User login
Reduction of Opioid Use With Enhanced Recovery Program for Total Knee Arthroplasty
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
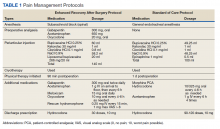
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
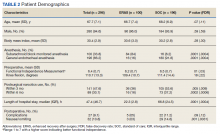
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
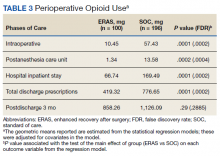
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
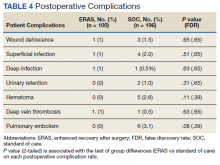
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002