User login
Association of stress biomarkers with 30-day unplanned readmission and death
It has been theorized that the physiologic stress that hospitalized patients experience accounts for their transient vulnerability after discharge, or posthospital syndrome.1 Their acute illness and life-habit changes during hospitalization result in continued impairment of physiologic systems after discharge, and this impairment might leave them more susceptible to new health threats.1 However, the theory that the stress experienced after a hospitalization might be associated with readmission has never been investigated.
Four biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis may help quantify posthospitalization stress: (1) midregional pro-adrenomedullin (ADM), a precursor reflecting adrenomedullin activity2; (2) copeptin (the C-terminal part of prepro-vasopressin, produced by the hypothalamus in response to stress3,4), the level of which closely correlates to the vasopressin level but is more stable and lacks circadian rhythm fluctuations5-7; (3) cortisol, released by the adrenal cortex in response to stress; and (4) prolactin, an indicator of HPA axis activity. These 4 stress biomarkers have been related to the severity, complications, or mortality of several diseases.3,5,8-17 Besides explaining the hypothetical association between posthospitalization stress and readmission and death, these biomarkers might be valuable in predicting which patients are at higher risk for readmission. Indeed, many prediction models have been developed to identify those patients, but most of these models underperform, target only very specific populations, or have not been externally validated.18
We hypothesized that the hospitalization stress measured by biomarkers is associated with readmission or death after discharge. In a prospective cohort study, we evaluated the association between 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) and 30-day unplanned readmissions and deaths after an acute-care medical hospitalization, and assessed their additive value to validated readmission prediction scores.
METHODS
Study Design and Population
Our prospective cohort study included all consecutive patients aged ≥50 years and admitted to the department of general internal medicine at Fribourg Cantonal Hospital in Switzerland between April 8, 2013 and September 23, 2013. Exclusion criteria were discharge on day of admission; death before discharge; discharge to another division, another acute-care hospital, a rehabilitation clinic, or a palliative-care clinic; and refusal or inability to give informed consent. In this hypothesis-generating observational study, we collected data on a convenience sample of patients and did not calculate sample size before data collection. The study was approved by the local ethics committee, and all patients gave informed consent.
Outcomes
The primary outcome was the composite of first unplanned readmission (to any division of any acute-care hospital) or death within 30 days after discharge from index admission. We also included deaths that occurred after discharge, hypothesizing that patients who died may have been readmitted had they lived. The secondary outcome was the same as the primary, but the period was 90 days. Planned readmission was defined as scheduled hospitalization for nonemergent treatment (eg, chemotherapy) or investigation (eg, elective coronarography). All patients were called 6 months after discharge, and readmissions and deaths recorded. If a patient could not be reached directly, we called his or her next of kin, primary care physician, or nursing home, depending on availability. Furthermore, we checked electronic health records for any readmission or death recorded within the Fribourg hospital network, which includes all 3 acute-care hospitals (Fribourg, Riaz, Tavel) in the same canton (state).
Independent Variables
Stress biomarkers. We measured serum levels of 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) at 8 am on an empty stomach on both day of admission and day of discharge. For a patient whose discharge decision was made after 8 hours for the same day, a blood sample was collected as soon as discharge was planned.
Clinical data. Collected data included demographics, history of hospitalization within 6 months before index admission, hospitalization diagnosis, and Charlson Comorbidity Index (CCI), which includes a list of medical conditions that are assigned a number of 1, 2, 3, or 6 points, according to their severity, and which has been associated with mortality.19
Causes of Admission, Unplanned Readmission, and Death
Causes of index admission, unplanned readmission, and death were obtained from medical records. We used our consensus opinion and a previous analysis20 to classify these causes by body system, and added 2 categories, cancer and infection (both associated with readmission20). The resulting 9 categories were (1) cancer, (2) respiratory disorder, (3) infectious disorder, (4) neurologic disorder (including dementia, psychiatric disorder, alcohol disorder, and intoxication), (5) gastrointestinal disorder, (6) osteoarticular disorder, (7) renal disorder, (8) cardiovascular disorder (including ischemic disease and heart failure), and (9) other.
Additional Performance With Existing Predictive Models
To better define the explanatory power of biomarkers to predict our outcome, we assessed the performance improvement of 2 validated readmission prediction scores by adding the stress biomarkers. As large effect sizes from additional predictors are needed to increase the power discrimination of a model, a significant performance improvement would further support the biomarkers’ important explanatory power. The 2 prediction scores tested were the LACE index (Length of stay, Admission Acuity, CCI, number of Emergency department visits within preceding 6 months21) and the HOSPITAL score (Hemoglobin level at discharge, discharge from Oncology service, Sodium level at discharge, any Procedure performed during index hospitalization, Index admission Type, number of Admissions within preceding 12 months, Length of stay). As we did not have an oncology service, we replaced “discharge from oncology service” with “active diagnosis of cancer.” “Length of stay” was tailored to the median in Switzerland (8 days instead of 5 days; Supplement Table 1).22,23
Data Analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) because of their non-normal distribution, and categorical variables were presented as frequencies and percentages. We compared medians using the nonparametric K-sample test on the equality of medians, and compared frequencies using the Pearson χ2 test. The discriminatory power of each biomarker in predicting readmission and death was calculated with the area under the receiver operating characteristic (ROC) curve (AUROC), using serum levels at discharge to better reflect the postdischarge period. Cutoff levels were selected by taking the best compromise between sensitivity and specificity according to the ROC curves (point nearest top left corner).24
Univariate logistic regression analysis was used to test the prediction of 30-day and 90-day unplanned readmission or death by each biomarker. We built 2 different multivariate models: one adjusting for age and LACE index points21 and the other adjusting for age and HOSPITAL score.22,23
To explore any association between reduction of stress during hospitalization and postdischarge outcome, we additionally calculated for each biomarker the difference between admission and discharge serum levels and assessed its association with readmission or death by logistic regression analysis. Because of the modification of cortisol serum levels during corticosteroid therapy, we excluded patients who underwent systemic corticosteroid therapy before or during hospitalization for the cortisol analysis (n = 105/346). Patients with a missing biomarker level were excluded from the respective analyses: discharge (ADM, 28 patients; copeptin, 27; cortisol, 24; prolactin, 24) and admission (ADM, 12 patients; copeptin, 15; cortisol, 8; prolactin, 8).
To assess an additional value of the biomarkers to prediction scores, we assessed the accuracy of the HOSPITAL score and LACE index in their original versions21,22 and after adding each biomarker. We used AUROC to assess the discriminatory power and used the method of DeLong et al.25 to compare results with and without adding each biomarker. Calibration was evaluated by comparing Hosmer-Lemeshow goodness-of-fit tests (P > 0.05 indicates good fit). Risk reclassification was assessed by Net Reclassification Improvement (NRI),26 quantifying how appropriately a new model reclassifies patients, compared with an old model. Basically, patients without outcome are assigned +1 if correctly reclassified to a lower risk category or –1 if incorrectly reclassified to a higher risk category. NRInonevent is the sum of all points/numbers of patients. Conversely, patients with outcome are assigned +1 if correctly reclassified to a higher risk category or –1 if incorrectly reclassified to a lower risk category. NRIevent is the sum of all points/numbers of patients. NRIoverall is the sum of NRIevent and NRInonevent ranging from –2 to 2, with a positive value indicating better classification with the new model.
Two-sided P < 0.05 was used for statistical significance. All statistical analyses were performed with Stata Release 13.0 (StataCorp).
RESULTS
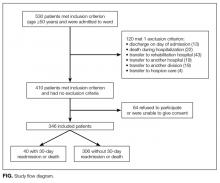
Among the 530 patients admitted to the ward, 184 were excluded (120 meeting exclusion criteria, 64 unable to give consent, Figure). Among the 346 patients included, 11.6% (n = 40) had a 30-day unplanned readmission or death (37 were readmitted, 2 died during readmission, 3 died without readmission). Within 90 days, 26.6% (n = 92) had a readmission or death (84 were readmitted, 10 died during or after readmission, 8 died without readmission).
Clinical Characteristics
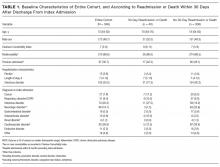
Table 1 lists the patients’ baseline characteristics. Median age was 73 years (IQR, 64-82 years). Of the 346 patients included, 172 (49.7%) were men. Median CCI was 7 (IQR, 5-9); according to this index, 310 patients (89.6%) had at least 2 comorbidities. Median length of stay was 7 days (IQR, 4-12 days).
Primary Diagnoses of Admission, Unplanned Readmission, and Death
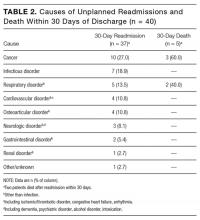
The 3 main causes of index admission were cardiovascular disorder (n = 92), infectious disorder (n = 70), and neurologic disorder (n = 66). Table 2 lists the causes of readmissions and deaths. A same-diagnosis category between index admission and readmission was found in 17 (45.9%) of the 37 readmitted patients and in 3 (60%) of the 5 patients who died.
Biomarkers and 30-Day Unplanned Readmission or Death
AUROC was 0.53 (95% confidence interval [CI], 0.43-0.63) for ADM, 0.60 (95% CI, 0.50-0.70) for copeptin, 0.59 (95% CI, 0.44-0.73) for cortisol, and 0.56 (95% CI, 0.45-0.66) for prolactin. The difference between admission and discharge levels was not associated with unplanned readmission or death for any of the biomarkers (Supplemental Table 2).
ADM and readmission or death. Median ADM level was not different between patients with and without readmission or death (1.0 nmol/L in each case; P = 1.00). The best cutoff level for ADM was 2 nmol/L (sensitivity, 16.7%; specificity, 91.8%). At this level, ADM was associated with a nonstatistically significant 130% increased odds of 30-day readmission or death (P = 0.09; Table 3, Supplemental Table 3). Conversely, the association with the 90-day outcome was significant (P = 0.02; Table 3, Supplemental Table 4).
Copeptin and readmission or death. Patients with 30-day readmission or death had a higher median copeptin level at discharge than patients without (10.4 pmol/L vs 7.3 pmol/L; P = 0.03). At a copeptin level higher than 9 pmol/L (to convert to pg/mL, divide by 0.249; sensitivity, 66.7%; specificity, 59.7%), both 30-day readmission or death (adjusted odds ratio [OR], 2.69; 95% CI, 1.29-5.64; P = 0.009) and 90-day readmission or death (adjusted OR, 2.76; 95% CI, 1.56-4.88; P < 0.001) were nearly 3 times as likely (Table 3, Supplemental Tables 3 and 4).
Cortisol and readmission or death. Median cortisol was not statistically different between patients with and without the primary outcome (431 nmol/L vs 465 nmol/L; P = 0.72). At a cortisol level higher than 590 nmol/L (to convert to μg/dL, divide by 27.59; sensitivity, 54.6%; specificity, 76.4%), 30-day outcome was more than 3 times as likely (adjusted OR, 3.43; 95% CI, 1.36-8.65; P = 0.009; Table 3, Supplemental Table 3). At 90 days, only the model that adjusted for age and LACE index points remained statistically significant (P = 0.02; Table 3, Supplemental Table 4).
Prolactin and readmission or death. Median prolactin was not statistically different between patients with and without the primary outcome (15.1 μg/L vs 14.1 μg/L; P = 0.24). The best cutoff level for prolactin was 23 μg/L (to convert to mIU/L, divide by 0.05; sensitivity, 27.8%; specificity, 82.9%). Prolactin was associated with a nonstatistically significant increased odds of 30-day (P = 0.16) and 90-day (P = 0.24) readmission or death (Table 3, Supplemental Tables 3 and 4).
Additive Value of Biomarkers to HOSPITAL Score and LACE Index
The AUROC for the original HOSPITAL score, 0.70 (95% CI, 0.60-0.80), nonsignificantly increased to 0.76 after adding the biomarkers (P > 0.14). For the LACE index, AUROC was 0.59 (95% CI, 0.49-0.68), with a significant 0.10 increase with cortisol (P = 0.04) and a near significant increase with copeptin (P = 0.08). Calibration remained almost unchanged after adding the biomarkers to both models (Supplemental Table 5). NRIoverall was positive for all biomarkers, with statistical significance for copeptin added to the HOSPITAL score (0.47; 95% CI, 0.13-0.79) and for cortisol added to the LACE index (0.62; 95% CI, 0.15-1.06).
DISCUSSION
In this prospective cohort study, 30-day and 90-day unplanned readmission or death was nearly 3 times as likely for patients with high copeptin levels on discharge from an acute-care medical hospitalization, and 30-day readmission or death was more than 3 times as likely for patients with high cortisol levels. High ADM and prolactin levels were not consistently associated with readmission or death. Adding such biomarkers to readmission prediction models improved their performance.
These findings support the theory of posthospital syndrome,1 which describes a period of vulnerability with increased stress after discharge from an acute-care hospitalization, and which may be associated with adverse outcome. The hormones cortisol and copeptin are strongly related to the stress response in humans.4,5 As copeptin level has been associated with adverse prognosis for several disorders affecting a wide range of physiologic systems,3,5,15,27 it may be a valuable biomarker of a stressful condition, even independent of the system affected by the acute illness, and its use may be widely generalizable, in contrast to predictive factors identified in other studies.18,28,29
Although cortisol was independently associated with 30-day readmission or death, and may be an interesting biomarker and less expensive than copeptin, its measurement is limited in patients treated with systemic corticosteroids. Compared with cortisol, copeptin does not undergo diurnal variation, is less affected by corticosteroid therapy, and mirrors stress levels better.5,7,30,31 Our results showed that, contrary to cortisol, copeptin was also associated with longer term outcome. High ADM level was associated with readmission or death at 90 days only; lack of a significant association at 30 days might be attributable to a lack of power (fewer outcomes at 30 days). Conversely, prolactin level was consistently not associated with outcome. Prolactin may be affected by many drugs that act on the dopaminergic system (eg, domperidone), and therefore its levels may be more difficult to interpret.
Levels of biomarkers were similar to those measured in patients without previously studied conditions (eg, myocardial infarction).5,8,10,13,14,16,17 In most previous analyses, levels were measured during a stressful event, whereas we measured them at discharge. Therefore, these biomarkers may constitute sensitive markers of remaining stress at discharge.
Our finding that copeptin level was independently associated with readmission or death supports its relevance as a possible simple measure of the risk of adverse postdischarge outcome, independently of disease type and independently of known predictors. Stress biomarkers may therefore be valuable predictors of which patients are at high risk. All these biomarkers can be measured within 30 minutes, extending their use beyond everyday practice, except for the possible need of an extra blood draw.
The most accurate and validated models are the HOSPITAL score (AUROC range, 0.68-0.7723,32-36) and the LACE index (AUROC range, 0.56-0.6823,34,35,37). Adding biomarkers to these models improved overall performance (up to 0.10 increase in AUROC), which is remarkable given that, once a particular level of discriminatory power is reached, extremely large effect sizes from additional markers are needed to increase AUROC.26 Incremental improvement is objectively supported by positive NRI. Our results suggest biomarkers added to prediction models may improve identification of high-risk patients.
We found that less than 50% of the primary diagnoses belonged to the same diagnosis category at readmission and at index admission. This result is in line with previous findings that readmissions were related to the primary diagnosis at index admission in only 22% to 46% of cases,20,38 and supports our study hypothesis that readmission is related to underlying stress factors often independent of the underlying illness.1
Study Limitations and Strengths
Our study had some limitations. First, it was a single-center study with a limited sample size. However, we found significant results within the sample. Second, we could not adjust for drugs that were acting on the dopaminergic system and might have affected prolactin levels. However, such interactions would limit the use of this biomarker in clinical practice anyway. Third, we used specific cutoffs, which might decrease analytical power, in comparison with continuous analyses. However, we followed a recognized method24 and found a significant association even with categorized levels. Furthermore, the distribution of biomarkers could not be normalized by logarithmic transformation, and cutoff values have the advantage of being integrable into score point systems (eg, HOSPITAL score, LACE index). Fourth, although in 2 models we found consistent associations with several potential confounders, we could not exclude residual confounding. Fifth, this study was not powered to assess the biomarkers’ predictive value for readmission and death, which might explain the lack of significant differences between AUROC with and without the biomarkers. For all these reasons, this study should be considered hypothesis-generating.
Our study also had its strengths. First, to our knowledge, this is the first study of the association between stress biomarkers at discharge and unplanned readmission or death. Second, the quality of our data was high, with a low percentage of missing biomarker levels. Third, we excluded planned readmissions. Fourth, we used an unselected medical patient population, which had the noteworthy advantage of widening the generalizability of results.
CONCLUSION
In this prospective cohort study, high copeptin and cortisol levels at discharge were significantly associated with increased odds, ranging from 2-fold to more than 3-fold, of unplanned readmission or death within 30 days after discharge from an internal medicine ward. This finding supports the theory that a physiologic stress that patients experience during hospitalization makes them more susceptible to new health threats (posthospital syndrome). These biomarkers, copeptin in particular, may help us better identify patients at high risk of early unplanned readmission or death.
Acknowledgment
Biomarker measurement was funded by the research fund of the Department of General Internal Medicine, Fribourg Cantonal Hospital, Fribourg, Switzerland.
Disclosure
Nothing to report.
1. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
2. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823-1829. PubMed
3. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172-190. PubMed
4. Yilman M, Erenler AK, Baydin A. Copeptin: a diagnostic factor for critical patients. Eur Rev Med Pharmacol Sci. 2015;19(16):3030-3036. PubMed
5. Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. PubMed
6. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-2504. PubMed
7. Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56(7):1190-1191. PubMed
8. Labad J, Stojanovic-Pérez A, Montalvo I, et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: roles for cortisol, prolactin and albumin. J Psychiatr Res. 2015;60:163-169. PubMed
9. Olsson T, Asplund K, Hagg E. Pituitary-thyroid axis, prolactin and growth hormone in patients with acute stroke. J Intern Med. 1990;228(3):287-290. PubMed
10. Parissis JT, Farmakis D, Fountoulaki K, et al. Clinical and neurohormonal correlates and prognostic value of serum prolactin levels in patients with chronic heart failure. Eur J Heart Fail. 2013;15(10):1122-1130. PubMed
11. Theodoropoulou A, Metallinos IC, Elloul J, et al. Prolactin, cortisol secretion and thyroid function in patients with stroke of mild severity. Horm Metab Res. 2006;38(9):587-591. PubMed
12. Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. Biomed Res Int. 2014;2014:803561. PubMed
13. Bahrmann P, Christ M, Hofner B, et al. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 2016;5(8):568-578. PubMed
14. Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96. PubMed
15. Artunc F, Nowak A, Mueller C, et al. Plasma concentrations of the vasoactive peptide fragments mid-regional pro-adrenomedullin, C-terminal pro-endothelin 1 and copeptin in hemodialysis patients: associated factors and prediction of mortality. PLoS One. 2014;9(1):e86148. PubMed
16. Rotman-Pikielny P, Roash V, Chen O, Limor R, Stern N, Gur HG. Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci. 2006;332(2):61-67. PubMed
17. Yamaji M, Tsutamoto T, Kawahara C, et al. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2(6):608-615. PubMed
18. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed
20. Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. PubMed
21. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
22. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
23. Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé J. Prospective validation and adaptation of the HOSPITAL score to predict high risk of unplanned readmission of medical patients. Swiss Med Wkly. 2016;146:w14335. PubMed
24. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-577. PubMed
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. PubMed
26. Pencina MJ, D’Agostino RB Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31(2):101-113. PubMed
27. Folli C, Consonni D, Spessot M, et al. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur J Intern Med. 2013;24(2):189-193. PubMed
28. Aujesky D, Mor MK, Geng M, Stone RA, Fine MJ, Ibrahim SA. Predictors of early hospital readmission after acute pulmonary embolism. Arch Intern Med. 2009;169(3):287-293. PubMed
29. Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4(1):60-67. PubMed
30. Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012;10:7. PubMed
31. Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341-346. PubMed
32. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502.PubMed
33. Burke RE, Schnipper JL, Williams MV, et al. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the Hospital Readmissions Reduction Program. Med Care. 2017;55(3):285-290. PubMed
34. Garrison GM, Robelia PM, Pecina JL, Dawson NL. Comparing performance of 30-day readmission risk classifiers among hospitalized primary care patients. J Eval Clin Pract. 2017;23(3):524-529. PubMed
35. Cooksley T, Nanayakkara PW, Nickel CH, et al. Readmissions of medical patients: an external validation of two existing prediction scores. QJM. 2016;109(4):245-248. PubMed
36. Robinson R. The HOSPITAL score as a predictor of 30 day readmission in a retrospective study at a university affiliated community hospital. PeerJ. 2016;4:e2441. PubMed
37. Wang H, Robinson RD, Johnson C, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. 2014;14:97. PubMed
38. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18. PubMed
It has been theorized that the physiologic stress that hospitalized patients experience accounts for their transient vulnerability after discharge, or posthospital syndrome.1 Their acute illness and life-habit changes during hospitalization result in continued impairment of physiologic systems after discharge, and this impairment might leave them more susceptible to new health threats.1 However, the theory that the stress experienced after a hospitalization might be associated with readmission has never been investigated.
Four biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis may help quantify posthospitalization stress: (1) midregional pro-adrenomedullin (ADM), a precursor reflecting adrenomedullin activity2; (2) copeptin (the C-terminal part of prepro-vasopressin, produced by the hypothalamus in response to stress3,4), the level of which closely correlates to the vasopressin level but is more stable and lacks circadian rhythm fluctuations5-7; (3) cortisol, released by the adrenal cortex in response to stress; and (4) prolactin, an indicator of HPA axis activity. These 4 stress biomarkers have been related to the severity, complications, or mortality of several diseases.3,5,8-17 Besides explaining the hypothetical association between posthospitalization stress and readmission and death, these biomarkers might be valuable in predicting which patients are at higher risk for readmission. Indeed, many prediction models have been developed to identify those patients, but most of these models underperform, target only very specific populations, or have not been externally validated.18
We hypothesized that the hospitalization stress measured by biomarkers is associated with readmission or death after discharge. In a prospective cohort study, we evaluated the association between 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) and 30-day unplanned readmissions and deaths after an acute-care medical hospitalization, and assessed their additive value to validated readmission prediction scores.
METHODS
Study Design and Population
Our prospective cohort study included all consecutive patients aged ≥50 years and admitted to the department of general internal medicine at Fribourg Cantonal Hospital in Switzerland between April 8, 2013 and September 23, 2013. Exclusion criteria were discharge on day of admission; death before discharge; discharge to another division, another acute-care hospital, a rehabilitation clinic, or a palliative-care clinic; and refusal or inability to give informed consent. In this hypothesis-generating observational study, we collected data on a convenience sample of patients and did not calculate sample size before data collection. The study was approved by the local ethics committee, and all patients gave informed consent.
Outcomes
The primary outcome was the composite of first unplanned readmission (to any division of any acute-care hospital) or death within 30 days after discharge from index admission. We also included deaths that occurred after discharge, hypothesizing that patients who died may have been readmitted had they lived. The secondary outcome was the same as the primary, but the period was 90 days. Planned readmission was defined as scheduled hospitalization for nonemergent treatment (eg, chemotherapy) or investigation (eg, elective coronarography). All patients were called 6 months after discharge, and readmissions and deaths recorded. If a patient could not be reached directly, we called his or her next of kin, primary care physician, or nursing home, depending on availability. Furthermore, we checked electronic health records for any readmission or death recorded within the Fribourg hospital network, which includes all 3 acute-care hospitals (Fribourg, Riaz, Tavel) in the same canton (state).
Independent Variables
Stress biomarkers. We measured serum levels of 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) at 8 am on an empty stomach on both day of admission and day of discharge. For a patient whose discharge decision was made after 8 hours for the same day, a blood sample was collected as soon as discharge was planned.
Clinical data. Collected data included demographics, history of hospitalization within 6 months before index admission, hospitalization diagnosis, and Charlson Comorbidity Index (CCI), which includes a list of medical conditions that are assigned a number of 1, 2, 3, or 6 points, according to their severity, and which has been associated with mortality.19
Causes of Admission, Unplanned Readmission, and Death
Causes of index admission, unplanned readmission, and death were obtained from medical records. We used our consensus opinion and a previous analysis20 to classify these causes by body system, and added 2 categories, cancer and infection (both associated with readmission20). The resulting 9 categories were (1) cancer, (2) respiratory disorder, (3) infectious disorder, (4) neurologic disorder (including dementia, psychiatric disorder, alcohol disorder, and intoxication), (5) gastrointestinal disorder, (6) osteoarticular disorder, (7) renal disorder, (8) cardiovascular disorder (including ischemic disease and heart failure), and (9) other.
Additional Performance With Existing Predictive Models
To better define the explanatory power of biomarkers to predict our outcome, we assessed the performance improvement of 2 validated readmission prediction scores by adding the stress biomarkers. As large effect sizes from additional predictors are needed to increase the power discrimination of a model, a significant performance improvement would further support the biomarkers’ important explanatory power. The 2 prediction scores tested were the LACE index (Length of stay, Admission Acuity, CCI, number of Emergency department visits within preceding 6 months21) and the HOSPITAL score (Hemoglobin level at discharge, discharge from Oncology service, Sodium level at discharge, any Procedure performed during index hospitalization, Index admission Type, number of Admissions within preceding 12 months, Length of stay). As we did not have an oncology service, we replaced “discharge from oncology service” with “active diagnosis of cancer.” “Length of stay” was tailored to the median in Switzerland (8 days instead of 5 days; Supplement Table 1).22,23
Data Analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) because of their non-normal distribution, and categorical variables were presented as frequencies and percentages. We compared medians using the nonparametric K-sample test on the equality of medians, and compared frequencies using the Pearson χ2 test. The discriminatory power of each biomarker in predicting readmission and death was calculated with the area under the receiver operating characteristic (ROC) curve (AUROC), using serum levels at discharge to better reflect the postdischarge period. Cutoff levels were selected by taking the best compromise between sensitivity and specificity according to the ROC curves (point nearest top left corner).24
Univariate logistic regression analysis was used to test the prediction of 30-day and 90-day unplanned readmission or death by each biomarker. We built 2 different multivariate models: one adjusting for age and LACE index points21 and the other adjusting for age and HOSPITAL score.22,23
To explore any association between reduction of stress during hospitalization and postdischarge outcome, we additionally calculated for each biomarker the difference between admission and discharge serum levels and assessed its association with readmission or death by logistic regression analysis. Because of the modification of cortisol serum levels during corticosteroid therapy, we excluded patients who underwent systemic corticosteroid therapy before or during hospitalization for the cortisol analysis (n = 105/346). Patients with a missing biomarker level were excluded from the respective analyses: discharge (ADM, 28 patients; copeptin, 27; cortisol, 24; prolactin, 24) and admission (ADM, 12 patients; copeptin, 15; cortisol, 8; prolactin, 8).
To assess an additional value of the biomarkers to prediction scores, we assessed the accuracy of the HOSPITAL score and LACE index in their original versions21,22 and after adding each biomarker. We used AUROC to assess the discriminatory power and used the method of DeLong et al.25 to compare results with and without adding each biomarker. Calibration was evaluated by comparing Hosmer-Lemeshow goodness-of-fit tests (P > 0.05 indicates good fit). Risk reclassification was assessed by Net Reclassification Improvement (NRI),26 quantifying how appropriately a new model reclassifies patients, compared with an old model. Basically, patients without outcome are assigned +1 if correctly reclassified to a lower risk category or –1 if incorrectly reclassified to a higher risk category. NRInonevent is the sum of all points/numbers of patients. Conversely, patients with outcome are assigned +1 if correctly reclassified to a higher risk category or –1 if incorrectly reclassified to a lower risk category. NRIevent is the sum of all points/numbers of patients. NRIoverall is the sum of NRIevent and NRInonevent ranging from –2 to 2, with a positive value indicating better classification with the new model.
Two-sided P < 0.05 was used for statistical significance. All statistical analyses were performed with Stata Release 13.0 (StataCorp).
RESULTS
Among the 530 patients admitted to the ward, 184 were excluded (120 meeting exclusion criteria, 64 unable to give consent, Figure). Among the 346 patients included, 11.6% (n = 40) had a 30-day unplanned readmission or death (37 were readmitted, 2 died during readmission, 3 died without readmission). Within 90 days, 26.6% (n = 92) had a readmission or death (84 were readmitted, 10 died during or after readmission, 8 died without readmission).
Clinical Characteristics
Table 1 lists the patients’ baseline characteristics. Median age was 73 years (IQR, 64-82 years). Of the 346 patients included, 172 (49.7%) were men. Median CCI was 7 (IQR, 5-9); according to this index, 310 patients (89.6%) had at least 2 comorbidities. Median length of stay was 7 days (IQR, 4-12 days).
Primary Diagnoses of Admission, Unplanned Readmission, and Death
The 3 main causes of index admission were cardiovascular disorder (n = 92), infectious disorder (n = 70), and neurologic disorder (n = 66). Table 2 lists the causes of readmissions and deaths. A same-diagnosis category between index admission and readmission was found in 17 (45.9%) of the 37 readmitted patients and in 3 (60%) of the 5 patients who died.
Biomarkers and 30-Day Unplanned Readmission or Death
AUROC was 0.53 (95% confidence interval [CI], 0.43-0.63) for ADM, 0.60 (95% CI, 0.50-0.70) for copeptin, 0.59 (95% CI, 0.44-0.73) for cortisol, and 0.56 (95% CI, 0.45-0.66) for prolactin. The difference between admission and discharge levels was not associated with unplanned readmission or death for any of the biomarkers (Supplemental Table 2).
ADM and readmission or death. Median ADM level was not different between patients with and without readmission or death (1.0 nmol/L in each case; P = 1.00). The best cutoff level for ADM was 2 nmol/L (sensitivity, 16.7%; specificity, 91.8%). At this level, ADM was associated with a nonstatistically significant 130% increased odds of 30-day readmission or death (P = 0.09; Table 3, Supplemental Table 3). Conversely, the association with the 90-day outcome was significant (P = 0.02; Table 3, Supplemental Table 4).
Copeptin and readmission or death. Patients with 30-day readmission or death had a higher median copeptin level at discharge than patients without (10.4 pmol/L vs 7.3 pmol/L; P = 0.03). At a copeptin level higher than 9 pmol/L (to convert to pg/mL, divide by 0.249; sensitivity, 66.7%; specificity, 59.7%), both 30-day readmission or death (adjusted odds ratio [OR], 2.69; 95% CI, 1.29-5.64; P = 0.009) and 90-day readmission or death (adjusted OR, 2.76; 95% CI, 1.56-4.88; P < 0.001) were nearly 3 times as likely (Table 3, Supplemental Tables 3 and 4).
Cortisol and readmission or death. Median cortisol was not statistically different between patients with and without the primary outcome (431 nmol/L vs 465 nmol/L; P = 0.72). At a cortisol level higher than 590 nmol/L (to convert to μg/dL, divide by 27.59; sensitivity, 54.6%; specificity, 76.4%), 30-day outcome was more than 3 times as likely (adjusted OR, 3.43; 95% CI, 1.36-8.65; P = 0.009; Table 3, Supplemental Table 3). At 90 days, only the model that adjusted for age and LACE index points remained statistically significant (P = 0.02; Table 3, Supplemental Table 4).
Prolactin and readmission or death. Median prolactin was not statistically different between patients with and without the primary outcome (15.1 μg/L vs 14.1 μg/L; P = 0.24). The best cutoff level for prolactin was 23 μg/L (to convert to mIU/L, divide by 0.05; sensitivity, 27.8%; specificity, 82.9%). Prolactin was associated with a nonstatistically significant increased odds of 30-day (P = 0.16) and 90-day (P = 0.24) readmission or death (Table 3, Supplemental Tables 3 and 4).
Additive Value of Biomarkers to HOSPITAL Score and LACE Index
The AUROC for the original HOSPITAL score, 0.70 (95% CI, 0.60-0.80), nonsignificantly increased to 0.76 after adding the biomarkers (P > 0.14). For the LACE index, AUROC was 0.59 (95% CI, 0.49-0.68), with a significant 0.10 increase with cortisol (P = 0.04) and a near significant increase with copeptin (P = 0.08). Calibration remained almost unchanged after adding the biomarkers to both models (Supplemental Table 5). NRIoverall was positive for all biomarkers, with statistical significance for copeptin added to the HOSPITAL score (0.47; 95% CI, 0.13-0.79) and for cortisol added to the LACE index (0.62; 95% CI, 0.15-1.06).
DISCUSSION
In this prospective cohort study, 30-day and 90-day unplanned readmission or death was nearly 3 times as likely for patients with high copeptin levels on discharge from an acute-care medical hospitalization, and 30-day readmission or death was more than 3 times as likely for patients with high cortisol levels. High ADM and prolactin levels were not consistently associated with readmission or death. Adding such biomarkers to readmission prediction models improved their performance.
These findings support the theory of posthospital syndrome,1 which describes a period of vulnerability with increased stress after discharge from an acute-care hospitalization, and which may be associated with adverse outcome. The hormones cortisol and copeptin are strongly related to the stress response in humans.4,5 As copeptin level has been associated with adverse prognosis for several disorders affecting a wide range of physiologic systems,3,5,15,27 it may be a valuable biomarker of a stressful condition, even independent of the system affected by the acute illness, and its use may be widely generalizable, in contrast to predictive factors identified in other studies.18,28,29
Although cortisol was independently associated with 30-day readmission or death, and may be an interesting biomarker and less expensive than copeptin, its measurement is limited in patients treated with systemic corticosteroids. Compared with cortisol, copeptin does not undergo diurnal variation, is less affected by corticosteroid therapy, and mirrors stress levels better.5,7,30,31 Our results showed that, contrary to cortisol, copeptin was also associated with longer term outcome. High ADM level was associated with readmission or death at 90 days only; lack of a significant association at 30 days might be attributable to a lack of power (fewer outcomes at 30 days). Conversely, prolactin level was consistently not associated with outcome. Prolactin may be affected by many drugs that act on the dopaminergic system (eg, domperidone), and therefore its levels may be more difficult to interpret.
Levels of biomarkers were similar to those measured in patients without previously studied conditions (eg, myocardial infarction).5,8,10,13,14,16,17 In most previous analyses, levels were measured during a stressful event, whereas we measured them at discharge. Therefore, these biomarkers may constitute sensitive markers of remaining stress at discharge.
Our finding that copeptin level was independently associated with readmission or death supports its relevance as a possible simple measure of the risk of adverse postdischarge outcome, independently of disease type and independently of known predictors. Stress biomarkers may therefore be valuable predictors of which patients are at high risk. All these biomarkers can be measured within 30 minutes, extending their use beyond everyday practice, except for the possible need of an extra blood draw.
The most accurate and validated models are the HOSPITAL score (AUROC range, 0.68-0.7723,32-36) and the LACE index (AUROC range, 0.56-0.6823,34,35,37). Adding biomarkers to these models improved overall performance (up to 0.10 increase in AUROC), which is remarkable given that, once a particular level of discriminatory power is reached, extremely large effect sizes from additional markers are needed to increase AUROC.26 Incremental improvement is objectively supported by positive NRI. Our results suggest biomarkers added to prediction models may improve identification of high-risk patients.
We found that less than 50% of the primary diagnoses belonged to the same diagnosis category at readmission and at index admission. This result is in line with previous findings that readmissions were related to the primary diagnosis at index admission in only 22% to 46% of cases,20,38 and supports our study hypothesis that readmission is related to underlying stress factors often independent of the underlying illness.1
Study Limitations and Strengths
Our study had some limitations. First, it was a single-center study with a limited sample size. However, we found significant results within the sample. Second, we could not adjust for drugs that were acting on the dopaminergic system and might have affected prolactin levels. However, such interactions would limit the use of this biomarker in clinical practice anyway. Third, we used specific cutoffs, which might decrease analytical power, in comparison with continuous analyses. However, we followed a recognized method24 and found a significant association even with categorized levels. Furthermore, the distribution of biomarkers could not be normalized by logarithmic transformation, and cutoff values have the advantage of being integrable into score point systems (eg, HOSPITAL score, LACE index). Fourth, although in 2 models we found consistent associations with several potential confounders, we could not exclude residual confounding. Fifth, this study was not powered to assess the biomarkers’ predictive value for readmission and death, which might explain the lack of significant differences between AUROC with and without the biomarkers. For all these reasons, this study should be considered hypothesis-generating.
Our study also had its strengths. First, to our knowledge, this is the first study of the association between stress biomarkers at discharge and unplanned readmission or death. Second, the quality of our data was high, with a low percentage of missing biomarker levels. Third, we excluded planned readmissions. Fourth, we used an unselected medical patient population, which had the noteworthy advantage of widening the generalizability of results.
CONCLUSION
In this prospective cohort study, high copeptin and cortisol levels at discharge were significantly associated with increased odds, ranging from 2-fold to more than 3-fold, of unplanned readmission or death within 30 days after discharge from an internal medicine ward. This finding supports the theory that a physiologic stress that patients experience during hospitalization makes them more susceptible to new health threats (posthospital syndrome). These biomarkers, copeptin in particular, may help us better identify patients at high risk of early unplanned readmission or death.
Acknowledgment
Biomarker measurement was funded by the research fund of the Department of General Internal Medicine, Fribourg Cantonal Hospital, Fribourg, Switzerland.
Disclosure
Nothing to report.
It has been theorized that the physiologic stress that hospitalized patients experience accounts for their transient vulnerability after discharge, or posthospital syndrome.1 Their acute illness and life-habit changes during hospitalization result in continued impairment of physiologic systems after discharge, and this impairment might leave them more susceptible to new health threats.1 However, the theory that the stress experienced after a hospitalization might be associated with readmission has never been investigated.
Four biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis may help quantify posthospitalization stress: (1) midregional pro-adrenomedullin (ADM), a precursor reflecting adrenomedullin activity2; (2) copeptin (the C-terminal part of prepro-vasopressin, produced by the hypothalamus in response to stress3,4), the level of which closely correlates to the vasopressin level but is more stable and lacks circadian rhythm fluctuations5-7; (3) cortisol, released by the adrenal cortex in response to stress; and (4) prolactin, an indicator of HPA axis activity. These 4 stress biomarkers have been related to the severity, complications, or mortality of several diseases.3,5,8-17 Besides explaining the hypothetical association between posthospitalization stress and readmission and death, these biomarkers might be valuable in predicting which patients are at higher risk for readmission. Indeed, many prediction models have been developed to identify those patients, but most of these models underperform, target only very specific populations, or have not been externally validated.18
We hypothesized that the hospitalization stress measured by biomarkers is associated with readmission or death after discharge. In a prospective cohort study, we evaluated the association between 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) and 30-day unplanned readmissions and deaths after an acute-care medical hospitalization, and assessed their additive value to validated readmission prediction scores.
METHODS
Study Design and Population
Our prospective cohort study included all consecutive patients aged ≥50 years and admitted to the department of general internal medicine at Fribourg Cantonal Hospital in Switzerland between April 8, 2013 and September 23, 2013. Exclusion criteria were discharge on day of admission; death before discharge; discharge to another division, another acute-care hospital, a rehabilitation clinic, or a palliative-care clinic; and refusal or inability to give informed consent. In this hypothesis-generating observational study, we collected data on a convenience sample of patients and did not calculate sample size before data collection. The study was approved by the local ethics committee, and all patients gave informed consent.
Outcomes
The primary outcome was the composite of first unplanned readmission (to any division of any acute-care hospital) or death within 30 days after discharge from index admission. We also included deaths that occurred after discharge, hypothesizing that patients who died may have been readmitted had they lived. The secondary outcome was the same as the primary, but the period was 90 days. Planned readmission was defined as scheduled hospitalization for nonemergent treatment (eg, chemotherapy) or investigation (eg, elective coronarography). All patients were called 6 months after discharge, and readmissions and deaths recorded. If a patient could not be reached directly, we called his or her next of kin, primary care physician, or nursing home, depending on availability. Furthermore, we checked electronic health records for any readmission or death recorded within the Fribourg hospital network, which includes all 3 acute-care hospitals (Fribourg, Riaz, Tavel) in the same canton (state).
Independent Variables
Stress biomarkers. We measured serum levels of 4 stress biomarkers (ADM, copeptin, cortisol, prolactin) at 8 am on an empty stomach on both day of admission and day of discharge. For a patient whose discharge decision was made after 8 hours for the same day, a blood sample was collected as soon as discharge was planned.
Clinical data. Collected data included demographics, history of hospitalization within 6 months before index admission, hospitalization diagnosis, and Charlson Comorbidity Index (CCI), which includes a list of medical conditions that are assigned a number of 1, 2, 3, or 6 points, according to their severity, and which has been associated with mortality.19
Causes of Admission, Unplanned Readmission, and Death
Causes of index admission, unplanned readmission, and death were obtained from medical records. We used our consensus opinion and a previous analysis20 to classify these causes by body system, and added 2 categories, cancer and infection (both associated with readmission20). The resulting 9 categories were (1) cancer, (2) respiratory disorder, (3) infectious disorder, (4) neurologic disorder (including dementia, psychiatric disorder, alcohol disorder, and intoxication), (5) gastrointestinal disorder, (6) osteoarticular disorder, (7) renal disorder, (8) cardiovascular disorder (including ischemic disease and heart failure), and (9) other.
Additional Performance With Existing Predictive Models
To better define the explanatory power of biomarkers to predict our outcome, we assessed the performance improvement of 2 validated readmission prediction scores by adding the stress biomarkers. As large effect sizes from additional predictors are needed to increase the power discrimination of a model, a significant performance improvement would further support the biomarkers’ important explanatory power. The 2 prediction scores tested were the LACE index (Length of stay, Admission Acuity, CCI, number of Emergency department visits within preceding 6 months21) and the HOSPITAL score (Hemoglobin level at discharge, discharge from Oncology service, Sodium level at discharge, any Procedure performed during index hospitalization, Index admission Type, number of Admissions within preceding 12 months, Length of stay). As we did not have an oncology service, we replaced “discharge from oncology service” with “active diagnosis of cancer.” “Length of stay” was tailored to the median in Switzerland (8 days instead of 5 days; Supplement Table 1).22,23
Data Analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) because of their non-normal distribution, and categorical variables were presented as frequencies and percentages. We compared medians using the nonparametric K-sample test on the equality of medians, and compared frequencies using the Pearson χ2 test. The discriminatory power of each biomarker in predicting readmission and death was calculated with the area under the receiver operating characteristic (ROC) curve (AUROC), using serum levels at discharge to better reflect the postdischarge period. Cutoff levels were selected by taking the best compromise between sensitivity and specificity according to the ROC curves (point nearest top left corner).24
Univariate logistic regression analysis was used to test the prediction of 30-day and 90-day unplanned readmission or death by each biomarker. We built 2 different multivariate models: one adjusting for age and LACE index points21 and the other adjusting for age and HOSPITAL score.22,23
To explore any association between reduction of stress during hospitalization and postdischarge outcome, we additionally calculated for each biomarker the difference between admission and discharge serum levels and assessed its association with readmission or death by logistic regression analysis. Because of the modification of cortisol serum levels during corticosteroid therapy, we excluded patients who underwent systemic corticosteroid therapy before or during hospitalization for the cortisol analysis (n = 105/346). Patients with a missing biomarker level were excluded from the respective analyses: discharge (ADM, 28 patients; copeptin, 27; cortisol, 24; prolactin, 24) and admission (ADM, 12 patients; copeptin, 15; cortisol, 8; prolactin, 8).
To assess an additional value of the biomarkers to prediction scores, we assessed the accuracy of the HOSPITAL score and LACE index in their original versions21,22 and after adding each biomarker. We used AUROC to assess the discriminatory power and used the method of DeLong et al.25 to compare results with and without adding each biomarker. Calibration was evaluated by comparing Hosmer-Lemeshow goodness-of-fit tests (P > 0.05 indicates good fit). Risk reclassification was assessed by Net Reclassification Improvement (NRI),26 quantifying how appropriately a new model reclassifies patients, compared with an old model. Basically, patients without outcome are assigned +1 if correctly reclassified to a lower risk category or –1 if incorrectly reclassified to a higher risk category. NRInonevent is the sum of all points/numbers of patients. Conversely, patients with outcome are assigned +1 if correctly reclassified to a higher risk category or –1 if incorrectly reclassified to a lower risk category. NRIevent is the sum of all points/numbers of patients. NRIoverall is the sum of NRIevent and NRInonevent ranging from –2 to 2, with a positive value indicating better classification with the new model.
Two-sided P < 0.05 was used for statistical significance. All statistical analyses were performed with Stata Release 13.0 (StataCorp).
RESULTS
Among the 530 patients admitted to the ward, 184 were excluded (120 meeting exclusion criteria, 64 unable to give consent, Figure). Among the 346 patients included, 11.6% (n = 40) had a 30-day unplanned readmission or death (37 were readmitted, 2 died during readmission, 3 died without readmission). Within 90 days, 26.6% (n = 92) had a readmission or death (84 were readmitted, 10 died during or after readmission, 8 died without readmission).
Clinical Characteristics
Table 1 lists the patients’ baseline characteristics. Median age was 73 years (IQR, 64-82 years). Of the 346 patients included, 172 (49.7%) were men. Median CCI was 7 (IQR, 5-9); according to this index, 310 patients (89.6%) had at least 2 comorbidities. Median length of stay was 7 days (IQR, 4-12 days).
Primary Diagnoses of Admission, Unplanned Readmission, and Death
The 3 main causes of index admission were cardiovascular disorder (n = 92), infectious disorder (n = 70), and neurologic disorder (n = 66). Table 2 lists the causes of readmissions and deaths. A same-diagnosis category between index admission and readmission was found in 17 (45.9%) of the 37 readmitted patients and in 3 (60%) of the 5 patients who died.
Biomarkers and 30-Day Unplanned Readmission or Death
AUROC was 0.53 (95% confidence interval [CI], 0.43-0.63) for ADM, 0.60 (95% CI, 0.50-0.70) for copeptin, 0.59 (95% CI, 0.44-0.73) for cortisol, and 0.56 (95% CI, 0.45-0.66) for prolactin. The difference between admission and discharge levels was not associated with unplanned readmission or death for any of the biomarkers (Supplemental Table 2).
ADM and readmission or death. Median ADM level was not different between patients with and without readmission or death (1.0 nmol/L in each case; P = 1.00). The best cutoff level for ADM was 2 nmol/L (sensitivity, 16.7%; specificity, 91.8%). At this level, ADM was associated with a nonstatistically significant 130% increased odds of 30-day readmission or death (P = 0.09; Table 3, Supplemental Table 3). Conversely, the association with the 90-day outcome was significant (P = 0.02; Table 3, Supplemental Table 4).
Copeptin and readmission or death. Patients with 30-day readmission or death had a higher median copeptin level at discharge than patients without (10.4 pmol/L vs 7.3 pmol/L; P = 0.03). At a copeptin level higher than 9 pmol/L (to convert to pg/mL, divide by 0.249; sensitivity, 66.7%; specificity, 59.7%), both 30-day readmission or death (adjusted odds ratio [OR], 2.69; 95% CI, 1.29-5.64; P = 0.009) and 90-day readmission or death (adjusted OR, 2.76; 95% CI, 1.56-4.88; P < 0.001) were nearly 3 times as likely (Table 3, Supplemental Tables 3 and 4).
Cortisol and readmission or death. Median cortisol was not statistically different between patients with and without the primary outcome (431 nmol/L vs 465 nmol/L; P = 0.72). At a cortisol level higher than 590 nmol/L (to convert to μg/dL, divide by 27.59; sensitivity, 54.6%; specificity, 76.4%), 30-day outcome was more than 3 times as likely (adjusted OR, 3.43; 95% CI, 1.36-8.65; P = 0.009; Table 3, Supplemental Table 3). At 90 days, only the model that adjusted for age and LACE index points remained statistically significant (P = 0.02; Table 3, Supplemental Table 4).
Prolactin and readmission or death. Median prolactin was not statistically different between patients with and without the primary outcome (15.1 μg/L vs 14.1 μg/L; P = 0.24). The best cutoff level for prolactin was 23 μg/L (to convert to mIU/L, divide by 0.05; sensitivity, 27.8%; specificity, 82.9%). Prolactin was associated with a nonstatistically significant increased odds of 30-day (P = 0.16) and 90-day (P = 0.24) readmission or death (Table 3, Supplemental Tables 3 and 4).
Additive Value of Biomarkers to HOSPITAL Score and LACE Index
The AUROC for the original HOSPITAL score, 0.70 (95% CI, 0.60-0.80), nonsignificantly increased to 0.76 after adding the biomarkers (P > 0.14). For the LACE index, AUROC was 0.59 (95% CI, 0.49-0.68), with a significant 0.10 increase with cortisol (P = 0.04) and a near significant increase with copeptin (P = 0.08). Calibration remained almost unchanged after adding the biomarkers to both models (Supplemental Table 5). NRIoverall was positive for all biomarkers, with statistical significance for copeptin added to the HOSPITAL score (0.47; 95% CI, 0.13-0.79) and for cortisol added to the LACE index (0.62; 95% CI, 0.15-1.06).
DISCUSSION
In this prospective cohort study, 30-day and 90-day unplanned readmission or death was nearly 3 times as likely for patients with high copeptin levels on discharge from an acute-care medical hospitalization, and 30-day readmission or death was more than 3 times as likely for patients with high cortisol levels. High ADM and prolactin levels were not consistently associated with readmission or death. Adding such biomarkers to readmission prediction models improved their performance.
These findings support the theory of posthospital syndrome,1 which describes a period of vulnerability with increased stress after discharge from an acute-care hospitalization, and which may be associated with adverse outcome. The hormones cortisol and copeptin are strongly related to the stress response in humans.4,5 As copeptin level has been associated with adverse prognosis for several disorders affecting a wide range of physiologic systems,3,5,15,27 it may be a valuable biomarker of a stressful condition, even independent of the system affected by the acute illness, and its use may be widely generalizable, in contrast to predictive factors identified in other studies.18,28,29
Although cortisol was independently associated with 30-day readmission or death, and may be an interesting biomarker and less expensive than copeptin, its measurement is limited in patients treated with systemic corticosteroids. Compared with cortisol, copeptin does not undergo diurnal variation, is less affected by corticosteroid therapy, and mirrors stress levels better.5,7,30,31 Our results showed that, contrary to cortisol, copeptin was also associated with longer term outcome. High ADM level was associated with readmission or death at 90 days only; lack of a significant association at 30 days might be attributable to a lack of power (fewer outcomes at 30 days). Conversely, prolactin level was consistently not associated with outcome. Prolactin may be affected by many drugs that act on the dopaminergic system (eg, domperidone), and therefore its levels may be more difficult to interpret.
Levels of biomarkers were similar to those measured in patients without previously studied conditions (eg, myocardial infarction).5,8,10,13,14,16,17 In most previous analyses, levels were measured during a stressful event, whereas we measured them at discharge. Therefore, these biomarkers may constitute sensitive markers of remaining stress at discharge.
Our finding that copeptin level was independently associated with readmission or death supports its relevance as a possible simple measure of the risk of adverse postdischarge outcome, independently of disease type and independently of known predictors. Stress biomarkers may therefore be valuable predictors of which patients are at high risk. All these biomarkers can be measured within 30 minutes, extending their use beyond everyday practice, except for the possible need of an extra blood draw.
The most accurate and validated models are the HOSPITAL score (AUROC range, 0.68-0.7723,32-36) and the LACE index (AUROC range, 0.56-0.6823,34,35,37). Adding biomarkers to these models improved overall performance (up to 0.10 increase in AUROC), which is remarkable given that, once a particular level of discriminatory power is reached, extremely large effect sizes from additional markers are needed to increase AUROC.26 Incremental improvement is objectively supported by positive NRI. Our results suggest biomarkers added to prediction models may improve identification of high-risk patients.
We found that less than 50% of the primary diagnoses belonged to the same diagnosis category at readmission and at index admission. This result is in line with previous findings that readmissions were related to the primary diagnosis at index admission in only 22% to 46% of cases,20,38 and supports our study hypothesis that readmission is related to underlying stress factors often independent of the underlying illness.1
Study Limitations and Strengths
Our study had some limitations. First, it was a single-center study with a limited sample size. However, we found significant results within the sample. Second, we could not adjust for drugs that were acting on the dopaminergic system and might have affected prolactin levels. However, such interactions would limit the use of this biomarker in clinical practice anyway. Third, we used specific cutoffs, which might decrease analytical power, in comparison with continuous analyses. However, we followed a recognized method24 and found a significant association even with categorized levels. Furthermore, the distribution of biomarkers could not be normalized by logarithmic transformation, and cutoff values have the advantage of being integrable into score point systems (eg, HOSPITAL score, LACE index). Fourth, although in 2 models we found consistent associations with several potential confounders, we could not exclude residual confounding. Fifth, this study was not powered to assess the biomarkers’ predictive value for readmission and death, which might explain the lack of significant differences between AUROC with and without the biomarkers. For all these reasons, this study should be considered hypothesis-generating.
Our study also had its strengths. First, to our knowledge, this is the first study of the association between stress biomarkers at discharge and unplanned readmission or death. Second, the quality of our data was high, with a low percentage of missing biomarker levels. Third, we excluded planned readmissions. Fourth, we used an unselected medical patient population, which had the noteworthy advantage of widening the generalizability of results.
CONCLUSION
In this prospective cohort study, high copeptin and cortisol levels at discharge were significantly associated with increased odds, ranging from 2-fold to more than 3-fold, of unplanned readmission or death within 30 days after discharge from an internal medicine ward. This finding supports the theory that a physiologic stress that patients experience during hospitalization makes them more susceptible to new health threats (posthospital syndrome). These biomarkers, copeptin in particular, may help us better identify patients at high risk of early unplanned readmission or death.
Acknowledgment
Biomarker measurement was funded by the research fund of the Department of General Internal Medicine, Fribourg Cantonal Hospital, Fribourg, Switzerland.
Disclosure
Nothing to report.
1. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
2. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823-1829. PubMed
3. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172-190. PubMed
4. Yilman M, Erenler AK, Baydin A. Copeptin: a diagnostic factor for critical patients. Eur Rev Med Pharmacol Sci. 2015;19(16):3030-3036. PubMed
5. Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. PubMed
6. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-2504. PubMed
7. Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56(7):1190-1191. PubMed
8. Labad J, Stojanovic-Pérez A, Montalvo I, et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: roles for cortisol, prolactin and albumin. J Psychiatr Res. 2015;60:163-169. PubMed
9. Olsson T, Asplund K, Hagg E. Pituitary-thyroid axis, prolactin and growth hormone in patients with acute stroke. J Intern Med. 1990;228(3):287-290. PubMed
10. Parissis JT, Farmakis D, Fountoulaki K, et al. Clinical and neurohormonal correlates and prognostic value of serum prolactin levels in patients with chronic heart failure. Eur J Heart Fail. 2013;15(10):1122-1130. PubMed
11. Theodoropoulou A, Metallinos IC, Elloul J, et al. Prolactin, cortisol secretion and thyroid function in patients with stroke of mild severity. Horm Metab Res. 2006;38(9):587-591. PubMed
12. Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. Biomed Res Int. 2014;2014:803561. PubMed
13. Bahrmann P, Christ M, Hofner B, et al. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 2016;5(8):568-578. PubMed
14. Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96. PubMed
15. Artunc F, Nowak A, Mueller C, et al. Plasma concentrations of the vasoactive peptide fragments mid-regional pro-adrenomedullin, C-terminal pro-endothelin 1 and copeptin in hemodialysis patients: associated factors and prediction of mortality. PLoS One. 2014;9(1):e86148. PubMed
16. Rotman-Pikielny P, Roash V, Chen O, Limor R, Stern N, Gur HG. Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci. 2006;332(2):61-67. PubMed
17. Yamaji M, Tsutamoto T, Kawahara C, et al. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2(6):608-615. PubMed
18. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed
20. Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. PubMed
21. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
22. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
23. Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé J. Prospective validation and adaptation of the HOSPITAL score to predict high risk of unplanned readmission of medical patients. Swiss Med Wkly. 2016;146:w14335. PubMed
24. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-577. PubMed
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. PubMed
26. Pencina MJ, D’Agostino RB Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31(2):101-113. PubMed
27. Folli C, Consonni D, Spessot M, et al. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur J Intern Med. 2013;24(2):189-193. PubMed
28. Aujesky D, Mor MK, Geng M, Stone RA, Fine MJ, Ibrahim SA. Predictors of early hospital readmission after acute pulmonary embolism. Arch Intern Med. 2009;169(3):287-293. PubMed
29. Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4(1):60-67. PubMed
30. Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012;10:7. PubMed
31. Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341-346. PubMed
32. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502.PubMed
33. Burke RE, Schnipper JL, Williams MV, et al. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the Hospital Readmissions Reduction Program. Med Care. 2017;55(3):285-290. PubMed
34. Garrison GM, Robelia PM, Pecina JL, Dawson NL. Comparing performance of 30-day readmission risk classifiers among hospitalized primary care patients. J Eval Clin Pract. 2017;23(3):524-529. PubMed
35. Cooksley T, Nanayakkara PW, Nickel CH, et al. Readmissions of medical patients: an external validation of two existing prediction scores. QJM. 2016;109(4):245-248. PubMed
36. Robinson R. The HOSPITAL score as a predictor of 30 day readmission in a retrospective study at a university affiliated community hospital. PeerJ. 2016;4:e2441. PubMed
37. Wang H, Robinson RD, Johnson C, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. 2014;14:97. PubMed
38. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18. PubMed
1. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
2. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823-1829. PubMed
3. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172-190. PubMed
4. Yilman M, Erenler AK, Baydin A. Copeptin: a diagnostic factor for critical patients. Eur Rev Med Pharmacol Sci. 2015;19(16):3030-3036. PubMed
5. Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. PubMed
6. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-2504. PubMed
7. Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56(7):1190-1191. PubMed
8. Labad J, Stojanovic-Pérez A, Montalvo I, et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: roles for cortisol, prolactin and albumin. J Psychiatr Res. 2015;60:163-169. PubMed
9. Olsson T, Asplund K, Hagg E. Pituitary-thyroid axis, prolactin and growth hormone in patients with acute stroke. J Intern Med. 1990;228(3):287-290. PubMed
10. Parissis JT, Farmakis D, Fountoulaki K, et al. Clinical and neurohormonal correlates and prognostic value of serum prolactin levels in patients with chronic heart failure. Eur J Heart Fail. 2013;15(10):1122-1130. PubMed
11. Theodoropoulou A, Metallinos IC, Elloul J, et al. Prolactin, cortisol secretion and thyroid function in patients with stroke of mild severity. Horm Metab Res. 2006;38(9):587-591. PubMed
12. Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. Biomed Res Int. 2014;2014:803561. PubMed
13. Bahrmann P, Christ M, Hofner B, et al. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 2016;5(8):568-578. PubMed
14. Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96. PubMed
15. Artunc F, Nowak A, Mueller C, et al. Plasma concentrations of the vasoactive peptide fragments mid-regional pro-adrenomedullin, C-terminal pro-endothelin 1 and copeptin in hemodialysis patients: associated factors and prediction of mortality. PLoS One. 2014;9(1):e86148. PubMed
16. Rotman-Pikielny P, Roash V, Chen O, Limor R, Stern N, Gur HG. Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci. 2006;332(2):61-67. PubMed
17. Yamaji M, Tsutamoto T, Kawahara C, et al. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2(6):608-615. PubMed
18. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688-1698. PubMed
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed
20. Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. PubMed
21. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
22. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
23. Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé J. Prospective validation and adaptation of the HOSPITAL score to predict high risk of unplanned readmission of medical patients. Swiss Med Wkly. 2016;146:w14335. PubMed
24. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-577. PubMed
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. PubMed
26. Pencina MJ, D’Agostino RB Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31(2):101-113. PubMed
27. Folli C, Consonni D, Spessot M, et al. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur J Intern Med. 2013;24(2):189-193. PubMed
28. Aujesky D, Mor MK, Geng M, Stone RA, Fine MJ, Ibrahim SA. Predictors of early hospital readmission after acute pulmonary embolism. Arch Intern Med. 2009;169(3):287-293. PubMed
29. Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4(1):60-67. PubMed
30. Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012;10:7. PubMed
31. Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341-346. PubMed
32. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502.PubMed
33. Burke RE, Schnipper JL, Williams MV, et al. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the Hospital Readmissions Reduction Program. Med Care. 2017;55(3):285-290. PubMed
34. Garrison GM, Robelia PM, Pecina JL, Dawson NL. Comparing performance of 30-day readmission risk classifiers among hospitalized primary care patients. J Eval Clin Pract. 2017;23(3):524-529. PubMed
35. Cooksley T, Nanayakkara PW, Nickel CH, et al. Readmissions of medical patients: an external validation of two existing prediction scores. QJM. 2016;109(4):245-248. PubMed
36. Robinson R. The HOSPITAL score as a predictor of 30 day readmission in a retrospective study at a university affiliated community hospital. PeerJ. 2016;4:e2441. PubMed
37. Wang H, Robinson RD, Johnson C, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. 2014;14:97. PubMed
38. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18. PubMed
© 2017 Society of Hospital Medicine