User login
Using Superficial Curettage to Diagnose Talon Noir
Using Superficial Curettage to Diagnose Talon Noir
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
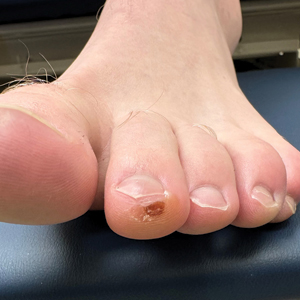
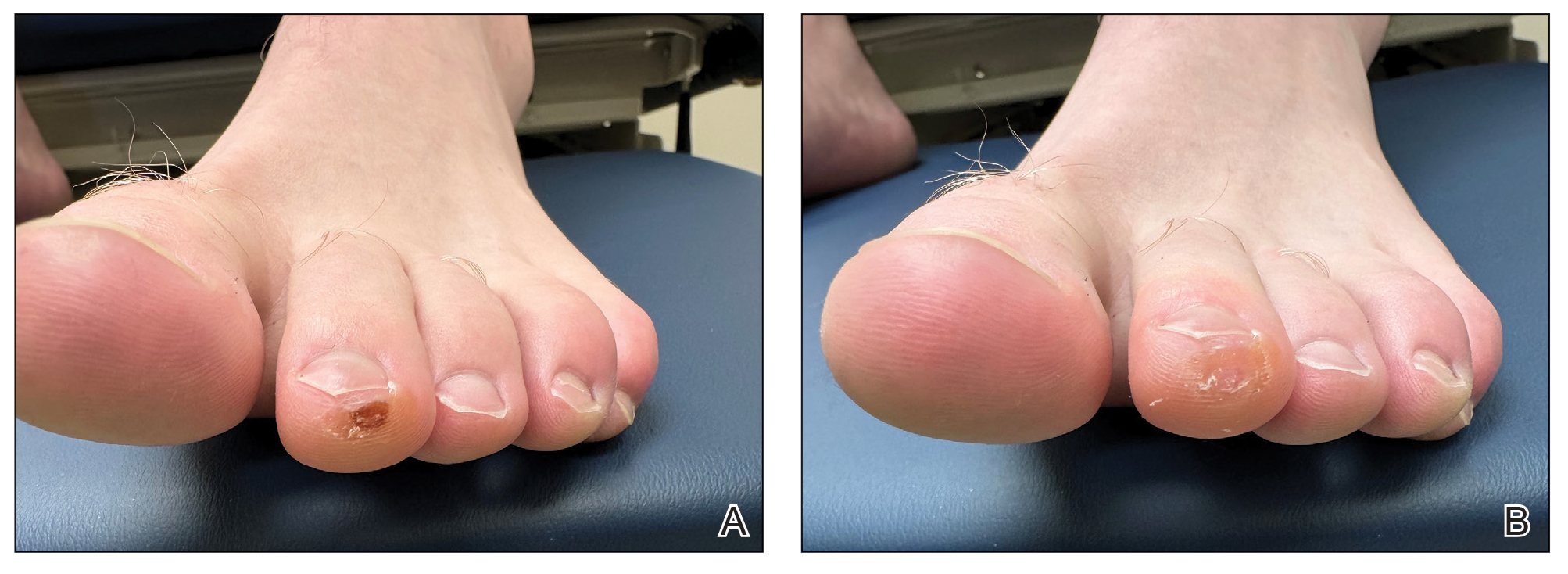
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.
Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.

Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.

Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
Using Superficial Curettage to Diagnose Talon Noir
Using Superficial Curettage to Diagnose Talon Noir
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).
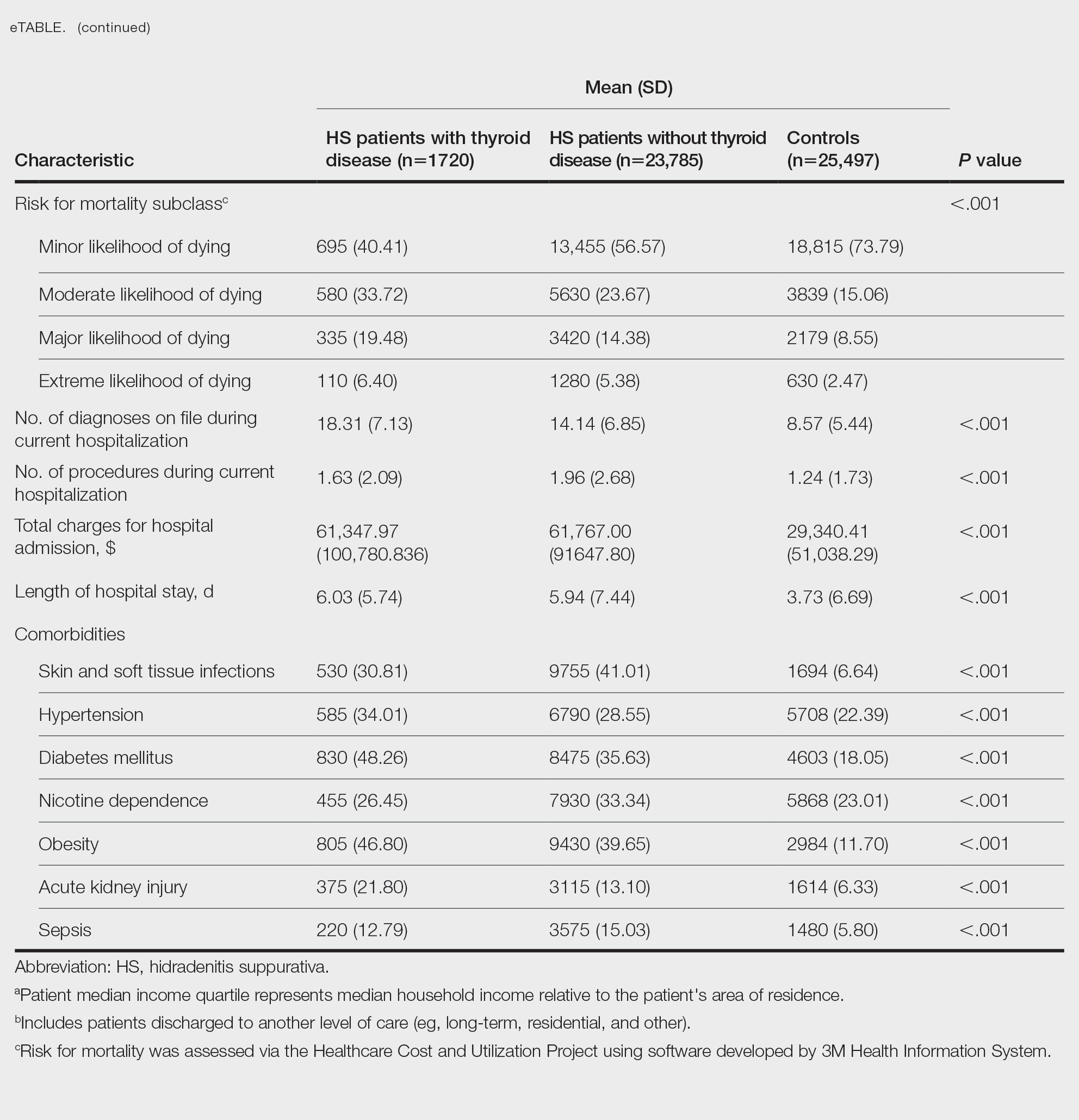
On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).
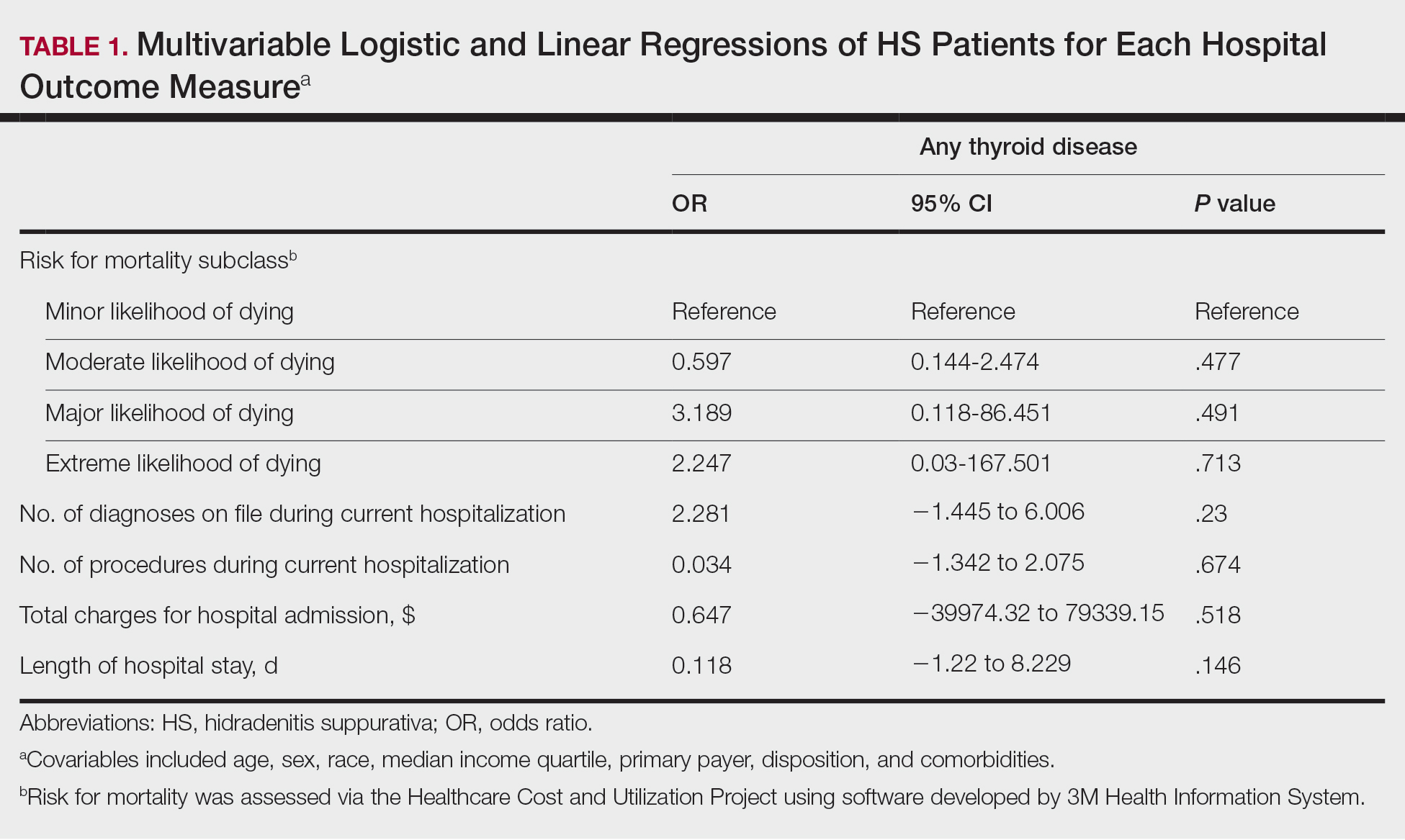

We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).


On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).


We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).


On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).


We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
PRACTICE
- Hidradenitis suppurativa (HS) is associated with autoimmune and endocrine conditions, but the association between HS and thyroid disorders is poorly characterized.
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Following exposure to potentially morally injurious events (PMIEs), some individuals may experience moral injury, which represents negative psychological, social, behavioral, and occasionally spiritual impacts.1 The consequences of PMIE exposure and moral injury are well documented. Individuals may begin to question the goodness and trustworthiness of oneself, others, or the world.1 Examples of other sequelae include guilt, demoralization, spiritual pain, loss of trust in the self or others, and difficulties with forgiveness.2-6 In addition, prior studies have found that moral injury is associated with an increased risk of suicidal thoughts and behaviors, posttraumatic stress disorder (PTSD) symptoms, spiritual distress, and interpersonal difficulties.7-11
Moral injury was first conceptualized in relation to combat trauma. However in recent years it has been examined in other groups such as health care practitioners, educators, refugees, and law enforcement personnel.12-17 Furthermore, there has been a recent call for the study of moral injury in other understudied groups. One such group is legal-involved individuals, defined as those who are currently involved or previously involved in the criminal justice system (ie, arrests, incarceration, parole, and probation).1,18-22
Many veterans are also involved with the legal system. Specifically, veterans currently comprise about 8% of the incarcerated US population, with an estimated > 180,000 veterans in prisons or jails and even more on parole or probation.23,24 Legal-involved veterans may be at heightened risk for homelessness, suicide, unemployment, and high prevalence rates of psychiatric diagnoses.25-28
Limited research has explored exposure to PMIEs as part of the legal process and the resulting expression of moral injury. The circumstances leading to incarceration, interactions with the US legal system, the environment of prison itself, and the subsequent challenges faced by legal-involved individuals after release all provide ample opportunity for PMIEs to occur.18 For example, engaging in a criminal act may represent a PMIE, particularly in violent offenses that involve harm to another individual. Moreover, the process of being convicted and charged with an offense may serve as a powerful reminder of the PMIE and tie this event to the individual’s identity and future. Furthermore, the physical and social environment of prison itself (eg, being surrounded by other offenders, witnessing the perpetration of violence, participating in violence for survival) presents a myriad of opportunities for PMIEs to occur.18
The consequences of PMIEs in the context of legal involvement may also have bearing on a touchstone of moral injury: changes in one’s schema of the self and world.4 At a societal level, legal-involved individuals are, by definition, deemed “guilty” and held culpable for their offense, which may reinforce a negative change in one’s view of self and the world.29 In line with identity theory, external negative appraisals about legal-involved individuals (eg, they are a danger to society, they cannot be trusted to do the right thing) may influence their self-perception.30 Furthermore, the affective characteristics often found in the context of moral injury (eg, guilt, shame, anger, contempt) may be exacerbated by legal involvement.29 Personal feelings of guilt and shame may be reinforced by receiving a verdict and sentence, as well as the negative perceptions of individuals around them (eg, disapproval from prior sources of social support). Additionally, feelings of betrayal and distrust towards the legal system may arise.
In sum, legal-involved veterans incur increased risk of moral injury due to the potential for exposure to PMIEs across multiple time points (eg, prior to military service, during military service, during arrest/sentencing, during imprisonment, and postincarceration). The stigma that accompanies legal involvement may limit access to treatment or a willingness to seek treatment for distress related to moral injury.29 Additionally, repeated exposure to PMIEs and resulting moral injury may compound over time, potentially exacerbating psychosocial functioning and increasing the risk for psychosocial stressors (eg, homelessness, unemployment) and mental health disorders (eg, depression, substance misuse).31
Although numerous measures of moral injury have been developed, most require that respondents consider a specific context (eg, military experiences).32 Therefore, study of legal-related moral injury requires adaptation of existing instruments to the legal context. The original and most commonly used scale of moral injury is the Moral Injury Events Scale (MIES).33 The MIES scales was originally developed to measure moral injury in military-related contexts but has since been adapted as a measure of exposure to context-specific PMIEs.34
Unfortunately, there are no validated measures for assessing legal-related moral injury. Such a gap in understanding is problematic, as it may impact measurement of the prevalence of PMIEs in both clinical and research settings for this at-risk population. The goal of this study was to conduct a psychometric evaluation of an adapted version of the MIES for legal-involved persons (MIES-LIP).
METHODS
A total of 177 veterans from the US Department of Veterans Affairs (VA) North Texas Health Care System were contacted for study enrollment between November 2020 and June 2021, yielding a final sample of 100 legal-involved veteran participants. Adults aged ≥ 18 years who were US military veterans and had ≥ 1 prior felony conviction resulting in incarceration were included. Participants were excluded if they had symptoms of psychosis that would preclude meaningful participation.
The study collected data on participants’ demographic and clinical characteristics using a semistructured survey instrument. Each participant completed an instructor-led questionnaire in a session that lasted about 1.5 hours. Participants who completed the visit in person received a $50 cash voucher for their time. Participants who were unable to meet with the study coordinator in person were able to complete the visit via telephone and received a $25 digital gift card. Of the total 100 participants, 79 participants completed the interview in person, and 21 completed by telephone. No significant differences were found in assessment measures between administration methods. Written informed consent was obtained during all in-person visits. For those completing via telephone, a waiver of written informed consent was obtained. This study was approved by the VA North Texas Health Care System’s Institutional Review Board.
Measures
The Moral Injury Events Scale (MIES) is a 9-item self-report measure that assesses exposure to PMIEs.33 Respondents rate their agreement with each item on a 6-point Likert scale (strongly disagree to strongly agree), with higher scores indicating greater moral injury. The MIES has a 2-factor structure: Factor 1 has 6 items on perceived transgressions and Factor 2 has 3 items on perceived betrayals.33
Creation of Legal-Involved Moral Injury Measure. To create the MIES-LIP, items and instructions from the MIES were modified to address moral injury in the context of legal involvement.33 Adaptations were finalized following consultation and approval by the authors of the original measure. Specifically, the instructions were changed to: “Please respond to these items based specifically in the context of your involvement with the legal system.” The instructions clarified that legal involvement could include experiences related to committing an offense, legal proceedings and sentencing, incarceration, or transitioning out of the legal system. This differs from the original measure, which focused on military experiences, with instructions stating: “Please respond to these items based specifically in the context of your military service (ie, events and experiences during enlistment, deployment, combat, etc).”
Other measures. The study collected data on demographic characteristics including sex, race and ethnicity, marital status, military service, combat experience, and legal involvement. PTSD symptom severity, based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), was assessed using the PTSD Checklist for DSM-5 (PCL-5).35,36 The PCL-5 is a 20-item self-report measure in which item scores are summed to create a total score. The PCL-5 has demonstrated strong psychometric properties, including good internal consistency, test-retest reliability convergent validity, and discriminant validity.37,38
Depressive symptom severity was measured using the Personal Health Questionnaire-9 (PHQ-9).39 The PHQ-9 is a 9-item self-report measure where item scores summed to create a total score. The PHQ-9 has demonstrated strong psychometric properties, including internal consistency and test-retest reliability.39
STATISTICAL METHODS
Descriptive statistics (mean and standard deviation for continuous variables; frequencies and percentages for categorical variables) were used to describe the study sample. Factor analysis was conducted to evaluate the psychometric properties of the MIES-LIP. Confirmatory factor analysis (CFA) was used to determine whether the MEIS-LIP had a similar factor structure to the MIES.40 Criteria for fit indices used for CFA include the Comparative Fit Index (CFI; values of > 0.95 suggest good fit), Tucker-Lewis index (TLI; values of > 0.95 suggest a good fit), root mean square error of approximation (RMSEA; values of ≥ 0.06 suggest good fit), and standardized root mean square residual (SRMR; values of ≥ 0.08 suggest good fit). With insufficient fit, subsequent exploratory factor analysis was conducted using maximum likelihood estimation with an Oblimin rotation. The Kaiser rule and a scree plot were considered when defining the factor structure. Reliability was evaluated using the McDonald omega coefficient test. Convergent validity was assessed through the association between adapted measures and other clinical measures (ie, PCL-5, PHQ-9). In addition, associations between the PCL-5 and PHQ-9 were examined as they related to the MIES and MIES-LIP.
RESULTS
Table 1 describes demographic characteristics of the study sample. Rates of potentially morally injurious experiences and the expression of moral injury in the legal context are presented in Table 2. Witnessing PMIEs while in the legal system was nearly ubiquitous, with > 90% of the sample endorsing this experience. More than half of the sample also endorsed engaging in morally injurious behavior by commission or omission, as well as experiencing betrayal while involved with the legal system.
Factor Analysis
Confirmatory factor analysis (CFA) was utilized to test the factor structure of the adapted MIES-LIP in our sample compared to the published factor structures of the MIES.33 Results did not support the established factor structure. Analysis yielded unacceptable CFI (0.79), TLI (0.70), SRMR (0.14), and RMSEA (0.21). The unsatisfactory results of CFA warranted follow-up exploratory factor analysis (EFA) to examine the factor structure of the moral injury scales in this sample.
EFA of MIES-LIP
The factor structure of the MIES-LIP was examined using EFA. The factorability of the data was examined using the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO value = 0.75) and Bartlett Test of Sphericity (X2 = 525.41; P < .001), both of which suggested that the data were appropriate for factor analysis. The number of factors to retain was selected based on the Kaiser criterion.41 After extraction, an Oblimin rotation was applied, given that we expected factors to be correlated. A 2-factor solution was found, explaining 65.76% of the common variance. All 9 items were retained as they had factor loadings > 0.30. Factor 1, comprised self-directed moral injury questions (3-6). Factor 2 comprised other directed moral injury questions (1, 2, 7-9) (Table 3). The factor correlation coefficient between Factor 1 and Factor 2 was 0.34, which supports utilizing an oblique rotation.
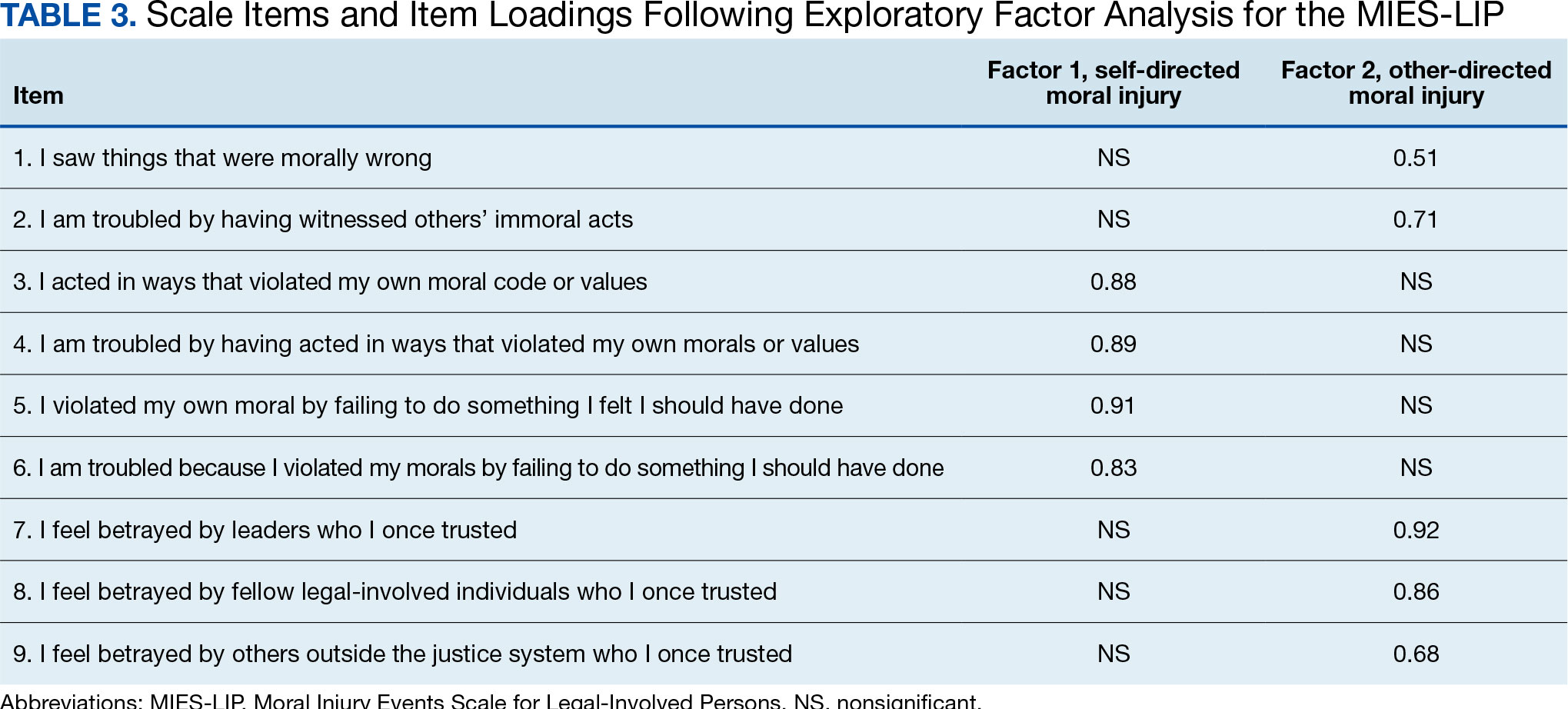
Reliability. We examined the reliability of the adapted MIES-LIP using measures of internal consistency, with both MIES-LIP factors demonstrating good reliability. The internal consistency of both factors of the MIES-LIP were found to be good (self-directed moral injury: Ω = 0.89; other-directed moral injury: Ω = 0.83).
Convergent Validity
Association between moral injury scales. A significant, moderate correlation was observed between all subscales of the MIES and MIES-LIP. Specifically, the self-directed moral injury factor of the MIES-LIP was associated with both the perceived transgressions (r = 0.41, P < .001) and the MIES perceived betrayals factors (r = 0.25, P < .05). Similarly, the other-directed moral injury factor of the MIES-LIP was associated with both the MIES perceived transgressions (r = 0.45, P < .001) and the MIES perceived betrayals factors (r = 0.45, P < .001).
Association with PTSD symptoms. All subscales of both the MIES and MIES-LIP were associated with PTSD symptom severity. The MIES perceived transgressions factor (r = 0.43, P < .001) and the perceived betrayals factor of the MIES (r = 0.39, P < .001) were moderately associated with the PCL-5. Mirroring this, the “self-directed moral injury” factor of the MIESLIP (r = 0.44, P < .001) and the “other-directed moral injury” factor of the MIES-LIP (r = 0.42, P < .001) were also positively associated with PCL-5.
Association with depression symptoms. All subscales of the MIES and MIES-LIP were also associated with depressive symptoms. The MIES perceived transgressions factor (r = 0.27, P < .01) and the MIES perceived betrayals factor (r = 0.23, P < .05) had a small association with the PHQ-9. In addition, the self-directed moral injury factor of the MIES-LIP (r = 0.40, P < .001) and the other-directed moral injury factor of the MIES-LIP (r = 0.31, P < .01) had small to moderate associations with the PCL-5.
DISCUSSION
Potentially morally injurious events appear to be a salient factor affecting legal-involved veterans. Among our sample, the vast majority of legal-involved veterans endorsed experiencing both legal- and military-related PMIEs. Witnessing or participating in a legal-related PMIE appears to be widespread among those who have experienced incarceration. The MIES-LIP yielded a 2-factor structure: self-directed moral injury and other-directed moral injury, in the evaluated population. The MIES-LIP showed similar psychometric performance to the MIES in our sample. Specifically, the MIES-LIP had good reliability and adequate convergent validity. While CFA did not confirm the anticipated factor structure of the MIES-LIP within our sample, EFA showed similarities in factor structure between the original and adapted measures. While further research and validation are needed, preliminary results show promise of the MIES-LIP in assessing legal-related moral injury.
Originally, the MIES was found to have a 2-factor structure, defined by perceived transgressions and perceived betrayals.33 However, additional research has identified a 3-factor structure, where the betrayal factor is maintained, and the transgressions factor is divided into transgressions by others and by self.8 The factor structure of the MIES-LIP was more closely related to the factor structure, with transgressions by others and betrayal mapped onto the same factor (ie, other-directed moral injury).8 While further research is needed, it is possible that the nature of morally injurious events experienced in legal contexts are experienced more in terms of self vs other, compared to morally injurious events experienced by veterans or active-duty service members.
Accurately identifying the types of moral injury experienced in a legal context may be important for determining the differences in drivers of legal-related moral injury compared to military-related moral injury. For example, self-directed moral injury in legal contexts may include a variety of actions the individual initiated that led to conviction and incarceration (eg, a criminal offense), as well as behaviors performed or witnessed while incarcerated (eg, engaging in violence). Inconsistent with military populations where other-directed moral injury clusters with self-directed moral injury, other-directed moral injury clustered with betrayal in legal contexts in our sample. This discrepancy may result from differences in identification with the military vs legal system. When veterans witness fellow service members engaging in PMIEs (eg, physical violence towards civilians in a military setting), this may be similar to self-directed moral injury due to the veteran’s identification with the same military system as the perpetrator.42 When legal-involved veterans witness other incarcerated individuals engaging in PMIEs (eg, physical violence toward other inmates), this may be experienced as similar to betrayal due to lack of personal identification with the criminal-legal system. Additional research is needed to better understand how self- and other-related moral injury are associated with betrayal in legal contexts.
Another potential driver of legal-related moral injury may be culpability. In order for moral injury to occur, an individual must perceive that something has taken place that deeply violated their sense of right and wrong.1 In terms of criminal offenses or even engaging in violent behavior while incarcerated, the potential for moral injury may differ based on whether an individual views themselves as culpable for the act(s).29 This may further distinguish between self-directed and other-directed moral injury in legal contexts. In situations where the individual views themselves as culpable, self-directed moral injury may be higher. In situations where the individual does not view themselves as culpable, other-directed moral injury may be higher based on the perception that the legal system is unfairly punishing them. Further research is needed to clarify how an individual’s view of their culpability relates to moral injury, as well as to elucidate which aspects of military service and legal involvement are most closely associated with moral injury among legal-involved veterans.
While this study treated legal-related and military-related moral injury as distinct, it is possible moral injury may have a cumulative effect over time with individuals experiencing morally injurious events across different contexts (eg, military, legal involvement). This, in turn, may compound risk for moral injury. These cumulative experiences may result in increased negative outcomes such as exacerbated psychiatric symptoms, substance misuse, and elevated suicide risk. Future studies should examine differences between groups who have experienced moral injury in differing contexts, as well as those with multiple sources of moral injury.
Limitations
The sample for this study included only veterans. The number of veterans incarcerated is large and the focus on veterans also allowed for a more robust comparison of moral injury related to the legal system and the more traditional military-related moral injury. However, the generalizability of the findings to nonveterans cannot be assured. The study used a relatively small sample (N = 100), which was overwhelmingly male. Although the PCL-5 was utilized to examine traumatic stress symptoms, this measure was not anchored to a specific criterion A trauma nor was it anchored specifically to a morally injurious experience. For all participants, their most recent military service preceded their most recent legal involvement which could affect the associations between variables. Furthermore, while all participants endorsed prior legal involvement, many participants reported no combat exposure.
CONCLUSIONS
This study resulted in several key findings. First, legal-involved veterans endorsed high rates of experiencing legal-related morally injurious experiences. Second, our adapted measure displayed adequate psychometric strength and suggests that legal-related moral injury is a salient and distinct phenomenon affecting legal-involved veterans. These items may not capture all the nuances of legal-related moral injury. Qualitative interviews with legal-involved persons may help identify relevant areas of legal-related moral injury not reflected in the current instrument. The MIES-LIP represents a practical measure that may help clinicians identify and address legal-related moral injury when working with legal-involved veterans. Given the high prevalence of PMIEs among legal-involved veterans, further examination of whether current interventions for moral injury and novel treatments being developed are effective for this population is needed.
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Currier JM, Holland JM, Malott J. Moral injury, meaning making, and mental health in returning veterans. J Clin Psychol. 2015;71(3):229-240. doi:10.1002/jclp.22134
- Jinkerson JD. Defining and assessing moral injury: a syndrome perspective. Traumatology. 2016;22(2):122-130. doi:10.1037/trm0000069
- Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
- Maguen S, Litz B. Moral injury in veterans of war. PTSD Res Q. 2012;23(1):1-6. www.vva1071.org/uploads/3/4/4/6/34460116/moral_injury_in_veterans_of_war.pdf
- Drescher KD, Foy DW, Kelly C, Leshner A, Schutz K, Litz B. An exploration of the viability and usefulness of the construct of moral injury in war veterans. Traumatology. 2011;17(1):8-13. doi:10.1177/1534765610395615
- Wisco BE, Marx BP, May CL, et al. Moral injury in U.S. combat veterans: results from the national health and resilience in veterans study. Depress Anxiety. 2017; 34(4):340-347. doi:10.1002/da.22614
- Bryan CJ, Bryan AO, Anestis MD, et al. Measuring moral injury: psychometric properties of the moral injury events scale in two military samples. Assessment. 2016;23(5):557- 570. doi:10.1177/1073191115590855
- Currier JM, Smith PN, Kuhlman S. Assessing the unique role of religious coping in suicidal behavior among U.S. Iraq and Afghanistan veterans. Psychol Relig Spiritual. 2017;9(1):118-123. doi:10.1037/rel0000055
- Kopacz MS, Connery AL, Bishop TM, et al. Moral injury: a new challenge for complementary and alternative medicine. Complement Ther Med. 2016;24:29-33. doi:10.1016/j.ctim.2015.11.003
- Vargas AF, Hanson T, Kraus D, Drescher K, Foy D. Moral injury themes in combat veterans’ narrative responses from the national vietnam veterans’ readjustment study. Traumatology. 2013;19(3):243-250. doi:10.1177/1534765613476099
- Borges LM, Barnes SM, Farnsworth JK, Bahraini NH, Brenner LA. A commentary on moral injury among health care providers during the COVID-19 pandemic. Psychol Trauma. 2020;12(S1):S138-S140. doi:10.1037/tra0000698
- Borges LM, Holliday R, Barnes SM, et al. A longitudinal analysis of the role of potentially morally injurious events on COVID-19-related psychosocial functioning among healthcare providers. PLoS One. 2021;16(11):e0260033. doi:10.1371/journal.pone.0260033
- Currier JM, Holland JM, Rojas-Flores L, Herrera S, Foy D. Morally injurious experiences and meaning in Salvadorian teachers exposed to violence. Psychol Trauma. 2015;7(1):24-33. doi:10.1037/a0034092
- Nickerson A, Schnyder U, Bryant RA, Schick M, Mueller J, Morina N. Moral injury in traumatized refugees. Psychother Psychosom. 2015;84(2):122-123. doi:10.1159/000369353
- Papazoglou K, Chopko B. The role of moral suffering (moral distress and moral injury) in police compassion fatigue and PTSD: An unexplored topic. Front Psychol. 2017;8:1999. doi:10.3389/fpsyg.2017.01999
- Papazoglou K, Blumberg DM, Chiongbian VB, et al. The role of moral injury in PTSD among law enforcement officers: a brief report. Front Psychol. 2020;11:310. doi:10.3389/fpsyg.2020.00310
- Martin WB, Holliday R, LePage JP. Trauma and diversity: moral injury among justice involved veterans: an understudied clinical concern. Stresspoints. 2020;33(5).
- Currier JM, Drescher KD, Nieuwsma J. Future directions for addressing moral injury in clinical practice: concluding comments. In: Currier JM, Drescher KD, Nieuwsma J, eds. Addressing Moral Injury in Clinical Practice. American Psychological Association; 2021:261-271. doi:10.1037/0000204-015
- Alexander AR, Mendez L, Kerig PK. Moral injury as a transdiagnostic risk factor for mental health problems in detained youth. Crim Justice Behav. 2023;51(2):194-212. doi:10.1177/00938548231208203
- DeCaro JB, Straka K, Malek N, Zalta AK. Sentenced to shame: moral injury exposure in former lifers. Psychol Trauma. 2024; 15(5):722-730. doi:10.1037/tra0001400
- Orak U, Kelton K, Vaughn MG, Tsai J, Pietrzak RH. Homelessness and contact with the criminal legal system among U.S. combat veterans: an exploration of potential mediating factors. Crim Justice Behav. 2022;50(3):392-409. doi:10.1177/00938548221140352
- Bronson J, Carson EA, Noonan M. Veterans in Prison and Jail, 2011-12. US Department of Justice, Bureau of Justice Statistics; Published December 2015. Accessed March 4, 2025. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
- Maruschak LM, Bronson J, Alper M. Veterans in Prison: Survey of Prison Inmates, 2016. US Department of Justice, Bureau of Justice Statistics; March 2021. Accessed March 4, 2025. https://bjs.ojp.gov/redirect-legacy/content/pub/pdf/vpspi16st.pdf
- Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justiceinvolved veterans. Epidemiol Rev. 2015;37:163-176. doi:10.1093/epirev/mxu003
- Finlay AK, Owens MD, Taylor E, et al. A scoping review of military veterans involved in the criminal justice system and their health and healthcare. Health Justice. 2019;7(1):6. doi:10.1186/s40352-019-0086-9
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Wortzel HS, Binswanger IA, Anderson CA, Adler LE. Suicide among incarcerated veterans. J Am Acad Psychiatry Law. 2009;37(1):82-91.
- Desai A, Holliday R, Borges LM, et al. Facilitating successful reentry among justice-involved veterans: the role of veteran and offender identity. J Psychiatr Pract. 2021;27(1):52-60. doi:10.1097/PRA.0000000000000520
- Asencio EK, Burke PJ. Does incarceration change the criminal identity? A synthesis of labeling and identity theory perspectives on identity change. Sociol Perspect. 2011;54(2):163-182. doi:10.1525/sop.2011.54.2.163
- Borges LM, Desai A, Barnes SM, Johnson JPS. The role of social determinants of health in moral injury: implications and future directions. Curr Treat Options Psychiatry. 2022;9(3):202-214. doi:10.1007/s40501-022-00272-4
- Houle SA, Ein N, Gervasio J, et al. Measuring moral distress and moral injury: a systematic review and content analysis of existing scales. Clin Psychol Rev. 2024;108:102377. doi:10.1016/j.cpr.2023.102377
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the moral injury events scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Zerach G, Ben-Yehuda A, Levi-Belz Y. Prospective associations between psychological factors, potentially morally injurious events, and psychiatric symptoms among Israeli combatants: the roles of ethical leadership and ethical preparation. Psychol Trauma. 2023;15(8):1367-1377. doi:10.1037/tra0001466
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013.
- Weathers FW, Litz BT, Keane TM, Palmeri PA, Marx BP. The PTSD Checklist for DSM-5 (PCL-5). National Center for PTSD. Accessed March 4, 2025. www.ptsd.va.gov
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379-1391. doi:10.1037/pas0000254
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The osttraumatic stress disorder checklist for DSM-5 (PCL- 5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Kroenke K, Spi tzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Brown TA. Confirmatory Factor Analysis for Applied Research. 2nd ed. Guilford Press; 2015.
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20(1):141-151. doi:10.1177/001316446002000116
- Schorr Y, Stein NR, Maguen S, Barnes JB, Bosch J, Litz BT. Sources of moral injury among war veterans: a qualitative evaluation. J Clin Psychol. 2018;74(12):2203-2218. doi:10.1002/jclp.22660
Following exposure to potentially morally injurious events (PMIEs), some individuals may experience moral injury, which represents negative psychological, social, behavioral, and occasionally spiritual impacts.1 The consequences of PMIE exposure and moral injury are well documented. Individuals may begin to question the goodness and trustworthiness of oneself, others, or the world.1 Examples of other sequelae include guilt, demoralization, spiritual pain, loss of trust in the self or others, and difficulties with forgiveness.2-6 In addition, prior studies have found that moral injury is associated with an increased risk of suicidal thoughts and behaviors, posttraumatic stress disorder (PTSD) symptoms, spiritual distress, and interpersonal difficulties.7-11
Moral injury was first conceptualized in relation to combat trauma. However in recent years it has been examined in other groups such as health care practitioners, educators, refugees, and law enforcement personnel.12-17 Furthermore, there has been a recent call for the study of moral injury in other understudied groups. One such group is legal-involved individuals, defined as those who are currently involved or previously involved in the criminal justice system (ie, arrests, incarceration, parole, and probation).1,18-22
Many veterans are also involved with the legal system. Specifically, veterans currently comprise about 8% of the incarcerated US population, with an estimated > 180,000 veterans in prisons or jails and even more on parole or probation.23,24 Legal-involved veterans may be at heightened risk for homelessness, suicide, unemployment, and high prevalence rates of psychiatric diagnoses.25-28
Limited research has explored exposure to PMIEs as part of the legal process and the resulting expression of moral injury. The circumstances leading to incarceration, interactions with the US legal system, the environment of prison itself, and the subsequent challenges faced by legal-involved individuals after release all provide ample opportunity for PMIEs to occur.18 For example, engaging in a criminal act may represent a PMIE, particularly in violent offenses that involve harm to another individual. Moreover, the process of being convicted and charged with an offense may serve as a powerful reminder of the PMIE and tie this event to the individual’s identity and future. Furthermore, the physical and social environment of prison itself (eg, being surrounded by other offenders, witnessing the perpetration of violence, participating in violence for survival) presents a myriad of opportunities for PMIEs to occur.18
The consequences of PMIEs in the context of legal involvement may also have bearing on a touchstone of moral injury: changes in one’s schema of the self and world.4 At a societal level, legal-involved individuals are, by definition, deemed “guilty” and held culpable for their offense, which may reinforce a negative change in one’s view of self and the world.29 In line with identity theory, external negative appraisals about legal-involved individuals (eg, they are a danger to society, they cannot be trusted to do the right thing) may influence their self-perception.30 Furthermore, the affective characteristics often found in the context of moral injury (eg, guilt, shame, anger, contempt) may be exacerbated by legal involvement.29 Personal feelings of guilt and shame may be reinforced by receiving a verdict and sentence, as well as the negative perceptions of individuals around them (eg, disapproval from prior sources of social support). Additionally, feelings of betrayal and distrust towards the legal system may arise.
In sum, legal-involved veterans incur increased risk of moral injury due to the potential for exposure to PMIEs across multiple time points (eg, prior to military service, during military service, during arrest/sentencing, during imprisonment, and postincarceration). The stigma that accompanies legal involvement may limit access to treatment or a willingness to seek treatment for distress related to moral injury.29 Additionally, repeated exposure to PMIEs and resulting moral injury may compound over time, potentially exacerbating psychosocial functioning and increasing the risk for psychosocial stressors (eg, homelessness, unemployment) and mental health disorders (eg, depression, substance misuse).31
Although numerous measures of moral injury have been developed, most require that respondents consider a specific context (eg, military experiences).32 Therefore, study of legal-related moral injury requires adaptation of existing instruments to the legal context. The original and most commonly used scale of moral injury is the Moral Injury Events Scale (MIES).33 The MIES scales was originally developed to measure moral injury in military-related contexts but has since been adapted as a measure of exposure to context-specific PMIEs.34
Unfortunately, there are no validated measures for assessing legal-related moral injury. Such a gap in understanding is problematic, as it may impact measurement of the prevalence of PMIEs in both clinical and research settings for this at-risk population. The goal of this study was to conduct a psychometric evaluation of an adapted version of the MIES for legal-involved persons (MIES-LIP).
METHODS
A total of 177 veterans from the US Department of Veterans Affairs (VA) North Texas Health Care System were contacted for study enrollment between November 2020 and June 2021, yielding a final sample of 100 legal-involved veteran participants. Adults aged ≥ 18 years who were US military veterans and had ≥ 1 prior felony conviction resulting in incarceration were included. Participants were excluded if they had symptoms of psychosis that would preclude meaningful participation.
The study collected data on participants’ demographic and clinical characteristics using a semistructured survey instrument. Each participant completed an instructor-led questionnaire in a session that lasted about 1.5 hours. Participants who completed the visit in person received a $50 cash voucher for their time. Participants who were unable to meet with the study coordinator in person were able to complete the visit via telephone and received a $25 digital gift card. Of the total 100 participants, 79 participants completed the interview in person, and 21 completed by telephone. No significant differences were found in assessment measures between administration methods. Written informed consent was obtained during all in-person visits. For those completing via telephone, a waiver of written informed consent was obtained. This study was approved by the VA North Texas Health Care System’s Institutional Review Board.
Measures
The Moral Injury Events Scale (MIES) is a 9-item self-report measure that assesses exposure to PMIEs.33 Respondents rate their agreement with each item on a 6-point Likert scale (strongly disagree to strongly agree), with higher scores indicating greater moral injury. The MIES has a 2-factor structure: Factor 1 has 6 items on perceived transgressions and Factor 2 has 3 items on perceived betrayals.33
Creation of Legal-Involved Moral Injury Measure. To create the MIES-LIP, items and instructions from the MIES were modified to address moral injury in the context of legal involvement.33 Adaptations were finalized following consultation and approval by the authors of the original measure. Specifically, the instructions were changed to: “Please respond to these items based specifically in the context of your involvement with the legal system.” The instructions clarified that legal involvement could include experiences related to committing an offense, legal proceedings and sentencing, incarceration, or transitioning out of the legal system. This differs from the original measure, which focused on military experiences, with instructions stating: “Please respond to these items based specifically in the context of your military service (ie, events and experiences during enlistment, deployment, combat, etc).”
Other measures. The study collected data on demographic characteristics including sex, race and ethnicity, marital status, military service, combat experience, and legal involvement. PTSD symptom severity, based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), was assessed using the PTSD Checklist for DSM-5 (PCL-5).35,36 The PCL-5 is a 20-item self-report measure in which item scores are summed to create a total score. The PCL-5 has demonstrated strong psychometric properties, including good internal consistency, test-retest reliability convergent validity, and discriminant validity.37,38
Depressive symptom severity was measured using the Personal Health Questionnaire-9 (PHQ-9).39 The PHQ-9 is a 9-item self-report measure where item scores summed to create a total score. The PHQ-9 has demonstrated strong psychometric properties, including internal consistency and test-retest reliability.39
STATISTICAL METHODS
Descriptive statistics (mean and standard deviation for continuous variables; frequencies and percentages for categorical variables) were used to describe the study sample. Factor analysis was conducted to evaluate the psychometric properties of the MIES-LIP. Confirmatory factor analysis (CFA) was used to determine whether the MEIS-LIP had a similar factor structure to the MIES.40 Criteria for fit indices used for CFA include the Comparative Fit Index (CFI; values of > 0.95 suggest good fit), Tucker-Lewis index (TLI; values of > 0.95 suggest a good fit), root mean square error of approximation (RMSEA; values of ≥ 0.06 suggest good fit), and standardized root mean square residual (SRMR; values of ≥ 0.08 suggest good fit). With insufficient fit, subsequent exploratory factor analysis was conducted using maximum likelihood estimation with an Oblimin rotation. The Kaiser rule and a scree plot were considered when defining the factor structure. Reliability was evaluated using the McDonald omega coefficient test. Convergent validity was assessed through the association between adapted measures and other clinical measures (ie, PCL-5, PHQ-9). In addition, associations between the PCL-5 and PHQ-9 were examined as they related to the MIES and MIES-LIP.
RESULTS
Table 1 describes demographic characteristics of the study sample. Rates of potentially morally injurious experiences and the expression of moral injury in the legal context are presented in Table 2. Witnessing PMIEs while in the legal system was nearly ubiquitous, with > 90% of the sample endorsing this experience. More than half of the sample also endorsed engaging in morally injurious behavior by commission or omission, as well as experiencing betrayal while involved with the legal system.


Factor Analysis
Confirmatory factor analysis (CFA) was utilized to test the factor structure of the adapted MIES-LIP in our sample compared to the published factor structures of the MIES.33 Results did not support the established factor structure. Analysis yielded unacceptable CFI (0.79), TLI (0.70), SRMR (0.14), and RMSEA (0.21). The unsatisfactory results of CFA warranted follow-up exploratory factor analysis (EFA) to examine the factor structure of the moral injury scales in this sample.
EFA of MIES-LIP
The factor structure of the MIES-LIP was examined using EFA. The factorability of the data was examined using the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO value = 0.75) and Bartlett Test of Sphericity (X2 = 525.41; P < .001), both of which suggested that the data were appropriate for factor analysis. The number of factors to retain was selected based on the Kaiser criterion.41 After extraction, an Oblimin rotation was applied, given that we expected factors to be correlated. A 2-factor solution was found, explaining 65.76% of the common variance. All 9 items were retained as they had factor loadings > 0.30. Factor 1, comprised self-directed moral injury questions (3-6). Factor 2 comprised other directed moral injury questions (1, 2, 7-9) (Table 3). The factor correlation coefficient between Factor 1 and Factor 2 was 0.34, which supports utilizing an oblique rotation.

Reliability. We examined the reliability of the adapted MIES-LIP using measures of internal consistency, with both MIES-LIP factors demonstrating good reliability. The internal consistency of both factors of the MIES-LIP were found to be good (self-directed moral injury: Ω = 0.89; other-directed moral injury: Ω = 0.83).
Convergent Validity
Association between moral injury scales. A significant, moderate correlation was observed between all subscales of the MIES and MIES-LIP. Specifically, the self-directed moral injury factor of the MIES-LIP was associated with both the perceived transgressions (r = 0.41, P < .001) and the MIES perceived betrayals factors (r = 0.25, P < .05). Similarly, the other-directed moral injury factor of the MIES-LIP was associated with both the MIES perceived transgressions (r = 0.45, P < .001) and the MIES perceived betrayals factors (r = 0.45, P < .001).
Association with PTSD symptoms. All subscales of both the MIES and MIES-LIP were associated with PTSD symptom severity. The MIES perceived transgressions factor (r = 0.43, P < .001) and the perceived betrayals factor of the MIES (r = 0.39, P < .001) were moderately associated with the PCL-5. Mirroring this, the “self-directed moral injury” factor of the MIESLIP (r = 0.44, P < .001) and the “other-directed moral injury” factor of the MIES-LIP (r = 0.42, P < .001) were also positively associated with PCL-5.
Association with depression symptoms. All subscales of the MIES and MIES-LIP were also associated with depressive symptoms. The MIES perceived transgressions factor (r = 0.27, P < .01) and the MIES perceived betrayals factor (r = 0.23, P < .05) had a small association with the PHQ-9. In addition, the self-directed moral injury factor of the MIES-LIP (r = 0.40, P < .001) and the other-directed moral injury factor of the MIES-LIP (r = 0.31, P < .01) had small to moderate associations with the PCL-5.
DISCUSSION
Potentially morally injurious events appear to be a salient factor affecting legal-involved veterans. Among our sample, the vast majority of legal-involved veterans endorsed experiencing both legal- and military-related PMIEs. Witnessing or participating in a legal-related PMIE appears to be widespread among those who have experienced incarceration. The MIES-LIP yielded a 2-factor structure: self-directed moral injury and other-directed moral injury, in the evaluated population. The MIES-LIP showed similar psychometric performance to the MIES in our sample. Specifically, the MIES-LIP had good reliability and adequate convergent validity. While CFA did not confirm the anticipated factor structure of the MIES-LIP within our sample, EFA showed similarities in factor structure between the original and adapted measures. While further research and validation are needed, preliminary results show promise of the MIES-LIP in assessing legal-related moral injury.
Originally, the MIES was found to have a 2-factor structure, defined by perceived transgressions and perceived betrayals.33 However, additional research has identified a 3-factor structure, where the betrayal factor is maintained, and the transgressions factor is divided into transgressions by others and by self.8 The factor structure of the MIES-LIP was more closely related to the factor structure, with transgressions by others and betrayal mapped onto the same factor (ie, other-directed moral injury).8 While further research is needed, it is possible that the nature of morally injurious events experienced in legal contexts are experienced more in terms of self vs other, compared to morally injurious events experienced by veterans or active-duty service members.
Accurately identifying the types of moral injury experienced in a legal context may be important for determining the differences in drivers of legal-related moral injury compared to military-related moral injury. For example, self-directed moral injury in legal contexts may include a variety of actions the individual initiated that led to conviction and incarceration (eg, a criminal offense), as well as behaviors performed or witnessed while incarcerated (eg, engaging in violence). Inconsistent with military populations where other-directed moral injury clusters with self-directed moral injury, other-directed moral injury clustered with betrayal in legal contexts in our sample. This discrepancy may result from differences in identification with the military vs legal system. When veterans witness fellow service members engaging in PMIEs (eg, physical violence towards civilians in a military setting), this may be similar to self-directed moral injury due to the veteran’s identification with the same military system as the perpetrator.42 When legal-involved veterans witness other incarcerated individuals engaging in PMIEs (eg, physical violence toward other inmates), this may be experienced as similar to betrayal due to lack of personal identification with the criminal-legal system. Additional research is needed to better understand how self- and other-related moral injury are associated with betrayal in legal contexts.
Another potential driver of legal-related moral injury may be culpability. In order for moral injury to occur, an individual must perceive that something has taken place that deeply violated their sense of right and wrong.1 In terms of criminal offenses or even engaging in violent behavior while incarcerated, the potential for moral injury may differ based on whether an individual views themselves as culpable for the act(s).29 This may further distinguish between self-directed and other-directed moral injury in legal contexts. In situations where the individual views themselves as culpable, self-directed moral injury may be higher. In situations where the individual does not view themselves as culpable, other-directed moral injury may be higher based on the perception that the legal system is unfairly punishing them. Further research is needed to clarify how an individual’s view of their culpability relates to moral injury, as well as to elucidate which aspects of military service and legal involvement are most closely associated with moral injury among legal-involved veterans.
While this study treated legal-related and military-related moral injury as distinct, it is possible moral injury may have a cumulative effect over time with individuals experiencing morally injurious events across different contexts (eg, military, legal involvement). This, in turn, may compound risk for moral injury. These cumulative experiences may result in increased negative outcomes such as exacerbated psychiatric symptoms, substance misuse, and elevated suicide risk. Future studies should examine differences between groups who have experienced moral injury in differing contexts, as well as those with multiple sources of moral injury.
Limitations
The sample for this study included only veterans. The number of veterans incarcerated is large and the focus on veterans also allowed for a more robust comparison of moral injury related to the legal system and the more traditional military-related moral injury. However, the generalizability of the findings to nonveterans cannot be assured. The study used a relatively small sample (N = 100), which was overwhelmingly male. Although the PCL-5 was utilized to examine traumatic stress symptoms, this measure was not anchored to a specific criterion A trauma nor was it anchored specifically to a morally injurious experience. For all participants, their most recent military service preceded their most recent legal involvement which could affect the associations between variables. Furthermore, while all participants endorsed prior legal involvement, many participants reported no combat exposure.
CONCLUSIONS
This study resulted in several key findings. First, legal-involved veterans endorsed high rates of experiencing legal-related morally injurious experiences. Second, our adapted measure displayed adequate psychometric strength and suggests that legal-related moral injury is a salient and distinct phenomenon affecting legal-involved veterans. These items may not capture all the nuances of legal-related moral injury. Qualitative interviews with legal-involved persons may help identify relevant areas of legal-related moral injury not reflected in the current instrument. The MIES-LIP represents a practical measure that may help clinicians identify and address legal-related moral injury when working with legal-involved veterans. Given the high prevalence of PMIEs among legal-involved veterans, further examination of whether current interventions for moral injury and novel treatments being developed are effective for this population is needed.
Following exposure to potentially morally injurious events (PMIEs), some individuals may experience moral injury, which represents negative psychological, social, behavioral, and occasionally spiritual impacts.1 The consequences of PMIE exposure and moral injury are well documented. Individuals may begin to question the goodness and trustworthiness of oneself, others, or the world.1 Examples of other sequelae include guilt, demoralization, spiritual pain, loss of trust in the self or others, and difficulties with forgiveness.2-6 In addition, prior studies have found that moral injury is associated with an increased risk of suicidal thoughts and behaviors, posttraumatic stress disorder (PTSD) symptoms, spiritual distress, and interpersonal difficulties.7-11
Moral injury was first conceptualized in relation to combat trauma. However in recent years it has been examined in other groups such as health care practitioners, educators, refugees, and law enforcement personnel.12-17 Furthermore, there has been a recent call for the study of moral injury in other understudied groups. One such group is legal-involved individuals, defined as those who are currently involved or previously involved in the criminal justice system (ie, arrests, incarceration, parole, and probation).1,18-22
Many veterans are also involved with the legal system. Specifically, veterans currently comprise about 8% of the incarcerated US population, with an estimated > 180,000 veterans in prisons or jails and even more on parole or probation.23,24 Legal-involved veterans may be at heightened risk for homelessness, suicide, unemployment, and high prevalence rates of psychiatric diagnoses.25-28
Limited research has explored exposure to PMIEs as part of the legal process and the resulting expression of moral injury. The circumstances leading to incarceration, interactions with the US legal system, the environment of prison itself, and the subsequent challenges faced by legal-involved individuals after release all provide ample opportunity for PMIEs to occur.18 For example, engaging in a criminal act may represent a PMIE, particularly in violent offenses that involve harm to another individual. Moreover, the process of being convicted and charged with an offense may serve as a powerful reminder of the PMIE and tie this event to the individual’s identity and future. Furthermore, the physical and social environment of prison itself (eg, being surrounded by other offenders, witnessing the perpetration of violence, participating in violence for survival) presents a myriad of opportunities for PMIEs to occur.18
The consequences of PMIEs in the context of legal involvement may also have bearing on a touchstone of moral injury: changes in one’s schema of the self and world.4 At a societal level, legal-involved individuals are, by definition, deemed “guilty” and held culpable for their offense, which may reinforce a negative change in one’s view of self and the world.29 In line with identity theory, external negative appraisals about legal-involved individuals (eg, they are a danger to society, they cannot be trusted to do the right thing) may influence their self-perception.30 Furthermore, the affective characteristics often found in the context of moral injury (eg, guilt, shame, anger, contempt) may be exacerbated by legal involvement.29 Personal feelings of guilt and shame may be reinforced by receiving a verdict and sentence, as well as the negative perceptions of individuals around them (eg, disapproval from prior sources of social support). Additionally, feelings of betrayal and distrust towards the legal system may arise.
In sum, legal-involved veterans incur increased risk of moral injury due to the potential for exposure to PMIEs across multiple time points (eg, prior to military service, during military service, during arrest/sentencing, during imprisonment, and postincarceration). The stigma that accompanies legal involvement may limit access to treatment or a willingness to seek treatment for distress related to moral injury.29 Additionally, repeated exposure to PMIEs and resulting moral injury may compound over time, potentially exacerbating psychosocial functioning and increasing the risk for psychosocial stressors (eg, homelessness, unemployment) and mental health disorders (eg, depression, substance misuse).31
Although numerous measures of moral injury have been developed, most require that respondents consider a specific context (eg, military experiences).32 Therefore, study of legal-related moral injury requires adaptation of existing instruments to the legal context. The original and most commonly used scale of moral injury is the Moral Injury Events Scale (MIES).33 The MIES scales was originally developed to measure moral injury in military-related contexts but has since been adapted as a measure of exposure to context-specific PMIEs.34
Unfortunately, there are no validated measures for assessing legal-related moral injury. Such a gap in understanding is problematic, as it may impact measurement of the prevalence of PMIEs in both clinical and research settings for this at-risk population. The goal of this study was to conduct a psychometric evaluation of an adapted version of the MIES for legal-involved persons (MIES-LIP).
METHODS
A total of 177 veterans from the US Department of Veterans Affairs (VA) North Texas Health Care System were contacted for study enrollment between November 2020 and June 2021, yielding a final sample of 100 legal-involved veteran participants. Adults aged ≥ 18 years who were US military veterans and had ≥ 1 prior felony conviction resulting in incarceration were included. Participants were excluded if they had symptoms of psychosis that would preclude meaningful participation.
The study collected data on participants’ demographic and clinical characteristics using a semistructured survey instrument. Each participant completed an instructor-led questionnaire in a session that lasted about 1.5 hours. Participants who completed the visit in person received a $50 cash voucher for their time. Participants who were unable to meet with the study coordinator in person were able to complete the visit via telephone and received a $25 digital gift card. Of the total 100 participants, 79 participants completed the interview in person, and 21 completed by telephone. No significant differences were found in assessment measures between administration methods. Written informed consent was obtained during all in-person visits. For those completing via telephone, a waiver of written informed consent was obtained. This study was approved by the VA North Texas Health Care System’s Institutional Review Board.
Measures
The Moral Injury Events Scale (MIES) is a 9-item self-report measure that assesses exposure to PMIEs.33 Respondents rate their agreement with each item on a 6-point Likert scale (strongly disagree to strongly agree), with higher scores indicating greater moral injury. The MIES has a 2-factor structure: Factor 1 has 6 items on perceived transgressions and Factor 2 has 3 items on perceived betrayals.33
Creation of Legal-Involved Moral Injury Measure. To create the MIES-LIP, items and instructions from the MIES were modified to address moral injury in the context of legal involvement.33 Adaptations were finalized following consultation and approval by the authors of the original measure. Specifically, the instructions were changed to: “Please respond to these items based specifically in the context of your involvement with the legal system.” The instructions clarified that legal involvement could include experiences related to committing an offense, legal proceedings and sentencing, incarceration, or transitioning out of the legal system. This differs from the original measure, which focused on military experiences, with instructions stating: “Please respond to these items based specifically in the context of your military service (ie, events and experiences during enlistment, deployment, combat, etc).”
Other measures. The study collected data on demographic characteristics including sex, race and ethnicity, marital status, military service, combat experience, and legal involvement. PTSD symptom severity, based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), was assessed using the PTSD Checklist for DSM-5 (PCL-5).35,36 The PCL-5 is a 20-item self-report measure in which item scores are summed to create a total score. The PCL-5 has demonstrated strong psychometric properties, including good internal consistency, test-retest reliability convergent validity, and discriminant validity.37,38
Depressive symptom severity was measured using the Personal Health Questionnaire-9 (PHQ-9).39 The PHQ-9 is a 9-item self-report measure where item scores summed to create a total score. The PHQ-9 has demonstrated strong psychometric properties, including internal consistency and test-retest reliability.39
STATISTICAL METHODS
Descriptive statistics (mean and standard deviation for continuous variables; frequencies and percentages for categorical variables) were used to describe the study sample. Factor analysis was conducted to evaluate the psychometric properties of the MIES-LIP. Confirmatory factor analysis (CFA) was used to determine whether the MEIS-LIP had a similar factor structure to the MIES.40 Criteria for fit indices used for CFA include the Comparative Fit Index (CFI; values of > 0.95 suggest good fit), Tucker-Lewis index (TLI; values of > 0.95 suggest a good fit), root mean square error of approximation (RMSEA; values of ≥ 0.06 suggest good fit), and standardized root mean square residual (SRMR; values of ≥ 0.08 suggest good fit). With insufficient fit, subsequent exploratory factor analysis was conducted using maximum likelihood estimation with an Oblimin rotation. The Kaiser rule and a scree plot were considered when defining the factor structure. Reliability was evaluated using the McDonald omega coefficient test. Convergent validity was assessed through the association between adapted measures and other clinical measures (ie, PCL-5, PHQ-9). In addition, associations between the PCL-5 and PHQ-9 were examined as they related to the MIES and MIES-LIP.
RESULTS
Table 1 describes demographic characteristics of the study sample. Rates of potentially morally injurious experiences and the expression of moral injury in the legal context are presented in Table 2. Witnessing PMIEs while in the legal system was nearly ubiquitous, with > 90% of the sample endorsing this experience. More than half of the sample also endorsed engaging in morally injurious behavior by commission or omission, as well as experiencing betrayal while involved with the legal system.


Factor Analysis
Confirmatory factor analysis (CFA) was utilized to test the factor structure of the adapted MIES-LIP in our sample compared to the published factor structures of the MIES.33 Results did not support the established factor structure. Analysis yielded unacceptable CFI (0.79), TLI (0.70), SRMR (0.14), and RMSEA (0.21). The unsatisfactory results of CFA warranted follow-up exploratory factor analysis (EFA) to examine the factor structure of the moral injury scales in this sample.
EFA of MIES-LIP
The factor structure of the MIES-LIP was examined using EFA. The factorability of the data was examined using the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO value = 0.75) and Bartlett Test of Sphericity (X2 = 525.41; P < .001), both of which suggested that the data were appropriate for factor analysis. The number of factors to retain was selected based on the Kaiser criterion.41 After extraction, an Oblimin rotation was applied, given that we expected factors to be correlated. A 2-factor solution was found, explaining 65.76% of the common variance. All 9 items were retained as they had factor loadings > 0.30. Factor 1, comprised self-directed moral injury questions (3-6). Factor 2 comprised other directed moral injury questions (1, 2, 7-9) (Table 3). The factor correlation coefficient between Factor 1 and Factor 2 was 0.34, which supports utilizing an oblique rotation.

Reliability. We examined the reliability of the adapted MIES-LIP using measures of internal consistency, with both MIES-LIP factors demonstrating good reliability. The internal consistency of both factors of the MIES-LIP were found to be good (self-directed moral injury: Ω = 0.89; other-directed moral injury: Ω = 0.83).
Convergent Validity
Association between moral injury scales. A significant, moderate correlation was observed between all subscales of the MIES and MIES-LIP. Specifically, the self-directed moral injury factor of the MIES-LIP was associated with both the perceived transgressions (r = 0.41, P < .001) and the MIES perceived betrayals factors (r = 0.25, P < .05). Similarly, the other-directed moral injury factor of the MIES-LIP was associated with both the MIES perceived transgressions (r = 0.45, P < .001) and the MIES perceived betrayals factors (r = 0.45, P < .001).
Association with PTSD symptoms. All subscales of both the MIES and MIES-LIP were associated with PTSD symptom severity. The MIES perceived transgressions factor (r = 0.43, P < .001) and the perceived betrayals factor of the MIES (r = 0.39, P < .001) were moderately associated with the PCL-5. Mirroring this, the “self-directed moral injury” factor of the MIESLIP (r = 0.44, P < .001) and the “other-directed moral injury” factor of the MIES-LIP (r = 0.42, P < .001) were also positively associated with PCL-5.
Association with depression symptoms. All subscales of the MIES and MIES-LIP were also associated with depressive symptoms. The MIES perceived transgressions factor (r = 0.27, P < .01) and the MIES perceived betrayals factor (r = 0.23, P < .05) had a small association with the PHQ-9. In addition, the self-directed moral injury factor of the MIES-LIP (r = 0.40, P < .001) and the other-directed moral injury factor of the MIES-LIP (r = 0.31, P < .01) had small to moderate associations with the PCL-5.
DISCUSSION
Potentially morally injurious events appear to be a salient factor affecting legal-involved veterans. Among our sample, the vast majority of legal-involved veterans endorsed experiencing both legal- and military-related PMIEs. Witnessing or participating in a legal-related PMIE appears to be widespread among those who have experienced incarceration. The MIES-LIP yielded a 2-factor structure: self-directed moral injury and other-directed moral injury, in the evaluated population. The MIES-LIP showed similar psychometric performance to the MIES in our sample. Specifically, the MIES-LIP had good reliability and adequate convergent validity. While CFA did not confirm the anticipated factor structure of the MIES-LIP within our sample, EFA showed similarities in factor structure between the original and adapted measures. While further research and validation are needed, preliminary results show promise of the MIES-LIP in assessing legal-related moral injury.
Originally, the MIES was found to have a 2-factor structure, defined by perceived transgressions and perceived betrayals.33 However, additional research has identified a 3-factor structure, where the betrayal factor is maintained, and the transgressions factor is divided into transgressions by others and by self.8 The factor structure of the MIES-LIP was more closely related to the factor structure, with transgressions by others and betrayal mapped onto the same factor (ie, other-directed moral injury).8 While further research is needed, it is possible that the nature of morally injurious events experienced in legal contexts are experienced more in terms of self vs other, compared to morally injurious events experienced by veterans or active-duty service members.
Accurately identifying the types of moral injury experienced in a legal context may be important for determining the differences in drivers of legal-related moral injury compared to military-related moral injury. For example, self-directed moral injury in legal contexts may include a variety of actions the individual initiated that led to conviction and incarceration (eg, a criminal offense), as well as behaviors performed or witnessed while incarcerated (eg, engaging in violence). Inconsistent with military populations where other-directed moral injury clusters with self-directed moral injury, other-directed moral injury clustered with betrayal in legal contexts in our sample. This discrepancy may result from differences in identification with the military vs legal system. When veterans witness fellow service members engaging in PMIEs (eg, physical violence towards civilians in a military setting), this may be similar to self-directed moral injury due to the veteran’s identification with the same military system as the perpetrator.42 When legal-involved veterans witness other incarcerated individuals engaging in PMIEs (eg, physical violence toward other inmates), this may be experienced as similar to betrayal due to lack of personal identification with the criminal-legal system. Additional research is needed to better understand how self- and other-related moral injury are associated with betrayal in legal contexts.
Another potential driver of legal-related moral injury may be culpability. In order for moral injury to occur, an individual must perceive that something has taken place that deeply violated their sense of right and wrong.1 In terms of criminal offenses or even engaging in violent behavior while incarcerated, the potential for moral injury may differ based on whether an individual views themselves as culpable for the act(s).29 This may further distinguish between self-directed and other-directed moral injury in legal contexts. In situations where the individual views themselves as culpable, self-directed moral injury may be higher. In situations where the individual does not view themselves as culpable, other-directed moral injury may be higher based on the perception that the legal system is unfairly punishing them. Further research is needed to clarify how an individual’s view of their culpability relates to moral injury, as well as to elucidate which aspects of military service and legal involvement are most closely associated with moral injury among legal-involved veterans.
While this study treated legal-related and military-related moral injury as distinct, it is possible moral injury may have a cumulative effect over time with individuals experiencing morally injurious events across different contexts (eg, military, legal involvement). This, in turn, may compound risk for moral injury. These cumulative experiences may result in increased negative outcomes such as exacerbated psychiatric symptoms, substance misuse, and elevated suicide risk. Future studies should examine differences between groups who have experienced moral injury in differing contexts, as well as those with multiple sources of moral injury.
Limitations
The sample for this study included only veterans. The number of veterans incarcerated is large and the focus on veterans also allowed for a more robust comparison of moral injury related to the legal system and the more traditional military-related moral injury. However, the generalizability of the findings to nonveterans cannot be assured. The study used a relatively small sample (N = 100), which was overwhelmingly male. Although the PCL-5 was utilized to examine traumatic stress symptoms, this measure was not anchored to a specific criterion A trauma nor was it anchored specifically to a morally injurious experience. For all participants, their most recent military service preceded their most recent legal involvement which could affect the associations between variables. Furthermore, while all participants endorsed prior legal involvement, many participants reported no combat exposure.
CONCLUSIONS
This study resulted in several key findings. First, legal-involved veterans endorsed high rates of experiencing legal-related morally injurious experiences. Second, our adapted measure displayed adequate psychometric strength and suggests that legal-related moral injury is a salient and distinct phenomenon affecting legal-involved veterans. These items may not capture all the nuances of legal-related moral injury. Qualitative interviews with legal-involved persons may help identify relevant areas of legal-related moral injury not reflected in the current instrument. The MIES-LIP represents a practical measure that may help clinicians identify and address legal-related moral injury when working with legal-involved veterans. Given the high prevalence of PMIEs among legal-involved veterans, further examination of whether current interventions for moral injury and novel treatments being developed are effective for this population is needed.
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Currier JM, Holland JM, Malott J. Moral injury, meaning making, and mental health in returning veterans. J Clin Psychol. 2015;71(3):229-240. doi:10.1002/jclp.22134
- Jinkerson JD. Defining and assessing moral injury: a syndrome perspective. Traumatology. 2016;22(2):122-130. doi:10.1037/trm0000069
- Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
- Maguen S, Litz B. Moral injury in veterans of war. PTSD Res Q. 2012;23(1):1-6. www.vva1071.org/uploads/3/4/4/6/34460116/moral_injury_in_veterans_of_war.pdf
- Drescher KD, Foy DW, Kelly C, Leshner A, Schutz K, Litz B. An exploration of the viability and usefulness of the construct of moral injury in war veterans. Traumatology. 2011;17(1):8-13. doi:10.1177/1534765610395615
- Wisco BE, Marx BP, May CL, et al. Moral injury in U.S. combat veterans: results from the national health and resilience in veterans study. Depress Anxiety. 2017; 34(4):340-347. doi:10.1002/da.22614
- Bryan CJ, Bryan AO, Anestis MD, et al. Measuring moral injury: psychometric properties of the moral injury events scale in two military samples. Assessment. 2016;23(5):557- 570. doi:10.1177/1073191115590855
- Currier JM, Smith PN, Kuhlman S. Assessing the unique role of religious coping in suicidal behavior among U.S. Iraq and Afghanistan veterans. Psychol Relig Spiritual. 2017;9(1):118-123. doi:10.1037/rel0000055
- Kopacz MS, Connery AL, Bishop TM, et al. Moral injury: a new challenge for complementary and alternative medicine. Complement Ther Med. 2016;24:29-33. doi:10.1016/j.ctim.2015.11.003
- Vargas AF, Hanson T, Kraus D, Drescher K, Foy D. Moral injury themes in combat veterans’ narrative responses from the national vietnam veterans’ readjustment study. Traumatology. 2013;19(3):243-250. doi:10.1177/1534765613476099
- Borges LM, Barnes SM, Farnsworth JK, Bahraini NH, Brenner LA. A commentary on moral injury among health care providers during the COVID-19 pandemic. Psychol Trauma. 2020;12(S1):S138-S140. doi:10.1037/tra0000698
- Borges LM, Holliday R, Barnes SM, et al. A longitudinal analysis of the role of potentially morally injurious events on COVID-19-related psychosocial functioning among healthcare providers. PLoS One. 2021;16(11):e0260033. doi:10.1371/journal.pone.0260033
- Currier JM, Holland JM, Rojas-Flores L, Herrera S, Foy D. Morally injurious experiences and meaning in Salvadorian teachers exposed to violence. Psychol Trauma. 2015;7(1):24-33. doi:10.1037/a0034092
- Nickerson A, Schnyder U, Bryant RA, Schick M, Mueller J, Morina N. Moral injury in traumatized refugees. Psychother Psychosom. 2015;84(2):122-123. doi:10.1159/000369353
- Papazoglou K, Chopko B. The role of moral suffering (moral distress and moral injury) in police compassion fatigue and PTSD: An unexplored topic. Front Psychol. 2017;8:1999. doi:10.3389/fpsyg.2017.01999
- Papazoglou K, Blumberg DM, Chiongbian VB, et al. The role of moral injury in PTSD among law enforcement officers: a brief report. Front Psychol. 2020;11:310. doi:10.3389/fpsyg.2020.00310
- Martin WB, Holliday R, LePage JP. Trauma and diversity: moral injury among justice involved veterans: an understudied clinical concern. Stresspoints. 2020;33(5).
- Currier JM, Drescher KD, Nieuwsma J. Future directions for addressing moral injury in clinical practice: concluding comments. In: Currier JM, Drescher KD, Nieuwsma J, eds. Addressing Moral Injury in Clinical Practice. American Psychological Association; 2021:261-271. doi:10.1037/0000204-015
- Alexander AR, Mendez L, Kerig PK. Moral injury as a transdiagnostic risk factor for mental health problems in detained youth. Crim Justice Behav. 2023;51(2):194-212. doi:10.1177/00938548231208203
- DeCaro JB, Straka K, Malek N, Zalta AK. Sentenced to shame: moral injury exposure in former lifers. Psychol Trauma. 2024; 15(5):722-730. doi:10.1037/tra0001400
- Orak U, Kelton K, Vaughn MG, Tsai J, Pietrzak RH. Homelessness and contact with the criminal legal system among U.S. combat veterans: an exploration of potential mediating factors. Crim Justice Behav. 2022;50(3):392-409. doi:10.1177/00938548221140352
- Bronson J, Carson EA, Noonan M. Veterans in Prison and Jail, 2011-12. US Department of Justice, Bureau of Justice Statistics; Published December 2015. Accessed March 4, 2025. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
- Maruschak LM, Bronson J, Alper M. Veterans in Prison: Survey of Prison Inmates, 2016. US Department of Justice, Bureau of Justice Statistics; March 2021. Accessed March 4, 2025. https://bjs.ojp.gov/redirect-legacy/content/pub/pdf/vpspi16st.pdf
- Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justiceinvolved veterans. Epidemiol Rev. 2015;37:163-176. doi:10.1093/epirev/mxu003
- Finlay AK, Owens MD, Taylor E, et al. A scoping review of military veterans involved in the criminal justice system and their health and healthcare. Health Justice. 2019;7(1):6. doi:10.1186/s40352-019-0086-9
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Wortzel HS, Binswanger IA, Anderson CA, Adler LE. Suicide among incarcerated veterans. J Am Acad Psychiatry Law. 2009;37(1):82-91.
- Desai A, Holliday R, Borges LM, et al. Facilitating successful reentry among justice-involved veterans: the role of veteran and offender identity. J Psychiatr Pract. 2021;27(1):52-60. doi:10.1097/PRA.0000000000000520
- Asencio EK, Burke PJ. Does incarceration change the criminal identity? A synthesis of labeling and identity theory perspectives on identity change. Sociol Perspect. 2011;54(2):163-182. doi:10.1525/sop.2011.54.2.163
- Borges LM, Desai A, Barnes SM, Johnson JPS. The role of social determinants of health in moral injury: implications and future directions. Curr Treat Options Psychiatry. 2022;9(3):202-214. doi:10.1007/s40501-022-00272-4
- Houle SA, Ein N, Gervasio J, et al. Measuring moral distress and moral injury: a systematic review and content analysis of existing scales. Clin Psychol Rev. 2024;108:102377. doi:10.1016/j.cpr.2023.102377
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the moral injury events scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Zerach G, Ben-Yehuda A, Levi-Belz Y. Prospective associations between psychological factors, potentially morally injurious events, and psychiatric symptoms among Israeli combatants: the roles of ethical leadership and ethical preparation. Psychol Trauma. 2023;15(8):1367-1377. doi:10.1037/tra0001466
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013.
- Weathers FW, Litz BT, Keane TM, Palmeri PA, Marx BP. The PTSD Checklist for DSM-5 (PCL-5). National Center for PTSD. Accessed March 4, 2025. www.ptsd.va.gov
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379-1391. doi:10.1037/pas0000254
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The osttraumatic stress disorder checklist for DSM-5 (PCL- 5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Kroenke K, Spi tzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Brown TA. Confirmatory Factor Analysis for Applied Research. 2nd ed. Guilford Press; 2015.
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20(1):141-151. doi:10.1177/001316446002000116
- Schorr Y, Stein NR, Maguen S, Barnes JB, Bosch J, Litz BT. Sources of moral injury among war veterans: a qualitative evaluation. J Clin Psychol. 2018;74(12):2203-2218. doi:10.1002/jclp.22660
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Currier JM, Holland JM, Malott J. Moral injury, meaning making, and mental health in returning veterans. J Clin Psychol. 2015;71(3):229-240. doi:10.1002/jclp.22134
- Jinkerson JD. Defining and assessing moral injury: a syndrome perspective. Traumatology. 2016;22(2):122-130. doi:10.1037/trm0000069
- Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
- Maguen S, Litz B. Moral injury in veterans of war. PTSD Res Q. 2012;23(1):1-6. www.vva1071.org/uploads/3/4/4/6/34460116/moral_injury_in_veterans_of_war.pdf
- Drescher KD, Foy DW, Kelly C, Leshner A, Schutz K, Litz B. An exploration of the viability and usefulness of the construct of moral injury in war veterans. Traumatology. 2011;17(1):8-13. doi:10.1177/1534765610395615
- Wisco BE, Marx BP, May CL, et al. Moral injury in U.S. combat veterans: results from the national health and resilience in veterans study. Depress Anxiety. 2017; 34(4):340-347. doi:10.1002/da.22614
- Bryan CJ, Bryan AO, Anestis MD, et al. Measuring moral injury: psychometric properties of the moral injury events scale in two military samples. Assessment. 2016;23(5):557- 570. doi:10.1177/1073191115590855
- Currier JM, Smith PN, Kuhlman S. Assessing the unique role of religious coping in suicidal behavior among U.S. Iraq and Afghanistan veterans. Psychol Relig Spiritual. 2017;9(1):118-123. doi:10.1037/rel0000055
- Kopacz MS, Connery AL, Bishop TM, et al. Moral injury: a new challenge for complementary and alternative medicine. Complement Ther Med. 2016;24:29-33. doi:10.1016/j.ctim.2015.11.003
- Vargas AF, Hanson T, Kraus D, Drescher K, Foy D. Moral injury themes in combat veterans’ narrative responses from the national vietnam veterans’ readjustment study. Traumatology. 2013;19(3):243-250. doi:10.1177/1534765613476099
- Borges LM, Barnes SM, Farnsworth JK, Bahraini NH, Brenner LA. A commentary on moral injury among health care providers during the COVID-19 pandemic. Psychol Trauma. 2020;12(S1):S138-S140. doi:10.1037/tra0000698
- Borges LM, Holliday R, Barnes SM, et al. A longitudinal analysis of the role of potentially morally injurious events on COVID-19-related psychosocial functioning among healthcare providers. PLoS One. 2021;16(11):e0260033. doi:10.1371/journal.pone.0260033
- Currier JM, Holland JM, Rojas-Flores L, Herrera S, Foy D. Morally injurious experiences and meaning in Salvadorian teachers exposed to violence. Psychol Trauma. 2015;7(1):24-33. doi:10.1037/a0034092
- Nickerson A, Schnyder U, Bryant RA, Schick M, Mueller J, Morina N. Moral injury in traumatized refugees. Psychother Psychosom. 2015;84(2):122-123. doi:10.1159/000369353
- Papazoglou K, Chopko B. The role of moral suffering (moral distress and moral injury) in police compassion fatigue and PTSD: An unexplored topic. Front Psychol. 2017;8:1999. doi:10.3389/fpsyg.2017.01999
- Papazoglou K, Blumberg DM, Chiongbian VB, et al. The role of moral injury in PTSD among law enforcement officers: a brief report. Front Psychol. 2020;11:310. doi:10.3389/fpsyg.2020.00310
- Martin WB, Holliday R, LePage JP. Trauma and diversity: moral injury among justice involved veterans: an understudied clinical concern. Stresspoints. 2020;33(5).
- Currier JM, Drescher KD, Nieuwsma J. Future directions for addressing moral injury in clinical practice: concluding comments. In: Currier JM, Drescher KD, Nieuwsma J, eds. Addressing Moral Injury in Clinical Practice. American Psychological Association; 2021:261-271. doi:10.1037/0000204-015
- Alexander AR, Mendez L, Kerig PK. Moral injury as a transdiagnostic risk factor for mental health problems in detained youth. Crim Justice Behav. 2023;51(2):194-212. doi:10.1177/00938548231208203
- DeCaro JB, Straka K, Malek N, Zalta AK. Sentenced to shame: moral injury exposure in former lifers. Psychol Trauma. 2024; 15(5):722-730. doi:10.1037/tra0001400
- Orak U, Kelton K, Vaughn MG, Tsai J, Pietrzak RH. Homelessness and contact with the criminal legal system among U.S. combat veterans: an exploration of potential mediating factors. Crim Justice Behav. 2022;50(3):392-409. doi:10.1177/00938548221140352
- Bronson J, Carson EA, Noonan M. Veterans in Prison and Jail, 2011-12. US Department of Justice, Bureau of Justice Statistics; Published December 2015. Accessed March 4, 2025. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
- Maruschak LM, Bronson J, Alper M. Veterans in Prison: Survey of Prison Inmates, 2016. US Department of Justice, Bureau of Justice Statistics; March 2021. Accessed March 4, 2025. https://bjs.ojp.gov/redirect-legacy/content/pub/pdf/vpspi16st.pdf
- Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justiceinvolved veterans. Epidemiol Rev. 2015;37:163-176. doi:10.1093/epirev/mxu003
- Finlay AK, Owens MD, Taylor E, et al. A scoping review of military veterans involved in the criminal justice system and their health and healthcare. Health Justice. 2019;7(1):6. doi:10.1186/s40352-019-0086-9
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Wortzel HS, Binswanger IA, Anderson CA, Adler LE. Suicide among incarcerated veterans. J Am Acad Psychiatry Law. 2009;37(1):82-91.
- Desai A, Holliday R, Borges LM, et al. Facilitating successful reentry among justice-involved veterans: the role of veteran and offender identity. J Psychiatr Pract. 2021;27(1):52-60. doi:10.1097/PRA.0000000000000520
- Asencio EK, Burke PJ. Does incarceration change the criminal identity? A synthesis of labeling and identity theory perspectives on identity change. Sociol Perspect. 2011;54(2):163-182. doi:10.1525/sop.2011.54.2.163
- Borges LM, Desai A, Barnes SM, Johnson JPS. The role of social determinants of health in moral injury: implications and future directions. Curr Treat Options Psychiatry. 2022;9(3):202-214. doi:10.1007/s40501-022-00272-4
- Houle SA, Ein N, Gervasio J, et al. Measuring moral distress and moral injury: a systematic review and content analysis of existing scales. Clin Psychol Rev. 2024;108:102377. doi:10.1016/j.cpr.2023.102377
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the moral injury events scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Zerach G, Ben-Yehuda A, Levi-Belz Y. Prospective associations between psychological factors, potentially morally injurious events, and psychiatric symptoms among Israeli combatants: the roles of ethical leadership and ethical preparation. Psychol Trauma. 2023;15(8):1367-1377. doi:10.1037/tra0001466
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013.
- Weathers FW, Litz BT, Keane TM, Palmeri PA, Marx BP. The PTSD Checklist for DSM-5 (PCL-5). National Center for PTSD. Accessed March 4, 2025. www.ptsd.va.gov
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379-1391. doi:10.1037/pas0000254
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The osttraumatic stress disorder checklist for DSM-5 (PCL- 5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Kroenke K, Spi tzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Brown TA. Confirmatory Factor Analysis for Applied Research. 2nd ed. Guilford Press; 2015.
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20(1):141-151. doi:10.1177/001316446002000116
- Schorr Y, Stein NR, Maguen S, Barnes JB, Bosch J, Litz BT. Sources of moral injury among war veterans: a qualitative evaluation. J Clin Psychol. 2018;74(12):2203-2218. doi:10.1002/jclp.22660
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Suicide prevention is the leading clinical priority for the US Department of Veterans Affairs (VA).1 An average of 18 veterans died by suicide each day in 2021.2 Numerous risk factors for veteran suicide have been identified, including mental health disorders, comorbidities, access to firearms, and potentially lethal medications.3-5 To better understand groups of patients at risk of suicide in medical settings, the authors have previously compared demographic and clinical risk factors between patients who died by suicide by using firearms or other means with matched patients who did not die by suicide (control group) to examine the impact of lack of social support, financial stress,6 legal problems,7 homelessness,8 and discrimination.9 The number of cooccurring risk factors a veteran experiences is associated with a greater likelihood of suicide attempts over time.10 In addition, some risk factors are social and environmental risk factors known as social determinants of health (SDoH), including financial stability and access to health care, food, housing, and education. 11 SDoH may influence health outcomes more broadly and are associated with greater risk of suicide.12,13
The VA offers programming to address suicide risk factors. However, not all veterans are eligible for VA care. Further, some veterans prefer to obtain non-VA services in their communities. Providing veterans with community resources that address risk factors, particularly SDoH, may be a worthwhile strategy for reducing suicide. Such resources have demonstrated success; for example, greater use of housing services was associated with a reduced risk for suicide-related mortality among unhoused veterans.12
The challenges that veterans experience can go beyond the scope of services the VA provides. For example, while the VA provides some services related to homelessness, justice involvement, and assistance with home loans, these services are often limited. Other services for veterans to address SDoH may require access to community resources, including food banks, employment assistance, respite and childcare services, and transportation assistance. Some veterans also may have experienced institutional betrayal, which could be a barrier to VA care and may motivate veterans to address their needs in the community.14 Veterans therefore may need a range of services beyond those within the VA. Leveraging community resources for veterans at risk for suicide is critical, as these resources may help to mitigate suicide risk.
An emerging emphasis of the VA is improving coordination with community partners to prevent veteran suicide. In 2019, the VA launched an improved Veterans Community Care Program, which implemented portions of the VA MISSION Act of 2018 to create additional connection to community care for VA-enrolled veterans. This includes assisting veterans in gaining access to specialty services not offered at a local VA medical center (VAMC), getting access to services sooner, and receiving care if they do not live near a VAMC.15 In addition, the COMPACT Act allows veterans in acute suicidal crisis to receive emergency health care through either VA or non-VA facilities at no cost.16 The VA National Strategy for Preventing Veteran Suicide 2018-2028 is a 10-year plan to reduce veteran suicide rates that includes initiatives to increase connections between VA and community agencies.17 A suicide prevention community toolkit is available online for health care professionals (HCPs) (and others, including employers) outside of the VA who may be unfamiliar with best practices for working with veterans at risk for suicide.18
The challenge, however, is that there is often a lack of “connectedness” between VA suicide prevention coordinators and community resources to address suicide risk factors and related social determinants of health. These services include, but are not limited to suicide prevention, mental health counseling (particularly no/low-cost services), unemployment resources, financial assistance and counseling, housing assistance, and identity-related supportive spaces. A major stumbling block in connecting resources with veterans (regardless of discharge status) who need them is there is no single, national organization with a comprehensive, community-based network that can serve in this intermediary role.
Community asset mapping (CAM), also known as asset mapping or environmental scanning, is a way to bridge the gap.19 CAM provides a method for identifying and aligning community resources relative to a specific need.20 CAM may be used to build community relationships in service of veteran suicide prevention. This process can help individuals learn about and make use of organizations and services within their communities. CAM also helps connect HCPs so they can network, exchange ideas, and collaborate with an eye toward increasing the availability of services and enhancing care coordination. CAM also allows community members (eg, leaders, organizations, individuals) to identify possible gaps in services that address suicide risk factors and solve these problems.
This article details CAM for suicide prevention, which can be utilized by the VA and community organizations alike. Within the VA, CAM can be used by HCPs and administrators, such as VA community engagement and partnership coordinators, to identify potential partnering organizations. For those who serve veterans outside of the VA, CAM can be used to connect at-risk individuals to resources that can enhance their care. This process can help increase the overall knowledge of, and access to, community resources.
COMMUNITY ASSET MAPPING
The University of California, Los Angeles Center for Health Policy Research provides 6 steps for the CAM process.21 These steps include: (1) defining the boundaries of people and places that comprise the community; (2) identifying people and organizations who share similar interests and goals; (3) determining the assets to include; (4) creating an inventory of all organizations’ assets; (5) creating an inventory of individuals’ assets; and (6) organizing the assets on a map. To address the needs of the veteran population, we’ve taken these 6 steps and adapted them to create a CAM for veterans at risk for suicide (Figure). The discussion that follows details how these steps can be implemented to identify community resources that address social determinants of health that may contribute to suicide risk. The goal is to prevent veteran suicide.
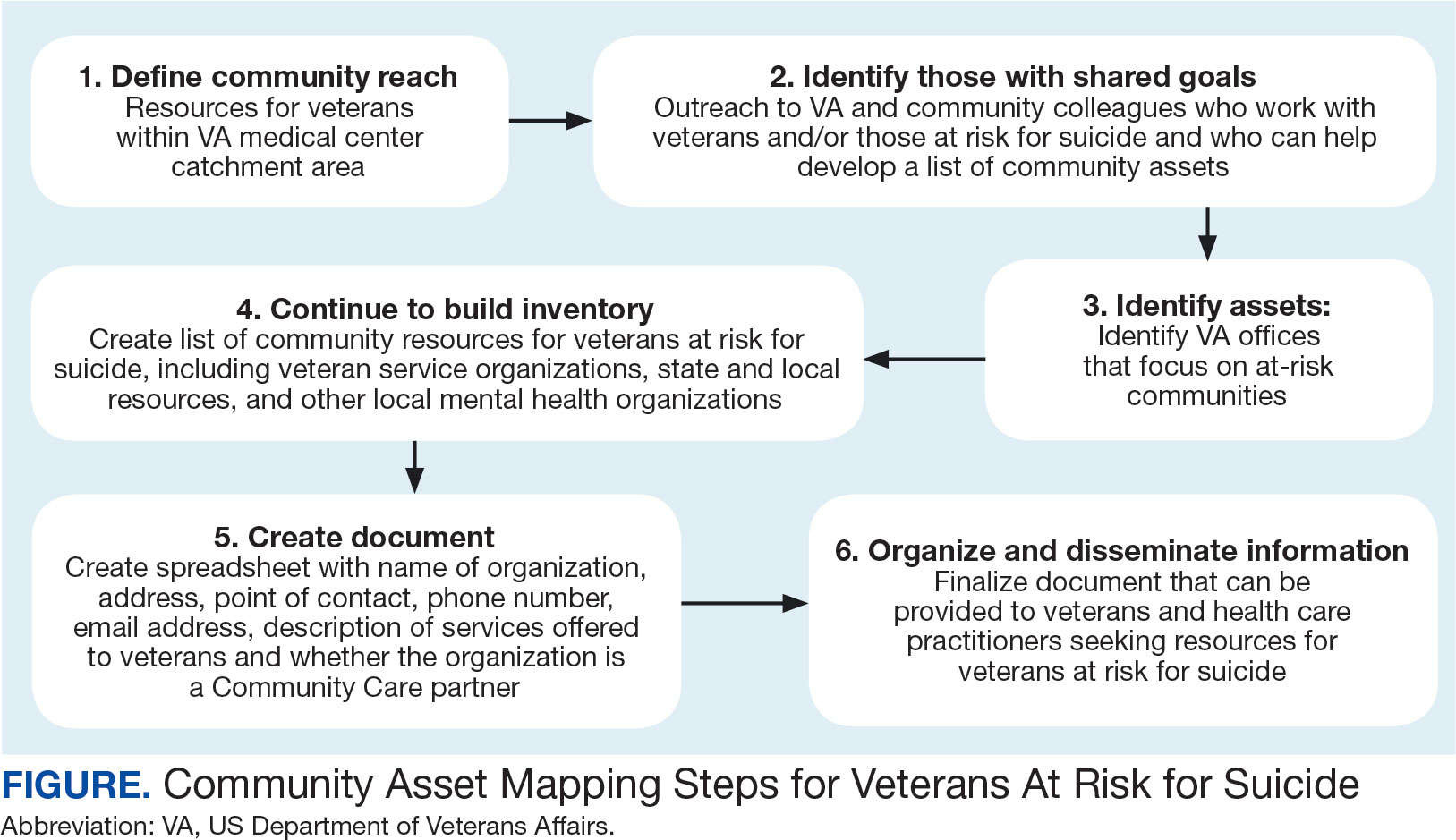
Step 1: Define Community Reach. The first step is to identify the geographical boundaries of the community. This may include all veterans within a catchment area (eg, veterans within 60 miles of a VAMC). Defining the geographical parameters of the community will provide structure to the effort so that the resource list is as comprehensive as possible.
Steps 2 and 3: Identify Community Members with Shared Goals; Identify Assets. It is important to identify community members who share similar interests and goals, including people with specific knowledge and skills, organizations with particular goals, and community partners with a broad reach. To begin building a list of referrals, reach out to colleagues within the VA system who are familiar with community resources for those with suicide risk factors. The local VA Transition and Care Management (TCM) office is a resource that connects those transitioning from military to civilian sectors with needed resources, and thus may be a helpful resource while building a CAM. Additionally, each office has a transition patient advocate, who is trained to resolve care-related concerns and may be familiar with community resources.
VA HCPs that can assist include Community Engagement and Partnership Coordinators, Suicide Prevention Coordinators, Local Recovery Coordinators, and substance abuse counselors. In addition, VA patient services, patient safety, and public affairs office staff—as well as VA Homeless Programs—may be good resources. Every VA health care system has care coordinators focused on military sexual trauma, intimate partner violence, and lesbian, gay, bisexual, transgender, queer+ care. These care coordinators may be able to provide information on community resources that address social determinants of health (eg, discrimination, violence).
Reaching out to key community resources and asking for recommendations of other groups that provide assistance to veterans can also be productive. You can start by connecting with veterans service organizations (VSOs), Vet Centers, Veterans Experience Offices (VEO), and Community Veterans Engagement Boards (CVEBs). The VEO is an office designed around VA and community engagement efforts. This office utilizes the CVEBs to foster a 2-way communication feedback loop between veterans and local VA facilities regarding community engagement efforts and outreach.22 CVEBs are particularly valuable sources of information because veterans directly contribute to the conversation about community engagement by describing the difficulties and successes they’ve experienced. Veteran feedback about how a particular resource met their needs can inform which community services are prioritized for inclusion in the resource list. In addition, CVEBs may have a listing of local government, military, and/or community resources that provide services for veterans. Consider, too, organizations that are unrelated to an individual’s veteran status, but speak to their race/ethnicity, sexual orientation, gender identity, spirituality, socioeconomic status, or disability.
Step 4: Continue to Build Inventory. Use online searches to identify additional resources in the community that are known to have local relationships. These include state suicide prevention coordinators, mental health organizations, and other resources that address social determinants of health (eg, public health and human service organizations, faith-based organizations, collegial organizations). A list of links and search tips are available in the Table.
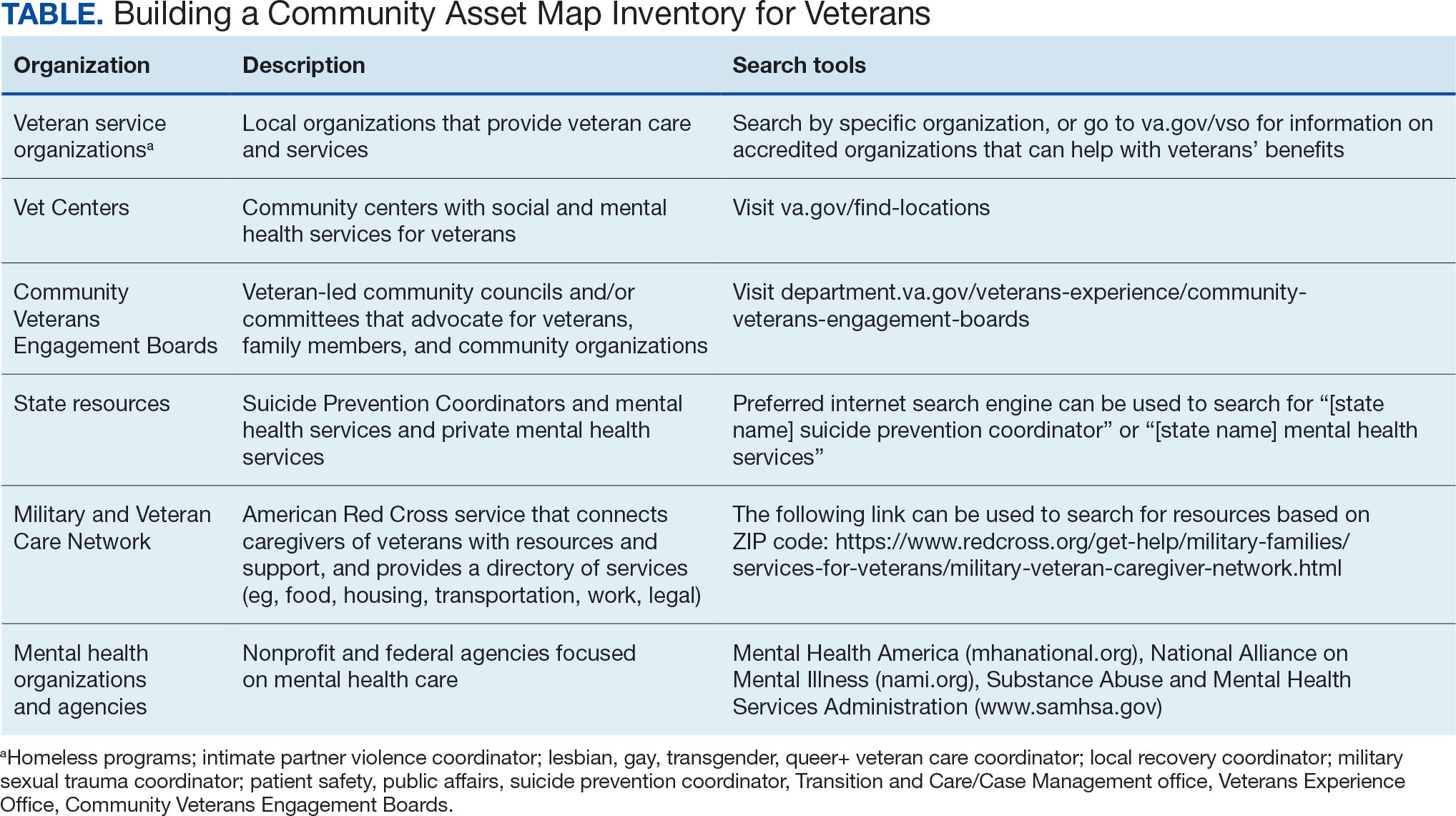
Steps 5 and 6: Create Document; Organize and Disseminate Information. A spreadsheet can be used to document organization information (Appendix). It is critical to record: (1) the name of the organization or individual; (2) the local address and a point of contact with contact information; (3) services offered to veterans; (4) services specific to suicide prevention, or that address risk factors for suicide; and (5) whether the referral organization is partnered with the VA Community Care Network, which is comprised of contracted HCPs who contract with the VA to provide care to veterans.23
Once a document is created, it can be disseminated through VA offices and among community partners who work with veterans at risk for suicide. It should also be stored in a centralized location such as a shared folder so that it can be continuously updated.
Regularly updating the list is vital so the resource list can continue to be helpful in addressing veterans’ needs and reducing suicide risk factors. Continued collaboration with those in the community can help ensure the resource list is up to date with all available services and pertinent contact information. It can also go far in strengthening collaborative bonds.
IMPLEMENTATION
To illustrate the use of CAM for veteran suicide prevention, we offer a case example of CAM conducted by the VA Patient Safety Center of Inquiry — Suicide Prevention Collaborative (VA PSCI-SPC) team, consisting of 4 team members. A veteran was included as a team member and assisted with the CAM process.
The VA PSCI-SPC sought to identify community services for veterans in Colorado who were not enrolled in VA health care and had risk factors for suicide. Next, the team reached out to colleagues and asked about community organizations that work with individuals at risk for suicide. VA PSCI-SPC outreach resulted in a list of assets that included resources to address mental health, legal concerns, employment, homelessness/housing, finances, religion, peer support, food insecurity, exercise, intimate partner violence, sexual and gender identity needs, and peer support. VSOs and CVEBs were also added to the list.
Next, the team continued to build on the inventory and identified state suicide prevention coordinators; health care systems; regional suicide prevention commissions; Colorado Department of Health and Human Services; program coordinators for Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and their Families; veterans councils; universities (eg, counseling clinics, legal clinics); and foundations devoted to general and veteran-specific suicide prevention within the region.
All the identified resources were inventoried. Details were gathered about each of the organizations, including addresses, points of contact and phone numbers, descriptions of services offered for veterans, descriptions of suicide prevention services offered, whether or not organizations were not-for-profit, the mission of the organizations, and whether or not the organizations were under contract for VA Community Care. Finally, the resource spreadsheet was created and disseminated among stakeholders to be used to enhance veteran suicide care. Stakeholders included social workers, psychologists, and nurse practitioners working with veterans. The list was circulated to VA and community partners as needed.
The VA PSCI-SPC resource document was only 1 benefit of CAM. The asset mapping also resulted in the creation of a learning collaborative comprised of VA and community partners, designed to share knowledge of best practices in suicide prevention and create an established referral network for veterans at risk for suicide.24 Ultimately, the goal of the CAM and the creation of the learning collaborative was to better connect veterans to care in order to decrease suicide risk. A secondary benefit of this community connectedness is that the list of resources produced by CAM became a living document that was, and continues to be, updated as the network became aware of new resources and resources that were no longer available. The VA PSCI-SPC learning collaborative met quarterly to discuss implementation of suicide prevention best practices within their organization.
Data from the VA PSCI-SPC learning collaborative via CAM revealed that organizations felt more efficacious in implementing suicide prevention best practices, noticed increased connections and collaborations with community organizations with the goal of providing services to veterans, and resulted in staff training that improved services provided to veterans.24 This is supported by other findings of a literature review of suicide prevention interventions, which indicated that programs with an established community support network were more effective at reducing suicide rates.25 CAM therefore may be a process through which greater community connection and increased knowledge of resources may help prevent suicide among veterans.
It seems reasonable that the CAM processes used by the VA PSCI-SPC can be implemented within the regional Veterans Integrated Service Networks to identify assets in a specific geographical area to address challenges with social determinants of health and potentially decrease veteran suicide risk.
CONCLUSIONS
CAM can be used to identify and build relationships with community resources that address the stressors that place veterans at risk for suicide. Six proposed steps to CAM for veterans at risk for suicide include: defining community reach (the map); identifying community members and organizations with shared goals; identifying assets within the community; continuing to build inventory; creating a document; and organizing and disseminating the information (while continuing to update the resources).21
CAM can be used to connect veterans with resources to address needs related to adverse social determinants of health that may heighten their risk for suicide. For example, veterans facing legal challenges can connect with a legal clinic; those having difficulties paying bills can obtain financial assistance; those who need help completing their VA claims can connect with the Veterans Benefits Administration or VSOs to assist them with their claims; and those experiencing discrimination can connect with organizations where they may experience acceptance, safety, and support. Broad community support surrounding suicide risk factors can be critical for effective suicide prevention.25
CAM may also be helpful for HCPs and others involved in veteran health care. For example, community mapping can be utilized by newly hired community engagement and partnership coordinators as a tool for outlining resources available for veterans in their community and as a framework to continually update their resource network. CAM develops community awareness, integrates resources, and enhances service utilization, which may assist in veteran suicide prevention by increasing care coordination.17 Finally, mapping community resources can create awareness of the many resources available to help veterans, even before suicide becomes a consideration.
- Rice L. VA Secretary Robert Wilkie says suicide prevention is his agency’s top ‘clinical’ priority. June 17, 2019. Accessed January 30, 2025. https://www.kut.org/post/va-secretary-robert-wilkie-says-suicide-prevention-his-agencys-top-clinical-priority
- US Department of Veterans Affairs. 2023 national veteran suicide prevention annual report. November 2023. Accessed January 30, 2025. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- DeBeer BB, Meyer EC, Kimbrel NA, Kittel JA, Gulliver SB, Morissette SB. Psychological inflexibility predicts of suicidal ideation over time in veterans of the conflicts in Iraq and Afghanistan. Suicide Life Threat Behav. 2018;48(6):627–641. doi:10.1111/sltb.12388
- Ilgen MA, Bohnert ASB, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67(11):1152–1158. doi:10.1001/archgenpsychiatry.2010.129
- Kimbrel NA, Meyer EC, DeBeer BB, Gulliver SB, Morissette SB. A 12-month prospective study of the effects of PTSD-depression comorbidity on suicidal behavior in Iraq/ Afghanistan-era veterans. Psychiatry Res. 2016;243:97–99. doi:10.1016/j.psychres.2016.06.011
- Hoffmire CA, Borowski S, Vogt D. Contribution of veterans’ initial post-separation vocational, financial, and social experiences to their suicidal ideation trajectories following military service. Suicide Life Threat Behav. 2023;53(3):443- 456. doi:10.1111/sltb.12955
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Holliday R, Liu S, Brenner LA, et al. Preventing suicide among homeless veterans: a consensus statement by the Veterans Affairs suicide prevention among veterans experiencing homelessness workgroup. Med Care. 2021;59(Suppl 2):S103- S105. doi:10.1097/MLR.0000000000001399
- Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: The role of social supsupport and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
- Lee DJ, Kearns JC, Wisco BE, et al. A longitudinal study of risk factors for suicide attempts among Operation Enduring Freedom and Operation Iraqi Freedom veterans. Depress Anxiety. 2018;35(7): 609-618. doi:10.1002/da.22736
- Center for Disease Control and Prevention. Social determinants of health (SDOH). Accessed January 30, 2025. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
- Montgomery AE, Dichter M, Byrne T, Blosnich J. Intervention to address homelessness and all-cause and suicide mortality among unstably housed US veterans, 2012- 2016. J Epidemiol Community Health. 2021;75:380-386. doi: 10.1136/jech-2020-214664
- Llamocca EN, Yeh HH, Miller-Matero LR, et al. Association between adverse social determinants of health and suicide death. Med Care. 2023;61(11):744-749. doi:10.1097/MLR.0000000000001918
- Monteith LL, Holliday R, Schneider AL, et al. Institutional betrayal and help-seeking among women survivors of military sexual trauma. Psychol Trauma. 2021;13(7):814-823. doi:10.1037/tra0001027
- VA launches new health care options under MISSION Act. News release. US Department of Veterans Affairs. June 6, 2019. Accessed January 31, 2025. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5264
- COMPACT Act expands free emergency suicide care for veterans. News release. US Department of Veterans Affairs. February 1, 2023. Accessed January 31,2025. https://www.va.gov/poplar-bluff-health-care/news-releases/compact-act-expands-free-emergency-suicide-care-for-veterans/
- US Department of Veterans Affairs. National strategy for preventing Veteran suicide 2018-2028. 2018. Accessed January 31, 2025. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. Veteran outreach toolkit: preventing veteran suicide is everyone’s business. A community call to action. Accessed February 3, 2025. https://floridavets.org/wp-content/uploads/2022/06/VA-Suicide-Prevention-Community-Outreach-Toolkit.pdf
- Crane K, Mooney M. Essential tools: community resource mapping. 2005. Accessed February 3, 2025. https://conservancy.umn.edu/bitstream/handle/11299/172995/NCSET_EssentialTools_ResourceMapping.pdf
- Community Tool Box. 2. Assessing Community Needs and Resources. Accessed February 3, 2025. https://ctb.ku.edu/en/assessing-community-needs-and-resources
- UCLA Center for Health Policy Research. Section 1: asset mapping. 2012. Accessed February 3, 2025. https://healthpolicy.ucla.edu/programs/healthdata/trainings/documents/tw_cba20.pdf
- US Department of Veterans Affairs, Veterans Experience Office. 4th quarter 2018 community engagement news. October 2, 2018. Accessed February 4, 2025. https://content.govdelivery.com/accounts/USVAVEO/bulletins/211836e
- US Department of Veterans Affairs. About our VA community care network and covered services. Accessed February 6, 2025. https://www.va.gov/resources/aboutour-va-community-care-network-and-covered-services/
- DeBeer B, Mignogna J, Borah E, et al. A pilot of a veteran suicide prevention learning collaborative among community organizations: Initial results and outcomes. Suicide Life Threat Behav. 2023;53(4):628-641. doi:10.1111/sltb.12969
- Fountoulakis KN, Gonda X, Rihmer Z. Suicide prevention programs through community intervention. J Affect Disord. 2011;130(1-2):10–16. doi:10.1016/j.jad.2010.06.009
Suicide prevention is the leading clinical priority for the US Department of Veterans Affairs (VA).1 An average of 18 veterans died by suicide each day in 2021.2 Numerous risk factors for veteran suicide have been identified, including mental health disorders, comorbidities, access to firearms, and potentially lethal medications.3-5 To better understand groups of patients at risk of suicide in medical settings, the authors have previously compared demographic and clinical risk factors between patients who died by suicide by using firearms or other means with matched patients who did not die by suicide (control group) to examine the impact of lack of social support, financial stress,6 legal problems,7 homelessness,8 and discrimination.9 The number of cooccurring risk factors a veteran experiences is associated with a greater likelihood of suicide attempts over time.10 In addition, some risk factors are social and environmental risk factors known as social determinants of health (SDoH), including financial stability and access to health care, food, housing, and education. 11 SDoH may influence health outcomes more broadly and are associated with greater risk of suicide.12,13
The VA offers programming to address suicide risk factors. However, not all veterans are eligible for VA care. Further, some veterans prefer to obtain non-VA services in their communities. Providing veterans with community resources that address risk factors, particularly SDoH, may be a worthwhile strategy for reducing suicide. Such resources have demonstrated success; for example, greater use of housing services was associated with a reduced risk for suicide-related mortality among unhoused veterans.12
The challenges that veterans experience can go beyond the scope of services the VA provides. For example, while the VA provides some services related to homelessness, justice involvement, and assistance with home loans, these services are often limited. Other services for veterans to address SDoH may require access to community resources, including food banks, employment assistance, respite and childcare services, and transportation assistance. Some veterans also may have experienced institutional betrayal, which could be a barrier to VA care and may motivate veterans to address their needs in the community.14 Veterans therefore may need a range of services beyond those within the VA. Leveraging community resources for veterans at risk for suicide is critical, as these resources may help to mitigate suicide risk.
An emerging emphasis of the VA is improving coordination with community partners to prevent veteran suicide. In 2019, the VA launched an improved Veterans Community Care Program, which implemented portions of the VA MISSION Act of 2018 to create additional connection to community care for VA-enrolled veterans. This includes assisting veterans in gaining access to specialty services not offered at a local VA medical center (VAMC), getting access to services sooner, and receiving care if they do not live near a VAMC.15 In addition, the COMPACT Act allows veterans in acute suicidal crisis to receive emergency health care through either VA or non-VA facilities at no cost.16 The VA National Strategy for Preventing Veteran Suicide 2018-2028 is a 10-year plan to reduce veteran suicide rates that includes initiatives to increase connections between VA and community agencies.17 A suicide prevention community toolkit is available online for health care professionals (HCPs) (and others, including employers) outside of the VA who may be unfamiliar with best practices for working with veterans at risk for suicide.18
The challenge, however, is that there is often a lack of “connectedness” between VA suicide prevention coordinators and community resources to address suicide risk factors and related social determinants of health. These services include, but are not limited to suicide prevention, mental health counseling (particularly no/low-cost services), unemployment resources, financial assistance and counseling, housing assistance, and identity-related supportive spaces. A major stumbling block in connecting resources with veterans (regardless of discharge status) who need them is there is no single, national organization with a comprehensive, community-based network that can serve in this intermediary role.
Community asset mapping (CAM), also known as asset mapping or environmental scanning, is a way to bridge the gap.19 CAM provides a method for identifying and aligning community resources relative to a specific need.20 CAM may be used to build community relationships in service of veteran suicide prevention. This process can help individuals learn about and make use of organizations and services within their communities. CAM also helps connect HCPs so they can network, exchange ideas, and collaborate with an eye toward increasing the availability of services and enhancing care coordination. CAM also allows community members (eg, leaders, organizations, individuals) to identify possible gaps in services that address suicide risk factors and solve these problems.
This article details CAM for suicide prevention, which can be utilized by the VA and community organizations alike. Within the VA, CAM can be used by HCPs and administrators, such as VA community engagement and partnership coordinators, to identify potential partnering organizations. For those who serve veterans outside of the VA, CAM can be used to connect at-risk individuals to resources that can enhance their care. This process can help increase the overall knowledge of, and access to, community resources.
COMMUNITY ASSET MAPPING
The University of California, Los Angeles Center for Health Policy Research provides 6 steps for the CAM process.21 These steps include: (1) defining the boundaries of people and places that comprise the community; (2) identifying people and organizations who share similar interests and goals; (3) determining the assets to include; (4) creating an inventory of all organizations’ assets; (5) creating an inventory of individuals’ assets; and (6) organizing the assets on a map. To address the needs of the veteran population, we’ve taken these 6 steps and adapted them to create a CAM for veterans at risk for suicide (Figure). The discussion that follows details how these steps can be implemented to identify community resources that address social determinants of health that may contribute to suicide risk. The goal is to prevent veteran suicide.

Step 1: Define Community Reach. The first step is to identify the geographical boundaries of the community. This may include all veterans within a catchment area (eg, veterans within 60 miles of a VAMC). Defining the geographical parameters of the community will provide structure to the effort so that the resource list is as comprehensive as possible.
Steps 2 and 3: Identify Community Members with Shared Goals; Identify Assets. It is important to identify community members who share similar interests and goals, including people with specific knowledge and skills, organizations with particular goals, and community partners with a broad reach. To begin building a list of referrals, reach out to colleagues within the VA system who are familiar with community resources for those with suicide risk factors. The local VA Transition and Care Management (TCM) office is a resource that connects those transitioning from military to civilian sectors with needed resources, and thus may be a helpful resource while building a CAM. Additionally, each office has a transition patient advocate, who is trained to resolve care-related concerns and may be familiar with community resources.
VA HCPs that can assist include Community Engagement and Partnership Coordinators, Suicide Prevention Coordinators, Local Recovery Coordinators, and substance abuse counselors. In addition, VA patient services, patient safety, and public affairs office staff—as well as VA Homeless Programs—may be good resources. Every VA health care system has care coordinators focused on military sexual trauma, intimate partner violence, and lesbian, gay, bisexual, transgender, queer+ care. These care coordinators may be able to provide information on community resources that address social determinants of health (eg, discrimination, violence).
Reaching out to key community resources and asking for recommendations of other groups that provide assistance to veterans can also be productive. You can start by connecting with veterans service organizations (VSOs), Vet Centers, Veterans Experience Offices (VEO), and Community Veterans Engagement Boards (CVEBs). The VEO is an office designed around VA and community engagement efforts. This office utilizes the CVEBs to foster a 2-way communication feedback loop between veterans and local VA facilities regarding community engagement efforts and outreach.22 CVEBs are particularly valuable sources of information because veterans directly contribute to the conversation about community engagement by describing the difficulties and successes they’ve experienced. Veteran feedback about how a particular resource met their needs can inform which community services are prioritized for inclusion in the resource list. In addition, CVEBs may have a listing of local government, military, and/or community resources that provide services for veterans. Consider, too, organizations that are unrelated to an individual’s veteran status, but speak to their race/ethnicity, sexual orientation, gender identity, spirituality, socioeconomic status, or disability.
Step 4: Continue to Build Inventory. Use online searches to identify additional resources in the community that are known to have local relationships. These include state suicide prevention coordinators, mental health organizations, and other resources that address social determinants of health (eg, public health and human service organizations, faith-based organizations, collegial organizations). A list of links and search tips are available in the Table.

Steps 5 and 6: Create Document; Organize and Disseminate Information. A spreadsheet can be used to document organization information (Appendix). It is critical to record: (1) the name of the organization or individual; (2) the local address and a point of contact with contact information; (3) services offered to veterans; (4) services specific to suicide prevention, or that address risk factors for suicide; and (5) whether the referral organization is partnered with the VA Community Care Network, which is comprised of contracted HCPs who contract with the VA to provide care to veterans.23

Once a document is created, it can be disseminated through VA offices and among community partners who work with veterans at risk for suicide. It should also be stored in a centralized location such as a shared folder so that it can be continuously updated.
Regularly updating the list is vital so the resource list can continue to be helpful in addressing veterans’ needs and reducing suicide risk factors. Continued collaboration with those in the community can help ensure the resource list is up to date with all available services and pertinent contact information. It can also go far in strengthening collaborative bonds.
IMPLEMENTATION
To illustrate the use of CAM for veteran suicide prevention, we offer a case example of CAM conducted by the VA Patient Safety Center of Inquiry — Suicide Prevention Collaborative (VA PSCI-SPC) team, consisting of 4 team members. A veteran was included as a team member and assisted with the CAM process.
The VA PSCI-SPC sought to identify community services for veterans in Colorado who were not enrolled in VA health care and had risk factors for suicide. Next, the team reached out to colleagues and asked about community organizations that work with individuals at risk for suicide. VA PSCI-SPC outreach resulted in a list of assets that included resources to address mental health, legal concerns, employment, homelessness/housing, finances, religion, peer support, food insecurity, exercise, intimate partner violence, sexual and gender identity needs, and peer support. VSOs and CVEBs were also added to the list.
Next, the team continued to build on the inventory and identified state suicide prevention coordinators; health care systems; regional suicide prevention commissions; Colorado Department of Health and Human Services; program coordinators for Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and their Families; veterans councils; universities (eg, counseling clinics, legal clinics); and foundations devoted to general and veteran-specific suicide prevention within the region.
All the identified resources were inventoried. Details were gathered about each of the organizations, including addresses, points of contact and phone numbers, descriptions of services offered for veterans, descriptions of suicide prevention services offered, whether or not organizations were not-for-profit, the mission of the organizations, and whether or not the organizations were under contract for VA Community Care. Finally, the resource spreadsheet was created and disseminated among stakeholders to be used to enhance veteran suicide care. Stakeholders included social workers, psychologists, and nurse practitioners working with veterans. The list was circulated to VA and community partners as needed.
The VA PSCI-SPC resource document was only 1 benefit of CAM. The asset mapping also resulted in the creation of a learning collaborative comprised of VA and community partners, designed to share knowledge of best practices in suicide prevention and create an established referral network for veterans at risk for suicide.24 Ultimately, the goal of the CAM and the creation of the learning collaborative was to better connect veterans to care in order to decrease suicide risk. A secondary benefit of this community connectedness is that the list of resources produced by CAM became a living document that was, and continues to be, updated as the network became aware of new resources and resources that were no longer available. The VA PSCI-SPC learning collaborative met quarterly to discuss implementation of suicide prevention best practices within their organization.
Data from the VA PSCI-SPC learning collaborative via CAM revealed that organizations felt more efficacious in implementing suicide prevention best practices, noticed increased connections and collaborations with community organizations with the goal of providing services to veterans, and resulted in staff training that improved services provided to veterans.24 This is supported by other findings of a literature review of suicide prevention interventions, which indicated that programs with an established community support network were more effective at reducing suicide rates.25 CAM therefore may be a process through which greater community connection and increased knowledge of resources may help prevent suicide among veterans.
It seems reasonable that the CAM processes used by the VA PSCI-SPC can be implemented within the regional Veterans Integrated Service Networks to identify assets in a specific geographical area to address challenges with social determinants of health and potentially decrease veteran suicide risk.
CONCLUSIONS
CAM can be used to identify and build relationships with community resources that address the stressors that place veterans at risk for suicide. Six proposed steps to CAM for veterans at risk for suicide include: defining community reach (the map); identifying community members and organizations with shared goals; identifying assets within the community; continuing to build inventory; creating a document; and organizing and disseminating the information (while continuing to update the resources).21
CAM can be used to connect veterans with resources to address needs related to adverse social determinants of health that may heighten their risk for suicide. For example, veterans facing legal challenges can connect with a legal clinic; those having difficulties paying bills can obtain financial assistance; those who need help completing their VA claims can connect with the Veterans Benefits Administration or VSOs to assist them with their claims; and those experiencing discrimination can connect with organizations where they may experience acceptance, safety, and support. Broad community support surrounding suicide risk factors can be critical for effective suicide prevention.25
CAM may also be helpful for HCPs and others involved in veteran health care. For example, community mapping can be utilized by newly hired community engagement and partnership coordinators as a tool for outlining resources available for veterans in their community and as a framework to continually update their resource network. CAM develops community awareness, integrates resources, and enhances service utilization, which may assist in veteran suicide prevention by increasing care coordination.17 Finally, mapping community resources can create awareness of the many resources available to help veterans, even before suicide becomes a consideration.
Suicide prevention is the leading clinical priority for the US Department of Veterans Affairs (VA).1 An average of 18 veterans died by suicide each day in 2021.2 Numerous risk factors for veteran suicide have been identified, including mental health disorders, comorbidities, access to firearms, and potentially lethal medications.3-5 To better understand groups of patients at risk of suicide in medical settings, the authors have previously compared demographic and clinical risk factors between patients who died by suicide by using firearms or other means with matched patients who did not die by suicide (control group) to examine the impact of lack of social support, financial stress,6 legal problems,7 homelessness,8 and discrimination.9 The number of cooccurring risk factors a veteran experiences is associated with a greater likelihood of suicide attempts over time.10 In addition, some risk factors are social and environmental risk factors known as social determinants of health (SDoH), including financial stability and access to health care, food, housing, and education. 11 SDoH may influence health outcomes more broadly and are associated with greater risk of suicide.12,13
The VA offers programming to address suicide risk factors. However, not all veterans are eligible for VA care. Further, some veterans prefer to obtain non-VA services in their communities. Providing veterans with community resources that address risk factors, particularly SDoH, may be a worthwhile strategy for reducing suicide. Such resources have demonstrated success; for example, greater use of housing services was associated with a reduced risk for suicide-related mortality among unhoused veterans.12
The challenges that veterans experience can go beyond the scope of services the VA provides. For example, while the VA provides some services related to homelessness, justice involvement, and assistance with home loans, these services are often limited. Other services for veterans to address SDoH may require access to community resources, including food banks, employment assistance, respite and childcare services, and transportation assistance. Some veterans also may have experienced institutional betrayal, which could be a barrier to VA care and may motivate veterans to address their needs in the community.14 Veterans therefore may need a range of services beyond those within the VA. Leveraging community resources for veterans at risk for suicide is critical, as these resources may help to mitigate suicide risk.
An emerging emphasis of the VA is improving coordination with community partners to prevent veteran suicide. In 2019, the VA launched an improved Veterans Community Care Program, which implemented portions of the VA MISSION Act of 2018 to create additional connection to community care for VA-enrolled veterans. This includes assisting veterans in gaining access to specialty services not offered at a local VA medical center (VAMC), getting access to services sooner, and receiving care if they do not live near a VAMC.15 In addition, the COMPACT Act allows veterans in acute suicidal crisis to receive emergency health care through either VA or non-VA facilities at no cost.16 The VA National Strategy for Preventing Veteran Suicide 2018-2028 is a 10-year plan to reduce veteran suicide rates that includes initiatives to increase connections between VA and community agencies.17 A suicide prevention community toolkit is available online for health care professionals (HCPs) (and others, including employers) outside of the VA who may be unfamiliar with best practices for working with veterans at risk for suicide.18
The challenge, however, is that there is often a lack of “connectedness” between VA suicide prevention coordinators and community resources to address suicide risk factors and related social determinants of health. These services include, but are not limited to suicide prevention, mental health counseling (particularly no/low-cost services), unemployment resources, financial assistance and counseling, housing assistance, and identity-related supportive spaces. A major stumbling block in connecting resources with veterans (regardless of discharge status) who need them is there is no single, national organization with a comprehensive, community-based network that can serve in this intermediary role.
Community asset mapping (CAM), also known as asset mapping or environmental scanning, is a way to bridge the gap.19 CAM provides a method for identifying and aligning community resources relative to a specific need.20 CAM may be used to build community relationships in service of veteran suicide prevention. This process can help individuals learn about and make use of organizations and services within their communities. CAM also helps connect HCPs so they can network, exchange ideas, and collaborate with an eye toward increasing the availability of services and enhancing care coordination. CAM also allows community members (eg, leaders, organizations, individuals) to identify possible gaps in services that address suicide risk factors and solve these problems.
This article details CAM for suicide prevention, which can be utilized by the VA and community organizations alike. Within the VA, CAM can be used by HCPs and administrators, such as VA community engagement and partnership coordinators, to identify potential partnering organizations. For those who serve veterans outside of the VA, CAM can be used to connect at-risk individuals to resources that can enhance their care. This process can help increase the overall knowledge of, and access to, community resources.
COMMUNITY ASSET MAPPING
The University of California, Los Angeles Center for Health Policy Research provides 6 steps for the CAM process.21 These steps include: (1) defining the boundaries of people and places that comprise the community; (2) identifying people and organizations who share similar interests and goals; (3) determining the assets to include; (4) creating an inventory of all organizations’ assets; (5) creating an inventory of individuals’ assets; and (6) organizing the assets on a map. To address the needs of the veteran population, we’ve taken these 6 steps and adapted them to create a CAM for veterans at risk for suicide (Figure). The discussion that follows details how these steps can be implemented to identify community resources that address social determinants of health that may contribute to suicide risk. The goal is to prevent veteran suicide.

Step 1: Define Community Reach. The first step is to identify the geographical boundaries of the community. This may include all veterans within a catchment area (eg, veterans within 60 miles of a VAMC). Defining the geographical parameters of the community will provide structure to the effort so that the resource list is as comprehensive as possible.
Steps 2 and 3: Identify Community Members with Shared Goals; Identify Assets. It is important to identify community members who share similar interests and goals, including people with specific knowledge and skills, organizations with particular goals, and community partners with a broad reach. To begin building a list of referrals, reach out to colleagues within the VA system who are familiar with community resources for those with suicide risk factors. The local VA Transition and Care Management (TCM) office is a resource that connects those transitioning from military to civilian sectors with needed resources, and thus may be a helpful resource while building a CAM. Additionally, each office has a transition patient advocate, who is trained to resolve care-related concerns and may be familiar with community resources.
VA HCPs that can assist include Community Engagement and Partnership Coordinators, Suicide Prevention Coordinators, Local Recovery Coordinators, and substance abuse counselors. In addition, VA patient services, patient safety, and public affairs office staff—as well as VA Homeless Programs—may be good resources. Every VA health care system has care coordinators focused on military sexual trauma, intimate partner violence, and lesbian, gay, bisexual, transgender, queer+ care. These care coordinators may be able to provide information on community resources that address social determinants of health (eg, discrimination, violence).
Reaching out to key community resources and asking for recommendations of other groups that provide assistance to veterans can also be productive. You can start by connecting with veterans service organizations (VSOs), Vet Centers, Veterans Experience Offices (VEO), and Community Veterans Engagement Boards (CVEBs). The VEO is an office designed around VA and community engagement efforts. This office utilizes the CVEBs to foster a 2-way communication feedback loop between veterans and local VA facilities regarding community engagement efforts and outreach.22 CVEBs are particularly valuable sources of information because veterans directly contribute to the conversation about community engagement by describing the difficulties and successes they’ve experienced. Veteran feedback about how a particular resource met their needs can inform which community services are prioritized for inclusion in the resource list. In addition, CVEBs may have a listing of local government, military, and/or community resources that provide services for veterans. Consider, too, organizations that are unrelated to an individual’s veteran status, but speak to their race/ethnicity, sexual orientation, gender identity, spirituality, socioeconomic status, or disability.
Step 4: Continue to Build Inventory. Use online searches to identify additional resources in the community that are known to have local relationships. These include state suicide prevention coordinators, mental health organizations, and other resources that address social determinants of health (eg, public health and human service organizations, faith-based organizations, collegial organizations). A list of links and search tips are available in the Table.

Steps 5 and 6: Create Document; Organize and Disseminate Information. A spreadsheet can be used to document organization information (Appendix). It is critical to record: (1) the name of the organization or individual; (2) the local address and a point of contact with contact information; (3) services offered to veterans; (4) services specific to suicide prevention, or that address risk factors for suicide; and (5) whether the referral organization is partnered with the VA Community Care Network, which is comprised of contracted HCPs who contract with the VA to provide care to veterans.23

Once a document is created, it can be disseminated through VA offices and among community partners who work with veterans at risk for suicide. It should also be stored in a centralized location such as a shared folder so that it can be continuously updated.
Regularly updating the list is vital so the resource list can continue to be helpful in addressing veterans’ needs and reducing suicide risk factors. Continued collaboration with those in the community can help ensure the resource list is up to date with all available services and pertinent contact information. It can also go far in strengthening collaborative bonds.
IMPLEMENTATION
To illustrate the use of CAM for veteran suicide prevention, we offer a case example of CAM conducted by the VA Patient Safety Center of Inquiry — Suicide Prevention Collaborative (VA PSCI-SPC) team, consisting of 4 team members. A veteran was included as a team member and assisted with the CAM process.
The VA PSCI-SPC sought to identify community services for veterans in Colorado who were not enrolled in VA health care and had risk factors for suicide. Next, the team reached out to colleagues and asked about community organizations that work with individuals at risk for suicide. VA PSCI-SPC outreach resulted in a list of assets that included resources to address mental health, legal concerns, employment, homelessness/housing, finances, religion, peer support, food insecurity, exercise, intimate partner violence, sexual and gender identity needs, and peer support. VSOs and CVEBs were also added to the list.
Next, the team continued to build on the inventory and identified state suicide prevention coordinators; health care systems; regional suicide prevention commissions; Colorado Department of Health and Human Services; program coordinators for Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and their Families; veterans councils; universities (eg, counseling clinics, legal clinics); and foundations devoted to general and veteran-specific suicide prevention within the region.
All the identified resources were inventoried. Details were gathered about each of the organizations, including addresses, points of contact and phone numbers, descriptions of services offered for veterans, descriptions of suicide prevention services offered, whether or not organizations were not-for-profit, the mission of the organizations, and whether or not the organizations were under contract for VA Community Care. Finally, the resource spreadsheet was created and disseminated among stakeholders to be used to enhance veteran suicide care. Stakeholders included social workers, psychologists, and nurse practitioners working with veterans. The list was circulated to VA and community partners as needed.
The VA PSCI-SPC resource document was only 1 benefit of CAM. The asset mapping also resulted in the creation of a learning collaborative comprised of VA and community partners, designed to share knowledge of best practices in suicide prevention and create an established referral network for veterans at risk for suicide.24 Ultimately, the goal of the CAM and the creation of the learning collaborative was to better connect veterans to care in order to decrease suicide risk. A secondary benefit of this community connectedness is that the list of resources produced by CAM became a living document that was, and continues to be, updated as the network became aware of new resources and resources that were no longer available. The VA PSCI-SPC learning collaborative met quarterly to discuss implementation of suicide prevention best practices within their organization.
Data from the VA PSCI-SPC learning collaborative via CAM revealed that organizations felt more efficacious in implementing suicide prevention best practices, noticed increased connections and collaborations with community organizations with the goal of providing services to veterans, and resulted in staff training that improved services provided to veterans.24 This is supported by other findings of a literature review of suicide prevention interventions, which indicated that programs with an established community support network were more effective at reducing suicide rates.25 CAM therefore may be a process through which greater community connection and increased knowledge of resources may help prevent suicide among veterans.
It seems reasonable that the CAM processes used by the VA PSCI-SPC can be implemented within the regional Veterans Integrated Service Networks to identify assets in a specific geographical area to address challenges with social determinants of health and potentially decrease veteran suicide risk.
CONCLUSIONS
CAM can be used to identify and build relationships with community resources that address the stressors that place veterans at risk for suicide. Six proposed steps to CAM for veterans at risk for suicide include: defining community reach (the map); identifying community members and organizations with shared goals; identifying assets within the community; continuing to build inventory; creating a document; and organizing and disseminating the information (while continuing to update the resources).21
CAM can be used to connect veterans with resources to address needs related to adverse social determinants of health that may heighten their risk for suicide. For example, veterans facing legal challenges can connect with a legal clinic; those having difficulties paying bills can obtain financial assistance; those who need help completing their VA claims can connect with the Veterans Benefits Administration or VSOs to assist them with their claims; and those experiencing discrimination can connect with organizations where they may experience acceptance, safety, and support. Broad community support surrounding suicide risk factors can be critical for effective suicide prevention.25
CAM may also be helpful for HCPs and others involved in veteran health care. For example, community mapping can be utilized by newly hired community engagement and partnership coordinators as a tool for outlining resources available for veterans in their community and as a framework to continually update their resource network. CAM develops community awareness, integrates resources, and enhances service utilization, which may assist in veteran suicide prevention by increasing care coordination.17 Finally, mapping community resources can create awareness of the many resources available to help veterans, even before suicide becomes a consideration.
- Rice L. VA Secretary Robert Wilkie says suicide prevention is his agency’s top ‘clinical’ priority. June 17, 2019. Accessed January 30, 2025. https://www.kut.org/post/va-secretary-robert-wilkie-says-suicide-prevention-his-agencys-top-clinical-priority
- US Department of Veterans Affairs. 2023 national veteran suicide prevention annual report. November 2023. Accessed January 30, 2025. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- DeBeer BB, Meyer EC, Kimbrel NA, Kittel JA, Gulliver SB, Morissette SB. Psychological inflexibility predicts of suicidal ideation over time in veterans of the conflicts in Iraq and Afghanistan. Suicide Life Threat Behav. 2018;48(6):627–641. doi:10.1111/sltb.12388
- Ilgen MA, Bohnert ASB, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67(11):1152–1158. doi:10.1001/archgenpsychiatry.2010.129
- Kimbrel NA, Meyer EC, DeBeer BB, Gulliver SB, Morissette SB. A 12-month prospective study of the effects of PTSD-depression comorbidity on suicidal behavior in Iraq/ Afghanistan-era veterans. Psychiatry Res. 2016;243:97–99. doi:10.1016/j.psychres.2016.06.011
- Hoffmire CA, Borowski S, Vogt D. Contribution of veterans’ initial post-separation vocational, financial, and social experiences to their suicidal ideation trajectories following military service. Suicide Life Threat Behav. 2023;53(3):443- 456. doi:10.1111/sltb.12955
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Holliday R, Liu S, Brenner LA, et al. Preventing suicide among homeless veterans: a consensus statement by the Veterans Affairs suicide prevention among veterans experiencing homelessness workgroup. Med Care. 2021;59(Suppl 2):S103- S105. doi:10.1097/MLR.0000000000001399
- Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: The role of social supsupport and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
- Lee DJ, Kearns JC, Wisco BE, et al. A longitudinal study of risk factors for suicide attempts among Operation Enduring Freedom and Operation Iraqi Freedom veterans. Depress Anxiety. 2018;35(7): 609-618. doi:10.1002/da.22736
- Center for Disease Control and Prevention. Social determinants of health (SDOH). Accessed January 30, 2025. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
- Montgomery AE, Dichter M, Byrne T, Blosnich J. Intervention to address homelessness and all-cause and suicide mortality among unstably housed US veterans, 2012- 2016. J Epidemiol Community Health. 2021;75:380-386. doi: 10.1136/jech-2020-214664
- Llamocca EN, Yeh HH, Miller-Matero LR, et al. Association between adverse social determinants of health and suicide death. Med Care. 2023;61(11):744-749. doi:10.1097/MLR.0000000000001918
- Monteith LL, Holliday R, Schneider AL, et al. Institutional betrayal and help-seeking among women survivors of military sexual trauma. Psychol Trauma. 2021;13(7):814-823. doi:10.1037/tra0001027
- VA launches new health care options under MISSION Act. News release. US Department of Veterans Affairs. June 6, 2019. Accessed January 31, 2025. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5264
- COMPACT Act expands free emergency suicide care for veterans. News release. US Department of Veterans Affairs. February 1, 2023. Accessed January 31,2025. https://www.va.gov/poplar-bluff-health-care/news-releases/compact-act-expands-free-emergency-suicide-care-for-veterans/
- US Department of Veterans Affairs. National strategy for preventing Veteran suicide 2018-2028. 2018. Accessed January 31, 2025. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. Veteran outreach toolkit: preventing veteran suicide is everyone’s business. A community call to action. Accessed February 3, 2025. https://floridavets.org/wp-content/uploads/2022/06/VA-Suicide-Prevention-Community-Outreach-Toolkit.pdf
- Crane K, Mooney M. Essential tools: community resource mapping. 2005. Accessed February 3, 2025. https://conservancy.umn.edu/bitstream/handle/11299/172995/NCSET_EssentialTools_ResourceMapping.pdf
- Community Tool Box. 2. Assessing Community Needs and Resources. Accessed February 3, 2025. https://ctb.ku.edu/en/assessing-community-needs-and-resources
- UCLA Center for Health Policy Research. Section 1: asset mapping. 2012. Accessed February 3, 2025. https://healthpolicy.ucla.edu/programs/healthdata/trainings/documents/tw_cba20.pdf
- US Department of Veterans Affairs, Veterans Experience Office. 4th quarter 2018 community engagement news. October 2, 2018. Accessed February 4, 2025. https://content.govdelivery.com/accounts/USVAVEO/bulletins/211836e
- US Department of Veterans Affairs. About our VA community care network and covered services. Accessed February 6, 2025. https://www.va.gov/resources/aboutour-va-community-care-network-and-covered-services/
- DeBeer B, Mignogna J, Borah E, et al. A pilot of a veteran suicide prevention learning collaborative among community organizations: Initial results and outcomes. Suicide Life Threat Behav. 2023;53(4):628-641. doi:10.1111/sltb.12969
- Fountoulakis KN, Gonda X, Rihmer Z. Suicide prevention programs through community intervention. J Affect Disord. 2011;130(1-2):10–16. doi:10.1016/j.jad.2010.06.009
- Rice L. VA Secretary Robert Wilkie says suicide prevention is his agency’s top ‘clinical’ priority. June 17, 2019. Accessed January 30, 2025. https://www.kut.org/post/va-secretary-robert-wilkie-says-suicide-prevention-his-agencys-top-clinical-priority
- US Department of Veterans Affairs. 2023 national veteran suicide prevention annual report. November 2023. Accessed January 30, 2025. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- DeBeer BB, Meyer EC, Kimbrel NA, Kittel JA, Gulliver SB, Morissette SB. Psychological inflexibility predicts of suicidal ideation over time in veterans of the conflicts in Iraq and Afghanistan. Suicide Life Threat Behav. 2018;48(6):627–641. doi:10.1111/sltb.12388
- Ilgen MA, Bohnert ASB, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67(11):1152–1158. doi:10.1001/archgenpsychiatry.2010.129
- Kimbrel NA, Meyer EC, DeBeer BB, Gulliver SB, Morissette SB. A 12-month prospective study of the effects of PTSD-depression comorbidity on suicidal behavior in Iraq/ Afghanistan-era veterans. Psychiatry Res. 2016;243:97–99. doi:10.1016/j.psychres.2016.06.011
- Hoffmire CA, Borowski S, Vogt D. Contribution of veterans’ initial post-separation vocational, financial, and social experiences to their suicidal ideation trajectories following military service. Suicide Life Threat Behav. 2023;53(3):443- 456. doi:10.1111/sltb.12955
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Holliday R, Liu S, Brenner LA, et al. Preventing suicide among homeless veterans: a consensus statement by the Veterans Affairs suicide prevention among veterans experiencing homelessness workgroup. Med Care. 2021;59(Suppl 2):S103- S105. doi:10.1097/MLR.0000000000001399
- Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: The role of social supsupport and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
- Lee DJ, Kearns JC, Wisco BE, et al. A longitudinal study of risk factors for suicide attempts among Operation Enduring Freedom and Operation Iraqi Freedom veterans. Depress Anxiety. 2018;35(7): 609-618. doi:10.1002/da.22736
- Center for Disease Control and Prevention. Social determinants of health (SDOH). Accessed January 30, 2025. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
- Montgomery AE, Dichter M, Byrne T, Blosnich J. Intervention to address homelessness and all-cause and suicide mortality among unstably housed US veterans, 2012- 2016. J Epidemiol Community Health. 2021;75:380-386. doi: 10.1136/jech-2020-214664
- Llamocca EN, Yeh HH, Miller-Matero LR, et al. Association between adverse social determinants of health and suicide death. Med Care. 2023;61(11):744-749. doi:10.1097/MLR.0000000000001918
- Monteith LL, Holliday R, Schneider AL, et al. Institutional betrayal and help-seeking among women survivors of military sexual trauma. Psychol Trauma. 2021;13(7):814-823. doi:10.1037/tra0001027
- VA launches new health care options under MISSION Act. News release. US Department of Veterans Affairs. June 6, 2019. Accessed January 31, 2025. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5264
- COMPACT Act expands free emergency suicide care for veterans. News release. US Department of Veterans Affairs. February 1, 2023. Accessed January 31,2025. https://www.va.gov/poplar-bluff-health-care/news-releases/compact-act-expands-free-emergency-suicide-care-for-veterans/
- US Department of Veterans Affairs. National strategy for preventing Veteran suicide 2018-2028. 2018. Accessed January 31, 2025. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. Veteran outreach toolkit: preventing veteran suicide is everyone’s business. A community call to action. Accessed February 3, 2025. https://floridavets.org/wp-content/uploads/2022/06/VA-Suicide-Prevention-Community-Outreach-Toolkit.pdf
- Crane K, Mooney M. Essential tools: community resource mapping. 2005. Accessed February 3, 2025. https://conservancy.umn.edu/bitstream/handle/11299/172995/NCSET_EssentialTools_ResourceMapping.pdf
- Community Tool Box. 2. Assessing Community Needs and Resources. Accessed February 3, 2025. https://ctb.ku.edu/en/assessing-community-needs-and-resources
- UCLA Center for Health Policy Research. Section 1: asset mapping. 2012. Accessed February 3, 2025. https://healthpolicy.ucla.edu/programs/healthdata/trainings/documents/tw_cba20.pdf
- US Department of Veterans Affairs, Veterans Experience Office. 4th quarter 2018 community engagement news. October 2, 2018. Accessed February 4, 2025. https://content.govdelivery.com/accounts/USVAVEO/bulletins/211836e
- US Department of Veterans Affairs. About our VA community care network and covered services. Accessed February 6, 2025. https://www.va.gov/resources/aboutour-va-community-care-network-and-covered-services/
- DeBeer B, Mignogna J, Borah E, et al. A pilot of a veteran suicide prevention learning collaborative among community organizations: Initial results and outcomes. Suicide Life Threat Behav. 2023;53(4):628-641. doi:10.1111/sltb.12969
- Fountoulakis KN, Gonda X, Rihmer Z. Suicide prevention programs through community intervention. J Affect Disord. 2011;130(1-2):10–16. doi:10.1016/j.jad.2010.06.009
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care
Personality disorders (PDs) are enduring patterns of internal experience and behavior that differ from cultural norms and expectations, are inflexible and pervasive, have their onset in adolescence or early adulthood, and lead to distress or impairment. Ten PDs are included in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition): paranoid, schizoid, schizotypal, borderline, antisocial, histrionic, narcissistic, avoidant, dependent, and obsessive-compulsive.1 These disorders impose a high burden on patients, families, health care systems, and broader economic systems.2,3 Up to 1 in 7 persons in the community and 50% of those receiving outpatient mental health treatment experience a PD.4,5 These conditions are associated with an increased risk of adverse events, including suicide attempt and death by suicide, criminal-legal involvement, homelessness, substance use, underemployment, relational issues, and high utilization of psychiatric services.6-9 PDs are routinely underassessed, underdocumented, and undertreated in clinical settings, and consistently receive less research funding than other, less prevalent forms of psychopathology. 10-12 As a result, there is limited understanding of clinical needs of individuals experiencing PDs.
MILITARY VETERANS WITH PERSONALITY DISORDERS
Underacknowledgment of PDs and their associated difficulties may be especially pronounced in veteran populations. Due to longstanding etiological theories that implicate childhood trauma and adolescent onset in pathology development, PDs are traditionally considered pre-existing conditions or developmental abnormalities by the US Department of Defense and US Department of Veterans Affairs (VA). As a result, PDs are therefore deemed incompatible with military service and ineligible for service-connected disability benefits.13-15 Such determinations allowed PD pathology to be used as grounds for discharge for 26,000 service members from 2001 to 2007, or 2.6% of total enlisted discharges during that period.13,15,16
Despite this structural discrimination, recent research suggests veterans may be more likely to experience PD pathology than the general population.17 For example, a 2021 epidemiological survey in a community-based veteran sample found elevated rates of borderline, antisocial, and schizotypal PDs (6%-13%).6 In contrast, only 0.8% to 5.0% of veteran electronic health records (EHRs) have a documented PD diagnosis.8,18,19 Such elevations in PD pathology within veteran samples imply either a disproportionately high prevalence among enlistees (and therefore missed during recruitment procedures) or onset following military service, possibly due to exposure to traumatic events and/ or occupational stress.17 Due to the relative infancy of research in this area and a lack of longitudinal studies, etiology and course of illness for personality pathology in veterans remains largely unclear.
Structural underacknowledgment of PDs among military personnel has contributed to their underrepresentation in research on veteran populations. PD-focused research with veterans is rare, despite a rapid increase in broader empirical attention paid to these conditions in nonveteran samples.20 A recent meta-analysis of veterans with PDs identified 27 studies that included basic prevalence statistics. PDs were rarely a primary focus for these studies, and most were limited to veterans seen in Veterans Health Administration (VHA) settings.17 The literature also paints a bleak picture, suggesting veterans who experience PDs are at higher risk for suicide attempt and death by suicide, criminal-legal involvement, and homelessness. They also tend to experience more severe comorbid psychopathological symptoms and more often use high-intensity mental health services (eg, care within emergency departments or psychiatric inpatient settings) than veterans without PD pathology.6,8,18,19,21 However, PD pathology does not appear to impede the effectiveness of treatment for veterans.22-24 The implications of PD pathology on broader psychosocial functioning and health care needs certify a need for additional research that examines patterns of personality pathology, particularly in veterans outside the VHA.
METHODS
This study aims to enhance understanding of veterans affected by PDs and offer insight and guidance for treatment of these conditions in federal and nonfederal treatment settings. Previous research has been largely limited to VHA care-receiving samples; the longstanding stigma against PDs by the US military and VA may contribute to biased diagnosis and documentation of PDs in these settings. A large sample of veterans receiving community-based mental health care was therefore used to explore aims of the current study. This study specifically examined demographic patterns, diagnostic comorbidity, psychosocial outcomes, and treatment care settings among veterans with and without a PD diagnosis. Consistent with previous research, we hypothesized that veterans with a PD diagnosis would have more severe mental health comorbidities, poorer psychosocial outcomes, and receive care in higher intensity settings relative to veterans without a diagnosis.
Data for the sample were drawn from the Mental Health Client-Level Data, a publicly available national dataset of nearly 7 million patients who received mental health treatment services provided or funded through state mental health agencies in 2022.25 The analytic sample included about 2.5 million patients for whom veteran status and data around the presence or absence of a PD diagnosis were available. Of these patients, 104,198 were identified as veterans. Veteran patients were identified as predominantly male (63%), White (71%), non-Hispanic (90%), and never married (54%).
Measures
The parent dataset included demographic, clinical, and psychosocial outcome information reported by treatment facilities to individual state administrative systems for each patient who received services. To protect patient privacy, only nonprotected health information is included, and efforts were made throughout compilation of the parent dataset to ensure patient privacy (eg, limiting detail of information disseminated for public access). Because the parent dataset does not include protected health information, studies using these data are considered exempt from institutional review board oversight.
Demographic information. This study reviewed veteran status, sex, race, ethnicity, age, education, and marital status. Veteran status was defined by whether the patient was aged ≥ 18 years and had previously served (but was not currently serving) in the military. Patients with a history of service in the National Guard or Military Reserves were only classified as veterans if they had been called or ordered to active duty while serving. Sex was operationalized dichotomously as male or female; no patients were identified as intersex, transgender, or other gender identities.
Clinical information. Up to 3 mental health diagnoses were reported for each patient and included the following disorders: personality, trauma and attention-deficit/hyperactivity, stressor, anxiety, conduct, delirium/dementia, bipolar, depressive, oppositional defiant, pervasive developmental, schizophrenia or other psychotic, and alcohol or substance use. Mental health diagnosis categories were generated for the parent dataset by grouping diagnostic codes corresponding to each category. To protect patient privacy, more detailed diagnostic information was not available as part of the parent dataset. Although the American Psychiatric Association recognizes 10 distinct PDs, the exact nature of PD diagnoses was not included within the parent dataset. PD diagnoses were coded to reflect the presence or absence of any such diagnosis.
A substance use problem designation was also provided for patients according to various identification methods, including substance use disorder (SUD) diagnosis, substance use screening results, enrollment in a substance use program, substance use survey, service claims information, and other related sources of information. A severe mental illness or serious emotional disturbance designation was provided for patients meeting state definitions of these designations. Context(s) of service provision were coded as inpatient state psychiatric hospital, community-based program, residential treatment center, judicial institution, or other psychiatric inpatient setting.
Psychosocial outcome information. Patient employment and residential status were also included in analyses. Each reflected status at the time of discharge from services or end of reporting period; employment status was only provided for patients receiving treatment in community-based programs.
Data Analysis
Descriptive statistics and X2 analyses were used to compare demographic, clinical, and psychosocial outcome variables between patients with and without PD diagnoses. These analyses were calculated for both the 104,198 veterans and the 2,222,306 nonveterans aged ≥ 18 years in the dataset. Given the sample size, a conservative α of .01 was used to determine statistical significance.
RESULTS
In this sample of persons receiving state-funded mental health care, veterans were significantly less likely than nonveterans to have a documented PD diagnosis (2.1% vs 3.6%, X2 [1] = 647.49; P < .01). PD diagnoses were more common among White (risk ratio [RR], 1.11), non-Hispanic (RR, 1.03) veterans who were in middle to late adulthood (RR, 1.16-1.40), more educated (RR, 1.35), and divorced or widowed (RR, 1.43), and less common among Black/African American (RR, 0.78) or Puerto Rican (RR, 0.32) veterans who were in early adulthood (RR, 0.31-0.79), less educated (RR, 0.64-0.89), and currently married (RR, 0.89) or never married (RR, 0.86). Veteran men and women were equally likely to have a PD diagnosis (RR, 1.03) (Table 1). Among nonveterans, men were less likely than women to have a PD diagnosis (RR, 0.79), and PD diagnoses were most common among persons in middle adulthood (RR, 1.06-1.15) (eAppendix 1).
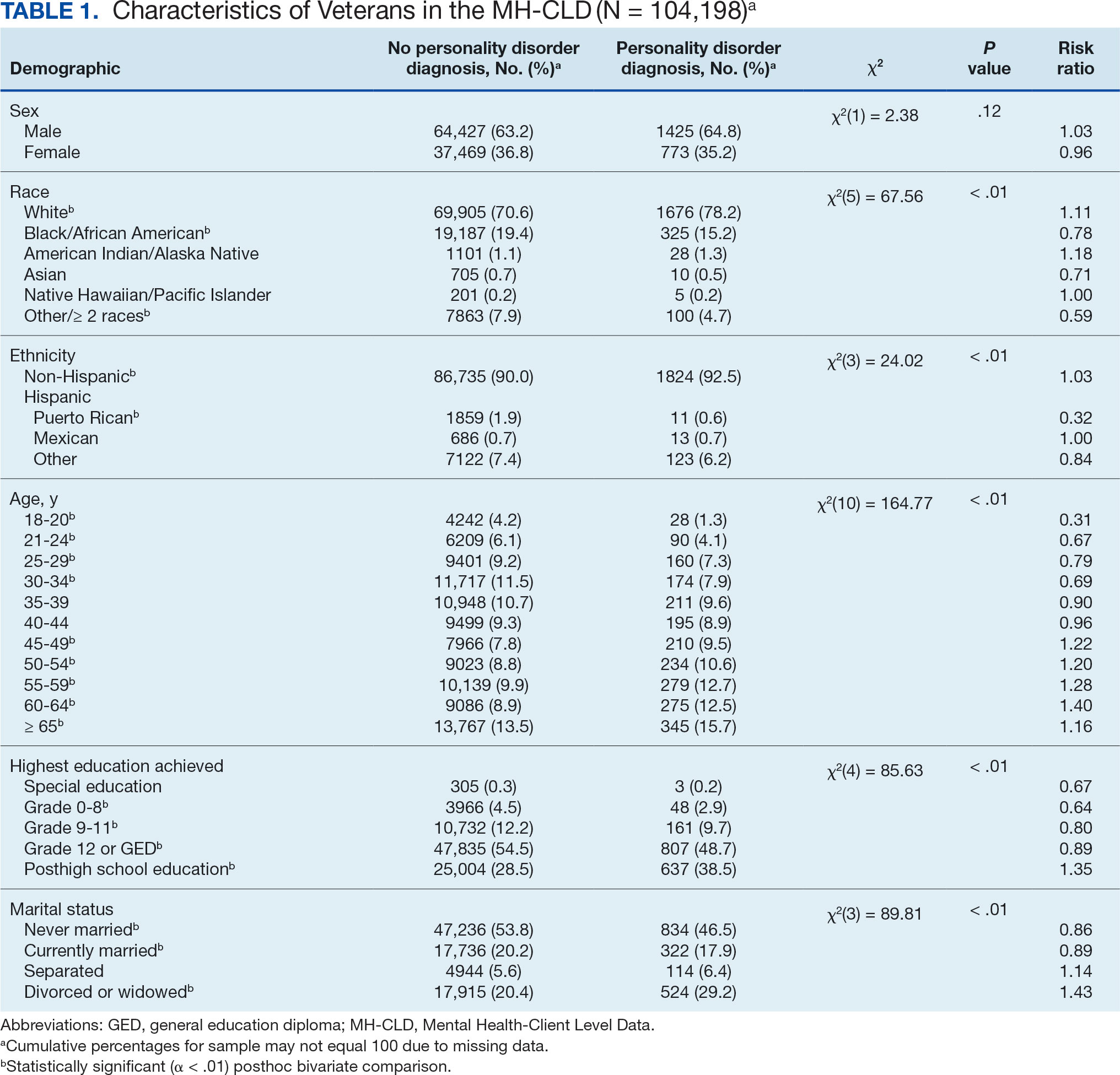
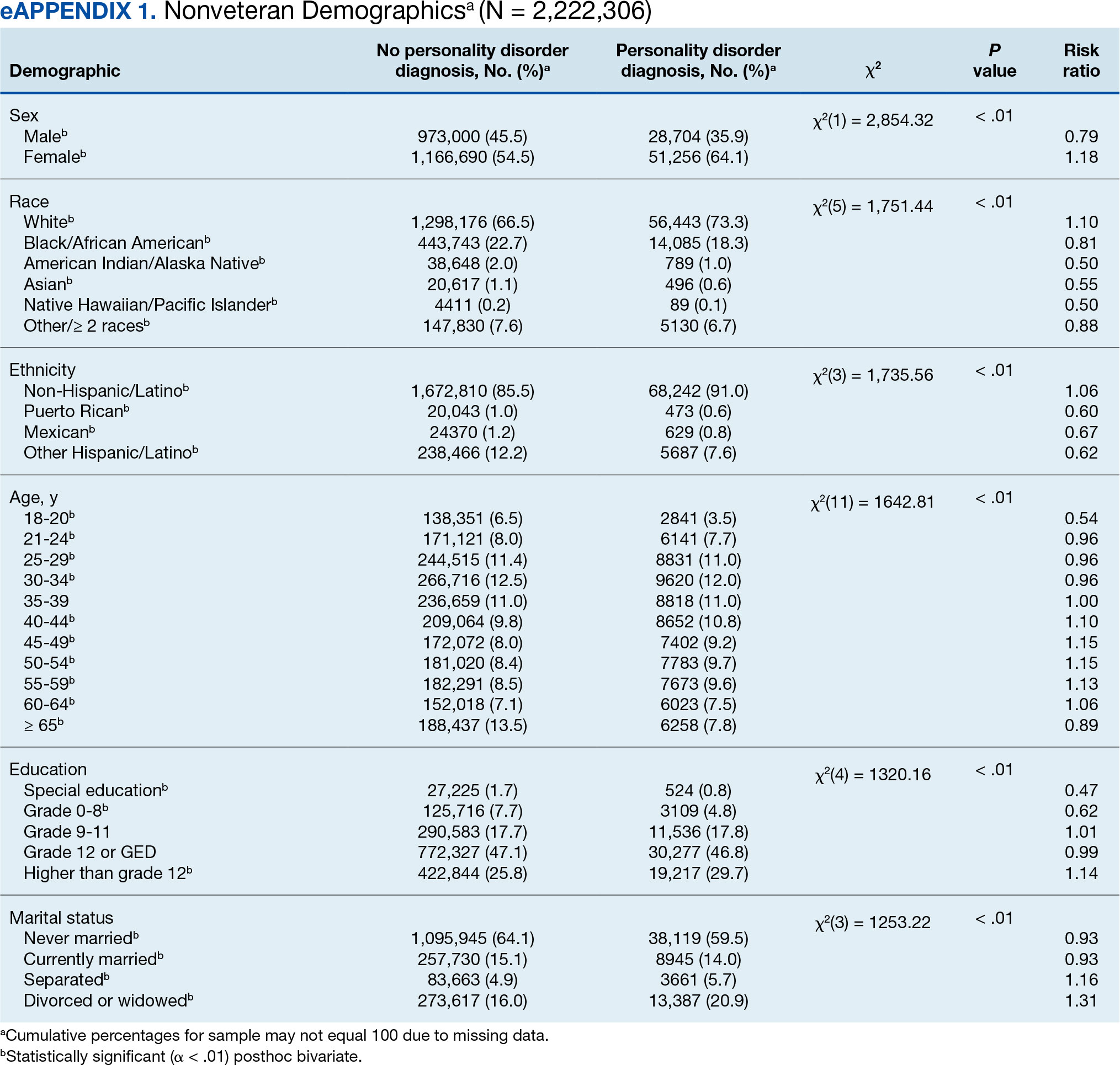
Veterans with a PD diagnosis were more likely than those without a diagnosis to have more diagnoses (RR, 2.96-8.49) and to have comorbid trauma or related stressor (RR, 1.33), or bipolar (RR, 1.56) or psychotic (RR, 1.15) disorder diagnoses, but less likely to have comorbid depressive disorder (RR, 0.82). Although veterans with and without a PD diagnosis were similarly likely to have a comorbid SUD (RR, 1.13), those with a PD diagnosis were significantly less likely to be assigned a substance use problem designation (RR, 0.78). PD diagnosis was also more common among veterans who received services in state psychiatric hospitals (RR, 3.05), community-based clinics (RR, 1.06), and judicial institutions (RR, 6.33) and less common among those who received services in other psychiatric inpatient settings (RR, 0.30). No differences were observed for residential treatment settings (RR, 0.79). Among nonveterans, a PD diagnosis was associated with slightly greater odds of a substance use designation (RR, 1.03) (eAppendix 2).
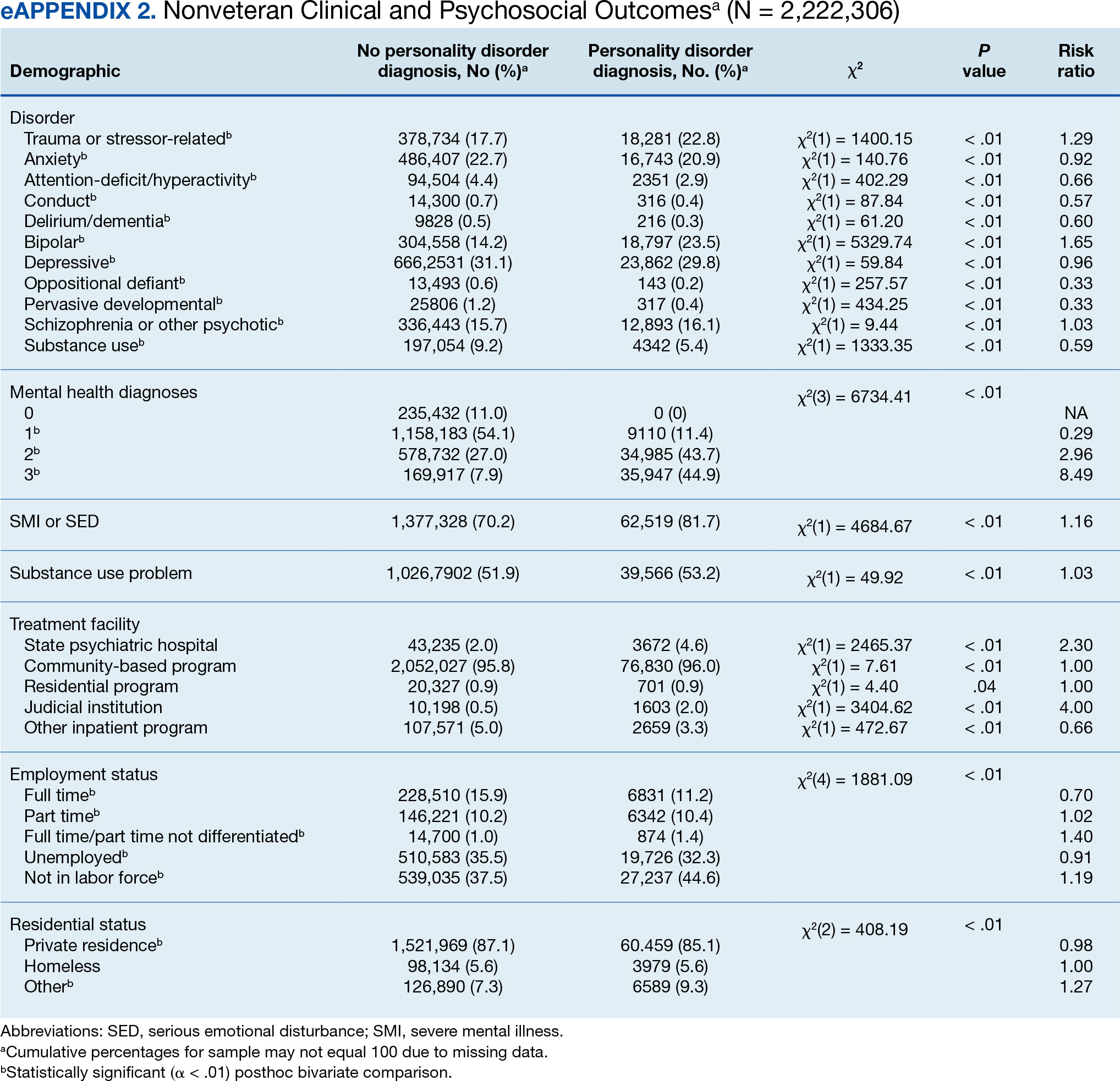
Veterans with a PD diagnosis were also less likely to have full-time employment (RR, 0.73) and more likely to have undifferentiated employment (RR, 2.00) or to be removed from the labor force (RR, 1.35). Veterans with a PD diagnosis were also more likely to reside in nontraditional living conditions (RR, 1.42) and less likely to be residing in a private residence (RR, 0.98), compared with those without PD diagnosis. The rates of homelessness were similar for veterans with and without a PD diagnosis (RR, 0.90) (Table 2). These patterns were similar among nonveterans.
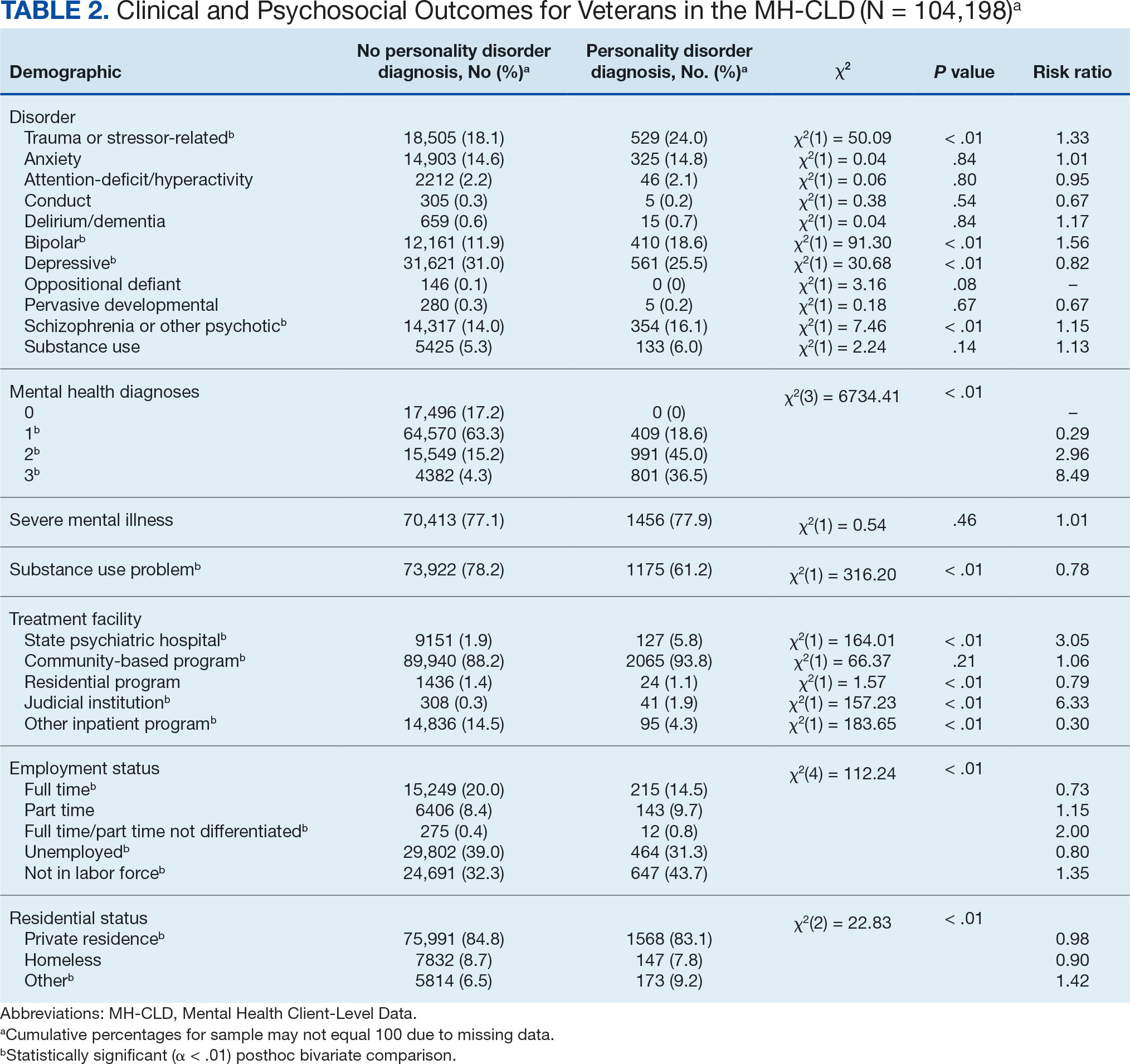
DISCUSSION
This study examined the rate and correlates of PD diagnosis among a large, community-based sample of veterans receiving state-funded mental health care. About 2% of veterans in this sample had a PD diagnosis, with diagnoses more common among veterans who were White, non-Hispanic, aged ≥ 45 years, with higher education, divorced or widowed, also diagnosed with trauma-related, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in state psychiatric hospital, community-based clinic, or judicial system settings.
The observed rate of PD diagnosis in this study aligns with what is typically observed in VHA EHRs.8,18,19 However, the rate is notably lower than prevalence estimates for psychiatric outpatient settings (about 50%) and in meta-analyses of prevalence among veterans (0.8%-23% for each of the 10 PDs).4,17,26 Longstanding stigma against PDs may contribute to underdiagnosis. For example, many clinicians are concerned that documentation or disclosure of a PD will interfere with the patient’s ability to access treatment due to stigma and discrimination.27,28 These fears are not unfounded; even among clinicians, PDs are commonly considered untreatable, and many individuals with PDs are denied access to evidence-based treatments due to the diagnosis.29 In a 2016 survey of community psychiatrists, nearly 1 in 4 reported that they avoid taking patients with a borderline PD diagnosis in their caseloads.28 To date, no studies have been conducted to explore clinicians’ willingness to accept patients with other PDs or, specifically, among veterans.
Despite such widespread stigma, research suggests clinicians' negative attitudes toward PDs can be decreased through antistigma campaigns.30 However, it remains unclear if such efforts also contribute to an increase in clinicians’ willingness to document PD diagnoses. Without accurate identification and documentation, the field’s understanding of PDs will remain limited.
In the current study, veterans with PD diagnoses tended to present with more complex and severe psychiatric comorbidities compared to veterans without such diagnoses. Observed comorbidity of PDs (particularly borderline PD) with trauma-related and bipolar disorders is well established.8 Conversely, co-occurring personality and psychotic disorders—which comprise 16% of veterans with a PD diagnosis in the sample in this study—are not consistently examined in the literature. A 2022 examination of veterans receiving VHA care suggested 12% and 13% of those with a PD diagnosis documented in their EHR also had documented schizophrenia or another psychotic disorder, respectively. PD diagnoses were associated with 6.88- and 9.80-fold increases in risk for comorbid schizophrenia and other psychotic disorder diagnoses, respectively.8 Similarly, a recent longitudinal study of nearly 2 million Swedish individuals suggested borderline PD is specifically associated with a > 24-times greater risk of having a comorbid psychotic disorder.31 It is therefore possible that the comorbidity between personality and psychotic disorders is quite common despite its relative lack of attention in empirical research.
Veterans with PD diagnoses in this study were also more likely to experience substandard housing, employment challenges, and receive treatment through judicial institutions than those without a PD diagnosis. Such findings are consistent with previous research demonstrating the substantial psychosocial challenges associated with PD diagnosis, even after controlling for comorbid conditions.7,9 Veterans with PDs may benefit from specialized case management and support to facilitate stable housing and employment and to mitigate the risk of judicial involvement. Some research suggests veterans with PDs may be less likely to gain competitive employment after participating in VA therapeutic and supportive employment services programs, suggesting standard programming may be less suitable for this population.32 Similarly, other research suggests individuals with PDs may benefit more from specialized, intensive services than standard clinical case management.33 Future research may therefore benefit from clarifying the degree to which adaptations to standard programming could yield beneficial effects for persons with PD diagnoses.
Implications
Cumulatively, the results of this study attest to the necessity for transdiagnostic treatment planning that includes close collaboration between psychotherapeutic, pharmacological, and case management services. Some psychotherapy models for PDs, such as dialectical behavior therapy (DBT), which includes a combination of group skills training, individual therapy, as-needed phone coaching, and therapist consultation, may be successfully adapted to include this collaboration.34-36 However, implementation of such comprehensive programming often requires extensive clinician training and coordination of resources, which poses implementation challenges.37-39 In 2021, the VHA began large-scale implementation of PD-specific psychotherapy for veterans with recent suicidal self-directed violence and borderline PD, including DBT, though to date results remain unclear.40 Generalist approaches, such as good psychiatric management (GPM), which emphasizes emotional validation, practical problem solving, realistic goal setting, and relationship functioning within the context of standard care appointments, may be more easily implemented in community care settings due to lesser training and resource requirements and can also be adapted to include needed elements of care coordination.41,42 Both DBT and GPM were initially developed for the treatment of borderline PD. Although DBT has also demonstrated some effectiveness in the treatment of antisocial PD, potential applications of DBT and GPM to other PDs remain largely underdeveloped.43-46
There are no widely accepted medications for the treatment of PDs. Pharmacotherapy for these conditions typically consists of individualized approaches informed by personal experience that attempt to balance targeting of specific symptoms while minimizing polypharmacy and potential risks (eg, overdose or addiction).47,48 Despite this, pharmacotherapy is often considered a necessary component in the treatment of bipolar and psychotic disorders, both common comorbidities of PDs found in veterans in this study.49,50 Careful consideration of complex comorbidities and pharmacotherapy needs is warranted in the treatment of veterans with PDs. Future research may benefit from clarifying clinical guidelines around pharmacotherapy, particularly for observed comorbidities of PDs to trauma, bipolar, and psychotic disorders.
It is important to note the discrepancies in the results of this study surrounding patient substance use. The results suggest a negligible or inverse association between the likelihood of a PD diagnosis and difficulties with substance use among the veterans in this study. However, the unexpectedly low rate of SUD diagnoses (< 6%) suggests that they were likely underdocumented. Research suggests a strong association between personality and SUDs in both veteran and civilian samples.6,51 Results suggesting a lower prevalence of substance use difficulties among treatment-seeking veterans with PDs should be interpreted with great caution.
Demographically, PD diagnoses were more common among veterans who were White, non-Hispanic, and aged ≥ 45 years, and less common among veterans who were Black/ African American, mixed/unspecified race, Puerto Rican or other non-Mexican Hispanic ethnicity, or aged < 35 years. No significant sex-based differences were observed. These patterns are consistent with research suggesting individuals who identify as Black may be less likely than individuals who identify as White to report PD symptoms, meet criteria for a PD, and have a PD diagnosed even when it is warranted.52
The findings observed in this study with respect to age, however, are notably inconsistent with the literature. Previous research typically suggests a negative association between age and PD pathology; however, a 2020 review of PDs in older adults by Penders et al suggests a prevalence of 11% to 15% in this population.53,54 Research into PDs most often focuses on adolescent and early adulthood developmental periods, limiting insight into the phenomenology of PDs in middle to late adulthood.55 Further, most research into PDs among geriatric populations has focused on psychometric assessment rather than practical treatment guidance.54 However, in this study, elevated risk for PD diagnoses was salient throughout middle to late adulthood among veterans; similar, albeit less pronounced patterns were also observed for elevated risk of PD diagnosis in middle adulthood among nonveterans. Such findings suggest clarifying the phenomenology and treatment needs of individuals with PDs in middle to late adulthood may have particularly salient implications for the mental health care of veterans affected by these conditions. As the veteran population advances in age, these needs will present unique challenges if health care systems are unprepared to effectively address them.
Limitations
This study is characterized by several strengths, most notably its use of a large dataset recently collected on a national scale. Few studies outside of the VHA system include samples of > 100,000 treatment-seeking veterans collected on a national scale. Nevertheless, results should be understood within the context of several methodological limitations. However, the dataset was limited to the first 3 diagnoses documented in patients’ EHRs, and many patients had no listed diagnoses. Patients with complex comorbidities may have > 3 diagnoses; for these individuals, data provided an incomplete picture of clinical presentation. This is especially relevant for individuals with PDs, who tend to meet criteria for a range of comorbid conditions.8,10 The now dated practice of listing PDs on Axis II also increases the chance of clinicians listing PDs after conditions traditionally listed on Axis I (eg, major depressive disorder) in patient charts.56 This study’s inclusion of only the first 3 listed diagnoses likely underestimated true PD diagnosis prevalence.
The results of this study must be interpreted as reflecting the prevalence and correlates of receiving a PD diagnosis rather than meeting diagnostic criteria for a PD. Relatedly, PD diagnoses were reported as a single construct, limiting insight into prevalence and correlates of individual PD diagnoses (eg, borderline vs paranoid PDs). Meta-analyses estimates suggest PD prevalence among veterans is likely much higher than observed in this study.17 Stigma continues to discourage clinicians from documenting and disclosing PD diagnoses even when warranted.27,28 Continued research should aim to clarify conditions (eg, patient presentation, stigma, or institutional culture) contributing to documentation of PD diagnoses. Given the cross-sectional nature of this study, results cannot speak to longitudinal treatment outcomes or prognosis of persons receiving a PD diagnosis.
Despite its large sample size and national representation, the sampling strategy of this study could have contributed to idiosyncrasies in the dataset. Restriction of data to the persons receiving state-funded mental health services introduces a notable bias to the composition of the sample, which is likely comprised of a disproportionately high number of Medicaid recipients, students, and individuals with chronic illnesses and underrepresentation of persons who pay for mental health services using private insurance or private pay arrangements. As such, although socioeconomic information was not provided within this dataset, one can presume a generally lower socioeconomic status among study participants compared to the community at large. This study also included a proportionally small sample of veterans (3.6% compared to about 6.2% in the broader US population), suggesting veterans may have been underrepresented or underidentified in surveyed mental health care settings.57 This study also did not include data around service in active-duty military, national guard, or military reserves; a greater proportion of the sample likely had a history of military service than was represented by veteran status designation. Further, the proportionally high sample of individuals with severe mental illness suggests a likely overrepresentation of such conditions in surveyed settings.
Institutional differences in the practice of assigning diagnoses likely limited statistical power to detect potentially meaningful associations and effects. Structural influences, such as stigma and institutional culture, may have notable effects on documentation practices, particularly for PDs. Future research should aim to replicate observed associations using more controlled diagnostic procedures.
Lastly, even with the use of a more conservative α and a focus on effect sizes to guide interpretation of results, use of multiple bivariate analyses can be presumed to have increased the likelihood of type I error. Given the limited prior research in this area, an exploratory approach to statistical analysis was considered warranted to maximize opportunity for identifying areas in need of additional empirical attention. Continued research using more conservative statistical approaches (eg, multivariate analyses) is needed to determine replicability and generalizability of observed results.
CONCLUSIONS
This study examined the prevalence and correlates of PD diagnoses in a national sample of veterans receiving community-based, state-funded mental health care. About 2% received a PD diagnosis, with diagnoses most common among veterans who were White, non-Hispanic, aged ≥ 45 years, also diagnosed with trauma-based, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in a state psychiatric hospital or judicial system setting. The results attest to a necessity for transdiagnostic treatment planning and care coordination for this population, with particular attention to psychosocial stressors.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
- Hastrup LH, Jennum P, Ibsen R, Kjellberg J, Simonsen E. Societal costs of borderline personality disorders: a matched-controlled nationwide study of patients and spouses. Acta Psychiatr Scand. 2019;140(5):458-467. doi:10.1111/acps.13094
- Sveen CA, Pedersen G, Ulvestad DA, Zahl KE, Wilberg T, Kvarstein EH. Societal costs of personality disorders: a cross-sectional multicenter study of treatment-seeking patients in mental health services in Norway. J Clin Psychol. 2023;79(8):1752-1769. doi:10.1002/jclp.23504
- Beckwith H, Moran PF, Reilly J. Personality disorder prevalence in psychiatric outpatients: a systematic literature review. Personal Ment Health. 2014;8(2):91-101. doi:10.1002/pmh.1252
- Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc. 2017.09.001
- Edwards ER, Barnes S, Govindarajulu U, Geraci J, Tsai J. Mental health and substance use patterns associated with lifetime suicide attempt, incarceration, and homelessness: a latent class analysis of a nationally representative sample of U.S. veterans. Psychol Serv. 2021;18(4):619-631. doi:10.1037/ser0000488
- Moran P, Romaniuk H, Coffey C, et al. The influence of personality disorder on the future mental health and social adjustment of young adults: a population-based cohort study. Lancet Psychiatry. 2016;3(7):636-645. doi:10.1016/S2215-0366(16)30029-3
- Nelson SM, Griffin CA, Hein TC, Bowersox N, McCarthy JF. Personality disorder and suicide risk among patients in the Veterans Affairs health system. Personal Disord. 2022;13(6):563-571. doi:10.1037/per0000521
- Skodol AE. Impact of personality pathology on psychosocial functioning. Curr Opin Psychol. 2018;21;33-38. doi:10.1016/j.copsyc.2017.09.006
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015;385(9969):717-726. doi:10.1016/S0140-6736(14)61995-4
- Fitzpatrick S, Goss S, Di Bartolomeo A, Varma S, Tissera T, Earle E. Follow the money: is borderline personality disorder research underfunded in Canada? Can Psychol. 2024;65(1):46-57. doi:10.1037/cap0000375
- Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944. doi:10.1016/j.psychres.2014.09.021
- Leroux TC. U.S. military discharges and pre-existing personality disorders: a health policy review. Adm Policy Ment Health. 2015;42(6):748-755. doi:10.1007/s10488-014-0611-z
- Monahan MC, Keener JK. Fitness-for-duty evaluations. In Kennedy CH, Zillmer EA, eds. Military Psychology: Clinical and Operational Applications. 2nd ed. Guilford Publications; 2012:25-49.
- Hearing Before the Committee on Veterans’ Affairs, 111th Congress 2nd Sess (2010). Personality disorder discharges: impact on veterans benefits. Accessed March 4, 2025. https://www.govinfo.gov/content/pkg/CHRG-111hhrg61755/html/CHRG-111hhrg61755.htm
- Ader M, Cuthbert R, Hoechst K, Simon EH, Strassburger Z, Wishnie M. Casting troops aside: the United States military’s illegal personality disorder discharge problem. Vietnam Veterans of America. March 2012. Accessed February 28, 2025. https://law.yale.edu/sites/default/files/documents/pdf/Clinics/VLSC_CastingTroopsAside.pdf
- Edwards ER, Tran H, Wrobleski J, Rabhan Y, Yin J, Chiodi C, Goodman M, Geraci J. Prevalence of personality disorders across veteran samples: A meta-analysis. J Pers Disord. 2022;36(3):339-358. doi:10.1521/ pedi.2022.36.3.339
- Holliday R, Desai A, Edwards E, Borges L. Personal i ty disorder diagnosis among just ice -involved veterans: an investigation of VA-using veterans. J Nerv Ment Dis. 2023;211(5):402-406 doi:10.1097/ NMD.0000000000001627
- McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942. doi:10.2105/AJPH.2015.302737
- Liu Y, Chen C, Zhou Y, Zhang N, Liu S. Twenty years of research on borderline personality disorder: a scientometric analysis of hotspots, bursts, and research trends. Front Psych. 2024;15:1361535. doi:10.3389/ fpsyt.2024.1361535
- Williams R, Holliday R, Clem M, Anderson E, Morris EE, Surís A. Borderline personality disorder and military sexual trauma: analysis of previous traumatization and current psychiatric presentation. J Interpers Violence. 2017;32(15):2223-2236. doi:10.1177/0886260515596149
- Holder N, Holliday R, Pai A, Surís A. Role of borderline personality disorder in the treatment of military sexual trauma-related posttraumatic stress disorder with cognitive processing therapy. Behav Med. 2017;43(3):184-190. doi:10.1080/08964289.2016.1276430
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. Am J Addict. 2007;16(6):443- 449. doi:10.1080/10550490701643336
- Walter KH, Bolte TA, Owens GP, Chard KM. The impact of personality disorders on treatment outcome for veterans in a posttraumatic stress disorder residential treatment program. Cognit Ther Res. 2012;36(5):576-584. doi:10.1007/s10608-011-9393-8
- Substance Abuse and Mental Health Services. Mental health client-level data (MH-CLD), 2022. Accessed February 28, 2025. https://www.datafiles.samhsa.gov/dataset/mental-health-client-level-data-2022-mh-cld-2022-ds0001
- Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918. doi:10.1176/appi.ajp.162.10.1911
- Campbell K, Clarke KA, Massey D, Lakeman R. Borderline personality disorder: To diagnose or not to diagnose? That is the question. Int J Mental Health Nurs. 2020;29(5):972-981. doi:10.1111/inm.12737
- Sisti D, Segal AG, Siegel AM, Johnson R, Gunderson J. Diagnosing, disclosing, and documenting borderline personality disorder: a survey of psychiatrists’ practices. J Pers Disord. 2016;30(6):848-856. doi:10.1521/ pedi_2015_29_228
- Klein P, Fairweather AK, Lawn S. Structural stigma and its impact on healthcare for borderline personality disorder: a scoping review. Int J Ment Health Syst. 2022;16(1):48. doi:10.1186/s13033-022-00558-3
- Knaak S, Szeto AC, Fitch K, Modgill G, Patten S. Stigma towards borderline personality disorder: effectiveness and generalizability of an anti-stigma program for healthcare providers using a pre-post randomized design. Borderline Personal Disord Emot Dysregul. 2015;2:9. doi:10.1186/s40479-015-0030-0
- Tate AE, Sahlin H, Liu S, et al. Borderline personality disorder: associations with psychiatric disorders, somatic illnesses, trauma, and adverse behaviors. Mol Psychiatry. 2022;27:2514-2521. doi:10.1038/s41380- 022-01503-z
- Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA Therapeutic and Supported Employment Services programs. Psychiatr Serv. 2017;68(9)938-946. doi:10.1176/appi. ps201600412
- Frisman LK, Mueser KT, Covell NH, et al. Use of integrated dual disorder treatment via Assertive Community Treatment versus clinical case management for persons with co-occurring disorders and antisocial personality disorder. J Nerv Ment Dis. 2009;197(11):822-828. doi:10.1097/NMD.0b013e3181beac52
- Edwards ER, Kober H, Rinne GR, Griffin SA, Axelrod S, Cooney EB. Skills]homework completion and phone coaching as predictors of therapeutic change and outcomes in completers of a DBT intensive outpatient programme. Psychol Psychother. 2021;94(3):504-522. doi:10.1111/papt.12325
- Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67(1):13-26. doi:10.1016/s0376-8716(02)00011-x
- Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry. 2015;72(5):475-482.doi:10.1001 /jamapsychiatry.2014.3039
- Carmel A, Rose ML, Fruzzetti AE. Barriers and solutions to implementing dialectical behavior therapy in a public behavioral health system. Adm Policy Ment Health. 2014;41(5):608-614. doi:10.1007/s10488-013-0504-6
- Decker SE, Matthieu MM, Smith BN, Landes SJ. Barriers and facilitators to dialectical behavior therapy skills groups in the Veterans Health Administration. Mil Med. 2024;189(5-6):1055-1063. doi:10.1093/milmed/ usad123
- Landes SJ, Rodriguez AL, Smith BN, et al. Barriers, facilitators, and benefits of implementation of dialectical behavior therapy in routine care: results from a national program evaluation survey in the Veterans Health Administration. Transl Behav Med. 2017;7(4):832-844. doi:10.1007/s13142-017-0465-5
- Walker J, Betthauser LM, Green K, Landes SJ, Stacy M. Suicide Prevention 2.0 Clinical Telehealth Program: Evidence- Based Treatment in the Veterans Health Administration. April 28, 2024. Accessed February 28, 2025. https://www.youtube.com/watch?v=fFsDzkg0SR0
- Gunderson J, Masland S, Choi-Kain L. Good psychiatric management: a review. Curr Opin Psychol. 2018;21:127- 131. doi:10.1016/j.copsyc.2017.12.006
- Kramer U. Good-enough therapy: a review of the empirical basis of good psychiatric management. Am J Psychother. 2025;78(1): 11-15. doi:10.1176/appi .psychotherapy.20230041
- Visdómine-Lozano JC. Contextualist perspectives in the treatment of antisocial behaviors and offending: a comparative review of FAP, ACT, DBT, and MDT. Trauma Violence Abuse. 2022;23(1):241-254. doi:10.1177/1524838020939509
- Drago A, Marogna C, Jørgen Søgaard H. A review of characteristics and treatments of the avoidant personality disorder. Could the DBT be an option? Int J Psychol Psychoanal. 2016;2(1):013.
- Finch EF, Choi-Kain LW, Iliakis EA, Eisen JL, Pinto A. Good psychiatric management for obsessive–compulsive personality disorder. Curr Behav Neurosci Rep. 2021;8:160-171. doi:10.1007/s40473-021-00239-4
- Miller TW, Kraus RF. Modified dialectical behavior therapy and problem solving for obsessive-compulsive personality disorder. Journal Contemp Psychother. 2007;37:79-85. doi:10.1007/s10879-006-9039-4
- Bozzatello P, Rocca P, De Rosa ML, Bellino S. Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert Opin Pharmacother. 2020;21(1):47-61.doi:10.1080/14656566 .2019.1686482
- Sand P, Derviososki E, Kollia S, Strand J, Di Leone F. Psychiatrists’ perspectives on prescription decisions for patients with personality disorders. J Pers Disord. 2024;38(3):225-240. doi:10.1521/pedi.2024.38.3.225
- Kane JM, Leucht S, Carpenter D, Docherty JP; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J Clin Psychiatry. 2003;64 Suppl 12:5-19.
- Nierenberg AA, Agustini B, Köhler-Forsberg O, et al. Diagnosis and treatment of bipolar disorder: a review. JAMA. 2023;330(14):1370-1380. doi:10.1001 /jama.2023.18588
- Köck P, Walter M. Personality disorder and substance use disorder–an update. Ment Health Prev. 2018;12:82- 89. doi:10.1016/J.MHP.2018.10.003
- Garb HN. Race bias and gender bias in the diagnosis of psychological disorders. Clin Psych Rev. 2021;90:102087. doi:10.1016/j.cpr.2021.102087
- Debast I, van Alphen SPJ, Rossi G, et al. Personality traits and personality disorders in late middle and old age: do they remain stable? A literature review. Clin Gerontol. 2014;37(3):253-271.doi:10.1080/07317115 .2014.885917
- Penders KAP, Peeters IGP, Metsemakers JFM, van Alphen SPJ. Personality disorders in older adults: a review of epidemiology, assessment, and treatment. Curr Psychiatry Rep. 2020;22(3):1-14. doi:10.1007/s11920-020- 1133-x
- Videler AC, Hutsebaut J, Schulkens JEM, Sobczak S, van Alphen SPJ. A life span perspective on borderline personality disorder. Curr Psychiatry Rep. 2019;21(7) :1-8. doi:10.1007/s11920-019-1040-1
- Wakefield JC. DSM-5 and the general definition of personality disorder. Clin Soc Work J. 2013;41(2):168-183. doi:10.1007/s10615-012-0402-5
- US Census Bureau. 2022 American Community Survey 1-year. Accessed February 28, 2025. https://data.census.gov/table/ACSST1Y2022.S2101?q=Veterans&y=2022comparison
Personality disorders (PDs) are enduring patterns of internal experience and behavior that differ from cultural norms and expectations, are inflexible and pervasive, have their onset in adolescence or early adulthood, and lead to distress or impairment. Ten PDs are included in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition): paranoid, schizoid, schizotypal, borderline, antisocial, histrionic, narcissistic, avoidant, dependent, and obsessive-compulsive.1 These disorders impose a high burden on patients, families, health care systems, and broader economic systems.2,3 Up to 1 in 7 persons in the community and 50% of those receiving outpatient mental health treatment experience a PD.4,5 These conditions are associated with an increased risk of adverse events, including suicide attempt and death by suicide, criminal-legal involvement, homelessness, substance use, underemployment, relational issues, and high utilization of psychiatric services.6-9 PDs are routinely underassessed, underdocumented, and undertreated in clinical settings, and consistently receive less research funding than other, less prevalent forms of psychopathology. 10-12 As a result, there is limited understanding of clinical needs of individuals experiencing PDs.
MILITARY VETERANS WITH PERSONALITY DISORDERS
Underacknowledgment of PDs and their associated difficulties may be especially pronounced in veteran populations. Due to longstanding etiological theories that implicate childhood trauma and adolescent onset in pathology development, PDs are traditionally considered pre-existing conditions or developmental abnormalities by the US Department of Defense and US Department of Veterans Affairs (VA). As a result, PDs are therefore deemed incompatible with military service and ineligible for service-connected disability benefits.13-15 Such determinations allowed PD pathology to be used as grounds for discharge for 26,000 service members from 2001 to 2007, or 2.6% of total enlisted discharges during that period.13,15,16
Despite this structural discrimination, recent research suggests veterans may be more likely to experience PD pathology than the general population.17 For example, a 2021 epidemiological survey in a community-based veteran sample found elevated rates of borderline, antisocial, and schizotypal PDs (6%-13%).6 In contrast, only 0.8% to 5.0% of veteran electronic health records (EHRs) have a documented PD diagnosis.8,18,19 Such elevations in PD pathology within veteran samples imply either a disproportionately high prevalence among enlistees (and therefore missed during recruitment procedures) or onset following military service, possibly due to exposure to traumatic events and/ or occupational stress.17 Due to the relative infancy of research in this area and a lack of longitudinal studies, etiology and course of illness for personality pathology in veterans remains largely unclear.
Structural underacknowledgment of PDs among military personnel has contributed to their underrepresentation in research on veteran populations. PD-focused research with veterans is rare, despite a rapid increase in broader empirical attention paid to these conditions in nonveteran samples.20 A recent meta-analysis of veterans with PDs identified 27 studies that included basic prevalence statistics. PDs were rarely a primary focus for these studies, and most were limited to veterans seen in Veterans Health Administration (VHA) settings.17 The literature also paints a bleak picture, suggesting veterans who experience PDs are at higher risk for suicide attempt and death by suicide, criminal-legal involvement, and homelessness. They also tend to experience more severe comorbid psychopathological symptoms and more often use high-intensity mental health services (eg, care within emergency departments or psychiatric inpatient settings) than veterans without PD pathology.6,8,18,19,21 However, PD pathology does not appear to impede the effectiveness of treatment for veterans.22-24 The implications of PD pathology on broader psychosocial functioning and health care needs certify a need for additional research that examines patterns of personality pathology, particularly in veterans outside the VHA.
METHODS
This study aims to enhance understanding of veterans affected by PDs and offer insight and guidance for treatment of these conditions in federal and nonfederal treatment settings. Previous research has been largely limited to VHA care-receiving samples; the longstanding stigma against PDs by the US military and VA may contribute to biased diagnosis and documentation of PDs in these settings. A large sample of veterans receiving community-based mental health care was therefore used to explore aims of the current study. This study specifically examined demographic patterns, diagnostic comorbidity, psychosocial outcomes, and treatment care settings among veterans with and without a PD diagnosis. Consistent with previous research, we hypothesized that veterans with a PD diagnosis would have more severe mental health comorbidities, poorer psychosocial outcomes, and receive care in higher intensity settings relative to veterans without a diagnosis.
Data for the sample were drawn from the Mental Health Client-Level Data, a publicly available national dataset of nearly 7 million patients who received mental health treatment services provided or funded through state mental health agencies in 2022.25 The analytic sample included about 2.5 million patients for whom veteran status and data around the presence or absence of a PD diagnosis were available. Of these patients, 104,198 were identified as veterans. Veteran patients were identified as predominantly male (63%), White (71%), non-Hispanic (90%), and never married (54%).
Measures
The parent dataset included demographic, clinical, and psychosocial outcome information reported by treatment facilities to individual state administrative systems for each patient who received services. To protect patient privacy, only nonprotected health information is included, and efforts were made throughout compilation of the parent dataset to ensure patient privacy (eg, limiting detail of information disseminated for public access). Because the parent dataset does not include protected health information, studies using these data are considered exempt from institutional review board oversight.
Demographic information. This study reviewed veteran status, sex, race, ethnicity, age, education, and marital status. Veteran status was defined by whether the patient was aged ≥ 18 years and had previously served (but was not currently serving) in the military. Patients with a history of service in the National Guard or Military Reserves were only classified as veterans if they had been called or ordered to active duty while serving. Sex was operationalized dichotomously as male or female; no patients were identified as intersex, transgender, or other gender identities.
Clinical information. Up to 3 mental health diagnoses were reported for each patient and included the following disorders: personality, trauma and attention-deficit/hyperactivity, stressor, anxiety, conduct, delirium/dementia, bipolar, depressive, oppositional defiant, pervasive developmental, schizophrenia or other psychotic, and alcohol or substance use. Mental health diagnosis categories were generated for the parent dataset by grouping diagnostic codes corresponding to each category. To protect patient privacy, more detailed diagnostic information was not available as part of the parent dataset. Although the American Psychiatric Association recognizes 10 distinct PDs, the exact nature of PD diagnoses was not included within the parent dataset. PD diagnoses were coded to reflect the presence or absence of any such diagnosis.
A substance use problem designation was also provided for patients according to various identification methods, including substance use disorder (SUD) diagnosis, substance use screening results, enrollment in a substance use program, substance use survey, service claims information, and other related sources of information. A severe mental illness or serious emotional disturbance designation was provided for patients meeting state definitions of these designations. Context(s) of service provision were coded as inpatient state psychiatric hospital, community-based program, residential treatment center, judicial institution, or other psychiatric inpatient setting.
Psychosocial outcome information. Patient employment and residential status were also included in analyses. Each reflected status at the time of discharge from services or end of reporting period; employment status was only provided for patients receiving treatment in community-based programs.
Data Analysis
Descriptive statistics and X2 analyses were used to compare demographic, clinical, and psychosocial outcome variables between patients with and without PD diagnoses. These analyses were calculated for both the 104,198 veterans and the 2,222,306 nonveterans aged ≥ 18 years in the dataset. Given the sample size, a conservative α of .01 was used to determine statistical significance.
RESULTS
In this sample of persons receiving state-funded mental health care, veterans were significantly less likely than nonveterans to have a documented PD diagnosis (2.1% vs 3.6%, X2 [1] = 647.49; P < .01). PD diagnoses were more common among White (risk ratio [RR], 1.11), non-Hispanic (RR, 1.03) veterans who were in middle to late adulthood (RR, 1.16-1.40), more educated (RR, 1.35), and divorced or widowed (RR, 1.43), and less common among Black/African American (RR, 0.78) or Puerto Rican (RR, 0.32) veterans who were in early adulthood (RR, 0.31-0.79), less educated (RR, 0.64-0.89), and currently married (RR, 0.89) or never married (RR, 0.86). Veteran men and women were equally likely to have a PD diagnosis (RR, 1.03) (Table 1). Among nonveterans, men were less likely than women to have a PD diagnosis (RR, 0.79), and PD diagnoses were most common among persons in middle adulthood (RR, 1.06-1.15) (eAppendix 1).


Veterans with a PD diagnosis were more likely than those without a diagnosis to have more diagnoses (RR, 2.96-8.49) and to have comorbid trauma or related stressor (RR, 1.33), or bipolar (RR, 1.56) or psychotic (RR, 1.15) disorder diagnoses, but less likely to have comorbid depressive disorder (RR, 0.82). Although veterans with and without a PD diagnosis were similarly likely to have a comorbid SUD (RR, 1.13), those with a PD diagnosis were significantly less likely to be assigned a substance use problem designation (RR, 0.78). PD diagnosis was also more common among veterans who received services in state psychiatric hospitals (RR, 3.05), community-based clinics (RR, 1.06), and judicial institutions (RR, 6.33) and less common among those who received services in other psychiatric inpatient settings (RR, 0.30). No differences were observed for residential treatment settings (RR, 0.79). Among nonveterans, a PD diagnosis was associated with slightly greater odds of a substance use designation (RR, 1.03) (eAppendix 2).

Veterans with a PD diagnosis were also less likely to have full-time employment (RR, 0.73) and more likely to have undifferentiated employment (RR, 2.00) or to be removed from the labor force (RR, 1.35). Veterans with a PD diagnosis were also more likely to reside in nontraditional living conditions (RR, 1.42) and less likely to be residing in a private residence (RR, 0.98), compared with those without PD diagnosis. The rates of homelessness were similar for veterans with and without a PD diagnosis (RR, 0.90) (Table 2). These patterns were similar among nonveterans.

DISCUSSION
This study examined the rate and correlates of PD diagnosis among a large, community-based sample of veterans receiving state-funded mental health care. About 2% of veterans in this sample had a PD diagnosis, with diagnoses more common among veterans who were White, non-Hispanic, aged ≥ 45 years, with higher education, divorced or widowed, also diagnosed with trauma-related, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in state psychiatric hospital, community-based clinic, or judicial system settings.
The observed rate of PD diagnosis in this study aligns with what is typically observed in VHA EHRs.8,18,19 However, the rate is notably lower than prevalence estimates for psychiatric outpatient settings (about 50%) and in meta-analyses of prevalence among veterans (0.8%-23% for each of the 10 PDs).4,17,26 Longstanding stigma against PDs may contribute to underdiagnosis. For example, many clinicians are concerned that documentation or disclosure of a PD will interfere with the patient’s ability to access treatment due to stigma and discrimination.27,28 These fears are not unfounded; even among clinicians, PDs are commonly considered untreatable, and many individuals with PDs are denied access to evidence-based treatments due to the diagnosis.29 In a 2016 survey of community psychiatrists, nearly 1 in 4 reported that they avoid taking patients with a borderline PD diagnosis in their caseloads.28 To date, no studies have been conducted to explore clinicians’ willingness to accept patients with other PDs or, specifically, among veterans.
Despite such widespread stigma, research suggests clinicians' negative attitudes toward PDs can be decreased through antistigma campaigns.30 However, it remains unclear if such efforts also contribute to an increase in clinicians’ willingness to document PD diagnoses. Without accurate identification and documentation, the field’s understanding of PDs will remain limited.
In the current study, veterans with PD diagnoses tended to present with more complex and severe psychiatric comorbidities compared to veterans without such diagnoses. Observed comorbidity of PDs (particularly borderline PD) with trauma-related and bipolar disorders is well established.8 Conversely, co-occurring personality and psychotic disorders—which comprise 16% of veterans with a PD diagnosis in the sample in this study—are not consistently examined in the literature. A 2022 examination of veterans receiving VHA care suggested 12% and 13% of those with a PD diagnosis documented in their EHR also had documented schizophrenia or another psychotic disorder, respectively. PD diagnoses were associated with 6.88- and 9.80-fold increases in risk for comorbid schizophrenia and other psychotic disorder diagnoses, respectively.8 Similarly, a recent longitudinal study of nearly 2 million Swedish individuals suggested borderline PD is specifically associated with a > 24-times greater risk of having a comorbid psychotic disorder.31 It is therefore possible that the comorbidity between personality and psychotic disorders is quite common despite its relative lack of attention in empirical research.
Veterans with PD diagnoses in this study were also more likely to experience substandard housing, employment challenges, and receive treatment through judicial institutions than those without a PD diagnosis. Such findings are consistent with previous research demonstrating the substantial psychosocial challenges associated with PD diagnosis, even after controlling for comorbid conditions.7,9 Veterans with PDs may benefit from specialized case management and support to facilitate stable housing and employment and to mitigate the risk of judicial involvement. Some research suggests veterans with PDs may be less likely to gain competitive employment after participating in VA therapeutic and supportive employment services programs, suggesting standard programming may be less suitable for this population.32 Similarly, other research suggests individuals with PDs may benefit more from specialized, intensive services than standard clinical case management.33 Future research may therefore benefit from clarifying the degree to which adaptations to standard programming could yield beneficial effects for persons with PD diagnoses.
Implications
Cumulatively, the results of this study attest to the necessity for transdiagnostic treatment planning that includes close collaboration between psychotherapeutic, pharmacological, and case management services. Some psychotherapy models for PDs, such as dialectical behavior therapy (DBT), which includes a combination of group skills training, individual therapy, as-needed phone coaching, and therapist consultation, may be successfully adapted to include this collaboration.34-36 However, implementation of such comprehensive programming often requires extensive clinician training and coordination of resources, which poses implementation challenges.37-39 In 2021, the VHA began large-scale implementation of PD-specific psychotherapy for veterans with recent suicidal self-directed violence and borderline PD, including DBT, though to date results remain unclear.40 Generalist approaches, such as good psychiatric management (GPM), which emphasizes emotional validation, practical problem solving, realistic goal setting, and relationship functioning within the context of standard care appointments, may be more easily implemented in community care settings due to lesser training and resource requirements and can also be adapted to include needed elements of care coordination.41,42 Both DBT and GPM were initially developed for the treatment of borderline PD. Although DBT has also demonstrated some effectiveness in the treatment of antisocial PD, potential applications of DBT and GPM to other PDs remain largely underdeveloped.43-46
There are no widely accepted medications for the treatment of PDs. Pharmacotherapy for these conditions typically consists of individualized approaches informed by personal experience that attempt to balance targeting of specific symptoms while minimizing polypharmacy and potential risks (eg, overdose or addiction).47,48 Despite this, pharmacotherapy is often considered a necessary component in the treatment of bipolar and psychotic disorders, both common comorbidities of PDs found in veterans in this study.49,50 Careful consideration of complex comorbidities and pharmacotherapy needs is warranted in the treatment of veterans with PDs. Future research may benefit from clarifying clinical guidelines around pharmacotherapy, particularly for observed comorbidities of PDs to trauma, bipolar, and psychotic disorders.
It is important to note the discrepancies in the results of this study surrounding patient substance use. The results suggest a negligible or inverse association between the likelihood of a PD diagnosis and difficulties with substance use among the veterans in this study. However, the unexpectedly low rate of SUD diagnoses (< 6%) suggests that they were likely underdocumented. Research suggests a strong association between personality and SUDs in both veteran and civilian samples.6,51 Results suggesting a lower prevalence of substance use difficulties among treatment-seeking veterans with PDs should be interpreted with great caution.
Demographically, PD diagnoses were more common among veterans who were White, non-Hispanic, and aged ≥ 45 years, and less common among veterans who were Black/ African American, mixed/unspecified race, Puerto Rican or other non-Mexican Hispanic ethnicity, or aged < 35 years. No significant sex-based differences were observed. These patterns are consistent with research suggesting individuals who identify as Black may be less likely than individuals who identify as White to report PD symptoms, meet criteria for a PD, and have a PD diagnosed even when it is warranted.52
The findings observed in this study with respect to age, however, are notably inconsistent with the literature. Previous research typically suggests a negative association between age and PD pathology; however, a 2020 review of PDs in older adults by Penders et al suggests a prevalence of 11% to 15% in this population.53,54 Research into PDs most often focuses on adolescent and early adulthood developmental periods, limiting insight into the phenomenology of PDs in middle to late adulthood.55 Further, most research into PDs among geriatric populations has focused on psychometric assessment rather than practical treatment guidance.54 However, in this study, elevated risk for PD diagnoses was salient throughout middle to late adulthood among veterans; similar, albeit less pronounced patterns were also observed for elevated risk of PD diagnosis in middle adulthood among nonveterans. Such findings suggest clarifying the phenomenology and treatment needs of individuals with PDs in middle to late adulthood may have particularly salient implications for the mental health care of veterans affected by these conditions. As the veteran population advances in age, these needs will present unique challenges if health care systems are unprepared to effectively address them.
Limitations
This study is characterized by several strengths, most notably its use of a large dataset recently collected on a national scale. Few studies outside of the VHA system include samples of > 100,000 treatment-seeking veterans collected on a national scale. Nevertheless, results should be understood within the context of several methodological limitations. However, the dataset was limited to the first 3 diagnoses documented in patients’ EHRs, and many patients had no listed diagnoses. Patients with complex comorbidities may have > 3 diagnoses; for these individuals, data provided an incomplete picture of clinical presentation. This is especially relevant for individuals with PDs, who tend to meet criteria for a range of comorbid conditions.8,10 The now dated practice of listing PDs on Axis II also increases the chance of clinicians listing PDs after conditions traditionally listed on Axis I (eg, major depressive disorder) in patient charts.56 This study’s inclusion of only the first 3 listed diagnoses likely underestimated true PD diagnosis prevalence.
The results of this study must be interpreted as reflecting the prevalence and correlates of receiving a PD diagnosis rather than meeting diagnostic criteria for a PD. Relatedly, PD diagnoses were reported as a single construct, limiting insight into prevalence and correlates of individual PD diagnoses (eg, borderline vs paranoid PDs). Meta-analyses estimates suggest PD prevalence among veterans is likely much higher than observed in this study.17 Stigma continues to discourage clinicians from documenting and disclosing PD diagnoses even when warranted.27,28 Continued research should aim to clarify conditions (eg, patient presentation, stigma, or institutional culture) contributing to documentation of PD diagnoses. Given the cross-sectional nature of this study, results cannot speak to longitudinal treatment outcomes or prognosis of persons receiving a PD diagnosis.
Despite its large sample size and national representation, the sampling strategy of this study could have contributed to idiosyncrasies in the dataset. Restriction of data to the persons receiving state-funded mental health services introduces a notable bias to the composition of the sample, which is likely comprised of a disproportionately high number of Medicaid recipients, students, and individuals with chronic illnesses and underrepresentation of persons who pay for mental health services using private insurance or private pay arrangements. As such, although socioeconomic information was not provided within this dataset, one can presume a generally lower socioeconomic status among study participants compared to the community at large. This study also included a proportionally small sample of veterans (3.6% compared to about 6.2% in the broader US population), suggesting veterans may have been underrepresented or underidentified in surveyed mental health care settings.57 This study also did not include data around service in active-duty military, national guard, or military reserves; a greater proportion of the sample likely had a history of military service than was represented by veteran status designation. Further, the proportionally high sample of individuals with severe mental illness suggests a likely overrepresentation of such conditions in surveyed settings.
Institutional differences in the practice of assigning diagnoses likely limited statistical power to detect potentially meaningful associations and effects. Structural influences, such as stigma and institutional culture, may have notable effects on documentation practices, particularly for PDs. Future research should aim to replicate observed associations using more controlled diagnostic procedures.
Lastly, even with the use of a more conservative α and a focus on effect sizes to guide interpretation of results, use of multiple bivariate analyses can be presumed to have increased the likelihood of type I error. Given the limited prior research in this area, an exploratory approach to statistical analysis was considered warranted to maximize opportunity for identifying areas in need of additional empirical attention. Continued research using more conservative statistical approaches (eg, multivariate analyses) is needed to determine replicability and generalizability of observed results.
CONCLUSIONS
This study examined the prevalence and correlates of PD diagnoses in a national sample of veterans receiving community-based, state-funded mental health care. About 2% received a PD diagnosis, with diagnoses most common among veterans who were White, non-Hispanic, aged ≥ 45 years, also diagnosed with trauma-based, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in a state psychiatric hospital or judicial system setting. The results attest to a necessity for transdiagnostic treatment planning and care coordination for this population, with particular attention to psychosocial stressors.
Personality disorders (PDs) are enduring patterns of internal experience and behavior that differ from cultural norms and expectations, are inflexible and pervasive, have their onset in adolescence or early adulthood, and lead to distress or impairment. Ten PDs are included in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition): paranoid, schizoid, schizotypal, borderline, antisocial, histrionic, narcissistic, avoidant, dependent, and obsessive-compulsive.1 These disorders impose a high burden on patients, families, health care systems, and broader economic systems.2,3 Up to 1 in 7 persons in the community and 50% of those receiving outpatient mental health treatment experience a PD.4,5 These conditions are associated with an increased risk of adverse events, including suicide attempt and death by suicide, criminal-legal involvement, homelessness, substance use, underemployment, relational issues, and high utilization of psychiatric services.6-9 PDs are routinely underassessed, underdocumented, and undertreated in clinical settings, and consistently receive less research funding than other, less prevalent forms of psychopathology. 10-12 As a result, there is limited understanding of clinical needs of individuals experiencing PDs.
MILITARY VETERANS WITH PERSONALITY DISORDERS
Underacknowledgment of PDs and their associated difficulties may be especially pronounced in veteran populations. Due to longstanding etiological theories that implicate childhood trauma and adolescent onset in pathology development, PDs are traditionally considered pre-existing conditions or developmental abnormalities by the US Department of Defense and US Department of Veterans Affairs (VA). As a result, PDs are therefore deemed incompatible with military service and ineligible for service-connected disability benefits.13-15 Such determinations allowed PD pathology to be used as grounds for discharge for 26,000 service members from 2001 to 2007, or 2.6% of total enlisted discharges during that period.13,15,16
Despite this structural discrimination, recent research suggests veterans may be more likely to experience PD pathology than the general population.17 For example, a 2021 epidemiological survey in a community-based veteran sample found elevated rates of borderline, antisocial, and schizotypal PDs (6%-13%).6 In contrast, only 0.8% to 5.0% of veteran electronic health records (EHRs) have a documented PD diagnosis.8,18,19 Such elevations in PD pathology within veteran samples imply either a disproportionately high prevalence among enlistees (and therefore missed during recruitment procedures) or onset following military service, possibly due to exposure to traumatic events and/ or occupational stress.17 Due to the relative infancy of research in this area and a lack of longitudinal studies, etiology and course of illness for personality pathology in veterans remains largely unclear.
Structural underacknowledgment of PDs among military personnel has contributed to their underrepresentation in research on veteran populations. PD-focused research with veterans is rare, despite a rapid increase in broader empirical attention paid to these conditions in nonveteran samples.20 A recent meta-analysis of veterans with PDs identified 27 studies that included basic prevalence statistics. PDs were rarely a primary focus for these studies, and most were limited to veterans seen in Veterans Health Administration (VHA) settings.17 The literature also paints a bleak picture, suggesting veterans who experience PDs are at higher risk for suicide attempt and death by suicide, criminal-legal involvement, and homelessness. They also tend to experience more severe comorbid psychopathological symptoms and more often use high-intensity mental health services (eg, care within emergency departments or psychiatric inpatient settings) than veterans without PD pathology.6,8,18,19,21 However, PD pathology does not appear to impede the effectiveness of treatment for veterans.22-24 The implications of PD pathology on broader psychosocial functioning and health care needs certify a need for additional research that examines patterns of personality pathology, particularly in veterans outside the VHA.
METHODS
This study aims to enhance understanding of veterans affected by PDs and offer insight and guidance for treatment of these conditions in federal and nonfederal treatment settings. Previous research has been largely limited to VHA care-receiving samples; the longstanding stigma against PDs by the US military and VA may contribute to biased diagnosis and documentation of PDs in these settings. A large sample of veterans receiving community-based mental health care was therefore used to explore aims of the current study. This study specifically examined demographic patterns, diagnostic comorbidity, psychosocial outcomes, and treatment care settings among veterans with and without a PD diagnosis. Consistent with previous research, we hypothesized that veterans with a PD diagnosis would have more severe mental health comorbidities, poorer psychosocial outcomes, and receive care in higher intensity settings relative to veterans without a diagnosis.
Data for the sample were drawn from the Mental Health Client-Level Data, a publicly available national dataset of nearly 7 million patients who received mental health treatment services provided or funded through state mental health agencies in 2022.25 The analytic sample included about 2.5 million patients for whom veteran status and data around the presence or absence of a PD diagnosis were available. Of these patients, 104,198 were identified as veterans. Veteran patients were identified as predominantly male (63%), White (71%), non-Hispanic (90%), and never married (54%).
Measures
The parent dataset included demographic, clinical, and psychosocial outcome information reported by treatment facilities to individual state administrative systems for each patient who received services. To protect patient privacy, only nonprotected health information is included, and efforts were made throughout compilation of the parent dataset to ensure patient privacy (eg, limiting detail of information disseminated for public access). Because the parent dataset does not include protected health information, studies using these data are considered exempt from institutional review board oversight.
Demographic information. This study reviewed veteran status, sex, race, ethnicity, age, education, and marital status. Veteran status was defined by whether the patient was aged ≥ 18 years and had previously served (but was not currently serving) in the military. Patients with a history of service in the National Guard or Military Reserves were only classified as veterans if they had been called or ordered to active duty while serving. Sex was operationalized dichotomously as male or female; no patients were identified as intersex, transgender, or other gender identities.
Clinical information. Up to 3 mental health diagnoses were reported for each patient and included the following disorders: personality, trauma and attention-deficit/hyperactivity, stressor, anxiety, conduct, delirium/dementia, bipolar, depressive, oppositional defiant, pervasive developmental, schizophrenia or other psychotic, and alcohol or substance use. Mental health diagnosis categories were generated for the parent dataset by grouping diagnostic codes corresponding to each category. To protect patient privacy, more detailed diagnostic information was not available as part of the parent dataset. Although the American Psychiatric Association recognizes 10 distinct PDs, the exact nature of PD diagnoses was not included within the parent dataset. PD diagnoses were coded to reflect the presence or absence of any such diagnosis.
A substance use problem designation was also provided for patients according to various identification methods, including substance use disorder (SUD) diagnosis, substance use screening results, enrollment in a substance use program, substance use survey, service claims information, and other related sources of information. A severe mental illness or serious emotional disturbance designation was provided for patients meeting state definitions of these designations. Context(s) of service provision were coded as inpatient state psychiatric hospital, community-based program, residential treatment center, judicial institution, or other psychiatric inpatient setting.
Psychosocial outcome information. Patient employment and residential status were also included in analyses. Each reflected status at the time of discharge from services or end of reporting period; employment status was only provided for patients receiving treatment in community-based programs.
Data Analysis
Descriptive statistics and X2 analyses were used to compare demographic, clinical, and psychosocial outcome variables between patients with and without PD diagnoses. These analyses were calculated for both the 104,198 veterans and the 2,222,306 nonveterans aged ≥ 18 years in the dataset. Given the sample size, a conservative α of .01 was used to determine statistical significance.
RESULTS
In this sample of persons receiving state-funded mental health care, veterans were significantly less likely than nonveterans to have a documented PD diagnosis (2.1% vs 3.6%, X2 [1] = 647.49; P < .01). PD diagnoses were more common among White (risk ratio [RR], 1.11), non-Hispanic (RR, 1.03) veterans who were in middle to late adulthood (RR, 1.16-1.40), more educated (RR, 1.35), and divorced or widowed (RR, 1.43), and less common among Black/African American (RR, 0.78) or Puerto Rican (RR, 0.32) veterans who were in early adulthood (RR, 0.31-0.79), less educated (RR, 0.64-0.89), and currently married (RR, 0.89) or never married (RR, 0.86). Veteran men and women were equally likely to have a PD diagnosis (RR, 1.03) (Table 1). Among nonveterans, men were less likely than women to have a PD diagnosis (RR, 0.79), and PD diagnoses were most common among persons in middle adulthood (RR, 1.06-1.15) (eAppendix 1).


Veterans with a PD diagnosis were more likely than those without a diagnosis to have more diagnoses (RR, 2.96-8.49) and to have comorbid trauma or related stressor (RR, 1.33), or bipolar (RR, 1.56) or psychotic (RR, 1.15) disorder diagnoses, but less likely to have comorbid depressive disorder (RR, 0.82). Although veterans with and without a PD diagnosis were similarly likely to have a comorbid SUD (RR, 1.13), those with a PD diagnosis were significantly less likely to be assigned a substance use problem designation (RR, 0.78). PD diagnosis was also more common among veterans who received services in state psychiatric hospitals (RR, 3.05), community-based clinics (RR, 1.06), and judicial institutions (RR, 6.33) and less common among those who received services in other psychiatric inpatient settings (RR, 0.30). No differences were observed for residential treatment settings (RR, 0.79). Among nonveterans, a PD diagnosis was associated with slightly greater odds of a substance use designation (RR, 1.03) (eAppendix 2).

Veterans with a PD diagnosis were also less likely to have full-time employment (RR, 0.73) and more likely to have undifferentiated employment (RR, 2.00) or to be removed from the labor force (RR, 1.35). Veterans with a PD diagnosis were also more likely to reside in nontraditional living conditions (RR, 1.42) and less likely to be residing in a private residence (RR, 0.98), compared with those without PD diagnosis. The rates of homelessness were similar for veterans with and without a PD diagnosis (RR, 0.90) (Table 2). These patterns were similar among nonveterans.

DISCUSSION
This study examined the rate and correlates of PD diagnosis among a large, community-based sample of veterans receiving state-funded mental health care. About 2% of veterans in this sample had a PD diagnosis, with diagnoses more common among veterans who were White, non-Hispanic, aged ≥ 45 years, with higher education, divorced or widowed, also diagnosed with trauma-related, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in state psychiatric hospital, community-based clinic, or judicial system settings.
The observed rate of PD diagnosis in this study aligns with what is typically observed in VHA EHRs.8,18,19 However, the rate is notably lower than prevalence estimates for psychiatric outpatient settings (about 50%) and in meta-analyses of prevalence among veterans (0.8%-23% for each of the 10 PDs).4,17,26 Longstanding stigma against PDs may contribute to underdiagnosis. For example, many clinicians are concerned that documentation or disclosure of a PD will interfere with the patient’s ability to access treatment due to stigma and discrimination.27,28 These fears are not unfounded; even among clinicians, PDs are commonly considered untreatable, and many individuals with PDs are denied access to evidence-based treatments due to the diagnosis.29 In a 2016 survey of community psychiatrists, nearly 1 in 4 reported that they avoid taking patients with a borderline PD diagnosis in their caseloads.28 To date, no studies have been conducted to explore clinicians’ willingness to accept patients with other PDs or, specifically, among veterans.
Despite such widespread stigma, research suggests clinicians' negative attitudes toward PDs can be decreased through antistigma campaigns.30 However, it remains unclear if such efforts also contribute to an increase in clinicians’ willingness to document PD diagnoses. Without accurate identification and documentation, the field’s understanding of PDs will remain limited.
In the current study, veterans with PD diagnoses tended to present with more complex and severe psychiatric comorbidities compared to veterans without such diagnoses. Observed comorbidity of PDs (particularly borderline PD) with trauma-related and bipolar disorders is well established.8 Conversely, co-occurring personality and psychotic disorders—which comprise 16% of veterans with a PD diagnosis in the sample in this study—are not consistently examined in the literature. A 2022 examination of veterans receiving VHA care suggested 12% and 13% of those with a PD diagnosis documented in their EHR also had documented schizophrenia or another psychotic disorder, respectively. PD diagnoses were associated with 6.88- and 9.80-fold increases in risk for comorbid schizophrenia and other psychotic disorder diagnoses, respectively.8 Similarly, a recent longitudinal study of nearly 2 million Swedish individuals suggested borderline PD is specifically associated with a > 24-times greater risk of having a comorbid psychotic disorder.31 It is therefore possible that the comorbidity between personality and psychotic disorders is quite common despite its relative lack of attention in empirical research.
Veterans with PD diagnoses in this study were also more likely to experience substandard housing, employment challenges, and receive treatment through judicial institutions than those without a PD diagnosis. Such findings are consistent with previous research demonstrating the substantial psychosocial challenges associated with PD diagnosis, even after controlling for comorbid conditions.7,9 Veterans with PDs may benefit from specialized case management and support to facilitate stable housing and employment and to mitigate the risk of judicial involvement. Some research suggests veterans with PDs may be less likely to gain competitive employment after participating in VA therapeutic and supportive employment services programs, suggesting standard programming may be less suitable for this population.32 Similarly, other research suggests individuals with PDs may benefit more from specialized, intensive services than standard clinical case management.33 Future research may therefore benefit from clarifying the degree to which adaptations to standard programming could yield beneficial effects for persons with PD diagnoses.
Implications
Cumulatively, the results of this study attest to the necessity for transdiagnostic treatment planning that includes close collaboration between psychotherapeutic, pharmacological, and case management services. Some psychotherapy models for PDs, such as dialectical behavior therapy (DBT), which includes a combination of group skills training, individual therapy, as-needed phone coaching, and therapist consultation, may be successfully adapted to include this collaboration.34-36 However, implementation of such comprehensive programming often requires extensive clinician training and coordination of resources, which poses implementation challenges.37-39 In 2021, the VHA began large-scale implementation of PD-specific psychotherapy for veterans with recent suicidal self-directed violence and borderline PD, including DBT, though to date results remain unclear.40 Generalist approaches, such as good psychiatric management (GPM), which emphasizes emotional validation, practical problem solving, realistic goal setting, and relationship functioning within the context of standard care appointments, may be more easily implemented in community care settings due to lesser training and resource requirements and can also be adapted to include needed elements of care coordination.41,42 Both DBT and GPM were initially developed for the treatment of borderline PD. Although DBT has also demonstrated some effectiveness in the treatment of antisocial PD, potential applications of DBT and GPM to other PDs remain largely underdeveloped.43-46
There are no widely accepted medications for the treatment of PDs. Pharmacotherapy for these conditions typically consists of individualized approaches informed by personal experience that attempt to balance targeting of specific symptoms while minimizing polypharmacy and potential risks (eg, overdose or addiction).47,48 Despite this, pharmacotherapy is often considered a necessary component in the treatment of bipolar and psychotic disorders, both common comorbidities of PDs found in veterans in this study.49,50 Careful consideration of complex comorbidities and pharmacotherapy needs is warranted in the treatment of veterans with PDs. Future research may benefit from clarifying clinical guidelines around pharmacotherapy, particularly for observed comorbidities of PDs to trauma, bipolar, and psychotic disorders.
It is important to note the discrepancies in the results of this study surrounding patient substance use. The results suggest a negligible or inverse association between the likelihood of a PD diagnosis and difficulties with substance use among the veterans in this study. However, the unexpectedly low rate of SUD diagnoses (< 6%) suggests that they were likely underdocumented. Research suggests a strong association between personality and SUDs in both veteran and civilian samples.6,51 Results suggesting a lower prevalence of substance use difficulties among treatment-seeking veterans with PDs should be interpreted with great caution.
Demographically, PD diagnoses were more common among veterans who were White, non-Hispanic, and aged ≥ 45 years, and less common among veterans who were Black/ African American, mixed/unspecified race, Puerto Rican or other non-Mexican Hispanic ethnicity, or aged < 35 years. No significant sex-based differences were observed. These patterns are consistent with research suggesting individuals who identify as Black may be less likely than individuals who identify as White to report PD symptoms, meet criteria for a PD, and have a PD diagnosed even when it is warranted.52
The findings observed in this study with respect to age, however, are notably inconsistent with the literature. Previous research typically suggests a negative association between age and PD pathology; however, a 2020 review of PDs in older adults by Penders et al suggests a prevalence of 11% to 15% in this population.53,54 Research into PDs most often focuses on adolescent and early adulthood developmental periods, limiting insight into the phenomenology of PDs in middle to late adulthood.55 Further, most research into PDs among geriatric populations has focused on psychometric assessment rather than practical treatment guidance.54 However, in this study, elevated risk for PD diagnoses was salient throughout middle to late adulthood among veterans; similar, albeit less pronounced patterns were also observed for elevated risk of PD diagnosis in middle adulthood among nonveterans. Such findings suggest clarifying the phenomenology and treatment needs of individuals with PDs in middle to late adulthood may have particularly salient implications for the mental health care of veterans affected by these conditions. As the veteran population advances in age, these needs will present unique challenges if health care systems are unprepared to effectively address them.
Limitations
This study is characterized by several strengths, most notably its use of a large dataset recently collected on a national scale. Few studies outside of the VHA system include samples of > 100,000 treatment-seeking veterans collected on a national scale. Nevertheless, results should be understood within the context of several methodological limitations. However, the dataset was limited to the first 3 diagnoses documented in patients’ EHRs, and many patients had no listed diagnoses. Patients with complex comorbidities may have > 3 diagnoses; for these individuals, data provided an incomplete picture of clinical presentation. This is especially relevant for individuals with PDs, who tend to meet criteria for a range of comorbid conditions.8,10 The now dated practice of listing PDs on Axis II also increases the chance of clinicians listing PDs after conditions traditionally listed on Axis I (eg, major depressive disorder) in patient charts.56 This study’s inclusion of only the first 3 listed diagnoses likely underestimated true PD diagnosis prevalence.
The results of this study must be interpreted as reflecting the prevalence and correlates of receiving a PD diagnosis rather than meeting diagnostic criteria for a PD. Relatedly, PD diagnoses were reported as a single construct, limiting insight into prevalence and correlates of individual PD diagnoses (eg, borderline vs paranoid PDs). Meta-analyses estimates suggest PD prevalence among veterans is likely much higher than observed in this study.17 Stigma continues to discourage clinicians from documenting and disclosing PD diagnoses even when warranted.27,28 Continued research should aim to clarify conditions (eg, patient presentation, stigma, or institutional culture) contributing to documentation of PD diagnoses. Given the cross-sectional nature of this study, results cannot speak to longitudinal treatment outcomes or prognosis of persons receiving a PD diagnosis.
Despite its large sample size and national representation, the sampling strategy of this study could have contributed to idiosyncrasies in the dataset. Restriction of data to the persons receiving state-funded mental health services introduces a notable bias to the composition of the sample, which is likely comprised of a disproportionately high number of Medicaid recipients, students, and individuals with chronic illnesses and underrepresentation of persons who pay for mental health services using private insurance or private pay arrangements. As such, although socioeconomic information was not provided within this dataset, one can presume a generally lower socioeconomic status among study participants compared to the community at large. This study also included a proportionally small sample of veterans (3.6% compared to about 6.2% in the broader US population), suggesting veterans may have been underrepresented or underidentified in surveyed mental health care settings.57 This study also did not include data around service in active-duty military, national guard, or military reserves; a greater proportion of the sample likely had a history of military service than was represented by veteran status designation. Further, the proportionally high sample of individuals with severe mental illness suggests a likely overrepresentation of such conditions in surveyed settings.
Institutional differences in the practice of assigning diagnoses likely limited statistical power to detect potentially meaningful associations and effects. Structural influences, such as stigma and institutional culture, may have notable effects on documentation practices, particularly for PDs. Future research should aim to replicate observed associations using more controlled diagnostic procedures.
Lastly, even with the use of a more conservative α and a focus on effect sizes to guide interpretation of results, use of multiple bivariate analyses can be presumed to have increased the likelihood of type I error. Given the limited prior research in this area, an exploratory approach to statistical analysis was considered warranted to maximize opportunity for identifying areas in need of additional empirical attention. Continued research using more conservative statistical approaches (eg, multivariate analyses) is needed to determine replicability and generalizability of observed results.
CONCLUSIONS
This study examined the prevalence and correlates of PD diagnoses in a national sample of veterans receiving community-based, state-funded mental health care. About 2% received a PD diagnosis, with diagnoses most common among veterans who were White, non-Hispanic, aged ≥ 45 years, also diagnosed with trauma-based, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in a state psychiatric hospital or judicial system setting. The results attest to a necessity for transdiagnostic treatment planning and care coordination for this population, with particular attention to psychosocial stressors.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
- Hastrup LH, Jennum P, Ibsen R, Kjellberg J, Simonsen E. Societal costs of borderline personality disorders: a matched-controlled nationwide study of patients and spouses. Acta Psychiatr Scand. 2019;140(5):458-467. doi:10.1111/acps.13094
- Sveen CA, Pedersen G, Ulvestad DA, Zahl KE, Wilberg T, Kvarstein EH. Societal costs of personality disorders: a cross-sectional multicenter study of treatment-seeking patients in mental health services in Norway. J Clin Psychol. 2023;79(8):1752-1769. doi:10.1002/jclp.23504
- Beckwith H, Moran PF, Reilly J. Personality disorder prevalence in psychiatric outpatients: a systematic literature review. Personal Ment Health. 2014;8(2):91-101. doi:10.1002/pmh.1252
- Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc. 2017.09.001
- Edwards ER, Barnes S, Govindarajulu U, Geraci J, Tsai J. Mental health and substance use patterns associated with lifetime suicide attempt, incarceration, and homelessness: a latent class analysis of a nationally representative sample of U.S. veterans. Psychol Serv. 2021;18(4):619-631. doi:10.1037/ser0000488
- Moran P, Romaniuk H, Coffey C, et al. The influence of personality disorder on the future mental health and social adjustment of young adults: a population-based cohort study. Lancet Psychiatry. 2016;3(7):636-645. doi:10.1016/S2215-0366(16)30029-3
- Nelson SM, Griffin CA, Hein TC, Bowersox N, McCarthy JF. Personality disorder and suicide risk among patients in the Veterans Affairs health system. Personal Disord. 2022;13(6):563-571. doi:10.1037/per0000521
- Skodol AE. Impact of personality pathology on psychosocial functioning. Curr Opin Psychol. 2018;21;33-38. doi:10.1016/j.copsyc.2017.09.006
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015;385(9969):717-726. doi:10.1016/S0140-6736(14)61995-4
- Fitzpatrick S, Goss S, Di Bartolomeo A, Varma S, Tissera T, Earle E. Follow the money: is borderline personality disorder research underfunded in Canada? Can Psychol. 2024;65(1):46-57. doi:10.1037/cap0000375
- Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944. doi:10.1016/j.psychres.2014.09.021
- Leroux TC. U.S. military discharges and pre-existing personality disorders: a health policy review. Adm Policy Ment Health. 2015;42(6):748-755. doi:10.1007/s10488-014-0611-z
- Monahan MC, Keener JK. Fitness-for-duty evaluations. In Kennedy CH, Zillmer EA, eds. Military Psychology: Clinical and Operational Applications. 2nd ed. Guilford Publications; 2012:25-49.
- Hearing Before the Committee on Veterans’ Affairs, 111th Congress 2nd Sess (2010). Personality disorder discharges: impact on veterans benefits. Accessed March 4, 2025. https://www.govinfo.gov/content/pkg/CHRG-111hhrg61755/html/CHRG-111hhrg61755.htm
- Ader M, Cuthbert R, Hoechst K, Simon EH, Strassburger Z, Wishnie M. Casting troops aside: the United States military’s illegal personality disorder discharge problem. Vietnam Veterans of America. March 2012. Accessed February 28, 2025. https://law.yale.edu/sites/default/files/documents/pdf/Clinics/VLSC_CastingTroopsAside.pdf
- Edwards ER, Tran H, Wrobleski J, Rabhan Y, Yin J, Chiodi C, Goodman M, Geraci J. Prevalence of personality disorders across veteran samples: A meta-analysis. J Pers Disord. 2022;36(3):339-358. doi:10.1521/ pedi.2022.36.3.339
- Holliday R, Desai A, Edwards E, Borges L. Personal i ty disorder diagnosis among just ice -involved veterans: an investigation of VA-using veterans. J Nerv Ment Dis. 2023;211(5):402-406 doi:10.1097/ NMD.0000000000001627
- McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942. doi:10.2105/AJPH.2015.302737
- Liu Y, Chen C, Zhou Y, Zhang N, Liu S. Twenty years of research on borderline personality disorder: a scientometric analysis of hotspots, bursts, and research trends. Front Psych. 2024;15:1361535. doi:10.3389/ fpsyt.2024.1361535
- Williams R, Holliday R, Clem M, Anderson E, Morris EE, Surís A. Borderline personality disorder and military sexual trauma: analysis of previous traumatization and current psychiatric presentation. J Interpers Violence. 2017;32(15):2223-2236. doi:10.1177/0886260515596149
- Holder N, Holliday R, Pai A, Surís A. Role of borderline personality disorder in the treatment of military sexual trauma-related posttraumatic stress disorder with cognitive processing therapy. Behav Med. 2017;43(3):184-190. doi:10.1080/08964289.2016.1276430
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. Am J Addict. 2007;16(6):443- 449. doi:10.1080/10550490701643336
- Walter KH, Bolte TA, Owens GP, Chard KM. The impact of personality disorders on treatment outcome for veterans in a posttraumatic stress disorder residential treatment program. Cognit Ther Res. 2012;36(5):576-584. doi:10.1007/s10608-011-9393-8
- Substance Abuse and Mental Health Services. Mental health client-level data (MH-CLD), 2022. Accessed February 28, 2025. https://www.datafiles.samhsa.gov/dataset/mental-health-client-level-data-2022-mh-cld-2022-ds0001
- Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918. doi:10.1176/appi.ajp.162.10.1911
- Campbell K, Clarke KA, Massey D, Lakeman R. Borderline personality disorder: To diagnose or not to diagnose? That is the question. Int J Mental Health Nurs. 2020;29(5):972-981. doi:10.1111/inm.12737
- Sisti D, Segal AG, Siegel AM, Johnson R, Gunderson J. Diagnosing, disclosing, and documenting borderline personality disorder: a survey of psychiatrists’ practices. J Pers Disord. 2016;30(6):848-856. doi:10.1521/ pedi_2015_29_228
- Klein P, Fairweather AK, Lawn S. Structural stigma and its impact on healthcare for borderline personality disorder: a scoping review. Int J Ment Health Syst. 2022;16(1):48. doi:10.1186/s13033-022-00558-3
- Knaak S, Szeto AC, Fitch K, Modgill G, Patten S. Stigma towards borderline personality disorder: effectiveness and generalizability of an anti-stigma program for healthcare providers using a pre-post randomized design. Borderline Personal Disord Emot Dysregul. 2015;2:9. doi:10.1186/s40479-015-0030-0
- Tate AE, Sahlin H, Liu S, et al. Borderline personality disorder: associations with psychiatric disorders, somatic illnesses, trauma, and adverse behaviors. Mol Psychiatry. 2022;27:2514-2521. doi:10.1038/s41380- 022-01503-z
- Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA Therapeutic and Supported Employment Services programs. Psychiatr Serv. 2017;68(9)938-946. doi:10.1176/appi. ps201600412
- Frisman LK, Mueser KT, Covell NH, et al. Use of integrated dual disorder treatment via Assertive Community Treatment versus clinical case management for persons with co-occurring disorders and antisocial personality disorder. J Nerv Ment Dis. 2009;197(11):822-828. doi:10.1097/NMD.0b013e3181beac52
- Edwards ER, Kober H, Rinne GR, Griffin SA, Axelrod S, Cooney EB. Skills]homework completion and phone coaching as predictors of therapeutic change and outcomes in completers of a DBT intensive outpatient programme. Psychol Psychother. 2021;94(3):504-522. doi:10.1111/papt.12325
- Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67(1):13-26. doi:10.1016/s0376-8716(02)00011-x
- Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry. 2015;72(5):475-482.doi:10.1001 /jamapsychiatry.2014.3039
- Carmel A, Rose ML, Fruzzetti AE. Barriers and solutions to implementing dialectical behavior therapy in a public behavioral health system. Adm Policy Ment Health. 2014;41(5):608-614. doi:10.1007/s10488-013-0504-6
- Decker SE, Matthieu MM, Smith BN, Landes SJ. Barriers and facilitators to dialectical behavior therapy skills groups in the Veterans Health Administration. Mil Med. 2024;189(5-6):1055-1063. doi:10.1093/milmed/ usad123
- Landes SJ, Rodriguez AL, Smith BN, et al. Barriers, facilitators, and benefits of implementation of dialectical behavior therapy in routine care: results from a national program evaluation survey in the Veterans Health Administration. Transl Behav Med. 2017;7(4):832-844. doi:10.1007/s13142-017-0465-5
- Walker J, Betthauser LM, Green K, Landes SJ, Stacy M. Suicide Prevention 2.0 Clinical Telehealth Program: Evidence- Based Treatment in the Veterans Health Administration. April 28, 2024. Accessed February 28, 2025. https://www.youtube.com/watch?v=fFsDzkg0SR0
- Gunderson J, Masland S, Choi-Kain L. Good psychiatric management: a review. Curr Opin Psychol. 2018;21:127- 131. doi:10.1016/j.copsyc.2017.12.006
- Kramer U. Good-enough therapy: a review of the empirical basis of good psychiatric management. Am J Psychother. 2025;78(1): 11-15. doi:10.1176/appi .psychotherapy.20230041
- Visdómine-Lozano JC. Contextualist perspectives in the treatment of antisocial behaviors and offending: a comparative review of FAP, ACT, DBT, and MDT. Trauma Violence Abuse. 2022;23(1):241-254. doi:10.1177/1524838020939509
- Drago A, Marogna C, Jørgen Søgaard H. A review of characteristics and treatments of the avoidant personality disorder. Could the DBT be an option? Int J Psychol Psychoanal. 2016;2(1):013.
- Finch EF, Choi-Kain LW, Iliakis EA, Eisen JL, Pinto A. Good psychiatric management for obsessive–compulsive personality disorder. Curr Behav Neurosci Rep. 2021;8:160-171. doi:10.1007/s40473-021-00239-4
- Miller TW, Kraus RF. Modified dialectical behavior therapy and problem solving for obsessive-compulsive personality disorder. Journal Contemp Psychother. 2007;37:79-85. doi:10.1007/s10879-006-9039-4
- Bozzatello P, Rocca P, De Rosa ML, Bellino S. Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert Opin Pharmacother. 2020;21(1):47-61.doi:10.1080/14656566 .2019.1686482
- Sand P, Derviososki E, Kollia S, Strand J, Di Leone F. Psychiatrists’ perspectives on prescription decisions for patients with personality disorders. J Pers Disord. 2024;38(3):225-240. doi:10.1521/pedi.2024.38.3.225
- Kane JM, Leucht S, Carpenter D, Docherty JP; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J Clin Psychiatry. 2003;64 Suppl 12:5-19.
- Nierenberg AA, Agustini B, Köhler-Forsberg O, et al. Diagnosis and treatment of bipolar disorder: a review. JAMA. 2023;330(14):1370-1380. doi:10.1001 /jama.2023.18588
- Köck P, Walter M. Personality disorder and substance use disorder–an update. Ment Health Prev. 2018;12:82- 89. doi:10.1016/J.MHP.2018.10.003
- Garb HN. Race bias and gender bias in the diagnosis of psychological disorders. Clin Psych Rev. 2021;90:102087. doi:10.1016/j.cpr.2021.102087
- Debast I, van Alphen SPJ, Rossi G, et al. Personality traits and personality disorders in late middle and old age: do they remain stable? A literature review. Clin Gerontol. 2014;37(3):253-271.doi:10.1080/07317115 .2014.885917
- Penders KAP, Peeters IGP, Metsemakers JFM, van Alphen SPJ. Personality disorders in older adults: a review of epidemiology, assessment, and treatment. Curr Psychiatry Rep. 2020;22(3):1-14. doi:10.1007/s11920-020- 1133-x
- Videler AC, Hutsebaut J, Schulkens JEM, Sobczak S, van Alphen SPJ. A life span perspective on borderline personality disorder. Curr Psychiatry Rep. 2019;21(7) :1-8. doi:10.1007/s11920-019-1040-1
- Wakefield JC. DSM-5 and the general definition of personality disorder. Clin Soc Work J. 2013;41(2):168-183. doi:10.1007/s10615-012-0402-5
- US Census Bureau. 2022 American Community Survey 1-year. Accessed February 28, 2025. https://data.census.gov/table/ACSST1Y2022.S2101?q=Veterans&y=2022comparison
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
- Hastrup LH, Jennum P, Ibsen R, Kjellberg J, Simonsen E. Societal costs of borderline personality disorders: a matched-controlled nationwide study of patients and spouses. Acta Psychiatr Scand. 2019;140(5):458-467. doi:10.1111/acps.13094
- Sveen CA, Pedersen G, Ulvestad DA, Zahl KE, Wilberg T, Kvarstein EH. Societal costs of personality disorders: a cross-sectional multicenter study of treatment-seeking patients in mental health services in Norway. J Clin Psychol. 2023;79(8):1752-1769. doi:10.1002/jclp.23504
- Beckwith H, Moran PF, Reilly J. Personality disorder prevalence in psychiatric outpatients: a systematic literature review. Personal Ment Health. 2014;8(2):91-101. doi:10.1002/pmh.1252
- Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc. 2017.09.001
- Edwards ER, Barnes S, Govindarajulu U, Geraci J, Tsai J. Mental health and substance use patterns associated with lifetime suicide attempt, incarceration, and homelessness: a latent class analysis of a nationally representative sample of U.S. veterans. Psychol Serv. 2021;18(4):619-631. doi:10.1037/ser0000488
- Moran P, Romaniuk H, Coffey C, et al. The influence of personality disorder on the future mental health and social adjustment of young adults: a population-based cohort study. Lancet Psychiatry. 2016;3(7):636-645. doi:10.1016/S2215-0366(16)30029-3
- Nelson SM, Griffin CA, Hein TC, Bowersox N, McCarthy JF. Personality disorder and suicide risk among patients in the Veterans Affairs health system. Personal Disord. 2022;13(6):563-571. doi:10.1037/per0000521
- Skodol AE. Impact of personality pathology on psychosocial functioning. Curr Opin Psychol. 2018;21;33-38. doi:10.1016/j.copsyc.2017.09.006
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015;385(9969):717-726. doi:10.1016/S0140-6736(14)61995-4
- Fitzpatrick S, Goss S, Di Bartolomeo A, Varma S, Tissera T, Earle E. Follow the money: is borderline personality disorder research underfunded in Canada? Can Psychol. 2024;65(1):46-57. doi:10.1037/cap0000375
- Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944. doi:10.1016/j.psychres.2014.09.021
- Leroux TC. U.S. military discharges and pre-existing personality disorders: a health policy review. Adm Policy Ment Health. 2015;42(6):748-755. doi:10.1007/s10488-014-0611-z
- Monahan MC, Keener JK. Fitness-for-duty evaluations. In Kennedy CH, Zillmer EA, eds. Military Psychology: Clinical and Operational Applications. 2nd ed. Guilford Publications; 2012:25-49.
- Hearing Before the Committee on Veterans’ Affairs, 111th Congress 2nd Sess (2010). Personality disorder discharges: impact on veterans benefits. Accessed March 4, 2025. https://www.govinfo.gov/content/pkg/CHRG-111hhrg61755/html/CHRG-111hhrg61755.htm
- Ader M, Cuthbert R, Hoechst K, Simon EH, Strassburger Z, Wishnie M. Casting troops aside: the United States military’s illegal personality disorder discharge problem. Vietnam Veterans of America. March 2012. Accessed February 28, 2025. https://law.yale.edu/sites/default/files/documents/pdf/Clinics/VLSC_CastingTroopsAside.pdf
- Edwards ER, Tran H, Wrobleski J, Rabhan Y, Yin J, Chiodi C, Goodman M, Geraci J. Prevalence of personality disorders across veteran samples: A meta-analysis. J Pers Disord. 2022;36(3):339-358. doi:10.1521/ pedi.2022.36.3.339
- Holliday R, Desai A, Edwards E, Borges L. Personal i ty disorder diagnosis among just ice -involved veterans: an investigation of VA-using veterans. J Nerv Ment Dis. 2023;211(5):402-406 doi:10.1097/ NMD.0000000000001627
- McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942. doi:10.2105/AJPH.2015.302737
- Liu Y, Chen C, Zhou Y, Zhang N, Liu S. Twenty years of research on borderline personality disorder: a scientometric analysis of hotspots, bursts, and research trends. Front Psych. 2024;15:1361535. doi:10.3389/ fpsyt.2024.1361535
- Williams R, Holliday R, Clem M, Anderson E, Morris EE, Surís A. Borderline personality disorder and military sexual trauma: analysis of previous traumatization and current psychiatric presentation. J Interpers Violence. 2017;32(15):2223-2236. doi:10.1177/0886260515596149
- Holder N, Holliday R, Pai A, Surís A. Role of borderline personality disorder in the treatment of military sexual trauma-related posttraumatic stress disorder with cognitive processing therapy. Behav Med. 2017;43(3):184-190. doi:10.1080/08964289.2016.1276430
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. Am J Addict. 2007;16(6):443- 449. doi:10.1080/10550490701643336
- Walter KH, Bolte TA, Owens GP, Chard KM. The impact of personality disorders on treatment outcome for veterans in a posttraumatic stress disorder residential treatment program. Cognit Ther Res. 2012;36(5):576-584. doi:10.1007/s10608-011-9393-8
- Substance Abuse and Mental Health Services. Mental health client-level data (MH-CLD), 2022. Accessed February 28, 2025. https://www.datafiles.samhsa.gov/dataset/mental-health-client-level-data-2022-mh-cld-2022-ds0001
- Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918. doi:10.1176/appi.ajp.162.10.1911
- Campbell K, Clarke KA, Massey D, Lakeman R. Borderline personality disorder: To diagnose or not to diagnose? That is the question. Int J Mental Health Nurs. 2020;29(5):972-981. doi:10.1111/inm.12737
- Sisti D, Segal AG, Siegel AM, Johnson R, Gunderson J. Diagnosing, disclosing, and documenting borderline personality disorder: a survey of psychiatrists’ practices. J Pers Disord. 2016;30(6):848-856. doi:10.1521/ pedi_2015_29_228
- Klein P, Fairweather AK, Lawn S. Structural stigma and its impact on healthcare for borderline personality disorder: a scoping review. Int J Ment Health Syst. 2022;16(1):48. doi:10.1186/s13033-022-00558-3
- Knaak S, Szeto AC, Fitch K, Modgill G, Patten S. Stigma towards borderline personality disorder: effectiveness and generalizability of an anti-stigma program for healthcare providers using a pre-post randomized design. Borderline Personal Disord Emot Dysregul. 2015;2:9. doi:10.1186/s40479-015-0030-0
- Tate AE, Sahlin H, Liu S, et al. Borderline personality disorder: associations with psychiatric disorders, somatic illnesses, trauma, and adverse behaviors. Mol Psychiatry. 2022;27:2514-2521. doi:10.1038/s41380- 022-01503-z
- Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA Therapeutic and Supported Employment Services programs. Psychiatr Serv. 2017;68(9)938-946. doi:10.1176/appi. ps201600412
- Frisman LK, Mueser KT, Covell NH, et al. Use of integrated dual disorder treatment via Assertive Community Treatment versus clinical case management for persons with co-occurring disorders and antisocial personality disorder. J Nerv Ment Dis. 2009;197(11):822-828. doi:10.1097/NMD.0b013e3181beac52
- Edwards ER, Kober H, Rinne GR, Griffin SA, Axelrod S, Cooney EB. Skills]homework completion and phone coaching as predictors of therapeutic change and outcomes in completers of a DBT intensive outpatient programme. Psychol Psychother. 2021;94(3):504-522. doi:10.1111/papt.12325
- Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67(1):13-26. doi:10.1016/s0376-8716(02)00011-x
- Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry. 2015;72(5):475-482.doi:10.1001 /jamapsychiatry.2014.3039
- Carmel A, Rose ML, Fruzzetti AE. Barriers and solutions to implementing dialectical behavior therapy in a public behavioral health system. Adm Policy Ment Health. 2014;41(5):608-614. doi:10.1007/s10488-013-0504-6
- Decker SE, Matthieu MM, Smith BN, Landes SJ. Barriers and facilitators to dialectical behavior therapy skills groups in the Veterans Health Administration. Mil Med. 2024;189(5-6):1055-1063. doi:10.1093/milmed/ usad123
- Landes SJ, Rodriguez AL, Smith BN, et al. Barriers, facilitators, and benefits of implementation of dialectical behavior therapy in routine care: results from a national program evaluation survey in the Veterans Health Administration. Transl Behav Med. 2017;7(4):832-844. doi:10.1007/s13142-017-0465-5
- Walker J, Betthauser LM, Green K, Landes SJ, Stacy M. Suicide Prevention 2.0 Clinical Telehealth Program: Evidence- Based Treatment in the Veterans Health Administration. April 28, 2024. Accessed February 28, 2025. https://www.youtube.com/watch?v=fFsDzkg0SR0
- Gunderson J, Masland S, Choi-Kain L. Good psychiatric management: a review. Curr Opin Psychol. 2018;21:127- 131. doi:10.1016/j.copsyc.2017.12.006
- Kramer U. Good-enough therapy: a review of the empirical basis of good psychiatric management. Am J Psychother. 2025;78(1): 11-15. doi:10.1176/appi .psychotherapy.20230041
- Visdómine-Lozano JC. Contextualist perspectives in the treatment of antisocial behaviors and offending: a comparative review of FAP, ACT, DBT, and MDT. Trauma Violence Abuse. 2022;23(1):241-254. doi:10.1177/1524838020939509
- Drago A, Marogna C, Jørgen Søgaard H. A review of characteristics and treatments of the avoidant personality disorder. Could the DBT be an option? Int J Psychol Psychoanal. 2016;2(1):013.
- Finch EF, Choi-Kain LW, Iliakis EA, Eisen JL, Pinto A. Good psychiatric management for obsessive–compulsive personality disorder. Curr Behav Neurosci Rep. 2021;8:160-171. doi:10.1007/s40473-021-00239-4
- Miller TW, Kraus RF. Modified dialectical behavior therapy and problem solving for obsessive-compulsive personality disorder. Journal Contemp Psychother. 2007;37:79-85. doi:10.1007/s10879-006-9039-4
- Bozzatello P, Rocca P, De Rosa ML, Bellino S. Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert Opin Pharmacother. 2020;21(1):47-61.doi:10.1080/14656566 .2019.1686482
- Sand P, Derviososki E, Kollia S, Strand J, Di Leone F. Psychiatrists’ perspectives on prescription decisions for patients with personality disorders. J Pers Disord. 2024;38(3):225-240. doi:10.1521/pedi.2024.38.3.225
- Kane JM, Leucht S, Carpenter D, Docherty JP; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J Clin Psychiatry. 2003;64 Suppl 12:5-19.
- Nierenberg AA, Agustini B, Köhler-Forsberg O, et al. Diagnosis and treatment of bipolar disorder: a review. JAMA. 2023;330(14):1370-1380. doi:10.1001 /jama.2023.18588
- Köck P, Walter M. Personality disorder and substance use disorder–an update. Ment Health Prev. 2018;12:82- 89. doi:10.1016/J.MHP.2018.10.003
- Garb HN. Race bias and gender bias in the diagnosis of psychological disorders. Clin Psych Rev. 2021;90:102087. doi:10.1016/j.cpr.2021.102087
- Debast I, van Alphen SPJ, Rossi G, et al. Personality traits and personality disorders in late middle and old age: do they remain stable? A literature review. Clin Gerontol. 2014;37(3):253-271.doi:10.1080/07317115 .2014.885917
- Penders KAP, Peeters IGP, Metsemakers JFM, van Alphen SPJ. Personality disorders in older adults: a review of epidemiology, assessment, and treatment. Curr Psychiatry Rep. 2020;22(3):1-14. doi:10.1007/s11920-020- 1133-x
- Videler AC, Hutsebaut J, Schulkens JEM, Sobczak S, van Alphen SPJ. A life span perspective on borderline personality disorder. Curr Psychiatry Rep. 2019;21(7) :1-8. doi:10.1007/s11920-019-1040-1
- Wakefield JC. DSM-5 and the general definition of personality disorder. Clin Soc Work J. 2013;41(2):168-183. doi:10.1007/s10615-012-0402-5
- US Census Bureau. 2022 American Community Survey 1-year. Accessed February 28, 2025. https://data.census.gov/table/ACSST1Y2022.S2101?q=Veterans&y=2022comparison
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
Evidence-based psychotherapy (EBP) for posttraumatic stress disorder (PTSD), such as prolonged exposure (PE), is supported by multiple clinical practice guidelines and is expected to be available to veterans served by the Veterans Health Administration (VHA).1-5 However, traditional models of EBP delivery with 1 or 2 sessions weekly have high dropout rates.6,7 Few veterans who could benefit from such EBPs receive them, and those who do have low completion rates.8,9 Over a 15-year period, VHA records review of > 265,500 veterans with PTSD showed only 9.1% completed EBP treatment that included but was not limited to PE.10
One empirically supported solution that has yet to be widely implemented is delivering EBPs for PTSD in a massed or accelerated format of ≥ 3 sessions weekly.11 While these massed models of EBP delivery for PTSD are promising, their implementation is limited in federal health care settings, such as the VHA.12 PE therapy is a first-line treatment for PTSD that has been evaluated in numerous clinical trials since the early 1990s and in a wide range of trauma populations.13,14 Massed PE is effective and PE has been found to be effective both in-person and via telehealth.11,15,16
Another approach to accelerated PE is the inclusion of a massed PE course within a broader treatment context that includes augmentation of the massed PE with additional services, this is referred to as an intensive outpatient model (IOP).17 PE-IOP has also been shown to be feasible, acceptable, and effective with increased completion rates in comparison to the traditional (1 or 2 sessions weekly) model of PE.12,16,18,19 Ragsdale et al describe a 2-week IOP with multiple treatment tracks, including a PTSD track. The PTSD treatment track includes massed PE and additional standard services including case management, wellness services, family services, and a single session effective behaviors group. Additional augmentation services are available when clinically indicated (eg, repetitive transcranial magnetic stimulation, transcranial direct current stimulation treatment, psychoeducation, motivational interviewing, and/or relapse prevention).17
Rauch et al studied the first 80 patients completing an IOP program that consisted of PE (5 sessions weekly) and complementary interventions (eg, mindfulness and yoga) and reported a 96% retention rate, significant reductions of self-reported PTSD symptoms, significant reduction in self-reported co-occurring depression symptoms, and significant increase in self-reported satisfaction with social functioning. 18 In another study, Sherril et al explored patient reactions to participation in massed PE (5 sessions weekly) and found that patients reported significantly more positive than negative reactions. Sherrill et al noted that according to patients, the benefits of massed PE included a structured format that limits avoidance and distraction. The resulting fast pace of progress enhanced motivation; however, drawbacks included short-term discomfort and time demands.19 Yamokoski et al explored the feasibility of massed PE in a larger study of PTSD treatment in an intensive outpatient track (IOT) in a VHA PTSD clinic with minimal staffing. The 48 patients who completed IOT PTSD treatment in 2 or 4 weeks (including 35 patients who received massed PE) had high retention rates (85%), reported high satisfaction, and had significantly reduced PTSD and depression symptoms.12
The massed IOT PE model implemented by Yamokoski et al included the primary EBP intervention of massed PE with adjunctive groups. The addition of these groups increased both retention and patient-reported satisfaction. The PE-IOP model implemented by Rauch et al and Sherrill et al also included wellness and educational groups, as well as access to complementary interventions such as mindfulness and yoga.18,19 The addition of wellness education along with a primary EBP aligned with the VHA focus on whole health well-being and wellness. The whole health approach includes understanding the factors that motivate a patient toward health and well-being, provision of health education, and providing access to complementary interventions such as mindfulness.20 Dryden et al describe the whole health transformation within VHA as a proactive approach to addressing employee and patient wellness and health. Their research found that the whole health model promoted well-being in patients and staff and was sustained even during the COVID-19 pandemic.21 Dryden et al also noted that use of virtual technologies facilitated and promoted continued whole health implementation. The literature illustrates that: (1) massed PE can be provided with complementary education and wellness offerings, and that such offerings may increase both retention and satisfaction by enriching the massed PE treatment (eg, delivering PE-IOP); (2) whole health including wellness education and complementary interventions (eg, mindfulness, motivational enhancement) promotes well-being in both patients and mental health professionals; and (3) whole health education and complementary interventions can be delivered virtually.
Health Care Need
Prior to the implementation of a massed EBP for PTSD program at US Department of Veterans Affairs (VA) Pacific Islands Health Care System (VAPIHCS), our setting included a traditional outpatient program for treatment of PTSD and a 12- bed residential program for treatment of PTSD for male-identified (self-identified and identified as male in the electronic medical record) veterans via a cohort model with an 8- or 9-week length of stay. Both programs were located on Oahu. Thus, veterans who received care at VAPIHCS had access to PE in both outpatient and residential settings and via in-person and telehealth modalities. However, their access to PE was limited to the traditional models of PE delivery (eg, 1 or 2 session per week) and very few veterans outside of the island of Oahu had accessed PE treatment for PTSD. Moreover, when looking at PE reach within VAPIHCS, in the fiscal year prior to the implementation of the massed EBP program, only 32 of the > 5000 eligible veterans with a PTSD diagnosis had received PE. VAPIHCS serves veterans in a catchment area across the Pacific Basin which includes 3 time zones: Hawaii Standard Time (HST), Chamorro Standard Time (ChST), and Samoa Standard Time (SST). ChST is 20 hours ahead of HST, making service delivery that is inclusive for patients in Guam and Saipan especially challenging when providing care from Hawaii or other US states or territories. Given all of this, implementation of a new program offering accelerated PE virtually to any veterans with PTSD within the VAPIHCS would increase access to and reduce barriers to receiving PE.
PROGRAM DESCRIPTION
The Intensive Virtual EBP Team (iVET) for PTSD consists of an accelerated course of PE therapy and whole health education provided via VA Video Connect (VVC). iVET is a 3-week program and includes 3 parts: (1) massed individual PE therapy for PTSD; (2) group whole health and wellness classes; and (3) individual health coaching to address personal wellness goals. Programming is offered over 10-hour days to increase access across multiple time zones, especially to allow for participation in Guam and Saipan.
When a patient is referred to the iVET, their first contact is a video (or telephone) appointment with a registered nurse (RN) for a screening session. The screening session is designed to educate the patient about the program, including interventions, time commitment, and resources required for participation. In addition, following the educational discussion, the RN completes screening for safety with the patient including suicidal ideation and risk, as well as intimate partner violence risk. If urgent safety concerns are present, a licensed social worker or psychologist will join the screening to complete further assessment of risk and to address any safety concerns. Following screening, patients are scheduled for a VVC intake with a licensed therapist (social worker or psychologist) to complete the Clinician-Administered PTSD Scale (CAPS-5) for the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), a clinical interview for PTSD assessment. Patients are also sent a secure link to complete a measurement-based care (MBC) battery of self-report measures including measures assessing demographics, PTSD symptoms, anxiety symptoms, depression symptoms, substance use, quality of life (QOL), and satisfaction with mental health care. The results of the CAPS-5 and self-report measures are discussed with the patient during the intake session when planning next steps and engaging in shared decision-making. This initial VVC intake not only allows for diagnostic goodness of fit but also provides the opportunity to troubleshoot any technical difficulties the patients might have with the virtual platforms.
There are minimal exclusion criteria for participation in iVET, which include active unmanaged psychosis or manic symptoms, recent suicidal crises (attempt within 8 weeks), active nonsuicidal self-injurious behaviors (within 8 weeks), and moderate-to-severe cognitive impairment. Following intake, patients are scheduled to begin their course of care with iVET. Upon completion of intake, patients are sent program materials for their individual and group classes, asked to obtain or request a recording device, and told they will receive email links for all VVC appointments. Patients are admitted to the iVET in a rolling admission fashion, thereby increasing access when compared to closed group and/or cohort models of care.
Patients receiving care in iVET attend 2 or 3 telehealth appointments daily with practice exercises daily between telehealth sessions. The primary EBP intervention in the iVET for PTSD program is a massed or accelerated course of PE, which includes 4 primary components: psychoeducation, in-vivo exposure, imaginal exposure, and breathing retraining. Specifically, PE is delivered in 4 90-minute individual sessions weekly allowing completion of the full PE protocol, to fidelity, in 3 weeks. In addition to receiving this primary intervention, patients also participate in four 50-minute group sessions per week of a whole health and wellness education class and have access to one 30- to 60-minute session weekly of individual health coaching should they wish to set wellness goals and receive coaching in support of attaining wellness goals. During iVET, patients are invited to complete MBC batteries of selfreport measures including measures assessing PTSD symptoms, anxiety symptoms, depression symptoms, substance use, QOL, and satisfaction with mental health care at sessions 1, 5, 9, and the final session of PE. Following discharge from the iVET, patients are offered 1-month, 3-month, and 6-month individual postdischarge check-up sessions with a therapist where they are invited to complete MBC measures and review relapse prevention and maintenance of treatment gains. Likewise, they are offered 1-month, 3-month, and 6-month postdischarge check-up sessions with an RN focused on maintaining wellness gains.
The iVET for PTSD staff includes 3 therapists (psychologists or social workers) and an RN. Additionally, the iVET for PTSD is supported by a program manager and a program support assistant. The primary cost of the program is salary for staff. Additional iVET for PTSD resources included computer equipment for staff and minimal supplies. Due to the virtual environment of care, iVET staff telework and do not require physical space within VAPIHCS.
OUTCOMES
All veterans receiving care in iVET for PTSD are invited to complete a MBC at multiple timepoints including pretreatment, during PE treatment, and posttreatment. The MBC measures included self-reported demographics, a 2-item measure of satisfaction with mental health services, the Brief Addiction Monitor-Intensive Outpatient Program questionnaire,22 the Generalized Anxiety Disorder-7 scale,23, the Patient Health Questionnaire (PHQ-9),24 the QOL Enjoyment and Satisfaction Questionnaire- Short Form,25 and the PTSD Checklist for DSM-5 (PCL-5), both weekly and monthly versions. 26,27
The retention rate has averaged 81% since the iVET for PTSD opened in 2022. To date, 132 veterans have completed the iVET for PTSD program, including a full course of massed PE (Table 1). Veterans experienced reduced PTSD (P < .005), depression (P < .005), anxiety (P < .005), and substance use risk (P < .005). Veterans experienced improved QOL (P < .005) and reported high satisfaction with mental health care in iVET for PTSD (Table 2). Veterans also experienced reduced thoughts of death or suicidal ideation (SI) based on PHQ-9 item 9 responses. When looking categorically at presence or absence of SI on PHQ-9 item 9, a significant relationship was found between the absence of suicidal ideation and completion of a course of massed PE: X2 (1, N = 132) = 13.75, P < .001. In addition, veterans who completed the program showed a significant decrease in severity of SI as measured continuously (range, 0-3) on PHQ-9 item 9 (P < .005).
Another important aspect to consider when implementing massed models of EBP is the impact on employee well-being and job satisfaction. The impact of EBP on staff was assessed following the initial EBP project. To explore this further, all staff members in the iVET for PTSD were invited to engage in a small program evaluation. iVET staff were guided through a visualization meditation intended to recall a typical workday 1 month prior to starting their new position with iVET. After the visualization meditation, staff completed the Professional Quality of Life (ProQOL) scale, a 30-item, self-reported questionnaire for health care workers that evaluates compassion satisfaction, perceived support, burnout, secondary traumatic stress, and moral distress.28 One week later, staff were asked to complete the ProQOL again to capture their state after the first 6 months into their tenure as iVET staff. iVET employees experienced significantly increased perceived support (P < .05), reduced burnout (P < .05), reduced secondary traumatic stress (P < .05), and reduced moral distress (P < .05). Team members also remarked on the rewarding nature of the work and care model.
Future Directions
Future research should aim to sustain these outcomes as the iVET program continues to serve more veterans. Another important line of inquiry is longer-term follow-up, as exploring if outcomes are maintained over time is an important question that has not been answered in this article. In addition, we hope to see the accelerated model of care applied to treatment of other presenting concerns in mental health treatment (eg, anxiety, depression, insomnia). Expansion of accelerated mental health treatment into other federal and non-federal health care settings is another worthy direction. Finally, while short term (6 months) assessment of staff satisfaction in iVET was promising, ongoing assessment staff satisfaction over a longer timeframe (1-5 years) is also important.
CONCLUSIONS
PE for PTSD has been demonstrated to be effective and improve functioning and is supported by multiple clinical practice guidelines.1-5 However, as federal practitioners, we must consider the reality that many of the individuals who could benefit are not engaging in PE and there is a high dropout rate for those that do. It is vital that we envision a future state where access to PE for PTSD is equitable and inclusive, retention rates are dramatically improved, and clinicians providing PE do not experience high rates of burnout.
We must continue exploring how we can better care for our patients and colleagues. We posit that the development of programs, or tracks within existing programs, that provide massed or accelerated PE for PTSD with virtual delivery options is an imperative step toward improved care. Federal health care settings treating trauma-exposed patients with PTSD, such as those within the US Department of Defense, Indian Health Services, Federal Bureau of Prisons, and VA, are well positioned to implement programs like iVET. We believe this model of care has great merit and foresee a future where all patients seeking PTSD treatment have the option to complete an accelerated or massed course of PE should they so desire. The experiences outlined in this article illustrate the feasibility, acceptability, and sustainability of such programs without requiring substantial staffing and financial resources.
- American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. February 24, 2017. Accessed February 27, 2025. https://www.apa.org/ptsd-guideline/ptsd.pdf
- US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. Veterans Health Administration (VHA) Handbook 1160.01. September 11, 2008. Accessed February 27, 2025. https://www.mentalhealth.va.gov/providers/sud/docs/UniformServicesHandbook1160-01.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3. 2017. Accessed February 27, 2025. https://www.healthquality.va.gov/guidelines/MH/ptsd/VA-DoD-CPG-PTSD-Full-CPG-Edited-11162024.pdf
- Hamblen JL, Bernardy NC, Sherrieb K, et al. VA PTSD clinic director perspectives: How perceptions of readiness influence delivery of evidence-based PTSD treatment. Prof Psychol Res Pract. 2015;46(2): 90-96. doi:10.1037/a0038535
- Schnurr PP, Chard KM, Ruzek JI, et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2136921. doi:10.1001/jamanetworkopen. 2021.36921
- Kehle-Forbes SM, Meis LA, Spoont MR, Polusny MA. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107-114. doi:10.1037/tra0000065
- Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273. doi:10.1002/jts.21927
- Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder. Adm Policy Ment Health. 2013;40(4):311-318. doi:10.1007/s10488-012-0421-0
- Maguen S, Holder N, Madden E, et al. Evidence-based psychotherapy trends among posttraumatic stress disorder patients in a national healthcare system, 2001-2014. Depress Anxiety. 2020;37(4):356-364. doi:10.1002/da.22983
- Maguen S, Li Y, Madden E, et al. Factors associated with completing evidence-based psychotherapy for PTSD among veterans in a national healthcare system. Psychiatry Res. 2019;274:112-128. doi:10.1016/j.psychres.2019.02.027
- Foa EB, McLean CP, Zang Y, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA. 2018;319(4):354-364. doi:10.1001/jama.2017.21242
- Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration. Psychol Serv. 2023;20(3):506-515. doi:10.1037/ser0000628
- McLean CP, Foa EB. State of the Science: Prolonged exposure therapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2024;37(4):535-550. doi:10.1002/jts.23046
- McLean CP, Levy HC, Miller ML, Tolin DF. Exposure therapy for PTSD: A meta-analysis. Clin Psychol Rev. 2022;91:102115. doi:10.1016/j.cpr.2021.102115
- Wells SY, Morland LA, Wilhite ER, et al. Delivering Prolonged Exposure Therapy via Videoconferencing During the COVID-19 Pandemic: An Overview of the Research and Special Considerations for Providers. J Trauma Stress. 2020;33(4):380-390. doi:10.1002/jts.22573
- Peterson AL, Blount TH, Foa EB, et al. Massed vs intensive outpatient prolonged exposure for combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Netw Open. 2023;6(1):e2249422. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.49422
- Ragsdale KA, Nichols AA, Mehta M, et al. Comorbid treatment of traumatic brain injury and mental health disorders. NeuroRehabilitation. 2024;55(3):375-384. doi:10.3233/NRE-230235
- Rauch SAM, Yasinski CW, Post LM, et al. An intensive outpatient program with prolonged exposure for veterans with posttraumatic stress disorder: retention, predictors, and patterns of change. Psychol Serv. 2021;18(4):606-618. doi:10.1037/ser0000422
- Sherrill AM, Maples-Keller JL, Yasinski CW, Loucks LA, Rothbaum BO, Rauch SAM. Perceived benefits and drawbacks of massed prolonged exposure: qualitative thematic analysis of reactions from treatment completers. Psychol Trauma. 2022;14(5):862-870. doi:10.1037/tra0000548
- Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
- Dryden EM, Bolton RE, Bokhour BG, et al. Leaning Into whole health: sustaining system transformation while supporting patients and employees during COVID-19. Glob Adv Health Med. 2021;10:21649561211021047. doi:10.1177/21649561211021047
- Cacciola JS, Alterman AI, Dephilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treat. 2013;44(3):256-263. doi:10.1016/j.jsat.2012.07.013
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
- Kroenke K, Spi tze r RL , Wi l l i ams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire-short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744-750. doi:10.1111/j.1365-2850.2011.01735.x
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL- 5). National Center for PTSD. Updated August 29, 2023. Accessed February 27, 2025. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.pdf
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Stamm BH. The Concise ProQOL Manual. 2nd ed. Pro- QOL.org; 2010.
Evidence-based psychotherapy (EBP) for posttraumatic stress disorder (PTSD), such as prolonged exposure (PE), is supported by multiple clinical practice guidelines and is expected to be available to veterans served by the Veterans Health Administration (VHA).1-5 However, traditional models of EBP delivery with 1 or 2 sessions weekly have high dropout rates.6,7 Few veterans who could benefit from such EBPs receive them, and those who do have low completion rates.8,9 Over a 15-year period, VHA records review of > 265,500 veterans with PTSD showed only 9.1% completed EBP treatment that included but was not limited to PE.10
One empirically supported solution that has yet to be widely implemented is delivering EBPs for PTSD in a massed or accelerated format of ≥ 3 sessions weekly.11 While these massed models of EBP delivery for PTSD are promising, their implementation is limited in federal health care settings, such as the VHA.12 PE therapy is a first-line treatment for PTSD that has been evaluated in numerous clinical trials since the early 1990s and in a wide range of trauma populations.13,14 Massed PE is effective and PE has been found to be effective both in-person and via telehealth.11,15,16
Another approach to accelerated PE is the inclusion of a massed PE course within a broader treatment context that includes augmentation of the massed PE with additional services, this is referred to as an intensive outpatient model (IOP).17 PE-IOP has also been shown to be feasible, acceptable, and effective with increased completion rates in comparison to the traditional (1 or 2 sessions weekly) model of PE.12,16,18,19 Ragsdale et al describe a 2-week IOP with multiple treatment tracks, including a PTSD track. The PTSD treatment track includes massed PE and additional standard services including case management, wellness services, family services, and a single session effective behaviors group. Additional augmentation services are available when clinically indicated (eg, repetitive transcranial magnetic stimulation, transcranial direct current stimulation treatment, psychoeducation, motivational interviewing, and/or relapse prevention).17
Rauch et al studied the first 80 patients completing an IOP program that consisted of PE (5 sessions weekly) and complementary interventions (eg, mindfulness and yoga) and reported a 96% retention rate, significant reductions of self-reported PTSD symptoms, significant reduction in self-reported co-occurring depression symptoms, and significant increase in self-reported satisfaction with social functioning. 18 In another study, Sherril et al explored patient reactions to participation in massed PE (5 sessions weekly) and found that patients reported significantly more positive than negative reactions. Sherrill et al noted that according to patients, the benefits of massed PE included a structured format that limits avoidance and distraction. The resulting fast pace of progress enhanced motivation; however, drawbacks included short-term discomfort and time demands.19 Yamokoski et al explored the feasibility of massed PE in a larger study of PTSD treatment in an intensive outpatient track (IOT) in a VHA PTSD clinic with minimal staffing. The 48 patients who completed IOT PTSD treatment in 2 or 4 weeks (including 35 patients who received massed PE) had high retention rates (85%), reported high satisfaction, and had significantly reduced PTSD and depression symptoms.12
The massed IOT PE model implemented by Yamokoski et al included the primary EBP intervention of massed PE with adjunctive groups. The addition of these groups increased both retention and patient-reported satisfaction. The PE-IOP model implemented by Rauch et al and Sherrill et al also included wellness and educational groups, as well as access to complementary interventions such as mindfulness and yoga.18,19 The addition of wellness education along with a primary EBP aligned with the VHA focus on whole health well-being and wellness. The whole health approach includes understanding the factors that motivate a patient toward health and well-being, provision of health education, and providing access to complementary interventions such as mindfulness.20 Dryden et al describe the whole health transformation within VHA as a proactive approach to addressing employee and patient wellness and health. Their research found that the whole health model promoted well-being in patients and staff and was sustained even during the COVID-19 pandemic.21 Dryden et al also noted that use of virtual technologies facilitated and promoted continued whole health implementation. The literature illustrates that: (1) massed PE can be provided with complementary education and wellness offerings, and that such offerings may increase both retention and satisfaction by enriching the massed PE treatment (eg, delivering PE-IOP); (2) whole health including wellness education and complementary interventions (eg, mindfulness, motivational enhancement) promotes well-being in both patients and mental health professionals; and (3) whole health education and complementary interventions can be delivered virtually.
Health Care Need
Prior to the implementation of a massed EBP for PTSD program at US Department of Veterans Affairs (VA) Pacific Islands Health Care System (VAPIHCS), our setting included a traditional outpatient program for treatment of PTSD and a 12- bed residential program for treatment of PTSD for male-identified (self-identified and identified as male in the electronic medical record) veterans via a cohort model with an 8- or 9-week length of stay. Both programs were located on Oahu. Thus, veterans who received care at VAPIHCS had access to PE in both outpatient and residential settings and via in-person and telehealth modalities. However, their access to PE was limited to the traditional models of PE delivery (eg, 1 or 2 session per week) and very few veterans outside of the island of Oahu had accessed PE treatment for PTSD. Moreover, when looking at PE reach within VAPIHCS, in the fiscal year prior to the implementation of the massed EBP program, only 32 of the > 5000 eligible veterans with a PTSD diagnosis had received PE. VAPIHCS serves veterans in a catchment area across the Pacific Basin which includes 3 time zones: Hawaii Standard Time (HST), Chamorro Standard Time (ChST), and Samoa Standard Time (SST). ChST is 20 hours ahead of HST, making service delivery that is inclusive for patients in Guam and Saipan especially challenging when providing care from Hawaii or other US states or territories. Given all of this, implementation of a new program offering accelerated PE virtually to any veterans with PTSD within the VAPIHCS would increase access to and reduce barriers to receiving PE.
PROGRAM DESCRIPTION
The Intensive Virtual EBP Team (iVET) for PTSD consists of an accelerated course of PE therapy and whole health education provided via VA Video Connect (VVC). iVET is a 3-week program and includes 3 parts: (1) massed individual PE therapy for PTSD; (2) group whole health and wellness classes; and (3) individual health coaching to address personal wellness goals. Programming is offered over 10-hour days to increase access across multiple time zones, especially to allow for participation in Guam and Saipan.
When a patient is referred to the iVET, their first contact is a video (or telephone) appointment with a registered nurse (RN) for a screening session. The screening session is designed to educate the patient about the program, including interventions, time commitment, and resources required for participation. In addition, following the educational discussion, the RN completes screening for safety with the patient including suicidal ideation and risk, as well as intimate partner violence risk. If urgent safety concerns are present, a licensed social worker or psychologist will join the screening to complete further assessment of risk and to address any safety concerns. Following screening, patients are scheduled for a VVC intake with a licensed therapist (social worker or psychologist) to complete the Clinician-Administered PTSD Scale (CAPS-5) for the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), a clinical interview for PTSD assessment. Patients are also sent a secure link to complete a measurement-based care (MBC) battery of self-report measures including measures assessing demographics, PTSD symptoms, anxiety symptoms, depression symptoms, substance use, quality of life (QOL), and satisfaction with mental health care. The results of the CAPS-5 and self-report measures are discussed with the patient during the intake session when planning next steps and engaging in shared decision-making. This initial VVC intake not only allows for diagnostic goodness of fit but also provides the opportunity to troubleshoot any technical difficulties the patients might have with the virtual platforms.
There are minimal exclusion criteria for participation in iVET, which include active unmanaged psychosis or manic symptoms, recent suicidal crises (attempt within 8 weeks), active nonsuicidal self-injurious behaviors (within 8 weeks), and moderate-to-severe cognitive impairment. Following intake, patients are scheduled to begin their course of care with iVET. Upon completion of intake, patients are sent program materials for their individual and group classes, asked to obtain or request a recording device, and told they will receive email links for all VVC appointments. Patients are admitted to the iVET in a rolling admission fashion, thereby increasing access when compared to closed group and/or cohort models of care.
Patients receiving care in iVET attend 2 or 3 telehealth appointments daily with practice exercises daily between telehealth sessions. The primary EBP intervention in the iVET for PTSD program is a massed or accelerated course of PE, which includes 4 primary components: psychoeducation, in-vivo exposure, imaginal exposure, and breathing retraining. Specifically, PE is delivered in 4 90-minute individual sessions weekly allowing completion of the full PE protocol, to fidelity, in 3 weeks. In addition to receiving this primary intervention, patients also participate in four 50-minute group sessions per week of a whole health and wellness education class and have access to one 30- to 60-minute session weekly of individual health coaching should they wish to set wellness goals and receive coaching in support of attaining wellness goals. During iVET, patients are invited to complete MBC batteries of selfreport measures including measures assessing PTSD symptoms, anxiety symptoms, depression symptoms, substance use, QOL, and satisfaction with mental health care at sessions 1, 5, 9, and the final session of PE. Following discharge from the iVET, patients are offered 1-month, 3-month, and 6-month individual postdischarge check-up sessions with a therapist where they are invited to complete MBC measures and review relapse prevention and maintenance of treatment gains. Likewise, they are offered 1-month, 3-month, and 6-month postdischarge check-up sessions with an RN focused on maintaining wellness gains.
The iVET for PTSD staff includes 3 therapists (psychologists or social workers) and an RN. Additionally, the iVET for PTSD is supported by a program manager and a program support assistant. The primary cost of the program is salary for staff. Additional iVET for PTSD resources included computer equipment for staff and minimal supplies. Due to the virtual environment of care, iVET staff telework and do not require physical space within VAPIHCS.
OUTCOMES
All veterans receiving care in iVET for PTSD are invited to complete a MBC at multiple timepoints including pretreatment, during PE treatment, and posttreatment. The MBC measures included self-reported demographics, a 2-item measure of satisfaction with mental health services, the Brief Addiction Monitor-Intensive Outpatient Program questionnaire,22 the Generalized Anxiety Disorder-7 scale,23, the Patient Health Questionnaire (PHQ-9),24 the QOL Enjoyment and Satisfaction Questionnaire- Short Form,25 and the PTSD Checklist for DSM-5 (PCL-5), both weekly and monthly versions. 26,27
The retention rate has averaged 81% since the iVET for PTSD opened in 2022. To date, 132 veterans have completed the iVET for PTSD program, including a full course of massed PE (Table 1). Veterans experienced reduced PTSD (P < .005), depression (P < .005), anxiety (P < .005), and substance use risk (P < .005). Veterans experienced improved QOL (P < .005) and reported high satisfaction with mental health care in iVET for PTSD (Table 2). Veterans also experienced reduced thoughts of death or suicidal ideation (SI) based on PHQ-9 item 9 responses. When looking categorically at presence or absence of SI on PHQ-9 item 9, a significant relationship was found between the absence of suicidal ideation and completion of a course of massed PE: X2 (1, N = 132) = 13.75, P < .001. In addition, veterans who completed the program showed a significant decrease in severity of SI as measured continuously (range, 0-3) on PHQ-9 item 9 (P < .005).


Another important aspect to consider when implementing massed models of EBP is the impact on employee well-being and job satisfaction. The impact of EBP on staff was assessed following the initial EBP project. To explore this further, all staff members in the iVET for PTSD were invited to engage in a small program evaluation. iVET staff were guided through a visualization meditation intended to recall a typical workday 1 month prior to starting their new position with iVET. After the visualization meditation, staff completed the Professional Quality of Life (ProQOL) scale, a 30-item, self-reported questionnaire for health care workers that evaluates compassion satisfaction, perceived support, burnout, secondary traumatic stress, and moral distress.28 One week later, staff were asked to complete the ProQOL again to capture their state after the first 6 months into their tenure as iVET staff. iVET employees experienced significantly increased perceived support (P < .05), reduced burnout (P < .05), reduced secondary traumatic stress (P < .05), and reduced moral distress (P < .05). Team members also remarked on the rewarding nature of the work and care model.
Future Directions
Future research should aim to sustain these outcomes as the iVET program continues to serve more veterans. Another important line of inquiry is longer-term follow-up, as exploring if outcomes are maintained over time is an important question that has not been answered in this article. In addition, we hope to see the accelerated model of care applied to treatment of other presenting concerns in mental health treatment (eg, anxiety, depression, insomnia). Expansion of accelerated mental health treatment into other federal and non-federal health care settings is another worthy direction. Finally, while short term (6 months) assessment of staff satisfaction in iVET was promising, ongoing assessment staff satisfaction over a longer timeframe (1-5 years) is also important.
CONCLUSIONS
PE for PTSD has been demonstrated to be effective and improve functioning and is supported by multiple clinical practice guidelines.1-5 However, as federal practitioners, we must consider the reality that many of the individuals who could benefit are not engaging in PE and there is a high dropout rate for those that do. It is vital that we envision a future state where access to PE for PTSD is equitable and inclusive, retention rates are dramatically improved, and clinicians providing PE do not experience high rates of burnout.
We must continue exploring how we can better care for our patients and colleagues. We posit that the development of programs, or tracks within existing programs, that provide massed or accelerated PE for PTSD with virtual delivery options is an imperative step toward improved care. Federal health care settings treating trauma-exposed patients with PTSD, such as those within the US Department of Defense, Indian Health Services, Federal Bureau of Prisons, and VA, are well positioned to implement programs like iVET. We believe this model of care has great merit and foresee a future where all patients seeking PTSD treatment have the option to complete an accelerated or massed course of PE should they so desire. The experiences outlined in this article illustrate the feasibility, acceptability, and sustainability of such programs without requiring substantial staffing and financial resources.
Evidence-based psychotherapy (EBP) for posttraumatic stress disorder (PTSD), such as prolonged exposure (PE), is supported by multiple clinical practice guidelines and is expected to be available to veterans served by the Veterans Health Administration (VHA).1-5 However, traditional models of EBP delivery with 1 or 2 sessions weekly have high dropout rates.6,7 Few veterans who could benefit from such EBPs receive them, and those who do have low completion rates.8,9 Over a 15-year period, VHA records review of > 265,500 veterans with PTSD showed only 9.1% completed EBP treatment that included but was not limited to PE.10
One empirically supported solution that has yet to be widely implemented is delivering EBPs for PTSD in a massed or accelerated format of ≥ 3 sessions weekly.11 While these massed models of EBP delivery for PTSD are promising, their implementation is limited in federal health care settings, such as the VHA.12 PE therapy is a first-line treatment for PTSD that has been evaluated in numerous clinical trials since the early 1990s and in a wide range of trauma populations.13,14 Massed PE is effective and PE has been found to be effective both in-person and via telehealth.11,15,16
Another approach to accelerated PE is the inclusion of a massed PE course within a broader treatment context that includes augmentation of the massed PE with additional services, this is referred to as an intensive outpatient model (IOP).17 PE-IOP has also been shown to be feasible, acceptable, and effective with increased completion rates in comparison to the traditional (1 or 2 sessions weekly) model of PE.12,16,18,19 Ragsdale et al describe a 2-week IOP with multiple treatment tracks, including a PTSD track. The PTSD treatment track includes massed PE and additional standard services including case management, wellness services, family services, and a single session effective behaviors group. Additional augmentation services are available when clinically indicated (eg, repetitive transcranial magnetic stimulation, transcranial direct current stimulation treatment, psychoeducation, motivational interviewing, and/or relapse prevention).17
Rauch et al studied the first 80 patients completing an IOP program that consisted of PE (5 sessions weekly) and complementary interventions (eg, mindfulness and yoga) and reported a 96% retention rate, significant reductions of self-reported PTSD symptoms, significant reduction in self-reported co-occurring depression symptoms, and significant increase in self-reported satisfaction with social functioning. 18 In another study, Sherril et al explored patient reactions to participation in massed PE (5 sessions weekly) and found that patients reported significantly more positive than negative reactions. Sherrill et al noted that according to patients, the benefits of massed PE included a structured format that limits avoidance and distraction. The resulting fast pace of progress enhanced motivation; however, drawbacks included short-term discomfort and time demands.19 Yamokoski et al explored the feasibility of massed PE in a larger study of PTSD treatment in an intensive outpatient track (IOT) in a VHA PTSD clinic with minimal staffing. The 48 patients who completed IOT PTSD treatment in 2 or 4 weeks (including 35 patients who received massed PE) had high retention rates (85%), reported high satisfaction, and had significantly reduced PTSD and depression symptoms.12
The massed IOT PE model implemented by Yamokoski et al included the primary EBP intervention of massed PE with adjunctive groups. The addition of these groups increased both retention and patient-reported satisfaction. The PE-IOP model implemented by Rauch et al and Sherrill et al also included wellness and educational groups, as well as access to complementary interventions such as mindfulness and yoga.18,19 The addition of wellness education along with a primary EBP aligned with the VHA focus on whole health well-being and wellness. The whole health approach includes understanding the factors that motivate a patient toward health and well-being, provision of health education, and providing access to complementary interventions such as mindfulness.20 Dryden et al describe the whole health transformation within VHA as a proactive approach to addressing employee and patient wellness and health. Their research found that the whole health model promoted well-being in patients and staff and was sustained even during the COVID-19 pandemic.21 Dryden et al also noted that use of virtual technologies facilitated and promoted continued whole health implementation. The literature illustrates that: (1) massed PE can be provided with complementary education and wellness offerings, and that such offerings may increase both retention and satisfaction by enriching the massed PE treatment (eg, delivering PE-IOP); (2) whole health including wellness education and complementary interventions (eg, mindfulness, motivational enhancement) promotes well-being in both patients and mental health professionals; and (3) whole health education and complementary interventions can be delivered virtually.
Health Care Need
Prior to the implementation of a massed EBP for PTSD program at US Department of Veterans Affairs (VA) Pacific Islands Health Care System (VAPIHCS), our setting included a traditional outpatient program for treatment of PTSD and a 12- bed residential program for treatment of PTSD for male-identified (self-identified and identified as male in the electronic medical record) veterans via a cohort model with an 8- or 9-week length of stay. Both programs were located on Oahu. Thus, veterans who received care at VAPIHCS had access to PE in both outpatient and residential settings and via in-person and telehealth modalities. However, their access to PE was limited to the traditional models of PE delivery (eg, 1 or 2 session per week) and very few veterans outside of the island of Oahu had accessed PE treatment for PTSD. Moreover, when looking at PE reach within VAPIHCS, in the fiscal year prior to the implementation of the massed EBP program, only 32 of the > 5000 eligible veterans with a PTSD diagnosis had received PE. VAPIHCS serves veterans in a catchment area across the Pacific Basin which includes 3 time zones: Hawaii Standard Time (HST), Chamorro Standard Time (ChST), and Samoa Standard Time (SST). ChST is 20 hours ahead of HST, making service delivery that is inclusive for patients in Guam and Saipan especially challenging when providing care from Hawaii or other US states or territories. Given all of this, implementation of a new program offering accelerated PE virtually to any veterans with PTSD within the VAPIHCS would increase access to and reduce barriers to receiving PE.
PROGRAM DESCRIPTION
The Intensive Virtual EBP Team (iVET) for PTSD consists of an accelerated course of PE therapy and whole health education provided via VA Video Connect (VVC). iVET is a 3-week program and includes 3 parts: (1) massed individual PE therapy for PTSD; (2) group whole health and wellness classes; and (3) individual health coaching to address personal wellness goals. Programming is offered over 10-hour days to increase access across multiple time zones, especially to allow for participation in Guam and Saipan.
When a patient is referred to the iVET, their first contact is a video (or telephone) appointment with a registered nurse (RN) for a screening session. The screening session is designed to educate the patient about the program, including interventions, time commitment, and resources required for participation. In addition, following the educational discussion, the RN completes screening for safety with the patient including suicidal ideation and risk, as well as intimate partner violence risk. If urgent safety concerns are present, a licensed social worker or psychologist will join the screening to complete further assessment of risk and to address any safety concerns. Following screening, patients are scheduled for a VVC intake with a licensed therapist (social worker or psychologist) to complete the Clinician-Administered PTSD Scale (CAPS-5) for the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), a clinical interview for PTSD assessment. Patients are also sent a secure link to complete a measurement-based care (MBC) battery of self-report measures including measures assessing demographics, PTSD symptoms, anxiety symptoms, depression symptoms, substance use, quality of life (QOL), and satisfaction with mental health care. The results of the CAPS-5 and self-report measures are discussed with the patient during the intake session when planning next steps and engaging in shared decision-making. This initial VVC intake not only allows for diagnostic goodness of fit but also provides the opportunity to troubleshoot any technical difficulties the patients might have with the virtual platforms.
There are minimal exclusion criteria for participation in iVET, which include active unmanaged psychosis or manic symptoms, recent suicidal crises (attempt within 8 weeks), active nonsuicidal self-injurious behaviors (within 8 weeks), and moderate-to-severe cognitive impairment. Following intake, patients are scheduled to begin their course of care with iVET. Upon completion of intake, patients are sent program materials for their individual and group classes, asked to obtain or request a recording device, and told they will receive email links for all VVC appointments. Patients are admitted to the iVET in a rolling admission fashion, thereby increasing access when compared to closed group and/or cohort models of care.
Patients receiving care in iVET attend 2 or 3 telehealth appointments daily with practice exercises daily between telehealth sessions. The primary EBP intervention in the iVET for PTSD program is a massed or accelerated course of PE, which includes 4 primary components: psychoeducation, in-vivo exposure, imaginal exposure, and breathing retraining. Specifically, PE is delivered in 4 90-minute individual sessions weekly allowing completion of the full PE protocol, to fidelity, in 3 weeks. In addition to receiving this primary intervention, patients also participate in four 50-minute group sessions per week of a whole health and wellness education class and have access to one 30- to 60-minute session weekly of individual health coaching should they wish to set wellness goals and receive coaching in support of attaining wellness goals. During iVET, patients are invited to complete MBC batteries of selfreport measures including measures assessing PTSD symptoms, anxiety symptoms, depression symptoms, substance use, QOL, and satisfaction with mental health care at sessions 1, 5, 9, and the final session of PE. Following discharge from the iVET, patients are offered 1-month, 3-month, and 6-month individual postdischarge check-up sessions with a therapist where they are invited to complete MBC measures and review relapse prevention and maintenance of treatment gains. Likewise, they are offered 1-month, 3-month, and 6-month postdischarge check-up sessions with an RN focused on maintaining wellness gains.
The iVET for PTSD staff includes 3 therapists (psychologists or social workers) and an RN. Additionally, the iVET for PTSD is supported by a program manager and a program support assistant. The primary cost of the program is salary for staff. Additional iVET for PTSD resources included computer equipment for staff and minimal supplies. Due to the virtual environment of care, iVET staff telework and do not require physical space within VAPIHCS.
OUTCOMES
All veterans receiving care in iVET for PTSD are invited to complete a MBC at multiple timepoints including pretreatment, during PE treatment, and posttreatment. The MBC measures included self-reported demographics, a 2-item measure of satisfaction with mental health services, the Brief Addiction Monitor-Intensive Outpatient Program questionnaire,22 the Generalized Anxiety Disorder-7 scale,23, the Patient Health Questionnaire (PHQ-9),24 the QOL Enjoyment and Satisfaction Questionnaire- Short Form,25 and the PTSD Checklist for DSM-5 (PCL-5), both weekly and monthly versions. 26,27
The retention rate has averaged 81% since the iVET for PTSD opened in 2022. To date, 132 veterans have completed the iVET for PTSD program, including a full course of massed PE (Table 1). Veterans experienced reduced PTSD (P < .005), depression (P < .005), anxiety (P < .005), and substance use risk (P < .005). Veterans experienced improved QOL (P < .005) and reported high satisfaction with mental health care in iVET for PTSD (Table 2). Veterans also experienced reduced thoughts of death or suicidal ideation (SI) based on PHQ-9 item 9 responses. When looking categorically at presence or absence of SI on PHQ-9 item 9, a significant relationship was found between the absence of suicidal ideation and completion of a course of massed PE: X2 (1, N = 132) = 13.75, P < .001. In addition, veterans who completed the program showed a significant decrease in severity of SI as measured continuously (range, 0-3) on PHQ-9 item 9 (P < .005).


Another important aspect to consider when implementing massed models of EBP is the impact on employee well-being and job satisfaction. The impact of EBP on staff was assessed following the initial EBP project. To explore this further, all staff members in the iVET for PTSD were invited to engage in a small program evaluation. iVET staff were guided through a visualization meditation intended to recall a typical workday 1 month prior to starting their new position with iVET. After the visualization meditation, staff completed the Professional Quality of Life (ProQOL) scale, a 30-item, self-reported questionnaire for health care workers that evaluates compassion satisfaction, perceived support, burnout, secondary traumatic stress, and moral distress.28 One week later, staff were asked to complete the ProQOL again to capture their state after the first 6 months into their tenure as iVET staff. iVET employees experienced significantly increased perceived support (P < .05), reduced burnout (P < .05), reduced secondary traumatic stress (P < .05), and reduced moral distress (P < .05). Team members also remarked on the rewarding nature of the work and care model.
Future Directions
Future research should aim to sustain these outcomes as the iVET program continues to serve more veterans. Another important line of inquiry is longer-term follow-up, as exploring if outcomes are maintained over time is an important question that has not been answered in this article. In addition, we hope to see the accelerated model of care applied to treatment of other presenting concerns in mental health treatment (eg, anxiety, depression, insomnia). Expansion of accelerated mental health treatment into other federal and non-federal health care settings is another worthy direction. Finally, while short term (6 months) assessment of staff satisfaction in iVET was promising, ongoing assessment staff satisfaction over a longer timeframe (1-5 years) is also important.
CONCLUSIONS
PE for PTSD has been demonstrated to be effective and improve functioning and is supported by multiple clinical practice guidelines.1-5 However, as federal practitioners, we must consider the reality that many of the individuals who could benefit are not engaging in PE and there is a high dropout rate for those that do. It is vital that we envision a future state where access to PE for PTSD is equitable and inclusive, retention rates are dramatically improved, and clinicians providing PE do not experience high rates of burnout.
We must continue exploring how we can better care for our patients and colleagues. We posit that the development of programs, or tracks within existing programs, that provide massed or accelerated PE for PTSD with virtual delivery options is an imperative step toward improved care. Federal health care settings treating trauma-exposed patients with PTSD, such as those within the US Department of Defense, Indian Health Services, Federal Bureau of Prisons, and VA, are well positioned to implement programs like iVET. We believe this model of care has great merit and foresee a future where all patients seeking PTSD treatment have the option to complete an accelerated or massed course of PE should they so desire. The experiences outlined in this article illustrate the feasibility, acceptability, and sustainability of such programs without requiring substantial staffing and financial resources.
- American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. February 24, 2017. Accessed February 27, 2025. https://www.apa.org/ptsd-guideline/ptsd.pdf
- US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. Veterans Health Administration (VHA) Handbook 1160.01. September 11, 2008. Accessed February 27, 2025. https://www.mentalhealth.va.gov/providers/sud/docs/UniformServicesHandbook1160-01.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3. 2017. Accessed February 27, 2025. https://www.healthquality.va.gov/guidelines/MH/ptsd/VA-DoD-CPG-PTSD-Full-CPG-Edited-11162024.pdf
- Hamblen JL, Bernardy NC, Sherrieb K, et al. VA PTSD clinic director perspectives: How perceptions of readiness influence delivery of evidence-based PTSD treatment. Prof Psychol Res Pract. 2015;46(2): 90-96. doi:10.1037/a0038535
- Schnurr PP, Chard KM, Ruzek JI, et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2136921. doi:10.1001/jamanetworkopen. 2021.36921
- Kehle-Forbes SM, Meis LA, Spoont MR, Polusny MA. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107-114. doi:10.1037/tra0000065
- Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273. doi:10.1002/jts.21927
- Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder. Adm Policy Ment Health. 2013;40(4):311-318. doi:10.1007/s10488-012-0421-0
- Maguen S, Holder N, Madden E, et al. Evidence-based psychotherapy trends among posttraumatic stress disorder patients in a national healthcare system, 2001-2014. Depress Anxiety. 2020;37(4):356-364. doi:10.1002/da.22983
- Maguen S, Li Y, Madden E, et al. Factors associated with completing evidence-based psychotherapy for PTSD among veterans in a national healthcare system. Psychiatry Res. 2019;274:112-128. doi:10.1016/j.psychres.2019.02.027
- Foa EB, McLean CP, Zang Y, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA. 2018;319(4):354-364. doi:10.1001/jama.2017.21242
- Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration. Psychol Serv. 2023;20(3):506-515. doi:10.1037/ser0000628
- McLean CP, Foa EB. State of the Science: Prolonged exposure therapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2024;37(4):535-550. doi:10.1002/jts.23046
- McLean CP, Levy HC, Miller ML, Tolin DF. Exposure therapy for PTSD: A meta-analysis. Clin Psychol Rev. 2022;91:102115. doi:10.1016/j.cpr.2021.102115
- Wells SY, Morland LA, Wilhite ER, et al. Delivering Prolonged Exposure Therapy via Videoconferencing During the COVID-19 Pandemic: An Overview of the Research and Special Considerations for Providers. J Trauma Stress. 2020;33(4):380-390. doi:10.1002/jts.22573
- Peterson AL, Blount TH, Foa EB, et al. Massed vs intensive outpatient prolonged exposure for combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Netw Open. 2023;6(1):e2249422. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.49422
- Ragsdale KA, Nichols AA, Mehta M, et al. Comorbid treatment of traumatic brain injury and mental health disorders. NeuroRehabilitation. 2024;55(3):375-384. doi:10.3233/NRE-230235
- Rauch SAM, Yasinski CW, Post LM, et al. An intensive outpatient program with prolonged exposure for veterans with posttraumatic stress disorder: retention, predictors, and patterns of change. Psychol Serv. 2021;18(4):606-618. doi:10.1037/ser0000422
- Sherrill AM, Maples-Keller JL, Yasinski CW, Loucks LA, Rothbaum BO, Rauch SAM. Perceived benefits and drawbacks of massed prolonged exposure: qualitative thematic analysis of reactions from treatment completers. Psychol Trauma. 2022;14(5):862-870. doi:10.1037/tra0000548
- Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
- Dryden EM, Bolton RE, Bokhour BG, et al. Leaning Into whole health: sustaining system transformation while supporting patients and employees during COVID-19. Glob Adv Health Med. 2021;10:21649561211021047. doi:10.1177/21649561211021047
- Cacciola JS, Alterman AI, Dephilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treat. 2013;44(3):256-263. doi:10.1016/j.jsat.2012.07.013
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
- Kroenke K, Spi tze r RL , Wi l l i ams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire-short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744-750. doi:10.1111/j.1365-2850.2011.01735.x
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL- 5). National Center for PTSD. Updated August 29, 2023. Accessed February 27, 2025. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.pdf
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Stamm BH. The Concise ProQOL Manual. 2nd ed. Pro- QOL.org; 2010.
- American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. February 24, 2017. Accessed February 27, 2025. https://www.apa.org/ptsd-guideline/ptsd.pdf
- US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. Veterans Health Administration (VHA) Handbook 1160.01. September 11, 2008. Accessed February 27, 2025. https://www.mentalhealth.va.gov/providers/sud/docs/UniformServicesHandbook1160-01.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3. 2017. Accessed February 27, 2025. https://www.healthquality.va.gov/guidelines/MH/ptsd/VA-DoD-CPG-PTSD-Full-CPG-Edited-11162024.pdf
- Hamblen JL, Bernardy NC, Sherrieb K, et al. VA PTSD clinic director perspectives: How perceptions of readiness influence delivery of evidence-based PTSD treatment. Prof Psychol Res Pract. 2015;46(2): 90-96. doi:10.1037/a0038535
- Schnurr PP, Chard KM, Ruzek JI, et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2136921. doi:10.1001/jamanetworkopen. 2021.36921
- Kehle-Forbes SM, Meis LA, Spoont MR, Polusny MA. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107-114. doi:10.1037/tra0000065
- Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273. doi:10.1002/jts.21927
- Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder. Adm Policy Ment Health. 2013;40(4):311-318. doi:10.1007/s10488-012-0421-0
- Maguen S, Holder N, Madden E, et al. Evidence-based psychotherapy trends among posttraumatic stress disorder patients in a national healthcare system, 2001-2014. Depress Anxiety. 2020;37(4):356-364. doi:10.1002/da.22983
- Maguen S, Li Y, Madden E, et al. Factors associated with completing evidence-based psychotherapy for PTSD among veterans in a national healthcare system. Psychiatry Res. 2019;274:112-128. doi:10.1016/j.psychres.2019.02.027
- Foa EB, McLean CP, Zang Y, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA. 2018;319(4):354-364. doi:10.1001/jama.2017.21242
- Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration. Psychol Serv. 2023;20(3):506-515. doi:10.1037/ser0000628
- McLean CP, Foa EB. State of the Science: Prolonged exposure therapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2024;37(4):535-550. doi:10.1002/jts.23046
- McLean CP, Levy HC, Miller ML, Tolin DF. Exposure therapy for PTSD: A meta-analysis. Clin Psychol Rev. 2022;91:102115. doi:10.1016/j.cpr.2021.102115
- Wells SY, Morland LA, Wilhite ER, et al. Delivering Prolonged Exposure Therapy via Videoconferencing During the COVID-19 Pandemic: An Overview of the Research and Special Considerations for Providers. J Trauma Stress. 2020;33(4):380-390. doi:10.1002/jts.22573
- Peterson AL, Blount TH, Foa EB, et al. Massed vs intensive outpatient prolonged exposure for combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Netw Open. 2023;6(1):e2249422. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.49422
- Ragsdale KA, Nichols AA, Mehta M, et al. Comorbid treatment of traumatic brain injury and mental health disorders. NeuroRehabilitation. 2024;55(3):375-384. doi:10.3233/NRE-230235
- Rauch SAM, Yasinski CW, Post LM, et al. An intensive outpatient program with prolonged exposure for veterans with posttraumatic stress disorder: retention, predictors, and patterns of change. Psychol Serv. 2021;18(4):606-618. doi:10.1037/ser0000422
- Sherrill AM, Maples-Keller JL, Yasinski CW, Loucks LA, Rothbaum BO, Rauch SAM. Perceived benefits and drawbacks of massed prolonged exposure: qualitative thematic analysis of reactions from treatment completers. Psychol Trauma. 2022;14(5):862-870. doi:10.1037/tra0000548
- Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
- Dryden EM, Bolton RE, Bokhour BG, et al. Leaning Into whole health: sustaining system transformation while supporting patients and employees during COVID-19. Glob Adv Health Med. 2021;10:21649561211021047. doi:10.1177/21649561211021047
- Cacciola JS, Alterman AI, Dephilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treat. 2013;44(3):256-263. doi:10.1016/j.jsat.2012.07.013
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
- Kroenke K, Spi tze r RL , Wi l l i ams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire-short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744-750. doi:10.1111/j.1365-2850.2011.01735.x
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL- 5). National Center for PTSD. Updated August 29, 2023. Accessed February 27, 2025. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.pdf
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Stamm BH. The Concise ProQOL Manual. 2nd ed. Pro- QOL.org; 2010.
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
VA is a Leader in Mental Health and Social Service Research and Operations
VA is a Leader in Mental Health and Social Service Research and Operations
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
VA is a Leader in Mental Health and Social Service Research and Operations
VA is a Leader in Mental Health and Social Service Research and Operations
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13
Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10
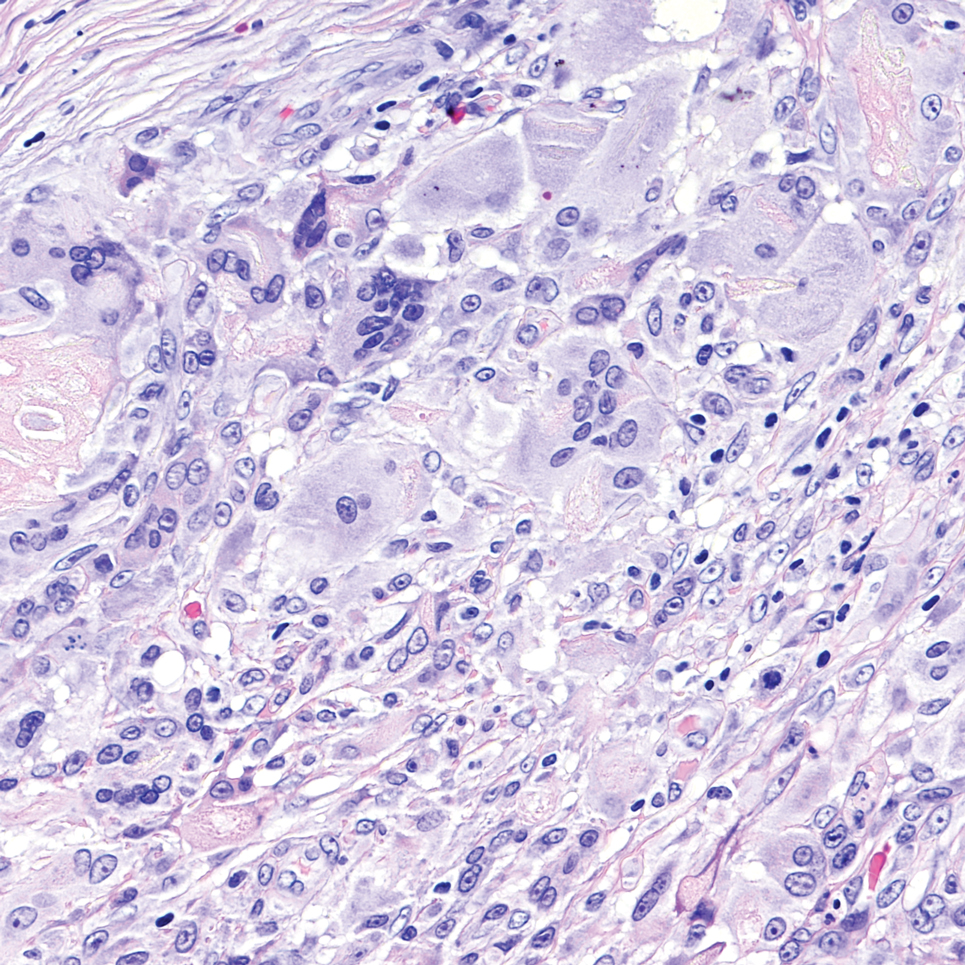
Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15
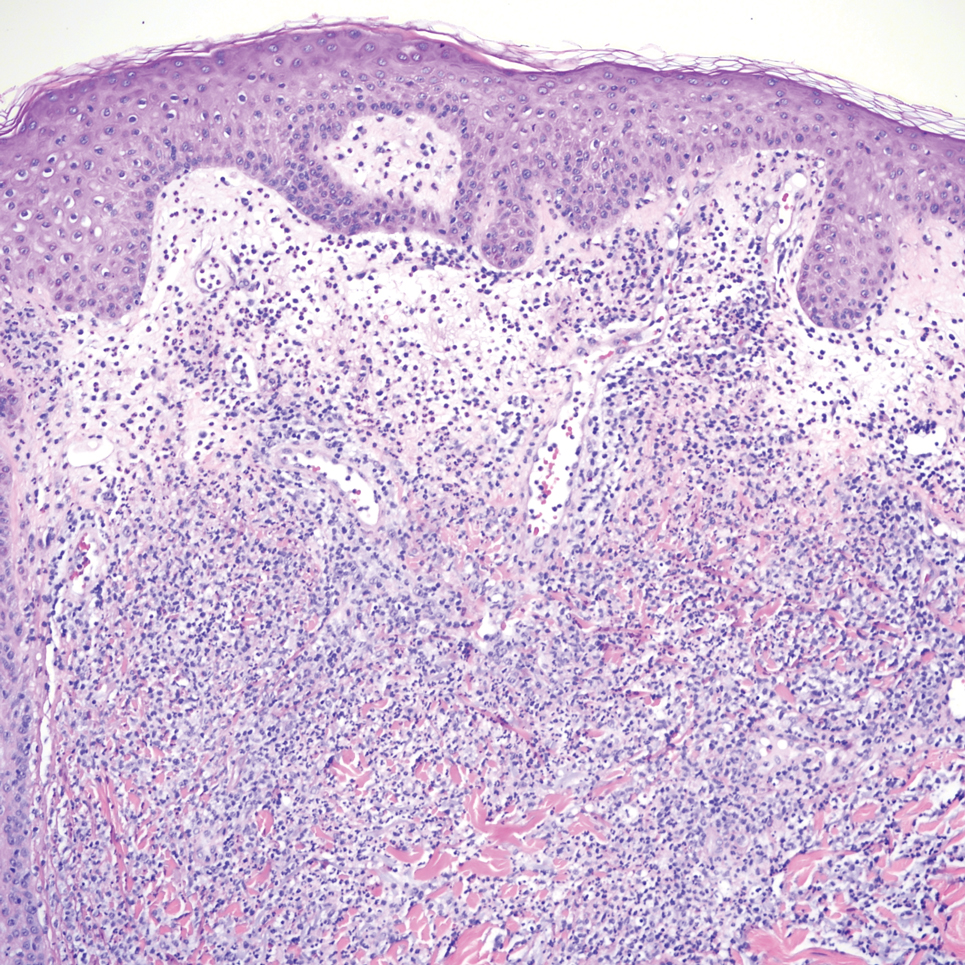
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
A 51-year-old man with a history of multiple sclerosis treated with fingolimod presented to the dermatology department with an ulcerated lesion on the left forearm of 2 to 3 months’ duration. The patient reported that he recently presented to the emergency department for drainage of the lesion, which was unsuccessful. Shortly after, he traumatized the lesion at his construction job. At the current presentation, physical examination revealed a 1-cm, flesh-colored to faintly pink, ulcerated nodule on the left forearm. A biopsy was performed.
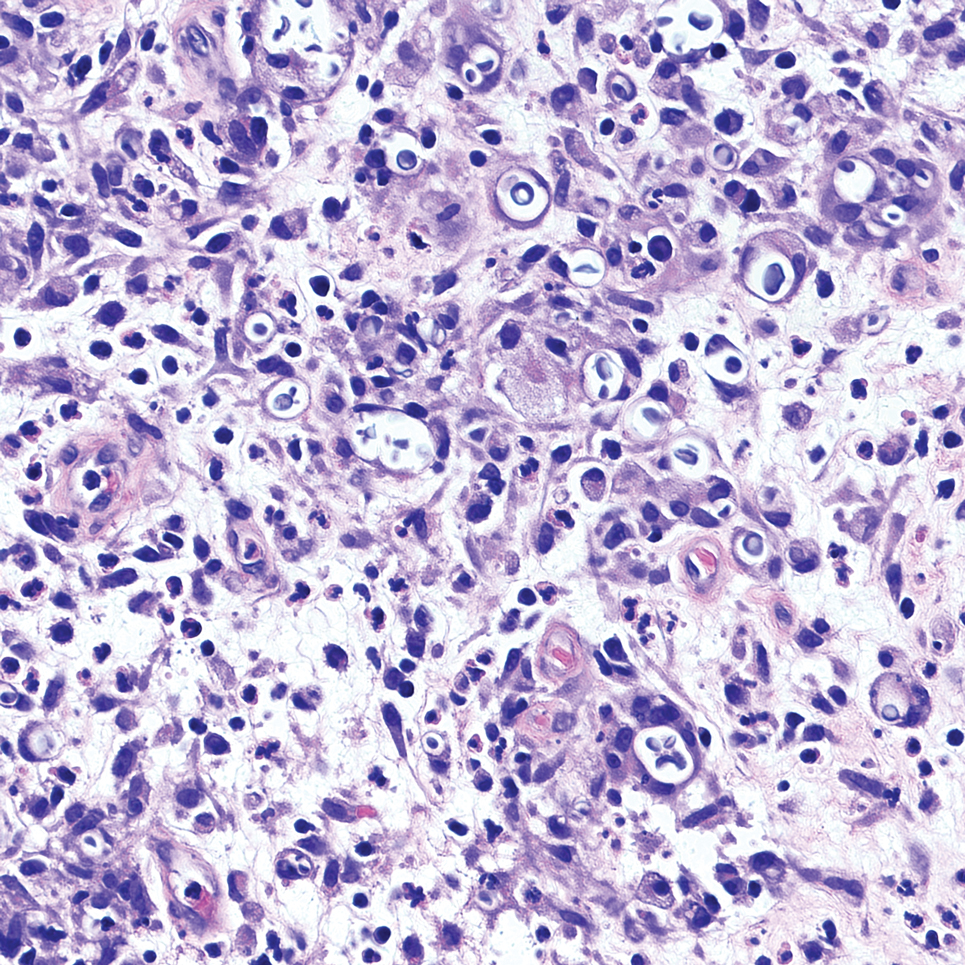
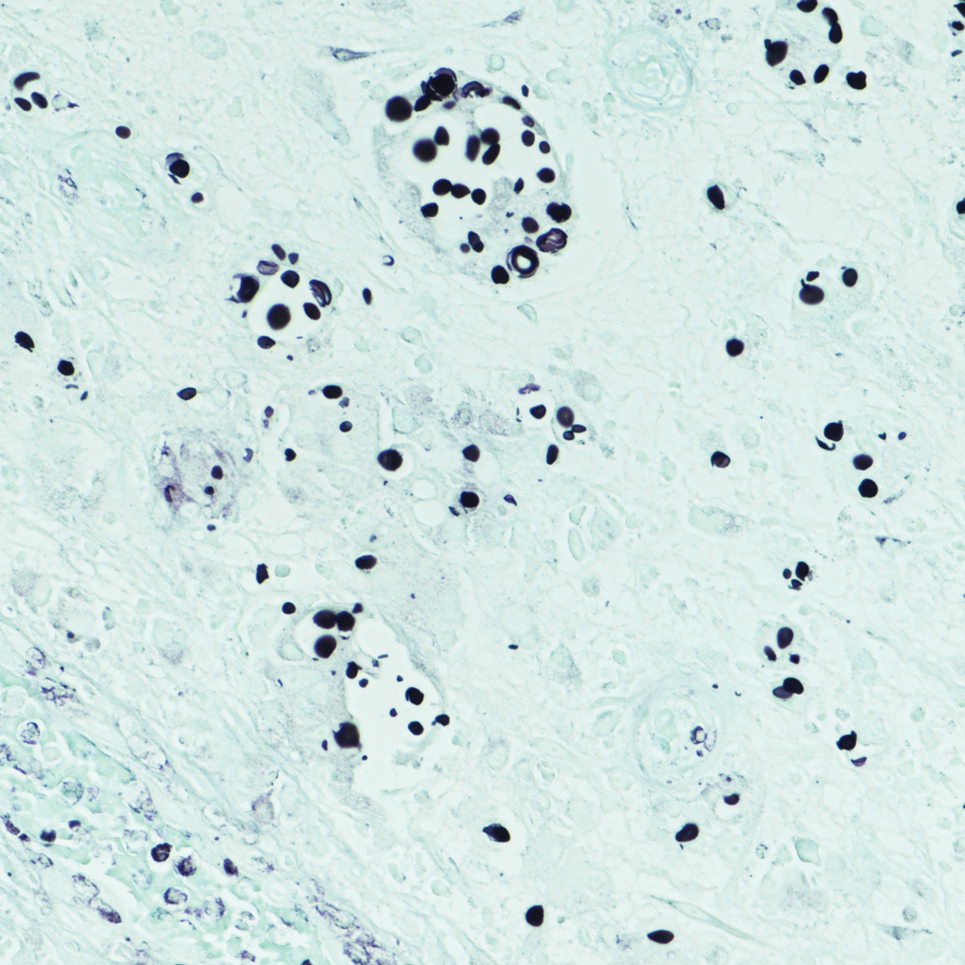
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.
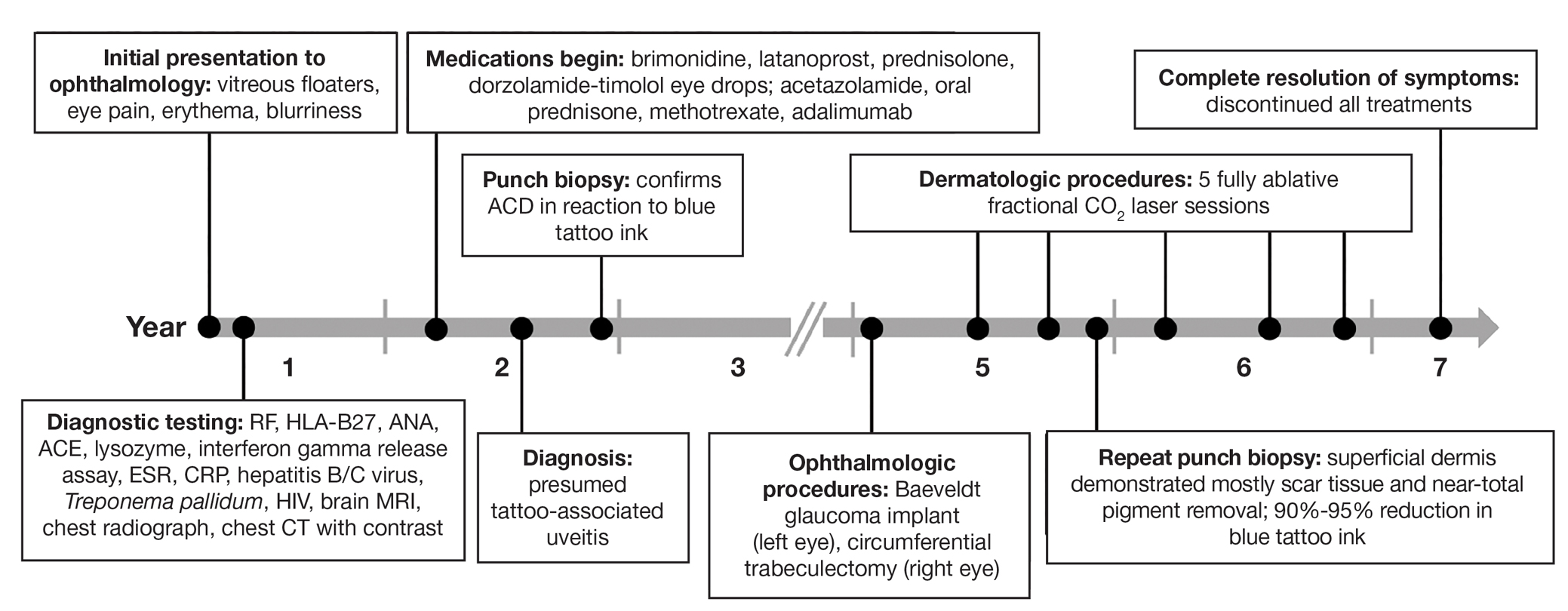
Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.
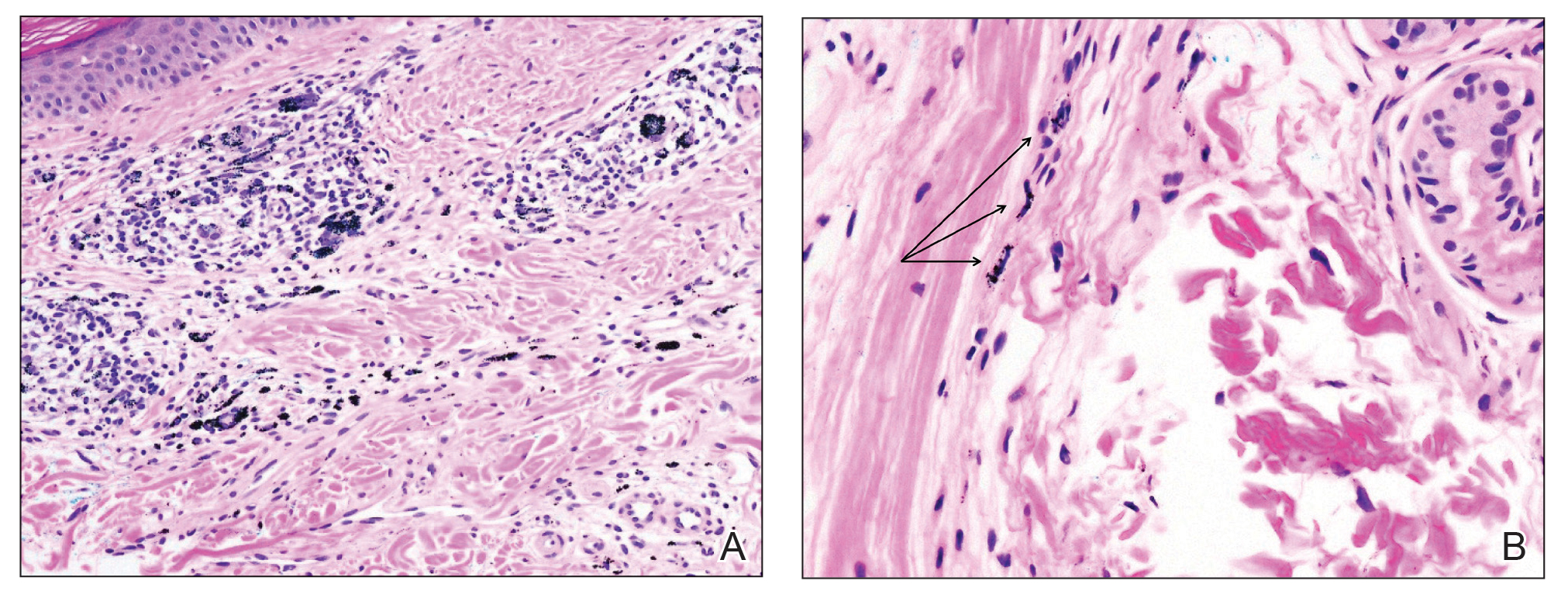

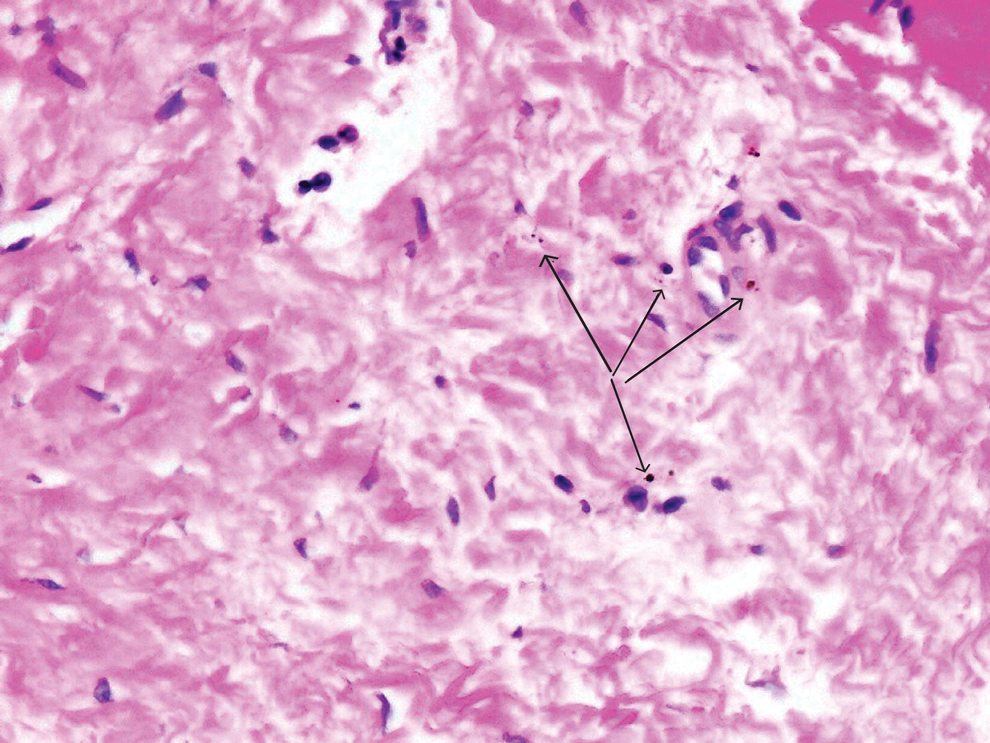
Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.

Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.



Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.

Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.



Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
PRACTICE POINTS
- Dermatologists should be aware that uveitis can develop as a delayed hypersensitivity reaction to tattoo ink, particularly in patients with blue ink tattoos.
- It is important to rule out systemic conditions such as sarcoidosis in patients presenting with uveitis and a history of tattoos.
- In a patient with progressive ocular symptoms, carbon dioxide laser ablation may be an effective treatment option if other potential rheumatologic conditions and sarcoidosis have been ruled out and other therapies have not resulted in improvement of symptoms.
- Continuous monitoring of ocular symptoms and intraocular pressure is vital to prevent complications such as glaucoma and potential blindness.