User login
The toxic zeitgeist of hyper-partisanship: A psychiatric perspective
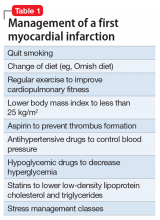
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
The puzzling relationship between cholesterol and psychopathology
Cholesterol generally is regarded as a cardiovascular risk factor when elevated. However, numerous studies suggest that cholesterol levels—both high and low—may be associated with various psychiatric brai
The relationship between cholesterol and mental illness is fascinating, complex, and perplexing. Whether elevated or reduced, cholesterol’s effects can be deleterious or salutary, but the literature is riddled with conflicting reports. Physicians should measure their patients’ serum cholesterol levels not only to assess cardiovascular risk, but because cholesterol can be associated with certain neuropsychiatric disorders or may predict the lack of response to psychopharmacotherapy.2
The fact that lowering total cholesterol levels in people with hypercholesterolemia reduces the risk of coronary heart disease is indisputable. Large-scale cardiology clinical trials have shown a significant reduction in mortality from heart disease or stroke with cholesterol-lowering drugs (statins). However, the same trials found an uptick in “unnatural deaths,” mostly suicide or homicide.3 Those findings triggered numerous intriguing reports of the association between cholesterol levels and psychopathology.
Consider the following:
- Low cholesterol levels have been associated with depression, antisocial personality disorder, borderline personality disorder, and dissociative disorder.4
- High cholesterol levels have been associated with schizophrenia, obsessive-compulsive disorder, panic disorder, generalized anxiety disorder, and posttraumatic stress disorder.4
- Some studies suggest that high cholesterol levels are associated with better mental health, mental processing speed, social skills, responsibility, self-control, and self-awareness.5
- In the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia study, better cognitive scores were found in patients with higher fasting cholesterol and triglyceride levels (H.A.N., unpublished data, 2017).
The brain is only 2% of body weight, but it contains 25% of the body’s cholesterol.6 Cholesterol is important for brain function and neurotransmission because neuroactive steroids (NASs) are synthesized from cholesterol and they modulate brain processes and interact with γ-aminobutyric acid, N-methyl-
Interestingly, both extremes in cholesterol levels represent a high risk for premature mortality.10 Hypercholesterolemia leads to early death from coronary artery disease. Studies that evaluated statins to lower cholesterol found increased mortality from suicide, accidents, and violence.11 Even without statin treatment, among persons with naturally low cholesterol, there is a significant increase in mortality from non-medical causes.12 However, some studies did not find an association between hypocholesterolemia and suicide.13,14
There also is some evidence that elevated cholesterol may play a role in dementia.15 Reducing cholesterol with statins decreases beta-amyloid in mice, while the opposite occurs with elevated cholesterol.2 Another possible mechanism by which high cholesterol worsens dementia is that neurodegeneration in Alzheimer’s disease (AD) breaks down neuronal cell membranes, which releases the neurotoxic metabolite of cholesterol (24-hydroxycholesterol), which leads to further neurodegeneration.16 Statins may decrease the production of 24-hydroxycholesterol in AD patients and slow down neurodegeneration.16
A large study of 4,444 consecutive patients in Taiwan found that those with low total cholesterol (<160 mg/dL) had higher scores of anxiety, phobia, psychoticism, and aggressive hostility.17 In the same study, women with low high-density lipoprotein cholesterol (<35 mg/dL) had significantly higher scores for depression, phobia, anxiety, interpersonal sensitivity, somatization, and aggressive hostility.17
Not surprisingly, low cholesterol has been proposed as a biomarker for mood dysregulation, depression, and suicidality,18 as well as a predictor of the depression severity and increased suicide risk.19 Clinical recovery in depression may be accompanied by a significant increase of total cholesterol20 but, interestingly, a decrease in cholesterol levels after treatment of mania. High cholesterol was reported to predict poorer response to selective serotonin reuptake inhibitors, and total cholesterol levels >200 mg/dL were associated with lack of response to fluoxetine and nortriptyline.2 Interestingly, clozapine, which elevates lipids, exerts a strong anti-suicide effect in schizophrenia and schizoaffective disorder, but that may not be the main reason for its efficacy in preventing suicide in patients with psychosis.
Cholesterol is an important lipid for brain function. At lower levels, it appears to be associated with depression, suicide, violence, anxiety, schizophrenia, and severe personality disorders (including antisocial personality disorder and borderline personality disorder). However, at high levels, it may improve cognition in schizophrenia and ameliorate the pace of AD and neurodegeneration. Psychiatrists should monitor patients for hypercholesterolemia and hypocholesterolemia, both of which are common among psychiatric patients. High levels may be genetic or the result of weight gain, hypercortisolemia, diabetes, or immune or inflammatory processes. Similarly, low levels may be genetic or secondary to statin therapy.
The bottom line: As psychiatric physicians, we should protect both the hearts and brains of our patients.
1. Hallahan B, Garland MR. Essential fatty acids and mental health. British J Psychiatry. 2005;186(4):275-277.
2. Papakostas GI, Ongür D, Iosifescu DV, et al. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004;14(2):135-142.
3. Muldoon MF, Manuck SB, Matthews KA, et al. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(647):309-314.
4. Jakovljevic
5. Rogers PJ. A healthy body, a healthy mind: long-term impact of diet on mood and cognitive function. Pro Nutr Soc. 2001;60(1):135-143.
6. Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493-508.
7. Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442.
8. Borroni MV, Vallés AS, Barrantes FJ. The lipid habitats of neurotransmitter receptors in the brain. Biochim Biophys Acta. 2016;1858(1):2662-2670.
9. Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60(6):1158-1171.
10. Graham I, Atar D, Borch-Johnsen K, et al; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of none societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):S1-S113.
11. Almeida-Montes LG, Valles-Sanchez V, Moreno-Aguilar J, et al. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J Psychiatry Neurosci. 2000;25(4):371-377.
12. Ryman A. Cholesterol, violent death, and mental disorder. BMJ. 1994;309(69525):421-422.
13. Wardle J. Cholesterol and psychological well-being. J Psychosom Res. 1995;39(5):549-562.
14. Irribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation. 1995;92(9):2396-2403.
15. Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211-223.
16. Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003;44(8):1423-1430.
17. Chen CC, Lu FH, Wu JS, et al. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2003;102(2):153-162.
18. Mössmer R, Mikova O, Koutsilieri E, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141-174.
19. Papakostas GI, Petersen T, Sonawalla SB, et al. Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003;47(3):146-151.
20. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
Cholesterol generally is regarded as a cardiovascular risk factor when elevated. However, numerous studies suggest that cholesterol levels—both high and low—may be associated with various psychiatric brai
The relationship between cholesterol and mental illness is fascinating, complex, and perplexing. Whether elevated or reduced, cholesterol’s effects can be deleterious or salutary, but the literature is riddled with conflicting reports. Physicians should measure their patients’ serum cholesterol levels not only to assess cardiovascular risk, but because cholesterol can be associated with certain neuropsychiatric disorders or may predict the lack of response to psychopharmacotherapy.2
The fact that lowering total cholesterol levels in people with hypercholesterolemia reduces the risk of coronary heart disease is indisputable. Large-scale cardiology clinical trials have shown a significant reduction in mortality from heart disease or stroke with cholesterol-lowering drugs (statins). However, the same trials found an uptick in “unnatural deaths,” mostly suicide or homicide.3 Those findings triggered numerous intriguing reports of the association between cholesterol levels and psychopathology.
Consider the following:
- Low cholesterol levels have been associated with depression, antisocial personality disorder, borderline personality disorder, and dissociative disorder.4
- High cholesterol levels have been associated with schizophrenia, obsessive-compulsive disorder, panic disorder, generalized anxiety disorder, and posttraumatic stress disorder.4
- Some studies suggest that high cholesterol levels are associated with better mental health, mental processing speed, social skills, responsibility, self-control, and self-awareness.5
- In the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia study, better cognitive scores were found in patients with higher fasting cholesterol and triglyceride levels (H.A.N., unpublished data, 2017).
The brain is only 2% of body weight, but it contains 25% of the body’s cholesterol.6 Cholesterol is important for brain function and neurotransmission because neuroactive steroids (NASs) are synthesized from cholesterol and they modulate brain processes and interact with γ-aminobutyric acid, N-methyl-
Interestingly, both extremes in cholesterol levels represent a high risk for premature mortality.10 Hypercholesterolemia leads to early death from coronary artery disease. Studies that evaluated statins to lower cholesterol found increased mortality from suicide, accidents, and violence.11 Even without statin treatment, among persons with naturally low cholesterol, there is a significant increase in mortality from non-medical causes.12 However, some studies did not find an association between hypocholesterolemia and suicide.13,14
There also is some evidence that elevated cholesterol may play a role in dementia.15 Reducing cholesterol with statins decreases beta-amyloid in mice, while the opposite occurs with elevated cholesterol.2 Another possible mechanism by which high cholesterol worsens dementia is that neurodegeneration in Alzheimer’s disease (AD) breaks down neuronal cell membranes, which releases the neurotoxic metabolite of cholesterol (24-hydroxycholesterol), which leads to further neurodegeneration.16 Statins may decrease the production of 24-hydroxycholesterol in AD patients and slow down neurodegeneration.16
A large study of 4,444 consecutive patients in Taiwan found that those with low total cholesterol (<160 mg/dL) had higher scores of anxiety, phobia, psychoticism, and aggressive hostility.17 In the same study, women with low high-density lipoprotein cholesterol (<35 mg/dL) had significantly higher scores for depression, phobia, anxiety, interpersonal sensitivity, somatization, and aggressive hostility.17
Not surprisingly, low cholesterol has been proposed as a biomarker for mood dysregulation, depression, and suicidality,18 as well as a predictor of the depression severity and increased suicide risk.19 Clinical recovery in depression may be accompanied by a significant increase of total cholesterol20 but, interestingly, a decrease in cholesterol levels after treatment of mania. High cholesterol was reported to predict poorer response to selective serotonin reuptake inhibitors, and total cholesterol levels >200 mg/dL were associated with lack of response to fluoxetine and nortriptyline.2 Interestingly, clozapine, which elevates lipids, exerts a strong anti-suicide effect in schizophrenia and schizoaffective disorder, but that may not be the main reason for its efficacy in preventing suicide in patients with psychosis.
Cholesterol is an important lipid for brain function. At lower levels, it appears to be associated with depression, suicide, violence, anxiety, schizophrenia, and severe personality disorders (including antisocial personality disorder and borderline personality disorder). However, at high levels, it may improve cognition in schizophrenia and ameliorate the pace of AD and neurodegeneration. Psychiatrists should monitor patients for hypercholesterolemia and hypocholesterolemia, both of which are common among psychiatric patients. High levels may be genetic or the result of weight gain, hypercortisolemia, diabetes, or immune or inflammatory processes. Similarly, low levels may be genetic or secondary to statin therapy.
The bottom line: As psychiatric physicians, we should protect both the hearts and brains of our patients.
Cholesterol generally is regarded as a cardiovascular risk factor when elevated. However, numerous studies suggest that cholesterol levels—both high and low—may be associated with various psychiatric brai
The relationship between cholesterol and mental illness is fascinating, complex, and perplexing. Whether elevated or reduced, cholesterol’s effects can be deleterious or salutary, but the literature is riddled with conflicting reports. Physicians should measure their patients’ serum cholesterol levels not only to assess cardiovascular risk, but because cholesterol can be associated with certain neuropsychiatric disorders or may predict the lack of response to psychopharmacotherapy.2
The fact that lowering total cholesterol levels in people with hypercholesterolemia reduces the risk of coronary heart disease is indisputable. Large-scale cardiology clinical trials have shown a significant reduction in mortality from heart disease or stroke with cholesterol-lowering drugs (statins). However, the same trials found an uptick in “unnatural deaths,” mostly suicide or homicide.3 Those findings triggered numerous intriguing reports of the association between cholesterol levels and psychopathology.
Consider the following:
- Low cholesterol levels have been associated with depression, antisocial personality disorder, borderline personality disorder, and dissociative disorder.4
- High cholesterol levels have been associated with schizophrenia, obsessive-compulsive disorder, panic disorder, generalized anxiety disorder, and posttraumatic stress disorder.4
- Some studies suggest that high cholesterol levels are associated with better mental health, mental processing speed, social skills, responsibility, self-control, and self-awareness.5
- In the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia study, better cognitive scores were found in patients with higher fasting cholesterol and triglyceride levels (H.A.N., unpublished data, 2017).
The brain is only 2% of body weight, but it contains 25% of the body’s cholesterol.6 Cholesterol is important for brain function and neurotransmission because neuroactive steroids (NASs) are synthesized from cholesterol and they modulate brain processes and interact with γ-aminobutyric acid, N-methyl-
Interestingly, both extremes in cholesterol levels represent a high risk for premature mortality.10 Hypercholesterolemia leads to early death from coronary artery disease. Studies that evaluated statins to lower cholesterol found increased mortality from suicide, accidents, and violence.11 Even without statin treatment, among persons with naturally low cholesterol, there is a significant increase in mortality from non-medical causes.12 However, some studies did not find an association between hypocholesterolemia and suicide.13,14
There also is some evidence that elevated cholesterol may play a role in dementia.15 Reducing cholesterol with statins decreases beta-amyloid in mice, while the opposite occurs with elevated cholesterol.2 Another possible mechanism by which high cholesterol worsens dementia is that neurodegeneration in Alzheimer’s disease (AD) breaks down neuronal cell membranes, which releases the neurotoxic metabolite of cholesterol (24-hydroxycholesterol), which leads to further neurodegeneration.16 Statins may decrease the production of 24-hydroxycholesterol in AD patients and slow down neurodegeneration.16
A large study of 4,444 consecutive patients in Taiwan found that those with low total cholesterol (<160 mg/dL) had higher scores of anxiety, phobia, psychoticism, and aggressive hostility.17 In the same study, women with low high-density lipoprotein cholesterol (<35 mg/dL) had significantly higher scores for depression, phobia, anxiety, interpersonal sensitivity, somatization, and aggressive hostility.17
Not surprisingly, low cholesterol has been proposed as a biomarker for mood dysregulation, depression, and suicidality,18 as well as a predictor of the depression severity and increased suicide risk.19 Clinical recovery in depression may be accompanied by a significant increase of total cholesterol20 but, interestingly, a decrease in cholesterol levels after treatment of mania. High cholesterol was reported to predict poorer response to selective serotonin reuptake inhibitors, and total cholesterol levels >200 mg/dL were associated with lack of response to fluoxetine and nortriptyline.2 Interestingly, clozapine, which elevates lipids, exerts a strong anti-suicide effect in schizophrenia and schizoaffective disorder, but that may not be the main reason for its efficacy in preventing suicide in patients with psychosis.
Cholesterol is an important lipid for brain function. At lower levels, it appears to be associated with depression, suicide, violence, anxiety, schizophrenia, and severe personality disorders (including antisocial personality disorder and borderline personality disorder). However, at high levels, it may improve cognition in schizophrenia and ameliorate the pace of AD and neurodegeneration. Psychiatrists should monitor patients for hypercholesterolemia and hypocholesterolemia, both of which are common among psychiatric patients. High levels may be genetic or the result of weight gain, hypercortisolemia, diabetes, or immune or inflammatory processes. Similarly, low levels may be genetic or secondary to statin therapy.
The bottom line: As psychiatric physicians, we should protect both the hearts and brains of our patients.
1. Hallahan B, Garland MR. Essential fatty acids and mental health. British J Psychiatry. 2005;186(4):275-277.
2. Papakostas GI, Ongür D, Iosifescu DV, et al. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004;14(2):135-142.
3. Muldoon MF, Manuck SB, Matthews KA, et al. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(647):309-314.
4. Jakovljevic
5. Rogers PJ. A healthy body, a healthy mind: long-term impact of diet on mood and cognitive function. Pro Nutr Soc. 2001;60(1):135-143.
6. Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493-508.
7. Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442.
8. Borroni MV, Vallés AS, Barrantes FJ. The lipid habitats of neurotransmitter receptors in the brain. Biochim Biophys Acta. 2016;1858(1):2662-2670.
9. Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60(6):1158-1171.
10. Graham I, Atar D, Borch-Johnsen K, et al; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of none societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):S1-S113.
11. Almeida-Montes LG, Valles-Sanchez V, Moreno-Aguilar J, et al. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J Psychiatry Neurosci. 2000;25(4):371-377.
12. Ryman A. Cholesterol, violent death, and mental disorder. BMJ. 1994;309(69525):421-422.
13. Wardle J. Cholesterol and psychological well-being. J Psychosom Res. 1995;39(5):549-562.
14. Irribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation. 1995;92(9):2396-2403.
15. Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211-223.
16. Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003;44(8):1423-1430.
17. Chen CC, Lu FH, Wu JS, et al. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2003;102(2):153-162.
18. Mössmer R, Mikova O, Koutsilieri E, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141-174.
19. Papakostas GI, Petersen T, Sonawalla SB, et al. Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003;47(3):146-151.
20. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
1. Hallahan B, Garland MR. Essential fatty acids and mental health. British J Psychiatry. 2005;186(4):275-277.
2. Papakostas GI, Ongür D, Iosifescu DV, et al. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004;14(2):135-142.
3. Muldoon MF, Manuck SB, Matthews KA, et al. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(647):309-314.
4. Jakovljevic
5. Rogers PJ. A healthy body, a healthy mind: long-term impact of diet on mood and cognitive function. Pro Nutr Soc. 2001;60(1):135-143.
6. Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493-508.
7. Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442.
8. Borroni MV, Vallés AS, Barrantes FJ. The lipid habitats of neurotransmitter receptors in the brain. Biochim Biophys Acta. 2016;1858(1):2662-2670.
9. Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60(6):1158-1171.
10. Graham I, Atar D, Borch-Johnsen K, et al; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of none societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):S1-S113.
11. Almeida-Montes LG, Valles-Sanchez V, Moreno-Aguilar J, et al. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J Psychiatry Neurosci. 2000;25(4):371-377.
12. Ryman A. Cholesterol, violent death, and mental disorder. BMJ. 1994;309(69525):421-422.
13. Wardle J. Cholesterol and psychological well-being. J Psychosom Res. 1995;39(5):549-562.
14. Irribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation. 1995;92(9):2396-2403.
15. Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211-223.
16. Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003;44(8):1423-1430.
17. Chen CC, Lu FH, Wu JS, et al. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2003;102(2):153-162.
18. Mössmer R, Mikova O, Koutsilieri E, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141-174.
19. Papakostas GI, Petersen T, Sonawalla SB, et al. Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003;47(3):146-151.
20. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
The dawn of precision psychiatry
Imagine being able to precisely select the medication with the optimal efficacy, safety, and tolerability at the outset of treatment for every psychiatric patient who needs pharmacotherapy. Imagine how much the patient would appreciate not receiving a series of drugs and suffering multiple adverse effects and unremitting symptoms until the “right medication” is identified. Imagine how gratifying it would be for you as a psychiatrist to watch every one of your patients improve rapidly with minimal complaints or adverse effects.
Precision psychiatry is the indispensable vehicle to achieve personalized medicine for psychiatric patients. Precision psychiatry is a cherished goal, but it remains an aspirational objective. Other medical specialties, especially oncology and cardiology, have made remarkable strides in precision medicine, but the journey to precision psychiatry is still in its early stages. Yet there is every reason to believe that we are making progress toward that cherished goal.
To implement precision psychiatry, we must be able to identify the biosignature of each patient’s psychiatric brain disorder. But there is a formidable challenge to overcome: the complex, extensive heterogeneity of psychiatric disorders, which requires intense and inspired neurobiology research. So, while clinicians go on with the mundane trial-and-error approach of contemporary psychopharmacology, psychiatric neuroscientists are diligently deconstructing major psychiatric disorders into specific biotypes with unique biosignatures that will one day guide accurate and prompt clinical management.
Psychiatric practitioners may be too busy to keep tabs on the progress being made in identifying various biomarkers that are the key ingredients to decoding the biosignature of each psychiatric patient. Take schizophrenia, for example. There are myriad clinical variations that comprise this heterogeneous brain syndrome, including level of premorbid functioning; acute vs gradual onset of psychosis; the type and severity of hallucinations or delusions; the dimensional spectrum of negative symptoms and cognitive impairments; the presence and intensity of suicidal or homicidal urges; and the type of medical and psychiatric comorbidities. No wonder every patient is a unique and fascinating clinical puzzle, and yet, patients with schizophrenia are still being homogenized under a single DSM diagnostic category.
There are hundreds of biomarkers in schizophrenia,1 but none can be used clinically until the biosignatures of the many diseases within the schizophrenia syndrome are identified. That grueling research quest will take time, given that so far >340 risk genes for schizophrenia have been discovered, along with countless copy number variants representing gene deletions or duplications, plus dozens of de novo mutations that preclude coding for any protein. Add to these the numerous prenatal pregnancy adverse events, delivery complications, and early childhood abuse—all of which are associated with neurodevelopmental disruptions that set up the brain for schizophrenia spectrum disorders in adulthood—and we have a perplexing conundrum to tackle.
Precision psychiatry will ultimately enable practitioners to recognize various psychotic diseases that are more specific than the current DSM psychosis categories. Further, precision psychiatry will provide guidance as to which member within a class of so-called “me-too” drugs is the optimal match for each patient. This will stand in stark contrast to the chaotic hit-or-miss approach.
Precision psychiatry also will reveal the absurdity of current FDA clinical trials design for drug development. How can a molecule with a putative mechanism of action relevant to a specific biotype be administered to a hodgepodge of heterogeneous biotypes that have been lumped in 1 clinical category, and yet be expected to exert efficacy in most biotypes? It is a small miracle that some new drugs beat placebo despite the extensive variability in both placebo responses and drug responses. But it is well known that in all FDA placebo-controlled trials, the therapeutic response across the patient population varies from extremely high to extremely low, and worsening may even occur in a subset of patients receiving either the active drug or placebo. Perhaps drug response should be used as 1 methodology to classify biotypes of patients encompassed within a heterogeneous syndrome such as schizophrenia.
Precision psychiatry will represent a huge paradigm shift in the science and practice of our specialty. In his landmark book, Thomas Kuhn defined a paradigm as “an entire worldview in which a theory exists and all the implications that come from that view.”2 Precision psychiatry will completely disrupt the current antiquated clinical paradigm, transforming psychiatry into the clinical neuroscience it is. Many “omics,” such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, and metagenomics, will inevitably find their way into the jargon of psychiatrists.3
A marriage of science and technology is essential for the emergence of precision psychiatry. To achieve this transformative amalgamation, we need to reconfigure our concepts, reengineer our methods, reinvent our models, and redesign our approaches to patient care.
As Peter Drucker said, “The best way to predict the future is to create it.”4 Precision psychiatry is our future. Let’s create it!
1. Nasrallah HA.
2. Kuhn TS. The structure of scientific revolutions. Chicago, IL: University of Chicago Press; 1964.
3. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
4. Cohen WA. Drucker on leadership: new lessons from the father of modern management. San Francisco, CA: Jossey-Bass; 2010.
Imagine being able to precisely select the medication with the optimal efficacy, safety, and tolerability at the outset of treatment for every psychiatric patient who needs pharmacotherapy. Imagine how much the patient would appreciate not receiving a series of drugs and suffering multiple adverse effects and unremitting symptoms until the “right medication” is identified. Imagine how gratifying it would be for you as a psychiatrist to watch every one of your patients improve rapidly with minimal complaints or adverse effects.
Precision psychiatry is the indispensable vehicle to achieve personalized medicine for psychiatric patients. Precision psychiatry is a cherished goal, but it remains an aspirational objective. Other medical specialties, especially oncology and cardiology, have made remarkable strides in precision medicine, but the journey to precision psychiatry is still in its early stages. Yet there is every reason to believe that we are making progress toward that cherished goal.
To implement precision psychiatry, we must be able to identify the biosignature of each patient’s psychiatric brain disorder. But there is a formidable challenge to overcome: the complex, extensive heterogeneity of psychiatric disorders, which requires intense and inspired neurobiology research. So, while clinicians go on with the mundane trial-and-error approach of contemporary psychopharmacology, psychiatric neuroscientists are diligently deconstructing major psychiatric disorders into specific biotypes with unique biosignatures that will one day guide accurate and prompt clinical management.
Psychiatric practitioners may be too busy to keep tabs on the progress being made in identifying various biomarkers that are the key ingredients to decoding the biosignature of each psychiatric patient. Take schizophrenia, for example. There are myriad clinical variations that comprise this heterogeneous brain syndrome, including level of premorbid functioning; acute vs gradual onset of psychosis; the type and severity of hallucinations or delusions; the dimensional spectrum of negative symptoms and cognitive impairments; the presence and intensity of suicidal or homicidal urges; and the type of medical and psychiatric comorbidities. No wonder every patient is a unique and fascinating clinical puzzle, and yet, patients with schizophrenia are still being homogenized under a single DSM diagnostic category.
There are hundreds of biomarkers in schizophrenia,1 but none can be used clinically until the biosignatures of the many diseases within the schizophrenia syndrome are identified. That grueling research quest will take time, given that so far >340 risk genes for schizophrenia have been discovered, along with countless copy number variants representing gene deletions or duplications, plus dozens of de novo mutations that preclude coding for any protein. Add to these the numerous prenatal pregnancy adverse events, delivery complications, and early childhood abuse—all of which are associated with neurodevelopmental disruptions that set up the brain for schizophrenia spectrum disorders in adulthood—and we have a perplexing conundrum to tackle.
Precision psychiatry will ultimately enable practitioners to recognize various psychotic diseases that are more specific than the current DSM psychosis categories. Further, precision psychiatry will provide guidance as to which member within a class of so-called “me-too” drugs is the optimal match for each patient. This will stand in stark contrast to the chaotic hit-or-miss approach.
Precision psychiatry also will reveal the absurdity of current FDA clinical trials design for drug development. How can a molecule with a putative mechanism of action relevant to a specific biotype be administered to a hodgepodge of heterogeneous biotypes that have been lumped in 1 clinical category, and yet be expected to exert efficacy in most biotypes? It is a small miracle that some new drugs beat placebo despite the extensive variability in both placebo responses and drug responses. But it is well known that in all FDA placebo-controlled trials, the therapeutic response across the patient population varies from extremely high to extremely low, and worsening may even occur in a subset of patients receiving either the active drug or placebo. Perhaps drug response should be used as 1 methodology to classify biotypes of patients encompassed within a heterogeneous syndrome such as schizophrenia.
Precision psychiatry will represent a huge paradigm shift in the science and practice of our specialty. In his landmark book, Thomas Kuhn defined a paradigm as “an entire worldview in which a theory exists and all the implications that come from that view.”2 Precision psychiatry will completely disrupt the current antiquated clinical paradigm, transforming psychiatry into the clinical neuroscience it is. Many “omics,” such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, and metagenomics, will inevitably find their way into the jargon of psychiatrists.3
A marriage of science and technology is essential for the emergence of precision psychiatry. To achieve this transformative amalgamation, we need to reconfigure our concepts, reengineer our methods, reinvent our models, and redesign our approaches to patient care.
As Peter Drucker said, “The best way to predict the future is to create it.”4 Precision psychiatry is our future. Let’s create it!
Imagine being able to precisely select the medication with the optimal efficacy, safety, and tolerability at the outset of treatment for every psychiatric patient who needs pharmacotherapy. Imagine how much the patient would appreciate not receiving a series of drugs and suffering multiple adverse effects and unremitting symptoms until the “right medication” is identified. Imagine how gratifying it would be for you as a psychiatrist to watch every one of your patients improve rapidly with minimal complaints or adverse effects.
Precision psychiatry is the indispensable vehicle to achieve personalized medicine for psychiatric patients. Precision psychiatry is a cherished goal, but it remains an aspirational objective. Other medical specialties, especially oncology and cardiology, have made remarkable strides in precision medicine, but the journey to precision psychiatry is still in its early stages. Yet there is every reason to believe that we are making progress toward that cherished goal.
To implement precision psychiatry, we must be able to identify the biosignature of each patient’s psychiatric brain disorder. But there is a formidable challenge to overcome: the complex, extensive heterogeneity of psychiatric disorders, which requires intense and inspired neurobiology research. So, while clinicians go on with the mundane trial-and-error approach of contemporary psychopharmacology, psychiatric neuroscientists are diligently deconstructing major psychiatric disorders into specific biotypes with unique biosignatures that will one day guide accurate and prompt clinical management.
Psychiatric practitioners may be too busy to keep tabs on the progress being made in identifying various biomarkers that are the key ingredients to decoding the biosignature of each psychiatric patient. Take schizophrenia, for example. There are myriad clinical variations that comprise this heterogeneous brain syndrome, including level of premorbid functioning; acute vs gradual onset of psychosis; the type and severity of hallucinations or delusions; the dimensional spectrum of negative symptoms and cognitive impairments; the presence and intensity of suicidal or homicidal urges; and the type of medical and psychiatric comorbidities. No wonder every patient is a unique and fascinating clinical puzzle, and yet, patients with schizophrenia are still being homogenized under a single DSM diagnostic category.
There are hundreds of biomarkers in schizophrenia,1 but none can be used clinically until the biosignatures of the many diseases within the schizophrenia syndrome are identified. That grueling research quest will take time, given that so far >340 risk genes for schizophrenia have been discovered, along with countless copy number variants representing gene deletions or duplications, plus dozens of de novo mutations that preclude coding for any protein. Add to these the numerous prenatal pregnancy adverse events, delivery complications, and early childhood abuse—all of which are associated with neurodevelopmental disruptions that set up the brain for schizophrenia spectrum disorders in adulthood—and we have a perplexing conundrum to tackle.
Precision psychiatry will ultimately enable practitioners to recognize various psychotic diseases that are more specific than the current DSM psychosis categories. Further, precision psychiatry will provide guidance as to which member within a class of so-called “me-too” drugs is the optimal match for each patient. This will stand in stark contrast to the chaotic hit-or-miss approach.
Precision psychiatry also will reveal the absurdity of current FDA clinical trials design for drug development. How can a molecule with a putative mechanism of action relevant to a specific biotype be administered to a hodgepodge of heterogeneous biotypes that have been lumped in 1 clinical category, and yet be expected to exert efficacy in most biotypes? It is a small miracle that some new drugs beat placebo despite the extensive variability in both placebo responses and drug responses. But it is well known that in all FDA placebo-controlled trials, the therapeutic response across the patient population varies from extremely high to extremely low, and worsening may even occur in a subset of patients receiving either the active drug or placebo. Perhaps drug response should be used as 1 methodology to classify biotypes of patients encompassed within a heterogeneous syndrome such as schizophrenia.
Precision psychiatry will represent a huge paradigm shift in the science and practice of our specialty. In his landmark book, Thomas Kuhn defined a paradigm as “an entire worldview in which a theory exists and all the implications that come from that view.”2 Precision psychiatry will completely disrupt the current antiquated clinical paradigm, transforming psychiatry into the clinical neuroscience it is. Many “omics,” such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, and metagenomics, will inevitably find their way into the jargon of psychiatrists.3
A marriage of science and technology is essential for the emergence of precision psychiatry. To achieve this transformative amalgamation, we need to reconfigure our concepts, reengineer our methods, reinvent our models, and redesign our approaches to patient care.
As Peter Drucker said, “The best way to predict the future is to create it.”4 Precision psychiatry is our future. Let’s create it!
1. Nasrallah HA.
2. Kuhn TS. The structure of scientific revolutions. Chicago, IL: University of Chicago Press; 1964.
3. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
4. Cohen WA. Drucker on leadership: new lessons from the father of modern management. San Francisco, CA: Jossey-Bass; 2010.
1. Nasrallah HA.
2. Kuhn TS. The structure of scientific revolutions. Chicago, IL: University of Chicago Press; 1964.
3. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
4. Cohen WA. Drucker on leadership: new lessons from the father of modern management. San Francisco, CA: Jossey-Bass; 2010.
Errors of omission and commission in psychiatric practice
There are many rewards for full-time academic psychiatrists such as myself, including didactic teaching, clinical supervision, and 1:1 mentorship of freshly minted medical school graduates, transforming them into accomplished clinical psychiatrists. The technical and personal growth of psychiatric residents over 4 years of post-MD training can be amazing and very gratifying to witness.
But the road to clinical competence often is littered with mistakes. It is the duty of the clinical supervisor to convert every error into a learning opportunity to hone the skills of a future psychiatrist. Over time, fewer mistakes occur, not only because of maturity and seasoning, but also because psychiatric residents learn how to thoughtfully deliberate about their clinical decision-making to select the best treatment option for their patients.
Yet, even with exemplary training, the rigors and constraints of clinical practice inevitably lead to some unforced errors, mostly minor but sometimes consequential. Even experienced practitioners are not immune from making a mistake in the hustle and bustle of daily work (exacerbated by the time-consuming pressures of electronic health record documentation). No one is infallible, but everyone must avoid making the same mistake twice, even if mounting demands lead to “shortcuts” that may not necessarily put the patient at risk but could lead to suboptimal outcomes. But once in a while, a serious complication may ensue.
Here are some common errors of omission or commission that even competent practitioners may make in a busy clinical practice.
Rushing to a diagnosis. To arrive at a primary psychiatric diagnosis, all potential secondary causes must be ruled out. This includes systematic screening for possible drug-induced psychopathology related not only to drugs of abuse, but also to prescription medications, some of which can have serious iatrogenic effects, including depression, anxiety, mania, psychosis, or cognitive dulling. The other important cause to rule out is the possibility of a general medical condition triggering psychiatric symptoms, which requires targeted questioning about medical history, a review of organ systems, and ordering key laboratory tests.
Skipping a baseline cognitive assessment. Cognitive impairment, especially memory and executive function, is now well recognized as an important component of major psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, anxiety, and attention-deficit/hyperactivity disorder. A standardized cognitive battery can provide a valuable profile of brain functions. Knowing the patient’s cognitive strengths and weaknesses before initiating pharmacotherapy is essential to assess the positive or negative impact of the medications. It also can help with patients’ vocational rehabilitation, matching them with jobs compatible with their cognitive strengths.
Inaccurate differential diagnosis. Is it borderline personality or bipolar disorder? Is it schizophrenia or psychotic bipolar disorder? Is it unipolar or bipolar depression? Is it a conversion reaction or a genuine medical condition? The answers to such questions are critical, because inaccurate diagnosis can lead to a lack of improvement and prolonged suffering for patients or adverse effects that could be avoided.
Using a high dose of a medication immediately for a first-episode psychiatric disorder. One of the least patient-friendly medical decisions is to start a first-episode patient on a high dose of a medication on day 1. Gradual titration can circumvent intolerable adverse effects and help establish the lowest effective dose. Patient acceptance and adherence are far more likely if the patient’s brain is not “abruptly medicated.”
Using combination therapy right away. There are a few psychiatric conditions for which combination therapy is FDA-approved and regarded as “rational polypharmacy.” However, it always makes sense to start with 1 (primary) medication and assess its efficacy, tolerability, and safety before adding an adjunctive agent. Some patients may improve substantially with monotherapy, which is always preferable. Using drug combinations as the initial intervention can be problematic, especially if they are not evidence-based and off-label.
Selecting an obesogenic drug as first-line. Many psychotropics, such as antipsychotics, antidepressants, or mood stabilizers, often come as a class of several agents. Clinicians can select any member of the same class (such as selective serotonin reuptake inhibitors [SSRIs] or atypical antipsychotics) because they are all FDA-approved for efficacy. However, the major difference among what often are called “me too” drugs is the adverse effects profile. For many psychotropic medications, significant weight gain is one of the worst medical adverse effects, because it often leads to metabolic dysregulation (hyperglycemia, dyslipidemia, and hypertension) and increases the risk of cardiovascular disease. Many psychiatric patients become obese and have great difficulty losing weight, especially if they are sedentary and have poor eating habits.
Using benzodiazepines as a first-line treatment for anxiety. Although certainly efficacious, and rapidly so, benzodiazepines must be avoided as a first-line treatment for anxiety. The addiction potential is significant, and patients with anxiety will subsequently not respond adequately to standard anxiolytic pharmacotherapy, such as an SSRI, because the anxiolytic effect of these other medications is gradual and not as rapid or potent. Some primary care providers (PCPs) resort to using strong benzodiazepines (such as alprazolam) as first-line, and then refer the patient to a psychiatrist, who finds it quite challenging to steer the patient to an evidence-based option that is less harmful for long-term management. The residents and I have encountered such situations often, sometimes leading to complex interactions with patients who demand renewal of a high dose of a benzodiazepine that had been prescribed to them by a different clinician.
Low utilization of some efficacious agents. It is surprising how some useful pharmacotherapeutic strategies are not employed as often as they should be. This includes lithium for a manic episode; a long-acting injectable antipsychotic in the early phase of schizophrenia; clozapine for patients who failed to respond to a couple of antipsychotics or have chronic suicidal tendencies; lurasidone or quetiapine for bipolar depression (the only FDA-approved medications for this condition); or monoamine oxidase inhibitors for treatment-resistant depression. These drugs can be useful, although some require ongoing blood-level measurements and monitoring for efficacy and adverse effects.
Not recognizing tardive dyskinesia (TD) earlier. TD is one of the most serious neurologic complications of dopamine-receptor working agents (antipsychotics). FDA-approved treatments finally arrived in 2017, but the recognition of the abnormal oro-bucco-lingual or facial choreiform movements remain low (and the use of the Abnormal Involuntary Movement Scale to screen for TD has faded since second-generation antipsychotics were introduced). It is essential to identify this adverse effect early and treat it promptly to avoid its worsening and potential irreversibility.
Other errors of omission or commission include:
- Not collaborating actively with the patient’s PCP to integrate the medical care to improve the patient’s overall health, not just mental health. Collaborative care improves clinical outcomes for most patients.
- Not using available pharmacogenetics testing to provide the patient with “personalized medicine,” such as establishing if they are poor or rapid metabolizers of certain cytochrome hepatic enzymes or checking whether they are less likely to respond to antidepressant medications.
- “Lowering expectations” for patients with severe psychiatric disorders, giving them the message (verbally or nonverbally) that their condition is “hopeless” and that recovery is beyond their reach. Giving hope and trying hard to find better treatment options are the foundation of good medical practice, especially for the sickest patients.
Psychiatrists always aim to do the right thing for their patients, even when the pressures of clinical practice are intense and palpable. But sometimes, an inadvertent slip may occur in the form of an error of omission or commission. These unforced errors are rarely dangerous, but they have the potential to delay response, increase the disease burden, or complicate the illness course. Compassion may be in generous supply, but it’s not enough: We must strive to make our patient-centered, evidence-based clinical practice an error-free zone.
There are many rewards for full-time academic psychiatrists such as myself, including didactic teaching, clinical supervision, and 1:1 mentorship of freshly minted medical school graduates, transforming them into accomplished clinical psychiatrists. The technical and personal growth of psychiatric residents over 4 years of post-MD training can be amazing and very gratifying to witness.
But the road to clinical competence often is littered with mistakes. It is the duty of the clinical supervisor to convert every error into a learning opportunity to hone the skills of a future psychiatrist. Over time, fewer mistakes occur, not only because of maturity and seasoning, but also because psychiatric residents learn how to thoughtfully deliberate about their clinical decision-making to select the best treatment option for their patients.
Yet, even with exemplary training, the rigors and constraints of clinical practice inevitably lead to some unforced errors, mostly minor but sometimes consequential. Even experienced practitioners are not immune from making a mistake in the hustle and bustle of daily work (exacerbated by the time-consuming pressures of electronic health record documentation). No one is infallible, but everyone must avoid making the same mistake twice, even if mounting demands lead to “shortcuts” that may not necessarily put the patient at risk but could lead to suboptimal outcomes. But once in a while, a serious complication may ensue.
Here are some common errors of omission or commission that even competent practitioners may make in a busy clinical practice.
Rushing to a diagnosis. To arrive at a primary psychiatric diagnosis, all potential secondary causes must be ruled out. This includes systematic screening for possible drug-induced psychopathology related not only to drugs of abuse, but also to prescription medications, some of which can have serious iatrogenic effects, including depression, anxiety, mania, psychosis, or cognitive dulling. The other important cause to rule out is the possibility of a general medical condition triggering psychiatric symptoms, which requires targeted questioning about medical history, a review of organ systems, and ordering key laboratory tests.
Skipping a baseline cognitive assessment. Cognitive impairment, especially memory and executive function, is now well recognized as an important component of major psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, anxiety, and attention-deficit/hyperactivity disorder. A standardized cognitive battery can provide a valuable profile of brain functions. Knowing the patient’s cognitive strengths and weaknesses before initiating pharmacotherapy is essential to assess the positive or negative impact of the medications. It also can help with patients’ vocational rehabilitation, matching them with jobs compatible with their cognitive strengths.
Inaccurate differential diagnosis. Is it borderline personality or bipolar disorder? Is it schizophrenia or psychotic bipolar disorder? Is it unipolar or bipolar depression? Is it a conversion reaction or a genuine medical condition? The answers to such questions are critical, because inaccurate diagnosis can lead to a lack of improvement and prolonged suffering for patients or adverse effects that could be avoided.
Using a high dose of a medication immediately for a first-episode psychiatric disorder. One of the least patient-friendly medical decisions is to start a first-episode patient on a high dose of a medication on day 1. Gradual titration can circumvent intolerable adverse effects and help establish the lowest effective dose. Patient acceptance and adherence are far more likely if the patient’s brain is not “abruptly medicated.”
Using combination therapy right away. There are a few psychiatric conditions for which combination therapy is FDA-approved and regarded as “rational polypharmacy.” However, it always makes sense to start with 1 (primary) medication and assess its efficacy, tolerability, and safety before adding an adjunctive agent. Some patients may improve substantially with monotherapy, which is always preferable. Using drug combinations as the initial intervention can be problematic, especially if they are not evidence-based and off-label.
Selecting an obesogenic drug as first-line. Many psychotropics, such as antipsychotics, antidepressants, or mood stabilizers, often come as a class of several agents. Clinicians can select any member of the same class (such as selective serotonin reuptake inhibitors [SSRIs] or atypical antipsychotics) because they are all FDA-approved for efficacy. However, the major difference among what often are called “me too” drugs is the adverse effects profile. For many psychotropic medications, significant weight gain is one of the worst medical adverse effects, because it often leads to metabolic dysregulation (hyperglycemia, dyslipidemia, and hypertension) and increases the risk of cardiovascular disease. Many psychiatric patients become obese and have great difficulty losing weight, especially if they are sedentary and have poor eating habits.
Using benzodiazepines as a first-line treatment for anxiety. Although certainly efficacious, and rapidly so, benzodiazepines must be avoided as a first-line treatment for anxiety. The addiction potential is significant, and patients with anxiety will subsequently not respond adequately to standard anxiolytic pharmacotherapy, such as an SSRI, because the anxiolytic effect of these other medications is gradual and not as rapid or potent. Some primary care providers (PCPs) resort to using strong benzodiazepines (such as alprazolam) as first-line, and then refer the patient to a psychiatrist, who finds it quite challenging to steer the patient to an evidence-based option that is less harmful for long-term management. The residents and I have encountered such situations often, sometimes leading to complex interactions with patients who demand renewal of a high dose of a benzodiazepine that had been prescribed to them by a different clinician.
Low utilization of some efficacious agents. It is surprising how some useful pharmacotherapeutic strategies are not employed as often as they should be. This includes lithium for a manic episode; a long-acting injectable antipsychotic in the early phase of schizophrenia; clozapine for patients who failed to respond to a couple of antipsychotics or have chronic suicidal tendencies; lurasidone or quetiapine for bipolar depression (the only FDA-approved medications for this condition); or monoamine oxidase inhibitors for treatment-resistant depression. These drugs can be useful, although some require ongoing blood-level measurements and monitoring for efficacy and adverse effects.
Not recognizing tardive dyskinesia (TD) earlier. TD is one of the most serious neurologic complications of dopamine-receptor working agents (antipsychotics). FDA-approved treatments finally arrived in 2017, but the recognition of the abnormal oro-bucco-lingual or facial choreiform movements remain low (and the use of the Abnormal Involuntary Movement Scale to screen for TD has faded since second-generation antipsychotics were introduced). It is essential to identify this adverse effect early and treat it promptly to avoid its worsening and potential irreversibility.
Other errors of omission or commission include:
- Not collaborating actively with the patient’s PCP to integrate the medical care to improve the patient’s overall health, not just mental health. Collaborative care improves clinical outcomes for most patients.
- Not using available pharmacogenetics testing to provide the patient with “personalized medicine,” such as establishing if they are poor or rapid metabolizers of certain cytochrome hepatic enzymes or checking whether they are less likely to respond to antidepressant medications.
- “Lowering expectations” for patients with severe psychiatric disorders, giving them the message (verbally or nonverbally) that their condition is “hopeless” and that recovery is beyond their reach. Giving hope and trying hard to find better treatment options are the foundation of good medical practice, especially for the sickest patients.
Psychiatrists always aim to do the right thing for their patients, even when the pressures of clinical practice are intense and palpable. But sometimes, an inadvertent slip may occur in the form of an error of omission or commission. These unforced errors are rarely dangerous, but they have the potential to delay response, increase the disease burden, or complicate the illness course. Compassion may be in generous supply, but it’s not enough: We must strive to make our patient-centered, evidence-based clinical practice an error-free zone.
There are many rewards for full-time academic psychiatrists such as myself, including didactic teaching, clinical supervision, and 1:1 mentorship of freshly minted medical school graduates, transforming them into accomplished clinical psychiatrists. The technical and personal growth of psychiatric residents over 4 years of post-MD training can be amazing and very gratifying to witness.
But the road to clinical competence often is littered with mistakes. It is the duty of the clinical supervisor to convert every error into a learning opportunity to hone the skills of a future psychiatrist. Over time, fewer mistakes occur, not only because of maturity and seasoning, but also because psychiatric residents learn how to thoughtfully deliberate about their clinical decision-making to select the best treatment option for their patients.
Yet, even with exemplary training, the rigors and constraints of clinical practice inevitably lead to some unforced errors, mostly minor but sometimes consequential. Even experienced practitioners are not immune from making a mistake in the hustle and bustle of daily work (exacerbated by the time-consuming pressures of electronic health record documentation). No one is infallible, but everyone must avoid making the same mistake twice, even if mounting demands lead to “shortcuts” that may not necessarily put the patient at risk but could lead to suboptimal outcomes. But once in a while, a serious complication may ensue.
Here are some common errors of omission or commission that even competent practitioners may make in a busy clinical practice.
Rushing to a diagnosis. To arrive at a primary psychiatric diagnosis, all potential secondary causes must be ruled out. This includes systematic screening for possible drug-induced psychopathology related not only to drugs of abuse, but also to prescription medications, some of which can have serious iatrogenic effects, including depression, anxiety, mania, psychosis, or cognitive dulling. The other important cause to rule out is the possibility of a general medical condition triggering psychiatric symptoms, which requires targeted questioning about medical history, a review of organ systems, and ordering key laboratory tests.
Skipping a baseline cognitive assessment. Cognitive impairment, especially memory and executive function, is now well recognized as an important component of major psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, anxiety, and attention-deficit/hyperactivity disorder. A standardized cognitive battery can provide a valuable profile of brain functions. Knowing the patient’s cognitive strengths and weaknesses before initiating pharmacotherapy is essential to assess the positive or negative impact of the medications. It also can help with patients’ vocational rehabilitation, matching them with jobs compatible with their cognitive strengths.
Inaccurate differential diagnosis. Is it borderline personality or bipolar disorder? Is it schizophrenia or psychotic bipolar disorder? Is it unipolar or bipolar depression? Is it a conversion reaction or a genuine medical condition? The answers to such questions are critical, because inaccurate diagnosis can lead to a lack of improvement and prolonged suffering for patients or adverse effects that could be avoided.
Using a high dose of a medication immediately for a first-episode psychiatric disorder. One of the least patient-friendly medical decisions is to start a first-episode patient on a high dose of a medication on day 1. Gradual titration can circumvent intolerable adverse effects and help establish the lowest effective dose. Patient acceptance and adherence are far more likely if the patient’s brain is not “abruptly medicated.”
Using combination therapy right away. There are a few psychiatric conditions for which combination therapy is FDA-approved and regarded as “rational polypharmacy.” However, it always makes sense to start with 1 (primary) medication and assess its efficacy, tolerability, and safety before adding an adjunctive agent. Some patients may improve substantially with monotherapy, which is always preferable. Using drug combinations as the initial intervention can be problematic, especially if they are not evidence-based and off-label.
Selecting an obesogenic drug as first-line. Many psychotropics, such as antipsychotics, antidepressants, or mood stabilizers, often come as a class of several agents. Clinicians can select any member of the same class (such as selective serotonin reuptake inhibitors [SSRIs] or atypical antipsychotics) because they are all FDA-approved for efficacy. However, the major difference among what often are called “me too” drugs is the adverse effects profile. For many psychotropic medications, significant weight gain is one of the worst medical adverse effects, because it often leads to metabolic dysregulation (hyperglycemia, dyslipidemia, and hypertension) and increases the risk of cardiovascular disease. Many psychiatric patients become obese and have great difficulty losing weight, especially if they are sedentary and have poor eating habits.
Using benzodiazepines as a first-line treatment for anxiety. Although certainly efficacious, and rapidly so, benzodiazepines must be avoided as a first-line treatment for anxiety. The addiction potential is significant, and patients with anxiety will subsequently not respond adequately to standard anxiolytic pharmacotherapy, such as an SSRI, because the anxiolytic effect of these other medications is gradual and not as rapid or potent. Some primary care providers (PCPs) resort to using strong benzodiazepines (such as alprazolam) as first-line, and then refer the patient to a psychiatrist, who finds it quite challenging to steer the patient to an evidence-based option that is less harmful for long-term management. The residents and I have encountered such situations often, sometimes leading to complex interactions with patients who demand renewal of a high dose of a benzodiazepine that had been prescribed to them by a different clinician.
Low utilization of some efficacious agents. It is surprising how some useful pharmacotherapeutic strategies are not employed as often as they should be. This includes lithium for a manic episode; a long-acting injectable antipsychotic in the early phase of schizophrenia; clozapine for patients who failed to respond to a couple of antipsychotics or have chronic suicidal tendencies; lurasidone or quetiapine for bipolar depression (the only FDA-approved medications for this condition); or monoamine oxidase inhibitors for treatment-resistant depression. These drugs can be useful, although some require ongoing blood-level measurements and monitoring for efficacy and adverse effects.
Not recognizing tardive dyskinesia (TD) earlier. TD is one of the most serious neurologic complications of dopamine-receptor working agents (antipsychotics). FDA-approved treatments finally arrived in 2017, but the recognition of the abnormal oro-bucco-lingual or facial choreiform movements remain low (and the use of the Abnormal Involuntary Movement Scale to screen for TD has faded since second-generation antipsychotics were introduced). It is essential to identify this adverse effect early and treat it promptly to avoid its worsening and potential irreversibility.
Other errors of omission or commission include:
- Not collaborating actively with the patient’s PCP to integrate the medical care to improve the patient’s overall health, not just mental health. Collaborative care improves clinical outcomes for most patients.
- Not using available pharmacogenetics testing to provide the patient with “personalized medicine,” such as establishing if they are poor or rapid metabolizers of certain cytochrome hepatic enzymes or checking whether they are less likely to respond to antidepressant medications.
- “Lowering expectations” for patients with severe psychiatric disorders, giving them the message (verbally or nonverbally) that their condition is “hopeless” and that recovery is beyond their reach. Giving hope and trying hard to find better treatment options are the foundation of good medical practice, especially for the sickest patients.
Psychiatrists always aim to do the right thing for their patients, even when the pressures of clinical practice are intense and palpable. But sometimes, an inadvertent slip may occur in the form of an error of omission or commission. These unforced errors are rarely dangerous, but they have the potential to delay response, increase the disease burden, or complicate the illness course. Compassion may be in generous supply, but it’s not enough: We must strive to make our patient-centered, evidence-based clinical practice an error-free zone.
Beyond DSM-5: Clinical and biologic features shared by major psychiatric syndromes
It does not adequately inform psychiatric practitioners about the many clinical and biologic features shared across the various diagnostic categories. It does not do justice to the galloping advances in the neurobiology of psychiatric brain disorders and the wealth of potential biomarkers that will eventually endow psychiatry with an objective and ultimately more valid, not just reliable, diagnostic model that is compatible with a future of precision medicine.
The Research Domain Criteria (RDoC) Project1 is a valiant attempt to transcend the DSM’s “Chinese menu” approach to diagnosis. It was championed by the former director of the National Institute of Mental Health (NIMH), who used his authority to encourage investigators applying for federal grants to employ the RDoC principles in their research programs. Who does not recall the awkward moment, a few weeks before the official baptism of DSM-5 as psychiatry’s latest diagnostic Bible in May 2013? The NIMH director’s unflattering portrayal of the incipient DSM-5 was a well-publicized shot across the bow. The kerfuffle was later resolved, but its effects linger among clinical researchers who relentlessly hope for neuroscience advances to translate into a more objective diagnostic approach to psychiatric diagnoses. The neurobiologic foundations of psychopathology are bound to guide us to a more valid set of diagnostic categories, yet the pace remains painfully slow.
However, the copious advances in brain research are providing other dividends beyond a better diagnostic forest. Many intriguing insights are emerging about the connectedness among major psychiatric “trees,” including schizophrenia, bipolar disorders, and major depressive disorder. The following are examples of neurobiologic, clinical, and treatment commonalities across those psychotic and mood disorders.
Shared neurobiology
Progressive brain tissue loss/neurodegeneration. Numerous studies have established that abnormal neuroplasticity is a common theme during psychotic, manic, and depressive episodes. These findings have demonstrated that the more recurrent the episodes, the more prominent the atrophy in either overall brain volume or specific brain regions, especially in the hippocampus, prefrontal cortex, and cerebellum as measured on MRI.
White matter pathology. Multiple studies have reported loss of myelin integrity in psychotic and mood disorders. Abnormalities are detected by using diffusion tensor imaging and measuring anisotropy and diffusivity of water flow in white matter traits. White matter pathology can be associated with intra- and inter-hemispheric disconnectivity and impairment of brain functional integration that may contribute to positive, negative, and cognitive symptoms.
Neuroinflammation. Acute psychotic and mood episodes have been shown to be associated with significant elevation in inflammatory cytokines in CSF and serum, including interleukins (such as interleukin-6), tumor necrosis factor-alpha, interferon gamma, and C-reactive protein. Those inflammatory biomarkers subside when the acute episodes are treated. It is believed that activation of the microglia leads to release of proinflammatory cytokines.
Mitochondrial dysfunction. Many studies document various dysfunctions of the mitochondria in schizophrenia, bipolar disorders, and major depressive disorder. The consequences include oxidative stress due to a decrease in the antioxidant glutathione, produced in the mitochondria, which is vital for neutralizing the reactive oxygen and nitrogen species referred to as free radicals. There is a substantial increase of free radicals during acute psychotic and mood episodes, which contributes to neurodegeneration.
Glutamate pathway abnormalities. A large body of literature has focused on the glutamate N-methyl-
Gene/environment interaction. Neurogenetic advances have demonstrated some shared genes among schizophrenia, bipolar disorders, and major depressive disorder (such as the CACNA1C gene).2 Also, environmental factors, such as severe childhood maltreatment, lead to high rates of psychosis and mood disorders in adulthood. Risk genes in schizophrenia and mood disorders are likely to be overexpressed with adverse environmental factors and epigenetics.
Shortened telomeres. Patients with psychotic and mood disorders have been reported to have shorter telomeres—proteins that cap the end of chromosomes and shorten with repeated cycles of mitosis and aging—at a younger age, predicting early senescence and mortality. Telomere shortening is associated with multiple factors, including chronic stress, smoking, poor diet, obesity, infections, inflammation, and free radicals, all shared by major psychiatric disorders.
Genetic heterogeneity. Schizophrenia, bipolar disorders, and major depressive disorder are associated with complex genetics (eg, risk genes, mutations, and copy number variants) and various perinatal complications (eg, infections, gestational diabetes, vitamin D deficiency, hypoxia at delivery), which makes them highly heterogeneous syndromes, comprised of hundreds of biotypes. There are many established endophenotypes that a future diagnostic system should adopt.
Elevated cortisol levels. Increased serum cortisol levels are found in depression and schizophrenia related to HPA axis dysregulation as well as life stress. Hypercortisolemia can contribute to neurodegeneration as well as to multiple systemic medical disorders often encountered in mood and psychotic disorders.
Shared clinical features
Psychotic and mood disorders share several key clinical features, including:
- cognitive deficits
- substance use disorders (especially Cannabis and alcohol) as a common comorbidity
- increased suicide risk
- high prevalence of smoking
- premature mortality, by 10 to 20 years
- anxiety as a common comorbidity
- elevated cardiometabolic risk factors, even before pharmacotherapy
- recurrent relapses lead to treatment resistance
- various degrees of fixed false beliefs (delusions)
- perceptional aberrations (various types of hallucinations)
- response to dopamine-serotonin antagonists (atypical antipsychotics) as monotherapy or adjunctive therapy.
While it is fair to say that a diagnostic manual like DSM-5 should focus on the diagnosis of individual psychiatric diseases and syndromes, it is also reasonable to say that focusing primarily on clinical features does not do justice to the biologic complexities of psychiatric disorders and the importance of including biomarkers to increase the validity of psychopathological categories. The shared neurobiologic and clinical features across major psychiatric syndromes, such as schizophrenia, bipolar disorders, and depression, indicate how multifaceted psychiatric diagnosis can be. The same approach is applicable to other psychiatric syndromes, such as anxiety, personality disorders, attention-deficit/hyperactivity disorder, or dementia. Our field should move firmly and steadily toward a diagnostic schema that incorporates ongoing breakthroughs in psychiatric neuroscience as soon as they are replicated.
If psychopathology is a forest, then DSM-5 is a simplistic depiction of each tree’s structure as roots, a trunk, branches, and leaves. Psychiatry needs to move to a far more sophisticated perspective of each tree as an amazingly complex, dynamic, and evolving organism, designed genetically but continuously influenced by its environment. Psychiatry also should keep an eye on the entire forest and detect distinctive patterns as well as idiosyncratic or shared features among the trees. Major insights will ensue about the etiology, course, and management of each diagnostic tree or the mosaic of related trees.
1. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395-397.
2. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
It does not adequately inform psychiatric practitioners about the many clinical and biologic features shared across the various diagnostic categories. It does not do justice to the galloping advances in the neurobiology of psychiatric brain disorders and the wealth of potential biomarkers that will eventually endow psychiatry with an objective and ultimately more valid, not just reliable, diagnostic model that is compatible with a future of precision medicine.
The Research Domain Criteria (RDoC) Project1 is a valiant attempt to transcend the DSM’s “Chinese menu” approach to diagnosis. It was championed by the former director of the National Institute of Mental Health (NIMH), who used his authority to encourage investigators applying for federal grants to employ the RDoC principles in their research programs. Who does not recall the awkward moment, a few weeks before the official baptism of DSM-5 as psychiatry’s latest diagnostic Bible in May 2013? The NIMH director’s unflattering portrayal of the incipient DSM-5 was a well-publicized shot across the bow. The kerfuffle was later resolved, but its effects linger among clinical researchers who relentlessly hope for neuroscience advances to translate into a more objective diagnostic approach to psychiatric diagnoses. The neurobiologic foundations of psychopathology are bound to guide us to a more valid set of diagnostic categories, yet the pace remains painfully slow.
However, the copious advances in brain research are providing other dividends beyond a better diagnostic forest. Many intriguing insights are emerging about the connectedness among major psychiatric “trees,” including schizophrenia, bipolar disorders, and major depressive disorder. The following are examples of neurobiologic, clinical, and treatment commonalities across those psychotic and mood disorders.
Shared neurobiology
Progressive brain tissue loss/neurodegeneration. Numerous studies have established that abnormal neuroplasticity is a common theme during psychotic, manic, and depressive episodes. These findings have demonstrated that the more recurrent the episodes, the more prominent the atrophy in either overall brain volume or specific brain regions, especially in the hippocampus, prefrontal cortex, and cerebellum as measured on MRI.
White matter pathology. Multiple studies have reported loss of myelin integrity in psychotic and mood disorders. Abnormalities are detected by using diffusion tensor imaging and measuring anisotropy and diffusivity of water flow in white matter traits. White matter pathology can be associated with intra- and inter-hemispheric disconnectivity and impairment of brain functional integration that may contribute to positive, negative, and cognitive symptoms.
Neuroinflammation. Acute psychotic and mood episodes have been shown to be associated with significant elevation in inflammatory cytokines in CSF and serum, including interleukins (such as interleukin-6), tumor necrosis factor-alpha, interferon gamma, and C-reactive protein. Those inflammatory biomarkers subside when the acute episodes are treated. It is believed that activation of the microglia leads to release of proinflammatory cytokines.
Mitochondrial dysfunction. Many studies document various dysfunctions of the mitochondria in schizophrenia, bipolar disorders, and major depressive disorder. The consequences include oxidative stress due to a decrease in the antioxidant glutathione, produced in the mitochondria, which is vital for neutralizing the reactive oxygen and nitrogen species referred to as free radicals. There is a substantial increase of free radicals during acute psychotic and mood episodes, which contributes to neurodegeneration.
Glutamate pathway abnormalities. A large body of literature has focused on the glutamate N-methyl-
Gene/environment interaction. Neurogenetic advances have demonstrated some shared genes among schizophrenia, bipolar disorders, and major depressive disorder (such as the CACNA1C gene).2 Also, environmental factors, such as severe childhood maltreatment, lead to high rates of psychosis and mood disorders in adulthood. Risk genes in schizophrenia and mood disorders are likely to be overexpressed with adverse environmental factors and epigenetics.
Shortened telomeres. Patients with psychotic and mood disorders have been reported to have shorter telomeres—proteins that cap the end of chromosomes and shorten with repeated cycles of mitosis and aging—at a younger age, predicting early senescence and mortality. Telomere shortening is associated with multiple factors, including chronic stress, smoking, poor diet, obesity, infections, inflammation, and free radicals, all shared by major psychiatric disorders.
Genetic heterogeneity. Schizophrenia, bipolar disorders, and major depressive disorder are associated with complex genetics (eg, risk genes, mutations, and copy number variants) and various perinatal complications (eg, infections, gestational diabetes, vitamin D deficiency, hypoxia at delivery), which makes them highly heterogeneous syndromes, comprised of hundreds of biotypes. There are many established endophenotypes that a future diagnostic system should adopt.
Elevated cortisol levels. Increased serum cortisol levels are found in depression and schizophrenia related to HPA axis dysregulation as well as life stress. Hypercortisolemia can contribute to neurodegeneration as well as to multiple systemic medical disorders often encountered in mood and psychotic disorders.
Shared clinical features
Psychotic and mood disorders share several key clinical features, including:
- cognitive deficits
- substance use disorders (especially Cannabis and alcohol) as a common comorbidity
- increased suicide risk
- high prevalence of smoking
- premature mortality, by 10 to 20 years
- anxiety as a common comorbidity
- elevated cardiometabolic risk factors, even before pharmacotherapy
- recurrent relapses lead to treatment resistance
- various degrees of fixed false beliefs (delusions)
- perceptional aberrations (various types of hallucinations)
- response to dopamine-serotonin antagonists (atypical antipsychotics) as monotherapy or adjunctive therapy.
While it is fair to say that a diagnostic manual like DSM-5 should focus on the diagnosis of individual psychiatric diseases and syndromes, it is also reasonable to say that focusing primarily on clinical features does not do justice to the biologic complexities of psychiatric disorders and the importance of including biomarkers to increase the validity of psychopathological categories. The shared neurobiologic and clinical features across major psychiatric syndromes, such as schizophrenia, bipolar disorders, and depression, indicate how multifaceted psychiatric diagnosis can be. The same approach is applicable to other psychiatric syndromes, such as anxiety, personality disorders, attention-deficit/hyperactivity disorder, or dementia. Our field should move firmly and steadily toward a diagnostic schema that incorporates ongoing breakthroughs in psychiatric neuroscience as soon as they are replicated.
If psychopathology is a forest, then DSM-5 is a simplistic depiction of each tree’s structure as roots, a trunk, branches, and leaves. Psychiatry needs to move to a far more sophisticated perspective of each tree as an amazingly complex, dynamic, and evolving organism, designed genetically but continuously influenced by its environment. Psychiatry also should keep an eye on the entire forest and detect distinctive patterns as well as idiosyncratic or shared features among the trees. Major insights will ensue about the etiology, course, and management of each diagnostic tree or the mosaic of related trees.
It does not adequately inform psychiatric practitioners about the many clinical and biologic features shared across the various diagnostic categories. It does not do justice to the galloping advances in the neurobiology of psychiatric brain disorders and the wealth of potential biomarkers that will eventually endow psychiatry with an objective and ultimately more valid, not just reliable, diagnostic model that is compatible with a future of precision medicine.
The Research Domain Criteria (RDoC) Project1 is a valiant attempt to transcend the DSM’s “Chinese menu” approach to diagnosis. It was championed by the former director of the National Institute of Mental Health (NIMH), who used his authority to encourage investigators applying for federal grants to employ the RDoC principles in their research programs. Who does not recall the awkward moment, a few weeks before the official baptism of DSM-5 as psychiatry’s latest diagnostic Bible in May 2013? The NIMH director’s unflattering portrayal of the incipient DSM-5 was a well-publicized shot across the bow. The kerfuffle was later resolved, but its effects linger among clinical researchers who relentlessly hope for neuroscience advances to translate into a more objective diagnostic approach to psychiatric diagnoses. The neurobiologic foundations of psychopathology are bound to guide us to a more valid set of diagnostic categories, yet the pace remains painfully slow.
However, the copious advances in brain research are providing other dividends beyond a better diagnostic forest. Many intriguing insights are emerging about the connectedness among major psychiatric “trees,” including schizophrenia, bipolar disorders, and major depressive disorder. The following are examples of neurobiologic, clinical, and treatment commonalities across those psychotic and mood disorders.
Shared neurobiology
Progressive brain tissue loss/neurodegeneration. Numerous studies have established that abnormal neuroplasticity is a common theme during psychotic, manic, and depressive episodes. These findings have demonstrated that the more recurrent the episodes, the more prominent the atrophy in either overall brain volume or specific brain regions, especially in the hippocampus, prefrontal cortex, and cerebellum as measured on MRI.
White matter pathology. Multiple studies have reported loss of myelin integrity in psychotic and mood disorders. Abnormalities are detected by using diffusion tensor imaging and measuring anisotropy and diffusivity of water flow in white matter traits. White matter pathology can be associated with intra- and inter-hemispheric disconnectivity and impairment of brain functional integration that may contribute to positive, negative, and cognitive symptoms.
Neuroinflammation. Acute psychotic and mood episodes have been shown to be associated with significant elevation in inflammatory cytokines in CSF and serum, including interleukins (such as interleukin-6), tumor necrosis factor-alpha, interferon gamma, and C-reactive protein. Those inflammatory biomarkers subside when the acute episodes are treated. It is believed that activation of the microglia leads to release of proinflammatory cytokines.
Mitochondrial dysfunction. Many studies document various dysfunctions of the mitochondria in schizophrenia, bipolar disorders, and major depressive disorder. The consequences include oxidative stress due to a decrease in the antioxidant glutathione, produced in the mitochondria, which is vital for neutralizing the reactive oxygen and nitrogen species referred to as free radicals. There is a substantial increase of free radicals during acute psychotic and mood episodes, which contributes to neurodegeneration.
Glutamate pathway abnormalities. A large body of literature has focused on the glutamate N-methyl-
Gene/environment interaction. Neurogenetic advances have demonstrated some shared genes among schizophrenia, bipolar disorders, and major depressive disorder (such as the CACNA1C gene).2 Also, environmental factors, such as severe childhood maltreatment, lead to high rates of psychosis and mood disorders in adulthood. Risk genes in schizophrenia and mood disorders are likely to be overexpressed with adverse environmental factors and epigenetics.
Shortened telomeres. Patients with psychotic and mood disorders have been reported to have shorter telomeres—proteins that cap the end of chromosomes and shorten with repeated cycles of mitosis and aging—at a younger age, predicting early senescence and mortality. Telomere shortening is associated with multiple factors, including chronic stress, smoking, poor diet, obesity, infections, inflammation, and free radicals, all shared by major psychiatric disorders.
Genetic heterogeneity. Schizophrenia, bipolar disorders, and major depressive disorder are associated with complex genetics (eg, risk genes, mutations, and copy number variants) and various perinatal complications (eg, infections, gestational diabetes, vitamin D deficiency, hypoxia at delivery), which makes them highly heterogeneous syndromes, comprised of hundreds of biotypes. There are many established endophenotypes that a future diagnostic system should adopt.
Elevated cortisol levels. Increased serum cortisol levels are found in depression and schizophrenia related to HPA axis dysregulation as well as life stress. Hypercortisolemia can contribute to neurodegeneration as well as to multiple systemic medical disorders often encountered in mood and psychotic disorders.
Shared clinical features
Psychotic and mood disorders share several key clinical features, including:
- cognitive deficits
- substance use disorders (especially Cannabis and alcohol) as a common comorbidity
- increased suicide risk
- high prevalence of smoking
- premature mortality, by 10 to 20 years
- anxiety as a common comorbidity
- elevated cardiometabolic risk factors, even before pharmacotherapy
- recurrent relapses lead to treatment resistance
- various degrees of fixed false beliefs (delusions)
- perceptional aberrations (various types of hallucinations)
- response to dopamine-serotonin antagonists (atypical antipsychotics) as monotherapy or adjunctive therapy.
While it is fair to say that a diagnostic manual like DSM-5 should focus on the diagnosis of individual psychiatric diseases and syndromes, it is also reasonable to say that focusing primarily on clinical features does not do justice to the biologic complexities of psychiatric disorders and the importance of including biomarkers to increase the validity of psychopathological categories. The shared neurobiologic and clinical features across major psychiatric syndromes, such as schizophrenia, bipolar disorders, and depression, indicate how multifaceted psychiatric diagnosis can be. The same approach is applicable to other psychiatric syndromes, such as anxiety, personality disorders, attention-deficit/hyperactivity disorder, or dementia. Our field should move firmly and steadily toward a diagnostic schema that incorporates ongoing breakthroughs in psychiatric neuroscience as soon as they are replicated.
If psychopathology is a forest, then DSM-5 is a simplistic depiction of each tree’s structure as roots, a trunk, branches, and leaves. Psychiatry needs to move to a far more sophisticated perspective of each tree as an amazingly complex, dynamic, and evolving organism, designed genetically but continuously influenced by its environment. Psychiatry also should keep an eye on the entire forest and detect distinctive patterns as well as idiosyncratic or shared features among the trees. Major insights will ensue about the etiology, course, and management of each diagnostic tree or the mosaic of related trees.
1. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395-397.
2. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
1. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395-397.
2. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
Advancing clinical neuroscience literacy among psychiatric practitioners
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
For first-episode psychosis, psychiatrists should behave like cardiologists
Myocardial infarction (MI) is the leading cause of death in the United States, and schizophrenia is the leading cause of disability. But while cardiologists manage the first heart attack very aggressively to prevent a second MI, we psychiatrists generally do not manage first-episode psychosis (FEP) as aggressively to prevent the more malignant second psychotic episode. Yet abundant evidence indicates that psychiatrists must behave like cardiologists at the onset of schizophrenia and other serious psychosis.
Individuals who survive the first heart attack, which permanently destroys part of the myocardium, are at high risk for a second MI, which may lead to death or weaken the heart so much that heart transplantation becomes necessary. Only implementation of aggressive medical intervention will prevent the likelihood of death due to a second MI in a person who has already suffered a first MI.
Similarly, the FEP of schizophrenia destroys brain tissue, about 10 to 12 cc containing millions of glial cells and billions of synapses.2 This neurotoxicity of psychosis is mediated by neuroinflammation and oxidative stress.3 In most FEP patients, the risk of a second psychotic episode is high, and the tissue destruction of the brain’s gray and white matter infrastructure is even more extensive, leading to clinical deterioration, treatment resistance, and functional disability. That is the grim turning point in the trajectory of schizophrenia.
Although most FEP patients respond well to antipsychotic medications and often return to their baseline social and vocational functioning, after a second episode, they are much more likely to become disabled. Unlike physical death, the mental, cognitive, social, and vocational death of chronic schizophrenia goes on for decades with much suffering, misery, and inability to have love and work, which is what life is all about (according to Freud).
But what is the most common psychiatric practice for a patient who suffers a FEP after he (she) is admitted to an acute inpatient ward? The patient is started on an oral antipsychotic but a long-acting injectable (LAI) antipsychotic, which is the best protection against future episodes, is never considered, let alone recommended. The patient is given a prescription for an oral antipsychotic at discharge and the family is told to find a private psychiatrist or a community mental health center for follow-up. This practice pattern will likely guarantee a relapse into a second psychotic episode for the following reasons:
- patients’ lack of insight (anosognosia) and refusal to believe they are sick or need medications
- adverse effects, especially extrapyramidal symptoms, to which FEP patients are particularly vulnerable unless they are started on small doses
- apathy and lack of motivation to take medication due to negative symptoms, which impair ability to initiate actions (avolition)
- severe memory impairment that leads to forgetting medications
- substance use, such as marijuana, stimulants, and hallucinogens, as well as alcohol, interferes with adherence.
Most patients and families are ignorant about FEP of schizophrenia and its recurrence and devastating effects.
Thus, because of the almost ubiquitous inability to adhere fully to antipsychotic medications after discharge, FEP patients are essentially destined (ie, doomed) to experience a destructive second psychotic episode, whose neurotoxicity starts the patient on a downhill journey of lifetime disability.4 LAI antipsychotics are the optimal solution to this serious problem, yet 99.99% of psychiatrists never start LAI during a FEP. This is inexplicable considering the body of evidence that supports early use of LAI to prevent relapse. Of the multipronged strategy that should be used for FEP patients to circumvent a second episode and avoid disability, starting LAI in FEP is the most important interventional tactic.5 Consider the following studies that support initiating LAIs during the FEP:
In South Africa, Emsley et al6 conducted the first study of LAI in FEP. In a 2-year follow-up, 64% of patients had complete remission and returned to their baseline functioning with restoration of insight and good quality of life. When the study ended and patients were returned to their referring psychiatrists after 2 years, all patients were switched to oral antipsychotics, because it was the standard practice among psychiatrists there. All patients relapsed within a few months due to poor adherence to oral medications. When they were placed back on the LAI they had received, a sobering (even shocking) clinical finding emerged: 16% of those who had responded so well to LAI for 2 years no longer responded!7 This rapid emergence of treatment resistance after only a second psychotic episode demonstrates how the brain changes drastically after a second episode and validates the recent adoption of “stages” in schizophrenia, similar to cancer stages.8 Many more patients will develop treatment resistance after subsequent episodes.
Subotnik et al9 compared LAI vs oral risperidone in 86 FEP patients. At the end of 1 year, they reported a 650% higher relapse rate in the oral medication group compared with the LAI group (33% vs 5%).9 This well-done study is a wake-up call for psychiatrists to help FEP patients avoid a brain-damaging second episode by using LAI as a first-line option in FEP.
In a separate study, Subotnik et al10 reported that when the blood level of a patient receiving an antipsychotic is measured at the time of discharge from FEP and every month for a year, all it took for a relapse was a drop of 25%.10 Thus, skipping an antipsychotic just 1 day out of 4 (partial nonadherence) is enough to cause a psychotic relapse.
So what should psychiatrists and nurse practitioners do to protect FEP patients from losing their lives to the permanent disability that begins with a second psychotic episode? They must simply change their attitude and their old-fashioned (antiquated?) prescribing habits that keep failing, and start administering LAI during the initial hospitalization right after a few (usually 3 or 4) days of receiving oral antipsychotics (with nursing-assured swallowing of pills) (Table 2). By starting the patient with oral antipsychotics, the presence of an allergic reaction is ruled out, and efficacy onset begins within 2 to 3 days.11 LAI can then be administered several days before discharge and continued in the outpatient setting.

However, various essential psychosocial interventions should be provided along with LAI to ensure progress toward remission and functional recovery after an FEP. The recently published National Institute of Mental Health-sponsored RAISE study12 is a prime example of the synergy between a multimodal and multidisciplinary team-based approach and antipsychotic medication to improve outcome and quality of life after emerging from FEP.
As psychiatric practitioners, we must be clinically aggressive during the “FEP window of opportunity” to avoid a second episode, thereby bending the curve of the downhill trajectory that occurs after second episodes. We must behave like cardiologists, and relentlessly protect patients who suffer a first “brain attack” from experiencing a relapse. No doubt, any psychiatrists who have a family member with FEP would channel their inner cardiologist and implement the evidence-based recommendations described above. But then, shouldn’t we apply the same standard of care to every FEP patient we see?
1. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
2. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
3. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog
4. Alvarez-Jiménoz M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33,38-45,e3.
6. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
7. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
8. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
9. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
10. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
11. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
12. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372.
Myocardial infarction (MI) is the leading cause of death in the United States, and schizophrenia is the leading cause of disability. But while cardiologists manage the first heart attack very aggressively to prevent a second MI, we psychiatrists generally do not manage first-episode psychosis (FEP) as aggressively to prevent the more malignant second psychotic episode. Yet abundant evidence indicates that psychiatrists must behave like cardiologists at the onset of schizophrenia and other serious psychosis.
Individuals who survive the first heart attack, which permanently destroys part of the myocardium, are at high risk for a second MI, which may lead to death or weaken the heart so much that heart transplantation becomes necessary. Only implementation of aggressive medical intervention will prevent the likelihood of death due to a second MI in a person who has already suffered a first MI.
Similarly, the FEP of schizophrenia destroys brain tissue, about 10 to 12 cc containing millions of glial cells and billions of synapses.2 This neurotoxicity of psychosis is mediated by neuroinflammation and oxidative stress.3 In most FEP patients, the risk of a second psychotic episode is high, and the tissue destruction of the brain’s gray and white matter infrastructure is even more extensive, leading to clinical deterioration, treatment resistance, and functional disability. That is the grim turning point in the trajectory of schizophrenia.
Although most FEP patients respond well to antipsychotic medications and often return to their baseline social and vocational functioning, after a second episode, they are much more likely to become disabled. Unlike physical death, the mental, cognitive, social, and vocational death of chronic schizophrenia goes on for decades with much suffering, misery, and inability to have love and work, which is what life is all about (according to Freud).
But what is the most common psychiatric practice for a patient who suffers a FEP after he (she) is admitted to an acute inpatient ward? The patient is started on an oral antipsychotic but a long-acting injectable (LAI) antipsychotic, which is the best protection against future episodes, is never considered, let alone recommended. The patient is given a prescription for an oral antipsychotic at discharge and the family is told to find a private psychiatrist or a community mental health center for follow-up. This practice pattern will likely guarantee a relapse into a second psychotic episode for the following reasons:
- patients’ lack of insight (anosognosia) and refusal to believe they are sick or need medications
- adverse effects, especially extrapyramidal symptoms, to which FEP patients are particularly vulnerable unless they are started on small doses
- apathy and lack of motivation to take medication due to negative symptoms, which impair ability to initiate actions (avolition)
- severe memory impairment that leads to forgetting medications
- substance use, such as marijuana, stimulants, and hallucinogens, as well as alcohol, interferes with adherence.
Most patients and families are ignorant about FEP of schizophrenia and its recurrence and devastating effects.
Thus, because of the almost ubiquitous inability to adhere fully to antipsychotic medications after discharge, FEP patients are essentially destined (ie, doomed) to experience a destructive second psychotic episode, whose neurotoxicity starts the patient on a downhill journey of lifetime disability.4 LAI antipsychotics are the optimal solution to this serious problem, yet 99.99% of psychiatrists never start LAI during a FEP. This is inexplicable considering the body of evidence that supports early use of LAI to prevent relapse. Of the multipronged strategy that should be used for FEP patients to circumvent a second episode and avoid disability, starting LAI in FEP is the most important interventional tactic.5 Consider the following studies that support initiating LAIs during the FEP:
In South Africa, Emsley et al6 conducted the first study of LAI in FEP. In a 2-year follow-up, 64% of patients had complete remission and returned to their baseline functioning with restoration of insight and good quality of life. When the study ended and patients were returned to their referring psychiatrists after 2 years, all patients were switched to oral antipsychotics, because it was the standard practice among psychiatrists there. All patients relapsed within a few months due to poor adherence to oral medications. When they were placed back on the LAI they had received, a sobering (even shocking) clinical finding emerged: 16% of those who had responded so well to LAI for 2 years no longer responded!7 This rapid emergence of treatment resistance after only a second psychotic episode demonstrates how the brain changes drastically after a second episode and validates the recent adoption of “stages” in schizophrenia, similar to cancer stages.8 Many more patients will develop treatment resistance after subsequent episodes.
Subotnik et al9 compared LAI vs oral risperidone in 86 FEP patients. At the end of 1 year, they reported a 650% higher relapse rate in the oral medication group compared with the LAI group (33% vs 5%).9 This well-done study is a wake-up call for psychiatrists to help FEP patients avoid a brain-damaging second episode by using LAI as a first-line option in FEP.
In a separate study, Subotnik et al10 reported that when the blood level of a patient receiving an antipsychotic is measured at the time of discharge from FEP and every month for a year, all it took for a relapse was a drop of 25%.10 Thus, skipping an antipsychotic just 1 day out of 4 (partial nonadherence) is enough to cause a psychotic relapse.
So what should psychiatrists and nurse practitioners do to protect FEP patients from losing their lives to the permanent disability that begins with a second psychotic episode? They must simply change their attitude and their old-fashioned (antiquated?) prescribing habits that keep failing, and start administering LAI during the initial hospitalization right after a few (usually 3 or 4) days of receiving oral antipsychotics (with nursing-assured swallowing of pills) (Table 2). By starting the patient with oral antipsychotics, the presence of an allergic reaction is ruled out, and efficacy onset begins within 2 to 3 days.11 LAI can then be administered several days before discharge and continued in the outpatient setting.

However, various essential psychosocial interventions should be provided along with LAI to ensure progress toward remission and functional recovery after an FEP. The recently published National Institute of Mental Health-sponsored RAISE study12 is a prime example of the synergy between a multimodal and multidisciplinary team-based approach and antipsychotic medication to improve outcome and quality of life after emerging from FEP.
As psychiatric practitioners, we must be clinically aggressive during the “FEP window of opportunity” to avoid a second episode, thereby bending the curve of the downhill trajectory that occurs after second episodes. We must behave like cardiologists, and relentlessly protect patients who suffer a first “brain attack” from experiencing a relapse. No doubt, any psychiatrists who have a family member with FEP would channel their inner cardiologist and implement the evidence-based recommendations described above. But then, shouldn’t we apply the same standard of care to every FEP patient we see?
Myocardial infarction (MI) is the leading cause of death in the United States, and schizophrenia is the leading cause of disability. But while cardiologists manage the first heart attack very aggressively to prevent a second MI, we psychiatrists generally do not manage first-episode psychosis (FEP) as aggressively to prevent the more malignant second psychotic episode. Yet abundant evidence indicates that psychiatrists must behave like cardiologists at the onset of schizophrenia and other serious psychosis.
Individuals who survive the first heart attack, which permanently destroys part of the myocardium, are at high risk for a second MI, which may lead to death or weaken the heart so much that heart transplantation becomes necessary. Only implementation of aggressive medical intervention will prevent the likelihood of death due to a second MI in a person who has already suffered a first MI.
Similarly, the FEP of schizophrenia destroys brain tissue, about 10 to 12 cc containing millions of glial cells and billions of synapses.2 This neurotoxicity of psychosis is mediated by neuroinflammation and oxidative stress.3 In most FEP patients, the risk of a second psychotic episode is high, and the tissue destruction of the brain’s gray and white matter infrastructure is even more extensive, leading to clinical deterioration, treatment resistance, and functional disability. That is the grim turning point in the trajectory of schizophrenia.
Although most FEP patients respond well to antipsychotic medications and often return to their baseline social and vocational functioning, after a second episode, they are much more likely to become disabled. Unlike physical death, the mental, cognitive, social, and vocational death of chronic schizophrenia goes on for decades with much suffering, misery, and inability to have love and work, which is what life is all about (according to Freud).
But what is the most common psychiatric practice for a patient who suffers a FEP after he (she) is admitted to an acute inpatient ward? The patient is started on an oral antipsychotic but a long-acting injectable (LAI) antipsychotic, which is the best protection against future episodes, is never considered, let alone recommended. The patient is given a prescription for an oral antipsychotic at discharge and the family is told to find a private psychiatrist or a community mental health center for follow-up. This practice pattern will likely guarantee a relapse into a second psychotic episode for the following reasons:
- patients’ lack of insight (anosognosia) and refusal to believe they are sick or need medications
- adverse effects, especially extrapyramidal symptoms, to which FEP patients are particularly vulnerable unless they are started on small doses
- apathy and lack of motivation to take medication due to negative symptoms, which impair ability to initiate actions (avolition)
- severe memory impairment that leads to forgetting medications
- substance use, such as marijuana, stimulants, and hallucinogens, as well as alcohol, interferes with adherence.
Most patients and families are ignorant about FEP of schizophrenia and its recurrence and devastating effects.
Thus, because of the almost ubiquitous inability to adhere fully to antipsychotic medications after discharge, FEP patients are essentially destined (ie, doomed) to experience a destructive second psychotic episode, whose neurotoxicity starts the patient on a downhill journey of lifetime disability.4 LAI antipsychotics are the optimal solution to this serious problem, yet 99.99% of psychiatrists never start LAI during a FEP. This is inexplicable considering the body of evidence that supports early use of LAI to prevent relapse. Of the multipronged strategy that should be used for FEP patients to circumvent a second episode and avoid disability, starting LAI in FEP is the most important interventional tactic.5 Consider the following studies that support initiating LAIs during the FEP:
In South Africa, Emsley et al6 conducted the first study of LAI in FEP. In a 2-year follow-up, 64% of patients had complete remission and returned to their baseline functioning with restoration of insight and good quality of life. When the study ended and patients were returned to their referring psychiatrists after 2 years, all patients were switched to oral antipsychotics, because it was the standard practice among psychiatrists there. All patients relapsed within a few months due to poor adherence to oral medications. When they were placed back on the LAI they had received, a sobering (even shocking) clinical finding emerged: 16% of those who had responded so well to LAI for 2 years no longer responded!7 This rapid emergence of treatment resistance after only a second psychotic episode demonstrates how the brain changes drastically after a second episode and validates the recent adoption of “stages” in schizophrenia, similar to cancer stages.8 Many more patients will develop treatment resistance after subsequent episodes.
Subotnik et al9 compared LAI vs oral risperidone in 86 FEP patients. At the end of 1 year, they reported a 650% higher relapse rate in the oral medication group compared with the LAI group (33% vs 5%).9 This well-done study is a wake-up call for psychiatrists to help FEP patients avoid a brain-damaging second episode by using LAI as a first-line option in FEP.
In a separate study, Subotnik et al10 reported that when the blood level of a patient receiving an antipsychotic is measured at the time of discharge from FEP and every month for a year, all it took for a relapse was a drop of 25%.10 Thus, skipping an antipsychotic just 1 day out of 4 (partial nonadherence) is enough to cause a psychotic relapse.
So what should psychiatrists and nurse practitioners do to protect FEP patients from losing their lives to the permanent disability that begins with a second psychotic episode? They must simply change their attitude and their old-fashioned (antiquated?) prescribing habits that keep failing, and start administering LAI during the initial hospitalization right after a few (usually 3 or 4) days of receiving oral antipsychotics (with nursing-assured swallowing of pills) (Table 2). By starting the patient with oral antipsychotics, the presence of an allergic reaction is ruled out, and efficacy onset begins within 2 to 3 days.11 LAI can then be administered several days before discharge and continued in the outpatient setting.

However, various essential psychosocial interventions should be provided along with LAI to ensure progress toward remission and functional recovery after an FEP. The recently published National Institute of Mental Health-sponsored RAISE study12 is a prime example of the synergy between a multimodal and multidisciplinary team-based approach and antipsychotic medication to improve outcome and quality of life after emerging from FEP.
As psychiatric practitioners, we must be clinically aggressive during the “FEP window of opportunity” to avoid a second episode, thereby bending the curve of the downhill trajectory that occurs after second episodes. We must behave like cardiologists, and relentlessly protect patients who suffer a first “brain attack” from experiencing a relapse. No doubt, any psychiatrists who have a family member with FEP would channel their inner cardiologist and implement the evidence-based recommendations described above. But then, shouldn’t we apply the same standard of care to every FEP patient we see?
1. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
2. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
3. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog
4. Alvarez-Jiménoz M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33,38-45,e3.
6. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
7. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
8. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
9. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
10. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
11. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
12. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372.
1. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
2. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
3. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog
4. Alvarez-Jiménoz M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33,38-45,e3.
6. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
7. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
8. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
9. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
10. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
11. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
12. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372.
Accelerated aging in schizophrenia
Glutamate’s exciting roles in body, brain, and mind: A fertile future pharmacotherapy target
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.
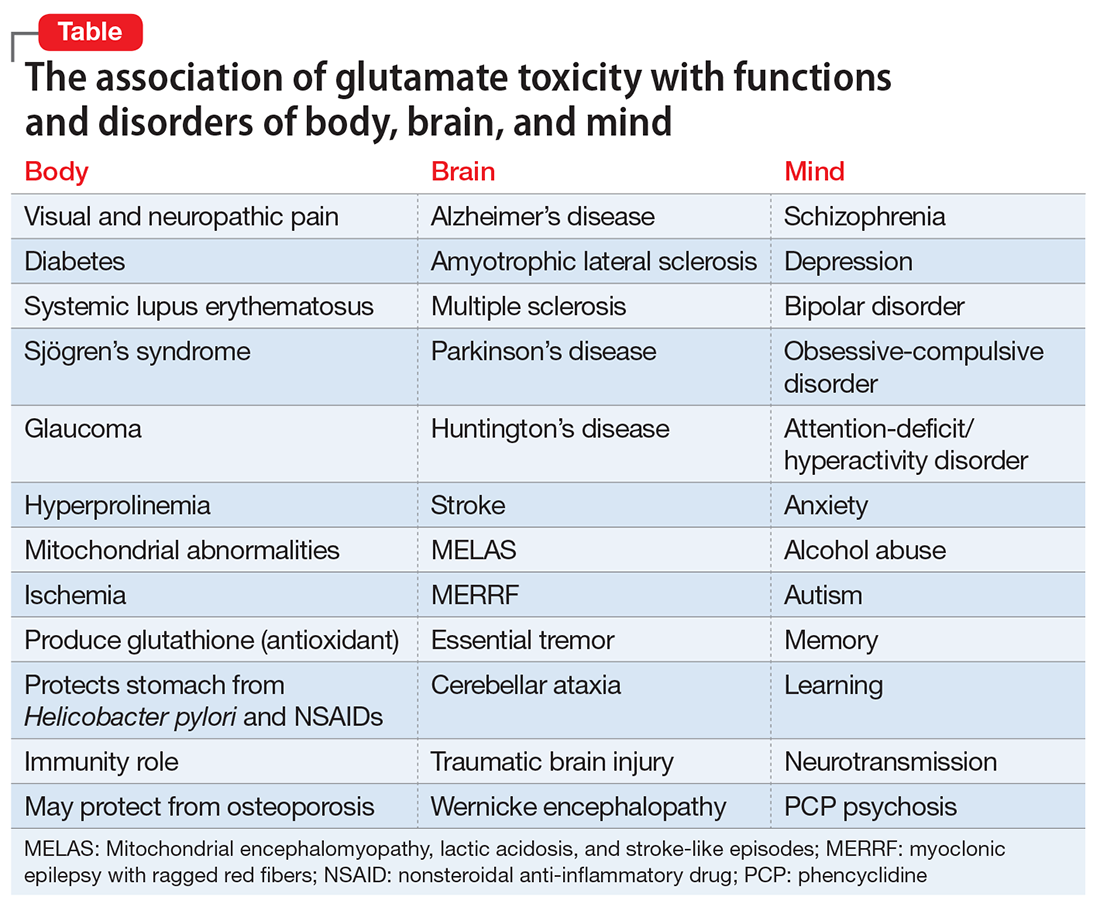
GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
Prescribing is the culmination of extensive medical training and psychologists don’t qualify
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Fourth: Laboratory ordering and monitoring. Ordering laboratory data during follow-up of a patient receiving a psychotropic drug is necessary to monitor serum concentrations and ensure a therapeutic range, or to check for serious adverse effects on various organ systems that could be affected by many psychiatric drugs (CNS, cardiovascular, gastrointestinal, sexual, endocrine, pulmonary, hepatic, renal, dermatologic, ophthalmologic, etc.).
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Fourth: Laboratory ordering and monitoring. Ordering laboratory data during follow-up of a patient receiving a psychotropic drug is necessary to monitor serum concentrations and ensure a therapeutic range, or to check for serious adverse effects on various organ systems that could be affected by many psychiatric drugs (CNS, cardiovascular, gastrointestinal, sexual, endocrine, pulmonary, hepatic, renal, dermatologic, ophthalmologic, etc.).
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Fourth: Laboratory ordering and monitoring. Ordering laboratory data during follow-up of a patient receiving a psychotropic drug is necessary to monitor serum concentrations and ensure a therapeutic range, or to check for serious adverse effects on various organ systems that could be affected by many psychiatric drugs (CNS, cardiovascular, gastrointestinal, sexual, endocrine, pulmonary, hepatic, renal, dermatologic, ophthalmologic, etc.).
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.