User login
A Single, Post-ACTH Cortisol Measurement to Screen for Adrenal Insufficiency in the Hospitalized Patient
Testing for adrenal insufficiency (AI) is common in the hospital setting. The gold standard remains the insulin tolerance test (ITT), in which cortisol concentration is measured after the induction of hypoglycemia to <35 mg/dL.1 Alternatively, metyrapone testing works by blocking cortisol synthesis. If pretest adrenocorticotropic hormone (ACTH) concentrations are low and ACTH concentrations do not rise after the administration of metyrapone, the patient is given a diagnosis of AI. Both assays pose some risk to patients with AI and are typically only performed as confirmatory tests. Morning random cortisol concentrations can be used to suggest AI if concentrations are <3 mcg/dL, but they often provide indeterminate results if concentrations are between 3 and 15 mcg/dL.2 Thus, morning cortisol concentrations in isolation are not diagnostic of AI. For these reasons, most experts recommend a dynamic, high-dose cosyntropin stimulation testing (CST) with 250 mcg of intravenous cosyntropin to screen for AI. The test can be done any time of day.3 Historically, an incremental response to cosyntropin, or “delta,” was also required to indicate a normal response to stimulation.4 However, the baseline cortisol concentration is dependent on circadian rhythm and level of stress. For this reason, a delta, whether large or small, has been abandoned as a requisite for the diagnosis of AI.5-7 A normal CST is widely accepted to be identified by any cortisol concentration >18 mcg/dL during the test (basal or poststimulation).8
The seminal studies by Lindholm, Kehlet, and coauthors9-11 validated the CST against the gold standard ITT and utilized only 0- and 30-minute cortisol concentrations. A later study in patients with pituitary disease demonstrated that 30-minute concentrations had a stronger correlation with the ITT than 60-minute concentrations (false-negative rate: 10% vs 27%).12 However, in that study, a higher threshold was used for the 60-minute concentration than for what was obtained at 30 minutes (25.4 vs 21.8 mcg/dL, respectively). Multiple studies have shown that the 60-minute concentration is higher than the 30-minute concentration after cosyntropin stimulation.4,5,13 Subsequent, small studies of patients who were known to have AI have shown that 60-minute concentrations are as useful as 30-minute concentrations.5,14,15 Because 30-minute cortisol concentrations are often lower than 60-minute concentrations, a single 30-minute result may lead to a falsely abnormal test.16,17 As such, the use of a single 60-minute test may be more appropriate. Indeed, some authors have suggested that measuring only 30-minute concentrations may lead to overdiagnosis of AI by missing an appropriate response, serum cortisol >18 mcg/dL, at 60 minutes.17-19 Peak cortisol concentrations after low-dose cosyntropin stimulation (1 mcg) are seen at 60 minutes, and low-dose stimulation has been shown to be more variable than in the high-dose test (250 mg).19,20
There is a lack of consensus to guide clinicians as to when cortisol concentrations should be measured after stimulation, and standard references lack uniformity. Commonly accessed medical resources—such as UpToDate and Jameson’s Endocrinology—recommend basal, 30-minute, and 60-minute cortisol concentrations, while Williams Textbook of Endocrinology recommends basal and 30-minute concentrations, and the Washington Manual recommends only a single 30-minute concentration.7,21,22 Goldman-Cecil Medicine8 recommends checking a cortisol concentration between 30 and 60 minutes and recommends the same 18 mcg/dL cutoff for any test obtained in this time period. As a result of these variable recommendations, all 3 time points are often obtained. Prominent review articles continue to recommend checking all 3 concentrations while presenting evidence of peak cortisol response at 60 minutes poststimulation.13
In this study, we retrospectively examined CSTs in hospitalized, adult patients both in the intensive care unit (ICU) and hospital ward and/or floor settings to evaluate for significant differences in 30- and 60-minute cortisol concentrations and compare the concordance of screening at each time point alone with traditional CST at all 3 time points. By using these results, we discuss the utility of obtaining 3 cortisol samples.
METHODS
After receiving approval from the institutional review board, we retrospectively reviewed all standard, high-dose CSTs performed on adult inpatients at the Barnes-Jewish Hospital laboratory from January 1, 2012, to August 31, 2013. All patients received the same standard dose (250 mcg cosyntropin, a synthetic ACTH, at a concentration of 1 mcg/mL administered over 2 minutes) regardless of age or weight. We collected patient gender; age; time of baseline cortisol measurement; cortisol results at baseline, 30, and 60 minutes; and patient location (inpatient floor vs ICU status). Tests were included if results from all 3 time points (0, 30, 60 minute) were available.
Cortisol concentrations were assessed by the laboratory according to the manufacturer’s instructions by using the ADVIA Centaur Cortisol assay (Siemens Healthcare Diagnostics Inc, Tarrytown, NY), a competitive chemiluminescent immunoassay. For the traditional CST, a cortisol concentration ≥18 mcg/dL at any time point during the test was used to define normal (negative). Patients with a positive (no results >18 mcg/mL) CST were defined as “screen positives” for the purposes of this analysis. Patient location data were available that allowed for an ICU vs non-ICU comparison.
Statistical analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). Continuous variables were compared by using a 2-tailed Student t test. Percentiles and proportions were compared by using χ2 tests or Fisher’s exact tests when appropriate. The concordance of screening at each time point compared with the traditional CST was calculated. Positive percent agreement (PPA) with the traditional CST in each subgroup (ICU and floor) and combined was also evaluated. A P value of .05 was used to determine significance.
RESULTS
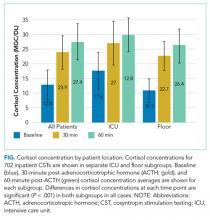
Cortisol concentrations obtained at 30 minutes were significantly higher than baseline cortisol concentrations (baseline: 12.8 mcg/dL; 30 minutes: 23.9 mcg/dL; P < .001) for all patients. The average cortisol concentrations obtained at 60 minutes (27.4 mcg/dL) were significantly higher than those at baseline and 30 minutes (P < .001). This trend was seen in each subgroup of patients in the ICU and on the floor (Figure). The average baseline cortisol concentration was higher for ICU patients compared to floor patients (17.6 mcg/dL vs 10.9 mcg/dL, respectively).
By using the traditional CST, there were 26 (13.1%) positive tests for AI in ICU patients and 84 (16.7%) positive tests in floor patients (Table).
Only 13% of CSTs were started in the recommended 3-hour window from 6:00
DISCUSSION
Our investigation of 702 CSTs, the largest retrospective analysis to date, finds that the 60-minute cortisol concentration is significantly higher than the 30-minute concentration in a standard, high-dose CST. Sixty-minute cortisol concentrations are more concordant with traditional CST results than the 30-minute concentrations in both critically ill ICU and noncritically ill floor patients. This suggests that a single 60-minute measurement is sufficient for AI screening. The use of only 30-minute concentrations would lead to a significant increase in false-positive screening tests and significantly lower PPA (98% vs 57%). With peak cortisol concentrations occurring at 60-minutes poststimulation, measuring both 30- and 60-minute poststimulation concentrations does not appear to be of significant clinical benefit. The cost-saving from reduced phlebotomy and laboratory expenses would be significant, especially in locations with limited staff or financial resources. Our findings are similar to other recent results by Chitale et al.,17 Mansoor et al.,16 and Zueger et al.18
Zueger et al.18 evaluated the results of high-dose CST in 73 patients and found 13.7% of patients with inadequate cortisol response (<18 mcg/dL) at 30 minutes had normal concentrations at 60 minutes (>18 mcg/dL). Their study did not identify a single case of normal cortisol concentration at 30 minutes that would have inappropriately screened positive for AI if cortisol concentrations were only checked at 60 minutes. Similarly, they suggested that the 30-minute test did not add any additional diagnostic value; however, no confirmatory testing was performed.
Higher cortisol concentrations at 60 minutes poststimulation may result in improved specificity for AI without reducing sensitivity, but it may also indicate that the cutoff value may need to be raised from 18 mcg/dL at 60 minutes to maintain an appropriate clinical sensitivity. Continued research should resolve this clinical question with gold-standard confirmatory testing. Furthermore, there is debate about an appropriate screening cortisol concentration threshold for critically ill patients. Researchers have compared concentrations of 25 mcg/dL to the traditional 18 mcg/dL to improve sensitivity for AI, but these studies do not involve comparisons to confirmatory testing and often result in reduced specificity.23,24
In our study, only a small fraction of testing was performed in the early-morning hours, when basal cortisol results are of value. There may be indications to perform traditional CSTs with a basal concentration, such as for suspected secondary AI, but testing must be performed in the early morning for interpretable results per current recommendations. However, poststimulation cortisol concentrations may be interpreted regardless of the time of day at which the test was initiated.3
Our study is limited by its scope because it is a retrospective analysis. It is also limited by a lack of gold-standard, clinical confirmatory testing or analysis of other clinical data. Our method of testing and interpretation is considered the screening standard and is often used to plan treatment for AI without confirmatory testing, as ITT is not routinely available for hospitalized patients. The validation of the traditional CST to the ITT has been performed extensively, but a randomized trial comparing a single 60-minute concentration to the ITT may be useful. The exact timing of blood draws may have introduced error in the concentration measurements, and this is critical to screening accuracy. Total serum cortisol is 10% bound to albumin,25 and medications such as steroids or opioids and medical conditions such as obesity or liver disease can affect cortisol concentrations.26 Albumin and free cortisol concentrations that may be used to adjust for these variables were not available.
CONCLUSION
We recommend changes to the standard CST to exclude a basal cortisol concentration unless it is indicated for the evaluation of secondary AI or obtained at the appropriate early-morning hour. A single 60-minute poststimulation cortisol concentration may be an appropriate screening test for AI and demonstrates high concordance with the traditional CST. The use of a 30-minute poststimulation concentration alone may lead to a significantly higher number of false-positive results. Alternatively, the stimulated cortisol threshold used to define a normal test may need to be higher at 60 minutes to maintain the appropriate sensitivity. Further study and comparison with confirmatory testing are needed.
Disclosure
The authors have no relevant conflicts of interest to disclose.
1. Ajala O, Lockett H, Twine G, Flanagan DE. Depth and duration of hypoglycaemia achieved during the insulin tolerance test. Eur J Endocrinol. 2012;167(1):59-65. PubMed
2. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350-2354. PubMed
3. Azziz R, Bradley E Jr, Huth J, et al. Acute adrenocorticotropin-(1-24) (ACTH) adrenal stimulation in eumenorrheic women: reproducibility and effect of ACTH dose, subject weight, and sampling time. J Clin Endocrinol Metab. 1990;70(5):1273-1279. PubMed
4. Wood, JB, Frankland AW, James VH, Landon J. A Rapid Test of Adrenocortical Function. Lancet. 1965;1(7379):243-245. PubMed
5. Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med. 1971;128(5):761-763. PubMed
6. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. PubMed
7. Melmed S, Polonksy K, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Elsevier: Amsterdam, Netherlands; 2016.
8. Nieman LK. Adrenal Cortex, in Goldman-Cecil Medicine. ed. L. Goldman. 2016, Elsevier: Amsterdam, Netherlands; 2016:1514-1521.
9. Kehlet H, Blichert-Toft M, Lindholm J, Rasmussen P. Short ACTH test in assessing hypothalamic-pituitary-adrenocortical function. Br Med J. 1976;1(6004):249-251. PubMed
10. Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26(1):53-59. PubMed
11. Lindholm J, Kehlet H, Blichert-Toft M, Dinesen B, Riishede J. Reliability of the 30-minute ACTH test in assessing hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 1978;47(2):272-274. PubMed
12. Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):141-146. PubMed
13. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194-204. PubMed
14. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75(1):259-264. PubMed
15. Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159(2):275-280. PubMed
16. Mansoor S, Islam N, Siddiqui I, Jabbar A. Sixty-minute post-Synacthen serum cortisol level: a reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singapore Med J. 2007;48(6):519-523. PubMed
17. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79(1):14-19. PubMed
18. Zueger T, Jordi M, Laimer M, Stettler C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med Wkly. 2014;144:w13987. PubMed
19. Cartaya J, Misra M. The low-dose ACTH stimulation test: is 30 minutes long enough? Endocr Pract. 2015;21(5):508-513. PubMed
20. Gonzálbez, Villabona, Ramon, et al. Establishment of reference values for standard dose short synacthen test (250 μg), low dose short synacthen test (1 μg) and insulin tolerance test for assessment of the hypothalamo–pituitary–adrenal axis in normal subjects. Clin Endocrinol. 2000;53(2):199-204.
21. McGill J, Clutter W, Baranski T. The Washington Manual of Endocrinology Subspecialty Consult. 3rd ed. Washington Manual, ed. Henderson K, De Fer T. Lippincott Williams and Wilkins: Philadelphia, PA; 2012:384.
22. Nieman L. Diagnosis of adrenal insufficiency in adults. In UpToDate, ed. Post T. Wolters Klewer: Waltham, MA; 2017.
23. Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267-1273. PubMed
24. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31(1):141-145. PubMed
25. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chemica Acta. 2005;359(1-2):189-194. PubMed
26. Torpy DJ, Ho JT. Value of Free Cortisol Measurement in Systemic Infection. Horm Metab Res. 2007;39(6):439-444. PubMed
Testing for adrenal insufficiency (AI) is common in the hospital setting. The gold standard remains the insulin tolerance test (ITT), in which cortisol concentration is measured after the induction of hypoglycemia to <35 mg/dL.1 Alternatively, metyrapone testing works by blocking cortisol synthesis. If pretest adrenocorticotropic hormone (ACTH) concentrations are low and ACTH concentrations do not rise after the administration of metyrapone, the patient is given a diagnosis of AI. Both assays pose some risk to patients with AI and are typically only performed as confirmatory tests. Morning random cortisol concentrations can be used to suggest AI if concentrations are <3 mcg/dL, but they often provide indeterminate results if concentrations are between 3 and 15 mcg/dL.2 Thus, morning cortisol concentrations in isolation are not diagnostic of AI. For these reasons, most experts recommend a dynamic, high-dose cosyntropin stimulation testing (CST) with 250 mcg of intravenous cosyntropin to screen for AI. The test can be done any time of day.3 Historically, an incremental response to cosyntropin, or “delta,” was also required to indicate a normal response to stimulation.4 However, the baseline cortisol concentration is dependent on circadian rhythm and level of stress. For this reason, a delta, whether large or small, has been abandoned as a requisite for the diagnosis of AI.5-7 A normal CST is widely accepted to be identified by any cortisol concentration >18 mcg/dL during the test (basal or poststimulation).8
The seminal studies by Lindholm, Kehlet, and coauthors9-11 validated the CST against the gold standard ITT and utilized only 0- and 30-minute cortisol concentrations. A later study in patients with pituitary disease demonstrated that 30-minute concentrations had a stronger correlation with the ITT than 60-minute concentrations (false-negative rate: 10% vs 27%).12 However, in that study, a higher threshold was used for the 60-minute concentration than for what was obtained at 30 minutes (25.4 vs 21.8 mcg/dL, respectively). Multiple studies have shown that the 60-minute concentration is higher than the 30-minute concentration after cosyntropin stimulation.4,5,13 Subsequent, small studies of patients who were known to have AI have shown that 60-minute concentrations are as useful as 30-minute concentrations.5,14,15 Because 30-minute cortisol concentrations are often lower than 60-minute concentrations, a single 30-minute result may lead to a falsely abnormal test.16,17 As such, the use of a single 60-minute test may be more appropriate. Indeed, some authors have suggested that measuring only 30-minute concentrations may lead to overdiagnosis of AI by missing an appropriate response, serum cortisol >18 mcg/dL, at 60 minutes.17-19 Peak cortisol concentrations after low-dose cosyntropin stimulation (1 mcg) are seen at 60 minutes, and low-dose stimulation has been shown to be more variable than in the high-dose test (250 mg).19,20
There is a lack of consensus to guide clinicians as to when cortisol concentrations should be measured after stimulation, and standard references lack uniformity. Commonly accessed medical resources—such as UpToDate and Jameson’s Endocrinology—recommend basal, 30-minute, and 60-minute cortisol concentrations, while Williams Textbook of Endocrinology recommends basal and 30-minute concentrations, and the Washington Manual recommends only a single 30-minute concentration.7,21,22 Goldman-Cecil Medicine8 recommends checking a cortisol concentration between 30 and 60 minutes and recommends the same 18 mcg/dL cutoff for any test obtained in this time period. As a result of these variable recommendations, all 3 time points are often obtained. Prominent review articles continue to recommend checking all 3 concentrations while presenting evidence of peak cortisol response at 60 minutes poststimulation.13
In this study, we retrospectively examined CSTs in hospitalized, adult patients both in the intensive care unit (ICU) and hospital ward and/or floor settings to evaluate for significant differences in 30- and 60-minute cortisol concentrations and compare the concordance of screening at each time point alone with traditional CST at all 3 time points. By using these results, we discuss the utility of obtaining 3 cortisol samples.
METHODS
After receiving approval from the institutional review board, we retrospectively reviewed all standard, high-dose CSTs performed on adult inpatients at the Barnes-Jewish Hospital laboratory from January 1, 2012, to August 31, 2013. All patients received the same standard dose (250 mcg cosyntropin, a synthetic ACTH, at a concentration of 1 mcg/mL administered over 2 minutes) regardless of age or weight. We collected patient gender; age; time of baseline cortisol measurement; cortisol results at baseline, 30, and 60 minutes; and patient location (inpatient floor vs ICU status). Tests were included if results from all 3 time points (0, 30, 60 minute) were available.
Cortisol concentrations were assessed by the laboratory according to the manufacturer’s instructions by using the ADVIA Centaur Cortisol assay (Siemens Healthcare Diagnostics Inc, Tarrytown, NY), a competitive chemiluminescent immunoassay. For the traditional CST, a cortisol concentration ≥18 mcg/dL at any time point during the test was used to define normal (negative). Patients with a positive (no results >18 mcg/mL) CST were defined as “screen positives” for the purposes of this analysis. Patient location data were available that allowed for an ICU vs non-ICU comparison.
Statistical analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). Continuous variables were compared by using a 2-tailed Student t test. Percentiles and proportions were compared by using χ2 tests or Fisher’s exact tests when appropriate. The concordance of screening at each time point compared with the traditional CST was calculated. Positive percent agreement (PPA) with the traditional CST in each subgroup (ICU and floor) and combined was also evaluated. A P value of .05 was used to determine significance.
RESULTS
Cortisol concentrations obtained at 30 minutes were significantly higher than baseline cortisol concentrations (baseline: 12.8 mcg/dL; 30 minutes: 23.9 mcg/dL; P < .001) for all patients. The average cortisol concentrations obtained at 60 minutes (27.4 mcg/dL) were significantly higher than those at baseline and 30 minutes (P < .001). This trend was seen in each subgroup of patients in the ICU and on the floor (Figure). The average baseline cortisol concentration was higher for ICU patients compared to floor patients (17.6 mcg/dL vs 10.9 mcg/dL, respectively).
By using the traditional CST, there were 26 (13.1%) positive tests for AI in ICU patients and 84 (16.7%) positive tests in floor patients (Table).
Only 13% of CSTs were started in the recommended 3-hour window from 6:00
DISCUSSION
Our investigation of 702 CSTs, the largest retrospective analysis to date, finds that the 60-minute cortisol concentration is significantly higher than the 30-minute concentration in a standard, high-dose CST. Sixty-minute cortisol concentrations are more concordant with traditional CST results than the 30-minute concentrations in both critically ill ICU and noncritically ill floor patients. This suggests that a single 60-minute measurement is sufficient for AI screening. The use of only 30-minute concentrations would lead to a significant increase in false-positive screening tests and significantly lower PPA (98% vs 57%). With peak cortisol concentrations occurring at 60-minutes poststimulation, measuring both 30- and 60-minute poststimulation concentrations does not appear to be of significant clinical benefit. The cost-saving from reduced phlebotomy and laboratory expenses would be significant, especially in locations with limited staff or financial resources. Our findings are similar to other recent results by Chitale et al.,17 Mansoor et al.,16 and Zueger et al.18
Zueger et al.18 evaluated the results of high-dose CST in 73 patients and found 13.7% of patients with inadequate cortisol response (<18 mcg/dL) at 30 minutes had normal concentrations at 60 minutes (>18 mcg/dL). Their study did not identify a single case of normal cortisol concentration at 30 minutes that would have inappropriately screened positive for AI if cortisol concentrations were only checked at 60 minutes. Similarly, they suggested that the 30-minute test did not add any additional diagnostic value; however, no confirmatory testing was performed.
Higher cortisol concentrations at 60 minutes poststimulation may result in improved specificity for AI without reducing sensitivity, but it may also indicate that the cutoff value may need to be raised from 18 mcg/dL at 60 minutes to maintain an appropriate clinical sensitivity. Continued research should resolve this clinical question with gold-standard confirmatory testing. Furthermore, there is debate about an appropriate screening cortisol concentration threshold for critically ill patients. Researchers have compared concentrations of 25 mcg/dL to the traditional 18 mcg/dL to improve sensitivity for AI, but these studies do not involve comparisons to confirmatory testing and often result in reduced specificity.23,24
In our study, only a small fraction of testing was performed in the early-morning hours, when basal cortisol results are of value. There may be indications to perform traditional CSTs with a basal concentration, such as for suspected secondary AI, but testing must be performed in the early morning for interpretable results per current recommendations. However, poststimulation cortisol concentrations may be interpreted regardless of the time of day at which the test was initiated.3
Our study is limited by its scope because it is a retrospective analysis. It is also limited by a lack of gold-standard, clinical confirmatory testing or analysis of other clinical data. Our method of testing and interpretation is considered the screening standard and is often used to plan treatment for AI without confirmatory testing, as ITT is not routinely available for hospitalized patients. The validation of the traditional CST to the ITT has been performed extensively, but a randomized trial comparing a single 60-minute concentration to the ITT may be useful. The exact timing of blood draws may have introduced error in the concentration measurements, and this is critical to screening accuracy. Total serum cortisol is 10% bound to albumin,25 and medications such as steroids or opioids and medical conditions such as obesity or liver disease can affect cortisol concentrations.26 Albumin and free cortisol concentrations that may be used to adjust for these variables were not available.
CONCLUSION
We recommend changes to the standard CST to exclude a basal cortisol concentration unless it is indicated for the evaluation of secondary AI or obtained at the appropriate early-morning hour. A single 60-minute poststimulation cortisol concentration may be an appropriate screening test for AI and demonstrates high concordance with the traditional CST. The use of a 30-minute poststimulation concentration alone may lead to a significantly higher number of false-positive results. Alternatively, the stimulated cortisol threshold used to define a normal test may need to be higher at 60 minutes to maintain the appropriate sensitivity. Further study and comparison with confirmatory testing are needed.
Disclosure
The authors have no relevant conflicts of interest to disclose.
Testing for adrenal insufficiency (AI) is common in the hospital setting. The gold standard remains the insulin tolerance test (ITT), in which cortisol concentration is measured after the induction of hypoglycemia to <35 mg/dL.1 Alternatively, metyrapone testing works by blocking cortisol synthesis. If pretest adrenocorticotropic hormone (ACTH) concentrations are low and ACTH concentrations do not rise after the administration of metyrapone, the patient is given a diagnosis of AI. Both assays pose some risk to patients with AI and are typically only performed as confirmatory tests. Morning random cortisol concentrations can be used to suggest AI if concentrations are <3 mcg/dL, but they often provide indeterminate results if concentrations are between 3 and 15 mcg/dL.2 Thus, morning cortisol concentrations in isolation are not diagnostic of AI. For these reasons, most experts recommend a dynamic, high-dose cosyntropin stimulation testing (CST) with 250 mcg of intravenous cosyntropin to screen for AI. The test can be done any time of day.3 Historically, an incremental response to cosyntropin, or “delta,” was also required to indicate a normal response to stimulation.4 However, the baseline cortisol concentration is dependent on circadian rhythm and level of stress. For this reason, a delta, whether large or small, has been abandoned as a requisite for the diagnosis of AI.5-7 A normal CST is widely accepted to be identified by any cortisol concentration >18 mcg/dL during the test (basal or poststimulation).8
The seminal studies by Lindholm, Kehlet, and coauthors9-11 validated the CST against the gold standard ITT and utilized only 0- and 30-minute cortisol concentrations. A later study in patients with pituitary disease demonstrated that 30-minute concentrations had a stronger correlation with the ITT than 60-minute concentrations (false-negative rate: 10% vs 27%).12 However, in that study, a higher threshold was used for the 60-minute concentration than for what was obtained at 30 minutes (25.4 vs 21.8 mcg/dL, respectively). Multiple studies have shown that the 60-minute concentration is higher than the 30-minute concentration after cosyntropin stimulation.4,5,13 Subsequent, small studies of patients who were known to have AI have shown that 60-minute concentrations are as useful as 30-minute concentrations.5,14,15 Because 30-minute cortisol concentrations are often lower than 60-minute concentrations, a single 30-minute result may lead to a falsely abnormal test.16,17 As such, the use of a single 60-minute test may be more appropriate. Indeed, some authors have suggested that measuring only 30-minute concentrations may lead to overdiagnosis of AI by missing an appropriate response, serum cortisol >18 mcg/dL, at 60 minutes.17-19 Peak cortisol concentrations after low-dose cosyntropin stimulation (1 mcg) are seen at 60 minutes, and low-dose stimulation has been shown to be more variable than in the high-dose test (250 mg).19,20
There is a lack of consensus to guide clinicians as to when cortisol concentrations should be measured after stimulation, and standard references lack uniformity. Commonly accessed medical resources—such as UpToDate and Jameson’s Endocrinology—recommend basal, 30-minute, and 60-minute cortisol concentrations, while Williams Textbook of Endocrinology recommends basal and 30-minute concentrations, and the Washington Manual recommends only a single 30-minute concentration.7,21,22 Goldman-Cecil Medicine8 recommends checking a cortisol concentration between 30 and 60 minutes and recommends the same 18 mcg/dL cutoff for any test obtained in this time period. As a result of these variable recommendations, all 3 time points are often obtained. Prominent review articles continue to recommend checking all 3 concentrations while presenting evidence of peak cortisol response at 60 minutes poststimulation.13
In this study, we retrospectively examined CSTs in hospitalized, adult patients both in the intensive care unit (ICU) and hospital ward and/or floor settings to evaluate for significant differences in 30- and 60-minute cortisol concentrations and compare the concordance of screening at each time point alone with traditional CST at all 3 time points. By using these results, we discuss the utility of obtaining 3 cortisol samples.
METHODS
After receiving approval from the institutional review board, we retrospectively reviewed all standard, high-dose CSTs performed on adult inpatients at the Barnes-Jewish Hospital laboratory from January 1, 2012, to August 31, 2013. All patients received the same standard dose (250 mcg cosyntropin, a synthetic ACTH, at a concentration of 1 mcg/mL administered over 2 minutes) regardless of age or weight. We collected patient gender; age; time of baseline cortisol measurement; cortisol results at baseline, 30, and 60 minutes; and patient location (inpatient floor vs ICU status). Tests were included if results from all 3 time points (0, 30, 60 minute) were available.
Cortisol concentrations were assessed by the laboratory according to the manufacturer’s instructions by using the ADVIA Centaur Cortisol assay (Siemens Healthcare Diagnostics Inc, Tarrytown, NY), a competitive chemiluminescent immunoassay. For the traditional CST, a cortisol concentration ≥18 mcg/dL at any time point during the test was used to define normal (negative). Patients with a positive (no results >18 mcg/mL) CST were defined as “screen positives” for the purposes of this analysis. Patient location data were available that allowed for an ICU vs non-ICU comparison.
Statistical analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). Continuous variables were compared by using a 2-tailed Student t test. Percentiles and proportions were compared by using χ2 tests or Fisher’s exact tests when appropriate. The concordance of screening at each time point compared with the traditional CST was calculated. Positive percent agreement (PPA) with the traditional CST in each subgroup (ICU and floor) and combined was also evaluated. A P value of .05 was used to determine significance.
RESULTS
Cortisol concentrations obtained at 30 minutes were significantly higher than baseline cortisol concentrations (baseline: 12.8 mcg/dL; 30 minutes: 23.9 mcg/dL; P < .001) for all patients. The average cortisol concentrations obtained at 60 minutes (27.4 mcg/dL) were significantly higher than those at baseline and 30 minutes (P < .001). This trend was seen in each subgroup of patients in the ICU and on the floor (Figure). The average baseline cortisol concentration was higher for ICU patients compared to floor patients (17.6 mcg/dL vs 10.9 mcg/dL, respectively).
By using the traditional CST, there were 26 (13.1%) positive tests for AI in ICU patients and 84 (16.7%) positive tests in floor patients (Table).
Only 13% of CSTs were started in the recommended 3-hour window from 6:00
DISCUSSION
Our investigation of 702 CSTs, the largest retrospective analysis to date, finds that the 60-minute cortisol concentration is significantly higher than the 30-minute concentration in a standard, high-dose CST. Sixty-minute cortisol concentrations are more concordant with traditional CST results than the 30-minute concentrations in both critically ill ICU and noncritically ill floor patients. This suggests that a single 60-minute measurement is sufficient for AI screening. The use of only 30-minute concentrations would lead to a significant increase in false-positive screening tests and significantly lower PPA (98% vs 57%). With peak cortisol concentrations occurring at 60-minutes poststimulation, measuring both 30- and 60-minute poststimulation concentrations does not appear to be of significant clinical benefit. The cost-saving from reduced phlebotomy and laboratory expenses would be significant, especially in locations with limited staff or financial resources. Our findings are similar to other recent results by Chitale et al.,17 Mansoor et al.,16 and Zueger et al.18
Zueger et al.18 evaluated the results of high-dose CST in 73 patients and found 13.7% of patients with inadequate cortisol response (<18 mcg/dL) at 30 minutes had normal concentrations at 60 minutes (>18 mcg/dL). Their study did not identify a single case of normal cortisol concentration at 30 minutes that would have inappropriately screened positive for AI if cortisol concentrations were only checked at 60 minutes. Similarly, they suggested that the 30-minute test did not add any additional diagnostic value; however, no confirmatory testing was performed.
Higher cortisol concentrations at 60 minutes poststimulation may result in improved specificity for AI without reducing sensitivity, but it may also indicate that the cutoff value may need to be raised from 18 mcg/dL at 60 minutes to maintain an appropriate clinical sensitivity. Continued research should resolve this clinical question with gold-standard confirmatory testing. Furthermore, there is debate about an appropriate screening cortisol concentration threshold for critically ill patients. Researchers have compared concentrations of 25 mcg/dL to the traditional 18 mcg/dL to improve sensitivity for AI, but these studies do not involve comparisons to confirmatory testing and often result in reduced specificity.23,24
In our study, only a small fraction of testing was performed in the early-morning hours, when basal cortisol results are of value. There may be indications to perform traditional CSTs with a basal concentration, such as for suspected secondary AI, but testing must be performed in the early morning for interpretable results per current recommendations. However, poststimulation cortisol concentrations may be interpreted regardless of the time of day at which the test was initiated.3
Our study is limited by its scope because it is a retrospective analysis. It is also limited by a lack of gold-standard, clinical confirmatory testing or analysis of other clinical data. Our method of testing and interpretation is considered the screening standard and is often used to plan treatment for AI without confirmatory testing, as ITT is not routinely available for hospitalized patients. The validation of the traditional CST to the ITT has been performed extensively, but a randomized trial comparing a single 60-minute concentration to the ITT may be useful. The exact timing of blood draws may have introduced error in the concentration measurements, and this is critical to screening accuracy. Total serum cortisol is 10% bound to albumin,25 and medications such as steroids or opioids and medical conditions such as obesity or liver disease can affect cortisol concentrations.26 Albumin and free cortisol concentrations that may be used to adjust for these variables were not available.
CONCLUSION
We recommend changes to the standard CST to exclude a basal cortisol concentration unless it is indicated for the evaluation of secondary AI or obtained at the appropriate early-morning hour. A single 60-minute poststimulation cortisol concentration may be an appropriate screening test for AI and demonstrates high concordance with the traditional CST. The use of a 30-minute poststimulation concentration alone may lead to a significantly higher number of false-positive results. Alternatively, the stimulated cortisol threshold used to define a normal test may need to be higher at 60 minutes to maintain the appropriate sensitivity. Further study and comparison with confirmatory testing are needed.
Disclosure
The authors have no relevant conflicts of interest to disclose.
1. Ajala O, Lockett H, Twine G, Flanagan DE. Depth and duration of hypoglycaemia achieved during the insulin tolerance test. Eur J Endocrinol. 2012;167(1):59-65. PubMed
2. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350-2354. PubMed
3. Azziz R, Bradley E Jr, Huth J, et al. Acute adrenocorticotropin-(1-24) (ACTH) adrenal stimulation in eumenorrheic women: reproducibility and effect of ACTH dose, subject weight, and sampling time. J Clin Endocrinol Metab. 1990;70(5):1273-1279. PubMed
4. Wood, JB, Frankland AW, James VH, Landon J. A Rapid Test of Adrenocortical Function. Lancet. 1965;1(7379):243-245. PubMed
5. Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med. 1971;128(5):761-763. PubMed
6. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. PubMed
7. Melmed S, Polonksy K, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Elsevier: Amsterdam, Netherlands; 2016.
8. Nieman LK. Adrenal Cortex, in Goldman-Cecil Medicine. ed. L. Goldman. 2016, Elsevier: Amsterdam, Netherlands; 2016:1514-1521.
9. Kehlet H, Blichert-Toft M, Lindholm J, Rasmussen P. Short ACTH test in assessing hypothalamic-pituitary-adrenocortical function. Br Med J. 1976;1(6004):249-251. PubMed
10. Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26(1):53-59. PubMed
11. Lindholm J, Kehlet H, Blichert-Toft M, Dinesen B, Riishede J. Reliability of the 30-minute ACTH test in assessing hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 1978;47(2):272-274. PubMed
12. Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):141-146. PubMed
13. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194-204. PubMed
14. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75(1):259-264. PubMed
15. Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159(2):275-280. PubMed
16. Mansoor S, Islam N, Siddiqui I, Jabbar A. Sixty-minute post-Synacthen serum cortisol level: a reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singapore Med J. 2007;48(6):519-523. PubMed
17. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79(1):14-19. PubMed
18. Zueger T, Jordi M, Laimer M, Stettler C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med Wkly. 2014;144:w13987. PubMed
19. Cartaya J, Misra M. The low-dose ACTH stimulation test: is 30 minutes long enough? Endocr Pract. 2015;21(5):508-513. PubMed
20. Gonzálbez, Villabona, Ramon, et al. Establishment of reference values for standard dose short synacthen test (250 μg), low dose short synacthen test (1 μg) and insulin tolerance test for assessment of the hypothalamo–pituitary–adrenal axis in normal subjects. Clin Endocrinol. 2000;53(2):199-204.
21. McGill J, Clutter W, Baranski T. The Washington Manual of Endocrinology Subspecialty Consult. 3rd ed. Washington Manual, ed. Henderson K, De Fer T. Lippincott Williams and Wilkins: Philadelphia, PA; 2012:384.
22. Nieman L. Diagnosis of adrenal insufficiency in adults. In UpToDate, ed. Post T. Wolters Klewer: Waltham, MA; 2017.
23. Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267-1273. PubMed
24. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31(1):141-145. PubMed
25. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chemica Acta. 2005;359(1-2):189-194. PubMed
26. Torpy DJ, Ho JT. Value of Free Cortisol Measurement in Systemic Infection. Horm Metab Res. 2007;39(6):439-444. PubMed
1. Ajala O, Lockett H, Twine G, Flanagan DE. Depth and duration of hypoglycaemia achieved during the insulin tolerance test. Eur J Endocrinol. 2012;167(1):59-65. PubMed
2. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350-2354. PubMed
3. Azziz R, Bradley E Jr, Huth J, et al. Acute adrenocorticotropin-(1-24) (ACTH) adrenal stimulation in eumenorrheic women: reproducibility and effect of ACTH dose, subject weight, and sampling time. J Clin Endocrinol Metab. 1990;70(5):1273-1279. PubMed
4. Wood, JB, Frankland AW, James VH, Landon J. A Rapid Test of Adrenocortical Function. Lancet. 1965;1(7379):243-245. PubMed
5. Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med. 1971;128(5):761-763. PubMed
6. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. PubMed
7. Melmed S, Polonksy K, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Elsevier: Amsterdam, Netherlands; 2016.
8. Nieman LK. Adrenal Cortex, in Goldman-Cecil Medicine. ed. L. Goldman. 2016, Elsevier: Amsterdam, Netherlands; 2016:1514-1521.
9. Kehlet H, Blichert-Toft M, Lindholm J, Rasmussen P. Short ACTH test in assessing hypothalamic-pituitary-adrenocortical function. Br Med J. 1976;1(6004):249-251. PubMed
10. Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26(1):53-59. PubMed
11. Lindholm J, Kehlet H, Blichert-Toft M, Dinesen B, Riishede J. Reliability of the 30-minute ACTH test in assessing hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 1978;47(2):272-274. PubMed
12. Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):141-146. PubMed
13. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194-204. PubMed
14. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75(1):259-264. PubMed
15. Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159(2):275-280. PubMed
16. Mansoor S, Islam N, Siddiqui I, Jabbar A. Sixty-minute post-Synacthen serum cortisol level: a reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singapore Med J. 2007;48(6):519-523. PubMed
17. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79(1):14-19. PubMed
18. Zueger T, Jordi M, Laimer M, Stettler C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med Wkly. 2014;144:w13987. PubMed
19. Cartaya J, Misra M. The low-dose ACTH stimulation test: is 30 minutes long enough? Endocr Pract. 2015;21(5):508-513. PubMed
20. Gonzálbez, Villabona, Ramon, et al. Establishment of reference values for standard dose short synacthen test (250 μg), low dose short synacthen test (1 μg) and insulin tolerance test for assessment of the hypothalamo–pituitary–adrenal axis in normal subjects. Clin Endocrinol. 2000;53(2):199-204.
21. McGill J, Clutter W, Baranski T. The Washington Manual of Endocrinology Subspecialty Consult. 3rd ed. Washington Manual, ed. Henderson K, De Fer T. Lippincott Williams and Wilkins: Philadelphia, PA; 2012:384.
22. Nieman L. Diagnosis of adrenal insufficiency in adults. In UpToDate, ed. Post T. Wolters Klewer: Waltham, MA; 2017.
23. Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267-1273. PubMed
24. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31(1):141-145. PubMed
25. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chemica Acta. 2005;359(1-2):189-194. PubMed
26. Torpy DJ, Ho JT. Value of Free Cortisol Measurement in Systemic Infection. Horm Metab Res. 2007;39(6):439-444. PubMed
© 2018 Society of Hospital Medicine