User login
Disparities of Cutaneous Malignancies in the US Military
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Practice Points
- Skin cancer is more prevalent among military personnel and veterans, especially those in the US Air Force. Frequent and/or prolonged sun exposure and lack of sun protection may be key factors.
- Future research should compare the prevalence of skin cancer in nonpilot military populations to the general US population; explore racial and ethnic differences by military branch and their influence on skin cancers; analyze each branch’s sun-protective measures, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure; and explore the effects of appropriate military skin cancer intervention and screening programs.
Association of BRAF V600E Status of Incident Melanoma and Risk for a Second Primary Malignancy: A Population-Based Study
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
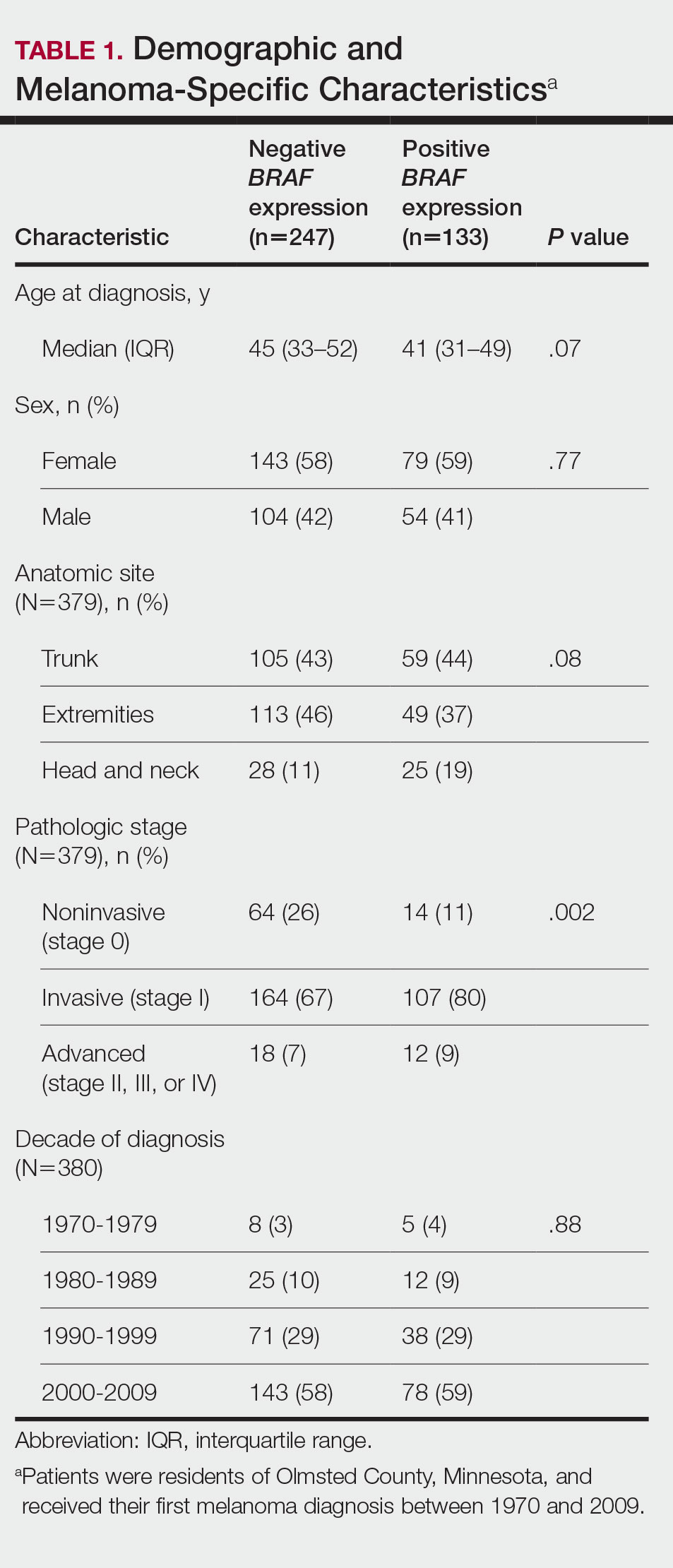
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results
Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.
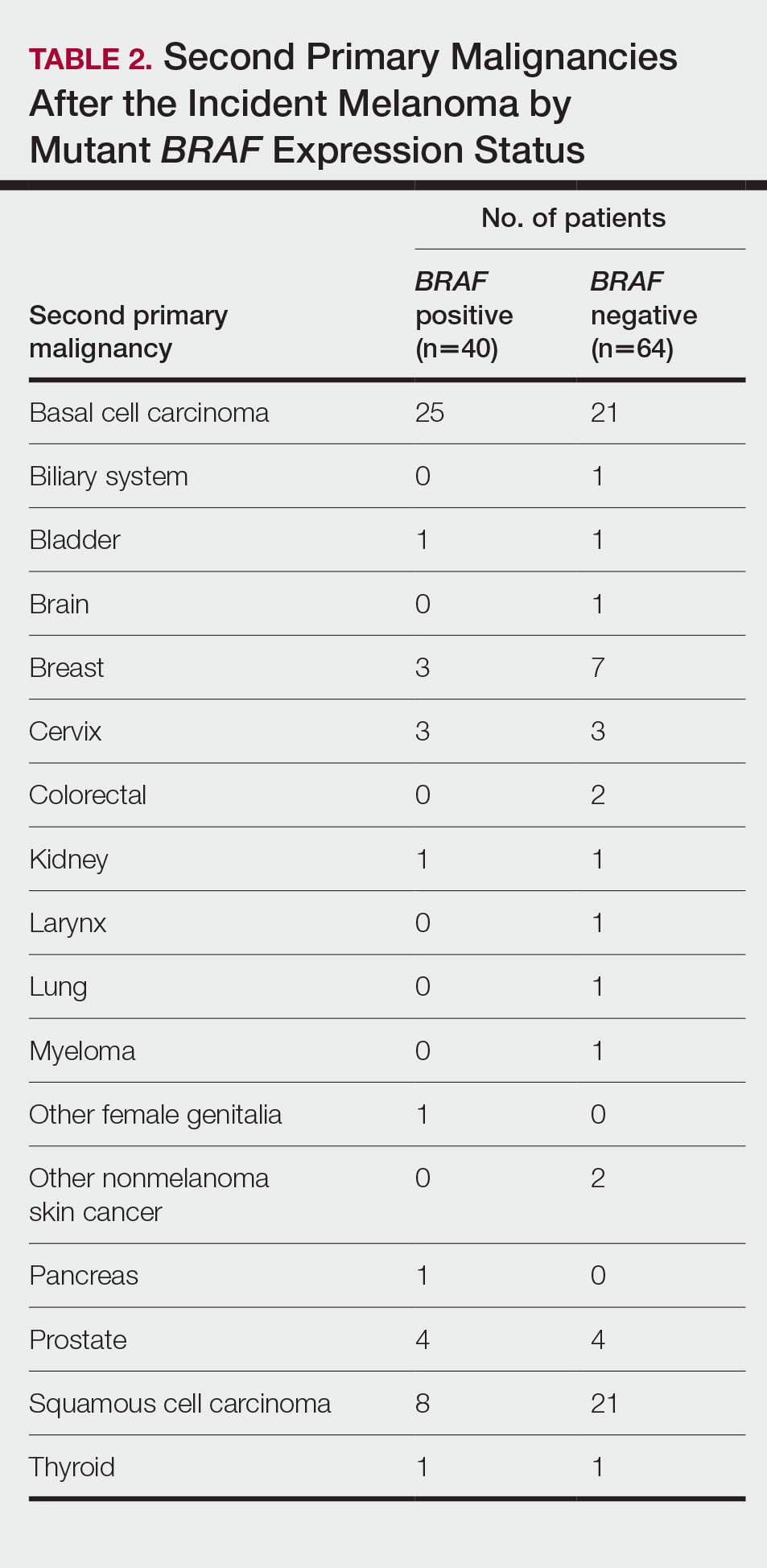
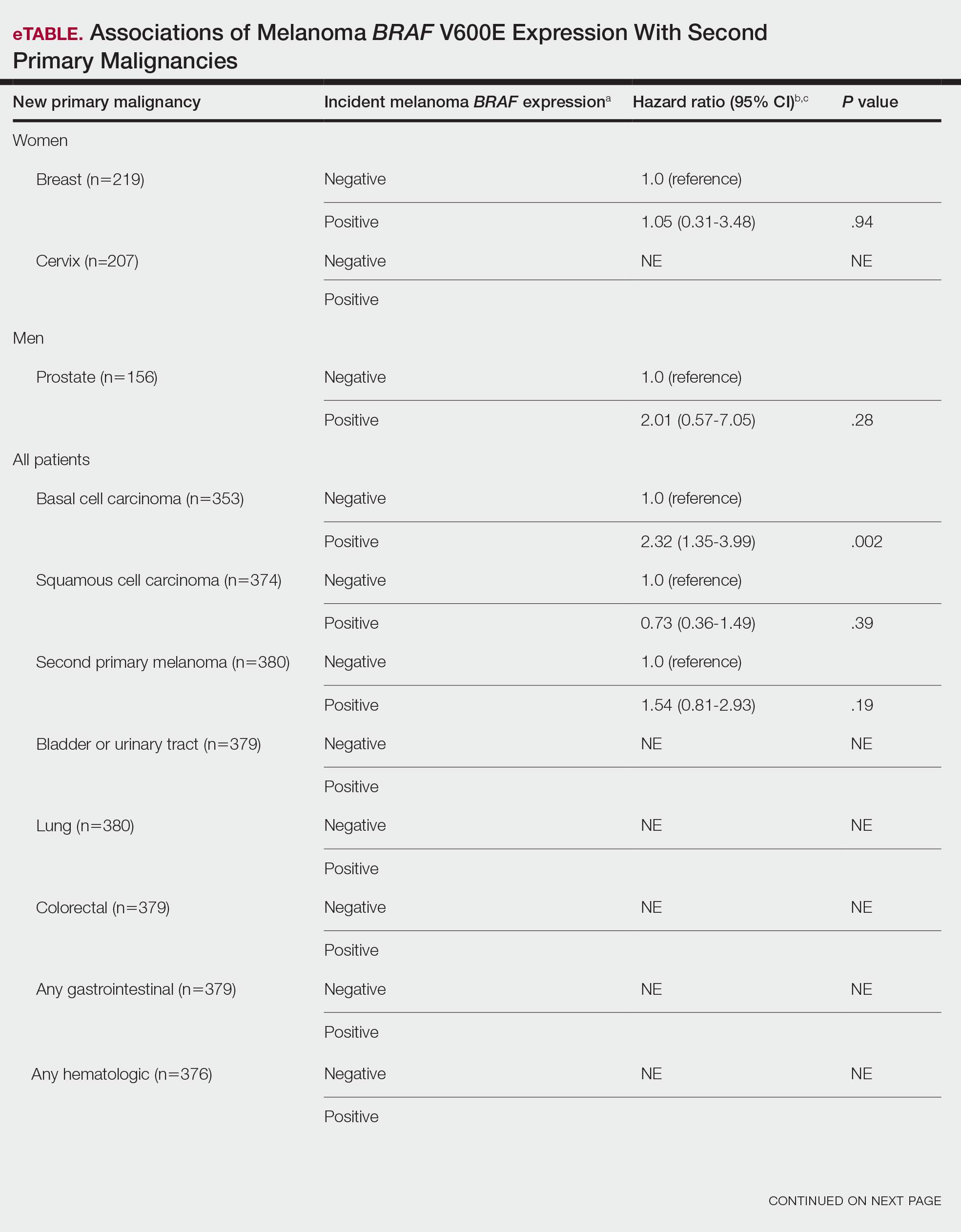
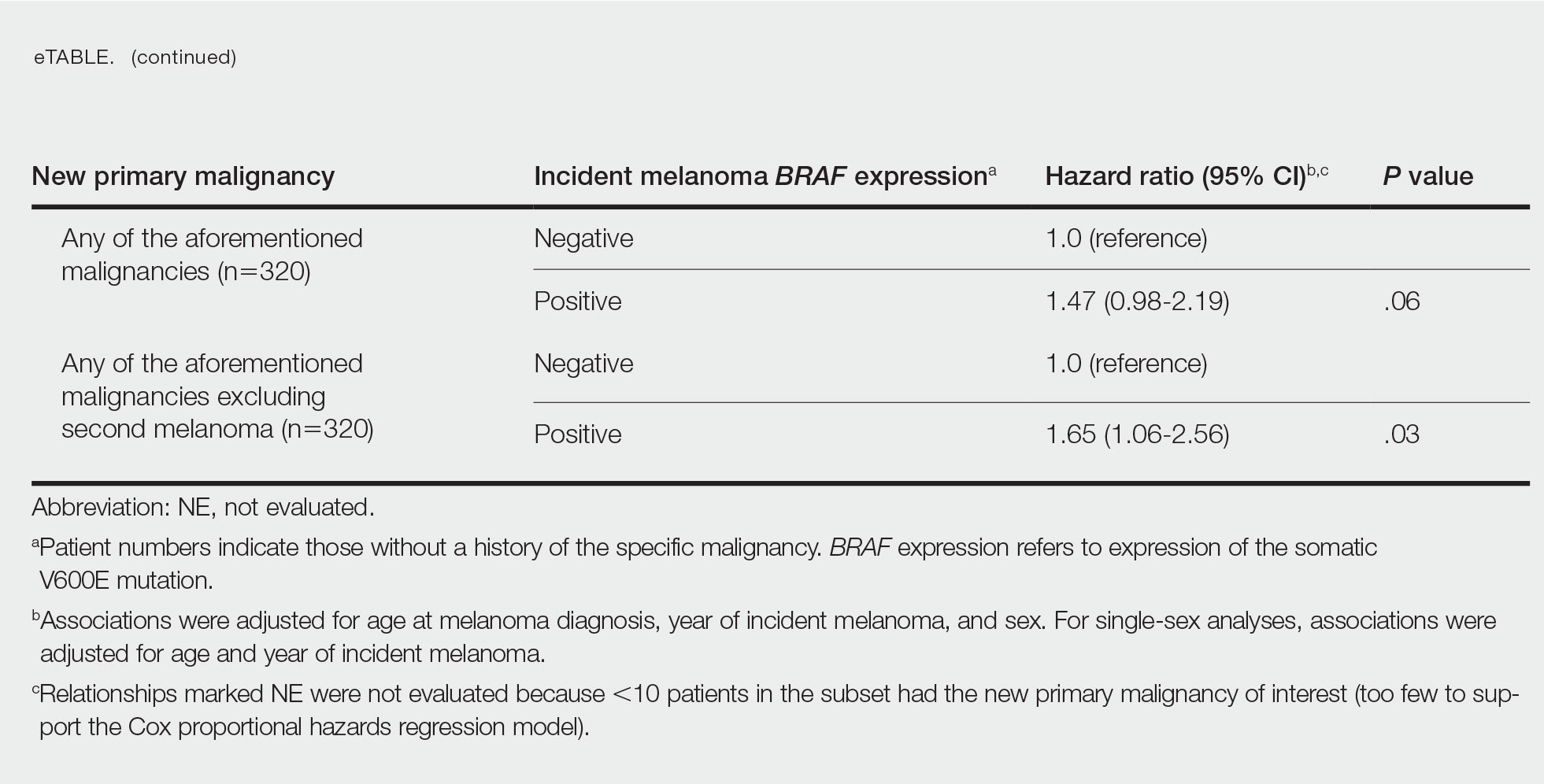
BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.
Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.
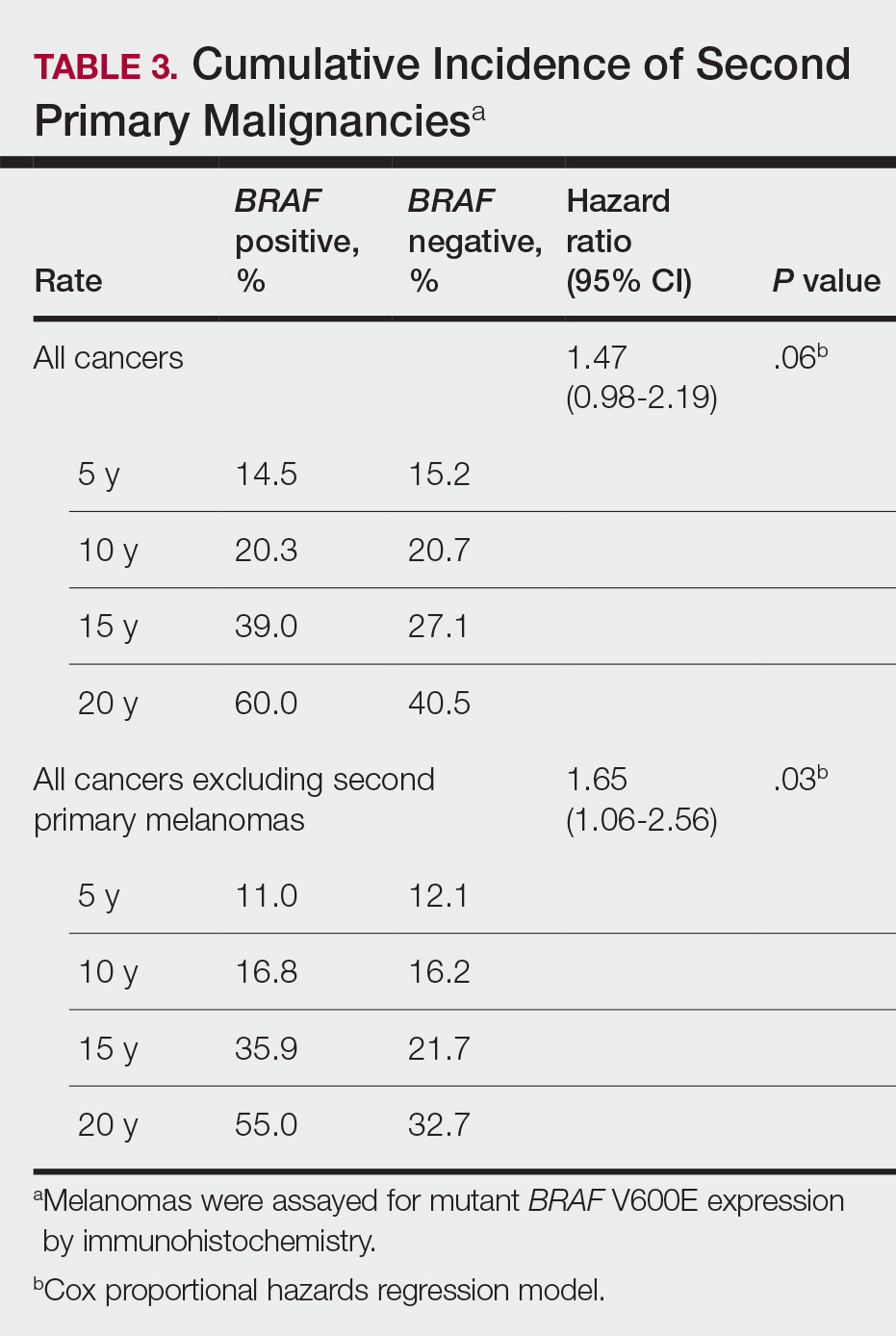
The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
Practice Points
- Dermatologists should be aware of the long-term risk of second primary malignancies after an incident melanoma.
- BRAF mutations occur in melanomas and several other cancers. Our study found that melanoma BRAF V600E expression is associated with an increased risk for basal cell carcinomas.
VA Amends Rule on Abortions
The US Department of Veterans Affairs (VA) has submitted an interim final rule amending its medical regulations to remove the exclusion on abortion counseling and establish exceptions to the exclusion on abortions.
With this rule, the VA will now offer veterans abortion counseling and abortions—specifically, when the life or health of the pregnant veteran would be endangered if the pregnancy were carried to term, or in cases of rape or incest. Beneficiaries enrolled in CHAMPVA will have the same access to care.
“As abortion bans come into force across the country,” the interim final rule indicates, “veterans in many states are no longer assured access to abortion services in their communities, even when those services are needed… Unless VA removes its existing prohibitions on abortion-related care and makes clear that needed abortion-related care is authorized, these veterans will face serious threats to their life and health.”
“This is a patient safety decision,” said Denis McDonough, Secretary of Veterans Affairs. “Pregnant veterans and VA beneficiaries deserve to have access to world-class reproductive care when they need it most. That’s what our nation owes them, and that’s what we at VA will deliver.”
The rule is the VA’s latest response to the June 24, 2022, Dobbs v Jackson Women’s Health Organization Supreme Court decision, which overruled Roe v Wade and Planned Parenthood of Southeastern Pennsylvania v Casey. “After Dobbs,” according to the rule summary, “certain States have begun to enforce existing abortion bans and restrictions on care, and are proposing and enacting new ones, creating urgent risks to the lives and health of pregnant veterans and CHAMPVA beneficiaries in these states.” The VA is “acting to help to ensure that, irrespective of what laws or policies States may impose” veterans will be able to receive needed care.
Restricting access to abortion care has well-documented adverse health consequences, including a higher risk of loss of future fertility, significant morbidity, or death. Veterans are also at greater risk of experiencing pregnancy-related complications due to increased rates of chronic health conditions. “We came to this decision after listening to VA health care providers and veterans across the country, who sounded the alarm that abortion restrictions are creating a medical emergency for those we serve,” said Under Secretary for Health Shereef Elnahal, MD, MBA. “Offering this care will save veterans’ health and lives, and there is nothing more important than that.”
Services will be authorized immediately after the interim final rule is published. VA is taking steps to guarantee abortion-related care anywhere in the country. VA employees, when working within the scope of their federal employment, may provide authorized services regardless of state restrictions.
The determination of whether the “life and health of the pregnant veteran would be endangered if the pregnancy were carried to term” will be made on a case-by-case basis after “careful consultation” between VA health care professionals and their patients, the VA says. In cases of rape or incest, self-reporting from a veteran or VA beneficiary will constitute sufficient evidence that an act of rape or incest occurred.
The interim final rule will be available for public comment for 30 days after it is published.
The US Department of Veterans Affairs (VA) has submitted an interim final rule amending its medical regulations to remove the exclusion on abortion counseling and establish exceptions to the exclusion on abortions.
With this rule, the VA will now offer veterans abortion counseling and abortions—specifically, when the life or health of the pregnant veteran would be endangered if the pregnancy were carried to term, or in cases of rape or incest. Beneficiaries enrolled in CHAMPVA will have the same access to care.
“As abortion bans come into force across the country,” the interim final rule indicates, “veterans in many states are no longer assured access to abortion services in their communities, even when those services are needed… Unless VA removes its existing prohibitions on abortion-related care and makes clear that needed abortion-related care is authorized, these veterans will face serious threats to their life and health.”
“This is a patient safety decision,” said Denis McDonough, Secretary of Veterans Affairs. “Pregnant veterans and VA beneficiaries deserve to have access to world-class reproductive care when they need it most. That’s what our nation owes them, and that’s what we at VA will deliver.”
The rule is the VA’s latest response to the June 24, 2022, Dobbs v Jackson Women’s Health Organization Supreme Court decision, which overruled Roe v Wade and Planned Parenthood of Southeastern Pennsylvania v Casey. “After Dobbs,” according to the rule summary, “certain States have begun to enforce existing abortion bans and restrictions on care, and are proposing and enacting new ones, creating urgent risks to the lives and health of pregnant veterans and CHAMPVA beneficiaries in these states.” The VA is “acting to help to ensure that, irrespective of what laws or policies States may impose” veterans will be able to receive needed care.
Restricting access to abortion care has well-documented adverse health consequences, including a higher risk of loss of future fertility, significant morbidity, or death. Veterans are also at greater risk of experiencing pregnancy-related complications due to increased rates of chronic health conditions. “We came to this decision after listening to VA health care providers and veterans across the country, who sounded the alarm that abortion restrictions are creating a medical emergency for those we serve,” said Under Secretary for Health Shereef Elnahal, MD, MBA. “Offering this care will save veterans’ health and lives, and there is nothing more important than that.”
Services will be authorized immediately after the interim final rule is published. VA is taking steps to guarantee abortion-related care anywhere in the country. VA employees, when working within the scope of their federal employment, may provide authorized services regardless of state restrictions.
The determination of whether the “life and health of the pregnant veteran would be endangered if the pregnancy were carried to term” will be made on a case-by-case basis after “careful consultation” between VA health care professionals and their patients, the VA says. In cases of rape or incest, self-reporting from a veteran or VA beneficiary will constitute sufficient evidence that an act of rape or incest occurred.
The interim final rule will be available for public comment for 30 days after it is published.
The US Department of Veterans Affairs (VA) has submitted an interim final rule amending its medical regulations to remove the exclusion on abortion counseling and establish exceptions to the exclusion on abortions.
With this rule, the VA will now offer veterans abortion counseling and abortions—specifically, when the life or health of the pregnant veteran would be endangered if the pregnancy were carried to term, or in cases of rape or incest. Beneficiaries enrolled in CHAMPVA will have the same access to care.
“As abortion bans come into force across the country,” the interim final rule indicates, “veterans in many states are no longer assured access to abortion services in their communities, even when those services are needed… Unless VA removes its existing prohibitions on abortion-related care and makes clear that needed abortion-related care is authorized, these veterans will face serious threats to their life and health.”
“This is a patient safety decision,” said Denis McDonough, Secretary of Veterans Affairs. “Pregnant veterans and VA beneficiaries deserve to have access to world-class reproductive care when they need it most. That’s what our nation owes them, and that’s what we at VA will deliver.”
The rule is the VA’s latest response to the June 24, 2022, Dobbs v Jackson Women’s Health Organization Supreme Court decision, which overruled Roe v Wade and Planned Parenthood of Southeastern Pennsylvania v Casey. “After Dobbs,” according to the rule summary, “certain States have begun to enforce existing abortion bans and restrictions on care, and are proposing and enacting new ones, creating urgent risks to the lives and health of pregnant veterans and CHAMPVA beneficiaries in these states.” The VA is “acting to help to ensure that, irrespective of what laws or policies States may impose” veterans will be able to receive needed care.
Restricting access to abortion care has well-documented adverse health consequences, including a higher risk of loss of future fertility, significant morbidity, or death. Veterans are also at greater risk of experiencing pregnancy-related complications due to increased rates of chronic health conditions. “We came to this decision after listening to VA health care providers and veterans across the country, who sounded the alarm that abortion restrictions are creating a medical emergency for those we serve,” said Under Secretary for Health Shereef Elnahal, MD, MBA. “Offering this care will save veterans’ health and lives, and there is nothing more important than that.”
Services will be authorized immediately after the interim final rule is published. VA is taking steps to guarantee abortion-related care anywhere in the country. VA employees, when working within the scope of their federal employment, may provide authorized services regardless of state restrictions.
The determination of whether the “life and health of the pregnant veteran would be endangered if the pregnancy were carried to term” will be made on a case-by-case basis after “careful consultation” between VA health care professionals and their patients, the VA says. In cases of rape or incest, self-reporting from a veteran or VA beneficiary will constitute sufficient evidence that an act of rape or incest occurred.
The interim final rule will be available for public comment for 30 days after it is published.
Risk Factors Predicting Cellulitis Diagnosis in a Prospective Cohort Undergoing Dermatology Consultation in the Emergency Department
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).
Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).
Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Practice Points
- Unilateral involvement and leukocytosis are both highly predictive of lower extremity cellulitis in uncomplicated presentations.
- Historical factors such as history of onychomycosis and trauma to the affected site are more predictive of lower extremity cellulitis than demographic factors such as age in uncomplicated presentations of cellulitis.
Health Literacy in Dermatology Patients: How to Level the Playing Field
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
Linear Hypopigmentation on the Right Arm
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
A 73-year-old woman presented to the dermatology clinic with hypopigmentation along the right arm. Her medical history was notable for prior treatment with intralesional triamcinolone injections for De Quervain tenosynovitis. Two months after receiving the steroid injections she noted progressive spreading of an asymptomatic white discoloration originating on the right wrist. Physical examination revealed a hypopigmented atrophic patch on the medial aspect of the right wrist (left) with linear hypopigmented patches extending proximally up the forearm (right).
AVAHO 2022: A Boost of Confidence
Lauren G. Cliffel, MSW, looks forward to the upcoming 2022 Association of VA Hematology/Oncology (AVAHO) annual meeting and the opportunity to connect in-person with social workers, physicians, nurses, cancer navigators, and other cancer care providers who share a mission to establish and expand oncology programs for veterans.
The theme of this year's annual meeting is "Your Best Version: Self-care in Cancer Care." Sessions include a discussion by a cancer care provider who was also a cancer patient, as well as presentations on the VA Whole Health initiative, survivorship, palliative care, and the Schwartz Rounds initiative. In addition, there will be sessions on self-care that include methods to address burnout and compassion fatigue.
Lauren G. Cliffel, MSW, looks forward to the upcoming 2022 Association of VA Hematology/Oncology (AVAHO) annual meeting and the opportunity to connect in-person with social workers, physicians, nurses, cancer navigators, and other cancer care providers who share a mission to establish and expand oncology programs for veterans.
The theme of this year's annual meeting is "Your Best Version: Self-care in Cancer Care." Sessions include a discussion by a cancer care provider who was also a cancer patient, as well as presentations on the VA Whole Health initiative, survivorship, palliative care, and the Schwartz Rounds initiative. In addition, there will be sessions on self-care that include methods to address burnout and compassion fatigue.
Lauren G. Cliffel, MSW, looks forward to the upcoming 2022 Association of VA Hematology/Oncology (AVAHO) annual meeting and the opportunity to connect in-person with social workers, physicians, nurses, cancer navigators, and other cancer care providers who share a mission to establish and expand oncology programs for veterans.
The theme of this year's annual meeting is "Your Best Version: Self-care in Cancer Care." Sessions include a discussion by a cancer care provider who was also a cancer patient, as well as presentations on the VA Whole Health initiative, survivorship, palliative care, and the Schwartz Rounds initiative. In addition, there will be sessions on self-care that include methods to address burnout and compassion fatigue.


AVAHO 2022: The Importance of Self-care
Bernadette Heron, PharmD, provides a glance at activities planned for the approaching AVAHO 2022 annual meeting and announces with enthusiasm this year's theme: "Your Best Version: Self-care in Cancer Care."
Bringing self-care into the theater of clinically relevant issues is central to optimizing the care of patients with cancer, Dr Heron suggests. To this purpose, the structured sessions and interventions to be presented at AVAHO 2022 include a deep dive into burnout among practitioners as well as everyday clinical care issues.
Bernadette Heron, PharmD, provides a glance at activities planned for the approaching AVAHO 2022 annual meeting and announces with enthusiasm this year's theme: "Your Best Version: Self-care in Cancer Care."
Bringing self-care into the theater of clinically relevant issues is central to optimizing the care of patients with cancer, Dr Heron suggests. To this purpose, the structured sessions and interventions to be presented at AVAHO 2022 include a deep dive into burnout among practitioners as well as everyday clinical care issues.
Bernadette Heron, PharmD, provides a glance at activities planned for the approaching AVAHO 2022 annual meeting and announces with enthusiasm this year's theme: "Your Best Version: Self-care in Cancer Care."
Bringing self-care into the theater of clinically relevant issues is central to optimizing the care of patients with cancer, Dr Heron suggests. To this purpose, the structured sessions and interventions to be presented at AVAHO 2022 include a deep dive into burnout among practitioners as well as everyday clinical care issues.
AVAHO 2022: Bringing Provider Well-Being Into Focus
Nick Burwick, MD, summarizes a series of topics to be addressed at the AVAHO 2022 annual meeting, including provider well-being, patient experience, multidisciplinary reflective care, and precision oncology.
In addition to the exciting presentations and sessions, Dr Burwick invokes the more informal aspects of AVAHO 2022, such as meeting colleagues and friends and taking the time to appreciate the host city of San Diego. Above all, Dr Burwick underscores this year’s theme of self-care and resilience in the cancer care setting.
Nick Burwick, MD, summarizes a series of topics to be addressed at the AVAHO 2022 annual meeting, including provider well-being, patient experience, multidisciplinary reflective care, and precision oncology.
In addition to the exciting presentations and sessions, Dr Burwick invokes the more informal aspects of AVAHO 2022, such as meeting colleagues and friends and taking the time to appreciate the host city of San Diego. Above all, Dr Burwick underscores this year’s theme of self-care and resilience in the cancer care setting.
Nick Burwick, MD, summarizes a series of topics to be addressed at the AVAHO 2022 annual meeting, including provider well-being, patient experience, multidisciplinary reflective care, and precision oncology.
In addition to the exciting presentations and sessions, Dr Burwick invokes the more informal aspects of AVAHO 2022, such as meeting colleagues and friends and taking the time to appreciate the host city of San Diego. Above all, Dr Burwick underscores this year’s theme of self-care and resilience in the cancer care setting.
MS Researchers Wonder Aloud: Is Remyelination Possible?
The 3 “Rs” of multiple sclerosis (MS)—repair, remyelinate, and restore—spell out the goals of patients and physicians alike. MS is an incurable, immune-mediated, neurodegenerative disease of the central nervous system (CNS), and is thought to develop from unexplained autoimmune attacks directed at myelin (the covering on neurons) and glial cells, or “oligodendrocytes.” Neurodegeneration is evident early in the disease process and is characterized by mitochondrial dysfunction, energy failure, and neuronal and glial death.
While most new and investigational therapies aim to address immune dysfunction, a new idea—
one not involving immune dysregulation—is being explored in various studies: Are there agents, outside of traditional MS therapies, able to help with remyelination?
Mitochondria, oxidative stress, and MS
Neurons, oligodendrocytes, and oligodendrocyte precursor cells (OPCs) are particularly sensitive to oxidative stress. In MS, chronic inflammation and autoimmunity are key drivers of oxidative stress and secondary mitochondrial dysfunction.
Mitochondrial dysfunction is particularly relevant for neurodegeneration in MS. The observed dysfunction includes mitochondrial DNA damage, deficiency in mitochondrial DNA repair, reduced levels of antioxidants, and increased free radicals. Furthermore, the structure and number of mitochondria temporarily increase to accommodate the increased energy needs. Despite the attempted adaptation, energy failure ultimately occurs, resulting in a mismatch between energy needs or consumption and energy production. Neuroinflammation and the imbalance between energy consumption and generation create a vicious, continuous cycle that is characteristic in progressive MS. The energy failure is then associated with neuronal death, Wallerian degeneration, and subsequent accumulation of neurologic disability.
Current therapeutic landscape
While the therapeutic landscape for MS continues to evolve, the approved 20-plus therapies are primarily directed at the immune system. The overall goal is to modulate immune dysregulation and decrease inflammation. Current therapies may be able to control this macroscopic inflammatory activity.
However, current treatments only show modest effects on disease progression, and do not help to repair neurons, remyelinate axons, or restore function that was impaired due to disease progression. Some US Food and Drug Administration (FDA)–approved therapies are thought to modulate mitochondrial functions. For example, the class of fumarates (eg, dimethyl fumarate, diroximel fumarate, monomethyl fumarate) activates the nuclear factor erythroid 2 -related factor 2 (Nrf2) pathway in treated MS patients. However, it is unclear whether activation of the Nrf2 pathway is involved in the therapeutic effects of fumarates. A recent study challenged the importance of the Nrf2 pathway as a therapeutic target for fumarates. It showed that in an MS animal model, the effects of fumarates on disease control were similar between Nrf2 knock-out mice and the wild type, suggesting that fumarates' therapeutic effects are independent of the Nrf2 pathway. Furthermore, fumarates failed to show benefits in progressive forms of MS both clinically and on a biomarker level.
Metformin, the mitochondria, and neurodegeneration
Metformin (1,1-dimethylbiguanide) is an oral medication used primarily as first-line treatment for type 2 diabetes. However, due to its pharmacologic properties, mitochondrial effects, and the ability to cross the blood-brain barrier, scientists have shown recent interest in studying metformin in neurodegenerative diseases, including MS. Some of the potential benefits of metformin in neurodegenerative diseases include reduction of oxidative stress and countering mitochondrial dysfunction. It is known that metformin inhibits mitochondrial complex 1. Also, several studies have shown a positive effect of metformin on the reduction of oxidative stress and mitochondrial DNA regulation. Therefore, could metformin help combat mitochondrial dysfunction in MS or rejuvenate certain elements within the CNS in people with neurodegenerative diseases, including MS?
Oligodendrocytes and remyelination
Oligodendrocytes are cells responsible for myelinating axons within the CNS. Those cells originate from progenitors called OPCs. Interestingly, in humans, OPCs can mature into oligodendrocytes throughout their lifecycle, although to a much lesser extent in adults compared with children. However, therapeutic efforts to facilitate OPC maturation in vivo in MS lesions have failed thus far. Examples include high-dose biotin, the anti-LINGO-1 opicinumab, and the anticancer, retinoid-analog drug bexarotene.
So, what is behind these unfortunate failures? Some molecules (eg, biotin, opicinumab) failed to meet their clinical endpoints in randomized clinical trials, while others had severe toxicity that halted further clinical testing (eg, bexarotene). On the other hand, some molecules (eg, clemastine fumarate), showed a modest yet promising effect on biomarkers in small clinical trials.
A discussion on molecule failures
What could explain the failure of molecules with such promising preclinical findings? One could argue that clinical trial designs may have been insufficient to detect small remyelinating effects. One could also argue that the maturation of OPCs into oligodendrocytes is too complex to facilitate using 1 molecule that may be an inhibitor of maturation or to activate/augment a facilitator of the maturation process. There are too many natural inhibitors and facilitators of OPC maturation, and an approach with combination therapy might have a better chance at achieving a favorable therapeutic effect.
Another piece of the complexity of OPC maturation is the recent discovery that, in humans, nonhuman primates, and other mammals, aged OPCs do not have the same capacity to mature into oligodendrocytes as young OPCs. There might be some clinical support here, as children with MS have more ability to recover from MS attacks than their adult counterparts. Also, the older the individual with MS is, the less likely they are to recover from MS attacks and the more likely they are to show signs of disease progression compared with their younger counterparts.
Theoretically, age-related recovery from clinical attacks may be partially explained by complications due to OPC aging. To this point, can we rejuvenate OPCs and restore their ability to mature into oligodendrocytes? Can metformin be the medicine that does so?
Interestingly, scientists could restore the ability of older OPCs to mature into oligodendrocytes, at least in the rodent model, through calorie restriction (eg, intermittent fasting) or by mimicking this state using metformin.
Metformin and the 3 “Rs”
One idea is to use metformin to create a biochemical state that allows OPCs to regain their ability to mature into oligodendrocytes in adult or aging individuals with MS. If that is achieved, other molecules may augment OPC' maturation or inhibit OPC maturation-inhibitors and become successful in promoting remyelination. A phase 2 clinical trial in the United Kingdom that is currently recruiting participants intends to investigate a combination of metformin and clemastine fumarate in 50 patients with relapsing-remitting MS. The goal is to learn whether metformin plus clemastine allows for therapeutic remyelination. In addition, a Canadian study is investigating metformin in children with MS. Two other studies are currently recruiting to study metformin in relapsing MS (Egypt) and progressive MS (United States).
Although testing metformin as a treatment for MS is still in the early stages, the scientific rationale is valid and supported by compelling preclinical evidence. Ongoing clinical trials will likely provide preliminary results on whether metformin will advance in clinical testing and provide clinically meaningful improvements for people living with MS.
If metformin is, in fact, a conditioning agent for use in remyelinating therapies, future clinical trials could be designed to administer metformin to rejuvenate OPCs before the administration of any molecule combination designed to facilitate OPC maturation. However, these trials will need to address an important issue: dosage. In type 2 diabetes, the typical daily dose is between 500 and 3000 mg per day. But in tests on rodents – which weigh about 10 grams – to rejuvenate OPCs, the doses of metformin were very high: 200 to 300 mg/kg. Given the body weight of humans and to avoid drug toxicity, the resulting smaller doses of metformin will take time to exert their potential therapeutic effect.
Should future research be successful in developing combination molecular therapies with diverse and synergistic therapeutic targets, then the 3 “Rs” in MS will allow for a fourth “R” to effectively succeed: repair, remyelinate, restore, and rehabilitate.
The 3 “Rs” of multiple sclerosis (MS)—repair, remyelinate, and restore—spell out the goals of patients and physicians alike. MS is an incurable, immune-mediated, neurodegenerative disease of the central nervous system (CNS), and is thought to develop from unexplained autoimmune attacks directed at myelin (the covering on neurons) and glial cells, or “oligodendrocytes.” Neurodegeneration is evident early in the disease process and is characterized by mitochondrial dysfunction, energy failure, and neuronal and glial death.
While most new and investigational therapies aim to address immune dysfunction, a new idea—
one not involving immune dysregulation—is being explored in various studies: Are there agents, outside of traditional MS therapies, able to help with remyelination?
Mitochondria, oxidative stress, and MS
Neurons, oligodendrocytes, and oligodendrocyte precursor cells (OPCs) are particularly sensitive to oxidative stress. In MS, chronic inflammation and autoimmunity are key drivers of oxidative stress and secondary mitochondrial dysfunction.
Mitochondrial dysfunction is particularly relevant for neurodegeneration in MS. The observed dysfunction includes mitochondrial DNA damage, deficiency in mitochondrial DNA repair, reduced levels of antioxidants, and increased free radicals. Furthermore, the structure and number of mitochondria temporarily increase to accommodate the increased energy needs. Despite the attempted adaptation, energy failure ultimately occurs, resulting in a mismatch between energy needs or consumption and energy production. Neuroinflammation and the imbalance between energy consumption and generation create a vicious, continuous cycle that is characteristic in progressive MS. The energy failure is then associated with neuronal death, Wallerian degeneration, and subsequent accumulation of neurologic disability.
Current therapeutic landscape
While the therapeutic landscape for MS continues to evolve, the approved 20-plus therapies are primarily directed at the immune system. The overall goal is to modulate immune dysregulation and decrease inflammation. Current therapies may be able to control this macroscopic inflammatory activity.
However, current treatments only show modest effects on disease progression, and do not help to repair neurons, remyelinate axons, or restore function that was impaired due to disease progression. Some US Food and Drug Administration (FDA)–approved therapies are thought to modulate mitochondrial functions. For example, the class of fumarates (eg, dimethyl fumarate, diroximel fumarate, monomethyl fumarate) activates the nuclear factor erythroid 2 -related factor 2 (Nrf2) pathway in treated MS patients. However, it is unclear whether activation of the Nrf2 pathway is involved in the therapeutic effects of fumarates. A recent study challenged the importance of the Nrf2 pathway as a therapeutic target for fumarates. It showed that in an MS animal model, the effects of fumarates on disease control were similar between Nrf2 knock-out mice and the wild type, suggesting that fumarates' therapeutic effects are independent of the Nrf2 pathway. Furthermore, fumarates failed to show benefits in progressive forms of MS both clinically and on a biomarker level.
Metformin, the mitochondria, and neurodegeneration
Metformin (1,1-dimethylbiguanide) is an oral medication used primarily as first-line treatment for type 2 diabetes. However, due to its pharmacologic properties, mitochondrial effects, and the ability to cross the blood-brain barrier, scientists have shown recent interest in studying metformin in neurodegenerative diseases, including MS. Some of the potential benefits of metformin in neurodegenerative diseases include reduction of oxidative stress and countering mitochondrial dysfunction. It is known that metformin inhibits mitochondrial complex 1. Also, several studies have shown a positive effect of metformin on the reduction of oxidative stress and mitochondrial DNA regulation. Therefore, could metformin help combat mitochondrial dysfunction in MS or rejuvenate certain elements within the CNS in people with neurodegenerative diseases, including MS?
Oligodendrocytes and remyelination
Oligodendrocytes are cells responsible for myelinating axons within the CNS. Those cells originate from progenitors called OPCs. Interestingly, in humans, OPCs can mature into oligodendrocytes throughout their lifecycle, although to a much lesser extent in adults compared with children. However, therapeutic efforts to facilitate OPC maturation in vivo in MS lesions have failed thus far. Examples include high-dose biotin, the anti-LINGO-1 opicinumab, and the anticancer, retinoid-analog drug bexarotene.
So, what is behind these unfortunate failures? Some molecules (eg, biotin, opicinumab) failed to meet their clinical endpoints in randomized clinical trials, while others had severe toxicity that halted further clinical testing (eg, bexarotene). On the other hand, some molecules (eg, clemastine fumarate), showed a modest yet promising effect on biomarkers in small clinical trials.
A discussion on molecule failures
What could explain the failure of molecules with such promising preclinical findings? One could argue that clinical trial designs may have been insufficient to detect small remyelinating effects. One could also argue that the maturation of OPCs into oligodendrocytes is too complex to facilitate using 1 molecule that may be an inhibitor of maturation or to activate/augment a facilitator of the maturation process. There are too many natural inhibitors and facilitators of OPC maturation, and an approach with combination therapy might have a better chance at achieving a favorable therapeutic effect.
Another piece of the complexity of OPC maturation is the recent discovery that, in humans, nonhuman primates, and other mammals, aged OPCs do not have the same capacity to mature into oligodendrocytes as young OPCs. There might be some clinical support here, as children with MS have more ability to recover from MS attacks than their adult counterparts. Also, the older the individual with MS is, the less likely they are to recover from MS attacks and the more likely they are to show signs of disease progression compared with their younger counterparts.
Theoretically, age-related recovery from clinical attacks may be partially explained by complications due to OPC aging. To this point, can we rejuvenate OPCs and restore their ability to mature into oligodendrocytes? Can metformin be the medicine that does so?
Interestingly, scientists could restore the ability of older OPCs to mature into oligodendrocytes, at least in the rodent model, through calorie restriction (eg, intermittent fasting) or by mimicking this state using metformin.
Metformin and the 3 “Rs”
One idea is to use metformin to create a biochemical state that allows OPCs to regain their ability to mature into oligodendrocytes in adult or aging individuals with MS. If that is achieved, other molecules may augment OPC' maturation or inhibit OPC maturation-inhibitors and become successful in promoting remyelination. A phase 2 clinical trial in the United Kingdom that is currently recruiting participants intends to investigate a combination of metformin and clemastine fumarate in 50 patients with relapsing-remitting MS. The goal is to learn whether metformin plus clemastine allows for therapeutic remyelination. In addition, a Canadian study is investigating metformin in children with MS. Two other studies are currently recruiting to study metformin in relapsing MS (Egypt) and progressive MS (United States).
Although testing metformin as a treatment for MS is still in the early stages, the scientific rationale is valid and supported by compelling preclinical evidence. Ongoing clinical trials will likely provide preliminary results on whether metformin will advance in clinical testing and provide clinically meaningful improvements for people living with MS.
If metformin is, in fact, a conditioning agent for use in remyelinating therapies, future clinical trials could be designed to administer metformin to rejuvenate OPCs before the administration of any molecule combination designed to facilitate OPC maturation. However, these trials will need to address an important issue: dosage. In type 2 diabetes, the typical daily dose is between 500 and 3000 mg per day. But in tests on rodents – which weigh about 10 grams – to rejuvenate OPCs, the doses of metformin were very high: 200 to 300 mg/kg. Given the body weight of humans and to avoid drug toxicity, the resulting smaller doses of metformin will take time to exert their potential therapeutic effect.
Should future research be successful in developing combination molecular therapies with diverse and synergistic therapeutic targets, then the 3 “Rs” in MS will allow for a fourth “R” to effectively succeed: repair, remyelinate, restore, and rehabilitate.
The 3 “Rs” of multiple sclerosis (MS)—repair, remyelinate, and restore—spell out the goals of patients and physicians alike. MS is an incurable, immune-mediated, neurodegenerative disease of the central nervous system (CNS), and is thought to develop from unexplained autoimmune attacks directed at myelin (the covering on neurons) and glial cells, or “oligodendrocytes.” Neurodegeneration is evident early in the disease process and is characterized by mitochondrial dysfunction, energy failure, and neuronal and glial death.
While most new and investigational therapies aim to address immune dysfunction, a new idea—
one not involving immune dysregulation—is being explored in various studies: Are there agents, outside of traditional MS therapies, able to help with remyelination?
Mitochondria, oxidative stress, and MS
Neurons, oligodendrocytes, and oligodendrocyte precursor cells (OPCs) are particularly sensitive to oxidative stress. In MS, chronic inflammation and autoimmunity are key drivers of oxidative stress and secondary mitochondrial dysfunction.
Mitochondrial dysfunction is particularly relevant for neurodegeneration in MS. The observed dysfunction includes mitochondrial DNA damage, deficiency in mitochondrial DNA repair, reduced levels of antioxidants, and increased free radicals. Furthermore, the structure and number of mitochondria temporarily increase to accommodate the increased energy needs. Despite the attempted adaptation, energy failure ultimately occurs, resulting in a mismatch between energy needs or consumption and energy production. Neuroinflammation and the imbalance between energy consumption and generation create a vicious, continuous cycle that is characteristic in progressive MS. The energy failure is then associated with neuronal death, Wallerian degeneration, and subsequent accumulation of neurologic disability.
Current therapeutic landscape
While the therapeutic landscape for MS continues to evolve, the approved 20-plus therapies are primarily directed at the immune system. The overall goal is to modulate immune dysregulation and decrease inflammation. Current therapies may be able to control this macroscopic inflammatory activity.
However, current treatments only show modest effects on disease progression, and do not help to repair neurons, remyelinate axons, or restore function that was impaired due to disease progression. Some US Food and Drug Administration (FDA)–approved therapies are thought to modulate mitochondrial functions. For example, the class of fumarates (eg, dimethyl fumarate, diroximel fumarate, monomethyl fumarate) activates the nuclear factor erythroid 2 -related factor 2 (Nrf2) pathway in treated MS patients. However, it is unclear whether activation of the Nrf2 pathway is involved in the therapeutic effects of fumarates. A recent study challenged the importance of the Nrf2 pathway as a therapeutic target for fumarates. It showed that in an MS animal model, the effects of fumarates on disease control were similar between Nrf2 knock-out mice and the wild type, suggesting that fumarates' therapeutic effects are independent of the Nrf2 pathway. Furthermore, fumarates failed to show benefits in progressive forms of MS both clinically and on a biomarker level.
Metformin, the mitochondria, and neurodegeneration
Metformin (1,1-dimethylbiguanide) is an oral medication used primarily as first-line treatment for type 2 diabetes. However, due to its pharmacologic properties, mitochondrial effects, and the ability to cross the blood-brain barrier, scientists have shown recent interest in studying metformin in neurodegenerative diseases, including MS. Some of the potential benefits of metformin in neurodegenerative diseases include reduction of oxidative stress and countering mitochondrial dysfunction. It is known that metformin inhibits mitochondrial complex 1. Also, several studies have shown a positive effect of metformin on the reduction of oxidative stress and mitochondrial DNA regulation. Therefore, could metformin help combat mitochondrial dysfunction in MS or rejuvenate certain elements within the CNS in people with neurodegenerative diseases, including MS?
Oligodendrocytes and remyelination
Oligodendrocytes are cells responsible for myelinating axons within the CNS. Those cells originate from progenitors called OPCs. Interestingly, in humans, OPCs can mature into oligodendrocytes throughout their lifecycle, although to a much lesser extent in adults compared with children. However, therapeutic efforts to facilitate OPC maturation in vivo in MS lesions have failed thus far. Examples include high-dose biotin, the anti-LINGO-1 opicinumab, and the anticancer, retinoid-analog drug bexarotene.
So, what is behind these unfortunate failures? Some molecules (eg, biotin, opicinumab) failed to meet their clinical endpoints in randomized clinical trials, while others had severe toxicity that halted further clinical testing (eg, bexarotene). On the other hand, some molecules (eg, clemastine fumarate), showed a modest yet promising effect on biomarkers in small clinical trials.
A discussion on molecule failures
What could explain the failure of molecules with such promising preclinical findings? One could argue that clinical trial designs may have been insufficient to detect small remyelinating effects. One could also argue that the maturation of OPCs into oligodendrocytes is too complex to facilitate using 1 molecule that may be an inhibitor of maturation or to activate/augment a facilitator of the maturation process. There are too many natural inhibitors and facilitators of OPC maturation, and an approach with combination therapy might have a better chance at achieving a favorable therapeutic effect.
Another piece of the complexity of OPC maturation is the recent discovery that, in humans, nonhuman primates, and other mammals, aged OPCs do not have the same capacity to mature into oligodendrocytes as young OPCs. There might be some clinical support here, as children with MS have more ability to recover from MS attacks than their adult counterparts. Also, the older the individual with MS is, the less likely they are to recover from MS attacks and the more likely they are to show signs of disease progression compared with their younger counterparts.
Theoretically, age-related recovery from clinical attacks may be partially explained by complications due to OPC aging. To this point, can we rejuvenate OPCs and restore their ability to mature into oligodendrocytes? Can metformin be the medicine that does so?
Interestingly, scientists could restore the ability of older OPCs to mature into oligodendrocytes, at least in the rodent model, through calorie restriction (eg, intermittent fasting) or by mimicking this state using metformin.
Metformin and the 3 “Rs”
One idea is to use metformin to create a biochemical state that allows OPCs to regain their ability to mature into oligodendrocytes in adult or aging individuals with MS. If that is achieved, other molecules may augment OPC' maturation or inhibit OPC maturation-inhibitors and become successful in promoting remyelination. A phase 2 clinical trial in the United Kingdom that is currently recruiting participants intends to investigate a combination of metformin and clemastine fumarate in 50 patients with relapsing-remitting MS. The goal is to learn whether metformin plus clemastine allows for therapeutic remyelination. In addition, a Canadian study is investigating metformin in children with MS. Two other studies are currently recruiting to study metformin in relapsing MS (Egypt) and progressive MS (United States).
Although testing metformin as a treatment for MS is still in the early stages, the scientific rationale is valid and supported by compelling preclinical evidence. Ongoing clinical trials will likely provide preliminary results on whether metformin will advance in clinical testing and provide clinically meaningful improvements for people living with MS.
If metformin is, in fact, a conditioning agent for use in remyelinating therapies, future clinical trials could be designed to administer metformin to rejuvenate OPCs before the administration of any molecule combination designed to facilitate OPC maturation. However, these trials will need to address an important issue: dosage. In type 2 diabetes, the typical daily dose is between 500 and 3000 mg per day. But in tests on rodents – which weigh about 10 grams – to rejuvenate OPCs, the doses of metformin were very high: 200 to 300 mg/kg. Given the body weight of humans and to avoid drug toxicity, the resulting smaller doses of metformin will take time to exert their potential therapeutic effect.
Should future research be successful in developing combination molecular therapies with diverse and synergistic therapeutic targets, then the 3 “Rs” in MS will allow for a fourth “R” to effectively succeed: repair, remyelinate, restore, and rehabilitate.