User login
Research and Publication Trends
In 1996, Wachter and Goldman heralded the arrival of hospitalists in the healthcare system. They recognized the need to link the clinical role of a hospitalist with other activities, both to provide a creative outlet and to assist in the creation of research and development arms.[1] The explosive growth of hospital medicine followed, and hospitalists rapidly entered the mainstream of the healthcare system.[2]
A consensus conference in 2009 identified the challenges faced by hospitalists in conducting research as a key obstacle in the evolution of the profession into an academic field.[3] Strategies for building and facilitating hospitalist research programs have been described.[4, 5, 6, 7] However, a survey of US academic hospitalist leaders found more than 40% feared their faculty was not developing sustainable nonclinical activities.[8]
Data describing research aspirations and support systems among hospitalists are sparse, and no previous study has described the trends in hospitalist publications. In this work we describe the current standing of hospital medicine research through a survey of both academic and non‐academic hospitalists and a review of hospitalist‐related publications.
METHODS
The Indiana University institutional review board approved this study.
Survey of Hospitalists
A 29‐item questionnaire that addressed research activities, barriers, and mentorship was designed and piloted with pediatrics trainees at Indiana University. The final version (see Supporting Survey in the online version of this article) was approved by the Society of Hospital Medicine (SHM) research committee and posted on Zoomerang (
Review of Publications
A PubMed search was conducted on October 8, 2013 for records with either hospital medicine or hospitalist in the affiliation field. This field provides the departmental name and address information for the first author, except for the not‐yet‐indexed publisher‐supplied records, which could include all author addresses.[10] Editorials and letters to the editor were excluded, and results were limited to English. All resulting articles were manually curated and retained only if the affiliation criteria of hospitalist or hospital medicine (as a relevant single phrase) were associated with the first author. All articles meeting the criteria were reviewed by 1 of the authors and categorized as a review, a case report, or as original research (when methodology was described in the abstract). Original research articles were assigned a category based on their methodology and research type, as defined in published literature. The categories included basic sciences, clinical, health information, health services, quality improvement (QI), education, and translational research.[11, 12, 13, 14, 15] If the article overlapped categories, a secondary category was also assigned. A second author independently evaluated a subset of articles. This subset was then used to calculate the overall concordance between the authors based on their agreement on either the primary or secondary category designations.
To capture data on research funding, each original research article was searched for statements directly linking the first author or the work to the funding source(s).
Publications in the Journal of Hospital Medicine (JHM) were reviewed to serve as a gauge of research interests in the field of hospital medicine that may not be reflected by the publications resulting from the PubMed search. JHM was selected as the journal best representing hospital medicine based on its stated mission of commitment to the advancement of the hospital medicine specialty.[16] All original research articles in JHM were assigned a category by 1 of the authors based on the methodology in the abstract.
Statistical Methods
The survey responses were summarized using descriptive statistics. Univariate tests of association between respondent characteristics and peer‐reviewed authorship were performed using the Fisher's exact test. P values of 0.05 were considered significant. Data from the publication searches were presented as descriptive statistics.
RESULTS
Survey
The survey link was emailed to 11,611 SHM members: 11,102 members received the link and 509 emails were returned as undeliverable. A total of 645 member responses were received (5.8% response rate).
The most common demographic characteristics identified included male gender, age 45 or younger, and white race. The locations of the current practices were distributed equally across the United States. Over half of the respondents were trained in internal medicine, and a quarter were trained in pediatrics. Eleven percent had undertaken fellowship training after residency. Thirty‐seven percent did not hold an academic rank, and among those who did, most were assistant professors. (Table 1)
| Characteristics | Responses, N (%) | |||
|---|---|---|---|---|
| All Responses | Responses With Funding | |||
| ||||
| Gender | 597 | 67 | ||
| Female | 248 (41) | 33 (49) | ||
| Male | 349 (58) | 34 (51) | ||
| Age, y | 599 | 67 | ||
| 2535 | 157 (26) | 17 (25) | ||
| 3645 | 274 (46) | 39 (58) | ||
| 4655 | 105 (17) | 6 (9) | ||
| 5665 | 56 (9) | 5 (7) | ||
| >65 | 7 (1) | 0 | ||
| Current practice location | 596 | 67 | ||
| Midwest | 147 (25) | 18 (27) | ||
| Northeast | 113 (19) | 12 (18) | ||
| South | 172 (29) | 14 (21) | ||
| West | 142 (4) | 16 (24) | ||
| Other | 22 (34) | 7 (10) | ||
| Race | 595 | 67 | ||
| White | 444 (75) | 58 (87) | ||
| Black | 18 (3) | 0 | ||
| Hispanic | 22 (4) | 1 (1) | ||
| Asian | 85 (14) | 8 (12) | ||
| Other | 26 (4) | |||
| Faculty appointment | 593 | 68 | ||
| Nonacademic | 221 (37) | 4 (6) | ||
| Instructor/lecturer | 60 (10) | 6 (9) | ||
| Assistant professor | 197 (33) | 32 (47) | ||
| Associate professor | 68 (11) | 19 (28) | ||
| Full professor | 14 (2) | 4 (6) | ||
| Other | 33 (6) | 3 (4) | ||
| Fellowship training | 68 | 14 | ||
| General IM/hospitalist | 15 (22) | 6 (43) | ||
| Pediatric hospital medicine | 7 (10) | 2 (14) | ||
| Other | 46 (68) | 6 (43) | ||
| Residency completed | 616 | 68 | ||
| IM | 340 (55) | 36 (53) | ||
| Pediatrics | 154 (25) | 27 (40) | ||
| Family medicine | 53 (9) | 1 (1) | ||
| IM/pediatrics | 48 (8) | 2 (3) | ||
| Other | 21 (3) | 2 (3) | ||
Overall availability of mentorship was low, but respondents with academic appointments were more likely to have a mentor than those without academic appointments (32% vs 2.7%, p<0.001). Hospitalists most likely identified their own mentors, and meetings between the hospitalist and mentor occurred more frequently than once every 3 months.
There were 213 (33%) respondents who identified themselves as currently conducting research, 96 (45%) of whom were trained in pediatrics. Ninety‐two (28%) of those with academic appointments and 157 (71%) of those without academic appointments had no current or future plans to engage in research. QI research, followed by clinical research, emerged as the most frequent type of research that hospitalists were either currently engaged in or planned to embark on. Most respondents identified factors other than age, family or financial issues, the grant process, or a lack of institutional support as the reason for not conducting research. (Table 2)
| Activity | Responses, N (%) | ||||
|---|---|---|---|---|---|
| Adult Medicine | Pediatric Medicine | ||||
| |||||
| No plan to conduct research | 245 | 26 | |||
| Reasons for not doing research | |||||
| Lack of institutional support | 42 (17) | 3 (12) | |||
| Family issues | 14 (6) | 1 (4) | |||
| Financial | 8 (3) | 0 | |||
| Grant process | 4 (2) | 2 (8) | |||
| Age | 5 (2) | 0 | |||
| Other | 171 (70) | 20 (77) | |||
| Currently doing research | 117 | 96 | |||
| Quality improvement | 79 (68) | 73 (76) | |||
| Clinical | 59 (50) | 62 (65) | |||
| Health services | 31 (26) | 30 (31) | |||
| Health informatics | 28 (24) | 11 (11) | |||
| Translational | 10 (8) | 7 (7) | |||
| Basic science | 3 (3) | 0 | |||
| Other | 17 (14) | 10 (10) | |||
| Plan on doing research | 183 | 30 | |||
| Quality improvement | 72 (39) | 25 (83) | |||
| Clinical | 65 (35) | 25 (83) | |||
| Health services | 20 (11) | 2 (7) | |||
| Health informatics | 25 (14) | 3 (10) | |||
| Translational | 8 (4) | 3 (10) | |||
| Basic science | 3 (2) | 0 | |||
| Other | 8 (4) | 0 | |||
| Peer‐review publications | 458 | 151 | |||
| No | 270 (59) | 62 (41) | |||
| Yes | 188 (41) | 89 (59) | |||
| Frequency | |||||
| Less than once/year | 111 (59) | 41 (46) | |||
| Once/year | 22 (12) | 20 (22) | |||
| Twice/year | 16 (8) | 16 (18) | |||
| More than twice/year | 23 (12) | 10 (11) | |||
| Other | 13 (7) | 1 (1) | |||
| Publication Type | |||||
| Original research | 97 (52) | 75 (84) | |||
| Case report/series | 80 (42) | 41 (46) | |||
| Reviews | 63 (34) | 25 (28) | |||
| Clinical trials | 36 (19) | 9 (10) | |||
| Practice guidelines | 18 (10) | 12 (13) | |||
| Meta‐analysis | 14 (7) | 8 (9) | |||
| Other | 23 (12) | 0 | |||
Sixty‐eight (10%) respondents held research funding, and 6 identified the grant process as an impediment to doing research. The most commonly reported funding source was from government and institutions, followed by support from foundations (see Supporting Figure 1A in the online version of this article). Responders with research funding were predominantly young, white, and assistant or associate professors. Fourteen hospitalists with funding reported completing a fellowship. (Table 1)
More than half of the respondents (n=332) had not authored peer‐reviewed publications. Of the 277 who had published successfully, 89 (31%) were trained in pediatrics. For those with publications, 152 (55%) reported publishing less than once per year. The type of article published most frequently was original research followed by case reports/series and reviews. (Table 2)
Variables individually associated with an increased likelihood of authoring peer‐reviewed publications included the completion of a fellowship, having an academic appointment, the availability of funding and mentorship, a background of pediatrics training, and more than 25% dedicated research time. (Table 3)
| Authored Peer‐Reviewed Publications, N (%) | |||||
|---|---|---|---|---|---|
| Characteristics | No | Yes | P | ||
| |||||
| Age, y | 327 | 272 | 0.437 | ||
| 2535 | 85 (26) | 72 (26) | |||
| 3645 | 146 (45) | 128 (47) | |||
| 4655 | 64 (20) | 41 (15) | |||
| 5665 | 30 (9) | 26 (10) | |||
| >65 | 2 (1) | 5 (2) | |||
| Gender | 327 | 270 | 0.067 | ||
| Female | 147 (45) | 101 (37) | |||
| Male | 180 (55) | 169 (63) | |||
| Faculty appointment | 301 | 247 | <0.001 | ||
| Nonacademic | 161 (53) | 63 (25) | |||
| Academic | 140 (46) | 184 (74) | |||
| Residency | 331 | 275 | <0.001 | ||
| Family | 39 (12) | 14 (5) | |||
| Internal medicine | 184 (56) | 151 (55) | |||
| Internal medicine (pediatrics) | 33 (10) | 15 (5) | |||
| Pediatrics | 62 (19) | 89 (32) | |||
| Other | 13 (4) | 6 (2) | |||
| Completed fellowship training | 332 | 19 (6) | 277 | 47 (17) | <0.001 |
| Current research/career mentor | 327 | 30 (9) | 272 | 96 (35) | <0.001 |
| Meet with mentor | 29 | 88 | 0.433 | ||
| More often than every 6 months | 21 (72) | 71 (81) | |||
| Every 6 months or less | 8 (28) | 17 (19) | |||
| Time for research | 54 | 153 | <0.001 | ||
| 25% or less | 53 (98) | 122 (80) | |||
| More than 25% | 1 (2) | 31 (20) | |||
| Has funding | 54 | 8 (15) | 156 | 60 (38) | <0.001 |
Publications Review
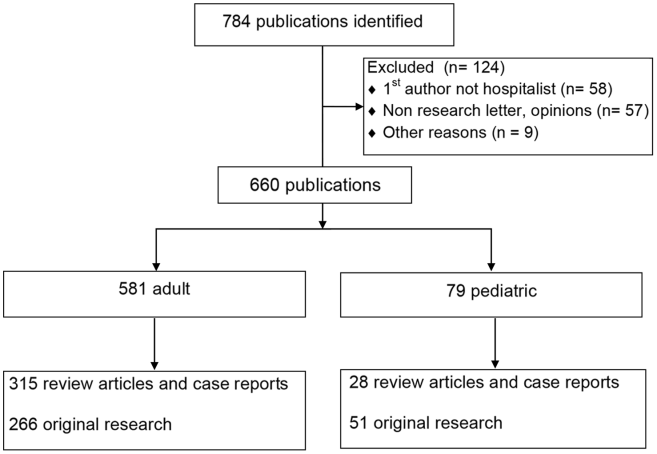
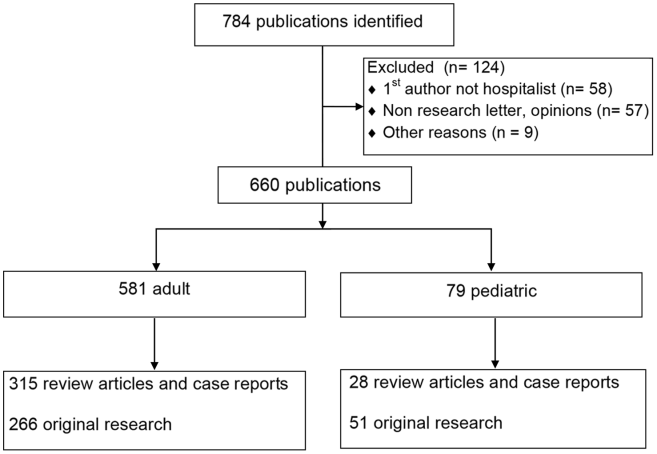
The PubMed search yielded 784 publications with hospital medicine or hospitalist in the affiliation field. After manual review, 660 articles were retained. (Figure 1)
The volume of hospitalist‐led publications has been increasing. Between 2006 and October 2013 there was a 5‐fold increase in hospitalist‐led publications (36 in 2006 to 179 in the first 10 months of 2013). Of the 660 articles culled from the PubMed search, 581 (88%) represented the work of authors affiliated with adult hospital medicine; 266 (46%) of these represented original research (the rest were reviews and case reports). Seventy‐nine (12%) of the 660 PubMed articles were related to pediatric hospital medicine; 51 (65%) of these represented original research. (Figure 1) In the period studied there was a variation from year to year in the proportion of publications representing original research, with a range of 37% to 71% comprising original research in adult hospital medicine publications and 50% to 81% in pediatric hospital medicine publications (Figure 2A).
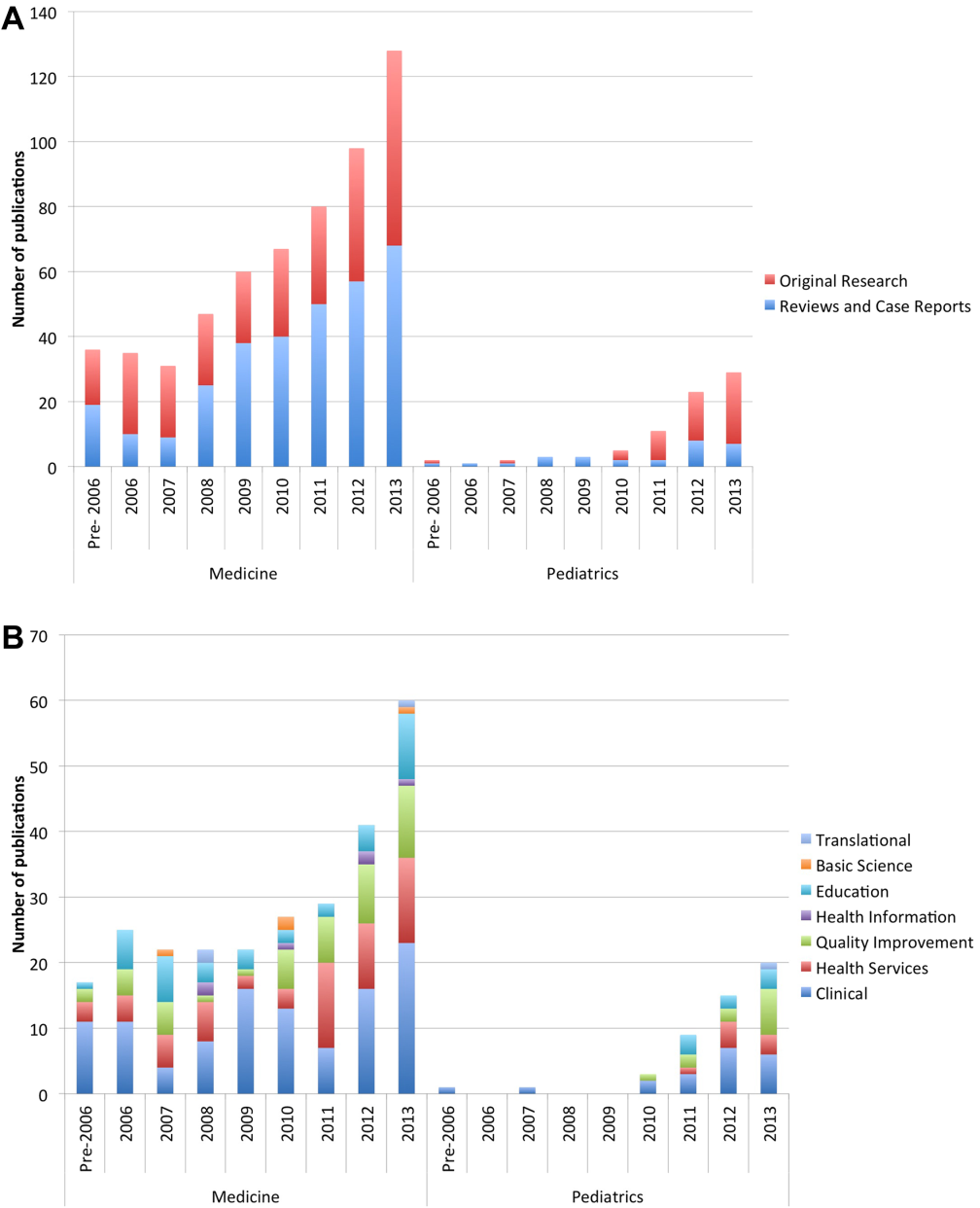
Nearly half (41%) of the original research in adult and pediatric medicine represented clinical research. Health services (21%) and QI (19%) were the next most frequent research categories published. Publications pertaining to research in education represented 15% of all original research. Health services and QI research are growing on a relatively stable base of clinical research. These trends were similar between adult and pediatric hospital medicine. (Figure 2B) The concordance rate on the assigned research categories was 82%, based on 67 publications that were independently reviewed by 2 authors.
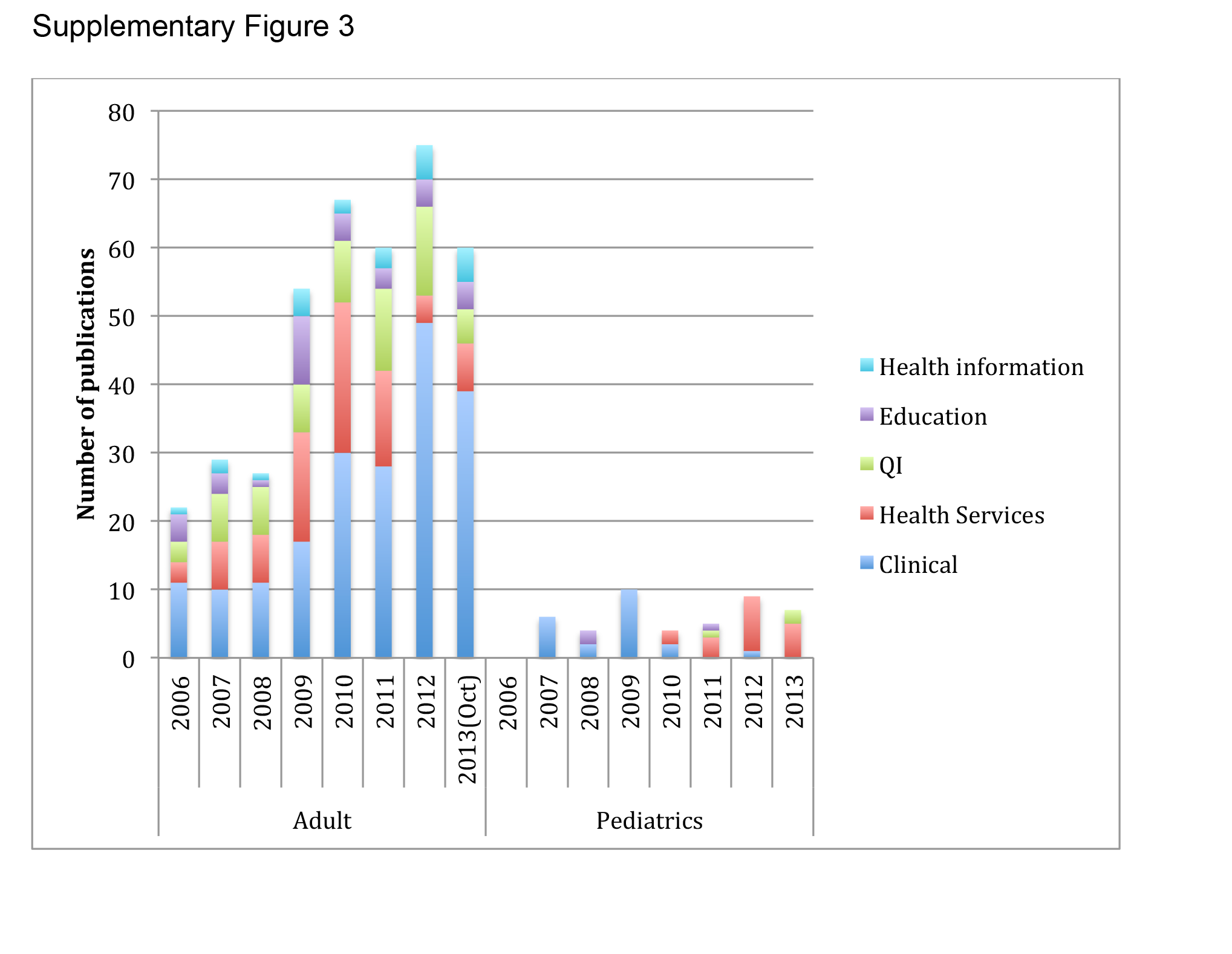
There were 457 original research articles published in JHM between 2006 and early October 2013. JHM publications followed a trend similar to the publications of hospitalist‐affiliated first authors from PubMed, with the majority (47%) reflecting clinical research followed by health services (25%) and QI (25%). (see Supporting Figure 3 in the online version of this article)
In our review, adult medicine hospitalist authors were affiliated with 124 different universities or centers. However, 5 centers represented nearly half the publication volume. The Cleveland Clinic Foundation, University of California San Francisco (UCSF), Harvard, Northwestern, and the University of Chicago were the top producers. Fewer centers produce original research, with 66 counted in our search. Centers most prolific in producing original research are UCSF, Northwestern, University of Chicago, Harvard, and Johns Hopkins. Their combined output represented 56% of all published original published research. (see Supporting Figure 2A,B in the online version of this article)
In our review, publications attributed to pediatric hospitalists were the product of 34 different centers. Cincinnati Children's Hospital Medical Center, Children's National Medical Center (Georgetown University), and the Monroe Carell Children's Hospital (Vanderbilt) were the most productive in publishing. The same centers were also the most productive in publishing original research. (see Supporting Figure 2C,D in the online version of this article)
Funding data from the 317 original research articles found in PubMed showed that 52% had funding listed for the first author and/or the work. These publications were the work of 181 different first authors, of whom 39 (22%) had 1 or more funding sources specifically associated with them in the publications. The majority of these authors reported government funding (n=24), followed by support from foundations (n=12), institutions (n=8), and industries (n=6) (see Supporting Figure 1B in the online version of this article).
DISCUSSION
Using results from both the survey and our review of publications in PubMed provided complementary information that has enriched our evaluation and reporting of the current state of research and publications in hospital medicine.
The initial growth of the field of hospital medicine can be attributed to its clinical contributions.[17] However, hospital medicine faces numerous challenges in its evolution into an academic specialty.[3] Job satisfaction rates among hospitalists may be falling,[18, 19] and pursuing intellectual outlets such as research may improve both satisfaction and productivity.[20, 21] Therefore, it is important to study the predictors of success for the nonclinical intellectual endeavors of hospitalists.
Across the career spectrum in academic medicine, effective mentorship has been found to be beneficial in enhancing teaching skills, productivity, and satisfaction.[22] Similar to prior studies, we found that mentorship was not readily accessible, and its absence was associated with a decreased likelihood of peer‐reviewed publications.[23, 24] Hospital medicine remains a youthful specialty, with the mean age of clinicians in the 40s.[18] In our survey, hospitalists aged 36 to 45 years reported the highest rates of publications and funding. If these hospitalists can be retained in the field, they may eventually serve as mentors to those entering the specialty. Strategies to provide mentorship have been described,[25] and continued efforts to innovate are needed in the development of mentorship potential.
Successfully promoted hospitalists identify peer‐reviewed publications as a key activity that supports promotion.[26] However, similar to Reid et al.,[23] our survey found that hospitalists reported low rates of peer‐reviewed publications. Hospitalists have unique access to the inpatient population, and setting up collaborative efforts between specialists and hospitalists, or participating in multi‐institutional projects that require patient recruitment,[27] may facilitate research and publication productivity. A specific emerging opportunity for this expertise is the need for collecting and identifying disease presentations to correlate with the exploding genetic data now available.[28]
QI research was identified from our survey results as the most frequent type of research that hospitalists were either engaged in or planned to pursue. However, based on our review of published research, the volume of QI research is surpassed by that of clinical research. Many factors contribute to this. First, an overlap between the categories of clinical and QI research may have led to lower numbers in QI. Second, there may be a lag between the interest in QI translating into publications. This may be related both to the dearth of QI mentorship and to the barriers in publishing QI. These barriers include increasing competition in target journals, the lack of generalizability of QI efforts, and the compressed time frames of rapid improvement cycles that differ from the slower pace of clinical research and its measurements.[29] Hospitalists may also perform QI that results in scholarly output other than publications (eg, grand rounds, posters, or presentations) that we did not address. In the absence of QI publications, the systematic documentation of QI efforts in a portfolio may assist career advancement.[30]
The review of publications in the PubMed database through early October 2013 showed a consistent increase in the number of publications produced by hospitalist first authors. Clinical research was represented most frequently followed by health services and QI research. The predominance of clinical research parallels the large clinical role of hospitalists; however, the diversity of research categories represented reflects the growing penetration and involvement of hospitalists in the arenas of QI, health services, and education. Although our search identified fewer pediatric hospitalist articles, pediatric hospitalist literature is also on the rise. There are other indicators of the enthusiasm for research among pediatric hospitalists, as nearly half the respondents in our survey who are currently engaged in research and nearly a third who had successfully published or had funding support were trained in pediatrics.
Publications by first authors who were hospitalists or affiliated with hospital medicine represented the effort of more than 100 institutions, implying a widespread engagement in hospital medicine‐related scholarship. However, fewer centers produce original research, and over half the original research output is the product of 8 centers. Strategies to select and support person‐job fit,[31] availability of mentorship, the presence of existing infrastructure, funding, and departmental priorities are all likely to affect an institution's publication productivity. To emulate the success of these centers, a closer study of the strategies they employ[5] would be instructive for the broader hospitalist community.
Although our survey data showed that the presence of funding is associated with success in publishing, the percentage of hospitalists who report funding both from the survey and PubMed publication reports is <25%. This underscores the need for innovations that help hospitalists obtain support and incentives for their work.
This study has limitations. A survey is a cross‐sectional snap shot, and associations do not imply causation. Survey response rates have been falling,[32] and our convenience sampling without incentives engendered a low response rate. This response rate is similar to that of other surveys administered through SHM (SHM membership and marketing data, October 2013). Although statistical significance is presented, the differences may not be generalizable given the low response rate. We cannot quantify all responder biases or comment on how the membership fee to SHM may affect the sample cohort. The demographics of our respondents parallel that of the SHM membership base in age and gender. However, 25% of our respondents were trained in pediatrics, whereas only 4.3% of the SHM membership base is pediatrics trained (SHM membership and marketing data, October 2013). We did not inquire about contributions from job dissatisfaction to the lack of participation in research activities, and this may represent an area for further research.
The search methodology used in this study is likely to under‐report hospitalist‐related research, because collaborative publications in which the lead author is not a hospitalist were not included. Furthermore, many hospitalists are associated with centers that do not have a hospitalist or hospital medicine title or department, and our search terms would have missed the publications stemming from these centers. Pediatric hospitalist literature is likely to be further under‐represented, as centers may not have separate pediatric hospitalist departments.
The assignment of each publication into a research category was based on definitions found in the literature. However, this designation ultimately remains a subjective process that may introduce bias.
Although the initial growth spurt of hospital medicine can be attributed to its clinical success, the increase in hospital medicine‐led peer‐reviewed publications in increasingly diverse domains provides evidence that supports the field's concomitant academic and scholarly maturation. Research into factors that impede or inspire hospitalists to participate in research, innovations that provide mentorship and funding for the specific interests of hospitalists, and the emulation of strategies employed by centers productive in publications are required to successfully foster the multidimensional growth of the field.
Acknowledgements
The authors thank Dr. Antoinette Laskey for her mentorship in survey development, Elaine Bammerlin for copyediting assistance, and the Society of Hospital Medicine members for taking the survey.
Disclosures: An Dang Do, MD, PhD, completed the major part of this work as a Morris Green Scholar at Indiana University School of Medicine. An N. Dang Do, MD, PhD, and Amy M. Munchhof, MD, PhD, contributed equally to this work. Areeba Kara, MD, is supported by a grant from the Methodist Health Foundation and by award number T15OC000047 from the Office of the National Coordinator for Health Information Technology, Office of the Secretary, US Department of Health & Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the National Coordinator For Health Information Technology, Office of the Secretary, US Department of Health & Human Services, or the National Institutes of Health.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- , , , . The status of hospital medicine groups in the United States. J Hosp Med. 2006;1(2):75–80.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308–313.
- , , , . An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314–318.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 25, 2013.
- US National Library of Medicine website. MEDLINE/PubMed data element (field) descriptions. Available at: http://www.nlm.nih.gov/bsd/mms/medlineelements.html. Accessed October 30, 2013.
- American Educational Research Association website. Available at: http://www.aera.net/EducationResearch/WhatisEducationResearch/tab id/13453/Default.aspx. Accessed October 30, 2013.
- National Institutes of Health website. Glossary of NIH terms. Available at: http://grants.nih.gov/grants/glossary.htm. Accessed February 26, 2013.
- US National Science Foundation website. National Center for Science and Engineering Statistics. Definitions of research and development: an annotated compilation of official sources. Available at: http://www.nsf.gov/statistics/randdef/fedgov.cfm. Accessed February 26, 2013.
- , . Health services research: an evolving definition of the field. US National Library of Medicine website. National Institutes of Health. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430351. Accessed February 26, 2013.
- Centers for Medicare and Medicaid Services website. Outcome measures. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/OutcomeMeasu res.html. Accessed August 22, 2013.
- Journal of Hospital Medicine. Available at: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1553–5606/homepage/ProductInformation.html. Accessed October 30, 2013.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , , . Preparing for “diastole”: Advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103–1115.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2011;27(1):23–27.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1–2.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , . The APA and the rise of pediatric generalist network research. Acad Pediatr. 2011;11(3):195–204.
- , , , et al. Phenotype harmonization and cross‐study collaboration in GWAS consortia: the GENEVA experience. Genet Epidemiol. 2011;35(3):159–173.
- , . Clinicians in quality improvement: a new career pathway in academic medicine. JAMA. 2009;301(7):766–768.
- , , , , Documenting quality improvement and patient safety efforts: the quality portfolio. A statement from the Academic Hospitalist Taskforce [published online ahead of print June 27, 2013]. J Gen Intern Med. doi: 10.1007/s11606‐013‐2532‐z.
- , , , , . Person‐job fit: an exploratory cross‐sectional analysis of hospitalists. J Hosp Med. 2012;8(2):96–101.
- , , , . Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40(1):213–226.
In 1996, Wachter and Goldman heralded the arrival of hospitalists in the healthcare system. They recognized the need to link the clinical role of a hospitalist with other activities, both to provide a creative outlet and to assist in the creation of research and development arms.[1] The explosive growth of hospital medicine followed, and hospitalists rapidly entered the mainstream of the healthcare system.[2]
A consensus conference in 2009 identified the challenges faced by hospitalists in conducting research as a key obstacle in the evolution of the profession into an academic field.[3] Strategies for building and facilitating hospitalist research programs have been described.[4, 5, 6, 7] However, a survey of US academic hospitalist leaders found more than 40% feared their faculty was not developing sustainable nonclinical activities.[8]
Data describing research aspirations and support systems among hospitalists are sparse, and no previous study has described the trends in hospitalist publications. In this work we describe the current standing of hospital medicine research through a survey of both academic and non‐academic hospitalists and a review of hospitalist‐related publications.
METHODS
The Indiana University institutional review board approved this study.
Survey of Hospitalists
A 29‐item questionnaire that addressed research activities, barriers, and mentorship was designed and piloted with pediatrics trainees at Indiana University. The final version (see Supporting Survey in the online version of this article) was approved by the Society of Hospital Medicine (SHM) research committee and posted on Zoomerang (
Review of Publications
A PubMed search was conducted on October 8, 2013 for records with either hospital medicine or hospitalist in the affiliation field. This field provides the departmental name and address information for the first author, except for the not‐yet‐indexed publisher‐supplied records, which could include all author addresses.[10] Editorials and letters to the editor were excluded, and results were limited to English. All resulting articles were manually curated and retained only if the affiliation criteria of hospitalist or hospital medicine (as a relevant single phrase) were associated with the first author. All articles meeting the criteria were reviewed by 1 of the authors and categorized as a review, a case report, or as original research (when methodology was described in the abstract). Original research articles were assigned a category based on their methodology and research type, as defined in published literature. The categories included basic sciences, clinical, health information, health services, quality improvement (QI), education, and translational research.[11, 12, 13, 14, 15] If the article overlapped categories, a secondary category was also assigned. A second author independently evaluated a subset of articles. This subset was then used to calculate the overall concordance between the authors based on their agreement on either the primary or secondary category designations.
To capture data on research funding, each original research article was searched for statements directly linking the first author or the work to the funding source(s).
Publications in the Journal of Hospital Medicine (JHM) were reviewed to serve as a gauge of research interests in the field of hospital medicine that may not be reflected by the publications resulting from the PubMed search. JHM was selected as the journal best representing hospital medicine based on its stated mission of commitment to the advancement of the hospital medicine specialty.[16] All original research articles in JHM were assigned a category by 1 of the authors based on the methodology in the abstract.
Statistical Methods
The survey responses were summarized using descriptive statistics. Univariate tests of association between respondent characteristics and peer‐reviewed authorship were performed using the Fisher's exact test. P values of 0.05 were considered significant. Data from the publication searches were presented as descriptive statistics.
RESULTS
Survey
The survey link was emailed to 11,611 SHM members: 11,102 members received the link and 509 emails were returned as undeliverable. A total of 645 member responses were received (5.8% response rate).
The most common demographic characteristics identified included male gender, age 45 or younger, and white race. The locations of the current practices were distributed equally across the United States. Over half of the respondents were trained in internal medicine, and a quarter were trained in pediatrics. Eleven percent had undertaken fellowship training after residency. Thirty‐seven percent did not hold an academic rank, and among those who did, most were assistant professors. (Table 1)
| Characteristics | Responses, N (%) | |||
|---|---|---|---|---|
| All Responses | Responses With Funding | |||
| ||||
| Gender | 597 | 67 | ||
| Female | 248 (41) | 33 (49) | ||
| Male | 349 (58) | 34 (51) | ||
| Age, y | 599 | 67 | ||
| 2535 | 157 (26) | 17 (25) | ||
| 3645 | 274 (46) | 39 (58) | ||
| 4655 | 105 (17) | 6 (9) | ||
| 5665 | 56 (9) | 5 (7) | ||
| >65 | 7 (1) | 0 | ||
| Current practice location | 596 | 67 | ||
| Midwest | 147 (25) | 18 (27) | ||
| Northeast | 113 (19) | 12 (18) | ||
| South | 172 (29) | 14 (21) | ||
| West | 142 (4) | 16 (24) | ||
| Other | 22 (34) | 7 (10) | ||
| Race | 595 | 67 | ||
| White | 444 (75) | 58 (87) | ||
| Black | 18 (3) | 0 | ||
| Hispanic | 22 (4) | 1 (1) | ||
| Asian | 85 (14) | 8 (12) | ||
| Other | 26 (4) | |||
| Faculty appointment | 593 | 68 | ||
| Nonacademic | 221 (37) | 4 (6) | ||
| Instructor/lecturer | 60 (10) | 6 (9) | ||
| Assistant professor | 197 (33) | 32 (47) | ||
| Associate professor | 68 (11) | 19 (28) | ||
| Full professor | 14 (2) | 4 (6) | ||
| Other | 33 (6) | 3 (4) | ||
| Fellowship training | 68 | 14 | ||
| General IM/hospitalist | 15 (22) | 6 (43) | ||
| Pediatric hospital medicine | 7 (10) | 2 (14) | ||
| Other | 46 (68) | 6 (43) | ||
| Residency completed | 616 | 68 | ||
| IM | 340 (55) | 36 (53) | ||
| Pediatrics | 154 (25) | 27 (40) | ||
| Family medicine | 53 (9) | 1 (1) | ||
| IM/pediatrics | 48 (8) | 2 (3) | ||
| Other | 21 (3) | 2 (3) | ||
Overall availability of mentorship was low, but respondents with academic appointments were more likely to have a mentor than those without academic appointments (32% vs 2.7%, p<0.001). Hospitalists most likely identified their own mentors, and meetings between the hospitalist and mentor occurred more frequently than once every 3 months.
There were 213 (33%) respondents who identified themselves as currently conducting research, 96 (45%) of whom were trained in pediatrics. Ninety‐two (28%) of those with academic appointments and 157 (71%) of those without academic appointments had no current or future plans to engage in research. QI research, followed by clinical research, emerged as the most frequent type of research that hospitalists were either currently engaged in or planned to embark on. Most respondents identified factors other than age, family or financial issues, the grant process, or a lack of institutional support as the reason for not conducting research. (Table 2)
| Activity | Responses, N (%) | ||||
|---|---|---|---|---|---|
| Adult Medicine | Pediatric Medicine | ||||
| |||||
| No plan to conduct research | 245 | 26 | |||
| Reasons for not doing research | |||||
| Lack of institutional support | 42 (17) | 3 (12) | |||
| Family issues | 14 (6) | 1 (4) | |||
| Financial | 8 (3) | 0 | |||
| Grant process | 4 (2) | 2 (8) | |||
| Age | 5 (2) | 0 | |||
| Other | 171 (70) | 20 (77) | |||
| Currently doing research | 117 | 96 | |||
| Quality improvement | 79 (68) | 73 (76) | |||
| Clinical | 59 (50) | 62 (65) | |||
| Health services | 31 (26) | 30 (31) | |||
| Health informatics | 28 (24) | 11 (11) | |||
| Translational | 10 (8) | 7 (7) | |||
| Basic science | 3 (3) | 0 | |||
| Other | 17 (14) | 10 (10) | |||
| Plan on doing research | 183 | 30 | |||
| Quality improvement | 72 (39) | 25 (83) | |||
| Clinical | 65 (35) | 25 (83) | |||
| Health services | 20 (11) | 2 (7) | |||
| Health informatics | 25 (14) | 3 (10) | |||
| Translational | 8 (4) | 3 (10) | |||
| Basic science | 3 (2) | 0 | |||
| Other | 8 (4) | 0 | |||
| Peer‐review publications | 458 | 151 | |||
| No | 270 (59) | 62 (41) | |||
| Yes | 188 (41) | 89 (59) | |||
| Frequency | |||||
| Less than once/year | 111 (59) | 41 (46) | |||
| Once/year | 22 (12) | 20 (22) | |||
| Twice/year | 16 (8) | 16 (18) | |||
| More than twice/year | 23 (12) | 10 (11) | |||
| Other | 13 (7) | 1 (1) | |||
| Publication Type | |||||
| Original research | 97 (52) | 75 (84) | |||
| Case report/series | 80 (42) | 41 (46) | |||
| Reviews | 63 (34) | 25 (28) | |||
| Clinical trials | 36 (19) | 9 (10) | |||
| Practice guidelines | 18 (10) | 12 (13) | |||
| Meta‐analysis | 14 (7) | 8 (9) | |||
| Other | 23 (12) | 0 | |||
Sixty‐eight (10%) respondents held research funding, and 6 identified the grant process as an impediment to doing research. The most commonly reported funding source was from government and institutions, followed by support from foundations (see Supporting Figure 1A in the online version of this article). Responders with research funding were predominantly young, white, and assistant or associate professors. Fourteen hospitalists with funding reported completing a fellowship. (Table 1)

More than half of the respondents (n=332) had not authored peer‐reviewed publications. Of the 277 who had published successfully, 89 (31%) were trained in pediatrics. For those with publications, 152 (55%) reported publishing less than once per year. The type of article published most frequently was original research followed by case reports/series and reviews. (Table 2)
Variables individually associated with an increased likelihood of authoring peer‐reviewed publications included the completion of a fellowship, having an academic appointment, the availability of funding and mentorship, a background of pediatrics training, and more than 25% dedicated research time. (Table 3)
| Authored Peer‐Reviewed Publications, N (%) | |||||
|---|---|---|---|---|---|
| Characteristics | No | Yes | P | ||
| |||||
| Age, y | 327 | 272 | 0.437 | ||
| 2535 | 85 (26) | 72 (26) | |||
| 3645 | 146 (45) | 128 (47) | |||
| 4655 | 64 (20) | 41 (15) | |||
| 5665 | 30 (9) | 26 (10) | |||
| >65 | 2 (1) | 5 (2) | |||
| Gender | 327 | 270 | 0.067 | ||
| Female | 147 (45) | 101 (37) | |||
| Male | 180 (55) | 169 (63) | |||
| Faculty appointment | 301 | 247 | <0.001 | ||
| Nonacademic | 161 (53) | 63 (25) | |||
| Academic | 140 (46) | 184 (74) | |||
| Residency | 331 | 275 | <0.001 | ||
| Family | 39 (12) | 14 (5) | |||
| Internal medicine | 184 (56) | 151 (55) | |||
| Internal medicine (pediatrics) | 33 (10) | 15 (5) | |||
| Pediatrics | 62 (19) | 89 (32) | |||
| Other | 13 (4) | 6 (2) | |||
| Completed fellowship training | 332 | 19 (6) | 277 | 47 (17) | <0.001 |
| Current research/career mentor | 327 | 30 (9) | 272 | 96 (35) | <0.001 |
| Meet with mentor | 29 | 88 | 0.433 | ||
| More often than every 6 months | 21 (72) | 71 (81) | |||
| Every 6 months or less | 8 (28) | 17 (19) | |||
| Time for research | 54 | 153 | <0.001 | ||
| 25% or less | 53 (98) | 122 (80) | |||
| More than 25% | 1 (2) | 31 (20) | |||
| Has funding | 54 | 8 (15) | 156 | 60 (38) | <0.001 |
Publications Review
The PubMed search yielded 784 publications with hospital medicine or hospitalist in the affiliation field. After manual review, 660 articles were retained. (Figure 1)
The volume of hospitalist‐led publications has been increasing. Between 2006 and October 2013 there was a 5‐fold increase in hospitalist‐led publications (36 in 2006 to 179 in the first 10 months of 2013). Of the 660 articles culled from the PubMed search, 581 (88%) represented the work of authors affiliated with adult hospital medicine; 266 (46%) of these represented original research (the rest were reviews and case reports). Seventy‐nine (12%) of the 660 PubMed articles were related to pediatric hospital medicine; 51 (65%) of these represented original research. (Figure 1) In the period studied there was a variation from year to year in the proportion of publications representing original research, with a range of 37% to 71% comprising original research in adult hospital medicine publications and 50% to 81% in pediatric hospital medicine publications (Figure 2A).

Nearly half (41%) of the original research in adult and pediatric medicine represented clinical research. Health services (21%) and QI (19%) were the next most frequent research categories published. Publications pertaining to research in education represented 15% of all original research. Health services and QI research are growing on a relatively stable base of clinical research. These trends were similar between adult and pediatric hospital medicine. (Figure 2B) The concordance rate on the assigned research categories was 82%, based on 67 publications that were independently reviewed by 2 authors.
There were 457 original research articles published in JHM between 2006 and early October 2013. JHM publications followed a trend similar to the publications of hospitalist‐affiliated first authors from PubMed, with the majority (47%) reflecting clinical research followed by health services (25%) and QI (25%). (see Supporting Figure 3 in the online version of this article)
In our review, adult medicine hospitalist authors were affiliated with 124 different universities or centers. However, 5 centers represented nearly half the publication volume. The Cleveland Clinic Foundation, University of California San Francisco (UCSF), Harvard, Northwestern, and the University of Chicago were the top producers. Fewer centers produce original research, with 66 counted in our search. Centers most prolific in producing original research are UCSF, Northwestern, University of Chicago, Harvard, and Johns Hopkins. Their combined output represented 56% of all published original published research. (see Supporting Figure 2A,B in the online version of this article)
In our review, publications attributed to pediatric hospitalists were the product of 34 different centers. Cincinnati Children's Hospital Medical Center, Children's National Medical Center (Georgetown University), and the Monroe Carell Children's Hospital (Vanderbilt) were the most productive in publishing. The same centers were also the most productive in publishing original research. (see Supporting Figure 2C,D in the online version of this article)
Funding data from the 317 original research articles found in PubMed showed that 52% had funding listed for the first author and/or the work. These publications were the work of 181 different first authors, of whom 39 (22%) had 1 or more funding sources specifically associated with them in the publications. The majority of these authors reported government funding (n=24), followed by support from foundations (n=12), institutions (n=8), and industries (n=6) (see Supporting Figure 1B in the online version of this article).
DISCUSSION
Using results from both the survey and our review of publications in PubMed provided complementary information that has enriched our evaluation and reporting of the current state of research and publications in hospital medicine.
The initial growth of the field of hospital medicine can be attributed to its clinical contributions.[17] However, hospital medicine faces numerous challenges in its evolution into an academic specialty.[3] Job satisfaction rates among hospitalists may be falling,[18, 19] and pursuing intellectual outlets such as research may improve both satisfaction and productivity.[20, 21] Therefore, it is important to study the predictors of success for the nonclinical intellectual endeavors of hospitalists.
Across the career spectrum in academic medicine, effective mentorship has been found to be beneficial in enhancing teaching skills, productivity, and satisfaction.[22] Similar to prior studies, we found that mentorship was not readily accessible, and its absence was associated with a decreased likelihood of peer‐reviewed publications.[23, 24] Hospital medicine remains a youthful specialty, with the mean age of clinicians in the 40s.[18] In our survey, hospitalists aged 36 to 45 years reported the highest rates of publications and funding. If these hospitalists can be retained in the field, they may eventually serve as mentors to those entering the specialty. Strategies to provide mentorship have been described,[25] and continued efforts to innovate are needed in the development of mentorship potential.
Successfully promoted hospitalists identify peer‐reviewed publications as a key activity that supports promotion.[26] However, similar to Reid et al.,[23] our survey found that hospitalists reported low rates of peer‐reviewed publications. Hospitalists have unique access to the inpatient population, and setting up collaborative efforts between specialists and hospitalists, or participating in multi‐institutional projects that require patient recruitment,[27] may facilitate research and publication productivity. A specific emerging opportunity for this expertise is the need for collecting and identifying disease presentations to correlate with the exploding genetic data now available.[28]
QI research was identified from our survey results as the most frequent type of research that hospitalists were either engaged in or planned to pursue. However, based on our review of published research, the volume of QI research is surpassed by that of clinical research. Many factors contribute to this. First, an overlap between the categories of clinical and QI research may have led to lower numbers in QI. Second, there may be a lag between the interest in QI translating into publications. This may be related both to the dearth of QI mentorship and to the barriers in publishing QI. These barriers include increasing competition in target journals, the lack of generalizability of QI efforts, and the compressed time frames of rapid improvement cycles that differ from the slower pace of clinical research and its measurements.[29] Hospitalists may also perform QI that results in scholarly output other than publications (eg, grand rounds, posters, or presentations) that we did not address. In the absence of QI publications, the systematic documentation of QI efforts in a portfolio may assist career advancement.[30]
The review of publications in the PubMed database through early October 2013 showed a consistent increase in the number of publications produced by hospitalist first authors. Clinical research was represented most frequently followed by health services and QI research. The predominance of clinical research parallels the large clinical role of hospitalists; however, the diversity of research categories represented reflects the growing penetration and involvement of hospitalists in the arenas of QI, health services, and education. Although our search identified fewer pediatric hospitalist articles, pediatric hospitalist literature is also on the rise. There are other indicators of the enthusiasm for research among pediatric hospitalists, as nearly half the respondents in our survey who are currently engaged in research and nearly a third who had successfully published or had funding support were trained in pediatrics.
Publications by first authors who were hospitalists or affiliated with hospital medicine represented the effort of more than 100 institutions, implying a widespread engagement in hospital medicine‐related scholarship. However, fewer centers produce original research, and over half the original research output is the product of 8 centers. Strategies to select and support person‐job fit,[31] availability of mentorship, the presence of existing infrastructure, funding, and departmental priorities are all likely to affect an institution's publication productivity. To emulate the success of these centers, a closer study of the strategies they employ[5] would be instructive for the broader hospitalist community.
Although our survey data showed that the presence of funding is associated with success in publishing, the percentage of hospitalists who report funding both from the survey and PubMed publication reports is <25%. This underscores the need for innovations that help hospitalists obtain support and incentives for their work.
This study has limitations. A survey is a cross‐sectional snap shot, and associations do not imply causation. Survey response rates have been falling,[32] and our convenience sampling without incentives engendered a low response rate. This response rate is similar to that of other surveys administered through SHM (SHM membership and marketing data, October 2013). Although statistical significance is presented, the differences may not be generalizable given the low response rate. We cannot quantify all responder biases or comment on how the membership fee to SHM may affect the sample cohort. The demographics of our respondents parallel that of the SHM membership base in age and gender. However, 25% of our respondents were trained in pediatrics, whereas only 4.3% of the SHM membership base is pediatrics trained (SHM membership and marketing data, October 2013). We did not inquire about contributions from job dissatisfaction to the lack of participation in research activities, and this may represent an area for further research.
The search methodology used in this study is likely to under‐report hospitalist‐related research, because collaborative publications in which the lead author is not a hospitalist were not included. Furthermore, many hospitalists are associated with centers that do not have a hospitalist or hospital medicine title or department, and our search terms would have missed the publications stemming from these centers. Pediatric hospitalist literature is likely to be further under‐represented, as centers may not have separate pediatric hospitalist departments.
The assignment of each publication into a research category was based on definitions found in the literature. However, this designation ultimately remains a subjective process that may introduce bias.
Although the initial growth spurt of hospital medicine can be attributed to its clinical success, the increase in hospital medicine‐led peer‐reviewed publications in increasingly diverse domains provides evidence that supports the field's concomitant academic and scholarly maturation. Research into factors that impede or inspire hospitalists to participate in research, innovations that provide mentorship and funding for the specific interests of hospitalists, and the emulation of strategies employed by centers productive in publications are required to successfully foster the multidimensional growth of the field.
Acknowledgements
The authors thank Dr. Antoinette Laskey for her mentorship in survey development, Elaine Bammerlin for copyediting assistance, and the Society of Hospital Medicine members for taking the survey.
Disclosures: An Dang Do, MD, PhD, completed the major part of this work as a Morris Green Scholar at Indiana University School of Medicine. An N. Dang Do, MD, PhD, and Amy M. Munchhof, MD, PhD, contributed equally to this work. Areeba Kara, MD, is supported by a grant from the Methodist Health Foundation and by award number T15OC000047 from the Office of the National Coordinator for Health Information Technology, Office of the Secretary, US Department of Health & Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the National Coordinator For Health Information Technology, Office of the Secretary, US Department of Health & Human Services, or the National Institutes of Health.
In 1996, Wachter and Goldman heralded the arrival of hospitalists in the healthcare system. They recognized the need to link the clinical role of a hospitalist with other activities, both to provide a creative outlet and to assist in the creation of research and development arms.[1] The explosive growth of hospital medicine followed, and hospitalists rapidly entered the mainstream of the healthcare system.[2]
A consensus conference in 2009 identified the challenges faced by hospitalists in conducting research as a key obstacle in the evolution of the profession into an academic field.[3] Strategies for building and facilitating hospitalist research programs have been described.[4, 5, 6, 7] However, a survey of US academic hospitalist leaders found more than 40% feared their faculty was not developing sustainable nonclinical activities.[8]
Data describing research aspirations and support systems among hospitalists are sparse, and no previous study has described the trends in hospitalist publications. In this work we describe the current standing of hospital medicine research through a survey of both academic and non‐academic hospitalists and a review of hospitalist‐related publications.
METHODS
The Indiana University institutional review board approved this study.
Survey of Hospitalists
A 29‐item questionnaire that addressed research activities, barriers, and mentorship was designed and piloted with pediatrics trainees at Indiana University. The final version (see Supporting Survey in the online version of this article) was approved by the Society of Hospital Medicine (SHM) research committee and posted on Zoomerang (
Review of Publications
A PubMed search was conducted on October 8, 2013 for records with either hospital medicine or hospitalist in the affiliation field. This field provides the departmental name and address information for the first author, except for the not‐yet‐indexed publisher‐supplied records, which could include all author addresses.[10] Editorials and letters to the editor were excluded, and results were limited to English. All resulting articles were manually curated and retained only if the affiliation criteria of hospitalist or hospital medicine (as a relevant single phrase) were associated with the first author. All articles meeting the criteria were reviewed by 1 of the authors and categorized as a review, a case report, or as original research (when methodology was described in the abstract). Original research articles were assigned a category based on their methodology and research type, as defined in published literature. The categories included basic sciences, clinical, health information, health services, quality improvement (QI), education, and translational research.[11, 12, 13, 14, 15] If the article overlapped categories, a secondary category was also assigned. A second author independently evaluated a subset of articles. This subset was then used to calculate the overall concordance between the authors based on their agreement on either the primary or secondary category designations.
To capture data on research funding, each original research article was searched for statements directly linking the first author or the work to the funding source(s).
Publications in the Journal of Hospital Medicine (JHM) were reviewed to serve as a gauge of research interests in the field of hospital medicine that may not be reflected by the publications resulting from the PubMed search. JHM was selected as the journal best representing hospital medicine based on its stated mission of commitment to the advancement of the hospital medicine specialty.[16] All original research articles in JHM were assigned a category by 1 of the authors based on the methodology in the abstract.
Statistical Methods
The survey responses were summarized using descriptive statistics. Univariate tests of association between respondent characteristics and peer‐reviewed authorship were performed using the Fisher's exact test. P values of 0.05 were considered significant. Data from the publication searches were presented as descriptive statistics.
RESULTS
Survey
The survey link was emailed to 11,611 SHM members: 11,102 members received the link and 509 emails were returned as undeliverable. A total of 645 member responses were received (5.8% response rate).
The most common demographic characteristics identified included male gender, age 45 or younger, and white race. The locations of the current practices were distributed equally across the United States. Over half of the respondents were trained in internal medicine, and a quarter were trained in pediatrics. Eleven percent had undertaken fellowship training after residency. Thirty‐seven percent did not hold an academic rank, and among those who did, most were assistant professors. (Table 1)
| Characteristics | Responses, N (%) | |||
|---|---|---|---|---|
| All Responses | Responses With Funding | |||
| ||||
| Gender | 597 | 67 | ||
| Female | 248 (41) | 33 (49) | ||
| Male | 349 (58) | 34 (51) | ||
| Age, y | 599 | 67 | ||
| 2535 | 157 (26) | 17 (25) | ||
| 3645 | 274 (46) | 39 (58) | ||
| 4655 | 105 (17) | 6 (9) | ||
| 5665 | 56 (9) | 5 (7) | ||
| >65 | 7 (1) | 0 | ||
| Current practice location | 596 | 67 | ||
| Midwest | 147 (25) | 18 (27) | ||
| Northeast | 113 (19) | 12 (18) | ||
| South | 172 (29) | 14 (21) | ||
| West | 142 (4) | 16 (24) | ||
| Other | 22 (34) | 7 (10) | ||
| Race | 595 | 67 | ||
| White | 444 (75) | 58 (87) | ||
| Black | 18 (3) | 0 | ||
| Hispanic | 22 (4) | 1 (1) | ||
| Asian | 85 (14) | 8 (12) | ||
| Other | 26 (4) | |||
| Faculty appointment | 593 | 68 | ||
| Nonacademic | 221 (37) | 4 (6) | ||
| Instructor/lecturer | 60 (10) | 6 (9) | ||
| Assistant professor | 197 (33) | 32 (47) | ||
| Associate professor | 68 (11) | 19 (28) | ||
| Full professor | 14 (2) | 4 (6) | ||
| Other | 33 (6) | 3 (4) | ||
| Fellowship training | 68 | 14 | ||
| General IM/hospitalist | 15 (22) | 6 (43) | ||
| Pediatric hospital medicine | 7 (10) | 2 (14) | ||
| Other | 46 (68) | 6 (43) | ||
| Residency completed | 616 | 68 | ||
| IM | 340 (55) | 36 (53) | ||
| Pediatrics | 154 (25) | 27 (40) | ||
| Family medicine | 53 (9) | 1 (1) | ||
| IM/pediatrics | 48 (8) | 2 (3) | ||
| Other | 21 (3) | 2 (3) | ||
Overall availability of mentorship was low, but respondents with academic appointments were more likely to have a mentor than those without academic appointments (32% vs 2.7%, p<0.001). Hospitalists most likely identified their own mentors, and meetings between the hospitalist and mentor occurred more frequently than once every 3 months.
There were 213 (33%) respondents who identified themselves as currently conducting research, 96 (45%) of whom were trained in pediatrics. Ninety‐two (28%) of those with academic appointments and 157 (71%) of those without academic appointments had no current or future plans to engage in research. QI research, followed by clinical research, emerged as the most frequent type of research that hospitalists were either currently engaged in or planned to embark on. Most respondents identified factors other than age, family or financial issues, the grant process, or a lack of institutional support as the reason for not conducting research. (Table 2)
| Activity | Responses, N (%) | ||||
|---|---|---|---|---|---|
| Adult Medicine | Pediatric Medicine | ||||
| |||||
| No plan to conduct research | 245 | 26 | |||
| Reasons for not doing research | |||||
| Lack of institutional support | 42 (17) | 3 (12) | |||
| Family issues | 14 (6) | 1 (4) | |||
| Financial | 8 (3) | 0 | |||
| Grant process | 4 (2) | 2 (8) | |||
| Age | 5 (2) | 0 | |||
| Other | 171 (70) | 20 (77) | |||
| Currently doing research | 117 | 96 | |||
| Quality improvement | 79 (68) | 73 (76) | |||
| Clinical | 59 (50) | 62 (65) | |||
| Health services | 31 (26) | 30 (31) | |||
| Health informatics | 28 (24) | 11 (11) | |||
| Translational | 10 (8) | 7 (7) | |||
| Basic science | 3 (3) | 0 | |||
| Other | 17 (14) | 10 (10) | |||
| Plan on doing research | 183 | 30 | |||
| Quality improvement | 72 (39) | 25 (83) | |||
| Clinical | 65 (35) | 25 (83) | |||
| Health services | 20 (11) | 2 (7) | |||
| Health informatics | 25 (14) | 3 (10) | |||
| Translational | 8 (4) | 3 (10) | |||
| Basic science | 3 (2) | 0 | |||
| Other | 8 (4) | 0 | |||
| Peer‐review publications | 458 | 151 | |||
| No | 270 (59) | 62 (41) | |||
| Yes | 188 (41) | 89 (59) | |||
| Frequency | |||||
| Less than once/year | 111 (59) | 41 (46) | |||
| Once/year | 22 (12) | 20 (22) | |||
| Twice/year | 16 (8) | 16 (18) | |||
| More than twice/year | 23 (12) | 10 (11) | |||
| Other | 13 (7) | 1 (1) | |||
| Publication Type | |||||
| Original research | 97 (52) | 75 (84) | |||
| Case report/series | 80 (42) | 41 (46) | |||
| Reviews | 63 (34) | 25 (28) | |||
| Clinical trials | 36 (19) | 9 (10) | |||
| Practice guidelines | 18 (10) | 12 (13) | |||
| Meta‐analysis | 14 (7) | 8 (9) | |||
| Other | 23 (12) | 0 | |||
Sixty‐eight (10%) respondents held research funding, and 6 identified the grant process as an impediment to doing research. The most commonly reported funding source was from government and institutions, followed by support from foundations (see Supporting Figure 1A in the online version of this article). Responders with research funding were predominantly young, white, and assistant or associate professors. Fourteen hospitalists with funding reported completing a fellowship. (Table 1)

More than half of the respondents (n=332) had not authored peer‐reviewed publications. Of the 277 who had published successfully, 89 (31%) were trained in pediatrics. For those with publications, 152 (55%) reported publishing less than once per year. The type of article published most frequently was original research followed by case reports/series and reviews. (Table 2)
Variables individually associated with an increased likelihood of authoring peer‐reviewed publications included the completion of a fellowship, having an academic appointment, the availability of funding and mentorship, a background of pediatrics training, and more than 25% dedicated research time. (Table 3)
| Authored Peer‐Reviewed Publications, N (%) | |||||
|---|---|---|---|---|---|
| Characteristics | No | Yes | P | ||
| |||||
| Age, y | 327 | 272 | 0.437 | ||
| 2535 | 85 (26) | 72 (26) | |||
| 3645 | 146 (45) | 128 (47) | |||
| 4655 | 64 (20) | 41 (15) | |||
| 5665 | 30 (9) | 26 (10) | |||
| >65 | 2 (1) | 5 (2) | |||
| Gender | 327 | 270 | 0.067 | ||
| Female | 147 (45) | 101 (37) | |||
| Male | 180 (55) | 169 (63) | |||
| Faculty appointment | 301 | 247 | <0.001 | ||
| Nonacademic | 161 (53) | 63 (25) | |||
| Academic | 140 (46) | 184 (74) | |||
| Residency | 331 | 275 | <0.001 | ||
| Family | 39 (12) | 14 (5) | |||
| Internal medicine | 184 (56) | 151 (55) | |||
| Internal medicine (pediatrics) | 33 (10) | 15 (5) | |||
| Pediatrics | 62 (19) | 89 (32) | |||
| Other | 13 (4) | 6 (2) | |||
| Completed fellowship training | 332 | 19 (6) | 277 | 47 (17) | <0.001 |
| Current research/career mentor | 327 | 30 (9) | 272 | 96 (35) | <0.001 |
| Meet with mentor | 29 | 88 | 0.433 | ||
| More often than every 6 months | 21 (72) | 71 (81) | |||
| Every 6 months or less | 8 (28) | 17 (19) | |||
| Time for research | 54 | 153 | <0.001 | ||
| 25% or less | 53 (98) | 122 (80) | |||
| More than 25% | 1 (2) | 31 (20) | |||
| Has funding | 54 | 8 (15) | 156 | 60 (38) | <0.001 |
Publications Review
The PubMed search yielded 784 publications with hospital medicine or hospitalist in the affiliation field. After manual review, 660 articles were retained. (Figure 1)
The volume of hospitalist‐led publications has been increasing. Between 2006 and October 2013 there was a 5‐fold increase in hospitalist‐led publications (36 in 2006 to 179 in the first 10 months of 2013). Of the 660 articles culled from the PubMed search, 581 (88%) represented the work of authors affiliated with adult hospital medicine; 266 (46%) of these represented original research (the rest were reviews and case reports). Seventy‐nine (12%) of the 660 PubMed articles were related to pediatric hospital medicine; 51 (65%) of these represented original research. (Figure 1) In the period studied there was a variation from year to year in the proportion of publications representing original research, with a range of 37% to 71% comprising original research in adult hospital medicine publications and 50% to 81% in pediatric hospital medicine publications (Figure 2A).

Nearly half (41%) of the original research in adult and pediatric medicine represented clinical research. Health services (21%) and QI (19%) were the next most frequent research categories published. Publications pertaining to research in education represented 15% of all original research. Health services and QI research are growing on a relatively stable base of clinical research. These trends were similar between adult and pediatric hospital medicine. (Figure 2B) The concordance rate on the assigned research categories was 82%, based on 67 publications that were independently reviewed by 2 authors.
There were 457 original research articles published in JHM between 2006 and early October 2013. JHM publications followed a trend similar to the publications of hospitalist‐affiliated first authors from PubMed, with the majority (47%) reflecting clinical research followed by health services (25%) and QI (25%). (see Supporting Figure 3 in the online version of this article)
In our review, adult medicine hospitalist authors were affiliated with 124 different universities or centers. However, 5 centers represented nearly half the publication volume. The Cleveland Clinic Foundation, University of California San Francisco (UCSF), Harvard, Northwestern, and the University of Chicago were the top producers. Fewer centers produce original research, with 66 counted in our search. Centers most prolific in producing original research are UCSF, Northwestern, University of Chicago, Harvard, and Johns Hopkins. Their combined output represented 56% of all published original published research. (see Supporting Figure 2A,B in the online version of this article)
In our review, publications attributed to pediatric hospitalists were the product of 34 different centers. Cincinnati Children's Hospital Medical Center, Children's National Medical Center (Georgetown University), and the Monroe Carell Children's Hospital (Vanderbilt) were the most productive in publishing. The same centers were also the most productive in publishing original research. (see Supporting Figure 2C,D in the online version of this article)
Funding data from the 317 original research articles found in PubMed showed that 52% had funding listed for the first author and/or the work. These publications were the work of 181 different first authors, of whom 39 (22%) had 1 or more funding sources specifically associated with them in the publications. The majority of these authors reported government funding (n=24), followed by support from foundations (n=12), institutions (n=8), and industries (n=6) (see Supporting Figure 1B in the online version of this article).
DISCUSSION
Using results from both the survey and our review of publications in PubMed provided complementary information that has enriched our evaluation and reporting of the current state of research and publications in hospital medicine.
The initial growth of the field of hospital medicine can be attributed to its clinical contributions.[17] However, hospital medicine faces numerous challenges in its evolution into an academic specialty.[3] Job satisfaction rates among hospitalists may be falling,[18, 19] and pursuing intellectual outlets such as research may improve both satisfaction and productivity.[20, 21] Therefore, it is important to study the predictors of success for the nonclinical intellectual endeavors of hospitalists.
Across the career spectrum in academic medicine, effective mentorship has been found to be beneficial in enhancing teaching skills, productivity, and satisfaction.[22] Similar to prior studies, we found that mentorship was not readily accessible, and its absence was associated with a decreased likelihood of peer‐reviewed publications.[23, 24] Hospital medicine remains a youthful specialty, with the mean age of clinicians in the 40s.[18] In our survey, hospitalists aged 36 to 45 years reported the highest rates of publications and funding. If these hospitalists can be retained in the field, they may eventually serve as mentors to those entering the specialty. Strategies to provide mentorship have been described,[25] and continued efforts to innovate are needed in the development of mentorship potential.
Successfully promoted hospitalists identify peer‐reviewed publications as a key activity that supports promotion.[26] However, similar to Reid et al.,[23] our survey found that hospitalists reported low rates of peer‐reviewed publications. Hospitalists have unique access to the inpatient population, and setting up collaborative efforts between specialists and hospitalists, or participating in multi‐institutional projects that require patient recruitment,[27] may facilitate research and publication productivity. A specific emerging opportunity for this expertise is the need for collecting and identifying disease presentations to correlate with the exploding genetic data now available.[28]
QI research was identified from our survey results as the most frequent type of research that hospitalists were either engaged in or planned to pursue. However, based on our review of published research, the volume of QI research is surpassed by that of clinical research. Many factors contribute to this. First, an overlap between the categories of clinical and QI research may have led to lower numbers in QI. Second, there may be a lag between the interest in QI translating into publications. This may be related both to the dearth of QI mentorship and to the barriers in publishing QI. These barriers include increasing competition in target journals, the lack of generalizability of QI efforts, and the compressed time frames of rapid improvement cycles that differ from the slower pace of clinical research and its measurements.[29] Hospitalists may also perform QI that results in scholarly output other than publications (eg, grand rounds, posters, or presentations) that we did not address. In the absence of QI publications, the systematic documentation of QI efforts in a portfolio may assist career advancement.[30]
The review of publications in the PubMed database through early October 2013 showed a consistent increase in the number of publications produced by hospitalist first authors. Clinical research was represented most frequently followed by health services and QI research. The predominance of clinical research parallels the large clinical role of hospitalists; however, the diversity of research categories represented reflects the growing penetration and involvement of hospitalists in the arenas of QI, health services, and education. Although our search identified fewer pediatric hospitalist articles, pediatric hospitalist literature is also on the rise. There are other indicators of the enthusiasm for research among pediatric hospitalists, as nearly half the respondents in our survey who are currently engaged in research and nearly a third who had successfully published or had funding support were trained in pediatrics.
Publications by first authors who were hospitalists or affiliated with hospital medicine represented the effort of more than 100 institutions, implying a widespread engagement in hospital medicine‐related scholarship. However, fewer centers produce original research, and over half the original research output is the product of 8 centers. Strategies to select and support person‐job fit,[31] availability of mentorship, the presence of existing infrastructure, funding, and departmental priorities are all likely to affect an institution's publication productivity. To emulate the success of these centers, a closer study of the strategies they employ[5] would be instructive for the broader hospitalist community.
Although our survey data showed that the presence of funding is associated with success in publishing, the percentage of hospitalists who report funding both from the survey and PubMed publication reports is <25%. This underscores the need for innovations that help hospitalists obtain support and incentives for their work.
This study has limitations. A survey is a cross‐sectional snap shot, and associations do not imply causation. Survey response rates have been falling,[32] and our convenience sampling without incentives engendered a low response rate. This response rate is similar to that of other surveys administered through SHM (SHM membership and marketing data, October 2013). Although statistical significance is presented, the differences may not be generalizable given the low response rate. We cannot quantify all responder biases or comment on how the membership fee to SHM may affect the sample cohort. The demographics of our respondents parallel that of the SHM membership base in age and gender. However, 25% of our respondents were trained in pediatrics, whereas only 4.3% of the SHM membership base is pediatrics trained (SHM membership and marketing data, October 2013). We did not inquire about contributions from job dissatisfaction to the lack of participation in research activities, and this may represent an area for further research.
The search methodology used in this study is likely to under‐report hospitalist‐related research, because collaborative publications in which the lead author is not a hospitalist were not included. Furthermore, many hospitalists are associated with centers that do not have a hospitalist or hospital medicine title or department, and our search terms would have missed the publications stemming from these centers. Pediatric hospitalist literature is likely to be further under‐represented, as centers may not have separate pediatric hospitalist departments.
The assignment of each publication into a research category was based on definitions found in the literature. However, this designation ultimately remains a subjective process that may introduce bias.
Although the initial growth spurt of hospital medicine can be attributed to its clinical success, the increase in hospital medicine‐led peer‐reviewed publications in increasingly diverse domains provides evidence that supports the field's concomitant academic and scholarly maturation. Research into factors that impede or inspire hospitalists to participate in research, innovations that provide mentorship and funding for the specific interests of hospitalists, and the emulation of strategies employed by centers productive in publications are required to successfully foster the multidimensional growth of the field.
Acknowledgements
The authors thank Dr. Antoinette Laskey for her mentorship in survey development, Elaine Bammerlin for copyediting assistance, and the Society of Hospital Medicine members for taking the survey.
Disclosures: An Dang Do, MD, PhD, completed the major part of this work as a Morris Green Scholar at Indiana University School of Medicine. An N. Dang Do, MD, PhD, and Amy M. Munchhof, MD, PhD, contributed equally to this work. Areeba Kara, MD, is supported by a grant from the Methodist Health Foundation and by award number T15OC000047 from the Office of the National Coordinator for Health Information Technology, Office of the Secretary, US Department of Health & Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the National Coordinator For Health Information Technology, Office of the Secretary, US Department of Health & Human Services, or the National Institutes of Health.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- , , , . The status of hospital medicine groups in the United States. J Hosp Med. 2006;1(2):75–80.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308–313.
- , , , . An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314–318.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 25, 2013.
- US National Library of Medicine website. MEDLINE/PubMed data element (field) descriptions. Available at: http://www.nlm.nih.gov/bsd/mms/medlineelements.html. Accessed October 30, 2013.
- American Educational Research Association website. Available at: http://www.aera.net/EducationResearch/WhatisEducationResearch/tab id/13453/Default.aspx. Accessed October 30, 2013.
- National Institutes of Health website. Glossary of NIH terms. Available at: http://grants.nih.gov/grants/glossary.htm. Accessed February 26, 2013.
- US National Science Foundation website. National Center for Science and Engineering Statistics. Definitions of research and development: an annotated compilation of official sources. Available at: http://www.nsf.gov/statistics/randdef/fedgov.cfm. Accessed February 26, 2013.
- , . Health services research: an evolving definition of the field. US National Library of Medicine website. National Institutes of Health. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430351. Accessed February 26, 2013.
- Centers for Medicare and Medicaid Services website. Outcome measures. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/OutcomeMeasu res.html. Accessed August 22, 2013.
- Journal of Hospital Medicine. Available at: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1553–5606/homepage/ProductInformation.html. Accessed October 30, 2013.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , , . Preparing for “diastole”: Advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103–1115.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2011;27(1):23–27.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1–2.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , . The APA and the rise of pediatric generalist network research. Acad Pediatr. 2011;11(3):195–204.
- , , , et al. Phenotype harmonization and cross‐study collaboration in GWAS consortia: the GENEVA experience. Genet Epidemiol. 2011;35(3):159–173.
- , . Clinicians in quality improvement: a new career pathway in academic medicine. JAMA. 2009;301(7):766–768.
- , , , , Documenting quality improvement and patient safety efforts: the quality portfolio. A statement from the Academic Hospitalist Taskforce [published online ahead of print June 27, 2013]. J Gen Intern Med. doi: 10.1007/s11606‐013‐2532‐z.
- , , , , . Person‐job fit: an exploratory cross‐sectional analysis of hospitalists. J Hosp Med. 2012;8(2):96–101.
- , , , . Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40(1):213–226.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- , , , . The status of hospital medicine groups in the United States. J Hosp Med. 2006;1(2):75–80.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308–313.
- , , , . An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314–318.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 25, 2013.
- US National Library of Medicine website. MEDLINE/PubMed data element (field) descriptions. Available at: http://www.nlm.nih.gov/bsd/mms/medlineelements.html. Accessed October 30, 2013.
- American Educational Research Association website. Available at: http://www.aera.net/EducationResearch/WhatisEducationResearch/tab id/13453/Default.aspx. Accessed October 30, 2013.
- National Institutes of Health website. Glossary of NIH terms. Available at: http://grants.nih.gov/grants/glossary.htm. Accessed February 26, 2013.
- US National Science Foundation website. National Center for Science and Engineering Statistics. Definitions of research and development: an annotated compilation of official sources. Available at: http://www.nsf.gov/statistics/randdef/fedgov.cfm. Accessed February 26, 2013.
- , . Health services research: an evolving definition of the field. US National Library of Medicine website. National Institutes of Health. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430351. Accessed February 26, 2013.
- Centers for Medicare and Medicaid Services website. Outcome measures. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/OutcomeMeasu res.html. Accessed August 22, 2013.
- Journal of Hospital Medicine. Available at: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1553–5606/homepage/ProductInformation.html. Accessed October 30, 2013.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , , . Preparing for “diastole”: Advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103–1115.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2011;27(1):23–27.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1–2.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , . The APA and the rise of pediatric generalist network research. Acad Pediatr. 2011;11(3):195–204.
- , , , et al. Phenotype harmonization and cross‐study collaboration in GWAS consortia: the GENEVA experience. Genet Epidemiol. 2011;35(3):159–173.
- , . Clinicians in quality improvement: a new career pathway in academic medicine. JAMA. 2009;301(7):766–768.
- , , , , Documenting quality improvement and patient safety efforts: the quality portfolio. A statement from the Academic Hospitalist Taskforce [published online ahead of print June 27, 2013]. J Gen Intern Med. doi: 10.1007/s11606‐013‐2532‐z.
- , , , , . Person‐job fit: an exploratory cross‐sectional analysis of hospitalists. J Hosp Med. 2012;8(2):96–101.
- , , , . Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40(1):213–226.
© 2014 Society of Hospital Medicine
A Multifaceted Case
Box
1
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Box
2
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
A 67‐year‐old male presented to an outside hospital with a 1‐day history of fevers up to 39.4C, bilateral upper extremity weakness, and confusion. Forty‐eight hours prior to his presentation he had undergone uncomplicated bilateral carpal tunnel release surgery for the complaint of bilateral upper extremity paresthesias.
Bilateral carpal tunnel syndrome should prompt consideration of systemic diseases that infiltrate or impinge both canals (eg, rheumatoid arthritis, acromegaly, hypothyroidism, amyloidosis), although it is most frequently explained by a bilateral repetitive stress (eg, workplace typing). The development of upper extremity weakness suggests that an alternative condition such as cervical myelopathy, bilateral radiculopathy, or a rapidly progressive peripheral neuropathy may be responsible for his paresthesias. It would be unusual for a central nervous system process to selectively cause bilateral upper extremity weakness. Occasionally, patients emerge from surgery with limb weakness caused by peripheral nerve injury sustained from malpositioning of the extremity, but this would have been evident immediately following the operation.
Postoperative fevers are frequently unexplained, but require a search for common healthcare‐associated infections, such as pneumonia, urinary tract infection, intravenous catheter thrombophlebitis, wound infection, or Clostridium difficile colitis. However, such complications are unlikely following an ambulatory procedure. Confusion and fever together point to a central nervous system infection (meningoencephalitis or brain abscess) or a systemic infection that has impaired cognition. Malignancies can cause fever and altered mental status, but these are typically asynchronous events.
His past medical history was notable for hypertension, dyslipidemia, gout, actinic keratosis, and gastroesophageal reflux. His surgical history included bilateral knee replacements, repair of a left rotator cuff injury, and a herniorrhaphy. He was a nonsmoker who consumed 4 to 6 beers daily. His medications included clonidine, colchicine, atorvastatin, extended release metoprolol, triamterene‐hydrochlorothiazide, probenecid, and as‐needed ibuprofen and omeprazole.
Upon presentation he was cooperative and in no distress. Temperature was 38.9C, pulse 119 beats per minute, blood pressure 140/90 mm Hg, and oxygen saturation 94% on room air. He was noted to have logical thinking but impaired concentration. His upper extremity movement was restricted because of postoperative discomfort and swelling rather than true weakness. The rest of the exam was normal.
Metabolic, infectious, structural (intracranial), and toxic disorders can cause altered mental status. His heavy alcohol use puts him at risk for alcohol withdrawal and infections (such as Listeria meningitis), both of which may explain his fever and altered mental status. Signs and symptoms of meningitis are absent at this time. His knee prostheses could have harbored an infection preoperatively and therefore warrant close examination. Patients sometimes have adverse reactions to medications they have been prescribed but are not exposed to until hospitalization, although his surgical procedure was likely done on an outpatient basis. Empiric thiamine should be administered early given his confusion and alcohol habits.
Basic laboratories revealed a hemoglobin of 11.2 g/dL, white blood cell (WBC) count of 6,900/mm3 with 75% neutrophils, platelets of 206,000/mm3. Mean corpuscular volume was 97 mm3. Serum albumin was 2.4 g/dl, sodium 134 mmol/L, potassium 3.9 mmol/L, blood urea nitrogen 12 mg/dL, and creatinine 0.9 mg/dL. The aspartate aminotransferase was 93 U/L, alanine aminotransferase 73 U/L, alkaline phosphatase 254 U/L, and total bilirubin 1.0 mg/dL. Urinalysis was normal. Over the next 16 days fevers and waxing and waning mentation continued. The following studies were normal or negative: blood and urine cultures; transthoracic echocardiogram, antinuclear antibodies, hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antibody; magnetic resonance imaging of the brain, electroencephalogram, and lower extremity venous ultrasound.
Hypoalbuminemia may signal chronic illness, hypoproduction from liver disease (caused by his heavy alcohol use), or losses from the kidney or gastrointestinal tract. His anemia may reflect chronic disease or point toward a specific underlying disorder. For example, fever and anemia could arise from hemolytic processes such as thrombotic thrombocytopenic purpura or clostridial infections.
An extensive workup has not revealed a cause for his prolonged fever (eg, infection, malignancy, autoimmune condition, or toxin). Likewise, an explanation for confusion is lacking. Because systemic illness and structural brain disease have not been uncovered, a lumbar puncture is indicated.
A lumbar puncture under fluoroscopic guidance revealed a cerebrospinal fluid (CSF) WBC count of 6/mm3, red blood cell count (RBC) 2255/mm3, protein 49 mg/dL, and glucose 54 mg/dL. The WBC differential was not reported. No growth was reported on bacterial cultures. Polymerase chain reactions for enterovirus and herpes simplex viruses 1 and 2 were negative. Cryptococcal antigen and Venereal Disease Research Laboratory serologies were also negative.
A CSF WBC count of 6 is out of the normal range, but could be explained by a traumatic tap given the elevated RBC; the protein and glucose are likewise at the border of normal. Collectively, these are nonspecific findings that could point to an infectious or noninfectious cause of intrathecal or paraspinous inflammation, but are not suggestive of bacterial meningitis.
The patient developed pneumonia, for which he received ertapenem. On hospital day 17 he was intubated for hypoxia and respiratory distress and was extubated after 4 days of mechanical ventilation. Increasing weakness in all extremities prompted magnetic resonance imaging of the spine, which revealed fluid and enhancement involving the soft tissues around C3‐C4 and C5‐C6, raising concerns for discitis and osteomyelitis. Possible septic arthritis at the C3‐C4 and C4‐C5 facets was noted. Ring enhancing fluid collections from T2‐T8 compatible with an epidural abscess with cord compression at T4‐T5 and T6‐T7 were seen. Enhancement and fluid involving the facet joints between T2‐T7 was also consistent with septic arthritis (Figure 1).
His pneumonia appears to have developed many days into his hospitalization, and therefore is unlikely to account for his initial fever and confusion. Blood cultures and echocardiogram have not suggested an endovascular infection that could account for such widespread vertebral and epidural deposition. A wide number of bacteria can cause epidural abscesses and septic arthritis, most commonly Staphylococcus aureus. Less common pathogens with a predilection for osteoarticular involvement, such as Brucella species, warrant consideration when there is appropriate epidemiologic risk.
Systemic bacterial infection remains a concern with his alcoholism rendering him partially immunosuppressed. However, a large number of adjacent spinal joints harboring a bacterial infection is unusual, and a working diagnosis of multilevel spinal infection, therefore, should prompt consideration of noninfectious processes. When a patient develops a swollen peripheral joint and fever in the postoperative setting, gout or pseudogout is a leading consideration. That same thinking should be applied to the vertebrae, where spinal gout can manifest. Surgery itself or associated changes in alcohol consumption patterns or changes in medications (at least 4 of which are relevant to goutcolchicine, hydrochlorothiazide, probenecid, and ibuprofen) could predispose him to a flare.
Aspiration of the epidural collection yielded a negative Gram stain and culture. He developed swelling in the bilateral proximal interphalangeal joints and was treated with steroids and colchicine for suspected gout flare. Vancomycin and piperacillin‐tazobactam were initiated, and on hospital day 22 the patient was transferred to another hospital for further evaluation by neurosurgery.
The negative Gram stain and culture argues against septic arthritis, but these are imperfect tests and will not detect atypical pathogens (eg, spinal tuberculosis). Reexamination of the aspirate for urate and calcium pyrophosphate crystals would be useful. Initiation of steroids in the setting of potentially undiagnosed infection requires a careful risk/benefit analysis. It may be reasonable to treat the patient with colchicine alone while withholding steroids and avoiding nonsteroidal agents in case invasive procedures are planned.
On exam his temperature was 36C, blood pressure 156/92 mm Hg, pulse 100 beats per minute, respirations 21 per minute, and oxygenation 97% on room air. He was not in acute distress and was only oriented to self. Bilateral 2+ lower extremity pitting edema up to the knees was noted. Examination of the heart and lungs was unremarkable. Gouty tophi were noted over both elbows. His joints were normal.
Cranial nerves IIXII were normal. Motor exam revealed normal muscle tone and bulk. Muscle strength was approximately 3/5 in the right upper extremity and 4+/5 in the left upper extremity. Bilateral lower extremity strength was 3/5 in hip flexion, knee flexion, and knee extension. Dorsiflexion and plantar flexion were approximately 2/5 bilaterally. Sensation was intact to light touch and pinprick, and proprioception was normal. Gait was not tested. A Foley catheter was in place.
This examination confirms ongoing encephalopathy and incomplete quadriplegia. The lower extremity weakness is nearly equal proximally and distally, which can be seen with an advanced peripheral neuropathy but is more characteristic of myelopathy. The expected concomitant sensory deficit of myelopathy is not present, although this may be difficult to detect in a confused patient. Reflex testing would help in distinguishing myelopathy (favored because of the imaging findings) from a rapid progressive peripheral motor neuropathy (eg, acute inflammatory demyelinating polyneuropathy or acute intermittent porphyria).
The pitting edema likely represents fluid overload, which can be nonspecific after prolonged immobility during hospitalization; hypoalbuminemia is oftentimes speculated to play a role when this develops. His alcohol use puts him at risk for heart failure (although there is no evidence of this on exam) and liver disease (which his liver function tests suggest). The tophi speak to the extent and chronicity of his hyperuricemia.
On arrival he reported recent onset diarrhea. Medications at transfer included metoprolol, omeprazole, prednisone, piperacillin/tazobactam, vancomycin, and colchicine; acetaminophen, bisacodyl, diphenhydramine, fentanyl, subcutaneous insulin, and labetalol were administered as needed. Laboratory studies included a hemoglobin of 9.5 g/dL, WBC count of 7,300/mm3 with 95% neutrophils, platelets 301,000/mm3, sodium 151 mmol/L, potassium 2.9 mmol/L, blood urea nitrogen 76 mg/dL, creatinine 2.0 mg/dL, aspartate aminotransferase 171 U/L, and alanine aminotransferase 127 U/L. Serum albumin was 1.7 g/dL.
At least 3 of his medicationsdiphenhydramine, fentanyl, and prednisonemay be contributing to his ongoing altered mental status, which may be further compounded by hypernatremia. Although his liver disease remains uncharacterized, hepatic encephalopathy may be contributing to his confusion as well.
Colchicine is likely responsible for his diarrhea, which would be the most readily available explanation for his hypernatremia, hypokalemia, and acute kidney injury (AKI). Acute kidney injury could result from progressive liver disease (hepatorenal syndrome), decreased arterial perfusion (suggested by third spacing or his diarrhea), acute tubular necrosis (from infection or medication), or urinary retention secondary to catheter obstruction. Acute hyperuricemia can also cause AKI (urate nephropathy).
Anemia has progressed and requires evaluation for blood loss as well as hemolysis. Hepatotoxicity from any of his medications (eg, acetaminophen) must be considered. Coagulation studies and review of the previous abdominal computed tomography would help determine the extent of his liver disease.
Neurosurgical consultation was obtained and the patient and his family elected to proceed with a thoracic laminectomy. Cheesy fluid was identified at the facet joints at T6‐T7, which was found to contain rare deposits of monosodium urate crystals. Surgical specimen cultures were sterile. His mental status and strength slowly improved to baseline following the surgery. He was discharged on postoperative day 7 to a rehabilitation facility. On the telephone follow‐up he reported that he has regained his strength completely.
The fluid analysis and clinical course confirms spinal gout. The presenting encephalopathy remains unexplained; I am unaware of gout leading to altered mental status.
COMMENTARY
Gout is an inflammatory condition triggered by the deposition of monosodium urate crystals in tissues in association with hyperuricemia.[1] Based on the 20072008 National Health and Nutrition Examination Survey, the prevalence of gout among US adults was 3.9% (8.3 million individuals).[2] These rates are increasing and are thought to be spurred by the aging population, increasing rates of obesity, and changing dietary habits including increases in the consumption of soft drinks and red meat.[3, 4, 5] The development of gout during hospitalization can prolong length of stay, and the implementation of a management protocol appears to help decrease treatment delays and the inappropriate discontinuation of gout prophylaxis.[6, 7] Surgery, with its associated physiologic stressors, can trigger gout, which is often polyarticular and presents with fever leading to testing and consultations for the febrile episode.[8]
Gout is an ancient disease that is familiar to most clinicians. In 1666, Daniel Sennert, a German physician, described gout as the physician's shame because of its infrequent recognition.[9] Clinical gout spans 3 stages: asymptomatic hyperuricemia, acute and intercritical gout, and chronic gouty arthritis. The typical acute presentation is monoarticular with the abrupt onset of pain, swelling, warmth, and erythema in a peripheral joint. It manifests most characteristically in the first metatarsophalangeal joint (podagra), but also frequently involves the midfoot, ankle, knee, and wrist and sometimes affects multiple joints simultaneously (polyarticular gout).[1, 10] The visualization of monosodium urate crystals either in synovial fluid or from a tophus is diagnostic of gout; however, guidelines recognize that a classic presentation of gout may be diagnosed based on clinical criteria alone.[11] Dual energy computerized tomography and ultrasonography are emerging as techniques for the visualization of monosodium urate crystals; however, they are not currently routinely recommended.[12]
There are many unusual presentations of gout, with an increase in such reports paralleling both the overall increase in the prevalence of gout and improvements in available imaging techniques.[13] Atypical presentations present diagnostic challenges and are often caused by tophaceous deposits in unusual locations. Reports of atypical gout have described entrapment neuropathies (eg, gouty deposits inducing carpal tunnel syndrome), ocular gout manifested as conjunctival deposits and uveitis, pancreatic gout presenting as a mass, and dermatologic manifestations including panniculitis.[13, 14]
Spinal gout (also known as axial gout) manifests when crystal‐induced inflammation, erosive arthritis, and tophaceous deposits occur along the spinal column. A cross‐sectional study of patients with poorly controlled gout reported the prevalence of spinal gout diagnosed by computerized tomography to be 35%. These radiographic findings were not consistently correlated with back pain.[15] Imaging features that are suggestive of spinal gout include intra‐articular and juxta‐articular erosions with sclerotic margins and density greater than the surrounding muscle. Periosteal new bone formation adjacent to bony destruction can form overhanging edges.[16] When retrospectively presented with the final diagnosis, the radiologist at our institution noted that the appearance was typical gout in an atypical location.
Spinal gout can be confused with spinal metastasis, infection, and stenosis. It can remain asymptomatic or present with back pain, radiculopathy, or cord compression. The lumbar spine is the most frequently affected site.[17, 18] Many patients with spinal gout have had chronic tophaceous gout with radiologic evidence of erosions in the peripheral joints.[15] Patients with spinal gout also have elevated urate levels and markers of inflammation.[18] Surgical decompression and stabilization is recommended when there is frank cord compression, progressive neurologic compromise, or lack of improvement with gout therapy alone.[18]
This patient's male gender, history of gout, hypertension, alcohol consumption, and thiazide diuretic use placed him at an increased risk of a gout attack.[19, 20] The possible interruption of urate‐lowering therapy for the surgical procedure and surgery itself further heightened his risk of suffering acute gouty arthritis in the perioperative period.[21] The patient's encephalopathy may have masked back pain and precluded an accurate neurologic exam. There is one case report to our knowledge describing encephalopathy that improved with colchicine and was possibly related to gout.[22] This patient's encephalopathy was deemed multifactorial and attributed to alcohol withdrawal, medications (including opioids and steroids), and infection (pneumonia).
Gout is best known for its peripheral arthritis and is rarely invoked in the consideration of spinal and myelopathic processes where more pressing competing diagnoses, such as infection and malignancy, are typically considered. In addition, when surgical specimens are submitted for examination for pathology in formaldehyde (rather than alcohol), monosodium urate crystals are dissolved and are thus difficult to identify in the specimen.
This case reminds us that gout remains a diagnostic challenge and should be considered in the differential of an inflammatory process. Recognition of the multifaceted nature of gout can allow for the earlier recognition and treatment of the less typical presentations of this ancient malady.
KEY TEACHING POINTS
- Crystalline disease is a common cause of postoperative arthritis.
- Gout (and pseudogout) should be considered in cases of focal inflammation (detected by examination or imaging) when the evidence or predisposition for infection is limited or nonexistent.
- Spinal gout presents with back pain, radiculopathy, or cord compression and may be confused with spinal metastasis, infection, and stenosis.
Acknowledgements
The authors thank Dr. Kari Waddell and Elaine Bammerlin for their assistance in the preparation of this manuscript.
Disclosure: Nothing to report.
- , . Clinical features and treatment of gout. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, eds. Kelley's Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013:1544–1575.
- , , . Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141.
- , , , . Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587.
- , , , , . Purine‐rich foods, dairy and protein intake, and the risk of gout in men. New Engl J Med. 2004;350(11):1093–1103.
- , , . Fructose‐rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–2278.
- , . Healthcare burden of in‐hospital gout. Intern Med J. 2012;42(11):1261–1263.
- , , , , , . Improved management of acute gout during hospitalization following introduction of a protocol. Int J Rheum Dis. 2012;15(6):512–520.
- , , . Postsurgical gout. Am Surg. 1995;61(1):56–59.
- , . Evolution of modern medicine. Arch Intern Med. 1960;105(4):640–644.
- . Clinical practice. Gout. N Engl J Med. 2011;364(5):443–452.
- . Management of gout: a 57‐year‐old man with a history of podagra, hyperuricemia, and mild renal insufficiency. JAMA. 2012;308(20):2133–2141.
- , , , et al. Diagnostic imaging of gout: comparison of high‐resolution US versus conventional X‐ray. Eur Radiol. 2008;18(3):621–630.
- , . The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42(2):146–154.
- , . Unusual clinical presentations of gout. Curr Opin Rheumatol. 2010;22(2):181–187.
- , , , , , . Correlates of axial gout: a cross‐sectional study. J Rheumatol. 2012;39(7):1445–1449.
- , , , , , . Axial gouty arthropathy. Am J Med Sci. 2009;338(2):140–146.
- , , . Axial (spinal) gout. Curr Rheumatol Rep. 2012;14(2):161–164.
- , , , . Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. 2007;67(1):65–73.
- , , , et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800.e11–800.e16.
- , , , , , . Recent diuretic use and the risk of recurrent gout attacks: the online case‐crossover gout study. J Rheumatol. 2006;33(7):1341–1345.
- , , , , . Clinical features and risk factors of postsurgical gout. Ann Rheum Dis. 2008;67(9):1271–1275.
- , , , . Gouty encephalopathy: myth or reality [in French]? Rev Med Interne. 1997;18(6):474–476.
Box
1
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Box
2
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
A 67‐year‐old male presented to an outside hospital with a 1‐day history of fevers up to 39.4C, bilateral upper extremity weakness, and confusion. Forty‐eight hours prior to his presentation he had undergone uncomplicated bilateral carpal tunnel release surgery for the complaint of bilateral upper extremity paresthesias.
Bilateral carpal tunnel syndrome should prompt consideration of systemic diseases that infiltrate or impinge both canals (eg, rheumatoid arthritis, acromegaly, hypothyroidism, amyloidosis), although it is most frequently explained by a bilateral repetitive stress (eg, workplace typing). The development of upper extremity weakness suggests that an alternative condition such as cervical myelopathy, bilateral radiculopathy, or a rapidly progressive peripheral neuropathy may be responsible for his paresthesias. It would be unusual for a central nervous system process to selectively cause bilateral upper extremity weakness. Occasionally, patients emerge from surgery with limb weakness caused by peripheral nerve injury sustained from malpositioning of the extremity, but this would have been evident immediately following the operation.
Postoperative fevers are frequently unexplained, but require a search for common healthcare‐associated infections, such as pneumonia, urinary tract infection, intravenous catheter thrombophlebitis, wound infection, or Clostridium difficile colitis. However, such complications are unlikely following an ambulatory procedure. Confusion and fever together point to a central nervous system infection (meningoencephalitis or brain abscess) or a systemic infection that has impaired cognition. Malignancies can cause fever and altered mental status, but these are typically asynchronous events.
His past medical history was notable for hypertension, dyslipidemia, gout, actinic keratosis, and gastroesophageal reflux. His surgical history included bilateral knee replacements, repair of a left rotator cuff injury, and a herniorrhaphy. He was a nonsmoker who consumed 4 to 6 beers daily. His medications included clonidine, colchicine, atorvastatin, extended release metoprolol, triamterene‐hydrochlorothiazide, probenecid, and as‐needed ibuprofen and omeprazole.
Upon presentation he was cooperative and in no distress. Temperature was 38.9C, pulse 119 beats per minute, blood pressure 140/90 mm Hg, and oxygen saturation 94% on room air. He was noted to have logical thinking but impaired concentration. His upper extremity movement was restricted because of postoperative discomfort and swelling rather than true weakness. The rest of the exam was normal.
Metabolic, infectious, structural (intracranial), and toxic disorders can cause altered mental status. His heavy alcohol use puts him at risk for alcohol withdrawal and infections (such as Listeria meningitis), both of which may explain his fever and altered mental status. Signs and symptoms of meningitis are absent at this time. His knee prostheses could have harbored an infection preoperatively and therefore warrant close examination. Patients sometimes have adverse reactions to medications they have been prescribed but are not exposed to until hospitalization, although his surgical procedure was likely done on an outpatient basis. Empiric thiamine should be administered early given his confusion and alcohol habits.
Basic laboratories revealed a hemoglobin of 11.2 g/dL, white blood cell (WBC) count of 6,900/mm3 with 75% neutrophils, platelets of 206,000/mm3. Mean corpuscular volume was 97 mm3. Serum albumin was 2.4 g/dl, sodium 134 mmol/L, potassium 3.9 mmol/L, blood urea nitrogen 12 mg/dL, and creatinine 0.9 mg/dL. The aspartate aminotransferase was 93 U/L, alanine aminotransferase 73 U/L, alkaline phosphatase 254 U/L, and total bilirubin 1.0 mg/dL. Urinalysis was normal. Over the next 16 days fevers and waxing and waning mentation continued. The following studies were normal or negative: blood and urine cultures; transthoracic echocardiogram, antinuclear antibodies, hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antibody; magnetic resonance imaging of the brain, electroencephalogram, and lower extremity venous ultrasound.
Hypoalbuminemia may signal chronic illness, hypoproduction from liver disease (caused by his heavy alcohol use), or losses from the kidney or gastrointestinal tract. His anemia may reflect chronic disease or point toward a specific underlying disorder. For example, fever and anemia could arise from hemolytic processes such as thrombotic thrombocytopenic purpura or clostridial infections.
An extensive workup has not revealed a cause for his prolonged fever (eg, infection, malignancy, autoimmune condition, or toxin). Likewise, an explanation for confusion is lacking. Because systemic illness and structural brain disease have not been uncovered, a lumbar puncture is indicated.
A lumbar puncture under fluoroscopic guidance revealed a cerebrospinal fluid (CSF) WBC count of 6/mm3, red blood cell count (RBC) 2255/mm3, protein 49 mg/dL, and glucose 54 mg/dL. The WBC differential was not reported. No growth was reported on bacterial cultures. Polymerase chain reactions for enterovirus and herpes simplex viruses 1 and 2 were negative. Cryptococcal antigen and Venereal Disease Research Laboratory serologies were also negative.
A CSF WBC count of 6 is out of the normal range, but could be explained by a traumatic tap given the elevated RBC; the protein and glucose are likewise at the border of normal. Collectively, these are nonspecific findings that could point to an infectious or noninfectious cause of intrathecal or paraspinous inflammation, but are not suggestive of bacterial meningitis.
The patient developed pneumonia, for which he received ertapenem. On hospital day 17 he was intubated for hypoxia and respiratory distress and was extubated after 4 days of mechanical ventilation. Increasing weakness in all extremities prompted magnetic resonance imaging of the spine, which revealed fluid and enhancement involving the soft tissues around C3‐C4 and C5‐C6, raising concerns for discitis and osteomyelitis. Possible septic arthritis at the C3‐C4 and C4‐C5 facets was noted. Ring enhancing fluid collections from T2‐T8 compatible with an epidural abscess with cord compression at T4‐T5 and T6‐T7 were seen. Enhancement and fluid involving the facet joints between T2‐T7 was also consistent with septic arthritis (Figure 1).

His pneumonia appears to have developed many days into his hospitalization, and therefore is unlikely to account for his initial fever and confusion. Blood cultures and echocardiogram have not suggested an endovascular infection that could account for such widespread vertebral and epidural deposition. A wide number of bacteria can cause epidural abscesses and septic arthritis, most commonly Staphylococcus aureus. Less common pathogens with a predilection for osteoarticular involvement, such as Brucella species, warrant consideration when there is appropriate epidemiologic risk.
Systemic bacterial infection remains a concern with his alcoholism rendering him partially immunosuppressed. However, a large number of adjacent spinal joints harboring a bacterial infection is unusual, and a working diagnosis of multilevel spinal infection, therefore, should prompt consideration of noninfectious processes. When a patient develops a swollen peripheral joint and fever in the postoperative setting, gout or pseudogout is a leading consideration. That same thinking should be applied to the vertebrae, where spinal gout can manifest. Surgery itself or associated changes in alcohol consumption patterns or changes in medications (at least 4 of which are relevant to goutcolchicine, hydrochlorothiazide, probenecid, and ibuprofen) could predispose him to a flare.
Aspiration of the epidural collection yielded a negative Gram stain and culture. He developed swelling in the bilateral proximal interphalangeal joints and was treated with steroids and colchicine for suspected gout flare. Vancomycin and piperacillin‐tazobactam were initiated, and on hospital day 22 the patient was transferred to another hospital for further evaluation by neurosurgery.
The negative Gram stain and culture argues against septic arthritis, but these are imperfect tests and will not detect atypical pathogens (eg, spinal tuberculosis). Reexamination of the aspirate for urate and calcium pyrophosphate crystals would be useful. Initiation of steroids in the setting of potentially undiagnosed infection requires a careful risk/benefit analysis. It may be reasonable to treat the patient with colchicine alone while withholding steroids and avoiding nonsteroidal agents in case invasive procedures are planned.
On exam his temperature was 36C, blood pressure 156/92 mm Hg, pulse 100 beats per minute, respirations 21 per minute, and oxygenation 97% on room air. He was not in acute distress and was only oriented to self. Bilateral 2+ lower extremity pitting edema up to the knees was noted. Examination of the heart and lungs was unremarkable. Gouty tophi were noted over both elbows. His joints were normal.
Cranial nerves IIXII were normal. Motor exam revealed normal muscle tone and bulk. Muscle strength was approximately 3/5 in the right upper extremity and 4+/5 in the left upper extremity. Bilateral lower extremity strength was 3/5 in hip flexion, knee flexion, and knee extension. Dorsiflexion and plantar flexion were approximately 2/5 bilaterally. Sensation was intact to light touch and pinprick, and proprioception was normal. Gait was not tested. A Foley catheter was in place.
This examination confirms ongoing encephalopathy and incomplete quadriplegia. The lower extremity weakness is nearly equal proximally and distally, which can be seen with an advanced peripheral neuropathy but is more characteristic of myelopathy. The expected concomitant sensory deficit of myelopathy is not present, although this may be difficult to detect in a confused patient. Reflex testing would help in distinguishing myelopathy (favored because of the imaging findings) from a rapid progressive peripheral motor neuropathy (eg, acute inflammatory demyelinating polyneuropathy or acute intermittent porphyria).
The pitting edema likely represents fluid overload, which can be nonspecific after prolonged immobility during hospitalization; hypoalbuminemia is oftentimes speculated to play a role when this develops. His alcohol use puts him at risk for heart failure (although there is no evidence of this on exam) and liver disease (which his liver function tests suggest). The tophi speak to the extent and chronicity of his hyperuricemia.
On arrival he reported recent onset diarrhea. Medications at transfer included metoprolol, omeprazole, prednisone, piperacillin/tazobactam, vancomycin, and colchicine; acetaminophen, bisacodyl, diphenhydramine, fentanyl, subcutaneous insulin, and labetalol were administered as needed. Laboratory studies included a hemoglobin of 9.5 g/dL, WBC count of 7,300/mm3 with 95% neutrophils, platelets 301,000/mm3, sodium 151 mmol/L, potassium 2.9 mmol/L, blood urea nitrogen 76 mg/dL, creatinine 2.0 mg/dL, aspartate aminotransferase 171 U/L, and alanine aminotransferase 127 U/L. Serum albumin was 1.7 g/dL.
At least 3 of his medicationsdiphenhydramine, fentanyl, and prednisonemay be contributing to his ongoing altered mental status, which may be further compounded by hypernatremia. Although his liver disease remains uncharacterized, hepatic encephalopathy may be contributing to his confusion as well.
Colchicine is likely responsible for his diarrhea, which would be the most readily available explanation for his hypernatremia, hypokalemia, and acute kidney injury (AKI). Acute kidney injury could result from progressive liver disease (hepatorenal syndrome), decreased arterial perfusion (suggested by third spacing or his diarrhea), acute tubular necrosis (from infection or medication), or urinary retention secondary to catheter obstruction. Acute hyperuricemia can also cause AKI (urate nephropathy).
Anemia has progressed and requires evaluation for blood loss as well as hemolysis. Hepatotoxicity from any of his medications (eg, acetaminophen) must be considered. Coagulation studies and review of the previous abdominal computed tomography would help determine the extent of his liver disease.
Neurosurgical consultation was obtained and the patient and his family elected to proceed with a thoracic laminectomy. Cheesy fluid was identified at the facet joints at T6‐T7, which was found to contain rare deposits of monosodium urate crystals. Surgical specimen cultures were sterile. His mental status and strength slowly improved to baseline following the surgery. He was discharged on postoperative day 7 to a rehabilitation facility. On the telephone follow‐up he reported that he has regained his strength completely.
The fluid analysis and clinical course confirms spinal gout. The presenting encephalopathy remains unexplained; I am unaware of gout leading to altered mental status.
COMMENTARY
Gout is an inflammatory condition triggered by the deposition of monosodium urate crystals in tissues in association with hyperuricemia.[1] Based on the 20072008 National Health and Nutrition Examination Survey, the prevalence of gout among US adults was 3.9% (8.3 million individuals).[2] These rates are increasing and are thought to be spurred by the aging population, increasing rates of obesity, and changing dietary habits including increases in the consumption of soft drinks and red meat.[3, 4, 5] The development of gout during hospitalization can prolong length of stay, and the implementation of a management protocol appears to help decrease treatment delays and the inappropriate discontinuation of gout prophylaxis.[6, 7] Surgery, with its associated physiologic stressors, can trigger gout, which is often polyarticular and presents with fever leading to testing and consultations for the febrile episode.[8]
Gout is an ancient disease that is familiar to most clinicians. In 1666, Daniel Sennert, a German physician, described gout as the physician's shame because of its infrequent recognition.[9] Clinical gout spans 3 stages: asymptomatic hyperuricemia, acute and intercritical gout, and chronic gouty arthritis. The typical acute presentation is monoarticular with the abrupt onset of pain, swelling, warmth, and erythema in a peripheral joint. It manifests most characteristically in the first metatarsophalangeal joint (podagra), but also frequently involves the midfoot, ankle, knee, and wrist and sometimes affects multiple joints simultaneously (polyarticular gout).[1, 10] The visualization of monosodium urate crystals either in synovial fluid or from a tophus is diagnostic of gout; however, guidelines recognize that a classic presentation of gout may be diagnosed based on clinical criteria alone.[11] Dual energy computerized tomography and ultrasonography are emerging as techniques for the visualization of monosodium urate crystals; however, they are not currently routinely recommended.[12]
There are many unusual presentations of gout, with an increase in such reports paralleling both the overall increase in the prevalence of gout and improvements in available imaging techniques.[13] Atypical presentations present diagnostic challenges and are often caused by tophaceous deposits in unusual locations. Reports of atypical gout have described entrapment neuropathies (eg, gouty deposits inducing carpal tunnel syndrome), ocular gout manifested as conjunctival deposits and uveitis, pancreatic gout presenting as a mass, and dermatologic manifestations including panniculitis.[13, 14]
Spinal gout (also known as axial gout) manifests when crystal‐induced inflammation, erosive arthritis, and tophaceous deposits occur along the spinal column. A cross‐sectional study of patients with poorly controlled gout reported the prevalence of spinal gout diagnosed by computerized tomography to be 35%. These radiographic findings were not consistently correlated with back pain.[15] Imaging features that are suggestive of spinal gout include intra‐articular and juxta‐articular erosions with sclerotic margins and density greater than the surrounding muscle. Periosteal new bone formation adjacent to bony destruction can form overhanging edges.[16] When retrospectively presented with the final diagnosis, the radiologist at our institution noted that the appearance was typical gout in an atypical location.
Spinal gout can be confused with spinal metastasis, infection, and stenosis. It can remain asymptomatic or present with back pain, radiculopathy, or cord compression. The lumbar spine is the most frequently affected site.[17, 18] Many patients with spinal gout have had chronic tophaceous gout with radiologic evidence of erosions in the peripheral joints.[15] Patients with spinal gout also have elevated urate levels and markers of inflammation.[18] Surgical decompression and stabilization is recommended when there is frank cord compression, progressive neurologic compromise, or lack of improvement with gout therapy alone.[18]
This patient's male gender, history of gout, hypertension, alcohol consumption, and thiazide diuretic use placed him at an increased risk of a gout attack.[19, 20] The possible interruption of urate‐lowering therapy for the surgical procedure and surgery itself further heightened his risk of suffering acute gouty arthritis in the perioperative period.[21] The patient's encephalopathy may have masked back pain and precluded an accurate neurologic exam. There is one case report to our knowledge describing encephalopathy that improved with colchicine and was possibly related to gout.[22] This patient's encephalopathy was deemed multifactorial and attributed to alcohol withdrawal, medications (including opioids and steroids), and infection (pneumonia).
Gout is best known for its peripheral arthritis and is rarely invoked in the consideration of spinal and myelopathic processes where more pressing competing diagnoses, such as infection and malignancy, are typically considered. In addition, when surgical specimens are submitted for examination for pathology in formaldehyde (rather than alcohol), monosodium urate crystals are dissolved and are thus difficult to identify in the specimen.
This case reminds us that gout remains a diagnostic challenge and should be considered in the differential of an inflammatory process. Recognition of the multifaceted nature of gout can allow for the earlier recognition and treatment of the less typical presentations of this ancient malady.
KEY TEACHING POINTS
- Crystalline disease is a common cause of postoperative arthritis.
- Gout (and pseudogout) should be considered in cases of focal inflammation (detected by examination or imaging) when the evidence or predisposition for infection is limited or nonexistent.
- Spinal gout presents with back pain, radiculopathy, or cord compression and may be confused with spinal metastasis, infection, and stenosis.
Acknowledgements
The authors thank Dr. Kari Waddell and Elaine Bammerlin for their assistance in the preparation of this manuscript.
Disclosure: Nothing to report.
Box
1
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Box
2
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
A 67‐year‐old male presented to an outside hospital with a 1‐day history of fevers up to 39.4C, bilateral upper extremity weakness, and confusion. Forty‐eight hours prior to his presentation he had undergone uncomplicated bilateral carpal tunnel release surgery for the complaint of bilateral upper extremity paresthesias.
Bilateral carpal tunnel syndrome should prompt consideration of systemic diseases that infiltrate or impinge both canals (eg, rheumatoid arthritis, acromegaly, hypothyroidism, amyloidosis), although it is most frequently explained by a bilateral repetitive stress (eg, workplace typing). The development of upper extremity weakness suggests that an alternative condition such as cervical myelopathy, bilateral radiculopathy, or a rapidly progressive peripheral neuropathy may be responsible for his paresthesias. It would be unusual for a central nervous system process to selectively cause bilateral upper extremity weakness. Occasionally, patients emerge from surgery with limb weakness caused by peripheral nerve injury sustained from malpositioning of the extremity, but this would have been evident immediately following the operation.
Postoperative fevers are frequently unexplained, but require a search for common healthcare‐associated infections, such as pneumonia, urinary tract infection, intravenous catheter thrombophlebitis, wound infection, or Clostridium difficile colitis. However, such complications are unlikely following an ambulatory procedure. Confusion and fever together point to a central nervous system infection (meningoencephalitis or brain abscess) or a systemic infection that has impaired cognition. Malignancies can cause fever and altered mental status, but these are typically asynchronous events.
His past medical history was notable for hypertension, dyslipidemia, gout, actinic keratosis, and gastroesophageal reflux. His surgical history included bilateral knee replacements, repair of a left rotator cuff injury, and a herniorrhaphy. He was a nonsmoker who consumed 4 to 6 beers daily. His medications included clonidine, colchicine, atorvastatin, extended release metoprolol, triamterene‐hydrochlorothiazide, probenecid, and as‐needed ibuprofen and omeprazole.
Upon presentation he was cooperative and in no distress. Temperature was 38.9C, pulse 119 beats per minute, blood pressure 140/90 mm Hg, and oxygen saturation 94% on room air. He was noted to have logical thinking but impaired concentration. His upper extremity movement was restricted because of postoperative discomfort and swelling rather than true weakness. The rest of the exam was normal.
Metabolic, infectious, structural (intracranial), and toxic disorders can cause altered mental status. His heavy alcohol use puts him at risk for alcohol withdrawal and infections (such as Listeria meningitis), both of which may explain his fever and altered mental status. Signs and symptoms of meningitis are absent at this time. His knee prostheses could have harbored an infection preoperatively and therefore warrant close examination. Patients sometimes have adverse reactions to medications they have been prescribed but are not exposed to until hospitalization, although his surgical procedure was likely done on an outpatient basis. Empiric thiamine should be administered early given his confusion and alcohol habits.
Basic laboratories revealed a hemoglobin of 11.2 g/dL, white blood cell (WBC) count of 6,900/mm3 with 75% neutrophils, platelets of 206,000/mm3. Mean corpuscular volume was 97 mm3. Serum albumin was 2.4 g/dl, sodium 134 mmol/L, potassium 3.9 mmol/L, blood urea nitrogen 12 mg/dL, and creatinine 0.9 mg/dL. The aspartate aminotransferase was 93 U/L, alanine aminotransferase 73 U/L, alkaline phosphatase 254 U/L, and total bilirubin 1.0 mg/dL. Urinalysis was normal. Over the next 16 days fevers and waxing and waning mentation continued. The following studies were normal or negative: blood and urine cultures; transthoracic echocardiogram, antinuclear antibodies, hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antibody; magnetic resonance imaging of the brain, electroencephalogram, and lower extremity venous ultrasound.
Hypoalbuminemia may signal chronic illness, hypoproduction from liver disease (caused by his heavy alcohol use), or losses from the kidney or gastrointestinal tract. His anemia may reflect chronic disease or point toward a specific underlying disorder. For example, fever and anemia could arise from hemolytic processes such as thrombotic thrombocytopenic purpura or clostridial infections.
An extensive workup has not revealed a cause for his prolonged fever (eg, infection, malignancy, autoimmune condition, or toxin). Likewise, an explanation for confusion is lacking. Because systemic illness and structural brain disease have not been uncovered, a lumbar puncture is indicated.
A lumbar puncture under fluoroscopic guidance revealed a cerebrospinal fluid (CSF) WBC count of 6/mm3, red blood cell count (RBC) 2255/mm3, protein 49 mg/dL, and glucose 54 mg/dL. The WBC differential was not reported. No growth was reported on bacterial cultures. Polymerase chain reactions for enterovirus and herpes simplex viruses 1 and 2 were negative. Cryptococcal antigen and Venereal Disease Research Laboratory serologies were also negative.
A CSF WBC count of 6 is out of the normal range, but could be explained by a traumatic tap given the elevated RBC; the protein and glucose are likewise at the border of normal. Collectively, these are nonspecific findings that could point to an infectious or noninfectious cause of intrathecal or paraspinous inflammation, but are not suggestive of bacterial meningitis.
The patient developed pneumonia, for which he received ertapenem. On hospital day 17 he was intubated for hypoxia and respiratory distress and was extubated after 4 days of mechanical ventilation. Increasing weakness in all extremities prompted magnetic resonance imaging of the spine, which revealed fluid and enhancement involving the soft tissues around C3‐C4 and C5‐C6, raising concerns for discitis and osteomyelitis. Possible septic arthritis at the C3‐C4 and C4‐C5 facets was noted. Ring enhancing fluid collections from T2‐T8 compatible with an epidural abscess with cord compression at T4‐T5 and T6‐T7 were seen. Enhancement and fluid involving the facet joints between T2‐T7 was also consistent with septic arthritis (Figure 1).

His pneumonia appears to have developed many days into his hospitalization, and therefore is unlikely to account for his initial fever and confusion. Blood cultures and echocardiogram have not suggested an endovascular infection that could account for such widespread vertebral and epidural deposition. A wide number of bacteria can cause epidural abscesses and septic arthritis, most commonly Staphylococcus aureus. Less common pathogens with a predilection for osteoarticular involvement, such as Brucella species, warrant consideration when there is appropriate epidemiologic risk.
Systemic bacterial infection remains a concern with his alcoholism rendering him partially immunosuppressed. However, a large number of adjacent spinal joints harboring a bacterial infection is unusual, and a working diagnosis of multilevel spinal infection, therefore, should prompt consideration of noninfectious processes. When a patient develops a swollen peripheral joint and fever in the postoperative setting, gout or pseudogout is a leading consideration. That same thinking should be applied to the vertebrae, where spinal gout can manifest. Surgery itself or associated changes in alcohol consumption patterns or changes in medications (at least 4 of which are relevant to goutcolchicine, hydrochlorothiazide, probenecid, and ibuprofen) could predispose him to a flare.
Aspiration of the epidural collection yielded a negative Gram stain and culture. He developed swelling in the bilateral proximal interphalangeal joints and was treated with steroids and colchicine for suspected gout flare. Vancomycin and piperacillin‐tazobactam were initiated, and on hospital day 22 the patient was transferred to another hospital for further evaluation by neurosurgery.
The negative Gram stain and culture argues against septic arthritis, but these are imperfect tests and will not detect atypical pathogens (eg, spinal tuberculosis). Reexamination of the aspirate for urate and calcium pyrophosphate crystals would be useful. Initiation of steroids in the setting of potentially undiagnosed infection requires a careful risk/benefit analysis. It may be reasonable to treat the patient with colchicine alone while withholding steroids and avoiding nonsteroidal agents in case invasive procedures are planned.
On exam his temperature was 36C, blood pressure 156/92 mm Hg, pulse 100 beats per minute, respirations 21 per minute, and oxygenation 97% on room air. He was not in acute distress and was only oriented to self. Bilateral 2+ lower extremity pitting edema up to the knees was noted. Examination of the heart and lungs was unremarkable. Gouty tophi were noted over both elbows. His joints were normal.
Cranial nerves IIXII were normal. Motor exam revealed normal muscle tone and bulk. Muscle strength was approximately 3/5 in the right upper extremity and 4+/5 in the left upper extremity. Bilateral lower extremity strength was 3/5 in hip flexion, knee flexion, and knee extension. Dorsiflexion and plantar flexion were approximately 2/5 bilaterally. Sensation was intact to light touch and pinprick, and proprioception was normal. Gait was not tested. A Foley catheter was in place.
This examination confirms ongoing encephalopathy and incomplete quadriplegia. The lower extremity weakness is nearly equal proximally and distally, which can be seen with an advanced peripheral neuropathy but is more characteristic of myelopathy. The expected concomitant sensory deficit of myelopathy is not present, although this may be difficult to detect in a confused patient. Reflex testing would help in distinguishing myelopathy (favored because of the imaging findings) from a rapid progressive peripheral motor neuropathy (eg, acute inflammatory demyelinating polyneuropathy or acute intermittent porphyria).
The pitting edema likely represents fluid overload, which can be nonspecific after prolonged immobility during hospitalization; hypoalbuminemia is oftentimes speculated to play a role when this develops. His alcohol use puts him at risk for heart failure (although there is no evidence of this on exam) and liver disease (which his liver function tests suggest). The tophi speak to the extent and chronicity of his hyperuricemia.
On arrival he reported recent onset diarrhea. Medications at transfer included metoprolol, omeprazole, prednisone, piperacillin/tazobactam, vancomycin, and colchicine; acetaminophen, bisacodyl, diphenhydramine, fentanyl, subcutaneous insulin, and labetalol were administered as needed. Laboratory studies included a hemoglobin of 9.5 g/dL, WBC count of 7,300/mm3 with 95% neutrophils, platelets 301,000/mm3, sodium 151 mmol/L, potassium 2.9 mmol/L, blood urea nitrogen 76 mg/dL, creatinine 2.0 mg/dL, aspartate aminotransferase 171 U/L, and alanine aminotransferase 127 U/L. Serum albumin was 1.7 g/dL.
At least 3 of his medicationsdiphenhydramine, fentanyl, and prednisonemay be contributing to his ongoing altered mental status, which may be further compounded by hypernatremia. Although his liver disease remains uncharacterized, hepatic encephalopathy may be contributing to his confusion as well.
Colchicine is likely responsible for his diarrhea, which would be the most readily available explanation for his hypernatremia, hypokalemia, and acute kidney injury (AKI). Acute kidney injury could result from progressive liver disease (hepatorenal syndrome), decreased arterial perfusion (suggested by third spacing or his diarrhea), acute tubular necrosis (from infection or medication), or urinary retention secondary to catheter obstruction. Acute hyperuricemia can also cause AKI (urate nephropathy).
Anemia has progressed and requires evaluation for blood loss as well as hemolysis. Hepatotoxicity from any of his medications (eg, acetaminophen) must be considered. Coagulation studies and review of the previous abdominal computed tomography would help determine the extent of his liver disease.
Neurosurgical consultation was obtained and the patient and his family elected to proceed with a thoracic laminectomy. Cheesy fluid was identified at the facet joints at T6‐T7, which was found to contain rare deposits of monosodium urate crystals. Surgical specimen cultures were sterile. His mental status and strength slowly improved to baseline following the surgery. He was discharged on postoperative day 7 to a rehabilitation facility. On the telephone follow‐up he reported that he has regained his strength completely.
The fluid analysis and clinical course confirms spinal gout. The presenting encephalopathy remains unexplained; I am unaware of gout leading to altered mental status.
COMMENTARY
Gout is an inflammatory condition triggered by the deposition of monosodium urate crystals in tissues in association with hyperuricemia.[1] Based on the 20072008 National Health and Nutrition Examination Survey, the prevalence of gout among US adults was 3.9% (8.3 million individuals).[2] These rates are increasing and are thought to be spurred by the aging population, increasing rates of obesity, and changing dietary habits including increases in the consumption of soft drinks and red meat.[3, 4, 5] The development of gout during hospitalization can prolong length of stay, and the implementation of a management protocol appears to help decrease treatment delays and the inappropriate discontinuation of gout prophylaxis.[6, 7] Surgery, with its associated physiologic stressors, can trigger gout, which is often polyarticular and presents with fever leading to testing and consultations for the febrile episode.[8]
Gout is an ancient disease that is familiar to most clinicians. In 1666, Daniel Sennert, a German physician, described gout as the physician's shame because of its infrequent recognition.[9] Clinical gout spans 3 stages: asymptomatic hyperuricemia, acute and intercritical gout, and chronic gouty arthritis. The typical acute presentation is monoarticular with the abrupt onset of pain, swelling, warmth, and erythema in a peripheral joint. It manifests most characteristically in the first metatarsophalangeal joint (podagra), but also frequently involves the midfoot, ankle, knee, and wrist and sometimes affects multiple joints simultaneously (polyarticular gout).[1, 10] The visualization of monosodium urate crystals either in synovial fluid or from a tophus is diagnostic of gout; however, guidelines recognize that a classic presentation of gout may be diagnosed based on clinical criteria alone.[11] Dual energy computerized tomography and ultrasonography are emerging as techniques for the visualization of monosodium urate crystals; however, they are not currently routinely recommended.[12]
There are many unusual presentations of gout, with an increase in such reports paralleling both the overall increase in the prevalence of gout and improvements in available imaging techniques.[13] Atypical presentations present diagnostic challenges and are often caused by tophaceous deposits in unusual locations. Reports of atypical gout have described entrapment neuropathies (eg, gouty deposits inducing carpal tunnel syndrome), ocular gout manifested as conjunctival deposits and uveitis, pancreatic gout presenting as a mass, and dermatologic manifestations including panniculitis.[13, 14]
Spinal gout (also known as axial gout) manifests when crystal‐induced inflammation, erosive arthritis, and tophaceous deposits occur along the spinal column. A cross‐sectional study of patients with poorly controlled gout reported the prevalence of spinal gout diagnosed by computerized tomography to be 35%. These radiographic findings were not consistently correlated with back pain.[15] Imaging features that are suggestive of spinal gout include intra‐articular and juxta‐articular erosions with sclerotic margins and density greater than the surrounding muscle. Periosteal new bone formation adjacent to bony destruction can form overhanging edges.[16] When retrospectively presented with the final diagnosis, the radiologist at our institution noted that the appearance was typical gout in an atypical location.
Spinal gout can be confused with spinal metastasis, infection, and stenosis. It can remain asymptomatic or present with back pain, radiculopathy, or cord compression. The lumbar spine is the most frequently affected site.[17, 18] Many patients with spinal gout have had chronic tophaceous gout with radiologic evidence of erosions in the peripheral joints.[15] Patients with spinal gout also have elevated urate levels and markers of inflammation.[18] Surgical decompression and stabilization is recommended when there is frank cord compression, progressive neurologic compromise, or lack of improvement with gout therapy alone.[18]
This patient's male gender, history of gout, hypertension, alcohol consumption, and thiazide diuretic use placed him at an increased risk of a gout attack.[19, 20] The possible interruption of urate‐lowering therapy for the surgical procedure and surgery itself further heightened his risk of suffering acute gouty arthritis in the perioperative period.[21] The patient's encephalopathy may have masked back pain and precluded an accurate neurologic exam. There is one case report to our knowledge describing encephalopathy that improved with colchicine and was possibly related to gout.[22] This patient's encephalopathy was deemed multifactorial and attributed to alcohol withdrawal, medications (including opioids and steroids), and infection (pneumonia).
Gout is best known for its peripheral arthritis and is rarely invoked in the consideration of spinal and myelopathic processes where more pressing competing diagnoses, such as infection and malignancy, are typically considered. In addition, when surgical specimens are submitted for examination for pathology in formaldehyde (rather than alcohol), monosodium urate crystals are dissolved and are thus difficult to identify in the specimen.
This case reminds us that gout remains a diagnostic challenge and should be considered in the differential of an inflammatory process. Recognition of the multifaceted nature of gout can allow for the earlier recognition and treatment of the less typical presentations of this ancient malady.
KEY TEACHING POINTS
- Crystalline disease is a common cause of postoperative arthritis.
- Gout (and pseudogout) should be considered in cases of focal inflammation (detected by examination or imaging) when the evidence or predisposition for infection is limited or nonexistent.
- Spinal gout presents with back pain, radiculopathy, or cord compression and may be confused with spinal metastasis, infection, and stenosis.
Acknowledgements
The authors thank Dr. Kari Waddell and Elaine Bammerlin for their assistance in the preparation of this manuscript.
Disclosure: Nothing to report.
- , . Clinical features and treatment of gout. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, eds. Kelley's Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013:1544–1575.
- , , . Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141.
- , , , . Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587.
- , , , , . Purine‐rich foods, dairy and protein intake, and the risk of gout in men. New Engl J Med. 2004;350(11):1093–1103.
- , , . Fructose‐rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–2278.
- , . Healthcare burden of in‐hospital gout. Intern Med J. 2012;42(11):1261–1263.
- , , , , , . Improved management of acute gout during hospitalization following introduction of a protocol. Int J Rheum Dis. 2012;15(6):512–520.
- , , . Postsurgical gout. Am Surg. 1995;61(1):56–59.
- , . Evolution of modern medicine. Arch Intern Med. 1960;105(4):640–644.
- . Clinical practice. Gout. N Engl J Med. 2011;364(5):443–452.
- . Management of gout: a 57‐year‐old man with a history of podagra, hyperuricemia, and mild renal insufficiency. JAMA. 2012;308(20):2133–2141.
- , , , et al. Diagnostic imaging of gout: comparison of high‐resolution US versus conventional X‐ray. Eur Radiol. 2008;18(3):621–630.
- , . The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42(2):146–154.
- , . Unusual clinical presentations of gout. Curr Opin Rheumatol. 2010;22(2):181–187.
- , , , , , . Correlates of axial gout: a cross‐sectional study. J Rheumatol. 2012;39(7):1445–1449.
- , , , , , . Axial gouty arthropathy. Am J Med Sci. 2009;338(2):140–146.
- , , . Axial (spinal) gout. Curr Rheumatol Rep. 2012;14(2):161–164.
- , , , . Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. 2007;67(1):65–73.
- , , , et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800.e11–800.e16.
- , , , , , . Recent diuretic use and the risk of recurrent gout attacks: the online case‐crossover gout study. J Rheumatol. 2006;33(7):1341–1345.
- , , , , . Clinical features and risk factors of postsurgical gout. Ann Rheum Dis. 2008;67(9):1271–1275.
- , , , . Gouty encephalopathy: myth or reality [in French]? Rev Med Interne. 1997;18(6):474–476.
- , . Clinical features and treatment of gout. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, eds. Kelley's Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013:1544–1575.
- , , . Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141.
- , , , . Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587.
- , , , , . Purine‐rich foods, dairy and protein intake, and the risk of gout in men. New Engl J Med. 2004;350(11):1093–1103.
- , , . Fructose‐rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–2278.
- , . Healthcare burden of in‐hospital gout. Intern Med J. 2012;42(11):1261–1263.
- , , , , , . Improved management of acute gout during hospitalization following introduction of a protocol. Int J Rheum Dis. 2012;15(6):512–520.
- , , . Postsurgical gout. Am Surg. 1995;61(1):56–59.
- , . Evolution of modern medicine. Arch Intern Med. 1960;105(4):640–644.
- . Clinical practice. Gout. N Engl J Med. 2011;364(5):443–452.
- . Management of gout: a 57‐year‐old man with a history of podagra, hyperuricemia, and mild renal insufficiency. JAMA. 2012;308(20):2133–2141.
- , , , et al. Diagnostic imaging of gout: comparison of high‐resolution US versus conventional X‐ray. Eur Radiol. 2008;18(3):621–630.
- , . The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42(2):146–154.
- , . Unusual clinical presentations of gout. Curr Opin Rheumatol. 2010;22(2):181–187.
- , , , , , . Correlates of axial gout: a cross‐sectional study. J Rheumatol. 2012;39(7):1445–1449.
- , , , , , . Axial gouty arthropathy. Am J Med Sci. 2009;338(2):140–146.
- , , . Axial (spinal) gout. Curr Rheumatol Rep. 2012;14(2):161–164.
- , , , . Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. 2007;67(1):65–73.
- , , , et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800.e11–800.e16.
- , , , , , . Recent diuretic use and the risk of recurrent gout attacks: the online case‐crossover gout study. J Rheumatol. 2006;33(7):1341–1345.
- , , , , . Clinical features and risk factors of postsurgical gout. Ann Rheum Dis. 2008;67(9):1271–1275.
- , , , . Gouty encephalopathy: myth or reality [in French]? Rev Med Interne. 1997;18(6):474–476.