User login
Onychomycosis: A simpler in-office technique for sampling specimens
Background In onychomycosis, proper specimen collection is essential for an accurate diagnosis and initiation of appropriate therapy. Several techniques and locations have been suggested for specimen collection.
Objective To investigate the optimal technique of fungal sampling in onychomycosis.
Methods We reexamined 106 patients with distal and lateral subungual onychomycosis (DLSO) of the toenails. (The diagnosis had previously been confirmed by a laboratory mycological examination—both potassium hydroxide [KOH] test and fungal culture—of samples obtained by the proximal sampling approach.) We collected fungal specimens from the distal nail bed first, and later from the distal underside of the nail plate. The collected specimens underwent laboratory mycological examination.
Results KOH testing was positive in 84 (79.2%) specimens from the distal nail bed and only in 60 (56.6%) from the distal underside of the nail plate (P=.0007); cultures were positive in 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations showed positive KOH test results in 92 (86.8%) of the 106 patients and positive cultures in 100 (94.3%) patients.
Conclusions Based on our study, we suggest that in cases of suspected DLSO, material should be obtained by scraping nail material from the distal underside of the nail and then collecting all the material from the distal part of the nail bed.
When assessing possible onychomycosis, conventional practice is to take samples from the most proximal infected area. But this approach is usually technically difficult and may cause discomfort to patients.1-6 We therefore sought to determine the optimal location for fungal sampling from the distal part of the affected nail.
Methods
To assess the accuracy of distal sampling in diagnosing distal and lateral subungual onychomycosis (DLSO) of the toenails, we reevaluated 106 patients with DLSO previously confirmed by microscopic visualization of fungi in potassium hydroxide (KOH) solution and by fungal culture of specimens obtained using the proximal sampling approach.
Before we obtained our samples, we cleaned the nails with alcohol and pared the most distal part of the nails in an effort to eliminate contaminant molds and bacteria. Using a 1- or 2-mm curette, we took specimens first from the distal nail bed and, second, from the distal underside of the nail plate ( FIGURE ). We separated specimens for use in either direct KOH visualization or in fungal culture using Sabouraud’s Dextrose agar (Novamed; Jerusalem, Israel), which contains chloramphenicol or streptomycin and penicillin to prevent contamination.
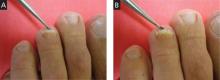
FIGURE
Distal sampling for distal and lateral subungual onychomycosis
Using a 2-mm curette, we collected specimens from the distal nail bed first (A), and then from the distal underside of the nail plate (B). However, our recommendation for clinical practice is to reverse this order of sampling to collect all possible material.
Statistical analyses
We recorded sociodemographic characteristics and fungal culture results in basic descriptive (prevalence) tables. In univariate analysis, we used t-tests to compare the means of continuous variables (eg, age, duration of fungal infection). To assess the distribution of categorical parameters (eg, sex) and to gauge the efficacy of the different probing techniques, we used chi-square (χ2) tests. We analyzed coded data using SPSS (Chicago, IL) for Windows software, version 12.
We conducted the study according to the rules of the local Helsinki Committee.
Results
We examined 106 patients with DLSO, of which 65 (61.3%) were male and 41 (38.7%) were female, ages 23 to 72 years (mean age, 44.6). The duration of fungal infection ranged from 3 to 30 years, with a mean of 14.9 years. In 70.8% of cases, the infection involved the first toenail. Duration of the fungal disease did not differ significantly between the sexes.
KOH test results were positive for 84 (79.2%) specimens from the distal nail bed, and for only 60 (56.6%) specimens from the distal underside of the nail plate (P=.0007); culture results were positive for 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations (all positive samples from the nail bed, plus positive samples from the nail underside when results from the nail bed were negative) yielded confirmation with KOH testing in 92 (86.8%) patients and with culture in 100 (94.3%) patients. There were no statistically significant differences between the combined results of both locations and the results from the distal nail bed alone (KOH, P=.143; culture, P=.149) ( TABLE ).
TABLE
Accuracy of distal sampling in 106 patients with confirmed DLSO
| Nail bed | Underside of nail plate | P value | Combined results | P value* | |
|---|---|---|---|---|---|
| Positive KOH | 84 (79.2%) | 60 (56.65%) | .0007 | 92 (86.8%) | .143 |
| Positive culture | 93 (87.7%) | 76 (71.7%) | .0063 | 100 (94.3%) | .149 |
| *Differences between results from sampling the nail bed alone and results from combined nail bed and nail plate sampling were not statistically significant. DLSO, distal and lateral subungual onychomycosis; KOH, potassium hydroxide. | |||||
Discussion
In DLSO, most dermatophyte species invade the middle and ventral layers of the nail plate adjacent to the nail bed, where the keratin is soft and close to the living cells below. In fact, the nail bed is probably the primary invasion site of dermatophytes, and it acts as a reservoir for continual reinfection of the growing nail.7 Obtaining confirmation of fungal infection before initiating antifungal treatment is the gold standard in clinical practice, given that antifungal agents have potentially serious adverse effects, that treatment is expensive, and that medicolegal issues exist.8
The standard methods used to detect a fungal nail infection are direct microscopy with KOH preparation and fungal culture. The KOH test is the simplest, least expensive method used in the detection of fungi, but it cannot identify the specific pathogen. Fungal speciation is done by culture. More accurate histopathologic evaluation is possible with periodic acid-Schiff stain, immunofluorescent microscopy with calcofluor stain, or polymerase chain reaction, but these techniques are more expensive and less feasible in outpatient clinics.9
The diagnostic accuracy of the KOH test and fungal culture ranges from 50% to 70%, depending on the experience of the laboratory technician and the methods used to collect and prepare the sample.8-10 It is better to take samples from the most proximal infected area by curettage or drilling, but this technique is usually more difficult than a distal approach, should be performed by skilled personnel, and may cause discomfort to patients.3,5,6
Our recommendation for practice. Earlier studies suggested that nail specimens should be taken from the nail bed.11-13 We sampled the nail bed first in our study because, in trying to determine an optimal location for sampling, we wanted to avoid contaminating nail-bed specimens with debris from the underside of the nail. In practice, however, we suggest that, in cases of suspected DLSO, clinicians first obtain specimens from the distal underside of the nail, and then collect all remaining material from the distal part of the nail bed. This technique is simple and can easily be performed in an office setting. If test results are negative but DLSO remains clinically likely, test a second sample after a week or 2.
CORRESPONDENCE Boaz Amichai, MD, Department of Dermatology, Sheba Medical Center, Tel-Hashomer, Israel; [email protected]
1. Lawry MA, Haneke E, Strobeck K, et al. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Arch Dermatol. 2000;136:1112-1116.
2. Elewski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3 pt 2):S6-S9.
3. Heikkila H. Isolation of fungi from onychomycosis-suspected nails by two methods: clipping and drilling. Mycoses. 1996;39:479-482.
4. Mochizuki T, Kawasaki M, Tanabe H, et al. A nail drilling method suitable for the diagnosis of onychomycosis. J Dermatol. 2005;32:108-113.
5. Shemer A, Trau H, Davidovici B, et al. Nail sampling in onychomycosis: comparative study of curettage from three sites of the infected nail. J Dtsch Dermatol Ges. 2007;5:1108-1111.
6. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
7. Hay RJ, Baran R, Haneke E. Fungal (onychomycosis) and other infections involving the nail apparatus. In: Baran R, Dawber RPR, eds. Disease of the Nails and Their Management. 2nd ed. Oxford, England: Blackwell Sciences Ltd; 1994: 97–134.
8. Daniel CR, 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
9. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
10. Arnold B, Kianifrad F, Tavakkol A. A comparison of KOH and culture results from two mycology laboratories for the diagnosis of onychomycosis during a randomized, multicenter clinical trial: a subset study. J Am Podiatr Med Assoc. 2005;95:421-423.
11. English MP. Nails and fungi. Br J Dermatol. 1976;94:697-701.
12. Elewski BE. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500-501.
13. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677–678.
Background In onychomycosis, proper specimen collection is essential for an accurate diagnosis and initiation of appropriate therapy. Several techniques and locations have been suggested for specimen collection.
Objective To investigate the optimal technique of fungal sampling in onychomycosis.
Methods We reexamined 106 patients with distal and lateral subungual onychomycosis (DLSO) of the toenails. (The diagnosis had previously been confirmed by a laboratory mycological examination—both potassium hydroxide [KOH] test and fungal culture—of samples obtained by the proximal sampling approach.) We collected fungal specimens from the distal nail bed first, and later from the distal underside of the nail plate. The collected specimens underwent laboratory mycological examination.
Results KOH testing was positive in 84 (79.2%) specimens from the distal nail bed and only in 60 (56.6%) from the distal underside of the nail plate (P=.0007); cultures were positive in 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations showed positive KOH test results in 92 (86.8%) of the 106 patients and positive cultures in 100 (94.3%) patients.
Conclusions Based on our study, we suggest that in cases of suspected DLSO, material should be obtained by scraping nail material from the distal underside of the nail and then collecting all the material from the distal part of the nail bed.
When assessing possible onychomycosis, conventional practice is to take samples from the most proximal infected area. But this approach is usually technically difficult and may cause discomfort to patients.1-6 We therefore sought to determine the optimal location for fungal sampling from the distal part of the affected nail.
Methods
To assess the accuracy of distal sampling in diagnosing distal and lateral subungual onychomycosis (DLSO) of the toenails, we reevaluated 106 patients with DLSO previously confirmed by microscopic visualization of fungi in potassium hydroxide (KOH) solution and by fungal culture of specimens obtained using the proximal sampling approach.
Before we obtained our samples, we cleaned the nails with alcohol and pared the most distal part of the nails in an effort to eliminate contaminant molds and bacteria. Using a 1- or 2-mm curette, we took specimens first from the distal nail bed and, second, from the distal underside of the nail plate ( FIGURE ). We separated specimens for use in either direct KOH visualization or in fungal culture using Sabouraud’s Dextrose agar (Novamed; Jerusalem, Israel), which contains chloramphenicol or streptomycin and penicillin to prevent contamination.
FIGURE
Distal sampling for distal and lateral subungual onychomycosis
Using a 2-mm curette, we collected specimens from the distal nail bed first (A), and then from the distal underside of the nail plate (B). However, our recommendation for clinical practice is to reverse this order of sampling to collect all possible material.
Statistical analyses
We recorded sociodemographic characteristics and fungal culture results in basic descriptive (prevalence) tables. In univariate analysis, we used t-tests to compare the means of continuous variables (eg, age, duration of fungal infection). To assess the distribution of categorical parameters (eg, sex) and to gauge the efficacy of the different probing techniques, we used chi-square (χ2) tests. We analyzed coded data using SPSS (Chicago, IL) for Windows software, version 12.
We conducted the study according to the rules of the local Helsinki Committee.
Results
We examined 106 patients with DLSO, of which 65 (61.3%) were male and 41 (38.7%) were female, ages 23 to 72 years (mean age, 44.6). The duration of fungal infection ranged from 3 to 30 years, with a mean of 14.9 years. In 70.8% of cases, the infection involved the first toenail. Duration of the fungal disease did not differ significantly between the sexes.
KOH test results were positive for 84 (79.2%) specimens from the distal nail bed, and for only 60 (56.6%) specimens from the distal underside of the nail plate (P=.0007); culture results were positive for 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations (all positive samples from the nail bed, plus positive samples from the nail underside when results from the nail bed were negative) yielded confirmation with KOH testing in 92 (86.8%) patients and with culture in 100 (94.3%) patients. There were no statistically significant differences between the combined results of both locations and the results from the distal nail bed alone (KOH, P=.143; culture, P=.149) ( TABLE ).
TABLE
Accuracy of distal sampling in 106 patients with confirmed DLSO
| Nail bed | Underside of nail plate | P value | Combined results | P value* | |
|---|---|---|---|---|---|
| Positive KOH | 84 (79.2%) | 60 (56.65%) | .0007 | 92 (86.8%) | .143 |
| Positive culture | 93 (87.7%) | 76 (71.7%) | .0063 | 100 (94.3%) | .149 |
| *Differences between results from sampling the nail bed alone and results from combined nail bed and nail plate sampling were not statistically significant. DLSO, distal and lateral subungual onychomycosis; KOH, potassium hydroxide. | |||||
Discussion
In DLSO, most dermatophyte species invade the middle and ventral layers of the nail plate adjacent to the nail bed, where the keratin is soft and close to the living cells below. In fact, the nail bed is probably the primary invasion site of dermatophytes, and it acts as a reservoir for continual reinfection of the growing nail.7 Obtaining confirmation of fungal infection before initiating antifungal treatment is the gold standard in clinical practice, given that antifungal agents have potentially serious adverse effects, that treatment is expensive, and that medicolegal issues exist.8
The standard methods used to detect a fungal nail infection are direct microscopy with KOH preparation and fungal culture. The KOH test is the simplest, least expensive method used in the detection of fungi, but it cannot identify the specific pathogen. Fungal speciation is done by culture. More accurate histopathologic evaluation is possible with periodic acid-Schiff stain, immunofluorescent microscopy with calcofluor stain, or polymerase chain reaction, but these techniques are more expensive and less feasible in outpatient clinics.9
The diagnostic accuracy of the KOH test and fungal culture ranges from 50% to 70%, depending on the experience of the laboratory technician and the methods used to collect and prepare the sample.8-10 It is better to take samples from the most proximal infected area by curettage or drilling, but this technique is usually more difficult than a distal approach, should be performed by skilled personnel, and may cause discomfort to patients.3,5,6
Our recommendation for practice. Earlier studies suggested that nail specimens should be taken from the nail bed.11-13 We sampled the nail bed first in our study because, in trying to determine an optimal location for sampling, we wanted to avoid contaminating nail-bed specimens with debris from the underside of the nail. In practice, however, we suggest that, in cases of suspected DLSO, clinicians first obtain specimens from the distal underside of the nail, and then collect all remaining material from the distal part of the nail bed. This technique is simple and can easily be performed in an office setting. If test results are negative but DLSO remains clinically likely, test a second sample after a week or 2.
CORRESPONDENCE Boaz Amichai, MD, Department of Dermatology, Sheba Medical Center, Tel-Hashomer, Israel; [email protected]
Background In onychomycosis, proper specimen collection is essential for an accurate diagnosis and initiation of appropriate therapy. Several techniques and locations have been suggested for specimen collection.
Objective To investigate the optimal technique of fungal sampling in onychomycosis.
Methods We reexamined 106 patients with distal and lateral subungual onychomycosis (DLSO) of the toenails. (The diagnosis had previously been confirmed by a laboratory mycological examination—both potassium hydroxide [KOH] test and fungal culture—of samples obtained by the proximal sampling approach.) We collected fungal specimens from the distal nail bed first, and later from the distal underside of the nail plate. The collected specimens underwent laboratory mycological examination.
Results KOH testing was positive in 84 (79.2%) specimens from the distal nail bed and only in 60 (56.6%) from the distal underside of the nail plate (P=.0007); cultures were positive in 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations showed positive KOH test results in 92 (86.8%) of the 106 patients and positive cultures in 100 (94.3%) patients.
Conclusions Based on our study, we suggest that in cases of suspected DLSO, material should be obtained by scraping nail material from the distal underside of the nail and then collecting all the material from the distal part of the nail bed.
When assessing possible onychomycosis, conventional practice is to take samples from the most proximal infected area. But this approach is usually technically difficult and may cause discomfort to patients.1-6 We therefore sought to determine the optimal location for fungal sampling from the distal part of the affected nail.
Methods
To assess the accuracy of distal sampling in diagnosing distal and lateral subungual onychomycosis (DLSO) of the toenails, we reevaluated 106 patients with DLSO previously confirmed by microscopic visualization of fungi in potassium hydroxide (KOH) solution and by fungal culture of specimens obtained using the proximal sampling approach.
Before we obtained our samples, we cleaned the nails with alcohol and pared the most distal part of the nails in an effort to eliminate contaminant molds and bacteria. Using a 1- or 2-mm curette, we took specimens first from the distal nail bed and, second, from the distal underside of the nail plate ( FIGURE ). We separated specimens for use in either direct KOH visualization or in fungal culture using Sabouraud’s Dextrose agar (Novamed; Jerusalem, Israel), which contains chloramphenicol or streptomycin and penicillin to prevent contamination.
FIGURE
Distal sampling for distal and lateral subungual onychomycosis
Using a 2-mm curette, we collected specimens from the distal nail bed first (A), and then from the distal underside of the nail plate (B). However, our recommendation for clinical practice is to reverse this order of sampling to collect all possible material.
Statistical analyses
We recorded sociodemographic characteristics and fungal culture results in basic descriptive (prevalence) tables. In univariate analysis, we used t-tests to compare the means of continuous variables (eg, age, duration of fungal infection). To assess the distribution of categorical parameters (eg, sex) and to gauge the efficacy of the different probing techniques, we used chi-square (χ2) tests. We analyzed coded data using SPSS (Chicago, IL) for Windows software, version 12.
We conducted the study according to the rules of the local Helsinki Committee.
Results
We examined 106 patients with DLSO, of which 65 (61.3%) were male and 41 (38.7%) were female, ages 23 to 72 years (mean age, 44.6). The duration of fungal infection ranged from 3 to 30 years, with a mean of 14.9 years. In 70.8% of cases, the infection involved the first toenail. Duration of the fungal disease did not differ significantly between the sexes.
KOH test results were positive for 84 (79.2%) specimens from the distal nail bed, and for only 60 (56.6%) specimens from the distal underside of the nail plate (P=.0007); culture results were positive for 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations (all positive samples from the nail bed, plus positive samples from the nail underside when results from the nail bed were negative) yielded confirmation with KOH testing in 92 (86.8%) patients and with culture in 100 (94.3%) patients. There were no statistically significant differences between the combined results of both locations and the results from the distal nail bed alone (KOH, P=.143; culture, P=.149) ( TABLE ).
TABLE
Accuracy of distal sampling in 106 patients with confirmed DLSO
| Nail bed | Underside of nail plate | P value | Combined results | P value* | |
|---|---|---|---|---|---|
| Positive KOH | 84 (79.2%) | 60 (56.65%) | .0007 | 92 (86.8%) | .143 |
| Positive culture | 93 (87.7%) | 76 (71.7%) | .0063 | 100 (94.3%) | .149 |
| *Differences between results from sampling the nail bed alone and results from combined nail bed and nail plate sampling were not statistically significant. DLSO, distal and lateral subungual onychomycosis; KOH, potassium hydroxide. | |||||
Discussion
In DLSO, most dermatophyte species invade the middle and ventral layers of the nail plate adjacent to the nail bed, where the keratin is soft and close to the living cells below. In fact, the nail bed is probably the primary invasion site of dermatophytes, and it acts as a reservoir for continual reinfection of the growing nail.7 Obtaining confirmation of fungal infection before initiating antifungal treatment is the gold standard in clinical practice, given that antifungal agents have potentially serious adverse effects, that treatment is expensive, and that medicolegal issues exist.8
The standard methods used to detect a fungal nail infection are direct microscopy with KOH preparation and fungal culture. The KOH test is the simplest, least expensive method used in the detection of fungi, but it cannot identify the specific pathogen. Fungal speciation is done by culture. More accurate histopathologic evaluation is possible with periodic acid-Schiff stain, immunofluorescent microscopy with calcofluor stain, or polymerase chain reaction, but these techniques are more expensive and less feasible in outpatient clinics.9
The diagnostic accuracy of the KOH test and fungal culture ranges from 50% to 70%, depending on the experience of the laboratory technician and the methods used to collect and prepare the sample.8-10 It is better to take samples from the most proximal infected area by curettage or drilling, but this technique is usually more difficult than a distal approach, should be performed by skilled personnel, and may cause discomfort to patients.3,5,6
Our recommendation for practice. Earlier studies suggested that nail specimens should be taken from the nail bed.11-13 We sampled the nail bed first in our study because, in trying to determine an optimal location for sampling, we wanted to avoid contaminating nail-bed specimens with debris from the underside of the nail. In practice, however, we suggest that, in cases of suspected DLSO, clinicians first obtain specimens from the distal underside of the nail, and then collect all remaining material from the distal part of the nail bed. This technique is simple and can easily be performed in an office setting. If test results are negative but DLSO remains clinically likely, test a second sample after a week or 2.
CORRESPONDENCE Boaz Amichai, MD, Department of Dermatology, Sheba Medical Center, Tel-Hashomer, Israel; [email protected]
1. Lawry MA, Haneke E, Strobeck K, et al. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Arch Dermatol. 2000;136:1112-1116.
2. Elewski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3 pt 2):S6-S9.
3. Heikkila H. Isolation of fungi from onychomycosis-suspected nails by two methods: clipping and drilling. Mycoses. 1996;39:479-482.
4. Mochizuki T, Kawasaki M, Tanabe H, et al. A nail drilling method suitable for the diagnosis of onychomycosis. J Dermatol. 2005;32:108-113.
5. Shemer A, Trau H, Davidovici B, et al. Nail sampling in onychomycosis: comparative study of curettage from three sites of the infected nail. J Dtsch Dermatol Ges. 2007;5:1108-1111.
6. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
7. Hay RJ, Baran R, Haneke E. Fungal (onychomycosis) and other infections involving the nail apparatus. In: Baran R, Dawber RPR, eds. Disease of the Nails and Their Management. 2nd ed. Oxford, England: Blackwell Sciences Ltd; 1994: 97–134.
8. Daniel CR, 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
9. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
10. Arnold B, Kianifrad F, Tavakkol A. A comparison of KOH and culture results from two mycology laboratories for the diagnosis of onychomycosis during a randomized, multicenter clinical trial: a subset study. J Am Podiatr Med Assoc. 2005;95:421-423.
11. English MP. Nails and fungi. Br J Dermatol. 1976;94:697-701.
12. Elewski BE. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500-501.
13. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677–678.
1. Lawry MA, Haneke E, Strobeck K, et al. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Arch Dermatol. 2000;136:1112-1116.
2. Elewski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3 pt 2):S6-S9.
3. Heikkila H. Isolation of fungi from onychomycosis-suspected nails by two methods: clipping and drilling. Mycoses. 1996;39:479-482.
4. Mochizuki T, Kawasaki M, Tanabe H, et al. A nail drilling method suitable for the diagnosis of onychomycosis. J Dermatol. 2005;32:108-113.
5. Shemer A, Trau H, Davidovici B, et al. Nail sampling in onychomycosis: comparative study of curettage from three sites of the infected nail. J Dtsch Dermatol Ges. 2007;5:1108-1111.
6. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
7. Hay RJ, Baran R, Haneke E. Fungal (onychomycosis) and other infections involving the nail apparatus. In: Baran R, Dawber RPR, eds. Disease of the Nails and Their Management. 2nd ed. Oxford, England: Blackwell Sciences Ltd; 1994: 97–134.
8. Daniel CR, 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
9. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
10. Arnold B, Kianifrad F, Tavakkol A. A comparison of KOH and culture results from two mycology laboratories for the diagnosis of onychomycosis during a randomized, multicenter clinical trial: a subset study. J Am Podiatr Med Assoc. 2005;95:421-423.
11. English MP. Nails and fungi. Br J Dermatol. 1976;94:697-701.
12. Elewski BE. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500-501.
13. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677–678.