User login
In reply: Managing bloodstream infections
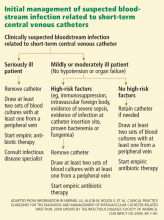
In Reply: We thank Dr. Dias for his careful read of our article, “Managing bloodstream infections in patients who have short-term central venous catheters,” and we acknowledge that he is correct to point out that, by definition, severe sepsis is sepsis associated with organ dysfunction, hypoperfusion, or hypotension. Given this, he is correct that patients with severe sepsis should be categorized in the “seriously ill” patient group in our Figure 1.
In effect, however, the recommendations for patients in the “high-risk-factor” group are the same as the recommendations for the “seriously ill” patient group, which are to remove the catheter, draw at least two sets of blood cultures with at least one from a peripheral vein, and start empiric antibiotic therapy.
In Reply: We thank Dr. Dias for his careful read of our article, “Managing bloodstream infections in patients who have short-term central venous catheters,” and we acknowledge that he is correct to point out that, by definition, severe sepsis is sepsis associated with organ dysfunction, hypoperfusion, or hypotension. Given this, he is correct that patients with severe sepsis should be categorized in the “seriously ill” patient group in our Figure 1.
In effect, however, the recommendations for patients in the “high-risk-factor” group are the same as the recommendations for the “seriously ill” patient group, which are to remove the catheter, draw at least two sets of blood cultures with at least one from a peripheral vein, and start empiric antibiotic therapy.
In Reply: We thank Dr. Dias for his careful read of our article, “Managing bloodstream infections in patients who have short-term central venous catheters,” and we acknowledge that he is correct to point out that, by definition, severe sepsis is sepsis associated with organ dysfunction, hypoperfusion, or hypotension. Given this, he is correct that patients with severe sepsis should be categorized in the “seriously ill” patient group in our Figure 1.
In effect, however, the recommendations for patients in the “high-risk-factor” group are the same as the recommendations for the “seriously ill” patient group, which are to remove the catheter, draw at least two sets of blood cultures with at least one from a peripheral vein, and start empiric antibiotic therapy.
Managing bloodstream infections in patients who have short-term central venous catheters
Vascular catheters are very common in everyday inpatient and, increasingly, outpatient care. Nearly 300 million catheters are estimated to be used annually in the United States, and approximately 3 million of these are central venous catheters (CVCs).1
Although significant gains have been made in preventing CVC-related bloodstream infections, these infections continue to occur, with estimated rates ranging from 1.3 per 1,000 catheter days on inpatient medical-surgical wards to 5.6 per 1,000 catheter days in intensive care burn units.2
CVCs are classified as either long-term or short-term. Long-term CVCs are surgically implanted or tunneled and used for prolonged chemotherapy, home infusion therapy, or hemodialysis. Short-term CVCs do not require surgical implantation. They are more common than long-term CVCs and account for most CVC-related bloodstream infections. Given the frequency of short-term CVC use, a growing number of health care providers from mid-level practitioners to intensivists are faced with deciding how to manage bloodstream infection related to short-term CVCs.
At baseline, management decisions about bloodstream infections from short-term CVCs can be challenging. Questions that regularly arise include:
- Should a potentially infected catheter be removed?
- Which empiric antibiotic therapy should be started pending a microbiologic diagnosis?
- How should therapy be tailored (eg, antibiotic choice and course and whether to remove or retain the catheter) based on the specific pathogen identified?
Adding to the complexity of these decisions are increasingly resistant microorganisms, heterogeneity of affected patient populations, and variability in the quality and availability of evidence.
This review provides a concise guide to managing bloodstream infections related to short-term CVCs in adults, based on updated guidelines from the Infectious Diseases Society of America (IDSA).3
DEFINITION AND DIAGNOSTIC CRITERIA
We have adapted the following definition and diagnostic criteria from the general definition and diagnostic criteria for catheter-related bloodstream infections proposed by the IDSA.
Bloodstream infection related to a short-term CVC is defined as bacteremia or fungemia in a patient with the CVC in place, clinical manifestations of infection (eg, fever, chills, hypotension), and no apparent source of the bloodstream infection aside from the catheter. At least one of the three diagnostic criteria should be met:
- Cultures of the catheter tip and of the peripheral blood grow the same organism. Catheter tip culture should be quantitative, with more than 102 colony-forming units (cfu) per catheter segment, or semiquantitative, with more than 15 cfu per catheter segment.
- Blood drawn from the catheter lumen grows the same organism as blood drawn from a peripheral vein (or less optimally, a different lumen), but at three times the amount by quantitative culture.
- Blood drawn simultaneously from the catheter lumen and from a peripheral vein (or less optimally, a different lumen) grows the same organism, and growth from the CVC lumen sample is detected (by automated blood culture system) at least 2 hours before growth from the peripheral vein sample.
MANAGING BLOODSTREAM INFECTIONS IN PATIENTS WITH SHORT-TERM CVCs
When to remove a potentially infected short-term CVC
In a nonneutropenic intensive care population, Bouza et al4 found that, of 204 episodes of clinically suspected bloodstream infection from a short-term CVC, only 28 (14%) were confirmed to be catheter-related, 27 (13%) were bloodstream infections that were not catheter-related, 36 (18%) involved catheter-tip colonization with negative blood cultures, and the remainder were cases with negative catheter-tip and blood cultures.
Rijnders et al,5 in a study of 100 adult medical-surgical intensive care patients with a clinically suspected bloodstream infection related to a short-term CVC, found only three confirmed cases.
A randomized clinical trial comparing early removal of short-term CVCs and watchful waiting in an adult intensive care population with clinically suspected bloodstream infections showed no difference between treatment groups in length of stay in the intensive care unit or in the mortality rate.6 This trial included a low-risk subset of adult medical-surgical intensive care patients (ie, immunocompetent, no intravascular foreign body, no evidence of severe sepsis or septic shock, no evidence of infection at the catheter insertion site, no proven bacteremia or fungemia). These results suggest that a similar subset of patients can be safely monitored without catheter removal while being assessed for possible catheter-related bloodstream infection.
Empiric catheter removal vs watchful waiting has not and likely will not be studied in higher-risk populations. In this group, clinical judgment should outweigh any specific management algorithm. In patients who are in shock or who are otherwise hemodynamically unstable, early catheter removal should be a priority; however, in some circumstances the risks of immediate catheter removal (eg, coagulopathy with risk of bleeding diathesis, or lack of site to replace the catheter) may outweigh the potential benefits.
Empiric antibiotic therapy for bloodstream infection from a short-term CVC
In order of prevalence, the four most common pathogens are coagulase-negative staphylococci, Staphylococcus aureus, Candida species, and enteric gram-negative bacilli.7
Gram-positive pathogens. A recent randomized clinical trial comparing vancomycin and linezolid (Zyvox) treatment for CVC-related bloodstream infections showed that 89 (57%) of 157 S aureus isolates and 95 (80%) of 119 coagulase-negative staphylococcal isolates were resistant to methicillin.8 Given the prevalence of gram-positive infections and the regularity of methicillin-resistant isolates, vancomycin should be started empirically in cases of suspected bloodstream infection related to short-term CVCs. In institutions where methicillin-resistant S aureus (MRSA) isolates regularly have a vancomycin minimum inhibitory concentration (MIC) of greater than 2 μg/mL, an alternative agent such as daptomycin (Cubicin) should be used.9,10
Gram-negative pathogens. Infections due to resistant gram-negative pathogens have become more common in the past 10 years.11,12 Prospective cohort studies have shown that resistant gram-negative infections and inadequate empiric antimicrobial therapy of bloodstream infections independently predict the risk of death.13,14 Risk factors for resistant gram-negative infections include critical illness, neutropenia, prior antibiotic therapy, and femoral insertion of the CVC.15–18 Patients with these risk factors should receive empiric antibiotic therapy for gram-negative bacilli.
No randomized controlled trial has been done to guide the choice of empiric gram-negative antibiotic coverage. The initial choice should be based on local antimicrobial patterns and susceptibility data and on the severity of the patient’s illness. Initial options include fourth-generation cephalosporins, carbapenems, or combined beta-lactam and beta-lactamase inhibitors. Patients with neutropenia, severe sepsis, or known multiple-drug-resistant gram-negative bacilli colonization or prior infection should receive empiric combination therapy with two different classes of antibiotics.
Candida. Risk factors for CVC-related bloodstream infections due to Candida species include total parenteral nutrition, prolonged use of broad-spectrum antibiotics, hematologic malignancy, solid organ or bone marrow transplantation, colonization with Candida species at multiple sites, and femoral catheter insertion. Empiric treatment with an echinocandin is recommended for patients with these risk factors. Fluconazole (Diflucan) can be substituted for an echinocandin in patients without azole exposure in the previous 3 months and in settings where the prevalence of Candida krusei and Candida glabrata is low.
PATHOGEN-SPECIFIC MANAGEMENT: RECOMMENDATIONS
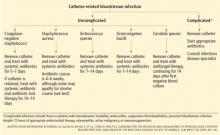
Coagulase-negative staphylococci
Most patients with coagulase-negative staphylococcal infections have a benign clinical course.
Although no randomized trial has evaluated different treatment approaches, most experts recommend removing the catheter and giving a short course of antibiotics (ie, 5–7 days). Longer courses of antibiotics may be required for patients with endovascular hardware in place or persistent fever or bacteremia after catheter removal. The IDSA guidelines recommend 5 to 7 days of antibiotic therapy if the catheter is removed, and 10 to 14 days of systemic antibiotic therapy in combination with “antibiotic lock therapy” if the catheter is retained. Antibiotic lock therapy involves instilling a high concentration of an antibiotic to which the organism is susceptible into the catheter lumen and allowing it to dwell.
Not all patients are good candidates for antibiotic lock therapy, and neither are all organisms. In general, patients should be at low risk (immunocompetent, without hardware in place), and organisms should have a low risk of causing metastatic infection.
Staphylococcus lugdunensis can cause endocarditis and metastatic infections similar to those caused by S aureus and so should be managed similarly to S aureus.19
Staphylococcus aureus
Short-term CVCs infected with S aureus should be removed immediately. Removal of vascular catheters infected with S aureus has been associated with more rapid clinical response and higher cure rates compared with catheter retention.20–23S aureus bacteremia results in hematogenous complications in 20% to 30% of patients, and failure to remove or a delay in removing the catheter increases the risk of complications.21,24–27
There are no data from randomized clinical trials on the optimal duration of antibiotic therapy for S aureus bloodstream infections related to short-term CVCs. Traditionally, 4 weeks have been recommended out of concern for the risk of infective endocarditis,28,29 and the IDSA recommends 4 to 6 weeks unless patients meet certain low-risk criteria.
Factors associated with a higher risk of hematogenous complications include the presence of a retained foreign body, an intravascular prosthetic device, retained catheter, immune suppression, diabetes, persistent bacteremia at 72 hours despite catheter removal and appropriate antibiotics, skin changes consistent with septic emboli, or evidence of endocarditis or suppurative thrombophlebitis on transesophageal echocardiography (TEE) or ultrasonography, respectively.21,25–27 TEE is superior to transthoracic echocardiography and is most sensitive when performed 5 to 7 days after the onset of bacteremia.28,30 Patients who have had the catheter removed and who do not have any of these risk factors, and in whom TEE performed 5 to 7 days after the onset of bacteremia is negative, can be considered for a shorter duration of therapy (but a minimum of 14 days).
Patients with catheters colonized with S aureus (ie, those with positive catheter-tip cultures and negative blood cultures) are at risk of subsequent bacteremia. This risk may be reduced with anti-staphylococcal therapy started within 24 hours of catheter removal.31,32 Therapy should be continued for 5 to 7 days, and patients should be closely monitored for signs or symptoms of ongoing infection.
Oxacillin or nafcillin should be the first-line therapy for susceptible S aureus isolates. Vancomycin should be used to treat MRSA. Patients with MRSA isolates with a vancomycin MIC greater than 2 μg/mL should receive daptomycin or linezolid, depending on susceptibility data.
Enterococcal species
Up to 10% of nosocomially acquired bloodstream infections are due to enterococci, and many are related to intravascular catheters.33,34 Although the risk of endocarditis as a complication of enterococcal CVC-related bloodstream infection is relatively low, estimated at 1.5% in a multicenter prospective study, enterococcal bacteremia lasting longer than 4 days has been independently associated with risk of death.35,36 These observational data support routine removal of short-term CVCs infected with enterococci.
The choice of antibiotics for enterococcal infections depends on the susceptibility of the isolate. Sixty percent of Enterococcus faecium isolates and 2% of Enterococcus faecalis isolates are vancomycin-resistant, and reports of resistance to newer agents, including linezolid, have been published.34,37,38 Ampicillin is the preferred antibiotic for treatment of ampicillin-susceptible enterococci. Vancomycin should be used if the pathogen is ampicillin-resistant and vancomycin-susceptible. Enterococci resistant to both ampicillin and vancomycin can be treated with linezolid or daptomycin, based on susceptibility data.
For combination therapy with an aminoglycoside, the data are mixed. Retrospective observational studies have shown no difference in outcomes in uncomplicated enterococcal bacteremia with combination therapy vs monotherapy.39,40 However, in a large series of patients with enterococcal infections in which the catheter was retained, the combination of gentamicin and ampicillin was more effective than monotherapy.41
No controlled trial has been done to define the optimal duration of antibiotic therapy for enterococcal bloodstream infections related to short-term CVCs, but the IDSA recommends 7 to 14 days. If catheter salvage is attempted, concurrent antimicrobial lock therapy is recommended based on expert opinion. Catheters should be removed if complications arise (eg, insertion site or pocket infection, suppurative thrombophlebitis, sepsis, endocarditis, persistent bacteremia, metastatic infection). Signs and symptoms of endocarditis, persistent bacteremia, or the presence of a prosthetic heart valve should prompt evaluation with TEE.42,43
Gram-negative bacilli
Given the propensity of many gram-negative bacilli to form a biofilm, a number of studies have advocated removing CVCs infected with gram-negative bacilli.15,16,44 Recent studies examining the role of combination systemic antibiotic therapy and antibiotic lock therapy of gram-negative infections have found high success rates.45,46
The IDSA recommends routine removal of short-term CVCs infected with gram-negative bacilli and 7 to 14 days of systemic antibiotic therapy based on microbial susceptibility data. Antibiotic options generally include fourth-generation cephalosporins, carbapenems, or a combination beta-lactam and beta-lactamase inhibitor. The first-line treatment for Stenotrophomonas maltophilia and Burkholderia cepacia is trimethoprim-sulfamethoxazole (Bactrim). Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli should not be treated with cephalosporins or piperacillin-tazobactam (Zosyn) even if the organisms are susceptible in vitro, as doing so has been associated with poor clinical outcomes.11,47
There is growing concern over multiple-drug-resistant gram-negative bacilli with carbapenemases that confer resistance to carbapenems. No controlled study has evaluated treatment of multiple-drug-resistant gram-negative bacilli that require therapy with polymyxin (Colistin).
Candida species
The benefit of removing the CVC in the setting of candidemia is supported by six prospective studies.48–53 Patients with catheter-related bloodstream infections due to Candida species should have the catheter removed. C albicans and azole-susceptible candidal strains can be effectively treated with fluconazole at a dosage of 400 mg daily, continued for 14 days following the first negative blood culture.54 Echinocandins as first-line therapy and lipid formulations of amphotericin B (Abelcet) as an alternative are both highly effective for the treatment of Candida species with decreased susceptibility to azoles (eg, C glabrata and C krusei).55–57
Other gram-positive microorganisms
The isolation of Corynebacterium, Bacillus, and Micrococcus species from a single blood culture does not prove bloodstream infection, and confirmation requires at least two positive results drawn from different sites. CVC infections with these organisms are difficult to treat unless the infected catheter is removed.58,59
ADDITIONAL RECOMMENDATIONS
Infectious disease consultation should be considered for patients with complicated bloodstream infection related to a short-term CVC. Complicated cases include catheter infections in patients with hemodynamic instability, endocarditis, suppurative thrombophlebitis, persistent bloodstream infection despite 72 hours of appropriate antimicrobial therapy, osteomyelitis, active malignancy, or immunosuppression.
Infectious disease consultation should also be sought for assistance with determining if a patient is a candidate for antibiotic lock therapy; for management, dosing, and course of antibiotic lock therapy; for assistance with antibiotic choice and course for multiple-drug-resistant gram-negative bacilli; and for recommendations on management of infections due to uncommon pathogens (eg, Corynebacterium jeikeium, Chryseobacterium species, Malassezia furfur, and Mycobacterium species).
- Edgeworth J. Intravascular catheter infections. J Hosp Infect 2009; 73:323–330.
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009; 37:783–805.
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45.
- Bouza E, Alvarado N, Alcalá L, Pérez MJ, Rincón C, Muñoz P. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin Infect Dis 2007; 44:820–826.
- Rijnders BJ, Verwaest C, Peetermans WE, et al. Difference in time to positivity of hub-blood versus nonhub-blood cultures is not useful for the diagnosis of catheter-related bloodstream infection in critically ill patients. Crit Care Med 2001; 29:1399–1403.
- Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Van Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter-related infection: a randomized trial. Intensive Care Med 2004; 30:1073–1080.
- Safdar A, Mermel LA, Maki DG. The epidemiology of catheter-related infection in the critically ill. In:O’Grady NP, Pittet D, editors. Catheter-Related Infections in the Critically Ill. Boston: Kluwer, 2004:1–22.
- Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009; 48:203–212.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007; 51:2582–2586.
- Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med 2005; 352:380–391.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470–485.
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:146–155.
- Raymond DP, Pelletier SJ, Crabtree TD, Evans HL, Pruett TL, Sawyer RG. Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 2003; 31:1035–1041.
- Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect 2002; 45:47–53.
- Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore) 1995; 74:340–349.
- Seifert H. Catheter-related infections due to gram-negative bacilli. In:Seifert H, Jansen B, Farr BM, editors. Catheter-Related infections. New York: M. Dekker, 1997:111–138.
- Lorente L, Jiménez A, Santana M, et al. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med 2007; 35:2424–2427.
- Zinkernagel AS, Zinkernagel MS, Elzi MV, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 2008; 36:314–321.
- Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med 1995; 155:1161–1166.
- Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703.
- Fowler VG, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–486.
- Reilly JJ, Steed DL, Ritter PS. Indwelling venous access catheters in patients with acute leukemia. Cancer 1984; 53:219–223.
- Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147:536–539.
- Fowler VG, Miro JM, Hoen B, et al; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021.
- Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–2072.
- Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–332.
- Rosen AB, Fowler VG, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 1999; 130:810–820.
- Pigrau C, Rodríguez D, Planes AM, et al. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis 2003; 22:713–719.
- Sochowski RA, Chan KL. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. J Am Coll Cardiol 1993; 21:216–221.
- Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med 2008; 36:397–402.
- Ruhe JJ, Menon A. Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect 2006; 12:933–936.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–317.
- Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn Microbiol Infect Dis 1997; 29:95–102.
- DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis 2005; 191:588–595.
- Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis 2000; 36:145–158.
- Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179.
- Kanafani ZA, Federspiel JJ, Fowler VG. Infective endocarditis caused by daptomycin-resistant Enterococcus faecalis: a case report. Scand J Infect Dis 2007; 39:75–77.
- Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988; 67:248–269.
- Gray J, Marsh PJ, Stewart D, Pedler SJ. Enterococcal bacteraemia: a prospective study of 125 episodes. J Hosp Infect 1994; 27:179–186.
- Sandoe JA, Witherden IR, Au-Yeung HK, Kite P, Kerr KG, Wilcox MH. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J Antimicrob Chemother 2002; 50:577–582.
- Anderson DJ, Murdoch DR, Sexton DJ, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 2004; 32:72–77.
- Fernández-Guerrero ML, Herrero L, Bellver M, Gadea I, Roblas RF, de Górgolas M. Nosocomial enterococcal endocarditis: a serious hazard for hospitalized patients with enterococcal bacteraemia. J Intern Med 2002; 252:510–515.
- Elting LS, Bodey GP. Septicemia due to Xanthomonas species and non-aeruginosa Pseudomonas species: increasing incidence of catheter-related infections. Medicine (Baltimore) 1990; 69:296–306.
- Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother 2006; 57:1172–1180.
- Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant 2004; 19:1237–1244.
- Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39:2206–2212.
- Nguyen MH, Peacock JE, Tanner DC, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med 1995; 155:2429–2435.
- Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc 1996; 95:19–28.
- Rex JH, Bennett JE, Sugar AM, et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 1995; 21:994–996.
- Karlowicz MG, Hashimoto LN, Kelly RE, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 2000; 106:E63.
- Nucci M, Colombo AL, Silveira F, et al. Risk factors for death in patients with candidemia. Infect Control Hosp Epidemiol 1998; 19:846–850.
- Almirante B, Rodríguez D, Park BJ, et al; Barcelona Candidemia Project Study Group. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005; 43:1829–1835.
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331:1325–1330.
- Kuse ER, Chetchotisakd P, da Cunha CA, et al; Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–1527.
- Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
- Mora-Duarte J, Betts R, Rotstein C, et al; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–2029.
- Peces R, Gago E, Tejada F, Laures AS, Alvarez-Grande J. Relapsing bacteraemia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant 1997; 12:2428–2429.
- Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzo PA. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol 1987; 25:672–674.
Vascular catheters are very common in everyday inpatient and, increasingly, outpatient care. Nearly 300 million catheters are estimated to be used annually in the United States, and approximately 3 million of these are central venous catheters (CVCs).1
Although significant gains have been made in preventing CVC-related bloodstream infections, these infections continue to occur, with estimated rates ranging from 1.3 per 1,000 catheter days on inpatient medical-surgical wards to 5.6 per 1,000 catheter days in intensive care burn units.2
CVCs are classified as either long-term or short-term. Long-term CVCs are surgically implanted or tunneled and used for prolonged chemotherapy, home infusion therapy, or hemodialysis. Short-term CVCs do not require surgical implantation. They are more common than long-term CVCs and account for most CVC-related bloodstream infections. Given the frequency of short-term CVC use, a growing number of health care providers from mid-level practitioners to intensivists are faced with deciding how to manage bloodstream infection related to short-term CVCs.
At baseline, management decisions about bloodstream infections from short-term CVCs can be challenging. Questions that regularly arise include:
- Should a potentially infected catheter be removed?
- Which empiric antibiotic therapy should be started pending a microbiologic diagnosis?
- How should therapy be tailored (eg, antibiotic choice and course and whether to remove or retain the catheter) based on the specific pathogen identified?
Adding to the complexity of these decisions are increasingly resistant microorganisms, heterogeneity of affected patient populations, and variability in the quality and availability of evidence.
This review provides a concise guide to managing bloodstream infections related to short-term CVCs in adults, based on updated guidelines from the Infectious Diseases Society of America (IDSA).3
DEFINITION AND DIAGNOSTIC CRITERIA
We have adapted the following definition and diagnostic criteria from the general definition and diagnostic criteria for catheter-related bloodstream infections proposed by the IDSA.
Bloodstream infection related to a short-term CVC is defined as bacteremia or fungemia in a patient with the CVC in place, clinical manifestations of infection (eg, fever, chills, hypotension), and no apparent source of the bloodstream infection aside from the catheter. At least one of the three diagnostic criteria should be met:
- Cultures of the catheter tip and of the peripheral blood grow the same organism. Catheter tip culture should be quantitative, with more than 102 colony-forming units (cfu) per catheter segment, or semiquantitative, with more than 15 cfu per catheter segment.
- Blood drawn from the catheter lumen grows the same organism as blood drawn from a peripheral vein (or less optimally, a different lumen), but at three times the amount by quantitative culture.
- Blood drawn simultaneously from the catheter lumen and from a peripheral vein (or less optimally, a different lumen) grows the same organism, and growth from the CVC lumen sample is detected (by automated blood culture system) at least 2 hours before growth from the peripheral vein sample.
MANAGING BLOODSTREAM INFECTIONS IN PATIENTS WITH SHORT-TERM CVCs
When to remove a potentially infected short-term CVC
In a nonneutropenic intensive care population, Bouza et al4 found that, of 204 episodes of clinically suspected bloodstream infection from a short-term CVC, only 28 (14%) were confirmed to be catheter-related, 27 (13%) were bloodstream infections that were not catheter-related, 36 (18%) involved catheter-tip colonization with negative blood cultures, and the remainder were cases with negative catheter-tip and blood cultures.
Rijnders et al,5 in a study of 100 adult medical-surgical intensive care patients with a clinically suspected bloodstream infection related to a short-term CVC, found only three confirmed cases.
A randomized clinical trial comparing early removal of short-term CVCs and watchful waiting in an adult intensive care population with clinically suspected bloodstream infections showed no difference between treatment groups in length of stay in the intensive care unit or in the mortality rate.6 This trial included a low-risk subset of adult medical-surgical intensive care patients (ie, immunocompetent, no intravascular foreign body, no evidence of severe sepsis or septic shock, no evidence of infection at the catheter insertion site, no proven bacteremia or fungemia). These results suggest that a similar subset of patients can be safely monitored without catheter removal while being assessed for possible catheter-related bloodstream infection.
Empiric catheter removal vs watchful waiting has not and likely will not be studied in higher-risk populations. In this group, clinical judgment should outweigh any specific management algorithm. In patients who are in shock or who are otherwise hemodynamically unstable, early catheter removal should be a priority; however, in some circumstances the risks of immediate catheter removal (eg, coagulopathy with risk of bleeding diathesis, or lack of site to replace the catheter) may outweigh the potential benefits.
Empiric antibiotic therapy for bloodstream infection from a short-term CVC
In order of prevalence, the four most common pathogens are coagulase-negative staphylococci, Staphylococcus aureus, Candida species, and enteric gram-negative bacilli.7
Gram-positive pathogens. A recent randomized clinical trial comparing vancomycin and linezolid (Zyvox) treatment for CVC-related bloodstream infections showed that 89 (57%) of 157 S aureus isolates and 95 (80%) of 119 coagulase-negative staphylococcal isolates were resistant to methicillin.8 Given the prevalence of gram-positive infections and the regularity of methicillin-resistant isolates, vancomycin should be started empirically in cases of suspected bloodstream infection related to short-term CVCs. In institutions where methicillin-resistant S aureus (MRSA) isolates regularly have a vancomycin minimum inhibitory concentration (MIC) of greater than 2 μg/mL, an alternative agent such as daptomycin (Cubicin) should be used.9,10
Gram-negative pathogens. Infections due to resistant gram-negative pathogens have become more common in the past 10 years.11,12 Prospective cohort studies have shown that resistant gram-negative infections and inadequate empiric antimicrobial therapy of bloodstream infections independently predict the risk of death.13,14 Risk factors for resistant gram-negative infections include critical illness, neutropenia, prior antibiotic therapy, and femoral insertion of the CVC.15–18 Patients with these risk factors should receive empiric antibiotic therapy for gram-negative bacilli.
No randomized controlled trial has been done to guide the choice of empiric gram-negative antibiotic coverage. The initial choice should be based on local antimicrobial patterns and susceptibility data and on the severity of the patient’s illness. Initial options include fourth-generation cephalosporins, carbapenems, or combined beta-lactam and beta-lactamase inhibitors. Patients with neutropenia, severe sepsis, or known multiple-drug-resistant gram-negative bacilli colonization or prior infection should receive empiric combination therapy with two different classes of antibiotics.
Candida. Risk factors for CVC-related bloodstream infections due to Candida species include total parenteral nutrition, prolonged use of broad-spectrum antibiotics, hematologic malignancy, solid organ or bone marrow transplantation, colonization with Candida species at multiple sites, and femoral catheter insertion. Empiric treatment with an echinocandin is recommended for patients with these risk factors. Fluconazole (Diflucan) can be substituted for an echinocandin in patients without azole exposure in the previous 3 months and in settings where the prevalence of Candida krusei and Candida glabrata is low.
PATHOGEN-SPECIFIC MANAGEMENT: RECOMMENDATIONS
Coagulase-negative staphylococci
Most patients with coagulase-negative staphylococcal infections have a benign clinical course.
Although no randomized trial has evaluated different treatment approaches, most experts recommend removing the catheter and giving a short course of antibiotics (ie, 5–7 days). Longer courses of antibiotics may be required for patients with endovascular hardware in place or persistent fever or bacteremia after catheter removal. The IDSA guidelines recommend 5 to 7 days of antibiotic therapy if the catheter is removed, and 10 to 14 days of systemic antibiotic therapy in combination with “antibiotic lock therapy” if the catheter is retained. Antibiotic lock therapy involves instilling a high concentration of an antibiotic to which the organism is susceptible into the catheter lumen and allowing it to dwell.
Not all patients are good candidates for antibiotic lock therapy, and neither are all organisms. In general, patients should be at low risk (immunocompetent, without hardware in place), and organisms should have a low risk of causing metastatic infection.
Staphylococcus lugdunensis can cause endocarditis and metastatic infections similar to those caused by S aureus and so should be managed similarly to S aureus.19
Staphylococcus aureus
Short-term CVCs infected with S aureus should be removed immediately. Removal of vascular catheters infected with S aureus has been associated with more rapid clinical response and higher cure rates compared with catheter retention.20–23S aureus bacteremia results in hematogenous complications in 20% to 30% of patients, and failure to remove or a delay in removing the catheter increases the risk of complications.21,24–27
There are no data from randomized clinical trials on the optimal duration of antibiotic therapy for S aureus bloodstream infections related to short-term CVCs. Traditionally, 4 weeks have been recommended out of concern for the risk of infective endocarditis,28,29 and the IDSA recommends 4 to 6 weeks unless patients meet certain low-risk criteria.
Factors associated with a higher risk of hematogenous complications include the presence of a retained foreign body, an intravascular prosthetic device, retained catheter, immune suppression, diabetes, persistent bacteremia at 72 hours despite catheter removal and appropriate antibiotics, skin changes consistent with septic emboli, or evidence of endocarditis or suppurative thrombophlebitis on transesophageal echocardiography (TEE) or ultrasonography, respectively.21,25–27 TEE is superior to transthoracic echocardiography and is most sensitive when performed 5 to 7 days after the onset of bacteremia.28,30 Patients who have had the catheter removed and who do not have any of these risk factors, and in whom TEE performed 5 to 7 days after the onset of bacteremia is negative, can be considered for a shorter duration of therapy (but a minimum of 14 days).
Patients with catheters colonized with S aureus (ie, those with positive catheter-tip cultures and negative blood cultures) are at risk of subsequent bacteremia. This risk may be reduced with anti-staphylococcal therapy started within 24 hours of catheter removal.31,32 Therapy should be continued for 5 to 7 days, and patients should be closely monitored for signs or symptoms of ongoing infection.
Oxacillin or nafcillin should be the first-line therapy for susceptible S aureus isolates. Vancomycin should be used to treat MRSA. Patients with MRSA isolates with a vancomycin MIC greater than 2 μg/mL should receive daptomycin or linezolid, depending on susceptibility data.
Enterococcal species
Up to 10% of nosocomially acquired bloodstream infections are due to enterococci, and many are related to intravascular catheters.33,34 Although the risk of endocarditis as a complication of enterococcal CVC-related bloodstream infection is relatively low, estimated at 1.5% in a multicenter prospective study, enterococcal bacteremia lasting longer than 4 days has been independently associated with risk of death.35,36 These observational data support routine removal of short-term CVCs infected with enterococci.
The choice of antibiotics for enterococcal infections depends on the susceptibility of the isolate. Sixty percent of Enterococcus faecium isolates and 2% of Enterococcus faecalis isolates are vancomycin-resistant, and reports of resistance to newer agents, including linezolid, have been published.34,37,38 Ampicillin is the preferred antibiotic for treatment of ampicillin-susceptible enterococci. Vancomycin should be used if the pathogen is ampicillin-resistant and vancomycin-susceptible. Enterococci resistant to both ampicillin and vancomycin can be treated with linezolid or daptomycin, based on susceptibility data.
For combination therapy with an aminoglycoside, the data are mixed. Retrospective observational studies have shown no difference in outcomes in uncomplicated enterococcal bacteremia with combination therapy vs monotherapy.39,40 However, in a large series of patients with enterococcal infections in which the catheter was retained, the combination of gentamicin and ampicillin was more effective than monotherapy.41
No controlled trial has been done to define the optimal duration of antibiotic therapy for enterococcal bloodstream infections related to short-term CVCs, but the IDSA recommends 7 to 14 days. If catheter salvage is attempted, concurrent antimicrobial lock therapy is recommended based on expert opinion. Catheters should be removed if complications arise (eg, insertion site or pocket infection, suppurative thrombophlebitis, sepsis, endocarditis, persistent bacteremia, metastatic infection). Signs and symptoms of endocarditis, persistent bacteremia, or the presence of a prosthetic heart valve should prompt evaluation with TEE.42,43
Gram-negative bacilli
Given the propensity of many gram-negative bacilli to form a biofilm, a number of studies have advocated removing CVCs infected with gram-negative bacilli.15,16,44 Recent studies examining the role of combination systemic antibiotic therapy and antibiotic lock therapy of gram-negative infections have found high success rates.45,46
The IDSA recommends routine removal of short-term CVCs infected with gram-negative bacilli and 7 to 14 days of systemic antibiotic therapy based on microbial susceptibility data. Antibiotic options generally include fourth-generation cephalosporins, carbapenems, or a combination beta-lactam and beta-lactamase inhibitor. The first-line treatment for Stenotrophomonas maltophilia and Burkholderia cepacia is trimethoprim-sulfamethoxazole (Bactrim). Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli should not be treated with cephalosporins or piperacillin-tazobactam (Zosyn) even if the organisms are susceptible in vitro, as doing so has been associated with poor clinical outcomes.11,47
There is growing concern over multiple-drug-resistant gram-negative bacilli with carbapenemases that confer resistance to carbapenems. No controlled study has evaluated treatment of multiple-drug-resistant gram-negative bacilli that require therapy with polymyxin (Colistin).
Candida species
The benefit of removing the CVC in the setting of candidemia is supported by six prospective studies.48–53 Patients with catheter-related bloodstream infections due to Candida species should have the catheter removed. C albicans and azole-susceptible candidal strains can be effectively treated with fluconazole at a dosage of 400 mg daily, continued for 14 days following the first negative blood culture.54 Echinocandins as first-line therapy and lipid formulations of amphotericin B (Abelcet) as an alternative are both highly effective for the treatment of Candida species with decreased susceptibility to azoles (eg, C glabrata and C krusei).55–57
Other gram-positive microorganisms
The isolation of Corynebacterium, Bacillus, and Micrococcus species from a single blood culture does not prove bloodstream infection, and confirmation requires at least two positive results drawn from different sites. CVC infections with these organisms are difficult to treat unless the infected catheter is removed.58,59
ADDITIONAL RECOMMENDATIONS
Infectious disease consultation should be considered for patients with complicated bloodstream infection related to a short-term CVC. Complicated cases include catheter infections in patients with hemodynamic instability, endocarditis, suppurative thrombophlebitis, persistent bloodstream infection despite 72 hours of appropriate antimicrobial therapy, osteomyelitis, active malignancy, or immunosuppression.
Infectious disease consultation should also be sought for assistance with determining if a patient is a candidate for antibiotic lock therapy; for management, dosing, and course of antibiotic lock therapy; for assistance with antibiotic choice and course for multiple-drug-resistant gram-negative bacilli; and for recommendations on management of infections due to uncommon pathogens (eg, Corynebacterium jeikeium, Chryseobacterium species, Malassezia furfur, and Mycobacterium species).
Vascular catheters are very common in everyday inpatient and, increasingly, outpatient care. Nearly 300 million catheters are estimated to be used annually in the United States, and approximately 3 million of these are central venous catheters (CVCs).1
Although significant gains have been made in preventing CVC-related bloodstream infections, these infections continue to occur, with estimated rates ranging from 1.3 per 1,000 catheter days on inpatient medical-surgical wards to 5.6 per 1,000 catheter days in intensive care burn units.2
CVCs are classified as either long-term or short-term. Long-term CVCs are surgically implanted or tunneled and used for prolonged chemotherapy, home infusion therapy, or hemodialysis. Short-term CVCs do not require surgical implantation. They are more common than long-term CVCs and account for most CVC-related bloodstream infections. Given the frequency of short-term CVC use, a growing number of health care providers from mid-level practitioners to intensivists are faced with deciding how to manage bloodstream infection related to short-term CVCs.
At baseline, management decisions about bloodstream infections from short-term CVCs can be challenging. Questions that regularly arise include:
- Should a potentially infected catheter be removed?
- Which empiric antibiotic therapy should be started pending a microbiologic diagnosis?
- How should therapy be tailored (eg, antibiotic choice and course and whether to remove or retain the catheter) based on the specific pathogen identified?
Adding to the complexity of these decisions are increasingly resistant microorganisms, heterogeneity of affected patient populations, and variability in the quality and availability of evidence.
This review provides a concise guide to managing bloodstream infections related to short-term CVCs in adults, based on updated guidelines from the Infectious Diseases Society of America (IDSA).3
DEFINITION AND DIAGNOSTIC CRITERIA
We have adapted the following definition and diagnostic criteria from the general definition and diagnostic criteria for catheter-related bloodstream infections proposed by the IDSA.
Bloodstream infection related to a short-term CVC is defined as bacteremia or fungemia in a patient with the CVC in place, clinical manifestations of infection (eg, fever, chills, hypotension), and no apparent source of the bloodstream infection aside from the catheter. At least one of the three diagnostic criteria should be met:
- Cultures of the catheter tip and of the peripheral blood grow the same organism. Catheter tip culture should be quantitative, with more than 102 colony-forming units (cfu) per catheter segment, or semiquantitative, with more than 15 cfu per catheter segment.
- Blood drawn from the catheter lumen grows the same organism as blood drawn from a peripheral vein (or less optimally, a different lumen), but at three times the amount by quantitative culture.
- Blood drawn simultaneously from the catheter lumen and from a peripheral vein (or less optimally, a different lumen) grows the same organism, and growth from the CVC lumen sample is detected (by automated blood culture system) at least 2 hours before growth from the peripheral vein sample.
MANAGING BLOODSTREAM INFECTIONS IN PATIENTS WITH SHORT-TERM CVCs
When to remove a potentially infected short-term CVC
In a nonneutropenic intensive care population, Bouza et al4 found that, of 204 episodes of clinically suspected bloodstream infection from a short-term CVC, only 28 (14%) were confirmed to be catheter-related, 27 (13%) were bloodstream infections that were not catheter-related, 36 (18%) involved catheter-tip colonization with negative blood cultures, and the remainder were cases with negative catheter-tip and blood cultures.
Rijnders et al,5 in a study of 100 adult medical-surgical intensive care patients with a clinically suspected bloodstream infection related to a short-term CVC, found only three confirmed cases.
A randomized clinical trial comparing early removal of short-term CVCs and watchful waiting in an adult intensive care population with clinically suspected bloodstream infections showed no difference between treatment groups in length of stay in the intensive care unit or in the mortality rate.6 This trial included a low-risk subset of adult medical-surgical intensive care patients (ie, immunocompetent, no intravascular foreign body, no evidence of severe sepsis or septic shock, no evidence of infection at the catheter insertion site, no proven bacteremia or fungemia). These results suggest that a similar subset of patients can be safely monitored without catheter removal while being assessed for possible catheter-related bloodstream infection.
Empiric catheter removal vs watchful waiting has not and likely will not be studied in higher-risk populations. In this group, clinical judgment should outweigh any specific management algorithm. In patients who are in shock or who are otherwise hemodynamically unstable, early catheter removal should be a priority; however, in some circumstances the risks of immediate catheter removal (eg, coagulopathy with risk of bleeding diathesis, or lack of site to replace the catheter) may outweigh the potential benefits.
Empiric antibiotic therapy for bloodstream infection from a short-term CVC
In order of prevalence, the four most common pathogens are coagulase-negative staphylococci, Staphylococcus aureus, Candida species, and enteric gram-negative bacilli.7
Gram-positive pathogens. A recent randomized clinical trial comparing vancomycin and linezolid (Zyvox) treatment for CVC-related bloodstream infections showed that 89 (57%) of 157 S aureus isolates and 95 (80%) of 119 coagulase-negative staphylococcal isolates were resistant to methicillin.8 Given the prevalence of gram-positive infections and the regularity of methicillin-resistant isolates, vancomycin should be started empirically in cases of suspected bloodstream infection related to short-term CVCs. In institutions where methicillin-resistant S aureus (MRSA) isolates regularly have a vancomycin minimum inhibitory concentration (MIC) of greater than 2 μg/mL, an alternative agent such as daptomycin (Cubicin) should be used.9,10
Gram-negative pathogens. Infections due to resistant gram-negative pathogens have become more common in the past 10 years.11,12 Prospective cohort studies have shown that resistant gram-negative infections and inadequate empiric antimicrobial therapy of bloodstream infections independently predict the risk of death.13,14 Risk factors for resistant gram-negative infections include critical illness, neutropenia, prior antibiotic therapy, and femoral insertion of the CVC.15–18 Patients with these risk factors should receive empiric antibiotic therapy for gram-negative bacilli.
No randomized controlled trial has been done to guide the choice of empiric gram-negative antibiotic coverage. The initial choice should be based on local antimicrobial patterns and susceptibility data and on the severity of the patient’s illness. Initial options include fourth-generation cephalosporins, carbapenems, or combined beta-lactam and beta-lactamase inhibitors. Patients with neutropenia, severe sepsis, or known multiple-drug-resistant gram-negative bacilli colonization or prior infection should receive empiric combination therapy with two different classes of antibiotics.
Candida. Risk factors for CVC-related bloodstream infections due to Candida species include total parenteral nutrition, prolonged use of broad-spectrum antibiotics, hematologic malignancy, solid organ or bone marrow transplantation, colonization with Candida species at multiple sites, and femoral catheter insertion. Empiric treatment with an echinocandin is recommended for patients with these risk factors. Fluconazole (Diflucan) can be substituted for an echinocandin in patients without azole exposure in the previous 3 months and in settings where the prevalence of Candida krusei and Candida glabrata is low.
PATHOGEN-SPECIFIC MANAGEMENT: RECOMMENDATIONS
Coagulase-negative staphylococci
Most patients with coagulase-negative staphylococcal infections have a benign clinical course.
Although no randomized trial has evaluated different treatment approaches, most experts recommend removing the catheter and giving a short course of antibiotics (ie, 5–7 days). Longer courses of antibiotics may be required for patients with endovascular hardware in place or persistent fever or bacteremia after catheter removal. The IDSA guidelines recommend 5 to 7 days of antibiotic therapy if the catheter is removed, and 10 to 14 days of systemic antibiotic therapy in combination with “antibiotic lock therapy” if the catheter is retained. Antibiotic lock therapy involves instilling a high concentration of an antibiotic to which the organism is susceptible into the catheter lumen and allowing it to dwell.
Not all patients are good candidates for antibiotic lock therapy, and neither are all organisms. In general, patients should be at low risk (immunocompetent, without hardware in place), and organisms should have a low risk of causing metastatic infection.
Staphylococcus lugdunensis can cause endocarditis and metastatic infections similar to those caused by S aureus and so should be managed similarly to S aureus.19
Staphylococcus aureus
Short-term CVCs infected with S aureus should be removed immediately. Removal of vascular catheters infected with S aureus has been associated with more rapid clinical response and higher cure rates compared with catheter retention.20–23S aureus bacteremia results in hematogenous complications in 20% to 30% of patients, and failure to remove or a delay in removing the catheter increases the risk of complications.21,24–27
There are no data from randomized clinical trials on the optimal duration of antibiotic therapy for S aureus bloodstream infections related to short-term CVCs. Traditionally, 4 weeks have been recommended out of concern for the risk of infective endocarditis,28,29 and the IDSA recommends 4 to 6 weeks unless patients meet certain low-risk criteria.
Factors associated with a higher risk of hematogenous complications include the presence of a retained foreign body, an intravascular prosthetic device, retained catheter, immune suppression, diabetes, persistent bacteremia at 72 hours despite catheter removal and appropriate antibiotics, skin changes consistent with septic emboli, or evidence of endocarditis or suppurative thrombophlebitis on transesophageal echocardiography (TEE) or ultrasonography, respectively.21,25–27 TEE is superior to transthoracic echocardiography and is most sensitive when performed 5 to 7 days after the onset of bacteremia.28,30 Patients who have had the catheter removed and who do not have any of these risk factors, and in whom TEE performed 5 to 7 days after the onset of bacteremia is negative, can be considered for a shorter duration of therapy (but a minimum of 14 days).
Patients with catheters colonized with S aureus (ie, those with positive catheter-tip cultures and negative blood cultures) are at risk of subsequent bacteremia. This risk may be reduced with anti-staphylococcal therapy started within 24 hours of catheter removal.31,32 Therapy should be continued for 5 to 7 days, and patients should be closely monitored for signs or symptoms of ongoing infection.
Oxacillin or nafcillin should be the first-line therapy for susceptible S aureus isolates. Vancomycin should be used to treat MRSA. Patients with MRSA isolates with a vancomycin MIC greater than 2 μg/mL should receive daptomycin or linezolid, depending on susceptibility data.
Enterococcal species
Up to 10% of nosocomially acquired bloodstream infections are due to enterococci, and many are related to intravascular catheters.33,34 Although the risk of endocarditis as a complication of enterococcal CVC-related bloodstream infection is relatively low, estimated at 1.5% in a multicenter prospective study, enterococcal bacteremia lasting longer than 4 days has been independently associated with risk of death.35,36 These observational data support routine removal of short-term CVCs infected with enterococci.
The choice of antibiotics for enterococcal infections depends on the susceptibility of the isolate. Sixty percent of Enterococcus faecium isolates and 2% of Enterococcus faecalis isolates are vancomycin-resistant, and reports of resistance to newer agents, including linezolid, have been published.34,37,38 Ampicillin is the preferred antibiotic for treatment of ampicillin-susceptible enterococci. Vancomycin should be used if the pathogen is ampicillin-resistant and vancomycin-susceptible. Enterococci resistant to both ampicillin and vancomycin can be treated with linezolid or daptomycin, based on susceptibility data.
For combination therapy with an aminoglycoside, the data are mixed. Retrospective observational studies have shown no difference in outcomes in uncomplicated enterococcal bacteremia with combination therapy vs monotherapy.39,40 However, in a large series of patients with enterococcal infections in which the catheter was retained, the combination of gentamicin and ampicillin was more effective than monotherapy.41
No controlled trial has been done to define the optimal duration of antibiotic therapy for enterococcal bloodstream infections related to short-term CVCs, but the IDSA recommends 7 to 14 days. If catheter salvage is attempted, concurrent antimicrobial lock therapy is recommended based on expert opinion. Catheters should be removed if complications arise (eg, insertion site or pocket infection, suppurative thrombophlebitis, sepsis, endocarditis, persistent bacteremia, metastatic infection). Signs and symptoms of endocarditis, persistent bacteremia, or the presence of a prosthetic heart valve should prompt evaluation with TEE.42,43
Gram-negative bacilli
Given the propensity of many gram-negative bacilli to form a biofilm, a number of studies have advocated removing CVCs infected with gram-negative bacilli.15,16,44 Recent studies examining the role of combination systemic antibiotic therapy and antibiotic lock therapy of gram-negative infections have found high success rates.45,46
The IDSA recommends routine removal of short-term CVCs infected with gram-negative bacilli and 7 to 14 days of systemic antibiotic therapy based on microbial susceptibility data. Antibiotic options generally include fourth-generation cephalosporins, carbapenems, or a combination beta-lactam and beta-lactamase inhibitor. The first-line treatment for Stenotrophomonas maltophilia and Burkholderia cepacia is trimethoprim-sulfamethoxazole (Bactrim). Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli should not be treated with cephalosporins or piperacillin-tazobactam (Zosyn) even if the organisms are susceptible in vitro, as doing so has been associated with poor clinical outcomes.11,47
There is growing concern over multiple-drug-resistant gram-negative bacilli with carbapenemases that confer resistance to carbapenems. No controlled study has evaluated treatment of multiple-drug-resistant gram-negative bacilli that require therapy with polymyxin (Colistin).
Candida species
The benefit of removing the CVC in the setting of candidemia is supported by six prospective studies.48–53 Patients with catheter-related bloodstream infections due to Candida species should have the catheter removed. C albicans and azole-susceptible candidal strains can be effectively treated with fluconazole at a dosage of 400 mg daily, continued for 14 days following the first negative blood culture.54 Echinocandins as first-line therapy and lipid formulations of amphotericin B (Abelcet) as an alternative are both highly effective for the treatment of Candida species with decreased susceptibility to azoles (eg, C glabrata and C krusei).55–57
Other gram-positive microorganisms
The isolation of Corynebacterium, Bacillus, and Micrococcus species from a single blood culture does not prove bloodstream infection, and confirmation requires at least two positive results drawn from different sites. CVC infections with these organisms are difficult to treat unless the infected catheter is removed.58,59
ADDITIONAL RECOMMENDATIONS
Infectious disease consultation should be considered for patients with complicated bloodstream infection related to a short-term CVC. Complicated cases include catheter infections in patients with hemodynamic instability, endocarditis, suppurative thrombophlebitis, persistent bloodstream infection despite 72 hours of appropriate antimicrobial therapy, osteomyelitis, active malignancy, or immunosuppression.
Infectious disease consultation should also be sought for assistance with determining if a patient is a candidate for antibiotic lock therapy; for management, dosing, and course of antibiotic lock therapy; for assistance with antibiotic choice and course for multiple-drug-resistant gram-negative bacilli; and for recommendations on management of infections due to uncommon pathogens (eg, Corynebacterium jeikeium, Chryseobacterium species, Malassezia furfur, and Mycobacterium species).
- Edgeworth J. Intravascular catheter infections. J Hosp Infect 2009; 73:323–330.
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009; 37:783–805.
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45.
- Bouza E, Alvarado N, Alcalá L, Pérez MJ, Rincón C, Muñoz P. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin Infect Dis 2007; 44:820–826.
- Rijnders BJ, Verwaest C, Peetermans WE, et al. Difference in time to positivity of hub-blood versus nonhub-blood cultures is not useful for the diagnosis of catheter-related bloodstream infection in critically ill patients. Crit Care Med 2001; 29:1399–1403.
- Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Van Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter-related infection: a randomized trial. Intensive Care Med 2004; 30:1073–1080.
- Safdar A, Mermel LA, Maki DG. The epidemiology of catheter-related infection in the critically ill. In:O’Grady NP, Pittet D, editors. Catheter-Related Infections in the Critically Ill. Boston: Kluwer, 2004:1–22.
- Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009; 48:203–212.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007; 51:2582–2586.
- Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med 2005; 352:380–391.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470–485.
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:146–155.
- Raymond DP, Pelletier SJ, Crabtree TD, Evans HL, Pruett TL, Sawyer RG. Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 2003; 31:1035–1041.
- Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect 2002; 45:47–53.
- Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore) 1995; 74:340–349.
- Seifert H. Catheter-related infections due to gram-negative bacilli. In:Seifert H, Jansen B, Farr BM, editors. Catheter-Related infections. New York: M. Dekker, 1997:111–138.
- Lorente L, Jiménez A, Santana M, et al. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med 2007; 35:2424–2427.
- Zinkernagel AS, Zinkernagel MS, Elzi MV, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 2008; 36:314–321.
- Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med 1995; 155:1161–1166.
- Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703.
- Fowler VG, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–486.
- Reilly JJ, Steed DL, Ritter PS. Indwelling venous access catheters in patients with acute leukemia. Cancer 1984; 53:219–223.
- Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147:536–539.
- Fowler VG, Miro JM, Hoen B, et al; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021.
- Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–2072.
- Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–332.
- Rosen AB, Fowler VG, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 1999; 130:810–820.
- Pigrau C, Rodríguez D, Planes AM, et al. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis 2003; 22:713–719.
- Sochowski RA, Chan KL. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. J Am Coll Cardiol 1993; 21:216–221.
- Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med 2008; 36:397–402.
- Ruhe JJ, Menon A. Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect 2006; 12:933–936.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–317.
- Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn Microbiol Infect Dis 1997; 29:95–102.
- DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis 2005; 191:588–595.
- Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis 2000; 36:145–158.
- Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179.
- Kanafani ZA, Federspiel JJ, Fowler VG. Infective endocarditis caused by daptomycin-resistant Enterococcus faecalis: a case report. Scand J Infect Dis 2007; 39:75–77.
- Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988; 67:248–269.
- Gray J, Marsh PJ, Stewart D, Pedler SJ. Enterococcal bacteraemia: a prospective study of 125 episodes. J Hosp Infect 1994; 27:179–186.
- Sandoe JA, Witherden IR, Au-Yeung HK, Kite P, Kerr KG, Wilcox MH. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J Antimicrob Chemother 2002; 50:577–582.
- Anderson DJ, Murdoch DR, Sexton DJ, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 2004; 32:72–77.
- Fernández-Guerrero ML, Herrero L, Bellver M, Gadea I, Roblas RF, de Górgolas M. Nosocomial enterococcal endocarditis: a serious hazard for hospitalized patients with enterococcal bacteraemia. J Intern Med 2002; 252:510–515.
- Elting LS, Bodey GP. Septicemia due to Xanthomonas species and non-aeruginosa Pseudomonas species: increasing incidence of catheter-related infections. Medicine (Baltimore) 1990; 69:296–306.
- Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother 2006; 57:1172–1180.
- Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant 2004; 19:1237–1244.
- Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39:2206–2212.
- Nguyen MH, Peacock JE, Tanner DC, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med 1995; 155:2429–2435.
- Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc 1996; 95:19–28.
- Rex JH, Bennett JE, Sugar AM, et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 1995; 21:994–996.
- Karlowicz MG, Hashimoto LN, Kelly RE, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 2000; 106:E63.
- Nucci M, Colombo AL, Silveira F, et al. Risk factors for death in patients with candidemia. Infect Control Hosp Epidemiol 1998; 19:846–850.
- Almirante B, Rodríguez D, Park BJ, et al; Barcelona Candidemia Project Study Group. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005; 43:1829–1835.
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331:1325–1330.
- Kuse ER, Chetchotisakd P, da Cunha CA, et al; Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–1527.
- Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
- Mora-Duarte J, Betts R, Rotstein C, et al; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–2029.
- Peces R, Gago E, Tejada F, Laures AS, Alvarez-Grande J. Relapsing bacteraemia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant 1997; 12:2428–2429.
- Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzo PA. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol 1987; 25:672–674.
- Edgeworth J. Intravascular catheter infections. J Hosp Infect 2009; 73:323–330.
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009; 37:783–805.
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45.
- Bouza E, Alvarado N, Alcalá L, Pérez MJ, Rincón C, Muñoz P. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin Infect Dis 2007; 44:820–826.
- Rijnders BJ, Verwaest C, Peetermans WE, et al. Difference in time to positivity of hub-blood versus nonhub-blood cultures is not useful for the diagnosis of catheter-related bloodstream infection in critically ill patients. Crit Care Med 2001; 29:1399–1403.
- Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Van Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter-related infection: a randomized trial. Intensive Care Med 2004; 30:1073–1080.
- Safdar A, Mermel LA, Maki DG. The epidemiology of catheter-related infection in the critically ill. In:O’Grady NP, Pittet D, editors. Catheter-Related Infections in the Critically Ill. Boston: Kluwer, 2004:1–22.
- Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009; 48:203–212.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007; 51:2582–2586.
- Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med 2005; 352:380–391.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470–485.
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:146–155.
- Raymond DP, Pelletier SJ, Crabtree TD, Evans HL, Pruett TL, Sawyer RG. Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 2003; 31:1035–1041.
- Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect 2002; 45:47–53.
- Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore) 1995; 74:340–349.
- Seifert H. Catheter-related infections due to gram-negative bacilli. In:Seifert H, Jansen B, Farr BM, editors. Catheter-Related infections. New York: M. Dekker, 1997:111–138.
- Lorente L, Jiménez A, Santana M, et al. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med 2007; 35:2424–2427.
- Zinkernagel AS, Zinkernagel MS, Elzi MV, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 2008; 36:314–321.
- Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med 1995; 155:1161–1166.
- Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703.
- Fowler VG, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–486.
- Reilly JJ, Steed DL, Ritter PS. Indwelling venous access catheters in patients with acute leukemia. Cancer 1984; 53:219–223.
- Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147:536–539.
- Fowler VG, Miro JM, Hoen B, et al; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021.
- Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–2072.
- Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–332.
- Rosen AB, Fowler VG, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 1999; 130:810–820.
- Pigrau C, Rodríguez D, Planes AM, et al. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis 2003; 22:713–719.
- Sochowski RA, Chan KL. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. J Am Coll Cardiol 1993; 21:216–221.
- Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med 2008; 36:397–402.
- Ruhe JJ, Menon A. Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect 2006; 12:933–936.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–317.
- Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn Microbiol Infect Dis 1997; 29:95–102.
- DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis 2005; 191:588–595.
- Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis 2000; 36:145–158.
- Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179.
- Kanafani ZA, Federspiel JJ, Fowler VG. Infective endocarditis caused by daptomycin-resistant Enterococcus faecalis: a case report. Scand J Infect Dis 2007; 39:75–77.
- Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988; 67:248–269.
- Gray J, Marsh PJ, Stewart D, Pedler SJ. Enterococcal bacteraemia: a prospective study of 125 episodes. J Hosp Infect 1994; 27:179–186.
- Sandoe JA, Witherden IR, Au-Yeung HK, Kite P, Kerr KG, Wilcox MH. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J Antimicrob Chemother 2002; 50:577–582.
- Anderson DJ, Murdoch DR, Sexton DJ, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 2004; 32:72–77.
- Fernández-Guerrero ML, Herrero L, Bellver M, Gadea I, Roblas RF, de Górgolas M. Nosocomial enterococcal endocarditis: a serious hazard for hospitalized patients with enterococcal bacteraemia. J Intern Med 2002; 252:510–515.
- Elting LS, Bodey GP. Septicemia due to Xanthomonas species and non-aeruginosa Pseudomonas species: increasing incidence of catheter-related infections. Medicine (Baltimore) 1990; 69:296–306.
- Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother 2006; 57:1172–1180.
- Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant 2004; 19:1237–1244.
- Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39:2206–2212.
- Nguyen MH, Peacock JE, Tanner DC, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med 1995; 155:2429–2435.
- Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc 1996; 95:19–28.
- Rex JH, Bennett JE, Sugar AM, et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 1995; 21:994–996.
- Karlowicz MG, Hashimoto LN, Kelly RE, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 2000; 106:E63.
- Nucci M, Colombo AL, Silveira F, et al. Risk factors for death in patients with candidemia. Infect Control Hosp Epidemiol 1998; 19:846–850.
- Almirante B, Rodríguez D, Park BJ, et al; Barcelona Candidemia Project Study Group. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005; 43:1829–1835.
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331:1325–1330.
- Kuse ER, Chetchotisakd P, da Cunha CA, et al; Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–1527.
- Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
- Mora-Duarte J, Betts R, Rotstein C, et al; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–2029.
- Peces R, Gago E, Tejada F, Laures AS, Alvarez-Grande J. Relapsing bacteraemia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant 1997; 12:2428–2429.
- Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzo PA. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol 1987; 25:672–674.
KEY POINTS
- Most bloodstream infections related to central venous catheters occur in patients with short-term central venous catheters; these infections result in significant morbidity and health care costs.
- Initial management of suspected cases requires decisions about whether to retain or remove the catheter and the choice of empiric antibiotic therapy.
- Management should be based on the specific pathogen isolated.
- An infectious disease specialist should be consulted in complicated cases or when multidrug-resistant bacteria or uncommon pathogens are isolated.