User login
Serum vitamin D levels, atopy not significantly linked
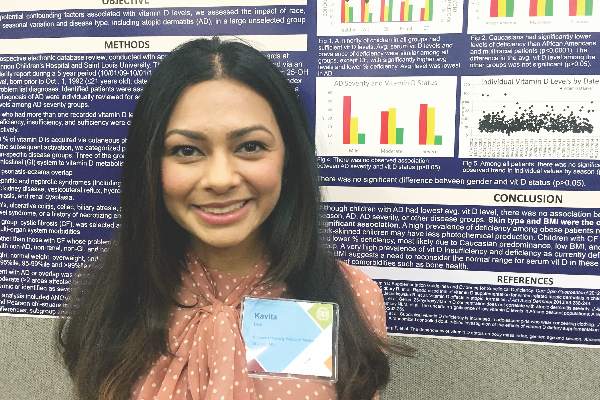
SCOTTSDALE, ARIZ. – Serum vitamin D level was not significantly associated with atopic dermatitis or disease severity in a single-center study of more than 600 children and adolescents.
However, “we did observe a strong correlation between average serum vitamin D levels and skin type, as well as body mass index,” said Kavita Darji, a medical student at Saint Louis (Mo.) University, who presented the findings in a poster at the annual meeting of the Society for Investigative Dermatology. Those findings challenge the logic of following universal definitions of vitamin D deficiency, especially given the phenotypic heterogeneity of patients in the United States, she added in an interview.
Serum vitamin D testing is one of most common laboratory assays in this country, but clinicians still debate the risks and benefits of supplementing children and adolescents who test below the Endocrine Society’s threshold for sufficiency (30.0 ng/mL).
To identify factors affecting vitamin D levels, Ms. Darji and her associates reviewed electronic medical charts for patients under age 22 years at Saint Louis University medical centers between 2009 and 2014. The cohort of 655 patients was primarily white (64%) or black (29%), and was nearly equally balanced by gender; their average age was 10 years. The researchers analyzed only the first vitamin D serum measurement for each patient, and defined deficiency as a level under 20 ng/mL, insufficiency as a level between 20 and 29.9 ng/mL, and sufficiency as a level of at least 30 ng/mL.
Serum vitamin D levels were slightly lower among atopic patients, compared with those without atopy, but the difference did not reach statistical significance (about 25 ng/mL vs. about 38 ng/mL; P greater than .05). “We also did not find an association between AD severity and vitamin D level,” Ms. Darji reported. Instead, race and body mass index were the most significant predictors of vitamin D deficiency, probably because these factors directly affect cutaneous photo-induced vitamin D synthesis and the sequestration of fat-soluble vitamins in adipose tissue, she said.
Using the standard definitions, more than 50% of black patients were vitamin D deficient, while less than 30% had sufficient vitamin D levels. In contrast, about 25% of white patients were vitamin D deficient, while nearly 40% had sufficient vitamin D levels (P less than .0001 for proportions of deficiency by race). Furthermore, only about 10% of obese children (those who exceeded the 99th percentile of BMI for age) had sufficient vitamin D levels, compared with more than 40% of underweight children and about 30% of normal-weight children (P less than .00001).
Since vitamin D deficiency was more common among black and obese patients, “maybe they could benefit from a different cut-off value than the standard 30 ng per mL that we used,” Ms. Darji said. “The question is, do they really require these supplements? It may be beneficial to look at the unique characteristics of each patient before supplementing, because the risks of supplementation are considerable in terms of bone health and cardiovascular disease.”
Vitamin D levels did not vary significantly by gender or by month or season measured, Ms. Darji noted. She reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Serum vitamin D level was not significantly associated with atopic dermatitis or disease severity in a single-center study of more than 600 children and adolescents.
However, “we did observe a strong correlation between average serum vitamin D levels and skin type, as well as body mass index,” said Kavita Darji, a medical student at Saint Louis (Mo.) University, who presented the findings in a poster at the annual meeting of the Society for Investigative Dermatology. Those findings challenge the logic of following universal definitions of vitamin D deficiency, especially given the phenotypic heterogeneity of patients in the United States, she added in an interview.
Serum vitamin D testing is one of most common laboratory assays in this country, but clinicians still debate the risks and benefits of supplementing children and adolescents who test below the Endocrine Society’s threshold for sufficiency (30.0 ng/mL).
To identify factors affecting vitamin D levels, Ms. Darji and her associates reviewed electronic medical charts for patients under age 22 years at Saint Louis University medical centers between 2009 and 2014. The cohort of 655 patients was primarily white (64%) or black (29%), and was nearly equally balanced by gender; their average age was 10 years. The researchers analyzed only the first vitamin D serum measurement for each patient, and defined deficiency as a level under 20 ng/mL, insufficiency as a level between 20 and 29.9 ng/mL, and sufficiency as a level of at least 30 ng/mL.
Serum vitamin D levels were slightly lower among atopic patients, compared with those without atopy, but the difference did not reach statistical significance (about 25 ng/mL vs. about 38 ng/mL; P greater than .05). “We also did not find an association between AD severity and vitamin D level,” Ms. Darji reported. Instead, race and body mass index were the most significant predictors of vitamin D deficiency, probably because these factors directly affect cutaneous photo-induced vitamin D synthesis and the sequestration of fat-soluble vitamins in adipose tissue, she said.
Using the standard definitions, more than 50% of black patients were vitamin D deficient, while less than 30% had sufficient vitamin D levels. In contrast, about 25% of white patients were vitamin D deficient, while nearly 40% had sufficient vitamin D levels (P less than .0001 for proportions of deficiency by race). Furthermore, only about 10% of obese children (those who exceeded the 99th percentile of BMI for age) had sufficient vitamin D levels, compared with more than 40% of underweight children and about 30% of normal-weight children (P less than .00001).
Since vitamin D deficiency was more common among black and obese patients, “maybe they could benefit from a different cut-off value than the standard 30 ng per mL that we used,” Ms. Darji said. “The question is, do they really require these supplements? It may be beneficial to look at the unique characteristics of each patient before supplementing, because the risks of supplementation are considerable in terms of bone health and cardiovascular disease.”
Vitamin D levels did not vary significantly by gender or by month or season measured, Ms. Darji noted. She reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Serum vitamin D level was not significantly associated with atopic dermatitis or disease severity in a single-center study of more than 600 children and adolescents.
However, “we did observe a strong correlation between average serum vitamin D levels and skin type, as well as body mass index,” said Kavita Darji, a medical student at Saint Louis (Mo.) University, who presented the findings in a poster at the annual meeting of the Society for Investigative Dermatology. Those findings challenge the logic of following universal definitions of vitamin D deficiency, especially given the phenotypic heterogeneity of patients in the United States, she added in an interview.
Serum vitamin D testing is one of most common laboratory assays in this country, but clinicians still debate the risks and benefits of supplementing children and adolescents who test below the Endocrine Society’s threshold for sufficiency (30.0 ng/mL).
To identify factors affecting vitamin D levels, Ms. Darji and her associates reviewed electronic medical charts for patients under age 22 years at Saint Louis University medical centers between 2009 and 2014. The cohort of 655 patients was primarily white (64%) or black (29%), and was nearly equally balanced by gender; their average age was 10 years. The researchers analyzed only the first vitamin D serum measurement for each patient, and defined deficiency as a level under 20 ng/mL, insufficiency as a level between 20 and 29.9 ng/mL, and sufficiency as a level of at least 30 ng/mL.
Serum vitamin D levels were slightly lower among atopic patients, compared with those without atopy, but the difference did not reach statistical significance (about 25 ng/mL vs. about 38 ng/mL; P greater than .05). “We also did not find an association between AD severity and vitamin D level,” Ms. Darji reported. Instead, race and body mass index were the most significant predictors of vitamin D deficiency, probably because these factors directly affect cutaneous photo-induced vitamin D synthesis and the sequestration of fat-soluble vitamins in adipose tissue, she said.
Using the standard definitions, more than 50% of black patients were vitamin D deficient, while less than 30% had sufficient vitamin D levels. In contrast, about 25% of white patients were vitamin D deficient, while nearly 40% had sufficient vitamin D levels (P less than .0001 for proportions of deficiency by race). Furthermore, only about 10% of obese children (those who exceeded the 99th percentile of BMI for age) had sufficient vitamin D levels, compared with more than 40% of underweight children and about 30% of normal-weight children (P less than .00001).
Since vitamin D deficiency was more common among black and obese patients, “maybe they could benefit from a different cut-off value than the standard 30 ng per mL that we used,” Ms. Darji said. “The question is, do they really require these supplements? It may be beneficial to look at the unique characteristics of each patient before supplementing, because the risks of supplementation are considerable in terms of bone health and cardiovascular disease.”
Vitamin D levels did not vary significantly by gender or by month or season measured, Ms. Darji noted. She reported no funding sources and had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Serum vitamin D was not a significant marker for pediatric atopic dermatitis or disease severity.
Major finding: The average serum vitamin D level was lower among patients with atopic dermatitis than healthy children, but the difference did not reach statistical significance.
Data source: A single-center retrospective review of electronic medical records from 655 patients aged 21 years and younger (average age, 10 years).
Disclosures: Ms. Darji reported no funding sources and had no disclosures.
Study Highlights Cardiovascular Benefits, Lower GI Risks of Low-dose Aspirin
Resuming low-dose aspirin after an initial lower gastrointestinal bleed significantly increased the chances of recurrence but protected against serious cardiovascular events, based on a single-center retrospective study published in the August issue of Gastroenterology.
In contrast, “we did not find concomitant use of anticoagulants, antiplatelets, and steroids as a predictor of recurrent lower GI bleeding,” said Dr. Francis Chan of the Prince of Wales Hospital in Hong Kong and his associates. “This may be due to the low percentage of concomitant drug use in both groups. Multicenter studies with a large number of patients will be required to identify additional risk factors for recurrent lower GI bleeding with aspirin use.”
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Low-dose aspirin has long been known to help prevent coronary artery and cerebrovascular disease, and more recently has been found to potentially reduce the risk of several types of cancer, the researchers noted. Aspirin is well known to increase the risk of upper GI bleeding, but some studies have also linked it to lower GI bleeding. However, “patients with underlying cardiovascular diseases often require lifelong aspirin,” they added. The risks and benefits of stopping or remaining on aspirin after an initial lower GI bleed are unclear (Gastroenterology 2016 Apr 26. doi: 10.1053/j.gastro.2016.04.013).
Accordingly, the researchers retrospectively studied 295 patients who had an initial aspirin-associated lower GI bleed, defined as 325 mg aspirin a day within a week of bleeding onset. All patients had melena or hematochezia documented by an attending physician and had no endoscopic evidence of upper GI bleeding.
For patients who continued using aspirin at least half the time, the 5-year cumulative incidence of recurrent lower GI bleeding was 19% (95% confidence interval [CI], 13%-25%) – more than double the rate among patients who used aspirin 20% or less of the time (5-year cumulative incidence, 7%; 95% CI, 3%-13%; P = .01). However, the 5-year cumulative incidence of serious cardiovascular events among nonusers was 37% (95% CI, 27%-46%), while the rate among aspirin users was 23% (95% CI, 17%-30%; P = .02). Mortality from noncardiovascular causes was also higher among nonusers (27%) than users (8%; P less than .001), probably because nonusers of aspirin tended to be older than users, but perhaps also because aspirin had a “nonvascular protective effect,” the researchers said.
A multivariate analysis confirmed these findings, linking lower GI bleeding to aspirin but not to use of steroids, anticoagulants, or antiplatelet drugs, or to age, sex, alcohol consumption, smoking, comorbidities, or cardiovascular risks. Indeed, continued aspirin use nearly tripled the chances of a recurrent lower GI bleed (hazard ratio, 2.76; 95% CI, 1.3-6.0; P = .01), but cut the risk of serious cardiovascular events by about 40% (HR, 0.59; 95% CI, 0.4-0.9; P = .02).
Deciding whether to resume aspirin after a severe lower GI bleed “presents a management dilemma for physicians, patients, and their families, particularly in the absence of risk-mitigating therapies and a lack of data on the risks and benefits of resuming aspirin,” the investigators emphasized. Their findings highlight the importance of weighing the cardiovascular benefits of aspirin against GI toxicity, they said. “Since there is substantial risk of recurrent bleeding, physicians should critically evaluate individual patients’ cardiovascular risk before resuming aspirin therapy. Our findings also suggest a need for a composite endpoint to evaluate clinically significant events throughout the GI tract in patients receiving antiplatelet drugs.”
The Chinese University of Hong Kong funded the study. Dr. Chan reported financial ties to Pfizer, Eisai, Takeda, Otsuka, and Astrazeneca.
Going back to low-dose aspirin after an index lower-gastrointestinal (LGI) bleed significantly increased recurrences of LGI bleeding while reducing rates of serious cardiovascular (CV) events and deaths, in a single-center, retrospective study of 295 patients from Hong Kong. Specifically, the 5-year cumulative risks of recurrent LGI bleeding were 19% in aspirin users vs. 7% for nonusers (P = .01), severe CV events (myocardial infarction or cerebrovascular accident) were 25% in aspirin users vs. 37% in nonusers (P = .02), and all-cause mortality rates for aspirin users vs. nonusers were 8.2% vs. 26.7% (P = .001).
This study complements what this research group previously published about resuming aspirin early in high-risk patients after severe ulcer hemorrhage to reduce the risk of fatal CV events (Ann Intern Med. 2010;151:1-9). Recurrent ulcer bleeding was more common in those who resumed aspirin, but fewer died of CV causes within 30 days. Until the investigators’ current publication, no reports on LGI hemorrhage were available to help clinicians weigh the risks and benefits of resuming aspirin. Despite some limitations of the current study, the message is clear about the benefits of aspirin even in the face of recurrent, nonfatal bleeding.
It seems wise to restart low-dose aspirin in patients who have a documented risk for CV events. Meanwhile, gastroenterologists can take care of the rebleeding and further study this problem.
Dennis Jensen, MD, is a digestive diseases specialist in the departments of medicine and gastroenterology at Ronald Reagan UCLA Medical Center, Los Angeles.
Going back to low-dose aspirin after an index lower-gastrointestinal (LGI) bleed significantly increased recurrences of LGI bleeding while reducing rates of serious cardiovascular (CV) events and deaths, in a single-center, retrospective study of 295 patients from Hong Kong. Specifically, the 5-year cumulative risks of recurrent LGI bleeding were 19% in aspirin users vs. 7% for nonusers (P = .01), severe CV events (myocardial infarction or cerebrovascular accident) were 25% in aspirin users vs. 37% in nonusers (P = .02), and all-cause mortality rates for aspirin users vs. nonusers were 8.2% vs. 26.7% (P = .001).
This study complements what this research group previously published about resuming aspirin early in high-risk patients after severe ulcer hemorrhage to reduce the risk of fatal CV events (Ann Intern Med. 2010;151:1-9). Recurrent ulcer bleeding was more common in those who resumed aspirin, but fewer died of CV causes within 30 days. Until the investigators’ current publication, no reports on LGI hemorrhage were available to help clinicians weigh the risks and benefits of resuming aspirin. Despite some limitations of the current study, the message is clear about the benefits of aspirin even in the face of recurrent, nonfatal bleeding.
It seems wise to restart low-dose aspirin in patients who have a documented risk for CV events. Meanwhile, gastroenterologists can take care of the rebleeding and further study this problem.
Dennis Jensen, MD, is a digestive diseases specialist in the departments of medicine and gastroenterology at Ronald Reagan UCLA Medical Center, Los Angeles.
Going back to low-dose aspirin after an index lower-gastrointestinal (LGI) bleed significantly increased recurrences of LGI bleeding while reducing rates of serious cardiovascular (CV) events and deaths, in a single-center, retrospective study of 295 patients from Hong Kong. Specifically, the 5-year cumulative risks of recurrent LGI bleeding were 19% in aspirin users vs. 7% for nonusers (P = .01), severe CV events (myocardial infarction or cerebrovascular accident) were 25% in aspirin users vs. 37% in nonusers (P = .02), and all-cause mortality rates for aspirin users vs. nonusers were 8.2% vs. 26.7% (P = .001).
This study complements what this research group previously published about resuming aspirin early in high-risk patients after severe ulcer hemorrhage to reduce the risk of fatal CV events (Ann Intern Med. 2010;151:1-9). Recurrent ulcer bleeding was more common in those who resumed aspirin, but fewer died of CV causes within 30 days. Until the investigators’ current publication, no reports on LGI hemorrhage were available to help clinicians weigh the risks and benefits of resuming aspirin. Despite some limitations of the current study, the message is clear about the benefits of aspirin even in the face of recurrent, nonfatal bleeding.
It seems wise to restart low-dose aspirin in patients who have a documented risk for CV events. Meanwhile, gastroenterologists can take care of the rebleeding and further study this problem.
Dennis Jensen, MD, is a digestive diseases specialist in the departments of medicine and gastroenterology at Ronald Reagan UCLA Medical Center, Los Angeles.
Resuming low-dose aspirin after an initial lower gastrointestinal bleed significantly increased the chances of recurrence but protected against serious cardiovascular events, based on a single-center retrospective study published in the August issue of Gastroenterology.
In contrast, “we did not find concomitant use of anticoagulants, antiplatelets, and steroids as a predictor of recurrent lower GI bleeding,” said Dr. Francis Chan of the Prince of Wales Hospital in Hong Kong and his associates. “This may be due to the low percentage of concomitant drug use in both groups. Multicenter studies with a large number of patients will be required to identify additional risk factors for recurrent lower GI bleeding with aspirin use.”
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Low-dose aspirin has long been known to help prevent coronary artery and cerebrovascular disease, and more recently has been found to potentially reduce the risk of several types of cancer, the researchers noted. Aspirin is well known to increase the risk of upper GI bleeding, but some studies have also linked it to lower GI bleeding. However, “patients with underlying cardiovascular diseases often require lifelong aspirin,” they added. The risks and benefits of stopping or remaining on aspirin after an initial lower GI bleed are unclear (Gastroenterology 2016 Apr 26. doi: 10.1053/j.gastro.2016.04.013).
Accordingly, the researchers retrospectively studied 295 patients who had an initial aspirin-associated lower GI bleed, defined as 325 mg aspirin a day within a week of bleeding onset. All patients had melena or hematochezia documented by an attending physician and had no endoscopic evidence of upper GI bleeding.
For patients who continued using aspirin at least half the time, the 5-year cumulative incidence of recurrent lower GI bleeding was 19% (95% confidence interval [CI], 13%-25%) – more than double the rate among patients who used aspirin 20% or less of the time (5-year cumulative incidence, 7%; 95% CI, 3%-13%; P = .01). However, the 5-year cumulative incidence of serious cardiovascular events among nonusers was 37% (95% CI, 27%-46%), while the rate among aspirin users was 23% (95% CI, 17%-30%; P = .02). Mortality from noncardiovascular causes was also higher among nonusers (27%) than users (8%; P less than .001), probably because nonusers of aspirin tended to be older than users, but perhaps also because aspirin had a “nonvascular protective effect,” the researchers said.
A multivariate analysis confirmed these findings, linking lower GI bleeding to aspirin but not to use of steroids, anticoagulants, or antiplatelet drugs, or to age, sex, alcohol consumption, smoking, comorbidities, or cardiovascular risks. Indeed, continued aspirin use nearly tripled the chances of a recurrent lower GI bleed (hazard ratio, 2.76; 95% CI, 1.3-6.0; P = .01), but cut the risk of serious cardiovascular events by about 40% (HR, 0.59; 95% CI, 0.4-0.9; P = .02).
Deciding whether to resume aspirin after a severe lower GI bleed “presents a management dilemma for physicians, patients, and their families, particularly in the absence of risk-mitigating therapies and a lack of data on the risks and benefits of resuming aspirin,” the investigators emphasized. Their findings highlight the importance of weighing the cardiovascular benefits of aspirin against GI toxicity, they said. “Since there is substantial risk of recurrent bleeding, physicians should critically evaluate individual patients’ cardiovascular risk before resuming aspirin therapy. Our findings also suggest a need for a composite endpoint to evaluate clinically significant events throughout the GI tract in patients receiving antiplatelet drugs.”
The Chinese University of Hong Kong funded the study. Dr. Chan reported financial ties to Pfizer, Eisai, Takeda, Otsuka, and Astrazeneca.
Resuming low-dose aspirin after an initial lower gastrointestinal bleed significantly increased the chances of recurrence but protected against serious cardiovascular events, based on a single-center retrospective study published in the August issue of Gastroenterology.
In contrast, “we did not find concomitant use of anticoagulants, antiplatelets, and steroids as a predictor of recurrent lower GI bleeding,” said Dr. Francis Chan of the Prince of Wales Hospital in Hong Kong and his associates. “This may be due to the low percentage of concomitant drug use in both groups. Multicenter studies with a large number of patients will be required to identify additional risk factors for recurrent lower GI bleeding with aspirin use.”
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Low-dose aspirin has long been known to help prevent coronary artery and cerebrovascular disease, and more recently has been found to potentially reduce the risk of several types of cancer, the researchers noted. Aspirin is well known to increase the risk of upper GI bleeding, but some studies have also linked it to lower GI bleeding. However, “patients with underlying cardiovascular diseases often require lifelong aspirin,” they added. The risks and benefits of stopping or remaining on aspirin after an initial lower GI bleed are unclear (Gastroenterology 2016 Apr 26. doi: 10.1053/j.gastro.2016.04.013).
Accordingly, the researchers retrospectively studied 295 patients who had an initial aspirin-associated lower GI bleed, defined as 325 mg aspirin a day within a week of bleeding onset. All patients had melena or hematochezia documented by an attending physician and had no endoscopic evidence of upper GI bleeding.
For patients who continued using aspirin at least half the time, the 5-year cumulative incidence of recurrent lower GI bleeding was 19% (95% confidence interval [CI], 13%-25%) – more than double the rate among patients who used aspirin 20% or less of the time (5-year cumulative incidence, 7%; 95% CI, 3%-13%; P = .01). However, the 5-year cumulative incidence of serious cardiovascular events among nonusers was 37% (95% CI, 27%-46%), while the rate among aspirin users was 23% (95% CI, 17%-30%; P = .02). Mortality from noncardiovascular causes was also higher among nonusers (27%) than users (8%; P less than .001), probably because nonusers of aspirin tended to be older than users, but perhaps also because aspirin had a “nonvascular protective effect,” the researchers said.
A multivariate analysis confirmed these findings, linking lower GI bleeding to aspirin but not to use of steroids, anticoagulants, or antiplatelet drugs, or to age, sex, alcohol consumption, smoking, comorbidities, or cardiovascular risks. Indeed, continued aspirin use nearly tripled the chances of a recurrent lower GI bleed (hazard ratio, 2.76; 95% CI, 1.3-6.0; P = .01), but cut the risk of serious cardiovascular events by about 40% (HR, 0.59; 95% CI, 0.4-0.9; P = .02).
Deciding whether to resume aspirin after a severe lower GI bleed “presents a management dilemma for physicians, patients, and their families, particularly in the absence of risk-mitigating therapies and a lack of data on the risks and benefits of resuming aspirin,” the investigators emphasized. Their findings highlight the importance of weighing the cardiovascular benefits of aspirin against GI toxicity, they said. “Since there is substantial risk of recurrent bleeding, physicians should critically evaluate individual patients’ cardiovascular risk before resuming aspirin therapy. Our findings also suggest a need for a composite endpoint to evaluate clinically significant events throughout the GI tract in patients receiving antiplatelet drugs.”
The Chinese University of Hong Kong funded the study. Dr. Chan reported financial ties to Pfizer, Eisai, Takeda, Otsuka, and Astrazeneca.
FROM GASTROENTEROLOGY
Celiac disease most common among Indians from Punjab study found
Americans from the Punjab region of India had the highest prevalence of celiac disease in a national cross-sectional study of duodenal mucosal biopsies according to a study reported in the August issue of Clinical Gastroenterology and Hepatology.
In contrast, persons of South Asian, East Asian, and Hispanic descent are significantly less likely to receive a biopsy-based diagnosis of celiac disease than were other Americans, Anna Krigel, MD, at Columbia University, New York, reported with her associates. The prevalence of celiac disease among Jewish and Middle Eastern individuals resembled that of other Americans and did not differ by sex, the researchers added. “These findings may have clinical relevance to gastroenterologists across the United States and may aid in their diagnostic practices.”
Dr. Krigel and her associates analyzed a national laboratory pathology registry of 454,885 patients who underwent esophagogastroduodenoscopy with duodenal biopsy between January 2008 and April 2015. Mucosal biopsy specimens were analyzed at three laboratories by histopathologists who had completed fellowships in gastrointestinal pathology. Patients were categorized based on their first and last names as North Indian, South Indian, East Asian, Hispanic, Middle Eastern, Jewish, or as “other Americans,” using a published algorithm. The researchers further validated this algorithm by adjusting it against a list of individuals of known ethnicities until its specificity reached 95%. Two-thirds of patients in the cohort were female, and the median age was 53 years (Clin Gastroenterol Hepatol. 2016 May 31. doi: 10.1016/j.cgh.2016.04.032).
Overall, 7,928 patients (1.7%) had duodenal villous atrophy indicative of disease, including 2% of North Indians, 1.8% of Jewish individuals, 1.8% of other Americans, 1.5% of Middle Easterners, 1.1% of Hispanics, and 0.15% of East Asians, said the investigators. Thus, Jewish persons and Middle Eastern persons had a prevalence of celiac disease similar to that of other Americans. Prevalence also was similar among Ashkenazi and Sephardic Jews. In contrast, none of the 177 South Indians in the study were diagnosed with celiac disease, and both East Asians and Hispanics were significantly less likely to be receive a diagnosis than other Americans, with odds ratios of 0.08 (95% confidence interval, 0.04-0.17) and 0.58 (0.52-0.64), respectively, and P values less than .0001.
Importantly, celiac disease was significantly more common among patients from the Punjab region of India (3.1%) than among other North Indians (1.5%; P = .02). Past studies of celiac disease in India reported similar prevalences of compatible human leukocyte antigen (HLA) haplotypes as in Western countries, without notable regional trends within India, the researchers noted. Substantial regional variations in wheat consumption in India are more likely to explain their findings and patterns of case reports in past studies, they added.
Because the registry lacked serology data, patients diagnosed with celiac disease could have actually had tropical sprue or sprue-like enteropathy due to olmesartan, the researchers acknowledged. “In particular, multiple studies have shown that tropical sprue is still the most common cause of malabsorption syndrome in India, whereas celiac disease is emerging as a more important cause of malabsorption than previously thought,” they said. “However, such cases of tropical sprue and sprue-like enteropathy due to olmesartan are far less common than celiac disease in the United States.” The study also did not account for patients whose celiac disease only was diagnosed by serology and clinical symptoms, and the algorithm probably assigned some individuals who were Hispanic to other ethnicities, because only 7% of the cohort was classified as Hispanic, compared with about 16% of Americans in the 2010 U.S. Census, they noted. But ethnic misclassification was unlikely to have differed by celiac disease status, they said.
The National Institutes of Health partially supported the work. The authors had no disclosures.
Americans from the Punjab region of India had the highest prevalence of celiac disease in a national cross-sectional study of duodenal mucosal biopsies according to a study reported in the August issue of Clinical Gastroenterology and Hepatology.
In contrast, persons of South Asian, East Asian, and Hispanic descent are significantly less likely to receive a biopsy-based diagnosis of celiac disease than were other Americans, Anna Krigel, MD, at Columbia University, New York, reported with her associates. The prevalence of celiac disease among Jewish and Middle Eastern individuals resembled that of other Americans and did not differ by sex, the researchers added. “These findings may have clinical relevance to gastroenterologists across the United States and may aid in their diagnostic practices.”
Dr. Krigel and her associates analyzed a national laboratory pathology registry of 454,885 patients who underwent esophagogastroduodenoscopy with duodenal biopsy between January 2008 and April 2015. Mucosal biopsy specimens were analyzed at three laboratories by histopathologists who had completed fellowships in gastrointestinal pathology. Patients were categorized based on their first and last names as North Indian, South Indian, East Asian, Hispanic, Middle Eastern, Jewish, or as “other Americans,” using a published algorithm. The researchers further validated this algorithm by adjusting it against a list of individuals of known ethnicities until its specificity reached 95%. Two-thirds of patients in the cohort were female, and the median age was 53 years (Clin Gastroenterol Hepatol. 2016 May 31. doi: 10.1016/j.cgh.2016.04.032).
Overall, 7,928 patients (1.7%) had duodenal villous atrophy indicative of disease, including 2% of North Indians, 1.8% of Jewish individuals, 1.8% of other Americans, 1.5% of Middle Easterners, 1.1% of Hispanics, and 0.15% of East Asians, said the investigators. Thus, Jewish persons and Middle Eastern persons had a prevalence of celiac disease similar to that of other Americans. Prevalence also was similar among Ashkenazi and Sephardic Jews. In contrast, none of the 177 South Indians in the study were diagnosed with celiac disease, and both East Asians and Hispanics were significantly less likely to be receive a diagnosis than other Americans, with odds ratios of 0.08 (95% confidence interval, 0.04-0.17) and 0.58 (0.52-0.64), respectively, and P values less than .0001.
Importantly, celiac disease was significantly more common among patients from the Punjab region of India (3.1%) than among other North Indians (1.5%; P = .02). Past studies of celiac disease in India reported similar prevalences of compatible human leukocyte antigen (HLA) haplotypes as in Western countries, without notable regional trends within India, the researchers noted. Substantial regional variations in wheat consumption in India are more likely to explain their findings and patterns of case reports in past studies, they added.
Because the registry lacked serology data, patients diagnosed with celiac disease could have actually had tropical sprue or sprue-like enteropathy due to olmesartan, the researchers acknowledged. “In particular, multiple studies have shown that tropical sprue is still the most common cause of malabsorption syndrome in India, whereas celiac disease is emerging as a more important cause of malabsorption than previously thought,” they said. “However, such cases of tropical sprue and sprue-like enteropathy due to olmesartan are far less common than celiac disease in the United States.” The study also did not account for patients whose celiac disease only was diagnosed by serology and clinical symptoms, and the algorithm probably assigned some individuals who were Hispanic to other ethnicities, because only 7% of the cohort was classified as Hispanic, compared with about 16% of Americans in the 2010 U.S. Census, they noted. But ethnic misclassification was unlikely to have differed by celiac disease status, they said.
The National Institutes of Health partially supported the work. The authors had no disclosures.
Americans from the Punjab region of India had the highest prevalence of celiac disease in a national cross-sectional study of duodenal mucosal biopsies according to a study reported in the August issue of Clinical Gastroenterology and Hepatology.
In contrast, persons of South Asian, East Asian, and Hispanic descent are significantly less likely to receive a biopsy-based diagnosis of celiac disease than were other Americans, Anna Krigel, MD, at Columbia University, New York, reported with her associates. The prevalence of celiac disease among Jewish and Middle Eastern individuals resembled that of other Americans and did not differ by sex, the researchers added. “These findings may have clinical relevance to gastroenterologists across the United States and may aid in their diagnostic practices.”
Dr. Krigel and her associates analyzed a national laboratory pathology registry of 454,885 patients who underwent esophagogastroduodenoscopy with duodenal biopsy between January 2008 and April 2015. Mucosal biopsy specimens were analyzed at three laboratories by histopathologists who had completed fellowships in gastrointestinal pathology. Patients were categorized based on their first and last names as North Indian, South Indian, East Asian, Hispanic, Middle Eastern, Jewish, or as “other Americans,” using a published algorithm. The researchers further validated this algorithm by adjusting it against a list of individuals of known ethnicities until its specificity reached 95%. Two-thirds of patients in the cohort were female, and the median age was 53 years (Clin Gastroenterol Hepatol. 2016 May 31. doi: 10.1016/j.cgh.2016.04.032).
Overall, 7,928 patients (1.7%) had duodenal villous atrophy indicative of disease, including 2% of North Indians, 1.8% of Jewish individuals, 1.8% of other Americans, 1.5% of Middle Easterners, 1.1% of Hispanics, and 0.15% of East Asians, said the investigators. Thus, Jewish persons and Middle Eastern persons had a prevalence of celiac disease similar to that of other Americans. Prevalence also was similar among Ashkenazi and Sephardic Jews. In contrast, none of the 177 South Indians in the study were diagnosed with celiac disease, and both East Asians and Hispanics were significantly less likely to be receive a diagnosis than other Americans, with odds ratios of 0.08 (95% confidence interval, 0.04-0.17) and 0.58 (0.52-0.64), respectively, and P values less than .0001.
Importantly, celiac disease was significantly more common among patients from the Punjab region of India (3.1%) than among other North Indians (1.5%; P = .02). Past studies of celiac disease in India reported similar prevalences of compatible human leukocyte antigen (HLA) haplotypes as in Western countries, without notable regional trends within India, the researchers noted. Substantial regional variations in wheat consumption in India are more likely to explain their findings and patterns of case reports in past studies, they added.
Because the registry lacked serology data, patients diagnosed with celiac disease could have actually had tropical sprue or sprue-like enteropathy due to olmesartan, the researchers acknowledged. “In particular, multiple studies have shown that tropical sprue is still the most common cause of malabsorption syndrome in India, whereas celiac disease is emerging as a more important cause of malabsorption than previously thought,” they said. “However, such cases of tropical sprue and sprue-like enteropathy due to olmesartan are far less common than celiac disease in the United States.” The study also did not account for patients whose celiac disease only was diagnosed by serology and clinical symptoms, and the algorithm probably assigned some individuals who were Hispanic to other ethnicities, because only 7% of the cohort was classified as Hispanic, compared with about 16% of Americans in the 2010 U.S. Census, they noted. But ethnic misclassification was unlikely to have differed by celiac disease status, they said.
The National Institutes of Health partially supported the work. The authors had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Duodenal villous atrophy consistent with celiac disease was most common among Americans from, or descended from, the Punjab region of India.
Major finding: The prevalence of celiac disease was highest among Punjabis (3.1%), and lowest among South Indians (0%).
Data source: A cross-sectional study of duodenal biopsies and associated demographic data for 454,885 patients in the United States.
Disclosures: The National Institutes of Health partially supported the work. The authors had no disclosures.
Study highlights cardiovascular benefits, lower GI risks of low-dose aspirin
Resuming low-dose aspirin after an initial lower gastrointestinal bleed significantly increased the chances of recurrence but protected against serious cardiovascular events, based on a single-center retrospective study published in the August issue of Gastroenterology.
In contrast, “we did not find concomitant use of anticoagulants, antiplatelets, and steroids as a predictor of recurrent lower GI bleeding,” said Dr. Francis Chan of the Prince of Wales Hospital in Hong Kong and his associates. “This may be due to the low percentage of concomitant drug use in both groups. Multicenter studies with a large number of patients will be required to identify additional risk factors for recurrent lower GI bleeding with aspirin use.”
Low-dose aspirin has long been known to help prevent coronary artery and cerebrovascular disease, and more recently has been found to potentially reduce the risk of several types of cancer, the researchers noted. Aspirin is well known to increase the risk of upper GI bleeding, but some studies have also linked it to lower GI bleeding. However, “patients with underlying cardiovascular diseases often require lifelong aspirin,” they added. The risks and benefits of stopping or remaining on aspirin after an initial lower GI bleed are unclear (Gastroenterology 2016 Apr 26. doi: 10.1053/j.gastro.2016.04.013).
Accordingly, the researchers retrospectively studied 295 patients who had an initial aspirin-associated lower GI bleed, defined as 325 mg aspirin a day within a week of bleeding onset. All patients had melena or hematochezia documented by an attending physician and had no endoscopic evidence of upper GI bleeding.
For patients who continued using aspirin at least half the time, the 5-year cumulative incidence of recurrent lower GI bleeding was 19% (95% confidence interval [CI], 13%-25%) – more than double the rate among patients who used aspirin 20% or less of the time (5-year cumulative incidence, 7%; 95% CI, 3%-13%; P = .01). However, the 5-year cumulative incidence of serious cardiovascular events among nonusers was 37% (95% CI, 27%-46%), while the rate among aspirin users was 23% (95% CI, 17%-30%; P = .02). Mortality from noncardiovascular causes was also higher among nonusers (27%) than users (8%; P less than .001), probably because nonusers of aspirin tended to be older than users, but perhaps also because aspirin had a “nonvascular protective effect,” the researchers said.
A multivariate analysis confirmed these findings, linking lower GI bleeding to aspirin but not to use of steroids, anticoagulants, or antiplatelet drugs, or to age, sex, alcohol consumption, smoking, comorbidities, or cardiovascular risks. Indeed, continued aspirin use nearly tripled the chances of a recurrent lower GI bleed (hazard ratio, 2.76; 95% CI, 1.3-6.0; P = .01), but cut the risk of serious cardiovascular events by about 40% (HR, 0.59; 95% CI, 0.4-0.9; P = .02).
Deciding whether to resume aspirin after a severe lower GI bleed “presents a management dilemma for physicians, patients, and their families, particularly in the absence of risk-mitigating therapies and a lack of data on the risks and benefits of resuming aspirin,” the investigators emphasized. Their findings highlight the importance of weighing the cardiovascular benefits of aspirin against GI toxicity, they said. “Since there is substantial risk of recurrent bleeding, physicians should critically evaluate individual patients’ cardiovascular risk before resuming aspirin therapy. Our findings also suggest a need for a composite endpoint to evaluate clinically significant events throughout the GI tract in patients receiving antiplatelet drugs.”
The Chinese University of Hong Kong funded the study. Dr. Chan reported financial ties to Pfizer, Eisai, Takeda, Otsuka, and Astrazeneca.
Resuming low-dose aspirin after an initial lower gastrointestinal bleed significantly increased the chances of recurrence but protected against serious cardiovascular events, based on a single-center retrospective study published in the August issue of Gastroenterology.
In contrast, “we did not find concomitant use of anticoagulants, antiplatelets, and steroids as a predictor of recurrent lower GI bleeding,” said Dr. Francis Chan of the Prince of Wales Hospital in Hong Kong and his associates. “This may be due to the low percentage of concomitant drug use in both groups. Multicenter studies with a large number of patients will be required to identify additional risk factors for recurrent lower GI bleeding with aspirin use.”
Low-dose aspirin has long been known to help prevent coronary artery and cerebrovascular disease, and more recently has been found to potentially reduce the risk of several types of cancer, the researchers noted. Aspirin is well known to increase the risk of upper GI bleeding, but some studies have also linked it to lower GI bleeding. However, “patients with underlying cardiovascular diseases often require lifelong aspirin,” they added. The risks and benefits of stopping or remaining on aspirin after an initial lower GI bleed are unclear (Gastroenterology 2016 Apr 26. doi: 10.1053/j.gastro.2016.04.013).
Accordingly, the researchers retrospectively studied 295 patients who had an initial aspirin-associated lower GI bleed, defined as 325 mg aspirin a day within a week of bleeding onset. All patients had melena or hematochezia documented by an attending physician and had no endoscopic evidence of upper GI bleeding.
For patients who continued using aspirin at least half the time, the 5-year cumulative incidence of recurrent lower GI bleeding was 19% (95% confidence interval [CI], 13%-25%) – more than double the rate among patients who used aspirin 20% or less of the time (5-year cumulative incidence, 7%; 95% CI, 3%-13%; P = .01). However, the 5-year cumulative incidence of serious cardiovascular events among nonusers was 37% (95% CI, 27%-46%), while the rate among aspirin users was 23% (95% CI, 17%-30%; P = .02). Mortality from noncardiovascular causes was also higher among nonusers (27%) than users (8%; P less than .001), probably because nonusers of aspirin tended to be older than users, but perhaps also because aspirin had a “nonvascular protective effect,” the researchers said.
A multivariate analysis confirmed these findings, linking lower GI bleeding to aspirin but not to use of steroids, anticoagulants, or antiplatelet drugs, or to age, sex, alcohol consumption, smoking, comorbidities, or cardiovascular risks. Indeed, continued aspirin use nearly tripled the chances of a recurrent lower GI bleed (hazard ratio, 2.76; 95% CI, 1.3-6.0; P = .01), but cut the risk of serious cardiovascular events by about 40% (HR, 0.59; 95% CI, 0.4-0.9; P = .02).
Deciding whether to resume aspirin after a severe lower GI bleed “presents a management dilemma for physicians, patients, and their families, particularly in the absence of risk-mitigating therapies and a lack of data on the risks and benefits of resuming aspirin,” the investigators emphasized. Their findings highlight the importance of weighing the cardiovascular benefits of aspirin against GI toxicity, they said. “Since there is substantial risk of recurrent bleeding, physicians should critically evaluate individual patients’ cardiovascular risk before resuming aspirin therapy. Our findings also suggest a need for a composite endpoint to evaluate clinically significant events throughout the GI tract in patients receiving antiplatelet drugs.”
The Chinese University of Hong Kong funded the study. Dr. Chan reported financial ties to Pfizer, Eisai, Takeda, Otsuka, and Astrazeneca.
Resuming low-dose aspirin after an initial lower gastrointestinal bleed significantly increased the chances of recurrence but protected against serious cardiovascular events, based on a single-center retrospective study published in the August issue of Gastroenterology.
In contrast, “we did not find concomitant use of anticoagulants, antiplatelets, and steroids as a predictor of recurrent lower GI bleeding,” said Dr. Francis Chan of the Prince of Wales Hospital in Hong Kong and his associates. “This may be due to the low percentage of concomitant drug use in both groups. Multicenter studies with a large number of patients will be required to identify additional risk factors for recurrent lower GI bleeding with aspirin use.”
Low-dose aspirin has long been known to help prevent coronary artery and cerebrovascular disease, and more recently has been found to potentially reduce the risk of several types of cancer, the researchers noted. Aspirin is well known to increase the risk of upper GI bleeding, but some studies have also linked it to lower GI bleeding. However, “patients with underlying cardiovascular diseases often require lifelong aspirin,” they added. The risks and benefits of stopping or remaining on aspirin after an initial lower GI bleed are unclear (Gastroenterology 2016 Apr 26. doi: 10.1053/j.gastro.2016.04.013).
Accordingly, the researchers retrospectively studied 295 patients who had an initial aspirin-associated lower GI bleed, defined as 325 mg aspirin a day within a week of bleeding onset. All patients had melena or hematochezia documented by an attending physician and had no endoscopic evidence of upper GI bleeding.
For patients who continued using aspirin at least half the time, the 5-year cumulative incidence of recurrent lower GI bleeding was 19% (95% confidence interval [CI], 13%-25%) – more than double the rate among patients who used aspirin 20% or less of the time (5-year cumulative incidence, 7%; 95% CI, 3%-13%; P = .01). However, the 5-year cumulative incidence of serious cardiovascular events among nonusers was 37% (95% CI, 27%-46%), while the rate among aspirin users was 23% (95% CI, 17%-30%; P = .02). Mortality from noncardiovascular causes was also higher among nonusers (27%) than users (8%; P less than .001), probably because nonusers of aspirin tended to be older than users, but perhaps also because aspirin had a “nonvascular protective effect,” the researchers said.
A multivariate analysis confirmed these findings, linking lower GI bleeding to aspirin but not to use of steroids, anticoagulants, or antiplatelet drugs, or to age, sex, alcohol consumption, smoking, comorbidities, or cardiovascular risks. Indeed, continued aspirin use nearly tripled the chances of a recurrent lower GI bleed (hazard ratio, 2.76; 95% CI, 1.3-6.0; P = .01), but cut the risk of serious cardiovascular events by about 40% (HR, 0.59; 95% CI, 0.4-0.9; P = .02).
Deciding whether to resume aspirin after a severe lower GI bleed “presents a management dilemma for physicians, patients, and their families, particularly in the absence of risk-mitigating therapies and a lack of data on the risks and benefits of resuming aspirin,” the investigators emphasized. Their findings highlight the importance of weighing the cardiovascular benefits of aspirin against GI toxicity, they said. “Since there is substantial risk of recurrent bleeding, physicians should critically evaluate individual patients’ cardiovascular risk before resuming aspirin therapy. Our findings also suggest a need for a composite endpoint to evaluate clinically significant events throughout the GI tract in patients receiving antiplatelet drugs.”
The Chinese University of Hong Kong funded the study. Dr. Chan reported financial ties to Pfizer, Eisai, Takeda, Otsuka, and Astrazeneca.
FROM GASTROENTEROLOGY
Key clinical point: Resuming low-dose aspirin after a lower gastrointestinal bleed increased the risk of recurrence but protected against cardiovascular events.
Major finding: At 5 years, the cumulative incidence of recurrent lower GI bleeding was 19% for patients who stayed on aspirin and 7% for patients who largely stopped it (P = .01). The cumulative incidence of serious cardiovascular events was 25% for users and 37% for nonusers (P = .02).
Data source: A single-center 5-year retrospective cohort study of 295 patients with aspirin-associated melena or hematochezia and no upper gastrointestinal bleeding.
Disclosures: The Chinese University of Hong Kong funded the study. Dr. Chan reported financial ties to Pfizer, Eisai, Takeda, Otsuka, and Astrazeneca.
Eating more fiber linked to lower odds of flares in Crohn’s disease
Patients with remitted Crohn’s disease who avoided dietary fiber were significantly more likely to flare than were those who fell into the highest quartile of fiber consumption, according to a 6-month prospective study published in the August issue of Clinical Gastroenterology and Hepatology.
Avoiding fiber was not linked to flare in ulcerative colitis, however, said Carol Brotherton, MD, from George Mason University, Fairfax, Va., and her associates. “The results of this study support findings reported in investigations occurring in the 1980s: Low-fiber eating does not result in improved outcomes for individuals with Crohn’s disease,” they concluded. They recommended studies exploring why patients with Crohn’s disease restrict their fiber intake, and exactly how fiber-rich foods might benefit certain phenotypes of inflammatory bowel disease (IBD).
Studies have linked IBD to an abnormal mucosal response to gastrointestinal microbiota and have shown that dietary fiber affects the microbiome. But clinicians lack rigorous data to guide recommendations about fiber intake for patients with IBD, according to the investigators. “Although there are reasons to think that fiber could have a beneficial influence through generation of short-chain fatty acids, such as butyrate, patients with IBD are often instructed to limit their fiber consumption,” they noted (Clin Gastroenterol Hepatol. 2015 Dec 31. doi: 10.1016/j.cgh.2015.12.029).
To help clarify the issue, Dr. Brotherton and her associates analyzed 26-item dietary surveys for 1,619 adult participants in the Crohn’s and Colitis Foundation of America Partners Internet cohort. In all, 1,130 patients had Crohn’s disease, and 489 had ulcerative colitis or indeterminate colitis. All were in remission at baseline. Patients completed surveys then and again 6 months later. They ranged from less than 30 to more than 60 years of age, and half reported having had IBD for at least 11 years.
Patients with Crohn’s disease reported consuming a median of 14.6 grams of fiber per day (interquartile range, 11.6-18.6 g). Those in the highest quartile, who consumed a median of nearly 24 g/fiber a day, were significantly less likely to report having flared at 6-month follow-up than were patients in the lowest quartile of fiber intake, who consumed less than half that amount (adjusted odds ratio, 0.58; 95% confidence interval, 0.37-0.90). Patients with Crohn’s disease who reported not avoiding high-fiber foods also were significantly less likely to subsequently flare than those who said they avoided high-fiber foods (adjusted OR, 0.59; 95% CI, 0.43-0.81).
Patients with ulcerative colitis consumed, on average, 2 more grams of fiber per day than patients with Crohn’s disease, and fiber intake was not linked to flares in ulcerative colitis, the researchers said (aOR for highest vs. lowest quartile of fiber intake, 1.82; 95% CI, 0.92-3.60). Males reported consuming significantly more fiber than females (aOR, 4.7; 95% CI, 3.3-6.7 and aOR, 2.6; 95% CI, 1.9-3.6), while a history of hospitalization and surgery were linked to lower fiber intake, as reported by a study from the late 1970s (BMJ 1979 Sep;2:764-6).
“Despite the large size of the study, the numbers of participants in certain subgroups was limited,” the researchers acknowledged. “We do not have accurate information from this Internet survey about disease phenotype, particularly stricturing disease in Crohn’s disease. However, in sensitivity analyses among patients with Crohn’s disease, effect estimates were very similar after excluding those with any history of IBD surgery or hospitalization, suggesting that our observations of protective effects of fiber intake on flare were not influenced by aggressive phenotypes.”
The research was supported by the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the Crohn’s and Colitis Foundation of America. One coinvestigator reported ties to Salix, AbbVie, and NPS Pharmaceuticals, while the rest had no disclosures.
Patients with remitted Crohn’s disease who avoided dietary fiber were significantly more likely to flare than were those who fell into the highest quartile of fiber consumption, according to a 6-month prospective study published in the August issue of Clinical Gastroenterology and Hepatology.
Avoiding fiber was not linked to flare in ulcerative colitis, however, said Carol Brotherton, MD, from George Mason University, Fairfax, Va., and her associates. “The results of this study support findings reported in investigations occurring in the 1980s: Low-fiber eating does not result in improved outcomes for individuals with Crohn’s disease,” they concluded. They recommended studies exploring why patients with Crohn’s disease restrict their fiber intake, and exactly how fiber-rich foods might benefit certain phenotypes of inflammatory bowel disease (IBD).
Studies have linked IBD to an abnormal mucosal response to gastrointestinal microbiota and have shown that dietary fiber affects the microbiome. But clinicians lack rigorous data to guide recommendations about fiber intake for patients with IBD, according to the investigators. “Although there are reasons to think that fiber could have a beneficial influence through generation of short-chain fatty acids, such as butyrate, patients with IBD are often instructed to limit their fiber consumption,” they noted (Clin Gastroenterol Hepatol. 2015 Dec 31. doi: 10.1016/j.cgh.2015.12.029).
To help clarify the issue, Dr. Brotherton and her associates analyzed 26-item dietary surveys for 1,619 adult participants in the Crohn’s and Colitis Foundation of America Partners Internet cohort. In all, 1,130 patients had Crohn’s disease, and 489 had ulcerative colitis or indeterminate colitis. All were in remission at baseline. Patients completed surveys then and again 6 months later. They ranged from less than 30 to more than 60 years of age, and half reported having had IBD for at least 11 years.
Patients with Crohn’s disease reported consuming a median of 14.6 grams of fiber per day (interquartile range, 11.6-18.6 g). Those in the highest quartile, who consumed a median of nearly 24 g/fiber a day, were significantly less likely to report having flared at 6-month follow-up than were patients in the lowest quartile of fiber intake, who consumed less than half that amount (adjusted odds ratio, 0.58; 95% confidence interval, 0.37-0.90). Patients with Crohn’s disease who reported not avoiding high-fiber foods also were significantly less likely to subsequently flare than those who said they avoided high-fiber foods (adjusted OR, 0.59; 95% CI, 0.43-0.81).
Patients with ulcerative colitis consumed, on average, 2 more grams of fiber per day than patients with Crohn’s disease, and fiber intake was not linked to flares in ulcerative colitis, the researchers said (aOR for highest vs. lowest quartile of fiber intake, 1.82; 95% CI, 0.92-3.60). Males reported consuming significantly more fiber than females (aOR, 4.7; 95% CI, 3.3-6.7 and aOR, 2.6; 95% CI, 1.9-3.6), while a history of hospitalization and surgery were linked to lower fiber intake, as reported by a study from the late 1970s (BMJ 1979 Sep;2:764-6).
“Despite the large size of the study, the numbers of participants in certain subgroups was limited,” the researchers acknowledged. “We do not have accurate information from this Internet survey about disease phenotype, particularly stricturing disease in Crohn’s disease. However, in sensitivity analyses among patients with Crohn’s disease, effect estimates were very similar after excluding those with any history of IBD surgery or hospitalization, suggesting that our observations of protective effects of fiber intake on flare were not influenced by aggressive phenotypes.”
The research was supported by the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the Crohn’s and Colitis Foundation of America. One coinvestigator reported ties to Salix, AbbVie, and NPS Pharmaceuticals, while the rest had no disclosures.
Patients with remitted Crohn’s disease who avoided dietary fiber were significantly more likely to flare than were those who fell into the highest quartile of fiber consumption, according to a 6-month prospective study published in the August issue of Clinical Gastroenterology and Hepatology.
Avoiding fiber was not linked to flare in ulcerative colitis, however, said Carol Brotherton, MD, from George Mason University, Fairfax, Va., and her associates. “The results of this study support findings reported in investigations occurring in the 1980s: Low-fiber eating does not result in improved outcomes for individuals with Crohn’s disease,” they concluded. They recommended studies exploring why patients with Crohn’s disease restrict their fiber intake, and exactly how fiber-rich foods might benefit certain phenotypes of inflammatory bowel disease (IBD).
Studies have linked IBD to an abnormal mucosal response to gastrointestinal microbiota and have shown that dietary fiber affects the microbiome. But clinicians lack rigorous data to guide recommendations about fiber intake for patients with IBD, according to the investigators. “Although there are reasons to think that fiber could have a beneficial influence through generation of short-chain fatty acids, such as butyrate, patients with IBD are often instructed to limit their fiber consumption,” they noted (Clin Gastroenterol Hepatol. 2015 Dec 31. doi: 10.1016/j.cgh.2015.12.029).
To help clarify the issue, Dr. Brotherton and her associates analyzed 26-item dietary surveys for 1,619 adult participants in the Crohn’s and Colitis Foundation of America Partners Internet cohort. In all, 1,130 patients had Crohn’s disease, and 489 had ulcerative colitis or indeterminate colitis. All were in remission at baseline. Patients completed surveys then and again 6 months later. They ranged from less than 30 to more than 60 years of age, and half reported having had IBD for at least 11 years.
Patients with Crohn’s disease reported consuming a median of 14.6 grams of fiber per day (interquartile range, 11.6-18.6 g). Those in the highest quartile, who consumed a median of nearly 24 g/fiber a day, were significantly less likely to report having flared at 6-month follow-up than were patients in the lowest quartile of fiber intake, who consumed less than half that amount (adjusted odds ratio, 0.58; 95% confidence interval, 0.37-0.90). Patients with Crohn’s disease who reported not avoiding high-fiber foods also were significantly less likely to subsequently flare than those who said they avoided high-fiber foods (adjusted OR, 0.59; 95% CI, 0.43-0.81).
Patients with ulcerative colitis consumed, on average, 2 more grams of fiber per day than patients with Crohn’s disease, and fiber intake was not linked to flares in ulcerative colitis, the researchers said (aOR for highest vs. lowest quartile of fiber intake, 1.82; 95% CI, 0.92-3.60). Males reported consuming significantly more fiber than females (aOR, 4.7; 95% CI, 3.3-6.7 and aOR, 2.6; 95% CI, 1.9-3.6), while a history of hospitalization and surgery were linked to lower fiber intake, as reported by a study from the late 1970s (BMJ 1979 Sep;2:764-6).
“Despite the large size of the study, the numbers of participants in certain subgroups was limited,” the researchers acknowledged. “We do not have accurate information from this Internet survey about disease phenotype, particularly stricturing disease in Crohn’s disease. However, in sensitivity analyses among patients with Crohn’s disease, effect estimates were very similar after excluding those with any history of IBD surgery or hospitalization, suggesting that our observations of protective effects of fiber intake on flare were not influenced by aggressive phenotypes.”
The research was supported by the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the Crohn’s and Colitis Foundation of America. One coinvestigator reported ties to Salix, AbbVie, and NPS Pharmaceuticals, while the rest had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Crohn’s disease patients who ate more fiber were significantly less likely to flare than were those who ate less fiber.
Major finding: Patients in the highest quartile of fiber consumption had about a 40% lower odds of flare at 6 months than did patients in the lowest quartile (adjusted odds ratio, 0.58). Fiber did not prevent flares in ulcerative colitis.
Data source: A prospective study of 1,619 participants in the Crohn’s and Colitis Foundation of America Partners Internet cohort.
Disclosures: The research was supported by the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the Crohn’s and Colitis Foundation of America. One coinvestigator reported ties to Salix, AbbVie, and NPS Pharmaceuticals, while the rest had no disclosures.
Rectal indomethacin cut odds of post-ERCP pancreatitis in real-world study
A single, 100-mg rectal dose of indomethacin cut the odds of moderate to severe pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) by 85% in a single-center retrospective study of more than 4,000 patients reported in the August issue of Gastroenterology.
The effect extended to low-risk patients and those with malignant biliary obstruction, who make up the majority of ERCP patients in community practice, said Nikhil R. Thiruvengadam, MD, and his associates at the University of Pennsylvania. “Usage of rectal indomethacin in current clinical practice is low, as most endoscopists outside of referral centers perform ERCP for indications that are considered low-risk for PEP [post-ERCP pancreatitis], and until now, there were no data to support a benefit of rectal NSAIDs in this population,” they wrote in Gastroenterology. Their “real-world analysis” clearly shows the benefits of rectal indomethacin in low-risk patients and supports its increased use after ERCP, they added.
Pancreatitis, the most common complication of ERCP, affected 2%-9% of patients in prior studies and costs about $200 million in the United States annually, the investigators noted. Pancreatic duct stents help prevent post-ERCP pancreatitis, but require experience to place and have their own complications that limit their use in low-risk patients. Past studies of rectal indomethacin after ERCP reported mixed results and mainly focused on high-risk patients, leaving questions about whether to routinely use this NSAID after ERCP, said the researchers (Gastroenterology. 2016 May 20. doi: 10.1053/j.gastro.2016.04.048). Their study included 4,017 patients who underwent ERCP at the University of Pennsylvania between 2009 and 2015. From 2012 onward, nearly all patients received 100 mg rectal indomethacin immediately after the duodenoscope was withdrawn. This indomethacin group included 2,007 patients, while 2,010 patients in the study did not receive rectal indomethacin. In all, 95 (4.73%) untreated patients developed post-ERCP pancreatitis, compared with only 40 (1.99%) patients who received indomethacin, for a 65% reduction in the odds of post-ERCP pancreatitis (odds ratio, 0.35; 95% confidence interval, 0.24-0.51; P less than .001). Rectal indomethacin also led to an 83% drop in the odds of moderate to severe post-ERCP pancreatitis (OR, 0.17; 95% CI, 0.09-0.32; P less than .001) and showed very similar protective effects for patients with malignant obstruction (OR, 0.35; 95% CI, 0.17-0.75; P less than .001] and 0.20; 95% CI, 0.07-0.63; P less than 0.001, respectively).
Rectal indomethacin was particularly beneficial for patients with malignant obstruction and pancreatic adenocarcinoma, the investigators noted. Such patients had post-ERCP rates of 2.31% when they received rectal indomethacin and 7.53% otherwise (P less than .001). They also had a nearly sevenfold lower rate of moderate to severe post-ERCP pancreatitis when they received rectal indomethacin (P = .001).
Treatment did not affect the chances of perforation and did not cause anaphylaxis, but was tied to a slightly higher rate of postprocedural gastrointestinal bleeding among sphincterotomy patients (0.65% with treatment versus 0.45% without; P = .52). However, sphincterotomy patients were much less likely to develop pancreatitis when they received rectal indomethacin than when they did not (0% and 9.58% of patients, respectively; P = .003).
“The majority of ERCPs were performed by experienced endoscopists at a tertiary care center, which may have limited the effects of variable procedural skills on the risk of PEP,” the researchers said. “Therefore, generalizability of our findings to other populations may be limited. However, it should be noted that the overall PEP rate in both the unexposed and indomethacin groups was fairly low and similar to large community-based estimates, suggesting that our overall patient population was of similar overall risk.” The study was not powered to assess the combined effects of rectal indomethacin and pancreatic duct stents, they noted.
The investigators reported no funding sources and had no disclosures.
A single, 100-mg rectal dose of indomethacin cut the odds of moderate to severe pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) by 85% in a single-center retrospective study of more than 4,000 patients reported in the August issue of Gastroenterology.
The effect extended to low-risk patients and those with malignant biliary obstruction, who make up the majority of ERCP patients in community practice, said Nikhil R. Thiruvengadam, MD, and his associates at the University of Pennsylvania. “Usage of rectal indomethacin in current clinical practice is low, as most endoscopists outside of referral centers perform ERCP for indications that are considered low-risk for PEP [post-ERCP pancreatitis], and until now, there were no data to support a benefit of rectal NSAIDs in this population,” they wrote in Gastroenterology. Their “real-world analysis” clearly shows the benefits of rectal indomethacin in low-risk patients and supports its increased use after ERCP, they added.
Pancreatitis, the most common complication of ERCP, affected 2%-9% of patients in prior studies and costs about $200 million in the United States annually, the investigators noted. Pancreatic duct stents help prevent post-ERCP pancreatitis, but require experience to place and have their own complications that limit their use in low-risk patients. Past studies of rectal indomethacin after ERCP reported mixed results and mainly focused on high-risk patients, leaving questions about whether to routinely use this NSAID after ERCP, said the researchers (Gastroenterology. 2016 May 20. doi: 10.1053/j.gastro.2016.04.048). Their study included 4,017 patients who underwent ERCP at the University of Pennsylvania between 2009 and 2015. From 2012 onward, nearly all patients received 100 mg rectal indomethacin immediately after the duodenoscope was withdrawn. This indomethacin group included 2,007 patients, while 2,010 patients in the study did not receive rectal indomethacin. In all, 95 (4.73%) untreated patients developed post-ERCP pancreatitis, compared with only 40 (1.99%) patients who received indomethacin, for a 65% reduction in the odds of post-ERCP pancreatitis (odds ratio, 0.35; 95% confidence interval, 0.24-0.51; P less than .001). Rectal indomethacin also led to an 83% drop in the odds of moderate to severe post-ERCP pancreatitis (OR, 0.17; 95% CI, 0.09-0.32; P less than .001) and showed very similar protective effects for patients with malignant obstruction (OR, 0.35; 95% CI, 0.17-0.75; P less than .001] and 0.20; 95% CI, 0.07-0.63; P less than 0.001, respectively).
Rectal indomethacin was particularly beneficial for patients with malignant obstruction and pancreatic adenocarcinoma, the investigators noted. Such patients had post-ERCP rates of 2.31% when they received rectal indomethacin and 7.53% otherwise (P less than .001). They also had a nearly sevenfold lower rate of moderate to severe post-ERCP pancreatitis when they received rectal indomethacin (P = .001).
Treatment did not affect the chances of perforation and did not cause anaphylaxis, but was tied to a slightly higher rate of postprocedural gastrointestinal bleeding among sphincterotomy patients (0.65% with treatment versus 0.45% without; P = .52). However, sphincterotomy patients were much less likely to develop pancreatitis when they received rectal indomethacin than when they did not (0% and 9.58% of patients, respectively; P = .003).
“The majority of ERCPs were performed by experienced endoscopists at a tertiary care center, which may have limited the effects of variable procedural skills on the risk of PEP,” the researchers said. “Therefore, generalizability of our findings to other populations may be limited. However, it should be noted that the overall PEP rate in both the unexposed and indomethacin groups was fairly low and similar to large community-based estimates, suggesting that our overall patient population was of similar overall risk.” The study was not powered to assess the combined effects of rectal indomethacin and pancreatic duct stents, they noted.
The investigators reported no funding sources and had no disclosures.
A single, 100-mg rectal dose of indomethacin cut the odds of moderate to severe pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) by 85% in a single-center retrospective study of more than 4,000 patients reported in the August issue of Gastroenterology.
The effect extended to low-risk patients and those with malignant biliary obstruction, who make up the majority of ERCP patients in community practice, said Nikhil R. Thiruvengadam, MD, and his associates at the University of Pennsylvania. “Usage of rectal indomethacin in current clinical practice is low, as most endoscopists outside of referral centers perform ERCP for indications that are considered low-risk for PEP [post-ERCP pancreatitis], and until now, there were no data to support a benefit of rectal NSAIDs in this population,” they wrote in Gastroenterology. Their “real-world analysis” clearly shows the benefits of rectal indomethacin in low-risk patients and supports its increased use after ERCP, they added.
Pancreatitis, the most common complication of ERCP, affected 2%-9% of patients in prior studies and costs about $200 million in the United States annually, the investigators noted. Pancreatic duct stents help prevent post-ERCP pancreatitis, but require experience to place and have their own complications that limit their use in low-risk patients. Past studies of rectal indomethacin after ERCP reported mixed results and mainly focused on high-risk patients, leaving questions about whether to routinely use this NSAID after ERCP, said the researchers (Gastroenterology. 2016 May 20. doi: 10.1053/j.gastro.2016.04.048). Their study included 4,017 patients who underwent ERCP at the University of Pennsylvania between 2009 and 2015. From 2012 onward, nearly all patients received 100 mg rectal indomethacin immediately after the duodenoscope was withdrawn. This indomethacin group included 2,007 patients, while 2,010 patients in the study did not receive rectal indomethacin. In all, 95 (4.73%) untreated patients developed post-ERCP pancreatitis, compared with only 40 (1.99%) patients who received indomethacin, for a 65% reduction in the odds of post-ERCP pancreatitis (odds ratio, 0.35; 95% confidence interval, 0.24-0.51; P less than .001). Rectal indomethacin also led to an 83% drop in the odds of moderate to severe post-ERCP pancreatitis (OR, 0.17; 95% CI, 0.09-0.32; P less than .001) and showed very similar protective effects for patients with malignant obstruction (OR, 0.35; 95% CI, 0.17-0.75; P less than .001] and 0.20; 95% CI, 0.07-0.63; P less than 0.001, respectively).
Rectal indomethacin was particularly beneficial for patients with malignant obstruction and pancreatic adenocarcinoma, the investigators noted. Such patients had post-ERCP rates of 2.31% when they received rectal indomethacin and 7.53% otherwise (P less than .001). They also had a nearly sevenfold lower rate of moderate to severe post-ERCP pancreatitis when they received rectal indomethacin (P = .001).
Treatment did not affect the chances of perforation and did not cause anaphylaxis, but was tied to a slightly higher rate of postprocedural gastrointestinal bleeding among sphincterotomy patients (0.65% with treatment versus 0.45% without; P = .52). However, sphincterotomy patients were much less likely to develop pancreatitis when they received rectal indomethacin than when they did not (0% and 9.58% of patients, respectively; P = .003).
“The majority of ERCPs were performed by experienced endoscopists at a tertiary care center, which may have limited the effects of variable procedural skills on the risk of PEP,” the researchers said. “Therefore, generalizability of our findings to other populations may be limited. However, it should be noted that the overall PEP rate in both the unexposed and indomethacin groups was fairly low and similar to large community-based estimates, suggesting that our overall patient population was of similar overall risk.” The study was not powered to assess the combined effects of rectal indomethacin and pancreatic duct stents, they noted.
The investigators reported no funding sources and had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: A single 100-mg rectal dose of indomethacin given immediately after endoscopic retrograde cholangiopancreatography significantly reduced the odds of postprocedural pancreatitis, including in low-risk patients and those with malignant obstruction.
Major finding: The odds of pancreatitis were 65% lower when patients received rectal indomethacin than otherwise.
Data source: A single-center retrospective cohort study of 4,017 patients undergoing ERCP.
Disclosures: The investigators reported no funding sources and had no disclosures.
Adding ipilimumab to chemotherapy did not boost overall survival in small-cell lung cancer
For patients with extensive-stage small-cell lung cancer, adding the anti–CTLA-4 antibody ipilimumab (Yervoy) to etoposide and platinum did not improve overall survival, compared with administering etoposide and platinum alone, investigators reported in the Journal of Clinical Oncology.
“Although exploratory in nature, chemotherapy plus ipilimumab did not demonstrate significant improvement in other endpoints, and no subgroups demonstrated greater benefit versus chemotherapy alone,” added Martin Reck, MD, of LungenClinic Grosshansdorf (Germany), and his associates.
The randomized phase III study enrolled 1,132 chemotherapy-naive patients who were randomly assigned to either chemotherapy plus ipilimumab (etoposide plus investigator’s choice of cisplatin or carboplatin during cycles one to four, and ipilimumab during cycles three to six) or to chemotherapy plus placebo (etoposide plus investigator’s choice of cisplatin or carboplatin during cycles one to four, and placebo during cycles three to six). Patients with a complete or partial response during induction could undergo prophylactic cranial irradiation before starting maintenance ipilimumab (10 mg/kg) or placebo administered every 12 weeks, the investigators reported (J Clin Oncol. 2016 July 26. doi:10.1200/JCO.2016.67.6601).
Among 954 patients who received at least one dose of study therapy, the primary endpoint, median overall survival, was 11.0 months for the ipilimumab-chemotherapy arm and 10.9 months for the placebo arm (hazard ratio, 0.9; 95% confidence interval, 0.8-1.1; P = .4). “Across most prespecified patient subgroups, hazard ratios for overall survival [also] did not seem to favor one treatment arm,” the researchers wrote.
Ipilimumab also failed to achieve a meaningful increase in median progression-free survival, compared with placebo (4.6 months and 4.4 months, respectively). There was only one complete response in the ipilimumab-chemotherapy group and none in the chemotherapy-placebo group. A total of 62% of patients achieved a partial response in each group, and the median duration of response was 4 months in the ipilimumab-chemotherapy group (95% CI, 3.3-4.2 months) versus 3.5 months (95% CI, 3.3-4.1 months) in the chemotherapy-placebo group.
There were no new or unexpected safety signals, but ipilimumab was associated with higher rates of overall and severe-grade diarrhea, colitis, and rash. The five treatment-related deaths in the ipilimumab-chemotherapy group included two from colitis, two from sepsis, and one from liver toxicity. “In the chemotherapy plus placebo arm, there were two treatment-related deaths, one resulting from sepsis and one from bone marrow suppression,” the researchers wrote. Treatment-related discontinuations also were more common with ipilimumab plus chemotherapy (18%) than with chemotherapy plus placebo (2%).
“To date, PD-1 inhibitors, alone or in combination with CTLA-4 inhibitors, show the most promise in small-cell lung cancer,” the investigators said. Multiple trials are exploring the use of these agents as maintenance therapy or in second-line settings, they added.
Bristol-Myers Squibb makes ipilimumab, funded the study, and helped collect and analyze the data. Dr. Reck disclosed financial ties to Bristol-Myers Squibb, Hoffmann-La Roche, Eli Lilly, Merck Sharp & Dohme, and several other pharmaceutical companies.
For patients with extensive-stage small-cell lung cancer, adding the anti–CTLA-4 antibody ipilimumab (Yervoy) to etoposide and platinum did not improve overall survival, compared with administering etoposide and platinum alone, investigators reported in the Journal of Clinical Oncology.
“Although exploratory in nature, chemotherapy plus ipilimumab did not demonstrate significant improvement in other endpoints, and no subgroups demonstrated greater benefit versus chemotherapy alone,” added Martin Reck, MD, of LungenClinic Grosshansdorf (Germany), and his associates.
The randomized phase III study enrolled 1,132 chemotherapy-naive patients who were randomly assigned to either chemotherapy plus ipilimumab (etoposide plus investigator’s choice of cisplatin or carboplatin during cycles one to four, and ipilimumab during cycles three to six) or to chemotherapy plus placebo (etoposide plus investigator’s choice of cisplatin or carboplatin during cycles one to four, and placebo during cycles three to six). Patients with a complete or partial response during induction could undergo prophylactic cranial irradiation before starting maintenance ipilimumab (10 mg/kg) or placebo administered every 12 weeks, the investigators reported (J Clin Oncol. 2016 July 26. doi:10.1200/JCO.2016.67.6601).
Among 954 patients who received at least one dose of study therapy, the primary endpoint, median overall survival, was 11.0 months for the ipilimumab-chemotherapy arm and 10.9 months for the placebo arm (hazard ratio, 0.9; 95% confidence interval, 0.8-1.1; P = .4). “Across most prespecified patient subgroups, hazard ratios for overall survival [also] did not seem to favor one treatment arm,” the researchers wrote.
Ipilimumab also failed to achieve a meaningful increase in median progression-free survival, compared with placebo (4.6 months and 4.4 months, respectively). There was only one complete response in the ipilimumab-chemotherapy group and none in the chemotherapy-placebo group. A total of 62% of patients achieved a partial response in each group, and the median duration of response was 4 months in the ipilimumab-chemotherapy group (95% CI, 3.3-4.2 months) versus 3.5 months (95% CI, 3.3-4.1 months) in the chemotherapy-placebo group.
There were no new or unexpected safety signals, but ipilimumab was associated with higher rates of overall and severe-grade diarrhea, colitis, and rash. The five treatment-related deaths in the ipilimumab-chemotherapy group included two from colitis, two from sepsis, and one from liver toxicity. “In the chemotherapy plus placebo arm, there were two treatment-related deaths, one resulting from sepsis and one from bone marrow suppression,” the researchers wrote. Treatment-related discontinuations also were more common with ipilimumab plus chemotherapy (18%) than with chemotherapy plus placebo (2%).
“To date, PD-1 inhibitors, alone or in combination with CTLA-4 inhibitors, show the most promise in small-cell lung cancer,” the investigators said. Multiple trials are exploring the use of these agents as maintenance therapy or in second-line settings, they added.
Bristol-Myers Squibb makes ipilimumab, funded the study, and helped collect and analyze the data. Dr. Reck disclosed financial ties to Bristol-Myers Squibb, Hoffmann-La Roche, Eli Lilly, Merck Sharp & Dohme, and several other pharmaceutical companies.
For patients with extensive-stage small-cell lung cancer, adding the anti–CTLA-4 antibody ipilimumab (Yervoy) to etoposide and platinum did not improve overall survival, compared with administering etoposide and platinum alone, investigators reported in the Journal of Clinical Oncology.
“Although exploratory in nature, chemotherapy plus ipilimumab did not demonstrate significant improvement in other endpoints, and no subgroups demonstrated greater benefit versus chemotherapy alone,” added Martin Reck, MD, of LungenClinic Grosshansdorf (Germany), and his associates.
The randomized phase III study enrolled 1,132 chemotherapy-naive patients who were randomly assigned to either chemotherapy plus ipilimumab (etoposide plus investigator’s choice of cisplatin or carboplatin during cycles one to four, and ipilimumab during cycles three to six) or to chemotherapy plus placebo (etoposide plus investigator’s choice of cisplatin or carboplatin during cycles one to four, and placebo during cycles three to six). Patients with a complete or partial response during induction could undergo prophylactic cranial irradiation before starting maintenance ipilimumab (10 mg/kg) or placebo administered every 12 weeks, the investigators reported (J Clin Oncol. 2016 July 26. doi:10.1200/JCO.2016.67.6601).
Among 954 patients who received at least one dose of study therapy, the primary endpoint, median overall survival, was 11.0 months for the ipilimumab-chemotherapy arm and 10.9 months for the placebo arm (hazard ratio, 0.9; 95% confidence interval, 0.8-1.1; P = .4). “Across most prespecified patient subgroups, hazard ratios for overall survival [also] did not seem to favor one treatment arm,” the researchers wrote.
Ipilimumab also failed to achieve a meaningful increase in median progression-free survival, compared with placebo (4.6 months and 4.4 months, respectively). There was only one complete response in the ipilimumab-chemotherapy group and none in the chemotherapy-placebo group. A total of 62% of patients achieved a partial response in each group, and the median duration of response was 4 months in the ipilimumab-chemotherapy group (95% CI, 3.3-4.2 months) versus 3.5 months (95% CI, 3.3-4.1 months) in the chemotherapy-placebo group.
There were no new or unexpected safety signals, but ipilimumab was associated with higher rates of overall and severe-grade diarrhea, colitis, and rash. The five treatment-related deaths in the ipilimumab-chemotherapy group included two from colitis, two from sepsis, and one from liver toxicity. “In the chemotherapy plus placebo arm, there were two treatment-related deaths, one resulting from sepsis and one from bone marrow suppression,” the researchers wrote. Treatment-related discontinuations also were more common with ipilimumab plus chemotherapy (18%) than with chemotherapy plus placebo (2%).
“To date, PD-1 inhibitors, alone or in combination with CTLA-4 inhibitors, show the most promise in small-cell lung cancer,” the investigators said. Multiple trials are exploring the use of these agents as maintenance therapy or in second-line settings, they added.
Bristol-Myers Squibb makes ipilimumab, funded the study, and helped collect and analyze the data. Dr. Reck disclosed financial ties to Bristol-Myers Squibb, Hoffmann-La Roche, Eli Lilly, Merck Sharp & Dohme, and several other pharmaceutical companies.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The anti–CTLA-4 antibody ipilimumab (Yervoy) missed its primary endpoint in a trial of patients with extensive-stage small-cell lung cancer.
Major finding: The median overall survival, was 11.0 months for the ipilimumab-chemotherapy arm and 10.9 months for the placebo arm (hazard ratio, 0.9; 95% confidence interval, 0.8-1.1; P = .4).
Data source: A randomized phase III trial of 954 patients.
Disclosures: Bristol-Myers Squibb funded the study and helped collect and analyze the study results. Dr. Reck disclosed financial ties to Bristol-Myers Squibb, Hoffmann-La Roche, Eli Lilly, Merck Sharp & Dohme, and several other pharmaceutical companies.
AAN recommends against routine closure of patent foramen ovale for secondary stroke prevention
An updated practice advisory from the American Academy of Neurology does not recommend the routine use of catheter-based closure of patent foramen ovale in patients with a history of cryptogenic ischemic stroke.
“Because of the limitations of the efficacy evidence and the potential for serious adverse effects, we judge the risk-benefit trade-offs of PFO [patent foramen ovale] closure by either the STARFlex or AMPLATZER PFO Occluder to be uncertain,” wrote Steven Messé, MD, associate professor of neurology at the University of Pennsylvania, Philadelphia, and his associates. “In rare circumstances, such as recurrent strokes despite adequate medical therapy with no other mechanism identified, clinicians may offer the AMPLATZER PFO Occluder if it is available,” they noted.
They also supported antiplatelet agents over anticoagulants unless patients have another indication for blood thinners, noting “the uncertainty surrounding the benefit of anticoagulation in the setting of PFO, and anticoagulation’s well-known harm profile.”
PFO affects about one in four individuals overall and up to half of cryptogenic stroke patients. The previous (2004) version of this practice advisory cited insufficient evidence to guide optimal therapy for secondary stroke prevention in these patients (Neurology. 2004;Apr 13;62[7]:1042-50). To update the guideline, Dr. Messé and his associates searched the literature for relevant randomized studies, excluding transient ischemic attacks when feasible because of their subjective nature, and focusing on intention-to-treat analyses to reduce bias (Neurology. 2016 Jul 27;doi: 10.1212/WNL.0000000000002961).
Among 809 initial articles, 5 were considered relevant – a randomized, open-label, multicenter study of the STARFlex device (CLOSURE I), two randomized, controlled trials of the AMPLATZER PFO Occluder (PC Trial and RESPECT), and two randomized studies of warfarin versus aspirin in cryptogenic stroke patients, the experts said.
Percutaneous PFO closure with the STARFlex device did not appear to prevent secondary stroke, compared with medical therapy alone, based on a small positive estimated difference in risk of about 0.1%, and a 95% confidence interval that crossed zero (–2.2% to 2.0%). In contrast, the AMPLATZER PFO Occluder decreased the risk of secondary stroke by about 1.7% (95% CI, –3.2% to –0.2%), but upped the risk of procedural complications by more than 3%, and also slightly increased the risk of new-onset atrial fibrillation (1.6%; 95% CI, 0.1% to 3.2%).
Efficacy data were insufficient to clearly support anticoagulants over antiplatelet therapy for recurrent stroke prevention, the experts concluded. Compared with antiplatelet therapy, anticoagulation was associated with a 2% increase in risk of recurrent stroke, but the 95% confidence interval for this estimate was wide and crossed zero. “In the absence of another indication for anticoagulation, clinicians may routinely offer antiplatelet medications instead of anticoagulation to patients with cryptogenic stroke and PFO,” they wrote. “In rare circumstances, such as stroke that recurs while a patient is undergoing antiplatelet therapy, clinicians may offer anticoagulation to patients with cryptogenic stroke and PFO.”
Their strongest recommendation was to counsel patients who are considering percutaneous PFO closure “that having a PFO is common; it occurs in about 1 in 4 people; it is impossible to determine with certainty whether their PFOs caused their strokes or TIAs; the effectiveness of the procedure for reducing stroke risk remains uncertain; and the procedure is associated with relatively uncommon, yet potentially serious, complications.”
The practice advisory was supported by the American Academy of Neurology. Dr. Messé disclosed ties to GlaxoSmithKline and WL Gore & Associates and has been an investigator for the REDUCE and CLOSURE-I trials. Five of his coauthors have been investigators for RESPECT, CLOSURE-I, and REDUCE, have been editors for Neurology, and have received compensation from Genentech, Pfizer, Gilead Sciences, and other pharmaceutical companies. One coauthor had no disclosures.
An updated practice advisory from the American Academy of Neurology does not recommend the routine use of catheter-based closure of patent foramen ovale in patients with a history of cryptogenic ischemic stroke.
“Because of the limitations of the efficacy evidence and the potential for serious adverse effects, we judge the risk-benefit trade-offs of PFO [patent foramen ovale] closure by either the STARFlex or AMPLATZER PFO Occluder to be uncertain,” wrote Steven Messé, MD, associate professor of neurology at the University of Pennsylvania, Philadelphia, and his associates. “In rare circumstances, such as recurrent strokes despite adequate medical therapy with no other mechanism identified, clinicians may offer the AMPLATZER PFO Occluder if it is available,” they noted.
They also supported antiplatelet agents over anticoagulants unless patients have another indication for blood thinners, noting “the uncertainty surrounding the benefit of anticoagulation in the setting of PFO, and anticoagulation’s well-known harm profile.”
PFO affects about one in four individuals overall and up to half of cryptogenic stroke patients. The previous (2004) version of this practice advisory cited insufficient evidence to guide optimal therapy for secondary stroke prevention in these patients (Neurology. 2004;Apr 13;62[7]:1042-50). To update the guideline, Dr. Messé and his associates searched the literature for relevant randomized studies, excluding transient ischemic attacks when feasible because of their subjective nature, and focusing on intention-to-treat analyses to reduce bias (Neurology. 2016 Jul 27;doi: 10.1212/WNL.0000000000002961).
Among 809 initial articles, 5 were considered relevant – a randomized, open-label, multicenter study of the STARFlex device (CLOSURE I), two randomized, controlled trials of the AMPLATZER PFO Occluder (PC Trial and RESPECT), and two randomized studies of warfarin versus aspirin in cryptogenic stroke patients, the experts said.
Percutaneous PFO closure with the STARFlex device did not appear to prevent secondary stroke, compared with medical therapy alone, based on a small positive estimated difference in risk of about 0.1%, and a 95% confidence interval that crossed zero (–2.2% to 2.0%). In contrast, the AMPLATZER PFO Occluder decreased the risk of secondary stroke by about 1.7% (95% CI, –3.2% to –0.2%), but upped the risk of procedural complications by more than 3%, and also slightly increased the risk of new-onset atrial fibrillation (1.6%; 95% CI, 0.1% to 3.2%).
Efficacy data were insufficient to clearly support anticoagulants over antiplatelet therapy for recurrent stroke prevention, the experts concluded. Compared with antiplatelet therapy, anticoagulation was associated with a 2% increase in risk of recurrent stroke, but the 95% confidence interval for this estimate was wide and crossed zero. “In the absence of another indication for anticoagulation, clinicians may routinely offer antiplatelet medications instead of anticoagulation to patients with cryptogenic stroke and PFO,” they wrote. “In rare circumstances, such as stroke that recurs while a patient is undergoing antiplatelet therapy, clinicians may offer anticoagulation to patients with cryptogenic stroke and PFO.”
Their strongest recommendation was to counsel patients who are considering percutaneous PFO closure “that having a PFO is common; it occurs in about 1 in 4 people; it is impossible to determine with certainty whether their PFOs caused their strokes or TIAs; the effectiveness of the procedure for reducing stroke risk remains uncertain; and the procedure is associated with relatively uncommon, yet potentially serious, complications.”
The practice advisory was supported by the American Academy of Neurology. Dr. Messé disclosed ties to GlaxoSmithKline and WL Gore & Associates and has been an investigator for the REDUCE and CLOSURE-I trials. Five of his coauthors have been investigators for RESPECT, CLOSURE-I, and REDUCE, have been editors for Neurology, and have received compensation from Genentech, Pfizer, Gilead Sciences, and other pharmaceutical companies. One coauthor had no disclosures.
An updated practice advisory from the American Academy of Neurology does not recommend the routine use of catheter-based closure of patent foramen ovale in patients with a history of cryptogenic ischemic stroke.
“Because of the limitations of the efficacy evidence and the potential for serious adverse effects, we judge the risk-benefit trade-offs of PFO [patent foramen ovale] closure by either the STARFlex or AMPLATZER PFO Occluder to be uncertain,” wrote Steven Messé, MD, associate professor of neurology at the University of Pennsylvania, Philadelphia, and his associates. “In rare circumstances, such as recurrent strokes despite adequate medical therapy with no other mechanism identified, clinicians may offer the AMPLATZER PFO Occluder if it is available,” they noted.
They also supported antiplatelet agents over anticoagulants unless patients have another indication for blood thinners, noting “the uncertainty surrounding the benefit of anticoagulation in the setting of PFO, and anticoagulation’s well-known harm profile.”
PFO affects about one in four individuals overall and up to half of cryptogenic stroke patients. The previous (2004) version of this practice advisory cited insufficient evidence to guide optimal therapy for secondary stroke prevention in these patients (Neurology. 2004;Apr 13;62[7]:1042-50). To update the guideline, Dr. Messé and his associates searched the literature for relevant randomized studies, excluding transient ischemic attacks when feasible because of their subjective nature, and focusing on intention-to-treat analyses to reduce bias (Neurology. 2016 Jul 27;doi: 10.1212/WNL.0000000000002961).
Among 809 initial articles, 5 were considered relevant – a randomized, open-label, multicenter study of the STARFlex device (CLOSURE I), two randomized, controlled trials of the AMPLATZER PFO Occluder (PC Trial and RESPECT), and two randomized studies of warfarin versus aspirin in cryptogenic stroke patients, the experts said.
Percutaneous PFO closure with the STARFlex device did not appear to prevent secondary stroke, compared with medical therapy alone, based on a small positive estimated difference in risk of about 0.1%, and a 95% confidence interval that crossed zero (–2.2% to 2.0%). In contrast, the AMPLATZER PFO Occluder decreased the risk of secondary stroke by about 1.7% (95% CI, –3.2% to –0.2%), but upped the risk of procedural complications by more than 3%, and also slightly increased the risk of new-onset atrial fibrillation (1.6%; 95% CI, 0.1% to 3.2%).
Efficacy data were insufficient to clearly support anticoagulants over antiplatelet therapy for recurrent stroke prevention, the experts concluded. Compared with antiplatelet therapy, anticoagulation was associated with a 2% increase in risk of recurrent stroke, but the 95% confidence interval for this estimate was wide and crossed zero. “In the absence of another indication for anticoagulation, clinicians may routinely offer antiplatelet medications instead of anticoagulation to patients with cryptogenic stroke and PFO,” they wrote. “In rare circumstances, such as stroke that recurs while a patient is undergoing antiplatelet therapy, clinicians may offer anticoagulation to patients with cryptogenic stroke and PFO.”
Their strongest recommendation was to counsel patients who are considering percutaneous PFO closure “that having a PFO is common; it occurs in about 1 in 4 people; it is impossible to determine with certainty whether their PFOs caused their strokes or TIAs; the effectiveness of the procedure for reducing stroke risk remains uncertain; and the procedure is associated with relatively uncommon, yet potentially serious, complications.”
The practice advisory was supported by the American Academy of Neurology. Dr. Messé disclosed ties to GlaxoSmithKline and WL Gore & Associates and has been an investigator for the REDUCE and CLOSURE-I trials. Five of his coauthors have been investigators for RESPECT, CLOSURE-I, and REDUCE, have been editors for Neurology, and have received compensation from Genentech, Pfizer, Gilead Sciences, and other pharmaceutical companies. One coauthor had no disclosures.
FROM NEUROLOGY
Study Links Severe Childhood Eczema to Sedentary Behaviors
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Study links severe childhood eczema to sedentary behaviors
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A large national study linked severe atopic dermatitis to sedentary behaviors and screen time.
Major finding: Compared with children without eczema, those with severe disease were about 60% less likely to exercise at least once a week (OR, 0.39).
Data source: An analysis of data for 131,783 children from the National Survey of Children’s Health.
Disclosures: The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.