User login
More evidence supporting ultra-low retreatment dose of rituximab in RA
Key clinical point: Retreatment with a lower rituximab dose of 200 mg or 500 mg was as effective as 1000 mg in patients with rheumatoid arthritis (RA) who responded well to standard rituximab dose.
Major finding: Treatment response was not maintained in 11%, 21%, and 13% of patients in the 1000 mg, 500 mg, and 200 mg rituximab groups, respectively. Ultra-low rituximab dosage was not associated with the presence of antidrug antibodies at 6 months, and B-cell counts were not significantly different between the dosing groups.
Study details: The data comes from a preplanned secondary analysis of the REDO trial involving 140 patients with RA who responded well to the standard rituximab dose for at least 6 months and were randomly assigned to receive 200 mg, 500 mg, or 1000 mg rituximab.
Disclosures: The REDO study was funded by health insurance companies Centraal Ziekenfonds and Menzis, and this secondary analysis did not receive any external funding. The Sint Maartenskliniek (employer of 6 authors) has a patent application filed for rituximab in the treatment of polymyalgia rheumatica.
Source: Wientjes MHM et al. Rheumatology (Oxford). 2022 (Jan 12). Doi: 10.1093/rheumatology/keac024
Key clinical point: Retreatment with a lower rituximab dose of 200 mg or 500 mg was as effective as 1000 mg in patients with rheumatoid arthritis (RA) who responded well to standard rituximab dose.
Major finding: Treatment response was not maintained in 11%, 21%, and 13% of patients in the 1000 mg, 500 mg, and 200 mg rituximab groups, respectively. Ultra-low rituximab dosage was not associated with the presence of antidrug antibodies at 6 months, and B-cell counts were not significantly different between the dosing groups.
Study details: The data comes from a preplanned secondary analysis of the REDO trial involving 140 patients with RA who responded well to the standard rituximab dose for at least 6 months and were randomly assigned to receive 200 mg, 500 mg, or 1000 mg rituximab.
Disclosures: The REDO study was funded by health insurance companies Centraal Ziekenfonds and Menzis, and this secondary analysis did not receive any external funding. The Sint Maartenskliniek (employer of 6 authors) has a patent application filed for rituximab in the treatment of polymyalgia rheumatica.
Source: Wientjes MHM et al. Rheumatology (Oxford). 2022 (Jan 12). Doi: 10.1093/rheumatology/keac024
Key clinical point: Retreatment with a lower rituximab dose of 200 mg or 500 mg was as effective as 1000 mg in patients with rheumatoid arthritis (RA) who responded well to standard rituximab dose.
Major finding: Treatment response was not maintained in 11%, 21%, and 13% of patients in the 1000 mg, 500 mg, and 200 mg rituximab groups, respectively. Ultra-low rituximab dosage was not associated with the presence of antidrug antibodies at 6 months, and B-cell counts were not significantly different between the dosing groups.
Study details: The data comes from a preplanned secondary analysis of the REDO trial involving 140 patients with RA who responded well to the standard rituximab dose for at least 6 months and were randomly assigned to receive 200 mg, 500 mg, or 1000 mg rituximab.
Disclosures: The REDO study was funded by health insurance companies Centraal Ziekenfonds and Menzis, and this secondary analysis did not receive any external funding. The Sint Maartenskliniek (employer of 6 authors) has a patent application filed for rituximab in the treatment of polymyalgia rheumatica.
Source: Wientjes MHM et al. Rheumatology (Oxford). 2022 (Jan 12). Doi: 10.1093/rheumatology/keac024
Rheumatoid arthritis: Higher risk for MACE and cancer with tofacitinib vs. TNF inhibitors
Key clinical point: Tofacitinib was associated with a higher risk for major adverse cardiovascular events (MACE) and cancer than tumor necrosis factor (TNF) inhibitors in a cardiovascular risk-enriched population of patients with active rheumatoid arthritis (RA).
Major finding: During a median follow-up of 4 years, the combined tofacitinib doses vs. TNF inhibitors were associated with a higher incidence of MACE (hazard ratio [HR] 1.33; 95% CI 0.91-1.94) and cancer (HR 1.48; 95% CI 1.04-2.09), not meeting the predefined criteria for noninferiority.
Study details: The findings come from the noninferiority, phase 3b-4, safety end-point ORAL Surveillance trial involving 4,362 patients aged 50 years or older with at least 1 additional cardiovascular risk factor who had active RA despite methotrexate treatment. The patients were randomly assigned to 5 mg or 10 mg tofacitinib twice daily or a TNF inhibitor.
Disclosures: This study was funded by Pfizer. Some of the authors declared being employees or holding stocks at Pfizer, whereas some others declared serving as a consultant or receiving grants from various sources.
Source: Ytterberg SR et al. N Engl J Med. 2022;386:316-326 (Jan 27). Doi: 10.1056/NEJMoa2109927
Key clinical point: Tofacitinib was associated with a higher risk for major adverse cardiovascular events (MACE) and cancer than tumor necrosis factor (TNF) inhibitors in a cardiovascular risk-enriched population of patients with active rheumatoid arthritis (RA).
Major finding: During a median follow-up of 4 years, the combined tofacitinib doses vs. TNF inhibitors were associated with a higher incidence of MACE (hazard ratio [HR] 1.33; 95% CI 0.91-1.94) and cancer (HR 1.48; 95% CI 1.04-2.09), not meeting the predefined criteria for noninferiority.
Study details: The findings come from the noninferiority, phase 3b-4, safety end-point ORAL Surveillance trial involving 4,362 patients aged 50 years or older with at least 1 additional cardiovascular risk factor who had active RA despite methotrexate treatment. The patients were randomly assigned to 5 mg or 10 mg tofacitinib twice daily or a TNF inhibitor.
Disclosures: This study was funded by Pfizer. Some of the authors declared being employees or holding stocks at Pfizer, whereas some others declared serving as a consultant or receiving grants from various sources.
Source: Ytterberg SR et al. N Engl J Med. 2022;386:316-326 (Jan 27). Doi: 10.1056/NEJMoa2109927
Key clinical point: Tofacitinib was associated with a higher risk for major adverse cardiovascular events (MACE) and cancer than tumor necrosis factor (TNF) inhibitors in a cardiovascular risk-enriched population of patients with active rheumatoid arthritis (RA).
Major finding: During a median follow-up of 4 years, the combined tofacitinib doses vs. TNF inhibitors were associated with a higher incidence of MACE (hazard ratio [HR] 1.33; 95% CI 0.91-1.94) and cancer (HR 1.48; 95% CI 1.04-2.09), not meeting the predefined criteria for noninferiority.
Study details: The findings come from the noninferiority, phase 3b-4, safety end-point ORAL Surveillance trial involving 4,362 patients aged 50 years or older with at least 1 additional cardiovascular risk factor who had active RA despite methotrexate treatment. The patients were randomly assigned to 5 mg or 10 mg tofacitinib twice daily or a TNF inhibitor.
Disclosures: This study was funded by Pfizer. Some of the authors declared being employees or holding stocks at Pfizer, whereas some others declared serving as a consultant or receiving grants from various sources.
Source: Ytterberg SR et al. N Engl J Med. 2022;386:316-326 (Jan 27). Doi: 10.1056/NEJMoa2109927
Clinical Edge Journal Scan Commentary: PsA March 2022
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1 assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13). doi: 10.1093/aje/kwac009. Epub ahead of print. PMID: 35029650.
5. Walscheid K, Rothaus K, Niewerth M, Klotsche J, Minden K, Heiligenhaus A. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15). doi: 10.3899/jrheum.210755. Epub ahead of print. PMID: 35034000.
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1 assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13). doi: 10.1093/aje/kwac009. Epub ahead of print. PMID: 35029650.
5. Walscheid K, Rothaus K, Niewerth M, Klotsche J, Minden K, Heiligenhaus A. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15). doi: 10.3899/jrheum.210755. Epub ahead of print. PMID: 35034000.
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1 assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13). doi: 10.1093/aje/kwac009. Epub ahead of print. PMID: 35029650.
5. Walscheid K, Rothaus K, Niewerth M, Klotsche J, Minden K, Heiligenhaus A. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15). doi: 10.3899/jrheum.210755. Epub ahead of print. PMID: 35034000.
Clinical Edge Journal Scan Commentary: HCC March 2022
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Clinical Edge Journal Scan Commentary: HCC March 2022
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
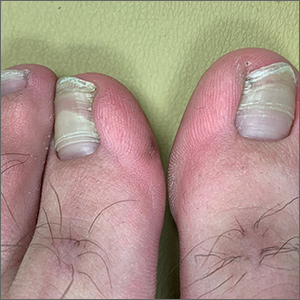
Toenail ridges
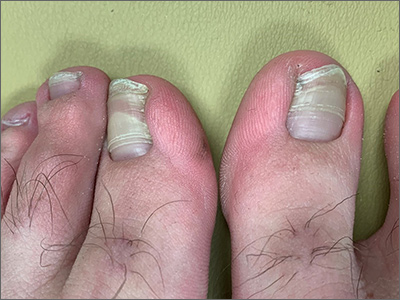
Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026

Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026
Leukemia Cutis Manifesting as Nonpalpable Purpura
To the Editor:
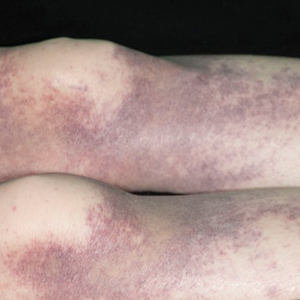
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.
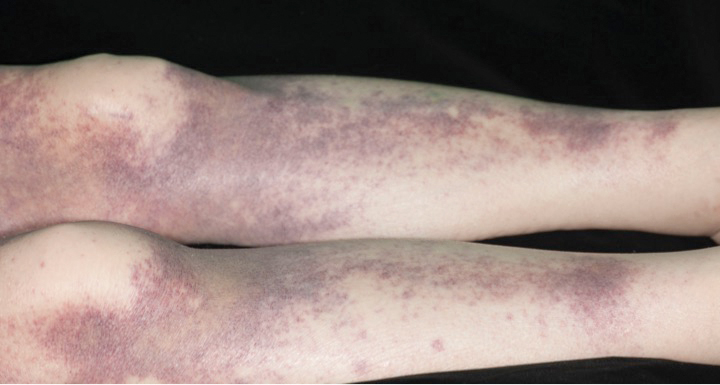
The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).
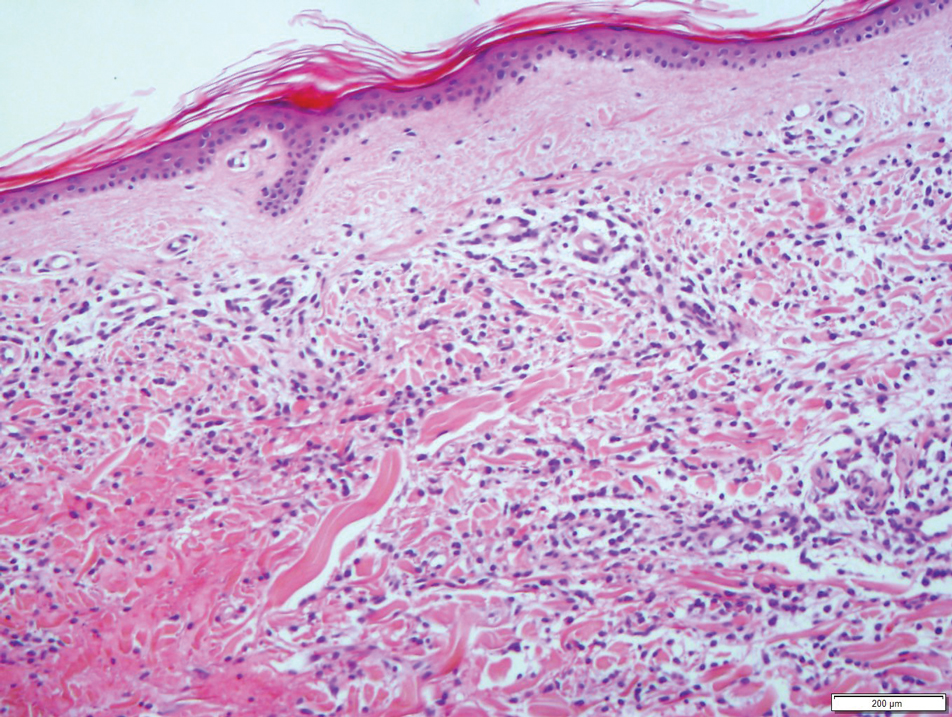
Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.
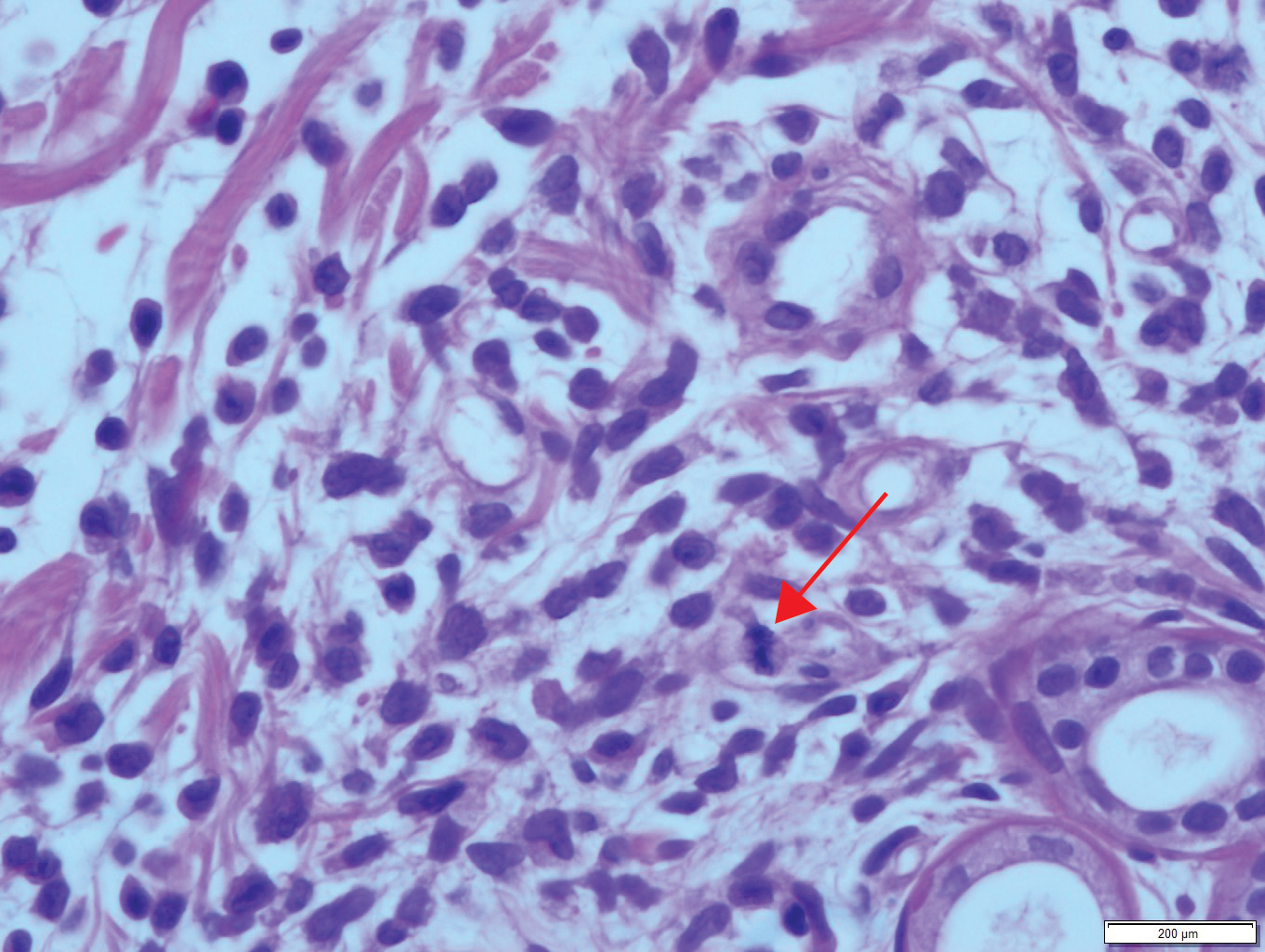
Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.
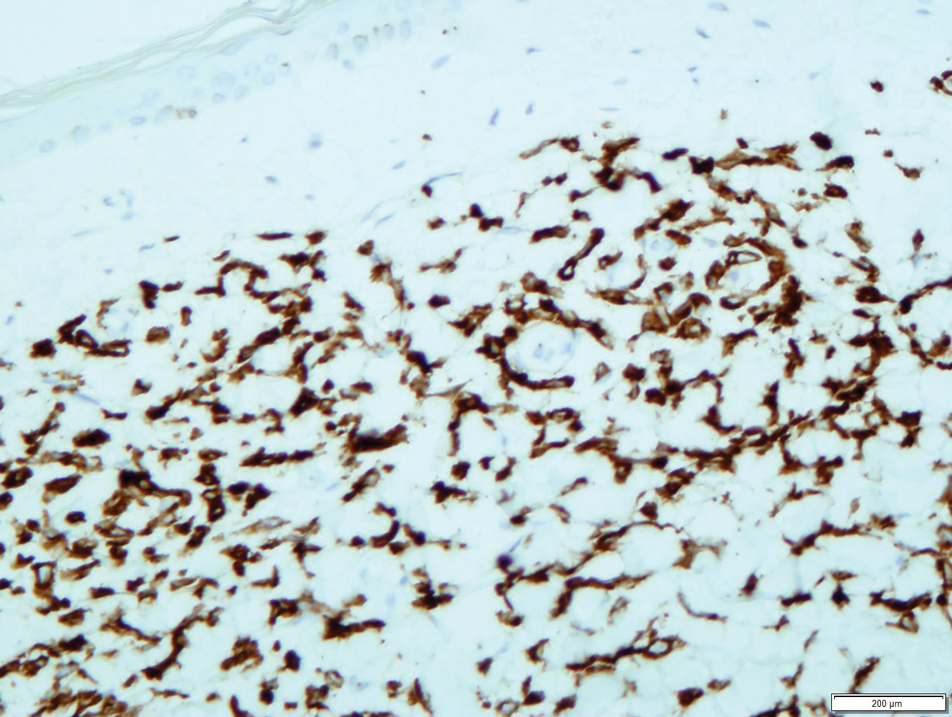
Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.

The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).

Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.

Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.

Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.

The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).

Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.

Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.

Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
Practice Points
- Leukemia cutis complicates 5% to 15% of all cases of acute myeloid leukemia (AML) in adults.
- The appearance of leukemia cutis may be highly variable. Therefore, it should be included in the differential diagnosis for any cutaneous presentation in patients with an existing diagnosis or high likelihood of AML.
- Leukemic infiltrates are associated with sites of inflammation.
Pulsating unilateral headache and nausea
The patient is probably experiencing migraine without aura. Migraines are a complex disorder characterized by recurrent episodes of headache, most often unilateral. These attacks are associated with symptoms related to the central nervous system. Approximately 15% of patients with migraine experience aura (temporary visual, sensory, speech, or other motor disturbances). More research is needed to determine whether migraine with and without aura are potentially different diagnostic entities.
Classic migraine is a clinical diagnosis. When patients experience migraine symptoms routinely, however, it is important to consider whether these signs and symptoms can be accounted for by another diagnosis. Neuroimaging and, less commonly, lumbar puncture may be indicated in some presentations; red flags that call for additional workup are captured in the acronym SNOOP: systemic symptoms, neurologic symptoms, onset is acute, older patients, and previous history. In addition, classic migraine should be distinguished from common headaches as well as rare subtypes of migraine. For instance, hemiplegic migraine typically presents with temporary unilateral hemiparesis, sometimes accompanied by speech disturbance, and may be inherited (familial hemiplegic migraine). Basilar migraine is another rare subtype of migraine that manifests with signs of vertebrobasilar insufficiency. Attacks of chronic paroxysmal hemicrania are unilateral (just as migraines can be in about half of all cases); they are marked by their high intensity but short duration, and are accompanied by same-side facial autonomic symptoms (eg, tearing, congestion). Such patients' history and presentation do not fulfill criteria put forth by the American Headache Society (AHS) for chronic migraine, which specify having headaches 15 or more days per month for more than 3 months, and in which on at least 8 days per month those attacks are consistent with migraine or are relieved by a triptan or ergot derivative.
Migraine management must be personalized for each patient and is often associated with a marked trial-and-error period. Migraine without aura and migraine with aura are treated via similar approaches. AHS guidelines include several medications that may be effective in mitigating migraines, including both migraine-specific agents (ergotamine, ergotamine derivatives, and lasmiditan), and nonspecific agents (NSAIDs, combination analgesics, intravenous magnesium, isometheptene-containing compounds, and antiemetics). Triptans represent first-line acute treatment for migraine, but the FDA has approved five acute migraine treatments in total: celecoxib, lasmiditan, remote electrical neuromodulation (REN), rimegepant, and ubrogepant. For moderate or severe attacks, migraine-specific agents are recommended: beyond triptans, dihydroergotamine (DHE), small-molecule calcitonin gene-related peptide receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Patients should limit medication use to an average of two headache days per week, and those who do not find relief within these parameters are candidates for preventive migraine treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
The patient is probably experiencing migraine without aura. Migraines are a complex disorder characterized by recurrent episodes of headache, most often unilateral. These attacks are associated with symptoms related to the central nervous system. Approximately 15% of patients with migraine experience aura (temporary visual, sensory, speech, or other motor disturbances). More research is needed to determine whether migraine with and without aura are potentially different diagnostic entities.
Classic migraine is a clinical diagnosis. When patients experience migraine symptoms routinely, however, it is important to consider whether these signs and symptoms can be accounted for by another diagnosis. Neuroimaging and, less commonly, lumbar puncture may be indicated in some presentations; red flags that call for additional workup are captured in the acronym SNOOP: systemic symptoms, neurologic symptoms, onset is acute, older patients, and previous history. In addition, classic migraine should be distinguished from common headaches as well as rare subtypes of migraine. For instance, hemiplegic migraine typically presents with temporary unilateral hemiparesis, sometimes accompanied by speech disturbance, and may be inherited (familial hemiplegic migraine). Basilar migraine is another rare subtype of migraine that manifests with signs of vertebrobasilar insufficiency. Attacks of chronic paroxysmal hemicrania are unilateral (just as migraines can be in about half of all cases); they are marked by their high intensity but short duration, and are accompanied by same-side facial autonomic symptoms (eg, tearing, congestion). Such patients' history and presentation do not fulfill criteria put forth by the American Headache Society (AHS) for chronic migraine, which specify having headaches 15 or more days per month for more than 3 months, and in which on at least 8 days per month those attacks are consistent with migraine or are relieved by a triptan or ergot derivative.
Migraine management must be personalized for each patient and is often associated with a marked trial-and-error period. Migraine without aura and migraine with aura are treated via similar approaches. AHS guidelines include several medications that may be effective in mitigating migraines, including both migraine-specific agents (ergotamine, ergotamine derivatives, and lasmiditan), and nonspecific agents (NSAIDs, combination analgesics, intravenous magnesium, isometheptene-containing compounds, and antiemetics). Triptans represent first-line acute treatment for migraine, but the FDA has approved five acute migraine treatments in total: celecoxib, lasmiditan, remote electrical neuromodulation (REN), rimegepant, and ubrogepant. For moderate or severe attacks, migraine-specific agents are recommended: beyond triptans, dihydroergotamine (DHE), small-molecule calcitonin gene-related peptide receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Patients should limit medication use to an average of two headache days per week, and those who do not find relief within these parameters are candidates for preventive migraine treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
The patient is probably experiencing migraine without aura. Migraines are a complex disorder characterized by recurrent episodes of headache, most often unilateral. These attacks are associated with symptoms related to the central nervous system. Approximately 15% of patients with migraine experience aura (temporary visual, sensory, speech, or other motor disturbances). More research is needed to determine whether migraine with and without aura are potentially different diagnostic entities.
Classic migraine is a clinical diagnosis. When patients experience migraine symptoms routinely, however, it is important to consider whether these signs and symptoms can be accounted for by another diagnosis. Neuroimaging and, less commonly, lumbar puncture may be indicated in some presentations; red flags that call for additional workup are captured in the acronym SNOOP: systemic symptoms, neurologic symptoms, onset is acute, older patients, and previous history. In addition, classic migraine should be distinguished from common headaches as well as rare subtypes of migraine. For instance, hemiplegic migraine typically presents with temporary unilateral hemiparesis, sometimes accompanied by speech disturbance, and may be inherited (familial hemiplegic migraine). Basilar migraine is another rare subtype of migraine that manifests with signs of vertebrobasilar insufficiency. Attacks of chronic paroxysmal hemicrania are unilateral (just as migraines can be in about half of all cases); they are marked by their high intensity but short duration, and are accompanied by same-side facial autonomic symptoms (eg, tearing, congestion). Such patients' history and presentation do not fulfill criteria put forth by the American Headache Society (AHS) for chronic migraine, which specify having headaches 15 or more days per month for more than 3 months, and in which on at least 8 days per month those attacks are consistent with migraine or are relieved by a triptan or ergot derivative.
Migraine management must be personalized for each patient and is often associated with a marked trial-and-error period. Migraine without aura and migraine with aura are treated via similar approaches. AHS guidelines include several medications that may be effective in mitigating migraines, including both migraine-specific agents (ergotamine, ergotamine derivatives, and lasmiditan), and nonspecific agents (NSAIDs, combination analgesics, intravenous magnesium, isometheptene-containing compounds, and antiemetics). Triptans represent first-line acute treatment for migraine, but the FDA has approved five acute migraine treatments in total: celecoxib, lasmiditan, remote electrical neuromodulation (REN), rimegepant, and ubrogepant. For moderate or severe attacks, migraine-specific agents are recommended: beyond triptans, dihydroergotamine (DHE), small-molecule calcitonin gene-related peptide receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Patients should limit medication use to an average of two headache days per week, and those who do not find relief within these parameters are candidates for preventive migraine treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
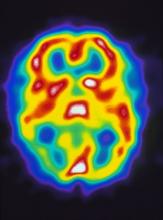
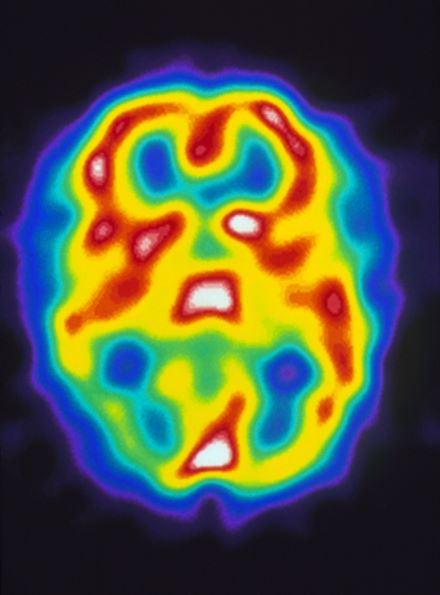
A 22-year-old woman presents with a pulsating unilateral headache (right side) and is very nauseated. The patient reports that since childhood, she has been prone to headaches, with no other significant medical history. Over the past year or so, the headaches have been occurring about once or twice a month, have taken on a throbbing quality, and usually last for several days without relief from nonsteroidal anti-inflammatory drugs (NSAIDs). While taking part in a clinical trial, the patient undergoes a single photon emission CT scan which shows reduced blood flow (lower left).
NSCLC Management: Advanced by Science, Challenged by Human Barriers
New therapies developed to treat non-small cell lung cancer are not reaching all patients with this disease. Human-created barriers bar the way for those experiencing real or perceived stigma and those who reside in remote places or live on little income.
In this supplement, Abbie Begnaud, MD, FCCP, hones in on this human-created dichotomy and discusses the problems and possible solutions, along with diagnostic and treatment options.
New therapies developed to treat non-small cell lung cancer are not reaching all patients with this disease. Human-created barriers bar the way for those experiencing real or perceived stigma and those who reside in remote places or live on little income.
In this supplement, Abbie Begnaud, MD, FCCP, hones in on this human-created dichotomy and discusses the problems and possible solutions, along with diagnostic and treatment options.
New therapies developed to treat non-small cell lung cancer are not reaching all patients with this disease. Human-created barriers bar the way for those experiencing real or perceived stigma and those who reside in remote places or live on little income.
In this supplement, Abbie Begnaud, MD, FCCP, hones in on this human-created dichotomy and discusses the problems and possible solutions, along with diagnostic and treatment options.
2021 in Review: Key Trials in Type 2 Diabetes (T2D)
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.