User login
Improved Pharmacogenomic Testing Process for Veterans in Outpatient Settings by Clinical Pharmacist Practitioners
Peer-review, evidence-based, detailed gene/drug clinical practice guidelines suggest that genetic variations can impact how individuals metabolize medications, which is sometimes included in medication prescribing information.1-3 Pharmacogenomic testing identifies genetic markers so medication selection and dosing can be tailored to each individual by identifying whether a specific medication is likely to be safe and effective prior to prescribing.4
Pharmacogenomics can be a valuable tool for personalizing medicine but has had suboptimal implementation since its discovery. The US Department of Veterans Affairs (VA) health care system reviewed the implementation of the Pharmacogenomic Testing for Veterans (PHASER) program. This review identified clinician barriers pre- and post-PHASER program implementation; staffing issues, competing clinical priorities, and inadequate PHASER program resources were the most frequently reported barriers to implementation of pharmacogenomic testing.5
Another evaluation of the implementation of the PHASER program that surveyed VA patients found that patients could be separated into 3 groups. Acceptors of pharmacogenomic testing emphasized potential health benefits of testing. Patients that declined testing often cited concerns for genetic information affecting insurance coverage, being misused, or being susceptible to data breach. The third group—identified as contemplators—reported the need for clinician outreach to impact their decision on whether or not to receive pharmacogenomic testing.6 These studies suggest that removing barriers by providing ample pharmacogenomics resources to clinicians, in addition to detailed training on how to offer and follow up with patients regarding pharmacogenomic testing, is crucial to successful implementation of the PHASER program.
PHASER
In 2019, the VA began working with Sanford Health to establish the PHASER program and offer pharmacogenomic testing. PHASER has since expanded to 25 VA medical centers, including the VA Central Ohio Healthcare System (VACOHCS).7,8 Pharmacogenomic testing through PHASER is conducted using a standardized laboratory panel that includes 12 different medication classes.9 The drug classes include certain anti-infective, anticoagulant, antiplatelet, cardiovascular, cholesterol, gastrointestinal, mental health, neurological, oncology, pain, transplant, and other miscellaneous medications. Medications are correlated to each class and assessed for therapeutic impacts based on gene panel results.
Clinical recommendations for medication-gene interactions can range from monitoring for increased risk of adverse effects or therapeutic failure to recommending avoiding a medication. For example, patients who test positive for the HLA-B gene have significantly increased risk of hypersensitivity to abacavir, an HIV treatment.10
Similarly, patients who cannot adequately metabolize cytochrome P450 2C19 should consider avoiding clopidogrel as they are unlikely to convert clopidogrel to its active prodrug, which reduces its effectiveness.11 Pharmacists can play a critical role educating patients about pharmacogenomic testing, especially within hematology and oncology.12 Patients can benefit from this testing even if they are not currently taking medications with known concerns as they could be prescribed in the future. The SLCO1B1 gene-drug test, for example, can identify risk for statin-associated muscle symptoms.13
Clinical pharmacist practitioners (CPPs) can increase access to genetic testing because they interact with patients in a variety of settings and can order this laboratory test.12,14 Recent research has demonstrated that most VA patients carry ≥ 1 genetic variant that may influence medication decisions and that half of veterans are prescribed a medication with known gene-drug interactions.15 CPP ordering of pharmacogenomic tests at the VACOHCS outpatient clinic was evaluated through collection of baseline data from March 8, 2023, to September 8, 2023. A goal was identified to increase orders by 50% for a patient care quality improvement initiative and use CPPs to increase access to pharmacogenomic testing. The purpose of this quality improvement initiative was to expand access to pharmacogenomic testing through process implementation and improvement within CPP-led clinic settings.
Gap Analysis
Lean Six Sigma A3 methodology was used to identify ways to increase the use of pharmacogenomic testing for veterans at VACOHCS and develop an improved process for increased ordering of pharmacogenomic testing. Lean Six Sigma A3 methodology is a stepwise approach to process improvement that helps identify gaps in efficiency, sustainable changes, and eliminate waste.16 Baseline data were collected from March 8, 2023, to September 8, 2023, to determine the frequency of CPPs ordering pharmacogenomic laboratory panels during clinic appointments. The ordering of pharmacogenomic panels was monitored by the VACOHCS PHASER coordinator.
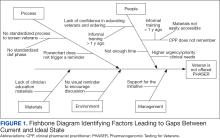
CPPs were surveyed to identify perceived barriers to PHASER implementation. A gap analysis was conducted using Lean Six Sigma A3 methodology. Gap analyses use lean tools such as a Fishbone Diagram to illustrate and identify the gap between current state and ideal state. (Figure 1).The following barriers were identified: lack of clinician education materials, lack of a standardized patient screening process, time constraints on patient education and ordering, higher priority clinical needs, forgetting to order, lack of comfort with pharmacogenomics ordering and education, lack of support for the initiative, and increased workload and burnout. Among these perceived barriers, higher priority clinical needs, forgetting to order, and time constraints ranked highest in importance among CPPs.
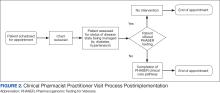
In line with Lean Six Sigma A3 methodology, several tests of change were used to improve pharmacogenomic testing ordering. These changes focused on increasing patient and clinician awareness, facilitating discussion, educating clinicians, and simplifying documentation to ease time constraints. Several strategies were employed postimplementation (Figure 2). Prefilled templates simplified documentation. These templates helped identify patients without pharmacogenomic testing, provided reminders, and saved documentation time during visits. CPPs also received training and materials on PHASER ordering and documentation within encounter notes. Additionally, patient-directed advertisements were displayed in CPP examination rooms to help inspire and facilitate discussion between veterans and CPPs.
Process Improvement Data
The quality improvement project goal was to increase PHASER orders by 50% after 3 months. PHASER orders increased from 87 at baseline (March 8, 2023, to September 8, 2023) to 196 during the intervention (November 16, 2023, to February 16, 2024), a 125% increase. Changes were consistent and sustained with 65 orders the first month, 67 orders the second month, and 64 orders the third month.
Discussion
Using Lean Six Sigma A3 methodology for a quality improvement process to increase PHASER orders by CPPs revealed barriers and guided potential solutions to overcome these barriers. Interventions included additional CPP training and ordering, tools for easier identification of potential patients, documentation best practices, patient-directed advertisements to facilitate conversations. These interventions required about 8 hours for preparation, distribution, development, and interpretation of surveys, education, and documentation materials. The financial impact of these interventions was already included in allotted office materials budgeted and provided. Additional funding was not needed to provide patient-directed advertisements or education materials. The VACOHCS pharmacogenomics CPP discusses PHASER test results with patients at a separate appointment.
Future directions include educating other CPPs to assist in discussing results with veterans. Overall, the changes implemented to improve the PHASER ordering process were low effort and exemplify the ease of streamlining future initiatives, allowing for sustained optimal implementation of pharmacogenomic testing.
Conclusions
A quality improvement initiative resulted in increased PHASER orders and a clearly defined process, allowing for a continued increase and sustained support. Perceived barriers were identified, and the changes implemented were often low effort but exhibited a sustained impact. The insights gleaned from this process will shape future process development initiatives and continue to sustain pharmacogenomic testing ordering by CPPs. This process will be extended to other VACOHCS clinical departments to further support increased access to pharmacogenomic testing, reduce medication trial and error, and reduce hospitalizations from adverse effects for veterans.
Cecchin E, Stocco G. Pharmacogenomics and personalized medicine. Genes (Basel). 2020;11(6):679. doi:10.3390/genes11060679
Guidelines. CPIC. Accessed April 16, 2025. https://cpicpgx.org/guidelines/
PharmGKB. PharmGKB. 2025. Accessed April 16, 2025. https://www.pharmgkb.org
Centers for Disease Control and Prevention. Pharmacogenomics. Updated November 13, 2024. Accessed April 16, 2024. https://www.cdc.gov/genomics-and-health/pharmacogenomics/
Dong OM, Roberts MC, Wu RR, et al. Evaluation of the Veterans Affairs Pharmacogenomic Testing for Veterans (PHASER) clinical program at initial test sites. Pharmacogenomics. 2021;22(17):1121-1133. doi:10.2217/pgs-2021-0089
Melendez K, Gutierrez-Meza D, Gavin KL, et al. Patient perspectives of barriers and facilitators for the uptake of pharmacogenomic testing in Veterans Affairs’ pharmacogenomic testing for the veterans (PHASER) program. J Pers Med. 2023;13(9):1367. doi:10.3390/jpm13091367
Sanford Health Imagenetics. FREQUENTLY ASKED QUESTIONS (FAQs) about the “Pharmacogenomic Teting for Vetans” (PHASER) Program. US Department of Veterans Affairs. December 20, 2019. Accessed April 16, 2025. https://www.va.gov/opa/publications/factsheets/PHASER-FLYER-VA-Patient-FAQ.pdf
Peterson H. PHASER program testing informs how you respond to medicines. VA News. September 6, 2022. Accessed April 16, 2025. https://news.va.gov/108091/phaser-program-testing-respond-medicines/
Pharmacogenomics (PGx). Sanford Health Imagenetics. 2025. Accessed April 16, 2025. https://imagenetics.sanfordhealth.org/pharmacogenomics/
Martin MA, Hoffman JM, Freimuth RR, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499-500. doi:10.1038/clpt.2014.38
Lee CR, Luzum JA, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112(5):959-967. doi:10.1002/cpt.2526
Dreischmeier E, Hecht H, Crocker E, et al. Integration of a clinical pharmacist practitioner-led pharmacogenomics service in a Veterans Affairs hematology/oncology clinic. Am J Health Syst Pharm. 2024;81(19):e634-e639. doi:10.1093/ajhp/zxae122
Tomcsanyi KM, Tran KA, Bates J, et al. Veterans Health Administration: implementation of pharmacogenomic clinical decision support with statin medications and the SLCO1B1 gene as an exemplar. Am J Health Syst Pharm. 2023;80(16):1082-1089. doi:10.1093/ajhp/zxad111
Gammal RS, Lee YM, Petry NJ, et al. Pharmacists leading the way to precision medicine: updates to the core pharmacist competencies in genomics. Am J Pharm Educ. 2022;86(4):8634. doi:10.5688/ajpe8634
Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi:10.1001/jamanetworkopen.2019.5345
Shaffie S, Shahbazi S. The McGraw-Hill 36-Hour Course: Lean Six Sigma. McGraw-Hill; 2012.
Peer-review, evidence-based, detailed gene/drug clinical practice guidelines suggest that genetic variations can impact how individuals metabolize medications, which is sometimes included in medication prescribing information.1-3 Pharmacogenomic testing identifies genetic markers so medication selection and dosing can be tailored to each individual by identifying whether a specific medication is likely to be safe and effective prior to prescribing.4
Pharmacogenomics can be a valuable tool for personalizing medicine but has had suboptimal implementation since its discovery. The US Department of Veterans Affairs (VA) health care system reviewed the implementation of the Pharmacogenomic Testing for Veterans (PHASER) program. This review identified clinician barriers pre- and post-PHASER program implementation; staffing issues, competing clinical priorities, and inadequate PHASER program resources were the most frequently reported barriers to implementation of pharmacogenomic testing.5
Another evaluation of the implementation of the PHASER program that surveyed VA patients found that patients could be separated into 3 groups. Acceptors of pharmacogenomic testing emphasized potential health benefits of testing. Patients that declined testing often cited concerns for genetic information affecting insurance coverage, being misused, or being susceptible to data breach. The third group—identified as contemplators—reported the need for clinician outreach to impact their decision on whether or not to receive pharmacogenomic testing.6 These studies suggest that removing barriers by providing ample pharmacogenomics resources to clinicians, in addition to detailed training on how to offer and follow up with patients regarding pharmacogenomic testing, is crucial to successful implementation of the PHASER program.
PHASER
In 2019, the VA began working with Sanford Health to establish the PHASER program and offer pharmacogenomic testing. PHASER has since expanded to 25 VA medical centers, including the VA Central Ohio Healthcare System (VACOHCS).7,8 Pharmacogenomic testing through PHASER is conducted using a standardized laboratory panel that includes 12 different medication classes.9 The drug classes include certain anti-infective, anticoagulant, antiplatelet, cardiovascular, cholesterol, gastrointestinal, mental health, neurological, oncology, pain, transplant, and other miscellaneous medications. Medications are correlated to each class and assessed for therapeutic impacts based on gene panel results.
Clinical recommendations for medication-gene interactions can range from monitoring for increased risk of adverse effects or therapeutic failure to recommending avoiding a medication. For example, patients who test positive for the HLA-B gene have significantly increased risk of hypersensitivity to abacavir, an HIV treatment.10
Similarly, patients who cannot adequately metabolize cytochrome P450 2C19 should consider avoiding clopidogrel as they are unlikely to convert clopidogrel to its active prodrug, which reduces its effectiveness.11 Pharmacists can play a critical role educating patients about pharmacogenomic testing, especially within hematology and oncology.12 Patients can benefit from this testing even if they are not currently taking medications with known concerns as they could be prescribed in the future. The SLCO1B1 gene-drug test, for example, can identify risk for statin-associated muscle symptoms.13
Clinical pharmacist practitioners (CPPs) can increase access to genetic testing because they interact with patients in a variety of settings and can order this laboratory test.12,14 Recent research has demonstrated that most VA patients carry ≥ 1 genetic variant that may influence medication decisions and that half of veterans are prescribed a medication with known gene-drug interactions.15 CPP ordering of pharmacogenomic tests at the VACOHCS outpatient clinic was evaluated through collection of baseline data from March 8, 2023, to September 8, 2023. A goal was identified to increase orders by 50% for a patient care quality improvement initiative and use CPPs to increase access to pharmacogenomic testing. The purpose of this quality improvement initiative was to expand access to pharmacogenomic testing through process implementation and improvement within CPP-led clinic settings.
Gap Analysis
Lean Six Sigma A3 methodology was used to identify ways to increase the use of pharmacogenomic testing for veterans at VACOHCS and develop an improved process for increased ordering of pharmacogenomic testing. Lean Six Sigma A3 methodology is a stepwise approach to process improvement that helps identify gaps in efficiency, sustainable changes, and eliminate waste.16 Baseline data were collected from March 8, 2023, to September 8, 2023, to determine the frequency of CPPs ordering pharmacogenomic laboratory panels during clinic appointments. The ordering of pharmacogenomic panels was monitored by the VACOHCS PHASER coordinator.
CPPs were surveyed to identify perceived barriers to PHASER implementation. A gap analysis was conducted using Lean Six Sigma A3 methodology. Gap analyses use lean tools such as a Fishbone Diagram to illustrate and identify the gap between current state and ideal state. (Figure 1).The following barriers were identified: lack of clinician education materials, lack of a standardized patient screening process, time constraints on patient education and ordering, higher priority clinical needs, forgetting to order, lack of comfort with pharmacogenomics ordering and education, lack of support for the initiative, and increased workload and burnout. Among these perceived barriers, higher priority clinical needs, forgetting to order, and time constraints ranked highest in importance among CPPs.
In line with Lean Six Sigma A3 methodology, several tests of change were used to improve pharmacogenomic testing ordering. These changes focused on increasing patient and clinician awareness, facilitating discussion, educating clinicians, and simplifying documentation to ease time constraints. Several strategies were employed postimplementation (Figure 2). Prefilled templates simplified documentation. These templates helped identify patients without pharmacogenomic testing, provided reminders, and saved documentation time during visits. CPPs also received training and materials on PHASER ordering and documentation within encounter notes. Additionally, patient-directed advertisements were displayed in CPP examination rooms to help inspire and facilitate discussion between veterans and CPPs.
Process Improvement Data
The quality improvement project goal was to increase PHASER orders by 50% after 3 months. PHASER orders increased from 87 at baseline (March 8, 2023, to September 8, 2023) to 196 during the intervention (November 16, 2023, to February 16, 2024), a 125% increase. Changes were consistent and sustained with 65 orders the first month, 67 orders the second month, and 64 orders the third month.
Discussion
Using Lean Six Sigma A3 methodology for a quality improvement process to increase PHASER orders by CPPs revealed barriers and guided potential solutions to overcome these barriers. Interventions included additional CPP training and ordering, tools for easier identification of potential patients, documentation best practices, patient-directed advertisements to facilitate conversations. These interventions required about 8 hours for preparation, distribution, development, and interpretation of surveys, education, and documentation materials. The financial impact of these interventions was already included in allotted office materials budgeted and provided. Additional funding was not needed to provide patient-directed advertisements or education materials. The VACOHCS pharmacogenomics CPP discusses PHASER test results with patients at a separate appointment.
Future directions include educating other CPPs to assist in discussing results with veterans. Overall, the changes implemented to improve the PHASER ordering process were low effort and exemplify the ease of streamlining future initiatives, allowing for sustained optimal implementation of pharmacogenomic testing.
Conclusions
A quality improvement initiative resulted in increased PHASER orders and a clearly defined process, allowing for a continued increase and sustained support. Perceived barriers were identified, and the changes implemented were often low effort but exhibited a sustained impact. The insights gleaned from this process will shape future process development initiatives and continue to sustain pharmacogenomic testing ordering by CPPs. This process will be extended to other VACOHCS clinical departments to further support increased access to pharmacogenomic testing, reduce medication trial and error, and reduce hospitalizations from adverse effects for veterans.
Peer-review, evidence-based, detailed gene/drug clinical practice guidelines suggest that genetic variations can impact how individuals metabolize medications, which is sometimes included in medication prescribing information.1-3 Pharmacogenomic testing identifies genetic markers so medication selection and dosing can be tailored to each individual by identifying whether a specific medication is likely to be safe and effective prior to prescribing.4
Pharmacogenomics can be a valuable tool for personalizing medicine but has had suboptimal implementation since its discovery. The US Department of Veterans Affairs (VA) health care system reviewed the implementation of the Pharmacogenomic Testing for Veterans (PHASER) program. This review identified clinician barriers pre- and post-PHASER program implementation; staffing issues, competing clinical priorities, and inadequate PHASER program resources were the most frequently reported barriers to implementation of pharmacogenomic testing.5
Another evaluation of the implementation of the PHASER program that surveyed VA patients found that patients could be separated into 3 groups. Acceptors of pharmacogenomic testing emphasized potential health benefits of testing. Patients that declined testing often cited concerns for genetic information affecting insurance coverage, being misused, or being susceptible to data breach. The third group—identified as contemplators—reported the need for clinician outreach to impact their decision on whether or not to receive pharmacogenomic testing.6 These studies suggest that removing barriers by providing ample pharmacogenomics resources to clinicians, in addition to detailed training on how to offer and follow up with patients regarding pharmacogenomic testing, is crucial to successful implementation of the PHASER program.
PHASER
In 2019, the VA began working with Sanford Health to establish the PHASER program and offer pharmacogenomic testing. PHASER has since expanded to 25 VA medical centers, including the VA Central Ohio Healthcare System (VACOHCS).7,8 Pharmacogenomic testing through PHASER is conducted using a standardized laboratory panel that includes 12 different medication classes.9 The drug classes include certain anti-infective, anticoagulant, antiplatelet, cardiovascular, cholesterol, gastrointestinal, mental health, neurological, oncology, pain, transplant, and other miscellaneous medications. Medications are correlated to each class and assessed for therapeutic impacts based on gene panel results.
Clinical recommendations for medication-gene interactions can range from monitoring for increased risk of adverse effects or therapeutic failure to recommending avoiding a medication. For example, patients who test positive for the HLA-B gene have significantly increased risk of hypersensitivity to abacavir, an HIV treatment.10
Similarly, patients who cannot adequately metabolize cytochrome P450 2C19 should consider avoiding clopidogrel as they are unlikely to convert clopidogrel to its active prodrug, which reduces its effectiveness.11 Pharmacists can play a critical role educating patients about pharmacogenomic testing, especially within hematology and oncology.12 Patients can benefit from this testing even if they are not currently taking medications with known concerns as they could be prescribed in the future. The SLCO1B1 gene-drug test, for example, can identify risk for statin-associated muscle symptoms.13
Clinical pharmacist practitioners (CPPs) can increase access to genetic testing because they interact with patients in a variety of settings and can order this laboratory test.12,14 Recent research has demonstrated that most VA patients carry ≥ 1 genetic variant that may influence medication decisions and that half of veterans are prescribed a medication with known gene-drug interactions.15 CPP ordering of pharmacogenomic tests at the VACOHCS outpatient clinic was evaluated through collection of baseline data from March 8, 2023, to September 8, 2023. A goal was identified to increase orders by 50% for a patient care quality improvement initiative and use CPPs to increase access to pharmacogenomic testing. The purpose of this quality improvement initiative was to expand access to pharmacogenomic testing through process implementation and improvement within CPP-led clinic settings.
Gap Analysis
Lean Six Sigma A3 methodology was used to identify ways to increase the use of pharmacogenomic testing for veterans at VACOHCS and develop an improved process for increased ordering of pharmacogenomic testing. Lean Six Sigma A3 methodology is a stepwise approach to process improvement that helps identify gaps in efficiency, sustainable changes, and eliminate waste.16 Baseline data were collected from March 8, 2023, to September 8, 2023, to determine the frequency of CPPs ordering pharmacogenomic laboratory panels during clinic appointments. The ordering of pharmacogenomic panels was monitored by the VACOHCS PHASER coordinator.
CPPs were surveyed to identify perceived barriers to PHASER implementation. A gap analysis was conducted using Lean Six Sigma A3 methodology. Gap analyses use lean tools such as a Fishbone Diagram to illustrate and identify the gap between current state and ideal state. (Figure 1).The following barriers were identified: lack of clinician education materials, lack of a standardized patient screening process, time constraints on patient education and ordering, higher priority clinical needs, forgetting to order, lack of comfort with pharmacogenomics ordering and education, lack of support for the initiative, and increased workload and burnout. Among these perceived barriers, higher priority clinical needs, forgetting to order, and time constraints ranked highest in importance among CPPs.
In line with Lean Six Sigma A3 methodology, several tests of change were used to improve pharmacogenomic testing ordering. These changes focused on increasing patient and clinician awareness, facilitating discussion, educating clinicians, and simplifying documentation to ease time constraints. Several strategies were employed postimplementation (Figure 2). Prefilled templates simplified documentation. These templates helped identify patients without pharmacogenomic testing, provided reminders, and saved documentation time during visits. CPPs also received training and materials on PHASER ordering and documentation within encounter notes. Additionally, patient-directed advertisements were displayed in CPP examination rooms to help inspire and facilitate discussion between veterans and CPPs.
Process Improvement Data
The quality improvement project goal was to increase PHASER orders by 50% after 3 months. PHASER orders increased from 87 at baseline (March 8, 2023, to September 8, 2023) to 196 during the intervention (November 16, 2023, to February 16, 2024), a 125% increase. Changes were consistent and sustained with 65 orders the first month, 67 orders the second month, and 64 orders the third month.
Discussion
Using Lean Six Sigma A3 methodology for a quality improvement process to increase PHASER orders by CPPs revealed barriers and guided potential solutions to overcome these barriers. Interventions included additional CPP training and ordering, tools for easier identification of potential patients, documentation best practices, patient-directed advertisements to facilitate conversations. These interventions required about 8 hours for preparation, distribution, development, and interpretation of surveys, education, and documentation materials. The financial impact of these interventions was already included in allotted office materials budgeted and provided. Additional funding was not needed to provide patient-directed advertisements or education materials. The VACOHCS pharmacogenomics CPP discusses PHASER test results with patients at a separate appointment.
Future directions include educating other CPPs to assist in discussing results with veterans. Overall, the changes implemented to improve the PHASER ordering process were low effort and exemplify the ease of streamlining future initiatives, allowing for sustained optimal implementation of pharmacogenomic testing.
Conclusions
A quality improvement initiative resulted in increased PHASER orders and a clearly defined process, allowing for a continued increase and sustained support. Perceived barriers were identified, and the changes implemented were often low effort but exhibited a sustained impact. The insights gleaned from this process will shape future process development initiatives and continue to sustain pharmacogenomic testing ordering by CPPs. This process will be extended to other VACOHCS clinical departments to further support increased access to pharmacogenomic testing, reduce medication trial and error, and reduce hospitalizations from adverse effects for veterans.
Cecchin E, Stocco G. Pharmacogenomics and personalized medicine. Genes (Basel). 2020;11(6):679. doi:10.3390/genes11060679
Guidelines. CPIC. Accessed April 16, 2025. https://cpicpgx.org/guidelines/
PharmGKB. PharmGKB. 2025. Accessed April 16, 2025. https://www.pharmgkb.org
Centers for Disease Control and Prevention. Pharmacogenomics. Updated November 13, 2024. Accessed April 16, 2024. https://www.cdc.gov/genomics-and-health/pharmacogenomics/
Dong OM, Roberts MC, Wu RR, et al. Evaluation of the Veterans Affairs Pharmacogenomic Testing for Veterans (PHASER) clinical program at initial test sites. Pharmacogenomics. 2021;22(17):1121-1133. doi:10.2217/pgs-2021-0089
Melendez K, Gutierrez-Meza D, Gavin KL, et al. Patient perspectives of barriers and facilitators for the uptake of pharmacogenomic testing in Veterans Affairs’ pharmacogenomic testing for the veterans (PHASER) program. J Pers Med. 2023;13(9):1367. doi:10.3390/jpm13091367
Sanford Health Imagenetics. FREQUENTLY ASKED QUESTIONS (FAQs) about the “Pharmacogenomic Teting for Vetans” (PHASER) Program. US Department of Veterans Affairs. December 20, 2019. Accessed April 16, 2025. https://www.va.gov/opa/publications/factsheets/PHASER-FLYER-VA-Patient-FAQ.pdf
Peterson H. PHASER program testing informs how you respond to medicines. VA News. September 6, 2022. Accessed April 16, 2025. https://news.va.gov/108091/phaser-program-testing-respond-medicines/
Pharmacogenomics (PGx). Sanford Health Imagenetics. 2025. Accessed April 16, 2025. https://imagenetics.sanfordhealth.org/pharmacogenomics/
Martin MA, Hoffman JM, Freimuth RR, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499-500. doi:10.1038/clpt.2014.38
Lee CR, Luzum JA, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112(5):959-967. doi:10.1002/cpt.2526
Dreischmeier E, Hecht H, Crocker E, et al. Integration of a clinical pharmacist practitioner-led pharmacogenomics service in a Veterans Affairs hematology/oncology clinic. Am J Health Syst Pharm. 2024;81(19):e634-e639. doi:10.1093/ajhp/zxae122
Tomcsanyi KM, Tran KA, Bates J, et al. Veterans Health Administration: implementation of pharmacogenomic clinical decision support with statin medications and the SLCO1B1 gene as an exemplar. Am J Health Syst Pharm. 2023;80(16):1082-1089. doi:10.1093/ajhp/zxad111
Gammal RS, Lee YM, Petry NJ, et al. Pharmacists leading the way to precision medicine: updates to the core pharmacist competencies in genomics. Am J Pharm Educ. 2022;86(4):8634. doi:10.5688/ajpe8634
Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi:10.1001/jamanetworkopen.2019.5345
Shaffie S, Shahbazi S. The McGraw-Hill 36-Hour Course: Lean Six Sigma. McGraw-Hill; 2012.
Cecchin E, Stocco G. Pharmacogenomics and personalized medicine. Genes (Basel). 2020;11(6):679. doi:10.3390/genes11060679
Guidelines. CPIC. Accessed April 16, 2025. https://cpicpgx.org/guidelines/
PharmGKB. PharmGKB. 2025. Accessed April 16, 2025. https://www.pharmgkb.org
Centers for Disease Control and Prevention. Pharmacogenomics. Updated November 13, 2024. Accessed April 16, 2024. https://www.cdc.gov/genomics-and-health/pharmacogenomics/
Dong OM, Roberts MC, Wu RR, et al. Evaluation of the Veterans Affairs Pharmacogenomic Testing for Veterans (PHASER) clinical program at initial test sites. Pharmacogenomics. 2021;22(17):1121-1133. doi:10.2217/pgs-2021-0089
Melendez K, Gutierrez-Meza D, Gavin KL, et al. Patient perspectives of barriers and facilitators for the uptake of pharmacogenomic testing in Veterans Affairs’ pharmacogenomic testing for the veterans (PHASER) program. J Pers Med. 2023;13(9):1367. doi:10.3390/jpm13091367
Sanford Health Imagenetics. FREQUENTLY ASKED QUESTIONS (FAQs) about the “Pharmacogenomic Teting for Vetans” (PHASER) Program. US Department of Veterans Affairs. December 20, 2019. Accessed April 16, 2025. https://www.va.gov/opa/publications/factsheets/PHASER-FLYER-VA-Patient-FAQ.pdf
Peterson H. PHASER program testing informs how you respond to medicines. VA News. September 6, 2022. Accessed April 16, 2025. https://news.va.gov/108091/phaser-program-testing-respond-medicines/
Pharmacogenomics (PGx). Sanford Health Imagenetics. 2025. Accessed April 16, 2025. https://imagenetics.sanfordhealth.org/pharmacogenomics/
Martin MA, Hoffman JM, Freimuth RR, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499-500. doi:10.1038/clpt.2014.38
Lee CR, Luzum JA, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112(5):959-967. doi:10.1002/cpt.2526
Dreischmeier E, Hecht H, Crocker E, et al. Integration of a clinical pharmacist practitioner-led pharmacogenomics service in a Veterans Affairs hematology/oncology clinic. Am J Health Syst Pharm. 2024;81(19):e634-e639. doi:10.1093/ajhp/zxae122
Tomcsanyi KM, Tran KA, Bates J, et al. Veterans Health Administration: implementation of pharmacogenomic clinical decision support with statin medications and the SLCO1B1 gene as an exemplar. Am J Health Syst Pharm. 2023;80(16):1082-1089. doi:10.1093/ajhp/zxad111
Gammal RS, Lee YM, Petry NJ, et al. Pharmacists leading the way to precision medicine: updates to the core pharmacist competencies in genomics. Am J Pharm Educ. 2022;86(4):8634. doi:10.5688/ajpe8634
Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi:10.1001/jamanetworkopen.2019.5345
Shaffie S, Shahbazi S. The McGraw-Hill 36-Hour Course: Lean Six Sigma. McGraw-Hill; 2012.