User login
Damian McNamara is a journalist for Medscape Medical News and MDedge. He worked full-time for MDedge as the Miami Bureau covering a dozen medical specialties during 2001-2012, then as a freelancer for Medscape and MDedge, before being hired on staff by Medscape in 2018. Now the two companies are one. He uses what he learned in school – Damian has a BS in chemistry and an MS in science, health and environmental reporting/journalism. He works out of a home office in Miami, with a 100-pound chocolate lab known to snore under his desk during work hours.
Study highlights need to investigate psoriasis treatment outcomes in skin of color patients
MIAMI – Psoriasis often presents differently in skin of color patients, but an unanswered question remains: Does response to treatment with an agent like a fixed-dose combination foam also differ by ethnicity?
Researchers at Mount Sinai St. Luke’s Hospital in New York addressed this question using phase 2 and 3 study data for 1,104 people with psoriasis, about half of whom were randomized to topical treatment with calcipotriene and betamethasone dipropionate foam 0.005%/0.064% (Enstilar); the rest received a single component or vehicle only. The data were obtained from LEO Pharma, the product’s manufacturer.
“We were very interested in knowing if there was any difference in efficacy between the specific ethnic groups – the skin of color and non–skin of color patients,” said Bridget Kaufman, MD, a dermatopharmacology fellow at Mount Sinai St. Luke’s Hospital. “So we went back to look at the data to see if there was any difference in side effects or efficacy between ethnic groups.”
Strength in numbers?
The three randomized, pooled clinical studies included many ethnic groups. However, only 6.5% of participants were black and even fewer were Asian, American Indian, or native Hawaiian, Dr. Kaufman said. “It’s hard to see meaningful differences when you don’t have a substantial skin of color population.”
As a result, no significant associations emerged from the pooled data. “That is the main take-home message of this study: We don’t have a great understanding now of the difference in efficacy between white and nonwhite ethnic groups,” Dr. Kaufman said.
The researchers defined treatment success at 4 weeks as a two-point improvement to “clear” or “almost clear” on the Investigator Global Assessment of psoriasis. Of the adult participants with chronic plaque psoriasis randomized to the combination foam product, 54% of the white patients; 30% of black patients; 69% of Asian patients; and one of the two Hawaiian/Pacific Islander patients achieved treatment success after 4 weeks of topical treatment.
“All subgroups analyzed had a good response to treatment at 4 weeks. Numerically it appears African Americans in particular did not do quite as well, but we can’t [definitively] draw that conclusion,” Dr. Kaufman said in an interview.
More data, please
The study is just the first step in investigating the efficacy of this particular product in diverse ethnic groups, Dr. Kaufman added. “That really emphasizes the importance of studying these medications in skin of color populations in particular.”
More guidance on psoriasis in skin of color patients is available in a published review article by Dr. Kaufman and Andrew F. Alexis, MD, director of the Skin of Color Center at Mount Sinai St. Luke’s and Mount Sinai West, New York (Am J Clin Dermatol. 2017 Dec 5. doi: 10.1007/s40257-017-0332-7).
Enstilar manufacturer LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
[email protected]
SOURCE: Kaufman B et al. ODAC 2018
MIAMI – Psoriasis often presents differently in skin of color patients, but an unanswered question remains: Does response to treatment with an agent like a fixed-dose combination foam also differ by ethnicity?
Researchers at Mount Sinai St. Luke’s Hospital in New York addressed this question using phase 2 and 3 study data for 1,104 people with psoriasis, about half of whom were randomized to topical treatment with calcipotriene and betamethasone dipropionate foam 0.005%/0.064% (Enstilar); the rest received a single component or vehicle only. The data were obtained from LEO Pharma, the product’s manufacturer.
“We were very interested in knowing if there was any difference in efficacy between the specific ethnic groups – the skin of color and non–skin of color patients,” said Bridget Kaufman, MD, a dermatopharmacology fellow at Mount Sinai St. Luke’s Hospital. “So we went back to look at the data to see if there was any difference in side effects or efficacy between ethnic groups.”
Strength in numbers?
The three randomized, pooled clinical studies included many ethnic groups. However, only 6.5% of participants were black and even fewer were Asian, American Indian, or native Hawaiian, Dr. Kaufman said. “It’s hard to see meaningful differences when you don’t have a substantial skin of color population.”
As a result, no significant associations emerged from the pooled data. “That is the main take-home message of this study: We don’t have a great understanding now of the difference in efficacy between white and nonwhite ethnic groups,” Dr. Kaufman said.
The researchers defined treatment success at 4 weeks as a two-point improvement to “clear” or “almost clear” on the Investigator Global Assessment of psoriasis. Of the adult participants with chronic plaque psoriasis randomized to the combination foam product, 54% of the white patients; 30% of black patients; 69% of Asian patients; and one of the two Hawaiian/Pacific Islander patients achieved treatment success after 4 weeks of topical treatment.
“All subgroups analyzed had a good response to treatment at 4 weeks. Numerically it appears African Americans in particular did not do quite as well, but we can’t [definitively] draw that conclusion,” Dr. Kaufman said in an interview.
More data, please
The study is just the first step in investigating the efficacy of this particular product in diverse ethnic groups, Dr. Kaufman added. “That really emphasizes the importance of studying these medications in skin of color populations in particular.”
More guidance on psoriasis in skin of color patients is available in a published review article by Dr. Kaufman and Andrew F. Alexis, MD, director of the Skin of Color Center at Mount Sinai St. Luke’s and Mount Sinai West, New York (Am J Clin Dermatol. 2017 Dec 5. doi: 10.1007/s40257-017-0332-7).
Enstilar manufacturer LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
[email protected]
SOURCE: Kaufman B et al. ODAC 2018
MIAMI – Psoriasis often presents differently in skin of color patients, but an unanswered question remains: Does response to treatment with an agent like a fixed-dose combination foam also differ by ethnicity?
Researchers at Mount Sinai St. Luke’s Hospital in New York addressed this question using phase 2 and 3 study data for 1,104 people with psoriasis, about half of whom were randomized to topical treatment with calcipotriene and betamethasone dipropionate foam 0.005%/0.064% (Enstilar); the rest received a single component or vehicle only. The data were obtained from LEO Pharma, the product’s manufacturer.
“We were very interested in knowing if there was any difference in efficacy between the specific ethnic groups – the skin of color and non–skin of color patients,” said Bridget Kaufman, MD, a dermatopharmacology fellow at Mount Sinai St. Luke’s Hospital. “So we went back to look at the data to see if there was any difference in side effects or efficacy between ethnic groups.”
Strength in numbers?
The three randomized, pooled clinical studies included many ethnic groups. However, only 6.5% of participants were black and even fewer were Asian, American Indian, or native Hawaiian, Dr. Kaufman said. “It’s hard to see meaningful differences when you don’t have a substantial skin of color population.”
As a result, no significant associations emerged from the pooled data. “That is the main take-home message of this study: We don’t have a great understanding now of the difference in efficacy between white and nonwhite ethnic groups,” Dr. Kaufman said.
The researchers defined treatment success at 4 weeks as a two-point improvement to “clear” or “almost clear” on the Investigator Global Assessment of psoriasis. Of the adult participants with chronic plaque psoriasis randomized to the combination foam product, 54% of the white patients; 30% of black patients; 69% of Asian patients; and one of the two Hawaiian/Pacific Islander patients achieved treatment success after 4 weeks of topical treatment.
“All subgroups analyzed had a good response to treatment at 4 weeks. Numerically it appears African Americans in particular did not do quite as well, but we can’t [definitively] draw that conclusion,” Dr. Kaufman said in an interview.
More data, please
The study is just the first step in investigating the efficacy of this particular product in diverse ethnic groups, Dr. Kaufman added. “That really emphasizes the importance of studying these medications in skin of color populations in particular.”
More guidance on psoriasis in skin of color patients is available in a published review article by Dr. Kaufman and Andrew F. Alexis, MD, director of the Skin of Color Center at Mount Sinai St. Luke’s and Mount Sinai West, New York (Am J Clin Dermatol. 2017 Dec 5. doi: 10.1007/s40257-017-0332-7).
Enstilar manufacturer LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
[email protected]
SOURCE: Kaufman B et al. ODAC 2018
REPORTING FROM ODAC 2018
Key clinical point: There were no significant differences in response to calcipotriene and betamethasone dipropionate foam in phase 2 and 3 trials, possibly because of small percentages of skin of color participants in these studies.
Major finding: Treatment success rates at 4 weeks were 30% among black patients, 54% among white patients, and 69% among Asian patients, but not enough skin of color patients were enrolled for differences to reach statistical significance.
Study details: A retrospective analysis of pooled phase 2 and phase 3 studies with 1,104 participants with psoriasis.
Disclosures: LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
Source: Kaufman B et al. ODAC 2018.
Alemtuzumab-induced autoimmunity: getting closer to answers
SAN DIEGO – The monoclonal antibody alemtuzumab can be an effective treatment for people living with multiple sclerosis, but there’s a catch — the agent is also associated with an increased risk for developing other autoimmune diseases, leaving clinicians with a conundrum.
“This is an efficacious treatment in multiple sclerosis” that can slow the rate of brain atrophy over the long-term, Alasdair Coles, MD, said at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “But 1 or 2 years after each cycle of alemtuzumab [Lemtrada], patients are at very high risk of autoimmune diseases. This is the not-too-worrying thyroid disease, but there are some very troubling and potentially highly threatening complications at lower frequency.”
Subsequent autoimmune thyroid disease can affect up to 40% of patients treated with alemtuzumab, but immune thrombocytopenia (3%) and autoimmune renal disease (0.1%) are also reported. About 1 in 10 people treated with the monoclonal antibody for MS can also develop de novo asymptomatic autoantibodies (10%).
“People ask: ‘Why doesn’t MS come back as part of this generic mechanism?’ and I don’t know the answer to that,” Dr. Coles said.
In the United States, alemtuzumab is indicated for treatment of relapsing multiple sclerosis in adults who have failed to respond adequately to two or more previous therapies. In contrast, “this has become a first-line treatment in the U.K.,” said Dr. Coles, a professor in the department of clinical neurosciences at the University of Cambridge (England).
“Unfortunately, we can offer no proven treatment to prevent this autoimmunity.”
Considering the prospects for different proposed mechanisms
Dr. Coles shared some encouraging news at ACTRIMS Forum 2018. His team and other researchers are getting closer to understanding the cellular mechanism underlying the paradoxical autoimmunity associated with alemtuzumab. Published reports in the literature from others suggest faulty immune B cells could be the culprit, pointing to a similar reconstitution of B cells after bone marrow transplantation. However, he said, “There is no difference in this reconstitution pattern between those who do and don’t get autoimmunity. So we do not think that autoimmunity after alemtuzumab is primarily a B cell problem.”
Other investigators have pointed to possible depletion of a key immune regulatory cell associated with alemtuzumab, such as alterations in CD52-positive T cells that cause depletion in T cells as part of an autoimmune cascade that involve CD52-high expressing cells and sialic acid-binding immunoglobulin-like lectin 10. “I’m not going to describe why we don’t believe any of this,” Dr. Coles said, but added, “We cannot replicate the data in type 1 diabetes or MS about the depletion of T cells.”
Along with his colleague Joanne Jones, PhD, a clinical fellow in the same department at the University of Cambridge, Dr. Coles and his team instead propose that autoimmunity after alemtuzumab therapy is associated with a homeostatic proliferation of T cells in the context of a defective thymus. “We see thymic function reduced after alemtuzumab for a few months. We don’t know if alemtuzumab is having a direct impact on the thymus or if it’s an indirect effect though a cytokine storm at the time of administering alemtuzumab.”
In addition, in contrast to B cells, both CD4-positive and CD8-positive T cells are clonally restricted after alemtuzumab treatment, Dr. Coles explained.
“These are the only changes that distinguish patients who do and do not develop autoimmunity,” he said. “Those who develop autoimmunity have reduced clonality and have impaired thymic function compared to those who don’t.”
As the theory goes, the limited clonal repertoire leads to expansion of the T cells, preferentially expanding autoreactive T cells, leading to B-cell- and antibody-mediated autoimmunity.
The bigger picture
The autoimmune phenomenon is not unique to alemtuzumab or multiple sclerosis. “This turns out to be one of a family of clinical situations where the reconstitution of the depleted lymphocyte repertoire leads to autoimmunity,” Dr. Coles said. A similar effect was seen years ago when very lymphopenic HIV patients were given antiviral therapy, he added, affecting about 10% of treated patients. About 10% of bone marrow transplant patients may experience similar autoimmune concerns.
“What we do think is true is we’ve tapped into a classical expression of autoimmunity,” Dr. Coles said. “Alemtuzumab is a fantastic opportunity to study the mechanisms underlying lymphopenia-associated autoimmunity.”
A ‘tantalizing prospect’
“It’s a tantalizing prospect that susceptible individuals might be identified in the future prior to treatment,” Dr. Coles said. One promising lead, he added, is “we also looked at IL-21. We showed that after treatment, and perhaps more interestingly, before treatment with alemtuzumab, serum IL-21 is greater in those who subsequently develop autoimmune disease. This suggests some individuals are prone to develop autoimmune disease, and could be identified potentially prior to treatment with alemtuzumab.”
More work is needed, including the development of more sensitive IL-21 assays for use in this population, Dr. Coles said. “Please do not attempt to predict the risk of autoimmunity after alemtuzumab using the current commercial assays. This is a source of some frustration for me.”
A potential route of lymphocyte repertoire reconstitution after alemtuzumab is thymic reconstitution, leading to a more diverse immune repertoire, Dr. Coles said. “The obvious corollary of this is if we can direct reconstitution through the thymic reconstitution, we should be able to prevent autoimmunity.”
Dr. Coles disclosed that he receives honoraria for travel and speaking from Sanofi Genzyme, which markets alemtuzumab.
SAN DIEGO – The monoclonal antibody alemtuzumab can be an effective treatment for people living with multiple sclerosis, but there’s a catch — the agent is also associated with an increased risk for developing other autoimmune diseases, leaving clinicians with a conundrum.
“This is an efficacious treatment in multiple sclerosis” that can slow the rate of brain atrophy over the long-term, Alasdair Coles, MD, said at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “But 1 or 2 years after each cycle of alemtuzumab [Lemtrada], patients are at very high risk of autoimmune diseases. This is the not-too-worrying thyroid disease, but there are some very troubling and potentially highly threatening complications at lower frequency.”
Subsequent autoimmune thyroid disease can affect up to 40% of patients treated with alemtuzumab, but immune thrombocytopenia (3%) and autoimmune renal disease (0.1%) are also reported. About 1 in 10 people treated with the monoclonal antibody for MS can also develop de novo asymptomatic autoantibodies (10%).
“People ask: ‘Why doesn’t MS come back as part of this generic mechanism?’ and I don’t know the answer to that,” Dr. Coles said.
In the United States, alemtuzumab is indicated for treatment of relapsing multiple sclerosis in adults who have failed to respond adequately to two or more previous therapies. In contrast, “this has become a first-line treatment in the U.K.,” said Dr. Coles, a professor in the department of clinical neurosciences at the University of Cambridge (England).
“Unfortunately, we can offer no proven treatment to prevent this autoimmunity.”
Considering the prospects for different proposed mechanisms
Dr. Coles shared some encouraging news at ACTRIMS Forum 2018. His team and other researchers are getting closer to understanding the cellular mechanism underlying the paradoxical autoimmunity associated with alemtuzumab. Published reports in the literature from others suggest faulty immune B cells could be the culprit, pointing to a similar reconstitution of B cells after bone marrow transplantation. However, he said, “There is no difference in this reconstitution pattern between those who do and don’t get autoimmunity. So we do not think that autoimmunity after alemtuzumab is primarily a B cell problem.”
Other investigators have pointed to possible depletion of a key immune regulatory cell associated with alemtuzumab, such as alterations in CD52-positive T cells that cause depletion in T cells as part of an autoimmune cascade that involve CD52-high expressing cells and sialic acid-binding immunoglobulin-like lectin 10. “I’m not going to describe why we don’t believe any of this,” Dr. Coles said, but added, “We cannot replicate the data in type 1 diabetes or MS about the depletion of T cells.”
Along with his colleague Joanne Jones, PhD, a clinical fellow in the same department at the University of Cambridge, Dr. Coles and his team instead propose that autoimmunity after alemtuzumab therapy is associated with a homeostatic proliferation of T cells in the context of a defective thymus. “We see thymic function reduced after alemtuzumab for a few months. We don’t know if alemtuzumab is having a direct impact on the thymus or if it’s an indirect effect though a cytokine storm at the time of administering alemtuzumab.”
In addition, in contrast to B cells, both CD4-positive and CD8-positive T cells are clonally restricted after alemtuzumab treatment, Dr. Coles explained.
“These are the only changes that distinguish patients who do and do not develop autoimmunity,” he said. “Those who develop autoimmunity have reduced clonality and have impaired thymic function compared to those who don’t.”
As the theory goes, the limited clonal repertoire leads to expansion of the T cells, preferentially expanding autoreactive T cells, leading to B-cell- and antibody-mediated autoimmunity.
The bigger picture
The autoimmune phenomenon is not unique to alemtuzumab or multiple sclerosis. “This turns out to be one of a family of clinical situations where the reconstitution of the depleted lymphocyte repertoire leads to autoimmunity,” Dr. Coles said. A similar effect was seen years ago when very lymphopenic HIV patients were given antiviral therapy, he added, affecting about 10% of treated patients. About 10% of bone marrow transplant patients may experience similar autoimmune concerns.
“What we do think is true is we’ve tapped into a classical expression of autoimmunity,” Dr. Coles said. “Alemtuzumab is a fantastic opportunity to study the mechanisms underlying lymphopenia-associated autoimmunity.”
A ‘tantalizing prospect’
“It’s a tantalizing prospect that susceptible individuals might be identified in the future prior to treatment,” Dr. Coles said. One promising lead, he added, is “we also looked at IL-21. We showed that after treatment, and perhaps more interestingly, before treatment with alemtuzumab, serum IL-21 is greater in those who subsequently develop autoimmune disease. This suggests some individuals are prone to develop autoimmune disease, and could be identified potentially prior to treatment with alemtuzumab.”
More work is needed, including the development of more sensitive IL-21 assays for use in this population, Dr. Coles said. “Please do not attempt to predict the risk of autoimmunity after alemtuzumab using the current commercial assays. This is a source of some frustration for me.”
A potential route of lymphocyte repertoire reconstitution after alemtuzumab is thymic reconstitution, leading to a more diverse immune repertoire, Dr. Coles said. “The obvious corollary of this is if we can direct reconstitution through the thymic reconstitution, we should be able to prevent autoimmunity.”
Dr. Coles disclosed that he receives honoraria for travel and speaking from Sanofi Genzyme, which markets alemtuzumab.
SAN DIEGO – The monoclonal antibody alemtuzumab can be an effective treatment for people living with multiple sclerosis, but there’s a catch — the agent is also associated with an increased risk for developing other autoimmune diseases, leaving clinicians with a conundrum.
“This is an efficacious treatment in multiple sclerosis” that can slow the rate of brain atrophy over the long-term, Alasdair Coles, MD, said at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “But 1 or 2 years after each cycle of alemtuzumab [Lemtrada], patients are at very high risk of autoimmune diseases. This is the not-too-worrying thyroid disease, but there are some very troubling and potentially highly threatening complications at lower frequency.”
Subsequent autoimmune thyroid disease can affect up to 40% of patients treated with alemtuzumab, but immune thrombocytopenia (3%) and autoimmune renal disease (0.1%) are also reported. About 1 in 10 people treated with the monoclonal antibody for MS can also develop de novo asymptomatic autoantibodies (10%).
“People ask: ‘Why doesn’t MS come back as part of this generic mechanism?’ and I don’t know the answer to that,” Dr. Coles said.
In the United States, alemtuzumab is indicated for treatment of relapsing multiple sclerosis in adults who have failed to respond adequately to two or more previous therapies. In contrast, “this has become a first-line treatment in the U.K.,” said Dr. Coles, a professor in the department of clinical neurosciences at the University of Cambridge (England).
“Unfortunately, we can offer no proven treatment to prevent this autoimmunity.”
Considering the prospects for different proposed mechanisms
Dr. Coles shared some encouraging news at ACTRIMS Forum 2018. His team and other researchers are getting closer to understanding the cellular mechanism underlying the paradoxical autoimmunity associated with alemtuzumab. Published reports in the literature from others suggest faulty immune B cells could be the culprit, pointing to a similar reconstitution of B cells after bone marrow transplantation. However, he said, “There is no difference in this reconstitution pattern between those who do and don’t get autoimmunity. So we do not think that autoimmunity after alemtuzumab is primarily a B cell problem.”
Other investigators have pointed to possible depletion of a key immune regulatory cell associated with alemtuzumab, such as alterations in CD52-positive T cells that cause depletion in T cells as part of an autoimmune cascade that involve CD52-high expressing cells and sialic acid-binding immunoglobulin-like lectin 10. “I’m not going to describe why we don’t believe any of this,” Dr. Coles said, but added, “We cannot replicate the data in type 1 diabetes or MS about the depletion of T cells.”
Along with his colleague Joanne Jones, PhD, a clinical fellow in the same department at the University of Cambridge, Dr. Coles and his team instead propose that autoimmunity after alemtuzumab therapy is associated with a homeostatic proliferation of T cells in the context of a defective thymus. “We see thymic function reduced after alemtuzumab for a few months. We don’t know if alemtuzumab is having a direct impact on the thymus or if it’s an indirect effect though a cytokine storm at the time of administering alemtuzumab.”
In addition, in contrast to B cells, both CD4-positive and CD8-positive T cells are clonally restricted after alemtuzumab treatment, Dr. Coles explained.
“These are the only changes that distinguish patients who do and do not develop autoimmunity,” he said. “Those who develop autoimmunity have reduced clonality and have impaired thymic function compared to those who don’t.”
As the theory goes, the limited clonal repertoire leads to expansion of the T cells, preferentially expanding autoreactive T cells, leading to B-cell- and antibody-mediated autoimmunity.
The bigger picture
The autoimmune phenomenon is not unique to alemtuzumab or multiple sclerosis. “This turns out to be one of a family of clinical situations where the reconstitution of the depleted lymphocyte repertoire leads to autoimmunity,” Dr. Coles said. A similar effect was seen years ago when very lymphopenic HIV patients were given antiviral therapy, he added, affecting about 10% of treated patients. About 10% of bone marrow transplant patients may experience similar autoimmune concerns.
“What we do think is true is we’ve tapped into a classical expression of autoimmunity,” Dr. Coles said. “Alemtuzumab is a fantastic opportunity to study the mechanisms underlying lymphopenia-associated autoimmunity.”
A ‘tantalizing prospect’
“It’s a tantalizing prospect that susceptible individuals might be identified in the future prior to treatment,” Dr. Coles said. One promising lead, he added, is “we also looked at IL-21. We showed that after treatment, and perhaps more interestingly, before treatment with alemtuzumab, serum IL-21 is greater in those who subsequently develop autoimmune disease. This suggests some individuals are prone to develop autoimmune disease, and could be identified potentially prior to treatment with alemtuzumab.”
More work is needed, including the development of more sensitive IL-21 assays for use in this population, Dr. Coles said. “Please do not attempt to predict the risk of autoimmunity after alemtuzumab using the current commercial assays. This is a source of some frustration for me.”
A potential route of lymphocyte repertoire reconstitution after alemtuzumab is thymic reconstitution, leading to a more diverse immune repertoire, Dr. Coles said. “The obvious corollary of this is if we can direct reconstitution through the thymic reconstitution, we should be able to prevent autoimmunity.”
Dr. Coles disclosed that he receives honoraria for travel and speaking from Sanofi Genzyme, which markets alemtuzumab.
EXPERT ANALYSIS FROM ACTRIMS FORUM 2018
Researchers find predictors of worse MS outcomes in post hoc study of three trials
SAN DIEGO – Baseline Expanded Disability Status Scale scores and number of relapses during the first year were the most consistent predictors of disability worsening or relapses over the subsequent 3 years in long-term analysis of three phase 3 fingolimod trials.
Those patients identified at higher risk for worse long-term clinical outcomes could benefit from an early review of multiple sclerosis (MS) treatment regimens to help prevent worsening disability, Pavle Repovic, MD, PhD, said in an interview.
“The idea for some time now has been to figure out what will tell whether a patient is responding to a therapy early on or not,” Dr. Repovic said at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
To explore long-term clinical predictors of disability progression and relapse risk, Dr. Repovic and his colleagues analyzed three phase 3 trials assessing fingolimod (Gilenya). They evaluated parameters at baseline and during the first year of the FREEDOMS, FREEDOMS II, and TRANSFORMS studies.
Investigators in the FREEDOMS studies enrolled 1,118 people with relapsing MS and randomized them to fingolimod or placebo for 2 years. The patients who were initially treated with placebo were then re-randomized to one of two fingolimod doses for an additional 18 months. Finally, all participants underwent treatment with the same fingolimod dose during an open-label phase. Similarly, in TRANSFORMS, researchers enrolled 1,292 people with relapsing MS and randomized them to either fingolimod or interferon beta-1a for 1 year. They then re-randomized the interferon beta-1a group to one of two fingolimod doses for a 1-year extension, then assigned all participants to one dose of fingolimod for an open-label phase.
At baseline in the FREEDOMS and FREEDOMS II studies, a longer duration of MS was associated with a higher risk for clinically definite progression or relapses at 6 months (odds ratio, 1.040), as was baseline Expanded Disability Status Scale (EDSS) score (OR, 1.229).
“In the FREEDOMS trials, what we learned is that the duration of multiple sclerosis since diagnosis and the baseline disability predict worsening between 1 and 4 years,” said Dr. Repovic, a neurologist with the Multiple Sclerosis Center at the Swedish Neuroscience Institute in Seattle.
Dr. Repovic added that “there is nothing you can do about them [the baseline predictors]. Patients are as disabled as they are, and they’ve had multiple sclerosis as long as they have.”
The researchers also looked at predictors of 6-month confirmed disability progression or relapses during the first year. “The idea is that year 1 might tell you what happens next,” Dr. Repovic said. The number of confirmed relapses (OR, 2.788) and MRI lesion activity (OR, 1.638), were predictive.
“So breakthrough disease in year 1 predicts more disease down the road.”
In the TRANSFORMS trial, previous treatment for MS (OR, 1.613) and EDSS score at baseline (OR, 1.228) predicted who would do worse. The number of confirmed relapses in the first year of the study was the only predictor (OR, 1.774). There was a trend for MRI lesion activity that approached statistical significance.
The confirmed number of relapses in the first year was the common predictor among all the phase 3 studies. “So with people who relapse, we really need to watch out for them,” Dr. Repovic said.
“The challenge with this particular study is we took everybody,” Dr. Repovic added. Next he would like to look at only those participants who received fingolimod throughout the trials to see if any specific predictors emerge.
Dr. Repovic disclosed he is on the speakers bureau for Novartis, the company that sponsored the study.
SOURCE: Repovic P et al. Abstract P210.
SAN DIEGO – Baseline Expanded Disability Status Scale scores and number of relapses during the first year were the most consistent predictors of disability worsening or relapses over the subsequent 3 years in long-term analysis of three phase 3 fingolimod trials.
Those patients identified at higher risk for worse long-term clinical outcomes could benefit from an early review of multiple sclerosis (MS) treatment regimens to help prevent worsening disability, Pavle Repovic, MD, PhD, said in an interview.
“The idea for some time now has been to figure out what will tell whether a patient is responding to a therapy early on or not,” Dr. Repovic said at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
To explore long-term clinical predictors of disability progression and relapse risk, Dr. Repovic and his colleagues analyzed three phase 3 trials assessing fingolimod (Gilenya). They evaluated parameters at baseline and during the first year of the FREEDOMS, FREEDOMS II, and TRANSFORMS studies.
Investigators in the FREEDOMS studies enrolled 1,118 people with relapsing MS and randomized them to fingolimod or placebo for 2 years. The patients who were initially treated with placebo were then re-randomized to one of two fingolimod doses for an additional 18 months. Finally, all participants underwent treatment with the same fingolimod dose during an open-label phase. Similarly, in TRANSFORMS, researchers enrolled 1,292 people with relapsing MS and randomized them to either fingolimod or interferon beta-1a for 1 year. They then re-randomized the interferon beta-1a group to one of two fingolimod doses for a 1-year extension, then assigned all participants to one dose of fingolimod for an open-label phase.
At baseline in the FREEDOMS and FREEDOMS II studies, a longer duration of MS was associated with a higher risk for clinically definite progression or relapses at 6 months (odds ratio, 1.040), as was baseline Expanded Disability Status Scale (EDSS) score (OR, 1.229).
“In the FREEDOMS trials, what we learned is that the duration of multiple sclerosis since diagnosis and the baseline disability predict worsening between 1 and 4 years,” said Dr. Repovic, a neurologist with the Multiple Sclerosis Center at the Swedish Neuroscience Institute in Seattle.
Dr. Repovic added that “there is nothing you can do about them [the baseline predictors]. Patients are as disabled as they are, and they’ve had multiple sclerosis as long as they have.”
The researchers also looked at predictors of 6-month confirmed disability progression or relapses during the first year. “The idea is that year 1 might tell you what happens next,” Dr. Repovic said. The number of confirmed relapses (OR, 2.788) and MRI lesion activity (OR, 1.638), were predictive.
“So breakthrough disease in year 1 predicts more disease down the road.”
In the TRANSFORMS trial, previous treatment for MS (OR, 1.613) and EDSS score at baseline (OR, 1.228) predicted who would do worse. The number of confirmed relapses in the first year of the study was the only predictor (OR, 1.774). There was a trend for MRI lesion activity that approached statistical significance.
The confirmed number of relapses in the first year was the common predictor among all the phase 3 studies. “So with people who relapse, we really need to watch out for them,” Dr. Repovic said.
“The challenge with this particular study is we took everybody,” Dr. Repovic added. Next he would like to look at only those participants who received fingolimod throughout the trials to see if any specific predictors emerge.
Dr. Repovic disclosed he is on the speakers bureau for Novartis, the company that sponsored the study.
SOURCE: Repovic P et al. Abstract P210.
SAN DIEGO – Baseline Expanded Disability Status Scale scores and number of relapses during the first year were the most consistent predictors of disability worsening or relapses over the subsequent 3 years in long-term analysis of three phase 3 fingolimod trials.
Those patients identified at higher risk for worse long-term clinical outcomes could benefit from an early review of multiple sclerosis (MS) treatment regimens to help prevent worsening disability, Pavle Repovic, MD, PhD, said in an interview.
“The idea for some time now has been to figure out what will tell whether a patient is responding to a therapy early on or not,” Dr. Repovic said at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
To explore long-term clinical predictors of disability progression and relapse risk, Dr. Repovic and his colleagues analyzed three phase 3 trials assessing fingolimod (Gilenya). They evaluated parameters at baseline and during the first year of the FREEDOMS, FREEDOMS II, and TRANSFORMS studies.
Investigators in the FREEDOMS studies enrolled 1,118 people with relapsing MS and randomized them to fingolimod or placebo for 2 years. The patients who were initially treated with placebo were then re-randomized to one of two fingolimod doses for an additional 18 months. Finally, all participants underwent treatment with the same fingolimod dose during an open-label phase. Similarly, in TRANSFORMS, researchers enrolled 1,292 people with relapsing MS and randomized them to either fingolimod or interferon beta-1a for 1 year. They then re-randomized the interferon beta-1a group to one of two fingolimod doses for a 1-year extension, then assigned all participants to one dose of fingolimod for an open-label phase.
At baseline in the FREEDOMS and FREEDOMS II studies, a longer duration of MS was associated with a higher risk for clinically definite progression or relapses at 6 months (odds ratio, 1.040), as was baseline Expanded Disability Status Scale (EDSS) score (OR, 1.229).
“In the FREEDOMS trials, what we learned is that the duration of multiple sclerosis since diagnosis and the baseline disability predict worsening between 1 and 4 years,” said Dr. Repovic, a neurologist with the Multiple Sclerosis Center at the Swedish Neuroscience Institute in Seattle.
Dr. Repovic added that “there is nothing you can do about them [the baseline predictors]. Patients are as disabled as they are, and they’ve had multiple sclerosis as long as they have.”
The researchers also looked at predictors of 6-month confirmed disability progression or relapses during the first year. “The idea is that year 1 might tell you what happens next,” Dr. Repovic said. The number of confirmed relapses (OR, 2.788) and MRI lesion activity (OR, 1.638), were predictive.
“So breakthrough disease in year 1 predicts more disease down the road.”
In the TRANSFORMS trial, previous treatment for MS (OR, 1.613) and EDSS score at baseline (OR, 1.228) predicted who would do worse. The number of confirmed relapses in the first year of the study was the only predictor (OR, 1.774). There was a trend for MRI lesion activity that approached statistical significance.
The confirmed number of relapses in the first year was the common predictor among all the phase 3 studies. “So with people who relapse, we really need to watch out for them,” Dr. Repovic said.
“The challenge with this particular study is we took everybody,” Dr. Repovic added. Next he would like to look at only those participants who received fingolimod throughout the trials to see if any specific predictors emerge.
Dr. Repovic disclosed he is on the speakers bureau for Novartis, the company that sponsored the study.
SOURCE: Repovic P et al. Abstract P210.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: Certain factors at baseline and over first year of three pooled studies were associated with worse long-term multiple sclerosis outcomes.
Major finding: The number of confirmed relapses in the first year of the FREEDOMS, FREEDOMS II, and TRANSFORMS studies predicted worse subsequent outcomes.
Study details: Pooled data from three phase 3 trials of fingolimod with 2,355 patients.
Disclosures: Dr. Repovic disclosed he is on the speakers bureau for Novartis, the company that sponsored the study.
Source: Repovic P et al. Abstract P210.
Fingolimod switch from an injectable linked to improved outcomes in relapsing MS
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
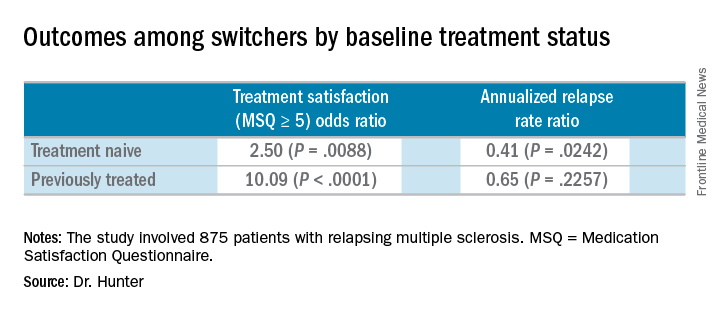
At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.

At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.

At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: Patients who switched from an injectable disease-modifying therapy to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001).
Study details: Post hoc analyses of the extension study of the PREFERMS trial.
Disclosures: Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
Source: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
Set realistic expectations prior to perioral rejuvenation procedures
MIAMI – The success of perioral rejuvenation depends in large part on setting realistic expectations. But there are also tips and tricks to individualizing the technique for each patient that can lead to better outcomes and greater satisfaction – whether patients receive injections into the fine lines above the lip, full-field erbium laser resurfacing, neuromodulator treatment, or a combination approach, according to Joel L. Cohen, MD.
When a patient presents with major lines in the perioral area, an “orange peel” texture, and/or elastotic changes, laser resurfacing can be an appropriate option. “Full field erbium laser resurfacing can give patients a nice improvement of upper lip lines and even a nice contraction of oral commissure,” Dr. Cohen said at the Orlando Dermatology Aesthetic and Clinical Conference.
Although each treatment is individualized to the patient, “I tend to do full field erbium resurfacing around the mouth and eyes, and fractional ablative resurfacing around the rest of the face,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in metropolitan Denver.
More downtime is associated with laser resurfacing compared with fillers or neuromodulator injections, but long-term patient satisfaction, even with improvement in quality of life for some patients (who become less anxious about these lines and more self-confident), can make this approach worthwhile. During his presentation, Dr. Cohen showed photos of many of his treated patients, including one woman whose grandchildren he said had been commenting about the “orange peel texture of her upper lip,” until she completed the resurfacing treatment.
Keep expectations realistic
Dr. Cohen recommended counseling patients about the potential benefits – and the caveats – associated with full-field erbium heavier resurfacing. “Make sure people understand they will look terrible for several days after heavy resurfacing, usually taking about 10-12 days to re-epithelialize,” he said. “We need to tell patients that the perioral area typically manifests more lines than other areas, so we need treat this area differently than just ablative fractional resurfacing in many cases.”
He explained that with heavier resurfacing procedures, it helps to show patients what is expected over the days to weeks in the healing process. They need to understand and see photos that show that the full-field erbium areas will have a yellow fibrinous healing response for the first week or so, which looks very different from the fractional ablative-treated areas (which are more typically red, weepy, and swollen).
He encourages these patients to come back a few days after the procedure to check their healing and review wound-care instructions, especially for patients who have deeper full-field perioral erbium resurfacing (those who are treated with 450-700 microns). Another tip he provided is to have these postresurfacing patients enter/exit through a separate entrance and also sit in a separate cosmetic waiting room at off-hours.
Re-epithelialization generally takes about 10-12 days for most people, with a maximal improvement at approximately 3 months, Dr. Cohen said. “Some patients can see not only significant improvement of upper lip lines, but often a nice contraction of the oral commissure even before fillers are performed to buttress the marionette area and oral commissure,” he said.
With full-field ablative resurfacing in specific areas, rather than simply fractional ablative resurfacing, it is also important to educate patients that some postinflammatory erythema is expected, which, in some cases, may persist for a few months. “In my experience, topical vasoconstrictors don’t seem to help minimize prolonged redness in the full-field erbium areas, but potent topical steroids can be beneficial,” Dr. Cohen said.
More tips for success
Injected local anesthesia is warranted prior to heavier laser resurfacing to keep patients as comfortable as possible. An infraorbital block with an added submucosal/sulcus block with plain lidocaine can be a good approach, he noted. Different perioral and facial areas have different degrees of lines, requiring different laser settings. He prefers to use plain lidocaine perioral blocks, “so that I can theoretically best see the endpoint pinpoint bleeding,” he said, adding that “significant pinpoint bleeding is a good place to stop.”
Typically, he uses a neuromodulator a week or two before full-field perioral erbium resurfacing. “I choose not to give a neuromodulator on the same day as I am concerned about swelling or manipulation of the skin causing unwanted spread to adjacent musculature,” Dr. Cohen said.
Another tip is to take photos with more than one device. “Standardized photos may lose detail of etched lines; we take both iPad and standardized camera system photos,” he said, adding that it is important that clinic staff are proficient at taking proper before-and-after photos, making sure, for example, that the patient does not have confounding makeup or lipstick on for photos, and patient positioning is consistent.
He said it is imperative to emphasize the importance of diligent sun protection for several months after the laser procedure. “Every patient reassures us they use sunscreen, but we often don’t know what sunscreen they are using or how frequently they are using it,” Dr. Cohen said. “If they don’t follow our specific instructions to use a physical block sunscreen, they will significantly increase their risk of developing postinflammatory hyperpigmentation. This caveat applies all year round, and isn’t just for those that go to the beach or play golf, but is also especially important for those patients that ski or hike at higher altitudes.”
Depending on the degree of etched-in lines in the perioral area, one perioral full-field laser resurfacing treatment is generally sufficient for most patients to see significant improvement. For those with more severe etched lines and/or bigger goals for improvement, additional treatments can be performed – but he generally waits about 3 months to see the overall effect of the initial treatment session.
If patients have just a few discrete etched-lines on each side of the upper lip, fillers can be helpful. But, the number and caliber of fine lines on the cutaneous lip limit how much a dermatologist can realistically treat. “So for people with many, many etched-in lines on the cutaneous lip, I explain that fillers are not the right tool for the job – and that they need heavier laser resurfacing.” And those patients really concerned about downtime need to understand that the bruising that can occur with fillers for several days can lead to some degree of social downtime as well.
Options to treat perioral lines not ‘etched in’
Sometimes younger patients, those in their late 20s to early 40s, present with concerns about their perioral appearance. Although they do not have lines at rest yet, they can be unhappy with the muscle columns that appear above their lips with animation that begin to cause lines at rest imprinted in the skin. And many of these women complain that their lipstick bleeds into this area,” Dr. Cohen said. “These patients without etched lines can be treated with a neuromodulator alone to soften the mechanical action of the orbicularis oris muscle,” he pointed out.
Dr. Cohen disclosed having participated in clinical trials and/or having served as a consultant for Merz, Allergan, Galderma, Suneva, Sciton, and Lutronic.
MIAMI – The success of perioral rejuvenation depends in large part on setting realistic expectations. But there are also tips and tricks to individualizing the technique for each patient that can lead to better outcomes and greater satisfaction – whether patients receive injections into the fine lines above the lip, full-field erbium laser resurfacing, neuromodulator treatment, or a combination approach, according to Joel L. Cohen, MD.
When a patient presents with major lines in the perioral area, an “orange peel” texture, and/or elastotic changes, laser resurfacing can be an appropriate option. “Full field erbium laser resurfacing can give patients a nice improvement of upper lip lines and even a nice contraction of oral commissure,” Dr. Cohen said at the Orlando Dermatology Aesthetic and Clinical Conference.
Although each treatment is individualized to the patient, “I tend to do full field erbium resurfacing around the mouth and eyes, and fractional ablative resurfacing around the rest of the face,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in metropolitan Denver.
More downtime is associated with laser resurfacing compared with fillers or neuromodulator injections, but long-term patient satisfaction, even with improvement in quality of life for some patients (who become less anxious about these lines and more self-confident), can make this approach worthwhile. During his presentation, Dr. Cohen showed photos of many of his treated patients, including one woman whose grandchildren he said had been commenting about the “orange peel texture of her upper lip,” until she completed the resurfacing treatment.
Keep expectations realistic
Dr. Cohen recommended counseling patients about the potential benefits – and the caveats – associated with full-field erbium heavier resurfacing. “Make sure people understand they will look terrible for several days after heavy resurfacing, usually taking about 10-12 days to re-epithelialize,” he said. “We need to tell patients that the perioral area typically manifests more lines than other areas, so we need treat this area differently than just ablative fractional resurfacing in many cases.”
He explained that with heavier resurfacing procedures, it helps to show patients what is expected over the days to weeks in the healing process. They need to understand and see photos that show that the full-field erbium areas will have a yellow fibrinous healing response for the first week or so, which looks very different from the fractional ablative-treated areas (which are more typically red, weepy, and swollen).
He encourages these patients to come back a few days after the procedure to check their healing and review wound-care instructions, especially for patients who have deeper full-field perioral erbium resurfacing (those who are treated with 450-700 microns). Another tip he provided is to have these postresurfacing patients enter/exit through a separate entrance and also sit in a separate cosmetic waiting room at off-hours.
Re-epithelialization generally takes about 10-12 days for most people, with a maximal improvement at approximately 3 months, Dr. Cohen said. “Some patients can see not only significant improvement of upper lip lines, but often a nice contraction of the oral commissure even before fillers are performed to buttress the marionette area and oral commissure,” he said.
With full-field ablative resurfacing in specific areas, rather than simply fractional ablative resurfacing, it is also important to educate patients that some postinflammatory erythema is expected, which, in some cases, may persist for a few months. “In my experience, topical vasoconstrictors don’t seem to help minimize prolonged redness in the full-field erbium areas, but potent topical steroids can be beneficial,” Dr. Cohen said.
More tips for success
Injected local anesthesia is warranted prior to heavier laser resurfacing to keep patients as comfortable as possible. An infraorbital block with an added submucosal/sulcus block with plain lidocaine can be a good approach, he noted. Different perioral and facial areas have different degrees of lines, requiring different laser settings. He prefers to use plain lidocaine perioral blocks, “so that I can theoretically best see the endpoint pinpoint bleeding,” he said, adding that “significant pinpoint bleeding is a good place to stop.”
Typically, he uses a neuromodulator a week or two before full-field perioral erbium resurfacing. “I choose not to give a neuromodulator on the same day as I am concerned about swelling or manipulation of the skin causing unwanted spread to adjacent musculature,” Dr. Cohen said.
Another tip is to take photos with more than one device. “Standardized photos may lose detail of etched lines; we take both iPad and standardized camera system photos,” he said, adding that it is important that clinic staff are proficient at taking proper before-and-after photos, making sure, for example, that the patient does not have confounding makeup or lipstick on for photos, and patient positioning is consistent.
He said it is imperative to emphasize the importance of diligent sun protection for several months after the laser procedure. “Every patient reassures us they use sunscreen, but we often don’t know what sunscreen they are using or how frequently they are using it,” Dr. Cohen said. “If they don’t follow our specific instructions to use a physical block sunscreen, they will significantly increase their risk of developing postinflammatory hyperpigmentation. This caveat applies all year round, and isn’t just for those that go to the beach or play golf, but is also especially important for those patients that ski or hike at higher altitudes.”
Depending on the degree of etched-in lines in the perioral area, one perioral full-field laser resurfacing treatment is generally sufficient for most patients to see significant improvement. For those with more severe etched lines and/or bigger goals for improvement, additional treatments can be performed – but he generally waits about 3 months to see the overall effect of the initial treatment session.
If patients have just a few discrete etched-lines on each side of the upper lip, fillers can be helpful. But, the number and caliber of fine lines on the cutaneous lip limit how much a dermatologist can realistically treat. “So for people with many, many etched-in lines on the cutaneous lip, I explain that fillers are not the right tool for the job – and that they need heavier laser resurfacing.” And those patients really concerned about downtime need to understand that the bruising that can occur with fillers for several days can lead to some degree of social downtime as well.
Options to treat perioral lines not ‘etched in’
Sometimes younger patients, those in their late 20s to early 40s, present with concerns about their perioral appearance. Although they do not have lines at rest yet, they can be unhappy with the muscle columns that appear above their lips with animation that begin to cause lines at rest imprinted in the skin. And many of these women complain that their lipstick bleeds into this area,” Dr. Cohen said. “These patients without etched lines can be treated with a neuromodulator alone to soften the mechanical action of the orbicularis oris muscle,” he pointed out.
Dr. Cohen disclosed having participated in clinical trials and/or having served as a consultant for Merz, Allergan, Galderma, Suneva, Sciton, and Lutronic.
MIAMI – The success of perioral rejuvenation depends in large part on setting realistic expectations. But there are also tips and tricks to individualizing the technique for each patient that can lead to better outcomes and greater satisfaction – whether patients receive injections into the fine lines above the lip, full-field erbium laser resurfacing, neuromodulator treatment, or a combination approach, according to Joel L. Cohen, MD.
When a patient presents with major lines in the perioral area, an “orange peel” texture, and/or elastotic changes, laser resurfacing can be an appropriate option. “Full field erbium laser resurfacing can give patients a nice improvement of upper lip lines and even a nice contraction of oral commissure,” Dr. Cohen said at the Orlando Dermatology Aesthetic and Clinical Conference.
Although each treatment is individualized to the patient, “I tend to do full field erbium resurfacing around the mouth and eyes, and fractional ablative resurfacing around the rest of the face,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in metropolitan Denver.
More downtime is associated with laser resurfacing compared with fillers or neuromodulator injections, but long-term patient satisfaction, even with improvement in quality of life for some patients (who become less anxious about these lines and more self-confident), can make this approach worthwhile. During his presentation, Dr. Cohen showed photos of many of his treated patients, including one woman whose grandchildren he said had been commenting about the “orange peel texture of her upper lip,” until she completed the resurfacing treatment.
Keep expectations realistic
Dr. Cohen recommended counseling patients about the potential benefits – and the caveats – associated with full-field erbium heavier resurfacing. “Make sure people understand they will look terrible for several days after heavy resurfacing, usually taking about 10-12 days to re-epithelialize,” he said. “We need to tell patients that the perioral area typically manifests more lines than other areas, so we need treat this area differently than just ablative fractional resurfacing in many cases.”
He explained that with heavier resurfacing procedures, it helps to show patients what is expected over the days to weeks in the healing process. They need to understand and see photos that show that the full-field erbium areas will have a yellow fibrinous healing response for the first week or so, which looks very different from the fractional ablative-treated areas (which are more typically red, weepy, and swollen).
He encourages these patients to come back a few days after the procedure to check their healing and review wound-care instructions, especially for patients who have deeper full-field perioral erbium resurfacing (those who are treated with 450-700 microns). Another tip he provided is to have these postresurfacing patients enter/exit through a separate entrance and also sit in a separate cosmetic waiting room at off-hours.
Re-epithelialization generally takes about 10-12 days for most people, with a maximal improvement at approximately 3 months, Dr. Cohen said. “Some patients can see not only significant improvement of upper lip lines, but often a nice contraction of the oral commissure even before fillers are performed to buttress the marionette area and oral commissure,” he said.
With full-field ablative resurfacing in specific areas, rather than simply fractional ablative resurfacing, it is also important to educate patients that some postinflammatory erythema is expected, which, in some cases, may persist for a few months. “In my experience, topical vasoconstrictors don’t seem to help minimize prolonged redness in the full-field erbium areas, but potent topical steroids can be beneficial,” Dr. Cohen said.
More tips for success
Injected local anesthesia is warranted prior to heavier laser resurfacing to keep patients as comfortable as possible. An infraorbital block with an added submucosal/sulcus block with plain lidocaine can be a good approach, he noted. Different perioral and facial areas have different degrees of lines, requiring different laser settings. He prefers to use plain lidocaine perioral blocks, “so that I can theoretically best see the endpoint pinpoint bleeding,” he said, adding that “significant pinpoint bleeding is a good place to stop.”
Typically, he uses a neuromodulator a week or two before full-field perioral erbium resurfacing. “I choose not to give a neuromodulator on the same day as I am concerned about swelling or manipulation of the skin causing unwanted spread to adjacent musculature,” Dr. Cohen said.
Another tip is to take photos with more than one device. “Standardized photos may lose detail of etched lines; we take both iPad and standardized camera system photos,” he said, adding that it is important that clinic staff are proficient at taking proper before-and-after photos, making sure, for example, that the patient does not have confounding makeup or lipstick on for photos, and patient positioning is consistent.
He said it is imperative to emphasize the importance of diligent sun protection for several months after the laser procedure. “Every patient reassures us they use sunscreen, but we often don’t know what sunscreen they are using or how frequently they are using it,” Dr. Cohen said. “If they don’t follow our specific instructions to use a physical block sunscreen, they will significantly increase their risk of developing postinflammatory hyperpigmentation. This caveat applies all year round, and isn’t just for those that go to the beach or play golf, but is also especially important for those patients that ski or hike at higher altitudes.”
Depending on the degree of etched-in lines in the perioral area, one perioral full-field laser resurfacing treatment is generally sufficient for most patients to see significant improvement. For those with more severe etched lines and/or bigger goals for improvement, additional treatments can be performed – but he generally waits about 3 months to see the overall effect of the initial treatment session.
If patients have just a few discrete etched-lines on each side of the upper lip, fillers can be helpful. But, the number and caliber of fine lines on the cutaneous lip limit how much a dermatologist can realistically treat. “So for people with many, many etched-in lines on the cutaneous lip, I explain that fillers are not the right tool for the job – and that they need heavier laser resurfacing.” And those patients really concerned about downtime need to understand that the bruising that can occur with fillers for several days can lead to some degree of social downtime as well.
Options to treat perioral lines not ‘etched in’
Sometimes younger patients, those in their late 20s to early 40s, present with concerns about their perioral appearance. Although they do not have lines at rest yet, they can be unhappy with the muscle columns that appear above their lips with animation that begin to cause lines at rest imprinted in the skin. And many of these women complain that their lipstick bleeds into this area,” Dr. Cohen said. “These patients without etched lines can be treated with a neuromodulator alone to soften the mechanical action of the orbicularis oris muscle,” he pointed out.
Dr. Cohen disclosed having participated in clinical trials and/or having served as a consultant for Merz, Allergan, Galderma, Suneva, Sciton, and Lutronic.
EXPERT ANALYSIS FROM ODAC 2018
Be alert for BAP1 mutations in hereditary melanomas
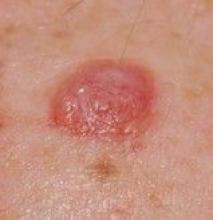
MIAMI – Although rare, patients who present with one or more skin cancers characteristic of those associated with loss of the BAP1 tumor suppressor protein may be at elevated risk for more aggressive uveal melanomas and other cancers such as kidney cancer and mesothelioma. For this reason, dermatologists who recognize the lesions and telltale pattern of this inherited mutation within families can do a great service, encouraging education, genetic counseling, and referral of patients to a nearby cancer center, according to Hensin Tsao, MD, PhD.
“ Dr. Tsao said at the 2018 Orlando Dermatology Aesthetic and Clinical Conference.
The BAP1-associated skin lesions can emerge when patients are relatively young, even as teenagers. The melanoma and renal cell cancers also can have an early onset, said Dr. Tsao, director of the melanoma genetics program at Massachusetts General Hospital, Boston. The skin lesion itself can be a tipoff for a BAP1 germline mutation. In general, they are small, dome shaped – not flat like a superficial basal cell – rarely pigmented and appear “orangey translucent.” Dr. Tsao added: “When you start seeing them, you’ll recognize them. However, to be sure, you’re going to have to biopsy to know what is going on.”
In one patient he described, the pattern of malignancies in the patient’s family was a hint that she had a BAP1 mutation, Dr. Tsao said. The proband had melanoma starting at age 31 years, a squamous cell carcinoma at 35 years, and basal cell carcinoma at age 40 years. “She had nine ‘nevoid melanomas’ over the years. Nevoid melanomas are rare, and with nine in a row, you know something odd is going on.” Dr. Tsao and his team performed a series of sentinel lymph node biopsies that ruled out metastasis. “What is also interesting is the father had ocular melanoma, which is what got us to thinking about BAP1 mutations in this family.” A sister who developed melanoma and a brother who also was diagnosed with melanoma plus kidney cancer at age 45 years were further clues to the germline mutation.
No longer ‘condemned proteins’
Under normal circumstances, BAP1 is a tumor suppressor protein involved in cellular process called “ubiquitination.” Often, ubiquitination serves to identify proteins “condemned” for destruction by the proteasome system. The BAP1 protein acts through a molecular relay and removes ubiquitin polypeptide groups on the protein. “In the absence of BAP1, proteins often linger longer because they accumulate ubiquitin groups, or alternatively, the protein’s function is somehow altered by mechanisms we don’t quite understand yet,” Dr. Tsao explained.
Once a dermatologist suspects a BAP1 mutation–associated cancer, they can order a BAP1 nuclear stain to confirm diagnosis. Formal documentation of a germline mutation, however, requires genetic testing of blood DNA.
A family history lesson
Ask patients not only about history of melanoma in their family, including if any close relative was diagnosed with eye melanoma, Dr. Tsao suggested. “We had an opportunity to look at cutaneous and ocular melanoma families. Overall, if your family has an ocular melanoma along with cutaneous melanoma, the risk of being a BAP1 mutation–bearing family is greater.” In addition, he and his colleagues did a case control study with Ivana K. Kim, MD, at the Massachusetts Eye and Ear Infirmary in Boston, and found people with metastatic ocular melanoma were more likely to have BAP1 mutations, compared with those with nonmetastatic ocular melanoma.
“The fear is, of course, patient who are BAP1 mutation carriers might be predisposed to more lethal variants of uveal melanoma.”
Although taking a family history is essential, some patients may be unfamiliar with mesothelioma. “So ask about any unusual lung cancers or eye cancers,” Dr. Tsao suggested. “And if it looks like there is an aggregation of rare tumors, get them to a nearby cancer center [for further work-up]. Mesothelioma is difficult to treat and a horrible disease,” he added. “So if there is any chance you can [catch] the mesothelioma early, that’s good.”
He also cautioned against over interpretation of patient reports about family malignancies, in part because lung and breast cancers are relatively common. “Sometimes, when you see a family with lung or breast cancers, it could just be a chance association since these are quite common in the general population.” In other words, determining if a lung cancer in a family with melanoma is an association beyond chance can take some “pretty large numbers to prove.”
In contrast, “the number of kidney cancers among BAP1 families I do believe are out of proportion with normal population expectations,” Dr. Tsao added.
Follow-up and genetic counseling
There is no standard protocol for follow-up once a patient is identified with a BAP1 mutation. “I refer them for uveal, kidney, and/or lung cancer evaluation and see them back two to four times a year for skin checks.”
A meeting attendee suggested that management of a patient with melanoma might not differ based on genetic-testing results. “I agree with you that I don’t need to know the genetic status within these families to help with their cutaneous melanomas,” Dr. Tsao replied. “But the question becomes, are there other internal malignancies you’re not screening for appropriately?”
Another attendee asked about genetic counseling. “I encourage genetic counseling since dermatologists often don’t have time to take at detailed family history of all cancers and ages of onset,” Dr. Tsao said. “Genetic counselors can help sort out the strength of the genetic pedigree in a family. My residents usually ask if someone has a history of melanoma in their family, and that’s it. But there is a big difference between having a cousin with melanoma and three brothers with melanoma.”
MIAMI – Although rare, patients who present with one or more skin cancers characteristic of those associated with loss of the BAP1 tumor suppressor protein may be at elevated risk for more aggressive uveal melanomas and other cancers such as kidney cancer and mesothelioma. For this reason, dermatologists who recognize the lesions and telltale pattern of this inherited mutation within families can do a great service, encouraging education, genetic counseling, and referral of patients to a nearby cancer center, according to Hensin Tsao, MD, PhD.
“ Dr. Tsao said at the 2018 Orlando Dermatology Aesthetic and Clinical Conference.
The BAP1-associated skin lesions can emerge when patients are relatively young, even as teenagers. The melanoma and renal cell cancers also can have an early onset, said Dr. Tsao, director of the melanoma genetics program at Massachusetts General Hospital, Boston. The skin lesion itself can be a tipoff for a BAP1 germline mutation. In general, they are small, dome shaped – not flat like a superficial basal cell – rarely pigmented and appear “orangey translucent.” Dr. Tsao added: “When you start seeing them, you’ll recognize them. However, to be sure, you’re going to have to biopsy to know what is going on.”
In one patient he described, the pattern of malignancies in the patient’s family was a hint that she had a BAP1 mutation, Dr. Tsao said. The proband had melanoma starting at age 31 years, a squamous cell carcinoma at 35 years, and basal cell carcinoma at age 40 years. “She had nine ‘nevoid melanomas’ over the years. Nevoid melanomas are rare, and with nine in a row, you know something odd is going on.” Dr. Tsao and his team performed a series of sentinel lymph node biopsies that ruled out metastasis. “What is also interesting is the father had ocular melanoma, which is what got us to thinking about BAP1 mutations in this family.” A sister who developed melanoma and a brother who also was diagnosed with melanoma plus kidney cancer at age 45 years were further clues to the germline mutation.
No longer ‘condemned proteins’
Under normal circumstances, BAP1 is a tumor suppressor protein involved in cellular process called “ubiquitination.” Often, ubiquitination serves to identify proteins “condemned” for destruction by the proteasome system. The BAP1 protein acts through a molecular relay and removes ubiquitin polypeptide groups on the protein. “In the absence of BAP1, proteins often linger longer because they accumulate ubiquitin groups, or alternatively, the protein’s function is somehow altered by mechanisms we don’t quite understand yet,” Dr. Tsao explained.
Once a dermatologist suspects a BAP1 mutation–associated cancer, they can order a BAP1 nuclear stain to confirm diagnosis. Formal documentation of a germline mutation, however, requires genetic testing of blood DNA.
A family history lesson
Ask patients not only about history of melanoma in their family, including if any close relative was diagnosed with eye melanoma, Dr. Tsao suggested. “We had an opportunity to look at cutaneous and ocular melanoma families. Overall, if your family has an ocular melanoma along with cutaneous melanoma, the risk of being a BAP1 mutation–bearing family is greater.” In addition, he and his colleagues did a case control study with Ivana K. Kim, MD, at the Massachusetts Eye and Ear Infirmary in Boston, and found people with metastatic ocular melanoma were more likely to have BAP1 mutations, compared with those with nonmetastatic ocular melanoma.
“The fear is, of course, patient who are BAP1 mutation carriers might be predisposed to more lethal variants of uveal melanoma.”
Although taking a family history is essential, some patients may be unfamiliar with mesothelioma. “So ask about any unusual lung cancers or eye cancers,” Dr. Tsao suggested. “And if it looks like there is an aggregation of rare tumors, get them to a nearby cancer center [for further work-up]. Mesothelioma is difficult to treat and a horrible disease,” he added. “So if there is any chance you can [catch] the mesothelioma early, that’s good.”
He also cautioned against over interpretation of patient reports about family malignancies, in part because lung and breast cancers are relatively common. “Sometimes, when you see a family with lung or breast cancers, it could just be a chance association since these are quite common in the general population.” In other words, determining if a lung cancer in a family with melanoma is an association beyond chance can take some “pretty large numbers to prove.”
In contrast, “the number of kidney cancers among BAP1 families I do believe are out of proportion with normal population expectations,” Dr. Tsao added.
Follow-up and genetic counseling
There is no standard protocol for follow-up once a patient is identified with a BAP1 mutation. “I refer them for uveal, kidney, and/or lung cancer evaluation and see them back two to four times a year for skin checks.”
A meeting attendee suggested that management of a patient with melanoma might not differ based on genetic-testing results. “I agree with you that I don’t need to know the genetic status within these families to help with their cutaneous melanomas,” Dr. Tsao replied. “But the question becomes, are there other internal malignancies you’re not screening for appropriately?”
Another attendee asked about genetic counseling. “I encourage genetic counseling since dermatologists often don’t have time to take at detailed family history of all cancers and ages of onset,” Dr. Tsao said. “Genetic counselors can help sort out the strength of the genetic pedigree in a family. My residents usually ask if someone has a history of melanoma in their family, and that’s it. But there is a big difference between having a cousin with melanoma and three brothers with melanoma.”
MIAMI – Although rare, patients who present with one or more skin cancers characteristic of those associated with loss of the BAP1 tumor suppressor protein may be at elevated risk for more aggressive uveal melanomas and other cancers such as kidney cancer and mesothelioma. For this reason, dermatologists who recognize the lesions and telltale pattern of this inherited mutation within families can do a great service, encouraging education, genetic counseling, and referral of patients to a nearby cancer center, according to Hensin Tsao, MD, PhD.
“ Dr. Tsao said at the 2018 Orlando Dermatology Aesthetic and Clinical Conference.
The BAP1-associated skin lesions can emerge when patients are relatively young, even as teenagers. The melanoma and renal cell cancers also can have an early onset, said Dr. Tsao, director of the melanoma genetics program at Massachusetts General Hospital, Boston. The skin lesion itself can be a tipoff for a BAP1 germline mutation. In general, they are small, dome shaped – not flat like a superficial basal cell – rarely pigmented and appear “orangey translucent.” Dr. Tsao added: “When you start seeing them, you’ll recognize them. However, to be sure, you’re going to have to biopsy to know what is going on.”
In one patient he described, the pattern of malignancies in the patient’s family was a hint that she had a BAP1 mutation, Dr. Tsao said. The proband had melanoma starting at age 31 years, a squamous cell carcinoma at 35 years, and basal cell carcinoma at age 40 years. “She had nine ‘nevoid melanomas’ over the years. Nevoid melanomas are rare, and with nine in a row, you know something odd is going on.” Dr. Tsao and his team performed a series of sentinel lymph node biopsies that ruled out metastasis. “What is also interesting is the father had ocular melanoma, which is what got us to thinking about BAP1 mutations in this family.” A sister who developed melanoma and a brother who also was diagnosed with melanoma plus kidney cancer at age 45 years were further clues to the germline mutation.
No longer ‘condemned proteins’
Under normal circumstances, BAP1 is a tumor suppressor protein involved in cellular process called “ubiquitination.” Often, ubiquitination serves to identify proteins “condemned” for destruction by the proteasome system. The BAP1 protein acts through a molecular relay and removes ubiquitin polypeptide groups on the protein. “In the absence of BAP1, proteins often linger longer because they accumulate ubiquitin groups, or alternatively, the protein’s function is somehow altered by mechanisms we don’t quite understand yet,” Dr. Tsao explained.
Once a dermatologist suspects a BAP1 mutation–associated cancer, they can order a BAP1 nuclear stain to confirm diagnosis. Formal documentation of a germline mutation, however, requires genetic testing of blood DNA.
A family history lesson
Ask patients not only about history of melanoma in their family, including if any close relative was diagnosed with eye melanoma, Dr. Tsao suggested. “We had an opportunity to look at cutaneous and ocular melanoma families. Overall, if your family has an ocular melanoma along with cutaneous melanoma, the risk of being a BAP1 mutation–bearing family is greater.” In addition, he and his colleagues did a case control study with Ivana K. Kim, MD, at the Massachusetts Eye and Ear Infirmary in Boston, and found people with metastatic ocular melanoma were more likely to have BAP1 mutations, compared with those with nonmetastatic ocular melanoma.
“The fear is, of course, patient who are BAP1 mutation carriers might be predisposed to more lethal variants of uveal melanoma.”
Although taking a family history is essential, some patients may be unfamiliar with mesothelioma. “So ask about any unusual lung cancers or eye cancers,” Dr. Tsao suggested. “And if it looks like there is an aggregation of rare tumors, get them to a nearby cancer center [for further work-up]. Mesothelioma is difficult to treat and a horrible disease,” he added. “So if there is any chance you can [catch] the mesothelioma early, that’s good.”
He also cautioned against over interpretation of patient reports about family malignancies, in part because lung and breast cancers are relatively common. “Sometimes, when you see a family with lung or breast cancers, it could just be a chance association since these are quite common in the general population.” In other words, determining if a lung cancer in a family with melanoma is an association beyond chance can take some “pretty large numbers to prove.”
In contrast, “the number of kidney cancers among BAP1 families I do believe are out of proportion with normal population expectations,” Dr. Tsao added.
Follow-up and genetic counseling
There is no standard protocol for follow-up once a patient is identified with a BAP1 mutation. “I refer them for uveal, kidney, and/or lung cancer evaluation and see them back two to four times a year for skin checks.”
A meeting attendee suggested that management of a patient with melanoma might not differ based on genetic-testing results. “I agree with you that I don’t need to know the genetic status within these families to help with their cutaneous melanomas,” Dr. Tsao replied. “But the question becomes, are there other internal malignancies you’re not screening for appropriately?”
Another attendee asked about genetic counseling. “I encourage genetic counseling since dermatologists often don’t have time to take at detailed family history of all cancers and ages of onset,” Dr. Tsao said. “Genetic counselors can help sort out the strength of the genetic pedigree in a family. My residents usually ask if someone has a history of melanoma in their family, and that’s it. But there is a big difference between having a cousin with melanoma and three brothers with melanoma.”
REPORTING FROM ODAC 2018
VIDEO: Managing the alemtuzumab paradox
SAN DIEGO – A paradox of treating people with the monoclonal antibody alemtuzumab is that the agent can be an effective therapy for many people living with multiple sclerosis, but in some patients is associated with the development of other autoimmune diseases.
“I counsel patients with multiple sclerosis that this is a high risk, high gain drug,” Alasdair Coles, MD, of the University of Cambridge (England), said at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Patients can make it safer through strict compliance with the drug’s risk monitoring program. But without patient compliance, alemtuzumab becomes a dangerous drug, he said in a video interview.
Knowing in advance which patients are at an elevated risk for subsequent autoimmune diseases has been difficult to predict. But researchers are getting closer, Dr. Coles said. In the future, measuring a serum factor, potentially interleukin 21, could produce a pretreatment risk assessment for each individual.
SAN DIEGO – A paradox of treating people with the monoclonal antibody alemtuzumab is that the agent can be an effective therapy for many people living with multiple sclerosis, but in some patients is associated with the development of other autoimmune diseases.
“I counsel patients with multiple sclerosis that this is a high risk, high gain drug,” Alasdair Coles, MD, of the University of Cambridge (England), said at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Patients can make it safer through strict compliance with the drug’s risk monitoring program. But without patient compliance, alemtuzumab becomes a dangerous drug, he said in a video interview.
Knowing in advance which patients are at an elevated risk for subsequent autoimmune diseases has been difficult to predict. But researchers are getting closer, Dr. Coles said. In the future, measuring a serum factor, potentially interleukin 21, could produce a pretreatment risk assessment for each individual.
SAN DIEGO – A paradox of treating people with the monoclonal antibody alemtuzumab is that the agent can be an effective therapy for many people living with multiple sclerosis, but in some patients is associated with the development of other autoimmune diseases.
“I counsel patients with multiple sclerosis that this is a high risk, high gain drug,” Alasdair Coles, MD, of the University of Cambridge (England), said at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Patients can make it safer through strict compliance with the drug’s risk monitoring program. But without patient compliance, alemtuzumab becomes a dangerous drug, he said in a video interview.
Knowing in advance which patients are at an elevated risk for subsequent autoimmune diseases has been difficult to predict. But researchers are getting closer, Dr. Coles said. In the future, measuring a serum factor, potentially interleukin 21, could produce a pretreatment risk assessment for each individual.
REPORTING FROM ACTRIMS FORUM 2018
Third course of alemtuzumab can improve MS outcomes
SAN DIEGO – Approximately 30% of people with active multiple sclerosis who initially responded well to two courses of alemtuzumab in the CARE-MS II trial experience relapse or MRI activity over time. But investigators set out to determine whether retreatment with a subsequent course of alemtuzumab is worthwhile.
“What we found is, after the third course, they continued to do well again – at this point for an average of another 3-4 years,” Ann D. Bass, MD, said in an interview at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The take-home message is if they do need to be retreated after those two courses, it doesn’t [necessarily] mean they are a treatment failure. Give them another chance and see if they will do well with a third course, which is what they did,” said Dr. Bass, of the Neurology Center of San Antonio.
Through year 6 of an extension study with 393 of the original CARE-MS II trial participants, 45% received one or more additional courses of alemtuzumab. Participants were required to wait 12 months or more after completion of the two initial courses of therapy. This figure includes 30% who received a third course of alemtuzumab, 12% who received a fourth course, and 2% and 1% who received a fifth or sixth course, respectively.
“The average time until they needed a third course was 2.5 years after the second course; so they didn’t need it right away,” Dr. Bass said. “The majority, even at 6 years, did not need to have a third course.”
When patients did require a subsequent course, “about half needed it because of clinical relapse; one-quarter needed it because of MRI relapse; and about one-quarter needed it because of both,” Dr. Bass said during a poster presentation.
In terms of effectiveness, the annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001). In these patients, the annual relapse rate remained low, at 0.17, up to 3 years later.
Investigators also tracked disability using the Expanded Disability Status Scale. They found that more than two-thirds, 68%, maintained stable or had improved scores after administration of a third alemtuzumab course. In addition, the percentage of patients with confirmed disability improvement increased from 4.4% in the 12 months prior to a third course to 14.4% in the year following pretreatment.
Retreatment was at the patient’s discretion. “The patients have the right to say ‘No, I’m doing great. I don’t want to be retreated’ or ‘I want to explore other options’ for whatever reason,” Dr. Bass said. “That’s rare though; most patients actually say yes.”
To qualify for retreatment based on MRI findings, patients had to have at least two lesions – one enlarging and one enhancing, two enlarging, or two new.
Only patients who opted for a subsequent course of alemtuzumab were included in the current analysis; those who chose a different disease-modifying therapy were excluded.
“Many achieve clinical and MRI remission. I never say cure – you don’t want to say that word,” Dr. Bass said.
Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
SOURCE: Bass A et al. ACTRIMS Forum 2018 Poster P035.
SAN DIEGO – Approximately 30% of people with active multiple sclerosis who initially responded well to two courses of alemtuzumab in the CARE-MS II trial experience relapse or MRI activity over time. But investigators set out to determine whether retreatment with a subsequent course of alemtuzumab is worthwhile.
“What we found is, after the third course, they continued to do well again – at this point for an average of another 3-4 years,” Ann D. Bass, MD, said in an interview at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The take-home message is if they do need to be retreated after those two courses, it doesn’t [necessarily] mean they are a treatment failure. Give them another chance and see if they will do well with a third course, which is what they did,” said Dr. Bass, of the Neurology Center of San Antonio.
Through year 6 of an extension study with 393 of the original CARE-MS II trial participants, 45% received one or more additional courses of alemtuzumab. Participants were required to wait 12 months or more after completion of the two initial courses of therapy. This figure includes 30% who received a third course of alemtuzumab, 12% who received a fourth course, and 2% and 1% who received a fifth or sixth course, respectively.
“The average time until they needed a third course was 2.5 years after the second course; so they didn’t need it right away,” Dr. Bass said. “The majority, even at 6 years, did not need to have a third course.”
When patients did require a subsequent course, “about half needed it because of clinical relapse; one-quarter needed it because of MRI relapse; and about one-quarter needed it because of both,” Dr. Bass said during a poster presentation.
In terms of effectiveness, the annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001). In these patients, the annual relapse rate remained low, at 0.17, up to 3 years later.
Investigators also tracked disability using the Expanded Disability Status Scale. They found that more than two-thirds, 68%, maintained stable or had improved scores after administration of a third alemtuzumab course. In addition, the percentage of patients with confirmed disability improvement increased from 4.4% in the 12 months prior to a third course to 14.4% in the year following pretreatment.
Retreatment was at the patient’s discretion. “The patients have the right to say ‘No, I’m doing great. I don’t want to be retreated’ or ‘I want to explore other options’ for whatever reason,” Dr. Bass said. “That’s rare though; most patients actually say yes.”
To qualify for retreatment based on MRI findings, patients had to have at least two lesions – one enlarging and one enhancing, two enlarging, or two new.
Only patients who opted for a subsequent course of alemtuzumab were included in the current analysis; those who chose a different disease-modifying therapy were excluded.
“Many achieve clinical and MRI remission. I never say cure – you don’t want to say that word,” Dr. Bass said.
Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
SOURCE: Bass A et al. ACTRIMS Forum 2018 Poster P035.
SAN DIEGO – Approximately 30% of people with active multiple sclerosis who initially responded well to two courses of alemtuzumab in the CARE-MS II trial experience relapse or MRI activity over time. But investigators set out to determine whether retreatment with a subsequent course of alemtuzumab is worthwhile.
“What we found is, after the third course, they continued to do well again – at this point for an average of another 3-4 years,” Ann D. Bass, MD, said in an interview at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The take-home message is if they do need to be retreated after those two courses, it doesn’t [necessarily] mean they are a treatment failure. Give them another chance and see if they will do well with a third course, which is what they did,” said Dr. Bass, of the Neurology Center of San Antonio.
Through year 6 of an extension study with 393 of the original CARE-MS II trial participants, 45% received one or more additional courses of alemtuzumab. Participants were required to wait 12 months or more after completion of the two initial courses of therapy. This figure includes 30% who received a third course of alemtuzumab, 12% who received a fourth course, and 2% and 1% who received a fifth or sixth course, respectively.
“The average time until they needed a third course was 2.5 years after the second course; so they didn’t need it right away,” Dr. Bass said. “The majority, even at 6 years, did not need to have a third course.”
When patients did require a subsequent course, “about half needed it because of clinical relapse; one-quarter needed it because of MRI relapse; and about one-quarter needed it because of both,” Dr. Bass said during a poster presentation.
In terms of effectiveness, the annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001). In these patients, the annual relapse rate remained low, at 0.17, up to 3 years later.
Investigators also tracked disability using the Expanded Disability Status Scale. They found that more than two-thirds, 68%, maintained stable or had improved scores after administration of a third alemtuzumab course. In addition, the percentage of patients with confirmed disability improvement increased from 4.4% in the 12 months prior to a third course to 14.4% in the year following pretreatment.
Retreatment was at the patient’s discretion. “The patients have the right to say ‘No, I’m doing great. I don’t want to be retreated’ or ‘I want to explore other options’ for whatever reason,” Dr. Bass said. “That’s rare though; most patients actually say yes.”
To qualify for retreatment based on MRI findings, patients had to have at least two lesions – one enlarging and one enhancing, two enlarging, or two new.
Only patients who opted for a subsequent course of alemtuzumab were included in the current analysis; those who chose a different disease-modifying therapy were excluded.
“Many achieve clinical and MRI remission. I never say cure – you don’t want to say that word,” Dr. Bass said.
Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
SOURCE: Bass A et al. ACTRIMS Forum 2018 Poster P035.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: The annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001).
Study details: An extension study of the CARE-MS II trial involving 393 of the original study participants.
Disclosures: Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
Source: Bass A et al. ACTRIMS Forum 2018 Poster P035.
VIDEO: New MS ambulatory measure could fill clinical gap
REPORTING FROM ACTRIMS FORUM 2018
SAN DIEGO – Although clinical tools to assess ambulatory function among people with multiple sclerosis exist, some measure it as part of a comprehensive assessment while others require the patient to answer many questions and then clinicians to calculate a score.
To devise a more targeted, simpler instrument, Emily Evans, MD, and her colleagues developed the PDAS or Patient Derived Ambulation Scale. They evaluated the correlation of this single-item scale to assess ambulation – an important measure of patient function – and evaluated how the results correlated with existing tools such as the Patient Determined Disease Steps and 12-item MS Walking Scale. Dr. Evans presented preliminary findings at the ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“We feel this is a quick test that can be readily implemented into clinical practice,” Dr. Evans, a neurologist at the John L. Trotter MS Center at Washington University in St. Louis, said in a video interview.
REPORTING FROM ACTRIMS FORUM 2018
SAN DIEGO – Although clinical tools to assess ambulatory function among people with multiple sclerosis exist, some measure it as part of a comprehensive assessment while others require the patient to answer many questions and then clinicians to calculate a score.
To devise a more targeted, simpler instrument, Emily Evans, MD, and her colleagues developed the PDAS or Patient Derived Ambulation Scale. They evaluated the correlation of this single-item scale to assess ambulation – an important measure of patient function – and evaluated how the results correlated with existing tools such as the Patient Determined Disease Steps and 12-item MS Walking Scale. Dr. Evans presented preliminary findings at the ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“We feel this is a quick test that can be readily implemented into clinical practice,” Dr. Evans, a neurologist at the John L. Trotter MS Center at Washington University in St. Louis, said in a video interview.
REPORTING FROM ACTRIMS FORUM 2018
SAN DIEGO – Although clinical tools to assess ambulatory function among people with multiple sclerosis exist, some measure it as part of a comprehensive assessment while others require the patient to answer many questions and then clinicians to calculate a score.
To devise a more targeted, simpler instrument, Emily Evans, MD, and her colleagues developed the PDAS or Patient Derived Ambulation Scale. They evaluated the correlation of this single-item scale to assess ambulation – an important measure of patient function – and evaluated how the results correlated with existing tools such as the Patient Determined Disease Steps and 12-item MS Walking Scale. Dr. Evans presented preliminary findings at the ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“We feel this is a quick test that can be readily implemented into clinical practice,” Dr. Evans, a neurologist at the John L. Trotter MS Center at Washington University in St. Louis, said in a video interview.
VIDEO: Alemtuzumab associated with long-term MS control in TOPAZ study
SAN DIEGO – A majority of patients with active relapsing-remitting multiple sclerosis and inadequate response to previous therapy achieved a durable response after treatment with alemtuzumab in the TOPAZ trial, a 5-year extension to the CARE-MS II study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Almost half of the 317 participants in TOPAZ received no further therapy beyond their initial two courses of alemtuzumab infusion therapy that they received as part of the CARE-MS II study.
“If you follow patients over time ... you’re seeing a significant group of patients who have improvement. It’s very unexpected, especially when you look at the patients who entered the clinical trial who had a fair amount of active disease,” said Barry A. Singer, MD, director of The MS Center for Innovations in Care at Missouri Baptist Medical Center in St. Louis.
At the 7-year evaluation of patients in TOPAZ, the annualized relapse rate was 0.14. In addition, 87% of patients remained relapse-free in year 7. Dr. Singer and his colleagues also reported that 73% of TOPAZ participants were stable or improved based on their Expanded Disability Status Scale (EDSS) scores.
“As we follow the data out and follow these patients out, we’re seeing how the clinical course for these patients is dramatically improving for the majority of patients,” Dr. Singer said in a video interview at ACTRIMS Forum 2018, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The TOPAZ study also revealed that 69% of patients were free of clinical disease worsening and 44% experienced clinical disease improvement in the 6 months before year 7. The majority also had no evidence of disease activity, Dr. Singer reported.
“One of the attributes that makes alemtuzumab so attractive as a clinician and for patients is you can go through a couple of series of medication [treatments] ... and really alter your disease course – that is the exciting thing,” he said.
The Food and Drug Administration approved alemtuzumab (Lemtrada) in November 2014 for the treatment of patients with relapsing forms of multiple sclerosis. Use of alemtuzumab is generally reserved for patients who have had an inadequate response to two or more previous drugs indicated for the treatment of multiple sclerosis.
In CARE-MS II, participants received two annual courses of alemtuzumab: intravenous infusion of 12 mg/day for 5 days at baseline and again for 3 days at 12 months. Additional treatment in TOPAZ for relapse or MRI evidence of disease was at the discretion of the investigator and could include alemtuzumab retreatment 12 mg/day on 3 consecutive days 12 months or more after a previous course, or another disease-modifying therapy at any time. Annual follow-up exams included an MRI scan.
A durable treatment effect was achieved by a majority of patients, even though 47% received no further treatment with alemtuzumab or another disease-modifying therapy after the initial two alemtuzumab courses.
The incidence of most adverse events, including infusion-associated reactions and infections, decreased over the course of the TOPAZ study and were lower than the incidence reported in the 2-year CARE-MS II trial. Of note, the incidence of thyroid-related adverse events peaked in the third year of the follow-up and continued to decline out to 7 years, Dr. Singer said. “We’re not seeing any new safety issues.”
Dr. Singer and his coinvestigators plan to continue the research, monitoring and scoring patients over time.
The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
SOURCE: Singer B et al. ACTRIMS Forum 2018, abstract P026.
SAN DIEGO – A majority of patients with active relapsing-remitting multiple sclerosis and inadequate response to previous therapy achieved a durable response after treatment with alemtuzumab in the TOPAZ trial, a 5-year extension to the CARE-MS II study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Almost half of the 317 participants in TOPAZ received no further therapy beyond their initial two courses of alemtuzumab infusion therapy that they received as part of the CARE-MS II study.
“If you follow patients over time ... you’re seeing a significant group of patients who have improvement. It’s very unexpected, especially when you look at the patients who entered the clinical trial who had a fair amount of active disease,” said Barry A. Singer, MD, director of The MS Center for Innovations in Care at Missouri Baptist Medical Center in St. Louis.
At the 7-year evaluation of patients in TOPAZ, the annualized relapse rate was 0.14. In addition, 87% of patients remained relapse-free in year 7. Dr. Singer and his colleagues also reported that 73% of TOPAZ participants were stable or improved based on their Expanded Disability Status Scale (EDSS) scores.
“As we follow the data out and follow these patients out, we’re seeing how the clinical course for these patients is dramatically improving for the majority of patients,” Dr. Singer said in a video interview at ACTRIMS Forum 2018, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The TOPAZ study also revealed that 69% of patients were free of clinical disease worsening and 44% experienced clinical disease improvement in the 6 months before year 7. The majority also had no evidence of disease activity, Dr. Singer reported.
“One of the attributes that makes alemtuzumab so attractive as a clinician and for patients is you can go through a couple of series of medication [treatments] ... and really alter your disease course – that is the exciting thing,” he said.
The Food and Drug Administration approved alemtuzumab (Lemtrada) in November 2014 for the treatment of patients with relapsing forms of multiple sclerosis. Use of alemtuzumab is generally reserved for patients who have had an inadequate response to two or more previous drugs indicated for the treatment of multiple sclerosis.
In CARE-MS II, participants received two annual courses of alemtuzumab: intravenous infusion of 12 mg/day for 5 days at baseline and again for 3 days at 12 months. Additional treatment in TOPAZ for relapse or MRI evidence of disease was at the discretion of the investigator and could include alemtuzumab retreatment 12 mg/day on 3 consecutive days 12 months or more after a previous course, or another disease-modifying therapy at any time. Annual follow-up exams included an MRI scan.
A durable treatment effect was achieved by a majority of patients, even though 47% received no further treatment with alemtuzumab or another disease-modifying therapy after the initial two alemtuzumab courses.
The incidence of most adverse events, including infusion-associated reactions and infections, decreased over the course of the TOPAZ study and were lower than the incidence reported in the 2-year CARE-MS II trial. Of note, the incidence of thyroid-related adverse events peaked in the third year of the follow-up and continued to decline out to 7 years, Dr. Singer said. “We’re not seeing any new safety issues.”
Dr. Singer and his coinvestigators plan to continue the research, monitoring and scoring patients over time.
The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
SOURCE: Singer B et al. ACTRIMS Forum 2018, abstract P026.
SAN DIEGO – A majority of patients with active relapsing-remitting multiple sclerosis and inadequate response to previous therapy achieved a durable response after treatment with alemtuzumab in the TOPAZ trial, a 5-year extension to the CARE-MS II study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Almost half of the 317 participants in TOPAZ received no further therapy beyond their initial two courses of alemtuzumab infusion therapy that they received as part of the CARE-MS II study.
“If you follow patients over time ... you’re seeing a significant group of patients who have improvement. It’s very unexpected, especially when you look at the patients who entered the clinical trial who had a fair amount of active disease,” said Barry A. Singer, MD, director of The MS Center for Innovations in Care at Missouri Baptist Medical Center in St. Louis.
At the 7-year evaluation of patients in TOPAZ, the annualized relapse rate was 0.14. In addition, 87% of patients remained relapse-free in year 7. Dr. Singer and his colleagues also reported that 73% of TOPAZ participants were stable or improved based on their Expanded Disability Status Scale (EDSS) scores.
“As we follow the data out and follow these patients out, we’re seeing how the clinical course for these patients is dramatically improving for the majority of patients,” Dr. Singer said in a video interview at ACTRIMS Forum 2018, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The TOPAZ study also revealed that 69% of patients were free of clinical disease worsening and 44% experienced clinical disease improvement in the 6 months before year 7. The majority also had no evidence of disease activity, Dr. Singer reported.
“One of the attributes that makes alemtuzumab so attractive as a clinician and for patients is you can go through a couple of series of medication [treatments] ... and really alter your disease course – that is the exciting thing,” he said.
The Food and Drug Administration approved alemtuzumab (Lemtrada) in November 2014 for the treatment of patients with relapsing forms of multiple sclerosis. Use of alemtuzumab is generally reserved for patients who have had an inadequate response to two or more previous drugs indicated for the treatment of multiple sclerosis.
In CARE-MS II, participants received two annual courses of alemtuzumab: intravenous infusion of 12 mg/day for 5 days at baseline and again for 3 days at 12 months. Additional treatment in TOPAZ for relapse or MRI evidence of disease was at the discretion of the investigator and could include alemtuzumab retreatment 12 mg/day on 3 consecutive days 12 months or more after a previous course, or another disease-modifying therapy at any time. Annual follow-up exams included an MRI scan.
A durable treatment effect was achieved by a majority of patients, even though 47% received no further treatment with alemtuzumab or another disease-modifying therapy after the initial two alemtuzumab courses.
The incidence of most adverse events, including infusion-associated reactions and infections, decreased over the course of the TOPAZ study and were lower than the incidence reported in the 2-year CARE-MS II trial. Of note, the incidence of thyroid-related adverse events peaked in the third year of the follow-up and continued to decline out to 7 years, Dr. Singer said. “We’re not seeing any new safety issues.”
Dr. Singer and his coinvestigators plan to continue the research, monitoring and scoring patients over time.
The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
SOURCE: Singer B et al. ACTRIMS Forum 2018, abstract P026.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: The annualized relapse rate was 0.14 at year 7 among the 87% of participants who remained in the TOPAZ study.
Study details: A 5-year extension study of 317 participants from the initial CARE-MS II trial.
Disclosures: The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
Source: Singer B et al. ACTRIMS Forum 2018, abstract P026.