User login
Groups launch CRC screening campaign as incidence declines
WASHINGTON – Several dozen organizations have joined together to help increase colorectal cancer screening rates to 80% of all Americans by 2018, citing the success of campaigns over the past decade that led to a 30% decline in incidence in individuals over age 50.
The National Colorectal Cancer Roundtable is leading the 80% by 2018 effort, saying that 1 in 3 adults between the ages of 50 and 75 years, or 23 million Americans, are not getting recommended screening.
Colorectal cancer is the third-leading cause of cancer death in the United States. In 2014, there will be an estimated 136,830 new diagnoses and 50,310 deaths. Sixty percent of the cases and 70% of the deaths are in those over age 65.
"Our goal today is absolutely clear: to eliminate colorectal cancer as a major public health threat," said Dr. Howard Koh, assistant secretary for health at the Health and Human Services department. HHS will be part of the campaign, along with state health departments; patient advocates; the American Association of Retired Persons (AARP); the American Cancer Society (ACS); and physician organizations, including the American College of Gastroenterology, the American College of Radiology, and the American Society for Gastrointestinal Endoscopy.
Dr. Koh spoke at a March 17 briefing sponsored by the ACS, which also published an analysis that day showing a decline in colorectal cancer incidence and mortality for most age groups, but for older Americans, in particular.
The study was published online in CA: A Cancer Journal for Clinicians, and was based on data from the Centers for Disease Control and Prevention’s National Center for Health Statistics; the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program; and the CDC’s National Program of Cancer Registries.
Overall, the incidence of colorectal cancer decreased by an average 3.4% per year from 2001 to 2010. The biggest declines were in people aged 50 years or older – at 3.9% per year, or a 30% decline over that decade. For people over age 65, the decline has been particularly notable—3.6% a year from 2001 to 2008 and 7.2% a year during 2008-2010.
The authors attribute that decline to a growth in screening. In 2000, 19% of Americans aged 50-75 years received some kind of screen. By 2010, the number had jumped to 55%.
"In other words, we are making real progress," said John R. Seffrin, chief executive officer of the ACS, at the briefing.
Some of the screening increase was due to Medicare coverage of all screening methods, including the fecal occult blood test, colonoscopy, and sigmoidoscopy. Sixty-four percent of Americans over age 65 had a screening test in 2010, compared with 55% of those in the 50- to 64-year-old group not covered by Medicare. Screening rates are highest in well-educated Americans who have health insurance, and in whites. Only a quarter of recent immigrants – those in the United States less than 10 years – were screened, compared with 62% of non-Hispanic whites. And only 18% of the uninsured had a screening exam in 2010, compared with 62% of those with insurance.
Just having insurance does not guarantee that screening will be cost-free, even though it is covered under Medicare and is an essential benefit under policies covered by the Affordable Care Act.
If polyps are found and removed, however, Medicare patients and others with private insurance often find themselves facing large copayments or lack of coverage for subsequent screenings. Gastroenterologists want to see an end to that "postpolypectomy surprise," said Dr. Ronald Vender, immediate past president of the American College of Gastroenterology, at the briefing.
The Removing Barriers to Colorectal Cancer Screening Act (H.R. 1070), which would cover any of those subsequent costs, was introduced in Congress in March 2013.
There are still major disparities in colorectal cancer incidence and mortality rates. Incidence and mortality rates are 30-40% higher in men than women. Incidence and mortality are highest in African Americans and lowest among Asian Pacific Islanders. From 2006 to 2010, the incidence in blacks was 25% higher than in whites and 50% higher than in Asian Pacific Islanders. Mortality rates are 50% higher for blacks than for whites, at 29.4 per 100,000. The higher incidence and mortality in blacks may be due to a lower socioeconomic status, but there are also other factors at play, said the authors, noting that rates are higher even for blacks in the same socioeconomic status as white peers.
Survival rates do not vary substantially by sex, according to the paper. While 5-year survival rates are relatively high – at 65% – only 40% of patients are diagnosed when the disease is localized. The 5-year survival at that stage is 90%.
But survival among blacks is the lowest among any race, 10% lower than for Asian Pacific Islanders, which have the best rates. Survival rates are lowest in high-poverty areas of America, including Appalachia and the mid-South, according to the ACS report.
On Twitter @aliciaault
WASHINGTON – Several dozen organizations have joined together to help increase colorectal cancer screening rates to 80% of all Americans by 2018, citing the success of campaigns over the past decade that led to a 30% decline in incidence in individuals over age 50.
The National Colorectal Cancer Roundtable is leading the 80% by 2018 effort, saying that 1 in 3 adults between the ages of 50 and 75 years, or 23 million Americans, are not getting recommended screening.
Colorectal cancer is the third-leading cause of cancer death in the United States. In 2014, there will be an estimated 136,830 new diagnoses and 50,310 deaths. Sixty percent of the cases and 70% of the deaths are in those over age 65.
"Our goal today is absolutely clear: to eliminate colorectal cancer as a major public health threat," said Dr. Howard Koh, assistant secretary for health at the Health and Human Services department. HHS will be part of the campaign, along with state health departments; patient advocates; the American Association of Retired Persons (AARP); the American Cancer Society (ACS); and physician organizations, including the American College of Gastroenterology, the American College of Radiology, and the American Society for Gastrointestinal Endoscopy.
Dr. Koh spoke at a March 17 briefing sponsored by the ACS, which also published an analysis that day showing a decline in colorectal cancer incidence and mortality for most age groups, but for older Americans, in particular.
The study was published online in CA: A Cancer Journal for Clinicians, and was based on data from the Centers for Disease Control and Prevention’s National Center for Health Statistics; the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program; and the CDC’s National Program of Cancer Registries.
Overall, the incidence of colorectal cancer decreased by an average 3.4% per year from 2001 to 2010. The biggest declines were in people aged 50 years or older – at 3.9% per year, or a 30% decline over that decade. For people over age 65, the decline has been particularly notable—3.6% a year from 2001 to 2008 and 7.2% a year during 2008-2010.
The authors attribute that decline to a growth in screening. In 2000, 19% of Americans aged 50-75 years received some kind of screen. By 2010, the number had jumped to 55%.
"In other words, we are making real progress," said John R. Seffrin, chief executive officer of the ACS, at the briefing.
Some of the screening increase was due to Medicare coverage of all screening methods, including the fecal occult blood test, colonoscopy, and sigmoidoscopy. Sixty-four percent of Americans over age 65 had a screening test in 2010, compared with 55% of those in the 50- to 64-year-old group not covered by Medicare. Screening rates are highest in well-educated Americans who have health insurance, and in whites. Only a quarter of recent immigrants – those in the United States less than 10 years – were screened, compared with 62% of non-Hispanic whites. And only 18% of the uninsured had a screening exam in 2010, compared with 62% of those with insurance.
Just having insurance does not guarantee that screening will be cost-free, even though it is covered under Medicare and is an essential benefit under policies covered by the Affordable Care Act.
If polyps are found and removed, however, Medicare patients and others with private insurance often find themselves facing large copayments or lack of coverage for subsequent screenings. Gastroenterologists want to see an end to that "postpolypectomy surprise," said Dr. Ronald Vender, immediate past president of the American College of Gastroenterology, at the briefing.
The Removing Barriers to Colorectal Cancer Screening Act (H.R. 1070), which would cover any of those subsequent costs, was introduced in Congress in March 2013.
There are still major disparities in colorectal cancer incidence and mortality rates. Incidence and mortality rates are 30-40% higher in men than women. Incidence and mortality are highest in African Americans and lowest among Asian Pacific Islanders. From 2006 to 2010, the incidence in blacks was 25% higher than in whites and 50% higher than in Asian Pacific Islanders. Mortality rates are 50% higher for blacks than for whites, at 29.4 per 100,000. The higher incidence and mortality in blacks may be due to a lower socioeconomic status, but there are also other factors at play, said the authors, noting that rates are higher even for blacks in the same socioeconomic status as white peers.
Survival rates do not vary substantially by sex, according to the paper. While 5-year survival rates are relatively high – at 65% – only 40% of patients are diagnosed when the disease is localized. The 5-year survival at that stage is 90%.
But survival among blacks is the lowest among any race, 10% lower than for Asian Pacific Islanders, which have the best rates. Survival rates are lowest in high-poverty areas of America, including Appalachia and the mid-South, according to the ACS report.
On Twitter @aliciaault
WASHINGTON – Several dozen organizations have joined together to help increase colorectal cancer screening rates to 80% of all Americans by 2018, citing the success of campaigns over the past decade that led to a 30% decline in incidence in individuals over age 50.
The National Colorectal Cancer Roundtable is leading the 80% by 2018 effort, saying that 1 in 3 adults between the ages of 50 and 75 years, or 23 million Americans, are not getting recommended screening.
Colorectal cancer is the third-leading cause of cancer death in the United States. In 2014, there will be an estimated 136,830 new diagnoses and 50,310 deaths. Sixty percent of the cases and 70% of the deaths are in those over age 65.
"Our goal today is absolutely clear: to eliminate colorectal cancer as a major public health threat," said Dr. Howard Koh, assistant secretary for health at the Health and Human Services department. HHS will be part of the campaign, along with state health departments; patient advocates; the American Association of Retired Persons (AARP); the American Cancer Society (ACS); and physician organizations, including the American College of Gastroenterology, the American College of Radiology, and the American Society for Gastrointestinal Endoscopy.
Dr. Koh spoke at a March 17 briefing sponsored by the ACS, which also published an analysis that day showing a decline in colorectal cancer incidence and mortality for most age groups, but for older Americans, in particular.
The study was published online in CA: A Cancer Journal for Clinicians, and was based on data from the Centers for Disease Control and Prevention’s National Center for Health Statistics; the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program; and the CDC’s National Program of Cancer Registries.
Overall, the incidence of colorectal cancer decreased by an average 3.4% per year from 2001 to 2010. The biggest declines were in people aged 50 years or older – at 3.9% per year, or a 30% decline over that decade. For people over age 65, the decline has been particularly notable—3.6% a year from 2001 to 2008 and 7.2% a year during 2008-2010.
The authors attribute that decline to a growth in screening. In 2000, 19% of Americans aged 50-75 years received some kind of screen. By 2010, the number had jumped to 55%.
"In other words, we are making real progress," said John R. Seffrin, chief executive officer of the ACS, at the briefing.
Some of the screening increase was due to Medicare coverage of all screening methods, including the fecal occult blood test, colonoscopy, and sigmoidoscopy. Sixty-four percent of Americans over age 65 had a screening test in 2010, compared with 55% of those in the 50- to 64-year-old group not covered by Medicare. Screening rates are highest in well-educated Americans who have health insurance, and in whites. Only a quarter of recent immigrants – those in the United States less than 10 years – were screened, compared with 62% of non-Hispanic whites. And only 18% of the uninsured had a screening exam in 2010, compared with 62% of those with insurance.
Just having insurance does not guarantee that screening will be cost-free, even though it is covered under Medicare and is an essential benefit under policies covered by the Affordable Care Act.
If polyps are found and removed, however, Medicare patients and others with private insurance often find themselves facing large copayments or lack of coverage for subsequent screenings. Gastroenterologists want to see an end to that "postpolypectomy surprise," said Dr. Ronald Vender, immediate past president of the American College of Gastroenterology, at the briefing.
The Removing Barriers to Colorectal Cancer Screening Act (H.R. 1070), which would cover any of those subsequent costs, was introduced in Congress in March 2013.
There are still major disparities in colorectal cancer incidence and mortality rates. Incidence and mortality rates are 30-40% higher in men than women. Incidence and mortality are highest in African Americans and lowest among Asian Pacific Islanders. From 2006 to 2010, the incidence in blacks was 25% higher than in whites and 50% higher than in Asian Pacific Islanders. Mortality rates are 50% higher for blacks than for whites, at 29.4 per 100,000. The higher incidence and mortality in blacks may be due to a lower socioeconomic status, but there are also other factors at play, said the authors, noting that rates are higher even for blacks in the same socioeconomic status as white peers.
Survival rates do not vary substantially by sex, according to the paper. While 5-year survival rates are relatively high – at 65% – only 40% of patients are diagnosed when the disease is localized. The 5-year survival at that stage is 90%.
But survival among blacks is the lowest among any race, 10% lower than for Asian Pacific Islanders, which have the best rates. Survival rates are lowest in high-poverty areas of America, including Appalachia and the mid-South, according to the ACS report.
On Twitter @aliciaault
House passes bill to repeal the SGR
The House of Representatives on March 14 approved a bill to repeal the Medicare Sustainable Growth Rate formula; the Senate is not likely to take up the bill.
The House voted mostly along party lines, with 226 Republicans and 12 Democrats voting in favor of approving H.R. 4015 and sending it to the Senate. Many Democrats who voted against it objected to the bill’s financing, which was achieved through a 5-year delay of the Affordable Care Act’s individual health insurance mandate.
The Congressional Budget Office estimated that delaying the mandate would save about $170 billion over 10 years, while the Sustainable Growth Rate (SGR) fix would cost $130 billion in the same period. But the CBO also said that the plan would increase the number of uninsured by 13 million and lead to 10%-20% premium increases.
"Political games are threatening to derail months of bipartisan, bicameral progress" on implementing the Affordable Care Act, House Minority Leader Nancy Pelosi (D-Calif.) said in a speech before the vote.
"We shouldn’t be wasting time on this foolishness and this recklessness," she added, noting that securing a permanent fix before a 24% physician pay cut takes effect on April 1 was increasingly less likely as the Senate was not likely to approve the plan with the accompanying financing.
The House now is on break until March 25.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that she was disappointed at the House action, given that the House and Senate had already agreed on the fundamentals of replacing the SGR. "It would be a shame for lawmakers to have done all of that hard work only to have it overcome by partisan politics over budgetary issues," she said in a statement.
"We thank all members who spoke on the floor in support of a return to bipartisan negotiations and encourage the United States Senate to proceed in a timely and bipartisan manner to advance legislation in that body," she said, adding that the AMA would continue to try to help forge a compromise.
"Continuing the cycle of kicking the can down the road through temporary patches in the months ahead simply wastes more taxpayer money to preserve a bad policy of Congress’ own making," Dr. Hoven said.
Leaders at other physicians’ organizations echoed Dr. Hoven’s disappointment.
"We’re dismayed that Congress sabotaged their own work by linking this legislation to unrelated, ideological issues – particularly in light of the nearly universal opposition to such action from patients, insurers, and the medical community," Dr. Reid Blackwelder, president of the American Academy of Family Physicians, said in a statement.
"It’s imperative that both parties come together to reach agreement on the budgetary payment that will pass both the House and the Senate before April 1," Dr. Blackwelder said.
Ms. Molly Cooke, president of the American College of Physicians, said the ACP stands by its statement from March 7. At that time, she said, "Congress knows that it is counterproductive for either the House or the Senate, Republicans or Democrats, to tie the bipartisan, bicameral SGR repeal bill to other policies that do not have the bipartisan support needed to pass both chambers, and be signed into law by the President."
Dr. Cooke added, "We cannot support linking SGR repeal to changes in current law that will result in fewer people getting health insurance coverage."
House Republican leaders called on the Senate to act.
"We have never come this far in finding a permanent solution," House Energy and Commerce Committee Chairman Fred Upton (R-Mich.) said in a statement. "But there is still much work to be done after today’s vote, and I call on my good friend Sen. Ron Wyden [D-Ore.] to pick up the torch and work with Majority Leader Harry Reid [D-Nev.] to put politics aside, stand up for our seniors and doctors, and solve SGR this year."
On Twitter @aliciaault
The House of Representatives on March 14 approved a bill to repeal the Medicare Sustainable Growth Rate formula; the Senate is not likely to take up the bill.
The House voted mostly along party lines, with 226 Republicans and 12 Democrats voting in favor of approving H.R. 4015 and sending it to the Senate. Many Democrats who voted against it objected to the bill’s financing, which was achieved through a 5-year delay of the Affordable Care Act’s individual health insurance mandate.
The Congressional Budget Office estimated that delaying the mandate would save about $170 billion over 10 years, while the Sustainable Growth Rate (SGR) fix would cost $130 billion in the same period. But the CBO also said that the plan would increase the number of uninsured by 13 million and lead to 10%-20% premium increases.
"Political games are threatening to derail months of bipartisan, bicameral progress" on implementing the Affordable Care Act, House Minority Leader Nancy Pelosi (D-Calif.) said in a speech before the vote.
"We shouldn’t be wasting time on this foolishness and this recklessness," she added, noting that securing a permanent fix before a 24% physician pay cut takes effect on April 1 was increasingly less likely as the Senate was not likely to approve the plan with the accompanying financing.
The House now is on break until March 25.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that she was disappointed at the House action, given that the House and Senate had already agreed on the fundamentals of replacing the SGR. "It would be a shame for lawmakers to have done all of that hard work only to have it overcome by partisan politics over budgetary issues," she said in a statement.
"We thank all members who spoke on the floor in support of a return to bipartisan negotiations and encourage the United States Senate to proceed in a timely and bipartisan manner to advance legislation in that body," she said, adding that the AMA would continue to try to help forge a compromise.
"Continuing the cycle of kicking the can down the road through temporary patches in the months ahead simply wastes more taxpayer money to preserve a bad policy of Congress’ own making," Dr. Hoven said.
Leaders at other physicians’ organizations echoed Dr. Hoven’s disappointment.
"We’re dismayed that Congress sabotaged their own work by linking this legislation to unrelated, ideological issues – particularly in light of the nearly universal opposition to such action from patients, insurers, and the medical community," Dr. Reid Blackwelder, president of the American Academy of Family Physicians, said in a statement.
"It’s imperative that both parties come together to reach agreement on the budgetary payment that will pass both the House and the Senate before April 1," Dr. Blackwelder said.
Ms. Molly Cooke, president of the American College of Physicians, said the ACP stands by its statement from March 7. At that time, she said, "Congress knows that it is counterproductive for either the House or the Senate, Republicans or Democrats, to tie the bipartisan, bicameral SGR repeal bill to other policies that do not have the bipartisan support needed to pass both chambers, and be signed into law by the President."
Dr. Cooke added, "We cannot support linking SGR repeal to changes in current law that will result in fewer people getting health insurance coverage."
House Republican leaders called on the Senate to act.
"We have never come this far in finding a permanent solution," House Energy and Commerce Committee Chairman Fred Upton (R-Mich.) said in a statement. "But there is still much work to be done after today’s vote, and I call on my good friend Sen. Ron Wyden [D-Ore.] to pick up the torch and work with Majority Leader Harry Reid [D-Nev.] to put politics aside, stand up for our seniors and doctors, and solve SGR this year."
On Twitter @aliciaault
The House of Representatives on March 14 approved a bill to repeal the Medicare Sustainable Growth Rate formula; the Senate is not likely to take up the bill.
The House voted mostly along party lines, with 226 Republicans and 12 Democrats voting in favor of approving H.R. 4015 and sending it to the Senate. Many Democrats who voted against it objected to the bill’s financing, which was achieved through a 5-year delay of the Affordable Care Act’s individual health insurance mandate.
The Congressional Budget Office estimated that delaying the mandate would save about $170 billion over 10 years, while the Sustainable Growth Rate (SGR) fix would cost $130 billion in the same period. But the CBO also said that the plan would increase the number of uninsured by 13 million and lead to 10%-20% premium increases.
"Political games are threatening to derail months of bipartisan, bicameral progress" on implementing the Affordable Care Act, House Minority Leader Nancy Pelosi (D-Calif.) said in a speech before the vote.
"We shouldn’t be wasting time on this foolishness and this recklessness," she added, noting that securing a permanent fix before a 24% physician pay cut takes effect on April 1 was increasingly less likely as the Senate was not likely to approve the plan with the accompanying financing.
The House now is on break until March 25.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that she was disappointed at the House action, given that the House and Senate had already agreed on the fundamentals of replacing the SGR. "It would be a shame for lawmakers to have done all of that hard work only to have it overcome by partisan politics over budgetary issues," she said in a statement.
"We thank all members who spoke on the floor in support of a return to bipartisan negotiations and encourage the United States Senate to proceed in a timely and bipartisan manner to advance legislation in that body," she said, adding that the AMA would continue to try to help forge a compromise.
"Continuing the cycle of kicking the can down the road through temporary patches in the months ahead simply wastes more taxpayer money to preserve a bad policy of Congress’ own making," Dr. Hoven said.
Leaders at other physicians’ organizations echoed Dr. Hoven’s disappointment.
"We’re dismayed that Congress sabotaged their own work by linking this legislation to unrelated, ideological issues – particularly in light of the nearly universal opposition to such action from patients, insurers, and the medical community," Dr. Reid Blackwelder, president of the American Academy of Family Physicians, said in a statement.
"It’s imperative that both parties come together to reach agreement on the budgetary payment that will pass both the House and the Senate before April 1," Dr. Blackwelder said.
Ms. Molly Cooke, president of the American College of Physicians, said the ACP stands by its statement from March 7. At that time, she said, "Congress knows that it is counterproductive for either the House or the Senate, Republicans or Democrats, to tie the bipartisan, bicameral SGR repeal bill to other policies that do not have the bipartisan support needed to pass both chambers, and be signed into law by the President."
Dr. Cooke added, "We cannot support linking SGR repeal to changes in current law that will result in fewer people getting health insurance coverage."
House Republican leaders called on the Senate to act.
"We have never come this far in finding a permanent solution," House Energy and Commerce Committee Chairman Fred Upton (R-Mich.) said in a statement. "But there is still much work to be done after today’s vote, and I call on my good friend Sen. Ron Wyden [D-Ore.] to pick up the torch and work with Majority Leader Harry Reid [D-Nev.] to put politics aside, stand up for our seniors and doctors, and solve SGR this year."
On Twitter @aliciaault
Cleveland Clinic first to get Joint Commission medical home certification
The Cleveland Clinic is the first health care provider to receive the Joint Commission’s Primary Care Medical Home certification.
The Joint Commission started its medical home certification option for hospitals in early 2013. The Cleveland Clinic is the first provider of any kind to receive the certification, according to the commission. It covers 39 practices at 29 Cleveland Clinic sites, involving 230 primary care physicians and advanced practice nurses.
"This certification recognizes our striving to be national leaders in population management and transformation of the care delivery model in the United States," said Dr. David L. Longworth, chair of the Medicine Institute at the Cleveland Clinic, in a statement.
To be certified by the Joint Commission, hospitals must already be accredited, and offer services from primary care clinicians – that can include physicians, nurse practitioners, and physician assistants. The hospitals are evaluated in five major areas: patient centeredness, comprehensiveness, coordinated care, access to care, and a systems-based approach to quality and safety. Patient centeredness, for instance, includes assessing a patient’s health literacy and including patients in their treatment plan. For access, a medical home has to provide care 24 hours a day, 7 days a week. In addition to urgent care, that can include clinical advice services by telephone.
The Cleveland Clinic had an on-site survey in September to determine if it met the parameters for certification.
The Joint Commission’s accreditation program is one of several aiming to give nationally recognized credentials to providers.
The National Committee on Quality Assurance (NCQA) has recognized about 7,000 primary care practices, encompassing almost 35,000 clinicians, as meeting its patient-centered medical home requirements. Thirty-seven states incorporate NCQA requirements into patient-centered medical home–related legislation, according to the organization. In late March, the NCQA will be unveiling updated medical home requirements.
The Accreditation Association for Ambulatory Health Care also offers medical home accreditation, as does URAC, which aligns its program with principles outlined by the major primary care societies.
On Twitter @aliciaault
The Cleveland Clinic is the first health care provider to receive the Joint Commission’s Primary Care Medical Home certification.
The Joint Commission started its medical home certification option for hospitals in early 2013. The Cleveland Clinic is the first provider of any kind to receive the certification, according to the commission. It covers 39 practices at 29 Cleveland Clinic sites, involving 230 primary care physicians and advanced practice nurses.
"This certification recognizes our striving to be national leaders in population management and transformation of the care delivery model in the United States," said Dr. David L. Longworth, chair of the Medicine Institute at the Cleveland Clinic, in a statement.
To be certified by the Joint Commission, hospitals must already be accredited, and offer services from primary care clinicians – that can include physicians, nurse practitioners, and physician assistants. The hospitals are evaluated in five major areas: patient centeredness, comprehensiveness, coordinated care, access to care, and a systems-based approach to quality and safety. Patient centeredness, for instance, includes assessing a patient’s health literacy and including patients in their treatment plan. For access, a medical home has to provide care 24 hours a day, 7 days a week. In addition to urgent care, that can include clinical advice services by telephone.
The Cleveland Clinic had an on-site survey in September to determine if it met the parameters for certification.
The Joint Commission’s accreditation program is one of several aiming to give nationally recognized credentials to providers.
The National Committee on Quality Assurance (NCQA) has recognized about 7,000 primary care practices, encompassing almost 35,000 clinicians, as meeting its patient-centered medical home requirements. Thirty-seven states incorporate NCQA requirements into patient-centered medical home–related legislation, according to the organization. In late March, the NCQA will be unveiling updated medical home requirements.
The Accreditation Association for Ambulatory Health Care also offers medical home accreditation, as does URAC, which aligns its program with principles outlined by the major primary care societies.
On Twitter @aliciaault
The Cleveland Clinic is the first health care provider to receive the Joint Commission’s Primary Care Medical Home certification.
The Joint Commission started its medical home certification option for hospitals in early 2013. The Cleveland Clinic is the first provider of any kind to receive the certification, according to the commission. It covers 39 practices at 29 Cleveland Clinic sites, involving 230 primary care physicians and advanced practice nurses.
"This certification recognizes our striving to be national leaders in population management and transformation of the care delivery model in the United States," said Dr. David L. Longworth, chair of the Medicine Institute at the Cleveland Clinic, in a statement.
To be certified by the Joint Commission, hospitals must already be accredited, and offer services from primary care clinicians – that can include physicians, nurse practitioners, and physician assistants. The hospitals are evaluated in five major areas: patient centeredness, comprehensiveness, coordinated care, access to care, and a systems-based approach to quality and safety. Patient centeredness, for instance, includes assessing a patient’s health literacy and including patients in their treatment plan. For access, a medical home has to provide care 24 hours a day, 7 days a week. In addition to urgent care, that can include clinical advice services by telephone.
The Cleveland Clinic had an on-site survey in September to determine if it met the parameters for certification.
The Joint Commission’s accreditation program is one of several aiming to give nationally recognized credentials to providers.
The National Committee on Quality Assurance (NCQA) has recognized about 7,000 primary care practices, encompassing almost 35,000 clinicians, as meeting its patient-centered medical home requirements. Thirty-seven states incorporate NCQA requirements into patient-centered medical home–related legislation, according to the organization. In late March, the NCQA will be unveiling updated medical home requirements.
The Accreditation Association for Ambulatory Health Care also offers medical home accreditation, as does URAC, which aligns its program with principles outlined by the major primary care societies.
On Twitter @aliciaault
CMS outlines how to get meaningful use exemptions
As promised, the Centers for Medicare & Medicaid Services has issued guidance on how physicians can apply for a hardship exemption on meeting Stage 2 of the meaningful use of electronic health records in 2014.
Administrator Marilyn Tavenner said at the annual meeting of the Healthcare Information and Management Systems Society on Feb. 27 that CMS would not extend the deadline for meeting Stage 2 criteria, but that it would allow more flexibility in how physicians and other eligible professionals reach the goals.
Physicians who participate in the meaningful use program receive incentive payments from Medicare. If they don’t participate this year, they’ll be penalized next year. On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
In a guidance document, the agency now says it is currently accepting applications for exemptions from penalties for 2015. Physicians who have not yet participated in meaningful use at all have until July 1 to apply.
If a physician has demonstrated meaningful use for the 2013 reporting year, there will be no penalty in 2015. If, however, a practice is having trouble implementing 2014 certified EHR technology for a 2014 reporting period, the practice or physician can apply for a hardship exception by July 1, 2015, to avoid a penalty in 2016.
Applications will be reviewed on a case-by-case basis, according to the guidance.
Meaningful use penalties only apply to physicians who participate in the Medicare incentive program, or in both the Medicare and Medicaid programs. There are no penalties for those who participate only in the Medicaid incentive program.
On Twitter @aliciaault
As promised, the Centers for Medicare & Medicaid Services has issued guidance on how physicians can apply for a hardship exemption on meeting Stage 2 of the meaningful use of electronic health records in 2014.
Administrator Marilyn Tavenner said at the annual meeting of the Healthcare Information and Management Systems Society on Feb. 27 that CMS would not extend the deadline for meeting Stage 2 criteria, but that it would allow more flexibility in how physicians and other eligible professionals reach the goals.
Physicians who participate in the meaningful use program receive incentive payments from Medicare. If they don’t participate this year, they’ll be penalized next year. On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
In a guidance document, the agency now says it is currently accepting applications for exemptions from penalties for 2015. Physicians who have not yet participated in meaningful use at all have until July 1 to apply.
If a physician has demonstrated meaningful use for the 2013 reporting year, there will be no penalty in 2015. If, however, a practice is having trouble implementing 2014 certified EHR technology for a 2014 reporting period, the practice or physician can apply for a hardship exception by July 1, 2015, to avoid a penalty in 2016.
Applications will be reviewed on a case-by-case basis, according to the guidance.
Meaningful use penalties only apply to physicians who participate in the Medicare incentive program, or in both the Medicare and Medicaid programs. There are no penalties for those who participate only in the Medicaid incentive program.
On Twitter @aliciaault
As promised, the Centers for Medicare & Medicaid Services has issued guidance on how physicians can apply for a hardship exemption on meeting Stage 2 of the meaningful use of electronic health records in 2014.
Administrator Marilyn Tavenner said at the annual meeting of the Healthcare Information and Management Systems Society on Feb. 27 that CMS would not extend the deadline for meeting Stage 2 criteria, but that it would allow more flexibility in how physicians and other eligible professionals reach the goals.
Physicians who participate in the meaningful use program receive incentive payments from Medicare. If they don’t participate this year, they’ll be penalized next year. On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
In a guidance document, the agency now says it is currently accepting applications for exemptions from penalties for 2015. Physicians who have not yet participated in meaningful use at all have until July 1 to apply.
If a physician has demonstrated meaningful use for the 2013 reporting year, there will be no penalty in 2015. If, however, a practice is having trouble implementing 2014 certified EHR technology for a 2014 reporting period, the practice or physician can apply for a hardship exception by July 1, 2015, to avoid a penalty in 2016.
Applications will be reviewed on a case-by-case basis, according to the guidance.
Meaningful use penalties only apply to physicians who participate in the Medicare incentive program, or in both the Medicare and Medicaid programs. There are no penalties for those who participate only in the Medicaid incentive program.
On Twitter @aliciaault
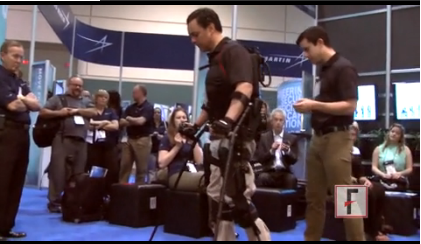
VIDEO: Bionic exoskeleton helps paralyzed patients walk
ORLANDO – It’s a bionic suit, a battery-powered exoskeleton, or as Chris Tagatac calls it, a wearable robot.
Mr. Tagatac, who is paralyzed from the lower ribs down, demonstrated the computer- and battery-operated Ekso Bionics suit at the annual meeting of the Healthcare Information and Management Systems Society, and he spoke with us about his experience. Watch the video to learn more about him and the technology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
On Twitter @naseemsmiller
ORLANDO – It’s a bionic suit, a battery-powered exoskeleton, or as Chris Tagatac calls it, a wearable robot.
Mr. Tagatac, who is paralyzed from the lower ribs down, demonstrated the computer- and battery-operated Ekso Bionics suit at the annual meeting of the Healthcare Information and Management Systems Society, and he spoke with us about his experience. Watch the video to learn more about him and the technology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
On Twitter @naseemsmiller
ORLANDO – It’s a bionic suit, a battery-powered exoskeleton, or as Chris Tagatac calls it, a wearable robot.
Mr. Tagatac, who is paralyzed from the lower ribs down, demonstrated the computer- and battery-operated Ekso Bionics suit at the annual meeting of the Healthcare Information and Management Systems Society, and he spoke with us about his experience. Watch the video to learn more about him and the technology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
On Twitter @naseemsmiller
AT HIMSS14
MedPAC commissioners weigh extension of primary care pay bump
WASHINGTON – Primary care physicians should continue to receive a bump up in pay for their efforts, according to the Medicare Payment Advisory Commission.
At their March 6 meeting, MedPAC commissioners weighed potential options for extending a 10% pay increase for primary care physicians that was established by the Affordable Care Act and began in 2011. The increase is due to expire at the end of 2015.
MedPAC Chairman Glenn Hackbarth said that the panel is looking for ways to continue to attract more physicians to primary care. "We have too little primary care for the population that needs to be served," said Mr. Hackbarth. He said the commission wants to address that mismatch as quickly as possible.
The country should "expand the capacity of our existing primary care practices to care for bigger populations," Mr. Hackbarth said, adding that waiting for more physicians to be trained will take too long.
Currently, to be eligible for the quarterly payments, primary care physicians have to show that at least 60% of their total Medicare allowed charges come from Current Procedural Terminology (CPT) codes 99201-99215 (for office and other outpatient visits).
In 2011, the Centers for Medicare and Medicaid Services (CMS) paid $560 million in bonuses, and in 2012, the agency paid $664 million in bonuses to 194,428 family physicians, internists, pediatricians, geriatricians, nurse practitioners, and physician assistants.
MedPAC wants to revamp the payment scheme in part because it’s based on the fee-for-service system. "It’s about changing what qualifies as productivity for payment," said Mr. Hackbarth.
Instead, the commission is considering a per-beneficiary payment that would acknowledge all of the intangibles that go with primary care, including care coordination, afterhours access, and non–face-to-face interactions, Mr. Hackbarth said.
MedPAC staff suggested several funding options. One would be to reduce payments for almost all services covered by the Physician Fee Schedule by about 1%. That would allow for a monthly per-beneficiary payment of about $2.60 a month.
Another option would be to reduce Medicare payments for services provided by specialists by 1.4%; that would provide the same $2.60 a month payment.
MedPAC staff also floated the idea of identifying and reducing payments for overpriced services. The CMS is currently evaluating many overpriced services, and the American Medical Association’s Relative Value Update Committee (RUC) has subsequently reduced time estimates and values for many of them. But the reductions in time and work values have not been proportionate, which means some services are still likely overvalued, said MedPAC staffer Kevin Hayes.
He estimated that targeting more of the overpriced services could allow a per-beneficiary pay boost to continue for 5 years, rising from $2.60 a month in the first year to $13 in the final year.
MedPAC meets again in April and submits its report to Congress in June. The next report will likely contain its final recommendations on the primary care incentive payment program.
[email protected] On Twitter @aliciaault
WASHINGTON – Primary care physicians should continue to receive a bump up in pay for their efforts, according to the Medicare Payment Advisory Commission.
At their March 6 meeting, MedPAC commissioners weighed potential options for extending a 10% pay increase for primary care physicians that was established by the Affordable Care Act and began in 2011. The increase is due to expire at the end of 2015.
MedPAC Chairman Glenn Hackbarth said that the panel is looking for ways to continue to attract more physicians to primary care. "We have too little primary care for the population that needs to be served," said Mr. Hackbarth. He said the commission wants to address that mismatch as quickly as possible.
The country should "expand the capacity of our existing primary care practices to care for bigger populations," Mr. Hackbarth said, adding that waiting for more physicians to be trained will take too long.
Currently, to be eligible for the quarterly payments, primary care physicians have to show that at least 60% of their total Medicare allowed charges come from Current Procedural Terminology (CPT) codes 99201-99215 (for office and other outpatient visits).
In 2011, the Centers for Medicare and Medicaid Services (CMS) paid $560 million in bonuses, and in 2012, the agency paid $664 million in bonuses to 194,428 family physicians, internists, pediatricians, geriatricians, nurse practitioners, and physician assistants.
MedPAC wants to revamp the payment scheme in part because it’s based on the fee-for-service system. "It’s about changing what qualifies as productivity for payment," said Mr. Hackbarth.
Instead, the commission is considering a per-beneficiary payment that would acknowledge all of the intangibles that go with primary care, including care coordination, afterhours access, and non–face-to-face interactions, Mr. Hackbarth said.
MedPAC staff suggested several funding options. One would be to reduce payments for almost all services covered by the Physician Fee Schedule by about 1%. That would allow for a monthly per-beneficiary payment of about $2.60 a month.
Another option would be to reduce Medicare payments for services provided by specialists by 1.4%; that would provide the same $2.60 a month payment.
MedPAC staff also floated the idea of identifying and reducing payments for overpriced services. The CMS is currently evaluating many overpriced services, and the American Medical Association’s Relative Value Update Committee (RUC) has subsequently reduced time estimates and values for many of them. But the reductions in time and work values have not been proportionate, which means some services are still likely overvalued, said MedPAC staffer Kevin Hayes.
He estimated that targeting more of the overpriced services could allow a per-beneficiary pay boost to continue for 5 years, rising from $2.60 a month in the first year to $13 in the final year.
MedPAC meets again in April and submits its report to Congress in June. The next report will likely contain its final recommendations on the primary care incentive payment program.
[email protected] On Twitter @aliciaault
WASHINGTON – Primary care physicians should continue to receive a bump up in pay for their efforts, according to the Medicare Payment Advisory Commission.
At their March 6 meeting, MedPAC commissioners weighed potential options for extending a 10% pay increase for primary care physicians that was established by the Affordable Care Act and began in 2011. The increase is due to expire at the end of 2015.
MedPAC Chairman Glenn Hackbarth said that the panel is looking for ways to continue to attract more physicians to primary care. "We have too little primary care for the population that needs to be served," said Mr. Hackbarth. He said the commission wants to address that mismatch as quickly as possible.
The country should "expand the capacity of our existing primary care practices to care for bigger populations," Mr. Hackbarth said, adding that waiting for more physicians to be trained will take too long.
Currently, to be eligible for the quarterly payments, primary care physicians have to show that at least 60% of their total Medicare allowed charges come from Current Procedural Terminology (CPT) codes 99201-99215 (for office and other outpatient visits).
In 2011, the Centers for Medicare and Medicaid Services (CMS) paid $560 million in bonuses, and in 2012, the agency paid $664 million in bonuses to 194,428 family physicians, internists, pediatricians, geriatricians, nurse practitioners, and physician assistants.
MedPAC wants to revamp the payment scheme in part because it’s based on the fee-for-service system. "It’s about changing what qualifies as productivity for payment," said Mr. Hackbarth.
Instead, the commission is considering a per-beneficiary payment that would acknowledge all of the intangibles that go with primary care, including care coordination, afterhours access, and non–face-to-face interactions, Mr. Hackbarth said.
MedPAC staff suggested several funding options. One would be to reduce payments for almost all services covered by the Physician Fee Schedule by about 1%. That would allow for a monthly per-beneficiary payment of about $2.60 a month.
Another option would be to reduce Medicare payments for services provided by specialists by 1.4%; that would provide the same $2.60 a month payment.
MedPAC staff also floated the idea of identifying and reducing payments for overpriced services. The CMS is currently evaluating many overpriced services, and the American Medical Association’s Relative Value Update Committee (RUC) has subsequently reduced time estimates and values for many of them. But the reductions in time and work values have not been proportionate, which means some services are still likely overvalued, said MedPAC staffer Kevin Hayes.
He estimated that targeting more of the overpriced services could allow a per-beneficiary pay boost to continue for 5 years, rising from $2.60 a month in the first year to $13 in the final year.
MedPAC meets again in April and submits its report to Congress in June. The next report will likely contain its final recommendations on the primary care incentive payment program.
[email protected] On Twitter @aliciaault
AT A MEDPAC MEETING
Administration to allow noncompliant health policies through 2017
The Obama Administration said that it will allow through 2016 renewals of health insurance policies that do not meet the minimum criteria established by the Affordable Care Act.
Each state has the final say on whether such policies can continue to be sold, but the move by the White House will help remove some of the hurdles, a senior administration official said March 5.
In November, President Obama said that, to avoid cancellations, the federal government would let insurers renew, for 1 year, policies that did not contain the essential health benefits outlined by the ACA. That would give policyholders who did renew time to find new coverage.
About half of the states agreed to allow renewals after the policy change, but some states with the largest number of such policyholders – including California, Washington, New York, and Maryland – refused to allow renewals.
Now, in a wide-ranging rule that covers many aspects of the ACA for 2015, the administration said that noncompliant policies can be renewed as late as Oct. 1, 2016. No new policies can be issued. States that chose not to allow the renewals before also can change their decisions, senior administration officials said.
In 2015, the administration also is giving consumers an additional month to shop for coverage on the federal and state health insurance exchanges for 2015: Open enrollment will run from Nov. 15, 2014, to Feb. 15, 2015.
The rule also reduces out-of-pocket limits for individuals and families who buy coverage through the exchanges. Depending on income, individuals will pay a maximum of $2,250 to $5,200, and families will pay $4,500 to $10,400.
"These policies implement the health care law in a common-sense way by continuing to smooth the transition for consumers and stakeholders and fixing problems wherever the law provides flexibility," HHS Secretary Kathleen Sebelius said in a statement. "This comprehensive guidance will help ensure that consumers, employers and insurers have the information they need to plan for next year and make it easier for families to make decisions to access quality, affordable coverage."
The administration said that it had worked on the guidance for 2015 with input from consumers, states, businesses, health professionals, insurance commissioners, insurers, and members of Congress.
On Twitter @aliciaault
The Obama Administration said that it will allow through 2016 renewals of health insurance policies that do not meet the minimum criteria established by the Affordable Care Act.
Each state has the final say on whether such policies can continue to be sold, but the move by the White House will help remove some of the hurdles, a senior administration official said March 5.
In November, President Obama said that, to avoid cancellations, the federal government would let insurers renew, for 1 year, policies that did not contain the essential health benefits outlined by the ACA. That would give policyholders who did renew time to find new coverage.
About half of the states agreed to allow renewals after the policy change, but some states with the largest number of such policyholders – including California, Washington, New York, and Maryland – refused to allow renewals.
Now, in a wide-ranging rule that covers many aspects of the ACA for 2015, the administration said that noncompliant policies can be renewed as late as Oct. 1, 2016. No new policies can be issued. States that chose not to allow the renewals before also can change their decisions, senior administration officials said.
In 2015, the administration also is giving consumers an additional month to shop for coverage on the federal and state health insurance exchanges for 2015: Open enrollment will run from Nov. 15, 2014, to Feb. 15, 2015.
The rule also reduces out-of-pocket limits for individuals and families who buy coverage through the exchanges. Depending on income, individuals will pay a maximum of $2,250 to $5,200, and families will pay $4,500 to $10,400.
"These policies implement the health care law in a common-sense way by continuing to smooth the transition for consumers and stakeholders and fixing problems wherever the law provides flexibility," HHS Secretary Kathleen Sebelius said in a statement. "This comprehensive guidance will help ensure that consumers, employers and insurers have the information they need to plan for next year and make it easier for families to make decisions to access quality, affordable coverage."
The administration said that it had worked on the guidance for 2015 with input from consumers, states, businesses, health professionals, insurance commissioners, insurers, and members of Congress.
On Twitter @aliciaault
The Obama Administration said that it will allow through 2016 renewals of health insurance policies that do not meet the minimum criteria established by the Affordable Care Act.
Each state has the final say on whether such policies can continue to be sold, but the move by the White House will help remove some of the hurdles, a senior administration official said March 5.
In November, President Obama said that, to avoid cancellations, the federal government would let insurers renew, for 1 year, policies that did not contain the essential health benefits outlined by the ACA. That would give policyholders who did renew time to find new coverage.
About half of the states agreed to allow renewals after the policy change, but some states with the largest number of such policyholders – including California, Washington, New York, and Maryland – refused to allow renewals.
Now, in a wide-ranging rule that covers many aspects of the ACA for 2015, the administration said that noncompliant policies can be renewed as late as Oct. 1, 2016. No new policies can be issued. States that chose not to allow the renewals before also can change their decisions, senior administration officials said.
In 2015, the administration also is giving consumers an additional month to shop for coverage on the federal and state health insurance exchanges for 2015: Open enrollment will run from Nov. 15, 2014, to Feb. 15, 2015.
The rule also reduces out-of-pocket limits for individuals and families who buy coverage through the exchanges. Depending on income, individuals will pay a maximum of $2,250 to $5,200, and families will pay $4,500 to $10,400.
"These policies implement the health care law in a common-sense way by continuing to smooth the transition for consumers and stakeholders and fixing problems wherever the law provides flexibility," HHS Secretary Kathleen Sebelius said in a statement. "This comprehensive guidance will help ensure that consumers, employers and insurers have the information they need to plan for next year and make it easier for families to make decisions to access quality, affordable coverage."
The administration said that it had worked on the guidance for 2015 with input from consumers, states, businesses, health professionals, insurance commissioners, insurers, and members of Congress.
On Twitter @aliciaault
Flexibility – but no passes – on meaningful use Stage 2
ORLANDO – The government can be a bit more flexible on physicians meeting Stage 2 of meaningful use of electronic health records, but won’t give blanket permission to slide on deadlines.
That’s according to Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services (CMS), who spoke Feb. 27 at the annual meeting of the Healthcare Information and Management Systems Society.
Physicians have been seeking more leeway from CMS on participating in meaningful use this year, in part because they must purchase or upgrade to the 2014 edition of certified EHR (electronic health records) technology, and in part because they must get ready to switch over to the ICD-10 coding set on Oct. 1.
Physicians who participate in meaningful use get incentive payments from Medicare or Medicaid. If they don’t participate this year, they’ll be penalized starting in 2015.
On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
Ms. Tavenner said that CMS officials had heard many concerns about moving forward with Stage 2, and "are sensitive to those concerns." She noted that over the past few years, the agency had delayed the start of Stage 1 and Stage 2, and most recently pushed back implementation of Stage 3 to 2017.
"But now is not the time to stop moving forward," she said. Ms. Tavenner said that it was understood that some health care providers and vendors "may legitimately have issues with establishing Stage 2 reporting deadlines."
Because of that, the CMS has "decided to permit flexibility in how hardship exemptions will be granted in the 2014 reporting year," she said.
The agency will look at hardship requests case-by-case, as is required by law. And it is expected to issue further guidance on what qualifies as a hardship very soon.
But Ms. Tavenner said the agency would not give everyone a pass.
"I must stress to you that we do expect all eligible Stage 2 providers to fully meet all requirements in 2015," Ms. Tavenner said. "And I urge all of you to do everything you can to meet the Stage 2 requirements this year."
[email protected]
On Twitter @aliciaault
ORLANDO – The government can be a bit more flexible on physicians meeting Stage 2 of meaningful use of electronic health records, but won’t give blanket permission to slide on deadlines.
That’s according to Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services (CMS), who spoke Feb. 27 at the annual meeting of the Healthcare Information and Management Systems Society.
Physicians have been seeking more leeway from CMS on participating in meaningful use this year, in part because they must purchase or upgrade to the 2014 edition of certified EHR (electronic health records) technology, and in part because they must get ready to switch over to the ICD-10 coding set on Oct. 1.
Physicians who participate in meaningful use get incentive payments from Medicare or Medicaid. If they don’t participate this year, they’ll be penalized starting in 2015.
On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
Ms. Tavenner said that CMS officials had heard many concerns about moving forward with Stage 2, and "are sensitive to those concerns." She noted that over the past few years, the agency had delayed the start of Stage 1 and Stage 2, and most recently pushed back implementation of Stage 3 to 2017.
"But now is not the time to stop moving forward," she said. Ms. Tavenner said that it was understood that some health care providers and vendors "may legitimately have issues with establishing Stage 2 reporting deadlines."
Because of that, the CMS has "decided to permit flexibility in how hardship exemptions will be granted in the 2014 reporting year," she said.
The agency will look at hardship requests case-by-case, as is required by law. And it is expected to issue further guidance on what qualifies as a hardship very soon.
But Ms. Tavenner said the agency would not give everyone a pass.
"I must stress to you that we do expect all eligible Stage 2 providers to fully meet all requirements in 2015," Ms. Tavenner said. "And I urge all of you to do everything you can to meet the Stage 2 requirements this year."
[email protected]
On Twitter @aliciaault
ORLANDO – The government can be a bit more flexible on physicians meeting Stage 2 of meaningful use of electronic health records, but won’t give blanket permission to slide on deadlines.
That’s according to Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services (CMS), who spoke Feb. 27 at the annual meeting of the Healthcare Information and Management Systems Society.
Physicians have been seeking more leeway from CMS on participating in meaningful use this year, in part because they must purchase or upgrade to the 2014 edition of certified EHR (electronic health records) technology, and in part because they must get ready to switch over to the ICD-10 coding set on Oct. 1.
Physicians who participate in meaningful use get incentive payments from Medicare or Medicaid. If they don’t participate this year, they’ll be penalized starting in 2015.
On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
Ms. Tavenner said that CMS officials had heard many concerns about moving forward with Stage 2, and "are sensitive to those concerns." She noted that over the past few years, the agency had delayed the start of Stage 1 and Stage 2, and most recently pushed back implementation of Stage 3 to 2017.
"But now is not the time to stop moving forward," she said. Ms. Tavenner said that it was understood that some health care providers and vendors "may legitimately have issues with establishing Stage 2 reporting deadlines."
Because of that, the CMS has "decided to permit flexibility in how hardship exemptions will be granted in the 2014 reporting year," she said.
The agency will look at hardship requests case-by-case, as is required by law. And it is expected to issue further guidance on what qualifies as a hardship very soon.
But Ms. Tavenner said the agency would not give everyone a pass.
"I must stress to you that we do expect all eligible Stage 2 providers to fully meet all requirements in 2015," Ms. Tavenner said. "And I urge all of you to do everything you can to meet the Stage 2 requirements this year."
[email protected]
On Twitter @aliciaault
AT HIMSS14
CMS launches ICD-10 website for small physician practices
ORLANDO – A new resource is available for small practices as they prepare to switch to the ICD-10 coding system.
Officials from the Centers for Medicare & Medicaid Services (CMS) launched a new website – Road to ICD-10 – to provide a central source of basic information as well as a place to start transitioning to the new code set, which becomes mandatory on Oct. 1.
The site provides fact sheets, training videos, sample codes, and a variety of other resources. It also provides training modules that are specific to certain specialties.
Importantly, it gives physicians a template for building an action plan, said Denesecia Green, director of the Administrative Simplification Group at the CMS Office of eHealth Standards and Services.
So far, templates have been tailored for internal medicine, family practice, pediatrics, cardiology, obstetrics/gynecology, and orthopedics. The site also offers a more general path for other specialties.
According to Ms. Green, the action plan gives physicians a pictorial road map and lists the steps that might help pinpoint where diagnosis codes are used in the practice, how to identify the transition team, how to prepare a budget for the transition to ICD-10, and how to arrange for training.
The site was developed by physicians and came out of a series of listening sessions the agency held with a variety of physicians, said Dr. Ricardo Martinez, a member of the CMS’s small physician practice team, and an emergency physician.
"Physicians are concerned that everything is happening to them, rather than with them or for them," Dr. Martinez said.
However, many in the listening sessions said that they did see some potential benefits with ICD-10. In the end, Dr. Martinez said, physicians had a big question: "If there is a better world ahead, how do you get there?"
The Road to ICD-10 website is designed to help doctors make the journey, Dr. Martinez said.
The site was announced Feb. 26 at the annual meeting of the Healthcare Information and Management Systems Society (HIMSS).
On Twitter @aliciaault
ORLANDO – A new resource is available for small practices as they prepare to switch to the ICD-10 coding system.
Officials from the Centers for Medicare & Medicaid Services (CMS) launched a new website – Road to ICD-10 – to provide a central source of basic information as well as a place to start transitioning to the new code set, which becomes mandatory on Oct. 1.
The site provides fact sheets, training videos, sample codes, and a variety of other resources. It also provides training modules that are specific to certain specialties.
Importantly, it gives physicians a template for building an action plan, said Denesecia Green, director of the Administrative Simplification Group at the CMS Office of eHealth Standards and Services.
So far, templates have been tailored for internal medicine, family practice, pediatrics, cardiology, obstetrics/gynecology, and orthopedics. The site also offers a more general path for other specialties.
According to Ms. Green, the action plan gives physicians a pictorial road map and lists the steps that might help pinpoint where diagnosis codes are used in the practice, how to identify the transition team, how to prepare a budget for the transition to ICD-10, and how to arrange for training.
The site was developed by physicians and came out of a series of listening sessions the agency held with a variety of physicians, said Dr. Ricardo Martinez, a member of the CMS’s small physician practice team, and an emergency physician.
"Physicians are concerned that everything is happening to them, rather than with them or for them," Dr. Martinez said.
However, many in the listening sessions said that they did see some potential benefits with ICD-10. In the end, Dr. Martinez said, physicians had a big question: "If there is a better world ahead, how do you get there?"
The Road to ICD-10 website is designed to help doctors make the journey, Dr. Martinez said.
The site was announced Feb. 26 at the annual meeting of the Healthcare Information and Management Systems Society (HIMSS).
On Twitter @aliciaault
ORLANDO – A new resource is available for small practices as they prepare to switch to the ICD-10 coding system.
Officials from the Centers for Medicare & Medicaid Services (CMS) launched a new website – Road to ICD-10 – to provide a central source of basic information as well as a place to start transitioning to the new code set, which becomes mandatory on Oct. 1.
The site provides fact sheets, training videos, sample codes, and a variety of other resources. It also provides training modules that are specific to certain specialties.
Importantly, it gives physicians a template for building an action plan, said Denesecia Green, director of the Administrative Simplification Group at the CMS Office of eHealth Standards and Services.
So far, templates have been tailored for internal medicine, family practice, pediatrics, cardiology, obstetrics/gynecology, and orthopedics. The site also offers a more general path for other specialties.
According to Ms. Green, the action plan gives physicians a pictorial road map and lists the steps that might help pinpoint where diagnosis codes are used in the practice, how to identify the transition team, how to prepare a budget for the transition to ICD-10, and how to arrange for training.
The site was developed by physicians and came out of a series of listening sessions the agency held with a variety of physicians, said Dr. Ricardo Martinez, a member of the CMS’s small physician practice team, and an emergency physician.
"Physicians are concerned that everything is happening to them, rather than with them or for them," Dr. Martinez said.
However, many in the listening sessions said that they did see some potential benefits with ICD-10. In the end, Dr. Martinez said, physicians had a big question: "If there is a better world ahead, how do you get there?"
The Road to ICD-10 website is designed to help doctors make the journey, Dr. Martinez said.
The site was announced Feb. 26 at the annual meeting of the Healthcare Information and Management Systems Society (HIMSS).
On Twitter @aliciaault
AT HIMSS14
Stage 2 of meaningful use: Expect tougher objectives, pre-payment audits
ORLANDO – Expect a more rigorous process of certification for Stage 2 of meaningful use and be prepared for audits that will withhold incentive payments until all issues are resolved, federal officials advised.
This year is the first that physicians can work to meet the requirements for Stage 2 of meaningful use. Stage 1 was about data capture, but Stage 2 is about sharing information across care settings and between patients and providers, Robert Anthony, deputy director of the Health IT Initiatives Group at the Centers for Medicare and Medicaid Services’ Office of E-Health Standards and Services, said at the annual meeting of the Healthcare Information and Management Systems Society.
Among the core requirements for Stage 2 are establishing a patient portal and ensuring and using secure messaging between patients and providers. Physicians must be able to link to imaging results, report to a registry, and record electronic progress notes.
Mr. Anthony said that CMS purposely set what he called a "low bar" for patient engagement in Stage 2. To demonstrate meaningful use, 5% of patients must use the practice’s patient portal and 5% must participate in secure messaging beyond appointment booking, he said.
Even so, meeting that target might not be easy. Mr. Anthony said that he had heard from some physicians that they are sitting down with patients at the end of a visit and walking them through use of the portal or the messaging process. Those encounters, he added, can be counted toward the target.
Requirements for documenting care transitions are stricter as well, Mr. Anthony said.
"In Stage 1, ‘transitions of care’ was a menu objective, and virtually none of you selected transitions of care," he said. "We know why everybody didn’t select it – because it’s a difficult objective to achieve."
Care transitions, though, are "the Holy Grail" for showing that systems and clinicians can talk to each other, so the measure was moved into the core objectives for stage 2, he said.
Under Stage 1, physicians could send a summary of care to the next provider by any method, as long as it arrived, said Mr. Anthony. Under Stage 2, summaries must be electronically transmitted 10% or more of the time. At least some have to be sent to clinicians using a different electronic health record system.
Finally, CMS will be performing more audits under Stage 2. The agency must be accountable for the $21 billion spent on incentive payments so far, he said. In stage 1, the audits for Medicare were primarily post payment. Now, payments will be withheld until the audits are resolved. Some 5%-10% of physicians will be subject to an audit. They will be chosen at random or through risk profiling.
And, "no, we’re not going to talk about what raises a red flag, because that’s the point of an oversight program," he added.
So far, documentation has been the primary deficiency found through the audit process, Mr. Anthony said.
"I am shocked by the number of people who do not retain any documentation related to their attestation figures," he said. Physicians need to document the numerators and denominators they use for attestation and then keep that documentation for 6 years.
There are no consultants or companies that have any special knowledge about how to avoid audits or resolve an audit more quickly. They also don’t know anything special about the appeals process. "The process is the same for everybody and is transparent and public for everybody," he said.
"Anybody who is telling you separately that they have some kind of inside line to CMS is drumming up business and nothing more," Mr. Anthony said.
He also said that there is no secret process to getting an appeal. The agency spells out what it is looking for in the appeals documentation available on the CMS ICD-10 website. There’s also a sample audit letter from the CMS contractor, Figliozzi and Co.
On Twitter @aliciaault
ORLANDO – Expect a more rigorous process of certification for Stage 2 of meaningful use and be prepared for audits that will withhold incentive payments until all issues are resolved, federal officials advised.
This year is the first that physicians can work to meet the requirements for Stage 2 of meaningful use. Stage 1 was about data capture, but Stage 2 is about sharing information across care settings and between patients and providers, Robert Anthony, deputy director of the Health IT Initiatives Group at the Centers for Medicare and Medicaid Services’ Office of E-Health Standards and Services, said at the annual meeting of the Healthcare Information and Management Systems Society.
Among the core requirements for Stage 2 are establishing a patient portal and ensuring and using secure messaging between patients and providers. Physicians must be able to link to imaging results, report to a registry, and record electronic progress notes.
Mr. Anthony said that CMS purposely set what he called a "low bar" for patient engagement in Stage 2. To demonstrate meaningful use, 5% of patients must use the practice’s patient portal and 5% must participate in secure messaging beyond appointment booking, he said.
Even so, meeting that target might not be easy. Mr. Anthony said that he had heard from some physicians that they are sitting down with patients at the end of a visit and walking them through use of the portal or the messaging process. Those encounters, he added, can be counted toward the target.
Requirements for documenting care transitions are stricter as well, Mr. Anthony said.
"In Stage 1, ‘transitions of care’ was a menu objective, and virtually none of you selected transitions of care," he said. "We know why everybody didn’t select it – because it’s a difficult objective to achieve."
Care transitions, though, are "the Holy Grail" for showing that systems and clinicians can talk to each other, so the measure was moved into the core objectives for stage 2, he said.
Under Stage 1, physicians could send a summary of care to the next provider by any method, as long as it arrived, said Mr. Anthony. Under Stage 2, summaries must be electronically transmitted 10% or more of the time. At least some have to be sent to clinicians using a different electronic health record system.
Finally, CMS will be performing more audits under Stage 2. The agency must be accountable for the $21 billion spent on incentive payments so far, he said. In stage 1, the audits for Medicare were primarily post payment. Now, payments will be withheld until the audits are resolved. Some 5%-10% of physicians will be subject to an audit. They will be chosen at random or through risk profiling.
And, "no, we’re not going to talk about what raises a red flag, because that’s the point of an oversight program," he added.
So far, documentation has been the primary deficiency found through the audit process, Mr. Anthony said.
"I am shocked by the number of people who do not retain any documentation related to their attestation figures," he said. Physicians need to document the numerators and denominators they use for attestation and then keep that documentation for 6 years.
There are no consultants or companies that have any special knowledge about how to avoid audits or resolve an audit more quickly. They also don’t know anything special about the appeals process. "The process is the same for everybody and is transparent and public for everybody," he said.
"Anybody who is telling you separately that they have some kind of inside line to CMS is drumming up business and nothing more," Mr. Anthony said.
He also said that there is no secret process to getting an appeal. The agency spells out what it is looking for in the appeals documentation available on the CMS ICD-10 website. There’s also a sample audit letter from the CMS contractor, Figliozzi and Co.
On Twitter @aliciaault
ORLANDO – Expect a more rigorous process of certification for Stage 2 of meaningful use and be prepared for audits that will withhold incentive payments until all issues are resolved, federal officials advised.
This year is the first that physicians can work to meet the requirements for Stage 2 of meaningful use. Stage 1 was about data capture, but Stage 2 is about sharing information across care settings and between patients and providers, Robert Anthony, deputy director of the Health IT Initiatives Group at the Centers for Medicare and Medicaid Services’ Office of E-Health Standards and Services, said at the annual meeting of the Healthcare Information and Management Systems Society.
Among the core requirements for Stage 2 are establishing a patient portal and ensuring and using secure messaging between patients and providers. Physicians must be able to link to imaging results, report to a registry, and record electronic progress notes.
Mr. Anthony said that CMS purposely set what he called a "low bar" for patient engagement in Stage 2. To demonstrate meaningful use, 5% of patients must use the practice’s patient portal and 5% must participate in secure messaging beyond appointment booking, he said.
Even so, meeting that target might not be easy. Mr. Anthony said that he had heard from some physicians that they are sitting down with patients at the end of a visit and walking them through use of the portal or the messaging process. Those encounters, he added, can be counted toward the target.
Requirements for documenting care transitions are stricter as well, Mr. Anthony said.
"In Stage 1, ‘transitions of care’ was a menu objective, and virtually none of you selected transitions of care," he said. "We know why everybody didn’t select it – because it’s a difficult objective to achieve."
Care transitions, though, are "the Holy Grail" for showing that systems and clinicians can talk to each other, so the measure was moved into the core objectives for stage 2, he said.
Under Stage 1, physicians could send a summary of care to the next provider by any method, as long as it arrived, said Mr. Anthony. Under Stage 2, summaries must be electronically transmitted 10% or more of the time. At least some have to be sent to clinicians using a different electronic health record system.
Finally, CMS will be performing more audits under Stage 2. The agency must be accountable for the $21 billion spent on incentive payments so far, he said. In stage 1, the audits for Medicare were primarily post payment. Now, payments will be withheld until the audits are resolved. Some 5%-10% of physicians will be subject to an audit. They will be chosen at random or through risk profiling.
And, "no, we’re not going to talk about what raises a red flag, because that’s the point of an oversight program," he added.
So far, documentation has been the primary deficiency found through the audit process, Mr. Anthony said.
"I am shocked by the number of people who do not retain any documentation related to their attestation figures," he said. Physicians need to document the numerators and denominators they use for attestation and then keep that documentation for 6 years.
There are no consultants or companies that have any special knowledge about how to avoid audits or resolve an audit more quickly. They also don’t know anything special about the appeals process. "The process is the same for everybody and is transparent and public for everybody," he said.
"Anybody who is telling you separately that they have some kind of inside line to CMS is drumming up business and nothing more," Mr. Anthony said.
He also said that there is no secret process to getting an appeal. The agency spells out what it is looking for in the appeals documentation available on the CMS ICD-10 website. There’s also a sample audit letter from the CMS contractor, Figliozzi and Co.
On Twitter @aliciaault
AT HIMSS14