User login
Gout Deserves Tender Treatment in Elderly
The prevalence of gout has increased in the United States, especially among the eldest population, according to the latest national data. That means that nursing home staff are caring for more hot, swollen, and inflamed joints than ever, said several experts on the topic.
In interviews, they added that clinicians’ potential success in preventing, diagnosing, and treating the disease in long-term care residents lies largely in fundamental practices and an individualized approach to medications.
Data from the National Health and Nutrition Examination Survey (NHANES) show an increase in gout prevalence among U.S. adults from 2.7% in the survey’s 1994-1998 reporting periods to 3.9% in 2007-2008. Meanwhile, the prevalence in adults aged 80 years and older jumped from 5.9% to 12.6%.
The prevalence of hyperuricemia, which usually precedes gout, was 31% in adults aged 65 years or older and 37% among those aged 80 years and older, according to the latest NHANES data. The increase in actual gout occurred mainly among men in NHANES. Other studies, however, have documented an increased prevalence of the disease among older women as well. Studies cited in a review of "Crystal-Associated Arthritis in the Elderly" showed women making up at least half of the cases in which gout first strikes after age 60 years. Among individuals who have a first episode after age 80, women seem to predominate (Rheum. Dis. Clin. N. Am. 2007;33:33-55).
The presentation of the disease, as well as its prevalence, is changing, said Dr. Arthur Weinstein, professor of medicine at Georgetown University Medical Center and director of rheumatology at Washington Hospital Center. "We’re seeing more and more polyarticular gout in older patients, for instance, either as an initial presentation or years after just a single monoarticular episode" with no subsequent recurrences, said Dr. Weinstein in an interview.
He and others said they believe that the common use of thiazide diuretics in older patients is a major driver of gout’s changing profile. Other factors could include genetic predispositions to hyperuricemia and gout and increasing obesity, insulin resistance, and metabolic syndrome in the aging population.
While often the best choice for hypertension management, thiazide diuretics can contribute to the development of chronic hyperuricemia, as can low-dose aspirin and cyclosporine. Also, increasing numbers of elderly people have chronic cardiac and renal disease – factors that have been associated with hyperuricemia and gout.
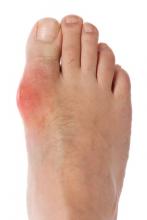
Other changes in gout’s presentation in the elderly include earlier and often-atypical development of the soft tissue masses known as tophi and more frequent and earlier involvement of the small joints of the fingers.
Diagnosis, Empiric Therapy
The differential diagnosis of a swollen, inflamed joint often involves ruling out the likelihood of septic arthritis, fracture, or other injury, and pseudogout – the other main form of crystal-induced arthritis.
Patients experiencing an acute gout attack can have a low-grade fever. "But with a fever of 101 or higher, you have to consider that it’s something that’s not crystal induced," said Dr. John W. Rachow, a geriatrician and rheumatologist at the University of Iowa, Iowa City.
Septic arthritis should also be suspected when patients have joint pain and tachycardia, hypotension, or signs of other acute illness. "And if there is hardware in the joint, even without a fever, patients should be evaluated at a higher level," said Dr. Rachow, who also serves as an attending physician in numerous nursing homes in the Iowa City area.
While the diagnostic standard for gout – synovial fluid or tophi aspiration with the identification of monosodium urate crystals under polarizing microscopy – can seem even more important in the older population, it is also more untenable given the stresses of transporting nursing home patients to hospitals.
A good long-term care mindset can preclude the need for crystal diagnosis in every case, said clinicians interviewed for this story.
"In health care, we tend to turn things into acute episodes when they’re really acute exacerbations of chronic conditions," said Dr. George Taler, director of long-term care in the department of medicine at the Washington Hospital Center and medical director of the Capitol Hill Nursing Center, both in Washington.
"Diagnosing and treating gout successfully "means remembering that our patients have a history," said Dr. Taler. He advised "making sure that when the nurse calls about a swollen knee, he or she has reviewed the medical record."
A host of factors can indicate the likelihood that joint pain is a gout attack. These include a history of gout, persistent elevated uric acid levels, and the use of medications or existence of medical conditions associated with gout. Blood testing at the time of an attack is not informative, said Dr. Rachow. While most patients with gout have chronically elevated uric acid levels, serum uric acid levels at the time of an attack are frequently normal, he explained.
Gout should also be considered in elderly patients when attacks of acute pain and swelling are seen in osteoarthritic joints of the fingers, especially in patients who have renal disease or are on chronic diuretic therapy.
An older patient without a history of gout and without any obvious risk for gout or sign of a septic joint probably has pseudogout, which is caused by the deposition of calcium pyrophosphate dihydrate rather than monosodium urate. Pseudogout often strikes the wrist or the knee and does not commonly involve polyarticular attacks, as gout does.
Clinical experience has shown that with a suspicion of either gout or pseudogout, a short empiric course of anti-inflammatory treatment should be considered, said Dr. Rachow. "If it really is crystal induced, you’ll know in 12 hours and definitely within 24 hours" because the pain will begin to subside, he said.
"And, at that point, if you’ve started with a nonsteroidal anti-inflammatory and it’s working well, you can add a gastroprotective agent and continue the NSAID, reducing the dose once symptoms begin to improve. Or you can switch to colchicine."
"At your leisure, you can consider getting an x-ray," Dr. Rachow added, as detectable calcium deposits in the cartilage are typical of pseudogout.
If the anti-inflammatory treatment is "not working spectacularly well within 24 hours, you need to put the brakes on, close your office door, and think things over again," he said.
Facilities that have a sizable number of patients with frequent flares probably should have a nurse practitioner or of physician assistant trained to aspirate joints and arrange the logistics for sending out samples, Dr. Taler said.
The Longer Term
With a correct approach, "gout is eminently preventable and treatable in 90% of nursing home residents," said Dr. Weinstein. "The principles are studied, reported, and well described," he said. The American College of Rheumatology plans to release its first practice guidelines on the management of gout in 2012.
Decisions about managing acute attacks – whether to use NSAIDs, glucocorticoids, or oral colchicine – are rightly driven by the severity of gout and consideration of the patient’s coexisting illnesses and the drugs’ side effects. While NSAID use carries the risk of gastropathy, colchicine can cause diarrhea and other potentially serious side effects and should be avoided in patients who have renal or hepatic insufficiency.
Many clinicians consider colchicine a second-line therapy for acute gout, after NSAIDs or corticosteroids. In very elderly people, however, the treatment decision might be different. Dr. Weinstein said he worries about possible cardiac risks with the use of NSAIDs in very old patients. He has had success with the early use of low-dose colchicine in very elderly patients with reasonable kidney function, and he said that the drug "works best in the first 48-72 hours."
Parenteral corticosteroids, intra-articular injections, or even an oral prednisone taper are good options, he emphasized. Issues of whether and how to move from acute management of gout attacks to long-term urate-lowering therapy are taking on added significance in nursing homes as the prevalence of gout increases there.
Dr. Rachow recommended that hypouricemic therapy be initiated for patients who have documented hyperuricemia and a history of multiple attacks, and for patients who have developed tophi, which the therapy can dissolve.
It can even be considered for a frail, ill nursing home resident for whom a second gout attack would be unusually complicating and traumatic, said Dr. Rachow. "There may be residents who we can’t imagine having to hospitalize in the next year [for another gout attack], for whom we want to take possible recurrence out of the picture once and for all."
When it is deemed beneficial, the urate-lowering therapy must be undertaken with care, he emphasized. Dr. Rachow said that hypouricemic therapy should start only after an acute attack is completely resolved (or even 2-4 weeks after flare resolution), with cautious dosing and careful monitoring for adverse effects and, when possible, under the cover of a prophylactic anti-inflammatory drug. Low-dose colchicine has long been used to prevent flares associated with the lowering of urate.
Allopurinol, the xanthine oxidase inhibitor most commonly prescribed to lower urate levels, should be "started low and increased slowly" in older patients with renal impairment, Dr. Weinstein said. He advised beginning with 100 mg/day and increasing by 100 mg/day each month until the patient’s uric acid level is below 6 mg/dL.
Another xanthine oxidase inhibitor called febuxostat (Uloric) was approved in 2009 by the FDA for treatment of hyperuricemia in patients with gout, but its efficacy and safety compared with allopurinol is not fully established (N. Engl. J. Med. 2011;364:443-52). The new drug may have advantages, however, in that it appears to be safe without dose adjustment in patients with renal insufficiency, Dr. Weinstein said.
Also on the medication front is a newly approved drug called pegloticase (Krystexxa), which might be a good option for lowering serum uric acid levels and preventing gout attacks in patients who cannot use allopurinol, said Dr. Weinstein.
If a gout attack strikes during hypouricemic therapy, Dr. Weinstein cautioned, "Don’t change anything – don’t stop the allopurinol, don’t change the diuretic. ... Just add the steroid or whatever you’ve chosen for an acute attack. Treat the attack like any other."
Colcrys, the Costly Alternative
Physicians who have long had colchicine as part of their armamentarium for the treatment of acute gout and, more important, for gout-flare prophylaxis, are coping with the loss of an array of traditional colchicine products and the current availability of only one, recently approved formulation – Colcrys – which is 50 times the cost of the earlier versions.
The winnowing of options is a result of the Food and Drug Administration’s Unapproved Drugs Initiative of 2006 that aims to bring previously grandfathered prescription drugs in compliance with current FDA approval requirements for safety and efficacy.
In July 2009, the FDA approved URL Pharma to market Colcrys for treatment of acute gout flares and prophylaxis of flares of familial Mediterranean fever. The agency gave the company 3 years of marketing exclusivity for the acute gout indication. The FDA approved an indication for gout flare prophylaxis several months later. Then, in the Oct. 1, 2010, Federal Register, the agency announced its intention to "take enforcement action" against the manufacture and distribution of unapproved single-ingredient oral colchicine products.
The cost of Colcrys caused an uproar in the rheumatology community, and the American College of Rheumatology worked with URL Pharma to cut prices to patients finding it hard to afford the drug.
College officials have also been encouraging manufacturers of unapproved colchicine products to submit new drug applications for the gout prophylaxis indication. According to the college, URL Pharma does not have exclusivity for this indication, and one other manufacturer has filed an application to market its product for this indication.
In the meantime, the FDA has been calling attention to safety concerns identified during its review of Colcrys, namely, a risk for severe drug interactions in certain patients treated with colchicine and concomitant P-gp or strong CYP3A4 inhibitors such as clarithromycin. Also, a dosing study submitted as part of the Colcrys application indicated that low-dose colchicine (1.8 mg over 1 hour) was a better treatment for acute gout than was a high-dose regimen (4.8 mg over 6 hours).
Dr. Rachow and Dr. Taler said they had no conflict of interest to disclose on this topic. Dr. Weinstein disclosed that he has received research grants from Savient Pharmaceuticals, the maker of pegloticase, for a clinical study, but not for any therapeutic studies.
The prevalence of gout has increased in the United States, especially among the eldest population, according to the latest national data. That means that nursing home staff are caring for more hot, swollen, and inflamed joints than ever, said several experts on the topic.
In interviews, they added that clinicians’ potential success in preventing, diagnosing, and treating the disease in long-term care residents lies largely in fundamental practices and an individualized approach to medications.
Data from the National Health and Nutrition Examination Survey (NHANES) show an increase in gout prevalence among U.S. adults from 2.7% in the survey’s 1994-1998 reporting periods to 3.9% in 2007-2008. Meanwhile, the prevalence in adults aged 80 years and older jumped from 5.9% to 12.6%.
The prevalence of hyperuricemia, which usually precedes gout, was 31% in adults aged 65 years or older and 37% among those aged 80 years and older, according to the latest NHANES data. The increase in actual gout occurred mainly among men in NHANES. Other studies, however, have documented an increased prevalence of the disease among older women as well. Studies cited in a review of "Crystal-Associated Arthritis in the Elderly" showed women making up at least half of the cases in which gout first strikes after age 60 years. Among individuals who have a first episode after age 80, women seem to predominate (Rheum. Dis. Clin. N. Am. 2007;33:33-55).
The presentation of the disease, as well as its prevalence, is changing, said Dr. Arthur Weinstein, professor of medicine at Georgetown University Medical Center and director of rheumatology at Washington Hospital Center. "We’re seeing more and more polyarticular gout in older patients, for instance, either as an initial presentation or years after just a single monoarticular episode" with no subsequent recurrences, said Dr. Weinstein in an interview.
He and others said they believe that the common use of thiazide diuretics in older patients is a major driver of gout’s changing profile. Other factors could include genetic predispositions to hyperuricemia and gout and increasing obesity, insulin resistance, and metabolic syndrome in the aging population.
While often the best choice for hypertension management, thiazide diuretics can contribute to the development of chronic hyperuricemia, as can low-dose aspirin and cyclosporine. Also, increasing numbers of elderly people have chronic cardiac and renal disease – factors that have been associated with hyperuricemia and gout.
Other changes in gout’s presentation in the elderly include earlier and often-atypical development of the soft tissue masses known as tophi and more frequent and earlier involvement of the small joints of the fingers.
Diagnosis, Empiric Therapy
The differential diagnosis of a swollen, inflamed joint often involves ruling out the likelihood of septic arthritis, fracture, or other injury, and pseudogout – the other main form of crystal-induced arthritis.
Patients experiencing an acute gout attack can have a low-grade fever. "But with a fever of 101 or higher, you have to consider that it’s something that’s not crystal induced," said Dr. John W. Rachow, a geriatrician and rheumatologist at the University of Iowa, Iowa City.
Septic arthritis should also be suspected when patients have joint pain and tachycardia, hypotension, or signs of other acute illness. "And if there is hardware in the joint, even without a fever, patients should be evaluated at a higher level," said Dr. Rachow, who also serves as an attending physician in numerous nursing homes in the Iowa City area.
While the diagnostic standard for gout – synovial fluid or tophi aspiration with the identification of monosodium urate crystals under polarizing microscopy – can seem even more important in the older population, it is also more untenable given the stresses of transporting nursing home patients to hospitals.
A good long-term care mindset can preclude the need for crystal diagnosis in every case, said clinicians interviewed for this story.
"In health care, we tend to turn things into acute episodes when they’re really acute exacerbations of chronic conditions," said Dr. George Taler, director of long-term care in the department of medicine at the Washington Hospital Center and medical director of the Capitol Hill Nursing Center, both in Washington.
"Diagnosing and treating gout successfully "means remembering that our patients have a history," said Dr. Taler. He advised "making sure that when the nurse calls about a swollen knee, he or she has reviewed the medical record."
A host of factors can indicate the likelihood that joint pain is a gout attack. These include a history of gout, persistent elevated uric acid levels, and the use of medications or existence of medical conditions associated with gout. Blood testing at the time of an attack is not informative, said Dr. Rachow. While most patients with gout have chronically elevated uric acid levels, serum uric acid levels at the time of an attack are frequently normal, he explained.
Gout should also be considered in elderly patients when attacks of acute pain and swelling are seen in osteoarthritic joints of the fingers, especially in patients who have renal disease or are on chronic diuretic therapy.
An older patient without a history of gout and without any obvious risk for gout or sign of a septic joint probably has pseudogout, which is caused by the deposition of calcium pyrophosphate dihydrate rather than monosodium urate. Pseudogout often strikes the wrist or the knee and does not commonly involve polyarticular attacks, as gout does.
Clinical experience has shown that with a suspicion of either gout or pseudogout, a short empiric course of anti-inflammatory treatment should be considered, said Dr. Rachow. "If it really is crystal induced, you’ll know in 12 hours and definitely within 24 hours" because the pain will begin to subside, he said.
"And, at that point, if you’ve started with a nonsteroidal anti-inflammatory and it’s working well, you can add a gastroprotective agent and continue the NSAID, reducing the dose once symptoms begin to improve. Or you can switch to colchicine."
"At your leisure, you can consider getting an x-ray," Dr. Rachow added, as detectable calcium deposits in the cartilage are typical of pseudogout.
If the anti-inflammatory treatment is "not working spectacularly well within 24 hours, you need to put the brakes on, close your office door, and think things over again," he said.
Facilities that have a sizable number of patients with frequent flares probably should have a nurse practitioner or of physician assistant trained to aspirate joints and arrange the logistics for sending out samples, Dr. Taler said.
The Longer Term
With a correct approach, "gout is eminently preventable and treatable in 90% of nursing home residents," said Dr. Weinstein. "The principles are studied, reported, and well described," he said. The American College of Rheumatology plans to release its first practice guidelines on the management of gout in 2012.
Decisions about managing acute attacks – whether to use NSAIDs, glucocorticoids, or oral colchicine – are rightly driven by the severity of gout and consideration of the patient’s coexisting illnesses and the drugs’ side effects. While NSAID use carries the risk of gastropathy, colchicine can cause diarrhea and other potentially serious side effects and should be avoided in patients who have renal or hepatic insufficiency.
Many clinicians consider colchicine a second-line therapy for acute gout, after NSAIDs or corticosteroids. In very elderly people, however, the treatment decision might be different. Dr. Weinstein said he worries about possible cardiac risks with the use of NSAIDs in very old patients. He has had success with the early use of low-dose colchicine in very elderly patients with reasonable kidney function, and he said that the drug "works best in the first 48-72 hours."
Parenteral corticosteroids, intra-articular injections, or even an oral prednisone taper are good options, he emphasized. Issues of whether and how to move from acute management of gout attacks to long-term urate-lowering therapy are taking on added significance in nursing homes as the prevalence of gout increases there.
Dr. Rachow recommended that hypouricemic therapy be initiated for patients who have documented hyperuricemia and a history of multiple attacks, and for patients who have developed tophi, which the therapy can dissolve.
It can even be considered for a frail, ill nursing home resident for whom a second gout attack would be unusually complicating and traumatic, said Dr. Rachow. "There may be residents who we can’t imagine having to hospitalize in the next year [for another gout attack], for whom we want to take possible recurrence out of the picture once and for all."
When it is deemed beneficial, the urate-lowering therapy must be undertaken with care, he emphasized. Dr. Rachow said that hypouricemic therapy should start only after an acute attack is completely resolved (or even 2-4 weeks after flare resolution), with cautious dosing and careful monitoring for adverse effects and, when possible, under the cover of a prophylactic anti-inflammatory drug. Low-dose colchicine has long been used to prevent flares associated with the lowering of urate.
Allopurinol, the xanthine oxidase inhibitor most commonly prescribed to lower urate levels, should be "started low and increased slowly" in older patients with renal impairment, Dr. Weinstein said. He advised beginning with 100 mg/day and increasing by 100 mg/day each month until the patient’s uric acid level is below 6 mg/dL.
Another xanthine oxidase inhibitor called febuxostat (Uloric) was approved in 2009 by the FDA for treatment of hyperuricemia in patients with gout, but its efficacy and safety compared with allopurinol is not fully established (N. Engl. J. Med. 2011;364:443-52). The new drug may have advantages, however, in that it appears to be safe without dose adjustment in patients with renal insufficiency, Dr. Weinstein said.
Also on the medication front is a newly approved drug called pegloticase (Krystexxa), which might be a good option for lowering serum uric acid levels and preventing gout attacks in patients who cannot use allopurinol, said Dr. Weinstein.
If a gout attack strikes during hypouricemic therapy, Dr. Weinstein cautioned, "Don’t change anything – don’t stop the allopurinol, don’t change the diuretic. ... Just add the steroid or whatever you’ve chosen for an acute attack. Treat the attack like any other."
Colcrys, the Costly Alternative
Physicians who have long had colchicine as part of their armamentarium for the treatment of acute gout and, more important, for gout-flare prophylaxis, are coping with the loss of an array of traditional colchicine products and the current availability of only one, recently approved formulation – Colcrys – which is 50 times the cost of the earlier versions.
The winnowing of options is a result of the Food and Drug Administration’s Unapproved Drugs Initiative of 2006 that aims to bring previously grandfathered prescription drugs in compliance with current FDA approval requirements for safety and efficacy.
In July 2009, the FDA approved URL Pharma to market Colcrys for treatment of acute gout flares and prophylaxis of flares of familial Mediterranean fever. The agency gave the company 3 years of marketing exclusivity for the acute gout indication. The FDA approved an indication for gout flare prophylaxis several months later. Then, in the Oct. 1, 2010, Federal Register, the agency announced its intention to "take enforcement action" against the manufacture and distribution of unapproved single-ingredient oral colchicine products.
The cost of Colcrys caused an uproar in the rheumatology community, and the American College of Rheumatology worked with URL Pharma to cut prices to patients finding it hard to afford the drug.
College officials have also been encouraging manufacturers of unapproved colchicine products to submit new drug applications for the gout prophylaxis indication. According to the college, URL Pharma does not have exclusivity for this indication, and one other manufacturer has filed an application to market its product for this indication.
In the meantime, the FDA has been calling attention to safety concerns identified during its review of Colcrys, namely, a risk for severe drug interactions in certain patients treated with colchicine and concomitant P-gp or strong CYP3A4 inhibitors such as clarithromycin. Also, a dosing study submitted as part of the Colcrys application indicated that low-dose colchicine (1.8 mg over 1 hour) was a better treatment for acute gout than was a high-dose regimen (4.8 mg over 6 hours).
Dr. Rachow and Dr. Taler said they had no conflict of interest to disclose on this topic. Dr. Weinstein disclosed that he has received research grants from Savient Pharmaceuticals, the maker of pegloticase, for a clinical study, but not for any therapeutic studies.
The prevalence of gout has increased in the United States, especially among the eldest population, according to the latest national data. That means that nursing home staff are caring for more hot, swollen, and inflamed joints than ever, said several experts on the topic.
In interviews, they added that clinicians’ potential success in preventing, diagnosing, and treating the disease in long-term care residents lies largely in fundamental practices and an individualized approach to medications.
Data from the National Health and Nutrition Examination Survey (NHANES) show an increase in gout prevalence among U.S. adults from 2.7% in the survey’s 1994-1998 reporting periods to 3.9% in 2007-2008. Meanwhile, the prevalence in adults aged 80 years and older jumped from 5.9% to 12.6%.
The prevalence of hyperuricemia, which usually precedes gout, was 31% in adults aged 65 years or older and 37% among those aged 80 years and older, according to the latest NHANES data. The increase in actual gout occurred mainly among men in NHANES. Other studies, however, have documented an increased prevalence of the disease among older women as well. Studies cited in a review of "Crystal-Associated Arthritis in the Elderly" showed women making up at least half of the cases in which gout first strikes after age 60 years. Among individuals who have a first episode after age 80, women seem to predominate (Rheum. Dis. Clin. N. Am. 2007;33:33-55).
The presentation of the disease, as well as its prevalence, is changing, said Dr. Arthur Weinstein, professor of medicine at Georgetown University Medical Center and director of rheumatology at Washington Hospital Center. "We’re seeing more and more polyarticular gout in older patients, for instance, either as an initial presentation or years after just a single monoarticular episode" with no subsequent recurrences, said Dr. Weinstein in an interview.
He and others said they believe that the common use of thiazide diuretics in older patients is a major driver of gout’s changing profile. Other factors could include genetic predispositions to hyperuricemia and gout and increasing obesity, insulin resistance, and metabolic syndrome in the aging population.
While often the best choice for hypertension management, thiazide diuretics can contribute to the development of chronic hyperuricemia, as can low-dose aspirin and cyclosporine. Also, increasing numbers of elderly people have chronic cardiac and renal disease – factors that have been associated with hyperuricemia and gout.
Other changes in gout’s presentation in the elderly include earlier and often-atypical development of the soft tissue masses known as tophi and more frequent and earlier involvement of the small joints of the fingers.
Diagnosis, Empiric Therapy
The differential diagnosis of a swollen, inflamed joint often involves ruling out the likelihood of septic arthritis, fracture, or other injury, and pseudogout – the other main form of crystal-induced arthritis.
Patients experiencing an acute gout attack can have a low-grade fever. "But with a fever of 101 or higher, you have to consider that it’s something that’s not crystal induced," said Dr. John W. Rachow, a geriatrician and rheumatologist at the University of Iowa, Iowa City.
Septic arthritis should also be suspected when patients have joint pain and tachycardia, hypotension, or signs of other acute illness. "And if there is hardware in the joint, even without a fever, patients should be evaluated at a higher level," said Dr. Rachow, who also serves as an attending physician in numerous nursing homes in the Iowa City area.
While the diagnostic standard for gout – synovial fluid or tophi aspiration with the identification of monosodium urate crystals under polarizing microscopy – can seem even more important in the older population, it is also more untenable given the stresses of transporting nursing home patients to hospitals.
A good long-term care mindset can preclude the need for crystal diagnosis in every case, said clinicians interviewed for this story.
"In health care, we tend to turn things into acute episodes when they’re really acute exacerbations of chronic conditions," said Dr. George Taler, director of long-term care in the department of medicine at the Washington Hospital Center and medical director of the Capitol Hill Nursing Center, both in Washington.
"Diagnosing and treating gout successfully "means remembering that our patients have a history," said Dr. Taler. He advised "making sure that when the nurse calls about a swollen knee, he or she has reviewed the medical record."
A host of factors can indicate the likelihood that joint pain is a gout attack. These include a history of gout, persistent elevated uric acid levels, and the use of medications or existence of medical conditions associated with gout. Blood testing at the time of an attack is not informative, said Dr. Rachow. While most patients with gout have chronically elevated uric acid levels, serum uric acid levels at the time of an attack are frequently normal, he explained.
Gout should also be considered in elderly patients when attacks of acute pain and swelling are seen in osteoarthritic joints of the fingers, especially in patients who have renal disease or are on chronic diuretic therapy.
An older patient without a history of gout and without any obvious risk for gout or sign of a septic joint probably has pseudogout, which is caused by the deposition of calcium pyrophosphate dihydrate rather than monosodium urate. Pseudogout often strikes the wrist or the knee and does not commonly involve polyarticular attacks, as gout does.
Clinical experience has shown that with a suspicion of either gout or pseudogout, a short empiric course of anti-inflammatory treatment should be considered, said Dr. Rachow. "If it really is crystal induced, you’ll know in 12 hours and definitely within 24 hours" because the pain will begin to subside, he said.
"And, at that point, if you’ve started with a nonsteroidal anti-inflammatory and it’s working well, you can add a gastroprotective agent and continue the NSAID, reducing the dose once symptoms begin to improve. Or you can switch to colchicine."
"At your leisure, you can consider getting an x-ray," Dr. Rachow added, as detectable calcium deposits in the cartilage are typical of pseudogout.
If the anti-inflammatory treatment is "not working spectacularly well within 24 hours, you need to put the brakes on, close your office door, and think things over again," he said.
Facilities that have a sizable number of patients with frequent flares probably should have a nurse practitioner or of physician assistant trained to aspirate joints and arrange the logistics for sending out samples, Dr. Taler said.
The Longer Term
With a correct approach, "gout is eminently preventable and treatable in 90% of nursing home residents," said Dr. Weinstein. "The principles are studied, reported, and well described," he said. The American College of Rheumatology plans to release its first practice guidelines on the management of gout in 2012.
Decisions about managing acute attacks – whether to use NSAIDs, glucocorticoids, or oral colchicine – are rightly driven by the severity of gout and consideration of the patient’s coexisting illnesses and the drugs’ side effects. While NSAID use carries the risk of gastropathy, colchicine can cause diarrhea and other potentially serious side effects and should be avoided in patients who have renal or hepatic insufficiency.
Many clinicians consider colchicine a second-line therapy for acute gout, after NSAIDs or corticosteroids. In very elderly people, however, the treatment decision might be different. Dr. Weinstein said he worries about possible cardiac risks with the use of NSAIDs in very old patients. He has had success with the early use of low-dose colchicine in very elderly patients with reasonable kidney function, and he said that the drug "works best in the first 48-72 hours."
Parenteral corticosteroids, intra-articular injections, or even an oral prednisone taper are good options, he emphasized. Issues of whether and how to move from acute management of gout attacks to long-term urate-lowering therapy are taking on added significance in nursing homes as the prevalence of gout increases there.
Dr. Rachow recommended that hypouricemic therapy be initiated for patients who have documented hyperuricemia and a history of multiple attacks, and for patients who have developed tophi, which the therapy can dissolve.
It can even be considered for a frail, ill nursing home resident for whom a second gout attack would be unusually complicating and traumatic, said Dr. Rachow. "There may be residents who we can’t imagine having to hospitalize in the next year [for another gout attack], for whom we want to take possible recurrence out of the picture once and for all."
When it is deemed beneficial, the urate-lowering therapy must be undertaken with care, he emphasized. Dr. Rachow said that hypouricemic therapy should start only after an acute attack is completely resolved (or even 2-4 weeks after flare resolution), with cautious dosing and careful monitoring for adverse effects and, when possible, under the cover of a prophylactic anti-inflammatory drug. Low-dose colchicine has long been used to prevent flares associated with the lowering of urate.
Allopurinol, the xanthine oxidase inhibitor most commonly prescribed to lower urate levels, should be "started low and increased slowly" in older patients with renal impairment, Dr. Weinstein said. He advised beginning with 100 mg/day and increasing by 100 mg/day each month until the patient’s uric acid level is below 6 mg/dL.
Another xanthine oxidase inhibitor called febuxostat (Uloric) was approved in 2009 by the FDA for treatment of hyperuricemia in patients with gout, but its efficacy and safety compared with allopurinol is not fully established (N. Engl. J. Med. 2011;364:443-52). The new drug may have advantages, however, in that it appears to be safe without dose adjustment in patients with renal insufficiency, Dr. Weinstein said.
Also on the medication front is a newly approved drug called pegloticase (Krystexxa), which might be a good option for lowering serum uric acid levels and preventing gout attacks in patients who cannot use allopurinol, said Dr. Weinstein.
If a gout attack strikes during hypouricemic therapy, Dr. Weinstein cautioned, "Don’t change anything – don’t stop the allopurinol, don’t change the diuretic. ... Just add the steroid or whatever you’ve chosen for an acute attack. Treat the attack like any other."
Colcrys, the Costly Alternative
Physicians who have long had colchicine as part of their armamentarium for the treatment of acute gout and, more important, for gout-flare prophylaxis, are coping with the loss of an array of traditional colchicine products and the current availability of only one, recently approved formulation – Colcrys – which is 50 times the cost of the earlier versions.
The winnowing of options is a result of the Food and Drug Administration’s Unapproved Drugs Initiative of 2006 that aims to bring previously grandfathered prescription drugs in compliance with current FDA approval requirements for safety and efficacy.
In July 2009, the FDA approved URL Pharma to market Colcrys for treatment of acute gout flares and prophylaxis of flares of familial Mediterranean fever. The agency gave the company 3 years of marketing exclusivity for the acute gout indication. The FDA approved an indication for gout flare prophylaxis several months later. Then, in the Oct. 1, 2010, Federal Register, the agency announced its intention to "take enforcement action" against the manufacture and distribution of unapproved single-ingredient oral colchicine products.
The cost of Colcrys caused an uproar in the rheumatology community, and the American College of Rheumatology worked with URL Pharma to cut prices to patients finding it hard to afford the drug.
College officials have also been encouraging manufacturers of unapproved colchicine products to submit new drug applications for the gout prophylaxis indication. According to the college, URL Pharma does not have exclusivity for this indication, and one other manufacturer has filed an application to market its product for this indication.
In the meantime, the FDA has been calling attention to safety concerns identified during its review of Colcrys, namely, a risk for severe drug interactions in certain patients treated with colchicine and concomitant P-gp or strong CYP3A4 inhibitors such as clarithromycin. Also, a dosing study submitted as part of the Colcrys application indicated that low-dose colchicine (1.8 mg over 1 hour) was a better treatment for acute gout than was a high-dose regimen (4.8 mg over 6 hours).
Dr. Rachow and Dr. Taler said they had no conflict of interest to disclose on this topic. Dr. Weinstein disclosed that he has received research grants from Savient Pharmaceuticals, the maker of pegloticase, for a clinical study, but not for any therapeutic studies.
Gout Deserves Tender Treatment in Elderly
The prevalence of gout has increased in the United States, especially among the eldest population, according to the latest national data. That means that nursing home staff are caring for more hot, swollen, and inflamed joints than ever, said several experts on the topic.
In interviews, they added that clinicians’ potential success in preventing, diagnosing, and treating the disease in long-term care residents lies largely in fundamental practices and an individualized approach to medications.
Data from the National Health and Nutrition Examination Survey (NHANES) show an increase in gout prevalence among U.S. adults from 2.7% in the survey’s 1994-1998 reporting periods to 3.9% in 2007-2008. Meanwhile, the prevalence in adults aged 80 years and older jumped from 5.9% to 12.6%.
The prevalence of hyperuricemia, which usually precedes gout, was 31% in adults aged 65 years or older and 37% among those aged 80 years and older, according to the latest NHANES data. The increase in actual gout occurred mainly among men in NHANES. Other studies, however, have documented an increased prevalence of the disease among older women as well. Studies cited in a review of "Crystal-Associated Arthritis in the Elderly" showed women making up at least half of the cases in which gout first strikes after age 60 years. Among individuals who have a first episode after age 80, women seem to predominate (Rheum. Dis. Clin. N. Am. 2007;33:33-55).
The presentation of the disease, as well as its prevalence, is changing, said Dr. Arthur Weinstein, professor of medicine at Georgetown University Medical Center and director of rheumatology at Washington Hospital Center. "We’re seeing more and more polyarticular gout in older patients, for instance, either as an initial presentation or years after just a single monoarticular episode" with no subsequent recurrences, said Dr. Weinstein in an interview.
He and others said they believe that the common use of thiazide diuretics in older patients is a major driver of gout’s changing profile. Other factors could include genetic predispositions to hyperuricemia and gout and increasing obesity, insulin resistance, and metabolic syndrome in the aging population.
While often the best choice for hypertension management, thiazide diuretics can contribute to the development of chronic hyperuricemia, as can low-dose aspirin and cyclosporine. Also, increasing numbers of elderly people have chronic cardiac and renal disease – factors that have been associated with hyperuricemia and gout.
Other changes in gout’s presentation in the elderly include earlier and often-atypical development of the soft tissue masses known as tophi and more frequent and earlier involvement of the small joints of the fingers.
Diagnosis, Empiric Therapy
The differential diagnosis of a swollen, inflamed joint often involves ruling out the likelihood of septic arthritis, fracture, or other injury, and pseudogout – the other main form of crystal-induced arthritis.
Patients experiencing an acute gout attack can have a low-grade fever. "But with a fever of 101 or higher, you have to consider that it’s something that’s not crystal induced," said Dr. John W. Rachow, a geriatrician and rheumatologist at the University of Iowa, Iowa City.
Septic arthritis should also be suspected when patients have joint pain and tachycardia, hypotension, or signs of other acute illness. "And if there is hardware in the joint, even without a fever, patients should be evaluated at a higher level," said Dr. Rachow, who also serves as an attending physician in numerous nursing homes in the Iowa City area.
While the diagnostic standard for gout – synovial fluid or tophi aspiration with the identification of monosodium urate crystals under polarizing microscopy – can seem even more important in the older population, it is also more untenable given the stresses of transporting nursing home patients to hospitals.
A good long-term care mindset can preclude the need for crystal diagnosis in every case, said clinicians interviewed for this story.
"In health care, we tend to turn things into acute episodes when they’re really acute exacerbations of chronic conditions," said Dr. George Taler, director of long-term care in the department of medicine at the Washington Hospital Center and medical director of the Capitol Hill Nursing Center, both in Washington.
"Diagnosing and treating gout successfully "means remembering that our patients have a history," said Dr. Taler. He advised "making sure that when the nurse calls about a swollen knee, he or she has reviewed the medical record."
A host of factors can indicate the likelihood that joint pain is a gout attack. These include a history of gout, persistent elevated uric acid levels, and the use of medications or existence of medical conditions associated with gout. Blood testing at the time of an attack is not informative, said Dr. Rachow. While most patients with gout have chronically elevated uric acid levels, serum uric acid levels at the time of an attack are frequently normal, he explained.
Gout should also be considered in elderly patients when attacks of acute pain and swelling are seen in osteoarthritic joints of the fingers, especially in patients who have renal disease or are on chronic diuretic therapy.
An older patient without a history of gout and without any obvious risk for gout or sign of a septic joint probably has pseudogout, which is caused by the deposition of calcium pyrophosphate dihydrate rather than monosodium urate. Pseudogout often strikes the wrist or the knee and does not commonly involve polyarticular attacks, as gout does.
Clinical experience has shown that with a suspicion of either gout or pseudogout, a short empiric course of anti-inflammatory treatment should be considered, said Dr. Rachow. "If it really is crystal induced, you’ll know in 12 hours and definitely within 24 hours" because the pain will begin to subside, he said.
"And, at that point, if you’ve started with a nonsteroidal anti-inflammatory and it’s working well, you can add a gastroprotective agent and continue the NSAID, reducing the dose once symptoms begin to improve. Or you can switch to colchicine."
"At your leisure, you can consider getting an x-ray," Dr. Rachow added, as detectable calcium deposits in the cartilage are typical of pseudogout.
If the anti-inflammatory treatment is "not working spectacularly well within 24 hours, you need to put the brakes on, close your office door, and think things over again," he said.
Facilities that have a sizable number of patients with frequent flares probably should have a nurse practitioner or of physician assistant trained to aspirate joints and arrange the logistics for sending out samples, Dr. Taler said.
The Longer Term
With a correct approach, "gout is eminently preventable and treatable in 90% of nursing home residents," said Dr. Weinstein. "The principles are studied, reported, and well described," he said. The American College of Rheumatology plans to release its first practice guidelines on the management of gout in 2012.
Decisions about managing acute attacks – whether to use NSAIDs, glucocorticoids, or oral colchicine – are rightly driven by the severity of gout and consideration of the patient’s coexisting illnesses and the drugs’ side effects. While NSAID use carries the risk of gastropathy, colchicine can cause diarrhea and other potentially serious side effects and should be avoided in patients who have renal or hepatic insufficiency.
Many clinicians consider colchicine a second-line therapy for acute gout, after NSAIDs or corticosteroids. In very elderly people, however, the treatment decision might be different. Dr. Weinstein said he worries about possible cardiac risks with the use of NSAIDs in very old patients. He has had success with the early use of low-dose colchicine in very elderly patients with reasonable kidney function, and he said that the drug "works best in the first 48-72 hours."
Parenteral corticosteroids, intra-articular injections, or even an oral prednisone taper are good options, he emphasized. Issues of whether and how to move from acute management of gout attacks to long-term urate-lowering therapy are taking on added significance in nursing homes as the prevalence of gout increases there.
Dr. Rachow recommended that hypouricemic therapy be initiated for patients who have documented hyperuricemia and a history of multiple attacks, and for patients who have developed tophi, which the therapy can dissolve.
It can even be considered for a frail, ill nursing home resident for whom a second gout attack would be unusually complicating and traumatic, said Dr. Rachow. "There may be residents who we can’t imagine having to hospitalize in the next year [for another gout attack], for whom we want to take possible recurrence out of the picture once and for all."
When it is deemed beneficial, the urate-lowering therapy must be undertaken with care, he emphasized. Dr. Rachow said that hypouricemic therapy should start only after an acute attack is completely resolved (or even 2-4 weeks after flare resolution), with cautious dosing and careful monitoring for adverse effects and, when possible, under the cover of a prophylactic anti-inflammatory drug. Low-dose colchicine has long been used to prevent flares associated with the lowering of urate.
Allopurinol, the xanthine oxidase inhibitor most commonly prescribed to lower urate levels, should be "started low and increased slowly" in older patients with renal impairment, Dr. Weinstein said. He advised beginning with 100 mg/day and increasing by 100 mg/day each month until the patient’s uric acid level is below 6 mg/dL.
Another xanthine oxidase inhibitor called febuxostat (Uloric) was approved in 2009 by the FDA for treatment of hyperuricemia in patients with gout, but its efficacy and safety compared with allopurinol is not fully established (N. Engl. J. Med. 2011;364:443-52). The new drug may have advantages, however, in that it appears to be safe without dose adjustment in patients with renal insufficiency, Dr. Weinstein said.
Also on the medication front is a newly approved drug called pegloticase (Krystexxa), which might be a good option for lowering serum uric acid levels and preventing gout attacks in patients who cannot use allopurinol, said Dr. Weinstein.
If a gout attack strikes during hypouricemic therapy, Dr. Weinstein cautioned, "Don’t change anything – don’t stop the allopurinol, don’t change the diuretic. ... Just add the steroid or whatever you’ve chosen for an acute attack. Treat the attack like any other."
Colcrys, the Costly Alternative
Physicians who have long had colchicine as part of their armamentarium for the treatment of acute gout and, more important, for gout-flare prophylaxis, are coping with the loss of an array of traditional colchicine products and the current availability of only one, recently approved formulation – Colcrys – which is 50 times the cost of the earlier versions.
The winnowing of options is a result of the Food and Drug Administration’s Unapproved Drugs Initiative of 2006 that aims to bring previously grandfathered prescription drugs in compliance with current FDA approval requirements for safety and efficacy.
In July 2009, the FDA approved URL Pharma to market Colcrys for treatment of acute gout flares and prophylaxis of flares of familial Mediterranean fever. The agency gave the company 3 years of marketing exclusivity for the acute gout indication. The FDA approved an indication for gout flare prophylaxis several months later. Then, in the Oct. 1, 2010, Federal Register, the agency announced its intention to "take enforcement action" against the manufacture and distribution of unapproved single-ingredient oral colchicine products.
The cost of Colcrys caused an uproar in the rheumatology community, and the American College of Rheumatology worked with URL Pharma to cut prices to patients finding it hard to afford the drug.
College officials have also been encouraging manufacturers of unapproved colchicine products to submit new drug applications for the gout prophylaxis indication. According to the college, URL Pharma does not have exclusivity for this indication, and one other manufacturer has filed an application to market its product for this indication.
In the meantime, the FDA has been calling attention to safety concerns identified during its review of Colcrys, namely, a risk for severe drug interactions in certain patients treated with colchicine and concomitant P-gp or strong CYP3A4 inhibitors such as clarithromycin. Also, a dosing study submitted as part of the Colcrys application indicated that low-dose colchicine (1.8 mg over 1 hour) was a better treatment for acute gout than was a high-dose regimen (4.8 mg over 6 hours).
Dr. Rachow and Dr. Taler said they had no conflict of interest to disclose on this topic. Dr. Weinstein disclosed that he has received research grants from Savient Pharmaceuticals, the maker of pegloticase, for a clinical study, but not for any therapeutic studies.
The prevalence of gout has increased in the United States, especially among the eldest population, according to the latest national data. That means that nursing home staff are caring for more hot, swollen, and inflamed joints than ever, said several experts on the topic.
In interviews, they added that clinicians’ potential success in preventing, diagnosing, and treating the disease in long-term care residents lies largely in fundamental practices and an individualized approach to medications.
Data from the National Health and Nutrition Examination Survey (NHANES) show an increase in gout prevalence among U.S. adults from 2.7% in the survey’s 1994-1998 reporting periods to 3.9% in 2007-2008. Meanwhile, the prevalence in adults aged 80 years and older jumped from 5.9% to 12.6%.
The prevalence of hyperuricemia, which usually precedes gout, was 31% in adults aged 65 years or older and 37% among those aged 80 years and older, according to the latest NHANES data. The increase in actual gout occurred mainly among men in NHANES. Other studies, however, have documented an increased prevalence of the disease among older women as well. Studies cited in a review of "Crystal-Associated Arthritis in the Elderly" showed women making up at least half of the cases in which gout first strikes after age 60 years. Among individuals who have a first episode after age 80, women seem to predominate (Rheum. Dis. Clin. N. Am. 2007;33:33-55).
The presentation of the disease, as well as its prevalence, is changing, said Dr. Arthur Weinstein, professor of medicine at Georgetown University Medical Center and director of rheumatology at Washington Hospital Center. "We’re seeing more and more polyarticular gout in older patients, for instance, either as an initial presentation or years after just a single monoarticular episode" with no subsequent recurrences, said Dr. Weinstein in an interview.
He and others said they believe that the common use of thiazide diuretics in older patients is a major driver of gout’s changing profile. Other factors could include genetic predispositions to hyperuricemia and gout and increasing obesity, insulin resistance, and metabolic syndrome in the aging population.
While often the best choice for hypertension management, thiazide diuretics can contribute to the development of chronic hyperuricemia, as can low-dose aspirin and cyclosporine. Also, increasing numbers of elderly people have chronic cardiac and renal disease – factors that have been associated with hyperuricemia and gout.
Other changes in gout’s presentation in the elderly include earlier and often-atypical development of the soft tissue masses known as tophi and more frequent and earlier involvement of the small joints of the fingers.
Diagnosis, Empiric Therapy
The differential diagnosis of a swollen, inflamed joint often involves ruling out the likelihood of septic arthritis, fracture, or other injury, and pseudogout – the other main form of crystal-induced arthritis.
Patients experiencing an acute gout attack can have a low-grade fever. "But with a fever of 101 or higher, you have to consider that it’s something that’s not crystal induced," said Dr. John W. Rachow, a geriatrician and rheumatologist at the University of Iowa, Iowa City.
Septic arthritis should also be suspected when patients have joint pain and tachycardia, hypotension, or signs of other acute illness. "And if there is hardware in the joint, even without a fever, patients should be evaluated at a higher level," said Dr. Rachow, who also serves as an attending physician in numerous nursing homes in the Iowa City area.
While the diagnostic standard for gout – synovial fluid or tophi aspiration with the identification of monosodium urate crystals under polarizing microscopy – can seem even more important in the older population, it is also more untenable given the stresses of transporting nursing home patients to hospitals.
A good long-term care mindset can preclude the need for crystal diagnosis in every case, said clinicians interviewed for this story.
"In health care, we tend to turn things into acute episodes when they’re really acute exacerbations of chronic conditions," said Dr. George Taler, director of long-term care in the department of medicine at the Washington Hospital Center and medical director of the Capitol Hill Nursing Center, both in Washington.
"Diagnosing and treating gout successfully "means remembering that our patients have a history," said Dr. Taler. He advised "making sure that when the nurse calls about a swollen knee, he or she has reviewed the medical record."
A host of factors can indicate the likelihood that joint pain is a gout attack. These include a history of gout, persistent elevated uric acid levels, and the use of medications or existence of medical conditions associated with gout. Blood testing at the time of an attack is not informative, said Dr. Rachow. While most patients with gout have chronically elevated uric acid levels, serum uric acid levels at the time of an attack are frequently normal, he explained.
Gout should also be considered in elderly patients when attacks of acute pain and swelling are seen in osteoarthritic joints of the fingers, especially in patients who have renal disease or are on chronic diuretic therapy.
An older patient without a history of gout and without any obvious risk for gout or sign of a septic joint probably has pseudogout, which is caused by the deposition of calcium pyrophosphate dihydrate rather than monosodium urate. Pseudogout often strikes the wrist or the knee and does not commonly involve polyarticular attacks, as gout does.
Clinical experience has shown that with a suspicion of either gout or pseudogout, a short empiric course of anti-inflammatory treatment should be considered, said Dr. Rachow. "If it really is crystal induced, you’ll know in 12 hours and definitely within 24 hours" because the pain will begin to subside, he said.
"And, at that point, if you’ve started with a nonsteroidal anti-inflammatory and it’s working well, you can add a gastroprotective agent and continue the NSAID, reducing the dose once symptoms begin to improve. Or you can switch to colchicine."
"At your leisure, you can consider getting an x-ray," Dr. Rachow added, as detectable calcium deposits in the cartilage are typical of pseudogout.
If the anti-inflammatory treatment is "not working spectacularly well within 24 hours, you need to put the brakes on, close your office door, and think things over again," he said.
Facilities that have a sizable number of patients with frequent flares probably should have a nurse practitioner or of physician assistant trained to aspirate joints and arrange the logistics for sending out samples, Dr. Taler said.
The Longer Term
With a correct approach, "gout is eminently preventable and treatable in 90% of nursing home residents," said Dr. Weinstein. "The principles are studied, reported, and well described," he said. The American College of Rheumatology plans to release its first practice guidelines on the management of gout in 2012.
Decisions about managing acute attacks – whether to use NSAIDs, glucocorticoids, or oral colchicine – are rightly driven by the severity of gout and consideration of the patient’s coexisting illnesses and the drugs’ side effects. While NSAID use carries the risk of gastropathy, colchicine can cause diarrhea and other potentially serious side effects and should be avoided in patients who have renal or hepatic insufficiency.
Many clinicians consider colchicine a second-line therapy for acute gout, after NSAIDs or corticosteroids. In very elderly people, however, the treatment decision might be different. Dr. Weinstein said he worries about possible cardiac risks with the use of NSAIDs in very old patients. He has had success with the early use of low-dose colchicine in very elderly patients with reasonable kidney function, and he said that the drug "works best in the first 48-72 hours."
Parenteral corticosteroids, intra-articular injections, or even an oral prednisone taper are good options, he emphasized. Issues of whether and how to move from acute management of gout attacks to long-term urate-lowering therapy are taking on added significance in nursing homes as the prevalence of gout increases there.
Dr. Rachow recommended that hypouricemic therapy be initiated for patients who have documented hyperuricemia and a history of multiple attacks, and for patients who have developed tophi, which the therapy can dissolve.
It can even be considered for a frail, ill nursing home resident for whom a second gout attack would be unusually complicating and traumatic, said Dr. Rachow. "There may be residents who we can’t imagine having to hospitalize in the next year [for another gout attack], for whom we want to take possible recurrence out of the picture once and for all."
When it is deemed beneficial, the urate-lowering therapy must be undertaken with care, he emphasized. Dr. Rachow said that hypouricemic therapy should start only after an acute attack is completely resolved (or even 2-4 weeks after flare resolution), with cautious dosing and careful monitoring for adverse effects and, when possible, under the cover of a prophylactic anti-inflammatory drug. Low-dose colchicine has long been used to prevent flares associated with the lowering of urate.
Allopurinol, the xanthine oxidase inhibitor most commonly prescribed to lower urate levels, should be "started low and increased slowly" in older patients with renal impairment, Dr. Weinstein said. He advised beginning with 100 mg/day and increasing by 100 mg/day each month until the patient’s uric acid level is below 6 mg/dL.
Another xanthine oxidase inhibitor called febuxostat (Uloric) was approved in 2009 by the FDA for treatment of hyperuricemia in patients with gout, but its efficacy and safety compared with allopurinol is not fully established (N. Engl. J. Med. 2011;364:443-52). The new drug may have advantages, however, in that it appears to be safe without dose adjustment in patients with renal insufficiency, Dr. Weinstein said.
Also on the medication front is a newly approved drug called pegloticase (Krystexxa), which might be a good option for lowering serum uric acid levels and preventing gout attacks in patients who cannot use allopurinol, said Dr. Weinstein.
If a gout attack strikes during hypouricemic therapy, Dr. Weinstein cautioned, "Don’t change anything – don’t stop the allopurinol, don’t change the diuretic. ... Just add the steroid or whatever you’ve chosen for an acute attack. Treat the attack like any other."
Colcrys, the Costly Alternative
Physicians who have long had colchicine as part of their armamentarium for the treatment of acute gout and, more important, for gout-flare prophylaxis, are coping with the loss of an array of traditional colchicine products and the current availability of only one, recently approved formulation – Colcrys – which is 50 times the cost of the earlier versions.
The winnowing of options is a result of the Food and Drug Administration’s Unapproved Drugs Initiative of 2006 that aims to bring previously grandfathered prescription drugs in compliance with current FDA approval requirements for safety and efficacy.
In July 2009, the FDA approved URL Pharma to market Colcrys for treatment of acute gout flares and prophylaxis of flares of familial Mediterranean fever. The agency gave the company 3 years of marketing exclusivity for the acute gout indication. The FDA approved an indication for gout flare prophylaxis several months later. Then, in the Oct. 1, 2010, Federal Register, the agency announced its intention to "take enforcement action" against the manufacture and distribution of unapproved single-ingredient oral colchicine products.
The cost of Colcrys caused an uproar in the rheumatology community, and the American College of Rheumatology worked with URL Pharma to cut prices to patients finding it hard to afford the drug.
College officials have also been encouraging manufacturers of unapproved colchicine products to submit new drug applications for the gout prophylaxis indication. According to the college, URL Pharma does not have exclusivity for this indication, and one other manufacturer has filed an application to market its product for this indication.
In the meantime, the FDA has been calling attention to safety concerns identified during its review of Colcrys, namely, a risk for severe drug interactions in certain patients treated with colchicine and concomitant P-gp or strong CYP3A4 inhibitors such as clarithromycin. Also, a dosing study submitted as part of the Colcrys application indicated that low-dose colchicine (1.8 mg over 1 hour) was a better treatment for acute gout than was a high-dose regimen (4.8 mg over 6 hours).
Dr. Rachow and Dr. Taler said they had no conflict of interest to disclose on this topic. Dr. Weinstein disclosed that he has received research grants from Savient Pharmaceuticals, the maker of pegloticase, for a clinical study, but not for any therapeutic studies.
The prevalence of gout has increased in the United States, especially among the eldest population, according to the latest national data. That means that nursing home staff are caring for more hot, swollen, and inflamed joints than ever, said several experts on the topic.
In interviews, they added that clinicians’ potential success in preventing, diagnosing, and treating the disease in long-term care residents lies largely in fundamental practices and an individualized approach to medications.
Data from the National Health and Nutrition Examination Survey (NHANES) show an increase in gout prevalence among U.S. adults from 2.7% in the survey’s 1994-1998 reporting periods to 3.9% in 2007-2008. Meanwhile, the prevalence in adults aged 80 years and older jumped from 5.9% to 12.6%.
The prevalence of hyperuricemia, which usually precedes gout, was 31% in adults aged 65 years or older and 37% among those aged 80 years and older, according to the latest NHANES data. The increase in actual gout occurred mainly among men in NHANES. Other studies, however, have documented an increased prevalence of the disease among older women as well. Studies cited in a review of "Crystal-Associated Arthritis in the Elderly" showed women making up at least half of the cases in which gout first strikes after age 60 years. Among individuals who have a first episode after age 80, women seem to predominate (Rheum. Dis. Clin. N. Am. 2007;33:33-55).
The presentation of the disease, as well as its prevalence, is changing, said Dr. Arthur Weinstein, professor of medicine at Georgetown University Medical Center and director of rheumatology at Washington Hospital Center. "We’re seeing more and more polyarticular gout in older patients, for instance, either as an initial presentation or years after just a single monoarticular episode" with no subsequent recurrences, said Dr. Weinstein in an interview.
He and others said they believe that the common use of thiazide diuretics in older patients is a major driver of gout’s changing profile. Other factors could include genetic predispositions to hyperuricemia and gout and increasing obesity, insulin resistance, and metabolic syndrome in the aging population.
While often the best choice for hypertension management, thiazide diuretics can contribute to the development of chronic hyperuricemia, as can low-dose aspirin and cyclosporine. Also, increasing numbers of elderly people have chronic cardiac and renal disease – factors that have been associated with hyperuricemia and gout.
Other changes in gout’s presentation in the elderly include earlier and often-atypical development of the soft tissue masses known as tophi and more frequent and earlier involvement of the small joints of the fingers.
Diagnosis, Empiric Therapy
The differential diagnosis of a swollen, inflamed joint often involves ruling out the likelihood of septic arthritis, fracture, or other injury, and pseudogout – the other main form of crystal-induced arthritis.
Patients experiencing an acute gout attack can have a low-grade fever. "But with a fever of 101 or higher, you have to consider that it’s something that’s not crystal induced," said Dr. John W. Rachow, a geriatrician and rheumatologist at the University of Iowa, Iowa City.
Septic arthritis should also be suspected when patients have joint pain and tachycardia, hypotension, or signs of other acute illness. "And if there is hardware in the joint, even without a fever, patients should be evaluated at a higher level," said Dr. Rachow, who also serves as an attending physician in numerous nursing homes in the Iowa City area.
While the diagnostic standard for gout – synovial fluid or tophi aspiration with the identification of monosodium urate crystals under polarizing microscopy – can seem even more important in the older population, it is also more untenable given the stresses of transporting nursing home patients to hospitals.
A good long-term care mindset can preclude the need for crystal diagnosis in every case, said clinicians interviewed for this story.
"In health care, we tend to turn things into acute episodes when they’re really acute exacerbations of chronic conditions," said Dr. George Taler, director of long-term care in the department of medicine at the Washington Hospital Center and medical director of the Capitol Hill Nursing Center, both in Washington.
"Diagnosing and treating gout successfully "means remembering that our patients have a history," said Dr. Taler. He advised "making sure that when the nurse calls about a swollen knee, he or she has reviewed the medical record."
A host of factors can indicate the likelihood that joint pain is a gout attack. These include a history of gout, persistent elevated uric acid levels, and the use of medications or existence of medical conditions associated with gout. Blood testing at the time of an attack is not informative, said Dr. Rachow. While most patients with gout have chronically elevated uric acid levels, serum uric acid levels at the time of an attack are frequently normal, he explained.
Gout should also be considered in elderly patients when attacks of acute pain and swelling are seen in osteoarthritic joints of the fingers, especially in patients who have renal disease or are on chronic diuretic therapy.
An older patient without a history of gout and without any obvious risk for gout or sign of a septic joint probably has pseudogout, which is caused by the deposition of calcium pyrophosphate dihydrate rather than monosodium urate. Pseudogout often strikes the wrist or the knee and does not commonly involve polyarticular attacks, as gout does.
Clinical experience has shown that with a suspicion of either gout or pseudogout, a short empiric course of anti-inflammatory treatment should be considered, said Dr. Rachow. "If it really is crystal induced, you’ll know in 12 hours and definitely within 24 hours" because the pain will begin to subside, he said.
"And, at that point, if you’ve started with a nonsteroidal anti-inflammatory and it’s working well, you can add a gastroprotective agent and continue the NSAID, reducing the dose once symptoms begin to improve. Or you can switch to colchicine."
"At your leisure, you can consider getting an x-ray," Dr. Rachow added, as detectable calcium deposits in the cartilage are typical of pseudogout.
If the anti-inflammatory treatment is "not working spectacularly well within 24 hours, you need to put the brakes on, close your office door, and think things over again," he said.
Facilities that have a sizable number of patients with frequent flares probably should have a nurse practitioner or of physician assistant trained to aspirate joints and arrange the logistics for sending out samples, Dr. Taler said.
The Longer Term
With a correct approach, "gout is eminently preventable and treatable in 90% of nursing home residents," said Dr. Weinstein. "The principles are studied, reported, and well described," he said. The American College of Rheumatology plans to release its first practice guidelines on the management of gout in 2012.
Decisions about managing acute attacks – whether to use NSAIDs, glucocorticoids, or oral colchicine – are rightly driven by the severity of gout and consideration of the patient’s coexisting illnesses and the drugs’ side effects. While NSAID use carries the risk of gastropathy, colchicine can cause diarrhea and other potentially serious side effects and should be avoided in patients who have renal or hepatic insufficiency.
Many clinicians consider colchicine a second-line therapy for acute gout, after NSAIDs or corticosteroids. In very elderly people, however, the treatment decision might be different. Dr. Weinstein said he worries about possible cardiac risks with the use of NSAIDs in very old patients. He has had success with the early use of low-dose colchicine in very elderly patients with reasonable kidney function, and he said that the drug "works best in the first 48-72 hours."
Parenteral corticosteroids, intra-articular injections, or even an oral prednisone taper are good options, he emphasized. Issues of whether and how to move from acute management of gout attacks to long-term urate-lowering therapy are taking on added significance in nursing homes as the prevalence of gout increases there.
Dr. Rachow recommended that hypouricemic therapy be initiated for patients who have documented hyperuricemia and a history of multiple attacks, and for patients who have developed tophi, which the therapy can dissolve.
It can even be considered for a frail, ill nursing home resident for whom a second gout attack would be unusually complicating and traumatic, said Dr. Rachow. "There may be residents who we can’t imagine having to hospitalize in the next year [for another gout attack], for whom we want to take possible recurrence out of the picture once and for all."
When it is deemed beneficial, the urate-lowering therapy must be undertaken with care, he emphasized. Dr. Rachow said that hypouricemic therapy should start only after an acute attack is completely resolved (or even 2-4 weeks after flare resolution), with cautious dosing and careful monitoring for adverse effects and, when possible, under the cover of a prophylactic anti-inflammatory drug. Low-dose colchicine has long been used to prevent flares associated with the lowering of urate.
Allopurinol, the xanthine oxidase inhibitor most commonly prescribed to lower urate levels, should be "started low and increased slowly" in older patients with renal impairment, Dr. Weinstein said. He advised beginning with 100 mg/day and increasing by 100 mg/day each month until the patient’s uric acid level is below 6 mg/dL.
Another xanthine oxidase inhibitor called febuxostat (Uloric) was approved in 2009 by the FDA for treatment of hyperuricemia in patients with gout, but its efficacy and safety compared with allopurinol is not fully established (N. Engl. J. Med. 2011;364:443-52). The new drug may have advantages, however, in that it appears to be safe without dose adjustment in patients with renal insufficiency, Dr. Weinstein said.
Also on the medication front is a newly approved drug called pegloticase (Krystexxa), which might be a good option for lowering serum uric acid levels and preventing gout attacks in patients who cannot use allopurinol, said Dr. Weinstein.
If a gout attack strikes during hypouricemic therapy, Dr. Weinstein cautioned, "Don’t change anything – don’t stop the allopurinol, don’t change the diuretic. ... Just add the steroid or whatever you’ve chosen for an acute attack. Treat the attack like any other."
Colcrys, the Costly Alternative
Physicians who have long had colchicine as part of their armamentarium for the treatment of acute gout and, more important, for gout-flare prophylaxis, are coping with the loss of an array of traditional colchicine products and the current availability of only one, recently approved formulation – Colcrys – which is 50 times the cost of the earlier versions.
The winnowing of options is a result of the Food and Drug Administration’s Unapproved Drugs Initiative of 2006 that aims to bring previously grandfathered prescription drugs in compliance with current FDA approval requirements for safety and efficacy.
In July 2009, the FDA approved URL Pharma to market Colcrys for treatment of acute gout flares and prophylaxis of flares of familial Mediterranean fever. The agency gave the company 3 years of marketing exclusivity for the acute gout indication. The FDA approved an indication for gout flare prophylaxis several months later. Then, in the Oct. 1, 2010, Federal Register, the agency announced its intention to "take enforcement action" against the manufacture and distribution of unapproved single-ingredient oral colchicine products.
The cost of Colcrys caused an uproar in the rheumatology community, and the American College of Rheumatology worked with URL Pharma to cut prices to patients finding it hard to afford the drug.
College officials have also been encouraging manufacturers of unapproved colchicine products to submit new drug applications for the gout prophylaxis indication. According to the college, URL Pharma does not have exclusivity for this indication, and one other manufacturer has filed an application to market its product for this indication.
In the meantime, the FDA has been calling attention to safety concerns identified during its review of Colcrys, namely, a risk for severe drug interactions in certain patients treated with colchicine and concomitant P-gp or strong CYP3A4 inhibitors such as clarithromycin. Also, a dosing study submitted as part of the Colcrys application indicated that low-dose colchicine (1.8 mg over 1 hour) was a better treatment for acute gout than was a high-dose regimen (4.8 mg over 6 hours).
Dr. Rachow and Dr. Taler said they had no conflict of interest to disclose on this topic. Dr. Weinstein disclosed that he has received research grants from Savient Pharmaceuticals, the maker of pegloticase, for a clinical study, but not for any therapeutic studies.
Robotic Techniques Show Promise for Pancreatic Procedures
Robotic surgery is continuing to expand its reach, with widespread interest in the technology for endometrial cancer staging and recent exploration of its viability for complex pancreatic resections and reconstructions.
Gynecologic oncologists and pancreatic surgeons aren’t the only ones vying for time with the da Vinci Surgical System (Intuitive Surgical) – the only such system currently on the market. Their experiences, however, offer perspective on some of the key issues – from operative time and cost effectiveness to training needs – that are being raised as robotic technology is adopted.
Pancreatic Surgery
"Robotic-assisted pancreatic resections and reconstructions can be performed safely with postoperative complication rates and fistula formation comparable to results observed with open techniques," according to Dr. Herbert J. Zeh III, and Dr. A. James Moser, codirectors of University of Pittsburgh Medical Center’s pancreatic cancer center, who reported their results with 30 robotic-assisted major pancreatic resections and reconstructions, including 24 pancreaticoduodenectomies (Arch. Surg. 2011:146:256-61).
By now, they said in an interview, they have used a robotic approach for more than 110 major pancreatic resections and reconstructions, including approximately 60 robotic-assisted pancreaticoduodenectomies, or Whipple procedures.
Together with surgeons at the Cleveland Clinic, the Mayo Clinic in Rochester, Minn., and the University of Pisa in Italy, Dr. Moser and Dr. Zeh have pooled outcomes data, and a combined report has been submitted for publication.
"We’ve established comparable safety, and we’re at the point now where our operating times are dropping steadily and we can start to see feasibility and applicability down the road," said Dr. Zeh of the department of surgery at the University of Pittsburgh. "In the next year or two, we’ll start to see whether there are real advantages such as decreased blood loss and better outcomes resulting from a diminished physiologic impact on the patient."
Reductions in postoperative morbidity could make the complex Whipple procedure "less fearsome" for many of the patients with early pancreatic cancer who currently refuse definitive treatment. Robotic surgery might also enable more patients to recover quickly and be well enough to complete chemotherapy, said Dr. Moser of the departments of surgery and cell biology at the university. With the open approach, almost 40%-50% of patients never recover enough to complete their chemotherapy regimens, he said.
Dr. Moser and Dr. Zeh, neither of whom has any financial relationship with Intuitive, began exploring minimally invasive approaches to the Whipple procedure about 2½ years ago. They have been using laparoscopy for distal pancreatectomies since 2001, but were concerned about their ability to take a traditional laparoscopic approach with the Whipple procedure, which involves complex reconstruction.
"The first laparoscopic pancreaticoduodenectomy was described in 1994, and between then and 2010 there were only about 150 cases reported. It didn’t catch on. I think it’s because it takes such a Herculean effort to overcome the limits of the technology [with its two-dimensional imaging and limited range of instrument motion]," said Dr. Zeh.
"We wanted to completely duplicate the open Whipple with a minimally invasive technique without making any compromises or altering any surgical principles because of the limitations of the technology," added Dr. Moser.
Dr. Michael L. Kendrick, who last year published one of the two largest series of totally laparoscopic Whipple procedures, covering 62 patients (Arch. Surg. 2010;145:19-23), maintains that he makes "no alternations or adaptations in surgical principle" with the purely laparoscopic approach, compared with open surgery.
Whether this approach or a robotic-assisted laparoscopic approach is best for patients needing a pancreaticoduodenectomy appears at this time to be "completely the surgeon’s choice" and not driven by any patient characteristics or clinical indications, according to Dr. Kendrick, who chairs the division of gastroenterologic and general surgery at the Mayo Clinic. Proving "any additional clinical advantage of robotic assistance over pure laparoscopy will be very difficult and is unlikely," he said, but "pancreas surgeons may prefer the robotic approach because it is easier to learn and master."
Dr. Kendrick’s experience might be unique. He performed his first laparoscopic Whipple procedure in 2007 and then added the robotic-assisted Whipple to his armamentarium in 2008. To date, he has performed approximately 135 minimally invasive pancreaticoduodenectomies, of which about 30 have been robotically assisted.
The Pittsburgh surgeons, who perform their robotic-assisted Whipple procedures together, place the learning curve for robotic-assisted Whipple at approximately 60 operations – about the same number experts consider necessary with the open approach. While the median operating time in their first reported series of robotic-assisted pancreaticoduodenectomies was almost 9 ½ hours (at least several hours longer than needed for an experienced surgeon to complete an open Whipple), it has steadily dropped to 7-8 hours, they said.
An early laparoscopic Whipple for Dr. Kendrick also took approximately 10 hours, "but within 20-30 cases, it was down to 4 or 5 hours, sometimes 6," he said. "There’s a lower average operative time for laparoscopic Whipple ... but [in the end], is that really an important factor if the patient still gains the benefits of [the minimally invasive surgery]?"
Gynecologic Surgery
In gynecologic oncology, where robotic surgery in recent years has been rapidly embraced as a tool for performing minimally invasive endometrial cancer staging, operating time and cost are factors in an "ongoing debate in over whether robotics provides an advantage over laparoscopy for lymph node dissection," said Dr. James E. Delmore, director of gynecologic oncology for the University of Kansas, Wichita.
At least a half-dozen studies have found that robotic-assisted hysterectomy and lymphadenectomy for endometrial cancer results in significantly shorter hospital stays as well as fewer wound infections and postoperative complications compared with an open approach, according to Dr. Delmore, who has served as a proctor for Intuitive. However, fewer studies have compared all three approaches (robotic assisted, traditional laparoscopy, and open).
"It’s hard to determine and analyze procedure costs in many hospital systems," he said. But "from what we can tell thus far, purely laparoscopic hysterectomy with removal of the lymph nodes is the least expensive approach, followed by robotics and then abdominal."
Such cost differentials were demonstrated in a study comparing the outcomes and cost of endometrial cancer staging performed via traditional laparotomy, standard laparoscopy, and robotic techniques. Dr. Maria C. Bell performed all of the procedures (40 robotic, 40 laparotomy, and 30 laparoscopic) at the Sanford Clinic in Sioux Falls, S.D.
Robotic hysterectomy took about an hour longer to perform than did hysterectomy completed via laparotomy (with no significant difference in operating time, compared with pure laparoscopy), but resulted in the lowest complication rate of the three approaches and the shortest average return to normal activity. Estimated blood loss and average length of stay were both significantly reduced for the robotic cohort, compared with laparotomy, and were comparable to laparoscopy. There were no differences in lymph node retrieval among the three groups (Gyn. Oncol. 2008;111:407-11).
When both direct and indirect costs were considered (including a measure by the Lewin Group of "societal/productivity" costs and the cost of the robot on a 5-year amortization schedule), the total average costs for hysterectomy with staging were $12,943 with laparotomy, $7,569 for standard laparoscopy, and $8,212 for robotic assistance.
The overall decreased cost for robotic surgery was unexpected and could be a result of money "invested up front [being] recouped by less time rounding ... and less time taking care of complications," as well as lower societal costs, wrote Dr. Bell, also of the University of South Dakota, Vermillion.
"I was very surprised that laparotomy was the most costly to the hospital," Dr. Bell said in an interview. (One of the coauthors of the report, Usha Seshadri-Kreaden, is employed by Intuitive, and Dr. Bell is a proctor for Intuitive, but the company did not sponsor the study.)
Other investigators who have compared robotic-assisted and conventional laparoscopic hysterectomies have reported higher per-case hospital costs with the robot. With hysterectomy having surpassed prostatectomy in 2010 as the highest-volume procedure for the da Vinci Surgical System, according to its manufacturer, the value of robotics for routine hysterectomy may well be increasingly scrutinized.
The da Vinci is used to treat more than 90% of endometrial cancer patients at Dr. Bell’s and Dr. Delmore’s institutions, but both surgeons are quick to emphasize the need for more data on outcomes and cost-effectiveness. "The temptation of ‘the patient wants it, so we need to offer it’ needs to be tempered with the question ‘are we making progress doing it?’ " said Dr. Delmore.
Training Needs
Institutions are individually grappling with how best to train residents in robotic-assisted surgery. The University of Kansas model includes an online tutorial, training with inanimate objects, animal lab training, and bedside assistance in real robotic-assisted hysterectomies. Dr. Delmore and his colleagues have a study underway to look at how graduate ob.gyns. use this training.
Within the realm of hepatobiliary and pancreatic surgery, where a minimally invasive approach is younger and where training models for the open Whipple procedure are still deliberated, "there is a dramatic appetite for minimally invasive skills," Dr. Kendrick said. HBP fellows at the Mayo Clinic currently are exposed to minimally invasive surgery, but "our goal over the next 2 years is to incorporate an even more significant focus" on robotics and laparoscopy, he said.
At the University of Pittsburgh, surgical oncology and HBP fellows and residents receive training with the robot in a "dry lab" format, Dr. Moser said. Trainees also can sit during actual cases at a second "teaching console" that is part of the current da Vinci Surgical System.
"We typically teach robotic cholecystectomy and sewing of the duodenojejunostomy during the Whipple procedure and allow them to do these portions of the procedure," Dr. Moser said.
Dr. Kendrick, who said he has no disclosures to make relevant to this article, hopes to see several fellowships established nationwide to address minimally invasive pancreas and liver surgery. However, "we have to be cautious about credentialing and assessing the adequacy of training. There’s a real mortality rate and a major complication rate [with the Whipple procedure] ... We’re trying to make it lower and lower with time, and not go backward."
Robotic surgery is continuing to expand its reach, with widespread interest in the technology for endometrial cancer staging and recent exploration of its viability for complex pancreatic resections and reconstructions.
Gynecologic oncologists and pancreatic surgeons aren’t the only ones vying for time with the da Vinci Surgical System (Intuitive Surgical) – the only such system currently on the market. Their experiences, however, offer perspective on some of the key issues – from operative time and cost effectiveness to training needs – that are being raised as robotic technology is adopted.
Pancreatic Surgery
"Robotic-assisted pancreatic resections and reconstructions can be performed safely with postoperative complication rates and fistula formation comparable to results observed with open techniques," according to Dr. Herbert J. Zeh III, and Dr. A. James Moser, codirectors of University of Pittsburgh Medical Center’s pancreatic cancer center, who reported their results with 30 robotic-assisted major pancreatic resections and reconstructions, including 24 pancreaticoduodenectomies (Arch. Surg. 2011:146:256-61).
By now, they said in an interview, they have used a robotic approach for more than 110 major pancreatic resections and reconstructions, including approximately 60 robotic-assisted pancreaticoduodenectomies, or Whipple procedures.
Together with surgeons at the Cleveland Clinic, the Mayo Clinic in Rochester, Minn., and the University of Pisa in Italy, Dr. Moser and Dr. Zeh have pooled outcomes data, and a combined report has been submitted for publication.
"We’ve established comparable safety, and we’re at the point now where our operating times are dropping steadily and we can start to see feasibility and applicability down the road," said Dr. Zeh of the department of surgery at the University of Pittsburgh. "In the next year or two, we’ll start to see whether there are real advantages such as decreased blood loss and better outcomes resulting from a diminished physiologic impact on the patient."
Reductions in postoperative morbidity could make the complex Whipple procedure "less fearsome" for many of the patients with early pancreatic cancer who currently refuse definitive treatment. Robotic surgery might also enable more patients to recover quickly and be well enough to complete chemotherapy, said Dr. Moser of the departments of surgery and cell biology at the university. With the open approach, almost 40%-50% of patients never recover enough to complete their chemotherapy regimens, he said.
Dr. Moser and Dr. Zeh, neither of whom has any financial relationship with Intuitive, began exploring minimally invasive approaches to the Whipple procedure about 2½ years ago. They have been using laparoscopy for distal pancreatectomies since 2001, but were concerned about their ability to take a traditional laparoscopic approach with the Whipple procedure, which involves complex reconstruction.
"The first laparoscopic pancreaticoduodenectomy was described in 1994, and between then and 2010 there were only about 150 cases reported. It didn’t catch on. I think it’s because it takes such a Herculean effort to overcome the limits of the technology [with its two-dimensional imaging and limited range of instrument motion]," said Dr. Zeh.
"We wanted to completely duplicate the open Whipple with a minimally invasive technique without making any compromises or altering any surgical principles because of the limitations of the technology," added Dr. Moser.
Dr. Michael L. Kendrick, who last year published one of the two largest series of totally laparoscopic Whipple procedures, covering 62 patients (Arch. Surg. 2010;145:19-23), maintains that he makes "no alternations or adaptations in surgical principle" with the purely laparoscopic approach, compared with open surgery.
Whether this approach or a robotic-assisted laparoscopic approach is best for patients needing a pancreaticoduodenectomy appears at this time to be "completely the surgeon’s choice" and not driven by any patient characteristics or clinical indications, according to Dr. Kendrick, who chairs the division of gastroenterologic and general surgery at the Mayo Clinic. Proving "any additional clinical advantage of robotic assistance over pure laparoscopy will be very difficult and is unlikely," he said, but "pancreas surgeons may prefer the robotic approach because it is easier to learn and master."
Dr. Kendrick’s experience might be unique. He performed his first laparoscopic Whipple procedure in 2007 and then added the robotic-assisted Whipple to his armamentarium in 2008. To date, he has performed approximately 135 minimally invasive pancreaticoduodenectomies, of which about 30 have been robotically assisted.
The Pittsburgh surgeons, who perform their robotic-assisted Whipple procedures together, place the learning curve for robotic-assisted Whipple at approximately 60 operations – about the same number experts consider necessary with the open approach. While the median operating time in their first reported series of robotic-assisted pancreaticoduodenectomies was almost 9 ½ hours (at least several hours longer than needed for an experienced surgeon to complete an open Whipple), it has steadily dropped to 7-8 hours, they said.
An early laparoscopic Whipple for Dr. Kendrick also took approximately 10 hours, "but within 20-30 cases, it was down to 4 or 5 hours, sometimes 6," he said. "There’s a lower average operative time for laparoscopic Whipple ... but [in the end], is that really an important factor if the patient still gains the benefits of [the minimally invasive surgery]?"
Gynecologic Surgery
In gynecologic oncology, where robotic surgery in recent years has been rapidly embraced as a tool for performing minimally invasive endometrial cancer staging, operating time and cost are factors in an "ongoing debate in over whether robotics provides an advantage over laparoscopy for lymph node dissection," said Dr. James E. Delmore, director of gynecologic oncology for the University of Kansas, Wichita.
At least a half-dozen studies have found that robotic-assisted hysterectomy and lymphadenectomy for endometrial cancer results in significantly shorter hospital stays as well as fewer wound infections and postoperative complications compared with an open approach, according to Dr. Delmore, who has served as a proctor for Intuitive. However, fewer studies have compared all three approaches (robotic assisted, traditional laparoscopy, and open).
"It’s hard to determine and analyze procedure costs in many hospital systems," he said. But "from what we can tell thus far, purely laparoscopic hysterectomy with removal of the lymph nodes is the least expensive approach, followed by robotics and then abdominal."
Such cost differentials were demonstrated in a study comparing the outcomes and cost of endometrial cancer staging performed via traditional laparotomy, standard laparoscopy, and robotic techniques. Dr. Maria C. Bell performed all of the procedures (40 robotic, 40 laparotomy, and 30 laparoscopic) at the Sanford Clinic in Sioux Falls, S.D.
Robotic hysterectomy took about an hour longer to perform than did hysterectomy completed via laparotomy (with no significant difference in operating time, compared with pure laparoscopy), but resulted in the lowest complication rate of the three approaches and the shortest average return to normal activity. Estimated blood loss and average length of stay were both significantly reduced for the robotic cohort, compared with laparotomy, and were comparable to laparoscopy. There were no differences in lymph node retrieval among the three groups (Gyn. Oncol. 2008;111:407-11).
When both direct and indirect costs were considered (including a measure by the Lewin Group of "societal/productivity" costs and the cost of the robot on a 5-year amortization schedule), the total average costs for hysterectomy with staging were $12,943 with laparotomy, $7,569 for standard laparoscopy, and $8,212 for robotic assistance.
The overall decreased cost for robotic surgery was unexpected and could be a result of money "invested up front [being] recouped by less time rounding ... and less time taking care of complications," as well as lower societal costs, wrote Dr. Bell, also of the University of South Dakota, Vermillion.
"I was very surprised that laparotomy was the most costly to the hospital," Dr. Bell said in an interview. (One of the coauthors of the report, Usha Seshadri-Kreaden, is employed by Intuitive, and Dr. Bell is a proctor for Intuitive, but the company did not sponsor the study.)
Other investigators who have compared robotic-assisted and conventional laparoscopic hysterectomies have reported higher per-case hospital costs with the robot. With hysterectomy having surpassed prostatectomy in 2010 as the highest-volume procedure for the da Vinci Surgical System, according to its manufacturer, the value of robotics for routine hysterectomy may well be increasingly scrutinized.
The da Vinci is used to treat more than 90% of endometrial cancer patients at Dr. Bell’s and Dr. Delmore’s institutions, but both surgeons are quick to emphasize the need for more data on outcomes and cost-effectiveness. "The temptation of ‘the patient wants it, so we need to offer it’ needs to be tempered with the question ‘are we making progress doing it?’ " said Dr. Delmore.
Training Needs
Institutions are individually grappling with how best to train residents in robotic-assisted surgery. The University of Kansas model includes an online tutorial, training with inanimate objects, animal lab training, and bedside assistance in real robotic-assisted hysterectomies. Dr. Delmore and his colleagues have a study underway to look at how graduate ob.gyns. use this training.
Within the realm of hepatobiliary and pancreatic surgery, where a minimally invasive approach is younger and where training models for the open Whipple procedure are still deliberated, "there is a dramatic appetite for minimally invasive skills," Dr. Kendrick said. HBP fellows at the Mayo Clinic currently are exposed to minimally invasive surgery, but "our goal over the next 2 years is to incorporate an even more significant focus" on robotics and laparoscopy, he said.
At the University of Pittsburgh, surgical oncology and HBP fellows and residents receive training with the robot in a "dry lab" format, Dr. Moser said. Trainees also can sit during actual cases at a second "teaching console" that is part of the current da Vinci Surgical System.
"We typically teach robotic cholecystectomy and sewing of the duodenojejunostomy during the Whipple procedure and allow them to do these portions of the procedure," Dr. Moser said.
Dr. Kendrick, who said he has no disclosures to make relevant to this article, hopes to see several fellowships established nationwide to address minimally invasive pancreas and liver surgery. However, "we have to be cautious about credentialing and assessing the adequacy of training. There’s a real mortality rate and a major complication rate [with the Whipple procedure] ... We’re trying to make it lower and lower with time, and not go backward."
Robotic surgery is continuing to expand its reach, with widespread interest in the technology for endometrial cancer staging and recent exploration of its viability for complex pancreatic resections and reconstructions.
Gynecologic oncologists and pancreatic surgeons aren’t the only ones vying for time with the da Vinci Surgical System (Intuitive Surgical) – the only such system currently on the market. Their experiences, however, offer perspective on some of the key issues – from operative time and cost effectiveness to training needs – that are being raised as robotic technology is adopted.
Pancreatic Surgery
"Robotic-assisted pancreatic resections and reconstructions can be performed safely with postoperative complication rates and fistula formation comparable to results observed with open techniques," according to Dr. Herbert J. Zeh III, and Dr. A. James Moser, codirectors of University of Pittsburgh Medical Center’s pancreatic cancer center, who reported their results with 30 robotic-assisted major pancreatic resections and reconstructions, including 24 pancreaticoduodenectomies (Arch. Surg. 2011:146:256-61).
By now, they said in an interview, they have used a robotic approach for more than 110 major pancreatic resections and reconstructions, including approximately 60 robotic-assisted pancreaticoduodenectomies, or Whipple procedures.
Together with surgeons at the Cleveland Clinic, the Mayo Clinic in Rochester, Minn., and the University of Pisa in Italy, Dr. Moser and Dr. Zeh have pooled outcomes data, and a combined report has been submitted for publication.
"We’ve established comparable safety, and we’re at the point now where our operating times are dropping steadily and we can start to see feasibility and applicability down the road," said Dr. Zeh of the department of surgery at the University of Pittsburgh. "In the next year or two, we’ll start to see whether there are real advantages such as decreased blood loss and better outcomes resulting from a diminished physiologic impact on the patient."
Reductions in postoperative morbidity could make the complex Whipple procedure "less fearsome" for many of the patients with early pancreatic cancer who currently refuse definitive treatment. Robotic surgery might also enable more patients to recover quickly and be well enough to complete chemotherapy, said Dr. Moser of the departments of surgery and cell biology at the university. With the open approach, almost 40%-50% of patients never recover enough to complete their chemotherapy regimens, he said.
Dr. Moser and Dr. Zeh, neither of whom has any financial relationship with Intuitive, began exploring minimally invasive approaches to the Whipple procedure about 2½ years ago. They have been using laparoscopy for distal pancreatectomies since 2001, but were concerned about their ability to take a traditional laparoscopic approach with the Whipple procedure, which involves complex reconstruction.
"The first laparoscopic pancreaticoduodenectomy was described in 1994, and between then and 2010 there were only about 150 cases reported. It didn’t catch on. I think it’s because it takes such a Herculean effort to overcome the limits of the technology [with its two-dimensional imaging and limited range of instrument motion]," said Dr. Zeh.
"We wanted to completely duplicate the open Whipple with a minimally invasive technique without making any compromises or altering any surgical principles because of the limitations of the technology," added Dr. Moser.
Dr. Michael L. Kendrick, who last year published one of the two largest series of totally laparoscopic Whipple procedures, covering 62 patients (Arch. Surg. 2010;145:19-23), maintains that he makes "no alternations or adaptations in surgical principle" with the purely laparoscopic approach, compared with open surgery.
Whether this approach or a robotic-assisted laparoscopic approach is best for patients needing a pancreaticoduodenectomy appears at this time to be "completely the surgeon’s choice" and not driven by any patient characteristics or clinical indications, according to Dr. Kendrick, who chairs the division of gastroenterologic and general surgery at the Mayo Clinic. Proving "any additional clinical advantage of robotic assistance over pure laparoscopy will be very difficult and is unlikely," he said, but "pancreas surgeons may prefer the robotic approach because it is easier to learn and master."
Dr. Kendrick’s experience might be unique. He performed his first laparoscopic Whipple procedure in 2007 and then added the robotic-assisted Whipple to his armamentarium in 2008. To date, he has performed approximately 135 minimally invasive pancreaticoduodenectomies, of which about 30 have been robotically assisted.
The Pittsburgh surgeons, who perform their robotic-assisted Whipple procedures together, place the learning curve for robotic-assisted Whipple at approximately 60 operations – about the same number experts consider necessary with the open approach. While the median operating time in their first reported series of robotic-assisted pancreaticoduodenectomies was almost 9 ½ hours (at least several hours longer than needed for an experienced surgeon to complete an open Whipple), it has steadily dropped to 7-8 hours, they said.
An early laparoscopic Whipple for Dr. Kendrick also took approximately 10 hours, "but within 20-30 cases, it was down to 4 or 5 hours, sometimes 6," he said. "There’s a lower average operative time for laparoscopic Whipple ... but [in the end], is that really an important factor if the patient still gains the benefits of [the minimally invasive surgery]?"
Gynecologic Surgery
In gynecologic oncology, where robotic surgery in recent years has been rapidly embraced as a tool for performing minimally invasive endometrial cancer staging, operating time and cost are factors in an "ongoing debate in over whether robotics provides an advantage over laparoscopy for lymph node dissection," said Dr. James E. Delmore, director of gynecologic oncology for the University of Kansas, Wichita.
At least a half-dozen studies have found that robotic-assisted hysterectomy and lymphadenectomy for endometrial cancer results in significantly shorter hospital stays as well as fewer wound infections and postoperative complications compared with an open approach, according to Dr. Delmore, who has served as a proctor for Intuitive. However, fewer studies have compared all three approaches (robotic assisted, traditional laparoscopy, and open).
"It’s hard to determine and analyze procedure costs in many hospital systems," he said. But "from what we can tell thus far, purely laparoscopic hysterectomy with removal of the lymph nodes is the least expensive approach, followed by robotics and then abdominal."
Such cost differentials were demonstrated in a study comparing the outcomes and cost of endometrial cancer staging performed via traditional laparotomy, standard laparoscopy, and robotic techniques. Dr. Maria C. Bell performed all of the procedures (40 robotic, 40 laparotomy, and 30 laparoscopic) at the Sanford Clinic in Sioux Falls, S.D.
Robotic hysterectomy took about an hour longer to perform than did hysterectomy completed via laparotomy (with no significant difference in operating time, compared with pure laparoscopy), but resulted in the lowest complication rate of the three approaches and the shortest average return to normal activity. Estimated blood loss and average length of stay were both significantly reduced for the robotic cohort, compared with laparotomy, and were comparable to laparoscopy. There were no differences in lymph node retrieval among the three groups (Gyn. Oncol. 2008;111:407-11).
When both direct and indirect costs were considered (including a measure by the Lewin Group of "societal/productivity" costs and the cost of the robot on a 5-year amortization schedule), the total average costs for hysterectomy with staging were $12,943 with laparotomy, $7,569 for standard laparoscopy, and $8,212 for robotic assistance.
The overall decreased cost for robotic surgery was unexpected and could be a result of money "invested up front [being] recouped by less time rounding ... and less time taking care of complications," as well as lower societal costs, wrote Dr. Bell, also of the University of South Dakota, Vermillion.
"I was very surprised that laparotomy was the most costly to the hospital," Dr. Bell said in an interview. (One of the coauthors of the report, Usha Seshadri-Kreaden, is employed by Intuitive, and Dr. Bell is a proctor for Intuitive, but the company did not sponsor the study.)
Other investigators who have compared robotic-assisted and conventional laparoscopic hysterectomies have reported higher per-case hospital costs with the robot. With hysterectomy having surpassed prostatectomy in 2010 as the highest-volume procedure for the da Vinci Surgical System, according to its manufacturer, the value of robotics for routine hysterectomy may well be increasingly scrutinized.
The da Vinci is used to treat more than 90% of endometrial cancer patients at Dr. Bell’s and Dr. Delmore’s institutions, but both surgeons are quick to emphasize the need for more data on outcomes and cost-effectiveness. "The temptation of ‘the patient wants it, so we need to offer it’ needs to be tempered with the question ‘are we making progress doing it?’ " said Dr. Delmore.
Training Needs
Institutions are individually grappling with how best to train residents in robotic-assisted surgery. The University of Kansas model includes an online tutorial, training with inanimate objects, animal lab training, and bedside assistance in real robotic-assisted hysterectomies. Dr. Delmore and his colleagues have a study underway to look at how graduate ob.gyns. use this training.
Within the realm of hepatobiliary and pancreatic surgery, where a minimally invasive approach is younger and where training models for the open Whipple procedure are still deliberated, "there is a dramatic appetite for minimally invasive skills," Dr. Kendrick said. HBP fellows at the Mayo Clinic currently are exposed to minimally invasive surgery, but "our goal over the next 2 years is to incorporate an even more significant focus" on robotics and laparoscopy, he said.
At the University of Pittsburgh, surgical oncology and HBP fellows and residents receive training with the robot in a "dry lab" format, Dr. Moser said. Trainees also can sit during actual cases at a second "teaching console" that is part of the current da Vinci Surgical System.
"We typically teach robotic cholecystectomy and sewing of the duodenojejunostomy during the Whipple procedure and allow them to do these portions of the procedure," Dr. Moser said.
Dr. Kendrick, who said he has no disclosures to make relevant to this article, hopes to see several fellowships established nationwide to address minimally invasive pancreas and liver surgery. However, "we have to be cautious about credentialing and assessing the adequacy of training. There’s a real mortality rate and a major complication rate [with the Whipple procedure] ... We’re trying to make it lower and lower with time, and not go backward."
Robotic Techniques Show Promise for Pancreatic Procedures
Robotic surgery is continuing to expand its reach, with widespread interest in the technology for endometrial cancer staging and recent exploration of its viability for complex pancreatic resections and reconstructions.
Gynecologic oncologists and pancreatic surgeons aren’t the only ones vying for time with the da Vinci Surgical System (Intuitive Surgical) – the only such system currently on the market. Their experiences, however, offer perspective on some of the key issues – from operative time and cost effectiveness to training needs – that are being raised as robotic technology is adopted.
Pancreatic Surgery
"Robotic-assisted pancreatic resections and reconstructions can be performed safely with postoperative complication rates and fistula formation comparable to results observed with open techniques," according to Dr. Herbert J. Zeh III, and Dr. A. James Moser, codirectors of University of Pittsburgh Medical Center’s pancreatic cancer center, who reported their results with 30 robotic-assisted major pancreatic resections and reconstructions, including 24 pancreaticoduodenectomies (Arch. Surg. 2011:146:256-61).
By now, they said in an interview, they have used a robotic approach for more than 110 major pancreatic resections and reconstructions, including approximately 60 robotic-assisted pancreaticoduodenectomies, or Whipple procedures.
Together with surgeons at the Cleveland Clinic, the Mayo Clinic in Rochester, Minn., and the University of Pisa in Italy, Dr. Moser and Dr. Zeh have pooled outcomes data, and a combined report has been submitted for publication.
"We’ve established comparable safety, and we’re at the point now where our operating times are dropping steadily and we can start to see feasibility and applicability down the road," said Dr. Zeh of the department of surgery at the University of Pittsburgh. "In the next year or two, we’ll start to see whether there are real advantages such as decreased blood loss and better outcomes resulting from a diminished physiologic impact on the patient."
Reductions in postoperative morbidity could make the complex Whipple procedure "less fearsome" for many of the patients with early pancreatic cancer who currently refuse definitive treatment. Robotic surgery might also enable more patients to recover quickly and be well enough to complete chemotherapy, said Dr. Moser of the departments of surgery and cell biology at the university. With the open approach, almost 40%-50% of patients never recover enough to complete their chemotherapy regimens, he said.
Dr. Moser and Dr. Zeh, neither of whom has any financial relationship with Intuitive, began exploring minimally invasive approaches to the Whipple procedure about 2½ years ago. They have been using laparoscopy for distal pancreatectomies since 2001, but were concerned about their ability to take a traditional laparoscopic approach with the Whipple procedure, which involves complex reconstruction.
"The first laparoscopic pancreaticoduodenectomy was described in 1994, and between then and 2010 there were only about 150 cases reported. It didn’t catch on. I think it’s because it takes such a Herculean effort to overcome the limits of the technology [with its two-dimensional imaging and limited range of instrument motion]," said Dr. Zeh.
"We wanted to completely duplicate the open Whipple with a minimally invasive technique without making any compromises or altering any surgical principles because of the limitations of the technology," added Dr. Moser.
Dr. Michael L. Kendrick, who last year published one of the two largest series of totally laparoscopic Whipple procedures, covering 62 patients (Arch. Surg. 2010;145:19-23), maintains that he makes "no alternations or adaptations in surgical principle" with the purely laparoscopic approach, compared with open surgery.
Whether this approach or a robotic-assisted laparoscopic approach is best for patients needing a pancreaticoduodenectomy appears at this time to be "completely the surgeon’s choice" and not driven by any patient characteristics or clinical indications, according to Dr. Kendrick, who chairs the division of gastroenterologic and general surgery at the Mayo Clinic. Proving "any additional clinical advantage of robotic assistance over pure laparoscopy will be very difficult and is unlikely," he said, but "pancreas surgeons may prefer the robotic approach because it is easier to learn and master."
Dr. Kendrick’s experience might be unique. He performed his first laparoscopic Whipple procedure in 2007 and then added the robotic-assisted Whipple to his armamentarium in 2008. To date, he has performed approximately 135 minimally invasive pancreaticoduodenectomies, of which about 30 have been robotically assisted.
The Pittsburgh surgeons, who perform their robotic-assisted Whipple procedures together, place the learning curve for robotic-assisted Whipple at approximately 60 operations – about the same number experts consider necessary with the open approach. While the median operating time in their first reported series of robotic-assisted pancreaticoduodenectomies was almost 9 ½ hours (at least several hours longer than needed for an experienced surgeon to complete an open Whipple), it has steadily dropped to 7-8 hours, they said.
An early laparoscopic Whipple for Dr. Kendrick also took approximately 10 hours, "but within 20-30 cases, it was down to 4 or 5 hours, sometimes 6," he said. "There’s a lower average operative time for laparoscopic Whipple ... but [in the end], is that really an important factor if the patient still gains the benefits of [the minimally invasive surgery]?"
Gynecologic Surgery
In gynecologic oncology, where robotic surgery in recent years has been rapidly embraced as a tool for performing minimally invasive endometrial cancer staging, operating time and cost are factors in an "ongoing debate in over whether robotics provides an advantage over laparoscopy for lymph node dissection," said Dr. James E. Delmore, director of gynecologic oncology for the University of Kansas, Wichita.
At least a half-dozen studies have found that robotic-assisted hysterectomy and lymphadenectomy for endometrial cancer results in significantly shorter hospital stays as well as fewer wound infections and postoperative complications compared with an open approach, according to Dr. Delmore, who has served as a proctor for Intuitive. However, fewer studies have compared all three approaches (robotic assisted, traditional laparoscopy, and open).
"It’s hard to determine and analyze procedure costs in many hospital systems," he said. But "from what we can tell thus far, purely laparoscopic hysterectomy with removal of the lymph nodes is the least expensive approach, followed by robotics and then abdominal."
Such cost differentials were demonstrated in a study comparing the outcomes and cost of endometrial cancer staging performed via traditional laparotomy, standard laparoscopy, and robotic techniques. Dr. Maria C. Bell performed all of the procedures (40 robotic, 40 laparotomy, and 30 laparoscopic) at the Sanford Clinic in Sioux Falls, S.D.
Robotic hysterectomy took about an hour longer to perform than did hysterectomy completed via laparotomy (with no significant difference in operating time, compared with pure laparoscopy), but resulted in the lowest complication rate of the three approaches and the shortest average return to normal activity. Estimated blood loss and average length of stay were both significantly reduced for the robotic cohort, compared with laparotomy, and were comparable to laparoscopy. There were no differences in lymph node retrieval among the three groups (Gyn. Oncol. 2008;111:407-11).
When both direct and indirect costs were considered (including a measure by the Lewin Group of "societal/productivity" costs and the cost of the robot on a 5-year amortization schedule), the total average costs for hysterectomy with staging were $12,943 with laparotomy, $7,569 for standard laparoscopy, and $8,212 for robotic assistance.
The overall decreased cost for robotic surgery was unexpected and could be a result of money "invested up front [being] recouped by less time rounding ... and less time taking care of complications," as well as lower societal costs, wrote Dr. Bell, also of the University of South Dakota, Vermillion.
"I was very surprised that laparotomy was the most costly to the hospital," Dr. Bell said in an interview. (One of the coauthors of the report, Usha Seshadri-Kreaden, is employed by Intuitive, and Dr. Bell is a proctor for Intuitive, but the company did not sponsor the study.)
Other investigators who have compared robotic-assisted and conventional laparoscopic hysterectomies have reported higher per-case hospital costs with the robot. With hysterectomy having surpassed prostatectomy in 2010 as the highest-volume procedure for the da Vinci Surgical System, according to its manufacturer, the value of robotics for routine hysterectomy may well be increasingly scrutinized.
The da Vinci is used to treat more than 90% of endometrial cancer patients at Dr. Bell’s and Dr. Delmore’s institutions, but both surgeons are quick to emphasize the need for more data on outcomes and cost-effectiveness. "The temptation of ‘the patient wants it, so we need to offer it’ needs to be tempered with the question ‘are we making progress doing it?’ " said Dr. Delmore.
Training Needs
Institutions are individually grappling with how best to train residents in robotic-assisted surgery. The University of Kansas model includes an online tutorial, training with inanimate objects, animal lab training, and bedside assistance in real robotic-assisted hysterectomies. Dr. Delmore and his colleagues have a study underway to look at how graduate ob.gyns. use this training.
Within the realm of hepatobiliary and pancreatic surgery, where a minimally invasive approach is younger and where training models for the open Whipple procedure are still deliberated, "there is a dramatic appetite for minimally invasive skills," Dr. Kendrick said. HBP fellows at the Mayo Clinic currently are exposed to minimally invasive surgery, but "our goal over the next 2 years is to incorporate an even more significant focus" on robotics and laparoscopy, he said.
At the University of Pittsburgh, surgical oncology and HBP fellows and residents receive training with the robot in a "dry lab" format, Dr. Moser said. Trainees also can sit during actual cases at a second "teaching console" that is part of the current da Vinci Surgical System.
"We typically teach robotic cholecystectomy and sewing of the duodenojejunostomy during the Whipple procedure and allow them to do these portions of the procedure," Dr. Moser said.
Dr. Kendrick, who said he has no disclosures to make relevant to this article, hopes to see several fellowships established nationwide to address minimally invasive pancreas and liver surgery. However, "we have to be cautious about credentialing and assessing the adequacy of training. There’s a real mortality rate and a major complication rate [with the Whipple procedure] ... We’re trying to make it lower and lower with time, and not go backward."
Robotic surgery is continuing to expand its reach, with widespread interest in the technology for endometrial cancer staging and recent exploration of its viability for complex pancreatic resections and reconstructions.
Gynecologic oncologists and pancreatic surgeons aren’t the only ones vying for time with the da Vinci Surgical System (Intuitive Surgical) – the only such system currently on the market. Their experiences, however, offer perspective on some of the key issues – from operative time and cost effectiveness to training needs – that are being raised as robotic technology is adopted.
Pancreatic Surgery
"Robotic-assisted pancreatic resections and reconstructions can be performed safely with postoperative complication rates and fistula formation comparable to results observed with open techniques," according to Dr. Herbert J. Zeh III, and Dr. A. James Moser, codirectors of University of Pittsburgh Medical Center’s pancreatic cancer center, who reported their results with 30 robotic-assisted major pancreatic resections and reconstructions, including 24 pancreaticoduodenectomies (Arch. Surg. 2011:146:256-61).
By now, they said in an interview, they have used a robotic approach for more than 110 major pancreatic resections and reconstructions, including approximately 60 robotic-assisted pancreaticoduodenectomies, or Whipple procedures.
Together with surgeons at the Cleveland Clinic, the Mayo Clinic in Rochester, Minn., and the University of Pisa in Italy, Dr. Moser and Dr. Zeh have pooled outcomes data, and a combined report has been submitted for publication.
"We’ve established comparable safety, and we’re at the point now where our operating times are dropping steadily and we can start to see feasibility and applicability down the road," said Dr. Zeh of the department of surgery at the University of Pittsburgh. "In the next year or two, we’ll start to see whether there are real advantages such as decreased blood loss and better outcomes resulting from a diminished physiologic impact on the patient."
Reductions in postoperative morbidity could make the complex Whipple procedure "less fearsome" for many of the patients with early pancreatic cancer who currently refuse definitive treatment. Robotic surgery might also enable more patients to recover quickly and be well enough to complete chemotherapy, said Dr. Moser of the departments of surgery and cell biology at the university. With the open approach, almost 40%-50% of patients never recover enough to complete their chemotherapy regimens, he said.
Dr. Moser and Dr. Zeh, neither of whom has any financial relationship with Intuitive, began exploring minimally invasive approaches to the Whipple procedure about 2½ years ago. They have been using laparoscopy for distal pancreatectomies since 2001, but were concerned about their ability to take a traditional laparoscopic approach with the Whipple procedure, which involves complex reconstruction.
"The first laparoscopic pancreaticoduodenectomy was described in 1994, and between then and 2010 there were only about 150 cases reported. It didn’t catch on. I think it’s because it takes such a Herculean effort to overcome the limits of the technology [with its two-dimensional imaging and limited range of instrument motion]," said Dr. Zeh.
"We wanted to completely duplicate the open Whipple with a minimally invasive technique without making any compromises or altering any surgical principles because of the limitations of the technology," added Dr. Moser.
Dr. Michael L. Kendrick, who last year published one of the two largest series of totally laparoscopic Whipple procedures, covering 62 patients (Arch. Surg. 2010;145:19-23), maintains that he makes "no alternations or adaptations in surgical principle" with the purely laparoscopic approach, compared with open surgery.
Whether this approach or a robotic-assisted laparoscopic approach is best for patients needing a pancreaticoduodenectomy appears at this time to be "completely the surgeon’s choice" and not driven by any patient characteristics or clinical indications, according to Dr. Kendrick, who chairs the division of gastroenterologic and general surgery at the Mayo Clinic. Proving "any additional clinical advantage of robotic assistance over pure laparoscopy will be very difficult and is unlikely," he said, but "pancreas surgeons may prefer the robotic approach because it is easier to learn and master."
Dr. Kendrick’s experience might be unique. He performed his first laparoscopic Whipple procedure in 2007 and then added the robotic-assisted Whipple to his armamentarium in 2008. To date, he has performed approximately 135 minimally invasive pancreaticoduodenectomies, of which about 30 have been robotically assisted.
The Pittsburgh surgeons, who perform their robotic-assisted Whipple procedures together, place the learning curve for robotic-assisted Whipple at approximately 60 operations – about the same number experts consider necessary with the open approach. While the median operating time in their first reported series of robotic-assisted pancreaticoduodenectomies was almost 9 ½ hours (at least several hours longer than needed for an experienced surgeon to complete an open Whipple), it has steadily dropped to 7-8 hours, they said.
An early laparoscopic Whipple for Dr. Kendrick also took approximately 10 hours, "but within 20-30 cases, it was down to 4 or 5 hours, sometimes 6," he said. "There’s a lower average operative time for laparoscopic Whipple ... but [in the end], is that really an important factor if the patient still gains the benefits of [the minimally invasive surgery]?"
Gynecologic Surgery
In gynecologic oncology, where robotic surgery in recent years has been rapidly embraced as a tool for performing minimally invasive endometrial cancer staging, operating time and cost are factors in an "ongoing debate in over whether robotics provides an advantage over laparoscopy for lymph node dissection," said Dr. James E. Delmore, director of gynecologic oncology for the University of Kansas, Wichita.
At least a half-dozen studies have found that robotic-assisted hysterectomy and lymphadenectomy for endometrial cancer results in significantly shorter hospital stays as well as fewer wound infections and postoperative complications compared with an open approach, according to Dr. Delmore, who has served as a proctor for Intuitive. However, fewer studies have compared all three approaches (robotic assisted, traditional laparoscopy, and open).
"It’s hard to determine and analyze procedure costs in many hospital systems," he said. But "from what we can tell thus far, purely laparoscopic hysterectomy with removal of the lymph nodes is the least expensive approach, followed by robotics and then abdominal."
Such cost differentials were demonstrated in a study comparing the outcomes and cost of endometrial cancer staging performed via traditional laparotomy, standard laparoscopy, and robotic techniques. Dr. Maria C. Bell performed all of the procedures (40 robotic, 40 laparotomy, and 30 laparoscopic) at the Sanford Clinic in Sioux Falls, S.D.
Robotic hysterectomy took about an hour longer to perform than did hysterectomy completed via laparotomy (with no significant difference in operating time, compared with pure laparoscopy), but resulted in the lowest complication rate of the three approaches and the shortest average return to normal activity. Estimated blood loss and average length of stay were both significantly reduced for the robotic cohort, compared with laparotomy, and were comparable to laparoscopy. There were no differences in lymph node retrieval among the three groups (Gyn. Oncol. 2008;111:407-11).
When both direct and indirect costs were considered (including a measure by the Lewin Group of "societal/productivity" costs and the cost of the robot on a 5-year amortization schedule), the total average costs for hysterectomy with staging were $12,943 with laparotomy, $7,569 for standard laparoscopy, and $8,212 for robotic assistance.
The overall decreased cost for robotic surgery was unexpected and could be a result of money "invested up front [being] recouped by less time rounding ... and less time taking care of complications," as well as lower societal costs, wrote Dr. Bell, also of the University of South Dakota, Vermillion.
"I was very surprised that laparotomy was the most costly to the hospital," Dr. Bell said in an interview. (One of the coauthors of the report, Usha Seshadri-Kreaden, is employed by Intuitive, and Dr. Bell is a proctor for Intuitive, but the company did not sponsor the study.)
Other investigators who have compared robotic-assisted and conventional laparoscopic hysterectomies have reported higher per-case hospital costs with the robot. With hysterectomy having surpassed prostatectomy in 2010 as the highest-volume procedure for the da Vinci Surgical System, according to its manufacturer, the value of robotics for routine hysterectomy may well be increasingly scrutinized.
The da Vinci is used to treat more than 90% of endometrial cancer patients at Dr. Bell’s and Dr. Delmore’s institutions, but both surgeons are quick to emphasize the need for more data on outcomes and cost-effectiveness. "The temptation of ‘the patient wants it, so we need to offer it’ needs to be tempered with the question ‘are we making progress doing it?’ " said Dr. Delmore.
Training Needs
Institutions are individually grappling with how best to train residents in robotic-assisted surgery. The University of Kansas model includes an online tutorial, training with inanimate objects, animal lab training, and bedside assistance in real robotic-assisted hysterectomies. Dr. Delmore and his colleagues have a study underway to look at how graduate ob.gyns. use this training.
Within the realm of hepatobiliary and pancreatic surgery, where a minimally invasive approach is younger and where training models for the open Whipple procedure are still deliberated, "there is a dramatic appetite for minimally invasive skills," Dr. Kendrick said. HBP fellows at the Mayo Clinic currently are exposed to minimally invasive surgery, but "our goal over the next 2 years is to incorporate an even more significant focus" on robotics and laparoscopy, he said.
At the University of Pittsburgh, surgical oncology and HBP fellows and residents receive training with the robot in a "dry lab" format, Dr. Moser said. Trainees also can sit during actual cases at a second "teaching console" that is part of the current da Vinci Surgical System.
"We typically teach robotic cholecystectomy and sewing of the duodenojejunostomy during the Whipple procedure and allow them to do these portions of the procedure," Dr. Moser said.
Dr. Kendrick, who said he has no disclosures to make relevant to this article, hopes to see several fellowships established nationwide to address minimally invasive pancreas and liver surgery. However, "we have to be cautious about credentialing and assessing the adequacy of training. There’s a real mortality rate and a major complication rate [with the Whipple procedure] ... We’re trying to make it lower and lower with time, and not go backward."
Robotic surgery is continuing to expand its reach, with widespread interest in the technology for endometrial cancer staging and recent exploration of its viability for complex pancreatic resections and reconstructions.
Gynecologic oncologists and pancreatic surgeons aren’t the only ones vying for time with the da Vinci Surgical System (Intuitive Surgical) – the only such system currently on the market. Their experiences, however, offer perspective on some of the key issues – from operative time and cost effectiveness to training needs – that are being raised as robotic technology is adopted.
Pancreatic Surgery
"Robotic-assisted pancreatic resections and reconstructions can be performed safely with postoperative complication rates and fistula formation comparable to results observed with open techniques," according to Dr. Herbert J. Zeh III, and Dr. A. James Moser, codirectors of University of Pittsburgh Medical Center’s pancreatic cancer center, who reported their results with 30 robotic-assisted major pancreatic resections and reconstructions, including 24 pancreaticoduodenectomies (Arch. Surg. 2011:146:256-61).
By now, they said in an interview, they have used a robotic approach for more than 110 major pancreatic resections and reconstructions, including approximately 60 robotic-assisted pancreaticoduodenectomies, or Whipple procedures.
Together with surgeons at the Cleveland Clinic, the Mayo Clinic in Rochester, Minn., and the University of Pisa in Italy, Dr. Moser and Dr. Zeh have pooled outcomes data, and a combined report has been submitted for publication.
"We’ve established comparable safety, and we’re at the point now where our operating times are dropping steadily and we can start to see feasibility and applicability down the road," said Dr. Zeh of the department of surgery at the University of Pittsburgh. "In the next year or two, we’ll start to see whether there are real advantages such as decreased blood loss and better outcomes resulting from a diminished physiologic impact on the patient."
Reductions in postoperative morbidity could make the complex Whipple procedure "less fearsome" for many of the patients with early pancreatic cancer who currently refuse definitive treatment. Robotic surgery might also enable more patients to recover quickly and be well enough to complete chemotherapy, said Dr. Moser of the departments of surgery and cell biology at the university. With the open approach, almost 40%-50% of patients never recover enough to complete their chemotherapy regimens, he said.
Dr. Moser and Dr. Zeh, neither of whom has any financial relationship with Intuitive, began exploring minimally invasive approaches to the Whipple procedure about 2½ years ago. They have been using laparoscopy for distal pancreatectomies since 2001, but were concerned about their ability to take a traditional laparoscopic approach with the Whipple procedure, which involves complex reconstruction.
"The first laparoscopic pancreaticoduodenectomy was described in 1994, and between then and 2010 there were only about 150 cases reported. It didn’t catch on. I think it’s because it takes such a Herculean effort to overcome the limits of the technology [with its two-dimensional imaging and limited range of instrument motion]," said Dr. Zeh.
"We wanted to completely duplicate the open Whipple with a minimally invasive technique without making any compromises or altering any surgical principles because of the limitations of the technology," added Dr. Moser.
Dr. Michael L. Kendrick, who last year published one of the two largest series of totally laparoscopic Whipple procedures, covering 62 patients (Arch. Surg. 2010;145:19-23), maintains that he makes "no alternations or adaptations in surgical principle" with the purely laparoscopic approach, compared with open surgery.
Whether this approach or a robotic-assisted laparoscopic approach is best for patients needing a pancreaticoduodenectomy appears at this time to be "completely the surgeon’s choice" and not driven by any patient characteristics or clinical indications, according to Dr. Kendrick, who chairs the division of gastroenterologic and general surgery at the Mayo Clinic. Proving "any additional clinical advantage of robotic assistance over pure laparoscopy will be very difficult and is unlikely," he said, but "pancreas surgeons may prefer the robotic approach because it is easier to learn and master."
Dr. Kendrick’s experience might be unique. He performed his first laparoscopic Whipple procedure in 2007 and then added the robotic-assisted Whipple to his armamentarium in 2008. To date, he has performed approximately 135 minimally invasive pancreaticoduodenectomies, of which about 30 have been robotically assisted.
The Pittsburgh surgeons, who perform their robotic-assisted Whipple procedures together, place the learning curve for robotic-assisted Whipple at approximately 60 operations – about the same number experts consider necessary with the open approach. While the median operating time in their first reported series of robotic-assisted pancreaticoduodenectomies was almost 9 ½ hours (at least several hours longer than needed for an experienced surgeon to complete an open Whipple), it has steadily dropped to 7-8 hours, they said.
An early laparoscopic Whipple for Dr. Kendrick also took approximately 10 hours, "but within 20-30 cases, it was down to 4 or 5 hours, sometimes 6," he said. "There’s a lower average operative time for laparoscopic Whipple ... but [in the end], is that really an important factor if the patient still gains the benefits of [the minimally invasive surgery]?"
Gynecologic Surgery
In gynecologic oncology, where robotic surgery in recent years has been rapidly embraced as a tool for performing minimally invasive endometrial cancer staging, operating time and cost are factors in an "ongoing debate in over whether robotics provides an advantage over laparoscopy for lymph node dissection," said Dr. James E. Delmore, director of gynecologic oncology for the University of Kansas, Wichita.
At least a half-dozen studies have found that robotic-assisted hysterectomy and lymphadenectomy for endometrial cancer results in significantly shorter hospital stays as well as fewer wound infections and postoperative complications compared with an open approach, according to Dr. Delmore, who has served as a proctor for Intuitive. However, fewer studies have compared all three approaches (robotic assisted, traditional laparoscopy, and open).
"It’s hard to determine and analyze procedure costs in many hospital systems," he said. But "from what we can tell thus far, purely laparoscopic hysterectomy with removal of the lymph nodes is the least expensive approach, followed by robotics and then abdominal."
Such cost differentials were demonstrated in a study comparing the outcomes and cost of endometrial cancer staging performed via traditional laparotomy, standard laparoscopy, and robotic techniques. Dr. Maria C. Bell performed all of the procedures (40 robotic, 40 laparotomy, and 30 laparoscopic) at the Sanford Clinic in Sioux Falls, S.D.
Robotic hysterectomy took about an hour longer to perform than did hysterectomy completed via laparotomy (with no significant difference in operating time, compared with pure laparoscopy), but resulted in the lowest complication rate of the three approaches and the shortest average return to normal activity. Estimated blood loss and average length of stay were both significantly reduced for the robotic cohort, compared with laparotomy, and were comparable to laparoscopy. There were no differences in lymph node retrieval among the three groups (Gyn. Oncol. 2008;111:407-11).
When both direct and indirect costs were considered (including a measure by the Lewin Group of "societal/productivity" costs and the cost of the robot on a 5-year amortization schedule), the total average costs for hysterectomy with staging were $12,943 with laparotomy, $7,569 for standard laparoscopy, and $8,212 for robotic assistance.
The overall decreased cost for robotic surgery was unexpected and could be a result of money "invested up front [being] recouped by less time rounding ... and less time taking care of complications," as well as lower societal costs, wrote Dr. Bell, also of the University of South Dakota, Vermillion.
"I was very surprised that laparotomy was the most costly to the hospital," Dr. Bell said in an interview. (One of the coauthors of the report, Usha Seshadri-Kreaden, is employed by Intuitive, and Dr. Bell is a proctor for Intuitive, but the company did not sponsor the study.)
Other investigators who have compared robotic-assisted and conventional laparoscopic hysterectomies have reported higher per-case hospital costs with the robot. With hysterectomy having surpassed prostatectomy in 2010 as the highest-volume procedure for the da Vinci Surgical System, according to its manufacturer, the value of robotics for routine hysterectomy may well be increasingly scrutinized.
The da Vinci is used to treat more than 90% of endometrial cancer patients at Dr. Bell’s and Dr. Delmore’s institutions, but both surgeons are quick to emphasize the need for more data on outcomes and cost-effectiveness. "The temptation of ‘the patient wants it, so we need to offer it’ needs to be tempered with the question ‘are we making progress doing it?’ " said Dr. Delmore.
Training Needs
Institutions are individually grappling with how best to train residents in robotic-assisted surgery. The University of Kansas model includes an online tutorial, training with inanimate objects, animal lab training, and bedside assistance in real robotic-assisted hysterectomies. Dr. Delmore and his colleagues have a study underway to look at how graduate ob.gyns. use this training.
Within the realm of hepatobiliary and pancreatic surgery, where a minimally invasive approach is younger and where training models for the open Whipple procedure are still deliberated, "there is a dramatic appetite for minimally invasive skills," Dr. Kendrick said. HBP fellows at the Mayo Clinic currently are exposed to minimally invasive surgery, but "our goal over the next 2 years is to incorporate an even more significant focus" on robotics and laparoscopy, he said.
At the University of Pittsburgh, surgical oncology and HBP fellows and residents receive training with the robot in a "dry lab" format, Dr. Moser said. Trainees also can sit during actual cases at a second "teaching console" that is part of the current da Vinci Surgical System.
"We typically teach robotic cholecystectomy and sewing of the duodenojejunostomy during the Whipple procedure and allow them to do these portions of the procedure," Dr. Moser said.
Dr. Kendrick, who said he has no disclosures to make relevant to this article, hopes to see several fellowships established nationwide to address minimally invasive pancreas and liver surgery. However, "we have to be cautious about credentialing and assessing the adequacy of training. There’s a real mortality rate and a major complication rate [with the Whipple procedure] ... We’re trying to make it lower and lower with time, and not go backward."
Improving Access to Pediatric Palliative Care : Neuromuscular and neurodegenerative disorders are among the leading life-limiting diseases
Dr. Stefan J. Friedrichsdorf has a list of “myths” about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children's Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the field of palliative care. He and pediatrician Savithri Nageswaran of Brenner Children's Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf's institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
“It's truly interdisciplinary, in that people need to really go beyond what they've been trained for,” said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children's. “I'm nothing without my team.”
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
“The vast majority of these children do not have access to pediatric palliative care in this country,” Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for “innovative symptom management, compassion, and family-centered care,” Dr. Friedrichsdorf said he and his team take “an extremely aggressive approach” to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the “myths” he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. “It's not one or the other. It's using the whole breadth [of therapies] at the same moment,” said Dr. Friedrichsdorf, who is trained in self-hypnosis.
“We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away,” he said. “Our goal is for children to live as long as possible, as well as possible.”
In addition to physicians and nurses, the pain and palliative care team at Children's includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care “rounding team” in the department's pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department's home-based component. The team can be called upon by anyone – a doctor, a patient, a relative, or a friend – for a consultation, and its members meet regularly to discuss patients.
“My physical therapist may tell me, for instance, that I need to change [a patient's] pain medications because she sees side effects,” Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children's hospitals, to be published soon, found that professionals in the teams had a “clear idea of what the other professionals offered to the patient and family,” said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient's medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
“Having shared goals of care and strong communication is also important so that everything doesn't have to be explained every time a shift changes or a patient is transferred to a different setting,” she said.
“Most of these pediatric palliative care teams are fairly newly established,” Dr. Berlinger noted.
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
“Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home,” Dr. Nageswaran said in an interview. “We weren't achieving this with the traditional consult model.”
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
“The family wanted end-of-life care to be delivered at home, but they didn't want to forgo medical care,” Dr. Nageswaran recalled. “We went step-by-step, aligning the family's wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home.”
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
“Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care,” said Dr. Nageswaran. “When we meet families, we meet them without a set agenda, and we make sure we don't impose our structure.”
Similarly, Dr. Friedrichsdorf said, “When I enter a room, the first thing I say is, 'How can I help you?' We start with that open question.” At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children. Currently, most families have to forgo home-health services to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. “Physician services are reimbursed, but not enough to account for the amount of time involved,” said Dr. Nageswaran. “And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed.”
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
One goal in the meantime, said Dr. Berlinger, is to “influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory.”
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
“Each family is unique in how they perceive illness and how they make decisions,” said Dr. Savithri Nageswaran.
Source Courtesy WFUBMC Photography
Dr. Stefan J. Friedrichsdorf has a list of “myths” about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children's Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the field of palliative care. He and pediatrician Savithri Nageswaran of Brenner Children's Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf's institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
“It's truly interdisciplinary, in that people need to really go beyond what they've been trained for,” said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children's. “I'm nothing without my team.”
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
“The vast majority of these children do not have access to pediatric palliative care in this country,” Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for “innovative symptom management, compassion, and family-centered care,” Dr. Friedrichsdorf said he and his team take “an extremely aggressive approach” to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the “myths” he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. “It's not one or the other. It's using the whole breadth [of therapies] at the same moment,” said Dr. Friedrichsdorf, who is trained in self-hypnosis.
“We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away,” he said. “Our goal is for children to live as long as possible, as well as possible.”
In addition to physicians and nurses, the pain and palliative care team at Children's includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care “rounding team” in the department's pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department's home-based component. The team can be called upon by anyone – a doctor, a patient, a relative, or a friend – for a consultation, and its members meet regularly to discuss patients.
“My physical therapist may tell me, for instance, that I need to change [a patient's] pain medications because she sees side effects,” Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children's hospitals, to be published soon, found that professionals in the teams had a “clear idea of what the other professionals offered to the patient and family,” said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient's medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
“Having shared goals of care and strong communication is also important so that everything doesn't have to be explained every time a shift changes or a patient is transferred to a different setting,” she said.
“Most of these pediatric palliative care teams are fairly newly established,” Dr. Berlinger noted.
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
“Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home,” Dr. Nageswaran said in an interview. “We weren't achieving this with the traditional consult model.”
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
“The family wanted end-of-life care to be delivered at home, but they didn't want to forgo medical care,” Dr. Nageswaran recalled. “We went step-by-step, aligning the family's wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home.”
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
“Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care,” said Dr. Nageswaran. “When we meet families, we meet them without a set agenda, and we make sure we don't impose our structure.”
Similarly, Dr. Friedrichsdorf said, “When I enter a room, the first thing I say is, 'How can I help you?' We start with that open question.” At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children. Currently, most families have to forgo home-health services to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. “Physician services are reimbursed, but not enough to account for the amount of time involved,” said Dr. Nageswaran. “And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed.”
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
One goal in the meantime, said Dr. Berlinger, is to “influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory.”
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
“Each family is unique in how they perceive illness and how they make decisions,” said Dr. Savithri Nageswaran.
Source Courtesy WFUBMC Photography
Dr. Stefan J. Friedrichsdorf has a list of “myths” about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children's Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the field of palliative care. He and pediatrician Savithri Nageswaran of Brenner Children's Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf's institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
“It's truly interdisciplinary, in that people need to really go beyond what they've been trained for,” said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children's. “I'm nothing without my team.”
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
“The vast majority of these children do not have access to pediatric palliative care in this country,” Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for “innovative symptom management, compassion, and family-centered care,” Dr. Friedrichsdorf said he and his team take “an extremely aggressive approach” to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the “myths” he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. “It's not one or the other. It's using the whole breadth [of therapies] at the same moment,” said Dr. Friedrichsdorf, who is trained in self-hypnosis.
“We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away,” he said. “Our goal is for children to live as long as possible, as well as possible.”
In addition to physicians and nurses, the pain and palliative care team at Children's includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care “rounding team” in the department's pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department's home-based component. The team can be called upon by anyone – a doctor, a patient, a relative, or a friend – for a consultation, and its members meet regularly to discuss patients.
“My physical therapist may tell me, for instance, that I need to change [a patient's] pain medications because she sees side effects,” Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children's hospitals, to be published soon, found that professionals in the teams had a “clear idea of what the other professionals offered to the patient and family,” said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient's medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
“Having shared goals of care and strong communication is also important so that everything doesn't have to be explained every time a shift changes or a patient is transferred to a different setting,” she said.
“Most of these pediatric palliative care teams are fairly newly established,” Dr. Berlinger noted.
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
“Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home,” Dr. Nageswaran said in an interview. “We weren't achieving this with the traditional consult model.”
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
“The family wanted end-of-life care to be delivered at home, but they didn't want to forgo medical care,” Dr. Nageswaran recalled. “We went step-by-step, aligning the family's wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home.”
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
“Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care,” said Dr. Nageswaran. “When we meet families, we meet them without a set agenda, and we make sure we don't impose our structure.”
Similarly, Dr. Friedrichsdorf said, “When I enter a room, the first thing I say is, 'How can I help you?' We start with that open question.” At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children. Currently, most families have to forgo home-health services to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. “Physician services are reimbursed, but not enough to account for the amount of time involved,” said Dr. Nageswaran. “And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed.”
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
One goal in the meantime, said Dr. Berlinger, is to “influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory.”
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
“Each family is unique in how they perceive illness and how they make decisions,” said Dr. Savithri Nageswaran.
Source Courtesy WFUBMC Photography
Improving Access to Pediatric Palliative Care
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation (pdf) and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation (pdf) and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation (pdf) and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Improving Access to Pediatric Palliative Care
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Improving Access to Pediatric Palliative Care
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation (pdf) and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation (pdf) and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
Dr. Stefan J. Friedrichsdorf has a list of "myths" about pediatric palliative care that he presents during lectures. Among them: that the death of a child in the United States is a rare event, that pediatric palliative care is just for children with cancer, and that care starts when treatment stops.
In his lectures – and in his work every day at Children’s Hospitals and Clinics of Minnesota, Minneapolis – Dr. Friedrichsdorf debunks these myths.
In January, he was one of two pediatricians who won national awards from the Hastings Center and a partnering foundation for their contributions to the broader field of palliative care. He and pediatrician Dr. Savithri Nageswaran of Brenner Children’s Hospital at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., joined two geriatricians and an internist in receiving the award.
The pain and palliative care program at Dr. Friedrichsdorf’s institution is a relatively long-standing program, but pediatric palliative care is a new subspecialty and is still a relatively new area of pediatric care and of palliative medicine – one for which delivery models and educational pathways are still evolving, and one for which reimbursement is poor and regulatory barriers are challenging.
"It’s truly interdisciplinary, in that people need to really go beyond what they’ve been trained for," said Dr. Friedrichsdorf, medical director of the department of pain medicine, palliative care & integrative medicine at Children’s. "I’m nothing without my team."
Pediatric palliative care has been defined and described by the World Health Organization, the Institute of Medicine, the American Academy of Pediatrics, and other bodies as individualized, integrative care that is provided for children with life-threatening conditions. The care starts at diagnosis, continues through the trajectory of the illness, and is directed at the underlying illness and at the physical, emotional, social, and spiritual needs of the child and family.
More than 15,000 children and teens die in the United States each year from life-limiting diseases – and less than a quarter of them have cancer, according to data cited by Dr. Friedrichsdorf. Neuromuscular or neurodegenerative disorders cause a significant proportion of those deaths, followed by congenital or genetic disorders, cardiovascular disorders, and metabolic disorders.
"The vast majority of these children do not have access to pediatric palliative care in this country," Dr. Friedrichsdorf said in an interview. Data show that these children are suffering needlessly from pain, breathlessness, nausea, and vomiting.
Praised by the awards committee for "innovative symptom management, compassion, and family-centered care," Dr. Friedrichsdorf said he and his team take "an extremely aggressive approach" to managing pain and distressing symptoms in children with either life-threatening or life-limiting conditions.
He believes strong pain medications are underused in children (and one of the "myths" he debunks is that increasing doses of opioids and/or benzodiazepines causes respiratory depression and quickens death), but also that pharmacology alone is insufficient.
His department employs both pharmacology and complementary therapies such as biofeedback, massage, hypnosis, acupuncture, and acupressure. Physicians and other staff are trained in such modalities. "It’s not one or the other. It’s using the whole breadth [of therapies] at the same moment," said Dr. Friedrichsdorf, who is trained in self-hypnosis.
"We want to promise each family, if your child is suffering from distressing symptoms like nausea, pain, or dyspnea, we can usually make these symptoms go away," he said. "Our goal is for children to live as long as possible, as well as possible."
In addition to physicians and nurses, the pain and palliative care team at Children’s includes social workers, psychologists, a physical therapist, a child-life specialist, massage therapists, and advanced practice nurses.
Each of these professionals can see patients as part of a hospital-based pain and palliative care "rounding team" in the department’s pain and palliative care clinic, or for patients in the Minneapolis/St. Paul area, in the home through the department’s home-based component. The team can be called upon by anyone – a doctor, a patient, a relative or friend – for a consultation, and its members meet regularly to discuss patients.
"My physical therapist may tell me, for instance, that I need to change [a patient’s] pain medications because she sees side effects," Dr. Friedrichsdorf said.
A pilot study of pediatric palliative care teams at eight children’s hospitals, to be published soon, found that professionals in the teams had a "clear idea of what the other professionals offered to the patient and family," said Nancy Berlinger, Ph.D., deputy director and research scholar at the Hastings Center, which conducted the study with researchers at Rush University, Chicago.
A chaplain knows, for instance, how the physician and nurse are addressing the patient’s medical needs, and the physician is aware that the chaplain is supporting the parents and, in some cases, the child, she said in an interview.
"Having shared goals of care and strong communication is also important so that everything doesn’t have to be explained every time a shift changes or a patient is transferred to a different setting," she said.
"Most of these pediatric palliative care teams are fairly newly established," she noted. "There was some pediatric palliative care before then, but not necessarily with a strong team approach."
Dr. Nageswaran, who led the establishment of the first pediatric palliative care program at her hospital in 2008, said she was struck by the amount of coordination needed to provide good palliative care and by the flexibility needed to design a good program.
She and her colleagues started the program as a consult service for children who were hospitalized with complicated, often life-limiting conditions. The service used a half-time nurse coordinator, a one-quarter full-time equivalent (FTE) clinician post to be shared by a handful of physicians for rotating on-call duty, and a 1% FTE post for a physician coordinator.
"Very soon, we realized that the biggest need was to facilitate collaboration between multiple providers and to ensure sufficient continuity of care as these children transition back and forth from the hospital to home," Dr. Nageswaran said in an interview. "We weren’t achieving this with the traditional consult model where we’d see patients in the hospital and leave recommendations for the primary medical team."
In a subsequent restructuring, physician time was consolidated into a one-third–time FTE coordinator post, which Dr. Nageswaran fills herself, and funding was obtained from the federal Maternal and Child Health Bureau to add another half-time nurse coordinator who could focus on making home visits and coordinating home-based care in one local county.
The flexibility to coordinate care outside the hospital is critical, Dr. Nageswaran said. One of the 235 children cared for under the palliative care program thus far was a child with a rare genetic disorder characterized by skeletal abnormalities, urologic abnormalities, and severe neurologic impairment and seizures.
"The family wanted end-of-life care to be delivered at home, but they didn’t want to forgo medical care," Dr. Nageswaran recalled. "We went step-by-step, aligning the family’s wishes with the care the child received. We worked with the primary care doctor, the subspecialists, the home health agency, and the parents to provide medical treatment, pain and symptom management, and other care at home."
Both she and Dr. Friedrichsdorf emphasized the value of open inquiry with parents, children and families.
"Each family is unique in how they perceive illness and how they make decisions about treatment and end-of-life care," said Dr. Nageswaran. "When we meet families, we meet them without a set agenda, and we make sure we don’t impose our structure."
Similarly, Dr. Friedrichsdorf said, "When I enter a room, the first thing I say is, ‘How can I help you?’ We start with that open question." At that point, he said, surveys or other structured tools can be used to help determine needs and care plans.
One of the thorns in the field of pediatric palliative care is the unavailability of hospice services for many children, given the prognostic uncertainty of most childhood life-threatening conditions and the desire for continued treatment. Currently, most families have to forgo home-health services in order to receive hospice services.
Some states have taken action; policy reform passed in California in 2006, for instance, makes it easier for parents to utilize the Medi-Cal hospice benefit for children. A section of the federal Patient Protection and Affordable Care Act, moreover, is expected to change the Medicaid system to allow children with life-limiting conditions to receive both hospice care and curative treatment.
Another problem is poor provider reimbursement. "Physician services are reimbursed, but not enough to account for the amount of time involved," said Dr. Nageswaran. "\"And the services of nurses and social workers, who are key to pediatric palliative care programs, are not reimbursed."
She jump-started her program with a grant from the Duke Endowment, a private foundation, but now relies primarily on financial support from the hospital. Dr. Friedrichsdorf estimates that his hospital is reimbursed for only about half of its costs, and says that it relies heavily on philanthropy to make up the difference.
Philanthropy recently benefited the pediatric palliative care program at Akron (Ohio) Children’s Hospital. With $1.2 million in donations from the Haslinger Family Foundation (pdf) and other leadership gifts, the hospital has created an endowed chair for its services, which began in 2002.]
One goal in the meantime, said Dr. Berlinger, is to "influence the culture of health care so that pediatric palliative care is recognized as ethically mandatory."
The $15,000 awards that Dr. Nageswaran and Dr. Friedrichsdorf received were given by the Hastings Center, a bioethics research institute based in Garrison, N.Y., in partnership with the Cunniff-Dixon Foundation, a foundation that focuses on the doctor-patient relationship near the end of life.
Pediatric Palliative Care Training
In terms of education, pediatric palliative care might be where pediatric subspecialties such as pulmonary care or neonatology were 25 years ago, Dr. Friedrichsdorf said, with an initial cadre of trained physicians having emerged.
In 2008, 47 physicians were certified in Hospice and Palliative Medicine (HPM) by the American Board of Pediatrics after taking the first American Board of Medical Specialties–recognized examination for the subspecialty. In total, 1,274 physicians were certified by various boards in the new subspecialty.
The American Board of Medical Specialties (ABMS) approved the creation of HPM as a subspecialty of 10 participating boards in 2006. Prior to 2006, board certification in hospice and palliative medicine was administered by the American Board of Hospice and Palliative Medicine but not recognized by the ABMS.
Other pediatricians have taken courses and attended educational retreats through organizations such as the Initiative for Pediatric Palliative Care, Dr. Berlinger said.
Ideally, she and Dr. Friedrichsdorf say, both educational tracks – fellowships and educational opportunities for mid-career pediatricians – will grow.
Starting in 2013, physicians who want to sit for the HPM board exam will have to have completed an Accreditation Council for Graduate Medical Education-accredited fellowship – a change that should spur the development of more fellowship programs. Children’s Hospitals and Clinics of Minnesota, Dr. Friedrichsdorf’s hospital, houses one of a handful of fellowship programs in pediatric palliative care. It has applied for ACGME approval.
Dr. Friedrichsdorf is the principal investigator of a National Institutes of Health/National Cancer Institute study on the creation and implementation of a pediatric palliative care curriculum that is slated to be offered to physicians who are in the midst of their careers and not seeking subspecialty training.
"Many professionals working in children’s hospitals are likely to care for a dying child, and need to be comfortable and knowledgeable," said Dr. Friedrichsdorf, who completed a fellowship in pediatric pain and palliative care at the Children’s Hospital at Westmead, Australia, after finishing his pediatric residency in Germany.
On-Site Availability of Long-Acting Contraceptives Not High
A national survey on contraceptive availability suggests that on-site availability of intrauterine devices and other long-acting, reversible contraceptive methods is less than maximal for both office-based physicians and Title X clinics, according to a report in the Jan. 14 Morbidity and Mortality Weekly Report.
Approximately one-quarter of the 635 office-based physicians and nearly one-third of the 1,368 Title X clinic providers who participated in the CDC survey reported that they refer patients to other providers for IUDs – and even higher proportions said they refer patients out for contraceptive implants.
Increasing on-site availability and thus improving access to such long-acting, reversible contraceptive (LARC) methods could reduce rates of unintended pregnancy in the United States, the report stated.
The high unintended pregnancy rate of nearly 50% is thought to result, in part, from less-frequent use of LARC methods, compared with other user-dependent methods such as condoms and oral contraceptives. LARCs have been shown to be more effective in preventing unintended pregnancies during typical use, according to the report (MMWR 2011;60:1-4).
Previous studies have similarly shown that contraceptive availability – through either on-site provision or through prescription or provider recommendation – is highest for oral contraceptives and lower for the patch, IUD, and vaginal ring. This is one of the few studies, however, to examine provider-specific availability of a wide range of contraceptive methods and the first national study to delineate on-site availability without combining hormonal methods into one category, according to the report.
The survey was mailed to physicians in three specialties: obstetrics/gynecology, family medicine, and adolescent medicine. Title X clinics represented a range of provider agencies, including public health departments and community health centers.
Overall, with the exception of the levonorgestrel-releasing intrauterine device (LNG-IUD, Mirena) – for which on-site availability was reported by 56% of office-based physicians and 47% of Title X clinic providers – office-based physicians were less likely than were Title X clinics to report on-site availability of a range of contraceptive methods.
While nearly all Title X clinics reported having on-site availability of combined oral contraceptives, for instance, approximately half of office-based physicians had them available on-site and half had them available by prescription. One-quarter of office-based physicians offered progestin-only oral contraceptives on-site, in comparison, and almost 30% offered the contraceptive patch on-site. Most of the others reported that these two methods were available by prescription.
Similarly, male condoms were available on-site in nearly all Title X clinics but only in one-quarter of physicians’ offices.
Survey participants were also asked whether specific contraceptive methods were simply "not available" to their patients. The methods most frequently reported as such were female condoms and implants.
Almost 18% of office-based physicians and 10% of Title X providers said female condoms were unavailable, and 8% and 9% of office-based physicians and Title X providers, respectively, said contraceptive implants were unavailable. On the other hand, almost half of the Title X clinic providers said female condoms were available on-site, compared with 7% of in-office physicians. Contraceptive implants were reported by about one-third of the providers in each category to be available on-site.
The survey findings are limited by a low response rate for office-based physicians (47%), the report noted.
The survey did not ascertain reasons why contraceptive methods were unavailable in some cases or not available on-site. Differences in availability can reflect various factors, from variations in reimbursable costs and health insurance trends, to federal and state policies, provider training and patient characteristics, according to the report.
A national survey on contraceptive availability suggests that on-site availability of intrauterine devices and other long-acting, reversible contraceptive methods is less than maximal for both office-based physicians and Title X clinics, according to a report in the Jan. 14 Morbidity and Mortality Weekly Report.
Approximately one-quarter of the 635 office-based physicians and nearly one-third of the 1,368 Title X clinic providers who participated in the CDC survey reported that they refer patients to other providers for IUDs – and even higher proportions said they refer patients out for contraceptive implants.
Increasing on-site availability and thus improving access to such long-acting, reversible contraceptive (LARC) methods could reduce rates of unintended pregnancy in the United States, the report stated.
The high unintended pregnancy rate of nearly 50% is thought to result, in part, from less-frequent use of LARC methods, compared with other user-dependent methods such as condoms and oral contraceptives. LARCs have been shown to be more effective in preventing unintended pregnancies during typical use, according to the report (MMWR 2011;60:1-4).
Previous studies have similarly shown that contraceptive availability – through either on-site provision or through prescription or provider recommendation – is highest for oral contraceptives and lower for the patch, IUD, and vaginal ring. This is one of the few studies, however, to examine provider-specific availability of a wide range of contraceptive methods and the first national study to delineate on-site availability without combining hormonal methods into one category, according to the report.
The survey was mailed to physicians in three specialties: obstetrics/gynecology, family medicine, and adolescent medicine. Title X clinics represented a range of provider agencies, including public health departments and community health centers.
Overall, with the exception of the levonorgestrel-releasing intrauterine device (LNG-IUD, Mirena) – for which on-site availability was reported by 56% of office-based physicians and 47% of Title X clinic providers – office-based physicians were less likely than were Title X clinics to report on-site availability of a range of contraceptive methods.
While nearly all Title X clinics reported having on-site availability of combined oral contraceptives, for instance, approximately half of office-based physicians had them available on-site and half had them available by prescription. One-quarter of office-based physicians offered progestin-only oral contraceptives on-site, in comparison, and almost 30% offered the contraceptive patch on-site. Most of the others reported that these two methods were available by prescription.
Similarly, male condoms were available on-site in nearly all Title X clinics but only in one-quarter of physicians’ offices.
Survey participants were also asked whether specific contraceptive methods were simply "not available" to their patients. The methods most frequently reported as such were female condoms and implants.
Almost 18% of office-based physicians and 10% of Title X providers said female condoms were unavailable, and 8% and 9% of office-based physicians and Title X providers, respectively, said contraceptive implants were unavailable. On the other hand, almost half of the Title X clinic providers said female condoms were available on-site, compared with 7% of in-office physicians. Contraceptive implants were reported by about one-third of the providers in each category to be available on-site.
The survey findings are limited by a low response rate for office-based physicians (47%), the report noted.
The survey did not ascertain reasons why contraceptive methods were unavailable in some cases or not available on-site. Differences in availability can reflect various factors, from variations in reimbursable costs and health insurance trends, to federal and state policies, provider training and patient characteristics, according to the report.
A national survey on contraceptive availability suggests that on-site availability of intrauterine devices and other long-acting, reversible contraceptive methods is less than maximal for both office-based physicians and Title X clinics, according to a report in the Jan. 14 Morbidity and Mortality Weekly Report.
Approximately one-quarter of the 635 office-based physicians and nearly one-third of the 1,368 Title X clinic providers who participated in the CDC survey reported that they refer patients to other providers for IUDs – and even higher proportions said they refer patients out for contraceptive implants.
Increasing on-site availability and thus improving access to such long-acting, reversible contraceptive (LARC) methods could reduce rates of unintended pregnancy in the United States, the report stated.
The high unintended pregnancy rate of nearly 50% is thought to result, in part, from less-frequent use of LARC methods, compared with other user-dependent methods such as condoms and oral contraceptives. LARCs have been shown to be more effective in preventing unintended pregnancies during typical use, according to the report (MMWR 2011;60:1-4).
Previous studies have similarly shown that contraceptive availability – through either on-site provision or through prescription or provider recommendation – is highest for oral contraceptives and lower for the patch, IUD, and vaginal ring. This is one of the few studies, however, to examine provider-specific availability of a wide range of contraceptive methods and the first national study to delineate on-site availability without combining hormonal methods into one category, according to the report.
The survey was mailed to physicians in three specialties: obstetrics/gynecology, family medicine, and adolescent medicine. Title X clinics represented a range of provider agencies, including public health departments and community health centers.
Overall, with the exception of the levonorgestrel-releasing intrauterine device (LNG-IUD, Mirena) – for which on-site availability was reported by 56% of office-based physicians and 47% of Title X clinic providers – office-based physicians were less likely than were Title X clinics to report on-site availability of a range of contraceptive methods.
While nearly all Title X clinics reported having on-site availability of combined oral contraceptives, for instance, approximately half of office-based physicians had them available on-site and half had them available by prescription. One-quarter of office-based physicians offered progestin-only oral contraceptives on-site, in comparison, and almost 30% offered the contraceptive patch on-site. Most of the others reported that these two methods were available by prescription.
Similarly, male condoms were available on-site in nearly all Title X clinics but only in one-quarter of physicians’ offices.
Survey participants were also asked whether specific contraceptive methods were simply "not available" to their patients. The methods most frequently reported as such were female condoms and implants.
Almost 18% of office-based physicians and 10% of Title X providers said female condoms were unavailable, and 8% and 9% of office-based physicians and Title X providers, respectively, said contraceptive implants were unavailable. On the other hand, almost half of the Title X clinic providers said female condoms were available on-site, compared with 7% of in-office physicians. Contraceptive implants were reported by about one-third of the providers in each category to be available on-site.
The survey findings are limited by a low response rate for office-based physicians (47%), the report noted.
The survey did not ascertain reasons why contraceptive methods were unavailable in some cases or not available on-site. Differences in availability can reflect various factors, from variations in reimbursable costs and health insurance trends, to federal and state policies, provider training and patient characteristics, according to the report.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major Finding: Approximately one-quarter of office-based physicians and one-third of Title X clinics refer patients to other providers for IUDs. And, with the exception of the LNG-IUD, fewer office-based physicians than Title X clinics offer a wide array of contraceptive methods on-site.
Data Source: A CDC survey reported in the Morbidity and Mortality Weekly Report.
Disclosures: NA
On-Site Availability of Long-Acting Contraceptives Not High
A national survey on contraceptive availability suggests that on-site availability of intrauterine devices and other long-acting, reversible contraceptive methods is less than maximal for both office-based physicians and Title X clinics, according to a report in the Jan. 14 Morbidity and Mortality Weekly Report.
Approximately one-quarter of the 635 office-based physicians and nearly one-third of the 1,368 Title X clinic providers who participated in the CDC survey reported that they refer patients to other providers for IUDs – and even higher proportions said they refer patients out for contraceptive implants.
Increasing on-site availability and thus improving access to such long-acting, reversible contraceptive (LARC) methods could reduce rates of unintended pregnancy in the United States, the report stated.
The high unintended pregnancy rate of nearly 50% is thought to result, in part, from less-frequent use of LARC methods, compared with other user-dependent methods such as condoms and oral contraceptives. LARCs have been shown to be more effective in preventing unintended pregnancies during typical use, according to the report (MMWR 2011;60:1-4).
Previous studies have similarly shown that contraceptive availability – through either on-site provision or through prescription or provider recommendation – is highest for oral contraceptives and lower for the patch, IUD, and vaginal ring. This is one of the few studies, however, to examine provider-specific availability of a wide range of contraceptive methods and the first national study to delineate on-site availability without combining hormonal methods into one category, according to the report.
The survey was mailed to physicians in three specialties: obstetrics/gynecology, family medicine, and adolescent medicine. Title X clinics represented a range of provider agencies, including public health departments and community health centers.
Overall, with the exception of the levonorgestrel-releasing intrauterine device (LNG-IUD, Mirena) – for which on-site availability was reported by 56% of office-based physicians and 47% of Title X clinic providers – office-based physicians were less likely than were Title X clinics to report on-site availability of a range of contraceptive methods.
While nearly all Title X clinics reported having on-site availability of combined oral contraceptives, for instance, approximately half of office-based physicians had them available on-site and half had them available by prescription. One-quarter of office-based physicians offered progestin-only oral contraceptives on-site, in comparison, and almost 30% offered the contraceptive patch on-site. Most of the others reported that these two methods were available by prescription.
Similarly, male condoms were available on-site in nearly all Title X clinics but only in one-quarter of physicians’ offices.
Survey participants were also asked whether specific contraceptive methods were simply "not available" to their patients. The methods most frequently reported as such were female condoms and implants.
Almost 18% of office-based physicians and 10% of Title X providers said female condoms were unavailable, and 8% and 9% of office-based physicians and Title X providers, respectively, said contraceptive implants were unavailable. On the other hand, almost half of the Title X clinic providers said female condoms were available on-site, compared with 7% of in-office physicians. Contraceptive implants were reported by about one-third of the providers in each category to be available on-site.
The survey findings are limited by a low response rate for office-based physicians (47%), the report noted.
The survey did not ascertain reasons why contraceptive methods were unavailable in some cases or not available on-site. Differences in availability can reflect various factors, from variations in reimbursable costs and health insurance trends, to federal and state policies, provider training and patient characteristics, according to the report.
A national survey on contraceptive availability suggests that on-site availability of intrauterine devices and other long-acting, reversible contraceptive methods is less than maximal for both office-based physicians and Title X clinics, according to a report in the Jan. 14 Morbidity and Mortality Weekly Report.
Approximately one-quarter of the 635 office-based physicians and nearly one-third of the 1,368 Title X clinic providers who participated in the CDC survey reported that they refer patients to other providers for IUDs – and even higher proportions said they refer patients out for contraceptive implants.
Increasing on-site availability and thus improving access to such long-acting, reversible contraceptive (LARC) methods could reduce rates of unintended pregnancy in the United States, the report stated.
The high unintended pregnancy rate of nearly 50% is thought to result, in part, from less-frequent use of LARC methods, compared with other user-dependent methods such as condoms and oral contraceptives. LARCs have been shown to be more effective in preventing unintended pregnancies during typical use, according to the report (MMWR 2011;60:1-4).
Previous studies have similarly shown that contraceptive availability – through either on-site provision or through prescription or provider recommendation – is highest for oral contraceptives and lower for the patch, IUD, and vaginal ring. This is one of the few studies, however, to examine provider-specific availability of a wide range of contraceptive methods and the first national study to delineate on-site availability without combining hormonal methods into one category, according to the report.
The survey was mailed to physicians in three specialties: obstetrics/gynecology, family medicine, and adolescent medicine. Title X clinics represented a range of provider agencies, including public health departments and community health centers.
Overall, with the exception of the levonorgestrel-releasing intrauterine device (LNG-IUD, Mirena) – for which on-site availability was reported by 56% of office-based physicians and 47% of Title X clinic providers – office-based physicians were less likely than were Title X clinics to report on-site availability of a range of contraceptive methods.
While nearly all Title X clinics reported having on-site availability of combined oral contraceptives, for instance, approximately half of office-based physicians had them available on-site and half had them available by prescription. One-quarter of office-based physicians offered progestin-only oral contraceptives on-site, in comparison, and almost 30% offered the contraceptive patch on-site. Most of the others reported that these two methods were available by prescription.
Similarly, male condoms were available on-site in nearly all Title X clinics but only in one-quarter of physicians’ offices.
Survey participants were also asked whether specific contraceptive methods were simply "not available" to their patients. The methods most frequently reported as such were female condoms and implants.
Almost 18% of office-based physicians and 10% of Title X providers said female condoms were unavailable, and 8% and 9% of office-based physicians and Title X providers, respectively, said contraceptive implants were unavailable. On the other hand, almost half of the Title X clinic providers said female condoms were available on-site, compared with 7% of in-office physicians. Contraceptive implants were reported by about one-third of the providers in each category to be available on-site.
The survey findings are limited by a low response rate for office-based physicians (47%), the report noted.
The survey did not ascertain reasons why contraceptive methods were unavailable in some cases or not available on-site. Differences in availability can reflect various factors, from variations in reimbursable costs and health insurance trends, to federal and state policies, provider training and patient characteristics, according to the report.
A national survey on contraceptive availability suggests that on-site availability of intrauterine devices and other long-acting, reversible contraceptive methods is less than maximal for both office-based physicians and Title X clinics, according to a report in the Jan. 14 Morbidity and Mortality Weekly Report.
Approximately one-quarter of the 635 office-based physicians and nearly one-third of the 1,368 Title X clinic providers who participated in the CDC survey reported that they refer patients to other providers for IUDs – and even higher proportions said they refer patients out for contraceptive implants.
Increasing on-site availability and thus improving access to such long-acting, reversible contraceptive (LARC) methods could reduce rates of unintended pregnancy in the United States, the report stated.
The high unintended pregnancy rate of nearly 50% is thought to result, in part, from less-frequent use of LARC methods, compared with other user-dependent methods such as condoms and oral contraceptives. LARCs have been shown to be more effective in preventing unintended pregnancies during typical use, according to the report (MMWR 2011;60:1-4).
Previous studies have similarly shown that contraceptive availability – through either on-site provision or through prescription or provider recommendation – is highest for oral contraceptives and lower for the patch, IUD, and vaginal ring. This is one of the few studies, however, to examine provider-specific availability of a wide range of contraceptive methods and the first national study to delineate on-site availability without combining hormonal methods into one category, according to the report.
The survey was mailed to physicians in three specialties: obstetrics/gynecology, family medicine, and adolescent medicine. Title X clinics represented a range of provider agencies, including public health departments and community health centers.
Overall, with the exception of the levonorgestrel-releasing intrauterine device (LNG-IUD, Mirena) – for which on-site availability was reported by 56% of office-based physicians and 47% of Title X clinic providers – office-based physicians were less likely than were Title X clinics to report on-site availability of a range of contraceptive methods.
While nearly all Title X clinics reported having on-site availability of combined oral contraceptives, for instance, approximately half of office-based physicians had them available on-site and half had them available by prescription. One-quarter of office-based physicians offered progestin-only oral contraceptives on-site, in comparison, and almost 30% offered the contraceptive patch on-site. Most of the others reported that these two methods were available by prescription.
Similarly, male condoms were available on-site in nearly all Title X clinics but only in one-quarter of physicians’ offices.
Survey participants were also asked whether specific contraceptive methods were simply "not available" to their patients. The methods most frequently reported as such were female condoms and implants.
Almost 18% of office-based physicians and 10% of Title X providers said female condoms were unavailable, and 8% and 9% of office-based physicians and Title X providers, respectively, said contraceptive implants were unavailable. On the other hand, almost half of the Title X clinic providers said female condoms were available on-site, compared with 7% of in-office physicians. Contraceptive implants were reported by about one-third of the providers in each category to be available on-site.
The survey findings are limited by a low response rate for office-based physicians (47%), the report noted.
The survey did not ascertain reasons why contraceptive methods were unavailable in some cases or not available on-site. Differences in availability can reflect various factors, from variations in reimbursable costs and health insurance trends, to federal and state policies, provider training and patient characteristics, according to the report.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major Finding: Approximately one-quarter of office-based physicians and one-third of Title X clinics refer patients to other providers for IUDs. And, with the exception of the LNG-IUD, fewer office-based physicians than Title X clinics offer a wide array of contraceptive methods on-site.
Data Source: A CDC survey reported in the Morbidity and Mortality Weekly Report.
Disclosures: NA