User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
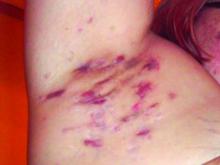
Spironolactone safe, effective option for women with hidradenitis suppurativa
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
AT CALDERM 2023
FDA issues letter regarding lebrikizumab review for atopic dermatitis
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
Does the number of primary melanomas affect survival?
TOPLINE:
.
METHODOLOGY:
- The difference in outcomes between people with multiple primary melanomas (MPMs) and a single primary melanoma (SPM) has not been established.
- To compare 10-year melanoma-specific mortality and overall mortality between people with MPMs and SPM, researchers drew from the Melanoma Patterns of Care study, a population-based observational analysis of residents in the state of New South Wales, Australia, who had a melanoma reported to the state cancer registry over 12 months in 2006-2007, and were followed up until 2018, for a median of almost 12 years.
- The researchers performed logistic regression analyses to assess 10-year melanoma-specific mortality differences between the two groups.
TAKEAWAY:
- Of 3,404 people included in the analysis, 2,830 had an SPM and 574 developed MPMs during follow-up.
- On multivariable regression adjusted for pathologic characteristics of the thickest lesion in the MPM group, no significant differences were seen in 10-year melanoma-specific mortality between the two groups (odds ratio, 0.85; 95% confidence interval, 0.58-1.24; P = .40).
- Sensitivity analyses adjusted for parameters of the first primary melanoma among patients with MPMs revealed similar findings (OR, 1.34; 95% CI, 0.92-1.96; P = .12).
- On multivariable analysis using data from the thickest lesion, factors independently associated with melanoma-specific mortality were male sex, disadvantaged socioeconomic status (based on location of residence), and Breslow thickness.
- Factors independently associated with 10-year overall mortality were like those seen in other studies and included sex, Breslow thickness, ulceration status, and socioeconomic disadvantage.
IN PRACTICE:
“The results of our study suggest that the number of primary melanomas is not an independent risk factor for mortality,” the researchers concluded. “In addition, the detection of melanoma at an early stage (with a thin Breslow thickness) rather than an intrinsic biologic factor remains the biggest influence on melanoma mortality after diagnosis of one or more melanomas.”
SOURCE:
Corresponding author Serigne N. Lo, PhD, of the Melanoma Institute Australia, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
No adjustments for treatment modality were made in the study, and at baseline survey, effective systemic treatments for melanoma were not available.
DISCLOSURES:
This study was supported by the Australian National Health and Medical Research Council, Cancer Institute New South Wales, and the New South Wale State Government via a grant to the New South Wales Melanoma Network. Additional support was provided by Melanoma Institute Australia and the New South Wales Melanoma Network.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- The difference in outcomes between people with multiple primary melanomas (MPMs) and a single primary melanoma (SPM) has not been established.
- To compare 10-year melanoma-specific mortality and overall mortality between people with MPMs and SPM, researchers drew from the Melanoma Patterns of Care study, a population-based observational analysis of residents in the state of New South Wales, Australia, who had a melanoma reported to the state cancer registry over 12 months in 2006-2007, and were followed up until 2018, for a median of almost 12 years.
- The researchers performed logistic regression analyses to assess 10-year melanoma-specific mortality differences between the two groups.
TAKEAWAY:
- Of 3,404 people included in the analysis, 2,830 had an SPM and 574 developed MPMs during follow-up.
- On multivariable regression adjusted for pathologic characteristics of the thickest lesion in the MPM group, no significant differences were seen in 10-year melanoma-specific mortality between the two groups (odds ratio, 0.85; 95% confidence interval, 0.58-1.24; P = .40).
- Sensitivity analyses adjusted for parameters of the first primary melanoma among patients with MPMs revealed similar findings (OR, 1.34; 95% CI, 0.92-1.96; P = .12).
- On multivariable analysis using data from the thickest lesion, factors independently associated with melanoma-specific mortality were male sex, disadvantaged socioeconomic status (based on location of residence), and Breslow thickness.
- Factors independently associated with 10-year overall mortality were like those seen in other studies and included sex, Breslow thickness, ulceration status, and socioeconomic disadvantage.
IN PRACTICE:
“The results of our study suggest that the number of primary melanomas is not an independent risk factor for mortality,” the researchers concluded. “In addition, the detection of melanoma at an early stage (with a thin Breslow thickness) rather than an intrinsic biologic factor remains the biggest influence on melanoma mortality after diagnosis of one or more melanomas.”
SOURCE:
Corresponding author Serigne N. Lo, PhD, of the Melanoma Institute Australia, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
No adjustments for treatment modality were made in the study, and at baseline survey, effective systemic treatments for melanoma were not available.
DISCLOSURES:
This study was supported by the Australian National Health and Medical Research Council, Cancer Institute New South Wales, and the New South Wale State Government via a grant to the New South Wales Melanoma Network. Additional support was provided by Melanoma Institute Australia and the New South Wales Melanoma Network.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- The difference in outcomes between people with multiple primary melanomas (MPMs) and a single primary melanoma (SPM) has not been established.
- To compare 10-year melanoma-specific mortality and overall mortality between people with MPMs and SPM, researchers drew from the Melanoma Patterns of Care study, a population-based observational analysis of residents in the state of New South Wales, Australia, who had a melanoma reported to the state cancer registry over 12 months in 2006-2007, and were followed up until 2018, for a median of almost 12 years.
- The researchers performed logistic regression analyses to assess 10-year melanoma-specific mortality differences between the two groups.
TAKEAWAY:
- Of 3,404 people included in the analysis, 2,830 had an SPM and 574 developed MPMs during follow-up.
- On multivariable regression adjusted for pathologic characteristics of the thickest lesion in the MPM group, no significant differences were seen in 10-year melanoma-specific mortality between the two groups (odds ratio, 0.85; 95% confidence interval, 0.58-1.24; P = .40).
- Sensitivity analyses adjusted for parameters of the first primary melanoma among patients with MPMs revealed similar findings (OR, 1.34; 95% CI, 0.92-1.96; P = .12).
- On multivariable analysis using data from the thickest lesion, factors independently associated with melanoma-specific mortality were male sex, disadvantaged socioeconomic status (based on location of residence), and Breslow thickness.
- Factors independently associated with 10-year overall mortality were like those seen in other studies and included sex, Breslow thickness, ulceration status, and socioeconomic disadvantage.
IN PRACTICE:
“The results of our study suggest that the number of primary melanomas is not an independent risk factor for mortality,” the researchers concluded. “In addition, the detection of melanoma at an early stage (with a thin Breslow thickness) rather than an intrinsic biologic factor remains the biggest influence on melanoma mortality after diagnosis of one or more melanomas.”
SOURCE:
Corresponding author Serigne N. Lo, PhD, of the Melanoma Institute Australia, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
No adjustments for treatment modality were made in the study, and at baseline survey, effective systemic treatments for melanoma were not available.
DISCLOSURES:
This study was supported by the Australian National Health and Medical Research Council, Cancer Institute New South Wales, and the New South Wale State Government via a grant to the New South Wales Melanoma Network. Additional support was provided by Melanoma Institute Australia and the New South Wales Melanoma Network.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
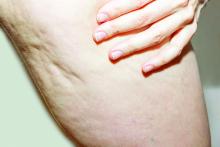
A glimpse at devices designed to tackle cellulite
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
AT MOAS 2023
Study spotlights paucity of black dermatologists in academia
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
‘Old school’ laser resurfacing remains an effective option for rejuvenation
SAN DIEGO – , according to Arisa E. Ortiz, MD.
“Fractional resurfacing is great because there is less downtime, but the results are not as dramatic as with fully ablative resurfacing,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. In her practice, she said, “we do a combination,” which can include “fully ablative around the mouth and eyes and fractional everywhere else.”
Key drawbacks to fully ablative laser resurfacing include significant downtime and extensive wound care, “so it’s not for everybody,” she said. Prolonged erythema following treatment is expected, “so patients need to plan for this. It can last 3-4 months, and it will continue to fade and can be covered up with makeup, but it does last a while,” she noted. “One of the things that made ablative resurfacing fall out of favor was the delayed and permanent hypopigmentation where there’s a stark line of demarcation because you can’t treat the neck [with this modality], so patients have this pearly white looking face that appears 6 months after the treatment,” she added.
Preoperatively, Dr. Ortiz asks patients what other cosmetic procedures they have had in the past. For example, if they have had a facelift, they might have neck skin on their jawline, which will react differently to fully ablative resurfacing than facial skin. “I don’t perform fully ablative resurfacing on the neck or body, or in patients with darker skin types,” she said.
To optimize results, she recommends pretreatment of the area with a neuromodulator a week or 2 before the procedure, “so that they’re not actively contracting and recreating creases,” she said. Studies, she noted, have shown that this approach results in better outcomes. She also asks patients to apply a tripeptide serum daily a week or 2 prior to their procedure to stimulate wound healing and collagen remodeling.
For antibiotic and antiviral prophylaxis, Dr. Ortiz typically prescribes doxycycline 100 mg b.i.d. for 7 days and valacyclovir 500 mg b.i.d. for 7 days and asks patients to start the course the night before the procedure. “If they break through the antiviral, I increase to zoster dosing,” she said. “I make sure they have my cell phone number and call me right away if that happens. I don’t routinely prescribe an antifungal, but you can if you want to.”
For anesthesia, Dr. Ortiz applies lidocaine 23%/tetracaine 7% an hour before the procedure and performs nerve blocks at the mentalis, infraorbital, supraorbital, and nasalis muscles. “I also do local infiltration with a three-pronged Mesoram adapter,” she said. “That has changed the comfort level for these patients. I don’t offer any sedation in my practice but that is an option if you have it available. If you’re going to be resurfacing within the orbital rim you need to know how to place corneal shields. Only use injectable lidocaine in this area because if topical lidocaine gets into the eye, it can cause a chemical corneal abrasion. Nothing happens to their vision permanently, but it’s extremely painful for 24-48 hours.”
Dr. Ortiz described postoperative wound care as “the hardest part” of fully ablative laser resurfacing treatments. The treated area will look “bloody and crusty” for 1-2 weeks. She instructs patients to do vinegar soaks four times per day for 2-3 weeks, “depending on how quickly they heal,” she said. She also counsels patients to apply petrolatum ointment to the area and provides them with a bottle of hypochlorous acid spray, an antiseptic – which also helps with the itching they may experience. “They need to avoid the sun, so I recommend full face visors,” she added.
In her clinical experience, postoperative pain medications are not required. “If the patient calls you on day 3 with increased pain, that’s usually a sign of infection; don’t ignore that,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. In a case of suspected infection, she asks the patient to come in right away, and obtains a bacterial culture. “If they break through the doxycycline, it’s usually a gram-negative infection, so I’ll treat them prophylactically for that,” she said.
“Significant itching may be a sign of Candida infection,” she noted. “Because the epidermis has been disrupted, if they have systemic symptoms then you want to consider IV antibiotics because the infection can spread rapidly.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – , according to Arisa E. Ortiz, MD.
“Fractional resurfacing is great because there is less downtime, but the results are not as dramatic as with fully ablative resurfacing,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. In her practice, she said, “we do a combination,” which can include “fully ablative around the mouth and eyes and fractional everywhere else.”
Key drawbacks to fully ablative laser resurfacing include significant downtime and extensive wound care, “so it’s not for everybody,” she said. Prolonged erythema following treatment is expected, “so patients need to plan for this. It can last 3-4 months, and it will continue to fade and can be covered up with makeup, but it does last a while,” she noted. “One of the things that made ablative resurfacing fall out of favor was the delayed and permanent hypopigmentation where there’s a stark line of demarcation because you can’t treat the neck [with this modality], so patients have this pearly white looking face that appears 6 months after the treatment,” she added.
Preoperatively, Dr. Ortiz asks patients what other cosmetic procedures they have had in the past. For example, if they have had a facelift, they might have neck skin on their jawline, which will react differently to fully ablative resurfacing than facial skin. “I don’t perform fully ablative resurfacing on the neck or body, or in patients with darker skin types,” she said.
To optimize results, she recommends pretreatment of the area with a neuromodulator a week or 2 before the procedure, “so that they’re not actively contracting and recreating creases,” she said. Studies, she noted, have shown that this approach results in better outcomes. She also asks patients to apply a tripeptide serum daily a week or 2 prior to their procedure to stimulate wound healing and collagen remodeling.
For antibiotic and antiviral prophylaxis, Dr. Ortiz typically prescribes doxycycline 100 mg b.i.d. for 7 days and valacyclovir 500 mg b.i.d. for 7 days and asks patients to start the course the night before the procedure. “If they break through the antiviral, I increase to zoster dosing,” she said. “I make sure they have my cell phone number and call me right away if that happens. I don’t routinely prescribe an antifungal, but you can if you want to.”
For anesthesia, Dr. Ortiz applies lidocaine 23%/tetracaine 7% an hour before the procedure and performs nerve blocks at the mentalis, infraorbital, supraorbital, and nasalis muscles. “I also do local infiltration with a three-pronged Mesoram adapter,” she said. “That has changed the comfort level for these patients. I don’t offer any sedation in my practice but that is an option if you have it available. If you’re going to be resurfacing within the orbital rim you need to know how to place corneal shields. Only use injectable lidocaine in this area because if topical lidocaine gets into the eye, it can cause a chemical corneal abrasion. Nothing happens to their vision permanently, but it’s extremely painful for 24-48 hours.”
Dr. Ortiz described postoperative wound care as “the hardest part” of fully ablative laser resurfacing treatments. The treated area will look “bloody and crusty” for 1-2 weeks. She instructs patients to do vinegar soaks four times per day for 2-3 weeks, “depending on how quickly they heal,” she said. She also counsels patients to apply petrolatum ointment to the area and provides them with a bottle of hypochlorous acid spray, an antiseptic – which also helps with the itching they may experience. “They need to avoid the sun, so I recommend full face visors,” she added.
In her clinical experience, postoperative pain medications are not required. “If the patient calls you on day 3 with increased pain, that’s usually a sign of infection; don’t ignore that,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. In a case of suspected infection, she asks the patient to come in right away, and obtains a bacterial culture. “If they break through the doxycycline, it’s usually a gram-negative infection, so I’ll treat them prophylactically for that,” she said.
“Significant itching may be a sign of Candida infection,” she noted. “Because the epidermis has been disrupted, if they have systemic symptoms then you want to consider IV antibiotics because the infection can spread rapidly.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – , according to Arisa E. Ortiz, MD.
“Fractional resurfacing is great because there is less downtime, but the results are not as dramatic as with fully ablative resurfacing,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. In her practice, she said, “we do a combination,” which can include “fully ablative around the mouth and eyes and fractional everywhere else.”
Key drawbacks to fully ablative laser resurfacing include significant downtime and extensive wound care, “so it’s not for everybody,” she said. Prolonged erythema following treatment is expected, “so patients need to plan for this. It can last 3-4 months, and it will continue to fade and can be covered up with makeup, but it does last a while,” she noted. “One of the things that made ablative resurfacing fall out of favor was the delayed and permanent hypopigmentation where there’s a stark line of demarcation because you can’t treat the neck [with this modality], so patients have this pearly white looking face that appears 6 months after the treatment,” she added.
Preoperatively, Dr. Ortiz asks patients what other cosmetic procedures they have had in the past. For example, if they have had a facelift, they might have neck skin on their jawline, which will react differently to fully ablative resurfacing than facial skin. “I don’t perform fully ablative resurfacing on the neck or body, or in patients with darker skin types,” she said.
To optimize results, she recommends pretreatment of the area with a neuromodulator a week or 2 before the procedure, “so that they’re not actively contracting and recreating creases,” she said. Studies, she noted, have shown that this approach results in better outcomes. She also asks patients to apply a tripeptide serum daily a week or 2 prior to their procedure to stimulate wound healing and collagen remodeling.
For antibiotic and antiviral prophylaxis, Dr. Ortiz typically prescribes doxycycline 100 mg b.i.d. for 7 days and valacyclovir 500 mg b.i.d. for 7 days and asks patients to start the course the night before the procedure. “If they break through the antiviral, I increase to zoster dosing,” she said. “I make sure they have my cell phone number and call me right away if that happens. I don’t routinely prescribe an antifungal, but you can if you want to.”
For anesthesia, Dr. Ortiz applies lidocaine 23%/tetracaine 7% an hour before the procedure and performs nerve blocks at the mentalis, infraorbital, supraorbital, and nasalis muscles. “I also do local infiltration with a three-pronged Mesoram adapter,” she said. “That has changed the comfort level for these patients. I don’t offer any sedation in my practice but that is an option if you have it available. If you’re going to be resurfacing within the orbital rim you need to know how to place corneal shields. Only use injectable lidocaine in this area because if topical lidocaine gets into the eye, it can cause a chemical corneal abrasion. Nothing happens to their vision permanently, but it’s extremely painful for 24-48 hours.”
Dr. Ortiz described postoperative wound care as “the hardest part” of fully ablative laser resurfacing treatments. The treated area will look “bloody and crusty” for 1-2 weeks. She instructs patients to do vinegar soaks four times per day for 2-3 weeks, “depending on how quickly they heal,” she said. She also counsels patients to apply petrolatum ointment to the area and provides them with a bottle of hypochlorous acid spray, an antiseptic – which also helps with the itching they may experience. “They need to avoid the sun, so I recommend full face visors,” she added.
In her clinical experience, postoperative pain medications are not required. “If the patient calls you on day 3 with increased pain, that’s usually a sign of infection; don’t ignore that,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. In a case of suspected infection, she asks the patient to come in right away, and obtains a bacterial culture. “If they break through the doxycycline, it’s usually a gram-negative infection, so I’ll treat them prophylactically for that,” she said.
“Significant itching may be a sign of Candida infection,” she noted. “Because the epidermis has been disrupted, if they have systemic symptoms then you want to consider IV antibiotics because the infection can spread rapidly.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
AT MOAS 2023
Thread lifts an option for noninvasive facial tightening
SAN DIEGO – .
In the 1990s, clinicians used nonabsorbable sutures for thread lifts, including polypropylene-barbed threads, which caused adverse events ranging from extrusion and migration to thread expulsion, dimpling, granuloma formation, and prolonged pain, Dr. DiGiorgio, a laser and cosmetic dermatologist who practices in Boston, said at the annual Masters of Aesthetics Symposium. As a result, the Food and Drug Administration withdrew its approval of contour thread aesthetic procedures in 2009. Since then, the development of absorbable threads made from a hybrid of poly-l-lactic acid (PLLA) and polyglycolide/l-lactide (PLGA), and from polydioxanone (PDO) has led to renewed interest in thread lift procedures.
While a surgical facelift remains the gold standard, “we have some options to offer patients for noninvasive tightening,” Dr. DiGiorgio said. “We have devices that provide minimal downtime and are less costly, but results are inconsistent. Thread lifts, or suspension sutures, also have minimal downtime and are less costly, but the [results are] subtle and not long lasting.”
PLGA/PLLA threads consist of an 18% PLGA and 82% PLLA monofilament with bidirectional cones that shift the tissue. They are available in 8, 12, or 16 cones spaced 5-8 mm apart on either side of a 2-cm central cone-free area. “There is a 12-cm, 23-gauge needle on either side of the thread, to allow for insertion,” she explained. “These cones stimulate types I and II collagen, which results in collagenesis. The skin encapsulates the cones, resulting in lasting volume and contour.”
PDO threads, meanwhile, are biodegradable by hydrolysis over 4-8 months. They are inserted with a cannula or a needle and vary based on length, diameter, twined vs. braided, coned vs. barbed, and twisted vs. smooth. “The barbed PDO threads are what I use the most,” Dr. DiGiorgio said. “They provide slight tissue repositioning by anchoring and gripping.”
In 2019, researchers in Korea published results of a study that evaluated the collagen-producing effects of powdered PDO injection, compared with PLLA injection, in a murine model. They found that while both PDO and PLLA induced granulomatous reactions and collagen formation, PDO resulted in slightly more collagen formation than PLLA.
Dr. DiGiorgio, who transitioned to using PDO threads after first using the PLLA/PLGA threads, said that both are effective. “I find PDO threads to be easier. They’re less costly for me, they’re less costly for the patient, and the results are about equivalent.”
Absorbable threads are indicated for the cheek, jawline, neck, lips, forehead, and brow. She finds them most useful “for the lower face, below the nasolabial fold down to the jawline, for improvement of the jowls,” she said. “I don’t think they really work on the neck.”
As with any cosmetic procedure, patient selection is key. According to Dr. DiGiorgio, the patient should have specific and segmental areas of facial laxity amenable to lifting and recontouring along a straight-line vector, adequate dermal thickness, and appropriate expectations for the level of correction. “I like to re-volumize with filler before performing thread lifts to make sure that volume is restored, because you can’t really provide lift to someone with significant volume loss,” she said.
Procedural tips
Prior to the procedure, Dr. DiGiorgio marks the area to be treated while the patient is seated upright and holding a mirror. Then, she pulls back the amount of skin laxity the thread is going to correct. The plane of insertion for barbed threads is at the superficial musculoaponeurotic system (SMAS), and she typically uses 3-4 threads on each side of the face.
“How do you know you’re in the right plane?” If the patient is experiencing significant pain, “you’re too deep, and it’s not going to work,” she said. “You can see if the thread is too superficial as you do more of these.”
After the procedure she asks the patient to sit up prior to trimming the threads. “I take a look in the mirror with them and have them smile and make funny faces to see if there is any dimpling or crimpling, which is probably the most common side effect,” she said. “If I see that, I will pull the thread immediately, so we don’t have a problem. It’s a little uncomfortable to pull the thread but not more uncomfortable than the procedure itself, but I think it’s worth doing to avoid having a dimple or a crimple that can last up to a year.”
In her clinical experience, thread lifts last about 8-10 months. “I find that my patients will come in about once a year for this procedure, and the treated area feels tight afterward,” Dr. DiGiorgio said. “I think that sensation of feeling tight also provides satisfaction to the patient. Results are very subtle. It’s tissue repositioning; it is not a facelift. There’s not really any downtime, but further studies are required to see if threads are safe and effective in the long-term.”
In an interview after the meeting, she noted that the learning curve for thread lifts is variable, as with any new procedure a physician chooses to add to his or her practice. “It’s important to see these patients in follow-up 2 weeks after the procedure consistently, especially when someone first starts performing the procedure,” she recommended. “These patients are usually coming in to see me for other treatments, so I see them at regular 3-month intervals regardless. You begin to get a feel for what angles work and why and how to optimize the results. As with any procedure, the more experience you have performing the procedure will result in improved outcomes and improved management.”
Dr. DiGiorgio disclosed that she has been an advisory board member for Quthero and she holds stock options in the company. She is a consultant for Revelle and has received equipment from Acclaro.
SAN DIEGO – .
In the 1990s, clinicians used nonabsorbable sutures for thread lifts, including polypropylene-barbed threads, which caused adverse events ranging from extrusion and migration to thread expulsion, dimpling, granuloma formation, and prolonged pain, Dr. DiGiorgio, a laser and cosmetic dermatologist who practices in Boston, said at the annual Masters of Aesthetics Symposium. As a result, the Food and Drug Administration withdrew its approval of contour thread aesthetic procedures in 2009. Since then, the development of absorbable threads made from a hybrid of poly-l-lactic acid (PLLA) and polyglycolide/l-lactide (PLGA), and from polydioxanone (PDO) has led to renewed interest in thread lift procedures.
While a surgical facelift remains the gold standard, “we have some options to offer patients for noninvasive tightening,” Dr. DiGiorgio said. “We have devices that provide minimal downtime and are less costly, but results are inconsistent. Thread lifts, or suspension sutures, also have minimal downtime and are less costly, but the [results are] subtle and not long lasting.”
PLGA/PLLA threads consist of an 18% PLGA and 82% PLLA monofilament with bidirectional cones that shift the tissue. They are available in 8, 12, or 16 cones spaced 5-8 mm apart on either side of a 2-cm central cone-free area. “There is a 12-cm, 23-gauge needle on either side of the thread, to allow for insertion,” she explained. “These cones stimulate types I and II collagen, which results in collagenesis. The skin encapsulates the cones, resulting in lasting volume and contour.”
PDO threads, meanwhile, are biodegradable by hydrolysis over 4-8 months. They are inserted with a cannula or a needle and vary based on length, diameter, twined vs. braided, coned vs. barbed, and twisted vs. smooth. “The barbed PDO threads are what I use the most,” Dr. DiGiorgio said. “They provide slight tissue repositioning by anchoring and gripping.”
In 2019, researchers in Korea published results of a study that evaluated the collagen-producing effects of powdered PDO injection, compared with PLLA injection, in a murine model. They found that while both PDO and PLLA induced granulomatous reactions and collagen formation, PDO resulted in slightly more collagen formation than PLLA.
Dr. DiGiorgio, who transitioned to using PDO threads after first using the PLLA/PLGA threads, said that both are effective. “I find PDO threads to be easier. They’re less costly for me, they’re less costly for the patient, and the results are about equivalent.”
Absorbable threads are indicated for the cheek, jawline, neck, lips, forehead, and brow. She finds them most useful “for the lower face, below the nasolabial fold down to the jawline, for improvement of the jowls,” she said. “I don’t think they really work on the neck.”
As with any cosmetic procedure, patient selection is key. According to Dr. DiGiorgio, the patient should have specific and segmental areas of facial laxity amenable to lifting and recontouring along a straight-line vector, adequate dermal thickness, and appropriate expectations for the level of correction. “I like to re-volumize with filler before performing thread lifts to make sure that volume is restored, because you can’t really provide lift to someone with significant volume loss,” she said.
Procedural tips
Prior to the procedure, Dr. DiGiorgio marks the area to be treated while the patient is seated upright and holding a mirror. Then, she pulls back the amount of skin laxity the thread is going to correct. The plane of insertion for barbed threads is at the superficial musculoaponeurotic system (SMAS), and she typically uses 3-4 threads on each side of the face.
“How do you know you’re in the right plane?” If the patient is experiencing significant pain, “you’re too deep, and it’s not going to work,” she said. “You can see if the thread is too superficial as you do more of these.”
After the procedure she asks the patient to sit up prior to trimming the threads. “I take a look in the mirror with them and have them smile and make funny faces to see if there is any dimpling or crimpling, which is probably the most common side effect,” she said. “If I see that, I will pull the thread immediately, so we don’t have a problem. It’s a little uncomfortable to pull the thread but not more uncomfortable than the procedure itself, but I think it’s worth doing to avoid having a dimple or a crimple that can last up to a year.”
In her clinical experience, thread lifts last about 8-10 months. “I find that my patients will come in about once a year for this procedure, and the treated area feels tight afterward,” Dr. DiGiorgio said. “I think that sensation of feeling tight also provides satisfaction to the patient. Results are very subtle. It’s tissue repositioning; it is not a facelift. There’s not really any downtime, but further studies are required to see if threads are safe and effective in the long-term.”
In an interview after the meeting, she noted that the learning curve for thread lifts is variable, as with any new procedure a physician chooses to add to his or her practice. “It’s important to see these patients in follow-up 2 weeks after the procedure consistently, especially when someone first starts performing the procedure,” she recommended. “These patients are usually coming in to see me for other treatments, so I see them at regular 3-month intervals regardless. You begin to get a feel for what angles work and why and how to optimize the results. As with any procedure, the more experience you have performing the procedure will result in improved outcomes and improved management.”
Dr. DiGiorgio disclosed that she has been an advisory board member for Quthero and she holds stock options in the company. She is a consultant for Revelle and has received equipment from Acclaro.
SAN DIEGO – .
In the 1990s, clinicians used nonabsorbable sutures for thread lifts, including polypropylene-barbed threads, which caused adverse events ranging from extrusion and migration to thread expulsion, dimpling, granuloma formation, and prolonged pain, Dr. DiGiorgio, a laser and cosmetic dermatologist who practices in Boston, said at the annual Masters of Aesthetics Symposium. As a result, the Food and Drug Administration withdrew its approval of contour thread aesthetic procedures in 2009. Since then, the development of absorbable threads made from a hybrid of poly-l-lactic acid (PLLA) and polyglycolide/l-lactide (PLGA), and from polydioxanone (PDO) has led to renewed interest in thread lift procedures.
While a surgical facelift remains the gold standard, “we have some options to offer patients for noninvasive tightening,” Dr. DiGiorgio said. “We have devices that provide minimal downtime and are less costly, but results are inconsistent. Thread lifts, or suspension sutures, also have minimal downtime and are less costly, but the [results are] subtle and not long lasting.”
PLGA/PLLA threads consist of an 18% PLGA and 82% PLLA monofilament with bidirectional cones that shift the tissue. They are available in 8, 12, or 16 cones spaced 5-8 mm apart on either side of a 2-cm central cone-free area. “There is a 12-cm, 23-gauge needle on either side of the thread, to allow for insertion,” she explained. “These cones stimulate types I and II collagen, which results in collagenesis. The skin encapsulates the cones, resulting in lasting volume and contour.”
PDO threads, meanwhile, are biodegradable by hydrolysis over 4-8 months. They are inserted with a cannula or a needle and vary based on length, diameter, twined vs. braided, coned vs. barbed, and twisted vs. smooth. “The barbed PDO threads are what I use the most,” Dr. DiGiorgio said. “They provide slight tissue repositioning by anchoring and gripping.”
In 2019, researchers in Korea published results of a study that evaluated the collagen-producing effects of powdered PDO injection, compared with PLLA injection, in a murine model. They found that while both PDO and PLLA induced granulomatous reactions and collagen formation, PDO resulted in slightly more collagen formation than PLLA.
Dr. DiGiorgio, who transitioned to using PDO threads after first using the PLLA/PLGA threads, said that both are effective. “I find PDO threads to be easier. They’re less costly for me, they’re less costly for the patient, and the results are about equivalent.”
Absorbable threads are indicated for the cheek, jawline, neck, lips, forehead, and brow. She finds them most useful “for the lower face, below the nasolabial fold down to the jawline, for improvement of the jowls,” she said. “I don’t think they really work on the neck.”
As with any cosmetic procedure, patient selection is key. According to Dr. DiGiorgio, the patient should have specific and segmental areas of facial laxity amenable to lifting and recontouring along a straight-line vector, adequate dermal thickness, and appropriate expectations for the level of correction. “I like to re-volumize with filler before performing thread lifts to make sure that volume is restored, because you can’t really provide lift to someone with significant volume loss,” she said.
Procedural tips
Prior to the procedure, Dr. DiGiorgio marks the area to be treated while the patient is seated upright and holding a mirror. Then, she pulls back the amount of skin laxity the thread is going to correct. The plane of insertion for barbed threads is at the superficial musculoaponeurotic system (SMAS), and she typically uses 3-4 threads on each side of the face.
“How do you know you’re in the right plane?” If the patient is experiencing significant pain, “you’re too deep, and it’s not going to work,” she said. “You can see if the thread is too superficial as you do more of these.”
After the procedure she asks the patient to sit up prior to trimming the threads. “I take a look in the mirror with them and have them smile and make funny faces to see if there is any dimpling or crimpling, which is probably the most common side effect,” she said. “If I see that, I will pull the thread immediately, so we don’t have a problem. It’s a little uncomfortable to pull the thread but not more uncomfortable than the procedure itself, but I think it’s worth doing to avoid having a dimple or a crimple that can last up to a year.”
In her clinical experience, thread lifts last about 8-10 months. “I find that my patients will come in about once a year for this procedure, and the treated area feels tight afterward,” Dr. DiGiorgio said. “I think that sensation of feeling tight also provides satisfaction to the patient. Results are very subtle. It’s tissue repositioning; it is not a facelift. There’s not really any downtime, but further studies are required to see if threads are safe and effective in the long-term.”
In an interview after the meeting, she noted that the learning curve for thread lifts is variable, as with any new procedure a physician chooses to add to his or her practice. “It’s important to see these patients in follow-up 2 weeks after the procedure consistently, especially when someone first starts performing the procedure,” she recommended. “These patients are usually coming in to see me for other treatments, so I see them at regular 3-month intervals regardless. You begin to get a feel for what angles work and why and how to optimize the results. As with any procedure, the more experience you have performing the procedure will result in improved outcomes and improved management.”
Dr. DiGiorgio disclosed that she has been an advisory board member for Quthero and she holds stock options in the company. She is a consultant for Revelle and has received equipment from Acclaro.
FROM MOAS 2023
Severe psoriasis linked to a higher risk for heart disease, study confirms
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
European Commission grants approval of ritlecitinib for severe alopecia areata
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
Combining lasers: A recipe for maximizing results and patient satisfaction
SAN DIEGO –
“Using a fractional laser as a solo treatment is missing an opportunity to achieve more dramatic improvement for your patients,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said at the annual Masters of Aesthetics Symposium. Among the laser treatments he performs, “combination fractional treatments, typically using the 1927-nm laser” are associated with the highest patient satisfaction, he said.
The order of device use matters, he noted. First, he recommended, use a pulsed dye laser, KTP, or intense pulsed light (IPL) for erythema and telangiectasias, and/or a Q-switched or picosecond laser for pigment. Second, use an ablative or nonablative fractional laser for resurfacing. “A lot of seborrheic keratoses don’t respond to selective photothermolysis well, so I’ll use liquid nitrogen at the time of treatment and before or after treat with a picosecond laser,” added Dr. Avram. “This combined treatment approach is less painful than ablative fractional treatment. You’re going to have downtime anyway, so why not maximize the results at that one treatment session?”
The fractional 1927 laser delivers hundreds of thousands of microscopic pulses and fosters high water absorption, so it targets superficial skin conditions such as actinic keratoses, lentigines, and ephelides at depths of 200-250 microns. It thermally coagulates 30%-40% of skin, which heals without affecting surrounding skin and leaves no perceptible scar, he said.
Clinicians can also combine devices to treat scars. “For red scars, it’s often best to treat both erythema and scar texture with two lasers at the same session,” Dr. Avram said. Again, the order matters. First, he recommended using the pulse dye laser, IPL, or KTP at low fluence and short pulse duration. Second, treat with an ablative or nonablative fractional laser at a low density. “In my experience the ablative fractional lasers are far more efficacious,” he said. “Then we typically add a little Kenalog and 5-FU via laser-assisted drug delivery.”
Dr. Avram disclosed that he has received consulting fees from Allergan. He also reported holding shareholder interest and intellectual property rights with Cytrellis Biosystems.
SAN DIEGO –
“Using a fractional laser as a solo treatment is missing an opportunity to achieve more dramatic improvement for your patients,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said at the annual Masters of Aesthetics Symposium. Among the laser treatments he performs, “combination fractional treatments, typically using the 1927-nm laser” are associated with the highest patient satisfaction, he said.
The order of device use matters, he noted. First, he recommended, use a pulsed dye laser, KTP, or intense pulsed light (IPL) for erythema and telangiectasias, and/or a Q-switched or picosecond laser for pigment. Second, use an ablative or nonablative fractional laser for resurfacing. “A lot of seborrheic keratoses don’t respond to selective photothermolysis well, so I’ll use liquid nitrogen at the time of treatment and before or after treat with a picosecond laser,” added Dr. Avram. “This combined treatment approach is less painful than ablative fractional treatment. You’re going to have downtime anyway, so why not maximize the results at that one treatment session?”
The fractional 1927 laser delivers hundreds of thousands of microscopic pulses and fosters high water absorption, so it targets superficial skin conditions such as actinic keratoses, lentigines, and ephelides at depths of 200-250 microns. It thermally coagulates 30%-40% of skin, which heals without affecting surrounding skin and leaves no perceptible scar, he said.
Clinicians can also combine devices to treat scars. “For red scars, it’s often best to treat both erythema and scar texture with two lasers at the same session,” Dr. Avram said. Again, the order matters. First, he recommended using the pulse dye laser, IPL, or KTP at low fluence and short pulse duration. Second, treat with an ablative or nonablative fractional laser at a low density. “In my experience the ablative fractional lasers are far more efficacious,” he said. “Then we typically add a little Kenalog and 5-FU via laser-assisted drug delivery.”
Dr. Avram disclosed that he has received consulting fees from Allergan. He also reported holding shareholder interest and intellectual property rights with Cytrellis Biosystems.
SAN DIEGO –
“Using a fractional laser as a solo treatment is missing an opportunity to achieve more dramatic improvement for your patients,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said at the annual Masters of Aesthetics Symposium. Among the laser treatments he performs, “combination fractional treatments, typically using the 1927-nm laser” are associated with the highest patient satisfaction, he said.
The order of device use matters, he noted. First, he recommended, use a pulsed dye laser, KTP, or intense pulsed light (IPL) for erythema and telangiectasias, and/or a Q-switched or picosecond laser for pigment. Second, use an ablative or nonablative fractional laser for resurfacing. “A lot of seborrheic keratoses don’t respond to selective photothermolysis well, so I’ll use liquid nitrogen at the time of treatment and before or after treat with a picosecond laser,” added Dr. Avram. “This combined treatment approach is less painful than ablative fractional treatment. You’re going to have downtime anyway, so why not maximize the results at that one treatment session?”
The fractional 1927 laser delivers hundreds of thousands of microscopic pulses and fosters high water absorption, so it targets superficial skin conditions such as actinic keratoses, lentigines, and ephelides at depths of 200-250 microns. It thermally coagulates 30%-40% of skin, which heals without affecting surrounding skin and leaves no perceptible scar, he said.
Clinicians can also combine devices to treat scars. “For red scars, it’s often best to treat both erythema and scar texture with two lasers at the same session,” Dr. Avram said. Again, the order matters. First, he recommended using the pulse dye laser, IPL, or KTP at low fluence and short pulse duration. Second, treat with an ablative or nonablative fractional laser at a low density. “In my experience the ablative fractional lasers are far more efficacious,” he said. “Then we typically add a little Kenalog and 5-FU via laser-assisted drug delivery.”
Dr. Avram disclosed that he has received consulting fees from Allergan. He also reported holding shareholder interest and intellectual property rights with Cytrellis Biosystems.
AT MOAS 2023