User login
Bilateral leg edema and difficulty swallowing
A 70-year-old man came to our medical center for treatment of painful bilateral leg swelling that had gotten progressively worse over the past week. He had no significant past medical or surgical history, took an aspirin daily, did not smoke tobacco or drink alcohol, and had not taken any trips recently. He denied any chest pain, dyspnea, or orthopnea, but indicated that he’d been having difficulty swallowing food for the past month.
The patient had a cachectic appearance, diminished breath sounds and dullness to percussion over the right middle and lower lung fields, and pitting edema up to the knees bilaterally.
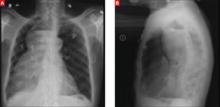
Lab studies showed a normal brain natriuretic peptide of 16 pg/mL, normocytic anemia (hemoglobin, 9.5 g/dL; hematocrit, 29.6%; and a mean corpuscular volume of 82.6 fL), potassium of 2.4 mEq/L, calcium of 5.4 mg/dL, and albumin of 1.4 g/dL. An electrocardiogram (EKG) revealed a bifascicular heart block, which was not new based on older EKGs. A plain chest radiograph was performed (FIGURES 1A AND 1B).
FIGURE 1
Chest x-rays reveal large mass
What is your diagnosis?
Diagnosis: Achalasia
Achalasia is characterized by an incomplete relaxation of the lower esophageal sphincter (LES) accompanied by aperistalsis of the esophageal body. The etiology of primary achalasia is unknown, but involves the degeneration of inhibitory neurons within the myenteric plexus responsible for LES relaxation and esophageal peristalsis.
Many potential causes. Infectious, inflammatory, autoimmune, and genetic causes have all been proposed. Achalasia has a prevalence of less than 1 in 10,000; its incidence is 0.3 to 1 per 100,000 per year, with peaks in the third and seventh decades of life. Men and women are affected equally.1
Conversely, pseudoachalasia—or secondary achalasia—has a known cause that either destroys the neurons of LES relaxation or has a mass effect that limits LES relaxation. Its most common cause is malignancy involving the gastroesophageal junction. Other causes include Chagas disease, an infection by the parasite Trypanosoma cruzi that affects the myenteric plexus, and amyloidosis.2
A disorder that goes undiagnosed for years
In primary achalasia symptom onset is insidious, with the disorder typically going undiagnosed for several years. Patients experience a gradual dysphagia for solids, progressing to liquids. Over time, patients adopt particular behaviors to aid the transit of food boluses down the esophagus and through the contracted LES, including eating slowly, stretching, moving side-to-side, or walking after meals.1 Regurgitation of food, chest pain, weight loss, heartburn, and difficulty belching are also common complaints.3 A more rapid onset and progression of symptoms (less than 6 months) is suggestive of pseudoachalasia and cancer.
Left untreated, primary achalasia leads to a progressively dilating esophagus with increased risk of aspiration, perforation, malnutrition, weight loss, and esophageal cancer.3 This case is remarkable for how far the patient’s disease progressed before he presented with signs and symptoms more indicative of secondary malnutrition than the primary disease.
Differential Dx includes hiatal hernia
The differential diagnosis for achalasia includes hiatal hernia, right middle lobe pneumonia, empyema, and lung abscess. Most patients with hiatal hernias are asymptomatic, and the diagnosis is made incidentally on chest x-ray as a retrocardiac air-fluid level or on upper gastrointestinal studies. Right middle lobe pneumonia would appear on chest x-ray as a well-demarcated opacity within the confines of that lobe, but would not have an air-fluid level. Empyema and lung abscess are complications of pneumonia, and patients present with persistent fever, cough, dyspnea, and malaise. With an empyema, chest x-ray reveals a pleural effusion. A lung abscess is an intrapulmonary cavitary lesion with an air-fluid level, most commonly due to aspiration pneumonia.
This patient’s chest x-ray showed a mass outside the lung tissue and pleural space that had an air-fluid level, was within the mediastinum, and displaced the trachea anteriorly, all suggestive of a dilated esophagus filled with undigested food unable to pass the LES.
Esophageal manometry clinches the diagnosis
Esophageal manometry is the gold standard for diagnosis. In achalasia, manometry shows poor peristalsis of the esophageal body and a constricted LES that does not relax sufficiently with swallowing.3 Manometry cannot, however, reliably distinguish primary achalasia from pseudoachalasia.
Barium swallow studies and endoscopy can assist in the diagnosis and help rule out pseudoachalasia. Timed barium studies can show a lack of peristalsis, an air-fluid level at the top of a barium column retained within the dilated esophagus, and the narrowing of the distal esophagus into the pathognomonic “bird’s beak” of primary achalasia.4 Endoscopy can visualize a mass beyond the LES, but cannot adequately assess esophageal peristalsis or LES relaxation.1
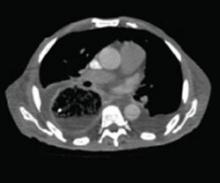
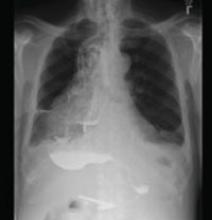
In our patient’s initial workup, a CT (FIGURE 2), barium study (FIGURE 3), and endoscopy were all used to rule out an obstructive lesion.
FIGURE 2
Massively distended esophagus
FIGURE 3
The “bird’s beak” of achalasia
Treatment targets the constricted LES
The goal of treatment in achalasia is to help food move through the constricted LES. Pharmacologic therapy, used to decrease LES resting tone, has limited benefit and becomes less effective as the disease progresses. Calcium channel blockers, particularly nifedipine, and nitrates are the most commonly used agents.5
Pneumatic dilation is the most effective nonsurgical treatment of achalasia. It involves the controlled rupture of LES muscle fibers to dilate the sphincter and allow gravity to move food from the esophagus into the stomach.6 Symptom improvement lasts for 60% of patients at 1 year, 50% at 5 years, and 36% at 10 years. Repeated dilations are possible and sometimes necessary, but have diminishing success rates. The most worrisome complication of pneumatic dilation is esophageal rupture, which occurs 2% of the time.5
Botulinum toxin injection is another accepted nonsurgical therapy and is recommended for patients who would like to avoid more invasive treatment. When injected into the LES, the toxin causes temporary atrophy of the neurons responsible for LES contraction. After the procedure, 70% of patients report improvement in symptoms, with half in remission at 6 months and one-third at 1 year. Patients may require repeat injections every 3 to 4 months, but the toxin’s effectiveness diminishes with each injection.6
The laparoscopic Heller myotomy involves cutting the LES muscle, making it incompetent and allowing food boluses to pass through the LES. Ninety percent of patients experience symptom relief after surgery,5 with 10-year remission rates of 67% to 85%.1 Comparisons to pneumatic dilation do not show significant differences in outcomes over time, although most studies favor myotomy over dilation.
Surgical treatment is indicated for patients who are younger than 40 years of age, those who have failed repeated nonsurgical therapies, and those at high risk of esophageal perforation.5
Patient’s course highlights Tx risks
Due to the severity of the patient’s achalasia, a Heller myotomy was performed. During the postoperative period he had several complications. He ultimately died from an uncontrollable hemorrhage after an esophageal perforation, highlighting the seriousness of this disease and the risks inherent to its treatment.
Correspondence
Drew C. Baird, MD, Department of Family and Community Medicine, D.D. Eisenhower Army Medical Center, 300 Hospital Road, Fort Gordon, GA 30905.
1. Pohl D, Tutuian R. Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis. 2007;16:297-303.
2. Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-151.
3. Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis. 2007;2:38.-
4. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology. 2005;237:414-427.
5. Roberts KE, Duffy AJ, Bell RL. Controversies in the treatment of gastroesophageal reflux and achalasia. World J Gastroenterol. 2006;12:3155-3161.
6. Annese V, Bassotti G. Non-surgical treatment of esophageal achalasia. World J Gastroenterol. 2006;12:5763-5766.
A 70-year-old man came to our medical center for treatment of painful bilateral leg swelling that had gotten progressively worse over the past week. He had no significant past medical or surgical history, took an aspirin daily, did not smoke tobacco or drink alcohol, and had not taken any trips recently. He denied any chest pain, dyspnea, or orthopnea, but indicated that he’d been having difficulty swallowing food for the past month.
The patient had a cachectic appearance, diminished breath sounds and dullness to percussion over the right middle and lower lung fields, and pitting edema up to the knees bilaterally.
Lab studies showed a normal brain natriuretic peptide of 16 pg/mL, normocytic anemia (hemoglobin, 9.5 g/dL; hematocrit, 29.6%; and a mean corpuscular volume of 82.6 fL), potassium of 2.4 mEq/L, calcium of 5.4 mg/dL, and albumin of 1.4 g/dL. An electrocardiogram (EKG) revealed a bifascicular heart block, which was not new based on older EKGs. A plain chest radiograph was performed (FIGURES 1A AND 1B).
FIGURE 1
Chest x-rays reveal large mass
What is your diagnosis?
Diagnosis: Achalasia
Achalasia is characterized by an incomplete relaxation of the lower esophageal sphincter (LES) accompanied by aperistalsis of the esophageal body. The etiology of primary achalasia is unknown, but involves the degeneration of inhibitory neurons within the myenteric plexus responsible for LES relaxation and esophageal peristalsis.
Many potential causes. Infectious, inflammatory, autoimmune, and genetic causes have all been proposed. Achalasia has a prevalence of less than 1 in 10,000; its incidence is 0.3 to 1 per 100,000 per year, with peaks in the third and seventh decades of life. Men and women are affected equally.1
Conversely, pseudoachalasia—or secondary achalasia—has a known cause that either destroys the neurons of LES relaxation or has a mass effect that limits LES relaxation. Its most common cause is malignancy involving the gastroesophageal junction. Other causes include Chagas disease, an infection by the parasite Trypanosoma cruzi that affects the myenteric plexus, and amyloidosis.2
A disorder that goes undiagnosed for years
In primary achalasia symptom onset is insidious, with the disorder typically going undiagnosed for several years. Patients experience a gradual dysphagia for solids, progressing to liquids. Over time, patients adopt particular behaviors to aid the transit of food boluses down the esophagus and through the contracted LES, including eating slowly, stretching, moving side-to-side, or walking after meals.1 Regurgitation of food, chest pain, weight loss, heartburn, and difficulty belching are also common complaints.3 A more rapid onset and progression of symptoms (less than 6 months) is suggestive of pseudoachalasia and cancer.
Left untreated, primary achalasia leads to a progressively dilating esophagus with increased risk of aspiration, perforation, malnutrition, weight loss, and esophageal cancer.3 This case is remarkable for how far the patient’s disease progressed before he presented with signs and symptoms more indicative of secondary malnutrition than the primary disease.
Differential Dx includes hiatal hernia
The differential diagnosis for achalasia includes hiatal hernia, right middle lobe pneumonia, empyema, and lung abscess. Most patients with hiatal hernias are asymptomatic, and the diagnosis is made incidentally on chest x-ray as a retrocardiac air-fluid level or on upper gastrointestinal studies. Right middle lobe pneumonia would appear on chest x-ray as a well-demarcated opacity within the confines of that lobe, but would not have an air-fluid level. Empyema and lung abscess are complications of pneumonia, and patients present with persistent fever, cough, dyspnea, and malaise. With an empyema, chest x-ray reveals a pleural effusion. A lung abscess is an intrapulmonary cavitary lesion with an air-fluid level, most commonly due to aspiration pneumonia.
This patient’s chest x-ray showed a mass outside the lung tissue and pleural space that had an air-fluid level, was within the mediastinum, and displaced the trachea anteriorly, all suggestive of a dilated esophagus filled with undigested food unable to pass the LES.
Esophageal manometry clinches the diagnosis
Esophageal manometry is the gold standard for diagnosis. In achalasia, manometry shows poor peristalsis of the esophageal body and a constricted LES that does not relax sufficiently with swallowing.3 Manometry cannot, however, reliably distinguish primary achalasia from pseudoachalasia.
Barium swallow studies and endoscopy can assist in the diagnosis and help rule out pseudoachalasia. Timed barium studies can show a lack of peristalsis, an air-fluid level at the top of a barium column retained within the dilated esophagus, and the narrowing of the distal esophagus into the pathognomonic “bird’s beak” of primary achalasia.4 Endoscopy can visualize a mass beyond the LES, but cannot adequately assess esophageal peristalsis or LES relaxation.1
In our patient’s initial workup, a CT (FIGURE 2), barium study (FIGURE 3), and endoscopy were all used to rule out an obstructive lesion.
FIGURE 2
Massively distended esophagus
FIGURE 3
The “bird’s beak” of achalasia
Treatment targets the constricted LES
The goal of treatment in achalasia is to help food move through the constricted LES. Pharmacologic therapy, used to decrease LES resting tone, has limited benefit and becomes less effective as the disease progresses. Calcium channel blockers, particularly nifedipine, and nitrates are the most commonly used agents.5
Pneumatic dilation is the most effective nonsurgical treatment of achalasia. It involves the controlled rupture of LES muscle fibers to dilate the sphincter and allow gravity to move food from the esophagus into the stomach.6 Symptom improvement lasts for 60% of patients at 1 year, 50% at 5 years, and 36% at 10 years. Repeated dilations are possible and sometimes necessary, but have diminishing success rates. The most worrisome complication of pneumatic dilation is esophageal rupture, which occurs 2% of the time.5
Botulinum toxin injection is another accepted nonsurgical therapy and is recommended for patients who would like to avoid more invasive treatment. When injected into the LES, the toxin causes temporary atrophy of the neurons responsible for LES contraction. After the procedure, 70% of patients report improvement in symptoms, with half in remission at 6 months and one-third at 1 year. Patients may require repeat injections every 3 to 4 months, but the toxin’s effectiveness diminishes with each injection.6
The laparoscopic Heller myotomy involves cutting the LES muscle, making it incompetent and allowing food boluses to pass through the LES. Ninety percent of patients experience symptom relief after surgery,5 with 10-year remission rates of 67% to 85%.1 Comparisons to pneumatic dilation do not show significant differences in outcomes over time, although most studies favor myotomy over dilation.
Surgical treatment is indicated for patients who are younger than 40 years of age, those who have failed repeated nonsurgical therapies, and those at high risk of esophageal perforation.5
Patient’s course highlights Tx risks
Due to the severity of the patient’s achalasia, a Heller myotomy was performed. During the postoperative period he had several complications. He ultimately died from an uncontrollable hemorrhage after an esophageal perforation, highlighting the seriousness of this disease and the risks inherent to its treatment.
Correspondence
Drew C. Baird, MD, Department of Family and Community Medicine, D.D. Eisenhower Army Medical Center, 300 Hospital Road, Fort Gordon, GA 30905.
A 70-year-old man came to our medical center for treatment of painful bilateral leg swelling that had gotten progressively worse over the past week. He had no significant past medical or surgical history, took an aspirin daily, did not smoke tobacco or drink alcohol, and had not taken any trips recently. He denied any chest pain, dyspnea, or orthopnea, but indicated that he’d been having difficulty swallowing food for the past month.
The patient had a cachectic appearance, diminished breath sounds and dullness to percussion over the right middle and lower lung fields, and pitting edema up to the knees bilaterally.
Lab studies showed a normal brain natriuretic peptide of 16 pg/mL, normocytic anemia (hemoglobin, 9.5 g/dL; hematocrit, 29.6%; and a mean corpuscular volume of 82.6 fL), potassium of 2.4 mEq/L, calcium of 5.4 mg/dL, and albumin of 1.4 g/dL. An electrocardiogram (EKG) revealed a bifascicular heart block, which was not new based on older EKGs. A plain chest radiograph was performed (FIGURES 1A AND 1B).
FIGURE 1
Chest x-rays reveal large mass
What is your diagnosis?
Diagnosis: Achalasia
Achalasia is characterized by an incomplete relaxation of the lower esophageal sphincter (LES) accompanied by aperistalsis of the esophageal body. The etiology of primary achalasia is unknown, but involves the degeneration of inhibitory neurons within the myenteric plexus responsible for LES relaxation and esophageal peristalsis.
Many potential causes. Infectious, inflammatory, autoimmune, and genetic causes have all been proposed. Achalasia has a prevalence of less than 1 in 10,000; its incidence is 0.3 to 1 per 100,000 per year, with peaks in the third and seventh decades of life. Men and women are affected equally.1
Conversely, pseudoachalasia—or secondary achalasia—has a known cause that either destroys the neurons of LES relaxation or has a mass effect that limits LES relaxation. Its most common cause is malignancy involving the gastroesophageal junction. Other causes include Chagas disease, an infection by the parasite Trypanosoma cruzi that affects the myenteric plexus, and amyloidosis.2
A disorder that goes undiagnosed for years
In primary achalasia symptom onset is insidious, with the disorder typically going undiagnosed for several years. Patients experience a gradual dysphagia for solids, progressing to liquids. Over time, patients adopt particular behaviors to aid the transit of food boluses down the esophagus and through the contracted LES, including eating slowly, stretching, moving side-to-side, or walking after meals.1 Regurgitation of food, chest pain, weight loss, heartburn, and difficulty belching are also common complaints.3 A more rapid onset and progression of symptoms (less than 6 months) is suggestive of pseudoachalasia and cancer.
Left untreated, primary achalasia leads to a progressively dilating esophagus with increased risk of aspiration, perforation, malnutrition, weight loss, and esophageal cancer.3 This case is remarkable for how far the patient’s disease progressed before he presented with signs and symptoms more indicative of secondary malnutrition than the primary disease.
Differential Dx includes hiatal hernia
The differential diagnosis for achalasia includes hiatal hernia, right middle lobe pneumonia, empyema, and lung abscess. Most patients with hiatal hernias are asymptomatic, and the diagnosis is made incidentally on chest x-ray as a retrocardiac air-fluid level or on upper gastrointestinal studies. Right middle lobe pneumonia would appear on chest x-ray as a well-demarcated opacity within the confines of that lobe, but would not have an air-fluid level. Empyema and lung abscess are complications of pneumonia, and patients present with persistent fever, cough, dyspnea, and malaise. With an empyema, chest x-ray reveals a pleural effusion. A lung abscess is an intrapulmonary cavitary lesion with an air-fluid level, most commonly due to aspiration pneumonia.
This patient’s chest x-ray showed a mass outside the lung tissue and pleural space that had an air-fluid level, was within the mediastinum, and displaced the trachea anteriorly, all suggestive of a dilated esophagus filled with undigested food unable to pass the LES.
Esophageal manometry clinches the diagnosis
Esophageal manometry is the gold standard for diagnosis. In achalasia, manometry shows poor peristalsis of the esophageal body and a constricted LES that does not relax sufficiently with swallowing.3 Manometry cannot, however, reliably distinguish primary achalasia from pseudoachalasia.
Barium swallow studies and endoscopy can assist in the diagnosis and help rule out pseudoachalasia. Timed barium studies can show a lack of peristalsis, an air-fluid level at the top of a barium column retained within the dilated esophagus, and the narrowing of the distal esophagus into the pathognomonic “bird’s beak” of primary achalasia.4 Endoscopy can visualize a mass beyond the LES, but cannot adequately assess esophageal peristalsis or LES relaxation.1
In our patient’s initial workup, a CT (FIGURE 2), barium study (FIGURE 3), and endoscopy were all used to rule out an obstructive lesion.
FIGURE 2
Massively distended esophagus
FIGURE 3
The “bird’s beak” of achalasia
Treatment targets the constricted LES
The goal of treatment in achalasia is to help food move through the constricted LES. Pharmacologic therapy, used to decrease LES resting tone, has limited benefit and becomes less effective as the disease progresses. Calcium channel blockers, particularly nifedipine, and nitrates are the most commonly used agents.5
Pneumatic dilation is the most effective nonsurgical treatment of achalasia. It involves the controlled rupture of LES muscle fibers to dilate the sphincter and allow gravity to move food from the esophagus into the stomach.6 Symptom improvement lasts for 60% of patients at 1 year, 50% at 5 years, and 36% at 10 years. Repeated dilations are possible and sometimes necessary, but have diminishing success rates. The most worrisome complication of pneumatic dilation is esophageal rupture, which occurs 2% of the time.5
Botulinum toxin injection is another accepted nonsurgical therapy and is recommended for patients who would like to avoid more invasive treatment. When injected into the LES, the toxin causes temporary atrophy of the neurons responsible for LES contraction. After the procedure, 70% of patients report improvement in symptoms, with half in remission at 6 months and one-third at 1 year. Patients may require repeat injections every 3 to 4 months, but the toxin’s effectiveness diminishes with each injection.6
The laparoscopic Heller myotomy involves cutting the LES muscle, making it incompetent and allowing food boluses to pass through the LES. Ninety percent of patients experience symptom relief after surgery,5 with 10-year remission rates of 67% to 85%.1 Comparisons to pneumatic dilation do not show significant differences in outcomes over time, although most studies favor myotomy over dilation.
Surgical treatment is indicated for patients who are younger than 40 years of age, those who have failed repeated nonsurgical therapies, and those at high risk of esophageal perforation.5
Patient’s course highlights Tx risks
Due to the severity of the patient’s achalasia, a Heller myotomy was performed. During the postoperative period he had several complications. He ultimately died from an uncontrollable hemorrhage after an esophageal perforation, highlighting the seriousness of this disease and the risks inherent to its treatment.
Correspondence
Drew C. Baird, MD, Department of Family and Community Medicine, D.D. Eisenhower Army Medical Center, 300 Hospital Road, Fort Gordon, GA 30905.
1. Pohl D, Tutuian R. Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis. 2007;16:297-303.
2. Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-151.
3. Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis. 2007;2:38.-
4. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology. 2005;237:414-427.
5. Roberts KE, Duffy AJ, Bell RL. Controversies in the treatment of gastroesophageal reflux and achalasia. World J Gastroenterol. 2006;12:3155-3161.
6. Annese V, Bassotti G. Non-surgical treatment of esophageal achalasia. World J Gastroenterol. 2006;12:5763-5766.
1. Pohl D, Tutuian R. Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis. 2007;16:297-303.
2. Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-151.
3. Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis. 2007;2:38.-
4. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology. 2005;237:414-427.
5. Roberts KE, Duffy AJ, Bell RL. Controversies in the treatment of gastroesophageal reflux and achalasia. World J Gastroenterol. 2006;12:3155-3161.
6. Annese V, Bassotti G. Non-surgical treatment of esophageal achalasia. World J Gastroenterol. 2006;12:5763-5766.