User login
Retro-orbital headache and nausea
On the basis of his presentation, this patient is probably experiencing migraine with visual aura. Migraine is a condition in children and adolescents whose prevalence increases with age: 1%-3% between age 3 and 7 years, 4%-11% between age 7 and 11 years, and 8%-23% by age 15 years. Although migraine without aura is relatively uncommon in the pediatric population, visual aura is a hallmark sign of migraine headache and excludes the other headache types in the differential diagnosis. Basilar migraine is unlikely because the patient has not experienced symptoms that suggest occipital or brainstem area dysfunction post-aura.
The diagnosis of migraine is largely clinical, but the American Academy of Neurology (AAN) guidelines for the acute treatment of pediatric migraine recommend that when assessing children and adolescents with headache, clinicians should diagnose a specific headache type: primary, secondary, or other headache syndrome. Migraine in pediatric patients is often related to triggering factors such as infection, physical or psychological stress, or dietary choices, but on the basis of the patient's history, this headache appears to be primary in nature.
Most pediatric patients can achieve control of their migraines with acute treatments and benefit from nonprescription oral analgesics, including acetaminophen, ibuprofen, and naproxen. Clinicians should prescribe ibuprofen orally (10 mg/kg) as an initial treatment for children and adolescents with migraine. The US Food and Drug Administration has only approved certain triptans for pediatric patients: almotriptan, sumatriptan-naproxen, and zolmitriptan nasal spray for patients aged 12 years or older and rizatriptan for patients aged 6-17 years.
The AAN guidelines for the pharmacologic treatment of pediatric migraine prevention report that for those who experience migraine with aura, taking a triptan during the aura is safe, though it may be more effective when taken at the onset of head pain, as is the case with other acute treatments.
In pediatric patients, avoidance of known headache triggers is generally sufficient for migraine prevention. This includes managing anxiety, depression, attention-deficit/hyperactivity disorder, and other psychiatric comorbidities that can exacerbate headache. Lifestyle management also includes ensuring adequate sleep, exercise, hydration, and stress management.
The guidelines conclude that although the majority of randomized controlled trials exploring the efficacy of preventive medications in the pediatric population fail to demonstrate superiority to placebo, migraine prophylaxis should be considered when headaches occur with high frequency and severity and cause migraine-related disability based on the Pediatric Migraine Disability Assessment (PedMIDAS).
Jasmin Harpe, MD, MPH, Headache Fellow, Department of Neurology, Harvard University, John R. Graham Headache Center, Mass General Brigham, Boston, MA
Jasmin Harpe, MD, MPH, has disclosed no relevant financial relationships
On the basis of his presentation, this patient is probably experiencing migraine with visual aura. Migraine is a condition in children and adolescents whose prevalence increases with age: 1%-3% between age 3 and 7 years, 4%-11% between age 7 and 11 years, and 8%-23% by age 15 years. Although migraine without aura is relatively uncommon in the pediatric population, visual aura is a hallmark sign of migraine headache and excludes the other headache types in the differential diagnosis. Basilar migraine is unlikely because the patient has not experienced symptoms that suggest occipital or brainstem area dysfunction post-aura.
The diagnosis of migraine is largely clinical, but the American Academy of Neurology (AAN) guidelines for the acute treatment of pediatric migraine recommend that when assessing children and adolescents with headache, clinicians should diagnose a specific headache type: primary, secondary, or other headache syndrome. Migraine in pediatric patients is often related to triggering factors such as infection, physical or psychological stress, or dietary choices, but on the basis of the patient's history, this headache appears to be primary in nature.
Most pediatric patients can achieve control of their migraines with acute treatments and benefit from nonprescription oral analgesics, including acetaminophen, ibuprofen, and naproxen. Clinicians should prescribe ibuprofen orally (10 mg/kg) as an initial treatment for children and adolescents with migraine. The US Food and Drug Administration has only approved certain triptans for pediatric patients: almotriptan, sumatriptan-naproxen, and zolmitriptan nasal spray for patients aged 12 years or older and rizatriptan for patients aged 6-17 years.
The AAN guidelines for the pharmacologic treatment of pediatric migraine prevention report that for those who experience migraine with aura, taking a triptan during the aura is safe, though it may be more effective when taken at the onset of head pain, as is the case with other acute treatments.
In pediatric patients, avoidance of known headache triggers is generally sufficient for migraine prevention. This includes managing anxiety, depression, attention-deficit/hyperactivity disorder, and other psychiatric comorbidities that can exacerbate headache. Lifestyle management also includes ensuring adequate sleep, exercise, hydration, and stress management.
The guidelines conclude that although the majority of randomized controlled trials exploring the efficacy of preventive medications in the pediatric population fail to demonstrate superiority to placebo, migraine prophylaxis should be considered when headaches occur with high frequency and severity and cause migraine-related disability based on the Pediatric Migraine Disability Assessment (PedMIDAS).
Jasmin Harpe, MD, MPH, Headache Fellow, Department of Neurology, Harvard University, John R. Graham Headache Center, Mass General Brigham, Boston, MA
Jasmin Harpe, MD, MPH, has disclosed no relevant financial relationships
On the basis of his presentation, this patient is probably experiencing migraine with visual aura. Migraine is a condition in children and adolescents whose prevalence increases with age: 1%-3% between age 3 and 7 years, 4%-11% between age 7 and 11 years, and 8%-23% by age 15 years. Although migraine without aura is relatively uncommon in the pediatric population, visual aura is a hallmark sign of migraine headache and excludes the other headache types in the differential diagnosis. Basilar migraine is unlikely because the patient has not experienced symptoms that suggest occipital or brainstem area dysfunction post-aura.
The diagnosis of migraine is largely clinical, but the American Academy of Neurology (AAN) guidelines for the acute treatment of pediatric migraine recommend that when assessing children and adolescents with headache, clinicians should diagnose a specific headache type: primary, secondary, or other headache syndrome. Migraine in pediatric patients is often related to triggering factors such as infection, physical or psychological stress, or dietary choices, but on the basis of the patient's history, this headache appears to be primary in nature.
Most pediatric patients can achieve control of their migraines with acute treatments and benefit from nonprescription oral analgesics, including acetaminophen, ibuprofen, and naproxen. Clinicians should prescribe ibuprofen orally (10 mg/kg) as an initial treatment for children and adolescents with migraine. The US Food and Drug Administration has only approved certain triptans for pediatric patients: almotriptan, sumatriptan-naproxen, and zolmitriptan nasal spray for patients aged 12 years or older and rizatriptan for patients aged 6-17 years.
The AAN guidelines for the pharmacologic treatment of pediatric migraine prevention report that for those who experience migraine with aura, taking a triptan during the aura is safe, though it may be more effective when taken at the onset of head pain, as is the case with other acute treatments.
In pediatric patients, avoidance of known headache triggers is generally sufficient for migraine prevention. This includes managing anxiety, depression, attention-deficit/hyperactivity disorder, and other psychiatric comorbidities that can exacerbate headache. Lifestyle management also includes ensuring adequate sleep, exercise, hydration, and stress management.
The guidelines conclude that although the majority of randomized controlled trials exploring the efficacy of preventive medications in the pediatric population fail to demonstrate superiority to placebo, migraine prophylaxis should be considered when headaches occur with high frequency and severity and cause migraine-related disability based on the Pediatric Migraine Disability Assessment (PedMIDAS).
Jasmin Harpe, MD, MPH, Headache Fellow, Department of Neurology, Harvard University, John R. Graham Headache Center, Mass General Brigham, Boston, MA
Jasmin Harpe, MD, MPH, has disclosed no relevant financial relationships
A 7-year-old boy presents with a retro-orbital headache, nausea, and photophobia. Height is 4 ft 2 in and weight is 70 lb (BMI 19.7). The patient's mother reports that he described seeing "rainbow shapes" in his line of vision about 20 minutes before the onset of head pain and notes that she herself has a history of headache. The patient is nonfebrile but sweating and drowsy. Physical examination is unrevealing. The patient has no known allergies and is not currently on medication.
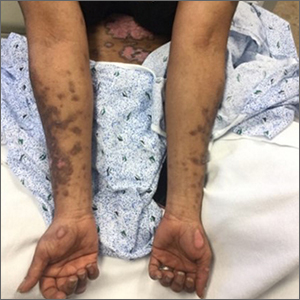
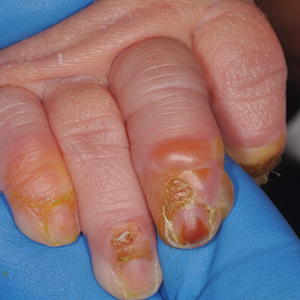
Scaly rash

Scaly plaques on sun-exposed skin with hyperpigmentation and dyspigmentation are classic signs of cutaneous lupus erythematosus (CLE). (The dyspigmentation seen in this case signaled that she likely had chronic cutaneous lupus erythematosus [CCLE]—a subtype of CLE.) At the patient’s follow-up primary care visit, her antinuclear antibodies titer was 1:1280 (≥ 1:160 is considered a positive test) and her 24-hour urine protein was 1188 mg (normal levels in adults, < 150 mg/d). In light of the patient’s joint pain, lab findings, and skin manifestations, she was also given a diagnosis of systemic lupus erythematosus (SLE).
Lupus erythematosus has an increased prevalence in women and typically occurs between the ages of 20 to 50 years.1 The incidence and prevalence of this condition is also greater in Black patients. CLE can either occur with SLE or independently. Patients with CLE should be monitored for the development of SLE. A diagnosis of CLE is based mainly on clinical features; biopsy is only indicated if there is a high degree of uncertainty.
Patients with CLE may suffer from a lower quality of life compared to patients with other dermatologic conditions due to the often disfiguring and disabling nature of the condition.1,2 Additionally, Black patients have an even higher chance of developing depressive symptoms associated with CCLE.2
Therapeutic management for CLE involves photoprotection by wearing sun-protective clothing, sunscreen, and limiting sun exposure.1 Initial treatment includes topical or intralesional corticosteroids, or topical calcineurin inhibitors. Systemic therapy is similar to that used for SLE. Oral glucocorticoids, and antimalarial agents are considered first-line systemic therapy.1 Second-line treatment includes methotrexate, mycophenolate mofetil, systemic retinoids, and azathioprine. Other immunosuppressive agents that are less commonly used include clofazimine, cyclophosphamide, and rituximab.1
The patient was treated sequentially with trials of oral azathioprine 50 mg bid, then prednisone 10 mg once daily, and then hydroxychloroquine 400 mg daily, without significant change in her condition. Additionally, topical steroids did not improve the patient’s symptoms. She was subsequently started on rituximab 1000 mg intravenously with a second dose repeated 2 weeks later, and another treatment 6 months after that. One year after her visit to the ED, the patient was experiencing marked improvement in her lesions.
Photo courtesy of Christy Nwankwo BA. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17:135-146. doi:10.1007/s40257-016-0173-9
2. Hong J, Aspey L, Bao G, et al. Chronic cutaneous lupus erythematosus: depression burden and associated factors. Am J Clin Dermatol. 2019;20:465-475. doi:10.1007/s40257-019-00429-7

Scaly plaques on sun-exposed skin with hyperpigmentation and dyspigmentation are classic signs of cutaneous lupus erythematosus (CLE). (The dyspigmentation seen in this case signaled that she likely had chronic cutaneous lupus erythematosus [CCLE]—a subtype of CLE.) At the patient’s follow-up primary care visit, her antinuclear antibodies titer was 1:1280 (≥ 1:160 is considered a positive test) and her 24-hour urine protein was 1188 mg (normal levels in adults, < 150 mg/d). In light of the patient’s joint pain, lab findings, and skin manifestations, she was also given a diagnosis of systemic lupus erythematosus (SLE).
Lupus erythematosus has an increased prevalence in women and typically occurs between the ages of 20 to 50 years.1 The incidence and prevalence of this condition is also greater in Black patients. CLE can either occur with SLE or independently. Patients with CLE should be monitored for the development of SLE. A diagnosis of CLE is based mainly on clinical features; biopsy is only indicated if there is a high degree of uncertainty.
Patients with CLE may suffer from a lower quality of life compared to patients with other dermatologic conditions due to the often disfiguring and disabling nature of the condition.1,2 Additionally, Black patients have an even higher chance of developing depressive symptoms associated with CCLE.2
Therapeutic management for CLE involves photoprotection by wearing sun-protective clothing, sunscreen, and limiting sun exposure.1 Initial treatment includes topical or intralesional corticosteroids, or topical calcineurin inhibitors. Systemic therapy is similar to that used for SLE. Oral glucocorticoids, and antimalarial agents are considered first-line systemic therapy.1 Second-line treatment includes methotrexate, mycophenolate mofetil, systemic retinoids, and azathioprine. Other immunosuppressive agents that are less commonly used include clofazimine, cyclophosphamide, and rituximab.1
The patient was treated sequentially with trials of oral azathioprine 50 mg bid, then prednisone 10 mg once daily, and then hydroxychloroquine 400 mg daily, without significant change in her condition. Additionally, topical steroids did not improve the patient’s symptoms. She was subsequently started on rituximab 1000 mg intravenously with a second dose repeated 2 weeks later, and another treatment 6 months after that. One year after her visit to the ED, the patient was experiencing marked improvement in her lesions.
Photo courtesy of Christy Nwankwo BA. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque

Scaly plaques on sun-exposed skin with hyperpigmentation and dyspigmentation are classic signs of cutaneous lupus erythematosus (CLE). (The dyspigmentation seen in this case signaled that she likely had chronic cutaneous lupus erythematosus [CCLE]—a subtype of CLE.) At the patient’s follow-up primary care visit, her antinuclear antibodies titer was 1:1280 (≥ 1:160 is considered a positive test) and her 24-hour urine protein was 1188 mg (normal levels in adults, < 150 mg/d). In light of the patient’s joint pain, lab findings, and skin manifestations, she was also given a diagnosis of systemic lupus erythematosus (SLE).
Lupus erythematosus has an increased prevalence in women and typically occurs between the ages of 20 to 50 years.1 The incidence and prevalence of this condition is also greater in Black patients. CLE can either occur with SLE or independently. Patients with CLE should be monitored for the development of SLE. A diagnosis of CLE is based mainly on clinical features; biopsy is only indicated if there is a high degree of uncertainty.
Patients with CLE may suffer from a lower quality of life compared to patients with other dermatologic conditions due to the often disfiguring and disabling nature of the condition.1,2 Additionally, Black patients have an even higher chance of developing depressive symptoms associated with CCLE.2
Therapeutic management for CLE involves photoprotection by wearing sun-protective clothing, sunscreen, and limiting sun exposure.1 Initial treatment includes topical or intralesional corticosteroids, or topical calcineurin inhibitors. Systemic therapy is similar to that used for SLE. Oral glucocorticoids, and antimalarial agents are considered first-line systemic therapy.1 Second-line treatment includes methotrexate, mycophenolate mofetil, systemic retinoids, and azathioprine. Other immunosuppressive agents that are less commonly used include clofazimine, cyclophosphamide, and rituximab.1
The patient was treated sequentially with trials of oral azathioprine 50 mg bid, then prednisone 10 mg once daily, and then hydroxychloroquine 400 mg daily, without significant change in her condition. Additionally, topical steroids did not improve the patient’s symptoms. She was subsequently started on rituximab 1000 mg intravenously with a second dose repeated 2 weeks later, and another treatment 6 months after that. One year after her visit to the ED, the patient was experiencing marked improvement in her lesions.
Photo courtesy of Christy Nwankwo BA. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17:135-146. doi:10.1007/s40257-016-0173-9
2. Hong J, Aspey L, Bao G, et al. Chronic cutaneous lupus erythematosus: depression burden and associated factors. Am J Clin Dermatol. 2019;20:465-475. doi:10.1007/s40257-019-00429-7
1. Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17:135-146. doi:10.1007/s40257-016-0173-9
2. Hong J, Aspey L, Bao G, et al. Chronic cutaneous lupus erythematosus: depression burden and associated factors. Am J Clin Dermatol. 2019;20:465-475. doi:10.1007/s40257-019-00429-7
What are the reasons to use the Gail risk assessment model?
Text copyright DenseBreast-info.org.
Answer
B. The Gail risk model1-3 is used to predict 5-year and lifetime risks of developing invasive breast cancer, and to identify women who may benefit from risk-reducing medications such as tamoxifen. The Gail model should not be used to determine risk for purposes of screening magnetic resonance imaging (MRI)4 (or genetic testing).
Breast cancer risk models are used to stratify patients into risk categories to facilitate personalized screening and surveillance plans for clinical management. Several breast cancer risk assessment tools have been developed that include different combinations of known risk factors and are used for the following purposes:
1. To identify women who may benefit from risk-reducing medications. The Gail model is used to determine risk for purposes of advising on use of risk-reducing medications. Any woman with a 5-year risk ≥1.67% by the Gail model may be considered for treatment with tamoxifen (pre or postmenopausal), raloxifene (postmenopausal), or aromatase inhibitors (postmenopausal).5
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 study,6 women at increased risk for breast cancer were defined as follows:
- age 35 to 59 years with at least a 1.66% 5-year risk for developing breast cancer by the Gail model
- personal history of lobular carcinoma in situ (LCIS)
- age over 60 years.
More than 13,000 such women were randomly assigned to receive tamoxifen or placebo daily for 5 years. Tamoxifen reduced the risk of invasive breast cancer by 49% and reduced the risk of noninvasive cancer by 50% compared with placebo. The reduced risk of breast cancer was only seen for estrogen-receptor–expressing tumors. There was a 2.5-fold increase in risk of endometrial cancer in women taking tamoxifen and a decrease in hip and spine fracture risk. Blood clots causing stroke and deep vein thrombosis are increased in women taking tamoxifen.7,8
2. To identify women who may carry a pathogenic mutation in BRCA1 or BRCA2. Some models (eg, Tyrer-Cuzick [IBIS],9 Penn II,10 BOADICEA,11 and BRCAPRO12) estimate the probability of a BRCA1/2 mutation; however, most testing guidelines are now criterion based (eg, National Comprehensive Cancer Network [NCCN]) as opposed to probability based. In practical terms, clinical decision making around genetic testing is rarely based on a priori probabilities.
3. To identify women who meet criteria for high-risk screening MRI. Current American Cancer Society (ACS) guidelines4 recommend annual screening MRI, in addition to mammography, beginning by age 25 to 30 in women who have a lifetime risk of breast cancer ≥20%. Any of the models used to predict risk of a pathogenic mutation (Tyrer-Cuzick [IBIS], Penn II, BOADICEA, BRCAPRO),or the Claus model,13 but not the Gail model, can be used to estimate lifetime risk for purposes of screening MRI guidelines. The ACS and NCCN guidelines specifically recommend against using the Gail model to determine risk for purposes of MRI screening or risk of pathogenic mutation, as it does not include detailed family history such as age at diagnosis or second-degree relatives.
ACS and NCCN guidelines also recommend annual screening MRI beginning by age 25, with the addition of mammography beginning at age 30, in women who are known to carry pathogenic mutations in BRCA1 or BRCA2 (unless the woman has had bilateral mastectomy), and in women who are first-degree relatives of known mutation carriers but who are themselves untested.14
Women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome, Bannayan-Riley-Ruvalcaba syndrome, hereditary diffuse gastric cancer, Peutz-Jeghers syndrome, Cowden syndrome, Neurofibromatosis type 1, or Fanconi anemia) are also recommended for annual screening MRI beginning between ages 20-35, depending on the mutation.14 Women with known pathogenic mutations in ATM, CHEK2, or NBN should consider annual MRI starting at age 40 or 5-10 years before the earliest known breast cancer in the family (whichever comes first).
Finally, women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,4,15,16 with risk similar in magnitude to pathogenic BRCA1 or BRCA2 carriers. These women are also recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
Currently the Tyrer-Cuzick Model (IBIS) version 817 and the Breast Cancer Surveillance Consortium (BCSC) models18 include breast density in risk calculations; the Gail, Penn II, and Claus models do not include breast density.
Adding polygenic risk scores based on single nucleotide polymorphisms to traditional comprehensive risk models such as the Tyrer-Cuzick model has been shown to improve model performance.19 In addition, artificial intelligence is being used to identify textural and other findings beyond breast density on mammograms that predict increased risk. Such information, which is complementary to the Tyrer-Cuzick model (v.8),20 has more accurately identified high-risk patients than the Tyrer-Cuzick v8 risk model and prior deep learning models.21
In a study from the Karolinska Institute, a model that included computer-aided detection of microcalcifications and masses in addition to other traditional risk factors (including breast density) successfully identified women who would develop interval or advanced cancer in the 2 years after a normal mammogram and improved short-term (2-to-3-year) risk assessment over TyrerCuzick (v.7) or Gail models.22 This model proved more accurate than traditional risk models and can augment genetic/family history to help identify women who should and, importantly, who should not, have supplemental screening after 2D mammography. Risk models that include detailed family history should be used rather than the Gail model to identify women who meet high risk criteria for MRI screening. Research also supports the benefits of MRI in women with dense breasts who are not otherwise considered “high risk,” and while not widely available, lower cost, abbreviated MRI protocols have been validated for all women with dense breasts.23 For more details on risk models, including a risk models table with live links to commonly used breast cancer risk assessment tools, visit https://densebreast-info .org/for-providers/risk-model-tutorial/. ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- The Breast Cancer Risk Assessment Tool. https://bcrisktool .cancer.gov/calculator.html. Accessed March 15, 2022.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
- Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782-1792.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Network NCC. Breast Cancer Risk Reduction (Version 1.2022). https://www.nccn.org/professionals/physician_gls /pdf/breast_risk.pdf. Published 2022. Accessed February 8, 2022.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen
treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442-4449. - Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Panchal SM, Ennis M, Canon S, et al. Selecting a BRCA risk assessment model for use in a familial cancer clinic. BMC Med Genet. 2008;9:116.
- Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580-1590.
- Berry DA, Iversen ES, Jr., Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes.
J Clin Oncol. 2002;20:2701-2712. - Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643-651.
- Network NCC. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022). https:// www.nccn.org/professionals/physician_gls/pdf/genetics _bop.pdf. Accessed February 9, 2022.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408-414.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301: 404-414.
- Brentnall AR, Cuzick J, Buist DSM, et al. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4:e180174.
- Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337-347.
- Brentnall AR, van Veen EM, Harkness EF, et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int J Cancer. 2020;146:2122-2129.
- Yala A, Lehman C, Schuster T, et al. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292:60-66.
- Yala A, Mikhael PG, Strand F, et al. Toward robust mammography-based models for breast cancer risk. Sci Transl Med. 2021;13.
- Eriksson M, Czene K, Pawitan Y, et al. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19:29.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756.
Text copyright DenseBreast-info.org.
Answer
B. The Gail risk model1-3 is used to predict 5-year and lifetime risks of developing invasive breast cancer, and to identify women who may benefit from risk-reducing medications such as tamoxifen. The Gail model should not be used to determine risk for purposes of screening magnetic resonance imaging (MRI)4 (or genetic testing).
Breast cancer risk models are used to stratify patients into risk categories to facilitate personalized screening and surveillance plans for clinical management. Several breast cancer risk assessment tools have been developed that include different combinations of known risk factors and are used for the following purposes:
1. To identify women who may benefit from risk-reducing medications. The Gail model is used to determine risk for purposes of advising on use of risk-reducing medications. Any woman with a 5-year risk ≥1.67% by the Gail model may be considered for treatment with tamoxifen (pre or postmenopausal), raloxifene (postmenopausal), or aromatase inhibitors (postmenopausal).5
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 study,6 women at increased risk for breast cancer were defined as follows:
- age 35 to 59 years with at least a 1.66% 5-year risk for developing breast cancer by the Gail model
- personal history of lobular carcinoma in situ (LCIS)
- age over 60 years.
More than 13,000 such women were randomly assigned to receive tamoxifen or placebo daily for 5 years. Tamoxifen reduced the risk of invasive breast cancer by 49% and reduced the risk of noninvasive cancer by 50% compared with placebo. The reduced risk of breast cancer was only seen for estrogen-receptor–expressing tumors. There was a 2.5-fold increase in risk of endometrial cancer in women taking tamoxifen and a decrease in hip and spine fracture risk. Blood clots causing stroke and deep vein thrombosis are increased in women taking tamoxifen.7,8
2. To identify women who may carry a pathogenic mutation in BRCA1 or BRCA2. Some models (eg, Tyrer-Cuzick [IBIS],9 Penn II,10 BOADICEA,11 and BRCAPRO12) estimate the probability of a BRCA1/2 mutation; however, most testing guidelines are now criterion based (eg, National Comprehensive Cancer Network [NCCN]) as opposed to probability based. In practical terms, clinical decision making around genetic testing is rarely based on a priori probabilities.
3. To identify women who meet criteria for high-risk screening MRI. Current American Cancer Society (ACS) guidelines4 recommend annual screening MRI, in addition to mammography, beginning by age 25 to 30 in women who have a lifetime risk of breast cancer ≥20%. Any of the models used to predict risk of a pathogenic mutation (Tyrer-Cuzick [IBIS], Penn II, BOADICEA, BRCAPRO),or the Claus model,13 but not the Gail model, can be used to estimate lifetime risk for purposes of screening MRI guidelines. The ACS and NCCN guidelines specifically recommend against using the Gail model to determine risk for purposes of MRI screening or risk of pathogenic mutation, as it does not include detailed family history such as age at diagnosis or second-degree relatives.
ACS and NCCN guidelines also recommend annual screening MRI beginning by age 25, with the addition of mammography beginning at age 30, in women who are known to carry pathogenic mutations in BRCA1 or BRCA2 (unless the woman has had bilateral mastectomy), and in women who are first-degree relatives of known mutation carriers but who are themselves untested.14
Women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome, Bannayan-Riley-Ruvalcaba syndrome, hereditary diffuse gastric cancer, Peutz-Jeghers syndrome, Cowden syndrome, Neurofibromatosis type 1, or Fanconi anemia) are also recommended for annual screening MRI beginning between ages 20-35, depending on the mutation.14 Women with known pathogenic mutations in ATM, CHEK2, or NBN should consider annual MRI starting at age 40 or 5-10 years before the earliest known breast cancer in the family (whichever comes first).
Finally, women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,4,15,16 with risk similar in magnitude to pathogenic BRCA1 or BRCA2 carriers. These women are also recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
Currently the Tyrer-Cuzick Model (IBIS) version 817 and the Breast Cancer Surveillance Consortium (BCSC) models18 include breast density in risk calculations; the Gail, Penn II, and Claus models do not include breast density.
Adding polygenic risk scores based on single nucleotide polymorphisms to traditional comprehensive risk models such as the Tyrer-Cuzick model has been shown to improve model performance.19 In addition, artificial intelligence is being used to identify textural and other findings beyond breast density on mammograms that predict increased risk. Such information, which is complementary to the Tyrer-Cuzick model (v.8),20 has more accurately identified high-risk patients than the Tyrer-Cuzick v8 risk model and prior deep learning models.21
In a study from the Karolinska Institute, a model that included computer-aided detection of microcalcifications and masses in addition to other traditional risk factors (including breast density) successfully identified women who would develop interval or advanced cancer in the 2 years after a normal mammogram and improved short-term (2-to-3-year) risk assessment over TyrerCuzick (v.7) or Gail models.22 This model proved more accurate than traditional risk models and can augment genetic/family history to help identify women who should and, importantly, who should not, have supplemental screening after 2D mammography. Risk models that include detailed family history should be used rather than the Gail model to identify women who meet high risk criteria for MRI screening. Research also supports the benefits of MRI in women with dense breasts who are not otherwise considered “high risk,” and while not widely available, lower cost, abbreviated MRI protocols have been validated for all women with dense breasts.23 For more details on risk models, including a risk models table with live links to commonly used breast cancer risk assessment tools, visit https://densebreast-info .org/for-providers/risk-model-tutorial/. ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
B. The Gail risk model1-3 is used to predict 5-year and lifetime risks of developing invasive breast cancer, and to identify women who may benefit from risk-reducing medications such as tamoxifen. The Gail model should not be used to determine risk for purposes of screening magnetic resonance imaging (MRI)4 (or genetic testing).
Breast cancer risk models are used to stratify patients into risk categories to facilitate personalized screening and surveillance plans for clinical management. Several breast cancer risk assessment tools have been developed that include different combinations of known risk factors and are used for the following purposes:
1. To identify women who may benefit from risk-reducing medications. The Gail model is used to determine risk for purposes of advising on use of risk-reducing medications. Any woman with a 5-year risk ≥1.67% by the Gail model may be considered for treatment with tamoxifen (pre or postmenopausal), raloxifene (postmenopausal), or aromatase inhibitors (postmenopausal).5
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 study,6 women at increased risk for breast cancer were defined as follows:
- age 35 to 59 years with at least a 1.66% 5-year risk for developing breast cancer by the Gail model
- personal history of lobular carcinoma in situ (LCIS)
- age over 60 years.
More than 13,000 such women were randomly assigned to receive tamoxifen or placebo daily for 5 years. Tamoxifen reduced the risk of invasive breast cancer by 49% and reduced the risk of noninvasive cancer by 50% compared with placebo. The reduced risk of breast cancer was only seen for estrogen-receptor–expressing tumors. There was a 2.5-fold increase in risk of endometrial cancer in women taking tamoxifen and a decrease in hip and spine fracture risk. Blood clots causing stroke and deep vein thrombosis are increased in women taking tamoxifen.7,8
2. To identify women who may carry a pathogenic mutation in BRCA1 or BRCA2. Some models (eg, Tyrer-Cuzick [IBIS],9 Penn II,10 BOADICEA,11 and BRCAPRO12) estimate the probability of a BRCA1/2 mutation; however, most testing guidelines are now criterion based (eg, National Comprehensive Cancer Network [NCCN]) as opposed to probability based. In practical terms, clinical decision making around genetic testing is rarely based on a priori probabilities.
3. To identify women who meet criteria for high-risk screening MRI. Current American Cancer Society (ACS) guidelines4 recommend annual screening MRI, in addition to mammography, beginning by age 25 to 30 in women who have a lifetime risk of breast cancer ≥20%. Any of the models used to predict risk of a pathogenic mutation (Tyrer-Cuzick [IBIS], Penn II, BOADICEA, BRCAPRO),or the Claus model,13 but not the Gail model, can be used to estimate lifetime risk for purposes of screening MRI guidelines. The ACS and NCCN guidelines specifically recommend against using the Gail model to determine risk for purposes of MRI screening or risk of pathogenic mutation, as it does not include detailed family history such as age at diagnosis or second-degree relatives.
ACS and NCCN guidelines also recommend annual screening MRI beginning by age 25, with the addition of mammography beginning at age 30, in women who are known to carry pathogenic mutations in BRCA1 or BRCA2 (unless the woman has had bilateral mastectomy), and in women who are first-degree relatives of known mutation carriers but who are themselves untested.14
Women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing mutations (such as those associated with Li-Fraumeni syndrome, Bannayan-Riley-Ruvalcaba syndrome, hereditary diffuse gastric cancer, Peutz-Jeghers syndrome, Cowden syndrome, Neurofibromatosis type 1, or Fanconi anemia) are also recommended for annual screening MRI beginning between ages 20-35, depending on the mutation.14 Women with known pathogenic mutations in ATM, CHEK2, or NBN should consider annual MRI starting at age 40 or 5-10 years before the earliest known breast cancer in the family (whichever comes first).
Finally, women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer,4,15,16 with risk similar in magnitude to pathogenic BRCA1 or BRCA2 carriers. These women are also recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
Currently the Tyrer-Cuzick Model (IBIS) version 817 and the Breast Cancer Surveillance Consortium (BCSC) models18 include breast density in risk calculations; the Gail, Penn II, and Claus models do not include breast density.
Adding polygenic risk scores based on single nucleotide polymorphisms to traditional comprehensive risk models such as the Tyrer-Cuzick model has been shown to improve model performance.19 In addition, artificial intelligence is being used to identify textural and other findings beyond breast density on mammograms that predict increased risk. Such information, which is complementary to the Tyrer-Cuzick model (v.8),20 has more accurately identified high-risk patients than the Tyrer-Cuzick v8 risk model and prior deep learning models.21
In a study from the Karolinska Institute, a model that included computer-aided detection of microcalcifications and masses in addition to other traditional risk factors (including breast density) successfully identified women who would develop interval or advanced cancer in the 2 years after a normal mammogram and improved short-term (2-to-3-year) risk assessment over TyrerCuzick (v.7) or Gail models.22 This model proved more accurate than traditional risk models and can augment genetic/family history to help identify women who should and, importantly, who should not, have supplemental screening after 2D mammography. Risk models that include detailed family history should be used rather than the Gail model to identify women who meet high risk criteria for MRI screening. Research also supports the benefits of MRI in women with dense breasts who are not otherwise considered “high risk,” and while not widely available, lower cost, abbreviated MRI protocols have been validated for all women with dense breasts.23 For more details on risk models, including a risk models table with live links to commonly used breast cancer risk assessment tools, visit https://densebreast-info .org/for-providers/risk-model-tutorial/. ●
For more information, visit medically sourced DenseBreastinfo.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- The Breast Cancer Risk Assessment Tool. https://bcrisktool .cancer.gov/calculator.html. Accessed March 15, 2022.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
- Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782-1792.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Network NCC. Breast Cancer Risk Reduction (Version 1.2022). https://www.nccn.org/professionals/physician_gls /pdf/breast_risk.pdf. Published 2022. Accessed February 8, 2022.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen
treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442-4449. - Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Panchal SM, Ennis M, Canon S, et al. Selecting a BRCA risk assessment model for use in a familial cancer clinic. BMC Med Genet. 2008;9:116.
- Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580-1590.
- Berry DA, Iversen ES, Jr., Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes.
J Clin Oncol. 2002;20:2701-2712. - Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643-651.
- Network NCC. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022). https:// www.nccn.org/professionals/physician_gls/pdf/genetics _bop.pdf. Accessed February 9, 2022.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408-414.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301: 404-414.
- Brentnall AR, Cuzick J, Buist DSM, et al. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4:e180174.
- Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337-347.
- Brentnall AR, van Veen EM, Harkness EF, et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int J Cancer. 2020;146:2122-2129.
- Yala A, Lehman C, Schuster T, et al. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292:60-66.
- Yala A, Mikhael PG, Strand F, et al. Toward robust mammography-based models for breast cancer risk. Sci Transl Med. 2021;13.
- Eriksson M, Czene K, Pawitan Y, et al. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19:29.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756.
- The Breast Cancer Risk Assessment Tool. https://bcrisktool .cancer.gov/calculator.html. Accessed March 15, 2022.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
- Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782-1792.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Network NCC. Breast Cancer Risk Reduction (Version 1.2022). https://www.nccn.org/professionals/physician_gls /pdf/breast_risk.pdf. Published 2022. Accessed February 8, 2022.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen
treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442-4449. - Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Panchal SM, Ennis M, Canon S, et al. Selecting a BRCA risk assessment model for use in a familial cancer clinic. BMC Med Genet. 2008;9:116.
- Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580-1590.
- Berry DA, Iversen ES, Jr., Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes.
J Clin Oncol. 2002;20:2701-2712. - Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643-651.
- Network NCC. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022). https:// www.nccn.org/professionals/physician_gls/pdf/genetics _bop.pdf. Accessed February 9, 2022.
- Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408-414.
- Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301: 404-414.
- Brentnall AR, Cuzick J, Buist DSM, et al. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4:e180174.
- Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337-347.
- Brentnall AR, van Veen EM, Harkness EF, et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int J Cancer. 2020;146:2122-2129.
- Yala A, Lehman C, Schuster T, et al. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292:60-66.
- Yala A, Mikhael PG, Strand F, et al. Toward robust mammography-based models for breast cancer risk. Sci Transl Med. 2021;13.
- Eriksson M, Czene K, Pawitan Y, et al. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19:29.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756.
Women in rheumatology: A look back, a look forward
Jean Liew, MD, recalls the long list of women mentors who have guided her career in rheumatology.
It started during her residency, when Jennifer Barton, MD, at Oregon Health & Science University, Portland, exposed her to new ways of conducting clinical research on patient outcomes.
In fellowship, she met Lianne Gensler, MD, a leader in axial spondyloarthritis, at the annual meeting of the American College of Rheumatology. Through Dr. Gensler’s mentorship and sponsorship, she was introduced to Maureen Dubreuil, MD, at Boston University, whose research focuses on pharmacoepidemiologic approaches using large databases.
Dr. Liew currently practices rheumatology under the leadership of Tuhina Neogi, MD, a world-renowned expert in osteoarthritis and gout. “She’s my research mentor,” Dr. Liew, an assistant professor of medicine at Boston University, said in an interview.
Her academic timeline reflects the powerful network and influence of women rheumatologists, who represent half of the adult rheumatology workforce in the United States. “In the research arena, many experts are women and they serve as role models and mentors to many,” Dr. Liew said.
But there’s more work to do, she and others acknowledged.
Rheumatology faces ongoing workforce shortages while struggling with a gender gap that’s closing but not as quickly as many women rheumatologists would like to see.
The gap persists, despite overall gains in the field of medicine, Vaneet Sandhu, MD, a rheumatologist with Loma Linda (Calif.) University, said in an interview. Women have exceeded men as enrollees in medical colleges, reported the Association of American Medical Colleges. And yet, “our colleagues reported last year that, in academic rheumatology, women are less likely to be full or associate professors than men,” she said.
The odds of being a fellowship program director or division director is similar in both males and females. “So, we’ve had some gains, but there’s always room for more,” Dr. Sandhu said.
Too few physicians
The next 10 years forecasts a dearth in American physicians.
AAMC projects a shortage of 124,000 doctors in the United States by 2034. Following on a similar trajectory, the ACR in 2015 anticipated a 25% drop in the supply of rheumatology clinical providers by 2030, with demand exceeding supply by more than 4,100 clinical employees.
The ACR’s workforce study projected that more women would come into rheumatology, noted Marcy Bolster, MD, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston. Women make up at least 50% of the workforce and 66% of fellows If these numbers hold, “we’ll definitely see an increase in the percent of women in the workforce” moving forward, Dr. Bolster said in an interview.
Women have helped the shortage to a great extent, said Nilanjana Bose, MD, a rheumatologist at Lonestar Rheumatology, Houston.
The work-life balance that rheumatology offers, combined with its focus on the cognitive part of internal medicine, explains why the field has attracted so many women. Rheumatology provides flexible work options. Women “get to teach or do rounds in the hospital or have a private practice where you’re mostly outpatient with some hospital work,” Dr. Bose said in an interview.
With anticipated shortages looming over the next decade, the profession needs to be cognizant of the different demands women face in their careers and how it can accommodate the workforce to meet the needs of its providers and maintain access for patients, Dr. Bolster said.
There are many innovative ways to match the demand for access. One thought is to create shared positions. Instead of employing four full-time physicians and one person part time, have two people who are working part time, Dr. Bolster suggested. “It is also important to not only expand our workforce with advanced practice providers, but to ensure their retention in the rheumatology workforce, to improve access to care for those with rheumatic diseases.”
Increasing the number of residency positions is another step toward addressing the shortage, Dr. Sandhu offered.
Women rheumatologists should make their voices heard by contacting members of Congress to support legislation that advocates for workforce shortage solutions, “in addition to generally supporting women’s rights and growth in the workplace,” she said.
The gender divide continues
Rosalind Ramsey-Goldman, MD, DrPH, remembers being the only woman in a group of five during her fellowship in the mid-1980s. Few women role models existed within the ACR, especially those in academic careers. “Now, most fellowships have more than 50% women, reflecting the number of women going to medical school,” said Dr. Ramsey-Goldman, Gallagher Research Professor in Rheumatology at Northwestern University and Northwestern Medicine, Chicago.
As more women enter the profession, women rheumatologists in academic rheumatology have started to outpace men in recent years. Some research suggests they’ve made headway in gaining leadership spots at institutions.
One recent paper, a cross-sectional national study of more than 6,100 rheumatologists, found that women had similar odds of attaining fellowship program or division director positions as men. As directors of training programs, women in rheumatology “instill this collaborative and growth mindset that encourages learners to self-reflect and work as a team,” Dr. Sandhu said.
Women bring a different perspective to training, and how curriculum works, Dr. Bose said. Studies have shown that women tend to be more empathic. They ask more questions. “That’s not to say men aren’t good. Women just have an inborn ability for connecting,” and this perspective helps to enrich the educational experience for trainees.
Women who lead training programs are also attuned to realities that female trainees confront, such as dealing with the challenges of achieving the best possible education while also raising a family, noted Graciela S. Alarcón, MD, MPH, who holds emeritus positions at the University of Alabama at Birmingham and the Universidad Peruana Cayetano Heredia in Lima, Peru.
“These program directors cultivate the ability to relate to women trainees in a very personal manner, supporting them in their efforts to achieve a balance between their training demands and their family/personal responsibilities,” she said.
Other research suggests the gender gap hasn’t gone away. Women continue to have lower odds of holding a higher-level professorship, receiving a federal grant, or speaking at academic conferences. They are also less likely to serve as first authors on rheumatology guidelines or recommendations.
Some studies suggest that women see fewer patients and earn less than their male counterparts. At peak difference, men can earn up to $100,000+ more than women. “My own impression is that it takes more efforts for women to reach the same level of recognition than men, and although overt discrimination is rare nowadays, subtle discrimination still occurs,” according to Dr. Alarcón.
Over a lifetime, female physicians can expect to earn less than their male counterparts, with clear implications for different retirement income levels, she said.
Fixing a leaky academic pipeline
The reality is the academic pipeline, and especially the physician-scientist pipeline, “continues to be leaky,” Dr. Liew said. “We know that caregivers to young children have larger barriers to surmount in academics and in research, and that there is a gender disparity present.” The toll of academic medicine on early career women who are parents is especially pronounced. While the pandemic has intensified this problem, it was around pre-COVID, she added.
Women who start in academia as academic clinicians or clinician researchers aren’t always able to meet their goals for promotion within the appropriate time frame. This is because of inequities in the system and lack of support related to maternity leave, childcare, and other issues. As a result, they leave academia and go into private practice or industry, Dr. Liew said.
The ACR in its 2015 survey projected that more women would be seeking part-time positions.
The good news is many academic institutions are taking a more equitable view about different career paths, offering equal parental leave to both men and women, Dr. Bolster noted. “It is essential that workforce planning encompasses the changing responsibilities within families and account for more parental leave by both men and women.” If certain projections come true, with 50% of the profession retiring between 2015 and 2030, combined with more men and women working part time, “it is requisite that workforce strategies plan for this.”
When Dr. Ramsey-Goldman was a trainee and junior faculty, there were no formal maternity leave policies.
Now, this benefit is available, she said. In another critical change, the ACR has made childcare services and a lactation room available for young mothers during its annual meeting. “Virtual meetings afford further ways to interact with colleagues,” she added.
Whether women choose to stay in academia or go into clinical practice is a very personal decision. “But it is also fair that, in some programs, training directors and faculty members can encourage trainees toward academia and its fascinating research possibilities,” Dr. Alarcón offered.
Making gains in research
Women are increasingly driving groundbreaking rheumatology research at all levels, Dr. Sandhu said. “And women empower women. Not infrequently, our female leaders, veterans in rheumatology research, seek younger female rheumatologists to help them grow in their niches. This has been one of the most beautiful things of the sisterhood in rheumatology that I have been blessed to be part of.”
In pediatric rheumatology, young female researchers are leading global research efforts. Some standouts include Kate Webb, MD, a pediatric rheumatologist in Cape Town, South Africa, and scientist who has worked on multisystem inflammatory syndrome during the pandemic. Sheila Angeles-Han, MD, who works on uveitis in juvenile idiopathic arthritis, had a role in recent ACR guidelines. Laura Lewandowski, MD, has also contributed to global rheumatology efforts, especially in low- and middle-income countries, Dr. Liew said.
The 2021 ACR annual meeting highlighted the research efforts of women rheumatologists from around the world. A global rheumatology summit at the meeting featured many women voices, including Dzifa Dey, MD, from Ghana, who received the ACR Distinguished International Rheumatology Professional Award. Ashira Blazer, MD, and Irene Blanco, MD, have spearheaded the ACR’s diversity, equity, and inclusion initiatives.
Women researchers have many opportunities to study rheumatologic diseases that disproportionately affect women, Dr. Alarcón said.
Lupus, for example, affects women in a much higher proportion than men (90% vs. 10%). This may be an attractive target for the best and brightest among future women researchers, Dr. Alarcón suggested. “It is a fact that publications related to lupus in leading internal medicine and rheumatology journals often include women either as first or senior authors. In that context, it can be said that several advances in the study of lupus worldwide can be attributed to women.”
This applies to disparities in social determinants of health that account for extremely complex outcomes in lupus among women of color, compared with White women, in addition to the costs associated with the disease and its impact on morbidity, mortality, and quality of life.
Women rheumatologists have advanced the work in reproductive management of rheumatic diseases, including a recent ACR-endorsed publication that provides formal guidance on managing reproductive health in women with rheumatic disease, Dr. Sandhu said. “One thing is clear: Without women, the work on reproductive diseases in rheumatology to date would not likely be where it is.”
Dr. Ramsey-Goldman added that “this critical work will not only set the stage for clinical care of both women and men regarding their reproductive health but will also inform education strategies for trainees and future research activities, and help direct policy regarding access to care, medication development, and costs of treatment.”
Obtaining grant funding to support salaries and researcher endeavors remains a challenge, Dr. Liew said. “It takes working evenings, weekends, and holidays to meet those goals within a set time frame. So you can see why a female faculty member with children might be disadvantaged, compared to a male counterpart without children.”
Competition for grant funding remains fierce as budgets become tighter, she added.
“We will lose a lot more brilliant and compassionate rheumatologists (clinicians, physician-scientists, and scientists alike) if we do not think of ways to make things more equitable or do not acknowledge the privileges that support some to continued career successes and leave others behind,” Dr. Liew said.
Women who choose a research field should seek out mentor and financial support that will allow them enough protected time to balance out research with other clinical activities, such as teaching and patient care, Dr. Alarcón said.
Training directors, mentors, and faculty should prioritize the needs of current and future women researchers, she said. “The guidance provided to young female trainees toward a successful research career is a formidable challenge that may provide, in turn, enormous satisfaction. There are established avenues to seek funding as new investigators.”
Progress in diversity
Rheumatology as a field is attracting more candidates and all races and genders, Dr. Bose said. “I think in the coming years we will see more and more women from minorities being incorporated into the rheumatology workforce.”
Others would like to see further improvements in diversity and attracting women from historically excluded backgrounds. Patients will benefit from rheumatologists who are able to connect with them through shared languages, cultures, and other life experiences, Dr. Liew said. “It is imperative that we work on recruitment, mentorship, and retention in this regard.”
While the representation of women of color is still inadequate, there has been some progress, Dr. Sandhu said. The number of female Hispanic, Latinx, and Black or African American graduates from medical school has seen a steady rise since 2017. And, AAMC has established task forces such as the Women of Color Initiative to identify strategies for furthering the careers of women of color in academic medicine.
“There’s still a lot of room to grow. I am, however, proud to say we will finally have a woman of color as the president of ACR in 2023,” said Dr. Sandhu, referring to Deborah Dyett Desir, MD.
Dr. Desir discussed the importance of diversifying the ACR in a recent interview.
All rheumatologists know that there is a place for them in the ACR, she stressed. “The demographics of our membership should reflect that of our population.”
As growth in diverse representation occurs, so will recruitment, retention, and a greater awareness and distribution of knowledge and means to address implicit biases and microaggressions, Dr. Sandhu said. “We will see a greater quality of health care, where patients may feel more connected to someone they can identify with.”
Looking ahead
Dr. Alarcón expects women to continue to play a major role in rheumatology, not just in research, education, and patient care but in leadership of academic societies and professional organizations.
“Women in rheumatology have come a long way – a piece of history that I have been fortunate to witness from my beginnings in the early 1970s. We have, I think, paved the way for the next generations of leaders in our beloved specialty field.”
Dr. Bolster is a member of the ACR board of directors and board liaison of the ACR Workforce Solutions Committee. Dr. Ramsey-Goldman has been a GlaxoSmithKline consultant for lupus studies, a consultant and site investigator with Exagen Diagnostics for lupus biomarker studies, and a site investigator for Xencor and Horizon Pharma lupus trials. Dr. Sandhu serves on the ACR’s Committee on Rheumatology Training and Workforce Issues.
Related article
Pioneer days of rheumatology: One veteran looks back
Patricia Woo, CBE, FMedSci, FRCP, has seen it all.
As a member of the British Rheumatology Society and fellow of the Royal College of Physicians, she presented the case for and obtained official training approval for pediatric rheumatology in the 1990s. She also set the wheels in motion to form the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology European Society.
Now 74, Dr. Woo remembers the discrimination she faced in the 1970s. “I was told I couldn’t become an investigator or consultant if I were to marry or have children.” Around the same time, she found out a male clinician researcher didn’t want to work with her, not because of her qualifications, but because she was a woman.
That wouldn’t happen now with all the antidiscrimination laws in place, noted Dr. Woo, an emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. Looking at the advances made by women in rheumatology, “there’s a major difference between 3 decades ago and today. If anyone discriminates today, they are called out.”
As the founding president of the Paediatric Rheumatology European Society, Dr. Woo is one of many early trailblazers who weathered many changes and made gains in the profession.
It’s important to recognize the work of Barbara Ansell, MD, the founder of pediatric rheumatology in the Canadian Red Cross Memorial Hospital, said Dr. Woo. Back in the 1960s, this wasn’t even a subspecialty. “Sick kids in general were taken either to pediatricians who didn’t know much about undescribed rheumatological conditions, and rheumatologists who didn’t know or have facilities for pediatric care.”
Dr. Ansell started this work, and Dr. Woo took over when she retired. With her colleagues, she set up a syllabus for pediatric rheumatology to formalize training for all junior doctors. This established a model of multidisciplinary clinical care and research. “Over the years, more women doctors have been attracted to pediatric rheumatology and have done well,” she said.
The rise of female leaders in rheumatology over the past few decades has been exponential, she continued. Women have become presidents of rheumatologic societies. Some established themselves as leaders in specific disciplines.
Carol Black, MD, from the United Kingdom is renowned for her international collaborative work in scleroderma research and clinical care. Patience White, MD in Washington, D.C., started research on the process of transitioning from childhood to adolescent to adult clinical care, a discipline that now has a strong international presence, Dr. Woo said.
The European Alliance of Associations for Rheumatology, which created a task force on gender equity in academic rheumatology, is evolving, she continued. The Academy of Medical Sciences in the United Kingdom also has active gender equality and mentoring programs, including a program to boost the careers of all researchers.
It’s also much easier now for women to become lead authors on papers since many are heads of lab or clinical services, Dr. Woo continued. “I don’t think there’s much discrimination if you’re a good clinician, and/or a good scientist. If women do their work well, they get the appropriate acknowledgment.”
Jean Liew, MD, recalls the long list of women mentors who have guided her career in rheumatology.
It started during her residency, when Jennifer Barton, MD, at Oregon Health & Science University, Portland, exposed her to new ways of conducting clinical research on patient outcomes.
In fellowship, she met Lianne Gensler, MD, a leader in axial spondyloarthritis, at the annual meeting of the American College of Rheumatology. Through Dr. Gensler’s mentorship and sponsorship, she was introduced to Maureen Dubreuil, MD, at Boston University, whose research focuses on pharmacoepidemiologic approaches using large databases.
Dr. Liew currently practices rheumatology under the leadership of Tuhina Neogi, MD, a world-renowned expert in osteoarthritis and gout. “She’s my research mentor,” Dr. Liew, an assistant professor of medicine at Boston University, said in an interview.
Her academic timeline reflects the powerful network and influence of women rheumatologists, who represent half of the adult rheumatology workforce in the United States. “In the research arena, many experts are women and they serve as role models and mentors to many,” Dr. Liew said.
But there’s more work to do, she and others acknowledged.
Rheumatology faces ongoing workforce shortages while struggling with a gender gap that’s closing but not as quickly as many women rheumatologists would like to see.
The gap persists, despite overall gains in the field of medicine, Vaneet Sandhu, MD, a rheumatologist with Loma Linda (Calif.) University, said in an interview. Women have exceeded men as enrollees in medical colleges, reported the Association of American Medical Colleges. And yet, “our colleagues reported last year that, in academic rheumatology, women are less likely to be full or associate professors than men,” she said.
The odds of being a fellowship program director or division director is similar in both males and females. “So, we’ve had some gains, but there’s always room for more,” Dr. Sandhu said.
Too few physicians
The next 10 years forecasts a dearth in American physicians.
AAMC projects a shortage of 124,000 doctors in the United States by 2034. Following on a similar trajectory, the ACR in 2015 anticipated a 25% drop in the supply of rheumatology clinical providers by 2030, with demand exceeding supply by more than 4,100 clinical employees.
The ACR’s workforce study projected that more women would come into rheumatology, noted Marcy Bolster, MD, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston. Women make up at least 50% of the workforce and 66% of fellows If these numbers hold, “we’ll definitely see an increase in the percent of women in the workforce” moving forward, Dr. Bolster said in an interview.
Women have helped the shortage to a great extent, said Nilanjana Bose, MD, a rheumatologist at Lonestar Rheumatology, Houston.
The work-life balance that rheumatology offers, combined with its focus on the cognitive part of internal medicine, explains why the field has attracted so many women. Rheumatology provides flexible work options. Women “get to teach or do rounds in the hospital or have a private practice where you’re mostly outpatient with some hospital work,” Dr. Bose said in an interview.
With anticipated shortages looming over the next decade, the profession needs to be cognizant of the different demands women face in their careers and how it can accommodate the workforce to meet the needs of its providers and maintain access for patients, Dr. Bolster said.
There are many innovative ways to match the demand for access. One thought is to create shared positions. Instead of employing four full-time physicians and one person part time, have two people who are working part time, Dr. Bolster suggested. “It is also important to not only expand our workforce with advanced practice providers, but to ensure their retention in the rheumatology workforce, to improve access to care for those with rheumatic diseases.”
Increasing the number of residency positions is another step toward addressing the shortage, Dr. Sandhu offered.
Women rheumatologists should make their voices heard by contacting members of Congress to support legislation that advocates for workforce shortage solutions, “in addition to generally supporting women’s rights and growth in the workplace,” she said.
The gender divide continues
Rosalind Ramsey-Goldman, MD, DrPH, remembers being the only woman in a group of five during her fellowship in the mid-1980s. Few women role models existed within the ACR, especially those in academic careers. “Now, most fellowships have more than 50% women, reflecting the number of women going to medical school,” said Dr. Ramsey-Goldman, Gallagher Research Professor in Rheumatology at Northwestern University and Northwestern Medicine, Chicago.
As more women enter the profession, women rheumatologists in academic rheumatology have started to outpace men in recent years. Some research suggests they’ve made headway in gaining leadership spots at institutions.
One recent paper, a cross-sectional national study of more than 6,100 rheumatologists, found that women had similar odds of attaining fellowship program or division director positions as men. As directors of training programs, women in rheumatology “instill this collaborative and growth mindset that encourages learners to self-reflect and work as a team,” Dr. Sandhu said.
Women bring a different perspective to training, and how curriculum works, Dr. Bose said. Studies have shown that women tend to be more empathic. They ask more questions. “That’s not to say men aren’t good. Women just have an inborn ability for connecting,” and this perspective helps to enrich the educational experience for trainees.
Women who lead training programs are also attuned to realities that female trainees confront, such as dealing with the challenges of achieving the best possible education while also raising a family, noted Graciela S. Alarcón, MD, MPH, who holds emeritus positions at the University of Alabama at Birmingham and the Universidad Peruana Cayetano Heredia in Lima, Peru.
“These program directors cultivate the ability to relate to women trainees in a very personal manner, supporting them in their efforts to achieve a balance between their training demands and their family/personal responsibilities,” she said.
Other research suggests the gender gap hasn’t gone away. Women continue to have lower odds of holding a higher-level professorship, receiving a federal grant, or speaking at academic conferences. They are also less likely to serve as first authors on rheumatology guidelines or recommendations.
Some studies suggest that women see fewer patients and earn less than their male counterparts. At peak difference, men can earn up to $100,000+ more than women. “My own impression is that it takes more efforts for women to reach the same level of recognition than men, and although overt discrimination is rare nowadays, subtle discrimination still occurs,” according to Dr. Alarcón.
Over a lifetime, female physicians can expect to earn less than their male counterparts, with clear implications for different retirement income levels, she said.
Fixing a leaky academic pipeline
The reality is the academic pipeline, and especially the physician-scientist pipeline, “continues to be leaky,” Dr. Liew said. “We know that caregivers to young children have larger barriers to surmount in academics and in research, and that there is a gender disparity present.” The toll of academic medicine on early career women who are parents is especially pronounced. While the pandemic has intensified this problem, it was around pre-COVID, she added.
Women who start in academia as academic clinicians or clinician researchers aren’t always able to meet their goals for promotion within the appropriate time frame. This is because of inequities in the system and lack of support related to maternity leave, childcare, and other issues. As a result, they leave academia and go into private practice or industry, Dr. Liew said.
The ACR in its 2015 survey projected that more women would be seeking part-time positions.
The good news is many academic institutions are taking a more equitable view about different career paths, offering equal parental leave to both men and women, Dr. Bolster noted. “It is essential that workforce planning encompasses the changing responsibilities within families and account for more parental leave by both men and women.” If certain projections come true, with 50% of the profession retiring between 2015 and 2030, combined with more men and women working part time, “it is requisite that workforce strategies plan for this.”
When Dr. Ramsey-Goldman was a trainee and junior faculty, there were no formal maternity leave policies.
Now, this benefit is available, she said. In another critical change, the ACR has made childcare services and a lactation room available for young mothers during its annual meeting. “Virtual meetings afford further ways to interact with colleagues,” she added.
Whether women choose to stay in academia or go into clinical practice is a very personal decision. “But it is also fair that, in some programs, training directors and faculty members can encourage trainees toward academia and its fascinating research possibilities,” Dr. Alarcón offered.
Making gains in research
Women are increasingly driving groundbreaking rheumatology research at all levels, Dr. Sandhu said. “And women empower women. Not infrequently, our female leaders, veterans in rheumatology research, seek younger female rheumatologists to help them grow in their niches. This has been one of the most beautiful things of the sisterhood in rheumatology that I have been blessed to be part of.”
In pediatric rheumatology, young female researchers are leading global research efforts. Some standouts include Kate Webb, MD, a pediatric rheumatologist in Cape Town, South Africa, and scientist who has worked on multisystem inflammatory syndrome during the pandemic. Sheila Angeles-Han, MD, who works on uveitis in juvenile idiopathic arthritis, had a role in recent ACR guidelines. Laura Lewandowski, MD, has also contributed to global rheumatology efforts, especially in low- and middle-income countries, Dr. Liew said.
The 2021 ACR annual meeting highlighted the research efforts of women rheumatologists from around the world. A global rheumatology summit at the meeting featured many women voices, including Dzifa Dey, MD, from Ghana, who received the ACR Distinguished International Rheumatology Professional Award. Ashira Blazer, MD, and Irene Blanco, MD, have spearheaded the ACR’s diversity, equity, and inclusion initiatives.
Women researchers have many opportunities to study rheumatologic diseases that disproportionately affect women, Dr. Alarcón said.
Lupus, for example, affects women in a much higher proportion than men (90% vs. 10%). This may be an attractive target for the best and brightest among future women researchers, Dr. Alarcón suggested. “It is a fact that publications related to lupus in leading internal medicine and rheumatology journals often include women either as first or senior authors. In that context, it can be said that several advances in the study of lupus worldwide can be attributed to women.”
This applies to disparities in social determinants of health that account for extremely complex outcomes in lupus among women of color, compared with White women, in addition to the costs associated with the disease and its impact on morbidity, mortality, and quality of life.
Women rheumatologists have advanced the work in reproductive management of rheumatic diseases, including a recent ACR-endorsed publication that provides formal guidance on managing reproductive health in women with rheumatic disease, Dr. Sandhu said. “One thing is clear: Without women, the work on reproductive diseases in rheumatology to date would not likely be where it is.”
Dr. Ramsey-Goldman added that “this critical work will not only set the stage for clinical care of both women and men regarding their reproductive health but will also inform education strategies for trainees and future research activities, and help direct policy regarding access to care, medication development, and costs of treatment.”
Obtaining grant funding to support salaries and researcher endeavors remains a challenge, Dr. Liew said. “It takes working evenings, weekends, and holidays to meet those goals within a set time frame. So you can see why a female faculty member with children might be disadvantaged, compared to a male counterpart without children.”
Competition for grant funding remains fierce as budgets become tighter, she added.
“We will lose a lot more brilliant and compassionate rheumatologists (clinicians, physician-scientists, and scientists alike) if we do not think of ways to make things more equitable or do not acknowledge the privileges that support some to continued career successes and leave others behind,” Dr. Liew said.
Women who choose a research field should seek out mentor and financial support that will allow them enough protected time to balance out research with other clinical activities, such as teaching and patient care, Dr. Alarcón said.
Training directors, mentors, and faculty should prioritize the needs of current and future women researchers, she said. “The guidance provided to young female trainees toward a successful research career is a formidable challenge that may provide, in turn, enormous satisfaction. There are established avenues to seek funding as new investigators.”
Progress in diversity
Rheumatology as a field is attracting more candidates and all races and genders, Dr. Bose said. “I think in the coming years we will see more and more women from minorities being incorporated into the rheumatology workforce.”
Others would like to see further improvements in diversity and attracting women from historically excluded backgrounds. Patients will benefit from rheumatologists who are able to connect with them through shared languages, cultures, and other life experiences, Dr. Liew said. “It is imperative that we work on recruitment, mentorship, and retention in this regard.”
While the representation of women of color is still inadequate, there has been some progress, Dr. Sandhu said. The number of female Hispanic, Latinx, and Black or African American graduates from medical school has seen a steady rise since 2017. And, AAMC has established task forces such as the Women of Color Initiative to identify strategies for furthering the careers of women of color in academic medicine.
“There’s still a lot of room to grow. I am, however, proud to say we will finally have a woman of color as the president of ACR in 2023,” said Dr. Sandhu, referring to Deborah Dyett Desir, MD.
Dr. Desir discussed the importance of diversifying the ACR in a recent interview.
All rheumatologists know that there is a place for them in the ACR, she stressed. “The demographics of our membership should reflect that of our population.”
As growth in diverse representation occurs, so will recruitment, retention, and a greater awareness and distribution of knowledge and means to address implicit biases and microaggressions, Dr. Sandhu said. “We will see a greater quality of health care, where patients may feel more connected to someone they can identify with.”
Looking ahead
Dr. Alarcón expects women to continue to play a major role in rheumatology, not just in research, education, and patient care but in leadership of academic societies and professional organizations.
“Women in rheumatology have come a long way – a piece of history that I have been fortunate to witness from my beginnings in the early 1970s. We have, I think, paved the way for the next generations of leaders in our beloved specialty field.”
Dr. Bolster is a member of the ACR board of directors and board liaison of the ACR Workforce Solutions Committee. Dr. Ramsey-Goldman has been a GlaxoSmithKline consultant for lupus studies, a consultant and site investigator with Exagen Diagnostics for lupus biomarker studies, and a site investigator for Xencor and Horizon Pharma lupus trials. Dr. Sandhu serves on the ACR’s Committee on Rheumatology Training and Workforce Issues.
Related article
Pioneer days of rheumatology: One veteran looks back
Patricia Woo, CBE, FMedSci, FRCP, has seen it all.
As a member of the British Rheumatology Society and fellow of the Royal College of Physicians, she presented the case for and obtained official training approval for pediatric rheumatology in the 1990s. She also set the wheels in motion to form the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology European Society.
Now 74, Dr. Woo remembers the discrimination she faced in the 1970s. “I was told I couldn’t become an investigator or consultant if I were to marry or have children.” Around the same time, she found out a male clinician researcher didn’t want to work with her, not because of her qualifications, but because she was a woman.
That wouldn’t happen now with all the antidiscrimination laws in place, noted Dr. Woo, an emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. Looking at the advances made by women in rheumatology, “there’s a major difference between 3 decades ago and today. If anyone discriminates today, they are called out.”
As the founding president of the Paediatric Rheumatology European Society, Dr. Woo is one of many early trailblazers who weathered many changes and made gains in the profession.
It’s important to recognize the work of Barbara Ansell, MD, the founder of pediatric rheumatology in the Canadian Red Cross Memorial Hospital, said Dr. Woo. Back in the 1960s, this wasn’t even a subspecialty. “Sick kids in general were taken either to pediatricians who didn’t know much about undescribed rheumatological conditions, and rheumatologists who didn’t know or have facilities for pediatric care.”
Dr. Ansell started this work, and Dr. Woo took over when she retired. With her colleagues, she set up a syllabus for pediatric rheumatology to formalize training for all junior doctors. This established a model of multidisciplinary clinical care and research. “Over the years, more women doctors have been attracted to pediatric rheumatology and have done well,” she said.
The rise of female leaders in rheumatology over the past few decades has been exponential, she continued. Women have become presidents of rheumatologic societies. Some established themselves as leaders in specific disciplines.
Carol Black, MD, from the United Kingdom is renowned for her international collaborative work in scleroderma research and clinical care. Patience White, MD in Washington, D.C., started research on the process of transitioning from childhood to adolescent to adult clinical care, a discipline that now has a strong international presence, Dr. Woo said.
The European Alliance of Associations for Rheumatology, which created a task force on gender equity in academic rheumatology, is evolving, she continued. The Academy of Medical Sciences in the United Kingdom also has active gender equality and mentoring programs, including a program to boost the careers of all researchers.
It’s also much easier now for women to become lead authors on papers since many are heads of lab or clinical services, Dr. Woo continued. “I don’t think there’s much discrimination if you’re a good clinician, and/or a good scientist. If women do their work well, they get the appropriate acknowledgment.”
Jean Liew, MD, recalls the long list of women mentors who have guided her career in rheumatology.
It started during her residency, when Jennifer Barton, MD, at Oregon Health & Science University, Portland, exposed her to new ways of conducting clinical research on patient outcomes.
In fellowship, she met Lianne Gensler, MD, a leader in axial spondyloarthritis, at the annual meeting of the American College of Rheumatology. Through Dr. Gensler’s mentorship and sponsorship, she was introduced to Maureen Dubreuil, MD, at Boston University, whose research focuses on pharmacoepidemiologic approaches using large databases.
Dr. Liew currently practices rheumatology under the leadership of Tuhina Neogi, MD, a world-renowned expert in osteoarthritis and gout. “She’s my research mentor,” Dr. Liew, an assistant professor of medicine at Boston University, said in an interview.
Her academic timeline reflects the powerful network and influence of women rheumatologists, who represent half of the adult rheumatology workforce in the United States. “In the research arena, many experts are women and they serve as role models and mentors to many,” Dr. Liew said.
But there’s more work to do, she and others acknowledged.
Rheumatology faces ongoing workforce shortages while struggling with a gender gap that’s closing but not as quickly as many women rheumatologists would like to see.
The gap persists, despite overall gains in the field of medicine, Vaneet Sandhu, MD, a rheumatologist with Loma Linda (Calif.) University, said in an interview. Women have exceeded men as enrollees in medical colleges, reported the Association of American Medical Colleges. And yet, “our colleagues reported last year that, in academic rheumatology, women are less likely to be full or associate professors than men,” she said.
The odds of being a fellowship program director or division director is similar in both males and females. “So, we’ve had some gains, but there’s always room for more,” Dr. Sandhu said.
Too few physicians
The next 10 years forecasts a dearth in American physicians.
AAMC projects a shortage of 124,000 doctors in the United States by 2034. Following on a similar trajectory, the ACR in 2015 anticipated a 25% drop in the supply of rheumatology clinical providers by 2030, with demand exceeding supply by more than 4,100 clinical employees.
The ACR’s workforce study projected that more women would come into rheumatology, noted Marcy Bolster, MD, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston. Women make up at least 50% of the workforce and 66% of fellows If these numbers hold, “we’ll definitely see an increase in the percent of women in the workforce” moving forward, Dr. Bolster said in an interview.
Women have helped the shortage to a great extent, said Nilanjana Bose, MD, a rheumatologist at Lonestar Rheumatology, Houston.
The work-life balance that rheumatology offers, combined with its focus on the cognitive part of internal medicine, explains why the field has attracted so many women. Rheumatology provides flexible work options. Women “get to teach or do rounds in the hospital or have a private practice where you’re mostly outpatient with some hospital work,” Dr. Bose said in an interview.
With anticipated shortages looming over the next decade, the profession needs to be cognizant of the different demands women face in their careers and how it can accommodate the workforce to meet the needs of its providers and maintain access for patients, Dr. Bolster said.
There are many innovative ways to match the demand for access. One thought is to create shared positions. Instead of employing four full-time physicians and one person part time, have two people who are working part time, Dr. Bolster suggested. “It is also important to not only expand our workforce with advanced practice providers, but to ensure their retention in the rheumatology workforce, to improve access to care for those with rheumatic diseases.”
Increasing the number of residency positions is another step toward addressing the shortage, Dr. Sandhu offered.
Women rheumatologists should make their voices heard by contacting members of Congress to support legislation that advocates for workforce shortage solutions, “in addition to generally supporting women’s rights and growth in the workplace,” she said.
The gender divide continues
Rosalind Ramsey-Goldman, MD, DrPH, remembers being the only woman in a group of five during her fellowship in the mid-1980s. Few women role models existed within the ACR, especially those in academic careers. “Now, most fellowships have more than 50% women, reflecting the number of women going to medical school,” said Dr. Ramsey-Goldman, Gallagher Research Professor in Rheumatology at Northwestern University and Northwestern Medicine, Chicago.
As more women enter the profession, women rheumatologists in academic rheumatology have started to outpace men in recent years. Some research suggests they’ve made headway in gaining leadership spots at institutions.
One recent paper, a cross-sectional national study of more than 6,100 rheumatologists, found that women had similar odds of attaining fellowship program or division director positions as men. As directors of training programs, women in rheumatology “instill this collaborative and growth mindset that encourages learners to self-reflect and work as a team,” Dr. Sandhu said.
Women bring a different perspective to training, and how curriculum works, Dr. Bose said. Studies have shown that women tend to be more empathic. They ask more questions. “That’s not to say men aren’t good. Women just have an inborn ability for connecting,” and this perspective helps to enrich the educational experience for trainees.
Women who lead training programs are also attuned to realities that female trainees confront, such as dealing with the challenges of achieving the best possible education while also raising a family, noted Graciela S. Alarcón, MD, MPH, who holds emeritus positions at the University of Alabama at Birmingham and the Universidad Peruana Cayetano Heredia in Lima, Peru.
“These program directors cultivate the ability to relate to women trainees in a very personal manner, supporting them in their efforts to achieve a balance between their training demands and their family/personal responsibilities,” she said.
Other research suggests the gender gap hasn’t gone away. Women continue to have lower odds of holding a higher-level professorship, receiving a federal grant, or speaking at academic conferences. They are also less likely to serve as first authors on rheumatology guidelines or recommendations.
Some studies suggest that women see fewer patients and earn less than their male counterparts. At peak difference, men can earn up to $100,000+ more than women. “My own impression is that it takes more efforts for women to reach the same level of recognition than men, and although overt discrimination is rare nowadays, subtle discrimination still occurs,” according to Dr. Alarcón.
Over a lifetime, female physicians can expect to earn less than their male counterparts, with clear implications for different retirement income levels, she said.
Fixing a leaky academic pipeline
The reality is the academic pipeline, and especially the physician-scientist pipeline, “continues to be leaky,” Dr. Liew said. “We know that caregivers to young children have larger barriers to surmount in academics and in research, and that there is a gender disparity present.” The toll of academic medicine on early career women who are parents is especially pronounced. While the pandemic has intensified this problem, it was around pre-COVID, she added.
Women who start in academia as academic clinicians or clinician researchers aren’t always able to meet their goals for promotion within the appropriate time frame. This is because of inequities in the system and lack of support related to maternity leave, childcare, and other issues. As a result, they leave academia and go into private practice or industry, Dr. Liew said.
The ACR in its 2015 survey projected that more women would be seeking part-time positions.
The good news is many academic institutions are taking a more equitable view about different career paths, offering equal parental leave to both men and women, Dr. Bolster noted. “It is essential that workforce planning encompasses the changing responsibilities within families and account for more parental leave by both men and women.” If certain projections come true, with 50% of the profession retiring between 2015 and 2030, combined with more men and women working part time, “it is requisite that workforce strategies plan for this.”
When Dr. Ramsey-Goldman was a trainee and junior faculty, there were no formal maternity leave policies.
Now, this benefit is available, she said. In another critical change, the ACR has made childcare services and a lactation room available for young mothers during its annual meeting. “Virtual meetings afford further ways to interact with colleagues,” she added.
Whether women choose to stay in academia or go into clinical practice is a very personal decision. “But it is also fair that, in some programs, training directors and faculty members can encourage trainees toward academia and its fascinating research possibilities,” Dr. Alarcón offered.
Making gains in research
Women are increasingly driving groundbreaking rheumatology research at all levels, Dr. Sandhu said. “And women empower women. Not infrequently, our female leaders, veterans in rheumatology research, seek younger female rheumatologists to help them grow in their niches. This has been one of the most beautiful things of the sisterhood in rheumatology that I have been blessed to be part of.”
In pediatric rheumatology, young female researchers are leading global research efforts. Some standouts include Kate Webb, MD, a pediatric rheumatologist in Cape Town, South Africa, and scientist who has worked on multisystem inflammatory syndrome during the pandemic. Sheila Angeles-Han, MD, who works on uveitis in juvenile idiopathic arthritis, had a role in recent ACR guidelines. Laura Lewandowski, MD, has also contributed to global rheumatology efforts, especially in low- and middle-income countries, Dr. Liew said.
The 2021 ACR annual meeting highlighted the research efforts of women rheumatologists from around the world. A global rheumatology summit at the meeting featured many women voices, including Dzifa Dey, MD, from Ghana, who received the ACR Distinguished International Rheumatology Professional Award. Ashira Blazer, MD, and Irene Blanco, MD, have spearheaded the ACR’s diversity, equity, and inclusion initiatives.
Women researchers have many opportunities to study rheumatologic diseases that disproportionately affect women, Dr. Alarcón said.
Lupus, for example, affects women in a much higher proportion than men (90% vs. 10%). This may be an attractive target for the best and brightest among future women researchers, Dr. Alarcón suggested. “It is a fact that publications related to lupus in leading internal medicine and rheumatology journals often include women either as first or senior authors. In that context, it can be said that several advances in the study of lupus worldwide can be attributed to women.”
This applies to disparities in social determinants of health that account for extremely complex outcomes in lupus among women of color, compared with White women, in addition to the costs associated with the disease and its impact on morbidity, mortality, and quality of life.
Women rheumatologists have advanced the work in reproductive management of rheumatic diseases, including a recent ACR-endorsed publication that provides formal guidance on managing reproductive health in women with rheumatic disease, Dr. Sandhu said. “One thing is clear: Without women, the work on reproductive diseases in rheumatology to date would not likely be where it is.”
Dr. Ramsey-Goldman added that “this critical work will not only set the stage for clinical care of both women and men regarding their reproductive health but will also inform education strategies for trainees and future research activities, and help direct policy regarding access to care, medication development, and costs of treatment.”
Obtaining grant funding to support salaries and researcher endeavors remains a challenge, Dr. Liew said. “It takes working evenings, weekends, and holidays to meet those goals within a set time frame. So you can see why a female faculty member with children might be disadvantaged, compared to a male counterpart without children.”
Competition for grant funding remains fierce as budgets become tighter, she added.
“We will lose a lot more brilliant and compassionate rheumatologists (clinicians, physician-scientists, and scientists alike) if we do not think of ways to make things more equitable or do not acknowledge the privileges that support some to continued career successes and leave others behind,” Dr. Liew said.
Women who choose a research field should seek out mentor and financial support that will allow them enough protected time to balance out research with other clinical activities, such as teaching and patient care, Dr. Alarcón said.
Training directors, mentors, and faculty should prioritize the needs of current and future women researchers, she said. “The guidance provided to young female trainees toward a successful research career is a formidable challenge that may provide, in turn, enormous satisfaction. There are established avenues to seek funding as new investigators.”
Progress in diversity
Rheumatology as a field is attracting more candidates and all races and genders, Dr. Bose said. “I think in the coming years we will see more and more women from minorities being incorporated into the rheumatology workforce.”
Others would like to see further improvements in diversity and attracting women from historically excluded backgrounds. Patients will benefit from rheumatologists who are able to connect with them through shared languages, cultures, and other life experiences, Dr. Liew said. “It is imperative that we work on recruitment, mentorship, and retention in this regard.”
While the representation of women of color is still inadequate, there has been some progress, Dr. Sandhu said. The number of female Hispanic, Latinx, and Black or African American graduates from medical school has seen a steady rise since 2017. And, AAMC has established task forces such as the Women of Color Initiative to identify strategies for furthering the careers of women of color in academic medicine.
“There’s still a lot of room to grow. I am, however, proud to say we will finally have a woman of color as the president of ACR in 2023,” said Dr. Sandhu, referring to Deborah Dyett Desir, MD.
Dr. Desir discussed the importance of diversifying the ACR in a recent interview.
All rheumatologists know that there is a place for them in the ACR, she stressed. “The demographics of our membership should reflect that of our population.”
As growth in diverse representation occurs, so will recruitment, retention, and a greater awareness and distribution of knowledge and means to address implicit biases and microaggressions, Dr. Sandhu said. “We will see a greater quality of health care, where patients may feel more connected to someone they can identify with.”
Looking ahead
Dr. Alarcón expects women to continue to play a major role in rheumatology, not just in research, education, and patient care but in leadership of academic societies and professional organizations.
“Women in rheumatology have come a long way – a piece of history that I have been fortunate to witness from my beginnings in the early 1970s. We have, I think, paved the way for the next generations of leaders in our beloved specialty field.”
Dr. Bolster is a member of the ACR board of directors and board liaison of the ACR Workforce Solutions Committee. Dr. Ramsey-Goldman has been a GlaxoSmithKline consultant for lupus studies, a consultant and site investigator with Exagen Diagnostics for lupus biomarker studies, and a site investigator for Xencor and Horizon Pharma lupus trials. Dr. Sandhu serves on the ACR’s Committee on Rheumatology Training and Workforce Issues.
Related article
Pioneer days of rheumatology: One veteran looks back
Patricia Woo, CBE, FMedSci, FRCP, has seen it all.
As a member of the British Rheumatology Society and fellow of the Royal College of Physicians, she presented the case for and obtained official training approval for pediatric rheumatology in the 1990s. She also set the wheels in motion to form the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology European Society.
Now 74, Dr. Woo remembers the discrimination she faced in the 1970s. “I was told I couldn’t become an investigator or consultant if I were to marry or have children.” Around the same time, she found out a male clinician researcher didn’t want to work with her, not because of her qualifications, but because she was a woman.
That wouldn’t happen now with all the antidiscrimination laws in place, noted Dr. Woo, an emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. Looking at the advances made by women in rheumatology, “there’s a major difference between 3 decades ago and today. If anyone discriminates today, they are called out.”
As the founding president of the Paediatric Rheumatology European Society, Dr. Woo is one of many early trailblazers who weathered many changes and made gains in the profession.
It’s important to recognize the work of Barbara Ansell, MD, the founder of pediatric rheumatology in the Canadian Red Cross Memorial Hospital, said Dr. Woo. Back in the 1960s, this wasn’t even a subspecialty. “Sick kids in general were taken either to pediatricians who didn’t know much about undescribed rheumatological conditions, and rheumatologists who didn’t know or have facilities for pediatric care.”
Dr. Ansell started this work, and Dr. Woo took over when she retired. With her colleagues, she set up a syllabus for pediatric rheumatology to formalize training for all junior doctors. This established a model of multidisciplinary clinical care and research. “Over the years, more women doctors have been attracted to pediatric rheumatology and have done well,” she said.
The rise of female leaders in rheumatology over the past few decades has been exponential, she continued. Women have become presidents of rheumatologic societies. Some established themselves as leaders in specific disciplines.
Carol Black, MD, from the United Kingdom is renowned for her international collaborative work in scleroderma research and clinical care. Patience White, MD in Washington, D.C., started research on the process of transitioning from childhood to adolescent to adult clinical care, a discipline that now has a strong international presence, Dr. Woo said.
The European Alliance of Associations for Rheumatology, which created a task force on gender equity in academic rheumatology, is evolving, she continued. The Academy of Medical Sciences in the United Kingdom also has active gender equality and mentoring programs, including a program to boost the careers of all researchers.
It’s also much easier now for women to become lead authors on papers since many are heads of lab or clinical services, Dr. Woo continued. “I don’t think there’s much discrimination if you’re a good clinician, and/or a good scientist. If women do their work well, they get the appropriate acknowledgment.”
Blistering Lesions in a Newborn
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
A 4-day-old infant boy presented with blisters on the skin. He was born at 36 weeks’ gestation by cesarean delivery to a nulliparous mother who received appropriate prenatal care. On day 2 of life, the patient developed bullae with breakdown of the skin on the bilateral heels and on the skin surrounding intravenous injection sites. Similar blisters subsequently developed on the fingers (top), thighs, groin, and toes (bottom), sparing the oral mucosa and trunk. He remained afebrile and stable and was started on ampicillin, gentamicin, and acyclovir with continued development of blisters. Two weeks later he developed painful ulcers on the tongue that bled upon scraping.
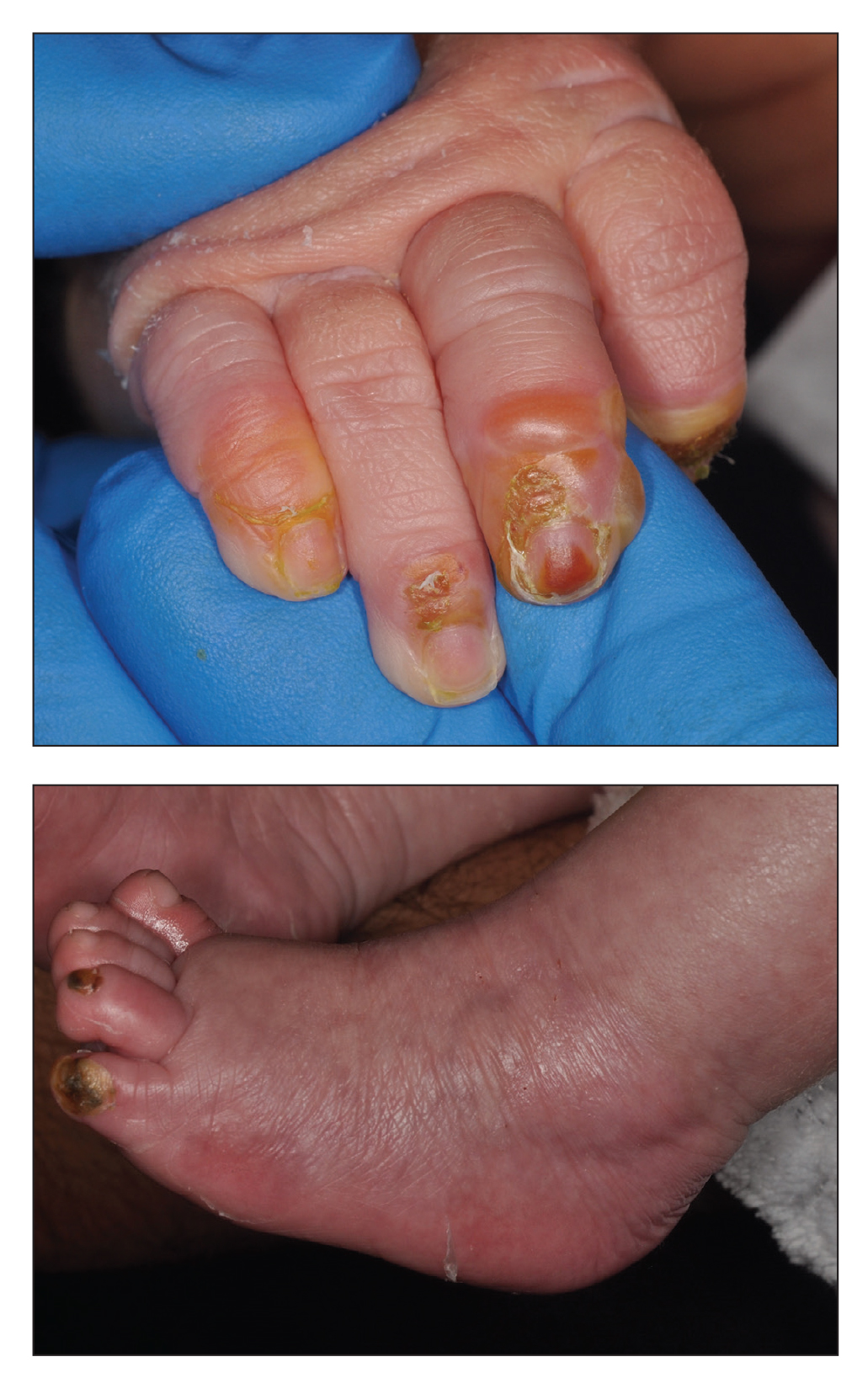
Infectious disease pop quiz: Clinical challenge #23 for the ObGyn
What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Continue to the answer...
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci).
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Continue to the answer...
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci).
What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Continue to the answer...
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci).
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Reducing Attacks and Neuropathic Pain in NMOSD
Patients with neuromyelitis optica spectrum disorder (NMOSD) experience unpredictable episodes of inflammation involving the optic nerve, spine, or both. Debilitating neuropathic pain accompanies the healing process, which lasts anywhere from 2 to 6 months after an attack.
In this ReCAP, Dr Michael Levy, of Harvard Medical School in Boston, Massachusetts, outlines the heavy psychological and economic burdens associated with NMOSD and reports on three new targeted therapies that have been approved to prevent relapse and delay disease progression.
He then looks at current pharmaceutical treatments for NMOSD pain, before reporting promising trial data exploring transcutaneous electrical nerve stimulation as a safe and cost-effective treatment for neuropathic pain.
--
Michael Levy, MD, PhD, Associate Professor, Department of Neurology, Harvard Medical School, Boston, Massachusetts
Michael Levy, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Alexion; Horizon; Genentech; UCB; Sanofi; Quest
Received research grant from: National Institutes of Health; Sanofi; Genentech; Horizon; Alexion
Patients with neuromyelitis optica spectrum disorder (NMOSD) experience unpredictable episodes of inflammation involving the optic nerve, spine, or both. Debilitating neuropathic pain accompanies the healing process, which lasts anywhere from 2 to 6 months after an attack.
In this ReCAP, Dr Michael Levy, of Harvard Medical School in Boston, Massachusetts, outlines the heavy psychological and economic burdens associated with NMOSD and reports on three new targeted therapies that have been approved to prevent relapse and delay disease progression.
He then looks at current pharmaceutical treatments for NMOSD pain, before reporting promising trial data exploring transcutaneous electrical nerve stimulation as a safe and cost-effective treatment for neuropathic pain.
--
Michael Levy, MD, PhD, Associate Professor, Department of Neurology, Harvard Medical School, Boston, Massachusetts
Michael Levy, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Alexion; Horizon; Genentech; UCB; Sanofi; Quest
Received research grant from: National Institutes of Health; Sanofi; Genentech; Horizon; Alexion
Patients with neuromyelitis optica spectrum disorder (NMOSD) experience unpredictable episodes of inflammation involving the optic nerve, spine, or both. Debilitating neuropathic pain accompanies the healing process, which lasts anywhere from 2 to 6 months after an attack.
In this ReCAP, Dr Michael Levy, of Harvard Medical School in Boston, Massachusetts, outlines the heavy psychological and economic burdens associated with NMOSD and reports on three new targeted therapies that have been approved to prevent relapse and delay disease progression.
He then looks at current pharmaceutical treatments for NMOSD pain, before reporting promising trial data exploring transcutaneous electrical nerve stimulation as a safe and cost-effective treatment for neuropathic pain.
--
Michael Levy, MD, PhD, Associate Professor, Department of Neurology, Harvard Medical School, Boston, Massachusetts
Michael Levy, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Alexion; Horizon; Genentech; UCB; Sanofi; Quest
Received research grant from: National Institutes of Health; Sanofi; Genentech; Horizon; Alexion

Managing Heavy Menstrual Bleeding Associated with Fibroids
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634