Administering an antihypertensive at night results in better ambulatory BP control and reduces cardiovascular mortality.12
CASE JR is on maximal doses of 2 antihypertensive agents, and his BP is 132/70 mm Hg. His physician must individualize care and decide if adding a third agent is worth the risk of another medication when clear benefit has not been demonstrated. It is reasonable to continue his current regimen with the exception of changing his lisinopril dose to the evening and reassessing his BP control at his next visit.
Cholesterol
Controlling LDL remains the top priority of T2DM lipid management. In addition to lifestyle changes, statins are the primary means of achieving LDL goals. All patients with overt CVD should receive a statin.4 Also prescribe a statin for patients with diabetes who do not have CVD but who are older than 40 and have one or more cardiac risk factors, regardless of their baseline LDL cholesterol.4 The recommended LDL goal in T2DM patients continues to be <100 mg/dL. However, <70 mg/dL is a reasonable goal for those with known CVD.13
Using additional lipid-lowering agents besides a statin may improve cholesterol numbers, but not CVD outcomes. In the ACCORD study, adding fenofibrate to simvastatin did not decrease fatal cardiovascular events or nonfatal myocardial infarction and stroke compared with simvastatin given alone.14 The AIM-High study showed no difference in cardiovascular outcomes and a possible increase in ischemic stroke with combination niacin and statin compared with statin therapy alone.15 For now, lifestyle changes and statins remain the ideal modalities to achieve LDL goals.
CASE Should a statin be initiated for our patient? Since JR is over 40 without known CVD and has a cardiac risk factor of hypertension, he should be started on statin therapy regardless of his baseline LDL (90 mg/dL), which is already at goal (<100 mg/dL).
Drug management
Let the glycemic goal for each patient guide your medication management. The 2012 ADA recommendation for most adults is an A1C of <7%.4 More strict control (A1C <6.5%) may be appropriate for certain individuals with a long life expectancy, short duration of diabetes, and no significant micro- or macrovascular disease.4 Less strict control (A1C <8%) may be appropriate for individuals with significant comorbidities, shorter life expectancy, severe hypoglycemia, or long-standing T2DM that’s been difficult to control despite multiple medications, including insulin.4
Individualize treatment. In April 2012, the ADA released a position statement encouraging a patient-centered approach to managing hyperglycemia in T2DM.16 This statement contains a new treatment algorithm (available at: http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) that is less prescriptive than the previous 2009 algorithm and balances provider judgment, patient preference, and susceptibility to adverse effects in order to attain an individualized A1C target.16 Although a comprehensive review of T2DM pharmacotherapy is beyond the scope of this article, we will discuss the importance of metformin, familiarize prescribers with incretin-based therapy, and highlight recent safety concerns regarding thiazolidinediones (TZDs).
Metformin is first line. The 2012 ADA guidelines recommend prescribing metformin at the time of diagnosis of T2DM, in addition to advising lifestyle changes.4,16 The American College of Physicians (ACP) also recommends metformin as the first agent in diabetes management, citing the benefits of weight loss, improved lipid profiles, and decreased cardiovascular mortality.17 Adding a second medication to metformin at the time of diagnosis may be considered if the initial A1C value is >9%.16 Because robust comparative trials are lacking, the selection of additional medications beyond metformin depends on a patient-centered approach, with consideration of efficacy, adverse effect profile, and cost.16 The TABLE provides a succinct review of the key properties of diabetic medications that clinicians may discuss with their patients. All of the listed agents are valid second-line treatments, and you should select one based on the individual’s needs.
Incretin-based therapy. Among newer antihyperglycemic agents, incretins have drawn much attention and thus warrant special focus. The emphasis on these agents should not be interpreted as an implied endorsement for their second-line use. There are 2 main classes: dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Both act on the gut peptide GLP-1 to enhance glucose-stimulated insulin secretion and glucagon suppression.16
DPP-4 inhibitors promote the effects of endogenous GLP-1 by inhibiting its breakdown by the enzyme DPP-4. By increasing GLP-1, these agents achieve mild glucose lowering while remaining weight neutral.16 DPP-4 inhibitors can be combined with metformin and other oral agents and are not associated with hypoglycemia.16
Injectable GLP-1 receptor agonists provide supraphysiologic levels of GLP-1, resulting in increased insulin secretion, reduced glucagon secretion, delayed gastric emptying, increased satiety, and weight loss.16 Research has shown that exenatide can decrease mean weight by 7 kg over 2.4 years.18,19 Exenatide is dosed subcutaneously twice daily, while liraglutide is administered once daily. Once-weekly exenatide was approved by the US Food and Drug Administration (FDA) in February 2012. A recent study showed once-weekly exenatide lowered A1C levels, reduced weight, and caused fewer episodes of hypoglycemia compared with adding insulin glargine to the regimen when diabetes was uncontrolled on metformin (with or without a sulfonylurea).20 Patients may experience nausea, vomiting, and diarrhea at the onset of use of GLP-1 agonists.21 Slow titration and forewarning the patient of these adverse effects will help with compliance.
In October 2011, the FDA approved the use of exenatide with basal insulin. For patients already taking basal insulin with or without metformin or pioglitazone, adding exenatide resulted in improved A1C values and weight loss over a 30-week period.22 Reducing the dose of basal insulin at the initiation of exenatide helps decrease the incidence of hypoglycemia when considering this combination.22 Basal insulin lowers fasting glucose levels, while exenatide reduces postprandial glucose.
Although gaining in popularity, incretin therapy is being monitored for long-term safety. Cases of pancreatitis have been reported in both classes of medicines.4 Liraglutide has been associated with medullary thyroid cancer (MTC) in rodents.23,24 The FDA has recommended against using liraglutide and extended-release exenatide in patients with a personal or family history of MTC.16 Although the long-term safety of GLP-1 agonists and DPP-4 inhibitors is unknown, their novel mechanisms of action can prove useful for the right patient.
Concerns over TZDs. In addition to the FDA recommendation to avoid TZDs in patients with symptomatic heart failure, 2 studies have recently found that pioglitazone may be associated with an increased risk of bladder cancer.25,26 The FDA recommends avoiding use of pioglitazone in patients with active bladder cancer, and that it should be used with caution in patients with a history of cured bladder cancer. The European Medicines Agency also recommends against pioglitazone use in patients with uninvestigated macroscopic hematuria.27 The potential association between pioglitazone and bladder cancer requires further study. At this point, TZDs remain a valid second- or third-line treatment option in patients only after they are made aware of the potential risks and benefits.
CASE JR’s A1C of 7.6% is above his individualized goal of 7%. He feels he has maximized his efforts in the realm of lifestyle changes and is interested in another medication. Using the recommended patient-centered approach, we discuss with him the risks and benefits of each medication in the TABLE and we select the medication best suited to him based on adverse-effect profile.
TABLE
Matching diabetic medication attributes to patient needs
| Class | Medications | Actions | Benefits | Possible adverse effects and disadvantages | A1C-lowering (%) | Cost* |
|---|---|---|---|---|---|---|
| Biguanides | Metformin | ↓ Hepatic glucose production | Weight neutral or loss No hypoglycemia ↓ CV mortality | GI side effects Lactic acidosis Impaired B12 absorption Use caution or avoid in renal dysfunction | 1-2 | $ |
| Sulfonylureas | Gliclazide Glimepiride Glipizide Glyburide | ↑ Insulin secretion | Fast-onset glucose lowering | Hypoglycemia Lack of durable glycemic control Weight gain | 1-2 | $ |
| Meglitinides | Repaglinide Nateglinide | ↑ Insulin secretion | Improve meal-related insulin release and postprandial glucose | Hypoglycemia Weight gain | 0.1-2.1 | $$-$$$ |
| Thiazolidinediones | Pioglitazone | ↑ Insulin sensitivity | No hypoglycemia ↑ HDL ↓ Triglycerides | Bladder cancer concerns Edema Fracture risk Heart failure Weight gain | 0.5-1.4 | $$$ |
| GLP-1 receptor agonists | Exenatide Liraglutide | ↑ Insulin secretion ↓ Glucagon secretion Delayed gastric emptying Early satiety | Possible beta-cell preservation Weight loss | GI (nausea, vomiting, diarrhea) Injectable Medullary thyroid tumors in rodents Pancreatitis | 0.5-1.5 | $$$ |
| DPP-4 inhibitors | Linagliptin Saxagliptin Sitagliptin Vildagliptin | ↓ Glucagon secretion ↑ Insulin secretion | No hypoglycemia Weight neutral | Angioedema Pancreatitis | 0.5-0.8 | $$$ |
| Alpha-glucosidase inhibitors | Acarbose Miglitol | Delays carbohydrate absorption | Nonsystemic medication Reduces postprandial glucose | Frequent dosing GI side effects (abdominal cramping, flatulence) | 0.5-0.8 | $$ |
| Insulin | Aspart Detemir Glargine Lispro NPH Regular | Replaces endogenous insulin | Mimics physiology Rapidly effective | Hypoglycemia Weight gain | 1.5-3.5 | $-$$$ |
| CV, cardiovascular; DPP, dipeptidyl peptidase; GI, gastrointestinal; GLP, glucagon-like peptide, HDL, high-density lipoprotein. *Monthly cost of an average daily maintenance dose of available products: $, <$50; $$, $50.01-$100; $$$, >$100. Source: www.drugstore.com; accessed October 10, 2012. Adapted from: Reid TS. Options for intensifying diabetes treatment. J Fam Pract. 2011;9(suppl 1):S7-S10; American Diabetes Association Position Statement. Standards of Medical Care in Type 2 Diabetes-2012. Diabetes Care. 2012;35(suppl 1):S11-S63. | ||||||
Immunizations
An often overlooked but important part of the diabetes visit is reviewing the patient’s immunization history. Unless there are contraindications, all individuals with diabetes should receive the pneumococcal and annual influenza vaccines.4 In addition, the Advisory Committee on Immunization Practices now recommends hepatitis B virus (HBV) vaccine for unvaccinated adults with diabetes from ages 19 to 59.28 Unvaccinated adults with diabetes over age 60 should be vaccinated at the discretion of the provider after risk assessment.28 Patients may be at risk of contracting HBV in long-term care facilities where assisted blood sugar monitoring commonly occurs.28 Studies have shown that patients with diabetes may progress to chronic hepatitis B infection more often than patients without diabetes, and are at higher risk for nonalcoholic liver disease and hepatocellular carcinoma.29
CASE JR’s history shows that he is current on his influenza and pneumococcal vaccines. However, he doesn’t recall whether he’s been vaccinated against HBV. Serum testing reveals no previous immunization, and recommending HBV vaccine is appropriate.
Surveillance
The 2012 ADA recommendations do not include any new surveillance practices for microvascular disease. Providers should continue to offer the following screening to T2DM patients annually: urine albumin excretion testing and serum creatinine to assess for nephropathy, a comprehensive dilated eye exam to assess for retinopathy, and a foot exam to assess for distal symmetric polyneuropathy.4
CASE Each of these tests were performed (or ordered) for JR. We’ll see him again in 2 to 3 months for diabetes follow-up.
Acknowledgement
The authors thank Pamela Williams, MD, and Brent Smith, MD, for their guidance in the preparation of this article.
CORRESPONDENCE Jason C. McCarthy, MD, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
1. Ogawa H, Nakayama M, Morimoto T, et al. Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141.
2. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.-
3. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
4. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
6. Turner R, Holman R, Stratton I, et al. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
7. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
8. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
9. Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
10. Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799-2810.
11. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
12. Hermida R, Ayala D, Mojon A, et al. Influence of time of day of blood-pressure lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2001;34:1270-1276.
13. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
14. Cushman W, Evans G, Byington R, et al. The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
15. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
16. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
17. Qaseem A, Humphrey L, Sweet D, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218-231.
18. Varanasi A, Chaudhuri A, Dhindsa S, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192-200.
19. Vilsboll T, Christensen M, Junker A, et al. Effects of glucagon-like peptide-1 receptor agonist on weight loss: systemic review and meta-analyses of randomized controlled trials. BMJ. 2012;344:d7771.-
20. Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35:683-689.
21. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
22. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized controlled trial. Ann Intern Med. 2011;154:103-112.
23. Drucker DJ, Sherman SI, Bergenstal RM, et al. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027-2031.
24. Elashoff M, Matveyenko A, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156.
25. Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371.
26. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
27. European Medicines Agency. European Medicines Agency recommends new contra-indications and warnings for pioglitazone to reduce small increased risk of bladder cancer, updated July 7, 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07. Accessed March 22, 2012.
28. Sawyer M, Hoerger T. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
29. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.
1. Ogawa H, Nakayama M, Morimoto T, et al. Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141.
2. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.-
3. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
4. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
6. Turner R, Holman R, Stratton I, et al. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
7. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
8. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
9. Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
10. Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799-2810.
11. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
12. Hermida R, Ayala D, Mojon A, et al. Influence of time of day of blood-pressure lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2001;34:1270-1276.
13. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
14. Cushman W, Evans G, Byington R, et al. The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
15. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
16. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
17. Qaseem A, Humphrey L, Sweet D, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218-231.
18. Varanasi A, Chaudhuri A, Dhindsa S, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192-200.
19. Vilsboll T, Christensen M, Junker A, et al. Effects of glucagon-like peptide-1 receptor agonist on weight loss: systemic review and meta-analyses of randomized controlled trials. BMJ. 2012;344:d7771.-
20. Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35:683-689.
21. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
22. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized controlled trial. Ann Intern Med. 2011;154:103-112.
23. Drucker DJ, Sherman SI, Bergenstal RM, et al. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027-2031.
24. Elashoff M, Matveyenko A, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156.
25. Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371.
26. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
27. European Medicines Agency. European Medicines Agency recommends new contra-indications and warnings for pioglitazone to reduce small increased risk of bladder cancer, updated July 7, 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07. Accessed March 22, 2012.
28. Sawyer M, Hoerger T. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
29. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.