User login
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
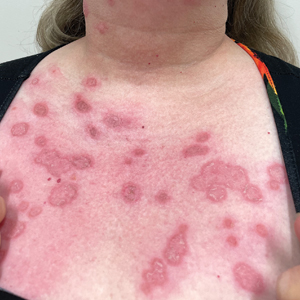
Celery (Apium graveolens)—that lowly vegetable that often languishes in the refrigerator crisper and apparently supplies fewer calories than are required to consume it—contains a myriad of photosensitizing chemicals known as furocoumarins and psoralens that can cause phytophotodermatitis (PPD) when handled prior to exposure to UV light.1 Individuals who are most likely to develop PPD caused by repeated contact with celery include food industry workers (eg, grocery store workers, farmers) who pick, handle, or prepare celery for consumption. While eating celery as part of a standard diet is highly unlikely to cause PPD, celery infected with Sclerotinia sclerotiorum (known as pink rot) causes more severe generalized sun sensitivity due to an increased amount of furocoumarins produced in response to the fungus.2 Contact with celery also can induce cutaneous manifestations unrelated to sun exposure in some individuals, including urticaria, allergic contact dermatitis, and anaphylaxis.3 In this article, we provide an overview of the life cycle and origin of celery as well as its irritant and allergic properties. We also describe cutaneous rashes associated with PPD caused by exposure to celery and highlight treatment options.
Morphology and Distribution
The Apiaceae family features aromatic flowering plants that comprise more than 3500 species, including many economically important vegetables, herbs, and spices.4 It also includes many alkaloid-containing species that are known to be poisonous to humans, such as poison hemlock (Conium maculatum) and water hemlock (Cicuta maculate). Most Apiaceae plants that are consumed by humans originate from the Mediterranean region.5 While known for their diversity of flavor and aroma, most of the plants from this family have low caloric value and provide minimal amounts of energy.
Members of the Apiaceae family have flowers that create a classic umbel shape mimicking the appearance of an upside-down umbrella (thus the former name for this family, Umbelliferae). The pedicles—the small stems attached to the base of each flower—spread from a common center to form the umbel.5 The Apiaceae family also includes the greatest number of plants that cause PPD due to their high concentration of furocoumarins, which deter fungus from harming the plants.6
A biennial plant, celery completes its life cycle in 2 years. During the first season, the stems, roots, and leaves sprout; in the second and final year, the flowers, fruits, and seeds proliferate, followed by decomposition. Apium graveolens approaches heights of 2 to 3 ft, growing upright and displaying grooved stems. Each stem terminates in a basal rosette of leaves. The second season brings white flower blooms in terminal or axillary umbels.7
Celery originated in the temperate Mediterranean regions of Europe, but farmers now cultivate it globally.8 It grows best in rich moist soil with full exposure to sunlight. Plants multiply their numbers through self-seeding. Celery commonly is found in suburban and rural homes, both in refrigerators for consumption as well as in medicine cabinets in capsule form for the treatment of arthritis.4
Irritant and Allergenic Properties
Despite the potential health benefits of celery, the Apiaceae family, which includes hogweed, dill, and fennel, prevails as the most common culprit for phytotoxic reactions. The Rutaceae family, including citrus plants and rue, remains runner-up for causes of PPD.9 Phytophotodermatitis is not an immunologic reaction, making anyone susceptible to formation of the cutaneous lesions when exposed to UV light after handling celery. Pruritis rarely occurs, unlike in allergic phytodermatitis.10 Upon photoexcitation from exposure to UVA light, individual psoralen molecules covalently bind to pyrimidine bases, causing interstrand cross-linking that prevents DNA replication and triggering a cascade leading to apoptosis of the cell. Apoptosis induces cell membrane edema, which manifests as cutaneous vesicles and bullae on the skin.10 Regardless of plant species, PPD reactions have similar appearance.
Celery roots contain the greatest concentration of psoralens, making it the most likely part of the plant to induce PPD.6 Phytophotodermatitis caused by celery can occur at any time of the year, but most eruptions occur during the summer months due to increased sunlight exposure and intensity. Among 320 randomly selected Michigan celery harvesters, 163 (51%) displayed evidence of vesicular and bullous dermatitis on the fingers, hands, and forearms.11 In this study, celery infected with pink rot fungus induced an erythematous eruption with vesicles and bullae within 48 hours of contact after just 30 seconds of summer sunlight exposure; however, eruptions are not limited to summer months, as the cutaneous presentation depends solely on exposure to UVA light, which can occur year-round.
Use of tanning beds is a major risk factor for PPD.12 Tanning beds utilize fluorescent bulbs that primarily emit UVA light, with UVB light emitted to a lesser degree. The UVA radiation produced by tanning beds is more than 3 times as intense as natural sunlight.12 Among grocery store employees, the combination of these 2 risk factors—regular contact with celery and tanning bed use—resulted in a prevalence ratio for PPD more than 40 times greater than that of individuals with neither risk factor.13
Cutaneous Manifestations of PPD
Phytophotodermatitis is a nonimmunologic dermatitis that forms via the interaction between UV light exposure and the photosensitizing chemicals inherent to some plant species. Development of PPD following contact with celery may be caused by the photoactive substances in celery, including the psoralens 8-methoxypsoralen and 5-methoxypsoralen.14 The psoralens must become activated by UV light with wavelengths between 320 nm and 400 nm (UVA) to initiate biologic effects.15
Once chemically activated, the photoactive mediators cause an erythematous and edematous sunburnlike reaction. Current hypotheses state that psoralen plus UVA generates reactive oxygen species, which damage the DNA within cells and alter receptors on cell membranes within the epidermis.14 The cutaneous eruption usually appears between 12 and 36 hours after sun exposure. Although they generally are not pruritic, the eruptions may induce pain. Within 7 to 10 days following development of the rash, hyperpigmentation occurs in the affected area and often persists for months to years.16 Ingestion of large amounts of celery has been cited to cause generalized phototoxic reactions; however, PPD rarely arises solely after ingestion, unless excessive amounts are consumed with concomitant exposure to psoralen plus UVA or tanning beds.17 In these cases, patients develop diffuse redness with superficial scaling, pain, and blistering if severe.
Treatment of PPD
Prevention remains the best form of treatment for PPD caused by exposure to celery. Postcontact management includes washing the affected area with soap and water and changing clothes promptly. Topical corticosteroids have mild utility in treatment of PPD.18 Oral steroid tapers, which reduce acute inflammation, also are an option for treatment. Alternatively, intramuscular triamcinolone acetonide 1 mg/kg mixed with budesonide 0.1 mg/kg is an option and is associated with a reduced risk for adverse effects compared to oral steroids. The resulting hyperpigmentation develops 1 to 2 weeks postepithelialization.19 Hyperpigmentation often fades slowly over several months in lighter-skinned individuals but may last for years or indefinitely in darker-skinned patients.
Final Thoughts
Dermatologists should be knowledgeable about the various plant culprits that can induce PPD. Understanding the mechanism and pathophysiology can help guide both therapeutic interventions and preventive counseling. Understanding that even readily available vegetables such as celery can induce cutaneous eruptions should put PPD in the differential diagnosis more commonly when unspecified dermatitides are present.
- Walansky A. Study finally confirms eating celery burns more calories than it contains. Food & Wine. June 22, 2017. Accessed January 17, 2025. https://www.foodandwine.com/news/study-finally-confirms-eating-celery-burns-more-caloriesit-contains
- Puig L. Enhancement of PUVA phototoxic effects following celery ingestion: cool broth also can burn. Arch Dermatol. 1994;130:809-810. doi:10.1001/archderm.130.6.809
- Perez-Pimiento AJ, Moneo I, Santaolalla M, et al. Anaphylactic reaction to young garlic. Allergy. 1999;54:626-629.
- The Editors of Encyclopaedia Britannica. Apiaceae. Britannica. Updated November 25, 2024. Accessed January 17, 2025. https://www.britannica.com/plant/Apiaceae
- Smith R. Celery. In: Geoffriau E, Simon PW, eds. Carrots and Related Apiaceae Crops. 2nd ed. CABI; 2021:272-282.
- Dijkstra JWE, Chang L. Severe phototoxic burn following celery ingestion. Arch Dermatol. 1992;128:1277.
- Tobyn G, Denham A, Whitelegg M. Apium graveolens, wild celery. The Western Herbal Tradition: 2000 years of Medicinal Plant Knowledge. Elsevier. 2011:79-89. doi:10.1016/b978-0-443-10344-5.00014-8
- Rademaker M. Celery. DermNet. Accessed January 17, 2025. https://dermnetnz.org/topics/celery
- Sasseville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
- Jin Goon AT, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710. doi:10.4103/0019-5154.91833
- Birmingham DJ, Key MM, Tublich GE. Phototoxic bullae among celery harvesters. Arch Dermatol. 1961;83:73-87.
- Robb-Nicholson C. By the way, doctor: is a tanning bed safer than sunlight? Harvard Health Publishing. Harvard Medical School. September 1, 2009. Accessed January 17, 2025. https://www.health.harvard.edu/staying-healthy/is-a-tanning-bed-saferthan-sunlight
- Vester L, Thyssen JP, Menne T, et al. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis. 2012;67:328-333.
- Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015. Br J Dermatol. 2016;174:24-55.
- Laskin JD. Cellular and molecular mechanisms in photochemical sensitization: studies on the mechanism of action of psoralens. Food Chem Toxicol. 1994;32:119-127. doi:10.1016/0278-6915(94)90172-4
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UpToDate. Updated February 23, 2023. Accessed January 17, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Boffa, MJ, Gilmour E, Ead RD. Celery soup causing severe phototoxity during PUVA therapy. Br J Dermatol. 1996;135:334. doi:10.1111/j.1365-2133.1996.tb01182.x
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Mosby; 2018:286-303.
Celery (Apium graveolens)—that lowly vegetable that often languishes in the refrigerator crisper and apparently supplies fewer calories than are required to consume it—contains a myriad of photosensitizing chemicals known as furocoumarins and psoralens that can cause phytophotodermatitis (PPD) when handled prior to exposure to UV light.1 Individuals who are most likely to develop PPD caused by repeated contact with celery include food industry workers (eg, grocery store workers, farmers) who pick, handle, or prepare celery for consumption. While eating celery as part of a standard diet is highly unlikely to cause PPD, celery infected with Sclerotinia sclerotiorum (known as pink rot) causes more severe generalized sun sensitivity due to an increased amount of furocoumarins produced in response to the fungus.2 Contact with celery also can induce cutaneous manifestations unrelated to sun exposure in some individuals, including urticaria, allergic contact dermatitis, and anaphylaxis.3 In this article, we provide an overview of the life cycle and origin of celery as well as its irritant and allergic properties. We also describe cutaneous rashes associated with PPD caused by exposure to celery and highlight treatment options.
Morphology and Distribution
The Apiaceae family features aromatic flowering plants that comprise more than 3500 species, including many economically important vegetables, herbs, and spices.4 It also includes many alkaloid-containing species that are known to be poisonous to humans, such as poison hemlock (Conium maculatum) and water hemlock (Cicuta maculate). Most Apiaceae plants that are consumed by humans originate from the Mediterranean region.5 While known for their diversity of flavor and aroma, most of the plants from this family have low caloric value and provide minimal amounts of energy.
Members of the Apiaceae family have flowers that create a classic umbel shape mimicking the appearance of an upside-down umbrella (thus the former name for this family, Umbelliferae). The pedicles—the small stems attached to the base of each flower—spread from a common center to form the umbel.5 The Apiaceae family also includes the greatest number of plants that cause PPD due to their high concentration of furocoumarins, which deter fungus from harming the plants.6
A biennial plant, celery completes its life cycle in 2 years. During the first season, the stems, roots, and leaves sprout; in the second and final year, the flowers, fruits, and seeds proliferate, followed by decomposition. Apium graveolens approaches heights of 2 to 3 ft, growing upright and displaying grooved stems. Each stem terminates in a basal rosette of leaves. The second season brings white flower blooms in terminal or axillary umbels.7
Celery originated in the temperate Mediterranean regions of Europe, but farmers now cultivate it globally.8 It grows best in rich moist soil with full exposure to sunlight. Plants multiply their numbers through self-seeding. Celery commonly is found in suburban and rural homes, both in refrigerators for consumption as well as in medicine cabinets in capsule form for the treatment of arthritis.4
Irritant and Allergenic Properties
Despite the potential health benefits of celery, the Apiaceae family, which includes hogweed, dill, and fennel, prevails as the most common culprit for phytotoxic reactions. The Rutaceae family, including citrus plants and rue, remains runner-up for causes of PPD.9 Phytophotodermatitis is not an immunologic reaction, making anyone susceptible to formation of the cutaneous lesions when exposed to UV light after handling celery. Pruritis rarely occurs, unlike in allergic phytodermatitis.10 Upon photoexcitation from exposure to UVA light, individual psoralen molecules covalently bind to pyrimidine bases, causing interstrand cross-linking that prevents DNA replication and triggering a cascade leading to apoptosis of the cell. Apoptosis induces cell membrane edema, which manifests as cutaneous vesicles and bullae on the skin.10 Regardless of plant species, PPD reactions have similar appearance.
Celery roots contain the greatest concentration of psoralens, making it the most likely part of the plant to induce PPD.6 Phytophotodermatitis caused by celery can occur at any time of the year, but most eruptions occur during the summer months due to increased sunlight exposure and intensity. Among 320 randomly selected Michigan celery harvesters, 163 (51%) displayed evidence of vesicular and bullous dermatitis on the fingers, hands, and forearms.11 In this study, celery infected with pink rot fungus induced an erythematous eruption with vesicles and bullae within 48 hours of contact after just 30 seconds of summer sunlight exposure; however, eruptions are not limited to summer months, as the cutaneous presentation depends solely on exposure to UVA light, which can occur year-round.
Use of tanning beds is a major risk factor for PPD.12 Tanning beds utilize fluorescent bulbs that primarily emit UVA light, with UVB light emitted to a lesser degree. The UVA radiation produced by tanning beds is more than 3 times as intense as natural sunlight.12 Among grocery store employees, the combination of these 2 risk factors—regular contact with celery and tanning bed use—resulted in a prevalence ratio for PPD more than 40 times greater than that of individuals with neither risk factor.13
Cutaneous Manifestations of PPD
Phytophotodermatitis is a nonimmunologic dermatitis that forms via the interaction between UV light exposure and the photosensitizing chemicals inherent to some plant species. Development of PPD following contact with celery may be caused by the photoactive substances in celery, including the psoralens 8-methoxypsoralen and 5-methoxypsoralen.14 The psoralens must become activated by UV light with wavelengths between 320 nm and 400 nm (UVA) to initiate biologic effects.15
Once chemically activated, the photoactive mediators cause an erythematous and edematous sunburnlike reaction. Current hypotheses state that psoralen plus UVA generates reactive oxygen species, which damage the DNA within cells and alter receptors on cell membranes within the epidermis.14 The cutaneous eruption usually appears between 12 and 36 hours after sun exposure. Although they generally are not pruritic, the eruptions may induce pain. Within 7 to 10 days following development of the rash, hyperpigmentation occurs in the affected area and often persists for months to years.16 Ingestion of large amounts of celery has been cited to cause generalized phototoxic reactions; however, PPD rarely arises solely after ingestion, unless excessive amounts are consumed with concomitant exposure to psoralen plus UVA or tanning beds.17 In these cases, patients develop diffuse redness with superficial scaling, pain, and blistering if severe.
Treatment of PPD
Prevention remains the best form of treatment for PPD caused by exposure to celery. Postcontact management includes washing the affected area with soap and water and changing clothes promptly. Topical corticosteroids have mild utility in treatment of PPD.18 Oral steroid tapers, which reduce acute inflammation, also are an option for treatment. Alternatively, intramuscular triamcinolone acetonide 1 mg/kg mixed with budesonide 0.1 mg/kg is an option and is associated with a reduced risk for adverse effects compared to oral steroids. The resulting hyperpigmentation develops 1 to 2 weeks postepithelialization.19 Hyperpigmentation often fades slowly over several months in lighter-skinned individuals but may last for years or indefinitely in darker-skinned patients.
Final Thoughts
Dermatologists should be knowledgeable about the various plant culprits that can induce PPD. Understanding the mechanism and pathophysiology can help guide both therapeutic interventions and preventive counseling. Understanding that even readily available vegetables such as celery can induce cutaneous eruptions should put PPD in the differential diagnosis more commonly when unspecified dermatitides are present.
Celery (Apium graveolens)—that lowly vegetable that often languishes in the refrigerator crisper and apparently supplies fewer calories than are required to consume it—contains a myriad of photosensitizing chemicals known as furocoumarins and psoralens that can cause phytophotodermatitis (PPD) when handled prior to exposure to UV light.1 Individuals who are most likely to develop PPD caused by repeated contact with celery include food industry workers (eg, grocery store workers, farmers) who pick, handle, or prepare celery for consumption. While eating celery as part of a standard diet is highly unlikely to cause PPD, celery infected with Sclerotinia sclerotiorum (known as pink rot) causes more severe generalized sun sensitivity due to an increased amount of furocoumarins produced in response to the fungus.2 Contact with celery also can induce cutaneous manifestations unrelated to sun exposure in some individuals, including urticaria, allergic contact dermatitis, and anaphylaxis.3 In this article, we provide an overview of the life cycle and origin of celery as well as its irritant and allergic properties. We also describe cutaneous rashes associated with PPD caused by exposure to celery and highlight treatment options.
Morphology and Distribution
The Apiaceae family features aromatic flowering plants that comprise more than 3500 species, including many economically important vegetables, herbs, and spices.4 It also includes many alkaloid-containing species that are known to be poisonous to humans, such as poison hemlock (Conium maculatum) and water hemlock (Cicuta maculate). Most Apiaceae plants that are consumed by humans originate from the Mediterranean region.5 While known for their diversity of flavor and aroma, most of the plants from this family have low caloric value and provide minimal amounts of energy.
Members of the Apiaceae family have flowers that create a classic umbel shape mimicking the appearance of an upside-down umbrella (thus the former name for this family, Umbelliferae). The pedicles—the small stems attached to the base of each flower—spread from a common center to form the umbel.5 The Apiaceae family also includes the greatest number of plants that cause PPD due to their high concentration of furocoumarins, which deter fungus from harming the plants.6
A biennial plant, celery completes its life cycle in 2 years. During the first season, the stems, roots, and leaves sprout; in the second and final year, the flowers, fruits, and seeds proliferate, followed by decomposition. Apium graveolens approaches heights of 2 to 3 ft, growing upright and displaying grooved stems. Each stem terminates in a basal rosette of leaves. The second season brings white flower blooms in terminal or axillary umbels.7
Celery originated in the temperate Mediterranean regions of Europe, but farmers now cultivate it globally.8 It grows best in rich moist soil with full exposure to sunlight. Plants multiply their numbers through self-seeding. Celery commonly is found in suburban and rural homes, both in refrigerators for consumption as well as in medicine cabinets in capsule form for the treatment of arthritis.4
Irritant and Allergenic Properties
Despite the potential health benefits of celery, the Apiaceae family, which includes hogweed, dill, and fennel, prevails as the most common culprit for phytotoxic reactions. The Rutaceae family, including citrus plants and rue, remains runner-up for causes of PPD.9 Phytophotodermatitis is not an immunologic reaction, making anyone susceptible to formation of the cutaneous lesions when exposed to UV light after handling celery. Pruritis rarely occurs, unlike in allergic phytodermatitis.10 Upon photoexcitation from exposure to UVA light, individual psoralen molecules covalently bind to pyrimidine bases, causing interstrand cross-linking that prevents DNA replication and triggering a cascade leading to apoptosis of the cell. Apoptosis induces cell membrane edema, which manifests as cutaneous vesicles and bullae on the skin.10 Regardless of plant species, PPD reactions have similar appearance.
Celery roots contain the greatest concentration of psoralens, making it the most likely part of the plant to induce PPD.6 Phytophotodermatitis caused by celery can occur at any time of the year, but most eruptions occur during the summer months due to increased sunlight exposure and intensity. Among 320 randomly selected Michigan celery harvesters, 163 (51%) displayed evidence of vesicular and bullous dermatitis on the fingers, hands, and forearms.11 In this study, celery infected with pink rot fungus induced an erythematous eruption with vesicles and bullae within 48 hours of contact after just 30 seconds of summer sunlight exposure; however, eruptions are not limited to summer months, as the cutaneous presentation depends solely on exposure to UVA light, which can occur year-round.
Use of tanning beds is a major risk factor for PPD.12 Tanning beds utilize fluorescent bulbs that primarily emit UVA light, with UVB light emitted to a lesser degree. The UVA radiation produced by tanning beds is more than 3 times as intense as natural sunlight.12 Among grocery store employees, the combination of these 2 risk factors—regular contact with celery and tanning bed use—resulted in a prevalence ratio for PPD more than 40 times greater than that of individuals with neither risk factor.13
Cutaneous Manifestations of PPD
Phytophotodermatitis is a nonimmunologic dermatitis that forms via the interaction between UV light exposure and the photosensitizing chemicals inherent to some plant species. Development of PPD following contact with celery may be caused by the photoactive substances in celery, including the psoralens 8-methoxypsoralen and 5-methoxypsoralen.14 The psoralens must become activated by UV light with wavelengths between 320 nm and 400 nm (UVA) to initiate biologic effects.15
Once chemically activated, the photoactive mediators cause an erythematous and edematous sunburnlike reaction. Current hypotheses state that psoralen plus UVA generates reactive oxygen species, which damage the DNA within cells and alter receptors on cell membranes within the epidermis.14 The cutaneous eruption usually appears between 12 and 36 hours after sun exposure. Although they generally are not pruritic, the eruptions may induce pain. Within 7 to 10 days following development of the rash, hyperpigmentation occurs in the affected area and often persists for months to years.16 Ingestion of large amounts of celery has been cited to cause generalized phototoxic reactions; however, PPD rarely arises solely after ingestion, unless excessive amounts are consumed with concomitant exposure to psoralen plus UVA or tanning beds.17 In these cases, patients develop diffuse redness with superficial scaling, pain, and blistering if severe.
Treatment of PPD
Prevention remains the best form of treatment for PPD caused by exposure to celery. Postcontact management includes washing the affected area with soap and water and changing clothes promptly. Topical corticosteroids have mild utility in treatment of PPD.18 Oral steroid tapers, which reduce acute inflammation, also are an option for treatment. Alternatively, intramuscular triamcinolone acetonide 1 mg/kg mixed with budesonide 0.1 mg/kg is an option and is associated with a reduced risk for adverse effects compared to oral steroids. The resulting hyperpigmentation develops 1 to 2 weeks postepithelialization.19 Hyperpigmentation often fades slowly over several months in lighter-skinned individuals but may last for years or indefinitely in darker-skinned patients.
Final Thoughts
Dermatologists should be knowledgeable about the various plant culprits that can induce PPD. Understanding the mechanism and pathophysiology can help guide both therapeutic interventions and preventive counseling. Understanding that even readily available vegetables such as celery can induce cutaneous eruptions should put PPD in the differential diagnosis more commonly when unspecified dermatitides are present.
- Walansky A. Study finally confirms eating celery burns more calories than it contains. Food & Wine. June 22, 2017. Accessed January 17, 2025. https://www.foodandwine.com/news/study-finally-confirms-eating-celery-burns-more-caloriesit-contains
- Puig L. Enhancement of PUVA phototoxic effects following celery ingestion: cool broth also can burn. Arch Dermatol. 1994;130:809-810. doi:10.1001/archderm.130.6.809
- Perez-Pimiento AJ, Moneo I, Santaolalla M, et al. Anaphylactic reaction to young garlic. Allergy. 1999;54:626-629.
- The Editors of Encyclopaedia Britannica. Apiaceae. Britannica. Updated November 25, 2024. Accessed January 17, 2025. https://www.britannica.com/plant/Apiaceae
- Smith R. Celery. In: Geoffriau E, Simon PW, eds. Carrots and Related Apiaceae Crops. 2nd ed. CABI; 2021:272-282.
- Dijkstra JWE, Chang L. Severe phototoxic burn following celery ingestion. Arch Dermatol. 1992;128:1277.
- Tobyn G, Denham A, Whitelegg M. Apium graveolens, wild celery. The Western Herbal Tradition: 2000 years of Medicinal Plant Knowledge. Elsevier. 2011:79-89. doi:10.1016/b978-0-443-10344-5.00014-8
- Rademaker M. Celery. DermNet. Accessed January 17, 2025. https://dermnetnz.org/topics/celery
- Sasseville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
- Jin Goon AT, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710. doi:10.4103/0019-5154.91833
- Birmingham DJ, Key MM, Tublich GE. Phototoxic bullae among celery harvesters. Arch Dermatol. 1961;83:73-87.
- Robb-Nicholson C. By the way, doctor: is a tanning bed safer than sunlight? Harvard Health Publishing. Harvard Medical School. September 1, 2009. Accessed January 17, 2025. https://www.health.harvard.edu/staying-healthy/is-a-tanning-bed-saferthan-sunlight
- Vester L, Thyssen JP, Menne T, et al. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis. 2012;67:328-333.
- Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015. Br J Dermatol. 2016;174:24-55.
- Laskin JD. Cellular and molecular mechanisms in photochemical sensitization: studies on the mechanism of action of psoralens. Food Chem Toxicol. 1994;32:119-127. doi:10.1016/0278-6915(94)90172-4
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UpToDate. Updated February 23, 2023. Accessed January 17, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Boffa, MJ, Gilmour E, Ead RD. Celery soup causing severe phototoxity during PUVA therapy. Br J Dermatol. 1996;135:334. doi:10.1111/j.1365-2133.1996.tb01182.x
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Mosby; 2018:286-303.
- Walansky A. Study finally confirms eating celery burns more calories than it contains. Food & Wine. June 22, 2017. Accessed January 17, 2025. https://www.foodandwine.com/news/study-finally-confirms-eating-celery-burns-more-caloriesit-contains
- Puig L. Enhancement of PUVA phototoxic effects following celery ingestion: cool broth also can burn. Arch Dermatol. 1994;130:809-810. doi:10.1001/archderm.130.6.809
- Perez-Pimiento AJ, Moneo I, Santaolalla M, et al. Anaphylactic reaction to young garlic. Allergy. 1999;54:626-629.
- The Editors of Encyclopaedia Britannica. Apiaceae. Britannica. Updated November 25, 2024. Accessed January 17, 2025. https://www.britannica.com/plant/Apiaceae
- Smith R. Celery. In: Geoffriau E, Simon PW, eds. Carrots and Related Apiaceae Crops. 2nd ed. CABI; 2021:272-282.
- Dijkstra JWE, Chang L. Severe phototoxic burn following celery ingestion. Arch Dermatol. 1992;128:1277.
- Tobyn G, Denham A, Whitelegg M. Apium graveolens, wild celery. The Western Herbal Tradition: 2000 years of Medicinal Plant Knowledge. Elsevier. 2011:79-89. doi:10.1016/b978-0-443-10344-5.00014-8
- Rademaker M. Celery. DermNet. Accessed January 17, 2025. https://dermnetnz.org/topics/celery
- Sasseville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
- Jin Goon AT, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710. doi:10.4103/0019-5154.91833
- Birmingham DJ, Key MM, Tublich GE. Phototoxic bullae among celery harvesters. Arch Dermatol. 1961;83:73-87.
- Robb-Nicholson C. By the way, doctor: is a tanning bed safer than sunlight? Harvard Health Publishing. Harvard Medical School. September 1, 2009. Accessed January 17, 2025. https://www.health.harvard.edu/staying-healthy/is-a-tanning-bed-saferthan-sunlight
- Vester L, Thyssen JP, Menne T, et al. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis. 2012;67:328-333.
- Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015. Br J Dermatol. 2016;174:24-55.
- Laskin JD. Cellular and molecular mechanisms in photochemical sensitization: studies on the mechanism of action of psoralens. Food Chem Toxicol. 1994;32:119-127. doi:10.1016/0278-6915(94)90172-4
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UpToDate. Updated February 23, 2023. Accessed January 17, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Boffa, MJ, Gilmour E, Ead RD. Celery soup causing severe phototoxity during PUVA therapy. Br J Dermatol. 1996;135:334. doi:10.1111/j.1365-2133.1996.tb01182.x
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Mosby; 2018:286-303.
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
PRACTICE POINTS
- Clinicians should consider phytophotodermatitis (PPD) in the differential diagnosis for erythematous eruptions with bullae and vesicles manifesting in sun-exposed distributions.
- A clinical history that includes the patient’s occupation, diet, and history of treatment with psoralen plus UVA and use of tanning beds may help diagnose PPD.
- It is important to educate patients who regularly handle celery and other plants containing furocoumarins and psoralens on how to prevent PPD and utilize effective photoprotection.
Vascular Nodule on the Upper Chest
Vascular Nodule on the Upper Chest
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
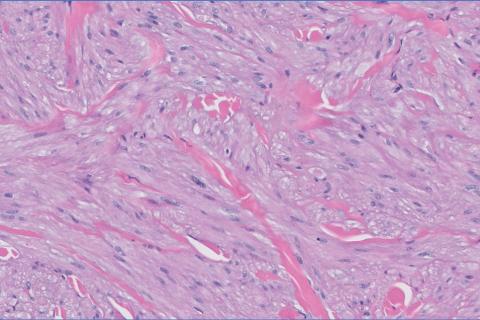
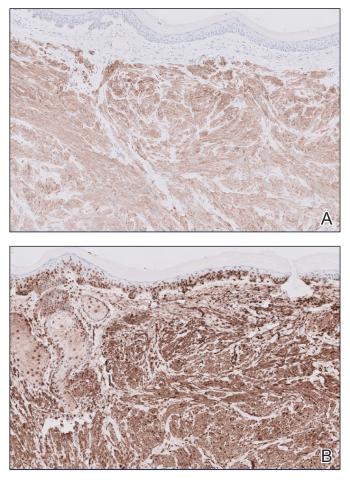
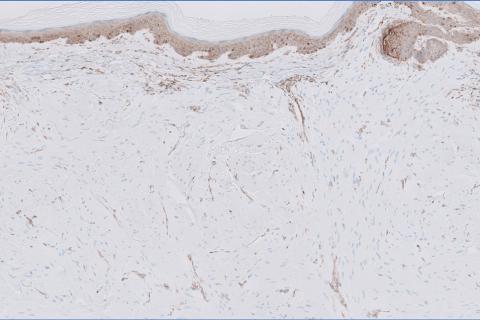
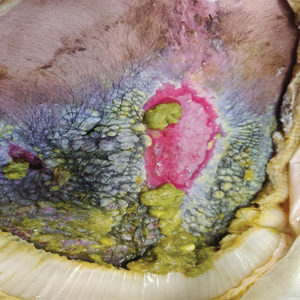
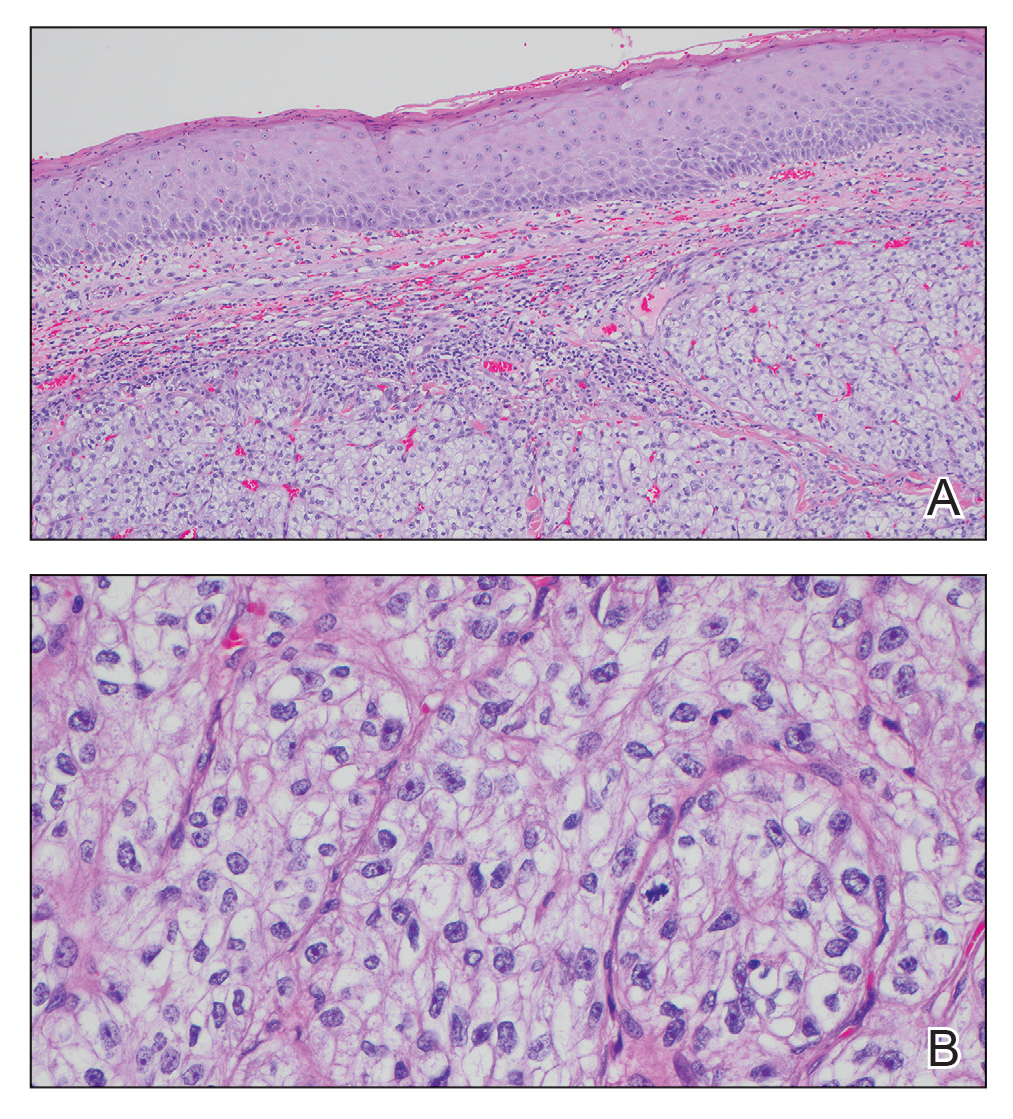
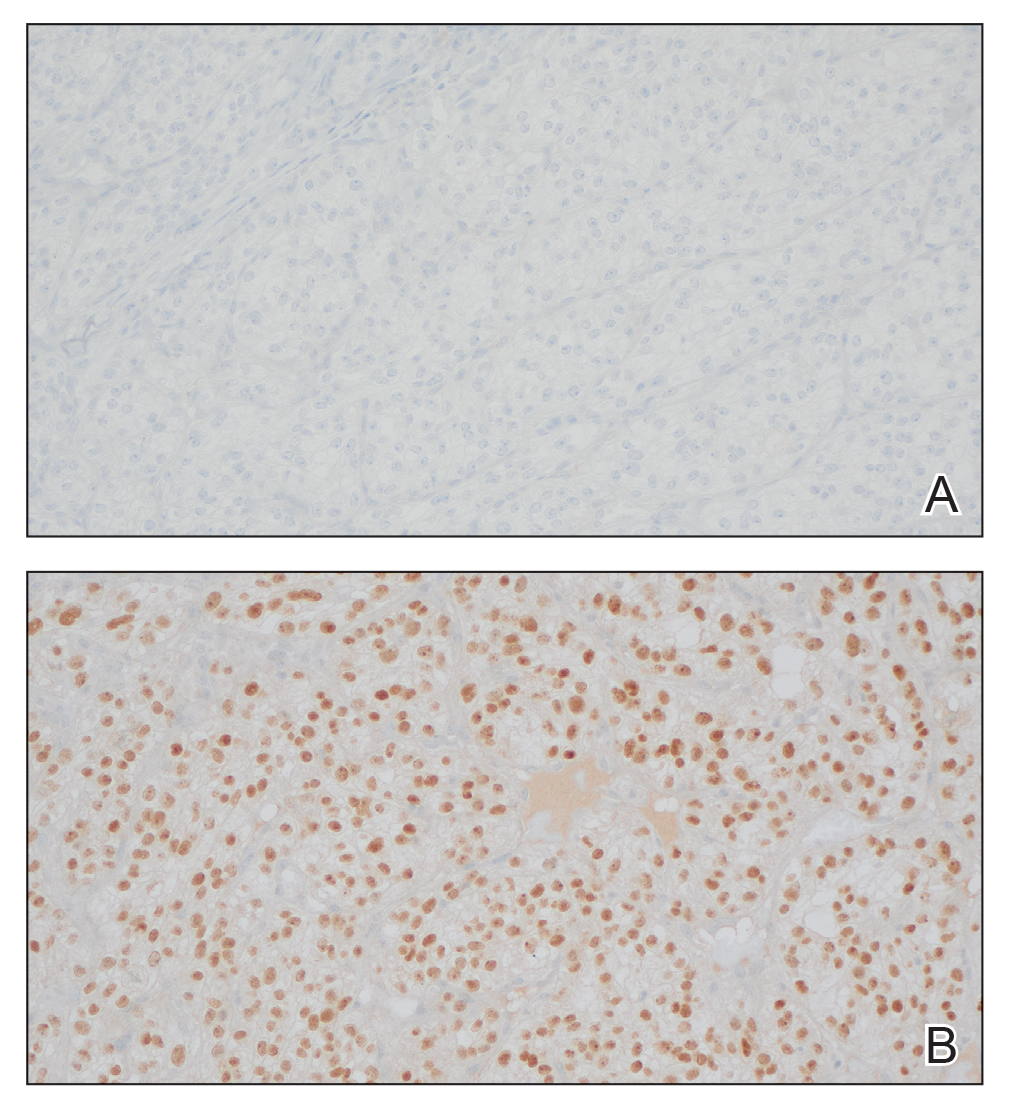
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.
Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.


Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.


Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
Vascular Nodule on the Upper Chest
Vascular Nodule on the Upper Chest
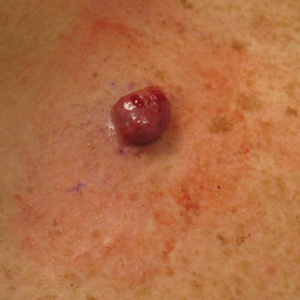
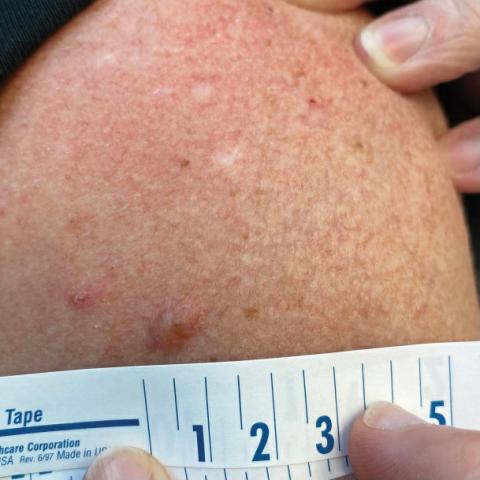
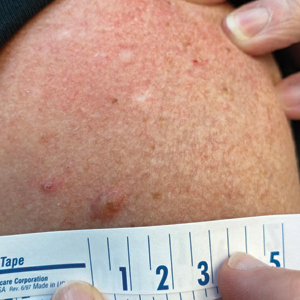
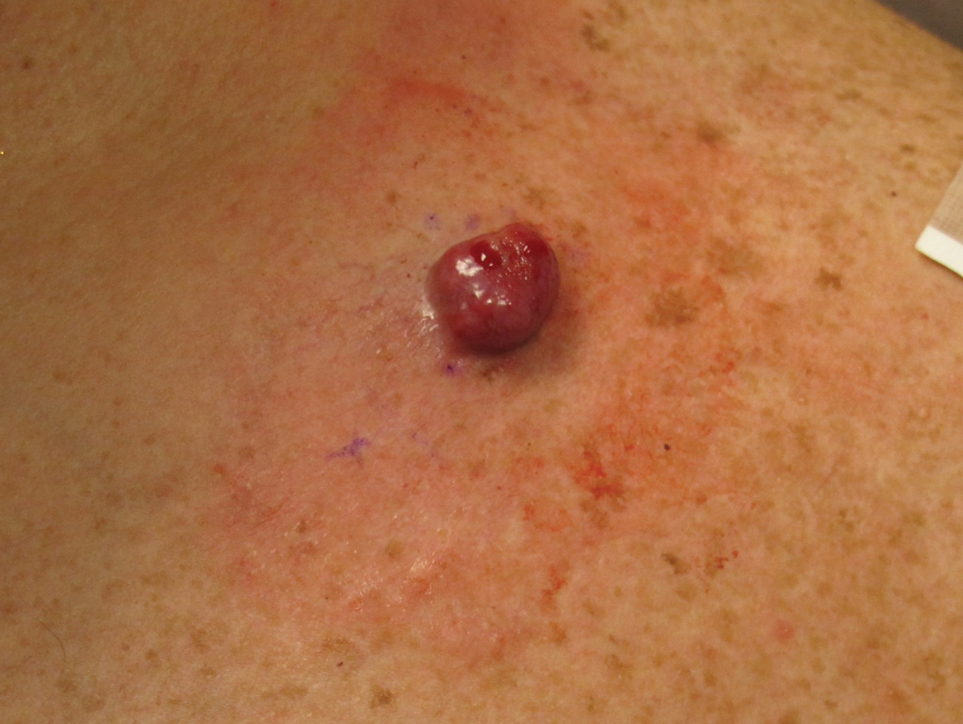
A 45-year-old man presented to the dermatology clinic with a bleeding nodule on the upper chest of 2 months’ duration. He had a history of a low-grade mucoepidermoid carcinoma of the left parotid gland that was diagnosed 14 years prior and was treated via parotidectomy with 1 positive lymph node removed. Two months prior to the current presentation, the patient presented to the emergency department with unintentional weight loss and fatigue and subsequently was diagnosed with clear cell renal cell carcinoma that was treated via radical nephrectomy.
At the current presentation, the patient denied any recent fatigue, fever, weight loss, shortness of breath, or abdominal pain but reported neck stiffness. Physical examination revealed a solitary, smooth, vascular, 1.5×1.5 cm nodule on the left upper chest with no overlying skin changes. The remainder of the skin examination was unremarkable. A shave biopsy of the nodule was performed.
Helping to Protect Our Children From Invasive Pneumococcal Disease
Invasive pneumococcal disease (IPD) remains a serious health threat for infants and can result in hospitalizations, serious complications, or even death.1-3 IPD rates peak at a critical stage in a child’s immune development, when maternal antibody protection wanes and the child has not yet received or is in the process of receiving their primary vaccination series.4 Pneumococcal vaccination is especially important during this vulnerable period to help protect against potentially severe consequences from IPD.2,4,5
Over the last 25 years, the widespread adoption of pneumococcal conjugate vaccines (PCVs) in children has led to a reduction in the spread of many different types of pneumococcal bacteria – referred to as serotypes.2 Although these vaccines have helped reduce the burden of disease, pneumococcal disease remains an issue, with specific serotypes presenting a greater threat to children’s health.6-10
Understanding the burden of IPD in children
According to the Centers for Disease Control and Prevention (CDC), the incidence of IPD is highest in the first year of life,3,* and the death rate due to IPD is higher in infants than in any other pediatric age group.11,† Infants' immune systems are still developing in the first year of life; therefore, protection during this time is critical.3,4,11
The CDC recommends routine pediatric pneumococcal vaccination as a four-dose series at months two, four, and six with a booster administered between 12-15 months.12 Despite the risks associated with invasive pneumococcal disease, some children do not receive all four doses.1-3,13 Many factors can contribute to incomplete childhood immunization coverage, including ethnicity, geographic location, and socioeconomic status.14 In fact, up to one in five babies within the Vaccines for Children Program have received only three of the four recommended PCV doses by two years of age, according to a CDC Morbidity and Mortality Weekly Report from 2021-2023.12,13 The immune response generated after the third dose of a pneumococcal conjugate vaccine is important when evaluating protection against IPD, especially for the children who don't receive their fourth dose.12,15,16
Additionally, certain serotypes, like Serotype 3, are responsible for more IPD cases and are associated with higher morbidity and mortality rates in children.7-10,a Despite being included in PCVs for over a decade, Serotype 3 continues to be a leading cause of IPD in children under five, as shown in a pooled analysis of national-level CDC data from 2018-2022.7,17 This particular serotype has resisted antibody-mediated clearance and continues to be associated with adverse effects.18
What should pediatricians consider when it comes to protecting children from IPD?
When it comes to protecting against IPD, it's important to consider factors in addition to the number of serotypes covered by a vaccine, such as early and robust protection against key serotypes that cause pediatric IPD in the first year of life.2,7,10,19
VAXNEUVANCE® (Pneumococcal 15-valent Conjugate Vaccine) is a pediatric pneumococcal conjugate vaccine that can help deliver strong protection against key disease-causing serotypes during infancy, when the threat of IPD is the highest.2,3,7,10,19-21
Indications and Usage
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
(Select Safety Information for VAXNEUVANCE continues below.)
VAXNEUVANCE delivers robust immune responses at seven months, following the third dose, for three key disease-causing serotypes: 3, 22F and 33F.7,10,19,b,c Clinical data showed that immune responses for VAXNEUVANCE were superior to PCV13 (pneumococcal 13-valent conjugate vaccine) for those three critical serotypes2,7,10,19,d and were comparable for the 12 shared serotypes between the vaccines.19
Further, VAXNEUVANCE showcased superior immune responses against Serotype 3 after the third dose with an immunoglobulin G (IgG) geometric mean concentrations (GMCs) response rate of 93.1% compared to PCV13, which demonstrated a 74% response rate.19,b
Although completing the full recommended immunization series remains the best way to help maximize protection,12,22 many children still receive fewer than the recommended four doses of a PCV.12,13 It is important to consider a vaccine that targets problematic serotypes and provides robust immune responses after three doses – of the four dose series – to help protect this vulnerable population from IPD.3,4,7,10,11,19
VAXNEUVANCE can help prevent pediatric IPD in the first year of life and beyond and is an important option for pediatricians to consider for their appropriate patients.7,19
###
Select Safety Information (continued)
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
* Based on pooled analysis of national-level CDC ABC surveillance data from 2018–2022, representing ~35 million people surveyed annually in 10 states across the US. IPD incidence rates were 10.3 in <1 year, 8.2 in 1 year, 4.0 in 2–4 years, 5.0 in 1–4 years, and 1.3 in 5–17 years (Regional variations may exist).3
† Based on national-level CDC ABC surveillance data from 2022, representing ~35 million people in 10 states across the US (Regional variations may exist).11
Key Study Details
GMC Ratios Postdose 3c
Primary endpoint: VAXNEUVANCE delivered comparable immune responses for 12 of the 13 shared serotypes found in PCV13. Shared Serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 vs >0.5.19,23
Study Design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.19
aBased on a pooled analysis of national-level CDC data from 2018–2021, the top 6 IPD-causing serotypes in children under 5 years of age were 15C, 33F, 19F, 3, 23B, and 22F. Serotypes 15C and 23B are not included in any recommended pediatric PCV in the US.7,17,19,22,24
bPostdose 3 superiority was demonstrated based on measurements taken 30 days after the 6-month dose (at 7 months).19
cMeasurements were taken 30 days postdose specified.19
dSecondary endpoint: Postdose 3 IgG response rate percentage point difference vs PCV13 (95% CI): for Serotype 3, 19.1 (14.4, 24.0); for Serotype 22F, 8.1 (5.1, 11.5); for Serotype 33F, -5.1 (-9.5, -0.7).19,23
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.19
References:
1Dalton M. Pneumoccal disease. National Foundation for Infectious Diseases. Published July 2024. https://www.nfid.org/infectious-disease/pneumococcal/
2Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Epidemiology and Prevention of Vaccine-Preventable Diseases. Published May 1, 2024. Accessed December 10, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
3Data available on request from the Merck National Service Center via email at [email protected]. Please specify information package US-PVC-02072.
4Mohanty S, Done N, Liu Q, et al. Incidence of pneumococcal disease in children ≤48 months old in the United States: 1998–2019. Vaccine. Published online March 1, 2024. doi: 10.1016/j.vaccine.2024.03.013
5Clinical overview of pneumococcal disease. Centers for Disease Control and Prevention. February 6, 2024. Accessed May 22, 2024. https://www.cdc.gov/pneumococcal/hcp/clinical-overview/
6Wasserman MD, Perdrizet J, Grant L, et al. Clinical and economic burden of pneumococcal disease due to serotypes contained in current and investigational pneumococcal conjugate vaccines in children under five years of age. Infect Dis Ther. 2021;10(4):2701-2720. doi:10.1007/s40121-021-00544-1
7Centers for Disease Control and Prevention (CDC). Visualization – Based on 1998-2022 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance (ABCs). Updated July 22, 2024. Accessed August 30, 2024. https://data.cdc.gov/Public-Health-Surveillance/1998-2022-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data
8Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512.e1-512.e10. doi:10.1016/j.cmi.2019.09.008
9Azarian T, Mitchell PK, Georgieva M, et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11):e1007438. doi:10.1371/journal.ppat.1007438
10Hu T, Weiss T, Owusu-Edusei K, Petigara T. Health and economic burden associated with 15-valent pneumococcal conjugate vaccine serotypes in children in the United States. J Med Econ. 2020;23(12):1653-1660. doi:10.1080/13696998.2020.184021613
11Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2022. Centers for Disease Control and Prevention. Updated July 5, 2024. Accessed October 15, 2024. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf
12Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Centers for Disease Control and Prevention. Addendum updated November 21, 2024. Accessed November 25, 2024. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
13Hill HA, et al. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021 — National Immunization Survey-Child, United States, 2021–2023. MMWR Morb Mortal Wkly Rep, pages 844–853.
14Feemster K, Weaver J, Buchwald U, Banniettis N, Cox KS, McIntosh ED, Spoulou V. Pneumococcal Vaccine Breakthrough and Failure in Infants and Children: A Narrative Review. Vaccines (Basel). 2023 Nov 24;11(12):1750. doi:10.3390/vaccines11121750. PMID: 38140155; PMCID: PMC10747311.
15Recommendations to assure the quality, safety and efficacy of pneumoccoccal conjugate vaccines. Annex 3. TRS no 977. World Health Organization. October 19, 2013. Accessed October 31, 2024. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977
16Guidelines on clinical evaluation of vaccines: regulatory expectations. Annex 9. TRA No 924.World Health Organization. Last reviewed October 21, 2020. Accessed October 31, 2024. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9
17Prevnar 13. Prescribing Information. Pfizer; 2019.
18Luck JN, Tettelin H, Orihuela CJ. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front Cell Infect Microbiol. 2020;10:613287. Published 2020 Dec 23. doi:10.3389/fcimb.2020.613287
19VAXNEUVANCE. Prescribing Information. Merck & Co., Inc., 2024.
20Moraes-Pinto MI, Suano-Souza F, Aranda CS. Immune system: development and acquisition of immunological competence. J Pediatr (Rio J). 2021;97(S1):S59-S66. doi:10.1016/j.jped.2020.10.006
21Wodi AP, Morelli V. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 1: Principles of vaccination. Centers for Disease Control and Prevention. Last reviewed March 2024. Accessed May 9, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-1-principles-of-vaccination.html
22Pneumococcal vaccination. Centers for Disease Control and Prevention. Last reviewed September 12, 2024. Accessed September 30, 2024. https://www.cdc.gov/pneumococcal/vaccines/index.html
23Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142-1152. doi:10.1016/j.vaccine.2022.12.054
24Prevnar 20. Prescribing Information. Pfizer; 2023.
Copyright © 2025 Frontline Medical Communications Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form, by any means, without prior written permission of the Publisher. Frontline Medical Communications Inc. will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein. The opinions expressed in this publication do not necessarily reflect the views of the Publisher. All other trademarks are property of their respective owners.
Neither the editors of Pediatric News nor the Editorial Advisory Board nor the reporting staff contributed to this content.
US-PVC-01998 03/25
Invasive pneumococcal disease (IPD) remains a serious health threat for infants and can result in hospitalizations, serious complications, or even death.1-3 IPD rates peak at a critical stage in a child’s immune development, when maternal antibody protection wanes and the child has not yet received or is in the process of receiving their primary vaccination series.4 Pneumococcal vaccination is especially important during this vulnerable period to help protect against potentially severe consequences from IPD.2,4,5
Over the last 25 years, the widespread adoption of pneumococcal conjugate vaccines (PCVs) in children has led to a reduction in the spread of many different types of pneumococcal bacteria – referred to as serotypes.2 Although these vaccines have helped reduce the burden of disease, pneumococcal disease remains an issue, with specific serotypes presenting a greater threat to children’s health.6-10
Understanding the burden of IPD in children
According to the Centers for Disease Control and Prevention (CDC), the incidence of IPD is highest in the first year of life,3,* and the death rate due to IPD is higher in infants than in any other pediatric age group.11,† Infants' immune systems are still developing in the first year of life; therefore, protection during this time is critical.3,4,11
The CDC recommends routine pediatric pneumococcal vaccination as a four-dose series at months two, four, and six with a booster administered between 12-15 months.12 Despite the risks associated with invasive pneumococcal disease, some children do not receive all four doses.1-3,13 Many factors can contribute to incomplete childhood immunization coverage, including ethnicity, geographic location, and socioeconomic status.14 In fact, up to one in five babies within the Vaccines for Children Program have received only three of the four recommended PCV doses by two years of age, according to a CDC Morbidity and Mortality Weekly Report from 2021-2023.12,13 The immune response generated after the third dose of a pneumococcal conjugate vaccine is important when evaluating protection against IPD, especially for the children who don't receive their fourth dose.12,15,16
Additionally, certain serotypes, like Serotype 3, are responsible for more IPD cases and are associated with higher morbidity and mortality rates in children.7-10,a Despite being included in PCVs for over a decade, Serotype 3 continues to be a leading cause of IPD in children under five, as shown in a pooled analysis of national-level CDC data from 2018-2022.7,17 This particular serotype has resisted antibody-mediated clearance and continues to be associated with adverse effects.18
What should pediatricians consider when it comes to protecting children from IPD?
When it comes to protecting against IPD, it's important to consider factors in addition to the number of serotypes covered by a vaccine, such as early and robust protection against key serotypes that cause pediatric IPD in the first year of life.2,7,10,19
VAXNEUVANCE® (Pneumococcal 15-valent Conjugate Vaccine) is a pediatric pneumococcal conjugate vaccine that can help deliver strong protection against key disease-causing serotypes during infancy, when the threat of IPD is the highest.2,3,7,10,19-21
Indications and Usage
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
(Select Safety Information for VAXNEUVANCE continues below.)
VAXNEUVANCE delivers robust immune responses at seven months, following the third dose, for three key disease-causing serotypes: 3, 22F and 33F.7,10,19,b,c Clinical data showed that immune responses for VAXNEUVANCE were superior to PCV13 (pneumococcal 13-valent conjugate vaccine) for those three critical serotypes2,7,10,19,d and were comparable for the 12 shared serotypes between the vaccines.19
Further, VAXNEUVANCE showcased superior immune responses against Serotype 3 after the third dose with an immunoglobulin G (IgG) geometric mean concentrations (GMCs) response rate of 93.1% compared to PCV13, which demonstrated a 74% response rate.19,b
Although completing the full recommended immunization series remains the best way to help maximize protection,12,22 many children still receive fewer than the recommended four doses of a PCV.12,13 It is important to consider a vaccine that targets problematic serotypes and provides robust immune responses after three doses – of the four dose series – to help protect this vulnerable population from IPD.3,4,7,10,11,19
VAXNEUVANCE can help prevent pediatric IPD in the first year of life and beyond and is an important option for pediatricians to consider for their appropriate patients.7,19
###
Select Safety Information (continued)
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
* Based on pooled analysis of national-level CDC ABC surveillance data from 2018–2022, representing ~35 million people surveyed annually in 10 states across the US. IPD incidence rates were 10.3 in <1 year, 8.2 in 1 year, 4.0 in 2–4 years, 5.0 in 1–4 years, and 1.3 in 5–17 years (Regional variations may exist).3
† Based on national-level CDC ABC surveillance data from 2022, representing ~35 million people in 10 states across the US (Regional variations may exist).11
Key Study Details
GMC Ratios Postdose 3c
Primary endpoint: VAXNEUVANCE delivered comparable immune responses for 12 of the 13 shared serotypes found in PCV13. Shared Serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 vs >0.5.19,23
Study Design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.19
aBased on a pooled analysis of national-level CDC data from 2018–2021, the top 6 IPD-causing serotypes in children under 5 years of age were 15C, 33F, 19F, 3, 23B, and 22F. Serotypes 15C and 23B are not included in any recommended pediatric PCV in the US.7,17,19,22,24
bPostdose 3 superiority was demonstrated based on measurements taken 30 days after the 6-month dose (at 7 months).19
cMeasurements were taken 30 days postdose specified.19
dSecondary endpoint: Postdose 3 IgG response rate percentage point difference vs PCV13 (95% CI): for Serotype 3, 19.1 (14.4, 24.0); for Serotype 22F, 8.1 (5.1, 11.5); for Serotype 33F, -5.1 (-9.5, -0.7).19,23
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.19
References:
1Dalton M. Pneumoccal disease. National Foundation for Infectious Diseases. Published July 2024. https://www.nfid.org/infectious-disease/pneumococcal/
2Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Epidemiology and Prevention of Vaccine-Preventable Diseases. Published May 1, 2024. Accessed December 10, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
3Data available on request from the Merck National Service Center via email at [email protected]. Please specify information package US-PVC-02072.
4Mohanty S, Done N, Liu Q, et al. Incidence of pneumococcal disease in children ≤48 months old in the United States: 1998–2019. Vaccine. Published online March 1, 2024. doi: 10.1016/j.vaccine.2024.03.013
5Clinical overview of pneumococcal disease. Centers for Disease Control and Prevention. February 6, 2024. Accessed May 22, 2024. https://www.cdc.gov/pneumococcal/hcp/clinical-overview/
6Wasserman MD, Perdrizet J, Grant L, et al. Clinical and economic burden of pneumococcal disease due to serotypes contained in current and investigational pneumococcal conjugate vaccines in children under five years of age. Infect Dis Ther. 2021;10(4):2701-2720. doi:10.1007/s40121-021-00544-1
7Centers for Disease Control and Prevention (CDC). Visualization – Based on 1998-2022 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance (ABCs). Updated July 22, 2024. Accessed August 30, 2024. https://data.cdc.gov/Public-Health-Surveillance/1998-2022-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data
8Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512.e1-512.e10. doi:10.1016/j.cmi.2019.09.008
9Azarian T, Mitchell PK, Georgieva M, et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11):e1007438. doi:10.1371/journal.ppat.1007438
10Hu T, Weiss T, Owusu-Edusei K, Petigara T. Health and economic burden associated with 15-valent pneumococcal conjugate vaccine serotypes in children in the United States. J Med Econ. 2020;23(12):1653-1660. doi:10.1080/13696998.2020.184021613
11Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2022. Centers for Disease Control and Prevention. Updated July 5, 2024. Accessed October 15, 2024. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf
12Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Centers for Disease Control and Prevention. Addendum updated November 21, 2024. Accessed November 25, 2024. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
13Hill HA, et al. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021 — National Immunization Survey-Child, United States, 2021–2023. MMWR Morb Mortal Wkly Rep, pages 844–853.
14Feemster K, Weaver J, Buchwald U, Banniettis N, Cox KS, McIntosh ED, Spoulou V. Pneumococcal Vaccine Breakthrough and Failure in Infants and Children: A Narrative Review. Vaccines (Basel). 2023 Nov 24;11(12):1750. doi:10.3390/vaccines11121750. PMID: 38140155; PMCID: PMC10747311.
15Recommendations to assure the quality, safety and efficacy of pneumoccoccal conjugate vaccines. Annex 3. TRS no 977. World Health Organization. October 19, 2013. Accessed October 31, 2024. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977
16Guidelines on clinical evaluation of vaccines: regulatory expectations. Annex 9. TRA No 924.World Health Organization. Last reviewed October 21, 2020. Accessed October 31, 2024. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9
17Prevnar 13. Prescribing Information. Pfizer; 2019.
18Luck JN, Tettelin H, Orihuela CJ. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front Cell Infect Microbiol. 2020;10:613287. Published 2020 Dec 23. doi:10.3389/fcimb.2020.613287
19VAXNEUVANCE. Prescribing Information. Merck & Co., Inc., 2024.
20Moraes-Pinto MI, Suano-Souza F, Aranda CS. Immune system: development and acquisition of immunological competence. J Pediatr (Rio J). 2021;97(S1):S59-S66. doi:10.1016/j.jped.2020.10.006
21Wodi AP, Morelli V. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 1: Principles of vaccination. Centers for Disease Control and Prevention. Last reviewed March 2024. Accessed May 9, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-1-principles-of-vaccination.html
22Pneumococcal vaccination. Centers for Disease Control and Prevention. Last reviewed September 12, 2024. Accessed September 30, 2024. https://www.cdc.gov/pneumococcal/vaccines/index.html
23Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142-1152. doi:10.1016/j.vaccine.2022.12.054
24Prevnar 20. Prescribing Information. Pfizer; 2023.
Copyright © 2025 Frontline Medical Communications Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form, by any means, without prior written permission of the Publisher. Frontline Medical Communications Inc. will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein. The opinions expressed in this publication do not necessarily reflect the views of the Publisher. All other trademarks are property of their respective owners.
Neither the editors of Pediatric News nor the Editorial Advisory Board nor the reporting staff contributed to this content.
US-PVC-01998 03/25
Invasive pneumococcal disease (IPD) remains a serious health threat for infants and can result in hospitalizations, serious complications, or even death.1-3 IPD rates peak at a critical stage in a child’s immune development, when maternal antibody protection wanes and the child has not yet received or is in the process of receiving their primary vaccination series.4 Pneumococcal vaccination is especially important during this vulnerable period to help protect against potentially severe consequences from IPD.2,4,5
Over the last 25 years, the widespread adoption of pneumococcal conjugate vaccines (PCVs) in children has led to a reduction in the spread of many different types of pneumococcal bacteria – referred to as serotypes.2 Although these vaccines have helped reduce the burden of disease, pneumococcal disease remains an issue, with specific serotypes presenting a greater threat to children’s health.6-10
Understanding the burden of IPD in children
According to the Centers for Disease Control and Prevention (CDC), the incidence of IPD is highest in the first year of life,3,* and the death rate due to IPD is higher in infants than in any other pediatric age group.11,† Infants' immune systems are still developing in the first year of life; therefore, protection during this time is critical.3,4,11
The CDC recommends routine pediatric pneumococcal vaccination as a four-dose series at months two, four, and six with a booster administered between 12-15 months.12 Despite the risks associated with invasive pneumococcal disease, some children do not receive all four doses.1-3,13 Many factors can contribute to incomplete childhood immunization coverage, including ethnicity, geographic location, and socioeconomic status.14 In fact, up to one in five babies within the Vaccines for Children Program have received only three of the four recommended PCV doses by two years of age, according to a CDC Morbidity and Mortality Weekly Report from 2021-2023.12,13 The immune response generated after the third dose of a pneumococcal conjugate vaccine is important when evaluating protection against IPD, especially for the children who don't receive their fourth dose.12,15,16
Additionally, certain serotypes, like Serotype 3, are responsible for more IPD cases and are associated with higher morbidity and mortality rates in children.7-10,a Despite being included in PCVs for over a decade, Serotype 3 continues to be a leading cause of IPD in children under five, as shown in a pooled analysis of national-level CDC data from 2018-2022.7,17 This particular serotype has resisted antibody-mediated clearance and continues to be associated with adverse effects.18
What should pediatricians consider when it comes to protecting children from IPD?
When it comes to protecting against IPD, it's important to consider factors in addition to the number of serotypes covered by a vaccine, such as early and robust protection against key serotypes that cause pediatric IPD in the first year of life.2,7,10,19
VAXNEUVANCE® (Pneumococcal 15-valent Conjugate Vaccine) is a pediatric pneumococcal conjugate vaccine that can help deliver strong protection against key disease-causing serotypes during infancy, when the threat of IPD is the highest.2,3,7,10,19-21
Indications and Usage
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
(Select Safety Information for VAXNEUVANCE continues below.)
VAXNEUVANCE delivers robust immune responses at seven months, following the third dose, for three key disease-causing serotypes: 3, 22F and 33F.7,10,19,b,c Clinical data showed that immune responses for VAXNEUVANCE were superior to PCV13 (pneumococcal 13-valent conjugate vaccine) for those three critical serotypes2,7,10,19,d and were comparable for the 12 shared serotypes between the vaccines.19
Further, VAXNEUVANCE showcased superior immune responses against Serotype 3 after the third dose with an immunoglobulin G (IgG) geometric mean concentrations (GMCs) response rate of 93.1% compared to PCV13, which demonstrated a 74% response rate.19,b
Although completing the full recommended immunization series remains the best way to help maximize protection,12,22 many children still receive fewer than the recommended four doses of a PCV.12,13 It is important to consider a vaccine that targets problematic serotypes and provides robust immune responses after three doses – of the four dose series – to help protect this vulnerable population from IPD.3,4,7,10,11,19
VAXNEUVANCE can help prevent pediatric IPD in the first year of life and beyond and is an important option for pediatricians to consider for their appropriate patients.7,19
###
Select Safety Information (continued)
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
* Based on pooled analysis of national-level CDC ABC surveillance data from 2018–2022, representing ~35 million people surveyed annually in 10 states across the US. IPD incidence rates were 10.3 in <1 year, 8.2 in 1 year, 4.0 in 2–4 years, 5.0 in 1–4 years, and 1.3 in 5–17 years (Regional variations may exist).3
† Based on national-level CDC ABC surveillance data from 2022, representing ~35 million people in 10 states across the US (Regional variations may exist).11
Key Study Details
GMC Ratios Postdose 3c
Primary endpoint: VAXNEUVANCE delivered comparable immune responses for 12 of the 13 shared serotypes found in PCV13. Shared Serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 vs >0.5.19,23
Study Design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.19
aBased on a pooled analysis of national-level CDC data from 2018–2021, the top 6 IPD-causing serotypes in children under 5 years of age were 15C, 33F, 19F, 3, 23B, and 22F. Serotypes 15C and 23B are not included in any recommended pediatric PCV in the US.7,17,19,22,24
bPostdose 3 superiority was demonstrated based on measurements taken 30 days after the 6-month dose (at 7 months).19
cMeasurements were taken 30 days postdose specified.19
dSecondary endpoint: Postdose 3 IgG response rate percentage point difference vs PCV13 (95% CI): for Serotype 3, 19.1 (14.4, 24.0); for Serotype 22F, 8.1 (5.1, 11.5); for Serotype 33F, -5.1 (-9.5, -0.7).19,23
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.19
References:
1Dalton M. Pneumoccal disease. National Foundation for Infectious Diseases. Published July 2024. https://www.nfid.org/infectious-disease/pneumococcal/
2Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Epidemiology and Prevention of Vaccine-Preventable Diseases. Published May 1, 2024. Accessed December 10, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
3Data available on request from the Merck National Service Center via email at [email protected]. Please specify information package US-PVC-02072.
4Mohanty S, Done N, Liu Q, et al. Incidence of pneumococcal disease in children ≤48 months old in the United States: 1998–2019. Vaccine. Published online March 1, 2024. doi: 10.1016/j.vaccine.2024.03.013
5Clinical overview of pneumococcal disease. Centers for Disease Control and Prevention. February 6, 2024. Accessed May 22, 2024. https://www.cdc.gov/pneumococcal/hcp/clinical-overview/
6Wasserman MD, Perdrizet J, Grant L, et al. Clinical and economic burden of pneumococcal disease due to serotypes contained in current and investigational pneumococcal conjugate vaccines in children under five years of age. Infect Dis Ther. 2021;10(4):2701-2720. doi:10.1007/s40121-021-00544-1
7Centers for Disease Control and Prevention (CDC). Visualization – Based on 1998-2022 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance (ABCs). Updated July 22, 2024. Accessed August 30, 2024. https://data.cdc.gov/Public-Health-Surveillance/1998-2022-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data
8Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512.e1-512.e10. doi:10.1016/j.cmi.2019.09.008
9Azarian T, Mitchell PK, Georgieva M, et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11):e1007438. doi:10.1371/journal.ppat.1007438
10Hu T, Weiss T, Owusu-Edusei K, Petigara T. Health and economic burden associated with 15-valent pneumococcal conjugate vaccine serotypes in children in the United States. J Med Econ. 2020;23(12):1653-1660. doi:10.1080/13696998.2020.184021613
11Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2022. Centers for Disease Control and Prevention. Updated July 5, 2024. Accessed October 15, 2024. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf
12Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Centers for Disease Control and Prevention. Addendum updated November 21, 2024. Accessed November 25, 2024. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
13Hill HA, et al. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021 — National Immunization Survey-Child, United States, 2021–2023. MMWR Morb Mortal Wkly Rep, pages 844–853.
14Feemster K, Weaver J, Buchwald U, Banniettis N, Cox KS, McIntosh ED, Spoulou V. Pneumococcal Vaccine Breakthrough and Failure in Infants and Children: A Narrative Review. Vaccines (Basel). 2023 Nov 24;11(12):1750. doi:10.3390/vaccines11121750. PMID: 38140155; PMCID: PMC10747311.
15Recommendations to assure the quality, safety and efficacy of pneumoccoccal conjugate vaccines. Annex 3. TRS no 977. World Health Organization. October 19, 2013. Accessed October 31, 2024. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977
16Guidelines on clinical evaluation of vaccines: regulatory expectations. Annex 9. TRA No 924.World Health Organization. Last reviewed October 21, 2020. Accessed October 31, 2024. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9
17Prevnar 13. Prescribing Information. Pfizer; 2019.
18Luck JN, Tettelin H, Orihuela CJ. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front Cell Infect Microbiol. 2020;10:613287. Published 2020 Dec 23. doi:10.3389/fcimb.2020.613287
19VAXNEUVANCE. Prescribing Information. Merck & Co., Inc., 2024.
20Moraes-Pinto MI, Suano-Souza F, Aranda CS. Immune system: development and acquisition of immunological competence. J Pediatr (Rio J). 2021;97(S1):S59-S66. doi:10.1016/j.jped.2020.10.006
21Wodi AP, Morelli V. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 1: Principles of vaccination. Centers for Disease Control and Prevention. Last reviewed March 2024. Accessed May 9, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-1-principles-of-vaccination.html
22Pneumococcal vaccination. Centers for Disease Control and Prevention. Last reviewed September 12, 2024. Accessed September 30, 2024. https://www.cdc.gov/pneumococcal/vaccines/index.html
23Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142-1152. doi:10.1016/j.vaccine.2022.12.054
24Prevnar 20. Prescribing Information. Pfizer; 2023.
Copyright © 2025 Frontline Medical Communications Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form, by any means, without prior written permission of the Publisher. Frontline Medical Communications Inc. will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein. The opinions expressed in this publication do not necessarily reflect the views of the Publisher. All other trademarks are property of their respective owners.
Neither the editors of Pediatric News nor the Editorial Advisory Board nor the reporting staff contributed to this content.
US-PVC-01998 03/25
A Painful Flesh-Colored Papule on the Shoulder
A Painful Flesh-Colored Papule on the Shoulder
The Diagnosis: Leiomyoma
Histopathology revealed a dermal mesenchymal tumor composed of fascicles of bland spindle cells with tapered nuclei, perinuclear vacuoles, eosinophilic cytoplasm, and low cellularity (Figure 1). Immunohistochemical studies of the cells stained strongly positive for smooth muscle actin and desmin, consistent with a smooth muscle neoplasm (Figure 2). Fumarate hydratase (FH) staining revealed loss of expression in tumor cells, consistent with FH deficiency (Figure 3). A diagnosis of cutaneous leiomyoma was made, and although the clinical and histologic findings suggested hereditary leiomyomatosis and renal cell cancer (HLRCC), genetic testing was negative for an FH gene mutation. This negative result indicated that HLRCC was unlikely despite the initial concerns based on the findings.
Leiomyomas are benign neoplasms that are challenging to diagnose based on the clinical picture alone. Leiomyomas most commonly are found in the genitourinary and gastrointestinal systems, with cutaneous manifestation being the second most common presentation.1 These benign smooth muscle tumors manifest as tender, firm, flesh-colored, pink or reddish-brown nodules that are subcategorized based on the derivation of the smooth muscle within the tumor.2 Angioleiomyomas, the most common type, arise from the tunica media of blood vessels, whereas piloleiomyomas and genital leiomyomas arise from the arrector pili musculature of the hair follicle and the smooth muscle found in the scrotum, labia, or nipple.2 Rare cases of cutaneous leiomyosarcomas and angioleiomyosarcomas have been reported in the literature.3,4 Solitary leiomyomas tend to develop on the lower extremities, whereas multiple lesions frequently manifest on the extensor surfaces of extremities and the trunk. Lesions often are painful, either spontaneously or in association with applied pressure, emotional stress, or exposure to cold temperatures.2
Although leiomyomas themselves are benign, patients with multiple cutaneous leiomyomas may have an underlying genetic mutation that increases their risk of developing HLRCC, an autosomal-dominant syndrome.5 Referral should be considered for individuals with a personal history of or a first-degree relative with cutaneous leiomyomas or renal cell carcinoma (RCC) with histology typical of hereditary leiomyomatosis and RCC, as recommended by the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors.6 In this case, the decision to refer the patient for genetic testing was based on her family history, specifically her paternal uncle having multiple similar lesions, which, while not a first-degree relative, still raised concerns about potential hereditary risks and warranted further evaluation. A germline mutation in the FH gene, which encodes an enzyme that converts fumarate to malate in the Krebs cycle and plays a role in tumor suppression, is the cause of HLRCC.2,7 When part of this genetic condition, cutaneous leiomyomas tend to occur around 25 years of age (range, 10-50 years).2 A diagnosis of HLRCC should be strongly considered if a patient displays multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion or at least 2 of the following: solitary cutaneous leiomyoma with family history of HLRCC, onset of severely symptomatic uterine fibroids before age 40 years, type II papillary or collecting duct renal cell cancer before age 40 years, or a first-degree family member who meets 1 of these criteria.5,8
Diagnosis of cutaneous leiomyoma may be accomplished by microscopic examination of a tissue sample; however, further diagnostic workup is warranted due to the strong correlation with HLRCC.2 A definitive diagnosis of HLRCC is confirmed with a germline mutation in the FH gene, and genetic screening should be offered to patients before renal cancer surveillance to avoid unwarranted investigations.8 Timely clinical diagnosis enables early genetic testing and enhanced outcomes for patients with confirmed HLRCC who may need a multidisciplinary approach of dermatologists, gynecologists, and urologic oncologists.5,8
Cutaneous leiomyomas can be excised, and this typically is the gold standard of care for small and localized lesions, although the use of cryosurgery and carbon dioxide lasers has been reported as well.2,9,10 For more widespread lesions or for patients who are not appropriate candidates for surgery, pharmacologic therapies (α-blockers, calcium channel blockers, nitroglycerin), intralesional corticosteroids, and/or botulinum toxin injections can be utilized.2,11
The acronym BLEND AN EGG encompasses the clinical differential diagnosis for painful skin tumors: blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor. Blue rubber bleb nevi are deep blue in color, and angiolipomas sit under the skin and present as subcutaneous swellings. Dermatofibromas and neurofibromas also are included in the differential.12 Dermatofibromas are firm solitary lesions that have a pathognomonic pinch sign. Neurofibromas are soft and rubbery, have a buttonhole sign, and stain positively for S-100 protein and SOX-10 but negatively for actin and desmin.12
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. In: StatPearls. StatPearls Publishing; 2023.
- Chayed Z, Kristensen LK, Ousager LB, et al. Hereditary leiomyomatosis and renal cell carcinoma: a case series and literature review. Orphanet J Rare Dis. 2021;16:34. doi:10.1186/s13023-020-01653-9
- Perkins J, Scarbrough C, Sammons D, et al. Reed syndrome: an atypical presentation of a rare disease. Dermatol Online J. 2014;21: 13030/qt5k35r5pn.
- Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014;7:253-260. doi:10.2147 /IJNRD.S42097
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70-87. doi:10.1038/gim.2014.147
- Alam NA, Barclay E, Rowan AJ, et al. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol. 2005;141:199-206. doi:10.1001 /archderm.141.2.199
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644. doi:10.1007/s10689-014-9735-2
- Uyar B, Acar EM, Subas¸ıog˘lu A. Treatment of three hereditary leiomyomatosis patients with cryotherapy. Dermatol Ther. 2020;33:e13226. doi:10.1111/dth.13226
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322. doi:10.1046/j.1524-4725.2000.99250.x
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma- related pain. J Am Acad Dermatol. 2009;60:325-328. doi:10.1016 /j.jaad.2008.05.044
- Clarey DD, Lauer SR, Adams JL. Painful papules on the arms. Cutis. 2020;106:232-249. doi:10.12788/cutis.0109
The Diagnosis: Leiomyoma
Histopathology revealed a dermal mesenchymal tumor composed of fascicles of bland spindle cells with tapered nuclei, perinuclear vacuoles, eosinophilic cytoplasm, and low cellularity (Figure 1). Immunohistochemical studies of the cells stained strongly positive for smooth muscle actin and desmin, consistent with a smooth muscle neoplasm (Figure 2). Fumarate hydratase (FH) staining revealed loss of expression in tumor cells, consistent with FH deficiency (Figure 3). A diagnosis of cutaneous leiomyoma was made, and although the clinical and histologic findings suggested hereditary leiomyomatosis and renal cell cancer (HLRCC), genetic testing was negative for an FH gene mutation. This negative result indicated that HLRCC was unlikely despite the initial concerns based on the findings.
Leiomyomas are benign neoplasms that are challenging to diagnose based on the clinical picture alone. Leiomyomas most commonly are found in the genitourinary and gastrointestinal systems, with cutaneous manifestation being the second most common presentation.1 These benign smooth muscle tumors manifest as tender, firm, flesh-colored, pink or reddish-brown nodules that are subcategorized based on the derivation of the smooth muscle within the tumor.2 Angioleiomyomas, the most common type, arise from the tunica media of blood vessels, whereas piloleiomyomas and genital leiomyomas arise from the arrector pili musculature of the hair follicle and the smooth muscle found in the scrotum, labia, or nipple.2 Rare cases of cutaneous leiomyosarcomas and angioleiomyosarcomas have been reported in the literature.3,4 Solitary leiomyomas tend to develop on the lower extremities, whereas multiple lesions frequently manifest on the extensor surfaces of extremities and the trunk. Lesions often are painful, either spontaneously or in association with applied pressure, emotional stress, or exposure to cold temperatures.2
Although leiomyomas themselves are benign, patients with multiple cutaneous leiomyomas may have an underlying genetic mutation that increases their risk of developing HLRCC, an autosomal-dominant syndrome.5 Referral should be considered for individuals with a personal history of or a first-degree relative with cutaneous leiomyomas or renal cell carcinoma (RCC) with histology typical of hereditary leiomyomatosis and RCC, as recommended by the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors.6 In this case, the decision to refer the patient for genetic testing was based on her family history, specifically her paternal uncle having multiple similar lesions, which, while not a first-degree relative, still raised concerns about potential hereditary risks and warranted further evaluation. A germline mutation in the FH gene, which encodes an enzyme that converts fumarate to malate in the Krebs cycle and plays a role in tumor suppression, is the cause of HLRCC.2,7 When part of this genetic condition, cutaneous leiomyomas tend to occur around 25 years of age (range, 10-50 years).2 A diagnosis of HLRCC should be strongly considered if a patient displays multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion or at least 2 of the following: solitary cutaneous leiomyoma with family history of HLRCC, onset of severely symptomatic uterine fibroids before age 40 years, type II papillary or collecting duct renal cell cancer before age 40 years, or a first-degree family member who meets 1 of these criteria.5,8
Diagnosis of cutaneous leiomyoma may be accomplished by microscopic examination of a tissue sample; however, further diagnostic workup is warranted due to the strong correlation with HLRCC.2 A definitive diagnosis of HLRCC is confirmed with a germline mutation in the FH gene, and genetic screening should be offered to patients before renal cancer surveillance to avoid unwarranted investigations.8 Timely clinical diagnosis enables early genetic testing and enhanced outcomes for patients with confirmed HLRCC who may need a multidisciplinary approach of dermatologists, gynecologists, and urologic oncologists.5,8
Cutaneous leiomyomas can be excised, and this typically is the gold standard of care for small and localized lesions, although the use of cryosurgery and carbon dioxide lasers has been reported as well.2,9,10 For more widespread lesions or for patients who are not appropriate candidates for surgery, pharmacologic therapies (α-blockers, calcium channel blockers, nitroglycerin), intralesional corticosteroids, and/or botulinum toxin injections can be utilized.2,11
The acronym BLEND AN EGG encompasses the clinical differential diagnosis for painful skin tumors: blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor. Blue rubber bleb nevi are deep blue in color, and angiolipomas sit under the skin and present as subcutaneous swellings. Dermatofibromas and neurofibromas also are included in the differential.12 Dermatofibromas are firm solitary lesions that have a pathognomonic pinch sign. Neurofibromas are soft and rubbery, have a buttonhole sign, and stain positively for S-100 protein and SOX-10 but negatively for actin and desmin.12
The Diagnosis: Leiomyoma
Histopathology revealed a dermal mesenchymal tumor composed of fascicles of bland spindle cells with tapered nuclei, perinuclear vacuoles, eosinophilic cytoplasm, and low cellularity (Figure 1). Immunohistochemical studies of the cells stained strongly positive for smooth muscle actin and desmin, consistent with a smooth muscle neoplasm (Figure 2). Fumarate hydratase (FH) staining revealed loss of expression in tumor cells, consistent with FH deficiency (Figure 3). A diagnosis of cutaneous leiomyoma was made, and although the clinical and histologic findings suggested hereditary leiomyomatosis and renal cell cancer (HLRCC), genetic testing was negative for an FH gene mutation. This negative result indicated that HLRCC was unlikely despite the initial concerns based on the findings.
Leiomyomas are benign neoplasms that are challenging to diagnose based on the clinical picture alone. Leiomyomas most commonly are found in the genitourinary and gastrointestinal systems, with cutaneous manifestation being the second most common presentation.1 These benign smooth muscle tumors manifest as tender, firm, flesh-colored, pink or reddish-brown nodules that are subcategorized based on the derivation of the smooth muscle within the tumor.2 Angioleiomyomas, the most common type, arise from the tunica media of blood vessels, whereas piloleiomyomas and genital leiomyomas arise from the arrector pili musculature of the hair follicle and the smooth muscle found in the scrotum, labia, or nipple.2 Rare cases of cutaneous leiomyosarcomas and angioleiomyosarcomas have been reported in the literature.3,4 Solitary leiomyomas tend to develop on the lower extremities, whereas multiple lesions frequently manifest on the extensor surfaces of extremities and the trunk. Lesions often are painful, either spontaneously or in association with applied pressure, emotional stress, or exposure to cold temperatures.2
Although leiomyomas themselves are benign, patients with multiple cutaneous leiomyomas may have an underlying genetic mutation that increases their risk of developing HLRCC, an autosomal-dominant syndrome.5 Referral should be considered for individuals with a personal history of or a first-degree relative with cutaneous leiomyomas or renal cell carcinoma (RCC) with histology typical of hereditary leiomyomatosis and RCC, as recommended by the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors.6 In this case, the decision to refer the patient for genetic testing was based on her family history, specifically her paternal uncle having multiple similar lesions, which, while not a first-degree relative, still raised concerns about potential hereditary risks and warranted further evaluation. A germline mutation in the FH gene, which encodes an enzyme that converts fumarate to malate in the Krebs cycle and plays a role in tumor suppression, is the cause of HLRCC.2,7 When part of this genetic condition, cutaneous leiomyomas tend to occur around 25 years of age (range, 10-50 years).2 A diagnosis of HLRCC should be strongly considered if a patient displays multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion or at least 2 of the following: solitary cutaneous leiomyoma with family history of HLRCC, onset of severely symptomatic uterine fibroids before age 40 years, type II papillary or collecting duct renal cell cancer before age 40 years, or a first-degree family member who meets 1 of these criteria.5,8
Diagnosis of cutaneous leiomyoma may be accomplished by microscopic examination of a tissue sample; however, further diagnostic workup is warranted due to the strong correlation with HLRCC.2 A definitive diagnosis of HLRCC is confirmed with a germline mutation in the FH gene, and genetic screening should be offered to patients before renal cancer surveillance to avoid unwarranted investigations.8 Timely clinical diagnosis enables early genetic testing and enhanced outcomes for patients with confirmed HLRCC who may need a multidisciplinary approach of dermatologists, gynecologists, and urologic oncologists.5,8
Cutaneous leiomyomas can be excised, and this typically is the gold standard of care for small and localized lesions, although the use of cryosurgery and carbon dioxide lasers has been reported as well.2,9,10 For more widespread lesions or for patients who are not appropriate candidates for surgery, pharmacologic therapies (α-blockers, calcium channel blockers, nitroglycerin), intralesional corticosteroids, and/or botulinum toxin injections can be utilized.2,11
The acronym BLEND AN EGG encompasses the clinical differential diagnosis for painful skin tumors: blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor. Blue rubber bleb nevi are deep blue in color, and angiolipomas sit under the skin and present as subcutaneous swellings. Dermatofibromas and neurofibromas also are included in the differential.12 Dermatofibromas are firm solitary lesions that have a pathognomonic pinch sign. Neurofibromas are soft and rubbery, have a buttonhole sign, and stain positively for S-100 protein and SOX-10 but negatively for actin and desmin.12
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. In: StatPearls. StatPearls Publishing; 2023.
- Chayed Z, Kristensen LK, Ousager LB, et al. Hereditary leiomyomatosis and renal cell carcinoma: a case series and literature review. Orphanet J Rare Dis. 2021;16:34. doi:10.1186/s13023-020-01653-9
- Perkins J, Scarbrough C, Sammons D, et al. Reed syndrome: an atypical presentation of a rare disease. Dermatol Online J. 2014;21: 13030/qt5k35r5pn.
- Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014;7:253-260. doi:10.2147 /IJNRD.S42097
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70-87. doi:10.1038/gim.2014.147
- Alam NA, Barclay E, Rowan AJ, et al. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol. 2005;141:199-206. doi:10.1001 /archderm.141.2.199
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644. doi:10.1007/s10689-014-9735-2
- Uyar B, Acar EM, Subas¸ıog˘lu A. Treatment of three hereditary leiomyomatosis patients with cryotherapy. Dermatol Ther. 2020;33:e13226. doi:10.1111/dth.13226
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322. doi:10.1046/j.1524-4725.2000.99250.x
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma- related pain. J Am Acad Dermatol. 2009;60:325-328. doi:10.1016 /j.jaad.2008.05.044
- Clarey DD, Lauer SR, Adams JL. Painful papules on the arms. Cutis. 2020;106:232-249. doi:10.12788/cutis.0109
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. In: StatPearls. StatPearls Publishing; 2023.
- Chayed Z, Kristensen LK, Ousager LB, et al. Hereditary leiomyomatosis and renal cell carcinoma: a case series and literature review. Orphanet J Rare Dis. 2021;16:34. doi:10.1186/s13023-020-01653-9
- Perkins J, Scarbrough C, Sammons D, et al. Reed syndrome: an atypical presentation of a rare disease. Dermatol Online J. 2014;21: 13030/qt5k35r5pn.
- Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014;7:253-260. doi:10.2147 /IJNRD.S42097
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70-87. doi:10.1038/gim.2014.147
- Alam NA, Barclay E, Rowan AJ, et al. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol. 2005;141:199-206. doi:10.1001 /archderm.141.2.199
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644. doi:10.1007/s10689-014-9735-2
- Uyar B, Acar EM, Subas¸ıog˘lu A. Treatment of three hereditary leiomyomatosis patients with cryotherapy. Dermatol Ther. 2020;33:e13226. doi:10.1111/dth.13226
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322. doi:10.1046/j.1524-4725.2000.99250.x
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma- related pain. J Am Acad Dermatol. 2009;60:325-328. doi:10.1016 /j.jaad.2008.05.044
- Clarey DD, Lauer SR, Adams JL. Painful papules on the arms. Cutis. 2020;106:232-249. doi:10.12788/cutis.0109
A Painful Flesh-Colored Papule on the Shoulder
A Painful Flesh-Colored Papule on the Shoulder
A 65-year-old woman with a history of metabolic syndrome presented to the family medicine clinic for evaluation of a papule on the right shoulder that had started small and increased in size over the past 3 years. Physical examination revealed a 1.0×0.8×0.1-cm, smooth, flesh-colored to light brown papule on the right shoulder that was notably tender to palpation. The patient reported that her paternal uncle had multiple skin lesions of similar morphology dispersed on the bilateral upper extremities. A shave biopsy of the lesion was performed.
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
To the Editor:
A 22-year-old man was referred to our dermatology outpatient department for wartlike growths that gradually developed around a postoperative enteroatmospheric fistula and stoma over the past 4 months. The patient presented for an emergency exploratory laparotomy with a history of perforation peritonitis 1.5 years prior to the current presentation. He also had a small bowel obstruction 5 months prior to the current presentation that resulted in the resection of a large segment of the small bowel. He underwent a diverting loop ileostomy when the abdominal closure was not achieved because of bowel edema, following which he developed a postoperative enteroatmospheric fistula. In addition, the stoma retracted and was followed by dermal dehiscence, which led to notable leakage and resulted in heavy fecal contamination of the midline wound.
At the current presentation, physical examination revealed multiple grayish-white, dome-shaped, moist papules coalescing to form a peristomal pseudoverrucous mass on the lower side of the stoma (Figure 1). The patient experienced mild itching. The lesion showed no signs of erosion, bleeding, or purulent discharge, and there were no nearby lumps or enlarged lymph nodes. The differential diagnosis included peristomal pyoderma gangrenosum, human papillomavirus (HPV) infection, pseudoverrucous papules and nodules (PPNs), squamous cell carcinoma, and exuberant granulation tissue. A skin biopsy was performed, and histopathology revealed hyperkeratosis, moderate papillomatosis, and marked acanthotic hyperplasia seen as downgrowths into the dermis (Figure 2). No koilocytes, atypia, or mitotic figures were present. Abundant neutrophils and few eosinophils were seen in the dermal infiltrate. A final diagnosis of PPN was made based on clinicopathologic correlation. The patient was advised to use a smaller stoma bag and to change the collection pouch frequently to reduce skin contact with fecal matter.
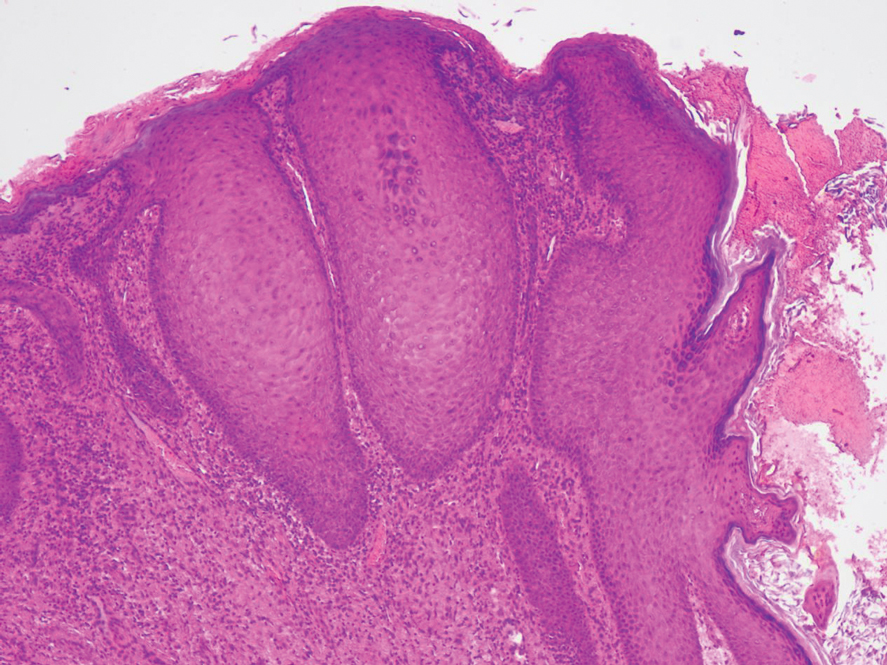
Peristomal skin conditions are reported in 18% to 55% of patients with stomas and include allergic contact dermatitis, mechanical dermatitis, infections, pyoderma gangrenosum, and irritant contact dermatitis.1,2 Pseudoverrucous papules (also called chronic papillomatous dermatitis or pseudoverrucous lesions) is a rare dermatologic complication found on the skin around stomas,3 most commonly around urostomy stomas. The presence of PPNs around colostomy stomas and the perianal region is extremely rare.2,4 This condition is the result of chronic irritant dermatitis from frequent exposure to urine or feces, leading to maceration and epidermal hyperplasia. It occurs because of improper sizing of the stoma bag or incorrect positioning or construction of the stoma.5
the overuse of topical benzocaine-resorcinol, leading to chronic irritation.6 It is clinically characterized by multiple grayish-white, wartlike, confluent papulonodules around areas chronically exposed to moisture. Differential diagnoses such as secondary neoplasms, HPV infection, exuberant granulation tissue, and candidal infections should be considered.3 Final diagnosis is based on clinicopathologic findings, similar to our case. Epidermal growth factor and transforming growth factor are thought to play a role in the pathophysiology of pseudoepitheliomatous hyperplasia. Increased expression of these mediators leads to proliferation of the epidermis into the dermis.7 The role of HPV in PPN remains unclear, as not all PPN lesions are positive for HPV and the cutaneous lesions resolve once the source of irritation is removed. Recommended treatment includes local skin care; stoma refitting; and, in severe cases, excision and revision of the stoma.2 Dermatologists must be aware of this often-underdiagnosed condition.
- Alslaim F, Al Farajat F, Alslaim HS, et al. Etiology and management of peristomal pseudoepitheliomatous hyperplasia. Cureus. 2021;13 :E20196. doi:10.7759/cureus.20196
- Rambhia PH, Conic RZ, Honda K, et al. Chronic papillomatous dermatitis in a patient with a urinary ileal diversion: a case report and review of the literature. Dermatol Arch. 2017;1:47-50. doi:10.36959/661/297
- Latour-Álvarez I, García-Peris E, Pestana-Eliche MM, et al. Nodular peristomal lesions. Actas Dermosifiliogr. 2016;108:363-364. doi:10.1016/j.ad.2016.02.018
- Dandale A, Dhurat R, Ghate S. Perianal pseudoverrucous papules and nodules. Indian J Sex Transm Dis AIDS. 2013;34:44-46. doi:10.4103/0253-7184.112939
- Brogna L. Prevention and management of pseudoverrucous lesions: a review and case scenarios. Adv Skin Wound Care. 2021;34:461-471. doi:10.1097/01.ASW.0000758620.93518.39
- Robson KJ, Maughan JA, Purcell SD, et al. Erosive papulonodular dermatosis associated with topical benzocaine: a report of two cases and evidence that granuloma gluteale, pseudoverrucous papules, and Jacquet’s erosive dermatitis are a disease spectrum. J Am Acad Dermatol. 2006;55(5 suppl):S74-S80. doi:10.1016/j .jaad.2005.12.025
- Oğuz ID, Vural S, Cinar E, et al. Peristomal pseudoverrucous lesions: a rare skin complication of colostomy. Cureus. 2023;15:E38068. doi:10.7759/cureus.38068
To the Editor:
A 22-year-old man was referred to our dermatology outpatient department for wartlike growths that gradually developed around a postoperative enteroatmospheric fistula and stoma over the past 4 months. The patient presented for an emergency exploratory laparotomy with a history of perforation peritonitis 1.5 years prior to the current presentation. He also had a small bowel obstruction 5 months prior to the current presentation that resulted in the resection of a large segment of the small bowel. He underwent a diverting loop ileostomy when the abdominal closure was not achieved because of bowel edema, following which he developed a postoperative enteroatmospheric fistula. In addition, the stoma retracted and was followed by dermal dehiscence, which led to notable leakage and resulted in heavy fecal contamination of the midline wound.
At the current presentation, physical examination revealed multiple grayish-white, dome-shaped, moist papules coalescing to form a peristomal pseudoverrucous mass on the lower side of the stoma (Figure 1). The patient experienced mild itching. The lesion showed no signs of erosion, bleeding, or purulent discharge, and there were no nearby lumps or enlarged lymph nodes. The differential diagnosis included peristomal pyoderma gangrenosum, human papillomavirus (HPV) infection, pseudoverrucous papules and nodules (PPNs), squamous cell carcinoma, and exuberant granulation tissue. A skin biopsy was performed, and histopathology revealed hyperkeratosis, moderate papillomatosis, and marked acanthotic hyperplasia seen as downgrowths into the dermis (Figure 2). No koilocytes, atypia, or mitotic figures were present. Abundant neutrophils and few eosinophils were seen in the dermal infiltrate. A final diagnosis of PPN was made based on clinicopathologic correlation. The patient was advised to use a smaller stoma bag and to change the collection pouch frequently to reduce skin contact with fecal matter.


Peristomal skin conditions are reported in 18% to 55% of patients with stomas and include allergic contact dermatitis, mechanical dermatitis, infections, pyoderma gangrenosum, and irritant contact dermatitis.1,2 Pseudoverrucous papules (also called chronic papillomatous dermatitis or pseudoverrucous lesions) is a rare dermatologic complication found on the skin around stomas,3 most commonly around urostomy stomas. The presence of PPNs around colostomy stomas and the perianal region is extremely rare.2,4 This condition is the result of chronic irritant dermatitis from frequent exposure to urine or feces, leading to maceration and epidermal hyperplasia. It occurs because of improper sizing of the stoma bag or incorrect positioning or construction of the stoma.5
the overuse of topical benzocaine-resorcinol, leading to chronic irritation.6 It is clinically characterized by multiple grayish-white, wartlike, confluent papulonodules around areas chronically exposed to moisture. Differential diagnoses such as secondary neoplasms, HPV infection, exuberant granulation tissue, and candidal infections should be considered.3 Final diagnosis is based on clinicopathologic findings, similar to our case. Epidermal growth factor and transforming growth factor are thought to play a role in the pathophysiology of pseudoepitheliomatous hyperplasia. Increased expression of these mediators leads to proliferation of the epidermis into the dermis.7 The role of HPV in PPN remains unclear, as not all PPN lesions are positive for HPV and the cutaneous lesions resolve once the source of irritation is removed. Recommended treatment includes local skin care; stoma refitting; and, in severe cases, excision and revision of the stoma.2 Dermatologists must be aware of this often-underdiagnosed condition.
To the Editor:
A 22-year-old man was referred to our dermatology outpatient department for wartlike growths that gradually developed around a postoperative enteroatmospheric fistula and stoma over the past 4 months. The patient presented for an emergency exploratory laparotomy with a history of perforation peritonitis 1.5 years prior to the current presentation. He also had a small bowel obstruction 5 months prior to the current presentation that resulted in the resection of a large segment of the small bowel. He underwent a diverting loop ileostomy when the abdominal closure was not achieved because of bowel edema, following which he developed a postoperative enteroatmospheric fistula. In addition, the stoma retracted and was followed by dermal dehiscence, which led to notable leakage and resulted in heavy fecal contamination of the midline wound.
At the current presentation, physical examination revealed multiple grayish-white, dome-shaped, moist papules coalescing to form a peristomal pseudoverrucous mass on the lower side of the stoma (Figure 1). The patient experienced mild itching. The lesion showed no signs of erosion, bleeding, or purulent discharge, and there were no nearby lumps or enlarged lymph nodes. The differential diagnosis included peristomal pyoderma gangrenosum, human papillomavirus (HPV) infection, pseudoverrucous papules and nodules (PPNs), squamous cell carcinoma, and exuberant granulation tissue. A skin biopsy was performed, and histopathology revealed hyperkeratosis, moderate papillomatosis, and marked acanthotic hyperplasia seen as downgrowths into the dermis (Figure 2). No koilocytes, atypia, or mitotic figures were present. Abundant neutrophils and few eosinophils were seen in the dermal infiltrate. A final diagnosis of PPN was made based on clinicopathologic correlation. The patient was advised to use a smaller stoma bag and to change the collection pouch frequently to reduce skin contact with fecal matter.


Peristomal skin conditions are reported in 18% to 55% of patients with stomas and include allergic contact dermatitis, mechanical dermatitis, infections, pyoderma gangrenosum, and irritant contact dermatitis.1,2 Pseudoverrucous papules (also called chronic papillomatous dermatitis or pseudoverrucous lesions) is a rare dermatologic complication found on the skin around stomas,3 most commonly around urostomy stomas. The presence of PPNs around colostomy stomas and the perianal region is extremely rare.2,4 This condition is the result of chronic irritant dermatitis from frequent exposure to urine or feces, leading to maceration and epidermal hyperplasia. It occurs because of improper sizing of the stoma bag or incorrect positioning or construction of the stoma.5
the overuse of topical benzocaine-resorcinol, leading to chronic irritation.6 It is clinically characterized by multiple grayish-white, wartlike, confluent papulonodules around areas chronically exposed to moisture. Differential diagnoses such as secondary neoplasms, HPV infection, exuberant granulation tissue, and candidal infections should be considered.3 Final diagnosis is based on clinicopathologic findings, similar to our case. Epidermal growth factor and transforming growth factor are thought to play a role in the pathophysiology of pseudoepitheliomatous hyperplasia. Increased expression of these mediators leads to proliferation of the epidermis into the dermis.7 The role of HPV in PPN remains unclear, as not all PPN lesions are positive for HPV and the cutaneous lesions resolve once the source of irritation is removed. Recommended treatment includes local skin care; stoma refitting; and, in severe cases, excision and revision of the stoma.2 Dermatologists must be aware of this often-underdiagnosed condition.
- Alslaim F, Al Farajat F, Alslaim HS, et al. Etiology and management of peristomal pseudoepitheliomatous hyperplasia. Cureus. 2021;13 :E20196. doi:10.7759/cureus.20196
- Rambhia PH, Conic RZ, Honda K, et al. Chronic papillomatous dermatitis in a patient with a urinary ileal diversion: a case report and review of the literature. Dermatol Arch. 2017;1:47-50. doi:10.36959/661/297
- Latour-Álvarez I, García-Peris E, Pestana-Eliche MM, et al. Nodular peristomal lesions. Actas Dermosifiliogr. 2016;108:363-364. doi:10.1016/j.ad.2016.02.018
- Dandale A, Dhurat R, Ghate S. Perianal pseudoverrucous papules and nodules. Indian J Sex Transm Dis AIDS. 2013;34:44-46. doi:10.4103/0253-7184.112939
- Brogna L. Prevention and management of pseudoverrucous lesions: a review and case scenarios. Adv Skin Wound Care. 2021;34:461-471. doi:10.1097/01.ASW.0000758620.93518.39
- Robson KJ, Maughan JA, Purcell SD, et al. Erosive papulonodular dermatosis associated with topical benzocaine: a report of two cases and evidence that granuloma gluteale, pseudoverrucous papules, and Jacquet’s erosive dermatitis are a disease spectrum. J Am Acad Dermatol. 2006;55(5 suppl):S74-S80. doi:10.1016/j .jaad.2005.12.025
- Oğuz ID, Vural S, Cinar E, et al. Peristomal pseudoverrucous lesions: a rare skin complication of colostomy. Cureus. 2023;15:E38068. doi:10.7759/cureus.38068
- Alslaim F, Al Farajat F, Alslaim HS, et al. Etiology and management of peristomal pseudoepitheliomatous hyperplasia. Cureus. 2021;13 :E20196. doi:10.7759/cureus.20196
- Rambhia PH, Conic RZ, Honda K, et al. Chronic papillomatous dermatitis in a patient with a urinary ileal diversion: a case report and review of the literature. Dermatol Arch. 2017;1:47-50. doi:10.36959/661/297
- Latour-Álvarez I, García-Peris E, Pestana-Eliche MM, et al. Nodular peristomal lesions. Actas Dermosifiliogr. 2016;108:363-364. doi:10.1016/j.ad.2016.02.018
- Dandale A, Dhurat R, Ghate S. Perianal pseudoverrucous papules and nodules. Indian J Sex Transm Dis AIDS. 2013;34:44-46. doi:10.4103/0253-7184.112939
- Brogna L. Prevention and management of pseudoverrucous lesions: a review and case scenarios. Adv Skin Wound Care. 2021;34:461-471. doi:10.1097/01.ASW.0000758620.93518.39
- Robson KJ, Maughan JA, Purcell SD, et al. Erosive papulonodular dermatosis associated with topical benzocaine: a report of two cases and evidence that granuloma gluteale, pseudoverrucous papules, and Jacquet’s erosive dermatitis are a disease spectrum. J Am Acad Dermatol. 2006;55(5 suppl):S74-S80. doi:10.1016/j .jaad.2005.12.025
- Oğuz ID, Vural S, Cinar E, et al. Peristomal pseudoverrucous lesions: a rare skin complication of colostomy. Cureus. 2023;15:E38068. doi:10.7759/cureus.38068
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
PRACTICE POINTS
- Pseudoverrucous papules and nodules (PPNs) can develop around stomas due to chronic irritant dermatitis from fecal or urinary exposure.
- Proper stoma management, including the use of appropriately sized stoma bags and frequent changes, is essential to prevent skin complications such as PPN.
- When evaluating peristomal lesions, consider a broad differential diagnosis, including infections, neoplasms, and dermatitis, and ensure thorough clinicopathologic correlation for accurate diagnosis and treatment.
Key Features of North American Venomous Snake Bites
Key Features of North American Venomous Snake Bites
North American venomous snakes traditionally are classified as members of either the Viperidae (eg, rattlesnakes, copperheads, cottonmouths) or Elapidae (eg, coral snakes) families and account for roughly 5000 to 10,000 reported envenomations annually.1,2 In 2021, America’s Poison Centers reported 2287 calls related to copperheads, 71 related to coral snakes, 229 related to cottonmouths, 1184 related to rattlesnakes, and 524 related to unknown snakes.3 The majority of calls related to snake bites were for adult patients, resulting in absent to minor outcomes. Only 1 death due to a rattlesnake bite was reported.3 Death by envenomation from a North American snake species is considered rare and typically is attributed to a lapse in medical attention; however, rattlesnakes are the most common reported cause of death by snake envenomation (Figure 1).1,3 A study comparing snake bites and hospital stays in the southeast vs southwest United States found that the southeast had the highest incidence of copperhead bites (37%), while the southwest had a higher incidence of rattlesnake bites (70%); those who were bitten by a rattlesnake were reported to have more severe symptoms and greater need for medical attention and antivenin.4 Some reports have linked pediatric and elderly patients to worse outcomes.5 However, one study examining 24,388 emergency department visits for snake bites from 2006 through 2014 found that the majority of pediatric cases were handled by non– trauma centers in the southern United States,6 supporting evidence found by Campbell et al7 indicating that most snake bites in children can be managed with conservative care. Though reported complications—including weakness, paralysis, hypovolemic shock, thrombocytopenia, and death—from North American venomous snake bites are low, they are still considered a medical emergency.8 It is essential for physicians to understand the clinical manifestations and treatment of North American venomous snake bites and to educate patients on how to protect themselves against and avoid provoking snakes, particularly in rural areas.2 In this article, we review the characteristics of common North American venomous snakes and the clinical manifestations of their bites. We also discuss the appropriate measures for staging, evaluating, and treating snake envenomation to improve patient management and care.

Features of North American Venomous Snakes
Individual snakes within the Viperidae family vary in size, markings and coloration, activity, and region, and physicians should consult their local health departments regarding snakes that are common in their area.2 Cottonmouth snakes are semiaquatic and traditionally are found within the southern and central United States. With a spade-shaped head and distinct two-tone coloration, cottonmouths may be mistaken for other nonvenomous water snakes in these regions (Figure 2).2 Copperheads, true to their name, are red in color; they inhabit a large portion of the southeastern United States and eastern Texas regions and are the cause of the majority of venomous snake bites in North America (Figure 3). Both cottonmouths and copperheads are believed to bite and envenomate as a defensive mechanism when provoked.


Coral snakes, found in the eastern United States and Texas regions, are the only subspecies of the Elapidae family (Figure 4).2,9 They can be distinguished from the nonvenomous milk snake by their characteristic banding, as coral snakes are patterned in a red-yellow-black band sequence and milk snakes are patterned in a red-black-yellow or white sequence. The differences in appearance of these snakes often is remembered by the phrase “red on yellow kills a fellow.”

Anatomic differences between the Viperidae and Elapidae families, including fang size, placement, and type, as well as venom composition, are directly linked to clinical manifestations of the bites. Viperidae fangs extend from the maxillary bones and are mobile, long, and hollow, making it easy for the snake to control fang movement and envenomation.9 Viperidae snakes are uniquely capable of inflicting puncture wounds without the injection of venom, known as dry bites. In contrast, Elapidae snakes have short, hollow, and fixed fangs, and thus patients can protect themselves by wearing appropriate clothing and covered footwear.9 Currently, identifying the type of snake responsible for the bite relies on visualization of the snake and/or the identification of clinical symptoms of envenomation by a dermatologist.
Clinical Manifestations of Venomous Snake Bites
Clinical manifestations and cutaneous findings often are used to grade the severity of venomous snake bites as well as to dictate treatment procedures. Grade 0 indicates a bite has occurred without envenomation, while grades I to V describe the progression and severity of envenomation.10 Grade I describes minimal erythema and edema around the site (fang marks may or may not be present) and no systemic symptoms. Grade II describes erythema and edema extending up the extremity to the first joint (eg, hand to wrist), pain, some systemic symptoms if there is rapid progression, and potential bleeding at the site. Grade III describes erythema and edema spreading to the second joint in the extremity, pain, and systemic symptoms, including coagulation defects. Grade IV describes erythema and edema of the whole extremity, a rapid reaction and progression following the bite, and risk for compartment syndrome. Grade V includes erythema and edema beyond the extremity and increasing systemic symptoms.10
Local pain and edema, usually on easily accessible or exposed extremities, are the most common clinical symptoms reported following a Viperidae snake bite.11 Due to their capability of producing a dry bite, puncture markings alone do not indicate envenomation. Patients will need to be monitored for several hours for signs of envenomation, which may include swelling, pain, ecchymosis, and indications of systemic manifestation (eg, weakness, dizziness, nausea, severe hypotension, thrombocytopenia).11 Viperidae venom hemorrhagic metalloproteinases act on capillary blood vessels by cleaving basement membrane proteins and allowing for extravasation of fluid into local tissue.12 The inflammatory response produced at the site of envenomation likely is due to the release of tumor necrosis factor á and endogenous matrix metalloprotein.12 There is a higher risk for death associated with bites from rattlesnakes within the Viperidae family because their venom contains a unique neurotoxin that works by blocking presynaptic junctions and causing a range of paralytic symptoms from ptosis to respiratory failure.13
The severity of Elapidae bites is thought to be related to the amount of venom injected, the size of the victim, and the length of the snake. Though clothing may offer protection, envenomation occurs in 75% of coral snake bites and can produce devastating consequences due to the venom content.14 In a retrospective study between 2002 and 2004, 90% of Elapidae snake bite patients (n=82) reported local pain, redness, and paresthesia, while around 7% developed systemic symptoms.15 Elapidae venom primarily is neurotoxic and is thought to spread via lymphatics.16 Delayed reactions are common and may take up to 12 hours to develop. Patients should be monitored, as local reactions may progress to weakness, fasciculations, extremity paralysis, and lastly, respiratory paralysis. Due to the risk for progression, all patients with likely coral snake bites should be given antivenin.8,15,17
Much like the North American coral snake, the venomous snake species Gloydius blomhoffii—referred to as the salmosa or mamushi snake depending on the region of origin (ie, Korea or Japan)—is a frequent source of devastating rural snake bites due to neurotoxins (Figure 5). The species’ slender fangs are thought to directly inject the snake’s potent venom, which contains hemorrhagic toxins and α-neurotoxins and Β-neurotoxins, into the bloodstream; however, the salmosa is considered a viper like the North American cottonmouth and copperhead because of its triangular head shape and hollow fangs, which allow for the accommodation of venom-containing glands and mechanism of venom injection. Salmosa venom shares both Viperidae and Elapidae characteristics. Cutaneous findings such as progressive edema, erythema, and bleeding frequently are reported and are attributed to the proteases and hemorrhagic toxins characteristic of vipers (Figure 6). α-Neurotoxins and Β-neurotoxins, similar to the proteolytic venom of the Elapidae family, are responsible for the unique visual disturbances (binocular diplopia) caused by the salmosa.12,18,19

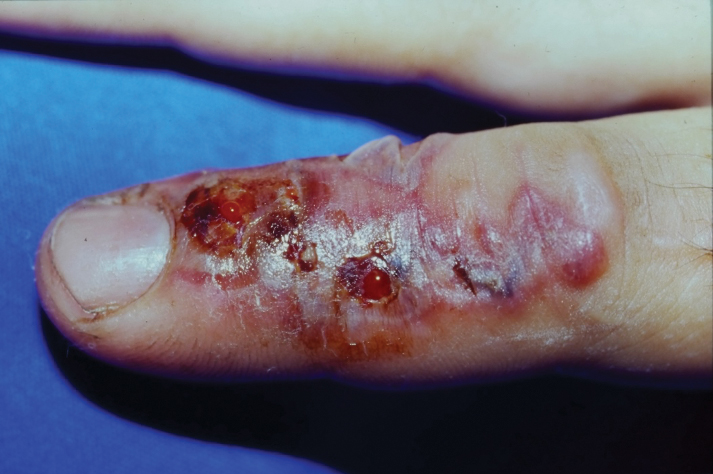
Treatment
Treating snake bites begins with assessing the patient’s airway, breathing, and circulation, followed by a thorough medical and encounter history (including description of how the bite occurred). Due to the range of Viperidae symptoms, it generally is recommended that patients remove any restrictive clothing or jewelry near the bite and/or over the affected limb or body part, place the affected body part at the level of the heart, and go to the nearest medical facility for prompt care. Historically, empiric antibiotics often were used to prevent wound infections; however, studies have since demonstrated that antibiotics are not necessary and lack efficacy in uncomplicated snake bites.16,20 In a study of 114 pediatric cases from 1995 to 2005, it was determined that most patients could be managed with conservative treatment directed at pain management and swelling reduction via elevation of the affected extremity.6 While conservative management may be all that is needed to care for the majority of cases, one retrospective study from Texas indicated that 70% of pediatric venomous snake bites were treated with either intravenous antibiotics and/or antivenin, highlighting the variability in management and opportunity for improvement.21
Antivenin, specifically antivenin (Crotalidae) polyvalent, is the indicated treatment for Viperidae hemorrhagic or coagulopathic envenomation.13,22 Per guidelines from the World Health Organization, physical examination will yield a grading of the snake bite based on cutaneous findings. Grades III to V are considered moderate to severe and should be given antivenin.23 Physicians should look for signs of progressive injury and coagulopathy, such as increased swelling, bruising, hypotension, or altered mental status.22 Due to the major neurotoxic risks associated with Elapidae venom, all coral snake bites should be treated with antivenin; early intubation and ventilation may be considered.13 Similarly, patients who report a salmosa snake bite require prompt treatment with antivenin and/or cepharanthine, an additive agent to reduce swelling and pain.18 Due to the nature of the neurotoxins contained in the salmosa venom (α-neurotoxin causing postsynaptic inhibition of the neuromuscular junction and Β-neurotoxin inhibiting neurotransmitter release from the presynaptic terminal), anticholinesterases, which work by blocking the enzymatic breakdown of the neurotransmitter acetylcholine, should not be used.19 While bleeding and skin and systemic changes may be reversed by antivenin, visual changes are unlikely to resolve with antivenin administration due to the presynaptic binding of Β-neurotoxin and the blockade of neuromuscular signaling.19
Antivenin should be administered intravenously for the fastest onset of action in a setting suitable for the management of anaphylaxis.24 In situations when the benefits may outweigh the risks (eg, if the patient has had a prior allergic reaction or is not in an environment where they can be watched for at least 8 hours for progression of envenomation or adverse reactions), premedication with an antihistamine or epinephrine may be considered.17 Per the World Allergy Organization and World Health Organization, adverse reactions should be treated with crystalloid solutions and antihistamines, corticosteroids, or epinephrine as indicated.25 In a qualitative analysis of emergency physicians’ attitudes toward antivenin, most expressed treatment hesitancy due to lack of knowledge and experience using the medication.26 When possible, snake bites should thus be managed in consultation with a toxicologist.2
Conclusion
Snake bites and envenomation occur commonly in the United States due to exposure to a variety of venomous snakes in the North American Viperidae and Elapidae families. Appropriate and successful management of snake bites by physicians requires general knowledge of regional snakes, the cutaneous and systemic manifestations of snake bites and envenomation, and current treatment methods.
- Greene SC, Folt J, Wyatt K, et al. Epidemiology of fatal snakebites in the United States 1981-2018. Am J Emerg Med. 2021;45:309-316.
- Wozniak EJ, Wisser J, Schwartz M. Venomous adversaries: a reference to snake identification, field safety, and bite-victim first aid for disaster-response personnel deploying into the hurricaneprone regions of North America. Wilderness Environ Med. 2006; 17:246-266.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2021 annual report of National Poison Data System (NPDS) from America’s Poison Centers: 39th Annual Report. Clin Toxicol (Phila). 2022;60:1381-1643.
- Chotai PN, Watlington J, Lewis S, et al. Pediatric snakebites: comparing patients in two geographic locations in the United States. J Surg Res. 2021;265:297-302.
- Johnson PN, McGoodwin L, Banner W Jr. Utilisation of Crotalidae polyvalent immune fab (ovine) for Viperidae envenomations in children. Emerg Med J. 2008;25:793-798.
- Tadros A, Sharon M, Davis S, et al. Emergency department visits by pediatric patients for snakebites. Pediatr Emerg Care. 2022; 38:279-282.
- Campbell BT, Corsi JM, Boneti C, et al. Pediatric snake bites: lessons learned from 114 cases. J Pediatr Surg. 2008;43:1338-1341.
- Peterson ME. Snake bites: coral snakes. Clin Tech Small Anim Pract. 2006;21:183-186.
- Porter KR. Herpetology. WB Saunders Company; 1972.
- Rana A, Kheora S. Grading and envenomation of the snake bite among the emergency cases in a medical college in rural India. Hmlyn Jr Appl Med Sci Res. 2021;2:33-36.
- Peterson ME. Snake bite: pit vipers. Clin Tech Small Anim Pract. 2006;21:174-182.
- Gutierrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841-850.
- Weinstein SA, Dart RC, Staples A, et al. Envenomations: an overview of clinical toxicology for the primary care physician. Am Fam Physician. 2009;80:793-802.
- Kitchens CS, Van Mierop LH. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): a study of 39 victims. JAMA. 1987;258:1615-1618.
- Morgan DL, Borys DJ, Stanford R, et al. Texas coral snake (Micrurus tener) bites. South Med J. 2007;100:152-156.
- Clark RF, Delden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993; 11:583-586.
- Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347-356.
- Hifumi T, Sakai A, Kondo Y, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015;3:16.
- Igari R, Iseki K, Abe S, et al. Binocular diplopia and ptosis due to snake bite (Agkistrodon blomhoffi “mamushi”) case report. Brain Nerve. 2010;62:273-277.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-372.
- Correa JA, Fallon SC, Cruz AT, et al. Management of pediatric snake bites: are we doing too much? J Pediatr Surg. 2014;49:1009-1015.
- Dart RC, McNally J. Efficacy, safety and use of snake antivenoms in the United States. Ann Emerg Med. 2001;47:181-188.
- World Health Organization Regional Office for South-East Asia. Guidelines for the Management of Snakebites. 2nd ed. World Health Organization; 2016.
- Clark RF, McKinney PE, Chase PB, et al. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (ovine) antivenom. Ann Emerg Med. 2002;39:671-676.
- World Health Organization. WHO Guidelines for the production, control, and regulation of snake antivenom immunoglobulins. Accessed November 25, 2024. https://extranet.who.int/prequal/vaccines/guidelines-production-control-and-regulation-snake-antivenom-immunoglobulins
- Tupetz A, Barcenas LK, Phillips AJ, et al. Bites study: a qualitive analysis among emergency medicine physicians on snake envenomation management practices. PloS One. 2022;17:E0262215.
North American venomous snakes traditionally are classified as members of either the Viperidae (eg, rattlesnakes, copperheads, cottonmouths) or Elapidae (eg, coral snakes) families and account for roughly 5000 to 10,000 reported envenomations annually.1,2 In 2021, America’s Poison Centers reported 2287 calls related to copperheads, 71 related to coral snakes, 229 related to cottonmouths, 1184 related to rattlesnakes, and 524 related to unknown snakes.3 The majority of calls related to snake bites were for adult patients, resulting in absent to minor outcomes. Only 1 death due to a rattlesnake bite was reported.3 Death by envenomation from a North American snake species is considered rare and typically is attributed to a lapse in medical attention; however, rattlesnakes are the most common reported cause of death by snake envenomation (Figure 1).1,3 A study comparing snake bites and hospital stays in the southeast vs southwest United States found that the southeast had the highest incidence of copperhead bites (37%), while the southwest had a higher incidence of rattlesnake bites (70%); those who were bitten by a rattlesnake were reported to have more severe symptoms and greater need for medical attention and antivenin.4 Some reports have linked pediatric and elderly patients to worse outcomes.5 However, one study examining 24,388 emergency department visits for snake bites from 2006 through 2014 found that the majority of pediatric cases were handled by non– trauma centers in the southern United States,6 supporting evidence found by Campbell et al7 indicating that most snake bites in children can be managed with conservative care. Though reported complications—including weakness, paralysis, hypovolemic shock, thrombocytopenia, and death—from North American venomous snake bites are low, they are still considered a medical emergency.8 It is essential for physicians to understand the clinical manifestations and treatment of North American venomous snake bites and to educate patients on how to protect themselves against and avoid provoking snakes, particularly in rural areas.2 In this article, we review the characteristics of common North American venomous snakes and the clinical manifestations of their bites. We also discuss the appropriate measures for staging, evaluating, and treating snake envenomation to improve patient management and care.

Features of North American Venomous Snakes
Individual snakes within the Viperidae family vary in size, markings and coloration, activity, and region, and physicians should consult their local health departments regarding snakes that are common in their area.2 Cottonmouth snakes are semiaquatic and traditionally are found within the southern and central United States. With a spade-shaped head and distinct two-tone coloration, cottonmouths may be mistaken for other nonvenomous water snakes in these regions (Figure 2).2 Copperheads, true to their name, are red in color; they inhabit a large portion of the southeastern United States and eastern Texas regions and are the cause of the majority of venomous snake bites in North America (Figure 3). Both cottonmouths and copperheads are believed to bite and envenomate as a defensive mechanism when provoked.


Coral snakes, found in the eastern United States and Texas regions, are the only subspecies of the Elapidae family (Figure 4).2,9 They can be distinguished from the nonvenomous milk snake by their characteristic banding, as coral snakes are patterned in a red-yellow-black band sequence and milk snakes are patterned in a red-black-yellow or white sequence. The differences in appearance of these snakes often is remembered by the phrase “red on yellow kills a fellow.”

Anatomic differences between the Viperidae and Elapidae families, including fang size, placement, and type, as well as venom composition, are directly linked to clinical manifestations of the bites. Viperidae fangs extend from the maxillary bones and are mobile, long, and hollow, making it easy for the snake to control fang movement and envenomation.9 Viperidae snakes are uniquely capable of inflicting puncture wounds without the injection of venom, known as dry bites. In contrast, Elapidae snakes have short, hollow, and fixed fangs, and thus patients can protect themselves by wearing appropriate clothing and covered footwear.9 Currently, identifying the type of snake responsible for the bite relies on visualization of the snake and/or the identification of clinical symptoms of envenomation by a dermatologist.
Clinical Manifestations of Venomous Snake Bites
Clinical manifestations and cutaneous findings often are used to grade the severity of venomous snake bites as well as to dictate treatment procedures. Grade 0 indicates a bite has occurred without envenomation, while grades I to V describe the progression and severity of envenomation.10 Grade I describes minimal erythema and edema around the site (fang marks may or may not be present) and no systemic symptoms. Grade II describes erythema and edema extending up the extremity to the first joint (eg, hand to wrist), pain, some systemic symptoms if there is rapid progression, and potential bleeding at the site. Grade III describes erythema and edema spreading to the second joint in the extremity, pain, and systemic symptoms, including coagulation defects. Grade IV describes erythema and edema of the whole extremity, a rapid reaction and progression following the bite, and risk for compartment syndrome. Grade V includes erythema and edema beyond the extremity and increasing systemic symptoms.10
Local pain and edema, usually on easily accessible or exposed extremities, are the most common clinical symptoms reported following a Viperidae snake bite.11 Due to their capability of producing a dry bite, puncture markings alone do not indicate envenomation. Patients will need to be monitored for several hours for signs of envenomation, which may include swelling, pain, ecchymosis, and indications of systemic manifestation (eg, weakness, dizziness, nausea, severe hypotension, thrombocytopenia).11 Viperidae venom hemorrhagic metalloproteinases act on capillary blood vessels by cleaving basement membrane proteins and allowing for extravasation of fluid into local tissue.12 The inflammatory response produced at the site of envenomation likely is due to the release of tumor necrosis factor á and endogenous matrix metalloprotein.12 There is a higher risk for death associated with bites from rattlesnakes within the Viperidae family because their venom contains a unique neurotoxin that works by blocking presynaptic junctions and causing a range of paralytic symptoms from ptosis to respiratory failure.13
The severity of Elapidae bites is thought to be related to the amount of venom injected, the size of the victim, and the length of the snake. Though clothing may offer protection, envenomation occurs in 75% of coral snake bites and can produce devastating consequences due to the venom content.14 In a retrospective study between 2002 and 2004, 90% of Elapidae snake bite patients (n=82) reported local pain, redness, and paresthesia, while around 7% developed systemic symptoms.15 Elapidae venom primarily is neurotoxic and is thought to spread via lymphatics.16 Delayed reactions are common and may take up to 12 hours to develop. Patients should be monitored, as local reactions may progress to weakness, fasciculations, extremity paralysis, and lastly, respiratory paralysis. Due to the risk for progression, all patients with likely coral snake bites should be given antivenin.8,15,17
Much like the North American coral snake, the venomous snake species Gloydius blomhoffii—referred to as the salmosa or mamushi snake depending on the region of origin (ie, Korea or Japan)—is a frequent source of devastating rural snake bites due to neurotoxins (Figure 5). The species’ slender fangs are thought to directly inject the snake’s potent venom, which contains hemorrhagic toxins and α-neurotoxins and Β-neurotoxins, into the bloodstream; however, the salmosa is considered a viper like the North American cottonmouth and copperhead because of its triangular head shape and hollow fangs, which allow for the accommodation of venom-containing glands and mechanism of venom injection. Salmosa venom shares both Viperidae and Elapidae characteristics. Cutaneous findings such as progressive edema, erythema, and bleeding frequently are reported and are attributed to the proteases and hemorrhagic toxins characteristic of vipers (Figure 6). α-Neurotoxins and Β-neurotoxins, similar to the proteolytic venom of the Elapidae family, are responsible for the unique visual disturbances (binocular diplopia) caused by the salmosa.12,18,19


Treatment
Treating snake bites begins with assessing the patient’s airway, breathing, and circulation, followed by a thorough medical and encounter history (including description of how the bite occurred). Due to the range of Viperidae symptoms, it generally is recommended that patients remove any restrictive clothing or jewelry near the bite and/or over the affected limb or body part, place the affected body part at the level of the heart, and go to the nearest medical facility for prompt care. Historically, empiric antibiotics often were used to prevent wound infections; however, studies have since demonstrated that antibiotics are not necessary and lack efficacy in uncomplicated snake bites.16,20 In a study of 114 pediatric cases from 1995 to 2005, it was determined that most patients could be managed with conservative treatment directed at pain management and swelling reduction via elevation of the affected extremity.6 While conservative management may be all that is needed to care for the majority of cases, one retrospective study from Texas indicated that 70% of pediatric venomous snake bites were treated with either intravenous antibiotics and/or antivenin, highlighting the variability in management and opportunity for improvement.21
Antivenin, specifically antivenin (Crotalidae) polyvalent, is the indicated treatment for Viperidae hemorrhagic or coagulopathic envenomation.13,22 Per guidelines from the World Health Organization, physical examination will yield a grading of the snake bite based on cutaneous findings. Grades III to V are considered moderate to severe and should be given antivenin.23 Physicians should look for signs of progressive injury and coagulopathy, such as increased swelling, bruising, hypotension, or altered mental status.22 Due to the major neurotoxic risks associated with Elapidae venom, all coral snake bites should be treated with antivenin; early intubation and ventilation may be considered.13 Similarly, patients who report a salmosa snake bite require prompt treatment with antivenin and/or cepharanthine, an additive agent to reduce swelling and pain.18 Due to the nature of the neurotoxins contained in the salmosa venom (α-neurotoxin causing postsynaptic inhibition of the neuromuscular junction and Β-neurotoxin inhibiting neurotransmitter release from the presynaptic terminal), anticholinesterases, which work by blocking the enzymatic breakdown of the neurotransmitter acetylcholine, should not be used.19 While bleeding and skin and systemic changes may be reversed by antivenin, visual changes are unlikely to resolve with antivenin administration due to the presynaptic binding of Β-neurotoxin and the blockade of neuromuscular signaling.19
Antivenin should be administered intravenously for the fastest onset of action in a setting suitable for the management of anaphylaxis.24 In situations when the benefits may outweigh the risks (eg, if the patient has had a prior allergic reaction or is not in an environment where they can be watched for at least 8 hours for progression of envenomation or adverse reactions), premedication with an antihistamine or epinephrine may be considered.17 Per the World Allergy Organization and World Health Organization, adverse reactions should be treated with crystalloid solutions and antihistamines, corticosteroids, or epinephrine as indicated.25 In a qualitative analysis of emergency physicians’ attitudes toward antivenin, most expressed treatment hesitancy due to lack of knowledge and experience using the medication.26 When possible, snake bites should thus be managed in consultation with a toxicologist.2
Conclusion
Snake bites and envenomation occur commonly in the United States due to exposure to a variety of venomous snakes in the North American Viperidae and Elapidae families. Appropriate and successful management of snake bites by physicians requires general knowledge of regional snakes, the cutaneous and systemic manifestations of snake bites and envenomation, and current treatment methods.
North American venomous snakes traditionally are classified as members of either the Viperidae (eg, rattlesnakes, copperheads, cottonmouths) or Elapidae (eg, coral snakes) families and account for roughly 5000 to 10,000 reported envenomations annually.1,2 In 2021, America’s Poison Centers reported 2287 calls related to copperheads, 71 related to coral snakes, 229 related to cottonmouths, 1184 related to rattlesnakes, and 524 related to unknown snakes.3 The majority of calls related to snake bites were for adult patients, resulting in absent to minor outcomes. Only 1 death due to a rattlesnake bite was reported.3 Death by envenomation from a North American snake species is considered rare and typically is attributed to a lapse in medical attention; however, rattlesnakes are the most common reported cause of death by snake envenomation (Figure 1).1,3 A study comparing snake bites and hospital stays in the southeast vs southwest United States found that the southeast had the highest incidence of copperhead bites (37%), while the southwest had a higher incidence of rattlesnake bites (70%); those who were bitten by a rattlesnake were reported to have more severe symptoms and greater need for medical attention and antivenin.4 Some reports have linked pediatric and elderly patients to worse outcomes.5 However, one study examining 24,388 emergency department visits for snake bites from 2006 through 2014 found that the majority of pediatric cases were handled by non– trauma centers in the southern United States,6 supporting evidence found by Campbell et al7 indicating that most snake bites in children can be managed with conservative care. Though reported complications—including weakness, paralysis, hypovolemic shock, thrombocytopenia, and death—from North American venomous snake bites are low, they are still considered a medical emergency.8 It is essential for physicians to understand the clinical manifestations and treatment of North American venomous snake bites and to educate patients on how to protect themselves against and avoid provoking snakes, particularly in rural areas.2 In this article, we review the characteristics of common North American venomous snakes and the clinical manifestations of their bites. We also discuss the appropriate measures for staging, evaluating, and treating snake envenomation to improve patient management and care.

Features of North American Venomous Snakes
Individual snakes within the Viperidae family vary in size, markings and coloration, activity, and region, and physicians should consult their local health departments regarding snakes that are common in their area.2 Cottonmouth snakes are semiaquatic and traditionally are found within the southern and central United States. With a spade-shaped head and distinct two-tone coloration, cottonmouths may be mistaken for other nonvenomous water snakes in these regions (Figure 2).2 Copperheads, true to their name, are red in color; they inhabit a large portion of the southeastern United States and eastern Texas regions and are the cause of the majority of venomous snake bites in North America (Figure 3). Both cottonmouths and copperheads are believed to bite and envenomate as a defensive mechanism when provoked.


Coral snakes, found in the eastern United States and Texas regions, are the only subspecies of the Elapidae family (Figure 4).2,9 They can be distinguished from the nonvenomous milk snake by their characteristic banding, as coral snakes are patterned in a red-yellow-black band sequence and milk snakes are patterned in a red-black-yellow or white sequence. The differences in appearance of these snakes often is remembered by the phrase “red on yellow kills a fellow.”

Anatomic differences between the Viperidae and Elapidae families, including fang size, placement, and type, as well as venom composition, are directly linked to clinical manifestations of the bites. Viperidae fangs extend from the maxillary bones and are mobile, long, and hollow, making it easy for the snake to control fang movement and envenomation.9 Viperidae snakes are uniquely capable of inflicting puncture wounds without the injection of venom, known as dry bites. In contrast, Elapidae snakes have short, hollow, and fixed fangs, and thus patients can protect themselves by wearing appropriate clothing and covered footwear.9 Currently, identifying the type of snake responsible for the bite relies on visualization of the snake and/or the identification of clinical symptoms of envenomation by a dermatologist.
Clinical Manifestations of Venomous Snake Bites
Clinical manifestations and cutaneous findings often are used to grade the severity of venomous snake bites as well as to dictate treatment procedures. Grade 0 indicates a bite has occurred without envenomation, while grades I to V describe the progression and severity of envenomation.10 Grade I describes minimal erythema and edema around the site (fang marks may or may not be present) and no systemic symptoms. Grade II describes erythema and edema extending up the extremity to the first joint (eg, hand to wrist), pain, some systemic symptoms if there is rapid progression, and potential bleeding at the site. Grade III describes erythema and edema spreading to the second joint in the extremity, pain, and systemic symptoms, including coagulation defects. Grade IV describes erythema and edema of the whole extremity, a rapid reaction and progression following the bite, and risk for compartment syndrome. Grade V includes erythema and edema beyond the extremity and increasing systemic symptoms.10
Local pain and edema, usually on easily accessible or exposed extremities, are the most common clinical symptoms reported following a Viperidae snake bite.11 Due to their capability of producing a dry bite, puncture markings alone do not indicate envenomation. Patients will need to be monitored for several hours for signs of envenomation, which may include swelling, pain, ecchymosis, and indications of systemic manifestation (eg, weakness, dizziness, nausea, severe hypotension, thrombocytopenia).11 Viperidae venom hemorrhagic metalloproteinases act on capillary blood vessels by cleaving basement membrane proteins and allowing for extravasation of fluid into local tissue.12 The inflammatory response produced at the site of envenomation likely is due to the release of tumor necrosis factor á and endogenous matrix metalloprotein.12 There is a higher risk for death associated with bites from rattlesnakes within the Viperidae family because their venom contains a unique neurotoxin that works by blocking presynaptic junctions and causing a range of paralytic symptoms from ptosis to respiratory failure.13
The severity of Elapidae bites is thought to be related to the amount of venom injected, the size of the victim, and the length of the snake. Though clothing may offer protection, envenomation occurs in 75% of coral snake bites and can produce devastating consequences due to the venom content.14 In a retrospective study between 2002 and 2004, 90% of Elapidae snake bite patients (n=82) reported local pain, redness, and paresthesia, while around 7% developed systemic symptoms.15 Elapidae venom primarily is neurotoxic and is thought to spread via lymphatics.16 Delayed reactions are common and may take up to 12 hours to develop. Patients should be monitored, as local reactions may progress to weakness, fasciculations, extremity paralysis, and lastly, respiratory paralysis. Due to the risk for progression, all patients with likely coral snake bites should be given antivenin.8,15,17
Much like the North American coral snake, the venomous snake species Gloydius blomhoffii—referred to as the salmosa or mamushi snake depending on the region of origin (ie, Korea or Japan)—is a frequent source of devastating rural snake bites due to neurotoxins (Figure 5). The species’ slender fangs are thought to directly inject the snake’s potent venom, which contains hemorrhagic toxins and α-neurotoxins and Β-neurotoxins, into the bloodstream; however, the salmosa is considered a viper like the North American cottonmouth and copperhead because of its triangular head shape and hollow fangs, which allow for the accommodation of venom-containing glands and mechanism of venom injection. Salmosa venom shares both Viperidae and Elapidae characteristics. Cutaneous findings such as progressive edema, erythema, and bleeding frequently are reported and are attributed to the proteases and hemorrhagic toxins characteristic of vipers (Figure 6). α-Neurotoxins and Β-neurotoxins, similar to the proteolytic venom of the Elapidae family, are responsible for the unique visual disturbances (binocular diplopia) caused by the salmosa.12,18,19


Treatment
Treating snake bites begins with assessing the patient’s airway, breathing, and circulation, followed by a thorough medical and encounter history (including description of how the bite occurred). Due to the range of Viperidae symptoms, it generally is recommended that patients remove any restrictive clothing or jewelry near the bite and/or over the affected limb or body part, place the affected body part at the level of the heart, and go to the nearest medical facility for prompt care. Historically, empiric antibiotics often were used to prevent wound infections; however, studies have since demonstrated that antibiotics are not necessary and lack efficacy in uncomplicated snake bites.16,20 In a study of 114 pediatric cases from 1995 to 2005, it was determined that most patients could be managed with conservative treatment directed at pain management and swelling reduction via elevation of the affected extremity.6 While conservative management may be all that is needed to care for the majority of cases, one retrospective study from Texas indicated that 70% of pediatric venomous snake bites were treated with either intravenous antibiotics and/or antivenin, highlighting the variability in management and opportunity for improvement.21
Antivenin, specifically antivenin (Crotalidae) polyvalent, is the indicated treatment for Viperidae hemorrhagic or coagulopathic envenomation.13,22 Per guidelines from the World Health Organization, physical examination will yield a grading of the snake bite based on cutaneous findings. Grades III to V are considered moderate to severe and should be given antivenin.23 Physicians should look for signs of progressive injury and coagulopathy, such as increased swelling, bruising, hypotension, or altered mental status.22 Due to the major neurotoxic risks associated with Elapidae venom, all coral snake bites should be treated with antivenin; early intubation and ventilation may be considered.13 Similarly, patients who report a salmosa snake bite require prompt treatment with antivenin and/or cepharanthine, an additive agent to reduce swelling and pain.18 Due to the nature of the neurotoxins contained in the salmosa venom (α-neurotoxin causing postsynaptic inhibition of the neuromuscular junction and Β-neurotoxin inhibiting neurotransmitter release from the presynaptic terminal), anticholinesterases, which work by blocking the enzymatic breakdown of the neurotransmitter acetylcholine, should not be used.19 While bleeding and skin and systemic changes may be reversed by antivenin, visual changes are unlikely to resolve with antivenin administration due to the presynaptic binding of Β-neurotoxin and the blockade of neuromuscular signaling.19
Antivenin should be administered intravenously for the fastest onset of action in a setting suitable for the management of anaphylaxis.24 In situations when the benefits may outweigh the risks (eg, if the patient has had a prior allergic reaction or is not in an environment where they can be watched for at least 8 hours for progression of envenomation or adverse reactions), premedication with an antihistamine or epinephrine may be considered.17 Per the World Allergy Organization and World Health Organization, adverse reactions should be treated with crystalloid solutions and antihistamines, corticosteroids, or epinephrine as indicated.25 In a qualitative analysis of emergency physicians’ attitudes toward antivenin, most expressed treatment hesitancy due to lack of knowledge and experience using the medication.26 When possible, snake bites should thus be managed in consultation with a toxicologist.2
Conclusion
Snake bites and envenomation occur commonly in the United States due to exposure to a variety of venomous snakes in the North American Viperidae and Elapidae families. Appropriate and successful management of snake bites by physicians requires general knowledge of regional snakes, the cutaneous and systemic manifestations of snake bites and envenomation, and current treatment methods.
- Greene SC, Folt J, Wyatt K, et al. Epidemiology of fatal snakebites in the United States 1981-2018. Am J Emerg Med. 2021;45:309-316.
- Wozniak EJ, Wisser J, Schwartz M. Venomous adversaries: a reference to snake identification, field safety, and bite-victim first aid for disaster-response personnel deploying into the hurricaneprone regions of North America. Wilderness Environ Med. 2006; 17:246-266.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2021 annual report of National Poison Data System (NPDS) from America’s Poison Centers: 39th Annual Report. Clin Toxicol (Phila). 2022;60:1381-1643.
- Chotai PN, Watlington J, Lewis S, et al. Pediatric snakebites: comparing patients in two geographic locations in the United States. J Surg Res. 2021;265:297-302.
- Johnson PN, McGoodwin L, Banner W Jr. Utilisation of Crotalidae polyvalent immune fab (ovine) for Viperidae envenomations in children. Emerg Med J. 2008;25:793-798.
- Tadros A, Sharon M, Davis S, et al. Emergency department visits by pediatric patients for snakebites. Pediatr Emerg Care. 2022; 38:279-282.
- Campbell BT, Corsi JM, Boneti C, et al. Pediatric snake bites: lessons learned from 114 cases. J Pediatr Surg. 2008;43:1338-1341.
- Peterson ME. Snake bites: coral snakes. Clin Tech Small Anim Pract. 2006;21:183-186.
- Porter KR. Herpetology. WB Saunders Company; 1972.
- Rana A, Kheora S. Grading and envenomation of the snake bite among the emergency cases in a medical college in rural India. Hmlyn Jr Appl Med Sci Res. 2021;2:33-36.
- Peterson ME. Snake bite: pit vipers. Clin Tech Small Anim Pract. 2006;21:174-182.
- Gutierrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841-850.
- Weinstein SA, Dart RC, Staples A, et al. Envenomations: an overview of clinical toxicology for the primary care physician. Am Fam Physician. 2009;80:793-802.
- Kitchens CS, Van Mierop LH. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): a study of 39 victims. JAMA. 1987;258:1615-1618.
- Morgan DL, Borys DJ, Stanford R, et al. Texas coral snake (Micrurus tener) bites. South Med J. 2007;100:152-156.
- Clark RF, Delden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993; 11:583-586.
- Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347-356.
- Hifumi T, Sakai A, Kondo Y, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015;3:16.
- Igari R, Iseki K, Abe S, et al. Binocular diplopia and ptosis due to snake bite (Agkistrodon blomhoffi “mamushi”) case report. Brain Nerve. 2010;62:273-277.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-372.
- Correa JA, Fallon SC, Cruz AT, et al. Management of pediatric snake bites: are we doing too much? J Pediatr Surg. 2014;49:1009-1015.
- Dart RC, McNally J. Efficacy, safety and use of snake antivenoms in the United States. Ann Emerg Med. 2001;47:181-188.
- World Health Organization Regional Office for South-East Asia. Guidelines for the Management of Snakebites. 2nd ed. World Health Organization; 2016.
- Clark RF, McKinney PE, Chase PB, et al. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (ovine) antivenom. Ann Emerg Med. 2002;39:671-676.
- World Health Organization. WHO Guidelines for the production, control, and regulation of snake antivenom immunoglobulins. Accessed November 25, 2024. https://extranet.who.int/prequal/vaccines/guidelines-production-control-and-regulation-snake-antivenom-immunoglobulins
- Tupetz A, Barcenas LK, Phillips AJ, et al. Bites study: a qualitive analysis among emergency medicine physicians on snake envenomation management practices. PloS One. 2022;17:E0262215.
- Greene SC, Folt J, Wyatt K, et al. Epidemiology of fatal snakebites in the United States 1981-2018. Am J Emerg Med. 2021;45:309-316.
- Wozniak EJ, Wisser J, Schwartz M. Venomous adversaries: a reference to snake identification, field safety, and bite-victim first aid for disaster-response personnel deploying into the hurricaneprone regions of North America. Wilderness Environ Med. 2006; 17:246-266.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2021 annual report of National Poison Data System (NPDS) from America’s Poison Centers: 39th Annual Report. Clin Toxicol (Phila). 2022;60:1381-1643.
- Chotai PN, Watlington J, Lewis S, et al. Pediatric snakebites: comparing patients in two geographic locations in the United States. J Surg Res. 2021;265:297-302.
- Johnson PN, McGoodwin L, Banner W Jr. Utilisation of Crotalidae polyvalent immune fab (ovine) for Viperidae envenomations in children. Emerg Med J. 2008;25:793-798.
- Tadros A, Sharon M, Davis S, et al. Emergency department visits by pediatric patients for snakebites. Pediatr Emerg Care. 2022; 38:279-282.
- Campbell BT, Corsi JM, Boneti C, et al. Pediatric snake bites: lessons learned from 114 cases. J Pediatr Surg. 2008;43:1338-1341.
- Peterson ME. Snake bites: coral snakes. Clin Tech Small Anim Pract. 2006;21:183-186.
- Porter KR. Herpetology. WB Saunders Company; 1972.
- Rana A, Kheora S. Grading and envenomation of the snake bite among the emergency cases in a medical college in rural India. Hmlyn Jr Appl Med Sci Res. 2021;2:33-36.
- Peterson ME. Snake bite: pit vipers. Clin Tech Small Anim Pract. 2006;21:174-182.
- Gutierrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841-850.
- Weinstein SA, Dart RC, Staples A, et al. Envenomations: an overview of clinical toxicology for the primary care physician. Am Fam Physician. 2009;80:793-802.
- Kitchens CS, Van Mierop LH. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): a study of 39 victims. JAMA. 1987;258:1615-1618.
- Morgan DL, Borys DJ, Stanford R, et al. Texas coral snake (Micrurus tener) bites. South Med J. 2007;100:152-156.
- Clark RF, Delden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993; 11:583-586.
- Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347-356.
- Hifumi T, Sakai A, Kondo Y, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015;3:16.
- Igari R, Iseki K, Abe S, et al. Binocular diplopia and ptosis due to snake bite (Agkistrodon blomhoffi “mamushi”) case report. Brain Nerve. 2010;62:273-277.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-372.
- Correa JA, Fallon SC, Cruz AT, et al. Management of pediatric snake bites: are we doing too much? J Pediatr Surg. 2014;49:1009-1015.
- Dart RC, McNally J. Efficacy, safety and use of snake antivenoms in the United States. Ann Emerg Med. 2001;47:181-188.
- World Health Organization Regional Office for South-East Asia. Guidelines for the Management of Snakebites. 2nd ed. World Health Organization; 2016.
- Clark RF, McKinney PE, Chase PB, et al. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (ovine) antivenom. Ann Emerg Med. 2002;39:671-676.
- World Health Organization. WHO Guidelines for the production, control, and regulation of snake antivenom immunoglobulins. Accessed November 25, 2024. https://extranet.who.int/prequal/vaccines/guidelines-production-control-and-regulation-snake-antivenom-immunoglobulins
- Tupetz A, Barcenas LK, Phillips AJ, et al. Bites study: a qualitive analysis among emergency medicine physicians on snake envenomation management practices. PloS One. 2022;17:E0262215.
Key Features of North American Venomous Snake Bites
Key Features of North American Venomous Snake Bites
PRACTICE POINTS
- Venomous snake bites require prompt medical attention and assessment of symptoms to determine the optimal course of management and need for antivenin.
- Envenomation may cause may cause discoloration and swelling of the skin as well as thrombotic or paralytic changes.
Erythematous Annular Scaly Plaques on the Upper Chest
Erythematous Annular Scaly Plaques on the Upper Chest
THE DIAGNOSIS: Tinea Corporis
Due to the scaly and acute nature of the rash, a potassium hydroxide (KOH) preparation was performed, and hyphal elements were floridly present. After further questioning, the patient reported finding a stray kitten a few weeks before the onset of the eruption and shared a picture of it lying on her chest in the area corresponding with the main distribution of the rash (Figure). Based on the patient’s personal history and the positive KOH preparation, a diagnosis of tinea corporis was made. She was immediately started on fluconazole 300 mg once weekly for 4 weeks and naftifine gel 1%, which she used for 6 to 8 weeks with complete resolution of the eruption.

Tinea corporis is a dermatophyte infection that typically affects exposed areas of the skin such as the chest, arms, and legs. Spread via human-to-human contact, Trichophyton rubrum is the most common cause worldwide. The second most common is Trichophyton mentagrophytes, which is spread through animal-to-human contact.1,2
Symptoms of tinea corporis usually appear 1 to 3 weeks after exposure and manifest as itchy scaly papules that spread outward, forming annular, circinate, and petaloid erythematous plaques with central clearing. The condition most commonly is diagnosed through the examination of scale from the affected area using a KOH preparation, which will reveal hyphae when positive.2-4 Cultures are the gold standard for identifying dermatophyte species,5 but results can take several weeks. Biopsy also can confirm the diagnosis by showing the presence of hyphae in the stratum corneum, which can be highlighted using periodic acid–Schiff or silver stains.3
Topical antifungals are the first-line treatment for cutaneous dermatophyte infections.3-5 The most effective topical therapies are allylamines and azoles, which work by inhibiting the growth of the fungus. Allylamines are more effective than azoles due to their fungicidal properties and ability to penetrate the skin more effectively.6,7 Topical medications should be applied at least 2 cm beyond the infected area for 2 to 4 weeks or until the infection has cleared.3 Systemic antifungals may be necessary in more complicated cases.
It is important to consider a broad differential and take into consideration the distribution of the plaques, the patient’s history, and other clinical features when differentiating tinea corporis from other conditions. Erythema annulare centrifugum more often presents as nonpruritic annular plaques with a trailing scale instead of a leading scale seen in tinea corporis. Biopsy exhibits a dense, perivascular, lymphocytic infiltrate in superficial vessels, resembling a coat sleeve.3,8 Pemphigus foliaceous can manifest with painful crusted scaly plaques and vesicles in a seborrheic distribution. Biopsy reveals subcorneal acantholytic vesicles and can be confirmed on direct immunofluorescence.3,8 Subacute cutaneous lupus erythematosus presents with annular plaques that often are symmetric and most prominent in sun-exposed areas, sparing the face.3,9,10 It can be associated with other autoimmune conditions as well as medications such as thiazides, terbinafine, and calcium channel blockers. Additionally, 76% to 90% of patients are Ro/SSA antibody positive.3 Biopsy often demonstrates follicular plugging, perivascular and periadnexal lymphocytic infiltrates, and mucin.3,10 Lastly, pityriasis rosea typically begins with a herald patch, followed by a widespread rash that often appears in a Christmas tree distribution.3
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51 (suppl 4):2-15. doi: 10.1111 /j.1439-0507.2008.01606.x
- Yee G, Al Aboud AM. Tinea corporis. 2022 Aug 8. In: StatPearls [Internet]. StatPearls Publishing; 2023
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Elsevier; 2018.
- Diseases resulting from fungal and yeast. In: James WD, Berger TG, Elston DM, et al, eds. Andrews’ Diseases of The Skin: Clinical Dermatology. 12th ed. Elsevier; 2016: 289-290.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6 . doi:10.7573/dic.2020-5-6
- El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;2014:CD009992. doi:10.1002/14651858 .CD009992.pub2
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2018.
- Burgdorf W. Erythema annulare centrifugum and other figurate erythemas. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill; 2008: 366-368.
- Modi GM, Maender JL, Coleman N, et al. Tinea corporis masquerading as subacute cutaneous lupus erythematosus. Dermatol Online J. 2008;14:8.
- Stavropoulos PG, Goules AV, Avgerinou G, et al. Pathogenesis of subacute cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2008;22:1281.
THE DIAGNOSIS: Tinea Corporis
Due to the scaly and acute nature of the rash, a potassium hydroxide (KOH) preparation was performed, and hyphal elements were floridly present. After further questioning, the patient reported finding a stray kitten a few weeks before the onset of the eruption and shared a picture of it lying on her chest in the area corresponding with the main distribution of the rash (Figure). Based on the patient’s personal history and the positive KOH preparation, a diagnosis of tinea corporis was made. She was immediately started on fluconazole 300 mg once weekly for 4 weeks and naftifine gel 1%, which she used for 6 to 8 weeks with complete resolution of the eruption.

Tinea corporis is a dermatophyte infection that typically affects exposed areas of the skin such as the chest, arms, and legs. Spread via human-to-human contact, Trichophyton rubrum is the most common cause worldwide. The second most common is Trichophyton mentagrophytes, which is spread through animal-to-human contact.1,2
Symptoms of tinea corporis usually appear 1 to 3 weeks after exposure and manifest as itchy scaly papules that spread outward, forming annular, circinate, and petaloid erythematous plaques with central clearing. The condition most commonly is diagnosed through the examination of scale from the affected area using a KOH preparation, which will reveal hyphae when positive.2-4 Cultures are the gold standard for identifying dermatophyte species,5 but results can take several weeks. Biopsy also can confirm the diagnosis by showing the presence of hyphae in the stratum corneum, which can be highlighted using periodic acid–Schiff or silver stains.3
Topical antifungals are the first-line treatment for cutaneous dermatophyte infections.3-5 The most effective topical therapies are allylamines and azoles, which work by inhibiting the growth of the fungus. Allylamines are more effective than azoles due to their fungicidal properties and ability to penetrate the skin more effectively.6,7 Topical medications should be applied at least 2 cm beyond the infected area for 2 to 4 weeks or until the infection has cleared.3 Systemic antifungals may be necessary in more complicated cases.
It is important to consider a broad differential and take into consideration the distribution of the plaques, the patient’s history, and other clinical features when differentiating tinea corporis from other conditions. Erythema annulare centrifugum more often presents as nonpruritic annular plaques with a trailing scale instead of a leading scale seen in tinea corporis. Biopsy exhibits a dense, perivascular, lymphocytic infiltrate in superficial vessels, resembling a coat sleeve.3,8 Pemphigus foliaceous can manifest with painful crusted scaly plaques and vesicles in a seborrheic distribution. Biopsy reveals subcorneal acantholytic vesicles and can be confirmed on direct immunofluorescence.3,8 Subacute cutaneous lupus erythematosus presents with annular plaques that often are symmetric and most prominent in sun-exposed areas, sparing the face.3,9,10 It can be associated with other autoimmune conditions as well as medications such as thiazides, terbinafine, and calcium channel blockers. Additionally, 76% to 90% of patients are Ro/SSA antibody positive.3 Biopsy often demonstrates follicular plugging, perivascular and periadnexal lymphocytic infiltrates, and mucin.3,10 Lastly, pityriasis rosea typically begins with a herald patch, followed by a widespread rash that often appears in a Christmas tree distribution.3
THE DIAGNOSIS: Tinea Corporis
Due to the scaly and acute nature of the rash, a potassium hydroxide (KOH) preparation was performed, and hyphal elements were floridly present. After further questioning, the patient reported finding a stray kitten a few weeks before the onset of the eruption and shared a picture of it lying on her chest in the area corresponding with the main distribution of the rash (Figure). Based on the patient’s personal history and the positive KOH preparation, a diagnosis of tinea corporis was made. She was immediately started on fluconazole 300 mg once weekly for 4 weeks and naftifine gel 1%, which she used for 6 to 8 weeks with complete resolution of the eruption.

Tinea corporis is a dermatophyte infection that typically affects exposed areas of the skin such as the chest, arms, and legs. Spread via human-to-human contact, Trichophyton rubrum is the most common cause worldwide. The second most common is Trichophyton mentagrophytes, which is spread through animal-to-human contact.1,2
Symptoms of tinea corporis usually appear 1 to 3 weeks after exposure and manifest as itchy scaly papules that spread outward, forming annular, circinate, and petaloid erythematous plaques with central clearing. The condition most commonly is diagnosed through the examination of scale from the affected area using a KOH preparation, which will reveal hyphae when positive.2-4 Cultures are the gold standard for identifying dermatophyte species,5 but results can take several weeks. Biopsy also can confirm the diagnosis by showing the presence of hyphae in the stratum corneum, which can be highlighted using periodic acid–Schiff or silver stains.3
Topical antifungals are the first-line treatment for cutaneous dermatophyte infections.3-5 The most effective topical therapies are allylamines and azoles, which work by inhibiting the growth of the fungus. Allylamines are more effective than azoles due to their fungicidal properties and ability to penetrate the skin more effectively.6,7 Topical medications should be applied at least 2 cm beyond the infected area for 2 to 4 weeks or until the infection has cleared.3 Systemic antifungals may be necessary in more complicated cases.
It is important to consider a broad differential and take into consideration the distribution of the plaques, the patient’s history, and other clinical features when differentiating tinea corporis from other conditions. Erythema annulare centrifugum more often presents as nonpruritic annular plaques with a trailing scale instead of a leading scale seen in tinea corporis. Biopsy exhibits a dense, perivascular, lymphocytic infiltrate in superficial vessels, resembling a coat sleeve.3,8 Pemphigus foliaceous can manifest with painful crusted scaly plaques and vesicles in a seborrheic distribution. Biopsy reveals subcorneal acantholytic vesicles and can be confirmed on direct immunofluorescence.3,8 Subacute cutaneous lupus erythematosus presents with annular plaques that often are symmetric and most prominent in sun-exposed areas, sparing the face.3,9,10 It can be associated with other autoimmune conditions as well as medications such as thiazides, terbinafine, and calcium channel blockers. Additionally, 76% to 90% of patients are Ro/SSA antibody positive.3 Biopsy often demonstrates follicular plugging, perivascular and periadnexal lymphocytic infiltrates, and mucin.3,10 Lastly, pityriasis rosea typically begins with a herald patch, followed by a widespread rash that often appears in a Christmas tree distribution.3
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51 (suppl 4):2-15. doi: 10.1111 /j.1439-0507.2008.01606.x
- Yee G, Al Aboud AM. Tinea corporis. 2022 Aug 8. In: StatPearls [Internet]. StatPearls Publishing; 2023
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Elsevier; 2018.
- Diseases resulting from fungal and yeast. In: James WD, Berger TG, Elston DM, et al, eds. Andrews’ Diseases of The Skin: Clinical Dermatology. 12th ed. Elsevier; 2016: 289-290.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6 . doi:10.7573/dic.2020-5-6
- El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;2014:CD009992. doi:10.1002/14651858 .CD009992.pub2
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2018.
- Burgdorf W. Erythema annulare centrifugum and other figurate erythemas. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill; 2008: 366-368.
- Modi GM, Maender JL, Coleman N, et al. Tinea corporis masquerading as subacute cutaneous lupus erythematosus. Dermatol Online J. 2008;14:8.
- Stavropoulos PG, Goules AV, Avgerinou G, et al. Pathogenesis of subacute cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2008;22:1281.
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51 (suppl 4):2-15. doi: 10.1111 /j.1439-0507.2008.01606.x
- Yee G, Al Aboud AM. Tinea corporis. 2022 Aug 8. In: StatPearls [Internet]. StatPearls Publishing; 2023
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Elsevier; 2018.
- Diseases resulting from fungal and yeast. In: James WD, Berger TG, Elston DM, et al, eds. Andrews’ Diseases of The Skin: Clinical Dermatology. 12th ed. Elsevier; 2016: 289-290.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6 . doi:10.7573/dic.2020-5-6
- El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;2014:CD009992. doi:10.1002/14651858 .CD009992.pub2
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2018.
- Burgdorf W. Erythema annulare centrifugum and other figurate erythemas. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill; 2008: 366-368.
- Modi GM, Maender JL, Coleman N, et al. Tinea corporis masquerading as subacute cutaneous lupus erythematosus. Dermatol Online J. 2008;14:8.
- Stavropoulos PG, Goules AV, Avgerinou G, et al. Pathogenesis of subacute cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2008;22:1281.
Erythematous Annular Scaly Plaques on the Upper Chest
Erythematous Annular Scaly Plaques on the Upper Chest
A 60-year-old woman with a history of keratinocyte carcinomas, hypertension, diabetes mellitus, and anxiety presented to the dermatology department with a widespread rash of more than 2 weeks’ duration. The patient had tried 1 to 2 days of self-treatment with triamcinolone cream that she had previously been prescribed for an unknown dermatitis and zinc oxide cream, which caused considerable inflammation of the rash and prompted her to discontinue use. She could not recall any recent use of new personal care products or medications or eating any new foods. She also denied any recent yard work, known arthropod bites, illnesses, prolonged sun exposure, or constitutional symptoms. Her medications included metformin, hydrochlorothiazide, losartan, and sertraline. She also reported taking daily supplements of vitamins D, K, and C as well as acetaminophen and ibuprofen as needed. Physical examination revealed several 2- to 4-cm, erythematous, annular, circinate, petaloid plaques with scale mostly on photodistributed areas of the central anterior chest, neck, lower cheeks, and chin as well as a few scattered lesions with similar morphology on the arms, lower abdomen, left buttock, and back.
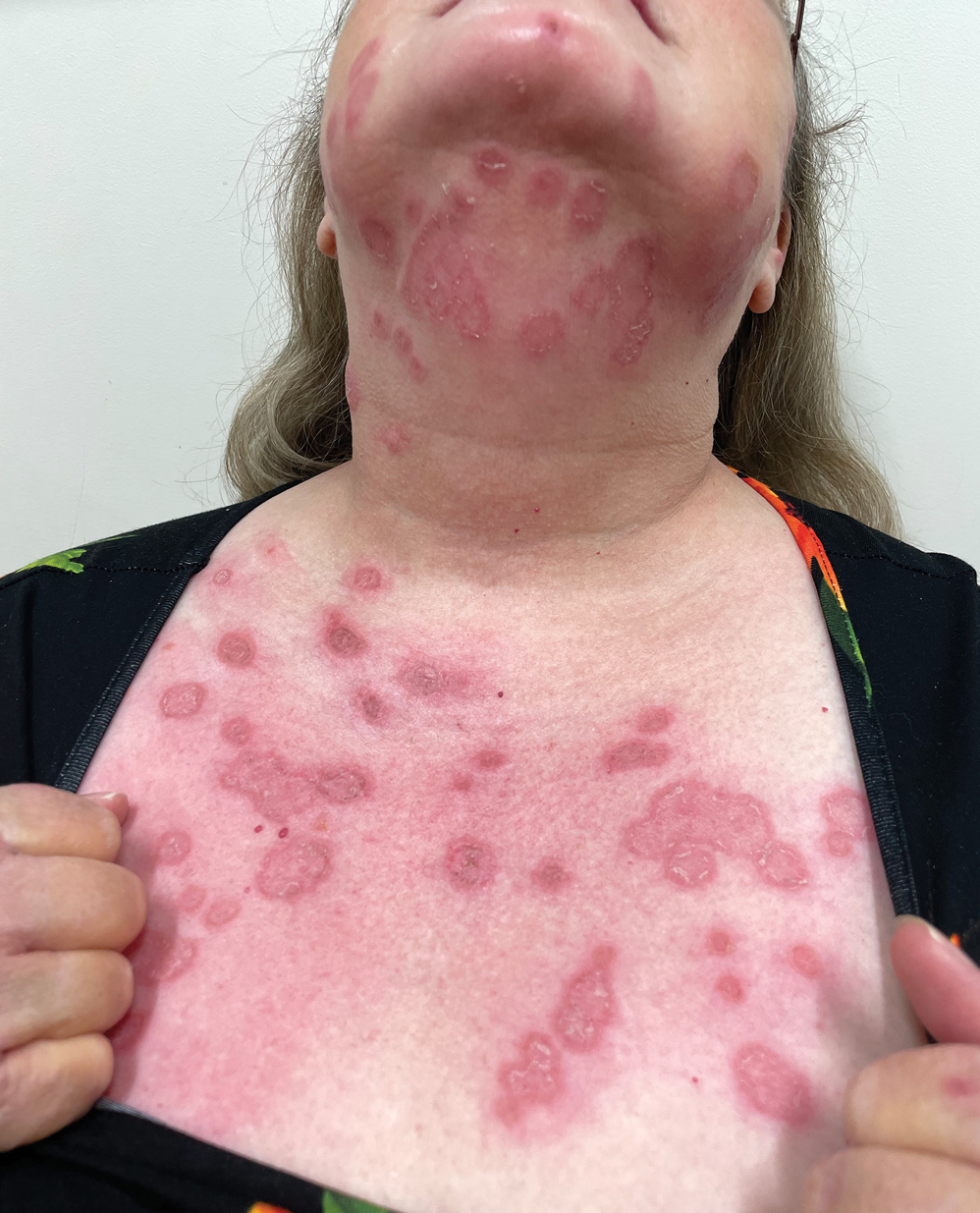
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
Electronic health records (EHRs) are an integral part of modern health care. The 2009, Health Information Technology for Economic and Clinical Health Act established financial incentives for US hospitals to adopt EHRs. In 2009 only 12% of nonfederal acute care hospitals had adopted a certified EHR system, which increased to 96% by 2021.1
EHRs have transformed the way patient data are stored and accessed, streamlining the process of providing quality patient care with improvements in efficiency, effectiveness, patient satisfaction, and safety.2 Despite their widespread adoption and benefits, EHRs have generally been met with mixed physician satisfaction.3 Interactions with EHRs are linked to disproportionate time at the computer and physician burnout.4-6
The US Department of Veterans Affairs (VA) was at the forefront of EHR development, establishing the Veterans Health Information Systems and Technology Architecture (VistA) in the 1970s. The VA released the Computerized Patient Record System (CPRS) in 1997, the first clinical user interface for VistA. In May 2018, the VA signed a $10 billion contract with Cerner (now Oracle Health) to modernize its EHR.7 This was later revised to $16.1 billion, and the Institute for Defense Analyses estimates it will cost $49.8 billion.8 The transition to Oracle Health has been faced with significant challenges, including patient safety risks and workflow inefficiencies, leading to a pause in rollout.9
Due to the known challenges with EHRs and the aging CPRS system (without a scheduled replacement date), innovations that facilitate the synthesis and display of clinical information are needed. To address this gap, the VA Ann Arbor Healthcare System (VAAAHS) developed the Inpatient Dashboard, an online EHR companion tool. The Inpatient Dashboard was designed to draw data from VistA to reduce time spent at the computer by streamlining clinical information presentation, standardizing inpatient notes, improving safety measures, and enhancing overall clinician satisfaction. This study evaluated the adoption and user experience with the Inpatient Dashboard.
INPATIENT DASHBOARD
The Inpatient Dashboard consists of several modules created by a contractor for the VAAAHS that is housed on VA servers with access restricted to individuals with patient health data privileges. As the Inpatient Dashboard draws data from VistA, it can display laboratory information, studies, and notes from all VA sites.
The main dashboard is a snapshot summary of patient information, including patient location, code status, last vital sign readings, vital sign ranges over the previous 24 hours, intake/output, deep vein thrombosis (DVT) prophylaxis, the presence of telemetry orders, or use of Foley or central catheters (Figure). It also includes a customizable to-do list and contact information for the patient’s clinician and nurse. Significant events, such as abnormal vital signs or speciation/sensitivities for blood cultures, are automatically populated on the to-do list. From this main dashboard overview, clinicians can customize which patients are displayed, create and print a rounding list, print a sign-out sheet, or select individual patients to open a progress note module.
Notes can be written in the patient history and physical module, progress note module, and discharge summary module. The patient history and physical module has text blocks allocated to the traditional components of a history and physical note (ie, chief complaint, history of present illness, review of systems, past medical history, family history, social history, allergies, medications, physical examination, assessment, and plan) (eAppendix 1). Some elements, such as past medical history, family history, and social history are prepopulated if the patient was previously admitted. Vital signs, laboratory results, studies, microbiology/ pathology reports, and other CPRS notes are displayed in this module.
The progress note module contains text blocks allocated to the traditional components of a progress note, such as subjective/interval events, physical examination, assessment, and plan (eAppendix 2). Vital signs, laboratory results, studies, microbiology/ pathology reports, other CPRS notes, and the patient’s medication administration record are also displayed in this module. Lastly, the discharge summary module includes patient follow-up, patient instructions, hospitalization summary, medication reconciliation, laboratory results, and studies/procedures, ensuring a comprehensive discharge summary for patients and clinicians (eAppendix 3).
A medication reconciliation tool was embedded within the history and physical and discharge summary modules. This tool has been shown to reduce medication errors in patients admitted from the emergency department to the hospital (eAppendix 4).10 The handoff/sign-out tool (eAppendix 5) accessible through the main dashboard page is modeled on the I-PASS handoff framework.11,12 This includes the patient identifier, interval events, inpatient medications, specific sign-out guidance, sign-out tasks/to-dos, and any other pertinent information.
The Inpatient Dashboard is a team-based construct shared by the attending physicians, residents, and medical students. Each team (eg, general medicine, general surgery) is its own entity; only team members can change the content or add to the documentation. Each facility can have multiple teams caring for the same patient (eg, primary and consulting teams). Additional care members can also be incorporated (eg, pharmacists assist with medication reconciliation for admission and discharge at VAAAHS). The Inpatient Dashboard can export information directly to CPRS for clinicians to review and sign. It can also generate a note that can be pasted into CPRS.
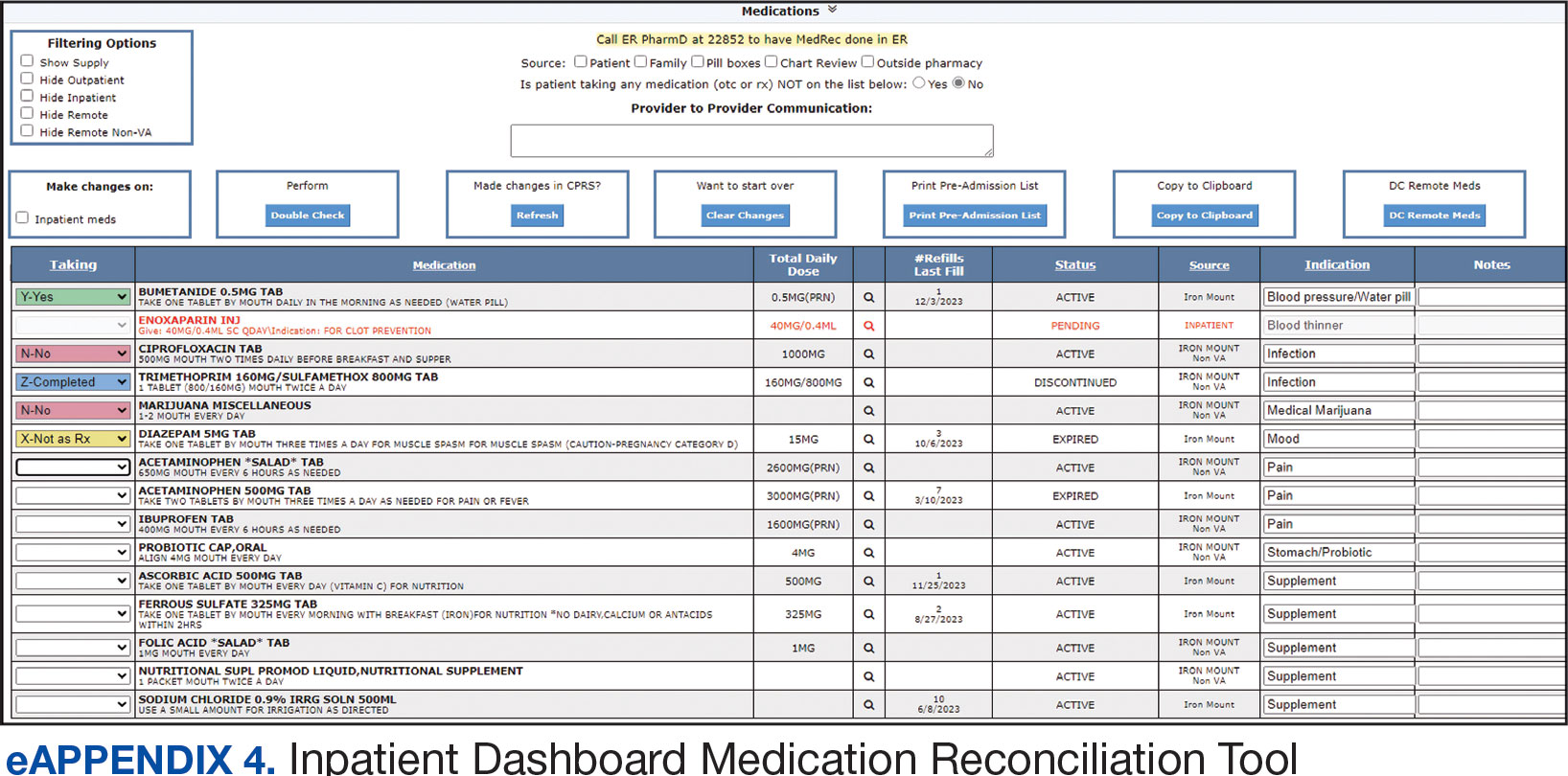
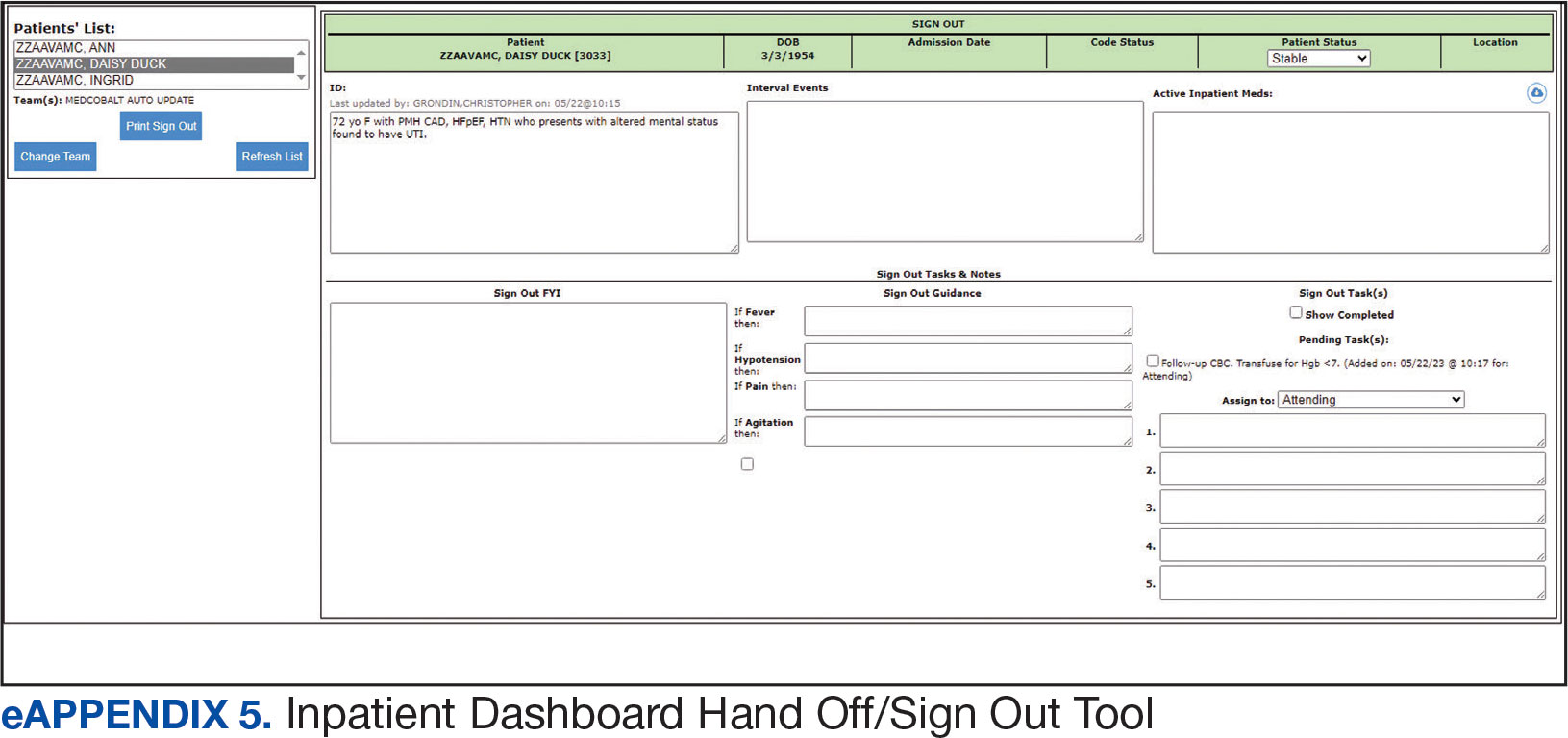
Clinician Feedback and Satisfaction
A survey was developed to evaluate clinician experiences with using the Inpatient Dashboard as an adjunct to the CPRS. The Inpatient Dashboard was made available to general medicine teams in November 2018. The survey was conducted from December 2018 to September 2019. The study was conducted at the VAAAHS and included 4 general medicine teams. Each team included an attending physician, a senior resident, 2 to 3 interns, and 3 to 4 medical students. Eligibility was extended to any team member who used both the CPRS and Inpatient Dashboard. Participation in the survey was voluntary. All respondents were informed of the study’s purpose and encouraged to provide candid feedback to ensure the reliability and validity of the findings.
Data were collected through a semistructured survey administered via the Qualtrics platform. The questionnaire was designed to capture multidimensional insights into clinician experience, with particular focus on satisfaction, efficiency, and perceived safety when using the tool as an adjunct to CPRS compared to using CPRS alone. The questionnaire primarily used a Likert scale for responses. Surveys were emailed at the completion of a team’s 1-month inpatient block. An answer was not required for every question, resulting in slightly different response numbers for some questions.
A question regarding the tool’s impact on workload stress was added halfway through the study period, which resulted in fewer responses. Adoption was assessed by counting the Inpatient Dashboard unique users. Descriptive statistics were used within individual survey responses to report the distribution of responses. Differences in response between levels of training were assessed using a X2 test of independence.
Survey Results
From September 2023 through November 2023, there were 1549 rounding printouts across 144 unique users (5 nurses, 40 medical students, 87 residents, and 12 attending physicians) and 1468 handoff printouts across 148 unique users (5 nurses, 10 medical students, 111 residents, and 22 attending physicians). The clinician survey received 68 responses from users at various levels of medical training: 23 medical students, 31 interns, 12 senior residents, and 2 attending physicians. All 68 participants confirmed they had used the Inpatient Dashboard.
User satisfaction and preference for the Inpatient Dashboard vs CPRS were assessed. Sixty-one respondents (90%) expressed overall satisfaction with the Inpatient Dashboard; 22 (32%) were extremely satisfied, and 39 (57%) were somewhat satisfied (Table 1). Three respondents (4%) were neutral, 2 (3%) were somewhat dissatisfied, and 2 (3%) were extremely dissatisfied with the Inpatient Dashboard. Responses differed by level of training (P = .03), with medical students trending towards higher satisfaction.
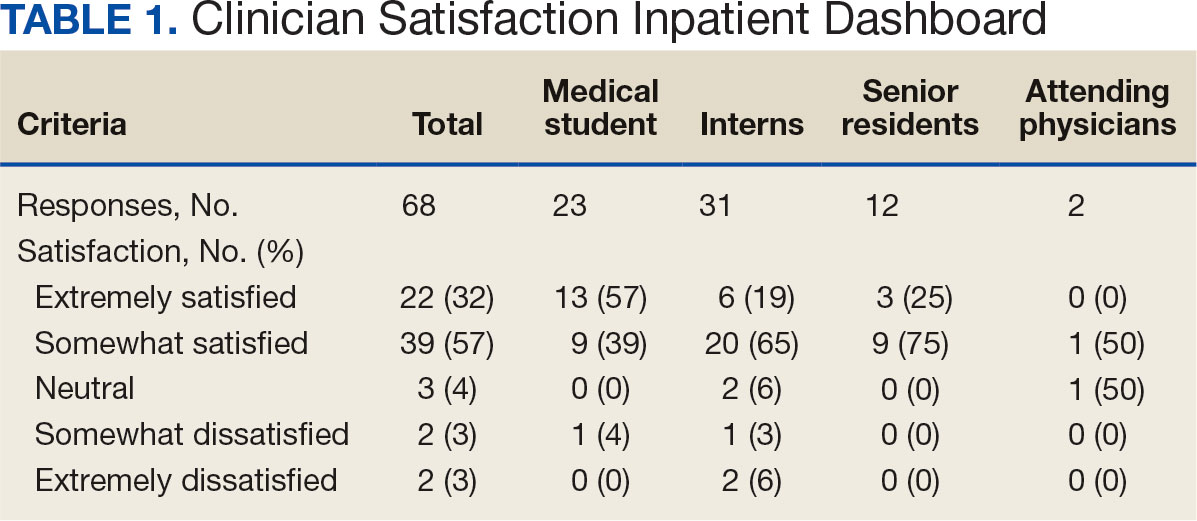
Respondents preferred the Inpatient Dashboard over CPRS for gathering information and writing progress notes; 42 (64%) respondents mostly favored the Inpatient Dashboard, 15 (23%) slightly favored the Inpatient Dashboard over CPRS, and 8 (12%) were neutral. One respondent (2%) slightly favored CPRS to the Inpatient Dashboard (Table 2).
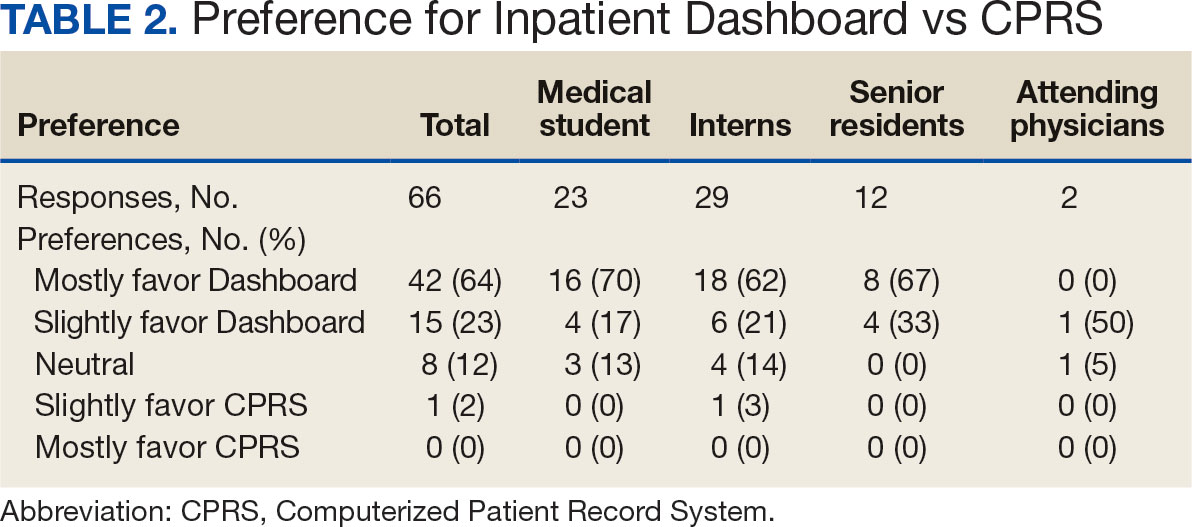
Sixty-five respondents (100%) found the Inpatient Dashboard’s ability to summarize patient information in a central place helpful (Table 3). Among them, 53 (82%) respondents reported it was very or extremely helpful, 10 (15%) respondents reported it was moderately helpful, and 2 (3%) respondents reported it was slightly helpful. This feature positively impacted users’ awareness of patients with DVT prophylaxis or a Foley catheter. Ten (15%) respondents reported being much more aware, and 29 (45%) respondents reporting they were slightly more aware. The remaining 26 (40%) respondents reported no change in awareness.
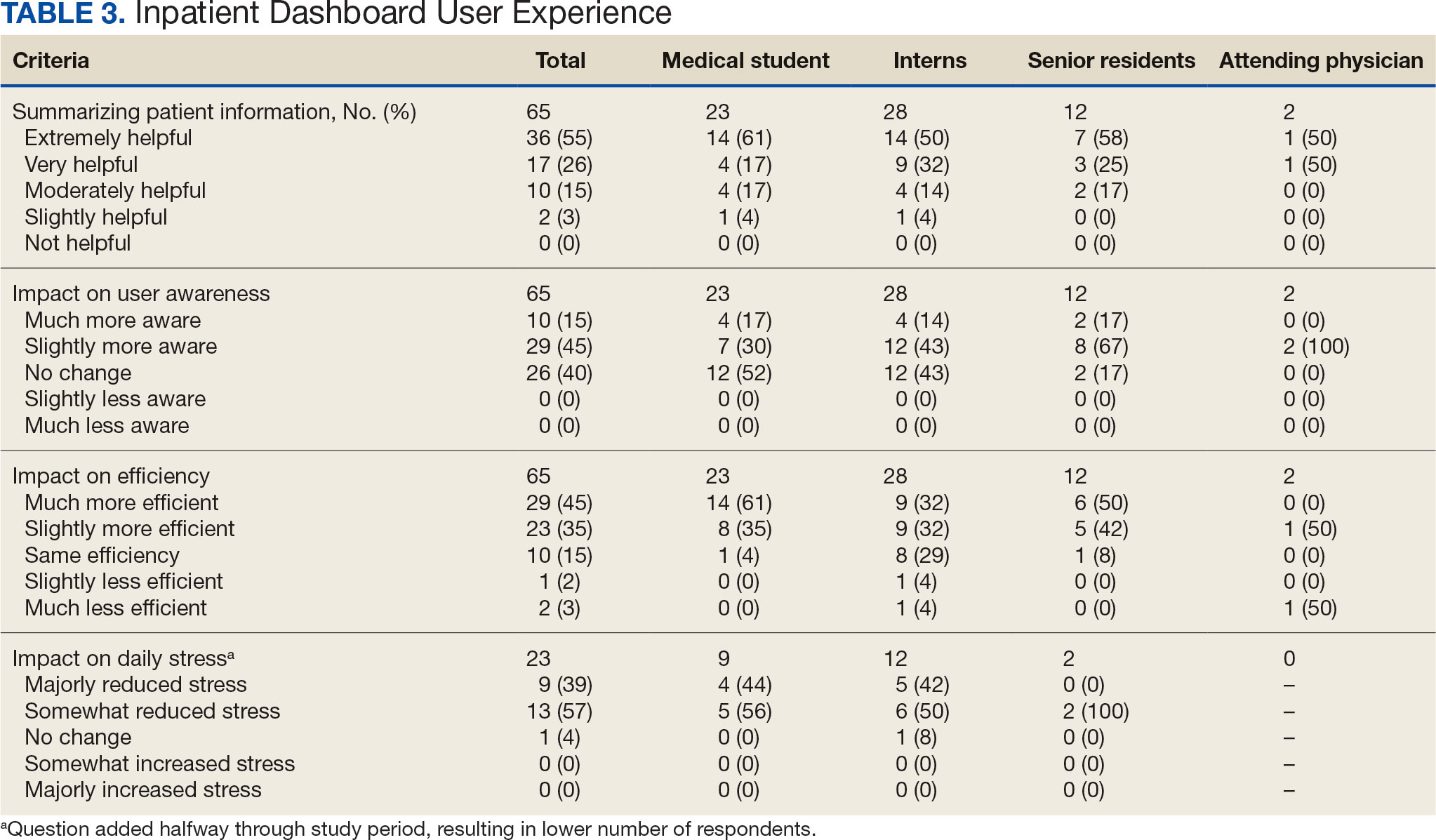
The Inpatient Dashboard was reported to save time preparing for physician rounds by 52 (80%) respondents, contributing to much greater efficiency for 29 (45%) respondents and slightly more efficiency for 23 (35%) respondents. However, 10 (15%) respondents reported no change in efficiency, and 3 (5%) respondents reduced efficiency, with 1 (2%) respondent reporting it slightly less efficient and 2 (3%) respondents reporting it much less efficient. Responses differed by level of training (P = .01), with medical students trending towards higher efficiency. Of the 23 respondents who reported on the Inpatient Dashboard’s impact on daily workload stress level, 22 (96%) indicated the tool had a stress-reducing effect, with 9 (39%) experiencing a major reduction in stress level, and 13 (57%) experiencing somewhat reduced stress level. Only 1 participant (4%) reported no change in stress. No participants reported an increase in stress.
DISCUSSION
The adoption of EHRs has transformed operational modalities in contemporary health care systems, heralding advancements in patient satisfaction, safety, and overall quality and efficiency of care.1,2 However, EHRs still present challenges, predominantly around clinician satisfaction, marked by instances of burnout and increased time spent on computers.2-6 In this context, the Inpatient Dashboard, an online companion to the CPRS, exemplifies how user-centered innovations in EHRs can address and mitigate associated challenges.
The Inpatient Dashboard has been well received with most respondents of the survey conducted in this study indicating they were both satisfied with the instrument and preferring it to CPRS. This high approval aligns with existing literature on the potential advantages of user-centered design in health care technology.13 The tool has gained widespread acceptance at the VAAAHS even in the absence of obligatory usage or institutional incentives. The appeal of the Inpatient Dashboard may stem from its increased efficiency, with most users affirming its timesaving nature. While CPRS can only display local notes, laboratory results, and studies, the Inpatient Dashboard can display data from across all VA sites. The VA Joint Longitudinal Viewer can similarly display data from across all sites, but the display is not streamlined as it is in the Inpatient Dashboard. The Inpatient Dashboard incorporates this clinical information into a single page to facilitate day-to-day workflow and dynamic documentation (ie, reviewing laboratory results, medications, writing notes, and signing out patients). This increased efficiency allows clinicians to counter 2 common barriers to EHR implementation: productivity loss and insufficient time.14
The association between EHRs and improved quality and safety in health care is well-documented.3 The Inpatient Dashboard fortifies this association by enhancing awareness around patient status, evidenced by a majority of respondents, and by integrating a medication reconciliation tool to decrease medication errors on transition from the emergency department to inpatient hospitalization.10
The Inpatient Dashboard’s impact on alleviating daily workload stress is noteworthy, with almost all respondents experiencing reduced stress levels and physician burnout, which has been linked to deteriorating well-being, compromised patient safety, and escalated health care costs.15,16 The heightened susceptibility of physicians to burnout compared to other professionals underscores the imperative for incorporating stress-mitigating interventions in the EHR.17,18
While responses to most questions did not significantly differ by training levels, overall satisfaction with the Inpatient Dashboard and its ability to save time preparing for rounds were rated higher by medical students. This may be attributable to a greater derived benefit from collating and presenting data to learners with less familiarity with the native EHR. It is also notable that the Inpatient Dashboard allows medical students to directly contribute to a patient’s note, which could be another driver in satisfaction. While most interns still felt the Inpatient Dashboard enabled them to save time preparing for rounds, there were a considerable number of ‘no change’ responses, which suggests some interns may not have modified their existing prerounding strategies. These associations are limited by the relatively small number of respondents by learner category, with senior medical residents and attending physicians being underrepresented.
While there are a multitude of dashboards available at the VA, most are made to track certain quality metrics and are used more by administrative and leadership staff. The Inpatient Dashboard was created specifically for frontline clinicians to facilitate their day-to-day workflow and dynamic documentation. This tool can additionally help with quality metrics, though its main purpose was and is to make clinician workflow easier and more efficient.
These results are especially timely because the VA is modernizing its EHR by transitioning to Oracle Health.7 Due to the numerous reports both from veterans and VA clinicians that the Oracle Health EHR is not meeting expectations, deployment at further sites has been halted while improving the experience of the 5 institutions using Oracle Health is prioritized.9 The Inpatient Dashboard, instead of being merely an enhancement to CPRS, could emerge as a potential bridge to Oracle Health if adapted to display data from Oracle Health as it does VistA. This would facilitate a smoother, more integrated transition for those health care institutions employing the Inpatient Dashboard.
Limitations
The reliance on self-reported data inherently carries the risk of bias, and the absence of objective measures, like time-tracking studies, limits the quantifiable assessment of the Inpatient Dashboard efficacy. The single-center nature of the study also may restrict the generalizability of the results.
CONCLUSIONS
Optimal integration of EHRs into health care delivery is critical to high-quality patient care and operational efficiency. The Inpatient Dashboard is an example of an innovative, user-centric solution that integrated and presented clinical information in a way that produced high satisfaction and adoption by users at a VA hospital.
- Office of the National Coordinator for Health Information Technology. National Trends in Hospital and Physician Adoption of Electronic Health Records. HealthIT.gov. Accessed February 5, 2025. https://www.healthit.gov/data/quickstats/national-trends-hospital-and-physician-adoption-electronic-health-records
- Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464-471. doi:10.1377/hlthaff.2011.0178
- Nguyen L, Bellucci E, Nguyen LT. Electronic health records implementation: an evaluation of information system impact and contingency factors. Int J Med Inf. 2014;83(11):779-796. doi:10.1016/j.ijmedinf.2014.06.011
- Alexander AG, Ballou KA. Work-life balance, burnout, and the electronic health record. Am J Med. 2018;131(8):857- 858. doi:10.1016/j.amjmed.2018.02.033
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753-760. doi:10.7326/M16-0961
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med. 2019;179(6):760-767. doi:10.1001/jamainternmed.2019.0095
- US Department of Veterans Affairs. Statement by Acting Secretary Robert Wilkie - VA signs contract with Cerner for an electronic health record system. New release. May 17, 2018. Accessed February 5, 2025. https://news.va.gov/press-room/statement-by-acting-secretary-robert-wilkie-va-signs-contract-with-cerner-for-an-electronic-health-record-system/
- US Government Publishing Office. VA’s Electronic health record modernization: an update on rollout, cost, and schedule. Subcommittee on Military Construction, Veterans Affairs, and Related Agencies, Committee on Appropriations, United States Senate. 117th Congress, 2nd Session. September 21, 2022. Accessed February 5, 2025. https://www.govinfo.gov/content/pkg/CHRG-117shrg52328/html/CHRG-117shrg52328.htm
- US Department of Veterans Affairs. VA announces reset of electronic health record project. Accessed December 21, 2023. https://news.va.gov/press-room/va-announces-reset-of-electronic-health-record-project/
- Grondin C, Gupta A, Houchens N, et al. Medication reconciliation tool reduces errors in patients admitted from the ED to hospital. Am J Med Qual. 2021;36(2):129. doi:10.1097/01.JMQ.0000741500.33781.eb
- Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi:10.1056/NEJMsa1405556
- Starmer AJ, O’Toole JK, Rosenbluth G, et al. Development, implementation, and dissemination of the I-PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876-884. doi:10.1097/ACM.0000000000000264
- Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
- Kruse CS, Kristof C, Jones B, Mitchell E, Martinez A. Barriers to electronic health record adoption: a systematic literature review. J Med Syst. 2016;40(12):252. doi:10.1007/s10916-016-0628-9
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516-529. doi:10.1111/joim.12752
- Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907- 912. doi:10.1016/j.amjsurg.2019.07.004
- Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385. doi:10.1001/archinternmed.2012.3199
- Budd J. Burnout related to electronic health record use in primary care. J Prim Care Community Health. 2023;14:21501319231166921. doi:10.1177/21501319231166921
Electronic health records (EHRs) are an integral part of modern health care. The 2009, Health Information Technology for Economic and Clinical Health Act established financial incentives for US hospitals to adopt EHRs. In 2009 only 12% of nonfederal acute care hospitals had adopted a certified EHR system, which increased to 96% by 2021.1
EHRs have transformed the way patient data are stored and accessed, streamlining the process of providing quality patient care with improvements in efficiency, effectiveness, patient satisfaction, and safety.2 Despite their widespread adoption and benefits, EHRs have generally been met with mixed physician satisfaction.3 Interactions with EHRs are linked to disproportionate time at the computer and physician burnout.4-6
The US Department of Veterans Affairs (VA) was at the forefront of EHR development, establishing the Veterans Health Information Systems and Technology Architecture (VistA) in the 1970s. The VA released the Computerized Patient Record System (CPRS) in 1997, the first clinical user interface for VistA. In May 2018, the VA signed a $10 billion contract with Cerner (now Oracle Health) to modernize its EHR.7 This was later revised to $16.1 billion, and the Institute for Defense Analyses estimates it will cost $49.8 billion.8 The transition to Oracle Health has been faced with significant challenges, including patient safety risks and workflow inefficiencies, leading to a pause in rollout.9
Due to the known challenges with EHRs and the aging CPRS system (without a scheduled replacement date), innovations that facilitate the synthesis and display of clinical information are needed. To address this gap, the VA Ann Arbor Healthcare System (VAAAHS) developed the Inpatient Dashboard, an online EHR companion tool. The Inpatient Dashboard was designed to draw data from VistA to reduce time spent at the computer by streamlining clinical information presentation, standardizing inpatient notes, improving safety measures, and enhancing overall clinician satisfaction. This study evaluated the adoption and user experience with the Inpatient Dashboard.
INPATIENT DASHBOARD
The Inpatient Dashboard consists of several modules created by a contractor for the VAAAHS that is housed on VA servers with access restricted to individuals with patient health data privileges. As the Inpatient Dashboard draws data from VistA, it can display laboratory information, studies, and notes from all VA sites.
The main dashboard is a snapshot summary of patient information, including patient location, code status, last vital sign readings, vital sign ranges over the previous 24 hours, intake/output, deep vein thrombosis (DVT) prophylaxis, the presence of telemetry orders, or use of Foley or central catheters (Figure). It also includes a customizable to-do list and contact information for the patient’s clinician and nurse. Significant events, such as abnormal vital signs or speciation/sensitivities for blood cultures, are automatically populated on the to-do list. From this main dashboard overview, clinicians can customize which patients are displayed, create and print a rounding list, print a sign-out sheet, or select individual patients to open a progress note module.

Notes can be written in the patient history and physical module, progress note module, and discharge summary module. The patient history and physical module has text blocks allocated to the traditional components of a history and physical note (ie, chief complaint, history of present illness, review of systems, past medical history, family history, social history, allergies, medications, physical examination, assessment, and plan) (eAppendix 1). Some elements, such as past medical history, family history, and social history are prepopulated if the patient was previously admitted. Vital signs, laboratory results, studies, microbiology/ pathology reports, and other CPRS notes are displayed in this module.

The progress note module contains text blocks allocated to the traditional components of a progress note, such as subjective/interval events, physical examination, assessment, and plan (eAppendix 2). Vital signs, laboratory results, studies, microbiology/ pathology reports, other CPRS notes, and the patient’s medication administration record are also displayed in this module. Lastly, the discharge summary module includes patient follow-up, patient instructions, hospitalization summary, medication reconciliation, laboratory results, and studies/procedures, ensuring a comprehensive discharge summary for patients and clinicians (eAppendix 3).


A medication reconciliation tool was embedded within the history and physical and discharge summary modules. This tool has been shown to reduce medication errors in patients admitted from the emergency department to the hospital (eAppendix 4).10 The handoff/sign-out tool (eAppendix 5) accessible through the main dashboard page is modeled on the I-PASS handoff framework.11,12 This includes the patient identifier, interval events, inpatient medications, specific sign-out guidance, sign-out tasks/to-dos, and any other pertinent information.
The Inpatient Dashboard is a team-based construct shared by the attending physicians, residents, and medical students. Each team (eg, general medicine, general surgery) is its own entity; only team members can change the content or add to the documentation. Each facility can have multiple teams caring for the same patient (eg, primary and consulting teams). Additional care members can also be incorporated (eg, pharmacists assist with medication reconciliation for admission and discharge at VAAAHS). The Inpatient Dashboard can export information directly to CPRS for clinicians to review and sign. It can also generate a note that can be pasted into CPRS.


Clinician Feedback and Satisfaction
A survey was developed to evaluate clinician experiences with using the Inpatient Dashboard as an adjunct to the CPRS. The Inpatient Dashboard was made available to general medicine teams in November 2018. The survey was conducted from December 2018 to September 2019. The study was conducted at the VAAAHS and included 4 general medicine teams. Each team included an attending physician, a senior resident, 2 to 3 interns, and 3 to 4 medical students. Eligibility was extended to any team member who used both the CPRS and Inpatient Dashboard. Participation in the survey was voluntary. All respondents were informed of the study’s purpose and encouraged to provide candid feedback to ensure the reliability and validity of the findings.
Data were collected through a semistructured survey administered via the Qualtrics platform. The questionnaire was designed to capture multidimensional insights into clinician experience, with particular focus on satisfaction, efficiency, and perceived safety when using the tool as an adjunct to CPRS compared to using CPRS alone. The questionnaire primarily used a Likert scale for responses. Surveys were emailed at the completion of a team’s 1-month inpatient block. An answer was not required for every question, resulting in slightly different response numbers for some questions.
A question regarding the tool’s impact on workload stress was added halfway through the study period, which resulted in fewer responses. Adoption was assessed by counting the Inpatient Dashboard unique users. Descriptive statistics were used within individual survey responses to report the distribution of responses. Differences in response between levels of training were assessed using a X2 test of independence.
Survey Results
From September 2023 through November 2023, there were 1549 rounding printouts across 144 unique users (5 nurses, 40 medical students, 87 residents, and 12 attending physicians) and 1468 handoff printouts across 148 unique users (5 nurses, 10 medical students, 111 residents, and 22 attending physicians). The clinician survey received 68 responses from users at various levels of medical training: 23 medical students, 31 interns, 12 senior residents, and 2 attending physicians. All 68 participants confirmed they had used the Inpatient Dashboard.
User satisfaction and preference for the Inpatient Dashboard vs CPRS were assessed. Sixty-one respondents (90%) expressed overall satisfaction with the Inpatient Dashboard; 22 (32%) were extremely satisfied, and 39 (57%) were somewhat satisfied (Table 1). Three respondents (4%) were neutral, 2 (3%) were somewhat dissatisfied, and 2 (3%) were extremely dissatisfied with the Inpatient Dashboard. Responses differed by level of training (P = .03), with medical students trending towards higher satisfaction.

Respondents preferred the Inpatient Dashboard over CPRS for gathering information and writing progress notes; 42 (64%) respondents mostly favored the Inpatient Dashboard, 15 (23%) slightly favored the Inpatient Dashboard over CPRS, and 8 (12%) were neutral. One respondent (2%) slightly favored CPRS to the Inpatient Dashboard (Table 2).

Sixty-five respondents (100%) found the Inpatient Dashboard’s ability to summarize patient information in a central place helpful (Table 3). Among them, 53 (82%) respondents reported it was very or extremely helpful, 10 (15%) respondents reported it was moderately helpful, and 2 (3%) respondents reported it was slightly helpful. This feature positively impacted users’ awareness of patients with DVT prophylaxis or a Foley catheter. Ten (15%) respondents reported being much more aware, and 29 (45%) respondents reporting they were slightly more aware. The remaining 26 (40%) respondents reported no change in awareness.

The Inpatient Dashboard was reported to save time preparing for physician rounds by 52 (80%) respondents, contributing to much greater efficiency for 29 (45%) respondents and slightly more efficiency for 23 (35%) respondents. However, 10 (15%) respondents reported no change in efficiency, and 3 (5%) respondents reduced efficiency, with 1 (2%) respondent reporting it slightly less efficient and 2 (3%) respondents reporting it much less efficient. Responses differed by level of training (P = .01), with medical students trending towards higher efficiency. Of the 23 respondents who reported on the Inpatient Dashboard’s impact on daily workload stress level, 22 (96%) indicated the tool had a stress-reducing effect, with 9 (39%) experiencing a major reduction in stress level, and 13 (57%) experiencing somewhat reduced stress level. Only 1 participant (4%) reported no change in stress. No participants reported an increase in stress.
DISCUSSION
The adoption of EHRs has transformed operational modalities in contemporary health care systems, heralding advancements in patient satisfaction, safety, and overall quality and efficiency of care.1,2 However, EHRs still present challenges, predominantly around clinician satisfaction, marked by instances of burnout and increased time spent on computers.2-6 In this context, the Inpatient Dashboard, an online companion to the CPRS, exemplifies how user-centered innovations in EHRs can address and mitigate associated challenges.
The Inpatient Dashboard has been well received with most respondents of the survey conducted in this study indicating they were both satisfied with the instrument and preferring it to CPRS. This high approval aligns with existing literature on the potential advantages of user-centered design in health care technology.13 The tool has gained widespread acceptance at the VAAAHS even in the absence of obligatory usage or institutional incentives. The appeal of the Inpatient Dashboard may stem from its increased efficiency, with most users affirming its timesaving nature. While CPRS can only display local notes, laboratory results, and studies, the Inpatient Dashboard can display data from across all VA sites. The VA Joint Longitudinal Viewer can similarly display data from across all sites, but the display is not streamlined as it is in the Inpatient Dashboard. The Inpatient Dashboard incorporates this clinical information into a single page to facilitate day-to-day workflow and dynamic documentation (ie, reviewing laboratory results, medications, writing notes, and signing out patients). This increased efficiency allows clinicians to counter 2 common barriers to EHR implementation: productivity loss and insufficient time.14
The association between EHRs and improved quality and safety in health care is well-documented.3 The Inpatient Dashboard fortifies this association by enhancing awareness around patient status, evidenced by a majority of respondents, and by integrating a medication reconciliation tool to decrease medication errors on transition from the emergency department to inpatient hospitalization.10
The Inpatient Dashboard’s impact on alleviating daily workload stress is noteworthy, with almost all respondents experiencing reduced stress levels and physician burnout, which has been linked to deteriorating well-being, compromised patient safety, and escalated health care costs.15,16 The heightened susceptibility of physicians to burnout compared to other professionals underscores the imperative for incorporating stress-mitigating interventions in the EHR.17,18
While responses to most questions did not significantly differ by training levels, overall satisfaction with the Inpatient Dashboard and its ability to save time preparing for rounds were rated higher by medical students. This may be attributable to a greater derived benefit from collating and presenting data to learners with less familiarity with the native EHR. It is also notable that the Inpatient Dashboard allows medical students to directly contribute to a patient’s note, which could be another driver in satisfaction. While most interns still felt the Inpatient Dashboard enabled them to save time preparing for rounds, there were a considerable number of ‘no change’ responses, which suggests some interns may not have modified their existing prerounding strategies. These associations are limited by the relatively small number of respondents by learner category, with senior medical residents and attending physicians being underrepresented.
While there are a multitude of dashboards available at the VA, most are made to track certain quality metrics and are used more by administrative and leadership staff. The Inpatient Dashboard was created specifically for frontline clinicians to facilitate their day-to-day workflow and dynamic documentation. This tool can additionally help with quality metrics, though its main purpose was and is to make clinician workflow easier and more efficient.
These results are especially timely because the VA is modernizing its EHR by transitioning to Oracle Health.7 Due to the numerous reports both from veterans and VA clinicians that the Oracle Health EHR is not meeting expectations, deployment at further sites has been halted while improving the experience of the 5 institutions using Oracle Health is prioritized.9 The Inpatient Dashboard, instead of being merely an enhancement to CPRS, could emerge as a potential bridge to Oracle Health if adapted to display data from Oracle Health as it does VistA. This would facilitate a smoother, more integrated transition for those health care institutions employing the Inpatient Dashboard.
Limitations
The reliance on self-reported data inherently carries the risk of bias, and the absence of objective measures, like time-tracking studies, limits the quantifiable assessment of the Inpatient Dashboard efficacy. The single-center nature of the study also may restrict the generalizability of the results.
CONCLUSIONS
Optimal integration of EHRs into health care delivery is critical to high-quality patient care and operational efficiency. The Inpatient Dashboard is an example of an innovative, user-centric solution that integrated and presented clinical information in a way that produced high satisfaction and adoption by users at a VA hospital.
Electronic health records (EHRs) are an integral part of modern health care. The 2009, Health Information Technology for Economic and Clinical Health Act established financial incentives for US hospitals to adopt EHRs. In 2009 only 12% of nonfederal acute care hospitals had adopted a certified EHR system, which increased to 96% by 2021.1
EHRs have transformed the way patient data are stored and accessed, streamlining the process of providing quality patient care with improvements in efficiency, effectiveness, patient satisfaction, and safety.2 Despite their widespread adoption and benefits, EHRs have generally been met with mixed physician satisfaction.3 Interactions with EHRs are linked to disproportionate time at the computer and physician burnout.4-6
The US Department of Veterans Affairs (VA) was at the forefront of EHR development, establishing the Veterans Health Information Systems and Technology Architecture (VistA) in the 1970s. The VA released the Computerized Patient Record System (CPRS) in 1997, the first clinical user interface for VistA. In May 2018, the VA signed a $10 billion contract with Cerner (now Oracle Health) to modernize its EHR.7 This was later revised to $16.1 billion, and the Institute for Defense Analyses estimates it will cost $49.8 billion.8 The transition to Oracle Health has been faced with significant challenges, including patient safety risks and workflow inefficiencies, leading to a pause in rollout.9
Due to the known challenges with EHRs and the aging CPRS system (without a scheduled replacement date), innovations that facilitate the synthesis and display of clinical information are needed. To address this gap, the VA Ann Arbor Healthcare System (VAAAHS) developed the Inpatient Dashboard, an online EHR companion tool. The Inpatient Dashboard was designed to draw data from VistA to reduce time spent at the computer by streamlining clinical information presentation, standardizing inpatient notes, improving safety measures, and enhancing overall clinician satisfaction. This study evaluated the adoption and user experience with the Inpatient Dashboard.
INPATIENT DASHBOARD
The Inpatient Dashboard consists of several modules created by a contractor for the VAAAHS that is housed on VA servers with access restricted to individuals with patient health data privileges. As the Inpatient Dashboard draws data from VistA, it can display laboratory information, studies, and notes from all VA sites.
The main dashboard is a snapshot summary of patient information, including patient location, code status, last vital sign readings, vital sign ranges over the previous 24 hours, intake/output, deep vein thrombosis (DVT) prophylaxis, the presence of telemetry orders, or use of Foley or central catheters (Figure). It also includes a customizable to-do list and contact information for the patient’s clinician and nurse. Significant events, such as abnormal vital signs or speciation/sensitivities for blood cultures, are automatically populated on the to-do list. From this main dashboard overview, clinicians can customize which patients are displayed, create and print a rounding list, print a sign-out sheet, or select individual patients to open a progress note module.

Notes can be written in the patient history and physical module, progress note module, and discharge summary module. The patient history and physical module has text blocks allocated to the traditional components of a history and physical note (ie, chief complaint, history of present illness, review of systems, past medical history, family history, social history, allergies, medications, physical examination, assessment, and plan) (eAppendix 1). Some elements, such as past medical history, family history, and social history are prepopulated if the patient was previously admitted. Vital signs, laboratory results, studies, microbiology/ pathology reports, and other CPRS notes are displayed in this module.

The progress note module contains text blocks allocated to the traditional components of a progress note, such as subjective/interval events, physical examination, assessment, and plan (eAppendix 2). Vital signs, laboratory results, studies, microbiology/ pathology reports, other CPRS notes, and the patient’s medication administration record are also displayed in this module. Lastly, the discharge summary module includes patient follow-up, patient instructions, hospitalization summary, medication reconciliation, laboratory results, and studies/procedures, ensuring a comprehensive discharge summary for patients and clinicians (eAppendix 3).


A medication reconciliation tool was embedded within the history and physical and discharge summary modules. This tool has been shown to reduce medication errors in patients admitted from the emergency department to the hospital (eAppendix 4).10 The handoff/sign-out tool (eAppendix 5) accessible through the main dashboard page is modeled on the I-PASS handoff framework.11,12 This includes the patient identifier, interval events, inpatient medications, specific sign-out guidance, sign-out tasks/to-dos, and any other pertinent information.
The Inpatient Dashboard is a team-based construct shared by the attending physicians, residents, and medical students. Each team (eg, general medicine, general surgery) is its own entity; only team members can change the content or add to the documentation. Each facility can have multiple teams caring for the same patient (eg, primary and consulting teams). Additional care members can also be incorporated (eg, pharmacists assist with medication reconciliation for admission and discharge at VAAAHS). The Inpatient Dashboard can export information directly to CPRS for clinicians to review and sign. It can also generate a note that can be pasted into CPRS.


Clinician Feedback and Satisfaction
A survey was developed to evaluate clinician experiences with using the Inpatient Dashboard as an adjunct to the CPRS. The Inpatient Dashboard was made available to general medicine teams in November 2018. The survey was conducted from December 2018 to September 2019. The study was conducted at the VAAAHS and included 4 general medicine teams. Each team included an attending physician, a senior resident, 2 to 3 interns, and 3 to 4 medical students. Eligibility was extended to any team member who used both the CPRS and Inpatient Dashboard. Participation in the survey was voluntary. All respondents were informed of the study’s purpose and encouraged to provide candid feedback to ensure the reliability and validity of the findings.
Data were collected through a semistructured survey administered via the Qualtrics platform. The questionnaire was designed to capture multidimensional insights into clinician experience, with particular focus on satisfaction, efficiency, and perceived safety when using the tool as an adjunct to CPRS compared to using CPRS alone. The questionnaire primarily used a Likert scale for responses. Surveys were emailed at the completion of a team’s 1-month inpatient block. An answer was not required for every question, resulting in slightly different response numbers for some questions.
A question regarding the tool’s impact on workload stress was added halfway through the study period, which resulted in fewer responses. Adoption was assessed by counting the Inpatient Dashboard unique users. Descriptive statistics were used within individual survey responses to report the distribution of responses. Differences in response between levels of training were assessed using a X2 test of independence.
Survey Results
From September 2023 through November 2023, there were 1549 rounding printouts across 144 unique users (5 nurses, 40 medical students, 87 residents, and 12 attending physicians) and 1468 handoff printouts across 148 unique users (5 nurses, 10 medical students, 111 residents, and 22 attending physicians). The clinician survey received 68 responses from users at various levels of medical training: 23 medical students, 31 interns, 12 senior residents, and 2 attending physicians. All 68 participants confirmed they had used the Inpatient Dashboard.
User satisfaction and preference for the Inpatient Dashboard vs CPRS were assessed. Sixty-one respondents (90%) expressed overall satisfaction with the Inpatient Dashboard; 22 (32%) were extremely satisfied, and 39 (57%) were somewhat satisfied (Table 1). Three respondents (4%) were neutral, 2 (3%) were somewhat dissatisfied, and 2 (3%) were extremely dissatisfied with the Inpatient Dashboard. Responses differed by level of training (P = .03), with medical students trending towards higher satisfaction.

Respondents preferred the Inpatient Dashboard over CPRS for gathering information and writing progress notes; 42 (64%) respondents mostly favored the Inpatient Dashboard, 15 (23%) slightly favored the Inpatient Dashboard over CPRS, and 8 (12%) were neutral. One respondent (2%) slightly favored CPRS to the Inpatient Dashboard (Table 2).

Sixty-five respondents (100%) found the Inpatient Dashboard’s ability to summarize patient information in a central place helpful (Table 3). Among them, 53 (82%) respondents reported it was very or extremely helpful, 10 (15%) respondents reported it was moderately helpful, and 2 (3%) respondents reported it was slightly helpful. This feature positively impacted users’ awareness of patients with DVT prophylaxis or a Foley catheter. Ten (15%) respondents reported being much more aware, and 29 (45%) respondents reporting they were slightly more aware. The remaining 26 (40%) respondents reported no change in awareness.

The Inpatient Dashboard was reported to save time preparing for physician rounds by 52 (80%) respondents, contributing to much greater efficiency for 29 (45%) respondents and slightly more efficiency for 23 (35%) respondents. However, 10 (15%) respondents reported no change in efficiency, and 3 (5%) respondents reduced efficiency, with 1 (2%) respondent reporting it slightly less efficient and 2 (3%) respondents reporting it much less efficient. Responses differed by level of training (P = .01), with medical students trending towards higher efficiency. Of the 23 respondents who reported on the Inpatient Dashboard’s impact on daily workload stress level, 22 (96%) indicated the tool had a stress-reducing effect, with 9 (39%) experiencing a major reduction in stress level, and 13 (57%) experiencing somewhat reduced stress level. Only 1 participant (4%) reported no change in stress. No participants reported an increase in stress.
DISCUSSION
The adoption of EHRs has transformed operational modalities in contemporary health care systems, heralding advancements in patient satisfaction, safety, and overall quality and efficiency of care.1,2 However, EHRs still present challenges, predominantly around clinician satisfaction, marked by instances of burnout and increased time spent on computers.2-6 In this context, the Inpatient Dashboard, an online companion to the CPRS, exemplifies how user-centered innovations in EHRs can address and mitigate associated challenges.
The Inpatient Dashboard has been well received with most respondents of the survey conducted in this study indicating they were both satisfied with the instrument and preferring it to CPRS. This high approval aligns with existing literature on the potential advantages of user-centered design in health care technology.13 The tool has gained widespread acceptance at the VAAAHS even in the absence of obligatory usage or institutional incentives. The appeal of the Inpatient Dashboard may stem from its increased efficiency, with most users affirming its timesaving nature. While CPRS can only display local notes, laboratory results, and studies, the Inpatient Dashboard can display data from across all VA sites. The VA Joint Longitudinal Viewer can similarly display data from across all sites, but the display is not streamlined as it is in the Inpatient Dashboard. The Inpatient Dashboard incorporates this clinical information into a single page to facilitate day-to-day workflow and dynamic documentation (ie, reviewing laboratory results, medications, writing notes, and signing out patients). This increased efficiency allows clinicians to counter 2 common barriers to EHR implementation: productivity loss and insufficient time.14
The association between EHRs and improved quality and safety in health care is well-documented.3 The Inpatient Dashboard fortifies this association by enhancing awareness around patient status, evidenced by a majority of respondents, and by integrating a medication reconciliation tool to decrease medication errors on transition from the emergency department to inpatient hospitalization.10
The Inpatient Dashboard’s impact on alleviating daily workload stress is noteworthy, with almost all respondents experiencing reduced stress levels and physician burnout, which has been linked to deteriorating well-being, compromised patient safety, and escalated health care costs.15,16 The heightened susceptibility of physicians to burnout compared to other professionals underscores the imperative for incorporating stress-mitigating interventions in the EHR.17,18
While responses to most questions did not significantly differ by training levels, overall satisfaction with the Inpatient Dashboard and its ability to save time preparing for rounds were rated higher by medical students. This may be attributable to a greater derived benefit from collating and presenting data to learners with less familiarity with the native EHR. It is also notable that the Inpatient Dashboard allows medical students to directly contribute to a patient’s note, which could be another driver in satisfaction. While most interns still felt the Inpatient Dashboard enabled them to save time preparing for rounds, there were a considerable number of ‘no change’ responses, which suggests some interns may not have modified their existing prerounding strategies. These associations are limited by the relatively small number of respondents by learner category, with senior medical residents and attending physicians being underrepresented.
While there are a multitude of dashboards available at the VA, most are made to track certain quality metrics and are used more by administrative and leadership staff. The Inpatient Dashboard was created specifically for frontline clinicians to facilitate their day-to-day workflow and dynamic documentation. This tool can additionally help with quality metrics, though its main purpose was and is to make clinician workflow easier and more efficient.
These results are especially timely because the VA is modernizing its EHR by transitioning to Oracle Health.7 Due to the numerous reports both from veterans and VA clinicians that the Oracle Health EHR is not meeting expectations, deployment at further sites has been halted while improving the experience of the 5 institutions using Oracle Health is prioritized.9 The Inpatient Dashboard, instead of being merely an enhancement to CPRS, could emerge as a potential bridge to Oracle Health if adapted to display data from Oracle Health as it does VistA. This would facilitate a smoother, more integrated transition for those health care institutions employing the Inpatient Dashboard.
Limitations
The reliance on self-reported data inherently carries the risk of bias, and the absence of objective measures, like time-tracking studies, limits the quantifiable assessment of the Inpatient Dashboard efficacy. The single-center nature of the study also may restrict the generalizability of the results.
CONCLUSIONS
Optimal integration of EHRs into health care delivery is critical to high-quality patient care and operational efficiency. The Inpatient Dashboard is an example of an innovative, user-centric solution that integrated and presented clinical information in a way that produced high satisfaction and adoption by users at a VA hospital.
- Office of the National Coordinator for Health Information Technology. National Trends in Hospital and Physician Adoption of Electronic Health Records. HealthIT.gov. Accessed February 5, 2025. https://www.healthit.gov/data/quickstats/national-trends-hospital-and-physician-adoption-electronic-health-records
- Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464-471. doi:10.1377/hlthaff.2011.0178
- Nguyen L, Bellucci E, Nguyen LT. Electronic health records implementation: an evaluation of information system impact and contingency factors. Int J Med Inf. 2014;83(11):779-796. doi:10.1016/j.ijmedinf.2014.06.011
- Alexander AG, Ballou KA. Work-life balance, burnout, and the electronic health record. Am J Med. 2018;131(8):857- 858. doi:10.1016/j.amjmed.2018.02.033
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753-760. doi:10.7326/M16-0961
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med. 2019;179(6):760-767. doi:10.1001/jamainternmed.2019.0095
- US Department of Veterans Affairs. Statement by Acting Secretary Robert Wilkie - VA signs contract with Cerner for an electronic health record system. New release. May 17, 2018. Accessed February 5, 2025. https://news.va.gov/press-room/statement-by-acting-secretary-robert-wilkie-va-signs-contract-with-cerner-for-an-electronic-health-record-system/
- US Government Publishing Office. VA’s Electronic health record modernization: an update on rollout, cost, and schedule. Subcommittee on Military Construction, Veterans Affairs, and Related Agencies, Committee on Appropriations, United States Senate. 117th Congress, 2nd Session. September 21, 2022. Accessed February 5, 2025. https://www.govinfo.gov/content/pkg/CHRG-117shrg52328/html/CHRG-117shrg52328.htm
- US Department of Veterans Affairs. VA announces reset of electronic health record project. Accessed December 21, 2023. https://news.va.gov/press-room/va-announces-reset-of-electronic-health-record-project/
- Grondin C, Gupta A, Houchens N, et al. Medication reconciliation tool reduces errors in patients admitted from the ED to hospital. Am J Med Qual. 2021;36(2):129. doi:10.1097/01.JMQ.0000741500.33781.eb
- Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi:10.1056/NEJMsa1405556
- Starmer AJ, O’Toole JK, Rosenbluth G, et al. Development, implementation, and dissemination of the I-PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876-884. doi:10.1097/ACM.0000000000000264
- Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
- Kruse CS, Kristof C, Jones B, Mitchell E, Martinez A. Barriers to electronic health record adoption: a systematic literature review. J Med Syst. 2016;40(12):252. doi:10.1007/s10916-016-0628-9
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516-529. doi:10.1111/joim.12752
- Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907- 912. doi:10.1016/j.amjsurg.2019.07.004
- Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385. doi:10.1001/archinternmed.2012.3199
- Budd J. Burnout related to electronic health record use in primary care. J Prim Care Community Health. 2023;14:21501319231166921. doi:10.1177/21501319231166921
- Office of the National Coordinator for Health Information Technology. National Trends in Hospital and Physician Adoption of Electronic Health Records. HealthIT.gov. Accessed February 5, 2025. https://www.healthit.gov/data/quickstats/national-trends-hospital-and-physician-adoption-electronic-health-records
- Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464-471. doi:10.1377/hlthaff.2011.0178
- Nguyen L, Bellucci E, Nguyen LT. Electronic health records implementation: an evaluation of information system impact and contingency factors. Int J Med Inf. 2014;83(11):779-796. doi:10.1016/j.ijmedinf.2014.06.011
- Alexander AG, Ballou KA. Work-life balance, burnout, and the electronic health record. Am J Med. 2018;131(8):857- 858. doi:10.1016/j.amjmed.2018.02.033
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753-760. doi:10.7326/M16-0961
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med. 2019;179(6):760-767. doi:10.1001/jamainternmed.2019.0095
- US Department of Veterans Affairs. Statement by Acting Secretary Robert Wilkie - VA signs contract with Cerner for an electronic health record system. New release. May 17, 2018. Accessed February 5, 2025. https://news.va.gov/press-room/statement-by-acting-secretary-robert-wilkie-va-signs-contract-with-cerner-for-an-electronic-health-record-system/
- US Government Publishing Office. VA’s Electronic health record modernization: an update on rollout, cost, and schedule. Subcommittee on Military Construction, Veterans Affairs, and Related Agencies, Committee on Appropriations, United States Senate. 117th Congress, 2nd Session. September 21, 2022. Accessed February 5, 2025. https://www.govinfo.gov/content/pkg/CHRG-117shrg52328/html/CHRG-117shrg52328.htm
- US Department of Veterans Affairs. VA announces reset of electronic health record project. Accessed December 21, 2023. https://news.va.gov/press-room/va-announces-reset-of-electronic-health-record-project/
- Grondin C, Gupta A, Houchens N, et al. Medication reconciliation tool reduces errors in patients admitted from the ED to hospital. Am J Med Qual. 2021;36(2):129. doi:10.1097/01.JMQ.0000741500.33781.eb
- Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi:10.1056/NEJMsa1405556
- Starmer AJ, O’Toole JK, Rosenbluth G, et al. Development, implementation, and dissemination of the I-PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876-884. doi:10.1097/ACM.0000000000000264
- Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
- Kruse CS, Kristof C, Jones B, Mitchell E, Martinez A. Barriers to electronic health record adoption: a systematic literature review. J Med Syst. 2016;40(12):252. doi:10.1007/s10916-016-0628-9
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516-529. doi:10.1111/joim.12752
- Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907- 912. doi:10.1016/j.amjsurg.2019.07.004
- Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385. doi:10.1001/archinternmed.2012.3199
- Budd J. Burnout related to electronic health record use in primary care. J Prim Care Community Health. 2023;14:21501319231166921. doi:10.1177/21501319231166921
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).
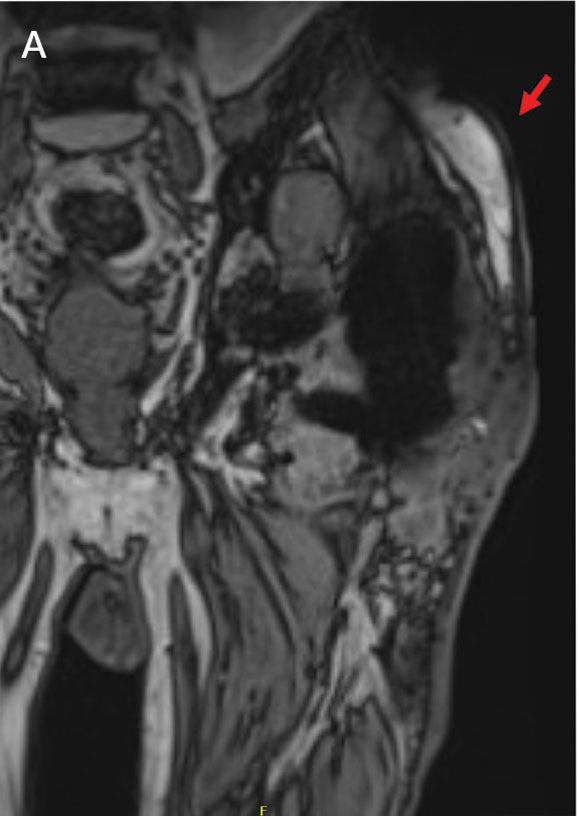
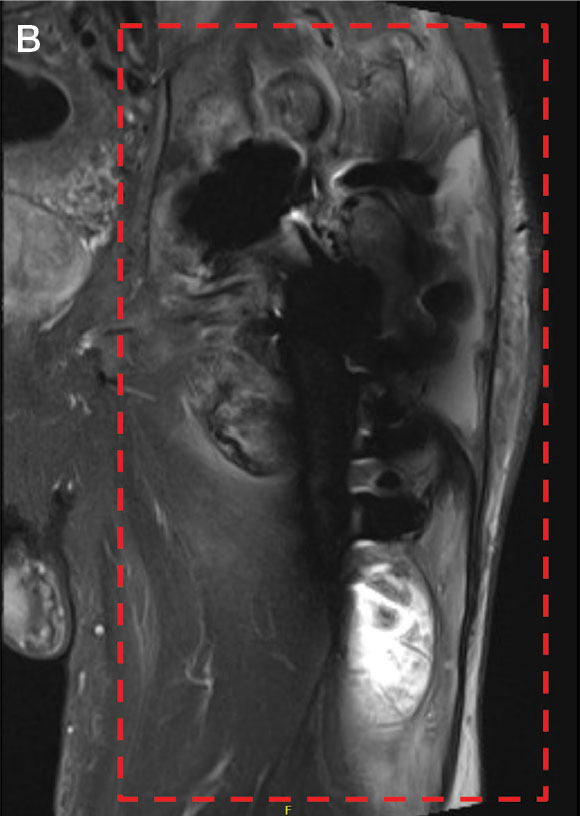
Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.
DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.

DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.

DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement