User login
The Wisconsin Upper Respiratory Symptom Survey (WURSS)
OBJECTIVE: To develop a sensitive, reliable, responsive, and easy-to-use instrument for assessing the severity and functional impact of the common cold.
STUDY DESIGN: We created an illness-specific health-related quality-of-life outcomes instrument using previous scales, expert opinion, and common knowledge. This original questionnaire was used in a 1999 randomized trial of echinacea for the common cold. In 2000 we employed cognitive interview and focus group qualitative methods to further develop the instrument. Semistructured interviews used open-ended questions to elicit symptoms, terminology, and perceived functional impact. Responses were used to improve the instrument.
POPULATION: The randomized trial watched 142 University of Wisconsin students for a total of 953 days of illness. The subsequent qualitative instrument development project recruited 74 adults with self-diagnosed colds for 56 in-person interviews and 3 focus groups.
OUTCOMES MEASURED: We measured specific symptoms, symptom clusters (dimensions), functional impact, and global severity.
RESULTS: The original questionnaire included 20 questions: a global severity indicator, 15 symptom-severity items using 9-point severity scales, and 4 yes/no functional assessments. Data from the trial provided evidence of 4 underlying dimensions: nasal, throat, cough, and fever and aches, with reliability coefficients of 0.663, 0.668, 0.794, and 0.753, respectively. Qualitative assessments from the interviews and focus groups led us to expand from 15 to 32 symptom-specific items and from 4 to 10 functional impairment items. The original 9-point severity scale was revised to 7 points. Two global severity questions bring the item count to 44. The instrument fits comfortably on the front and back of a single sheet of paper and takes 5 to 10 minutes to complete.
CONCLUSIONS: The Wisconsin Upper Respiratory Symptom Survey (WURSS) is now ready for formal validity testing or practical use in common cold research.
The common cold, usually caused by viral infection of the upper respiratory tract, is a very prevalent illness. On average, US adults suffer from 1 to 4 episodes per year.1-3 This high incidence, along with significant symptomatic and functional impairment, combine to make this syndrome an important health problem. Hundreds of trials have attempted to demonstrate effective treatments.4,5 Unfortunately, few efforts have been made to develop and validate instruments to measure the symptomatic and functional impact of the common cold.
The term “upper respiratory infection” (URI) is a nosologic category constructed by physicians and other health professionals to reflect an upper airway, mucus-producing, inflammatory reaction to infection, usually viral. It is a disease category. The terms rhinitis, rhinosinusitis, pharyngitis, and bronchitis are often used to indicate the anatomic area most affected. The term “common cold” is an illness term constructed and used by the general populace. This distinction between professional (disease) and popular (illness) conceptions6 provides the reasoning for participant-based, patient-oriented qualitative development of measurement tools. While many medical professionals may choose to measure URI disease by physical examination, viral culture, or laboratory analysis of blood or nasal discharge, we believe that most people are more interested in how they can reduce the severity and duration of their symptoms and the functional impairments that result from their illness.
George Gee Jackson and colleagues7 began experimental work in the 1950s, observing and recording the cold symptoms produced by challenging more than 1,000 volunteers with filtered nasal secretions obtained from cold-sufferers. Eight symptoms–sneezing, headache, malaise, chilliness, nasal discharge, nasal obstruction, sore throat, and cough–were selected for evaluation and graded as absent (0), mild (1), moderate (2), or severe (3) every day for 6 days after inoculation. A score of 14 or higher was chosen as the cutoff value that best distinguished infected from noninfected participants. Thus, the original Jackson scale was apparently designed to discriminate between those with and without demonstrable viral infection, and not as a measure of severity. The tables and graphics in Jackson’s seminal works point toward reasonable internal consistency and discriminate validity.7-9 However, other important measurement properties, such as precision, reliability, responsiveness, and stability, were not reported. Despite these limitations, Jackson’s scale has been used for decades by most of the major common cold research groups.10-15
Using various modifications of the Jackson scale, researchers of the cold have characterized the frequency and severity of the 8 symptoms noted above in both natural colds and experimentally induced rhinovirus infections. Variability in symptom expression remains a hallmark of URI. Although specific pathogens are associated with the severity and distribution of symptoms at the population level, symptoms are poor predictors of etiology at the individual level. Infection itself is an imperfect predictor of symptom expression, as asymptomatic infections occur frequently, and as URI-like symptoms occur in people in whom it is not possible to demonstrate infections.16 Even among people with documented experimental infections of single strains of virus, variance outweighs central tendency in all symptom measurements.17,18
The search for objective disease measures with which to compare symptom scores has also progressed. To date, the following measures have been evaluated: detection of virus with culture or polymerase chain reaction,16,19 cytokine measurement,20-22 serologic markers,23 physical examination,9,24 radiologic imaging,25,26 rhinomanometry,27,28 mucus weight,29 mucus velocity, and number of tissues used.30 None have been shown to be superior to self-reported symptoms in terms of precision, reliability, or responsiveness or in their ability to predict functional impairment or subsequent illness. Perhaps more important, none have been shown to reflect the values of the people who experience colds. Although a number of quality-of-life instruments have been developed to assess allergic rhinitis,31-36 we have been unable to locate any specifically developed to assess URI.
We therefore decided to develop the Wisconsin Upper Respiratory Symptom Survey (WURSS) to provide a standardized measure for evaluating the negative consequences of the common cold. We were particularly interested in developing a health-related quality of life instrument that would represent the symptomatic and functional dimensions that are important to cold-sufferers.37-40 The instrument should be able to discriminate accurately between active intervention and placebo effects in randomized therapeutic trials and should balance brevity and ease of use with optimal precision, reliability, and responsiveness.41-43 It should be based on self-diagnosis and self-assessment because neither accepted criteria nor adequate tests are available to diagnose “upper respiratory infection” or “acute infectious rhinosinusitis”(with or without “pharyngitis”) and because the vast majority of cold treatments will be taken without professional input after self-diagnosis.
Methods
Phase 1: Initial development during a randomized trial
The development of this study began in 1998 during the design of a randomized controlled trial (RCT) of echinacea as a cold treatment. We created our first instrument by showing successive drafts to friends and colleagues (mostly family physicians), stopping once we were satisfied that the questionnaire had reasonable face validity. This initial instrument rated global severity of illness (“How sick do you feel today?”) and 15 individual symptoms on a 9-point Likert-type scale. The 15 symptom-measuring items were complemented by 4 dichotomous (yes/no) functional outcome questions, adapted with permission from the validated Medical Outcomes Study 36-item Short-Form Health Survey (SF-36).44
This initial instrument was used in the spring of 1999 in the echinacea RCT.45 This experience provided a good initial test of our instrument, as the participants were recruited within 36 hours of their first symptom and monitored each day until they had answered “No” to the question, “Do you think that you are still sick today?” for 2 days in a row. Each participant was asked to fill out the questionnaire both on paper and on a computerized data-collecting facsimile (available at http://www.fammed.wisc.edu/samplecold).
Phase 2: Further instrument development using qualitative methods
After the echinacea RCT was completed, our primary concern was that we might be overlooking or under-representing important illness domains. We also suspected that wording, question order, response range, and other formatting concerns could be improved. To achieve these goals, we used qualitative instrument-development methodologies, involving the people we wanted to measure–cold-sufferers–in the development process.40,46-50
After obtaining approval from the University of Wisconsin Medical School Human Subjects Committee, we began interviewing Madison-area adults who responded to community advertising asking for volunteers with colds. Inclusion criteria required answering “Yes” to the question, “Do you believe that you have a cold?” For an interview to be arranged, at least one cold symptom had to be present, and the research assistant had to be convinced that the caller was indeed suffering from a common cold. Prospective participants with itchy eyes, sneezing, or a history of allergy were excluded if either the participant or the interviewer felt that any current symptoms might have been caused by allergy. Interviews were held in a location of mutual convenience and with the aid of an interview guide developed by our research team. Interviewers were carefully trained in the research protocol and used interview guides for both the initial telephone screen and the in-person interviews. Interviewers included both clinicians and nonclinicians.
The semistructured interview guide used open-ended questions designed to elicit the participants’ own terminology for describing their colds (Table 1).51-53 We aimed for an understanding of how the experience of the cold influenced the lives of the participants. Participants were first asked to list all their symptoms, then to describe how each symptom bothered them. Next, we asked which symptom(s) appeared first and which one(s) followed. We then asked which symptoms were most bothersome and why. Participants were asked to describe what they did to relieve their cold symptoms, why, and whether the therapy provided any relief. Participants were then asked about how their cold affected their lifestyle with regard to work, relationships, activities, and so forth. Additionally, we asked about symptoms and effects of previous colds. This exploratory phase of the interview lasted approximately 20 to 30 minutes.
Once the interviewer had a thorough description and understanding of the participant’s cold, the participant was asked to complete the questionnaire-in-development. After marking answers on the questionnaire (which took 3 to 5 minutes), each participant was asked to comment on its ease of use, item wording, formatting, and response range as well as whether it accurately and comprehensively measured the symptoms and functional impact they were experiencing. The instrument development phase of the interview lasted for another 20 to 30 minutes.
We used focus group methods in the final month of the study as an additional window into participants’ experiences.54-56 The focus groups used the same inclusion criteria as the long interviews and followed the same general format, first using open-ended questions to elicit symptoms and their impact, then administrating the questionnaire and discussing item inclusion and formatting. However, we encouraged discussion rather than self-assessment, as the focus group methodology derives its strength from the interactive nature of conversation. For instance, a statement made by one participant would spark interest or recall in another, thereby generating a richer, fuller, and more representative description of symptoms and functional impact.
Individual interviews were held by 1 of 5 trained interviewers (B.B., L.L., R.M., E.S., J.S.). All 3 focus groups were run by the lead author, with at least 1 other research team member assisting. Interviews and focus groups were arranged as soon as possible after the initial telephone contact so that participants would still have cold symptoms while being interviewed. All interviews and focus groups were discussed in biweekly group meetings. Decisions on item inclusion, wording, and questionnaire format were made by research group consensus. Several versions of the questionnaire were brought back to cold-sufferers for further cognitive testing. The diversity of interviewers and respondents provided protection against personal bias in ascertaining and interpreting symptoms and impairments.
TABLE 1
QUESTIONS ASKED DURING INTERVIEW
| Current Symptom History and Evaluation |
|---|
| List and describe all symptoms you have with this cold. |
| How do these symptoms bother you? |
| What is the first symptom you noticed when getting this cold? The Next? |
| The next? |
| Which cold symptom bothers you the most? How and why? |
| Are there other symptoms that bother you? How and why? |
| Interventions |
| What do you do to relieve cold symptoms? Why? |
| What over-the-counter medicines would you use? Why? Did it help? |
| What herbal medicines would you use? Why? Did it help? |
| Do you do anything else to relieve symptoms or treat your cold? Why? |
| Did it help? |
| When would you see a doctor or other health care provider? Why? |
| Lifestyle |
| Has this cold interfered with your normal activities? How? |
| When does a cold keep you from doing what you want or need to do? How? |
| Describe what things are harder to do? |
| Previous Symptom History and Evaluation |
| How many colds did you have this past year? |
| How long did they usually last? |
| List and describe what symptoms you usually get with your colds? |
| How do these symptoms bother you? |
| Survey Evaluation (After Participant Has Completed the Questionnaire) |
| Is this form easy to read? |
| Are there any other symptoms that should be on this questionnaire? |
| Are there any questions that shouldn’t be there? |
| Are there any questions that could be worded better? |
| Is the 7-point scale appropriate? Why or why not? |
Results
Phase 1
Of the 148 college students enrolled, 142 followed protocol and were included in the analysis. Of the 853 person-days documented, 546 (64%) were covered by both data systems; 287 (33.6%) came from paper surveys only; and 18 (2.1%) were filled out via computer only. Because only 2 (0.2%) questionnaires were missing any data, our data capture rate was 99.8%. Comparing data from the computerized and paper data sources provided evidence of consistency. Of the 546 days in which both paper and computer instruments provided data, 512 yielded identical responses (94% concordant) to the global severity of illness question. Of the 34 (6%) discrepancies, 29 were off by 1 point on the 9-point Likert-type scale and 5 discrepancies were off by 2 points. Comparing computer and paper responses with the 15 specific symptom questions also yielded high levels of concordance. Of 8190 item responses, 7777 (95%) were concordant, while 413 (5%) were classified as data discrepancies. Of these, 293 were off by 1 point on the 9-point scale; 68 were off by 2 points; 27, by 3 points; 17, by 4 points; 7, by 5 points; and 1 by 6 points.
Factor analysis of the data provided further evidence of internal validity. Structural equation modeling techniques57,58 were used to model symptom severities over time. A 4-dimensional symptom-recovery model (df = 71; P = .000025) provided a goodness of fit index of 0.88, a root mean square residual of .095, and a chi-squared/df ratio of 139/71 = 1.95. From the pool of 15 scaled symptom scores, 14 items contributed significantly to the model. (In this data set, loss of appetite was an infrequent symptom contributing insignificantly toward the model, and was dropped.) The 14 symptoms naturally aggregated into 4 underlying symptomatic dimensions: cough, throat, nasal, and fever and aches. Table 2 provides the reliability coefficients, standardized item loading coefficients, and standard errors of these loadings for the 4 dimensions. The reliability coefficients of the symptom dimensions were calculated using a procedure proposed by Dillon and Goldstein.59 Scale recovery curves, generated using a mixed modeling approach,60,61 were internally predictive, responsive,37,62 and consistent with what is known about the natural history of URI.
TABLE 2
RELIABILITY OF SYMPTOM DIMENSION MODELS
| Item Loading (SE)* | |
|---|---|
| Cough Dimension (Reliability = 0.794) | |
| Coughing | 2.01 (0.20) |
| Coughing stuff up | 1.75 (0.18) |
| Cough interfering with sleep | 1.16 (0.17) |
| Fever and Aches Dimension (Reliability = 0.753) | |
| Headache | 1.28 (0.23) |
| Fever | 1.07 (0.13) |
| Sweats | 1.25 (0.16) |
| Muscle aches | 1.76 (0.19) |
| Feeling run down | 1.17 (0.19) |
| Throat Dimension (Reliability = 0.668) | |
| Sore throat | 1.10 (0.22) |
| Scratchy throat | 1.73 (0.23) |
| Hoarseness | 1.68 (0.24) |
| Nasal Dimension (Reliability = 0.663) | |
| Runny nose | 1.93 (0.28) |
| Stuffy nose | 1.05 (0.23) |
| Sneezing | 1.63 (0.26) |
| *All significant at P < .05. | |
| SE denotes standard error. | |
Phase 2
Between July and December 2000, 108 persons from the general population responded to advertising by calling a telephone number listed on posted flyers and in the newspaper. Of these 108 callers, 27 were eligible but declined to participate; 7 did not meet inclusion criteria (were younger than 18 years of age, had current allergy symptoms, or did not have cold symptoms); and 74 met study criteria and elected to participate (Table 3). Those declining to participate usually did so because of inconvenience in arranging an immediate interview or because compensation ($10 for interview, $15 for focus group) was insufficient. Participants were met in person for semistructured individual interviews (n = 56) or focus groups (3 groups, 20 individuals total). Two people were interviewed both individually and in focus group.
Based on the information gained during interviews, the instrument-in-development underwent 6 revisions during 2000. Each modification was tested with additional interviews. A final version was created in December 2000. A few items from the initial instrument used in the echinacea trial were modified in response to participants’ descriptions and insights. Several other items were added to reflect symptoms and functional impairments described by participants in response to our open-ended questions. All items used wording provided by participants or tested during subsequent interviews and focus groups.
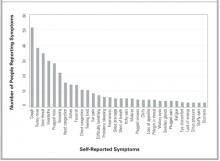
All symptoms spontaneously reported by at least 3 participants in either individual interview or focus group were included in the final version (Table 4). Figure 1 provides a frequency distribution of the symptoms described during the individual interviews.
On the basis of our participants’ comments, the distribution of severity data from the echinacea trial, and recommendations from published studies, we decided to decrease the response range from a 9-point to a 7-point Likert-type severity scale. The resulting severity range was marked at 1 (very mild), 3 (mild), 5 (moderate), and 7 (severe), following the majority opinion of our respondents. Unmarked (even-numbered) options were included, as most of the respondents felt they should have “in-between” choices. The functional outcome questions adapted from the SF-36 were replaced with participant-generated items, which were then scaled in a 7-point format similar to that used for the symptoms. A final question comparing today’s global severity with yesterday’s was added to provide a comparative measure of change over time (responsiveness). Figure 2 displays the final format of WURSS items.
The qualitative data provided by our informants improved our understanding of the symptomatic and functional impact of the common cold and assisted the development of the WURSS questionnaire. Although it is clear that people experience colds in different ways, several common threads emerged. For instance, we found that our original instrument (like the Jackson criteria) had overrated individual symptoms and had underrated functional impact, interference with social relationships, and general malaise. Informants often told us that it was not necessarily the individual symptoms that bothered them, but the general feelings, described as “sick feeling,” “loss of energy,” “run down,” “tired,” “fatigue,” “malaise,” “lousy,” “lazy,” “spacey," "blah," "yucky," "foggy," "lightheaded," "fuzzy brain,” “cloudy,” “disoriented,” “uncomfortable,” “distracted,” and “miserable.” Our informants also told us that they were bothered by the way their cold interfered with day-to-day activities and relationships. Colds affected physical activities such as breathing or walking, performance at work or in the home, and interactions with friends, family, and coworkers. Terms describing the most frequent and bothersome effects were incorporated into the final WURSS instrument.
TABLE 3
INTERVIEW AND FOCUS GROUP PARTICIPANT DEMOGRAPHICS
| Number | |
|---|---|
| Method of Data Collection | |
| Individual interviews | 56 |
| Focus groups | 20 |
| Total | 74* |
| Sex | |
| Women | 49 |
| Men | 25 |
| Ethnicity | |
| Native American | 2 |
| Black | 12 |
| Hispanic | 2 |
| White | 57 |
| No response | 1 |
| Annual Income | |
| < $10,000 | 28 |
| $10,000–19,999 | 17 |
| $20,000–29,999 | 13 |
| $30,000–49,999 | 9 |
| $50,000–75,000 | 3 |
| $75,000 | 2 |
| No response | 2 |
| Education | |
| Some high school | 13 |
| High school or equivalent | 11 |
| Some college | 11 |
| Associate or technical degree | 6 |
| Bachelor’s degree | 21 |
| Master’s degree | 6 |
| Professional degree | 4 |
| No response | 2 |
| Tobacco Use | |
| Current | 26 |
| Past | 19 |
| Never smoker | 28 |
| No response | 1 |
| *Two participants were used in both data collection methods. | |
| NOTE: Age range was 19 to 71 years, mean = 35.9 years (standard deviation, 11.9). | |
TABLE 4
SYMPTOMS AND FUNCTIONAL IMPAIRMENTS EVALUATED BY THE WISCONSIN UPPER RESPIRATORY SYMPTOM SURVEY
| Symptoms | Plugged ears |
| Cough | Ear discomfort |
| “Coughing stuff up” | Watery eyes |
| Cough interfering with sleep | Eye discomfort |
| Sore throat | Head congestion |
| Scratchy throat | Chest congestion |
| Hoarseness | Chest tightness |
| Runny nose | Heaviness in chest |
| Plugged nose | Lack of energy |
| Sneezing | Loss of appetite |
| Headache | |
| Body aches | Functional Impairments |
| Feeling “run down” | Think clearly |
| Sweats | Speak clearly |
| Chills | Sleep well |
| Feeling feverish | Breathe easily |
| Feeling dizzy | Walk, climb stairs, exercise |
| Feeling tired | Accomplish daily activities |
| Irritability | Work outside the home |
| Sinus pain | Work inside the home |
| Sinus pressure | Interact with others |
| Sinus drainage | Live your personal life |
| Swollen glands |
FIGURE 1
SYMPTOMS REPORTED IN INDIVIDUAL INTERVIEWS
FIGURE 2
ITEM FORMAT FOR THE WISCONSIN UPPER RESPIRATORY SYMPTOM SURVEY
Discussion
Researchers of URIs and the common cold need a well-developed, standardized, validated outcomes instrument that reflects the experience and values of cold sufferers. While the Jackson scale and various modifications have been widely used, few data support the validity of these scales. Although correlations with external measures, such as physical examinations, mucus weight, and the ability to culture virus have been reported, the symptomatic and functional impact of colds has largely been neglected. Perhaps more important, the symptomatic measures used to date were apparently developed without significant input from the people whose illnesses were measured. Questionnaire development and cognitive testing methods have not been described, nor have adequate tests of psychometric properties been reported. Although the Jackson scale may demonstrate marginal face validity in terms of symptoms, it does not do so in terms of functional impact.
This article describes the first steps taken in the development and validation of a new illness-specific quality-of-life instrument for measuring the common cold. The WURSS instrument is more comprehensive than existing alternatives and better reflects cold-sufferers’ experiences and values. Therefore, it provides greater face validity. The length (44 items) reflects a compromise between ease of use and comprehensiveness.41 It is possible that a subset of the items will prove nearly as effective and that a short-form WURSS will eventually be available. Item reduction will need to be guided by both internal (factor analysis) and external (frequency and perceived value) considerations. The standardized 7-point severity scale used throughout the WURSS makes the instrument very easy to use.49 It also provides a severity range that our informants and previous researchers40,63,64 agree is optimal. The WURSS allows a cold-sufferer to swiftly and accurately assess his or her common cold. We hope that WURSS will prove worthy in terms of standard psychometric properties such as precision, reliability, and responsiveness.65-68 A large prospective study will be necessary for those assessments.
Limitations
The work described here has a number of limitations. The WURSS was developed in Madison, Wis., largely among people with self-diagnosed colds during the period from July to December 2000. The RCT occurred over several months in the spring of 1999 and was limited to college students. Our results are therefore limited by both population and etiologic agent, which in turn may influence the symptom and severity spectrums assessed. Although we aimed for and achieved a moderate degree of socioeconomic diversity (Table 3), our participants’ responses may not be representative of the larger universe of cold-sufferers. The symptom distribution in Figure 1, for example, is unlikely to represent global cold symptom frequency accurately. Previous research with both natural and induced colds suggests that nasal symptoms and sore throat are usually more frequent than cough.2,3,6-18 The comprehensiveness of the instrument is more important for instrument development than are the specific item frequencies. Here, we feel that we succeeded in representing a sufficient range of items.
Another important limitation is the inherent variability and subjectivity of information generated from qualitative research. A similar instrument development effort carried out by different researchers would inevitably yield a somewhat different questionnaire. Eliciting and formatting terminology that reflects symptomatic and functional impact presents a number of challenges. Future research could employ a quantitative importance scale for participants to use in assessing the value of symptoms and functional impacts. Such value scales could be used alongside factor analysis models of item and dimension frequency and severity. These could in turn be compared with external criteria such as physician assessment, tissue counts, and nasal mucus weights, measurements of inflammatory cytokines, and quantitative viral cultures. Because no gold standard exists, single-criterion validity assessment will not be sufficient. Instead, the concept of construct validity will need to be invoked for future attempts at validation. Construct validity has been defined as “validity assessed by comparing the results of several contrasting tests of validity (including concurrent, convergent, and divergent validation studies) with predictions from a theoretical model.”64 Our work so far has only begun to scratch the surface of such rigorous validity assessment.
Conclusions
We have developed an instrument that measures patient-oriented outcomes identified as important by people with self-diagnosed common colds. We expect that the WURSS will do well with physician-diagnosed “bronchitis,” “sinusitis,” or “pharyngitis,” but as yet have no data with which to evaluate that supposition. We hope that the development of the WURSS stimulates other researchers to undergo similar efforts at aimed at patient-oriented outcome measurement and that the efforts can be compared. We have made the WURSS available for general use by placing a printable facsimile online at http://www.fammed.wisc.edu/wurss/. University-based health care researchers and other nonprofit entities may use the WURSS freely, but we do ask to be notified of such use. For-profit entities should contact us before using this copyrighted instrument.
The next step will be for WURSS to undergo large-scale psychometric testing with the goal of assessing its internal and external validity properties more accurately. We welcome comments, consultation, and collaboration and hope to involve other researchers as we move further in the direction of an accurate and reliable method for assessing the impact of the common cold.
Acknowledgments
The authors would like to acknowledge the participants who contributed their time and energy while they were sick. We also thank the many physicians and coworkers who contributed their knowledge and opinions, especially Nora Cate Schaeffer, PhD; Mary Beth Plane, PhD; Jon Temte, MD, PhD; Donn D’Alessio, MD; and William Scheckler, MD, in Madison, Wis., and Jack Gwaltney, MD, in Charlottesville, Va. During most of this project, Dr Barrett has had support from the National Center for Complementary and Alter native Medicine at the National Institutes of Health, Grant #K23 AT00051-01.
1. Dingle JH, Badger GF, Jordan WS. Illness in the home: a study of 25,000 illnesses in a group of Cleveland families. Cleveland, Ohio: Western Reserve University Press; 1964.
2. Gwaltney JM, Hendley JO, Simon G, Jordan WS. Rhinovirus infections in an industrial population. JAMA 1967;202:158-64.
3. Monto AS, Ullman BM. Acute respiratory illness in an American community. JAMA 1974;227:164-9.
4. Smith MBH, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA 1993;269:2258-63.
5. Turner RB. The treatment of rhinovirus infections: progress and potential. Antiviral Res 2001;49:1-14.
6. Kleinman A. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med 1978;88:251-8.
7. Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. Arch Intern Med 1958;101:267-78.
8. Jackson GG, Dowling HF, Anderson TO, Riff L, Saporta J, Turck M. Susceptibility and immunity to common upper respiratory viral infections–the common cold. Ann Intern Med 1960;55:719-38.
9. Jackson GG, Dowling HF, Muldoon RL. Present concepts of the common cold. Am J Public Health 1962;52:940-5.
10. D’Alessio D, Peterson JA, Dick CR, Dick EC. Transmission of experimental rhinovirus colds in volunteer married couples. J Infect Dis 1976;133:28-36.
11. Dick EC, Jennings LC, Mink KA, Wartgrow CD, Inhorn SL. Aerosol transmission of rhinovirus colds. J Infect Dis 1987;156:442-8.
12. Gwaltney JM, Hendley JO. Transmission of experimental rhinovirus infection by contaminated surfaces. Am J Epidemiol 1982;116:828-33.
13. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds. Ann Intern Med 1996;125:89-97.
14. Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol 1998;17:214-23.
15. Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Microbiol Rev 1999;12:9-18.
16. Arruda E, Pitkäranta A, Witek TJ, Doyle CA, Hayden FG. Frequency and history of rhinovirus infections in adults during autumn. J Clin Microbiol 1997;35:2864-8.
17. Gwaltney JM, Buier RM, Rogers JL. The influence of signal variation, bias, noise and effect size on statistical significance in treatment studies of the common cold. Antiviral Res 1996;29:287-95.
18. Rao SS, Hendley JO, Hayden FG, Gwaltney JM. Symptom expression in natural and experimental rhinovirus colds. Am J Rhinol 1995;9:49-52.
19. Freymuth F, Vabret A, Brouard J, et al. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol 1999;13:131-9.
20. Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol 1993;92:722-31.
21. Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol 1998;160:6172-81.
22. Teran LM, Johnston SL, Schroder JM, Church MK, Holgate ST. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus induced asthma. Am J Respir Crit Care Med 1997;155:1362-6.
23. Korppi M, Kröger L. Laitinen. White blood cell and differential counts in acute respiratory viral and bacterial infections in children. Scand J Infect Dis 1993;25:435-40.
24. McBride TP, Doyle WJ, Hayden FG, Gwaltney JM. Alterations of the Eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg 1989;115:1054-9.
25. Gwaltney JM, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med 1994;330:25-30.
26. Turner BW, Cail WS, Hendley JO, et al. Physiologic abnormalities in the paranasal sinuses during experimental rhinovirus colds. J Allergy Clin Immunol 1992;90:474-8.
27. Dressler WE, Myers T, London SJ, Rankell AS, Poetsch CE. A system of rhinomanometry in the clinical evaluation of nasal decongestants. Ann Otol Rhinol Laryngol 1977;86:310-6.
28. Tomkinson A, Eccles R. Comparison of the relative abilities of acoustic rhinometry, rhinomanometry, and the visual analogue scale in detecting change in the nasal cavity in a healthy adult population. Am J Rhinol 1996;10:161-5.
29. Parekh HH, Cragun KT, Hayden FG, Hendley JO, Gwaltney JM. Nasal mucus weights in experimental rhinovirus infection. Am J Rhinol 1992;6:107-10.
30. Scaglione F, Lund B. Efficacy in the treatment of the common cold of a preparation containing an echinacea extract. Int J Immunopharmacol 1995;11:163-6.
31. Juniper EF, Guyatt GH, Griffith LE, Ferrie PJ. Interpretation of rhinoconjuctivitis quality of life questionnaire data. J Allergy Clin Immunol 1996;98:843-5.
32. Juniper EF. Measuring health-related quality of life in rhinitis. J Allergy Clin Immunol 1997;99:S742-9.
33. Kozma CM, Sadik MK, Watrous ML. Economic outcomes for the treatment of allergic rhinitis. PharmacoEconomics 1996;410:4-13.
34. Meltzer EO, Nathan RA, Selner JC, Storms W. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol 1997;99:S807-28.
35. Meltzer EO, Nathan RA, Selner JC, Storms W. Quality of life and rhinitic symptoms: Result of a nationwide survey with the SF-36 and RQLQ questionnaires. J Allergy Clin Immunol 1997;99:815-9.
36. Piccirillo JF, Edwards D, Haiduk A, Yonan C, Thawley SE. Psychometric and clinimetric validity of the 31-item rhinosinusitis outcome measure (RSOM-31). Am J Rhinol 1995;9:297-306.
37. Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Controlled Clin Trials 1991;12(suppl 4):142S-158S.
38. Guyatt GH, Kirshner B, Jaeschke R. Measuring health status: What are the necessary measurement properties? J Clin Epidemiol 1992;45:1341-5.
39. Guyatt GH. Health status, quality of life, and the individual. JAMA 1994;272:630-1.
40. Juniper EF, Guyatt GH, Jaeschke R. How to develop and validate a new health-related quality of life instrument. In Spilker B, ed. Quality of life and pharmacoeconomics in clinical trials. Philadelphia, Pa: Lippincott-Raven; 1996;49-56.
41. Katz JN, Larson MG, Phillips CB. Comparative measurement sensitivity of short and longer health status instruments. Med Care 1992;30:917-25.
42. Schaeffer NC, Charng H-W. Two experiments in simplifying response categories: intensity and frequency categories. Sociol Perspect 1991;34:165-82.
43. Shrout PE, Yager TJ. Reliability and validity of screening scales: Effect of reducing scale length. J Clin Epidemiol 1989;42:69-78.
44. McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1998;31:247-63.
45. Barrett B, Locken L, Maberry R, Brown RL, Bobula JA, D’Alessio D. A randomized double-blind trial of an unrefined mixture of Echinacea purpurea and E angustifolia used to treat the common cold: no benefit detected. Submitted for publication 2001.
46. Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res 1993;2:451-9.
47. Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient’s view of change as a clinical outcome measure. JAMA 1999;282:1157-62.
48. Harris-Kojetin LD, Fowler FJ, Brown JA, Schnaier JA, Sweeny SF. The use of cognitive testing to develop and evaluate CAHPS 1.0 core survey items. Consumer assessment of health plans study. Med Care 1999;37:MS10-21.
49. Mullin PA, Lohr KN, Bresnahan BW, McNulty P. Applying cognitive design principles to formatting HRQOL instruments. Qual Life Res 2000;9:13-27.
50. Schaeffer NC. Conversation with a purpose or conversation? Interaction in the standardized interview. In: Biemer P, Groves RM, Lyberg LE, Mathiowitz NA, Sudman S, eds. Measurement errors in surveys. New York, NY: Wiley; 1991;367-91.
51. Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3.
52. Crabtree BF, Miller WL. Qualitative approach to primary care research: the long interview. Fam Med 1991;23:145-51.
53. McCracken G. The long interview. Thousand Oaks, Calif: Sage Publications; 1988.
54. Hughes D, DuMont K. Using focus groups to facilitate culturally anchored research. Am J Community Psychol 1993;21:775-807.
55. Kitzinger J. Qualitative research: Introducing focus groups. BMJ 1995;311:299-302.
56. Morgan D. Focus groups and qualitative research. Thousand Oaks, Calif: Sage Publications; 1988.
57. Bolan KA. Structural equations with latent variables. New York, NY: John Wiley & Sons; 1989.
58. Joreskog KG, Sorbom D. LISREL 8 User’s Reference Guide. Chicago, Ill: Scientific Software International, Inc; 1993.
59. Dillon WR, Goldstein M. Multivariate analysis: methods and applications. New York, NY: John Wiley & Sons; 1984.
60. Davis CS. Semi-parametric and non-parametric methods for the analysis of repeated measurements with applications to clinical trials. Stat Med 1991;1:1959-80.
61. Goldstein H. Multilevel statistical models. New York: Halsted, 1995.
62. Hays RD, Hadom D. Responsiveness to change: an aspect of validity, not a separate dimension. Qual Life Res 1992;1:73-5.
63. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15.
64. McDowell I, Newell C. Measuring health: a guide to rating scales and questionnaires. Oxford, England: Oxford University Press; 1996.
65. Deyo RA, Centor RM. Assessing the responsiveness of functional scales to clinical change: an analogy to diagnostic test performance. J Chronic Dis 1986;39:897-906.
66. Guyatt GH, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 1987;40:171-8.
67. Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res 1993;2:441-9.
68. Wright JG, Feinstein AR. A comparative contrast of clinimetric and psychometric methods for constructing indexes and rating scales. J Clin Epidemiol 1992;45:1201-18.
OBJECTIVE: To develop a sensitive, reliable, responsive, and easy-to-use instrument for assessing the severity and functional impact of the common cold.
STUDY DESIGN: We created an illness-specific health-related quality-of-life outcomes instrument using previous scales, expert opinion, and common knowledge. This original questionnaire was used in a 1999 randomized trial of echinacea for the common cold. In 2000 we employed cognitive interview and focus group qualitative methods to further develop the instrument. Semistructured interviews used open-ended questions to elicit symptoms, terminology, and perceived functional impact. Responses were used to improve the instrument.
POPULATION: The randomized trial watched 142 University of Wisconsin students for a total of 953 days of illness. The subsequent qualitative instrument development project recruited 74 adults with self-diagnosed colds for 56 in-person interviews and 3 focus groups.
OUTCOMES MEASURED: We measured specific symptoms, symptom clusters (dimensions), functional impact, and global severity.
RESULTS: The original questionnaire included 20 questions: a global severity indicator, 15 symptom-severity items using 9-point severity scales, and 4 yes/no functional assessments. Data from the trial provided evidence of 4 underlying dimensions: nasal, throat, cough, and fever and aches, with reliability coefficients of 0.663, 0.668, 0.794, and 0.753, respectively. Qualitative assessments from the interviews and focus groups led us to expand from 15 to 32 symptom-specific items and from 4 to 10 functional impairment items. The original 9-point severity scale was revised to 7 points. Two global severity questions bring the item count to 44. The instrument fits comfortably on the front and back of a single sheet of paper and takes 5 to 10 minutes to complete.
CONCLUSIONS: The Wisconsin Upper Respiratory Symptom Survey (WURSS) is now ready for formal validity testing or practical use in common cold research.
The common cold, usually caused by viral infection of the upper respiratory tract, is a very prevalent illness. On average, US adults suffer from 1 to 4 episodes per year.1-3 This high incidence, along with significant symptomatic and functional impairment, combine to make this syndrome an important health problem. Hundreds of trials have attempted to demonstrate effective treatments.4,5 Unfortunately, few efforts have been made to develop and validate instruments to measure the symptomatic and functional impact of the common cold.
The term “upper respiratory infection” (URI) is a nosologic category constructed by physicians and other health professionals to reflect an upper airway, mucus-producing, inflammatory reaction to infection, usually viral. It is a disease category. The terms rhinitis, rhinosinusitis, pharyngitis, and bronchitis are often used to indicate the anatomic area most affected. The term “common cold” is an illness term constructed and used by the general populace. This distinction between professional (disease) and popular (illness) conceptions6 provides the reasoning for participant-based, patient-oriented qualitative development of measurement tools. While many medical professionals may choose to measure URI disease by physical examination, viral culture, or laboratory analysis of blood or nasal discharge, we believe that most people are more interested in how they can reduce the severity and duration of their symptoms and the functional impairments that result from their illness.
George Gee Jackson and colleagues7 began experimental work in the 1950s, observing and recording the cold symptoms produced by challenging more than 1,000 volunteers with filtered nasal secretions obtained from cold-sufferers. Eight symptoms–sneezing, headache, malaise, chilliness, nasal discharge, nasal obstruction, sore throat, and cough–were selected for evaluation and graded as absent (0), mild (1), moderate (2), or severe (3) every day for 6 days after inoculation. A score of 14 or higher was chosen as the cutoff value that best distinguished infected from noninfected participants. Thus, the original Jackson scale was apparently designed to discriminate between those with and without demonstrable viral infection, and not as a measure of severity. The tables and graphics in Jackson’s seminal works point toward reasonable internal consistency and discriminate validity.7-9 However, other important measurement properties, such as precision, reliability, responsiveness, and stability, were not reported. Despite these limitations, Jackson’s scale has been used for decades by most of the major common cold research groups.10-15
Using various modifications of the Jackson scale, researchers of the cold have characterized the frequency and severity of the 8 symptoms noted above in both natural colds and experimentally induced rhinovirus infections. Variability in symptom expression remains a hallmark of URI. Although specific pathogens are associated with the severity and distribution of symptoms at the population level, symptoms are poor predictors of etiology at the individual level. Infection itself is an imperfect predictor of symptom expression, as asymptomatic infections occur frequently, and as URI-like symptoms occur in people in whom it is not possible to demonstrate infections.16 Even among people with documented experimental infections of single strains of virus, variance outweighs central tendency in all symptom measurements.17,18
The search for objective disease measures with which to compare symptom scores has also progressed. To date, the following measures have been evaluated: detection of virus with culture or polymerase chain reaction,16,19 cytokine measurement,20-22 serologic markers,23 physical examination,9,24 radiologic imaging,25,26 rhinomanometry,27,28 mucus weight,29 mucus velocity, and number of tissues used.30 None have been shown to be superior to self-reported symptoms in terms of precision, reliability, or responsiveness or in their ability to predict functional impairment or subsequent illness. Perhaps more important, none have been shown to reflect the values of the people who experience colds. Although a number of quality-of-life instruments have been developed to assess allergic rhinitis,31-36 we have been unable to locate any specifically developed to assess URI.
We therefore decided to develop the Wisconsin Upper Respiratory Symptom Survey (WURSS) to provide a standardized measure for evaluating the negative consequences of the common cold. We were particularly interested in developing a health-related quality of life instrument that would represent the symptomatic and functional dimensions that are important to cold-sufferers.37-40 The instrument should be able to discriminate accurately between active intervention and placebo effects in randomized therapeutic trials and should balance brevity and ease of use with optimal precision, reliability, and responsiveness.41-43 It should be based on self-diagnosis and self-assessment because neither accepted criteria nor adequate tests are available to diagnose “upper respiratory infection” or “acute infectious rhinosinusitis”(with or without “pharyngitis”) and because the vast majority of cold treatments will be taken without professional input after self-diagnosis.
Methods
Phase 1: Initial development during a randomized trial
The development of this study began in 1998 during the design of a randomized controlled trial (RCT) of echinacea as a cold treatment. We created our first instrument by showing successive drafts to friends and colleagues (mostly family physicians), stopping once we were satisfied that the questionnaire had reasonable face validity. This initial instrument rated global severity of illness (“How sick do you feel today?”) and 15 individual symptoms on a 9-point Likert-type scale. The 15 symptom-measuring items were complemented by 4 dichotomous (yes/no) functional outcome questions, adapted with permission from the validated Medical Outcomes Study 36-item Short-Form Health Survey (SF-36).44
This initial instrument was used in the spring of 1999 in the echinacea RCT.45 This experience provided a good initial test of our instrument, as the participants were recruited within 36 hours of their first symptom and monitored each day until they had answered “No” to the question, “Do you think that you are still sick today?” for 2 days in a row. Each participant was asked to fill out the questionnaire both on paper and on a computerized data-collecting facsimile (available at http://www.fammed.wisc.edu/samplecold).
Phase 2: Further instrument development using qualitative methods
After the echinacea RCT was completed, our primary concern was that we might be overlooking or under-representing important illness domains. We also suspected that wording, question order, response range, and other formatting concerns could be improved. To achieve these goals, we used qualitative instrument-development methodologies, involving the people we wanted to measure–cold-sufferers–in the development process.40,46-50
After obtaining approval from the University of Wisconsin Medical School Human Subjects Committee, we began interviewing Madison-area adults who responded to community advertising asking for volunteers with colds. Inclusion criteria required answering “Yes” to the question, “Do you believe that you have a cold?” For an interview to be arranged, at least one cold symptom had to be present, and the research assistant had to be convinced that the caller was indeed suffering from a common cold. Prospective participants with itchy eyes, sneezing, or a history of allergy were excluded if either the participant or the interviewer felt that any current symptoms might have been caused by allergy. Interviews were held in a location of mutual convenience and with the aid of an interview guide developed by our research team. Interviewers were carefully trained in the research protocol and used interview guides for both the initial telephone screen and the in-person interviews. Interviewers included both clinicians and nonclinicians.
The semistructured interview guide used open-ended questions designed to elicit the participants’ own terminology for describing their colds (Table 1).51-53 We aimed for an understanding of how the experience of the cold influenced the lives of the participants. Participants were first asked to list all their symptoms, then to describe how each symptom bothered them. Next, we asked which symptom(s) appeared first and which one(s) followed. We then asked which symptoms were most bothersome and why. Participants were asked to describe what they did to relieve their cold symptoms, why, and whether the therapy provided any relief. Participants were then asked about how their cold affected their lifestyle with regard to work, relationships, activities, and so forth. Additionally, we asked about symptoms and effects of previous colds. This exploratory phase of the interview lasted approximately 20 to 30 minutes.
Once the interviewer had a thorough description and understanding of the participant’s cold, the participant was asked to complete the questionnaire-in-development. After marking answers on the questionnaire (which took 3 to 5 minutes), each participant was asked to comment on its ease of use, item wording, formatting, and response range as well as whether it accurately and comprehensively measured the symptoms and functional impact they were experiencing. The instrument development phase of the interview lasted for another 20 to 30 minutes.
We used focus group methods in the final month of the study as an additional window into participants’ experiences.54-56 The focus groups used the same inclusion criteria as the long interviews and followed the same general format, first using open-ended questions to elicit symptoms and their impact, then administrating the questionnaire and discussing item inclusion and formatting. However, we encouraged discussion rather than self-assessment, as the focus group methodology derives its strength from the interactive nature of conversation. For instance, a statement made by one participant would spark interest or recall in another, thereby generating a richer, fuller, and more representative description of symptoms and functional impact.
Individual interviews were held by 1 of 5 trained interviewers (B.B., L.L., R.M., E.S., J.S.). All 3 focus groups were run by the lead author, with at least 1 other research team member assisting. Interviews and focus groups were arranged as soon as possible after the initial telephone contact so that participants would still have cold symptoms while being interviewed. All interviews and focus groups were discussed in biweekly group meetings. Decisions on item inclusion, wording, and questionnaire format were made by research group consensus. Several versions of the questionnaire were brought back to cold-sufferers for further cognitive testing. The diversity of interviewers and respondents provided protection against personal bias in ascertaining and interpreting symptoms and impairments.
TABLE 1
QUESTIONS ASKED DURING INTERVIEW
| Current Symptom History and Evaluation |
|---|
| List and describe all symptoms you have with this cold. |
| How do these symptoms bother you? |
| What is the first symptom you noticed when getting this cold? The Next? |
| The next? |
| Which cold symptom bothers you the most? How and why? |
| Are there other symptoms that bother you? How and why? |
| Interventions |
| What do you do to relieve cold symptoms? Why? |
| What over-the-counter medicines would you use? Why? Did it help? |
| What herbal medicines would you use? Why? Did it help? |
| Do you do anything else to relieve symptoms or treat your cold? Why? |
| Did it help? |
| When would you see a doctor or other health care provider? Why? |
| Lifestyle |
| Has this cold interfered with your normal activities? How? |
| When does a cold keep you from doing what you want or need to do? How? |
| Describe what things are harder to do? |
| Previous Symptom History and Evaluation |
| How many colds did you have this past year? |
| How long did they usually last? |
| List and describe what symptoms you usually get with your colds? |
| How do these symptoms bother you? |
| Survey Evaluation (After Participant Has Completed the Questionnaire) |
| Is this form easy to read? |
| Are there any other symptoms that should be on this questionnaire? |
| Are there any questions that shouldn’t be there? |
| Are there any questions that could be worded better? |
| Is the 7-point scale appropriate? Why or why not? |
Results
Phase 1
Of the 148 college students enrolled, 142 followed protocol and were included in the analysis. Of the 853 person-days documented, 546 (64%) were covered by both data systems; 287 (33.6%) came from paper surveys only; and 18 (2.1%) were filled out via computer only. Because only 2 (0.2%) questionnaires were missing any data, our data capture rate was 99.8%. Comparing data from the computerized and paper data sources provided evidence of consistency. Of the 546 days in which both paper and computer instruments provided data, 512 yielded identical responses (94% concordant) to the global severity of illness question. Of the 34 (6%) discrepancies, 29 were off by 1 point on the 9-point Likert-type scale and 5 discrepancies were off by 2 points. Comparing computer and paper responses with the 15 specific symptom questions also yielded high levels of concordance. Of 8190 item responses, 7777 (95%) were concordant, while 413 (5%) were classified as data discrepancies. Of these, 293 were off by 1 point on the 9-point scale; 68 were off by 2 points; 27, by 3 points; 17, by 4 points; 7, by 5 points; and 1 by 6 points.
Factor analysis of the data provided further evidence of internal validity. Structural equation modeling techniques57,58 were used to model symptom severities over time. A 4-dimensional symptom-recovery model (df = 71; P = .000025) provided a goodness of fit index of 0.88, a root mean square residual of .095, and a chi-squared/df ratio of 139/71 = 1.95. From the pool of 15 scaled symptom scores, 14 items contributed significantly to the model. (In this data set, loss of appetite was an infrequent symptom contributing insignificantly toward the model, and was dropped.) The 14 symptoms naturally aggregated into 4 underlying symptomatic dimensions: cough, throat, nasal, and fever and aches. Table 2 provides the reliability coefficients, standardized item loading coefficients, and standard errors of these loadings for the 4 dimensions. The reliability coefficients of the symptom dimensions were calculated using a procedure proposed by Dillon and Goldstein.59 Scale recovery curves, generated using a mixed modeling approach,60,61 were internally predictive, responsive,37,62 and consistent with what is known about the natural history of URI.
TABLE 2
RELIABILITY OF SYMPTOM DIMENSION MODELS
| Item Loading (SE)* | |
|---|---|
| Cough Dimension (Reliability = 0.794) | |
| Coughing | 2.01 (0.20) |
| Coughing stuff up | 1.75 (0.18) |
| Cough interfering with sleep | 1.16 (0.17) |
| Fever and Aches Dimension (Reliability = 0.753) | |
| Headache | 1.28 (0.23) |
| Fever | 1.07 (0.13) |
| Sweats | 1.25 (0.16) |
| Muscle aches | 1.76 (0.19) |
| Feeling run down | 1.17 (0.19) |
| Throat Dimension (Reliability = 0.668) | |
| Sore throat | 1.10 (0.22) |
| Scratchy throat | 1.73 (0.23) |
| Hoarseness | 1.68 (0.24) |
| Nasal Dimension (Reliability = 0.663) | |
| Runny nose | 1.93 (0.28) |
| Stuffy nose | 1.05 (0.23) |
| Sneezing | 1.63 (0.26) |
| *All significant at P < .05. | |
| SE denotes standard error. | |
Phase 2
Between July and December 2000, 108 persons from the general population responded to advertising by calling a telephone number listed on posted flyers and in the newspaper. Of these 108 callers, 27 were eligible but declined to participate; 7 did not meet inclusion criteria (were younger than 18 years of age, had current allergy symptoms, or did not have cold symptoms); and 74 met study criteria and elected to participate (Table 3). Those declining to participate usually did so because of inconvenience in arranging an immediate interview or because compensation ($10 for interview, $15 for focus group) was insufficient. Participants were met in person for semistructured individual interviews (n = 56) or focus groups (3 groups, 20 individuals total). Two people were interviewed both individually and in focus group.
Based on the information gained during interviews, the instrument-in-development underwent 6 revisions during 2000. Each modification was tested with additional interviews. A final version was created in December 2000. A few items from the initial instrument used in the echinacea trial were modified in response to participants’ descriptions and insights. Several other items were added to reflect symptoms and functional impairments described by participants in response to our open-ended questions. All items used wording provided by participants or tested during subsequent interviews and focus groups.
All symptoms spontaneously reported by at least 3 participants in either individual interview or focus group were included in the final version (Table 4). Figure 1 provides a frequency distribution of the symptoms described during the individual interviews.
On the basis of our participants’ comments, the distribution of severity data from the echinacea trial, and recommendations from published studies, we decided to decrease the response range from a 9-point to a 7-point Likert-type severity scale. The resulting severity range was marked at 1 (very mild), 3 (mild), 5 (moderate), and 7 (severe), following the majority opinion of our respondents. Unmarked (even-numbered) options were included, as most of the respondents felt they should have “in-between” choices. The functional outcome questions adapted from the SF-36 were replaced with participant-generated items, which were then scaled in a 7-point format similar to that used for the symptoms. A final question comparing today’s global severity with yesterday’s was added to provide a comparative measure of change over time (responsiveness). Figure 2 displays the final format of WURSS items.
The qualitative data provided by our informants improved our understanding of the symptomatic and functional impact of the common cold and assisted the development of the WURSS questionnaire. Although it is clear that people experience colds in different ways, several common threads emerged. For instance, we found that our original instrument (like the Jackson criteria) had overrated individual symptoms and had underrated functional impact, interference with social relationships, and general malaise. Informants often told us that it was not necessarily the individual symptoms that bothered them, but the general feelings, described as “sick feeling,” “loss of energy,” “run down,” “tired,” “fatigue,” “malaise,” “lousy,” “lazy,” “spacey," "blah," "yucky," "foggy," "lightheaded," "fuzzy brain,” “cloudy,” “disoriented,” “uncomfortable,” “distracted,” and “miserable.” Our informants also told us that they were bothered by the way their cold interfered with day-to-day activities and relationships. Colds affected physical activities such as breathing or walking, performance at work or in the home, and interactions with friends, family, and coworkers. Terms describing the most frequent and bothersome effects were incorporated into the final WURSS instrument.
TABLE 3
INTERVIEW AND FOCUS GROUP PARTICIPANT DEMOGRAPHICS
| Number | |
|---|---|
| Method of Data Collection | |
| Individual interviews | 56 |
| Focus groups | 20 |
| Total | 74* |
| Sex | |
| Women | 49 |
| Men | 25 |
| Ethnicity | |
| Native American | 2 |
| Black | 12 |
| Hispanic | 2 |
| White | 57 |
| No response | 1 |
| Annual Income | |
| < $10,000 | 28 |
| $10,000–19,999 | 17 |
| $20,000–29,999 | 13 |
| $30,000–49,999 | 9 |
| $50,000–75,000 | 3 |
| $75,000 | 2 |
| No response | 2 |
| Education | |
| Some high school | 13 |
| High school or equivalent | 11 |
| Some college | 11 |
| Associate or technical degree | 6 |
| Bachelor’s degree | 21 |
| Master’s degree | 6 |
| Professional degree | 4 |
| No response | 2 |
| Tobacco Use | |
| Current | 26 |
| Past | 19 |
| Never smoker | 28 |
| No response | 1 |
| *Two participants were used in both data collection methods. | |
| NOTE: Age range was 19 to 71 years, mean = 35.9 years (standard deviation, 11.9). | |
TABLE 4
SYMPTOMS AND FUNCTIONAL IMPAIRMENTS EVALUATED BY THE WISCONSIN UPPER RESPIRATORY SYMPTOM SURVEY
| Symptoms | Plugged ears |
| Cough | Ear discomfort |
| “Coughing stuff up” | Watery eyes |
| Cough interfering with sleep | Eye discomfort |
| Sore throat | Head congestion |
| Scratchy throat | Chest congestion |
| Hoarseness | Chest tightness |
| Runny nose | Heaviness in chest |
| Plugged nose | Lack of energy |
| Sneezing | Loss of appetite |
| Headache | |
| Body aches | Functional Impairments |
| Feeling “run down” | Think clearly |
| Sweats | Speak clearly |
| Chills | Sleep well |
| Feeling feverish | Breathe easily |
| Feeling dizzy | Walk, climb stairs, exercise |
| Feeling tired | Accomplish daily activities |
| Irritability | Work outside the home |
| Sinus pain | Work inside the home |
| Sinus pressure | Interact with others |
| Sinus drainage | Live your personal life |
| Swollen glands |
FIGURE 1
SYMPTOMS REPORTED IN INDIVIDUAL INTERVIEWS
FIGURE 2
ITEM FORMAT FOR THE WISCONSIN UPPER RESPIRATORY SYMPTOM SURVEY
Discussion
Researchers of URIs and the common cold need a well-developed, standardized, validated outcomes instrument that reflects the experience and values of cold sufferers. While the Jackson scale and various modifications have been widely used, few data support the validity of these scales. Although correlations with external measures, such as physical examinations, mucus weight, and the ability to culture virus have been reported, the symptomatic and functional impact of colds has largely been neglected. Perhaps more important, the symptomatic measures used to date were apparently developed without significant input from the people whose illnesses were measured. Questionnaire development and cognitive testing methods have not been described, nor have adequate tests of psychometric properties been reported. Although the Jackson scale may demonstrate marginal face validity in terms of symptoms, it does not do so in terms of functional impact.
This article describes the first steps taken in the development and validation of a new illness-specific quality-of-life instrument for measuring the common cold. The WURSS instrument is more comprehensive than existing alternatives and better reflects cold-sufferers’ experiences and values. Therefore, it provides greater face validity. The length (44 items) reflects a compromise between ease of use and comprehensiveness.41 It is possible that a subset of the items will prove nearly as effective and that a short-form WURSS will eventually be available. Item reduction will need to be guided by both internal (factor analysis) and external (frequency and perceived value) considerations. The standardized 7-point severity scale used throughout the WURSS makes the instrument very easy to use.49 It also provides a severity range that our informants and previous researchers40,63,64 agree is optimal. The WURSS allows a cold-sufferer to swiftly and accurately assess his or her common cold. We hope that WURSS will prove worthy in terms of standard psychometric properties such as precision, reliability, and responsiveness.65-68 A large prospective study will be necessary for those assessments.
Limitations
The work described here has a number of limitations. The WURSS was developed in Madison, Wis., largely among people with self-diagnosed colds during the period from July to December 2000. The RCT occurred over several months in the spring of 1999 and was limited to college students. Our results are therefore limited by both population and etiologic agent, which in turn may influence the symptom and severity spectrums assessed. Although we aimed for and achieved a moderate degree of socioeconomic diversity (Table 3), our participants’ responses may not be representative of the larger universe of cold-sufferers. The symptom distribution in Figure 1, for example, is unlikely to represent global cold symptom frequency accurately. Previous research with both natural and induced colds suggests that nasal symptoms and sore throat are usually more frequent than cough.2,3,6-18 The comprehensiveness of the instrument is more important for instrument development than are the specific item frequencies. Here, we feel that we succeeded in representing a sufficient range of items.
Another important limitation is the inherent variability and subjectivity of information generated from qualitative research. A similar instrument development effort carried out by different researchers would inevitably yield a somewhat different questionnaire. Eliciting and formatting terminology that reflects symptomatic and functional impact presents a number of challenges. Future research could employ a quantitative importance scale for participants to use in assessing the value of symptoms and functional impacts. Such value scales could be used alongside factor analysis models of item and dimension frequency and severity. These could in turn be compared with external criteria such as physician assessment, tissue counts, and nasal mucus weights, measurements of inflammatory cytokines, and quantitative viral cultures. Because no gold standard exists, single-criterion validity assessment will not be sufficient. Instead, the concept of construct validity will need to be invoked for future attempts at validation. Construct validity has been defined as “validity assessed by comparing the results of several contrasting tests of validity (including concurrent, convergent, and divergent validation studies) with predictions from a theoretical model.”64 Our work so far has only begun to scratch the surface of such rigorous validity assessment.
Conclusions
We have developed an instrument that measures patient-oriented outcomes identified as important by people with self-diagnosed common colds. We expect that the WURSS will do well with physician-diagnosed “bronchitis,” “sinusitis,” or “pharyngitis,” but as yet have no data with which to evaluate that supposition. We hope that the development of the WURSS stimulates other researchers to undergo similar efforts at aimed at patient-oriented outcome measurement and that the efforts can be compared. We have made the WURSS available for general use by placing a printable facsimile online at http://www.fammed.wisc.edu/wurss/. University-based health care researchers and other nonprofit entities may use the WURSS freely, but we do ask to be notified of such use. For-profit entities should contact us before using this copyrighted instrument.
The next step will be for WURSS to undergo large-scale psychometric testing with the goal of assessing its internal and external validity properties more accurately. We welcome comments, consultation, and collaboration and hope to involve other researchers as we move further in the direction of an accurate and reliable method for assessing the impact of the common cold.
Acknowledgments
The authors would like to acknowledge the participants who contributed their time and energy while they were sick. We also thank the many physicians and coworkers who contributed their knowledge and opinions, especially Nora Cate Schaeffer, PhD; Mary Beth Plane, PhD; Jon Temte, MD, PhD; Donn D’Alessio, MD; and William Scheckler, MD, in Madison, Wis., and Jack Gwaltney, MD, in Charlottesville, Va. During most of this project, Dr Barrett has had support from the National Center for Complementary and Alter native Medicine at the National Institutes of Health, Grant #K23 AT00051-01.
OBJECTIVE: To develop a sensitive, reliable, responsive, and easy-to-use instrument for assessing the severity and functional impact of the common cold.
STUDY DESIGN: We created an illness-specific health-related quality-of-life outcomes instrument using previous scales, expert opinion, and common knowledge. This original questionnaire was used in a 1999 randomized trial of echinacea for the common cold. In 2000 we employed cognitive interview and focus group qualitative methods to further develop the instrument. Semistructured interviews used open-ended questions to elicit symptoms, terminology, and perceived functional impact. Responses were used to improve the instrument.
POPULATION: The randomized trial watched 142 University of Wisconsin students for a total of 953 days of illness. The subsequent qualitative instrument development project recruited 74 adults with self-diagnosed colds for 56 in-person interviews and 3 focus groups.
OUTCOMES MEASURED: We measured specific symptoms, symptom clusters (dimensions), functional impact, and global severity.
RESULTS: The original questionnaire included 20 questions: a global severity indicator, 15 symptom-severity items using 9-point severity scales, and 4 yes/no functional assessments. Data from the trial provided evidence of 4 underlying dimensions: nasal, throat, cough, and fever and aches, with reliability coefficients of 0.663, 0.668, 0.794, and 0.753, respectively. Qualitative assessments from the interviews and focus groups led us to expand from 15 to 32 symptom-specific items and from 4 to 10 functional impairment items. The original 9-point severity scale was revised to 7 points. Two global severity questions bring the item count to 44. The instrument fits comfortably on the front and back of a single sheet of paper and takes 5 to 10 minutes to complete.
CONCLUSIONS: The Wisconsin Upper Respiratory Symptom Survey (WURSS) is now ready for formal validity testing or practical use in common cold research.
The common cold, usually caused by viral infection of the upper respiratory tract, is a very prevalent illness. On average, US adults suffer from 1 to 4 episodes per year.1-3 This high incidence, along with significant symptomatic and functional impairment, combine to make this syndrome an important health problem. Hundreds of trials have attempted to demonstrate effective treatments.4,5 Unfortunately, few efforts have been made to develop and validate instruments to measure the symptomatic and functional impact of the common cold.
The term “upper respiratory infection” (URI) is a nosologic category constructed by physicians and other health professionals to reflect an upper airway, mucus-producing, inflammatory reaction to infection, usually viral. It is a disease category. The terms rhinitis, rhinosinusitis, pharyngitis, and bronchitis are often used to indicate the anatomic area most affected. The term “common cold” is an illness term constructed and used by the general populace. This distinction between professional (disease) and popular (illness) conceptions6 provides the reasoning for participant-based, patient-oriented qualitative development of measurement tools. While many medical professionals may choose to measure URI disease by physical examination, viral culture, or laboratory analysis of blood or nasal discharge, we believe that most people are more interested in how they can reduce the severity and duration of their symptoms and the functional impairments that result from their illness.
George Gee Jackson and colleagues7 began experimental work in the 1950s, observing and recording the cold symptoms produced by challenging more than 1,000 volunteers with filtered nasal secretions obtained from cold-sufferers. Eight symptoms–sneezing, headache, malaise, chilliness, nasal discharge, nasal obstruction, sore throat, and cough–were selected for evaluation and graded as absent (0), mild (1), moderate (2), or severe (3) every day for 6 days after inoculation. A score of 14 or higher was chosen as the cutoff value that best distinguished infected from noninfected participants. Thus, the original Jackson scale was apparently designed to discriminate between those with and without demonstrable viral infection, and not as a measure of severity. The tables and graphics in Jackson’s seminal works point toward reasonable internal consistency and discriminate validity.7-9 However, other important measurement properties, such as precision, reliability, responsiveness, and stability, were not reported. Despite these limitations, Jackson’s scale has been used for decades by most of the major common cold research groups.10-15
Using various modifications of the Jackson scale, researchers of the cold have characterized the frequency and severity of the 8 symptoms noted above in both natural colds and experimentally induced rhinovirus infections. Variability in symptom expression remains a hallmark of URI. Although specific pathogens are associated with the severity and distribution of symptoms at the population level, symptoms are poor predictors of etiology at the individual level. Infection itself is an imperfect predictor of symptom expression, as asymptomatic infections occur frequently, and as URI-like symptoms occur in people in whom it is not possible to demonstrate infections.16 Even among people with documented experimental infections of single strains of virus, variance outweighs central tendency in all symptom measurements.17,18
The search for objective disease measures with which to compare symptom scores has also progressed. To date, the following measures have been evaluated: detection of virus with culture or polymerase chain reaction,16,19 cytokine measurement,20-22 serologic markers,23 physical examination,9,24 radiologic imaging,25,26 rhinomanometry,27,28 mucus weight,29 mucus velocity, and number of tissues used.30 None have been shown to be superior to self-reported symptoms in terms of precision, reliability, or responsiveness or in their ability to predict functional impairment or subsequent illness. Perhaps more important, none have been shown to reflect the values of the people who experience colds. Although a number of quality-of-life instruments have been developed to assess allergic rhinitis,31-36 we have been unable to locate any specifically developed to assess URI.
We therefore decided to develop the Wisconsin Upper Respiratory Symptom Survey (WURSS) to provide a standardized measure for evaluating the negative consequences of the common cold. We were particularly interested in developing a health-related quality of life instrument that would represent the symptomatic and functional dimensions that are important to cold-sufferers.37-40 The instrument should be able to discriminate accurately between active intervention and placebo effects in randomized therapeutic trials and should balance brevity and ease of use with optimal precision, reliability, and responsiveness.41-43 It should be based on self-diagnosis and self-assessment because neither accepted criteria nor adequate tests are available to diagnose “upper respiratory infection” or “acute infectious rhinosinusitis”(with or without “pharyngitis”) and because the vast majority of cold treatments will be taken without professional input after self-diagnosis.
Methods
Phase 1: Initial development during a randomized trial
The development of this study began in 1998 during the design of a randomized controlled trial (RCT) of echinacea as a cold treatment. We created our first instrument by showing successive drafts to friends and colleagues (mostly family physicians), stopping once we were satisfied that the questionnaire had reasonable face validity. This initial instrument rated global severity of illness (“How sick do you feel today?”) and 15 individual symptoms on a 9-point Likert-type scale. The 15 symptom-measuring items were complemented by 4 dichotomous (yes/no) functional outcome questions, adapted with permission from the validated Medical Outcomes Study 36-item Short-Form Health Survey (SF-36).44
This initial instrument was used in the spring of 1999 in the echinacea RCT.45 This experience provided a good initial test of our instrument, as the participants were recruited within 36 hours of their first symptom and monitored each day until they had answered “No” to the question, “Do you think that you are still sick today?” for 2 days in a row. Each participant was asked to fill out the questionnaire both on paper and on a computerized data-collecting facsimile (available at http://www.fammed.wisc.edu/samplecold).
Phase 2: Further instrument development using qualitative methods
After the echinacea RCT was completed, our primary concern was that we might be overlooking or under-representing important illness domains. We also suspected that wording, question order, response range, and other formatting concerns could be improved. To achieve these goals, we used qualitative instrument-development methodologies, involving the people we wanted to measure–cold-sufferers–in the development process.40,46-50
After obtaining approval from the University of Wisconsin Medical School Human Subjects Committee, we began interviewing Madison-area adults who responded to community advertising asking for volunteers with colds. Inclusion criteria required answering “Yes” to the question, “Do you believe that you have a cold?” For an interview to be arranged, at least one cold symptom had to be present, and the research assistant had to be convinced that the caller was indeed suffering from a common cold. Prospective participants with itchy eyes, sneezing, or a history of allergy were excluded if either the participant or the interviewer felt that any current symptoms might have been caused by allergy. Interviews were held in a location of mutual convenience and with the aid of an interview guide developed by our research team. Interviewers were carefully trained in the research protocol and used interview guides for both the initial telephone screen and the in-person interviews. Interviewers included both clinicians and nonclinicians.
The semistructured interview guide used open-ended questions designed to elicit the participants’ own terminology for describing their colds (Table 1).51-53 We aimed for an understanding of how the experience of the cold influenced the lives of the participants. Participants were first asked to list all their symptoms, then to describe how each symptom bothered them. Next, we asked which symptom(s) appeared first and which one(s) followed. We then asked which symptoms were most bothersome and why. Participants were asked to describe what they did to relieve their cold symptoms, why, and whether the therapy provided any relief. Participants were then asked about how their cold affected their lifestyle with regard to work, relationships, activities, and so forth. Additionally, we asked about symptoms and effects of previous colds. This exploratory phase of the interview lasted approximately 20 to 30 minutes.
Once the interviewer had a thorough description and understanding of the participant’s cold, the participant was asked to complete the questionnaire-in-development. After marking answers on the questionnaire (which took 3 to 5 minutes), each participant was asked to comment on its ease of use, item wording, formatting, and response range as well as whether it accurately and comprehensively measured the symptoms and functional impact they were experiencing. The instrument development phase of the interview lasted for another 20 to 30 minutes.
We used focus group methods in the final month of the study as an additional window into participants’ experiences.54-56 The focus groups used the same inclusion criteria as the long interviews and followed the same general format, first using open-ended questions to elicit symptoms and their impact, then administrating the questionnaire and discussing item inclusion and formatting. However, we encouraged discussion rather than self-assessment, as the focus group methodology derives its strength from the interactive nature of conversation. For instance, a statement made by one participant would spark interest or recall in another, thereby generating a richer, fuller, and more representative description of symptoms and functional impact.
Individual interviews were held by 1 of 5 trained interviewers (B.B., L.L., R.M., E.S., J.S.). All 3 focus groups were run by the lead author, with at least 1 other research team member assisting. Interviews and focus groups were arranged as soon as possible after the initial telephone contact so that participants would still have cold symptoms while being interviewed. All interviews and focus groups were discussed in biweekly group meetings. Decisions on item inclusion, wording, and questionnaire format were made by research group consensus. Several versions of the questionnaire were brought back to cold-sufferers for further cognitive testing. The diversity of interviewers and respondents provided protection against personal bias in ascertaining and interpreting symptoms and impairments.
TABLE 1
QUESTIONS ASKED DURING INTERVIEW
| Current Symptom History and Evaluation |
|---|
| List and describe all symptoms you have with this cold. |
| How do these symptoms bother you? |
| What is the first symptom you noticed when getting this cold? The Next? |
| The next? |
| Which cold symptom bothers you the most? How and why? |
| Are there other symptoms that bother you? How and why? |
| Interventions |
| What do you do to relieve cold symptoms? Why? |
| What over-the-counter medicines would you use? Why? Did it help? |
| What herbal medicines would you use? Why? Did it help? |
| Do you do anything else to relieve symptoms or treat your cold? Why? |
| Did it help? |
| When would you see a doctor or other health care provider? Why? |
| Lifestyle |
| Has this cold interfered with your normal activities? How? |
| When does a cold keep you from doing what you want or need to do? How? |
| Describe what things are harder to do? |
| Previous Symptom History and Evaluation |
| How many colds did you have this past year? |
| How long did they usually last? |
| List and describe what symptoms you usually get with your colds? |
| How do these symptoms bother you? |
| Survey Evaluation (After Participant Has Completed the Questionnaire) |
| Is this form easy to read? |
| Are there any other symptoms that should be on this questionnaire? |
| Are there any questions that shouldn’t be there? |
| Are there any questions that could be worded better? |
| Is the 7-point scale appropriate? Why or why not? |
Results
Phase 1
Of the 148 college students enrolled, 142 followed protocol and were included in the analysis. Of the 853 person-days documented, 546 (64%) were covered by both data systems; 287 (33.6%) came from paper surveys only; and 18 (2.1%) were filled out via computer only. Because only 2 (0.2%) questionnaires were missing any data, our data capture rate was 99.8%. Comparing data from the computerized and paper data sources provided evidence of consistency. Of the 546 days in which both paper and computer instruments provided data, 512 yielded identical responses (94% concordant) to the global severity of illness question. Of the 34 (6%) discrepancies, 29 were off by 1 point on the 9-point Likert-type scale and 5 discrepancies were off by 2 points. Comparing computer and paper responses with the 15 specific symptom questions also yielded high levels of concordance. Of 8190 item responses, 7777 (95%) were concordant, while 413 (5%) were classified as data discrepancies. Of these, 293 were off by 1 point on the 9-point scale; 68 were off by 2 points; 27, by 3 points; 17, by 4 points; 7, by 5 points; and 1 by 6 points.
Factor analysis of the data provided further evidence of internal validity. Structural equation modeling techniques57,58 were used to model symptom severities over time. A 4-dimensional symptom-recovery model (df = 71; P = .000025) provided a goodness of fit index of 0.88, a root mean square residual of .095, and a chi-squared/df ratio of 139/71 = 1.95. From the pool of 15 scaled symptom scores, 14 items contributed significantly to the model. (In this data set, loss of appetite was an infrequent symptom contributing insignificantly toward the model, and was dropped.) The 14 symptoms naturally aggregated into 4 underlying symptomatic dimensions: cough, throat, nasal, and fever and aches. Table 2 provides the reliability coefficients, standardized item loading coefficients, and standard errors of these loadings for the 4 dimensions. The reliability coefficients of the symptom dimensions were calculated using a procedure proposed by Dillon and Goldstein.59 Scale recovery curves, generated using a mixed modeling approach,60,61 were internally predictive, responsive,37,62 and consistent with what is known about the natural history of URI.
TABLE 2
RELIABILITY OF SYMPTOM DIMENSION MODELS
| Item Loading (SE)* | |
|---|---|
| Cough Dimension (Reliability = 0.794) | |
| Coughing | 2.01 (0.20) |
| Coughing stuff up | 1.75 (0.18) |
| Cough interfering with sleep | 1.16 (0.17) |
| Fever and Aches Dimension (Reliability = 0.753) | |
| Headache | 1.28 (0.23) |
| Fever | 1.07 (0.13) |
| Sweats | 1.25 (0.16) |
| Muscle aches | 1.76 (0.19) |
| Feeling run down | 1.17 (0.19) |
| Throat Dimension (Reliability = 0.668) | |
| Sore throat | 1.10 (0.22) |
| Scratchy throat | 1.73 (0.23) |
| Hoarseness | 1.68 (0.24) |
| Nasal Dimension (Reliability = 0.663) | |
| Runny nose | 1.93 (0.28) |
| Stuffy nose | 1.05 (0.23) |
| Sneezing | 1.63 (0.26) |
| *All significant at P < .05. | |
| SE denotes standard error. | |
Phase 2
Between July and December 2000, 108 persons from the general population responded to advertising by calling a telephone number listed on posted flyers and in the newspaper. Of these 108 callers, 27 were eligible but declined to participate; 7 did not meet inclusion criteria (were younger than 18 years of age, had current allergy symptoms, or did not have cold symptoms); and 74 met study criteria and elected to participate (Table 3). Those declining to participate usually did so because of inconvenience in arranging an immediate interview or because compensation ($10 for interview, $15 for focus group) was insufficient. Participants were met in person for semistructured individual interviews (n = 56) or focus groups (3 groups, 20 individuals total). Two people were interviewed both individually and in focus group.
Based on the information gained during interviews, the instrument-in-development underwent 6 revisions during 2000. Each modification was tested with additional interviews. A final version was created in December 2000. A few items from the initial instrument used in the echinacea trial were modified in response to participants’ descriptions and insights. Several other items were added to reflect symptoms and functional impairments described by participants in response to our open-ended questions. All items used wording provided by participants or tested during subsequent interviews and focus groups.
All symptoms spontaneously reported by at least 3 participants in either individual interview or focus group were included in the final version (Table 4). Figure 1 provides a frequency distribution of the symptoms described during the individual interviews.
On the basis of our participants’ comments, the distribution of severity data from the echinacea trial, and recommendations from published studies, we decided to decrease the response range from a 9-point to a 7-point Likert-type severity scale. The resulting severity range was marked at 1 (very mild), 3 (mild), 5 (moderate), and 7 (severe), following the majority opinion of our respondents. Unmarked (even-numbered) options were included, as most of the respondents felt they should have “in-between” choices. The functional outcome questions adapted from the SF-36 were replaced with participant-generated items, which were then scaled in a 7-point format similar to that used for the symptoms. A final question comparing today’s global severity with yesterday’s was added to provide a comparative measure of change over time (responsiveness). Figure 2 displays the final format of WURSS items.
The qualitative data provided by our informants improved our understanding of the symptomatic and functional impact of the common cold and assisted the development of the WURSS questionnaire. Although it is clear that people experience colds in different ways, several common threads emerged. For instance, we found that our original instrument (like the Jackson criteria) had overrated individual symptoms and had underrated functional impact, interference with social relationships, and general malaise. Informants often told us that it was not necessarily the individual symptoms that bothered them, but the general feelings, described as “sick feeling,” “loss of energy,” “run down,” “tired,” “fatigue,” “malaise,” “lousy,” “lazy,” “spacey," "blah," "yucky," "foggy," "lightheaded," "fuzzy brain,” “cloudy,” “disoriented,” “uncomfortable,” “distracted,” and “miserable.” Our informants also told us that they were bothered by the way their cold interfered with day-to-day activities and relationships. Colds affected physical activities such as breathing or walking, performance at work or in the home, and interactions with friends, family, and coworkers. Terms describing the most frequent and bothersome effects were incorporated into the final WURSS instrument.
TABLE 3
INTERVIEW AND FOCUS GROUP PARTICIPANT DEMOGRAPHICS
| Number | |
|---|---|
| Method of Data Collection | |
| Individual interviews | 56 |
| Focus groups | 20 |
| Total | 74* |
| Sex | |
| Women | 49 |
| Men | 25 |
| Ethnicity | |
| Native American | 2 |
| Black | 12 |
| Hispanic | 2 |
| White | 57 |
| No response | 1 |
| Annual Income | |
| < $10,000 | 28 |
| $10,000–19,999 | 17 |
| $20,000–29,999 | 13 |
| $30,000–49,999 | 9 |
| $50,000–75,000 | 3 |
| $75,000 | 2 |
| No response | 2 |
| Education | |
| Some high school | 13 |
| High school or equivalent | 11 |
| Some college | 11 |
| Associate or technical degree | 6 |
| Bachelor’s degree | 21 |
| Master’s degree | 6 |
| Professional degree | 4 |
| No response | 2 |
| Tobacco Use | |
| Current | 26 |
| Past | 19 |
| Never smoker | 28 |
| No response | 1 |
| *Two participants were used in both data collection methods. | |
| NOTE: Age range was 19 to 71 years, mean = 35.9 years (standard deviation, 11.9). | |
TABLE 4
SYMPTOMS AND FUNCTIONAL IMPAIRMENTS EVALUATED BY THE WISCONSIN UPPER RESPIRATORY SYMPTOM SURVEY
| Symptoms | Plugged ears |
| Cough | Ear discomfort |
| “Coughing stuff up” | Watery eyes |
| Cough interfering with sleep | Eye discomfort |
| Sore throat | Head congestion |
| Scratchy throat | Chest congestion |
| Hoarseness | Chest tightness |
| Runny nose | Heaviness in chest |
| Plugged nose | Lack of energy |
| Sneezing | Loss of appetite |
| Headache | |
| Body aches | Functional Impairments |
| Feeling “run down” | Think clearly |
| Sweats | Speak clearly |
| Chills | Sleep well |
| Feeling feverish | Breathe easily |
| Feeling dizzy | Walk, climb stairs, exercise |
| Feeling tired | Accomplish daily activities |
| Irritability | Work outside the home |
| Sinus pain | Work inside the home |
| Sinus pressure | Interact with others |
| Sinus drainage | Live your personal life |
| Swollen glands |
FIGURE 1
SYMPTOMS REPORTED IN INDIVIDUAL INTERVIEWS
FIGURE 2
ITEM FORMAT FOR THE WISCONSIN UPPER RESPIRATORY SYMPTOM SURVEY
Discussion
Researchers of URIs and the common cold need a well-developed, standardized, validated outcomes instrument that reflects the experience and values of cold sufferers. While the Jackson scale and various modifications have been widely used, few data support the validity of these scales. Although correlations with external measures, such as physical examinations, mucus weight, and the ability to culture virus have been reported, the symptomatic and functional impact of colds has largely been neglected. Perhaps more important, the symptomatic measures used to date were apparently developed without significant input from the people whose illnesses were measured. Questionnaire development and cognitive testing methods have not been described, nor have adequate tests of psychometric properties been reported. Although the Jackson scale may demonstrate marginal face validity in terms of symptoms, it does not do so in terms of functional impact.
This article describes the first steps taken in the development and validation of a new illness-specific quality-of-life instrument for measuring the common cold. The WURSS instrument is more comprehensive than existing alternatives and better reflects cold-sufferers’ experiences and values. Therefore, it provides greater face validity. The length (44 items) reflects a compromise between ease of use and comprehensiveness.41 It is possible that a subset of the items will prove nearly as effective and that a short-form WURSS will eventually be available. Item reduction will need to be guided by both internal (factor analysis) and external (frequency and perceived value) considerations. The standardized 7-point severity scale used throughout the WURSS makes the instrument very easy to use.49 It also provides a severity range that our informants and previous researchers40,63,64 agree is optimal. The WURSS allows a cold-sufferer to swiftly and accurately assess his or her common cold. We hope that WURSS will prove worthy in terms of standard psychometric properties such as precision, reliability, and responsiveness.65-68 A large prospective study will be necessary for those assessments.
Limitations
The work described here has a number of limitations. The WURSS was developed in Madison, Wis., largely among people with self-diagnosed colds during the period from July to December 2000. The RCT occurred over several months in the spring of 1999 and was limited to college students. Our results are therefore limited by both population and etiologic agent, which in turn may influence the symptom and severity spectrums assessed. Although we aimed for and achieved a moderate degree of socioeconomic diversity (Table 3), our participants’ responses may not be representative of the larger universe of cold-sufferers. The symptom distribution in Figure 1, for example, is unlikely to represent global cold symptom frequency accurately. Previous research with both natural and induced colds suggests that nasal symptoms and sore throat are usually more frequent than cough.2,3,6-18 The comprehensiveness of the instrument is more important for instrument development than are the specific item frequencies. Here, we feel that we succeeded in representing a sufficient range of items.
Another important limitation is the inherent variability and subjectivity of information generated from qualitative research. A similar instrument development effort carried out by different researchers would inevitably yield a somewhat different questionnaire. Eliciting and formatting terminology that reflects symptomatic and functional impact presents a number of challenges. Future research could employ a quantitative importance scale for participants to use in assessing the value of symptoms and functional impacts. Such value scales could be used alongside factor analysis models of item and dimension frequency and severity. These could in turn be compared with external criteria such as physician assessment, tissue counts, and nasal mucus weights, measurements of inflammatory cytokines, and quantitative viral cultures. Because no gold standard exists, single-criterion validity assessment will not be sufficient. Instead, the concept of construct validity will need to be invoked for future attempts at validation. Construct validity has been defined as “validity assessed by comparing the results of several contrasting tests of validity (including concurrent, convergent, and divergent validation studies) with predictions from a theoretical model.”64 Our work so far has only begun to scratch the surface of such rigorous validity assessment.
Conclusions
We have developed an instrument that measures patient-oriented outcomes identified as important by people with self-diagnosed common colds. We expect that the WURSS will do well with physician-diagnosed “bronchitis,” “sinusitis,” or “pharyngitis,” but as yet have no data with which to evaluate that supposition. We hope that the development of the WURSS stimulates other researchers to undergo similar efforts at aimed at patient-oriented outcome measurement and that the efforts can be compared. We have made the WURSS available for general use by placing a printable facsimile online at http://www.fammed.wisc.edu/wurss/. University-based health care researchers and other nonprofit entities may use the WURSS freely, but we do ask to be notified of such use. For-profit entities should contact us before using this copyrighted instrument.
The next step will be for WURSS to undergo large-scale psychometric testing with the goal of assessing its internal and external validity properties more accurately. We welcome comments, consultation, and collaboration and hope to involve other researchers as we move further in the direction of an accurate and reliable method for assessing the impact of the common cold.
Acknowledgments
The authors would like to acknowledge the participants who contributed their time and energy while they were sick. We also thank the many physicians and coworkers who contributed their knowledge and opinions, especially Nora Cate Schaeffer, PhD; Mary Beth Plane, PhD; Jon Temte, MD, PhD; Donn D’Alessio, MD; and William Scheckler, MD, in Madison, Wis., and Jack Gwaltney, MD, in Charlottesville, Va. During most of this project, Dr Barrett has had support from the National Center for Complementary and Alter native Medicine at the National Institutes of Health, Grant #K23 AT00051-01.
1. Dingle JH, Badger GF, Jordan WS. Illness in the home: a study of 25,000 illnesses in a group of Cleveland families. Cleveland, Ohio: Western Reserve University Press; 1964.
2. Gwaltney JM, Hendley JO, Simon G, Jordan WS. Rhinovirus infections in an industrial population. JAMA 1967;202:158-64.
3. Monto AS, Ullman BM. Acute respiratory illness in an American community. JAMA 1974;227:164-9.
4. Smith MBH, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA 1993;269:2258-63.
5. Turner RB. The treatment of rhinovirus infections: progress and potential. Antiviral Res 2001;49:1-14.
6. Kleinman A. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med 1978;88:251-8.
7. Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. Arch Intern Med 1958;101:267-78.
8. Jackson GG, Dowling HF, Anderson TO, Riff L, Saporta J, Turck M. Susceptibility and immunity to common upper respiratory viral infections–the common cold. Ann Intern Med 1960;55:719-38.
9. Jackson GG, Dowling HF, Muldoon RL. Present concepts of the common cold. Am J Public Health 1962;52:940-5.
10. D’Alessio D, Peterson JA, Dick CR, Dick EC. Transmission of experimental rhinovirus colds in volunteer married couples. J Infect Dis 1976;133:28-36.
11. Dick EC, Jennings LC, Mink KA, Wartgrow CD, Inhorn SL. Aerosol transmission of rhinovirus colds. J Infect Dis 1987;156:442-8.
12. Gwaltney JM, Hendley JO. Transmission of experimental rhinovirus infection by contaminated surfaces. Am J Epidemiol 1982;116:828-33.
13. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds. Ann Intern Med 1996;125:89-97.
14. Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol 1998;17:214-23.
15. Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Microbiol Rev 1999;12:9-18.
16. Arruda E, Pitkäranta A, Witek TJ, Doyle CA, Hayden FG. Frequency and history of rhinovirus infections in adults during autumn. J Clin Microbiol 1997;35:2864-8.
17. Gwaltney JM, Buier RM, Rogers JL. The influence of signal variation, bias, noise and effect size on statistical significance in treatment studies of the common cold. Antiviral Res 1996;29:287-95.
18. Rao SS, Hendley JO, Hayden FG, Gwaltney JM. Symptom expression in natural and experimental rhinovirus colds. Am J Rhinol 1995;9:49-52.
19. Freymuth F, Vabret A, Brouard J, et al. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol 1999;13:131-9.
20. Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol 1993;92:722-31.
21. Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol 1998;160:6172-81.
22. Teran LM, Johnston SL, Schroder JM, Church MK, Holgate ST. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus induced asthma. Am J Respir Crit Care Med 1997;155:1362-6.
23. Korppi M, Kröger L. Laitinen. White blood cell and differential counts in acute respiratory viral and bacterial infections in children. Scand J Infect Dis 1993;25:435-40.
24. McBride TP, Doyle WJ, Hayden FG, Gwaltney JM. Alterations of the Eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg 1989;115:1054-9.
25. Gwaltney JM, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med 1994;330:25-30.
26. Turner BW, Cail WS, Hendley JO, et al. Physiologic abnormalities in the paranasal sinuses during experimental rhinovirus colds. J Allergy Clin Immunol 1992;90:474-8.
27. Dressler WE, Myers T, London SJ, Rankell AS, Poetsch CE. A system of rhinomanometry in the clinical evaluation of nasal decongestants. Ann Otol Rhinol Laryngol 1977;86:310-6.
28. Tomkinson A, Eccles R. Comparison of the relative abilities of acoustic rhinometry, rhinomanometry, and the visual analogue scale in detecting change in the nasal cavity in a healthy adult population. Am J Rhinol 1996;10:161-5.
29. Parekh HH, Cragun KT, Hayden FG, Hendley JO, Gwaltney JM. Nasal mucus weights in experimental rhinovirus infection. Am J Rhinol 1992;6:107-10.
30. Scaglione F, Lund B. Efficacy in the treatment of the common cold of a preparation containing an echinacea extract. Int J Immunopharmacol 1995;11:163-6.
31. Juniper EF, Guyatt GH, Griffith LE, Ferrie PJ. Interpretation of rhinoconjuctivitis quality of life questionnaire data. J Allergy Clin Immunol 1996;98:843-5.
32. Juniper EF. Measuring health-related quality of life in rhinitis. J Allergy Clin Immunol 1997;99:S742-9.
33. Kozma CM, Sadik MK, Watrous ML. Economic outcomes for the treatment of allergic rhinitis. PharmacoEconomics 1996;410:4-13.
34. Meltzer EO, Nathan RA, Selner JC, Storms W. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol 1997;99:S807-28.
35. Meltzer EO, Nathan RA, Selner JC, Storms W. Quality of life and rhinitic symptoms: Result of a nationwide survey with the SF-36 and RQLQ questionnaires. J Allergy Clin Immunol 1997;99:815-9.
36. Piccirillo JF, Edwards D, Haiduk A, Yonan C, Thawley SE. Psychometric and clinimetric validity of the 31-item rhinosinusitis outcome measure (RSOM-31). Am J Rhinol 1995;9:297-306.
37. Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Controlled Clin Trials 1991;12(suppl 4):142S-158S.
38. Guyatt GH, Kirshner B, Jaeschke R. Measuring health status: What are the necessary measurement properties? J Clin Epidemiol 1992;45:1341-5.
39. Guyatt GH. Health status, quality of life, and the individual. JAMA 1994;272:630-1.
40. Juniper EF, Guyatt GH, Jaeschke R. How to develop and validate a new health-related quality of life instrument. In Spilker B, ed. Quality of life and pharmacoeconomics in clinical trials. Philadelphia, Pa: Lippincott-Raven; 1996;49-56.
41. Katz JN, Larson MG, Phillips CB. Comparative measurement sensitivity of short and longer health status instruments. Med Care 1992;30:917-25.
42. Schaeffer NC, Charng H-W. Two experiments in simplifying response categories: intensity and frequency categories. Sociol Perspect 1991;34:165-82.
43. Shrout PE, Yager TJ. Reliability and validity of screening scales: Effect of reducing scale length. J Clin Epidemiol 1989;42:69-78.
44. McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1998;31:247-63.
45. Barrett B, Locken L, Maberry R, Brown RL, Bobula JA, D’Alessio D. A randomized double-blind trial of an unrefined mixture of Echinacea purpurea and E angustifolia used to treat the common cold: no benefit detected. Submitted for publication 2001.
46. Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res 1993;2:451-9.
47. Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient’s view of change as a clinical outcome measure. JAMA 1999;282:1157-62.
48. Harris-Kojetin LD, Fowler FJ, Brown JA, Schnaier JA, Sweeny SF. The use of cognitive testing to develop and evaluate CAHPS 1.0 core survey items. Consumer assessment of health plans study. Med Care 1999;37:MS10-21.
49. Mullin PA, Lohr KN, Bresnahan BW, McNulty P. Applying cognitive design principles to formatting HRQOL instruments. Qual Life Res 2000;9:13-27.
50. Schaeffer NC. Conversation with a purpose or conversation? Interaction in the standardized interview. In: Biemer P, Groves RM, Lyberg LE, Mathiowitz NA, Sudman S, eds. Measurement errors in surveys. New York, NY: Wiley; 1991;367-91.
51. Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3.
52. Crabtree BF, Miller WL. Qualitative approach to primary care research: the long interview. Fam Med 1991;23:145-51.
53. McCracken G. The long interview. Thousand Oaks, Calif: Sage Publications; 1988.
54. Hughes D, DuMont K. Using focus groups to facilitate culturally anchored research. Am J Community Psychol 1993;21:775-807.
55. Kitzinger J. Qualitative research: Introducing focus groups. BMJ 1995;311:299-302.
56. Morgan D. Focus groups and qualitative research. Thousand Oaks, Calif: Sage Publications; 1988.
57. Bolan KA. Structural equations with latent variables. New York, NY: John Wiley & Sons; 1989.
58. Joreskog KG, Sorbom D. LISREL 8 User’s Reference Guide. Chicago, Ill: Scientific Software International, Inc; 1993.
59. Dillon WR, Goldstein M. Multivariate analysis: methods and applications. New York, NY: John Wiley & Sons; 1984.
60. Davis CS. Semi-parametric and non-parametric methods for the analysis of repeated measurements with applications to clinical trials. Stat Med 1991;1:1959-80.
61. Goldstein H. Multilevel statistical models. New York: Halsted, 1995.
62. Hays RD, Hadom D. Responsiveness to change: an aspect of validity, not a separate dimension. Qual Life Res 1992;1:73-5.
63. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15.
64. McDowell I, Newell C. Measuring health: a guide to rating scales and questionnaires. Oxford, England: Oxford University Press; 1996.
65. Deyo RA, Centor RM. Assessing the responsiveness of functional scales to clinical change: an analogy to diagnostic test performance. J Chronic Dis 1986;39:897-906.
66. Guyatt GH, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 1987;40:171-8.
67. Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res 1993;2:441-9.
68. Wright JG, Feinstein AR. A comparative contrast of clinimetric and psychometric methods for constructing indexes and rating scales. J Clin Epidemiol 1992;45:1201-18.
1. Dingle JH, Badger GF, Jordan WS. Illness in the home: a study of 25,000 illnesses in a group of Cleveland families. Cleveland, Ohio: Western Reserve University Press; 1964.
2. Gwaltney JM, Hendley JO, Simon G, Jordan WS. Rhinovirus infections in an industrial population. JAMA 1967;202:158-64.
3. Monto AS, Ullman BM. Acute respiratory illness in an American community. JAMA 1974;227:164-9.
4. Smith MBH, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA 1993;269:2258-63.
5. Turner RB. The treatment of rhinovirus infections: progress and potential. Antiviral Res 2001;49:1-14.
6. Kleinman A. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med 1978;88:251-8.
7. Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. Arch Intern Med 1958;101:267-78.
8. Jackson GG, Dowling HF, Anderson TO, Riff L, Saporta J, Turck M. Susceptibility and immunity to common upper respiratory viral infections–the common cold. Ann Intern Med 1960;55:719-38.
9. Jackson GG, Dowling HF, Muldoon RL. Present concepts of the common cold. Am J Public Health 1962;52:940-5.
10. D’Alessio D, Peterson JA, Dick CR, Dick EC. Transmission of experimental rhinovirus colds in volunteer married couples. J Infect Dis 1976;133:28-36.
11. Dick EC, Jennings LC, Mink KA, Wartgrow CD, Inhorn SL. Aerosol transmission of rhinovirus colds. J Infect Dis 1987;156:442-8.
12. Gwaltney JM, Hendley JO. Transmission of experimental rhinovirus infection by contaminated surfaces. Am J Epidemiol 1982;116:828-33.
13. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds. Ann Intern Med 1996;125:89-97.
14. Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol 1998;17:214-23.
15. Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Microbiol Rev 1999;12:9-18.
16. Arruda E, Pitkäranta A, Witek TJ, Doyle CA, Hayden FG. Frequency and history of rhinovirus infections in adults during autumn. J Clin Microbiol 1997;35:2864-8.
17. Gwaltney JM, Buier RM, Rogers JL. The influence of signal variation, bias, noise and effect size on statistical significance in treatment studies of the common cold. Antiviral Res 1996;29:287-95.
18. Rao SS, Hendley JO, Hayden FG, Gwaltney JM. Symptom expression in natural and experimental rhinovirus colds. Am J Rhinol 1995;9:49-52.
19. Freymuth F, Vabret A, Brouard J, et al. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol 1999;13:131-9.
20. Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol 1993;92:722-31.
21. Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol 1998;160:6172-81.
22. Teran LM, Johnston SL, Schroder JM, Church MK, Holgate ST. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus induced asthma. Am J Respir Crit Care Med 1997;155:1362-6.
23. Korppi M, Kröger L. Laitinen. White blood cell and differential counts in acute respiratory viral and bacterial infections in children. Scand J Infect Dis 1993;25:435-40.
24. McBride TP, Doyle WJ, Hayden FG, Gwaltney JM. Alterations of the Eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg 1989;115:1054-9.
25. Gwaltney JM, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med 1994;330:25-30.
26. Turner BW, Cail WS, Hendley JO, et al. Physiologic abnormalities in the paranasal sinuses during experimental rhinovirus colds. J Allergy Clin Immunol 1992;90:474-8.
27. Dressler WE, Myers T, London SJ, Rankell AS, Poetsch CE. A system of rhinomanometry in the clinical evaluation of nasal decongestants. Ann Otol Rhinol Laryngol 1977;86:310-6.
28. Tomkinson A, Eccles R. Comparison of the relative abilities of acoustic rhinometry, rhinomanometry, and the visual analogue scale in detecting change in the nasal cavity in a healthy adult population. Am J Rhinol 1996;10:161-5.
29. Parekh HH, Cragun KT, Hayden FG, Hendley JO, Gwaltney JM. Nasal mucus weights in experimental rhinovirus infection. Am J Rhinol 1992;6:107-10.
30. Scaglione F, Lund B. Efficacy in the treatment of the common cold of a preparation containing an echinacea extract. Int J Immunopharmacol 1995;11:163-6.
31. Juniper EF, Guyatt GH, Griffith LE, Ferrie PJ. Interpretation of rhinoconjuctivitis quality of life questionnaire data. J Allergy Clin Immunol 1996;98:843-5.
32. Juniper EF. Measuring health-related quality of life in rhinitis. J Allergy Clin Immunol 1997;99:S742-9.
33. Kozma CM, Sadik MK, Watrous ML. Economic outcomes for the treatment of allergic rhinitis. PharmacoEconomics 1996;410:4-13.
34. Meltzer EO, Nathan RA, Selner JC, Storms W. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol 1997;99:S807-28.
35. Meltzer EO, Nathan RA, Selner JC, Storms W. Quality of life and rhinitic symptoms: Result of a nationwide survey with the SF-36 and RQLQ questionnaires. J Allergy Clin Immunol 1997;99:815-9.
36. Piccirillo JF, Edwards D, Haiduk A, Yonan C, Thawley SE. Psychometric and clinimetric validity of the 31-item rhinosinusitis outcome measure (RSOM-31). Am J Rhinol 1995;9:297-306.
37. Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Controlled Clin Trials 1991;12(suppl 4):142S-158S.
38. Guyatt GH, Kirshner B, Jaeschke R. Measuring health status: What are the necessary measurement properties? J Clin Epidemiol 1992;45:1341-5.
39. Guyatt GH. Health status, quality of life, and the individual. JAMA 1994;272:630-1.
40. Juniper EF, Guyatt GH, Jaeschke R. How to develop and validate a new health-related quality of life instrument. In Spilker B, ed. Quality of life and pharmacoeconomics in clinical trials. Philadelphia, Pa: Lippincott-Raven; 1996;49-56.
41. Katz JN, Larson MG, Phillips CB. Comparative measurement sensitivity of short and longer health status instruments. Med Care 1992;30:917-25.
42. Schaeffer NC, Charng H-W. Two experiments in simplifying response categories: intensity and frequency categories. Sociol Perspect 1991;34:165-82.
43. Shrout PE, Yager TJ. Reliability and validity of screening scales: Effect of reducing scale length. J Clin Epidemiol 1989;42:69-78.
44. McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1998;31:247-63.
45. Barrett B, Locken L, Maberry R, Brown RL, Bobula JA, D’Alessio D. A randomized double-blind trial of an unrefined mixture of Echinacea purpurea and E angustifolia used to treat the common cold: no benefit detected. Submitted for publication 2001.
46. Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res 1993;2:451-9.
47. Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient’s view of change as a clinical outcome measure. JAMA 1999;282:1157-62.
48. Harris-Kojetin LD, Fowler FJ, Brown JA, Schnaier JA, Sweeny SF. The use of cognitive testing to develop and evaluate CAHPS 1.0 core survey items. Consumer assessment of health plans study. Med Care 1999;37:MS10-21.
49. Mullin PA, Lohr KN, Bresnahan BW, McNulty P. Applying cognitive design principles to formatting HRQOL instruments. Qual Life Res 2000;9:13-27.
50. Schaeffer NC. Conversation with a purpose or conversation? Interaction in the standardized interview. In: Biemer P, Groves RM, Lyberg LE, Mathiowitz NA, Sudman S, eds. Measurement errors in surveys. New York, NY: Wiley; 1991;367-91.
51. Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3.
52. Crabtree BF, Miller WL. Qualitative approach to primary care research: the long interview. Fam Med 1991;23:145-51.
53. McCracken G. The long interview. Thousand Oaks, Calif: Sage Publications; 1988.
54. Hughes D, DuMont K. Using focus groups to facilitate culturally anchored research. Am J Community Psychol 1993;21:775-807.
55. Kitzinger J. Qualitative research: Introducing focus groups. BMJ 1995;311:299-302.
56. Morgan D. Focus groups and qualitative research. Thousand Oaks, Calif: Sage Publications; 1988.
57. Bolan KA. Structural equations with latent variables. New York, NY: John Wiley & Sons; 1989.
58. Joreskog KG, Sorbom D. LISREL 8 User’s Reference Guide. Chicago, Ill: Scientific Software International, Inc; 1993.
59. Dillon WR, Goldstein M. Multivariate analysis: methods and applications. New York, NY: John Wiley & Sons; 1984.
60. Davis CS. Semi-parametric and non-parametric methods for the analysis of repeated measurements with applications to clinical trials. Stat Med 1991;1:1959-80.
61. Goldstein H. Multilevel statistical models. New York: Halsted, 1995.
62. Hays RD, Hadom D. Responsiveness to change: an aspect of validity, not a separate dimension. Qual Life Res 1992;1:73-5.
63. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15.
64. McDowell I, Newell C. Measuring health: a guide to rating scales and questionnaires. Oxford, England: Oxford University Press; 1996.
65. Deyo RA, Centor RM. Assessing the responsiveness of functional scales to clinical change: an analogy to diagnostic test performance. J Chronic Dis 1986;39:897-906.
66. Guyatt GH, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 1987;40:171-8.
67. Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res 1993;2:441-9.
68. Wright JG, Feinstein AR. A comparative contrast of clinimetric and psychometric methods for constructing indexes and rating scales. J Clin Epidemiol 1992;45:1201-18.
Bridging the Gap Between Conventional and Alternative Medicine
METHODS: We conducted semistructured in-depth interviews focused on the knowledge, attitudes, and behaviors of a random sample of 17 patients who had used both CAM and conventional therapies during the past year. Participants were recruited using telephone listings. Twenty alternative practitioners were selected to represent the major modalities. The topics discussed included healing philosophy, choices of therapeutic methods, and ideas concerning concurrent use of differing therapeutic modalities. An 8-member multidisciplinary team analyzed the transcripts individually and in group meetings.
RESULTS: Four major themes emerged from the interview data: (1) holism, (2) empowerment, (3) access, and (4) legitimization. Both patients and providers distinguished between the socially legitimized and widely accessible but disempowering and mechanistic attributes of conventional medicine and the holistic and empowering but relatively less accessible and less legitimate nature of alternative healing. There was a strong call for integrating the best aspects of both.
CONCLUSIONS: Practitioners and users of alternative therapies in the Madison area confirmed both the alternative and complementary natures of unconventional health care, called for more integrated and accessible health care, and provided insights that could be useful in bridging the gap between conventional and alternative medicine.
The use of complementary and alternative medicine (CAM) is on the rise. Various CAM modalities, including acupuncture, Chinese medicine, homeopathy, massage, naturopathy, spiritual healing, and the use of herbal medicines and supplements compete with, provide an alternative to, or complement the more conventional forms of medicine available in hospitals and licensed physicians’ offices. There is an emerging literature on various aspects of this growing phenomenon with hundreds of articles and dozens of texts already on the market.1-7 The Journal of the American Medical Association and associated Archives journals chose alternative medicine for their 1998 theme issue. The boundaries of CAM are being defined and redefined.8-13 A fair amount of research has explored physicians’ attitudes and practices regarding this increasingly prevalent phenomenon.14-18 However, very little is known about how patients think about and choose among the many alternatives or how alternative practitioners situate themselves in this process.19-22 To map out this relatively unknown but clearly expanding social territory we conducted, transcribed, and reviewed 37 in-person semistructured long interviews with providers of alternative therapies and people who use those therapies. Our respondents provided us with coherent and intriguing depictions of the many issues involved. Perhaps most important, our participants highlighted a number of ideas and issues that together form themes defining and demarcating “complementary”, “alternative”, and “conventional” health care.
Methods
Our goal was to investigate the knowledge, attitudes, and practices of patients and providers of complementary and alternative therapies. Eisenberg and colleagues23 defined complementary and alternative medicine as “unconventional.” In their framework, CAM therapies are those that are not taught in US medical schools or widely available in hospitals and licensed physicians’ offices. Our operational definition of CAM was consistent with that definition. Our inclusion criteria were focused on issues of reimbursement. Therapies such as herbal medicine, homeopathy, and mind-body medicine are not generally reimbursable, while most therapies prescribed by physicians are covered by third-party payers. We considered osteopathy and chiropractic as conventional medicine but included acupuncture in the alternative category. These broad definitions were not rigid. Instead, we let patients and providers describe to us their definitions and understandings of “complementary” and “alternative.” CAM therapies represented in our study are listed in Table 1. As a qualitative interview-based study, our research was designed to be exploratory, descriptive, integrative, multirelational, and hypothesis-generating. We followed the standard qualitative research method of formal multidisciplinary review of transcribed in-person long interviews.24-26 Each transcribed interview was reviewed by each member of an 8-person multidisciplinary research team, using a standardized worksheet. Transcriptions were reviewed individually and then discussed in 4 face-to-face group meetings. The research team consisted of an anthropologist and family physician research fellow, a faculty family physician, a biocultural anthropologist, a medical-education nurse and the faculty coordinator for an alternative medicine course for medical students, an alternative psychotherapist, 2 premedical students, and the research assistant interviewer who recently graduated in sociology and women’s studies.
Alternative therapy providers were recruited using a key informant sampling method. We began by generating a near-complete list of alternative providers in the Madison, Wisconsin, area. We used telephone listings, informal interviews with knowledgable informants, and looked for notices and business cards posted at pharmacies, health food stores, and alternative healing centers. Once we felt confident that our list was sufficiently comprehensive (approximately 150 individuals), we sampled the CAM providers to include a wide representation of healing modalities. Following informed consent procedures approved by our institutional review board, 20 healers were recruited and interviewed using a semistructured format aimed at understanding the nature of practice, philosophy of healing, and attitudes and practices with regard to patients’ use of conventional medicine. Examples of the questions asked are listed in Table 2. The interviews were semistructured, and the interviewer was allowed flexibility to explore ideas brought up by the respondent.
We recruited patients randomly using the Madison telephone listings. Our inclusion criteria required that they were aged 18 years or older, used both alternative and conventional medicine in the past year, and were willing to meet for an unpaid hourlong interview. Out of 237 phone numbers randomly chosen from the telephone book, 29 were disconnected, and 58 went unanswered after at least 3 attempts. Of the remaining 150, 25 fit inclusion criteria, 19 agreed to an interview, and 17 were actually interviewed. The location of the interview was open to the participants’ choice and included home, office, and a public place, such as a restaurant or library. The semistructured format allowed free-flowing interviews aimed at probing health beliefs, choices, and practices. We were specifically interested in how patients thought about both alternative and conventional health services, how and why they made their health care choices, and how they personally valued various aspects of CAM and conventional medicine that came to light during the interview. Although our initial goal was to interview 20 patients, we stopped after 17 because we felt we had reached the point of data saturation (we ceased to learn new things with our interviews).
Results
During the 37 interviews in the practitioner and patient categories, a number of points emerged. Issues contrasting conventional and alternative health care rose to prominence during the interviews. Respondents noted differences of style, cost, training, institutional structure, and philosophy, orientation, and world-view. Vague, subtle, implicit, and subjective differences were described as well as obvious, clear, and objective differences. Among the medley of points that surfaced, a number of issues were raised repeatedly. After discussion and deliberation we organized that data into 4 major thematic categories: holism, empowerment, access, and legitimization. Nearly every idea, talking point, or issue that we noted—several hundred in all—fit comfortably within at least one of these major themes. Some of the more salient ideas and issues are shown in Table 3. A number of points, issues, or subthemes related to more than one of the themes and provided important interconnections or overlaps.
Holism
Patients and practitioners of alternative therapy stressed the importance of a grounded integrated whole-person approach. Patients noted that they preferred to be treated as whole people rather than as composites of numerous biomedical attributes. Practitioners stressed the multidimensional nature of their work and contrasted their integrated approach from what they considered the mechanistic reductionist methods of biomedicine. One practitioner said, “I think conventional medicine tries to fix a problem…rather than get to the heart of the issue.” One patient described it as “healing and staying healthy in a proactive sense.” Another said it was “amazing, your mind and body working together.” The importance of incorporating emotional, physical, psychological, and social factors in both diagnosis and therapy was repeatedly stressed.
Empowerment
Our participants told us that conventional biomedical practitioners often disempower their patients by acting in condescending, disparaging, chauvinistic, or paternalistic manners. In contrast, alternative healers were characterized as facilitating rather than directing the healing process, relying on self-empowerment and personal responsibility for health. One patient said, “I have to be part of the process for it to work.” Another defined conventional medicine as: “You broke it, so let’s fix you.” A third, describing her interaction with conventional physicians said, “And every time I bring it up they blow it off. So I didn’t get very far when I voiced my concerns.” A fourth went so far as to say, “I think the doctors’ way of being is phasing out because people are getting more responsible for their health care.” Most respondents felt that personal empowerment was important for health and told us that alternative healers tend to focus on personal empowerment more than their conventional biomedical counterparts.
Access
The thematic category we called “access” combines issues of insurance coverage with physical, economic, and social availability. As a whole, patients noted that conventional health care was relatively accessible because it was paid for by insurance that was usually available through employment. Additionally, conventional medicine was noted to be physically available in most areas; CAM services were often harder to locate and visit. Alternative medicine was described as expensive from an out-of-pocket perspective and less economically accessible from the patient’s point of view. Several patients noted that they tried conventional methods before moving on to alternative healing. When asked why, one said “I thought I would exhaust the route of things that are free.” Another noted the relative high cost of alternative therapy by saying, “So basically out of sheer monetary restraints, I’ll go back to the physician.” Alternative providers were in consensus that “Most people pay out of pocket…. It is a detriment for people to pay out of pocket.”
Legitimization
Our respondents differentiated between the officially recognized nature of conventional medicine and the less legitimated but increasingly recognized status of CAM. They also differentiated between official or legal legitimacy and legitimacy originating from credible evidence. Practitioners and patients believed in the effectiveness of the modes they used. Referring to the evidence base of alternative medicine, one patient said, “It is just as sound as conventional medicine. It’s just that there haven’t been enough studies yet.” However, one practitioner claimed that “What I teach (and practice) is research based and backed up by studies.” A patient, however, felt that the evidence of effectiveness was often “anecdotal and that doesn’t work in health care.” Both patients and practitioners noted the current lack of standards and felt that standardization (and legitimization) should occur. One said, “They (alternative practitioners) are not under any regulated umbrella, and I think there are a lot of exaggerated claims about what they can do for you.” Another put it more bluntly, “I think there are many quacks out there without review and standards, and people are skeptical of them.”
Discussion
Alternative, complementary, or unconventional therapies are increasingly prevalent throughout the industrialized world.27-33 In the United States, Eisenberg and coworkers estimated that in 1990 approximately 34% of Americans used an unconventional therapy, and made an estimated 425 million visits to alternative practitioners.23 By 1997, those figures had climbed to 42% of Americans using an unconventional therapy, and 629 million visits to CAM providers.34 Other studies12,35-37 provide similar estimates. The number of visits to CAM providers clearly outnumbers the number of visits to primary care physicians, estimated at 388 million in 1997. It is an interesting situation, considering the relatively high (estimated at $27 billion in 1997) out-of-pocket costs of alternative medicine and the greater credibility and legitimacy of conventional medicine. The literature provides few insights. A number of hypotheses have been proposed, but few have been tested.18-20,22 Socioeconomic indicators are mildly predictive at best. There is a slight positive association with education, income, and female gender and a moderate negative association with African American ethnicity, but overall these are poor predictors, with odds ratios ranging from 1.2 to 1.4.19,22,23,34,35 Dissatisfaction with conventional medicine does not seem to significantly predict CAM use. In contrast, most patients who use CAM continue to utilize and appreciate conventional medicine, although they often do not tell their physician about their unconventional choices.
Our research project was designed to begin to map out the attitudinal and behavioral territory of CAM and between alternative and conventional medicine. Both patients and providers distinguished between the socially legitimized and widely accessible but disempowering and mechanistic attributes of conventional medicine and the holistic and empowering but relatively less accessible and less legitimate nature of alternative healing. They believe conventional medicine has legitimate strength in both diagnosis and treatment but often fails to take account of the complexity of the whole person. Many noted that because of its power, conventional medicine can easily do harm. One practitioner described conventional medicine as “like using a boulder to kill an ant.” Another used a velocity metaphor in describing the difference, “Chinese medicine has a top speed of 30 miles per hour and if your disease is going 45 to 50, you need to go to an allopathic (conventional) physician because they can go 120.”
Of the 4 thematic categories identified, access is perhaps the most complex, because it includes health system issues (eg, insurance coverage) and patient-provider communication issues (eg, language, culture, and socioeconomic accessibility). Alternative therapies cost less to society than conventional ones. A person paying out of pocket will receive more provider time per dollar with an alternative practitioner than with most conventional providers. However, for the majority of people in the United States who have health care coverage, conventional medicine costs very little out of pocket, certainly less than the $25 to $50 per hour charged by many alternative providers. So we are left with the contradictory situation in which the direct costs to patients using the relatively expensive official health care system are often less than the price that alternative healers charge. However, for the substantial minority that lack insurance coverage (15% to 20% of the United States), conventional medicine is often beyond their financial reach, perhaps increasing the relative accessibility of alternative medicine.
The incorporation of a more holistic and empowering healing philosophy can be seen as a natural step in the growth of medicine, a step that has already been taken by many persons. This type of healing strategy is consistent with the adoption of the biopsychosocial model, a theory-based health care strategy first proposed by Engel in the 1970s.38-40 In conventional health care, family physicians have perhaps most embraced holism, humanism, and biopsychosocial medicine.41-45 Our results can also be seen as consistent with the behavioral model of health care developed by Andersen and colleagues.46 This behavioral model postulates that choices of health care arise from predisposing characteristics (health beliefs and social structure) interacting with enabling resources (access), as well as health needs.47 The behavioral model has been applied to alternative health care by Kelner and Wellman,21 who after analyzing in-depth interviews with 300 patients concluded that “the choice of type of practitioner(s) is multidimensional and cannot solely be explained either by disenchantment with medicine or by an ‘alternative ideology.’”
People seek either conventional or alternative health care for a variety of reasons, from perceived health need to accessibility to perceived effectiveness.48,49 Astin35 has shown that philosophical orientation—as defined by an interest in personal and spiritual growth and a commitment to environmentalism and feminism—is a significant predictor of use of CAM. In a national representative survey, patients within this defined group of “cultural creatives” were twice as likely to choose CAM therapies than other patients; it was a stronger statistical predictor than either education or global health status. Astin’s conclusions infer that the values of this segment of society have permeated the social matrix enough to influence health care behaviors, leading to the dramatic increase in CAM therapy during the 1990s.
Conclusions
The patients and providers interviewed for our study called for integration of the best aspects of conventional and alternative care. They suggested that the apparent strengths of CAM—especially holism and empowerment—could be used to help improve the quality of conventional health care. We suggest that some ideas and methods from CAM should be investigated and, if found deserving, either adopted or adapted by the official medical system. CAM therapists should be credentialed and incorporated into the health care team and some CAM methods should be learned by conventional providers. Our respondents felt that an integrative effort, if backed by credible evidence and implemented with high-quality standards, could help heal a powerful, but sometimes dangerous, health care system. We feel that this type of integrative effort requires regulation as well as recognition, and should include outcomes-based research. Such an endeavor should be based both on principles and on outcomes data. One such principle was clearly articulated by our respondents: All people should have access to legitimate, holistic, and empowering health care.
1. Benjamin SA, Benson H, Gordon JS, Sullivan M. Mind-body medicine: expanding the health model. Patient Care 1997;15:127-45.
2. Cant S, Sharma U. Complementary and alternative medicines: knowledge in practice. New York, NY: Free Association Books; 1996.
3. Cohen MH. Complementary and alternative medicine: legal boundaries and regulatory perspectives. Baltimore, Md: Johns Hopkins University Press; 1998.
4. Lewith G, Kenyon J, Lewis P. Complementary medicine: an integrated approach. Oxford general practice series, no. 34. Oxford, England: Oxford University Press; 1996.
5. Marshall E. The politics of alternative medicine. Science 1994;265:2000-2.
6. National Institutes of Health. Alternative medicine: expanding medical horizons. A report to the National Institutes of Health on alternative medical systems and practices in the United States. Washington, DC: US Government Printing Office; 1992.
7. Wardwell WI. Alternative medicine in the United States. Soc Sci Med 1994;38:1061-8.
8. Abbot NC, White AR, Ernst E. Complementary medicine. Nature 1996;381:361.-
9. Alpert JS. The relativity of alternative medicine. Arch Intern Med 1995;155:2385.-
10. CAM Panel. Defining and describing complementary and alternative medicine: panel consensus following CAM research methodology conference, April 1995. Alternative Ther Health Med 1997;3:49-57.
11. Ernst E. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996.
12. Pavek RR, Trachtenberg AI. Current status of alternative health practices in the United States. Contemp Intern Med 1995;7:61-72.
13. Spencer JW, Jacobs JJ. Complementary/alternative medicine: an evidence-based approach. St. Louis, Mo: Mosby, Inc; 1999.
14. Bernam BM, Singh BK, Lao L, Singh BB, Ferentz KS, Hartnoll SM. Physicians attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract 1995;8:361-6.
15. Borkan J, Neher JO, Anson O, Smoker B. Referrals for alternative therapies. J Fam Pract 1994;39:545-50.
16. Bower H. Double standards exist in judging traditional and alternative medicine. BMJ 1998;316:1694.-
17. Ernst E, Resch K-L, White A. Complementary medicine: what physicians think of it. A meta-analysis. Arch Intern Med 1995;155:2405-8.
18. White AR, Resch K-L, Ernst E. Complementary medicine: use and attitudes among GPs. Fam Pract 1997;14:302-6.
19. Ernst E, Wiloughby M, Weihmayr T. Nine possible reasons for choosing complementary medicine. Perfusion 1995;11:356-9.
20. Furnham A. Why do people choose and use complementary therapies? In: Ernst E, ed. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996;71-88.
21. Kelner M, Wellman B. Health care and consumer choice: medical and alternative therapies. Soc Sci Med 1997;45:203-12.
22. Vincent C, Furnham A. Why do patients turn to complementary medicine? An empirical study. Br Psychological Society 1996;35:37-48.
23. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med 1993;328:246-52.
24. Crabtree BF, Miller WF. Doing qualitative research. Sage publications series on research methods for primary care, volume 3. Thousand Oaks, Calif: Sage Publications; 1992.
25. Denzin NK, Lincoln YS. Handbook of qualitative research. Thousand Oaks, Calif: Sage Publications; 1993.
26. McCracken G. The long interview. Thousand Oaks, Calif: Sage Publications; 1988.
27. Dickinson DPS. The growth of complementary therapies: a consumer-led boom. In Ernst E, ed. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996;150-62.
28. Fisher P, Ward A. Complementary medicine in Europe. BMJ 1994;309:107-10.
29. Gray C. Growing popularity of complementary medicine leads to national organization for MDs. Can Med Assoc J 1997;157:186-8.
30. MacLennan AH, Wilson DH, Taylor AW. Prevalence and cost of alternative medicine in Australia. Lancet 1996;347:569-73.
31. Millar WJ. Use of alternative health care practitioners by Canadians. Can J Public Health 1997;88:154-8.
32. Shenfield GM, Atkin PA, Kristoffersen SS. Alternative medicine: an expanding health industry. Med J Australia 1997;166:516-7.
33. Thomas KJ, Carr J, Westlake L, Williams BT. The use of non-orthodox and conventional health care in Great Britain. BMJ 1991;302:207-10.
34. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA 1998;280:1569-75.
35. Astin JA. Why patients use alternative medicine: results of a national study. JAMA 1998;279:1548-53.
36. Brevoort P. The booming US botanical market: a new overview. HerbalGram 1998;44:33-46.
37. Landmark Healthcare. The Landmark report on public perceptions of alternative care. 1998 nationwide study of alternative care. Random telephone survey of 1500 households. Sacramento, Calif: Landmark Healthcare, Inc; 1998.
38. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129-36.
39. Sadler JZ, Hulgus Y. Knowing, valuing, acting: clues to revising the biopsychosocial model. Compr Psychiatry 1990;31:185-95.
40. Smith KC, Kleinman A. Beyond the biomedical model: integration of psychosocial and cultural orientations. In: Taylor RB, David AK, Johnson TA, eds. Family medicine: principles and practice. New York, NY: Springer-Verlag; 1983.
41. Goldstein M, Sutherland C, Jaffe D, Wilson J. Holistic physicians and family practitioners: similarities, differences and implications for health policy. Soc Sci Med 1988;26:853-61.
42. Kuzel AJ. Naturalistic inquiry: an appropriate model for family medicine. Fam Med 1986;18:369-74.
43. Miller WL. Models of health, illness, and health care. In: Taylor RB, David AK, Johnson TA, eds. Family Medicine: Principles and Practice. New York, NY: Springer-Verlag; 1988.
44. Stephens GG. The intellectual basis of family practice. J Fam Pract 1975;2:423-8.
45. Stephens GG. Family medicine as counter-culture. STFM annual meeting, Denver, Colo. May 9, 1979.
46. Andersen RM. Behavioral model of families’ use of health services. University of Chicago Center for Health Services research series no 250 Chicago, Ill: University of Chicago Press; 1968.
47. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1-10.
48. Campbell S, Roland MO. Why do people consult the doctor? Fam Pract 1996;13:75-83.
49. Zola IK. Pathways to the doctor: from person to patient. Soc Sci Med 1973;7:677-89.
METHODS: We conducted semistructured in-depth interviews focused on the knowledge, attitudes, and behaviors of a random sample of 17 patients who had used both CAM and conventional therapies during the past year. Participants were recruited using telephone listings. Twenty alternative practitioners were selected to represent the major modalities. The topics discussed included healing philosophy, choices of therapeutic methods, and ideas concerning concurrent use of differing therapeutic modalities. An 8-member multidisciplinary team analyzed the transcripts individually and in group meetings.
RESULTS: Four major themes emerged from the interview data: (1) holism, (2) empowerment, (3) access, and (4) legitimization. Both patients and providers distinguished between the socially legitimized and widely accessible but disempowering and mechanistic attributes of conventional medicine and the holistic and empowering but relatively less accessible and less legitimate nature of alternative healing. There was a strong call for integrating the best aspects of both.
CONCLUSIONS: Practitioners and users of alternative therapies in the Madison area confirmed both the alternative and complementary natures of unconventional health care, called for more integrated and accessible health care, and provided insights that could be useful in bridging the gap between conventional and alternative medicine.
The use of complementary and alternative medicine (CAM) is on the rise. Various CAM modalities, including acupuncture, Chinese medicine, homeopathy, massage, naturopathy, spiritual healing, and the use of herbal medicines and supplements compete with, provide an alternative to, or complement the more conventional forms of medicine available in hospitals and licensed physicians’ offices. There is an emerging literature on various aspects of this growing phenomenon with hundreds of articles and dozens of texts already on the market.1-7 The Journal of the American Medical Association and associated Archives journals chose alternative medicine for their 1998 theme issue. The boundaries of CAM are being defined and redefined.8-13 A fair amount of research has explored physicians’ attitudes and practices regarding this increasingly prevalent phenomenon.14-18 However, very little is known about how patients think about and choose among the many alternatives or how alternative practitioners situate themselves in this process.19-22 To map out this relatively unknown but clearly expanding social territory we conducted, transcribed, and reviewed 37 in-person semistructured long interviews with providers of alternative therapies and people who use those therapies. Our respondents provided us with coherent and intriguing depictions of the many issues involved. Perhaps most important, our participants highlighted a number of ideas and issues that together form themes defining and demarcating “complementary”, “alternative”, and “conventional” health care.
Methods
Our goal was to investigate the knowledge, attitudes, and practices of patients and providers of complementary and alternative therapies. Eisenberg and colleagues23 defined complementary and alternative medicine as “unconventional.” In their framework, CAM therapies are those that are not taught in US medical schools or widely available in hospitals and licensed physicians’ offices. Our operational definition of CAM was consistent with that definition. Our inclusion criteria were focused on issues of reimbursement. Therapies such as herbal medicine, homeopathy, and mind-body medicine are not generally reimbursable, while most therapies prescribed by physicians are covered by third-party payers. We considered osteopathy and chiropractic as conventional medicine but included acupuncture in the alternative category. These broad definitions were not rigid. Instead, we let patients and providers describe to us their definitions and understandings of “complementary” and “alternative.” CAM therapies represented in our study are listed in Table 1. As a qualitative interview-based study, our research was designed to be exploratory, descriptive, integrative, multirelational, and hypothesis-generating. We followed the standard qualitative research method of formal multidisciplinary review of transcribed in-person long interviews.24-26 Each transcribed interview was reviewed by each member of an 8-person multidisciplinary research team, using a standardized worksheet. Transcriptions were reviewed individually and then discussed in 4 face-to-face group meetings. The research team consisted of an anthropologist and family physician research fellow, a faculty family physician, a biocultural anthropologist, a medical-education nurse and the faculty coordinator for an alternative medicine course for medical students, an alternative psychotherapist, 2 premedical students, and the research assistant interviewer who recently graduated in sociology and women’s studies.
Alternative therapy providers were recruited using a key informant sampling method. We began by generating a near-complete list of alternative providers in the Madison, Wisconsin, area. We used telephone listings, informal interviews with knowledgable informants, and looked for notices and business cards posted at pharmacies, health food stores, and alternative healing centers. Once we felt confident that our list was sufficiently comprehensive (approximately 150 individuals), we sampled the CAM providers to include a wide representation of healing modalities. Following informed consent procedures approved by our institutional review board, 20 healers were recruited and interviewed using a semistructured format aimed at understanding the nature of practice, philosophy of healing, and attitudes and practices with regard to patients’ use of conventional medicine. Examples of the questions asked are listed in Table 2. The interviews were semistructured, and the interviewer was allowed flexibility to explore ideas brought up by the respondent.
We recruited patients randomly using the Madison telephone listings. Our inclusion criteria required that they were aged 18 years or older, used both alternative and conventional medicine in the past year, and were willing to meet for an unpaid hourlong interview. Out of 237 phone numbers randomly chosen from the telephone book, 29 were disconnected, and 58 went unanswered after at least 3 attempts. Of the remaining 150, 25 fit inclusion criteria, 19 agreed to an interview, and 17 were actually interviewed. The location of the interview was open to the participants’ choice and included home, office, and a public place, such as a restaurant or library. The semistructured format allowed free-flowing interviews aimed at probing health beliefs, choices, and practices. We were specifically interested in how patients thought about both alternative and conventional health services, how and why they made their health care choices, and how they personally valued various aspects of CAM and conventional medicine that came to light during the interview. Although our initial goal was to interview 20 patients, we stopped after 17 because we felt we had reached the point of data saturation (we ceased to learn new things with our interviews).
Results
During the 37 interviews in the practitioner and patient categories, a number of points emerged. Issues contrasting conventional and alternative health care rose to prominence during the interviews. Respondents noted differences of style, cost, training, institutional structure, and philosophy, orientation, and world-view. Vague, subtle, implicit, and subjective differences were described as well as obvious, clear, and objective differences. Among the medley of points that surfaced, a number of issues were raised repeatedly. After discussion and deliberation we organized that data into 4 major thematic categories: holism, empowerment, access, and legitimization. Nearly every idea, talking point, or issue that we noted—several hundred in all—fit comfortably within at least one of these major themes. Some of the more salient ideas and issues are shown in Table 3. A number of points, issues, or subthemes related to more than one of the themes and provided important interconnections or overlaps.
Holism
Patients and practitioners of alternative therapy stressed the importance of a grounded integrated whole-person approach. Patients noted that they preferred to be treated as whole people rather than as composites of numerous biomedical attributes. Practitioners stressed the multidimensional nature of their work and contrasted their integrated approach from what they considered the mechanistic reductionist methods of biomedicine. One practitioner said, “I think conventional medicine tries to fix a problem…rather than get to the heart of the issue.” One patient described it as “healing and staying healthy in a proactive sense.” Another said it was “amazing, your mind and body working together.” The importance of incorporating emotional, physical, psychological, and social factors in both diagnosis and therapy was repeatedly stressed.
Empowerment
Our participants told us that conventional biomedical practitioners often disempower their patients by acting in condescending, disparaging, chauvinistic, or paternalistic manners. In contrast, alternative healers were characterized as facilitating rather than directing the healing process, relying on self-empowerment and personal responsibility for health. One patient said, “I have to be part of the process for it to work.” Another defined conventional medicine as: “You broke it, so let’s fix you.” A third, describing her interaction with conventional physicians said, “And every time I bring it up they blow it off. So I didn’t get very far when I voiced my concerns.” A fourth went so far as to say, “I think the doctors’ way of being is phasing out because people are getting more responsible for their health care.” Most respondents felt that personal empowerment was important for health and told us that alternative healers tend to focus on personal empowerment more than their conventional biomedical counterparts.
Access
The thematic category we called “access” combines issues of insurance coverage with physical, economic, and social availability. As a whole, patients noted that conventional health care was relatively accessible because it was paid for by insurance that was usually available through employment. Additionally, conventional medicine was noted to be physically available in most areas; CAM services were often harder to locate and visit. Alternative medicine was described as expensive from an out-of-pocket perspective and less economically accessible from the patient’s point of view. Several patients noted that they tried conventional methods before moving on to alternative healing. When asked why, one said “I thought I would exhaust the route of things that are free.” Another noted the relative high cost of alternative therapy by saying, “So basically out of sheer monetary restraints, I’ll go back to the physician.” Alternative providers were in consensus that “Most people pay out of pocket…. It is a detriment for people to pay out of pocket.”
Legitimization
Our respondents differentiated between the officially recognized nature of conventional medicine and the less legitimated but increasingly recognized status of CAM. They also differentiated between official or legal legitimacy and legitimacy originating from credible evidence. Practitioners and patients believed in the effectiveness of the modes they used. Referring to the evidence base of alternative medicine, one patient said, “It is just as sound as conventional medicine. It’s just that there haven’t been enough studies yet.” However, one practitioner claimed that “What I teach (and practice) is research based and backed up by studies.” A patient, however, felt that the evidence of effectiveness was often “anecdotal and that doesn’t work in health care.” Both patients and practitioners noted the current lack of standards and felt that standardization (and legitimization) should occur. One said, “They (alternative practitioners) are not under any regulated umbrella, and I think there are a lot of exaggerated claims about what they can do for you.” Another put it more bluntly, “I think there are many quacks out there without review and standards, and people are skeptical of them.”
Discussion
Alternative, complementary, or unconventional therapies are increasingly prevalent throughout the industrialized world.27-33 In the United States, Eisenberg and coworkers estimated that in 1990 approximately 34% of Americans used an unconventional therapy, and made an estimated 425 million visits to alternative practitioners.23 By 1997, those figures had climbed to 42% of Americans using an unconventional therapy, and 629 million visits to CAM providers.34 Other studies12,35-37 provide similar estimates. The number of visits to CAM providers clearly outnumbers the number of visits to primary care physicians, estimated at 388 million in 1997. It is an interesting situation, considering the relatively high (estimated at $27 billion in 1997) out-of-pocket costs of alternative medicine and the greater credibility and legitimacy of conventional medicine. The literature provides few insights. A number of hypotheses have been proposed, but few have been tested.18-20,22 Socioeconomic indicators are mildly predictive at best. There is a slight positive association with education, income, and female gender and a moderate negative association with African American ethnicity, but overall these are poor predictors, with odds ratios ranging from 1.2 to 1.4.19,22,23,34,35 Dissatisfaction with conventional medicine does not seem to significantly predict CAM use. In contrast, most patients who use CAM continue to utilize and appreciate conventional medicine, although they often do not tell their physician about their unconventional choices.
Our research project was designed to begin to map out the attitudinal and behavioral territory of CAM and between alternative and conventional medicine. Both patients and providers distinguished between the socially legitimized and widely accessible but disempowering and mechanistic attributes of conventional medicine and the holistic and empowering but relatively less accessible and less legitimate nature of alternative healing. They believe conventional medicine has legitimate strength in both diagnosis and treatment but often fails to take account of the complexity of the whole person. Many noted that because of its power, conventional medicine can easily do harm. One practitioner described conventional medicine as “like using a boulder to kill an ant.” Another used a velocity metaphor in describing the difference, “Chinese medicine has a top speed of 30 miles per hour and if your disease is going 45 to 50, you need to go to an allopathic (conventional) physician because they can go 120.”
Of the 4 thematic categories identified, access is perhaps the most complex, because it includes health system issues (eg, insurance coverage) and patient-provider communication issues (eg, language, culture, and socioeconomic accessibility). Alternative therapies cost less to society than conventional ones. A person paying out of pocket will receive more provider time per dollar with an alternative practitioner than with most conventional providers. However, for the majority of people in the United States who have health care coverage, conventional medicine costs very little out of pocket, certainly less than the $25 to $50 per hour charged by many alternative providers. So we are left with the contradictory situation in which the direct costs to patients using the relatively expensive official health care system are often less than the price that alternative healers charge. However, for the substantial minority that lack insurance coverage (15% to 20% of the United States), conventional medicine is often beyond their financial reach, perhaps increasing the relative accessibility of alternative medicine.
The incorporation of a more holistic and empowering healing philosophy can be seen as a natural step in the growth of medicine, a step that has already been taken by many persons. This type of healing strategy is consistent with the adoption of the biopsychosocial model, a theory-based health care strategy first proposed by Engel in the 1970s.38-40 In conventional health care, family physicians have perhaps most embraced holism, humanism, and biopsychosocial medicine.41-45 Our results can also be seen as consistent with the behavioral model of health care developed by Andersen and colleagues.46 This behavioral model postulates that choices of health care arise from predisposing characteristics (health beliefs and social structure) interacting with enabling resources (access), as well as health needs.47 The behavioral model has been applied to alternative health care by Kelner and Wellman,21 who after analyzing in-depth interviews with 300 patients concluded that “the choice of type of practitioner(s) is multidimensional and cannot solely be explained either by disenchantment with medicine or by an ‘alternative ideology.’”
People seek either conventional or alternative health care for a variety of reasons, from perceived health need to accessibility to perceived effectiveness.48,49 Astin35 has shown that philosophical orientation—as defined by an interest in personal and spiritual growth and a commitment to environmentalism and feminism—is a significant predictor of use of CAM. In a national representative survey, patients within this defined group of “cultural creatives” were twice as likely to choose CAM therapies than other patients; it was a stronger statistical predictor than either education or global health status. Astin’s conclusions infer that the values of this segment of society have permeated the social matrix enough to influence health care behaviors, leading to the dramatic increase in CAM therapy during the 1990s.
Conclusions
The patients and providers interviewed for our study called for integration of the best aspects of conventional and alternative care. They suggested that the apparent strengths of CAM—especially holism and empowerment—could be used to help improve the quality of conventional health care. We suggest that some ideas and methods from CAM should be investigated and, if found deserving, either adopted or adapted by the official medical system. CAM therapists should be credentialed and incorporated into the health care team and some CAM methods should be learned by conventional providers. Our respondents felt that an integrative effort, if backed by credible evidence and implemented with high-quality standards, could help heal a powerful, but sometimes dangerous, health care system. We feel that this type of integrative effort requires regulation as well as recognition, and should include outcomes-based research. Such an endeavor should be based both on principles and on outcomes data. One such principle was clearly articulated by our respondents: All people should have access to legitimate, holistic, and empowering health care.
METHODS: We conducted semistructured in-depth interviews focused on the knowledge, attitudes, and behaviors of a random sample of 17 patients who had used both CAM and conventional therapies during the past year. Participants were recruited using telephone listings. Twenty alternative practitioners were selected to represent the major modalities. The topics discussed included healing philosophy, choices of therapeutic methods, and ideas concerning concurrent use of differing therapeutic modalities. An 8-member multidisciplinary team analyzed the transcripts individually and in group meetings.
RESULTS: Four major themes emerged from the interview data: (1) holism, (2) empowerment, (3) access, and (4) legitimization. Both patients and providers distinguished between the socially legitimized and widely accessible but disempowering and mechanistic attributes of conventional medicine and the holistic and empowering but relatively less accessible and less legitimate nature of alternative healing. There was a strong call for integrating the best aspects of both.
CONCLUSIONS: Practitioners and users of alternative therapies in the Madison area confirmed both the alternative and complementary natures of unconventional health care, called for more integrated and accessible health care, and provided insights that could be useful in bridging the gap between conventional and alternative medicine.
The use of complementary and alternative medicine (CAM) is on the rise. Various CAM modalities, including acupuncture, Chinese medicine, homeopathy, massage, naturopathy, spiritual healing, and the use of herbal medicines and supplements compete with, provide an alternative to, or complement the more conventional forms of medicine available in hospitals and licensed physicians’ offices. There is an emerging literature on various aspects of this growing phenomenon with hundreds of articles and dozens of texts already on the market.1-7 The Journal of the American Medical Association and associated Archives journals chose alternative medicine for their 1998 theme issue. The boundaries of CAM are being defined and redefined.8-13 A fair amount of research has explored physicians’ attitudes and practices regarding this increasingly prevalent phenomenon.14-18 However, very little is known about how patients think about and choose among the many alternatives or how alternative practitioners situate themselves in this process.19-22 To map out this relatively unknown but clearly expanding social territory we conducted, transcribed, and reviewed 37 in-person semistructured long interviews with providers of alternative therapies and people who use those therapies. Our respondents provided us with coherent and intriguing depictions of the many issues involved. Perhaps most important, our participants highlighted a number of ideas and issues that together form themes defining and demarcating “complementary”, “alternative”, and “conventional” health care.
Methods
Our goal was to investigate the knowledge, attitudes, and practices of patients and providers of complementary and alternative therapies. Eisenberg and colleagues23 defined complementary and alternative medicine as “unconventional.” In their framework, CAM therapies are those that are not taught in US medical schools or widely available in hospitals and licensed physicians’ offices. Our operational definition of CAM was consistent with that definition. Our inclusion criteria were focused on issues of reimbursement. Therapies such as herbal medicine, homeopathy, and mind-body medicine are not generally reimbursable, while most therapies prescribed by physicians are covered by third-party payers. We considered osteopathy and chiropractic as conventional medicine but included acupuncture in the alternative category. These broad definitions were not rigid. Instead, we let patients and providers describe to us their definitions and understandings of “complementary” and “alternative.” CAM therapies represented in our study are listed in Table 1. As a qualitative interview-based study, our research was designed to be exploratory, descriptive, integrative, multirelational, and hypothesis-generating. We followed the standard qualitative research method of formal multidisciplinary review of transcribed in-person long interviews.24-26 Each transcribed interview was reviewed by each member of an 8-person multidisciplinary research team, using a standardized worksheet. Transcriptions were reviewed individually and then discussed in 4 face-to-face group meetings. The research team consisted of an anthropologist and family physician research fellow, a faculty family physician, a biocultural anthropologist, a medical-education nurse and the faculty coordinator for an alternative medicine course for medical students, an alternative psychotherapist, 2 premedical students, and the research assistant interviewer who recently graduated in sociology and women’s studies.
Alternative therapy providers were recruited using a key informant sampling method. We began by generating a near-complete list of alternative providers in the Madison, Wisconsin, area. We used telephone listings, informal interviews with knowledgable informants, and looked for notices and business cards posted at pharmacies, health food stores, and alternative healing centers. Once we felt confident that our list was sufficiently comprehensive (approximately 150 individuals), we sampled the CAM providers to include a wide representation of healing modalities. Following informed consent procedures approved by our institutional review board, 20 healers were recruited and interviewed using a semistructured format aimed at understanding the nature of practice, philosophy of healing, and attitudes and practices with regard to patients’ use of conventional medicine. Examples of the questions asked are listed in Table 2. The interviews were semistructured, and the interviewer was allowed flexibility to explore ideas brought up by the respondent.
We recruited patients randomly using the Madison telephone listings. Our inclusion criteria required that they were aged 18 years or older, used both alternative and conventional medicine in the past year, and were willing to meet for an unpaid hourlong interview. Out of 237 phone numbers randomly chosen from the telephone book, 29 were disconnected, and 58 went unanswered after at least 3 attempts. Of the remaining 150, 25 fit inclusion criteria, 19 agreed to an interview, and 17 were actually interviewed. The location of the interview was open to the participants’ choice and included home, office, and a public place, such as a restaurant or library. The semistructured format allowed free-flowing interviews aimed at probing health beliefs, choices, and practices. We were specifically interested in how patients thought about both alternative and conventional health services, how and why they made their health care choices, and how they personally valued various aspects of CAM and conventional medicine that came to light during the interview. Although our initial goal was to interview 20 patients, we stopped after 17 because we felt we had reached the point of data saturation (we ceased to learn new things with our interviews).
Results
During the 37 interviews in the practitioner and patient categories, a number of points emerged. Issues contrasting conventional and alternative health care rose to prominence during the interviews. Respondents noted differences of style, cost, training, institutional structure, and philosophy, orientation, and world-view. Vague, subtle, implicit, and subjective differences were described as well as obvious, clear, and objective differences. Among the medley of points that surfaced, a number of issues were raised repeatedly. After discussion and deliberation we organized that data into 4 major thematic categories: holism, empowerment, access, and legitimization. Nearly every idea, talking point, or issue that we noted—several hundred in all—fit comfortably within at least one of these major themes. Some of the more salient ideas and issues are shown in Table 3. A number of points, issues, or subthemes related to more than one of the themes and provided important interconnections or overlaps.
Holism
Patients and practitioners of alternative therapy stressed the importance of a grounded integrated whole-person approach. Patients noted that they preferred to be treated as whole people rather than as composites of numerous biomedical attributes. Practitioners stressed the multidimensional nature of their work and contrasted their integrated approach from what they considered the mechanistic reductionist methods of biomedicine. One practitioner said, “I think conventional medicine tries to fix a problem…rather than get to the heart of the issue.” One patient described it as “healing and staying healthy in a proactive sense.” Another said it was “amazing, your mind and body working together.” The importance of incorporating emotional, physical, psychological, and social factors in both diagnosis and therapy was repeatedly stressed.
Empowerment
Our participants told us that conventional biomedical practitioners often disempower their patients by acting in condescending, disparaging, chauvinistic, or paternalistic manners. In contrast, alternative healers were characterized as facilitating rather than directing the healing process, relying on self-empowerment and personal responsibility for health. One patient said, “I have to be part of the process for it to work.” Another defined conventional medicine as: “You broke it, so let’s fix you.” A third, describing her interaction with conventional physicians said, “And every time I bring it up they blow it off. So I didn’t get very far when I voiced my concerns.” A fourth went so far as to say, “I think the doctors’ way of being is phasing out because people are getting more responsible for their health care.” Most respondents felt that personal empowerment was important for health and told us that alternative healers tend to focus on personal empowerment more than their conventional biomedical counterparts.
Access
The thematic category we called “access” combines issues of insurance coverage with physical, economic, and social availability. As a whole, patients noted that conventional health care was relatively accessible because it was paid for by insurance that was usually available through employment. Additionally, conventional medicine was noted to be physically available in most areas; CAM services were often harder to locate and visit. Alternative medicine was described as expensive from an out-of-pocket perspective and less economically accessible from the patient’s point of view. Several patients noted that they tried conventional methods before moving on to alternative healing. When asked why, one said “I thought I would exhaust the route of things that are free.” Another noted the relative high cost of alternative therapy by saying, “So basically out of sheer monetary restraints, I’ll go back to the physician.” Alternative providers were in consensus that “Most people pay out of pocket…. It is a detriment for people to pay out of pocket.”
Legitimization
Our respondents differentiated between the officially recognized nature of conventional medicine and the less legitimated but increasingly recognized status of CAM. They also differentiated between official or legal legitimacy and legitimacy originating from credible evidence. Practitioners and patients believed in the effectiveness of the modes they used. Referring to the evidence base of alternative medicine, one patient said, “It is just as sound as conventional medicine. It’s just that there haven’t been enough studies yet.” However, one practitioner claimed that “What I teach (and practice) is research based and backed up by studies.” A patient, however, felt that the evidence of effectiveness was often “anecdotal and that doesn’t work in health care.” Both patients and practitioners noted the current lack of standards and felt that standardization (and legitimization) should occur. One said, “They (alternative practitioners) are not under any regulated umbrella, and I think there are a lot of exaggerated claims about what they can do for you.” Another put it more bluntly, “I think there are many quacks out there without review and standards, and people are skeptical of them.”
Discussion
Alternative, complementary, or unconventional therapies are increasingly prevalent throughout the industrialized world.27-33 In the United States, Eisenberg and coworkers estimated that in 1990 approximately 34% of Americans used an unconventional therapy, and made an estimated 425 million visits to alternative practitioners.23 By 1997, those figures had climbed to 42% of Americans using an unconventional therapy, and 629 million visits to CAM providers.34 Other studies12,35-37 provide similar estimates. The number of visits to CAM providers clearly outnumbers the number of visits to primary care physicians, estimated at 388 million in 1997. It is an interesting situation, considering the relatively high (estimated at $27 billion in 1997) out-of-pocket costs of alternative medicine and the greater credibility and legitimacy of conventional medicine. The literature provides few insights. A number of hypotheses have been proposed, but few have been tested.18-20,22 Socioeconomic indicators are mildly predictive at best. There is a slight positive association with education, income, and female gender and a moderate negative association with African American ethnicity, but overall these are poor predictors, with odds ratios ranging from 1.2 to 1.4.19,22,23,34,35 Dissatisfaction with conventional medicine does not seem to significantly predict CAM use. In contrast, most patients who use CAM continue to utilize and appreciate conventional medicine, although they often do not tell their physician about their unconventional choices.
Our research project was designed to begin to map out the attitudinal and behavioral territory of CAM and between alternative and conventional medicine. Both patients and providers distinguished between the socially legitimized and widely accessible but disempowering and mechanistic attributes of conventional medicine and the holistic and empowering but relatively less accessible and less legitimate nature of alternative healing. They believe conventional medicine has legitimate strength in both diagnosis and treatment but often fails to take account of the complexity of the whole person. Many noted that because of its power, conventional medicine can easily do harm. One practitioner described conventional medicine as “like using a boulder to kill an ant.” Another used a velocity metaphor in describing the difference, “Chinese medicine has a top speed of 30 miles per hour and if your disease is going 45 to 50, you need to go to an allopathic (conventional) physician because they can go 120.”
Of the 4 thematic categories identified, access is perhaps the most complex, because it includes health system issues (eg, insurance coverage) and patient-provider communication issues (eg, language, culture, and socioeconomic accessibility). Alternative therapies cost less to society than conventional ones. A person paying out of pocket will receive more provider time per dollar with an alternative practitioner than with most conventional providers. However, for the majority of people in the United States who have health care coverage, conventional medicine costs very little out of pocket, certainly less than the $25 to $50 per hour charged by many alternative providers. So we are left with the contradictory situation in which the direct costs to patients using the relatively expensive official health care system are often less than the price that alternative healers charge. However, for the substantial minority that lack insurance coverage (15% to 20% of the United States), conventional medicine is often beyond their financial reach, perhaps increasing the relative accessibility of alternative medicine.
The incorporation of a more holistic and empowering healing philosophy can be seen as a natural step in the growth of medicine, a step that has already been taken by many persons. This type of healing strategy is consistent with the adoption of the biopsychosocial model, a theory-based health care strategy first proposed by Engel in the 1970s.38-40 In conventional health care, family physicians have perhaps most embraced holism, humanism, and biopsychosocial medicine.41-45 Our results can also be seen as consistent with the behavioral model of health care developed by Andersen and colleagues.46 This behavioral model postulates that choices of health care arise from predisposing characteristics (health beliefs and social structure) interacting with enabling resources (access), as well as health needs.47 The behavioral model has been applied to alternative health care by Kelner and Wellman,21 who after analyzing in-depth interviews with 300 patients concluded that “the choice of type of practitioner(s) is multidimensional and cannot solely be explained either by disenchantment with medicine or by an ‘alternative ideology.’”
People seek either conventional or alternative health care for a variety of reasons, from perceived health need to accessibility to perceived effectiveness.48,49 Astin35 has shown that philosophical orientation—as defined by an interest in personal and spiritual growth and a commitment to environmentalism and feminism—is a significant predictor of use of CAM. In a national representative survey, patients within this defined group of “cultural creatives” were twice as likely to choose CAM therapies than other patients; it was a stronger statistical predictor than either education or global health status. Astin’s conclusions infer that the values of this segment of society have permeated the social matrix enough to influence health care behaviors, leading to the dramatic increase in CAM therapy during the 1990s.
Conclusions
The patients and providers interviewed for our study called for integration of the best aspects of conventional and alternative care. They suggested that the apparent strengths of CAM—especially holism and empowerment—could be used to help improve the quality of conventional health care. We suggest that some ideas and methods from CAM should be investigated and, if found deserving, either adopted or adapted by the official medical system. CAM therapists should be credentialed and incorporated into the health care team and some CAM methods should be learned by conventional providers. Our respondents felt that an integrative effort, if backed by credible evidence and implemented with high-quality standards, could help heal a powerful, but sometimes dangerous, health care system. We feel that this type of integrative effort requires regulation as well as recognition, and should include outcomes-based research. Such an endeavor should be based both on principles and on outcomes data. One such principle was clearly articulated by our respondents: All people should have access to legitimate, holistic, and empowering health care.
1. Benjamin SA, Benson H, Gordon JS, Sullivan M. Mind-body medicine: expanding the health model. Patient Care 1997;15:127-45.
2. Cant S, Sharma U. Complementary and alternative medicines: knowledge in practice. New York, NY: Free Association Books; 1996.
3. Cohen MH. Complementary and alternative medicine: legal boundaries and regulatory perspectives. Baltimore, Md: Johns Hopkins University Press; 1998.
4. Lewith G, Kenyon J, Lewis P. Complementary medicine: an integrated approach. Oxford general practice series, no. 34. Oxford, England: Oxford University Press; 1996.
5. Marshall E. The politics of alternative medicine. Science 1994;265:2000-2.
6. National Institutes of Health. Alternative medicine: expanding medical horizons. A report to the National Institutes of Health on alternative medical systems and practices in the United States. Washington, DC: US Government Printing Office; 1992.
7. Wardwell WI. Alternative medicine in the United States. Soc Sci Med 1994;38:1061-8.
8. Abbot NC, White AR, Ernst E. Complementary medicine. Nature 1996;381:361.-
9. Alpert JS. The relativity of alternative medicine. Arch Intern Med 1995;155:2385.-
10. CAM Panel. Defining and describing complementary and alternative medicine: panel consensus following CAM research methodology conference, April 1995. Alternative Ther Health Med 1997;3:49-57.
11. Ernst E. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996.
12. Pavek RR, Trachtenberg AI. Current status of alternative health practices in the United States. Contemp Intern Med 1995;7:61-72.
13. Spencer JW, Jacobs JJ. Complementary/alternative medicine: an evidence-based approach. St. Louis, Mo: Mosby, Inc; 1999.
14. Bernam BM, Singh BK, Lao L, Singh BB, Ferentz KS, Hartnoll SM. Physicians attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract 1995;8:361-6.
15. Borkan J, Neher JO, Anson O, Smoker B. Referrals for alternative therapies. J Fam Pract 1994;39:545-50.
16. Bower H. Double standards exist in judging traditional and alternative medicine. BMJ 1998;316:1694.-
17. Ernst E, Resch K-L, White A. Complementary medicine: what physicians think of it. A meta-analysis. Arch Intern Med 1995;155:2405-8.
18. White AR, Resch K-L, Ernst E. Complementary medicine: use and attitudes among GPs. Fam Pract 1997;14:302-6.
19. Ernst E, Wiloughby M, Weihmayr T. Nine possible reasons for choosing complementary medicine. Perfusion 1995;11:356-9.
20. Furnham A. Why do people choose and use complementary therapies? In: Ernst E, ed. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996;71-88.
21. Kelner M, Wellman B. Health care and consumer choice: medical and alternative therapies. Soc Sci Med 1997;45:203-12.
22. Vincent C, Furnham A. Why do patients turn to complementary medicine? An empirical study. Br Psychological Society 1996;35:37-48.
23. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med 1993;328:246-52.
24. Crabtree BF, Miller WF. Doing qualitative research. Sage publications series on research methods for primary care, volume 3. Thousand Oaks, Calif: Sage Publications; 1992.
25. Denzin NK, Lincoln YS. Handbook of qualitative research. Thousand Oaks, Calif: Sage Publications; 1993.
26. McCracken G. The long interview. Thousand Oaks, Calif: Sage Publications; 1988.
27. Dickinson DPS. The growth of complementary therapies: a consumer-led boom. In Ernst E, ed. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996;150-62.
28. Fisher P, Ward A. Complementary medicine in Europe. BMJ 1994;309:107-10.
29. Gray C. Growing popularity of complementary medicine leads to national organization for MDs. Can Med Assoc J 1997;157:186-8.
30. MacLennan AH, Wilson DH, Taylor AW. Prevalence and cost of alternative medicine in Australia. Lancet 1996;347:569-73.
31. Millar WJ. Use of alternative health care practitioners by Canadians. Can J Public Health 1997;88:154-8.
32. Shenfield GM, Atkin PA, Kristoffersen SS. Alternative medicine: an expanding health industry. Med J Australia 1997;166:516-7.
33. Thomas KJ, Carr J, Westlake L, Williams BT. The use of non-orthodox and conventional health care in Great Britain. BMJ 1991;302:207-10.
34. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA 1998;280:1569-75.
35. Astin JA. Why patients use alternative medicine: results of a national study. JAMA 1998;279:1548-53.
36. Brevoort P. The booming US botanical market: a new overview. HerbalGram 1998;44:33-46.
37. Landmark Healthcare. The Landmark report on public perceptions of alternative care. 1998 nationwide study of alternative care. Random telephone survey of 1500 households. Sacramento, Calif: Landmark Healthcare, Inc; 1998.
38. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129-36.
39. Sadler JZ, Hulgus Y. Knowing, valuing, acting: clues to revising the biopsychosocial model. Compr Psychiatry 1990;31:185-95.
40. Smith KC, Kleinman A. Beyond the biomedical model: integration of psychosocial and cultural orientations. In: Taylor RB, David AK, Johnson TA, eds. Family medicine: principles and practice. New York, NY: Springer-Verlag; 1983.
41. Goldstein M, Sutherland C, Jaffe D, Wilson J. Holistic physicians and family practitioners: similarities, differences and implications for health policy. Soc Sci Med 1988;26:853-61.
42. Kuzel AJ. Naturalistic inquiry: an appropriate model for family medicine. Fam Med 1986;18:369-74.
43. Miller WL. Models of health, illness, and health care. In: Taylor RB, David AK, Johnson TA, eds. Family Medicine: Principles and Practice. New York, NY: Springer-Verlag; 1988.
44. Stephens GG. The intellectual basis of family practice. J Fam Pract 1975;2:423-8.
45. Stephens GG. Family medicine as counter-culture. STFM annual meeting, Denver, Colo. May 9, 1979.
46. Andersen RM. Behavioral model of families’ use of health services. University of Chicago Center for Health Services research series no 250 Chicago, Ill: University of Chicago Press; 1968.
47. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1-10.
48. Campbell S, Roland MO. Why do people consult the doctor? Fam Pract 1996;13:75-83.
49. Zola IK. Pathways to the doctor: from person to patient. Soc Sci Med 1973;7:677-89.
1. Benjamin SA, Benson H, Gordon JS, Sullivan M. Mind-body medicine: expanding the health model. Patient Care 1997;15:127-45.
2. Cant S, Sharma U. Complementary and alternative medicines: knowledge in practice. New York, NY: Free Association Books; 1996.
3. Cohen MH. Complementary and alternative medicine: legal boundaries and regulatory perspectives. Baltimore, Md: Johns Hopkins University Press; 1998.
4. Lewith G, Kenyon J, Lewis P. Complementary medicine: an integrated approach. Oxford general practice series, no. 34. Oxford, England: Oxford University Press; 1996.
5. Marshall E. The politics of alternative medicine. Science 1994;265:2000-2.
6. National Institutes of Health. Alternative medicine: expanding medical horizons. A report to the National Institutes of Health on alternative medical systems and practices in the United States. Washington, DC: US Government Printing Office; 1992.
7. Wardwell WI. Alternative medicine in the United States. Soc Sci Med 1994;38:1061-8.
8. Abbot NC, White AR, Ernst E. Complementary medicine. Nature 1996;381:361.-
9. Alpert JS. The relativity of alternative medicine. Arch Intern Med 1995;155:2385.-
10. CAM Panel. Defining and describing complementary and alternative medicine: panel consensus following CAM research methodology conference, April 1995. Alternative Ther Health Med 1997;3:49-57.
11. Ernst E. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996.
12. Pavek RR, Trachtenberg AI. Current status of alternative health practices in the United States. Contemp Intern Med 1995;7:61-72.
13. Spencer JW, Jacobs JJ. Complementary/alternative medicine: an evidence-based approach. St. Louis, Mo: Mosby, Inc; 1999.
14. Bernam BM, Singh BK, Lao L, Singh BB, Ferentz KS, Hartnoll SM. Physicians attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract 1995;8:361-6.
15. Borkan J, Neher JO, Anson O, Smoker B. Referrals for alternative therapies. J Fam Pract 1994;39:545-50.
16. Bower H. Double standards exist in judging traditional and alternative medicine. BMJ 1998;316:1694.-
17. Ernst E, Resch K-L, White A. Complementary medicine: what physicians think of it. A meta-analysis. Arch Intern Med 1995;155:2405-8.
18. White AR, Resch K-L, Ernst E. Complementary medicine: use and attitudes among GPs. Fam Pract 1997;14:302-6.
19. Ernst E, Wiloughby M, Weihmayr T. Nine possible reasons for choosing complementary medicine. Perfusion 1995;11:356-9.
20. Furnham A. Why do people choose and use complementary therapies? In: Ernst E, ed. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996;71-88.
21. Kelner M, Wellman B. Health care and consumer choice: medical and alternative therapies. Soc Sci Med 1997;45:203-12.
22. Vincent C, Furnham A. Why do patients turn to complementary medicine? An empirical study. Br Psychological Society 1996;35:37-48.
23. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med 1993;328:246-52.
24. Crabtree BF, Miller WF. Doing qualitative research. Sage publications series on research methods for primary care, volume 3. Thousand Oaks, Calif: Sage Publications; 1992.
25. Denzin NK, Lincoln YS. Handbook of qualitative research. Thousand Oaks, Calif: Sage Publications; 1993.
26. McCracken G. The long interview. Thousand Oaks, Calif: Sage Publications; 1988.
27. Dickinson DPS. The growth of complementary therapies: a consumer-led boom. In Ernst E, ed. Complementary medicine: an objective appraisal. Oxford, England: Butterworth-Heinemann; 1996;150-62.
28. Fisher P, Ward A. Complementary medicine in Europe. BMJ 1994;309:107-10.
29. Gray C. Growing popularity of complementary medicine leads to national organization for MDs. Can Med Assoc J 1997;157:186-8.
30. MacLennan AH, Wilson DH, Taylor AW. Prevalence and cost of alternative medicine in Australia. Lancet 1996;347:569-73.
31. Millar WJ. Use of alternative health care practitioners by Canadians. Can J Public Health 1997;88:154-8.
32. Shenfield GM, Atkin PA, Kristoffersen SS. Alternative medicine: an expanding health industry. Med J Australia 1997;166:516-7.
33. Thomas KJ, Carr J, Westlake L, Williams BT. The use of non-orthodox and conventional health care in Great Britain. BMJ 1991;302:207-10.
34. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA 1998;280:1569-75.
35. Astin JA. Why patients use alternative medicine: results of a national study. JAMA 1998;279:1548-53.
36. Brevoort P. The booming US botanical market: a new overview. HerbalGram 1998;44:33-46.
37. Landmark Healthcare. The Landmark report on public perceptions of alternative care. 1998 nationwide study of alternative care. Random telephone survey of 1500 households. Sacramento, Calif: Landmark Healthcare, Inc; 1998.
38. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129-36.
39. Sadler JZ, Hulgus Y. Knowing, valuing, acting: clues to revising the biopsychosocial model. Compr Psychiatry 1990;31:185-95.
40. Smith KC, Kleinman A. Beyond the biomedical model: integration of psychosocial and cultural orientations. In: Taylor RB, David AK, Johnson TA, eds. Family medicine: principles and practice. New York, NY: Springer-Verlag; 1983.
41. Goldstein M, Sutherland C, Jaffe D, Wilson J. Holistic physicians and family practitioners: similarities, differences and implications for health policy. Soc Sci Med 1988;26:853-61.
42. Kuzel AJ. Naturalistic inquiry: an appropriate model for family medicine. Fam Med 1986;18:369-74.
43. Miller WL. Models of health, illness, and health care. In: Taylor RB, David AK, Johnson TA, eds. Family Medicine: Principles and Practice. New York, NY: Springer-Verlag; 1988.
44. Stephens GG. The intellectual basis of family practice. J Fam Pract 1975;2:423-8.
45. Stephens GG. Family medicine as counter-culture. STFM annual meeting, Denver, Colo. May 9, 1979.
46. Andersen RM. Behavioral model of families’ use of health services. University of Chicago Center for Health Services research series no 250 Chicago, Ill: University of Chicago Press; 1968.
47. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1-10.
48. Campbell S, Roland MO. Why do people consult the doctor? Fam Pract 1996;13:75-83.
49. Zola IK. Pathways to the doctor: from person to patient. Soc Sci Med 1973;7:677-89.
Echinacea for Upper Respiratory Infection
SEARCH STRATEGIES: Information from a wide range of sources was used as background material. More than 100 articles, books, and book chapters were reviewed for content and further references. Database searches, bibliographic reviews, and conversations with experts were carried out iteratively from January 1997 to February 1999.
SELECTION CRITERIA: Published or unpublished reports of all blinded placebo-controlled randomized trials of any Echinacea formulation used as a treatment or for the prevention of URIs.
DATA COLLECTION AND ANALYSIS: Review considerations included randomization, blinding, power, validity and clinical relevance of outcome measurements, inclusion and exclusion criteria, indistinguishability of treatment and placebo, and appropriateness of conclusions for the data presented.
MAIN RESULTS: Nine treatment trials and 4 prevention trials fitting the selection criteria were found. Eight of the treatment trials reported generally positive results, and 3 of the prevention trials reported marginal benefit. Methodologic quality of the majority of the trials was modest.
CONCLUSIONS: Evidence from published trials suggests that Echinacea may be beneficial for the early treatment of acute URIs. The influence of publication bias on those results is unknown. Echinacea preparations vary widely in composition, and are often found in combination with other potentially active constituents, making specific dose recommendations problematic. There is very little evidence supporting the prolonged use of Echinacea for the prevention of URIs.
Clinical question
Are orally ingested Echinacea extracts effective in reducing the incidence, severity, or duration of acute upper respiratory infections?
Upper respiratory infection (URI), usually viral, with its common variants rhinosinusitis and pharyngitis, is the highest-incidence acute illness in the developed world.1-3 According to estimates, the average adult in the United States has 2 to 4 colds per year; the average schoolchild has 6 to 10.4 Although patients with complications, such as bacterial sinusitis, otitis media, streptococcal pharyngitis, bronchospasm, or pneumonia may benefit from antibiotic or inhaler treatment, medical science has little to offer for uncomplicated infections.5-10 Nevertheless, antibiotics are frequently prescribed, despite convincing evidence of little or no benefit.11-17 Clearly, there is great need for effective, safe, and affordable treatment.
Botanical extracts from plants of the genus Echinacea are among the most widely used herbal medicines throughout Europe and North America and are most commonly used for the prevention or treatment of URIs. Echinacea extracts are believed to affect URIs through “immunostimulating” activity. Symptom reduction through immunomodulation holds some theoretical and empirical promise.18,19 If effective, such treatment could have an impact on the morbidity and loss of productivity associated with URIs, and the overuse of antibiotics and the effects of their sequelae in terms of costs, adverse effects, and antibiotic resistance.
Background
Echinacea was first used by Native Americans as a remedy for a wide variety of illnesses. It was mentioned in the Flora Virginica in 1762, the Eclectic Dispensatory of the United States of America in 1852, and the National Formulary of the United States from 1916 until 1950.20,21 A 1909 editorial in the Journal of the American Medical Association stated that Echinacea was “deemed unworthy of future consideration,” and it subsequently fell into many decades of disuse in the United States.22 In Europe, however, Echinacea grew in popularity from its introduction in the 1920s to the present. Extracts from the leaves, flowers, and roots of Echinacea purpurea and its cousins E pallida and E angustifolia are currently sold under hundreds of brand names throughout Europe and North America. In Germany, Echinacea has been approved by the German regulatory Commission E for treating respiratory and urinary tract infections.23 More than 3 million physician prescriptions for Echinacea preparations are written each year.24,25 More than 400 scientific studies, mostly German, have detailed Echinacea’s botany, chemistry, pharmacology, and clinical effects.26-29
In the United States, perhaps because of the regulatory climate,30 herbal medicines are usually used without the advice or knowledge of a physician. Although precise estimates of the scope of Echinacea use in the United States are not available, several indicators point toward a large and growing pattern of use. Eisenberg and colleagues,31 using a randomized national telephone survey, estimated that in 1990 34% of Americans had used some type of unconventional medicine, and 10% had seen a provider of herbal therapy. Using the same methods, these researchers32 put the 1997 estimates at 42% and 15%, respectively. Another randomized national telephone survey in 1997 estimated that 17% of Americans used some type of herbal therapy.33 A Gallup poll in 1997 estimated that 32% of Americans used herbal medicines, and a Harris poll in 1998 placed the figure at 37%.34 According to recent market surveys, Americans spend close to $4 billion a year on herbal supplements.34 Several surveys have indicated that Echinacea preparations are the leading botanical medicines in the United States, with close to 10% of the total herbal market.34,35 Given its current popularity and reputation as scientifically justified, Echinacea will likely continue to be widely used.
Echinacea extracts are thought to have immunomodulating pharmacologic activity. Most notably, macrophage activation and enhanced phagocytosis have been reported in a number of studies.36-42 Serum levels of properdin, a member of the complement system, increase after Echinacea administration.43 Increased levels of tumor necrosis factor alpha, interleukins 1, 6, and 10, and of several other cytokines have also been variously reported.44,45 Leukocytosis (especially granulocytes and macrophages) has been variably observed in tissue culture and live animal experiments.43 Echinacea extracts given to mice before an injection with Candida and Listeria species have improved survival rates.46,47 Anti-inflammatory effects have also been reported,48-50 as have antibacterial, antiviral, and antiparasitical activities.43,51 Echinacea’s pharmacologic effects appear to result from a combination of active ingredients rather than from a single agent. Various chemical constituents, including alkamides, caffeic acid derivatives (cicchoric acid), flavonoids, glycoproteins, isobutylamides, polyenes, and polysaccharides, have been identified and implicated as active constituents.52,53 These phytochemicals occur at variable levels among the flowers, leaves, stems, and roots of the 3 medicinal species, E purpurea, E angustifolia, and E pallida.
Methods
The goal of our search strategy was to locate, retrieve, and review the original reports of all blinded randomized trials of Echinacea for the prevention or treatment of acute URI. Throughout 1997 and 1998, we used MEDLINE and other bibliographic reference services to find relevant articles. Searches using variants of the key word “echinacea” were repeated on multiple occasions, covering all years available. More than 100 articles, books, and book chapters were reviewed for content and further references. Herbal medicine experts in the United States and Germany were contacted and questioned concerning their knowledge of published and unpublished controlled trials. All relevant original reports of randomized controlled trials (RCTs) were requested and reviewed in detail. Several of the RCTs we reviewed were not cited in MEDLINE. Retrieval of a few of the older German studies required personal contact with physicians and researchers in Germany, as medical libraries in the United States were unable to locate the studies. Seven of the RCTs were reviewed in the original German by a family physician fluent in the language. Review considerations included randomization, blinding, power, validity and clinical relevance of outcome measurements, inclusion and exclusion criteria, indistinguishability of treatment and placebo, and appropriateness of conclusions for the data presented. Because of dissimilarities in products, methods, and outcome measurements, meta-analysis was not a viable option.
Results
Following the search strategy outlined above, reports of 13 blinded randomized studies were obtained and reviewed Table 1. We found no meta-analyses of Echinacea trials. However, Melchart and colleagues54 reviewed 26 prospective trials (18 randomized, 11 double-blind) testing Echinacea for a variety of indications. Some 30 of 34 reported outcomes in treatment groups were claimed to be superior to controls by the original authors. However, Melchart and coworkers concluded that only 22 of the 34 outcomes were reasonably demonstrated. Further, only 8 of the 26 trials earned 50% or better on the researchers’ quality point-scoring system. Of the 12 URI trials, (6 prevention, 6 treatment), 9 were double-blinded,55-63 but only 5 of these earned more than 50 quality points.55-57,60,63 We reviewed all 9 randomized blinded URI trials identified by Melchart and coworkers, as well as 4 trials conducted subsequently.64-66 Of the 13 trials we reviewed, 9 were treatment trials, and 4 were prevention trials. All studies were randomized and double-blinded. Eight of 9 treatment trials reported benefit. The study reporting no treatment benefit remains unpublished.64 Two of the prevention trials reported marginal benefit.58,60 A third was reported in 1992 to show benefit61—subgroup analyses found statistical significance—but was later reported as largely negative.65 The authors of the fourth study (which we judged to be of the highest quality) found no statistically significant benefit, but noted that a 15% reduction in URI incidence attributable to Echinacea was consistent with their findings.66
Treatment Trials
The most recently reported Echinacea treatment trial was published by Brinkeborn and colleagues66 in 1998. Approximately 119 participants were treated for 8 days with 3 doses of 2 tablets each of Echinaforce, a dried ethanolic extract of E purpurea (95% herb, 5% root). Ten symptoms and the “overall clinical picture” were assessed on a severity scale of 0 to 3 with a physician visit at the beginning of an acute URI (day 1 or 2 of symptoms) and again at day 8. An intention-to-treat analysis showed statistically significant benefit, with an indexed score dropping from 9.0 to 4.1 in the treatment group compared with 8.8 to 5.3 in the placebo group (P = .045). A per-protocol analysis of 87 of the participants yielded highly significant results (P = .007). Construction of the index was not described, and inclusion criteria, exclusion criteria, and verification of randomization and blinding were not properly reported.
Also recently published, and perhaps most convincing in its reported benefit, was the study by Hoheisel and coworkers66 in 1997. This was a double-blind randomized placebo-controlled single-center clinical trial among adult factory workers in Sweden. The 120 participants were recruited at the first sign of URI, but before a full cold had developed. Participants were randomly given either placebo or active drug, and were followed up until symptoms had resolved. The active drug used was Echinagard, also called Echinacin, a commercial preparation made of juice from the above-ground parts of E purpurea. Participants were instructed to take 20 drops every 2 hours for the first day, and 3 times per day thereafter until symptoms resolved. The authors reported that 60% of the placebo groups, but only 40% of the Echinacea group, developed a “real cold.” Among those who had a “real cold,” the median time to resolution was 4 days in the Echinacea group and 8 days in the placebo group. Statistical significance was reached among all reported outcomes in an intention-to-treat analysis. The limitations of this study include: poorly defined inclusion and exclusion criteria, use of retrospectively defined criteria for progression from “first sign of a cold” to “real cold,”65 and lack of evidence of indistinguishability between Echinacea and placebo.
In their 1992 article, Bräunig and coworkers55 reported the results of a randomized double-blind trial of E purpurea root extract among 180 volunteers presenting with recent-onset influenza-like respiratory symptoms. There were 60 participants in each of the 3 groups: placebo, low-dose, and high-dose. The 2 treatment groups received twice daily doses of either 1 dropperful (about 4.5 mL) or 2 dropperfuls (about 9 mL) of juice extracted from E purpurea root. Primary end points were 8 symptoms (cough, sore throat, nasal symptoms, tearing, headache, fatigue, chills or sweats, and muscle aches) and 1 global indicator of severity, all rated on a 0 to 3 scale as either absent, mild, moderate, or severe, with measurements taken at time 0, after 3 to 4 days, and after 8 to 10 days. Although the low-dose regimen showed little improvement over placebo, the higher-dose group showed statistically significant improvement over placebo in several symptom scores, with positive trends in all measurements. Symptom scores in the treated group were 24% to 50% lower in the placebo group at 3 to 4 days, with the gap widening to 36% to 75% at 8 to 10 days. This study is singular in reporting a dose-dependency effect.
In their 1993 article, Bräunig and colleagues56 described the results of a randomized double-blind clinical trial of E pallida root extract among 160 volunteers presenting with influenza-like respiratory infection. The dose used was equivalent to approximately 900 mg per day of dried extract. Symptom assessment was similar to that described above, with a 4-point none-to-severe rating scale assessed at days 3 to 4 and 8 to 10. The median duration of illness in the Echinacea group was 9.8 days, a statistically significant (P <.001) improvement over placebo (13 days). Interestingly, a physician assessment attempted to classify infections as viral or bacterial. A subgroup analysis showed greater benefit among patients with viral infections. White blood counts and differentials were not clearly different between the placebo and verum groups or the viral and bacterial groups.
Dorn’s57 trial consisted of recruiting 100 participants within 2 days of URI onset, and treating with either placebo or Resistan, a commercial preparation made primarily from E angustifolia herb and root, but also containing extracts from Eupatorium perfoliatum, Baptisia, and Arnica. Dosage was 30 cc for the first and second days, followed by 15 cc on the third through sixth days. Outcomes scored on a 0- to 3-point scale (none, mild, moderate, severe) included 7 self-reported symptoms and several physician-documented signs. These were assessed twice, at days 2 to 4 and 6 to 8. Three symptoms (sore throat, nasal drainage, and cough), and 1 sign (pharyngeal erythema), were reported as significantly superior to placebo (P <.01).
Vorberg and Schneider63 reported a treatment trial in 1989 of Resistan among 100 participants suffering from URI. Patients were enrolled in the first 2 days of URI symptoms and randomly treated with either Resistan or an identical placebo. Symptom scores at days 3 and 8 were significantly improved (P < .01) in the treatment group when compared with placebo, with some benefit found in all assessed symptoms. An approximately 20% benefit at day 3 widened to an average 50% reduction at day 8. The authors concluded that there was a clear severity and duration benefit to Echinacea when compared with placebo.
Reitz59 described the results of a trial of Esberitox-N among 150 participants with respiratory infections. Esberitox-N is a commercial preparation containing extracts from E angustifolia and E pallida roots, along with small amounts of Baptisia and Thuja occidentalis extracts. Participants were randomized to treatment or placebo (containing Vitamin C) for 8 weeks and were followed in a double-blinded manner for approximately 1 year. Outcomes measured at 7 and 14 days and monthly thereafter included 8 symptoms, 3 signs, and blood work, including a complete blood cell count and an immunoglobulin measurement. Reitz reported that the majority of symptoms and signs at 7 and 14 days were significantly better in the Esberitox group than with placebo, but provided little statistical analysis to support this claim. Relative improvements in nasal symptoms were noted most prominently. No differences in laboratory measurements were reported.
The 1984 trial by Vorberg62 included 100 participants treated with either vitamin C as placebo or Esberitox. In addition to the ingredients in Esberitox-N, Esberitox contains homeopathic dilutions of Apis, Crotal, Silicea, and Lachesis. Outcomes were self-reported symptoms and physician-reported signs, all assessed on a 0 to 3 scale of severity at days 3 and 10. Headache (P <.001), cough (P <.05), and subfebrile temperature (P <.01) differed significantly, favoring the Esberitox over the vitamin C group. Fatigue, sore throat, difficulty swallowing, nasal drainage, and physician-reported pharyngeal erythema and edema all trended toward benefit in the Esberitox group.
Of the 13 studies we reviewed, the one by Galea and Thacker64 was singular because it reported no measurable benefit, was conducted in North America, and so far remains unpublished. This study treated a total of 190 undergraduate Canadian students with either placebo or a 250-mg capsule preparation of dried E angustifolia 3 times per day. Participants were recruited at first sign of URI and followed up by presence or absence of 8 symptoms for 10 days. No clear trends or statistically significant differences were found between the Echinacea and placebo groups. The relatively low dose and the lack of measures of severity may account for these negative findings.
A treatment trial of a capsulized mixture of dried powder made from the herb (25%) and root (25%) of E purpurea and the root of E angustifolia (50%) is currently under way at the Department of Family Medicine at the University of Wisconsin-Madison. Participants are recruited within 36 hours of first symptoms of URI. Capsulized dried plant material is taken in 1 g doses, 6 times on the first day and 3 times on each subsequent day. Symptom-based outcomes are measured daily using Likert-scale severity measures.
Prevention Trials
Melchart and colleagues68 conducted a 3-arm prevention trial in which 302 volunteers took 50 drops of either placebo or 1 of 2 alcohol extracts from the root of either E angustifolia or E purpurea twice daily for 12 weeks. This was the first head-to-head trial of Echinacea preparations. Median time to onset of first URI was similar among the 3 groups. Compared with placebo, the relative risk of an infection was 0.80 in the E purpurea group and 0.87 in the E angustifolia group. These differences were not statistically significant, hence the null hypothesis that Echinacea is no better than placebo at preventing URI could not be rejected. The authors speculated that a larger study might be able to show an effect, as their study did not have the power to demonstrate a hypothetical 10% to 20% relative risk benefit.
Schöneberger and coworkers61 conducted a trial in which 108 patients “with increased susceptibility to colds” were divided into treatment and placebo groups and followed for 8 weeks. Doses of 4 mL juice from the above-ground parts of E purpurea (Echinacin or Echinagard) were given twice daily for the entire 8-week period. The treatment group had 19 people (35%) without infections compared with 14 (26%) in the placebo group. Average duration of infection was 5.3 days in the treatment group compared with 7.5 days in the placebo group. When infections were grouped into 3 classes according to severity, the treatment group had fewer individuals in all 3 classes (33 vs 34 in mild; 8 vs 13 in moderate; 0 vs 3 in severe). Although these results were not statistically significant, all trends reported were in favor of Echinacea treatment. Grimm and Muller’s 1999 analysis and interpretation of this trial65 was less optimistic than Schöneberger’s original report.
Schmidt and colleagues60 reported a trial of Resistan as prevention of URI among 646 college students at the University of Cologne. Resistan was taken daily for 8 weeks, and students were monitored every 2 weeks and during each URI or flu-like infection. The symptoms assessed on a 0 to 3 scale were cough, sore throat, difficulty swallowing, nasal drainage, congestion, headache, muscle aches, and fatigue. Overall frequency of infection was 15% lower in the Echinacea group than in the placebo group, trending toward statistical significance (P = .08). A subgroup analysis of those patients judged to be especially prone to infection (3 or more colds per year for each of the previous 3 years) showed a statistically significant (P <.05) relative risk reduction in verum (27%) compared with placebo (15%).
Forth and colleagues58 reported a study of 95 patients with URIs randomized to either Esberitox liquid, Esberitox tablet, or identical placebo. Participants took treatments 3 times daily from November until late February, filling out incidence and severity questionnaires every 14 days. A relative risk reduction of 38% (P <.005) was reported for nasal symptoms in the Echinacea tablet group compared with placebo. Other outcomes were reported as similar in all groups.
Data collection for a trial designed to study the efficacy of Echinacea for URI prevention was recently completed at Bastyr University in Seattle, Washington. Subjects who had experienced at least 3 respiratory infections in the 6 months before enrollment were treated with 8 mL E purpurea juice on an intermittent basis over 6 months and were followed in terms of incidence of URI and severity and duration of symptoms. Granolocyte and monocyte phagocytosis differences were assessed using an ex vivo laboratory model. Data analysis is currently under way, with results forthcoming.
Another prevention trial is under way at Oregon Health Sciences University. Explicit methods are not available.
Discussion
In the treatment of acute URI, 8 of 9 randomized trials report some evidence of benefit of Echinacea. Although we attempted to review all trials, including those that were not yet published, we only located 1 unpublished trial, which reported a negative result; therefore, the influence of publication bias remains unknown. Although there is a moderate degree of methodologic deficiency in all of the reviewed studies, and statistical significance is not reached for all outcomes, the published evidence supports the ability of Echinacea to decrease the severity and duration of acute URI. This evidence is not conclusive, however, and higher quality trials are needed. Future trials should include: (1) larger, more representative populations; (2) more precisely defined inclusion and exclusion criteria; (3) more precisely defined objective and validated outcomes measurement; (4) data to verify the inability of participants to distinguish placebo from drug; and (5) better characterization of the active constituents and mechanism of action.
Nevertheless, the current evidence suggests that Echinacea may work as an early treatment for uncomplicated acute URI. Hoheisel and coworkers65 reported a 50% reduction in the proportion of people with first sign of a cold who went on to have a “real cold” (from 60% to 40%). Of those subjects who had a “real cold,” those taking Echinacea had markedly shorter lengths of illness (from a median duration of 8 days to a median duration of 4 days). Brinkeborn and colleagues66 reported a modest but statistically significant reduction in severity in what appears to be a moderately well-designed trial. The study by Dorn57 and the 2 studies by Bräunig and coworkers55,56 reported comparable clinical benefits, with 20% to 50% reductions in severity claimed. We interpret evidence from the highest quality trials to suggest that early dosing is important,67 as is sufficient dosing.55 The clinical significance of expected benefits cannot be precisely estimated.
The evidence for Echinacea’s ability to prevent rather than treat URI is not as promising. Published studies are few, of moderate quality, and report trends rather than statistically significant differences.58,60,61 The most recent and best designed of these prevention trials reported nonsignificant trends toward benefits consistent with a 10% to 20% reduction in incidence.68 We feel that the safety of long-term prophylactic dosing has not been sufficiently demonstrated, at least when valued against uncertain trends toward minor benefit. Neither expected benefits nor risks have been characterized properly, so no recommendations on preventive treatment can be made.
Despite equivocal clinical effects, the safety data on Echinacea are relatively strong, at least when compared with many other herbal medicines. In oral doses greater than 15 g per kg and intravenous doses greater than 5 g per kg, it has proved impossible to kill either a rat or a mouse, hence median lethal dose is so far incalculable.67 Extended (4-week) dosing of rats and mice up to 8 g per kg per day has similarly failed to show adverse effects, with red blood cells, white blood cells, platelets, liver enzymes, creatinine, urea, cholesterol, triglycerides, blood glucose, and body weight as measured end points.69 In a number of mutagenicity studies, no adverse effects were noted. An open-label trial with more than 1000 patients found the following side effects: unpleasant taste (1.7%), nausea or vomiting (0.5%), abdominal pain (0.3%), and diarrhea (0.3%).29 During the years 1989 to 1995, 4 of 13 adverse events reported in association with Echinacea were thought to be causally related by the German authority. When compared with a denominator of several million patient courses, the reported adverse effect rate, and hence the estimated risk, is quite small. Serious allergic or anaphylactic events have been reported, however, so some caution is needed.70
There is currently no universally accepted standardization procedure to ensure comparability among products. Unfortunately, given the apparent multiple chemical nature of Echinacea’s mode of action and the unequal distribution of active constituents in the flowers, leaves, stems, and roots of the 3 medicinal species, it is difficult to determine exactly what kind of standardization would be optimal.71 As there are a number of substances and mechanisms underlying Echinacea’s observed clinical effects, it seems possible that whole-extract dosing might indeed remain preferable to isolation and purification of single chemical entities. Still, as the concentrations of active ingredients are known to vary by species, among roots, leaves, and flowers, and most likely by season, soil type, and climate, and as there is very little research that tests one formulation against another, no recommendations regarding specific Echinacea products can be made.
Recommendations for clinical practice
The use of Echinacea for the early treatment of the common cold can be cautiously supported. More evidence is needed before clear recommendations can be made regarding specific formulations or dosing. Extracts from E purpurea, E angustifolia, and E pallida roots, leaves, and flowers cannot at this point be distinguished from each other in terms of their apparent beneficial activity. If the decision is made to use an Echinacea product, we recommend that it be taken early in the course of a cold, several times per day, and discontinued as symptoms abate. We recommend that Echinacea not be taken routinely, chronically, or on a preventive basis. We note that no trials have included infants, children, or pregnant women, and recommend caution among those populations. We also note the theoretical contraindication among persons suffering from serious autoimmune disorders.
At the present time we conclude that the evidence suggests Echinacea taken early in the course of an illness may be safe and effective in reducing the severity and duration of the common cold. The evidence of Echinacea’s ability to prevent infection is inadequate to make any recommendations in this regard.
1. Campbell H. Acute respiratory infection: a global challenge. Arch Dis Childhood 1995;73:281-3.
2. Murray CJL, Lopez AD. The global burden of disease. Cambridge, Mass: Harvard University Press, 1996.
3. Oh HM. Upper respiratory tract infections—otitis media, sinusitis and pharyngitis. Singapore Med J 1995;36:428-31.
4. Spector SL. The common cold: current therapy and natural history. J Allergy Clin Immunol 1995;95:1133-8.
5. De Lange de Klerk ESM, Blommers JKDJ, Bezemer PD, Feenstra L. Effects of homeopathic medicines on daily burden of symptoms in children with recurrent upper respiratory tract infections. BMJ 1994;309:1329-32.
6. Fireman P. Pathophysiology and pharmacotherapy of common upper respiratory diseases. Pharmacotherapy 1993;3:101S-9S.
7. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-40.
8. McMillan JA. Upper respiratory tract infections in children. Current Opin Pediatr 1993;5:50-4.
9. Monto AS. Viral respiratory infections in the community: epidemiology, agents, and interventions. Am J Med 1995;99:24S-7S.
10. Walker TA, Khurana S, Tilden SJ. Viral respiratory infections. Pediatr Clin North Am 1995;41:1365-81.
11. Dere WH. Acute bronchitis: results of US and European trials of antibiotic therapy. Am J Med 1992;92:53S-7S.
12. Fahey T, Stocks N, Thomas T. Quantitative systematic review of randomized controlled trials comparing antibiotic with placebo for acute cough in adults. BMJ 1998;316:906-10.
13. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901-4.
14. Mainous AG, III, Hueston WJ, Clark JR. Antibiotics and upper respiratory infection: do some folks think there is a cure for the common cold? J Fam Pract 1996;42:357-61.
15. Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA 1998;279:875-7.
16. Oeffinger KC, Snell LM, Foster BM, Panico KG, Archer RK. Diagnosis of acute bronchitis in adults: a national survey of family physicians. J Fam Pract 1997;45:402-9.
17. Orr PH, Scherer K, Macdonald A, Moffatt MEK. Randomized placebo-controlled trials of antibiotics for acute bronchitis: a critical review of the literature. J Fam Pract 1993;36:507-12.
18. Bergmann KC. Assessment of the clinical value of using an immunomodulator in recurrent respiratory infections. Adv Exp Med Biol 1995;371B:795-7.
19. Riedl-Seifert RJ, van Aubel A, Kammereit A, Elsasser U. Reduction of the number and severity of respiratory tract infections in children by oral immunostimulation. Adv Exp Med Biol 1995;371B:799-802.
20. Foster S. Echinacea: nature’s immune enhancer. Rochester, Vt: Healing Arts Press, 1991.
21. Hobbs C. Echinacea: a literature review. HerbalGram 1994;30(suppl):33-47.
22. Puckner WA. Echinacea considered valueless: report of the council on pharmacy and chemistry. JAMA 1909;1836.-
23. Blumenthal M, Goldberg A, Gruenwald J, et al. German Commission E monographs: therapeutic monographs on medicinal plants for human use. Austin, Tex: American Botanical Council, 1998.
24. Keller K. Legal requirements for the use of phytopharmaceutical drugs in the Federal Republic of Germany. J Ethnopharmacol 1991;32:225-9.
25. Tyler VE. Phytomedicines in Western Europe: their potential impact on herbal medicine in the United States. HerbalGram 1994;30:24-31.
26. Awang DVC, Kindack DG. Herbal medicine: Echinacea. Can Pharma J 1991;124:512-6.
27. Foster S. Echinacea: the purple coneflowers. Austin, Tex: American Botanical Council, 1996.
28. Hobbs C. Echinacea: the immune herb! Santa Cruz, Calif: Botanica Press, 1995.
29. Parnham MJ. Benefit-risk assessment of the squeezed sap of the purple coneflower (Echinacea purpurea) for long-term oral immunostimulation. Phytomedicine 1996;3:95-102.
30. Cohen MH. Complementary and alternative medicine: legal boundaries and regulatory perspectives. Baltimore, Md: Johns Hopkins University Press, 1998.
31. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med 1993;328:246-52.
32. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA 1998;280:1569-75.
33. Landmark Healthcare. The Landmark report on public perceptions of alternative care: 1998 nationwide study of alternative care. Random telephone survey of 1500 households. Sacramento, Calif: Landmark Healthcare, Inc, 1998.
34. Brevoort P. The booming US botanical market: a new overview. HerbalGram 1998;44:33-46.
35. Brevoort P. The US botanical market: an overview. HerbalGram 1996;36:49-57.
36. Bukovsky M, Vaverkova S, Kostalova D. Immunomodulating activity of Echinacea gloriosa L, Echinacea angustifolia DC, and Rudbeckia speciosa Wenderoth ethanol-water extracts. Pol J Pharmacol 1995;47:175-7.
37. Coeugniet EG, Elek E. Immunomodulation with Viscum album and Echinacea purpurea extracts. Onkologie 1987;10 (suppl):27-33.
38. Lersch C, Zeuner M, Bauer A, et al. Stimulation of the immune response in outpatients with hepatocellular carcinomas by low doses of cyclophosphamide (LDCY), Echinacea purpurea extracts (Echinacin) and thymostimulin. Arch Geschwulstforsch 1990;60:379-83.
39. Lersch C, Zeuner M, Bauer A, et al. Nonspecific immunomodulation with low doses of cyclophosphamide (LDCY), thymostimulin, and Echinacea purpurea extracts (Echinacin) in patients with far advanced colorectal cancers. Cancer Invest 1992;10:343-8.
40. Luettig B, Steinmuller C, Gifford GE, Wagner H, Lohman-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst 1989;
41. Melchart D, Linde K, Worku F, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J Altern Complement Med 1995;1:145-60.
42. Stimpel M. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun 1984;46:845-9.
43. Bauer R, Wagner H. Echinacea species as potential immunostimulatory drugs. In: Wagner H, Farnsworth NR, eds. Economic and medicinal plant research. New York, NY: Academic Press Limited, 1991.
44. Burger RA, Torres AR, Warren RP, Caldwell VD, Hughes BG. Echinacea-induced cytokine production by human macrophages. Int J Immunopharmacol 1997;19:371-9.
45. Elsasser-Beile U, Willenbacher W, Bartsch HH, Gallati H, Schulte Monting J, von Kleist S. Cytokine production in leukocyte cultures during therapy with Echinacea extract. J Clin Lab Anal 1996;10:441-5.
46. Roesler J, Steinmuller C, Kiderlen A, Emmendorffer A, Wagner H, Lohmann ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int J Immunopharmacol 1991;13:27-37.
47. Steinmuller C, Roesler J, Grottrup E, Franke G, Wagner H, Lohmann-Matth M. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria. Int J Immunopharmacol 1993;15:605-14.
48. Tragni E. Evidence from two classic irritation tests for an anti-inflammatory action of a natural extract, Echinacea. Food Chem Toxicol 1985;23:317-9.
49. Tragni E, Galli CL, Tubaro A, del Negro P, Della Loggia R. Anti-inflammatory activity of Echinacea angustifolia fractions separated on the basis of molecular weight. Pharmaceut Res Comm 1998;20(suppl):87-91.
50. Tubaro A, Tragni E, del Negro P, Galli CL, Della Loggia R. Anti-inflammatory activity of a polysaccharide fraction of Echinacea angustifolia. J Pharm Pharmacol 1987;39:567-9.
51. Wacker A. Virus inhibition by Echinacea purpurea. Planta Medica 1978;33:89-102.
52. Bauer R. Echinacea: biological effects and active principles. In: Lawson LD, Bauer R, eds. Phytomedicines of Europe: chemistry and biological activity. Washington, DC: American Chemists Society 1998;140-157.
53. Bone K. Echinacea: what makes it work? Altern Med Rev 1997;2:87-9.
54. Melchart D, Linde K, Worku F, Bauer R, Wagner H. Immunomodulation with Echinacea: a systematic review of controlled trials. Phytomedicine 1994;1:245-54.
55. Bräunig B, Dorn M, Limburg E, Knick E. Bausendorf: Echinaceae purpureae radix: zur Stärkung der körpereigenen Abwehr bei grippalen Infekten. Zeitschrift fur Phytotherapie 1992;13:7-13.
56. Bräunig B, Knick E. Therapeutische Erfahrungen mit Echinaceae pallidae bei grippalen Infekten. Naturheilpraxis 1993;1:72-5.
57. Dorn M. Milerung grippaler Effeckte durch ein pflanzliches Immunstimulans. Nutur Ganzheitsmedizin 1989;2:314-9.
58. Forth H, Beuscher N. Beeinflussing der Häufigkeit banaler Erkältungsinfekte durch Esberitox. Zeitschrift für Allgemeinmedizin 1981;57:2272-5.
59. Reitz HD. Immunmodulatoren mit pflanzlichen Wirkstoffen: eine wissenschaftliche Studie am Beispiel Esberitox N. Notabene medici 1990;20:362-6.
60. Schmidt U, Albrecht M, Schenk N. Pflankliches Immunstimulans senkt Häufigkeit grippaler Infekte: Plazebokontrollierte Doppelblindstudie mit einem kombinierten Echinacea-Präparat mit 646 Studenten der Kölner Universität. Natur Ganzheits Medizin 1990;3:277-81.
61. Schöneberger D. Einfluß der immunstimulierenden Wirkung von Preßsaft aus Herba Echinaceae purpureae auf Verlauf und Schweregrad von Erkältungskrankheiten. Ergebnisse einer Doppelblindstudie. Forum Immunologie 1992;2:18-22.
62. Vorberg G. Bei Erkältung unspezifische Immunabwehr stimulieran: Doppelblindstudie zeigt: Das bewährte Phytotherapeutikum Esberitox verkürzt die Symptommatik. Ärztliche Praxis 1984;36:97-8.
63. Vorberg G, Schneider B. Pflanzliches Immunstimulans verkürzt grippalen Infeckt. Doppelblindstudie belegt die Steigerung der unspezifischen Infektabwehr. Ärztliche Forschung 1989;36:3-8.
64. Galea S, Thacker K. Double-blind prospective trial investigating the effectiveness of a commonly prescribed herbal remedy in altering the duration, severity and symptoms of the common cold. Unpublished manuscript, 1996.
65. Grimm W, Muller HH. A randomized controlled trail of the effect of fluid extract of Echinacea purpurea on incidence and severity of colds and respiratory infections. Am J Med 1999;106:138-144.
66. Brinkeborn R, Shah D, Geissbühler S, Degenring FH. Echinaforce in the treatment of acute colds. Schweiz Zschr GanzheitsMedizin 1998;10:26-9.
67. Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schäfer M. Echinagard treatment shortens the course of the common cold: a double-blind, placebo-controlled clinical trial. Eur J Clin Res 1997;9:261-8.
68. Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med 1998;7:541-5.
69. Mengs U, Clare CB, Poiley JA. Toxicity of Echinacea pur
70. Mullins RJ. Echinacea-associated anaphylaxis Med J Aust 1998;168:170-1.
71. Reichling J, Saller R. Control in the manufacturing of modern herbal remedies. Q Rev Nat Med 1998;Spring:21-8.
SEARCH STRATEGIES: Information from a wide range of sources was used as background material. More than 100 articles, books, and book chapters were reviewed for content and further references. Database searches, bibliographic reviews, and conversations with experts were carried out iteratively from January 1997 to February 1999.
SELECTION CRITERIA: Published or unpublished reports of all blinded placebo-controlled randomized trials of any Echinacea formulation used as a treatment or for the prevention of URIs.
DATA COLLECTION AND ANALYSIS: Review considerations included randomization, blinding, power, validity and clinical relevance of outcome measurements, inclusion and exclusion criteria, indistinguishability of treatment and placebo, and appropriateness of conclusions for the data presented.
MAIN RESULTS: Nine treatment trials and 4 prevention trials fitting the selection criteria were found. Eight of the treatment trials reported generally positive results, and 3 of the prevention trials reported marginal benefit. Methodologic quality of the majority of the trials was modest.
CONCLUSIONS: Evidence from published trials suggests that Echinacea may be beneficial for the early treatment of acute URIs. The influence of publication bias on those results is unknown. Echinacea preparations vary widely in composition, and are often found in combination with other potentially active constituents, making specific dose recommendations problematic. There is very little evidence supporting the prolonged use of Echinacea for the prevention of URIs.
Clinical question
Are orally ingested Echinacea extracts effective in reducing the incidence, severity, or duration of acute upper respiratory infections?
Upper respiratory infection (URI), usually viral, with its common variants rhinosinusitis and pharyngitis, is the highest-incidence acute illness in the developed world.1-3 According to estimates, the average adult in the United States has 2 to 4 colds per year; the average schoolchild has 6 to 10.4 Although patients with complications, such as bacterial sinusitis, otitis media, streptococcal pharyngitis, bronchospasm, or pneumonia may benefit from antibiotic or inhaler treatment, medical science has little to offer for uncomplicated infections.5-10 Nevertheless, antibiotics are frequently prescribed, despite convincing evidence of little or no benefit.11-17 Clearly, there is great need for effective, safe, and affordable treatment.
Botanical extracts from plants of the genus Echinacea are among the most widely used herbal medicines throughout Europe and North America and are most commonly used for the prevention or treatment of URIs. Echinacea extracts are believed to affect URIs through “immunostimulating” activity. Symptom reduction through immunomodulation holds some theoretical and empirical promise.18,19 If effective, such treatment could have an impact on the morbidity and loss of productivity associated with URIs, and the overuse of antibiotics and the effects of their sequelae in terms of costs, adverse effects, and antibiotic resistance.
Background
Echinacea was first used by Native Americans as a remedy for a wide variety of illnesses. It was mentioned in the Flora Virginica in 1762, the Eclectic Dispensatory of the United States of America in 1852, and the National Formulary of the United States from 1916 until 1950.20,21 A 1909 editorial in the Journal of the American Medical Association stated that Echinacea was “deemed unworthy of future consideration,” and it subsequently fell into many decades of disuse in the United States.22 In Europe, however, Echinacea grew in popularity from its introduction in the 1920s to the present. Extracts from the leaves, flowers, and roots of Echinacea purpurea and its cousins E pallida and E angustifolia are currently sold under hundreds of brand names throughout Europe and North America. In Germany, Echinacea has been approved by the German regulatory Commission E for treating respiratory and urinary tract infections.23 More than 3 million physician prescriptions for Echinacea preparations are written each year.24,25 More than 400 scientific studies, mostly German, have detailed Echinacea’s botany, chemistry, pharmacology, and clinical effects.26-29
In the United States, perhaps because of the regulatory climate,30 herbal medicines are usually used without the advice or knowledge of a physician. Although precise estimates of the scope of Echinacea use in the United States are not available, several indicators point toward a large and growing pattern of use. Eisenberg and colleagues,31 using a randomized national telephone survey, estimated that in 1990 34% of Americans had used some type of unconventional medicine, and 10% had seen a provider of herbal therapy. Using the same methods, these researchers32 put the 1997 estimates at 42% and 15%, respectively. Another randomized national telephone survey in 1997 estimated that 17% of Americans used some type of herbal therapy.33 A Gallup poll in 1997 estimated that 32% of Americans used herbal medicines, and a Harris poll in 1998 placed the figure at 37%.34 According to recent market surveys, Americans spend close to $4 billion a year on herbal supplements.34 Several surveys have indicated that Echinacea preparations are the leading botanical medicines in the United States, with close to 10% of the total herbal market.34,35 Given its current popularity and reputation as scientifically justified, Echinacea will likely continue to be widely used.
Echinacea extracts are thought to have immunomodulating pharmacologic activity. Most notably, macrophage activation and enhanced phagocytosis have been reported in a number of studies.36-42 Serum levels of properdin, a member of the complement system, increase after Echinacea administration.43 Increased levels of tumor necrosis factor alpha, interleukins 1, 6, and 10, and of several other cytokines have also been variously reported.44,45 Leukocytosis (especially granulocytes and macrophages) has been variably observed in tissue culture and live animal experiments.43 Echinacea extracts given to mice before an injection with Candida and Listeria species have improved survival rates.46,47 Anti-inflammatory effects have also been reported,48-50 as have antibacterial, antiviral, and antiparasitical activities.43,51 Echinacea’s pharmacologic effects appear to result from a combination of active ingredients rather than from a single agent. Various chemical constituents, including alkamides, caffeic acid derivatives (cicchoric acid), flavonoids, glycoproteins, isobutylamides, polyenes, and polysaccharides, have been identified and implicated as active constituents.52,53 These phytochemicals occur at variable levels among the flowers, leaves, stems, and roots of the 3 medicinal species, E purpurea, E angustifolia, and E pallida.
Methods
The goal of our search strategy was to locate, retrieve, and review the original reports of all blinded randomized trials of Echinacea for the prevention or treatment of acute URI. Throughout 1997 and 1998, we used MEDLINE and other bibliographic reference services to find relevant articles. Searches using variants of the key word “echinacea” were repeated on multiple occasions, covering all years available. More than 100 articles, books, and book chapters were reviewed for content and further references. Herbal medicine experts in the United States and Germany were contacted and questioned concerning their knowledge of published and unpublished controlled trials. All relevant original reports of randomized controlled trials (RCTs) were requested and reviewed in detail. Several of the RCTs we reviewed were not cited in MEDLINE. Retrieval of a few of the older German studies required personal contact with physicians and researchers in Germany, as medical libraries in the United States were unable to locate the studies. Seven of the RCTs were reviewed in the original German by a family physician fluent in the language. Review considerations included randomization, blinding, power, validity and clinical relevance of outcome measurements, inclusion and exclusion criteria, indistinguishability of treatment and placebo, and appropriateness of conclusions for the data presented. Because of dissimilarities in products, methods, and outcome measurements, meta-analysis was not a viable option.
Results
Following the search strategy outlined above, reports of 13 blinded randomized studies were obtained and reviewed Table 1. We found no meta-analyses of Echinacea trials. However, Melchart and colleagues54 reviewed 26 prospective trials (18 randomized, 11 double-blind) testing Echinacea for a variety of indications. Some 30 of 34 reported outcomes in treatment groups were claimed to be superior to controls by the original authors. However, Melchart and coworkers concluded that only 22 of the 34 outcomes were reasonably demonstrated. Further, only 8 of the 26 trials earned 50% or better on the researchers’ quality point-scoring system. Of the 12 URI trials, (6 prevention, 6 treatment), 9 were double-blinded,55-63 but only 5 of these earned more than 50 quality points.55-57,60,63 We reviewed all 9 randomized blinded URI trials identified by Melchart and coworkers, as well as 4 trials conducted subsequently.64-66 Of the 13 trials we reviewed, 9 were treatment trials, and 4 were prevention trials. All studies were randomized and double-blinded. Eight of 9 treatment trials reported benefit. The study reporting no treatment benefit remains unpublished.64 Two of the prevention trials reported marginal benefit.58,60 A third was reported in 1992 to show benefit61—subgroup analyses found statistical significance—but was later reported as largely negative.65 The authors of the fourth study (which we judged to be of the highest quality) found no statistically significant benefit, but noted that a 15% reduction in URI incidence attributable to Echinacea was consistent with their findings.66
Treatment Trials
The most recently reported Echinacea treatment trial was published by Brinkeborn and colleagues66 in 1998. Approximately 119 participants were treated for 8 days with 3 doses of 2 tablets each of Echinaforce, a dried ethanolic extract of E purpurea (95% herb, 5% root). Ten symptoms and the “overall clinical picture” were assessed on a severity scale of 0 to 3 with a physician visit at the beginning of an acute URI (day 1 or 2 of symptoms) and again at day 8. An intention-to-treat analysis showed statistically significant benefit, with an indexed score dropping from 9.0 to 4.1 in the treatment group compared with 8.8 to 5.3 in the placebo group (P = .045). A per-protocol analysis of 87 of the participants yielded highly significant results (P = .007). Construction of the index was not described, and inclusion criteria, exclusion criteria, and verification of randomization and blinding were not properly reported.
Also recently published, and perhaps most convincing in its reported benefit, was the study by Hoheisel and coworkers66 in 1997. This was a double-blind randomized placebo-controlled single-center clinical trial among adult factory workers in Sweden. The 120 participants were recruited at the first sign of URI, but before a full cold had developed. Participants were randomly given either placebo or active drug, and were followed up until symptoms had resolved. The active drug used was Echinagard, also called Echinacin, a commercial preparation made of juice from the above-ground parts of E purpurea. Participants were instructed to take 20 drops every 2 hours for the first day, and 3 times per day thereafter until symptoms resolved. The authors reported that 60% of the placebo groups, but only 40% of the Echinacea group, developed a “real cold.” Among those who had a “real cold,” the median time to resolution was 4 days in the Echinacea group and 8 days in the placebo group. Statistical significance was reached among all reported outcomes in an intention-to-treat analysis. The limitations of this study include: poorly defined inclusion and exclusion criteria, use of retrospectively defined criteria for progression from “first sign of a cold” to “real cold,”65 and lack of evidence of indistinguishability between Echinacea and placebo.
In their 1992 article, Bräunig and coworkers55 reported the results of a randomized double-blind trial of E purpurea root extract among 180 volunteers presenting with recent-onset influenza-like respiratory symptoms. There were 60 participants in each of the 3 groups: placebo, low-dose, and high-dose. The 2 treatment groups received twice daily doses of either 1 dropperful (about 4.5 mL) or 2 dropperfuls (about 9 mL) of juice extracted from E purpurea root. Primary end points were 8 symptoms (cough, sore throat, nasal symptoms, tearing, headache, fatigue, chills or sweats, and muscle aches) and 1 global indicator of severity, all rated on a 0 to 3 scale as either absent, mild, moderate, or severe, with measurements taken at time 0, after 3 to 4 days, and after 8 to 10 days. Although the low-dose regimen showed little improvement over placebo, the higher-dose group showed statistically significant improvement over placebo in several symptom scores, with positive trends in all measurements. Symptom scores in the treated group were 24% to 50% lower in the placebo group at 3 to 4 days, with the gap widening to 36% to 75% at 8 to 10 days. This study is singular in reporting a dose-dependency effect.
In their 1993 article, Bräunig and colleagues56 described the results of a randomized double-blind clinical trial of E pallida root extract among 160 volunteers presenting with influenza-like respiratory infection. The dose used was equivalent to approximately 900 mg per day of dried extract. Symptom assessment was similar to that described above, with a 4-point none-to-severe rating scale assessed at days 3 to 4 and 8 to 10. The median duration of illness in the Echinacea group was 9.8 days, a statistically significant (P <.001) improvement over placebo (13 days). Interestingly, a physician assessment attempted to classify infections as viral or bacterial. A subgroup analysis showed greater benefit among patients with viral infections. White blood counts and differentials were not clearly different between the placebo and verum groups or the viral and bacterial groups.
Dorn’s57 trial consisted of recruiting 100 participants within 2 days of URI onset, and treating with either placebo or Resistan, a commercial preparation made primarily from E angustifolia herb and root, but also containing extracts from Eupatorium perfoliatum, Baptisia, and Arnica. Dosage was 30 cc for the first and second days, followed by 15 cc on the third through sixth days. Outcomes scored on a 0- to 3-point scale (none, mild, moderate, severe) included 7 self-reported symptoms and several physician-documented signs. These were assessed twice, at days 2 to 4 and 6 to 8. Three symptoms (sore throat, nasal drainage, and cough), and 1 sign (pharyngeal erythema), were reported as significantly superior to placebo (P <.01).
Vorberg and Schneider63 reported a treatment trial in 1989 of Resistan among 100 participants suffering from URI. Patients were enrolled in the first 2 days of URI symptoms and randomly treated with either Resistan or an identical placebo. Symptom scores at days 3 and 8 were significantly improved (P < .01) in the treatment group when compared with placebo, with some benefit found in all assessed symptoms. An approximately 20% benefit at day 3 widened to an average 50% reduction at day 8. The authors concluded that there was a clear severity and duration benefit to Echinacea when compared with placebo.
Reitz59 described the results of a trial of Esberitox-N among 150 participants with respiratory infections. Esberitox-N is a commercial preparation containing extracts from E angustifolia and E pallida roots, along with small amounts of Baptisia and Thuja occidentalis extracts. Participants were randomized to treatment or placebo (containing Vitamin C) for 8 weeks and were followed in a double-blinded manner for approximately 1 year. Outcomes measured at 7 and 14 days and monthly thereafter included 8 symptoms, 3 signs, and blood work, including a complete blood cell count and an immunoglobulin measurement. Reitz reported that the majority of symptoms and signs at 7 and 14 days were significantly better in the Esberitox group than with placebo, but provided little statistical analysis to support this claim. Relative improvements in nasal symptoms were noted most prominently. No differences in laboratory measurements were reported.
The 1984 trial by Vorberg62 included 100 participants treated with either vitamin C as placebo or Esberitox. In addition to the ingredients in Esberitox-N, Esberitox contains homeopathic dilutions of Apis, Crotal, Silicea, and Lachesis. Outcomes were self-reported symptoms and physician-reported signs, all assessed on a 0 to 3 scale of severity at days 3 and 10. Headache (P <.001), cough (P <.05), and subfebrile temperature (P <.01) differed significantly, favoring the Esberitox over the vitamin C group. Fatigue, sore throat, difficulty swallowing, nasal drainage, and physician-reported pharyngeal erythema and edema all trended toward benefit in the Esberitox group.
Of the 13 studies we reviewed, the one by Galea and Thacker64 was singular because it reported no measurable benefit, was conducted in North America, and so far remains unpublished. This study treated a total of 190 undergraduate Canadian students with either placebo or a 250-mg capsule preparation of dried E angustifolia 3 times per day. Participants were recruited at first sign of URI and followed up by presence or absence of 8 symptoms for 10 days. No clear trends or statistically significant differences were found between the Echinacea and placebo groups. The relatively low dose and the lack of measures of severity may account for these negative findings.
A treatment trial of a capsulized mixture of dried powder made from the herb (25%) and root (25%) of E purpurea and the root of E angustifolia (50%) is currently under way at the Department of Family Medicine at the University of Wisconsin-Madison. Participants are recruited within 36 hours of first symptoms of URI. Capsulized dried plant material is taken in 1 g doses, 6 times on the first day and 3 times on each subsequent day. Symptom-based outcomes are measured daily using Likert-scale severity measures.
Prevention Trials
Melchart and colleagues68 conducted a 3-arm prevention trial in which 302 volunteers took 50 drops of either placebo or 1 of 2 alcohol extracts from the root of either E angustifolia or E purpurea twice daily for 12 weeks. This was the first head-to-head trial of Echinacea preparations. Median time to onset of first URI was similar among the 3 groups. Compared with placebo, the relative risk of an infection was 0.80 in the E purpurea group and 0.87 in the E angustifolia group. These differences were not statistically significant, hence the null hypothesis that Echinacea is no better than placebo at preventing URI could not be rejected. The authors speculated that a larger study might be able to show an effect, as their study did not have the power to demonstrate a hypothetical 10% to 20% relative risk benefit.
Schöneberger and coworkers61 conducted a trial in which 108 patients “with increased susceptibility to colds” were divided into treatment and placebo groups and followed for 8 weeks. Doses of 4 mL juice from the above-ground parts of E purpurea (Echinacin or Echinagard) were given twice daily for the entire 8-week period. The treatment group had 19 people (35%) without infections compared with 14 (26%) in the placebo group. Average duration of infection was 5.3 days in the treatment group compared with 7.5 days in the placebo group. When infections were grouped into 3 classes according to severity, the treatment group had fewer individuals in all 3 classes (33 vs 34 in mild; 8 vs 13 in moderate; 0 vs 3 in severe). Although these results were not statistically significant, all trends reported were in favor of Echinacea treatment. Grimm and Muller’s 1999 analysis and interpretation of this trial65 was less optimistic than Schöneberger’s original report.
Schmidt and colleagues60 reported a trial of Resistan as prevention of URI among 646 college students at the University of Cologne. Resistan was taken daily for 8 weeks, and students were monitored every 2 weeks and during each URI or flu-like infection. The symptoms assessed on a 0 to 3 scale were cough, sore throat, difficulty swallowing, nasal drainage, congestion, headache, muscle aches, and fatigue. Overall frequency of infection was 15% lower in the Echinacea group than in the placebo group, trending toward statistical significance (P = .08). A subgroup analysis of those patients judged to be especially prone to infection (3 or more colds per year for each of the previous 3 years) showed a statistically significant (P <.05) relative risk reduction in verum (27%) compared with placebo (15%).
Forth and colleagues58 reported a study of 95 patients with URIs randomized to either Esberitox liquid, Esberitox tablet, or identical placebo. Participants took treatments 3 times daily from November until late February, filling out incidence and severity questionnaires every 14 days. A relative risk reduction of 38% (P <.005) was reported for nasal symptoms in the Echinacea tablet group compared with placebo. Other outcomes were reported as similar in all groups.
Data collection for a trial designed to study the efficacy of Echinacea for URI prevention was recently completed at Bastyr University in Seattle, Washington. Subjects who had experienced at least 3 respiratory infections in the 6 months before enrollment were treated with 8 mL E purpurea juice on an intermittent basis over 6 months and were followed in terms of incidence of URI and severity and duration of symptoms. Granolocyte and monocyte phagocytosis differences were assessed using an ex vivo laboratory model. Data analysis is currently under way, with results forthcoming.
Another prevention trial is under way at Oregon Health Sciences University. Explicit methods are not available.
Discussion
In the treatment of acute URI, 8 of 9 randomized trials report some evidence of benefit of Echinacea. Although we attempted to review all trials, including those that were not yet published, we only located 1 unpublished trial, which reported a negative result; therefore, the influence of publication bias remains unknown. Although there is a moderate degree of methodologic deficiency in all of the reviewed studies, and statistical significance is not reached for all outcomes, the published evidence supports the ability of Echinacea to decrease the severity and duration of acute URI. This evidence is not conclusive, however, and higher quality trials are needed. Future trials should include: (1) larger, more representative populations; (2) more precisely defined inclusion and exclusion criteria; (3) more precisely defined objective and validated outcomes measurement; (4) data to verify the inability of participants to distinguish placebo from drug; and (5) better characterization of the active constituents and mechanism of action.
Nevertheless, the current evidence suggests that Echinacea may work as an early treatment for uncomplicated acute URI. Hoheisel and coworkers65 reported a 50% reduction in the proportion of people with first sign of a cold who went on to have a “real cold” (from 60% to 40%). Of those subjects who had a “real cold,” those taking Echinacea had markedly shorter lengths of illness (from a median duration of 8 days to a median duration of 4 days). Brinkeborn and colleagues66 reported a modest but statistically significant reduction in severity in what appears to be a moderately well-designed trial. The study by Dorn57 and the 2 studies by Bräunig and coworkers55,56 reported comparable clinical benefits, with 20% to 50% reductions in severity claimed. We interpret evidence from the highest quality trials to suggest that early dosing is important,67 as is sufficient dosing.55 The clinical significance of expected benefits cannot be precisely estimated.
The evidence for Echinacea’s ability to prevent rather than treat URI is not as promising. Published studies are few, of moderate quality, and report trends rather than statistically significant differences.58,60,61 The most recent and best designed of these prevention trials reported nonsignificant trends toward benefits consistent with a 10% to 20% reduction in incidence.68 We feel that the safety of long-term prophylactic dosing has not been sufficiently demonstrated, at least when valued against uncertain trends toward minor benefit. Neither expected benefits nor risks have been characterized properly, so no recommendations on preventive treatment can be made.
Despite equivocal clinical effects, the safety data on Echinacea are relatively strong, at least when compared with many other herbal medicines. In oral doses greater than 15 g per kg and intravenous doses greater than 5 g per kg, it has proved impossible to kill either a rat or a mouse, hence median lethal dose is so far incalculable.67 Extended (4-week) dosing of rats and mice up to 8 g per kg per day has similarly failed to show adverse effects, with red blood cells, white blood cells, platelets, liver enzymes, creatinine, urea, cholesterol, triglycerides, blood glucose, and body weight as measured end points.69 In a number of mutagenicity studies, no adverse effects were noted. An open-label trial with more than 1000 patients found the following side effects: unpleasant taste (1.7%), nausea or vomiting (0.5%), abdominal pain (0.3%), and diarrhea (0.3%).29 During the years 1989 to 1995, 4 of 13 adverse events reported in association with Echinacea were thought to be causally related by the German authority. When compared with a denominator of several million patient courses, the reported adverse effect rate, and hence the estimated risk, is quite small. Serious allergic or anaphylactic events have been reported, however, so some caution is needed.70
There is currently no universally accepted standardization procedure to ensure comparability among products. Unfortunately, given the apparent multiple chemical nature of Echinacea’s mode of action and the unequal distribution of active constituents in the flowers, leaves, stems, and roots of the 3 medicinal species, it is difficult to determine exactly what kind of standardization would be optimal.71 As there are a number of substances and mechanisms underlying Echinacea’s observed clinical effects, it seems possible that whole-extract dosing might indeed remain preferable to isolation and purification of single chemical entities. Still, as the concentrations of active ingredients are known to vary by species, among roots, leaves, and flowers, and most likely by season, soil type, and climate, and as there is very little research that tests one formulation against another, no recommendations regarding specific Echinacea products can be made.
Recommendations for clinical practice
The use of Echinacea for the early treatment of the common cold can be cautiously supported. More evidence is needed before clear recommendations can be made regarding specific formulations or dosing. Extracts from E purpurea, E angustifolia, and E pallida roots, leaves, and flowers cannot at this point be distinguished from each other in terms of their apparent beneficial activity. If the decision is made to use an Echinacea product, we recommend that it be taken early in the course of a cold, several times per day, and discontinued as symptoms abate. We recommend that Echinacea not be taken routinely, chronically, or on a preventive basis. We note that no trials have included infants, children, or pregnant women, and recommend caution among those populations. We also note the theoretical contraindication among persons suffering from serious autoimmune disorders.
At the present time we conclude that the evidence suggests Echinacea taken early in the course of an illness may be safe and effective in reducing the severity and duration of the common cold. The evidence of Echinacea’s ability to prevent infection is inadequate to make any recommendations in this regard.
SEARCH STRATEGIES: Information from a wide range of sources was used as background material. More than 100 articles, books, and book chapters were reviewed for content and further references. Database searches, bibliographic reviews, and conversations with experts were carried out iteratively from January 1997 to February 1999.
SELECTION CRITERIA: Published or unpublished reports of all blinded placebo-controlled randomized trials of any Echinacea formulation used as a treatment or for the prevention of URIs.
DATA COLLECTION AND ANALYSIS: Review considerations included randomization, blinding, power, validity and clinical relevance of outcome measurements, inclusion and exclusion criteria, indistinguishability of treatment and placebo, and appropriateness of conclusions for the data presented.
MAIN RESULTS: Nine treatment trials and 4 prevention trials fitting the selection criteria were found. Eight of the treatment trials reported generally positive results, and 3 of the prevention trials reported marginal benefit. Methodologic quality of the majority of the trials was modest.
CONCLUSIONS: Evidence from published trials suggests that Echinacea may be beneficial for the early treatment of acute URIs. The influence of publication bias on those results is unknown. Echinacea preparations vary widely in composition, and are often found in combination with other potentially active constituents, making specific dose recommendations problematic. There is very little evidence supporting the prolonged use of Echinacea for the prevention of URIs.
Clinical question
Are orally ingested Echinacea extracts effective in reducing the incidence, severity, or duration of acute upper respiratory infections?
Upper respiratory infection (URI), usually viral, with its common variants rhinosinusitis and pharyngitis, is the highest-incidence acute illness in the developed world.1-3 According to estimates, the average adult in the United States has 2 to 4 colds per year; the average schoolchild has 6 to 10.4 Although patients with complications, such as bacterial sinusitis, otitis media, streptococcal pharyngitis, bronchospasm, or pneumonia may benefit from antibiotic or inhaler treatment, medical science has little to offer for uncomplicated infections.5-10 Nevertheless, antibiotics are frequently prescribed, despite convincing evidence of little or no benefit.11-17 Clearly, there is great need for effective, safe, and affordable treatment.
Botanical extracts from plants of the genus Echinacea are among the most widely used herbal medicines throughout Europe and North America and are most commonly used for the prevention or treatment of URIs. Echinacea extracts are believed to affect URIs through “immunostimulating” activity. Symptom reduction through immunomodulation holds some theoretical and empirical promise.18,19 If effective, such treatment could have an impact on the morbidity and loss of productivity associated with URIs, and the overuse of antibiotics and the effects of their sequelae in terms of costs, adverse effects, and antibiotic resistance.
Background
Echinacea was first used by Native Americans as a remedy for a wide variety of illnesses. It was mentioned in the Flora Virginica in 1762, the Eclectic Dispensatory of the United States of America in 1852, and the National Formulary of the United States from 1916 until 1950.20,21 A 1909 editorial in the Journal of the American Medical Association stated that Echinacea was “deemed unworthy of future consideration,” and it subsequently fell into many decades of disuse in the United States.22 In Europe, however, Echinacea grew in popularity from its introduction in the 1920s to the present. Extracts from the leaves, flowers, and roots of Echinacea purpurea and its cousins E pallida and E angustifolia are currently sold under hundreds of brand names throughout Europe and North America. In Germany, Echinacea has been approved by the German regulatory Commission E for treating respiratory and urinary tract infections.23 More than 3 million physician prescriptions for Echinacea preparations are written each year.24,25 More than 400 scientific studies, mostly German, have detailed Echinacea’s botany, chemistry, pharmacology, and clinical effects.26-29
In the United States, perhaps because of the regulatory climate,30 herbal medicines are usually used without the advice or knowledge of a physician. Although precise estimates of the scope of Echinacea use in the United States are not available, several indicators point toward a large and growing pattern of use. Eisenberg and colleagues,31 using a randomized national telephone survey, estimated that in 1990 34% of Americans had used some type of unconventional medicine, and 10% had seen a provider of herbal therapy. Using the same methods, these researchers32 put the 1997 estimates at 42% and 15%, respectively. Another randomized national telephone survey in 1997 estimated that 17% of Americans used some type of herbal therapy.33 A Gallup poll in 1997 estimated that 32% of Americans used herbal medicines, and a Harris poll in 1998 placed the figure at 37%.34 According to recent market surveys, Americans spend close to $4 billion a year on herbal supplements.34 Several surveys have indicated that Echinacea preparations are the leading botanical medicines in the United States, with close to 10% of the total herbal market.34,35 Given its current popularity and reputation as scientifically justified, Echinacea will likely continue to be widely used.
Echinacea extracts are thought to have immunomodulating pharmacologic activity. Most notably, macrophage activation and enhanced phagocytosis have been reported in a number of studies.36-42 Serum levels of properdin, a member of the complement system, increase after Echinacea administration.43 Increased levels of tumor necrosis factor alpha, interleukins 1, 6, and 10, and of several other cytokines have also been variously reported.44,45 Leukocytosis (especially granulocytes and macrophages) has been variably observed in tissue culture and live animal experiments.43 Echinacea extracts given to mice before an injection with Candida and Listeria species have improved survival rates.46,47 Anti-inflammatory effects have also been reported,48-50 as have antibacterial, antiviral, and antiparasitical activities.43,51 Echinacea’s pharmacologic effects appear to result from a combination of active ingredients rather than from a single agent. Various chemical constituents, including alkamides, caffeic acid derivatives (cicchoric acid), flavonoids, glycoproteins, isobutylamides, polyenes, and polysaccharides, have been identified and implicated as active constituents.52,53 These phytochemicals occur at variable levels among the flowers, leaves, stems, and roots of the 3 medicinal species, E purpurea, E angustifolia, and E pallida.
Methods
The goal of our search strategy was to locate, retrieve, and review the original reports of all blinded randomized trials of Echinacea for the prevention or treatment of acute URI. Throughout 1997 and 1998, we used MEDLINE and other bibliographic reference services to find relevant articles. Searches using variants of the key word “echinacea” were repeated on multiple occasions, covering all years available. More than 100 articles, books, and book chapters were reviewed for content and further references. Herbal medicine experts in the United States and Germany were contacted and questioned concerning their knowledge of published and unpublished controlled trials. All relevant original reports of randomized controlled trials (RCTs) were requested and reviewed in detail. Several of the RCTs we reviewed were not cited in MEDLINE. Retrieval of a few of the older German studies required personal contact with physicians and researchers in Germany, as medical libraries in the United States were unable to locate the studies. Seven of the RCTs were reviewed in the original German by a family physician fluent in the language. Review considerations included randomization, blinding, power, validity and clinical relevance of outcome measurements, inclusion and exclusion criteria, indistinguishability of treatment and placebo, and appropriateness of conclusions for the data presented. Because of dissimilarities in products, methods, and outcome measurements, meta-analysis was not a viable option.
Results
Following the search strategy outlined above, reports of 13 blinded randomized studies were obtained and reviewed Table 1. We found no meta-analyses of Echinacea trials. However, Melchart and colleagues54 reviewed 26 prospective trials (18 randomized, 11 double-blind) testing Echinacea for a variety of indications. Some 30 of 34 reported outcomes in treatment groups were claimed to be superior to controls by the original authors. However, Melchart and coworkers concluded that only 22 of the 34 outcomes were reasonably demonstrated. Further, only 8 of the 26 trials earned 50% or better on the researchers’ quality point-scoring system. Of the 12 URI trials, (6 prevention, 6 treatment), 9 were double-blinded,55-63 but only 5 of these earned more than 50 quality points.55-57,60,63 We reviewed all 9 randomized blinded URI trials identified by Melchart and coworkers, as well as 4 trials conducted subsequently.64-66 Of the 13 trials we reviewed, 9 were treatment trials, and 4 were prevention trials. All studies were randomized and double-blinded. Eight of 9 treatment trials reported benefit. The study reporting no treatment benefit remains unpublished.64 Two of the prevention trials reported marginal benefit.58,60 A third was reported in 1992 to show benefit61—subgroup analyses found statistical significance—but was later reported as largely negative.65 The authors of the fourth study (which we judged to be of the highest quality) found no statistically significant benefit, but noted that a 15% reduction in URI incidence attributable to Echinacea was consistent with their findings.66
Treatment Trials
The most recently reported Echinacea treatment trial was published by Brinkeborn and colleagues66 in 1998. Approximately 119 participants were treated for 8 days with 3 doses of 2 tablets each of Echinaforce, a dried ethanolic extract of E purpurea (95% herb, 5% root). Ten symptoms and the “overall clinical picture” were assessed on a severity scale of 0 to 3 with a physician visit at the beginning of an acute URI (day 1 or 2 of symptoms) and again at day 8. An intention-to-treat analysis showed statistically significant benefit, with an indexed score dropping from 9.0 to 4.1 in the treatment group compared with 8.8 to 5.3 in the placebo group (P = .045). A per-protocol analysis of 87 of the participants yielded highly significant results (P = .007). Construction of the index was not described, and inclusion criteria, exclusion criteria, and verification of randomization and blinding were not properly reported.
Also recently published, and perhaps most convincing in its reported benefit, was the study by Hoheisel and coworkers66 in 1997. This was a double-blind randomized placebo-controlled single-center clinical trial among adult factory workers in Sweden. The 120 participants were recruited at the first sign of URI, but before a full cold had developed. Participants were randomly given either placebo or active drug, and were followed up until symptoms had resolved. The active drug used was Echinagard, also called Echinacin, a commercial preparation made of juice from the above-ground parts of E purpurea. Participants were instructed to take 20 drops every 2 hours for the first day, and 3 times per day thereafter until symptoms resolved. The authors reported that 60% of the placebo groups, but only 40% of the Echinacea group, developed a “real cold.” Among those who had a “real cold,” the median time to resolution was 4 days in the Echinacea group and 8 days in the placebo group. Statistical significance was reached among all reported outcomes in an intention-to-treat analysis. The limitations of this study include: poorly defined inclusion and exclusion criteria, use of retrospectively defined criteria for progression from “first sign of a cold” to “real cold,”65 and lack of evidence of indistinguishability between Echinacea and placebo.
In their 1992 article, Bräunig and coworkers55 reported the results of a randomized double-blind trial of E purpurea root extract among 180 volunteers presenting with recent-onset influenza-like respiratory symptoms. There were 60 participants in each of the 3 groups: placebo, low-dose, and high-dose. The 2 treatment groups received twice daily doses of either 1 dropperful (about 4.5 mL) or 2 dropperfuls (about 9 mL) of juice extracted from E purpurea root. Primary end points were 8 symptoms (cough, sore throat, nasal symptoms, tearing, headache, fatigue, chills or sweats, and muscle aches) and 1 global indicator of severity, all rated on a 0 to 3 scale as either absent, mild, moderate, or severe, with measurements taken at time 0, after 3 to 4 days, and after 8 to 10 days. Although the low-dose regimen showed little improvement over placebo, the higher-dose group showed statistically significant improvement over placebo in several symptom scores, with positive trends in all measurements. Symptom scores in the treated group were 24% to 50% lower in the placebo group at 3 to 4 days, with the gap widening to 36% to 75% at 8 to 10 days. This study is singular in reporting a dose-dependency effect.
In their 1993 article, Bräunig and colleagues56 described the results of a randomized double-blind clinical trial of E pallida root extract among 160 volunteers presenting with influenza-like respiratory infection. The dose used was equivalent to approximately 900 mg per day of dried extract. Symptom assessment was similar to that described above, with a 4-point none-to-severe rating scale assessed at days 3 to 4 and 8 to 10. The median duration of illness in the Echinacea group was 9.8 days, a statistically significant (P <.001) improvement over placebo (13 days). Interestingly, a physician assessment attempted to classify infections as viral or bacterial. A subgroup analysis showed greater benefit among patients with viral infections. White blood counts and differentials were not clearly different between the placebo and verum groups or the viral and bacterial groups.
Dorn’s57 trial consisted of recruiting 100 participants within 2 days of URI onset, and treating with either placebo or Resistan, a commercial preparation made primarily from E angustifolia herb and root, but also containing extracts from Eupatorium perfoliatum, Baptisia, and Arnica. Dosage was 30 cc for the first and second days, followed by 15 cc on the third through sixth days. Outcomes scored on a 0- to 3-point scale (none, mild, moderate, severe) included 7 self-reported symptoms and several physician-documented signs. These were assessed twice, at days 2 to 4 and 6 to 8. Three symptoms (sore throat, nasal drainage, and cough), and 1 sign (pharyngeal erythema), were reported as significantly superior to placebo (P <.01).
Vorberg and Schneider63 reported a treatment trial in 1989 of Resistan among 100 participants suffering from URI. Patients were enrolled in the first 2 days of URI symptoms and randomly treated with either Resistan or an identical placebo. Symptom scores at days 3 and 8 were significantly improved (P < .01) in the treatment group when compared with placebo, with some benefit found in all assessed symptoms. An approximately 20% benefit at day 3 widened to an average 50% reduction at day 8. The authors concluded that there was a clear severity and duration benefit to Echinacea when compared with placebo.
Reitz59 described the results of a trial of Esberitox-N among 150 participants with respiratory infections. Esberitox-N is a commercial preparation containing extracts from E angustifolia and E pallida roots, along with small amounts of Baptisia and Thuja occidentalis extracts. Participants were randomized to treatment or placebo (containing Vitamin C) for 8 weeks and were followed in a double-blinded manner for approximately 1 year. Outcomes measured at 7 and 14 days and monthly thereafter included 8 symptoms, 3 signs, and blood work, including a complete blood cell count and an immunoglobulin measurement. Reitz reported that the majority of symptoms and signs at 7 and 14 days were significantly better in the Esberitox group than with placebo, but provided little statistical analysis to support this claim. Relative improvements in nasal symptoms were noted most prominently. No differences in laboratory measurements were reported.
The 1984 trial by Vorberg62 included 100 participants treated with either vitamin C as placebo or Esberitox. In addition to the ingredients in Esberitox-N, Esberitox contains homeopathic dilutions of Apis, Crotal, Silicea, and Lachesis. Outcomes were self-reported symptoms and physician-reported signs, all assessed on a 0 to 3 scale of severity at days 3 and 10. Headache (P <.001), cough (P <.05), and subfebrile temperature (P <.01) differed significantly, favoring the Esberitox over the vitamin C group. Fatigue, sore throat, difficulty swallowing, nasal drainage, and physician-reported pharyngeal erythema and edema all trended toward benefit in the Esberitox group.
Of the 13 studies we reviewed, the one by Galea and Thacker64 was singular because it reported no measurable benefit, was conducted in North America, and so far remains unpublished. This study treated a total of 190 undergraduate Canadian students with either placebo or a 250-mg capsule preparation of dried E angustifolia 3 times per day. Participants were recruited at first sign of URI and followed up by presence or absence of 8 symptoms for 10 days. No clear trends or statistically significant differences were found between the Echinacea and placebo groups. The relatively low dose and the lack of measures of severity may account for these negative findings.
A treatment trial of a capsulized mixture of dried powder made from the herb (25%) and root (25%) of E purpurea and the root of E angustifolia (50%) is currently under way at the Department of Family Medicine at the University of Wisconsin-Madison. Participants are recruited within 36 hours of first symptoms of URI. Capsulized dried plant material is taken in 1 g doses, 6 times on the first day and 3 times on each subsequent day. Symptom-based outcomes are measured daily using Likert-scale severity measures.
Prevention Trials
Melchart and colleagues68 conducted a 3-arm prevention trial in which 302 volunteers took 50 drops of either placebo or 1 of 2 alcohol extracts from the root of either E angustifolia or E purpurea twice daily for 12 weeks. This was the first head-to-head trial of Echinacea preparations. Median time to onset of first URI was similar among the 3 groups. Compared with placebo, the relative risk of an infection was 0.80 in the E purpurea group and 0.87 in the E angustifolia group. These differences were not statistically significant, hence the null hypothesis that Echinacea is no better than placebo at preventing URI could not be rejected. The authors speculated that a larger study might be able to show an effect, as their study did not have the power to demonstrate a hypothetical 10% to 20% relative risk benefit.
Schöneberger and coworkers61 conducted a trial in which 108 patients “with increased susceptibility to colds” were divided into treatment and placebo groups and followed for 8 weeks. Doses of 4 mL juice from the above-ground parts of E purpurea (Echinacin or Echinagard) were given twice daily for the entire 8-week period. The treatment group had 19 people (35%) without infections compared with 14 (26%) in the placebo group. Average duration of infection was 5.3 days in the treatment group compared with 7.5 days in the placebo group. When infections were grouped into 3 classes according to severity, the treatment group had fewer individuals in all 3 classes (33 vs 34 in mild; 8 vs 13 in moderate; 0 vs 3 in severe). Although these results were not statistically significant, all trends reported were in favor of Echinacea treatment. Grimm and Muller’s 1999 analysis and interpretation of this trial65 was less optimistic than Schöneberger’s original report.
Schmidt and colleagues60 reported a trial of Resistan as prevention of URI among 646 college students at the University of Cologne. Resistan was taken daily for 8 weeks, and students were monitored every 2 weeks and during each URI or flu-like infection. The symptoms assessed on a 0 to 3 scale were cough, sore throat, difficulty swallowing, nasal drainage, congestion, headache, muscle aches, and fatigue. Overall frequency of infection was 15% lower in the Echinacea group than in the placebo group, trending toward statistical significance (P = .08). A subgroup analysis of those patients judged to be especially prone to infection (3 or more colds per year for each of the previous 3 years) showed a statistically significant (P <.05) relative risk reduction in verum (27%) compared with placebo (15%).
Forth and colleagues58 reported a study of 95 patients with URIs randomized to either Esberitox liquid, Esberitox tablet, or identical placebo. Participants took treatments 3 times daily from November until late February, filling out incidence and severity questionnaires every 14 days. A relative risk reduction of 38% (P <.005) was reported for nasal symptoms in the Echinacea tablet group compared with placebo. Other outcomes were reported as similar in all groups.
Data collection for a trial designed to study the efficacy of Echinacea for URI prevention was recently completed at Bastyr University in Seattle, Washington. Subjects who had experienced at least 3 respiratory infections in the 6 months before enrollment were treated with 8 mL E purpurea juice on an intermittent basis over 6 months and were followed in terms of incidence of URI and severity and duration of symptoms. Granolocyte and monocyte phagocytosis differences were assessed using an ex vivo laboratory model. Data analysis is currently under way, with results forthcoming.
Another prevention trial is under way at Oregon Health Sciences University. Explicit methods are not available.
Discussion
In the treatment of acute URI, 8 of 9 randomized trials report some evidence of benefit of Echinacea. Although we attempted to review all trials, including those that were not yet published, we only located 1 unpublished trial, which reported a negative result; therefore, the influence of publication bias remains unknown. Although there is a moderate degree of methodologic deficiency in all of the reviewed studies, and statistical significance is not reached for all outcomes, the published evidence supports the ability of Echinacea to decrease the severity and duration of acute URI. This evidence is not conclusive, however, and higher quality trials are needed. Future trials should include: (1) larger, more representative populations; (2) more precisely defined inclusion and exclusion criteria; (3) more precisely defined objective and validated outcomes measurement; (4) data to verify the inability of participants to distinguish placebo from drug; and (5) better characterization of the active constituents and mechanism of action.
Nevertheless, the current evidence suggests that Echinacea may work as an early treatment for uncomplicated acute URI. Hoheisel and coworkers65 reported a 50% reduction in the proportion of people with first sign of a cold who went on to have a “real cold” (from 60% to 40%). Of those subjects who had a “real cold,” those taking Echinacea had markedly shorter lengths of illness (from a median duration of 8 days to a median duration of 4 days). Brinkeborn and colleagues66 reported a modest but statistically significant reduction in severity in what appears to be a moderately well-designed trial. The study by Dorn57 and the 2 studies by Bräunig and coworkers55,56 reported comparable clinical benefits, with 20% to 50% reductions in severity claimed. We interpret evidence from the highest quality trials to suggest that early dosing is important,67 as is sufficient dosing.55 The clinical significance of expected benefits cannot be precisely estimated.
The evidence for Echinacea’s ability to prevent rather than treat URI is not as promising. Published studies are few, of moderate quality, and report trends rather than statistically significant differences.58,60,61 The most recent and best designed of these prevention trials reported nonsignificant trends toward benefits consistent with a 10% to 20% reduction in incidence.68 We feel that the safety of long-term prophylactic dosing has not been sufficiently demonstrated, at least when valued against uncertain trends toward minor benefit. Neither expected benefits nor risks have been characterized properly, so no recommendations on preventive treatment can be made.
Despite equivocal clinical effects, the safety data on Echinacea are relatively strong, at least when compared with many other herbal medicines. In oral doses greater than 15 g per kg and intravenous doses greater than 5 g per kg, it has proved impossible to kill either a rat or a mouse, hence median lethal dose is so far incalculable.67 Extended (4-week) dosing of rats and mice up to 8 g per kg per day has similarly failed to show adverse effects, with red blood cells, white blood cells, platelets, liver enzymes, creatinine, urea, cholesterol, triglycerides, blood glucose, and body weight as measured end points.69 In a number of mutagenicity studies, no adverse effects were noted. An open-label trial with more than 1000 patients found the following side effects: unpleasant taste (1.7%), nausea or vomiting (0.5%), abdominal pain (0.3%), and diarrhea (0.3%).29 During the years 1989 to 1995, 4 of 13 adverse events reported in association with Echinacea were thought to be causally related by the German authority. When compared with a denominator of several million patient courses, the reported adverse effect rate, and hence the estimated risk, is quite small. Serious allergic or anaphylactic events have been reported, however, so some caution is needed.70
There is currently no universally accepted standardization procedure to ensure comparability among products. Unfortunately, given the apparent multiple chemical nature of Echinacea’s mode of action and the unequal distribution of active constituents in the flowers, leaves, stems, and roots of the 3 medicinal species, it is difficult to determine exactly what kind of standardization would be optimal.71 As there are a number of substances and mechanisms underlying Echinacea’s observed clinical effects, it seems possible that whole-extract dosing might indeed remain preferable to isolation and purification of single chemical entities. Still, as the concentrations of active ingredients are known to vary by species, among roots, leaves, and flowers, and most likely by season, soil type, and climate, and as there is very little research that tests one formulation against another, no recommendations regarding specific Echinacea products can be made.
Recommendations for clinical practice
The use of Echinacea for the early treatment of the common cold can be cautiously supported. More evidence is needed before clear recommendations can be made regarding specific formulations or dosing. Extracts from E purpurea, E angustifolia, and E pallida roots, leaves, and flowers cannot at this point be distinguished from each other in terms of their apparent beneficial activity. If the decision is made to use an Echinacea product, we recommend that it be taken early in the course of a cold, several times per day, and discontinued as symptoms abate. We recommend that Echinacea not be taken routinely, chronically, or on a preventive basis. We note that no trials have included infants, children, or pregnant women, and recommend caution among those populations. We also note the theoretical contraindication among persons suffering from serious autoimmune disorders.
At the present time we conclude that the evidence suggests Echinacea taken early in the course of an illness may be safe and effective in reducing the severity and duration of the common cold. The evidence of Echinacea’s ability to prevent infection is inadequate to make any recommendations in this regard.
1. Campbell H. Acute respiratory infection: a global challenge. Arch Dis Childhood 1995;73:281-3.
2. Murray CJL, Lopez AD. The global burden of disease. Cambridge, Mass: Harvard University Press, 1996.
3. Oh HM. Upper respiratory tract infections—otitis media, sinusitis and pharyngitis. Singapore Med J 1995;36:428-31.
4. Spector SL. The common cold: current therapy and natural history. J Allergy Clin Immunol 1995;95:1133-8.
5. De Lange de Klerk ESM, Blommers JKDJ, Bezemer PD, Feenstra L. Effects of homeopathic medicines on daily burden of symptoms in children with recurrent upper respiratory tract infections. BMJ 1994;309:1329-32.
6. Fireman P. Pathophysiology and pharmacotherapy of common upper respiratory diseases. Pharmacotherapy 1993;3:101S-9S.
7. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-40.
8. McMillan JA. Upper respiratory tract infections in children. Current Opin Pediatr 1993;5:50-4.
9. Monto AS. Viral respiratory infections in the community: epidemiology, agents, and interventions. Am J Med 1995;99:24S-7S.
10. Walker TA, Khurana S, Tilden SJ. Viral respiratory infections. Pediatr Clin North Am 1995;41:1365-81.
11. Dere WH. Acute bronchitis: results of US and European trials of antibiotic therapy. Am J Med 1992;92:53S-7S.
12. Fahey T, Stocks N, Thomas T. Quantitative systematic review of randomized controlled trials comparing antibiotic with placebo for acute cough in adults. BMJ 1998;316:906-10.
13. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901-4.
14. Mainous AG, III, Hueston WJ, Clark JR. Antibiotics and upper respiratory infection: do some folks think there is a cure for the common cold? J Fam Pract 1996;42:357-61.
15. Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA 1998;279:875-7.
16. Oeffinger KC, Snell LM, Foster BM, Panico KG, Archer RK. Diagnosis of acute bronchitis in adults: a national survey of family physicians. J Fam Pract 1997;45:402-9.
17. Orr PH, Scherer K, Macdonald A, Moffatt MEK. Randomized placebo-controlled trials of antibiotics for acute bronchitis: a critical review of the literature. J Fam Pract 1993;36:507-12.
18. Bergmann KC. Assessment of the clinical value of using an immunomodulator in recurrent respiratory infections. Adv Exp Med Biol 1995;371B:795-7.
19. Riedl-Seifert RJ, van Aubel A, Kammereit A, Elsasser U. Reduction of the number and severity of respiratory tract infections in children by oral immunostimulation. Adv Exp Med Biol 1995;371B:799-802.
20. Foster S. Echinacea: nature’s immune enhancer. Rochester, Vt: Healing Arts Press, 1991.
21. Hobbs C. Echinacea: a literature review. HerbalGram 1994;30(suppl):33-47.
22. Puckner WA. Echinacea considered valueless: report of the council on pharmacy and chemistry. JAMA 1909;1836.-
23. Blumenthal M, Goldberg A, Gruenwald J, et al. German Commission E monographs: therapeutic monographs on medicinal plants for human use. Austin, Tex: American Botanical Council, 1998.
24. Keller K. Legal requirements for the use of phytopharmaceutical drugs in the Federal Republic of Germany. J Ethnopharmacol 1991;32:225-9.
25. Tyler VE. Phytomedicines in Western Europe: their potential impact on herbal medicine in the United States. HerbalGram 1994;30:24-31.
26. Awang DVC, Kindack DG. Herbal medicine: Echinacea. Can Pharma J 1991;124:512-6.
27. Foster S. Echinacea: the purple coneflowers. Austin, Tex: American Botanical Council, 1996.
28. Hobbs C. Echinacea: the immune herb! Santa Cruz, Calif: Botanica Press, 1995.
29. Parnham MJ. Benefit-risk assessment of the squeezed sap of the purple coneflower (Echinacea purpurea) for long-term oral immunostimulation. Phytomedicine 1996;3:95-102.
30. Cohen MH. Complementary and alternative medicine: legal boundaries and regulatory perspectives. Baltimore, Md: Johns Hopkins University Press, 1998.
31. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med 1993;328:246-52.
32. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA 1998;280:1569-75.
33. Landmark Healthcare. The Landmark report on public perceptions of alternative care: 1998 nationwide study of alternative care. Random telephone survey of 1500 households. Sacramento, Calif: Landmark Healthcare, Inc, 1998.
34. Brevoort P. The booming US botanical market: a new overview. HerbalGram 1998;44:33-46.
35. Brevoort P. The US botanical market: an overview. HerbalGram 1996;36:49-57.
36. Bukovsky M, Vaverkova S, Kostalova D. Immunomodulating activity of Echinacea gloriosa L, Echinacea angustifolia DC, and Rudbeckia speciosa Wenderoth ethanol-water extracts. Pol J Pharmacol 1995;47:175-7.
37. Coeugniet EG, Elek E. Immunomodulation with Viscum album and Echinacea purpurea extracts. Onkologie 1987;10 (suppl):27-33.
38. Lersch C, Zeuner M, Bauer A, et al. Stimulation of the immune response in outpatients with hepatocellular carcinomas by low doses of cyclophosphamide (LDCY), Echinacea purpurea extracts (Echinacin) and thymostimulin. Arch Geschwulstforsch 1990;60:379-83.
39. Lersch C, Zeuner M, Bauer A, et al. Nonspecific immunomodulation with low doses of cyclophosphamide (LDCY), thymostimulin, and Echinacea purpurea extracts (Echinacin) in patients with far advanced colorectal cancers. Cancer Invest 1992;10:343-8.
40. Luettig B, Steinmuller C, Gifford GE, Wagner H, Lohman-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst 1989;
41. Melchart D, Linde K, Worku F, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J Altern Complement Med 1995;1:145-60.
42. Stimpel M. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun 1984;46:845-9.
43. Bauer R, Wagner H. Echinacea species as potential immunostimulatory drugs. In: Wagner H, Farnsworth NR, eds. Economic and medicinal plant research. New York, NY: Academic Press Limited, 1991.
44. Burger RA, Torres AR, Warren RP, Caldwell VD, Hughes BG. Echinacea-induced cytokine production by human macrophages. Int J Immunopharmacol 1997;19:371-9.
45. Elsasser-Beile U, Willenbacher W, Bartsch HH, Gallati H, Schulte Monting J, von Kleist S. Cytokine production in leukocyte cultures during therapy with Echinacea extract. J Clin Lab Anal 1996;10:441-5.
46. Roesler J, Steinmuller C, Kiderlen A, Emmendorffer A, Wagner H, Lohmann ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int J Immunopharmacol 1991;13:27-37.
47. Steinmuller C, Roesler J, Grottrup E, Franke G, Wagner H, Lohmann-Matth M. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria. Int J Immunopharmacol 1993;15:605-14.
48. Tragni E. Evidence from two classic irritation tests for an anti-inflammatory action of a natural extract, Echinacea. Food Chem Toxicol 1985;23:317-9.
49. Tragni E, Galli CL, Tubaro A, del Negro P, Della Loggia R. Anti-inflammatory activity of Echinacea angustifolia fractions separated on the basis of molecular weight. Pharmaceut Res Comm 1998;20(suppl):87-91.
50. Tubaro A, Tragni E, del Negro P, Galli CL, Della Loggia R. Anti-inflammatory activity of a polysaccharide fraction of Echinacea angustifolia. J Pharm Pharmacol 1987;39:567-9.
51. Wacker A. Virus inhibition by Echinacea purpurea. Planta Medica 1978;33:89-102.
52. Bauer R. Echinacea: biological effects and active principles. In: Lawson LD, Bauer R, eds. Phytomedicines of Europe: chemistry and biological activity. Washington, DC: American Chemists Society 1998;140-157.
53. Bone K. Echinacea: what makes it work? Altern Med Rev 1997;2:87-9.
54. Melchart D, Linde K, Worku F, Bauer R, Wagner H. Immunomodulation with Echinacea: a systematic review of controlled trials. Phytomedicine 1994;1:245-54.
55. Bräunig B, Dorn M, Limburg E, Knick E. Bausendorf: Echinaceae purpureae radix: zur Stärkung der körpereigenen Abwehr bei grippalen Infekten. Zeitschrift fur Phytotherapie 1992;13:7-13.
56. Bräunig B, Knick E. Therapeutische Erfahrungen mit Echinaceae pallidae bei grippalen Infekten. Naturheilpraxis 1993;1:72-5.
57. Dorn M. Milerung grippaler Effeckte durch ein pflanzliches Immunstimulans. Nutur Ganzheitsmedizin 1989;2:314-9.
58. Forth H, Beuscher N. Beeinflussing der Häufigkeit banaler Erkältungsinfekte durch Esberitox. Zeitschrift für Allgemeinmedizin 1981;57:2272-5.
59. Reitz HD. Immunmodulatoren mit pflanzlichen Wirkstoffen: eine wissenschaftliche Studie am Beispiel Esberitox N. Notabene medici 1990;20:362-6.
60. Schmidt U, Albrecht M, Schenk N. Pflankliches Immunstimulans senkt Häufigkeit grippaler Infekte: Plazebokontrollierte Doppelblindstudie mit einem kombinierten Echinacea-Präparat mit 646 Studenten der Kölner Universität. Natur Ganzheits Medizin 1990;3:277-81.
61. Schöneberger D. Einfluß der immunstimulierenden Wirkung von Preßsaft aus Herba Echinaceae purpureae auf Verlauf und Schweregrad von Erkältungskrankheiten. Ergebnisse einer Doppelblindstudie. Forum Immunologie 1992;2:18-22.
62. Vorberg G. Bei Erkältung unspezifische Immunabwehr stimulieran: Doppelblindstudie zeigt: Das bewährte Phytotherapeutikum Esberitox verkürzt die Symptommatik. Ärztliche Praxis 1984;36:97-8.
63. Vorberg G, Schneider B. Pflanzliches Immunstimulans verkürzt grippalen Infeckt. Doppelblindstudie belegt die Steigerung der unspezifischen Infektabwehr. Ärztliche Forschung 1989;36:3-8.
64. Galea S, Thacker K. Double-blind prospective trial investigating the effectiveness of a commonly prescribed herbal remedy in altering the duration, severity and symptoms of the common cold. Unpublished manuscript, 1996.
65. Grimm W, Muller HH. A randomized controlled trail of the effect of fluid extract of Echinacea purpurea on incidence and severity of colds and respiratory infections. Am J Med 1999;106:138-144.
66. Brinkeborn R, Shah D, Geissbühler S, Degenring FH. Echinaforce in the treatment of acute colds. Schweiz Zschr GanzheitsMedizin 1998;10:26-9.
67. Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schäfer M. Echinagard treatment shortens the course of the common cold: a double-blind, placebo-controlled clinical trial. Eur J Clin Res 1997;9:261-8.
68. Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med 1998;7:541-5.
69. Mengs U, Clare CB, Poiley JA. Toxicity of Echinacea pur
70. Mullins RJ. Echinacea-associated anaphylaxis Med J Aust 1998;168:170-1.
71. Reichling J, Saller R. Control in the manufacturing of modern herbal remedies. Q Rev Nat Med 1998;Spring:21-8.
1. Campbell H. Acute respiratory infection: a global challenge. Arch Dis Childhood 1995;73:281-3.
2. Murray CJL, Lopez AD. The global burden of disease. Cambridge, Mass: Harvard University Press, 1996.
3. Oh HM. Upper respiratory tract infections—otitis media, sinusitis and pharyngitis. Singapore Med J 1995;36:428-31.
4. Spector SL. The common cold: current therapy and natural history. J Allergy Clin Immunol 1995;95:1133-8.
5. De Lange de Klerk ESM, Blommers JKDJ, Bezemer PD, Feenstra L. Effects of homeopathic medicines on daily burden of symptoms in children with recurrent upper respiratory tract infections. BMJ 1994;309:1329-32.
6. Fireman P. Pathophysiology and pharmacotherapy of common upper respiratory diseases. Pharmacotherapy 1993;3:101S-9S.
7. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-40.
8. McMillan JA. Upper respiratory tract infections in children. Current Opin Pediatr 1993;5:50-4.
9. Monto AS. Viral respiratory infections in the community: epidemiology, agents, and interventions. Am J Med 1995;99:24S-7S.
10. Walker TA, Khurana S, Tilden SJ. Viral respiratory infections. Pediatr Clin North Am 1995;41:1365-81.
11. Dere WH. Acute bronchitis: results of US and European trials of antibiotic therapy. Am J Med 1992;92:53S-7S.
12. Fahey T, Stocks N, Thomas T. Quantitative systematic review of randomized controlled trials comparing antibiotic with placebo for acute cough in adults. BMJ 1998;316:906-10.
13. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901-4.
14. Mainous AG, III, Hueston WJ, Clark JR. Antibiotics and upper respiratory infection: do some folks think there is a cure for the common cold? J Fam Pract 1996;42:357-61.
15. Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA 1998;279:875-7.
16. Oeffinger KC, Snell LM, Foster BM, Panico KG, Archer RK. Diagnosis of acute bronchitis in adults: a national survey of family physicians. J Fam Pract 1997;45:402-9.
17. Orr PH, Scherer K, Macdonald A, Moffatt MEK. Randomized placebo-controlled trials of antibiotics for acute bronchitis: a critical review of the literature. J Fam Pract 1993;36:507-12.
18. Bergmann KC. Assessment of the clinical value of using an immunomodulator in recurrent respiratory infections. Adv Exp Med Biol 1995;371B:795-7.
19. Riedl-Seifert RJ, van Aubel A, Kammereit A, Elsasser U. Reduction of the number and severity of respiratory tract infections in children by oral immunostimulation. Adv Exp Med Biol 1995;371B:799-802.
20. Foster S. Echinacea: nature’s immune enhancer. Rochester, Vt: Healing Arts Press, 1991.
21. Hobbs C. Echinacea: a literature review. HerbalGram 1994;30(suppl):33-47.
22. Puckner WA. Echinacea considered valueless: report of the council on pharmacy and chemistry. JAMA 1909;1836.-
23. Blumenthal M, Goldberg A, Gruenwald J, et al. German Commission E monographs: therapeutic monographs on medicinal plants for human use. Austin, Tex: American Botanical Council, 1998.
24. Keller K. Legal requirements for the use of phytopharmaceutical drugs in the Federal Republic of Germany. J Ethnopharmacol 1991;32:225-9.
25. Tyler VE. Phytomedicines in Western Europe: their potential impact on herbal medicine in the United States. HerbalGram 1994;30:24-31.
26. Awang DVC, Kindack DG. Herbal medicine: Echinacea. Can Pharma J 1991;124:512-6.
27. Foster S. Echinacea: the purple coneflowers. Austin, Tex: American Botanical Council, 1996.
28. Hobbs C. Echinacea: the immune herb! Santa Cruz, Calif: Botanica Press, 1995.
29. Parnham MJ. Benefit-risk assessment of the squeezed sap of the purple coneflower (Echinacea purpurea) for long-term oral immunostimulation. Phytomedicine 1996;3:95-102.
30. Cohen MH. Complementary and alternative medicine: legal boundaries and regulatory perspectives. Baltimore, Md: Johns Hopkins University Press, 1998.
31. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med 1993;328:246-52.
32. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA 1998;280:1569-75.
33. Landmark Healthcare. The Landmark report on public perceptions of alternative care: 1998 nationwide study of alternative care. Random telephone survey of 1500 households. Sacramento, Calif: Landmark Healthcare, Inc, 1998.
34. Brevoort P. The booming US botanical market: a new overview. HerbalGram 1998;44:33-46.
35. Brevoort P. The US botanical market: an overview. HerbalGram 1996;36:49-57.
36. Bukovsky M, Vaverkova S, Kostalova D. Immunomodulating activity of Echinacea gloriosa L, Echinacea angustifolia DC, and Rudbeckia speciosa Wenderoth ethanol-water extracts. Pol J Pharmacol 1995;47:175-7.
37. Coeugniet EG, Elek E. Immunomodulation with Viscum album and Echinacea purpurea extracts. Onkologie 1987;10 (suppl):27-33.
38. Lersch C, Zeuner M, Bauer A, et al. Stimulation of the immune response in outpatients with hepatocellular carcinomas by low doses of cyclophosphamide (LDCY), Echinacea purpurea extracts (Echinacin) and thymostimulin. Arch Geschwulstforsch 1990;60:379-83.
39. Lersch C, Zeuner M, Bauer A, et al. Nonspecific immunomodulation with low doses of cyclophosphamide (LDCY), thymostimulin, and Echinacea purpurea extracts (Echinacin) in patients with far advanced colorectal cancers. Cancer Invest 1992;10:343-8.
40. Luettig B, Steinmuller C, Gifford GE, Wagner H, Lohman-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst 1989;
41. Melchart D, Linde K, Worku F, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J Altern Complement Med 1995;1:145-60.
42. Stimpel M. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun 1984;46:845-9.
43. Bauer R, Wagner H. Echinacea species as potential immunostimulatory drugs. In: Wagner H, Farnsworth NR, eds. Economic and medicinal plant research. New York, NY: Academic Press Limited, 1991.
44. Burger RA, Torres AR, Warren RP, Caldwell VD, Hughes BG. Echinacea-induced cytokine production by human macrophages. Int J Immunopharmacol 1997;19:371-9.
45. Elsasser-Beile U, Willenbacher W, Bartsch HH, Gallati H, Schulte Monting J, von Kleist S. Cytokine production in leukocyte cultures during therapy with Echinacea extract. J Clin Lab Anal 1996;10:441-5.
46. Roesler J, Steinmuller C, Kiderlen A, Emmendorffer A, Wagner H, Lohmann ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int J Immunopharmacol 1991;13:27-37.
47. Steinmuller C, Roesler J, Grottrup E, Franke G, Wagner H, Lohmann-Matth M. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria. Int J Immunopharmacol 1993;15:605-14.
48. Tragni E. Evidence from two classic irritation tests for an anti-inflammatory action of a natural extract, Echinacea. Food Chem Toxicol 1985;23:317-9.
49. Tragni E, Galli CL, Tubaro A, del Negro P, Della Loggia R. Anti-inflammatory activity of Echinacea angustifolia fractions separated on the basis of molecular weight. Pharmaceut Res Comm 1998;20(suppl):87-91.
50. Tubaro A, Tragni E, del Negro P, Galli CL, Della Loggia R. Anti-inflammatory activity of a polysaccharide fraction of Echinacea angustifolia. J Pharm Pharmacol 1987;39:567-9.
51. Wacker A. Virus inhibition by Echinacea purpurea. Planta Medica 1978;33:89-102.
52. Bauer R. Echinacea: biological effects and active principles. In: Lawson LD, Bauer R, eds. Phytomedicines of Europe: chemistry and biological activity. Washington, DC: American Chemists Society 1998;140-157.
53. Bone K. Echinacea: what makes it work? Altern Med Rev 1997;2:87-9.
54. Melchart D, Linde K, Worku F, Bauer R, Wagner H. Immunomodulation with Echinacea: a systematic review of controlled trials. Phytomedicine 1994;1:245-54.
55. Bräunig B, Dorn M, Limburg E, Knick E. Bausendorf: Echinaceae purpureae radix: zur Stärkung der körpereigenen Abwehr bei grippalen Infekten. Zeitschrift fur Phytotherapie 1992;13:7-13.
56. Bräunig B, Knick E. Therapeutische Erfahrungen mit Echinaceae pallidae bei grippalen Infekten. Naturheilpraxis 1993;1:72-5.
57. Dorn M. Milerung grippaler Effeckte durch ein pflanzliches Immunstimulans. Nutur Ganzheitsmedizin 1989;2:314-9.
58. Forth H, Beuscher N. Beeinflussing der Häufigkeit banaler Erkältungsinfekte durch Esberitox. Zeitschrift für Allgemeinmedizin 1981;57:2272-5.
59. Reitz HD. Immunmodulatoren mit pflanzlichen Wirkstoffen: eine wissenschaftliche Studie am Beispiel Esberitox N. Notabene medici 1990;20:362-6.
60. Schmidt U, Albrecht M, Schenk N. Pflankliches Immunstimulans senkt Häufigkeit grippaler Infekte: Plazebokontrollierte Doppelblindstudie mit einem kombinierten Echinacea-Präparat mit 646 Studenten der Kölner Universität. Natur Ganzheits Medizin 1990;3:277-81.
61. Schöneberger D. Einfluß der immunstimulierenden Wirkung von Preßsaft aus Herba Echinaceae purpureae auf Verlauf und Schweregrad von Erkältungskrankheiten. Ergebnisse einer Doppelblindstudie. Forum Immunologie 1992;2:18-22.
62. Vorberg G. Bei Erkältung unspezifische Immunabwehr stimulieran: Doppelblindstudie zeigt: Das bewährte Phytotherapeutikum Esberitox verkürzt die Symptommatik. Ärztliche Praxis 1984;36:97-8.
63. Vorberg G, Schneider B. Pflanzliches Immunstimulans verkürzt grippalen Infeckt. Doppelblindstudie belegt die Steigerung der unspezifischen Infektabwehr. Ärztliche Forschung 1989;36:3-8.
64. Galea S, Thacker K. Double-blind prospective trial investigating the effectiveness of a commonly prescribed herbal remedy in altering the duration, severity and symptoms of the common cold. Unpublished manuscript, 1996.
65. Grimm W, Muller HH. A randomized controlled trail of the effect of fluid extract of Echinacea purpurea on incidence and severity of colds and respiratory infections. Am J Med 1999;106:138-144.
66. Brinkeborn R, Shah D, Geissbühler S, Degenring FH. Echinaforce in the treatment of acute colds. Schweiz Zschr GanzheitsMedizin 1998;10:26-9.
67. Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schäfer M. Echinagard treatment shortens the course of the common cold: a double-blind, placebo-controlled clinical trial. Eur J Clin Res 1997;9:261-8.
68. Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med 1998;7:541-5.
69. Mengs U, Clare CB, Poiley JA. Toxicity of Echinacea pur
70. Mullins RJ. Echinacea-associated anaphylaxis Med J Aust 1998;168:170-1.
71. Reichling J, Saller R. Control in the manufacturing of modern herbal remedies. Q Rev Nat Med 1998;Spring:21-8.