User login
Automated Sepsis Alert Systems
Sepsis is the most expensive condition treated in the hospital, resulting in an aggregate cost of $20.3 billion or 5.2% of total aggregate cost for all hospitalizations in the United States.[1] Rates of sepsis and sepsis‐related mortality are rising in the United States.[2, 3] Timely treatment of sepsis, including adequate fluid resuscitation and appropriate antibiotic administration, decreases morbidity, mortality, and costs.[4, 5, 6] Consequently, the Surviving Sepsis Campaign recommends timely care with the implementation of sepsis bundles and protocols.[4] Though effective, sepsis protocols require dedicated personnel with specialized training, who must be highly vigilant and constantly monitor a patient's condition for the course of an entire hospitalization.[7, 8] As such, delays in administering evidence‐based therapies are common.[8, 9]
Automated electronic sepsis alerts are being developed and implemented to facilitate the delivery of timely sepsis care. Electronic alert systems synthesize electronic health data routinely collected for clinical purposes in real time or near real time to automatically identify sepsis based on prespecified diagnostic criteria, and immediately alert providers that their patient may meet sepsis criteria via electronic notifications (eg, through electronic health record [EHR], e‐mail, or pager alerts).
However, little data exist to describe whether automated, electronic systems achieve their intended goal of earlier, more effective sepsis care. To examine this question, we performed a systematic review on automated electronic sepsis alerts to assess their suitability for clinical use. Our 2 objectives were: (1) to describe the diagnostic accuracy of alert systems in identifying sepsis using electronic data available in real‐time or near real‐time, and (2) to evaluate the effectiveness of sepsis alert systems on sepsis care process measures and clinical outcomes.
MATERIALS AND METHODS
Data Sources and Search Strategies
We searched PubMed MEDLINE, Embase, The Cochrane Library, and the Cumulative Index to Nursing and Allied Health Literature from database inception through June 27, 2014, for all studies that contained the following 3 concepts: sepsis, electronic systems, and alerts (or identification). All citations were imported into an electronic database (EndNote X5; Thomson‐Reuters Corp., New York, NY) (see Supporting Information, Appendix, in the online version of this article for our complete search strategy).
Study Selection
Two authors (A.N.M. and O.K.N.) reviewed the citation titles, abstracts, and full‐text articles of potentially relevant references identified from the literature search for eligibility. References of selected articles were hand searched to identify additional eligible studies. Inclusion criteria for eligible studies were: (1) adult patients (aged 18 years) receiving care either in the emergency department or hospital, (2) outcomes of interest including diagnostic accuracy in identification of sepsis, and/or effectiveness of sepsis alerts on process measures and clinical outcomes evaluated using empiric data, and (3) sepsis alert systems used real time or near real time electronically available data to enable proactive, timely management. We excluded studies that: (1) tested the effect of other electronic interventions that were not sepsis alerts (ie, computerized order sets) for sepsis management; (2) studies solely focused on detecting and treating central line‐associated bloodstream infections, shock (not otherwise specified), bacteremia, or other device‐related infections; and (3) studies evaluating the effectiveness of sepsis alerts without a control group.
Data Extraction and Quality Assessment
Two reviewers (A.N.M. and O.K.N.) extracted data on the clinical setting, study design, dates of enrollment, definition of sepsis, details of the identification and alert systems, diagnostic accuracy of the alert system, and the incidence of process measures and clinical outcomes using a standardized form. Discrepancies between reviewers were resolved by discussion and consensus. Data discrepancies identified in 1 study were resolved by contacting the corresponding author.[10]
For studies assessing the diagnostic accuracy of sepsis identification, study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies revised tool.[11] For studies evaluating the effectiveness of sepsis alert systems, studies were considered high quality if a contemporaneous control group was present to account for temporal trends (eg, randomized controlled trial or observational analysis with a concurrent control). Fair‐quality studies were before‐and‐after studies that adjusted for potential confounders between time periods. Low‐quality studies included those that did not account for temporal trends, such as before‐and‐after studies using only historical controls without adjustment. Studies that did not use an intention‐to‐treat analysis were also considered low quality. The strength of the overall body of evidence, including risk of bias, was guided by the Grading of Recommendations Assessment, Development, and Evaluation Working Group Criteria adapted by the Agency of Healthcare Research and Quality.[12]
Data Synthesis
To analyze the diagnostic accuracy of automated sepsis alert systems to identify sepsis and to evaluate the effect on outcomes, we performed a qualitative assessment of all studies. We were unable to perform a meta‐analysis due to significant heterogeneity in study quality, clinical setting, and definition of the sepsis alert. Diagnostic accuracy of sepsis identification was measured by sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio (LR). Effectiveness was assessed by changes in sepsis care process measures (ie, time to antibiotics) and outcomes (length of stay, mortality).
RESULTS
Description of Studies
Of 1293 titles, 183 qualified for abstract review, 84 for full‐text review, and 8 articles met our inclusion criteria (see Supporting Figure in the online version of this article). Five articles evaluated the diagnostic accuracy of sepsis identification,[10, 13, 14, 15, 16] and 5 articles[10, 14, 17, 18, 19] evaluated the effectiveness of automated electronic sepsis alerts on sepsis process measures and patient outcomes. All articles were published between 2009 and 2014 and were single‐site studies conducted at academic medical centers (Tables 1 and 2). The clinical settings in the included studies varied and included the emergency department (ED), hospital wards, and the intensive care unit (ICU).
| Source | Site No./Type | Setting | Alert Threshold | Gold Standard Definition | Gold Standard Measurement | No. | Study Qualitya |
|---|---|---|---|---|---|---|---|
| |||||||
| Hooper et al., 201210 | 1/academic | MICU | 2 SIRS criteriab | Reviewer judgment, not otherwise specified | Chart review | 560 | High |
| Meurer et al., 200913 | 1/academic | ED | 2 SIRS criteria | Reviewer judgment whether diagnosis of infection present in ED plus SIRS criteria | Chart review | 248 | Low |
| Nelson J. et al., 201114 | 1/academic | ED | 2 SIRS criteria and 2 SBP measurements 90 mm Hg | Reviewer judgment whether infection present, requiring hospitalization with at least 1 organ system involved | Chart review | 1,386 | High |
| Nguyen et al., 201415 | 1/academic | ED | 2 SIRS criteria and 1 sign of shock (SBP 90 mm Hg or lactic acid 2.0 mmol/L) | Reviewer judgment to confirm SIRS, shock, and presence of a serious infection | Chart review | 1,095 | Low |
| Thiel et al., 201016 | 1/academic | Wards | Recursive partitioning tree analysis including vitals and laboratory resultsc | Admitted to the hospital wards and subsequently transferred to the ICU for septic shock and treated with vasopressor therapy | ICD‐9 discharge codes for acute infection, acute organ dysfunction, and need for vasopressors within 24 hours of ICU transfer | 27,674 | Low |
| Source | Design | Site No./ Type | Setting | No. | Alert System Type | Alert Threshold | Alert Notificationa | Treatment Recommendation | Study Qualityb |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Berger et al., 201017 | Before‐after (6 months pre and 6 months post) | 1/academic | ED | 5796c | CPOE system | 2 SIRS criteria | CPOE passive alert | Yes: lactate collection | Low |
| Hooper et al., 201210 | RCT | 1/academic | MICU | 443 | EHR | 2 SIRS criteriad | Text page and EHR passive alert | No | High |
| McRee et al., 201418 | Before‐after (6 months pre and 6 months post) | 1/academic | Wards | 171e | EHR | 2 SIRS criteria | Notified nurse, specifics unclear | No, but the nurse completed a sepsis risk evaluation flow sheet | Low |
| Nelson et al., 201114 | Before‐after (3 months pre and 3 months post) | 1/academic | ED | 184f | EHR | 2 SIRS criteria and 2 or more SBP readings 90 mm Hg | Text page and EHR passive alert | Yes: fluid resuscitation, blood culture collection, antibiotic administration, among others | Low |
| Sawyer et al., 201119 | Prospective, nonrandomized (2 intervention and 4 control wards) | 1/academic | Wards | 300 | EHR | Recursive partitioning regression tree algorithm including vitals and lab valuesg | Text page to charge nurse who then assessed patient and informed treating physicianh | No | High |
Among the 8 included studies, there was significant heterogeneity in threshold criteria for sepsis identification and subsequent alert activation. The most commonly defined threshold was the presence of 2 or more systemic inflammatory response syndrome (SIRS) criteria.[10, 13, 17, 18]
Diagnostic Accuracy of Automated Electronic Sepsis Alert Systems
The prevalence of sepsis varied substantially between the studies depending on the gold standard definition of sepsis used and the clinical setting (ED, wards, or ICU) of the study (Table 3). The 2 studies[14, 16] that defined sepsis as requiring evidence of shock had a substantially lower prevalence (0.8%4.7%) compared to the 2 studies[10, 13] that defined sepsis as having only 2 or more SIRS criteria with a presumed diagnosis of an infection (27.8%32.5%).
| Source | Setting | Alert Threshold | Prevalence, % | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | LR+, (95% CI) | LR, (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Hooper et al., 201210 | MICU | 2 SIRS criteriaa | 36.3 | 98.9 (95.799.8) | 18.1 (14.222.9) | 40.7 (36.145.5) | 96.7 (87.599.4) | 1.21 (1.14‐1.27) | 0.06 (0.01‐0.25) |
| Meurer et al., 200913 | ED | 2 SIRS criteria | 27.8 | 36.2 (25.348.8) | 79.9 (73.185.3) | 41.0 (28.854.3) | 76.5 (69.682.2) | 1.80 (1.17‐2.76) | 0.80 (0.67‐0.96) |
| Nelson et al., 201114 | ED | 2 SIRS criteria and 2 SBP measurements90 mm Hg | 0.8 | 63.6 (31.687.8) | 99.6 (99.099.8) | 53.8 (26.179.6) | 99.7 (99.299.9) | 145.8 (58.4364.1) | 0.37 (0.17‐0.80) |
| Nguyen et al., 201415 | ED | 2 SIRS criteria and 1 sign of shock (SBP 90 mm Hg or lactic acid 2.0 mmol/L) | Unable to estimateb | Unable to estimateb | Unable to estimateb | 44.7 (41.248.2) | 100.0c (98.8100.0) | Unable to estimateb | Unable to estimateb |
| Thiel et al., 201016 | Wards | Recursive partitioning tree analysis including vitals and laboratory resultsd | 4.7 | 17.1 (15.119.3) | 96.7 (96.596.9) | 20.5 (18.223.0) | 95.9 (95.796.2) | 5.22 (4.56‐5.98) | 0.86 (0.84‐0.88) |
All alert systems had suboptimal PPV (20.5%‐53.8%). The 2 studies that designed the sepsis alert to activate by SIRS criteria alone[10, 13] had a positive predictive value of 41% and a positive LR of 1.21 to 1.80. The ability to exclude the presence of sepsis varied considerably depending on the clinical setting. The study by Hooper et al.[10] that examined the alert among patients in the medical ICU appeared more effective at ruling out sepsis (NPV=96.7%; negative LR=0.06) compared to a similar alert system used by Meurer et al.[13] that studied patients in the ED (NPV=76.5%, negative LR=0.80).
There were also differences in the diagnostic accuracy of the sepsis alert systems depending on how the threshold for activating the sepsis alert was defined and applied in the study. Two studies evaluated a sepsis alert system among patients presenting to the ED at the same academic medical center.[13, 14] The alert system (Nelson et al.) that was triggered by a combination of SIRS criteria and hypotension (PPV=53.8%, LR+=145.8; NPV=99.7%, LR=0.37) outperformed the alert system (Meurer et al.) that was triggered by SIRS criteria alone (PPV=41.0%, LR+=1.80; NPV=76.5%, LR=0.80). Furthermore, the study by Meurer and colleagues evaluated the accuracy of the alert system only among patients who were hospitalized after presenting to the ED, rather than all consecutive patients presenting to the ED. This selection bias likely falsely inflated the diagnostic accuracy of the alert system used by Meurer et al., suggesting the alert system that was triggered by a combination of SIRS criteria and hypotension was comparatively even more accurate.
Two studies evaluating the diagnostic accuracy of the alert system were deemed to be high quality (Table 4). Three studies were considered low quality1 study did not include all patients in their assessment of diagnostic accuracy13; 1 study consecutively selected alert cases but randomly selected nonalert cases, greatly limiting the assessment of diagnostic accuracy15; and the other study applied a gold standard that was unlikely to correctly classify sepsis (septic shock requiring ICU transfer with vasopressor support in the first 24 hours was defined by discharge International Classification of Diseases, Ninth Revision diagnoses without chart review), with a considerable delay from the alert system trigger (alert identification was compared to the discharge diagnosis rather than physician review of real‐time data).[16]
| Study | Patient Selection | Index Test | Reference Standard | Flow and Timing |
|---|---|---|---|---|
| ||||
| Hooper et al., 201210 | +++ | +++ | ++b | +++ |
| Meurer et al., 200913 | +++ | +++ | ++b | +c |
| Nelson et al., 201114 | +++ | +++ | ++b | +++ |
| Nguyen et al., 201415 | +d | +++ | +e | +++ |
| Thiel et al., 201016 | +++ | +++ | +f | +g |
Effectiveness of Automated Electronic Sepsis Alert Systems
Characteristics of the studies evaluating the effectiveness of automated electronic sepsis alert systems are summarized in Table 2. Regarding activation of the sepsis alert, 2 studies notified the provider directly by an automated text page and a passive EHR alert (not requiring the provider to acknowledge the alert or take action),[10, 14] 1 study notified the provider by a passive electronic alert alone,[17] and 1 study only employed an automated text page.[19] Furthermore, if the sepsis alert was activated, 2 studies suggested specific clinical management decisions,[14, 17] 2 studies left clinical management decisions solely to the discretion of the treating provider,[10, 19] and 1 study assisted the diagnosis of sepsis by prompting nurses to complete a second manual sepsis risk evaluation.[18]
Table 5 summarizes the effectiveness of automated electronic sepsis alert systems. Two studies evaluating the effectiveness of the sepsis alert system were considered to be high‐quality studies based on the use of a contemporaneous control group to account for temporal trends and an intention‐to‐treat analysis.[10, 19] The 2 studies evaluating the effectiveness of a sepsis alert system in the ED were considered low quality due to before‐and‐after designs without an intention‐to‐treat analysis.[14, 17]
| Source | Outcomes Evaluated | Key Findings | Quality |
|---|---|---|---|
| |||
| Hooper et al., 201210 | Primary: time to receipt of antibiotic (new or changed) | No difference (6.1 hours for control vs 6.0 hours for intervention, P=0.95) | High |
| Secondary: sepsis‐related process measures and outcomes | No difference in amount of 6 hour IV fluid administration (964 mL vs 1,019 mL, P=0.6), collection of blood cultures (adjusted HR 1.01; 95% CI, 0.76 to 1.35), collection of lactate (adjusted HR 0.84; 95% CI, 0.54 to 1.30), ICU length of stay (3.0 vs 3.0 days, P=0.2), hospital length of stay (4.7 vs 5.7 days, P=0.08), and hospital mortality (10% for control vs 14% for intervention, P=0.3) | ||
| Sawyer et al., 201119 | Primary: sepsis‐related process measures (antibiotic escalation, IV fluids, oxygen therapy, vasopressor initiation, diagnostic testing (blood culture, CXR) within 12 hours of alert | Increases in receiving 1 measure (56% for control vs 71% for intervention, P=0.02), antibiotic escalation (24% vs 36%, P=0.04), IV fluid administration (24% vs 38%, P=0.01), and oxygen therapy (8% vs 20%, P=0.005). There was a nonsignificant increase in obtaining diagnostic tests (40% vs 52%, P=0.06) and vasopressor initiation (3% vs 6%, P=0.4) | High |
| Secondary: ICU transfer, hospital length of stay, hospital length of stay after alert, in‐hospital mortality | Similar rate of ICU transfer (23% for control vs 26% for intervention, P=0.6), hospital length of stay (7 vs 9 days, median, P=0.8), hospital length of stay after alert (5 vs 6 days, median, P=0.7), and in‐hospital mortality (12% vs 10%, P=0.7) | ||
| Berger et al., 201017 | Primary: lactate collection in ED | Increase in lactate collection in the ED (5.2% before vs 12.7% after alert implemented, absolute increase of 7.5%, 95% CI, 6.0% to 9.0%) | Low |
| Secondary: lactate collection among hospitalized patients, proportion of patients with abnormal lactate (4 mmol/L), and in‐hospital mortality among hospitalized patients | Increase in lactate collection among hospitalized patients (15.3% vs 34.2%, absolute increase of 18.9%, 95% CI, 15.0% to 22.8%); decrease in the proportion of abnormal lactate values (21.9% vs 14.8%, absolute decrease of 7.6%, 95% CI, 15.8% to 0.6%), and no significant difference in mortality (5.7% vs 5.2%, absolute decrease of 0.5%, 95% CI, 1.6% to 2.6%, P=0.6) | ||
| McRee et al., 201418 | Stage of sepsis, length of stay, mortality, discharge location | Nonsignificant decrease in stage of sepsis (34.7% with septic shock before vs 21.9% after, P>0.05); no difference in length‐of‐stay (8.5 days before vs 8.7 days after, P>0.05). Decrease in mortality (9.3% before vs 1.0% after, P0.05) and proportion of patients discharged home (25.3% before vs 49.0% after, P0.05) | Low |
| Nelson et al., 201114 | Frequency and time to completion of process measures: lactate, blood culture, CXR, and antibiotic initiation | Increases in blood culture collection (OR 2.9; 95% CI, 1.1 to 7.7) and CXR (OR 3.2; 95% CI, 1.1 to 9.5); nonsignificant increases in lactate collection (OR 1.7; 95% CI, 0.9 to 3.2) and antibiotic administration (OR 2.8; 95% CI, 0.9 to 8.3). Only blood cultures were collected in a more timely manner (median of 86 minutes before vs 81 minutes after alert implementation, P=0.03). | Low |
Neither of the 2 high‐quality studies that included a contemporaneous control found evidence for improving inpatient mortality or hospital and ICU length of stay.[10, 19] The impact of sepsis alert systems on improving process measures for sepsis management depended on the clinical setting. In a randomized controlled trial of patients admitted to a medical ICU, Hooper et al. did not find any benefit of implementing a sepsis alert system on improving intermediate outcome measures such as antibiotic escalation, fluid resuscitation, and collection of blood cultures and lactate.[10] However, in a well‐designed observational study, Sawyer et al. found significant increases in antibiotic escalation, fluid resuscitation, and diagnostic testing in patients admitted to the medical wards.[19] Both studies that evaluated the effectiveness of sepsis alert systems in the ED showed improvements in various process measures,[14, 17] but without improvement in mortality.[17] The single study that showed improvement in clinical outcomes (in‐hospital mortality and disposition location) was of low quality due to the prestudypoststudy design without adjustment for potential confounders and lack of an intention‐to‐treat analysis (only individuals with a discharge diagnosis of sepsis were included, rather than all individuals who triggered the alert).[18] Additionally, the preintervention group had a higher proportion of individuals with septic shock compared to the postintervention group, raising the possibility that the observed improvement was due to difference in severity of illness between the 2 groups rather than due to the intervention.
None of the studies included in this review explicitly reported on the potential harms (eg, excess antimicrobial use or alert fatigue) after implementation of sepsis alerts, but Hooper et al. found a nonsignificant increase in mortality, and Sawyer et al. showed a nonsignificant increase in the length of stay in the intervention group compared to the control group.[10, 19] Berger et al. showed an overall increase in the number of lactate tests performed, but with a decrease in the proportion of abnormal lactate values (21.9% vs 14.8%, absolute decrease of 7.6%, 95% confidence interval, 15.8% to 0.6%), suggesting potential overtesting in patients at low risk for septic shock. In the study by Hooper et al., 88% (442/502) of the patients in the medical intensive care unit triggered an alert, raising the concern for alert fatigue.[10] Furthermore, 3 studies did not perform intention‐to‐treat analyses; rather, they included only patients who triggered the alert and also had provider‐suspected or confirmed sepsis,[14, 17] or had a discharge diagnosis for sepsis.[18]
DISCUSSION
The use of sepsis alert systems derived from electronic health data and targeting hospitalized patients improve a subset of sepsis process of care measures, but at the cost of poor positive predictive value and no clear improvement in mortality or length of stay. There is insufficient evidence for the effectiveness of automated electronic sepsis alert systems in the emergency department.
We found considerable variability in the diagnostic accuracy of automated electronic sepsis alert systems. There was moderate evidence that alert systems designed to identify severe sepsis (eg, SIRS criteria plus measures of shock) had greater diagnostic accuracy than alert systems that detected sepsis based on SIRS criteria alone. Given that SIRS criteria are highly prevalent among hospitalized patients with noninfectious diseases,[20] sepsis alert systems triggered by standard SIRS criteria may have poorer predictive value with an increased risk of alert fatigueexcessive electronic warnings resulting in physicians disregarding clinically useful alerts.[21] The potential for alert fatigue is even greater in critical care settings. A retrospective analysis of physiological alarms in the ICU estimated on average 6 alarms per hour with only 15% of alarms considered to be clinically relevant.[22]
The fact that sepsis alert systems improve intermediate process measures among ward and ED patients but not ICU patients likely reflects differences in both the patients and the clinical settings.[23] First, patients in the ICU may already be prescribed broad spectrum antibiotics, aggressively fluid resuscitated, and have other diagnostic testing performed before the activation of a sepsis alert, so it would be less likely to see an improvement in the rates of process measures assessing initiation or escalation of therapy compared to patients treated on the wards or in the ED. The apparent lack of benefit of these systems in the ICU may merely represent a ceiling effect. Second, nurses and physicians are already vigilantly monitoring patients in the ICU for signs of clinical deterioration, so additional alert systems may be redundant. Third, patients in the ICU are connected to standard bedside monitors that continuously monitor for the presence of abnormal vital signs. An additional sepsis alert system triggered by SIRS criteria alone may be superfluous to the existing infrastructure. Fourth, the majority of patients in the ICU will trigger the sepsis alert system,[10] so there likely is a high noise‐to‐signal ratio with resultant alert fatigue.[21]
In addition to greater emphasis on alert systems of greater diagnostic accuracy and effectiveness, our review notes several important gaps that limit evidence supporting the usefulness of automated sepsis alert systems. First, there are little data to describe the optimal design of sepsis alerts[24, 25] or the frequency with which they are appropriately acted upon or dismissed. In addition, we found little data to support whether effectiveness of alert systems differed based on whether clinical decision support was included with the alert itself (eg, direct prompting with specific clinical management recommendations) or the configuration of the alert (eg, interruptive alert or informational).[24, 25] Most of the studies we reviewed employed alerts primarily targeting physicians; we found little evidence for systems that also alerted other providers (eg, nurses or rapid response teams). Few studies provided data on harms of these systems (eg, excess antimicrobial use, fluid overload due to aggressive fluid resuscitation) or how often these treatments were administered to patients who did not eventually have sepsis. Few studies employed study designs that limited biases (eg, randomized or quasiexperimental designs) or used an intention‐to‐treat approach. Studies that exclude false positive alerts in analyses could bias estimates toward making sepsis alert systems appear more effective than they actually were. Finally, although presumably, deploying automated sepsis alerts in the ED would facilitate more timely recognition and treatment, more rigorously conducted studies are needed to identify whether using these alerts in the ED are of greater value compared to the wards and ICU. Given the limited number of studies included in this review, we were unable to make strong conclusions regarding the clinical benefits and cost‐effectiveness of implementing automated sepsis alerts.
Our review has certain limitations. First, despite our extensive literature search strategy, we may have missed studies published in the grey literature or in non‐English languages. Second, there is potential publication bias given the number of abstracts that we identified addressing 1 of our prespecified research questions compared to the number of peer‐reviewed publications identified by our search strategy.
CONCLUSION
Automated electronic sepsis alert systems have promise in delivering early goal‐directed therapies to patients. However, at present, automated sepsis alerts derived from electronic health data may improve care processes but tend to have poor PPV and have not been shown to improve mortality or length of stay. Future efforts should develop and study methods for sepsis alert systems that avoid the potential for alert fatigue while improving outcomes.
Acknowledgements
The authors thank Gloria Won, MLIS, for her assistance with developing and performing the literature search strategy and wish her a long and joyous retirement.
Disclosures: Part of Dr. Makam's work on this project was completed while he was a primary care research fellow at the University of California, San Francisco, funded by a National Research Service Award (training grant T32HP19025‐07‐00). Dr. Makam is currently supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001103). Dr. Nguyen was supported by the Agency for Healthcare Research and Quality (R24HS022428‐01). Dr. Auerbach was supported by an NHLBI K24 grant (K24HL098372). Dr. Makam had full access to the data in the study and takes responsibility for the integrity of the date and accuracy of the data analysis. Study concept and design: all authors. Acquisition of data: Makam and Nguyen. Analysis and interpretation of data: all authors. Drafting of the manuscript: Makam. Critical revision of the manuscript: all authors. Statistical analysis: Makam and Nguyen. The authors have no conflicts of interest to disclose.
- , . National inpatient hospital costs: the most expensive conditions by payer, 2011: statistical brief #160. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , . Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;(62):1–8.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693.
- , . Implementation of early goal‐directed therapy for septic patients in the emergency department: a review of the literature. J Emerg Nurs. 2013;39(1):13–19.
- , , , , , . Factors influencing variability in compliance rates and clinical outcomes among three different severe sepsis bundles. Ann Pharmacother. 2007;41(6):929–936.
- , , , et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303.
- , , , et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med. 2012;40(7):2096–2101.
- , , , et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , et al. Real‐time identification of serious infection in geriatric patients using clinical information system surveillance. J Am Geriatr Soc. 2009;57(1):40–45.
- , , , . Prospective trial of real‐time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500–504.
- , , , et al. Automated electronic medical record sepsis detection in the emergency department. PeerJ. 2014;2:e343.
- , , , , , . Early prediction of septic shock in hospitalized patients. J Hosp Med. 2010;5(1):19–25.
- , , , , . A Computerized alert screening for severe sepsis in emergency department patients increases lactate testing but does not improve inpatient mortality. Appl Clin Inform. 2010;1(4):394–407.
- , , , , . The impact of an electronic medical record surveillance program on outcomes for patients with sepsis. Heart Lung. 2014;43(6):546–549.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- . The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26(suppl 1):S64–S74.
- , , , et al. Overrides of medication‐related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21(3):487–491.
- , , , , , . Intensive care unit alarms–how many do we need? Crit Care Med. 2010;38(2):451–456.
- , . How can we best use electronic data to find and treat the critically ill?*. Crit Care Med. 2012;40(7):2242–2243.
- , , , et al. Identifying best practices for clinical decision support and knowledge management in the field. Stud Health Technol Inform. 2010;160(pt 2):806–810.
- , , , et al. Best practices in clinical decision support: the case of preventive care reminders. Appl Clin Inform. 2010;1(3):331–345.
Sepsis is the most expensive condition treated in the hospital, resulting in an aggregate cost of $20.3 billion or 5.2% of total aggregate cost for all hospitalizations in the United States.[1] Rates of sepsis and sepsis‐related mortality are rising in the United States.[2, 3] Timely treatment of sepsis, including adequate fluid resuscitation and appropriate antibiotic administration, decreases morbidity, mortality, and costs.[4, 5, 6] Consequently, the Surviving Sepsis Campaign recommends timely care with the implementation of sepsis bundles and protocols.[4] Though effective, sepsis protocols require dedicated personnel with specialized training, who must be highly vigilant and constantly monitor a patient's condition for the course of an entire hospitalization.[7, 8] As such, delays in administering evidence‐based therapies are common.[8, 9]
Automated electronic sepsis alerts are being developed and implemented to facilitate the delivery of timely sepsis care. Electronic alert systems synthesize electronic health data routinely collected for clinical purposes in real time or near real time to automatically identify sepsis based on prespecified diagnostic criteria, and immediately alert providers that their patient may meet sepsis criteria via electronic notifications (eg, through electronic health record [EHR], e‐mail, or pager alerts).
However, little data exist to describe whether automated, electronic systems achieve their intended goal of earlier, more effective sepsis care. To examine this question, we performed a systematic review on automated electronic sepsis alerts to assess their suitability for clinical use. Our 2 objectives were: (1) to describe the diagnostic accuracy of alert systems in identifying sepsis using electronic data available in real‐time or near real‐time, and (2) to evaluate the effectiveness of sepsis alert systems on sepsis care process measures and clinical outcomes.
MATERIALS AND METHODS
Data Sources and Search Strategies
We searched PubMed MEDLINE, Embase, The Cochrane Library, and the Cumulative Index to Nursing and Allied Health Literature from database inception through June 27, 2014, for all studies that contained the following 3 concepts: sepsis, electronic systems, and alerts (or identification). All citations were imported into an electronic database (EndNote X5; Thomson‐Reuters Corp., New York, NY) (see Supporting Information, Appendix, in the online version of this article for our complete search strategy).
Study Selection
Two authors (A.N.M. and O.K.N.) reviewed the citation titles, abstracts, and full‐text articles of potentially relevant references identified from the literature search for eligibility. References of selected articles were hand searched to identify additional eligible studies. Inclusion criteria for eligible studies were: (1) adult patients (aged 18 years) receiving care either in the emergency department or hospital, (2) outcomes of interest including diagnostic accuracy in identification of sepsis, and/or effectiveness of sepsis alerts on process measures and clinical outcomes evaluated using empiric data, and (3) sepsis alert systems used real time or near real time electronically available data to enable proactive, timely management. We excluded studies that: (1) tested the effect of other electronic interventions that were not sepsis alerts (ie, computerized order sets) for sepsis management; (2) studies solely focused on detecting and treating central line‐associated bloodstream infections, shock (not otherwise specified), bacteremia, or other device‐related infections; and (3) studies evaluating the effectiveness of sepsis alerts without a control group.
Data Extraction and Quality Assessment
Two reviewers (A.N.M. and O.K.N.) extracted data on the clinical setting, study design, dates of enrollment, definition of sepsis, details of the identification and alert systems, diagnostic accuracy of the alert system, and the incidence of process measures and clinical outcomes using a standardized form. Discrepancies between reviewers were resolved by discussion and consensus. Data discrepancies identified in 1 study were resolved by contacting the corresponding author.[10]
For studies assessing the diagnostic accuracy of sepsis identification, study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies revised tool.[11] For studies evaluating the effectiveness of sepsis alert systems, studies were considered high quality if a contemporaneous control group was present to account for temporal trends (eg, randomized controlled trial or observational analysis with a concurrent control). Fair‐quality studies were before‐and‐after studies that adjusted for potential confounders between time periods. Low‐quality studies included those that did not account for temporal trends, such as before‐and‐after studies using only historical controls without adjustment. Studies that did not use an intention‐to‐treat analysis were also considered low quality. The strength of the overall body of evidence, including risk of bias, was guided by the Grading of Recommendations Assessment, Development, and Evaluation Working Group Criteria adapted by the Agency of Healthcare Research and Quality.[12]
Data Synthesis
To analyze the diagnostic accuracy of automated sepsis alert systems to identify sepsis and to evaluate the effect on outcomes, we performed a qualitative assessment of all studies. We were unable to perform a meta‐analysis due to significant heterogeneity in study quality, clinical setting, and definition of the sepsis alert. Diagnostic accuracy of sepsis identification was measured by sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio (LR). Effectiveness was assessed by changes in sepsis care process measures (ie, time to antibiotics) and outcomes (length of stay, mortality).
RESULTS
Description of Studies
Of 1293 titles, 183 qualified for abstract review, 84 for full‐text review, and 8 articles met our inclusion criteria (see Supporting Figure in the online version of this article). Five articles evaluated the diagnostic accuracy of sepsis identification,[10, 13, 14, 15, 16] and 5 articles[10, 14, 17, 18, 19] evaluated the effectiveness of automated electronic sepsis alerts on sepsis process measures and patient outcomes. All articles were published between 2009 and 2014 and were single‐site studies conducted at academic medical centers (Tables 1 and 2). The clinical settings in the included studies varied and included the emergency department (ED), hospital wards, and the intensive care unit (ICU).
| Source | Site No./Type | Setting | Alert Threshold | Gold Standard Definition | Gold Standard Measurement | No. | Study Qualitya |
|---|---|---|---|---|---|---|---|
| |||||||
| Hooper et al., 201210 | 1/academic | MICU | 2 SIRS criteriab | Reviewer judgment, not otherwise specified | Chart review | 560 | High |
| Meurer et al., 200913 | 1/academic | ED | 2 SIRS criteria | Reviewer judgment whether diagnosis of infection present in ED plus SIRS criteria | Chart review | 248 | Low |
| Nelson J. et al., 201114 | 1/academic | ED | 2 SIRS criteria and 2 SBP measurements 90 mm Hg | Reviewer judgment whether infection present, requiring hospitalization with at least 1 organ system involved | Chart review | 1,386 | High |
| Nguyen et al., 201415 | 1/academic | ED | 2 SIRS criteria and 1 sign of shock (SBP 90 mm Hg or lactic acid 2.0 mmol/L) | Reviewer judgment to confirm SIRS, shock, and presence of a serious infection | Chart review | 1,095 | Low |
| Thiel et al., 201016 | 1/academic | Wards | Recursive partitioning tree analysis including vitals and laboratory resultsc | Admitted to the hospital wards and subsequently transferred to the ICU for septic shock and treated with vasopressor therapy | ICD‐9 discharge codes for acute infection, acute organ dysfunction, and need for vasopressors within 24 hours of ICU transfer | 27,674 | Low |
| Source | Design | Site No./ Type | Setting | No. | Alert System Type | Alert Threshold | Alert Notificationa | Treatment Recommendation | Study Qualityb |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Berger et al., 201017 | Before‐after (6 months pre and 6 months post) | 1/academic | ED | 5796c | CPOE system | 2 SIRS criteria | CPOE passive alert | Yes: lactate collection | Low |
| Hooper et al., 201210 | RCT | 1/academic | MICU | 443 | EHR | 2 SIRS criteriad | Text page and EHR passive alert | No | High |
| McRee et al., 201418 | Before‐after (6 months pre and 6 months post) | 1/academic | Wards | 171e | EHR | 2 SIRS criteria | Notified nurse, specifics unclear | No, but the nurse completed a sepsis risk evaluation flow sheet | Low |
| Nelson et al., 201114 | Before‐after (3 months pre and 3 months post) | 1/academic | ED | 184f | EHR | 2 SIRS criteria and 2 or more SBP readings 90 mm Hg | Text page and EHR passive alert | Yes: fluid resuscitation, blood culture collection, antibiotic administration, among others | Low |
| Sawyer et al., 201119 | Prospective, nonrandomized (2 intervention and 4 control wards) | 1/academic | Wards | 300 | EHR | Recursive partitioning regression tree algorithm including vitals and lab valuesg | Text page to charge nurse who then assessed patient and informed treating physicianh | No | High |
Among the 8 included studies, there was significant heterogeneity in threshold criteria for sepsis identification and subsequent alert activation. The most commonly defined threshold was the presence of 2 or more systemic inflammatory response syndrome (SIRS) criteria.[10, 13, 17, 18]
Diagnostic Accuracy of Automated Electronic Sepsis Alert Systems
The prevalence of sepsis varied substantially between the studies depending on the gold standard definition of sepsis used and the clinical setting (ED, wards, or ICU) of the study (Table 3). The 2 studies[14, 16] that defined sepsis as requiring evidence of shock had a substantially lower prevalence (0.8%4.7%) compared to the 2 studies[10, 13] that defined sepsis as having only 2 or more SIRS criteria with a presumed diagnosis of an infection (27.8%32.5%).
| Source | Setting | Alert Threshold | Prevalence, % | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | LR+, (95% CI) | LR, (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Hooper et al., 201210 | MICU | 2 SIRS criteriaa | 36.3 | 98.9 (95.799.8) | 18.1 (14.222.9) | 40.7 (36.145.5) | 96.7 (87.599.4) | 1.21 (1.14‐1.27) | 0.06 (0.01‐0.25) |
| Meurer et al., 200913 | ED | 2 SIRS criteria | 27.8 | 36.2 (25.348.8) | 79.9 (73.185.3) | 41.0 (28.854.3) | 76.5 (69.682.2) | 1.80 (1.17‐2.76) | 0.80 (0.67‐0.96) |
| Nelson et al., 201114 | ED | 2 SIRS criteria and 2 SBP measurements90 mm Hg | 0.8 | 63.6 (31.687.8) | 99.6 (99.099.8) | 53.8 (26.179.6) | 99.7 (99.299.9) | 145.8 (58.4364.1) | 0.37 (0.17‐0.80) |
| Nguyen et al., 201415 | ED | 2 SIRS criteria and 1 sign of shock (SBP 90 mm Hg or lactic acid 2.0 mmol/L) | Unable to estimateb | Unable to estimateb | Unable to estimateb | 44.7 (41.248.2) | 100.0c (98.8100.0) | Unable to estimateb | Unable to estimateb |
| Thiel et al., 201016 | Wards | Recursive partitioning tree analysis including vitals and laboratory resultsd | 4.7 | 17.1 (15.119.3) | 96.7 (96.596.9) | 20.5 (18.223.0) | 95.9 (95.796.2) | 5.22 (4.56‐5.98) | 0.86 (0.84‐0.88) |
All alert systems had suboptimal PPV (20.5%‐53.8%). The 2 studies that designed the sepsis alert to activate by SIRS criteria alone[10, 13] had a positive predictive value of 41% and a positive LR of 1.21 to 1.80. The ability to exclude the presence of sepsis varied considerably depending on the clinical setting. The study by Hooper et al.[10] that examined the alert among patients in the medical ICU appeared more effective at ruling out sepsis (NPV=96.7%; negative LR=0.06) compared to a similar alert system used by Meurer et al.[13] that studied patients in the ED (NPV=76.5%, negative LR=0.80).
There were also differences in the diagnostic accuracy of the sepsis alert systems depending on how the threshold for activating the sepsis alert was defined and applied in the study. Two studies evaluated a sepsis alert system among patients presenting to the ED at the same academic medical center.[13, 14] The alert system (Nelson et al.) that was triggered by a combination of SIRS criteria and hypotension (PPV=53.8%, LR+=145.8; NPV=99.7%, LR=0.37) outperformed the alert system (Meurer et al.) that was triggered by SIRS criteria alone (PPV=41.0%, LR+=1.80; NPV=76.5%, LR=0.80). Furthermore, the study by Meurer and colleagues evaluated the accuracy of the alert system only among patients who were hospitalized after presenting to the ED, rather than all consecutive patients presenting to the ED. This selection bias likely falsely inflated the diagnostic accuracy of the alert system used by Meurer et al., suggesting the alert system that was triggered by a combination of SIRS criteria and hypotension was comparatively even more accurate.
Two studies evaluating the diagnostic accuracy of the alert system were deemed to be high quality (Table 4). Three studies were considered low quality1 study did not include all patients in their assessment of diagnostic accuracy13; 1 study consecutively selected alert cases but randomly selected nonalert cases, greatly limiting the assessment of diagnostic accuracy15; and the other study applied a gold standard that was unlikely to correctly classify sepsis (septic shock requiring ICU transfer with vasopressor support in the first 24 hours was defined by discharge International Classification of Diseases, Ninth Revision diagnoses without chart review), with a considerable delay from the alert system trigger (alert identification was compared to the discharge diagnosis rather than physician review of real‐time data).[16]
| Study | Patient Selection | Index Test | Reference Standard | Flow and Timing |
|---|---|---|---|---|
| ||||
| Hooper et al., 201210 | +++ | +++ | ++b | +++ |
| Meurer et al., 200913 | +++ | +++ | ++b | +c |
| Nelson et al., 201114 | +++ | +++ | ++b | +++ |
| Nguyen et al., 201415 | +d | +++ | +e | +++ |
| Thiel et al., 201016 | +++ | +++ | +f | +g |
Effectiveness of Automated Electronic Sepsis Alert Systems
Characteristics of the studies evaluating the effectiveness of automated electronic sepsis alert systems are summarized in Table 2. Regarding activation of the sepsis alert, 2 studies notified the provider directly by an automated text page and a passive EHR alert (not requiring the provider to acknowledge the alert or take action),[10, 14] 1 study notified the provider by a passive electronic alert alone,[17] and 1 study only employed an automated text page.[19] Furthermore, if the sepsis alert was activated, 2 studies suggested specific clinical management decisions,[14, 17] 2 studies left clinical management decisions solely to the discretion of the treating provider,[10, 19] and 1 study assisted the diagnosis of sepsis by prompting nurses to complete a second manual sepsis risk evaluation.[18]
Table 5 summarizes the effectiveness of automated electronic sepsis alert systems. Two studies evaluating the effectiveness of the sepsis alert system were considered to be high‐quality studies based on the use of a contemporaneous control group to account for temporal trends and an intention‐to‐treat analysis.[10, 19] The 2 studies evaluating the effectiveness of a sepsis alert system in the ED were considered low quality due to before‐and‐after designs without an intention‐to‐treat analysis.[14, 17]
| Source | Outcomes Evaluated | Key Findings | Quality |
|---|---|---|---|
| |||
| Hooper et al., 201210 | Primary: time to receipt of antibiotic (new or changed) | No difference (6.1 hours for control vs 6.0 hours for intervention, P=0.95) | High |
| Secondary: sepsis‐related process measures and outcomes | No difference in amount of 6 hour IV fluid administration (964 mL vs 1,019 mL, P=0.6), collection of blood cultures (adjusted HR 1.01; 95% CI, 0.76 to 1.35), collection of lactate (adjusted HR 0.84; 95% CI, 0.54 to 1.30), ICU length of stay (3.0 vs 3.0 days, P=0.2), hospital length of stay (4.7 vs 5.7 days, P=0.08), and hospital mortality (10% for control vs 14% for intervention, P=0.3) | ||
| Sawyer et al., 201119 | Primary: sepsis‐related process measures (antibiotic escalation, IV fluids, oxygen therapy, vasopressor initiation, diagnostic testing (blood culture, CXR) within 12 hours of alert | Increases in receiving 1 measure (56% for control vs 71% for intervention, P=0.02), antibiotic escalation (24% vs 36%, P=0.04), IV fluid administration (24% vs 38%, P=0.01), and oxygen therapy (8% vs 20%, P=0.005). There was a nonsignificant increase in obtaining diagnostic tests (40% vs 52%, P=0.06) and vasopressor initiation (3% vs 6%, P=0.4) | High |
| Secondary: ICU transfer, hospital length of stay, hospital length of stay after alert, in‐hospital mortality | Similar rate of ICU transfer (23% for control vs 26% for intervention, P=0.6), hospital length of stay (7 vs 9 days, median, P=0.8), hospital length of stay after alert (5 vs 6 days, median, P=0.7), and in‐hospital mortality (12% vs 10%, P=0.7) | ||
| Berger et al., 201017 | Primary: lactate collection in ED | Increase in lactate collection in the ED (5.2% before vs 12.7% after alert implemented, absolute increase of 7.5%, 95% CI, 6.0% to 9.0%) | Low |
| Secondary: lactate collection among hospitalized patients, proportion of patients with abnormal lactate (4 mmol/L), and in‐hospital mortality among hospitalized patients | Increase in lactate collection among hospitalized patients (15.3% vs 34.2%, absolute increase of 18.9%, 95% CI, 15.0% to 22.8%); decrease in the proportion of abnormal lactate values (21.9% vs 14.8%, absolute decrease of 7.6%, 95% CI, 15.8% to 0.6%), and no significant difference in mortality (5.7% vs 5.2%, absolute decrease of 0.5%, 95% CI, 1.6% to 2.6%, P=0.6) | ||
| McRee et al., 201418 | Stage of sepsis, length of stay, mortality, discharge location | Nonsignificant decrease in stage of sepsis (34.7% with septic shock before vs 21.9% after, P>0.05); no difference in length‐of‐stay (8.5 days before vs 8.7 days after, P>0.05). Decrease in mortality (9.3% before vs 1.0% after, P0.05) and proportion of patients discharged home (25.3% before vs 49.0% after, P0.05) | Low |
| Nelson et al., 201114 | Frequency and time to completion of process measures: lactate, blood culture, CXR, and antibiotic initiation | Increases in blood culture collection (OR 2.9; 95% CI, 1.1 to 7.7) and CXR (OR 3.2; 95% CI, 1.1 to 9.5); nonsignificant increases in lactate collection (OR 1.7; 95% CI, 0.9 to 3.2) and antibiotic administration (OR 2.8; 95% CI, 0.9 to 8.3). Only blood cultures were collected in a more timely manner (median of 86 minutes before vs 81 minutes after alert implementation, P=0.03). | Low |
Neither of the 2 high‐quality studies that included a contemporaneous control found evidence for improving inpatient mortality or hospital and ICU length of stay.[10, 19] The impact of sepsis alert systems on improving process measures for sepsis management depended on the clinical setting. In a randomized controlled trial of patients admitted to a medical ICU, Hooper et al. did not find any benefit of implementing a sepsis alert system on improving intermediate outcome measures such as antibiotic escalation, fluid resuscitation, and collection of blood cultures and lactate.[10] However, in a well‐designed observational study, Sawyer et al. found significant increases in antibiotic escalation, fluid resuscitation, and diagnostic testing in patients admitted to the medical wards.[19] Both studies that evaluated the effectiveness of sepsis alert systems in the ED showed improvements in various process measures,[14, 17] but without improvement in mortality.[17] The single study that showed improvement in clinical outcomes (in‐hospital mortality and disposition location) was of low quality due to the prestudypoststudy design without adjustment for potential confounders and lack of an intention‐to‐treat analysis (only individuals with a discharge diagnosis of sepsis were included, rather than all individuals who triggered the alert).[18] Additionally, the preintervention group had a higher proportion of individuals with septic shock compared to the postintervention group, raising the possibility that the observed improvement was due to difference in severity of illness between the 2 groups rather than due to the intervention.
None of the studies included in this review explicitly reported on the potential harms (eg, excess antimicrobial use or alert fatigue) after implementation of sepsis alerts, but Hooper et al. found a nonsignificant increase in mortality, and Sawyer et al. showed a nonsignificant increase in the length of stay in the intervention group compared to the control group.[10, 19] Berger et al. showed an overall increase in the number of lactate tests performed, but with a decrease in the proportion of abnormal lactate values (21.9% vs 14.8%, absolute decrease of 7.6%, 95% confidence interval, 15.8% to 0.6%), suggesting potential overtesting in patients at low risk for septic shock. In the study by Hooper et al., 88% (442/502) of the patients in the medical intensive care unit triggered an alert, raising the concern for alert fatigue.[10] Furthermore, 3 studies did not perform intention‐to‐treat analyses; rather, they included only patients who triggered the alert and also had provider‐suspected or confirmed sepsis,[14, 17] or had a discharge diagnosis for sepsis.[18]
DISCUSSION
The use of sepsis alert systems derived from electronic health data and targeting hospitalized patients improve a subset of sepsis process of care measures, but at the cost of poor positive predictive value and no clear improvement in mortality or length of stay. There is insufficient evidence for the effectiveness of automated electronic sepsis alert systems in the emergency department.
We found considerable variability in the diagnostic accuracy of automated electronic sepsis alert systems. There was moderate evidence that alert systems designed to identify severe sepsis (eg, SIRS criteria plus measures of shock) had greater diagnostic accuracy than alert systems that detected sepsis based on SIRS criteria alone. Given that SIRS criteria are highly prevalent among hospitalized patients with noninfectious diseases,[20] sepsis alert systems triggered by standard SIRS criteria may have poorer predictive value with an increased risk of alert fatigueexcessive electronic warnings resulting in physicians disregarding clinically useful alerts.[21] The potential for alert fatigue is even greater in critical care settings. A retrospective analysis of physiological alarms in the ICU estimated on average 6 alarms per hour with only 15% of alarms considered to be clinically relevant.[22]
The fact that sepsis alert systems improve intermediate process measures among ward and ED patients but not ICU patients likely reflects differences in both the patients and the clinical settings.[23] First, patients in the ICU may already be prescribed broad spectrum antibiotics, aggressively fluid resuscitated, and have other diagnostic testing performed before the activation of a sepsis alert, so it would be less likely to see an improvement in the rates of process measures assessing initiation or escalation of therapy compared to patients treated on the wards or in the ED. The apparent lack of benefit of these systems in the ICU may merely represent a ceiling effect. Second, nurses and physicians are already vigilantly monitoring patients in the ICU for signs of clinical deterioration, so additional alert systems may be redundant. Third, patients in the ICU are connected to standard bedside monitors that continuously monitor for the presence of abnormal vital signs. An additional sepsis alert system triggered by SIRS criteria alone may be superfluous to the existing infrastructure. Fourth, the majority of patients in the ICU will trigger the sepsis alert system,[10] so there likely is a high noise‐to‐signal ratio with resultant alert fatigue.[21]
In addition to greater emphasis on alert systems of greater diagnostic accuracy and effectiveness, our review notes several important gaps that limit evidence supporting the usefulness of automated sepsis alert systems. First, there are little data to describe the optimal design of sepsis alerts[24, 25] or the frequency with which they are appropriately acted upon or dismissed. In addition, we found little data to support whether effectiveness of alert systems differed based on whether clinical decision support was included with the alert itself (eg, direct prompting with specific clinical management recommendations) or the configuration of the alert (eg, interruptive alert or informational).[24, 25] Most of the studies we reviewed employed alerts primarily targeting physicians; we found little evidence for systems that also alerted other providers (eg, nurses or rapid response teams). Few studies provided data on harms of these systems (eg, excess antimicrobial use, fluid overload due to aggressive fluid resuscitation) or how often these treatments were administered to patients who did not eventually have sepsis. Few studies employed study designs that limited biases (eg, randomized or quasiexperimental designs) or used an intention‐to‐treat approach. Studies that exclude false positive alerts in analyses could bias estimates toward making sepsis alert systems appear more effective than they actually were. Finally, although presumably, deploying automated sepsis alerts in the ED would facilitate more timely recognition and treatment, more rigorously conducted studies are needed to identify whether using these alerts in the ED are of greater value compared to the wards and ICU. Given the limited number of studies included in this review, we were unable to make strong conclusions regarding the clinical benefits and cost‐effectiveness of implementing automated sepsis alerts.
Our review has certain limitations. First, despite our extensive literature search strategy, we may have missed studies published in the grey literature or in non‐English languages. Second, there is potential publication bias given the number of abstracts that we identified addressing 1 of our prespecified research questions compared to the number of peer‐reviewed publications identified by our search strategy.
CONCLUSION
Automated electronic sepsis alert systems have promise in delivering early goal‐directed therapies to patients. However, at present, automated sepsis alerts derived from electronic health data may improve care processes but tend to have poor PPV and have not been shown to improve mortality or length of stay. Future efforts should develop and study methods for sepsis alert systems that avoid the potential for alert fatigue while improving outcomes.
Acknowledgements
The authors thank Gloria Won, MLIS, for her assistance with developing and performing the literature search strategy and wish her a long and joyous retirement.
Disclosures: Part of Dr. Makam's work on this project was completed while he was a primary care research fellow at the University of California, San Francisco, funded by a National Research Service Award (training grant T32HP19025‐07‐00). Dr. Makam is currently supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001103). Dr. Nguyen was supported by the Agency for Healthcare Research and Quality (R24HS022428‐01). Dr. Auerbach was supported by an NHLBI K24 grant (K24HL098372). Dr. Makam had full access to the data in the study and takes responsibility for the integrity of the date and accuracy of the data analysis. Study concept and design: all authors. Acquisition of data: Makam and Nguyen. Analysis and interpretation of data: all authors. Drafting of the manuscript: Makam. Critical revision of the manuscript: all authors. Statistical analysis: Makam and Nguyen. The authors have no conflicts of interest to disclose.
Sepsis is the most expensive condition treated in the hospital, resulting in an aggregate cost of $20.3 billion or 5.2% of total aggregate cost for all hospitalizations in the United States.[1] Rates of sepsis and sepsis‐related mortality are rising in the United States.[2, 3] Timely treatment of sepsis, including adequate fluid resuscitation and appropriate antibiotic administration, decreases morbidity, mortality, and costs.[4, 5, 6] Consequently, the Surviving Sepsis Campaign recommends timely care with the implementation of sepsis bundles and protocols.[4] Though effective, sepsis protocols require dedicated personnel with specialized training, who must be highly vigilant and constantly monitor a patient's condition for the course of an entire hospitalization.[7, 8] As such, delays in administering evidence‐based therapies are common.[8, 9]
Automated electronic sepsis alerts are being developed and implemented to facilitate the delivery of timely sepsis care. Electronic alert systems synthesize electronic health data routinely collected for clinical purposes in real time or near real time to automatically identify sepsis based on prespecified diagnostic criteria, and immediately alert providers that their patient may meet sepsis criteria via electronic notifications (eg, through electronic health record [EHR], e‐mail, or pager alerts).
However, little data exist to describe whether automated, electronic systems achieve their intended goal of earlier, more effective sepsis care. To examine this question, we performed a systematic review on automated electronic sepsis alerts to assess their suitability for clinical use. Our 2 objectives were: (1) to describe the diagnostic accuracy of alert systems in identifying sepsis using electronic data available in real‐time or near real‐time, and (2) to evaluate the effectiveness of sepsis alert systems on sepsis care process measures and clinical outcomes.
MATERIALS AND METHODS
Data Sources and Search Strategies
We searched PubMed MEDLINE, Embase, The Cochrane Library, and the Cumulative Index to Nursing and Allied Health Literature from database inception through June 27, 2014, for all studies that contained the following 3 concepts: sepsis, electronic systems, and alerts (or identification). All citations were imported into an electronic database (EndNote X5; Thomson‐Reuters Corp., New York, NY) (see Supporting Information, Appendix, in the online version of this article for our complete search strategy).
Study Selection
Two authors (A.N.M. and O.K.N.) reviewed the citation titles, abstracts, and full‐text articles of potentially relevant references identified from the literature search for eligibility. References of selected articles were hand searched to identify additional eligible studies. Inclusion criteria for eligible studies were: (1) adult patients (aged 18 years) receiving care either in the emergency department or hospital, (2) outcomes of interest including diagnostic accuracy in identification of sepsis, and/or effectiveness of sepsis alerts on process measures and clinical outcomes evaluated using empiric data, and (3) sepsis alert systems used real time or near real time electronically available data to enable proactive, timely management. We excluded studies that: (1) tested the effect of other electronic interventions that were not sepsis alerts (ie, computerized order sets) for sepsis management; (2) studies solely focused on detecting and treating central line‐associated bloodstream infections, shock (not otherwise specified), bacteremia, or other device‐related infections; and (3) studies evaluating the effectiveness of sepsis alerts without a control group.
Data Extraction and Quality Assessment
Two reviewers (A.N.M. and O.K.N.) extracted data on the clinical setting, study design, dates of enrollment, definition of sepsis, details of the identification and alert systems, diagnostic accuracy of the alert system, and the incidence of process measures and clinical outcomes using a standardized form. Discrepancies between reviewers were resolved by discussion and consensus. Data discrepancies identified in 1 study were resolved by contacting the corresponding author.[10]
For studies assessing the diagnostic accuracy of sepsis identification, study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies revised tool.[11] For studies evaluating the effectiveness of sepsis alert systems, studies were considered high quality if a contemporaneous control group was present to account for temporal trends (eg, randomized controlled trial or observational analysis with a concurrent control). Fair‐quality studies were before‐and‐after studies that adjusted for potential confounders between time periods. Low‐quality studies included those that did not account for temporal trends, such as before‐and‐after studies using only historical controls without adjustment. Studies that did not use an intention‐to‐treat analysis were also considered low quality. The strength of the overall body of evidence, including risk of bias, was guided by the Grading of Recommendations Assessment, Development, and Evaluation Working Group Criteria adapted by the Agency of Healthcare Research and Quality.[12]
Data Synthesis
To analyze the diagnostic accuracy of automated sepsis alert systems to identify sepsis and to evaluate the effect on outcomes, we performed a qualitative assessment of all studies. We were unable to perform a meta‐analysis due to significant heterogeneity in study quality, clinical setting, and definition of the sepsis alert. Diagnostic accuracy of sepsis identification was measured by sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio (LR). Effectiveness was assessed by changes in sepsis care process measures (ie, time to antibiotics) and outcomes (length of stay, mortality).
RESULTS
Description of Studies
Of 1293 titles, 183 qualified for abstract review, 84 for full‐text review, and 8 articles met our inclusion criteria (see Supporting Figure in the online version of this article). Five articles evaluated the diagnostic accuracy of sepsis identification,[10, 13, 14, 15, 16] and 5 articles[10, 14, 17, 18, 19] evaluated the effectiveness of automated electronic sepsis alerts on sepsis process measures and patient outcomes. All articles were published between 2009 and 2014 and were single‐site studies conducted at academic medical centers (Tables 1 and 2). The clinical settings in the included studies varied and included the emergency department (ED), hospital wards, and the intensive care unit (ICU).
| Source | Site No./Type | Setting | Alert Threshold | Gold Standard Definition | Gold Standard Measurement | No. | Study Qualitya |
|---|---|---|---|---|---|---|---|
| |||||||
| Hooper et al., 201210 | 1/academic | MICU | 2 SIRS criteriab | Reviewer judgment, not otherwise specified | Chart review | 560 | High |
| Meurer et al., 200913 | 1/academic | ED | 2 SIRS criteria | Reviewer judgment whether diagnosis of infection present in ED plus SIRS criteria | Chart review | 248 | Low |
| Nelson J. et al., 201114 | 1/academic | ED | 2 SIRS criteria and 2 SBP measurements 90 mm Hg | Reviewer judgment whether infection present, requiring hospitalization with at least 1 organ system involved | Chart review | 1,386 | High |
| Nguyen et al., 201415 | 1/academic | ED | 2 SIRS criteria and 1 sign of shock (SBP 90 mm Hg or lactic acid 2.0 mmol/L) | Reviewer judgment to confirm SIRS, shock, and presence of a serious infection | Chart review | 1,095 | Low |
| Thiel et al., 201016 | 1/academic | Wards | Recursive partitioning tree analysis including vitals and laboratory resultsc | Admitted to the hospital wards and subsequently transferred to the ICU for septic shock and treated with vasopressor therapy | ICD‐9 discharge codes for acute infection, acute organ dysfunction, and need for vasopressors within 24 hours of ICU transfer | 27,674 | Low |
| Source | Design | Site No./ Type | Setting | No. | Alert System Type | Alert Threshold | Alert Notificationa | Treatment Recommendation | Study Qualityb |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Berger et al., 201017 | Before‐after (6 months pre and 6 months post) | 1/academic | ED | 5796c | CPOE system | 2 SIRS criteria | CPOE passive alert | Yes: lactate collection | Low |
| Hooper et al., 201210 | RCT | 1/academic | MICU | 443 | EHR | 2 SIRS criteriad | Text page and EHR passive alert | No | High |
| McRee et al., 201418 | Before‐after (6 months pre and 6 months post) | 1/academic | Wards | 171e | EHR | 2 SIRS criteria | Notified nurse, specifics unclear | No, but the nurse completed a sepsis risk evaluation flow sheet | Low |
| Nelson et al., 201114 | Before‐after (3 months pre and 3 months post) | 1/academic | ED | 184f | EHR | 2 SIRS criteria and 2 or more SBP readings 90 mm Hg | Text page and EHR passive alert | Yes: fluid resuscitation, blood culture collection, antibiotic administration, among others | Low |
| Sawyer et al., 201119 | Prospective, nonrandomized (2 intervention and 4 control wards) | 1/academic | Wards | 300 | EHR | Recursive partitioning regression tree algorithm including vitals and lab valuesg | Text page to charge nurse who then assessed patient and informed treating physicianh | No | High |
Among the 8 included studies, there was significant heterogeneity in threshold criteria for sepsis identification and subsequent alert activation. The most commonly defined threshold was the presence of 2 or more systemic inflammatory response syndrome (SIRS) criteria.[10, 13, 17, 18]
Diagnostic Accuracy of Automated Electronic Sepsis Alert Systems
The prevalence of sepsis varied substantially between the studies depending on the gold standard definition of sepsis used and the clinical setting (ED, wards, or ICU) of the study (Table 3). The 2 studies[14, 16] that defined sepsis as requiring evidence of shock had a substantially lower prevalence (0.8%4.7%) compared to the 2 studies[10, 13] that defined sepsis as having only 2 or more SIRS criteria with a presumed diagnosis of an infection (27.8%32.5%).
| Source | Setting | Alert Threshold | Prevalence, % | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | LR+, (95% CI) | LR, (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Hooper et al., 201210 | MICU | 2 SIRS criteriaa | 36.3 | 98.9 (95.799.8) | 18.1 (14.222.9) | 40.7 (36.145.5) | 96.7 (87.599.4) | 1.21 (1.14‐1.27) | 0.06 (0.01‐0.25) |
| Meurer et al., 200913 | ED | 2 SIRS criteria | 27.8 | 36.2 (25.348.8) | 79.9 (73.185.3) | 41.0 (28.854.3) | 76.5 (69.682.2) | 1.80 (1.17‐2.76) | 0.80 (0.67‐0.96) |
| Nelson et al., 201114 | ED | 2 SIRS criteria and 2 SBP measurements90 mm Hg | 0.8 | 63.6 (31.687.8) | 99.6 (99.099.8) | 53.8 (26.179.6) | 99.7 (99.299.9) | 145.8 (58.4364.1) | 0.37 (0.17‐0.80) |
| Nguyen et al., 201415 | ED | 2 SIRS criteria and 1 sign of shock (SBP 90 mm Hg or lactic acid 2.0 mmol/L) | Unable to estimateb | Unable to estimateb | Unable to estimateb | 44.7 (41.248.2) | 100.0c (98.8100.0) | Unable to estimateb | Unable to estimateb |
| Thiel et al., 201016 | Wards | Recursive partitioning tree analysis including vitals and laboratory resultsd | 4.7 | 17.1 (15.119.3) | 96.7 (96.596.9) | 20.5 (18.223.0) | 95.9 (95.796.2) | 5.22 (4.56‐5.98) | 0.86 (0.84‐0.88) |
All alert systems had suboptimal PPV (20.5%‐53.8%). The 2 studies that designed the sepsis alert to activate by SIRS criteria alone[10, 13] had a positive predictive value of 41% and a positive LR of 1.21 to 1.80. The ability to exclude the presence of sepsis varied considerably depending on the clinical setting. The study by Hooper et al.[10] that examined the alert among patients in the medical ICU appeared more effective at ruling out sepsis (NPV=96.7%; negative LR=0.06) compared to a similar alert system used by Meurer et al.[13] that studied patients in the ED (NPV=76.5%, negative LR=0.80).
There were also differences in the diagnostic accuracy of the sepsis alert systems depending on how the threshold for activating the sepsis alert was defined and applied in the study. Two studies evaluated a sepsis alert system among patients presenting to the ED at the same academic medical center.[13, 14] The alert system (Nelson et al.) that was triggered by a combination of SIRS criteria and hypotension (PPV=53.8%, LR+=145.8; NPV=99.7%, LR=0.37) outperformed the alert system (Meurer et al.) that was triggered by SIRS criteria alone (PPV=41.0%, LR+=1.80; NPV=76.5%, LR=0.80). Furthermore, the study by Meurer and colleagues evaluated the accuracy of the alert system only among patients who were hospitalized after presenting to the ED, rather than all consecutive patients presenting to the ED. This selection bias likely falsely inflated the diagnostic accuracy of the alert system used by Meurer et al., suggesting the alert system that was triggered by a combination of SIRS criteria and hypotension was comparatively even more accurate.
Two studies evaluating the diagnostic accuracy of the alert system were deemed to be high quality (Table 4). Three studies were considered low quality1 study did not include all patients in their assessment of diagnostic accuracy13; 1 study consecutively selected alert cases but randomly selected nonalert cases, greatly limiting the assessment of diagnostic accuracy15; and the other study applied a gold standard that was unlikely to correctly classify sepsis (septic shock requiring ICU transfer with vasopressor support in the first 24 hours was defined by discharge International Classification of Diseases, Ninth Revision diagnoses without chart review), with a considerable delay from the alert system trigger (alert identification was compared to the discharge diagnosis rather than physician review of real‐time data).[16]
| Study | Patient Selection | Index Test | Reference Standard | Flow and Timing |
|---|---|---|---|---|
| ||||
| Hooper et al., 201210 | +++ | +++ | ++b | +++ |
| Meurer et al., 200913 | +++ | +++ | ++b | +c |
| Nelson et al., 201114 | +++ | +++ | ++b | +++ |
| Nguyen et al., 201415 | +d | +++ | +e | +++ |
| Thiel et al., 201016 | +++ | +++ | +f | +g |
Effectiveness of Automated Electronic Sepsis Alert Systems
Characteristics of the studies evaluating the effectiveness of automated electronic sepsis alert systems are summarized in Table 2. Regarding activation of the sepsis alert, 2 studies notified the provider directly by an automated text page and a passive EHR alert (not requiring the provider to acknowledge the alert or take action),[10, 14] 1 study notified the provider by a passive electronic alert alone,[17] and 1 study only employed an automated text page.[19] Furthermore, if the sepsis alert was activated, 2 studies suggested specific clinical management decisions,[14, 17] 2 studies left clinical management decisions solely to the discretion of the treating provider,[10, 19] and 1 study assisted the diagnosis of sepsis by prompting nurses to complete a second manual sepsis risk evaluation.[18]
Table 5 summarizes the effectiveness of automated electronic sepsis alert systems. Two studies evaluating the effectiveness of the sepsis alert system were considered to be high‐quality studies based on the use of a contemporaneous control group to account for temporal trends and an intention‐to‐treat analysis.[10, 19] The 2 studies evaluating the effectiveness of a sepsis alert system in the ED were considered low quality due to before‐and‐after designs without an intention‐to‐treat analysis.[14, 17]
| Source | Outcomes Evaluated | Key Findings | Quality |
|---|---|---|---|
| |||
| Hooper et al., 201210 | Primary: time to receipt of antibiotic (new or changed) | No difference (6.1 hours for control vs 6.0 hours for intervention, P=0.95) | High |
| Secondary: sepsis‐related process measures and outcomes | No difference in amount of 6 hour IV fluid administration (964 mL vs 1,019 mL, P=0.6), collection of blood cultures (adjusted HR 1.01; 95% CI, 0.76 to 1.35), collection of lactate (adjusted HR 0.84; 95% CI, 0.54 to 1.30), ICU length of stay (3.0 vs 3.0 days, P=0.2), hospital length of stay (4.7 vs 5.7 days, P=0.08), and hospital mortality (10% for control vs 14% for intervention, P=0.3) | ||
| Sawyer et al., 201119 | Primary: sepsis‐related process measures (antibiotic escalation, IV fluids, oxygen therapy, vasopressor initiation, diagnostic testing (blood culture, CXR) within 12 hours of alert | Increases in receiving 1 measure (56% for control vs 71% for intervention, P=0.02), antibiotic escalation (24% vs 36%, P=0.04), IV fluid administration (24% vs 38%, P=0.01), and oxygen therapy (8% vs 20%, P=0.005). There was a nonsignificant increase in obtaining diagnostic tests (40% vs 52%, P=0.06) and vasopressor initiation (3% vs 6%, P=0.4) | High |
| Secondary: ICU transfer, hospital length of stay, hospital length of stay after alert, in‐hospital mortality | Similar rate of ICU transfer (23% for control vs 26% for intervention, P=0.6), hospital length of stay (7 vs 9 days, median, P=0.8), hospital length of stay after alert (5 vs 6 days, median, P=0.7), and in‐hospital mortality (12% vs 10%, P=0.7) | ||
| Berger et al., 201017 | Primary: lactate collection in ED | Increase in lactate collection in the ED (5.2% before vs 12.7% after alert implemented, absolute increase of 7.5%, 95% CI, 6.0% to 9.0%) | Low |
| Secondary: lactate collection among hospitalized patients, proportion of patients with abnormal lactate (4 mmol/L), and in‐hospital mortality among hospitalized patients | Increase in lactate collection among hospitalized patients (15.3% vs 34.2%, absolute increase of 18.9%, 95% CI, 15.0% to 22.8%); decrease in the proportion of abnormal lactate values (21.9% vs 14.8%, absolute decrease of 7.6%, 95% CI, 15.8% to 0.6%), and no significant difference in mortality (5.7% vs 5.2%, absolute decrease of 0.5%, 95% CI, 1.6% to 2.6%, P=0.6) | ||
| McRee et al., 201418 | Stage of sepsis, length of stay, mortality, discharge location | Nonsignificant decrease in stage of sepsis (34.7% with septic shock before vs 21.9% after, P>0.05); no difference in length‐of‐stay (8.5 days before vs 8.7 days after, P>0.05). Decrease in mortality (9.3% before vs 1.0% after, P0.05) and proportion of patients discharged home (25.3% before vs 49.0% after, P0.05) | Low |
| Nelson et al., 201114 | Frequency and time to completion of process measures: lactate, blood culture, CXR, and antibiotic initiation | Increases in blood culture collection (OR 2.9; 95% CI, 1.1 to 7.7) and CXR (OR 3.2; 95% CI, 1.1 to 9.5); nonsignificant increases in lactate collection (OR 1.7; 95% CI, 0.9 to 3.2) and antibiotic administration (OR 2.8; 95% CI, 0.9 to 8.3). Only blood cultures were collected in a more timely manner (median of 86 minutes before vs 81 minutes after alert implementation, P=0.03). | Low |
Neither of the 2 high‐quality studies that included a contemporaneous control found evidence for improving inpatient mortality or hospital and ICU length of stay.[10, 19] The impact of sepsis alert systems on improving process measures for sepsis management depended on the clinical setting. In a randomized controlled trial of patients admitted to a medical ICU, Hooper et al. did not find any benefit of implementing a sepsis alert system on improving intermediate outcome measures such as antibiotic escalation, fluid resuscitation, and collection of blood cultures and lactate.[10] However, in a well‐designed observational study, Sawyer et al. found significant increases in antibiotic escalation, fluid resuscitation, and diagnostic testing in patients admitted to the medical wards.[19] Both studies that evaluated the effectiveness of sepsis alert systems in the ED showed improvements in various process measures,[14, 17] but without improvement in mortality.[17] The single study that showed improvement in clinical outcomes (in‐hospital mortality and disposition location) was of low quality due to the prestudypoststudy design without adjustment for potential confounders and lack of an intention‐to‐treat analysis (only individuals with a discharge diagnosis of sepsis were included, rather than all individuals who triggered the alert).[18] Additionally, the preintervention group had a higher proportion of individuals with septic shock compared to the postintervention group, raising the possibility that the observed improvement was due to difference in severity of illness between the 2 groups rather than due to the intervention.
None of the studies included in this review explicitly reported on the potential harms (eg, excess antimicrobial use or alert fatigue) after implementation of sepsis alerts, but Hooper et al. found a nonsignificant increase in mortality, and Sawyer et al. showed a nonsignificant increase in the length of stay in the intervention group compared to the control group.[10, 19] Berger et al. showed an overall increase in the number of lactate tests performed, but with a decrease in the proportion of abnormal lactate values (21.9% vs 14.8%, absolute decrease of 7.6%, 95% confidence interval, 15.8% to 0.6%), suggesting potential overtesting in patients at low risk for septic shock. In the study by Hooper et al., 88% (442/502) of the patients in the medical intensive care unit triggered an alert, raising the concern for alert fatigue.[10] Furthermore, 3 studies did not perform intention‐to‐treat analyses; rather, they included only patients who triggered the alert and also had provider‐suspected or confirmed sepsis,[14, 17] or had a discharge diagnosis for sepsis.[18]
DISCUSSION
The use of sepsis alert systems derived from electronic health data and targeting hospitalized patients improve a subset of sepsis process of care measures, but at the cost of poor positive predictive value and no clear improvement in mortality or length of stay. There is insufficient evidence for the effectiveness of automated electronic sepsis alert systems in the emergency department.
We found considerable variability in the diagnostic accuracy of automated electronic sepsis alert systems. There was moderate evidence that alert systems designed to identify severe sepsis (eg, SIRS criteria plus measures of shock) had greater diagnostic accuracy than alert systems that detected sepsis based on SIRS criteria alone. Given that SIRS criteria are highly prevalent among hospitalized patients with noninfectious diseases,[20] sepsis alert systems triggered by standard SIRS criteria may have poorer predictive value with an increased risk of alert fatigueexcessive electronic warnings resulting in physicians disregarding clinically useful alerts.[21] The potential for alert fatigue is even greater in critical care settings. A retrospective analysis of physiological alarms in the ICU estimated on average 6 alarms per hour with only 15% of alarms considered to be clinically relevant.[22]
The fact that sepsis alert systems improve intermediate process measures among ward and ED patients but not ICU patients likely reflects differences in both the patients and the clinical settings.[23] First, patients in the ICU may already be prescribed broad spectrum antibiotics, aggressively fluid resuscitated, and have other diagnostic testing performed before the activation of a sepsis alert, so it would be less likely to see an improvement in the rates of process measures assessing initiation or escalation of therapy compared to patients treated on the wards or in the ED. The apparent lack of benefit of these systems in the ICU may merely represent a ceiling effect. Second, nurses and physicians are already vigilantly monitoring patients in the ICU for signs of clinical deterioration, so additional alert systems may be redundant. Third, patients in the ICU are connected to standard bedside monitors that continuously monitor for the presence of abnormal vital signs. An additional sepsis alert system triggered by SIRS criteria alone may be superfluous to the existing infrastructure. Fourth, the majority of patients in the ICU will trigger the sepsis alert system,[10] so there likely is a high noise‐to‐signal ratio with resultant alert fatigue.[21]
In addition to greater emphasis on alert systems of greater diagnostic accuracy and effectiveness, our review notes several important gaps that limit evidence supporting the usefulness of automated sepsis alert systems. First, there are little data to describe the optimal design of sepsis alerts[24, 25] or the frequency with which they are appropriately acted upon or dismissed. In addition, we found little data to support whether effectiveness of alert systems differed based on whether clinical decision support was included with the alert itself (eg, direct prompting with specific clinical management recommendations) or the configuration of the alert (eg, interruptive alert or informational).[24, 25] Most of the studies we reviewed employed alerts primarily targeting physicians; we found little evidence for systems that also alerted other providers (eg, nurses or rapid response teams). Few studies provided data on harms of these systems (eg, excess antimicrobial use, fluid overload due to aggressive fluid resuscitation) or how often these treatments were administered to patients who did not eventually have sepsis. Few studies employed study designs that limited biases (eg, randomized or quasiexperimental designs) or used an intention‐to‐treat approach. Studies that exclude false positive alerts in analyses could bias estimates toward making sepsis alert systems appear more effective than they actually were. Finally, although presumably, deploying automated sepsis alerts in the ED would facilitate more timely recognition and treatment, more rigorously conducted studies are needed to identify whether using these alerts in the ED are of greater value compared to the wards and ICU. Given the limited number of studies included in this review, we were unable to make strong conclusions regarding the clinical benefits and cost‐effectiveness of implementing automated sepsis alerts.
Our review has certain limitations. First, despite our extensive literature search strategy, we may have missed studies published in the grey literature or in non‐English languages. Second, there is potential publication bias given the number of abstracts that we identified addressing 1 of our prespecified research questions compared to the number of peer‐reviewed publications identified by our search strategy.
CONCLUSION
Automated electronic sepsis alert systems have promise in delivering early goal‐directed therapies to patients. However, at present, automated sepsis alerts derived from electronic health data may improve care processes but tend to have poor PPV and have not been shown to improve mortality or length of stay. Future efforts should develop and study methods for sepsis alert systems that avoid the potential for alert fatigue while improving outcomes.
Acknowledgements
The authors thank Gloria Won, MLIS, for her assistance with developing and performing the literature search strategy and wish her a long and joyous retirement.
Disclosures: Part of Dr. Makam's work on this project was completed while he was a primary care research fellow at the University of California, San Francisco, funded by a National Research Service Award (training grant T32HP19025‐07‐00). Dr. Makam is currently supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001103). Dr. Nguyen was supported by the Agency for Healthcare Research and Quality (R24HS022428‐01). Dr. Auerbach was supported by an NHLBI K24 grant (K24HL098372). Dr. Makam had full access to the data in the study and takes responsibility for the integrity of the date and accuracy of the data analysis. Study concept and design: all authors. Acquisition of data: Makam and Nguyen. Analysis and interpretation of data: all authors. Drafting of the manuscript: Makam. Critical revision of the manuscript: all authors. Statistical analysis: Makam and Nguyen. The authors have no conflicts of interest to disclose.
- , . National inpatient hospital costs: the most expensive conditions by payer, 2011: statistical brief #160. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , . Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;(62):1–8.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693.
- , . Implementation of early goal‐directed therapy for septic patients in the emergency department: a review of the literature. J Emerg Nurs. 2013;39(1):13–19.
- , , , , , . Factors influencing variability in compliance rates and clinical outcomes among three different severe sepsis bundles. Ann Pharmacother. 2007;41(6):929–936.
- , , , et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303.
- , , , et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med. 2012;40(7):2096–2101.
- , , , et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , et al. Real‐time identification of serious infection in geriatric patients using clinical information system surveillance. J Am Geriatr Soc. 2009;57(1):40–45.
- , , , . Prospective trial of real‐time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500–504.
- , , , et al. Automated electronic medical record sepsis detection in the emergency department. PeerJ. 2014;2:e343.
- , , , , , . Early prediction of septic shock in hospitalized patients. J Hosp Med. 2010;5(1):19–25.
- , , , , . A Computerized alert screening for severe sepsis in emergency department patients increases lactate testing but does not improve inpatient mortality. Appl Clin Inform. 2010;1(4):394–407.
- , , , , . The impact of an electronic medical record surveillance program on outcomes for patients with sepsis. Heart Lung. 2014;43(6):546–549.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- . The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26(suppl 1):S64–S74.
- , , , et al. Overrides of medication‐related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21(3):487–491.
- , , , , , . Intensive care unit alarms–how many do we need? Crit Care Med. 2010;38(2):451–456.
- , . How can we best use electronic data to find and treat the critically ill?*. Crit Care Med. 2012;40(7):2242–2243.
- , , , et al. Identifying best practices for clinical decision support and knowledge management in the field. Stud Health Technol Inform. 2010;160(pt 2):806–810.
- , , , et al. Best practices in clinical decision support: the case of preventive care reminders. Appl Clin Inform. 2010;1(3):331–345.
- , . National inpatient hospital costs: the most expensive conditions by payer, 2011: statistical brief #160. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , . Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;(62):1–8.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693.
- , . Implementation of early goal‐directed therapy for septic patients in the emergency department: a review of the literature. J Emerg Nurs. 2013;39(1):13–19.
- , , , , , . Factors influencing variability in compliance rates and clinical outcomes among three different severe sepsis bundles. Ann Pharmacother. 2007;41(6):929–936.
- , , , et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303.
- , , , et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med. 2012;40(7):2096–2101.
- , , , et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , et al. Real‐time identification of serious infection in geriatric patients using clinical information system surveillance. J Am Geriatr Soc. 2009;57(1):40–45.
- , , , . Prospective trial of real‐time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500–504.
- , , , et al. Automated electronic medical record sepsis detection in the emergency department. PeerJ. 2014;2:e343.
- , , , , , . Early prediction of septic shock in hospitalized patients. J Hosp Med. 2010;5(1):19–25.
- , , , , . A Computerized alert screening for severe sepsis in emergency department patients increases lactate testing but does not improve inpatient mortality. Appl Clin Inform. 2010;1(4):394–407.
- , , , , . The impact of an electronic medical record surveillance program on outcomes for patients with sepsis. Heart Lung. 2014;43(6):546–549.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- . The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26(suppl 1):S64–S74.
- , , , et al. Overrides of medication‐related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21(3):487–491.
- , , , , , . Intensive care unit alarms–how many do we need? Crit Care Med. 2010;38(2):451–456.
- , . How can we best use electronic data to find and treat the critically ill?*. Crit Care Med. 2012;40(7):2242–2243.
- , , , et al. Identifying best practices for clinical decision support and knowledge management in the field. Stud Health Technol Inform. 2010;160(pt 2):806–810.
- , , , et al. Best practices in clinical decision support: the case of preventive care reminders. Appl Clin Inform. 2010;1(3):331–345.
Medication Reconciliation Perspectives
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P0.001), or educate patients on the postdischarge medication regimen (P0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P0.001), or educate patients on the postdischarge medication regimen (P0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P0.001), or educate patients on the postdischarge medication regimen (P0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
Hospitalists and Liability
In this issue of the Journal of Hospital Medicine, Schaffer and colleagues report their analysis of malpractice claims against hospitalists compared to other physician specialties.[1] In contrast to previous work examining medical liability,[2, 3] Schaffer and colleagues have been able to identify hospitalists specifically.[2, 3]
Perhaps surprisingly, their main finding was that the rate of claims against hospitalists was significantly lower than for nonhospitalist internists, emergency medicine physicians, general surgeons, and obstetriciansgynecologists. We say surprisingly, because health systems utilizing hospitalists generally include features that might increase the risk for malpractice claims.
For example, new patients are typically assigned to whichever hospitalist is up for the next admission. Research shows that strained patientphysician relationships increase the risk for malpractice claims.[4, 5] Schaffer's data suggest that lack of a preexisting relationship is a challenge, but one to which most hospitalists have grown accustomed. Hospitalists develop and hone skills that allow them to quickly establish rapport with patients and families. Moreover, though patients seldom choose their hospitalist, they often do select the hospital in which they receive their care. The research group of 1 of the authors was recently surprised to find patients had high levels of trust with their hospital physicians, despite frequently being unable to name them or identify their role.[6] We suspect patients in the study had high levels of trust with the hospital and transferred this trust to their assigned physicians as representatives of the organization. Certainly, this hypothesis needs to be tested, and in no way does it minimize the importance of a strong patient‐physician relationship.
In addition, patientphysician continuity has long been felt to be paramount to safe and effective care; however, it is difficult to achieve in hospitalist systems. Hospitalized patients experience multiple handoffs, including those at admission, for night coverage, and at the time of service change (ie, end of rotation/stint). The potential for loss of information is enormous. Though increased attention has been dedicated to handoffs among housestaff, little work has been done to describe issues related to handoffs among practicing physicians. However, some discontinuity may be advantageous. A physician newly taking over patient care from another may not be anchored to the initial diagnosis and treatment plan established by the first. This free second look may actually prevent missed/delayed diagnoses and optimize plans of care, further reducing harm from care and risk of malpractice.[7]
Hospital discharge is another highly risky time, due to issues such as tests pending at the time of discharge and the need to manage ongoing workup and treatment of unresolved issues.[8, 9] The responsibility for tying up these loose ends may be unclear as patients are transitioned from the care of hospitalists to outpatient physicians. Prior research has shown that patients are at particularly high risk for preventable adverse events after hospital discharge.[10, 11] More recently, healthcare policy has focused on measuring and incentivizing the reduction of readmissions.[12] Although only a portion of readmissions are truly preventable,[13] and many patients who suffer adverse events after discharge are not readmitted,[11] the efforts resulting from these policy initiatives may have improved the overall safety of transitions of care.
A particularly important contribution of Schaffer and colleagues' study is that it helps us identify patient safety issues related to hospital medicine. Despite intense national efforts over the past 10 to 15 years, progress has been slow in reducing the rate of adverse events among hospitalized patients.[14, 15, 16] Although adverse events and medical liability do not always correlate,[17, 18] the contributing factors identified in Schaffer and colleagues' study help direct our patient safety efforts.
Clinical judgment was the most common factor associated with hospitalist malpractice claims, with examples including failure or delay in ordering a necessary diagnostic test or specialist consultation. These results may be misinterpreted by some to suggest that ordering more tests and services may reduce risk for malpractice claims. However, there is no evidence to support the belief that these defensive medicine behaviors actually reduce risk. In fact, the opposite may be true. Research shows that abnormal tests are frequently overlooked,[9, 19] and failure to act on abnormal results is a common cause of diagnostic error.[20] Experts have called for the development of diagnosis‐related quality measures and better strategies to enhance trainees' clinical reasoning skills.[21] We suggest that future research also clarify the effect of interruptions, distractions, and workload on cognitive errors in hospital settings.
Communication failures were the second most common contributing factor. As previously mentioned, communication failures may occur between hospitalists during handoffs. We also have major opportunities to improve interprofessional teamwork, especially between physicians and nurses.[22, 23] An increasing number of hospitalist groups are collaborating with other hospital‐based professionals to implement novel strategies to improve teamwork,[24, 25] many of which were recently summarized in a review published in this journal.[26]
Documentation was the third most common contributing factor. Most malpractice claims are filed long after the alleged injury has occurred.[18] Unless the clinicians involved and the hospital in which they work are aware of an event that might result in a malpractice claim, the investigation may be severely delayed. As time goes on, professionals are less able to recall details pertinent to understanding contributing factors to an event. Thus, documentation is critical. As the saying goes, if it wasn't documented, it didn't happen. The flipside of too little documentation is, of course, too much. The increasing use of electronic health records makes it easy to copy and paste outdated information, the sloppiness of which can only hurt when attempting to defend a malpractice claim.[27]
In conclusion, despite a model with inherent features that might contribute to medical malpractice risk, hospital medicine has a claim rate lower than many other specialties. Though reassuring, hospitalists should remember that the most productive way to approach malpractice risk is reframe the problem as one that attempts to reduce risk for patients, rather than for physicians. Improving patient safety is a core value for hospital medicine. Schaffer and colleagues' study identifies factors contributing to patient safety risk in hospital medicine, allowing us to renew our efforts in focused areas.
- , , , . Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750–755.
- , , . Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305(23):2427–2431.
- , , , . Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629–636.
- , , , . The doctor‐patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365–1370.
- , , , , . Physician‐patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553–559.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175(1):5.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305–1311.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- U.S. Department of Health 183(7):E391–E402.
- U.S. Department of Health 363(22):2124–2134.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370(4):341–351.
- , , , et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245–251.
- , , , et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024–2033.
- , , , , , . “I wish I had seen this test result earlier!”: Dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223–2228.
- , , , et al. Diagnostic error in medicine: analysis of 583 physician‐reported errors. Arch Intern Med. 2009;169(20):1881–1887.
- , , . Bringing diagnosis into the quality and safety equations. JAMA. 2012;308(12):1211–1212.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Effects of a multicentre teamwork and communication programme on patient outcomes: results from the Triad for Optimal Patient Safety (TOPS) project. BMJ Qual Saf. 2012;21(2):118–126.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
- , , , . Interdisciplinary teamwork in hospitals: A review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , . Legal, ethical, and financial dilemmas in electronic health record adoption and use. Pediatrics. 2011;127(4):e1042–e1047.
In this issue of the Journal of Hospital Medicine, Schaffer and colleagues report their analysis of malpractice claims against hospitalists compared to other physician specialties.[1] In contrast to previous work examining medical liability,[2, 3] Schaffer and colleagues have been able to identify hospitalists specifically.[2, 3]
Perhaps surprisingly, their main finding was that the rate of claims against hospitalists was significantly lower than for nonhospitalist internists, emergency medicine physicians, general surgeons, and obstetriciansgynecologists. We say surprisingly, because health systems utilizing hospitalists generally include features that might increase the risk for malpractice claims.
For example, new patients are typically assigned to whichever hospitalist is up for the next admission. Research shows that strained patientphysician relationships increase the risk for malpractice claims.[4, 5] Schaffer's data suggest that lack of a preexisting relationship is a challenge, but one to which most hospitalists have grown accustomed. Hospitalists develop and hone skills that allow them to quickly establish rapport with patients and families. Moreover, though patients seldom choose their hospitalist, they often do select the hospital in which they receive their care. The research group of 1 of the authors was recently surprised to find patients had high levels of trust with their hospital physicians, despite frequently being unable to name them or identify their role.[6] We suspect patients in the study had high levels of trust with the hospital and transferred this trust to their assigned physicians as representatives of the organization. Certainly, this hypothesis needs to be tested, and in no way does it minimize the importance of a strong patient‐physician relationship.
In addition, patientphysician continuity has long been felt to be paramount to safe and effective care; however, it is difficult to achieve in hospitalist systems. Hospitalized patients experience multiple handoffs, including those at admission, for night coverage, and at the time of service change (ie, end of rotation/stint). The potential for loss of information is enormous. Though increased attention has been dedicated to handoffs among housestaff, little work has been done to describe issues related to handoffs among practicing physicians. However, some discontinuity may be advantageous. A physician newly taking over patient care from another may not be anchored to the initial diagnosis and treatment plan established by the first. This free second look may actually prevent missed/delayed diagnoses and optimize plans of care, further reducing harm from care and risk of malpractice.[7]
Hospital discharge is another highly risky time, due to issues such as tests pending at the time of discharge and the need to manage ongoing workup and treatment of unresolved issues.[8, 9] The responsibility for tying up these loose ends may be unclear as patients are transitioned from the care of hospitalists to outpatient physicians. Prior research has shown that patients are at particularly high risk for preventable adverse events after hospital discharge.[10, 11] More recently, healthcare policy has focused on measuring and incentivizing the reduction of readmissions.[12] Although only a portion of readmissions are truly preventable,[13] and many patients who suffer adverse events after discharge are not readmitted,[11] the efforts resulting from these policy initiatives may have improved the overall safety of transitions of care.
A particularly important contribution of Schaffer and colleagues' study is that it helps us identify patient safety issues related to hospital medicine. Despite intense national efforts over the past 10 to 15 years, progress has been slow in reducing the rate of adverse events among hospitalized patients.[14, 15, 16] Although adverse events and medical liability do not always correlate,[17, 18] the contributing factors identified in Schaffer and colleagues' study help direct our patient safety efforts.
Clinical judgment was the most common factor associated with hospitalist malpractice claims, with examples including failure or delay in ordering a necessary diagnostic test or specialist consultation. These results may be misinterpreted by some to suggest that ordering more tests and services may reduce risk for malpractice claims. However, there is no evidence to support the belief that these defensive medicine behaviors actually reduce risk. In fact, the opposite may be true. Research shows that abnormal tests are frequently overlooked,[9, 19] and failure to act on abnormal results is a common cause of diagnostic error.[20] Experts have called for the development of diagnosis‐related quality measures and better strategies to enhance trainees' clinical reasoning skills.[21] We suggest that future research also clarify the effect of interruptions, distractions, and workload on cognitive errors in hospital settings.
Communication failures were the second most common contributing factor. As previously mentioned, communication failures may occur between hospitalists during handoffs. We also have major opportunities to improve interprofessional teamwork, especially between physicians and nurses.[22, 23] An increasing number of hospitalist groups are collaborating with other hospital‐based professionals to implement novel strategies to improve teamwork,[24, 25] many of which were recently summarized in a review published in this journal.[26]
Documentation was the third most common contributing factor. Most malpractice claims are filed long after the alleged injury has occurred.[18] Unless the clinicians involved and the hospital in which they work are aware of an event that might result in a malpractice claim, the investigation may be severely delayed. As time goes on, professionals are less able to recall details pertinent to understanding contributing factors to an event. Thus, documentation is critical. As the saying goes, if it wasn't documented, it didn't happen. The flipside of too little documentation is, of course, too much. The increasing use of electronic health records makes it easy to copy and paste outdated information, the sloppiness of which can only hurt when attempting to defend a malpractice claim.[27]
In conclusion, despite a model with inherent features that might contribute to medical malpractice risk, hospital medicine has a claim rate lower than many other specialties. Though reassuring, hospitalists should remember that the most productive way to approach malpractice risk is reframe the problem as one that attempts to reduce risk for patients, rather than for physicians. Improving patient safety is a core value for hospital medicine. Schaffer and colleagues' study identifies factors contributing to patient safety risk in hospital medicine, allowing us to renew our efforts in focused areas.
In this issue of the Journal of Hospital Medicine, Schaffer and colleagues report their analysis of malpractice claims against hospitalists compared to other physician specialties.[1] In contrast to previous work examining medical liability,[2, 3] Schaffer and colleagues have been able to identify hospitalists specifically.[2, 3]
Perhaps surprisingly, their main finding was that the rate of claims against hospitalists was significantly lower than for nonhospitalist internists, emergency medicine physicians, general surgeons, and obstetriciansgynecologists. We say surprisingly, because health systems utilizing hospitalists generally include features that might increase the risk for malpractice claims.
For example, new patients are typically assigned to whichever hospitalist is up for the next admission. Research shows that strained patientphysician relationships increase the risk for malpractice claims.[4, 5] Schaffer's data suggest that lack of a preexisting relationship is a challenge, but one to which most hospitalists have grown accustomed. Hospitalists develop and hone skills that allow them to quickly establish rapport with patients and families. Moreover, though patients seldom choose their hospitalist, they often do select the hospital in which they receive their care. The research group of 1 of the authors was recently surprised to find patients had high levels of trust with their hospital physicians, despite frequently being unable to name them or identify their role.[6] We suspect patients in the study had high levels of trust with the hospital and transferred this trust to their assigned physicians as representatives of the organization. Certainly, this hypothesis needs to be tested, and in no way does it minimize the importance of a strong patient‐physician relationship.
In addition, patientphysician continuity has long been felt to be paramount to safe and effective care; however, it is difficult to achieve in hospitalist systems. Hospitalized patients experience multiple handoffs, including those at admission, for night coverage, and at the time of service change (ie, end of rotation/stint). The potential for loss of information is enormous. Though increased attention has been dedicated to handoffs among housestaff, little work has been done to describe issues related to handoffs among practicing physicians. However, some discontinuity may be advantageous. A physician newly taking over patient care from another may not be anchored to the initial diagnosis and treatment plan established by the first. This free second look may actually prevent missed/delayed diagnoses and optimize plans of care, further reducing harm from care and risk of malpractice.[7]
Hospital discharge is another highly risky time, due to issues such as tests pending at the time of discharge and the need to manage ongoing workup and treatment of unresolved issues.[8, 9] The responsibility for tying up these loose ends may be unclear as patients are transitioned from the care of hospitalists to outpatient physicians. Prior research has shown that patients are at particularly high risk for preventable adverse events after hospital discharge.[10, 11] More recently, healthcare policy has focused on measuring and incentivizing the reduction of readmissions.[12] Although only a portion of readmissions are truly preventable,[13] and many patients who suffer adverse events after discharge are not readmitted,[11] the efforts resulting from these policy initiatives may have improved the overall safety of transitions of care.
A particularly important contribution of Schaffer and colleagues' study is that it helps us identify patient safety issues related to hospital medicine. Despite intense national efforts over the past 10 to 15 years, progress has been slow in reducing the rate of adverse events among hospitalized patients.[14, 15, 16] Although adverse events and medical liability do not always correlate,[17, 18] the contributing factors identified in Schaffer and colleagues' study help direct our patient safety efforts.
Clinical judgment was the most common factor associated with hospitalist malpractice claims, with examples including failure or delay in ordering a necessary diagnostic test or specialist consultation. These results may be misinterpreted by some to suggest that ordering more tests and services may reduce risk for malpractice claims. However, there is no evidence to support the belief that these defensive medicine behaviors actually reduce risk. In fact, the opposite may be true. Research shows that abnormal tests are frequently overlooked,[9, 19] and failure to act on abnormal results is a common cause of diagnostic error.[20] Experts have called for the development of diagnosis‐related quality measures and better strategies to enhance trainees' clinical reasoning skills.[21] We suggest that future research also clarify the effect of interruptions, distractions, and workload on cognitive errors in hospital settings.
Communication failures were the second most common contributing factor. As previously mentioned, communication failures may occur between hospitalists during handoffs. We also have major opportunities to improve interprofessional teamwork, especially between physicians and nurses.[22, 23] An increasing number of hospitalist groups are collaborating with other hospital‐based professionals to implement novel strategies to improve teamwork,[24, 25] many of which were recently summarized in a review published in this journal.[26]
Documentation was the third most common contributing factor. Most malpractice claims are filed long after the alleged injury has occurred.[18] Unless the clinicians involved and the hospital in which they work are aware of an event that might result in a malpractice claim, the investigation may be severely delayed. As time goes on, professionals are less able to recall details pertinent to understanding contributing factors to an event. Thus, documentation is critical. As the saying goes, if it wasn't documented, it didn't happen. The flipside of too little documentation is, of course, too much. The increasing use of electronic health records makes it easy to copy and paste outdated information, the sloppiness of which can only hurt when attempting to defend a malpractice claim.[27]
In conclusion, despite a model with inherent features that might contribute to medical malpractice risk, hospital medicine has a claim rate lower than many other specialties. Though reassuring, hospitalists should remember that the most productive way to approach malpractice risk is reframe the problem as one that attempts to reduce risk for patients, rather than for physicians. Improving patient safety is a core value for hospital medicine. Schaffer and colleagues' study identifies factors contributing to patient safety risk in hospital medicine, allowing us to renew our efforts in focused areas.
- , , , . Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750–755.
- , , . Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305(23):2427–2431.
- , , , . Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629–636.
- , , , . The doctor‐patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365–1370.
- , , , , . Physician‐patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553–559.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175(1):5.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305–1311.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- U.S. Department of Health 183(7):E391–E402.
- U.S. Department of Health 363(22):2124–2134.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370(4):341–351.
- , , , et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245–251.
- , , , et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024–2033.
- , , , , , . “I wish I had seen this test result earlier!”: Dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223–2228.
- , , , et al. Diagnostic error in medicine: analysis of 583 physician‐reported errors. Arch Intern Med. 2009;169(20):1881–1887.
- , , . Bringing diagnosis into the quality and safety equations. JAMA. 2012;308(12):1211–1212.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Effects of a multicentre teamwork and communication programme on patient outcomes: results from the Triad for Optimal Patient Safety (TOPS) project. BMJ Qual Saf. 2012;21(2):118–126.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
- , , , . Interdisciplinary teamwork in hospitals: A review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , . Legal, ethical, and financial dilemmas in electronic health record adoption and use. Pediatrics. 2011;127(4):e1042–e1047.
- , , , . Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750–755.
- , , . Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305(23):2427–2431.
- , , , . Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629–636.
- , , , . The doctor‐patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365–1370.
- , , , , . Physician‐patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553–559.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175(1):5.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305–1311.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- U.S. Department of Health 183(7):E391–E402.
- U.S. Department of Health 363(22):2124–2134.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370(4):341–351.
- , , , et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245–251.
- , , , et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024–2033.
- , , , , , . “I wish I had seen this test result earlier!”: Dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223–2228.
- , , , et al. Diagnostic error in medicine: analysis of 583 physician‐reported errors. Arch Intern Med. 2009;169(20):1881–1887.
- , , . Bringing diagnosis into the quality and safety equations. JAMA. 2012;308(12):1211–1212.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Effects of a multicentre teamwork and communication programme on patient outcomes: results from the Triad for Optimal Patient Safety (TOPS) project. BMJ Qual Saf. 2012;21(2):118–126.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
- , , , . Interdisciplinary teamwork in hospitals: A review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , . Legal, ethical, and financial dilemmas in electronic health record adoption and use. Pediatrics. 2011;127(4):e1042–e1047.
Letter to the Editor
We agree with Drs. Arora and Mahmud that emerging mobile health (mHealth) approaches to improving patient engagement will need to demonstrate their value to advance health and healthcare. The potential for mHealth to do this has been often described[1, 2] but, so far, rarely measured or demonstrated.
The technology costs of our tablet‐based intervention[3] were low: 2 iPads at $400 each. The real expense was for personnel: research assistants needed to teach patients how to use the technology effectively. In the future, we hope to shift device and software orientation to patient‐care assistants, nurses, or even digital assistants, nonmedical personnel who have technical expertise with the health‐related devices and software needed to engage with the electronic health record and educational materials. Thus, at least part of the challenge of cost‐effectiveness aside from improved outcomeswill be demonstrating eventual time savings for providers who no longer need to hand deliver or explain paper pamphlets or printouts, or shepherd patients through their digitally assisted education.
One day we may muse, what did we do before mHealth? as we might do now when using mobile technologies for nonhealth‐related tasks like getting directions or making a call. Indeed, who can remember the last time they routinely used a paper map or phonebook for these daily tasks? Our prescription for tablets is a step in that direction, but we will need to also reimagine patient education and related daily tasks at the hospital and system level to realize the potential of lower costs and higher quality care we can achieve using mHealth.[4]
- , , . Can mobile health technologies transform health care? JAMA. 2013;310(22):2395–2396.
- , , , et al. The effectiveness of mobile‐health technologies to improve health care service delivery processes: a systematic review and meta‐analysis. PLoS Med. 2013;10(1):e1001363.
- , , , , . Tablet computers for hospitalized patients: a pilot study to improve inpatient engagement [published online ahead of print February 13, 2013]. J Hosp Med. doi: 10.1002/jhm.2169.
- , , , et al. Patient engagement in the inpatient setting: a systematic review [published online ahead of print November 22, 2013]. J Am Med Inform Assoc. doi: 10.1136/amiajnl‐2013‐002141.
We agree with Drs. Arora and Mahmud that emerging mobile health (mHealth) approaches to improving patient engagement will need to demonstrate their value to advance health and healthcare. The potential for mHealth to do this has been often described[1, 2] but, so far, rarely measured or demonstrated.
The technology costs of our tablet‐based intervention[3] were low: 2 iPads at $400 each. The real expense was for personnel: research assistants needed to teach patients how to use the technology effectively. In the future, we hope to shift device and software orientation to patient‐care assistants, nurses, or even digital assistants, nonmedical personnel who have technical expertise with the health‐related devices and software needed to engage with the electronic health record and educational materials. Thus, at least part of the challenge of cost‐effectiveness aside from improved outcomeswill be demonstrating eventual time savings for providers who no longer need to hand deliver or explain paper pamphlets or printouts, or shepherd patients through their digitally assisted education.
One day we may muse, what did we do before mHealth? as we might do now when using mobile technologies for nonhealth‐related tasks like getting directions or making a call. Indeed, who can remember the last time they routinely used a paper map or phonebook for these daily tasks? Our prescription for tablets is a step in that direction, but we will need to also reimagine patient education and related daily tasks at the hospital and system level to realize the potential of lower costs and higher quality care we can achieve using mHealth.[4]
We agree with Drs. Arora and Mahmud that emerging mobile health (mHealth) approaches to improving patient engagement will need to demonstrate their value to advance health and healthcare. The potential for mHealth to do this has been often described[1, 2] but, so far, rarely measured or demonstrated.
The technology costs of our tablet‐based intervention[3] were low: 2 iPads at $400 each. The real expense was for personnel: research assistants needed to teach patients how to use the technology effectively. In the future, we hope to shift device and software orientation to patient‐care assistants, nurses, or even digital assistants, nonmedical personnel who have technical expertise with the health‐related devices and software needed to engage with the electronic health record and educational materials. Thus, at least part of the challenge of cost‐effectiveness aside from improved outcomeswill be demonstrating eventual time savings for providers who no longer need to hand deliver or explain paper pamphlets or printouts, or shepherd patients through their digitally assisted education.
One day we may muse, what did we do before mHealth? as we might do now when using mobile technologies for nonhealth‐related tasks like getting directions or making a call. Indeed, who can remember the last time they routinely used a paper map or phonebook for these daily tasks? Our prescription for tablets is a step in that direction, but we will need to also reimagine patient education and related daily tasks at the hospital and system level to realize the potential of lower costs and higher quality care we can achieve using mHealth.[4]
- , , . Can mobile health technologies transform health care? JAMA. 2013;310(22):2395–2396.
- , , , et al. The effectiveness of mobile‐health technologies to improve health care service delivery processes: a systematic review and meta‐analysis. PLoS Med. 2013;10(1):e1001363.
- , , , , . Tablet computers for hospitalized patients: a pilot study to improve inpatient engagement [published online ahead of print February 13, 2013]. J Hosp Med. doi: 10.1002/jhm.2169.
- , , , et al. Patient engagement in the inpatient setting: a systematic review [published online ahead of print November 22, 2013]. J Am Med Inform Assoc. doi: 10.1136/amiajnl‐2013‐002141.
- , , . Can mobile health technologies transform health care? JAMA. 2013;310(22):2395–2396.
- , , , et al. The effectiveness of mobile‐health technologies to improve health care service delivery processes: a systematic review and meta‐analysis. PLoS Med. 2013;10(1):e1001363.
- , , , , . Tablet computers for hospitalized patients: a pilot study to improve inpatient engagement [published online ahead of print February 13, 2013]. J Hosp Med. doi: 10.1002/jhm.2169.
- , , , et al. Patient engagement in the inpatient setting: a systematic review [published online ahead of print November 22, 2013]. J Am Med Inform Assoc. doi: 10.1136/amiajnl‐2013‐002141.
Blood Cultures in Nonpneumonia Illness
In 2002, based on consensus practice guidelines,[1] the Centers for Medicare and Medicaid Services (CMS) and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) announced a core measure mandating the collection of routine blood cultures in the emergency department (ED) for all patients hospitalized with community‐acquired pneumonia (CAP) to benchmark the quality of hospital care. However, due to the limited utility and false‐positive results of routine blood cultures,[2, 3, 4, 5, 6] performance measures and practice guidelines were modified in 2005 and 2007, respectively, to recommend routine collection in only the sickest patients with CAP.[2, 7] Despite recommendations for a more narrow set of indications, the collection of blood cultures in patients hospitalized with CAP continued to increase.[8]
Distinguishing CAP from other respiratory illnesses may be challenging. Among patients presenting to the ED with an acute respiratory illness, only a minority of patients (10%30%) are diagnosed with pneumonia.[9] Therefore, the harms and costs of inappropriate diagnostic tests for CAP may be further magnified if applied to a larger population of patients who present to the ED with similar clinical signs and symptoms as pneumonia. Using a national sample of ED visits, we examined whether there was a similar increase in the frequency of blood culture collection among patients who were hospitalized with respiratory symptoms due to an illness other than pneumonia.
METHOD
Study Design, Setting, and Participants
We performed a cross‐sectional analysis using data from the 2002 to 2010 National Hospital Ambulatory Medical Care Surveys (NHAMCS), a probability sample of visits to EDs of noninstitutional general and short‐stay hospitals in the United States, excluding federal, military, and Veterans Administration hospitals.[10] The NHAMCS data are derived through multistage sampling and estimation procedures that produce unbiased national estimates.[11] Further details regarding the sampling and estimation procedures can be found on the US Centers for Disease Control and Prevention website.[10, 11] Years 2005 and 2006 are omitted because NHAMCS did not collect blood culture use during this period. We included all visits by patients aged 18 years or older who were subsequently hospitalized.
Measurements
Trained hospital staff collected data with oversight from US Census Bureau field representatives.[12] Blood culture collection during the visit was recorded as a checkbox on the NHAMCS data collection form if at least 1 culture was ordered or collected in the ED. Visits for conditions that may resemble pneumonia were defined as visits with a respiratory symptom listed for at least 1 of the 3 reason for visit fields, excluding those visits admitted with a diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes 481.xx‐486.xx). The reason for visit field captures the patient's complaints, symptoms, or other reasons for the visit in the patient's own words. CAP was defined by having 1 of the 3 ED provider's diagnosis fields coded as pneumonia (ICD‐9‐CM 481486), excluding patients with suspected hospital‐acquired pneumonia (nursing home or institutionalized resident, seen in the ED in the past 72 hours, or discharged from any hospital within the past 7 days) or those with a follow‐up visit for the same problem.[8]
Data Analysis
All analyses accounted for the complex survey design, including weights, to reflect national estimates. To examine for potential spillover effects of the blood culture recommendations for CAP on other conditions that may present similarly, we used linear regression to examine the trend in collecting blood cultures in patients admitted to the hospital with respiratory symptoms due to a nonpneumonia illness.
The data were analyzed using Stata statistical software, version 12.0 (StataCorp, College Station, TX). This study was exempt from review by the institutional review board of the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center.
RESULTS
This study included 4854 ED visits, representing approximately 17 million visits by adult patients hospitalized with respiratory symptoms due to a nonpneumonia illness. The most common primary ED provider's diagnoses for these visits included heart failure (15.9%), chronic obstructive pulmonary disease (12.6%), chest pain (11.9%), respiratory insufficiency or failure (8.8%), and asthma (5.5%). The characteristics of these visits are shown in Table 1.
| Years 20022004, Weighted % (Unweighted N=2,175)b | Years 20072008, Weighted % (Unweighted N=1,346)b | Years 20092010, Weighted % (Unweighted N=1,333)b | |
|---|---|---|---|
| |||
| Blood culture collected | 9.8 | 14.4 | 19.9 |
| Demographics | |||
| Age 65 years | 56.9 | 55.1 | 50.9 |
| Female | 54.0 | 57.5 | 51.3 |
| Race/ethnicity | |||
| White, non‐Hispanic | 71.5c | 69.5 | 67.2 |
| Black, non‐Hispanic | 17.1c | 20.8 | 22.2 |
| Other | 11.3c | 9.7 | 10.6 |
| Primary payer | |||
| Private insurance | 23.4 | 19.1 | 19.1 |
| Medicare | 55.2 | 58.0 | 54.2 |
| Medicaid | 10.0 | 10.5 | 13.8 |
| Other/unknown | 11.4 | 12.4 | 13.0 |
| Visit characteristics | |||
| Disposition status | |||
| Non‐ICU | 86.8 | 85.5 | 83.3 |
| ICU | 13.2 | 14.5 | 16.7 |
| Fever (38.0C) | 6.1 | 5.3 | 4.8 |
| Hypoxia (90%)d | 11.5 | 10.9 | |
| Emergent status by triage | 46.1 | 44.5 | 35.8 |
| Administered antibiotics | 19.6 | 24.6 | 24.8 |
| Tests/services ordered in ED | |||
| 05 | 29.9 | 29.1 | 22.3 |
| 610 | 43.5 | 58.3 | 56.1 |
| >10 | 26.6 | 12.6 | 21.6 |
| ED characteristics | |||
| Region | |||
| West | 16.6 | 18.2 | 15.8 |
| Midwest | 27.1 | 25.2 | 22.8 |
| South | 32.8 | 36.4 | 38.6 |
| Northeast | 23.5 | 20.2 | 22.7 |
| Hospital owner | |||
| Nonprofit | 80.6 | 84.6 | 80.7 |
| Government | 12.1 | 6.4 | 13.0 |
| Private | 7.4 | 9.0 | 6.3 |
The proportion of blood cultures collected in the ED for patients hospitalized with respiratory symptoms due to a nonpneumonia illness increased from 9.9% (95% confidence interval [CI]: 7.1%‐13.5%) in 2002 to 20.4% (95% CI: 16.1%‐25.6%) in 2010 (P0.001 for the trend). This observed increase paralleled the increase in the frequency of culture collection in patients hospitalized with CAP (P=0.12 for the difference in temporal trends). The estimated absolute number of visits for respiratory symptoms due a nonpneumonia illness with a blood culture collected increased from 211,000 (95% CI: 126,000296,000) in 2002 to 526,000 (95% CI: 361,000692,000) in 2010, which was similar in magnitude to the estimated number of visits for CAP with a culture collected (Table 2).
| National Weighted Estimates (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Condition | 2002 | 2003 | 2004 | 2007 | 2008 | 2009 | 2010 | P Valueb |
| Respiratory symptomc | ||||||||
| % | 9.9 (7.113.5) | 9.2 (6.912.2) | 10.6 (7.914.1) | 13.5 (10.117.8) | 15.2 (12.118.8) | 19.4 (15.923.5) | 20.4 (16.125.6) | 0.001 |
| No., thousands | 211 (126296) | 229 (140319) | 212 (140285) | 287 (191382) | 418 (288548) | 486 (344627) | 526 (361692) | |
| CAP | ||||||||
| % | 29.4 (21.938.3) | 34.2 (25.943.6) | 38.4 (31.045.4) | 45.7 (35.456.4) | 44.1 (34.154.6) | 46.7 (37.456.1) | 51.1 (41.860.3) | 0.027 |
| No., thousands | 155 (100210) | 287 (177397) | 276 (192361) | 277 (173381) | 361 (255467) | 350 (237464) | 428 (283574) | |
DISCUSSION
In this national study of ED visits, we found that the collection of blood cultures in patients hospitalized with respiratory symptoms due to an illness other than pneumonia continued to increase from 2002 to 2010 in a parallel fashion to the trend observed for patients hospitalized with CAP. Our findings suggest that the heightened attention of collecting blood cultures for suspected pneumonia had unintended consequences, which led to an increase in the collection of blood cultures in patients hospitalized with conditions that mimic pneumonia in the ED.
There can be a great deal of diagnostic uncertainty when treating patients in the ED who present with acute respiratory symptoms. Unfortunately, the initial history and physical exam are often insufficient to effectively rule in CAP.[13] Furthermore, the challenge of diagnosing pneumonia is amplified in the subset of patients who present with evolving, atypical, or occult disease. Faced with this diagnostic uncertainty, ED providers may feel pressured to comply with performance measures for CAP, promoting the overuse of inappropriate diagnostic tests and treatments. For instance, efforts to comply with early antibiotic administration in patients with CAP have led to an increase in unnecessary antibiotic use among patients with a diagnosis other than CAP.[14] Due to concerns for these unintended consequences, the core measure for early antibiotic administration was effectively retired in 2012.
Although a smaller percentage of ED visits for respiratory symptoms had a blood culture collected compared to CAP visits, there was a similar absolute number of visits with a blood culture collected during the study period. While a fraction of these patients may present with an infectious etiology aside from pneumonia, the majority of these cases likely represent situations where blood cultures add little diagnostic value at the expense of potentially longer hospital stays and broad spectrum antimicrobial use due to false‐positive results,[5, 15] as well as higher costs incurred by the test itself.[15, 16]
Although recommendations for routine culture collection for all patients hospitalized with CAP have been revised, the JCAHO/CMS core measure (PN‐3b) announced in 2002 mandates that if a culture is collected in the ED, it should be collected prior to antibiotic administration. Due to inherent uncertainty and challenges in making a timely diagnosis of pneumonia, this measure may encourage providers to reflexively order cultures in all patients presenting with respiratory symptoms in whom antibiotic administration is anticipated. The observed increasing trend in culture collection in patients hospitalized with respiratory symptoms due to a nonpneumonia illness should prompt JCAHO and CMS to reevaluate the risks and benefits of this core measure, with consideration of eliminating it altogether to discourage overuse in this population.
Our study had certain limitations. First, the omission of 2005 and 2006 data prohibited an evaluation of whether culture rates slowed down among patients hospitalized with respiratory symptoms due to a nonpneumonia illness after revisions in recommendations for obtaining cultures in patients with CAP. Second, there may have been misclassification of culture collection due to errors in chart abstraction. However, abstraction errors in the NHAMCS typically result in undercoding.[17] Therefore, our findings likely underestimate the magnitude and frequency of culture collection in this population.
In conclusion, collecting blood cultures in the ED for patients hospitalized with respiratory symptoms due to a nonpneumonia illness has increased in a parallel fashion compared to the trend in culture collection in patients hospitalized with CAP from 2002 to 2010. This suggests an important potential unintended consequence of blood culture recommendations for CAP on patients who present with conditions that resemble pneumonia. More attention to the judicious use of blood cultures in these patients to reduce harm and costs is needed.
ACKNOWLEDGEMENT
Disclosures: Dr. Makam's work on this project was completed while he was a Primary Care Research Fellow at the University of California San Francisco, funded by an NRSA training grant (T32HP19025‐07‐00). The authors report no conflicts of interest.
- , , , , , . Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31(2):347–382.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , , . The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study. Chest. 2003;123(4):1142–1150.
- , , , , , . Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005;46(5):393–400.
- , , , . Predicting bacteremia in patients with community‐acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347.
- , . The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78–82.
- , . The Joint Commission on Accreditation of Healthcare Organizations and Center for Medicare and Medicaid Services community‐acquired pneumonia initiative: what went wrong? Ann Emerg Med. 2005;46(5):409–411.
- , , . Blood culture use in the emergency department in patients hospitalized for community‐acquired pneumonia [published online ahead of print March 10, 2014]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.13808.
- , , , et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113(9):664–670.
- Centers for Disease Control and Prevention. NHAMCS scope and sample design. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_scope.htm#nhamcs_scope. Accessed May 27, 2013.
- Centers for Disease Control and Prevention. NHAMCS estimation procedures. http://www.cdc.gov/nchs/ahcd/ahcd_estimation_procedures.htm#nhamcs_procedures. Updated January 15, 2010. Accessed May 27, 2013.
- , , , et al. NHAMCS: does it hold up to scrutiny? Ann Emerg Med. 2013;62(5):549–551.
- , , . Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA. 1997;278(17):1440–1445.
- , , , . Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4‐h antibiotic administration rule. Chest. 2007;131(6):1865–1869.
- , , . Contaminant blood cultures and resource utilization. The true consequences of false‐positive results. JAMA. 1991;265(3):365–369.
- , . Analysis of strategies to improve cost effectiveness of blood cultures. J Hosp Med. 2006;1(5):272–276.
- . NHAMCS: does it hold up to scrutiny? Ann Emerg Med. 2012;60(6):722–725.
In 2002, based on consensus practice guidelines,[1] the Centers for Medicare and Medicaid Services (CMS) and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) announced a core measure mandating the collection of routine blood cultures in the emergency department (ED) for all patients hospitalized with community‐acquired pneumonia (CAP) to benchmark the quality of hospital care. However, due to the limited utility and false‐positive results of routine blood cultures,[2, 3, 4, 5, 6] performance measures and practice guidelines were modified in 2005 and 2007, respectively, to recommend routine collection in only the sickest patients with CAP.[2, 7] Despite recommendations for a more narrow set of indications, the collection of blood cultures in patients hospitalized with CAP continued to increase.[8]
Distinguishing CAP from other respiratory illnesses may be challenging. Among patients presenting to the ED with an acute respiratory illness, only a minority of patients (10%30%) are diagnosed with pneumonia.[9] Therefore, the harms and costs of inappropriate diagnostic tests for CAP may be further magnified if applied to a larger population of patients who present to the ED with similar clinical signs and symptoms as pneumonia. Using a national sample of ED visits, we examined whether there was a similar increase in the frequency of blood culture collection among patients who were hospitalized with respiratory symptoms due to an illness other than pneumonia.
METHOD
Study Design, Setting, and Participants
We performed a cross‐sectional analysis using data from the 2002 to 2010 National Hospital Ambulatory Medical Care Surveys (NHAMCS), a probability sample of visits to EDs of noninstitutional general and short‐stay hospitals in the United States, excluding federal, military, and Veterans Administration hospitals.[10] The NHAMCS data are derived through multistage sampling and estimation procedures that produce unbiased national estimates.[11] Further details regarding the sampling and estimation procedures can be found on the US Centers for Disease Control and Prevention website.[10, 11] Years 2005 and 2006 are omitted because NHAMCS did not collect blood culture use during this period. We included all visits by patients aged 18 years or older who were subsequently hospitalized.
Measurements
Trained hospital staff collected data with oversight from US Census Bureau field representatives.[12] Blood culture collection during the visit was recorded as a checkbox on the NHAMCS data collection form if at least 1 culture was ordered or collected in the ED. Visits for conditions that may resemble pneumonia were defined as visits with a respiratory symptom listed for at least 1 of the 3 reason for visit fields, excluding those visits admitted with a diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes 481.xx‐486.xx). The reason for visit field captures the patient's complaints, symptoms, or other reasons for the visit in the patient's own words. CAP was defined by having 1 of the 3 ED provider's diagnosis fields coded as pneumonia (ICD‐9‐CM 481486), excluding patients with suspected hospital‐acquired pneumonia (nursing home or institutionalized resident, seen in the ED in the past 72 hours, or discharged from any hospital within the past 7 days) or those with a follow‐up visit for the same problem.[8]
Data Analysis
All analyses accounted for the complex survey design, including weights, to reflect national estimates. To examine for potential spillover effects of the blood culture recommendations for CAP on other conditions that may present similarly, we used linear regression to examine the trend in collecting blood cultures in patients admitted to the hospital with respiratory symptoms due to a nonpneumonia illness.
The data were analyzed using Stata statistical software, version 12.0 (StataCorp, College Station, TX). This study was exempt from review by the institutional review board of the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center.
RESULTS
This study included 4854 ED visits, representing approximately 17 million visits by adult patients hospitalized with respiratory symptoms due to a nonpneumonia illness. The most common primary ED provider's diagnoses for these visits included heart failure (15.9%), chronic obstructive pulmonary disease (12.6%), chest pain (11.9%), respiratory insufficiency or failure (8.8%), and asthma (5.5%). The characteristics of these visits are shown in Table 1.
| Years 20022004, Weighted % (Unweighted N=2,175)b | Years 20072008, Weighted % (Unweighted N=1,346)b | Years 20092010, Weighted % (Unweighted N=1,333)b | |
|---|---|---|---|
| |||
| Blood culture collected | 9.8 | 14.4 | 19.9 |
| Demographics | |||
| Age 65 years | 56.9 | 55.1 | 50.9 |
| Female | 54.0 | 57.5 | 51.3 |
| Race/ethnicity | |||
| White, non‐Hispanic | 71.5c | 69.5 | 67.2 |
| Black, non‐Hispanic | 17.1c | 20.8 | 22.2 |
| Other | 11.3c | 9.7 | 10.6 |
| Primary payer | |||
| Private insurance | 23.4 | 19.1 | 19.1 |
| Medicare | 55.2 | 58.0 | 54.2 |
| Medicaid | 10.0 | 10.5 | 13.8 |
| Other/unknown | 11.4 | 12.4 | 13.0 |
| Visit characteristics | |||
| Disposition status | |||
| Non‐ICU | 86.8 | 85.5 | 83.3 |
| ICU | 13.2 | 14.5 | 16.7 |
| Fever (38.0C) | 6.1 | 5.3 | 4.8 |
| Hypoxia (90%)d | 11.5 | 10.9 | |
| Emergent status by triage | 46.1 | 44.5 | 35.8 |
| Administered antibiotics | 19.6 | 24.6 | 24.8 |
| Tests/services ordered in ED | |||
| 05 | 29.9 | 29.1 | 22.3 |
| 610 | 43.5 | 58.3 | 56.1 |
| >10 | 26.6 | 12.6 | 21.6 |
| ED characteristics | |||
| Region | |||
| West | 16.6 | 18.2 | 15.8 |
| Midwest | 27.1 | 25.2 | 22.8 |
| South | 32.8 | 36.4 | 38.6 |
| Northeast | 23.5 | 20.2 | 22.7 |
| Hospital owner | |||
| Nonprofit | 80.6 | 84.6 | 80.7 |
| Government | 12.1 | 6.4 | 13.0 |
| Private | 7.4 | 9.0 | 6.3 |
The proportion of blood cultures collected in the ED for patients hospitalized with respiratory symptoms due to a nonpneumonia illness increased from 9.9% (95% confidence interval [CI]: 7.1%‐13.5%) in 2002 to 20.4% (95% CI: 16.1%‐25.6%) in 2010 (P0.001 for the trend). This observed increase paralleled the increase in the frequency of culture collection in patients hospitalized with CAP (P=0.12 for the difference in temporal trends). The estimated absolute number of visits for respiratory symptoms due a nonpneumonia illness with a blood culture collected increased from 211,000 (95% CI: 126,000296,000) in 2002 to 526,000 (95% CI: 361,000692,000) in 2010, which was similar in magnitude to the estimated number of visits for CAP with a culture collected (Table 2).
| National Weighted Estimates (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Condition | 2002 | 2003 | 2004 | 2007 | 2008 | 2009 | 2010 | P Valueb |
| Respiratory symptomc | ||||||||
| % | 9.9 (7.113.5) | 9.2 (6.912.2) | 10.6 (7.914.1) | 13.5 (10.117.8) | 15.2 (12.118.8) | 19.4 (15.923.5) | 20.4 (16.125.6) | 0.001 |
| No., thousands | 211 (126296) | 229 (140319) | 212 (140285) | 287 (191382) | 418 (288548) | 486 (344627) | 526 (361692) | |
| CAP | ||||||||
| % | 29.4 (21.938.3) | 34.2 (25.943.6) | 38.4 (31.045.4) | 45.7 (35.456.4) | 44.1 (34.154.6) | 46.7 (37.456.1) | 51.1 (41.860.3) | 0.027 |
| No., thousands | 155 (100210) | 287 (177397) | 276 (192361) | 277 (173381) | 361 (255467) | 350 (237464) | 428 (283574) | |
DISCUSSION
In this national study of ED visits, we found that the collection of blood cultures in patients hospitalized with respiratory symptoms due to an illness other than pneumonia continued to increase from 2002 to 2010 in a parallel fashion to the trend observed for patients hospitalized with CAP. Our findings suggest that the heightened attention of collecting blood cultures for suspected pneumonia had unintended consequences, which led to an increase in the collection of blood cultures in patients hospitalized with conditions that mimic pneumonia in the ED.
There can be a great deal of diagnostic uncertainty when treating patients in the ED who present with acute respiratory symptoms. Unfortunately, the initial history and physical exam are often insufficient to effectively rule in CAP.[13] Furthermore, the challenge of diagnosing pneumonia is amplified in the subset of patients who present with evolving, atypical, or occult disease. Faced with this diagnostic uncertainty, ED providers may feel pressured to comply with performance measures for CAP, promoting the overuse of inappropriate diagnostic tests and treatments. For instance, efforts to comply with early antibiotic administration in patients with CAP have led to an increase in unnecessary antibiotic use among patients with a diagnosis other than CAP.[14] Due to concerns for these unintended consequences, the core measure for early antibiotic administration was effectively retired in 2012.
Although a smaller percentage of ED visits for respiratory symptoms had a blood culture collected compared to CAP visits, there was a similar absolute number of visits with a blood culture collected during the study period. While a fraction of these patients may present with an infectious etiology aside from pneumonia, the majority of these cases likely represent situations where blood cultures add little diagnostic value at the expense of potentially longer hospital stays and broad spectrum antimicrobial use due to false‐positive results,[5, 15] as well as higher costs incurred by the test itself.[15, 16]
Although recommendations for routine culture collection for all patients hospitalized with CAP have been revised, the JCAHO/CMS core measure (PN‐3b) announced in 2002 mandates that if a culture is collected in the ED, it should be collected prior to antibiotic administration. Due to inherent uncertainty and challenges in making a timely diagnosis of pneumonia, this measure may encourage providers to reflexively order cultures in all patients presenting with respiratory symptoms in whom antibiotic administration is anticipated. The observed increasing trend in culture collection in patients hospitalized with respiratory symptoms due to a nonpneumonia illness should prompt JCAHO and CMS to reevaluate the risks and benefits of this core measure, with consideration of eliminating it altogether to discourage overuse in this population.
Our study had certain limitations. First, the omission of 2005 and 2006 data prohibited an evaluation of whether culture rates slowed down among patients hospitalized with respiratory symptoms due to a nonpneumonia illness after revisions in recommendations for obtaining cultures in patients with CAP. Second, there may have been misclassification of culture collection due to errors in chart abstraction. However, abstraction errors in the NHAMCS typically result in undercoding.[17] Therefore, our findings likely underestimate the magnitude and frequency of culture collection in this population.
In conclusion, collecting blood cultures in the ED for patients hospitalized with respiratory symptoms due to a nonpneumonia illness has increased in a parallel fashion compared to the trend in culture collection in patients hospitalized with CAP from 2002 to 2010. This suggests an important potential unintended consequence of blood culture recommendations for CAP on patients who present with conditions that resemble pneumonia. More attention to the judicious use of blood cultures in these patients to reduce harm and costs is needed.
ACKNOWLEDGEMENT
Disclosures: Dr. Makam's work on this project was completed while he was a Primary Care Research Fellow at the University of California San Francisco, funded by an NRSA training grant (T32HP19025‐07‐00). The authors report no conflicts of interest.
In 2002, based on consensus practice guidelines,[1] the Centers for Medicare and Medicaid Services (CMS) and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) announced a core measure mandating the collection of routine blood cultures in the emergency department (ED) for all patients hospitalized with community‐acquired pneumonia (CAP) to benchmark the quality of hospital care. However, due to the limited utility and false‐positive results of routine blood cultures,[2, 3, 4, 5, 6] performance measures and practice guidelines were modified in 2005 and 2007, respectively, to recommend routine collection in only the sickest patients with CAP.[2, 7] Despite recommendations for a more narrow set of indications, the collection of blood cultures in patients hospitalized with CAP continued to increase.[8]
Distinguishing CAP from other respiratory illnesses may be challenging. Among patients presenting to the ED with an acute respiratory illness, only a minority of patients (10%30%) are diagnosed with pneumonia.[9] Therefore, the harms and costs of inappropriate diagnostic tests for CAP may be further magnified if applied to a larger population of patients who present to the ED with similar clinical signs and symptoms as pneumonia. Using a national sample of ED visits, we examined whether there was a similar increase in the frequency of blood culture collection among patients who were hospitalized with respiratory symptoms due to an illness other than pneumonia.
METHOD
Study Design, Setting, and Participants
We performed a cross‐sectional analysis using data from the 2002 to 2010 National Hospital Ambulatory Medical Care Surveys (NHAMCS), a probability sample of visits to EDs of noninstitutional general and short‐stay hospitals in the United States, excluding federal, military, and Veterans Administration hospitals.[10] The NHAMCS data are derived through multistage sampling and estimation procedures that produce unbiased national estimates.[11] Further details regarding the sampling and estimation procedures can be found on the US Centers for Disease Control and Prevention website.[10, 11] Years 2005 and 2006 are omitted because NHAMCS did not collect blood culture use during this period. We included all visits by patients aged 18 years or older who were subsequently hospitalized.
Measurements
Trained hospital staff collected data with oversight from US Census Bureau field representatives.[12] Blood culture collection during the visit was recorded as a checkbox on the NHAMCS data collection form if at least 1 culture was ordered or collected in the ED. Visits for conditions that may resemble pneumonia were defined as visits with a respiratory symptom listed for at least 1 of the 3 reason for visit fields, excluding those visits admitted with a diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes 481.xx‐486.xx). The reason for visit field captures the patient's complaints, symptoms, or other reasons for the visit in the patient's own words. CAP was defined by having 1 of the 3 ED provider's diagnosis fields coded as pneumonia (ICD‐9‐CM 481486), excluding patients with suspected hospital‐acquired pneumonia (nursing home or institutionalized resident, seen in the ED in the past 72 hours, or discharged from any hospital within the past 7 days) or those with a follow‐up visit for the same problem.[8]
Data Analysis
All analyses accounted for the complex survey design, including weights, to reflect national estimates. To examine for potential spillover effects of the blood culture recommendations for CAP on other conditions that may present similarly, we used linear regression to examine the trend in collecting blood cultures in patients admitted to the hospital with respiratory symptoms due to a nonpneumonia illness.
The data were analyzed using Stata statistical software, version 12.0 (StataCorp, College Station, TX). This study was exempt from review by the institutional review board of the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center.
RESULTS
This study included 4854 ED visits, representing approximately 17 million visits by adult patients hospitalized with respiratory symptoms due to a nonpneumonia illness. The most common primary ED provider's diagnoses for these visits included heart failure (15.9%), chronic obstructive pulmonary disease (12.6%), chest pain (11.9%), respiratory insufficiency or failure (8.8%), and asthma (5.5%). The characteristics of these visits are shown in Table 1.
| Years 20022004, Weighted % (Unweighted N=2,175)b | Years 20072008, Weighted % (Unweighted N=1,346)b | Years 20092010, Weighted % (Unweighted N=1,333)b | |
|---|---|---|---|
| |||
| Blood culture collected | 9.8 | 14.4 | 19.9 |
| Demographics | |||
| Age 65 years | 56.9 | 55.1 | 50.9 |
| Female | 54.0 | 57.5 | 51.3 |
| Race/ethnicity | |||
| White, non‐Hispanic | 71.5c | 69.5 | 67.2 |
| Black, non‐Hispanic | 17.1c | 20.8 | 22.2 |
| Other | 11.3c | 9.7 | 10.6 |
| Primary payer | |||
| Private insurance | 23.4 | 19.1 | 19.1 |
| Medicare | 55.2 | 58.0 | 54.2 |
| Medicaid | 10.0 | 10.5 | 13.8 |
| Other/unknown | 11.4 | 12.4 | 13.0 |
| Visit characteristics | |||
| Disposition status | |||
| Non‐ICU | 86.8 | 85.5 | 83.3 |
| ICU | 13.2 | 14.5 | 16.7 |
| Fever (38.0C) | 6.1 | 5.3 | 4.8 |
| Hypoxia (90%)d | 11.5 | 10.9 | |
| Emergent status by triage | 46.1 | 44.5 | 35.8 |
| Administered antibiotics | 19.6 | 24.6 | 24.8 |
| Tests/services ordered in ED | |||
| 05 | 29.9 | 29.1 | 22.3 |
| 610 | 43.5 | 58.3 | 56.1 |
| >10 | 26.6 | 12.6 | 21.6 |
| ED characteristics | |||
| Region | |||
| West | 16.6 | 18.2 | 15.8 |
| Midwest | 27.1 | 25.2 | 22.8 |
| South | 32.8 | 36.4 | 38.6 |
| Northeast | 23.5 | 20.2 | 22.7 |
| Hospital owner | |||
| Nonprofit | 80.6 | 84.6 | 80.7 |
| Government | 12.1 | 6.4 | 13.0 |
| Private | 7.4 | 9.0 | 6.3 |
The proportion of blood cultures collected in the ED for patients hospitalized with respiratory symptoms due to a nonpneumonia illness increased from 9.9% (95% confidence interval [CI]: 7.1%‐13.5%) in 2002 to 20.4% (95% CI: 16.1%‐25.6%) in 2010 (P0.001 for the trend). This observed increase paralleled the increase in the frequency of culture collection in patients hospitalized with CAP (P=0.12 for the difference in temporal trends). The estimated absolute number of visits for respiratory symptoms due a nonpneumonia illness with a blood culture collected increased from 211,000 (95% CI: 126,000296,000) in 2002 to 526,000 (95% CI: 361,000692,000) in 2010, which was similar in magnitude to the estimated number of visits for CAP with a culture collected (Table 2).
| National Weighted Estimates (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Condition | 2002 | 2003 | 2004 | 2007 | 2008 | 2009 | 2010 | P Valueb |
| Respiratory symptomc | ||||||||
| % | 9.9 (7.113.5) | 9.2 (6.912.2) | 10.6 (7.914.1) | 13.5 (10.117.8) | 15.2 (12.118.8) | 19.4 (15.923.5) | 20.4 (16.125.6) | 0.001 |
| No., thousands | 211 (126296) | 229 (140319) | 212 (140285) | 287 (191382) | 418 (288548) | 486 (344627) | 526 (361692) | |
| CAP | ||||||||
| % | 29.4 (21.938.3) | 34.2 (25.943.6) | 38.4 (31.045.4) | 45.7 (35.456.4) | 44.1 (34.154.6) | 46.7 (37.456.1) | 51.1 (41.860.3) | 0.027 |
| No., thousands | 155 (100210) | 287 (177397) | 276 (192361) | 277 (173381) | 361 (255467) | 350 (237464) | 428 (283574) | |
DISCUSSION
In this national study of ED visits, we found that the collection of blood cultures in patients hospitalized with respiratory symptoms due to an illness other than pneumonia continued to increase from 2002 to 2010 in a parallel fashion to the trend observed for patients hospitalized with CAP. Our findings suggest that the heightened attention of collecting blood cultures for suspected pneumonia had unintended consequences, which led to an increase in the collection of blood cultures in patients hospitalized with conditions that mimic pneumonia in the ED.
There can be a great deal of diagnostic uncertainty when treating patients in the ED who present with acute respiratory symptoms. Unfortunately, the initial history and physical exam are often insufficient to effectively rule in CAP.[13] Furthermore, the challenge of diagnosing pneumonia is amplified in the subset of patients who present with evolving, atypical, or occult disease. Faced with this diagnostic uncertainty, ED providers may feel pressured to comply with performance measures for CAP, promoting the overuse of inappropriate diagnostic tests and treatments. For instance, efforts to comply with early antibiotic administration in patients with CAP have led to an increase in unnecessary antibiotic use among patients with a diagnosis other than CAP.[14] Due to concerns for these unintended consequences, the core measure for early antibiotic administration was effectively retired in 2012.
Although a smaller percentage of ED visits for respiratory symptoms had a blood culture collected compared to CAP visits, there was a similar absolute number of visits with a blood culture collected during the study period. While a fraction of these patients may present with an infectious etiology aside from pneumonia, the majority of these cases likely represent situations where blood cultures add little diagnostic value at the expense of potentially longer hospital stays and broad spectrum antimicrobial use due to false‐positive results,[5, 15] as well as higher costs incurred by the test itself.[15, 16]
Although recommendations for routine culture collection for all patients hospitalized with CAP have been revised, the JCAHO/CMS core measure (PN‐3b) announced in 2002 mandates that if a culture is collected in the ED, it should be collected prior to antibiotic administration. Due to inherent uncertainty and challenges in making a timely diagnosis of pneumonia, this measure may encourage providers to reflexively order cultures in all patients presenting with respiratory symptoms in whom antibiotic administration is anticipated. The observed increasing trend in culture collection in patients hospitalized with respiratory symptoms due to a nonpneumonia illness should prompt JCAHO and CMS to reevaluate the risks and benefits of this core measure, with consideration of eliminating it altogether to discourage overuse in this population.
Our study had certain limitations. First, the omission of 2005 and 2006 data prohibited an evaluation of whether culture rates slowed down among patients hospitalized with respiratory symptoms due to a nonpneumonia illness after revisions in recommendations for obtaining cultures in patients with CAP. Second, there may have been misclassification of culture collection due to errors in chart abstraction. However, abstraction errors in the NHAMCS typically result in undercoding.[17] Therefore, our findings likely underestimate the magnitude and frequency of culture collection in this population.
In conclusion, collecting blood cultures in the ED for patients hospitalized with respiratory symptoms due to a nonpneumonia illness has increased in a parallel fashion compared to the trend in culture collection in patients hospitalized with CAP from 2002 to 2010. This suggests an important potential unintended consequence of blood culture recommendations for CAP on patients who present with conditions that resemble pneumonia. More attention to the judicious use of blood cultures in these patients to reduce harm and costs is needed.
ACKNOWLEDGEMENT
Disclosures: Dr. Makam's work on this project was completed while he was a Primary Care Research Fellow at the University of California San Francisco, funded by an NRSA training grant (T32HP19025‐07‐00). The authors report no conflicts of interest.
- , , , , , . Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31(2):347–382.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , , . The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study. Chest. 2003;123(4):1142–1150.
- , , , , , . Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005;46(5):393–400.
- , , , . Predicting bacteremia in patients with community‐acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347.
- , . The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78–82.
- , . The Joint Commission on Accreditation of Healthcare Organizations and Center for Medicare and Medicaid Services community‐acquired pneumonia initiative: what went wrong? Ann Emerg Med. 2005;46(5):409–411.
- , , . Blood culture use in the emergency department in patients hospitalized for community‐acquired pneumonia [published online ahead of print March 10, 2014]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.13808.
- , , , et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113(9):664–670.
- Centers for Disease Control and Prevention. NHAMCS scope and sample design. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_scope.htm#nhamcs_scope. Accessed May 27, 2013.
- Centers for Disease Control and Prevention. NHAMCS estimation procedures. http://www.cdc.gov/nchs/ahcd/ahcd_estimation_procedures.htm#nhamcs_procedures. Updated January 15, 2010. Accessed May 27, 2013.
- , , , et al. NHAMCS: does it hold up to scrutiny? Ann Emerg Med. 2013;62(5):549–551.
- , , . Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA. 1997;278(17):1440–1445.
- , , , . Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4‐h antibiotic administration rule. Chest. 2007;131(6):1865–1869.
- , , . Contaminant blood cultures and resource utilization. The true consequences of false‐positive results. JAMA. 1991;265(3):365–369.
- , . Analysis of strategies to improve cost effectiveness of blood cultures. J Hosp Med. 2006;1(5):272–276.
- . NHAMCS: does it hold up to scrutiny? Ann Emerg Med. 2012;60(6):722–725.
- , , , , , . Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31(2):347–382.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , , . The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study. Chest. 2003;123(4):1142–1150.
- , , , , , . Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005;46(5):393–400.
- , , , . Predicting bacteremia in patients with community‐acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347.
- , . The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78–82.
- , . The Joint Commission on Accreditation of Healthcare Organizations and Center for Medicare and Medicaid Services community‐acquired pneumonia initiative: what went wrong? Ann Emerg Med. 2005;46(5):409–411.
- , , . Blood culture use in the emergency department in patients hospitalized for community‐acquired pneumonia [published online ahead of print March 10, 2014]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.13808.
- , , , et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113(9):664–670.
- Centers for Disease Control and Prevention. NHAMCS scope and sample design. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_scope.htm#nhamcs_scope. Accessed May 27, 2013.
- Centers for Disease Control and Prevention. NHAMCS estimation procedures. http://www.cdc.gov/nchs/ahcd/ahcd_estimation_procedures.htm#nhamcs_procedures. Updated January 15, 2010. Accessed May 27, 2013.
- , , , et al. NHAMCS: does it hold up to scrutiny? Ann Emerg Med. 2013;62(5):549–551.
- , , . Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA. 1997;278(17):1440–1445.
- , , , . Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4‐h antibiotic administration rule. Chest. 2007;131(6):1865–1869.
- , , . Contaminant blood cultures and resource utilization. The true consequences of false‐positive results. JAMA. 1991;265(3):365–369.
- , . Analysis of strategies to improve cost effectiveness of blood cultures. J Hosp Med. 2006;1(5):272–276.
- . NHAMCS: does it hold up to scrutiny? Ann Emerg Med. 2012;60(6):722–725.
Peer‐Reviewed Journals and Social Media
Only 20 years ago, science from peer‐reviewed journals was still distributed and consumed in the same fashion that evolved from the earliest days of medical science: in print at monthly or weekly intervals. The Internet radically accelerated this paradigm but left the essential processes intact; journals could publish the information and readers could read it more easily, but the basic forums for interaction and discussion over the content remained the same. Enter Web 2.0 and the era of social media. Authors, editors, and readers can now interact easily with each other over the content in real time and across great distances.
Social media may not have changed the way science is produced and reviewed, but it is certainly changing how people consume and use the science. Some have suggested that social media activity around particular articles or journals may be a more important measure of impact than traditional measures of citation,[1] and others have suggested that Twitter activity in particular has changed both the speed and quality of discussion about new studies within the scientific community.[2] In the face of these trends, the Journal of Hospital Medicine (JHM) has decided to develop a bold strategy for leadership in this emerging area, with an initial focus on increasing JHM's activity and visibility on Twitter.
As part of this initial focus, JHM has successfully developed and implemented a protocol for use by authors to compose 2 Tweets describing their publications: the first announces the article's publication (e.g., New evidence on white coats and risk for hospital‐acquired infections), and the second promotes a key point from the article (e.g., Does the doctor's white coat spread hospital infection?). These Tweets are encouraged (but not required) from the corresponding author for every article in every edition, and JHM's editorial staff works with individual authors to refine their message and maximize their impact. To help authors, we have developed several tips for effective tweeting (Table 1).
| 1. Make it short:The limit is 140 characters, but getting retweets requires additional room for others to add their 2 cents, so try to get it under 100 characters. |
| 2. Make it simple: If your tweet includes complex terminology or analytic methods, it is not likely to get picked up. Make it easy to read for the lay public. |
| 3. Make it clear: Your article may have several conclusions, but pick the most newsworthy for the general public. It is usually best to focus on the main finding. |
| 4. Pose a question: Raise interest by piquing the curiosity of potential readers. A good question can motivate readers to click on your article to find the answer. |
| 5. Add a hashtag: Hashtags index tweets on Twitter. It is best to pick 1 or 2 existing tags from the healthcare hashtag project that fit the focus of your article ( |
| 6. Build your following: Include your Twitter handle to alert current/prospective followers of your publication. |
Even after just 1 year of this Twitter‐focused strategy, we are already seeing noteworthy impact and have learned several lessons.
AUTHORS CAN AND WILL GENERATE TWEETS FOR THEIR ARTICLES
When we started asking authors to generate tweets for their articles, Twitter was relatively new, and we were unsure if authors would be willing and able to participate. Since we started, we have noticed a steady increase in the number of author‐generated tweets. Today, more than three‐quarters of tweets per issue are author generated. Anecdotal feedback has been very positive, and authors have expressed interest in the plan for tweeting as well as feedback on how well their tweets were written. If authors or institutions are on Twitter, we also encourage using the Twitter name or handle in the tweet, which serves as a way for others on Twitter to identify directly with the author or institution and often results in greater interest in a particular tweet. Of note, authors have no obligation to become regular users of Twitter or engage with followers of JHM's Twitter feed, but many find themselves following the journal's feed more closely (and responding to posts by other authors) once they have joined Twitter and tweeted about their own work via JHM.
#HASHTAGS MAKE IT HAPPEN
Because Twitter users are a very large crowd of people with diverse interests, it is important to target tweets to the groups that would be most interested in studies. The use of hashtags makes it easy to index tweets. One of the major edits of author‐generated tweets that we provide is to index the articles to the most popular hashtags. For example, medical education studies can be indexed under #meded, which is a popular hashtag for clinician educators. Other important hashtags for hospitalists include #ptsafety, #readmissions, #healthpolicy, #healthcosts, or #infectiousdisease. To select hashtags, we have found the healthcare hashtag directory maintained by Symplur (Upland, CA;
HIGH IMPACT STUDIES MAKE A BIGGER IMPACT ON TWITTER
We observed a high number of retweets and comments about articles that were the most viewed studies on JHM online, referring to Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) and the Society of Hospital Medicine's Choosing Wisely campaign. This is not surprising given the national focus on readmissions as well as cost‐conscious care. Moreover, our experience is in line with observations that Twitter provides an additional source of page views and article downloads for medical journals[3] and research, which demonstrates that studies that are tweeted will eventually be cited more.[4, 5]
TECHNOLOGY STUDIES ARE ADORED BY TWITTER
Studies and research examining the use of smartphones, apps, or social media in healthcare draw a lot of attention on Twitter, particularly from other technophiles in healthcare who often use the #hscm healthcare social media hashtag. Such studies often resonate with Twitter users, who tend to be engaged in technology at a high level and are interested in how to advance the use of technology in the healthcare workplace.
JHM's social media strategy has already been very successful in its early implementation; the JHM twitter feed has >600 followers. Although most authors submit their own tweets (71/117 or 61% of articles over the last year), JHM has also created social media roles for editors to fill in tweets when missing and ensure timely and consistent output from the JHM feed. We have also started a Facebook page, with a rapidly growing number of followers, and we continue to see our social media influence scores rise. In the next year we hope to develop a JHM blog, with invited commentary as well as a process for unsolicited submissions from our readership.
Increasingly, a journal's impact (small i) is measured not only in the traditional metric of impact factor (a representation of the number of papers cited in a given journal publication year), but also by the journal's ability to disseminate knowledge and awareness of issues key to the field. Social media is a major element of the next phase of evidence dissemination, and JHM is pleased to be developing and growing its footprint in the digital world.
- , . Exploring the use of social media to measure journal article impact. AMIA Annu Symp Proc. 2011;2011:374–381.
- . Peer review: trial by Twitter. Nature. 2011;469(7330):286–287.
- , , , . Social media release increases dissemination of original articles in the clinical pain sciences. PLoS One. 2013;8(7):e68914.
- . Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123.
- , , , . Do altmetrics work? Twitter and ten other social web services. PLoS One. 2013;8(5):e64841.
Only 20 years ago, science from peer‐reviewed journals was still distributed and consumed in the same fashion that evolved from the earliest days of medical science: in print at monthly or weekly intervals. The Internet radically accelerated this paradigm but left the essential processes intact; journals could publish the information and readers could read it more easily, but the basic forums for interaction and discussion over the content remained the same. Enter Web 2.0 and the era of social media. Authors, editors, and readers can now interact easily with each other over the content in real time and across great distances.
Social media may not have changed the way science is produced and reviewed, but it is certainly changing how people consume and use the science. Some have suggested that social media activity around particular articles or journals may be a more important measure of impact than traditional measures of citation,[1] and others have suggested that Twitter activity in particular has changed both the speed and quality of discussion about new studies within the scientific community.[2] In the face of these trends, the Journal of Hospital Medicine (JHM) has decided to develop a bold strategy for leadership in this emerging area, with an initial focus on increasing JHM's activity and visibility on Twitter.
As part of this initial focus, JHM has successfully developed and implemented a protocol for use by authors to compose 2 Tweets describing their publications: the first announces the article's publication (e.g., New evidence on white coats and risk for hospital‐acquired infections), and the second promotes a key point from the article (e.g., Does the doctor's white coat spread hospital infection?). These Tweets are encouraged (but not required) from the corresponding author for every article in every edition, and JHM's editorial staff works with individual authors to refine their message and maximize their impact. To help authors, we have developed several tips for effective tweeting (Table 1).
| 1. Make it short:The limit is 140 characters, but getting retweets requires additional room for others to add their 2 cents, so try to get it under 100 characters. |
| 2. Make it simple: If your tweet includes complex terminology or analytic methods, it is not likely to get picked up. Make it easy to read for the lay public. |
| 3. Make it clear: Your article may have several conclusions, but pick the most newsworthy for the general public. It is usually best to focus on the main finding. |
| 4. Pose a question: Raise interest by piquing the curiosity of potential readers. A good question can motivate readers to click on your article to find the answer. |
| 5. Add a hashtag: Hashtags index tweets on Twitter. It is best to pick 1 or 2 existing tags from the healthcare hashtag project that fit the focus of your article ( |
| 6. Build your following: Include your Twitter handle to alert current/prospective followers of your publication. |
Even after just 1 year of this Twitter‐focused strategy, we are already seeing noteworthy impact and have learned several lessons.
AUTHORS CAN AND WILL GENERATE TWEETS FOR THEIR ARTICLES
When we started asking authors to generate tweets for their articles, Twitter was relatively new, and we were unsure if authors would be willing and able to participate. Since we started, we have noticed a steady increase in the number of author‐generated tweets. Today, more than three‐quarters of tweets per issue are author generated. Anecdotal feedback has been very positive, and authors have expressed interest in the plan for tweeting as well as feedback on how well their tweets were written. If authors or institutions are on Twitter, we also encourage using the Twitter name or handle in the tweet, which serves as a way for others on Twitter to identify directly with the author or institution and often results in greater interest in a particular tweet. Of note, authors have no obligation to become regular users of Twitter or engage with followers of JHM's Twitter feed, but many find themselves following the journal's feed more closely (and responding to posts by other authors) once they have joined Twitter and tweeted about their own work via JHM.
#HASHTAGS MAKE IT HAPPEN
Because Twitter users are a very large crowd of people with diverse interests, it is important to target tweets to the groups that would be most interested in studies. The use of hashtags makes it easy to index tweets. One of the major edits of author‐generated tweets that we provide is to index the articles to the most popular hashtags. For example, medical education studies can be indexed under #meded, which is a popular hashtag for clinician educators. Other important hashtags for hospitalists include #ptsafety, #readmissions, #healthpolicy, #healthcosts, or #infectiousdisease. To select hashtags, we have found the healthcare hashtag directory maintained by Symplur (Upland, CA;
HIGH IMPACT STUDIES MAKE A BIGGER IMPACT ON TWITTER
We observed a high number of retweets and comments about articles that were the most viewed studies on JHM online, referring to Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) and the Society of Hospital Medicine's Choosing Wisely campaign. This is not surprising given the national focus on readmissions as well as cost‐conscious care. Moreover, our experience is in line with observations that Twitter provides an additional source of page views and article downloads for medical journals[3] and research, which demonstrates that studies that are tweeted will eventually be cited more.[4, 5]
TECHNOLOGY STUDIES ARE ADORED BY TWITTER
Studies and research examining the use of smartphones, apps, or social media in healthcare draw a lot of attention on Twitter, particularly from other technophiles in healthcare who often use the #hscm healthcare social media hashtag. Such studies often resonate with Twitter users, who tend to be engaged in technology at a high level and are interested in how to advance the use of technology in the healthcare workplace.
JHM's social media strategy has already been very successful in its early implementation; the JHM twitter feed has >600 followers. Although most authors submit their own tweets (71/117 or 61% of articles over the last year), JHM has also created social media roles for editors to fill in tweets when missing and ensure timely and consistent output from the JHM feed. We have also started a Facebook page, with a rapidly growing number of followers, and we continue to see our social media influence scores rise. In the next year we hope to develop a JHM blog, with invited commentary as well as a process for unsolicited submissions from our readership.
Increasingly, a journal's impact (small i) is measured not only in the traditional metric of impact factor (a representation of the number of papers cited in a given journal publication year), but also by the journal's ability to disseminate knowledge and awareness of issues key to the field. Social media is a major element of the next phase of evidence dissemination, and JHM is pleased to be developing and growing its footprint in the digital world.
Only 20 years ago, science from peer‐reviewed journals was still distributed and consumed in the same fashion that evolved from the earliest days of medical science: in print at monthly or weekly intervals. The Internet radically accelerated this paradigm but left the essential processes intact; journals could publish the information and readers could read it more easily, but the basic forums for interaction and discussion over the content remained the same. Enter Web 2.0 and the era of social media. Authors, editors, and readers can now interact easily with each other over the content in real time and across great distances.
Social media may not have changed the way science is produced and reviewed, but it is certainly changing how people consume and use the science. Some have suggested that social media activity around particular articles or journals may be a more important measure of impact than traditional measures of citation,[1] and others have suggested that Twitter activity in particular has changed both the speed and quality of discussion about new studies within the scientific community.[2] In the face of these trends, the Journal of Hospital Medicine (JHM) has decided to develop a bold strategy for leadership in this emerging area, with an initial focus on increasing JHM's activity and visibility on Twitter.
As part of this initial focus, JHM has successfully developed and implemented a protocol for use by authors to compose 2 Tweets describing their publications: the first announces the article's publication (e.g., New evidence on white coats and risk for hospital‐acquired infections), and the second promotes a key point from the article (e.g., Does the doctor's white coat spread hospital infection?). These Tweets are encouraged (but not required) from the corresponding author for every article in every edition, and JHM's editorial staff works with individual authors to refine their message and maximize their impact. To help authors, we have developed several tips for effective tweeting (Table 1).
| 1. Make it short:The limit is 140 characters, but getting retweets requires additional room for others to add their 2 cents, so try to get it under 100 characters. |
| 2. Make it simple: If your tweet includes complex terminology or analytic methods, it is not likely to get picked up. Make it easy to read for the lay public. |
| 3. Make it clear: Your article may have several conclusions, but pick the most newsworthy for the general public. It is usually best to focus on the main finding. |
| 4. Pose a question: Raise interest by piquing the curiosity of potential readers. A good question can motivate readers to click on your article to find the answer. |
| 5. Add a hashtag: Hashtags index tweets on Twitter. It is best to pick 1 or 2 existing tags from the healthcare hashtag project that fit the focus of your article ( |
| 6. Build your following: Include your Twitter handle to alert current/prospective followers of your publication. |
Even after just 1 year of this Twitter‐focused strategy, we are already seeing noteworthy impact and have learned several lessons.
AUTHORS CAN AND WILL GENERATE TWEETS FOR THEIR ARTICLES
When we started asking authors to generate tweets for their articles, Twitter was relatively new, and we were unsure if authors would be willing and able to participate. Since we started, we have noticed a steady increase in the number of author‐generated tweets. Today, more than three‐quarters of tweets per issue are author generated. Anecdotal feedback has been very positive, and authors have expressed interest in the plan for tweeting as well as feedback on how well their tweets were written. If authors or institutions are on Twitter, we also encourage using the Twitter name or handle in the tweet, which serves as a way for others on Twitter to identify directly with the author or institution and often results in greater interest in a particular tweet. Of note, authors have no obligation to become regular users of Twitter or engage with followers of JHM's Twitter feed, but many find themselves following the journal's feed more closely (and responding to posts by other authors) once they have joined Twitter and tweeted about their own work via JHM.
#HASHTAGS MAKE IT HAPPEN
Because Twitter users are a very large crowd of people with diverse interests, it is important to target tweets to the groups that would be most interested in studies. The use of hashtags makes it easy to index tweets. One of the major edits of author‐generated tweets that we provide is to index the articles to the most popular hashtags. For example, medical education studies can be indexed under #meded, which is a popular hashtag for clinician educators. Other important hashtags for hospitalists include #ptsafety, #readmissions, #healthpolicy, #healthcosts, or #infectiousdisease. To select hashtags, we have found the healthcare hashtag directory maintained by Symplur (Upland, CA;
HIGH IMPACT STUDIES MAKE A BIGGER IMPACT ON TWITTER
We observed a high number of retweets and comments about articles that were the most viewed studies on JHM online, referring to Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) and the Society of Hospital Medicine's Choosing Wisely campaign. This is not surprising given the national focus on readmissions as well as cost‐conscious care. Moreover, our experience is in line with observations that Twitter provides an additional source of page views and article downloads for medical journals[3] and research, which demonstrates that studies that are tweeted will eventually be cited more.[4, 5]
TECHNOLOGY STUDIES ARE ADORED BY TWITTER
Studies and research examining the use of smartphones, apps, or social media in healthcare draw a lot of attention on Twitter, particularly from other technophiles in healthcare who often use the #hscm healthcare social media hashtag. Such studies often resonate with Twitter users, who tend to be engaged in technology at a high level and are interested in how to advance the use of technology in the healthcare workplace.
JHM's social media strategy has already been very successful in its early implementation; the JHM twitter feed has >600 followers. Although most authors submit their own tweets (71/117 or 61% of articles over the last year), JHM has also created social media roles for editors to fill in tweets when missing and ensure timely and consistent output from the JHM feed. We have also started a Facebook page, with a rapidly growing number of followers, and we continue to see our social media influence scores rise. In the next year we hope to develop a JHM blog, with invited commentary as well as a process for unsolicited submissions from our readership.
Increasingly, a journal's impact (small i) is measured not only in the traditional metric of impact factor (a representation of the number of papers cited in a given journal publication year), but also by the journal's ability to disseminate knowledge and awareness of issues key to the field. Social media is a major element of the next phase of evidence dissemination, and JHM is pleased to be developing and growing its footprint in the digital world.
- , . Exploring the use of social media to measure journal article impact. AMIA Annu Symp Proc. 2011;2011:374–381.
- . Peer review: trial by Twitter. Nature. 2011;469(7330):286–287.
- , , , . Social media release increases dissemination of original articles in the clinical pain sciences. PLoS One. 2013;8(7):e68914.
- . Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123.
- , , , . Do altmetrics work? Twitter and ten other social web services. PLoS One. 2013;8(5):e64841.
- , . Exploring the use of social media to measure journal article impact. AMIA Annu Symp Proc. 2011;2011:374–381.
- . Peer review: trial by Twitter. Nature. 2011;469(7330):286–287.
- , , , . Social media release increases dissemination of original articles in the clinical pain sciences. PLoS One. 2013;8(7):e68914.
- . Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123.
- , , , . Do altmetrics work? Twitter and ten other social web services. PLoS One. 2013;8(5):e64841.
Tablet Computers to Engage Patients
BACKGROUND
Many hospitals have initiated intense efforts to improve transitions of care[1] such as discharge coordinators or transition coaches,[2, 3] but use of mobile devices as approaches to add or extend the value of human interventions have been understudied.[4] Additionally, many hospitalized patients experience substantial inactive time between provider visits, tests, and treatments. This time could be used to engage patients in their care through interactive health education modules and by learning to use their PHR to manage medications and postdischarge appointments.
Greater understanding of the advantages and limitations of mobile devices may be important for improving transitions of care and may help leverage existing hospital personnel resources. However, prior studies have focused on healthcare provider uses of tablet computers for medical education,[5] to collect clinical registration data,[6] or to do clinical work (eg, check labs, write notes)[7, 8, 9] primarily in outpatient settings; few studies have focused on patient uses for this technology in hospital settings.[10, 11] To address these knowledge gaps, we conducted a pilot project to explore inpatient satisfaction with bedside tablets and barriers to usability. Additionally, we evaluated use of these devices to deliver 2 specific Web‐based programs: (1) an interactive video to improve inpatient education about hospital safety, and (2) PHR access to promote inpatient engagement in discharge planning.
METHODS
Study Design, Patient Selection, and Devices/Programs
We conducted a prospective study of tablet computers to engage patients in their care and discharge planning through Web‐based interactive health education modules and use of PHRs. We used 2 tablets, distributed daily by research assistants (RAs) to eligible patients after morning rounds. Inclusion criteria for patients were ability to speak English and admission to the medical (hospitalist) service at University of California San Francisco (UCSF) Medical Center. Exclusion criteria were intensive care unit admission, contact isolation, or inability to complete the consent process due to altered mental status or cognitive impairment.
RAs screened patients for inclusion/exclusion via the electronic medical record and then approached them after rounds for enrollment (11:00 am1:00 pm). RAs then performed a tiered orientation tailored to individual patient experience and needs. First, they delivered a brief tutorial focused on the tablet itself and its basic functions (touchscreen, keypad, and Internet browser use). Second, RAs showed patients how to access the online educational health module and how to navigate content within the module. RAs next explained what the PHR is and demonstrated how to login, how to navigate tabs within the PHR, and how to perform basic tasks (view/refill medications, view/modify appointments, and view/send messages to providers). The RAs left devices with patients for 3 to 5 hours and returned to collect them and perform debriefing interviews. After each device was returned, RAs cleaned devices with disinfectant wipes available in patient rooms and checked for physical damage or software malfunctions (eg, unable to turn on or unlock). Finally, RAs used the reset function to erase any personal data or setting modifications made by patients and docked the devices overnight to resynchronize the original settings and recharge the batteries.
We used the 16 gigabyte Apple iPad2 (Apple Inc., Cupertino, CA) without any enclosures, cases, or security devices. Our educational health module was Patient Safety in the Hospital, which was professionally developed by Emmi Solutions (
Survey Instruments and Data Collection
We developed pre‐ and postintervention surveys to characterize patients' demographics, device ownership, and health‐related Internet activities in the last year based on questions used in the Centers for Disease Control and Prevention National Health Interview Study (
Analyses
We used frequency analysis to describe patient demographics, ability to complete online health educational modules, and utilization of their PHR. We performed bivariate analyses (Fisher exact test) to assess correlations between demographics (age, device ownership, Internet use) and key pilot program performance measures (orientation time 15 minutes, online health module completion, and completion of 1 essential function in the PHR). All analyses were performed in SAS 9.2 (SAS Institute Inc., Cary, NC). The institutional review board of record for UCSF approved this study.
RESULTS
As shown in Table 1, we enrolled 30 patients. Most participants (60%) were aged 40 years or older, and most (87%) owned a mobile device; 70% owned a laptop and 60% owned a smartphone, but few (22%) owned a computer tablet. Most participants accessed the Internet daily, but fewer reported Internet use for health tasks; about half (52%) communicated with a provider, but few refilled a prescription (27%) or scheduled an appointment (21%) online over the last year.
| Characteristic | No. (%) |
|---|---|
| Age, y | |
| 1839 | 11 (38%) |
| 4049 | 5 (18%) |
| 5059 | 4 (14%) |
| 6069 | 5 (18%) |
| 7079 | 3 (10%) |
| Gender, female | 17 (60%) |
| Device ownership | |
| Desktop computer | 12 (44%) |
| Laptop computer | 19 (70%) |
| Smart phone | 17 (60%) |
| Tablet computer | 6 (22%) |
| Any mobile device (laptop, smartphone, or tablet) | 26 (87%) |
| Internet use | |
| Daily | 21 (72%) |
| Several times a week | 3 (10%) |
| Once a week or less | 5 (18%) |
| Prestudy online health tasks | |
| Looked up health information | 21 (72%) |
| Communicated with provider | 15 (52%) |
| Refilled prescription | 8 (27%) |
| Scheduled medical appointment | 6 (21%) |
Nearly all participants (90%) were satisfied or very satisfied with their experience using the tablet in the hospital (Figure 1). Most (87%) required 30 minutes or less for basic orientation, and 70% required only 15 minutes or less. Most participants (83%) were able to independently complete an interactive health education module on hospital safety and were highly satisfied with the module. Despite the fact that 73% of participants were first‐time users of our PHR, the majority were able to login and independently access their medication list, verify scheduled appointments, or send a secure message to their primary care provider.
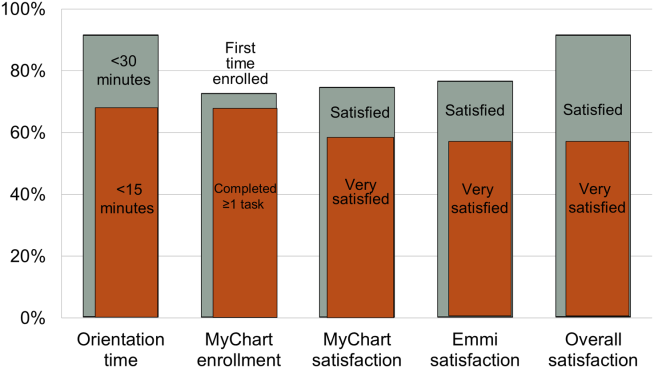
Participants aged 50 years or older were less likely to complete orientation in 15 minutes or less compared to those under 50 years old (25% vs 79%, P=0.025); however, age was not a significant factor in ability to complete the online health educational module or perform at least 1 essential PHR function. Other demographic features, such as device ownership and daily Internet use, did not correlate with shorter orientation time, educational module completion, or PHR use (data available on request).
Participants also made suggestions for improvement during the debrief interviews. Several suggested applications for entertainment (gaming, magazines, or music) and 2 suggested that all patients should be introduced to their PHR during hospitalization (data available on request). No device software malfunction (eg, device freezes, Internet connection failures), hardware issues (eg, damage from falls, wetness, or repeated disinfectant exposure), or theft or misappropriation were reported by patients or observed by the RAs to date.
DISCUSSION
Our pilot study suggests that tablet‐based access to educational modules and PHRs can increase inpatient engagement in care with high satisfaction and minimal time for orientation. Surprisingly, even older patients and those who might be considered less tech savvy in terms of Internet use and device ownership were equally able to utilize our tablet interventions. Furthermore, we did not experience any hardware issues in the harsh physical environment of inpatient wards. These preliminary findings suggest the potential utility of tablets for clinically meaningful tasks by inpatients with a low rate of technical issues.
From a technical standpoint, our experience suggests several next steps. First, although orientation time was minimal, it might be even less if patients used their own mobile devices because most patients already owned one. This bring your own device (BYOD) approach could also promote postdischarge patient engagement. Second, the flexibility of a BYOD approach raises the question of whether to also allow patients a choice of application‐based versus browser‐based platforms for specific programs such as the PHR and educational video we used. Indeed, although we used a browser‐based approach, several other teams have developed patient‐facing engagement applications (or apps) for mobile devices,[4, 12] and hospitalists could prescribe apps at discharge as a more providers are now doing in outpatient settings.[13] Of course, maximizing flexibility for BYOD and Web‐based versus app‐based approaches would likely increase patient engagement but would come at the cost of more time and effort for hospital providers to vet apps/websites and educate patients about their use. Third, regardless of the devices and programs used, broader engagement of patients, nurses, hospitalists, and other providers will be needed in the future to identify key areas for development to avoid overburdening patients and providers.
From a quality‐improvement perspective, recent literature has considered broad clinical uses for tablets by hospital providers,[14, 15] but our experience suggests more specific opportunities to improve transitions of care though direct patient engagement. Tablets and other mobile devices may help improve discharge education for patients taking high‐risk medications such as warfarin or insulin using interactive educational modules similar to the hospital safety modules we used. Additionally, clinical staff, such as nurses and pharmacists, can be trained to deliver mobile device interventions such as education on high‐risk medications.[16] Ultimately, scale up for our intervention will require that mobile devices and content eventually improve and replace current practices by hospital staff (especially nurses) in a way that streamlines, rather than compounds, current workflow. This could increase efficiency in these discharge tasks and extend contributions of these providers to high‐quality transitions.
Our study has several limitations. First, although this is a pilot study with only 30 patients, it adds needed scale to much smaller (N=58) published feasibility studies of tablet computer use by inpatients.[11, 12] Beyond more robust feasibility testing, our study adds new data about mobile device use for specific clinical tasks in the hospital such as patient education and PHR use. Second, we did not track postdischarge outcomes to test the effects of our intervention on transition care quality; this will be a focus of our future research. Third, we used existing platforms for interactive educational modules and PHR access at our site; participant satisfaction in our study may not generalize to other platforms. Furthermore, most PHR platforms (including ours) are not optimally configured to engage patients during transitions of care, but we plan to integrate existing functions (such as ability to refill medications or change appointments) into discharge education and planning. Finally, we have not engaged caregivers as surrogates for cognitively impaired patients or adapted our platform for non‐English speakers; these are areas for development in our ongoing work. Overall, our pilot results help set the stage to deploy mobile devices for better patient monitoring, engagement, and quality of care in the inpatient setting.[17]
In conclusion, our pilot project demonstrates that tablet computers can be used to improve inpatient education and patient engagement in discharge planning. Inpatients are highly satisfied with the use of tablets to complete health education modules and access their PHR, with minimal time required for patient training and device management by hospital staff. Tablets and other mobile devices have significant potential to improve patients' education and engagement in their hospital care.
Acknowledgements
The authors thank the UCSF mHealth group and Center for Digital Health Innovation for advice and for providing tablet computers for this pilot project.
Disclosures: This article was presented as a finalist in the Research, Innovations, and Clinical Vignettes competition (Innovations category) at the 2013 Annual Meeting of the Society for Hospital Medicine. Dr. Auerbach was supported by grant K24HL098372 (NHLBI). Dr. Greysen was supported by a career development award (KL‐2) from the UCSF Clinical Translational Sciences Institute. The authors have declared they have no financial, personal, or other conflicts of interest relevant to this study.
- , . Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306(16):1794–1795.
- , , , et al. A reengineered hospital discharge program to decrease re‐hospitalization. Ann Intern Med. 2009;150:178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
- Project RED. Meet Louise…and virtual patient advocates. Available at: http://www.bu.edu/fammed/projectred/publications/VirtualPatientAdvocateWebsiteInfo2.pdf. Accessed July 12, 2013.
- , , , . Use of handheld computers in medical education. A systematic review. J Gen Intern Med. 2006;21(5):531–537.
- , , , . Ongoing evaluation of ease‐of‐use and usefulness of wireless tablet computers within an ambulatory care unit. Stud Health Tech Inform. 2009;143:459–464.
- . Use of a tablet personal computer to enhance patient care on multidisciplinary rounds. Am J Health Syst Pharm. 2009;66(21):1909–1911.
- , . Experiences incorporating Tablet PCs into clinical pharmacists' workflow. J Healthc Inf Manag. 2005;19(4):32–37.
- , , . The impact of mobile handheld technology on hospital physicians' work practices and patient care: a systematic review. J Am Med Inform Assoc. 2009;16(6):792–801.
- , , , et al. An investigation of the efficacy of electronic consenting interfaces of research permissions management system in a hospital setting. Int J Med Inform. 2013;82(9):854–863.
- , , , et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428–1435.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39(1):15–19.
- . How apps are changing family medicine. J Fam Pract. 2013Jul;62(7):362–367.
- . The iPad: gadget or medical godsend? Ann Emerg Med. 2010;56(1):A21–A22.
- , , , et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233–241.
- . Keeping the patient focus: using tablet technology to enhance education and practice. J Contin Educ Nurs. 2012;43(6):249–250.
- , , , et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5–10.
BACKGROUND
Many hospitals have initiated intense efforts to improve transitions of care[1] such as discharge coordinators or transition coaches,[2, 3] but use of mobile devices as approaches to add or extend the value of human interventions have been understudied.[4] Additionally, many hospitalized patients experience substantial inactive time between provider visits, tests, and treatments. This time could be used to engage patients in their care through interactive health education modules and by learning to use their PHR to manage medications and postdischarge appointments.
Greater understanding of the advantages and limitations of mobile devices may be important for improving transitions of care and may help leverage existing hospital personnel resources. However, prior studies have focused on healthcare provider uses of tablet computers for medical education,[5] to collect clinical registration data,[6] or to do clinical work (eg, check labs, write notes)[7, 8, 9] primarily in outpatient settings; few studies have focused on patient uses for this technology in hospital settings.[10, 11] To address these knowledge gaps, we conducted a pilot project to explore inpatient satisfaction with bedside tablets and barriers to usability. Additionally, we evaluated use of these devices to deliver 2 specific Web‐based programs: (1) an interactive video to improve inpatient education about hospital safety, and (2) PHR access to promote inpatient engagement in discharge planning.
METHODS
Study Design, Patient Selection, and Devices/Programs
We conducted a prospective study of tablet computers to engage patients in their care and discharge planning through Web‐based interactive health education modules and use of PHRs. We used 2 tablets, distributed daily by research assistants (RAs) to eligible patients after morning rounds. Inclusion criteria for patients were ability to speak English and admission to the medical (hospitalist) service at University of California San Francisco (UCSF) Medical Center. Exclusion criteria were intensive care unit admission, contact isolation, or inability to complete the consent process due to altered mental status or cognitive impairment.
RAs screened patients for inclusion/exclusion via the electronic medical record and then approached them after rounds for enrollment (11:00 am1:00 pm). RAs then performed a tiered orientation tailored to individual patient experience and needs. First, they delivered a brief tutorial focused on the tablet itself and its basic functions (touchscreen, keypad, and Internet browser use). Second, RAs showed patients how to access the online educational health module and how to navigate content within the module. RAs next explained what the PHR is and demonstrated how to login, how to navigate tabs within the PHR, and how to perform basic tasks (view/refill medications, view/modify appointments, and view/send messages to providers). The RAs left devices with patients for 3 to 5 hours and returned to collect them and perform debriefing interviews. After each device was returned, RAs cleaned devices with disinfectant wipes available in patient rooms and checked for physical damage or software malfunctions (eg, unable to turn on or unlock). Finally, RAs used the reset function to erase any personal data or setting modifications made by patients and docked the devices overnight to resynchronize the original settings and recharge the batteries.
We used the 16 gigabyte Apple iPad2 (Apple Inc., Cupertino, CA) without any enclosures, cases, or security devices. Our educational health module was Patient Safety in the Hospital, which was professionally developed by Emmi Solutions (
Survey Instruments and Data Collection
We developed pre‐ and postintervention surveys to characterize patients' demographics, device ownership, and health‐related Internet activities in the last year based on questions used in the Centers for Disease Control and Prevention National Health Interview Study (
Analyses
We used frequency analysis to describe patient demographics, ability to complete online health educational modules, and utilization of their PHR. We performed bivariate analyses (Fisher exact test) to assess correlations between demographics (age, device ownership, Internet use) and key pilot program performance measures (orientation time 15 minutes, online health module completion, and completion of 1 essential function in the PHR). All analyses were performed in SAS 9.2 (SAS Institute Inc., Cary, NC). The institutional review board of record for UCSF approved this study.
RESULTS
As shown in Table 1, we enrolled 30 patients. Most participants (60%) were aged 40 years or older, and most (87%) owned a mobile device; 70% owned a laptop and 60% owned a smartphone, but few (22%) owned a computer tablet. Most participants accessed the Internet daily, but fewer reported Internet use for health tasks; about half (52%) communicated with a provider, but few refilled a prescription (27%) or scheduled an appointment (21%) online over the last year.
| Characteristic | No. (%) |
|---|---|
| Age, y | |
| 1839 | 11 (38%) |
| 4049 | 5 (18%) |
| 5059 | 4 (14%) |
| 6069 | 5 (18%) |
| 7079 | 3 (10%) |
| Gender, female | 17 (60%) |
| Device ownership | |
| Desktop computer | 12 (44%) |
| Laptop computer | 19 (70%) |
| Smart phone | 17 (60%) |
| Tablet computer | 6 (22%) |
| Any mobile device (laptop, smartphone, or tablet) | 26 (87%) |
| Internet use | |
| Daily | 21 (72%) |
| Several times a week | 3 (10%) |
| Once a week or less | 5 (18%) |
| Prestudy online health tasks | |
| Looked up health information | 21 (72%) |
| Communicated with provider | 15 (52%) |
| Refilled prescription | 8 (27%) |
| Scheduled medical appointment | 6 (21%) |
Nearly all participants (90%) were satisfied or very satisfied with their experience using the tablet in the hospital (Figure 1). Most (87%) required 30 minutes or less for basic orientation, and 70% required only 15 minutes or less. Most participants (83%) were able to independently complete an interactive health education module on hospital safety and were highly satisfied with the module. Despite the fact that 73% of participants were first‐time users of our PHR, the majority were able to login and independently access their medication list, verify scheduled appointments, or send a secure message to their primary care provider.

Participants aged 50 years or older were less likely to complete orientation in 15 minutes or less compared to those under 50 years old (25% vs 79%, P=0.025); however, age was not a significant factor in ability to complete the online health educational module or perform at least 1 essential PHR function. Other demographic features, such as device ownership and daily Internet use, did not correlate with shorter orientation time, educational module completion, or PHR use (data available on request).
Participants also made suggestions for improvement during the debrief interviews. Several suggested applications for entertainment (gaming, magazines, or music) and 2 suggested that all patients should be introduced to their PHR during hospitalization (data available on request). No device software malfunction (eg, device freezes, Internet connection failures), hardware issues (eg, damage from falls, wetness, or repeated disinfectant exposure), or theft or misappropriation were reported by patients or observed by the RAs to date.
DISCUSSION
Our pilot study suggests that tablet‐based access to educational modules and PHRs can increase inpatient engagement in care with high satisfaction and minimal time for orientation. Surprisingly, even older patients and those who might be considered less tech savvy in terms of Internet use and device ownership were equally able to utilize our tablet interventions. Furthermore, we did not experience any hardware issues in the harsh physical environment of inpatient wards. These preliminary findings suggest the potential utility of tablets for clinically meaningful tasks by inpatients with a low rate of technical issues.
From a technical standpoint, our experience suggests several next steps. First, although orientation time was minimal, it might be even less if patients used their own mobile devices because most patients already owned one. This bring your own device (BYOD) approach could also promote postdischarge patient engagement. Second, the flexibility of a BYOD approach raises the question of whether to also allow patients a choice of application‐based versus browser‐based platforms for specific programs such as the PHR and educational video we used. Indeed, although we used a browser‐based approach, several other teams have developed patient‐facing engagement applications (or apps) for mobile devices,[4, 12] and hospitalists could prescribe apps at discharge as a more providers are now doing in outpatient settings.[13] Of course, maximizing flexibility for BYOD and Web‐based versus app‐based approaches would likely increase patient engagement but would come at the cost of more time and effort for hospital providers to vet apps/websites and educate patients about their use. Third, regardless of the devices and programs used, broader engagement of patients, nurses, hospitalists, and other providers will be needed in the future to identify key areas for development to avoid overburdening patients and providers.
From a quality‐improvement perspective, recent literature has considered broad clinical uses for tablets by hospital providers,[14, 15] but our experience suggests more specific opportunities to improve transitions of care though direct patient engagement. Tablets and other mobile devices may help improve discharge education for patients taking high‐risk medications such as warfarin or insulin using interactive educational modules similar to the hospital safety modules we used. Additionally, clinical staff, such as nurses and pharmacists, can be trained to deliver mobile device interventions such as education on high‐risk medications.[16] Ultimately, scale up for our intervention will require that mobile devices and content eventually improve and replace current practices by hospital staff (especially nurses) in a way that streamlines, rather than compounds, current workflow. This could increase efficiency in these discharge tasks and extend contributions of these providers to high‐quality transitions.
Our study has several limitations. First, although this is a pilot study with only 30 patients, it adds needed scale to much smaller (N=58) published feasibility studies of tablet computer use by inpatients.[11, 12] Beyond more robust feasibility testing, our study adds new data about mobile device use for specific clinical tasks in the hospital such as patient education and PHR use. Second, we did not track postdischarge outcomes to test the effects of our intervention on transition care quality; this will be a focus of our future research. Third, we used existing platforms for interactive educational modules and PHR access at our site; participant satisfaction in our study may not generalize to other platforms. Furthermore, most PHR platforms (including ours) are not optimally configured to engage patients during transitions of care, but we plan to integrate existing functions (such as ability to refill medications or change appointments) into discharge education and planning. Finally, we have not engaged caregivers as surrogates for cognitively impaired patients or adapted our platform for non‐English speakers; these are areas for development in our ongoing work. Overall, our pilot results help set the stage to deploy mobile devices for better patient monitoring, engagement, and quality of care in the inpatient setting.[17]
In conclusion, our pilot project demonstrates that tablet computers can be used to improve inpatient education and patient engagement in discharge planning. Inpatients are highly satisfied with the use of tablets to complete health education modules and access their PHR, with minimal time required for patient training and device management by hospital staff. Tablets and other mobile devices have significant potential to improve patients' education and engagement in their hospital care.
Acknowledgements
The authors thank the UCSF mHealth group and Center for Digital Health Innovation for advice and for providing tablet computers for this pilot project.
Disclosures: This article was presented as a finalist in the Research, Innovations, and Clinical Vignettes competition (Innovations category) at the 2013 Annual Meeting of the Society for Hospital Medicine. Dr. Auerbach was supported by grant K24HL098372 (NHLBI). Dr. Greysen was supported by a career development award (KL‐2) from the UCSF Clinical Translational Sciences Institute. The authors have declared they have no financial, personal, or other conflicts of interest relevant to this study.
BACKGROUND
Many hospitals have initiated intense efforts to improve transitions of care[1] such as discharge coordinators or transition coaches,[2, 3] but use of mobile devices as approaches to add or extend the value of human interventions have been understudied.[4] Additionally, many hospitalized patients experience substantial inactive time between provider visits, tests, and treatments. This time could be used to engage patients in their care through interactive health education modules and by learning to use their PHR to manage medications and postdischarge appointments.
Greater understanding of the advantages and limitations of mobile devices may be important for improving transitions of care and may help leverage existing hospital personnel resources. However, prior studies have focused on healthcare provider uses of tablet computers for medical education,[5] to collect clinical registration data,[6] or to do clinical work (eg, check labs, write notes)[7, 8, 9] primarily in outpatient settings; few studies have focused on patient uses for this technology in hospital settings.[10, 11] To address these knowledge gaps, we conducted a pilot project to explore inpatient satisfaction with bedside tablets and barriers to usability. Additionally, we evaluated use of these devices to deliver 2 specific Web‐based programs: (1) an interactive video to improve inpatient education about hospital safety, and (2) PHR access to promote inpatient engagement in discharge planning.
METHODS
Study Design, Patient Selection, and Devices/Programs
We conducted a prospective study of tablet computers to engage patients in their care and discharge planning through Web‐based interactive health education modules and use of PHRs. We used 2 tablets, distributed daily by research assistants (RAs) to eligible patients after morning rounds. Inclusion criteria for patients were ability to speak English and admission to the medical (hospitalist) service at University of California San Francisco (UCSF) Medical Center. Exclusion criteria were intensive care unit admission, contact isolation, or inability to complete the consent process due to altered mental status or cognitive impairment.
RAs screened patients for inclusion/exclusion via the electronic medical record and then approached them after rounds for enrollment (11:00 am1:00 pm). RAs then performed a tiered orientation tailored to individual patient experience and needs. First, they delivered a brief tutorial focused on the tablet itself and its basic functions (touchscreen, keypad, and Internet browser use). Second, RAs showed patients how to access the online educational health module and how to navigate content within the module. RAs next explained what the PHR is and demonstrated how to login, how to navigate tabs within the PHR, and how to perform basic tasks (view/refill medications, view/modify appointments, and view/send messages to providers). The RAs left devices with patients for 3 to 5 hours and returned to collect them and perform debriefing interviews. After each device was returned, RAs cleaned devices with disinfectant wipes available in patient rooms and checked for physical damage or software malfunctions (eg, unable to turn on or unlock). Finally, RAs used the reset function to erase any personal data or setting modifications made by patients and docked the devices overnight to resynchronize the original settings and recharge the batteries.
We used the 16 gigabyte Apple iPad2 (Apple Inc., Cupertino, CA) without any enclosures, cases, or security devices. Our educational health module was Patient Safety in the Hospital, which was professionally developed by Emmi Solutions (
Survey Instruments and Data Collection
We developed pre‐ and postintervention surveys to characterize patients' demographics, device ownership, and health‐related Internet activities in the last year based on questions used in the Centers for Disease Control and Prevention National Health Interview Study (
Analyses
We used frequency analysis to describe patient demographics, ability to complete online health educational modules, and utilization of their PHR. We performed bivariate analyses (Fisher exact test) to assess correlations between demographics (age, device ownership, Internet use) and key pilot program performance measures (orientation time 15 minutes, online health module completion, and completion of 1 essential function in the PHR). All analyses were performed in SAS 9.2 (SAS Institute Inc., Cary, NC). The institutional review board of record for UCSF approved this study.
RESULTS
As shown in Table 1, we enrolled 30 patients. Most participants (60%) were aged 40 years or older, and most (87%) owned a mobile device; 70% owned a laptop and 60% owned a smartphone, but few (22%) owned a computer tablet. Most participants accessed the Internet daily, but fewer reported Internet use for health tasks; about half (52%) communicated with a provider, but few refilled a prescription (27%) or scheduled an appointment (21%) online over the last year.
| Characteristic | No. (%) |
|---|---|
| Age, y | |
| 1839 | 11 (38%) |
| 4049 | 5 (18%) |
| 5059 | 4 (14%) |
| 6069 | 5 (18%) |
| 7079 | 3 (10%) |
| Gender, female | 17 (60%) |
| Device ownership | |
| Desktop computer | 12 (44%) |
| Laptop computer | 19 (70%) |
| Smart phone | 17 (60%) |
| Tablet computer | 6 (22%) |
| Any mobile device (laptop, smartphone, or tablet) | 26 (87%) |
| Internet use | |
| Daily | 21 (72%) |
| Several times a week | 3 (10%) |
| Once a week or less | 5 (18%) |
| Prestudy online health tasks | |
| Looked up health information | 21 (72%) |
| Communicated with provider | 15 (52%) |
| Refilled prescription | 8 (27%) |
| Scheduled medical appointment | 6 (21%) |
Nearly all participants (90%) were satisfied or very satisfied with their experience using the tablet in the hospital (Figure 1). Most (87%) required 30 minutes or less for basic orientation, and 70% required only 15 minutes or less. Most participants (83%) were able to independently complete an interactive health education module on hospital safety and were highly satisfied with the module. Despite the fact that 73% of participants were first‐time users of our PHR, the majority were able to login and independently access their medication list, verify scheduled appointments, or send a secure message to their primary care provider.

Participants aged 50 years or older were less likely to complete orientation in 15 minutes or less compared to those under 50 years old (25% vs 79%, P=0.025); however, age was not a significant factor in ability to complete the online health educational module or perform at least 1 essential PHR function. Other demographic features, such as device ownership and daily Internet use, did not correlate with shorter orientation time, educational module completion, or PHR use (data available on request).
Participants also made suggestions for improvement during the debrief interviews. Several suggested applications for entertainment (gaming, magazines, or music) and 2 suggested that all patients should be introduced to their PHR during hospitalization (data available on request). No device software malfunction (eg, device freezes, Internet connection failures), hardware issues (eg, damage from falls, wetness, or repeated disinfectant exposure), or theft or misappropriation were reported by patients or observed by the RAs to date.
DISCUSSION
Our pilot study suggests that tablet‐based access to educational modules and PHRs can increase inpatient engagement in care with high satisfaction and minimal time for orientation. Surprisingly, even older patients and those who might be considered less tech savvy in terms of Internet use and device ownership were equally able to utilize our tablet interventions. Furthermore, we did not experience any hardware issues in the harsh physical environment of inpatient wards. These preliminary findings suggest the potential utility of tablets for clinically meaningful tasks by inpatients with a low rate of technical issues.
From a technical standpoint, our experience suggests several next steps. First, although orientation time was minimal, it might be even less if patients used their own mobile devices because most patients already owned one. This bring your own device (BYOD) approach could also promote postdischarge patient engagement. Second, the flexibility of a BYOD approach raises the question of whether to also allow patients a choice of application‐based versus browser‐based platforms for specific programs such as the PHR and educational video we used. Indeed, although we used a browser‐based approach, several other teams have developed patient‐facing engagement applications (or apps) for mobile devices,[4, 12] and hospitalists could prescribe apps at discharge as a more providers are now doing in outpatient settings.[13] Of course, maximizing flexibility for BYOD and Web‐based versus app‐based approaches would likely increase patient engagement but would come at the cost of more time and effort for hospital providers to vet apps/websites and educate patients about their use. Third, regardless of the devices and programs used, broader engagement of patients, nurses, hospitalists, and other providers will be needed in the future to identify key areas for development to avoid overburdening patients and providers.
From a quality‐improvement perspective, recent literature has considered broad clinical uses for tablets by hospital providers,[14, 15] but our experience suggests more specific opportunities to improve transitions of care though direct patient engagement. Tablets and other mobile devices may help improve discharge education for patients taking high‐risk medications such as warfarin or insulin using interactive educational modules similar to the hospital safety modules we used. Additionally, clinical staff, such as nurses and pharmacists, can be trained to deliver mobile device interventions such as education on high‐risk medications.[16] Ultimately, scale up for our intervention will require that mobile devices and content eventually improve and replace current practices by hospital staff (especially nurses) in a way that streamlines, rather than compounds, current workflow. This could increase efficiency in these discharge tasks and extend contributions of these providers to high‐quality transitions.
Our study has several limitations. First, although this is a pilot study with only 30 patients, it adds needed scale to much smaller (N=58) published feasibility studies of tablet computer use by inpatients.[11, 12] Beyond more robust feasibility testing, our study adds new data about mobile device use for specific clinical tasks in the hospital such as patient education and PHR use. Second, we did not track postdischarge outcomes to test the effects of our intervention on transition care quality; this will be a focus of our future research. Third, we used existing platforms for interactive educational modules and PHR access at our site; participant satisfaction in our study may not generalize to other platforms. Furthermore, most PHR platforms (including ours) are not optimally configured to engage patients during transitions of care, but we plan to integrate existing functions (such as ability to refill medications or change appointments) into discharge education and planning. Finally, we have not engaged caregivers as surrogates for cognitively impaired patients or adapted our platform for non‐English speakers; these are areas for development in our ongoing work. Overall, our pilot results help set the stage to deploy mobile devices for better patient monitoring, engagement, and quality of care in the inpatient setting.[17]
In conclusion, our pilot project demonstrates that tablet computers can be used to improve inpatient education and patient engagement in discharge planning. Inpatients are highly satisfied with the use of tablets to complete health education modules and access their PHR, with minimal time required for patient training and device management by hospital staff. Tablets and other mobile devices have significant potential to improve patients' education and engagement in their hospital care.
Acknowledgements
The authors thank the UCSF mHealth group and Center for Digital Health Innovation for advice and for providing tablet computers for this pilot project.
Disclosures: This article was presented as a finalist in the Research, Innovations, and Clinical Vignettes competition (Innovations category) at the 2013 Annual Meeting of the Society for Hospital Medicine. Dr. Auerbach was supported by grant K24HL098372 (NHLBI). Dr. Greysen was supported by a career development award (KL‐2) from the UCSF Clinical Translational Sciences Institute. The authors have declared they have no financial, personal, or other conflicts of interest relevant to this study.
- , . Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306(16):1794–1795.
- , , , et al. A reengineered hospital discharge program to decrease re‐hospitalization. Ann Intern Med. 2009;150:178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
- Project RED. Meet Louise…and virtual patient advocates. Available at: http://www.bu.edu/fammed/projectred/publications/VirtualPatientAdvocateWebsiteInfo2.pdf. Accessed July 12, 2013.
- , , , . Use of handheld computers in medical education. A systematic review. J Gen Intern Med. 2006;21(5):531–537.
- , , , . Ongoing evaluation of ease‐of‐use and usefulness of wireless tablet computers within an ambulatory care unit. Stud Health Tech Inform. 2009;143:459–464.
- . Use of a tablet personal computer to enhance patient care on multidisciplinary rounds. Am J Health Syst Pharm. 2009;66(21):1909–1911.
- , . Experiences incorporating Tablet PCs into clinical pharmacists' workflow. J Healthc Inf Manag. 2005;19(4):32–37.
- , , . The impact of mobile handheld technology on hospital physicians' work practices and patient care: a systematic review. J Am Med Inform Assoc. 2009;16(6):792–801.
- , , , et al. An investigation of the efficacy of electronic consenting interfaces of research permissions management system in a hospital setting. Int J Med Inform. 2013;82(9):854–863.
- , , , et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428–1435.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39(1):15–19.
- . How apps are changing family medicine. J Fam Pract. 2013Jul;62(7):362–367.
- . The iPad: gadget or medical godsend? Ann Emerg Med. 2010;56(1):A21–A22.
- , , , et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233–241.
- . Keeping the patient focus: using tablet technology to enhance education and practice. J Contin Educ Nurs. 2012;43(6):249–250.
- , , , et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5–10.
- , . Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306(16):1794–1795.
- , , , et al. A reengineered hospital discharge program to decrease re‐hospitalization. Ann Intern Med. 2009;150:178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
- Project RED. Meet Louise…and virtual patient advocates. Available at: http://www.bu.edu/fammed/projectred/publications/VirtualPatientAdvocateWebsiteInfo2.pdf. Accessed July 12, 2013.
- , , , . Use of handheld computers in medical education. A systematic review. J Gen Intern Med. 2006;21(5):531–537.
- , , , . Ongoing evaluation of ease‐of‐use and usefulness of wireless tablet computers within an ambulatory care unit. Stud Health Tech Inform. 2009;143:459–464.
- . Use of a tablet personal computer to enhance patient care on multidisciplinary rounds. Am J Health Syst Pharm. 2009;66(21):1909–1911.
- , . Experiences incorporating Tablet PCs into clinical pharmacists' workflow. J Healthc Inf Manag. 2005;19(4):32–37.
- , , . The impact of mobile handheld technology on hospital physicians' work practices and patient care: a systematic review. J Am Med Inform Assoc. 2009;16(6):792–801.
- , , , et al. An investigation of the efficacy of electronic consenting interfaces of research permissions management system in a hospital setting. Int J Med Inform. 2013;82(9):854–863.
- , , , et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428–1435.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39(1):15–19.
- . How apps are changing family medicine. J Fam Pract. 2013Jul;62(7):362–367.
- . The iPad: gadget or medical godsend? Ann Emerg Med. 2010;56(1):A21–A22.
- , , , et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233–241.
- . Keeping the patient focus: using tablet technology to enhance education and practice. J Contin Educ Nurs. 2012;43(6):249–250.
- , , , et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5–10.
Journal of hospital medicine in 2014 and beyond
2013 WAS A GREAT YEAR FOR JHM
As the field of hospital medicine continues to grow and prosper, so does the Journal of Hospital Medicine (JHM). For JHM, 2013 reflected the field's growth with continued excellence, as manifested in a number of ways.
First, submissions to JHM rose more than 25% over 2012, with the majority of this growth coming in the form of original research, a key indication of vigorous growth in hospital medicine. Growth in submissions was accommodated through a switch to monthly publication frequency, allowing the journal to keep acceptance rates equivalent over time.
Second, peer review time has markedly improved, with average times to first decision falling from more than 35 days in 2011 to fewer than 26 days in 2013. At the same time, the time to papers appearing in Early View fell from more than 3 months to under 2 months, and the time to appearance in print fell to 2 months. Time to decision and time to publication are important measures for the journal, as they represent JHM's service to authors while also ensuring timely publication of articles that may have relevant external context.
Third, the journal continues to garner attention from the press and frequent downloads by readers (Table 1). The most widely downloaded papers of the last 12 months provided evidence‐based guidelines for medication reconciliation and transitions programs, key features of hospital medicine practice. At least as importantly, clinical research articles were also frequently mentioned in the press and downloaded, and many of these important papers were published in the last year.
| Article | No. of Downloads |
|---|---|
| |
| Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists[1] | 4,010 |
| Making inpatient medication reconciliation patient centered, clinically relevant and implementable:A consensus statement on key principles and necessary first steps[2] | 3,580 |
| Hospital performance trends on national quality measures and the association with joint commission accreditation[3] | 3,357 |
| Zolpidem is independently associated with increased risk of inpatient falls[4] | 2,376 |
| Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization[5] | 2,271 |
| Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis[6] | 1,466 |
| BOOST and readmissions: Thinking beyond the walls of the hospital[7] | 1,182 |
| Nutrition in the hospitalized patient[8] | 1,181 |
| The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond?[9] | 1,003 |
| Nurse staffing ratios: Trends and policy implications for hospitalists and the safety net[10] | 1,003 |
Fourth, JHM implemented a social media strategy including Twitter and Facebook efforts that have resulted in rapid follower growth; the JHM twitter feed has more than 600 followers and a rapidly improving social media influence score.
Finally, the JHM editors remain deeply thankful to the many outstanding peer reviewers who contribute their time and expertise to the journal. Through their efforts, each article submitted to JHM is improved, whether published or not. Our peer reviewers help the journal, but also play a key role in ensuring the continued growth of the field of hospital medicine. We single out a select few of our most highly regarded reviewers in this editorial (Table 2), and all of our peer reviewers are acknowledged following this editorial.
| Gerry Barber, University of Colorado | Luke Hansen, Northwestern University | Jim Pile, Case Western ReserveUniversity |
| Joshua Baru, John Stroger Hospital of Cook County | Keiki Hinami Northwestern University | Jennifer Quartarolo, University of California San Diego |
| Arpi Bekmezian, University of California Los Angeles | Guibenson Hyppolite, Massachusetts General Hospital | Alvin Rajkomar, University of California San Francisco |
| Jacob Blazo, Virginia Tech Carilion School of Medicine and Research Institute | Devan Kansagara, Portland VA Medical Center | Maria Raven, University of California San Francisco |
| Christopher Bonafide, The Children's Hospital ofPhiladelphia | A. Scott Keller, Mayo Clinic | Allen Repp, Fletcher Allen Health Care |
| Elizabeth Cerceo, Cooper University Hospital | Scott Lorch, The Children's Hospital of Philadelphia and University of Pennsylvania | Stephen Schmaltz, The Joint Commission Health Services Research |
| Chayan Chakraborti, George Washington University Hospital | Henry Michtalik, Johns Hopkins University | Gregory Seymann, University of California San Diego |
| Chase Coffey, Henry Ford Health System | Hilary Mosher, University of Iowa Hospitals and Clinics | Ann Sheehy, University of Wisconsin |
| Lauren Doctoroff, Beth Israel Deaconess Medical Center | Stephanie Mueller, Brigham and Women's Hospital | Daniel Shine, New York University Langone Medical Center |
| Honora Englander, Oregon Health & Science University | Andrew Odden, University of Michigan | Kevin Smith, Loyola University Medical Center |
| Matt Garber, Palmetto Health | Vikas Parekh, University of Michigan | Brett Stauffer, Baylor University |
| Zachary Goldberger, University of Washington | Henry Perkins, University of Texas | Cecelia Theobald, VA Tennessee Valley Healthcare System |
| Paul Grant, University of Michigan | Jason Persoff, University of Colorado |
SO WHAT WILL 2014 BRING?
JHM continues to anticipate growth in submissions and will be working to accommodate need and maintain acceptance rates at a reasonable level. We feel this is a critical strategy for the journal as we seek to increase the level of academic discourse in hospital medicine. The editors will continue to work to ensure that authors receive a fair and expeditious review, one that will produce an article that is improved, whether or not it is accepted in JHM.
We are also pleased to continue to support the Clinical Cases and Conundrums (CCC) series in JHM. The CCC series is a highly respected part of the journal's offerings, and we have sought to improve JHM's ability to solicit and publish outstanding clinical cases by enlisting the help of a group of outstanding national correspondents who will work with the CCC series editor, Brian Harte, to turn fascinating clinical cases into outstanding publications.
JHM will continue to work to make as many articles open access as possible. Even though Society of Hospital Medicine members have free full‐text access to the journal, many other readers do not have direct access to the JHM articles; we will announce articles that are freely available through our Twitter (@JHospMedicine) and Facebook pages.
In addition, JHM will be announcing new criteria for reporting initial experiences with our evaluations of health system innovations. These criteria will help JHM authors and readers understand whether a quality improvement (or value improvement) program was innovative, whether it is implementable, and whether and how it has impact on patient outcomes.
Finally, JHM will be announcing a new series on healthcare value, to begin in the spring of 2014. More details about this series, which will include reviews of key topics in value improvement written by prominent authors, will be forthcoming. We view this as an incredible opportunity for JHM, and one that will confirm hospital medicine's role as a specialty focused on providing the highest quality and highest value care to its patients.
You should be proud of your journal, and we are pleased to have continued to shepherd its growth over the last 2 years. We look forward to your help in charting JHM's course in 2014 and to continuing to shape the future of hospital medicine.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477–485.
- , , , , . Hospital performance trends on national quality measures and the association with Ioint Commission accreditation. J Hosp Med. 2011;6:454–461.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5:E12–E3.
- . BOOST and readmissions: thinking beyond the walls of the hospital. J Hosp Med. 2013;8:470–471.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8:52–58.
- , , , , . The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–E16.
- , , , , . Nurse staffing ratios: trends and policy implications for hospitalists and the safety net. J Hosp Med. 2008;3:193–199.
2013 WAS A GREAT YEAR FOR JHM
As the field of hospital medicine continues to grow and prosper, so does the Journal of Hospital Medicine (JHM). For JHM, 2013 reflected the field's growth with continued excellence, as manifested in a number of ways.
First, submissions to JHM rose more than 25% over 2012, with the majority of this growth coming in the form of original research, a key indication of vigorous growth in hospital medicine. Growth in submissions was accommodated through a switch to monthly publication frequency, allowing the journal to keep acceptance rates equivalent over time.
Second, peer review time has markedly improved, with average times to first decision falling from more than 35 days in 2011 to fewer than 26 days in 2013. At the same time, the time to papers appearing in Early View fell from more than 3 months to under 2 months, and the time to appearance in print fell to 2 months. Time to decision and time to publication are important measures for the journal, as they represent JHM's service to authors while also ensuring timely publication of articles that may have relevant external context.
Third, the journal continues to garner attention from the press and frequent downloads by readers (Table 1). The most widely downloaded papers of the last 12 months provided evidence‐based guidelines for medication reconciliation and transitions programs, key features of hospital medicine practice. At least as importantly, clinical research articles were also frequently mentioned in the press and downloaded, and many of these important papers were published in the last year.
| Article | No. of Downloads |
|---|---|
| |
| Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists[1] | 4,010 |
| Making inpatient medication reconciliation patient centered, clinically relevant and implementable:A consensus statement on key principles and necessary first steps[2] | 3,580 |
| Hospital performance trends on national quality measures and the association with joint commission accreditation[3] | 3,357 |
| Zolpidem is independently associated with increased risk of inpatient falls[4] | 2,376 |
| Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization[5] | 2,271 |
| Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis[6] | 1,466 |
| BOOST and readmissions: Thinking beyond the walls of the hospital[7] | 1,182 |
| Nutrition in the hospitalized patient[8] | 1,181 |
| The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond?[9] | 1,003 |
| Nurse staffing ratios: Trends and policy implications for hospitalists and the safety net[10] | 1,003 |
Fourth, JHM implemented a social media strategy including Twitter and Facebook efforts that have resulted in rapid follower growth; the JHM twitter feed has more than 600 followers and a rapidly improving social media influence score.
Finally, the JHM editors remain deeply thankful to the many outstanding peer reviewers who contribute their time and expertise to the journal. Through their efforts, each article submitted to JHM is improved, whether published or not. Our peer reviewers help the journal, but also play a key role in ensuring the continued growth of the field of hospital medicine. We single out a select few of our most highly regarded reviewers in this editorial (Table 2), and all of our peer reviewers are acknowledged following this editorial.
| Gerry Barber, University of Colorado | Luke Hansen, Northwestern University | Jim Pile, Case Western ReserveUniversity |
| Joshua Baru, John Stroger Hospital of Cook County | Keiki Hinami Northwestern University | Jennifer Quartarolo, University of California San Diego |
| Arpi Bekmezian, University of California Los Angeles | Guibenson Hyppolite, Massachusetts General Hospital | Alvin Rajkomar, University of California San Francisco |
| Jacob Blazo, Virginia Tech Carilion School of Medicine and Research Institute | Devan Kansagara, Portland VA Medical Center | Maria Raven, University of California San Francisco |
| Christopher Bonafide, The Children's Hospital ofPhiladelphia | A. Scott Keller, Mayo Clinic | Allen Repp, Fletcher Allen Health Care |
| Elizabeth Cerceo, Cooper University Hospital | Scott Lorch, The Children's Hospital of Philadelphia and University of Pennsylvania | Stephen Schmaltz, The Joint Commission Health Services Research |
| Chayan Chakraborti, George Washington University Hospital | Henry Michtalik, Johns Hopkins University | Gregory Seymann, University of California San Diego |
| Chase Coffey, Henry Ford Health System | Hilary Mosher, University of Iowa Hospitals and Clinics | Ann Sheehy, University of Wisconsin |
| Lauren Doctoroff, Beth Israel Deaconess Medical Center | Stephanie Mueller, Brigham and Women's Hospital | Daniel Shine, New York University Langone Medical Center |
| Honora Englander, Oregon Health & Science University | Andrew Odden, University of Michigan | Kevin Smith, Loyola University Medical Center |
| Matt Garber, Palmetto Health | Vikas Parekh, University of Michigan | Brett Stauffer, Baylor University |
| Zachary Goldberger, University of Washington | Henry Perkins, University of Texas | Cecelia Theobald, VA Tennessee Valley Healthcare System |
| Paul Grant, University of Michigan | Jason Persoff, University of Colorado |
SO WHAT WILL 2014 BRING?
JHM continues to anticipate growth in submissions and will be working to accommodate need and maintain acceptance rates at a reasonable level. We feel this is a critical strategy for the journal as we seek to increase the level of academic discourse in hospital medicine. The editors will continue to work to ensure that authors receive a fair and expeditious review, one that will produce an article that is improved, whether or not it is accepted in JHM.
We are also pleased to continue to support the Clinical Cases and Conundrums (CCC) series in JHM. The CCC series is a highly respected part of the journal's offerings, and we have sought to improve JHM's ability to solicit and publish outstanding clinical cases by enlisting the help of a group of outstanding national correspondents who will work with the CCC series editor, Brian Harte, to turn fascinating clinical cases into outstanding publications.
JHM will continue to work to make as many articles open access as possible. Even though Society of Hospital Medicine members have free full‐text access to the journal, many other readers do not have direct access to the JHM articles; we will announce articles that are freely available through our Twitter (@JHospMedicine) and Facebook pages.
In addition, JHM will be announcing new criteria for reporting initial experiences with our evaluations of health system innovations. These criteria will help JHM authors and readers understand whether a quality improvement (or value improvement) program was innovative, whether it is implementable, and whether and how it has impact on patient outcomes.
Finally, JHM will be announcing a new series on healthcare value, to begin in the spring of 2014. More details about this series, which will include reviews of key topics in value improvement written by prominent authors, will be forthcoming. We view this as an incredible opportunity for JHM, and one that will confirm hospital medicine's role as a specialty focused on providing the highest quality and highest value care to its patients.
You should be proud of your journal, and we are pleased to have continued to shepherd its growth over the last 2 years. We look forward to your help in charting JHM's course in 2014 and to continuing to shape the future of hospital medicine.
2013 WAS A GREAT YEAR FOR JHM
As the field of hospital medicine continues to grow and prosper, so does the Journal of Hospital Medicine (JHM). For JHM, 2013 reflected the field's growth with continued excellence, as manifested in a number of ways.
First, submissions to JHM rose more than 25% over 2012, with the majority of this growth coming in the form of original research, a key indication of vigorous growth in hospital medicine. Growth in submissions was accommodated through a switch to monthly publication frequency, allowing the journal to keep acceptance rates equivalent over time.
Second, peer review time has markedly improved, with average times to first decision falling from more than 35 days in 2011 to fewer than 26 days in 2013. At the same time, the time to papers appearing in Early View fell from more than 3 months to under 2 months, and the time to appearance in print fell to 2 months. Time to decision and time to publication are important measures for the journal, as they represent JHM's service to authors while also ensuring timely publication of articles that may have relevant external context.
Third, the journal continues to garner attention from the press and frequent downloads by readers (Table 1). The most widely downloaded papers of the last 12 months provided evidence‐based guidelines for medication reconciliation and transitions programs, key features of hospital medicine practice. At least as importantly, clinical research articles were also frequently mentioned in the press and downloaded, and many of these important papers were published in the last year.
| Article | No. of Downloads |
|---|---|
| |
| Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists[1] | 4,010 |
| Making inpatient medication reconciliation patient centered, clinically relevant and implementable:A consensus statement on key principles and necessary first steps[2] | 3,580 |
| Hospital performance trends on national quality measures and the association with joint commission accreditation[3] | 3,357 |
| Zolpidem is independently associated with increased risk of inpatient falls[4] | 2,376 |
| Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization[5] | 2,271 |
| Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis[6] | 1,466 |
| BOOST and readmissions: Thinking beyond the walls of the hospital[7] | 1,182 |
| Nutrition in the hospitalized patient[8] | 1,181 |
| The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond?[9] | 1,003 |
| Nurse staffing ratios: Trends and policy implications for hospitalists and the safety net[10] | 1,003 |
Fourth, JHM implemented a social media strategy including Twitter and Facebook efforts that have resulted in rapid follower growth; the JHM twitter feed has more than 600 followers and a rapidly improving social media influence score.
Finally, the JHM editors remain deeply thankful to the many outstanding peer reviewers who contribute their time and expertise to the journal. Through their efforts, each article submitted to JHM is improved, whether published or not. Our peer reviewers help the journal, but also play a key role in ensuring the continued growth of the field of hospital medicine. We single out a select few of our most highly regarded reviewers in this editorial (Table 2), and all of our peer reviewers are acknowledged following this editorial.
| Gerry Barber, University of Colorado | Luke Hansen, Northwestern University | Jim Pile, Case Western ReserveUniversity |
| Joshua Baru, John Stroger Hospital of Cook County | Keiki Hinami Northwestern University | Jennifer Quartarolo, University of California San Diego |
| Arpi Bekmezian, University of California Los Angeles | Guibenson Hyppolite, Massachusetts General Hospital | Alvin Rajkomar, University of California San Francisco |
| Jacob Blazo, Virginia Tech Carilion School of Medicine and Research Institute | Devan Kansagara, Portland VA Medical Center | Maria Raven, University of California San Francisco |
| Christopher Bonafide, The Children's Hospital ofPhiladelphia | A. Scott Keller, Mayo Clinic | Allen Repp, Fletcher Allen Health Care |
| Elizabeth Cerceo, Cooper University Hospital | Scott Lorch, The Children's Hospital of Philadelphia and University of Pennsylvania | Stephen Schmaltz, The Joint Commission Health Services Research |
| Chayan Chakraborti, George Washington University Hospital | Henry Michtalik, Johns Hopkins University | Gregory Seymann, University of California San Diego |
| Chase Coffey, Henry Ford Health System | Hilary Mosher, University of Iowa Hospitals and Clinics | Ann Sheehy, University of Wisconsin |
| Lauren Doctoroff, Beth Israel Deaconess Medical Center | Stephanie Mueller, Brigham and Women's Hospital | Daniel Shine, New York University Langone Medical Center |
| Honora Englander, Oregon Health & Science University | Andrew Odden, University of Michigan | Kevin Smith, Loyola University Medical Center |
| Matt Garber, Palmetto Health | Vikas Parekh, University of Michigan | Brett Stauffer, Baylor University |
| Zachary Goldberger, University of Washington | Henry Perkins, University of Texas | Cecelia Theobald, VA Tennessee Valley Healthcare System |
| Paul Grant, University of Michigan | Jason Persoff, University of Colorado |
SO WHAT WILL 2014 BRING?
JHM continues to anticipate growth in submissions and will be working to accommodate need and maintain acceptance rates at a reasonable level. We feel this is a critical strategy for the journal as we seek to increase the level of academic discourse in hospital medicine. The editors will continue to work to ensure that authors receive a fair and expeditious review, one that will produce an article that is improved, whether or not it is accepted in JHM.
We are also pleased to continue to support the Clinical Cases and Conundrums (CCC) series in JHM. The CCC series is a highly respected part of the journal's offerings, and we have sought to improve JHM's ability to solicit and publish outstanding clinical cases by enlisting the help of a group of outstanding national correspondents who will work with the CCC series editor, Brian Harte, to turn fascinating clinical cases into outstanding publications.
JHM will continue to work to make as many articles open access as possible. Even though Society of Hospital Medicine members have free full‐text access to the journal, many other readers do not have direct access to the JHM articles; we will announce articles that are freely available through our Twitter (@JHospMedicine) and Facebook pages.
In addition, JHM will be announcing new criteria for reporting initial experiences with our evaluations of health system innovations. These criteria will help JHM authors and readers understand whether a quality improvement (or value improvement) program was innovative, whether it is implementable, and whether and how it has impact on patient outcomes.
Finally, JHM will be announcing a new series on healthcare value, to begin in the spring of 2014. More details about this series, which will include reviews of key topics in value improvement written by prominent authors, will be forthcoming. We view this as an incredible opportunity for JHM, and one that will confirm hospital medicine's role as a specialty focused on providing the highest quality and highest value care to its patients.
You should be proud of your journal, and we are pleased to have continued to shepherd its growth over the last 2 years. We look forward to your help in charting JHM's course in 2014 and to continuing to shape the future of hospital medicine.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477–485.
- , , , , . Hospital performance trends on national quality measures and the association with Ioint Commission accreditation. J Hosp Med. 2011;6:454–461.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5:E12–E3.
- . BOOST and readmissions: thinking beyond the walls of the hospital. J Hosp Med. 2013;8:470–471.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8:52–58.
- , , , , . The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–E16.
- , , , , . Nurse staffing ratios: trends and policy implications for hospitalists and the safety net. J Hosp Med. 2008;3:193–199.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477–485.
- , , , , . Hospital performance trends on national quality measures and the association with Ioint Commission accreditation. J Hosp Med. 2011;6:454–461.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5:E12–E3.
- . BOOST and readmissions: thinking beyond the walls of the hospital. J Hosp Med. 2013;8:470–471.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8:52–58.
- , , , , . The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–E16.
- , , , , . Nurse staffing ratios: trends and policy implications for hospitalists and the safety net. J Hosp Med. 2008;3:193–199.
Focusing on Value
Over the last 30 years, rounds of therapeutic treatments with cost consciousness and cost containment have been administered to the healthcare industry, with generally disappointing clinical response. The last treatment cycle came in the 1990s, with the combination therapy of prospective payment and managed care, treatments that produced a transient remission in cost inflation but that left the healthcare system spent and decidedly unenthusiastic about another round of intensive therapy. For the next 15 years or so, the underlying conditions remained untreated, and unsurprisingly, runaway healthcare inflation returned. To continue this metaphor only a bit further, in 2013 the healthcare system is again facing intensive treatments, but in this case the treatments seem more likely to produce a strong and durable clinical response.
Although some argue that current efforts shall also pass, we believe that the present day is clearly different. A major difference is the implementation of the Affordable Care Act, which creates new structures to facilitate and incentives to promote cost reductions. More importantly, there has been a sea change in how the publicnot just payors or employersview healthcare costs. The ideas that care is too expensive and that much of it adds no value to patients have gained wide acceptance across the political spectrum, among patients, and increasingly among physicians.
It was in this context that the American Board of Internal Medicine Foundation (ABIMF) launched its Choosing Wisely campaign in 2011.[1] The stated goal of the campaign was to promote important conversations [between doctors and patients] necessary to ensure the right care is delivered at the right time. Importantly, this careful framing successfully avoided the caricatures of rationing or death panels, reactions that doomed prior efforts to engage all stakeholders in a reasoned national dialogue about costs and value.
The ABIMF chose an approach of having physicians identify tests and procedures that may be unnecessary in certain situations. Working with Consumer Reports, the Foundation asked a wide range of medical specialty societies to develop their own list of tests and procedures that could potentially be avoided with no harm to patients. The vast majority, 25 as of July 2013, chose to participate.
In February 2013, the Society of Hospital Medicine (SHM) joined the initiative when it posted adult and pediatric versions of Five Things Physicians and Patients Should Question.[2] We are pleased to publish summaries of the recommendations and the processes by which the 2 working groups produced their lists in the Journal of Hospital Medicine.[3, 4]
In reading these articles, we are struck by the importance of the SHM's work to reduce costs and improve value. However, it really is a first step: both articles must now catalyze a body of work to create and sustain meaningful change.
Although many of the 10 targets have strong face validity, it is not clear whether they are in fact the most common, costly, or low‐value practices under the purview of hospitalists. Given the fact that the selection process involved both evidence‐based reviews and consensus, it is possible that other, potentially more contentious, practices may provide even more bang for the buck, or in this case, nonbuck.
Nevertheless, these are quibbles. These lists are good starting points, and in fact many hospitalist groups, including our own, are using the SHM practices as a foundation for our waste‐reduction efforts. The next challenge will be translating these recommendations into actionable measures and then clinical practice. For example, 1 of the adult recommendations is to avoid repeat blood counts and chemistries in patients who are clinically stable. Concepts of clinical stability are notoriously difficult to define within specific patient subgroups, much less across the diverse patient populations seen by hospitalists. One approach here would be to narrow the focus (eg, do not order repeated blood counts in patients with gastrointestinal bleeding whose labs have been stable for 48 hours), but this step would limit the cost savings. Other measures, such as those related to urinary catheters, are more clearly defined and seem closer to being widely adoptable.
For all these measures, the ultimate question remains: How much can actually be saved and how do we measure the savings? The marginal cost of a complete blood count is extraordinarily small in comparison to an entire hospital stay, but it is possible that eliminating redundant testing also reduces the costs related to follow‐up of false positive findings. Reducing the use of urinary catheters can cut the costs of urinary tract infections and the complications of treatment, but these costs could be offset by the higher‐level nursing care needed to mobilize patients earlier or assist patients in toileting, squeezing the proverbial balloon. For all these measures, it is unclear whether what might be relatively small variable cost reductions related to specific tests/procedures can lead to subsequent reduction in fixed costs related to facilities and equipment, where more than 70% of healthcare costs lie.[5] In other words, reducing the number of lab technicians and the amount of laboratory equipment needed will lead to far greater cost reductions than reducing individual test utilization.
None of this is to say that the Choosing Wisely campaign is without merit. To the contrary, the campaign and the efforts of the SHM are early and critical steps in changing the behavior of a profession. Since the early days of hospital medicine, hospitalists have embraced cost reduction and value improvement as a central focus. By successfully engaging consumers and the community of medical specialties, Choosing Wisely has created a language and a framework that will allow our field and others to tackle the crucial work of resource stewardship with new purpose, and we hope, unprecedented success.
Disclosures
Dr. Wachter is immediate past‐chair of the American Board of Internal Medicine (ABIM) and serves on the ABIM Foundation's Board of Trustees. Dr. Auerbach receives honoraria from the American Board of Internal Medicine as a contributor to the Maintenance of Certification question pool.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Are you choosing wisely? 2013. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement8:486–492.
- , , , et al. Choosing Wisely in inpatient pediatrics: 5 opportunities for improved healthcare value. J Hosp Med. 2013;8:479–485.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281:644–649.
Over the last 30 years, rounds of therapeutic treatments with cost consciousness and cost containment have been administered to the healthcare industry, with generally disappointing clinical response. The last treatment cycle came in the 1990s, with the combination therapy of prospective payment and managed care, treatments that produced a transient remission in cost inflation but that left the healthcare system spent and decidedly unenthusiastic about another round of intensive therapy. For the next 15 years or so, the underlying conditions remained untreated, and unsurprisingly, runaway healthcare inflation returned. To continue this metaphor only a bit further, in 2013 the healthcare system is again facing intensive treatments, but in this case the treatments seem more likely to produce a strong and durable clinical response.
Although some argue that current efforts shall also pass, we believe that the present day is clearly different. A major difference is the implementation of the Affordable Care Act, which creates new structures to facilitate and incentives to promote cost reductions. More importantly, there has been a sea change in how the publicnot just payors or employersview healthcare costs. The ideas that care is too expensive and that much of it adds no value to patients have gained wide acceptance across the political spectrum, among patients, and increasingly among physicians.
It was in this context that the American Board of Internal Medicine Foundation (ABIMF) launched its Choosing Wisely campaign in 2011.[1] The stated goal of the campaign was to promote important conversations [between doctors and patients] necessary to ensure the right care is delivered at the right time. Importantly, this careful framing successfully avoided the caricatures of rationing or death panels, reactions that doomed prior efforts to engage all stakeholders in a reasoned national dialogue about costs and value.
The ABIMF chose an approach of having physicians identify tests and procedures that may be unnecessary in certain situations. Working with Consumer Reports, the Foundation asked a wide range of medical specialty societies to develop their own list of tests and procedures that could potentially be avoided with no harm to patients. The vast majority, 25 as of July 2013, chose to participate.
In February 2013, the Society of Hospital Medicine (SHM) joined the initiative when it posted adult and pediatric versions of Five Things Physicians and Patients Should Question.[2] We are pleased to publish summaries of the recommendations and the processes by which the 2 working groups produced their lists in the Journal of Hospital Medicine.[3, 4]
In reading these articles, we are struck by the importance of the SHM's work to reduce costs and improve value. However, it really is a first step: both articles must now catalyze a body of work to create and sustain meaningful change.
Although many of the 10 targets have strong face validity, it is not clear whether they are in fact the most common, costly, or low‐value practices under the purview of hospitalists. Given the fact that the selection process involved both evidence‐based reviews and consensus, it is possible that other, potentially more contentious, practices may provide even more bang for the buck, or in this case, nonbuck.
Nevertheless, these are quibbles. These lists are good starting points, and in fact many hospitalist groups, including our own, are using the SHM practices as a foundation for our waste‐reduction efforts. The next challenge will be translating these recommendations into actionable measures and then clinical practice. For example, 1 of the adult recommendations is to avoid repeat blood counts and chemistries in patients who are clinically stable. Concepts of clinical stability are notoriously difficult to define within specific patient subgroups, much less across the diverse patient populations seen by hospitalists. One approach here would be to narrow the focus (eg, do not order repeated blood counts in patients with gastrointestinal bleeding whose labs have been stable for 48 hours), but this step would limit the cost savings. Other measures, such as those related to urinary catheters, are more clearly defined and seem closer to being widely adoptable.
For all these measures, the ultimate question remains: How much can actually be saved and how do we measure the savings? The marginal cost of a complete blood count is extraordinarily small in comparison to an entire hospital stay, but it is possible that eliminating redundant testing also reduces the costs related to follow‐up of false positive findings. Reducing the use of urinary catheters can cut the costs of urinary tract infections and the complications of treatment, but these costs could be offset by the higher‐level nursing care needed to mobilize patients earlier or assist patients in toileting, squeezing the proverbial balloon. For all these measures, it is unclear whether what might be relatively small variable cost reductions related to specific tests/procedures can lead to subsequent reduction in fixed costs related to facilities and equipment, where more than 70% of healthcare costs lie.[5] In other words, reducing the number of lab technicians and the amount of laboratory equipment needed will lead to far greater cost reductions than reducing individual test utilization.
None of this is to say that the Choosing Wisely campaign is without merit. To the contrary, the campaign and the efforts of the SHM are early and critical steps in changing the behavior of a profession. Since the early days of hospital medicine, hospitalists have embraced cost reduction and value improvement as a central focus. By successfully engaging consumers and the community of medical specialties, Choosing Wisely has created a language and a framework that will allow our field and others to tackle the crucial work of resource stewardship with new purpose, and we hope, unprecedented success.
Disclosures
Dr. Wachter is immediate past‐chair of the American Board of Internal Medicine (ABIM) and serves on the ABIM Foundation's Board of Trustees. Dr. Auerbach receives honoraria from the American Board of Internal Medicine as a contributor to the Maintenance of Certification question pool.
Over the last 30 years, rounds of therapeutic treatments with cost consciousness and cost containment have been administered to the healthcare industry, with generally disappointing clinical response. The last treatment cycle came in the 1990s, with the combination therapy of prospective payment and managed care, treatments that produced a transient remission in cost inflation but that left the healthcare system spent and decidedly unenthusiastic about another round of intensive therapy. For the next 15 years or so, the underlying conditions remained untreated, and unsurprisingly, runaway healthcare inflation returned. To continue this metaphor only a bit further, in 2013 the healthcare system is again facing intensive treatments, but in this case the treatments seem more likely to produce a strong and durable clinical response.
Although some argue that current efforts shall also pass, we believe that the present day is clearly different. A major difference is the implementation of the Affordable Care Act, which creates new structures to facilitate and incentives to promote cost reductions. More importantly, there has been a sea change in how the publicnot just payors or employersview healthcare costs. The ideas that care is too expensive and that much of it adds no value to patients have gained wide acceptance across the political spectrum, among patients, and increasingly among physicians.
It was in this context that the American Board of Internal Medicine Foundation (ABIMF) launched its Choosing Wisely campaign in 2011.[1] The stated goal of the campaign was to promote important conversations [between doctors and patients] necessary to ensure the right care is delivered at the right time. Importantly, this careful framing successfully avoided the caricatures of rationing or death panels, reactions that doomed prior efforts to engage all stakeholders in a reasoned national dialogue about costs and value.
The ABIMF chose an approach of having physicians identify tests and procedures that may be unnecessary in certain situations. Working with Consumer Reports, the Foundation asked a wide range of medical specialty societies to develop their own list of tests and procedures that could potentially be avoided with no harm to patients. The vast majority, 25 as of July 2013, chose to participate.
In February 2013, the Society of Hospital Medicine (SHM) joined the initiative when it posted adult and pediatric versions of Five Things Physicians and Patients Should Question.[2] We are pleased to publish summaries of the recommendations and the processes by which the 2 working groups produced their lists in the Journal of Hospital Medicine.[3, 4]
In reading these articles, we are struck by the importance of the SHM's work to reduce costs and improve value. However, it really is a first step: both articles must now catalyze a body of work to create and sustain meaningful change.
Although many of the 10 targets have strong face validity, it is not clear whether they are in fact the most common, costly, or low‐value practices under the purview of hospitalists. Given the fact that the selection process involved both evidence‐based reviews and consensus, it is possible that other, potentially more contentious, practices may provide even more bang for the buck, or in this case, nonbuck.
Nevertheless, these are quibbles. These lists are good starting points, and in fact many hospitalist groups, including our own, are using the SHM practices as a foundation for our waste‐reduction efforts. The next challenge will be translating these recommendations into actionable measures and then clinical practice. For example, 1 of the adult recommendations is to avoid repeat blood counts and chemistries in patients who are clinically stable. Concepts of clinical stability are notoriously difficult to define within specific patient subgroups, much less across the diverse patient populations seen by hospitalists. One approach here would be to narrow the focus (eg, do not order repeated blood counts in patients with gastrointestinal bleeding whose labs have been stable for 48 hours), but this step would limit the cost savings. Other measures, such as those related to urinary catheters, are more clearly defined and seem closer to being widely adoptable.
For all these measures, the ultimate question remains: How much can actually be saved and how do we measure the savings? The marginal cost of a complete blood count is extraordinarily small in comparison to an entire hospital stay, but it is possible that eliminating redundant testing also reduces the costs related to follow‐up of false positive findings. Reducing the use of urinary catheters can cut the costs of urinary tract infections and the complications of treatment, but these costs could be offset by the higher‐level nursing care needed to mobilize patients earlier or assist patients in toileting, squeezing the proverbial balloon. For all these measures, it is unclear whether what might be relatively small variable cost reductions related to specific tests/procedures can lead to subsequent reduction in fixed costs related to facilities and equipment, where more than 70% of healthcare costs lie.[5] In other words, reducing the number of lab technicians and the amount of laboratory equipment needed will lead to far greater cost reductions than reducing individual test utilization.
None of this is to say that the Choosing Wisely campaign is without merit. To the contrary, the campaign and the efforts of the SHM are early and critical steps in changing the behavior of a profession. Since the early days of hospital medicine, hospitalists have embraced cost reduction and value improvement as a central focus. By successfully engaging consumers and the community of medical specialties, Choosing Wisely has created a language and a framework that will allow our field and others to tackle the crucial work of resource stewardship with new purpose, and we hope, unprecedented success.
Disclosures
Dr. Wachter is immediate past‐chair of the American Board of Internal Medicine (ABIM) and serves on the ABIM Foundation's Board of Trustees. Dr. Auerbach receives honoraria from the American Board of Internal Medicine as a contributor to the Maintenance of Certification question pool.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Are you choosing wisely? 2013. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement8:486–492.
- , , , et al. Choosing Wisely in inpatient pediatrics: 5 opportunities for improved healthcare value. J Hosp Med. 2013;8:479–485.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281:644–649.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Are you choosing wisely? 2013. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement8:486–492.
- , , , et al. Choosing Wisely in inpatient pediatrics: 5 opportunities for improved healthcare value. J Hosp Med. 2013;8:479–485.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281:644–649.
Proactive Rounding by RRT
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.
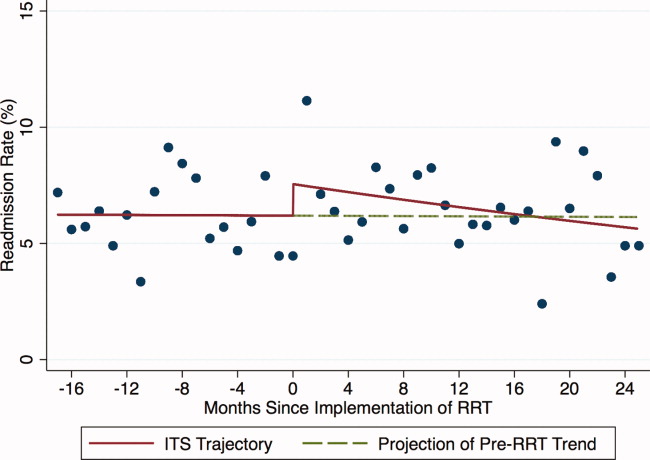
| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.
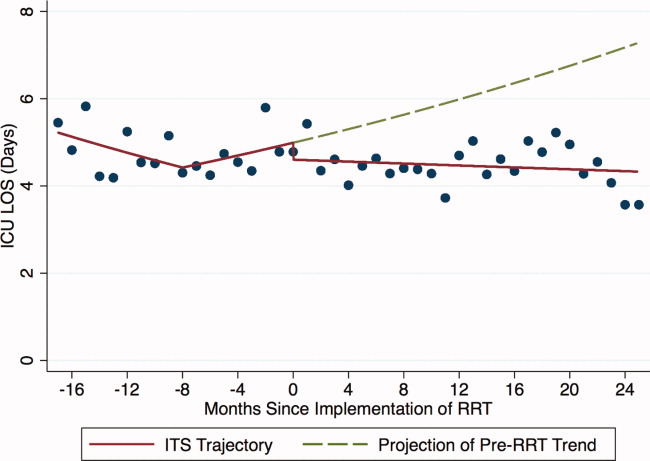
In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).
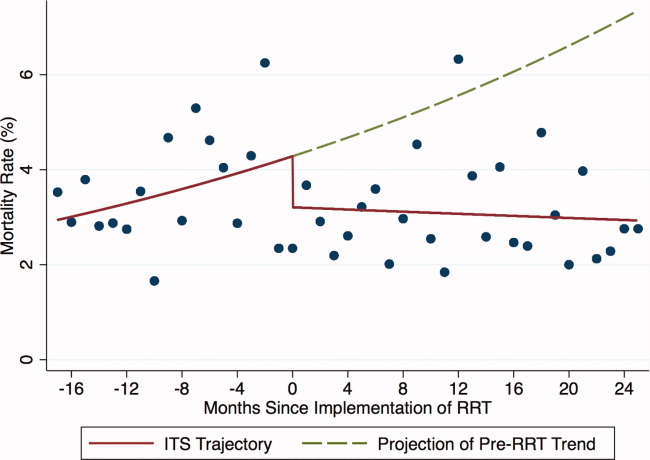
Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.

| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.

In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).

Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.

| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.

In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).

Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Copyright © 2012 Society of Hospital Medicine