User login
COVID-19: ‘Record’ spike in Internet anxiety, panic queries
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
More evidence links gum disease and dementia risk
especially in those with severe gum inflammation and edentulism, new research suggests.
Over a 20-year period, investigators prospectively followed more than 8,000 individuals aged around 63 years who did not have cognitive impairment or dementia at baseline, grouping them based on the extent and severity of their periodontal disease and number of lost teeth.
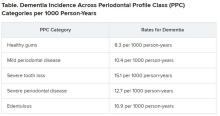
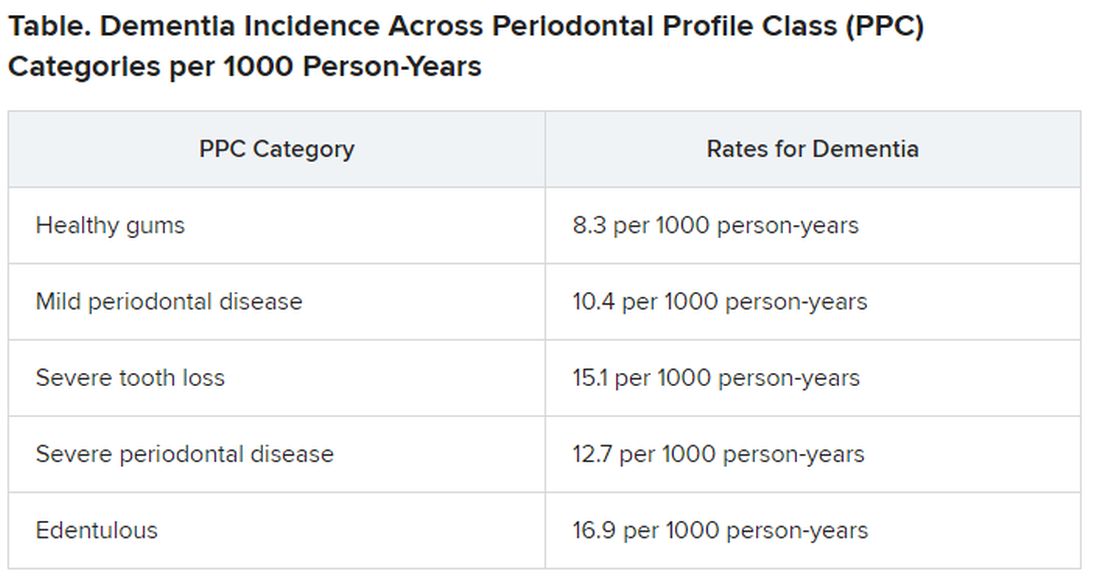
Results showed that 14% of participants with healthy gums and all their teeth at baseline developed dementia, compared with 18% of those with mild periodontal disease and 22% who had severe periodontal disease. The highest percentage (23%) of participants who developed dementia was found in those who were edentulous.
After accounting for comorbidities that might affect dementia risk, edentulous participants had a 20% higher risk for developing MCI or dementia, compared with the healthy group.
Because the study was observational, “we don’t have knowledge of causality so we cannot state that if you treat periodontal disease you can prevent or treat dementia,” said lead author Ryan T. Demmer, PhD, MPH, associate professor, division of epidemiology and community health, University of Minnesota, Minneapolis. However, “the take-home message from this paper is that it further supports the possibility that oral infections could be a risk factor for dementia.”
The study was published online July 29 in Neurology.
The ARIC trial
Prior studies have “described the interrelation of tooth loss or periodontal disease and cognitive outcomes, although many reports were cross-sectional or case-control … and often lacked robust confounder adjustment,” the investigators noted. Additionally, lack of longitudinal data impedes the “potential for baseline periodontal status to predict incident MCI.”
To explore the associations between periodontal status and incident MCI and dementia, the researchers studied participants in the ARIC study, a community-based longitudinal cohort consisting of 15,792 predominantly Black and White participants aged 45-64 years. The current analysis included 8,275 individuals (55% women; 21% black; mean age, 63 years) who at baseline did not meet criteria for dementia or MCI.
A full-mouth periodontal examination was conducted at baseline and participants were categorized according to the severity and extent of gingival inflammation and tooth attachment loss based on the Periodontal Profile Class (PPC) seven-category model. Potential confounding variables included age, race, education level, physical activity, smoking status, oral hygiene and access to care, plasma lipid levels, APOE genotype, body mass index, blood pressure, type 2 diabetes, and heart failure.
Based on PPC categorization, 22% of the patients had healthy gums, 12% had mild periodontal disease, 8% had a high gingival inflammation index, and 12% had posterior disease (with 6% having severe disease). In addition, 9% had tooth loss, 11% had severe tooth loss, and 20% were edentulous.
Infection hypothesis
Results showed that participants with worse periodontal status were more likely to have risk factors for vascular disease and dementia, such as smoking, hypertension, diabetes, and coronary heart disease. During median follow-up of 18.4 years, 19% of participants overall (n = 1,569) developed dementia, translating into 11.8 cases per 1,000 person-years. There were notable differences between the PPC categories in rates of incident dementia, with edentulous participants at twice the risk for developing dementia, compared with those who had healthy gums.
For participants with severe PPC, including severe tooth loss and severe disease, the multivariable-adjusted hazard ratio for incident dementia was 1.22 (95% confidence interval, 1.01-1.47) versus those who were periodontally healthy. For participants with edentulism, the HR was 1.21 (95% CI, 0.99-1.48). The adjusted risk ratios for the combined dementia/MCI outcome among participants with mild to intermediate PPC, severe PPC, or edentulism versus the periodontal healthy group were 1.22 (95% CI, 1.00-1.48), 1.15 (95% CI, 0.88-1.51), and 1.90 (95% CI, 1.40-2.58), respectively.
These findings were most pronounced among younger (median age at dental exam, younger than 62) versus older (62 years and older) participants (P = .02). Severe disease or total tooth loss were associated with an approximately 20% greater dementia incidence during the follow-up period, compared with healthy gums.
The investigators noted that the findings were “generally consistent” when considering the combined outcome of MCI and dementia. However, they noted that the association between edentulism and MCI was “markedly stronger,” with an approximate 100% increase in MCI or MCI plus dementia.
The association between periodontal disease and MCI or dementia “is rooted in the infection hypothesis, meaning adverse microbial exposures in the mucosal surfaces of the mouth, especially the subgingival space,” Dr. Demmer said. “One notion is that there could somehow be a direct infection of the brain with oral organisms, which posits that the oral organism could travel to the brain, colonize there, and cause damage that impairs cognition.”
Another possible mechanism is that chronic systemic inflammation in response to oral infections can eventually lead to vascular disease which, in turn, is a known risk factor for future dementia, he noted.
“Brush and floss”
Commenting on the research findings, James M. Noble, MD, associate professor of neurology, Taub Institute for Research on Alzheimer’s and the Aging Brain, Columbia University, New York, called the study “well characterized both by whole-mouth assessments and cognitive assessments performed in a standardized manner.” Moreover, “the study was sufficiently sized to allow for exploration of age and suggests that oral health may be a more important factor earlier in the course of aging, in late adulthood,” said Dr. Noble, who was not involved with the research.
The study also “makes an important contribution to this field through a rigorously followed cohort and robust design for both periodontal predictor and cognitive outcome assessments,” he said, noting that, “as always, the take-home message is ‘brush and floss.’
“Although we don’t know if treating periodontal disease can help treat dementia, this study suggests that we have to pay attention to good oral hygiene and make referrals to dentists when appropriate,” Dr. Demmer added.
The ARIC trial is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute. Dr. Demmer, the study coauthors, and Dr. Noble have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
especially in those with severe gum inflammation and edentulism, new research suggests.
Over a 20-year period, investigators prospectively followed more than 8,000 individuals aged around 63 years who did not have cognitive impairment or dementia at baseline, grouping them based on the extent and severity of their periodontal disease and number of lost teeth.
Results showed that 14% of participants with healthy gums and all their teeth at baseline developed dementia, compared with 18% of those with mild periodontal disease and 22% who had severe periodontal disease. The highest percentage (23%) of participants who developed dementia was found in those who were edentulous.
After accounting for comorbidities that might affect dementia risk, edentulous participants had a 20% higher risk for developing MCI or dementia, compared with the healthy group.
Because the study was observational, “we don’t have knowledge of causality so we cannot state that if you treat periodontal disease you can prevent or treat dementia,” said lead author Ryan T. Demmer, PhD, MPH, associate professor, division of epidemiology and community health, University of Minnesota, Minneapolis. However, “the take-home message from this paper is that it further supports the possibility that oral infections could be a risk factor for dementia.”
The study was published online July 29 in Neurology.
The ARIC trial
Prior studies have “described the interrelation of tooth loss or periodontal disease and cognitive outcomes, although many reports were cross-sectional or case-control … and often lacked robust confounder adjustment,” the investigators noted. Additionally, lack of longitudinal data impedes the “potential for baseline periodontal status to predict incident MCI.”
To explore the associations between periodontal status and incident MCI and dementia, the researchers studied participants in the ARIC study, a community-based longitudinal cohort consisting of 15,792 predominantly Black and White participants aged 45-64 years. The current analysis included 8,275 individuals (55% women; 21% black; mean age, 63 years) who at baseline did not meet criteria for dementia or MCI.
A full-mouth periodontal examination was conducted at baseline and participants were categorized according to the severity and extent of gingival inflammation and tooth attachment loss based on the Periodontal Profile Class (PPC) seven-category model. Potential confounding variables included age, race, education level, physical activity, smoking status, oral hygiene and access to care, plasma lipid levels, APOE genotype, body mass index, blood pressure, type 2 diabetes, and heart failure.
Based on PPC categorization, 22% of the patients had healthy gums, 12% had mild periodontal disease, 8% had a high gingival inflammation index, and 12% had posterior disease (with 6% having severe disease). In addition, 9% had tooth loss, 11% had severe tooth loss, and 20% were edentulous.
Infection hypothesis
Results showed that participants with worse periodontal status were more likely to have risk factors for vascular disease and dementia, such as smoking, hypertension, diabetes, and coronary heart disease. During median follow-up of 18.4 years, 19% of participants overall (n = 1,569) developed dementia, translating into 11.8 cases per 1,000 person-years. There were notable differences between the PPC categories in rates of incident dementia, with edentulous participants at twice the risk for developing dementia, compared with those who had healthy gums.
For participants with severe PPC, including severe tooth loss and severe disease, the multivariable-adjusted hazard ratio for incident dementia was 1.22 (95% confidence interval, 1.01-1.47) versus those who were periodontally healthy. For participants with edentulism, the HR was 1.21 (95% CI, 0.99-1.48). The adjusted risk ratios for the combined dementia/MCI outcome among participants with mild to intermediate PPC, severe PPC, or edentulism versus the periodontal healthy group were 1.22 (95% CI, 1.00-1.48), 1.15 (95% CI, 0.88-1.51), and 1.90 (95% CI, 1.40-2.58), respectively.
These findings were most pronounced among younger (median age at dental exam, younger than 62) versus older (62 years and older) participants (P = .02). Severe disease or total tooth loss were associated with an approximately 20% greater dementia incidence during the follow-up period, compared with healthy gums.
The investigators noted that the findings were “generally consistent” when considering the combined outcome of MCI and dementia. However, they noted that the association between edentulism and MCI was “markedly stronger,” with an approximate 100% increase in MCI or MCI plus dementia.
The association between periodontal disease and MCI or dementia “is rooted in the infection hypothesis, meaning adverse microbial exposures in the mucosal surfaces of the mouth, especially the subgingival space,” Dr. Demmer said. “One notion is that there could somehow be a direct infection of the brain with oral organisms, which posits that the oral organism could travel to the brain, colonize there, and cause damage that impairs cognition.”
Another possible mechanism is that chronic systemic inflammation in response to oral infections can eventually lead to vascular disease which, in turn, is a known risk factor for future dementia, he noted.
“Brush and floss”
Commenting on the research findings, James M. Noble, MD, associate professor of neurology, Taub Institute for Research on Alzheimer’s and the Aging Brain, Columbia University, New York, called the study “well characterized both by whole-mouth assessments and cognitive assessments performed in a standardized manner.” Moreover, “the study was sufficiently sized to allow for exploration of age and suggests that oral health may be a more important factor earlier in the course of aging, in late adulthood,” said Dr. Noble, who was not involved with the research.
The study also “makes an important contribution to this field through a rigorously followed cohort and robust design for both periodontal predictor and cognitive outcome assessments,” he said, noting that, “as always, the take-home message is ‘brush and floss.’
“Although we don’t know if treating periodontal disease can help treat dementia, this study suggests that we have to pay attention to good oral hygiene and make referrals to dentists when appropriate,” Dr. Demmer added.
The ARIC trial is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute. Dr. Demmer, the study coauthors, and Dr. Noble have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
especially in those with severe gum inflammation and edentulism, new research suggests.
Over a 20-year period, investigators prospectively followed more than 8,000 individuals aged around 63 years who did not have cognitive impairment or dementia at baseline, grouping them based on the extent and severity of their periodontal disease and number of lost teeth.
Results showed that 14% of participants with healthy gums and all their teeth at baseline developed dementia, compared with 18% of those with mild periodontal disease and 22% who had severe periodontal disease. The highest percentage (23%) of participants who developed dementia was found in those who were edentulous.
After accounting for comorbidities that might affect dementia risk, edentulous participants had a 20% higher risk for developing MCI or dementia, compared with the healthy group.
Because the study was observational, “we don’t have knowledge of causality so we cannot state that if you treat periodontal disease you can prevent or treat dementia,” said lead author Ryan T. Demmer, PhD, MPH, associate professor, division of epidemiology and community health, University of Minnesota, Minneapolis. However, “the take-home message from this paper is that it further supports the possibility that oral infections could be a risk factor for dementia.”
The study was published online July 29 in Neurology.
The ARIC trial
Prior studies have “described the interrelation of tooth loss or periodontal disease and cognitive outcomes, although many reports were cross-sectional or case-control … and often lacked robust confounder adjustment,” the investigators noted. Additionally, lack of longitudinal data impedes the “potential for baseline periodontal status to predict incident MCI.”
To explore the associations between periodontal status and incident MCI and dementia, the researchers studied participants in the ARIC study, a community-based longitudinal cohort consisting of 15,792 predominantly Black and White participants aged 45-64 years. The current analysis included 8,275 individuals (55% women; 21% black; mean age, 63 years) who at baseline did not meet criteria for dementia or MCI.
A full-mouth periodontal examination was conducted at baseline and participants were categorized according to the severity and extent of gingival inflammation and tooth attachment loss based on the Periodontal Profile Class (PPC) seven-category model. Potential confounding variables included age, race, education level, physical activity, smoking status, oral hygiene and access to care, plasma lipid levels, APOE genotype, body mass index, blood pressure, type 2 diabetes, and heart failure.
Based on PPC categorization, 22% of the patients had healthy gums, 12% had mild periodontal disease, 8% had a high gingival inflammation index, and 12% had posterior disease (with 6% having severe disease). In addition, 9% had tooth loss, 11% had severe tooth loss, and 20% were edentulous.
Infection hypothesis
Results showed that participants with worse periodontal status were more likely to have risk factors for vascular disease and dementia, such as smoking, hypertension, diabetes, and coronary heart disease. During median follow-up of 18.4 years, 19% of participants overall (n = 1,569) developed dementia, translating into 11.8 cases per 1,000 person-years. There were notable differences between the PPC categories in rates of incident dementia, with edentulous participants at twice the risk for developing dementia, compared with those who had healthy gums.
For participants with severe PPC, including severe tooth loss and severe disease, the multivariable-adjusted hazard ratio for incident dementia was 1.22 (95% confidence interval, 1.01-1.47) versus those who were periodontally healthy. For participants with edentulism, the HR was 1.21 (95% CI, 0.99-1.48). The adjusted risk ratios for the combined dementia/MCI outcome among participants with mild to intermediate PPC, severe PPC, or edentulism versus the periodontal healthy group were 1.22 (95% CI, 1.00-1.48), 1.15 (95% CI, 0.88-1.51), and 1.90 (95% CI, 1.40-2.58), respectively.
These findings were most pronounced among younger (median age at dental exam, younger than 62) versus older (62 years and older) participants (P = .02). Severe disease or total tooth loss were associated with an approximately 20% greater dementia incidence during the follow-up period, compared with healthy gums.
The investigators noted that the findings were “generally consistent” when considering the combined outcome of MCI and dementia. However, they noted that the association between edentulism and MCI was “markedly stronger,” with an approximate 100% increase in MCI or MCI plus dementia.
The association between periodontal disease and MCI or dementia “is rooted in the infection hypothesis, meaning adverse microbial exposures in the mucosal surfaces of the mouth, especially the subgingival space,” Dr. Demmer said. “One notion is that there could somehow be a direct infection of the brain with oral organisms, which posits that the oral organism could travel to the brain, colonize there, and cause damage that impairs cognition.”
Another possible mechanism is that chronic systemic inflammation in response to oral infections can eventually lead to vascular disease which, in turn, is a known risk factor for future dementia, he noted.
“Brush and floss”
Commenting on the research findings, James M. Noble, MD, associate professor of neurology, Taub Institute for Research on Alzheimer’s and the Aging Brain, Columbia University, New York, called the study “well characterized both by whole-mouth assessments and cognitive assessments performed in a standardized manner.” Moreover, “the study was sufficiently sized to allow for exploration of age and suggests that oral health may be a more important factor earlier in the course of aging, in late adulthood,” said Dr. Noble, who was not involved with the research.
The study also “makes an important contribution to this field through a rigorously followed cohort and robust design for both periodontal predictor and cognitive outcome assessments,” he said, noting that, “as always, the take-home message is ‘brush and floss.’
“Although we don’t know if treating periodontal disease can help treat dementia, this study suggests that we have to pay attention to good oral hygiene and make referrals to dentists when appropriate,” Dr. Demmer added.
The ARIC trial is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute. Dr. Demmer, the study coauthors, and Dr. Noble have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Frequent cannabis use in depression tripled over past decade
Not only are individuals with depression at significantly higher risk for cannabis use, compared with those without depression, this trend has increased dramatically over the last decade, new research shows.
Investigators analyzed data from more than 16,000 U.S. adults between the ages of 20 and 59 years and found that those with depression had almost twice the odds of any past-month cannabis use compared with those without depression. Odds rose from 1.5 in the 2005-2006 period to 2.3 in the 2015-2016 period.
Moreover, the odds ratio for daily or near-daily use almost tripled for those with versus without depression between the two periods.
“Clinicians should screen their depressed patients for cannabis use, since this is becoming more common and could actually make their depressive symptoms worse rather than better,” senior author Deborah Hasin, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York City, told Medscape Medical News.
The results were published online August 18 in JAMA Network Open.
Misleading advertising
“Cannabis use is increasing in the U.S. and the potency of cannabis products is increasing as well,” Dr. Hasin said.
“Misleading media information and advertising suggests that cannabis is a good treatment for depression, although studies show that cannabis use may actually worsen depression symptoms, [so] we were interested in whether U.S. adults were increasingly likely to be cannabis users if they were depressed,” she reported.
To investigate, the researchers assessed data from the National Health and Nutrition Examination Survey (NHANES), with a final study sample consisting of 16,216 U.S. adults. The mean age was 39.12 years, 48.9% were men, 66.4% were non-Hispanic White, 65.6% had at least some college education, and 62.4% had an annual family income of less than $75,000.
Of these participants, 7.5% had “probable depression,” based on the Patient Health Questionnaire–9, the investigators report.
Past-month cannabis use was defined as using cannabis at least once during the past 20 days. Daily or near-daily past-month use was defined as using cannabis at least 20 times in the past 30 days.
Covariates included age, gender, race, education, marital status, annual family income, and past-year use of other substances, such as alcohol, heroin, and methamphetamine.
The researchers note that because the NHANES data were divided into six survey years (2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, and 2015-2016), their analysis was based on a “new sample weight” that combined the datasets.
Especially pronounced
Results showed that the prevalence of any past-month cannabis use in the overall sample group increased from 12.2% in the 2005-2006 period to 17.3% in the 2015-2016 period (P < .001).
The investigators characterized this change as “significant,” adding that the estimated odds of cannabis use increased by approximately 9% between every 2-year time period.
The change was even more dramatic when the increase was examined across survey time periods (OR, 1.12; P < .001). The estimated odds of daily or near-daily use increased by approximately 12% between every 2-year period.
Interestingly, however, there were no significant changes in odds for depression when consecutive survey years were compared.
When the researchers specifically focused on the association between any past-month cannabis use and depression versus no depression, they found an adjusted OR of 1.90 (95% CI, 1.62-2.12; P < .001).
Individuals with depression also had 2.29 (95% CI, 1.80-2.92) times the odds for daily or near-daily cannabis use, compared with those without depression.
A post-hoc analysis looked at time trends in a sample group that included those missing information on at least one covariate (n = 17,724 participants). It showed similar results to those in the final sample that included no missing data.
People with depression have increased risk of using “most substances that can be abused,” Dr. Hasin said. “However, with the overall rates of cannabis use increasing in the general population, this is becoming especially pronounced for cannabis.”
Clear implications
Commenting on the findings for Medscape Medical News, Deepak D’Souza, MD, professor of psychiatry, Yale University, New Haven, Conn., said there is “concern about the unsubstantiated claims of cannabis having a beneficial effect in psychiatric disorders, the most common being depression.”
Dr. D’Souza, who was not involved with the study, called it “yet another piece of evidence suggesting that over the period of time during which cannabis laws have been liberalized, rates of past-month and daily cannabis use have increased, whereas rates of other substances, including alcohol, have remained stable.”
He suggested that a common limitation of epidemiological studies is that it is difficult to tell the direction of the association, “and it could be bidirectional.”
Nevertheless, there are clear implications for the practicing clinician, he added.
“If people have a history of depression, one should ask patients about the use of cannabis and also remind them about potential psychiatric negative effects of use,” Dr. D’Souza noted.
For the general public, “the point is that there is no good evidence to support cannabis use in depression treatment and, in fact, people with depression might be more likely to use it in problematic way,” he said.
Dr. Hasin agreed that it is “certainly possible that the relationship between cannabis use and depression is bidirectional, but the mechanism of this association requires more study.”
The study was supported by a grant from the National Institute on Drug Abuse to Dr. Hasin and by the New York State Psychiatric Institute. The study authors and Dr. D’Souza disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Not only are individuals with depression at significantly higher risk for cannabis use, compared with those without depression, this trend has increased dramatically over the last decade, new research shows.
Investigators analyzed data from more than 16,000 U.S. adults between the ages of 20 and 59 years and found that those with depression had almost twice the odds of any past-month cannabis use compared with those without depression. Odds rose from 1.5 in the 2005-2006 period to 2.3 in the 2015-2016 period.
Moreover, the odds ratio for daily or near-daily use almost tripled for those with versus without depression between the two periods.
“Clinicians should screen their depressed patients for cannabis use, since this is becoming more common and could actually make their depressive symptoms worse rather than better,” senior author Deborah Hasin, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York City, told Medscape Medical News.
The results were published online August 18 in JAMA Network Open.
Misleading advertising
“Cannabis use is increasing in the U.S. and the potency of cannabis products is increasing as well,” Dr. Hasin said.
“Misleading media information and advertising suggests that cannabis is a good treatment for depression, although studies show that cannabis use may actually worsen depression symptoms, [so] we were interested in whether U.S. adults were increasingly likely to be cannabis users if they were depressed,” she reported.
To investigate, the researchers assessed data from the National Health and Nutrition Examination Survey (NHANES), with a final study sample consisting of 16,216 U.S. adults. The mean age was 39.12 years, 48.9% were men, 66.4% were non-Hispanic White, 65.6% had at least some college education, and 62.4% had an annual family income of less than $75,000.
Of these participants, 7.5% had “probable depression,” based on the Patient Health Questionnaire–9, the investigators report.
Past-month cannabis use was defined as using cannabis at least once during the past 20 days. Daily or near-daily past-month use was defined as using cannabis at least 20 times in the past 30 days.
Covariates included age, gender, race, education, marital status, annual family income, and past-year use of other substances, such as alcohol, heroin, and methamphetamine.
The researchers note that because the NHANES data were divided into six survey years (2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, and 2015-2016), their analysis was based on a “new sample weight” that combined the datasets.
Especially pronounced
Results showed that the prevalence of any past-month cannabis use in the overall sample group increased from 12.2% in the 2005-2006 period to 17.3% in the 2015-2016 period (P < .001).
The investigators characterized this change as “significant,” adding that the estimated odds of cannabis use increased by approximately 9% between every 2-year time period.
The change was even more dramatic when the increase was examined across survey time periods (OR, 1.12; P < .001). The estimated odds of daily or near-daily use increased by approximately 12% between every 2-year period.
Interestingly, however, there were no significant changes in odds for depression when consecutive survey years were compared.
When the researchers specifically focused on the association between any past-month cannabis use and depression versus no depression, they found an adjusted OR of 1.90 (95% CI, 1.62-2.12; P < .001).
Individuals with depression also had 2.29 (95% CI, 1.80-2.92) times the odds for daily or near-daily cannabis use, compared with those without depression.
A post-hoc analysis looked at time trends in a sample group that included those missing information on at least one covariate (n = 17,724 participants). It showed similar results to those in the final sample that included no missing data.
People with depression have increased risk of using “most substances that can be abused,” Dr. Hasin said. “However, with the overall rates of cannabis use increasing in the general population, this is becoming especially pronounced for cannabis.”
Clear implications
Commenting on the findings for Medscape Medical News, Deepak D’Souza, MD, professor of psychiatry, Yale University, New Haven, Conn., said there is “concern about the unsubstantiated claims of cannabis having a beneficial effect in psychiatric disorders, the most common being depression.”
Dr. D’Souza, who was not involved with the study, called it “yet another piece of evidence suggesting that over the period of time during which cannabis laws have been liberalized, rates of past-month and daily cannabis use have increased, whereas rates of other substances, including alcohol, have remained stable.”
He suggested that a common limitation of epidemiological studies is that it is difficult to tell the direction of the association, “and it could be bidirectional.”
Nevertheless, there are clear implications for the practicing clinician, he added.
“If people have a history of depression, one should ask patients about the use of cannabis and also remind them about potential psychiatric negative effects of use,” Dr. D’Souza noted.
For the general public, “the point is that there is no good evidence to support cannabis use in depression treatment and, in fact, people with depression might be more likely to use it in problematic way,” he said.
Dr. Hasin agreed that it is “certainly possible that the relationship between cannabis use and depression is bidirectional, but the mechanism of this association requires more study.”
The study was supported by a grant from the National Institute on Drug Abuse to Dr. Hasin and by the New York State Psychiatric Institute. The study authors and Dr. D’Souza disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Not only are individuals with depression at significantly higher risk for cannabis use, compared with those without depression, this trend has increased dramatically over the last decade, new research shows.
Investigators analyzed data from more than 16,000 U.S. adults between the ages of 20 and 59 years and found that those with depression had almost twice the odds of any past-month cannabis use compared with those without depression. Odds rose from 1.5 in the 2005-2006 period to 2.3 in the 2015-2016 period.
Moreover, the odds ratio for daily or near-daily use almost tripled for those with versus without depression between the two periods.
“Clinicians should screen their depressed patients for cannabis use, since this is becoming more common and could actually make their depressive symptoms worse rather than better,” senior author Deborah Hasin, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York City, told Medscape Medical News.
The results were published online August 18 in JAMA Network Open.
Misleading advertising
“Cannabis use is increasing in the U.S. and the potency of cannabis products is increasing as well,” Dr. Hasin said.
“Misleading media information and advertising suggests that cannabis is a good treatment for depression, although studies show that cannabis use may actually worsen depression symptoms, [so] we were interested in whether U.S. adults were increasingly likely to be cannabis users if they were depressed,” she reported.
To investigate, the researchers assessed data from the National Health and Nutrition Examination Survey (NHANES), with a final study sample consisting of 16,216 U.S. adults. The mean age was 39.12 years, 48.9% were men, 66.4% were non-Hispanic White, 65.6% had at least some college education, and 62.4% had an annual family income of less than $75,000.
Of these participants, 7.5% had “probable depression,” based on the Patient Health Questionnaire–9, the investigators report.
Past-month cannabis use was defined as using cannabis at least once during the past 20 days. Daily or near-daily past-month use was defined as using cannabis at least 20 times in the past 30 days.
Covariates included age, gender, race, education, marital status, annual family income, and past-year use of other substances, such as alcohol, heroin, and methamphetamine.
The researchers note that because the NHANES data were divided into six survey years (2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, and 2015-2016), their analysis was based on a “new sample weight” that combined the datasets.
Especially pronounced
Results showed that the prevalence of any past-month cannabis use in the overall sample group increased from 12.2% in the 2005-2006 period to 17.3% in the 2015-2016 period (P < .001).
The investigators characterized this change as “significant,” adding that the estimated odds of cannabis use increased by approximately 9% between every 2-year time period.
The change was even more dramatic when the increase was examined across survey time periods (OR, 1.12; P < .001). The estimated odds of daily or near-daily use increased by approximately 12% between every 2-year period.
Interestingly, however, there were no significant changes in odds for depression when consecutive survey years were compared.
When the researchers specifically focused on the association between any past-month cannabis use and depression versus no depression, they found an adjusted OR of 1.90 (95% CI, 1.62-2.12; P < .001).
Individuals with depression also had 2.29 (95% CI, 1.80-2.92) times the odds for daily or near-daily cannabis use, compared with those without depression.
A post-hoc analysis looked at time trends in a sample group that included those missing information on at least one covariate (n = 17,724 participants). It showed similar results to those in the final sample that included no missing data.
People with depression have increased risk of using “most substances that can be abused,” Dr. Hasin said. “However, with the overall rates of cannabis use increasing in the general population, this is becoming especially pronounced for cannabis.”
Clear implications
Commenting on the findings for Medscape Medical News, Deepak D’Souza, MD, professor of psychiatry, Yale University, New Haven, Conn., said there is “concern about the unsubstantiated claims of cannabis having a beneficial effect in psychiatric disorders, the most common being depression.”
Dr. D’Souza, who was not involved with the study, called it “yet another piece of evidence suggesting that over the period of time during which cannabis laws have been liberalized, rates of past-month and daily cannabis use have increased, whereas rates of other substances, including alcohol, have remained stable.”
He suggested that a common limitation of epidemiological studies is that it is difficult to tell the direction of the association, “and it could be bidirectional.”
Nevertheless, there are clear implications for the practicing clinician, he added.
“If people have a history of depression, one should ask patients about the use of cannabis and also remind them about potential psychiatric negative effects of use,” Dr. D’Souza noted.
For the general public, “the point is that there is no good evidence to support cannabis use in depression treatment and, in fact, people with depression might be more likely to use it in problematic way,” he said.
Dr. Hasin agreed that it is “certainly possible that the relationship between cannabis use and depression is bidirectional, but the mechanism of this association requires more study.”
The study was supported by a grant from the National Institute on Drug Abuse to Dr. Hasin and by the New York State Psychiatric Institute. The study authors and Dr. D’Souza disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
AHA statement recommends dietary screening at routine checkups
A new scientific statement from the American Heart Association recommends incorporating a rapid diet-screening tool into routine primary care visits to inform dietary counseling and integrating the tool into patients’ electronic health record platforms across all healthcare settings.
The statement authors evaluated 15 existing screening tools and, although they did not recommend a specific tool, they did present advantages and disadvantages of some of the tools and encouraged “critical conversations” among clinicians and other specialists to arrive at a tool that would be most appropriate for use in a particular health care setting.
“The key takeaway is for clinicians to incorporate discussion of dietary patterns into routine preventive care appointments because a suboptimal diet is the No. 1 risk factor for cardiovascular disease,” Maya Vadiveloo, PhD, RD, chair of the statement group, said in an interview.
“We also wanted to touch on the fact the screening tool could be incorporated into the EHR and then used for clinical support and for tracking and monitoring the patient’s dietary patterns over time,” said Dr. Vadiveloo, assistant professor of nutrition and food sciences in the College of Health Science, University of Rhode Island, Kingston.
The statement was published online Aug. 7 in Circulation: Cardiovascular Quality and Outcomes.
Competing demands
Poor dietary quality has “surpassed all other mortality risk factors, accounting for 11 million deaths and about 50% of cardiovascular disease (CVD) deaths globally,” the authors wrote.
Diets deficient in fruits, vegetables, and whole grains and high in red and processed meat, added sugars, sodium, and total energy are the “leading determinants” of the risks for CVD and other conditions, so “strategies that promote holistically healthier dietary patterns to reduce chronic disease risk are of contemporary importance.”
Most clinicians and other members of health care teams “do not currently assess or counsel patients about their food and beverage intake during routine clinical care,” the authors observed.
Reasons for this may include lack of training and knowledge, insufficient time, insufficient integration of nutrition services into health care settings, insufficient reimbursement, and “competing demands during the visit,” they noted.
Dr. Vadiveloo said that an evidence-based rapid screening tool can go a long way toward helping to overcome these barriers.
“Research shows that when primary care practitioners discuss diet with patients, the patients are receptive, but we also know that clinical workloads are already very compressed, and adding another thing to a routine preventive care appointment is challenging,” she said. “So we wanted to look and see if there were already screening tools that showed promise as valid, reliable, reflective of the best science, and easy to incorporate into various types of practice settings.”
Top picks
The authors established “theoretical and practice-based criteria” for an optimal diet screening tool for use in the adult population (aged 20 to 75 years). The tool had to:
- Be developed or used within clinical practice in the past 10 years.
- Be evidence-based, reliable, and valid.
- Assess total dietary pattern rather than focusing on a single food or nutrient.
- Be able to be completed and scored at administration without special knowledge or software.
- Give actionable next steps and support to patients.
- Be able track and monitor dietary change over time.
- Be brief.
- Be useful for chronic disease management.
Of the 15 tools reviewed, the three that met the most theoretical and practice-based validity criteria were the Mediterranean Diet Adherence Screener (MEDAS) and its variations; the modified, shortened Rapid Eating Assessment for Participants (REAP), and the modified version of the Starting the Conversation Tool. However, the authors noted that the Powell and Greenberg Screening Tool was the “least time-intensive.”
One size does not fit all
No single tool will be appropriate for all practice settings, so “we would like clinicians to discuss what will work in their particular setting,” Dr. Vadiveloo emphasized.
For example, should the screening tool be completed by the clinician, a member of the health care team, or the patient? Advantages of a tool completed by clinicians or team members include collection of the information in real time, where it can be used in shared decision-making during the encounter and increased reliability because the screen has been completed by a clinician. On the other hand, the clinician might not be able to prioritize administering the screening tool during a short clinical encounter.
Advantages of a tool completed by the patient via an EHR portal is that the patient may feel less risk of judgment by the clinician or health care professional and patients can complete the screen at their convenience. Disadvantages are limited reach into underserved populations and, potentially, less reliability than clinician-administered tools.
“It is advantageous to have tools that can be administered by multiple members of health care teams to ease the demand on clinicians, if such staff is available, but in other settings, self-administration might be better, so we tried to leave it open-ended,” Dr. Vadiveloo explained.
‘Ideal platform’
“The EHR is the ideal platform to prompt clinicians and other members of the health care team to capture dietary data and deliver dietary advice to patients,” the authors observed.
EHRs allow secure storage of data and also enable access to these data when needed at the point of care. They are also important for documentation purposes.
The authors noted that the use of “myriad EHR platforms and versions of platforms” have created “technical challenges.” They recommended “standardized approaches” for transmitting health data that will “more seamlessly allow rapid diet screeners to be implemented in the EHR.”
They also recommended that the prototypes of rapid diet screeners be tested by end users prior to implementation within particular clinics. “Gathering these data ahead of time can improve the uptake of the application in the real world,” they stated.
Dr. Vadiveloo added that dietary counseling can be conducted by several members of a health care team, such as a dietitian, not just by the physician. Or the patient may need to be referred to a dietitian for counseling and follow-up.
The authors concluded by characterizing the AHA statement as “a call to action ... designed to accelerate efforts to make diet quality assessment an integral part of office-based care delivery by encouraging critical conversations among clinicians, individuals with diet/lifestyle expertise, and specialists in information technology.”
Dr. Vadiveloo has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association recommends incorporating a rapid diet-screening tool into routine primary care visits to inform dietary counseling and integrating the tool into patients’ electronic health record platforms across all healthcare settings.
The statement authors evaluated 15 existing screening tools and, although they did not recommend a specific tool, they did present advantages and disadvantages of some of the tools and encouraged “critical conversations” among clinicians and other specialists to arrive at a tool that would be most appropriate for use in a particular health care setting.
“The key takeaway is for clinicians to incorporate discussion of dietary patterns into routine preventive care appointments because a suboptimal diet is the No. 1 risk factor for cardiovascular disease,” Maya Vadiveloo, PhD, RD, chair of the statement group, said in an interview.
“We also wanted to touch on the fact the screening tool could be incorporated into the EHR and then used for clinical support and for tracking and monitoring the patient’s dietary patterns over time,” said Dr. Vadiveloo, assistant professor of nutrition and food sciences in the College of Health Science, University of Rhode Island, Kingston.
The statement was published online Aug. 7 in Circulation: Cardiovascular Quality and Outcomes.
Competing demands
Poor dietary quality has “surpassed all other mortality risk factors, accounting for 11 million deaths and about 50% of cardiovascular disease (CVD) deaths globally,” the authors wrote.
Diets deficient in fruits, vegetables, and whole grains and high in red and processed meat, added sugars, sodium, and total energy are the “leading determinants” of the risks for CVD and other conditions, so “strategies that promote holistically healthier dietary patterns to reduce chronic disease risk are of contemporary importance.”
Most clinicians and other members of health care teams “do not currently assess or counsel patients about their food and beverage intake during routine clinical care,” the authors observed.
Reasons for this may include lack of training and knowledge, insufficient time, insufficient integration of nutrition services into health care settings, insufficient reimbursement, and “competing demands during the visit,” they noted.
Dr. Vadiveloo said that an evidence-based rapid screening tool can go a long way toward helping to overcome these barriers.
“Research shows that when primary care practitioners discuss diet with patients, the patients are receptive, but we also know that clinical workloads are already very compressed, and adding another thing to a routine preventive care appointment is challenging,” she said. “So we wanted to look and see if there were already screening tools that showed promise as valid, reliable, reflective of the best science, and easy to incorporate into various types of practice settings.”
Top picks
The authors established “theoretical and practice-based criteria” for an optimal diet screening tool for use in the adult population (aged 20 to 75 years). The tool had to:
- Be developed or used within clinical practice in the past 10 years.
- Be evidence-based, reliable, and valid.
- Assess total dietary pattern rather than focusing on a single food or nutrient.
- Be able to be completed and scored at administration without special knowledge or software.
- Give actionable next steps and support to patients.
- Be able track and monitor dietary change over time.
- Be brief.
- Be useful for chronic disease management.
Of the 15 tools reviewed, the three that met the most theoretical and practice-based validity criteria were the Mediterranean Diet Adherence Screener (MEDAS) and its variations; the modified, shortened Rapid Eating Assessment for Participants (REAP), and the modified version of the Starting the Conversation Tool. However, the authors noted that the Powell and Greenberg Screening Tool was the “least time-intensive.”
One size does not fit all
No single tool will be appropriate for all practice settings, so “we would like clinicians to discuss what will work in their particular setting,” Dr. Vadiveloo emphasized.
For example, should the screening tool be completed by the clinician, a member of the health care team, or the patient? Advantages of a tool completed by clinicians or team members include collection of the information in real time, where it can be used in shared decision-making during the encounter and increased reliability because the screen has been completed by a clinician. On the other hand, the clinician might not be able to prioritize administering the screening tool during a short clinical encounter.
Advantages of a tool completed by the patient via an EHR portal is that the patient may feel less risk of judgment by the clinician or health care professional and patients can complete the screen at their convenience. Disadvantages are limited reach into underserved populations and, potentially, less reliability than clinician-administered tools.
“It is advantageous to have tools that can be administered by multiple members of health care teams to ease the demand on clinicians, if such staff is available, but in other settings, self-administration might be better, so we tried to leave it open-ended,” Dr. Vadiveloo explained.
‘Ideal platform’
“The EHR is the ideal platform to prompt clinicians and other members of the health care team to capture dietary data and deliver dietary advice to patients,” the authors observed.
EHRs allow secure storage of data and also enable access to these data when needed at the point of care. They are also important for documentation purposes.
The authors noted that the use of “myriad EHR platforms and versions of platforms” have created “technical challenges.” They recommended “standardized approaches” for transmitting health data that will “more seamlessly allow rapid diet screeners to be implemented in the EHR.”
They also recommended that the prototypes of rapid diet screeners be tested by end users prior to implementation within particular clinics. “Gathering these data ahead of time can improve the uptake of the application in the real world,” they stated.
Dr. Vadiveloo added that dietary counseling can be conducted by several members of a health care team, such as a dietitian, not just by the physician. Or the patient may need to be referred to a dietitian for counseling and follow-up.
The authors concluded by characterizing the AHA statement as “a call to action ... designed to accelerate efforts to make diet quality assessment an integral part of office-based care delivery by encouraging critical conversations among clinicians, individuals with diet/lifestyle expertise, and specialists in information technology.”
Dr. Vadiveloo has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association recommends incorporating a rapid diet-screening tool into routine primary care visits to inform dietary counseling and integrating the tool into patients’ electronic health record platforms across all healthcare settings.
The statement authors evaluated 15 existing screening tools and, although they did not recommend a specific tool, they did present advantages and disadvantages of some of the tools and encouraged “critical conversations” among clinicians and other specialists to arrive at a tool that would be most appropriate for use in a particular health care setting.
“The key takeaway is for clinicians to incorporate discussion of dietary patterns into routine preventive care appointments because a suboptimal diet is the No. 1 risk factor for cardiovascular disease,” Maya Vadiveloo, PhD, RD, chair of the statement group, said in an interview.
“We also wanted to touch on the fact the screening tool could be incorporated into the EHR and then used for clinical support and for tracking and monitoring the patient’s dietary patterns over time,” said Dr. Vadiveloo, assistant professor of nutrition and food sciences in the College of Health Science, University of Rhode Island, Kingston.
The statement was published online Aug. 7 in Circulation: Cardiovascular Quality and Outcomes.
Competing demands
Poor dietary quality has “surpassed all other mortality risk factors, accounting for 11 million deaths and about 50% of cardiovascular disease (CVD) deaths globally,” the authors wrote.
Diets deficient in fruits, vegetables, and whole grains and high in red and processed meat, added sugars, sodium, and total energy are the “leading determinants” of the risks for CVD and other conditions, so “strategies that promote holistically healthier dietary patterns to reduce chronic disease risk are of contemporary importance.”
Most clinicians and other members of health care teams “do not currently assess or counsel patients about their food and beverage intake during routine clinical care,” the authors observed.
Reasons for this may include lack of training and knowledge, insufficient time, insufficient integration of nutrition services into health care settings, insufficient reimbursement, and “competing demands during the visit,” they noted.
Dr. Vadiveloo said that an evidence-based rapid screening tool can go a long way toward helping to overcome these barriers.
“Research shows that when primary care practitioners discuss diet with patients, the patients are receptive, but we also know that clinical workloads are already very compressed, and adding another thing to a routine preventive care appointment is challenging,” she said. “So we wanted to look and see if there were already screening tools that showed promise as valid, reliable, reflective of the best science, and easy to incorporate into various types of practice settings.”
Top picks
The authors established “theoretical and practice-based criteria” for an optimal diet screening tool for use in the adult population (aged 20 to 75 years). The tool had to:
- Be developed or used within clinical practice in the past 10 years.
- Be evidence-based, reliable, and valid.
- Assess total dietary pattern rather than focusing on a single food or nutrient.
- Be able to be completed and scored at administration without special knowledge or software.
- Give actionable next steps and support to patients.
- Be able track and monitor dietary change over time.
- Be brief.
- Be useful for chronic disease management.
Of the 15 tools reviewed, the three that met the most theoretical and practice-based validity criteria were the Mediterranean Diet Adherence Screener (MEDAS) and its variations; the modified, shortened Rapid Eating Assessment for Participants (REAP), and the modified version of the Starting the Conversation Tool. However, the authors noted that the Powell and Greenberg Screening Tool was the “least time-intensive.”
One size does not fit all
No single tool will be appropriate for all practice settings, so “we would like clinicians to discuss what will work in their particular setting,” Dr. Vadiveloo emphasized.
For example, should the screening tool be completed by the clinician, a member of the health care team, or the patient? Advantages of a tool completed by clinicians or team members include collection of the information in real time, where it can be used in shared decision-making during the encounter and increased reliability because the screen has been completed by a clinician. On the other hand, the clinician might not be able to prioritize administering the screening tool during a short clinical encounter.
Advantages of a tool completed by the patient via an EHR portal is that the patient may feel less risk of judgment by the clinician or health care professional and patients can complete the screen at their convenience. Disadvantages are limited reach into underserved populations and, potentially, less reliability than clinician-administered tools.
“It is advantageous to have tools that can be administered by multiple members of health care teams to ease the demand on clinicians, if such staff is available, but in other settings, self-administration might be better, so we tried to leave it open-ended,” Dr. Vadiveloo explained.
‘Ideal platform’
“The EHR is the ideal platform to prompt clinicians and other members of the health care team to capture dietary data and deliver dietary advice to patients,” the authors observed.
EHRs allow secure storage of data and also enable access to these data when needed at the point of care. They are also important for documentation purposes.
The authors noted that the use of “myriad EHR platforms and versions of platforms” have created “technical challenges.” They recommended “standardized approaches” for transmitting health data that will “more seamlessly allow rapid diet screeners to be implemented in the EHR.”
They also recommended that the prototypes of rapid diet screeners be tested by end users prior to implementation within particular clinics. “Gathering these data ahead of time can improve the uptake of the application in the real world,” they stated.
Dr. Vadiveloo added that dietary counseling can be conducted by several members of a health care team, such as a dietitian, not just by the physician. Or the patient may need to be referred to a dietitian for counseling and follow-up.
The authors concluded by characterizing the AHA statement as “a call to action ... designed to accelerate efforts to make diet quality assessment an integral part of office-based care delivery by encouraging critical conversations among clinicians, individuals with diet/lifestyle expertise, and specialists in information technology.”
Dr. Vadiveloo has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article originally appeared on Medscape.com.
Chloroquine linked to serious psychiatric side effects
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
ED visits for mental health, substance use doubled in 1 decade
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
‘Staggering’ increase in COVID-linked depression, anxiety
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Ticagrelor/aspirin combo: Fewer repeat strokes and deaths, but more bleeds
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From New England Journal of Medicine
Prior beta-blockers predict extra burden of heart failure in women with ACS
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
PCI or not, mortality climbs with post-ACS bleeding complications
Patients with acute coronary syndromes (ACS) with later bleeding complications that were at least moderate in severity showed a 15-fold increased risk of dying within 30 days, compared with those without such bleeding, in a pooled analysis of four randomized antithrombotic-therapy trials.

Mortality 1 month to 1 year after a bleeding event was not as sharply increased, but there was still almost triple the risk seen in patients without bleeding complications.
In both cases, the risk increase was independent of whether percutaneous coronary intervention (PCI) had been part of the management of ACS, concludes the study, published in the July 14 issue of the Journal of the American College of Cardiology.
“We showed that postdischarge bleeding was associated with a pretty bad prognosis, in terms of all-cause mortality, regardless of the index treatment – PCI or medical therapy,” lead author Guillaume Marquis-Gravel, MD, MSc, Duke Clinical Research Institute, Durham, N.C., said in an interview.
“Our data suggest that we should care about bleeding prevention in patients who had a previous ACS, regardless of the treatment strategy, as much as we care for prevention of future ischemic events,” said Dr. Marquis-Gravel, who is also an interventional cardiologist at the Montreal Heart Institute.
“This large-scale analysis clearly demonstrates that bleeding events occurring among ACS patients with coronary stents carry the same prognostic significance in magnitude and time course as among patients who do not undergo PCI,” observed Derek Chew, MBBS, MPH, PhD, of Flinders University, Adelaide, Australia, and Jack Wei Chieh Tan, MBBS, MBA, of National Heart Centre, Singapore, in an accompanying editorial.
“Therefore, at least in the later phases of planning antithrombotic therapy, when weighting bleeding risk in these conditions, these estimates should not be ‘discounted’ for the absence or presence of PCI during the initial ACS management,” they wrote.
A “proven assumption”
“A great deal of research has previously been conducted to tailor DAPT [dual-antiplatelet therapy] and to minimize bleeding risk following PCI based on the proven assumption that bleeding is associated with adverse clinical outcomes,” Dr. Marques-Gravel explained.
“The prognostic impact of postdischarge bleeding has not been studied thoroughly in patients with ACS who were only treated medically with DAPT without PCI.” Yet this population makes up a large proportion of the ACS population, and patients are “generally older and sicker” and therefore at increased risk for both ischemic and bleeding events, he said.
The researchers explored those issues in a post hoc pooled analysis of four randomized comparisons of antithrombotic strategies in patients with ACS: APPRAISE-2, PLATO, TRACER, and TRILOGY ACS. The analyses tracked bleeding events that took place from a landmark time of 7 days after presentation with ACS over a median follow-up of 1 year in 45,011 patients (31.3% female), 48% of whom were managed with PCI.
Those treated with PCI, compared with those medically managed only, tended to be younger, more often male, more likely to have ST-segment elevation myocardial infarction (STEMI) as their ACS, and less likely to have cardiovascular comorbidities.
During the total follow-up of 48,717 person-years, the postdischarge rate of moderate, severe, or life-threatening bleeding defined by GUSTO criteria reached 2.6 events per 100 patient-years. A total of 2,149 patients died, and mortality was consistently higher in patients who had such bleeding complications. They showed an adjusted hazard ratio of 15.7 (95% confidence interval, 12.3-20.0) for mortality within 30 days, compared with patients without bleeds. Their HR for mortality at 30 days to 1 year was 2.7 (95% CI, 2.1-3.4).
The association between bleeding complications and mortality remained consistent, regardless of whether patients had undergone PCI for their ACS (interaction P = .240).
A pragmatic interpretation
Although an observational study can’t show causality between bleeding and mortality, Dr. Marquis-Gravel cautioned, “the fact that the majority of deaths occurred early after the bleeding event, within 30 days, is strongly suggestive of a causal relationship.”
He recommended a “pragmatic interpretation” of the study: “Bleeding avoidance strategies tested in PCI populations, including short-term DAPT or aspirin-free strategies, should also be considered in medically treated patients with ACS deemed at higher risk of bleeding.”
“It is clear that bleeding events after successful PCI for an ACS are independently associated with increased mortality and morbidity,” Debabrata Mukherjee, MD, of Texas Tech University, El Paso, said in an interview.
“Every effort should be made to minimize bleeding events with the use of appropriate access site for PCI, dosing, selection, and duration of antiplatelet and antithrombotic agents, and use of proton pump inhibitors when appropriate,” he said.
The clinical decision-making involved in this individualized approach “is often not easy,” said Dr. Mukherjee, who was not involved in the current study. “Integrating patients and clinical pharmacists in choosing optimal antithrombotic therapies post-MI is likely to be helpful” in the process.
Although “major bleeding following ACS increases the risk of mortality for both medically managed and PCI-managed patients with ACS, the vast majority of deaths, 90%, occur in those that have not had a bleed,” Mamas A. Mamas, DPhil, Keele University, Staffordshire, England, said in an interview.
“It is important to understand the causes of death in this population and think about how interventions may impact on this,” agreed Dr. Mamas, who was not involved in the study.
Dr. Marquis-Gravel reported receiving speaking fees and honoraria from Servier and Novartis; disclosures for the other authors are in the report. Dr. Chew reported receiving speaking fees and institutional grants in aid from Roche Diagnostics, AstraZeneca, and Edwards Lifesciences. Dr. Tan discloses receiving speaking fees and educational grants from Amgen, Roche Diagnostics, AstraZeneca, Bayer, and Abbott Vascular. Dr. Mukherjee and Dr. Mamas report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with acute coronary syndromes (ACS) with later bleeding complications that were at least moderate in severity showed a 15-fold increased risk of dying within 30 days, compared with those without such bleeding, in a pooled analysis of four randomized antithrombotic-therapy trials.

Mortality 1 month to 1 year after a bleeding event was not as sharply increased, but there was still almost triple the risk seen in patients without bleeding complications.
In both cases, the risk increase was independent of whether percutaneous coronary intervention (PCI) had been part of the management of ACS, concludes the study, published in the July 14 issue of the Journal of the American College of Cardiology.
“We showed that postdischarge bleeding was associated with a pretty bad prognosis, in terms of all-cause mortality, regardless of the index treatment – PCI or medical therapy,” lead author Guillaume Marquis-Gravel, MD, MSc, Duke Clinical Research Institute, Durham, N.C., said in an interview.
“Our data suggest that we should care about bleeding prevention in patients who had a previous ACS, regardless of the treatment strategy, as much as we care for prevention of future ischemic events,” said Dr. Marquis-Gravel, who is also an interventional cardiologist at the Montreal Heart Institute.
“This large-scale analysis clearly demonstrates that bleeding events occurring among ACS patients with coronary stents carry the same prognostic significance in magnitude and time course as among patients who do not undergo PCI,” observed Derek Chew, MBBS, MPH, PhD, of Flinders University, Adelaide, Australia, and Jack Wei Chieh Tan, MBBS, MBA, of National Heart Centre, Singapore, in an accompanying editorial.
“Therefore, at least in the later phases of planning antithrombotic therapy, when weighting bleeding risk in these conditions, these estimates should not be ‘discounted’ for the absence or presence of PCI during the initial ACS management,” they wrote.
A “proven assumption”
“A great deal of research has previously been conducted to tailor DAPT [dual-antiplatelet therapy] and to minimize bleeding risk following PCI based on the proven assumption that bleeding is associated with adverse clinical outcomes,” Dr. Marques-Gravel explained.
“The prognostic impact of postdischarge bleeding has not been studied thoroughly in patients with ACS who were only treated medically with DAPT without PCI.” Yet this population makes up a large proportion of the ACS population, and patients are “generally older and sicker” and therefore at increased risk for both ischemic and bleeding events, he said.
The researchers explored those issues in a post hoc pooled analysis of four randomized comparisons of antithrombotic strategies in patients with ACS: APPRAISE-2, PLATO, TRACER, and TRILOGY ACS. The analyses tracked bleeding events that took place from a landmark time of 7 days after presentation with ACS over a median follow-up of 1 year in 45,011 patients (31.3% female), 48% of whom were managed with PCI.
Those treated with PCI, compared with those medically managed only, tended to be younger, more often male, more likely to have ST-segment elevation myocardial infarction (STEMI) as their ACS, and less likely to have cardiovascular comorbidities.
During the total follow-up of 48,717 person-years, the postdischarge rate of moderate, severe, or life-threatening bleeding defined by GUSTO criteria reached 2.6 events per 100 patient-years. A total of 2,149 patients died, and mortality was consistently higher in patients who had such bleeding complications. They showed an adjusted hazard ratio of 15.7 (95% confidence interval, 12.3-20.0) for mortality within 30 days, compared with patients without bleeds. Their HR for mortality at 30 days to 1 year was 2.7 (95% CI, 2.1-3.4).
The association between bleeding complications and mortality remained consistent, regardless of whether patients had undergone PCI for their ACS (interaction P = .240).
A pragmatic interpretation
Although an observational study can’t show causality between bleeding and mortality, Dr. Marquis-Gravel cautioned, “the fact that the majority of deaths occurred early after the bleeding event, within 30 days, is strongly suggestive of a causal relationship.”
He recommended a “pragmatic interpretation” of the study: “Bleeding avoidance strategies tested in PCI populations, including short-term DAPT or aspirin-free strategies, should also be considered in medically treated patients with ACS deemed at higher risk of bleeding.”
“It is clear that bleeding events after successful PCI for an ACS are independently associated with increased mortality and morbidity,” Debabrata Mukherjee, MD, of Texas Tech University, El Paso, said in an interview.
“Every effort should be made to minimize bleeding events with the use of appropriate access site for PCI, dosing, selection, and duration of antiplatelet and antithrombotic agents, and use of proton pump inhibitors when appropriate,” he said.
The clinical decision-making involved in this individualized approach “is often not easy,” said Dr. Mukherjee, who was not involved in the current study. “Integrating patients and clinical pharmacists in choosing optimal antithrombotic therapies post-MI is likely to be helpful” in the process.
Although “major bleeding following ACS increases the risk of mortality for both medically managed and PCI-managed patients with ACS, the vast majority of deaths, 90%, occur in those that have not had a bleed,” Mamas A. Mamas, DPhil, Keele University, Staffordshire, England, said in an interview.
“It is important to understand the causes of death in this population and think about how interventions may impact on this,” agreed Dr. Mamas, who was not involved in the study.
Dr. Marquis-Gravel reported receiving speaking fees and honoraria from Servier and Novartis; disclosures for the other authors are in the report. Dr. Chew reported receiving speaking fees and institutional grants in aid from Roche Diagnostics, AstraZeneca, and Edwards Lifesciences. Dr. Tan discloses receiving speaking fees and educational grants from Amgen, Roche Diagnostics, AstraZeneca, Bayer, and Abbott Vascular. Dr. Mukherjee and Dr. Mamas report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with acute coronary syndromes (ACS) with later bleeding complications that were at least moderate in severity showed a 15-fold increased risk of dying within 30 days, compared with those without such bleeding, in a pooled analysis of four randomized antithrombotic-therapy trials.

Mortality 1 month to 1 year after a bleeding event was not as sharply increased, but there was still almost triple the risk seen in patients without bleeding complications.
In both cases, the risk increase was independent of whether percutaneous coronary intervention (PCI) had been part of the management of ACS, concludes the study, published in the July 14 issue of the Journal of the American College of Cardiology.
“We showed that postdischarge bleeding was associated with a pretty bad prognosis, in terms of all-cause mortality, regardless of the index treatment – PCI or medical therapy,” lead author Guillaume Marquis-Gravel, MD, MSc, Duke Clinical Research Institute, Durham, N.C., said in an interview.
“Our data suggest that we should care about bleeding prevention in patients who had a previous ACS, regardless of the treatment strategy, as much as we care for prevention of future ischemic events,” said Dr. Marquis-Gravel, who is also an interventional cardiologist at the Montreal Heart Institute.
“This large-scale analysis clearly demonstrates that bleeding events occurring among ACS patients with coronary stents carry the same prognostic significance in magnitude and time course as among patients who do not undergo PCI,” observed Derek Chew, MBBS, MPH, PhD, of Flinders University, Adelaide, Australia, and Jack Wei Chieh Tan, MBBS, MBA, of National Heart Centre, Singapore, in an accompanying editorial.
“Therefore, at least in the later phases of planning antithrombotic therapy, when weighting bleeding risk in these conditions, these estimates should not be ‘discounted’ for the absence or presence of PCI during the initial ACS management,” they wrote.
A “proven assumption”
“A great deal of research has previously been conducted to tailor DAPT [dual-antiplatelet therapy] and to minimize bleeding risk following PCI based on the proven assumption that bleeding is associated with adverse clinical outcomes,” Dr. Marques-Gravel explained.
“The prognostic impact of postdischarge bleeding has not been studied thoroughly in patients with ACS who were only treated medically with DAPT without PCI.” Yet this population makes up a large proportion of the ACS population, and patients are “generally older and sicker” and therefore at increased risk for both ischemic and bleeding events, he said.
The researchers explored those issues in a post hoc pooled analysis of four randomized comparisons of antithrombotic strategies in patients with ACS: APPRAISE-2, PLATO, TRACER, and TRILOGY ACS. The analyses tracked bleeding events that took place from a landmark time of 7 days after presentation with ACS over a median follow-up of 1 year in 45,011 patients (31.3% female), 48% of whom were managed with PCI.
Those treated with PCI, compared with those medically managed only, tended to be younger, more often male, more likely to have ST-segment elevation myocardial infarction (STEMI) as their ACS, and less likely to have cardiovascular comorbidities.
During the total follow-up of 48,717 person-years, the postdischarge rate of moderate, severe, or life-threatening bleeding defined by GUSTO criteria reached 2.6 events per 100 patient-years. A total of 2,149 patients died, and mortality was consistently higher in patients who had such bleeding complications. They showed an adjusted hazard ratio of 15.7 (95% confidence interval, 12.3-20.0) for mortality within 30 days, compared with patients without bleeds. Their HR for mortality at 30 days to 1 year was 2.7 (95% CI, 2.1-3.4).
The association between bleeding complications and mortality remained consistent, regardless of whether patients had undergone PCI for their ACS (interaction P = .240).
A pragmatic interpretation
Although an observational study can’t show causality between bleeding and mortality, Dr. Marquis-Gravel cautioned, “the fact that the majority of deaths occurred early after the bleeding event, within 30 days, is strongly suggestive of a causal relationship.”
He recommended a “pragmatic interpretation” of the study: “Bleeding avoidance strategies tested in PCI populations, including short-term DAPT or aspirin-free strategies, should also be considered in medically treated patients with ACS deemed at higher risk of bleeding.”
“It is clear that bleeding events after successful PCI for an ACS are independently associated with increased mortality and morbidity,” Debabrata Mukherjee, MD, of Texas Tech University, El Paso, said in an interview.
“Every effort should be made to minimize bleeding events with the use of appropriate access site for PCI, dosing, selection, and duration of antiplatelet and antithrombotic agents, and use of proton pump inhibitors when appropriate,” he said.
The clinical decision-making involved in this individualized approach “is often not easy,” said Dr. Mukherjee, who was not involved in the current study. “Integrating patients and clinical pharmacists in choosing optimal antithrombotic therapies post-MI is likely to be helpful” in the process.
Although “major bleeding following ACS increases the risk of mortality for both medically managed and PCI-managed patients with ACS, the vast majority of deaths, 90%, occur in those that have not had a bleed,” Mamas A. Mamas, DPhil, Keele University, Staffordshire, England, said in an interview.
“It is important to understand the causes of death in this population and think about how interventions may impact on this,” agreed Dr. Mamas, who was not involved in the study.
Dr. Marquis-Gravel reported receiving speaking fees and honoraria from Servier and Novartis; disclosures for the other authors are in the report. Dr. Chew reported receiving speaking fees and institutional grants in aid from Roche Diagnostics, AstraZeneca, and Edwards Lifesciences. Dr. Tan discloses receiving speaking fees and educational grants from Amgen, Roche Diagnostics, AstraZeneca, Bayer, and Abbott Vascular. Dr. Mukherjee and Dr. Mamas report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.