User login
Commentary: Treating Chronic Migraine and Providing Temporary Relief, July 2022
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
Most headache specialists are familiar with the "standard" or PREEMPT onabotulinumtoxinA (Botox) paradigm used preventively for migraine. This protocol uses 155 units of onabotulinumtoxinA over 31 sites in seven muscle groups. OnabotulinumtoxinA vials typically come in 100 or 200 units, and when preparing onabotulinumtoxinA for patients who are being injected most providers are forced to discard most or all of the remaining 45 units. Anecdotally, some providers do inject the entire 200-unit vial, and the additional injection sites are either given in another standard protocol or in a follow-the-pain manner.
The study by Zandieh and colleagues followed 175 patients with chronic migraine who first received three injections of 150 units of onabotulinumtoxinA, then three injections of 200 units of this agent. The additional 50 units were injected into the temporalis and occipitalis muscles — the standard sites were used, but additional units were injected into each of the sites. The majority of patients experienced primarily frontal pain; the injections were not given in specific areas where more pain was manifesting.
The average number of headache days per month decreased significantly when the onabotulinumtoxinA dose was increased; patients tolerated the medication over the 3-month period as well. In practice, many providers use the additional units of onabotulinumtoxinA. This study argues that there is a minimal risk, and probably a potential significant benefit, when using up to 200 units every 3 months. Providers should, however, be aware that in rare instances, some insurances will only cover a 155-unit injection, and the use of additional units may jeopardize reimbursement for those plans.
Many patients anecdotally will use cold or heat as a treatment for acute migraine pain; however, the topical use of temperature has not been well studied for this purpose. Cold stimulus has, importantly, been known to be a trigger of migraine as well as other headache disorders classified in the International Classification of Headache Disorders, third edition (ICHD-3), including external cold stimulus headache and "brain freeze" or internal cold stimulus headache. Hsu and colleagues produced a meta-analysis and systematic review on the use of cold for acute treatment of migraine.
Six studies were found to be eligible for this review. The cold stimulus could be placed anywhere on the head, and the studies could have considered its use for any migraine-associated symptom. This includes headache, eye pain, nausea, or vomiting. The interventions used cold somewhat differently, including as ice packing, cooling compression, soaking, and as a rinse. Both randomized and nonrandomized trials were included in the systematic review; however, only randomized controlled trials were used for the meta-analysis.
The primary outcome evaluated by the authors was pain intensity; secondary outcomes were duration of migraine pain as well as associated symptoms (eg, nausea, vomiting). The meta-analysis revealed that cold interventions reduce migraine pain by 3.21 points on an analog scale, and this was found to be effective within 30 minutes. At 1-2 hours after the intervention, the effect was not seen to be significant. At 24 hours, the effect of cold intervention was marginal. Cold was not seen to significantly reduce nausea or vomiting at 2 hours after intervention.
Although cold treatments are commonly used by patients, there appears to be benefit only early in the onset of a migraine attack. Headache specialists typically recommend early treatment with a migraine-specific acute medication; however, the medication may take minutes to hours before taking effect. Cold can be recommended to patients during that intervening period, and it may help until the time that their acute medications take effect.
Chronic refractory migraine remains one of the most debilitating neurologic disorders and is a challenge even for the best trained neurologist or headache specialist. There are few headache centers with inpatient headache units around the United States, and those that remain use treatments that most neurologists are not familiar with. Schwenk and colleagues retrospectively reviewed the data of a major academic headache center and revealed impressive outcomes in this very difficult-to-treat population.
This study reviewed the outcomes of 609 consecutive patients admitted to the Thomas Jefferson University inpatient headache unit from 2017 to 2021. These patients all received continuous lidocaine infusions that were titrated according to an internal protocol that balanced daily plasma lidocaine levels, tolerability, and pain relief. Hospital discharge occurred when patients were pain-free for 12-24 hours or had a minimal response after 5 days of treatment. All patients had at least eight severe headaches per month for at least 6 consecutive months and had tried one to seven preventive medications, with the result of either intolerance or ineffectiveness.
The primary outcome was change from baseline to discharge pain level. Patients were admitted with an average score of 7.0 of 10 on admission and were discharged at a score of 1.0 of 10. Secondary outcomes were average pain at post-discharge appointment vs baseline (5.5 vs 7.0), number of monthly headache days at post-discharge appointment (22.5 vs 26.8), and current and average pain levels at the post-discharge appointment, which were both significantly lower as well. The most common adverse effect was nausea; others noted were cardiovascular changes, hallucinations or nightmares, sedation, anxiety, and chest pain.
This is an important retrospective on the effectiveness of an inpatient lidocaine protocol for refractory chronic migraine. When considering this population, especially if multiple lines of preventive and acute medications are not effective, referral to an academic inpatient headache center should definitely be considered. This patient population does not respond effectively to most treatment modalities, and this is cause to give them hope.
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
Most headache specialists are familiar with the "standard" or PREEMPT onabotulinumtoxinA (Botox) paradigm used preventively for migraine. This protocol uses 155 units of onabotulinumtoxinA over 31 sites in seven muscle groups. OnabotulinumtoxinA vials typically come in 100 or 200 units, and when preparing onabotulinumtoxinA for patients who are being injected most providers are forced to discard most or all of the remaining 45 units. Anecdotally, some providers do inject the entire 200-unit vial, and the additional injection sites are either given in another standard protocol or in a follow-the-pain manner.
The study by Zandieh and colleagues followed 175 patients with chronic migraine who first received three injections of 150 units of onabotulinumtoxinA, then three injections of 200 units of this agent. The additional 50 units were injected into the temporalis and occipitalis muscles — the standard sites were used, but additional units were injected into each of the sites. The majority of patients experienced primarily frontal pain; the injections were not given in specific areas where more pain was manifesting.
The average number of headache days per month decreased significantly when the onabotulinumtoxinA dose was increased; patients tolerated the medication over the 3-month period as well. In practice, many providers use the additional units of onabotulinumtoxinA. This study argues that there is a minimal risk, and probably a potential significant benefit, when using up to 200 units every 3 months. Providers should, however, be aware that in rare instances, some insurances will only cover a 155-unit injection, and the use of additional units may jeopardize reimbursement for those plans.
Many patients anecdotally will use cold or heat as a treatment for acute migraine pain; however, the topical use of temperature has not been well studied for this purpose. Cold stimulus has, importantly, been known to be a trigger of migraine as well as other headache disorders classified in the International Classification of Headache Disorders, third edition (ICHD-3), including external cold stimulus headache and "brain freeze" or internal cold stimulus headache. Hsu and colleagues produced a meta-analysis and systematic review on the use of cold for acute treatment of migraine.
Six studies were found to be eligible for this review. The cold stimulus could be placed anywhere on the head, and the studies could have considered its use for any migraine-associated symptom. This includes headache, eye pain, nausea, or vomiting. The interventions used cold somewhat differently, including as ice packing, cooling compression, soaking, and as a rinse. Both randomized and nonrandomized trials were included in the systematic review; however, only randomized controlled trials were used for the meta-analysis.
The primary outcome evaluated by the authors was pain intensity; secondary outcomes were duration of migraine pain as well as associated symptoms (eg, nausea, vomiting). The meta-analysis revealed that cold interventions reduce migraine pain by 3.21 points on an analog scale, and this was found to be effective within 30 minutes. At 1-2 hours after the intervention, the effect was not seen to be significant. At 24 hours, the effect of cold intervention was marginal. Cold was not seen to significantly reduce nausea or vomiting at 2 hours after intervention.
Although cold treatments are commonly used by patients, there appears to be benefit only early in the onset of a migraine attack. Headache specialists typically recommend early treatment with a migraine-specific acute medication; however, the medication may take minutes to hours before taking effect. Cold can be recommended to patients during that intervening period, and it may help until the time that their acute medications take effect.
Chronic refractory migraine remains one of the most debilitating neurologic disorders and is a challenge even for the best trained neurologist or headache specialist. There are few headache centers with inpatient headache units around the United States, and those that remain use treatments that most neurologists are not familiar with. Schwenk and colleagues retrospectively reviewed the data of a major academic headache center and revealed impressive outcomes in this very difficult-to-treat population.
This study reviewed the outcomes of 609 consecutive patients admitted to the Thomas Jefferson University inpatient headache unit from 2017 to 2021. These patients all received continuous lidocaine infusions that were titrated according to an internal protocol that balanced daily plasma lidocaine levels, tolerability, and pain relief. Hospital discharge occurred when patients were pain-free for 12-24 hours or had a minimal response after 5 days of treatment. All patients had at least eight severe headaches per month for at least 6 consecutive months and had tried one to seven preventive medications, with the result of either intolerance or ineffectiveness.
The primary outcome was change from baseline to discharge pain level. Patients were admitted with an average score of 7.0 of 10 on admission and were discharged at a score of 1.0 of 10. Secondary outcomes were average pain at post-discharge appointment vs baseline (5.5 vs 7.0), number of monthly headache days at post-discharge appointment (22.5 vs 26.8), and current and average pain levels at the post-discharge appointment, which were both significantly lower as well. The most common adverse effect was nausea; others noted were cardiovascular changes, hallucinations or nightmares, sedation, anxiety, and chest pain.
This is an important retrospective on the effectiveness of an inpatient lidocaine protocol for refractory chronic migraine. When considering this population, especially if multiple lines of preventive and acute medications are not effective, referral to an academic inpatient headache center should definitely be considered. This patient population does not respond effectively to most treatment modalities, and this is cause to give them hope.
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
Most headache specialists are familiar with the "standard" or PREEMPT onabotulinumtoxinA (Botox) paradigm used preventively for migraine. This protocol uses 155 units of onabotulinumtoxinA over 31 sites in seven muscle groups. OnabotulinumtoxinA vials typically come in 100 or 200 units, and when preparing onabotulinumtoxinA for patients who are being injected most providers are forced to discard most or all of the remaining 45 units. Anecdotally, some providers do inject the entire 200-unit vial, and the additional injection sites are either given in another standard protocol or in a follow-the-pain manner.
The study by Zandieh and colleagues followed 175 patients with chronic migraine who first received three injections of 150 units of onabotulinumtoxinA, then three injections of 200 units of this agent. The additional 50 units were injected into the temporalis and occipitalis muscles — the standard sites were used, but additional units were injected into each of the sites. The majority of patients experienced primarily frontal pain; the injections were not given in specific areas where more pain was manifesting.
The average number of headache days per month decreased significantly when the onabotulinumtoxinA dose was increased; patients tolerated the medication over the 3-month period as well. In practice, many providers use the additional units of onabotulinumtoxinA. This study argues that there is a minimal risk, and probably a potential significant benefit, when using up to 200 units every 3 months. Providers should, however, be aware that in rare instances, some insurances will only cover a 155-unit injection, and the use of additional units may jeopardize reimbursement for those plans.
Many patients anecdotally will use cold or heat as a treatment for acute migraine pain; however, the topical use of temperature has not been well studied for this purpose. Cold stimulus has, importantly, been known to be a trigger of migraine as well as other headache disorders classified in the International Classification of Headache Disorders, third edition (ICHD-3), including external cold stimulus headache and "brain freeze" or internal cold stimulus headache. Hsu and colleagues produced a meta-analysis and systematic review on the use of cold for acute treatment of migraine.
Six studies were found to be eligible for this review. The cold stimulus could be placed anywhere on the head, and the studies could have considered its use for any migraine-associated symptom. This includes headache, eye pain, nausea, or vomiting. The interventions used cold somewhat differently, including as ice packing, cooling compression, soaking, and as a rinse. Both randomized and nonrandomized trials were included in the systematic review; however, only randomized controlled trials were used for the meta-analysis.
The primary outcome evaluated by the authors was pain intensity; secondary outcomes were duration of migraine pain as well as associated symptoms (eg, nausea, vomiting). The meta-analysis revealed that cold interventions reduce migraine pain by 3.21 points on an analog scale, and this was found to be effective within 30 minutes. At 1-2 hours after the intervention, the effect was not seen to be significant. At 24 hours, the effect of cold intervention was marginal. Cold was not seen to significantly reduce nausea or vomiting at 2 hours after intervention.
Although cold treatments are commonly used by patients, there appears to be benefit only early in the onset of a migraine attack. Headache specialists typically recommend early treatment with a migraine-specific acute medication; however, the medication may take minutes to hours before taking effect. Cold can be recommended to patients during that intervening period, and it may help until the time that their acute medications take effect.
Chronic refractory migraine remains one of the most debilitating neurologic disorders and is a challenge even for the best trained neurologist or headache specialist. There are few headache centers with inpatient headache units around the United States, and those that remain use treatments that most neurologists are not familiar with. Schwenk and colleagues retrospectively reviewed the data of a major academic headache center and revealed impressive outcomes in this very difficult-to-treat population.
This study reviewed the outcomes of 609 consecutive patients admitted to the Thomas Jefferson University inpatient headache unit from 2017 to 2021. These patients all received continuous lidocaine infusions that were titrated according to an internal protocol that balanced daily plasma lidocaine levels, tolerability, and pain relief. Hospital discharge occurred when patients were pain-free for 12-24 hours or had a minimal response after 5 days of treatment. All patients had at least eight severe headaches per month for at least 6 consecutive months and had tried one to seven preventive medications, with the result of either intolerance or ineffectiveness.
The primary outcome was change from baseline to discharge pain level. Patients were admitted with an average score of 7.0 of 10 on admission and were discharged at a score of 1.0 of 10. Secondary outcomes were average pain at post-discharge appointment vs baseline (5.5 vs 7.0), number of monthly headache days at post-discharge appointment (22.5 vs 26.8), and current and average pain levels at the post-discharge appointment, which were both significantly lower as well. The most common adverse effect was nausea; others noted were cardiovascular changes, hallucinations or nightmares, sedation, anxiety, and chest pain.
This is an important retrospective on the effectiveness of an inpatient lidocaine protocol for refractory chronic migraine. When considering this population, especially if multiple lines of preventive and acute medications are not effective, referral to an academic inpatient headache center should definitely be considered. This patient population does not respond effectively to most treatment modalities, and this is cause to give them hope.
No more injections after one-off gene therapy in hemophilia B
Patients with hemophilia B face a lifelong need for regular factor IX injections.
“Removing the need for hemophilia patients to regularly inject themselves with the missing protein is an important step in improving their quality of life,” lead author Pratima Chowdary, MD, of the Royal Free Hospital, University College London Cancer Institute, commented in a press statement.
The team reported new results with the investigational gene therapy FLT180a in a study published in the New England Journal of Medicine.
“We found that normal factor IX levels can be achieved in patients with severe or moderately severe hemophilia B with the use of relatively low vector doses of FLT180a,” the authors reported. “In all but one patient, gene therapy led to durable factor IX expression, eliminated the need for factor IX prophylaxis, and eliminated spontaneous bleeding leading to factor IX replacement.”
FLT180a (Freeline Therapeutics) is a liver-directed, adeno-associated virus (AAV) gene therapy designed to normalize levels of the factor IX protein that is needed for coagulation; however, it is produced in dangerously low levels in people with hemophilia B as a result of gene mutations.
Under the current standard of care, patients with hemophilia B require lifelong prophylaxis of regular intravenous injections with recombinant factor IX replacement therapy, and they commonly continue to experience potentially severe joint pain.
While factor-replacement therapies with longer half-lives have emerged, the prophylaxis is still invasive and extremely expensive, with the average price tag in the United States of $397,491 a year for the conventional treatment and an average of $788,861 a year for an extended half-life treatment, according to a 2019 report.
Novel gene therapy
Hemophilia B is a rare and inherited genetic bleeding disorder caused by defects in the gene responsible for factor IX protein, which is needed for blood clotting.
AAV gene therapy delivers a functional copy of this gene directly to patient tissues to compensate for one that is not working properly. It leads to the synthesis of factor IX proteins and a one-time gene therapy infusion can achieve long-lasting effects, the team explained in a press release.
The results they reported come from the phase 1/2 multicenter B-AMAZE open-label trial. It involved 10 patients (all age 18 and older) with severe or moderately severe hemophilia B, defined as having a factor IX level of 2% or less that of normal values.
All patients received one-off gene therapy infusion, at one of four FLT180a doses.
All patients also received immunosuppression to prevent the body from rejecting the vector gene therapy. This consisted of glucocorticoids with or without tacrolimus for a period of ranging from several weeks to several months.
Following the FLT180a infusion, all patients showed dose-dependent increases in factor IX levels. After a median follow-up of 27.2 months (range, 19.1-42.4 months), nearly all the patients (9 of 10) continued to show sustained factor IX activity.
Steady production of factor IX activity started at month 12, with low bleeding frequency that allowed these nine patients to no longer require weekly injections of the protein.
Five of the patients had factor IX levels in the normal range, from 51% to 78%; three patients had lower increases of 23%-43% of the normal range, and one patient who had received the highest dose, had a level that was 260% of normal.
The exception was one patient who required a return to factor IX prophylaxis. He had experienced a failure in the immunosuppression regimen due to a delay in the recognition of an immune response at approximately 22 weeks after treatment, the authors reported.
The therapy was generally well tolerated, with no infusion reactions or discontinuations of infusions. As of the study cutoff, no inhibitors of factor IX were detected.
Of the adverse events, about 10% were determined to be related to the gene therapy. The most common event associated with the gene therapy was increases in liver aminotransferase, which is a concern with AAV gene therapies, the authors commented.
Otherwise, 24% of adverse events were determined to be related to the immunosuppression, and were consistent with the known safety profiles of glucocorticoids and tacrolimus.
Late increases in aminotransferase levels were reported among patients who had received prolonged tacrolimus beyond the tapering of glucocorticoid treatment.
The one serious adverse event that was reported involved an arteriovenous fistula thrombosis, which occurred in the patient who had received the highest dose of gene therapy and who showed the highest factor IX levels.
The current findings, along with data from another recent study involving gene therapy for patients with hemophilia A, emphasized that “immune responses can occur later than previously expected and may coincide with the withdrawal of immunosuppression,” the authors cautioned.
“Consistent best practices for monitoring aminotransferase levels and deciding when ALT increases warrant intervention remain a critical topic for the field,” they noted.
Meanwhile, the patients in this B-AMAZE trial all remain enrolled in a long-term follow-up study to assess the safety and durability of FLT180a over 15 years.
The trial was sponsored by University College London and funded by Freeline Therapeutics. Dr. Chowdary disclosed various relationships with industry.
A version of this article first appeared on Medscape.com.
Patients with hemophilia B face a lifelong need for regular factor IX injections.
“Removing the need for hemophilia patients to regularly inject themselves with the missing protein is an important step in improving their quality of life,” lead author Pratima Chowdary, MD, of the Royal Free Hospital, University College London Cancer Institute, commented in a press statement.
The team reported new results with the investigational gene therapy FLT180a in a study published in the New England Journal of Medicine.
“We found that normal factor IX levels can be achieved in patients with severe or moderately severe hemophilia B with the use of relatively low vector doses of FLT180a,” the authors reported. “In all but one patient, gene therapy led to durable factor IX expression, eliminated the need for factor IX prophylaxis, and eliminated spontaneous bleeding leading to factor IX replacement.”
FLT180a (Freeline Therapeutics) is a liver-directed, adeno-associated virus (AAV) gene therapy designed to normalize levels of the factor IX protein that is needed for coagulation; however, it is produced in dangerously low levels in people with hemophilia B as a result of gene mutations.
Under the current standard of care, patients with hemophilia B require lifelong prophylaxis of regular intravenous injections with recombinant factor IX replacement therapy, and they commonly continue to experience potentially severe joint pain.
While factor-replacement therapies with longer half-lives have emerged, the prophylaxis is still invasive and extremely expensive, with the average price tag in the United States of $397,491 a year for the conventional treatment and an average of $788,861 a year for an extended half-life treatment, according to a 2019 report.
Novel gene therapy
Hemophilia B is a rare and inherited genetic bleeding disorder caused by defects in the gene responsible for factor IX protein, which is needed for blood clotting.
AAV gene therapy delivers a functional copy of this gene directly to patient tissues to compensate for one that is not working properly. It leads to the synthesis of factor IX proteins and a one-time gene therapy infusion can achieve long-lasting effects, the team explained in a press release.
The results they reported come from the phase 1/2 multicenter B-AMAZE open-label trial. It involved 10 patients (all age 18 and older) with severe or moderately severe hemophilia B, defined as having a factor IX level of 2% or less that of normal values.
All patients received one-off gene therapy infusion, at one of four FLT180a doses.
All patients also received immunosuppression to prevent the body from rejecting the vector gene therapy. This consisted of glucocorticoids with or without tacrolimus for a period of ranging from several weeks to several months.
Following the FLT180a infusion, all patients showed dose-dependent increases in factor IX levels. After a median follow-up of 27.2 months (range, 19.1-42.4 months), nearly all the patients (9 of 10) continued to show sustained factor IX activity.
Steady production of factor IX activity started at month 12, with low bleeding frequency that allowed these nine patients to no longer require weekly injections of the protein.
Five of the patients had factor IX levels in the normal range, from 51% to 78%; three patients had lower increases of 23%-43% of the normal range, and one patient who had received the highest dose, had a level that was 260% of normal.
The exception was one patient who required a return to factor IX prophylaxis. He had experienced a failure in the immunosuppression regimen due to a delay in the recognition of an immune response at approximately 22 weeks after treatment, the authors reported.
The therapy was generally well tolerated, with no infusion reactions or discontinuations of infusions. As of the study cutoff, no inhibitors of factor IX were detected.
Of the adverse events, about 10% were determined to be related to the gene therapy. The most common event associated with the gene therapy was increases in liver aminotransferase, which is a concern with AAV gene therapies, the authors commented.
Otherwise, 24% of adverse events were determined to be related to the immunosuppression, and were consistent with the known safety profiles of glucocorticoids and tacrolimus.
Late increases in aminotransferase levels were reported among patients who had received prolonged tacrolimus beyond the tapering of glucocorticoid treatment.
The one serious adverse event that was reported involved an arteriovenous fistula thrombosis, which occurred in the patient who had received the highest dose of gene therapy and who showed the highest factor IX levels.
The current findings, along with data from another recent study involving gene therapy for patients with hemophilia A, emphasized that “immune responses can occur later than previously expected and may coincide with the withdrawal of immunosuppression,” the authors cautioned.
“Consistent best practices for monitoring aminotransferase levels and deciding when ALT increases warrant intervention remain a critical topic for the field,” they noted.
Meanwhile, the patients in this B-AMAZE trial all remain enrolled in a long-term follow-up study to assess the safety and durability of FLT180a over 15 years.
The trial was sponsored by University College London and funded by Freeline Therapeutics. Dr. Chowdary disclosed various relationships with industry.
A version of this article first appeared on Medscape.com.
Patients with hemophilia B face a lifelong need for regular factor IX injections.
“Removing the need for hemophilia patients to regularly inject themselves with the missing protein is an important step in improving their quality of life,” lead author Pratima Chowdary, MD, of the Royal Free Hospital, University College London Cancer Institute, commented in a press statement.
The team reported new results with the investigational gene therapy FLT180a in a study published in the New England Journal of Medicine.
“We found that normal factor IX levels can be achieved in patients with severe or moderately severe hemophilia B with the use of relatively low vector doses of FLT180a,” the authors reported. “In all but one patient, gene therapy led to durable factor IX expression, eliminated the need for factor IX prophylaxis, and eliminated spontaneous bleeding leading to factor IX replacement.”
FLT180a (Freeline Therapeutics) is a liver-directed, adeno-associated virus (AAV) gene therapy designed to normalize levels of the factor IX protein that is needed for coagulation; however, it is produced in dangerously low levels in people with hemophilia B as a result of gene mutations.
Under the current standard of care, patients with hemophilia B require lifelong prophylaxis of regular intravenous injections with recombinant factor IX replacement therapy, and they commonly continue to experience potentially severe joint pain.
While factor-replacement therapies with longer half-lives have emerged, the prophylaxis is still invasive and extremely expensive, with the average price tag in the United States of $397,491 a year for the conventional treatment and an average of $788,861 a year for an extended half-life treatment, according to a 2019 report.
Novel gene therapy
Hemophilia B is a rare and inherited genetic bleeding disorder caused by defects in the gene responsible for factor IX protein, which is needed for blood clotting.
AAV gene therapy delivers a functional copy of this gene directly to patient tissues to compensate for one that is not working properly. It leads to the synthesis of factor IX proteins and a one-time gene therapy infusion can achieve long-lasting effects, the team explained in a press release.
The results they reported come from the phase 1/2 multicenter B-AMAZE open-label trial. It involved 10 patients (all age 18 and older) with severe or moderately severe hemophilia B, defined as having a factor IX level of 2% or less that of normal values.
All patients received one-off gene therapy infusion, at one of four FLT180a doses.
All patients also received immunosuppression to prevent the body from rejecting the vector gene therapy. This consisted of glucocorticoids with or without tacrolimus for a period of ranging from several weeks to several months.
Following the FLT180a infusion, all patients showed dose-dependent increases in factor IX levels. After a median follow-up of 27.2 months (range, 19.1-42.4 months), nearly all the patients (9 of 10) continued to show sustained factor IX activity.
Steady production of factor IX activity started at month 12, with low bleeding frequency that allowed these nine patients to no longer require weekly injections of the protein.
Five of the patients had factor IX levels in the normal range, from 51% to 78%; three patients had lower increases of 23%-43% of the normal range, and one patient who had received the highest dose, had a level that was 260% of normal.
The exception was one patient who required a return to factor IX prophylaxis. He had experienced a failure in the immunosuppression regimen due to a delay in the recognition of an immune response at approximately 22 weeks after treatment, the authors reported.
The therapy was generally well tolerated, with no infusion reactions or discontinuations of infusions. As of the study cutoff, no inhibitors of factor IX were detected.
Of the adverse events, about 10% were determined to be related to the gene therapy. The most common event associated with the gene therapy was increases in liver aminotransferase, which is a concern with AAV gene therapies, the authors commented.
Otherwise, 24% of adverse events were determined to be related to the immunosuppression, and were consistent with the known safety profiles of glucocorticoids and tacrolimus.
Late increases in aminotransferase levels were reported among patients who had received prolonged tacrolimus beyond the tapering of glucocorticoid treatment.
The one serious adverse event that was reported involved an arteriovenous fistula thrombosis, which occurred in the patient who had received the highest dose of gene therapy and who showed the highest factor IX levels.
The current findings, along with data from another recent study involving gene therapy for patients with hemophilia A, emphasized that “immune responses can occur later than previously expected and may coincide with the withdrawal of immunosuppression,” the authors cautioned.
“Consistent best practices for monitoring aminotransferase levels and deciding when ALT increases warrant intervention remain a critical topic for the field,” they noted.
Meanwhile, the patients in this B-AMAZE trial all remain enrolled in a long-term follow-up study to assess the safety and durability of FLT180a over 15 years.
The trial was sponsored by University College London and funded by Freeline Therapeutics. Dr. Chowdary disclosed various relationships with industry.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
In the Quest for Migraine Relief, The Search for Biomarkers Intensifies
The Health Terminology/Ontology Portal (HeTOP), on which the curious can discover information about off-label use, lists 645 medications prescribed for migraine worldwide. Treatments ranging from blood pressure medications to antidepressants, and anticonvulsants to antiepileptics, along with their doses and administrations, are all listed. The number of migraine-indicated medications is 114. Dominated by triptans and topiramate, the list also includes erenumab, the calcitonin gene-related peptide CGRP agonist. The difference in figures between the predominately off label and migraine-approved lists is a good indicator of the struggle that health care providers have had through the years to help their patients.
The idea now is to make that list even longer by finding biomarkers that lead to new therapies.
But first, a conversation about the trigeminal ganglia.
The trigeminal ganglia
The trigeminal ganglia sit on either side of the head, in front of the ears. Their primary role is to receive stimuli and convey it to the brain. The human trigeminal ganglia contain 20,000 to 35,000 neurons and express an array of neuropeptides, including CGRP. Some neuropeptides, like CGRP and pituitary adenylate cyclase–activating peptide 38 (PACAP38) are vasodilators. Others, like substance P, are vasoconstrictors. Edvinsson and Goadsby discussed in 1994 how CGRP was released simultaneously in those with “spontaneous attacks of migraine.”
Over the past 30 years, researchers in our institution and elsewhere have shown repeatedly that migraine develops in individuals who are exposed to certain signaling molecules, namely nitroglycerin, CGRP, cyclic guanosine monophosphate (cGMP), intracellular cyclic adenosine monophosphate (cAMP), potassium, and PACAP38, among others. Such exposure reinforces the notion that peripheral sensitization of trigeminal sensory neurons brings on headache. The attack could occur due to vasodilation, mast cell degranulation, involvement of the parasympathetic system, or activation of nerve fibers.
Some examples from the literature:
- In our research, results from a small study of patients under spontaneous migraine attack, who underwent a 3-Tesla MRI scan, showed that cortical thickness diminishes in the prefrontal and pericalcarine cortices. The analysis we performed involving individuals with migraine without aura revealed that these patients experience reduced cortical thickness and volume when migraine attacks come on, suggesting that cortical thickness and volume may serve as a potential biomarker.
- A comparison of 20 individuals with chronic migraine and 20 healthy controls by way of 3-Tesla magnetic resonance imaging scans revealed that those with headache appeared to have substantially increased neural connectivity between the hypothalamus and certain brain areas – yet there appeared to be no connectivity irregularities between the hypothalamus and brainstem, which as the authors noted, is the “migraine generator.”
In other words, vasodilation might be a secondary symptom of migraine but likely isn’t its source.
Other migraine makers
Neurochemicals and nucleotides play a role in migraine formation, too:
- Nitric oxide. Can open blood vessels in the head and brain and has been shown to set migraine in motion. It leads to peak headache intensity 5.5 hours after infusion and causes migraine without aura.
- GRP. Gastrin-releasing peptide receptors cause delayed headache, including what qualifies as an induced migraine attack. Researchers also note that similar pathways trigger migraine with and without aura.
- Intracellular cGMP and intracellular cAMP. These 2 cyclic nucleotides are found extensively in the trigeminovascular system and have a role in the pathogenesis of migraine. Studies demonstrate that cGMP levels increase after nitroglycerin administration and cAMP increases after CGRP and PACAP38 exposure.
- Levcromakalim. This potassium channel opener is sensitive to ATP. In a trial published in 2019, researchers showed that modulating potassium channels could cause some headache pain, even in those without migraine. They infused 20 healthy volunteers with levcromakalim; over the next 5-plus hours, the middle meningeal artery of all 20 became and remained dilated. Later research showed that this dilation is linked to substance P.
Identifying migraine types
Diagnosing migraine is 1 step; determining its type is another.
Consider that a person with a posttraumatic headache can have migraine-like symptoms. To find objective separate characteristics, researchers at Mayo Clinic designed a headache classification model using questionnaires, which were then paired with the patient’s MRI data. The questionnaires delved into headache characteristics, sensory hypersensitivities, cognitive functioning, and mood. The system worked well with primary migraine, with 97% accuracy. But with posttraumatic headache, the system was 65% accurate. What proved to differentiate persistent posttraumatic headache were questions regarding decision making and anxiety. These patients had severe symptoms of anxiety, depression, physical issues, and mild brain injury attributed to blasts.
All of which explains why we and others are actively looking for biomarkers.
The biomarkers
A look at clinicaltrials.gov shows that 15 trials are recruiting patients (including us) in the search for biomarkers. One wants to identify a computational algorithm using AI, based on 9 types of markers in hopes of identifying those predictive elements that will respond to CGRP-targeting monoclonal antibodies (mABs). The factors range from the clinical to epigenetic to structural and functional brain imaging. Another registered study is using ocular coherence tomography, among other technologies, to identify photophobia.
Our interests are in identifying CGRP as a definitive biomarker; finding structural and functional cerebral changes, using MRI, in study subjects before and after they are given erenumab. We also want to create a registry for migraine based on the structural and functional MRI findings.
Another significant reason for finding biomarkers is to identify the alteration that accompany progression from episodic to chronic migraine. Pozo-Rosich et al write that these imaging, neurophysiological, and biochemical changes that occur with this progression could be used “for developing chronic migraine biomarkers that might assist with diagnosis, prognosticating individual patient outcomes, and predicting responses to migraine therapies.” And, ultimately, in practicing precision medicine to improve care of patients.
Significant barriers still exist in declaring a molecule is a biomarker. For example, a meta-analysis points to the replication challenge observed in neuroimaging research. Additionally, several genetic variants produce small effect sizes, which also might be impacted by environmental factors. This makes it difficult to map genetic biomarkers. Large prospective studies are needed to bring this area of research out of infancy to a place where treatment response can be clinically assessed. Additionally, while research evaluating provocation biomarkers has already contributed to the treatment landscape, large-scale registry studies may help uncover a predictive biomarker of treatment response. Blood biomarker research still needs a standardized protocol. Imaging-based biomarkers show much potential, but standardized imaging protocols and improved characterization and data integration are necessary going forward.
The patients
The discovery of the CGRPs couldn’t have been more timely.
Those of us who have been treating patients with migraine for years have seen the prevalence of this disease slowly rise. In 2018, the age-adjusted prevalence was 15.9% for all adults in the United States; in 2010, it was 13.2%. Worldwide, in 2019, it was 14%. In 2015, it was 11.6%.
In the past few years, journal articles have appeared regarding the connection between obesity, diabetes, hypertension, and migraine severity. Numerous other comorbidities affect our patients – not just the well-known psychiatric disorders – but also the respiratory, digestive, and central nervous system illnesses.
In other words, many of our patients come to us sicker than in years past.
Some cannot take one or more medications designed for acute migraine attacks due to comorbidities, including cardiovascular disease or related risk factors, and gastrointestinal bleeding.
A large survey of 15,133 people with migraine confirmed the findings on these numerous comorbidities; they reported that they have more insomnia, depression, and anxiety. As the authors point out, identifying these comorbidities can help with accurate diagnosis, treatment and its adherence, and prognosis. The authors also noted that as migraine days increase per month, so do the rates of comorbidities.
But the CGRPs are showing how beneficial they can be. One study assessing medication overuse showed how 60% of the enrolled patients no longer fit that description 6 months after receiving erenumab or galcanezumab. Some patients who contend with episodic migraine showed a complete response after receiving eptinezumab and galcanezumab. They also have helped patients with menstrual migraine and refractory migraine.
But they are not complete responses to these medications, which is an excellent reason to continue viewing, recording, and assessing the migraine brain, for all it can tell us.
The Health Terminology/Ontology Portal (HeTOP), on which the curious can discover information about off-label use, lists 645 medications prescribed for migraine worldwide. Treatments ranging from blood pressure medications to antidepressants, and anticonvulsants to antiepileptics, along with their doses and administrations, are all listed. The number of migraine-indicated medications is 114. Dominated by triptans and topiramate, the list also includes erenumab, the calcitonin gene-related peptide CGRP agonist. The difference in figures between the predominately off label and migraine-approved lists is a good indicator of the struggle that health care providers have had through the years to help their patients.
The idea now is to make that list even longer by finding biomarkers that lead to new therapies.
But first, a conversation about the trigeminal ganglia.
The trigeminal ganglia
The trigeminal ganglia sit on either side of the head, in front of the ears. Their primary role is to receive stimuli and convey it to the brain. The human trigeminal ganglia contain 20,000 to 35,000 neurons and express an array of neuropeptides, including CGRP. Some neuropeptides, like CGRP and pituitary adenylate cyclase–activating peptide 38 (PACAP38) are vasodilators. Others, like substance P, are vasoconstrictors. Edvinsson and Goadsby discussed in 1994 how CGRP was released simultaneously in those with “spontaneous attacks of migraine.”
Over the past 30 years, researchers in our institution and elsewhere have shown repeatedly that migraine develops in individuals who are exposed to certain signaling molecules, namely nitroglycerin, CGRP, cyclic guanosine monophosphate (cGMP), intracellular cyclic adenosine monophosphate (cAMP), potassium, and PACAP38, among others. Such exposure reinforces the notion that peripheral sensitization of trigeminal sensory neurons brings on headache. The attack could occur due to vasodilation, mast cell degranulation, involvement of the parasympathetic system, or activation of nerve fibers.
Some examples from the literature:
- In our research, results from a small study of patients under spontaneous migraine attack, who underwent a 3-Tesla MRI scan, showed that cortical thickness diminishes in the prefrontal and pericalcarine cortices. The analysis we performed involving individuals with migraine without aura revealed that these patients experience reduced cortical thickness and volume when migraine attacks come on, suggesting that cortical thickness and volume may serve as a potential biomarker.
- A comparison of 20 individuals with chronic migraine and 20 healthy controls by way of 3-Tesla magnetic resonance imaging scans revealed that those with headache appeared to have substantially increased neural connectivity between the hypothalamus and certain brain areas – yet there appeared to be no connectivity irregularities between the hypothalamus and brainstem, which as the authors noted, is the “migraine generator.”
In other words, vasodilation might be a secondary symptom of migraine but likely isn’t its source.
Other migraine makers
Neurochemicals and nucleotides play a role in migraine formation, too:
- Nitric oxide. Can open blood vessels in the head and brain and has been shown to set migraine in motion. It leads to peak headache intensity 5.5 hours after infusion and causes migraine without aura.
- GRP. Gastrin-releasing peptide receptors cause delayed headache, including what qualifies as an induced migraine attack. Researchers also note that similar pathways trigger migraine with and without aura.
- Intracellular cGMP and intracellular cAMP. These 2 cyclic nucleotides are found extensively in the trigeminovascular system and have a role in the pathogenesis of migraine. Studies demonstrate that cGMP levels increase after nitroglycerin administration and cAMP increases after CGRP and PACAP38 exposure.
- Levcromakalim. This potassium channel opener is sensitive to ATP. In a trial published in 2019, researchers showed that modulating potassium channels could cause some headache pain, even in those without migraine. They infused 20 healthy volunteers with levcromakalim; over the next 5-plus hours, the middle meningeal artery of all 20 became and remained dilated. Later research showed that this dilation is linked to substance P.
Identifying migraine types
Diagnosing migraine is 1 step; determining its type is another.
Consider that a person with a posttraumatic headache can have migraine-like symptoms. To find objective separate characteristics, researchers at Mayo Clinic designed a headache classification model using questionnaires, which were then paired with the patient’s MRI data. The questionnaires delved into headache characteristics, sensory hypersensitivities, cognitive functioning, and mood. The system worked well with primary migraine, with 97% accuracy. But with posttraumatic headache, the system was 65% accurate. What proved to differentiate persistent posttraumatic headache were questions regarding decision making and anxiety. These patients had severe symptoms of anxiety, depression, physical issues, and mild brain injury attributed to blasts.
All of which explains why we and others are actively looking for biomarkers.
The biomarkers
A look at clinicaltrials.gov shows that 15 trials are recruiting patients (including us) in the search for biomarkers. One wants to identify a computational algorithm using AI, based on 9 types of markers in hopes of identifying those predictive elements that will respond to CGRP-targeting monoclonal antibodies (mABs). The factors range from the clinical to epigenetic to structural and functional brain imaging. Another registered study is using ocular coherence tomography, among other technologies, to identify photophobia.
Our interests are in identifying CGRP as a definitive biomarker; finding structural and functional cerebral changes, using MRI, in study subjects before and after they are given erenumab. We also want to create a registry for migraine based on the structural and functional MRI findings.
Another significant reason for finding biomarkers is to identify the alteration that accompany progression from episodic to chronic migraine. Pozo-Rosich et al write that these imaging, neurophysiological, and biochemical changes that occur with this progression could be used “for developing chronic migraine biomarkers that might assist with diagnosis, prognosticating individual patient outcomes, and predicting responses to migraine therapies.” And, ultimately, in practicing precision medicine to improve care of patients.
Significant barriers still exist in declaring a molecule is a biomarker. For example, a meta-analysis points to the replication challenge observed in neuroimaging research. Additionally, several genetic variants produce small effect sizes, which also might be impacted by environmental factors. This makes it difficult to map genetic biomarkers. Large prospective studies are needed to bring this area of research out of infancy to a place where treatment response can be clinically assessed. Additionally, while research evaluating provocation biomarkers has already contributed to the treatment landscape, large-scale registry studies may help uncover a predictive biomarker of treatment response. Blood biomarker research still needs a standardized protocol. Imaging-based biomarkers show much potential, but standardized imaging protocols and improved characterization and data integration are necessary going forward.
The patients
The discovery of the CGRPs couldn’t have been more timely.
Those of us who have been treating patients with migraine for years have seen the prevalence of this disease slowly rise. In 2018, the age-adjusted prevalence was 15.9% for all adults in the United States; in 2010, it was 13.2%. Worldwide, in 2019, it was 14%. In 2015, it was 11.6%.
In the past few years, journal articles have appeared regarding the connection between obesity, diabetes, hypertension, and migraine severity. Numerous other comorbidities affect our patients – not just the well-known psychiatric disorders – but also the respiratory, digestive, and central nervous system illnesses.
In other words, many of our patients come to us sicker than in years past.
Some cannot take one or more medications designed for acute migraine attacks due to comorbidities, including cardiovascular disease or related risk factors, and gastrointestinal bleeding.
A large survey of 15,133 people with migraine confirmed the findings on these numerous comorbidities; they reported that they have more insomnia, depression, and anxiety. As the authors point out, identifying these comorbidities can help with accurate diagnosis, treatment and its adherence, and prognosis. The authors also noted that as migraine days increase per month, so do the rates of comorbidities.
But the CGRPs are showing how beneficial they can be. One study assessing medication overuse showed how 60% of the enrolled patients no longer fit that description 6 months after receiving erenumab or galcanezumab. Some patients who contend with episodic migraine showed a complete response after receiving eptinezumab and galcanezumab. They also have helped patients with menstrual migraine and refractory migraine.
But they are not complete responses to these medications, which is an excellent reason to continue viewing, recording, and assessing the migraine brain, for all it can tell us.
The Health Terminology/Ontology Portal (HeTOP), on which the curious can discover information about off-label use, lists 645 medications prescribed for migraine worldwide. Treatments ranging from blood pressure medications to antidepressants, and anticonvulsants to antiepileptics, along with their doses and administrations, are all listed. The number of migraine-indicated medications is 114. Dominated by triptans and topiramate, the list also includes erenumab, the calcitonin gene-related peptide CGRP agonist. The difference in figures between the predominately off label and migraine-approved lists is a good indicator of the struggle that health care providers have had through the years to help their patients.
The idea now is to make that list even longer by finding biomarkers that lead to new therapies.
But first, a conversation about the trigeminal ganglia.
The trigeminal ganglia
The trigeminal ganglia sit on either side of the head, in front of the ears. Their primary role is to receive stimuli and convey it to the brain. The human trigeminal ganglia contain 20,000 to 35,000 neurons and express an array of neuropeptides, including CGRP. Some neuropeptides, like CGRP and pituitary adenylate cyclase–activating peptide 38 (PACAP38) are vasodilators. Others, like substance P, are vasoconstrictors. Edvinsson and Goadsby discussed in 1994 how CGRP was released simultaneously in those with “spontaneous attacks of migraine.”
Over the past 30 years, researchers in our institution and elsewhere have shown repeatedly that migraine develops in individuals who are exposed to certain signaling molecules, namely nitroglycerin, CGRP, cyclic guanosine monophosphate (cGMP), intracellular cyclic adenosine monophosphate (cAMP), potassium, and PACAP38, among others. Such exposure reinforces the notion that peripheral sensitization of trigeminal sensory neurons brings on headache. The attack could occur due to vasodilation, mast cell degranulation, involvement of the parasympathetic system, or activation of nerve fibers.
Some examples from the literature:
- In our research, results from a small study of patients under spontaneous migraine attack, who underwent a 3-Tesla MRI scan, showed that cortical thickness diminishes in the prefrontal and pericalcarine cortices. The analysis we performed involving individuals with migraine without aura revealed that these patients experience reduced cortical thickness and volume when migraine attacks come on, suggesting that cortical thickness and volume may serve as a potential biomarker.
- A comparison of 20 individuals with chronic migraine and 20 healthy controls by way of 3-Tesla magnetic resonance imaging scans revealed that those with headache appeared to have substantially increased neural connectivity between the hypothalamus and certain brain areas – yet there appeared to be no connectivity irregularities between the hypothalamus and brainstem, which as the authors noted, is the “migraine generator.”
In other words, vasodilation might be a secondary symptom of migraine but likely isn’t its source.
Other migraine makers
Neurochemicals and nucleotides play a role in migraine formation, too:
- Nitric oxide. Can open blood vessels in the head and brain and has been shown to set migraine in motion. It leads to peak headache intensity 5.5 hours after infusion and causes migraine without aura.
- GRP. Gastrin-releasing peptide receptors cause delayed headache, including what qualifies as an induced migraine attack. Researchers also note that similar pathways trigger migraine with and without aura.
- Intracellular cGMP and intracellular cAMP. These 2 cyclic nucleotides are found extensively in the trigeminovascular system and have a role in the pathogenesis of migraine. Studies demonstrate that cGMP levels increase after nitroglycerin administration and cAMP increases after CGRP and PACAP38 exposure.
- Levcromakalim. This potassium channel opener is sensitive to ATP. In a trial published in 2019, researchers showed that modulating potassium channels could cause some headache pain, even in those without migraine. They infused 20 healthy volunteers with levcromakalim; over the next 5-plus hours, the middle meningeal artery of all 20 became and remained dilated. Later research showed that this dilation is linked to substance P.
Identifying migraine types
Diagnosing migraine is 1 step; determining its type is another.
Consider that a person with a posttraumatic headache can have migraine-like symptoms. To find objective separate characteristics, researchers at Mayo Clinic designed a headache classification model using questionnaires, which were then paired with the patient’s MRI data. The questionnaires delved into headache characteristics, sensory hypersensitivities, cognitive functioning, and mood. The system worked well with primary migraine, with 97% accuracy. But with posttraumatic headache, the system was 65% accurate. What proved to differentiate persistent posttraumatic headache were questions regarding decision making and anxiety. These patients had severe symptoms of anxiety, depression, physical issues, and mild brain injury attributed to blasts.
All of which explains why we and others are actively looking for biomarkers.
The biomarkers
A look at clinicaltrials.gov shows that 15 trials are recruiting patients (including us) in the search for biomarkers. One wants to identify a computational algorithm using AI, based on 9 types of markers in hopes of identifying those predictive elements that will respond to CGRP-targeting monoclonal antibodies (mABs). The factors range from the clinical to epigenetic to structural and functional brain imaging. Another registered study is using ocular coherence tomography, among other technologies, to identify photophobia.
Our interests are in identifying CGRP as a definitive biomarker; finding structural and functional cerebral changes, using MRI, in study subjects before and after they are given erenumab. We also want to create a registry for migraine based on the structural and functional MRI findings.
Another significant reason for finding biomarkers is to identify the alteration that accompany progression from episodic to chronic migraine. Pozo-Rosich et al write that these imaging, neurophysiological, and biochemical changes that occur with this progression could be used “for developing chronic migraine biomarkers that might assist with diagnosis, prognosticating individual patient outcomes, and predicting responses to migraine therapies.” And, ultimately, in practicing precision medicine to improve care of patients.
Significant barriers still exist in declaring a molecule is a biomarker. For example, a meta-analysis points to the replication challenge observed in neuroimaging research. Additionally, several genetic variants produce small effect sizes, which also might be impacted by environmental factors. This makes it difficult to map genetic biomarkers. Large prospective studies are needed to bring this area of research out of infancy to a place where treatment response can be clinically assessed. Additionally, while research evaluating provocation biomarkers has already contributed to the treatment landscape, large-scale registry studies may help uncover a predictive biomarker of treatment response. Blood biomarker research still needs a standardized protocol. Imaging-based biomarkers show much potential, but standardized imaging protocols and improved characterization and data integration are necessary going forward.
The patients
The discovery of the CGRPs couldn’t have been more timely.
Those of us who have been treating patients with migraine for years have seen the prevalence of this disease slowly rise. In 2018, the age-adjusted prevalence was 15.9% for all adults in the United States; in 2010, it was 13.2%. Worldwide, in 2019, it was 14%. In 2015, it was 11.6%.
In the past few years, journal articles have appeared regarding the connection between obesity, diabetes, hypertension, and migraine severity. Numerous other comorbidities affect our patients – not just the well-known psychiatric disorders – but also the respiratory, digestive, and central nervous system illnesses.
In other words, many of our patients come to us sicker than in years past.
Some cannot take one or more medications designed for acute migraine attacks due to comorbidities, including cardiovascular disease or related risk factors, and gastrointestinal bleeding.
A large survey of 15,133 people with migraine confirmed the findings on these numerous comorbidities; they reported that they have more insomnia, depression, and anxiety. As the authors point out, identifying these comorbidities can help with accurate diagnosis, treatment and its adherence, and prognosis. The authors also noted that as migraine days increase per month, so do the rates of comorbidities.
But the CGRPs are showing how beneficial they can be. One study assessing medication overuse showed how 60% of the enrolled patients no longer fit that description 6 months after receiving erenumab or galcanezumab. Some patients who contend with episodic migraine showed a complete response after receiving eptinezumab and galcanezumab. They also have helped patients with menstrual migraine and refractory migraine.
But they are not complete responses to these medications, which is an excellent reason to continue viewing, recording, and assessing the migraine brain, for all it can tell us.
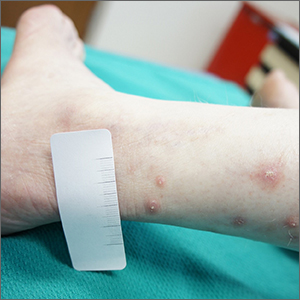
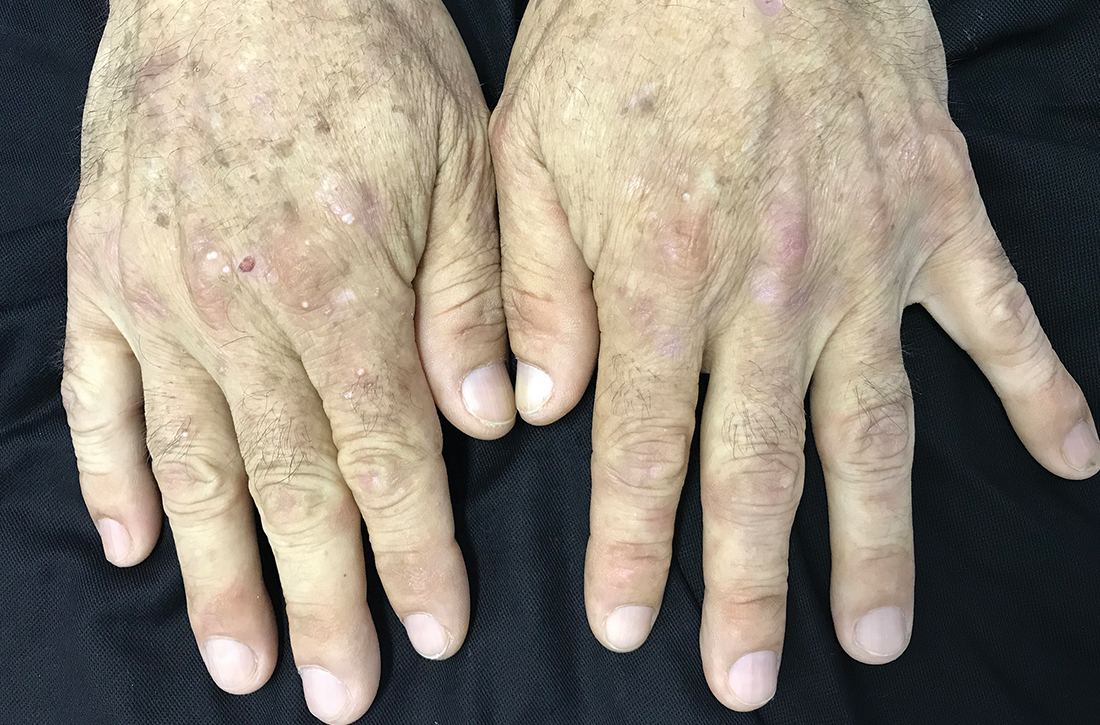
Leg lesions

A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561

A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561
The testing we order should help, not hurt
Ordering and interpreting tests is at the heart of what we do as family physicians. Ordering tests judiciously and interpreting them accurately is not easy. The Choosing Wisely campaign1 has focused our attention on the need to think carefully before ordering tests, whether they be laboratory tests or imaging. Before ordering any test, one should always ask: Is the result of this test going to help me make better decisions about managing this patient?
I would like to highlight and expand on 2 problematic issues Kaminski and Venkat raise in their excellent article on testing in this issue of JFP.2
First, they advise us to know the pretest probability of a disease before we order a test. If we order a test on a patient for whom the probability of disease is very low, a positive result is likely to be a false-positive and mislead us into thinking the patient has the disease when he does not. If we order a test for a patient with a high probability of disease and the result is negative, there is great danger of a false-negative. We might think the patient does not have the disease, but she does.
There is a deeper problem here, however. Primary care physicians are notorious for overestimating disease probability. In a recent study, primary care clinicians overestimated the pretest probability of disease 2- to 10-fold in scenarios involving 4 common diagnoses: breast cancer, coronary artery disease (CAD), pneumonia, and urinary tract infection.3 Even after receiving a negative test result, clinicians still overestimated the chance of disease in all the scenarios. For example, when presented with a 43-year-old premenopausal woman with atypical chest pain and a normal electrocardiogram, clinicians’ average estimate of the probability of CAD was 10%—considerably higher than true estimates of 1% to 4.4%.3
To improve your accuracy in judging pretest probabilities, see the diagnostic test calculators in Essential Evidence Plus (www.essentialevidenceplus.com/).
Secondly, Kaminski and Venkat advise us to try to avoid the testing cascade.2 The associated dangers to patients are considerable. For a cautionary tale, I recommend you read the essay by Michael B. Rothberg, MD, MPH, called “The $50,000 Physical”.4 Dr. Rothberg describes the testing cascade his 85-year-old father experienced, which led to a liver biopsy that nearly killed him from post-biopsy bleeding. Always remember: Testing is a double-edged sword. It can help—or harm—your patients.
1. American Board of Internal Medicine Foundation. Choosing Wisely. Accessed June 30, 2022. www.choosingwisely.org/
2. Kaminski M, Venkat N. A judicious approach to ordering lab tests. J Fam Pract. 2022;71:245-250. doi: 10.12788/jfp.0444
3. Morgan DJ, Pineles L, Owczarzak J, et al. Accuracy of practitioner estimates of probability of diagnosis before and after testing. JAMA Intern Med. 2021;181:747-755. doi: 10.1001/jamainternmed.2021.0269
4. Rothberg MB. The $50 000 physical. JAMA. 2020;323:1682-1683. doi: 10.1001/jama.2020.2866
Ordering and interpreting tests is at the heart of what we do as family physicians. Ordering tests judiciously and interpreting them accurately is not easy. The Choosing Wisely campaign1 has focused our attention on the need to think carefully before ordering tests, whether they be laboratory tests or imaging. Before ordering any test, one should always ask: Is the result of this test going to help me make better decisions about managing this patient?
I would like to highlight and expand on 2 problematic issues Kaminski and Venkat raise in their excellent article on testing in this issue of JFP.2
First, they advise us to know the pretest probability of a disease before we order a test. If we order a test on a patient for whom the probability of disease is very low, a positive result is likely to be a false-positive and mislead us into thinking the patient has the disease when he does not. If we order a test for a patient with a high probability of disease and the result is negative, there is great danger of a false-negative. We might think the patient does not have the disease, but she does.
There is a deeper problem here, however. Primary care physicians are notorious for overestimating disease probability. In a recent study, primary care clinicians overestimated the pretest probability of disease 2- to 10-fold in scenarios involving 4 common diagnoses: breast cancer, coronary artery disease (CAD), pneumonia, and urinary tract infection.3 Even after receiving a negative test result, clinicians still overestimated the chance of disease in all the scenarios. For example, when presented with a 43-year-old premenopausal woman with atypical chest pain and a normal electrocardiogram, clinicians’ average estimate of the probability of CAD was 10%—considerably higher than true estimates of 1% to 4.4%.3
To improve your accuracy in judging pretest probabilities, see the diagnostic test calculators in Essential Evidence Plus (www.essentialevidenceplus.com/).
Secondly, Kaminski and Venkat advise us to try to avoid the testing cascade.2 The associated dangers to patients are considerable. For a cautionary tale, I recommend you read the essay by Michael B. Rothberg, MD, MPH, called “The $50,000 Physical”.4 Dr. Rothberg describes the testing cascade his 85-year-old father experienced, which led to a liver biopsy that nearly killed him from post-biopsy bleeding. Always remember: Testing is a double-edged sword. It can help—or harm—your patients.
Ordering and interpreting tests is at the heart of what we do as family physicians. Ordering tests judiciously and interpreting them accurately is not easy. The Choosing Wisely campaign1 has focused our attention on the need to think carefully before ordering tests, whether they be laboratory tests or imaging. Before ordering any test, one should always ask: Is the result of this test going to help me make better decisions about managing this patient?
I would like to highlight and expand on 2 problematic issues Kaminski and Venkat raise in their excellent article on testing in this issue of JFP.2
First, they advise us to know the pretest probability of a disease before we order a test. If we order a test on a patient for whom the probability of disease is very low, a positive result is likely to be a false-positive and mislead us into thinking the patient has the disease when he does not. If we order a test for a patient with a high probability of disease and the result is negative, there is great danger of a false-negative. We might think the patient does not have the disease, but she does.
There is a deeper problem here, however. Primary care physicians are notorious for overestimating disease probability. In a recent study, primary care clinicians overestimated the pretest probability of disease 2- to 10-fold in scenarios involving 4 common diagnoses: breast cancer, coronary artery disease (CAD), pneumonia, and urinary tract infection.3 Even after receiving a negative test result, clinicians still overestimated the chance of disease in all the scenarios. For example, when presented with a 43-year-old premenopausal woman with atypical chest pain and a normal electrocardiogram, clinicians’ average estimate of the probability of CAD was 10%—considerably higher than true estimates of 1% to 4.4%.3
To improve your accuracy in judging pretest probabilities, see the diagnostic test calculators in Essential Evidence Plus (www.essentialevidenceplus.com/).
Secondly, Kaminski and Venkat advise us to try to avoid the testing cascade.2 The associated dangers to patients are considerable. For a cautionary tale, I recommend you read the essay by Michael B. Rothberg, MD, MPH, called “The $50,000 Physical”.4 Dr. Rothberg describes the testing cascade his 85-year-old father experienced, which led to a liver biopsy that nearly killed him from post-biopsy bleeding. Always remember: Testing is a double-edged sword. It can help—or harm—your patients.
1. American Board of Internal Medicine Foundation. Choosing Wisely. Accessed June 30, 2022. www.choosingwisely.org/
2. Kaminski M, Venkat N. A judicious approach to ordering lab tests. J Fam Pract. 2022;71:245-250. doi: 10.12788/jfp.0444
3. Morgan DJ, Pineles L, Owczarzak J, et al. Accuracy of practitioner estimates of probability of diagnosis before and after testing. JAMA Intern Med. 2021;181:747-755. doi: 10.1001/jamainternmed.2021.0269
4. Rothberg MB. The $50 000 physical. JAMA. 2020;323:1682-1683. doi: 10.1001/jama.2020.2866
1. American Board of Internal Medicine Foundation. Choosing Wisely. Accessed June 30, 2022. www.choosingwisely.org/
2. Kaminski M, Venkat N. A judicious approach to ordering lab tests. J Fam Pract. 2022;71:245-250. doi: 10.12788/jfp.0444
3. Morgan DJ, Pineles L, Owczarzak J, et al. Accuracy of practitioner estimates of probability of diagnosis before and after testing. JAMA Intern Med. 2021;181:747-755. doi: 10.1001/jamainternmed.2021.0269
4. Rothberg MB. The $50 000 physical. JAMA. 2020;323:1682-1683. doi: 10.1001/jama.2020.2866
Milium cysts on hands; hypertrichosis on face
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda

The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda

The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda

The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
Can early introduction of gluten reduce risk of celiac disease?
ILLUSTRATIVE CASE
You are seeing a 2-month-old female infant for a routine well-child visit. The birth history was unremarkable. The infant is meeting appropriate developmental milestones. Growth is appropriate at the 40th percentile. The infant is exclusively breastfed. The parents report that they have heard confusing information about when to introduce solid foods, including bread, to their child’s diet. There is no known family history of CD. What anticipatory guidance can you offer regarding gluten introduction and the risk of CD?
CD is an inflammatory disease of the small intestine caused by an immune-based reaction to dietary gluten. The worldwide incidence of CD in children younger than 15 years is 21.3 per 100,000 person-years; this incidence has increased by 7.5% per year over the past several decades.2 CD has a range of both gastrointestinal and nongastrointestinal manifestations, including diarrhea, weight loss, abdominal pain, abnormal liver function test results, and iron deficiency anemia.
Diagnosis of CD in adults is based on a combination of clinical symptoms, elevated levels of immunoglobulin A anti-tissue transglutaminase antibody (tTG-IgA), and biopsy-confirmed villous atrophy of the duodenum on upper endoscopy.3 European pediatric guidelines suggest that use of certain criteria, including very high results of tTG-IgA antibody testing (> 10 times the upper limit of normal), can help to avoid endoscopic biopsies and/or human leukocyte antigens (HLA) testing for diagnosis in children.4
The mainstay of CD management is strict adherence to a gluten-free diet.3 Because this can be difficult, and yield an incomplete disease response, emphasis has been placed on primary prevention by modifying introduction of dietary gluten. Multiple prior studies examining the risk of CD have failed to demonstrate a significant association between timing of gluten introduction and development of CD among high-risk infants (eg, those with HLA-DR3 alleles or first-degree relatives with CD or type 1 diabetes).5-7 A 2016 meta-analysis concluded that there was not enough evidence to support early introduction of gluten (at 4-6 months).8 RCTs have not previously been conducted to examine the timing of gluten introduction on CD prevalence for infants at average risk, using age-appropriate doses of gluten prior to age 6 months.
Current dietary guidelines in the United States and the United Kingdom recommend introduction of nutrient-dense foods, including potentially allergenic foods, at about age 6 months to complement human milk or infant formula feedings.9,10 These guidelines do not specify the exact timing or quantity of gluten- containing food introduction for infants. A 2016 position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition indicated that gluten could be introduced into the infant’s diet any time between 4 and 12 months. They did indicate that the amount of gluten introduced into the diet should be low to start and then increased, and that infants at high risk for CD should wait longer for gluten introduction (4 vs 6 months or 6 vs 12 months).11
STUDY SUMMARY
Gluten introduced at 4 months may be linked to lower occurrence of CD
The Enquiring About Tolerance (EAT) Study was an open-label RCT (N = 1303) with children from the general population in England and Wales. The EAT Study sought to test the prevention of food allergy by introducing allergenic foods to infants at age 4 months compared with exclusively breastfeeding until age 6 months. The median age at enrollment was 3.4 months, but allergenic food was not started until age 4 months.1,12 Most patients were White (84.%-85.4%) and lived in an urban area (77.3%-77.4%). The mean gestational age at delivery was 39.7 to 39.9 weeks.12
Infants were exclusively breastfed until age 13 weeks, at which time they were randomized into an early introduction group (EIG) or a standard introduction group (SIG). In addition to breast milk, infants in the EIG consumed 6 allergenic foods (peanut, sesame, hen’s egg, cow’s milk, cod fish, and wheat [gluten]) in a specified pattern per protocol, starting at age 4 months. Wheat (gluten) was introduced during Week 5 of the EIG protocol (median age, 20.6 weeks).12 The recommended minimum dose of gluten was 3.2 g/wk from age 16 weeks, or 4 g/wk of wheat protein (given as 2 cereal biscuits or the equivalent). Infants in the SIG avoided allergenic foods, following UK infant feeding recommendations for exclusive breastfeeding until about age 6 months. The EIG had a significantly higher rate of cesarean births than the SIG, but the study groups were otherwise balanced.13
Continue to: Families completed monthly...
Families completed monthly questionnaires on infant gluten intake and symptoms (eg, gastrointestinal, fatigue) through age 1 year, and then every 3 months through age 3 years. All children were tested for anti-transglutaminase type 2 (anti-TG2) antibodies at age 3 years as a screen for CD. Children with antibody levels > 20 IU/L were referred to independent gastroenterologists for further evaluation, which could include HLA (DQ-2/DQ-8) testing and biopsy in accordance with current European diagnostic guidelines.4
In an intention-to-treat analysis for the primary outcome, 595 children in the SIG (91.4%) and 567 in the EIG (87.0%) were included. Between ages 4 and 6 months, the mean (SD) quantity of gluten consumed in the SIG was 0.49 (1.40) g/wk; in the EIG, the mean quantity was 2.66 (1.85) g/wk (P < .001). At age 3 years, of a total of 1004 children tested for anti-TG2 antibodies, 9 had anti-TG2 levels requiring referral (7 in the SIG and 2 in the EIG). A diagnosis of CD was confirmed in 7 of 516 children in the SIG (1.4%) vs none of the 488 children in the EIG (P = .02). Using bootstrap resampling, the risk difference between the groups was 1.4% (95% CI, 0.6%-2.6%).
WHAT’S NEW
Findings have potential to change nutritional guidance
This study demonstrated that introduction of age-appropriate portions of gluten-containing products at age 4 months, in addition to breast milk, may reduce the risk of CD at 3 years in children at average risk. This finding has the potential to change anticipatory guidance given to parents regarding infant nutrition recommendations.
CAVEATS
More studies needed to confirm prevention vs delay of CD
The homogeneous study population may limit generalizability. Infants in this study were from England and Wales (84.3% were White), born at term, and were exclusively breastfed until age 13 weeks. Further studies are required to determine whether these findings can be applied to infants who are no longer breastfeeding, are more racially diverse, or are preterm in gestational age at birth. Additionally, the study followed the participants only until age 3 years. Given that the onset of CD after this age is likely, further research is needed to support that CD is truly prevented rather than delayed.
CHALLENGES TO IMPLEMENTATION
Guidance on allergen introduction may be unclear
The EAT Study protocol required parents in the EIG to sequentially introduce a minimum amount of the 6 allergenic foods specified. Only 42% of the EIG cohort reported adherence to the protocol.12 It is unclear how important this specific regimen is to the study results and whether introduction of all 6 allergenic foods simultaneously modifies the immune response to gluten. Therefore, there may be challenges to implementation if physicians do not know how to provide anticipatory guidance on the appropriate steps for allergen introduction.
1. Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047. doi: 10.1001/jamapediatrics.2020.2893
2. King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115:507-525. doi: 10.14309/ajg.0000000000000523
3. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
4. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
5. Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304-1315. doi: 10.1056/NEJMoa1404172
6. Beyerlein A, Chmiel R, Hummel S, et al. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. 2014;37:e194-e195. doi: 10.2337/dc14-1208
7. Lionetti E, Castellaneta S, Francavilla R, et al; SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295-1303. doi: 10.1056/NEJMoa1400697
8. Pinto-Sánchez MI, Verdu EF, Liu E, et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J Pediatr. 2016;168:132-143.e3. doi: 10.1016/j.jpeds.2015.09.032
9. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. December 2020. Accessed June 8, 2022. www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
10. NHS. Food allergies in babies and young children. Last reviewed November 5, 2021. Accessed June 8, 2022. www.nhs.uk/conditions/baby/weaning-and-feeding/food-allergies-in-babies-and-young-children/
11. Szajewska H, Shamir R, Mearin L, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016;62:507-513. doi: 10.1097/MPG.0000000000001105
12. Perkin MR, Logan K, Marrs T, et al; EAT Study Team. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477-1486.e8. doi: 10.1016/j.jaci.2015.12.1322
13. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743. doi: 10.1056/NEJMoa1514210
ILLUSTRATIVE CASE
You are seeing a 2-month-old female infant for a routine well-child visit. The birth history was unremarkable. The infant is meeting appropriate developmental milestones. Growth is appropriate at the 40th percentile. The infant is exclusively breastfed. The parents report that they have heard confusing information about when to introduce solid foods, including bread, to their child’s diet. There is no known family history of CD. What anticipatory guidance can you offer regarding gluten introduction and the risk of CD?
CD is an inflammatory disease of the small intestine caused by an immune-based reaction to dietary gluten. The worldwide incidence of CD in children younger than 15 years is 21.3 per 100,000 person-years; this incidence has increased by 7.5% per year over the past several decades.2 CD has a range of both gastrointestinal and nongastrointestinal manifestations, including diarrhea, weight loss, abdominal pain, abnormal liver function test results, and iron deficiency anemia.
Diagnosis of CD in adults is based on a combination of clinical symptoms, elevated levels of immunoglobulin A anti-tissue transglutaminase antibody (tTG-IgA), and biopsy-confirmed villous atrophy of the duodenum on upper endoscopy.3 European pediatric guidelines suggest that use of certain criteria, including very high results of tTG-IgA antibody testing (> 10 times the upper limit of normal), can help to avoid endoscopic biopsies and/or human leukocyte antigens (HLA) testing for diagnosis in children.4
The mainstay of CD management is strict adherence to a gluten-free diet.3 Because this can be difficult, and yield an incomplete disease response, emphasis has been placed on primary prevention by modifying introduction of dietary gluten. Multiple prior studies examining the risk of CD have failed to demonstrate a significant association between timing of gluten introduction and development of CD among high-risk infants (eg, those with HLA-DR3 alleles or first-degree relatives with CD or type 1 diabetes).5-7 A 2016 meta-analysis concluded that there was not enough evidence to support early introduction of gluten (at 4-6 months).8 RCTs have not previously been conducted to examine the timing of gluten introduction on CD prevalence for infants at average risk, using age-appropriate doses of gluten prior to age 6 months.
Current dietary guidelines in the United States and the United Kingdom recommend introduction of nutrient-dense foods, including potentially allergenic foods, at about age 6 months to complement human milk or infant formula feedings.9,10 These guidelines do not specify the exact timing or quantity of gluten- containing food introduction for infants. A 2016 position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition indicated that gluten could be introduced into the infant’s diet any time between 4 and 12 months. They did indicate that the amount of gluten introduced into the diet should be low to start and then increased, and that infants at high risk for CD should wait longer for gluten introduction (4 vs 6 months or 6 vs 12 months).11
STUDY SUMMARY
Gluten introduced at 4 months may be linked to lower occurrence of CD
The Enquiring About Tolerance (EAT) Study was an open-label RCT (N = 1303) with children from the general population in England and Wales. The EAT Study sought to test the prevention of food allergy by introducing allergenic foods to infants at age 4 months compared with exclusively breastfeeding until age 6 months. The median age at enrollment was 3.4 months, but allergenic food was not started until age 4 months.1,12 Most patients were White (84.%-85.4%) and lived in an urban area (77.3%-77.4%). The mean gestational age at delivery was 39.7 to 39.9 weeks.12
Infants were exclusively breastfed until age 13 weeks, at which time they were randomized into an early introduction group (EIG) or a standard introduction group (SIG). In addition to breast milk, infants in the EIG consumed 6 allergenic foods (peanut, sesame, hen’s egg, cow’s milk, cod fish, and wheat [gluten]) in a specified pattern per protocol, starting at age 4 months. Wheat (gluten) was introduced during Week 5 of the EIG protocol (median age, 20.6 weeks).12 The recommended minimum dose of gluten was 3.2 g/wk from age 16 weeks, or 4 g/wk of wheat protein (given as 2 cereal biscuits or the equivalent). Infants in the SIG avoided allergenic foods, following UK infant feeding recommendations for exclusive breastfeeding until about age 6 months. The EIG had a significantly higher rate of cesarean births than the SIG, but the study groups were otherwise balanced.13
Continue to: Families completed monthly...
Families completed monthly questionnaires on infant gluten intake and symptoms (eg, gastrointestinal, fatigue) through age 1 year, and then every 3 months through age 3 years. All children were tested for anti-transglutaminase type 2 (anti-TG2) antibodies at age 3 years as a screen for CD. Children with antibody levels > 20 IU/L were referred to independent gastroenterologists for further evaluation, which could include HLA (DQ-2/DQ-8) testing and biopsy in accordance with current European diagnostic guidelines.4
In an intention-to-treat analysis for the primary outcome, 595 children in the SIG (91.4%) and 567 in the EIG (87.0%) were included. Between ages 4 and 6 months, the mean (SD) quantity of gluten consumed in the SIG was 0.49 (1.40) g/wk; in the EIG, the mean quantity was 2.66 (1.85) g/wk (P < .001). At age 3 years, of a total of 1004 children tested for anti-TG2 antibodies, 9 had anti-TG2 levels requiring referral (7 in the SIG and 2 in the EIG). A diagnosis of CD was confirmed in 7 of 516 children in the SIG (1.4%) vs none of the 488 children in the EIG (P = .02). Using bootstrap resampling, the risk difference between the groups was 1.4% (95% CI, 0.6%-2.6%).
WHAT’S NEW
Findings have potential to change nutritional guidance
This study demonstrated that introduction of age-appropriate portions of gluten-containing products at age 4 months, in addition to breast milk, may reduce the risk of CD at 3 years in children at average risk. This finding has the potential to change anticipatory guidance given to parents regarding infant nutrition recommendations.
CAVEATS
More studies needed to confirm prevention vs delay of CD
The homogeneous study population may limit generalizability. Infants in this study were from England and Wales (84.3% were White), born at term, and were exclusively breastfed until age 13 weeks. Further studies are required to determine whether these findings can be applied to infants who are no longer breastfeeding, are more racially diverse, or are preterm in gestational age at birth. Additionally, the study followed the participants only until age 3 years. Given that the onset of CD after this age is likely, further research is needed to support that CD is truly prevented rather than delayed.
CHALLENGES TO IMPLEMENTATION
Guidance on allergen introduction may be unclear
The EAT Study protocol required parents in the EIG to sequentially introduce a minimum amount of the 6 allergenic foods specified. Only 42% of the EIG cohort reported adherence to the protocol.12 It is unclear how important this specific regimen is to the study results and whether introduction of all 6 allergenic foods simultaneously modifies the immune response to gluten. Therefore, there may be challenges to implementation if physicians do not know how to provide anticipatory guidance on the appropriate steps for allergen introduction.
ILLUSTRATIVE CASE
You are seeing a 2-month-old female infant for a routine well-child visit. The birth history was unremarkable. The infant is meeting appropriate developmental milestones. Growth is appropriate at the 40th percentile. The infant is exclusively breastfed. The parents report that they have heard confusing information about when to introduce solid foods, including bread, to their child’s diet. There is no known family history of CD. What anticipatory guidance can you offer regarding gluten introduction and the risk of CD?
CD is an inflammatory disease of the small intestine caused by an immune-based reaction to dietary gluten. The worldwide incidence of CD in children younger than 15 years is 21.3 per 100,000 person-years; this incidence has increased by 7.5% per year over the past several decades.2 CD has a range of both gastrointestinal and nongastrointestinal manifestations, including diarrhea, weight loss, abdominal pain, abnormal liver function test results, and iron deficiency anemia.
Diagnosis of CD in adults is based on a combination of clinical symptoms, elevated levels of immunoglobulin A anti-tissue transglutaminase antibody (tTG-IgA), and biopsy-confirmed villous atrophy of the duodenum on upper endoscopy.3 European pediatric guidelines suggest that use of certain criteria, including very high results of tTG-IgA antibody testing (> 10 times the upper limit of normal), can help to avoid endoscopic biopsies and/or human leukocyte antigens (HLA) testing for diagnosis in children.4
The mainstay of CD management is strict adherence to a gluten-free diet.3 Because this can be difficult, and yield an incomplete disease response, emphasis has been placed on primary prevention by modifying introduction of dietary gluten. Multiple prior studies examining the risk of CD have failed to demonstrate a significant association between timing of gluten introduction and development of CD among high-risk infants (eg, those with HLA-DR3 alleles or first-degree relatives with CD or type 1 diabetes).5-7 A 2016 meta-analysis concluded that there was not enough evidence to support early introduction of gluten (at 4-6 months).8 RCTs have not previously been conducted to examine the timing of gluten introduction on CD prevalence for infants at average risk, using age-appropriate doses of gluten prior to age 6 months.
Current dietary guidelines in the United States and the United Kingdom recommend introduction of nutrient-dense foods, including potentially allergenic foods, at about age 6 months to complement human milk or infant formula feedings.9,10 These guidelines do not specify the exact timing or quantity of gluten- containing food introduction for infants. A 2016 position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition indicated that gluten could be introduced into the infant’s diet any time between 4 and 12 months. They did indicate that the amount of gluten introduced into the diet should be low to start and then increased, and that infants at high risk for CD should wait longer for gluten introduction (4 vs 6 months or 6 vs 12 months).11
STUDY SUMMARY
Gluten introduced at 4 months may be linked to lower occurrence of CD
The Enquiring About Tolerance (EAT) Study was an open-label RCT (N = 1303) with children from the general population in England and Wales. The EAT Study sought to test the prevention of food allergy by introducing allergenic foods to infants at age 4 months compared with exclusively breastfeeding until age 6 months. The median age at enrollment was 3.4 months, but allergenic food was not started until age 4 months.1,12 Most patients were White (84.%-85.4%) and lived in an urban area (77.3%-77.4%). The mean gestational age at delivery was 39.7 to 39.9 weeks.12
Infants were exclusively breastfed until age 13 weeks, at which time they were randomized into an early introduction group (EIG) or a standard introduction group (SIG). In addition to breast milk, infants in the EIG consumed 6 allergenic foods (peanut, sesame, hen’s egg, cow’s milk, cod fish, and wheat [gluten]) in a specified pattern per protocol, starting at age 4 months. Wheat (gluten) was introduced during Week 5 of the EIG protocol (median age, 20.6 weeks).12 The recommended minimum dose of gluten was 3.2 g/wk from age 16 weeks, or 4 g/wk of wheat protein (given as 2 cereal biscuits or the equivalent). Infants in the SIG avoided allergenic foods, following UK infant feeding recommendations for exclusive breastfeeding until about age 6 months. The EIG had a significantly higher rate of cesarean births than the SIG, but the study groups were otherwise balanced.13
Continue to: Families completed monthly...
Families completed monthly questionnaires on infant gluten intake and symptoms (eg, gastrointestinal, fatigue) through age 1 year, and then every 3 months through age 3 years. All children were tested for anti-transglutaminase type 2 (anti-TG2) antibodies at age 3 years as a screen for CD. Children with antibody levels > 20 IU/L were referred to independent gastroenterologists for further evaluation, which could include HLA (DQ-2/DQ-8) testing and biopsy in accordance with current European diagnostic guidelines.4
In an intention-to-treat analysis for the primary outcome, 595 children in the SIG (91.4%) and 567 in the EIG (87.0%) were included. Between ages 4 and 6 months, the mean (SD) quantity of gluten consumed in the SIG was 0.49 (1.40) g/wk; in the EIG, the mean quantity was 2.66 (1.85) g/wk (P < .001). At age 3 years, of a total of 1004 children tested for anti-TG2 antibodies, 9 had anti-TG2 levels requiring referral (7 in the SIG and 2 in the EIG). A diagnosis of CD was confirmed in 7 of 516 children in the SIG (1.4%) vs none of the 488 children in the EIG (P = .02). Using bootstrap resampling, the risk difference between the groups was 1.4% (95% CI, 0.6%-2.6%).
WHAT’S NEW
Findings have potential to change nutritional guidance
This study demonstrated that introduction of age-appropriate portions of gluten-containing products at age 4 months, in addition to breast milk, may reduce the risk of CD at 3 years in children at average risk. This finding has the potential to change anticipatory guidance given to parents regarding infant nutrition recommendations.
CAVEATS
More studies needed to confirm prevention vs delay of CD
The homogeneous study population may limit generalizability. Infants in this study were from England and Wales (84.3% were White), born at term, and were exclusively breastfed until age 13 weeks. Further studies are required to determine whether these findings can be applied to infants who are no longer breastfeeding, are more racially diverse, or are preterm in gestational age at birth. Additionally, the study followed the participants only until age 3 years. Given that the onset of CD after this age is likely, further research is needed to support that CD is truly prevented rather than delayed.
CHALLENGES TO IMPLEMENTATION
Guidance on allergen introduction may be unclear
The EAT Study protocol required parents in the EIG to sequentially introduce a minimum amount of the 6 allergenic foods specified. Only 42% of the EIG cohort reported adherence to the protocol.12 It is unclear how important this specific regimen is to the study results and whether introduction of all 6 allergenic foods simultaneously modifies the immune response to gluten. Therefore, there may be challenges to implementation if physicians do not know how to provide anticipatory guidance on the appropriate steps for allergen introduction.
1. Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047. doi: 10.1001/jamapediatrics.2020.2893
2. King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115:507-525. doi: 10.14309/ajg.0000000000000523
3. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
4. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
5. Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304-1315. doi: 10.1056/NEJMoa1404172
6. Beyerlein A, Chmiel R, Hummel S, et al. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. 2014;37:e194-e195. doi: 10.2337/dc14-1208
7. Lionetti E, Castellaneta S, Francavilla R, et al; SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295-1303. doi: 10.1056/NEJMoa1400697
8. Pinto-Sánchez MI, Verdu EF, Liu E, et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J Pediatr. 2016;168:132-143.e3. doi: 10.1016/j.jpeds.2015.09.032
9. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. December 2020. Accessed June 8, 2022. www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
10. NHS. Food allergies in babies and young children. Last reviewed November 5, 2021. Accessed June 8, 2022. www.nhs.uk/conditions/baby/weaning-and-feeding/food-allergies-in-babies-and-young-children/
11. Szajewska H, Shamir R, Mearin L, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016;62:507-513. doi: 10.1097/MPG.0000000000001105
12. Perkin MR, Logan K, Marrs T, et al; EAT Study Team. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477-1486.e8. doi: 10.1016/j.jaci.2015.12.1322
13. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743. doi: 10.1056/NEJMoa1514210
1. Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047. doi: 10.1001/jamapediatrics.2020.2893
2. King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115:507-525. doi: 10.14309/ajg.0000000000000523
3. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
4. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
5. Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304-1315. doi: 10.1056/NEJMoa1404172
6. Beyerlein A, Chmiel R, Hummel S, et al. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. 2014;37:e194-e195. doi: 10.2337/dc14-1208
7. Lionetti E, Castellaneta S, Francavilla R, et al; SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295-1303. doi: 10.1056/NEJMoa1400697
8. Pinto-Sánchez MI, Verdu EF, Liu E, et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J Pediatr. 2016;168:132-143.e3. doi: 10.1016/j.jpeds.2015.09.032
9. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. December 2020. Accessed June 8, 2022. www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
10. NHS. Food allergies in babies and young children. Last reviewed November 5, 2021. Accessed June 8, 2022. www.nhs.uk/conditions/baby/weaning-and-feeding/food-allergies-in-babies-and-young-children/
11. Szajewska H, Shamir R, Mearin L, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016;62:507-513. doi: 10.1097/MPG.0000000000001105
12. Perkin MR, Logan K, Marrs T, et al; EAT Study Team. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477-1486.e8. doi: 10.1016/j.jaci.2015.12.1322
13. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743. doi: 10.1056/NEJMoa1514210
PRACTICE CHANGER
Consider introducing gluten (wheat) in addition to breast milk or infant formula from age 4 months to potentially reduce the risk of celiac disease (CD) at age 3 years.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT) with a patient-oriented outcome of CD diagnosis.1
Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047.
A judicious approach to ordering lab tests
CASE
A 35-year-old man arrives for an annual wellness visit with no specific complaints and no significant personal or family history. His normal exam includes a blood pressure of 110/74 mm Hg and a body mass index (BMI) of 23.6. You order “routine labs” for prevention, which include a comprehensive metabolic panel (CMP), fasting lipid profile, and thyroid-stimulating hormone (TSH) and 25(OH) vitamin D tests. Are you practicing value-based laboratory testing?
The answer to this question appears in the Case discussion at the end of the article.
Value-based care, including care provided through laboratory testing, can achieve the Institute for Healthcare Improvement’s Triple Aim of improving population health, improving the patient experience of care (including quality and satisfaction), and reducing cost: Value = (Quality x Patient experience) / Cost.1
As quality and patient experience rise and cost falls, the value of care increases. Unnecessary lab testing, however, can negatively impact this equation:
- Error introduced by unnecessary testing can adversely affect quality.
- Patients experience inconvenience and sometimes cascades of testing, in addition to financial responsibility, from unnecessary testing.
- Low-value testing also contributes to work burden and provider burnout by requiring additional review and follow-up.
Rising health care costs are approaching 18% of the US gross domestic product, driven in large part by a wasteful and inefficient care delivery system.2 One review of “waste domains” identified by the Institute of Medicine estimates that approximately one-quarter of health care costs represent waste, including overtreatment, breakdowns of care coordination, and pricing that fails to correlate to the level of care received.3 High-volume, low-cost testing contributes more to total cost than low-volume, high-cost tests.4
Provider and system factors that contribute to ongoing waste
A lack of awareness of waste and how to reduce it contribute to the problem, as does an underappreciation of the harmful effects caused by incidental abnormal results.
Provider intolerance of diagnostic uncertainty often leads to ordering even more tests.
Continue to: Also, a hope of avoiding...
Also, a hope of avoiding missed diagnoses and potential lawsuits leads to defensive practice and more testing. In addition, patients and family members can exert pressure based on a belief that more testing represents better care. Of course, financial revenues from testing may come into play, with few disincentives to forgo testing. Something that also comes into play is that evidence-based guidance on cost-effective laboratory testing may be lacking, or there may be a lack of knowledge on how to access existing evidence.
Automated systems can facilitate wasteful laboratory testing, and the heavy testing practices of hospitals and specialists may be inappropriately applied to outpatient primary care.
Factors affecting the cost of laboratory testing
Laboratory test results drive 70% of today’s medical decisions.5 Negotiated insurance payment for tests is usually much less than the direct out-of-pocket costs charged to the patient. Without insurance, lab tests can cost patients between $100 and $1000.6 If multiple tests are ordered, the costs could likely be many thousands of dollars.
Actual costs typically vary by the testing facility, the patient’s health plan, and location in the United States; hospital-based testing tends to be the most expensive. Insurers will pay for lab tests with appropriate indications that are covered in the contract with the provider.6
Choosing Wisely initiative weighs in on lab testing
Choosing Wisely, a prominent initiative of the American Board of Internal Medicine Foundation, promotes appropriate resource utilization through educational campaigns that detail how to avoid unnecessary medical tests, treatments, and procedures.7 Recommendations are based largely on specialty society consensus and disease-oriented evidence. Choosing Wisely recommendations advise against the following7:
- performing laboratory blood testing unless clinically indicated or necessary for diagnosis or management, in order to avoid iatrogenic anemia. (American Academy of Family Physicians; Society for the Advancement of Patient Blood Management)
- requesting just a serum creatinine to test adult patients with diabetes and/or hypertension for chronic kidney disease. Use the kidney profile: serum creatinine with estimated glomerular filtration rate and urinary albumin-creatinine ratio. (American Society for Clinical Pathology)
- routinely screening for prostate cancer using a prostate-specific antigen test. It should be performed only after engaging in shared decision-making with the patient. (American Academy of Family Physicians; American Urological Association)
- screening for genital herpes simplex virus infectionFrutiger LT Std in asymptomatic adults, including pregnant women. (American Academy of Family Physicians)
- performing preoperative medical tests for eye surgery unless there are specific medical indications. (American Academy of Ophthalmology)
Sequential steps to takefor value-based lab ordering
Ask the question: “How will ordering this specific test change the management of my patient?” From there, take sequential steps using sound, evidence-based pathways. Morgan and colleagues8 outline the following practical approaches to rational test ordering:
- Perform a thorough clinical assessment.
- Consider the probability and implications of a positive test result.
- Practice patient-centered communication: address the patient’s concerns and discuss the risks and benefits of tests and how they will influence management.
- Follow clinical guidelines when available.
- Avoid ordering tests to reassure the patient; unnecessary tests with insignificant results do little to reduce patient anxieties.
- Avoid letting uncertainty drive unnecessary testing. Watchful waiting can allow time for the illness to resolve or declare itself.
Let’s consider this approach in the context of 4 areas: preventive care, diagnostic evaluation, ongoing management of chronic conditions, and preoperative testing.
Continue to: Preventive guidance from the USPSTF
Preventive guidance from the USPSTF
An independent volunteer panel of 16 national experts in prevention and evidence-based medicine develop recommendations for the US Preventive Services Task Force (USPSTF).9 These guidelines are based on evidence and are updated as new evidence surfaces. Thirteen recommendations, some of which advise avoiding preventive procedures that could cause harm to patients, cover laboratory tests used in screening for conditions such as hyperlipidemia10 and prostate cancer.11 We review the ones pertinent to our patient later at the end of the Case.
While the target audience for USPSTF recommendations is clinicians who provide preventive care, the recommendations are widely followed by policymakers, managed care organizations, public and private payers, quality improvement organizations, research institutions, and patients.
Take a critical look at how you approach the diagnostic evaluation
To reduce unnecessary testing in the diagnostic evaluation of patients, first consider pretest probability, test sensitivity and specificity, narrowly out-of-range tests, habitually paired tests, and repetitive laboratory testing.
Pretest probability, and test sensitivity and specificity. Pretest probability is the estimated chance that the patient has the disease before the test result is known. In a patient with low pretest probability of a disease, the ability to conclusively arrive at the diagnosis with one positive result is limited. Similarly, for tests in patients with high pretest probability of disease, a negative test cannot be used to firmly rule out a diagnosis.12
Reliability also depends on test sensitivity (the proportion of true positive results) and specificity (the proportion of true negative results). A test with high sensitivity but low specificity will generate more false-positive results, with potential harm to patients who do not have a disease.
The pretest probability along with test sensitivity and specificity help a clinician to interpret a test result, and even decide whether to order the test at all. For example, the anti-nuclear antibody (ANA) test for systemic lupus erythematosus (SLE) has a sensitivity of 100% and a specificity of 86%13; it will always be positive in a patient with SLE. But when applied to individuals with low likelihood of SLE, false-positives are more common; the ANA is falsely positive in up to 14% of healthy individuals, depending on the population studied.13
Ordering a test may be unnecessary if the results will not change the treatment plan. For example, in a female patient with classic symptoms of an uncomplicated urinary tract infection, a urine culture and even a urinalysis may not change treatment.
Continue to: Narrowly out-of-range tests
Narrowly out-of-range tests. Test results that fall just outside the “normal” range may be of questionable significance. When an asymptomatic patient has mildly elevated liver enzymes, should additional tests be ordered to avoid missing a treatable disorder? In these scenarios, a history, including possible contributing factors such as alcohol or substance misuse, must be paired with the clinical presentation to assess pre-test probability of a particular condition.14 Repeating a narrowly out-of-range test is an option in patients when follow-up is possible. Alternatively, you could pursue watchful waiting and monitor a minor abnormality over time while being vigilant for clinical changes. This whole-patient approach will guide the decision of whether to order additional testing.
Habitually paired tests. Reflexively ordering tests together often contributes to unnecessary testing. Examples of commonly paired tests are serum lipase with amylase, C-reactive protein (CRP) with erythrocyte sedimentation rate (ESR), and TSH with free T4 to monitor patients with treated hypothyroidism. These tests add minimal value together and can be decoupled.15-17 Evidence supports ordering serum lipase alone, CRP instead of ESR, and TSH alone for monitoring thyroid status.
Some commonly paired tests may not even be necessary for diagnosis. The well-established Rotterdam Criteria for diagnosing polycystic ovary syndrome specify clinical features and ovarian ultrasound for diagnosis.18 They do not require measurement of commonly ordered follicle-stimulating hormone and luteinizing hormone for diagnosis.
Serial rather than parallel testing, a “2-step approach,” is a strategy made easier with the advent of the electronic medical record (EMR) and computerized lab systems.8 These records and lab systems allow providers to order reflex tests, and to add on additional tests, if necessary, to an existing blood specimen.
Repetitive laboratory testing. Repetitive inpatient laboratory testing in patients who are clinically stable is wasteful and potentially harmful. Interventions involving physician education alone show mixed results, but combining education with clinician audit and feedback, along with EMR-enabled restrictive ordering, have resulted in significant and sustained reductions in repetitive laboratory testing.19
Continue to: Ongoing management of chronic conditions
Ongoing management of chronic conditions
Evidence-based guidelines support choices of tests and testing intervals for ongoing management of chronic conditions such as diabetes, hyperlipidemia, and hypertension.
Diabetes. Guidelines also define quality standards that are applied to value-based contracts. For example, the American Diabetes Association recommends assessing A1C every 6 months in patients whose type 2 diabetes is under stable control.20
Hyperlipidemia. For patients diagnosed with hyperlipidemia, 2018 clinical practice guidelines published by multiple specialty societies recommend assessing adherence and response to lifestyle changes and LDL-C–lowering medications with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed.21
Hypertension. With a new diagnosis of hypertension, guidelines advise an initial assessment for comorbidities and end-organ damage with an electrocardiogram, urinalysis, glucose level, blood count, electrolytes, creatinine, calcium, lipids, and urinary albumin/creatinine ratio. For ongoing monitoring, guidelines recommend assessment for end-organ damage through regular measurements of creatinine, glomerular filtration rate, and urinary microalbumin/creatinine ratio. Initiation and alteration of medications should prompt appropriate additional lab follow-up—eg, a measurement of serum potassium after starting a diuretic.22
Preoperative testing
Preoperative testing is overused in low-risk, ambulatory surgery. And testing, even with abnormal results, does not affect postoperative outcomes.23
Continue to: The American Society of Anesthesiologists (ASA) Physical Status Classification System
The American Society of Anesthesiologists (ASA) Physical Status Classification System, which has been in use for more than 60 years, considers the patient’s physical status (ASA grades I-VI),24 and when paired with surgery grades of minor, intermediate, and major/complex, can help assess preoperative risk and guide preoperative testing (TABLE).24-26
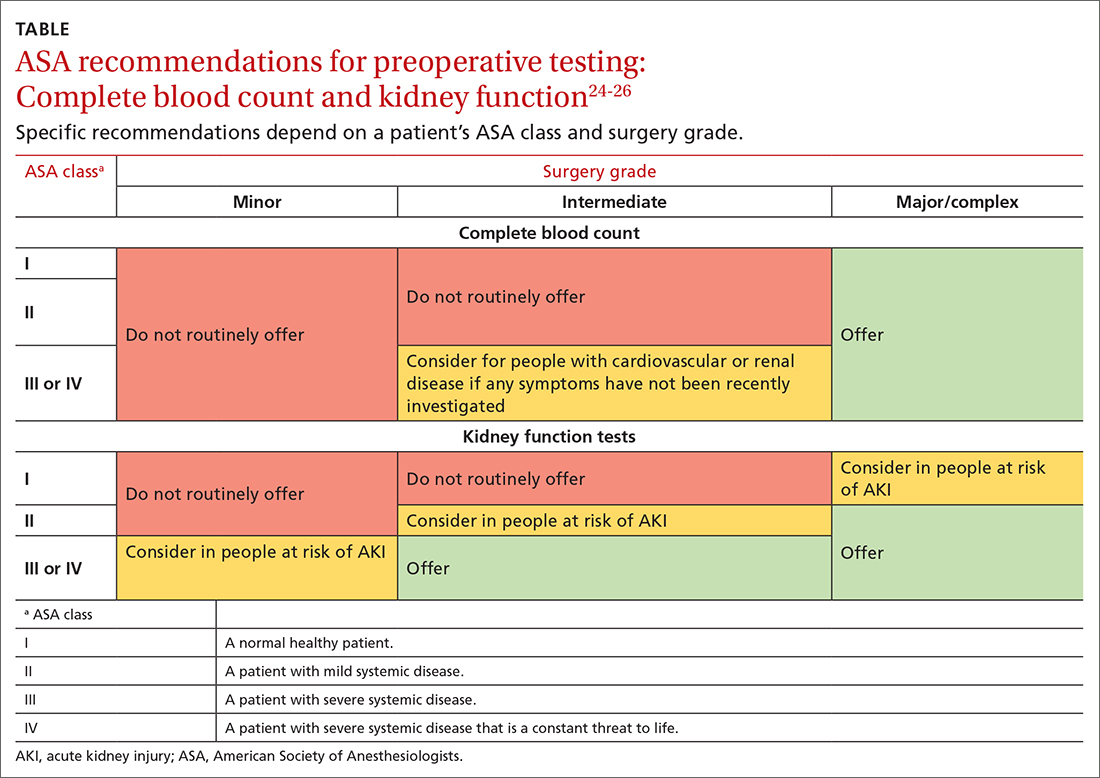
Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared with selective or no testing.27 Unnecessary preoperative testing can lead to a nonproductive cascade of additional investigations. In a 2018 study of Medicare beneficiaries, unnecessary routine preoperative testing and testing sequelae for cataract surgery was calculated to cost Medicare up to $45.4 million annually.28
CASE
You would not be practicing value-based laboratory testing, according to the USPSTF, if you ordered a CMP, fasting lipid profile, and TSH and 25(OH) vitamin D tests for this healthy 35-year-old man whose family history, blood pressure, and BMI do not put him at elevated risk. Universal lipid screening (Grade Ba) is recommended for all adults ages 40 to 75. Thyroid screening tests and measurement of 25(OH) vitamin D level (I statementsa) are not recommended. The USPSTF has not evaluated chemistry panels for screening.
The USPSTF would recommend the following actions for this patient:
- Screen for HIV (ages 15 to 65 years; and younger or older if patient is at risk). (A recommendationa,29)
- Screen for hepatitis C virus (in those ages 18 to 79). (B recommendation30)
The following USPSTF recommendations might have come into play if this patient had certain risk factors, or if the patient had been a woman:
- Screen for diabetes if the patient is overweight or obese (B recommendation).
- Screen for hepatitis B in adults at risk (B recommendation).
- Screen for gonorrhea and chlamydia in women at risk (B recommendation). Such screening has an “I”statement for screening men at risk.
Continue to: As noted, costs of laboratory...
As noted, costs of laboratory testing vary widely, depending upon what tests are ordered, what type of insurance the patient has, and which tests the patient’s insurance covers. Who performs the testing also factors into the cost. Payers negotiate reduced fees for commercial lab testing, but potential out-of-pocket costs to patients are much higher.
For our healthy 35-year-old man, the cost of the initially proposed testing (CMP, lipid panel, TSH, and 25[OH] vitamin D level) ranges from a negotiated payer cost of $85 to potential patient out-of-pocket cost of more than $400.6
Insurance would cover the USPSTF-recommended testing (HIV and hepatitis C screening tests), which might incur only a patient co-pay, and cost the system about $65.
The USPSTF home page, found at www.uspreventiveservicestaskforce.org/uspstf/ includes recommendations that can be sorted for your patients. A web and mobile device application is also available through the website.
a USPSTF grade definitions:
A: There is high certainty that the net benefit is substantial. Offer service.
B: There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial. Offer service.
C: There is at least moderate certainty that the net benefit is small. Offer service selectively.
D: There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. Don’t offer service.
I: Current evidence is insufficient to assess the balance of benefits and harms of the service.
CORRESPONDENCE
Mitchell Kaminski, MD, MBA, 901 Walnut Street, 10th Floor, Jefferson College of Population Health, Philadelphia, PA 19107; [email protected]
1. IHI. What is the Triple Aim? Accessed June 20, 2022. http://www.ihi.org/Topics/TripleAim/Pages/Overview.aspx#:~:text=It%20is%20IHI’s%20belief%20that,capita%20cost%20of%20health%20care
2. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
3. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system estimated costs and potential for savings. JAMA. 2019;322:1501-1509. doi:10.1001/jama.2019.13978
4. Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704. doi: 10.1377/hlthaff.2017.0385
5. CDC. Strengthening clinical laboratories. 2018. Accessed June 2020, 2022. www.cdc.gov/csels/dls/strengthening-clinical-labs.html
6. Vuong KT. How much do lab tests cost without insurance in 2022? Accessed May 11, 2022. www.talktomira.com/post/how-much-do-lab-test-cost-without-insurance
7. Choosing Wisely: Promoting conversations between providers and patients. Accessed June 20, 2022. www.choosingwisely.org
8. Morgan S, van Driel M, Coleman J, et al. Rational test ordering in family medicine. Can Fam Physician. 2015;61:535-537.
9. US Preventive Services Taskforce. Screening for glaucoma and impaired vision. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf
10. Arnold MJ, O’Malley PG, Downs JR. Key recommendations on managing dyslipidemia for cardiovascular risk reduction: stopping where the evidence does. Am Fam Physician. 2021;103:455-458.
11. Welch HG, Albertsen PC. Reconsidering prostate cancer mortality—the future of PSA screening. N Engl J Med. 2020;382:1557-1563. doi: 10.1056/NEJMms1914228
12. American Society for Microbiology. Why pretest and posttest probability matter in the time of COVID-19. Accessed June 20, 2022. https://asm.org/Articles/2020/June/Why-Pretest-and-Posttest-Probability-Matter-in-the
13. Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med. 1996;156:1421-1425.
14. Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med. 2010;77:195-204. doi: 10.3949/ccjm.77a.09064
15. Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275-1280. doi: 10.1016/j.clinbiochem.2017.07.003.
16. Gottheil S, Khemani E, Copley K, et al. Reducing inappropriate ESR testing with computerized clinical decision support. BMJ Quality Improvement Reports, 2016;5:u211376.w4582. doi: 10.1136/bmjquality.u211376.w4582
17. Schneider C, Feller M, Bauer DC, et al. Initial evaluation of thyroid dysfunction - are simultaneous TSH and fT4 tests necessary? PloS One. 2018;13:e0196631–e0196631. doi: 10.1371/journal.pone.0196631
18. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94:106-113.
19. Eaton KP, Levy K, Soong C et.al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177:1833-1839. doi: 10.1001/jamainternmed.2017.5152
20. ADA. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73-S84. doi: 10.2337/dc21-S006
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
22. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
23. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528. doi: 10.1097/SLA.0b013e318265bcdb
24. ASA. ASA physical status classification system. Accessed June 22,2022. www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
25. NLM. Preoperative tests (update): routine preoperative tests for elective surgery. Accessed June 22, 2022. www.ncbi.nlm.nih.gov/books/NBK367919/
26. ASA. American Society of Anesthesiologists releases list of commonly used tests and treatments to question-AS PART OF CHOOSING WISELY® CAMPAIGN. Accessed June 22, 2022. www.asahq.org/about-asa/newsroom/news-releases/2013/10/choosing-wisely
27. Keay L, Lindsley K, Tielsch J, et al. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2019;1:CD007293. doi: 10.1002/14651858.CD007293.pub4
28. Chen CL, Clay TH, McLeod S, et al. A revised estimate of costs associated with routine preoperative testing in Medicare cataract patients with a procedure-specific indicator. JAMA Ophthalmol. 2018;136:231-238. doi:10.1001/jamaophthalmol.2017.6372
29. USPSTF. Human immunodeficiency virus (HIV) infection: screening. Accessed May 16, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/human-immunodeficiency-virus-hiv-infection-screening
30. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
CASE
A 35-year-old man arrives for an annual wellness visit with no specific complaints and no significant personal or family history. His normal exam includes a blood pressure of 110/74 mm Hg and a body mass index (BMI) of 23.6. You order “routine labs” for prevention, which include a comprehensive metabolic panel (CMP), fasting lipid profile, and thyroid-stimulating hormone (TSH) and 25(OH) vitamin D tests. Are you practicing value-based laboratory testing?
The answer to this question appears in the Case discussion at the end of the article.
Value-based care, including care provided through laboratory testing, can achieve the Institute for Healthcare Improvement’s Triple Aim of improving population health, improving the patient experience of care (including quality and satisfaction), and reducing cost: Value = (Quality x Patient experience) / Cost.1
As quality and patient experience rise and cost falls, the value of care increases. Unnecessary lab testing, however, can negatively impact this equation:
- Error introduced by unnecessary testing can adversely affect quality.
- Patients experience inconvenience and sometimes cascades of testing, in addition to financial responsibility, from unnecessary testing.
- Low-value testing also contributes to work burden and provider burnout by requiring additional review and follow-up.
Rising health care costs are approaching 18% of the US gross domestic product, driven in large part by a wasteful and inefficient care delivery system.2 One review of “waste domains” identified by the Institute of Medicine estimates that approximately one-quarter of health care costs represent waste, including overtreatment, breakdowns of care coordination, and pricing that fails to correlate to the level of care received.3 High-volume, low-cost testing contributes more to total cost than low-volume, high-cost tests.4
Provider and system factors that contribute to ongoing waste
A lack of awareness of waste and how to reduce it contribute to the problem, as does an underappreciation of the harmful effects caused by incidental abnormal results.
Provider intolerance of diagnostic uncertainty often leads to ordering even more tests.
Continue to: Also, a hope of avoiding...
Also, a hope of avoiding missed diagnoses and potential lawsuits leads to defensive practice and more testing. In addition, patients and family members can exert pressure based on a belief that more testing represents better care. Of course, financial revenues from testing may come into play, with few disincentives to forgo testing. Something that also comes into play is that evidence-based guidance on cost-effective laboratory testing may be lacking, or there may be a lack of knowledge on how to access existing evidence.
Automated systems can facilitate wasteful laboratory testing, and the heavy testing practices of hospitals and specialists may be inappropriately applied to outpatient primary care.
Factors affecting the cost of laboratory testing
Laboratory test results drive 70% of today’s medical decisions.5 Negotiated insurance payment for tests is usually much less than the direct out-of-pocket costs charged to the patient. Without insurance, lab tests can cost patients between $100 and $1000.6 If multiple tests are ordered, the costs could likely be many thousands of dollars.
Actual costs typically vary by the testing facility, the patient’s health plan, and location in the United States; hospital-based testing tends to be the most expensive. Insurers will pay for lab tests with appropriate indications that are covered in the contract with the provider.6
Choosing Wisely initiative weighs in on lab testing
Choosing Wisely, a prominent initiative of the American Board of Internal Medicine Foundation, promotes appropriate resource utilization through educational campaigns that detail how to avoid unnecessary medical tests, treatments, and procedures.7 Recommendations are based largely on specialty society consensus and disease-oriented evidence. Choosing Wisely recommendations advise against the following7:
- performing laboratory blood testing unless clinically indicated or necessary for diagnosis or management, in order to avoid iatrogenic anemia. (American Academy of Family Physicians; Society for the Advancement of Patient Blood Management)
- requesting just a serum creatinine to test adult patients with diabetes and/or hypertension for chronic kidney disease. Use the kidney profile: serum creatinine with estimated glomerular filtration rate and urinary albumin-creatinine ratio. (American Society for Clinical Pathology)
- routinely screening for prostate cancer using a prostate-specific antigen test. It should be performed only after engaging in shared decision-making with the patient. (American Academy of Family Physicians; American Urological Association)
- screening for genital herpes simplex virus infectionFrutiger LT Std in asymptomatic adults, including pregnant women. (American Academy of Family Physicians)
- performing preoperative medical tests for eye surgery unless there are specific medical indications. (American Academy of Ophthalmology)
Sequential steps to takefor value-based lab ordering
Ask the question: “How will ordering this specific test change the management of my patient?” From there, take sequential steps using sound, evidence-based pathways. Morgan and colleagues8 outline the following practical approaches to rational test ordering:
- Perform a thorough clinical assessment.
- Consider the probability and implications of a positive test result.
- Practice patient-centered communication: address the patient’s concerns and discuss the risks and benefits of tests and how they will influence management.
- Follow clinical guidelines when available.
- Avoid ordering tests to reassure the patient; unnecessary tests with insignificant results do little to reduce patient anxieties.
- Avoid letting uncertainty drive unnecessary testing. Watchful waiting can allow time for the illness to resolve or declare itself.
Let’s consider this approach in the context of 4 areas: preventive care, diagnostic evaluation, ongoing management of chronic conditions, and preoperative testing.
Continue to: Preventive guidance from the USPSTF
Preventive guidance from the USPSTF
An independent volunteer panel of 16 national experts in prevention and evidence-based medicine develop recommendations for the US Preventive Services Task Force (USPSTF).9 These guidelines are based on evidence and are updated as new evidence surfaces. Thirteen recommendations, some of which advise avoiding preventive procedures that could cause harm to patients, cover laboratory tests used in screening for conditions such as hyperlipidemia10 and prostate cancer.11 We review the ones pertinent to our patient later at the end of the Case.
While the target audience for USPSTF recommendations is clinicians who provide preventive care, the recommendations are widely followed by policymakers, managed care organizations, public and private payers, quality improvement organizations, research institutions, and patients.
Take a critical look at how you approach the diagnostic evaluation
To reduce unnecessary testing in the diagnostic evaluation of patients, first consider pretest probability, test sensitivity and specificity, narrowly out-of-range tests, habitually paired tests, and repetitive laboratory testing.
Pretest probability, and test sensitivity and specificity. Pretest probability is the estimated chance that the patient has the disease before the test result is known. In a patient with low pretest probability of a disease, the ability to conclusively arrive at the diagnosis with one positive result is limited. Similarly, for tests in patients with high pretest probability of disease, a negative test cannot be used to firmly rule out a diagnosis.12
Reliability also depends on test sensitivity (the proportion of true positive results) and specificity (the proportion of true negative results). A test with high sensitivity but low specificity will generate more false-positive results, with potential harm to patients who do not have a disease.
The pretest probability along with test sensitivity and specificity help a clinician to interpret a test result, and even decide whether to order the test at all. For example, the anti-nuclear antibody (ANA) test for systemic lupus erythematosus (SLE) has a sensitivity of 100% and a specificity of 86%13; it will always be positive in a patient with SLE. But when applied to individuals with low likelihood of SLE, false-positives are more common; the ANA is falsely positive in up to 14% of healthy individuals, depending on the population studied.13
Ordering a test may be unnecessary if the results will not change the treatment plan. For example, in a female patient with classic symptoms of an uncomplicated urinary tract infection, a urine culture and even a urinalysis may not change treatment.
Continue to: Narrowly out-of-range tests
Narrowly out-of-range tests. Test results that fall just outside the “normal” range may be of questionable significance. When an asymptomatic patient has mildly elevated liver enzymes, should additional tests be ordered to avoid missing a treatable disorder? In these scenarios, a history, including possible contributing factors such as alcohol or substance misuse, must be paired with the clinical presentation to assess pre-test probability of a particular condition.14 Repeating a narrowly out-of-range test is an option in patients when follow-up is possible. Alternatively, you could pursue watchful waiting and monitor a minor abnormality over time while being vigilant for clinical changes. This whole-patient approach will guide the decision of whether to order additional testing.
Habitually paired tests. Reflexively ordering tests together often contributes to unnecessary testing. Examples of commonly paired tests are serum lipase with amylase, C-reactive protein (CRP) with erythrocyte sedimentation rate (ESR), and TSH with free T4 to monitor patients with treated hypothyroidism. These tests add minimal value together and can be decoupled.15-17 Evidence supports ordering serum lipase alone, CRP instead of ESR, and TSH alone for monitoring thyroid status.
Some commonly paired tests may not even be necessary for diagnosis. The well-established Rotterdam Criteria for diagnosing polycystic ovary syndrome specify clinical features and ovarian ultrasound for diagnosis.18 They do not require measurement of commonly ordered follicle-stimulating hormone and luteinizing hormone for diagnosis.
Serial rather than parallel testing, a “2-step approach,” is a strategy made easier with the advent of the electronic medical record (EMR) and computerized lab systems.8 These records and lab systems allow providers to order reflex tests, and to add on additional tests, if necessary, to an existing blood specimen.
Repetitive laboratory testing. Repetitive inpatient laboratory testing in patients who are clinically stable is wasteful and potentially harmful. Interventions involving physician education alone show mixed results, but combining education with clinician audit and feedback, along with EMR-enabled restrictive ordering, have resulted in significant and sustained reductions in repetitive laboratory testing.19
Continue to: Ongoing management of chronic conditions
Ongoing management of chronic conditions
Evidence-based guidelines support choices of tests and testing intervals for ongoing management of chronic conditions such as diabetes, hyperlipidemia, and hypertension.
Diabetes. Guidelines also define quality standards that are applied to value-based contracts. For example, the American Diabetes Association recommends assessing A1C every 6 months in patients whose type 2 diabetes is under stable control.20
Hyperlipidemia. For patients diagnosed with hyperlipidemia, 2018 clinical practice guidelines published by multiple specialty societies recommend assessing adherence and response to lifestyle changes and LDL-C–lowering medications with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed.21
Hypertension. With a new diagnosis of hypertension, guidelines advise an initial assessment for comorbidities and end-organ damage with an electrocardiogram, urinalysis, glucose level, blood count, electrolytes, creatinine, calcium, lipids, and urinary albumin/creatinine ratio. For ongoing monitoring, guidelines recommend assessment for end-organ damage through regular measurements of creatinine, glomerular filtration rate, and urinary microalbumin/creatinine ratio. Initiation and alteration of medications should prompt appropriate additional lab follow-up—eg, a measurement of serum potassium after starting a diuretic.22
Preoperative testing
Preoperative testing is overused in low-risk, ambulatory surgery. And testing, even with abnormal results, does not affect postoperative outcomes.23
Continue to: The American Society of Anesthesiologists (ASA) Physical Status Classification System
The American Society of Anesthesiologists (ASA) Physical Status Classification System, which has been in use for more than 60 years, considers the patient’s physical status (ASA grades I-VI),24 and when paired with surgery grades of minor, intermediate, and major/complex, can help assess preoperative risk and guide preoperative testing (TABLE).24-26

Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared with selective or no testing.27 Unnecessary preoperative testing can lead to a nonproductive cascade of additional investigations. In a 2018 study of Medicare beneficiaries, unnecessary routine preoperative testing and testing sequelae for cataract surgery was calculated to cost Medicare up to $45.4 million annually.28
CASE
You would not be practicing value-based laboratory testing, according to the USPSTF, if you ordered a CMP, fasting lipid profile, and TSH and 25(OH) vitamin D tests for this healthy 35-year-old man whose family history, blood pressure, and BMI do not put him at elevated risk. Universal lipid screening (Grade Ba) is recommended for all adults ages 40 to 75. Thyroid screening tests and measurement of 25(OH) vitamin D level (I statementsa) are not recommended. The USPSTF has not evaluated chemistry panels for screening.
The USPSTF would recommend the following actions for this patient:
- Screen for HIV (ages 15 to 65 years; and younger or older if patient is at risk). (A recommendationa,29)
- Screen for hepatitis C virus (in those ages 18 to 79). (B recommendation30)
The following USPSTF recommendations might have come into play if this patient had certain risk factors, or if the patient had been a woman:
- Screen for diabetes if the patient is overweight or obese (B recommendation).
- Screen for hepatitis B in adults at risk (B recommendation).
- Screen for gonorrhea and chlamydia in women at risk (B recommendation). Such screening has an “I”statement for screening men at risk.
Continue to: As noted, costs of laboratory...
As noted, costs of laboratory testing vary widely, depending upon what tests are ordered, what type of insurance the patient has, and which tests the patient’s insurance covers. Who performs the testing also factors into the cost. Payers negotiate reduced fees for commercial lab testing, but potential out-of-pocket costs to patients are much higher.
For our healthy 35-year-old man, the cost of the initially proposed testing (CMP, lipid panel, TSH, and 25[OH] vitamin D level) ranges from a negotiated payer cost of $85 to potential patient out-of-pocket cost of more than $400.6
Insurance would cover the USPSTF-recommended testing (HIV and hepatitis C screening tests), which might incur only a patient co-pay, and cost the system about $65.
The USPSTF home page, found at www.uspreventiveservicestaskforce.org/uspstf/ includes recommendations that can be sorted for your patients. A web and mobile device application is also available through the website.
a USPSTF grade definitions:
A: There is high certainty that the net benefit is substantial. Offer service.
B: There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial. Offer service.
C: There is at least moderate certainty that the net benefit is small. Offer service selectively.
D: There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. Don’t offer service.
I: Current evidence is insufficient to assess the balance of benefits and harms of the service.
CORRESPONDENCE
Mitchell Kaminski, MD, MBA, 901 Walnut Street, 10th Floor, Jefferson College of Population Health, Philadelphia, PA 19107; [email protected]
CASE
A 35-year-old man arrives for an annual wellness visit with no specific complaints and no significant personal or family history. His normal exam includes a blood pressure of 110/74 mm Hg and a body mass index (BMI) of 23.6. You order “routine labs” for prevention, which include a comprehensive metabolic panel (CMP), fasting lipid profile, and thyroid-stimulating hormone (TSH) and 25(OH) vitamin D tests. Are you practicing value-based laboratory testing?
The answer to this question appears in the Case discussion at the end of the article.
Value-based care, including care provided through laboratory testing, can achieve the Institute for Healthcare Improvement’s Triple Aim of improving population health, improving the patient experience of care (including quality and satisfaction), and reducing cost: Value = (Quality x Patient experience) / Cost.1
As quality and patient experience rise and cost falls, the value of care increases. Unnecessary lab testing, however, can negatively impact this equation:
- Error introduced by unnecessary testing can adversely affect quality.
- Patients experience inconvenience and sometimes cascades of testing, in addition to financial responsibility, from unnecessary testing.
- Low-value testing also contributes to work burden and provider burnout by requiring additional review and follow-up.
Rising health care costs are approaching 18% of the US gross domestic product, driven in large part by a wasteful and inefficient care delivery system.2 One review of “waste domains” identified by the Institute of Medicine estimates that approximately one-quarter of health care costs represent waste, including overtreatment, breakdowns of care coordination, and pricing that fails to correlate to the level of care received.3 High-volume, low-cost testing contributes more to total cost than low-volume, high-cost tests.4
Provider and system factors that contribute to ongoing waste
A lack of awareness of waste and how to reduce it contribute to the problem, as does an underappreciation of the harmful effects caused by incidental abnormal results.
Provider intolerance of diagnostic uncertainty often leads to ordering even more tests.
Continue to: Also, a hope of avoiding...
Also, a hope of avoiding missed diagnoses and potential lawsuits leads to defensive practice and more testing. In addition, patients and family members can exert pressure based on a belief that more testing represents better care. Of course, financial revenues from testing may come into play, with few disincentives to forgo testing. Something that also comes into play is that evidence-based guidance on cost-effective laboratory testing may be lacking, or there may be a lack of knowledge on how to access existing evidence.
Automated systems can facilitate wasteful laboratory testing, and the heavy testing practices of hospitals and specialists may be inappropriately applied to outpatient primary care.
Factors affecting the cost of laboratory testing
Laboratory test results drive 70% of today’s medical decisions.5 Negotiated insurance payment for tests is usually much less than the direct out-of-pocket costs charged to the patient. Without insurance, lab tests can cost patients between $100 and $1000.6 If multiple tests are ordered, the costs could likely be many thousands of dollars.
Actual costs typically vary by the testing facility, the patient’s health plan, and location in the United States; hospital-based testing tends to be the most expensive. Insurers will pay for lab tests with appropriate indications that are covered in the contract with the provider.6
Choosing Wisely initiative weighs in on lab testing
Choosing Wisely, a prominent initiative of the American Board of Internal Medicine Foundation, promotes appropriate resource utilization through educational campaigns that detail how to avoid unnecessary medical tests, treatments, and procedures.7 Recommendations are based largely on specialty society consensus and disease-oriented evidence. Choosing Wisely recommendations advise against the following7:
- performing laboratory blood testing unless clinically indicated or necessary for diagnosis or management, in order to avoid iatrogenic anemia. (American Academy of Family Physicians; Society for the Advancement of Patient Blood Management)
- requesting just a serum creatinine to test adult patients with diabetes and/or hypertension for chronic kidney disease. Use the kidney profile: serum creatinine with estimated glomerular filtration rate and urinary albumin-creatinine ratio. (American Society for Clinical Pathology)
- routinely screening for prostate cancer using a prostate-specific antigen test. It should be performed only after engaging in shared decision-making with the patient. (American Academy of Family Physicians; American Urological Association)
- screening for genital herpes simplex virus infectionFrutiger LT Std in asymptomatic adults, including pregnant women. (American Academy of Family Physicians)
- performing preoperative medical tests for eye surgery unless there are specific medical indications. (American Academy of Ophthalmology)
Sequential steps to takefor value-based lab ordering
Ask the question: “How will ordering this specific test change the management of my patient?” From there, take sequential steps using sound, evidence-based pathways. Morgan and colleagues8 outline the following practical approaches to rational test ordering:
- Perform a thorough clinical assessment.
- Consider the probability and implications of a positive test result.
- Practice patient-centered communication: address the patient’s concerns and discuss the risks and benefits of tests and how they will influence management.
- Follow clinical guidelines when available.
- Avoid ordering tests to reassure the patient; unnecessary tests with insignificant results do little to reduce patient anxieties.
- Avoid letting uncertainty drive unnecessary testing. Watchful waiting can allow time for the illness to resolve or declare itself.
Let’s consider this approach in the context of 4 areas: preventive care, diagnostic evaluation, ongoing management of chronic conditions, and preoperative testing.
Continue to: Preventive guidance from the USPSTF
Preventive guidance from the USPSTF
An independent volunteer panel of 16 national experts in prevention and evidence-based medicine develop recommendations for the US Preventive Services Task Force (USPSTF).9 These guidelines are based on evidence and are updated as new evidence surfaces. Thirteen recommendations, some of which advise avoiding preventive procedures that could cause harm to patients, cover laboratory tests used in screening for conditions such as hyperlipidemia10 and prostate cancer.11 We review the ones pertinent to our patient later at the end of the Case.
While the target audience for USPSTF recommendations is clinicians who provide preventive care, the recommendations are widely followed by policymakers, managed care organizations, public and private payers, quality improvement organizations, research institutions, and patients.
Take a critical look at how you approach the diagnostic evaluation
To reduce unnecessary testing in the diagnostic evaluation of patients, first consider pretest probability, test sensitivity and specificity, narrowly out-of-range tests, habitually paired tests, and repetitive laboratory testing.
Pretest probability, and test sensitivity and specificity. Pretest probability is the estimated chance that the patient has the disease before the test result is known. In a patient with low pretest probability of a disease, the ability to conclusively arrive at the diagnosis with one positive result is limited. Similarly, for tests in patients with high pretest probability of disease, a negative test cannot be used to firmly rule out a diagnosis.12
Reliability also depends on test sensitivity (the proportion of true positive results) and specificity (the proportion of true negative results). A test with high sensitivity but low specificity will generate more false-positive results, with potential harm to patients who do not have a disease.
The pretest probability along with test sensitivity and specificity help a clinician to interpret a test result, and even decide whether to order the test at all. For example, the anti-nuclear antibody (ANA) test for systemic lupus erythematosus (SLE) has a sensitivity of 100% and a specificity of 86%13; it will always be positive in a patient with SLE. But when applied to individuals with low likelihood of SLE, false-positives are more common; the ANA is falsely positive in up to 14% of healthy individuals, depending on the population studied.13
Ordering a test may be unnecessary if the results will not change the treatment plan. For example, in a female patient with classic symptoms of an uncomplicated urinary tract infection, a urine culture and even a urinalysis may not change treatment.
Continue to: Narrowly out-of-range tests
Narrowly out-of-range tests. Test results that fall just outside the “normal” range may be of questionable significance. When an asymptomatic patient has mildly elevated liver enzymes, should additional tests be ordered to avoid missing a treatable disorder? In these scenarios, a history, including possible contributing factors such as alcohol or substance misuse, must be paired with the clinical presentation to assess pre-test probability of a particular condition.14 Repeating a narrowly out-of-range test is an option in patients when follow-up is possible. Alternatively, you could pursue watchful waiting and monitor a minor abnormality over time while being vigilant for clinical changes. This whole-patient approach will guide the decision of whether to order additional testing.
Habitually paired tests. Reflexively ordering tests together often contributes to unnecessary testing. Examples of commonly paired tests are serum lipase with amylase, C-reactive protein (CRP) with erythrocyte sedimentation rate (ESR), and TSH with free T4 to monitor patients with treated hypothyroidism. These tests add minimal value together and can be decoupled.15-17 Evidence supports ordering serum lipase alone, CRP instead of ESR, and TSH alone for monitoring thyroid status.
Some commonly paired tests may not even be necessary for diagnosis. The well-established Rotterdam Criteria for diagnosing polycystic ovary syndrome specify clinical features and ovarian ultrasound for diagnosis.18 They do not require measurement of commonly ordered follicle-stimulating hormone and luteinizing hormone for diagnosis.
Serial rather than parallel testing, a “2-step approach,” is a strategy made easier with the advent of the electronic medical record (EMR) and computerized lab systems.8 These records and lab systems allow providers to order reflex tests, and to add on additional tests, if necessary, to an existing blood specimen.
Repetitive laboratory testing. Repetitive inpatient laboratory testing in patients who are clinically stable is wasteful and potentially harmful. Interventions involving physician education alone show mixed results, but combining education with clinician audit and feedback, along with EMR-enabled restrictive ordering, have resulted in significant and sustained reductions in repetitive laboratory testing.19
Continue to: Ongoing management of chronic conditions
Ongoing management of chronic conditions
Evidence-based guidelines support choices of tests and testing intervals for ongoing management of chronic conditions such as diabetes, hyperlipidemia, and hypertension.
Diabetes. Guidelines also define quality standards that are applied to value-based contracts. For example, the American Diabetes Association recommends assessing A1C every 6 months in patients whose type 2 diabetes is under stable control.20
Hyperlipidemia. For patients diagnosed with hyperlipidemia, 2018 clinical practice guidelines published by multiple specialty societies recommend assessing adherence and response to lifestyle changes and LDL-C–lowering medications with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed.21
Hypertension. With a new diagnosis of hypertension, guidelines advise an initial assessment for comorbidities and end-organ damage with an electrocardiogram, urinalysis, glucose level, blood count, electrolytes, creatinine, calcium, lipids, and urinary albumin/creatinine ratio. For ongoing monitoring, guidelines recommend assessment for end-organ damage through regular measurements of creatinine, glomerular filtration rate, and urinary microalbumin/creatinine ratio. Initiation and alteration of medications should prompt appropriate additional lab follow-up—eg, a measurement of serum potassium after starting a diuretic.22
Preoperative testing
Preoperative testing is overused in low-risk, ambulatory surgery. And testing, even with abnormal results, does not affect postoperative outcomes.23
Continue to: The American Society of Anesthesiologists (ASA) Physical Status Classification System
The American Society of Anesthesiologists (ASA) Physical Status Classification System, which has been in use for more than 60 years, considers the patient’s physical status (ASA grades I-VI),24 and when paired with surgery grades of minor, intermediate, and major/complex, can help assess preoperative risk and guide preoperative testing (TABLE).24-26

Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared with selective or no testing.27 Unnecessary preoperative testing can lead to a nonproductive cascade of additional investigations. In a 2018 study of Medicare beneficiaries, unnecessary routine preoperative testing and testing sequelae for cataract surgery was calculated to cost Medicare up to $45.4 million annually.28
CASE
You would not be practicing value-based laboratory testing, according to the USPSTF, if you ordered a CMP, fasting lipid profile, and TSH and 25(OH) vitamin D tests for this healthy 35-year-old man whose family history, blood pressure, and BMI do not put him at elevated risk. Universal lipid screening (Grade Ba) is recommended for all adults ages 40 to 75. Thyroid screening tests and measurement of 25(OH) vitamin D level (I statementsa) are not recommended. The USPSTF has not evaluated chemistry panels for screening.
The USPSTF would recommend the following actions for this patient:
- Screen for HIV (ages 15 to 65 years; and younger or older if patient is at risk). (A recommendationa,29)
- Screen for hepatitis C virus (in those ages 18 to 79). (B recommendation30)
The following USPSTF recommendations might have come into play if this patient had certain risk factors, or if the patient had been a woman:
- Screen for diabetes if the patient is overweight or obese (B recommendation).
- Screen for hepatitis B in adults at risk (B recommendation).
- Screen for gonorrhea and chlamydia in women at risk (B recommendation). Such screening has an “I”statement for screening men at risk.
Continue to: As noted, costs of laboratory...
As noted, costs of laboratory testing vary widely, depending upon what tests are ordered, what type of insurance the patient has, and which tests the patient’s insurance covers. Who performs the testing also factors into the cost. Payers negotiate reduced fees for commercial lab testing, but potential out-of-pocket costs to patients are much higher.
For our healthy 35-year-old man, the cost of the initially proposed testing (CMP, lipid panel, TSH, and 25[OH] vitamin D level) ranges from a negotiated payer cost of $85 to potential patient out-of-pocket cost of more than $400.6
Insurance would cover the USPSTF-recommended testing (HIV and hepatitis C screening tests), which might incur only a patient co-pay, and cost the system about $65.
The USPSTF home page, found at www.uspreventiveservicestaskforce.org/uspstf/ includes recommendations that can be sorted for your patients. A web and mobile device application is also available through the website.
a USPSTF grade definitions:
A: There is high certainty that the net benefit is substantial. Offer service.
B: There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial. Offer service.
C: There is at least moderate certainty that the net benefit is small. Offer service selectively.
D: There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. Don’t offer service.
I: Current evidence is insufficient to assess the balance of benefits and harms of the service.
CORRESPONDENCE
Mitchell Kaminski, MD, MBA, 901 Walnut Street, 10th Floor, Jefferson College of Population Health, Philadelphia, PA 19107; [email protected]
1. IHI. What is the Triple Aim? Accessed June 20, 2022. http://www.ihi.org/Topics/TripleAim/Pages/Overview.aspx#:~:text=It%20is%20IHI’s%20belief%20that,capita%20cost%20of%20health%20care
2. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
3. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system estimated costs and potential for savings. JAMA. 2019;322:1501-1509. doi:10.1001/jama.2019.13978
4. Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704. doi: 10.1377/hlthaff.2017.0385
5. CDC. Strengthening clinical laboratories. 2018. Accessed June 2020, 2022. www.cdc.gov/csels/dls/strengthening-clinical-labs.html
6. Vuong KT. How much do lab tests cost without insurance in 2022? Accessed May 11, 2022. www.talktomira.com/post/how-much-do-lab-test-cost-without-insurance
7. Choosing Wisely: Promoting conversations between providers and patients. Accessed June 20, 2022. www.choosingwisely.org
8. Morgan S, van Driel M, Coleman J, et al. Rational test ordering in family medicine. Can Fam Physician. 2015;61:535-537.
9. US Preventive Services Taskforce. Screening for glaucoma and impaired vision. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf
10. Arnold MJ, O’Malley PG, Downs JR. Key recommendations on managing dyslipidemia for cardiovascular risk reduction: stopping where the evidence does. Am Fam Physician. 2021;103:455-458.
11. Welch HG, Albertsen PC. Reconsidering prostate cancer mortality—the future of PSA screening. N Engl J Med. 2020;382:1557-1563. doi: 10.1056/NEJMms1914228
12. American Society for Microbiology. Why pretest and posttest probability matter in the time of COVID-19. Accessed June 20, 2022. https://asm.org/Articles/2020/June/Why-Pretest-and-Posttest-Probability-Matter-in-the
13. Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med. 1996;156:1421-1425.
14. Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med. 2010;77:195-204. doi: 10.3949/ccjm.77a.09064
15. Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275-1280. doi: 10.1016/j.clinbiochem.2017.07.003.
16. Gottheil S, Khemani E, Copley K, et al. Reducing inappropriate ESR testing with computerized clinical decision support. BMJ Quality Improvement Reports, 2016;5:u211376.w4582. doi: 10.1136/bmjquality.u211376.w4582
17. Schneider C, Feller M, Bauer DC, et al. Initial evaluation of thyroid dysfunction - are simultaneous TSH and fT4 tests necessary? PloS One. 2018;13:e0196631–e0196631. doi: 10.1371/journal.pone.0196631
18. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94:106-113.
19. Eaton KP, Levy K, Soong C et.al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177:1833-1839. doi: 10.1001/jamainternmed.2017.5152
20. ADA. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73-S84. doi: 10.2337/dc21-S006
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
22. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
23. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528. doi: 10.1097/SLA.0b013e318265bcdb
24. ASA. ASA physical status classification system. Accessed June 22,2022. www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
25. NLM. Preoperative tests (update): routine preoperative tests for elective surgery. Accessed June 22, 2022. www.ncbi.nlm.nih.gov/books/NBK367919/
26. ASA. American Society of Anesthesiologists releases list of commonly used tests and treatments to question-AS PART OF CHOOSING WISELY® CAMPAIGN. Accessed June 22, 2022. www.asahq.org/about-asa/newsroom/news-releases/2013/10/choosing-wisely
27. Keay L, Lindsley K, Tielsch J, et al. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2019;1:CD007293. doi: 10.1002/14651858.CD007293.pub4
28. Chen CL, Clay TH, McLeod S, et al. A revised estimate of costs associated with routine preoperative testing in Medicare cataract patients with a procedure-specific indicator. JAMA Ophthalmol. 2018;136:231-238. doi:10.1001/jamaophthalmol.2017.6372
29. USPSTF. Human immunodeficiency virus (HIV) infection: screening. Accessed May 16, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/human-immunodeficiency-virus-hiv-infection-screening
30. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
1. IHI. What is the Triple Aim? Accessed June 20, 2022. http://www.ihi.org/Topics/TripleAim/Pages/Overview.aspx#:~:text=It%20is%20IHI’s%20belief%20that,capita%20cost%20of%20health%20care
2. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
3. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system estimated costs and potential for savings. JAMA. 2019;322:1501-1509. doi:10.1001/jama.2019.13978
4. Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704. doi: 10.1377/hlthaff.2017.0385
5. CDC. Strengthening clinical laboratories. 2018. Accessed June 2020, 2022. www.cdc.gov/csels/dls/strengthening-clinical-labs.html
6. Vuong KT. How much do lab tests cost without insurance in 2022? Accessed May 11, 2022. www.talktomira.com/post/how-much-do-lab-test-cost-without-insurance
7. Choosing Wisely: Promoting conversations between providers and patients. Accessed June 20, 2022. www.choosingwisely.org
8. Morgan S, van Driel M, Coleman J, et al. Rational test ordering in family medicine. Can Fam Physician. 2015;61:535-537.
9. US Preventive Services Taskforce. Screening for glaucoma and impaired vision. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf
10. Arnold MJ, O’Malley PG, Downs JR. Key recommendations on managing dyslipidemia for cardiovascular risk reduction: stopping where the evidence does. Am Fam Physician. 2021;103:455-458.
11. Welch HG, Albertsen PC. Reconsidering prostate cancer mortality—the future of PSA screening. N Engl J Med. 2020;382:1557-1563. doi: 10.1056/NEJMms1914228
12. American Society for Microbiology. Why pretest and posttest probability matter in the time of COVID-19. Accessed June 20, 2022. https://asm.org/Articles/2020/June/Why-Pretest-and-Posttest-Probability-Matter-in-the
13. Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med. 1996;156:1421-1425.
14. Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med. 2010;77:195-204. doi: 10.3949/ccjm.77a.09064
15. Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275-1280. doi: 10.1016/j.clinbiochem.2017.07.003.
16. Gottheil S, Khemani E, Copley K, et al. Reducing inappropriate ESR testing with computerized clinical decision support. BMJ Quality Improvement Reports, 2016;5:u211376.w4582. doi: 10.1136/bmjquality.u211376.w4582
17. Schneider C, Feller M, Bauer DC, et al. Initial evaluation of thyroid dysfunction - are simultaneous TSH and fT4 tests necessary? PloS One. 2018;13:e0196631–e0196631. doi: 10.1371/journal.pone.0196631
18. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94:106-113.
19. Eaton KP, Levy K, Soong C et.al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177:1833-1839. doi: 10.1001/jamainternmed.2017.5152
20. ADA. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73-S84. doi: 10.2337/dc21-S006
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
22. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
23. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528. doi: 10.1097/SLA.0b013e318265bcdb
24. ASA. ASA physical status classification system. Accessed June 22,2022. www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
25. NLM. Preoperative tests (update): routine preoperative tests for elective surgery. Accessed June 22, 2022. www.ncbi.nlm.nih.gov/books/NBK367919/
26. ASA. American Society of Anesthesiologists releases list of commonly used tests and treatments to question-AS PART OF CHOOSING WISELY® CAMPAIGN. Accessed June 22, 2022. www.asahq.org/about-asa/newsroom/news-releases/2013/10/choosing-wisely
27. Keay L, Lindsley K, Tielsch J, et al. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2019;1:CD007293. doi: 10.1002/14651858.CD007293.pub4
28. Chen CL, Clay TH, McLeod S, et al. A revised estimate of costs associated with routine preoperative testing in Medicare cataract patients with a procedure-specific indicator. JAMA Ophthalmol. 2018;136:231-238. doi:10.1001/jamaophthalmol.2017.6372
29. USPSTF. Human immunodeficiency virus (HIV) infection: screening. Accessed May 16, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/human-immunodeficiency-virus-hiv-infection-screening
30. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
PRACTICE RECOMMENDATIONS
› Follow US Preventive Services Task Force and professional society recommendations for laboratory testing, including choice and frequency of tests. A
› Consider the pretest probability of your patient having a disease, and order the most sensitive and specific test to diagnose a new condition. Employ a 2-step approach with a second laboratory test when the first is outside the reference range. B
› Refrain from ordering routine preoperative testing for patients undergoing low-risk surgeries; these data do not improve postoperative outcomes, can lead to costly testing cascades, and may delay necessary surgical care for patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Focal Palmoplantar Keratoderma and Gingival Keratosis Caused by a KRT16 Mutation
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
Practice Points
- Focal palmoplantar keratoderma and gingival keratosis (FPGK) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma (PPK) and gingival hyperkeratosis presenting as leukokeratosis.
- Focal pressure-related PPK and oral hyperkeratosis also are seen in pachyonychia congenita (PC), which is caused by mutations in keratin genes and is distinguished from FPGK by characteristic nail changes.
- A shared causative gene suggests that FPGK should be considered part of the PC spectrum.
Erenumab effective and well-tolerated in chronic migraine
Key clinical point: Erenumab is effective and well tolerated in patients with chronic migraine who did not respond to previous migraine treatments.
Major finding: Overall, 71.4% of patients treated with erenumab achieved ≥30% reduction in monthly migraine days from baseline to 9-12 weeks and 34.0% of patients at all assessment periods through 52 weeks. Constipation was the most common adverse event and 13.7% of patients discontinued treatment because of a lack of tolerability.
Study details: The data come from a 52-week, prospective, observational study including 300 patients with chronic migraine who received ≥1 dose of erenumab, of which 273 and 119 patients completed 12 and 52 weeks of treatment, respectively.
Disclosures: This study was funded by and conducted in collaboration with Novartis Pharma AG, Basel, Switzerland. Some authors reported being consultants, speakers, or scientific advisors for or receiving personal fees from various sources, including Novartis. Two authors declared being employees of and holding stocks in Novartis.
Source: Cullum CK et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: A 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61 Jun 2). Doi:10.1186/s10194-022-01433-9
Key clinical point: Erenumab is effective and well tolerated in patients with chronic migraine who did not respond to previous migraine treatments.
Major finding: Overall, 71.4% of patients treated with erenumab achieved ≥30% reduction in monthly migraine days from baseline to 9-12 weeks and 34.0% of patients at all assessment periods through 52 weeks. Constipation was the most common adverse event and 13.7% of patients discontinued treatment because of a lack of tolerability.
Study details: The data come from a 52-week, prospective, observational study including 300 patients with chronic migraine who received ≥1 dose of erenumab, of which 273 and 119 patients completed 12 and 52 weeks of treatment, respectively.
Disclosures: This study was funded by and conducted in collaboration with Novartis Pharma AG, Basel, Switzerland. Some authors reported being consultants, speakers, or scientific advisors for or receiving personal fees from various sources, including Novartis. Two authors declared being employees of and holding stocks in Novartis.
Source: Cullum CK et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: A 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61 Jun 2). Doi:10.1186/s10194-022-01433-9
Key clinical point: Erenumab is effective and well tolerated in patients with chronic migraine who did not respond to previous migraine treatments.
Major finding: Overall, 71.4% of patients treated with erenumab achieved ≥30% reduction in monthly migraine days from baseline to 9-12 weeks and 34.0% of patients at all assessment periods through 52 weeks. Constipation was the most common adverse event and 13.7% of patients discontinued treatment because of a lack of tolerability.
Study details: The data come from a 52-week, prospective, observational study including 300 patients with chronic migraine who received ≥1 dose of erenumab, of which 273 and 119 patients completed 12 and 52 weeks of treatment, respectively.
Disclosures: This study was funded by and conducted in collaboration with Novartis Pharma AG, Basel, Switzerland. Some authors reported being consultants, speakers, or scientific advisors for or receiving personal fees from various sources, including Novartis. Two authors declared being employees of and holding stocks in Novartis.
Source: Cullum CK et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: A 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61 Jun 2). Doi:10.1186/s10194-022-01433-9