User login
Evaluation of Pharmacologic Interventions for Weight Management in a Veteran Population
The American Heart Association, the American College of Cardiology, and the Obesity Society define overweight as a body mass index (BMI) of 25 to 29.9 and obesity as a BMI ≥ 30. Morbid obesity is defined as a BMI ≥ 35 or 40.2,3 Based on these BMI cutoffs, the Endocrine Society recommends diet and lifestyle as the foundation of weight management and pharmacotherapy for those with a BMI ≥ 30 without comorbidities. In patients with a BMI ≥ 27, weight management medications may be considered if a patient has comorbid hypertension, T2DM, dyslipidemia, metabolic syndrome, obstructive sleep apnea, or nonalcoholic fatty liver disease. Patients with BMI > 40 are eligible for weight loss surgery.4
Lifestyle and dietary interventions are the foundation of current weight management guidelines from the Endocrine Society.4 At a minimum, guidelines recommended enrolling motivated patients in a high-intensity lifestyle intervention class of at least 14 sessions in the first 6 months to reach a goal weight loss of 5 to 10% from baseline and to maintain a reduction of 3 to 5% from baseline.3 Medications are recommended as an adjunct to lifestyle and dietary changes. Most weight management medications work in the brain to stimulate satiety signaling, which helps motivated patients adhere to their dietary interventions, assist those who have been unsuccessful in earlier weight loss attempts, and help maintain weight.3,4
Guidelines recommend 7 weight management medications, including orlistat (both prescription strength and over-the-counter), liraglutide, phentermine, phentermine/topiramate, lorcaserin, and naltrexone/bupropion. Using medications to assist with weight loss increases likelihood that patients will achieve 5 to 10% weight loss from baseline.5,6 Studies looking at long-term effects of these medications on weight loss have found improvements in blood pressure (BP), biomarkers for cardiovascular disease, and T2DM-related comorbidities.3,5,7
Positive effects on comorbidities have been found to be related to drug class and mechanism of action (MOA); those that also are approved for T2DM have demonstrated the most favorable cardiovascular effects.7 Other medications that work as stimulants or as modulators of serotonin pathways are associated with increased risks, prompting the US Food and Drug Administration (FDA) to remove some medications from the market.7,8 In January 2020, lorcaserin was taken off the market because of increased risk of cancer found in postmarketing surveillance.9 The benefit of weight loss must be weighed against the risk of medication use.
Monthly follow-up is recommended with weight management medications in the beginning to assess safety and efficacy; medications should be discontinued if weight loss is inadequate in the first 3 months.1,3,4 Limited studies have assessed the long-term use of weight management medications in a real-world setting. Medications are prescribed for weight management at Veteran Health Indiana (VHI) in outpatient clinics, including primary care, endocrinology, and gastrointestinal (GI) specialties. However, prescribing practices, outcomes, and adherence to guideline recommendations have not been studied. Data from this study will be used to better understand how VHI can serve its veterans through diet, lifestyle, and pharmacologic interventions.
Methods
We conducted a single-center, retrospective chart review for patients started on weight management medications at VHI. A patient list was generated based on prescription fills from June 1, 2017 to June 30, 2019. All data were obtained using the Computerized Patient Record System and patients were not contacted. This study was approved by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
At the time of study, orlistat, liraglutide, phentermine/topiramate,
Patients were included in the study if they received a prescription of any 1 of the 5 available medications during the enrollment period. Patients were excluded if they received a prescription from or were treated by a civilian health care provider, if they never used the medication, or if their weight loss was attributed to a cancer diagnosis. These criteria produced 86 patients of whom 96 unique weight loss prescriptions were generated. Data were collected for each instance of medication use so that some patients were included multiple times. In this case, data collection for the failed medication ended when failure was documented, and new data points began when new medication was prescribed; all data collected were per medication, not per patient. This method was used to account for medication failure and provide accurate weight loss results based on medication choice within this institution.
The primary outcomes included total weight loss and weight loss as a percentage of baseline weight at 3, 6, 12, and > 12 months of therapy. Secondary outcomes included weight loss of 5% from baseline, rate of successful weight maintenance after initial weight loss of 5% from baseline, adverse drug reaction (ADR) monitoring, and use of weight management medications across clinics at VHI.
Demographic data included race, age, sex, baseline weight, BMI, and comorbid medical conditions. Comorbidities were collected based on the most recent primary care clinical note before initiating medication. Medication data collected included medications used to manage comorbidities. Data related to weight management medication included prescribing clinic, reason for medication discontinuation, or bariatric surgery intervention if applicable.
Efficacy outcome data included weight and BMI across therapy duration. Safety outcomes data included heart rate, BP, and ADRs that resulted in medication discontinuation as documented in the electronic health record (EHR).
We used descriptive statistics, including mean, standard deviation (SD), range, and percentage. For continuous data, Kruskal-Wallis tests were used because of nonparametric data distribution among the different medications with a prespecified α = 0.05. With the observed sample sizes and SDs in this study, post hoc poststudy power calculations showed that the study had 80% power at a 5% significance level to detect weight changes of 8.6 kg, 7.3 kg, and 12.4 kg at 3, 6, and 12 months, respectively, using nonparametric tests.
Results
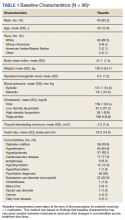
A total of 86 patients were identified based on prescription fills, which produced 99 unique instances of medication use. Of the 99 identified, 3 met exclusion criteria and were not included in the final analysis. Among included veterans, 16 were female and 80 were male (Table 1). Most of those included identified as White race (86%), male (83%), and mean age 53 years. At baseline, mean weight was 130 kg and mean BMI 41.
Comorbidities and Medication Use
Hypertension (66%), hyperlipidemia (64%), and psychiatric diagnoses (50%) were most common comorbid conditions. Substance use (23%) and T2DM (40%) were the most common comorbidities influencing medication choice. Substance use evaluation included amphetamines and cocaine for this analysis.
Phentermine/topiramate is the preferred first-line agent unless patients have contraindications for use, in which case naltrexone/bupropion is recommended, based on guidelines for weight management medications within the VHI system. However, for patients with comorbid T2DM, liraglutide is preferred because of its beneficial effects for both weight loss and blood glucose control.2 Most patients at VHI were started on liraglutide (44%) or phentermine/topiramate (42%), which was in line with recommendations. Our sample included ≥ 1 prescription for each medication available at our facility, although the number of patients on each medication was not equal. Of note, the one patient taking lorcaserin at the time of study discontinued therapy in response to recent FDA guidance.9
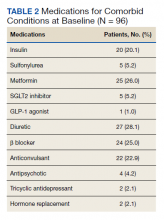
Medications for comorbid conditions could contribute to weight gain. Of the patient sample, β blockers (n = 24) and anticonvulsants, including gabapentin and pregabalin (n = 22) were the most common Other medications that could have contributed to weight gain included sulfonylureas (n = 5), antipsychotics (n = 4), tricyclic antidepressants (n = 2), and hormone replacement therapies (n = 2).
Primary Outcomes
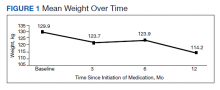
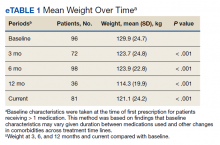
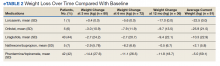
The mean weight of participants dropped from 129.9 to 114.2 kg over the 12 months of weight management medication therapy for a absolute difference of 15.8 kg (Figure 1 and eTable 1 available at doi:10.12788/fp.0117). Weight loss was recorded at 3, 6, 12, and > 12 months of weight management therapy. At each time point, weight loss was statistically significant (P < .001) compared with baseline (Table 2), even though not every patient had weight loss records at each time point.
When classified by medication choice,
Secondary Outcomes
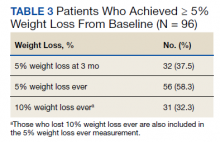
More than one-half of the patients analyzed lost 5 to 10% from baseline while taking weight management medication.
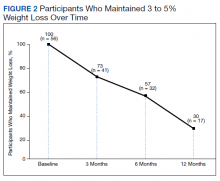
Among patients who lost at least 5% from baseline, we performed further analysis to assess weight maintenance of 3 to 5% from baseline for 12 months.
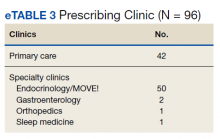
We found that most of our prescriptions (n = 50) were entered by the endocrinology department in conjunction with the MOVE! program (eTable 3 available at doi:10.12788/fp.0117). All 4 of our primary care clinics prescribed weight loss medication; however, 1 clinic prescribed the most. Other prescriptions came from community-based outpatient clinics or other specialties, including gastroenterology, orthopedics, and sleep medicine.
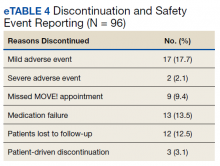
Nineteen (18%) patients experienced an adverse event (AE) that led to medication discontinuation, which was recorded in their chart (eTable 4 available at doi:10.12788/fp.0117). Most common AEs were GI upset with liraglutide or orlistat or dull aching and pain with phentermine/topiramate. Two severe AEs occurred: One patient experienced a change in mental health status and suicide attempt with naltrexone/bupropion; and 1 patient discontinued phentermine/topiramate because of a change in neurologic status.
Primarily medications were stopped because of inadequate weight loss (n = 13), and most patients tried additional medications. However, 1 medication failure resulted in sleeve gastrectomy. Other reasons for medication discontinuation included missed MOVE! appointments, patient lost to follow-up, and patient-elected discontinuation.
Discussion
This study evaluated the use and outcomes of weight management medication among veterans at VHI. The study aimed to better understand the efficacy and safety of these medications while exposing potential weaknesses in care and to promote avenues to improve weight loss and maintenance.
Clinical trials for weight management medications reported weight loss of 8 to 10 kg over 56 weeks: 21 to 63% of patients losing at least 5% from baseline weight.10-14 The findings from our study found a higher average weight loss (−15.8 kg) than that reported in trials and a consistent percentage of patients (58.3%) who achieved at least 5% weight loss. It is promising to see that when used in a noncontrolled setting, these medications were able to produce weight loss consistent with results seen in large, controlled trials.
Pi-Sunyer and colleagues found continued weight loss after the initial 5% weight loss to an eventual 10% weight loss in many patients.10 Additionally, Smith and colleagues found that nearly 68% of their participants who took lorcaserin were able to maintain 3 to 5% weight loss over 12 months.13 Sjöström and colleagues acknowledged that many patients taking orlistat for an extended period began to gain weight, although at one-half the rate than that seen in the placebo group.12 This study found that fewer patients were able to maintain their weight loss over 12 months, with only 30% of patients maintaining 3 to 5% weight loss from baseline. This difference in weight maintenance likely was because of the uncontrolled nature of this study. Once patients reach their initial weight loss goal, even the most motivated patients will have trouble maintaining that weight.4 Despite the challenges associated with maintaining weight loss, the quality of life benefits patients gained and potential reductions in health care spending support using resources to improve these outcomes.2,14,15
Pi-Sunyer and colleagues reported high incidences of nausea (40%), vomiting (16%), diarrhea (21%), and constipation (20%) with liraglutide.10 Sjöström and colleagues reported 7% of patients experienced GI upset with orlistat.12 Comparatively, only 17% of our patients reported AEs that required discontinuation, including GI upset. One patient in our study discontinued naltrexone/bupropion because of a significant change in mental status and suicide attempt. Clinical trials did not report a greater risk of depression or suicidality compared with placebo; however, there is a warning on the labeling of naltrexone/bupropion for increased suicidality with the use of antidepressant agents.16,17 The neurologic AE that required discontinuation of phentermine/topiramate at our institution is unique based on published information.11,18
The data from this study reinforced the observation that weight maintenance is the most challenging aspect of weight loss. Although our data showed clinically meaningful weight loss from baseline, many patients regained their weight, and some exceeded their baseline weight. Beyond providing these medications, this evidence suggests the need for close, continued follow-up through patients’ weight loss journey.
Limitations
Because this is a retrospective chart review, data collection was influenced by and limited to information that had been recorded in the EHR. AEs that resulted in medication discontinuation were assessed from the patient’s chart, which might not be correct if providers did not update the records. Follow-up was not always scheduled at regular intervals after medication initiation, resulting in varying sample numbers at each time point, potentially interfering with true weight loss averages. Although not included in this analysis, it might be beneficial to evaluate adherence to recommendations for follow-up with laboratory and weight monitoring to better capture where future monitoring can be improved. Second, there was an unbalanced number of patients taking each medication. Specifically, we saw a change in weight with orlistat that exceeded what is consistently seen in larger, more controlled trials. Although this is an effect of the real world, small sample sizes cannot be generalized to the larger population and might result in data reflecting that of an outlier. Last, there is a lack of generalizability because of the veteran population demographic, which is more male and lacks ethnic diversity. This study also was carried out at a single, educational tertiary medical center, which might not apply to all populations.
Conclusions
Despite the limitations discussed, this study shows that the use of weight management medications in a general veteran population produces initial weight loss consistent with previous studies. However, there is room for continued improvement in follow-up strategies to promote greater weight maintenance after initial weight loss. Considering the high health care costs, personal burden, and potential long-term complications associated with obesity, efforts to promote development of programs that support weight management and maintenance are imperative.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Centers for Disease Control and Prevention. Adult obesity facts. Accessed April 2020. https://www.cdc.gov/obesity/data/adult.html
2. The Management of Overweight and Obesity Working Group. VA/DoD Clinical Practice Guideline for Screening and Management of Overweight and Obesity. Accessed March 13, 2021. https://www.healthquality.va.gov/guidelines/CD/obesity/VADoDCPGManagementOfOverweightAndObesityFinal.pdf
3. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25, pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
4. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100(2):342-362. doi:10.1210/jc.2014-3415
5. Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194-1199. doi:10.1136/bmj.39385.413113.25
6. Siebenhofer A, Winterholer, S, Jeitler K, et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev 2021;1:CD007654. doi:10.1002/14651858.CD007654.pub5
7. Bramante CT, Raatz S, Bomber EM, Oberle MM, Ryder JR. Cardiovascular risks and benefits of medications used for weight loss. Front Endocrinol (Lausanne). 2020;10:883. doi:10.3389/fendo.2019.00883
8. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomized trials. Lancet. 2007;370(9600):1706-1713. doi:10.1016/S0140-6736(07)61721-8
9. US Food and Drug Administration. FDA requests the withdrawal of the weight-loss drug Blevique, Belvique XR (lorcaserin) from the market. Accessed April 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
10. Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
11. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
12. Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016/s0140-6736(97)11509-4
13. Smith SR, Weissman NJ, Anderson CM, et al; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight loss. N Engl J Med. 2010;363(3):245-256. doi:10.1056/NEJMoa0909809
14. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev. 2014;15(3):169-182. doi:10.1111/obr.12113
15. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822-831. doi:10.1377/hlthaff.28.5.w822
16. Greenway FL, Fujioka K, Plodkowski RA, et al; COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicenter, randomized, double-blind, placebo-controlled phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
17. Contrave. Prescribing information. Nalpropion Pharmaceuticals, Inc; 2019.
18. Qsymia. Prescribing information. VIVUS Inc; 2018.
The American Heart Association, the American College of Cardiology, and the Obesity Society define overweight as a body mass index (BMI) of 25 to 29.9 and obesity as a BMI ≥ 30. Morbid obesity is defined as a BMI ≥ 35 or 40.2,3 Based on these BMI cutoffs, the Endocrine Society recommends diet and lifestyle as the foundation of weight management and pharmacotherapy for those with a BMI ≥ 30 without comorbidities. In patients with a BMI ≥ 27, weight management medications may be considered if a patient has comorbid hypertension, T2DM, dyslipidemia, metabolic syndrome, obstructive sleep apnea, or nonalcoholic fatty liver disease. Patients with BMI > 40 are eligible for weight loss surgery.4
Lifestyle and dietary interventions are the foundation of current weight management guidelines from the Endocrine Society.4 At a minimum, guidelines recommended enrolling motivated patients in a high-intensity lifestyle intervention class of at least 14 sessions in the first 6 months to reach a goal weight loss of 5 to 10% from baseline and to maintain a reduction of 3 to 5% from baseline.3 Medications are recommended as an adjunct to lifestyle and dietary changes. Most weight management medications work in the brain to stimulate satiety signaling, which helps motivated patients adhere to their dietary interventions, assist those who have been unsuccessful in earlier weight loss attempts, and help maintain weight.3,4
Guidelines recommend 7 weight management medications, including orlistat (both prescription strength and over-the-counter), liraglutide, phentermine, phentermine/topiramate, lorcaserin, and naltrexone/bupropion. Using medications to assist with weight loss increases likelihood that patients will achieve 5 to 10% weight loss from baseline.5,6 Studies looking at long-term effects of these medications on weight loss have found improvements in blood pressure (BP), biomarkers for cardiovascular disease, and T2DM-related comorbidities.3,5,7
Positive effects on comorbidities have been found to be related to drug class and mechanism of action (MOA); those that also are approved for T2DM have demonstrated the most favorable cardiovascular effects.7 Other medications that work as stimulants or as modulators of serotonin pathways are associated with increased risks, prompting the US Food and Drug Administration (FDA) to remove some medications from the market.7,8 In January 2020, lorcaserin was taken off the market because of increased risk of cancer found in postmarketing surveillance.9 The benefit of weight loss must be weighed against the risk of medication use.
Monthly follow-up is recommended with weight management medications in the beginning to assess safety and efficacy; medications should be discontinued if weight loss is inadequate in the first 3 months.1,3,4 Limited studies have assessed the long-term use of weight management medications in a real-world setting. Medications are prescribed for weight management at Veteran Health Indiana (VHI) in outpatient clinics, including primary care, endocrinology, and gastrointestinal (GI) specialties. However, prescribing practices, outcomes, and adherence to guideline recommendations have not been studied. Data from this study will be used to better understand how VHI can serve its veterans through diet, lifestyle, and pharmacologic interventions.
Methods
We conducted a single-center, retrospective chart review for patients started on weight management medications at VHI. A patient list was generated based on prescription fills from June 1, 2017 to June 30, 2019. All data were obtained using the Computerized Patient Record System and patients were not contacted. This study was approved by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
At the time of study, orlistat, liraglutide, phentermine/topiramate,
Patients were included in the study if they received a prescription of any 1 of the 5 available medications during the enrollment period. Patients were excluded if they received a prescription from or were treated by a civilian health care provider, if they never used the medication, or if their weight loss was attributed to a cancer diagnosis. These criteria produced 86 patients of whom 96 unique weight loss prescriptions were generated. Data were collected for each instance of medication use so that some patients were included multiple times. In this case, data collection for the failed medication ended when failure was documented, and new data points began when new medication was prescribed; all data collected were per medication, not per patient. This method was used to account for medication failure and provide accurate weight loss results based on medication choice within this institution.
The primary outcomes included total weight loss and weight loss as a percentage of baseline weight at 3, 6, 12, and > 12 months of therapy. Secondary outcomes included weight loss of 5% from baseline, rate of successful weight maintenance after initial weight loss of 5% from baseline, adverse drug reaction (ADR) monitoring, and use of weight management medications across clinics at VHI.
Demographic data included race, age, sex, baseline weight, BMI, and comorbid medical conditions. Comorbidities were collected based on the most recent primary care clinical note before initiating medication. Medication data collected included medications used to manage comorbidities. Data related to weight management medication included prescribing clinic, reason for medication discontinuation, or bariatric surgery intervention if applicable.
Efficacy outcome data included weight and BMI across therapy duration. Safety outcomes data included heart rate, BP, and ADRs that resulted in medication discontinuation as documented in the electronic health record (EHR).
We used descriptive statistics, including mean, standard deviation (SD), range, and percentage. For continuous data, Kruskal-Wallis tests were used because of nonparametric data distribution among the different medications with a prespecified α = 0.05. With the observed sample sizes and SDs in this study, post hoc poststudy power calculations showed that the study had 80% power at a 5% significance level to detect weight changes of 8.6 kg, 7.3 kg, and 12.4 kg at 3, 6, and 12 months, respectively, using nonparametric tests.
Results
A total of 86 patients were identified based on prescription fills, which produced 99 unique instances of medication use. Of the 99 identified, 3 met exclusion criteria and were not included in the final analysis. Among included veterans, 16 were female and 80 were male (Table 1). Most of those included identified as White race (86%), male (83%), and mean age 53 years. At baseline, mean weight was 130 kg and mean BMI 41.
Comorbidities and Medication Use
Hypertension (66%), hyperlipidemia (64%), and psychiatric diagnoses (50%) were most common comorbid conditions. Substance use (23%) and T2DM (40%) were the most common comorbidities influencing medication choice. Substance use evaluation included amphetamines and cocaine for this analysis.
Phentermine/topiramate is the preferred first-line agent unless patients have contraindications for use, in which case naltrexone/bupropion is recommended, based on guidelines for weight management medications within the VHI system. However, for patients with comorbid T2DM, liraglutide is preferred because of its beneficial effects for both weight loss and blood glucose control.2 Most patients at VHI were started on liraglutide (44%) or phentermine/topiramate (42%), which was in line with recommendations. Our sample included ≥ 1 prescription for each medication available at our facility, although the number of patients on each medication was not equal. Of note, the one patient taking lorcaserin at the time of study discontinued therapy in response to recent FDA guidance.9
Medications for comorbid conditions could contribute to weight gain. Of the patient sample, β blockers (n = 24) and anticonvulsants, including gabapentin and pregabalin (n = 22) were the most common Other medications that could have contributed to weight gain included sulfonylureas (n = 5), antipsychotics (n = 4), tricyclic antidepressants (n = 2), and hormone replacement therapies (n = 2).
Primary Outcomes
The mean weight of participants dropped from 129.9 to 114.2 kg over the 12 months of weight management medication therapy for a absolute difference of 15.8 kg (Figure 1 and eTable 1 available at doi:10.12788/fp.0117). Weight loss was recorded at 3, 6, 12, and > 12 months of weight management therapy. At each time point, weight loss was statistically significant (P < .001) compared with baseline (Table 2), even though not every patient had weight loss records at each time point.
When classified by medication choice,
Secondary Outcomes
More than one-half of the patients analyzed lost 5 to 10% from baseline while taking weight management medication.
Among patients who lost at least 5% from baseline, we performed further analysis to assess weight maintenance of 3 to 5% from baseline for 12 months.
We found that most of our prescriptions (n = 50) were entered by the endocrinology department in conjunction with the MOVE! program (eTable 3 available at doi:10.12788/fp.0117). All 4 of our primary care clinics prescribed weight loss medication; however, 1 clinic prescribed the most. Other prescriptions came from community-based outpatient clinics or other specialties, including gastroenterology, orthopedics, and sleep medicine.
Nineteen (18%) patients experienced an adverse event (AE) that led to medication discontinuation, which was recorded in their chart (eTable 4 available at doi:10.12788/fp.0117). Most common AEs were GI upset with liraglutide or orlistat or dull aching and pain with phentermine/topiramate. Two severe AEs occurred: One patient experienced a change in mental health status and suicide attempt with naltrexone/bupropion; and 1 patient discontinued phentermine/topiramate because of a change in neurologic status.
Primarily medications were stopped because of inadequate weight loss (n = 13), and most patients tried additional medications. However, 1 medication failure resulted in sleeve gastrectomy. Other reasons for medication discontinuation included missed MOVE! appointments, patient lost to follow-up, and patient-elected discontinuation.
Discussion
This study evaluated the use and outcomes of weight management medication among veterans at VHI. The study aimed to better understand the efficacy and safety of these medications while exposing potential weaknesses in care and to promote avenues to improve weight loss and maintenance.
Clinical trials for weight management medications reported weight loss of 8 to 10 kg over 56 weeks: 21 to 63% of patients losing at least 5% from baseline weight.10-14 The findings from our study found a higher average weight loss (−15.8 kg) than that reported in trials and a consistent percentage of patients (58.3%) who achieved at least 5% weight loss. It is promising to see that when used in a noncontrolled setting, these medications were able to produce weight loss consistent with results seen in large, controlled trials.
Pi-Sunyer and colleagues found continued weight loss after the initial 5% weight loss to an eventual 10% weight loss in many patients.10 Additionally, Smith and colleagues found that nearly 68% of their participants who took lorcaserin were able to maintain 3 to 5% weight loss over 12 months.13 Sjöström and colleagues acknowledged that many patients taking orlistat for an extended period began to gain weight, although at one-half the rate than that seen in the placebo group.12 This study found that fewer patients were able to maintain their weight loss over 12 months, with only 30% of patients maintaining 3 to 5% weight loss from baseline. This difference in weight maintenance likely was because of the uncontrolled nature of this study. Once patients reach their initial weight loss goal, even the most motivated patients will have trouble maintaining that weight.4 Despite the challenges associated with maintaining weight loss, the quality of life benefits patients gained and potential reductions in health care spending support using resources to improve these outcomes.2,14,15
Pi-Sunyer and colleagues reported high incidences of nausea (40%), vomiting (16%), diarrhea (21%), and constipation (20%) with liraglutide.10 Sjöström and colleagues reported 7% of patients experienced GI upset with orlistat.12 Comparatively, only 17% of our patients reported AEs that required discontinuation, including GI upset. One patient in our study discontinued naltrexone/bupropion because of a significant change in mental status and suicide attempt. Clinical trials did not report a greater risk of depression or suicidality compared with placebo; however, there is a warning on the labeling of naltrexone/bupropion for increased suicidality with the use of antidepressant agents.16,17 The neurologic AE that required discontinuation of phentermine/topiramate at our institution is unique based on published information.11,18
The data from this study reinforced the observation that weight maintenance is the most challenging aspect of weight loss. Although our data showed clinically meaningful weight loss from baseline, many patients regained their weight, and some exceeded their baseline weight. Beyond providing these medications, this evidence suggests the need for close, continued follow-up through patients’ weight loss journey.
Limitations
Because this is a retrospective chart review, data collection was influenced by and limited to information that had been recorded in the EHR. AEs that resulted in medication discontinuation were assessed from the patient’s chart, which might not be correct if providers did not update the records. Follow-up was not always scheduled at regular intervals after medication initiation, resulting in varying sample numbers at each time point, potentially interfering with true weight loss averages. Although not included in this analysis, it might be beneficial to evaluate adherence to recommendations for follow-up with laboratory and weight monitoring to better capture where future monitoring can be improved. Second, there was an unbalanced number of patients taking each medication. Specifically, we saw a change in weight with orlistat that exceeded what is consistently seen in larger, more controlled trials. Although this is an effect of the real world, small sample sizes cannot be generalized to the larger population and might result in data reflecting that of an outlier. Last, there is a lack of generalizability because of the veteran population demographic, which is more male and lacks ethnic diversity. This study also was carried out at a single, educational tertiary medical center, which might not apply to all populations.
Conclusions
Despite the limitations discussed, this study shows that the use of weight management medications in a general veteran population produces initial weight loss consistent with previous studies. However, there is room for continued improvement in follow-up strategies to promote greater weight maintenance after initial weight loss. Considering the high health care costs, personal burden, and potential long-term complications associated with obesity, efforts to promote development of programs that support weight management and maintenance are imperative.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
The American Heart Association, the American College of Cardiology, and the Obesity Society define overweight as a body mass index (BMI) of 25 to 29.9 and obesity as a BMI ≥ 30. Morbid obesity is defined as a BMI ≥ 35 or 40.2,3 Based on these BMI cutoffs, the Endocrine Society recommends diet and lifestyle as the foundation of weight management and pharmacotherapy for those with a BMI ≥ 30 without comorbidities. In patients with a BMI ≥ 27, weight management medications may be considered if a patient has comorbid hypertension, T2DM, dyslipidemia, metabolic syndrome, obstructive sleep apnea, or nonalcoholic fatty liver disease. Patients with BMI > 40 are eligible for weight loss surgery.4
Lifestyle and dietary interventions are the foundation of current weight management guidelines from the Endocrine Society.4 At a minimum, guidelines recommended enrolling motivated patients in a high-intensity lifestyle intervention class of at least 14 sessions in the first 6 months to reach a goal weight loss of 5 to 10% from baseline and to maintain a reduction of 3 to 5% from baseline.3 Medications are recommended as an adjunct to lifestyle and dietary changes. Most weight management medications work in the brain to stimulate satiety signaling, which helps motivated patients adhere to their dietary interventions, assist those who have been unsuccessful in earlier weight loss attempts, and help maintain weight.3,4
Guidelines recommend 7 weight management medications, including orlistat (both prescription strength and over-the-counter), liraglutide, phentermine, phentermine/topiramate, lorcaserin, and naltrexone/bupropion. Using medications to assist with weight loss increases likelihood that patients will achieve 5 to 10% weight loss from baseline.5,6 Studies looking at long-term effects of these medications on weight loss have found improvements in blood pressure (BP), biomarkers for cardiovascular disease, and T2DM-related comorbidities.3,5,7
Positive effects on comorbidities have been found to be related to drug class and mechanism of action (MOA); those that also are approved for T2DM have demonstrated the most favorable cardiovascular effects.7 Other medications that work as stimulants or as modulators of serotonin pathways are associated with increased risks, prompting the US Food and Drug Administration (FDA) to remove some medications from the market.7,8 In January 2020, lorcaserin was taken off the market because of increased risk of cancer found in postmarketing surveillance.9 The benefit of weight loss must be weighed against the risk of medication use.
Monthly follow-up is recommended with weight management medications in the beginning to assess safety and efficacy; medications should be discontinued if weight loss is inadequate in the first 3 months.1,3,4 Limited studies have assessed the long-term use of weight management medications in a real-world setting. Medications are prescribed for weight management at Veteran Health Indiana (VHI) in outpatient clinics, including primary care, endocrinology, and gastrointestinal (GI) specialties. However, prescribing practices, outcomes, and adherence to guideline recommendations have not been studied. Data from this study will be used to better understand how VHI can serve its veterans through diet, lifestyle, and pharmacologic interventions.
Methods
We conducted a single-center, retrospective chart review for patients started on weight management medications at VHI. A patient list was generated based on prescription fills from June 1, 2017 to June 30, 2019. All data were obtained using the Computerized Patient Record System and patients were not contacted. This study was approved by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
At the time of study, orlistat, liraglutide, phentermine/topiramate,
Patients were included in the study if they received a prescription of any 1 of the 5 available medications during the enrollment period. Patients were excluded if they received a prescription from or were treated by a civilian health care provider, if they never used the medication, or if their weight loss was attributed to a cancer diagnosis. These criteria produced 86 patients of whom 96 unique weight loss prescriptions were generated. Data were collected for each instance of medication use so that some patients were included multiple times. In this case, data collection for the failed medication ended when failure was documented, and new data points began when new medication was prescribed; all data collected were per medication, not per patient. This method was used to account for medication failure and provide accurate weight loss results based on medication choice within this institution.
The primary outcomes included total weight loss and weight loss as a percentage of baseline weight at 3, 6, 12, and > 12 months of therapy. Secondary outcomes included weight loss of 5% from baseline, rate of successful weight maintenance after initial weight loss of 5% from baseline, adverse drug reaction (ADR) monitoring, and use of weight management medications across clinics at VHI.
Demographic data included race, age, sex, baseline weight, BMI, and comorbid medical conditions. Comorbidities were collected based on the most recent primary care clinical note before initiating medication. Medication data collected included medications used to manage comorbidities. Data related to weight management medication included prescribing clinic, reason for medication discontinuation, or bariatric surgery intervention if applicable.
Efficacy outcome data included weight and BMI across therapy duration. Safety outcomes data included heart rate, BP, and ADRs that resulted in medication discontinuation as documented in the electronic health record (EHR).
We used descriptive statistics, including mean, standard deviation (SD), range, and percentage. For continuous data, Kruskal-Wallis tests were used because of nonparametric data distribution among the different medications with a prespecified α = 0.05. With the observed sample sizes and SDs in this study, post hoc poststudy power calculations showed that the study had 80% power at a 5% significance level to detect weight changes of 8.6 kg, 7.3 kg, and 12.4 kg at 3, 6, and 12 months, respectively, using nonparametric tests.
Results
A total of 86 patients were identified based on prescription fills, which produced 99 unique instances of medication use. Of the 99 identified, 3 met exclusion criteria and were not included in the final analysis. Among included veterans, 16 were female and 80 were male (Table 1). Most of those included identified as White race (86%), male (83%), and mean age 53 years. At baseline, mean weight was 130 kg and mean BMI 41.
Comorbidities and Medication Use
Hypertension (66%), hyperlipidemia (64%), and psychiatric diagnoses (50%) were most common comorbid conditions. Substance use (23%) and T2DM (40%) were the most common comorbidities influencing medication choice. Substance use evaluation included amphetamines and cocaine for this analysis.
Phentermine/topiramate is the preferred first-line agent unless patients have contraindications for use, in which case naltrexone/bupropion is recommended, based on guidelines for weight management medications within the VHI system. However, for patients with comorbid T2DM, liraglutide is preferred because of its beneficial effects for both weight loss and blood glucose control.2 Most patients at VHI were started on liraglutide (44%) or phentermine/topiramate (42%), which was in line with recommendations. Our sample included ≥ 1 prescription for each medication available at our facility, although the number of patients on each medication was not equal. Of note, the one patient taking lorcaserin at the time of study discontinued therapy in response to recent FDA guidance.9
Medications for comorbid conditions could contribute to weight gain. Of the patient sample, β blockers (n = 24) and anticonvulsants, including gabapentin and pregabalin (n = 22) were the most common Other medications that could have contributed to weight gain included sulfonylureas (n = 5), antipsychotics (n = 4), tricyclic antidepressants (n = 2), and hormone replacement therapies (n = 2).
Primary Outcomes
The mean weight of participants dropped from 129.9 to 114.2 kg over the 12 months of weight management medication therapy for a absolute difference of 15.8 kg (Figure 1 and eTable 1 available at doi:10.12788/fp.0117). Weight loss was recorded at 3, 6, 12, and > 12 months of weight management therapy. At each time point, weight loss was statistically significant (P < .001) compared with baseline (Table 2), even though not every patient had weight loss records at each time point.
When classified by medication choice,
Secondary Outcomes
More than one-half of the patients analyzed lost 5 to 10% from baseline while taking weight management medication.
Among patients who lost at least 5% from baseline, we performed further analysis to assess weight maintenance of 3 to 5% from baseline for 12 months.
We found that most of our prescriptions (n = 50) were entered by the endocrinology department in conjunction with the MOVE! program (eTable 3 available at doi:10.12788/fp.0117). All 4 of our primary care clinics prescribed weight loss medication; however, 1 clinic prescribed the most. Other prescriptions came from community-based outpatient clinics or other specialties, including gastroenterology, orthopedics, and sleep medicine.
Nineteen (18%) patients experienced an adverse event (AE) that led to medication discontinuation, which was recorded in their chart (eTable 4 available at doi:10.12788/fp.0117). Most common AEs were GI upset with liraglutide or orlistat or dull aching and pain with phentermine/topiramate. Two severe AEs occurred: One patient experienced a change in mental health status and suicide attempt with naltrexone/bupropion; and 1 patient discontinued phentermine/topiramate because of a change in neurologic status.
Primarily medications were stopped because of inadequate weight loss (n = 13), and most patients tried additional medications. However, 1 medication failure resulted in sleeve gastrectomy. Other reasons for medication discontinuation included missed MOVE! appointments, patient lost to follow-up, and patient-elected discontinuation.
Discussion
This study evaluated the use and outcomes of weight management medication among veterans at VHI. The study aimed to better understand the efficacy and safety of these medications while exposing potential weaknesses in care and to promote avenues to improve weight loss and maintenance.
Clinical trials for weight management medications reported weight loss of 8 to 10 kg over 56 weeks: 21 to 63% of patients losing at least 5% from baseline weight.10-14 The findings from our study found a higher average weight loss (−15.8 kg) than that reported in trials and a consistent percentage of patients (58.3%) who achieved at least 5% weight loss. It is promising to see that when used in a noncontrolled setting, these medications were able to produce weight loss consistent with results seen in large, controlled trials.
Pi-Sunyer and colleagues found continued weight loss after the initial 5% weight loss to an eventual 10% weight loss in many patients.10 Additionally, Smith and colleagues found that nearly 68% of their participants who took lorcaserin were able to maintain 3 to 5% weight loss over 12 months.13 Sjöström and colleagues acknowledged that many patients taking orlistat for an extended period began to gain weight, although at one-half the rate than that seen in the placebo group.12 This study found that fewer patients were able to maintain their weight loss over 12 months, with only 30% of patients maintaining 3 to 5% weight loss from baseline. This difference in weight maintenance likely was because of the uncontrolled nature of this study. Once patients reach their initial weight loss goal, even the most motivated patients will have trouble maintaining that weight.4 Despite the challenges associated with maintaining weight loss, the quality of life benefits patients gained and potential reductions in health care spending support using resources to improve these outcomes.2,14,15
Pi-Sunyer and colleagues reported high incidences of nausea (40%), vomiting (16%), diarrhea (21%), and constipation (20%) with liraglutide.10 Sjöström and colleagues reported 7% of patients experienced GI upset with orlistat.12 Comparatively, only 17% of our patients reported AEs that required discontinuation, including GI upset. One patient in our study discontinued naltrexone/bupropion because of a significant change in mental status and suicide attempt. Clinical trials did not report a greater risk of depression or suicidality compared with placebo; however, there is a warning on the labeling of naltrexone/bupropion for increased suicidality with the use of antidepressant agents.16,17 The neurologic AE that required discontinuation of phentermine/topiramate at our institution is unique based on published information.11,18
The data from this study reinforced the observation that weight maintenance is the most challenging aspect of weight loss. Although our data showed clinically meaningful weight loss from baseline, many patients regained their weight, and some exceeded their baseline weight. Beyond providing these medications, this evidence suggests the need for close, continued follow-up through patients’ weight loss journey.
Limitations
Because this is a retrospective chart review, data collection was influenced by and limited to information that had been recorded in the EHR. AEs that resulted in medication discontinuation were assessed from the patient’s chart, which might not be correct if providers did not update the records. Follow-up was not always scheduled at regular intervals after medication initiation, resulting in varying sample numbers at each time point, potentially interfering with true weight loss averages. Although not included in this analysis, it might be beneficial to evaluate adherence to recommendations for follow-up with laboratory and weight monitoring to better capture where future monitoring can be improved. Second, there was an unbalanced number of patients taking each medication. Specifically, we saw a change in weight with orlistat that exceeded what is consistently seen in larger, more controlled trials. Although this is an effect of the real world, small sample sizes cannot be generalized to the larger population and might result in data reflecting that of an outlier. Last, there is a lack of generalizability because of the veteran population demographic, which is more male and lacks ethnic diversity. This study also was carried out at a single, educational tertiary medical center, which might not apply to all populations.
Conclusions
Despite the limitations discussed, this study shows that the use of weight management medications in a general veteran population produces initial weight loss consistent with previous studies. However, there is room for continued improvement in follow-up strategies to promote greater weight maintenance after initial weight loss. Considering the high health care costs, personal burden, and potential long-term complications associated with obesity, efforts to promote development of programs that support weight management and maintenance are imperative.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Centers for Disease Control and Prevention. Adult obesity facts. Accessed April 2020. https://www.cdc.gov/obesity/data/adult.html
2. The Management of Overweight and Obesity Working Group. VA/DoD Clinical Practice Guideline for Screening and Management of Overweight and Obesity. Accessed March 13, 2021. https://www.healthquality.va.gov/guidelines/CD/obesity/VADoDCPGManagementOfOverweightAndObesityFinal.pdf
3. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25, pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
4. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100(2):342-362. doi:10.1210/jc.2014-3415
5. Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194-1199. doi:10.1136/bmj.39385.413113.25
6. Siebenhofer A, Winterholer, S, Jeitler K, et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev 2021;1:CD007654. doi:10.1002/14651858.CD007654.pub5
7. Bramante CT, Raatz S, Bomber EM, Oberle MM, Ryder JR. Cardiovascular risks and benefits of medications used for weight loss. Front Endocrinol (Lausanne). 2020;10:883. doi:10.3389/fendo.2019.00883
8. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomized trials. Lancet. 2007;370(9600):1706-1713. doi:10.1016/S0140-6736(07)61721-8
9. US Food and Drug Administration. FDA requests the withdrawal of the weight-loss drug Blevique, Belvique XR (lorcaserin) from the market. Accessed April 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
10. Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
11. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
12. Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016/s0140-6736(97)11509-4
13. Smith SR, Weissman NJ, Anderson CM, et al; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight loss. N Engl J Med. 2010;363(3):245-256. doi:10.1056/NEJMoa0909809
14. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev. 2014;15(3):169-182. doi:10.1111/obr.12113
15. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822-831. doi:10.1377/hlthaff.28.5.w822
16. Greenway FL, Fujioka K, Plodkowski RA, et al; COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicenter, randomized, double-blind, placebo-controlled phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
17. Contrave. Prescribing information. Nalpropion Pharmaceuticals, Inc; 2019.
18. Qsymia. Prescribing information. VIVUS Inc; 2018.
1. Centers for Disease Control and Prevention. Adult obesity facts. Accessed April 2020. https://www.cdc.gov/obesity/data/adult.html
2. The Management of Overweight and Obesity Working Group. VA/DoD Clinical Practice Guideline for Screening and Management of Overweight and Obesity. Accessed March 13, 2021. https://www.healthquality.va.gov/guidelines/CD/obesity/VADoDCPGManagementOfOverweightAndObesityFinal.pdf
3. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25, pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
4. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100(2):342-362. doi:10.1210/jc.2014-3415
5. Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194-1199. doi:10.1136/bmj.39385.413113.25
6. Siebenhofer A, Winterholer, S, Jeitler K, et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev 2021;1:CD007654. doi:10.1002/14651858.CD007654.pub5
7. Bramante CT, Raatz S, Bomber EM, Oberle MM, Ryder JR. Cardiovascular risks and benefits of medications used for weight loss. Front Endocrinol (Lausanne). 2020;10:883. doi:10.3389/fendo.2019.00883
8. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomized trials. Lancet. 2007;370(9600):1706-1713. doi:10.1016/S0140-6736(07)61721-8
9. US Food and Drug Administration. FDA requests the withdrawal of the weight-loss drug Blevique, Belvique XR (lorcaserin) from the market. Accessed April 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
10. Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
11. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
12. Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016/s0140-6736(97)11509-4
13. Smith SR, Weissman NJ, Anderson CM, et al; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight loss. N Engl J Med. 2010;363(3):245-256. doi:10.1056/NEJMoa0909809
14. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev. 2014;15(3):169-182. doi:10.1111/obr.12113
15. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822-831. doi:10.1377/hlthaff.28.5.w822
16. Greenway FL, Fujioka K, Plodkowski RA, et al; COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicenter, randomized, double-blind, placebo-controlled phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
17. Contrave. Prescribing information. Nalpropion Pharmaceuticals, Inc; 2019.
18. Qsymia. Prescribing information. VIVUS Inc; 2018.