User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Questions about optimal dosing of isotretinoin persist, expert says
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
FROM ODAC 2022
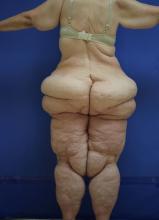
Lipedema: A potentially devastating, often unrecognized disease
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
FROM ODAC 2022
Gowning up: Is it necessary when examining patients with atopic dermatitis?
When evaluating patients with atopic dermatitis (AD), it is essential to do a full body exam, rather than simply asking patients to roll up their sleeves to examine the antecubital fossa, advised Jonathan I. Silverberg, MD, PhD, MPH.
Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, recommends that patients with AD should be asked to gown up for clinical encounters so that their body surface area (BSA) can be assessed. “Whether you use the palmar method or use the rule of nines (a chart that divides the body into sections representing 9% BSA) ... you need to look at BSA because lesion severity in a localized area doesn’t tell you the whole story,” he said during the Revolutionizing Atopic Dermatitis virtual symposium.
He described his anecdotal experiences with patients objecting to being asked by office staff to wear a gown for exams, who often say they have never been asked by a doctor to do so, and often tell him that with previous exams, they were asked to roll up their sleeves only. But there are many patients with AD who do not have flexural disease “and if they just roll up their sleeve for an exam, you would miss the fact that they might be covered over their trunk or legs or other parts of the body,” Dr. Silverberg said. “Make a concerted effort to look not just at lesion severity but to assess body surface area. We need to assess both.”
Capturing the patient perspective
From a patient-reported standpoint, Dr. Silverberg favors asking patients to verbally rate the severity of their disease. Clear or almost clear? Mild, moderate, or severe? “This approach correlates beautifully with validated outcome measures for AD,” he said.
“You could use a numeric rating scale (NRS) for itch, pain, or sleep disturbance. I would argue that it’s best to use a 7-day recall period; 24 hours is too short. They may be clear yesterday but may have been bad 3 days earlier.” The NRS will soon be a reportable item on the AAD DataDerm Clinical Registry, he said, but noted that “the NRS by itself does not accurately predict the full severity of AD.”
A tool he finds useful is the Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD), which was developed in 2017 by an international panel of experts. “It’s free, feasible to use, and a great option for clinical practice.” Dr. Silverberg said. “It’s highly clinically relevant, but it doesn’t take into account BSA. So, BSA is a separate tool that you want to use as well.”
Another tool he mentioned is the Atopic Dermatitis Control Tool (ADCT), developed by industry in 2018. It uses six questions about AD control intended to be used during a 1-week recall period.
“To maximize efficiency, consider having patients complete patient-reported outcomes through patient portals prior to the office visit,” he advised. “Collecting this information prior to the encounter can speed up the clinical encounter and improve quality of care.”
Dr. Silverberg disclosed that he is a consultant to numerous pharmaceutical companies, and receives fees for non-CME/CE services from Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, as well as contracted research fees from Galderma.
When evaluating patients with atopic dermatitis (AD), it is essential to do a full body exam, rather than simply asking patients to roll up their sleeves to examine the antecubital fossa, advised Jonathan I. Silverberg, MD, PhD, MPH.
Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, recommends that patients with AD should be asked to gown up for clinical encounters so that their body surface area (BSA) can be assessed. “Whether you use the palmar method or use the rule of nines (a chart that divides the body into sections representing 9% BSA) ... you need to look at BSA because lesion severity in a localized area doesn’t tell you the whole story,” he said during the Revolutionizing Atopic Dermatitis virtual symposium.
He described his anecdotal experiences with patients objecting to being asked by office staff to wear a gown for exams, who often say they have never been asked by a doctor to do so, and often tell him that with previous exams, they were asked to roll up their sleeves only. But there are many patients with AD who do not have flexural disease “and if they just roll up their sleeve for an exam, you would miss the fact that they might be covered over their trunk or legs or other parts of the body,” Dr. Silverberg said. “Make a concerted effort to look not just at lesion severity but to assess body surface area. We need to assess both.”
Capturing the patient perspective
From a patient-reported standpoint, Dr. Silverberg favors asking patients to verbally rate the severity of their disease. Clear or almost clear? Mild, moderate, or severe? “This approach correlates beautifully with validated outcome measures for AD,” he said.
“You could use a numeric rating scale (NRS) for itch, pain, or sleep disturbance. I would argue that it’s best to use a 7-day recall period; 24 hours is too short. They may be clear yesterday but may have been bad 3 days earlier.” The NRS will soon be a reportable item on the AAD DataDerm Clinical Registry, he said, but noted that “the NRS by itself does not accurately predict the full severity of AD.”
A tool he finds useful is the Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD), which was developed in 2017 by an international panel of experts. “It’s free, feasible to use, and a great option for clinical practice.” Dr. Silverberg said. “It’s highly clinically relevant, but it doesn’t take into account BSA. So, BSA is a separate tool that you want to use as well.”
Another tool he mentioned is the Atopic Dermatitis Control Tool (ADCT), developed by industry in 2018. It uses six questions about AD control intended to be used during a 1-week recall period.
“To maximize efficiency, consider having patients complete patient-reported outcomes through patient portals prior to the office visit,” he advised. “Collecting this information prior to the encounter can speed up the clinical encounter and improve quality of care.”
Dr. Silverberg disclosed that he is a consultant to numerous pharmaceutical companies, and receives fees for non-CME/CE services from Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, as well as contracted research fees from Galderma.
When evaluating patients with atopic dermatitis (AD), it is essential to do a full body exam, rather than simply asking patients to roll up their sleeves to examine the antecubital fossa, advised Jonathan I. Silverberg, MD, PhD, MPH.
Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, recommends that patients with AD should be asked to gown up for clinical encounters so that their body surface area (BSA) can be assessed. “Whether you use the palmar method or use the rule of nines (a chart that divides the body into sections representing 9% BSA) ... you need to look at BSA because lesion severity in a localized area doesn’t tell you the whole story,” he said during the Revolutionizing Atopic Dermatitis virtual symposium.
He described his anecdotal experiences with patients objecting to being asked by office staff to wear a gown for exams, who often say they have never been asked by a doctor to do so, and often tell him that with previous exams, they were asked to roll up their sleeves only. But there are many patients with AD who do not have flexural disease “and if they just roll up their sleeve for an exam, you would miss the fact that they might be covered over their trunk or legs or other parts of the body,” Dr. Silverberg said. “Make a concerted effort to look not just at lesion severity but to assess body surface area. We need to assess both.”
Capturing the patient perspective
From a patient-reported standpoint, Dr. Silverberg favors asking patients to verbally rate the severity of their disease. Clear or almost clear? Mild, moderate, or severe? “This approach correlates beautifully with validated outcome measures for AD,” he said.
“You could use a numeric rating scale (NRS) for itch, pain, or sleep disturbance. I would argue that it’s best to use a 7-day recall period; 24 hours is too short. They may be clear yesterday but may have been bad 3 days earlier.” The NRS will soon be a reportable item on the AAD DataDerm Clinical Registry, he said, but noted that “the NRS by itself does not accurately predict the full severity of AD.”
A tool he finds useful is the Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD), which was developed in 2017 by an international panel of experts. “It’s free, feasible to use, and a great option for clinical practice.” Dr. Silverberg said. “It’s highly clinically relevant, but it doesn’t take into account BSA. So, BSA is a separate tool that you want to use as well.”
Another tool he mentioned is the Atopic Dermatitis Control Tool (ADCT), developed by industry in 2018. It uses six questions about AD control intended to be used during a 1-week recall period.
“To maximize efficiency, consider having patients complete patient-reported outcomes through patient portals prior to the office visit,” he advised. “Collecting this information prior to the encounter can speed up the clinical encounter and improve quality of care.”
Dr. Silverberg disclosed that he is a consultant to numerous pharmaceutical companies, and receives fees for non-CME/CE services from Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, as well as contracted research fees from Galderma.
FROM REVOLUTIONIZING AD 2021
New AAD guidelines eye comorbidities in adults with atopic dermatitis
While it’s well established that atopic dermatitis (AD) in adults is associated with asthma, allergic rhinitis, and other atopic conditions, the links between AD and other comorbidities are coming into clearer focus.
, published evidence supports an association between AD and comorbidities that may not be on the radar of clinicians and patients, including substance use, attention-deficit/hyperactivity disorder (ADHD), elements of metabolic syndrome, and various cardiovascular conditions.
“There are more comorbidities with AD than we anticipated, that are supported by data in the literature,” Dawn M.R. Davis, MD, cochair and an author of the guidelines, told this news organization. “We are learning more about the interconnectivity of various medical conditions,” she continued. “Many skin diseases over time have been noted to be impactful to the whole person and not only the skin. A classic example of that is psoriasis. We now understand that psoriasis is a multisystem inflammatory disorder.”
As for AD, “we’ve always appreciated that AD patients tend to be at higher risk for other atopic diseases such as asthma, allergic rhinoconjunctivitis, and food allergies,” said Dr. Davis, of the departments of dermatology and pediatrics at the Mayo Clinic, Rochester, Minn. “With further research, we are now able to delineate those associations more intimately and have data to support our suspicions. Additionally, we’re now understanding that these inflammatory conditions can impact more than the end organ involved, such as the skin and AD. We wanted to look at how AD can affect the whole patient.”
For the guidelines, which are the first of their kind and were published online in the Journal of the American Academy of Dermatology, Dr. Davis and project cochair Robert Sidbury, MD, MPH, chief of dermatology at Seattle Children’s Hospital, led a multidisciplinary group of 12 experts to review the association between AD and selected comorbidities. They applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for prognosis approach for assessing the certainty of the evidence and provided statements of association based on the available evidence.
With respect to highlights for atopic and allergic conditions, the guideline authors found high-quality evidence that AD in adults is associated with food allergies, moderate-quality evidence that AD is associated with asthma, and low-quality evidence that AD in adults may be associated with eosinophilic esophagitis.
In the realm of mental health and substance use, ample evidence exists to support an association between AD and mental health conditions such as depression and anxiety, the guidelines state. “For many patients, low mood may be driven by the symptoms of AD, including chronic itch and poor sleep,” Dr. Davis and her coauthors wrote. “Successfully treating AD may alleviate depressive symptoms for some patients; for others, assessment and treatment specific to their mental health may be needed.”
The guidelines also state that low-quality evidence exists to suggest that AD in adults may be associated with alcohol abuse disorders and cigarette smoking.
The authors noted “limited but consistent evidence” supporting a link between AD and adverse bone health, including osteoporosis and fractures, while associations between AD and cardiovascular risk factors and comorbidities, including hypertension, myocardial infarction, and stroke, are more controversial.
“I have published on bone health and AD so that was not as surprising to me,” Dr. Davis said in the interview. “I found a lot of the evidence in the guidelines to be validating of patterns that we see in our patients. The most significant learning point for me was [the link to] cardiovascular disease and the link to specific mental health and substance use disorders. It validates how impactful AD is to the individual.”
According to the guidelines, moderate-quality evidence exists linking AD in adults to both alopecia areata and urticaria. “Because we are dermatologists and take care of both of those diseases, be mindful of that in your daily practice,” Dr. Davis advised. “I would also encourage our colleagues to remember to educate patients on the comorbidities of AD so that they are empowered, and to screen for those comorbidities in your office based on the patient and their history and physical exam, to the level that you think is appropriate for that person’s individual’s care.”
Christine Ko, MD, who was asked to comment on the guidelines, characterized some of the reported comorbidity associations as predictable, such as asthma, food allergy, allergic rhinitis, and skin infections. “As the authors comment, ‘associations between AD and other atopic and allergic conditions have been recognized for decades and even contribute to diagnostic criteria for AD,’ ” said Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn, who was not involved with the guidelines. “I was a bit surprised to see that atopic dermatitis in adults is associated with osteoporosis and fractures. As the authors suggest, this could be secondary to treatment with oral prednisone, and it is possible that use of dupilumab and JAK inhibitors may lessen this association.”
Shawn G. Kwatra, MD, of the department of dermatology at Johns Hopkins University, Baltimore, who was not involved with the guidelines, and was also asked to comment, said that the guidelines underscore the importance of informing adults with AD “of the risks of unchecked inflammation and the potential for multiple disease comorbidities.” Dr. Kwatra, who has AD, added that “these results make me want to be more proactive in treating my eczema to reduce the potential for development of these comorbidities.”
He pointed out that the guidelines did not address racial and ethnic differences in the observed comorbidities. “Unfortunately, minority populations have a greater comorbidity burden in many inflammatory skin diseases so this will be another area needing further investigation,” he said. “As an example, our group found from multicenter data that black patients with atopic dermatitis have higher levels of C-reactive protein, blood eosinophils, and other inflammatory biomarkers.”
The AAD guidelines are the first in a four-part series on AD expected to be published over the next 1-2 years, Dr. Davis said. The subsequent guidelines will address topicals, phototherapy/systemics, and pediatrics.
The study was funded by internal funds from the AAD. Dr. Davis reported having no financial disclosures. Dr. Sidbury disclosed that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, and an investigator for Brickell Biotech and Galderma. He is also a consultant for Galderma Global and Microes. Dr. Ko reported having no financial disclosures. Dr. Kwatra is a member of the board of directors of the Skin of Color Society. He is also an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron Pharmaceuticals, and Sanofi, and has served as an investigator for Galderma, Pfizer, and Sanofi.
While it’s well established that atopic dermatitis (AD) in adults is associated with asthma, allergic rhinitis, and other atopic conditions, the links between AD and other comorbidities are coming into clearer focus.
, published evidence supports an association between AD and comorbidities that may not be on the radar of clinicians and patients, including substance use, attention-deficit/hyperactivity disorder (ADHD), elements of metabolic syndrome, and various cardiovascular conditions.
“There are more comorbidities with AD than we anticipated, that are supported by data in the literature,” Dawn M.R. Davis, MD, cochair and an author of the guidelines, told this news organization. “We are learning more about the interconnectivity of various medical conditions,” she continued. “Many skin diseases over time have been noted to be impactful to the whole person and not only the skin. A classic example of that is psoriasis. We now understand that psoriasis is a multisystem inflammatory disorder.”
As for AD, “we’ve always appreciated that AD patients tend to be at higher risk for other atopic diseases such as asthma, allergic rhinoconjunctivitis, and food allergies,” said Dr. Davis, of the departments of dermatology and pediatrics at the Mayo Clinic, Rochester, Minn. “With further research, we are now able to delineate those associations more intimately and have data to support our suspicions. Additionally, we’re now understanding that these inflammatory conditions can impact more than the end organ involved, such as the skin and AD. We wanted to look at how AD can affect the whole patient.”
For the guidelines, which are the first of their kind and were published online in the Journal of the American Academy of Dermatology, Dr. Davis and project cochair Robert Sidbury, MD, MPH, chief of dermatology at Seattle Children’s Hospital, led a multidisciplinary group of 12 experts to review the association between AD and selected comorbidities. They applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for prognosis approach for assessing the certainty of the evidence and provided statements of association based on the available evidence.
With respect to highlights for atopic and allergic conditions, the guideline authors found high-quality evidence that AD in adults is associated with food allergies, moderate-quality evidence that AD is associated with asthma, and low-quality evidence that AD in adults may be associated with eosinophilic esophagitis.
In the realm of mental health and substance use, ample evidence exists to support an association between AD and mental health conditions such as depression and anxiety, the guidelines state. “For many patients, low mood may be driven by the symptoms of AD, including chronic itch and poor sleep,” Dr. Davis and her coauthors wrote. “Successfully treating AD may alleviate depressive symptoms for some patients; for others, assessment and treatment specific to their mental health may be needed.”
The guidelines also state that low-quality evidence exists to suggest that AD in adults may be associated with alcohol abuse disorders and cigarette smoking.
The authors noted “limited but consistent evidence” supporting a link between AD and adverse bone health, including osteoporosis and fractures, while associations between AD and cardiovascular risk factors and comorbidities, including hypertension, myocardial infarction, and stroke, are more controversial.
“I have published on bone health and AD so that was not as surprising to me,” Dr. Davis said in the interview. “I found a lot of the evidence in the guidelines to be validating of patterns that we see in our patients. The most significant learning point for me was [the link to] cardiovascular disease and the link to specific mental health and substance use disorders. It validates how impactful AD is to the individual.”
According to the guidelines, moderate-quality evidence exists linking AD in adults to both alopecia areata and urticaria. “Because we are dermatologists and take care of both of those diseases, be mindful of that in your daily practice,” Dr. Davis advised. “I would also encourage our colleagues to remember to educate patients on the comorbidities of AD so that they are empowered, and to screen for those comorbidities in your office based on the patient and their history and physical exam, to the level that you think is appropriate for that person’s individual’s care.”
Christine Ko, MD, who was asked to comment on the guidelines, characterized some of the reported comorbidity associations as predictable, such as asthma, food allergy, allergic rhinitis, and skin infections. “As the authors comment, ‘associations between AD and other atopic and allergic conditions have been recognized for decades and even contribute to diagnostic criteria for AD,’ ” said Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn, who was not involved with the guidelines. “I was a bit surprised to see that atopic dermatitis in adults is associated with osteoporosis and fractures. As the authors suggest, this could be secondary to treatment with oral prednisone, and it is possible that use of dupilumab and JAK inhibitors may lessen this association.”
Shawn G. Kwatra, MD, of the department of dermatology at Johns Hopkins University, Baltimore, who was not involved with the guidelines, and was also asked to comment, said that the guidelines underscore the importance of informing adults with AD “of the risks of unchecked inflammation and the potential for multiple disease comorbidities.” Dr. Kwatra, who has AD, added that “these results make me want to be more proactive in treating my eczema to reduce the potential for development of these comorbidities.”
He pointed out that the guidelines did not address racial and ethnic differences in the observed comorbidities. “Unfortunately, minority populations have a greater comorbidity burden in many inflammatory skin diseases so this will be another area needing further investigation,” he said. “As an example, our group found from multicenter data that black patients with atopic dermatitis have higher levels of C-reactive protein, blood eosinophils, and other inflammatory biomarkers.”
The AAD guidelines are the first in a four-part series on AD expected to be published over the next 1-2 years, Dr. Davis said. The subsequent guidelines will address topicals, phototherapy/systemics, and pediatrics.
The study was funded by internal funds from the AAD. Dr. Davis reported having no financial disclosures. Dr. Sidbury disclosed that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, and an investigator for Brickell Biotech and Galderma. He is also a consultant for Galderma Global and Microes. Dr. Ko reported having no financial disclosures. Dr. Kwatra is a member of the board of directors of the Skin of Color Society. He is also an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron Pharmaceuticals, and Sanofi, and has served as an investigator for Galderma, Pfizer, and Sanofi.
While it’s well established that atopic dermatitis (AD) in adults is associated with asthma, allergic rhinitis, and other atopic conditions, the links between AD and other comorbidities are coming into clearer focus.
, published evidence supports an association between AD and comorbidities that may not be on the radar of clinicians and patients, including substance use, attention-deficit/hyperactivity disorder (ADHD), elements of metabolic syndrome, and various cardiovascular conditions.
“There are more comorbidities with AD than we anticipated, that are supported by data in the literature,” Dawn M.R. Davis, MD, cochair and an author of the guidelines, told this news organization. “We are learning more about the interconnectivity of various medical conditions,” she continued. “Many skin diseases over time have been noted to be impactful to the whole person and not only the skin. A classic example of that is psoriasis. We now understand that psoriasis is a multisystem inflammatory disorder.”
As for AD, “we’ve always appreciated that AD patients tend to be at higher risk for other atopic diseases such as asthma, allergic rhinoconjunctivitis, and food allergies,” said Dr. Davis, of the departments of dermatology and pediatrics at the Mayo Clinic, Rochester, Minn. “With further research, we are now able to delineate those associations more intimately and have data to support our suspicions. Additionally, we’re now understanding that these inflammatory conditions can impact more than the end organ involved, such as the skin and AD. We wanted to look at how AD can affect the whole patient.”
For the guidelines, which are the first of their kind and were published online in the Journal of the American Academy of Dermatology, Dr. Davis and project cochair Robert Sidbury, MD, MPH, chief of dermatology at Seattle Children’s Hospital, led a multidisciplinary group of 12 experts to review the association between AD and selected comorbidities. They applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for prognosis approach for assessing the certainty of the evidence and provided statements of association based on the available evidence.
With respect to highlights for atopic and allergic conditions, the guideline authors found high-quality evidence that AD in adults is associated with food allergies, moderate-quality evidence that AD is associated with asthma, and low-quality evidence that AD in adults may be associated with eosinophilic esophagitis.
In the realm of mental health and substance use, ample evidence exists to support an association between AD and mental health conditions such as depression and anxiety, the guidelines state. “For many patients, low mood may be driven by the symptoms of AD, including chronic itch and poor sleep,” Dr. Davis and her coauthors wrote. “Successfully treating AD may alleviate depressive symptoms for some patients; for others, assessment and treatment specific to their mental health may be needed.”
The guidelines also state that low-quality evidence exists to suggest that AD in adults may be associated with alcohol abuse disorders and cigarette smoking.
The authors noted “limited but consistent evidence” supporting a link between AD and adverse bone health, including osteoporosis and fractures, while associations between AD and cardiovascular risk factors and comorbidities, including hypertension, myocardial infarction, and stroke, are more controversial.
“I have published on bone health and AD so that was not as surprising to me,” Dr. Davis said in the interview. “I found a lot of the evidence in the guidelines to be validating of patterns that we see in our patients. The most significant learning point for me was [the link to] cardiovascular disease and the link to specific mental health and substance use disorders. It validates how impactful AD is to the individual.”
According to the guidelines, moderate-quality evidence exists linking AD in adults to both alopecia areata and urticaria. “Because we are dermatologists and take care of both of those diseases, be mindful of that in your daily practice,” Dr. Davis advised. “I would also encourage our colleagues to remember to educate patients on the comorbidities of AD so that they are empowered, and to screen for those comorbidities in your office based on the patient and their history and physical exam, to the level that you think is appropriate for that person’s individual’s care.”
Christine Ko, MD, who was asked to comment on the guidelines, characterized some of the reported comorbidity associations as predictable, such as asthma, food allergy, allergic rhinitis, and skin infections. “As the authors comment, ‘associations between AD and other atopic and allergic conditions have been recognized for decades and even contribute to diagnostic criteria for AD,’ ” said Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn, who was not involved with the guidelines. “I was a bit surprised to see that atopic dermatitis in adults is associated with osteoporosis and fractures. As the authors suggest, this could be secondary to treatment with oral prednisone, and it is possible that use of dupilumab and JAK inhibitors may lessen this association.”
Shawn G. Kwatra, MD, of the department of dermatology at Johns Hopkins University, Baltimore, who was not involved with the guidelines, and was also asked to comment, said that the guidelines underscore the importance of informing adults with AD “of the risks of unchecked inflammation and the potential for multiple disease comorbidities.” Dr. Kwatra, who has AD, added that “these results make me want to be more proactive in treating my eczema to reduce the potential for development of these comorbidities.”
He pointed out that the guidelines did not address racial and ethnic differences in the observed comorbidities. “Unfortunately, minority populations have a greater comorbidity burden in many inflammatory skin diseases so this will be another area needing further investigation,” he said. “As an example, our group found from multicenter data that black patients with atopic dermatitis have higher levels of C-reactive protein, blood eosinophils, and other inflammatory biomarkers.”
The AAD guidelines are the first in a four-part series on AD expected to be published over the next 1-2 years, Dr. Davis said. The subsequent guidelines will address topicals, phototherapy/systemics, and pediatrics.
The study was funded by internal funds from the AAD. Dr. Davis reported having no financial disclosures. Dr. Sidbury disclosed that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, and an investigator for Brickell Biotech and Galderma. He is also a consultant for Galderma Global and Microes. Dr. Ko reported having no financial disclosures. Dr. Kwatra is a member of the board of directors of the Skin of Color Society. He is also an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron Pharmaceuticals, and Sanofi, and has served as an investigator for Galderma, Pfizer, and Sanofi.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Perception of atopic dermatitis severity often differs between patients, physicians
It’s no secret that atopic dermatitis (AD) is associated with a high burden of disease, with an impact on sleep disturbance, increased anxiety, depression, reduced function and productivity at work and school, and overall decreased quality of life.
But to complicate matters, , according to Zelma Chiesa Fuxench, MD, a dermatologist at the University of Pennsylvania, Philadelphia. For example, a cross-sectional study of 678 patients with AD, which assessed disease severity based on self-reports and physician-reported disease severity using components of the Eczema Area and Severity Index score, found that the level of agreement matched in about 68% of the cases. However, in about 32% of cases, there was a mismatch between how patients and physicians rated disease severity. In about 11% of the cases, patients reported a higher degree of disease severity, compared with physicians, while in about 20% of cases, patients reported lower disease severity, compared with the physician assessment.
“This has potential implications for overestimating or underestimating disease burden and could impact our treatment of AD patients,” Dr. Chiesa Fuxench said at the Revolutionizing Atopic Dermatitis symposium.
The study also found that, while the pattern of agreement was not affected by the extent of AD in terms of the body surface area, the use of immunomodulatory drugs, or the Eczema Area and Severity Index (EASI) score, increased sleep disturbance did have an influence. Also, quality of life was lower and a higher impact on work productivity was observed when patients rated their disease severity higher than the rating of physicians.
Measures to assess disease severity
“If we understand that there is mismatch between how a patient experiences their disease and how physicians rate it, what can we do to be better at assessing disease severity in AD to truly capture the full disease burden in patients with AD?” Dr. Chiesa Fuxench asked. She noted that different validated measures have been described in the literature, and objective assessment tools often used in clinical trials include the EASI and the SCORing Atopic Dermatitis (SCORAD). “These are measures that are done by the physician that take into account the extent of the body surface area involvement and also the intensity of the lesions such as how red or thick they are,” she said. “In addition, the SCORAD will also take into account the patient-reported intensity level of itch and sleep loss.”
The Patient-Oriented SCORAD (PO-SCORAD) is similar to the SCORAD except that it is completed by the patient or the patient’s caregiver. In all three outcome measures, a higher score indicates a higher level of disease severity. Other measures that have been frequently described in the literature include the Patient-Oriented Eczema Measure (POEM), which takes into account seven symptoms scored over the last week (itch, sleep, weeping/oozing, cracking, flaking, and dryness/roughness), with higher scores indicating increased disease severity, and the Dermatology Life Quality Index (DLQI), which is a generic measure to assess the burden of skin diseases including AD. The DLQI “asks 10 questions as they relate to the impact of health-related quality of life over the last week, with higher scores indicating more severe disease,” Dr. Chiesa Fuxench said.
There are also symptom-specific scales such as the Pruritus Numerical Rating Scale (Pruritus-NRS) that measures the impact of itch on a scale of 0 to 10, and the Three-Item Severity Scale (TIS) and the Validated Investigator Global Assessment (v-IGA) that are used to assess different measures in terms of intensity of the lesions.”
However, the study that looked at the discordance between AD severity reported by physicians and patients also found that awareness and use of clinical and patient-reported measures for assessing AD disease severity among physicians was low. The authors further divided their findings among primary care physicians, dermatologists, and allergists/immunologists. “While dermatologists and allergists/immunologists reported being more aware of these outcome measures, a high proportion of physicians within this group were not using these outcomes measures in daily clinical practice,” Dr. Chiesa Fuxench said.
“Is there a need for us to use more than one outcome measure instrument when trying to assess the impact of AD, understanding that many of us practice in a very busy clinical setting? The answer is probably yes. The use of multiple assessment tools that measure different domains could potentially help better capture the broad manifestations of AD, because of the complex nature of disease burden in this population. In addition, there are studies showing poor correlation between patient-reported and physician-assessed disease severity for various instruments, emphasizing the point that these measures may be capturing very different things.”
With so many measures to choose from and limited time in the office, which ones should clinicians use? Harmonizing Outcome Measures for Eczema (HOME), based at the Center of Evidence-based Dermatology, at the University of Nottingham (England), is a consortium of patients and other key stakeholders in AD aiming to develop a consensus-based core outcome set for clinical trials and clinical practice. At a consensus meeting in 2018, the consortium reported that the PO-SCORAD and the POEM could be used in the clinical setting to better capture the true level of disease severity and burden in patients with AD.
The PO-SCORAD is also available as an App. A PO-SCORAD of less than 25 is associated with mild disease; a PO-SCORAD between 25 and 50 is associated with moderate disease, and a PO-SCORAD of greater than 50 is associated with severe disease.
“It’s recommended that patients capture the PO-SCORAD once or twice a week,” Dr. Chiesa Fuxench said, noting that the newer version of the App includes photos of different skin types to make it more relevant for a larger number of patients.
Another advantage of using the App is that a patient can track their disease severity through time. They can upload photographs, or they can send you a graphical input of their disease severity either through e-mail or print it out and bring it to their office visit to share the results with you.”
A prospective observational European study of 471 adult and pediatric patients with AD found a statistically significant correlation between SCORAD and PO-SCORAD results at day 0 and day 28. A separate large study conducted in 12 countries found that PO-SCORAD was the only self-assessment score to be highly correlated with the SCORAD index and POEM (A Spearman’s correlation coefficient of greater than or equal to 0.70). In that study, PO-SCORAD also correlated most closely with the results of the DLQI (r = 0.67) and the Dermatitis Family Questionnaire Impact DFQI (r = 0.56).
A more recent study of almost 300 adults with AD that examined the correlations between PO-SCORAD, POEM, and DLQI yielded similar findings.
Other researchers are aiming to assess the full burden of AD at the patient level. Drawing from a cross-sectional study called AWARE (Adults With Atopic Dermatitis Reporting on their Experience), an international observational study, investigators sought to identify what terms AD patients were using to describe their disease. The most commonly used terms were itch (37%), embarrassed (37%), annoyed (35%), pain (25%), and frustration (22%). “Although our study did not identify all patient-reported consequences of AD, such as the known impact of AD on sexual health, our qualitative approach has provided an understanding of patient perceptions and the underlying range of physical and emotional consequences of AD, which can inform shared decision-making,” the authors wrote. “These findings suggest the need for broader assessment of the impact of AD on patients’ lives,” they added.
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
The study on AD severity reported by physicians and patients was funded by Sanofi and Regeneron Pharmaceuticals, and several authors were employees of those companies.
It’s no secret that atopic dermatitis (AD) is associated with a high burden of disease, with an impact on sleep disturbance, increased anxiety, depression, reduced function and productivity at work and school, and overall decreased quality of life.
But to complicate matters, , according to Zelma Chiesa Fuxench, MD, a dermatologist at the University of Pennsylvania, Philadelphia. For example, a cross-sectional study of 678 patients with AD, which assessed disease severity based on self-reports and physician-reported disease severity using components of the Eczema Area and Severity Index score, found that the level of agreement matched in about 68% of the cases. However, in about 32% of cases, there was a mismatch between how patients and physicians rated disease severity. In about 11% of the cases, patients reported a higher degree of disease severity, compared with physicians, while in about 20% of cases, patients reported lower disease severity, compared with the physician assessment.
“This has potential implications for overestimating or underestimating disease burden and could impact our treatment of AD patients,” Dr. Chiesa Fuxench said at the Revolutionizing Atopic Dermatitis symposium.
The study also found that, while the pattern of agreement was not affected by the extent of AD in terms of the body surface area, the use of immunomodulatory drugs, or the Eczema Area and Severity Index (EASI) score, increased sleep disturbance did have an influence. Also, quality of life was lower and a higher impact on work productivity was observed when patients rated their disease severity higher than the rating of physicians.
Measures to assess disease severity
“If we understand that there is mismatch between how a patient experiences their disease and how physicians rate it, what can we do to be better at assessing disease severity in AD to truly capture the full disease burden in patients with AD?” Dr. Chiesa Fuxench asked. She noted that different validated measures have been described in the literature, and objective assessment tools often used in clinical trials include the EASI and the SCORing Atopic Dermatitis (SCORAD). “These are measures that are done by the physician that take into account the extent of the body surface area involvement and also the intensity of the lesions such as how red or thick they are,” she said. “In addition, the SCORAD will also take into account the patient-reported intensity level of itch and sleep loss.”
The Patient-Oriented SCORAD (PO-SCORAD) is similar to the SCORAD except that it is completed by the patient or the patient’s caregiver. In all three outcome measures, a higher score indicates a higher level of disease severity. Other measures that have been frequently described in the literature include the Patient-Oriented Eczema Measure (POEM), which takes into account seven symptoms scored over the last week (itch, sleep, weeping/oozing, cracking, flaking, and dryness/roughness), with higher scores indicating increased disease severity, and the Dermatology Life Quality Index (DLQI), which is a generic measure to assess the burden of skin diseases including AD. The DLQI “asks 10 questions as they relate to the impact of health-related quality of life over the last week, with higher scores indicating more severe disease,” Dr. Chiesa Fuxench said.
There are also symptom-specific scales such as the Pruritus Numerical Rating Scale (Pruritus-NRS) that measures the impact of itch on a scale of 0 to 10, and the Three-Item Severity Scale (TIS) and the Validated Investigator Global Assessment (v-IGA) that are used to assess different measures in terms of intensity of the lesions.”
However, the study that looked at the discordance between AD severity reported by physicians and patients also found that awareness and use of clinical and patient-reported measures for assessing AD disease severity among physicians was low. The authors further divided their findings among primary care physicians, dermatologists, and allergists/immunologists. “While dermatologists and allergists/immunologists reported being more aware of these outcome measures, a high proportion of physicians within this group were not using these outcomes measures in daily clinical practice,” Dr. Chiesa Fuxench said.
“Is there a need for us to use more than one outcome measure instrument when trying to assess the impact of AD, understanding that many of us practice in a very busy clinical setting? The answer is probably yes. The use of multiple assessment tools that measure different domains could potentially help better capture the broad manifestations of AD, because of the complex nature of disease burden in this population. In addition, there are studies showing poor correlation between patient-reported and physician-assessed disease severity for various instruments, emphasizing the point that these measures may be capturing very different things.”
With so many measures to choose from and limited time in the office, which ones should clinicians use? Harmonizing Outcome Measures for Eczema (HOME), based at the Center of Evidence-based Dermatology, at the University of Nottingham (England), is a consortium of patients and other key stakeholders in AD aiming to develop a consensus-based core outcome set for clinical trials and clinical practice. At a consensus meeting in 2018, the consortium reported that the PO-SCORAD and the POEM could be used in the clinical setting to better capture the true level of disease severity and burden in patients with AD.
The PO-SCORAD is also available as an App. A PO-SCORAD of less than 25 is associated with mild disease; a PO-SCORAD between 25 and 50 is associated with moderate disease, and a PO-SCORAD of greater than 50 is associated with severe disease.
“It’s recommended that patients capture the PO-SCORAD once or twice a week,” Dr. Chiesa Fuxench said, noting that the newer version of the App includes photos of different skin types to make it more relevant for a larger number of patients.
Another advantage of using the App is that a patient can track their disease severity through time. They can upload photographs, or they can send you a graphical input of their disease severity either through e-mail or print it out and bring it to their office visit to share the results with you.”
A prospective observational European study of 471 adult and pediatric patients with AD found a statistically significant correlation between SCORAD and PO-SCORAD results at day 0 and day 28. A separate large study conducted in 12 countries found that PO-SCORAD was the only self-assessment score to be highly correlated with the SCORAD index and POEM (A Spearman’s correlation coefficient of greater than or equal to 0.70). In that study, PO-SCORAD also correlated most closely with the results of the DLQI (r = 0.67) and the Dermatitis Family Questionnaire Impact DFQI (r = 0.56).
A more recent study of almost 300 adults with AD that examined the correlations between PO-SCORAD, POEM, and DLQI yielded similar findings.
Other researchers are aiming to assess the full burden of AD at the patient level. Drawing from a cross-sectional study called AWARE (Adults With Atopic Dermatitis Reporting on their Experience), an international observational study, investigators sought to identify what terms AD patients were using to describe their disease. The most commonly used terms were itch (37%), embarrassed (37%), annoyed (35%), pain (25%), and frustration (22%). “Although our study did not identify all patient-reported consequences of AD, such as the known impact of AD on sexual health, our qualitative approach has provided an understanding of patient perceptions and the underlying range of physical and emotional consequences of AD, which can inform shared decision-making,” the authors wrote. “These findings suggest the need for broader assessment of the impact of AD on patients’ lives,” they added.
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
The study on AD severity reported by physicians and patients was funded by Sanofi and Regeneron Pharmaceuticals, and several authors were employees of those companies.
It’s no secret that atopic dermatitis (AD) is associated with a high burden of disease, with an impact on sleep disturbance, increased anxiety, depression, reduced function and productivity at work and school, and overall decreased quality of life.
But to complicate matters, , according to Zelma Chiesa Fuxench, MD, a dermatologist at the University of Pennsylvania, Philadelphia. For example, a cross-sectional study of 678 patients with AD, which assessed disease severity based on self-reports and physician-reported disease severity using components of the Eczema Area and Severity Index score, found that the level of agreement matched in about 68% of the cases. However, in about 32% of cases, there was a mismatch between how patients and physicians rated disease severity. In about 11% of the cases, patients reported a higher degree of disease severity, compared with physicians, while in about 20% of cases, patients reported lower disease severity, compared with the physician assessment.
“This has potential implications for overestimating or underestimating disease burden and could impact our treatment of AD patients,” Dr. Chiesa Fuxench said at the Revolutionizing Atopic Dermatitis symposium.
The study also found that, while the pattern of agreement was not affected by the extent of AD in terms of the body surface area, the use of immunomodulatory drugs, or the Eczema Area and Severity Index (EASI) score, increased sleep disturbance did have an influence. Also, quality of life was lower and a higher impact on work productivity was observed when patients rated their disease severity higher than the rating of physicians.
Measures to assess disease severity
“If we understand that there is mismatch between how a patient experiences their disease and how physicians rate it, what can we do to be better at assessing disease severity in AD to truly capture the full disease burden in patients with AD?” Dr. Chiesa Fuxench asked. She noted that different validated measures have been described in the literature, and objective assessment tools often used in clinical trials include the EASI and the SCORing Atopic Dermatitis (SCORAD). “These are measures that are done by the physician that take into account the extent of the body surface area involvement and also the intensity of the lesions such as how red or thick they are,” she said. “In addition, the SCORAD will also take into account the patient-reported intensity level of itch and sleep loss.”
The Patient-Oriented SCORAD (PO-SCORAD) is similar to the SCORAD except that it is completed by the patient or the patient’s caregiver. In all three outcome measures, a higher score indicates a higher level of disease severity. Other measures that have been frequently described in the literature include the Patient-Oriented Eczema Measure (POEM), which takes into account seven symptoms scored over the last week (itch, sleep, weeping/oozing, cracking, flaking, and dryness/roughness), with higher scores indicating increased disease severity, and the Dermatology Life Quality Index (DLQI), which is a generic measure to assess the burden of skin diseases including AD. The DLQI “asks 10 questions as they relate to the impact of health-related quality of life over the last week, with higher scores indicating more severe disease,” Dr. Chiesa Fuxench said.
There are also symptom-specific scales such as the Pruritus Numerical Rating Scale (Pruritus-NRS) that measures the impact of itch on a scale of 0 to 10, and the Three-Item Severity Scale (TIS) and the Validated Investigator Global Assessment (v-IGA) that are used to assess different measures in terms of intensity of the lesions.”
However, the study that looked at the discordance between AD severity reported by physicians and patients also found that awareness and use of clinical and patient-reported measures for assessing AD disease severity among physicians was low. The authors further divided their findings among primary care physicians, dermatologists, and allergists/immunologists. “While dermatologists and allergists/immunologists reported being more aware of these outcome measures, a high proportion of physicians within this group were not using these outcomes measures in daily clinical practice,” Dr. Chiesa Fuxench said.
“Is there a need for us to use more than one outcome measure instrument when trying to assess the impact of AD, understanding that many of us practice in a very busy clinical setting? The answer is probably yes. The use of multiple assessment tools that measure different domains could potentially help better capture the broad manifestations of AD, because of the complex nature of disease burden in this population. In addition, there are studies showing poor correlation between patient-reported and physician-assessed disease severity for various instruments, emphasizing the point that these measures may be capturing very different things.”
With so many measures to choose from and limited time in the office, which ones should clinicians use? Harmonizing Outcome Measures for Eczema (HOME), based at the Center of Evidence-based Dermatology, at the University of Nottingham (England), is a consortium of patients and other key stakeholders in AD aiming to develop a consensus-based core outcome set for clinical trials and clinical practice. At a consensus meeting in 2018, the consortium reported that the PO-SCORAD and the POEM could be used in the clinical setting to better capture the true level of disease severity and burden in patients with AD.
The PO-SCORAD is also available as an App. A PO-SCORAD of less than 25 is associated with mild disease; a PO-SCORAD between 25 and 50 is associated with moderate disease, and a PO-SCORAD of greater than 50 is associated with severe disease.
“It’s recommended that patients capture the PO-SCORAD once or twice a week,” Dr. Chiesa Fuxench said, noting that the newer version of the App includes photos of different skin types to make it more relevant for a larger number of patients.
Another advantage of using the App is that a patient can track their disease severity through time. They can upload photographs, or they can send you a graphical input of their disease severity either through e-mail or print it out and bring it to their office visit to share the results with you.”
A prospective observational European study of 471 adult and pediatric patients with AD found a statistically significant correlation between SCORAD and PO-SCORAD results at day 0 and day 28. A separate large study conducted in 12 countries found that PO-SCORAD was the only self-assessment score to be highly correlated with the SCORAD index and POEM (A Spearman’s correlation coefficient of greater than or equal to 0.70). In that study, PO-SCORAD also correlated most closely with the results of the DLQI (r = 0.67) and the Dermatitis Family Questionnaire Impact DFQI (r = 0.56).
A more recent study of almost 300 adults with AD that examined the correlations between PO-SCORAD, POEM, and DLQI yielded similar findings.
Other researchers are aiming to assess the full burden of AD at the patient level. Drawing from a cross-sectional study called AWARE (Adults With Atopic Dermatitis Reporting on their Experience), an international observational study, investigators sought to identify what terms AD patients were using to describe their disease. The most commonly used terms were itch (37%), embarrassed (37%), annoyed (35%), pain (25%), and frustration (22%). “Although our study did not identify all patient-reported consequences of AD, such as the known impact of AD on sexual health, our qualitative approach has provided an understanding of patient perceptions and the underlying range of physical and emotional consequences of AD, which can inform shared decision-making,” the authors wrote. “These findings suggest the need for broader assessment of the impact of AD on patients’ lives,” they added.
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
The study on AD severity reported by physicians and patients was funded by Sanofi and Regeneron Pharmaceuticals, and several authors were employees of those companies.
FROM REVOLUTIONIZING AD 2021
Pruritus in elderly patients: Not a diagnosis
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2021
More frequent secukinumab dosing found to benefit overweight psoriasis patients
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Phase 2 studies of novel JAK1 inhibitor for HS show promise
results from two small phase 2 studies showed.
“INCB054707 is an oral, small-molecule JAK1 inhibitor with approximately 52-fold greater selectivity for JAK1 versus JAK2,” researchers led by Afsaneh Alavi, MD, of the Mayo Clinic in Rochester, Minn., wrote in an article published recently in the British Journal of Dermatology. “Specifically targeting JAK1, a critical regulator of proinflammatory cytokine signaling implicated in several immune-related diseases, may reduce cytokine signaling involved in HS pathogenesis while limiting JAK2-mediated cytopenias.”
For the first study, 10 patients received 15 mg INCB054707 once daily for 8 weeks (NCT03569371). For the second study, 35 patients were randomized to 30, 60, or 90 mg INCB054707 once daily or placebo (3:1 within each cohort) for 8 weeks (NCT03607487). Eligibility criteria for both studies included patients with Hurley stage II/III HS who aged 18-75 years with lesions present in two or more anatomic locations, and a total abscess and inflammatory nodule count of three or more. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
The researchers reported that 30% of patients in study 1 and 42.3% of patients who received INCB054707 in study 2 experienced one or more treatment-emergent adverse event, most commonly upper respiratory tract infection. Among evaluable patients, 3 patients (42.9%) in study 1 and 17 patients in study 2 (65.4%) achieved HiSCR at week 8, compared with 57.1% of those in the placebo group. By dosing, 55.6% in the 30-mg group achieved HiSCR at week 8, compared with 55.6% in the 60-mg group and 87.5% in the 90-mg group.
“In conclusion, safety and efficacy findings from these two phase 2 studies establish proof of concept for the JAK1 inhibitor INCB054707 in the treatment of moderate to severe HS,” the authors wrote. “A phase 2, dose-ranging, placebo-controlled study exploring three dose levels and including approximately 200 patients is ongoing (NCT04476043) and expected to provide additional evidence of the safety and efficacy profile of INCB054707 in patients with HS.”
INCB054707 is being developed by Incyte Corporation. Dr. Alavi disclosed that she has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRX, and UCB, and received honoraria as an investigator for Boehringer Ingelheim and Processa.
results from two small phase 2 studies showed.
“INCB054707 is an oral, small-molecule JAK1 inhibitor with approximately 52-fold greater selectivity for JAK1 versus JAK2,” researchers led by Afsaneh Alavi, MD, of the Mayo Clinic in Rochester, Minn., wrote in an article published recently in the British Journal of Dermatology. “Specifically targeting JAK1, a critical regulator of proinflammatory cytokine signaling implicated in several immune-related diseases, may reduce cytokine signaling involved in HS pathogenesis while limiting JAK2-mediated cytopenias.”
For the first study, 10 patients received 15 mg INCB054707 once daily for 8 weeks (NCT03569371). For the second study, 35 patients were randomized to 30, 60, or 90 mg INCB054707 once daily or placebo (3:1 within each cohort) for 8 weeks (NCT03607487). Eligibility criteria for both studies included patients with Hurley stage II/III HS who aged 18-75 years with lesions present in two or more anatomic locations, and a total abscess and inflammatory nodule count of three or more. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
The researchers reported that 30% of patients in study 1 and 42.3% of patients who received INCB054707 in study 2 experienced one or more treatment-emergent adverse event, most commonly upper respiratory tract infection. Among evaluable patients, 3 patients (42.9%) in study 1 and 17 patients in study 2 (65.4%) achieved HiSCR at week 8, compared with 57.1% of those in the placebo group. By dosing, 55.6% in the 30-mg group achieved HiSCR at week 8, compared with 55.6% in the 60-mg group and 87.5% in the 90-mg group.
“In conclusion, safety and efficacy findings from these two phase 2 studies establish proof of concept for the JAK1 inhibitor INCB054707 in the treatment of moderate to severe HS,” the authors wrote. “A phase 2, dose-ranging, placebo-controlled study exploring three dose levels and including approximately 200 patients is ongoing (NCT04476043) and expected to provide additional evidence of the safety and efficacy profile of INCB054707 in patients with HS.”
INCB054707 is being developed by Incyte Corporation. Dr. Alavi disclosed that she has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRX, and UCB, and received honoraria as an investigator for Boehringer Ingelheim and Processa.
results from two small phase 2 studies showed.
“INCB054707 is an oral, small-molecule JAK1 inhibitor with approximately 52-fold greater selectivity for JAK1 versus JAK2,” researchers led by Afsaneh Alavi, MD, of the Mayo Clinic in Rochester, Minn., wrote in an article published recently in the British Journal of Dermatology. “Specifically targeting JAK1, a critical regulator of proinflammatory cytokine signaling implicated in several immune-related diseases, may reduce cytokine signaling involved in HS pathogenesis while limiting JAK2-mediated cytopenias.”
For the first study, 10 patients received 15 mg INCB054707 once daily for 8 weeks (NCT03569371). For the second study, 35 patients were randomized to 30, 60, or 90 mg INCB054707 once daily or placebo (3:1 within each cohort) for 8 weeks (NCT03607487). Eligibility criteria for both studies included patients with Hurley stage II/III HS who aged 18-75 years with lesions present in two or more anatomic locations, and a total abscess and inflammatory nodule count of three or more. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
The researchers reported that 30% of patients in study 1 and 42.3% of patients who received INCB054707 in study 2 experienced one or more treatment-emergent adverse event, most commonly upper respiratory tract infection. Among evaluable patients, 3 patients (42.9%) in study 1 and 17 patients in study 2 (65.4%) achieved HiSCR at week 8, compared with 57.1% of those in the placebo group. By dosing, 55.6% in the 30-mg group achieved HiSCR at week 8, compared with 55.6% in the 60-mg group and 87.5% in the 90-mg group.
“In conclusion, safety and efficacy findings from these two phase 2 studies establish proof of concept for the JAK1 inhibitor INCB054707 in the treatment of moderate to severe HS,” the authors wrote. “A phase 2, dose-ranging, placebo-controlled study exploring three dose levels and including approximately 200 patients is ongoing (NCT04476043) and expected to provide additional evidence of the safety and efficacy profile of INCB054707 in patients with HS.”
INCB054707 is being developed by Incyte Corporation. Dr. Alavi disclosed that she has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRX, and UCB, and received honoraria as an investigator for Boehringer Ingelheim and Processa.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
FDA approves two JAK-1 inhibitors for moderate to severe atopic dermatitis
The available for this indication in the United States.
“It’s big news because a few years ago we didn’t have any systemic treatments that are safer than the classical immunosuppressants like cyclosporine and methotrexate,” Emma Guttman-Yassky, MD, PhD, Waldman professor and system chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York, told this news organization commenting on upadacitinib’s approval.
“The only oral approved drug for AD up to now was oral prednisone, which has terrible safety concerns. This is basically the first oral medication that we can provide our patients for long-term use.”
Upadacitinib
The approval of upadacitinib (Rinvoq), marketed by AbbVie, for moderate to severe AD in patients ages 12 and older, comes on the heels of findings from three pivotal phase 3 studies involving more than 2,500 adults and children 12 years of age and older with moderate to severe AD: Measure Up 1 and 2, led by Dr. Guttman-Yassky, which evaluated upadacitinib compared with placebo, and AD UP, which compared upadacitinib along with topical corticosteroids, compared with placebo.
Across the three studies, upadacitinib – both 15 mg and 30 mg once daily monotherapy – met all primary and secondary endpoints at week 16, with some patients achieving higher levels of skin clearance based on the Eczema Area and Severity Index 90 (EASI-90) and EASI-100.
“I always say that patients with AD need options,” Dr. Guttman-Yassky said. “We need biologics. We need oral medications. Not everybody likes an injectable. The plus of the class of JAK inhibitors in general is the quick onset of action.” Many patients in her clinic are maintained on upadacitinib more than two years later “and are super happy,” she said. “Many of them failed cyclosporine and other immunosuppressants such as methotrexate and prednisone.”
She predicted that health insurance companies will find coverage cost-effective “because it sets a new bar for efficacy, and because many patients have failed other treatments.”
Abrocitinib
Abrocitinib (Cibinqo), marketed by Pfizer, was approved for adults with moderate to severe AD. The approval was based on results of five clinical trials from a large-scale clinical trial program of more than 1,600 patients. The recommended doses are 100 mg and 200 mg, with the 200 mg dose recommended for patients who are not responding to the 100 mg dose.
The labeling of abrocitinib and upadacitinib include a boxed warning for JAK inhibitors, regarding the risk of serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Dr. Guttman-Yassky has served as a principal investigator for AbbVie and has received consulting fees from the company.
The available for this indication in the United States.
“It’s big news because a few years ago we didn’t have any systemic treatments that are safer than the classical immunosuppressants like cyclosporine and methotrexate,” Emma Guttman-Yassky, MD, PhD, Waldman professor and system chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York, told this news organization commenting on upadacitinib’s approval.
“The only oral approved drug for AD up to now was oral prednisone, which has terrible safety concerns. This is basically the first oral medication that we can provide our patients for long-term use.”
Upadacitinib
The approval of upadacitinib (Rinvoq), marketed by AbbVie, for moderate to severe AD in patients ages 12 and older, comes on the heels of findings from three pivotal phase 3 studies involving more than 2,500 adults and children 12 years of age and older with moderate to severe AD: Measure Up 1 and 2, led by Dr. Guttman-Yassky, which evaluated upadacitinib compared with placebo, and AD UP, which compared upadacitinib along with topical corticosteroids, compared with placebo.
Across the three studies, upadacitinib – both 15 mg and 30 mg once daily monotherapy – met all primary and secondary endpoints at week 16, with some patients achieving higher levels of skin clearance based on the Eczema Area and Severity Index 90 (EASI-90) and EASI-100.
“I always say that patients with AD need options,” Dr. Guttman-Yassky said. “We need biologics. We need oral medications. Not everybody likes an injectable. The plus of the class of JAK inhibitors in general is the quick onset of action.” Many patients in her clinic are maintained on upadacitinib more than two years later “and are super happy,” she said. “Many of them failed cyclosporine and other immunosuppressants such as methotrexate and prednisone.”
She predicted that health insurance companies will find coverage cost-effective “because it sets a new bar for efficacy, and because many patients have failed other treatments.”
Abrocitinib
Abrocitinib (Cibinqo), marketed by Pfizer, was approved for adults with moderate to severe AD. The approval was based on results of five clinical trials from a large-scale clinical trial program of more than 1,600 patients. The recommended doses are 100 mg and 200 mg, with the 200 mg dose recommended for patients who are not responding to the 100 mg dose.
The labeling of abrocitinib and upadacitinib include a boxed warning for JAK inhibitors, regarding the risk of serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Dr. Guttman-Yassky has served as a principal investigator for AbbVie and has received consulting fees from the company.
The available for this indication in the United States.
“It’s big news because a few years ago we didn’t have any systemic treatments that are safer than the classical immunosuppressants like cyclosporine and methotrexate,” Emma Guttman-Yassky, MD, PhD, Waldman professor and system chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York, told this news organization commenting on upadacitinib’s approval.
“The only oral approved drug for AD up to now was oral prednisone, which has terrible safety concerns. This is basically the first oral medication that we can provide our patients for long-term use.”
Upadacitinib
The approval of upadacitinib (Rinvoq), marketed by AbbVie, for moderate to severe AD in patients ages 12 and older, comes on the heels of findings from three pivotal phase 3 studies involving more than 2,500 adults and children 12 years of age and older with moderate to severe AD: Measure Up 1 and 2, led by Dr. Guttman-Yassky, which evaluated upadacitinib compared with placebo, and AD UP, which compared upadacitinib along with topical corticosteroids, compared with placebo.
Across the three studies, upadacitinib – both 15 mg and 30 mg once daily monotherapy – met all primary and secondary endpoints at week 16, with some patients achieving higher levels of skin clearance based on the Eczema Area and Severity Index 90 (EASI-90) and EASI-100.
“I always say that patients with AD need options,” Dr. Guttman-Yassky said. “We need biologics. We need oral medications. Not everybody likes an injectable. The plus of the class of JAK inhibitors in general is the quick onset of action.” Many patients in her clinic are maintained on upadacitinib more than two years later “and are super happy,” she said. “Many of them failed cyclosporine and other immunosuppressants such as methotrexate and prednisone.”
She predicted that health insurance companies will find coverage cost-effective “because it sets a new bar for efficacy, and because many patients have failed other treatments.”
Abrocitinib
Abrocitinib (Cibinqo), marketed by Pfizer, was approved for adults with moderate to severe AD. The approval was based on results of five clinical trials from a large-scale clinical trial program of more than 1,600 patients. The recommended doses are 100 mg and 200 mg, with the 200 mg dose recommended for patients who are not responding to the 100 mg dose.
The labeling of abrocitinib and upadacitinib include a boxed warning for JAK inhibitors, regarding the risk of serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Dr. Guttman-Yassky has served as a principal investigator for AbbVie and has received consulting fees from the company.
Hand eczema and atopic dermatitis closely linked
An estimated (AD), according to Jacob P. Thyssen, MD, PhD.
“If we look at individuals with AD, the lifetime prevalence of hand eczema reaches 50%, so we see a strong association between hand eczema and AD,” Dr. Thyssen, professor of dermatology at the University of Copenhagen, said at the Revolutionizing Atopic Dermatitis symposium.
Risk factors for hand eczema – defined as eczema on the hand and/or wrists – include AD, which increases the risk by two- to threefold, as well as genetic predisposition beyond AD, exposure to irritants and allergens, female gender, young age, low socioeconomic group, high risk occupations (including construction workers and hairdressers), and tobacco smoking.
“As clinicians, we sometimes need to rule out a few differentials, including psoriasis and T-cell lymphoma. As an example, 10% of T-cell lymphoma patients, a very rare condition, have first onset of the disease on their hands,” Dr. Thyssen said. “Once we see persistent hand eczema, we need to obtain a history of irritant exposure and allergen exposure, both at home and at work, perform a patch test, sometimes a skin prick test, and ask about personal and family history of AD and psoriasis.”
He noted that while formal classification of hand eczema has been a struggle for decades, he favors the “straightforward” clinical approach from the European Environmental and Contact Dermatitis Research Group. Atopic hand eczema, he said, “is very much characterized by dorsal involvement of the hands and fingers and sparse involvement of the palmar aspects of the hands.”
The cheeks and hands are predilection sites for AD in filaggrin mutation carriers (as they are sites of low filaggrin levels), and sometimes harsh environmental exposures, such as cold and dry air. In a study of 3,335 patients in Denmark, Dr. Thyssen and colleagues found that filaggrin mutations and AD were associated with early-onset and persistent hand eczema. In another study of 3,834 adults with AD or psoriasis, he and colleagues found that among those with AD, the wrists, back of the hands, and interdigital areas were often sites of severe eczema, while palmar involvement was more uncommon.
The same findings apply for the feet in filaggrin mutation carriers with AD; the dorsal aspect of the feet was more commonly affected, compared with plantar aspects of the feet.
Medical literature regarding foot eczema is scarce, but a retrospective cohort study from Germany found that foot eczema and hand eczema often co-occur. Among 723 hand eczema patients, 201 (28%) had concomitant foot eczema. The same morphological features were found on the hands and feet in 71% of patients. Foot eczema was significantly associated with male sex, atopic hand eczema, hyperhidrosis, wearing of safety shoes/boots at work, and tobacco smoking.
In addition, a systematic review and meta-analysis of studies of hand eczema and AD found that there was a 4.29-fold increased risk of hand eczema in individuals with AD, and the risk (lifetime prevalence) of occupational hand eczema was increased by more than twofold. “However, this study could not differentiate between irritant contact dermatitis on the hands and atopic dermatitis,” Dr. Thyssen said. “The studies were not accurate enough to allow for any conclusions.”
A multicenter study of adults with hand eczema in Italy found that the proportion of patients with AD was the highest among those with severe and refractory chronic hand eczema. In addition, certain professions, including those of hairdressers, health professionals, and those in trade work, such as plumbing, were more often associated with chronic hand eczema. “This teaches us that we should be very careful about steering these patients from at-risk occupations,” Dr. Thyssen said. “Also, we should remember to treat them aggressively in the beginning to reduce the risk of severe and refractory chronic hand eczema.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Asian, Arena, Almirall, AbbVie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme.
An estimated (AD), according to Jacob P. Thyssen, MD, PhD.
“If we look at individuals with AD, the lifetime prevalence of hand eczema reaches 50%, so we see a strong association between hand eczema and AD,” Dr. Thyssen, professor of dermatology at the University of Copenhagen, said at the Revolutionizing Atopic Dermatitis symposium.
Risk factors for hand eczema – defined as eczema on the hand and/or wrists – include AD, which increases the risk by two- to threefold, as well as genetic predisposition beyond AD, exposure to irritants and allergens, female gender, young age, low socioeconomic group, high risk occupations (including construction workers and hairdressers), and tobacco smoking.
“As clinicians, we sometimes need to rule out a few differentials, including psoriasis and T-cell lymphoma. As an example, 10% of T-cell lymphoma patients, a very rare condition, have first onset of the disease on their hands,” Dr. Thyssen said. “Once we see persistent hand eczema, we need to obtain a history of irritant exposure and allergen exposure, both at home and at work, perform a patch test, sometimes a skin prick test, and ask about personal and family history of AD and psoriasis.”
He noted that while formal classification of hand eczema has been a struggle for decades, he favors the “straightforward” clinical approach from the European Environmental and Contact Dermatitis Research Group. Atopic hand eczema, he said, “is very much characterized by dorsal involvement of the hands and fingers and sparse involvement of the palmar aspects of the hands.”
The cheeks and hands are predilection sites for AD in filaggrin mutation carriers (as they are sites of low filaggrin levels), and sometimes harsh environmental exposures, such as cold and dry air. In a study of 3,335 patients in Denmark, Dr. Thyssen and colleagues found that filaggrin mutations and AD were associated with early-onset and persistent hand eczema. In another study of 3,834 adults with AD or psoriasis, he and colleagues found that among those with AD, the wrists, back of the hands, and interdigital areas were often sites of severe eczema, while palmar involvement was more uncommon.
The same findings apply for the feet in filaggrin mutation carriers with AD; the dorsal aspect of the feet was more commonly affected, compared with plantar aspects of the feet.
Medical literature regarding foot eczema is scarce, but a retrospective cohort study from Germany found that foot eczema and hand eczema often co-occur. Among 723 hand eczema patients, 201 (28%) had concomitant foot eczema. The same morphological features were found on the hands and feet in 71% of patients. Foot eczema was significantly associated with male sex, atopic hand eczema, hyperhidrosis, wearing of safety shoes/boots at work, and tobacco smoking.
In addition, a systematic review and meta-analysis of studies of hand eczema and AD found that there was a 4.29-fold increased risk of hand eczema in individuals with AD, and the risk (lifetime prevalence) of occupational hand eczema was increased by more than twofold. “However, this study could not differentiate between irritant contact dermatitis on the hands and atopic dermatitis,” Dr. Thyssen said. “The studies were not accurate enough to allow for any conclusions.”
A multicenter study of adults with hand eczema in Italy found that the proportion of patients with AD was the highest among those with severe and refractory chronic hand eczema. In addition, certain professions, including those of hairdressers, health professionals, and those in trade work, such as plumbing, were more often associated with chronic hand eczema. “This teaches us that we should be very careful about steering these patients from at-risk occupations,” Dr. Thyssen said. “Also, we should remember to treat them aggressively in the beginning to reduce the risk of severe and refractory chronic hand eczema.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Asian, Arena, Almirall, AbbVie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme.
An estimated (AD), according to Jacob P. Thyssen, MD, PhD.
“If we look at individuals with AD, the lifetime prevalence of hand eczema reaches 50%, so we see a strong association between hand eczema and AD,” Dr. Thyssen, professor of dermatology at the University of Copenhagen, said at the Revolutionizing Atopic Dermatitis symposium.
Risk factors for hand eczema – defined as eczema on the hand and/or wrists – include AD, which increases the risk by two- to threefold, as well as genetic predisposition beyond AD, exposure to irritants and allergens, female gender, young age, low socioeconomic group, high risk occupations (including construction workers and hairdressers), and tobacco smoking.
“As clinicians, we sometimes need to rule out a few differentials, including psoriasis and T-cell lymphoma. As an example, 10% of T-cell lymphoma patients, a very rare condition, have first onset of the disease on their hands,” Dr. Thyssen said. “Once we see persistent hand eczema, we need to obtain a history of irritant exposure and allergen exposure, both at home and at work, perform a patch test, sometimes a skin prick test, and ask about personal and family history of AD and psoriasis.”
He noted that while formal classification of hand eczema has been a struggle for decades, he favors the “straightforward” clinical approach from the European Environmental and Contact Dermatitis Research Group. Atopic hand eczema, he said, “is very much characterized by dorsal involvement of the hands and fingers and sparse involvement of the palmar aspects of the hands.”
The cheeks and hands are predilection sites for AD in filaggrin mutation carriers (as they are sites of low filaggrin levels), and sometimes harsh environmental exposures, such as cold and dry air. In a study of 3,335 patients in Denmark, Dr. Thyssen and colleagues found that filaggrin mutations and AD were associated with early-onset and persistent hand eczema. In another study of 3,834 adults with AD or psoriasis, he and colleagues found that among those with AD, the wrists, back of the hands, and interdigital areas were often sites of severe eczema, while palmar involvement was more uncommon.
The same findings apply for the feet in filaggrin mutation carriers with AD; the dorsal aspect of the feet was more commonly affected, compared with plantar aspects of the feet.
Medical literature regarding foot eczema is scarce, but a retrospective cohort study from Germany found that foot eczema and hand eczema often co-occur. Among 723 hand eczema patients, 201 (28%) had concomitant foot eczema. The same morphological features were found on the hands and feet in 71% of patients. Foot eczema was significantly associated with male sex, atopic hand eczema, hyperhidrosis, wearing of safety shoes/boots at work, and tobacco smoking.
In addition, a systematic review and meta-analysis of studies of hand eczema and AD found that there was a 4.29-fold increased risk of hand eczema in individuals with AD, and the risk (lifetime prevalence) of occupational hand eczema was increased by more than twofold. “However, this study could not differentiate between irritant contact dermatitis on the hands and atopic dermatitis,” Dr. Thyssen said. “The studies were not accurate enough to allow for any conclusions.”
A multicenter study of adults with hand eczema in Italy found that the proportion of patients with AD was the highest among those with severe and refractory chronic hand eczema. In addition, certain professions, including those of hairdressers, health professionals, and those in trade work, such as plumbing, were more often associated with chronic hand eczema. “This teaches us that we should be very careful about steering these patients from at-risk occupations,” Dr. Thyssen said. “Also, we should remember to treat them aggressively in the beginning to reduce the risk of severe and refractory chronic hand eczema.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Asian, Arena, Almirall, AbbVie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme.
FROM REVOLUTIONIZING AD 2021