User login
Case Report: High-Pressure Injection Hand Injury
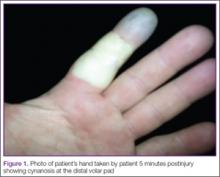
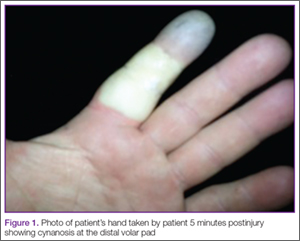
A 42-year-old healthy man presented to the ED 3 hours after sustaining an injury to the tip of his left index finger from a pressurized washer. He stated that while at work, he had touched the jet nozzle of the washer to “test” its pressure, and had experienced immediate pain in his entire finger as well as blanching in his mid-finger. He took a picture of his finger with his cell phone approximately 5 minutes postinjury, which showed cynanosis at the distal volar pad (Figure 1).
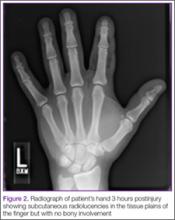
On presentation to the ED, physical examination revealed only an innocuous 1-mm puncture wound to the middle of the volar pad of the distal phalanx of his left index finger, with mild tenderness along the length of the finger but no swelling. The rest of the finger and hand appeared intact with normal color and sensation.
Three weeks after discharge, however, the patient developed pain, diffuse swelling, and purulent drainage from the same injured finger and presented again to the ED. He was immediately taken to the operating room where a broad dissection was performed and drains were placed. Two months later, he reported a complete resolution of the infection and was still working on regaining full functioning of his finger by attending physical therapy.
High-Pressure Injection injuries
Diagnosis
At the time of presentation, most high-pressure injection injuries to the hand appear innocuous but frequently result in severe sequelae, including functional disability and amputation. High-pressure injuries occur when soft tissues are placed in contact or near the opening of a high-pressure device or malfunctioning equipment (eg, pinhole rupture in a hydraulic hose).
While the literature reports that 100 psi or greater is required to break the skin,1 patients presenting to the ED typically report operating machinery shooting between 2,000 and 12,000 psi.2,3 Case-report reviews of patients with high-pressure injection injuries by Schoo et al4 and Hogan and Ruland5 found this type of injury most often occurred in the nondominant hand of male laborers—primarily in the index finger—with 30% to 48% of such injuries resulting in amputation of the digit.
Mechanical and Chemical Injuries
High-pressure injection injuries can be both mechanical as well as chemical. A mechanical high-pressure injury depends upon the magnitude of the injection force, with higher pressures associated with increased dysfunction and incidence of amputation. A chemical-related injury depends upon the duration of exposure and the volume of the material injected.
A high-pressure injury results from a tearing of the soft tissues, with shearing and dissection within and among the tissue plains down the finger and into the palm and proximal tissues.4,6 In this type of injury, there is often direct injury to the neurovascular bundles.
Along with the mechanical aspect of injury, a concurrent chemical injury depends upon the type of material injected (ie, gas or liquid), cytotoxicity, concentration, and inflammatory properties.6 Hogan and Ruland5 described the incidence of amputation associated with injected materials to be greater than 40% with diesel fuel, paint thinner, oil, and paint; 20% to 40% with undercoating, hydraulic fluid, and grease; and 0% with air and water. Oil-based paint injections were associated with a 58% amputation rate comapred to only 6% for latex paints.5
In general, evaluation of high-pressure-related injuries begins with a thorough history to identify the injected material and its attributes, pressure of injection, mechanism of exposure, time elapsed since the event, and the patient’s tetanus immunization status. Interventions include radiographs to evaluate for proximal spread of injected material; tetanus immunization, if required; prophylactic antibiotic therapy7; analgesia; and often emergency surgical consultation. The use of corticosteroid therapy has not been shown to impact the amputation rate or the incidence of infection.5
Type of Injury and Treatment
Wong et al8 developed the following guidelines categorizing the type and recommended treatment of mild, moderate, and severe high-pressure injection injuries:
Mild Injuries. In mild high-pressure injection injuries, patients tend to seek immediate treatment. These types of injuries typically involve oil or water, with a relatively low-pressure exposure, and preserved circulation in the injured area.8 Treatment includes conservative management with an option for surgery.5,8
Moderate Injuries. In moderate injection injuries, there is more significant tissue damage from any of the aforementioned materials, but with preserved neurovascular structures. Treatment often includes debridement in addition to antibiotic therapy.
Severe Injuries. These types of injection injuries often involve paints and solvents, high-pressure exposure, and usually present with an abnormal neurovasculature. The best chance for salvage injuries involves rapid surgical intervention for debridement and reconstruction.9,10
Surgical Evaluation and Debridement
Despite optimal care, amputation and dysfunction rates with high-risk injection injuries (due to higher psi, organic solvents, and/or delayed definitive care) have been reported from 48% to 80%.10-13 Immediate surgical evaluation is often recommended when injection injuries are encountered.10,13 Patients who receive immediate surgical debridement (ie, within 6 hours from injury), specifically those injected with organic solvents, have a lower amputation rate than those who do not have immediate surgical intervention.13
In some reports, more than 80% of those injected with organic solvents who did not have immediate washout and debridement required amputation.4 Other effects of postponed debridement include delayed return to work time and decreased functionality of the hand.13,14
Conclusion
Although high-pressure injection injuries often appear mild at initial presentation since the immediate symptoms of the neurovascular injury often resolves, patients often experience severe sequelae including infection, disability, or amputation. The severity of the injury not only depends upon the psi, but also the type and amount of chemical injected. Because of the high morbidity, it is imperative that emergency physicians are aware of and identify these types of injuries and their sequelae.10,11,13,14
Dr Wilson is a third year resident in the department of emergency medicine at the Alpert Medical School, Brown University, Providence, Rhode Island. Dr Hack is the director for the division of medical toxicology at Brown University and the director of the educational program in medical toxicology. He is an associate professor at Warren Alpert Medical School; and an attending physician in the department of emergency medicine at Brown University, Rhode Island Hospital, Miriam Hospital, Providence.
Disclosure: The authors report no conflicts of interest.
- Scott AR. Occupational high-pressure injection injuries: pathogenesis and prevention. J Soc Occup Med. 1983;33(2):56-59.
- Pappou IP, Deal DN. High-pressure injection injuries. J Hand Surg Am. 2012;37(11):2404-2407.
- Soyuncu S, Bektas F, Dinc S. High-pressure air injection injury to the upper extremity. J Emerg Med. 2013;45(1):96-98.
- Schoo MJ, Scott FA, Boswick JA Jr. High-pressure injection injuries of the hand. J Trauma. 1980;20(3):229-238.
- Hogan CJ, Ruland RT. High-pressure injection injuries to the upper extremity: a review of the literature. J Orthop Trauma. 2006;20(7):503-511.
- Rosenwasser MP, Wei DH. High-pressure injection injuries to the hand. J Am Acad Orthop Surg. 2014;22(1):38-45.
- Mirzayan R, Schnall SB, Chon JH, Holtom PD, Pazakis MJ, Stevanovic MV. Culture results and amputation rates in high-pressure paint gun injuries of the hand. Orthopedics. 2001;24(6):587-589.
- Wong TC, Ip FK, Wu WC. High-pressure injection injuries of the hand in a Chinese population. J Hand Surg Br. 2005;30(6):588-592.
- Pinto MR, Turkula-Pinto LD, Cooney WP, Wood MB, Dobyns JH: High-pressure injection injuries of the hand: review of 25 patients managed by open wound technique. J Hand Surg Am. 1993;18(1):125-130.
- Kaufman, HD. High pressure injection injuries, the problems, pathogenesis and management. Hand. 1970;2(1);63-73.
- Vasilevski D, Noorbergen M, Depierreux M, Lafontaine M. High-pressure injection injuries to the hand. Am J Emerg Med. 2000;18(7):820-824.
- Bekler H, Gokce A, Beyzadeoglu T, Parmaksizoglu F. The surgical treatment and outcomes of high pressure injection injuries of the hand. J Hand Surg Eur Vol. 2007;32(4):394-399.
- Amsdell SL, Hammert WC. High-pressure injection injuries in the hand: current treatment concepts. Plast Reconstr Surg. 2013;132(4):586e-591e.
- Hart RG, Smith GD, Haq A. Prevention of high-pressure injection injuries to the hand. Am J Emerg Med. 2005;24(1):73-76.
A 42-year-old healthy man presented to the ED 3 hours after sustaining an injury to the tip of his left index finger from a pressurized washer. He stated that while at work, he had touched the jet nozzle of the washer to “test” its pressure, and had experienced immediate pain in his entire finger as well as blanching in his mid-finger. He took a picture of his finger with his cell phone approximately 5 minutes postinjury, which showed cynanosis at the distal volar pad (Figure 1).
On presentation to the ED, physical examination revealed only an innocuous 1-mm puncture wound to the middle of the volar pad of the distal phalanx of his left index finger, with mild tenderness along the length of the finger but no swelling. The rest of the finger and hand appeared intact with normal color and sensation.
Three weeks after discharge, however, the patient developed pain, diffuse swelling, and purulent drainage from the same injured finger and presented again to the ED. He was immediately taken to the operating room where a broad dissection was performed and drains were placed. Two months later, he reported a complete resolution of the infection and was still working on regaining full functioning of his finger by attending physical therapy.
High-Pressure Injection injuries
Diagnosis
At the time of presentation, most high-pressure injection injuries to the hand appear innocuous but frequently result in severe sequelae, including functional disability and amputation. High-pressure injuries occur when soft tissues are placed in contact or near the opening of a high-pressure device or malfunctioning equipment (eg, pinhole rupture in a hydraulic hose).
While the literature reports that 100 psi or greater is required to break the skin,1 patients presenting to the ED typically report operating machinery shooting between 2,000 and 12,000 psi.2,3 Case-report reviews of patients with high-pressure injection injuries by Schoo et al4 and Hogan and Ruland5 found this type of injury most often occurred in the nondominant hand of male laborers—primarily in the index finger—with 30% to 48% of such injuries resulting in amputation of the digit.
Mechanical and Chemical Injuries
High-pressure injection injuries can be both mechanical as well as chemical. A mechanical high-pressure injury depends upon the magnitude of the injection force, with higher pressures associated with increased dysfunction and incidence of amputation. A chemical-related injury depends upon the duration of exposure and the volume of the material injected.
A high-pressure injury results from a tearing of the soft tissues, with shearing and dissection within and among the tissue plains down the finger and into the palm and proximal tissues.4,6 In this type of injury, there is often direct injury to the neurovascular bundles.
Along with the mechanical aspect of injury, a concurrent chemical injury depends upon the type of material injected (ie, gas or liquid), cytotoxicity, concentration, and inflammatory properties.6 Hogan and Ruland5 described the incidence of amputation associated with injected materials to be greater than 40% with diesel fuel, paint thinner, oil, and paint; 20% to 40% with undercoating, hydraulic fluid, and grease; and 0% with air and water. Oil-based paint injections were associated with a 58% amputation rate comapred to only 6% for latex paints.5
In general, evaluation of high-pressure-related injuries begins with a thorough history to identify the injected material and its attributes, pressure of injection, mechanism of exposure, time elapsed since the event, and the patient’s tetanus immunization status. Interventions include radiographs to evaluate for proximal spread of injected material; tetanus immunization, if required; prophylactic antibiotic therapy7; analgesia; and often emergency surgical consultation. The use of corticosteroid therapy has not been shown to impact the amputation rate or the incidence of infection.5
Type of Injury and Treatment
Wong et al8 developed the following guidelines categorizing the type and recommended treatment of mild, moderate, and severe high-pressure injection injuries:
Mild Injuries. In mild high-pressure injection injuries, patients tend to seek immediate treatment. These types of injuries typically involve oil or water, with a relatively low-pressure exposure, and preserved circulation in the injured area.8 Treatment includes conservative management with an option for surgery.5,8
Moderate Injuries. In moderate injection injuries, there is more significant tissue damage from any of the aforementioned materials, but with preserved neurovascular structures. Treatment often includes debridement in addition to antibiotic therapy.
Severe Injuries. These types of injection injuries often involve paints and solvents, high-pressure exposure, and usually present with an abnormal neurovasculature. The best chance for salvage injuries involves rapid surgical intervention for debridement and reconstruction.9,10
Surgical Evaluation and Debridement
Despite optimal care, amputation and dysfunction rates with high-risk injection injuries (due to higher psi, organic solvents, and/or delayed definitive care) have been reported from 48% to 80%.10-13 Immediate surgical evaluation is often recommended when injection injuries are encountered.10,13 Patients who receive immediate surgical debridement (ie, within 6 hours from injury), specifically those injected with organic solvents, have a lower amputation rate than those who do not have immediate surgical intervention.13
In some reports, more than 80% of those injected with organic solvents who did not have immediate washout and debridement required amputation.4 Other effects of postponed debridement include delayed return to work time and decreased functionality of the hand.13,14
Conclusion
Although high-pressure injection injuries often appear mild at initial presentation since the immediate symptoms of the neurovascular injury often resolves, patients often experience severe sequelae including infection, disability, or amputation. The severity of the injury not only depends upon the psi, but also the type and amount of chemical injected. Because of the high morbidity, it is imperative that emergency physicians are aware of and identify these types of injuries and their sequelae.10,11,13,14
Dr Wilson is a third year resident in the department of emergency medicine at the Alpert Medical School, Brown University, Providence, Rhode Island. Dr Hack is the director for the division of medical toxicology at Brown University and the director of the educational program in medical toxicology. He is an associate professor at Warren Alpert Medical School; and an attending physician in the department of emergency medicine at Brown University, Rhode Island Hospital, Miriam Hospital, Providence.
Disclosure: The authors report no conflicts of interest.
A 42-year-old healthy man presented to the ED 3 hours after sustaining an injury to the tip of his left index finger from a pressurized washer. He stated that while at work, he had touched the jet nozzle of the washer to “test” its pressure, and had experienced immediate pain in his entire finger as well as blanching in his mid-finger. He took a picture of his finger with his cell phone approximately 5 minutes postinjury, which showed cynanosis at the distal volar pad (Figure 1).
On presentation to the ED, physical examination revealed only an innocuous 1-mm puncture wound to the middle of the volar pad of the distal phalanx of his left index finger, with mild tenderness along the length of the finger but no swelling. The rest of the finger and hand appeared intact with normal color and sensation.
Three weeks after discharge, however, the patient developed pain, diffuse swelling, and purulent drainage from the same injured finger and presented again to the ED. He was immediately taken to the operating room where a broad dissection was performed and drains were placed. Two months later, he reported a complete resolution of the infection and was still working on regaining full functioning of his finger by attending physical therapy.
High-Pressure Injection injuries
Diagnosis
At the time of presentation, most high-pressure injection injuries to the hand appear innocuous but frequently result in severe sequelae, including functional disability and amputation. High-pressure injuries occur when soft tissues are placed in contact or near the opening of a high-pressure device or malfunctioning equipment (eg, pinhole rupture in a hydraulic hose).
While the literature reports that 100 psi or greater is required to break the skin,1 patients presenting to the ED typically report operating machinery shooting between 2,000 and 12,000 psi.2,3 Case-report reviews of patients with high-pressure injection injuries by Schoo et al4 and Hogan and Ruland5 found this type of injury most often occurred in the nondominant hand of male laborers—primarily in the index finger—with 30% to 48% of such injuries resulting in amputation of the digit.
Mechanical and Chemical Injuries
High-pressure injection injuries can be both mechanical as well as chemical. A mechanical high-pressure injury depends upon the magnitude of the injection force, with higher pressures associated with increased dysfunction and incidence of amputation. A chemical-related injury depends upon the duration of exposure and the volume of the material injected.
A high-pressure injury results from a tearing of the soft tissues, with shearing and dissection within and among the tissue plains down the finger and into the palm and proximal tissues.4,6 In this type of injury, there is often direct injury to the neurovascular bundles.
Along with the mechanical aspect of injury, a concurrent chemical injury depends upon the type of material injected (ie, gas or liquid), cytotoxicity, concentration, and inflammatory properties.6 Hogan and Ruland5 described the incidence of amputation associated with injected materials to be greater than 40% with diesel fuel, paint thinner, oil, and paint; 20% to 40% with undercoating, hydraulic fluid, and grease; and 0% with air and water. Oil-based paint injections were associated with a 58% amputation rate comapred to only 6% for latex paints.5
In general, evaluation of high-pressure-related injuries begins with a thorough history to identify the injected material and its attributes, pressure of injection, mechanism of exposure, time elapsed since the event, and the patient’s tetanus immunization status. Interventions include radiographs to evaluate for proximal spread of injected material; tetanus immunization, if required; prophylactic antibiotic therapy7; analgesia; and often emergency surgical consultation. The use of corticosteroid therapy has not been shown to impact the amputation rate or the incidence of infection.5
Type of Injury and Treatment
Wong et al8 developed the following guidelines categorizing the type and recommended treatment of mild, moderate, and severe high-pressure injection injuries:
Mild Injuries. In mild high-pressure injection injuries, patients tend to seek immediate treatment. These types of injuries typically involve oil or water, with a relatively low-pressure exposure, and preserved circulation in the injured area.8 Treatment includes conservative management with an option for surgery.5,8
Moderate Injuries. In moderate injection injuries, there is more significant tissue damage from any of the aforementioned materials, but with preserved neurovascular structures. Treatment often includes debridement in addition to antibiotic therapy.
Severe Injuries. These types of injection injuries often involve paints and solvents, high-pressure exposure, and usually present with an abnormal neurovasculature. The best chance for salvage injuries involves rapid surgical intervention for debridement and reconstruction.9,10
Surgical Evaluation and Debridement
Despite optimal care, amputation and dysfunction rates with high-risk injection injuries (due to higher psi, organic solvents, and/or delayed definitive care) have been reported from 48% to 80%.10-13 Immediate surgical evaluation is often recommended when injection injuries are encountered.10,13 Patients who receive immediate surgical debridement (ie, within 6 hours from injury), specifically those injected with organic solvents, have a lower amputation rate than those who do not have immediate surgical intervention.13
In some reports, more than 80% of those injected with organic solvents who did not have immediate washout and debridement required amputation.4 Other effects of postponed debridement include delayed return to work time and decreased functionality of the hand.13,14
Conclusion
Although high-pressure injection injuries often appear mild at initial presentation since the immediate symptoms of the neurovascular injury often resolves, patients often experience severe sequelae including infection, disability, or amputation. The severity of the injury not only depends upon the psi, but also the type and amount of chemical injected. Because of the high morbidity, it is imperative that emergency physicians are aware of and identify these types of injuries and their sequelae.10,11,13,14
Dr Wilson is a third year resident in the department of emergency medicine at the Alpert Medical School, Brown University, Providence, Rhode Island. Dr Hack is the director for the division of medical toxicology at Brown University and the director of the educational program in medical toxicology. He is an associate professor at Warren Alpert Medical School; and an attending physician in the department of emergency medicine at Brown University, Rhode Island Hospital, Miriam Hospital, Providence.
Disclosure: The authors report no conflicts of interest.
- Scott AR. Occupational high-pressure injection injuries: pathogenesis and prevention. J Soc Occup Med. 1983;33(2):56-59.
- Pappou IP, Deal DN. High-pressure injection injuries. J Hand Surg Am. 2012;37(11):2404-2407.
- Soyuncu S, Bektas F, Dinc S. High-pressure air injection injury to the upper extremity. J Emerg Med. 2013;45(1):96-98.
- Schoo MJ, Scott FA, Boswick JA Jr. High-pressure injection injuries of the hand. J Trauma. 1980;20(3):229-238.
- Hogan CJ, Ruland RT. High-pressure injection injuries to the upper extremity: a review of the literature. J Orthop Trauma. 2006;20(7):503-511.
- Rosenwasser MP, Wei DH. High-pressure injection injuries to the hand. J Am Acad Orthop Surg. 2014;22(1):38-45.
- Mirzayan R, Schnall SB, Chon JH, Holtom PD, Pazakis MJ, Stevanovic MV. Culture results and amputation rates in high-pressure paint gun injuries of the hand. Orthopedics. 2001;24(6):587-589.
- Wong TC, Ip FK, Wu WC. High-pressure injection injuries of the hand in a Chinese population. J Hand Surg Br. 2005;30(6):588-592.
- Pinto MR, Turkula-Pinto LD, Cooney WP, Wood MB, Dobyns JH: High-pressure injection injuries of the hand: review of 25 patients managed by open wound technique. J Hand Surg Am. 1993;18(1):125-130.
- Kaufman, HD. High pressure injection injuries, the problems, pathogenesis and management. Hand. 1970;2(1);63-73.
- Vasilevski D, Noorbergen M, Depierreux M, Lafontaine M. High-pressure injection injuries to the hand. Am J Emerg Med. 2000;18(7):820-824.
- Bekler H, Gokce A, Beyzadeoglu T, Parmaksizoglu F. The surgical treatment and outcomes of high pressure injection injuries of the hand. J Hand Surg Eur Vol. 2007;32(4):394-399.
- Amsdell SL, Hammert WC. High-pressure injection injuries in the hand: current treatment concepts. Plast Reconstr Surg. 2013;132(4):586e-591e.
- Hart RG, Smith GD, Haq A. Prevention of high-pressure injection injuries to the hand. Am J Emerg Med. 2005;24(1):73-76.
- Scott AR. Occupational high-pressure injection injuries: pathogenesis and prevention. J Soc Occup Med. 1983;33(2):56-59.
- Pappou IP, Deal DN. High-pressure injection injuries. J Hand Surg Am. 2012;37(11):2404-2407.
- Soyuncu S, Bektas F, Dinc S. High-pressure air injection injury to the upper extremity. J Emerg Med. 2013;45(1):96-98.
- Schoo MJ, Scott FA, Boswick JA Jr. High-pressure injection injuries of the hand. J Trauma. 1980;20(3):229-238.
- Hogan CJ, Ruland RT. High-pressure injection injuries to the upper extremity: a review of the literature. J Orthop Trauma. 2006;20(7):503-511.
- Rosenwasser MP, Wei DH. High-pressure injection injuries to the hand. J Am Acad Orthop Surg. 2014;22(1):38-45.
- Mirzayan R, Schnall SB, Chon JH, Holtom PD, Pazakis MJ, Stevanovic MV. Culture results and amputation rates in high-pressure paint gun injuries of the hand. Orthopedics. 2001;24(6):587-589.
- Wong TC, Ip FK, Wu WC. High-pressure injection injuries of the hand in a Chinese population. J Hand Surg Br. 2005;30(6):588-592.
- Pinto MR, Turkula-Pinto LD, Cooney WP, Wood MB, Dobyns JH: High-pressure injection injuries of the hand: review of 25 patients managed by open wound technique. J Hand Surg Am. 1993;18(1):125-130.
- Kaufman, HD. High pressure injection injuries, the problems, pathogenesis and management. Hand. 1970;2(1);63-73.
- Vasilevski D, Noorbergen M, Depierreux M, Lafontaine M. High-pressure injection injuries to the hand. Am J Emerg Med. 2000;18(7):820-824.
- Bekler H, Gokce A, Beyzadeoglu T, Parmaksizoglu F. The surgical treatment and outcomes of high pressure injection injuries of the hand. J Hand Surg Eur Vol. 2007;32(4):394-399.
- Amsdell SL, Hammert WC. High-pressure injection injuries in the hand: current treatment concepts. Plast Reconstr Surg. 2013;132(4):586e-591e.
- Hart RG, Smith GD, Haq A. Prevention of high-pressure injection injuries to the hand. Am J Emerg Med. 2005;24(1):73-76.
Clostridium Perfringens Septicemia: A Critical Emergency Department Identification
Intravascular hemolysis in the presence of infection should prompt emergency physicians (EPs) to consider Clostridium perfringens septicemia and to act quickly to treat the infection. C perfringens septicemia is a rare, rapidly fatal disease with a reported mortality rate of at least 70%.1 Its expeditious lethality is due to a combination 7-minute doubling time of the organism and its production of a multitude of virulent toxins.1
This disease is deceptive: Patients may not appear to be severely ill and may be hemodynamically stable, not meeting systemic inflammatory response syndrome (SIRS) criteria, yet they rapidly decompensate. No interventions have been shown to reliably change outcome; however, the best hope for survival lays in early identification and definitive treatment with intravenous (IV) antibiotics and surgical intervention for source control. The authors present a fatal ED case of massive acute intravascular hemolysis due to C perfringens septicemia, the result of a cryptic liver abscess.
Case
A 74-year-old man with noninsulin-dependent diabetes mellitus (DM) and hypertension was transferred from an outside hospital to a tertiary-care referral center for leukocytosis and hyperbilirubinemia, where he had presented with fatigue and jaundice. A year prior, he had a cholecystectomy complicated by accidental hepatic artery ligation necessitating biliary tract reconstruction, but had been well since that event.
At the outside hospital, blood tests revealed a white blood cell (WBC) count of 21.0 x 109/L and elevated values on liver function tests with an elevated indirect hyperbilirubinemia. A right upper quadrant ultrasound showed a poorly defined hyperechoic mass behind the liver, but no bile-duct dilation or stones.
On arrival, the patient’s vital signs were: temperature, 100.4°F; heart rate, 92 beats/minute; blood pressure, 187/98 mm Hg; respiratory rate, 18 breaths/minute. His oxygen saturation was 97% on room air. An electrocardiogram showed sinus rhythm with a nonspecific intraventricular conduction delay with strain pattern. He was awake, comfortable, and conversant and oriented to place and person, but not to time. He was markedly jaundiced with scleral icterus, dry mucosal membranes, and foul breath. His neck was supple and nontender. His heart had a regular rate and rhythm, without murmur, rubs, or gallops, and his lungs were bilaterally clear to auscultation. The patient’s obese abdomen was soft and nontender, without rebound or guarding. He moved in a coordinated manner, and had no clonus or asterixis.
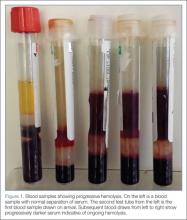
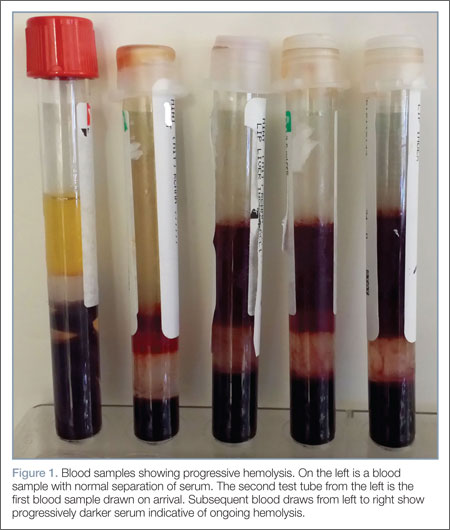
Repeat laboratory evaluation revealed a WBC of 32.2 x109/L, an elevated troponin level of 0.30 ng/mL, and an increased brain natriuretic peptide (BNP) of 636.2 pg/mL. Prothrombin time (PT) and international normalized ratio (INR) were normal. The chemistry test was reported as hemolyzed. Blood samples were redrawn three additional times, but each time the laboratory reported that each sample appeared progressively more hemolyzed and they were unable to obtain testable serum (Figure 1).
During his ED workup, the patient became more pale and jaundiced and began to produce bright red urine. The authors suspected that his hemolyzed blood samples were not due to blood draw artifact, but to intravascular hemolysis. A review of the blood smear showed gram-positive positive bacilli and ghost cells, commonly observed in hemolysis. Repeat laboratory tests showed interval increases in partial thromboplastin time, PT, and INR, and a D-dimer value of 3,621 ng/mL. Fibrinogen could not be measured due to gross hemolysis.
Empiric IV vancomycin and piperacillin/tazobactam were administered, and computed tomography (CT) studies of the brain and abdomen were ordered. The CT of the brain was normal; however, CT of the abdomen revealed an air-filled abscess in the right hepatic lobe and air in the left hepatic lobe and biliary tree without biliary dilation or wall thickening (Figure 2).
After discussion with cardiology, the medical intensive care team, and general surgery services, the patient was admitted to the surgical intensive care unit (SICU) with a plan to percutaneously drain the abscess the next morning.
Six and a half hours after arriving at the ED, the patient became acutely confused, tachypneic (RR, 22 breaths/minute) and tachycardic (HR, 102 beats/minute). On arrival to the SICU, he became unresponsive and pulseless. Resuscitation attempts were unsuccessful and the patient died.
Discussion
C perfringens is an anaerobic gram-positive bacillus known for causing gas gangrene and is normally found in the human gastrointestinal (GI) and genital tracts. Individuals most at risk for C perfringens septicemia have underlying hepatobiliary or genitourinary tract disease, malignancy, immunosuppression, DM, or recent history of GI or genitourinary surgery.2,3
Hemolysis has been observed in 7% to 15% of C perfringens bacteremias.3 This is caused by C perfringens’ primary toxin, phospholipase C lecithinase (α-toxin), which splits lecithin in the red blood-cell membrane thereby damaging its structural integrity and causing hemolysis.4 Other virulent factors of C perfringens are β-, ε-, and τ- toxins, all of which cause capillary leakage by damaging the vascular endothelium.5
Hemolysis with signs of septic shock due to C perfringens infection has been almost invariably fatal in several small case series reviews.2,3 While definitive identification of C perfringens is often delayed, it may be identified on gram stain; a DNA polymerase chain reaction (PCR) test has been developed, but is not widely available.6
The deceptive severity of this patient’s illness is a hallmark of C perfringens sepsis. Many patients may appear calm and report they feel “fine.” This lack of concern, “la belle indifférence,” is also seen in patients with necrotizing soft-tissue infections.7
Patients may be hemodynamically normal or fail to meet SIRS criteria. Case series have revealed no difference in SIRS criteria between survivors and nonsurvivors of C perfringens septicemia2; however, survivors were observed to have higher plasma fibrinogen levels than nonsurvivors.2 Fibrinogen, which is a known risk factor for the development of shock,8 may be a useful prognostic indicator given the association between shock and death in C perfringens septicemia.2
These factors were evident in this case. During the first 5 hours in the ED, the patient was hemodynamically normal, meeting only one of the SIRS criteria (elevated WBC). He also exhibited a nonchalant attitude, saying he “felt fine” before his rapid decline and death. Although fibrinogen was not available, this case is a clear reminder that exclusive use of SIRS criteria and patient reporting as barometers for severity of illness can be misleading.
Treatment
First-line treatment for C Perfringens includes high-dose IV penicillin G (10-24 million units daily), clindamycin for suppression of toxin synthesis, and surgical debridement.1,10 Second-line antibiotics include penicillin derivatives, chloramphenicol, doxycycline, carbapenems, tetracycline, and metronidazole.10,11
Immediate surgical intervention may be needed for survival. Limited review studies from Tokyo and the Netherlands indicate that surgical intervention is a strong prognostic indicator of survival and should be pursued expediently.2,3 A Dutch review 3 of 40 cases in the English medical literature published since 1990 demonstrated an overall mortality rate of 80% (32 of 40 patients). Among eight patients who had a surgical intervention (eg, hysterectomy, drainage of liver abscess) two deaths (25%) occurred. Those patients medically managed had a mortality rate of 93.7% (30/32 patients). While there is an impressive difference between these two groups—the authors assert a relative risk of mortality with surgical intervention of 0.27 (95% CI 0.08 to 0.89)—they are incomparable as many individuals in the medically managed group were not candidates for surgical intervention due to multiorgan failure or death prior to diagnosis.
While the strength of evidence for the efficacy of other interventions is limited, observational data suggest novel interventions are worth considering in an attempt to save these patients. Hyperbaric oxygen therapy has been used with some success in combination with surgery for gas gangrene and necrotizing soft-tissue infections; a few observational studies have shown benefit in sepsis.11 In the setting of massive hemolysis, blood transfusion may be required.12 If hemolysis is caught in early stages, exchange transfusion may prevent further complications.13 The α-toxin antitoxin, historically used for gas gangrene, has been abandoned in the United States due to severe allergic reactions and poor efficacy.14 However, researchers in Japan are investigating the efficacy of antitoxin for C perfringens liver abscesses when multiorgan failure prohibits surgical intervention.15
Conclusion
C perfringens septicemia should be considered when intravascular hemolysis is encountered, even in patients not meeting SIRS criteria. Treatment with appropriate antibiotics and an expedited search for a source (with subsequent immediate intervention) must be initiated prior to onset of shock if there is any hope of survival. If C perfringens septicemia is suspected, clear communication with family members and consultants about the seriousness of the patient’s condition is of the upmost importance as all parties involved must be made aware of the aggressive and unrelenting course of this disease and high likelihood of death.
Dr Samuels is a third-year resident in the department of emergency medicine at Brown University, Providence, Rhode Island. Dr Hack is the division director of medical toxicology at the University of Emergency Medicine Foundation; director of the educational program in medical toxicology and an associate professor at Warren Alpert Medical School; and an attending physician in the department of emergency medicine at Brown University, Rhode Island Hospital, Miriam Hospital, Providence.
- Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol. 2012;4(8):252-255.
- Fujita H, Nishimura S, Kurosawa S, Akiya I, Nakamura-Uchiyama F, Ohnishi K. Clinical and epidemiological features of Clostridium perfringens bacteremia: a review of 18 cases over 8 year-period in a tertiary care center in metropolitan Tokyo area in Japan. Intern Med. 2010;49(22):2433-2437.
- van Bunderen CC, Bomers MK, Wesdorp E, Peerbooms P, Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68(9):343-346.
- Hübl W, Mostbeck B, Hartleb H, Pointner H, Kofler K, Bayer PM. Investigation of the pathogenesis of massive hemolysis in a case of Clostridium perfringens septicemia. Ann Hematol. 1993;67(3):145-147.
- Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3(1):66-98.
- Bhatnagar J, Deleon-Carnes M, Kellar KL, et al. Rapid, simultaneous detection of Clostridium sordellii and Clostridium perfringens in archived tissues by a novel PCR-based microsphere assay: diagnostic implications for pregnancy-associated toxic shock syndrome cases. Infect Dis Obstet Gynecol. 2012;2012:972845.
- Herbert M and Swadron S. Necrotizing Fasciitis [Audio Podcast]. January 2009. Emergency Medicine: Reviews and Perspectives. EM:RAP Web site. http://www.emrap.org/episode/2009/january/necrotizing. Accessed November 1, 2013.
- Lissalde-Lavigne G1, Combescure C, Muller L, et al. Simple coagulation tests improve survival reduction in patients with septic shock. J Thromb Haemost. 2008;6(4):645-653.
- Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31(2):213-218.
- Clostridium perfringens. (2012) In: Chambers HF, Eliopoulos GM, eds. The Sanford Guide to Antimicrobial Therapy. v2.02 for Android [Mobile application software]. Sperryville, VA: Antimicrobial Therapy, Inc. Retrieved from http://www.sanfordguide.com/publications/the-sanford-guide-to-antimicrobial-therapy/mobile-applications.
- Rajendran G, Bothma P, Brodbeck A. Intravascular haemolysis and septicaemia due to Clostridium perfringens liver abscess. Anaesth Intensive Care. 2010;38(5):942-945.
- Watt J, Amini A, Mosier J, et al. Treatment of severe hemolytic anemia caused by Clostridium perfringens sepsis in a liver transplant recipient. Surg Infect. (Larchmont). 2012;13(1):60-62.
- Rubenberg ML, Baker LR, McBride JA, Sevitt LH, Brain MC. Intravascular coagulation in a case of Clostridium perfringens septicaemia: treatment by exchange transfusion and heparin. Br Med J. 1967;4(5574):271-274.
- Lober B. Gas gangrene and other clostridium associated diseases. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 5th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2000:2549-2561.
- Hifumi T, Koido Y, Takahashi M, Yamamoto A. Antitoxin treatment for liver abscess caused by Clostridium perfringens. Clin Mol Hepatol. 2013;19(1):97-98.
Intravascular hemolysis in the presence of infection should prompt emergency physicians (EPs) to consider Clostridium perfringens septicemia and to act quickly to treat the infection. C perfringens septicemia is a rare, rapidly fatal disease with a reported mortality rate of at least 70%.1 Its expeditious lethality is due to a combination 7-minute doubling time of the organism and its production of a multitude of virulent toxins.1
This disease is deceptive: Patients may not appear to be severely ill and may be hemodynamically stable, not meeting systemic inflammatory response syndrome (SIRS) criteria, yet they rapidly decompensate. No interventions have been shown to reliably change outcome; however, the best hope for survival lays in early identification and definitive treatment with intravenous (IV) antibiotics and surgical intervention for source control. The authors present a fatal ED case of massive acute intravascular hemolysis due to C perfringens septicemia, the result of a cryptic liver abscess.
Case
A 74-year-old man with noninsulin-dependent diabetes mellitus (DM) and hypertension was transferred from an outside hospital to a tertiary-care referral center for leukocytosis and hyperbilirubinemia, where he had presented with fatigue and jaundice. A year prior, he had a cholecystectomy complicated by accidental hepatic artery ligation necessitating biliary tract reconstruction, but had been well since that event.
At the outside hospital, blood tests revealed a white blood cell (WBC) count of 21.0 x 109/L and elevated values on liver function tests with an elevated indirect hyperbilirubinemia. A right upper quadrant ultrasound showed a poorly defined hyperechoic mass behind the liver, but no bile-duct dilation or stones.
On arrival, the patient’s vital signs were: temperature, 100.4°F; heart rate, 92 beats/minute; blood pressure, 187/98 mm Hg; respiratory rate, 18 breaths/minute. His oxygen saturation was 97% on room air. An electrocardiogram showed sinus rhythm with a nonspecific intraventricular conduction delay with strain pattern. He was awake, comfortable, and conversant and oriented to place and person, but not to time. He was markedly jaundiced with scleral icterus, dry mucosal membranes, and foul breath. His neck was supple and nontender. His heart had a regular rate and rhythm, without murmur, rubs, or gallops, and his lungs were bilaterally clear to auscultation. The patient’s obese abdomen was soft and nontender, without rebound or guarding. He moved in a coordinated manner, and had no clonus or asterixis.
Repeat laboratory evaluation revealed a WBC of 32.2 x109/L, an elevated troponin level of 0.30 ng/mL, and an increased brain natriuretic peptide (BNP) of 636.2 pg/mL. Prothrombin time (PT) and international normalized ratio (INR) were normal. The chemistry test was reported as hemolyzed. Blood samples were redrawn three additional times, but each time the laboratory reported that each sample appeared progressively more hemolyzed and they were unable to obtain testable serum (Figure 1).
During his ED workup, the patient became more pale and jaundiced and began to produce bright red urine. The authors suspected that his hemolyzed blood samples were not due to blood draw artifact, but to intravascular hemolysis. A review of the blood smear showed gram-positive positive bacilli and ghost cells, commonly observed in hemolysis. Repeat laboratory tests showed interval increases in partial thromboplastin time, PT, and INR, and a D-dimer value of 3,621 ng/mL. Fibrinogen could not be measured due to gross hemolysis.
Empiric IV vancomycin and piperacillin/tazobactam were administered, and computed tomography (CT) studies of the brain and abdomen were ordered. The CT of the brain was normal; however, CT of the abdomen revealed an air-filled abscess in the right hepatic lobe and air in the left hepatic lobe and biliary tree without biliary dilation or wall thickening (Figure 2).
After discussion with cardiology, the medical intensive care team, and general surgery services, the patient was admitted to the surgical intensive care unit (SICU) with a plan to percutaneously drain the abscess the next morning.
Six and a half hours after arriving at the ED, the patient became acutely confused, tachypneic (RR, 22 breaths/minute) and tachycardic (HR, 102 beats/minute). On arrival to the SICU, he became unresponsive and pulseless. Resuscitation attempts were unsuccessful and the patient died.
Discussion
C perfringens is an anaerobic gram-positive bacillus known for causing gas gangrene and is normally found in the human gastrointestinal (GI) and genital tracts. Individuals most at risk for C perfringens septicemia have underlying hepatobiliary or genitourinary tract disease, malignancy, immunosuppression, DM, or recent history of GI or genitourinary surgery.2,3
Hemolysis has been observed in 7% to 15% of C perfringens bacteremias.3 This is caused by C perfringens’ primary toxin, phospholipase C lecithinase (α-toxin), which splits lecithin in the red blood-cell membrane thereby damaging its structural integrity and causing hemolysis.4 Other virulent factors of C perfringens are β-, ε-, and τ- toxins, all of which cause capillary leakage by damaging the vascular endothelium.5
Hemolysis with signs of septic shock due to C perfringens infection has been almost invariably fatal in several small case series reviews.2,3 While definitive identification of C perfringens is often delayed, it may be identified on gram stain; a DNA polymerase chain reaction (PCR) test has been developed, but is not widely available.6
The deceptive severity of this patient’s illness is a hallmark of C perfringens sepsis. Many patients may appear calm and report they feel “fine.” This lack of concern, “la belle indifférence,” is also seen in patients with necrotizing soft-tissue infections.7
Patients may be hemodynamically normal or fail to meet SIRS criteria. Case series have revealed no difference in SIRS criteria between survivors and nonsurvivors of C perfringens septicemia2; however, survivors were observed to have higher plasma fibrinogen levels than nonsurvivors.2 Fibrinogen, which is a known risk factor for the development of shock,8 may be a useful prognostic indicator given the association between shock and death in C perfringens septicemia.2
These factors were evident in this case. During the first 5 hours in the ED, the patient was hemodynamically normal, meeting only one of the SIRS criteria (elevated WBC). He also exhibited a nonchalant attitude, saying he “felt fine” before his rapid decline and death. Although fibrinogen was not available, this case is a clear reminder that exclusive use of SIRS criteria and patient reporting as barometers for severity of illness can be misleading.
Treatment
First-line treatment for C Perfringens includes high-dose IV penicillin G (10-24 million units daily), clindamycin for suppression of toxin synthesis, and surgical debridement.1,10 Second-line antibiotics include penicillin derivatives, chloramphenicol, doxycycline, carbapenems, tetracycline, and metronidazole.10,11
Immediate surgical intervention may be needed for survival. Limited review studies from Tokyo and the Netherlands indicate that surgical intervention is a strong prognostic indicator of survival and should be pursued expediently.2,3 A Dutch review 3 of 40 cases in the English medical literature published since 1990 demonstrated an overall mortality rate of 80% (32 of 40 patients). Among eight patients who had a surgical intervention (eg, hysterectomy, drainage of liver abscess) two deaths (25%) occurred. Those patients medically managed had a mortality rate of 93.7% (30/32 patients). While there is an impressive difference between these two groups—the authors assert a relative risk of mortality with surgical intervention of 0.27 (95% CI 0.08 to 0.89)—they are incomparable as many individuals in the medically managed group were not candidates for surgical intervention due to multiorgan failure or death prior to diagnosis.
While the strength of evidence for the efficacy of other interventions is limited, observational data suggest novel interventions are worth considering in an attempt to save these patients. Hyperbaric oxygen therapy has been used with some success in combination with surgery for gas gangrene and necrotizing soft-tissue infections; a few observational studies have shown benefit in sepsis.11 In the setting of massive hemolysis, blood transfusion may be required.12 If hemolysis is caught in early stages, exchange transfusion may prevent further complications.13 The α-toxin antitoxin, historically used for gas gangrene, has been abandoned in the United States due to severe allergic reactions and poor efficacy.14 However, researchers in Japan are investigating the efficacy of antitoxin for C perfringens liver abscesses when multiorgan failure prohibits surgical intervention.15
Conclusion
C perfringens septicemia should be considered when intravascular hemolysis is encountered, even in patients not meeting SIRS criteria. Treatment with appropriate antibiotics and an expedited search for a source (with subsequent immediate intervention) must be initiated prior to onset of shock if there is any hope of survival. If C perfringens septicemia is suspected, clear communication with family members and consultants about the seriousness of the patient’s condition is of the upmost importance as all parties involved must be made aware of the aggressive and unrelenting course of this disease and high likelihood of death.
Dr Samuels is a third-year resident in the department of emergency medicine at Brown University, Providence, Rhode Island. Dr Hack is the division director of medical toxicology at the University of Emergency Medicine Foundation; director of the educational program in medical toxicology and an associate professor at Warren Alpert Medical School; and an attending physician in the department of emergency medicine at Brown University, Rhode Island Hospital, Miriam Hospital, Providence.
Intravascular hemolysis in the presence of infection should prompt emergency physicians (EPs) to consider Clostridium perfringens septicemia and to act quickly to treat the infection. C perfringens septicemia is a rare, rapidly fatal disease with a reported mortality rate of at least 70%.1 Its expeditious lethality is due to a combination 7-minute doubling time of the organism and its production of a multitude of virulent toxins.1
This disease is deceptive: Patients may not appear to be severely ill and may be hemodynamically stable, not meeting systemic inflammatory response syndrome (SIRS) criteria, yet they rapidly decompensate. No interventions have been shown to reliably change outcome; however, the best hope for survival lays in early identification and definitive treatment with intravenous (IV) antibiotics and surgical intervention for source control. The authors present a fatal ED case of massive acute intravascular hemolysis due to C perfringens septicemia, the result of a cryptic liver abscess.
Case
A 74-year-old man with noninsulin-dependent diabetes mellitus (DM) and hypertension was transferred from an outside hospital to a tertiary-care referral center for leukocytosis and hyperbilirubinemia, where he had presented with fatigue and jaundice. A year prior, he had a cholecystectomy complicated by accidental hepatic artery ligation necessitating biliary tract reconstruction, but had been well since that event.
At the outside hospital, blood tests revealed a white blood cell (WBC) count of 21.0 x 109/L and elevated values on liver function tests with an elevated indirect hyperbilirubinemia. A right upper quadrant ultrasound showed a poorly defined hyperechoic mass behind the liver, but no bile-duct dilation or stones.
On arrival, the patient’s vital signs were: temperature, 100.4°F; heart rate, 92 beats/minute; blood pressure, 187/98 mm Hg; respiratory rate, 18 breaths/minute. His oxygen saturation was 97% on room air. An electrocardiogram showed sinus rhythm with a nonspecific intraventricular conduction delay with strain pattern. He was awake, comfortable, and conversant and oriented to place and person, but not to time. He was markedly jaundiced with scleral icterus, dry mucosal membranes, and foul breath. His neck was supple and nontender. His heart had a regular rate and rhythm, without murmur, rubs, or gallops, and his lungs were bilaterally clear to auscultation. The patient’s obese abdomen was soft and nontender, without rebound or guarding. He moved in a coordinated manner, and had no clonus or asterixis.
Repeat laboratory evaluation revealed a WBC of 32.2 x109/L, an elevated troponin level of 0.30 ng/mL, and an increased brain natriuretic peptide (BNP) of 636.2 pg/mL. Prothrombin time (PT) and international normalized ratio (INR) were normal. The chemistry test was reported as hemolyzed. Blood samples were redrawn three additional times, but each time the laboratory reported that each sample appeared progressively more hemolyzed and they were unable to obtain testable serum (Figure 1).
During his ED workup, the patient became more pale and jaundiced and began to produce bright red urine. The authors suspected that his hemolyzed blood samples were not due to blood draw artifact, but to intravascular hemolysis. A review of the blood smear showed gram-positive positive bacilli and ghost cells, commonly observed in hemolysis. Repeat laboratory tests showed interval increases in partial thromboplastin time, PT, and INR, and a D-dimer value of 3,621 ng/mL. Fibrinogen could not be measured due to gross hemolysis.
Empiric IV vancomycin and piperacillin/tazobactam were administered, and computed tomography (CT) studies of the brain and abdomen were ordered. The CT of the brain was normal; however, CT of the abdomen revealed an air-filled abscess in the right hepatic lobe and air in the left hepatic lobe and biliary tree without biliary dilation or wall thickening (Figure 2).
After discussion with cardiology, the medical intensive care team, and general surgery services, the patient was admitted to the surgical intensive care unit (SICU) with a plan to percutaneously drain the abscess the next morning.
Six and a half hours after arriving at the ED, the patient became acutely confused, tachypneic (RR, 22 breaths/minute) and tachycardic (HR, 102 beats/minute). On arrival to the SICU, he became unresponsive and pulseless. Resuscitation attempts were unsuccessful and the patient died.
Discussion
C perfringens is an anaerobic gram-positive bacillus known for causing gas gangrene and is normally found in the human gastrointestinal (GI) and genital tracts. Individuals most at risk for C perfringens septicemia have underlying hepatobiliary or genitourinary tract disease, malignancy, immunosuppression, DM, or recent history of GI or genitourinary surgery.2,3
Hemolysis has been observed in 7% to 15% of C perfringens bacteremias.3 This is caused by C perfringens’ primary toxin, phospholipase C lecithinase (α-toxin), which splits lecithin in the red blood-cell membrane thereby damaging its structural integrity and causing hemolysis.4 Other virulent factors of C perfringens are β-, ε-, and τ- toxins, all of which cause capillary leakage by damaging the vascular endothelium.5
Hemolysis with signs of septic shock due to C perfringens infection has been almost invariably fatal in several small case series reviews.2,3 While definitive identification of C perfringens is often delayed, it may be identified on gram stain; a DNA polymerase chain reaction (PCR) test has been developed, but is not widely available.6
The deceptive severity of this patient’s illness is a hallmark of C perfringens sepsis. Many patients may appear calm and report they feel “fine.” This lack of concern, “la belle indifférence,” is also seen in patients with necrotizing soft-tissue infections.7
Patients may be hemodynamically normal or fail to meet SIRS criteria. Case series have revealed no difference in SIRS criteria between survivors and nonsurvivors of C perfringens septicemia2; however, survivors were observed to have higher plasma fibrinogen levels than nonsurvivors.2 Fibrinogen, which is a known risk factor for the development of shock,8 may be a useful prognostic indicator given the association between shock and death in C perfringens septicemia.2
These factors were evident in this case. During the first 5 hours in the ED, the patient was hemodynamically normal, meeting only one of the SIRS criteria (elevated WBC). He also exhibited a nonchalant attitude, saying he “felt fine” before his rapid decline and death. Although fibrinogen was not available, this case is a clear reminder that exclusive use of SIRS criteria and patient reporting as barometers for severity of illness can be misleading.
Treatment
First-line treatment for C Perfringens includes high-dose IV penicillin G (10-24 million units daily), clindamycin for suppression of toxin synthesis, and surgical debridement.1,10 Second-line antibiotics include penicillin derivatives, chloramphenicol, doxycycline, carbapenems, tetracycline, and metronidazole.10,11
Immediate surgical intervention may be needed for survival. Limited review studies from Tokyo and the Netherlands indicate that surgical intervention is a strong prognostic indicator of survival and should be pursued expediently.2,3 A Dutch review 3 of 40 cases in the English medical literature published since 1990 demonstrated an overall mortality rate of 80% (32 of 40 patients). Among eight patients who had a surgical intervention (eg, hysterectomy, drainage of liver abscess) two deaths (25%) occurred. Those patients medically managed had a mortality rate of 93.7% (30/32 patients). While there is an impressive difference between these two groups—the authors assert a relative risk of mortality with surgical intervention of 0.27 (95% CI 0.08 to 0.89)—they are incomparable as many individuals in the medically managed group were not candidates for surgical intervention due to multiorgan failure or death prior to diagnosis.
While the strength of evidence for the efficacy of other interventions is limited, observational data suggest novel interventions are worth considering in an attempt to save these patients. Hyperbaric oxygen therapy has been used with some success in combination with surgery for gas gangrene and necrotizing soft-tissue infections; a few observational studies have shown benefit in sepsis.11 In the setting of massive hemolysis, blood transfusion may be required.12 If hemolysis is caught in early stages, exchange transfusion may prevent further complications.13 The α-toxin antitoxin, historically used for gas gangrene, has been abandoned in the United States due to severe allergic reactions and poor efficacy.14 However, researchers in Japan are investigating the efficacy of antitoxin for C perfringens liver abscesses when multiorgan failure prohibits surgical intervention.15
Conclusion
C perfringens septicemia should be considered when intravascular hemolysis is encountered, even in patients not meeting SIRS criteria. Treatment with appropriate antibiotics and an expedited search for a source (with subsequent immediate intervention) must be initiated prior to onset of shock if there is any hope of survival. If C perfringens septicemia is suspected, clear communication with family members and consultants about the seriousness of the patient’s condition is of the upmost importance as all parties involved must be made aware of the aggressive and unrelenting course of this disease and high likelihood of death.
Dr Samuels is a third-year resident in the department of emergency medicine at Brown University, Providence, Rhode Island. Dr Hack is the division director of medical toxicology at the University of Emergency Medicine Foundation; director of the educational program in medical toxicology and an associate professor at Warren Alpert Medical School; and an attending physician in the department of emergency medicine at Brown University, Rhode Island Hospital, Miriam Hospital, Providence.
- Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol. 2012;4(8):252-255.
- Fujita H, Nishimura S, Kurosawa S, Akiya I, Nakamura-Uchiyama F, Ohnishi K. Clinical and epidemiological features of Clostridium perfringens bacteremia: a review of 18 cases over 8 year-period in a tertiary care center in metropolitan Tokyo area in Japan. Intern Med. 2010;49(22):2433-2437.
- van Bunderen CC, Bomers MK, Wesdorp E, Peerbooms P, Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68(9):343-346.
- Hübl W, Mostbeck B, Hartleb H, Pointner H, Kofler K, Bayer PM. Investigation of the pathogenesis of massive hemolysis in a case of Clostridium perfringens septicemia. Ann Hematol. 1993;67(3):145-147.
- Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3(1):66-98.
- Bhatnagar J, Deleon-Carnes M, Kellar KL, et al. Rapid, simultaneous detection of Clostridium sordellii and Clostridium perfringens in archived tissues by a novel PCR-based microsphere assay: diagnostic implications for pregnancy-associated toxic shock syndrome cases. Infect Dis Obstet Gynecol. 2012;2012:972845.
- Herbert M and Swadron S. Necrotizing Fasciitis [Audio Podcast]. January 2009. Emergency Medicine: Reviews and Perspectives. EM:RAP Web site. http://www.emrap.org/episode/2009/january/necrotizing. Accessed November 1, 2013.
- Lissalde-Lavigne G1, Combescure C, Muller L, et al. Simple coagulation tests improve survival reduction in patients with septic shock. J Thromb Haemost. 2008;6(4):645-653.
- Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31(2):213-218.
- Clostridium perfringens. (2012) In: Chambers HF, Eliopoulos GM, eds. The Sanford Guide to Antimicrobial Therapy. v2.02 for Android [Mobile application software]. Sperryville, VA: Antimicrobial Therapy, Inc. Retrieved from http://www.sanfordguide.com/publications/the-sanford-guide-to-antimicrobial-therapy/mobile-applications.
- Rajendran G, Bothma P, Brodbeck A. Intravascular haemolysis and septicaemia due to Clostridium perfringens liver abscess. Anaesth Intensive Care. 2010;38(5):942-945.
- Watt J, Amini A, Mosier J, et al. Treatment of severe hemolytic anemia caused by Clostridium perfringens sepsis in a liver transplant recipient. Surg Infect. (Larchmont). 2012;13(1):60-62.
- Rubenberg ML, Baker LR, McBride JA, Sevitt LH, Brain MC. Intravascular coagulation in a case of Clostridium perfringens septicaemia: treatment by exchange transfusion and heparin. Br Med J. 1967;4(5574):271-274.
- Lober B. Gas gangrene and other clostridium associated diseases. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 5th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2000:2549-2561.
- Hifumi T, Koido Y, Takahashi M, Yamamoto A. Antitoxin treatment for liver abscess caused by Clostridium perfringens. Clin Mol Hepatol. 2013;19(1):97-98.
- Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol. 2012;4(8):252-255.
- Fujita H, Nishimura S, Kurosawa S, Akiya I, Nakamura-Uchiyama F, Ohnishi K. Clinical and epidemiological features of Clostridium perfringens bacteremia: a review of 18 cases over 8 year-period in a tertiary care center in metropolitan Tokyo area in Japan. Intern Med. 2010;49(22):2433-2437.
- van Bunderen CC, Bomers MK, Wesdorp E, Peerbooms P, Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68(9):343-346.
- Hübl W, Mostbeck B, Hartleb H, Pointner H, Kofler K, Bayer PM. Investigation of the pathogenesis of massive hemolysis in a case of Clostridium perfringens septicemia. Ann Hematol. 1993;67(3):145-147.
- Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3(1):66-98.
- Bhatnagar J, Deleon-Carnes M, Kellar KL, et al. Rapid, simultaneous detection of Clostridium sordellii and Clostridium perfringens in archived tissues by a novel PCR-based microsphere assay: diagnostic implications for pregnancy-associated toxic shock syndrome cases. Infect Dis Obstet Gynecol. 2012;2012:972845.
- Herbert M and Swadron S. Necrotizing Fasciitis [Audio Podcast]. January 2009. Emergency Medicine: Reviews and Perspectives. EM:RAP Web site. http://www.emrap.org/episode/2009/january/necrotizing. Accessed November 1, 2013.
- Lissalde-Lavigne G1, Combescure C, Muller L, et al. Simple coagulation tests improve survival reduction in patients with septic shock. J Thromb Haemost. 2008;6(4):645-653.
- Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31(2):213-218.
- Clostridium perfringens. (2012) In: Chambers HF, Eliopoulos GM, eds. The Sanford Guide to Antimicrobial Therapy. v2.02 for Android [Mobile application software]. Sperryville, VA: Antimicrobial Therapy, Inc. Retrieved from http://www.sanfordguide.com/publications/the-sanford-guide-to-antimicrobial-therapy/mobile-applications.
- Rajendran G, Bothma P, Brodbeck A. Intravascular haemolysis and septicaemia due to Clostridium perfringens liver abscess. Anaesth Intensive Care. 2010;38(5):942-945.
- Watt J, Amini A, Mosier J, et al. Treatment of severe hemolytic anemia caused by Clostridium perfringens sepsis in a liver transplant recipient. Surg Infect. (Larchmont). 2012;13(1):60-62.
- Rubenberg ML, Baker LR, McBride JA, Sevitt LH, Brain MC. Intravascular coagulation in a case of Clostridium perfringens septicaemia: treatment by exchange transfusion and heparin. Br Med J. 1967;4(5574):271-274.
- Lober B. Gas gangrene and other clostridium associated diseases. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 5th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2000:2549-2561.
- Hifumi T, Koido Y, Takahashi M, Yamamoto A. Antitoxin treatment for liver abscess caused by Clostridium perfringens. Clin Mol Hepatol. 2013;19(1):97-98.