User login
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates
Blood cultures provide crucial evidence for diagnostic medicine, specifically aimed at identifying the presence of microbial infections in the bloodstream. Blood culturing is instrumental in diagnosing conditions such as sepsis, bacteremia, or fungemia, where the identification of the causative agent is necessary for targeted and effective treatment.1
The process involves aseptically drawing blood into sterile culture bottles, minimizing the risk of contamination with well-established guidelines. These culture bottles contain specific growth media that support the replication of microorganisms if they are present. Once the blood specimen is collected, it incubates, allowing any potential pathogens to grow. Subsequent analysis and identification of these microorganisms enable health care professionals (HCPs) to prescribe appropriate antimicrobial therapies to treat specific infections, contributing to more effective and targeted patient care.2
The reliability of blood culture results depends on minimizing contamination risk, a challenge inherent in the procedure. Contamination can lead to false-positive results, potentially misguiding treatment.3 HCPs must adhere to strict aseptic techniques during blood draws, ensuring proper skin preparation with antiseptic solutions. The use of sterile equipment and avoiding prolonged tourniquet application helps maintain the integrity of the blood specimen. Timely inoculation of blood into culture bottles and careful handling are essential to mitigate contamination risk.2 Regular training and reinforcement of proper techniques is important to uphold the accuracy of blood culture results and enhance the reliability of diagnoses and treatment decisions.3 Despite diligent contamination prevention efforts, health care systems struggle to maintain contamination rates below the 3.0% national benchmark set by the Clinical & Laboratory Standards Institute (CLSI).4
Blood culture contamination is a critical concern in clinical practice; it can lead to misdiagnosis, prolonged hospital stays, unnecessary antibiotic use, and increased health care costs.5 Monitoring blood culture contamination is integral to patient safety, avoiding inappropriate and potentially harmful treatment, providing efficient care, contributing to antibiotic stewardship, supporting cost efficiency, and maintaining quality assurance and clinical research practices for public health.6
The initial specimen diversion technique (ISDT) recently emerged as a potential strategy to reduce blood culture contamination rates. This technique involves diverting a small portion of the initial blood plus the skin plug from the hollow needle away from the primary collection site before filling the culture bottles. This process minimizes skin surface contaminants, providing a cleaner blood specimen for culturing.7
The ISDT was introduced as a result of historically elevated contamination rates.8 Despite implementing various mitigation methods, the US Department of Veterans Affairs (VA) Central Texas Healthcare System (VACTHCS) has struggled to meet the national benchmark of maintaining blood culture contamination < 3.0%. The VACTHCS is a 146-bed teaching hospital with about 30,000 annual visits at the Olin E. Teague Veterans Affairs Medical Center (OETVMC) emergency department (ED). VACTHCS conducted a 16-month pilot study using 2 commercially available ISDT devices and published the findings.8
The Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022 (MilCon-VA Act) committee report prioritized the reduction of blood culture contamination to < 1% to prevent health risks and harm to veterans undergoing blood testing for the diagnosis of sepsis.9 Because it had been 5 years since OETVMC began using an ISDT in the ED, the ISDT adaptation strategy for mitigating blood culture contamination was revisited per institution policy.
The objective of this quality improvement project was to analyze retrospective data to understand the long-term impact of ISDT use on blood culture contamination rates. We hypothesized that ISDT use would contribute to efforts to maintain OETVMC ED blood culture contamination rate below the national (3.0%) and VACTHCS (2.5%) thresholds. This project assessed the progress for reducing blood culture contamination compared with the pre-ISDT era.8
METHODS
This retrospective analysis compared the blood culture contamination rates 36 months before and after the introduction of the ISDT device at the OETVMC ED. The preimplementation period was from December 2014 through November 2017 (36 months) and the postimplementation period was December 2017 through November 2020 (36 months). Data were collected from the Department of Pathology and Microbiology blood culture records of all adult patients admitted to the hospital through the ED and required blood cultures for suspicion of infection. Protected health information and VA sensitive information were not collected: all data were deidentified. A total of 18,541 blood cultures were collected 36 months preimplementation and 14,865 blood cultures were collected up to 36 months postimplementation. For comparison purposes, a similar dataset was collected from patients’ blood samples drawn by phlebotomists in the laboratory, where there had been no previous issues with overcontamination; no ISDT devices were used in the collection of these samples.
Blood Culture Contamination Variable
Blood cultures were monitored using the BACT/ALERT 3D (bioMérieux) and subsequently BACT/ALERT VIRTUO (bioMérieux), with positive bottles characterized by VITEK MS Matrix Assisted Laser Desorption Ionization Time-of-Flight technology (bioMérieux) and automated susceptibility testing (VITEK 2 [bioMérieux]).10 In an updated review of blood culture contamination, the American Society for Microbiology used the College of American Pathologists' Q-Probes quality improvement studies as a guideline for classifying contamination. A sample was determined to be contaminated if ≥ 1 of the following organisms were found in only 1 bottle in a series of blood culture sets: coagulase-negative staphylococci, Micrococcus species, α-hemolytic viridans group streptococci, Corynebacterium species, Propionibacterium acnes, and Bacillus species.11 The contamination assessment criteria remained unchanged, except for use of an ISDT device in blood culture collection at the ED.
The VACTHCS Infection Prevention Department ensured that the ISDT device was available and that ED nurses were trained annually on its use to collect blood cultures. Monthly reports of contamination were sent to the nursing supervisor for corrective action and retraining. The initial performance improvement project was slated for 16 months but was expanded to a 6-year period of retrospective data to obtain strong correlation.
Statistical Analysis
Contamination rates were recorded monthly from the hospital laboratory information management system for 36 months both before and after ISDT adoption. Statistical analysis was performed using a 2-tailed unpaired t-test to compare monthly contamination rates for the 2 periods with GraphPad Prism version 10.0.0 for Windows.
RESULTS
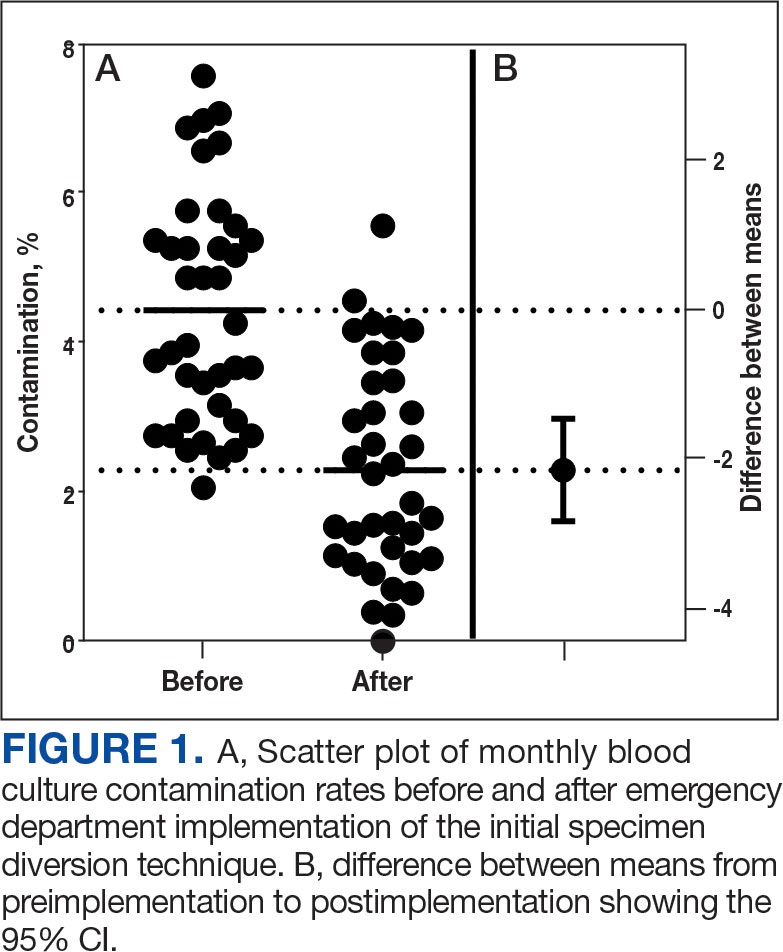
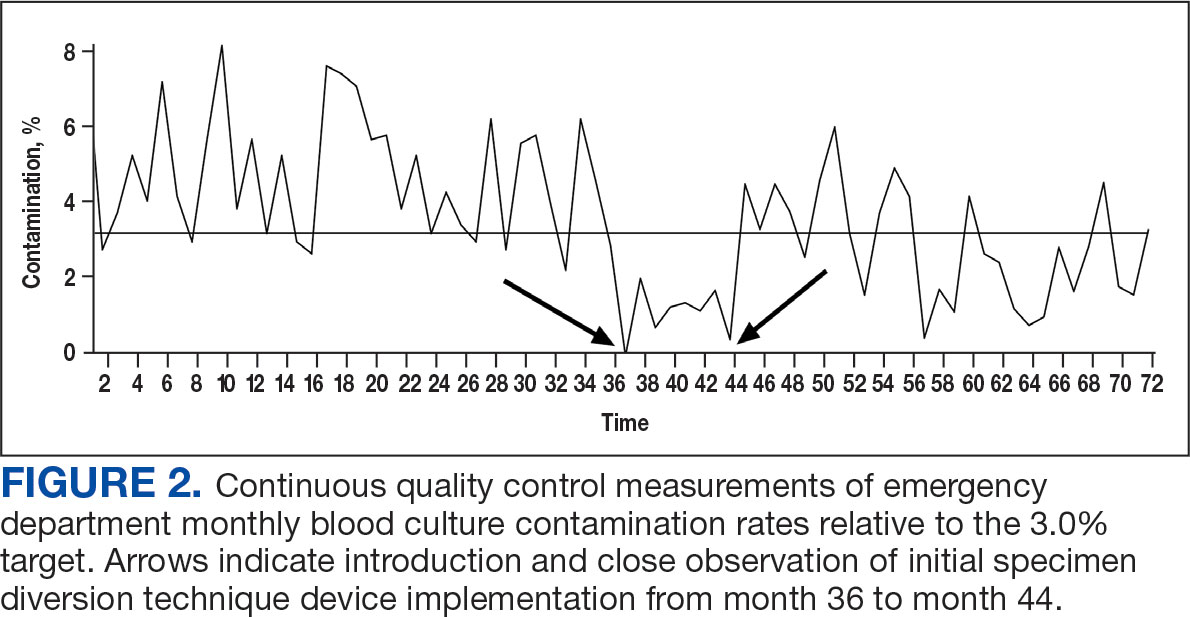
Prior to 2017, the ED reported contamination rates above the national (3.0%) and OETVMC thresholds (2.5%), with a mean of 4.5% (95% CI, 3.90-4.90).8 After ISDT implementation, the ED showed significant improvement with a reduction to mean 2.6% (95% CI, 2.10-3.20) (P < .001) (Figure 1). Figure 2 shows monthly blood culture contamination rates at the ED from December 2014 through November 2020. Month 36 (November 2017) shows a clear dip in contamination rate when the ISDT was introduced and month 37 to month 44 show remarkably low contamination rates. During this time, the institute experimented with 2 ISDT devices, and closer scrutiny may reveal this period as an outlier due to the monitoring of ISDT application, as previously reported.8
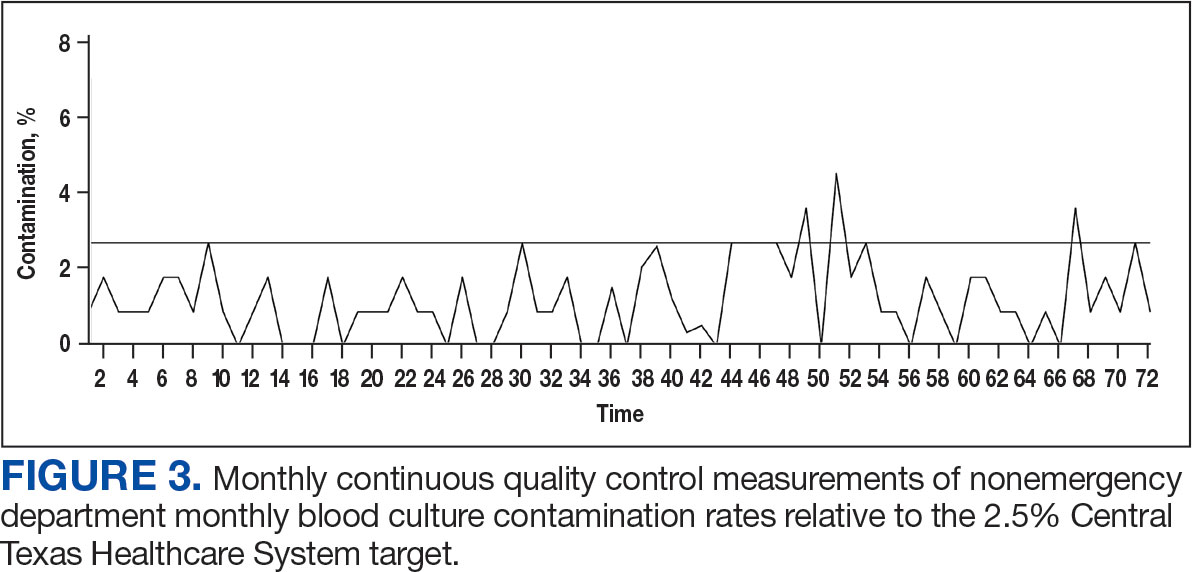
The blood culture contamination rate for samples drawn by the phlebotomists in the laboratory (excluding the ED) was calculated during the same time period (Figure 3). Non-ED contamination rates remained below 2.5% for 69 of 72 months.
DISCUSSION
The blood culture contamination rate in the OETVMC ED dropped following ISDT implementation and continued to show long-term benefits. For the 36-month period following ISDT implementation, the mean contamination rate was 2.6%, which was below the national target threshold of 3.0% and close to the OETVMC target of 2.5%. These results suggest that ISDT can have a positive impact on patient care and laboratory efficiency. Improvements in the blood contamination rates in the ED can have a positive impact on the overall hospital contamination rates.
Blood drawn by phlebotomists in the hospital laboratory infrequently had contamination rates that exceeded the 2.5% target threshold. Because the non-ED contamination rates did not change throughout the comparison period, other factors were likely not involved in the improvements seen in the ED. The decision to implement ISDT exclusively in the ED was based on its historically elevated contamination rate.8 Issues with blood culture contamination in EDs across various hospital systems are well documented and not unique to VACTHCS.12
Contamination in blood cultures can be a significant issue in the hospital. It occurs when microorganisms from the skin or environment enter the blood culture sample during collection. Moreover, it can contribute to antibiotic resistance when patients are prescribed inappropriate antibiotics. It is also important to ensure HCPs are well-trained and consistently follow standardized protocols and understand the implications of false-positive results.13
ISDT helps reduce false-positive results and is a significant advancement in the field of blood culture collection.8,14 By discarding the initial blood, it ensures that only the true bloodstream sample is cultured, leading to more accurate results.15 It also may minimize the risk of contamination-related delays in diagnosis and treatment and benefits patients and health care institutions by potentially reducing hospital stays, unnecessary antibiotic use, and health care costs.
One of the ISDT device manufacturers estimated the financial impact on OETVMC based on the pilot project.8 While this study did not calculate the direct and indirect cost savings associated with this process improvement, the manufacturer’s website suggests that VACTHCS could annually save about $486,000.16 Furthermore, implementation of ISDT may improve laboratory efficiency, as they reduce the workload associated with identifying and reporting false-positive cultures. 6 ISDT devices represent a valuable tool in the efforts to reduce blood culture contamination and its wide-ranging implications in clinical settings. While ISDT alone will not be sufficient in achieving a lower threshold (< 1%) of blood culture contamination, it can be part of a multiprong effort that optimizes best practices in the collection, handling, and management of blood cultures.
Continuous quality improvement efforts and monitoring of blood culture contamination rates can help health care institutions identify problem areas and implement necessary changes. Addressing blood culture contamination can improve patient care, reduce costs, and address antibiotic resistance.
Limitations
This study was limited by its study design, which did not use a side-by-side comparison of blood cultures from groups with and without ISDT. All blood cultures from patients in the region were processed at OETVMC, which may not be representative of non-VA EDs. Part of this study took place during the COVID-19 pandemic, which may have skewed data. Additionally, hospital data were collected from a veteran population in Central Texas, and the lack of demographic diversity may not be generalizable to the greater population.
CONCLUSIONS
The findings of this study suggest ISDT may be effective in reducing blood culture contamination rates in the high-risk ED environment, which aligns with previous research. 5,14 The ISDT may help reduce blood culture contamination rates, improving the quality of patient care and reducing health care costs. MilCon-VA mandated that all VA facilities have blood culture contamination as a metric with a goal of blood culture contamination rates < 1%.8 However, achieving this goal remains a challenge. Further research and continuous quality improvement efforts are necessary to achieve it. Consistently achieving a contamination threshold of < 1% may require minimizing human error. An automated robotic venipuncture device, as recently designed and reported, may be necessary to reduce human error in blood draw and contamination.16
- Chela HK, Vasudevan A, Rojas-Moreno C, Naqvi SH. Approach to positive blood cultures in the hospitalized patient: a review. Mo Med. 2019;116(4):313-317.
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi:10.3389/fmicb.2016.00697
- Doern GV, Carroll KC, Diekema DJ, et al. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33:e00009-19. doi:10.1128/CMR.00009-19
- Wilson ML, Kirn Jr TJ, Antonara S, et al. Clinical and Laboratory Standards Institute Guideline M47—Principles and Procedures for Blood Cultures. Clinical and Laboratory Standards Institute. April 22, 2022. Accessed May 21, 2025. https://clsi.org/shop/standards/m47/
- Hancock JA, Campbell S, Jones MM, Wang-Rodriguez J, VHA Microbiology SME Workgroup, Klutts JS. Development and validation of a standardized blood culture contamination definition and metric dashboard for a large health care system. Am J Clin Pathol. 2023;160(3):255-260. doi:10.1093/ajcp/aqad044
- Shinozaki T, Deane RS, Mazuzan JE Jr, Hamel AJ, Hazelton D. Bacterial contamination of arterial lines. A prospective study. JAMA. 1983;249(2):223-225.
- Al Mohajer M, Lasco T. The impact of initial specimen diversion systems on blood culture contamination. Open Forum Infect Dis. 2023;10:ofad182. doi:10.1093/ofid/ofad182
- Arenas M, Boseman GM, Coppin JD, Lukey J, Jinadatha C, Navarathna DH. Asynchronous testing of 2 specimen-diversion devices to reduce blood culture contamination: a single-site product supply quality improvement project. J Emerg Nurs. 2021;47(2):256-264. e6. doi:10.1016/j.jen.2020.11.008
- Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022, HR 4355, 117th Cong (2021-2022). Accessed May 12, 2025. https://www.congress.gov/bill/117th-congress/house-bill/4355?
- Altun O, Almuhayawi M, Lüthje P, Taha R, Ullberg M, Özenci V. Controlled evaluation of the New BacT/ Alert Virtuo blood culture system for detection and time to detection of bacteria and yeasts. J Clin Microbiol. 2016;54(4):1148-1151. doi:10.1128/JCM.03362-15
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. doi:10.1128/CMR.00062-05
- Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47(4):1021-1024. doi:10.1128/JCM.02162-08
- Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central lineassociated bloodstream infections. Am J Infect Control. 2015;43(11):1222-1237. doi:10.1016/j.ajic.2015.06.030
- Callado GY, Lin V, Thottacherry E, et al. Diagnostic stewardship: a systematic review and meta-analysis of blood collection diversion devices used to reduce blood culture contamination and improve the accuracy of diagnosis in clinical settings. Open Forum Infect Dis. 2023;10(9):ofad433. doi:10.1093/ofid/ofad433
- Patton RG, Schmitt T. Innovation for reducing blood culture contamination: initial specimen diversion technique. J Clin Microbiol. 2010;48:4501-4503. doi:10.1128/JCM.00910-10
- Kurin. Clinical evidence: published Kurin studies. 2024. Accessed May 12, 2025. https://www.kurin.com/studies
- Leipheimer JM, Balter ML, Chen AI, et al. First-in-human evaluation of a hand-held automated venipuncture device for rapid venous blood draws. Technology (Singap World Sci). 2019;7(3-4):98-107. doi:10.1142/S2339547819500067?
Blood cultures provide crucial evidence for diagnostic medicine, specifically aimed at identifying the presence of microbial infections in the bloodstream. Blood culturing is instrumental in diagnosing conditions such as sepsis, bacteremia, or fungemia, where the identification of the causative agent is necessary for targeted and effective treatment.1
The process involves aseptically drawing blood into sterile culture bottles, minimizing the risk of contamination with well-established guidelines. These culture bottles contain specific growth media that support the replication of microorganisms if they are present. Once the blood specimen is collected, it incubates, allowing any potential pathogens to grow. Subsequent analysis and identification of these microorganisms enable health care professionals (HCPs) to prescribe appropriate antimicrobial therapies to treat specific infections, contributing to more effective and targeted patient care.2
The reliability of blood culture results depends on minimizing contamination risk, a challenge inherent in the procedure. Contamination can lead to false-positive results, potentially misguiding treatment.3 HCPs must adhere to strict aseptic techniques during blood draws, ensuring proper skin preparation with antiseptic solutions. The use of sterile equipment and avoiding prolonged tourniquet application helps maintain the integrity of the blood specimen. Timely inoculation of blood into culture bottles and careful handling are essential to mitigate contamination risk.2 Regular training and reinforcement of proper techniques is important to uphold the accuracy of blood culture results and enhance the reliability of diagnoses and treatment decisions.3 Despite diligent contamination prevention efforts, health care systems struggle to maintain contamination rates below the 3.0% national benchmark set by the Clinical & Laboratory Standards Institute (CLSI).4
Blood culture contamination is a critical concern in clinical practice; it can lead to misdiagnosis, prolonged hospital stays, unnecessary antibiotic use, and increased health care costs.5 Monitoring blood culture contamination is integral to patient safety, avoiding inappropriate and potentially harmful treatment, providing efficient care, contributing to antibiotic stewardship, supporting cost efficiency, and maintaining quality assurance and clinical research practices for public health.6
The initial specimen diversion technique (ISDT) recently emerged as a potential strategy to reduce blood culture contamination rates. This technique involves diverting a small portion of the initial blood plus the skin plug from the hollow needle away from the primary collection site before filling the culture bottles. This process minimizes skin surface contaminants, providing a cleaner blood specimen for culturing.7
The ISDT was introduced as a result of historically elevated contamination rates.8 Despite implementing various mitigation methods, the US Department of Veterans Affairs (VA) Central Texas Healthcare System (VACTHCS) has struggled to meet the national benchmark of maintaining blood culture contamination < 3.0%. The VACTHCS is a 146-bed teaching hospital with about 30,000 annual visits at the Olin E. Teague Veterans Affairs Medical Center (OETVMC) emergency department (ED). VACTHCS conducted a 16-month pilot study using 2 commercially available ISDT devices and published the findings.8
The Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022 (MilCon-VA Act) committee report prioritized the reduction of blood culture contamination to < 1% to prevent health risks and harm to veterans undergoing blood testing for the diagnosis of sepsis.9 Because it had been 5 years since OETVMC began using an ISDT in the ED, the ISDT adaptation strategy for mitigating blood culture contamination was revisited per institution policy.
The objective of this quality improvement project was to analyze retrospective data to understand the long-term impact of ISDT use on blood culture contamination rates. We hypothesized that ISDT use would contribute to efforts to maintain OETVMC ED blood culture contamination rate below the national (3.0%) and VACTHCS (2.5%) thresholds. This project assessed the progress for reducing blood culture contamination compared with the pre-ISDT era.8
METHODS
This retrospective analysis compared the blood culture contamination rates 36 months before and after the introduction of the ISDT device at the OETVMC ED. The preimplementation period was from December 2014 through November 2017 (36 months) and the postimplementation period was December 2017 through November 2020 (36 months). Data were collected from the Department of Pathology and Microbiology blood culture records of all adult patients admitted to the hospital through the ED and required blood cultures for suspicion of infection. Protected health information and VA sensitive information were not collected: all data were deidentified. A total of 18,541 blood cultures were collected 36 months preimplementation and 14,865 blood cultures were collected up to 36 months postimplementation. For comparison purposes, a similar dataset was collected from patients’ blood samples drawn by phlebotomists in the laboratory, where there had been no previous issues with overcontamination; no ISDT devices were used in the collection of these samples.
Blood Culture Contamination Variable
Blood cultures were monitored using the BACT/ALERT 3D (bioMérieux) and subsequently BACT/ALERT VIRTUO (bioMérieux), with positive bottles characterized by VITEK MS Matrix Assisted Laser Desorption Ionization Time-of-Flight technology (bioMérieux) and automated susceptibility testing (VITEK 2 [bioMérieux]).10 In an updated review of blood culture contamination, the American Society for Microbiology used the College of American Pathologists' Q-Probes quality improvement studies as a guideline for classifying contamination. A sample was determined to be contaminated if ≥ 1 of the following organisms were found in only 1 bottle in a series of blood culture sets: coagulase-negative staphylococci, Micrococcus species, α-hemolytic viridans group streptococci, Corynebacterium species, Propionibacterium acnes, and Bacillus species.11 The contamination assessment criteria remained unchanged, except for use of an ISDT device in blood culture collection at the ED.
The VACTHCS Infection Prevention Department ensured that the ISDT device was available and that ED nurses were trained annually on its use to collect blood cultures. Monthly reports of contamination were sent to the nursing supervisor for corrective action and retraining. The initial performance improvement project was slated for 16 months but was expanded to a 6-year period of retrospective data to obtain strong correlation.
Statistical Analysis
Contamination rates were recorded monthly from the hospital laboratory information management system for 36 months both before and after ISDT adoption. Statistical analysis was performed using a 2-tailed unpaired t-test to compare monthly contamination rates for the 2 periods with GraphPad Prism version 10.0.0 for Windows.
RESULTS
Prior to 2017, the ED reported contamination rates above the national (3.0%) and OETVMC thresholds (2.5%), with a mean of 4.5% (95% CI, 3.90-4.90).8 After ISDT implementation, the ED showed significant improvement with a reduction to mean 2.6% (95% CI, 2.10-3.20) (P < .001) (Figure 1). Figure 2 shows monthly blood culture contamination rates at the ED from December 2014 through November 2020. Month 36 (November 2017) shows a clear dip in contamination rate when the ISDT was introduced and month 37 to month 44 show remarkably low contamination rates. During this time, the institute experimented with 2 ISDT devices, and closer scrutiny may reveal this period as an outlier due to the monitoring of ISDT application, as previously reported.8


The blood culture contamination rate for samples drawn by the phlebotomists in the laboratory (excluding the ED) was calculated during the same time period (Figure 3). Non-ED contamination rates remained below 2.5% for 69 of 72 months.

DISCUSSION
The blood culture contamination rate in the OETVMC ED dropped following ISDT implementation and continued to show long-term benefits. For the 36-month period following ISDT implementation, the mean contamination rate was 2.6%, which was below the national target threshold of 3.0% and close to the OETVMC target of 2.5%. These results suggest that ISDT can have a positive impact on patient care and laboratory efficiency. Improvements in the blood contamination rates in the ED can have a positive impact on the overall hospital contamination rates.
Blood drawn by phlebotomists in the hospital laboratory infrequently had contamination rates that exceeded the 2.5% target threshold. Because the non-ED contamination rates did not change throughout the comparison period, other factors were likely not involved in the improvements seen in the ED. The decision to implement ISDT exclusively in the ED was based on its historically elevated contamination rate.8 Issues with blood culture contamination in EDs across various hospital systems are well documented and not unique to VACTHCS.12
Contamination in blood cultures can be a significant issue in the hospital. It occurs when microorganisms from the skin or environment enter the blood culture sample during collection. Moreover, it can contribute to antibiotic resistance when patients are prescribed inappropriate antibiotics. It is also important to ensure HCPs are well-trained and consistently follow standardized protocols and understand the implications of false-positive results.13
ISDT helps reduce false-positive results and is a significant advancement in the field of blood culture collection.8,14 By discarding the initial blood, it ensures that only the true bloodstream sample is cultured, leading to more accurate results.15 It also may minimize the risk of contamination-related delays in diagnosis and treatment and benefits patients and health care institutions by potentially reducing hospital stays, unnecessary antibiotic use, and health care costs.
One of the ISDT device manufacturers estimated the financial impact on OETVMC based on the pilot project.8 While this study did not calculate the direct and indirect cost savings associated with this process improvement, the manufacturer’s website suggests that VACTHCS could annually save about $486,000.16 Furthermore, implementation of ISDT may improve laboratory efficiency, as they reduce the workload associated with identifying and reporting false-positive cultures. 6 ISDT devices represent a valuable tool in the efforts to reduce blood culture contamination and its wide-ranging implications in clinical settings. While ISDT alone will not be sufficient in achieving a lower threshold (< 1%) of blood culture contamination, it can be part of a multiprong effort that optimizes best practices in the collection, handling, and management of blood cultures.
Continuous quality improvement efforts and monitoring of blood culture contamination rates can help health care institutions identify problem areas and implement necessary changes. Addressing blood culture contamination can improve patient care, reduce costs, and address antibiotic resistance.
Limitations
This study was limited by its study design, which did not use a side-by-side comparison of blood cultures from groups with and without ISDT. All blood cultures from patients in the region were processed at OETVMC, which may not be representative of non-VA EDs. Part of this study took place during the COVID-19 pandemic, which may have skewed data. Additionally, hospital data were collected from a veteran population in Central Texas, and the lack of demographic diversity may not be generalizable to the greater population.
CONCLUSIONS
The findings of this study suggest ISDT may be effective in reducing blood culture contamination rates in the high-risk ED environment, which aligns with previous research. 5,14 The ISDT may help reduce blood culture contamination rates, improving the quality of patient care and reducing health care costs. MilCon-VA mandated that all VA facilities have blood culture contamination as a metric with a goal of blood culture contamination rates < 1%.8 However, achieving this goal remains a challenge. Further research and continuous quality improvement efforts are necessary to achieve it. Consistently achieving a contamination threshold of < 1% may require minimizing human error. An automated robotic venipuncture device, as recently designed and reported, may be necessary to reduce human error in blood draw and contamination.16
Blood cultures provide crucial evidence for diagnostic medicine, specifically aimed at identifying the presence of microbial infections in the bloodstream. Blood culturing is instrumental in diagnosing conditions such as sepsis, bacteremia, or fungemia, where the identification of the causative agent is necessary for targeted and effective treatment.1
The process involves aseptically drawing blood into sterile culture bottles, minimizing the risk of contamination with well-established guidelines. These culture bottles contain specific growth media that support the replication of microorganisms if they are present. Once the blood specimen is collected, it incubates, allowing any potential pathogens to grow. Subsequent analysis and identification of these microorganisms enable health care professionals (HCPs) to prescribe appropriate antimicrobial therapies to treat specific infections, contributing to more effective and targeted patient care.2
The reliability of blood culture results depends on minimizing contamination risk, a challenge inherent in the procedure. Contamination can lead to false-positive results, potentially misguiding treatment.3 HCPs must adhere to strict aseptic techniques during blood draws, ensuring proper skin preparation with antiseptic solutions. The use of sterile equipment and avoiding prolonged tourniquet application helps maintain the integrity of the blood specimen. Timely inoculation of blood into culture bottles and careful handling are essential to mitigate contamination risk.2 Regular training and reinforcement of proper techniques is important to uphold the accuracy of blood culture results and enhance the reliability of diagnoses and treatment decisions.3 Despite diligent contamination prevention efforts, health care systems struggle to maintain contamination rates below the 3.0% national benchmark set by the Clinical & Laboratory Standards Institute (CLSI).4
Blood culture contamination is a critical concern in clinical practice; it can lead to misdiagnosis, prolonged hospital stays, unnecessary antibiotic use, and increased health care costs.5 Monitoring blood culture contamination is integral to patient safety, avoiding inappropriate and potentially harmful treatment, providing efficient care, contributing to antibiotic stewardship, supporting cost efficiency, and maintaining quality assurance and clinical research practices for public health.6
The initial specimen diversion technique (ISDT) recently emerged as a potential strategy to reduce blood culture contamination rates. This technique involves diverting a small portion of the initial blood plus the skin plug from the hollow needle away from the primary collection site before filling the culture bottles. This process minimizes skin surface contaminants, providing a cleaner blood specimen for culturing.7
The ISDT was introduced as a result of historically elevated contamination rates.8 Despite implementing various mitigation methods, the US Department of Veterans Affairs (VA) Central Texas Healthcare System (VACTHCS) has struggled to meet the national benchmark of maintaining blood culture contamination < 3.0%. The VACTHCS is a 146-bed teaching hospital with about 30,000 annual visits at the Olin E. Teague Veterans Affairs Medical Center (OETVMC) emergency department (ED). VACTHCS conducted a 16-month pilot study using 2 commercially available ISDT devices and published the findings.8
The Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022 (MilCon-VA Act) committee report prioritized the reduction of blood culture contamination to < 1% to prevent health risks and harm to veterans undergoing blood testing for the diagnosis of sepsis.9 Because it had been 5 years since OETVMC began using an ISDT in the ED, the ISDT adaptation strategy for mitigating blood culture contamination was revisited per institution policy.
The objective of this quality improvement project was to analyze retrospective data to understand the long-term impact of ISDT use on blood culture contamination rates. We hypothesized that ISDT use would contribute to efforts to maintain OETVMC ED blood culture contamination rate below the national (3.0%) and VACTHCS (2.5%) thresholds. This project assessed the progress for reducing blood culture contamination compared with the pre-ISDT era.8
METHODS
This retrospective analysis compared the blood culture contamination rates 36 months before and after the introduction of the ISDT device at the OETVMC ED. The preimplementation period was from December 2014 through November 2017 (36 months) and the postimplementation period was December 2017 through November 2020 (36 months). Data were collected from the Department of Pathology and Microbiology blood culture records of all adult patients admitted to the hospital through the ED and required blood cultures for suspicion of infection. Protected health information and VA sensitive information were not collected: all data were deidentified. A total of 18,541 blood cultures were collected 36 months preimplementation and 14,865 blood cultures were collected up to 36 months postimplementation. For comparison purposes, a similar dataset was collected from patients’ blood samples drawn by phlebotomists in the laboratory, where there had been no previous issues with overcontamination; no ISDT devices were used in the collection of these samples.
Blood Culture Contamination Variable
Blood cultures were monitored using the BACT/ALERT 3D (bioMérieux) and subsequently BACT/ALERT VIRTUO (bioMérieux), with positive bottles characterized by VITEK MS Matrix Assisted Laser Desorption Ionization Time-of-Flight technology (bioMérieux) and automated susceptibility testing (VITEK 2 [bioMérieux]).10 In an updated review of blood culture contamination, the American Society for Microbiology used the College of American Pathologists' Q-Probes quality improvement studies as a guideline for classifying contamination. A sample was determined to be contaminated if ≥ 1 of the following organisms were found in only 1 bottle in a series of blood culture sets: coagulase-negative staphylococci, Micrococcus species, α-hemolytic viridans group streptococci, Corynebacterium species, Propionibacterium acnes, and Bacillus species.11 The contamination assessment criteria remained unchanged, except for use of an ISDT device in blood culture collection at the ED.
The VACTHCS Infection Prevention Department ensured that the ISDT device was available and that ED nurses were trained annually on its use to collect blood cultures. Monthly reports of contamination were sent to the nursing supervisor for corrective action and retraining. The initial performance improvement project was slated for 16 months but was expanded to a 6-year period of retrospective data to obtain strong correlation.
Statistical Analysis
Contamination rates were recorded monthly from the hospital laboratory information management system for 36 months both before and after ISDT adoption. Statistical analysis was performed using a 2-tailed unpaired t-test to compare monthly contamination rates for the 2 periods with GraphPad Prism version 10.0.0 for Windows.
RESULTS
Prior to 2017, the ED reported contamination rates above the national (3.0%) and OETVMC thresholds (2.5%), with a mean of 4.5% (95% CI, 3.90-4.90).8 After ISDT implementation, the ED showed significant improvement with a reduction to mean 2.6% (95% CI, 2.10-3.20) (P < .001) (Figure 1). Figure 2 shows monthly blood culture contamination rates at the ED from December 2014 through November 2020. Month 36 (November 2017) shows a clear dip in contamination rate when the ISDT was introduced and month 37 to month 44 show remarkably low contamination rates. During this time, the institute experimented with 2 ISDT devices, and closer scrutiny may reveal this period as an outlier due to the monitoring of ISDT application, as previously reported.8


The blood culture contamination rate for samples drawn by the phlebotomists in the laboratory (excluding the ED) was calculated during the same time period (Figure 3). Non-ED contamination rates remained below 2.5% for 69 of 72 months.

DISCUSSION
The blood culture contamination rate in the OETVMC ED dropped following ISDT implementation and continued to show long-term benefits. For the 36-month period following ISDT implementation, the mean contamination rate was 2.6%, which was below the national target threshold of 3.0% and close to the OETVMC target of 2.5%. These results suggest that ISDT can have a positive impact on patient care and laboratory efficiency. Improvements in the blood contamination rates in the ED can have a positive impact on the overall hospital contamination rates.
Blood drawn by phlebotomists in the hospital laboratory infrequently had contamination rates that exceeded the 2.5% target threshold. Because the non-ED contamination rates did not change throughout the comparison period, other factors were likely not involved in the improvements seen in the ED. The decision to implement ISDT exclusively in the ED was based on its historically elevated contamination rate.8 Issues with blood culture contamination in EDs across various hospital systems are well documented and not unique to VACTHCS.12
Contamination in blood cultures can be a significant issue in the hospital. It occurs when microorganisms from the skin or environment enter the blood culture sample during collection. Moreover, it can contribute to antibiotic resistance when patients are prescribed inappropriate antibiotics. It is also important to ensure HCPs are well-trained and consistently follow standardized protocols and understand the implications of false-positive results.13
ISDT helps reduce false-positive results and is a significant advancement in the field of blood culture collection.8,14 By discarding the initial blood, it ensures that only the true bloodstream sample is cultured, leading to more accurate results.15 It also may minimize the risk of contamination-related delays in diagnosis and treatment and benefits patients and health care institutions by potentially reducing hospital stays, unnecessary antibiotic use, and health care costs.
One of the ISDT device manufacturers estimated the financial impact on OETVMC based on the pilot project.8 While this study did not calculate the direct and indirect cost savings associated with this process improvement, the manufacturer’s website suggests that VACTHCS could annually save about $486,000.16 Furthermore, implementation of ISDT may improve laboratory efficiency, as they reduce the workload associated with identifying and reporting false-positive cultures. 6 ISDT devices represent a valuable tool in the efforts to reduce blood culture contamination and its wide-ranging implications in clinical settings. While ISDT alone will not be sufficient in achieving a lower threshold (< 1%) of blood culture contamination, it can be part of a multiprong effort that optimizes best practices in the collection, handling, and management of blood cultures.
Continuous quality improvement efforts and monitoring of blood culture contamination rates can help health care institutions identify problem areas and implement necessary changes. Addressing blood culture contamination can improve patient care, reduce costs, and address antibiotic resistance.
Limitations
This study was limited by its study design, which did not use a side-by-side comparison of blood cultures from groups with and without ISDT. All blood cultures from patients in the region were processed at OETVMC, which may not be representative of non-VA EDs. Part of this study took place during the COVID-19 pandemic, which may have skewed data. Additionally, hospital data were collected from a veteran population in Central Texas, and the lack of demographic diversity may not be generalizable to the greater population.
CONCLUSIONS
The findings of this study suggest ISDT may be effective in reducing blood culture contamination rates in the high-risk ED environment, which aligns with previous research. 5,14 The ISDT may help reduce blood culture contamination rates, improving the quality of patient care and reducing health care costs. MilCon-VA mandated that all VA facilities have blood culture contamination as a metric with a goal of blood culture contamination rates < 1%.8 However, achieving this goal remains a challenge. Further research and continuous quality improvement efforts are necessary to achieve it. Consistently achieving a contamination threshold of < 1% may require minimizing human error. An automated robotic venipuncture device, as recently designed and reported, may be necessary to reduce human error in blood draw and contamination.16
- Chela HK, Vasudevan A, Rojas-Moreno C, Naqvi SH. Approach to positive blood cultures in the hospitalized patient: a review. Mo Med. 2019;116(4):313-317.
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi:10.3389/fmicb.2016.00697
- Doern GV, Carroll KC, Diekema DJ, et al. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33:e00009-19. doi:10.1128/CMR.00009-19
- Wilson ML, Kirn Jr TJ, Antonara S, et al. Clinical and Laboratory Standards Institute Guideline M47—Principles and Procedures for Blood Cultures. Clinical and Laboratory Standards Institute. April 22, 2022. Accessed May 21, 2025. https://clsi.org/shop/standards/m47/
- Hancock JA, Campbell S, Jones MM, Wang-Rodriguez J, VHA Microbiology SME Workgroup, Klutts JS. Development and validation of a standardized blood culture contamination definition and metric dashboard for a large health care system. Am J Clin Pathol. 2023;160(3):255-260. doi:10.1093/ajcp/aqad044
- Shinozaki T, Deane RS, Mazuzan JE Jr, Hamel AJ, Hazelton D. Bacterial contamination of arterial lines. A prospective study. JAMA. 1983;249(2):223-225.
- Al Mohajer M, Lasco T. The impact of initial specimen diversion systems on blood culture contamination. Open Forum Infect Dis. 2023;10:ofad182. doi:10.1093/ofid/ofad182
- Arenas M, Boseman GM, Coppin JD, Lukey J, Jinadatha C, Navarathna DH. Asynchronous testing of 2 specimen-diversion devices to reduce blood culture contamination: a single-site product supply quality improvement project. J Emerg Nurs. 2021;47(2):256-264. e6. doi:10.1016/j.jen.2020.11.008
- Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022, HR 4355, 117th Cong (2021-2022). Accessed May 12, 2025. https://www.congress.gov/bill/117th-congress/house-bill/4355?
- Altun O, Almuhayawi M, Lüthje P, Taha R, Ullberg M, Özenci V. Controlled evaluation of the New BacT/ Alert Virtuo blood culture system for detection and time to detection of bacteria and yeasts. J Clin Microbiol. 2016;54(4):1148-1151. doi:10.1128/JCM.03362-15
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. doi:10.1128/CMR.00062-05
- Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47(4):1021-1024. doi:10.1128/JCM.02162-08
- Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central lineassociated bloodstream infections. Am J Infect Control. 2015;43(11):1222-1237. doi:10.1016/j.ajic.2015.06.030
- Callado GY, Lin V, Thottacherry E, et al. Diagnostic stewardship: a systematic review and meta-analysis of blood collection diversion devices used to reduce blood culture contamination and improve the accuracy of diagnosis in clinical settings. Open Forum Infect Dis. 2023;10(9):ofad433. doi:10.1093/ofid/ofad433
- Patton RG, Schmitt T. Innovation for reducing blood culture contamination: initial specimen diversion technique. J Clin Microbiol. 2010;48:4501-4503. doi:10.1128/JCM.00910-10
- Kurin. Clinical evidence: published Kurin studies. 2024. Accessed May 12, 2025. https://www.kurin.com/studies
- Leipheimer JM, Balter ML, Chen AI, et al. First-in-human evaluation of a hand-held automated venipuncture device for rapid venous blood draws. Technology (Singap World Sci). 2019;7(3-4):98-107. doi:10.1142/S2339547819500067?
- Chela HK, Vasudevan A, Rojas-Moreno C, Naqvi SH. Approach to positive blood cultures in the hospitalized patient: a review. Mo Med. 2019;116(4):313-317.
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi:10.3389/fmicb.2016.00697
- Doern GV, Carroll KC, Diekema DJ, et al. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33:e00009-19. doi:10.1128/CMR.00009-19
- Wilson ML, Kirn Jr TJ, Antonara S, et al. Clinical and Laboratory Standards Institute Guideline M47—Principles and Procedures for Blood Cultures. Clinical and Laboratory Standards Institute. April 22, 2022. Accessed May 21, 2025. https://clsi.org/shop/standards/m47/
- Hancock JA, Campbell S, Jones MM, Wang-Rodriguez J, VHA Microbiology SME Workgroup, Klutts JS. Development and validation of a standardized blood culture contamination definition and metric dashboard for a large health care system. Am J Clin Pathol. 2023;160(3):255-260. doi:10.1093/ajcp/aqad044
- Shinozaki T, Deane RS, Mazuzan JE Jr, Hamel AJ, Hazelton D. Bacterial contamination of arterial lines. A prospective study. JAMA. 1983;249(2):223-225.
- Al Mohajer M, Lasco T. The impact of initial specimen diversion systems on blood culture contamination. Open Forum Infect Dis. 2023;10:ofad182. doi:10.1093/ofid/ofad182
- Arenas M, Boseman GM, Coppin JD, Lukey J, Jinadatha C, Navarathna DH. Asynchronous testing of 2 specimen-diversion devices to reduce blood culture contamination: a single-site product supply quality improvement project. J Emerg Nurs. 2021;47(2):256-264. e6. doi:10.1016/j.jen.2020.11.008
- Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022, HR 4355, 117th Cong (2021-2022). Accessed May 12, 2025. https://www.congress.gov/bill/117th-congress/house-bill/4355?
- Altun O, Almuhayawi M, Lüthje P, Taha R, Ullberg M, Özenci V. Controlled evaluation of the New BacT/ Alert Virtuo blood culture system for detection and time to detection of bacteria and yeasts. J Clin Microbiol. 2016;54(4):1148-1151. doi:10.1128/JCM.03362-15
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. doi:10.1128/CMR.00062-05
- Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47(4):1021-1024. doi:10.1128/JCM.02162-08
- Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central lineassociated bloodstream infections. Am J Infect Control. 2015;43(11):1222-1237. doi:10.1016/j.ajic.2015.06.030
- Callado GY, Lin V, Thottacherry E, et al. Diagnostic stewardship: a systematic review and meta-analysis of blood collection diversion devices used to reduce blood culture contamination and improve the accuracy of diagnosis in clinical settings. Open Forum Infect Dis. 2023;10(9):ofad433. doi:10.1093/ofid/ofad433
- Patton RG, Schmitt T. Innovation for reducing blood culture contamination: initial specimen diversion technique. J Clin Microbiol. 2010;48:4501-4503. doi:10.1128/JCM.00910-10
- Kurin. Clinical evidence: published Kurin studies. 2024. Accessed May 12, 2025. https://www.kurin.com/studies
- Leipheimer JM, Balter ML, Chen AI, et al. First-in-human evaluation of a hand-held automated venipuncture device for rapid venous blood draws. Technology (Singap World Sci). 2019;7(3-4):98-107. doi:10.1142/S2339547819500067?
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates