User login
New ustekinumab response predictor in Crohn’s called ‘brilliant’
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.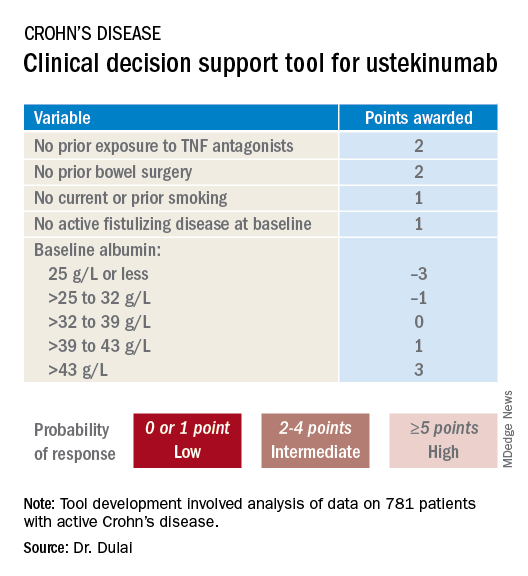
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
REPORTING FROM ACG 2019
New ustekinumab response predictor in Crohn’s called ‘brilliant’
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
REPORTING FROM ACG 2019
Adult atopic dermatitis brings increased osteoporosis risk
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
REPORTING FROM EADV 2019
Which children are at greatest risk for atopic dermatitis?
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
REPORTING FROM The EADV CONGRESS
Key clinical point: Pediatric atopic dermatitis risk varies according to type of parental history of atopic disease.
Major finding: A parental history of atopic dermatitis is associated with a 3.3-fold increased risk of atopic dermatitis in the child, twice the risk associated with parental asthma or allergic rhinitis.
Study details: This was a systematic review and meta-analysis of 149 published studies with 656,711 participants.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
Source: Ravn NH. The EADV Congress.
Human milk oligosaccharides quell IBS symptoms
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
REPORTING FROM ACG 2019
Biofeedback corrects dyssynergic constipation in elderly
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
REPORTING FROM ACG 2019
CTS5 score partially validated for predicting late distant breast cancer recurrences
SAN ANTONIO – The Clinical Treatment Score post 5 years (CTS5) has been validated for the prediction of late distant recurrences in a large contemporary cohort of breast cancer patients drawn from the landmark TAILORx study – but only provided they’re over age 50 at the time of their initial breast cancer diagnosis, Ivana Sestak, PhD, reported at the San Antonio Breast Cancer Symposium.
“The CTS5 was much less prognostic in younger patients, and we did not observe good discrimination for the CTS5 in this cohort,” she said. “Further evaluation in premenopausal cohorts is needed before CTS5 can be applied to younger patients.”
She and her coworkers developed the CTS5 as a simple, expeditious tool to identify women at high risk of late distance recurrence of estrogen receptor–positive breast cancer after successfully completing 5 years of endocrine therapy. It’s designed to serve as an aid to physicians and patients in clinical decision making: Women who are CTS5 high risk are likely to benefit from extended endocrine therapy beyond the 5-year mark, while those at low risk are not.
“Trials so far have shown only a modest risk reduction of around 5% with extended endocrine therapy. This may be partly due to the fact that none of these trials had specifically selected patients who were at high risk of developing a late recurrence. It is therefore crucial that we identify those patients who are at high risk of late recurrence, as they will benefit most from extended endocrine therapy,” explained Dr. Sestak, a medical statistician at Queen Mary University, London.
The CTS5 calculator is freely available online at www.cts5-calculator.com. Clinicians simply plug in readily available information on four specific variables for their patients who have completed 5 years of endocrine therapy free of distant recurrence: age at breast cancer diagnosis, tumor size in millimeters, tumor grade, and number of involved nodes. The calculator promptly spits out a CTS5 score and the associated risk of distant recurrence during years 5-10 after initial diagnosis. That risk is categorized as low if it’s 5% or less in years 5-10, and high if it’s greater than 10%.
The CTS5 was developed and validated using long-term follow-up data on more than 11,000 postmenopausal breast cancer patients in the ATAC and BIG1-98 randomized trials. The CTS5 performed well in those tests. But those studies were completed more than a decade ago and were limited to postmenopausal patients. Dr. Sestak and coinvestigators wanted to assess the tool’s discriminatory powers in a contemporary population of breast cancer patients that included large numbers of premenopausal women. So they tapped into the National Cancer Institute–sponsored TAILORx study, which included 7,353 breast cancer patients who were distant recurrence free after 5 years. All had early-stage, hormone receptor–positive, HER2-negative, and axillary node–negative breast cancer. And all underwent baseline testing using Genomic Health’s Oncotype DX Breast Recurrence Score to assess expression of 21 genes associated with breast cancer recurrence.
The CTS5 proved to be highly prognostic in the overall TAILORx population. But upon drilling down further, Dr. Sestak and coworkers determined that CTS5 had only marginal prognostic value in the 2,259 women age 50 years or younger. Indeed, not a single patient in that age group was categorized as CTS5 high risk, and the actual distant recurrence rates during years 5-9 weren’t significantly different between the low- and intermediate-risk CTS5 groups.
In contrast, CTS5 performed well as a prognosticator in the 2,257 TAILORx participants over age 50 who received both chemotherapy and endocrine therapy during their first 5 years following diagnosis. For a fast and simple test with zero cost, it displayed impressive discriminatory power: The 63.8% of women classified as CTS5 low risk had a 2.6% distant recurrence rate – and thus constituted a group who could reasonably avoid extended endocrine therapy – while the 3.5% who were CTS5 high risk had a 9.5% event rate, and the intermediate-risk group had a 7.3% event rate. The prognostic power of CTS5 in the 2,837 women aged over 50 years who received only hormonal therapy was less robust, albeit still statistically significant.
In women classified as being at low risk of recurrence based upon an Oncotype DX score of 0-10, the CTS5 was not a significant prognosticator for the prediction of late distant recurrences. However, in those who were at intermediate or high risk as determined by a score of 11-100 on the Oncotype test, CTS5 was highly prognostic.
A significant limitation of this CTS5 validation study in the TAILORx population was that only a median 2.86 years of follow-up data after the 5-year mark was available – not sufficient time for a large number of distant recurrences. The rate was 3.1% in women treated with only endocrine therapy who had an Oncotype DX score of 0-25, and 3.8% in those with a score of 11-100 who received both chemotherapy and endocrine therapy.
Dr. Sestak shrugged off the less than stellar performance of the CTS5 in women aged age 50 years or younger in the TAILORx analysis.
“We developed the CTS5 specifically in postmenopausal women, so we’re not really surprised that it’s less prognostic in young women,” she said.
Her group next plans to evaluate the CTS5 in another large premenopausal cohort of breast cancer patients.
“If it’s not prognostic there, then we’ll have to adjust the algorithm and recalibrate it specifically for younger patients,” according to Dr. Sestak.
The TAILORx validation study was supported by Breast Cancer Now, Cancer Research UK, Exact Sciences, and the University of London. Dr. Sestak reported having received honoraria from Myriad Genetics, Nanostring Technology, and Pfizer Oncology.
SOURCE: Sestak I et al. SABCS 2019, Abstract GS4-03.
SAN ANTONIO – The Clinical Treatment Score post 5 years (CTS5) has been validated for the prediction of late distant recurrences in a large contemporary cohort of breast cancer patients drawn from the landmark TAILORx study – but only provided they’re over age 50 at the time of their initial breast cancer diagnosis, Ivana Sestak, PhD, reported at the San Antonio Breast Cancer Symposium.
“The CTS5 was much less prognostic in younger patients, and we did not observe good discrimination for the CTS5 in this cohort,” she said. “Further evaluation in premenopausal cohorts is needed before CTS5 can be applied to younger patients.”
She and her coworkers developed the CTS5 as a simple, expeditious tool to identify women at high risk of late distance recurrence of estrogen receptor–positive breast cancer after successfully completing 5 years of endocrine therapy. It’s designed to serve as an aid to physicians and patients in clinical decision making: Women who are CTS5 high risk are likely to benefit from extended endocrine therapy beyond the 5-year mark, while those at low risk are not.
“Trials so far have shown only a modest risk reduction of around 5% with extended endocrine therapy. This may be partly due to the fact that none of these trials had specifically selected patients who were at high risk of developing a late recurrence. It is therefore crucial that we identify those patients who are at high risk of late recurrence, as they will benefit most from extended endocrine therapy,” explained Dr. Sestak, a medical statistician at Queen Mary University, London.
The CTS5 calculator is freely available online at www.cts5-calculator.com. Clinicians simply plug in readily available information on four specific variables for their patients who have completed 5 years of endocrine therapy free of distant recurrence: age at breast cancer diagnosis, tumor size in millimeters, tumor grade, and number of involved nodes. The calculator promptly spits out a CTS5 score and the associated risk of distant recurrence during years 5-10 after initial diagnosis. That risk is categorized as low if it’s 5% or less in years 5-10, and high if it’s greater than 10%.
The CTS5 was developed and validated using long-term follow-up data on more than 11,000 postmenopausal breast cancer patients in the ATAC and BIG1-98 randomized trials. The CTS5 performed well in those tests. But those studies were completed more than a decade ago and were limited to postmenopausal patients. Dr. Sestak and coinvestigators wanted to assess the tool’s discriminatory powers in a contemporary population of breast cancer patients that included large numbers of premenopausal women. So they tapped into the National Cancer Institute–sponsored TAILORx study, which included 7,353 breast cancer patients who were distant recurrence free after 5 years. All had early-stage, hormone receptor–positive, HER2-negative, and axillary node–negative breast cancer. And all underwent baseline testing using Genomic Health’s Oncotype DX Breast Recurrence Score to assess expression of 21 genes associated with breast cancer recurrence.
The CTS5 proved to be highly prognostic in the overall TAILORx population. But upon drilling down further, Dr. Sestak and coworkers determined that CTS5 had only marginal prognostic value in the 2,259 women age 50 years or younger. Indeed, not a single patient in that age group was categorized as CTS5 high risk, and the actual distant recurrence rates during years 5-9 weren’t significantly different between the low- and intermediate-risk CTS5 groups.
In contrast, CTS5 performed well as a prognosticator in the 2,257 TAILORx participants over age 50 who received both chemotherapy and endocrine therapy during their first 5 years following diagnosis. For a fast and simple test with zero cost, it displayed impressive discriminatory power: The 63.8% of women classified as CTS5 low risk had a 2.6% distant recurrence rate – and thus constituted a group who could reasonably avoid extended endocrine therapy – while the 3.5% who were CTS5 high risk had a 9.5% event rate, and the intermediate-risk group had a 7.3% event rate. The prognostic power of CTS5 in the 2,837 women aged over 50 years who received only hormonal therapy was less robust, albeit still statistically significant.
In women classified as being at low risk of recurrence based upon an Oncotype DX score of 0-10, the CTS5 was not a significant prognosticator for the prediction of late distant recurrences. However, in those who were at intermediate or high risk as determined by a score of 11-100 on the Oncotype test, CTS5 was highly prognostic.
A significant limitation of this CTS5 validation study in the TAILORx population was that only a median 2.86 years of follow-up data after the 5-year mark was available – not sufficient time for a large number of distant recurrences. The rate was 3.1% in women treated with only endocrine therapy who had an Oncotype DX score of 0-25, and 3.8% in those with a score of 11-100 who received both chemotherapy and endocrine therapy.
Dr. Sestak shrugged off the less than stellar performance of the CTS5 in women aged age 50 years or younger in the TAILORx analysis.
“We developed the CTS5 specifically in postmenopausal women, so we’re not really surprised that it’s less prognostic in young women,” she said.
Her group next plans to evaluate the CTS5 in another large premenopausal cohort of breast cancer patients.
“If it’s not prognostic there, then we’ll have to adjust the algorithm and recalibrate it specifically for younger patients,” according to Dr. Sestak.
The TAILORx validation study was supported by Breast Cancer Now, Cancer Research UK, Exact Sciences, and the University of London. Dr. Sestak reported having received honoraria from Myriad Genetics, Nanostring Technology, and Pfizer Oncology.
SOURCE: Sestak I et al. SABCS 2019, Abstract GS4-03.
SAN ANTONIO – The Clinical Treatment Score post 5 years (CTS5) has been validated for the prediction of late distant recurrences in a large contemporary cohort of breast cancer patients drawn from the landmark TAILORx study – but only provided they’re over age 50 at the time of their initial breast cancer diagnosis, Ivana Sestak, PhD, reported at the San Antonio Breast Cancer Symposium.
“The CTS5 was much less prognostic in younger patients, and we did not observe good discrimination for the CTS5 in this cohort,” she said. “Further evaluation in premenopausal cohorts is needed before CTS5 can be applied to younger patients.”
She and her coworkers developed the CTS5 as a simple, expeditious tool to identify women at high risk of late distance recurrence of estrogen receptor–positive breast cancer after successfully completing 5 years of endocrine therapy. It’s designed to serve as an aid to physicians and patients in clinical decision making: Women who are CTS5 high risk are likely to benefit from extended endocrine therapy beyond the 5-year mark, while those at low risk are not.
“Trials so far have shown only a modest risk reduction of around 5% with extended endocrine therapy. This may be partly due to the fact that none of these trials had specifically selected patients who were at high risk of developing a late recurrence. It is therefore crucial that we identify those patients who are at high risk of late recurrence, as they will benefit most from extended endocrine therapy,” explained Dr. Sestak, a medical statistician at Queen Mary University, London.
The CTS5 calculator is freely available online at www.cts5-calculator.com. Clinicians simply plug in readily available information on four specific variables for their patients who have completed 5 years of endocrine therapy free of distant recurrence: age at breast cancer diagnosis, tumor size in millimeters, tumor grade, and number of involved nodes. The calculator promptly spits out a CTS5 score and the associated risk of distant recurrence during years 5-10 after initial diagnosis. That risk is categorized as low if it’s 5% or less in years 5-10, and high if it’s greater than 10%.
The CTS5 was developed and validated using long-term follow-up data on more than 11,000 postmenopausal breast cancer patients in the ATAC and BIG1-98 randomized trials. The CTS5 performed well in those tests. But those studies were completed more than a decade ago and were limited to postmenopausal patients. Dr. Sestak and coinvestigators wanted to assess the tool’s discriminatory powers in a contemporary population of breast cancer patients that included large numbers of premenopausal women. So they tapped into the National Cancer Institute–sponsored TAILORx study, which included 7,353 breast cancer patients who were distant recurrence free after 5 years. All had early-stage, hormone receptor–positive, HER2-negative, and axillary node–negative breast cancer. And all underwent baseline testing using Genomic Health’s Oncotype DX Breast Recurrence Score to assess expression of 21 genes associated with breast cancer recurrence.
The CTS5 proved to be highly prognostic in the overall TAILORx population. But upon drilling down further, Dr. Sestak and coworkers determined that CTS5 had only marginal prognostic value in the 2,259 women age 50 years or younger. Indeed, not a single patient in that age group was categorized as CTS5 high risk, and the actual distant recurrence rates during years 5-9 weren’t significantly different between the low- and intermediate-risk CTS5 groups.
In contrast, CTS5 performed well as a prognosticator in the 2,257 TAILORx participants over age 50 who received both chemotherapy and endocrine therapy during their first 5 years following diagnosis. For a fast and simple test with zero cost, it displayed impressive discriminatory power: The 63.8% of women classified as CTS5 low risk had a 2.6% distant recurrence rate – and thus constituted a group who could reasonably avoid extended endocrine therapy – while the 3.5% who were CTS5 high risk had a 9.5% event rate, and the intermediate-risk group had a 7.3% event rate. The prognostic power of CTS5 in the 2,837 women aged over 50 years who received only hormonal therapy was less robust, albeit still statistically significant.
In women classified as being at low risk of recurrence based upon an Oncotype DX score of 0-10, the CTS5 was not a significant prognosticator for the prediction of late distant recurrences. However, in those who were at intermediate or high risk as determined by a score of 11-100 on the Oncotype test, CTS5 was highly prognostic.
A significant limitation of this CTS5 validation study in the TAILORx population was that only a median 2.86 years of follow-up data after the 5-year mark was available – not sufficient time for a large number of distant recurrences. The rate was 3.1% in women treated with only endocrine therapy who had an Oncotype DX score of 0-25, and 3.8% in those with a score of 11-100 who received both chemotherapy and endocrine therapy.
Dr. Sestak shrugged off the less than stellar performance of the CTS5 in women aged age 50 years or younger in the TAILORx analysis.
“We developed the CTS5 specifically in postmenopausal women, so we’re not really surprised that it’s less prognostic in young women,” she said.
Her group next plans to evaluate the CTS5 in another large premenopausal cohort of breast cancer patients.
“If it’s not prognostic there, then we’ll have to adjust the algorithm and recalibrate it specifically for younger patients,” according to Dr. Sestak.
The TAILORx validation study was supported by Breast Cancer Now, Cancer Research UK, Exact Sciences, and the University of London. Dr. Sestak reported having received honoraria from Myriad Genetics, Nanostring Technology, and Pfizer Oncology.
SOURCE: Sestak I et al. SABCS 2019, Abstract GS4-03.
REPORTING FROM SABCS 2019
Promising new neoadjuvant strategy in luminal B breast cancer
SAN ANTONIO – The combination of ribociclib and letrozole proved to be an attractive alternative to standard multidrug neoadjuvant chemotherapy for women with high-risk luminal B breast cancer in the exploratory phase 2 SOLTI-1402/CORALLEEN trial.
Neoadjuvant therapy with ribociclib (Kisqali), an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), in combination with the aromatase inhibitor letrozole (Femara) proved as effective for presurgical molecular disease downstaging as standard multiagent chemotherapy, but with considerably less toxicity, Joaquín Gavilá, MD, reported at the San Antonio Breast Cancer Symposium.
“We believe that these results suggest that in clinically high-risk luminal B disease, a chemotherapy-free treatment strategy based upon CDK4/6 inhibition is worth exploring in future neoadjuvant trials,” declared Dr. Gavilá, a medical oncologist at the Valencia (Spain) Institute of Oncology.
SOLTI-1402/CORALLEEN was an open-label, multicenter trial involving 106 postmenopausal women with hormone receptor–positive and HER2-negative stage I-IIIA breast cancer, an operable tumor size of at least 2 cm measured by MRI, and high-risk luminal B subtype disease as defined via the Prosigna genomic tumor profiling test, also known as PAM50, on which they had a baseline median Risk of Recurrence (ROR) score of 74 out of a possible 100 points. The luminal B subtype accounts for 30%-40% of all hormone receptor–positive/HER2-negative breast cancer and carries a greater than 10% risk of distant recurrence at 10 years.
The women were randomized to 6 months of neoadjuvant therapy involving one of two regimens: six 28-day cycles of oral ribociclib at 600 mg once daily for 3 weeks followed by 1 week off plus daily oral letrozole at 2.5 mg/day; or four cycles of intravenous doxorubicin at 60 mg/m2 and cyclophosphamide at 600 mg/m2 every 21 days, then weekly intravenous paclitaxel at 80 mg/m2 for 12 weeks.
The primary study endpoint was achievement of a low ROR score at the time of surgery: that is, a score below 40 points if pathologically node-negative at surgery, and below 15 with one to three positive nodes, which are the cutoffs for a less than 10% risk of distant recurrence at 10 years. A low ROR score was accomplished in 47% of the ribociclib/letrozole group and 46% of patients on standard multiagent chemotherapy. The median ROR score improved from 74 points at baseline to 18 in the investigational treatment arm and 25 in the standard chemotherapy arm.
“In other words, we observed that nearly half of the patients were downstaged from high risk to low risk in both treatment arms,” Dr. Gavilá noted.
Another 31% of patients in both treatment arms were ROR-intermediate at surgery.
The reduction in ROR score at day 15 of the study was more pronounced in the ribociclib/letrozole group than in the chemotherapy arm.
Turning to secondary outcomes, Dr. Gavilá noted that a Residual Cancer Burden score of 0 or 1, correlating with a pathologic complete response or minimal residual disease at time of surgery, was documented in 6% of the ribociclib/letrozole group and 12% of the chemotherapy group. A Preoperative Endocrine Prognostic Index (PEPI) score of 0 was attained in 22% of the novel treatment group and similarly in 17% of those on chemotherapy. Median levels of the tumor cell proliferation biomarker Ki 67 improved from 32% at baseline to 3% in the ribociclib/letrozole group and 10% in the chemotherapy arm. Eighty-eight percent of the ribociclib/letrozole group converted from luminal B to the less aggressive luminal A intrinsic subtype, as did 83% of patients following neoadjuvant chemotherapy.
The rate of serious adverse events was 4% in the ribociclib/letrozole group and 15% in the chemotherapy arm. The most common grade 3 or higher adverse event was neutropenia in both study arms, followed by increased transaminase levels in the ribociclib/letrozole group and febrile neutropenia in the chemotherapy arm. Fifty-nine percent of the ribociclib/letrozole group experienced an adverse event leading to dose reduction or temporary interruption of treatment, as did 83% of the chemotherapy group.
The SOLTI-1402/CORALLEEN trial was sponsored by Novartis, the Breast Cancer Research Foundation, The American Association for Cancer Research, and the Breast Cancer Now Career Catalyst. Dr. Gavilá reported serving as a consultant to Novartis, Roche, and MSD.
Simultaneously with Dr. Gavilá’s presentation in San Antonio, the study results were published online in The Lancet Oncology.
In an accompanying editorial, Massimo Cristofanilli, MD, opined that while the novel neoadjuvant treatment strategy tested in SOLTI-1402/CORALLEEN is promising, the most important concept introduced in the study is that a molecular subtyping tool such the Prosigna test can be used to assess the success of neoadjuvant therapy.
“The increasing availability of molecular testing in both the primary and metastatic setting is contributing to a change in the ability to stratify, select, and monitor disease biology and molecular evolution. This is resulting in the introduction of new frameworks in breast cancer treatments with potentially profound effects on patient outcomes and quality of life,” according to Dr. Cristofanilli, professor of medicine at Northwestern University, Chicago.
SOURCE: Gavilá J. SABCS 2019 Abstract GS2-06.
SAN ANTONIO – The combination of ribociclib and letrozole proved to be an attractive alternative to standard multidrug neoadjuvant chemotherapy for women with high-risk luminal B breast cancer in the exploratory phase 2 SOLTI-1402/CORALLEEN trial.
Neoadjuvant therapy with ribociclib (Kisqali), an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), in combination with the aromatase inhibitor letrozole (Femara) proved as effective for presurgical molecular disease downstaging as standard multiagent chemotherapy, but with considerably less toxicity, Joaquín Gavilá, MD, reported at the San Antonio Breast Cancer Symposium.
“We believe that these results suggest that in clinically high-risk luminal B disease, a chemotherapy-free treatment strategy based upon CDK4/6 inhibition is worth exploring in future neoadjuvant trials,” declared Dr. Gavilá, a medical oncologist at the Valencia (Spain) Institute of Oncology.
SOLTI-1402/CORALLEEN was an open-label, multicenter trial involving 106 postmenopausal women with hormone receptor–positive and HER2-negative stage I-IIIA breast cancer, an operable tumor size of at least 2 cm measured by MRI, and high-risk luminal B subtype disease as defined via the Prosigna genomic tumor profiling test, also known as PAM50, on which they had a baseline median Risk of Recurrence (ROR) score of 74 out of a possible 100 points. The luminal B subtype accounts for 30%-40% of all hormone receptor–positive/HER2-negative breast cancer and carries a greater than 10% risk of distant recurrence at 10 years.
The women were randomized to 6 months of neoadjuvant therapy involving one of two regimens: six 28-day cycles of oral ribociclib at 600 mg once daily for 3 weeks followed by 1 week off plus daily oral letrozole at 2.5 mg/day; or four cycles of intravenous doxorubicin at 60 mg/m2 and cyclophosphamide at 600 mg/m2 every 21 days, then weekly intravenous paclitaxel at 80 mg/m2 for 12 weeks.
The primary study endpoint was achievement of a low ROR score at the time of surgery: that is, a score below 40 points if pathologically node-negative at surgery, and below 15 with one to three positive nodes, which are the cutoffs for a less than 10% risk of distant recurrence at 10 years. A low ROR score was accomplished in 47% of the ribociclib/letrozole group and 46% of patients on standard multiagent chemotherapy. The median ROR score improved from 74 points at baseline to 18 in the investigational treatment arm and 25 in the standard chemotherapy arm.
“In other words, we observed that nearly half of the patients were downstaged from high risk to low risk in both treatment arms,” Dr. Gavilá noted.
Another 31% of patients in both treatment arms were ROR-intermediate at surgery.
The reduction in ROR score at day 15 of the study was more pronounced in the ribociclib/letrozole group than in the chemotherapy arm.
Turning to secondary outcomes, Dr. Gavilá noted that a Residual Cancer Burden score of 0 or 1, correlating with a pathologic complete response or minimal residual disease at time of surgery, was documented in 6% of the ribociclib/letrozole group and 12% of the chemotherapy group. A Preoperative Endocrine Prognostic Index (PEPI) score of 0 was attained in 22% of the novel treatment group and similarly in 17% of those on chemotherapy. Median levels of the tumor cell proliferation biomarker Ki 67 improved from 32% at baseline to 3% in the ribociclib/letrozole group and 10% in the chemotherapy arm. Eighty-eight percent of the ribociclib/letrozole group converted from luminal B to the less aggressive luminal A intrinsic subtype, as did 83% of patients following neoadjuvant chemotherapy.
The rate of serious adverse events was 4% in the ribociclib/letrozole group and 15% in the chemotherapy arm. The most common grade 3 or higher adverse event was neutropenia in both study arms, followed by increased transaminase levels in the ribociclib/letrozole group and febrile neutropenia in the chemotherapy arm. Fifty-nine percent of the ribociclib/letrozole group experienced an adverse event leading to dose reduction or temporary interruption of treatment, as did 83% of the chemotherapy group.
The SOLTI-1402/CORALLEEN trial was sponsored by Novartis, the Breast Cancer Research Foundation, The American Association for Cancer Research, and the Breast Cancer Now Career Catalyst. Dr. Gavilá reported serving as a consultant to Novartis, Roche, and MSD.
Simultaneously with Dr. Gavilá’s presentation in San Antonio, the study results were published online in The Lancet Oncology.
In an accompanying editorial, Massimo Cristofanilli, MD, opined that while the novel neoadjuvant treatment strategy tested in SOLTI-1402/CORALLEEN is promising, the most important concept introduced in the study is that a molecular subtyping tool such the Prosigna test can be used to assess the success of neoadjuvant therapy.
“The increasing availability of molecular testing in both the primary and metastatic setting is contributing to a change in the ability to stratify, select, and monitor disease biology and molecular evolution. This is resulting in the introduction of new frameworks in breast cancer treatments with potentially profound effects on patient outcomes and quality of life,” according to Dr. Cristofanilli, professor of medicine at Northwestern University, Chicago.
SOURCE: Gavilá J. SABCS 2019 Abstract GS2-06.
SAN ANTONIO – The combination of ribociclib and letrozole proved to be an attractive alternative to standard multidrug neoadjuvant chemotherapy for women with high-risk luminal B breast cancer in the exploratory phase 2 SOLTI-1402/CORALLEEN trial.
Neoadjuvant therapy with ribociclib (Kisqali), an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), in combination with the aromatase inhibitor letrozole (Femara) proved as effective for presurgical molecular disease downstaging as standard multiagent chemotherapy, but with considerably less toxicity, Joaquín Gavilá, MD, reported at the San Antonio Breast Cancer Symposium.
“We believe that these results suggest that in clinically high-risk luminal B disease, a chemotherapy-free treatment strategy based upon CDK4/6 inhibition is worth exploring in future neoadjuvant trials,” declared Dr. Gavilá, a medical oncologist at the Valencia (Spain) Institute of Oncology.
SOLTI-1402/CORALLEEN was an open-label, multicenter trial involving 106 postmenopausal women with hormone receptor–positive and HER2-negative stage I-IIIA breast cancer, an operable tumor size of at least 2 cm measured by MRI, and high-risk luminal B subtype disease as defined via the Prosigna genomic tumor profiling test, also known as PAM50, on which they had a baseline median Risk of Recurrence (ROR) score of 74 out of a possible 100 points. The luminal B subtype accounts for 30%-40% of all hormone receptor–positive/HER2-negative breast cancer and carries a greater than 10% risk of distant recurrence at 10 years.
The women were randomized to 6 months of neoadjuvant therapy involving one of two regimens: six 28-day cycles of oral ribociclib at 600 mg once daily for 3 weeks followed by 1 week off plus daily oral letrozole at 2.5 mg/day; or four cycles of intravenous doxorubicin at 60 mg/m2 and cyclophosphamide at 600 mg/m2 every 21 days, then weekly intravenous paclitaxel at 80 mg/m2 for 12 weeks.
The primary study endpoint was achievement of a low ROR score at the time of surgery: that is, a score below 40 points if pathologically node-negative at surgery, and below 15 with one to three positive nodes, which are the cutoffs for a less than 10% risk of distant recurrence at 10 years. A low ROR score was accomplished in 47% of the ribociclib/letrozole group and 46% of patients on standard multiagent chemotherapy. The median ROR score improved from 74 points at baseline to 18 in the investigational treatment arm and 25 in the standard chemotherapy arm.
“In other words, we observed that nearly half of the patients were downstaged from high risk to low risk in both treatment arms,” Dr. Gavilá noted.
Another 31% of patients in both treatment arms were ROR-intermediate at surgery.
The reduction in ROR score at day 15 of the study was more pronounced in the ribociclib/letrozole group than in the chemotherapy arm.
Turning to secondary outcomes, Dr. Gavilá noted that a Residual Cancer Burden score of 0 or 1, correlating with a pathologic complete response or minimal residual disease at time of surgery, was documented in 6% of the ribociclib/letrozole group and 12% of the chemotherapy group. A Preoperative Endocrine Prognostic Index (PEPI) score of 0 was attained in 22% of the novel treatment group and similarly in 17% of those on chemotherapy. Median levels of the tumor cell proliferation biomarker Ki 67 improved from 32% at baseline to 3% in the ribociclib/letrozole group and 10% in the chemotherapy arm. Eighty-eight percent of the ribociclib/letrozole group converted from luminal B to the less aggressive luminal A intrinsic subtype, as did 83% of patients following neoadjuvant chemotherapy.
The rate of serious adverse events was 4% in the ribociclib/letrozole group and 15% in the chemotherapy arm. The most common grade 3 or higher adverse event was neutropenia in both study arms, followed by increased transaminase levels in the ribociclib/letrozole group and febrile neutropenia in the chemotherapy arm. Fifty-nine percent of the ribociclib/letrozole group experienced an adverse event leading to dose reduction or temporary interruption of treatment, as did 83% of the chemotherapy group.
The SOLTI-1402/CORALLEEN trial was sponsored by Novartis, the Breast Cancer Research Foundation, The American Association for Cancer Research, and the Breast Cancer Now Career Catalyst. Dr. Gavilá reported serving as a consultant to Novartis, Roche, and MSD.
Simultaneously with Dr. Gavilá’s presentation in San Antonio, the study results were published online in The Lancet Oncology.
In an accompanying editorial, Massimo Cristofanilli, MD, opined that while the novel neoadjuvant treatment strategy tested in SOLTI-1402/CORALLEEN is promising, the most important concept introduced in the study is that a molecular subtyping tool such the Prosigna test can be used to assess the success of neoadjuvant therapy.
“The increasing availability of molecular testing in both the primary and metastatic setting is contributing to a change in the ability to stratify, select, and monitor disease biology and molecular evolution. This is resulting in the introduction of new frameworks in breast cancer treatments with potentially profound effects on patient outcomes and quality of life,” according to Dr. Cristofanilli, professor of medicine at Northwestern University, Chicago.
SOURCE: Gavilá J. SABCS 2019 Abstract GS2-06.
REPORTING FROM SABCS 2019
Tucatinib called game-changer in HER2-positive metastatic breast cancer
SAN ANTONIO – Tucatinib, an investigational oral inhibitor of human epidermal growth factor receptor 2 (HER2) tyrosine kinase, constitutes a major advance in the treatment of heavily pretreated HER2-positive metastatic breast cancer – including patients with brain metastases, Rashmi K. Murthy, MD, declared at the San Antonio Breast Cancer Symposium.
She presented the results of the pivotal HER2CLIMB trial, in which 612 women with heavily pretreated HER2-positive metastatic breast cancer were randomized two-to-one to oral tucatinib at 300 mg twice daily or placebo in combination with standard guideline-recommended treatment with trastuzumab and capecitabine. This landmark double-blind study, conducted at 155 sites in 15 countries, was the first-ever randomized trial to include HER2-positive metastatic breast cancer patients with baseline untreated or previously treated but progressing brain metastases; indeed, nearly half of participants had baseline brain metastases, 40% of which were untreated or treated and progressing.
Not only did tucatinib on top of background trastuzumab and capecitabine reduce the risk of death by 34% compared with placebo, it also more than doubled progression-free survival. And most encouragingly, it did so in patients with or without baseline brain metastases.
“I would like to take a minute here to highlight that this overall survival benefit was seen in patients who had already received trastuzumab, pertuzumab, and T-DM1 [trastuzumab emtansine] and included patients who had brain metastases. Tucatinib in combination with trastuzumab and capecitabine has the potential to become a new standard of care in this population with and without brain metastases,” said Dr. Murthy, a medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
Tucatinib, which crosses the blood-brain barrier, is highly selective for the kinase domain of HER2, with only miniscule inhibition of epidermal growth factor receptor. Its high selectivity is reflected in a favorable tolerability profile: Only 6% of patients discontinued tucatinib because of adverse events. This low discontinuation rate permitted longer treatment, even in this heavily pretreated population.
The most common adverse events in the tucatinib study arm were diarrhea, hand-foot syndrome, nausea, fatigue, and vomiting, with most cases being low grade. Diarrhea, the most common adverse event, occurred in 81% of tucatinib-treated patients and 53% of controls. However, only 13% of the tucatinib group experienced grade 3 or worse diarrhea. Notably, antidiarrheal prophylaxis wasn’t mandated in HER2CLIMB. Antidiarrheal medications were utilized in less than half of the treatment cycles where diarrhea was reported, and even then, for a median of only 3 days per cycle.
Median study follow-up was 14 months. The primary study endpoint – 1-year progression-free survival assessed by blinded independent central review – was 33% in the tucatinib group and 12% in controls. Thus, the risk of disease progression or death was reduced by 46% in the tucatinib group. The median duration of progression-free survival was 7.8 months in tucatinib-treated patients, compared with 5.6 months in controls.
Two-year estimated overall survival was 45% with tucatinib and 27% with placebo. The tucatinib group’s 21.9-month median overall survival was 4.5 months greater than in controls.
One-year estimated progression-free survival in patients with baseline brain metastases was 25% in the tucatinib group and zero in controls.
The confirmed objective response rate was 41% in the tucatinib group, nearly double the 23% figure in controls.
The overall and progression-free survival results were consistent across all prespecified subgroups based upon age, race, hormone receptor status, geographic location, and other factors.
The study met with an extremely favorable reception peppered with comments such as “tremendous results.”
“I think that the HER2CLIMB study is practice-changing,” Hope S. Rugo, MD, said in an interview.
“The overall survival benefit in all patients and in those who have brain metastases is clinically relevant for our patients who have HER2-positive metastatic breast cancer treated with trastuzumab, pertuzumab, and T-DMI. In addition, the toxicity profile is superior to prior oral TKIs, which is a huge issue for our patients with metastatic disease,” observed Dr. Rugo, professor of medicine and director of breast oncology and clinical trials education at the University of California, San Francisco.
The HER2CLIMB results raise an important question for future research: Namely, could tucatinib have a role in preventing brain metastases by giving the drug earlier in the course of treatment of patients who are at high risk for developing brain metastases, she added.
Simultaneously with Dr. Murthy’s presentation in San Antonio, the HER2CLIMB results were published online in the New England Journal of Medicine.
Dr. Murthy reported receiving institutional research support from and serving as a consultant to Seattle Genetics, sponsor of the HER2CLIMB trial, as well as from several other pharmaceutical companies.
SOURCE: Murthy RK. SABCS 2019 Abstract GS1-01.
SAN ANTONIO – Tucatinib, an investigational oral inhibitor of human epidermal growth factor receptor 2 (HER2) tyrosine kinase, constitutes a major advance in the treatment of heavily pretreated HER2-positive metastatic breast cancer – including patients with brain metastases, Rashmi K. Murthy, MD, declared at the San Antonio Breast Cancer Symposium.
She presented the results of the pivotal HER2CLIMB trial, in which 612 women with heavily pretreated HER2-positive metastatic breast cancer were randomized two-to-one to oral tucatinib at 300 mg twice daily or placebo in combination with standard guideline-recommended treatment with trastuzumab and capecitabine. This landmark double-blind study, conducted at 155 sites in 15 countries, was the first-ever randomized trial to include HER2-positive metastatic breast cancer patients with baseline untreated or previously treated but progressing brain metastases; indeed, nearly half of participants had baseline brain metastases, 40% of which were untreated or treated and progressing.
Not only did tucatinib on top of background trastuzumab and capecitabine reduce the risk of death by 34% compared with placebo, it also more than doubled progression-free survival. And most encouragingly, it did so in patients with or without baseline brain metastases.
“I would like to take a minute here to highlight that this overall survival benefit was seen in patients who had already received trastuzumab, pertuzumab, and T-DM1 [trastuzumab emtansine] and included patients who had brain metastases. Tucatinib in combination with trastuzumab and capecitabine has the potential to become a new standard of care in this population with and without brain metastases,” said Dr. Murthy, a medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
Tucatinib, which crosses the blood-brain barrier, is highly selective for the kinase domain of HER2, with only miniscule inhibition of epidermal growth factor receptor. Its high selectivity is reflected in a favorable tolerability profile: Only 6% of patients discontinued tucatinib because of adverse events. This low discontinuation rate permitted longer treatment, even in this heavily pretreated population.
The most common adverse events in the tucatinib study arm were diarrhea, hand-foot syndrome, nausea, fatigue, and vomiting, with most cases being low grade. Diarrhea, the most common adverse event, occurred in 81% of tucatinib-treated patients and 53% of controls. However, only 13% of the tucatinib group experienced grade 3 or worse diarrhea. Notably, antidiarrheal prophylaxis wasn’t mandated in HER2CLIMB. Antidiarrheal medications were utilized in less than half of the treatment cycles where diarrhea was reported, and even then, for a median of only 3 days per cycle.
Median study follow-up was 14 months. The primary study endpoint – 1-year progression-free survival assessed by blinded independent central review – was 33% in the tucatinib group and 12% in controls. Thus, the risk of disease progression or death was reduced by 46% in the tucatinib group. The median duration of progression-free survival was 7.8 months in tucatinib-treated patients, compared with 5.6 months in controls.
Two-year estimated overall survival was 45% with tucatinib and 27% with placebo. The tucatinib group’s 21.9-month median overall survival was 4.5 months greater than in controls.
One-year estimated progression-free survival in patients with baseline brain metastases was 25% in the tucatinib group and zero in controls.
The confirmed objective response rate was 41% in the tucatinib group, nearly double the 23% figure in controls.
The overall and progression-free survival results were consistent across all prespecified subgroups based upon age, race, hormone receptor status, geographic location, and other factors.
The study met with an extremely favorable reception peppered with comments such as “tremendous results.”
“I think that the HER2CLIMB study is practice-changing,” Hope S. Rugo, MD, said in an interview.
“The overall survival benefit in all patients and in those who have brain metastases is clinically relevant for our patients who have HER2-positive metastatic breast cancer treated with trastuzumab, pertuzumab, and T-DMI. In addition, the toxicity profile is superior to prior oral TKIs, which is a huge issue for our patients with metastatic disease,” observed Dr. Rugo, professor of medicine and director of breast oncology and clinical trials education at the University of California, San Francisco.
The HER2CLIMB results raise an important question for future research: Namely, could tucatinib have a role in preventing brain metastases by giving the drug earlier in the course of treatment of patients who are at high risk for developing brain metastases, she added.
Simultaneously with Dr. Murthy’s presentation in San Antonio, the HER2CLIMB results were published online in the New England Journal of Medicine.
Dr. Murthy reported receiving institutional research support from and serving as a consultant to Seattle Genetics, sponsor of the HER2CLIMB trial, as well as from several other pharmaceutical companies.
SOURCE: Murthy RK. SABCS 2019 Abstract GS1-01.
SAN ANTONIO – Tucatinib, an investigational oral inhibitor of human epidermal growth factor receptor 2 (HER2) tyrosine kinase, constitutes a major advance in the treatment of heavily pretreated HER2-positive metastatic breast cancer – including patients with brain metastases, Rashmi K. Murthy, MD, declared at the San Antonio Breast Cancer Symposium.
She presented the results of the pivotal HER2CLIMB trial, in which 612 women with heavily pretreated HER2-positive metastatic breast cancer were randomized two-to-one to oral tucatinib at 300 mg twice daily or placebo in combination with standard guideline-recommended treatment with trastuzumab and capecitabine. This landmark double-blind study, conducted at 155 sites in 15 countries, was the first-ever randomized trial to include HER2-positive metastatic breast cancer patients with baseline untreated or previously treated but progressing brain metastases; indeed, nearly half of participants had baseline brain metastases, 40% of which were untreated or treated and progressing.
Not only did tucatinib on top of background trastuzumab and capecitabine reduce the risk of death by 34% compared with placebo, it also more than doubled progression-free survival. And most encouragingly, it did so in patients with or without baseline brain metastases.
“I would like to take a minute here to highlight that this overall survival benefit was seen in patients who had already received trastuzumab, pertuzumab, and T-DM1 [trastuzumab emtansine] and included patients who had brain metastases. Tucatinib in combination with trastuzumab and capecitabine has the potential to become a new standard of care in this population with and without brain metastases,” said Dr. Murthy, a medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
Tucatinib, which crosses the blood-brain barrier, is highly selective for the kinase domain of HER2, with only miniscule inhibition of epidermal growth factor receptor. Its high selectivity is reflected in a favorable tolerability profile: Only 6% of patients discontinued tucatinib because of adverse events. This low discontinuation rate permitted longer treatment, even in this heavily pretreated population.
The most common adverse events in the tucatinib study arm were diarrhea, hand-foot syndrome, nausea, fatigue, and vomiting, with most cases being low grade. Diarrhea, the most common adverse event, occurred in 81% of tucatinib-treated patients and 53% of controls. However, only 13% of the tucatinib group experienced grade 3 or worse diarrhea. Notably, antidiarrheal prophylaxis wasn’t mandated in HER2CLIMB. Antidiarrheal medications were utilized in less than half of the treatment cycles where diarrhea was reported, and even then, for a median of only 3 days per cycle.
Median study follow-up was 14 months. The primary study endpoint – 1-year progression-free survival assessed by blinded independent central review – was 33% in the tucatinib group and 12% in controls. Thus, the risk of disease progression or death was reduced by 46% in the tucatinib group. The median duration of progression-free survival was 7.8 months in tucatinib-treated patients, compared with 5.6 months in controls.
Two-year estimated overall survival was 45% with tucatinib and 27% with placebo. The tucatinib group’s 21.9-month median overall survival was 4.5 months greater than in controls.
One-year estimated progression-free survival in patients with baseline brain metastases was 25% in the tucatinib group and zero in controls.
The confirmed objective response rate was 41% in the tucatinib group, nearly double the 23% figure in controls.
The overall and progression-free survival results were consistent across all prespecified subgroups based upon age, race, hormone receptor status, geographic location, and other factors.
The study met with an extremely favorable reception peppered with comments such as “tremendous results.”
“I think that the HER2CLIMB study is practice-changing,” Hope S. Rugo, MD, said in an interview.
“The overall survival benefit in all patients and in those who have brain metastases is clinically relevant for our patients who have HER2-positive metastatic breast cancer treated with trastuzumab, pertuzumab, and T-DMI. In addition, the toxicity profile is superior to prior oral TKIs, which is a huge issue for our patients with metastatic disease,” observed Dr. Rugo, professor of medicine and director of breast oncology and clinical trials education at the University of California, San Francisco.
The HER2CLIMB results raise an important question for future research: Namely, could tucatinib have a role in preventing brain metastases by giving the drug earlier in the course of treatment of patients who are at high risk for developing brain metastases, she added.
Simultaneously with Dr. Murthy’s presentation in San Antonio, the HER2CLIMB results were published online in the New England Journal of Medicine.
Dr. Murthy reported receiving institutional research support from and serving as a consultant to Seattle Genetics, sponsor of the HER2CLIMB trial, as well as from several other pharmaceutical companies.
SOURCE: Murthy RK. SABCS 2019 Abstract GS1-01.
REPORTING FROM SABCS 2019
Some with mild Crohn’s disease need no treatment
Kim L. Isaacs, MD, PhD, said at the annual meeting of the American College of Gastroenterology.
First, it’s essential to establish that a patient truly falls into the mild disease category. And second, during that initial evaluation it’s important to look for the features that signal a high risk of subsequent disease progression.
“Patients with risk factors for disease progression should not be treated as mild Crohn’s disease. These are people that we need to treat perhaps more aggressively up front,” said Dr. Isaacs, professor of medicine and codirector of the Multidisciplinary Center for IBD Research and Treatment at the University of North Carolina, Chapel Hill.
Management of patients with severe Crohn’s disease can be challenging, but Dr. Isaacs finds patients with mild disease flat out “terrifying.”
“The reason I find this situation terrifying is we want to prevent the surgery, the progression from inflammatory disease to more stricturing and penetrating disease. And how can I know that my patient a year from now is not going to be very, very ill and I’ve lost my window to use some of our more potent therapies?” she explained.
Mild Crohn’s disease is characterized clinically by less than 10% weight loss; no fever, tachycardia, or other symptoms of systemic disease; lack of abdominal tenderness; and no signs or symptoms of obstruction. When reading the literature, mild disease is defined by a Crohn’s Disease Activity Index score of 150-220. However, nobody uses that metric outside of research studies; it’s just too cumbersome. More useful in routine clinical practice is the Harvey-Bradshaw Index, where a score of 5-7 indicates mild disease.
Many patients with mild Crohn’s disease will not progress over time. In a recent prospective 29-center European study, just 14% of patients progressed from mild to stricturing and/or penetrating disease within 5 years after diagnosis (Gut. 2018 Jan 23. doi: 10.1136/gutjnl-2017-315568).
Who is likely to progress
Risk factors for disease progression include patients with ileocolonic or perianal disease, smokers, those with inflammatory arthritis or other associated immune-mediated disease, and patients who require corticosteroids in order to maintain remission.
On the other hand, patients with mild Crohn’s disease at low risk for progression have no or only mild symptoms, a limited distribution of bowel inflammation, normal or merely slightly elevated C-reactive protein and/or fecal calprotectin levels, no or only superficial bowel ulceration on colonoscopy, and were diagnosed with inflammatory bowel disease after age 30.
Treatment options
“Our goal in a patient we think is low risk is symptomatic management while avoiding high therapeutic risks in someone we don’t think is going to be progressing,” according to the gastroenterologist.
In patients with mild disease who have symptoms in the ileum or right colon, a good strategy is to induce remission using controlled ileal-release budesonide at 9 mg/day, then follow up with colonoscopy 6-12 months later. The high-dose budesonide regimen has been shown in multiple studies to be more effective than placebo at inducing remission. It’s less effective than conventional oral corticosteroids, but it also causes fewer steroid-related side effects. In fact, the evidence shows that the side effect profile of controlled-release budesonide is no different from that of placebo.
In low-risk, mildly symptomatic patients with diffuse Crohn’s colitis, Dr. Isaacs recommends initial treatment with prednisone and/or sulfasalazine or 5-aminosalicylates. The 5-aminosalicylates aren’t part of most practice guidelines because of conflicting study results. However, a meta-analysis of 22 randomized controlled trials has shown that high-dose mesalamine was 2.29-fold more effective than placebo at inducing remission and is a good option for patients who would rather avoid corticosteroids (Inflamm Bowel Dis. 2017 Mar;23(3):461-72). Prednisone should be tapered after achieving clinical remission. Inability to stop steroids within 6 months after entering remission warrants a switch to maintenance therapy with a biologic or immunomodulatory agent.
Smoking cessation should be a top priority
“I spend a good portion of my time in clinic telling patients, ‘You can do more with stopping smoking than I can do with any of my medications,’ ” Dr. Isaacs said.
Another worthwhile intervention is to check for and correct low serum vitamin D levels. Vitamin D is a potent immunomodulator, and severely low levels below 15 ng/mL are associated with increased risk of Crohn’s disease relapse, more hospitalizations, more active disease activity, and heavier use of corticosteroids than in patients with moderate deficiency in the 15- to 30-ng/mL range (Nutrients. 2019 May 11;11[5]. doi: 10.3390/nu11051059).
Monitoring patients with mild Crohn’s disease
“Monitor closely for mucosal inflammation because if they start to progress, we want to get to them early so they get a good response to their first therapies. If the CRP starts moving up, consider doing something,” she advised.
A CRP above 5 mg/L is associated with a markedly increased risk of relapse. And Spanish investigators have shown in a prospective study of 95 patients in clinical remission for at least 6 months while on a tumor necrosis factor inhibitor that thereafter a fecal calprotein level greater than 300 mcg/g at any point was strongly predictive of relapse within the next 4 months (J Clin Gastroenterol. 2018 Mar;52[3]:229-34). Following a patient with mild Crohn’s disease over time endoscopically, it’s of value to utilize the Simple Endoscopic Score for Crohn’s Disease (SES-CD) to document that in fact the disease is mild. Dr. Isaacs recommended IG-IBD Scores – Calculators in Gastroenterology as “a great site” for assistance in calculating the SES-CD, the Harvey-Bradshaw Index, and a plethora of other inflammatory bowel disease scores.
She reported having no financial conflicts of interest.
Kim L. Isaacs, MD, PhD, said at the annual meeting of the American College of Gastroenterology.
First, it’s essential to establish that a patient truly falls into the mild disease category. And second, during that initial evaluation it’s important to look for the features that signal a high risk of subsequent disease progression.
“Patients with risk factors for disease progression should not be treated as mild Crohn’s disease. These are people that we need to treat perhaps more aggressively up front,” said Dr. Isaacs, professor of medicine and codirector of the Multidisciplinary Center for IBD Research and Treatment at the University of North Carolina, Chapel Hill.
Management of patients with severe Crohn’s disease can be challenging, but Dr. Isaacs finds patients with mild disease flat out “terrifying.”
“The reason I find this situation terrifying is we want to prevent the surgery, the progression from inflammatory disease to more stricturing and penetrating disease. And how can I know that my patient a year from now is not going to be very, very ill and I’ve lost my window to use some of our more potent therapies?” she explained.
Mild Crohn’s disease is characterized clinically by less than 10% weight loss; no fever, tachycardia, or other symptoms of systemic disease; lack of abdominal tenderness; and no signs or symptoms of obstruction. When reading the literature, mild disease is defined by a Crohn’s Disease Activity Index score of 150-220. However, nobody uses that metric outside of research studies; it’s just too cumbersome. More useful in routine clinical practice is the Harvey-Bradshaw Index, where a score of 5-7 indicates mild disease.
Many patients with mild Crohn’s disease will not progress over time. In a recent prospective 29-center European study, just 14% of patients progressed from mild to stricturing and/or penetrating disease within 5 years after diagnosis (Gut. 2018 Jan 23. doi: 10.1136/gutjnl-2017-315568).
Who is likely to progress
Risk factors for disease progression include patients with ileocolonic or perianal disease, smokers, those with inflammatory arthritis or other associated immune-mediated disease, and patients who require corticosteroids in order to maintain remission.
On the other hand, patients with mild Crohn’s disease at low risk for progression have no or only mild symptoms, a limited distribution of bowel inflammation, normal or merely slightly elevated C-reactive protein and/or fecal calprotectin levels, no or only superficial bowel ulceration on colonoscopy, and were diagnosed with inflammatory bowel disease after age 30.
Treatment options
“Our goal in a patient we think is low risk is symptomatic management while avoiding high therapeutic risks in someone we don’t think is going to be progressing,” according to the gastroenterologist.
In patients with mild disease who have symptoms in the ileum or right colon, a good strategy is to induce remission using controlled ileal-release budesonide at 9 mg/day, then follow up with colonoscopy 6-12 months later. The high-dose budesonide regimen has been shown in multiple studies to be more effective than placebo at inducing remission. It’s less effective than conventional oral corticosteroids, but it also causes fewer steroid-related side effects. In fact, the evidence shows that the side effect profile of controlled-release budesonide is no different from that of placebo.
In low-risk, mildly symptomatic patients with diffuse Crohn’s colitis, Dr. Isaacs recommends initial treatment with prednisone and/or sulfasalazine or 5-aminosalicylates. The 5-aminosalicylates aren’t part of most practice guidelines because of conflicting study results. However, a meta-analysis of 22 randomized controlled trials has shown that high-dose mesalamine was 2.29-fold more effective than placebo at inducing remission and is a good option for patients who would rather avoid corticosteroids (Inflamm Bowel Dis. 2017 Mar;23(3):461-72). Prednisone should be tapered after achieving clinical remission. Inability to stop steroids within 6 months after entering remission warrants a switch to maintenance therapy with a biologic or immunomodulatory agent.
Smoking cessation should be a top priority
“I spend a good portion of my time in clinic telling patients, ‘You can do more with stopping smoking than I can do with any of my medications,’ ” Dr. Isaacs said.
Another worthwhile intervention is to check for and correct low serum vitamin D levels. Vitamin D is a potent immunomodulator, and severely low levels below 15 ng/mL are associated with increased risk of Crohn’s disease relapse, more hospitalizations, more active disease activity, and heavier use of corticosteroids than in patients with moderate deficiency in the 15- to 30-ng/mL range (Nutrients. 2019 May 11;11[5]. doi: 10.3390/nu11051059).
Monitoring patients with mild Crohn’s disease
“Monitor closely for mucosal inflammation because if they start to progress, we want to get to them early so they get a good response to their first therapies. If the CRP starts moving up, consider doing something,” she advised.
A CRP above 5 mg/L is associated with a markedly increased risk of relapse. And Spanish investigators have shown in a prospective study of 95 patients in clinical remission for at least 6 months while on a tumor necrosis factor inhibitor that thereafter a fecal calprotein level greater than 300 mcg/g at any point was strongly predictive of relapse within the next 4 months (J Clin Gastroenterol. 2018 Mar;52[3]:229-34). Following a patient with mild Crohn’s disease over time endoscopically, it’s of value to utilize the Simple Endoscopic Score for Crohn’s Disease (SES-CD) to document that in fact the disease is mild. Dr. Isaacs recommended IG-IBD Scores – Calculators in Gastroenterology as “a great site” for assistance in calculating the SES-CD, the Harvey-Bradshaw Index, and a plethora of other inflammatory bowel disease scores.
She reported having no financial conflicts of interest.
Kim L. Isaacs, MD, PhD, said at the annual meeting of the American College of Gastroenterology.
First, it’s essential to establish that a patient truly falls into the mild disease category. And second, during that initial evaluation it’s important to look for the features that signal a high risk of subsequent disease progression.
“Patients with risk factors for disease progression should not be treated as mild Crohn’s disease. These are people that we need to treat perhaps more aggressively up front,” said Dr. Isaacs, professor of medicine and codirector of the Multidisciplinary Center for IBD Research and Treatment at the University of North Carolina, Chapel Hill.
Management of patients with severe Crohn’s disease can be challenging, but Dr. Isaacs finds patients with mild disease flat out “terrifying.”
“The reason I find this situation terrifying is we want to prevent the surgery, the progression from inflammatory disease to more stricturing and penetrating disease. And how can I know that my patient a year from now is not going to be very, very ill and I’ve lost my window to use some of our more potent therapies?” she explained.
Mild Crohn’s disease is characterized clinically by less than 10% weight loss; no fever, tachycardia, or other symptoms of systemic disease; lack of abdominal tenderness; and no signs or symptoms of obstruction. When reading the literature, mild disease is defined by a Crohn’s Disease Activity Index score of 150-220. However, nobody uses that metric outside of research studies; it’s just too cumbersome. More useful in routine clinical practice is the Harvey-Bradshaw Index, where a score of 5-7 indicates mild disease.
Many patients with mild Crohn’s disease will not progress over time. In a recent prospective 29-center European study, just 14% of patients progressed from mild to stricturing and/or penetrating disease within 5 years after diagnosis (Gut. 2018 Jan 23. doi: 10.1136/gutjnl-2017-315568).
Who is likely to progress
Risk factors for disease progression include patients with ileocolonic or perianal disease, smokers, those with inflammatory arthritis or other associated immune-mediated disease, and patients who require corticosteroids in order to maintain remission.
On the other hand, patients with mild Crohn’s disease at low risk for progression have no or only mild symptoms, a limited distribution of bowel inflammation, normal or merely slightly elevated C-reactive protein and/or fecal calprotectin levels, no or only superficial bowel ulceration on colonoscopy, and were diagnosed with inflammatory bowel disease after age 30.
Treatment options
“Our goal in a patient we think is low risk is symptomatic management while avoiding high therapeutic risks in someone we don’t think is going to be progressing,” according to the gastroenterologist.
In patients with mild disease who have symptoms in the ileum or right colon, a good strategy is to induce remission using controlled ileal-release budesonide at 9 mg/day, then follow up with colonoscopy 6-12 months later. The high-dose budesonide regimen has been shown in multiple studies to be more effective than placebo at inducing remission. It’s less effective than conventional oral corticosteroids, but it also causes fewer steroid-related side effects. In fact, the evidence shows that the side effect profile of controlled-release budesonide is no different from that of placebo.
In low-risk, mildly symptomatic patients with diffuse Crohn’s colitis, Dr. Isaacs recommends initial treatment with prednisone and/or sulfasalazine or 5-aminosalicylates. The 5-aminosalicylates aren’t part of most practice guidelines because of conflicting study results. However, a meta-analysis of 22 randomized controlled trials has shown that high-dose mesalamine was 2.29-fold more effective than placebo at inducing remission and is a good option for patients who would rather avoid corticosteroids (Inflamm Bowel Dis. 2017 Mar;23(3):461-72). Prednisone should be tapered after achieving clinical remission. Inability to stop steroids within 6 months after entering remission warrants a switch to maintenance therapy with a biologic or immunomodulatory agent.
Smoking cessation should be a top priority
“I spend a good portion of my time in clinic telling patients, ‘You can do more with stopping smoking than I can do with any of my medications,’ ” Dr. Isaacs said.
Another worthwhile intervention is to check for and correct low serum vitamin D levels. Vitamin D is a potent immunomodulator, and severely low levels below 15 ng/mL are associated with increased risk of Crohn’s disease relapse, more hospitalizations, more active disease activity, and heavier use of corticosteroids than in patients with moderate deficiency in the 15- to 30-ng/mL range (Nutrients. 2019 May 11;11[5]. doi: 10.3390/nu11051059).
Monitoring patients with mild Crohn’s disease
“Monitor closely for mucosal inflammation because if they start to progress, we want to get to them early so they get a good response to their first therapies. If the CRP starts moving up, consider doing something,” she advised.
A CRP above 5 mg/L is associated with a markedly increased risk of relapse. And Spanish investigators have shown in a prospective study of 95 patients in clinical remission for at least 6 months while on a tumor necrosis factor inhibitor that thereafter a fecal calprotein level greater than 300 mcg/g at any point was strongly predictive of relapse within the next 4 months (J Clin Gastroenterol. 2018 Mar;52[3]:229-34). Following a patient with mild Crohn’s disease over time endoscopically, it’s of value to utilize the Simple Endoscopic Score for Crohn’s Disease (SES-CD) to document that in fact the disease is mild. Dr. Isaacs recommended IG-IBD Scores – Calculators in Gastroenterology as “a great site” for assistance in calculating the SES-CD, the Harvey-Bradshaw Index, and a plethora of other inflammatory bowel disease scores.
She reported having no financial conflicts of interest.
REPORTING FROM ACG 2019