User login
The Effect of an Inpatient Smoking Cessation Treatment Program on Hospital Readmissions and Length of Stay
Successful smoking cessation interventions result in substantial gains in health and life expectancy by reducing smoking-related illnesses and preventing premature deaths.1,2 The Department of Health and Human Services recommends clinicians use hospitalization as “an opportunity to promote smoking cessation’’ and ‘‘to prescribe medications to alleviate withdrawal symptoms”3 because individual readiness to quit may be high during hospitalizations. A meta-analysis of 50 studies (21 from the United States) examining the efficacy of hospital-initiated smoking cessation interventions concluded that smoking cessation support programs that began in the hospital and continued for at least 1 month postdischarge significantly increase the likelihood of patients being smoke-free in the long term.4 The most efficacious strategies included counseling and pharmacotherapy rather than counseling alone.3 Most inpatient smoking cessation studies have focused on quit-rates or medical outcomes, while fewer studies have looked at healthcare utilization.
However, previous research has shown that smoking cessation for inpatients has relatively immediate economic and health benefits. Patients who quit smoking during hospitalizations for cardiovascular disease are less likely to be readmitted or to die during follow-up.5,6 Patients with acute myocardial infarction (AMI), unstable angina, heart failure, and chronic obstructive pulmonary disease who received an inpatient smoking cessation intervention had reductions in inpatient readmission rates.7 A 1% reduction in overall smoking rates would lead to an annual reduction of 3,022 hospitalizations for stroke and 1,684 hospitalizations for AMI.8 One comprehensive program, the Ottawa Model for Smoking Cessation (OMSC), found that a hospital-initiated intervention increased long-term cessation rates by 15% in cardiac patients and by 11% in general hospital populations.9,10 The applicability of this result to US healthcare systems is unknown. This paper adds to the existing literature by evaluating the impact of an inpatient smoking cessation program on healthcare utilization among patients hospitalized for any reason, rather than solely focused on those with cardiopulmonary diagnoses.
The current study focuses on an inpatient smoking cessation program at a teaching hospital in the Rocky Mountain region. The hospital implemented a smoking cessation treatment program on July 1, 2013, based on the OMSC. The goal was to identify and support inpatient adult smokers who wanted to make a quit attempt and help them remain smoke-free after discharge. The objective of the current study was to determine the effect of the program on 30-day readmission rates and length of stay (LOS) of the index hospitalization. Although the general cost effectiveness of properly structured smoking cessation programs are well established,11-13 the healthcare utilization effects of inpatient smoking cessation programs are not well understood.
METHODS
Data
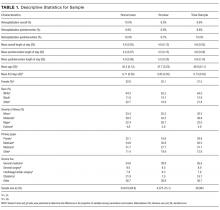
The study population consists of patients over age 18 who were admitted to the hospital between July 1, 2012, and July 1, 2014. Baseline smoking status was assessed at hospital admission and recorded in Epic (Epic Systems Corporation, Verona, Wisconsin), the electronic medical records system, as a current smoker (every day and some days), former smoker, never smoker, and never assessed. To check the accuracy of recorded smoking status, a random sample of 819 inpatients was selected and contacted via telephone for verification; 93% of Epic-identified smokers confirmed that they were smokers at hospital admission.14
Intervention
The intervention, which launched July 13, 2014, modified the Epic system to automatically alert providers viewing a tobacco user’s medical record that the patient should receive standardized orders for a bedside consultation with a Tobacco Treatment Specialist (TTS) and a prescription for nicotine replacement therapy (NRT) while in the hospital.15 Previously, referrals for tobacco treatment were done on an ad-hoc basis by the physician, and NRT was not routinely available. This system-level intervention standardized and automated the referral process. For patients with a bedside consultation order, TTS used a patient-centered approach (motivational interviewing) to explore patients’ motivation to quit smoking and offered NRT to improve comfort and safety while in the hospital. Patients who chose to make a quit attempt received a free 2-week supply of NRT at discharge and 6 months of free follow-up counseling by interactive voice response (IVR) telephone technology that included (a) prerecorded advice keyed to individual patient needs, (b) a warm-transfer option to speak with a live TTS (later dropped), and (c) a collection of patient smoking and cessation treatment measures.15
Statistical Analysis
We used an intent-to-treat (ITT) framework for the analysis, which considers everyone eligible for the treatment to be in the treatment group. The approach ignores treatment nonacceptance, nonadherence, protocol deviations, withdrawal from treatment, and cessation outcomes,
Readmission rates and LOS were estimated by using a “difference-in-differences” model, comparing outcomes between smokers before versus after the introduction of the cessation treatment program with nonsmokers before versus after program introduction. The difference-in-differences method looks at the difference pre-and-post in the exposed group (smokers) and unexposed group (nonsmokers). Subtracting the difference between the 2 groups gives an estimate of the policy effect controlling for background trends.19 The smoking cessation treatment effect on readmission is measured by the coefficient on the interaction term between the smoking variable and an indicator that the program is operational. The coefficient is the “difference-in-differences.”
Other control variables include demographic factors (gender, age, race), hospitalization payer (Medicare, Medicaid, commercial), and the service line of the admission. We also included a severity of illness variable from the APR-DRG Grouper (3M, Maplewood, Minnesota)20 and the number of days spent in the intensive care unit. For the readmission model, we included LOS as a control variable, because individuals with longer LOS had a better opportunity to access the intervention.
For readmissions, the model was estimated by using a probit model, predicting the effect of each of the intervention variables and the control variables on the marginal probability of a readmission. Because patients can appear in both the pre- and postyears, clustered standard errors were used, which correct for the lack of independence from multiple observations from the same individual.21 For LOS, a truncated negative binomial model was used. The negative binomial model is a specification for count models with a mass of observations plus a long right tail. The truncation is because zero and negative values for LOS are not possible. The dependent variable represents the number of days the individual was hospitalized. For both models, the reported coefficients represent the marginal effect of the independent variable on the dependent variable. This was calculated using the “margins” command in Stata version 13 (StataCorp LLC, College Station, Texas).
RESULTS
In the probit analysis, the smoking cessation intervention (Smoker*post intervention) showed no significant effect on the probability of readmission (Table 2). The coefficient is positive (β = 0.008) and statistically insignificant (P = 0.36). This indicates that we failed to reject the null hypothesis that there was not a systematic difference in the probability of readmission because of the smoking cessation intervention. Other significant variables generally had the expected relationship with readmission rates. Smokers were 1.6% less likely to be readmitted than nonsmokers (P = 0.01), controlling for other factors.
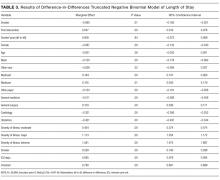
The program effect on smoker LOS was statistically insignificant (β = 0.008; P = 0.36). Smokers overall had a shorter LOS than nonsmokers (β = −0.090; P = 0.01), controlling for other factors. Overall LOS was longer postintervention (β = 0.047; P < 0.01). The control variables generally had the same relationship for the LOS model as for the readmission model.
DISCUSSION
This study investigated the effect of an inpatient smoking cessation program, based on a successful Canadian model, on inpatient readmission rates and LOS. The program showed no effect on 30-day readmission rates or LOS. We see several potential explanations for the absence of a detectable impact.
First, the ITT approach reflected real-world implementation of smoking cessation services. The ITT approach adopts the hospital’s perspective because the hospital will assess overall effectiveness without regard to programmatic limitations. The intervention group for this analysis included individuals who were offered but declined treatment, individuals who accepted treatment but failed to quit smoking, and individuals who both accepted treatment and quit smoking. If the analysis had focused only on the latter group, an effect would have been more likely to be found. Further analysis of the subset of patients who accepted the intervention and quit smoking is warranted. Nevertheless, hospitals cannot expect all inpatient smokers, or even a majority, to embrace an offer of cessation treatment. This also emphasizes the challenges hospitals will face in offering tobacco cessation programs to smokers in a timely way. Reasons for patients not receiving orders varied but included issues with weekend admissions.
Second, the timeframe of the analysis is limited to the inpatient stay (for LOS) and 30 days (for readmission). A longer-term analysis might have found an effect. However, we examined this from the hospital perspective. For the hospital, LOS is a key cost driver; thus, reductions in LOS would create a strong financial incentive for hospitals to implement smoking cessation programs. Similarly, reducing readmissions is now a priority for hospitals because of new Medicare rules that penalize hospitals for readmissions. Thus, the 2 outcomes we examined are outcomes that are financially important to hospitals.
There are several limitations to our analysis. First, the difference-in-differences model assumes that in the absence of treatment, the average change in the dependent variables would have been the same for both the treatment and control groups, also known as the parallel trends assumption. Specification tests showed this assumption was met for the preperiod. Second, our study relies on electronic health record data to identify smokers. However, 93% of individuals who were identified as smokers confirmed their smoking status upon interview. Finally, we looked at all categories of inpatient admissions. Improvement in LOS and short-term readmission rates may be limited to patients admitted for specific conditions, such as cardiovascular and respiratory conditions.
There are a number of plausible reasons for our null finding. First, the “dose” of intervention may have been too weak; that is, the number of smokers who were offered the treatment, accepted the treatment, and adhered to the treatment may have been too low, leading to too few smokers quitting smoking and, thus, no effect of the intervention on our outcomes. This follows directly from the ITT design of the study.23 This suggests that hospitals who wish to adopt smoking cessation programs need to focus on ensuring a timely offering of treatment and encouragement of uptake by smokers.
A second reason for the null finding may have been the short duration for the NRT, which was only offered for 2 weeks. Research suggests that use of NRT for less than 4 weeks is associated with a reduced likelihood of smoking cessation.24 However, a review of the literature concludes that the duration of NRT is less important than the dosage and the combination of NRT with other forms of smoking cessation therapy.25 It is important to note that this study used NRT; other treatments such as Chantix could have different effectiveness.26,27 Further research on different treatment approaches, including longer duration of NRT, would be appropriate.
Disclosure
The authors have no competing interests or conflicts to report. The study was supported by contract number 15FLA68717 from the Colorado Department of Public Health and Environment.
1. Taylor DH Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of Smoking Cessation for Longevity. Am J Public Health. 2002;92(6):990-996. PubMed
2. Weitkunat R, Coggins CRE, Sponsiello-Wang Z, Kallischnigg G, Dempsey R. Assessment of Cigarette Smoking in Epidemiologic Studies. Tobacco Research. 2013;25(7).
3. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville (MD): US Department of Health and Human Services. 2008. https://www.ncbi.nlm.nih.gov/books/NBK63952/.
4. Rigotti, NA, Clair, C, Munafo, MR, et al., Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012(5): CD001837. PubMed
5. Ladapo JA, Jaffer FA, Weinstein MC, Froelicher ES. Projected cost-effectiveness of smoking cessation interventions in patients hospitalized with myocardial infarction. Arch Intern Med. 2011;171:39-45. PubMed
6. Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446-452. PubMed
7. Mullen K, Coyle D, Manuel D, et al. Economic evaluation of a hospital-initiated intervention for smokers with chronic disease, in Ontario, Canada. Tob Control. 2015;24(5):489-496. PubMed
8. Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96(4):1089-1096. PubMed
9. Reid RD, Mullen KA, Slovinec D’Angelo ME, et al. Smoking cessation for hospitalized smokers: an evaluation of the “Ottawa Model.” Nicotine Tob Res. 2010;12:11-18. PubMed
10. Reid RD, Pipe AL, Quinlan B. Promoting smoking cessation during hospitalization for coronary artery disease. Can J Cardiol. 2006;22:775-780. PubMed
11. Krumholz H, Cohen B, Tsevat J, Pasternak R, Weinstein M. Cost-effectiveness of a smoking cessation program after myocardial infarction. J Am Coll Cardiol. 1993;22(6):1697-1702. PubMed
12. Curry S, Grothaus L, McAfee T, Pabiniak C. Use and Cost Effectiveness of Smoking-Cessation Services under Four Insurance Plans in a Health Maintenance Organization. N Engl J Med. 1998;339:673-679. PubMed
12. Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA. 1996;275:1247-1251. PubMed
13. CEPEG. Independent Evaluation: University of Colorado Hospital’s Smoking Cessation Treatment Program, preliminary report. Aurora: Colorado School of Public Health; 2014.
15. Cooper S, Pray S. Independent Evaluation: Hospital systems change to improve inpatient tobacco dependence treatment final results. Aurora: Colorado School of Public Health; June 2015.
16. Gupta S. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109-112. DOI:10.4103/2229-3485.83221 PubMed
17. Fisher LD, Dixon DO, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to treat in clinical trials. In: Peace KE, editor. Statistical Issues in Drug Research and Development. New York: Marcel Dekker; 1990:331-350
18. Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21(5):837-841. PubMed
19. Dimick JB, Ryan AM. Methods for Evaluating Changes in Health Care Policy: The Difference-in-Differences Approach. JAMA. 2014;312(22):2401-2402. DOI:10.1001/jama.2014.16153 PubMed
20. Averill R, Goldfield N, Steinbeck B, et al. Development of the All Patient Refined DRGs (APR-DRGs). Maplewood: 3M Health Information Systems; 1997. Report 8-9. PubMed
21. Cameron A, Miller D. A Practitioner’s Guide to Cluster-Robust Inference. J Hum Resour. 2015;50(2):317-372.
22. Cooper S, Pray S. Independent Evaluation: Hospital systems change to improve inpatient tobacco dependence treatment Final Results. Aurora: Colorado School of Public Health; April 2015.
23. Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109-112. DOI:10.4103/2229-3485.83221. PubMed
24. Zhang B, Cohen J, Bondy S. Duration of Nicotine Replacement Therapy Use and Smoking Cessation: A Population-Based Longitudinal Study. Am J Epidemiol. 2015;181(7):513-520. PubMed
25. Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;3:CD000146. DOI:10.1002/14651858.CD000146 PubMed
26. C, , . Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008;3:CD006103. DOI:10.1002/14651858.CD006103.pub3. PubMed
27. Hurt R, Sachs D, Glover E, et al. A Comparison of Sustained-Release Bupropion and Placebo for Smoking Cessation. N Engl J Med. 1997;337:1195-1202. PubMed
Successful smoking cessation interventions result in substantial gains in health and life expectancy by reducing smoking-related illnesses and preventing premature deaths.1,2 The Department of Health and Human Services recommends clinicians use hospitalization as “an opportunity to promote smoking cessation’’ and ‘‘to prescribe medications to alleviate withdrawal symptoms”3 because individual readiness to quit may be high during hospitalizations. A meta-analysis of 50 studies (21 from the United States) examining the efficacy of hospital-initiated smoking cessation interventions concluded that smoking cessation support programs that began in the hospital and continued for at least 1 month postdischarge significantly increase the likelihood of patients being smoke-free in the long term.4 The most efficacious strategies included counseling and pharmacotherapy rather than counseling alone.3 Most inpatient smoking cessation studies have focused on quit-rates or medical outcomes, while fewer studies have looked at healthcare utilization.
However, previous research has shown that smoking cessation for inpatients has relatively immediate economic and health benefits. Patients who quit smoking during hospitalizations for cardiovascular disease are less likely to be readmitted or to die during follow-up.5,6 Patients with acute myocardial infarction (AMI), unstable angina, heart failure, and chronic obstructive pulmonary disease who received an inpatient smoking cessation intervention had reductions in inpatient readmission rates.7 A 1% reduction in overall smoking rates would lead to an annual reduction of 3,022 hospitalizations for stroke and 1,684 hospitalizations for AMI.8 One comprehensive program, the Ottawa Model for Smoking Cessation (OMSC), found that a hospital-initiated intervention increased long-term cessation rates by 15% in cardiac patients and by 11% in general hospital populations.9,10 The applicability of this result to US healthcare systems is unknown. This paper adds to the existing literature by evaluating the impact of an inpatient smoking cessation program on healthcare utilization among patients hospitalized for any reason, rather than solely focused on those with cardiopulmonary diagnoses.
The current study focuses on an inpatient smoking cessation program at a teaching hospital in the Rocky Mountain region. The hospital implemented a smoking cessation treatment program on July 1, 2013, based on the OMSC. The goal was to identify and support inpatient adult smokers who wanted to make a quit attempt and help them remain smoke-free after discharge. The objective of the current study was to determine the effect of the program on 30-day readmission rates and length of stay (LOS) of the index hospitalization. Although the general cost effectiveness of properly structured smoking cessation programs are well established,11-13 the healthcare utilization effects of inpatient smoking cessation programs are not well understood.
METHODS
Data
The study population consists of patients over age 18 who were admitted to the hospital between July 1, 2012, and July 1, 2014. Baseline smoking status was assessed at hospital admission and recorded in Epic (Epic Systems Corporation, Verona, Wisconsin), the electronic medical records system, as a current smoker (every day and some days), former smoker, never smoker, and never assessed. To check the accuracy of recorded smoking status, a random sample of 819 inpatients was selected and contacted via telephone for verification; 93% of Epic-identified smokers confirmed that they were smokers at hospital admission.14
Intervention
The intervention, which launched July 13, 2014, modified the Epic system to automatically alert providers viewing a tobacco user’s medical record that the patient should receive standardized orders for a bedside consultation with a Tobacco Treatment Specialist (TTS) and a prescription for nicotine replacement therapy (NRT) while in the hospital.15 Previously, referrals for tobacco treatment were done on an ad-hoc basis by the physician, and NRT was not routinely available. This system-level intervention standardized and automated the referral process. For patients with a bedside consultation order, TTS used a patient-centered approach (motivational interviewing) to explore patients’ motivation to quit smoking and offered NRT to improve comfort and safety while in the hospital. Patients who chose to make a quit attempt received a free 2-week supply of NRT at discharge and 6 months of free follow-up counseling by interactive voice response (IVR) telephone technology that included (a) prerecorded advice keyed to individual patient needs, (b) a warm-transfer option to speak with a live TTS (later dropped), and (c) a collection of patient smoking and cessation treatment measures.15
Statistical Analysis
We used an intent-to-treat (ITT) framework for the analysis, which considers everyone eligible for the treatment to be in the treatment group. The approach ignores treatment nonacceptance, nonadherence, protocol deviations, withdrawal from treatment, and cessation outcomes,
Readmission rates and LOS were estimated by using a “difference-in-differences” model, comparing outcomes between smokers before versus after the introduction of the cessation treatment program with nonsmokers before versus after program introduction. The difference-in-differences method looks at the difference pre-and-post in the exposed group (smokers) and unexposed group (nonsmokers). Subtracting the difference between the 2 groups gives an estimate of the policy effect controlling for background trends.19 The smoking cessation treatment effect on readmission is measured by the coefficient on the interaction term between the smoking variable and an indicator that the program is operational. The coefficient is the “difference-in-differences.”
Other control variables include demographic factors (gender, age, race), hospitalization payer (Medicare, Medicaid, commercial), and the service line of the admission. We also included a severity of illness variable from the APR-DRG Grouper (3M, Maplewood, Minnesota)20 and the number of days spent in the intensive care unit. For the readmission model, we included LOS as a control variable, because individuals with longer LOS had a better opportunity to access the intervention.
For readmissions, the model was estimated by using a probit model, predicting the effect of each of the intervention variables and the control variables on the marginal probability of a readmission. Because patients can appear in both the pre- and postyears, clustered standard errors were used, which correct for the lack of independence from multiple observations from the same individual.21 For LOS, a truncated negative binomial model was used. The negative binomial model is a specification for count models with a mass of observations plus a long right tail. The truncation is because zero and negative values for LOS are not possible. The dependent variable represents the number of days the individual was hospitalized. For both models, the reported coefficients represent the marginal effect of the independent variable on the dependent variable. This was calculated using the “margins” command in Stata version 13 (StataCorp LLC, College Station, Texas).
RESULTS
In the probit analysis, the smoking cessation intervention (Smoker*post intervention) showed no significant effect on the probability of readmission (Table 2). The coefficient is positive (β = 0.008) and statistically insignificant (P = 0.36). This indicates that we failed to reject the null hypothesis that there was not a systematic difference in the probability of readmission because of the smoking cessation intervention. Other significant variables generally had the expected relationship with readmission rates. Smokers were 1.6% less likely to be readmitted than nonsmokers (P = 0.01), controlling for other factors.
The program effect on smoker LOS was statistically insignificant (β = 0.008; P = 0.36). Smokers overall had a shorter LOS than nonsmokers (β = −0.090; P = 0.01), controlling for other factors. Overall LOS was longer postintervention (β = 0.047; P < 0.01). The control variables generally had the same relationship for the LOS model as for the readmission model.
DISCUSSION
This study investigated the effect of an inpatient smoking cessation program, based on a successful Canadian model, on inpatient readmission rates and LOS. The program showed no effect on 30-day readmission rates or LOS. We see several potential explanations for the absence of a detectable impact.
First, the ITT approach reflected real-world implementation of smoking cessation services. The ITT approach adopts the hospital’s perspective because the hospital will assess overall effectiveness without regard to programmatic limitations. The intervention group for this analysis included individuals who were offered but declined treatment, individuals who accepted treatment but failed to quit smoking, and individuals who both accepted treatment and quit smoking. If the analysis had focused only on the latter group, an effect would have been more likely to be found. Further analysis of the subset of patients who accepted the intervention and quit smoking is warranted. Nevertheless, hospitals cannot expect all inpatient smokers, or even a majority, to embrace an offer of cessation treatment. This also emphasizes the challenges hospitals will face in offering tobacco cessation programs to smokers in a timely way. Reasons for patients not receiving orders varied but included issues with weekend admissions.
Second, the timeframe of the analysis is limited to the inpatient stay (for LOS) and 30 days (for readmission). A longer-term analysis might have found an effect. However, we examined this from the hospital perspective. For the hospital, LOS is a key cost driver; thus, reductions in LOS would create a strong financial incentive for hospitals to implement smoking cessation programs. Similarly, reducing readmissions is now a priority for hospitals because of new Medicare rules that penalize hospitals for readmissions. Thus, the 2 outcomes we examined are outcomes that are financially important to hospitals.
There are several limitations to our analysis. First, the difference-in-differences model assumes that in the absence of treatment, the average change in the dependent variables would have been the same for both the treatment and control groups, also known as the parallel trends assumption. Specification tests showed this assumption was met for the preperiod. Second, our study relies on electronic health record data to identify smokers. However, 93% of individuals who were identified as smokers confirmed their smoking status upon interview. Finally, we looked at all categories of inpatient admissions. Improvement in LOS and short-term readmission rates may be limited to patients admitted for specific conditions, such as cardiovascular and respiratory conditions.
There are a number of plausible reasons for our null finding. First, the “dose” of intervention may have been too weak; that is, the number of smokers who were offered the treatment, accepted the treatment, and adhered to the treatment may have been too low, leading to too few smokers quitting smoking and, thus, no effect of the intervention on our outcomes. This follows directly from the ITT design of the study.23 This suggests that hospitals who wish to adopt smoking cessation programs need to focus on ensuring a timely offering of treatment and encouragement of uptake by smokers.
A second reason for the null finding may have been the short duration for the NRT, which was only offered for 2 weeks. Research suggests that use of NRT for less than 4 weeks is associated with a reduced likelihood of smoking cessation.24 However, a review of the literature concludes that the duration of NRT is less important than the dosage and the combination of NRT with other forms of smoking cessation therapy.25 It is important to note that this study used NRT; other treatments such as Chantix could have different effectiveness.26,27 Further research on different treatment approaches, including longer duration of NRT, would be appropriate.
Disclosure
The authors have no competing interests or conflicts to report. The study was supported by contract number 15FLA68717 from the Colorado Department of Public Health and Environment.
Successful smoking cessation interventions result in substantial gains in health and life expectancy by reducing smoking-related illnesses and preventing premature deaths.1,2 The Department of Health and Human Services recommends clinicians use hospitalization as “an opportunity to promote smoking cessation’’ and ‘‘to prescribe medications to alleviate withdrawal symptoms”3 because individual readiness to quit may be high during hospitalizations. A meta-analysis of 50 studies (21 from the United States) examining the efficacy of hospital-initiated smoking cessation interventions concluded that smoking cessation support programs that began in the hospital and continued for at least 1 month postdischarge significantly increase the likelihood of patients being smoke-free in the long term.4 The most efficacious strategies included counseling and pharmacotherapy rather than counseling alone.3 Most inpatient smoking cessation studies have focused on quit-rates or medical outcomes, while fewer studies have looked at healthcare utilization.
However, previous research has shown that smoking cessation for inpatients has relatively immediate economic and health benefits. Patients who quit smoking during hospitalizations for cardiovascular disease are less likely to be readmitted or to die during follow-up.5,6 Patients with acute myocardial infarction (AMI), unstable angina, heart failure, and chronic obstructive pulmonary disease who received an inpatient smoking cessation intervention had reductions in inpatient readmission rates.7 A 1% reduction in overall smoking rates would lead to an annual reduction of 3,022 hospitalizations for stroke and 1,684 hospitalizations for AMI.8 One comprehensive program, the Ottawa Model for Smoking Cessation (OMSC), found that a hospital-initiated intervention increased long-term cessation rates by 15% in cardiac patients and by 11% in general hospital populations.9,10 The applicability of this result to US healthcare systems is unknown. This paper adds to the existing literature by evaluating the impact of an inpatient smoking cessation program on healthcare utilization among patients hospitalized for any reason, rather than solely focused on those with cardiopulmonary diagnoses.
The current study focuses on an inpatient smoking cessation program at a teaching hospital in the Rocky Mountain region. The hospital implemented a smoking cessation treatment program on July 1, 2013, based on the OMSC. The goal was to identify and support inpatient adult smokers who wanted to make a quit attempt and help them remain smoke-free after discharge. The objective of the current study was to determine the effect of the program on 30-day readmission rates and length of stay (LOS) of the index hospitalization. Although the general cost effectiveness of properly structured smoking cessation programs are well established,11-13 the healthcare utilization effects of inpatient smoking cessation programs are not well understood.
METHODS
Data
The study population consists of patients over age 18 who were admitted to the hospital between July 1, 2012, and July 1, 2014. Baseline smoking status was assessed at hospital admission and recorded in Epic (Epic Systems Corporation, Verona, Wisconsin), the electronic medical records system, as a current smoker (every day and some days), former smoker, never smoker, and never assessed. To check the accuracy of recorded smoking status, a random sample of 819 inpatients was selected and contacted via telephone for verification; 93% of Epic-identified smokers confirmed that they were smokers at hospital admission.14
Intervention
The intervention, which launched July 13, 2014, modified the Epic system to automatically alert providers viewing a tobacco user’s medical record that the patient should receive standardized orders for a bedside consultation with a Tobacco Treatment Specialist (TTS) and a prescription for nicotine replacement therapy (NRT) while in the hospital.15 Previously, referrals for tobacco treatment were done on an ad-hoc basis by the physician, and NRT was not routinely available. This system-level intervention standardized and automated the referral process. For patients with a bedside consultation order, TTS used a patient-centered approach (motivational interviewing) to explore patients’ motivation to quit smoking and offered NRT to improve comfort and safety while in the hospital. Patients who chose to make a quit attempt received a free 2-week supply of NRT at discharge and 6 months of free follow-up counseling by interactive voice response (IVR) telephone technology that included (a) prerecorded advice keyed to individual patient needs, (b) a warm-transfer option to speak with a live TTS (later dropped), and (c) a collection of patient smoking and cessation treatment measures.15
Statistical Analysis
We used an intent-to-treat (ITT) framework for the analysis, which considers everyone eligible for the treatment to be in the treatment group. The approach ignores treatment nonacceptance, nonadherence, protocol deviations, withdrawal from treatment, and cessation outcomes,
Readmission rates and LOS were estimated by using a “difference-in-differences” model, comparing outcomes between smokers before versus after the introduction of the cessation treatment program with nonsmokers before versus after program introduction. The difference-in-differences method looks at the difference pre-and-post in the exposed group (smokers) and unexposed group (nonsmokers). Subtracting the difference between the 2 groups gives an estimate of the policy effect controlling for background trends.19 The smoking cessation treatment effect on readmission is measured by the coefficient on the interaction term between the smoking variable and an indicator that the program is operational. The coefficient is the “difference-in-differences.”
Other control variables include demographic factors (gender, age, race), hospitalization payer (Medicare, Medicaid, commercial), and the service line of the admission. We also included a severity of illness variable from the APR-DRG Grouper (3M, Maplewood, Minnesota)20 and the number of days spent in the intensive care unit. For the readmission model, we included LOS as a control variable, because individuals with longer LOS had a better opportunity to access the intervention.
For readmissions, the model was estimated by using a probit model, predicting the effect of each of the intervention variables and the control variables on the marginal probability of a readmission. Because patients can appear in both the pre- and postyears, clustered standard errors were used, which correct for the lack of independence from multiple observations from the same individual.21 For LOS, a truncated negative binomial model was used. The negative binomial model is a specification for count models with a mass of observations plus a long right tail. The truncation is because zero and negative values for LOS are not possible. The dependent variable represents the number of days the individual was hospitalized. For both models, the reported coefficients represent the marginal effect of the independent variable on the dependent variable. This was calculated using the “margins” command in Stata version 13 (StataCorp LLC, College Station, Texas).
RESULTS
In the probit analysis, the smoking cessation intervention (Smoker*post intervention) showed no significant effect on the probability of readmission (Table 2). The coefficient is positive (β = 0.008) and statistically insignificant (P = 0.36). This indicates that we failed to reject the null hypothesis that there was not a systematic difference in the probability of readmission because of the smoking cessation intervention. Other significant variables generally had the expected relationship with readmission rates. Smokers were 1.6% less likely to be readmitted than nonsmokers (P = 0.01), controlling for other factors.
The program effect on smoker LOS was statistically insignificant (β = 0.008; P = 0.36). Smokers overall had a shorter LOS than nonsmokers (β = −0.090; P = 0.01), controlling for other factors. Overall LOS was longer postintervention (β = 0.047; P < 0.01). The control variables generally had the same relationship for the LOS model as for the readmission model.
DISCUSSION
This study investigated the effect of an inpatient smoking cessation program, based on a successful Canadian model, on inpatient readmission rates and LOS. The program showed no effect on 30-day readmission rates or LOS. We see several potential explanations for the absence of a detectable impact.
First, the ITT approach reflected real-world implementation of smoking cessation services. The ITT approach adopts the hospital’s perspective because the hospital will assess overall effectiveness without regard to programmatic limitations. The intervention group for this analysis included individuals who were offered but declined treatment, individuals who accepted treatment but failed to quit smoking, and individuals who both accepted treatment and quit smoking. If the analysis had focused only on the latter group, an effect would have been more likely to be found. Further analysis of the subset of patients who accepted the intervention and quit smoking is warranted. Nevertheless, hospitals cannot expect all inpatient smokers, or even a majority, to embrace an offer of cessation treatment. This also emphasizes the challenges hospitals will face in offering tobacco cessation programs to smokers in a timely way. Reasons for patients not receiving orders varied but included issues with weekend admissions.
Second, the timeframe of the analysis is limited to the inpatient stay (for LOS) and 30 days (for readmission). A longer-term analysis might have found an effect. However, we examined this from the hospital perspective. For the hospital, LOS is a key cost driver; thus, reductions in LOS would create a strong financial incentive for hospitals to implement smoking cessation programs. Similarly, reducing readmissions is now a priority for hospitals because of new Medicare rules that penalize hospitals for readmissions. Thus, the 2 outcomes we examined are outcomes that are financially important to hospitals.
There are several limitations to our analysis. First, the difference-in-differences model assumes that in the absence of treatment, the average change in the dependent variables would have been the same for both the treatment and control groups, also known as the parallel trends assumption. Specification tests showed this assumption was met for the preperiod. Second, our study relies on electronic health record data to identify smokers. However, 93% of individuals who were identified as smokers confirmed their smoking status upon interview. Finally, we looked at all categories of inpatient admissions. Improvement in LOS and short-term readmission rates may be limited to patients admitted for specific conditions, such as cardiovascular and respiratory conditions.
There are a number of plausible reasons for our null finding. First, the “dose” of intervention may have been too weak; that is, the number of smokers who were offered the treatment, accepted the treatment, and adhered to the treatment may have been too low, leading to too few smokers quitting smoking and, thus, no effect of the intervention on our outcomes. This follows directly from the ITT design of the study.23 This suggests that hospitals who wish to adopt smoking cessation programs need to focus on ensuring a timely offering of treatment and encouragement of uptake by smokers.
A second reason for the null finding may have been the short duration for the NRT, which was only offered for 2 weeks. Research suggests that use of NRT for less than 4 weeks is associated with a reduced likelihood of smoking cessation.24 However, a review of the literature concludes that the duration of NRT is less important than the dosage and the combination of NRT with other forms of smoking cessation therapy.25 It is important to note that this study used NRT; other treatments such as Chantix could have different effectiveness.26,27 Further research on different treatment approaches, including longer duration of NRT, would be appropriate.
Disclosure
The authors have no competing interests or conflicts to report. The study was supported by contract number 15FLA68717 from the Colorado Department of Public Health and Environment.
1. Taylor DH Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of Smoking Cessation for Longevity. Am J Public Health. 2002;92(6):990-996. PubMed
2. Weitkunat R, Coggins CRE, Sponsiello-Wang Z, Kallischnigg G, Dempsey R. Assessment of Cigarette Smoking in Epidemiologic Studies. Tobacco Research. 2013;25(7).
3. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville (MD): US Department of Health and Human Services. 2008. https://www.ncbi.nlm.nih.gov/books/NBK63952/.
4. Rigotti, NA, Clair, C, Munafo, MR, et al., Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012(5): CD001837. PubMed
5. Ladapo JA, Jaffer FA, Weinstein MC, Froelicher ES. Projected cost-effectiveness of smoking cessation interventions in patients hospitalized with myocardial infarction. Arch Intern Med. 2011;171:39-45. PubMed
6. Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446-452. PubMed
7. Mullen K, Coyle D, Manuel D, et al. Economic evaluation of a hospital-initiated intervention for smokers with chronic disease, in Ontario, Canada. Tob Control. 2015;24(5):489-496. PubMed
8. Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96(4):1089-1096. PubMed
9. Reid RD, Mullen KA, Slovinec D’Angelo ME, et al. Smoking cessation for hospitalized smokers: an evaluation of the “Ottawa Model.” Nicotine Tob Res. 2010;12:11-18. PubMed
10. Reid RD, Pipe AL, Quinlan B. Promoting smoking cessation during hospitalization for coronary artery disease. Can J Cardiol. 2006;22:775-780. PubMed
11. Krumholz H, Cohen B, Tsevat J, Pasternak R, Weinstein M. Cost-effectiveness of a smoking cessation program after myocardial infarction. J Am Coll Cardiol. 1993;22(6):1697-1702. PubMed
12. Curry S, Grothaus L, McAfee T, Pabiniak C. Use and Cost Effectiveness of Smoking-Cessation Services under Four Insurance Plans in a Health Maintenance Organization. N Engl J Med. 1998;339:673-679. PubMed
12. Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA. 1996;275:1247-1251. PubMed
13. CEPEG. Independent Evaluation: University of Colorado Hospital’s Smoking Cessation Treatment Program, preliminary report. Aurora: Colorado School of Public Health; 2014.
15. Cooper S, Pray S. Independent Evaluation: Hospital systems change to improve inpatient tobacco dependence treatment final results. Aurora: Colorado School of Public Health; June 2015.
16. Gupta S. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109-112. DOI:10.4103/2229-3485.83221 PubMed
17. Fisher LD, Dixon DO, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to treat in clinical trials. In: Peace KE, editor. Statistical Issues in Drug Research and Development. New York: Marcel Dekker; 1990:331-350
18. Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21(5):837-841. PubMed
19. Dimick JB, Ryan AM. Methods for Evaluating Changes in Health Care Policy: The Difference-in-Differences Approach. JAMA. 2014;312(22):2401-2402. DOI:10.1001/jama.2014.16153 PubMed
20. Averill R, Goldfield N, Steinbeck B, et al. Development of the All Patient Refined DRGs (APR-DRGs). Maplewood: 3M Health Information Systems; 1997. Report 8-9. PubMed
21. Cameron A, Miller D. A Practitioner’s Guide to Cluster-Robust Inference. J Hum Resour. 2015;50(2):317-372.
22. Cooper S, Pray S. Independent Evaluation: Hospital systems change to improve inpatient tobacco dependence treatment Final Results. Aurora: Colorado School of Public Health; April 2015.
23. Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109-112. DOI:10.4103/2229-3485.83221. PubMed
24. Zhang B, Cohen J, Bondy S. Duration of Nicotine Replacement Therapy Use and Smoking Cessation: A Population-Based Longitudinal Study. Am J Epidemiol. 2015;181(7):513-520. PubMed
25. Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;3:CD000146. DOI:10.1002/14651858.CD000146 PubMed
26. C, , . Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008;3:CD006103. DOI:10.1002/14651858.CD006103.pub3. PubMed
27. Hurt R, Sachs D, Glover E, et al. A Comparison of Sustained-Release Bupropion and Placebo for Smoking Cessation. N Engl J Med. 1997;337:1195-1202. PubMed
1. Taylor DH Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of Smoking Cessation for Longevity. Am J Public Health. 2002;92(6):990-996. PubMed
2. Weitkunat R, Coggins CRE, Sponsiello-Wang Z, Kallischnigg G, Dempsey R. Assessment of Cigarette Smoking in Epidemiologic Studies. Tobacco Research. 2013;25(7).
3. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville (MD): US Department of Health and Human Services. 2008. https://www.ncbi.nlm.nih.gov/books/NBK63952/.
4. Rigotti, NA, Clair, C, Munafo, MR, et al., Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012(5): CD001837. PubMed
5. Ladapo JA, Jaffer FA, Weinstein MC, Froelicher ES. Projected cost-effectiveness of smoking cessation interventions in patients hospitalized with myocardial infarction. Arch Intern Med. 2011;171:39-45. PubMed
6. Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446-452. PubMed
7. Mullen K, Coyle D, Manuel D, et al. Economic evaluation of a hospital-initiated intervention for smokers with chronic disease, in Ontario, Canada. Tob Control. 2015;24(5):489-496. PubMed
8. Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96(4):1089-1096. PubMed
9. Reid RD, Mullen KA, Slovinec D’Angelo ME, et al. Smoking cessation for hospitalized smokers: an evaluation of the “Ottawa Model.” Nicotine Tob Res. 2010;12:11-18. PubMed
10. Reid RD, Pipe AL, Quinlan B. Promoting smoking cessation during hospitalization for coronary artery disease. Can J Cardiol. 2006;22:775-780. PubMed
11. Krumholz H, Cohen B, Tsevat J, Pasternak R, Weinstein M. Cost-effectiveness of a smoking cessation program after myocardial infarction. J Am Coll Cardiol. 1993;22(6):1697-1702. PubMed
12. Curry S, Grothaus L, McAfee T, Pabiniak C. Use and Cost Effectiveness of Smoking-Cessation Services under Four Insurance Plans in a Health Maintenance Organization. N Engl J Med. 1998;339:673-679. PubMed
12. Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA. 1996;275:1247-1251. PubMed
13. CEPEG. Independent Evaluation: University of Colorado Hospital’s Smoking Cessation Treatment Program, preliminary report. Aurora: Colorado School of Public Health; 2014.
15. Cooper S, Pray S. Independent Evaluation: Hospital systems change to improve inpatient tobacco dependence treatment final results. Aurora: Colorado School of Public Health; June 2015.
16. Gupta S. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109-112. DOI:10.4103/2229-3485.83221 PubMed
17. Fisher LD, Dixon DO, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to treat in clinical trials. In: Peace KE, editor. Statistical Issues in Drug Research and Development. New York: Marcel Dekker; 1990:331-350
18. Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21(5):837-841. PubMed
19. Dimick JB, Ryan AM. Methods for Evaluating Changes in Health Care Policy: The Difference-in-Differences Approach. JAMA. 2014;312(22):2401-2402. DOI:10.1001/jama.2014.16153 PubMed
20. Averill R, Goldfield N, Steinbeck B, et al. Development of the All Patient Refined DRGs (APR-DRGs). Maplewood: 3M Health Information Systems; 1997. Report 8-9. PubMed
21. Cameron A, Miller D. A Practitioner’s Guide to Cluster-Robust Inference. J Hum Resour. 2015;50(2):317-372.
22. Cooper S, Pray S. Independent Evaluation: Hospital systems change to improve inpatient tobacco dependence treatment Final Results. Aurora: Colorado School of Public Health; April 2015.
23. Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109-112. DOI:10.4103/2229-3485.83221. PubMed
24. Zhang B, Cohen J, Bondy S. Duration of Nicotine Replacement Therapy Use and Smoking Cessation: A Population-Based Longitudinal Study. Am J Epidemiol. 2015;181(7):513-520. PubMed
25. Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;3:CD000146. DOI:10.1002/14651858.CD000146 PubMed
26. C, , . Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008;3:CD006103. DOI:10.1002/14651858.CD006103.pub3. PubMed
27. Hurt R, Sachs D, Glover E, et al. A Comparison of Sustained-Release Bupropion and Placebo for Smoking Cessation. N Engl J Med. 1997;337:1195-1202. PubMed
© 2017 Society of Hospital Medicine